Genomics-driven discovery of taiwachelin, a lipopeptide siderophore from Cupriavidus taiwanensis
|
|
|
- Ambrose Pierce
- 5 years ago
- Views:
Transcription
1 Supporting information Genomics-driven discovery of taiwachelin, a lipopeptide siderophore from Cupriavidus taiwanensis Martin F. Kreutzer and Markus Nett * Table of contents SI-2, SI-3 SI-4 SI-5 SI-6 SI-7 SI-8 SI-9 SI-10 SI-11 SI-12 SI-13 SI-14 SI-15 SI-16 SI-17 SI-18 SI-19 Table S1 Table S2 Figure S1 Figure S2 Figure S3 Table S3 Figure S4 Figure S5 Figure S6 Figure S7 Figure S8 Figure S9 Figure S10 Figure S11 Figure S12 Table S4 Figure S13 Environmental bacteria affiliated with the Burkholderiaceae and their siderophore biosynthentic potential Annotation of the tai biosynthesis genes MALDI-TOF/TOF fragmentation of taiwachelin HR-ESI-MS spectrum of taiwachelin HR-ESI-MS spectrum of Fe(III)-taiwachelin NMR spectroscopic data of taiwachelin in DMSO-d 6 COSY and HMBC correlations in taiwachelin 1 H NMR spectrum of taiwachelin in DMSO-d 6 13 C NMR spectrum of taiwachelin in DMSO-d 6 DEPT135 spectrum of taiwachelin in DMSO-d 6 1 H, 1 H COSY spectrum of taiwachelin in DMSO-d 6 HSQC spectrum of taiwachelin in DMSO-d 6 HMBC spectrum of taiwachelin in DMSO-d 6 1 H, 15 N HMBC spectrum of a crude extract from C. taiwanensis LMG19424 in DMSO-d 6 FT-IR spectrum of taiwachelin Marfey s analysis of the amino acid moieties in taiwachelin Chiral GC-MS analysis of the Hbu moiety in taiwachelin SI-1
2 Table S1. Environmental bacteria affiliated with the Burkholderiaceae and their siderophore biosynthetic potential. Organism Genome size [Mbp] # of identified NRPS clusters 1 # of identified NIS clusters 1 genomic location of siderophore biosynthetic loci and their products 2 Burkholderia gladioli BSR3 Burkholderia glumae BGR1 Burkholderia phymatum STM815 Burkholderia phytofirmans PsJN Burkholderia thailandensis E264 Burkholderia xenovorans LB400 Burkholderia sp. YI (2) 0 bgla_2g16840 bgla_2g16980 (unknown) bgla_4p2050 bgla_4p2140 (micacocidin) (1) 0 bglu_2g09700 bglu_2g09840 (unknown) (1) 0 Bphy_4035 Bphy_4047 (ornibactin) (1) 0 Bphyt_4063 Bphyt_4079 (ornibactin) (2) 0 BTH_I2414 BTH_I2427 (ornibactin) BTH_II1820 BTH_II1833 (pyochelin) (1) 0 Bxe_B0517 Bxe_B0531 (ornibactin) (2) 0 BYI23_C BYI23_C (unknown) BYI23_D BYI23_D (ornibactin) Cupriavidus metallidurans CH34 Cupriavidus necator H16 Cupriavidus necator JMP134 Cupriavidus necator N-1 Cupriavidus taiwanensis LMG (1) Rmet_1110 Rmet_1118 (staphyloferrin B) (1) 1 (1) H16_B1676 H16_B1692 (cupriachelin) PHG120 PHG126 (vibrioferrin) (1) Reut_B3681 Reut_B3687 (unknown) (1) 2c c14900 (staphyloferrin B) (1) 0 RALTA_B1222 RALTA_B1239 (taiwachelin) 3 SI-2
3 Table S1. continued. Organism Genome size [Mbp] # of identified NRPS clusters 1 # of identified NIS clusters 1 genomic location of siderophore biosynthetic loci and their products Ralstonia pickettii 12D Ralstonia pickettii 12J Ralstonia solanacearum CFBP2957 Ralstonia solanacearum GMI1000 Ralstonia solanacearum Po82 Ralstonia solanacearum PSI (1) Rpic12D_0180 Rpic12D_0181 (unknown) (1) Rpic_0173 Rpic_0174 (unknown) (1) 1 (1) (1) 1 (1) (1) 1 (1) (1) 1 (1) RCFBP_mp10439 RCFBP_mp10449 (staphyloferrin B) RCFBP_mp20475 RCFBP_mp20485 (yersiniabactin) RSc1804 RSc1813 (micacocidin) RSp0414 RSp0424 (staphyloferrin B) RSPO_m01293 RSPO_m01304 (yersiniabactin) RSPO_m01404 RSPO_m01415 (staphyloferrin B) RPSI07_1865 RPSI07_1874 (micacocidin) RPSI07_mp0375 RPSI07_mp0385 (staphyloferrin B) Polynucleobacter necessarius subsp. asymbioticus QLW Polynucleobacter necessarius subsp. necessarius STIR The number of gene clusters that are putatively involved in siderophore biosynthesis is set in parentheses. NRPS, nonribosomal peptide synthetase; NIS, NRPSindependent siderophore synthetase. 2 Predicted products are set in parentheses. Compounds that have actually been observed are highlighted in bold. 3 Taiwachelin is first described in this study. SI-3
4 Table S2. Annotation of the tai biosynthesis genes. Gene designation C. taiwanensis Protein Accession no. Size (aa) Proposed function LMG19424 gene no. (GenBank) taia RALTA_B1222 YP_ Cyclase/dehydrase taib RALTA_B1223 YP_ MbtH-like taic RALTA_B1224 YP_ Type-II thioesterase taid RALTA_B1225 YP_ Loading module: FAAL-ACP-C-TauD taie RALTA_B1226 YP_ NRPS: C-A-PCP taif RALTA_B1227 YP_ NRPS: C-A-PCP-E-C-A-PCP taig RALTA_B1228 YP_ NRPS: C-A-PCP-E-C-A-PCP-C-A-PCP-TE taih RALTA_B1229 YP_ TonB-dependent siderophore receptor taii RALTA_B1230 YP_ Iron-regulated membrane protein taij RALTA_B1231 YP_ Hypothetical protein taik RALTA_B1232 YP_ Siderophore-iron reductase tail RALTA_B1233 n/a 244 Transposase taim RALTA_B1234 YP_ ABC-type siderophore exporter tain RALTA_B1235 YP_ Esterase/lipase taio RALTA_B1236 YP_ N δ -hydroxyornithine O-acyltransferase taip RALTA_B1237 YP_ Ornithine N δ -monooxygenase taiq RALTA_B1238 YP_ Phosphopantetheinyl transferase tair RALTA_B1239 YP_ Iron-regulated transcription activating factor SI-4
5 Figure S1. MALDI-TOF/TOF fragmentation of taiwachelin. SI-5
6 Figure S2. HR-ESI-MS spectrum of taiwachelin. Theoretical mass (m/z): [M+H] + Delta (ppm): 0.17 Composition (calculated): C 41 H 71 O 18 N 8 SI-6
7 Figure S3. HR-ESI-MS spectrum of Fe(III)-taiwachelin. Theoretical mass (m/z): [M-H] - Delta (ppm): Composition (calculated): C 41 H 66 O 18 N 8 Fe SI-7
8 Table S3. NMR spectroscopic data of taiwachelin in dimethylsulfoxide-d 6. residue pos. δ C δ H, M (J in Hz) residue pos. δ C δ H, M (J in Hz) residue pos. δ C δ H, M (J in Hz) Fatty acid C L-Asp NH 8.15, d (7.8) L -N δ - NH 8.09, d (8.4) C , t (7.3) CO OH-Orn CO C , m Cα , ddd (7.8, 7.2, 6.8) (cyclic) Cα , ddd (10.7, 8.4, 5.1) C , m Cβ 36.1 a: 2.70, dd (16.5, 6.8) Cβ 27.3 a: 1.92, m C , m b: 2.53, dd (16.5, 7.2) b: 1.70, m C , m Cγ Cγ , m C-7 C-8 C-9 C-10 C-11 C , m 1.23, m 1.23, m 1.23, m 1.26, m 0.85, t (7.0) D-N δ - OH-Orn NH CO Cα Cβ Cγ Cδ , d (7.8) 4.29, m 1.47, m 1.55, m a: 3.50, m Cδ , m L-threo- NH 7.85, d (9.2) b: 3.42, m β-oh-asp CO Cα Cβ Cγ , dd (9.2, 2.7) 4.48, d (2.7) L-Hbu C-1 C-2 C a: 2.52, dd (15.0, 6.3) b: 2.37, dd (15.0, 6.3) 4.00, sext (6.3) D-allo-Thr NH 7.73, d (8.0) C , d (6.3) CO Cα Cβ Cγ , dd (8.0, 6.4) 3.82, quint (6.4) 1.03, d (6.4) L-Ser NH CO Cα Cβ , d (7.6) 4.31, m 3.59, d (7.2) SI-8
9 Figure S4. NMR-based structure elucidation of taiwachelin: COSY (bold lines) and selected HMBC correlations (arrows) observed in dimethylsulfoxide-d 6. SI-9
10 Figure S MHz 1 H NMR spectrum of taiwachelin in dimethylsulfoxide-d 6. SI-10
11 Figure S MHz 13 C NMR spectrum of taiwachelin in dimethylsulfoxide-d 6. SI-11
12 Figure S7. DEPT135 spectrum of taiwachelin in dimethylsulfoxide-d 6. SI-12
13 Figure S8. 1 H, 1 H COSY spectrum of taiwachelin in dimethylsulfoxide-d 6. SI-13
14 Figure S9. HSQC spectrum of taiwachelin in dimethylsulfoxide-d 6. SI-14
15 Figure S10. HMBC spectrum of taiwachelin in dimethylsulfoxide-d 6. SI-15
16 Figure S11. 1 H, 15 N HMBC spectrum of a crude extract from an iron-deficient culture of C. taiwanensis LMG19424 in dimethylsulfoxide-d 6. putative proton-to-hydroxamate nitrogen correlations SI-16
