Postnatal requirement of the epithelial sodium channel ENaC for the maintenance of the epidermal barrier function
|
|
|
- Priscilla George
- 6 years ago
- Views:
Transcription
1 JBC Papers in Press. Published on November 26, 2007 as Manuscript M The latest version is at Postnatal requirement of the epithelial sodium channel ENaC for the maintenance of the epidermal barrier function Roch-Philippe Charles *, Marjorie Guitard *, Céline Leyvraz *, Bernadette Breiden, Marek Haftek, Zofia Haftek-Terreau, Jean-Christophe Stehle #, Konrad Sandhoff, and Edith Hummler * * Département de Pharmacologie & de Toxicologie, Université de Lausanne, Lausanne, CH-1005 Lausanne, Switzerland Current address: Cutaneous Biology Research Center, Charlestown, MA 02129, USA # Département de Pathologie, Université de Lausanne, CH-1005 Lausanne, Switzerland LIMES, Membrane Biology and Lipid Biochemistry Unit, c/o, Kekulé-Institut für Organische Chemie und Biochemie der Universität Bonn, D Bonn, Germany Laboratoire Joliot-Curie, IFR128, Ecole Normale Supérieure de Lyon, Lyon, France Corresponding author: Dr. Edith Hummler, Département de Pharmacologie & de Toxicologie, Université de Lausanne, Rue du Bugnon 27, CH-1005 Lausanne, Switzerland; Phone: ; Fax: ; Edith.Hummler@unil.ch Keywords Impaired skin barrier function, tight junctions, stratum corneum lipids, skin surface ph, knockout, Scnn1a In skin, the physiological consequence of an ENaC-deficiency is not obvious directly at birth. Nevertheless, within hours after birth, mice deficient for the alpha subunit of the highly amiloride epithelial sodium channel (αenac/scnn1a) suffer from a significant increased dehydration. This is characterized by a loss of body weight (by 6% in 6 hours) and an increased transepidermal water loss, which is accompanied by a higher skin surface ph in one day-old pups. While early and late differentiation markers, as well as tight junction protein distribution and function seem not affected, deficiency of αenac severely disturbs the stratum corneum lipid composition with decreased ceramide and cholesterol levels, and increased pro-barrier lipids, while covalently-bound lipids are drastically reduced. Ultrastructural analysis revealed morphological changes in the formation of intercellular lamellar lipids and the lamellar body secretion. Extracellular formation of the lamellar lipids proved to be abnormal in the knockouts. In conclusion, ENaC-deficiency results in progressive dehydration and consequently weight loss due to severe impairment of lipid formation and secretion. Our data demonstrate that ENaC expression is required for the postnatal maintenance of the epidermal barrier function but not for its generation. Introduction The highly amiloride-sensitive epithelial sodium channel (ENaC) is a membrane constituent of many salt-absorbing epithelia. It facilitates Na + movement across tight epithelia and is therefore involved in whole net sodium ion balance of the body (1). ENaC is a member of the superfamily of ion channels implicated in mechanoperception and acid sensing (2). It is composed of three highly homologous subunits (α, β and γenac) encoding Scnn1a, Scnn1b and Scnn1g and characterized in several species, including man and mouse (3-5). In vitro experiments clearly demonstrate that in the absence of the α subunit, channels made of β and γ subunits alone do not confer ENaC activity (4,6). In vivo, the constitutive inactivation of the α subunit of ENaC leads to complete abolishment of ENaC activity and αenac knockout animals die within 40 hours after birth due to lung clearance failure (7). Further gene targeting experiments clearly demonstrated that ß and γenac (Scnn1b and Copyright 2007 by The American Society for Biochemistry and Molecular Biology, Inc.
2 Scnn1g) subunits are important for survival with the channel composed of the remaining subunits (αβ or αγ) conferring less channel activity (4,6,8). This may also explain the distinct clinical phenotypes observed (9,10). In human, mutations in all three ENaC subunits are reported to result in either ENaC hyper- or hypo- function associated with hypertension (Liddle s syndrome) or salt-wasting syndrome (pseudohypoaldosteronism type-1, PHA-1) (see for review 1). ENaC expression has been demonstrated in skin of amphibians and in hair follicles, interfollicular epidermis, and sweat glands of mammals (11-13). All three subunits (Scnn1a, Scnn1b and Scnn1g) are found in mouse and human keratinocytes (12,13). Interestingly, their expression level is increased in more differentiated keratinocytes and only found in the later stages of fetal epidermal development. In human epidermis, ENaC mrna is expressed throughout adulthood, but is absent in 10 week-old fetal epidermis (12,13). Patch clamp recordings on human keratinocytes reveal a sodium channel conductance that is blocked by benzamil with similar affinity and voltage dependence of the amiloride block as previously described for ENaC (13). Further evidence that ENaCmediated Na + transport may be implicated in keratinocyte and epidermal differentiation comes from the previous analysis of newborn αenac knockout mice which exhibit epidermal thickening and premature lipid secretion in the upper epidermis, suggesting that ENaC-mediated sodium ion fluxes control selective aspects of keratinocyte differentiation (14). The skin is a physical barrier at the interface between an organism and its environment preventing water loss and withstanding mechanical, chemical and microbial assaults. To perform these functions, the epidermis as outer layer of the skin undergoes keratinization, a process in which epidermal cells progressively mature from basal cells with proliferative potential to lifeless flattened squames of the stratum corneum. This differentiation is accompanied by alterations in gene expression, affecting structural proteins, expression and activation of enzymes that control post-translational modifications, metabolic changes, and lipid synthesis (15). A defect in any one of these structural components or enzymatic processes has therefore the potential to impair the barrier function of the skin and to cause diseases. Given the important role played by ENaC in the whole body sodium ion homeostasis, we sought to reveal its physiological role in skin. At birth, no differences in physiological parameters could be identified, but few hours later, αenac knockout mice show a severe life-threatening transepidermal water loss, and major impairment of the barrier function due to highly disturbed lipid secretion and formation. This dehydration is accompanied by impaired acidification of the skin surface ph. Our data demonstrate that this sodium channel is not required for establishment of the epidermal barrier function, but rather for maintaining this barrier function in postnatal life. Experimental Procedures Animals (source, genotyping) Transgenic αenac homozygous mutant (-/-, knockout), heterozygous mutant (+/-) and wild-type (+/+) littermates were obtained by interbreeding mice heterozygous mutant for the αenac allele [Scnn1a tm1 (21)] and analyzed after birth. Genotyping was performed by PCR essentially as described (56). All experiments were performed coded. Animals were kept on a 14:10-h light-dark cycle. Experimental procedures and animal maintenance followed federal guidelines and were approved by local authorities. At birth, (α)enac knockout animals present a metabolic acidosis as found by Hummler and coworkers (56), but for our present skin analysis, only animals without apparent respiratory distress syndrome have been considered for further analysis. Functional analyses of the epidermal barrier Skin permeability assay. Newborn mice were killed and subjected to methanol dehydration and subsequent rehydration as described previously (57). They were further washed in PBS, stained overnight at 4 C in 0.1% toluidin blue/ PBS (Merck), destained in PBS, and photographed with a digital camera (Coolpix 950, Nikon; (58) Measurement of the transepidermal water loss (TEWL). The rate of TEWL from the ventral skin of newborn mice was - 2 -
3 determined by using a Tewameter (Courage and Khazaka) as described previously (59). Dehydration assay. To determine the rate of fluid loss (58), newborns from 5 independent litters were separated from their mother to prevent fluid intake, and the rate of epithelial water loss was calculated by measuring the reduction of body weight as a function of time. Tight junction permeability assay. The Tight junction functional test was performed as described (60,61). Following subcutaneous injection of biotin (~300D, Pierce, USA), skin samples were snap-frozen. 10-μm-thick cryosections were prepared, fixed in 4% paraformaldehyde/pbs buffer and incubated with streptavidin FC630 overnight at 4 C (Fluoprobes, Interchim, Montluçon, France). Fluorescence was visualized using LSM confocal microscopy (model LSM510 Meta; Carl Zeiss MicroImaging, Inc.). For each genotype (wild-type and knockout), three pups were independently analyzed. Nuclei were counterstained with 0.2μg/ml DAPI (Roche, Basel, Switzerland) in mounting medium (Dako Schweiz AG, Switzerland, Baar). Skin surface ph. Skin surface ph of littermates was determined with a ph meter (PH900, Courage and Khazaka, Cologne, Germany). Briefly, a few μl of bi-distilled water were applied on the skin surface and a flat glass surface electrode (Mettler-Toledo, Giessen, Germany) was put on the top to equilibrate. The skin surface ph was then measured according to the manufacturer s guidelines. Immunohistochemistry Immunohistochemistry was performed on littermates. Tissue from knockout and wildtype mice was fixed overnight in 4.5% phosphate-buffered formalin (ph 7) and embedded in paraffin. Following deparaffination in xylene and progressive rehydration, 4-μm sagittal sections were incubated with primary antibodies for 1h at RT after treatment with unmasking solution (10mM Tris, 0.5mM EGTA, ph 9, boiling for 10min). Staining was visualized by laser scanning microscopy (LSM) after 1h of incubation at RT with alexa633-conjugated anti rabbit IgG for involucrin, loricrin and filaggrin or alexa488-conjugated anti rabbit IgG (Molecular Probes, Invitrogen) for K1, K6 and K14. All primary rabbit antibodies were purchased from Covance and were used at a dilution of 1:1000 except for K14 (1:4000). Nuclei were counterstained with DAPI. To analyze tight junction proteins, skin was dissected and frozen in optimum cutting temperature compound (OCT, Tissue-Tek, Sakura, Zoeterwoude, Netherland). 10-μmthick cryosections were processed to further fixation (30 min in 95% ethanol at 4 C and 1 min in acetone at RT) and permeabilization (10 min in 0.2% Triton X-100/PBS). Following 30 min in PBS/ 3% BSA blocking solution, sections were incubated with primary antibody at RT for 1h. Rabbit anti claudin-1 pab (Zymed Laboratories) was used (1:100 dilution) in combination with alexa633- conjugated anti rabbit IgG (Molecular Probes, Invitrogen) on paraffin sections (as described above). Rat anti-occludin (MOC37) and mouse anti-zo-1 (T8-754) pab (kindly provided by M. Furuse, Kyoto University, Kyoto, Japan) were combined with anti-rat FC488-conjugated (Fluoprobes, Interchim, Montluçon, France) and anti mouse alexa546-conjugated antibodies (Molecular Probes, Invitrogen), respectively. Nuclei were counterstained with DAPI. Immunofluorescence labeling was visualized by laser scanning microscopy. Western blot analysis Whole skin of newborns was homogenized in ice-cold 8M Urea, 50 mm Tris (ph 8.0), 10 mm EDTA using the tissue lyzer 4x 30 sec (Quiagen, Basel, Switzerland). Following 30 min. incubation on ice, lysates were centrifugated (13000g for 15 min. at 0 C) and quantified using the protein assay (BCA, Pierce, USA). For SDS-PAGE, 50 μg of proteins were loaded and separated on a 10% acrylamide gel. Western blot analysis was performed using rabbit antibodies to K14 (1:10000), K1 (1:10000), K6 (1:10000), filaggrin (1:5000), loricrin (1:5000), and involucrin (1:5000; Covance, Berkeley, USA). Signals were revealed with anti-rabbit IgG from donkey (1:2000) as secondary antibody and the Supersignal detection system (West Dura System, Pierce, USA). Lipid analysis Whole skin from newborns was removed at autopsy, frozen, and stored at 20 C until further treatment. Stratum corneum preparations, lipid analysis, and recovery of - 3 -
