The Subcellular Distribution of Chromosome 6-encoded Dystrophin-related Protein in the Brain
|
|
|
- Ellen Golden
- 5 years ago
- Views:
Transcription
1 The Subcellular Distribution of Chromosome 6-encoded Dystrophin-related Protein in the Brain Tejvir S. Khurana, Simon C. Watkins,* and Louis M. Kunkel Program of Neuroscience and Department of Pediatrics, Harvard Medical School and The Howard Hughes Medical Institute, Division of Genetics, Children's Hospital, Boston, Massachusetts 02115; and * Department of Neurobiology, Anatomy and Cell Science, University of Pittsburgh, Pennsylvania Abstract. Chromosome 6-encoded dystrophin-relatedprotein (DRP) shows significant structural similarities to dystrophin at the carboxyl terminus, though the two proteins are encoded on different chromosomes. Both DRP and dystrophin are expressed in muscle and brain and show some similarity in their subcellular localization. For example, in skeletal muscle both are expressed at neuromuscular and myotendinous junctions. However, while dystrophin is absent or severely reduced in Duchenne/Becker muscular dystrophy, DRP continues to be expressed. Within the brain, dystrophin is enriched at the postsynaptic regions of specific subsets of YSTROPHIN is a large cytoskeletal protein belonging to the superfamily of spectrin-like proteins. It is closely associated with the inner face of the plasmalemma in muscle and postsynaptic region of neurons in the brain (Hoffman et al., 1987; Koenig et al., 1988; Arahata et al., 1988; Bonilla et al., 1988; Watldns et al., 1988; Zubrzycka-Gaarn et al., 1988; Miike et al., 1989; Lidov et al., 1990). The role of dystrophin in muscle or neurons remains unclear, however mutations in the gene encoding dystrophin result in Duchenne/Becker Muscular Dystrophy (D/BMD), a disease characterized by progressive muscle wasting, cognitive impairment, and death by the third decade of life in humans (Duchenne, 1868; Engel, 1986; Hoffman and Kunkel, 1989). The spectrin superfamily includes a class of proteins sharing limited structural similarity and organizational motifs including an actin-binding amino terminus and a 'rod' like central domain, predicted to be composed of triple o~-helical repeat motifs (Davison and Critchley, 1988; Koenig et al., 1988; Byers et al., 1989). While the carboxyl terminus of dystrophin bears no homology to either the spectrins or o~-actinins, it shares considerable homology with chromosome 6 encoded transcript of the DMDL gene (Love et al., 1989). The protein product of this autosomal transcript, dystrophin-related protein (DRP) 1 was identified by ex- 1. Abbreviation used in this paper: DRP, dystrophin-related protein. neurons, while the distribution of DRP is yet to be described. In this study we demonstrate a distinct though highly specific pattern of distribution of DRP in the brain. DRP is enriched in the choroid plexus, pia mater, intracerebral vasculature, and ependymal lining. Within the parenchyma proper, DRP is located at the inner plasma face of astrocytic foot processes at the abluminal aspect of the blood-brain barrier. The distribution of DRP is conserved across a large evolutionary distance, from mammals to elasmobranchs, suggesting that DRP may play a role in the maintenance of regional specializations in the brain. pressing the cloned transcript as a recombinant bacterial protein and generating antibodies against the purified fusion protein (Khurana et al., 1990). DRP and dystrophin are similar in size and present at relatively low abundance in normal skeletal muscle (Hoffman et al., 1987; Khurana et al., 1990). However, DRP is more widely distributed than dystrophin, being detected in brain, blood vessels, nerves, kidney, spleen, liver, testis, stomach, and muscle. Additionally, the expression of DRP is unaltered in age matched D/BMD skeletal muscle, in which dystrophin is either not detectable or severely reduced (Hoffman et al., 1988; Khurana et al., 1990). The subcellular distribution of DRP in skeletal muscle has recently been elucidated using immunohistochemistry (Khurana et al., 1991; Man et al., 1991; Ohlendieck et al., 1991; Takemitsu et al., 1991; Cartaud et al., 1992). DRP is detected in intramuscular nerves, blood vessels, and found to be enriched at the neuromuscular and myotendinous junctional areas of the adult dystrophic myofiber (Khurana et al., 1991). Using antibodies of varying sensitivity and specificity, other investigators have detected DRP at some but not all these sites (Man et al., 1991; Ohiendieck et al., 1991; Takemitsu et al., 1991). Minimal sarcolemmal labeling of dystrophic muscle has also been noted when the sensitivity of DRP immunolabeling is optimized (Khurana et al., 1991; Man et al., 1991; Tanaka et al., 1991; Voit et al., 1991). However, during muscle regeneration intense and extended sarcolemmal immunolabeling is seen to completely encircle 9 The Rockefeller University Press, /92/10/357/10 $2.00 The Journal of Cell Biology, Volume 119, Number 2, October
2 the myofibers. Additionally, greater amounts of DRP are found during embryonic and perinatal development than in adult dystrophic muscle. Developmental studies suggest that DRP may play a role similar to that of dystrophin during certain developmental stages in the mdx mouse muscle (Khurana et al., 1991; Takemitsu et al., 1991). Whereas recent studies surveying the distribution of dystrophin and DRP have found some similarity in their distribution in myofibres (Byers et al., 1991; Khurana et al., 1991), the distribution of D RP in the brain is as yet unknown. However, anomalous immunolabeling of the cerebral vasculature and pial surfaces of the mdx brain using some dystrophin antisera has been reported and provides hints about the localization of DRP in the brain (Ishiura et al., 1990; Yoshioka et al., 1992), in much the same way that such cross reactivity did in skeletal muscle (Fardeau et al., 1990; Samitt and Bonilla, 1990). To address basic questions of the physiological role of DRP it is important to determine the subcellular distribution of DRP in the brain. We have therefore used a combined approach of immunoblotting, immunohistochemistry, immunoelectron microscopy, and tissue culture to define the distribution of DRP in the brain of mdx mice. Materials and Methods Antibody Specificity edna clones encoding the carboxyl termini of dystrophin (clone dll encompassing nucleotide position 9,786-11,555 of the dystrophin sequence, Koenig et al., 1988) and DRP (nucleotide position 690-1,353, Love et al., 1989: homologous to 10,457-11,518 of the dystrophin sequence, Koenig et al., 1988) were expressed as bacterial fusion peptides and purified as previously described (Koenig and Kunkel, 1990; Khurana et al., 1990). To determine the specificity of DRP antibodies on immunoblotting, equivalent amounts of purified fusion proteins were electrophoresed alongside each other and electro-transferred to nitrocellulose. The immunoblots were processed using affinity purified antibodies to the carboxyl terminus of DRP according to previously described methods (Hoffman et al., 1987; Khurana et al., 1990). (Note: for sake of brevity in this manuscript, we will refer to the dystrophin and DRP fusion peptides described above, as dl l fusion or DYS-pep and DRP fusion or DRP-pep, respectively). Peptide Competition An excess ('~1 mg) of either DRP peptide (DRP pep- encoding the carboxyl terminus of DRP) or dystrophin peptide (DYS pep- encoding the carboxyl terminus of dystrophin) was resuspended in 20/~1 of water. This was added to 100/xl of affinity purified DRP antibodies at a concentration of 1 ~tg/ml (final antibody concentration r~800 ng/t~l) and the mixture used for immunohistochemistry on cryostat sections of mdx brain. For comparison, 10- ttm-thick serial sections were incubated in parallel with (a) preimmune sera from the same rabbit and (b) affinity purified DRP antibodies without the DRP peptide at ~1 ~g/mi. Photographs were taken on Kodak Tri-X Pan film (Eastman Kodak Co., Rochester, NY) using the same camera settings and processed in parallel. Immunoblotting The methods used for immunoblotting have been previously described (Hoffman et al., 1987). Briefly, 20-~m cryostat sections, aliquots of tissue, or pellets of mechanically harvested tissue culture cells were placed in preweighed 0.5- or 1.5-ml tubes and chilled on dry ice.,'~20 mg of tissue were solubilized in 20 volumes of sample buffer containing 10 % SDS, 0.1 M Tris, ph 8.0, 10 mm EDTA, bromopbenol blue, and 50 mm DTT. Tissue lysates were made using a metallic or teflon-coated tissue homogenizer to thoroughly crush the sample inside the tube. The sample was boiled for 2 rain and cooled to room temperature. Protein concentration was measured using a colorimetric assay by staining an aliquot of the sample with Amido black (Nakamura et al., 1985). Appropriate dilutions were loaded in each well to achieve uniform loading. These aliquots were fractionated on a % gradient SDS-polyacrylamide get. Electrophoresis of the 1.5- mm-thick gel was for 3 h at 100 V or in some cases 60 V for 12 h (constant voltage). After electrophoresis, proteins were electro-transferred onto nitrocellulose filters, and the filters dried overnight. To control for uniformity of loading, the posttransfer gels were stained with Coomassie blue dye for residual myosin. In addition the filters were stained with Ponceau S solution (Sigma Chemical Co., St. Louis, MO), which together with the transfer of prestained protein molecular weight standards served to control the efficiency of transfer. DRP and dystrophin antibody complexes were detected using alkaline phosphatase staining as previously described (Hoffman et al., 1987; Khurana et al., 1990). Multiple filters were tested for each experiment. For quantification, the DRP band was subjected to computerized laser densitometric analysis by scanning on an LKB Ultroscan XL (LKB Instruments Inc., Brornma, Sweden) machine and results plotted as previously described (Khurana et al., 1991). Immunohistochemistry Immunohistochemistry was performed using previously described