Blood-borne Pathogens Exposure Policy
|
|
|
- Julianna Cain
- 5 years ago
- Views:
Transcription
1 Blood-borne Pathogens Exposure Policy Contents Blood Borne Pathogen(s) Exposure (BBPE) -Post-Exposure Prophylaxis... 1 STUDENT CHECKLIST... 3 PROGRAM CHECKLIST... 4 PROVIDER CHECKLIST... 5 POST-EXPOSURE PROCEDURE... 6 EXPOSURE NOTIFICATION FORM BLOOD BORNE PATHOGENS EXPOSURE MANAGEMENT FORM HIV EXPOSURE INFORMED CONSENT FOR PROPHYLAXIS AFTER BLOOD BORNE PATHOGENS EXPOSURE... 21
2 Blood Borne Pathogen(s) Exposure (BBPE) -Post-Exposure Prophylaxis Purpose Audience The purpose of this document is to establish University of La Verne s policy for the initiation of prophylaxis after an exposure to the human immunodeficiency virus (HIV) and hepatitis B virus (HBV) and early treatment of infection with the hepatitis C virus (at time of seroconversion) to prevent chronic infection. This policy has been developed from the most current medical literature, US Public Health Service Guidelines and CDC documents on prevention of Hepatitis B infection in Health-Care Personnel. This prophylaxis protocol and regimen will be continuously updated with the most recent medical information. University of La Verne's PA program students Definitions Occupational exposures requiring the initiation of prophylaxis are defined as: Percutaneous injury (e.g. needle stick, laceration with a sharp object) Contact of mucous membranes or ocular membranes Contact of non-intact skin (e.g. skin that is chapped, abraded) with Blood or other potentially infectious fluid (semen; vaginal secretions; and cerebrospinal, synovial, pleural, peritoneal, pericardial and amniotic fluids; bloody body fluids and unfixed tissue). Important Links Occupational exposures requiring monitoring include the three above requiring prophylaxis and: Contact with intact skin that is prolonged or involves an extensive area with Blood or other potentially infectious fluid (semen; vaginal secretions; and cerebrospinal, synovial, pleural, peritoneal, pericardial and amniotic fluids; bloody body fluids and unfixed tissue). CDC website for BBPE: OSHA website for IPPE: Clinical Consultation Center for Post-Exposure Prophylaxis (PEP): PEP Quick Reference Guide: 1
3 Occupational Exposure Monitoring Prophylaxis All La Verne s students with a documented occupational exposure shall have the exposure evaluated and documented by a healthcare provider following the standard protocol. At the Student Health/nearest Emergency Room, the healthcare provider will recommend prophylaxis for percutaneous exposures, contact of mucous membranes or non-intact skin. with Blood or other potentially infectious fluid (semen; vaginal secretions; and cerebrospinal, synovial, pleural, peritoneal, pericardial and amniotic fluids; bloody body fluids and unfixed tissue). Healthcare providers in the Emergency Department or in outlying clinics will recommend initial prophylaxis and refer exposed persons to Student Health Services associated with University of La Verne on the next business day for follow up. Prophylaxis shall be recommended to all La Verne s students: HIV: For percutaneous exposures, contact of mucous membranes or nonintact skin. with Blood or other potentially infectious fluid (semen; vaginal secretions; and cerebrospinal, synovial, pleural, peritoneal, pericardial and amniotic fluids; bloody body fluids and unfixed tissue). Ideally within two hours after exposures, but may be initiated up to 72 hours after exposure. With appropriate drug therapy. Until the source-patient blood has been obtained and analyzed. If the source-patient HIV status is determined to be negative, prophylaxis will be discontinued. HBV-Prophylaxis for HBV prevention will be evaluated on an individual basis. HCV-Employees/students testing positive for Hepatitis C Qualitative RNA (PCR) at 6 weeks, 3 months or 6 months, will be referred immediately to a hematologist for early treatment to prevent chronic hepatitis C infection Important Telephone Numbers University of La Verne Campus Safety: (909) University of La Verne Risk Management: (909) University of La Verne Student Health Center: (909) PA Program Clinical Coordinator: Lindsey Hoffmann, (909) PA Program: (909) Emergency or Police:
4 STUDENT CHECKLIST Wash exposed area immediately. Notify supervisor/preceptor immediately to assist with obtaining source consent & refer both the source and student to a designated medical provider or nearest emergency room. Have supervisor/preceptor document in source s medical record source of occupational exposure and that labs were drawn for HIV, HCV, and HBsAg (Hepatitis B surface antigen) with source s consent. Seek post-exposure care: For students on campus during regular business hours report to Week Days (8am-4pm) during traditional academic school year Student Health Center (909) Report to Emergency Department, after hours, weekends, or holidays (Follow-up in Student Health Center on the next business day). For students who are less than 2 hours away from the PA program, he/she should report to Student Health or Emergency Department as soon as possible, but at least within 2 hours of exposure. Off-site Exposures - Follow clinic specific policy. Immediately report to assigned supervisor/preceptor and clinical coordinator Complete Blood Borne Pathogen (BBP) Exposure Notification Form located below. - Off-site Students are to follow the clinical facility s protocol for initial evaluation or go to the nearest emergency room. - On-campus The appropriate form will be completed at the Student Health Center. After the medical evaluation/treatment, notify the PA Program s Clinical Coordinator. - If seen in the Emergency Department, form should be completed there and faxed - Send form(s) to Student Health and Clinical Coordinator Student Health Center Clinical Coordinator Fax (909) Fax: (909) Follow up with Student Health Center on the next business day 3
5 PROGRAM CHECKLIST Refer the student for post exposure care immediately. For students on campus during regular business hours report to Student Health Center - during traditional academic school year Phone: (909) Students should report to Emergency Department for after hours, weekends, or holidays (Follow-up with Student Health the next business day) Students should report to Student Health or Emergency Department as soon as possible, but at least within 2 hours of exposure. Assist the student with obtaining source consent and refer both the source and student to designated medical facility or local emergency room. Document in source s medical record source of occupational exposure and that labs were drawn for HIV, HCV, and HBsAg with source s consent. Remind the student to report exposure by completing BBP Exposure Notification Form. - Off-site Complete form and fax to Student Health and Clinical Coordinator. - On-campus (less than 2 hours away) Report to Student Health Center (form will be completed there). - If seen in the Emergency Department, form should be completed there and faxed to Student Health Center and Clinical Coordinator. For students with related questions about exposures, call Student Health Center at (909) or the PA program Clinical Coordinator at (909) Additional Information, visit 4
6 PROVIDER CHECKLIST Provide necessary medical care to treat the injury/exposure per site protocols If needed, refer to the enclosed procedures as a guide for post-exposure care Contact Clinical Consultation Center for Post-Exposure Prophylaxis (PEP) for additional recommendations: Refer to PEP Quick Reference Guide: Complete the Exposure Notification Form [enclosed] and fax to Student Health and Clinical Coordinator - Clinical Coordinator- FAX # (909) Student Health Center FAX # (909) Provide patient education information per site protocol or use [Appendix A]. Complete Informed Consent for Prophylaxis form [enclosed]. Billing instructions for Students Off Campus Exposures - Student s Insurance should be billed Student: Reimbursement will be for reasonable & customary charges for authorized or medically necessary tests as noted in enclosed information. Any additional charges will not be reimbursed by the PA program. 5
7 POST-EXPOSURE PROCEDURE I. MANAGE THE EXPOSURE A. Wash the area immediately with soap and water. B. For exposure to eyes, mouth, and/or nose flush area with water. II. NOTIFY SUPERVISOR/PRECEPTOR/CLINICAL COORDINATOR IMMEDIATELY A. Supervisor/preceptor/Clinical Coordinator will assist in obtaining source consent and refer both the source and the student to designated medical facility- onsite site treatment, Student Health, or closest emergency room. B. Supervisor/Preceptor or Clinical Coordinator shall release the student from their duties immediately to seek post-exposure care. III. PROCEDURE FOR SOURCE TESTING A. Obtain source consent & provide appropriate referrals to designated medical facility or emergency room B. General consent for comatose/general anesthesia patients will suffice but needs to be documented on either the enclosed Exposure Reporting or Exposure Management form. C. Refusals should be documented on the enclosed Exposure Reporting or Exposure Management form. Notify the PA program s Clinical Coordinator as soon as possible. The Clinical Coordinator will make the decision on how the source blood will be obtained. D. Direct the student to the medical facility or emergency room for an evaluation/treatment E. The Clinical Coordinator will inform the Student Health Center and assist the student with scheduling follow-up appointments at the Student Health Center on the next business day. IV. STUDENT POST EXPOSURE CARE A. For on-campus (less than 2 hours away) exposures: Monday through Friday, during traditional academic school year, Students are seen in Student Health Center 8AM to 4PM. B. Holidays, weekends, or after hours Go to the Emergency Department immediately. 1. Triage to ensure initiation of prophylaxis within 2 hours of exposure. 2. A consent is needed for HIV and other bloodborne pathogen testing. 3. Assure consent for testing is documented 4. The following laboratory tests for the designated medical facility, if La Verne s
