|
|
|
- Sheila Pierce
- 6 years ago
- Views:
Transcription
1 This article was originally published in a journal published by Elsevier, and the attached copy is provided by Elsevier for the author s benefit and for the benefit of the author s institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues that you know, and providing a copy to your institution s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier s permissions site at:
2 Basal ganglia oscillations and pathophysiology of movement disorders Michal Rivlin-Etzion 1,2, Odeya Marmor 1, Gali Heimer 1,4, Aeyal Raz 1,5, Asaph Nini 1,6 and Hagai Bergman 1,2,3 Low frequency rest tremor is one of the cardinal signs of Parkinson s disease and some of its animal models. Current physiological studies and models of the basal ganglia differ as to which aspects of neuronal activity are crucial to the pathophysiology of Parkinson s disease. There is evidence that neural oscillations and synchronization play a central role in the generation of the disease. However, parkinsonian tremor is not strictly correlated with the synchronous oscillations in the basal ganglia networks. Rather, abnormal basal ganglia output enforces abnormal thalamo-cortical processing leading to akinesia, the main negative symptom of Parkinson s disease. Parkinsonian tremor has probably evolved as a downstream compensatory mechanism. Addresses 1 Department of Physiology 2 The Interdisciplinary Center for Neural Computation 3 The Eric Roland Center for Neurodegenerative Diseases, The Hebrew University-Hadassah Medical School, Jerusalem, Israel, Department of Pediatrics, Hadassah Hebrew University Medical Center, Jerusalem, Israel, Department of Anesthesia, Rabin Medical Center-Beilinson Campus, Petach-Tikva, Israel 6 Department of intensive care, Division of Anesthesiology, Shiba Medical center, Tel-Hashomer, Israel Corresponding author: Bergman, Hagai (hagaibe@ekmd.huji.ac.il) Current Opinion in Neurobiology 2006, 16: This review comes from a themed issue on Motor systems Edited by Eve Marder and Peter L Strick Available online 3rd November /$ see front matter # 2006 Elsevier Ltd. All rights reserved. DOI /j.conb Introduction: Parkinson s disease clinical symptoms and pathology In 1817, almost two hundred years ago, the English physician James Parkinson wrote Essay on the Shaking Palsy, providing the first clinical description of the motor symptoms of the disease now bearing his name [1]. Today, Parkinson s disease (PD) is the most common basal ganglia movement disorder, and affects from 1% of those aged 65 to 4 5% of the 85 year old population [2]. attributed to metabolic or toxic causes either, and are, therefore, classified as idiopathic PD. PD is the result of a neurodegenerative process that causes damage to multiple neuronal circuits. The dopaminergic system is the most seriously damaged, but the noradrenergic, serotonergic and cholinergic systems are also affected [5]. On the basis of clinical observations of six patients (including two whom he met on the street and a third he observed at a distance), Parkinson described two of the most important and paradoxically related symptoms of PD: shaking now defined as a low frequency (4 7 Hz, but higher frequencies, up to 9 Hz, are encountered at early disease stages) tremor at rest (tremor amplitudes decrease during voluntary action and increase during mental stress), a hyperkinetic disorder; and palsy (or akinesia in modern terminology) characterized by a poverty of voluntary and especially involuntary movements, a hypokinetic disorder. The other cardinal motor symptoms of PD include bradykinesia (slowness of voluntary movements), rigidity (increased muscular tonus), and postural abnormalities. Cognitive and mood (emotional) deficits frequently accompany the motor symptoms. However, in this review we focus on the pathophysiology of the two main motor symptoms of PD as outlined by Parkinson: akinesia and tremor at rest. Note that we consider bradykinesia and related hypokinetic PD clinical features as akinetic symptoms. PD rigidity is characterized by a uniform resistance to passive movements owing to increased muscle response to passive stretch, and is not associated with changes of spinal alpha motor neuron excitability. Thus, and based on the clinical similarities of akinesia and rigidity as outlined below, we associate akinesia and rigidity. Finally, we limit our discussion to tremor at rest, although other non-harmonically related forms (e.g. postural and/or kinetic tremor) are very common in PD [6,7]. Akinesia versus tremor in Parkinson s disease PD is not a homogenous disease, either across patients or even within a single patient s disease course. Temporally, tremor is not a consistent feature of the disease, but rather is episodic, as opposed to akinesia. Unlike rigidity and akinesia, there is no correlation between the clinical severity of PD tremor and the severity of the dopaminergic deficit in the striatum or the clinical progression of the disease [7]. Only 5% of PD cases can be attributed to specific genetic causes [3,4]. Most of the remaining cases cannot be Human PD covers a broad spectrum of symptoms and can present as a predominant resting tremor (T-subtype) or Current Opinion in Neurobiology 2006, 16:
3 630 Motor Systems Glossary Direct indirect rate model of the basal ganglia: The circuitry of the basal ganglia is often divided into two major pathways, the direct pathway and the indirect pathway. The direct pathway directly connects the striatum to the GPi and SNr by GABAergic (inhibitory) projections. The indirect pathway connects the striatum to the GPi and SNr through the GPe and the STN, with net excitatory effects. Disinhibition: Removal of neuronal inhibition by inhibition. For example, cells in the striatum can inhibit neurons of the GPi and SNr, which in turn removes their tonic inhibition from the thalamus. Essential tremor: The most common movement disorder (10 20 times more prevalent than PD), characterized by a slowly progressive postural and/or kinetic tremor with no known cause. Negative symptoms: Normal behaviors or body states that are absent or diminished in a person with a mental or neurological disorder (e.g. akinesia of PD). Positive symptoms: These are the opposite of negative symptoms and refer to behaviors or body states that are practically absent in people in the general population but are present or enhanced in persons with the neurological disorder (e.g. PD tremor). Spectral harmonics: Spectral harmonics are other spectral peaks at frequencies equal to integer multiples of the fundamental frequency, usually as a result of distortions to the pure sinus generating the fundamental frequency. Spectral sidebands: In spectral analysis, a sideband is a band of frequencies higher or lower than the fundamental frequency, usually containing energy as a result of the amplitude modulation process. primarily as marked akinesia and rigidity (AR-subtype) [8], sometimes defined as the postural instability gait difficulty subtype. As early as 1877, the great French neurologist Jean-Martin Charcot noted that tremor is not always present in human PD patients, and, therefore, suggested changing the name of the disease from paralysis agitans (Latin for shaking palsy) to la maladie de Parkinson (Parkinson s disease). T-subtype PD patients have a better prognosis and slower disease progression than AR-subtype patients [8]. Interestingly, most patients with non-idiopathic PD display akinesia and rigidity but not rest tremor [9]. Anti-cholinergic agents, which were the first drugs available for the symptomatic treatment of PD, tend to have better effects on tremor than on akinetic-rigid symptoms, whereas akinesia might show better and earlier response to dopamine replacement therapy [10]. Several studies have indicated that the pathology of human T-type PD differs from that of the AR-type PD, with the retrorubral area (A8) more severely affected in the tremor-dominant form [11]. The frequency of tremor in a given PD patient is often remarkably similar in different muscles of the extremities and trunk [12]. These observations led to the assumption that a common single central oscillator controls all tremulous muscles. Coherence analysis, however, has shown that although the muscles within one body part (e.g. a limb) are mostly coherent, the tremor in different extremities, even on the same body side, is almost never coherent [13,14], indicating that different oscillators underlie parkinsonian tremor in the different extremities. This absence of tremor coherence could hint at mechanical or spinal reflex mechanisms rather than a single central oscillator. Nevertheless, several studies have failed to demonstrate any frequency reduction of the tremor as a result of load addition to the trembling limb in PD patients [6,7]. Resetting experiments, in which the tremulous limb is reset by mechanical perturbation, have been less conclusive. Initial studies indicated that resetting of tremor is much more easily achieved in essential tremor (see Glossary) than in PD tremor. However, more recent studies have shown that the resetting index varies significantly with the magnitude of the mechanical perturbation and with the tremor amplitude. When these factors were equalized, however, no significant difference was found in mean resetting indexes among PD tremor, essential tremor and normal subjects mimicking tremor. Resetting experiments with electrical stimulation of the median nerve or transcranial magnetic stimulation of the motor cortex did not show consistent resetting of the tremor rhythm when the periphery (median nerve) was stimulated, but did result in complete resetting when the cortex was stimulated [7]. In line with the central nervous system (CNS) hypothesis on the origin of PD tremor, it has long been known that different lesions within the CNS can suppress parkinsonian tremor. Early attempts to remove parts of the motor cortex or its downstream projections were successful in suppressing tremor but produced unacceptable side effects. The cerebellar receiving nuclei of the thalamus (e.g. the ventralis-intermedius, Vim) have traditionally been considered the optimal target for stereotaxic procedures for amelioration of PD and other tremors. Recently, it has been demonstrated that chronic highfrequency stimulation of these same thalamic targets, in addition to subthalamic or pallidal stimulations, are all able to efficiently suppress parkinsonian tremor and other motor symptoms [15 ]. In summary, most clinical human studies indicate that PD tremor and akinesia, although they share common origins and similarities, have significantly distinct characteristics. The role of striatal dopamine depletion and the central generators seem to be much more important in akinesia. PD tremor might be modulated by peripheral manipulation and by the activity of other central neuronal systems. It is possible that transmitter systems other than dopamine (e.g. cholinergic, serotonergic), or neural circuits other than the basal ganglia (e.g. cerebellum [16], red nucleus), play a crucial additive role in underlying this symptom. Parkinson s disease animal models Early animal models of PD were based on lesions of midbrain areas in monkeys. These anatomical lesions mainly produce rigidity and only rarely result in a spontaneous, sustained tremor. Careful analysis of the correlation between the clinical symptoms and the extent of the lesion led to the conclusion that experimental rest tremor Current Opinion in Neurobiology 2006, 16:
4 Basal ganglia oscillations and pathophysiology of movement disorders Rivlin-Etzion, Marmor, Heimer, Raz, Nini and Bergman 631 is the result of damage to both the nigro-striatal dopaminergic projections and the cerebellar outflow (to the red nucleus and thalamus). Damage to only one of these neuronal systems was not sufficient for reliable generation of tremor [17]. More modern animal models of PD have shifted from anatomical to chemical lesions. Early chemical animal models of PD for example, the 6-hydroxydopamine (6- OHDA) model were limited to dopaminergic damage, and mainly reproduced the main negative symptoms of PD; namely, akinesia (see Glossary) [18]. The more recently introduced primate 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) model of PD [19] better mimics the clinical and the pathological picture of PD. Post-mortem examination of the brains of MPTP-treated primates reveals that the primary damage is to the dopaminergic system. However, as in human PD, other neuromodulators are also affected [20]. Monkeys treated with MPTP mainly exhibit the akinetic rigid symptoms of PD [19]. Low frequency (4 7 Hz) resting tremor is not readily replicated in MPTP-treated macaque monkeys, but other species, notably the vervet (African green) monkey, often develop a prominent lowfrequency tremor following MPTP injections [21,22]. It is important to note that the tremor usually appears several days after the development of clinical akinesia and rigidity [21,23 ]. This reversed order of presentation of clinical symptoms compared with that of the human disease could be due to the fast induction of dopamine depletion in the MPTP model that might impede the development of compensatory processes found in the slow-evolving human disease. Yet, tremor is a much more overt phenomenon than akinesia and rigidity. A human patient or his/her family might first be made aware of the slow development of PD by the more easily recognizable tremor. As in human studies, there is a low coherence between the tremors of the limbs of MPTP-treated vervet monkeys following dopamine replacement therapy [23 ]. Basal ganglia anatomy The cumulative clinical and experimental evidence outlined above strongly indicates that the major pathological event leading to the motor symptoms of PD, and especially to akinesia, is the death of midbrain dopaminergic neurons and their striatal projections. The striatum (composed of caudate, putamen and ventral striatum) is the main input stage of the basal ganglia, receiving inputs from all cortical areas, from many thalamic nuclei and even from the cerebellum [24 ]. Therefore, a good grasp of the pathophysiology of PD depends on understanding the anatomy and physiology of the basal ganglia and dopamine networks. disorders of motor function explains why the basal ganglia were classified as part of the extra-pyramidal system. The pyramidal system starts at the motor cortices, and through the brainstem pyramids projects to a-motoneurons, innervating the distal parts of the limb, and controlling the execution of accurate and voluntary movements. By contrast, it was assumed that the extra-pyramidal system originated at the basal ganglia and the cerebellum, descended parallel to the pyramidal system, and innervated the spinal circuits involved with more axial (postural), automatic non-voluntary movements. The revolution in anatomical methods during the second half of the 20 th century led researchers to the conclusion that the basal ganglia are part of a closed loop connecting all cortical areas sequentially through the striatum, pallidum and thalamus with the frontal cortex (Figure 1). The frontal cortex projects downstream to the spinal level. The direct projection of the basal ganglia to upper brainstem nuclei (e.g. superior colliculus and peduncolopontine nucleus [16]) will be considered here, for the sake of simplicity, as part of this descending system. The new view of the basal ganglia networks assumes that there are two segregated internal pathways that start in the striatum and converge on the output structures of the basal ganglia (the internal segment of the globus pallidus [GPi] and the substantia nigra pars reticulata [SNr]). The direct pathway is a direct GABAergic inhibitory pathway, whereas the indirect pathway is a polysynaptic disinhibitory pathway (see Glossary) through the external segment of the globus pallidus (GPe) and the subthalamic nucleus (STN). The projection striatal neurons in the direct pathway express D1 dopamine receptors, whereas those in the indirect pathway express D2 dopamine receptors [25]. Midbrain dopamine (DA) has differential effects on the two striato-pallidal pathways: it facilitates transmission along the direct pathway through the D1 receptors and inhibits transmission along the indirect pathway through the D2 receptors [25,26]. Note that in this schematic description the crucial roles of both the cholinergic and the dopaminergic innervation of the striatum on Figure 1 The realization, at the turn of the 20 th century, that lesions involving the basal ganglia often result in severe Schematic view of the recurrent connectivity among the cortex, basal ganglia and muscle networks. Current Opinion in Neurobiology 2006, 16:
5 632 Motor Systems plasticity and learning in the cortico-striatal synapse have been neglected [27,28 ]. Recently, single axon tracing anatomical studies have revealed an even more complex map of basal ganglia connectivity. Striatal neurons projecting to GPi and SNr send collaterals to GPe [29,30]. The physiological evidence for the importance of the direct projections from the motor cortex to the STN (the hyper-direct pathway [31]) indicate that like the striatum, the STN is an input stage of the basal ganglia [32]. Moreover, the recently described feedback projections from the GPe to the striatum [33,34], in addition to the GPe to GPi projection, strongly suggest that the GPe is a central nucleus in the basal ganglia circuitry, rather than a simple relay station in the indirect pathway. Figure 2 summarizes the current view of the complex connectivity among the basal ganglia nuclei. Figure 2 Physiological studies of the basal ganglia in normal primates Single unit recording and analysis of neuronal activity at the level of single spikes of single cells are probably the main ways to study a neuronal network. The different nuclei of the basal ganglia have a diverse background (during a quiet, awake state) spiking activity. The striatal neurons are characterized by a low frequency discharge rate (<1 spikes/s by the projection neurons and 4 10 spikes/s by the tonically active neurons [TANs], the cholinergic interneurons). This slow discharge is striking in contrast to the high (50 80 spikes/s) frequency discharge of the pallidal and SNr neurons. In all these structures the firing rate is irregular (Poisson-like), and neuronal oscillations are seldom observed in normal awake subjects. Studies exploring the relationship between spiking activity of basal ganglia neurons and body movements have revealed even more unexpected results. The akinesia associated with PD suggests that the basal ganglia play a crucial role in movement initiation. Nevertheless, most basal ganglia neurons change their firing rate after initiation of movements (Putamen: [35,36], GP: [37 39], SNr: [40]), and do not have any exclusive or consistent relationship to movement parameters such as start and/or end, velocity or amplitude [41]. Taken together with the minor impairment of motor control following focal inactivation and lesions of the output structures of the basal ganglia [42 44], the physiological results lead to the surprising conclusion that the basal ganglia do not initiate movements [42,45]. Physiological studies in the dopaminedepleted basal ganglia networks Early physiological studies of parkinsonian MPTP-treated monkeys reported changes in the discharge rate within the GPe, GPi [46,47] and STN [21]. Reversed trends of pallidal discharge rates in response to dopamine replacement therapy have been reported in both human patients [48,49] and primates [23,50,51]. The crucial role of these rate changes in the pathophysiology of PD has been verified by the subsequent findings showing that inactivation of STN and GPi could improve the motor symptoms in parkinsonian animals [52] and in human patients [15 ]. These findings contributed to the formulation and the popularity of the direct indirect model of the basal ganglia (see Glossary) [26]. Nevertheless, several studies have failed to find the expected significant changes of firing rates in the pallidum [53], thalamus [54] or motor cortical areas [55] of MPTP monkeys. This and other inconsistencies with the assumptions and the predictions of the direct indirect rate model have attracted more attention to the potential roles of other aspects of neuronal activity, such as firing patterns and neuronal synchronization, in Detailed linear view of the connectivity of the cortex, basal ganglia and muscle networks. Color coding as in Figure 1. The basal ganglia inter-connectivity is shown in detail, with gray arrows representing excitatory glutamatergic projections and black round-head arrows for inhibitory GABAergic projections. Abbreviations: GPe, external segment of the globus pallidus; GPi, internal segment of the globus pallidus; STN, subthalamic nucleus. Current Opinion in Neurobiology 2006, 16:
6 Basal ganglia oscillations and pathophysiology of movement disorders Rivlin-Etzion, Marmor, Heimer, Raz, Nini and Bergman 633 the pathophysiology of PD. MPTP monkeys show an increase in the fraction of basal ganglia neurons that discharge in bursts. These bursts are either irregular or oscillatory and have been found in STN, GPe, GPi and primary motor cortex [21,22,46,47,55,56,57 ]. In most cases, the cells tend to oscillate at the tremor frequency or at double or even triple the tremor frequency [21]. Nevertheless, these studies repeatedly failed to reveal neurons with oscillations that are consistently coherent with the tremor [22,23 ]. Both STN inactivation [58] and dopamine replacement therapy [23 ] ameliorate the 4 7 Hz tremor and reduce the GPi 8 20 Hz oscillations, supporting the crucial role of double rather than the tremor frequency oscillations in tremor generation. Physiological studies and cross-correlation analysis of the activity of simultaneously recorded neurons in the basal ganglia and cortex of monkeys before and after MPTP treatment enable the assessment of the changes in neural synchronization in these areas. These studies have been conducted in the pallidum [22], as well as in the primary motor cortex [55], among striatal TANs and between TANs and pallidal neurons [59,60]. Cross correlation functions become peaked and oscillatory following MPTP treatments, suggesting that striatal dopamine depletion induces abnormal coupling of basal ganglia loops. The abnormal pallidal synchronization decreases in response to dopamine replacement therapy [23 ]. In most cases, the maximal power of the synchronous oscillations was at double the tremor frequency [22,23,59,60]. As in the MPTP primate, single unit studies of the basal ganglia of human PD patients (performed during electrophysiological mapping of the target area for therapeutic implantation of stimulating electrodes) report a high fraction of GPi cells oscillating at the tremor frequency [61]. However, as in the primate, the human studies [62,63 ] show that these oscillations are not fully coherent with the simultaneous recorded tremor. The sharp contrast between this transient, inconsistent pallidal-tremor synchronization and the high synchronicity found between thalamic Vim neurons and the tremor [64] suggests that pallidal neurons cannot be viewed as the tremor generators, or as reflecting the proprioceptive feedback of the tremor. Physiological studies of population neural activity in the dopamine-depleted basal ganglia networks Synchronization of basal ganglia neuronal activity is also evident in the local field potentials (LFPs) recorded in the subthalamic region of PD patients by the macroelectrodes used for high-frequency stimulation of these structures. These oscillations occur mainly in the b range (15 30 Hz) and following treatment with levodopa shift to higher frequencies in the gamma range [65 ]. Another study found that a significant reduction of the b range oscillations preceded the onset of the rest tremor [66 ]. In line with both the single unit and the LFP studies, magnetoencephalographic (MEG) studies [67] of T-type PD patients have revealed a strong coherence between the tremor and the activity in the motor and sensory cortices and the cerebellum at tremor frequency, and an even stronger coherency at double tremor frequency. Spectra of coherence between thalamic activity and cerebellum as well as between other brain areas have revealed additional broad peaks around 20 Hz. Studies of LFPs recorded from frontal cortex and STN of rats following 6-OHDA lesions of midbrain dopamine neurons [68] have revealed significant increases in the power and coherence of b-frequency oscillatory activity. Administration of apomorphine, the dopamine receptor agonist, to these dopamine-depleted animals suppressed the b-frequency oscillations, and increased coherent activity at gamma frequencies in the cortex and STN. Thus, the pattern of synchronization between population activity in the STN and that in the cortex in the 6- OHDA-lesioned rodent model of PD is parallel to that seen in the parkinsonian human. Recordings of both LFPs and multi-neuronal activity from microelectrodes inserted into STN in PD patients during functional neurosurgery suggest that the discharges of some of the neurons in STN are locked to b oscillations in the LFP [69]. LFP probably represents the synaptic input to a neural structure and its subthreshold slow activity. The discrepancies between LFP oscillatory activity and neuronal activity (in their frequency domain, prevalence and power) are probably due to the fact that even quite strong synchronized inputs can lead to weak neuronal correlations. Alternatively, correlations can be very low at the single-unit level, but still sum and become substantial at the population (LFP) level [70,71]. On correlation, causality and harmonics in spectral analysis Basal ganglia researchers are aware that depletion of dopamine in the striatum (the input stage of the basal ganglia) is the major event leading to clinical symptoms, including tremor, of PD. It is, thus, tempting to assume a causal relationship between the neural oscillations that are found in the STN and the globus pallidus (output stage of the basal ganglia) and the tremor. However, the closed loop structure of the cortex basal ganglia muscle networks (Figure 1), including inputs from other structures (e.g. cerebellum), suggests that basal ganglia oscillations are the result of proprioceptive feedback to the basal ganglia. The high-level energy at double the tremor frequency in many single unit correlation studies [22,59] and MEG studies [67] might suggest a 2:1 filtering mechanism downstream of the basal ganglia output [72,73]. However, there are important limitations to spectral analysis Current Opinion in Neurobiology 2006, 16:
7 634 Motor Systems [66,74 76]. In particular, any non-linear transformation (e.g. the addition of a derivative or high-pass filtered version of the original signal) might add higher harmonics (see Glossary) to the original peak. The amplitude of these higher harmonics can be larger than that of the original peak (Figure 3). Amplitude fluctuation of the tremor or neuronal analog signal (such as LFP) could add sidebands (see Glossary) both above and below the central frequency peak. Moreover, if the amplitude and the frequency fluctuation follow a complex pattern, and enough spectra are averaged (as is the common practice), the additional peaks can be distributed out over a broad frequency domain [77]. Thus, although neuronal oscillations can be detected at several levels of the basal ganglia network subsequent to dopamine depletion and the emergence of clinical PD tremor, there is not enough Figure 3 evidence to support the notion that the tremor follows the basal ganglia oscillations. Conclusions and future directions In this review we have explored the possible relationships between basal ganglia oscillatory activity and PD tremor. PD is the result of dopamine depletion in the striatum the input stage of the basal ganglia. Akinesia and rest tremor are two major symptoms of PD. Nevertheless, cumulative clinical and experimental evidence support the view that akinesia and rest tremor are not generated by identical neuronal mechanisms. Following striatal dopamine depletion, many basal ganglia neurons develop synchronous oscillations at the tremor frequency and at their higher harmonics in addition to in the b range. However, the PD tremor does not strictly follow the basal ganglia Simulated analog signals and their power spectrums. The analog signal (left column) is composed of a pure sinus wave plus its derivative (cosine function with the same frequency). K1 and K2 are scaling factors of the relative weights of the sinus and its derivate (cosine) function. The scaling factors were chosen to normalize all peak-to-peak amplitudes to the same value. Current Opinion in Neurobiology 2006, 16:
8 Basal ganglia oscillations and pathophysiology of movement disorders Rivlin-Etzion, Marmor, Heimer, Raz, Nini and Bergman 635 oscillatory activity. The recent demonstration of anatomical connections between the cerebellum and the basal ganglia, at the cortical, striatal and brainstem levels might suggest that the cerebellum is associated with the movement disorders classically described as basal ganglia disorders. The crucial role of cerebellar output in the generation of PD tremor has been demonstrated by lesion studies and the efficacy of Vim intervention in treatment of PD tremor. These findings, along with the physiological studies of the normal basal ganglia indicating that the basal ganglia do not initiate movements, strengthen the supposition that the abnormal synchronous oscillations in the basal ganglia provide noisy input to the frontal cortex, and hence lead to PD akinesia. In contrast to the akinesia, we suggest that PD tremor (Parkinson s shaking ) is the result of compensation mechanisms generated downstream of the basal ganglia in order to compensate for PD akinesia ( palsy ). This hypothesis is in accordance with the fact that T-subtype PD is slower to progress than AR-subtype PD, and with the late appearance of the tremor in the MPTP-treated monkeys. Moreover, the peripheral feedback from the tremulous body segments might interrupt the noisy activity of the basal ganglia cortical networks in a similar manner to subcortical DBS or lesions. Future studies of the complex neural network of the basal ganglia and their related neuronal structures will hopefully shed more light on their role and function in health and disease. Acknowledgements This research was supported in part by a Center of Excellence grant from the Israel Science Foundation (ISF) and the Fighting against Parkinson Foundation of the Hebrew University Netherlands Association (HUNA). We thank E Singer for critical reading and language editing. References and recommended reading Papers of particular interest, published within the annual period of review, have been highlighted as: of special interest of outstanding interest 1. Parkinson J: An essay on the shaking palsy. London: Sherwood, Neely and Jones; Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM: Incidence of Parkinson s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 2003, 157: Farrer MJ: Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet 2006, 7: An excellent review of the current view of the complex, multi-factorial heritable basis of PD. 4. Benmoyal-Segal L, Soreq H: Gene environment interactions in sporadic Parkinson s disease. J Neurochem 2006, 97: Jellinger KA: Pathology of Parkinson s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol 1991, 14: Elble RJ, Koller WC: Tremor. The Johns Hopkins University Press; Deuschl G, Raethjen J, Baron R, Lindemann M, Wilms H, Krack P: The pathophysiology of parkinsonian tremor: a review. J Neurol 2000, 247(Suppl 5):V33-V Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I et al.: Variable expression of Parkinson s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 1990, 40: Rajput AH: Clinical features of tremor in extrapyramidal syndromes. In Handbook of Tremor Disorders. Edited by Findley LJ, Koller WC. Marcel Dekker, Inc.; 1995: Tolosa ES, Marin C: Medical management of parkinsonian tremor. In Handbook of Tremor Disorders. Edited by Findley LJ, Koller WC. Marcel Dekker, Inc.; 1995: Paulus W, Jellinger K: The neuropathologic basis of different clinical subgroups of Parkinson s disease. J Neuropathol Exp Neurol 1991, 50: Hunker CJ, Abbs JH: Uniform frequency of parkinsonian resting tremor in the lips, jaw, tongue, and index finger. Mov Disord 1990, 5: Raethjen J, Lindemann M, Schmaljohann H, Wenzelburger R, Pfister G, Deuschl G: Multiple oscillators are causing parkinsonian and essential tremor. Mov Disord 2000, 15: Ben-Pazi H, Bergman H, Goldber JA, Giladi N, Hansel D, Reches A, Simon ES: Synchrony of rest tremor in multiple limbs in Parkinson s disease: evidence for multiple oscillators. J Neural Transm 2001, 108: Machado A, Rezai AR, Kopell BH, Gross RE, Sharan AD, Benabid AL: Deep brain stimulation for Parkinson s disease: Surgical technique and perioperative management. Mov Disord 2006, 21:S247-S258. An updated description of surgical techniques and patient management during deep brain stimulation (DBS) surgery for advanced PD. Since 1993, there have been more than DBS implants at more than 500 medical centers worldwide. This is part of a comprehensive review composed of nine manuscripts written by international experts in the field, published as Supplement 14 to volume 21 of Movement Disorders. 16. Stein JF, Aziz TZ: Does imbalance between basal ganglia and cerebellar outputs cause movement disorders? Curr Opin Neurol 1999, 12: Jenner P, Marsden CD: Neurochemical basis of parkinsonian tremor. In Movement disorders: tremor. Edited by Findley LJ, Capildeo R. Oxford University Press; 1984: Wilms H, Sievers J, Deuschl G: Animal models of tremor. Mov Disord 1999, 14: Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ: A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6- tetrahydropyridine. Proc Natl Acad Sci USA 1983, 80: Pifl C, Schingnitz G, Hornykiewicz O: Effect of 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience 1991, 44: Bergman H, Wichmann T, Karmon B, DeLong MR: The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol 1994, 72: Raz A, Vaadia E, Bergman H: Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci 2000, 20: Heimer G, Rivlin-Etzion M, Bar-Gad I, Goldberg JA, Haber SN, Bergman H: Dopamine replacement therapy does not restore the full spectrum of normal pallidal activity in the 1-methyl-4- phenyl-1,2,3,6-tetra-hydropyridine primate model of parkinsonism. J Neurosci 2006, 26: A recent study of the tremor, synchronous oscillations and bursts in the GPe and GPi of normal and MPTP-treated rhesus and vervet monkeys. 24. Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL: The cerebellum communicates with the basal ganglia. Nat Neurosci 2005, 8: Using the novel technique of transneuronal transport of the rabies virus, this manuscript describes a newly discovered communication pathway Current Opinion in Neurobiology 2006, 16:
9 636 Motor Systems from the cerebellum to the input stage of the basal ganglia. Previous studies by the same group revealed interactions between the cerebellum and the basal ganglia output at the level of the frontal cortex, and other studies have suggested such an interaction at the level of the brainstem. The novel findings of this study reveal that close association between the basal ganglia and the cerebellum also exists at the level of the input stage of the basal ganglia the striatum. 25. Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, Sibley DR: D1 and D2 dopamine receptorregulated gene expression of striatonigral and striatopallidal neurons. Science 1990, 250: Albin RL, Young AB, Penney JB: The functional anatomy of basal ganglia disorders. Trends Neurosci 1989, 12: Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ: Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron 2006, 50: Many previous studies have shown that activation of dopamine receptors can induce long-term potentiation (LTP) or long-term depression (LTD) in the cortico-striatal synapses. However, the role of dopamine D1 and D2 receptors in cortico-striatal plasticity was considered incompatible with their differential distribution to the direct and indirect pathway striatal neurons. Wang et al. show that the D2 dependence of LTD is not mediated by dopamine receptors of the striatal projection medium-spiny neurons. Rather, this plasticity is achieved through dopamine-induced changes in the release of acetylcholine by the cholinergic interneurons of the striatum. Besides providing a coherent hypothesis for the role of D1 and D2 dopamine receptors in cortico-striatal plasticity, this paper highlights the crucial role played by the cholinergic interneurons in the physiology of the striatum. 28. Graybiel AM: The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol 2005, 15: An excellent review of the current models and views on the role of the basal ganglia in reinforcement learning. A short review of current models of the basal ganglia is followed by a series of queries facing this field. 29. Levesque M, Parent A: The striatofugal fiber system in primates: a reevaluation of its organization based on single-axon tracing studies. Proc Natl Acad Sci USA 2005, 102: Neurons located in either striosomes or the extrastriosomal matrix in squirrel monkeys were injected with biotin dextran amine, and their labeled axons were entirely reconstructed with a camera lucida. 24 of 27 reconstructed axons arborized into the three main striatal targets (GPe, GPi and SNr), in sharp contrast with the predictions of the direct indirect model of the basal ganglia circuitry. Future studies should reveal whether or how the key concept of the direct or indirect basal ganglia, originally described in rodent studies, should be incorporated with these new primate findings. 