ISLET STUDIES. Linda M. Nguyen, 1 Marina Pozzoli, 1 Thomas H. Hraha, 1 and Richard K.P. Benninger 1,2. Diabetes Volume 63, May
|
|
|
- Clare Garrison
- 5 years ago
- Views:
Transcription
1 Diabetes Volume 63, May Linda M. Nguyen, 1 Marina Pozzoli, 1 Thomas H. Hraha, 1 and Richard K.P. Benninger 1,2 Decreasing Cx36 Gap Junction Coupling Compensates for Overactive K ATP Channels to Restore Insulin Secretion and Prevent Hyperglycemia in a Mouse Model of Neonatal Diabetes Mutations to the ATP-sensitive K + channel (K ATP channel) that reduce the sensitivity of ATP inhibition cause neonatal diabetes mellitus via suppression of b-cell glucose-stimulated free calcium activity ([Ca 2+ ] i ) and insulin secretion. Connexin-36 (Cx36) gap junctions also regulate islet electrical activity; upon knockout of Cx36, b-cells show [Ca 2+ ] i elevations at basal glucose. We hypothesized that in the presence of overactive ATP-insensitive K ATP channels, a reduction in Cx36 would allow elevations in glucose-stimulated [Ca 2+ ] i and insulin secretion to improve glucose homeostasis. To test this, we introduced a genetic knockout of Cx36 into mice that express ATP-insensitive K ATP channels and measured glucose homeostasis and islet metabolic, electrical, and insulin secretion responses. In the normal presence of Cx36, after expression of ATPinsensitive K ATP channels, blood glucose levels rapidly rose to >500 mg/dl. Islets from these mice showed reduced glucose-stimulated [Ca 2+ ] i and no insulin secretion. In mice lacking Cx36 after expression of ATP-insensitive K ATP channels, normal glucose levels were maintained. Islets from these mice had near-normal glucose-stimulated [Ca 2+ ] i and insulin secretion. We therefore demonstrate a novel mechanism by which islet function can be recovered in a monogenic model of diabetes. A reduction of gap junction coupling allows sufficient glucose-stimulated [Ca 2+ ] i and insulin secretion to prevent the emergence of diabetes. Diabetes 2014;63: DOI: /db Glucose-stimulated insulin secretion from b-cells in the islet is regulated via a series of metabolic and electrical events. The ATP-sensitive K + channel (K ATP channel) provides a central role in coupling increases in the ATP/ ADP ratio after the metabolism of glucose, to membrane depolarization, elevated intracellular free calcium activity ([Ca 2+ ] i ), and insulin granule exocytosis (1). The K ATP channel is made up of inward-rectifying K + channel Kir6.2 and sulfonylurea receptor 1 (Sur1). In humans, ISLET STUDIES 1 Department of Bioengineering, University of Colorado, Anschutz Medical Campus, Aurora, CO 2 Barbara Davis Center for Childhood Diabetes, University of Colorado, Anschutz Medical Campus, Aurora, CO Corresponding author: Richard K.P. Benninger, richard.benninger@ucdenver.edu. Received 3 July 2013 and accepted 8 January This article contains Supplementary Data online at L.M.N. and M.P. contributed equally to this study by the American Diabetes Association. See for details.
2 1686 Modulating Gap Junctions to Prevent Diabetes Diabetes Volume 63, May 2014 mutations in the genes encoding Kir6.2 (KCNJ11) and Sur1 (ABCC8), which reduce the sensitivity of ATP inhibition and lead to overactive K ATP channels (gain-offunction mutations), are the most common cause of neonatal diabetes mellitus (NDM) (2). Transgenic mice that express ATP-insensitive K ATP channels in b-cells can recapitulate the human disease (3 5). These mice exhibit marked hyperglycemia and reduced plasma insulin, and islets from these mice show a suppression of glucosestimulated [Ca 2+ ] i and insulin release (4,6). This demonstrates that a reduction of glucose-dependent excitability underlies the development of diabetes caused by K ATP channel mutations. Inhibition of K ATP channels with sulfonylureas can recover elevations in [Ca 2+ ] i and insulin secretion in islets of these mouse models (3,4,6). As such, sulfonylureas can be applied to treat patients with NDM associated with K ATP channel mutations (7,8). Nevertheless, some mutations render the K ATP channel insensitive to sulfonylureas (7,9,10). Cellular interactions within the islet have long been known to be important for enhancing the regulation of insulin release (11) and are also important for regulating electrical activity (12). Connexin-36 (Cx36) gap junction channels regulate islet electrical activity by coupling K ATP -regulated membrane depolarization between b-cells of the islet. This synchronizes oscillations in membrane depolarization and [Ca 2+ ] i at elevated glucose (13,14), leading to a coordination of first-phase insulin release and pulsatile second-phase insulin release (15). Under normal conditions, Cx36 gap junction channels also enhance a suppression of spontaneous elevations in [Ca 2+ ] i at basal glucose (16,17); upon a knockout of Cx36, elevated [Ca 2+ ] i is observed at lower glucose levels (14,18). This suppression likely occurs as a result of the longestablished presence of b-cell heterogeneity (19), where inexcitable cells in the islet suppress membrane depolarization and [Ca 2+ ] i in more excitable cells via gap junction coupling (16,18). Given that Cx36 gap junction channels coordinate K ATP -regulated membrane potential, we hypothesized that their action in suppressing [Ca 2+ ] i and insulin occurs more generally under conditions of K ATP channel opening. Therefore, similar to their normal action at lower glucose levels, we hypothesized that Cx36 gap junctions would inappropriately enhance the suppression of [Ca 2+ ] i and insulin secretion at elevated glucose in the presence of overactive ATP-insensitive K ATP channels in NDM. Therefore, we hypothesized that an absence of Cx36 gap junction coupling upon expression of ATP-insensitive K ATP channels would reduce this suppression and lead to spontaneous elevations in [Ca 2+ ] i at elevated glucose. We anticipated that this [Ca 2+ ] i elevation would stimulate sufficient insulin release to prevent the severe hyperglycemia that emerges in these animals. However, we anticipated that suppression of [Ca 2+ ] i and insulin release would be maintained at low glucose levels; in essence, a decrease in Cx36 will left shift the dose response to compensate for a right shift due to overactive K ATP channels. Here, we tested this by introducing a knockout of Cx36 gap junction channels into a mouse model of NDM that expresses ATP-insensitive K ATP channels in the b-cell under control of an inducible Cre ER - recombinase (3). We tested whether mice with reduced or absent Cx36 gap junction coupling showed an improvement in glucose homeostasis and whether islets from these mice showed a recovery of glucose-stimulated [Ca 2+ ] i and insulin secretion. RESEARCH DESIGN AND METHODS Animal Care Experiments were performed in compliance with the relevant laws and institutional guidelines and were approved by the University of Colorado Institutional Animal Care and Use Committee. Mice expressing Rosa26-Kir6.2 [DN30,K185Q] (Kir6.2 [DN30,K185Q] gain-offunction mutant), Pdx-Cre ER (pancreas-specific inducible Cre), and Cx36 2/2 (global Cx36 knockout) were generated as described previously (3,20,21) and supplied by collaborating laboratories. b-cell conditional expression of Kir6.2 [K185Q,DN30] is achieved through crossing Rosa26-Kir6.2 [DN30,K185Q] and Pdx-Cre ER mice to excise a loxp-flanked stop codon to drive Kir6.2 [K185Q,DN30] expression. Green fluorescent protein (GFP) is coexpressed via an internal ribosome entry site. Rosa26- Kir6.2 [DN30,K185Q] and Pdx-Cre ER mice either had normal gap junction coupling (to generate Cx36 +/+ ; Kir6.2 [DN30,K185Q] mice) or were first separately crossed with Cx36 2/2 mice to achieve a homozygous deletion and then bred together (to generate Cx36 2/2 ; Kir6.2 [DN30,K185Q] mice). Cx36 +/+ or Cx36 2/2 littermate mice lacking Rosa26-Kir6.2 [DN30,K185Q] and/or Pdx-Cre ER were used as controls. Mice were studied at generation F3 F5. To prevent genetic drift, new breeders were generated by crossing Cx36 +/+ - and Cx36 2/2 -expressing breeders every two to three generations. In Vivo Measurements Each experimental group (age-matched Cx36 +/+ ; Kir6.2 [DN30,K185Q] and Cx36 2/2 ;Kir6.2 [DN30,K185Q] with respective littermate Cx36 +/+ and Cx36 2/2 controls) received five daily doses of tamoxifen (50 mg/g body weight) at experimental day 1 5. Blood glucose was measured daily as previously performed (3) with a glucometer (Ascensia Contour; Bayer). Plasma insulin was measured at day 29 from blood samples centrifuged at 14 krev/min for 10 min and assayed using mouse ultrasensitive insulin ELISA (Alpco). Glucose tolerance tests were performed at day Littermate or agematched mice were fasted overnight for 16 h and received intraperitoneal injection of 2 g/kg body weight of glucose, and blood glucose was measured on tail vein blood samples preinjection (0 min) and 15, 30, 60, 90, and 120 min post glucose delivery. Insulin tolerance tests (ITTs) were performed at day Littermate
3 diabetes.diabetesjournals.org Nguyen and Associates 1687 mice were fasted for 6 h and received intraperitoneal injection of 0.75 units/kg body weight of human recombinant insulin (Novolin; Novo Nordisk), and blood glucose was measured on tail vein blood samples preinjection (0 min) and 15, 30, 45, 60, and 90 min postinjection. Islet Isolation and Insulin Secretion At day 30 36, islets were isolated from pancreata of each experimental mouse and maintained in islet medium (RPMI medium, 10% fetal bovine serum, 11 mmol/l glucose, 100 units/ml penicillin, 100 mg/ml streptomycin) at 37 C under humidified 5% CO 2 for 24 h. For static insulin secretion measurements, islets (five per column, in duplicate) were preincubated for 60 min at 37 C in Krebs-Ringer buffer (128.8 mmol/l NaCl, 5 mmol/l NaHCO 3, 5.8 mmol/l KCl, 1.2 mmol/l KH 2 PO 4, 2.5 CaCl 2, 1.2 mmol/l MgSO 4, 10 mmol/l HEPES, 0.1% BSA, ph 7.4) plus 2 mmol/l glucose and then incubated for 60 min at 37 C in Krebs-Ringer buffer plus different glucose concentrations and/or reagents as indicated. After the incubation period, the medium was removed and insulin concentration assayed using mouse ultrasensitive insulin ELISA. To estimate insulin content, islets were lysed in 1% Triton X-100 and frozen at 220 C overnight. Microscopy All isolated islets were imaged using established methods (13), in polydimethylsiloxane microfluidic devices (16), maintained at 37 C, with imaging medium (125 mmol/l NaCl, 5.7 mmol/l KCl, 2.5 mmol/l CaCl 2, 1.2 mmol/l MgCl 2, 10 mmol/l HEPES, 2 mmol/l glucose, 0.1% BSA, ph 7.4). To measure [Ca 2+ ] i response and dynamics, islets were loaded with 4 mmol/l Fura Red-AM (Invitrogen) for 2 h at room temperature and imaged on a Marianas spinning-disk microscope (3I) with a NA Plan- Neofluar oil-immersion objective (Zeiss). Images were acquired 10 min after glucose stimulation. Fura Red was imaged using a 488-nm diode laser for excitation and a 580- to 655-nm band-pass emission filter (Semrock). GFP was imaged using a 488-nm diode laser for excitation and a 500- to 550-nm band-pass emission filter. There was no detectable cross-talk between Fura Red and GFP. To measure [Ca 2+ ] i concentration, islets were loaded with 2 mmol/l Fura 2-AM for 30 min at room temperature and imaged on an Eclipse-Ti widefield microscope (Nikon) with a NA Plan Apo objective. Images were acquired 10 min after glucose stimulation or immediately after KCl stimulation. Fura 2 was imaged sequentially using 340- and 380-nm (65 nm) Arc-lamp excitation and a 470- to 550-nm band-pass emission filter (Chroma). To measure NAD(P)H, islets were imaged on an LSM510 microscope (Zeiss), with a NA C- Apochromatic water-immersion objective. NAD(P)H autofluorescence was imaged with two-photon excitation using a 710-nm mode-locked Ti:sapphire laser (Coherent) and a 400- to 500-nm band-pass emission filter (Chroma) and nondescanned detector. GFP fluorescence was imaged using a 488-nm Ar + laser line for excitation and a 500- to 550-nm band-pass emission filter. Z-stacks of six images were acquired at 2-mm spacing. No GFP fluorescence was detected in the NAD(P)H channel. To measure mitochondrial membrane potential changes, islets were loaded with 50 nmol/l Rhodamine 123 for 20 min at 37 C and imaged on an LSM510 microscope with a NA C-Apochromatic waterimmersion