methods (Watkins et al., t989), mdx mice were anaesthetized and sacrificed by cervical dislocation. Raja erinacea fish were obtained from the Marine Biological Laboratories (Woods Hole, MA). Fish were anaesthetized with chloral hydrate and sacrificed by cervical transsection. Brains were dissected out and various orientations of the brain placed on a marked piece of cardboard and plunged into isopentane cooled in liquid nitrogen and stored at -70~ After mounting on cold metal chucks with Tissue-Tek O.C.T. Compound (Miles Laboratories Inc., Elkhart, IN), 5-10-/zm sections were cut and lifted onto gelatin or polylysine-coated slides. Sections were fixed with cold methanol for 5 min and washed with PBS before being labeled with affinity purified DRP antibody in PBS with 10% calf serum (final concentration '~1 ~g/ml) for 1 h at room temperature. After washing in PBS, sections were incubated with FITC or TRITC anti-sheep or anti-rabbit antibody (Sigma Chemical Co.) for 1 h. Washed sections were mounted in Gelvatol (Monsanto Chemical Co., St. Louis, MO) or Aqua-mount (Lerner Laboratories, Pittsburgh, PA). In some cases immunolabeling of serial sections was done with antibodies against the vascular marker, yon Willebrands Factor (Sigma Chemical Co.) as described above. Sections were examined using either a Nikon FXA (Nikon Inc., Melville, NY) or Zeiss Axiophot microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with epi-fluorescence optics. Immunoelectron Microscopy mdx mice brains were fixed by cardiac perfusion with 2 % paraformaldehyde, 0.01% glutaraidehyde in PBS. Brains were removed from the cranial cavity and the cerebrum and cerebellum were dissected out and further fixed in the same buffer for a total of I h. Samples were cut into l-ram cubes and immersed in 2.3 M Sucrose in PBS overnight. Blocks were mounted on cutring slabs, shock frozen in liquid nitrogen, and stored under liquid nitrogen. 70-nm sections were cut using a Reichert Ultracut microtome fitted with a FC4D cryo-attachment (Reichert Scientific Instruments, Buffalo, NY). Sections were mounted on 200 mesh carbon-formvar grids, labeled with antibodies to DRP and revealed with a 5-urn gold conjugate directed against the rabbit primary antibodies as previously described (Byers et al., 1991). EM was performed with a Jeol 100-CX II (JEOL USA Inc., Peabody, MA). Micrographs were taken at a magnification of 29,000. Tissue Culture Anaesthetized mdx mice were sacrificed by cervical dissociation. For neuronal cultures embryonic day 18 mice were used and for glial cultures l-dold mice were used. Cells were cultured by standard protocols, as described by Freshney (1987). Briefly, brains were removed from the cranium and the meninges dissected out under a ;Zeiss stereo microscope (Carl Zeiss, Inc.). The cerebral hemispheres were minced, trypsinized in 10 volumes of PBS and dissociated by forced passage through flame-polished glass pipettes. The supernatant pools were decanted, and the separated tissue clumps discarded. The supernatant was spun at low speed and pellet resuspended in culture medium. Viable cells in the cell suspension were counted using trypan blue exclusion. ~10 ~ cells were plated on polylysine-coated cover slips. Neuronal cultures were enriched by treatment with the pyrimidine analogue cytosine arabinoside for 4-8 d before use. Antibodies against glial fibrillary acidic protein (GFAP) (Sigma Chemical Co.) were used as control for glial contaminants. Glial cultures were stained to evaluate the purity of cultures which were found to be >90% positive. The Journal of Cell Biology, Volume 119,
3 Results Specificity and Sensitivity of A~inity-Purified DRP Antibodies The domain of DRP used for expression of fusion proteins and antibody production is highly conserved between dystrophin and DRP. We have previously demonstrated that DRP antibodies are specific for DRP since they recognize DRP but not the internally truncated, smaller dystrophin in biopsies of Becker muscular dystrophy patients (Khurana et al., 1990; Khurana et al., 1991). To strengthen these original controls we performed experiments to determine whether DRP antibodies could recognize extremely small amounts of DRP fusion peptides and distinguish DRP peptides from the structurally similar dystrophin fusion peptides. DRP antibodies were used to probe an immunoblot containing increasing amounts of fusion protein representing the carboxyl termini of DRP and dystrophin. As demonstrated in Fig. 1, DRP antibody detected DRP fusion proteins at extremely low concentrations while not recognizing equivalent or greater amounts of fusion protein containing the corresponding carboxyl terminus of dystrophin. This observation coupled with our previous patient studies demonstrates that, on immunoblots, the affinity-purified DRP antibodies used in this study were sensitive and specific for DRP. Having established that the DRP antibodies were specific for DRP on Western blots we wished to extend these findings and characterize the specificity of the DRP antibodies by immunottuorescence microscopy. This was especially important in light of the possible existence of an alternative, shorter transcript of the dystrophin locus, that is predicted to encode the carboxyl terminus of dystrophin alone (Bar et al., 1990; Rapaport et al., 1992). This short transcript and encoded protein are predicted to be present in mdx mouse brain though full length dystrophin is absent in the mdx mouse (Hoffman et al., 1987). To demonstrate specificity by immunofluorescent microscopy, we competed the binding of DRP antibodies by either DRP or dystrophin carboxylterminal fusion peptides. Serial cryostat sections of mdx mouse brain were incubated with DRP antibody either with an excess of DRP carboxyl-terminal fusion protein or with excess of a dystrophin carboxy-terminai fusion protein. As shown in Fig. 2, DRP immunolabeling was successfully competed by the presence of DRP fusion protein but not by dystrophin fusion protein. This suggests that the affinitypurified DRP antibodies used were specific for DRP and capable of recognizing DRP-peptide despite the presence of the peptides encoding the carboxyl terminus of dystrophin, by immunofluorescence. Enrichment of DRP in Blood Vessels and the Choroid Plexus Having ensured that the DRP antibodies were specific and sensitive enough to distinguish DRP from dystrophin, we used these affinity-purified antibodies to quantitate regional differences in the amount of DRP in the (full length) dystrophin null mutant mdx mouse brain. Various regions of adult mdx mouse brain were dissected out, protein content quantitared, and equivalent amounts fractionated by SDS-PAGE, electro-transferred and immunoblotted with DRP antibodies. As shown in Fig. 3, DRP was detected in blood vessels, choroid plexus, cerebral cortex, cerebellum, internal cap- Figure 1. Specificity of affinity purified DRP antibodies by immunoblotting. Equivalent amounts of purified fusion peptides containing the carboxyl termini of DRP (DRP fusion: nucleotide position 690-1,353, Love et al., 1989; homologous to 10,457-11,518 of the dystrophin sequence, Koenig et al., 1988) and dystrophin (dll fusion: nucleotide position 9,786-11,555 of the dystrophin sequence, Koenig et al., 1988) were fractionated by SDS-PAGE and electrotransferred to nitrocellulose. Immunoblots were processed using affinity purified antibodies to the carboxyl termini of DRP. As shown above, DRP antibodies recognized minute amounts of DRP fusion peptides ('~60 kd) but not fusion peptides expressed by the dl 1 clone encoding the carboxyl terminus of dystrophin (predicted weight '~100 kd). sule, spinal cord, and leptomeninges. The amount of DRP in vascular tissue and the highly vascularized choroid plexus of the brain was observed to be significantly greater than elsewhere. To quantitate the relative amount of DRP in various regions of the brain, the immunoblot was subjected to densitometric scanning and the level of DRP was found to be approximately threefold greater in cerebral blood vessels and choroid plexus compared with other regions of the brain. Similar results were obtained on three independent trials using the brains from different mice (data not shown). Immunolocalization of DRP in the mdx Mouse Brain Having determined by Western blotting areas of the brain that were enriched in DRP, we extended these findings using immunofluorescent microscopy. A survey of cross sections of brain tissue revealed intense DRP immunolabeling at the pia mater, choroid plexus, and intracerebrai vasculature, Khurana et al. Location of Dystrophin-related Protein in the Brain 359
4 Figure 2. Specificity of affinity purified DRP antibodies by immunofluorescence. 10-#m-thick serial sections of mdx mouse brains were incubated for I h with (A) DRP antibodies alone, (8) DILP antibodies + I mg dystrophin fusion peptide (DYS-pep: dll fusion: nucleotide position 9, ,555 of the dystrophin sequence, Koenig et al., 1988), (C) DRP antibodies + DRP fusion protein (DRP-pep: nuclcotide position 690-1,353, Love et al., 1989; homologous to 10,45%11,518 of the dystrophin sequence, Kocnig et al., 1988) and (D) Prcimmune scra (PRE). Slides were processed as described in Materials and Methods and photographed. The immunolabeling pattern was unchanged despite the presence of DYS-pop. The immunolabeling was competed with DRP-pep itself as evidenced by precipitated complexes, consistent with previous results obtained while affinity purifying the DRP antibodies against the DRP-pep (Khurana et al., 1990). Bar, 50 #tm. The Journal of Cell Biology, Volume 119,