4 covalently bound lipids were performed as described (62). Ultrastructural analysis. Standard electron microscopy approach consisted of tissue fixation in 2% glutaraldehyde solution in sodium cacodylate buffer, followed by washing and post-fixation in 1% osmium tetroxide (OsO4; Sigma, France) in the same buffer. For lamellar lipid visualization, skin biopsies were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS; ph 7.2), washed in PBS, and post-fixed in 0.25% ruthenium tetroxide (RuO4; Polysciences Inc., Warrington, PA, USA) instead of OsO4. Thus prepared small tissue fragments were dehydrated in graded ethanol series and embedded in Epon at 60 C. Ultrathin sections recovered on copper grids were counterstained in uranyl acetate and lead citrate (63). Calculation and statistics All data are expressed as means ± SEM. Values of n refer to the number of mice in each group. Individual groups were compared by using the t test for pair-wise comparisons. To test the independence of groups (dehydration assay), the Kruskal and Wallis statistical test was used. A level of P< 0.05 was accepted as statistically significant for all comparisons. Results Time-dependent impairment of the epidermal barrier function in αenacdeficient mice In order to study the physiological consequences of ENaC-deficiency in the skin, we first tested the functional integrity of the epidermis by a toluidin blue penetration test across the entire epidermal surface. Directly at birth, pups from the knockout group did not show increased blue staining compared to the heterozygous and the wild-type mice (Fig.1A). We then tested the outwards barrier function by a dehydration test, thus determining the reduction in body weight as a function of time. The dehydration rate (mg/h/g of body weight) was significantly different in the knockout group (P< 0.001; Fig.1B). When following one litter over 6.5 hours after birth, the dehydration is evident at 2 hours after birth (3% water loss versus 1% in the control littermates; P< 0.01) and, at 6 hours after birth, the difference in dehydration reaches almost 7% of their body weight (compared to 2% in the control (+/+ and +/-) littermates (P< 0.001; Fig.1C). Dehydration through the skin was confirmed by TEWL. While the values are not different within the first 12 hours amongst the three groups (Fig.1D), knockout neonates measured between 12 and 24 hours after birth do display a significantly increased TEWL value (Fig.1E; P< 0.05). When measuring TEWL values in pups from the same litter at an early (4h) and late (24h) time point after birth, we have clearly seen a reduction in the TEWL values in the heterozygous and wild-type groups with time whereas the knockout group fail to reach the same basal levels (Fig.1F; P> 0.05). This is indicating that the skin of the knockout mice may not adapt normally to the external environment (Fig.1F, P< 0.05). These data strongly suggest that αenac expression is postnatally required to adapt the epidermal permeability barrier function. αenac is required for proper expression of the differentiation marker K6, an unspecific marker for proliferation To unveil defects in epidermal differentiation, we studied the distribution as well as the expression levels of differentiation markers (Fig.Supp1-2). Immunohistochemistry revealed that keratin 14 (K14), a specific marker for the stratum basale (SB) was similarly expressed in epidermal basal cells of wild-type and knockout neonates. Equally, the distribution of an intermediate (keratin 1) and the epidermal terminal differentiation markers (loricrin, involucrin and filaggrin) is similar in skin from both wild-type and knockout mice (Fig.Supp1). Loricrin staining is observed in the top stratum granulosum (SG) and continues in the stratum corneum (SC). Involucrin staining is strong in the SC, but is also found in the SG and slightly in the SP (stratum spinosum). In contrast, the expression of keratin 6 (K6), that is normally restricted to the outer root sheath of the hair follicle in wildtype mice, is expressed throughout the interfollicular epidermis in knockout animals indicative for a hyperproliferation (Fig.Supp1). The near-normal expression of the early and late differentiation markers is confirmed by Western blot analysis - 4 -
5 (Fig.Supp2). Additionally, processing of filaggrin, from profilaggrin (300kD) to the two-domain intermediates [2DI] (50kD) and [1DI] (25kD) monomers is not disturbed (Fig.Supp2). In summary, except for keratin 6, none of the analyzed early, intermediate and late differentiation markers is altered in the knockout group. Tight junction protein distribution and functionality are not altered The impaired skin barrier function may be caused by leakiness of tight junction proteins in the epidermis. However, immunohistochemical analysis shows a normal distribution of tight junction proteins (Fig.2). Claudin-1 is localized in the whole epidermis except for the stratum corneum and the last layer of stratum granulosum (Fig.2A). ZO-1 is detected in the mid-stratum granulosum and occludin shows a dotted staining, as expected for the presence of tight junctions (Fig.2A; arrows). We then examined whether the barrier function of the tight junctions is affected. Injection of biotin into the dermis and streptavidin revelation on the sections showed normal diffusion through the paracellular spaces of the epidermis from stratum basale to the supposed localization of the tight junction complexes at the mid-stratum granulosum (Fig.2B; arrows). These data clearly indicate that the tight junctions are unchanged in the knockout animals. This experiment has been performed at 4h and 24h after birth and showed the same results at both timepoints. αenac knockout mice exhibit severe defects in lipid matrix composition and highly impaired lamellar lipids Since content and localization of differentiation markers (see Fig.supp1-2) seem not to be affected, nor the tight junctions, we focused our analysis on lipid composition. We analyzed the lipid profile (probarrier lipids, lamellar lipids and covalently-bound lipids) at 5h after birth, and compared it with the lipid profile at 31h after birth (Fig.3A-F). In the αenac-knockout mice, ceramides and cholesterol levels based on dry weight are significantly decreased while the level of the fatty acids is unchanged (Fig.3A-D). The ceramides are preferentially formed of probarrier lipids, glucosylceramide (16-18), sphingomyelin (19) and cholesterol sulfate (20). The ceramide precursors glucosylceramide and sphingomyelin are both accumulated in the knockout mice (Fig.3B-E). In αenac-deficient skin, the level of cholesterol sulfate is increased to nearly 2 fold (at 5h) and 1.6 fold of wild-type mice at 24h after birth. Interestingly, the heterozygous mutants (+/-) mice display an intermediate phenotype between the wild-type and knockout group, although dehydration and body weight loss are not different from the control (wildtype) group (Fig.1B-F, and Fig.3A-D). Further densitometric quantification of the ceramides (see Suppl.Fig.2, and Table2) showed that the decrease observed in total ceramides affects all the ceramides and seems to be improving with time (31h compared to 5h). As the covalentlybound lipids are important for the lamellar lipid organization and therefore the barrier function, the extractable lipids of the stratum corneum samples were removed and covalentbound lipids were released by alkaline hydrolysis. The levels of ω hydroxylated fatty acid, Cer(OS), Cer(OP) and GlcCer(OS) are significantly decreased in the knockout group and this at both time points (Fig.3C-F and Table1), whereas the Cer(OH) lipid fraction is unchanged (Table1). Altogether, these data support a disturbed lipid composition with impaired formation of the lamellar lipids of the extracellular lipid matrix in the αenac-knockout mice. Ultrastructural analysis of (α)enac knockout mouse skin ( 24h after birth) shows morphological changes in the formation of intercellular lamellar lipids at the interface between the granular layer and stratum corneum. Lamellar body secretion and extracellular formation of the lamellar lipids proved abnormal (Fig.4A,C) when compared to wild-type pups (Fig.4B,D). The apical region of the most superficial granular keratinocyte layer contains highly disorganized lamellar lipids in the knockouts (Fig.4A). These lipids fail to be correctly arranged in long lamellar lipid structures filling out the space between the stratum corneum cells. This results in the formation of abnormal lamellar lipid deprived-spaces at the interface between the living and horny layers (Fig.4C, asterisks). The lamellar body-derived vesicular structures persist in the intercorneocyte spaces of the lower stratum corneum (Fig.4C). In contrast, in wildtype animals (Fig.4B,D), the lipid lamellae are tightly packed and adhere to the well visible corneocyte lipid envelopes
6 Increased skin surface ph in αenac knockout mice When we followed skin acidification in heterozygous and wild-type αenac mice, we found a gradual decrease of skin surface ph within the first week of life, from almost 7 to 5 (data not shown). The skin surface ph finally reached values as found in adulthood (ph=5). Interestingly, around 24h after birth, a time point at which all three genotypes (αenac+/+, +/- and -/- mice) are still present within the same litter, the αenac-/- pups showed a significant delay in acidification (Fig.5, P< 0.01 vs. heterozygotes, P< vs. wildtypes). Here, a skin surface ph of 6.35 was measured in the knockout group compared to 6.03 in the control littermates indicating a failure to complete acidification. Discussion ENaC is important in the maintenance of the epidermal permeability barrier function Skin as the outermost organ is continuously confronted to the external environment and serves as a primary defense system against infections and primary regulator of body temperature. The epidermal barrier function of the skin impedes the escape of water and electrolytes and thereby presents the physical barrier at the interface between inand outside. In this study, gene inactivation of the alpha subunit of the highly amiloride epithelial sodium channel (αenac, Scnn1a) leads to distinct perinatal effects on epidermal development and homeostasis, which culminates in a barrier defect within the first 24h. At birth, αenac newborns are not distinguishable from their heterozygous mutant and wild-type littermates and the barrier seems not yet impaired (see Fig.1 and 21). Consistently, at the same time point, the toluidine-blue whole-mount assay revealed no impaired skin permeability of αenac-/- newborns (Fig.1). Usually, models with barrier defect at birth like e.g., the Klf4-/- mice show