8 BBPE protocol is requested two serum separator tubes labeled appropriately. a. Order labs for exposed. In addition, HBV surface antibody should be ordered for students who have a history of HBV immunization b. Additional laboratory tests required if Student is starting prophylaxis for HIV exposure/possible exposure. Females must have a pregnancy test. c. Provide the following La Verne s BBPE protocol to the designated medical facility for prophylaxis treatment if requested (see subsequent paragraphs documenting post-exposure prophylaxis) 5. Post-exposure Prophylaxis for Tetanus/Diphtheria, Hepatitis B and Hepatitis C a. Offer tetanus/diphtheria booster following percutaneous injury if none within last 10 years. Offer Tdap or Td vaccine for the booster. b. For vaccinated students (who have written documentation of Tetanus/Diphtheria, Hepatitis B or Hepatitis C) testing the source patient for HBsAg is recommended, but not required. A. Exposed Student has never received Hepatitis B vaccine a. Offer HBV vaccine if source is known to be positive for hepatitis B or is high risk for hepatitis B or source is unknown and student has not been vaccinated against hepatitis B. b. Offer Hepatitis B vaccine if source is known to be positive for hepatitis B, or is high risk for hepatitis B or source is unknown and student has not been vaccinated against hepatitis B. B. Exposed Student has received 3 doses of Hepatitis B vaccine twice and titer of anti-hbs < 10 miu/ml a. For vaccinated Students (who have written documentation of Hepatitis B vaccination) the source patient should be tested for HBsAg as soon as possible after the exposure. If the source patient is HBsAg-positive or has unknown HbsAg status, Student should receive 2 doses of HBIG. The first dose should be administered as soon as possible after exposure and the second dose should be administered 1 month later. If the source patient is HBsAgnegative neither HBIG nor Hepatitis B vaccine is necessary. C. Exposed Student unvaccinated or incompletely vaccinated (including those who refused vaccination) a. For unvaccinated or incompletely vaccinated Student (including those who refused vaccination because of personal beliefs or religious practices), the source patient should be tested for HBsAg as soon as possible after the exposure. b. Students testing positive for Hepatitis C Qualitative RNA (PCR) at 6 weeks, 3 months or 6 months, will be referred immediately to a hematologist for treatment. c. Recommend HIV prophylaxis following percutaneous injury or contamination of mucous
9 membranes or non-intact skin with blood, body fluids visibly contaminated with blood, unfixed tissue, semen, vaginal secretions, and cerebrospinal, synovial, pleural, peritoneal, pericardial, and amniotic fluids (goal is to begin prophylaxis within 2 hours of exposure). d. Prophylaxis medications write Occupational Post-Exposure-Prophylaxis on prescription. 1. If Student is Pregnant prophylaxis should be reserved for those with HIGH RISK exposures. V. OFF-CAMPUS EXPOSURE OF LA VERNE S STUDENTS A. Maternal-Child Health Clinics refer to treating clinic specific policies for initial management. B. Outpatient Clinic refer to clinic specific policies for initial management or provide La Verne s BBPE policy, if requested Student Health (8AM-4PM) (909) C. Students who are LESS than 2 hours from the PA program should: 1. Notify your Clinical Coordinator immediately 2. Between 8am-4pm (M-F), come directly to La Verne s Student Health Center or to the nearest emergency room. Seek treatment within 2 hours. 3. After hours, weekends, or holidays go to the nearest emergency room for the initial evaluation and then follow up with Student Health the business next day. Seek treatment within 2 hours of the exposure. Follow up with Student Health Center the next business day. Student baseline laboratory tests to be drawn are HIV, HCV, total anti-hbc and anti- HBs. 4. Have the clinical facility order the source laboratory tests for HIV, HCV, and HBsAg. Follow the clinical facility s protocol for source laboratory tests and find out the contact person at the facility for the source laboratory test results. Have the Clinical Coordinator assist in this process. D. Students who are MORE than 2 hours away from the PA program: 1. Notify the Clinical Coordinator immediately 2. Follow the clinical facility s protocol for initial evaluation or go to the nearest emergency room for an evaluation. Seek treatment within 2 hours of the exposure. Always follow up with Student Health Center. Student s insurance should be billed for the evaluation/treatment. 3. If after hours, weekends, or holidays, follow the clinical facility s protocol for initial evaluation or go to the nearest Emergency room. Seek treatment within 2 hours of the exposure. Follow up with Student Health the next business day. Student s insurance should be billed if treatment is necessary. Student baseline laboratory tests to be drawn
10 are HIV, HCV, total anti-hbc and anti-hbs. 4. Have the clinical facility draw and run the source laboratory tests for HIV, HCV, and HBsAg. Follow the clinical facility s protocol for source laboratory tests and find out the contact person at the facility for the source laboratory test results. Have your Clinical Coordinator assist in this process. References: 1. Kuhar DT, Henderson DK, Struble KA, Heneine W, et al. Updated US Public Health Service Guidelines for the Management of Occupational Exposures to Human Immunodeficiency Virus and Recommendations for Post exposure Prophylaxis. Infect Control Hosp Epidemiol 2013;34: Centers for Disease Control and Prevention. CDC Guidance for Evaluating Health-Care Personnel for Hepatitis B Virus Protection and for Administering Post exposure Management, 2013;62(No. RR-10): Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis C with interferon alfa- 2b. N Engl J Med 2001; 345: Tivicay package insert. Research Triangle Park, NC: GlaxoSmithKline; 2013 Aug. 5. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at: Accessed January 29, 2008.
11 EXPOSURE NOTIFICATION FORM Person completing form Signature Date/Time Name Dept/School Supervisor/Faculty Home # Work # Pager # Student SSN Date of Exposure / / Time am_ pm _ Location where exposure occurred (Building, Floor, Rm) Personal Protective Equipment Used: Gloves Goggles/Mask/Faceshield Gown Other Was a safety device being used? Yes No If so, did it work? Yes No Type & Brand of safety device Body part exposed (circle one) hand, eye, mouth, other (please identify) Describe how exposure occurred. Type of body substance exposed to: blood body fluid contaminated by blood semen, vaginal secretions and cerebrospinal, synovial, pleural, peritoneal, pericardial and amniotic fluids and unfixed human tissue Type of exposure: needlestick..depth of injury (check all that apply) cut.depth of injury Fluid injected Yes No Estimated volume: Mucous membranes Non-intact skin (e.g., chapped, abraded, or otherwise non-intact) Did this exposure occur during the student s normal work activities? Yes No Is patient source known? Yes No Was source consent obtained? Yes No Source lab testing done? Yes No Source on antiretroviral therapy? Yes No List Drugs Was source blood sent to lab? Yes No Source name MR# Location Exposed student lab testing done? Yes No (For Females)- Pregnancy test result Was prophylaxis initiated? Yes No Date/Time of 1st dose / / Time am pm _ Have you had training on Standard Precautions within the last 12 months? Yes No Fax this form to the University of La Verne s Student Health Center at (909) For questions call the Student Health Center at (909) or the PA Program s Clinical Coordinator at (909)
12 BLOOD BORNE PATHOGENS EXPOSURE MANAGEMENT FORM For Student Use Name Phone: (Last) (First) (MI) (Home) (Pager) (Work) Date of Exposure Time of Exposure am/pm Initial Care of Injury/Exposure Site Date of Evaluation (Initial) Time of Evaluation (Initial) am/pm Completed BBP Notification Form attached. SOURCE INFORMATION AT TIME OF INCIDENT Known Source Unknown Source Name Source MR # SOURCE LAB RESULTS HIV HBsAg (Hep B surface antigen) HCV Location Date Drawn Student ID# Results HEPATITIS AND TETANUS Hepatitis B Vaccine [ 0 ] [ 1 ] [ 2 ] [ 3 ] Date Last Dose: Antibody Response: anti-hbs 10 miu/ml anti-hbs <10 miu/ml Unknown Hepatitis B Vaccine Given: Yes No Date Given: Immunoprophylaxis: HBIG Yes No Date Given: Tetanus/Diphtheria Booster (Tdap ): Date of Last Dose: Booster Given: Yes No Date Given: If patient refuses Tdap, offer Td.