objective. Images were acquired 10 min after glucose stimulation. Rhodamine 123 was imaged using a488-nmar + laser line for excitation and a 500- to 550-nm band-pass emission filter. Z-stacks of six images were acquired at 2-mm spacing. Image Analysis Images collected on the different microscope systems (3I and Zeiss; Nikon) were analyzed offline in Matlab (Mathworks) using established methods (6,18,22). For Fura 2, 340- and 380-nm intensities were averaged across each islet, and the background intensity averaged over an unstained islet subtracted. Time-averaged [Ca 2+ ] i concentration was calibrated from the backgroundsubtracted time-averaged 340-nm/380-nm intensity ratio using the Fura 2 calibration kit (Invitrogen). NAD(P)H and Rhodamine 123 fluorescence were averaged across each islet, with Z positions corrected for focal drift. The mean NAD(P)H autofluorescence for each experimental group was normalized to the average autofluorescence in Cx36 +/+ islets at 2 mmol/l. The mean Rhodamine 123 fluorescence of each islet was normalized to the fluorescence at 2 mmol/l glucose. To estimate the proportion of the islet showing elevations in [Ca 2+ ] i, Fura Red images were first smoothed using a 3 3 3averagefilter. The variance (intensity fluctuation) was calculated over the time course of each pixel. A threshold variance was calculated for a silent cell, identified as quiescent at 2 mmol/l glucose, which represents image noise. An active area showing [Ca 2+ ] i fluctuations was defined as having a variance significantly greater (95% confidence) than the variance of the silent cell. To determine high-gfp (GFP + ) cells, GFP images were first smoothed using a average filter. A threshold intensity was calculated from the autofluorescence in the GFP channel from control (GFP 2 ) Cx36 +/+ or Cx36 2/2 islets. A GFP + region was identified as having GFP fluorescence greater than the threshold intensity. RESULTS Inducible b-cell expression of mutant ATP-insensitive K ATP channel subunit (Kir6.2 [DN30,K185Q] ) forms K ATP channels with mixed mutant and wild-type Kir6.2 (3), which reduces the sensitivity of ATP inhibition. To test whether reduced Cx36 gap junction coupling could recover sufficient elevation in [Ca 2+ ] i and insulin secretion
4 1688 Modulating Gap Junctions to Prevent Diabetes Diabetes Volume 63, May 2014 in the presence of ATP-insensitive K ATP channels, agematched Rosa-Kir6.2 [DN30,K185Q] ;Pdx-Cre ER expressing mice with normal Cx36 gap junction coupling (Cx36 +/+ ; Kir6.2 [DN30,K185Q] )androsa-kir6.2 [DN30,K185Q] ;Pdx- Cre ER expressing mice with a lack of Cx36 gap junction coupling (Cx36 2/2 ;Kir6.2 [DN30,K185Q] ) were selected along with littermate control mice (Cx36 +/+ and Cx36 2/2, respectively). Each group of these four mice underwent tamoxifen injections to induce Kir6.2 [DN30,K185Q] expression and were studied in parallel. Cx36 Knockout Prevents Hyperglycemia Upon Mutant K ATP Channel Expression In Cx36 +/+ ;Kir6.2 [DN30,K185Q] mice, after the induction of Kir6.2 [DN30, K185Q] expression, ad libitum fed blood glucose levels rapidly rose to.500 mg/dl compared with littermate Cx36 +/+ control animals (Fig. 1A). Plasma insulin levels were substantially and significantly diminished in Cx36 +/+ ;Kir6.2 [DN30,K185Q] mice compared with littermate Cx36 +/+ controls (Fig. 1B). In Cx36 2/2 ; Kir6.2 [DN30,K185Q] mice, which lack b-cell gap junction coupling, after the induction of Kir6.2 [DN30,K185Q] expression, ad libitum fed blood glucose levels remained unchanged compared with Cx36 2/2 littermate control animals (Fig. 1C). Insulin levels were not significantly reduced in Cx36 2/2 ;Kir6.2 [DN30,K185Q] mice compared with littermate Cx36 2/2 controls (Fig. 1D). At day 30 postinduction, Cx36 +/+ ;Kir6.2 [DN30,K185Q] mice showed substantially greater blood glucose compared with Cx36 +/+ controls, whereas Cx36 2/2 ;Kir6.2 [DN30,K185Q] showed no Figure 1 Prevention of Kir6.2 [DN30,K185Q] -induced hyperglycemia. A: Mean time course of ad libitum fed blood glucose levels after tamoxifen injection in Cx36 +/+ ;Kir6.2 [DN30,K185Q] mice (open diamond) and control Cx36 +/+ mice (solid square). Duration of daily tamoxifen injections indicated by solid line. B: Mean plasma insulin levels at day 29 after tamoxifen injection in Cx36 +/+ ;Kir6.2 [DN30,K185Q] mice (hashed bar) and control Cx36 +/+ mice (solid bar). C:AsinA for Cx36 2/2 ;Kir6.2 [DN30,K185Q] mice (open diamond) and control Cx36 2/2 mice (solid square). D: AsinB for Cx36 2/2 ;Kir6.2 [DN30,K185Q] mice (hashed bar) and control Cx36 2/2 mice (solid bar). E: Mean ad libitum blood glucose levels for all groups of mice, averaged over days postinduction. F: Mean weight change for all groups of mice, between days 1 and 29 postinduction. Data in A, C, E, and F averaged over n = 22 (Cx36 +/+ ;Kir6.2 [DN30,K185Q] ) and n = 21 (Cx36 +/+ ) littermates. Data in B, D, E, and F averaged over n = 21 (Cx36 2/2 ;Kir6.2 [DN30,K185Q] ) and n = 24 (Cx36 2/2 ) littermates. *Significant difference (P < 0.05, twotailed Student t test); ns, no significant difference (P > 0.05) between each experimental group as indicated. Error bars represent mean 6 SEM.
5 diabetes.diabetesjournals.org Nguyen and Associates 1689 significant increase compared with littermate Cx36 2/2 controls, and similar to Cx36 +/+ controls (Fig. 1E). Over the 30 days postinduction, Cx36 +/+ ;Kir6.2 [DN30,K185Q] mice showed significantly less weight gain compared with Cx36 +/+ littermate controls, whereas Cx36 2/2 ;Kir6.2 [DN30,K185Q] showed no significant difference compared with Cx36 2/2 littermate controls (Fig. 1F). In Cx36 +/2 ;Kir6.2 [DN30,K185Q] mice with ;50% gap junction coupling, after the induction of Kir6.2 [DN30,K185Q] expression, ad libitum fed blood glucose levels also rose compared with littermate control animals (Supplementary Fig. 1). This initial elevation was slightly reduced compared with that in Cx36 +/+ ;Kir6.2 [DN30,K185Q] mice studied in parallel. However, mice ultimately progressed to similar high blood glucose levels.500 mg/dl. Plasma insulin levels were also significantly diminished in Cx36 +/2 ;Kir6.2 [DN30,K185Q] mice compared with control mice. Cx36 +/+ ;Kir6.2 [DN30,K185Q] mice also showed substantially greater fasting blood glucose compared with Cx36 +/+ controls and poor glucose tolerance during an intraperitoneal glucose tolerance test (IPGTT), as expected given the marked elevation in fed glucose levels (Fig. 2A). Whereas Cx36 2/2 ;Kir6.2 [DN30,K185Q] mice showed similar fasting blood glucose compared with Cx36 2/2 controls, they showed significantly elevated glucose levels during an IPGTT, indicating a reduced glucose tolerance (Fig. 2B). During an ITT, Cx36 2/2 ;Kir6.2 [DN30,K185Q] mice showed only a small elevation in blood glucose levels compared with Cx36 2/2 controls, indicating a small reduction in insulin sensitivity (Fig. 2C). Therefore, in the presence of Cx36 gap junction coupling, expression of ATP-insensitive K ATP channels results in severe diabetes, whereas in the absence of Cx36 gap junction coupling, only glucose intolerance occurs. Cx36 Knockout Improves [Ca 2+ ] i and Insulin Secretion Upon Mutant K ATP Channel Expression To discover whether the improved glycemic control originated from improvements in stimulus secretion coupling within the islet, we isolated islets from Cx36 +/+ ; Kir6.2 [DN30,K185Q] and Cx36 2/2 ;Kir6.2 [DN30,K185Q] mice with their respective littermate controls at 30 days postinduction. Islets isolated from Cx36 +/+ ;Kir6.2 [DN30,K185Q] and Cx36 2/2 ;Kir6.2 [DN30,K185Q] mice showed similar levels of GFP coexpression above the level of autofluorescence (Fig. 3A), with on average 50% (each ranging from 30 to 65%) of the islet GFP + (Fig. 3B). This indicates similar levels of high mutant Kir6.2 [DN30,K185Q] expression in each case. Significantly elevated insulin secretion was observed at elevated glucose levels (20 mmol/l) in islets from Cx36 2/2 ;Kir6.2 [DN30,K185Q] mice compared with low glucose levels (2 mmol/l), whereas no significant elevation in insulin secretion was observed in islets from Cx36 +/+ ;Kir6.2 [DN30,K185Q] mice (Fig. 3C). At elevated glucose levels (20 mmol/l), insulin secretion from Cx36 +/+ ; Kir6.2 [DN30,K185Q] islets was significantly lower compared with Cx36 2/2 ;Kir6.2 [DN30,K185Q] islets,aswellascompared Figure 2 Improved glucose tolerance. A: IPGTT on Cx36 +/+ ; Kir6.2 [DN30,K185Q] mice (open diamond) and control Cx36 +/+ mice (solid square) after 2 g/kg body weight intraperitoneal glucose injection. Right: Area under the curve (AUC) of the glucose excursion. n = 6 littermate mice in each group. B: AsinA for Cx36 2/2 ;Kir6.2 [DN30,K185Q] mice and control Cx36 2/2 mice. n =11 littermate mice in each group. C: ITT for Cx36 2/2 ;Kir6.2 [DN30,K185Q] mice and control Cx36 2/2 mice after units/kg intraperitoneal insulin injection after 0 time point. n = 13 littermate mice in each group. *Significant difference (P < 0.05, two-tailed paired Student t test) between each experimental group as indicated. Error bars represent mean 6 SEM. with Cx36 +/+ and Cx36 2/2 control islets. Whereas mean glucose-stimulated insulin secretion from Cx36 2/2 ; Kir6.2 [DN30,K185Q] islets was significantly greater than Cx36 +/+ ;Kir6.2 [DN30,K185Q] islets, it was ;50% less than Cx36 +/+ and Cx36 2/2 control islets. Under elevated KCl stimulation, similar levels of insulin secretion were observed in all cases (Fig. 3C). Insulin content was also slightly reduced in Cx36 +/+ ;Kir6.2 [DN30,K185Q] islets compared with Cx36 +/+ controls, whereas similar insulin content was observed in both Cx36 2/2 ;Kir6.2 [DN30,K185Q] and Cx36 2/2 islets (Fig. 3D). Therefore, in the absence of Cx36 gap junction coupling, ATP-insensitive K ATP channels have a reduced effect in suppressing glucose-stimulated insulin secretion. We next examined potential alterations in [Ca 2+ ] i in islets from these mice. At elevated (11 mmol/l) glucose
6 1690 Modulating Gap Junctions to Prevent Diabetes Diabetes Volume 63, May 2014 Figure 3 Recovery of glucose-stimulated insulin secretion. A: Representative images of GFP (green) in islets from Cx36 +/+ ;Kir6.2 [DN30,K185Q], Cx36 2/2 ;Kir6.2 [DN30,K185Q], and Cx36 +/+ mice. B: Mean percent coverage of GFP in islets from Cx36 +/+ ;Kir6.2 [DN30,K185Q] and Cx36 2/2 ; Kir6.2 [DN30,K185Q] mice. n = 9 age-matched mice in each group, two to six islets per mouse. C: Insulin secretion under low glucose (gluc.) (2 mmol/l), high glucose (20 mmol/l), and high glucose plus KCl (+20 mmol/l) in islets isolated from Cx36 +/+ ;Kir6.2 [DN30,K185Q] and Cx36 +/+ littermate control mice and Cx36 2/2 ;Kir6.2 [DN30,K185Q] and Cx36 2/2 littermate control mice. D: Insulin content in islets isolated from Cx36 +/+ ;Kir6.2 [DN30,K185Q] and Cx36 +/+ littermate control mice and Cx36 2/2 ;Kir6.2 [DN30,K185Q] and Cx36 2/2 littermate control mice. Data in C and D averaged over n = 6 age-matched mice. *Significant difference (P < 0.05, two-tailed Student t test); ns, no significant difference (P > 0.05) between each experimental group as indicated. Error bars represent mean 6 SEM. levels, transient elevations (oscillations) in [Ca 2+ ] i were observed in Cx36 +/+ control islets (Fig. 4A, top). These oscillations were rarely observed in Cx36 +/+ ;Kir6.2 [DN30,K185Q] islets at elevated glucose levels (Fig. 4A, middle) but observed frequently in many cells of Cx36 2/2 ;Kir6.2 [DN30,K185Q] islets at elevated (20 mmol/l) glucose (Fig. 4A, bottom). The proportion of cells showing transient elevations in [Ca 2+ ] i was significantly greater in Cx36 2/2 ;Kir6.2 [DN30,K185Q] islets compared with Cx36 +/+ ;Kir6.2 [DN30,K185Q] islets at elevated glucose (Fig. 4B). [Ca 2+ ] i oscillations were observed at a greater extent in low-gfp 2 cells in Cx36 2/2 ;Kir6.2 [DN30,K185Q] islets compared with high-gfp + cells (Fig. 4B and Supplementary Fig. 2), and to a similar extent as Cx36 +/+ control islets. Interestingly, a large proportion of high- GFP + cells still showed [Ca 2+ ] i oscillations in Cx36 2/2 ; Kir6.2 [DN30,K185Q] islets, albeit with a plateau fraction of,10%, compared with between 30 and 90% in low-gfp 2 cells (Fig. 4A). Upon elevated glucose levels, the timeaveraged free-calcium concentration was also significantly reduced in Cx36 +/+ ;Kir6.2 [DN30,K185Q] islets compared with Cx36 +/+ islets (Fig. 4C). At high glucose levels, Cx36 2/2 islets normally show a reduced time-average free-calcium concentration compared with wild-type islets (18), and no significant difference was observed between Cx36 2/2 ; Kir6.2 [DN30,K185Q] and Cx36 2/2 islets. Again, similar elevations in free-calcium concentration were observed in all groups upon elevated KCl. Therefore, in the absence of Cx36 gap junction coupling, ATP-insensitive K ATP channels have a reduced effect in suppressing glucose-stimulated [Ca 2+ ] i, consistent with the observed recovery of glucosestimulated insulin secretion. Cx36 Knockout Improves [Ca 2+ ] i and Insulin Secretion Upon Diazoxide-Induced K ATP Opening To further test whether modulating gap junction coupling can enhance [Ca 2+ ] i and insulin secretion upon K ATP channel opening, this time in the presence of normal b-cell heterogeneity, we applied varying concentrations of the K ATP activator diazoxide. At 11 mmol/l glucose in Cx36 +/+ islets, 100 mmol/l diazoxide suppressed [Ca 2+ ] i elevations across most of the islet (Fig. 5A, top), whereas in Cx36 2/2 islets, many cells still showed [Ca 2+ ] i elevations (Fig. 5A, bottom). [Ca 2+ ] i elevations were significantly greater in Cx36 2/2 islets upon 100 mmol/l diazoxide but not upon 250 mmol/l diazoxide (Fig. 5B). Similarly, upon 100 mmol/l diazoxide at 11 mmol/l glucose, insulin secretion from Cx36 2/2 islets was significantly greater than Cx36 +/+ islets. Therefore, in a more general case of K ATP channel opening, a reduction in gap junction coupling elevates [Ca 2+ ] i and insulin secretion.