17 Figure S12. FT-IR spectrum of taiwachelin. SI-17
18 Table S4. Marfey s analysis of the amino acid moieties in taiwachelin. amino acid m/z [M+H] + retention time (min): Marfey product of L-amino acid standard retention time (min): Marfey product of D-amino acid standard retention time (min): Marfey product of amino acid from taiwachelin hydrolysis threo-β-hydroxyaspartic acid allo-threonine aspartic acid ornithine / 9.4 serine* *HPLC separation using 10 mm ammonium formate buffer SI-18
19 Figure S13. Chiral GC-MS analysis of trifluoroacetylated Hbu samples. A: S-Hbu derivative; B: R,S-Hbu derivative; C: taiwachelin derivative after cleavage with 0.3 M HCl; D: EI-MS fragmentation of the Hbu peak in chromatogram C the fragmentation pattern is identical to the derivatised commercial standards of Hbu. D SI-19
Supporting Information for: New aspercryptins, lipopeptide natural products, revealed by HDAC inhibition in Aspergillus nidulans
 Supporting Information for: New aspercryptins, lipopeptide natural products, revealed by HDAC inhibition in Aspergillus nidulans Matthew T. Henke a, Alexandra A. Soukup b, Anthony W. Goering a, Ryan A.
Supporting Information for: New aspercryptins, lipopeptide natural products, revealed by HDAC inhibition in Aspergillus nidulans Matthew T. Henke a, Alexandra A. Soukup b, Anthony W. Goering a, Ryan A.
Supplementary Figure 1. Amino acid sequences of GodA and GodA*. Inserted. residues are colored red. Numbers indicate the position of each residue.
 Supplementary Figure 1. Amino acid sequences of GodA and GodA*. Inserted residues are colored red. Numbers indicate the position of each residue. Supplementary Figure 2. SDS-PAGE analysis of purified recombinant
Supplementary Figure 1. Amino acid sequences of GodA and GodA*. Inserted residues are colored red. Numbers indicate the position of each residue. Supplementary Figure 2. SDS-PAGE analysis of purified recombinant
Supporting information
 Supporting information Rhabdopeptide/Xenortide-like Peptides from Xenorhabdus innexi with Terminal Amines Showing Potent Anti-protozoal Activity Lei Zhao,, Marcel Kaiser,, Helge B. Bode *,, Molekulare
Supporting information Rhabdopeptide/Xenortide-like Peptides from Xenorhabdus innexi with Terminal Amines Showing Potent Anti-protozoal Activity Lei Zhao,, Marcel Kaiser,, Helge B. Bode *,, Molekulare
Supplementary Information
 Supplementary Information New Highly Oxygenated Germacranolides from Carpesium divaricatum and their Cytotoxic Activity Tao Zhang, 1 Jin-Guang Si, 1, 2 Qiu-Bo Zhang, 1 Gang Ding, 1 and Zhong-Mei Zou 1*
Supplementary Information New Highly Oxygenated Germacranolides from Carpesium divaricatum and their Cytotoxic Activity Tao Zhang, 1 Jin-Guang Si, 1, 2 Qiu-Bo Zhang, 1 Gang Ding, 1 and Zhong-Mei Zou 1*
Supplementary Figure 1. The proposed biosynthetic pathways for the A-ring transformation of the
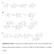 Supplementary Figure 1. The proposed biosynthetic pathways for the A-ring transformation of the kinamycin and lomaiviticin antibiotics. A, by Steven J. Gould; B, by Emily P. Balskus; C, by Bradley S. Moore.
Supplementary Figure 1. The proposed biosynthetic pathways for the A-ring transformation of the kinamycin and lomaiviticin antibiotics. A, by Steven J. Gould; B, by Emily P. Balskus; C, by Bradley S. Moore.
L-Carnosine-Derived Fmoc-Tripeptides Forming ph- Sensitive and Proteolytically Stable Supramolecular
 Supporting Information: L-Carnosine-Derived Fmoc-Tripeptides Forming ph- Sensitive and Proteolytically Stable Supramolecular Hydrogels Rita Das Mahapatra, a Joykrishna Dey* a, and Richard G. Weiss b a
Supporting Information: L-Carnosine-Derived Fmoc-Tripeptides Forming ph- Sensitive and Proteolytically Stable Supramolecular Hydrogels Rita Das Mahapatra, a Joykrishna Dey* a, and Richard G. Weiss b a
Supporting Information
 Supporting Information Wiley-VCH 2006 69451 Weinheim, Germany A Convergent Synthesis of N-Linked Glycopeptides Clyde M. Kaneshiro, Katja Michael* 1. LC-MS analysis of crude glycopeptide 16. 2. ESI-TOF
Supporting Information Wiley-VCH 2006 69451 Weinheim, Germany A Convergent Synthesis of N-Linked Glycopeptides Clyde M. Kaneshiro, Katja Michael* 1. LC-MS analysis of crude glycopeptide 16. 2. ESI-TOF
Supporting Information
 Supporting Information Asperphenins A and B, Lipopeptidyl Benzophenones from a Marinederived Aspergillus sp. Fungus Lijuan Liao, Song Yi Bae, Tae Hyung Won, Minjung You, Seong-Hwan Kim, Dong-Chan Oh, Sang
Supporting Information Asperphenins A and B, Lipopeptidyl Benzophenones from a Marinederived Aspergillus sp. Fungus Lijuan Liao, Song Yi Bae, Tae Hyung Won, Minjung You, Seong-Hwan Kim, Dong-Chan Oh, Sang
Preparation and Characterization of Cysteine Adducts of Deoxynivalenol
 Preparation and Characterization of Cysteine Adducts of Deoxynivalenol Ana Stanic, Silvio Uhlig, Anita Solhaug, Frode Rise, Alistair L. Wilkins, Christopher O. Miles S1 Figure S1. 1 H spectrum of 1 (DON)
Preparation and Characterization of Cysteine Adducts of Deoxynivalenol Ana Stanic, Silvio Uhlig, Anita Solhaug, Frode Rise, Alistair L. Wilkins, Christopher O. Miles S1 Figure S1. 1 H spectrum of 1 (DON)
Supporting Information
 Supporting Information A new series of cytotoxic pyrazoline derivatives as potential anticancer agents induces cell cycle arrest and apoptosis Hong Wang 1,, Jinhong Zheng 1,, Weijie Xu 1, Cheng Chen 1,
Supporting Information A new series of cytotoxic pyrazoline derivatives as potential anticancer agents induces cell cycle arrest and apoptosis Hong Wang 1,, Jinhong Zheng 1,, Weijie Xu 1, Cheng Chen 1,
Cytochalasins from an Australian marine sediment-derived Phomopsis sp. (CMB-M0042F): Acid-mediated intra-molecular cycloadditions enhance
 SUPPORTING INFORMATION Cytochalasins from an Australian marine sediment-derived Phomopsis sp. (CMB-M42F): Acid-mediated intra-molecular cycloadditions enhance chemical diversity Zhuo Shang, Ritesh Raju,
SUPPORTING INFORMATION Cytochalasins from an Australian marine sediment-derived Phomopsis sp. (CMB-M42F): Acid-mediated intra-molecular cycloadditions enhance chemical diversity Zhuo Shang, Ritesh Raju,
Identification of novel endophenaside antibiotics produced by Kitasatospora sp. MBT66
 SUPPORTING INFORMATION belonging to the manuscript: Identification of novel endophenaside antibiotics produced by Kitasatospora sp. MBT66 by Changsheng Wu 1, 2, Gilles P. van Wezel 1, *, and Young Hae
SUPPORTING INFORMATION belonging to the manuscript: Identification of novel endophenaside antibiotics produced by Kitasatospora sp. MBT66 by Changsheng Wu 1, 2, Gilles P. van Wezel 1, *, and Young Hae
THE JOURNAL OF ANTIBIOTICS. Polyketomycin, a New Antibiotic from Streptomyces sp. MK277-AF1. II. Structure Determination
 THE JOURNAL OF ANTIBIOTICS Polyketomycin, a New Antibiotic from Streptomyces sp. MK277-AF1 II. Structure Determination ISAO MOMOSE, WEI CHEN, HIKARU NAKAMURA, HIROSHI NAGANAWA, HIRONOBU IINUMA and TOMIO