5 Figure 3. Immunoblot analysis of DRP content in various regions of the mdx mouse brain. Various regions of the brain were dissected, solubilized, and proteins quantified as described in Materials and Methods. Equivalent amounts were fractionated by electrophoresis and immunoblotted with DRP antibodies. Different regions analyzed were (1) cerebral blood vessels, (2) ehoroid plexus, (3) cerebral cortex (4) cerebellum, (5) internal capsule, (6) spinal cord (cervical level), (7) skeletal muscle (control), and (8) leptomeninges (separate blot, same quantity of total protein). As demonstrated the DRP content (marked DRP; 427 kd) of cerebral blood vessels and choroid plexus is greater than other regions of the brain. The experiment was repeated three times and one representative blot subjected to densitometric analysis. DRP content of vessels and choroid was 3 times greater than other regions. however, only minimal DRP immunolabeling was detected in neuronal or glial elements of brain parenchyma (Fig. 4 A). The pia mater was immunolabeled, both at the surface of the brain and within infolding of the sulci. Intracerebral vascular structures such as veins, arteries, smaller arterioles and capillaries were immtmolabeled uniformly with the antibody (Fig. 4 B). The choroid plexus was found to label intensely, as were the specialized vessels forming the plexus, vessels associated with the ependyma and the ependyma itself (Fig. 4 C). The immunolabeling associated with the pial surfaces on the exterior surface of the brain was seen to follow invaginations of vasculature into the brain parenchyma along the anatomical space of Virchow-Robin (Fig. 4 D), however, it was not possible to distinguish, at the level of light microscopy if the leptomeningial immunolabeling was at the outer pial layer or associated with the inner, closely associated glia limitans. The identity of immunolabeled structures was verified by analyzing their characteristic morphology with phase-contrast microscopy. In some cases serial sections were also stained with haematoxylin and eosin (H&E), crystal violet or labeled with antibodies against the endothelial marker, yon Willebrand's factor to further validate the nature of identified structures. Additionally, similar immunolabeling results were obtained using normal B10 mice (data not shown). Preimmune sera from the same rabbit was used as a negative control to exclude nonspecific cross-reactivity in all experiments for both immunolabeling (Fig. 4 E), and immunoblots of dissected mdr cerebral blood vessels (data not shown). DRP Immunolabeling Across Species After definition of DRP distribution in the mammalian brain by immunofiuorescence we addressed the question of whether the distribution of DRP in the brain was conserved across species, since such conservation is often indicative of significant physiological function. Previously it has been demonstrated that the gene encoding DRP, the protein and the subcellular distribution of DRP in the muscle is conserved across species (Love et al., 1989; Khurana et al., 1990; Cartaud et al., 1992). We therefore analyzed by immunofluorescent microscopy, the distribution of DRP in cryosections of brain from Raja erinacea, an elasmobranch fish that is evolutionarily quite divergent from mammals. Significant, uniform immunolabeling was visualized at intracerebral vasculature with DRP antibodies (Fig. 5 A). Since the Raja erinacea intracerebral vessels possess a fenestrated endothelial lining rather than a relatively impermeable one, an ideal comparison would entail using the few rare regions of the mammalian brain parenchyma proper that have fenestrated endothelium as well. We therefore analyzed the area postrema from the mdx mouse brain (an area with fenestrated endothelium) for this comparison, and found similar patterns of immunolabeling with DRP antibodies as well (Fig. 5 B). The overall pattern of DRP immunolabeling in both regions was similar to that seen in other regions of the mdx mouse brain (Fig. 4). DRP Is Expressed in Tissue Culture While DRP immunolabeling was consistently associated with intracerebral capillaries, owing to the limited resolution by immunofluorescence microscopy, it is possible that DRP is distributed with components of the brain that lie in close proximity to the capillaries rather than the capillaries themselves. To address the possibility of immunolabeling either perivascular glia or neurons, these two major components of the brain were cultured in relative isolation from each other and analyzed for DRP content. Thus, we generated primary cultures of neurons and glia from mdx mouse brains, quantified proteins and analyzed equal amounts by immunoblotting with DRP antibodies. As shown in Fig. 6, both neurons and glial cultures expressed DRP however, glial cultures expressed significantly greater amounts of DRP than neuronal cultures. The lower expression of DRP in neurons is consistent with the minimal immunolabeling of identified neurons observed by immunofluorescence. The high levels of DRP expression in glial cultures may reflect the increased expression of DRP in the pool of perivascular glia, consistent with the possibility that perivascular regions of the brain were immunolabeled by DRP antibodies (Fig. 4). DRP Is Selectively Expressed at the Foot Processes of Perivascular Astrocytes Both immunofluorescent microscopy and tissue culture experiments using DRP antibodies coupled with the anatomical structure of the intracerebral vasculature suggest the possibility that perivascular glia are enriched in DRP. To Khurana r al Location of Dystrophin=related Protein in the Brain 361
6 The Journal of Cell Biology, Volume 119,
7 Figure 5. Immunolocalization of DRP in Elasmobranch fish brain and the area postrema of mdx mouse brain. 10-#m cryosections of brain tissue from Raja erinacea (A) and mdx mouse brain (B) were labeled with DRP antibodies. (A) Vascular labeling was similar to that seen in murine brain (compare with Fig. 4). (B) Transverse section at the level of the area postrema shows immunolabeled blood vessels, the overall pattern of both panels is similar to that seen in the rest of the mdx mouse brain. Bar, 50 #m. address this issue directly and define the subceuular localization of DRP we used the higher resolution offered by immunogold EM to examine the distribution of DRP in mdx brain sections. DRP was seen to selectively label the end-feet or foot processes of astrocytes closely apposed to intracerebral blood vessels (Fig. 7). Astrocytes were identified by their characteristic morphology and presence of glial filaments within the foot process (Fig. 7). The immunolabeling was seen to be maximal at the inner plasma face of astrocytes, regions forming the glio-vascular interface. Since the perivascular glia completely surround capillaries, this location of DRP is consistent with the uniform perivascular immunolabeling seen by fluorescent microscopy using DRP antibodies (Fig. 4). No immunolabeling was noted at the luminal aspect of basal lamina surrounding the endothelial cells. Sections labeled with either no primary or control antibodies were negative (data not shown). Discussion Using complementary immunoassays, cell biological, and microscopic techniques, we have determined the distribution of the chromosome 6 encoded DRP in the mdx mouse brain. DRP is expressed in neuronal, glial, and vascular cells of the brain and is enriched in neuroglial cells. DRP is localized at the end-feet of astrocytes closely apposed to capillaries, pia mater, intracerebral vasculature, the ependyma, and choroid plexus. Since proteins structurally similar to DRP are known to exist (e.g., dystrophin), it is of crucial importance to exclude their inadvertent visualization in studies such as this. To avoid anomalous cross-reaction with dystrophin we used the mdx mouse. This mouse strain harbors a point mutation in the dystrophin gene that precludes the translation of dystrophin, hence analysis of brain tissue from this mouse excludes the possibility of inadvertent visualization of full length dystrophin (Sicinski et al. 1989). Importantly, while no full length dystrophin is made in the mdx mouse, an alternative, smaller transcript encoding dystrophin's carboxyl terminus alone has recently been suggested to exist and to be expressed in nonmuscular tissues of this mouse (Bar et al., 1990; Rapaport et al., 1992). However, the antibodies used here only recognize the full length DRP and do not detect the 71-kD, smaller, alternative DMD gene product in the brain of mdx mice (Lederfein et al., 1992) (Fig. 3). Furthermore, peptide competition experiments clearly demonstrate that DRP antibodies can recognize DRP even in the presence of an excess of dystrophin carboxylterminal epitopes, both on immunoblotting and immunohistochemistry (Figs. 1 and 2). The use of specific and sensitive DRP antibodies, correlation of immunohistochemical and immunoblot results and tissue culture experiments strongly suggest that we are describing the distribution of DRP itself rather than dystrophin or its known isoforms at the aforementioned sites, although this possibility cannot be formally ruled out in the absence of a null mutant for DRP. In mammals the term "blood-brain barrier" refers to the endothelial cell-based permeability barrier that prevents the Figure 4. Immunolocalization of DRP in the mdx mouse brain #m cryosections of mdx mouse brain were labeled with DRP antibodies and visualized by indirect immunofluorescence. (A) DRP antibodies label the chomid plexus and pia mater (open arrows). A muscular artery is also labeled (closed arrow). All calibers of cerebral vessels were labeled and visualized in different orientations (i.e., lengthwise, tangentially, and end-on) within the substance of both the cerebral (Crm) and cerebellar (Cbm) hemispheres. (B) Irnmunolabeling of arterioles (closed arrows) and invaginations of the pia mater around the vessels (open arrows). (C) Intense immunolabeling is seen in the choroid plexus (open arrows) and ependyma lining the ventricular cavity (closed arrows). (D) The immunolabeling associated with blood vessels is seen to be colocalized with investments of the pia mater from the surface of the cerebellum. E demonstrates a lack of immunolabeling with preimmune antisera from the same rabbit. (C) 100 #m; (A, B, and D) 50 #m. Khurana et al. Location of Dystrophin-related Protein in the Brain 363