an extensive blue staining upon toluidine-blue treatment that penetrate efficiently the skin of the newborns (22). The establishment of the epidermal permeability barrier initiates normally at E16.5 on the dorsal surface and spreads ventrally and was proposed to be terminated before the end of gestation (23). In our model, we could show that αenac knockout pups loose significantly more weight through dehydration which becomes life-threatening (Fig.1). Interestingly, the epidermal barrier function is not different between knockouts and wild-types right after birth but seem to evolve during the following hours becoming more efficient in wild-types. This is the first report that demonstrates the post-natal epidermal barrier adaptation in mice which evolves in parallel with the skin surface acidification. Moreover, we show that this adaptation requires the participation of the sodium channel ENaC. Recently, Indra and co-workers identified the TAF10 as a gene required for the establishment of skin barrier function in embryonic, but not in adult mouse epidermis (24). We propose that ENaC is not required for the generation of the epidermal barrier, like e.g. the Klf4, or TAF10, (22,24), but rather in the maintenance of this skin barrier function after birth. Severely impaired SC lipid composition is most likely causative for the barrier defect The epidermal barrier function is the result of the combination of three mechanisms: terminal differentiation of keratinocytes forming the corneocytes, an embedding matrix of extracellular lipid membranes mainly consisting of ceramides, cholesterol and fatty acids, and the tight junctions (25,26). Here, we demonstrate that αenac knockout mice exhibit an altered epidermal barrier function. To investigate the causality we studied first the corneocytes differentiation markers. In our αenac knockout mice, we found a nearnormal pattern of the protein differentiation markers with the exception of the ectopic interfollicular expression of keratin 6, which may be indicative for a secondary hyperproliferation (hyperkeratosis) (27). Furthermore, analysis of the tight junction proteins (claudin-1, zona occludens-1, and occludin), known to be part of the tight junction complex in the SG, showed no difference in distribution nor in functionality as evidenced by the tight junction functional assay (Fig.2B). Stratum corneum intercellular lipids play an important role in the regulation of skin water barrier homeostasis and water-holding capacity, and any modification of intercellular - 6 -
7 lipid organization and composition may therefore impair these properties. The unique lamellar, bilayer organization of this lipid matrix provides the structural basis for the extraordinary lower permeability to water and other electrolytes (28). In a previous study, electron microscopy in mice deficient for αenac demonstrated a premature lipid secretion indicative for a disturbed lipid processing (14). We now performed a detailed lipid analysis and found a significant decrease in the major lipid species, namely ceramides and cholesterol, whereas the fatty acid levels are unchanged (Fig.3A-C). As these main lipid species are normally present in an approximately equimolar (1:1:1) ratio to form the extracellular lamellar membranes. The observed modifications in stoechiometry of the lamellar lipid composition in knockouts at 5h and 31h most likely modify the lamellar organization and therefore impair the epidermal barrier function. Interestingly, patients with skin diseases such as atopic dermatitis and psoriasis exhibit diminished skin barrier function and present decreased levels of ceramide and altered ceramide profiles (29,30). To verify the consequence of the lipids profile on the barrier function, we further performed a detailed ultrastructural (EM) study. Knockouts exhibit morphological changes in the formation of intercellular lamellar structures at the interface between the granular layer and stratum corneum (Fig.4). These lamellar lipids fail to form a stack of lipid membranes. These barrier lipids are formed by the mice only few days before birth (31), and their deficiency in psap- and β- glucocerebrosidase knockout mice results in disruption of the water permeability barrier and in early death (17,18). Equally, mice with a targeted disruption of the fatty acid transport protein 4 (Fatp 4) show a disturbed fatty acid composition of epidermal ceramides (32). Ceramides derive in large part from hydrolysis of glucosylceramides (GlcCers) of the lamellar bodies mediated by β-glucocerebrosidase (β- GlcCerase) (33). Therefore, the significant accumulation of all main three probarrier lipids, namely glucosylceramide, sphingomyelin and cholesterol sulfate in the αenac knockout group explains the reduction of mature lipids observed in knockouts (cholesterol and ceramides) (Fig.3B,E). Similarly, a drastic modification was observed in the composition of the very long chain lipids covalently attached by an ester linkage with their ω-hydroxyl groups to proteins on the surfaces of the corneocytes (Fig.3D,F). Taken together, the level of barrier forming lipids attached to proteins was significantly reduced in knockouts suggesting a potential cause for the observed defect in postnatal epidermal barrier (Fig.3D,F; Table1). This covalently bound lipidic envelope is thought to be essential for the interaction of highly cross-linked proteins of the corneocytes within the extracellular lipid matrix (34). To further analyze the epidermal barrier adaptation during the 24h after birth, we analyzed the lipid profile (probarrier lipids, lamellar lipids and covalently-bound lipids) at 5h after birth, an early time point (Fig.3A,B,C), where we do see high transepidermal water loss in wild-types and knockouts. Interestingly, the lipid profile as analyzed at 5h after birth resembles the profile as found at 31h after birth (Fig.3D,E,F). Here, the transepidermal water loss stays higher in knockouts. It is interesting to note that the ω- hydroxy-fatty acids are important for the stacking of the lamellar lipids around the corneocytes and decrease with time in the knockouts (Fig.3C,F). The observed reduction correlates very well with the disorganization observed in the lamellar bodies by electron microscopy, where the ω-hydroxy-fatty acids are attached to the membrane prior to the extrusion (18,35). Several mouse models have been described in which distinct lipid defects have been demonstrated (see review 36). For example, mice homozygous mutant for ElovlL4 display scaly, wrinkled skin and have severely compromised epidermal barrier function, and die within a few hours after birth. Skin histology showed an abnormally compacted outer epidermis while electron microscopy revealed deficient epidermal lamellar body contents and lack of normal stratum corneum lamellar membranes. The lipid analyses of epidermis revealed a global decrease in very long chain fatty acids (VLFA) (37,38). It is likely that reduced β- glucocerebrosidase activity is causative for the ceramides modifications in the αenac knockout group. Furthermore, a neutral skin surface ph has been proposed to cause incomplete lipid processing within the early - 7 -
8 post-natal period (39). Our data rather indicate that the lipid abnormalities are the primary defect (Fig.3). αenac knockout mice show an impaired postnatal skin surface acidification The initial observation that the skin has an acidic surface ( acid mantle ) was made a long time ago. Normally, late in gestation, fetal skin develops a permeability barrier competent for life in a terrestrial environment(40). In fetal rat and mouse skin the key steps leading to development of a competent barrier were thought to occur between day E20 and E21 in mice. Both, normal cornified envelope and mature lamellar bilayers are present by day E21, accounting for barrier competence (41). Yet, certain developmental processes take place after birth in human as well as in rodents, namely stratum corneum hydration, water loss, and skin surface acidification indicating that the stratum corneum barrier function is still in the process of adapting to extra-uterine life. In our present study, we found that wild-type and knockout mice display a near-neutral ph right at birth. Wild-type skin acidifies rapidly within 48h after birth reaching near-adult levels (data not shown). In contrary, the αenac knockout mice show a delay in the acidification of their skin surface ph at 24h after birth (Fig.5). This might have a direct consequence on the lipid abnormalities (Fig.3) since the enzymes responsible for the processing of sphingomyelin and glycosylceramide into ceramides is ph dependent (42,43). Thus, our finding of an impaired acidification in αenac knockout skin coincides well with the impaired lipid maturation. Skin surface acidification is more rapidly achieved in the neonatal mouse ( 2days), than in the rat ( 7days) or even human newborns, where this takes several weeks to months (44,45). A number of mechanisms have been proposed for the generation of stratum corneum acidity, like e.g, H + generated by Na/H exchanger in the stratum granulosum (46) and catabolic processes within the stratum corneum that generate acidic end-products. Intrinsic processes are (i) the breakdown of proteins such as filaggrin resulting in urocanic acid production (47), and (ii) the hydrolysis of lipids through specific phospholipases to yield free fatty acids (47,48). The epidermal Na/H exchanger antiporter activity certainly contributes to postnatal acidification although the skin phenotype of the Na/H exchanger knockout mice is not the primary cause for the death (49). Other ion transporters may be implicated as well. In human infants, the delay in acidification could explain the increased infantile risk for irritant/allergic contact dermatitis, infection, and percutanous absorption of toxic chemicals (45). We propose here that the absence of ENaC in the epidermis may be crucial for the sodium concentrations in the different strata composing the epidermis and if absent interfere with the proton movement needed for the establishment of the ph gradient. This implies that the different strata are functionally separated from each other and that the ions are not moving freely between the layers. The idea that there could be other structures than the classical tight junctions has been recently proposed (26), defining compartments in the epidermis that can arise from the presence of many tight junctions proteins all across the epidermis (50). During the stratification of human skin equivalents, an early and broadened synthesis of tight junction proteins precedes the formation of the stratum corneum and it has been proposed that tight junctions and tight junction like proteins are involved in barrier formation, serving as a rescue system when the stratum corneum is missing or impaired (25). Transmembrane ionic fluxes might control keratinocyte differentiation including the synthesis of cornified envelope and other differentiation-specific proteins (14,51). Expression of ENaC channel subunits is indeed increased in more differentiated keratinocytes (12,13), although we could not find major changes in the expression profile of the differentiation proteins in the αenac knockout mice (Suppl.Fig.2). Ionic fluxes further play an important role in skin differentiation processes as shown by identification of mutations in two calcium pumps leading to severe skin pathologies like Hailey-Hailey disease and Darier disease (52,53). Thus, ENaC activity may play a role in the regulation of diverse cellular processes in the skin like e.g. barrier function, galvanotaxis and wound healing (54,55)