THE JOURNAL OF ANTIBIOTICS Polyketomycin, a New Antibiotic from Streptomyces sp. MK277-AF1 II. Structure Determination ISAO MOMOSE, WEI CHEN, HIKARU NAKAMURA, HIROSHI NAGANAWA, HIRONOBU IINUMA and TOMIO
VOL. 56 NO. 2, FEB THE JOURNAL OF ANTIBIOTICS pp YM and YM , Novel Thiopeptide Antibiotics Produced
 VOL. 56 NO. 2, FEB. 2003 THE JOURNAL OF ANTIBIOTICS pp. 129-134 YM-266183 and YM-266184, Novel Thiopeptide Antibiotics Produced by Bacillus cereus Isolated from a Marine Sponge II. Structure Elucidation
VOL. 56 NO. 2, FEB. 2003 THE JOURNAL OF ANTIBIOTICS pp. 129-134 YM-266183 and YM-266184, Novel Thiopeptide Antibiotics Produced by Bacillus cereus Isolated from a Marine Sponge II. Structure Elucidation
Pyrrolizidine Alkaloids from Onosma kaheirei Teppner (Boraginaceae)
 Supporting Information Rec. Nat. Prod. 10:2 (2016) 221-227 Pyrrolizidine Alkaloids from nosma kaheirei Teppner (Boraginaceae) Ioanna-Maria rfanou 1, Harilaos Damianakos 1, Ioannis Bazos 2, Konstantia Graikou1
Supporting Information Rec. Nat. Prod. 10:2 (2016) 221-227 Pyrrolizidine Alkaloids from nosma kaheirei Teppner (Boraginaceae) Ioanna-Maria rfanou 1, Harilaos Damianakos 1, Ioannis Bazos 2, Konstantia Graikou1
2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked. amino-modification products by acrolein
![2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked. amino-modification products by acrolein 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked. amino-modification products by acrolein](/thumbs/86/94743397.jpg) Supplementary Information 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked amino-modification products by acrolein Ayumi Tsutsui and Katsunori Tanaka* Biofunctional Synthetic Chemistry Laboratory, RIKEN
Supplementary Information 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked amino-modification products by acrolein Ayumi Tsutsui and Katsunori Tanaka* Biofunctional Synthetic Chemistry Laboratory, RIKEN
Table S9A: List of taurine regulated genes in Bp K96243 Chr 1 (up regulated >=2 fold) Cluster no GENE ID Start Stop Strand Function
 Table S9A: List of taurine regulated genes in Bp K96243 Chr 1 (up regulated >=2 fold) Cluster no GENE ID Start Stop Strand Function 1 BPSL0024 26223 26621 + LrgA family BPSL0025 26690 27412 + hypothetical
Table S9A: List of taurine regulated genes in Bp K96243 Chr 1 (up regulated >=2 fold) Cluster no GENE ID Start Stop Strand Function 1 BPSL0024 26223 26621 + LrgA family BPSL0025 26690 27412 + hypothetical
Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2008
 Experimental Details Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich Chemical Company and were used as received. 2-DOS and neamine were kindly provided by Dr. F. Huang. Paromamine
Experimental Details Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich Chemical Company and were used as received. 2-DOS and neamine were kindly provided by Dr. F. Huang. Paromamine
Ynamides as racemization-free coupling reagents for amide and peptide synthesis
 Ynamides as racemization-free coupling reagents for amide and peptide synthesis Long Hu, Silin Xu, Zhenguang Zhao, Yang Yang, Zhiyuan Peng, Ming Yang, Changliu Wang, Junfeng Zhao* Key Laboratory of Chemical
Ynamides as racemization-free coupling reagents for amide and peptide synthesis Long Hu, Silin Xu, Zhenguang Zhao, Yang Yang, Zhiyuan Peng, Ming Yang, Changliu Wang, Junfeng Zhao* Key Laboratory of Chemical
Table removed due to copyright reasons.
 atural Product Biosynthesis Relevance of natural products in the pharmaceutical industry (see Table 2 in Butler J. at. Prod. 2004, 67, 2141-2153.)) Table removed due to copyright reasons. 2 * a phosphopantetheinyl
atural Product Biosynthesis Relevance of natural products in the pharmaceutical industry (see Table 2 in Butler J. at. Prod. 2004, 67, 2141-2153.)) Table removed due to copyright reasons. 2 * a phosphopantetheinyl
Lysoquinone-TH1, a new polyphenolic tridecaketide produced by expressing the lysolipin minimal PKS II in Streptomyces albus
 Supplemental Information Lysoquinone-TH1, a new polyphenolic tridecaketide produced by expressing the lysolipin minimal PKS II in Streptomyces albus Torben Hofeditz, 1 Claudia Eva-Maria Unsin 2, Jutta
Supplemental Information Lysoquinone-TH1, a new polyphenolic tridecaketide produced by expressing the lysolipin minimal PKS II in Streptomyces albus Torben Hofeditz, 1 Claudia Eva-Maria Unsin 2, Jutta
Supplementary Material. Efficient Synthesis of an Indinavir Precursor from Biomass Derived (-)- Levoglucosenone
 1.171/CH17227_AC CSIRO 217 Australian Journal of Chemistry 217, 7(1), 1146-115 Supplementary Material Efficient Synthesis of an Indinavir Precursor from Biomass Derived (-)- Levoglucosenone Edward T. Ledingham,
1.171/CH17227_AC CSIRO 217 Australian Journal of Chemistry 217, 7(1), 1146-115 Supplementary Material Efficient Synthesis of an Indinavir Precursor from Biomass Derived (-)- Levoglucosenone Edward T. Ledingham,
Acaulins A and B, Trimeric Macrodiolides from Acaulium. Table of Contents
 Supporting Information Acaulins A and B, Trimeric Macrodiolides from Acaulium sp. H-JQSF Ting Ting Wang, Ying Jie Wei, Hui Ming Ge, Rui Hua Jiao, Ren Xiang Tan* * Corresponding author. E-mail: rxtan@nju.edu.cn
Supporting Information Acaulins A and B, Trimeric Macrodiolides from Acaulium sp. H-JQSF Ting Ting Wang, Ying Jie Wei, Hui Ming Ge, Rui Hua Jiao, Ren Xiang Tan* * Corresponding author. E-mail: rxtan@nju.edu.cn
Study of On-Resin Convergent Synthesis of N-Linked. Glycopeptides Containing a Large High Mannose N- Linked Oligosaccharide
 Supporting Information Study of On-Resin Convergent Synthesis of N-Linked Glycopeptides Containing a Large High Mannose N- Linked Oligosaccharide Rui Chen and Thomas J. Tolbert* Department of Chemistry,
Supporting Information Study of On-Resin Convergent Synthesis of N-Linked Glycopeptides Containing a Large High Mannose N- Linked Oligosaccharide Rui Chen and Thomas J. Tolbert* Department of Chemistry,
Applying Molecular Networking for the Detection of
 Supporting Information Applying Molecular Networking for the Detection of Natural Sources and Analogues of the Selective Gq Protein Inhibitor FR900359 Raphael Reher, Markus Kuschak, Nina Heycke, Suvi Annala,
Supporting Information Applying Molecular Networking for the Detection of Natural Sources and Analogues of the Selective Gq Protein Inhibitor FR900359 Raphael Reher, Markus Kuschak, Nina Heycke, Suvi Annala,
Supporting Information. An Efficient Synthesis of Optically Active Physostigmine from Tryptophan via Alkylative Cyclization
 Supporting Information An Efficient Synthesis of Optically Active Physostigmine from Tryptophan via Alkylative Cyclization Michiaki, Kawahara, Atsushi Nishida, Masako Nakagawa* Faculty of Pharmaceutical
Supporting Information An Efficient Synthesis of Optically Active Physostigmine from Tryptophan via Alkylative Cyclization Michiaki, Kawahara, Atsushi Nishida, Masako Nakagawa* Faculty of Pharmaceutical
Your Name: Question 1. Spectrum Prediction I: Ethyl Acetoacetate. (15 points) ppm ppm ppm ppm. J(A,D) = 8 Hz = 0.