30. Nadjar A, Brotchie JM, Guigoni C, Li Q, Zhou SB, Wang GJ, Ravenscroft P, Georges F, Crossman AR, Bezard E: Phenotype of striatofugal medium spiny neurons in parkinsonian and dyskinetic nonhuman primates: a call for a reappraisal of the functional organization of the basal ganglia. J Neurosci 2006, 26: Nambu A: A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res 2004, 143: Feger J, Bevan M, Crossman AR: The projections from the parafascicular thalamic nucleus to the subthalamic nucleus and the striatum arise from separate neuronal populations: a comparison with the corticostriatal and corticosubthalamic efferents in a retrograde fluorescent double- labelling study. Neuroscience 1994, 60: Bolam JP, Hanley JJ, Booth PA, Bevan MD: Synaptic organisation of the basal ganglia. J Anat 2000, 196: Koos T, Tepper JM: Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 1999, 2: Crutcher MD, DeLong MR: Single cell studies of the primate putamen. II. Relations to direction of movement and pattern of muscular activity. Exp Brain Res 1984, 53: Alexander GE, Crutcher MD: Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol 1990, 64: DeLong MR: Activity of pallidal neurons during movement. J Neurophysiol 1971, 34: Anderson ME, Horak FB: Influence of the globus pallidus on arm movements in monkeys. III. Timing of movement-related information. J Neurophysiol 1985, 54: Mink JW, Thach WT: Basal ganglia motor control. II. Late pallidal timing relative to movement onset and inconsistent pallidal coding of movement parameters. J Neurophysiol 1991, 65: Schultz W: Activity of pars reticulata neurons of monkey substantia nigra in relation to motor, sensory, and complex events. J Neurophysiol 1986, 55: Mink JW, Thach WT: Basal ganglia motor control. I. Nonexclusive relation of pallidal discharge to five movement modes. J Neurophysiol 1991, 65: Horak FB, Anderson ME: Influence of globus pallidus on arm movements in monkeys. I. Effects of kainic acid-induced lesions. J Neurophysiol 1984, 52: Mink JW, Thach WT: Basal ganglia motor control. III. Pallidal ablation: normal reaction time, muscle cocontraction, and slow movement. J Neurophysiol 1991, 65: Kato M, Kimura M: Effects of reversible blockade of basal ganglia on voluntary arm movement. J Neurophysiol 1992, 68: Mink JW: The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 1996, 50: Miller WC, DeLong MR: Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of parkinsonism. In The Basal Ganglia II. Edited by Carpenter MB, Jayaraman A. Plenum Press; 1987: Filion M, Tremblay L: Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res 1991, 547: Hutchinson WD, Levy R, Dostrovsky JO, Lozano AM, Lang AE: Effects of apomorphine on globus pallidus neurons in parkinsonian patients. Ann Neurol 1997, 42: Merello M, Balej J, Delfino M, Cammarota A, Betti O, Leiguarda R: Apomorphine induces changes in GPi spontaneous outflow in patients with Parkinson s disease. Mov Disord 1999, 14: Filion M, Tremblay L, Bedard PJ: Effects of dopamine agonists on the spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism.. Brain Res 1991, 547: Papa SM, DeSimone R, Fiorani M, Oldfield EH: Internal globus pallidus discharge is nearly suppressed during levodopainduced dyskinesias. Ann Neurol 1999, 46: Bergman H, Wichmann T, DeLong MR: Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 1990, 249: Boraud T, Bezard E, Bioulac B, Gross CE: From single extracellular unit recording in experimental and human Parkinsonism to the development of a functional concept of the role played by the basal ganglia in motor control. Prog Neurobiol 2002, 66: Pessiglione M, Guehl D, Rolland AS, Francois C, Hirsch EC, Feger J, Tremblay L: Thalamic neuronal activity in dopaminedepleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci 2005, 25: Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H: Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson s disease. J Neurosci 2002, 22: Boraud T, Bezard E, Bioulac B, Gross CE: Dopamine agonistinduced dyskinesias are correlated to both firing pattern and frequency alterations of pallidal neurones in the MPTP-treated monkey. Brain 2001, 124: Current Opinion in Neurobiology 2006, 16:
10 Basal ganglia oscillations and pathophysiology of movement disorders Rivlin-Etzion, Marmor, Heimer, Raz, Nini and Bergman Wichmann T, Soares J: Neuronal firing before and after burst discharges in the monkey Basal Ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol 2006, 95: It is known that burst discharges in basal ganglia neurons are more common in PD than under normal conditions, but this study reveals significant changes in the structure of burst or peri-burst discharge in the STN, GPe and GPi of MPTP-treated macaque monkeys. Thus, complex changes in the burst structure, and in burst frequency, might contribute to abnormal information processing in PD. 58. Wichmann T, Bergman H, DeLong MR: The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J Neurophysiol 1994, 72: Raz A, Feingold A, Zelanskaya V, Vaadia E, Bergman H: Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. J Neurophysiol 1996, 76: Raz A, Frechter-Mazar V, Feingold A, Abeles M, Vaadia E, Bergman H: Activity of pallidal and striatal tonically active neurons is correlated in MPTP-treated monkeys but not in normal monkeys. J Neurosci 2001, 21:RC Hutchison WD, Lozano AM, Tasker RR, Lang AE, Dostrovsky JO: Identification and characterization of neurons with tremorfrequency activity in human globus pallidus. Exp Brain Res 1997, 113: Lemstra AW, Verhagen ML, Lee JI, Dougherty PM, Lenz FA: Tremor-frequency (3 6 Hz) activity in the sensorimotor arm representation of the internal segment of the globus pallidus in patients with Parkinson s disease. Neurosci Lett 1999, 267: Hurtado JM, Rubchinsky LL, Sigvardt KA, Wheelock VL, Pappas CT: Temporal evolution of oscillations and synchrony in GPi/muscle pairs in Parkinson s disease. J Neurophysiol 2005, 93: Advanced time-dependent phase correlation techniques were applied to 27 pairs of tremor-related GPi single units and EMG of PD patients undergoing stereotactic neurosurgery. Analysis using short (2s) sliding windows shows that oscillatory activity in both GPi oscillatory units and muscles occurs intermittently over time. There was partial overlap in the times of oscillatory activity but, in most cases, no correlation was found between the times of oscillatory episodes in the two signals. Phaselocking analysis revealed that pallidal oscillations and tremor are punctuated by phase slips, which were classified as synchronizing or desynchronizing. The results of this high-level quantitative characterization of parkinsonian tremor and pallidal oscillations can be explained by either a dynamical connectivity structure from the basal ganglia to the periphery or tremor generators downstream of the basal ganglia. 64. Lenz FA, Tasker RR, Kwan HC, Schnider S, Kwong R, Murayama Y, Dostrovsky JO, Murphy JT: Single unit analysis of the human ventral thalamic nuclear group: correlation of thalamic tremor cells with the 3-6 Hz component of parkinsonian tremor. J Neurosci 1988, 8: Kuhn AA, Kupsch A, Schneider GH, Brown P: Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson s disease. Eur J Neurosci 2006, 23: Previous studies by this group revealed strong 8-35 Hz LFP oscillations in the subthalamic nucleus (STN) of PD patients. In this study, they report a reduction in peak activity in the 8-35 Hz band with levodopa that is positively correlated with the improvement in the contralateral akinesia and rigidity (but not with tremor). A trend towards negative correlations was found between gamma band LFP power and PD symptoms, suggesting that positive correlations were relatively frequency-specific 66. Wang SY, Aziz TZ, Stein JF, Liu X: Time-frequency analysis of transient neuromuscular events: dynamic changes in activity of the subthalamic nucleus and forearm muscles related to the intermittent resting tremor. J Neurosci Methods 2005, 145: This study implemented a highly advanced dynamic analysis (short-time Fourier transform and continuous wavelet transform) of the functional correlation between both neural and muscular activity and the STN LFP over several episodes of transient resting tremor from a PD patient. A significant suppression in the power of the STN LFPs in the b band (10-30 Hz) preceded the onset of resting tremor. During the tremor episode the power at the tremor frequency ( Hz) in STN LFPs increased significantly. 67. Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A: The cerebral oscillatory network of parkinsonian resting tremor. Brain 2003, 126: Sharott A, Magill PJ, Harnack D, Kupsch A, Meissner W, Brown P: Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur J Neurosci 2005, 21: Kuhn AA, Trottenberg T, Kivi A, Kupsch A, Schneider GH, Brown P: The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson s disease. Exp Neurol 2005, 194: Goldberg JA, Rokni U, Boraud T, Vaadia E, Bergman H: Spike synchronization in the cortex-basal Ganglia networks of parkinsonian primates reflects global dynamics of the local field potentials. J Neurosci 2004, 24: Schneidman E, Berry MJ, Segev R, Bialek W: Weak pairwise correlations imply strongly correlated network states in a neural population. Nature 2006, 440: Pare D, Curro Dossi R, Steriade M: Neuronal basis of the parkinsonian resting tremor: a hypothesis and its implications for treatment. Neuroscience 1990, 35: Tass P, Rosenblum MG, Weule J, Kurths J, Pikovsky A, Volkmann J, Schnitzler A, Freund H-J: Detection of n:m phase locking from noisy data: application to magnetoencephalography. Phys Rev Lett 1998, 81: Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF: A framework for the analysis of mixed time series/ point process data theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 1995, 64: Mehta MR, Bergman H: Loss of frequencies in autocorrelations and a procedure to recover them. J Neurosci Methods 1995, 62: Rivlin-Etzion M, Ritov Y, Heimer G, Bergman H, Bar-Gad I: Local shuffling of spike trains boosts the accuracy of spike train spectral analysis. J Neurophysiol 2006, 95: Gresty M, Buckwell D: Spectral analysis of tremor: understanding the results. J Neurol Neurosurg Psychiatry 1990, 53: Current Opinion in Neurobiology 2006, 16:
SYNCHRONIZATION OF PALLIDAL ACTIVITY IN THE MPTP PRIMATE MODEL OF PARKINSONISM IS NOT LIMITED TO OSCILLATORY ACTIVITY
 SYNCHRONIZATION OF PALLIDAL ACTIVITY IN THE MPTP PRIMATE MODEL OF PARKINSONISM IS NOT LIMITED TO OSCILLATORY ACTIVITY Gali Heimer, Izhar Bar-Gad, Joshua A. Goldberg and Hagai Bergman * 1. INTRODUCTION
SYNCHRONIZATION OF PALLIDAL ACTIVITY IN THE MPTP PRIMATE MODEL OF PARKINSONISM IS NOT LIMITED TO OSCILLATORY ACTIVITY Gali Heimer, Izhar Bar-Gad, Joshua A. Goldberg and Hagai Bergman * 1. INTRODUCTION
GBME graduate course. Chapter 43. The Basal Ganglia
 GBME graduate course Chapter 43. The Basal Ganglia Basal ganglia in history Parkinson s disease Huntington s disease Parkinson s disease 1817 Parkinson's disease (PD) is a degenerative disorder of the
GBME graduate course Chapter 43. The Basal Ganglia Basal ganglia in history Parkinson s disease Huntington s disease Parkinson s disease 1817 Parkinson's disease (PD) is a degenerative disorder of the
COGNITIVE SCIENCE 107A. Motor Systems: Basal Ganglia. Jaime A. Pineda, Ph.D.
 COGNITIVE SCIENCE 107A Motor Systems: Basal Ganglia Jaime A. Pineda, Ph.D. Two major descending s Pyramidal vs. extrapyramidal Motor cortex Pyramidal system Pathway for voluntary movement Most fibers originate
COGNITIVE SCIENCE 107A Motor Systems: Basal Ganglia Jaime A. Pineda, Ph.D. Two major descending s Pyramidal vs. extrapyramidal Motor cortex Pyramidal system Pathway for voluntary movement Most fibers originate
Parkinson s Disease and Cortico-Basal Ganglia Circuits
 Continuing Medical Education 213 Parkinson s Disease and Cortico-Basal Ganglia Circuits Ming-Kai Pan, Chun-Hwei Tai, Chung-Chin Kuo Abstract- Cortico-basal ganglia circuit model has been studied extensively
Continuing Medical Education 213 Parkinson s Disease and Cortico-Basal Ganglia Circuits Ming-Kai Pan, Chun-Hwei Tai, Chung-Chin Kuo Abstract- Cortico-basal ganglia circuit model has been studied extensively
Anatomy of the basal ganglia. Dana Cohen Gonda Brain Research Center, room 410
 Anatomy of the basal ganglia Dana Cohen Gonda Brain Research Center, room 410 danacoh@gmail.com The basal ganglia The nuclei form a small minority of the brain s neuronal population. Little is known about
Anatomy of the basal ganglia Dana Cohen Gonda Brain Research Center, room 410 danacoh@gmail.com The basal ganglia The nuclei form a small minority of the brain s neuronal population. Little is known about
Basal Ganglia. Introduction. Basal Ganglia at a Glance. Role of the BG
 Basal Ganglia Shepherd (2004) Chapter 9 Charles J. Wilson Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Introduction A set of nuclei in the forebrain and midbrain area in mammals, birds, and reptiles.