9 In conclusion, our results unveil the physiological consequences of the mammalian epithelial sodium channel deficiency in skin that leads to distinct phenotypes in prenatal versus postnatal period. We propose that ENaC plays an important role in the maturation of the postnatal epidermal permeability barrier function. Thereby, the physiological consequences of αenac-deficiency in mouse skin will contribute to a better understanding of the developmental adjustments to achieve optimal epidermal barrier function in the dry ex utero environment. References 1. Hummler, E., and Vallon, V. (2005) J Am Soc Nephrol 16: Kellenberger, S., and Schild, L. E. r. (2002) Physiol Rev 82(3), Canessa, C. M., Horisberger, J. D., and Rossier, B. C. (1993) Nature 361, Canessa, C. M., Schild, L., Buell, G., Thorens, B., Gautschi, I., Horisberger, J. D., and Rossier, B. C. (1994) Nature 367(6462), Rossier, B. C., Pradervand, S., Schild, L., and Hummler, E. (2002) Ann Rev of Physiol 64, Firsov, D., Schild, L., Gautschi, I., Merillat, A. M., Schneeberger, E., and Rossier, B. C. (1996) Proc Natl Acad Sci U S A 93(26), Hummler, E., and Rossier, B. C. (1996) Kid. Blood Press. Res. 19, Pradervand, S., Barker, P., Wang, Q., Ernst, S. A., Beermann, F., Grubb, B., Burnier, M., Schmidt, A., Bindels, R. J. M., Gatzy, J., Rossier, B. C., and Hummler, E. (1999) Proc Natl Acad Sci USA 96, Barker, P. M., Ngugen, M. S., Gatzy, J. T., Grubb, B., Norman, H., Hummler, E., Rossier, B., Boucher, R. C., and Koller, B. (1998) J. Clin. Invest. 102, McDonald, F. J., Yang, B., Hrstka, R. F., Drummond, H. A., Tarr, D. E., McCray, P. B., Jr., Stokes, J. B., Welsh, M. J., and Williamson, R. A. S. b. k. (1999) Proc Natl Acad Sci U S A 96(4), Puoti, A., May, A., Canessa, C. M., Horisberger, J.-D., Schild, L., and Rossier, B. C. (1995) Am J Physiol 269, C Oda, Y., Imanzahrai, A., Kwong, A., Komuves, L., Elias, P. M., Largman, C., and Mauro, T. P. E. p. (1999) J Invest Dermatol 113(5), Brouard, M., Casado, M., Djelidi, S., Barrandon, Y., and Farman, N. P. E. p. (1999) J Cell Sci 112, Mauro, T., Guitard, M., Behne, M., Oda, Y., Crumrine, D., Komuves, L., Rassner, U., Elias, P. M., and Hummler, E. (2002) J Invest Dermatol 118(4), Roop, D. (1995) Science 267, Hamanaka, S., Hara, M., Nishio, H., Otsuka, F., Suzuki, A., and Uchida, Y. (2002) J Invest Dermatol 119(2), Doering, T., Holleran, W. M., Potratz, A., Vielhaber, G., Elias, P. M., Suzuki, K., and Sandhoff, K. (1999) J Biol Chem 274(16), Doering, T., Proia, R. L., and Sandhoff, K. (1999) FEBS Lett 447(2-3), Uchida, Y., Hara, M., Nishio, H., Sidransky, E., Inoue, S., Otsuka, F., Suzuki, A., Elias, P. M., Holleran, W. M., and Hamanaka, S. (2000) J Lipid Res 41(12), Zettersten, E., Man, M. Q., Sato, J., Denda, M., Farrell, A., Ghadially, R., Williams, M. L., Feingold, K. R., and Elias, P. M. C. d. (1998) J Invest Dermatol 111(5), Hummler, E., Barker, P., Gatzy, J., Beermann, F., Verdumo, C., Schmidt, A., Boucher, R., and Rossier, B. C. (1996) Nat Genet 12(3), Segre, J. A., Bauer, C., and Fuchs, E. (1999) Nat Genet 22(4), Hardman, M. J., Sisi, P., Banbury, D. N., and Byrne, C. (1998) Development 125(8), Indra, A. K., Dupe, V., Bornert, J. M., Messaddeq, N., Yaniv, M., Mark, M., Chambon, P., and Metzger, D. (2005) Development 132(20),
10 25. Brandner, J. M., Kief, S., Grund, C., Rendl, M., Houdek, P., Kuhn, C., Tschachler, E., Franke, W. W., and Moll, I. (2002) Eur J Cell Biol 81(5), Schluter, H., Moll, I., Wolburg, H., and Franke, W. W. (2007) Eur J Cell Biol (Epub ahead of print) 27. Navarro, J. M., Casatorres, J., and Jorcano, J. L. K. (1995) J Biol Chem 270(36), Park, B. D., Youm, J. K., Jeong, S. K., Choi, E. H., Ahn, S. K., and Lee, S. H. (2003) J Invest Dermatol 121(4), Choi, M. J., and Maibach, H. I. (2005) Am J Clin Dermatol 6(4), Macheleidt, O., Kaiser, H. W., and Sandhoff, K. (2002) J. Invest. Dermatol. 119, Doering, T., Brade, H., and Sandhoff, K. (2002) J. Lipid Res. 43, Herrmann, T., Van der Hoeven, F., Gröne, H.-J., Stewart, A. F., Langbein, L., Kaiser, I., Liebisch, G., Gosch, I., Buchkremer, F., Drobnik, W., Schmitz, G., and Stremmel, W. (2003) J. Cell Biol. 161, Holleran, W. M., Takagi, Y., Menon, G. K., Jackson, S. M., Lee, J. M., Feingold, K. R., and Elias, P. M. (1994) J Lipid Res 35(5), Liu, Y., Suzuki, K., Reed, J. D., Grinberg, A., Westphal, H., Hoffmann, A., Doring, T., Sandhoff, K., and Proia, R. L. (1998) Proc Natl Acad Sci U S A 95(5), Nemes, Z., Marekov, L. N., Fesus, L., and Steinert, P. M. (1999) Proc Natl Acad Sci U S A 96(15), Segre, J. A. (2006) J Clin Invest 116(5), Vasireddy, V., Uchida, Y., Salem, N., Jr., Kim, S. Y., Mandal, M. N., Reddy, G. B., Bodepudi, R., Alderson, N. L., Brown, J. C., Hama, H., Dlugosz, A., Elias, P. M., Holleran, W. M., and Ayyagari, R. (2007) Hum Mol Genet 16(5), Li, W., Sandhoff, R., Kono, M., Zerfas, P., Hoffmann, V., Ding, B. C., Proia, R. L., and Deng, C. X. (2007) Int J Biol Sci 3(2), Behne, M. J., Barry, N. P., Hanson, K. M., Aronchik, I., Clegg, R. W., Gratton, E., Feingold, K., Holleran, W. M., Elias, P. M., and Mauro, T. M. (2003) J Invest Dermatol 120(6), Aszterbaum, M., Menon, G. K., Feingold, K. R., and Williams, M. L. (1992) Pediatr Res 31(4 Pt 1), Aszterbaum, M., Feingold, K. R., Menon, G. K., and Williams, M. L. (1993) J Clin Invest 91(6), Jensen, J. M., Schutze, S., Forl, M., Kronke, M., and Proksch, E. S. a. r. a. p. (1999) J Clin Invest 104(12), Holleran, W. M., Takagi, Y., Imokawa, G., Jackson, S., Lee, J. M., and Elias, P. M. b.-g.-c. w. a. p. (1992) J Lipid Res 33(8), Fluhr, J. W., Behne, M. J., Brown, B. E., Moskowitz, D. G., Selden, C., Mao-Qiang, M., Mauro, T. M., Elias, P. M., and Feingold, K. R. (2004) J Invest Dermatol 122(2), Yosipovitch, G., Maayan-Metzger, A., Merlob, P., and Sirota, L. (2000) Pediatrics 106(1 Pt 1), Behne, M. J., Meyer, J. W., Hanson, K. M., Barry, N. P., Murata, S., Crumrine, D., Clegg, R. W., Gratton, E., Holleran, W. M., Elias, P. M., and Mauro, T. M. (2002) J Biol Chem 277(49), Krien, P. M., and Kermici, M. (2000) J Invest Dermatol 115(3), Schadow, A., Scholz-Pedretti, K., Lambeau, G., Gelb, M. H., Furstenberger, G., Pfeilschifter, J., and Kaszkin, M. (2001) J Invest Dermatol 116(1), Bell, S. M., Schreiner, C. M., Schultheis, P. J., Miller, M. L., Evans, R. L., Vorhees, C. V., Shull, G. E., and Scott, W. J. (1999) Am J Physiol 276(4 Pt 1), C Brandner, J. M., Kief, S., Wladykowski, E., Houdek, P., and Moll, I. (2006) Skin Pharmacol Physiol 19(2), Mauro, T., Cixon, D. B., Hanley, K., Isseroff, R. R., and Pappone, P. A. (1995) J Invest Dermatol 105, Hu, Z., Bionifas, J. M., Beech, J., Bench, G., Shigihara, T., Ogawa, H., Ikeda, S., Mauro, T., and Epstein, E. H. (2000) Nat. Genet. 24,
11 53. Sakuntabhai, A., Ruiz-Perez, V., Carter, S., Jacobsen, N., Burge, S., Monk, S., Smith, M., Munro, C. S., O'Donovan, M., Craddock, N., Kucherlapati, R., Rees, J. L., Owen, M., Lathrop, G. M., Monaco, A. P., Stachan, T., and Hovnanian, A. (1999) Nat Genet 21, Chifflet, S., Hernandez, J. A., and Grasso, S. (2005) Am J Physiol Cell Physiol 288(6), C Guitard, M., Leyvraz, C., and Hummler, E. (2004) News Physiol Sci 19, Hummler, E., Barker, P., Talbot, C., Wang, Q., Verdumo, C., Grubb, B., Gatzy, J., Burnier, M., Horisberger, J.-D., Beermann, F., Boucher, R., and Rossier, B. C. (1997) Proc. Natl. Acad. Sci. USA 94, Koch, P. J., de Viragh, P. A., Scharer, E., Bundman, D., Longley, M. A., Bickenbach, J., Kawachi, Y., Suga, Y., Zhou, z., Huber, M., Hohl, D., Kartasova, T., Jarnik, M., Steven, A. C., and Roop, D. R. (2000) J. Cell Biol. 151, Leyvraz, C., Charles, R. P., Rubera, I., Guitard, M., Rotman, S., Breiden, B., Sandhoff, K., and Hummler, E. (2005) J Cell Biol 170(3), Matsuki, M., Yamashita, F., Ishida-Yamamoto, A., Yamada, K., Kinoshita, C., Fushiki, S., Ueda, E., Morishima, Y., Tabata, K., Yasuno, H., Hashida, M., Iizuka, H., Ikawa, M., Okabe, M., Kondoh, G., Kinoshita, T., Takeda, J., and Yamanishi, K. T. k. (1998) Proc Natl Acad Sci U S A 95(3), Chen, Y., Merzdorf, C., Paul, D. L., and Goodenough, D. A. (1997) J Cell Biol 138(4), Furuse, M., Hata, M., Furuse, K., Yoshida, Y., Haratake, A., Sugitani, Y., Noda, T., Kubo, A., and Tsukita, S. (2002) J Cell Biol 156(6), Reichelt, J., Breiden, B., Sandhoff, K., and Magin, T. M. (2004) Eur J Cell Biol 83(11-12), Haftek, M., Teillon, M. H., and Schmitt, D. (1998) Microsc Res Techn 43, 1-8 Acknowlegdements We like to thank Mikio Furuse for the rat anti-mouse occludin mab(moc37) and ZO-1 mouse monoclonal antisera (T8-754). We wish to thank the Mouse Pathology platform (University of Lausanne) for histopathological analysis and the Cellular Imaging Facility platform from the University of Lausanne for help with the cellular imaging. The EM samples were observed at the Centre Technique des Microscopies, University Lyon 1 by M. Haftek. We also thank Anne-Marie Mérillat for technical assistance and Friedrich Beermann, Jean-Daniel Horisberger, Martin Behne and Roman Chrast for helpul suggestions on the manuscript, and also Bernard Rossier for his constant support on the project. We also thank members of the Hummler lab for helpful suggestions and discussions. This work was supported by the Swiss National Science Foundation (grants 3100A /1 and 3100A /1 to E. Hummler) and the Deutsche Forschungsgemeinschaft (SFB 645 to B. Breiden and K. Sandhoff). Figure Legends Fig.1 The postnatal barrier is impaired in αenac deficient mice (A) Barrier-dependent dye exclusion assay. Representative photographs from αenac (-/-, +/- and +/+) newborns (6 hours old) reveal no difference in dye exclusion from all epidermal surfaces. (B) Weight loss in αenac homozygous (-/-) and heterozygous (+/-) mutant, and wild-type (+/+) newborns determined from a total of 51 pups (n= 5 litters) analyzed shortly after birth (until 24h). The mean value for each group is indicated (bar)
