A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis
|
|
|
- Merryl Abigayle Stokes
- 6 years ago
- Views:
Transcription
1 Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, Developmental Cell (2013), Article A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis Mohammad Samie, 1 Xiang Wang, 1 Xiaoli Zhang, 1 Andrew Goschka, 1 Xinran Li, 1 Xiping Cheng, 1 Evan Gregg, 1 Marlene Azar, 1 Yue Zhuo, 1 Abigail G. Garrity, 1 Qiong Gao, 1 Susan Slaugenhaupt, 4 Jim Pickel, 5 Sergey N. Zolov, 3 Lois S. Weisman, 3 Guy M. Lenk, 2 Steve Titus, 6 Marthe Bryant-Genevier, 6 Noel Southall, 6 Marugan Juan, 6 Marc Ferrer, 6 and Haoxing Xu 1, * 1 Department of Molecular, Cellular, and Developmental Biology 2 Department of Human Genetics 3 Department of Cell and Developmental Biology and Life Sciences Institute University of Michigan, Ann Arbor, MI 48109, USA 4 Department of Neurology (Genetics), Center for Human Genetic Research, Massachusetts General Hospital/Harvard Medical School, CPZN-5254, 185 Cambridge Street, Boston, MA 02114, USA 5 NIMH Transgenics, National Institutes of Health, 49 Convent Drive Room B1C25, Bethesda, MD 20892, USA 6 NIH/NCATS/NCGC, 9800 Medical Center Drive, Rockville, MD 20850, USA *Correspondence: haoxingx@umich.edu SUMMARY Phagocytosis of large extracellular particles such as apoptotic bodies requires delivery of the intracellular endosomal and lysosomal membranes to form plasmalemmal pseudopods. Here, we identified mucolipin TRP channel 1 (TRPML1) as the key lysosomal Ca 2+ channel regulating focal exocytosis and phagosome biogenesis. Both particle ingestion and lysosomal exocytosis are inhibited by synthetic TRPML1 blockers and are defective in macrophages isolated from TRPML1 knockout mice. Furthermore, TRPML1 overexpression and TRPML1 agonists facilitate both lysosomal exocytosis and particle uptake. Using time-lapse confocal imaging and direct patch clamping of phagosomal membranes, we found that particle binding induces lysosomal PI(3,5)P 2 elevation to trigger TRPML1-mediated lysosomal Ca 2+ release specifically at the site of uptake, rapidly delivering TRPML1-resident lysosomal membranes to nascent phagosomes via lysosomal exocytosis. Thus phagocytic ingestion of large particles activates a phosphoinositide- and Ca 2+ -dependent exocytosis pathway to provide membranes necessary for pseudopod extension, leading to clearance of senescent and apoptotic cells in vivo. INTRODUCTION Macrophages participate in tissue remodeling and cellular clearance by engulfing large extracellular particles, such as apoptotic cells or senescent red blood cells (RBCs), through a highly regulated process called phagocytosis (Flannagan et al., 2012). Particle binding to specific cell surface receptors, such as IgG Fc receptors, triggers a cascade of signaling events to rapidly extend the plasma membrane around the particle(s) (Aderem and Underhill, 1999). Plasma membrane extensions, called plasmalemmal pseudopods, form phagocytic cups to ingest particles into vacuole-like structures called phagosomes within several minutes after particle binding (Niedergang and Chavrier, 2004) (see Figure 6I). Nascent phagosomes then undergo membrane fusion and fission events, collectively called phagosomal maturation, in a time course of tens of minutes to become phagolysosomes to mediate particle digestion (Aderem and Underhill, 1999; Vieira et al., 2002). Although a large amount of plasma membrane is internalized during phagocytosis (Aderem and Underhill, 1999; Touret et al., 2005), paradoxically, the overall surface area of the phagocytosing cells does not decrease (Braun and Niedergang, 2006; Huynh et al., 2007) and indeed increases significantly before the closing of the phagocytic cups (Holevinsky and Nelson, 1998). Consistently, membrane fusion machinery is shown to be required for phagosome formation/ biogenesis and efficient phagocytosis (Hackam et al., 1998), and a substantial amount of membrane from intracellular compartments are found to be added to the cell surface at the sites of particle uptake (so called focal exocytosis) (Braun et al., 2004; Czibener et al., 2006). Recycling endosomes have been shown as a supply of intracellular membrane for phagosome formation and particle uptake in a vesicle-associated membrane protein 3 (VAMP3)-dependent manner (Cox et al., 1999; Bajno et al., 2000). More recently, lysosomes have been identified as another major source of intracellular membrane required for particle uptake, particularly under high load conditions, i.e., when large (>5 mm) and/or multiple particles are ingested by a single phagocyte (Huynh et al., 2007). Under such conditions, lysosomes are involved in the very early steps of phagosome biogenesis/formation, prior to the closing of the phagocytic cups (see Figure 6I) (Braun et al., 2004; Czibener et al., 2006). Fusion of lysosomes with the plasma membrane (lysosomal exocytosis) and the delivery of lysosomal membranes/proteins to the surface of the newly formed phagosomes are observed at the sites of particle ingestion (Swanson et al., 1987; Tapper and Sundler, 1995), and manipulations blocking lysosomal exocytosis significantly reduce particle uptake in macrophages (Braun et al., 2004; Czibener et al., 2006). 26, 1 14, September 16, 2013 ª2013 Elsevier Inc. 1
2 Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, Developmental Cell (2013), TRPML1 Channels Regulate Phagosome Formation Ca 2+ is the only known trigger that has been identified for lysosomal exocytosis (Andrews, 2000), with Synaptotagmin VII (Syt-VII) being the primary Ca 2+ sensor in the lysosome (Czibener et al., 2006). Vesicle-associated membrane protein 7 (VAMP7), a SNARE protein in the late endosome and lysosome, is required for lysosomal exocytosis (Braun et al., 2004). Consistent with the role of lysosomal exocytosis in particle ingestion, macrophages lacking VAMP7 or Syt VII exhibit defects in both lysosomal exocytosis and particle uptake, but preferentially under high particle loads (Braun et al., 2004; Czibener et al., 2006). Intracellular Ca 2+ ([Ca 2+ ] i ) elevation was observed in the vicinity of the particle uptake sites in neutrophils (Theler et al., 1995; Tapper et al., 2002), but not in macrophages (Nunes and Demaurex, 2010). However, preloading cells with the fast membrane-permeant Ca 2+ chelator BAPTA-AM dramatically reduces lysosomal exocytosis and particle uptake in macrophages (Tapper et al., 2002; Czibener et al., 2006), suggesting that a small amount of Ca 2+ release from nonconventional internal stores that might have escaped detection by using conventional Ca 2+ imaging methods may play a crucial role in macrophage phagocytosis. Because the lysosomal lumen is believed to be the main source of Ca 2+ for Ca 2+ -dependent vesicular trafficking in the late endocytic pathways (Pryor et al., 2000), it is possible that lysosomes themselves provide the Ca 2+ required for particle-induced exocytosis. However, definitive evidence to support this hypothesis is still lacking, and more importantly, the ion channel(s) responsible for Ca 2+ release from lysosomes still remains elusive. Transient receptor potential mucolipin-1 (TRPML1 or ML1) is a member of the TRP cation channel superfamily and is primarily localized on the lysosome membrane (Cheng et al., 2010). Human mutations of TRPML1 cause mucolipidosis type IV (MLIV), a childhood neurodegenerative disorder with lysosomal trafficking defects at the cellular level (Sun et al., 2000; Cheng et al., 2010). In a Drosophila model of MLIV, it has been proposed that the defective clearance of late apoptotic neurons by phagocytes contributes significantly to neurodegeneration (Venkatachalam et al., 2008). By performing patch-clamp recordings directly on lysosomal membranes and by measuring lysosomal Ca 2+ release using genetically encoded Ca 2+ sensors, we have characterized ML1 as a Ca 2+ -permeable channel on the lysosomal membrane (Dong et al., 2010; Shen et al., 2012). ML1 conducts Ca 2+ from the lysosome lumen into the cytosol and is specifically activated by phosphatidylinositol 3,5-bisphosphate [PI(3,5)P 2 ], a late endosome and lysosome-specific low-abundance phosphoinositide (Dong et al., 2010). In the current study, using mouse knockouts and synthetic agonists/antagonists of ML1, we investigated the roles of ML1 in phagocytic particle uptake in bone marrow-derived macrophages. RESULTS Expression of ML1 Is Necessary for Efficient Uptake of Large Particles in Mouse Macrophages To study particle uptake/ingestion, we isolated bone marrow macrophages (BMMs) (Chow et al., 2004) from wild-type (WT) and ML1 knockout (KO) mice (Venugopal et al., 2007). ML1 KO BMMs contained no detectable level of full-length ML1 transcript, as shown by RT-PCR analysis (Figure 1A). Consistent with this, direct patch-clamping the endolysosomal membranes (Dong et al., 2008) showed that ML-SA1, a membrane-permeable TRPML-specific synthetic agonist (Shen et al., 2012), and PI(3,5)P 2, an endogenous activator of ML1 (Cheng et al., 2010; Dong et al., 2010), activated whole-endolysosome ML1-like currents (I ML1 ) in WT, but not ML1 KO BMMs (Figure 1B). In contrast, no significant differences were noted in cell morphology, cell size, and CD11b (macrophage-specific surface receptor) immunoreactivity between WT and ML1 KO BMMs (Figure S1A available online). The use of animals was approved by the University Committee on Use and Care for Animals (UCUCA) at the University of Michigan. To investigate the role of ML1 in particle ingestion, WT and ML1 KO BMMs were exposed to IgG-opsonized sheep red blood cells (IgG-RBCs), all 5 mm in size, for different periods of time (15 90 min; Figure 1C). IgG-RBC uptake was quantified from at least 150 BMMs per time point for each genotype; uningested IgG-RBCs were hypotonically lysed by briefly (1 2 min) incubating the cells in 4 C water (Chow et al., 2004). Ingested IgG-RBCs were counted individually by experimenters who were blind to the genotypes and experimental conditions. Significantly fewer IgG-RBCs were internalized by ML1 KO BMMs compared with WT controls at the latter three time points (30, 60, and 90 min; Figures 1C 1E). Based on distribution histograms of the number of ingested particles per cell (Figure S1B), thresholds were set (4 to 10 or more particles per cell) based on the cell type and particle size to compare the phagocytic capability. Based on a threshold of 10 or more (10+) IgG-RBCs for BMMs (Figure 1D), a significant difference in particle uptake was noted between WT and ML1 KO BMMs, with the uptake defects being more severe as time progressed (Figure 1C 1E and S1B). After 90 min, 50% of WT, but less than 20% of ML1 KO BMMs contained 10+ internalized IgG-RBCs (Figure 1D). The progressive inhibition of uptake caused by ML1 deficiency suggested that ML1 is necessary for the ongoing uptake of large particles, which is consistent with the reported, preferential requirement of lysosomal membrane delivery for large particle uptake (Huynh et al., 2007). To further probe this possibility, BMMs were tested for their ability to ingest different-sized IgGopsonized polystyrene beads. Interestingly, although the internalization of 3 mm beads (a threshold of mm beads set for BMMs) was similar for WT and ML1 KO BMMs, uptake of 6 mm beads (a threshold of 4+ 6 mm beads for BMMs) was significantly reduced in ML1 KO BMMs at all time points (Figures 1F and S1C). Uptake of zymosan particles (diameter = 2 3 mm) was also normal for ML1 KO BMMs (Figure S1D). These observations are reminiscent of the particle size-dependent uptake defect seen in Syt VII / BMMs (Czibener et al., 2006). Taken together, these results suggest that ML1 is required for efficient uptake of large particles, i.e., when the need for membranes from internal sources is high (Huynh et al., 2007). The Channel Activity of ML1 Regulates Large Particle Uptake in Macrophages Next, we investigated whether increasing the expression/ activity of ML1 can facilitate particle uptake in macrophages. Incubating WT BMMs with a TRPML agonist, ML-SA1 (1 10 mm), for 15 min caused more than a 2-fold increase in the percentage of cells containing 10+ IgG-RBCs (Figure 2A). In 2 26, 1 14, September 16, 2013 ª2013 Elsevier Inc.