Basal Ganglia Shepherd (2004) Chapter 9 Charles J. Wilson Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Introduction A set of nuclei in the forebrain and midbrain area in mammals, birds, and reptiles.
Basal Ganglia George R. Leichnetz, Ph.D.
 Basal Ganglia George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the brain structures which constitute the basal ganglia, and their interconnections 2. To understand the consequences (clinical manifestations)
Basal Ganglia George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the brain structures which constitute the basal ganglia, and their interconnections 2. To understand the consequences (clinical manifestations)
Visualization and simulated animations of pathology and symptoms of Parkinson s disease
 Visualization and simulated animations of pathology and symptoms of Parkinson s disease Prof. Yifan HAN Email: bctycan@ust.hk 1. Introduction 2. Biochemistry of Parkinson s disease 3. Course Design 4.
Visualization and simulated animations of pathology and symptoms of Parkinson s disease Prof. Yifan HAN Email: bctycan@ust.hk 1. Introduction 2. Biochemistry of Parkinson s disease 3. Course Design 4.
Teach-SHEET Basal Ganglia
 Teach-SHEET Basal Ganglia Purves D, et al. Neuroscience, 5 th Ed., Sinauer Associates, 2012 Common organizational principles Basic Circuits or Loops: Motor loop concerned with learned movements (scaling
Teach-SHEET Basal Ganglia Purves D, et al. Neuroscience, 5 th Ed., Sinauer Associates, 2012 Common organizational principles Basic Circuits or Loops: Motor loop concerned with learned movements (scaling
Connections of basal ganglia
 Connections of basal ganglia Introduction The basal ganglia, or basal nuclei, are areas of subcortical grey matter that play a prominent role in modulating movement, as well as cognitive and emotional
Connections of basal ganglia Introduction The basal ganglia, or basal nuclei, are areas of subcortical grey matter that play a prominent role in modulating movement, as well as cognitive and emotional
Lecture XIII. Brain Diseases I - Parkinsonism! Brain Diseases I!
 Lecture XIII. Brain Diseases I - Parkinsonism! Bio 3411! Wednesday!! Lecture XIII. Brain Diseases - I.! 1! Brain Diseases I! NEUROSCIENCE 5 th ed! Page!!Figure!!Feature! 408 18.9 A!!Substantia Nigra in
Lecture XIII. Brain Diseases I - Parkinsonism! Bio 3411! Wednesday!! Lecture XIII. Brain Diseases - I.! 1! Brain Diseases I! NEUROSCIENCE 5 th ed! Page!!Figure!!Feature! 408 18.9 A!!Substantia Nigra in
Synchronizing activity of basal ganglia and pathophysiology of Parkinson s disease
 JNT-Suppl 70-0/5 For Author s Correction Only J Neural Transm (2006) [Suppl] 70: 1 4 # Springer-Verlag 2006 Synchronizing activity of basal ganglia and pathophysiology of Parkinson s disease G. Heimer
JNT-Suppl 70-0/5 For Author s Correction Only J Neural Transm (2006) [Suppl] 70: 1 4 # Springer-Verlag 2006 Synchronizing activity of basal ganglia and pathophysiology of Parkinson s disease G. Heimer
A. General features of the basal ganglia, one of our 3 major motor control centers:
 Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. April 22, 2010 THE BASAL GANGLIA Objectives: 1. What are the
Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. April 22, 2010 THE BASAL GANGLIA Objectives: 1. What are the
Basal ganglia Sujata Sofat, class of 2009
 Basal ganglia Sujata Sofat, class of 2009 Basal ganglia Objectives Describe the function of the Basal Ganglia in movement Define the BG components and their locations Describe the motor loop of the BG
Basal ganglia Sujata Sofat, class of 2009 Basal ganglia Objectives Describe the function of the Basal Ganglia in movement Define the BG components and their locations Describe the motor loop of the BG
Making Things Happen 2: Motor Disorders
 Making Things Happen 2: Motor Disorders How Your Brain Works Prof. Jan Schnupp wschnupp@cityu.edu.hk HowYourBrainWorks.net On the Menu in This Lecture In the previous lecture we saw how motor cortex and
Making Things Happen 2: Motor Disorders How Your Brain Works Prof. Jan Schnupp wschnupp@cityu.edu.hk HowYourBrainWorks.net On the Menu in This Lecture In the previous lecture we saw how motor cortex and
A. General features of the basal ganglia, one of our 3 major motor control centers:
 Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. March 1, 2012 THE BASAL GANGLIA Objectives: 1. What are the main
Reading: Waxman pp. 141-146 are not very helpful! Computer Resources: HyperBrain, Chapter 12 Dental Neuroanatomy Suzanne S. Stensaas, Ph.D. March 1, 2012 THE BASAL GANGLIA Objectives: 1. What are the main
Synchronous Oscillations in the Basal-Ganglia-Cortical Network: Do They Generate Tremor and Other Symptoms of Parkinson's Disease?
 Synchronous Oscillations in the Basal-Ganglia-Cortical Network: Do They Generate Tremor and Other Symptoms of Parkinson's Disease? Thesis submitted for the degree of Doctor of Philosophy by Michal Rivlin-Etzion
Synchronous Oscillations in the Basal-Ganglia-Cortical Network: Do They Generate Tremor and Other Symptoms of Parkinson's Disease? Thesis submitted for the degree of Doctor of Philosophy by Michal Rivlin-Etzion
Effects of nicotine on neuronal firing patterns in human subthalamic nucleus. Kim Scott Mentor: Henry Lester SURF seminar, January 15, 2009
 Effects of nicotine on neuronal firing patterns in human subthalamic nucleus Kim Scott Mentor: Henry Lester SURF seminar, January 15, 2009 Smoking tobacco protects against Parkinson s Disease (PD). Identical
Effects of nicotine on neuronal firing patterns in human subthalamic nucleus Kim Scott Mentor: Henry Lester SURF seminar, January 15, 2009 Smoking tobacco protects against Parkinson s Disease (PD). Identical
Computational cognitive neuroscience: 8. Motor Control and Reinforcement Learning
 1 Computational cognitive neuroscience: 8. Motor Control and Reinforcement Learning Lubica Beňušková Centre for Cognitive Science, FMFI Comenius University in Bratislava 2 Sensory-motor loop The essence
1 Computational cognitive neuroscience: 8. Motor Control and Reinforcement Learning Lubica Beňušková Centre for Cognitive Science, FMFI Comenius University in Bratislava 2 Sensory-motor loop The essence
VL VA BASAL GANGLIA. FUNCTIONAl COMPONENTS. Function Component Deficits Start/initiation Basal Ganglia Spontan movements
 BASAL GANGLIA Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University at Buffalo I) Overview How do Basal Ganglia affect movement Basal ganglia enhance cortical motor activity and facilitate movement.
BASAL GANGLIA Chris Cohan, Ph.D. Dept. of Pathology/Anat Sci University at Buffalo I) Overview How do Basal Ganglia affect movement Basal ganglia enhance cortical motor activity and facilitate movement.
Strick Lecture 4 March 29, 2006 Page 1
 Strick Lecture 4 March 29, 2006 Page 1 Basal Ganglia OUTLINE- I. Structures included in the basal ganglia II. III. IV. Skeleton diagram of Basal Ganglia Loops with cortex Similarity with Cerebellar Loops
Strick Lecture 4 March 29, 2006 Page 1 Basal Ganglia OUTLINE- I. Structures included in the basal ganglia II. III. IV. Skeleton diagram of Basal Ganglia Loops with cortex Similarity with Cerebellar Loops
Temporal patterning of neural synchrony in the basal ganglia in Parkinson s disease
 Temporal patterning of neural synchrony in the basal ganglia in Parkinson s disease Shivakeshavan Ratnadurai-Giridharan 1, S. Elizabeth Zauber 2, Robert M. Worth 1,3, Thomas Witt 3, Sungwoo Ahn 1,5, Leonid
Temporal patterning of neural synchrony in the basal ganglia in Parkinson s disease Shivakeshavan Ratnadurai-Giridharan 1, S. Elizabeth Zauber 2, Robert M. Worth 1,3, Thomas Witt 3, Sungwoo Ahn 1,5, Leonid
Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817.
 Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817. Four (4) hallmark clinical signs: 1) Tremor: (Note -
Parkinsonism or Parkinson s Disease I. Symptoms: Main disorder of movement. Named after, an English physician who described the then known, in 1817. Four (4) hallmark clinical signs: 1) Tremor: (Note -
Damage on one side.. (Notes) Just remember: Unilateral damage to basal ganglia causes contralateral symptoms.