 Question 1. Spectrum Prediction I: Ethyl Acetoacetate. (15 points) A B C D 4.202 ppm 3.451 ppm 2.273 ppm 1.288 ppm J(A,D) = 8 Hz = 0.08 ppm (in CDCl 3 ) Draw the 100 MHz H-NMR spectrum to scale. Draw splitting
Question 1. Spectrum Prediction I: Ethyl Acetoacetate. (15 points) A B C D 4.202 ppm 3.451 ppm 2.273 ppm 1.288 ppm J(A,D) = 8 Hz = 0.08 ppm (in CDCl 3 ) Draw the 100 MHz H-NMR spectrum to scale. Draw splitting
SalenCo(OAc)/chiral ionic liquid catalyzed the asymmetric cycloaddition of CO 2 to epoxides
 Supporting information SalenCo(OAc)/chiral ionic liquid catalyzed the asymmetric cycloaddition of CO 2 to epoxides Suling Zhang, Yongzhong Huang, Huanwang Jing*, Weixuan Yao, Peng Yan State Key Laboratory
Supporting information SalenCo(OAc)/chiral ionic liquid catalyzed the asymmetric cycloaddition of CO 2 to epoxides Suling Zhang, Yongzhong Huang, Huanwang Jing*, Weixuan Yao, Peng Yan State Key Laboratory
Supporting Information
 Supporting Information For Heterologous production of thiostrepton A and biosynthetic engineering of thiostrepton analogs Chaoxuan Li, Feifei Zhang, and Wendy L. Kelly* Table of Contents Figure S1. PCR
Supporting Information For Heterologous production of thiostrepton A and biosynthetic engineering of thiostrepton analogs Chaoxuan Li, Feifei Zhang, and Wendy L. Kelly* Table of Contents Figure S1. PCR
SUPPLEMENTAL DATA. Cytochrome P450-type hydroxylation and epoxidation in a tyrosine-liganded hemoprotein, catalase-related allene oxide synthase
 SUPPLEMENTAL DATA Cytochrome P450-type hydroxylation and epoxidation in a tyrosine-liganded hemoprotein, catalase-related allene oxide synthase William E. Boeglin & Alan R. Brash Fig. S1 SP-HPLC separation
SUPPLEMENTAL DATA Cytochrome P450-type hydroxylation and epoxidation in a tyrosine-liganded hemoprotein, catalase-related allene oxide synthase William E. Boeglin & Alan R. Brash Fig. S1 SP-HPLC separation
Preparation, isolation and characterization of N α -Fmoc-peptide isocyanates: Solution synthesis of oligo-α-peptidyl ureas
 SUPPORTING INFORMATION Preparation, isolation and characterization of N α -Fmoc-peptide isocyanates: Solution synthesis of oligo-α-peptidyl ureas Vommina V. Suresh Babu*, Basanagoud S. Patil, and Rao Venkataramanarao
SUPPORTING INFORMATION Preparation, isolation and characterization of N α -Fmoc-peptide isocyanates: Solution synthesis of oligo-α-peptidyl ureas Vommina V. Suresh Babu*, Basanagoud S. Patil, and Rao Venkataramanarao
Supplementary Information
 Supplementary Information The conifer biomarkers dehydroabietic and abietic acids are widespread in Cyanobacteria Maria Sofia Costa, Adriana Rego, Vitor Ramos, Tiago Afonso, Sara Freitas, Marco Preto,
Supplementary Information The conifer biomarkers dehydroabietic and abietic acids are widespread in Cyanobacteria Maria Sofia Costa, Adriana Rego, Vitor Ramos, Tiago Afonso, Sara Freitas, Marco Preto,
Supplementary Figure 1. ESI/MS/MS analyses of native and de-acetylated S2A Supplementary Figure 2. Partial 1D 1H NMR spectrum of S2A
 Supplementary Figure 1. ESI/MS/MS analyses of native and de-acetylated S2A. Panel A, Positive ESI mass spectra of native (N) and de-acetylated (DA) non-active S2A were obtained, and demonstrated a shift
Supplementary Figure 1. ESI/MS/MS analyses of native and de-acetylated S2A. Panel A, Positive ESI mass spectra of native (N) and de-acetylated (DA) non-active S2A were obtained, and demonstrated a shift
2. Ionization Sources 3. Mass Analyzers 4. Tandem Mass Spectrometry
 Dr. Sanjeeva Srivastava 1. Fundamental of Mass Spectrometry Role of MS and basic concepts 2. Ionization Sources 3. Mass Analyzers 4. Tandem Mass Spectrometry 2 1 MS basic concepts Mass spectrometry - technique
Dr. Sanjeeva Srivastava 1. Fundamental of Mass Spectrometry Role of MS and basic concepts 2. Ionization Sources 3. Mass Analyzers 4. Tandem Mass Spectrometry 2 1 MS basic concepts Mass spectrometry - technique
Characterizing fatty acids with advanced multinuclear NMR methods
 Characterizing fatty acids with advanced multinuclear NMR methods Fatty acids consist of long carbon chains ending with a carboxylic acid on one side and a methyl group on the other. Most naturally occurring
Characterizing fatty acids with advanced multinuclear NMR methods Fatty acids consist of long carbon chains ending with a carboxylic acid on one side and a methyl group on the other. Most naturally occurring
Marine Streptomyces sp. derived antimycin analogues. suppress HeLa cells via depletion HPV E6/E7 mediated by
 Marine Streptomyces sp. derived antimycin analogues suppress HeLa cells via depletion HPV E6/E7 mediated by ROS-dependent ubiquitin proteasome system Weiyi Zhang 1, +, Qian Che 1, 2, +, Hongsheng Tan 1,
Marine Streptomyces sp. derived antimycin analogues suppress HeLa cells via depletion HPV E6/E7 mediated by ROS-dependent ubiquitin proteasome system Weiyi Zhang 1, +, Qian Che 1, 2, +, Hongsheng Tan 1,
This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is worth 2 points.