3 Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, Developmental Cell (2013), TRPML1 Channels Regulate Phagosome Formation A C RT- PCR L32 WT BMMs WT ML1 KO ML1 KO E per 100 BMMs B 15 min 30 min 60 min 90 min srbc/dapi F BMMs D BMMs BMMs Figure 1. TRPML1 Is Necessary for Optimal Phagocytosis of Large Particles in Bone Marrow Macrophages (A) RT-PCR analysis of wild-type (WT) and TRPML1 KO (ML1 KO) bone marrow-derived macrophages (BMMs) using a primer pair targeting the deleted region (exons 3, 4, and 5) of the TRPML1 gene (Venugopal et al., 2007). The housekeeping gene L32 served as a loading control. (B) ML-SA1 robustly activated endogenous wholeendolysosome ML1-like currents in WT, but not ML1 KO BMMs. (C) WT and ML1 KO BMMs were exposed to IgG-opsonized red blood cells (IgG-RBCs; red colored) at a ratio of 50 RBCs/BMM for time periods indicated (15, 30, 60, and 90 min). Noningested IgG-RBCs were lysed by briefly (1 2 min) incubating the cells in water at 4 C. Samples were then fixed and processed for confocal microscopy. (D) Average particle ingestion for WT and ML1 KO BMMs. Ingested IgG-RBCs were quantified for BMMs per experiment, by experimenters who were blind to the genotype. (E) ML1 KO BMMs had a lower uptake index compared with WT BMMs. Uptake index was calculated based on the total number of RBCs ingested for 100 BMMs. (F) Particle-size-dependent phagocytosis defect of ML1 KO BMMs. BMMs were exposed to 3 or 6 mm IgG-coated polystyrene beads for indicated periods of time. Samples were washed extensively and briefly trypsinized to dissociate noningested beads attached to the cell surface or coverslips. The number of ingested particles was determined as described in (D). For all panels, unless otherwise indicated, the data represent the mean ± SEM from at least three independent experiments. See also Figure S1. contrast, the increase was negligible for ML1 KO BMMs (Figure 2A). Moreover, heterologous expression of ML1-GFP in ML1 KO BMMs was sufficient to restore the uptake efficiency to the same level of WT BMMs, whereas expression of ML1- KK-GFP, a nonconducting pore mutant of ML1 (Dong et al., 2010), had no effect (Figure 2B). These results established a positive correlation between the channel activity of ML1 and IgG-RBC uptake. We also examined particle uptake in RAW macrophage cell line, as well as RAW cell lines stably expressing ML1 (ML1 overexpression or O/E) or ML1-specfic RNAi (ML1 knockdown or KD) (Thompson et al., 2007). ML1 expression levels in these three cell lines were confirmed using wholeendolysosome recordings (Figure 2C) and RT-PCR analysis (Figure 2D). Interestingly, particle uptake efficiency in these lines correlated with their respective expression levels of ML1, with an even greater correlation in the presence of ML-SA1 (Figures 2E and S2A). The threshold of 5+ ingested IgG-RBCs was set to reflect the smaller size of RAW cells compared to BMMs. Although 30% of the ML1 O/E cells ingested 5+ IgG-RBCs, less than 10% of ML1 KD cells contained 5+ IgG-RBCs (Figure 2E). Collectively, these data suggest that the efficiency of particle uptake in macrophages is positively correlated with the expression level and channel activity of ML1. Consistent with previous studies showing that uptake of large particles is a Ca 2+ -dependent process in BMMs (Czibener et al., 2006), and consistent with ML1 being a lysosomal Ca 2+ -permeable channel (Cheng et al., 2010), pretreatment of BMMs with a membrane-permeable, fast Ca 2+ chelator BAPTA-AM (Shen et al., 2011) inhibited particle uptake in WT macrophages but to a lower degree in ML1 KO cells (Figure 2F). In contrast, a membrane-permeable, slow Ca 2+ chelator EGTA-AM had little or no effect (Figure S2B), suggesting a local source of Ca 2+ (Shen et al., 2011). To further investigate the role of ML1 in particle uptake, we also employed pharmacological tools to acutely inhibit the channel function of ML1. Using a Ca 2+ imaging-based highthroughput screening method (Shen et al., 2012), we identified three synthetic small molecule inhibitors for ML1 (mucolipin synthetic inhibitor 1-3 or ML-SI1-3; see Figures S2C and S2F). Using whole-endolysosome patch-clamp recordings, we confirmed that ML-SI1 strongly inhibited basal (Figure 2G), ML-SA1, or PI(3,5)P 2 -activated I ML1 (Figure S2D). In contrast, ML-SI1 failed to inhibit PI(3,5)P 2 -activated whole-endolysosome TPC2 currents (Wang et al., 2012) (Figure S2E), demonstrating a relative specificity. ML-SI1 (10 mm) treatment was sufficient to reduce IgG-RBC uptake in WT BMMs to the same level as ML1 KO BMMs, without significantly affecting the uptake in ML1 KO cells (Figure 2H). Furthermore, all three ML1 inhibitors significantly reduced the uptake of IgGopsonized 6 mm beads (Figure S2G). These results suggest that the channel activity of ML1 is required for large particle uptake in macrophages. 26, 1 14, September 16, 2013 ª2013 Elsevier Inc. 3
4 Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, Developmental Cell (2013), TRPML1 Channels Regulate Phagosome Formation A C D F BMMs G B E H BMMs Figure 2. The Expression Level and Channel Activity of ML1 Regulate Particle Ingestion in Macrophages (A) Micromolar concentrations of ML-SA1 (0.1, 1, and 10 mm; preincubation for 15 min) increased particle uptake in WT, but not ML1 KO BMMs. BMMs were exposed to IgG-RBCs in the presence of ML-SA1 for 15 min. (B) Transfection of ML1-GFP, but not ML1-KK- GFP (nonconducting pore mutation) (Dong et al., 2010), rescued the uptake defect in ML1 KO BMMs. BMMs were exposed to IgG-RBCs for 30 min in the presence or absence of the small molecule TRPML agonist, ML-SA1 (10 mm). (C) ML-SA1-activated whole-endolysosome TRPML-like currents in RAW macrophage cell lines, RAW cells stably expressing ML1-GFP (ML1 overexpression or O/E), and RAW cells stably expressing ML1-specific RNAi (ML1 knockdown or KD) (Thompson et al., 2007). (D) Real-time RT-PCR analysis of ML1 RNA expression level (relative to L32) in RAW macrophages (n = 3 batches of independent experiments for each cell type). (E) Particle uptake in RAW 264.7, ML1 O/E, and ML1 KD RAW macrophages. (F) Particle uptake was sensitive to intracellular Ca 2+. BMMs were preincubated with the membrane-permeable fast Ca 2+ chelator BAPTA-AM (1 mm; 15 min) prior to IgG-RBC exposure. (G) ML-SI1 inhibited large basal whole-endolysosome I ML1 that was seen in a subset of ML1- expressing Cos1 cells. (H) ML-SI1 (10 mm; preincubation for 15 min) inhibited particle ingestion in WT BMMs to a similar level of that in ML1 KO BMMs. BMMs were exposed to IgG-RBCs in the presence of ML-SI1 for 30 min. For all panels, unless otherwise indicated, the data represent the mean ± SEM from at least three independent experiments; BMMs were analyzed for each experiment. See also Figure S2. ML1 Is Rapidly Recruited to Phagocytic Cups and Nascent Phagosomes To investigate the mechanisms by which ML1 contributes to particle uptake, we performed time-lapse confocal microscopy of live RAW cells during the particle uptake process. Strikingly, we observed that ML1-GFP was rapidly (within 1 3 min) recruited to the sites of phagosome formation during uptake of 6 mm beads (Figure 3A; Movie S1) and IgG-RBCs (Figure S3A; Movie S2). In contrast, ML1-GFP was not recruited to the sites of phagosome formation during uptake of 3 mm beads (Figure S3B; see also Figure 3C). Next, we compared the localization of ML1 with other endosomal and lysosomal proteins that are known to be involved in phagosome formation and maturation. Syt VII is a lysosomal Ca 2+ sensor, but has been shown to be recruited to the nascent phagosomes upon particle binding (Czibener et al., 2006). BMMs isolated from Syt VII KO mice exhibit a similar phenotype as ML1 KO BMMs: the defect is proportional to the particle size (Czibener et al., 2006). Both ML1-GFP and Syt VII-mCherry were found to be colocalized at the tip of the pseudopods in doubly transfected RAW cells (Figure S3C). Within 2 3 min after exposing the RAW cells to IgG-RBCs, both ML1-GFP and Syt VII-mCherry appeared on the membranes of nascent phagosomes (Figure 3B; Movie S3). ML1-GFP and Syt VII-mCherrypositive (lysosomal) tubules were then observed wrapping themselves around the newly formed phagosomes (Figure 3B, frame 3 min 53 s); the recruitment kinetics was similar for both proteins (Movie S3). Similar to ML1 and Syt-VII, lysosome-associated membrane protein 1 (Lamp-1) was also rapidly recruited to newly formed large-particle-containing phagosomes in RAW macrophages BMMs (Figure 3C). However, rapid recruitment of ML1 and Lamp1 were not seen in the presence of ML-SI1 (Figure S3E), or for small particle uptake (Figure S3D). The recruitment of ML1 and Lamp1 to forming phagosomes occurred prior to the recruitment of Rab5, an early phagosome maturation marker (Kitano et al., 2008; Guo et al., 2010), or Rab7, a late phagosome maturation marker (Henry et al., 2004; Guo et al., 2010) (Figures 3C and 3D). Consistently, during large particle uptake, the newly formed phagosomes (5 min after particle binding) in WT BMMs, compared with ML1 KO BMMs, contained significantly more Lamp1 proteins (Figure S3F). Phagosomes are still wrapped 4 26, 1 14, September 16, 2013 ª2013 Elsevier Inc.
5 Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, Developmental Cell (2013), TRPML1 Channels Regulate Phagosome Formation with polymerized actin within 5 10 min after particle binding (Figure S3G; see also Czibener et al., 2006). Collectively, these results suggest that during large particle uptake, recruitment of ML1 and other lysosomal membrane proteins occurred in the very early stages of phagosome formation, prior to Rab5- mediated early phagosome maturation. The recruitment of Lamp1 was significantly delayed in the ML1 KO BMMs, or in the presence of ML1 inhibitors (Figure S3E), suggestive of a crucial role of ML1 in delivering lysosomal proteins and membranes to phagocytic cups. To further separate phagosome formation from late phagosome maturation, we monitored the ML1-GFP recruitment in macrophages that were exposed to small particles. In contrast to large particle uptake, ML1 was recruited to small particlecontaining phagosomes only after min of phagocytosis initiation (Figures 3C and 3D), which was after Rab5 recruitment, but at a similar time course to Rab7 recruitment and phagosomal acidification (Figures S3D and S3E). Hence, ML1 might also play a role in late phagosome maturation. To further confirm the presence of ML1 on the newly formed phagosomes, we developed a patch-clamp method to directly record from phagosomal membranes (Figures 4A and 4B). Phagosomes were isolated from Lamp1-GFP or ML1-GFPtransfected RAW cells after exposure to IgG-RBCs or beads for 5 min. In Lamp1-GFP-transfected RAW cells, bath application of PI(3,5)P 2 (100 nm), or ML-SA1 (25 mm) readily activated endogenous whole-phagosome ML1-like currents (Figure 4C). Much larger whole-phagosome ML-SA1-activated currents were seen in ML1-GFP-transfected RAW cells. In WT BMMs, whole-phagosome I ML1 was activated by ML-SA1 and inhibited by ML-SI1 (Figure 4D). No significant whole-phagosome I ML1 was seen in ML1 KO BMMs (Figure 4D). In contrast, PI(3,5)P 2 - activated TPC currents (Wang et al., 2012) were present in a subset of WT and ML1 KO BMMs (Figure 4D). These results thus provide functional evidence that ML1 is recruited to nascent phagosomes. Particle Binding Induces ML1-Mediated Lysosomal Ca 2+ Release and Lysosomal Exocytosis in Macrophages Lysosome fusion with the plasma membrane (lysosomal exocytosis) has been shown to be required for the uptake of large particles in macrophages (Czibener et al., 2006). Fibroblasts from ML4 patients exhibit impaired lysosomal exocytosis induced by ionomycin and increased expression levels of the TFEB lysosome biogenesis transcription factor (LaPlante et al., 2006; Medina et al., 2011). To directly investigate the role of ML1 in lysosomal exocytosis, we acutely activated ML1 using ML-SA1 and evaluated lysosomal exocytosis by using Lamp1 surface immunostaining with the ML1 KO as the negative control. After lysosomal exocytosis, luminal lysosomal membrane proteins can be detected on the extracellular side of the plasma membrane by measuring surface expression of Lamp1 with a monoclonal antibody (1D4B) against a luminal epitope of Lamp1 (Reddy et al., 2001). After incubation with the agonist ML-SA1 for 30 min, WT, but not ML1 KO BMMs, exhibited a marked increase in Lamp1 staining (Figures 5A, S4A, and S4B). Pretreatment with the fast Ca 2+ chelator BAPTA-AM almost completely abolished ML-SA1-induced Lamp1 surface staining in WT BMMs (Figure S4A). Collectively, these results suggest that the channel activity of ML1 is required for lysosomal exocytosis in macrophages in a Ca 2+ -dependent manner. Upon lysosomal exocytosis, lysosomal hydrolytic enzymes such as acid phosphatases (APs) are released into the extracellular medium (Reddy et al., 2001). Incubation with ML-SA1 (1 or 10 mm for 15 and 30 min) caused a significant increase in the AP release/activity in WT, but not ML1 KO BMMs (Figures S4C and S4D). Treating cells with Ca 2+ ionophore ionomycin induced a large AP release in ML1 KO BMMs, suggesting that the exocytosis machinery was operative (Figure S4E). In RAW macrophage cell lines, the level of ML-SA1-induced AP release was correlated with the expression level and channel activity of ML1 (Figure S4C). ML-SA1-induced AP release in WT BMMs was largely abolished by BAPTA-AM treatment, but not by removing extracellular Ca 2+ (Figure S4F). Lysosomal membrane fusion events are presumed to be dependent on [Ca 2+ ] i increase in the close vicinity of fusion spots, though direct evidence has not been reported (Pryor et al., 2000; Piper and Luzio 2004; Shen et al., 2011). Because ML1-GFP was colocalized with Lamp1 in the tips of pseudopods in RAW cells (Figure S3C), we used a lysosome-targeted genetically encoded Ca 2+ Indicator, GCaMP3-ML1, to specifically measure lysosomal Ca 2+ release in intact cells (Shen et al., 2012). Transient and localized Ca 2+ increases, measured with GCaMP3 fluorescence changes, were observed preferentially at the uptake sites of Syt VII-mCherry-positive lysosomes within several minutes of particle binding for both IgG-RBCs (Figure 5B, frames 45 s and 3 min and 15 s) and 6 mm beads (Figure S4G). In contrast, little or no GCaMP3 fluorescence increase was seen in other areas of the phagocytosing macrophages within the same time course (Figures 5B, S4H, and S4I). These results suggest that particle binding induces ML1-mediated lysosomal Ca 2+ release, locally at the sites of uptake. To investigate the role of ML1 in lysosomal exocytosis and particle uptake, BMMs were exposed to IgG-RBCs for various lengths of time, and acid phosphatase (AP) release was measured. Compared with WT BMMs, ML1 KO cells exhibited significantly less IgG-RBC-induced AP release (Figure 5C). Interestingly, this difference was abolished by either BAPTA-AM or inhibition of ML1 using ML-SI1 (Figure 5C). Next, we directly investigated the insertion of ML1 onto the plasma membrane during particle uptake. Because current antibodies are inadequate for detecting endogenous ML1 proteins, we developed a whole-cell patch-clamp method to detect the plasma membrane insertion of ML1 during particle uptake. This electrophysiology-based exocytosis assay provides temporal resolution far superior to other exocytosis/secretion assays. In order to facilitate detection (by decreasing the turnover time of ML1 at the plasma membrane), we used a cellpermeable dynamin inhibitor, dynasore (Macia et al., 2006), to block endocytosis that is presumed to be coupled with focal exocytosis (Lee et al., 2007; Tam et al., 2010). Large ML-SA1- induced whole-cell ML1-like currents were observed upon exposure of WT BMMs to IgG-RBCs for 10 min in the presence of dynasore (Figures 5D and 5E). In contrast, no significant ML- SA1-induced whole-cell currents were observed in RBC-treated ML1 KO BMMs, or WT BMMs treated with dynasore alone (Figures 5D and 5E). IgG-RBC-induced whole-cell I ML1 was completely inhibited by BAPTA-AM pretreatment (Figure 5E), 26, 1 14, September 16, 2013 ª2013 Elsevier Inc. 5
6 Small Particle ML1-GFP ML1-mCheery Rab5-GF P Large Particle LysoTr acke r Lamp 1-GF P ML1-mCheery Rab7-GF P ML1-mCheery Lamp 1-GF P ML1 GFP Syt-VII Merged mcherry Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, Developmental Cell (2013), TRPML1 Channels Regulate Phagosome Formation A Imaging time zero 0:00 1:15 3:53 6:40 Adding RBCs B ML1-GFP RBC 0:00 2:36 3:53 6:49 C Phagocytosis initiation Time (min) * * * * * * * * * * * * * * * * * * * * * * * * * D Large Particle Phagocytosis initiation ML1 Lamp1 Syt-VII Rab7 LysoTracker Phagosome Formation/ Maturation min Small Particle Rab5 ML1 Figure 3. Particle Binding to Macrophages Rapidly Recruits ML1-GFP to the Phagocytic Cups and Nascent Phagosomes (A) Rapid recruitment of ML1-GFP to the sites of particle ingestion (white arrow) and to expanding membrane extensions. Selected frames from time-lapse confocal microscopy of a ML1-GFP transfected RAW macrophage that was exposed to 6 mm IgG-coated polystyrene beads. Approximately 1 min after the attachment of beads to the cell surface, ML1-GFP was recruited to the membrane extension surrounding the particle (Frame 1 min 15 s; Movie S1). Note that phagocytosis may have been initiated before the imaging time zero due to a technical difficulty in identifying phagocytosing macrophages and capturing phagocytosing events, which typically took 5 10 min after RBCs were added to the recording chamber. Scale bar represents 5 mm. (legend continued on next page) 6 26, 1 14, September 16, 2013 ª2013 Elsevier Inc.