12 (C) Data from one representative litter are presented as percentages of initial body weight in the knockout (open triangles; n= 4), heterozygous mutant (+/-, grey diamonds; n= 12) and wild-type (black squares; n= 9) mice. (D - F) Time-dependent transepidermal water loss (TEWL) as measured on ventral skin of αenac knockout (-/-, n= 5, white column), heterozygous mutant (+/-, n= 18, grey column) and wild-type (+/+, n= 9, black column; in total 3 litters) mice within 0-12h (D), and (E) within 12 24h following birth. (from 5 -/-, 8 +/-, and 5 +/+; in total 2 litters, respectively). (F) TEWL measurements at 4h and 24h after birth (-/-, n= 4, white column); (+/-, n= 4, grey column), (+/+, n= 2, black column).*, P< 0.05;, P< 0.01;, P< 0.001; n.s., not significant. Error bars represent SEM. Fig.2 Expression of tight junction proteins and TJ permeability assay (A) LSM immunofluorescence for claudin-1 (claudin), Zona occludens 1 (ZO-1), and occludin. For ZO-1 and occludin, the DAPI counterstaining is added. The two dotted lines indicate the basal membrane (bottom) and the limit SG/SC (top). Arrows indicate the localization of occludin within the granular layer. (B) TJ permeability assay visualized by LSM. For each genotype (+/+ and -/-), the staining for streptavidin/alexafluor488 is shown with DAPI conterstaining, and brightfield/dapi of the same picture is added for localization. The bottom dotted line indicates the basal membrane, and the top line indicates the SG/SC border. In animals of both groups, there is no difference in the diffusion of biotin that is blocked abruptly between the second and the last layer of the SG (arrows). Fig.3 Severe impairment of SC lipid composition in αenac-deficient mice (A-F) Analysis of SC lipid content and composition of αenac -/- (white), +/- (grey) and +/+ littermates (black columns). (A,D) Levels of free SC lipids at 5 or 31h. Chol, cholesterol; FFA, free fatty acid; Cer, ceramides. Note that cholesterol and ceramide levels are significantly decreased in knockouts (*, P < 0.05;, P< 0.01, compared to the corresponding control (+/+) group). (B,E) Analysis of probarrier lipids at 5 or 31h. Knockouts show significantly increased levels for all probarrier lipids glucosylceramide (GlcCer), sphingomyelin (SM) and cholesterol sulfate (CSO4). (C,F) Levels of covalently-bound lipids at 5 or 31h. Note the overall reduction of ω-hydroxylated fatty acid (ω-oh-fa) and the overall reduction in ceramides (Cer[OS];) levels in knockout (-/-) mice) compared to the wild-type (+/+) group. Note that heterozygotes (+/-) present an intermediate phenotype for all measured parameters (*, P< 0.05;, P< 0.01;, P< 0.001). Each value is the mean of at least five independent animals ±SEM (error bars). Fig.4 Ultrastructural analysis of ENaC wildtype and knockout mice Lamellar body secretion and extracellular formation of the lamellar lipids proved abnormal in the knockout epidermis (A,C; asterisks) compared to wildtype pups (B,D). Note that the apical region of the most superficial granular keratinocyte layer (SG, indicated with arrows) in the knockouts contains highly disorganized lipid lamellae (LL; A) and that the lamellar body-derived vesicular structures persist in the intercorneocyte spaces of the lower stratum corneum (D). In contrast, in wildtype animals (B,D), the lipid lamellae are tightly packed and adhere to the well visible corneocyte lipid envelopes. A,A,B,B : ruthenium tetroxide post-fixation; C,D: osmium tetroxide fixation. A,A,C: (α)enac knockout epidermis; B,B,C: wildtype epidermis. SC: stratum corneum; SG: stratum granulosum; N: nucleus; KHG: keratohyalin granule; Bars= 200nm in A, 500 nm in C and D. (A and B are enlarged version of A and B.) Fig.5 Elevated skin surface ph in αenac knockout mice Skin surface ph measurements at 24h after birth. Note the failure of the knockout group to acidify the surface ph (, P< 0.001, compared to the +/+ group). The heterozygotes display a phenotype not different from the wild-type (+/+) group, but different from the knockout group (, P< 0.01)
13 * P< 0.05; P< 0.01; P< Table 1. Covalently-bound lipids composition 5h Genotypes -/- +/- +/+ [ng/mg dry SC] [ng/mg dry SC] [ng/mg dry SC] GlcCer(OS) ± ± ± Cer(OH) ± ± ± Cer(OP) ± ± ± Cer(OS) ± ± ± ω-oh-fa * ± * ± ± h Genotypes -/- +/- +/+ [ng/mg dry SC] [ng/mg dry SC] [ng/mg dry SC] GlcCer(OS) ± ± ± Cer(OH) ± ± ± Cer(OP) ± ± ± Cer(OS) ± ± ± ω-oh-fa ± ± ±
14 Table2. Ceramides composition 5h Cer(EOS) ± ± ± Cer(C26-NS) ± ± ± Cer(C18-NS) ± ± ± x ± ± ± Cer(NP) ± ± ± Cer(C26-AS) ± ± ± Cer(C18-AS) ± ± ± Cer(C26-AP)/Cer(C26-AH) ± ± ± Cer(C18-AP)/Cer(C18-AH) ± ± ± x ± ± ± h Genotypes -/- +/- +/+ [ng/mg dry SC] [ng/mg dry SC] [ng/mg dry SC] Genotypes -/- +/- +/+ [ng/mg dry SC] [ng/mg dry SC] [ng/mg dry SC] Cer(EOS) ± ± ± Cer(C26-NS) ± ± ± Cer(C18-NS) ± ± ± x ± ± ± Cer(NP) ± ± ± Cer(C26-AS) ± ± ± Cer(C18-AS) ± ± ± Cer(C26-AP)/Cer(C26-AH) ± * ± ± Cer(C18-AP)/Cer(C18-AH) ± * ± ± x2 * ± ± ± * P< 0.05; P< 0.01; P< 0.001
15 A B C /- +/- +/+ Genotype /+ +/- -/ Time (h) -/- Genotype +/- +/+ mg/h/g of bodyweight % of initial bodyweight D E F 0 to 12h n.s. n.s. 12 to 24h 140 * * /- +/- +/+ Genotype n.s. n.s. -/- +/- +/+ * * n.s. 4h 24h Normalized TEWL Normalized TEWL Normalized TEWL /- +/- +/+ Genotype Figure 1
16 A Claudin ZO-1 20µm Occludin Knockout Wildtype Knockout Wildtype B Figure 2
17 A 80 5h * D 80 31h Lamellar Lipids µg/mg dry SC * µg/mg dry SC Chol FFA Cer 0 Chol FFA Cer B 3.5 E 3.5 Probarrier Lipids µg/mg dry SC * * GlcCer SM CSO4 µg/mg dry SC GlcCer SM CSO4 Covalently-bound Lipids C µg/mg dry SC Cer(OS) * * ω-oh-fa F µg/mg dry SC Cer(OS) ω-oh-fa Figure 3 Genotype -/- +/- +/+
18 Figure 4
Skin Barrier Function as a Self-Organizing System
 Review Forma, 15, 227 232, 2000 Skin Barrier Function as a Self-Organizing System Mitsuhiro DENDA Shiseido Research Center, 2-12-1 Fukuura, Kanazawa-ku, Yokohama, Kanagawa 236-8643, Japan E-mail: mitsuhiro.denda@to.shiseido.co.jp
Review Forma, 15, 227 232, 2000 Skin Barrier Function as a Self-Organizing System Mitsuhiro DENDA Shiseido Research Center, 2-12-1 Fukuura, Kanazawa-ku, Yokohama, Kanagawa 236-8643, Japan E-mail: mitsuhiro.denda@to.shiseido.co.jp
Sphingolipid metabolism during epidermal barrier development in mice
 Sphingolipid metabolism during epidermal barrier development in mice Thomas Doering, 1, * Helmut Brade, and Konrad Sandhoff*,2 Kekulé-Institut für Organische Chemie und Biochemie,* Universität Bonn, Gerhard-Domagk-Strasse
Sphingolipid metabolism during epidermal barrier development in mice Thomas Doering, 1, * Helmut Brade, and Konrad Sandhoff*,2 Kekulé-Institut für Organische Chemie und Biochemie,* Universität Bonn, Gerhard-Domagk-Strasse
Induction of Selected Lipid Metabolic Enzymes and Differentiation-Linked Structural Proteins by Air Exposure in Fetal Rat Skin Explants
 Induction of Selected Lipid Metabolic Enzymes and Differentiation-Linked Structural Proteins by Air Exposure in Fetal Rat Skin Explants László G. Kömüves,* Karen Hanley,*, Yan Jiang,* Chika Katagiri,*
Induction of Selected Lipid Metabolic Enzymes and Differentiation-Linked Structural Proteins by Air Exposure in Fetal Rat Skin Explants László G. Kömüves,* Karen Hanley,*, Yan Jiang,* Chika Katagiri,*
The Changes of Epidermal Calcium Gradient and Transitional Cells after Prolonged Occlusion Following Tape Stripping in the Murine Epidermis
 The Changes of Epidermal Calcium Gradient and Transitional Cells after Prolonged Occlusion Following Tape Stripping in the Murine Epidermis Sung Ku Ahn, Sang Min Hwang, Shao Jun Jiang, Eung Ho Choi, and
The Changes of Epidermal Calcium Gradient and Transitional Cells after Prolonged Occlusion Following Tape Stripping in the Murine Epidermis Sung Ku Ahn, Sang Min Hwang, Shao Jun Jiang, Eung Ho Choi, and
Patterned acquisition of skin barrier function during development
 Development 125, 1541-1552 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV1249 1541 Patterned acquisition of skin barrier function during development Matthew J. Hardman*, Paraskevi
Development 125, 1541-1552 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV1249 1541 Patterned acquisition of skin barrier function during development Matthew J. Hardman*, Paraskevi
The epidermal barrier function is dependent on the serine protease CAP1/Prss8
 JCB: ARTICLE The epidermal barrier function is dependent on the serine protease CAP1/Prss8 Céline Leyvraz, 1 Roch-Philippe Charles, 1 Isabelle Rubera, 1 Marjorie Guitard, 1 Samuel Rotman, 2 Bernadette
JCB: ARTICLE The epidermal barrier function is dependent on the serine protease CAP1/Prss8 Céline Leyvraz, 1 Roch-Philippe Charles, 1 Isabelle Rubera, 1 Marjorie Guitard, 1 Samuel Rotman, 2 Bernadette
The epidermis displays a characteristic calcium (Ca )
 Formation of the Epidermal Calcium Gradient Coincides with Key Milestones of Barrier Ontogenesis in the Rodent Peter M. Elias, Patricia Nau, Karen Hanley, Chris Cullander,* Debra Crumrine, Graham Bench,
Formation of the Epidermal Calcium Gradient Coincides with Key Milestones of Barrier Ontogenesis in the Rodent Peter M. Elias, Patricia Nau, Karen Hanley, Chris Cullander,* Debra Crumrine, Graham Bench,
TCF/Lef1-Mediated Control of Lipid Metabolism Regulates Skin Barrier Function
 ORIGINAL ARTICLE TCF/Lef1-Mediated Control of Lipid Metabolism Regulates Skin Barrier Function Dagmar Fehrenschild 1,2, Uwe Galli 1,2,8, Bernadette Breiden 3, Wilhelm Bloch 4, Peter Schettina 1,2, Susanne
ORIGINAL ARTICLE TCF/Lef1-Mediated Control of Lipid Metabolism Regulates Skin Barrier Function Dagmar Fehrenschild 1,2, Uwe Galli 1,2,8, Bernadette Breiden 3, Wilhelm Bloch 4, Peter Schettina 1,2, Susanne
Epidermis. Integumentary system
 Epidermis the doctor mentioned at the begging of the lecture that the slides is from different sources and has information and details that is enough for us so we don t have to go back and read from the
Epidermis the doctor mentioned at the begging of the lecture that the slides is from different sources and has information and details that is enough for us so we don t have to go back and read from the
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Application of Skin-Identical Ceramide 3 for Enhanced Skin Moisturization and Smoothness: Latest Results
 Application of Skin-Identical Ceramide 3 for Enhanced Skin Moisturization and Smoothness: Latest Results By Ute Wollenweber* and Dr. Mike Farwick* Keywords: Ceramides, Stratum corneum, skin moisturization,
Application of Skin-Identical Ceramide 3 for Enhanced Skin Moisturization and Smoothness: Latest Results By Ute Wollenweber* and Dr. Mike Farwick* Keywords: Ceramides, Stratum corneum, skin moisturization,
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2],
![Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2], Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2],](/thumbs/86/93801604.jpg) Supplementary information Methods Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2], and A/Hong Kong/213/03 [H5N1]) were grown in Madin-Darby canine kidney (MDCK) cells
Supplementary information Methods Cells and viruses. Human isolates (A/Kawasaki/173/01 [H1N1], A/Yokohama/2057/03 [H3N2], and A/Hong Kong/213/03 [H5N1]) were grown in Madin-Darby canine kidney (MDCK) cells
Akemi Ishida-Yamamoto
 Journal of Dermatological Science (2003) 31(1):3-8. Loricrin keratoderma: a novel disease entity characterized by nuclear accumulation of mutant loricrin Akemi Ishida-Yamamoto Loricrin Keratoderma. A novel
Journal of Dermatological Science (2003) 31(1):3-8. Loricrin keratoderma: a novel disease entity characterized by nuclear accumulation of mutant loricrin Akemi Ishida-Yamamoto Loricrin Keratoderma. A novel
Cytological and Histological Study of Adult and Neonate Epidermis in Thick and Thin Skin of Various Anatomical Sites
 Available online on www.ijpqa.com International Journal of Pharmaceutical Quality Assurance 218; 9(2); 174-179 doi: 1.25258/ijpqa.v9i2.13642 ISSN 975 956 Research Article Cytological and Histological Study
Available online on www.ijpqa.com International Journal of Pharmaceutical Quality Assurance 218; 9(2); 174-179 doi: 1.25258/ijpqa.v9i2.13642 ISSN 975 956 Research Article Cytological and Histological Study
SUPPLEMENTARY MATERIAL. Sample preparation for light microscopy
 SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated,
 1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated, embedded in matrigel and exposed
1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated, embedded in matrigel and exposed
PHYTOBIOACTIVES Cariciline CARICILINE GREENTECH S.A
 1 Natural Gel MOISTURIZING AND REFRESHING The main function of skin is : To ensure a protective function in relation to external aggressions : mechanical, chemical and microbial aggressions. To ensure
1 Natural Gel MOISTURIZING AND REFRESHING The main function of skin is : To ensure a protective function in relation to external aggressions : mechanical, chemical and microbial aggressions. To ensure
Topical Preparations
 Topical Preparations One of the functions of the skin is to protect the internal body components against the external environment and thus to control the passage of chemicals into and out of the body.