13 INITIAL VISIT HIV EXPOSURE BP P R T WT HT Medication(s) Drug Allergy(s) Clinical Findings: STUDENT BASELINE LAB Consent for HIV Testing Date Yes No Prophylaxis Baseline Labs: CBC Total bilirubin, ALT, AST, GGT Pregnancy Test (serum) Creatinine, BUN Blood glucose CPK RESULTS STUDENT RESULTS HIV1/HIV2 anti-hbs* (Hep B surface antibody) Total anti-hbc HCV antibody Date Drawn Results anti-hbs 10mlU/mL anti-hbs < 10mlU/mL *For students who have never received vaccine or declined vaccinations (e.g. personal beliefs or religious practices) or who have had a history of HBV immunization prior to coming to University of La Verne, an HBsAg (Hepatitis B surface antigen) test should be done Results Reported by: Via: Date: Fax this form to the University of La Verne s Student Health Center at (909) For questions call the Student Health Center at (909) or the PA Program s Clinical Coordinator at (909)
14 CHECK FOLLOW-UP NEEDED: 2 Weeks (If on prophylaxis) 4 Weeks (If on prophylaxis) 6 Weeks (HIV+source. HCV+source) 3 Months (HIV+source. HCV+source. Unknown source) 6 Months 12 Months (HIV+source) (HIV+source. HCV+source. Unknown source). FOLLOW-UP DIRECTIONS: HIV+source = HIV drawn HCV+source = HCV Qual-RNA (PCR) drawn HIV/HCV (-) = HIV drawn Unknown source = HIV and HCV drawn 2 WEEK FOLLOW-UP / / RESULTS 4 WEEK FOLLOW-UP / / RESULTS If placed on prophylaxis: CBC Total bilirubin, ALT, AST, GGT Pregnancy Test (urine or serum) Creatinine, BUN Blood glucose CPK If placed on prophylaxis: CBC Total bilirubin, ALT, AST, GGT Creatinine, BUN Blood glucose CPK Results Results Reported / / Via By Reported / / Via By 6 WEEK FOLLOW- UP / / RESULTS HIV, CBC Total bilirubin, ALT, AST, GGT Creatinine, BUN, blood glucose HCV Qual RNA (PCR) (only if source blood Hep C+) CPK Results Reported / / Via By
15 3 MONTH FOLLOW-UP / / RESULTS 6 MONTH FOLLOW-UP / / RESULTS HIV HIV HCV Qual RNA (PCR) (only if source blood Hep C+) HCV Qual RNA (PCR) (only if source blood Hep C+) HBsAg Total anti-hbc Results Results Reported / / Via By Reported / / Via By 12 MONTH FOLLOW UP / / RESULTS HIV Results: Reported / / Via By ASSESSMENT/RECOMMENDATIONS: Warnings discussed to avoid pregnancy and/or breast feeding during treatment (females) Yes No Assistance in Counseling Referral Made Declined Post-Exposure Treatment: Recommended Not Recommended Informed Consent: Obtained Refused Post-Exposure Prophylaxis: Declined Provided Date/Time of 1st dose / / - AM/PM Printed Materials Provided: Yes No Comments: Nurse: Healthcare Provider: Provider Information: Name: Clinic: Address: Date: Date: Phone: After follow-up is completed, Provider is to forward to: Student Health Center Cindy Denne 2147 E Street La Verne, CA FAX # (909)
16 For questions call: (909) or (909) Provision of PEP should not be delayed while awaiting expert consultation. Situations for which Infectious Diseases Consultation for Human Immunodeficiency Virus (HIV) post exposure prophylaxis (PEP) is recommended. Delayed (ie, later than 72 hours) exposure report. Interval after which benefits from PEP are undefined. Breastfeeding in the exposed person If source person s virus is known or suspected to be resistant to one or more of the drugs considered for PEP, selection of drugs to which the source person s virus is unlikely to be resistant is recommended. Toxicity of the initial PEP regimen Symptoms (eg, gastrointestinal symptoms and others) are often manageable without changing the PEP regimen by prescribing antimotility or antiemetic agents. Serious medical illness in the exposed person who is already taking multiple medications may increase the risk of drug toxicity and drug-drug interactions.
17 INFORMED CONSENT FOR PROPHYLAXIS AFTER BLOOD BORNE PATHOGENS EXPOSURE As a patient, you have the right to be informed about your risk after a blood borne pathogens (BBP) exposure and the recommended prophylaxis. This disclosure is not meant to alarm you; however, there are certain side effects which are associated with prophylaxis. I have read and understand the patient information entitled Occupational Exposures to Blood Borne Pathogens- Patient Information, which explains risks of infection, prophylaxis, medication schedule, pregnancy precautions, follow-up and special precautions. I hereby voluntarily consent to prophylaxis for exposure to blood and body fluids infected or possibly infected with human immunodeficiency virus-1(hiv-1). The medications include: I hereby decline prophylaxis following my exposure to blood borne pathogens. The healthcare provider has informed me of the possible risks associated with refusing this medication. The nature and purpose of the proposed prophylaxis & the risks and hazards if the treatment is withheld, have been explained to me by a healthcare provider. I have had an opportunity to discuss these matters with a healthcare provider and to ask questions about my exposure, alternatives, and the proposed treatment. STUDENT SIGNATURE Date Time HEALTHCARE PROVIDER Date Time WITNESS Date Time ***** Please forward to Student Health Services ****
18 The following laminated attachment is provided to all PA students to accompany their student ID badge as BBPE reporting reminders WIN WASH exposed area, IDENTIFY source, NOTIFY supervisor PACT Protect, Act, Clean, Tell Important Telephone Numbers: Emergency: Pomona Valley Hospital: (909) Program Clinical Coordinator: (909) PA Program: (909) Student Health Center: (909) Campus Safety: (909) Laverne.edu WIN WASH exposed area, IDENTIFY source, NOTIFY supervisor PACT Protect, Act, Clean, Tell Important Telephone Numbers: Emergency: Pomona Valley Hospital: (909) Program Clinical Coordinator: (909) PA Program: (909) Student Health Center: (909) Campus Safety: (909) Laverne.edu
Bloodbourne Pathogens (BBP) Occupational Post-Exposure Chemophrophylaxis
 Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
Bloodbourne Pathogens (BBP) Audience: All personnel in the. Purpose: The purpose of this document is to establish UTMB policy for the initiation of prophylaxis after occupational exposure to the human
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Prophylaxis
 01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Establish protocol for management of occupational exposures to blood or potentially infectious materials. All employees of
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Establish protocol for management of occupational exposures to blood or potentially infectious materials. All employees of
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Management
 01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Policy Definitions Establish protocol for management of occupational exposures to blood or potentially infectious materials.
01.02 Blood Borne Pathogens (BBP) Occupational Post Exposure Purpose Audience Policy Definitions Establish protocol for management of occupational exposures to blood or potentially infectious materials.