7 Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, (2013), TRPML1 Channels Regulate Phagosome Formation A RBC Pipette Pipette solution Bath solution Lysosome ML1 B Newly-formed phagosome Lamp1 Isolated phagosome Nucleus Nucleus Macrophage Slicing the cell membrane Intact macrophage Phagosome isolation DIC DIC Release of the phagosome Whole-phagosome configuration Particle ML1-GFP Merged Particle Lamp1-GFP Merged * * DIC DIC * * C D WT BMM ML1 KO BMM suggesting that membrane insertion during phagocytosis is a Ca2+-dependent process. ML1 Regulates Particle Ingestion via Lysosomal Exocytosis To directly investigate whether the involvement of ML1 in large particle uptake is mediated by lysosomal exocytosis, we assessed particle uptake upon increasing the expression level and channel activity of ML1 while simultaneously blocking lysosomal exocytosis. Three approaches were employed to block lysosomal exocytosis. First, lysosomal exocytosis and particle uptake are inhibited by dominant-negative (DN) forms of SytVII (Reddy et al., 2001). We found that ML-SA1-induced large particle uptake was significantly reduced in Syt-VII-DN-transfected ML1 O/E RAW cells (Figure 6A). Second, VAMP7 is a lysosomal SNARE protein required for lysosome-plasma membrane fusion (Braun et al., 2004). VAMP7 belongs to the Longin family of SNARE proteins, characterized by the presence of an autoinhibi- Figure 4. Whole-Phagosome Recording of ML1 Channels in the Isolated Nascent Phagosomes (A) Cartoon illustrations of whole-phagosome recording procedures. Upon RBC uptake (left panel), patch electrodes are used to slice open the phagocytosing macrophage within 5 min of particle binding and ingestion. After RBC-containing phagosomes are isolated, a patch pipette is used to achieve the whole-phagosome configuration. (B) An illustration of whole-phagosome configuration. Cells were transfected with Lamp-1-GFP or ML1-GFP and then exposed to IgG-coated beads on ice for 20 min; after phagocytosis was induced by transferring the cells to 37 C for 5 min, the newly formed phagosomes were isolated for electrophysiology. (C) ML-SA1- or PI(3,5)P2-activated endogenous whole-phagosome TRPML-like currents in RAW cells. (D) Whole-phagosome IML1 in WT, but not ML1 KO BMMs. ML-SA1 (25 mm)-activated IML1 was inhibited by ML-SI1 (50 mm) in WT phagosomes (left panel). No ML-SA1- activated IML1 was seen in ML1 KO phagosomes, in which PI(3,5)P2 (1 mm) robustly activated ITPC. tory domain at their N terminus (MartinezArca et al., 2003). Overexpression of the Longin domain of VAMP7 alone in macrophages is known to exert a dominant negative effect on both lysosomal exocytosis and particle uptake (Braun et al., 2004). ML-SA1 treatment failed to enhance particle uptake in cells transfected with Longin-GFP including ML1 O/E RAW cells (Figure 6B) and WT BMMs (Figure S5A), whereas overexpression of VAMP7-GFP had no inhibitory effect. Third, lysosomal exocytosis can also be reduced by treating the cells with colchicine, a known inhibitor of microtubule polymerization (Lee et al., 2007). We found that colchicine treatment significantly inhibited ML-SA1-induced large particle uptake (Figure 6C) and lysosomal AP release (Figure S5B) in ML1 O/E RAW cells. Taken together, these data suggest that ML1 participates in large particle uptake by regulating Ca2+-dependent lysosomal exocytosis in macrophages. The residual particle uptake in ML1 KO BMMs was further inhibited by tetanus toxin (TeNT, an inhibitor of several VAMP proteins including VAMP3; Figure S5C), which is consistent with an additional contribution of membranes/exocytosis from recycling endosomes as reported previously (Braun et al., 2004). (B) ML1-GFP and Syt VII-mCherry were simultaneously recruited to nascent phagosomes. Selected frames from time-lapse confocal microscopy (Movie S3) showed a RAW cell doubly transfected with ML1-GFP and Syt VII-mCherry that was exposed to IgG-RBCs. White box indicates the site of particle ingestion; both ML1-GFP and Syt VII-mCherry were concurrently recruited to the site of phagosome formation. Scale bar represents 10 mm. (C) The recruitment kinetics of different endolysosomal markers to the particle-containing phagosomes. RAW macrophage cells were transfected with various molecular endosomal and lysosomal markers. Upon particle binding, the recruitment of these markers to phagosomes was monitored using live-cell imaging over the course of 40 min. For large particles, ML1-mCherry and Lamp1-GFP were recruited to the forming phagosomes within 5 min after phagocytosis initiation; Rab7-GFP and LysoTracker were observed in the phagosomes 20 min after phagocytosis initiation; Rab5-GFP appeared on the phagosomes 10 min after phagocytosis initiation and then disappeared min later. For small particles (bottom row), ML1-GFP was recruited to the small particle-containing phagosomes min after phagocytosis initiation. The center of the particle is indicated with an asterisk. Scale bars represent 5 mm. (D) Summary of recruitment kinetics of various endolysosomal markers to phagosomes. See also Figure S3. 26, 1 14, September 16, 2013 ª2013 Elsevier Inc. 7
8 Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, Developmental Cell (2013), TRPML1 Channels Regulate Phagosome Formation A Lamp1 (total) Lamp1 (surface) C D Merged WT BMMs ML1 KO BMMs DMSO ML-SA1 DMSO ML-SA1 BMMs E B WT BMM phagocytic region Syt-VII GCaMP3 mcherry ML1 Merged 0:00 0:45 3:15 6:15 Figure 5. Particle Binding Induces ML1- Dependent Lysosomal Ca 2+ Release and Lysosomal Exocytosis in Macrophages (A) ML-SA1 (10 mm for 30 min) treatment resulted in localization of Lamp1 (red) on the plasma membrane in nonpermeabilized WT, but not ML1 KO BMMs. Lamp1 surface expression was detected using an antibody recognizing a luminal epitope (1D4B). After the cells were permeabilized, total (cell surface + intracellular; green) Lamp1 proteins were detected by using the same antibody. Scale bar represents 5 mm. (B) IgG-RBC binding triggered a localized Ca 2+ increase at the site of uptake, shown with selected image frames of a RAW cell doubly transfected with GCaMP3-ML1 and Syt VII-mCherry. Upon IgG-RBC binding, GCaMP3-ML1 fluorescence increased rapidly (frame 0 min 45 s) near the site of uptake and around the Syt VII-mCherry-positive compartments in the membrane extension and then decreased after phagosome formation (frame 6 min 15 s). Lower panel shows the time-dependent changes of GCaMP3-ML1 fluorescence upon particle binding, at the site of uptake (phagocytic region) and in the cell body (whole-cell region). White arrow indicates the phagocytic cup. Scale bar represents 10 mm. (C) RBC-ingestion-induced lysosomal acid phosphatase (AP) release in WT BMMs was reduced by treatment of BAPTA-AM (500 nm, preincubation for 15 min) or ML-SI1 (10 mm, preincubation for 10 min). (D) Whole-cell ML1-like currents in WT BMMs that were exposed to IgG-RBCs for 10 min. Dynasore (Dyn, 100 mm) was used to block Dynamindependent endocytosis to facilitate the detection of whole-cell I ML1. No significant whole-cell I ML1 was detected in ML1 KO BMMs (IgG-RBCs for 10 min). (E) Summary of whole-cell I ML1 under different experimental conditions. For all panels, unless otherwise indicated, the data represent the mean ± SEM from at least three independent experiments. See also Figure S4. ML1 Regulates Particle Ingestion in a PI(3,5)P 2 - Dependent Manner PI(3,5)P 2 is the only known endogenous activator of ML1 (Dong et al., 2010). PI(3,5)P 2 is generated from PI(3)P by a protein complex that contains PIKfyve/Fab1, a PI-5 kinase that is localized in late endosomes and lysosomes. The activity of PIKfyve/Fab1 is dependent on the FIG4 protein in the complex (Lenk et al., 2011). Thus, macrophages isolated from FIG4 KO mice are predicted to have a reduced PI(3,5)P 2 level compared with WT cells (Chow et al., 2007). FIG4 KO BMMs contained enlarged Lamp1- positive compartments (Figure S5D), as seen in other cells types from FIG4 KO mice (Chow et al., 2007; Lenk et al., 2011). Furthermore, FIG4 KO BMMs were defective in IgG-RBC uptake (Figure 6D) and ML-SA1- or IgG-RBC-induced lysosomal AP release (Figure S5E). Large (6 mm), but not small (3 mm) bead uptake, was also defective in WT BMMs treated with YM (1 mm; Figures 6E and 6F), a PIKfyve inhibitor (Jefferies et al., 2008). To directly test the possibility that an elevation of PI(3,5)P 2 is locally triggered by large particle binding, we have generated a PI(3,5)P 2 -specific probe, ML1-2N-GFP, made by the fusion of GFP to the phosphoinositide-binding domain of ML1 (X.L., X.W., X.Z., M. Zhao, Y. Zhang, R. Yau, L.S.W., and H.X., unpublished data). The probe was mainly localized to Lamp1-positive compartments, and this localization can be altered by mutations in the probe, or by genetically or pharmacologically manipulating the PI(3,5)P 2 level, suggestive of the specificity of the probe. Strikingly, ML1-2N-GFP was rapidly recruited to the forming phagosomes (Figure 6G) in RAW cells that were doubly transfected with ML1-2N-GFP and Syt-VII-mCherry. A transient and localized increase in ML1-2N-GFP fluorescent intensity was observed preferentially at the uptake sites of Syt-VII-mCherrypositive lysosomes within 3 min of particle binding (Figure 6G, frame 2 min and 54 s; Movie S4). In contrast, no increase in GFP fluorescence was observed in cells transfected with 8 26, 1 14, September 16, 2013 ª2013 Elsevier Inc.
9 Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, Developmental Cell (2013), TRPML1 Channels Regulate Phagosome Formation ML1-7Q-2N-GFP (a PI(3,5)P 2 unbinding control probe; X.L., X.W., X.Z., M. Zhao, Y. Zhang, R. Yau, L.S.W., and H.X., unpublished data) (Figure S5F). We also analyzed the kinetics of two other phosphoinositides that are known to accumulate at the site of phagocytosis. PI(3)P was detected using the 2X-FYVE- GFP probe, and PI(3,4)P 2 /PI(3,4,5)P 3 was detected using the AKT-PH-GFP probe (Yeung et al., 2006). The AKT-PH-GFP probe accumulated rapidly on forming phagosomes, but quickly disappeared upon closing of phagocytic cups (Figure S5H), suggesting that the time course of PI(3,4)P 2 /PI(3,4,5)P 3 transient matches phagosome formation and closure as shown previously (Yeung et al., 2006). In contrast, the PI(3)P probe accumulated on the early phagosomes 10 min after phagosome initiation (Figures S5G and S5H; see also Yeung et al., 2006), which was similar to Rab5 recruitment to early phagosomes. Note that PI(3)P increase occurred after Syt-VII recruitment (Figure S5G). Because PI(3,5)P 2 accumulates at the phagocytic site in a time course similar to PI(3,4)P 2 /PI(3,4,5)P 3, but prior to PI(3)P, it is very likely that PI(3,5)P 2 increase occurs during phagosome formation. We also measured the radio-labeled phosphoinositides using high-performance liquid chromatography (HPLC) (Zolov et al., 2012). We found that PI(3,5)P 2 levels increased significantly during the first 5 min of phagocytosis upon particle binding, which corresponds to the time necessary for phagosomes formation (Figure 6H). These results suggest that localized PI(3,5)P 2 generation may be one of the initial steps during large particle ingestion (Figure 6H). ML1 Deficiency Results in Defective Clearance of Senescent and Apoptotic Cells In Vivo Senescent RBCs are normally phagocytosed by macrophages in the red pulp region of the spleen, followed by the degradation of their hemoglobin and recycling of iron into the circulating blood (Bratosin et al., 1998). Considering that ML1 KO BMMs were defective in the uptake of large cellular particles, we investigated RBC clearance in the spleens of ML1 KO mice. In ML1 KO spleens, although Lamp1 expression was elevated (Figures S6A and S6B), immunoreactivity of the control macrophage marker CD11b was normal (Figures S6C). ML1 KO mice exhibited enlarged spleens, but had normal kidney size, compared with WT controls (Figures S6D and S6E), suggestive of compensatory changes secondary to defective clearance of senescent RBCs (Kohyama et al., 2009). Consistently, hematoxylin and eosin (H&E) staining of spleen sections revealed an accumulation of RBCs in both white and red pulps of ML1 KO but not WT spleens (Figure 7A). In addition, ML1 KO mice had a higher level of RBCs in the blood compared with WT controls, which had a red blood cell count within the normal range (Figure S6F). Accumulation of RBCs in the spleen reportedly leads to a buildup of splenic iron stores (Kohyama et al., 2009). Indeed, Perl s Prussian blue staining (for ferric iron) revealed an accumulation of iron stores in the spleens of ML1 KO mice, which was largely confined to the red pulp region (Figure 7B). Consistently, inductively coupled plasma mass spectrometry (ICP-MS) analysis revealed that iron content was selectively increased in the ML1 KO spleens (Figure 7C). Taken together, these results suggest that RBC clearance is defective in ML1 KO mice. Defective phagocytosis in the brain may lead to inflammation and microglia (macrophage-like cells in the brain) activation (Venkatachalam et al., 2008; Vitner et al., 2010). Consistently, Iba1, a 17 kda EF hand protein that is specifically expressed in activated macrophage/microglia (Ferguson et al., 2009), was upregulated in the brain of ML1 and FIG4 KO mice (Figures 7D and 7E). Further, massive cell death was observed in the brains of ML1 and FIG4 KO mice (Figure 7F). DISCUSSION Using pharmacological, genetic, and biochemical approaches, we demonstrate that the channel activity of ML1 is necessary for efficient uptake of large particles in macrophages. Live imaging and electrophysiological recordings reveal that ML1 is rapidly activated and recruited to the membrane extensions surrounding the particles in the process of phagocytic internalization. Our results are compatible with the following working model: large particle binding to macrophages triggers PI(3,5)P 2 elevation, which induces ML1-mediated lysosomal Ca 2+ release and lysosomal exocytosis at the sites of uptake (see Figure 6I). The activation of IgG Fc receptors during phagocytosis has been known to induce a transient increase in [Ca 2+ ] i in neutrophils (Myers and Swanson 2002; Nunes and Demaurex 2010). This was not seen in macrophages (Nunes and Demaurex 2010). Using our recently established lysosome-specific Ca 2+ imaging method with lysosome-targeted genetically encoded Ca 2+ sensor GCaMP3, which is more sensitive in detecting lysosomal Ca 2+ release than conventional methods, we indeed found that particle binding to macrophages also induces Ca 2+ increase, but exclusively at the site of the particle uptake. Furthermore, the present study has extended the earlier observations by demonstrating that transient Ca 2+ release from the lysosomes through ML1 is responsible for triggering lysosomal exocytosis during the uptake of large particles. Additionally, acidic granules in neutrophils (equivalent to lysosomes in macrophages) have been shown to redistribute toward the site of particle uptake, suggesting a source of membrane (Stendahl et al., 1994; Tapper et al., 2002). Using time-lapse live imaging, we have demonstrated that lysosomes and their resident proteins are also redistributed toward the site of uptake in macrophages. Thus, lysosomes serve uniquely as sources not only for membranes, but also for Ca 2+ that is required for Syt-VII activation/recruitment. What differentiates the uptake of large versus small particles is not clear. A small increase in the diameter of particle may result in a much larger demand for membranes, receptors, signaling molecules, and cytoskeleton machinery. It is known that the uptake of large (>4.5 mm) IgG-coated particles depends on phosphatidylinositol 3-kinase (PI3K) activity, whereas the uptake of smaller (<3 mm) particles does not require PI3K activity (Cox et al., 1999). For example, Wortmannin and LY294002, two nonspecific type I and II PI3K inhibitors, preferentially inhibit the uptake of large particles (Cox et al., 1999; Hoppe and Swanson 2004). Although type I PI3K has been linked to an effect on cytoskeleton rearrangement during large particle uptake (Cox et al., 1999; Hoppe and Swanson 2004), type II PI3K could contribute to large particle uptake via the production of PI(3)P, the precursor of PI(3,5)P 2, which in turn is the endogenous 26, 1 14, September 16, 2013 ª2013 Elsevier Inc. 9
10 Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, Developmental Cell (2013), TRPML1 Channels Regulate Phagosome Formation Figure 6. ML1-Mediated Facilitation of Particle Ingestion Is Dependent on Lysosomal Exocytosis (A) ML-SA1 (10 mm) failed to increase particle ingestion in ML1 O/E RAW cells transfected with a Syt VII dominant-negative (DN) construct. Cells were preincubated with DMSO or ML-SA1 for 15 min before they were exposed to IgG-RBCs for 30 min. (B) ML-SA1 (10 mm) failed to increase particle ingestion in ML1 O/E RAW cells transfected with a VAMP7 dominant-negative (VAMP7-DN) construct. (C) A microtubule-disrupting agent colchicine (10 mm) inhibited ML-SA1-enhanced particle ingestion in WT BMMs. (D) FIG4 KO macrophages were defective in IgG-RBC ingestion. WT and FIG4 KO BMMs were exposed to IgG-RBCs for time periods indicated. (E) YM (1 mm for 2 hr) treatment inhibited uptake of 6 mm beads, but not 3 mm beads in WT BMMs. (F) Differential effects of YM on large and small particle uptake. (legend continued on next page) 10 26, 1 14, September 16, 2013 ª2013 Elsevier Inc.
