Pericytes (PCs) are specialized cells that are located around. Neural Crest Origin of Retinal and Choroidal Pericytes. Anatomy and Pathology
|
|
|
- Solomon Floyd
- 5 years ago
- Views:
Transcription
1 Anatomy and Pathology Neural Crest Origin of Retinal and Choroidal Pericytes Andrea Trost, 1 3 Falk Schroedl, 1,3,4 Simona Lange, 2,3 Francisco J. Rivera, 2,3,5 Herbert Tempfer, 3,6,7 Stefanie Korntner, 4,6 Claus C. Stolt, 8 Michael Wegner, 8 Barbara Bogner, 1,3 Alexandra Kaser-Eichberger, 1,3 Karolina Krefft, 1,3 Christian Runge, 1,3 Ludwig Aigner, 2,3 and Herbert A. Reitsamer 1,3 1 Ophthalmology/Optometry and Research Program for Experimental Ophthalmology, Paracelsus Medical University, Salzburg, Austria 2 Molecular Regenerative Medicine, Paracelsus Medical University, Salzburg, Austria 3 Spinal Cord Injury and Tissue Regeneration Center Salzburg, Paracelsus Medical University, Salzburg, Austria 4 Anatomy, Paracelsus Medical University, Salzburg, Austria 5 Wellcome Trust and MRC Cambridge Stem Cell Institute & Department of Veterinary Medicine, University of Cambridge, Cambridge, United Kingdom 6 Tendon and Bone Regeneration, Paracelsus Medical University, Salzburg, Austria 7 Department of Organismic Biology, University Salzburg, Salzburg, Austria 8 Institut für Biochemie, Emil-Fischer-Zentrum, Universität Erlangen-Nürnberg, Erlangen, Germany Correspondence: Herbert A. Reitsamer, Ophthalmology/Optometry and Research Program for Experimental Ophthalmology, Paracelsus Medical University, Müllner Hauptstrae 48, 5020 Salzburg, Austria; h.reitsamer@salk.at. Submitted: July 30, 2013 Accepted: November 4, 2013 Citation: Trost A, Schroedl F, Lange S, et al. Neural crest origin of retinal and choroidal pericytes. Invest Ophthalmol Vis Sci. 2013;54: DOI: /iovs PURPOSE. The origin of pericytes (PCs) has been controversially discussed and at least three different sources of PCs are proposed: a neural crest, mesodermal, or bone marrow origin. In the present study we investigated a potential neural crest origin of ocular PCs in a transgenic Rosa26-YFP-Sox10-Cre neural crest specific reporter mouse model at different developmental stages. METHODS. The Rosa26-YFP-Sox10-Cre mouse model expresses the yellow fluorescent protein (YFP) reporter in cells with an active Sox10 promoter and was here used for cell fate studies of Sox10-positive neural crest derived progeny cells. Detection of the YFP signal in combination with double and triple immunohistochemistry of chondroitin sulfate proteoglycan (NG2), platelet derived growth factor receptor b (PDGFRb), a smooth muscle actin (asma), oligodendrocyte transcription factor 2 (Olig2), and lectin was performed and analyzed by confocal microscopy. RESULTS. Sox10-YFP positive cells and profiles were detected in the inner nuclear layer, the ganglionic cell layer, and the axons of the nerve fiber layer in postnatal retinas. An additional population has been identified in the retina, optic nerve, and choroid that displays strong perivascular localization. These cells were colocalized with the PC-specific markers NG2 and PDGFRb in embryonic (E14.5) as well as postnatal (P4, P12, 6-week-old) vasculature. Beside PCs, vascular smooth muscle cells (vsmcs) were also labeled by the Sox10-YFP reporter protein in all ocular tissues investigated. CONCLUSIONS. Since YFP-positive PCs and vsmcs are colocalized with NG2 and PDGFRb, we propose that capillary PCs and vsmcs in the retina and the optic nerve, both parts of the central nervous system, as well as in the choroid, a tissue of mesodermal origin, derive from the neural crest. Keywords: pericytes, neural crest, Sox10-Cre-YFP mouse, retina, choroid Pericytes (PCs) are specialized cells that are located around blood vessels and have numerous important functions. 1 They are active in angiogenesis, 2 regulate endothelial cell proliferation, 3 and are part of the blood brain barrier. 4 In the eye, they represent the outer boundary of retinal capillaries and share a common basement membrane with endothelial cells. PCs play a key role in tissue homeostasis by stabilizing blood vessels, 5 participating in blood flow regulation, 6 and by mediating the formation of the blood retina barrier. 4,7 9 In addition, PCs possess multipotency (i.e., they are able to differentiate into other cell types) 1,10 ; therefore, they are a promising target for regenerative approaches. 11 The ontogeny of perivascular cells, including both PCs and vascular smooth muscle cells (vsmcs), was studied using fate mapping techniques in developing embryos, and diverse origins were revealed. For vsmcs, at least seven origins are described in vertebrate embryos, such as the neural crest or the somites (reviewed by Majesky 12 ). Whether PCs and microvascular SMCs are recruited from the same lineage as the SMCs of large arteries remains unclear. At least three sources of PCs have been described so far. Performing neural crest quail-chick transplantations, Etchevers et al. 13 showed that vessels with PCs of diencephalic and mesencephalic neural fold origin supply the forebrain, while vessels with PCs of mesodermal origin supply the rest of the Copyright 2013 The Association for Research in Vision and Ophthalmology, Inc. j ISSN:
2 Neural Crest Origin of Pericytes IOVS j December 2013 j Vol. 54 j No. 13 j 7911 central nervous system (CNS). Quail-chick transplantations of brain anlagen and mesoderm verified that dorsal and ventral neuroectoderm can differentiate into PCs and vsmcs of embryonic cerebral blood vessels. 14 Further, under the control of the forkhead box S1 (Foxs1) promoter, neural crest origin could only be proven for PCs located on vessels of the brain surface, 15 while a neural crest origin for PCs in the eye 16 has been claimed using a Wnt-1 mouse model. 17 Beside neural crest origin of PCs, there are also data indicating a bone marrow (BM) origin, at least during postnatal neoangiogenesis: BM-derived green fluorescent protein (GFP)- positive cells have been recruited to vascular endothelial growth factor induced and tumor-induced vessels, suggesting that progenitors of mural cells are mobilized from the BM. 18 In contrast, in GFP reporter mice under the control of the bone marrow specific stem cell antigen-1 promoter (sca-1), a vascular endothelial pattern of GFP expression was detected in the brain. 19 However, sca-1 positive cells were found to migrate to the angiogenic front and sites of vascular remodeling, suggesting that they contribute to PC recruitment of developing retinal capillaries. 9 As a third source of PC origin the mesoderm has been suggested. Using a XlacZ4 reporter under the control of an adipose tissue specific promoter (ap2), Tidhar et al. 20 demonstrated lacz staining in vsmcs and PCs throughout the vascular bed. They further described vsmcs in the retina and the choroid; however, they did not specifically comment on retinal PCs. Since the origin of PCs is controversially discussed in the literature, we investigated a potential neural crest origin of ocular PCs located in the retina and the optic nerve, both parts of the CNS, as well as in the choroid, a tissue of mesodermal origin. For that, we characterized ocular tissue of embryonic (E10.5, E14.5) and postnatal (P4, P12, and 6-week-old) transgenic Rosa26-yellow fluorescent protein (YFP)-Sox10-Cre mice 21 using immunohistochemistry. MATERIALS AND METHODS Transgenic Mice and Controls Rosa26-YFP-Sox10-Cre transgenic mice were kindly provided by M. Wegner and C. Stolt (Erlangen, Germany). Sox10-Cre mice 22 and Rosa-YFP reporter mice 23 were bred to generate the Sox10-Cre Rosa-YFP mice. 21 Wild-type littermates (Sox10- wt Rosa-wt) were used as negative controls. Since the Rosa26- YFP-Sox10-Cre mice are on a C3H background, they suffer from a degenerated photoreceptor layer starting about 8 days after birth. As a result, adult animals show intact ganglion cell and bipolar layers but virtually no photoreceptor layer. 24 Therefore, transgenic mice were analyzed at different developmental stages: embryonic day 10.5 (E10.5, n ¼ 2), E14.5 (n ¼ 2), postnatal day 4 (P4, n ¼ 4), and P12 (n ¼ 2) and at 6 weeks old (n ¼ 3). Age-matched wild-type controls were screened at E10.5, P12, and 6 weeks. Tissue Preparation Eyes of Rosa26-YFP-Sox10-Cre (strain C57/bl6) transgenic mice and age-matched wild-type controls were prepared for retinal whole mounts and cross sections followed by immunohistochemistry. For that, the mice were deeply anesthetized with an overdose of ketamine and xylazine (100 mg/kg bodyweight and 5 mg/kg bodyweight intraperitoneally) and perfused via the left ventricle with phosphate-buffered saline (PBS) containing 4% paraformaldehyde (PFA). Eyes were dissected free, opened along the ora serrata, and immersed in PBS containing 4% PFA for an additional hour at room temperature (RT). They were rinsed in PBS (24 48 hours) and transferred into PBS containing 15% sucrose (24 hours at 48C). Eyes were embedded in tissue embedding medium (NEG50, Fisher Scientific, Wien, Austria) and frozen at 808C using liquid nitrogen-cooled methylbutane. Tissue was stored at 208C until further processing. Retinal whole mounts and cross sections were prepared from Rosa26-YFP-Sox10-Cre mice at E.10.5, E14.5 (cross sections), P4 (n ¼ 4), P12 (n ¼ 2), and 6 weeks (n ¼ 3) and from age-matched wild-type littermates. All animal procedures were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Immunohistochemistry The retina was dissected free and prepared for cross sections and whole mounts. Cross Sections. Eye cups were mounted in a cryostat (HM 550; Microm, Walldorf, Germany), cross sections of 12 lm were collected on adhesion slides (Superfrost Plus; Thermo Scientific, Wien, Austria), and air-dried for 1 hour at RT. After a 5-minute rinse in tris-buffered saline (TBS; Roth, Karlsruhe, Germany) slides were