Bloodborne Pathogen Exposure STUDENT. Blood Borne Pathogen Exposure. Student. Rev:
 Blood Borne Pathogen Exposure Student INSTRUCTIONS 1. Student: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Blood Borne Pathogen Exposure Student INSTRUCTIONS 1. Student: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Bloodborne Pathogens Exposure Procedure
 Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
Bloodborne Pathogens Exposure Procedure Background: Bloodborne pathogens are infectious microorganisms present in blood that can cause disease in humans. These pathogens include, but are not limited to,
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet. Instructions for Employees/Students:
 MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
MUSC Occupational Bloodborne Pathogen Protocol Off Campus Procedure Packet MUSC has established these protocols in accordance with the OSHA Bloodborne Pathogen Standard and Center for Disease Control recommendations
Blood Borne Pathogen Exposure Employee
 Blood Borne Pathogen Exposure Employee INSTRUCTIONS 1. Employee: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
Blood Borne Pathogen Exposure Employee INSTRUCTIONS 1. Employee: Get name, DOB and, if available, health record number of source patient Obtain form and assist your supervisor with providing information
To provide the guidelines for the management of healthcare workers who have had an occupational exposure to blood and/or body fluids.
 TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
TITLE/DESCRIPTION: MANAGEMENT OF OCCUPATIONAL EXPOSURE TO HBV, HCV, and HIV INDEX NUMBER: EFFECTIVE DATE: APPLIES TO: ISSUING AUTHORITY: 01/01/2009 01/01/2013 All GCC Countries GULF COOPERATION COUNCIL
EXPOSURE (HIV/HEPATITIS) BLOOD & BODY FLUIDS
 Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
Page(s): 1 of 11 PURPOSE To set a standardized procedure to ensure that employees are evaluated in a consistent and timely manner.. POLICY A. The treatment of Team Member exposure to bloodborne pathogens
Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May Contains information for:
 APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
APPENDIX A Safety Regulations and Procedures Occupational Health Bloodborne Pathogens Exposure Control Plan S80.10, updated, May 2018 BLOODBORNE PATHOGEN EXPOSURE INCIDENT PACKET Contains information for:
Exposures at Non-MUSC Clinical Sites
 BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
BLOOD BORNE PATHOGEN EXPOSURE CHECKLIST Exposures at Non-MUSC Clinical Sites (Students on clinical rotations at Roper Hospital, Trident Hospital, Affiliated Local Clinical Sites, Out of Town Clinical Sites,
Needlestick and Splash Exposure Flow Chart Page 1 Clinical Practice Guidelines
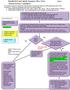 Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
Needlestick and Splash Exposure Flow Chart Page 1 If ANY student experiences a needlestick or splash exposure ANY time of day/night, they need to page #11709 which will be forwarded to: (**in the event
INITIAL EVALUATION AND MANAGEMENT FOLLOWING EXPOSURE TO BLOOD OR BODY FLUIDS
 Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Page 1 of 11 Original Date of Issue: June 1991 Reviewed 2/96 5/97 11/98 11/99 5/01 5/02 12/05 Revised 9/95 5/97 5/00 7/02 3/06 6/08 6/10 The following are the procedures to be followed when a person sustains
Policy S- 15 FLORIDA STATE UNIVERSITY COLLEGE OF NURSING BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN FOR NURSING STUDENTS
 TITLE: Policy S- 15 FLORIDA STATE UNIVERSITY COLLEGE OF NURSING BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN FOR NURSING STUDENTS Page 1 of 2 POLICY: The College of Nursing shall maintain a bloodborne pathogen
TITLE: Policy S- 15 FLORIDA STATE UNIVERSITY COLLEGE OF NURSING BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN FOR NURSING STUDENTS Page 1 of 2 POLICY: The College of Nursing shall maintain a bloodborne pathogen
Clinical Education Initiative OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS. Antonio E. Urbina, MD
 Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
Clinical Education Initiative Support@ceitraining.org OCCUPATIONAL POST- EXPOSURE PROPHYLAXIS Antonio E. Urbina, MD 5/22/2013 Occupational Post-Exposure Prophylaxis [Video Transcript] 00:00:15 - [Tony]
Blood borne Pathogen Exposure Policy for Students
 Blood borne Pathogen Exposure Policy for Students A University of Rhode Island (URI) student or intern who sustains an exposure from a needle stick, instrument stick, or mucous membranes to non-intact
Blood borne Pathogen Exposure Policy for Students A University of Rhode Island (URI) student or intern who sustains an exposure from a needle stick, instrument stick, or mucous membranes to non-intact
Blood/Body Fluid Exposure Option
 Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
Introduction: Transmission of bloodborne pathogens [e.g., Hepatitis B virus (HBV), Hepatitis C virus (HBC), Human Immunodeficiency Virus (HIV)] from patients to healthcare workers (HCW) is an important
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK)
 POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
Bloodborne Pathogens. Exposure Control Plan
 Bloodborne Pathogens Exposure Control Plan Maryland Institute College of Art Revision Date(s): January 2007/January 2008 Maryland Institute College of Art (MICA) Subject: Occupational/Non-occupational
Bloodborne Pathogens Exposure Control Plan Maryland Institute College of Art Revision Date(s): January 2007/January 2008 Maryland Institute College of Art (MICA) Subject: Occupational/Non-occupational
Effective Date: 6/10/2013 Review Date: 6/10/2016
 Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Policy Title: Sterilization and Disinfection of Patient-Care Items Policy Number: 11 6.2.2. Examples of useful items to maintain in the office sterilization log are as following: o Date and time of cycle
Infection Control Standard Precautions. CDC Recommendations: Application of Standard Precautions for All Patients
 Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
Infection Control Standard Precautions Standard Precautions Hand Hygiene CDC Recommendations: Application of Standard Precautions for All Patients Component Personal Protective Equipment (PPE) Gloves Mask,
B. Tasks and Procedures where employees, students or contractors can be exposed to bloodborne pathogens:
 Page 1 of 6 BLOODBORNE PATHOGEN PROGRAM INTRODUCTION The intended purpose of this document is to comply with OSHA s Occupational Exposures to Bloodborne Pathogens in Title 29 Code of Federal Regulations
Page 1 of 6 BLOODBORNE PATHOGEN PROGRAM INTRODUCTION The intended purpose of this document is to comply with OSHA s Occupational Exposures to Bloodborne Pathogens in Title 29 Code of Federal Regulations
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS
 STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
STUDENT EXPOSURE TO BLOOD OR OTHER POTENTIALLY INFECTIOUS MATERIALS (See Bloodborne Pathogen Worksheet and Post Exposure Prophylaxis consent immediately following this policy ) 1. Student must report immediately
Bloodborne Pathogens Training (OHS_BIO500) Course Material
 Introduction (OHS_BIO500) Course Material Welcome to the Bloodborne Pathogens (BBP) Training Course (OHS_BIO500). UAB Campus Employees whose job duties put them at increased risk for exposure to bloodborne
Introduction (OHS_BIO500) Course Material Welcome to the Bloodborne Pathogens (BBP) Training Course (OHS_BIO500). UAB Campus Employees whose job duties put them at increased risk for exposure to bloodborne
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY
 CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
Post Blood & Body Fluid Exposure Checklist
 Post Blood & Body Fluid Exposure Checklist Employee Name: : First Aid completed (If exposure was to eye, irrigate for 20 minutes at the nearest eye wash station. If exposure was to skin, wash affected
Post Blood & Body Fluid Exposure Checklist Employee Name: : First Aid completed (If exposure was to eye, irrigate for 20 minutes at the nearest eye wash station. If exposure was to skin, wash affected
Exposure. What Healthcare Personnel Need to Know
 Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Information from the Centers for Disease Control and Prevention National Center for Infectious Diseases Divison of Healthcare Quality Promotion and Division of Viral Hepatitis For additional brochures
Management of Workplace Exposure to Blood-borne Pathogens
 Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