8 Figure 6. DRP expression in tissue culture. Primary cultures of neurons and glia were made from mdx mouse brains. The cells were harvested, proteins quantified, and equivalent amounts electrophoresed and immunoblotted with DRP antibodies. DRP was detected in (1) mdx cerebral vessels (control), (2) mdx cultured neurons, (3) mdx cultured gila, and (4) mdx skeletal muscle (control). Note: background staining for various lanes with these DRP antibodies was dependent on the complexity of tissue type rather than loading differences (compare lanes 1 and 4; see Khurana et ai., 1990). As shown, DRP expression was greater in cultured glia as compared with neurons. entry of many blood-borne molecules into the brain parenchyma, thus helping maintain the milieu interieur necessary for neuronal function. Additionally, vascular pericytes and perivascular neuroglia, while not forming the actual barrier, have been ascribed a supportive role in the maintenance of blood-brain barrier integrity (Peters et al., 1991; Risau and Wolburg, 1990; Cserr and Bundgaard, 1984). Indeed, cocultured astrocytes are considered crucial for the induction of polarity and barrier properties among endothelial cells in murine models (Beck et al., 1984; Janzer and Raft, 1987; Tao-Cheng et al., 1987). Interestingly, in submammalian vertebrates (Elasmobranchs, e.g., Torpedo fish, skates and sharks) and invertebrates the endothelial cell layer is "leaky" and the barrier function is carried out exclusively by the endfeet of perivascular astrocytes (Bundgaard and Cserr, 1981). Such comparative studies have led to the suggestion that the mammalian (endothelial) blood-brain barrier may have evolved from an ancient one based solely on glial end-feet ensheathing blood vessels as they coursed through the primitive brain (Cserr and Bundgaard, 1984). Our demonstration that DRP is located within these perivascular astrocytes and conservation of its distribution over 400 million years of evolution strengthens the suggestion that perivascular astrocytes and proteins present in them (such as DRP) may play a role in maintaining the cellular specializations associated with the blood-brain barrier, perhaps by influencing the spatial regulation of intrarnembranous proteins such as ion channels or the orthogonal array of particles (OAPs) that cluster in this region of the astrocyte (Risau and Wolburg, 1990). Other members of the spectrin superfamily of cytoskeletal proteins have previously been demonstrated to interact with and stabilize integral membrane proteins, thus helping to form and maintain regional cellular specializations (Srinivasan et al., 1988; Coleman et al., 1989; Bennet 1990). Ohlendieck and coauthors drew an analogy between the localization of DRP and certain isoforms of laminin in the adult skeletal muscle (Ohlendieck et al., 1991). The laminins are constituents of the basal lamina which surround mammalian muscle and are known to be particularly well Figure 7. Immunoelectron microscopy of DRP at the bloodbrain interface. Ultrathin cryosections of mdx mouse brain were incubated with DRP antibodies and distribution of DRP revealed by 5-nm goat antirabbit gold conjugates. The figure demonstrates the ultrastructure of the blood-brain barrier. An astrocytic foot process (Ast) is seen to be closely apposed to an intracerebral capillary. Glial filaments (gf) present in the astrocytic foot process axe marked. '~lo-fold more gold particles (arrowheads) are seen to be distributed within astrocytic end-feet at the glio-vascular interface, than at the endothelial cells (End) or lumen of intracerebral capillaries. Bar, 0.2 tzm. The Journal of Cell Biology, Volume 119,
9 defined and somewhat distinct, at intramuscular nerves, vessels, and neuromuscular and myotendinous junctions of the muscle (Sanes, 1986; Sanes et al., 1990). The overall similarity of distribution of laminin(s) with DRP and dystrophin in the skeletal muscle is quite striking (Sanes and Hall, 1979; Sanes et al., 1990; Byers et al., 1991; Khurana et al., 1991; Man et al., 1991; Ohlendieck et al., 1991). In the brain, the basal lamina and laminin are known to be well defined at the distinct locales of the ependymal lining, choroid plexus, glia limitans, and the blood-brain barrier (Risau and Wolburg, 1990; Peters et al., 1991; Hagg et al., 1989; Chiu et al., 1991). We find it noteworthy that both the laminin(s) and DRP are similarly distributed in the brain as well. However, since DRP immunolabeling is wholly intracellular and laminin isoforms are distributed extracellularly, our study suggests that their interaction, if any, is likely to be indirect as has been demonstrated in case of dystrophin (Ibraghimov-Beskrovnaya et al., 1992). In conclusion, we have used a variety of techniques to describe the distribution of DRP in the mdx mouse brain. Consistent with predictions based on cross-reactivity of dystrophin antisera in mdx mice (Ishiura et al., 1990), we detected DRP in all regions of the brain tested including the pia mater, choroid plexus, cerebral vasculature, and spinal cord, a distribution which is quite different from that described for dystrophin (Miike et al., 1989; Lidov et al., 1990). The distribution of DRP in the brain was conserved over a significant period of evolution, from elasmobranch to mammals, suggesting that DRP plays an important physiological role. DRP was located by immunoelectron microscopy to the end-feet of perivascular astrocytes, thus its function is likely to be related to the maintenance of blood-brain barrier integrity. In view of the possible structural role(s) for DRP, we believe that further cell biological and tissue culture experiments are needed to address the issue of possible interactive/inductive properties of DRP and other proteins located at the bloodbrain barrier. Thanks to Drs. Manuel Graeber, Hart Lidov, Helen Cserr, and Kiichi Araham for invaluable advice and insightful comments on neuroanatomy, neuropathology, and the blood-brain barrier. To Drs. A. H. Beggs, T. J. Byers, and M. S. Anderson for constructive criticism, to Alan Frederikson for technical support, and to Dixon Yun for photography. This work was supported by grants from the National Institutes of Health (L. M. Kunkel), Muscular Dystrophy Association of U.S.A. (L. M. Kunkel), the National Science Foundation (S. C. Watkins) and the Division of Medical Sciences, Harvard Medical School (T. S. Khurana). L. M. Kunkel is an investigator of the Howard Hughes Medical Institute. Received for publication 10 April 1992 and in revised form 6 July References Arahata, K., S. Ishiura, T. Ishiguro, T. Tsukahara, Y. Suhara, C. Eguchi, T. Ishihara, L Nonaka, E. Ozawa, and H. Sugita Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptidc. Nature (Lond.). 333: Bar, S., E. Bamea, Z. Levy, S. Neuman, D. Yaffe, and U. Nudel A novel product of the Duchenne muscular dystrophy gene which greatly differs from known isoforms in its structure and tissue distribution. Biochem. J. 272: Beck, D. W., H. V. Vinters, M. N. Hart, and P. A. Cancilla (31ial cells infiuence polarity of the blood-brain barrier. J. Neuropathol. & Exp. Neurol. 43: Bennet, V Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol. Rev. 70: Bonilla, E., C. E. Samitt, A. F. Miranda, A. P. Hays, (3. Salviati, S. DiMauro, L. M. Kunkel, E. P. Hoffraan, and L. P. Rowland Duchenne Muscular Dystrophy: deficiency of dystrophin at the muscle celt surface. Cell. 54: Byers, T. L, A. Husain-Chishti, R. R. Dubreuil, D. Branton, and L. S. B. Goldstein Sequence similarity of the amino-terminal domain of Drosophila beta spectrin to alpha actinin and dystrophin. J. Cell. Biol. 109: Byers, T. L, L. M. Knnkel, and S. C. Watkins The subcellular distribution ofdystrophin in mouse skeletal, cardiac and smooth muscle. J. Cell Biol. 115: Bundgaard, M., and H. Cserr A glial blood-brain barrier in elasmobranchs. Brain Res, 226: Cartaod, A., M. A. Lodosky, F. M. S. Tomt, H. Collin, F. Stetzkowski- Marden, T. S. Khurana, L. M. Kunkel, M. Fardeau, J. P. Changeux, and J. Cartaud Localization of dystrophin and dystrophin related protein at the ele~tromotor synapse and neuromuscular junction in Torpedo Marmorata. Neuroscience. 48: Chiu, A. Y., A. E. D. L. Montems, R. A. Cole, S. Loera, andj. D. Vellis Lamiuln and s-lammin are produced and released by astrocytes, schwann cells and schwannomas in culture. Gila. 4: Coleman, T. R., D. J. Fishkind, M. S. Mooseker, and J. S. Morrow Functional diversity among spectrin isoforms. Cell MotiL Cytoskeleton. 12: Cserr, H. F., and M. Bundgaard. 1984, Blood-brain interfaces in vertebrates: a comparative approach. Am. J. Physiol. 246: Davison, M. D., and D. R. Critchley Alpha Actinin and the DMD protein contain spectrin-like repeats. Cell. 52: Ducheane de Boulogne Recherches sur la paralysie pseudo-hypertrophique ou paralysie myo-scltrosique, Arch. Gen. Medicine 1:5-23, Engel, A. G Ducherme dystrophy. In Myology. A. (3. Engel and B. Q. Banker, editors. McGraw Hill, New York Fardeau, M., F. M. S. Tomt, H. Collin, N. Augier, F. Pons, J. Ltger, and J. L~ger Prdsence d'une prottine de type dystrophine au niveau de la jonction neuromusculalre clans la dystrophie musculaire de Duchenne et la souris mutante<<mdx>>. C. R. Acad. Sci. Paris. 311: Freshney, R. I Culture of specific cell types. In Culture of Animal Cells. A Manual of Basic Techniques. R. L Freshney, editor. Alan R. Liss, Inc., New York. Hagg, T., D. Muir, E. Engvall, S. Varon, and M. Manthrope Lamininlike antigen in rat CNS neurons: distribution and changes upon brain injury and nerve growth factor treatment. Neuron. 3: Hoffman, E. P., and L. M. Kunkel Dystrophin abnormalities in Duchenne/Becker muscular dystrophy. Neuron. 2: Hoffman, E. P., R. H. Brown, Jr., and L. M. Kunkel Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 51: Hoffman, E. P., K. H. Fischbeck, R. H. Brown, Jr., M. Johnson, R. Medori, J. D. Loike, J. B. Harris, R. Waterston, M. Brooke, L. Specht, W. Kupsky, J. Chamberlain, C. T. Caskey, F. Shapiro, and L. M. Kunkel Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne or 13ecker's muscular dystrophy. N. Engl. J. Med, 318: Ibraghimov-Beskrovnaya, O., J. M. Ervasti, C. L Leveille, C. A. Slaughter, S. W. Sernett, and K. P. Campbell Primary structure of dystrophinassociated glycoproteins linking dystrophin to the extracellular matrix. Nature (Lond.). 