incubated for 1 hour at RT in TBS containing 5% donkey serum (Sigma-Aldrich, Wien, Austria), 1% bovine serum albumin (BSA; Sigma-Aldrich), and 0.5% Triton X-100 (Merck, Darmstadt, Germany). After a 5- minute rinse, slides were incubated with antibodies (Table) for single, double, and triple staining experiments in TBS/ BSA/Triton X-100 overnight at RT. After a rinse in TBS (three times, 5 minutes) binding sites of primary antibodies were visualized by incubation of the corresponding AF488-, AF555-, and AF647-tagged antisera (Invitrogen, Karlsruhe, Germany; 1:1000 in TBS, containing 1% BSA and 0.5% Triton X-100) for 1 hour at RT. After another rinse in TBS (three times, 5 minutes), slides were incubated 10 minutes with 4 0,6-diamidino-2 phenylindol dihydrochloride (DAPI, 1:4000, stock 1 mg/ml; VWR, Vienna, Austria), rinsed three times 5 minutes in PBS and were embedded in TBS:glycerol (1:1 at ph 8.6). Retinal Whole Mounts. Retinal whole mounts were processed in eight-well chamber slides in 250-lL incubation volume. The immunohistochemical procedure was identical to that already described; however the incubation times were prolonged. Blocking was performed overnight at 48C, all washing steps were conducted for 60 minutes, and the incubation with the primary antibodies was performed for 3 days at 48C. Antisera incubation was overnight at 48C and DAPI incubation for 2 hours at RT. Documentation For documentation, a confocal laser scanning unit (LSM710; Zeiss, Göttingen, Germany; 320 dry or 340 and 360 oil immersion objective lenses, with numeric apertures 0.8, 1.30, and 1.4, respectively; Zeiss) was used. Sections were imaged using the appropriate filter settings for AF488 (499-nm excitation), dsred2 (563-nm excitation), AF647 (652-nm excitation), and DAPI (345-nm excitation) and up to four channels were detected simultaneously. RESULTS YFP Reporter Protein Expression in the Mouse Retina In retinal cross sections of Rosa26-YFP-Sox10-Cre transgenic mice, a bright fluorescent reporter positive signal (YFP) was
3 Neural Crest Origin of Pericytes IOVS j December 2013 j Vol. 54 j No. 13 j 7912 TABLE. Primary Antibodies Protein Abbreviation Source Company Dilution Cell Type Chondroitin sulfate proteoglycan NG2 Rabbit Millipore, Vienna, Austria; AB5320 1:400 Pericyte, oligodendrocytes in the optic nerve head, smooth muscle cell Platelet-derived growth factor PDGFRb Goat R&D, Abingdon, UK; AF1042 1:100 Pericyte receptor b a Smooth muscle actin asma Goat Abcam, Cambridge, UK; ab :200 Smooth muscle cell, Pericyte Lectin from Bandeiraea Lectin-biotin Sigma, Vienna, Austria; L3759 1:1000 Endothelial cells simplicifolia biotin conjugate Oligodendrocyte transcription Olig2 Rabbit Millipore, AB9610 1:200 Oligodendrocytes factor 2 Green fluorescent protein GFP Chicken Life Technologies, Vienna, Austria; no :500 YFP reporter transgene detected in cells with perivascular localization starting at E14.5 (Fig. 1B F, arrowheads). Although sporadic sprouts of the hyaloid artery were identified by lectin staining in E10.5 embryos, Z-stack analysis of confocal images revealed solely nuclei of endothelial cells, displaying no pericytes (Fig. 1A and inset), indicated by absence of a pericyte nucleus and YFP signal. Even though the developing hyaloid vessels were devoid of PCs by E10.5, YFP-positive PCs were detected on vessels surrounding ocular structures (data not shown). In E14.5 transgenic embryos a positive YFP signal was detected in PCs located at hyaloid vessels (Fig. 1B, 2C, arrowheads). In both E10.5 and E14.5 embryos, YFP-positive cells were absent in cells of the developing retina. Since the Rosa26-YFP-Sox10-Cre mice are bred on a C3H background, a known degeneration of the photoreceptor cells starts progressively at postnatal day 8 (P8). Retinal vascularization develops normally until P12; however, degeneration of the deep vasculature starts at P13. 25,26 To analyze the vasculature in intact as well as degenerated retinas we investigated P4, P12, and 6-week-old mice. YFP-positive perivascular cells were detected on regressing hyaloid (Fig. 1D, open arrowheads) and developing retinal vessels (Fig. 1D, arrowheads) in the eye of P4 mice. Unlike in the embryonic retina, YFP-positive cells were also detected in the ganglionic cell layer (GCL) of P4 retinas (Fig. 1D, arrows), indicating a non neural crest activation of the YFP reporter via Sox10 promoter activity at a later time point in development. At P12, YFP-positive perivascular cells were detected in the superficial (Fig. 1E, arrowheads) as well as in the deep capillary plexus (Fig. 1E, open arrowheads), and additionally a bright YFP signal was detected in cells and profiles of the inner nuclear layer (INL; Fig. 1E, open arrows), the GCL, and axons of the nerve fiber layer (NFL; Fig. 1E, arrows). As in P4 and P12 Rosa26-YFP-Sox10-Cre mice, YFPpositive signals were detected in perivascular cells (Fig. 1F, arrowhead) as well as in cells of the INL (Fig. 1F, open arrows), and GCL (Fig. 1F, arrows) of the 6-week-old retina. It was not possible to evaluate the YFP signals in the outer nuclear layer (ONL) of the 6-week-old mice, since the photoreceptor layer in transgenic mice and littermates was degenerated due to the C3H background. In wild-type littermates no YFP signal was detectable (data not shown). Neural Crest Origin of Retinal PCs Retinal whole mount preparations displayed a distinct YFP labeling of perivascular cells in the superficial capillary plexus (Fig. 2A, 2C, 2E, arrowheads) in P4, P12, and 6-week-old mice, and furthermore in the deep capillary plexus of P12 mice. Again these Sox10-driven YFP structures were not detectable in the wild-type littermates (data not shown). Colocalization experiments with chondroitin sulfate proteoglycan (NG2), 27 specifically labeling PCs and vsmcs, revealed an almost total overlap with the YFP-positive perivascular cells (Fig. 2B, 2D, 2F, arrowheads). In subsequent triple labeling experiments these NG2- and YFP-positive retinal perivascular cells revealed also a colocalization with the PC-specific marker platelet-derived growth factor receptor b (PDGFRb; Fig. 3B, 3D, 3F, arrowheads). 28,29 While the majority of retinal PCs were positive for the Sox10-driven YFP reporter protein, a subset of PCs was devoid of Sox10-YFP reporter expression (Fig. 4A D, open arrowheads), suggesting that at least one additional cell lineage may contribute to PCs in the retina. Lectin labeling of endothelial cells (Fig. 4D, arrows) revealed no colocalization with YFP expression, excluding their neural crest origin. Neural Crest Reporter Expression in Retinal vsmcs In the 6-week-old mice, a smooth muscle actin (asma)-positive arteriolar smooth muscle cells (Fig. 5A) showed also a complete overlap with YFP-positive cells (Fig. 5B, 5C). While asma immunopositivity was observed in vessels > 10 lm, it was absent in perivascular cells covering capillaries (Fig. 5D, arrows). These cells represent YFP-positive capillary PCs, colocalizing also for NG2 (Fig. 5E, 5F, arrows). However, despite continuous asma labeling of retinal arteries and arterioles, YFP-negative segments were observed in a single animal (Fig. 5G I, arrowheads). This indicates that some vsmcs have a different origin than neural crest (Fig. 5I, merged picture). Neural Crest Origin of PCs in the Choroid and Optic Nerve In the choroid YFP-positive perivascular cells displayed colocalization with the PC markers NG2 and PDGFRb (Fig. 6A, 6B, arrowheads). When YFP-positive cells in the optic nerve were analyzed, these were immunoreactive for PDGFRb (Fig. 6D, arrowheads), thus representing neural crest derived PCs. Combining NG2 and oligodendrocyte transcription factor 2 (Olig2) 30 labeling, PCs could be differentiated from oligodendrocytes because they were devoid of Olig2 immunoreactivity. Olig2-positive oligodendrocytes were located posterior to the lamina cribrosa, showing nearly complete colocalization with YFP expression (Fig. 6E, 6F, arrows). DISCUSSION The neural crest harbors a population of multipotent cells that arises at the border of the nonneural ectoderm and the neural plate. 31,32 Mouse neural crest migration is initiated before the
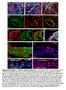
4 Neural Crest Origin of Pericytes IOVS j December 2013 j Vol. 54 j No. 13 j 7913 FIGURE 1. Analysis of YFP reporter protein expression at the developmental stages: E10.5, E14.5, P4, P12, and 6 weeks in the Rosa26-YFP-Sox10- Cre transgenic mice (SOX10-Cre-YFP): (A) YFP-positive cells were absent in the E10.5 retina. Per confocal visual field, only one to two sprouting hyaloid vessels were detectable by lectin immunoreactivity (red, boxed area). Note that these hyaloid vessels are not covered by pericytes at that time point, as indicated by absence of a pericyte nucleus and YFP signal. (B, C) At E14.5, the retina was again devoid of YFP-positive signals, whereas hyaloid vessels displayed YFP-positive pericytes (green). (C) Magnification of boxed area in (B): YFP-positive pericytes (green, arrowheads) attached to the hyaloids vessels were detected in this cross section. (D) P4 transgenic mice displayed YFP-positive PCs (green) in the developing retinal vasculature (arrowheads) and on regressing hyaloid vessel (open arrowheads; inset: magnification of the boxed area in D). Additionally YFP-positive signals were detected in cells of the GCL (arrows). (E) At P12, YFP-positive perivascular cells (green) were detected in the deep (open arrowheads) and superficial capillary plexus (arrowheads) of the retina. Additionally YFP-positive signals were detected in cells of the inner nuclear layer (INL; open arrows) and the ganglion cell layer (GCL; arrows). (F) In 6-week-old mice YFP-positive signals were detected in retinal perivascular cells (arrowhead) as wells as cells of the INL (open arrows) and GCL (arrows).
