Introduction ORIGINAL PAPER. Christian Zuber James C. Paulson Valeriu Toma Harry C. Winter Irwin J. Goldstein Jürgen Roth
|
|
|
- Andrea Dean
- 6 years ago
- Views:
Transcription
1 Histochem Cell Biol (2003) 120: DOI /s ORIGINAL PAPER Christian Zuber James C. Paulson Valeriu Toma Harry C. Winter Irwin J. Goldstein Jürgen Roth Spatiotemporal expression patterns of sialoglycoconjugates during nephron morphogenesis and their regional and cell type-specific distribution in adult rat kidney Accepted: 27 June 2003 / Published online: 26 July 2003 Springer-Verlag 2003 Abstract The expression of a2,6- and a2,3-linked sialic acids on N-glycans was studied in embryonic, postnatal, and adult rat kidney. Histochemistry and blotting using Polyporus squamosus and Sambucus nigra lectins for a2,6-linked sialic acids and the Maackia amurensis lectin for a2,3-linked sialic acids were performed and sialyltransferase activity was assayed. N-glycans with a2,6- and a2,3-linked sialic acid were differently expressed in the two embryonic anlagen and early stages of nephron. Metanephrogenic mesenchyme was positive for a2,3- linked sialic acid but not for the a2,6-linked one, which became detectable initially in the proximal part of S- shaped bodies. Collecting ducts were positive for a2,6- linked sialic acid, whereas a2,3-linked sialic acid was restricted to their ampullae. Although positive in embryonic kidney, S1 and S2 of proximal tubules became unreactive for a2,3-linked sialic acid in postnatal and adult kidneys. In adult kidney, intercalated but not principal cells of collecting ducts were reactive for a2,3-linked sialic acid. In contrast, a2,6-linked sialic acids were detected in all cells of adult kidney nephron. Blot analysis revealed a different but steady pattern of bands reactive for a2,6- and a2,3-linked sialic acid in embryonic, postnatal, and adult kidney. Activity of a2,6 C. Zuber V. Toma J. Roth () ) Division of Cell and Molecular Pathology, Department of Pathology, University of Zürich, Schmelzbergstrasse 12, 8091 Zürich, Switzerland juergen.roth@usz.ch Tel.: Fax: J. C. Paulson The Scripps Research Institute, Department of Molecular Biology, La Jolla, CA 92037, USA H. C. Winter I. J. Goldstein Department of Biological Chemistry, School of Medicine, University of Michigan, Ann Arbor, Michigan, , USA and a2,3 sialyltransferases was highest in embryonic kidney and decreased over postnatal to adult kidney with the activity of a2,6 sialyltransferase always being three to fourfold that of a2,3 sialyltransferase. Thus, a2,6- and a2,3-linked sialic acids are differently expressed in embryonic anlagen and mesenchyme-derived early stages of nephron and show regional and cell type-specific differences in adult kidney. Keywords Kidney Kidney development Sialic acid Sialyltransferase Histochemistry Polyporus squamosus lectin Maackia amurensis lectin Introduction The kidney belongs to the organs with an exceptional degree of complexity in terms of both morphological organization and function. With regard to the former, three different regions, the cortex and the outer and the inner medulla, can be distinguished because of the presence of different parts of the nephron (Kaissling and Dorup 1995; Kriz and Kaissling 2000). Along the nephron, various cell types can be distinguished fulfilling specific functions in filtering, reabsorption and secretion (Al-Awqati 1996, 2003; Kriz and Kaissling 2000; Pavenstädt et al. 2003). Thus, adult kidney has been subjected to studies on cell type-specific and plasma membrane domain-related expression of N- and O- glycans present in glycoproteins. For instance, specific expression patterns of sialic acid residues as detected with the Limax flavus lectin and of N-acetylgalactosamineterminated oligosaccharides reactive with the Helix pomatia lectin were found in the podocyte plasma membrane (Charest and Roth 1985; Roth et al. 1983). Sialoglycoproteins such as podocalyxin and podoplanin have been shown to be involved in establishing and maintaining the peculiar shape of podocytes (Kerjaschki et al. 1984; Matsui et al. 1999; Pavenstädt et al. 2003). Histochemical lectin-binding studies have also revealed a