 MBB 407/511 Molecular Biology and Biochemistry First Examination - October 1, 2002 Name Social Security Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is
MBB 407/511 Molecular Biology and Biochemistry First Examination - October 1, 2002 Name Social Security Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is
Supporting Information
 Investigation of self-immolative linkers in the design of hydrogen peroxide metalloprotein inhibitors Jody L. Major Jourden, Kevin B. Daniel, and Seth M. Cohen* Department of Chemistry and Biochemistry,
Investigation of self-immolative linkers in the design of hydrogen peroxide metalloprotein inhibitors Jody L. Major Jourden, Kevin B. Daniel, and Seth M. Cohen* Department of Chemistry and Biochemistry,
Supplementary Information
 Supplementary Information Efficient use of the Dmab Protecting Group: Applications for the Solid-Phase Synthesis of N- Linked Glycopeptides Trent Conroy, Katrina A. Jolliffe and Richard J. Payne* School
Supplementary Information Efficient use of the Dmab Protecting Group: Applications for the Solid-Phase Synthesis of N- Linked Glycopeptides Trent Conroy, Katrina A. Jolliffe and Richard J. Payne* School
Electronic Supplementary Information. Quinine/Selectfluor Combination Induced Asymmetric Semipinacol Rearrangement of
 Electronic Supplementary Information Quinine/Selectfluor Combination Induced Asymmetric Semipinacol Rearrangement of Allylic Alcohols: An Effective and Enantioselective Approach to α Quaternary β Fluoro
Electronic Supplementary Information Quinine/Selectfluor Combination Induced Asymmetric Semipinacol Rearrangement of Allylic Alcohols: An Effective and Enantioselective Approach to α Quaternary β Fluoro
CHAPTER-6 IDENTIFICATION, AND CHARACTERISATION OF DEGRADATION IMPURITY IN VALSARTAN TABLETS
 129 CHAPTER-6 IDENTIFICATION, AND CHARACTERISATION OF DEGRADATION IMPURITY IN VALSARTAN TABLETS 130 6.1. Introduction Valsartan is an orally active specific angiotensin II blocker effective in lowering
129 CHAPTER-6 IDENTIFICATION, AND CHARACTERISATION OF DEGRADATION IMPURITY IN VALSARTAN TABLETS 130 6.1. Introduction Valsartan is an orally active specific angiotensin II blocker effective in lowering
Supporting Information
 Supporting Information Wiley-VCH 2008 69451 Weinheim, Germany Supporting Information Enantioselective Cu-catalyzed 1,4-Addition of Various Grignard Reagents to Cyclohexenone using Taddol-derived Phosphine-Phosphite
Supporting Information Wiley-VCH 2008 69451 Weinheim, Germany Supporting Information Enantioselective Cu-catalyzed 1,4-Addition of Various Grignard Reagents to Cyclohexenone using Taddol-derived Phosphine-Phosphite
The efficient and selective catalytic oxidation of para-substituted cinnamic acid. derivatives by the cytochrome P450 monooxygenase, CYP199A4
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Supporting information for The efficient and selective catalytic oxidation of para-substituted
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Supporting information for The efficient and selective catalytic oxidation of para-substituted
Supporting Information
 Supporting Information Bisebromoamide, a Potent Cytotoxic Peptide from the Marine Cyanobacterium Lyngbya sp.: Isolation, Stereostructure, and Biological Activity Toshiaki Teruya, Hiroaki Sasaki, Hidesuke
Supporting Information Bisebromoamide, a Potent Cytotoxic Peptide from the Marine Cyanobacterium Lyngbya sp.: Isolation, Stereostructure, and Biological Activity Toshiaki Teruya, Hiroaki Sasaki, Hidesuke
Supplementary Results
 upplementary Results upplementary Table 1. Primers used in this study. upplementary Figure 1. PCP3-TE assay in the absence of ul. upplementary Figure 2. Multiple alignment of the sulfazecin TE, highlighting
upplementary Results upplementary Table 1. Primers used in this study. upplementary Figure 1. PCP3-TE assay in the absence of ul. upplementary Figure 2. Multiple alignment of the sulfazecin TE, highlighting
Facile Cu(II) mediated conjugation of thioesters and thioacids to peptides and proteins under mild conditions
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Facile Cu(II) mediated conjugation of thioesters and thioacids to peptides
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Facile Cu(II) mediated conjugation of thioesters and thioacids to peptides
Supporting Information
 Supporting Information Wiley-VCH 2008 69451 Weinheim, Germany Chemoselective Peptide Cyclization via Induced Traceless Staudinger Ligation Rolf Kleineweischede, Christian P.R. Hackenberger* Institute for
Supporting Information Wiley-VCH 2008 69451 Weinheim, Germany Chemoselective Peptide Cyclization via Induced Traceless Staudinger Ligation Rolf Kleineweischede, Christian P.R. Hackenberger* Institute for
Supporting Information. Recyclable hypervalent-iodine-mediated solid-phase peptide
 Supporting Information Recyclable hypervalent-iodine-mediated solid-phase peptide synthesis and cyclic peptide synthesis Dan Liu, Ya-Li Guo, Jin Qu and Chi Zhang* for Address: State Key Laboratory of Elemento-Organic
Supporting Information Recyclable hypervalent-iodine-mediated solid-phase peptide synthesis and cyclic peptide synthesis Dan Liu, Ya-Li Guo, Jin Qu and Chi Zhang* for Address: State Key Laboratory of Elemento-Organic
Level and activity of D-amino acids in mouse brain tissue and blood
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 SUPPLEMENTARY INFORMATION Level and activity of D-amino acids in mouse brain tissue and blood Choyce A. Weatherly 1, Siqi Du 1, Curran Parpia 1, Polan T. Santos 2, Adam
1 2 3 4 5 6 7 8 9 10 11 12 13 14 SUPPLEMENTARY INFORMATION Level and activity of D-amino acids in mouse brain tissue and blood Choyce A. Weatherly 1, Siqi Du 1, Curran Parpia 1, Polan T. Santos 2, Adam
Lecture 3. Tandem MS & Protein Sequencing
 Lecture 3 Tandem MS & Protein Sequencing Nancy Allbritton, M.D., Ph.D. Department of Physiology & Biophysics 824-9137 (office) nlallbri@uci.edu Office- Rm D349 Medical Science D Bldg. Tandem MS Steps:
Lecture 3 Tandem MS & Protein Sequencing Nancy Allbritton, M.D., Ph.D. Department of Physiology & Biophysics 824-9137 (office) nlallbri@uci.edu Office- Rm D349 Medical Science D Bldg. Tandem MS Steps:
Supplementary Materials
 Supplementary Materials Relative enthalpies (H) and Gibbs free energy (G) in kcal/mol for the cyclization process. The relative energies are calculated taking IIb as the reference. The solvent values are
Supplementary Materials Relative enthalpies (H) and Gibbs free energy (G) in kcal/mol for the cyclization process. The relative energies are calculated taking IIb as the reference. The solvent values are
ph Switchable and Fluorescent Ratiometric Squarylium Indocyanine Dyes as Extremely Alkaline Sensors
 ph Switchable and Fluorescent Ratiometric Squarylium Indocyanine Dyes as Extremely Alkaline Sensors Jie Li, Chendong Ji, Wantai Yang, Meizhen Yin* State Key Laboratory of Chemical Resource Engineering,
ph Switchable and Fluorescent Ratiometric Squarylium Indocyanine Dyes as Extremely Alkaline Sensors Jie Li, Chendong Ji, Wantai Yang, Meizhen Yin* State Key Laboratory of Chemical Resource Engineering,
mm C3a. 1 mm C3a Time (s) C5a. C3a. Blank. 10 mm Time (s) Time (s)