5 Neural Crest Origin of Pericytes IOVS j December 2013 j Vol. 54 j No. 13 j 7914 FIGURE 2. Retinal whole mount preparations of P4, P12, and 6-week-old transgenic mice. (A) A distinct YFP-positive labeling of perivascular cells (green, arrowheads) was detectable in the superficial capillary plexus at P4, resembling the GCL. (B) These YFP-positive perivascular cells were colocalized with the PC-specific marker NG2 (white, arrowheads). (C) Whole mount preparations of P12 retinas show YFP-positive PCs (green, arrowheads) in the superficial capillary plexus. (D) These PCs were colocalized with the PC-specific markers NG2 (white, arrowheads). (E) Perivascular cells in 6-week-old mice displayed YFP labeling (green, arrowheads) identical to P4 and P12. (F) YFP-positive PCs were colocalized with NG2 (white, arrowheads), confirming their pericyte identity. (A F) Additional YFP signals were detected in retinal ganglion cells (open arrows) for all ages shown here. neural tube is fully closed. 33 After neural crest induction, gene activation is performed by several transcription factors limited to the neural crest. Among these, the expression of the highmobility group (HMG) box-containing genes Sox8, Sox9, and Sox10 has been reported. 31 To investigate a putative neural crest origin of ocular perivascular cells, we used the Rosa26- YFP-Sox10-Cre transgenic mouse model, 21 in which neural crest derived cells are labeled via the expression of a Sox10 promoter mediated YFP reporter. With this work we propose that ocular perivascular cells, including PCs and vsmcs in both the retina and choroid, originate from the neural crest.
6 Neural Crest Origin of Pericytes IOVS j December 2013 j Vol. 54 j No. 13 j 7915 FIGURE 3. YFP-positive PCs (green, arrowheads) in the GCL at different developmental stages: (A) P4, (C) P12, and (E) 6 weeks. Subsequent multilabeling experiments (B, D, F) revealed a colocalization with the PC-specific marker NG2 (white) and PDGFRb (red); arrows here point towards endothelial cell nuclei (blue, DAPI). Endothelial cells are easily identified by the lack of NG2 and PDGFRb, as well as their elongated nuclei located at the luminal side of the capillaries. Note that nuclei of retinal ganglion cells likewise express the reporter protein YFP (open arrows). Reliability of the Sox10-Cre-YFP Reporter as a Neural Crest Marker SOX10 is a member of the SOX gene family, by homology related to the HMG box region of the testis-determining gene SRY and is described as one of the most general markers of early neural crest cells. 34 In mice and rats, Sox10 seems to be selectively expressed during early stages of development; in glial cells of the periphery and central nervous system Sox10 is expressed during maturation and adulthood. While Ferguson and Graham 35 described a strong Sox10 expression in the entire postmigratory postotic neural crest population during early chick embryonic development, they denied a mesodermal origin. Further, Bondurand et al. 36 verified Sox10 expression in human neural crest cells and derivatives that contribute to the formation of peripheral nervous system and later in the adult central nervous system. Mutations in the Sox10 gene have been identified in patients with Shah- Waardenburg syndrome, which affects melanocytes and
7 Neural Crest Origin of Pericytes IOVS j December 2013 j Vol. 54 j No. 13 j 7916 FIGURE 4. (A) The majority of perivascular cells in the GCL show YFP reporter expression (green, arrowheads), while a subpopulation of perivascular cells is lacking YFP expression (open arrowheads). (B) Although the majority of PCs, as identified by the expression of NG2 (white), show colocalization with YFP (arrowheads), a subpopulation was lacking YFP (open arrowheads). This arrangement indicates different PC origin for the YFP-negative population. (C) In retinal cross section, YFP-positive perivascular cells (green, arrowhead) are colocalized with the PC marker NG2 (D, white, arrowhead, inlay). Single NG2-positive PCs were negative for YFP (open arrowhead in C, D). Lectin was used to detect endothelial cells (D, red, arrow), this lectin immunoreactivity did not overlap with NG2-positive PCs or with the YFP-positive signal. Note that due to fixation, the retina forms a loop here, thus CGLs are facing each other (dotted line). intestinal ganglia cells and results in pigmentation abnormalities and defects in the enteric nervous system The Sox10 transcription factor is a reliable and commonly used target to detect neural crest derived cells, and the Rosa26-YFP-Sox10-Cre transgenic mouse model therefore facilitates the identification of neural crest progeny cells in the ocular vasculature. The Rosa26-YFP-Sox10-Cre transgenic mouse model used in this study was first reported by Müller et al. 21 in a study characterizing the neural crest origin of perivascular mesenchyme in the adult thymus. The neural crest specific expression of Sox10 is reported in the mouse from E8.5 to E12.5, 40 and specific labeling of neural crest derived tissue in E11.5 Rosa26-YFP-Sox10-Cre mouse embryos has been shown by Müller et al. 21 Jacques-Fricke et al. 41 analyzed the Sox10-Cre expression pattern in mouse embryos that were crossbred with a Rosa26-lacZ reporter mouse 42 strain. By somite stage 13 (13s), Sox10-Cre driven LacZ expression was high in the first branchial arch and lower in neural crest cells emerging from the neural folds into the first and second branchial arch streams. 41 These authors also reported a delay in Sox10-Cre driven lacz expression compared to Sox10 mrna expression in early migrating neural crest cells in the trunk. They further compared the Sox10-Cre to Wnt1-Cre expression pattern and concluded Sox10-Cre expression to be more lineage specific and restricted to neural crest derivatives. In this study, we used the Sox10-Cre mouse crossed with Rosa26-YFP mice, 23 in which Cre recombinase is activated in cells with active Sox10 regulatory sequences, enabling the expression of the reporter protein YFP. Once activated, YFP reporter expression is controlled by the ubiquitous ROSA26 locus and is sustained in Sox10 promoter active cells through excision of the stop sequence by Cre recombinase. Therefore, fate mapping studies and lineage tracing experiments can be conducted in progeny cells. To evaluate the YFP reporter protein expression at different developmental stages, we analyzed YFP expression in E10.5, E14.5, P4, P12, and 6-week-old Rosa26-YFP-Sox10-Cre transgenic mice. Neural Crest Origin of Perivascular Cells Neural crest cell fates have been extensively characterized in birds using quail-chick chimeras. In these experiments, a neuroectodermal origin of cranial PCs/vSMCs was shown by Korn et al., 14 and they subsequently speculated about a possible diencephalic neuroectodermal origin of retinal PCs. In another study, it was shown that the origin of cephalic blood vessels is mesodermal in dorsal/posterior (i.e., neck) vascular
8 Neural Crest Origin of Pericytes IOVS j December 2013 j Vol. 54 j No. 13 j 7917 FIGURE 5. Neural crest origin of vsmcs in retinal whole mounts. (A C) asma-positive arteriolar smooth muscle cells (A, red) colocalize with YFP (B, green; C, merged picture). Nuclei are visualized by DAPI labeling (blue). (D F) asma-positive arteriolar smooth muscle cells (D, red), colocalize with NG2 (E, white). Note that NG2-positive PCs on capillaries only lack the asma signal (F, arrows). (G I) On arterioles, the asma signal is homogenously expressed (G, red); however, at some segments this signal does not overlap with YFP (H, green, arrowheads). This indicates that some vsmcs have a different origin than neural crest (I, merged picture). compartments, while ventral/anterior compartments (i.e., face) derive their PCs and vsmcs from neural crest cells. 13 It was concluded that the forebrain and retina are supplied by capillaries of both mesoderm and neural crest origin, but there was no particular statement on PCs. For long-term fate studies, Cre-mediated DNA recombination labeling systems under the control of neural crest gene promoters are used, labeling neural crest cells as well as all subsequent progeny cells. Wnt1 and Sox10 promoter elements are two commonly used neural crest specific genes to study neural crest cell fate. Wnt1 is expressed in premigratory postotic neural crest cells, 17,43 whereas Sox10 is expressed in postmigratory neural crest cells. 31,35 Using a Wnt1 transgenic mouse model, 17 Foster et al. 44 proposed a neural crest origin of thymic PCs and vsmcs. In addition they compared the neural crest YFP expression pattern of Wnt1-Cre mice with Sox10-Cre mice and detected an identical distribution around thymic vessels. Müller et al. 21 described neural crest derived PCs along the entire thymus (micro-) vasculature using the Rosa26- YFP-Sox10-Cre mouse model, and asma was used to identify PCs in that study. We showed here that asma is absent in microvascular PCs indicating differences in asma expression pattern in the thymic versus retinal microvasculature. However, we detected YFP-positive signals in asma-positive vsmcs in arterioles and arteries of the retina and the choroid, corroborating those earlier results. Simon et al. 45 used for the first time an inducible Sox10-CreERT-GFP mouse model to study the contribution of neural crest cells to vascular development. They detected GFP-positive PCs in the cortical gray matter of adult mice after tamoxifen administration at