355: Ishiura, S., K. Arahata, T. Tsukahara, R. Koga, H. Anraku, M. Yamagochi, T. Kikuchi, I. Nonaka, and H. Sugita Antibody against the C-terminal portion of dystropin erossreacts with the 400 kda protein in the pia mater of dystrophin-deficiant mdx mouse brain. J. Biochem. 107: Janzer, R. C., and M. C. Raft Astrocytes induce blood-brain barrier properties in endothelial cells, Nature (Lond.). 325: Khurana, T. S., E. P. Hoffraan, and L. M. Kunkel Identification of a Chromosome 6 encoded Dystrophin-Related Protein. J. Biol. Chem. 265: Khurana, T. S., S. C. Watldns, P. Chafey, J. Chelly, F. M. S. Tomt, M. Fardeau, J. C. Kaplan, and L. M. Kunkel Immunolocalization and developmental expression of dystrophin-related protein in skeletal muscle. Neuromuscular Disorders. 1:185-t94. Koenig, M., and L. M. Kunkel. I990. Detailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility. J. Biol. Chem. 265: Koonig, M., A. P. Monaco, and L. M. Kunkel The complete sequence of dystrophin predicts a rod-shaped cytosketetal protein. Cell. 53: Lederfein, D., Z. Levy, N. Augier, D. Morned, G. Morris, O. Fuchs, U. Nodel, and D. Yaffe A 71-kilodalton protein is a major product of the Ducherme muscular dystrophy gene in brain and other non muscle tissues. P.N.A.S. 89: Lidov, H. G. W.,T. J. Byers, S. C. Watkins, andl. M. Kunkel Localization of dystrophin to postsynaptic regions of the central nervous system cortical neurons. Nature (Lond.). 348: Love, D. R., D. F. Hill, G. Dickson, N. K. Spurr, B. C. Byth, R. F. Marsdan, F. S. Walsh, Y. H. Edwards, and K. E. Davies An autosomal transcript in skeletal muscle with homology to dystrophin. Nature (Lond.). 339: Khurana et al. Location of Dystrophin-related Protein in the Brain 365
10 Man, N., J. M. Ellis, D. R. Love, K. E. Davies, K. C. Gatter, G. Dickson, and G. E. Morris Localization of the DMDL gene-encoded dystrophin-related protein using a panel of nineteen monoclonal antibodies: presence at neuromuscular junction, in the sarcolemma of dystrophic skeletal muscle, in vascular and other smooth muscles, and in proliferating brain cell lines. J. Cell Biol. 115: Miike, T., M. Miyatake, J. Zhao, M. T. Yoshioka, and M. Uchino Immunohistochemical Dystrophin Reaction in Synaptic Regions. Brain Dev. 11: Nakamura, K., T. Tanaka, A. Kuwahara, and K. Takeo Microassay for proteins on nitrocellulose filter using protein dye-staining procedure. Anal. Biochem. 148: Ohlendieck, K., J. M. Ervasti, K. Matsumura, S. D. Kahl, C. J. Leveille, and K. P. Campbell Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron. 7: Peters, A., S. L. Palay, and H. D. Webster The meninges. In The Fine Structure of the Nervous System. Neurons and Their Supporting Cells. A. Peters, S. L. Palay, and H. D. Webster. Oxford University Press, New York Rapaport, D., D. Lederfein, J. T. den Dunnen, P. M. Grootscolten, G. B. Van Ommen, O. Fuchs, U. Nuclei, and D. Yaffe Characterization and cell type distribution of a novel, major transcript of the Duchenne Muscular Dystrophy gene. Differentiation. 49: Risau, W., and H. Wolburg Development of the blood-brain barrier. Trends. Neurosci. 13: Samitt, C. E., and E. Bonilla Immunocytochemical study of dystrophin at the myotendinous junction. Muscle & Nerve. 13: Sanes, J. R The extracellular matrix. In Myology. A. G. Engel and B. Q. Banker, editors. McGraw Hill, New York. Sanes, J. R., and Z. W. Hall Antibodies that bind specifically to synaptic sites on muscle fiber basal Lamina. J. Cell Biol. 83: Sanes, J. R., E. Engvall, R. Butkowski, and D. D. Hunter Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J. Cell Biol. 111: Sicinski, P., Y. Geng, A. S. Ryder-Cook, E. A. Barnard, M. G. Darlison, and P. J. Barnard The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science (Wash. DC). 244: Srinivasan, Y., L. Elmer, J. Davis, V. Bennet, and K. Angelides Ankyrin and spectrin associate with voltage-depondent sodium channels in brain. Nature (Lond.). 333: Tao-Cheng, J., Z Nagy, and M. W. Brightman Tight junctions of brain endothelium in vitro are enhanced by astroglia. J. Neurosci. 7: Takemitsu, M., S. Ishiura, R. Koga, K. Kamakura, K. Arahata, I. Nonaka, and H. Sugita Dystrophin-Related Protein in the fetal and denervated skeletal muscles of normal and mdx muscle. Biochem. Biophys. Res. Commun. 180: Tanaka, H., T. Ishiguro, C. Eguchi, K. Salto, and E. Ozawa Expression of a dystrophin-related protein associated with the skeletal muscle cell membrane. Histochemistry. 96:1-5. Volt, T., K. Haas, J. O. Leger, F. Pons, andj. J. I.,r Xp21 dystrophin and 6q dystrophin-related protein. Comparative immunolocalization using multiple antibodies. Am. J. Path. 139: Watldns, S. C In situ hybridization and immunohistochemistry. In Current Protocols in Molecular Biology. F. Ausubel, R. Blent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl, editors. John Wiley & Sons, New York. Watldns, S. C., E. P. Hoffman, H. S. Slayter, and L. M. Kunkel Immunoelectron microscopic localization of dystrophin in myofibres. Nature (Lond.). 333: Yoshioka, K., J. Zhao, M. Uchino, and T. Miike Dystrophin isoforms and/or cross-reactive proteins on neurons and glial cells in control and mdx central nervous systems. J. Neurol. Sci. 108: Zubrzycka-Gaarn, E. E., D. E. Bulman, G. Karpati, A. H. M. Burghes, B. Belfall, H. J. Klamut, J. Talbot, R. S. Hodges, P. N. Ray, and R. G. Worton The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature (Lond.). 333: The Journal of Cell Biology, Volume 119,
Muscular Dystrophy. Biol 405 Molecular Medicine
 Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Dystrophy Patients Lacking COOH-terminal Domains of Dystrophin
 Deficiency of Dystrophin-associated Proteins in Duchenne Muscular Dystrophy Patients Lacking COOH-terminal Domains of Dystrophin Kiichiro Matsumura, * Fernando M. S. Tome,t Victor lonasescu, James M. Ervasti,
Deficiency of Dystrophin-associated Proteins in Duchenne Muscular Dystrophy Patients Lacking COOH-terminal Domains of Dystrophin Kiichiro Matsumura, * Fernando M. S. Tome,t Victor lonasescu, James M. Ervasti,
Functional significance of dystrophin positive fibres
 632 Muscular Dystrophy Group Research Laboratories, Regional Neurosciences Centre, Newcastle General Hospital, Newcastle upon Tyne L V B Nicholson M A Johnson K M D Bushby D Gardner-Medwin Correspondence
632 Muscular Dystrophy Group Research Laboratories, Regional Neurosciences Centre, Newcastle General Hospital, Newcastle upon Tyne L V B Nicholson M A Johnson K M D Bushby D Gardner-Medwin Correspondence
What Cell Make Up the Brain and Spinal Cord
 What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
Distribution of type IV collagen, laminin, nidogen and fibronectin in the haemodynamically stressed vascular wall
 Histol Histopath (1 990) 5: 161-1 67 Histology and Histopathology Distribution of type IV collagen, laminin, nidogen and fibronectin in the haemodynamically stressed vascular wall Reinhold Kittelberger,
Histol Histopath (1 990) 5: 161-1 67 Histology and Histopathology Distribution of type IV collagen, laminin, nidogen and fibronectin in the haemodynamically stressed vascular wall Reinhold Kittelberger,
Localization of Dystrophin COOH-terminal Domain by the Fracture-label Technique
 Localization of Dystrophin COOH-terminal Domain by the Fracture-label Technique Stefano Squarzoni,* Patrizia Sabatelli,* Maria Cristina Maltarello,* Amelia Cataldi,w Roberto Di Primio,w and Nadir Mario
Localization of Dystrophin COOH-terminal Domain by the Fracture-label Technique Stefano Squarzoni,* Patrizia Sabatelli,* Maria Cristina Maltarello,* Amelia Cataldi,w Roberto Di Primio,w and Nadir Mario
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
10.1: Introduction. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial cells) Dendrites.
 10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
Nerve tissue & the Nervous System
 Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
Major Structures of the Nervous System. Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors
 Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
Differentiation of Duchenne and Becker Muscular Dystrophy Phenotypes with Amino- and Carboxy-Terminal Antisera Specific for Dystrophin
 Am. J. Hum. Genet. 48:295-304, 1991 Differentiation of Duchenne and Becker Muscular Dystrophy Phenotypes with Amino- and Carboxy-Terminal Antisera Specific for Dystrophin Dennis E. Bulman,*,t E. Gordon
Am. J. Hum. Genet. 48:295-304, 1991 Differentiation of Duchenne and Becker Muscular Dystrophy Phenotypes with Amino- and Carboxy-Terminal Antisera Specific for Dystrophin Dennis E. Bulman,*,t E. Gordon
Predicted and observed sizes of dystrophin in some patients with gene deletions that disrupt the open reading frame
 892 8 Med Genet 1992; 29: 892-896 Muscular Dystrophy Group Research Laboratories, Regional Neurosciences Centre, Newcastle General Hospital, Newcastle upon Tyne NE4 6BE. L V B Nicholson K M D Bushby M
892 8 Med Genet 1992; 29: 892-896 Muscular Dystrophy Group Research Laboratories, Regional Neurosciences Centre, Newcastle General Hospital, Newcastle upon Tyne NE4 6BE. L V B Nicholson K M D Bushby M
Muscular Tissue. Functions of Muscular Tissue. Types of Muscular Tissue. Skeletal Muscular Tissue. Properties of Muscular Tissue
 Muscular Tissue Functions of Muscular Tissue Muscle makes up a large percentage of the body s weight (40-50%) Their main functions are to: Create motion muscles work with nerves, bones, and joints to produce
Muscular Tissue Functions of Muscular Tissue Muscle makes up a large percentage of the body s weight (40-50%) Their main functions are to: Create motion muscles work with nerves, bones, and joints to produce
TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet
 Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
Website: thermofisher.com Customer Service (US): 1 800 955 6288 ext. 1 Technical Support (US): 1 800 955 6288 ext. 441 TSH Receptor Monoclonal Antibody (49) Catalog Number MA3-218 Product data sheet Details
LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:!
 LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:! Spinal cord and peripheral nerves! Eyes, Inner ear, nasal
LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:! Spinal cord and peripheral nerves! Eyes, Inner ear, nasal
Nervous system. Dr. Rawaa Salim Hameed
 Nervous system Dr. Rawaa Salim Hameed Central nervous system (CNS) CNS consists of the brain (cerebrum, cerebellum, and brainstem) and spinal cord CNS is covered by connective tissue layers, the meninges
Nervous system Dr. Rawaa Salim Hameed Central nervous system (CNS) CNS consists of the brain (cerebrum, cerebellum, and brainstem) and spinal cord CNS is covered by connective tissue layers, the meninges
Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by
 Nakano et al. Supplementary information 1. Supplementary Figure 2. Methods 3. References Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into
Nakano et al. Supplementary information 1. Supplementary Figure 2. Methods 3. References Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into
Cellular components of CNS
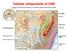 Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
Three Muscular Dystrophies: Loss of Cytoskeleton-Extracellular Matrix Linkage
 Cell, Vol. 80, 675-679, March 10, 1995, Copyright 1995 by Cell Press Three Muscular Dystrophies: Loss of Cytoskeleton-Extracellular Matrix Linkage Review Kevin P. Campbell Howard Hughes Medical Institute
Cell, Vol. 80, 675-679, March 10, 1995, Copyright 1995 by Cell Press Three Muscular Dystrophies: Loss of Cytoskeleton-Extracellular Matrix Linkage Review Kevin P. Campbell Howard Hughes Medical Institute
Cells of the nervous system
 Neurobiology Cells of the nervous system Anthony Heape 2011 1 Cells of the nervous system Neuroglia : part 2 The non excitable cells of the nervous system that provide support to neuronal survival and
Neurobiology Cells of the nervous system Anthony Heape 2011 1 Cells of the nervous system Neuroglia : part 2 The non excitable cells of the nervous system that provide support to neuronal survival and
FOCUS SubCell. For the Enrichment of Subcellular Fractions. (Cat. # ) think proteins! think G-Biosciences
 169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
Nature Neuroscience: doi: /nn.2275
 Supplementary Figure S1. The presence of MeCP2 in enriched primary glial cultures from rat or mouse brains is not neuronal. Western blot analysis of protein extracts from (a) rat glial and neuronal cultures.
Supplementary Figure S1. The presence of MeCP2 in enriched primary glial cultures from rat or mouse brains is not neuronal. Western blot analysis of protein extracts from (a) rat glial and neuronal cultures.
The 7 th lecture. Anatomy and Physiology For the. 1 st Class. By Dr. Ala a Hassan Mirza
 The 7 th lecture In Anatomy and Physiology For the 1 st Class By Dr. Ala a Hassan Mirza Nervous System (part I) The Nerve Tissue and the Nervous System The Tissues of the Body There are 4 types of tissues
The 7 th lecture In Anatomy and Physiology For the 1 st Class By Dr. Ala a Hassan Mirza Nervous System (part I) The Nerve Tissue and the Nervous System The Tissues of the Body There are 4 types of tissues
Supplementary Figure 1
 Supplementary Figure 1 The average sigmoid parametric curves of capillary dilation time courses and average time to 50% peak capillary diameter dilation computed from individual capillary responses averaged
Supplementary Figure 1 The average sigmoid parametric curves of capillary dilation time courses and average time to 50% peak capillary diameter dilation computed from individual capillary responses averaged
Dystrophin analysis using a panel of anti-dystrophin antibodies in Duchenne and Becker muscular dystrophy
 26 6Journal of Neurology, Neurosurgery, and Psychiatry 1993;56:26-31 Institute of Child Neurology and Psychiatry, Cagliari, Italy F Muntoni A Mateddu C Cianchetti M Marrosu Jerry Lewis Muscle Centre, Hammersmith
26 6Journal of Neurology, Neurosurgery, and Psychiatry 1993;56:26-31 Institute of Child Neurology and Psychiatry, Cagliari, Italy F Muntoni A Mateddu C Cianchetti M Marrosu Jerry Lewis Muscle Centre, Hammersmith
Nervous system Overview ( The master communication system)
 Nervous system Overview ( The master communication system) Neuron process Cell body nucleus Neuroglia Nerve Tissue COMPOSITION OF NERVE TISSUE Two principal types of cells, neurons and supporting cells
Nervous system Overview ( The master communication system) Neuron process Cell body nucleus Neuroglia Nerve Tissue COMPOSITION OF NERVE TISSUE Two principal types of cells, neurons and supporting cells
Chapter 7 Nerve tissue 1 Liu Jiamei
 Chapter 7 Nerve tissue 1 Liu Jiamei General description: nerve tissue nerve cells (neurons): show numerous long processes receive the stimulation make contact with each other, conduct the nerve impulse
Chapter 7 Nerve tissue 1 Liu Jiamei General description: nerve tissue nerve cells (neurons): show numerous long processes receive the stimulation make contact with each other, conduct the nerve impulse
Mammalian Membrane Protein Extraction Kit
 Mammalian Membrane Protein Extraction Kit Catalog number: AR0155 Boster s Mammalian Membrane Protein Extraction Kit is a simple, rapid and reproducible method to prepare cellular protein fractions highly
Mammalian Membrane Protein Extraction Kit Catalog number: AR0155 Boster s Mammalian Membrane Protein Extraction Kit is a simple, rapid and reproducible method to prepare cellular protein fractions highly
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Nervous System. Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI
 Nervous System Overview.I Histology.II Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI Repairs.VII Pathology.VIII.IV 1 Controls and integrates all body activities
Nervous System Overview.I Histology.II Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI Repairs.VII Pathology.VIII.IV 1 Controls and integrates all body activities
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Supplementary Figure 1 Subcellular segregation of VGluT2-IR and TH-IR within the same VGluT2-TH axon (wild type rats). (a-e) Serial sections of a dual VGluT2-TH labeled axon. This axon (blue outline) has
Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit
 PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
action potential afferent neuron Weblike; specifically, the weblike middle layer of the three meninges. arachnoid astrocytes autonomic nervous system
 action potential A large transient depolarization event, including polarity reversal, that is conducted along the membrane of a muscle cell or a nerve fiber. afferent neuron Nerve cell that carries impulses
action potential A large transient depolarization event, including polarity reversal, that is conducted along the membrane of a muscle cell or a nerve fiber. afferent neuron Nerve cell that carries impulses
HiPer Western Blotting Teaching Kit
 HiPer Western Blotting Teaching Kit Product Code: HTI009 Number of experiments that can be performed: 5/20 Duration of Experiment: ~ 2 days Day 1: 6-8 hours (SDS- PAGE and Electroblotting) Day 2: 3 hours
HiPer Western Blotting Teaching Kit Product Code: HTI009 Number of experiments that can be performed: 5/20 Duration of Experiment: ~ 2 days Day 1: 6-8 hours (SDS- PAGE and Electroblotting) Day 2: 3 hours
MOLECULAR AND CELLULAR NEUROSCIENCE
 MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
Neurons vs. glia. Traditionally, glia have been viewed as passive cells that help to maintain the function of neurons.
 GLIA Neurons vs. glia The defining characteristic of a neuron is its ability to transmit rapid electrical signals in the form of action potentials. All other neural cells that lack this property are broadly
GLIA Neurons vs. glia The defining characteristic of a neuron is its ability to transmit rapid electrical signals in the form of action potentials. All other neural cells that lack this property are broadly
PRODUCT INFORMATION & MANUAL
 PRODUCT INFORMATION & MANUAL Mitochondrial Extraction Kit NBP2-29448 Research use only. Not for diagnostic or therapeutic procedures www.novusbio.com P: 303.760.1950 P: 888.506.6887 F: 303.730.1966 technical@novusbio.com
PRODUCT INFORMATION & MANUAL Mitochondrial Extraction Kit NBP2-29448 Research use only. Not for diagnostic or therapeutic procedures www.novusbio.com P: 303.760.1950 P: 888.506.6887 F: 303.730.1966 technical@novusbio.com
Supplementary Materials for
 www.sciencetranslationalmedicine.org/cgi/content/full/4/117/117ra8/dc1 Supplementary Materials for Notch4 Normalization Reduces Blood Vessel Size in Arteriovenous Malformations Patrick A. Murphy, Tyson
www.sciencetranslationalmedicine.org/cgi/content/full/4/117/117ra8/dc1 Supplementary Materials for Notch4 Normalization Reduces Blood Vessel Size in Arteriovenous Malformations Patrick A. Murphy, Tyson
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 7 THE NERVOUS SYSTEM
 ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 7 THE NERVOUS SYSTEM Introduction The nervous system is the major controlling, regulatory, and communicating system in the body. It is the center of all mental
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 7 THE NERVOUS SYSTEM Introduction The nervous system is the major controlling, regulatory, and communicating system in the body. It is the center of all mental
Bio11: The Nervous System. Body control systems. The human brain. The human brain. The Cerebrum. What parts of your brain are you using right now?