7 diabetes.diabetesjournals.org Nguyen and Associates 1691 Figure 4 Recovery of glucose-stimulated [Ca 2+ ] i. A: Representative time courses of [Ca 2+ ] i, as measured from Fura Red fluorescence, in islets isolated from Cx36 +/+ mice (top), Cx36 +/+ ;Kir6.2 [DN30,K185Q] mice (middle), and Cx36 2/2 ;Kir6.2 [DN30,K185Q] mice (bottom). Displayed is the inverted, normalized Fura Red fluorescence from a number of nonadjacent cells with low GFP expression (black) or high GFP expression (green) at 2 mmol/l glucose (left) and 10 min after elevation to 20 mmol/l glucose (right), or at 2 and 11 mmol/l glucose in Cx36 +/+ islets. Time courses are offset for clarity, and vertical scale bar indicates 50% change in Fura Red fluorescence. B: Mean percent of cells displaying dynamic changes in [Ca 2+ ] i at 20 mmol/l glucose in Cx36 +/+ ;Kir6.2 [DN30,K185Q] islets and Cx36 2/2 ;Kir6.2 [DN30,K185Q] islets, and at 11 mmol/l glucose in Cx36 +/+ islets, averaged over high GFP expression only or low GFP expression only. Data averaged over n = 3 agematched mice in each group, three to seven islets per mouse. C: Mean [Ca 2+ ] i concentration measured from Fura 2 fluorescence, under low glucose (gluc.) (2 mmol/l), high glucose (20 mmol/l), and high glucose plus KCl (+20 mmol/l) in islets isolated from Cx36 +/+ ; Kir6.2 [DN30,K185Q] and Cx36 +/+ littermate control mice and Cx36 2/2 ;Kir6.2 [DN30,K185Q] and Cx36 2/2 littermate control mice. Data averaged over n = 6 age-matched mice, two to eight islets per mouse. *Significant difference (P < 0.05, two-tailed Student t test); significant difference (P < 0.05, two-tailed paired Student t test); ns, no significant difference (P > 0.05) between each experimental group as indicated. Error bars represent mean 6 SEM. Cx36 Knockout Reduces Metabolic Dysfunction After Mutant K ATP Channel Expression A secondary consequence of the chronic hyperglycemia and hypoinsulinemia that occurs in Kir6.2 [DN30,K185Q] - expressing mice is marked mitochondrial dysfunction (6), characterized by reduced mitochondrial NAD(P)H accumulation and reduced mitochondrial membrane depolarization at elevated glucose. We quantified these parameters in mice with reduced gap junction coupling that are protected from diabetes. Cx36 +/+ ;Kir6.2 [DN30,K185Q] islets showed elevated NAD(P)H at low levels of glucose compared with control Cx36 +/+ islets, but similar low levels of NAD(P)H were observed in Cx36 2/2 ;Kir6.2 [DN30,K185Q] islets and control Cx36 2/2 islets (Fig. 6A and B). Similar levels of NAD(P)H were observed at elevated glucose in all sets of islets (Fig. 6A). As a result, Cx36 +/+ ;Kir6.2 [DN30,K185Q] islets showed significantly reduced glucose-stimulated accumulation of NAD(P)H compared with other experimental groups (Fig. 6C). Cx36 +/+ ;Kir6.2 [DN30,K185Q] islets also showed significantly reduced mitochondrial membrane
8 1692 Modulating Gap Junctions to Prevent Diabetes Diabetes Volume 63, May 2014 Figure 5 Recovery of [Ca 2+ ] i in diazoxide-treated islets. A: Representative time courses of [Ca 2+ ] i, as measured from Fluo4 fluorescence, in islets isolated from Cx36 +/+ mice (top) and Cx36 2/2 mice (bottom) at 11 mmol/l glucose before and after diazoxide treatment. Displayed is the normalized Fluo4 fluorescence from a number of nonadjacent cells within an islet at 11 mmol/l glucose alone (left) and 11 mmol/l glucose 10 min after treatment with 100 mmol/l diazoxide. Time courses are offset for clarity, and vertical scale bar indicates 50% change in Fluo4 fluorescence. B: Mean percent of cells displaying dynamic changes in [Ca 2+ ] i at 11 mmol/l glucose in Cx36 +/+ and Cx36 2/2 islets at several diazoxide concentrations. Data averaged over n = 3 age-matched mice in each group, two to five islets per mouse. C: Insulin secretion under high glucose (11 mmol/l) in Cx36 +/+ and Cx36 2/2 islets at several diazoxide concentrations. Data averaged over n = 6 age-matched mice in each group. *Significant difference (P < 0.05, two-tailed Student t test); significant difference (P < 0.05, one-tailed paired Student t test). Error bars represent mean 6 SEM. depolarization compared with Cx36 2/2 ;Kir6.2 [DN30,K185Q] islets at elevated glucose, as indicated by Rhodamine 123 fluorescence (Fig. 6D F). Therefore, reducing gap junction coupling in Kir6.2 [DN30,K185Q] -expressing mice leads to an absence of secondary mitochondrial dysfunction. DISCUSSION In this study, we tested whether a reduction in islet Cx36 gap junction coupling could compensate for overactive ATP-insensitive K ATP channels in a model of NDM, and therefore prevent the emergence of diabetes. Upon a knockout of Cx36, the elevation in glucose-stimulated [Ca 2+ ] i and insulin secretion that we measured explains the normalization in blood glucose levels. Based on results presented here and prior studies, we propose the following mechanisms of action, schematically represented in Fig. 7A. A reduction in Cx36 gap junctions effectively left shifts the dose response of [Ca 2+ ] i (although insulin remains suppressed at low glucose due to [Ca 2+ ] i -independent mechanisms of suppression [18]), whereas expression of overactive K ATP channels effectively right shifts the dose response of [Ca 2+ ] i and insulin secretion (with a largely suppressed response over the physiological glucose range in the presence of Cx36). Therefore,inthepresenceofoveractiveK ATP channels, a reduction in Cx36 partially normalized the dose response, albeit with disrupted insulin secretion dynamics associated with a loss of Cx36. This is further detailed in Fig. 7B, where in the normal presence of Cx36, inexcitable cells that are present due to heterogeneity prevent membrane depolarization and [Ca 2+ ] i elevations in normally excitable cells at elevated glucose, via a gap
9 diabetes.diabetesjournals.org Nguyen and Associates 1693 Figure 6 Prevention of secondary mitochondrial defects. A: Representative NAD(P)H autofluorescence image at 2 mmol/l glucose (top) and 20 mmol/l glucose (bottom) in a Cx36 +/+ islet. B: Mean NAD(P)H autofluorescence in islets isolated from Cx36 +/+ ;Kir6.2 [DN30,K185Q] and Cx36 +/+ littermate control mice and Cx36 2/2 ;Kir6.2 [DN30,K185Q] and Cx36 2/2 littermate control mice. Data are normalized (norm.) to mean NAD(P)H fluorescence measured in Cx36 +/+ islets at 2 mmol/l glucose. C: Change in NAD(P)H from 2 to 20 mmol/l glucose in islets from each experimental group in B. Data in A and B averaged over n = 5 age-matched mice in each group, two to four islets per mouse. D: Representative Rhodamine 123 fluorescence image at 2 (top) and 20 mmol/l glucose (bottom) in a Cx36 +/+ islet. E: Mean Rhodamine 123 fluorescence in islets isolated from Cx36 +/+ ;Kir6.2 [DN30,K185Q] and Cx36 +/+ littermate control mice and Cx36 2/2 ;Kir6.2 [DN30,K185Q] and Cx36 2/2 littermate control mice. Data are normalized to fluorescence at 2 mmol/l glucose in each experimental group. F: Change in Rhodamine 123 fluorescence from 2 to 20 mmol/l glucose in islets from each experimental group in E. Data in E and F averaged over n =5 age-matched mice in each group, two to six islets per mouse. *Significant difference (P < 0.05, two-tailed Student t test) between each experimental group as indicated. Error bars represent mean 6 SEM. junction mediated current. This suppresses [Ca 2+ ] i elevations and insulin release across the islet. In the absence of Cx36 gap junction coupling, this current is absent (17) and therefore normally excitable cells are free to depolarize, elevate [Ca 2+ ] i, and release insulin at elevated glucose levels. Gap Junction Suppression of [Ca 2+ ] i Is Physiologically Important Previous work has shown that membrane depolarization and [Ca 2+ ] i elevations in excitable cells of an islet can be suppressed by gap junction coupling to inexcitable cells. This was demonstrated in islets expressing a K ATP lossof-function mutation (closes in the absence of ATP) at low (;2 mmol/l) glucose (16), where a reduction of gap junction coupling elevated [Ca 2+ ] i. Similar observations have been made in normal islets at basal (;5 mmol/l) glucose (17,18). Here, in the presence overactive ATPinsensitive K ATP channels (Fig. 4), or diazoxide-induced K ATP channel opening (Fig. 5), a reduction of gap junction coupling also elevated [Ca 2+ ] i. This shows that a general principle exists whereby gap junction channel closure/ deletion can (at least partially) counteract the effect of K ATP channel opening to elevate [Ca 2+ ] i. Importantly, in the presence of a gain-of-function mutation to the K ATP channels, there was no elevation in [Ca 2+ ] and insulin release at lower glucose levels. Asdescribedabove,likelyareductioninCx36imparts a left shift to the glucose-stimulated [Ca 2+ ] i response rather than a constitutive elevation, counteracting the right shift after ATP-insensitive mutant K ATP channel expression. Thus, a reduction in Cx36 cannot counteract the very strong K ATP opening that results from low glucose and ATP-insensitive K ATP channels. This suggests that a Cx36 gap junction reduction would be unlikely to counteract very strong mutations to the K ATP channel, as is also suggested by very high diazoxide treatment (Fig. 5). Interestingly, in islets from Cx36 2/2 ;Kir6.2 [DN30,K185Q] mice, even those high-gfp + cells that were inexcitable in