9 Neural Crest Origin of Pericytes IOVS j December 2013 j Vol. 54 j No. 13 j 7918 FIGURE 6. (A) In the choroid, PCs and vsmcs are immunoreactive for YFP (green, arrowheads) and are colocalized with the PC markers NG2 and PDGFRb (B, white and red, respectively). Asterisks indicate lumen of choroidal blood vessels, nuclei are visualized with DAPI (blue). (C, D) In the optic nerve, the microvasculature is surrounded by YFP-positive PCs (green, arrowheads), these also colocalize with the PC marker PDGFRb (D, red). (E) Posterior to the lamina cribrosa (indicated by the dotted line), numerous Olig2-positive oligodendrocytes (red) were detected. (F) Close-up of the optic nerve: oligodendrocytes labeled with Olig2 (red) show almost complete colocalization with the YFP reporter protein (green) as indicated by yellow mixed color (arrows). embryonic day They did not report GFP-positive cells in the retina after embryonic induction, which accords with our findings. We did not detect YFP-positive retinal cells at either E10.5 or E14.5; however, YFP-positive signals were detected in INL and GCL cells in P4, P12, and 6-week-old mice. This postnatal signal indicates a non neural crest derived YFP expression in the retina via Sox10 promoter activity at a later time point in development. While Simon et al. 45 induced reporter expression in Sox10 promoter active cells through tamoxifen administration in pregnant mice to target embryonic stages and in adult mice (2 3 months old) to target Sox10 expressing cells in a mature animal, only a subpopulation of neural crest cells at specific time points was hit in this model. This may have contributed to different findings in comparison
10 Neural Crest Origin of Pericytes IOVS j December 2013 j Vol. 54 j No. 13 j 7919 with our Sox10 mouse model, which expressed the reporter protein in Sox10-positive cells and progeny over the whole life span. Neural Crest Origin of Ocular PCs The mammalian retina is assigned to the CNS since it derives from the neural tube and is formed through evagination from the diencephalon. The choroid on the other hand is of mesodermal origin, thus representing a non CNS-derived tissue. To compare the origin of ocular PCs in CNS and non- CNS tissues, the choroid was studied in addition to the retina and the optic nerve. Sox10-Cre-driven YFP reporter expression was detected in PCs of the GCL and INL of the retina, the optic nerve, and the choroidal microvasculature in our experiments, therefore suggestion a neural crest origin of ocular PCs. Further, a neural crest origin of vsmcs could be demonstrated in larger vessels. In 2005 Gage et al. 16 used two transgenic mouse models to study the fate of cells derived from the neural crest (Wnt1- Cre 17 ) and mesodermal tissue (agsu 46 ) in the mammalian eye and proposed a neural crest origin of PCs. The authors focused on the evaluation of PCs in the embryonic hyaloid vessel and concluded that ocular PCs as well as vsmcs are neural crest derived. The b-gal expression in that Wnt1 mouse model indicated a possible neural crest origin of PCs. However, appropriate markers for PCs, vsmcs, as well as endothelial cells were not provided in the aforementioned study. 16 In the study presented here, the retinal (micro)vasculature of P4, P12, and 6-week-old mice was analyzed for the expression of markers specific for the neural crest (YFP), PCs (NG2, PDGFRb), and vsmcs (asma). Newborn mice possess an immature retinal vasculature and persistent hyaloid vessels. The superficial retinal vascular plexus, originating from optic nerve vessels, develops during the first postnatal week and reaches the retinal periphery by P8. Vertical sprouting of the superficial vessel starts around P7 and forms the deep (at P12) and later the intermediate vascular plexus (at P12 P15). 47 With increasing retinal vascularization, the hyaloid vessels degenerate and disappear by P13 to P16. 48,49 Although we identified sporadic sprouts of the hyaloid artery by lectin staining in E10.5 embryos, pericyte recruitment could not be proven in our set of experiments at that early time point. Analyzing E14.5 and P4 transgenic mice, we could confirm a neural crest origin of hyaloid vessel associated PCs, as proposed earlier in the Wnt1 model. 16 Since a colocalization of YFP-positive PCs with corresponding markers (NG2, PDGFRb) was detected in P12 and 6-week-old transgenic mice in the retinal vasculature, a neural crest origin was confirmed here as well. Neural Crest Origin of vsmcs in the Retina, a PCvSMC Continuum PCs and vsmcs are classified as the two major perivascular cell types. To investigate whether retinal PCs and vsmcs have the same origin, asma-positive vsmcs were studied for their colocalization with YFP expression. Since capillary PCs and vsmcs both express the YFP reporter, we suggest a common neural crest origin. Furthermore, we were able to distinguish PCs and vsmcs by their different asma expression pattern: vsmcs on vessels with diameters larger than 10 lm were asma immunopositive, while capillary PCs were lacking the asma signal. Because NG2 is reported to be specific for PCs and vsmcs, 27 and our experiments show a homogenous NG2 expression in PCs and vsmcs, the idea of a morphological and biochemical continuum from vsmcs to PCs 50,51 is supported by our data. This implies that PCs and vsmcs originate from a common precursor, expressing different marker profiles depending on the site of location (i.e., microvessels versus bigger vessels) and differentiation state. Neural Crest Origin of Choroidal PCs and vsmcs Studying human fetal eyes (8 40 weeks gestation) Chan-Ling et al. 52 suggested a common mesenchymal precursor cell for choroidal PCs and vsmcs. These findings are in contrast to our results proposing a neural crest origin of choroidal PCs and vsmcs. The different results obtained may be caused by species differences as well as differences in methodology (antibody analysis versus Sox10 promoter mediated reporter expression in this study). However, our findings are supported by Gage et al., 16 who suggested a neural crest origin of PCs and vsmcs in the choroid by using a Wnt1-Cre mouse model. Neural Crest Derived PCs in the Optic Nerve The present findings clearly demonstrate the neural crest origin of PCs in the optic nerve by colocalization of PDGFRb- andyfpreporter positive vascular structures. Due to NG2 expression of oligodendrocyte precursor cells (OPCs) in the optic nerve, 53,54 differentiation of PCs and OPCs is difficult. Therefore, we visualized PCs in the optic nerve primarily by PDGFRb immunohistochemistry. Stolt et al. 55 showed a distinct staining of oligodendrocytes in the optic nerve by using a Sox10 lacz knockin mouse model but did not comment on optic nerve vasculature. The expression of both Sox9 56 and Sox10 55 in OPCs in the spinal cord has been shown to be crucial for migration and survival of OPCs. 57 The reciprocal regulation of Olig2 and Sox10 expression in oligodendrocyte differentiation was demonstrated in chicken spinal cord. 58 As Olig2 expression persists even after differentiation into mature oligodendrocytes 59,60 a colocalization of Sox10-mediated YFP and Olig2 seemed plausible and was confirmed in double staining experiments in the Rosa26-YFP-Sox10-Cre transgenic mouse optic nerve. Degeneration of the Vasculature in rd Mutant Mice Mutation in the retinal degeneration (rd) gene in mice leads to photoreceptor degeneration starting at P8, which results in a single layer of photoreceptor nuclei at P20. 24,61 As a consequence of this photoreceptor degeneration, retinal vessels also degenerate. Retinal vascularization apparently develops normal until P12 in the rd mouse. A degeneration of the deep vasculature starts at P13, characterized by a rapid loss of endothelial cells, 62 while the intermediate capillary plexus degeneration starts at P21, although slower than observed in the deep plexus. 25,26,62 Retinal vasculature in the mouse is absent at birth (P0) and develops during the first three postnatal weeks. 63,64 Since the transgenic mice used in our study are on a C3H background (rd mutation), we cannot exclude rd-induced alterations of the retinal vasculature. Therefore, we additionally investigated the neural crest origin of PCs in P4 and P12 mice, which allowed us to study retinal vasculature under still normal conditions. This minimizes the possibility that Sox10 expression is induced as a consequence of retinal degeneration, leading to a ( wrong-positive ) non neural crest derived YFP reporter signal. However, since we demonstrate that the YFP signal pattern was identical in the vasculature of P4, P12, and 6-week-old retina, we therefore exclude this possibility. CONCLUSION Using the Sox10-YFP transgenic mouse model, we propose a neural crest origin of retinal PCs and vsmcs. Furthermore, a
11 Neural Crest Origin of Pericytes IOVS j December 2013 j Vol. 54 j No. 13 j 7920 neural crest origin of perivascular cells was demonstrated in the choroid and the optic nerve. A common neural crest origin of vsmcs and PCs supports the idea of a developmental and differentiation continuum from vsmcs to PCs. Acknowledgments We thank William D. Richardson and Nicoletta Kessaris (UCL, London, UK) for kindly providing the Sox10-Cre transgenic mice and Michael Wegner and Claus Stolt for providing the Rosa26-YFP- Sox10-Cre mouse (University Erlangen, Germany). Supported by Adele Rabensteiner Foundation, Fuchs-Foundation, Lotte Schwarz Endowment for Experimental Ophthalmology and Glaucoma Research, and a research funding grant of Paracelsus Medical University (PMU-FFF). H.T. and S.K. acknowledge support by the Austrian Cluster for Tissue Regeneration. Disclosure: A. Trost, None; F. Schroedl, None; S. Lange, None; F.J. Rivera, None; H. Tempfer, None; S. Korntner, None; C.C. Stolt, None; M. Wegner, None; B. Bogner, None; A. Kaser- Eichberger, None; K. Krefft, None; C. Runge, None; L. Aigner, None; H.A. Reitsamer, None References 1. Diaz-Flores L, Gutierrez R, Madrid JF, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24: Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314: Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97: Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68: von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312: Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2. 7. Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57: Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14: Pfister F, Przybyt E, Harmsen MC, Hammes HP. Pericytes in the eye. Pflugers Arch. 2013;465: Crisan M, Chen CW, Corselli M, Andriolo G, Lazzari L, Peault B. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci. 2009;1176: Ozen I, Boix J, Paul G. Perivascular mesenchymal stem cells in the adult human brain: a future target for neuroregeneration? Clin Transl Med. 2012;1: Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27: Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128: Korn J, Christ B, Kurz H. Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J Comp Neurol. 2002;442: Heglind M, Cederberg A, Aquino J, Lucas G, Ernfors P, Enerback S. Lack of the central nervous system- and neural crest-expressed forkhead gene Foxs1 affects motor function and body weight. Mol Cell Biol. 2005;25: Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46: Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8: Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104: Ma X, Robin C, Ottersbach K, Dzierzak E. The Ly-6A (Sca-1) GFP transgene is expressed in all adult mouse hematopoietic stem cells. Stem Cells. 2002;20: Tidhar A, Reichenstein M, Cohen D, et al. A novel transgenic marker for migrating limb muscle precursors and for vascular smooth muscle cells. Dev Dyn. 2001;220: Müller SM, Stolt CC, Terszowski G, et al. Neural crest origin of perivascular mesenchyme in the adult thymus. J Immunol. 2008;180: Matsuoka T, Ahlberg PE, Kessaris N, et al. Neural crest origins of the neck and shoulder. Nature. 2005;436: Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1: Sidman RL, Green MC. Retinal degeneration in the mouse: location of the rd locus in linkage group XVII. J Hered. 1965; 56: Matthes MT, Bok D. Blood vascular abnormalities in the degenerative mouse retina (C57BL/6J-rd le). Invest Ophthalmol Vis Sci. 1984;25: Blanks JC, Johnson LV. Vascular atrophy in the retinal degenerative rd mouse. J Comp Neurol. 1986;254: Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222: Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277: Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener. 2010;5: Ligon KL, Alberta JA, Kho AT, et al. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63: Huang X, Saint-Jeannet JP. Induction of the neural crest and the opportunities of life on the edge. Dev Biol. 2004;275: Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Dev Cell. 2004;7: Kulesa P, Ellies DL, Trainor PA. Comparative analysis of neural crest cell death, migration, and function during vertebrate embryogenesis. Dev Dyn. 2004;229: Cheng Y, Cheung M, Abu-Elmagd MM, Orme A, Scotting PJ. Chick sox10, a transcription factor expressed in both early neural crest cells and central nervous system. Brain Res Dev Brain Res. 2000;121: Ferguson CA, Graham A. Redefining the head-trunk interface for the neural crest. Dev Biol. 2004;269: Bondurand N, Kobetz A, Pingault V, et al. Expression of the SOX10 gene during human development. FEBS Lett. 1998;432: Read AP, Newton VE. Waardenburg syndrome. J Med Genet. 1997;34:
12 Neural Crest Origin of Pericytes IOVS j December 2013 j Vol. 54 j No. 13 j Verheij JB, Sival DA, van der Hoeven JH, et al. Shah- Waardenburg syndrome and PCWH associated with SOX10 mutations: a case report and review of the literature. Eur J Paediatr Neurol. 2006;10: Chan KK, Wong CK, Lui VC, Tam PK, Sham MH. Analysis of SOX10 mutations identified in Waardenburg-Hirschsprung patients: Differential effects on target gene regulation. J Cell Biochem. 2003;90: Hong CS, Saint-Jeannet JP. Sox proteins and neural crest development. Semin Cell Dev Biol. 2005;16: Jacques-Fricke BT, Roffers-Agarwal J, Gammill LS. DNA methyltransferase 3b is dispensable for mouse neural crest development. PLoS One. 2012;7:e Soriano P. Generalized lacz expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21: Echelard Y, Vassileva G, McMahon AP. Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development. 1994;120: Foster K, Sheridan J, Veiga-Fernandes H, et al. Contribution of neural crest-derived cells in the embryonic and adult thymus. J Immunol. 2008;180: Simon C, Lickert H, Gotz M, Dimou L. Sox10-iCreERT2: a mouse line to inducibly trace the neural crest and oligodendrocyte lineage. Genesis. 2012;50: Cushman LJ, Burrows HL, Seasholtz AF, Lewandoski M, Muzyczka N, Camper SA. Cre-mediated recombination in the pituitary gland. Genesis. 2000;28: Stahl A, Connor KM, Sapieha P, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51: Ito M, Yoshioka M. Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat Embryol (Berl). 1999;200: Brown AS, Leamen L, Cucevic V, Foster FS. Quantitation of hemodynamic function during developmental vascular regression in the mouse eye. Invest Ophthalmol Vis Sci. 2005;46: Nakamura A, Isoyama S, Goto K. Vessel size-dependent expression of intermediate-sized filaments, calponin, and h- caldesmon in smooth muscle cells of human coronary arteries. Heart Vessels. 1999;14: Hughes S, Chan-Ling T. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest Ophthalmol Vis Sci. 2004;45: Chan-Ling T, Dahlstrom JE, Koina ME, et al. Evidence of hematopoietic differentiation, vasculogenesis and angiogenesis in the formation of human choroidal blood vessels. Exp Eye Res. 2011;92: Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987; 7: Stallcup WB, Huang FJ. A role for the NG2 proteoglycan in glioma progression. Cell Adh Migr. 2008;2: Stolt CC, Rehberg S, Ader M, et al. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16: Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17: Finzsch M, Stolt CC, Lommes P, Wegner M. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor alpha expression. Development. 2008;135: Liu Z, Hu X, Cai J, et al. Induction of oligodendrocyte differentiation by Olig2 and Sox10: evidence for reciprocal interactions and dosage-dependent mechanisms. Dev Biol. 2007;302: Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25: Lu QR, Yuk D, Alberta JA, et al. Sonic hedgehog regulated oligodendrocyte lineage genes encoding bhlh proteins in the mammalian central nervous system. Neuron. 2000;25: Carter-Dawson LD, LaVail MM, Sidman RL. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. 1978;17: Otani A, Dorrell MI, Kinder K, et al. Rescue of retinal degeneration by intravitreally injected adult bone marrowderived lineage-negative hematopoietic stem cells. J Clin Invest. 2004;114: Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43: Fruttiger M. Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci. 2002;43:
Supplementary Figure 1
 Supplementary Figure 1 The average sigmoid parametric curves of capillary dilation time courses and average time to 50% peak capillary diameter dilation computed from individual capillary responses averaged
Supplementary Figure 1 The average sigmoid parametric curves of capillary dilation time courses and average time to 50% peak capillary diameter dilation computed from individual capillary responses averaged
SUPPLEMENTARY FIG. S2. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of dnscs.
 Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2610 Figure S1 FSMCs derived from MSLN CLN transgenic mice express smooth muscle-specific proteins. Beta-galactosidase is ubiquitously expressed within cultured FSMCs derived from MSLN
DOI: 10.1038/ncb2610 Figure S1 FSMCs derived from MSLN CLN transgenic mice express smooth muscle-specific proteins. Beta-galactosidase is ubiquitously expressed within cultured FSMCs derived from MSLN
Supplemental Information. Tissue Myeloid Progenitors Differentiate. into Pericytes through TGF-b Signaling. in Developing Skin Vasculature
 Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
Cell Reports, Volume 18 Supplemental Information Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-b Signaling in Developing Skin Vasculature Tomoko Yamazaki, Ani Nalbandian, Yutaka Uchida,
Neuroepithelial Cells and Neural Differentiation
 Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Chapter 5. Summary and Future directions
 95 Chapter 5 Summary and Future directions 96 Much of our knowledge about glial development in the vertebrate CNS comes from studies of purified oligodendrocyte precursor cells (Raff 1989; Pfeiffer et
95 Chapter 5 Summary and Future directions 96 Much of our knowledge about glial development in the vertebrate CNS comes from studies of purified oligodendrocyte precursor cells (Raff 1989; Pfeiffer et
Supplementary Materials for
 www.sciencetranslationalmedicine.org/cgi/content/full/4/117/117ra8/dc1 Supplementary Materials for Notch4 Normalization Reduces Blood Vessel Size in Arteriovenous Malformations Patrick A. Murphy, Tyson
www.sciencetranslationalmedicine.org/cgi/content/full/4/117/117ra8/dc1 Supplementary Materials for Notch4 Normalization Reduces Blood Vessel Size in Arteriovenous Malformations Patrick A. Murphy, Tyson
THE EYE: RETINA AND GLOBE
 Neuroanatomy Suzanne Stensaas February 24, 2011, 10:00-12:00 p.m. Reading: Waxman Ch. 15. Your histology and gross anatomy books should be useful. Reading: Histology of the Eye from any histology book
Neuroanatomy Suzanne Stensaas February 24, 2011, 10:00-12:00 p.m. Reading: Waxman Ch. 15. Your histology and gross anatomy books should be useful. Reading: Histology of the Eye from any histology book
Review of Nervous System Anatomy
 For the real amazement, if you wish to be amazed, is this process. You start out as a single cell derived from the coupling of a sperm and an egg; this divides in two, then four, then eight, and so on,
For the real amazement, if you wish to be amazed, is this process. You start out as a single cell derived from the coupling of a sperm and an egg; this divides in two, then four, then eight, and so on,
Chapter 6. Villous Growth
 Core Curriculum in Perinatal Pathology Chapter 6 Villous Growth Overview of vasculogenesis and angiogenesis Vasculogenesis Extraembryonic Vasculogenesis Angiogenesis Branching angiogenesis Sprouting angiogenesis
Core Curriculum in Perinatal Pathology Chapter 6 Villous Growth Overview of vasculogenesis and angiogenesis Vasculogenesis Extraembryonic Vasculogenesis Angiogenesis Branching angiogenesis Sprouting angiogenesis
CNS Developmental. Anke van Eekelen, PhD. Telethon Institute for Child Health Research
 CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at
 Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at E10.5 were double-stained for TUNEL (red) and PECAM-1 (green).
Supplementary Figure 1. EC-specific Deletion of Snail1 Does Not Affect EC Apoptosis. (a,b) Cryo-sections of WT (a) and Snail1 LOF (b) embryos at E10.5 were double-stained for TUNEL (red) and PECAM-1 (green).