 Bio11: The Nervous System Body control systems Nervous system Quick Sends message directly to target organ Endocrine system Sends a hormone as a messenger to the target organ Can target several organs
Bio11: The Nervous System Body control systems Nervous system Quick Sends message directly to target organ Endocrine system Sends a hormone as a messenger to the target organ Can target several organs
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Localization of talin in skeletal and cardiac muscles
 Volume 200, number 1 FEBS 3591 May 1986 Localization of talin in skeletal and cardiac muscles A.M. Belkin, N.I. Zhidkova and V.E. Koteliansky* Laboratory of Molecular and Cellular Cardiology, Institute
Volume 200, number 1 FEBS 3591 May 1986 Localization of talin in skeletal and cardiac muscles A.M. Belkin, N.I. Zhidkova and V.E. Koteliansky* Laboratory of Molecular and Cellular Cardiology, Institute
Instructions. Fuse-It-Color. Overview. Specifications
 Membrane fusion is a novel and highly superior method for incorporating various molecules and particles into mammalian cells. Cargo-specific liposomal carriers are able to attach and rapidly fuse with
Membrane fusion is a novel and highly superior method for incorporating various molecules and particles into mammalian cells. Cargo-specific liposomal carriers are able to attach and rapidly fuse with
RayBio Annexin V-Cy5 Apoptosis Detection Kit
 RayBio Annexin V-Cy5 Apoptosis Detection Kit User Manual Version 1.0 Mar 20, 2014 (Cat#: 68C5-AnnV-S) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll Free)1-888-494-8555 or
RayBio Annexin V-Cy5 Apoptosis Detection Kit User Manual Version 1.0 Mar 20, 2014 (Cat#: 68C5-AnnV-S) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll Free)1-888-494-8555 or
Minute TM Plasma Membrane Protein Isolation and Cell Fractionation Kit User Manual (v5)
 Minute TM Plasma Membrane Protein Isolation and Cell Fractionation Kit Catalog number: SM-005 Description Minute TM plasma membrane (PM) protein isolation kit is a novel and patented native PM protein
Minute TM Plasma Membrane Protein Isolation and Cell Fractionation Kit Catalog number: SM-005 Description Minute TM plasma membrane (PM) protein isolation kit is a novel and patented native PM protein
BI 232: Human Anatomy & Physiology
 BI 232: Human Anatomy & Physiology Roster Business Course Introduction and Syllabus Notecard Name E-mail Why you are taking the course Something interesting you did over break Lecture Tips Use the Study
BI 232: Human Anatomy & Physiology Roster Business Course Introduction and Syllabus Notecard Name E-mail Why you are taking the course Something interesting you did over break Lecture Tips Use the Study
Organization of The Nervous System PROF. MOUSAED ALFAYEZ & DR. SANAA ALSHAARAWY
 Organization of The Nervous System PROF. MOUSAED ALFAYEZ & DR. SANAA ALSHAARAWY Objectives At the end of the lecture, the students should be able to: List the parts of the nervous system. List the function
Organization of The Nervous System PROF. MOUSAED ALFAYEZ & DR. SANAA ALSHAARAWY Objectives At the end of the lecture, the students should be able to: List the parts of the nervous system. List the function
E.Z.N.A. SQ Blood DNA Kit II. Table of Contents
 E.Z.N.A. SQ Blood DNA Kit II Table of Contents Introduction and Overview...2 Kit Contents/Storage and Stability...3 Blood Storage and DNA Yield...4 Preparing Reagents...5 100-500 μl Whole Blood Protocol...6
E.Z.N.A. SQ Blood DNA Kit II Table of Contents Introduction and Overview...2 Kit Contents/Storage and Stability...3 Blood Storage and DNA Yield...4 Preparing Reagents...5 100-500 μl Whole Blood Protocol...6
Chapter 17 Nervous System
 Chapter 17 Nervous System 1 The Nervous System Two Anatomical Divisions Central Nervous System (CNS) Brain and Spinal Cord Peripheral Nervous System (PNS) Two Types of Cells Neurons Transmit nerve impulses
Chapter 17 Nervous System 1 The Nervous System Two Anatomical Divisions Central Nervous System (CNS) Brain and Spinal Cord Peripheral Nervous System (PNS) Two Types of Cells Neurons Transmit nerve impulses
Trypsin Mass Spectrometry Grade
 058PR-03 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Trypsin Mass Spectrometry Grade A Chemically Modified, TPCK treated, Affinity Purified
058PR-03 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Trypsin Mass Spectrometry Grade A Chemically Modified, TPCK treated, Affinity Purified
Skeletal Muscle and the Molecular Basis of Contraction. Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry
 Skeletal Muscle and the Molecular Basis of Contraction Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Like neurons, all muscle cells can be excited chemically, electrically, and
Skeletal Muscle and the Molecular Basis of Contraction Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Like neurons, all muscle cells can be excited chemically, electrically, and
PREPARATION OF IF- ENRICHED CYTOSKELETAL PROTEINS
 TMM,5-2011 PREPARATION OF IF- ENRICHED CYTOSKELETAL PROTEINS Ice-cold means cooled in ice water. In order to prevent proteolysis, make sure to perform all steps on ice. Pre-cool glass homogenizers, buffers
TMM,5-2011 PREPARATION OF IF- ENRICHED CYTOSKELETAL PROTEINS Ice-cold means cooled in ice water. In order to prevent proteolysis, make sure to perform all steps on ice. Pre-cool glass homogenizers, buffers
NERVOUS TISSUE. 1. Functional units of the nervous system; receive, process, store and transmit information to other neurons, muscle cells or glands.
 NERVOUS TISSUE LEARNING OBJECTIVES 1. Characterize and contrast the structure of neuronal cell bodies, dendrites and axons 2. List the classification of synapses and identify the basic structures of a
NERVOUS TISSUE LEARNING OBJECTIVES 1. Characterize and contrast the structure of neuronal cell bodies, dendrites and axons 2. List the classification of synapses and identify the basic structures of a
Nervous system is the most complex system in our body. It is formed by a network of more than 100 million nerve cells (neurons) assisted by many more
 Nervous system Nervous system is the most complex system in our body. It is formed by a network of more than 100 million nerve cells (neurons) assisted by many more glial cells. Devoid from connective
Nervous system Nervous system is the most complex system in our body. It is formed by a network of more than 100 million nerve cells (neurons) assisted by many more glial cells. Devoid from connective
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein
 Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Supplementary Figure 1. AdipoR1 silencing and overexpression controls. (a) Representative blots (upper and lower panels) showing the AdipoR1 protein content relative to GAPDH in two independent experiments.
Organization of The Nervous System PROF. SAEED ABUEL MAKAREM
 Organization of The Nervous System PROF. SAEED ABUEL MAKAREM Objectives By the end of the lecture, you should be able to: List the parts of the nervous system. List the function of the nervous system.
Organization of The Nervous System PROF. SAEED ABUEL MAKAREM Objectives By the end of the lecture, you should be able to: List the parts of the nervous system. List the function of the nervous system.
Biology Dr. Khalida Ibrahim Nervous system The nervous system is responsible for communication between different regions of the body, it is divided
 Biology Dr. Khalida Ibrahim Nervous system The nervous system is responsible for communication between different regions of the body, it is divided into: CNS (central nervous system) = brain + spinal cord
Biology Dr. Khalida Ibrahim Nervous system The nervous system is responsible for communication between different regions of the body, it is divided into: CNS (central nervous system) = brain + spinal cord
Chapter 9. Nervous System
 Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
Protein MultiColor Stable, Low Range
 Product Name: DynaMarker Protein MultiColor Stable, Low Range Code No: DM670L Lot No: ******* Size: 200 μl x 3 (DM670 x 3) (120 mini-gel lanes) Storage: 4 C Stability: 12 months at 4 C Storage Buffer:
Product Name: DynaMarker Protein MultiColor Stable, Low Range Code No: DM670L Lot No: ******* Size: 200 μl x 3 (DM670 x 3) (120 mini-gel lanes) Storage: 4 C Stability: 12 months at 4 C Storage Buffer:
Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Introduction Kit Components Cat. # # of vials Reagent Quantity Storage
 Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Catalog #5901 Introduction Human pluripotent stem cells (hpsc), including embryonic stem cells (ESC) and induced pluripotent stem
Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Catalog #5901 Introduction Human pluripotent stem cells (hpsc), including embryonic stem cells (ESC) and induced pluripotent stem
Product Use HPSC-CC are for research use only. It is not approved for human or animal use, or for application in in vitro diagnostic procedures.
 HPSC-derived Cardiomyocyte Cells (HPSC-CC) Catalog #6240 Cell Specification Human primary cardiomyocytes and cardiac tissue are superior modeling systems for heart disease studies, drug discovery and toxicity
HPSC-derived Cardiomyocyte Cells (HPSC-CC) Catalog #6240 Cell Specification Human primary cardiomyocytes and cardiac tissue are superior modeling systems for heart disease studies, drug discovery and toxicity
Instructions. Fuse-It-mRNA easy. Shipping and Storage. Overview. Kit Contents. Specifications. Note: Important Guidelines
 Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Membrane fusion is a highly efficient method for transfecting various molecules and particles into mammalian cells, even into sensitive and primary cells. The Fuse-It reagents are cargo-specific liposomal
Cells & Tissues. Chapter 3
 Cells & Tissues Chapter 3 Cell Theory Cell is structural and functional unit of life Activity of an organism is dependent upon its cells Principle of Complementarity functions of cells are dependent upon
Cells & Tissues Chapter 3 Cell Theory Cell is structural and functional unit of life Activity of an organism is dependent upon its cells Principle of Complementarity functions of cells are dependent upon
Mouse C-peptide EIA. Cat. No. YII-YK013-EX FOR LABORATORY USE ONLY
 Mouse C-peptide EIA Cat. No. YII-YK013-EX FOR LABORATORY USE ONLY TOYO 2CHOME, KOTO-KU, TOKYO, 135-0016, JAPAN http://www.cosmobio.co.jp e-mail : export@cosmobio.co.jp Phone : +81-3-5632-9617 FAX : +81-3-5632-9618
Mouse C-peptide EIA Cat. No. YII-YK013-EX FOR LABORATORY USE ONLY TOYO 2CHOME, KOTO-KU, TOKYO, 135-0016, JAPAN http://www.cosmobio.co.jp e-mail : export@cosmobio.co.jp Phone : +81-3-5632-9617 FAX : +81-3-5632-9618
Sheep Brain Dissection
 Sheep Brain Dissection Mammalian brains have many features in common. Human brains may not be available, so sheep brains often are dissected as an aid to understanding the mammalian brain since he general
Sheep Brain Dissection Mammalian brains have many features in common. Human brains may not be available, so sheep brains often are dissected as an aid to understanding the mammalian brain since he general
Human Anatomy and Physiology - Problem Drill 11: Neural Tissue & The Nervous System
 Human Anatomy and Physiology - Problem Drill 11: Neural Tissue & The Nervous System Question No. 1 of 10 The human body contains different types of tissue. The tissue is formed into organs and organ systems.
Human Anatomy and Physiology - Problem Drill 11: Neural Tissue & The Nervous System Question No. 1 of 10 The human body contains different types of tissue. The tissue is formed into organs and organ systems.
International Graduate Research Programme in Cardiovascular Science
 1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
1 International Graduate Research Programme in Cardiovascular Science This work has been supported by the European Community s Sixth Framework Programme under grant agreement n LSHM-CT-2005-01883 EUGeneHeart.
Good Morning! Take out your notes and vocab 1-10! Copyright 2003 Pearson Education, Inc. publishing as Benjamin Cummings
 Good Morning! Take out your notes and vocab 1-10! Functions of the Nervous System 1. Sensory input gathering information To monitor changes occurring inside and outside the body (changes = stimuli) 2.
Good Morning! Take out your notes and vocab 1-10! Functions of the Nervous System 1. Sensory input gathering information To monitor changes occurring inside and outside the body (changes = stimuli) 2.