10 1694 Modulating Gap Junctions to Prevent Diabetes Diabetes Volume 63, May 2014 Figure 7 Schematic describing recovery of insulin secretion. A: Schematic representation for glucose-stimulated [Ca 2+ ] i as a result of changes to Cx36 and K ATP activity. Top: Reduction in Cx36 activity due to genetic knockout leads to a left shift and more gradual dose response of [Ca 2+ ] i (solid red). Disrupted dynamics not shown. Note insulin at low glucose is still suppressed due to action of another communication mechanism (dotted red). Middle: Increase in K ATP activity due to mutations that reduce the sensitivity of ATP inhibition leads to a right shift in dose response in [Ca 2+ ] i (dashed black), such that it is largely suppressed within experimental glucose ranges. Insulin follows this dose response. Bottom: In the presence of increased K ATP activity, a reduction in Cx36 activity leads to a left shift in the dose response of [Ca 2+ ] i back to within a physiological range (dashed red), albeit with glucose-stimulated activity slightly blunted compared with a reduction in Cx36 activity alone. Insulin follows this dose response, although at low glucose it is still suppressed due to action of another communication mechanism (dotted red). B: Two representative, neighboring cells in an islet, with one inexcitable cell (intrinsically inexcitable or expressing high levels of ATP-insensitive K ATP channels: lower, green) and one excitable cell (with near-normal K ATP channels: upper, gray). At elevated glucose, the inexcitable cell remains hyperpolarized (V m low). In isolation, the excitable cell would depolarize (V m increase), elevate [Ca 2+ ] i, and release insulin. However, Cx36 gap junction coupling mediates a hyperpolarizing current (I h-pol ) from the inexcitable cell to the excitable cell, preventing depolarization and [Ca 2+ ] i elevations, thereby suppressing Ca 2+ triggering of insulin secretion across the islet. In the absence of Cx36 gap junction coupling, the hyperpolarizing current is abolished and the more excitable cell depolarizes and elevates [Ca 2+ ] i, which triggers insulin secretion. the presence of gap junctions showed some [Ca 2+ ] i elevations. These were less frequent than in the more excitable low-gfp 2 cells but significantly more than in islets of Cx36 +/+ ;Kir6.2 [DN30,K185Q] mice (Fig. 4). Electrically uncoupled cells will likely show some stochastic channel noise, as suggested by modeling studies (23,24), which may be sufficient to transiently depolarize and elevate [Ca 2+ ] i. This suggests that electrically uncoupled cells may inherently behave different to b-cells in normal islets, warranting further study. Importantly, the elevated [Ca 2+ ] i at elevated glucose levels that follows a reduction of gap junction coupling also led to elevated insulin secretion (Figs. 3 and 5). This contrasts with the behavior at lower glucose levels in normal islets (18), where this effect is normally masked due to other mechanisms of cell-cell communication that suppress insulin release independent of [Ca 2+ ] i. However, it was suggested that this other communication mechanism(s) that suppresses insulin is inactive at elevated glucose (18,25), as we have demonstrated. Of further importance, the gap junction mediated suppression of islet [Ca 2+ ] i and insulin release is physiologically important. By eliminating gap junction coupling in mice expressing ATP-insensitive K ATP channels, we eliminated hyperglycemia (Fig. 1). Gap junction coupling in the islet has been shown to play an important physiological role: to coordinate and enhance the first phase of insulin secretion and coordinate the pulsatile second phase of insulin secretion (15). In its absence, glucose intolerance occurs, and we observed glucose intolerance in Cx36 2/2 ;Kir6.2 [DN30,K185Q] mice, albeit slightly less than previously observed (15), likely due to the younger age of the mice. This physiological role gives a new fundamental understanding of the multicellular properties of the islet, specifically how the electrical coupling of b-cells via gap junction channels can be critically important to coordinate K ATP channel regulated
11 diabetes.diabetesjournals.org Nguyen and Associates 1695 electrical activity to regulate insulin secretion and glucose homeostasis. Relevance to Human NDM The majority of cases of NDM (;60%) occur as a result of gain-of-function mutations to the Kir6.2 or Sur1 subunits of the K ATP channel (26). In the normal presence of Cx36, we observed similar results to a prior mouse model that expresses a Kir6.2 mutation associated with NDM (4), including marked hyperglycemia, reduced weight gain, reduced islet insulin content, and suppression of glucose-stimulated [Ca 2+ ] i and insulin. Despite the reduced insulin content, normal depolarizationinduced [Ca 2+ ] i and insulin release was observed, also as previously observed (4). Given that a reduction of Cx36 gap junction coupling so dramatically normalized glycemic control, we anticipate an improvement in islet function would occur in humans with NDM. However, some subtle differences must be discussed. The distribution of Kir6.2 [DN30,K185Q] expression is likely more heterogeneous in islets from this mouse model compared with islets of humans with the disease, due to the stochastic and variable nature of Pdx- Cre ER mediated recombination (21). Therefore, the heterogeneity in b-cell function, from which the gap junction mediated suppression of [Ca 2+ ] i and insulin partly occurs, will not be exactly the same. However, it is well established that human and mouse b-cells lacking cellular proximity are very heterogeneous in their glucose response (27,28). This suggests that a Cx36 gap junction reduction will elevate glucose-stimulated [Ca 2+ ] i and insulin even in the presence of uniform Kir6.2 [DN30,K185Q] expression. Results from diazoxide-treated islets (Fig. 5) support this, as diazoxide treatment likely provides uniform K ATP opening across the islet. In this case, the endogenous b-cell heterogeneity would be closer to that of human islets with NDM mutations. Further, gap junction coupling suppresses excitability in other conditions of K ATP channel opening at lower glucose levels. In Cx36 2/2 islets, elevated [Ca 2+ ] i is observed in ;50% of b-cells at 5 mmol/l glucose (18), and in ;40% of b-cells upon 100 mmol/l diazoxide (Fig. 5), each similar to that after expression of Kir6.2 [DN30,K185Q] (Fig. 4). The precise quantitative balance between b-cell heterogeneity and gap junction coupling in determining islet function remains to be determined. Nevertheless, the presence of any heterogeneity will lead to gap junction coupling suppressing [Ca 2+ ] i over a certain range of glucose levels. Likely, the broader the heterogeneity, the greater the difference in the presence and absence of gap junction coupling (18) and the more dramatic the effect in modulating gap junction coupling. The discovery that NDM can be caused by gain-offunction mutations to the Kir6.2 and Sur1 subunits of the K ATP channel has meant that patients can switch from insulin therapy to oral sulfonylurea treatment with improved glycemic control (7). Although successful for treating NDM, sulfonylurea treatment has been reported to be associated with hypoglycemic episodes (29), which may occur due to constitutive glucose-independent K ATP channel closure, which reduces the low glucose regulation of insulin secretion. Chronic sulfonylurea therapy can also cause glucose intolerance (30), possibly resulting from overstimulation of [Ca 2+ ] i. As discussed above, a modulation of gap junction coupling retains a suppression of [Ca 2+ ] i and insulin secretion at low glucose (Figs. 3, 4, and 7) and thus would be anticipated to lessen potential hypoglycemia. More importantly, some Kir6.2 and Sur1 mutations reduce the sensitivity of sulfonylurea inhibition (7,9,10), reducing sulfonylurea effectiveness. We speculate that modulating Cx36 gap junction coupling may provide an alternative route to elevate glucose-stimulated [Ca 2+ ] i and insulin secretion, particularly in the presence of sulfonylurea-insensitive mutations. Here, a near-complete reduction in gap junction coupling (.95%, Cx36 2/2 ) normalized blood glucose levels, but a partial reduction (;50%, Cx36 +/2 )had a minimal effect (Supplementary Fig. 1). This Cx36 dose response is similar to how reducing Cx36 gap junction coupling disrupts glucose tolerance via firstphase and second-phase insulin dynamics (15). As a potential therapeutic target, we therefore anticipate that a gap junction inhibition of.50% would be required. Current inhibitors are weak with nonspecific effects, although a recent study developed a novel screen for gap junction modulators (31), which may yield more potent and specific inhibitors. A partial disruption to gap junction coupling can also result from hyperglycemia (32). However, in Cx36 +/+ ; Kir6.2 [DN30,K185Q] islets, significant coupling is still present, as shown by the coordinated residual [Ca 2+ ] i. Therefore, any Cx36 decrease resulting from hyperglycemia would likely be insufficient to significantly alter insulin release in this model. In principle, we anticipate that reducing gap junction coupling could similarly correct defects in insulin release caused by mutations proximal to [Ca 2+ ] i influx, including glucokinase (33,34) and genes that regulate mitochondrial function (35,36). Defects in proximal steps could affect the amplifying pathways that regulate insulin release (37), and reduce the effect of gap junction modulation. However, altering the K ATP regulation of membrane potential can compensate for an absence of glucokinase (38). Therefore, modulating Cx36 gap junction coupling may be more broadly applicable to normalize islet function. To summarize, we have shown that by reducing gap junction coupling between b-cells in a model of NDM caused by expression of ATP-insensitive K ATP channels,wecaneliminateseverehyperglycemiaand islet dysfunction. This is achieved through a novel pathway where a reduction in Cx36 can partially
12 1696 Modulating Gap Junctions to Prevent Diabetes Diabetes Volume 63, May 2014 compensate for overactive K ATP channels and prevent suppression of electrical activity across the islet. This restores glucose-stimulated [Ca 2+ ] i, insulin secretion, and glucose homeostasis. This yields a better understanding of how the islet functions as a coupled unit of b-cells and may ultimately provide a potential therapeutic target for treating NDM and other monogenic forms of diabetes. Acknowledgments. The authors thank Colin G. Nichols (Washington University, St. Louis, MO) and Maria S. Remedi (Washington University, St. Louis, MO) for helpful comments and suggestions in performing this study and for reviewing this manuscript. Funding. This study was primarily supported by National Science Foundation grant DGE (T.H.H.), National Institutes of Health grant R00-DK (to R.K.P.B.), and University of Colorado internal funds. Experiments on the 3I Marianas spinning disk microscope and the Zeiss LSM510 two-photon microscope were performed through the use of the University of Colorado Anschutz Medical campus advanced light microscopy core (P30-NS and UL1-RR ). Duality of Interest. No potential conflicts of interest relevant to this article were reported. Author Contributions. L.M.N., M.P., and T.H.H. researched data. R.K.P.B. designed experiments, researched data, and wrote the manuscript. R.K.P.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Prior Presentation. Part of this study was presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, June References 1. Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 2006;440: Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 2004;350: Remedi MS, Kurata HT, Scott A, et al. Secondary consequences of beta cell inexcitability: identificationandpreventioninamurinemodel of K(ATP)-induced neonatal diabetes mellitus. Cell Metab 2009;9: Girard CA, Wunderlich FT, Shimomura K, et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest 2009; 119: Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell 2000;100: Benninger RKP, Remedi MS, Head WS, Ustione A, Piston DW, Nichols CG. Defects in beta cell Ca²+ signalling, glucose metabolism and insulin secretion in a murine model of K(ATP) channel-induced neonatal diabetes mellitus. Diabetologia 2011;54: Pearson ER, Flechtner I, Njølstad PR, et al.; Neonatal Diabetes International Collaborative Group. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med 2006;355: Wambach JA, Marshall BA, Koster JC, White NH, Nichols CG. Successful sulfonylurea treatment of an insulin-naïve neonate with diabetes mellitus due to a KCNJ11 mutation. Pediatr Diabetes 2010;11: Proks P, Antcliff JF, Lippiat J, Gloyn AL, Hattersley AT, Ashcroft FM. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc Natl Acad Sci USA 2004; 101: Koster JC, Remedi MS, Dao C, Nichols CG. ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes 2005;54: Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia 1974;10: Zhang M, Goforth P, Bertram R, Sherman A, Satin L. The Ca2+ dynamics of isolated mouse beta-cells and islets: implications for mathematical models. Biophys J 2003;84: Benninger RKP, Zhang M, Head WS, Satin LS, Piston DW. Gap junction coupling and calcium waves in the pancreatic islet. Biophys J 2008;95: Ravier MA, Güldenagel M, Charollais A, et al. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes 2005;54: Head WS, Orseth ML, Nunemaker CS, Satin LS, Piston DW, Benninger RKP. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes 2012;61: Rocheleau JV, Remedi MS, Granada B, et al. Critical role of gap junction coupled KATP channel activity for regulated insulin secretion. PLoS Biol 2006;4:e Speier S, Gjinovci A, Charollais A, Meda P, Rupnik M. Cx36-mediated coupling reduces beta-cell heterogeneity, confines the stimulating glucose concentration range, and affects insulin release kinetics. Diabetes 2007; 56: Benninger RKP, Head WS, Zhang M, Satin LS, Piston DW. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J Physiol 2011;589: Pipeleers DG. Heterogeneity in pancreatic beta-cell population. Diabetes 1992;41: Degen J, Meier C, Van Der Giessen RS, et al. Expression pattern of lacz reporter gene representing connexin36 in transgenic mice. J Comp Neurol 2004;473: Zhang HJ, Fujitani Y, Wright CVE, Gannon M. Efficient recombination in pancreatic islets by a tamoxifen-inducible Cre-recombinase. Genesis 2005; 42: Hraha TH, Bernard AB, Nguyen LM, Anseth KS, Benninger RKP. Dimensionality and size scaling of coordinated Ca2+ dynamics in MIN6 b-cell clusters. Biophys J 2014;106: Jo J, Kang H, Choi MY, Koh DS. How noise and coupling induce bursting action potentials in pancreatic beta-cells. Biophys J 2005;89: Bertram R, Sherman A. Dynamical complexity and temporal plasticity in pancreatic beta-cells. J Biosci 2000;25: Konstantinova I, Nikolova G, Ohara-Imaizumi M, et al. EphA-Ephrin- A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 2007;129: Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes 2005;54:
Glucose Homeostasis. Liver. Glucose. Muscle, Fat. Pancreatic Islet. Glucose utilization. Glucose production, storage Insulin Glucagon
 Glucose Homeostasis Liver Glucose Glucose utilization Glucose production, storage Insulin Glucagon Muscle, Fat Pancreatic Islet Classification of Diabetes Type 1 diabetes Type 2 diabetes Other types of
Glucose Homeostasis Liver Glucose Glucose utilization Glucose production, storage Insulin Glucagon Muscle, Fat Pancreatic Islet Classification of Diabetes Type 1 diabetes Type 2 diabetes Other types of
Permanent neonatal diabetes mellitus. Case Report
 Rawal Medical Journal An official publication of Pakistan Medical Association Rawalpindi Islamabad branch Established 1975 Volume 36 Number 4 October - December 2011 Case Report Permanent neonatal diabetes
Rawal Medical Journal An official publication of Pakistan Medical Association Rawalpindi Islamabad branch Established 1975 Volume 36 Number 4 October - December 2011 Case Report Permanent neonatal diabetes
Numerical investigation of phase transition in a cellular network and disease onset
 Numerical investigation of phase transition in a cellular network and disease onset Xujing Wang, Associate Professor Dept of Physics xujingw@uab.edu 934-8186 The question: Is (chronic) disease onset a
Numerical investigation of phase transition in a cellular network and disease onset Xujing Wang, Associate Professor Dept of Physics xujingw@uab.edu 934-8186 The question: Is (chronic) disease onset a
Age-Dependent Decline in the Coordinated [Ca 2+ ] and Insulin Secretory Dynamics in Human Pancreatic Islets
![Age-Dependent Decline in the Coordinated [Ca 2+ ] and Insulin Secretory Dynamics in Human Pancreatic Islets Age-Dependent Decline in the Coordinated [Ca 2+ ] and Insulin Secretory Dynamics in Human Pancreatic Islets](/thumbs/93/113461582.jpg) 2436 Diabetes Volume 66, September 2017 Age-Dependent Decline in the Coordinated [Ca 2+ ] and Insulin Secretory Dynamics in Human Pancreatic Islets Matthew J. Westacott, 1 Nikki L. Farnsworth, 2 Joshua
2436 Diabetes Volume 66, September 2017 Age-Dependent Decline in the Coordinated [Ca 2+ ] and Insulin Secretory Dynamics in Human Pancreatic Islets Matthew J. Westacott, 1 Nikki L. Farnsworth, 2 Joshua
Supplementary Figure 1: Steviol and stevioside potentiate TRPM5 in a cell-free environment. (a) TRPM5 currents are activated in inside-out patches
 Supplementary Figure 1: Steviol and stevioside potentiate TRPM5 in a cell-free environment. (a) TRPM5 currents are activated in inside-out patches during application of 500 µm Ca 2+ at the intracellular
Supplementary Figure 1: Steviol and stevioside potentiate TRPM5 in a cell-free environment. (a) TRPM5 currents are activated in inside-out patches during application of 500 µm Ca 2+ at the intracellular
The Journal of Physiology
 J Physiol 592.20 (2014) pp 4431 4446 4431 Fluorescence recovery after photobleaching reveals regulation and distribution of connexin36 gap junction coupling within mouse islets of Langerhans Nikki L. Farnsworth
J Physiol 592.20 (2014) pp 4431 4446 4431 Fluorescence recovery after photobleaching reveals regulation and distribution of connexin36 gap junction coupling within mouse islets of Langerhans Nikki L. Farnsworth
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Relative expression of K IR2.1 transcript to enos was reduced 29-fold in capillaries from knockout animals. Relative expression of K IR2.1 transcript to enos was reduced 29-fold
Supplementary Figure 1 Relative expression of K IR2.1 transcript to enos was reduced 29-fold in capillaries from knockout animals. Relative expression of K IR2.1 transcript to enos was reduced 29-fold
Dynamics of Computational Islet Simulations: Islets with Majority Mutated Open K ATP Channels Retain Bursting
 An International Journal Volume NO, issue no (YEAR) http://www.lettersinbiomath.org Research Article Dynamics of Computational Islet Simulations: Islets with Majority Mutated Open K ATP Channels Retain
An International Journal Volume NO, issue no (YEAR) http://www.lettersinbiomath.org Research Article Dynamics of Computational Islet Simulations: Islets with Majority Mutated Open K ATP Channels Retain
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
2-Deoxyglucose Assay Kit (Colorimetric)
 2-Deoxyglucose Assay Kit (Colorimetric) Catalog Number KA3753 100 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
2-Deoxyglucose Assay Kit (Colorimetric) Catalog Number KA3753 100 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information...
UMBC REU Site: Computational Simulations of Pancreatic Beta Cells
 UMBC REU Site: Computational Simulations of Pancreatic Beta Cells Sidafa Conde 1, Teresa Lebair 2, Christopher Raastad 3, Virginia Smith 4 Kyle Stern, 5 David Trott 5 Dr. Matthias Gobbert 5, Dr. Bradford
UMBC REU Site: Computational Simulations of Pancreatic Beta Cells Sidafa Conde 1, Teresa Lebair 2, Christopher Raastad 3, Virginia Smith 4 Kyle Stern, 5 David Trott 5 Dr. Matthias Gobbert 5, Dr. Bradford
By: Dr. Doaa Khater Yassin, MM and M.D Paed Sr. specialist of Pediatrics SQUH
 By: Dr. Doaa Khater Yassin, MM and M.D Paed Sr. specialist of Pediatrics SQUH Born SGA with birth weight of 2.4 kg, had IUGR Hospitalized at the age of 2 month with severe dehydration Diagnosed as DKA
By: Dr. Doaa Khater Yassin, MM and M.D Paed Sr. specialist of Pediatrics SQUH Born SGA with birth weight of 2.4 kg, had IUGR Hospitalized at the age of 2 month with severe dehydration Diagnosed as DKA
Supporting Information
 Supporting Information Gerasimenko et al..73/pnas.39 SI Materials and Methods Reagents used in this study include Fluo-4/Fura- (Invitrogen), thapsigargin (albiochem), collagenase (Worthington), palmitoleic
Supporting Information Gerasimenko et al..73/pnas.39 SI Materials and Methods Reagents used in this study include Fluo-4/Fura- (Invitrogen), thapsigargin (albiochem), collagenase (Worthington), palmitoleic
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Report Reference Guide. THERAPY MANAGEMENT SOFTWARE FOR DIABETES CareLink Report Reference Guide 1
 Report Reference Guide THERAPY MANAGEMENT SOFTWARE FOR DIABETES CareLink Report Reference Guide 1 How to use this guide Each type of CareLink report and its components are described in the following sections.
Report Reference Guide THERAPY MANAGEMENT SOFTWARE FOR DIABETES CareLink Report Reference Guide 1 How to use this guide Each type of CareLink report and its components are described in the following sections.
Results and discussion
 Supplementary Figure 1. Assessment of the cross-talk between FuraPE3 and D4ER fluorescent spectra. Cells were perifused with 15 mmol/l glucose (G15) in the presence of 250 µmol/l of the KATP channel opener
Supplementary Figure 1. Assessment of the cross-talk between FuraPE3 and D4ER fluorescent spectra. Cells were perifused with 15 mmol/l glucose (G15) in the presence of 250 µmol/l of the KATP channel opener
Supplemental Information. Octopamine Neurons Mediate Flight-Induced Modulation of Visual Processing in Drosophila. Supplemental Inventory
 1 Current Biology, Volume 22 Supplemental Information Octopamine Neurons Mediate Flight-Induced Modulation of Visual Processing in Drosophila Marie P. Suver, Akira Mamiya, and Michael H. Dickinson Supplemental
1 Current Biology, Volume 22 Supplemental Information Octopamine Neurons Mediate Flight-Induced Modulation of Visual Processing in Drosophila Marie P. Suver, Akira Mamiya, and Michael H. Dickinson Supplemental
Sulfonylurea Treatment in Young Children with Neonatal Diabetes: Dealing with Hyperglycaemia, Hypoglycaemia and Sickdays
 Diabetes Care In Press, published online March 2, 2007 Sulfonylurea Treatment in Young Children with Neonatal Diabetes: Dealing with Hyperglycaemia, Hypoglycaemia and Sickdays Received for publication
Diabetes Care In Press, published online March 2, 2007 Sulfonylurea Treatment in Young Children with Neonatal Diabetes: Dealing with Hyperglycaemia, Hypoglycaemia and Sickdays Received for publication
SUPPORTING ONLINE MATERIAL
 Supporting Online Material Dishinger, J. F., Reid, K. R., Kennedy, R. T. SUPPORTING ONLINE MATERIAL Quantitative Sampling of Biphasic and Pulsatile Insulin Release from Pancreatic Islets with High Throughput
Supporting Online Material Dishinger, J. F., Reid, K. R., Kennedy, R. T. SUPPORTING ONLINE MATERIAL Quantitative Sampling of Biphasic and Pulsatile Insulin Release from Pancreatic Islets with High Throughput
Human TRPC6 Ion Channel Cell Line
 TECHNICAL DATA SHEET ValiScreen Ion Channel Cell Line Caution: For Laboratory Use. A research product for research purposes only Human TRPC6 Ion Channel Cell Line Product No.: AX-012-C Lot No.: 512-548-A
TECHNICAL DATA SHEET ValiScreen Ion Channel Cell Line Caution: For Laboratory Use. A research product for research purposes only Human TRPC6 Ion Channel Cell Line Product No.: AX-012-C Lot No.: 512-548-A
Glucose Uptake Colorimetric Assay Kit
 ab136955 Glucose Uptake Colorimetric Assay Kit Instructions for Use For the sensitive and accurate measurement of Glucose uptake in various samples This product is for research use only and is not intended
ab136955 Glucose Uptake Colorimetric Assay Kit Instructions for Use For the sensitive and accurate measurement of Glucose uptake in various samples This product is for research use only and is not intended
THERAPY MANAGEMENT SOFTWARE FOR DIABETES
 THERAPY MANAGEMENT SOFTWARE FOR DIABETES Report Report Interpretation Reference Guide Guide 2007 Medtronic MiniMed. All rights reserved. 6025274-0U2 120707 CareLink Pro Report Reference Guide 0 p.2 Sensor
THERAPY MANAGEMENT SOFTWARE FOR DIABETES Report Report Interpretation Reference Guide Guide 2007 Medtronic MiniMed. All rights reserved. 6025274-0U2 120707 CareLink Pro Report Reference Guide 0 p.2 Sensor
Neonatal Diabetes: A Special Case of Type 1 Diabetes
 Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/clinicians-roundtable/neonatal-diabetes-a-special-case-of-type-1-
Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/clinicians-roundtable/neonatal-diabetes-a-special-case-of-type-1-
SUPPLEMENTARY DATA. Supplementary Table 1. Primers used for PCR and qpcr Primer Name
 Supplementary Table. Primers used for PCR and qpcr Primer Name ccession Number Fwd Rev Type of PCR Cre NC_8 GGCGTCTTCCGC GTGCCCCTCGTTTG Standard PCR LoUcp CCGGGCTGTCTCCGCGG GGCTGTTCGCCCGGCC Standard PCR
Supplementary Table. Primers used for PCR and qpcr Primer Name ccession Number Fwd Rev Type of PCR Cre NC_8 GGCGTCTTCCGC GTGCCCCTCGTTTG Standard PCR LoUcp CCGGGCTGTCTCCGCGG GGCTGTTCGCCCGGCC Standard PCR
Supplementary Material and Methods
 Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
Online Supplement Kockx et al, Secretion of Apolipoprotein E from Macrophages 1 Supplementary Material and Methods Cloning of ApoE-GFP Full-length human apoe3 cdna (pcdna3.1/zeo + -apoe) was kindly provided
Alternative insulin delivery systems: how demanding should the patient be?