Supplementary Information
 Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
Angiogenesis in Human Development. Vascular Development
 Angiogenesis in Human Development Jan Kitajewski ICRC 217B, ph 851-4688, email: jkk9 BACKGROUND READING: Vascular Development Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan. Science 277:
Angiogenesis in Human Development Jan Kitajewski ICRC 217B, ph 851-4688, email: jkk9 BACKGROUND READING: Vascular Development Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan. Science 277:
Brain Development III
 Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Brain Development III Neural Development In the developing nervous system there must be: 1. The formation of different regions of the brain. 2. The ability of a neuron to differentiate. 3. The ability
Development of the Nervous System 1 st month
 Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Nature Neuroscience: doi: /nn Supplementary Figure 1. MADM labeling of thalamic clones.
 Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1.
 Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Cell Birth and Death. Chapter Three
 Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Embryology of the Nervous System. Steven McLoon Department of Neuroscience University of Minnesota
 Embryology of the Nervous System Steven McLoon Department of Neuroscience University of Minnesota In the blastula stage embryo, the embryonic disk has two layers. During gastrulation, epiblast cells migrate
Embryology of the Nervous System Steven McLoon Department of Neuroscience University of Minnesota In the blastula stage embryo, the embryonic disk has two layers. During gastrulation, epiblast cells migrate
Supplementary Figure 1. Chimeric analysis of inner ears. (A-H) Chimeric inner ears with fluorescent ES cells and (I,J) Rainbow inner ears.
 Supplementary Figure 1. himeric analysis of inner ears. (A-H) himeric inner ears with fluorescent ES cells and (I,J) Rainbow inner ears. (A,B) omposite images showing three colors in different vestibular
Supplementary Figure 1. himeric analysis of inner ears. (A-H) himeric inner ears with fluorescent ES cells and (I,J) Rainbow inner ears. (A,B) omposite images showing three colors in different vestibular
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
Neurons vs. glia. Traditionally, glia have been viewed as passive cells that help to maintain the function of neurons.
 GLIA Neurons vs. glia The defining characteristic of a neuron is its ability to transmit rapid electrical signals in the form of action potentials. All other neural cells that lack this property are broadly
GLIA Neurons vs. glia The defining characteristic of a neuron is its ability to transmit rapid electrical signals in the form of action potentials. All other neural cells that lack this property are broadly
Heart 3: Organogenesis, CHD, prenatal circulation
 Heart 3: Organogenesis, CHD, prenatal circulation Heart development Development of vasculature Pathogenesis of CHD Prenatal circulation and its remodeling after birth David Sedmera Charles University First
Heart 3: Organogenesis, CHD, prenatal circulation Heart development Development of vasculature Pathogenesis of CHD Prenatal circulation and its remodeling after birth David Sedmera Charles University First
Development of the Nervous System. Leah Militello, class of 2018
 Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
Chemokine Regulation of Oligodendrocyte Development in the Spinal Cord. Bob Avino Saint Louis University Senior Honors Thesis April 19, 2011
 Chemokine Regulation of Oligodendrocyte Development in the Spinal Cord Bob Avino Saint Louis University Senior Honors Thesis April 19, 2011 Richard J. Miller, PhD Northwestern University Feinberg School
Chemokine Regulation of Oligodendrocyte Development in the Spinal Cord Bob Avino Saint Louis University Senior Honors Thesis April 19, 2011 Richard J. Miller, PhD Northwestern University Feinberg School
Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System. Assoc.Prof. E.Elif Güzel, M.D.
 Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System Assoc.Prof. E.Elif Güzel, M.D. Third week of Embryogenesis Primitive streak/pit appears on the epiblast (day
Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System Assoc.Prof. E.Elif Güzel, M.D. Third week of Embryogenesis Primitive streak/pit appears on the epiblast (day
!! INL!!!! ONL!! IS/OS!!
 SUPPLEMENTARY FIGURES GCL INL ONL IS/OS RPE Choroid GR MR A B C HSD2 Cc RPE CV D Cc RPE CV E RPE Cc CV F RPE Cc CV Figure S1 Figure S1: Immunohistochemistry eidence for glucocorticoid receptor (GR), mineralocorticoid
SUPPLEMENTARY FIGURES GCL INL ONL IS/OS RPE Choroid GR MR A B C HSD2 Cc RPE CV D Cc RPE CV E RPE Cc CV F RPE Cc CV Figure S1 Figure S1: Immunohistochemistry eidence for glucocorticoid receptor (GR), mineralocorticoid
Meeting Report. From December 8 to 11, 2012 at Atlanta, GA, U.S.A
 Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
What Cell Make Up the Brain and Spinal Cord
 What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Supplemental Experimental Procedures
 Cell Stem Cell, Volume 2 Supplemental Data A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis Andrew S. Brack, Irina M. Conboy, Michael J. Conboy,
Cell Stem Cell, Volume 2 Supplemental Data A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis Andrew S. Brack, Irina M. Conboy, Michael J. Conboy,
Neural stem cells and the neurobiology of ageing. Chen Siyun 1, Dawe G.S. 2
 ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
The Nervous System. Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes =
 The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
CHAPTER 48: NERVOUS SYSTEMS
 CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
CHAPTER 48: NERVOUS SYSTEMS Name I. AN OVERVIEW OF NERVOUS SYSTEMS A. Nervous systems perform the three overlapping functions of sensory input, integration, and motor output B. Networks of neurons with
A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines
 Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Supplemental Figure 1. Quantification of proliferation in thyroid of WT, Ctns -/- and grafted
 Supplemental Figure 1. Quantification of proliferation in thyroid of WT, Ctns -/- and grafted Ctns -/- mice. Cells immunolabeled for the proliferation marker (Ki-67) were counted in sections (n=3 WT, n=4
Supplemental Figure 1. Quantification of proliferation in thyroid of WT, Ctns -/- and grafted Ctns -/- mice. Cells immunolabeled for the proliferation marker (Ki-67) were counted in sections (n=3 WT, n=4
Dr/ Marwa Abdellah EOS /16/2018. Dr/ Marwa Abdellah EOS When do you ask Fluorescein angiography for optic disc diseases???
 When do you ask Fluorescein angiography for optic disc diseases??? 1 NORMAL OPTIC DISC The normal optic disc on fluorescein angiography is fluorescent due to filling of vessels arising from the posterior
When do you ask Fluorescein angiography for optic disc diseases??? 1 NORMAL OPTIC DISC The normal optic disc on fluorescein angiography is fluorescent due to filling of vessels arising from the posterior
NG2 + CNS Glial Progenitors Remain Committed to the Oligodendrocyte Lineage in Postnatal Life and following Neurodegeneration
 Article NG2 + CNS Glial Progenitors Remain Committed to the Oligodendrocyte Lineage in Postnatal Life and following Neurodegeneration Shin H. Kang, 1 Masahiro Fukaya, 2,4 Jason K. Yang, 1 Jeffrey D. Rothstein,
Article NG2 + CNS Glial Progenitors Remain Committed to the Oligodendrocyte Lineage in Postnatal Life and following Neurodegeneration Shin H. Kang, 1 Masahiro Fukaya, 2,4 Jason K. Yang, 1 Jeffrey D. Rothstein,
High sensitivity rod photoreceptor input to blue-yellow color opponent pathway in macaque retina
 High sensitivity rod photoreceptor input to blue-yellow color opponent pathway in macaque retina Greg D. Field 1, Martin Greschner 1, Jeffrey L. Gauthier 1, Carolina Rangel 2, Jonathon Shlens 1,3, Alexander
High sensitivity rod photoreceptor input to blue-yellow color opponent pathway in macaque retina Greg D. Field 1, Martin Greschner 1, Jeffrey L. Gauthier 1, Carolina Rangel 2, Jonathon Shlens 1,3, Alexander
Option A: Neurobiology & Behavior HL BIOLOGY 2 ND EDITION DAMON, MCGONEGAL, TOSTO, AND
 Option A: Neurobiology & Behavior A1: NEURAL DEVELOPMENT USE THE INFO IN THE PRESENTATION TO COMPLETE A1 NOTES GUIDE INFORMATION TAKEN FROM: HL BIOLOGY 2 ND EDITION DAMON, MCGONEGAL, TOSTO, AND WARD BIOLOGY
Option A: Neurobiology & Behavior A1: NEURAL DEVELOPMENT USE THE INFO IN THE PRESENTATION TO COMPLETE A1 NOTES GUIDE INFORMATION TAKEN FROM: HL BIOLOGY 2 ND EDITION DAMON, MCGONEGAL, TOSTO, AND WARD BIOLOGY
Neuroanatomy. Assistant Professor of Anatomy Faculty of Medicine The University of Jordan Dr Maha ELBeltagy
 Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Development of the Central Nervous System Development of the nervous system Development
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Development of the Central Nervous System Development of the nervous system Development
4/18/2011. Physiology 67 Lecture on Neural Development
 Physiology 67 Lecture on Neural Development 1 2 3 4 5 6 Neural cell categories After the ectodermal tissue has folded into the neural tube, another series of signaling interactions determine the type of
Physiology 67 Lecture on Neural Development 1 2 3 4 5 6 Neural cell categories After the ectodermal tissue has folded into the neural tube, another series of signaling interactions determine the type of
Heart Development. Origins of congenital heart defects Properties of cardiac progenitor cells. Robert G. Kelly
 ESC CBCS Summer School on Cardiovascular Sciences Heart Development 19th June 2013 Origins of congenital heart defects Properties of cardiac progenitor cells Robert G. Kelly Animal models of heart development