11 Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, Developmental Cell (2013), TRPML1 Channels Regulate Phagosome Formation Figure 7. ML1 Deficiency Results in Defective Clearance of Senescent Red Blood Cells in the Spleen and Microglia Activation in the Brain (A) Accumulation of RBCs in the red (RP) and white (WP) pulps of a ML1 KO spleen revealed by H&E staining. (B) Perl s (Prussian blue) staining for ferric iron in the splenic red pulp (RP) from 4-month-old mice. Scale bar represents 200 mm. (C) ICP-MS analysis of the splenic iron content using digested whole spleens from WT and ML1 KO mice. Data were presented as ratios of different ions. (D) Propidium iodide (PI, 20 mg/ml)-labeled cells in the cerebral cortex and hippocampus of WT, ML1 KO, and FIG4 KO mouse brain sections. (E) Iba1 was upregulated in the whole-brain lysates of ML1 and FIG4 KO mice. (F) Iba1-positive cells were upregulated in the sections of the brain cortex of ML1 KO mice. For all panels, unless otherwise indicated, the data represent the mean ± SEM from at least three independent experiments. See also Figure S6. activator of ML1 (Shen et al., 2012). BMMs with reduced PI(3,5)P 2 levels also exhibit the size-dependent uptake phenotype. It is possible that PI(3,5)P 2 production, as detected using our genetically encoded PI(3,5)P 2 sensor, may be the key signaling event that leads to ML1 activation and subsequent lysosomal exocytosis (Figures 6H and 6G). We have shown that during large particle uptake, ML1, Syt-VII, and Lamp1 are rapidly delivered to forming phagosomes. The early recruitment of Syt-VII and Lamp1 within 5 min of phagocytosis initiation is impaired when the activity of ML1 is inhibited and is not seen for small particle uptake. In late phagosome maturation, lysosomal membrane proteins are also expected to appear on the phagosomal membranes through phagosome-lysosome fusion (Flannagan et al., 2012). Indeed, in contrast to large particle uptake, ML1-GFP was recruited only after 20 min of phagocytosis initiation in small particle uptake. Therefore, lysosomes play dual roles sequentially in (nascent) phagosome formation and (late) phagosome formation and are delivered to the phagosomes at two different stages. First, for large particles, lysosomes fuse with the plasma membrane at the phagocytic cup, which provides the membranes necessary for phagosome formation. As a result, lysosomal membrane proteins such as ML1 and Lamp1 are delivered to the newly formed phagosomes, prior to or during phagocytic cup closing. This is a path via lysosomal exocytosis that occurs early during phagosome formation. Second, for both large and small particles, lysosomes are also delivered to the late phagosomes through late phagosome maturation (phagosome-lysosome fusion), which is required for the degradation of phagocytic materials. This second stage is accompanied by Rab7 recruitment and phagosomal acidification, as well as further recruitment of lysosomal membrane proteins including ML1 and Lamp1. This is a path that occurs late during phagosome-lysosome fusion. As discussed in a previous study (Czibener et al., (G) Upon particle binding, ML1-2N-GFP and Syt VII-mCherry accumulated at the site of uptake within minutes (see Movie S4). Note that phagocytosis initiation may have occurred before the imaging time zero due to a technical difficulty in identifying macrophages undergoing phagocytosis. (H) HPLC analysis of the levels of PI(3,5)P 2 and PI(4,5)P 2 during phagosome formation. Myo-[2-3 H] inositol-labeled RAW cells were exposed to IgG-coated beads (6 mm) for 0, 1, 2, 5, and 10 min at 37 C. At each time point, cells were centrifuged at 4 C and prepared for HPLC analysis. Data were from three independent experiments. (I) Particle binding activates lysosomal ML1 to initiate Ca 2+ -dependent lysosomal exocytosis. Upon particle (IgG-RBC) binding, the activity of PIKfyve/Fab1 (not shown) is stimulated at the site of particle uptake. The subsequent increase of PI(3,5)P 2 level can then activate lysosomal ML1 to induce lysosomal Ca 2+ release. Juxtaorganellar Ca 2+ can then bind the Ca 2+ sensor Syt VII to trigger lysosome-plasma membrane fusion, which provides substantial amount of lysosomal membranes for the pseudopod extension around IgG-RBCs. As a result, lysosomal membrane proteins, such as ML1 and Syt-VII, are inserted into the plasma membrane. Pseudopod extension continues until the IgG-RBCs are completely internalized. Phagocytic cups are then closed, and nascent phagosomes are formed. Newly formed phagosomes then undergo a series of membrane fusion and fission processes, collectively called phagosome maturation, to become late phagosomes. Lysosomes are also delivered to the late phagosomes through late phagosome maturation (phagosome-lysosome fusion), which is required for the degradation of phagocytic materials. ML1 may also play a role in phagosome-lysosome fusion, but this requires further investigation. For all panels, unless otherwise indicated, the data represent the mean ± SEM from at least three independent experiments; macrophages were analyzed for each experiment. For all panels, scale bars represent 10 mm. See also Figure S5. 26, 1 14, September 16, 2013 ª2013 Elsevier Inc. 11
12 Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, Developmental Cell (2013), TRPML1 Channels Regulate Phagosome Formation 2006), the early recruitment of lysosomal membrane proteins may serve as a rather early preparation for phagosome maturation, especially for large particle uptake, but is not completely essential for phagosome maturation. Our results in the present study are consistent with previous reports showing that the clearance of late apoptotic neurons is impaired in the Drosophila TRPML mutant (Venkatachalam et al., 2008), suggesting that TRPML channels play a role in eliminating pathogenic extracellular particles. Extracellular accumulation of large particles such as apoptotic cells may cause brain inflammation and microglia activation, which are common features in most neurodegenerative lysosome storage diseases (LSDs) (Vitner et al., 2010). It is possible that defective phagocytosis of large particle is a general pathogenic factor for many LSDs, with ML1 channel deregulation in phagocytes being a primary cause. EXPERIMENTAL PROCEDURES Phagocytosis Assay Sheep RBCs (Lampire Biological Laboratories) were washed twice with PBS and then fixed with 4% paraformaldehyde (PFA) overnight at 4 C in an end-over-end rotator. Free aldehyde groups were quenched by resuspension in PBS containing 100 mm glycine for 30 min at 4 C; RBCs were opsonized with rabbit IgG antibody (Equitech-Bio) at 37 C for 1 hr. Phagocytosis was initiated by adding IgG-RBCs onto adherent BMMs at a ratio of 50:1 (RBC: BMM). To synchronize binding and internalization, IgG-RBCs were centrifuged at 300 rpm for 3 min together with adherent BMMs. BMMs were placed at 37 C and 5% CO 2 for various time points (15 90 min). Noningested IgG-RBCs were lysed by incubating the cells with water (1 ml) for 2 3 min at 4 C (Chow et al., 2004). RBC-containing BMMs were fixed in 2% PFA and stained with goat-anti-rabbit IgG AlexaFluor488 (1:500 dilution, Invitrogen). Approximately BMMs were typically analyzed for each experiment. Ingested RBCs were counted by experimenters who were blinded to the genotype and treatment. Whole-Phagosome Electrophysiology RAW macrophages and BMMs were transfected with Lamp1-GFP or ML1-GFP using the Neon electroporation system (Invitrogen, MPK 5000). IgG-RBCs were first incubated with macrophages at 4 C for 20 min to synchronize the binding of IgG-RBCs to cells. After several gentle washes, cells were placed at 37 C and 5% CO 2 for 5 min to initiate the phagocytosis, and then transferred to room temperature to slow down the phagocytosis (Holevinsky and Nelson 1998). Within 5 min of phagocytosis initiation, the phagosomes formed at this stage were not fused with lysosomes (Vieira et al., 2002) and were considered as newly formed or nascent phagosomes. Because the majority of RBC-containing phagosomes (5 mm, roughly as the size of RBCs) were also Lamp1-GFP or ML1-GFP-positive (see Figure 4), the GFP-positive vesicles (5 mm) were identified as nascent phagosomes. Patch-clamp recordings were performed on the isolated newly formed phagosomes (see Figure 4). To isolate phagosomes, a patch pipette was used to open the cell by slicing the cell membrane. Then phagosomes were released into the dish and recognized by GFP fluorescence. The bath (cytoplasmic) solution contained 140 mm K-gluconate, 4 mm NaCl, 1 mm EGTA, 2 mm MgCl 2, 0.39 mm CaCl 2, and 10 mm HEPES (ph adjusted with KOH to 7.2; free [Ca 2+ ] 100 nm). The pipette (luminal) solution contained 145 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl 2, 10 mm HEPES, 10 mm MES, and 10 mm glucose (ph 6.5, adjusted with NaOH). Data were collected and analyzed as described in the Supplemental Experimental Procedures. GCaMP3 and Fura-2 Ca 2+ Imaging Ca 2+ imaging was performed as described in Supplemental Experimental Procedures. Lysosomal Enzyme Release/Activity Lysosomal enzyme release was measured as described in Supplemental Experimental Procedures. HPLC Measurement of Phosphoinositide Levels Phosphoinositides are radio-labeled and measured as described in Supplemental Experimental Procedures. Data Analysis Data are presented as the mean ± SEM. Statistical comparisons were made using ANOVA. A p value < 0.05 was considered statistically significant. SUPPLEMENTAL INFORMATION Supplemental Information includes Supplemental Experimental Procedures, six figures, and four movies and can be found with this article online at ACKNOWLEDGMENTS This work was supported by National Institutes of Health (NIH) (NS062792, MH096595, AR to H.X, GM24872 to M. Meisler, and NS to L.S.W). We are grateful to Dr. Loren Looger for the GCaMP3 construct, Drs. Norma Andrews and Thomas Sudhof for the Syt-VII construct, Florence Niedergang and Robert Edwards for VAMP7 constructs, Ed Stuenkel for the TeNT construct, Johnny Fares for ML1-overexpressing or knockdown RAW macrophages, Gregg Sobocinski for assistance in confocal imaging, and Richard Hume and Miriam Meisler for comments on an earlier version of the manuscript. We appreciate the encouragement and helpful comments from other members of the Xu laboratory. Received: February 6, 2013 Revised: May 22, 2013 Accepted: August 5, 2013 Published: August 29, 2013 REFERENCES Aderem, A., and Underhill, D.M. (1999). Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17, Andrews, N.W. (2000). Regulated secretion of conventional lysosomes. Trends Cell Biol. 10, Bajno, L., Peng, X.R., Schreiber, A.D., Moore, H.P., Trimble, W.S., and Grinstein, S. (2000). Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J. Cell Biol. 149, Bratosin, D., Mazurier, J., Tissier, J.P., Estaquier, J., Huart, J.J., Ameisen, J.C., Aminoff, D., and Montreuil, J. (1998). Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie 80, Braun, V., and Niedergang, F. (2006). Linking exocytosis and endocytosis during phagocytosis. Biol. Cell 98, Braun, V., Fraisier, V., Raposo, G., Hurbain, I., Sibarita, J.B., Chavrier, P., Galli, T., and Niedergang, F. (2004). TI-VAMP/VAMP7 is required for optimal phagocytosis of opsonised particles in macrophages. EMBO J. 23, Cheng, X., Shen, D., Samie, M., and Xu, H. (2010). Mucolipins: intracellular TRPML1-3 channels. FEBS Lett. 584, Chow, C.W., Downey, G.P., and Grinstein, S. (2004). Measurements of phagocytosis and phagosomal maturation. Curr. Protoc. Cell Biol. 15, Unit Chow, C.Y., Zhang, Y., Dowling, J.J., Jin, N., Adamska, M., Shiga, K., Szigeti, K., Shy, M.E., Li, J., Zhang, X., et al. (2007). Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature 448, Cox, D., Tseng, C.C., Bjekic, G., and Greenberg, S. (1999). A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 274, , 1 14, September 16, 2013 ª2013 Elsevier Inc.