Nervous System. Master controlling and communicating system of the body. Secrete chemicals called neurotransmitters
 Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Chapter Skeletal Muscle Structure and Function
 Chapter 10.2 Skeletal Muscle Structure and Function Introduction to Muscle Physiology Movement is a fundamental characteristic of all living things All muscle cells (skeletal, cardiac, and smooth) are
Chapter 10.2 Skeletal Muscle Structure and Function Introduction to Muscle Physiology Movement is a fundamental characteristic of all living things All muscle cells (skeletal, cardiac, and smooth) are
Histology of the CNS
 Histology of the CNS Lecture Objectives Describe the histology of the cerebral cortex layers. Describe the histological features of the cerebellum; layers and cells of cerebellar cortex. Describe the elements
Histology of the CNS Lecture Objectives Describe the histology of the cerebral cortex layers. Describe the histological features of the cerebellum; layers and cells of cerebellar cortex. Describe the elements
Muscle Tissue. General concepts. Classification of muscle. I. Functional classification is based on the type of neural control.
 Muscle Tissue LEARNING OBJECTIVES 1. Identify the three types of muscle tissue at the light microscopic level. 2. List and compare the structural and functional features of each of the three muscle fiber
Muscle Tissue LEARNING OBJECTIVES 1. Identify the three types of muscle tissue at the light microscopic level. 2. List and compare the structural and functional features of each of the three muscle fiber
Electron microscopy in the investigation and diagnosis of muscle disease
 Electron microscopy in the investigation and diagnosis of muscle disease Roy Weller Clinical Neurosciences University of Southampton School of Medicine Normal Muscle Normal Muscle The Sarcomere The names
Electron microscopy in the investigation and diagnosis of muscle disease Roy Weller Clinical Neurosciences University of Southampton School of Medicine Normal Muscle Normal Muscle The Sarcomere The names
Biology 218 Human Anatomy
 Chapter 17 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Overview of the Nervous System (p. 537) 1. The nervous system and the endocrine system are the body s major control and integrating centers.
Chapter 17 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Overview of the Nervous System (p. 537) 1. The nervous system and the endocrine system are the body s major control and integrating centers.
Ventricles, CSF & Meninges. Steven McLoon Department of Neuroscience University of Minnesota
 Ventricles, CSF & Meninges Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Thursday (Sept 14) 8:30-9:30am Surdyk s Café in Northrop Auditorium Stop by for a minute or an
Ventricles, CSF & Meninges Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Thursday (Sept 14) 8:30-9:30am Surdyk s Café in Northrop Auditorium Stop by for a minute or an
Medical Biology. Dr. Khalida Ibrahim
 Dr. Khalida Ibrahim Medical Biology MUSCLE TISSUE 1. Muscle tissue is characterized by its well-developed properties of contraction. 2. Muscle is responsible for the movements of the body and the various
Dr. Khalida Ibrahim Medical Biology MUSCLE TISSUE 1. Muscle tissue is characterized by its well-developed properties of contraction. 2. Muscle is responsible for the movements of the body and the various
Procaspase-3. Cleaved caspase-3. actin. Cytochrome C (10 M) Z-VAD-fmk. Procaspase-3. Cleaved caspase-3. actin. Z-VAD-fmk
 A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization
 Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Immunohistochemical Analysis of Dystrophin-associated Proteins in Becker/Duchenne Muscular Dystrophy with Huge In-frame
 Immunohistochemical Analysis of Dystrophin-associated Proteins in Becker/Duchenne Muscular Dystrophy with Huge In-frame Deletions in the NH2-Terminal and Rod Domains of Dystrophin Kiichiro Matsumura,*
Immunohistochemical Analysis of Dystrophin-associated Proteins in Becker/Duchenne Muscular Dystrophy with Huge In-frame Deletions in the NH2-Terminal and Rod Domains of Dystrophin Kiichiro Matsumura,*
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Skeletal Muscle : Structure
 1 Skeletal Muscle : Structure Dr.Viral I. Champaneri, MD Assistant Professor Department of Physiology 2 Learning objectives 1. Gross anatomy of the skeletal muscle 2. Myofilaments & their molecular structure
1 Skeletal Muscle : Structure Dr.Viral I. Champaneri, MD Assistant Professor Department of Physiology 2 Learning objectives 1. Gross anatomy of the skeletal muscle 2. Myofilaments & their molecular structure
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Evaluation of directed and random motility in microslides Assessment of leukocyte adhesion in flow chambers
 Evaluation of directed and random motility in microslides Motility experiments in IBIDI microslides, image acquisition and processing were performed as described. PMN, which ended up in an angle < 180
Evaluation of directed and random motility in microslides Motility experiments in IBIDI microslides, image acquisition and processing were performed as described. PMN, which ended up in an angle < 180
Peripheral nerve dystroglycan: its function and potential role in the molecular pathogenesis of neuromuscular diseases
 Y. Fukuyama, M. Osawa and K. Saito (Eds.), Congenital Muscular Dyrfrophies O 1997 Elsevier Science B.V. All rights reserved CHAPTER 22 Peripheral nerve dystroglycan: its function and potential role in
Y. Fukuyama, M. Osawa and K. Saito (Eds.), Congenital Muscular Dyrfrophies O 1997 Elsevier Science B.V. All rights reserved CHAPTER 22 Peripheral nerve dystroglycan: its function and potential role in
Yara Saddam. Amr Alkhatib. Ihsan
 1 Yara Saddam Amr Alkhatib Ihsan NOTE: Yellow highlighting=correction/addition to the previous version of the sheet. Histology (micro anatomy) :- the study of tissues and how they are arranged into organs.
1 Yara Saddam Amr Alkhatib Ihsan NOTE: Yellow highlighting=correction/addition to the previous version of the sheet. Histology (micro anatomy) :- the study of tissues and how they are arranged into organs.
Zool 3200: Cell Biology Exam 4 Part II 2/3/15
 Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Name:Key Trask Zool 3200: Cell Biology Exam 4 Part II 2/3/15 Answer each of the following questions in the space provided, explaining your answers when asked to do so; circle the correct answer or answers
Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples:
 Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
The Nervous System. Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output
 The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
Olfactory ensheathing glia
 Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Lesson 14. The Nervous System. Introduction to Life Processes - SCI 102 1
 Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Lesson 14 The Nervous System Introduction to Life Processes - SCI 102 1 Structures and Functions of Nerve Cells The nervous system has two principal cell types: Neurons (nerve cells) Glia The functions
Central Nervous System (CNS) -> brain and spinal cord. Major Divisions of the nervous system:
 Central Nervous System (CNS) -> brain and spinal cord Major Divisions of the nervous system: Afferent (sensory input) -> cell bodies outside of the central nervous system (CNS), carry info into the CNS
Central Nervous System (CNS) -> brain and spinal cord Major Divisions of the nervous system: Afferent (sensory input) -> cell bodies outside of the central nervous system (CNS), carry info into the CNS
Nervous tissue, charachteristics, neurons, glial cells
 Nervous tissue, charachteristics, neurons, glial cells Functional Organization of Nervous Tissue The Nervous System Components Brain, spinal cord, nerves, sensory receptors Responsible for Sensory perceptions,
Nervous tissue, charachteristics, neurons, glial cells Functional Organization of Nervous Tissue The Nervous System Components Brain, spinal cord, nerves, sensory receptors Responsible for Sensory perceptions,
Functional Organization of Nervous Tissue. Nervous tissue, charachteristics, neurons, glial cells. The Nervous System. The Nervous System 21/12/2010
 Nervous tissue, charachteristics, neurons, glial cells Functional Organization of Nervous Tissue The Nervous System Components Brain, spinal cord, nerves, sensory receptors Responsible for Sensory perceptions,
Nervous tissue, charachteristics, neurons, glial cells Functional Organization of Nervous Tissue The Nervous System Components Brain, spinal cord, nerves, sensory receptors Responsible for Sensory perceptions,
Supporting Information. Maximizing the Supported Bilayer Phenomenon: LCP Liposomes Comprised Exclusively
 S-1 Supporting Information Maximizing the Supported Bilayer Phenomenon: LCP Liposomes Comprised Exclusively of PEGylated Phospholipids for Enhanced Systemic and Lymphatic Delivery Matthew T. Haynes and
S-1 Supporting Information Maximizing the Supported Bilayer Phenomenon: LCP Liposomes Comprised Exclusively of PEGylated Phospholipids for Enhanced Systemic and Lymphatic Delivery Matthew T. Haynes and
Neurophysiology scripts. Slide 2
 Neurophysiology scripts Slide 2 Nervous system and Endocrine system both maintain homeostasis in the body. Nervous system by nerve impulse and Endocrine system by hormones. Since the nerve impulse is an
Neurophysiology scripts Slide 2 Nervous system and Endocrine system both maintain homeostasis in the body. Nervous system by nerve impulse and Endocrine system by hormones. Since the nerve impulse is an
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
Ultrastructural Contributions to Desensitization at the Cerebellar Mossy Fiber to Granule Cell Synapse
 Ultrastructural Contributions to Desensitization at the Cerebellar Mossy Fiber to Granule Cell Synapse Matthew A.Xu-Friedman and Wade G. Regehr Department of Neurobiology, Harvard Medical School, Boston,
Ultrastructural Contributions to Desensitization at the Cerebellar Mossy Fiber to Granule Cell Synapse Matthew A.Xu-Friedman and Wade G. Regehr Department of Neurobiology, Harvard Medical School, Boston,
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
The dystrophin glycoprotein complex (DGC)1 consists
 Molecular Organization of Sarcoglycan Complex in Mouse Myotubes in Culture Yiu-mo Chan,* Carsten G. Bönnemann,* Hart G.W. Lidov, and Louis M. Kunkel* *Howard Hughes Medical Institute, Division of Genetics,
Molecular Organization of Sarcoglycan Complex in Mouse Myotubes in Culture Yiu-mo Chan,* Carsten G. Bönnemann,* Hart G.W. Lidov, and Louis M. Kunkel* *Howard Hughes Medical Institute, Division of Genetics,