 Diabetologia (1997) 4: S97 S11 Springer-Verlag 1997 Alternative insulin delivery systems: how demanding should the patient be? K.S. Polonsky, M. M. Byrne, J. Sturis Department of Medicine, The University
Diabetologia (1997) 4: S97 S11 Springer-Verlag 1997 Alternative insulin delivery systems: how demanding should the patient be? K.S. Polonsky, M. M. Byrne, J. Sturis Department of Medicine, The University
Maturity-onset diabetes of the young (MODY) is a heterogeneous group
 Over the years, different forms of maturity-onset diabetes of the young (MODY) have been identified, with mutations in a number of different genes associated with a MODY-like phenotype. Depending on the
Over the years, different forms of maturity-onset diabetes of the young (MODY) have been identified, with mutations in a number of different genes associated with a MODY-like phenotype. Depending on the
A novel role for vitamin D: modulation of expression and function of the local renin angiotensin system in mouse pancreatic islets
 Diabetologia () 5:77 DOI.7/s5--- SHORT COMMUNICATION A novel role for vitamin D: modulation of expression and function of the local renin angiotensin system in mouse pancreatic islets Q. Cheng & Y. C.
Diabetologia () 5:77 DOI.7/s5--- SHORT COMMUNICATION A novel role for vitamin D: modulation of expression and function of the local renin angiotensin system in mouse pancreatic islets Q. Cheng & Y. C.
THERAPY MANAGEMENT SOFTWARE FOR DIABETES
 THERAPY MANAGEMENT SOFTWARE FOR DIABETES Report Report Interpretation Reference Guide Guide 2009 Medtronic MiniMed. All rights reserved. 6025274-012_a CareLink Pro Report Reference Guide 0 p.2 Adherence
THERAPY MANAGEMENT SOFTWARE FOR DIABETES Report Report Interpretation Reference Guide Guide 2009 Medtronic MiniMed. All rights reserved. 6025274-012_a CareLink Pro Report Reference Guide 0 p.2 Adherence
Cross-talk between Two Partners Regulates the Gating of the K ATP Channel Pore
 International Conference on Metabolic Syndromes Cross-talk between Two Partners Regulates the Gating of the K ATP Channel Pore Dr Hussein N Rubaiy h.rubaiy@leeds.ac.uk School of Medicine, University of
International Conference on Metabolic Syndromes Cross-talk between Two Partners Regulates the Gating of the K ATP Channel Pore Dr Hussein N Rubaiy h.rubaiy@leeds.ac.uk School of Medicine, University of
Islets of Langerhans consist mostly of insulin-producing β cells. They will appear to be densely labeled
 Are adult pancreatic beta cells formed by self-duplication or stem cell differentiation? Introduction Researchers have long been interested in how tissues produce and maintain the correct number of cells
Are adult pancreatic beta cells formed by self-duplication or stem cell differentiation? Introduction Researchers have long been interested in how tissues produce and maintain the correct number of cells
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/317/5841/183/dc1 Supporting Online Material for Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses Gertrudis Perea and Alfonso Araque* *To whom
www.sciencemag.org/cgi/content/full/317/5841/183/dc1 Supporting Online Material for Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses Gertrudis Perea and Alfonso Araque* *To whom
Chapter 3. Expression of α5-megfp in Mouse Cortical Neurons. on the β subunit. Signal sequences in the M3-M4 loop of β nachrs bind protein factors to
 22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
22 Chapter 3 Expression of α5-megfp in Mouse Cortical Neurons Subcellular localization of the neuronal nachr subtypes α4β2 and α4β4 depends on the β subunit. Signal sequences in the M3-M4 loop of β nachrs
BIOL212- Biochemistry of Disease. Metabolic Disorders: Diabetes
 BIOL212- Biochemistry of Disease Metabolic Disorders: Diabetes Diabetes mellitus is, after heart disease and cancer, the third leading cause of death in the west. Insulin is either not secreted in sufficient
BIOL212- Biochemistry of Disease Metabolic Disorders: Diabetes Diabetes mellitus is, after heart disease and cancer, the third leading cause of death in the west. Insulin is either not secreted in sufficient
Metabolic Oscillations in Pancreatic Islets Depend on the Intracellular Ca2+ Level but Not Ca2+ Oscillations
 Virginia Commonwealth University VCU Scholars Compass Pharmacology and Toxicology Publications Dept. of Pharmacology and Toxicology 21 Metabolic Oscillations in Pancreatic Islets Depend on the Intracellular
Virginia Commonwealth University VCU Scholars Compass Pharmacology and Toxicology Publications Dept. of Pharmacology and Toxicology 21 Metabolic Oscillations in Pancreatic Islets Depend on the Intracellular
Nature Biotechnology: doi: /nbt.3828
 Supplementary Figure 1 Development of a FRET-based MCS. (a) Linker and MA2 modification are indicated by single letter amino acid code. indicates deletion of amino acids and N or C indicate the terminus
Supplementary Figure 1 Development of a FRET-based MCS. (a) Linker and MA2 modification are indicated by single letter amino acid code. indicates deletion of amino acids and N or C indicate the terminus
Nature Immunology: doi: /ni.3631
 Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1 SPT analyses of Zap70 at the T cell plasma membrane. (a) Total internal reflection fluorescent (TIRF) excitation at 64-68 degrees limits single molecule detection to 100-150 nm above
Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane
 Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane potential recorded from POMC neurons following treatment with
Supplementary Figure 1) GABAergic enhancement by leptin hyperpolarizes POMC neurons A) Representative recording samples showing the membrane potential recorded from POMC neurons following treatment with
Insulin Delivery and Glucose Monitoring Methods for Diabetes Mellitus: Comparative Effectiveness
 Insulin Delivery and Glucose Monitoring Methods for Diabetes Mellitus: Comparative Effectiveness Prepared for: Agency for Healthcare Research and Quality (AHRQ) www.ahrq.gov Outline of Material Introduction
Insulin Delivery and Glucose Monitoring Methods for Diabetes Mellitus: Comparative Effectiveness Prepared for: Agency for Healthcare Research and Quality (AHRQ) www.ahrq.gov Outline of Material Introduction
The camp Sensor Epac2 Is a Direct Target of Antidiabetic Sulfonylurea Drugs
 www.sciencemag.org/cgi/content/full/325/594/67/dc1 Supporting Online Material for The camp Sensor Epac2 Is a Direct Target of Antidiabetic Sulfonylurea Drugs Chang-Liang Zhang, Megumi Katoh, Tadao Shibasaki,
www.sciencemag.org/cgi/content/full/325/594/67/dc1 Supporting Online Material for The camp Sensor Epac2 Is a Direct Target of Antidiabetic Sulfonylurea Drugs Chang-Liang Zhang, Megumi Katoh, Tadao Shibasaki,
Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells.
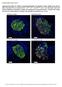 Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells. Pancreata from 16-weeks-old 6J +/+, 6J db/db, KS +/+ and KS db/db mice were harvested, fixed with
Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells. Pancreata from 16-weeks-old 6J +/+, 6J db/db, KS +/+ and KS db/db mice were harvested, fixed with
Supplemental Fig. 1. Changes in fat and lean mass determined by DEXA. Fat mass in high fat-fed
 Supplemental Fig. 1. hanges in fat and lean mass determined by EXA. Fat mass in high fat-fed mice (A) and chow-fed controls (). Lean mass in high fat-fed mice () and chow-fed controls (). ata represent
Supplemental Fig. 1. hanges in fat and lean mass determined by EXA. Fat mass in high fat-fed mice (A) and chow-fed controls (). Lean mass in high fat-fed mice () and chow-fed controls (). ata represent
Food Intake Regulation & the Clock. Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD
 Food Intake Regulation & the Clock Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Circadian disruption affect multiple organ systems: The diagram provides examples of how circadian disruption
Food Intake Regulation & the Clock Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD Circadian disruption affect multiple organ systems: The diagram provides examples of how circadian disruption
ATP-sensitive potassium channelopathies: focus on insulin secretion
 Review series ATP-sensitive potassium channelopathies: focus on insulin secretion Frances M. Ashcroft University Laboratory of Physiology, Oxford University, Oxford, United Kingdom. ATP-sensitive potassium
Review series ATP-sensitive potassium channelopathies: focus on insulin secretion Frances M. Ashcroft University Laboratory of Physiology, Oxford University, Oxford, United Kingdom. ATP-sensitive potassium
Pancreatic beta-cell response to increased metabolic demand and to pharmacologic secretagogues requires EPAC2A
 Page 1 of 48 Pancreatic beta-cell response to increased metabolic demand and to pharmacologic secretagogues requires EPAC2A Woo-Jin Song 1 *, Prosenjit Mondal 1, Yuanyuan Li 1, Suh Eun Lee 1, Mehboob A.
Page 1 of 48 Pancreatic beta-cell response to increased metabolic demand and to pharmacologic secretagogues requires EPAC2A Woo-Jin Song 1 *, Prosenjit Mondal 1, Yuanyuan Li 1, Suh Eun Lee 1, Mehboob A.
! acts via the autonomic nervous system. ! maintaining body weight within tight limits. ! ventromedial (VMN) ! arcuate (ARC) ! neuropeptide Y (NPY)
 Brain Regulates energy homeostatis Glucose Sensing in the Brain Seminar 2012 Gareth Price! acts in response to circulating signals of nutrient states! acts via the autonomic nervous system Ensures a balance
Brain Regulates energy homeostatis Glucose Sensing in the Brain Seminar 2012 Gareth Price! acts in response to circulating signals of nutrient states! acts via the autonomic nervous system Ensures a balance
Remission in Non-Operated Patients with Diffuse Disease and Long-Term Conservative Treatment.