Management of Workplace Exposure to Blood-borne Pathogens 11/22/2017 Management of Workplace Exposure to Blood-borne Pathogens BY SOLYMOLE KURUVILLA, PHD, RN, ACNP-BC DIRECTOR, OCCUPATIONAL HEALTH SERVICES
El Paso - Ambulatory Clinic Policy and Procedure
 Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
Regulation Reference: Title: NEEDLESTICK INJURIES/EXPOSURES TO BODY FLUIDS, CARE & FOLLOW UP Policy Number: EP 7.3 Effective Date: 6/2010 Policy Statement: A system is established and maintained to assure
PEDIATRIC EMERGENCY DEPARTMENT CLINICAL GUIDELINE: NON- OCCUPATIONAL EXPOSURE TO BLOOD-BORNE PATHOGENS (HIV, Hepatitis B, AND Hepatitis C)
 General Definition: Blood-borne pathogens are infectious agents that can be transmitted through contact with blood or certain other body fluids. The primary pathogens are HIV, Hepatitis B, and C. Before
General Definition: Blood-borne pathogens are infectious agents that can be transmitted through contact with blood or certain other body fluids. The primary pathogens are HIV, Hepatitis B, and C. Before
Bloodborne Pathogens Across the Continuum of Care Sue Sebazco, Arlington, Texas A Webber Training Teleclass
 Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Bloodborne Pathogens Across the Continuum of Care Objectives Sue Sebazco, RN, BS, CIC Infection Control/Employee Health Director Arlington, TX Pittsburg Mercy Health System skrystofiak@mercy.pmhs.org Define
Bloodborne Pathogens. Montclair Kimberley Academy 1
 Bloodborne Pathogens Montclair Kimberley Academy 1 Introduction! Approximately 5.6 million workers in health care and other facilities are at risk of exposure to bloodborne pathogens such as human immunodeficiency
Bloodborne Pathogens Montclair Kimberley Academy 1 Introduction! Approximately 5.6 million workers in health care and other facilities are at risk of exposure to bloodborne pathogens such as human immunodeficiency
3/23/2016. Managing Bloodborne Pathogen Exposures. Managing Bloodborne Pathogen Exposures
 This webinar begins at 11 a.m., Eastern. You will not hear anything over your telephone line until the program starts. If the system did not prompt you to enter your phone number and receive a call back,
This webinar begins at 11 a.m., Eastern. You will not hear anything over your telephone line until the program starts. If the system did not prompt you to enter your phone number and receive a call back,
Colgate University. Bloodborne Pathogens Exposure Control Plan
 Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
Colgate University Bloodborne Pathogens Exposure Control Plan COLGATE UNIVERSITY BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN I. STATEMENT OF POLICY It is the policy of Colgate University (CU) to limit or
Bloodborne Pathogens. Post-Exposure Incident Packet. An Informational Guide
 Bloodborne Pathogens Post-Exposure Incident Packet An Informational Guide Faribault Public Schools Bloodborne Pathogens Post-Exposure Incident Packet This packet has been developed as an informational
Bloodborne Pathogens Post-Exposure Incident Packet An Informational Guide Faribault Public Schools Bloodborne Pathogens Post-Exposure Incident Packet This packet has been developed as an informational
Occupational Exposures to Bloodborne Pathogen (BBP) Training
 Occupational Exposures to Bloodborne Pathogen (BBP) Training OSHA 29 CFR 1910.1030 Protects workers exposed to blood or other potentially infectious diseases Who are at Risk? Workers in many different
Occupational Exposures to Bloodborne Pathogen (BBP) Training OSHA 29 CFR 1910.1030 Protects workers exposed to blood or other potentially infectious diseases Who are at Risk? Workers in many different
Laboratory Exposure Control Plan WAC
 A. Introduction Because laboratories and classrooms working directly with blood or other potentially infectious materials have different bloodborne pathogen exposure concerns than the general university,
A. Introduction Because laboratories and classrooms working directly with blood or other potentially infectious materials have different bloodborne pathogen exposure concerns than the general university,
SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison
 SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
SIV/SHIV SIV/SHIV Exposure Medical Response Guidance for the University of Wisconsin-Madison 1.0 Instructions: Information in this guidance is meant to inform both laboratory staff and health professionals
Definitions. Appendix A
 Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
Definitions Appendix A 1. Blood means human blood, human blood components, and products made from human blood. 2. Bloodborne Pathogens means pathogenic microorganisms that are present in human blood and
Introduction to Blood Borne Pathogens
 Introduction to Blood Borne Pathogens What are blood pathogens? Any infectious microorganism in the human blood that can cause disease is a Blood borne pathogen. Three of these pathogens include hepatitis
Introduction to Blood Borne Pathogens What are blood pathogens? Any infectious microorganism in the human blood that can cause disease is a Blood borne pathogen. Three of these pathogens include hepatitis
Bloodborne Pathogens Training
 Bloodborne Pathogens Training OSHA S Bloodborne Pathogen Standard 29CFR 1910.1030 Employers must: Develop an Exposure Control Plan (ECP) that details their Bloodborne Pathogens (BBP) Program Provide employees
Bloodborne Pathogens Training OSHA S Bloodborne Pathogen Standard 29CFR 1910.1030 Employers must: Develop an Exposure Control Plan (ECP) that details their Bloodborne Pathogens (BBP) Program Provide employees
PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1
 PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1 RELATED POLICY: 5.21REV PAGE NO.: 1 OF 9 RESPONSIBLE ADMINISTRATOR(S): VPF&A/EHS EFECTIVE DATE: 07/11/14 NEXT REVIEW DATE:
PROCEDURE TITLE: BLOOD BORNE PATHOGENS EXPOSURE CONTROL PLAN PROCEDURE NO.: 5.21:1 RELATED POLICY: 5.21REV PAGE NO.: 1 OF 9 RESPONSIBLE ADMINISTRATOR(S): VPF&A/EHS EFECTIVE DATE: 07/11/14 NEXT REVIEW DATE:
University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: F:
 University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: 502-852-2708 F: 502-852-0660 hlthoff@louisville.edu Training Objectives Understand the OSHA Bloodborne Pathogens Regulation
University of Louisville Campus Health Services 401 East Chestnut Street Suite110 P: 502-852-2708 F: 502-852-0660 hlthoff@louisville.edu Training Objectives Understand the OSHA Bloodborne Pathogens Regulation
Bloodborne Pathogens and Exposure Control
 Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
Bloodborne Pathogens and Exposure Control 2016 Information in the Exposure Control Plan The Bloodborne Pathogen Exposure Control Plan was developed to communicate information to you about: - Your risk
NEOMED ACADEMIC POLICY
 (A) PURPOSE The purpose of this Policy is to delineate the management of incidents of exposure to blood-borne pathogens that occur to students while they are in the educational setting. (B) SCOPE This
(A) PURPOSE The purpose of this Policy is to delineate the management of incidents of exposure to blood-borne pathogens that occur to students while they are in the educational setting. (B) SCOPE This
Blood Borne Pathogens (BBP)
 Blood Borne Pathogens (BBP) Healthcare facilities are high-risk areas for exposure to bloodborne pathogens, so protect yourself and remind others to do the same. There are three bloodborne pathogens of
Blood Borne Pathogens (BBP) Healthcare facilities are high-risk areas for exposure to bloodborne pathogens, so protect yourself and remind others to do the same. There are three bloodborne pathogens of
BAY-ARENAC BEHAVIORAL HEALTH AUTHORITY POLICIES AND PROCEDURES MANUAL
 Page: 1 of 8 Policy Bay-Arenac Behavioral Health Authority (BABHA) is committed to high standards of employee and consumer safety practices. This standard will include the prevention, surveillance, identification
Page: 1 of 8 Policy Bay-Arenac Behavioral Health Authority (BABHA) is committed to high standards of employee and consumer safety practices. This standard will include the prevention, surveillance, identification
Contamination Incidents Frequently Asked Questions
 Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
Contamination Incidents Frequently Asked Questions WWR- 004 Index 1. What is a contamination injury? 2. What should I do immediately if I have sustained a contamination injury? 3. What should I do immediately
You will now begin the Bloodborne Pathogen Refresher Training.