 Lecture 20 - Basal Ganglia Basal Ganglia (Nolte 5 th Ed pp 464) Damage to the basal ganglia produces involuntary movements. Although the basal ganglia do not influence LMN directly (to cause this involuntary
Lecture 20 - Basal Ganglia Basal Ganglia (Nolte 5 th Ed pp 464) Damage to the basal ganglia produces involuntary movements. Although the basal ganglia do not influence LMN directly (to cause this involuntary
Basal ganglia macrocircuits
 Tepper, Abercrombie & Bolam (Eds.) Progress in Brain Research, Vol. 160 ISSN 0079-6123 Copyright r 2007 Elsevier B.V. All rights reserved CHAPTER 1 Basal ganglia macrocircuits J.M. Tepper 1,, E.D. Abercrombie
Tepper, Abercrombie & Bolam (Eds.) Progress in Brain Research, Vol. 160 ISSN 0079-6123 Copyright r 2007 Elsevier B.V. All rights reserved CHAPTER 1 Basal ganglia macrocircuits J.M. Tepper 1,, E.D. Abercrombie
Network Effects of Deep Brain Stimulation for Parkinson s Disease A Computational. Modeling Study. Karthik Kumaravelu
 Network Effects of Deep Brain Stimulation for Parkinson s Disease A Computational Modeling Study by Karthik Kumaravelu Department of Biomedical Engineering Duke University Date: Approved: Warren M. Grill,
Network Effects of Deep Brain Stimulation for Parkinson s Disease A Computational Modeling Study by Karthik Kumaravelu Department of Biomedical Engineering Duke University Date: Approved: Warren M. Grill,
Effects of Apomorphine on Subthalamic Nucleus and Globus Pallidus Internus Neurons in Patients With Parkinson s Disease
 Effects of Apomorphine on Subthalamic Nucleus and Globus Pallidus Internus Neurons in Patients With Parkinson s Disease R. LEVY, 1 J. O. DOSTROVSKY, 1,3 A. E. LANG, 3,4 E. SIME, 3 W. D. HUTCHISON, 1 3
Effects of Apomorphine on Subthalamic Nucleus and Globus Pallidus Internus Neurons in Patients With Parkinson s Disease R. LEVY, 1 J. O. DOSTROVSKY, 1,3 A. E. LANG, 3,4 E. SIME, 3 W. D. HUTCHISON, 1 3
Modeling facilitation and inhibition of competing motor programs in basal ganglia subthalamic nucleus pallidal circuits
 Modeling facilitation and inhibition of competing motor programs in basal ganglia subthalamic nucleus pallidal circuits Leonid L. Rubchinsky*, Nancy Kopell, and Karen A. Sigvardt* *Center for Neuroscience
Modeling facilitation and inhibition of competing motor programs in basal ganglia subthalamic nucleus pallidal circuits Leonid L. Rubchinsky*, Nancy Kopell, and Karen A. Sigvardt* *Center for Neuroscience
Modeling the interplay of short-term memory and the basal ganglia in sequence processing
 Neurocomputing 26}27 (1999) 687}692 Modeling the interplay of short-term memory and the basal ganglia in sequence processing Tomoki Fukai* Department of Electronics, Tokai University, Hiratsuka, Kanagawa
Neurocomputing 26}27 (1999) 687}692 Modeling the interplay of short-term memory and the basal ganglia in sequence processing Tomoki Fukai* Department of Electronics, Tokai University, Hiratsuka, Kanagawa
Electrophysiology of Subthalamic Nucleus in Normal and Parkinson s Disease
 Continuing Medical Education 206 Electrophysiology of Subthalamic Nucleus in Normal and Parkinson s Disease Chun-Hwei Tai and Chung-Chin Kuo Abstract- Subthalamic nucleus (STN) has been known to play an
Continuing Medical Education 206 Electrophysiology of Subthalamic Nucleus in Normal and Parkinson s Disease Chun-Hwei Tai and Chung-Chin Kuo Abstract- Subthalamic nucleus (STN) has been known to play an
Basal Ganglia. Steven McLoon Department of Neuroscience University of Minnesota
 Basal Ganglia Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Graduate School Discussion Wednesday, Nov 1, 11:00am MoosT 2-690 with Paul Mermelstein (invite your friends)
Basal Ganglia Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Graduate School Discussion Wednesday, Nov 1, 11:00am MoosT 2-690 with Paul Mermelstein (invite your friends)
The Effect of Weighted Compression Sleeves on Tremor Suppression in Individuals with Parkinson s Disease
 The Effect of Weighted Compression Sleeves on Tremor Suppression in Individuals with Parkinson s Disease Jennifer Iannello, Garry Johnson, Lauren Pilla Parkinson s Disease Second most common chronic, progressive,
The Effect of Weighted Compression Sleeves on Tremor Suppression in Individuals with Parkinson s Disease Jennifer Iannello, Garry Johnson, Lauren Pilla Parkinson s Disease Second most common chronic, progressive,
NS219: Basal Ganglia Anatomy
 NS219: Basal Ganglia Anatomy Human basal ganglia anatomy Analagous rodent basal ganglia nuclei Basal ganglia circuits: the classical model of direct and indirect pathways + Glutamate + - GABA - Gross anatomy
NS219: Basal Ganglia Anatomy Human basal ganglia anatomy Analagous rodent basal ganglia nuclei Basal ganglia circuits: the classical model of direct and indirect pathways + Glutamate + - GABA - Gross anatomy
Levodopa vs. deep brain stimulation: computational models of treatments for Parkinson's disease
 Levodopa vs. deep brain stimulation: computational models of treatments for Parkinson's disease Abstract Parkinson's disease (PD) is a neurodegenerative disease affecting the dopaminergic neurons of the
Levodopa vs. deep brain stimulation: computational models of treatments for Parkinson's disease Abstract Parkinson's disease (PD) is a neurodegenerative disease affecting the dopaminergic neurons of the
Movement Disorders. Psychology 372 Physiological Psychology. Background. Myasthenia Gravis. Many Types
 Background Movement Disorders Psychology 372 Physiological Psychology Steven E. Meier, Ph.D. Listen to the audio lecture while viewing these slides Early Studies Found some patients with progressive weakness
Background Movement Disorders Psychology 372 Physiological Psychology Steven E. Meier, Ph.D. Listen to the audio lecture while viewing these slides Early Studies Found some patients with progressive weakness
Concurrent Parkinson tremors
 Keywords: 9974 Journal of Physiology (2000), 529.1, pp. 273 281 273 Concurrent Parkinson tremors G. P. Moore, L. Ding* and H. M. Bronte-Stewart Department of Neurological Sciences, Stanford University
Keywords: 9974 Journal of Physiology (2000), 529.1, pp. 273 281 273 Concurrent Parkinson tremors G. P. Moore, L. Ding* and H. M. Bronte-Stewart Department of Neurological Sciences, Stanford University
Basal Ganglia General Info
 Basal Ganglia General Info Neural clusters in peripheral nervous system are ganglia. In the central nervous system, they are called nuclei. Should be called Basal Nuclei but usually called Basal Ganglia.
Basal Ganglia General Info Neural clusters in peripheral nervous system are ganglia. In the central nervous system, they are called nuclei. Should be called Basal Nuclei but usually called Basal Ganglia.
The motor regulator. 1) Basal ganglia/nucleus
 The motor regulator 1) Basal ganglia/nucleus Neural structures involved in the control of movement Basal Ganglia - Components of the basal ganglia - Function of the basal ganglia - Connection and circuits
The motor regulator 1) Basal ganglia/nucleus Neural structures involved in the control of movement Basal Ganglia - Components of the basal ganglia - Function of the basal ganglia - Connection and circuits
Biological Bases of Behavior. 8: Control of Movement
 Biological Bases of Behavior 8: Control of Movement m d Skeletal Muscle Movements of our body are accomplished by contraction of the skeletal muscles Flexion: contraction of a flexor muscle draws in a
Biological Bases of Behavior 8: Control of Movement m d Skeletal Muscle Movements of our body are accomplished by contraction of the skeletal muscles Flexion: contraction of a flexor muscle draws in a
Neurodegenerative Disease. April 12, Cunningham. Department of Neurosciences
 Neurodegenerative Disease April 12, 2017 Cunningham Department of Neurosciences NEURODEGENERATIVE DISEASE Any of a group of hereditary and sporadic conditions characterized by progressive dysfunction,
Neurodegenerative Disease April 12, 2017 Cunningham Department of Neurosciences NEURODEGENERATIVE DISEASE Any of a group of hereditary and sporadic conditions characterized by progressive dysfunction,
For more information about how to cite these materials visit
 Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Neurobiology of Disease
 The Journal of Neuroscience, August 2, 2006 26(31):8101 8114 8101 Neurobiology of Disease Dopamine Replacement Therapy Does Not Restore the Full Spectrum of Normal Pallidal Activity in the 1-Methyl-4-
The Journal of Neuroscience, August 2, 2006 26(31):8101 8114 8101 Neurobiology of Disease Dopamine Replacement Therapy Does Not Restore the Full Spectrum of Normal Pallidal Activity in the 1-Methyl-4-
Dr. Farah Nabil Abbas. MBChB, MSc, PhD
 Dr. Farah Nabil Abbas MBChB, MSc, PhD The Basal Ganglia *Functions in association with motor cortex and corticospinal pathways. *Regarded as accessory motor system besides cerebellum. *Receive most of
Dr. Farah Nabil Abbas MBChB, MSc, PhD The Basal Ganglia *Functions in association with motor cortex and corticospinal pathways. *Regarded as accessory motor system besides cerebellum. *Receive most of
Basal nuclei, cerebellum and movement
 Basal nuclei, cerebellum and movement MSTN121 - Neurophysiology Session 9 Department of Myotherapy Basal Nuclei (Ganglia) Basal Nuclei (Ganglia) Role: Predict the effects of various actions, then make
Basal nuclei, cerebellum and movement MSTN121 - Neurophysiology Session 9 Department of Myotherapy Basal Nuclei (Ganglia) Basal Nuclei (Ganglia) Role: Predict the effects of various actions, then make
Gangli della Base: un network multifunzionale
 Gangli della Base: un network multifunzionale Prof. Giovanni Abbruzzese Centro per la Malattia di Parkinson e i Disordini del Movimento DiNOGMI, Università di Genova IRCCS AOU San Martino IST Basal Ganglia
Gangli della Base: un network multifunzionale Prof. Giovanni Abbruzzese Centro per la Malattia di Parkinson e i Disordini del Movimento DiNOGMI, Università di Genova IRCCS AOU San Martino IST Basal Ganglia
NEURAL CONTROL OF MOVEMENT: ENGINEERING THE RHYTHMS OF THE BRAIN
 NEURAL CONTROL OF MOVEMENT: ENGINEERING THE RHYTHMS OF THE BRAIN Madeleine Lowery School of Electrical and Electronic Engineering Centre for Biomedical Engineering University College Dublin Parkinson s
NEURAL CONTROL OF MOVEMENT: ENGINEERING THE RHYTHMS OF THE BRAIN Madeleine Lowery School of Electrical and Electronic Engineering Centre for Biomedical Engineering University College Dublin Parkinson s
Beta and Tremor-Related Oscillations in the Motor Thalamus of Essential Tremor Patients
 IETF - WD Hutchison Feb 214 requesting $25, Beta and Tremor-Related Oscillations in the Motor Thalamus of Essential Tremor Patients 1. Specific aims of proposal The proposed study will examine beta oscillations
IETF - WD Hutchison Feb 214 requesting $25, Beta and Tremor-Related Oscillations in the Motor Thalamus of Essential Tremor Patients 1. Specific aims of proposal The proposed study will examine beta oscillations
Functional Correlations between Neighboring Neurons in the Primate Globus Pallidus Are Weak or Nonexistent
 4012 The Journal of Neuroscience, May 15, 2003 23(10):4012 4016 Brief Communication Functional Correlations between Neighboring Neurons in the Primate Globus Pallidus Are Weak or Nonexistent Izhar Bar-Gad,
4012 The Journal of Neuroscience, May 15, 2003 23(10):4012 4016 Brief Communication Functional Correlations between Neighboring Neurons in the Primate Globus Pallidus Are Weak or Nonexistent Izhar Bar-Gad,
DISORDERS OF THE MOTOR SYSTEM. Jeanette J. Norden, Ph.D. Professor Emerita Vanderbilt University School of Medicine
 DISORDERS OF THE MOTOR SYSTEM Jeanette J. Norden, Ph.D. Professor Emerita Vanderbilt University School of Medicine THE MOTOR SYSTEM To understand disorders of the motor system, we need to review how a
DISORDERS OF THE MOTOR SYSTEM Jeanette J. Norden, Ph.D. Professor Emerita Vanderbilt University School of Medicine THE MOTOR SYSTEM To understand disorders of the motor system, we need to review how a
The oscillatory activity in the Parkinsonian subthalamic nucleus investigated using the macro-electrodes for deep brain stimulation
 Clinical Neurophysiology 113 (2002) 1 6 www.elsevier.com/locate/clinph The oscillatory activity in the Parkinsonian subthalamic nucleus investigated using the macro-electrodes for deep brain stimulation
Clinical Neurophysiology 113 (2002) 1 6 www.elsevier.com/locate/clinph The oscillatory activity in the Parkinsonian subthalamic nucleus investigated using the macro-electrodes for deep brain stimulation
Whether deep brain stimulation can dramatically. Deep brain stimulation: How does it work?