ESC CBCS Summer School on Cardiovascular Sciences Heart Development 19th June 2013 Origins of congenital heart defects Properties of cardiac progenitor cells Robert G. Kelly Animal models of heart development
Cells of the nervous system
 Neurobiology Cells of the nervous system Anthony Heape 2011 1 Cells of the nervous system Neuroglia : part 2 The non excitable cells of the nervous system that provide support to neuronal survival and
Neurobiology Cells of the nervous system Anthony Heape 2011 1 Cells of the nervous system Neuroglia : part 2 The non excitable cells of the nervous system that provide support to neuronal survival and
Prss56, a novel marker of adult neurogenesis in the mouse brain. - Supplemental Figures 1 to 5- Brain Structure and Function
 Prss56, a novel marker of adult neurogenesis in the mouse brain - Supplemental Figures 1 to 5- Brain Structure and Function Alexandre Jourdon 1,2, Aurélie Gresset 1, Nathalie Spassky 1, Patrick Charnay
Prss56, a novel marker of adult neurogenesis in the mouse brain - Supplemental Figures 1 to 5- Brain Structure and Function Alexandre Jourdon 1,2, Aurélie Gresset 1, Nathalie Spassky 1, Patrick Charnay
Distribution of type IV collagen, laminin, nidogen and fibronectin in the haemodynamically stressed vascular wall
 Histol Histopath (1 990) 5: 161-1 67 Histology and Histopathology Distribution of type IV collagen, laminin, nidogen and fibronectin in the haemodynamically stressed vascular wall Reinhold Kittelberger,
Histol Histopath (1 990) 5: 161-1 67 Histology and Histopathology Distribution of type IV collagen, laminin, nidogen and fibronectin in the haemodynamically stressed vascular wall Reinhold Kittelberger,
4) Modification of Development by Sensory Experience (Nurture)
 Lecture 7 (Jan 29 th ): BRAIN DEVELOPMENT and EVOLUTION Lecture Outline 1) Overview of Neural Development 2) Stages of Neural Development 3) The Nature vs. Nurture Issue 4) Modification of Development
Lecture 7 (Jan 29 th ): BRAIN DEVELOPMENT and EVOLUTION Lecture Outline 1) Overview of Neural Development 2) Stages of Neural Development 3) The Nature vs. Nurture Issue 4) Modification of Development
Heart Development. Robert G. Kelly Developmental Biology Institute of Marseilles - Luminy
 ESC CBCS Summer School on Cardiovascular Sciences 15th June 2011 Heart Development Robert G. Kelly Developmental Biology Institute of Marseilles - Luminy Animal models of heart development Tinman/Nkx2.5
ESC CBCS Summer School on Cardiovascular Sciences 15th June 2011 Heart Development Robert G. Kelly Developmental Biology Institute of Marseilles - Luminy Animal models of heart development Tinman/Nkx2.5
Supplementary Figure S1 Enlarged coronary artery branches in Edn1-knockout mice. a-d, Coronary angiography by ink injection in wild-type (a, b) and
 Supplementary Figure S1 Enlarged coronary artery branches in Edn1-knockout mice. a-d, Coronary angiography by ink injection in wild-type (a, b) and Edn1-knockout (Edn1-KO) (c, d) hearts. The boxed areas
Supplementary Figure S1 Enlarged coronary artery branches in Edn1-knockout mice. a-d, Coronary angiography by ink injection in wild-type (a, b) and Edn1-knockout (Edn1-KO) (c, d) hearts. The boxed areas
Vertebrate Limb Patterning
 Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Ahtiainen et al., http :// /cgi /content /full /jcb /DC1
 Supplemental material JCB Ahtiainen et al., http ://www.jcb.org /cgi /content /full /jcb.201512074 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Distinct distribution of different cell cycle phases in the
Supplemental material JCB Ahtiainen et al., http ://www.jcb.org /cgi /content /full /jcb.201512074 /DC1 THE JOURNAL OF CELL BIOLOGY Figure S1. Distinct distribution of different cell cycle phases in the
Signaling Vascular Morphogenesis and Maintenance
 Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Supporting Information
 Supporting Information Rock et al. 10.1073/pnas.1117988108 Fig. S1. Heterogeneity of stromal cells in normal and fibrotic mouse lungs. Sections of normal mouse lungs (A and D) and fibrotic lungs collected
Supporting Information Rock et al. 10.1073/pnas.1117988108 Fig. S1. Heterogeneity of stromal cells in normal and fibrotic mouse lungs. Sections of normal mouse lungs (A and D) and fibrotic lungs collected
Cellular components of CNS
 Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
Bone Marrow Pop. (% Total) Mature Pool (Absolute %) Immature Pool (Absolute %) A10 EC Control A10 EC Control A10 EC Control
 Bone Marrow Pop. (% Total) Mature Pool (Asolute %) Immature Pool (Asolute %) A10 EC A10 EC A10 EC Myeloid 50.7 57.5 37.5 46.2 13.2 11.3 Erythroid 38.3 23.2 33.3 16.8 9.3 6.3 Lymphocytes 13.8 19.0 - - -
Bone Marrow Pop. (% Total) Mature Pool (Asolute %) Immature Pool (Asolute %) A10 EC A10 EC A10 EC Myeloid 50.7 57.5 37.5 46.2 13.2 11.3 Erythroid 38.3 23.2 33.3 16.8 9.3 6.3 Lymphocytes 13.8 19.0 - - -
Evaluation of directed and random motility in microslides Assessment of leukocyte adhesion in flow chambers
 Evaluation of directed and random motility in microslides Motility experiments in IBIDI microslides, image acquisition and processing were performed as described. PMN, which ended up in an angle < 180
Evaluation of directed and random motility in microslides Motility experiments in IBIDI microslides, image acquisition and processing were performed as described. PMN, which ended up in an angle < 180
Material and Methods Production and analysis of -gal+ clones Immunostaining Optical projection tomography Statistical analysis
 Material and Methods Production and analysis of β-gal+ clones The α-cardiac actin nlaacz/+ and Cx40 egfp mouse lines used in this study were genotyped as previously reported 1, 2. Double transgenic knock-in
Material and Methods Production and analysis of β-gal+ clones The α-cardiac actin nlaacz/+ and Cx40 egfp mouse lines used in this study were genotyped as previously reported 1, 2. Double transgenic knock-in
Patterning the Embryo
 Patterning the Embryo Anteroposterior axis Regional Identity in the Vertebrate Neural Tube Fig. 2.2 1 Brain and Segmental Ganglia in Drosophila Fig. 2.1 Genes that create positional and segment identity
Patterning the Embryo Anteroposterior axis Regional Identity in the Vertebrate Neural Tube Fig. 2.2 1 Brain and Segmental Ganglia in Drosophila Fig. 2.1 Genes that create positional and segment identity
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Plasticity of Cerebral Cortex in Development
 Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization
 Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Lesson 33. Objectives: References: Chapter 16: Reading for Next Lesson: Chapter 16:
 Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Supplemental Data. Wnt/β-Catenin Signaling in Mesenchymal Progenitors. Controls Osteoblast and Chondrocyte
 Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Contact: Course outline: Contact for other times.
 Contact: kdelaney@uvic.ca Course outline: http://web.uvic.ca/~kdelaney/b367 Scheduled office hours: 1:00-3:00, M&Th Cunn. 259A Contact kdelaney@uvic.ca for other times. Quiz (0.5 hrs) midterm (1.4 hrs)
Contact: kdelaney@uvic.ca Course outline: http://web.uvic.ca/~kdelaney/b367 Scheduled office hours: 1:00-3:00, M&Th Cunn. 259A Contact kdelaney@uvic.ca for other times. Quiz (0.5 hrs) midterm (1.4 hrs)
Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse
 Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Supplementary Figure 1. Nature Neuroscience: doi: /nn.4547
 Supplementary Figure 1 Characterization of the Microfetti mouse model. (a) Gating strategy for 8-color flow analysis of peripheral Ly-6C + monocytes from Microfetti mice 5-7 days after TAM treatment. Living
Supplementary Figure 1 Characterization of the Microfetti mouse model. (a) Gating strategy for 8-color flow analysis of peripheral Ly-6C + monocytes from Microfetti mice 5-7 days after TAM treatment. Living
Nervous system is the most complex system in our body. It is formed by a network of more than 100 million nerve cells (neurons) assisted by many more
 Nervous system Nervous system is the most complex system in our body. It is formed by a network of more than 100 million nerve cells (neurons) assisted by many more glial cells. Devoid from connective
Nervous system Nervous system is the most complex system in our body. It is formed by a network of more than 100 million nerve cells (neurons) assisted by many more glial cells. Devoid from connective
Major Structures of the Nervous System. Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors
 Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal
 glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
glial cells missing and gcm2 Cell-autonomously Regulate Both Glial and Neuronal Development in the Visual System of Drosophila Carole Chotard, Wendy Leung and Iris Salecker Supplemental Data Supplemental
Differentiation of the facial-vestibulocochlear ganglionic complex in human embryos of developmental stages 13 15
 O R I G I N A L A R T I C L E Folia Morphol. Vol. 68, No. 3, pp. 167 173 Copyright 2009 Via Medica ISSN 0015 5659 www.fm.viamedica.pl Differentiation of the facial-vestibulocochlear ganglionic complex
O R I G I N A L A R T I C L E Folia Morphol. Vol. 68, No. 3, pp. 167 173 Copyright 2009 Via Medica ISSN 0015 5659 www.fm.viamedica.pl Differentiation of the facial-vestibulocochlear ganglionic complex
Supplementary Figure S1: Tanycytes are restricted to the central/posterior hypothalamus
 Supplementary Figure S1: Tanycytes are restricted to the central/posterior hypothalamus a: Expression of Vimentin, GFAP, Sox2 and Nestin in anterior, central and posterior hypothalamus. In the anterior
Supplementary Figure S1: Tanycytes are restricted to the central/posterior hypothalamus a: Expression of Vimentin, GFAP, Sox2 and Nestin in anterior, central and posterior hypothalamus. In the anterior
BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE. Thomas Marino, Ph.D.
 BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE Thomas Marino, Ph.D. Development of the Brain I. Competencies: Upon completion of this section of the course, the student must be able to: 1. Understand
BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE Thomas Marino, Ph.D. Development of the Brain I. Competencies: Upon completion of this section of the course, the student must be able to: 1. Understand
Nerve tissue & the Nervous System
 Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
Organogenesis of Heart, Kidney, Nervous System & Sense Organs. [ GLOSSARY ]
![Organogenesis of Heart, Kidney, Nervous System & Sense Organs. [ GLOSSARY ] Organogenesis of Heart, Kidney, Nervous System & Sense Organs. [ GLOSSARY ]](/thumbs/86/94287046.jpg) Organogenesis of Heart, Kidney, Nervous System & Sense Organs. [ GLOSSARY ] Subject : Zoology Course : 3rd Year, B.Sc. Undergraduate UGC Syllabus, Model - 1 Paper No. : Z-305B & Title : Developmental Biology
Organogenesis of Heart, Kidney, Nervous System & Sense Organs. [ GLOSSARY ] Subject : Zoology Course : 3rd Year, B.Sc. Undergraduate UGC Syllabus, Model - 1 Paper No. : Z-305B & Title : Developmental Biology
Rescue of mutant rhodopsin traffic by metformin-induced AMPK activation accelerates photoreceptor degeneration Athanasiou et al
 Supplementary Material Rescue of mutant rhodopsin traffic by metformin-induced AMPK activation accelerates photoreceptor degeneration Athanasiou et al Supplementary Figure 1. AICAR improves P23H rod opsin
Supplementary Material Rescue of mutant rhodopsin traffic by metformin-induced AMPK activation accelerates photoreceptor degeneration Athanasiou et al Supplementary Figure 1. AICAR improves P23H rod opsin
The developmental history of a sensory ganglion cell
 (bozza v1, luglio 2016 per VC) The developmental history of a sensory ganglion cell by Brian Freeman, School of Medical Sciences, UNSW (b.freeman@unsw.edu.au) The sensory ganglion cell (e.g., in spinal
(bozza v1, luglio 2016 per VC) The developmental history of a sensory ganglion cell by Brian Freeman, School of Medical Sciences, UNSW (b.freeman@unsw.edu.au) The sensory ganglion cell (e.g., in spinal
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
Histology of the CNS
 Histology of the CNS Lecture Objectives Describe the histology of the cerebral cortex layers. Describe the histological features of the cerebellum; layers and cells of cerebellar cortex. Describe the elements
Histology of the CNS Lecture Objectives Describe the histology of the cerebral cortex layers. Describe the histological features of the cerebellum; layers and cells of cerebellar cortex. Describe the elements
Nervous System. Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI
 Nervous System Overview.I Histology.II Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI Repairs.VII Pathology.VIII.IV 1 Controls and integrates all body activities
Nervous System Overview.I Histology.II Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI Repairs.VII Pathology.VIII.IV 1 Controls and integrates all body activities
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70%
 Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70% Pictures: 9 days after electroporation Red: MAP2 Blue: GFAP Green: GFP The cells were from Embryonic Day 14 Mouse Cerebral Cortex Primary Mouse Hippocampal
Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70% Pictures: 9 days after electroporation Red: MAP2 Blue: GFAP Green: GFP The cells were from Embryonic Day 14 Mouse Cerebral Cortex Primary Mouse Hippocampal
Supplementary Table 1. List of primers used in this study
 Supplementary Table 1. List of primers used in this study Gene Forward primer Reverse primer Rat Met 5 -aggtcgcttcatgcaggt-3 5 -tccggagacacaggatgg-3 Rat Runx1 5 -cctccttgaaccactccact-3 5 -ctggatctgcctggcatc-3
Supplementary Table 1. List of primers used in this study Gene Forward primer Reverse primer Rat Met 5 -aggtcgcttcatgcaggt-3 5 -tccggagacacaggatgg-3 Rat Runx1 5 -cctccttgaaccactccact-3 5 -ctggatctgcctggcatc-3
Histology of the Eye
 Histology of the Eye Objectives By the end of this lecture, the student should be able to describe: The general structure of the eye. The microscopic structure of:»cornea.»retina. EYE BULB Three coats
Histology of the Eye Objectives By the end of this lecture, the student should be able to describe: The general structure of the eye. The microscopic structure of:»cornea.»retina. EYE BULB Three coats
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Nervous System. Lecture 4
 Nervous System Lecture 4 Neurons Functional unit of the nervous system Also called the nerve cell Soma or body Axon Dendrites Neuroglial cells support cells Schwann cells produce myelin in PNS Oligodendrocytes
Nervous System Lecture 4 Neurons Functional unit of the nervous system Also called the nerve cell Soma or body Axon Dendrites Neuroglial cells support cells Schwann cells produce myelin in PNS Oligodendrocytes
SUPPLEMENTARY INFORMATION
 b 350 300 250 200 150 100 50 0 E0 E10 E50 E0 E10 E50 E0 E10 E50 E0 E10 E50 Number of organoids per well 350 300 250 200 150 100 50 0 R0 R50 R100 R500 1st 2nd 3rd Noggin 100 ng/ml Noggin 10 ng/ml Noggin
b 350 300 250 200 150 100 50 0 E0 E10 E50 E0 E10 E50 E0 E10 E50 E0 E10 E50 Number of organoids per well 350 300 250 200 150 100 50 0 R0 R50 R100 R500 1st 2nd 3rd Noggin 100 ng/ml Noggin 10 ng/ml Noggin
Extending Injury- and Disease-Tolerant Phenotypes by Repetitive Conditioning
 Extending Injury- and Disease-Tolerant Phenotypes by Repetitive Conditioning Promoting Long-Lasting Protection in the CNS Jeff Gidday PhD Neurosurgery Ophthalmology & Visual Sciences Cell Biology & Physiology
Extending Injury- and Disease-Tolerant Phenotypes by Repetitive Conditioning Promoting Long-Lasting Protection in the CNS Jeff Gidday PhD Neurosurgery Ophthalmology & Visual Sciences Cell Biology & Physiology
The Nervous System PART C. PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Nervous System 7 PART C Protection of the Central Nervous System Scalp and skin Skull and vertebral
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Nervous System 7 PART C Protection of the Central Nervous System Scalp and skin Skull and vertebral
Fig.9.2. Structure of embryonic brain
 T Chapter 9 Development of Ectodermal Organs he ectoderm gives rise to 3 separate cell populations: neural(plate) ectoderm, neural crest cells, and epiderm (general body ectoderm). A primordium (anlage)
T Chapter 9 Development of Ectodermal Organs he ectoderm gives rise to 3 separate cell populations: neural(plate) ectoderm, neural crest cells, and epiderm (general body ectoderm). A primordium (anlage)
Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated,
 1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated, embedded in matrigel and exposed
1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated, embedded in matrigel and exposed
Organogenesis Part 2. V. Lateral Plate Mesoderm VI. Endoderm VII. Development of the Tetrapod Limb VIII. Sex Determination. V. Lateral Plate Mesoderm
 Organogenesis Part 2 V. Lateral Plate Mesoderm VI. Endoderm VII. Development of the Tetrapod Limb VIII. Sex Determination V. Lateral Plate Mesoderm chordamesoderm paraxial mesoderm intermediate mesoderm
Organogenesis Part 2 V. Lateral Plate Mesoderm VI. Endoderm VII. Development of the Tetrapod Limb VIII. Sex Determination V. Lateral Plate Mesoderm chordamesoderm paraxial mesoderm intermediate mesoderm
Cells of the Nervous System
 Cells of the Nervous System Layout of the Nervous System Central Nervous System (CNS) Brain (in the skull) Spinal Cord (in the spine) Interprets sensory input, initiates movement, and mediates complex
Cells of the Nervous System Layout of the Nervous System Central Nervous System (CNS) Brain (in the skull) Spinal Cord (in the spine) Interprets sensory input, initiates movement, and mediates complex
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Huntington s Disease & MARY ET BOYLE, PH.D. DEPARTMENT OF COGNITIVE SCIENCE
 Huntington s Disease & Early Nervous System Development MARY ET BOYLE, PH.D. DEPARTMENT OF COGNITIVE SCIENCE UCSD The cups fell to the floor with a crash. Was this the alarm signal? Or was it forgetting
Huntington s Disease & Early Nervous System Development MARY ET BOYLE, PH.D. DEPARTMENT OF COGNITIVE SCIENCE UCSD The cups fell to the floor with a crash. Was this the alarm signal? Or was it forgetting
Polarity and Segmentation. Chapter Two
 Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
NERVOUS TISSUE. 1. Functional units of the nervous system; receive, process, store and transmit information to other neurons, muscle cells or glands.
 NERVOUS TISSUE LEARNING OBJECTIVES 1. Characterize and contrast the structure of neuronal cell bodies, dendrites and axons 2. List the classification of synapses and identify the basic structures of a
NERVOUS TISSUE LEARNING OBJECTIVES 1. Characterize and contrast the structure of neuronal cell bodies, dendrites and axons 2. List the classification of synapses and identify the basic structures of a
( neural progeni2 tor cell), ,,,, , (neural crest) (radial glial cell) mrna [2 ]
![( neural progeni2 tor cell), ,,,, , (neural crest) (radial glial cell) mrna [2 ] ( neural progeni2 tor cell), ,,,, , (neural crest) (radial glial cell) mrna [2 ]](/thumbs/92/110608454.jpg) 246, 1999 6, 51 (3), 246 252 Acta Physiologica Sinica 3 P19 3 3 (, 200031 ;, 200031) (nestin),, RA P19,, (neural precursor cell) BMP4, (NF160), ( neural progeni2 tor cell),, : ; P19 ; BMP4 ; : Q71,,,,
246, 1999 6, 51 (3), 246 252 Acta Physiologica Sinica 3 P19 3 3 (, 200031 ;, 200031) (nestin),, RA P19,, (neural precursor cell) BMP4, (NF160), ( neural progeni2 tor cell),, : ; P19 ; BMP4 ; : Q71,,,,