2 144 remarkable degree of variability in glycocalyx composition of the various epithelia present in the distal and collecting tubules (Brown et al. 1985; Kaneko et al. 1995; LeHir and Dubach 1982; LeHir et al. 1982; Roth and Taatjes 1985; Taatjes et al. 1988; Toma et al. 1999), although the functional importance of these differences remains unknown. The permanent kidney, in contrast to other organs, develops from two different embryonic anlagen, the metanephrogenic mesenchyme and the epithelial ureteric bud, by interactive processes inducing morphogenesis and differentiation (Horster et al. 1999; SaxØn 1987). The blind ends of the sprouting and dichotomously branching ureteric bud induce clustering and condensation of the mesenchymal cells. Through growth and differentiation, they initially form the renal vesicle, which is the first epithelial structure and which further differentiates through the S-shaped body and capillary loop stage into maturing glomeruli. This is accompanied by segmental morphogenesis resulting in the formation of proximal and distal tubules with the loop of HenlØ in between, and connecting tubules. Thus, the entire epithelium of the nephron is derived from mesenchyme which undergoes a transition into epithelium, whereas the collecting duct system is primarily epithelia-derived. A variety of studies related to the inductive and morphogenetic events during nephrogenesis revealed spatiotemporal expression patterns of cell adhesion molecules, extracellular matrix components, growth factors, transcription factors, and proto-oncogene encoded receptor tyrosine kinases (Bard et al. 1994; Birchmeier and Birchmeier 1993; Hammerman et al. 1992; Horster et al. 1999; Kumar et al. 1997; Parr and McMahon 1994; Rauscher 1993). Spatiotemporal patterns of lectin-binding sites and of polysialic acids have been observed during the formation and differentiation of the nephron (Holthöfer and Virtanen 1987; Kunz et al. 1984; Lackie et al. 1990; Laitinen et al. 1987; Roth et al. 1987; Toma et al. 2000; Wagner and Roth 1988; Ziak and Roth 1999). The studies on polysialic acids disclosed remarkable correlations between their expression and particular stages of nephron development (Lackie et al. 1990; Roth et al. 1987; Ziak and Roth 1999), which were not as obvious for sialoglycoconjugates terminated by a single sialic acid residue as detected with the Limax flavus lectin (Wagner and Roth 1988), Sambucus nigra lectin, or Maackia amurensis lectin (Sata et al. 1989) and Amaranthin (Toma et al. 2000). The present study was conducted to analyze the presence and distribution of N-glycans terminated in sialic acid either in a2,6- or in a2,3-linkage to galactose b1,4 N-acetylglucosamine during embryonic development and postnatal differentiation of rat kidney and in the adult organ. For this purpose, the Polyporus squamosus lectin, highly reactive with the NeuAca2,6Galb1,4Glc- NAc trisaccharide sequence (Mo et al. 2000; Zhang et al. 2001), the Sambucus nigra lectin reactive with the NeuAca2,6Gal/GalNAc disaccharide sequence (Shibuya et al. 1987), and the Maackia amurensis lectin reactive with the NeuAca2,3Galb1,4GlcNAc trisaccharide sequence (Knibbs et al. 1991; Wang and Cummings 1988) were used for histochemistry and blot analysis. Furthermore, the specific activity of the respective sialyltransferases, b-galactoside a2,6 sialyltransferase (ST6Gal-I) and b-galactoside a2,3 sialyltransferase (ST3Gal-III), was determined in tissue extracts. Although sialic acid linked a2,6 or a2,3 to penultimate galactose of oligosaccharides is commonly found in various organs including the kidney (Kelm and Schauer 1997; Paulson and Colley 1989; Rosenberg 1995), our results show hitherto unrecognized differences in their cellular and regional expression during embryonic development and postnatal kidney differentiation and