 5th Congenital Hyperinsulinism International Family Conference Milan, September 17-18 Remission in Non-Operated Patients with Diffuse Disease and Long-Term Conservative Treatment. PD Dr. Thomas Meissner
5th Congenital Hyperinsulinism International Family Conference Milan, September 17-18 Remission in Non-Operated Patients with Diffuse Disease and Long-Term Conservative Treatment. PD Dr. Thomas Meissner
Division of Hypothalamic Research, Departments of Internal Medicine and
 1 5-HT 2C Rs Expressed by Pro-opiomelanocortin Neurons Regulate Insulin Sensitivity in Liver Yong Xu 1, 3*, Eric D. Berglund 1*, Jong-Woo Sohn 1, William L. Holland 2, Jen-Chieh Chuang 1, Makoto Fukuda
1 5-HT 2C Rs Expressed by Pro-opiomelanocortin Neurons Regulate Insulin Sensitivity in Liver Yong Xu 1, 3*, Eric D. Berglund 1*, Jong-Woo Sohn 1, William L. Holland 2, Jen-Chieh Chuang 1, Makoto Fukuda
ab Glucose Uptake Assay Kit (colorimetric) 1
 Version 16 Last updated 10 January 2018 ab136955 Glucose Uptake Assay Kit (Colorimetric) For the measurement of Glucose uptake in a variety of cells. This product is for research use only and is not intended
Version 16 Last updated 10 January 2018 ab136955 Glucose Uptake Assay Kit (Colorimetric) For the measurement of Glucose uptake in a variety of cells. This product is for research use only and is not intended
Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h)
 Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h) after feeding. A small slice (~5-1 mm 3 ) was taken
Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h) after feeding. A small slice (~5-1 mm 3 ) was taken
Report Reference Guide
 Report Reference Guide How to use this guide Each type of CareLink report and its components are described in the following sections. Report data used to generate the sample reports was from sample patient
Report Reference Guide How to use this guide Each type of CareLink report and its components are described in the following sections. Report data used to generate the sample reports was from sample patient
Closing in on the Mechanisms of Pulsatile Insulin Secretion
 Diabetes Volume 67, March 2018 351 Closing in on the Mechanisms of Pulsatile Insulin Secretion Richard Bertram, 1 Leslie S. Satin, 2 and Arthur S. Sherman 3 Diabetes 2018;67:351 359 https://doi.org/10.2337/dbi17-0004
Diabetes Volume 67, March 2018 351 Closing in on the Mechanisms of Pulsatile Insulin Secretion Richard Bertram, 1 Leslie S. Satin, 2 and Arthur S. Sherman 3 Diabetes 2018;67:351 359 https://doi.org/10.2337/dbi17-0004
SUPPLEMENTAL MATERIAL. Supplementary Methods
 SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
SUPPLEMENTAL MATERIAL Supplementary Methods Culture of cardiomyocytes, fibroblasts and cardiac microvascular endothelial cells The isolation and culturing of neonatal rat ventricular cardiomyocytes was
A MODEL OF GAP JUNCTION CONDUCTANCE AND VENTRICULAR TACHYARRHYTHMIA
 A MODEL OF GAP JUNCTION CONDUCTANCE AND VENTRICULAR TACHYARRHYTHMIA X. D. Wu, Y. L. Shen, J. L. Bao, C. M. Cao, W. H. Xu, Q. Xia Department of Physiology, Zhejiang University School of Medicine, Hangzhou,
A MODEL OF GAP JUNCTION CONDUCTANCE AND VENTRICULAR TACHYARRHYTHMIA X. D. Wu, Y. L. Shen, J. L. Bao, C. M. Cao, W. H. Xu, Q. Xia Department of Physiology, Zhejiang University School of Medicine, Hangzhou,
Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets
 Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets Gene 5 Forward 3 5 Reverse 3.T. Product (bp) ( C) mnox1 GTTCTTGGGCTGCCTTGG GCTGGGGCGGCGG 60 300 mnoxa1 GCTTTGCCGCGTGC GGTTCGGGTCCTTTGTGC
Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets Gene 5 Forward 3 5 Reverse 3.T. Product (bp) ( C) mnox1 GTTCTTGGGCTGCCTTGG GCTGGGGCGGCGG 60 300 mnoxa1 GCTTTGCCGCGTGC GGTTCGGGTCCTTTGTGC
European Medicines Agency decision
 EMA/482989/2013 European Medicines Agency decision P/0209/2013 of 3 September 2013 on the agreement of a paediatric investigation plan for glibenclamide, (EMEA-001324-PIP01-12) in accordance with Regulation
EMA/482989/2013 European Medicines Agency decision P/0209/2013 of 3 September 2013 on the agreement of a paediatric investigation plan for glibenclamide, (EMEA-001324-PIP01-12) in accordance with Regulation
Modulating Glucose Uptake in Skeletal Myotubes: Insulin Induction with Bioluminescent Glucose Uptake Analysis
 icell Skeletal Myoblasts Application Protocol Modulating Glucose Uptake in Skeletal Myotubes: Insulin Induction with Bioluminescent Glucose Uptake Analysis Introduction The skeletal muscle is one of the
icell Skeletal Myoblasts Application Protocol Modulating Glucose Uptake in Skeletal Myotubes: Insulin Induction with Bioluminescent Glucose Uptake Analysis Introduction The skeletal muscle is one of the
CareLink. software REPORT REFERENCE GUIDE. Management Software for Diabetes
 CareLink Management Software for Diabetes software REPORT REFERENCE GUIDE How to use this guide Each type of CareLink report and its components are described in the following sections. Report data used
CareLink Management Software for Diabetes software REPORT REFERENCE GUIDE How to use this guide Each type of CareLink report and its components are described in the following sections. Report data used
Supplementary Figure 1
 Supplementary Figure 1 how HFD how HFD Epi WT p p Hypothalamus p p Inguinal WT T Liver Lean mouse adipocytes p p p p p p Obese mouse adipocytes Kidney Muscle Spleen Heart p p p p p p p p Extracellular
Supplementary Figure 1 how HFD how HFD Epi WT p p Hypothalamus p p Inguinal WT T Liver Lean mouse adipocytes p p p p p p Obese mouse adipocytes Kidney Muscle Spleen Heart p p p p p p p p Extracellular
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/312/5779/1533/dc1 Supporting Online Material for Long-Term Potentiation of Neuron-Glia Synapses Mediated by Ca 2+ - Permeable AMPA Receptors Woo-Ping Ge, Xiu-Juan Yang,
www.sciencemag.org/cgi/content/full/312/5779/1533/dc1 Supporting Online Material for Long-Term Potentiation of Neuron-Glia Synapses Mediated by Ca 2+ - Permeable AMPA Receptors Woo-Ping Ge, Xiu-Juan Yang,
arxiv: v1 [physics.bio-ph] 29 Jun 2013
![arxiv: v1 [physics.bio-ph] 29 Jun 2013 arxiv: v1 [physics.bio-ph] 29 Jun 2013](/thumbs/94/121879928.jpg) 1 arxiv:1307.0131v1 [physics.bio-ph] 29 Jun 2013 Learning theories reveal loss of pancreatic electrical connectivity in diabetes as an adaptive response Pranay Goel 1,, Anita Mehta 2 1 Mathematics and
1 arxiv:1307.0131v1 [physics.bio-ph] 29 Jun 2013 Learning theories reveal loss of pancreatic electrical connectivity in diabetes as an adaptive response Pranay Goel 1,, Anita Mehta 2 1 Mathematics and
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11464 Supplemental Figure S1. The expression of Vegfb is increased in obese and diabetic mice as compared to lean mice. a-b, Body weight and postprandial blood
SUPPLEMENTARY INFORMATION doi:10.1038/nature11464 Supplemental Figure S1. The expression of Vegfb is increased in obese and diabetic mice as compared to lean mice. a-b, Body weight and postprandial blood
were isolated from the freshly drawn blood of healthy donors and ACS patients using the
 Supplemental Figure 1. Quality control of CD4 + T-cell purification. CD4 + T cells were isolated from the freshly drawn blood of healthy donors and ACS patients using the RosetteSep CD4 + T Cell Enrichment
Supplemental Figure 1. Quality control of CD4 + T-cell purification. CD4 + T cells were isolated from the freshly drawn blood of healthy donors and ACS patients using the RosetteSep CD4 + T Cell Enrichment
Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12
 SUPPLEMENTARY METHODS Cell cultures Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12 Ham with 25 mm HEPES and NaHCO 3 (1:1) and supplemented with 10% (v/v) FBS, 1.0
SUPPLEMENTARY METHODS Cell cultures Human SH-SY5Y neuroblastoma cells (A.T.C.C., Manassas, VA) were cultured in DMEM, F-12 Ham with 25 mm HEPES and NaHCO 3 (1:1) and supplemented with 10% (v/v) FBS, 1.0
SUPPLEMENTARY INFORMATION. Rett Syndrome Mutation MeCP2 T158A Disrupts DNA Binding, Protein Stability and ERP Responses
 SUPPLEMENTARY INFORMATION Rett Syndrome Mutation T158A Disrupts DNA Binding, Protein Stability and ERP Responses Darren Goffin, Megan Allen, Le Zhang, Maria Amorim, I-Ting Judy Wang, Arith-Ruth S. Reyes,
SUPPLEMENTARY INFORMATION Rett Syndrome Mutation T158A Disrupts DNA Binding, Protein Stability and ERP Responses Darren Goffin, Megan Allen, Le Zhang, Maria Amorim, I-Ting Judy Wang, Arith-Ruth S. Reyes,
Nature Neuroscience: doi: /nn Supplementary Figure 1. Trial structure for go/no-go behavior
 Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
Intravital Microscopic Interrogation of Peripheral Taste Sensation
 Supplementary Information Intravital Microscopic Interrogation of Peripheral Taste Sensation Myunghwan Choi 1, Woei Ming Lee 1,2, and Seok-Hyun Yun 1 * 1 Harvard Medical School and Wellman Center for Photomedicine,
Supplementary Information Intravital Microscopic Interrogation of Peripheral Taste Sensation Myunghwan Choi 1, Woei Ming Lee 1,2, and Seok-Hyun Yun 1 * 1 Harvard Medical School and Wellman Center for Photomedicine,
Slow oscillations of K ATP conductance in mouse pancreatic islets provide support for electrical bursting driven by metabolic oscillations
 Am J Physiol Endocrinol Metab 305: E805 E817, 2013. First published August 6, 2013; doi:10.1152/ajpendo.00046.2013. Slow oscillations of K ATP conductance in mouse pancreatic islets provide support for
Am J Physiol Endocrinol Metab 305: E805 E817, 2013. First published August 6, 2013; doi:10.1152/ajpendo.00046.2013. Slow oscillations of K ATP conductance in mouse pancreatic islets provide support for
Identification of GLP1R agonists using a novel high throughput screening assay Wan Namkung, Ph.D.
 Identification of GLP1R agonists using a novel high throughput screening assay Wan Namkung, Ph.D. College of Pharmacy, Yonsei University Contents High-throughput screening (HTS) HTS assays for identification
Identification of GLP1R agonists using a novel high throughput screening assay Wan Namkung, Ph.D. College of Pharmacy, Yonsei University Contents High-throughput screening (HTS) HTS assays for identification
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Advances in pancreatic islet monolayer culture on glass surfaces enable superresolution microscopy and insights into beta cell ciliogenesis and proliferation Edward A. Phelps,
SUPPLEMENTARY INFORMATION Advances in pancreatic islet monolayer culture on glass surfaces enable superresolution microscopy and insights into beta cell ciliogenesis and proliferation Edward A. Phelps,
Research Article A TRPM4 Inhibitor 9-Phenanthrol Inhibits Glucose- and Glucagon-Like Peptide 1-Induced Insulin Secretion from Rat Islets of Langerhans
 Hindawi Diabetes Research Volume 217, Article ID 5131785, 5 pages https://doi.org/1.1155/217/5131785 Research Article A TRPM4 Inhibitor 9-Phenanthrol Inhibits Glucose- and Glucagon-Like Peptide 1-Induced
Hindawi Diabetes Research Volume 217, Article ID 5131785, 5 pages https://doi.org/1.1155/217/5131785 Research Article A TRPM4 Inhibitor 9-Phenanthrol Inhibits Glucose- and Glucagon-Like Peptide 1-Induced
Is action potential threshold lowest in the axon?
 Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
Neurons of the Bed Nucleus of the Stria Terminalis (BNST)
 Neurons of the Bed Nucleus of the Stria Terminalis (BNST) Electrophysiological Properties and Their Response to Serotonin DONALD G. RAINNIE a Harvard Medical School and Department of Psychiatry, Brockton
Neurons of the Bed Nucleus of the Stria Terminalis (BNST) Electrophysiological Properties and Their Response to Serotonin DONALD G. RAINNIE a Harvard Medical School and Department of Psychiatry, Brockton
Dep. Control Time (min)
 aa Control Dep. RP 1s 1 mv 2s 1 mv b % potentiation of IPSP 2 15 1 5 Dep. * 1 2 3 4 Time (min) Supplementary Figure 1. Rebound potentiation of IPSPs in PCs. a, IPSPs recorded with a K + gluconate pipette
aa Control Dep. RP 1s 1 mv 2s 1 mv b % potentiation of IPSP 2 15 1 5 Dep. * 1 2 3 4 Time (min) Supplementary Figure 1. Rebound potentiation of IPSPs in PCs. a, IPSPs recorded with a K + gluconate pipette
Rescue IVF protocol for legacy stock
 Rescue IVF protocol for legacy stock Sperm thawing/ivf protocol for MTG sperm samples (80ul per straw) from straw and conventional CPA from Vial (100ml per vial) This protocol is based on methods developed
Rescue IVF protocol for legacy stock Sperm thawing/ivf protocol for MTG sperm samples (80ul per straw) from straw and conventional CPA from Vial (100ml per vial) This protocol is based on methods developed
marked secretion ofcatecholamines and a subsequent inhibition ofsecretion although the basal secretion shows an initial rise.