 You will now begin the Bloodborne Pathogen Refresher Training. The following program will review your occupational risks and the steps that you and your Client must take to reduce your risks of exposure.
You will now begin the Bloodborne Pathogen Refresher Training. The following program will review your occupational risks and the steps that you and your Client must take to reduce your risks of exposure.
HIV and PEP. LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED
 HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
HIV and PEP LTC Rose Ressner WRNMMC ID staff Oct 2014 UNCLASSIFIED Disclaimer The views expressed in this presentation are those of the speaker and do not reflect the official policy of the Department
Bloodborne Pathogens and Universal Precautions
 Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
Bloodborne Pathogens and Universal Precautions Parkway School District 2012-2013 Revised 9/19/2012 What Are Bloodborne Pathogens(BBPs) Bloodborne pathogens (BBPs) are disease causing microorganisms carried
Drew University Bloodborne Pathogens Exposure Control Plan and Procedures
 PURPOSE To provide a written plan for preventing and/or minimizing exposure to bloodborne pathogens for those Drew University personnel who may be involved in the handling of human blood, blood products,
PURPOSE To provide a written plan for preventing and/or minimizing exposure to bloodborne pathogens for those Drew University personnel who may be involved in the handling of human blood, blood products,
Healthcare Professionals
 EMPLOYEE HEALTH Employee Health: Screening and immunization programs Counseling, follow up work restrictions Analysis and trending of occupational exposure incidents Assess risk for occupational exposure
EMPLOYEE HEALTH Employee Health: Screening and immunization programs Counseling, follow up work restrictions Analysis and trending of occupational exposure incidents Assess risk for occupational exposure
Goldenrod Hills Community Action. Bloodborne Pathogen (BBP) Training according to OSHA Standard 29 CFR
 Goldenrod Hills Community Action Bloodborne Pathogen (BBP) Training according to OSHA Standard 29 CFR 1910.1030 Welcome to GHCA s Bloodborne Pathogen Training based upon the Occupational Safety and Health
Goldenrod Hills Community Action Bloodborne Pathogen (BBP) Training according to OSHA Standard 29 CFR 1910.1030 Welcome to GHCA s Bloodborne Pathogen Training based upon the Occupational Safety and Health
Revised April WGH ER #5 Hospital Rd. Whitehorse Phone: (867) Outside of Whitehorse: Local Community Health Centre/Clinic/ER
 Where to access care Who to consult and when Please consult whenever questions arise on how to proceed with postexposure management and when exposure is beyond the scope of guidelines. Blood and Body Fluid
Where to access care Who to consult and when Please consult whenever questions arise on how to proceed with postexposure management and when exposure is beyond the scope of guidelines. Blood and Body Fluid
2017 BLOODBORNE PATHOGENS
 2017 BLOODBORNE PATHOGENS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE To have a basic understanding of bloodborne pathogens and the role of Greenwood School District 50 and OSHA.
2017 BLOODBORNE PATHOGENS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE To have a basic understanding of bloodborne pathogens and the role of Greenwood School District 50 and OSHA.
Greenwood School District 50 OSHA UPDATE BLOODBORNE PATHOGENS
 Greenwood School District 50 OSHA UPDATE 2012 BLOODBORNE PATHOGENS TOPICS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE OBJECTIVES To have a basic understanding of bloodborne pathogens
Greenwood School District 50 OSHA UPDATE 2012 BLOODBORNE PATHOGENS TOPICS OSHA TERMS UPDATES HEPATITIS B HEPATITIS C HIV REPORTING AN EXPOSURE OBJECTIVES To have a basic understanding of bloodborne pathogens
Central Franciscan Health Rensselaer Blood and Body Fluid Exposures Procedure COPY
 Current Status: Active PolicyStat ID: 2625043 Original: 7/18/2011 Last Reviewed: 8/19/2016 Last Revised: 8/19/2016 Next Review: 8/19/2019 Responsible Party: Kristin Deno: Manager Policy Area: References:
Current Status: Active PolicyStat ID: 2625043 Original: 7/18/2011 Last Reviewed: 8/19/2016 Last Revised: 8/19/2016 Next Review: 8/19/2019 Responsible Party: Kristin Deno: Manager Policy Area: References:
QUALITY LIFE CONCEPTS Policy on Bloodborne Pathogens
 QUALITY LIFE CONCEPTS Policy on Bloodborne Pathogens A24 TUBERCULOSIS TESTING Quality Life Concepts is committed to protecting its employees and individuals who will be served by vigilance and regular
QUALITY LIFE CONCEPTS Policy on Bloodborne Pathogens A24 TUBERCULOSIS TESTING Quality Life Concepts is committed to protecting its employees and individuals who will be served by vigilance and regular
FULL-TIME ADULT STUDENT Acceptance Package Phase II
 Revised 6/2013 FULL-TIME ADULT STUDENT Acceptance Package Phase II THE FOLLOWING FORMS ARE NOT TO BE COMPLETED AND RETURNED UNLESS YOU ARE ACCEPTED INTO A PROGRAM Connecticut Technical High School System
Revised 6/2013 FULL-TIME ADULT STUDENT Acceptance Package Phase II THE FOLLOWING FORMS ARE NOT TO BE COMPLETED AND RETURNED UNLESS YOU ARE ACCEPTED INTO A PROGRAM Connecticut Technical High School System
Bloodborne Pathogen Module. Chelmsford Public Schools September, 2016
 Bloodborne Pathogen Module Chelmsford Public Schools September, 2016 Why is this important? v OSHA BB Pathogen Standard anyone whose job requires exposure to BB pathogens is required to complete training
Bloodborne Pathogen Module Chelmsford Public Schools September, 2016 Why is this important? v OSHA BB Pathogen Standard anyone whose job requires exposure to BB pathogens is required to complete training
One of your office personnel
 Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
Doug Campos-Outcalt, MD, MPA Department of Family and Community Medicine, University of Arizona College of Medicine, Phoenix HIV postexposure prophylaxis: Who should get it? CORRESPONDENCE Doug Campos-Outcalt,
Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV
 Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV By Dr. Sinan Butrus F.I.C.M.S Clinical Standards & Guidelines Kurdistan Board For Medical Specialties An exposure that might place health
Guidelines for Post- Exposure Prophylaxis for HAV, HBV, HCV & HIV By Dr. Sinan Butrus F.I.C.M.S Clinical Standards & Guidelines Kurdistan Board For Medical Specialties An exposure that might place health
An International Antiviral Society-USA
 Doug Campos-Outcalt, MD, MPA University of Arizona, Phoenix dougco@email.arizona. edu A look at new guidelines for HIV treatment and prevention Start antiretroviral therapy as soon as possible after HIV
Doug Campos-Outcalt, MD, MPA University of Arizona, Phoenix dougco@email.arizona. edu A look at new guidelines for HIV treatment and prevention Start antiretroviral therapy as soon as possible after HIV
Bloodborne Pathogens. By Deborah Massarella RN, MSN
 Bloodborne Pathogens By Deborah Massarella RN, MSN 2010, 2014 Objectives At conclusion of in-service learners will: 1. Identify two types of personal protective equipment. 2. Identify two prevention measures
Bloodborne Pathogens By Deborah Massarella RN, MSN 2010, 2014 Objectives At conclusion of in-service learners will: 1. Identify two types of personal protective equipment. 2. Identify two prevention measures
Needle Stick/Blood Borne Pathogen Exposure Guidelines
 Needle Stick/Blood Borne Pathogen Exposure Guidelines Purpose: To outline the expected behavior to be followed by all student pharmacists who have received an accidental exposure incident while in an educational
Needle Stick/Blood Borne Pathogen Exposure Guidelines Purpose: To outline the expected behavior to be followed by all student pharmacists who have received an accidental exposure incident while in an educational
Exposure. Blood. Department of Health & Human Services
 Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
Exposure to Blood What Health-Care Workers Need to Know Department of Health & Human Services OCCUPATIONAL EXPOSURES TO BLOOD Introduction Health-care workers are at risk for occupational exposure to bloodborne
Emergency Department Blood/Body Fluid Exposure Instructions