 JERROLD L. VITEK, MD, PhD Director, Neuromodulation Research Center, Department of Neurosciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH Deep brain stimulation: How does it work? ABSTRACT
JERROLD L. VITEK, MD, PhD Director, Neuromodulation Research Center, Department of Neurosciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH Deep brain stimulation: How does it work? ABSTRACT
1/2/2019. Basal Ganglia & Cerebellum a quick overview. Outcomes you want to accomplish. MHD-Neuroanatomy Neuroscience Block. Basal ganglia review
 This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the
This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the
doi: /brain/aws023 Brain 2012: 135; Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits?
 doi:10.1093/brain/aws023 Brain 2012: 135; 3206 3226 3206 BRAIN A JOURNAL OF NEUROLOGY REVIEW ARTICLE Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Rick C. Helmich,
doi:10.1093/brain/aws023 Brain 2012: 135; 3206 3226 3206 BRAIN A JOURNAL OF NEUROLOGY REVIEW ARTICLE Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Rick C. Helmich,
Deep Brain Stimulation: Surgical Process
 Deep Brain Stimulation: Surgical Process Kia Shahlaie, MD, PhD Assistant Professor Bronte Endowed Chair in Epilepsy Research Director of Functional Neurosurgery Minimally Invasive Neurosurgery Department
Deep Brain Stimulation: Surgical Process Kia Shahlaie, MD, PhD Assistant Professor Bronte Endowed Chair in Epilepsy Research Director of Functional Neurosurgery Minimally Invasive Neurosurgery Department
Bifurcation analysis points towards the source of beta neuronal oscillations in Parkinson s disease
 2011 50th IEEE Conference on Decision and Control and European Control Conference (CDC-ECC) Orlando, FL, USA, December 12-15, 2011 Bifurcation analysis points towards the source of beta neuronal oscillations
2011 50th IEEE Conference on Decision and Control and European Control Conference (CDC-ECC) Orlando, FL, USA, December 12-15, 2011 Bifurcation analysis points towards the source of beta neuronal oscillations
Voluntary Movement. Ch. 14: Supplemental Images
 Voluntary Movement Ch. 14: Supplemental Images Skeletal Motor Unit: The basics Upper motor neuron: Neurons that supply input to lower motor neurons. Lower motor neuron: neuron that innervates muscles,
Voluntary Movement Ch. 14: Supplemental Images Skeletal Motor Unit: The basics Upper motor neuron: Neurons that supply input to lower motor neurons. Lower motor neuron: neuron that innervates muscles,
The control of spiking by synaptic input in striatal and pallidal neurons
 The control of spiking by synaptic input in striatal and pallidal neurons Dieter Jaeger Department of Biology, Emory University, Atlanta, GA 30322 Key words: Abstract: rat, slice, whole cell, dynamic current
The control of spiking by synaptic input in striatal and pallidal neurons Dieter Jaeger Department of Biology, Emory University, Atlanta, GA 30322 Key words: Abstract: rat, slice, whole cell, dynamic current
Basal Ganglia. Today s lecture is about Basal Ganglia and it covers:
 Basal Ganglia Motor system is complex interaction between Lower motor neurons (spinal cord and brainstem circuits) and Upper motor neurons (pyramidal and extrapyramidal tracts) plus two main regulators
Basal Ganglia Motor system is complex interaction between Lower motor neurons (spinal cord and brainstem circuits) and Upper motor neurons (pyramidal and extrapyramidal tracts) plus two main regulators
Extrapyramidal Motor System. Basal Ganglia or Striatum. Basal Ganglia or Striatum 3/3/2010
 Extrapyramidal Motor System Basal Ganglia or Striatum Descending extrapyramidal paths receive input from other parts of motor system: From the cerebellum From the basal ganglia or corpus striatum Caudate
Extrapyramidal Motor System Basal Ganglia or Striatum Descending extrapyramidal paths receive input from other parts of motor system: From the cerebellum From the basal ganglia or corpus striatum Caudate
TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE
 TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE Angel Lago-Rodriguez 1, Binith Cheeran 2 and Miguel Fernández-Del-Olmo 3 1. Prism Lab, Behavioural Brain Sciences, School of
TREATMENT-SPECIFIC ABNORMAL SYNAPTIC PLASTICITY IN EARLY PARKINSON S DISEASE Angel Lago-Rodriguez 1, Binith Cheeran 2 and Miguel Fernández-Del-Olmo 3 1. Prism Lab, Behavioural Brain Sciences, School of
Dynamic Behaviour of a Spiking Model of Action Selection in the Basal Ganglia
 Dynamic Behaviour of a Spiking Model of Action Selection in the Basal Ganglia Terrence C. Stewart (tcstewar@uwaterloo.ca) Xuan Choo (fchoo@uwaterloo.ca) Chris Eliasmith (celiasmith@uwaterloo.ca) Centre
Dynamic Behaviour of a Spiking Model of Action Selection in the Basal Ganglia Terrence C. Stewart (tcstewar@uwaterloo.ca) Xuan Choo (fchoo@uwaterloo.ca) Chris Eliasmith (celiasmith@uwaterloo.ca) Centre
I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts.
 Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
On the Origin of Tremor in Parkinson s Disease
 On the Origin of Tremor in Parkinson s Disease Andrey Dovzhenok 1, Leonid L Rubchinsky 1,2 1 Department of Mathematical Sciences and Center for Mathematical Biosciences, Indiana University Purdue University
On the Origin of Tremor in Parkinson s Disease Andrey Dovzhenok 1, Leonid L Rubchinsky 1,2 1 Department of Mathematical Sciences and Center for Mathematical Biosciences, Indiana University Purdue University
Circuits & Behavior. Daniel Huber
 Circuits & Behavior Daniel Huber How to study circuits? Anatomy (boundaries, tracers, viral tools) Inactivations (lesions, optogenetic, pharma, accidents) Activations (electrodes, magnets, optogenetic)
Circuits & Behavior Daniel Huber How to study circuits? Anatomy (boundaries, tracers, viral tools) Inactivations (lesions, optogenetic, pharma, accidents) Activations (electrodes, magnets, optogenetic)
Deep Brain Stimulation Surgery for Parkinson s Disease
 Deep Brain Stimulation Surgery for Parkinson s Disease Demystifying Medicine 24 January 2012 Kareem A. Zaghloul, MD, PhD Staff Physician, Surgical Neurology Branch NINDS Surgery for Parkinson s Disease
Deep Brain Stimulation Surgery for Parkinson s Disease Demystifying Medicine 24 January 2012 Kareem A. Zaghloul, MD, PhD Staff Physician, Surgical Neurology Branch NINDS Surgery for Parkinson s Disease
Motor System Hierarchy
 Motor Pathways Lectures Objectives Define the terms upper and lower motor neurons with examples. Describe the corticospinal (pyramidal) tract and the direct motor pathways from the cortex to the trunk
Motor Pathways Lectures Objectives Define the terms upper and lower motor neurons with examples. Describe the corticospinal (pyramidal) tract and the direct motor pathways from the cortex to the trunk
DBS efficacia, complicanze in cronico e nuovi orizzonti terapeutici
 DBS efficacia, complicanze in cronico e nuovi orizzonti terapeutici TECNICHE DI NEUROMODULAZIONE Invasiva: odeep Brain Stimulation Non Invasiva: o Transcranial Magnetic Stimulation (TMS) o Transcranial
DBS efficacia, complicanze in cronico e nuovi orizzonti terapeutici TECNICHE DI NEUROMODULAZIONE Invasiva: odeep Brain Stimulation Non Invasiva: o Transcranial Magnetic Stimulation (TMS) o Transcranial
BASAL GANGLIA. Dr JAMILA EL MEDANY
 BASAL GANGLIA Dr JAMILA EL MEDANY OBJECTIVES At the end of the lecture, the student should be able to: Define basal ganglia and enumerate its components. Enumerate parts of Corpus Striatum and their important
BASAL GANGLIA Dr JAMILA EL MEDANY OBJECTIVES At the end of the lecture, the student should be able to: Define basal ganglia and enumerate its components. Enumerate parts of Corpus Striatum and their important
PETER PAZMANY CATHOLIC UNIVERSITY Consortium members SEMMELWEIS UNIVERSITY, DIALOG CAMPUS PUBLISHER
 PETER PAZMANY CATHOLIC UNIVERSITY SEMMELWEIS UNIVERSITY Development of Complex Curricula for Molecular Bionics and Infobionics Programs within a consortial* framework** Consortium leader PETER PAZMANY
PETER PAZMANY CATHOLIC UNIVERSITY SEMMELWEIS UNIVERSITY Development of Complex Curricula for Molecular Bionics and Infobionics Programs within a consortial* framework** Consortium leader PETER PAZMANY
Basal ganglia activity patterns in parkinsonism and computational modeling of their downstream effects
 Basal ganglia activity patterns in parkinsonism and computational modeling of their downstream effects Jonathan E. Rubin, University of Pittsburgh Cameron C. McIntyre, Cleveland Clinic Robert S Turner,
Basal ganglia activity patterns in parkinsonism and computational modeling of their downstream effects Jonathan E. Rubin, University of Pittsburgh Cameron C. McIntyre, Cleveland Clinic Robert S Turner,
Making Every Little Bit Count: Parkinson s Disease. SHP Neurobiology of Development and Disease
 Making Every Little Bit Count: Parkinson s Disease SHP Neurobiology of Development and Disease Parkinson s Disease Initially described symptomatically by Dr. James Parkinson in 1817 in An Essay on the
Making Every Little Bit Count: Parkinson s Disease SHP Neurobiology of Development and Disease Parkinson s Disease Initially described symptomatically by Dr. James Parkinson in 1817 in An Essay on the
The Wonders of the Basal Ganglia
 Basal Ganglia The Wonders of the Basal Ganglia by Mackenzie Breton and Laura Strong /// https://kin450- neurophysiology.wikispaces.com/basal+ganglia Introduction The basal ganglia are a group of nuclei
Basal Ganglia The Wonders of the Basal Ganglia by Mackenzie Breton and Laura Strong /// https://kin450- neurophysiology.wikispaces.com/basal+ganglia Introduction The basal ganglia are a group of nuclei
Deep Brain Stimulation: Indications and Ethical Applications
 Deep Brain Stimulation Overview Kara D. Beasley, DO, MBe, FACOS Boulder Neurosurgical and Spine Associates (303) 562-1372 Deep Brain Stimulation: Indications and Ethical Applications Instrument of Change
Deep Brain Stimulation Overview Kara D. Beasley, DO, MBe, FACOS Boulder Neurosurgical and Spine Associates (303) 562-1372 Deep Brain Stimulation: Indications and Ethical Applications Instrument of Change
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
神經解剖學 NEUROANATOMY BASAL NUCLEI 盧家鋒助理教授臺北醫學大學醫學系解剖學暨細胞生物學科臺北醫學大學醫學院轉譯影像研究中心.