 J. Physiol. (1969), 2, pp. 797-85 797 With 7 text-ftgurem Printed in Great Britain SODIUM IONS AND THE SECRETION OF CATECHOLAMINES By P. BANKS, ROSEMARY BIGGINS, R. BISHOP, B. CHRISTIAN AND N. CURRIE From
J. Physiol. (1969), 2, pp. 797-85 797 With 7 text-ftgurem Printed in Great Britain SODIUM IONS AND THE SECRETION OF CATECHOLAMINES By P. BANKS, ROSEMARY BIGGINS, R. BISHOP, B. CHRISTIAN AND N. CURRIE From
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/278/rs11/dc1 Supplementary Materials for In Vivo Phosphoproteomics Analysis Reveals the Cardiac Targets of β-adrenergic Receptor Signaling Alicia Lundby,* Martin
www.sciencesignaling.org/cgi/content/full/6/278/rs11/dc1 Supplementary Materials for In Vivo Phosphoproteomics Analysis Reveals the Cardiac Targets of β-adrenergic Receptor Signaling Alicia Lundby,* Martin
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) 108 (b-c) (d) (e) (f) (g)
 Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Glucose Uptake Assay Kit (Fluorometric)
 ab136956 Glucose Uptake Assay Kit (Fluorometric) Instructions for Use For the sensitive and accurate measurement of Glucose uptake in various samples This product is for research use only and is not intended
ab136956 Glucose Uptake Assay Kit (Fluorometric) Instructions for Use For the sensitive and accurate measurement of Glucose uptake in various samples This product is for research use only and is not intended
Shareque T. Shaikh, DD; Swati S. Jadhav, MD; Vyankatesh K. Shivane, DD; Anurag R. Lila, DM; Tushar R. Bandgar, DM; Nalini S.
 Case Report CHILDHOOD ONSET OF SULFONYLUREA RESPONSIVE NEONATAL DIABETES DUE TO A NOVEL HOMOZYGOUS AUTOSOMAL RECESSIVE MUTATION IN THE ABCC8 GENE WHICH WAS PRESUMED TO BE TYPE 1B DIABETES BEFORE GENETIC
Case Report CHILDHOOD ONSET OF SULFONYLUREA RESPONSIVE NEONATAL DIABETES DUE TO A NOVEL HOMOZYGOUS AUTOSOMAL RECESSIVE MUTATION IN THE ABCC8 GENE WHICH WAS PRESUMED TO BE TYPE 1B DIABETES BEFORE GENETIC
Surgery in Congenital Hyperinsulinismless. Winfried Barthlen
 Surgery in Congenital Hyperinsulinismless may be more Winfried Barthlen congenital hyperinsulinism - very rare (1:40.000) - uncontrolled insulin secretion - life threatening hypoglycemia symptoms - unconsciousness,
Surgery in Congenital Hyperinsulinismless may be more Winfried Barthlen congenital hyperinsulinism - very rare (1:40.000) - uncontrolled insulin secretion - life threatening hypoglycemia symptoms - unconsciousness,
Theta sequences are essential for internally generated hippocampal firing fields.
 Theta sequences are essential for internally generated hippocampal firing fields. Yingxue Wang, Sandro Romani, Brian Lustig, Anthony Leonardo, Eva Pastalkova Supplementary Materials Supplementary Modeling
Theta sequences are essential for internally generated hippocampal firing fields. Yingxue Wang, Sandro Romani, Brian Lustig, Anthony Leonardo, Eva Pastalkova Supplementary Materials Supplementary Modeling
Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo
 Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo Shannon L. Macauley, 1 Molly Stanley, 1 Emily E. Caesar, 1 Steven A. Yamada, 1 Marcus E. Raichle, 1,2 Ronaldo
Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo Shannon L. Macauley, 1 Molly Stanley, 1 Emily E. Caesar, 1 Steven A. Yamada, 1 Marcus E. Raichle, 1,2 Ronaldo
Supporting Information. A Two-In-One Fluorescent Sensor With Dual Channels to. Discriminate Zn 2+ and Cd 2+
 Electronic Supplementary Material (ESI) for RS Advances Supporting Information A Two-In-One Fluorescent Sensor With Dual hannels to Discriminate Zn 2 and d 2 Li-Kun Zhang, a Guang-Fu Wu, a Ying Zhang,
Electronic Supplementary Material (ESI) for RS Advances Supporting Information A Two-In-One Fluorescent Sensor With Dual hannels to Discriminate Zn 2 and d 2 Li-Kun Zhang, a Guang-Fu Wu, a Ying Zhang,
Biology Open (2014) 000, 1 10 doi: /bio
 (2014) 000, 1 10 doi:10.1242/bio.201410041 Supplementary Material Michael Brauchle et al. doi: 10.1242/bio.201410041 Fig. S1. Alignment of GFP, sfgfp, egfp, eyfp, mcherry and mruby2. Sequence-based alignment
(2014) 000, 1 10 doi:10.1242/bio.201410041 Supplementary Material Michael Brauchle et al. doi: 10.1242/bio.201410041 Fig. S1. Alignment of GFP, sfgfp, egfp, eyfp, mcherry and mruby2. Sequence-based alignment
Pharmacologyonline 2: (2009) Pharmacodynamic Drug Interaction of Gliclazide and Mexiletine in Rats
 Pharmacodynamic Drug Interaction of Gliclazide and Mexiletine in Rats K. Abedulla Khan 1, S. Satyanarayana 2, K. Eswar Kumar 2, K. Pavan Kumar 1, K. Anupama 1 1 Sultan-ul-uloom College of pharmacy, Banjarahills,
Pharmacodynamic Drug Interaction of Gliclazide and Mexiletine in Rats K. Abedulla Khan 1, S. Satyanarayana 2, K. Eswar Kumar 2, K. Pavan Kumar 1, K. Anupama 1 1 Sultan-ul-uloom College of pharmacy, Banjarahills,
High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lyososomes) in the heart.
 High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lyososomes) in the heart. Daniel Aston, Rebecca A. Capel, Kerrie L. Ford, Helen C. Christian,
High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lyososomes) in the heart. Daniel Aston, Rebecca A. Capel, Kerrie L. Ford, Helen C. Christian,
Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity.
 Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity. (a) Relative amounts of adiponectin, Ppar 2, C/ebp, and Tnf mrna
Supplementary Figure 1. DNA methylation of the adiponectin promoter R1, Pparg2, and Tnfa promoter in adipocytes is not affected by obesity. (a) Relative amounts of adiponectin, Ppar 2, C/ebp, and Tnf mrna
SUR1, sulfonylurea receptor 1; [Ca 2 ] c, cytosolic Ca 2 concentration; KO, knock-out.
![SUR1, sulfonylurea receptor 1; [Ca 2 ] c, cytosolic Ca 2 concentration; KO, knock-out. SUR1, sulfonylurea receptor 1; [Ca 2 ] c, cytosolic Ca 2 concentration; KO, knock-out.](/thumbs/76/73249559.jpg) THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 20, pp. 14768 14776, May 18, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Overnight Culture Unmasks
THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 282, NO. 20, pp. 14768 14776, May 18, 2007 2007 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in the U.S.A. Overnight Culture Unmasks
Relaxation responses of aortic rings from salt-loaded high calcium fed rats to potassium chloride, calcium chloride and magnesium sulphate
 Pathophysiology 4 (1998) 275 280 Relaxation responses of aortic rings from salt-loaded high calcium fed rats to potassium chloride, calcium chloride and magnesium sulphate B.J. Adegunloye, O.A. Sofola
Pathophysiology 4 (1998) 275 280 Relaxation responses of aortic rings from salt-loaded high calcium fed rats to potassium chloride, calcium chloride and magnesium sulphate B.J. Adegunloye, O.A. Sofola
Prasanna K. Dadi, Nicholas C. Vierra, and David A. Jacobson
 DIABETES-INSULIN-GLUCAGON-GASTROINTESTINAL Pancreatic -Cell specific Ablation of TASK-1 Channels Augments Glucose-stimulated Calcium Entry and Insulin Secretion, Improving Glucose Tolerance Prasanna K.
DIABETES-INSULIN-GLUCAGON-GASTROINTESTINAL Pancreatic -Cell specific Ablation of TASK-1 Channels Augments Glucose-stimulated Calcium Entry and Insulin Secretion, Improving Glucose Tolerance Prasanna K.
Maternal diet induced micrornas and mtor underlie b cell dysfunction in offspring
 Maternal diet induced micrornas and mtor underlie b cell dysfunction in offspring Emilyn U. Alejandro,, Peter Arvan, Ernesto Bernal- Mizrachi J Clin Invest. 2014;124(10):4395-4410. https://doi.org/10.1172/jci74237.
Maternal diet induced micrornas and mtor underlie b cell dysfunction in offspring Emilyn U. Alejandro,, Peter Arvan, Ernesto Bernal- Mizrachi J Clin Invest. 2014;124(10):4395-4410. https://doi.org/10.1172/jci74237.
THE UNIVERSITY OF NEWCASTLE- DISCIPLINE OF MEDICAL BIOCHEMISTRY
 Page: 1 of 5 1. Risk Assessment: This Risk Assessment is to be used as a general guide and as such, cannot accommodate all the varying factors that may be encountered when using this procedure. Therefore,
Page: 1 of 5 1. Risk Assessment: This Risk Assessment is to be used as a general guide and as such, cannot accommodate all the varying factors that may be encountered when using this procedure. Therefore,
Overview. o Limitations o Normal regulation of blood glucose o Definition o Symptoms o Clinical forms o Pathophysiology o Treatment.
 Pål R. Njølstad MD PhD KG Jebsen Center for Diabetes Research University of Bergen, Norway Depertment of Pediatrics Haukeland University Hospital Broad Institute of Harvard & MIT Cambridge, MA, USA Hypoglycemia
Pål R. Njølstad MD PhD KG Jebsen Center for Diabetes Research University of Bergen, Norway Depertment of Pediatrics Haukeland University Hospital Broad Institute of Harvard & MIT Cambridge, MA, USA Hypoglycemia
Human Immunodeficiency Virus type 1 (HIV-1) p24 / Capsid Protein p24 ELISA Pair Set
 Human Immunodeficiency Virus type 1 (HIV-1) p24 / Capsid Protein p24 ELISA Pair Set Catalog Number : SEK11695 To achieve the best assay results, this manual must be read carefully before using this product
Human Immunodeficiency Virus type 1 (HIV-1) p24 / Capsid Protein p24 ELISA Pair Set Catalog Number : SEK11695 To achieve the best assay results, this manual must be read carefully before using this product
Supplementary Information
 Hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K + channels Meena S. George, L.F. Abbott, Steven A. Siegelbaum Supplementary Information Part 1: Supplementary Figures
Hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K + channels Meena S. George, L.F. Abbott, Steven A. Siegelbaum Supplementary Information Part 1: Supplementary Figures
THE EFFECT OF COUPLING, NOISE, AND HETEROGENEITY ON BURSTING OSCILLATIONS
 CANADIAN APPLIED MATHEMATICS QUARTERLY Volume 11, Number 1, Spring 2003 THE EFFECT OF COUPLING, NOISE, AND HETEROGENEITY ON BURSTING OSCILLATIONS GERDA DE VRIES ABSTRACT. Bursting refers to a complex oscillation
CANADIAN APPLIED MATHEMATICS QUARTERLY Volume 11, Number 1, Spring 2003 THE EFFECT OF COUPLING, NOISE, AND HETEROGENEITY ON BURSTING OSCILLATIONS GERDA DE VRIES ABSTRACT. Bursting refers to a complex oscillation
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): Overall, this is an interesting study. The authors used a custom microfluidic device to deliver precise concentrations of bacterial food to worms
Reviewers' comments: Reviewer #1 (Remarks to the Author): Overall, this is an interesting study. The authors used a custom microfluidic device to deliver precise concentrations of bacterial food to worms