 Emergency Department Blood/Body Fluid Exposure Instructions For employees sent to the Emergency Department after hours to be evaluated for needle stick / body fluid exposure 1. Floor or unit Charge RN
Emergency Department Blood/Body Fluid Exposure Instructions For employees sent to the Emergency Department after hours to be evaluated for needle stick / body fluid exposure 1. Floor or unit Charge RN
EMPLOYEE HEALTH TABLE OF CONTENTS
 EMPLOYEE HEALTH TABLE OF CONTENTS PHYSICAL ASSESSMENT.2 REPORTING EMPLOYEE INFECTIONS...3 SUMMARY OF IMPORTANT RECOMMENDATIONS AND WORK RESTRICTIONS FOR PERSONNEL WITH INFECTIOUS DISEASES.4 HEPATITIS B
EMPLOYEE HEALTH TABLE OF CONTENTS PHYSICAL ASSESSMENT.2 REPORTING EMPLOYEE INFECTIONS...3 SUMMARY OF IMPORTANT RECOMMENDATIONS AND WORK RESTRICTIONS FOR PERSONNEL WITH INFECTIOUS DISEASES.4 HEPATITIS B
Infection Prevention and Control - General Orientation
 Infection Prevention and Control - General Orientation Hand Hygiene-CDC Isolation Precautions - CDC Medical Waste - OSHA Environmental Cleaning - CDC Safe Injection Practices - CDC Bloodborne Pathogens
Infection Prevention and Control - General Orientation Hand Hygiene-CDC Isolation Precautions - CDC Medical Waste - OSHA Environmental Cleaning - CDC Safe Injection Practices - CDC Bloodborne Pathogens
ADMINISTRATIVE SERVICES MANUAL
 1 of 10 Purpose Scope University of Alaska Anchorage departments will develop plans and procedures to limit occupational exposure to blood and other potentially infectious materials (PIM) in compliance
1 of 10 Purpose Scope University of Alaska Anchorage departments will develop plans and procedures to limit occupational exposure to blood and other potentially infectious materials (PIM) in compliance
SUBJECT: Management of Human Body Fluids/Waste (Bloodborne Pathogens)
 Page 1 of 6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 PURPOSE To establish uniform procedures for the safe management of human body fluids
Page 1 of 6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 PURPOSE To establish uniform procedures for the safe management of human body fluids
Dare County Schools. Bloodborne Pathogens Exposure Control Plan
 Dare County Schools Bloodborne Pathogens Exposure Control Plan 2017 Dare County Schools Bloodborne Pathogens Exposure Control Page 1 of 12 Dare County Schools Bloodborne Pathogen Program Purpose An infection
Dare County Schools Bloodborne Pathogens Exposure Control Plan 2017 Dare County Schools Bloodborne Pathogens Exposure Control Page 1 of 12 Dare County Schools Bloodborne Pathogen Program Purpose An infection
City of Montpelier, Vermont The Smallest Capital City in the United States BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN AND PROCEDURES
 City of Montpelier, Vermont The Smallest Capital City in the United States BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN AND PROCEDURES Last Updated June 19, 2003 1 Bloodborne Pathogens Exposure Control Plan
City of Montpelier, Vermont The Smallest Capital City in the United States BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN AND PROCEDURES Last Updated June 19, 2003 1 Bloodborne Pathogens Exposure Control Plan
BLOOD-BORNE PATHOGENS EXPOSURE PROTOCOL ON-CAMPUS LABORATORY EXPERIENCES STUDENT PROCEDURES
 BLOOD-BORNE PATHOGENS EXPOSURE PROTOCOL ON-CAMPUS LABORATORY EXPERIENCES STUDENT PROCEDURES MARCH 2015 STUDENT COMPLIANCE OFFICE 651.690.7781 Page 1 of 9 Page 2 of 9 The following is the student responsibilities
BLOOD-BORNE PATHOGENS EXPOSURE PROTOCOL ON-CAMPUS LABORATORY EXPERIENCES STUDENT PROCEDURES MARCH 2015 STUDENT COMPLIANCE OFFICE 651.690.7781 Page 1 of 9 Page 2 of 9 The following is the student responsibilities
(cf / / Exposure Control Plan for Bloodborne Pathogens)
 All Personnel BP 4119.43 UNIVERSAL PRECAUTIONS 4319.43 In order to protect employees from contact with potentially infectious blood or other body fluids, the Governing Board requires that universal precautions
All Personnel BP 4119.43 UNIVERSAL PRECAUTIONS 4319.43 In order to protect employees from contact with potentially infectious blood or other body fluids, the Governing Board requires that universal precautions
A. Background for Trainer: B. What OSHA Requires: Bloodborne Pathogens. Lesson Plan 6080a
 Lesson Plan 6080a This training session outline is designed to follow the accompanying booklet, OSHA s Bloodborne Pathogens Standard. The booklet reviews what employees who are potentially exposed to the
Lesson Plan 6080a This training session outline is designed to follow the accompanying booklet, OSHA s Bloodborne Pathogens Standard. The booklet reviews what employees who are potentially exposed to the
Pre and Post Exposure Prophylaxis. Laila AL Dabal MD FRCP MSc Infectious Diseases Unit Dubai Health Authority 15 March 2018
 Pre and Post Exposure Prophylaxis Laila AL Dabal MD FRCP MSc Infectious Diseases Unit Dubai Health Authority 15 March 2018 Indications for Pre and Post Exposure Prophylaxis: occupational and non-occupational
Pre and Post Exposure Prophylaxis Laila AL Dabal MD FRCP MSc Infectious Diseases Unit Dubai Health Authority 15 March 2018 Indications for Pre and Post Exposure Prophylaxis: occupational and non-occupational
Communicable Diseases
 Policy 1016 Elk Grove Police Department 1016.1 PURPOSE AND SCOPE This policy provides general guidelines to assist in minimizing the risk of department members contracting and/or spreading communicable
Policy 1016 Elk Grove Police Department 1016.1 PURPOSE AND SCOPE This policy provides general guidelines to assist in minimizing the risk of department members contracting and/or spreading communicable
The Bloodborne Pathogen Standard. An Overview
 The Bloodborne Pathogen Standard An Overview The Standard l In 1990, OSHA (Occupational Safety and Health Administration), developed the Bloodborne Pathogen Standard to protect workers by limiting occupational
The Bloodborne Pathogen Standard An Overview The Standard l In 1990, OSHA (Occupational Safety and Health Administration), developed the Bloodborne Pathogen Standard to protect workers by limiting occupational
Bloodborne Pathogens: Recognition and Treatment 2011
 Bloodborne Pathogens: Recognition and Treatment 2011 Occupational Health Services at The Summit Rita M. Lopez, APRN-BC, MSN 751-4189 rlopez@krmc.org OBJECTIVES DEFINE Bloodborne Pathogen Exposure WHO must
Bloodborne Pathogens: Recognition and Treatment 2011 Occupational Health Services at The Summit Rita M. Lopez, APRN-BC, MSN 751-4189 rlopez@krmc.org OBJECTIVES DEFINE Bloodborne Pathogen Exposure WHO must
BLOODBORNEPATHOGENS. CAP Safety Meetings. Revision: CAP Safety Meetings [Bloodborne Pathogens]
![BLOODBORNEPATHOGENS. CAP Safety Meetings. Revision: CAP Safety Meetings [Bloodborne Pathogens] BLOODBORNEPATHOGENS. CAP Safety Meetings. Revision: CAP Safety Meetings [Bloodborne Pathogens]](/thumbs/73/68341667.jpg) BLOODBORNEPATHOGENS CAP Safety Meetings Revision: 10-2011 2011 Copyright - PEC/Premier Safety Management, Inc. All Rights Reserved Revision: [10-2011] 1 THEBLOODBORNEPATHOGENSSTANDARD The Bloodborne Pathogens
BLOODBORNEPATHOGENS CAP Safety Meetings Revision: 10-2011 2011 Copyright - PEC/Premier Safety Management, Inc. All Rights Reserved Revision: [10-2011] 1 THEBLOODBORNEPATHOGENSSTANDARD The Bloodborne Pathogens
Bloodborne Pathogens LVHN s Annual Safety Course
 Slide 1 Bloodborne Pathogens LVHN s Annual Safety Course Nucleus Medical Media (2010). Blood Carries Nutrients. Smart Imagebase. Lehigh Valley Health Network cares about your safety especially about protecting
Slide 1 Bloodborne Pathogens LVHN s Annual Safety Course Nucleus Medical Media (2010). Blood Carries Nutrients. Smart Imagebase. Lehigh Valley Health Network cares about your safety especially about protecting
Code: GBGAA-R Amended: 08/09/10 Amended: 08/06/18 HERMON SCHOOL DEPARTMENT BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN
 Code: GBGAA-R Amended: 08/09/10 Amended: 08/06/18 HERMON SCHOOL DEPARTMENT BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN April 2018 Table of Contents Exposure Determination... 3 Hepatitis B Vaccine... 4 Universal
Code: GBGAA-R Amended: 08/09/10 Amended: 08/06/18 HERMON SCHOOL DEPARTMENT BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN April 2018 Table of Contents Exposure Determination... 3 Hepatitis B Vaccine... 4 Universal
Bloodborne Pathogens Training. IEA, Inc.