 神經解剖學 NEUROANATOMY BASAL NUCLEI 盧家鋒助理教授臺北醫學大學醫學系解剖學暨細胞生物學科臺北醫學大學醫學院轉譯影像研究中心 http://www.ym.edu.tw/~cflu OUTLINE Components and Pathways of the Basal Nuclei Functions and Related Disorders of the Basal Nuclei
神經解剖學 NEUROANATOMY BASAL NUCLEI 盧家鋒助理教授臺北醫學大學醫學系解剖學暨細胞生物學科臺北醫學大學醫學院轉譯影像研究中心 http://www.ym.edu.tw/~cflu OUTLINE Components and Pathways of the Basal Nuclei Functions and Related Disorders of the Basal Nuclei
Oscillations: From Neuron to MEG
 Oscillations: From Neuron to MEG Educational Symposium, MEG UK 2014, Nottingham, Jan 8th 2014 Krish Singh CUBRIC, School of Psychology Cardiff University What are we trying to achieve? Bridge the gap from
Oscillations: From Neuron to MEG Educational Symposium, MEG UK 2014, Nottingham, Jan 8th 2014 Krish Singh CUBRIC, School of Psychology Cardiff University What are we trying to achieve? Bridge the gap from
Data Mining Tools used in Deep Brain Stimulation Analysis Results
 Data Mining Tools used in Deep Brain Stimulation Analysis Results Oana Geman PhD, Faculty of Electrical Engineering and Computer Science, Stefan cel Mare University, Suceava, Romania geman@eed.usv.ro Abstract.
Data Mining Tools used in Deep Brain Stimulation Analysis Results Oana Geman PhD, Faculty of Electrical Engineering and Computer Science, Stefan cel Mare University, Suceava, Romania geman@eed.usv.ro Abstract.
Basal Ganglia Anatomy, Physiology, and Function. NS201c
 Basal Ganglia Anatomy, Physiology, and Function NS201c Human Basal Ganglia Anatomy Basal Ganglia Circuits: The Classical Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate
Basal Ganglia Anatomy, Physiology, and Function NS201c Human Basal Ganglia Anatomy Basal Ganglia Circuits: The Classical Model of Direct and Indirect Pathway Function Motor Cortex Premotor Cortex + Glutamate
III./3.1. Movement disorders with akinetic rigid symptoms
 III./3.1. Movement disorders with akinetic rigid symptoms III./3.1.1. Parkinson s disease Parkinson s disease (PD) is the second most common neurodegenerative disorder worldwide after Alzheimer s disease.
III./3.1. Movement disorders with akinetic rigid symptoms III./3.1.1. Parkinson s disease Parkinson s disease (PD) is the second most common neurodegenerative disorder worldwide after Alzheimer s disease.
Functional Distinctions
 Functional Distinctions FUNCTION COMPONENT DEFICITS Start Basal Ganglia Spontaneous Movements Move UMN/LMN Cerebral Cortex Brainstem, Spinal cord Roots/peripheral nerves Plan Cerebellum Ataxia Adjust Cerebellum
Functional Distinctions FUNCTION COMPONENT DEFICITS Start Basal Ganglia Spontaneous Movements Move UMN/LMN Cerebral Cortex Brainstem, Spinal cord Roots/peripheral nerves Plan Cerebellum Ataxia Adjust Cerebellum
Chapter 8. Control of movement
 Chapter 8 Control of movement 1st Type: Skeletal Muscle Skeletal Muscle: Ones that moves us Muscles contract, limb flex Flexion: a movement of a limb that tends to bend its joints, contraction of a flexor
Chapter 8 Control of movement 1st Type: Skeletal Muscle Skeletal Muscle: Ones that moves us Muscles contract, limb flex Flexion: a movement of a limb that tends to bend its joints, contraction of a flexor
Clinical Features and Treatment of Parkinson s Disease
 Clinical Features and Treatment of Parkinson s Disease Richard Camicioli, MD, FRCPC Cognitive and Movement Disorders Department of Medicine University of Alberta 1 Objectives To review the diagnosis and
Clinical Features and Treatment of Parkinson s Disease Richard Camicioli, MD, FRCPC Cognitive and Movement Disorders Department of Medicine University of Alberta 1 Objectives To review the diagnosis and
Kinematic Modeling in Parkinson s Disease
 Kinematic Modeling in Parkinson s Disease Alexander Hui Department of Bioengineering University of California, San Diego La Jolla, CA 92093 alexhui@ucsd.edu Abstract Parkinson s disease is a slowly progressing
Kinematic Modeling in Parkinson s Disease Alexander Hui Department of Bioengineering University of California, San Diego La Jolla, CA 92093 alexhui@ucsd.edu Abstract Parkinson s disease is a slowly progressing
Electrical recording with micro- and macroelectrodes from the cerebellum of man
 Electrical recording with micro- and macroelectrodes from the cerebellum of man D. GRAHAM SLAUGHTER, M.D., BLAINE S. NASHOLD, Jn., M.D., AND GEORGE G. SOMJEN, M.D. The Division of Neurosurgery, and the
Electrical recording with micro- and macroelectrodes from the cerebellum of man D. GRAHAM SLAUGHTER, M.D., BLAINE S. NASHOLD, Jn., M.D., AND GEORGE G. SOMJEN, M.D. The Division of Neurosurgery, and the
Stimulation of the Subthalamic Nucleus Changes the Firing Pattern of Pallidal Neurons
 1916 The Journal of Neuroscience, March 1, 2003 23(5):1916 1923 Stimulation of the Subthalamic Nucleus Changes the Firing Pattern of Pallidal Neurons Takao Hashimoto, 1,2 Christopher M. Elder, 1 Michael
1916 The Journal of Neuroscience, March 1, 2003 23(5):1916 1923 Stimulation of the Subthalamic Nucleus Changes the Firing Pattern of Pallidal Neurons Takao Hashimoto, 1,2 Christopher M. Elder, 1 Michael
This is a repository copy of Basal Ganglia. White Rose Research Online URL for this paper:
 This is a repository copy of. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/114159/ Version: Accepted Version Book Section: Prescott, A.J. orcid.org/0000-0003-4927-5390,
This is a repository copy of. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/114159/ Version: Accepted Version Book Section: Prescott, A.J. orcid.org/0000-0003-4927-5390,
CN V! touch! pain! Touch! P/T!
 CN V! touch! pain! Touch! P/T! Visual Pathways! L! R! B! A! C! D! LT! E! F! RT! G! hypothalamospinal! and! ALS! Vestibular Pathways! 1. Posture/Balance!!falling! 2. Head Position! 3. Eye-Head Movements
CN V! touch! pain! Touch! P/T! Visual Pathways! L! R! B! A! C! D! LT! E! F! RT! G! hypothalamospinal! and! ALS! Vestibular Pathways! 1. Posture/Balance!!falling! 2. Head Position! 3. Eye-Head Movements
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and
 This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
Huntington s Disease COGS 172
 Huntington s Disease COGS 172 Overview Part I: What is HD? - Clinical description and features - Genetic basis and neuropathology - Cell biology, mouse models and therapeutics Part II: HD as a model in
Huntington s Disease COGS 172 Overview Part I: What is HD? - Clinical description and features - Genetic basis and neuropathology - Cell biology, mouse models and therapeutics Part II: HD as a model in
Towards a Better Treatment of Parkinson's disease
 Loma Linda University TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works Loma Linda University Electronic Theses, Dissertations & Projects 3-2014 Towards a Better Treatment
Loma Linda University TheScholarsRepository@LLU: Digital Archive of Research, Scholarship & Creative Works Loma Linda University Electronic Theses, Dissertations & Projects 3-2014 Towards a Better Treatment
A3.1.7 Motor Control. 10 November 2016 Institute of Psychiatry,Psychology and Neuroscience Marinela Vavla
 A3.1.7 Motor Control 10 November 2016 Institute of Psychiatry,Psychology and Neuroscience Marinela Vavla marinela.vavla@kcl.ac.uk Learning objectives Motor systems: components & organization Spinal cord
A3.1.7 Motor Control 10 November 2016 Institute of Psychiatry,Psychology and Neuroscience Marinela Vavla marinela.vavla@kcl.ac.uk Learning objectives Motor systems: components & organization Spinal cord
UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL) Spring, 2014
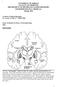 UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL) Spring, 2014 Textbook of Medical Physiology by: Guyton & Hall, 11 th edition 2006 Eman Al-Khateeb,
UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL) Spring, 2014 Textbook of Medical Physiology by: Guyton & Hall, 11 th edition 2006 Eman Al-Khateeb,
This manuscript version is distributed under the CC-BY-NC-ND (Creative Commons) license.
 This manuscript version is distributed under the CC-BY-NC-ND (Creative Commons) license. It has appeared in final form as: Van Albada SJ, Gray RT, Drysdale PM, Robinson PA. Mean-field modeling of the basal
This manuscript version is distributed under the CC-BY-NC-ND (Creative Commons) license. It has appeared in final form as: Van Albada SJ, Gray RT, Drysdale PM, Robinson PA. Mean-field modeling of the basal
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 1/1/2019 Section: SUR Policy No: 395 1/1/19 Medical Policy Committee Approved Date: 8/17; 2/18; 12/18 Medical Officer Date APPLIES TO: Medicare Only See Policy CPT/HCPCS CODE section below
Effective Date: 1/1/2019 Section: SUR Policy No: 395 1/1/19 Medical Policy Committee Approved Date: 8/17; 2/18; 12/18 Medical Officer Date APPLIES TO: Medicare Only See Policy CPT/HCPCS CODE section below
HUMAN MOTOR CONTROL. Emmanuel Guigon
 HUMAN MOTOR CONTROL Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris, France emmanuel.guigon@upmc.fr e.guigon.free.fr/teaching.html
HUMAN MOTOR CONTROL Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris, France emmanuel.guigon@upmc.fr e.guigon.free.fr/teaching.html
nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
 Nucleus accumbens From Wikipedia, the free encyclopedia Brain: Nucleus accumbens Nucleus accumbens visible in red. Latin NeuroNames MeSH NeuroLex ID nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
Nucleus accumbens From Wikipedia, the free encyclopedia Brain: Nucleus accumbens Nucleus accumbens visible in red. Latin NeuroNames MeSH NeuroLex ID nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
Motor systems.... the only thing mankind can do is to move things... whether whispering or felling a forest. C. Sherrington
 Motor systems... the only thing mankind can do is to move things... whether whispering or felling a forest. C. Sherrington 1 Descending pathways: CS corticospinal; TS tectospinal; RS reticulospinal; VS
Motor systems... the only thing mankind can do is to move things... whether whispering or felling a forest. C. Sherrington 1 Descending pathways: CS corticospinal; TS tectospinal; RS reticulospinal; VS
OSCILLATIONS IN THE BASAL GANGLIA: The good, the bad, and the unexpected
 In: The Basal Ganglia VIII (Editors: Bolam, J.P., Ingham, C.A. and Magill, P.J.) Springer Science and Business Media, New York. Published in 2005. OSCILLATIONS IN THE BASAL GANGLIA: The good, the bad,
In: The Basal Ganglia VIII (Editors: Bolam, J.P., Ingham, C.A. and Magill, P.J.) Springer Science and Business Media, New York. Published in 2005. OSCILLATIONS IN THE BASAL GANGLIA: The good, the bad,
Systems Neuroscience Dan Kiper. Today: Wolfger von der Behrens
 Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
Motor Functions of Cerebral Cortex
 Motor Functions of Cerebral Cortex I: To list the functions of different cortical laminae II: To describe the four motor areas of the cerebral cortex. III: To discuss the functions and dysfunctions of
Motor Functions of Cerebral Cortex I: To list the functions of different cortical laminae II: To describe the four motor areas of the cerebral cortex. III: To discuss the functions and dysfunctions of