Topical Preparations One of the functions of the skin is to protect the internal body components against the external environment and thus to control the passage of chemicals into and out of the body.
Update on emollients
 Update on emollients Amal Mhanna, MD Pediatric Dermatologist Clemenceau Medical Center Disclosure: I was a member of an advisory board y for J&J and received honoraria. Emollients and moisturizers are
Update on emollients Amal Mhanna, MD Pediatric Dermatologist Clemenceau Medical Center Disclosure: I was a member of an advisory board y for J&J and received honoraria. Emollients and moisturizers are
Integumentary System-Skin and Body Coverings
 Integumentary System-Skin and Body Coverings List the four types of epithelial or connective membranes. The epithelial cutaneous includes your and is exposed to the. Its function is to. An example is..
Integumentary System-Skin and Body Coverings List the four types of epithelial or connective membranes. The epithelial cutaneous includes your and is exposed to the. Its function is to. An example is..
This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur.
 Background Knowledge Functions of normal skin Background Knowledge This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur. Learning
Background Knowledge Functions of normal skin Background Knowledge This section covers the basic knowledge of normal skin structure and function required to help understand how skin diseases occur. Learning
DEBRIDEMENT: ANATOMY and PHYSIOLOGY. Professor Donald G. MacLellan Executive Director Health Education & Management Innovations
 DEBRIDEMENT: ANATOMY and PHYSIOLOGY Professor Donald G. MacLellan Executive Director Health Education & Management Innovations ANATOMY and PHYSIOLOGY Epidermal Layers ECM Structure Dermis Structure Skin
DEBRIDEMENT: ANATOMY and PHYSIOLOGY Professor Donald G. MacLellan Executive Director Health Education & Management Innovations ANATOMY and PHYSIOLOGY Epidermal Layers ECM Structure Dermis Structure Skin
BARNET ACTIVE INTELLIGENT MOISTURIZATION AESTHIGEL. Aesthetic Moisturizer acting from the bottom to the surface in every layer of the skin
 BARNET ACTIVE INTELLIGENT MOISTURIZATION AESTHIGEL Aesthetic Moisturizer acting from the bottom to the surface in every layer of the skin The information contained in this technical bulletin is, to the
BARNET ACTIVE INTELLIGENT MOISTURIZATION AESTHIGEL Aesthetic Moisturizer acting from the bottom to the surface in every layer of the skin The information contained in this technical bulletin is, to the
Dr Narmeen S. Ahmad. Lab 1
 Dr Narmeen S. Ahmad Lab 1 1 Tissues are groups of cells with a common structure (form) and function (job). There are (4) types of tissue: 1. Epithelial 2. Connective 3. Muscle 4. Nervous 2 Epithelial cells
Dr Narmeen S. Ahmad Lab 1 1 Tissues are groups of cells with a common structure (form) and function (job). There are (4) types of tissue: 1. Epithelial 2. Connective 3. Muscle 4. Nervous 2 Epithelial cells
Introduction. Skin and Body Membranes. Cutaneous Membranes Skin 9/14/2017. Classification of Body Membranes. Classification of Body Membranes
 Introduction Skin and Body Membranes Body membranes Cover surfaces Line body cavities Form protective and lubricating sheets around organs Classified in 5 categories Epithelial membranes 3 types- cutaneous,
Introduction Skin and Body Membranes Body membranes Cover surfaces Line body cavities Form protective and lubricating sheets around organs Classified in 5 categories Epithelial membranes 3 types- cutaneous,
Diffusion across cell membrane
 The Cell Membrane and Cellular Transport Diffusion across cell membrane Cell membrane is the boundary between inside & outside separates cell from its environment Can it be an impenetrable boundary? NO!
The Cell Membrane and Cellular Transport Diffusion across cell membrane Cell membrane is the boundary between inside & outside separates cell from its environment Can it be an impenetrable boundary? NO!
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the
 Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
Supplementary Figure 1 Expression of Crb3 in mouse sciatic nerve: biochemical analysis (a) Schematic of Crb3 isoforms, ERLI and CLPI, indicating the location of the transmembrane (TM), FRM binding (FB)
The Role of Epidermal Lipids in Cutaneous Permeability Barrier Homeostasis
 The Role of Epidermal Lipids in Cutaneous Permeability Barrier Homeostasis Kenneth R. Feingold Metabolism Section, Medical Service, Department of Veterans Affairs Medical Center, University of California
The Role of Epidermal Lipids in Cutaneous Permeability Barrier Homeostasis Kenneth R. Feingold Metabolism Section, Medical Service, Department of Veterans Affairs Medical Center, University of California
NHE1 REGULATES THE STRATUM CORNEUM PERMEABILITY BARRIER HOMEOSTASIS: MICROENVIRONMENT ACIDIFICATION ASSESSED WITH FLIM
 JBC Papers in Press. Published on September 7, 2002 as Manuscript M204759200 1 NHE1 REGULATES THE STRATUM CORNEUM PERMEABILITY BARRIER HOMEOSTASIS: MICROENVIRONMENT ACIDIFICATION ASSESSED WITH FLIM Martin
JBC Papers in Press. Published on September 7, 2002 as Manuscript M204759200 1 NHE1 REGULATES THE STRATUM CORNEUM PERMEABILITY BARRIER HOMEOSTASIS: MICROENVIRONMENT ACIDIFICATION ASSESSED WITH FLIM Martin
Skin barrier function in infants - new challenges and new opportunities
 Skin barrier function in infants - new challenges and new opportunities Georgios N Stamatas, Ph.D. Research Associate Director and Fellow Johnson & Johnson Santé Beauté France Any third party trademarks
Skin barrier function in infants - new challenges and new opportunities Georgios N Stamatas, Ph.D. Research Associate Director and Fellow Johnson & Johnson Santé Beauté France Any third party trademarks
Active Beauty Rubixyl Anti stress-ageing peptide. Crafted by synthesis
 Active Beauty Rubixyl Anti stress-ageing peptide Crafted by synthesis Focus on the product The skin barrier function The most crucial part of the skin barrier functions is the stratum corneum. The stratum
Active Beauty Rubixyl Anti stress-ageing peptide Crafted by synthesis Focus on the product The skin barrier function The most crucial part of the skin barrier functions is the stratum corneum. The stratum
Supplementary Information
 Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
Supplementary Information Supplementary Figure 1. CD4 + T cell activation and lack of apoptosis after crosslinking with anti-cd3 + anti-cd28 + anti-cd160. (a) Flow cytometry of anti-cd160 (5D.10A11) binding
A mouse model for the renal salt-wasting syndrome pseudohypoaldosteronism
 Proc. Natl. Acad. Sci. USA Vol. 94, pp. 11710 11715, October 1997 Physiology A mouse model for the renal salt-wasting syndrome pseudohypoaldosteronism EDITH HUMMLER*, PIERRE BARKER, COLLEEN TALBOT, QING
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 11710 11715, October 1997 Physiology A mouse model for the renal salt-wasting syndrome pseudohypoaldosteronism EDITH HUMMLER*, PIERRE BARKER, COLLEEN TALBOT, QING
Epigenetic Control of ENaC
 Epigenetic Control of ENaC Transcription, Na + Metabolism and Blood Pressure Wenzheng Zhang, Ph.D. Department of Internal Medicine The University of Texas Medical School at Houston International Conference
Epigenetic Control of ENaC Transcription, Na + Metabolism and Blood Pressure Wenzheng Zhang, Ph.D. Department of Internal Medicine The University of Texas Medical School at Houston International Conference
Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by
 Nakano et al. Supplementary information 1. Supplementary Figure 2. Methods 3. References Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into
Nakano et al. Supplementary information 1. Supplementary Figure 2. Methods 3. References Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into
Histopathology: skin pathology
 Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
Histopathology: skin pathology These presentations are to help you identify, and to test yourself on identifying, basic histopathological features. They do not contain the additional factual information
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
SUPPLEMENTARY INFORMATION FOR Liver X Receptor α mediates hepatic triglyceride accumulation through upregulation of G0/G1 Switch Gene 2 (G0S2) expression I: SUPPLEMENTARY METHODS II: SUPPLEMENTARY FIGURES
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
TFEB-mediated increase in peripheral lysosomes regulates. Store Operated Calcium Entry
 TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
TFEB-mediated increase in peripheral lysosomes regulates Store Operated Calcium Entry Luigi Sbano, Massimo Bonora, Saverio Marchi, Federica Baldassari, Diego L. Medina, Andrea Ballabio, Carlotta Giorgi
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Trimoist KMF Biomimetic 24 h hydrator
 Biomimetic 24 h hydrator Biomimetic 24 h hydrator An Effective Hydro-Complex with a Skin-Like Moisturizing System Trimoist KMF is a blend of lamellar lipids, CM-Glucan, humectant agents and the anti-aging
Biomimetic 24 h hydrator Biomimetic 24 h hydrator An Effective Hydro-Complex with a Skin-Like Moisturizing System Trimoist KMF is a blend of lamellar lipids, CM-Glucan, humectant agents and the anti-aging
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
Using Infrared and Raman Microspectroscopies to Compare Ex Vivo Involved Psoriatic Skin and Normal Human Skin
 Using Infrared and Raman Microspectroscopies to Compare Ex Vivo Involved Psoriatic Skin and Normal Human Skin Leroy, Marie (Laboratoire d'ingénierie de Surface (LIS)) Lefèvre, Thierry (Groupe de recherche
Using Infrared and Raman Microspectroscopies to Compare Ex Vivo Involved Psoriatic Skin and Normal Human Skin Leroy, Marie (Laboratoire d'ingénierie de Surface (LIS)) Lefèvre, Thierry (Groupe de recherche
Assessment of the in vitro skin irritation of chemicals using the Vitrolife-Skin human skin model
 AATEX 14, Special Issue, 417-423 Proc. 6th World Congress on Alternatives & Animal Use in the Life Sciences August 21-25, 2007, Tokyo, Japan Assessment of the in vitro skin irritation of chemicals using
AATEX 14, Special Issue, 417-423 Proc. 6th World Congress on Alternatives & Animal Use in the Life Sciences August 21-25, 2007, Tokyo, Japan Assessment of the in vitro skin irritation of chemicals using
(From The Rockefeller Institute) Materials and Methods. Observations with the Electron Microscope
 ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF THE PAPILLOMA VIRUS IN THE SKIN OF THE RABBIT* BY ROBERT S. STONE,~ M.D., RICHARD E. SHOPE, M.D., DAN H. MOORE, P,~.D. (From The Rockefeller Institute) PLATES
Anatomy Ch 6: Integumentary System
 Anatomy Ch 6: Integumentary System Introduction: A. Organs are body structures composed of two or more different tissues. B. The skin and its accessory organs make up the integumentary system. Types of
Anatomy Ch 6: Integumentary System Introduction: A. Organs are body structures composed of two or more different tissues. B. The skin and its accessory organs make up the integumentary system. Types of
THE sebaceous glands of the rabbit consist of clusters of about ten cells
 79 On the Relationship between Mammary, Sweat, and Sebaceous Glands By D. B. CARLISLE (From the Department of Zoology and Comparative Anatomy, Oxford, and the Plymouth Laboratory of the Marine Biological
79 On the Relationship between Mammary, Sweat, and Sebaceous Glands By D. B. CARLISLE (From the Department of Zoology and Comparative Anatomy, Oxford, and the Plymouth Laboratory of the Marine Biological
Structural and Functional Consequences of Loricrin Mutations in Human Loricrin Keratoderma (Vohwinkel Syndrome with Ichthyosis)
 Structural and Functional Consequences of Loricrin Mutations in Human Loricrin Keratoderma (Vohwinkel Syndrome with Ichthyosis) Matthias Schmuth, 1 Joachim W. Fluhr, 2 Debra C. Crumrine, Yoshikazu Uchida,
Structural and Functional Consequences of Loricrin Mutations in Human Loricrin Keratoderma (Vohwinkel Syndrome with Ichthyosis) Matthias Schmuth, 1 Joachim W. Fluhr, 2 Debra C. Crumrine, Yoshikazu Uchida,
LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:!
 LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:! Spinal cord and peripheral nerves! Eyes, Inner ear, nasal
LIST OF ORGANS FOR HISTOPATHOLOGICAL ANALYSIS:!! Neural!!!!!!Respiratory:! Brain : Cerebrum,!!! Lungs and trachea! Olfactory, Cerebellum!!!!Other:! Spinal cord and peripheral nerves! Eyes, Inner ear, nasal
8 Influence of permeation modulators on the behaviour of a SC lipid model mixture
 8 Influence of permeation modulators on the behaviour of a SC lipid model mixture 8.1 Introduction In the foregoing parts of this thesis, a model membrane system of SC lipids has been developed and characterized.
8 Influence of permeation modulators on the behaviour of a SC lipid model mixture 8.1 Introduction In the foregoing parts of this thesis, a model membrane system of SC lipids has been developed and characterized.
A ph-dependent Charge Reversal Peptide for Cancer Targeting
 Supporting Information A ph-dependent Charge Reversal Peptide for Cancer Targeting Naoko Wakabayashi 1, Yoshiaki Yano 1, Kenichi Kawano 1, and Katsumi Matsuzaki 1 1 Graduate School of Pharmaceutical Sciences,
Supporting Information A ph-dependent Charge Reversal Peptide for Cancer Targeting Naoko Wakabayashi 1, Yoshiaki Yano 1, Kenichi Kawano 1, and Katsumi Matsuzaki 1 1 Graduate School of Pharmaceutical Sciences,
Supplementary table and figures
 3D single molecule tracking with multifocal plane microscopy reveals rapid intercellular transferrin transport at epithelial cell barriers Sripad Ram, Dongyoung Kim, Raimund J. Ober and E. Sally Ward Supplementary
3D single molecule tracking with multifocal plane microscopy reveals rapid intercellular transferrin transport at epithelial cell barriers Sripad Ram, Dongyoung Kim, Raimund J. Ober and E. Sally Ward Supplementary
Supplementary Figure 1.
 Supplementary Figure 1. Visualization of endoplasmic reticulum-mitochondria interaction by in situ proximity ligation assay. A) Illustration of targeted proteins in mitochondria (M), endoplasmic reticulum
Supplementary Figure 1. Visualization of endoplasmic reticulum-mitochondria interaction by in situ proximity ligation assay. A) Illustration of targeted proteins in mitochondria (M), endoplasmic reticulum
The Beauty of the Skin
 The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
Skin barrier function and stratum corneum hydration
 LONG-TERM PARTNERS PRE-CONGRESS SYMPOSIUM / ESVD - ECVD 2011 ICF session Skin barrier function and stratum corneum hydration A major function of the skin is to provide a protective barrier at the interface
LONG-TERM PARTNERS PRE-CONGRESS SYMPOSIUM / ESVD - ECVD 2011 ICF session Skin barrier function and stratum corneum hydration A major function of the skin is to provide a protective barrier at the interface
The Cell Membrane (Ch. 7)
 The Cell Membrane (Ch. 7) Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those structure
The Cell Membrane (Ch. 7) Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those structure
Lecture Series 4 Cellular Membranes. Reading Assignments. Selective and Semi-permeable Barriers
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Skin. Lecture #14. Ref:
 Skin Lecture #14 Ref: http://www.ccunix.ccu.edu.tw/~chenmsl/tea/skin_910721.htm Structure of Skin 1. Epidermis 2. Dermis 3. Subcutis 4. Hair follicle 5. Sebaceous gland 6. Sweat gland Skin Largest human
Skin Lecture #14 Ref: http://www.ccunix.ccu.edu.tw/~chenmsl/tea/skin_910721.htm Structure of Skin 1. Epidermis 2. Dermis 3. Subcutis 4. Hair follicle 5. Sebaceous gland 6. Sweat gland Skin Largest human
An Aquaporin-inspired Lipid Concentrate for Mature Skin
 An Aquaporin-inspired Lipid Concentrate for Mature Skin Mike Farwick, Betty Santonnat and Peter Lersch EVONIK Goldschmidt GmbH, Essen, Germany Kees Korevaar EVONIK Cosmoferm B.V., Delft, Netherlands Anthony
An Aquaporin-inspired Lipid Concentrate for Mature Skin Mike Farwick, Betty Santonnat and Peter Lersch EVONIK Goldschmidt GmbH, Essen, Germany Kees Korevaar EVONIK Cosmoferm B.V., Delft, Netherlands Anthony
Integumentary System
 Integumentary System Overview Functions 1. Protection 2. Excretion of wastes 3. Maintenance of T b 4. Synthesis of vitamin D 3 5. Storage of lipids 6. Detection of sensory stimuli Epidermis Tissue types
Integumentary System Overview Functions 1. Protection 2. Excretion of wastes 3. Maintenance of T b 4. Synthesis of vitamin D 3 5. Storage of lipids 6. Detection of sensory stimuli Epidermis Tissue types
HHS Public Access Author manuscript J Invest Dermatol. Author manuscript; available in PMC 2015 January 01.
 RNA-seq Permits a Closer Look at Normal Skin and Psoriasis Gene Networks David Quigley 1,2,3 1 Helen Diller Family Comprehensive Cancer Center, University of California at San Francisco, San Francisco,
RNA-seq Permits a Closer Look at Normal Skin and Psoriasis Gene Networks David Quigley 1,2,3 1 Helen Diller Family Comprehensive Cancer Center, University of California at San Francisco, San Francisco,
Unit 4 The Integumentary System
 Unit 4 The Integumentary System I. Classification of Body Membranes A. Epithelial Membranes (3) 1. Cutaneous Membrane > Stratified Squamous > Sits on Dense Connective Tissue > Skin: Epidermis & Dermis
Unit 4 The Integumentary System I. Classification of Body Membranes A. Epithelial Membranes (3) 1. Cutaneous Membrane > Stratified Squamous > Sits on Dense Connective Tissue > Skin: Epidermis & Dermis
Histology: Epithelial tissue
 Histology: Epithelial tissue Epithelial Tissue is presented in two forms: 1. Covering Epithelia: 2. Glandular Epithelia: 1. Simple Epithelium: contain only one layer of cells. 2. Stratified Epithelium:
Histology: Epithelial tissue Epithelial Tissue is presented in two forms: 1. Covering Epithelia: 2. Glandular Epithelia: 1. Simple Epithelium: contain only one layer of cells. 2. Stratified Epithelium:
2/5/2019. Organ System: Skin or Integumentary System. Hypodermis (or superficial fascia) Integumentary System - Learn and Understand
 Integumentary System - Learn and Understand Skin is an organ comprised of all four tissues Each layer of the skin contributes to one or more of its numerous functions Skin is both strong and flexible Keratinization
Integumentary System - Learn and Understand Skin is an organ comprised of all four tissues Each layer of the skin contributes to one or more of its numerous functions Skin is both strong and flexible Keratinization
BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice
 SUPPLEMENTARY MATERIALS BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice Elena Corradini, Paul J. Schmidt, Delphine Meynard, Cinzia Garuti, Giuliana
SUPPLEMENTARY MATERIALS BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice Elena Corradini, Paul J. Schmidt, Delphine Meynard, Cinzia Garuti, Giuliana
Skin and Body Membranes
 4 Skin and Body Membranes PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Skin and Body Membranes
4 Skin and Body Membranes PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Skin and Body Membranes
Lecture Series 4 Cellular Membranes
 Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Lecture Series 4 Cellular Membranes Reading Assignments Read Chapter 11 Membrane Structure Review Chapter 12 Membrane Transport Review Chapter 15 regarding Endocytosis and Exocytosis Read Chapter 20 (Cell
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 5 The Integumentary System Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails,
Principles of Anatomy and Physiology 14 th Edition CHAPTER 5 The Integumentary System Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails,
Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs
 Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs Classification of Body Membranes Epithelial membranes Cutaneous
Skin and Body Membranes Body Membranes Function of body membranes Cover body surfaces Line body cavities Form protective sheets around organs Classification of Body Membranes Epithelial membranes Cutaneous
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Unit 4 - The Skin and Body Membranes 1
 Unit 4 - The Skin and Body Membranes 1 I. Unit 4: Skin and Body Membranes A. Body Membranes 1. Function of body membranes a) Cover body surfaces b) Line body cavities c) Form protective sheets around organs
Unit 4 - The Skin and Body Membranes 1 I. Unit 4: Skin and Body Membranes A. Body Membranes 1. Function of body membranes a) Cover body surfaces b) Line body cavities c) Form protective sheets around organs
Lecture Series 5 Cellular Membranes
 Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
Lecture Series 5 Cellular Membranes Cellular Membranes A. Membrane Composition and Structure B. Animal Cell Adhesion C. Passive Processes of Membrane Transport D. Active Transport E. Endocytosis and Exocytosis
A. Membrane Composition and Structure. B. Animal Cell Adhesion. C. Passive Processes of Membrane Transport. D. Active Transport
 Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Cellular Membranes A. Membrane Composition and Structure Lecture Series 5 Cellular Membranes B. Animal Cell Adhesion E. Endocytosis and Exocytosis A. Membrane Composition and Structure The Fluid Mosaic
Chapter 5: The Integumentary System - Introduction and Epidermis
 Chapter 5: The Integumentary System - Introduction and Epidermis The Integument Means Covering Composed: Skin Hair Nails Sweat glands Oil glands The Integument Thickness 1.5 4 mm (or more) Weight 9 11
Chapter 5: The Integumentary System - Introduction and Epidermis The Integument Means Covering Composed: Skin Hair Nails Sweat glands Oil glands The Integument Thickness 1.5 4 mm (or more) Weight 9 11
Integumentary System. Integumentary System
 1. General aspects a. The integumentary system consists of several organs major organ of the system is the skin other organs are relatively small and they can be considered as specialized structures of
1. General aspects a. The integumentary system consists of several organs major organ of the system is the skin other organs are relatively small and they can be considered as specialized structures of