13 Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, Developmental Cell (2013), TRPML1 Channels Regulate Phagosome Formation Czibener, C., Sherer, N.M., Becker, S.M., Pypaert, M., Hui, E., Chapman, E.R., Mothes, W., and Andrews, N.W. (2006). Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes. J. Cell Biol. 174, Dong, X.P., Cheng, X., Mills, E., Delling, M., Wang, F., Kurz, T., and Xu, H. (2008). The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 455, Dong, X.P., Shen, D., Wang, X., Dawson, T., Li, X., Zhang, Q., Cheng, X., Zhang, Y., Weisman, L.S., Delling, M., and Xu, H. (2010). PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun 1, 38. Ferguson, C.J., Lenk, G.M., and Meisler, M.H. (2009). Defective autophagy in neurons and astrocytes from mice deficient in PI(3,5)P2. Hum. Mol. Genet. 18, Flannagan, R.S., Jaumouillé, V., and Grinstein, S. (2012). The cell biology of phagocytosis. Annu. Rev. Pathol. 7, Guo, P., Hu, T., Zhang, J., Jiang, S., and Wang, X. (2010). Sequential action of Caenorhabditis elegans Rab GTPases regulates phagolysosome formation during apoptotic cell degradation. Proc. Natl. Acad. Sci. USA 107, Hackam, D.J., Rotstein, O.D., Sjolin, C., Schreiber, A.D., Trimble, W.S., and Grinstein, S. (1998). v-snare-dependent secretion is required for phagocytosis. Proc. Natl. Acad. Sci. USA 95, Henry, R.M., Hoppe, A.D., Joshi, N., and Swanson, J.A. (2004). The uniformity of phagosome maturation in macrophages. J. Cell Biol. 164, Holevinsky, K.O., and Nelson, D.J. (1998). Membrane capacitance changes associated with particle uptake during phagocytosis in macrophages. Biophys. J. 75, Hoppe, A.D., and Swanson, J.A. (2004). Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol. Biol. Cell 15, Huynh, K.K., Kay, J.G., Stow, J.L., and Grinstein, S. (2007). Fusion, fission, and secretion during phagocytosis. Physiology (Bethesda) 22, Jefferies, H.B., Cooke, F.T., Jat, P., Boucheron, C., Koizumi, T., Hayakawa, M., Kaizawa, H., Ohishi, T., Workman, P., Waterfield, M.D., and Parker, P.J. (2008). A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 9, Kitano, M., Nakaya, M., Nakamura, T., Nagata, S., and Matsuda, M. (2008). Imaging of Rab5 activity identifies essential regulators for phagosome maturation. Nature 453, Kohyama, M., Ise, W., Edelson, B.T., Wilker, P.R., Hildner, K., Mejia, C., Frazier, W.A., Murphy, T.L., and Murphy, K.M. (2009). Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature 457, LaPlante, J.M., Sun, M., Falardeau, J., Dai, D., Brown, E.M., Slaugenhaupt, S.A., and Vassilev, P.M. (2006). Lysosomal exocytosis is impaired in mucolipidosis type IV. Mol. Genet. Metab. 89, Lee, W.L., Mason, D., Schreiber, A.D., and Grinstein, S. (2007). Quantitative analysis of membrane remodeling at the phagocytic cup. Mol. Biol. Cell 18, Lenk, G.M., Ferguson, C.J., Chow, C.Y., Jin, N., Jones, J.M., Grant, A.E., Zolov, S.N., Winters, J.J., Giger, R.J., Dowling, J.J., et al. (2011). Pathogenic mechanism of the FIG4 mutation responsible for Charcot-Marie-Tooth disease CMT4J. PLoS Genet. 7, e Macia, E., Ehrlich, M., Massol, R., Boucrot, E., Brunner, C., and Kirchhausen, T. (2006). Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10, Martinez-Arca, S., Rudge, R., Vacca, M., Raposo, G., Camonis, J., Proux- Gillardeaux, V., Daviet, L., Formstecher, E., Hamburger, A., Filippini, F., et al. (2003). A dual mechanism controlling the localization and function of exocytic v-snares. Proc. Natl. Acad. Sci. USA 100, Medina, D.L., Fraldi, A., Bouche, V., Annunziata, F., Mansueto, G., Spampanato, C., Puri, C., Pignata, A., Martina, J.A., Sardiello, M., et al. (2011). Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell 21, Myers, J.T., and Swanson, J.A. (2002). Calcium spikes in activated macrophages during Fcgamma receptor-mediated phagocytosis. J. Leukoc. Biol. 72, Niedergang, F., and Chavrier, P. (2004). Signaling and membrane dynamics during phagocytosis: many roads lead to the phagos(r)ome. Curr. Opin. Cell Biol. 16, Nunes, P., and Demaurex, N. (2010). The role of calcium signaling in phagocytosis. J. Leukoc. Biol. 88, Piper, R.C., and Luzio, J.P. (2004). CUPpling calcium to lysosomal biogenesis. Trends Cell Biol. 14, Pryor, P.R., Mullock, B.M., Bright, N.A., Gray, S.R., and Luzio, J.P. (2000). The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 149, Reddy, A., Caler, E.V., and Andrews, N.W. (2001). Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell 106, Shen, D., Wang, X., and Xu, H. (2011). Pairing phosphoinositides with calcium ions in endolysosomal dynamics: phosphoinositides control the direction and specificity of membrane trafficking by regulating the activity of calcium channels in the endolysosomes. Bioessays 33, Shen, D., Wang, X., Li, X., Zhang, X., Yao, Z., Dibble, S., Dong, X.P., Yu, T., Lieberman, A.P., Showalter, H.D., and Xu, H. (2012). Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun 3, 731. Stendahl, O., Krause, K.H., Krischer, J., Jerström, P., Theler, J.M., Clark, R.A., Carpentier, J.L., and Lew, D.P. (1994). Redistribution of intracellular Ca2+ stores during phagocytosis in human neutrophils. Science 265, Sun, M., Goldin, E., Stahl, S., Falardeau, J.L., Kennedy, J.C., Acierno, J.S., Jr., Bove, C., Kaneski, C.R., Nagle, J., Bromley, M.C., et al. (2000). Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum. Mol. Genet. 9, Swanson, J., Bushnell, A., and Silverstein, S.C. (1987). Tubular lysosome morphology and distribution within macrophages depend on the integrity of cytoplasmic microtubules. Proc. Natl. Acad. Sci. USA 84, Tam, C., Idone, V., Devlin, C., Fernandes, M.C., Flannery, A., He, X., Schuchman, E., Tabas, I., and Andrews, N.W. (2010). Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J. Cell Biol. 189, Tapper, H., and Sundler, R. (1995). Glucan receptor and zymosan-induced lysosomal enzyme secretion in macrophages. Biochem. J. 306, Tapper, H., Furuya, W., and Grinstein, S. (2002). Localized exocytosis of primary (lysosomal) granules during phagocytosis: role of Ca2+-dependent tyrosine phosphorylation and microtubules. J. Immunol. 168, Theler, J.M., Lew, D.P., Jaconi, M.E., Krause, K.H., Wollheim, C.B., and Schlegel, W. (1995). Intracellular pattern of cytosolic Ca2+ changes during adhesion and multiple phagocytosis in human neutrophils. Dynamics of intracellular Ca2+ stores. Blood 85, Thompson, E.G., Schaheen, L., Dang, H., and Fares, H. (2007). Lysosomal trafficking functions of mucolipin-1 in murine macrophages. BMC Cell Biol. 8, 54. Touret, N., Paroutis, P., Terebiznik, M., Harrison, R.E., Trombetta, S., Pypaert, M., Chow, A., Jiang, A., Shaw, J., Yip, C., et al. (2005). Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell 123, Venkatachalam, K., Long, A.A., Elsaesser, R., Nikolaeva, D., Broadie, K., and Montell, C. (2008). Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell 135, Venugopal, B., Browning, M.F., Curcio-Morelli, C., Varro, A., Michaud, N., Nanthakumar, N., Walkley, S.U., Pickel, J., and Slaugenhaupt, S.A. (2007). Neurologic, gastric, and opthalmologic pathologies in a murine model of mucolipidosis type IV. Am. J. Hum. Genet. 81, , 1 14, September 16, 2013 ª2013 Elsevier Inc. 13
14 Please cite this article in press as: Samie et al., A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis, Developmental Cell (2013), TRPML1 Channels Regulate Phagosome Formation Vieira, O.V., Botelho, R.J., and Grinstein, S. (2002). Phagosome maturation: aging gracefully. Biochem. J. 366, Vitner, E.B., Platt, F.M., and Futerman, A.H. (2010). Common and uncommon pathogenic cascades in lysosomal storage diseases. J. Biol. Chem. 285, Wang, X., Zhang, X., Dong, X.P., Samie, M., Li, X., Cheng, X., Goschka, A., Shen, D., Zhou, Y., Harlow, J., et al. (2012). TPC proteins are phosphoinositideactivated sodium-selective ion channels in endosomes and lysosomes. Cell 151, Yeung, T., Ozdamar, B., Paroutis, P., and Grinstein, S. (2006). Lipid metabolism and dynamics during phagocytosis. Curr. Opin. Cell Biol. 18, Zolov, S.N., Bridges, D., Zhang, Y., Lee, W.W., Riehle, E., Verma, R., Lenk, G.M., Converso-Baran, K., Weide, T., Albin, R.L., et al. (2012). In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc. Natl. Acad. Sci. USA 109, , 1 14, September 16, 2013 ª2013 Elsevier Inc.
15 , Volume 26 Supplemental Information A TRP Channel in the Lysosome Regulates Large Particle Phagocytosis via Focal Exocytosis Mohammad Samie, Xiang Wang, Xiaoli Zhang, Andrew Goschka, Xinran Li, Xiping Cheng, Evan Gregg, Marlene Azar, Yue Zhuo, Abigail G. Garrity, Qiong Gao, Susan Slaugenhaupt, Jim Pickel, Sergey N. Zolov, Lois S. Weisman, Guy M. Lenk, Steve Titus, Marthe Bryant-Genevier, Noel Southall, Marugan Juan, Marc Ferrer, and Haoxing Xu Inventory of Supplemental Information Supplemental Figures and Legends Figure S1. Related to Figure 1. Uptake of large particles is reduced in ML1-null macrophages. Figure S2. Related to Figure 2. Phagocytosis of large particles in macrophages is inhibited by ML1 antagonists. Figure S3. Related to Figure 3. Rapid particle-size-dependent recruitment of lysosomal membrane proteins to the pseudopods of phagocytosing macrophages. Figure S4. Related to Figure 5. Lysosomal Ca 2+ release and lysosomal exocytosis in the phagocytic sites upon particle binding. Figure S5. Related to Figure 6. Requirement of PI(3,5)P 2 and lysosomal exocytosis in particle uptake. Figure S6. Related to Figure 7. Defective clearance of senescent red blood cells in the spleen of ML1 KO mice. Supplemental Movie Legends Movie S1. A time-lapse video of a ML1-GFP-transfected RAW cells that were exposed to IgGopsonized 6 μm beads (experiments conditions and snapshot images see Fig. 3A and figure legends).
16 Movie S2. A time-lapse video of a ML1-GFP-transfected RAW cells that were exposed to IgG- RBCs (experiments conditions and snapshot images see Suppl. Fig. S3A and figure legends). Movie S3. Simultaneous recruitment of ML1-GFP and Syt-VII-mCherry to the IgG-RBC uptake sites (experiments conditions and snapshot images see Fig. 3B and figure legends). Movie S4. Transient accumulation of ML1-2N-GFP to the phagocytic cups (experiments conditions and snapshot images see Fig. 6G and figure legends). Supplemental Experimental Procedures Supplemental References
17 Supplemental Figures Suppl. Fig. S1. Uptake of large particles is reduced in ML1-null macrophages-- Related to Figure 1. (A) Mature monocytes isolated from WT and ML1 KO mouse bone marrow had similar morphological characteristics as revealed by CD11b (mature macrophage-specific surface receptor) staining. Scale bar = 30 µm. Isolated monocytes were cultured in DMEM/F12 and recombinant colony stimulating factor for 5-7 days, and then fixed and stained with anti-cd11b antibody. (B) ML1 KO BMMs were defective in large particle uptake. Histograms represent the distribution (in percentage) of WT and ML1 KO BMMs containing different numbers of ingested RBCs (x-axis) for each time point. (C) ML1 KO BMMs had a lower uptake index for large, but not small beads compared with WT BMMs. Uptake index was calculated based on the total number of particles ingested for 100 BMMs. (D) Zymosan particle uptake was normal in ML1 KO BMMs. BMMs were exposed to Zymosan particles at 1:50 ratio for time periods indicated. For all panels, unless otherwise indicated, the data represent the mean ± the standard error of the mean (SEM) from at least three independent experiments.
18 Suppl. Fig. S2. Phagocytosis of large particles in macrophages is inhibited by ML1 antagonists-- Related to Figure 2. (A) Particle uptake index in RAW 264.7, ML1 O/E, and ML1 KD RAW macrophages. (B) Dosedependent inhibition of bead (6 m) uptake in WT BMMs by BAPTA-AM, but not EGTA-AM at 30 min upon particle binding. (C) ML-SI1 (20 M) inhibited ML-SA1-induced lysosomal Ca 2+ increase in HEK293 cells stably expressing GCaMP3-ML1 (left panel). ML-SA1 induced rapid increases in GCaMP3 fluorescence (measured as change of GCaMP3 fluorescence ΔF over basal fluorescence F 0 ; ΔF/F0) under low (<10 nm) external Ca 2+ in GCaMP3-ML1 stable cell lines. Co-application of ML- SI1 abolished the ML-SA1-induced lysosomal Ca 2+ release. Right panel shows the dose-dependent inhibition of Ca 2+ increase by ML1-SI1 in the micromolar range. (D) ML-SI1 inhibited ML-SA1 or PI(3,5)P 2 -activated whole-endolysosome I ML1. (E) Insensitivity of whole-endolysosome I TPC2 and sensitivity of whole-cell I TRPML3 to ML-SI1. I TPC2 and I TRPML3 were activated by PI(3,5)P 2 and ML- SA1, respectively. (F) ML-SI2 and ML-SI3 (20 M each) inhibited ML-SA1-induced lysosomal Ca 2+ increase in HEK293 cells stably expressing GCaMP3-ML1. (G) ML-SI1, ML-SI2, and ML-SI3 (10 M each) inhibited the uptake of large IgG-beads (6 m) in WT BMMs. For all panels, unless otherwise indicated, the data represent the mean ± the standard error of the mean (SEM) from at least three independent experiments.