 125 I-C5a (cpm) Fluorescnece Em 520nm a 4000 3000 2000 1000 c 0 5000 4000 3000 2000 Blank C5a C3a 6 0.3 mm C3a 7 9 10 11 12 13 15 16 0.3 mm C5a 0 300 600 900 1200 Time (s) 17 Fluorescnece Em 520nm Fluorescnece
125 I-C5a (cpm) Fluorescnece Em 520nm a 4000 3000 2000 1000 c 0 5000 4000 3000 2000 Blank C5a C3a 6 0.3 mm C3a 7 9 10 11 12 13 15 16 0.3 mm C5a 0 300 600 900 1200 Time (s) 17 Fluorescnece Em 520nm Fluorescnece
Supplementary Materials: An NMR Guided Screening Method for Selective Fragment Docking and Synthesis of a Warhead Inhibitor
 Molecules 216, 21, 846; doi:1.339/molecules217846 S1 of S14 Supplementary Materials: An NMR Guided Screening Method for Selective Fragment Docking and Synthesis of a Warhead Inhibitor Ram B. Khattri, Daniel
Molecules 216, 21, 846; doi:1.339/molecules217846 S1 of S14 Supplementary Materials: An NMR Guided Screening Method for Selective Fragment Docking and Synthesis of a Warhead Inhibitor Ram B. Khattri, Daniel
ANALYTICAL REPORT. 1-(1,3-diphenylpropan-2-yl)pyrrolidine (C19H23N) 1-(1,3-diphenylpropan-2-yl)pyrrolidine
 Vodovodna 95, 1000 LJUBLJANA, SLOVENIA E: nfl@policija.si Remark other NPS detected: none ANALYTICAL REPORT 1-(1,3-diphenylpropan-2-yl)pyrrolidine (C19H23N) 1-(1,3-diphenylpropan-2-yl)pyrrolidine Sample
Vodovodna 95, 1000 LJUBLJANA, SLOVENIA E: nfl@policija.si Remark other NPS detected: none ANALYTICAL REPORT 1-(1,3-diphenylpropan-2-yl)pyrrolidine (C19H23N) 1-(1,3-diphenylpropan-2-yl)pyrrolidine Sample
STILBENOIDS FROM THE LIANAS OF GNETUM PENDULUM
 Journal of Asian Natural Products Research, 2003 Vol. 5 (2), pp. 113 119 STILBENOIDS FROM THE LIANAS OF GNETUM PENDULUM XIAO-MEI LI, MAO LIN* and YING-HONG WANG Institute of Materia Medica, Chinese Academy
Journal of Asian Natural Products Research, 2003 Vol. 5 (2), pp. 113 119 STILBENOIDS FROM THE LIANAS OF GNETUM PENDULUM XIAO-MEI LI, MAO LIN* and YING-HONG WANG Institute of Materia Medica, Chinese Academy
Supporting Information
 Ferrocene Amino Acid Macrocycles as Hydrazone Based Receptors for Anions Sophie R. Beeren and Jeremy K. M. Sanders Supporting Information S1 Experimental 2 S1.1 General Experimental Procedures 2 S1.2 Synthesis
Ferrocene Amino Acid Macrocycles as Hydrazone Based Receptors for Anions Sophie R. Beeren and Jeremy K. M. Sanders Supporting Information S1 Experimental 2 S1.1 General Experimental Procedures 2 S1.2 Synthesis
Catalytic decarboxylative alkylation of β-keto acids with sulfonamides via the cleavage of carbon nitrogen and carbon carbon bonds
 Catalytic decarboxylative alkylation of β-keto acids with sulfonamides via the cleavage of carbon nitrogen and carbon carbon bonds Cui-Feng Yang, Jian-Yong Wang and Shi-Kai Tian* Joint Laboratory of Green
Catalytic decarboxylative alkylation of β-keto acids with sulfonamides via the cleavage of carbon nitrogen and carbon carbon bonds Cui-Feng Yang, Jian-Yong Wang and Shi-Kai Tian* Joint Laboratory of Green
Supporting Information for. Use of the Curtius Rearrangement of Acryloyl Azides in the Synthesis of. 3,5-Disubstituted Pyridines: Mechanistic Studies
 Supporting Information for Use of the Curtius Rearrangement of Acryloyl Azides in the Synthesis of 3,5-Disubstituted Pyridines: Mechanistic Studies Ta-Hsien Chuang* a, Yu-Chi Chen b and Someshwar Pola
Supporting Information for Use of the Curtius Rearrangement of Acryloyl Azides in the Synthesis of 3,5-Disubstituted Pyridines: Mechanistic Studies Ta-Hsien Chuang* a, Yu-Chi Chen b and Someshwar Pola
MICROBIAL METABOLIC EXCHANGE IN 3D SUPPLEMENTARY INFORMATION
 MICROBIAL METABOLIC EXCHANGE IN 3D SUPPLEMENTARY INFORMATION Jeramie Watrous 1,2*, Vanessa V. Phelan 2*, Cheng-Chih Hsu 1*, Wilna Moree 2, Brendan M. Duggan 2, Theodore Alexandrov 2,3 and Pieter C. Dorrestein
MICROBIAL METABOLIC EXCHANGE IN 3D SUPPLEMENTARY INFORMATION Jeramie Watrous 1,2*, Vanessa V. Phelan 2*, Cheng-Chih Hsu 1*, Wilna Moree 2, Brendan M. Duggan 2, Theodore Alexandrov 2,3 and Pieter C. Dorrestein
ANALYTICAL REPORT 1. Mexedrone (C12H17NO2) 3-methoxy-2-(methylamino)-1-(4-methylphenyl)propan-1-one
 + Vodovodna 95 1000 Ljubljana SLOVENIJA T: +386 (0)1 428 44 93 E: nfl@policija.si www.policija.si Remark other NPS detected: none ANALYTICAL REPORT 1 Mexedrone (C12H17NO2) 3-methoxy-2-(methylamino)-1-(4-methylphenyl)propan-1-one
+ Vodovodna 95 1000 Ljubljana SLOVENIJA T: +386 (0)1 428 44 93 E: nfl@policija.si www.policija.si Remark other NPS detected: none ANALYTICAL REPORT 1 Mexedrone (C12H17NO2) 3-methoxy-2-(methylamino)-1-(4-methylphenyl)propan-1-one
Biomimetic Chirality Sensing with Pyridoxal-5 -phosphate
 Supporting Information Biomimetic Chirality Sensing with Pyridoxal-5 -phosphate Samantha L. Pilicer, Pegah R. Bakhshi, Keith W. Bentley, Christian Wolf Department of Chemistry, Georgetown University, Washington,
Supporting Information Biomimetic Chirality Sensing with Pyridoxal-5 -phosphate Samantha L. Pilicer, Pegah R. Bakhshi, Keith W. Bentley, Christian Wolf Department of Chemistry, Georgetown University, Washington,
An Unusual Glycosylation Product from a Partially Protected Fucosyl Donor. under Silver Triflate activation conditions. Supporting information
 An Unusual Glycosylation Product from a Partially Protected Fucosyl Donor under Silver Triflate activation conditions Robin Daly a and Eoin M. Scanlan* a e-mail: eoin.scanlan@tcd.ie a Trinity Biomedical
An Unusual Glycosylation Product from a Partially Protected Fucosyl Donor under Silver Triflate activation conditions Robin Daly a and Eoin M. Scanlan* a e-mail: eoin.scanlan@tcd.ie a Trinity Biomedical
Two New Glycosides from the Fruits of Morinda citrifolia L.
 Molecules 2012, 17, 12651-12656; doi:10.3390/molecules171112651 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Two New Glycosides from the Fruits of Morinda citrifolia L. Ming-Xu
Molecules 2012, 17, 12651-12656; doi:10.3390/molecules171112651 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Two New Glycosides from the Fruits of Morinda citrifolia L. Ming-Xu
Supporting Information. as the nitro source
 Supporting Information Efficient ipso-nitration of arylboronic acids with iron nitrate as the nitro source Min Jiang, a,b Haijun Yang,* a,b Yong Li, a,b Zhiying Jia b and Hua Fu b a Beijing Key Laboratory
Supporting Information Efficient ipso-nitration of arylboronic acids with iron nitrate as the nitro source Min Jiang, a,b Haijun Yang,* a,b Yong Li, a,b Zhiying Jia b and Hua Fu b a Beijing Key Laboratory
Synthesis of an Alleged Byproduct Precursor in Iodixanol Preparation
 SUPPORTING INFORMATION Synthesis of an Alleged Byproduct Precursor in Iodixanol Preparation Marianne Bore Haarr, Emil Lindbäck, Torfinn Håland, * and Magne O. Sydnes * Department of Mathematics and Natural
SUPPORTING INFORMATION Synthesis of an Alleged Byproduct Precursor in Iodixanol Preparation Marianne Bore Haarr, Emil Lindbäck, Torfinn Håland, * and Magne O. Sydnes * Department of Mathematics and Natural
The N-terminal loop of IRAK-4 death domain regulates ordered assembly of the Myddosome signalling scaffold
 The N-terminal loop of IRAK-4 death domain regulates ordered assembly of the Myddosome signalling scaffold Anthony C.G. Dossang * 1, Precious G. Motshwene * 1, Yang Yang* 1, Martyn F. Symmons*, Clare E.
The N-terminal loop of IRAK-4 death domain regulates ordered assembly of the Myddosome signalling scaffold Anthony C.G. Dossang * 1, Precious G. Motshwene * 1, Yang Yang* 1, Martyn F. Symmons*, Clare E.