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: van Seters M, van Beurden M, ten Kate FJW, et al. Treatment
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: van Seters M, van Beurden M, ten Kate FJW, et al. Treatment
Comparative Anatomical Factors Affecting Topical Delivery
 Comparative Anatomical Factors Affecting Topical Delivery Nancy A. Monteiro-Riviere, Ph.D., Fellow ATS Professor of Investigative Dermatology and Toxicology Center for Chemical Toxicology Research and
Comparative Anatomical Factors Affecting Topical Delivery Nancy A. Monteiro-Riviere, Ph.D., Fellow ATS Professor of Investigative Dermatology and Toxicology Center for Chemical Toxicology Research and
Ch 4. Skin and Body Membranes
 Ch 4 Skin and Body Membranes TITLE HISTOLOGY SLIDES & NOTES ESSENTIAL QUESTION What tissues compose the integumentary system? Stratified Squamous Epithelium Stratified = several layers; Squamous = shape
Ch 4 Skin and Body Membranes TITLE HISTOLOGY SLIDES & NOTES ESSENTIAL QUESTION What tissues compose the integumentary system? Stratified Squamous Epithelium Stratified = several layers; Squamous = shape
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
INTEGUMENTARY 1-Epidermis, 2-Dermis, Structure of thick and thin skin I- Epidermis . Stratum basale
 INTEGUMENTARY The skin (integument, cutis ) and its derivatives constitute the integumentary system. It form the external covering of the body and is the largest organ of the body. The skin consists of
INTEGUMENTARY The skin (integument, cutis ) and its derivatives constitute the integumentary system. It form the external covering of the body and is the largest organ of the body. The skin consists of
Tissues. Tissues - Overview. Bio 101 Laboratory 3. Epithelial Tissues and Integument
 Bio 101 Laboratory 3 Epithelial Tissues and Integument 1 Tissues Tissues to be examined under the microscope Epithelial Tissue Integument Connective Tissue **We will be doing muscle and nervous tissues
Bio 101 Laboratory 3 Epithelial Tissues and Integument 1 Tissues Tissues to be examined under the microscope Epithelial Tissue Integument Connective Tissue **We will be doing muscle and nervous tissues
Figure S1. PMVs from THP-1 cells expose phosphatidylserine and carry actin. A) Flow
 SUPPLEMENTARY DATA Supplementary Figure Legends Figure S1. PMVs from THP-1 cells expose phosphatidylserine and carry actin. A) Flow cytometry analysis of PMVs labelled with annexin-v-pe (Guava technologies)
SUPPLEMENTARY DATA Supplementary Figure Legends Figure S1. PMVs from THP-1 cells expose phosphatidylserine and carry actin. A) Flow cytometry analysis of PMVs labelled with annexin-v-pe (Guava technologies)
Biophysical profile of a case of Peeling Skin Syndrome A model for study of Stratum Corneum function
 Biophysical profile of a case of Peeling Skin Syndrome A model for study of Stratum Corneum function Dr. Siddhi Tiwari 1, Dr. Ridhima Lakhani 2, Dr. Mahesh Prajapat 3, Dr. Puneet Bhargava 4$ 1,2,3 Resident,
Biophysical profile of a case of Peeling Skin Syndrome A model for study of Stratum Corneum function Dr. Siddhi Tiwari 1, Dr. Ridhima Lakhani 2, Dr. Mahesh Prajapat 3, Dr. Puneet Bhargava 4$ 1,2,3 Resident,
BIOL 158: BIOLOGICAL CHEMISTRY II
 BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 1: Membranes Lecturer: Christopher Larbie, PhD Introduction Introduction Cells and Organelles have membranes Membranes contain lipids, proteins and polysaccharides
BIOL 158: BIOLOGICAL CHEMISTRY II Lecture 1: Membranes Lecturer: Christopher Larbie, PhD Introduction Introduction Cells and Organelles have membranes Membranes contain lipids, proteins and polysaccharides
Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or
 Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or control nontargeting sirnas. At 90 hr after transfection,
Figure S1. Western blot analysis of clathrin RNA interference in human DCs Human immature DCs were transfected with 100 nm Clathrin SMARTpool or control nontargeting sirnas. At 90 hr after transfection,
Chapter 4 Opener Pearson Education, Inc.
 Chapter 4 Opener Introduction The integumentary system is composed of: Skin Hair Nails Sweat glands Oil glands Mammary glands The skin is the most visible organ of the body Clinicians can tell a lot about
Chapter 4 Opener Introduction The integumentary system is composed of: Skin Hair Nails Sweat glands Oil glands Mammary glands The skin is the most visible organ of the body Clinicians can tell a lot about
Through this induced natural adaptive process, also known as elicitation, Exsymol has foreseen an innovative way to produce natural peptides.
 Cold induces a general metabolism slowdown in every living organism. As a result, in arctic regions, various defensive strategies have been developed in order to resist these extreme conditions. Exsymol
Cold induces a general metabolism slowdown in every living organism. As a result, in arctic regions, various defensive strategies have been developed in order to resist these extreme conditions. Exsymol
(A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and a
 Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Experiment Note the locations of the epidermis, dermis, dermal papillae, and the sweat glands. Note that fat cells that comprise the
 Experiment 1 Examining Skin, Bones and Muscle Histology Experiment Inventory Skin Digital Slide Images Cortical (Compact) Bone Digital Slide Image Trabecular (Spongy) Bone Digital Slide Image Cardiac Muscle
Experiment 1 Examining Skin, Bones and Muscle Histology Experiment Inventory Skin Digital Slide Images Cortical (Compact) Bone Digital Slide Image Trabecular (Spongy) Bone Digital Slide Image Cardiac Muscle
The amiloride-sensitive epithelial sodium channel
 Lessons from Mouse Mutants of Epithelial Sodium Channel and Its Regulatory Proteins Edith Hummler* and Volker Vallon *Département de Pharmacologie et de Toxicologie, Université de Lausanne, Lausanne, Switzerland;
Lessons from Mouse Mutants of Epithelial Sodium Channel and Its Regulatory Proteins Edith Hummler* and Volker Vallon *Département de Pharmacologie et de Toxicologie, Université de Lausanne, Lausanne, Switzerland;
Overview on the identification of different classes of. lipids by HPTLC (High Performance Thin Layer. Chromatography) and ITLC (Immuno Thin Layer
 Overview on the identification of different classes of lipids by HPTLC (High Performance Thin Layer Chromatography) and ITLC (Immuno Thin Layer Chromatography) Iuliana Popa 1, Marie-Jeanne David 2, Daniel
Overview on the identification of different classes of lipids by HPTLC (High Performance Thin Layer Chromatography) and ITLC (Immuno Thin Layer Chromatography) Iuliana Popa 1, Marie-Jeanne David 2, Daniel
Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells.
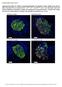 Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells. Pancreata from 16-weeks-old 6J +/+, 6J db/db, KS +/+ and KS db/db mice were harvested, fixed with
Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells. Pancreata from 16-weeks-old 6J +/+, 6J db/db, KS +/+ and KS db/db mice were harvested, fixed with
Epithelial cell death is an important contributor to oxidant-mediated acute lung injury SUPPORTING INFORMATION 60611, USA
 Epithelial cell death is an important contributor to oxidant-mediated acute lung injury SUPPORTING INFORMATION G.R. Scott Budinger 1,2 *, Gökhan M. Mutlu 1 *, Daniela Urich 2, Saul Soberanes 1, Leonard
Epithelial cell death is an important contributor to oxidant-mediated acute lung injury SUPPORTING INFORMATION G.R. Scott Budinger 1,2 *, Gökhan M. Mutlu 1 *, Daniela Urich 2, Saul Soberanes 1, Leonard
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
mm Distance (mm)
 b a Magnet Illumination Coverslips MPs Objective 2575 µm 1875 µm 1575 µm 1075 µm 875 µm 545 µm 20µm 2 3 0.5 0.3mm 1 1000 100 10 1 0.1 1000 100 10 1 0.1 Field Induction (Gauss) 1.5 0 5 10 15 20 Distance
b a Magnet Illumination Coverslips MPs Objective 2575 µm 1875 µm 1575 µm 1075 µm 875 µm 545 µm 20µm 2 3 0.5 0.3mm 1 1000 100 10 1 0.1 1000 100 10 1 0.1 Field Induction (Gauss) 1.5 0 5 10 15 20 Distance
Chapter 5 The Integumentary System. Copyright 2009, John Wiley & Sons, Inc. 1
 Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Chapter 5 The Integumentary System Copyright 2009, John Wiley & Sons, Inc. 1 Introduction The organs of the integumentary system include the skin and its accessory structures including hair, nails, and
Integumentary System (Script) Slide 1: Integumentary System. Slide 2: An overview of the integumentary system
 Integumentary System (Script) Slide 1: Integumentary System Slide 2: An overview of the integumentary system Skin is the body s largest and heaviest organ making up 15% of body weight. Most skin is 1 to
Integumentary System (Script) Slide 1: Integumentary System Slide 2: An overview of the integumentary system Skin is the body s largest and heaviest organ making up 15% of body weight. Most skin is 1 to
STUDIES ON MUSTARD-STIMULATED PROTEASES AND INHIBITORS IN HUMAN EPIDERMAL KERATINOCYTES (HEK): DEVELOPMENT OF ANTIVESICANT DRUGS
 STUDIES ON MUSTARD-STIMULATED PROTEASES AND INHIBITORS IN HUMAN EPIDERMAL KERATINOCYTES (HEK): DEVELOPMENT OF ANTIVESICANT DRUGS Xiannu Jin 1, Radharaman Ray 2, Guang Xu 1 and Prabhati Ray 1 1 Department
STUDIES ON MUSTARD-STIMULATED PROTEASES AND INHIBITORS IN HUMAN EPIDERMAL KERATINOCYTES (HEK): DEVELOPMENT OF ANTIVESICANT DRUGS Xiannu Jin 1, Radharaman Ray 2, Guang Xu 1 and Prabhati Ray 1 1 Department
Evaluation of the wound healing response post deep dermal heating by fractional RF: INTRAcel
 12th symposium of the Association of Korean Dermatologists (2009) 1 Evaluation of the wound healing response post deep dermal heating by fractional RF: INTRAcel Un-Cheol.Yeo, M.D. S&U Dermatologic Clinic,
12th symposium of the Association of Korean Dermatologists (2009) 1 Evaluation of the wound healing response post deep dermal heating by fractional RF: INTRAcel Un-Cheol.Yeo, M.D. S&U Dermatologic Clinic,