19 Suppl. Fig. S3. Rapid particle-size-dependent recruitment of lysosomal membrane proteins to the pseudopods of phagocytosing BMMs-- Related to Figure 3. (A) Rapid recruitment of ML1-GFP to the sites of particle ingestion and to expanding membrane extensions. Selected frames from time-lapse confocal microscopy of a ML1-GFP transfected RAW macrophage that was exposed to IgG-RBCs. Approximately one min after the attachment of RBCs to the cell surface, ML1-GFP was recruited to the membrane extension surrounding the particle
20 (Frame 1 min 12 sec; Video 2). Scale bar = 10 µm. (B). Particle-size-dependent recruitment of ML1- GFP to the uptake site. Time-lapse imaging of a ML1-GFP-transfected RAW macrophage that was exposed to both large (6 m) and small (3 m) IgG-coated beads simultaneously. Approximately 5 min after the attachment of large beads to the cell surface (phagocytosis initiation), ML1-GFP was present at the membrane extensions surrounding a newly formed phagosome (red arrow, frame 5 min 40 sec). In contrast, for small beads, no significant ML1-GFP accumulation was seen surrounding the forming phagosome (blue arrow, frame 6 min 33 sec). The center of the particle is indicated with an *. Bottom panel showed the time-dependent changes of ML1-GFP fluorescence at the site of phagosome formation upon particle binding. N=3 for both large and small particles. Scale bar = 10 µm. (C) In RAW cells doubly-transfected with ML1-GFP and Lamp1-mCherry, both ML1 and Lamp1 were co-localized at the tip of the pseudopods (arrows). (D) The effect of ML-SI1 on recruitment kinetics of different endolysosomal markers to the small particle-containing phagosomes. RAW macrophage cells were electroporated or labeled with various endosomal and lysosomal markers. Cells were pre-incubated with 20 µm ML-SI1 for 30 min, and then exposed to small (3 m) IgG-coated beads in the continued presence of ML-SI1. Upon particle binding, the recruitment of molecular markers to phagosomes was monitored using live-cell imaging over the course of 40 min. The center of the particle is indicated with an *. Scale bars = 5 µm. Low panel summarizes the recruitment kinetics of various markers. (E) The effect of ML-SI1 on recruitment kinetics of different endolysosomal markers to the large particle-containing phagosomes. Compared with DMSO control, ML-SI1 prevented the early localization of Lamp1 to the forming early phagosomes, delayed the appearance of Rab7-GFP and LysoTracker in the late phagosomes, but without significantly affecting the recruitment of Rab5-GFP. The center of the particle is indicated with an *. Low panel summarizes the recruitment kinetics of various markers. Scale bars = 5 µm. (F) Early recruitment of Lamp1 to nascent phagosomes was defective in ML1 KO BMMs. BMMs were exposed to IgG-RBCs for 5 min, fixed, and stained with an anti-lamp1 antibody (ID4B). Left panels: images represent the morphological criteria used to determine phagosome formation. Scale bar = 10 µm. Right panel: percentage of newly-formed phagosomes (5 min after particle binding) in WT and ML1 KO BMMs that were Lamp1-positive. Scale bar = 10 µm. (G). Actin polymerization of newly formed phagosomes, shown with phalloidin staining, was comparable in WT and ML1 KO BMMs. WT and ML1 KO BMMs were exposed to IgG-RBCs for 5 min, fixed, and labeled with phalloidin- Alexa- Fluor633 for 30 min. Left panel images represent the morphological criteria that were used to determine phagosome formation. The arrows point to RBC-containing phagosomes surrounded by phalloidin-stained polymerized actin. Right panel showed that about 60% of newly-formed phagosomes (5 min after particle binding) were Phalloidin positive in both WT and ML1 KO BMMs. For all panels, unless otherwise indicated, the data represent the mean ± the standard error of the mean (SEM) from at least three independent experiments.
21 Suppl. Fig. S4. Lysosomal Ca 2+ release and lysosomal exocytosis in the phagocytic sites upon particle binding--- Related to Figure 5. (A) Flow cytometric analysis of ML-SA1-induced Lamp1 surface expression in WT and ML1 KO BMMs. Pre-treatment with BAPTA-AM (1 µm for 30 min) completely abolished Lamp1 surface staining in WT BMMs. Lower panels: histograms showing the flow cytometric analysis of Lamp1 surface staining. (B) Lamp1 surface staining was co-localized with the plasma membrane marker Dil- C18 (Dong, Wang et al. 2009). Scale bar = 5 µm. (C) ML-SA1 ( µm for 15 or 30 min) treatment increased the release of lysosomal enzymes into the culture medium in BMMs and RAW cells. The activity of lysosomal enzyme acid phosphatase (AP) was determined using an AP activity colorimetric assay kit. The data are presented as the percentage of the activity of the released vs. total (released + cell-associated) enzymes. Left panels: lysosomal AP release in WT and ML1 KO BMMs. Right panels: lysosomal AP release in RAW 264.7, ML1 O/E, and ML1 KD RAW macrophages. (D) Lack of an effect of ML-SA1 on the release of cytosolic enzyme LDH. (E) Ionomycin-induced lysosomal enzyme acid phosphatase (AP) release/activity was comparable but slightly reduced in ML1 KO BMMs. The data are presented as the percentage of the activity of the released enzymes versus total (released + cell-associated) enzymes. (F) The effects of intracellular and extracellular Ca 2+ on ML-SA1 (10 M for 30 min) -induced lysosomal AP release. BAPTA-AM (500 nm) and EGTA (2 mm with no added extracellular Ca 2+ ) were used to chelate intracellular and extracellular Ca 2+, respectively. (G) An increase in GCaMP3 fluorescence was observed at the uptake site of large beads (6 m). (H) Selected image frames of a RAW cell doubly-transfected with GCaMP3-ML1-KK and Syt VII-mCherry. No significant GCaMP3 fluorescence increase was observed at the site of IgG-RBC uptake and cell body (whole-cell region). (I) Time-dependent change of the GCaMP3-ML1-KK fluorescence signal upon particle binding. For all panels, scale bars = 10 µm. For all panels, unless otherwise indicated, the data represent the mean ± the standard error of the mean (SEM) from at least three independent experiments.
22 Suppl. Fig. S5. Requirement of PI(3,5)P 2 and lysosomal exocytosis in particle uptake--- Related to Figure 6. (A) ML-SA1 (10 µm) failed to increase particle ingestion in BMMs transfected with a VAMP7 dominant-negative (VAMP7-DN) construct. (B) Colchicine (10 µm) inhibited ML-SA1-induced acid phosphatase (AP) release in WT BMMs. (C) TeNT toxin reduced particle uptake in both WT and ML1 KO BMMs. (D) Enlargement of Lamp1-positive compartments in FIG4 KO BMMs. Scale bar = 5 µm. (E) ML-SA1 (10 µm; left panel)-induced and IgG-RBC (30 min; right panel)-induced lysosomal AP release was reduced in BMMs from FIG4 KO mice. (F) Lack of ML1-7Q-2N-GFP accumulation at the site of uptake. Scale bar = 5 µm. (G) While Syt-VII-mCherry appeared on the forming phagosomes within 3-5 min of phagocytosis initiation, 2X-FYVE-GFP (PI(3)P probe) appeared on phagosomal membranes several minutes later (9 min 40 sec). The center of the particle is indicated with an *. (H) Distinct recruitment kinetics of AKT-PH-GFP (PI(3,4,5)P 3 /PI(3,4)P 2 probe ) and 2X-FYVE-GFP (PI(3)P probe) to forming phagosomes. Scale bars = 10 µm. For all panels, unless otherwise indicated, the data represent the mean ± the standard error of the mean (SEM) from at least three independent experiments.
23 Suppl. Fig. S6. Defective clearance of senescent red blood cells in the spleen of ML1 KO mice--- Related to Figure 7. (A) An increased expression of Lamp1 proteins in ML1 KO spleens. (B, C) Immunofluorescence staining of Lamp1 and CD11b in the spleen sections of WT and ML1 KO mice. While Lamp1 immunefluorescence was elevated in the red pulp (RP) region of the ML1 KO spleen sections (B), the CD11b staining was comparable between WT and ML1 KO mice (C). DAPI: nucleus staining. (D) Representative images of spleens from 4-month-old WT and ML1 KO mice. (E) Normalized weight of spleens and kidneys from 4-month-old mice (N=5 animals for each). (F) ML1 KO mice have an elevated level of total red blood cell count compared with WT controls. Blood samples (100 µl) were collected from the mouse tails, and then placed into EDTA-containing tubes before counting (N=5 animals for each genotype). For all panels, scale bars = 200 µm. For all panels, unless otherwise indicated, the data represent the mean ± the standard error of the mean (SEM) from at least three independent experiments.
Supplemental Materials Molecular Biology of the Cell
 Supplemental Materials Molecular Biology of the Cell Gilberti et al. SUPPLEMENTAL FIGURE LEGENDS: Figure S1: The effect of pharmacological inhibitors on particle uptake. The data presented in Figure 1
Supplemental Materials Molecular Biology of the Cell Gilberti et al. SUPPLEMENTAL FIGURE LEGENDS: Figure S1: The effect of pharmacological inhibitors on particle uptake. The data presented in Figure 1
Project report October 2012 March 2013 CRF fellow: Principal Investigator: Project title:
 Project report October 2012 March 2013 CRF fellow: Gennaro Napolitano Principal Investigator: Sergio Daniel Catz Project title: Small molecule regulators of vesicular trafficking to enhance lysosomal exocytosis
Project report October 2012 March 2013 CRF fellow: Gennaro Napolitano Principal Investigator: Sergio Daniel Catz Project title: Small molecule regulators of vesicular trafficking to enhance lysosomal exocytosis
a 10 4 Link et al. Supplementary Figure 1 Nature Immunology: doi: /ni.1842 Cells per mouse ( 10 5 ) TRPV2KO anti-gr1 anti-gr anti-f4/80
 a 10 4 WT 10 4 TRPV2KO 10 3 10 3 anti-gr1 10 2 10 1 anti-gr1 10 2 10 1 10 0 10 0 10 1 10 2 10 3 10 4 anti-f4/80 42.3 45.2 10 0 10 0 10 1 10 2 10 3 10 4 anti-f4/80 10 4 10 4 40 42.5 anti-cd11b 10 3 10 2
a 10 4 WT 10 4 TRPV2KO 10 3 10 3 anti-gr1 10 2 10 1 anti-gr1 10 2 10 1 10 0 10 0 10 1 10 2 10 3 10 4 anti-f4/80 42.3 45.2 10 0 10 0 10 1 10 2 10 3 10 4 anti-f4/80 10 4 10 4 40 42.5 anti-cd11b 10 3 10 2
THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS
 Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Research Foundation, 18 month progress report THE ROLE OF ALTERED CALCIUM AND mtor SIGNALING IN THE PATHOGENESIS OF CYSTINOSIS Ekaterina Ivanova, doctoral student Elena Levtchenko, MD, PhD, PI Antonella
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson
 Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
Lysosomes and endocytic pathways 9/27/2012 Phyllis Hanson General principles Properties of lysosomes Delivery of enzymes to lysosomes Endocytic uptake clathrin, others Endocytic pathways recycling vs.
General aspects of this review - specific examples were addressed in class.
 General aspects of this review - specific examples were addressed in class. 1 Exam 1 Lecture 2: Discussed intracellular killing mechanisms Important maturation steps Rapid development into a microbicidal
General aspects of this review - specific examples were addressed in class. 1 Exam 1 Lecture 2: Discussed intracellular killing mechanisms Important maturation steps Rapid development into a microbicidal
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplemental information contains 7 movies and 4 supplemental Figures
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplemental information contains 7 movies and 4 supplemental Figures Movies: Movie 1. Single virus tracking of A4-mCherry-WR MV
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplemental information contains 7 movies and 4 supplemental Figures Movies: Movie 1. Single virus tracking of A4-mCherry-WR MV
Module 3 Lecture 7 Endocytosis and Exocytosis
 Module 3 Lecture 7 Endocytosis and Exocytosis Endocytosis: Endocytosis is the process by which cells absorb larger molecules and particles from the surrounding by engulfing them. It is used by most of
Module 3 Lecture 7 Endocytosis and Exocytosis Endocytosis: Endocytosis is the process by which cells absorb larger molecules and particles from the surrounding by engulfing them. It is used by most of
Supplementary Figure S1 (a) (b)
 Supplementary Figure S1: IC87114 does not affect basal Ca 2+ level nor nicotineinduced Ca 2+ influx. (a) Bovine chromaffin cells were loaded with Fluo-4AM (1 μm) in buffer A containing 0.02% of pluronic
Supplementary Figure S1: IC87114 does not affect basal Ca 2+ level nor nicotineinduced Ca 2+ influx. (a) Bovine chromaffin cells were loaded with Fluo-4AM (1 μm) in buffer A containing 0.02% of pluronic
Thematic Review Series: Recent Advances in the Treatment of Lysosomal Storage Diseases. Lysosomal exocytosis and lipid storage disorders
 thematic review Thematic Review Series: Recent Advances in the Treatment of Lysosomal Storage Diseases Lysosomal exocytosis and lipid storage disorders Mohammad Ali Samie and Haoxing Xu 1 Department of
thematic review Thematic Review Series: Recent Advances in the Treatment of Lysosomal Storage Diseases Lysosomal exocytosis and lipid storage disorders Mohammad Ali Samie and Haoxing Xu 1 Department of
Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells.
 Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells. a. Schematic of the V-ATPase proton pump macro-complex structure. The V1 complex is composed of
Supplementary Fig. 1 V-ATPase depletion induces unique and robust phenotype in Drosophila fat body cells. a. Schematic of the V-ATPase proton pump macro-complex structure. The V1 complex is composed of
Modulation of Rab5 and Rab7 Recruitment to Phagosomes by Phosphatidylinositol 3-Kinase
 MOLECULAR AND CELLULAR BIOLOGY, Apr. 2003, p. 2501 2514 Vol. 23, No. 7 0270-7306/03/$08.00 0 DOI: 10.1128/MCB.23.7.2501 2514.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved.