Supporting Information
 Supporting Information Zheng, Y. et al, Lipoxygenases mediate the effect of essential fatty acid in skin barrier formation: A proposed role in releasing omega-hydroxyceramide for construction of the corneocyte
Supporting Information Zheng, Y. et al, Lipoxygenases mediate the effect of essential fatty acid in skin barrier formation: A proposed role in releasing omega-hydroxyceramide for construction of the corneocyte
Lecture 11 - Biosynthesis of Amino Acids
 Lecture 11 - Biosynthesis of Amino Acids Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction Biosynthetic pathways for amino acids, nucleotides and lipids
Lecture 11 - Biosynthesis of Amino Acids Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction Biosynthetic pathways for amino acids, nucleotides and lipids
Analysis of fatty acid metabolism using Click-Chemistry and HPLC-MS
 Analysis of fatty acid metabolism using Click-Chemistry and HPLC-MS Alexander J. Pérez and Helge B. Bode -Supporting Information- Contents Experimental section Supplementary figures NMR spectra Page S2
Analysis of fatty acid metabolism using Click-Chemistry and HPLC-MS Alexander J. Pérez and Helge B. Bode -Supporting Information- Contents Experimental section Supplementary figures NMR spectra Page S2
Ornicorrugatin, a New Siderophore from Pseudomonas fluorescens AF76
 Ornicorrugatin, a New Siderophore from Pseudomonas fluorescens AF76 Sandra Matthijs a, Herbert Budzikiewicz b, *, Mathias Schäfer b, Bernard Wathelet c, and Pierre Cornelis a a Laboratory of Microbial
Ornicorrugatin, a New Siderophore from Pseudomonas fluorescens AF76 Sandra Matthijs a, Herbert Budzikiewicz b, *, Mathias Schäfer b, Bernard Wathelet c, and Pierre Cornelis a a Laboratory of Microbial
Supplementary Information
 Supplementary Information Assembly and Clustering of Natural Antibiotics Guides Target Identification Chad W. Johnston 1,2, Michael A. Skinnider 1,2, Chris A. Dejong 1,2, Philip N. Rees 1,2, Gregory M.
Supplementary Information Assembly and Clustering of Natural Antibiotics Guides Target Identification Chad W. Johnston 1,2, Michael A. Skinnider 1,2, Chris A. Dejong 1,2, Philip N. Rees 1,2, Gregory M.
Scheme S1. Synthesis of glycose-amino ligand.
 Scheme S1. Synthesis of glycose-amino ligand. 5-Chloro-1-pentyl-2,3,4,6-tetra-O-acetyl-ß-D-glucopyranoside S2 To a solution of penta-o-acetyl-ß-d-glucopyranoside S1 (3.0 g, 7.69 mmol) and 5-chloropentan-1-ol
Scheme S1. Synthesis of glycose-amino ligand. 5-Chloro-1-pentyl-2,3,4,6-tetra-O-acetyl-ß-D-glucopyranoside S2 To a solution of penta-o-acetyl-ß-d-glucopyranoside S1 (3.0 g, 7.69 mmol) and 5-chloropentan-1-ol
Supporting Information. Chemoenzymatic Synthesis of Galectin Binding. Glycopolymers
 Supporting Information Chemoenzymatic Synthesis of Galectin Binding Glycopolymers Jessica H. Ennist, Henry R. Termuehlen, Samuel P. Bernhard, Mackenzie S. Fricke, and Mary J. Cloninger* Department of Chemistry
Supporting Information Chemoenzymatic Synthesis of Galectin Binding Glycopolymers Jessica H. Ennist, Henry R. Termuehlen, Samuel P. Bernhard, Mackenzie S. Fricke, and Mary J. Cloninger* Department of Chemistry
Preparation of Stable Aziridinium Ions and Their Ring Openings
 Supplementary Information Preparation of Stable Aziridinium Ions and Their Ring Openings Yongeun Kim a Hyun-Joon Ha*, a Sae Young Yun b and Won Koo Lee,*,b a Department of Chemistry and Protein Research
Supplementary Information Preparation of Stable Aziridinium Ions and Their Ring Openings Yongeun Kim a Hyun-Joon Ha*, a Sae Young Yun b and Won Koo Lee,*,b a Department of Chemistry and Protein Research
Naoya Takahashi, Keiya Hirota and Yoshitaka Saga* Supplementary material
 Supplementary material Facile transformation of the five-membered exocyclic E-ring in 13 2 -demethoxycarbonyl chlorophyll derivatives by molecular oxygen with titanium oxide in the dark Naoya Takahashi,
Supplementary material Facile transformation of the five-membered exocyclic E-ring in 13 2 -demethoxycarbonyl chlorophyll derivatives by molecular oxygen with titanium oxide in the dark Naoya Takahashi,
Supplementary information
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supplementary information Genomics-Driven Discovery of a Linear Lipopeptide
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supplementary information Genomics-Driven Discovery of a Linear Lipopeptide
Supporting Information for:
 Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Electronic supplementary information Poly(vinyl)chloride supported palladium nanoparticles: Catalyst for rapid hydrogenation reactions
 Electronic supplementary information Poly(vinyl)chloride supported palladium nanoparticles: Catalyst for rapid hydrogenation reactions Hosahalli P. Hemantha and Vommina V. Sureshbabu* Peptide Research
Electronic supplementary information Poly(vinyl)chloride supported palladium nanoparticles: Catalyst for rapid hydrogenation reactions Hosahalli P. Hemantha and Vommina V. Sureshbabu* Peptide Research
TWO NEW ELLAGIC ACID GLYCOSIDES FROM LEAVES OF DIPLOPANAX STACHYANTHUS
 Journal of Asian Natural Products Research, December 2004, Vol. 6 (4), pp. 271 276 TWO NEW ELLAGIC ACID GLYCOSIDES FROM LEAVES OF DIPLOPANAX STACHYANTHUS XIAO-HONG YAN and YUE-WEI GUO* State Key Laboratory
Journal of Asian Natural Products Research, December 2004, Vol. 6 (4), pp. 271 276 TWO NEW ELLAGIC ACID GLYCOSIDES FROM LEAVES OF DIPLOPANAX STACHYANTHUS XIAO-HONG YAN and YUE-WEI GUO* State Key Laboratory
PHOTOCATALYTIC DECONTAMINATION OF CHLORANTRANILIPROLE RESIDUES IN WATER USING ZnO NANOPARTICLES. DR. A. RAMESH, Ph.D, D.Sc.,
 PHOTOCATALYTIC DECONTAMINATION OF CHLORANTRANILIPROLE RESIDUES IN WATER USING ZnO NANOPARTICLES DR. A. RAMESH, Ph.D, D.Sc., raamesh_a@yahoo.co.in 1 OBJECTIVES Determination of persistence and photolysis
PHOTOCATALYTIC DECONTAMINATION OF CHLORANTRANILIPROLE RESIDUES IN WATER USING ZnO NANOPARTICLES DR. A. RAMESH, Ph.D, D.Sc., raamesh_a@yahoo.co.in 1 OBJECTIVES Determination of persistence and photolysis
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Chemical constituents from Agrimonia pilosa Ledeb. and their chemotaxonomic significance Wei-jie Liu, Xue-qian Hou, Hao Chen, Jing-yu Liang*, Jian-bo Sun** Department of Natural
SUPPLEMENTARY MATERIAL Chemical constituents from Agrimonia pilosa Ledeb. and their chemotaxonomic significance Wei-jie Liu, Xue-qian Hou, Hao Chen, Jing-yu Liang*, Jian-bo Sun** Department of Natural
Very-Long Chain Fatty Acid Biosynthesis
 Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Very-Long Chain Fatty Acid Biosynthesis Objectives: 1. Review information on the isolation of mutants deficient in VLCFA biosynthesis 2. Generate hypotheses to explain the absence of mutants with lesions
Chemical composition, antioxidant and antibacterial activities of Tamarix balansae J. Gay aerial parts
 SUPPLEMENTARY MATERIAL Chemical composition, antioxidant and antibacterial activities of Tamarix balansae J. Gay aerial parts Abbes Benmerache a, Mounira Benteldjoune a, Abdulmagid Alabdul Magid b, Amin
SUPPLEMENTARY MATERIAL Chemical composition, antioxidant and antibacterial activities of Tamarix balansae J. Gay aerial parts Abbes Benmerache a, Mounira Benteldjoune a, Abdulmagid Alabdul Magid b, Amin
Rapid Detection and Identification of Bacteria in Milk Products by Matrix-Assisted Laser Desorption/Ionization Tandem Mass Spectrometry (MALDI-MS/MS)