 Bloodborne Pathogens Training IEA, Inc. Review the potential hazard of exposure to blood and other potentially infectious materials (OPIMs). Review safe work practices to prevent occupational exposure
Bloodborne Pathogens Training IEA, Inc. Review the potential hazard of exposure to blood and other potentially infectious materials (OPIMs). Review safe work practices to prevent occupational exposure
2014 OSHA Blood-borne Pathogens (BBP) Update JHS Annual Mandatory Education
 2014 OSHA Blood-borne Pathogens (BBP) Update 2014 JHS Annual Mandatory Education Objectives Discuss the epidemiology of Bloodborne Pathogens. List the statistics of HIV/AIDS cases Identify the correlation
2014 OSHA Blood-borne Pathogens (BBP) Update 2014 JHS Annual Mandatory Education Objectives Discuss the epidemiology of Bloodborne Pathogens. List the statistics of HIV/AIDS cases Identify the correlation
BLOODBORNE PATHOGEN PROGRAM (OHS-0005) For
 () For Issued: July 2006 PAGE 1 OF 1 Table of Contents 1.0 Purpose...2 2.0 Document Control...2 2.1 Approvals...2 2.2 Responsibility...2 3.0 Definitions...3 4.0 Objective...7 5.0 Scope...8 6.0 Responsibilities...8
() For Issued: July 2006 PAGE 1 OF 1 Table of Contents 1.0 Purpose...2 2.0 Document Control...2 2.1 Approvals...2 2.2 Responsibility...2 3.0 Definitions...3 4.0 Objective...7 5.0 Scope...8 6.0 Responsibilities...8
Training for Employees of Taylor Special Care Services, Inc.
 Training for Employees of Taylor Special Care Services, Inc. TSCS Taylor Special Care Services housing staffing counseling on-going support Simon Pop, MBA Chief Operating Officer 2015 2016 Guidelines:
Training for Employees of Taylor Special Care Services, Inc. TSCS Taylor Special Care Services housing staffing counseling on-going support Simon Pop, MBA Chief Operating Officer 2015 2016 Guidelines:
School of Nursing. Bloodborne Pathogens Exposure Control Plan BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN
 DEPAUL UNIVERSITY S DePaul University School of Nursing Bloodborne Pathogens Exposure Control Plan BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN IF YOU ARE READING THIS PLAN BECAUSE YOU HAVE BEEN EXPOSED
DEPAUL UNIVERSITY S DePaul University School of Nursing Bloodborne Pathogens Exposure Control Plan BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN IF YOU ARE READING THIS PLAN BECAUSE YOU HAVE BEEN EXPOSED
Blood-Borne Pathogens and Post-Exposure Prophylaxis
 Blood-Borne Pathogens and Post-Exposure Prophylaxis Christopher Behrens MD Northwest Association of Occupational and Environmental Medicine October 2017 with thanks to Shireesha Dhanireddy MD Disclosures
Blood-Borne Pathogens and Post-Exposure Prophylaxis Christopher Behrens MD Northwest Association of Occupational and Environmental Medicine October 2017 with thanks to Shireesha Dhanireddy MD Disclosures
Bloodborne Pathogens. Kathleen Stefek, RN, MSN
 Bloodborne Pathogens Kathleen Stefek, RN, MSN What are Bloodborne Pathogens? Infectious agents carried in the blood and other body fluids that are capable of infecting a host (people like you and me) with
Bloodborne Pathogens Kathleen Stefek, RN, MSN What are Bloodborne Pathogens? Infectious agents carried in the blood and other body fluids that are capable of infecting a host (people like you and me) with
43. Guidelines on Needle stick Injury
 43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
43. Guidelines on Needle stick Injury The following information is abstracted from the South African Department of Health guidelines entitled: Management of Occupational Exposure to the Human Immunodeficiency
Bloodborne Pathogens
 Bloodborne Pathogens Disclaimer This training material presents very important information. Your organization must do an evaluation of all exposures, applicable codes and regulations, and establish proper
Bloodborne Pathogens Disclaimer This training material presents very important information. Your organization must do an evaluation of all exposures, applicable codes and regulations, and establish proper
Infectious Disease and Bloodborne Pathogens Training St. Michael-Albertville Public Schools
 Infectious Disease and Bloodborne Pathogens Training St. Michael-Albertville Public Schools Questions? Any time throughout the slide show or throughout the school year: Contact Jake Baxter at IEA Phone:
Infectious Disease and Bloodborne Pathogens Training St. Michael-Albertville Public Schools Questions? Any time throughout the slide show or throughout the school year: Contact Jake Baxter at IEA Phone:
BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN (ECP)
 Harvey Ingham 30 2804 Forest Ave Des Moines, IA 50311 515-271-3804 ehs@drake.edu www.drake.edu/ehs May 2016 BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN (ECP) PURPOSE This document serves as the written
Harvey Ingham 30 2804 Forest Ave Des Moines, IA 50311 515-271-3804 ehs@drake.edu www.drake.edu/ehs May 2016 BLOODBORNE PATHOGENS EXPOSURE CONTROL PLAN (ECP) PURPOSE This document serves as the written
The University of Texas at El Paso BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN
 The University of Texas at El Paso BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN Table of Contents SCOPE... 3 DEFINITIONS... 3 EXPOSURE DETERMINATION... 3 METHODS OF COMPLIANCE... 4 HEPATITIS B VACCINATION
The University of Texas at El Paso BLOODBORNE PATHOGEN EXPOSURE CONTROL PLAN Table of Contents SCOPE... 3 DEFINITIONS... 3 EXPOSURE DETERMINATION... 3 METHODS OF COMPLIANCE... 4 HEPATITIS B VACCINATION
Non-Occupational Post-Exposure HIV Prophylaxis npep
 Non-Occupational Post-Exposure HIV Prophylaxis npep Peter Meacher MD (Chief Medical Officer) Anthony Vavasis MD (Director of Medicine) Callen-Lorde Community Health Center Objectives - at the end of the
Non-Occupational Post-Exposure HIV Prophylaxis npep Peter Meacher MD (Chief Medical Officer) Anthony Vavasis MD (Director of Medicine) Callen-Lorde Community Health Center Objectives - at the end of the
OCCUPATIONAL HEALTH PROTOCOL
 Faculty of Medicine and Surgery OCCUPATIONAL HEALTH PROTOCOL (Applicable to applicants for the Doctor of Medicine and Surgery M.D. - Degree Course) Applicability for Courses commencing in October 2015
Faculty of Medicine and Surgery OCCUPATIONAL HEALTH PROTOCOL (Applicable to applicants for the Doctor of Medicine and Surgery M.D. - Degree Course) Applicability for Courses commencing in October 2015