MOLECULAR AND CELLULAR BIOLOGY, Apr. 2003, p. 2501 2514 Vol. 23, No. 7 0270-7306/03/$08.00 0 DOI: 10.1128/MCB.23.7.2501 2514.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved.
genome edited transient transfection, CMV promoter
 Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
Supplementary Figure 1. In the absence of new protein translation, overexpressed caveolin-1-gfp is degraded faster than caveolin-1-gfp expressed from the endogenous caveolin 1 locus % loss of total caveolin-1-gfp
7.06 Cell Biology EXAM #3 April 24, 2003
 7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
Protein Trafficking in the Secretory and Endocytic Pathways
 Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Legionella pneumophila: an intracellular pathogen of phagocytes Prof. Craig Roy
 an intracellular pathogen of phagocytes Section of Microbial Pathogenesis, Yale University School of Medicine 1 Legionella pneumophila Gram-negative bacterium Facultative intracellular pathogen Protozoa
an intracellular pathogen of phagocytes Section of Microbial Pathogenesis, Yale University School of Medicine 1 Legionella pneumophila Gram-negative bacterium Facultative intracellular pathogen Protozoa
El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb /DC1
 Supplemental material JCB El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb.201510043 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Acquisition of -phluorin correlates negatively with podosome
Supplemental material JCB El Azzouzi et al., http ://www.jcb.org /cgi /content /full /jcb.201510043 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Acquisition of -phluorin correlates negatively with podosome
2.4 : Cell Transport
 2.4 : Cell Transport At the end of the lesson, you should be able to : Explain the various transport mechanisms across the membrane : Active transport - Sodium-Potassium Pump Endocytosis and Exocytosis
2.4 : Cell Transport At the end of the lesson, you should be able to : Explain the various transport mechanisms across the membrane : Active transport - Sodium-Potassium Pump Endocytosis and Exocytosis
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/334/rs4/dc1 Supplementary Materials for Rapidly rendering cells phagocytic through a cell surface display technique and concurrent Rac activation Hiroki Onuma,
www.sciencesignaling.org/cgi/content/full/7/334/rs4/dc1 Supplementary Materials for Rapidly rendering cells phagocytic through a cell surface display technique and concurrent Rac activation Hiroki Onuma,
Nature Biotechnology: doi: /nbt.3828
 Supplementary Figure 1 Development of a FRET-based MCS. (a) Linker and MA2 modification are indicated by single letter amino acid code. indicates deletion of amino acids and N or C indicate the terminus
Supplementary Figure 1 Development of a FRET-based MCS. (a) Linker and MA2 modification are indicated by single letter amino acid code. indicates deletion of amino acids and N or C indicate the terminus
Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or
 Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or control nontargeting sirnas. At 90 hr after transfection,
Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or control nontargeting sirnas. At 90 hr after transfection,
04_polarity. The formation of synaptic vesicles
 Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
Brefeldin prevents assembly of the coats required for budding Nocodazole disrupts microtubules Constitutive: coatomer-coated Selected: clathrin-coated The formation of synaptic vesicles Nerve cells (and
A LYSOSOMAL CALCIUM CHANNEL REGULATES MEMBRANE TRAFFICKING DURING PHAGOCYTOSIS. Mohammad Ali Samie
 A LYSOSOMAL CALCIUM CHANNEL REGULATES MEMBRANE TRAFFICKING DURING PHAGOCYTOSIS By Mohammad Ali Samie A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy
A LYSOSOMAL CALCIUM CHANNEL REGULATES MEMBRANE TRAFFICKING DURING PHAGOCYTOSIS By Mohammad Ali Samie A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy
Phagocytosis: An Evolutionarily Conserved Mechanism to Remove Apoptotic Bodies and Microbial Pathogens
 Phagocytosis of IgG-coated Targets by s Phagocytosis: An Evolutionarily Conserved Mechanism to Remove Apoptotic Bodies and Microbial s 3 min 10 min Mast Cells Can Phagocytose Too! Extension of an F-actin-rich
Phagocytosis of IgG-coated Targets by s Phagocytosis: An Evolutionarily Conserved Mechanism to Remove Apoptotic Bodies and Microbial s 3 min 10 min Mast Cells Can Phagocytose Too! Extension of an F-actin-rich
293T cells were transfected with indicated expression vectors and the whole-cell extracts were subjected
 SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
SUPPLEMENTARY INFORMATION Supplementary Figure 1. Formation of a complex between Slo1 and CRL4A CRBN E3 ligase. (a) HEK 293T cells were transfected with indicated expression vectors and the whole-cell
Vesicle Transport. Vesicle pathway: many compartments, interconnected by trafficking routes 3/17/14
 Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
Vesicle Transport Vesicle Formation Curvature (Self Assembly of Coat complex) Sorting (Sorting Complex formation) Regulation (Sar1/Arf1 GTPases) Fission () Membrane Fusion SNARE combinations Tethers Regulation
Supplemental information
 Supplemental information PI(3)K p11δ controls the sucellular compartmentalization of TLR4 signaling and protects from endotoxic shock Ezra Aksoy, Salma Taoui, David Torres, Sandrine Delauve, Aderrahman
Supplemental information PI(3)K p11δ controls the sucellular compartmentalization of TLR4 signaling and protects from endotoxic shock Ezra Aksoy, Salma Taoui, David Torres, Sandrine Delauve, Aderrahman
SUPPLEMENTARY INFORMATION
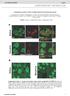 doi: 10.1038/nature06994 A phosphatase cascade by which rewarding stimuli control nucleosomal response A. Stipanovich*, E. Valjent*, M. Matamales*, A. Nishi, J.H. Ahn, M. Maroteaux, J. Bertran-Gonzalez,
doi: 10.1038/nature06994 A phosphatase cascade by which rewarding stimuli control nucleosomal response A. Stipanovich*, E. Valjent*, M. Matamales*, A. Nishi, J.H. Ahn, M. Maroteaux, J. Bertran-Gonzalez,
7.06 Spring of PROBLEM SET #6
 7.6 Spring 23 1 of 6 7.6 PROBLEM SET #6 1. You are studying a mouse model of hypercholesterolemia, a disease characterized by high levels of cholesterol in the blood. In normal cells, LDL particles in
7.6 Spring 23 1 of 6 7.6 PROBLEM SET #6 1. You are studying a mouse model of hypercholesterolemia, a disease characterized by high levels of cholesterol in the blood. In normal cells, LDL particles in
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Quantification of myelin fragments in the aging brain (a) Electron microscopy on corpus callosum is shown for a 18-month-old wild type mice. Myelin fragments (arrows) were detected
Supplementary Figure 1 Quantification of myelin fragments in the aging brain (a) Electron microscopy on corpus callosum is shown for a 18-month-old wild type mice. Myelin fragments (arrows) were detected
SUPPLEMENTARY FIGURE LEGENDS
 SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
SUPPLEMENTARY FIGURE LEGENDS Supplemental FIG. 1. Localization of myosin Vb in cultured neurons varies with maturation stage. A and B, localization of myosin Vb in cultured hippocampal neurons. A, in DIV
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Advanced Cell Biology. Lecture 33
 Advanced Cell Biology. Lecture 33 Alexey Shipunov Minot State University April 22, 2013 Shipunov (MSU) Advanced Cell Biology. Lecture 33 April 22, 2013 1 / 38 Outline Questions and answers Intracellular
Advanced Cell Biology. Lecture 33 Alexey Shipunov Minot State University April 22, 2013 Shipunov (MSU) Advanced Cell Biology. Lecture 33 April 22, 2013 1 / 38 Outline Questions and answers Intracellular
Chapter 3. Expression of α5-megfp in Mouse Cortical Neurons. on the β subunit. Signal sequences in the M3-M4 loop of β nachrs bind protein factors to
 22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
A Closer Look at Cell Membranes. Chapter 5 Part 2
 A Closer Look at Cell Membranes Chapter 5 Part 2 5.5 Membrane Trafficking By processes of endocytosis and exocytosis, vesicles help cells take in and expel particles that are too big for transport proteins,
A Closer Look at Cell Membranes Chapter 5 Part 2 5.5 Membrane Trafficking By processes of endocytosis and exocytosis, vesicles help cells take in and expel particles that are too big for transport proteins,
Phosphoinositide Signaling
 Phosphoinositide Signaling Pietro De Camilli Department of Cell Biology Howard Hughes Medical Institute Program in Cellular Neuroscience, Neurodegeneration and Repair Yale University School of Medicine
Phosphoinositide Signaling Pietro De Camilli Department of Cell Biology Howard Hughes Medical Institute Program in Cellular Neuroscience, Neurodegeneration and Repair Yale University School of Medicine
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
lysosomes Ingested materials Defective cell components Degrades macromolecules of all types:
 lysosomes Digests Ingested materials Defective cell components Degrades macromolecules of all types: Proteins Nucleic acids Carbohydrates Lipids Single membrane bound vesicle, contains up to 50 digestive
lysosomes Digests Ingested materials Defective cell components Degrades macromolecules of all types: Proteins Nucleic acids Carbohydrates Lipids Single membrane bound vesicle, contains up to 50 digestive
F-actin VWF Vinculin. F-actin. Vinculin VWF
 a F-actin VWF Vinculin b F-actin VWF Vinculin Supplementary Fig. 1. WPBs in HUVECs are located along stress fibers and at focal adhesions. (a) Immunofluorescence images of f-actin (cyan), VWF (yellow),
a F-actin VWF Vinculin b F-actin VWF Vinculin Supplementary Fig. 1. WPBs in HUVECs are located along stress fibers and at focal adhesions. (a) Immunofluorescence images of f-actin (cyan), VWF (yellow),
Lysosomes, Peroxisomes and Centrioles. Hüseyin Çağsın
 Lysosomes, Peroxisomes and Centrioles Hüseyin Çağsın Lysosomes Outline Endosomes Molecule transport to the lysosomes Endocytosis Exocytosis Autophagy Vacuoles Peroxisomes Centrioles Lysosomes Lysosomes
Lysosomes, Peroxisomes and Centrioles Hüseyin Çağsın Lysosomes Outline Endosomes Molecule transport to the lysosomes Endocytosis Exocytosis Autophagy Vacuoles Peroxisomes Centrioles Lysosomes Lysosomes
A genetically targeted optical sensor to monitor calcium signals in astrocyte processes
 A genetically targeted optical sensor to monitor calcium signals in astrocyte processes 1 Eiji Shigetomi, 1 Sebastian Kracun, 2 Michael V. Sofroniew & 1,2 *Baljit S. Khakh Ψ 1 Departments of Physiology
A genetically targeted optical sensor to monitor calcium signals in astrocyte processes 1 Eiji Shigetomi, 1 Sebastian Kracun, 2 Michael V. Sofroniew & 1,2 *Baljit S. Khakh Ψ 1 Departments of Physiology
Supporting Information
 ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD- 95 multi- protein complex U.Lalo, O.Palygin, A.Verkhratsky, S.G.N. Grant and Y. Pankratov Supporting
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human
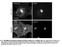 Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
Fig. S1. Subcellular localization of overexpressed LPP3wt-GFP in COS-7 and HeLa cells. Cos7 (top) and HeLa (bottom) cells expressing for 24 h human LPP3wt-GFP, fixed and stained for GM130 (A) or Golgi97
MCB130 Midterm. GSI s Name:
 1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
1. Peroxisomes are small, membrane-enclosed organelles that function in the degradation of fatty acids and in the degradation of H 2 O 2. Peroxisomes are not part of the secretory pathway and peroxisomal
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Data. Hao et al. (2014). Plant Cell /tpc
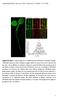 Supplemental Figure 1. Confocal Images and VA-TIRFM Analysis of GFP-RbohD in Arabidopsis Seedlings. (A) RbohD expression in whole Arabidopsis seedlings. RbohD was expressed in the leaves, hypocotyl, and
Supplemental Figure 1. Confocal Images and VA-TIRFM Analysis of GFP-RbohD in Arabidopsis Seedlings. (A) RbohD expression in whole Arabidopsis seedlings. RbohD was expressed in the leaves, hypocotyl, and
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Supplemental Information. Ca V 2.2 Gates Calcium-Independent. but Voltage-Dependent Secretion. in Mammalian Sensory Neurons
 Neuron, Volume 96 Supplemental Information Ca V 2.2 Gates Calcium-Independent but Voltage-Dependent Secretion in Mammalian Sensory Neurons Zuying Chai, Changhe Wang, Rong Huang, Yuan Wang, Xiaoyu Zhang,
Neuron, Volume 96 Supplemental Information Ca V 2.2 Gates Calcium-Independent but Voltage-Dependent Secretion in Mammalian Sensory Neurons Zuying Chai, Changhe Wang, Rong Huang, Yuan Wang, Xiaoyu Zhang,
Chapter 3 subtitles Action potentials
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
Expanded View Figures
 Shao-Ming Shen et al Role of I in MT of cancers MO reports xpanded View igures igure V1. nalysis of the expression of I isoforms in cancer cells and their interaction with PTN. RT PR detection of Ish and
Shao-Ming Shen et al Role of I in MT of cancers MO reports xpanded View igures igure V1. nalysis of the expression of I isoforms in cancer cells and their interaction with PTN. RT PR detection of Ish and
Chloride Channel Blockers Suppress Formation of Engulfment Pseudopodia in Microglial Cells
 Original Paper www.karger.com/cpb 319 Harl/Schmölzer/Jakab/Ritter/Kerschbaum: Accepted: February 07, 2013 Chloride Channel 1421-9778/13/0313-0319$38.00/0 Blockers Suppress This is an Open Access article
Original Paper www.karger.com/cpb 319 Harl/Schmölzer/Jakab/Ritter/Kerschbaum: Accepted: February 07, 2013 Chloride Channel 1421-9778/13/0313-0319$38.00/0 Blockers Suppress This is an Open Access article
Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary Materials and Methods
 Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
Procaspase-3. Cleaved caspase-3. actin. Cytochrome C (10 M) Z-VAD-fmk. Procaspase-3. Cleaved caspase-3. actin. Z-VAD-fmk
 A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
IP: anti-gfp VPS29-GFP. IP: anti-vps26. IP: anti-gfp - + +
 FAM21 Strump. WASH1 IP: anti- 1 2 3 4 5 6 FAM21 Strump. FKBP IP: anti-gfp VPS29- GFP GFP-FAM21 tail H H/P P H H/P P c FAM21 FKBP Strump. VPS29-GFP IP: anti-gfp 1 2 3 FKBP VPS VPS VPS VPS29 1 = VPS29-GFP
FAM21 Strump. WASH1 IP: anti- 1 2 3 4 5 6 FAM21 Strump. FKBP IP: anti-gfp VPS29- GFP GFP-FAM21 tail H H/P P H H/P P c FAM21 FKBP Strump. VPS29-GFP IP: anti-gfp 1 2 3 FKBP VPS VPS VPS VPS29 1 = VPS29-GFP
MISSION: understanding the mechanisms of therapeutic strategies
 Telethon Institute of Genetics and Medicine MISSION: understanding the mechanisms of genetic diseases to develop preventive and therapeutic strategies G E N O T Y P E G E Researcher N 1 3 5 O T Y P E 8
Telethon Institute of Genetics and Medicine MISSION: understanding the mechanisms of genetic diseases to develop preventive and therapeutic strategies G E N O T Y P E G E Researcher N 1 3 5 O T Y P E 8
(A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and a
 Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Cell Size. More Cell Notes. Limits. Why can t organisms be one big giant cell? DNA limits cell size. Diffusion limits cell size
 More Cell Notes Pre-AP Biology Cell Size Why are cells so small? Why can t organisms be one big giant cell? Most cells are between 2µm and 200µm A micrometer is 1 millionth of a meter! Too small to be
More Cell Notes Pre-AP Biology Cell Size Why are cells so small? Why can t organisms be one big giant cell? Most cells are between 2µm and 200µm A micrometer is 1 millionth of a meter! Too small to be
Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD
 ? Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Christian S Lobsiger & Don W Cleveland (2007) Nature Neuroscience 10, 1355-1360 Astrocytes: interlinked gatekeepers of glutamate astrocytes
? Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Christian S Lobsiger & Don W Cleveland (2007) Nature Neuroscience 10, 1355-1360 Astrocytes: interlinked gatekeepers of glutamate astrocytes
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
Supplementary Figure 1. Rab27a-KD inhibits speed and persistence of HEp3 cells migrating in the chick CAM. (a) Western blot analysis of Rab27a
 Supplementary Figure 1. Rab27a-KD inhibits speed and persistence of HEp3 cells migrating in the chick CAM. (a) Western blot analysis of Rab27a expression in GFP-expressing HEp3 cells. (b) Representative
Supplementary Figure 1. Rab27a-KD inhibits speed and persistence of HEp3 cells migrating in the chick CAM. (a) Western blot analysis of Rab27a expression in GFP-expressing HEp3 cells. (b) Representative
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission
 Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
Supplementary Materials for VAMP4 directs synaptic vesicles to a pool that selectively maintains asynchronous neurotransmission Jesica Raingo, Mikhail Khvotchev, Pei Liu, Frederic Darios, Ying C. Li, Denise
endomembrane system internal membranes origins transport of proteins chapter 15 endomembrane system
 endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
endo system chapter 15 internal s endo system functions as a coordinated unit divide cytoplasm into distinct compartments controls exocytosis and endocytosis movement of molecules which cannot pass through
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) The hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
Anatomy Chapter 2 - Cells
 Cells Cells are the basic living structural, functional unit of the body Cytology is the branch of science that studies cells The human body has 100 trillion cells 200 different cell types with a variety
Cells Cells are the basic living structural, functional unit of the body Cytology is the branch of science that studies cells The human body has 100 trillion cells 200 different cell types with a variety
Intracellular Vesicular Traffic Chapter 13, Alberts et al.
 Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
Intracellular Vesicular Traffic Chapter 13, Alberts et al. The endocytic and biosynthetic-secretory pathways The intracellular compartments of the eucaryotic ell involved in the biosynthetic-secretory
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/308/ra4/dc1 Supplementary Materials for Antipsychotics Activate mtorc1-dependent Translation to Enhance Neuronal Morphological Complexity Heather Bowling, Guoan
www.sciencesignaling.org/cgi/content/full/7/308/ra4/dc1 Supplementary Materials for Antipsychotics Activate mtorc1-dependent Translation to Enhance Neuronal Morphological Complexity Heather Bowling, Guoan
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Lu et al., http://www.jcb.org/cgi/content/full/jcb.201012063/dc1 Figure S1. Kinetics of nuclear envelope assembly, recruitment of Nup133
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Lu et al., http://www.jcb.org/cgi/content/full/jcb.201012063/dc1 Figure S1. Kinetics of nuclear envelope assembly, recruitment of Nup133
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/278/rs11/dc1 Supplementary Materials for In Vivo Phosphoproteomics Analysis Reveals the Cardiac Targets of β-adrenergic Receptor Signaling Alicia Lundby,* Martin
www.sciencesignaling.org/cgi/content/full/6/278/rs11/dc1 Supplementary Materials for In Vivo Phosphoproteomics Analysis Reveals the Cardiac Targets of β-adrenergic Receptor Signaling Alicia Lundby,* Martin
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
Molecular Cell Biology 5068 In class Exam 1 October 2, Please print your name: Instructions:
 Molecular Cell Biology 5068 In class Exam 1 October 2, 2012 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your number
Molecular Cell Biology 5068 In class Exam 1 October 2, 2012 Exam Number: Please print your name: Instructions: Please write only on these pages, in the spaces allotted and not on the back. Write your number
Structure. Lysosomes are membrane-enclosed organelles. Hydrolytic enzymes. Variable in size & shape need
 Lysosomes Structure Lysosomes are membrane-enclosed organelles Hydrolytic enzymes Variable in size & shape need Degrade material taken up from outside and inside the cell Variable in size and shape Lysosomal
Lysosomes Structure Lysosomes are membrane-enclosed organelles Hydrolytic enzymes Variable in size & shape need Degrade material taken up from outside and inside the cell Variable in size and shape Lysosomal
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Chen et al., http://www.jcb.org/cgi/content/full/jcb.201210119/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Lack of fast reversibility of UVR8 dissociation. (A) HEK293T
Supplemental material Chen et al., http://www.jcb.org/cgi/content/full/jcb.201210119/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Lack of fast reversibility of UVR8 dissociation. (A) HEK293T
Supplementary Figures
 J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
J. Cell Sci. 128: doi:10.1242/jcs.173807: Supplementary Material Supplementary Figures Fig. S1 Fig. S1. Description and/or validation of reagents used. All panels show Drosophila tissues oriented with
Molecular Trafficking
 SCBM 251 Molecular Trafficking Assoc. Prof. Rutaiwan Tohtong Department of Biochemistry Faculty of Science rutaiwan.toh@mahidol.ac.th Lecture outline 1. What is molecular trafficking? Why is it important?
SCBM 251 Molecular Trafficking Assoc. Prof. Rutaiwan Tohtong Department of Biochemistry Faculty of Science rutaiwan.toh@mahidol.ac.th Lecture outline 1. What is molecular trafficking? Why is it important?
Cell Quality Control. Peter Takizawa Department of Cell Biology
 Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Cells: The Living Units
 Chapter 3 Part B Cells: The Living Units Annie Leibovitz/Contact Press Images PowerPoint Lecture Slides prepared by Karen Dunbar Kareiva Ivy Tech Community College 3.4 Active Membrane Transport Two major
Chapter 3 Part B Cells: The Living Units Annie Leibovitz/Contact Press Images PowerPoint Lecture Slides prepared by Karen Dunbar Kareiva Ivy Tech Community College 3.4 Active Membrane Transport Two major
Supplementary Figure 1. Properties of various IZUMO1 monoclonal antibodies and behavior of SPACA6. (a) (b) (c) (d) (e) (f) (g) .
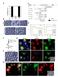 Supplementary Figure 1. Properties of various IZUMO1 monoclonal antibodies and behavior of SPACA6. (a) The inhibitory effects of new antibodies (Mab17 and Mab18). They were investigated in in vitro fertilization
Supplementary Figure 1. Properties of various IZUMO1 monoclonal antibodies and behavior of SPACA6. (a) The inhibitory effects of new antibodies (Mab17 and Mab18). They were investigated in in vitro fertilization
Chapter 3 Neurotransmitter release
 NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
Supplemental Figures:
 Supplemental Figures: Figure 1: Intracellular distribution of VWF by electron microscopy in human endothelial cells. a) Immunogold labeling of LC3 demonstrating an LC3-positive autophagosome (white arrow)
Supplemental Figures: Figure 1: Intracellular distribution of VWF by electron microscopy in human endothelial cells. a) Immunogold labeling of LC3 demonstrating an LC3-positive autophagosome (white arrow)
Supplementary Information
 Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
Supplementary Information D-Serine regulates cerebellar LTD and motor coordination through the 2 glutamate receptor Wataru Kakegawa, Yurika Miyoshi, Kenji Hamase, Shinji Matsuda, Keiko Matsuda, Kazuhisa
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
www.sciencesignaling.org/cgi/content/full/6/283/ra57/dc1 Supplementary Materials for JNK3 Couples the Neuronal Stress Response to Inhibition of Secretory Trafficking Guang Yang,* Xun Zhou, Jingyan Zhu,
Localized Biphasic Changes in Phosphatidylinositol-4,5-Bisphosphate at Sites of Phagocytosis
 Localized Biphasic Changes in Phosphatidylinositol-4,5-Bisphosphate at Sites of Phagocytosis Roberto J. Botelho,* Mary Teruel, Renee Dierckman, Richard Anderson, Alan Wells, John D. York, Tobias Meyer,
Localized Biphasic Changes in Phosphatidylinositol-4,5-Bisphosphate at Sites of Phagocytosis Roberto J. Botelho,* Mary Teruel, Renee Dierckman, Richard Anderson, Alan Wells, John D. York, Tobias Meyer,
Supplementary Figure 1 Maschalidi et al.
 a 1% 5% % 1% 5% % OVAb βgal activity A.U. (x1 4 ) 2 1 5 βgal activity A.U. (x1 4 ) 2 1 BSAb 2 hours 4 hours OVAb BSAb OVAb BSAb,1 1 1 1 SIINFEKL (ng/ml) CFSE b Beads Alexa488 (%) 8 6 4 2 ** ** 1:1 5:1
a 1% 5% % 1% 5% % OVAb βgal activity A.U. (x1 4 ) 2 1 5 βgal activity A.U. (x1 4 ) 2 1 BSAb 2 hours 4 hours OVAb BSAb OVAb BSAb,1 1 1 1 SIINFEKL (ng/ml) CFSE b Beads Alexa488 (%) 8 6 4 2 ** ** 1:1 5:1
BIPN 140 Problem Set 6
 BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
BIPN 140 Problem Set 6 1) Hippocampus is a cortical structure in the medial portion of the temporal lobe (medial temporal lobe in primates. a) What is the main function of the hippocampus? The hippocampus
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2294 Figure S1 Localization and function of cell wall polysaccharides in root hair cells. (a) Spinning-disk confocal sections of seven day-old A. thaliana seedlings stained with 0.1% S4B
DOI: 10.1038/ncb2294 Figure S1 Localization and function of cell wall polysaccharides in root hair cells. (a) Spinning-disk confocal sections of seven day-old A. thaliana seedlings stained with 0.1% S4B
Supplementary Figure 1
 Supplementary Figure 1 AAV-GFP injection in the MEC of the mouse brain C57Bl/6 mice at 4 months of age were injected with AAV-GFP into the MEC and sacrificed at 7 days post injection (dpi). (a) Brains
Supplementary Figure 1 AAV-GFP injection in the MEC of the mouse brain C57Bl/6 mice at 4 months of age were injected with AAV-GFP into the MEC and sacrificed at 7 days post injection (dpi). (a) Brains
LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role in Alzheimer s disease
 Brodeur et al. Molecular Neurodegeneration 2012, 7:31 RESEARCH ARTICLE Open Access LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role
Brodeur et al. Molecular Neurodegeneration 2012, 7:31 RESEARCH ARTICLE Open Access LDLR-related protein 10 (LRP10) regulates amyloid precursor protein (APP) trafficking and processing: evidence for a role
Hematopoiesis. Hematopoiesis. Hematopoiesis
 Chapter. Cells and Organs of the Immune System Hematopoiesis Hematopoiesis- formation and development of WBC and RBC bone marrow. Hematopoietic stem cell- give rise to any blood cells (constant number,
Chapter. Cells and Organs of the Immune System Hematopoiesis Hematopoiesis- formation and development of WBC and RBC bone marrow. Hematopoietic stem cell- give rise to any blood cells (constant number,
Supplementary figure legends
 Supplementary figure legends Supplementary Figure 1. Exposure of CRT occurs independently from the apoptosisassociated loss of the mitochondrial membrane potential (MMP). (A) HeLa cells treated with MTX
Supplementary figure legends Supplementary Figure 1. Exposure of CRT occurs independently from the apoptosisassociated loss of the mitochondrial membrane potential (MMP). (A) HeLa cells treated with MTX
Chapter 2. Investigation into mir-346 Regulation of the nachr α5 Subunit
 15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
15 Chapter 2 Investigation into mir-346 Regulation of the nachr α5 Subunit MicroRNA s (mirnas) are small (< 25 base pairs), single stranded, non-coding RNAs that regulate gene expression at the post transcriptional
Chapter 13: Vesicular Traffic
 Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
Chapter 13: Vesicular Traffic Know the terminology: ER, Golgi, vesicle, clathrin, COP-I, COP-II, BiP, glycosylation, KDEL, microtubule, SNAREs, dynamin, mannose-6-phosphate, M6P receptor, endocytosis,
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1. (A) Left, western blot analysis of ISGylated proteins in Jurkat T cells treated with 1000U ml -1 IFN for 16h (IFN) or left untreated (CONT); right, western
SUPPLEMENTARY FIGURES Supplementary Figure 1. (A) Left, western blot analysis of ISGylated proteins in Jurkat T cells treated with 1000U ml -1 IFN for 16h (IFN) or left untreated (CONT); right, western
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained
 Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
Exocytosis of late endosomes does not directly contribute membrane to the formation of phagocytic cups or pseudopods in Dictyostelium
 Exocytosis of late endosomes does not directly contribute membrane to the formation of phagocytic cups or pseudopods in Dictyostelium Steve J. Charette *, Pierre Cosson Université de Genève, Centre Médical
Exocytosis of late endosomes does not directly contribute membrane to the formation of phagocytic cups or pseudopods in Dictyostelium Steve J. Charette *, Pierre Cosson Université de Genève, Centre Médical
Chapter 6. Antigen Presentation to T lymphocytes
 Chapter 6 Antigen Presentation to T lymphocytes Generation of T-cell Receptor Ligands T cells only recognize Ags displayed on cell surfaces These Ags may be derived from pathogens that replicate within
Chapter 6 Antigen Presentation to T lymphocytes Generation of T-cell Receptor Ligands T cells only recognize Ags displayed on cell surfaces These Ags may be derived from pathogens that replicate within
BMDCs were generated in vitro from bone marrow cells cultured in 10 % RPMI supplemented
 Supplemental Materials Figure S1. Cultured BMDCs express CD11c BMDCs were generated in vitro from bone marrow cells cultured in 10 % RPMI supplemented with 15 ng/ml GM-CSF. Media was changed and fresh
Supplemental Materials Figure S1. Cultured BMDCs express CD11c BMDCs were generated in vitro from bone marrow cells cultured in 10 % RPMI supplemented with 15 ng/ml GM-CSF. Media was changed and fresh
Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death
 www.impactjournals.com/oncotarget/ Oncotarget, Supplementary Materials 2016 Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death Supplementary
www.impactjournals.com/oncotarget/ Oncotarget, Supplementary Materials 2016 Tumor suppressor Spred2 interaction with LC3 promotes autophagosome maturation and induces autophagy-dependent cell death Supplementary
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous
 Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
Supplementary Figure 1. Spatial distribution of LRP5 and β-catenin in intact cardiomyocytes. (a) and (b) Immunofluorescence staining of endogenous LRP5 in intact adult mouse ventricular myocytes (AMVMs)