 Rapid Detection and Identification of Bacteria in Milk Products by Matrix-Assisted Laser Desorption/Ionization Tandem Mass Spectrometry (MALDI-MS/MS) Nyesa Enakaya It is essential to be able to identify
Rapid Detection and Identification of Bacteria in Milk Products by Matrix-Assisted Laser Desorption/Ionization Tandem Mass Spectrometry (MALDI-MS/MS) Nyesa Enakaya It is essential to be able to identify
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Chemosystematic significance of flavonoids isolated from Diplotaxis acris (Brassicaceae) and related taxa Sameh R Hussein 1*, Mona M Marzouk 1, Mona ES Kassem 1, Rasha R Abdel Latif
SUPPLEMENTARY MATERIAL Chemosystematic significance of flavonoids isolated from Diplotaxis acris (Brassicaceae) and related taxa Sameh R Hussein 1*, Mona M Marzouk 1, Mona ES Kassem 1, Rasha R Abdel Latif
First Detection of Unprotected 1,2-Anhydro Aldopyranoses
 First Detection of Unprotected 1,2-Anhydro Aldopyranoses Kazunari Serizawa, Masato Noguchi, Gefei Li, and Shin-ichiro Shoda* Department of Biomolecular Engineering, Graduate School of Engineering, Tohoku
First Detection of Unprotected 1,2-Anhydro Aldopyranoses Kazunari Serizawa, Masato Noguchi, Gefei Li, and Shin-ichiro Shoda* Department of Biomolecular Engineering, Graduate School of Engineering, Tohoku
In vitro conversion of chanoclavine-i aldehyde to the stereoisomers festuclavine and pyroclavine was controlled by the second reduction step
 1 Electronic Supporting Information to: In vitro conversion of chanoclavinei aldehyde to the stereoisomers festuclavine and pyroclavine was controlled by the second reduction step Marco Matuschek a, Christiane
1 Electronic Supporting Information to: In vitro conversion of chanoclavinei aldehyde to the stereoisomers festuclavine and pyroclavine was controlled by the second reduction step Marco Matuschek a, Christiane
Synthesis, evaluation of anti-hiv-1 and anti-hcv activity of novel 2,3 -dideoxy- 2,2 -difluoro-4 -azanucleosides
 Synthesis, evaluation of anti-hiv-1 and anti-hcv activity of novel 2,3 -dideoxy- 2,2 -difluoro-4 -azanucleosides Saúl Martínez-Montero, a,b Susana Fernández, a Yogesh S. Sanghvi, c Emmanuel A. Theodorakis,*
Synthesis, evaluation of anti-hiv-1 and anti-hcv activity of novel 2,3 -dideoxy- 2,2 -difluoro-4 -azanucleosides Saúl Martínez-Montero, a,b Susana Fernández, a Yogesh S. Sanghvi, c Emmanuel A. Theodorakis,*
Supporting Information. Enzymatic synthesis of electrospinnable poly(butylene succinate-co-dilinoleic. succinate) thermoplastic elastomer
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Supporting Information Enzymatic synthesis of electrospinnable poly(butylene succinate-co-dilinoleic
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Supporting Information Enzymatic synthesis of electrospinnable poly(butylene succinate-co-dilinoleic
Chapter 3: Amino Acids and Peptides
 Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
Chapter 3: Amino Acids and Peptides BINF 6101/8101, Spring 2018 Outline 1. Overall amino acid structure 2. Amino acid stereochemistry 3. Amino acid sidechain structure & classification 4. Non-standard
ANALYTICAL REPORT 1. U ( C16H22Cl2N2O) 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide
![ANALYTICAL REPORT 1. U ( C16H22Cl2N2O) 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide ANALYTICAL REPORT 1. U ( C16H22Cl2N2O) 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide](/thumbs/96/126993301.jpg) Vodovodna 95 1000 Ljubljana SLOVENIJA T: +386 (0)1 428 44 93 E: nfl@policija.si www.policija.si Remark other NPS detected: none ANALYTICAL REPORT 1 U-47700 ( C16H22Cl2N2O) 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide
Vodovodna 95 1000 Ljubljana SLOVENIJA T: +386 (0)1 428 44 93 E: nfl@policija.si www.policija.si Remark other NPS detected: none ANALYTICAL REPORT 1 U-47700 ( C16H22Cl2N2O) 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide
Structure Elucidation of Verucopeptin, a HIF-1 Inhibitory Polyketide-Hexapeptide Hybrid Metabolite from an Actinomycete.
 Structure Elucidation of Verucopeptin, a HIF-1 Inhibitory Polyketide-Hexapeptide Hybrid Metabolite from an Actinomycete. Aya Yoshimura, Shinichi Nishimura, Saori Otsuka, Akira Hattori & Hideaki Kakeya*
Structure Elucidation of Verucopeptin, a HIF-1 Inhibitory Polyketide-Hexapeptide Hybrid Metabolite from an Actinomycete. Aya Yoshimura, Shinichi Nishimura, Saori Otsuka, Akira Hattori & Hideaki Kakeya*
Figure S7: ATR FTIR spectra of enantiomeric UP-PLA-UPy and sc-upy-pla-upy. Figure S9: SEM microphotographs sc-upy-pla-oh in 1,4-dioxane and chloroform
 Supporting Information for Macromolecules, DOI: Supramolecular Polylactides by the Cooperative Interaction of the End Groups and Stereocomplexation By M. Brzeziński*, T. Biela Table of Contents Figure
Supporting Information for Macromolecules, DOI: Supramolecular Polylactides by the Cooperative Interaction of the End Groups and Stereocomplexation By M. Brzeziński*, T. Biela Table of Contents Figure
Bioactivity Based Molecular Networking for the Discovery of Drug Lead in Natural Product Bioassay-Guided Fractionation
 Bioactivity Based Molecular Networking for the Discovery of Drug Lead in Natural Product Bioassay-Guided Fractionation Louis-Félix Nothias,,, Mélissa Nothias-Esposito,, # Ricardo da Silva,, Mingxun Wang,,,
Bioactivity Based Molecular Networking for the Discovery of Drug Lead in Natural Product Bioassay-Guided Fractionation Louis-Félix Nothias,,, Mélissa Nothias-Esposito,, # Ricardo da Silva,, Mingxun Wang,,,
Supplementary Information
 Supplementary Information J. Braz. Chem. Soc., Vol. 24, No. 12, S1-S21, 2013. Printed in Brazil - 2013 Sociedade Brasileira de Química 0103-5053 $6.00+0.00 SI Rui He, a,b,c Bochu Wang,*,b Toshiyuki Wakimoto,
Supplementary Information J. Braz. Chem. Soc., Vol. 24, No. 12, S1-S21, 2013. Printed in Brazil - 2013 Sociedade Brasileira de Química 0103-5053 $6.00+0.00 SI Rui He, a,b,c Bochu Wang,*,b Toshiyuki Wakimoto,
N-terminal charge-driven de novo sequencing by using ASDF-incorporated Curved Field Reflectron
 PO-CON1534E N-terminal charge-driven de novo sequencing by using ASDF-incorporated Curved Field Reflectron ASMS 2015 ThP 361 Yuzo Yamazaki 1 ; Keisuke Shima 1 ; Atsushi Kitanaka 2 ; Masahiro Miyashita
PO-CON1534E N-terminal charge-driven de novo sequencing by using ASDF-incorporated Curved Field Reflectron ASMS 2015 ThP 361 Yuzo Yamazaki 1 ; Keisuke Shima 1 ; Atsushi Kitanaka 2 ; Masahiro Miyashita
Gade Hyldgaard, Mette; Purup, Stig; Bond, Andrew David; Frete, Xavier; Qu, Haiyan; Teglgaard Jensen, Katrine; Christensen, Lars Porskjær
 Syddansk Universitet Guaianolides and a seco-eudesmane from the resinous exudates of cushion bush (Leucophyta brownii) and evaluation of their cytostatic and anti-inflammatory activity Gade Hyldgaard,
Syddansk Universitet Guaianolides and a seco-eudesmane from the resinous exudates of cushion bush (Leucophyta brownii) and evaluation of their cytostatic and anti-inflammatory activity Gade Hyldgaard,
Thiol-Ene Photoimmobilization of Chymotrypsin on Polysiloxane Gels for Enzymatic Peptide Synthesis
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2018 Supporting information for: Thiol-Ene Photoimmobilization of Chymotrypsin on Polysiloxane Gels
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2018 Supporting information for: Thiol-Ene Photoimmobilization of Chymotrypsin on Polysiloxane Gels