in the adult rat kidney. Materials and methods Reagents Polyporus squamosus lectin (PSL) was affinity purified as described (Mo et al. 2000). Lectin digoxigenin conjugates were prepared in the presence of 0.2 M galactose and 0.1% Triton X-100 using a digoxigenin conjugation kit from Roche Molecular Biochemicals (Mannheim, Germany) according to the manufacturer s instructions. Digoxigenin conjugates of Sambucus nigra lectin (SNL) and Maackia amurensis lectin (MAL) were available from previous studies. Horseradish peroxidase or alkaline phosphataseconjugated sheep anti-digoxigenin antibodies (Fab fragments), neuraminidase from Vibrio cholerae, and N-glycanase F were from Roche Molecular Biochemicals, and a 4 nm gold-labeled rabbit anti-horseradish peroxidase antibody was from Immunoresearch Laboratories (West Grove, PA). All other reagents were of analytical grade. Tissue preparation Sprague-Dawley rats and pregnant rats were obtained from the Central Animal Facility of the University of Zurich. Kidneys from adult rats were fixed by perfusion from the left cardiac ventricle with freshly prepared 3% formaldehyde 0.1% glutaraldehyde or 4% formaldehyde in Hank s balanced salt solution (ph 7.4, 37C) for 15 min, followed by immersion fixation at ambient temperature for 1 h 45 min. Kidneys from embryonic day 16, 18, and 20 as well as kidney slices from postnatal days 2, 5, 7, 14, and 21 were fixed by immersion in the above fixatives. Free aldehyde groups were blocked by immersing the tissues in 50 mm NH 4 Cl in PBS (10 mm phosphate buffer, 0.15 M NaCl, ph 7.4) for 1 h. Formaldehyde-fixed tissues were embedded in paraffin according to standard protocol and sections of approximately 5 mm thickness were prepared. Large pieces from formaldehyde/glutaraldehyde-fixed adult kidney were embedded at progressively lowered temperature (Ÿ42C finally) in Lowicryl K4 M and used to prepare large mm semithin sections containing cortex and adjacent outer medulla or inner medulla with adjacent outer medulla. Small pieces from kidney cortex and inner medulla were embedded in Lowicryl K4 M for the preparation of ultrathin sections (Roth 1989). Lectin histochemistry For light microscopy, a three-step labeling protocol followed by photochemical silver amplification was performed as described in detail previously (Roth et al. 1998; Toma et al. 2001). In brief, deparaffinized and rehydrated paraffin sections or semithin sections of Lowicryl K4 M-embedded tissue were incubated with digoxigenin-conjugated PSL (1.5 mg/ml), SNL (1.25 mg/ml), or MAL
3 145 (2.5 mg/ml) in PBS (ph 7.4) containing 1% BSA, 0.05% Tween 20, and 0.005% Triton X-100 for 2 h at ambient temperature, rinsed in buffer, and incubated with horseradish peroxidase-conjugated sheep anti-digoxigenin Fab (0.3 U/ml) for 1 h. After rinses with buffer, sections were incubated with 4 nm gold-labeled antihorseradish peroxidase antibody (Roth et al. 1992) adjusted to an absorbance of 0.05 at 525 nm. Finally, silver intensification using silver acetate (Roth et al. 1998) was performed followed by counterstaining with nuclear fast red (paraffin sections). Both paraffin and Lowicryl K4 M semithin sections were also incubated with directly gold-labeled SNL and MAL followed by silver intensification of the lectin gold labeling (Sata et al. 1989). For electron microscopy, ultrathin sections were incubated with directly gold-labeled MAL (Sata et al. 1989) or with digoxigeninconjugated MAL followed by 8 nm gold-labeled anti-digoxigenin antibody (Sata et al. 1990). The specificity of staining was established by incubating the lectins with varying amounts (0.1 mm to 0.2 M) of either 6 0 or 3 0 sialyllactose for 30 min prior to use to stain paraffin sections from kidney. Furthermore, specificity of lectin staining was corroborated by enzymatic removal of oligosaccharide side chains at the asparagine- N-acetylglucosamine core linkage. For this, paraffin sections were treated with 0.1% sodium dodecyl sulfate in TRIS-buffered saline at 70C for 30 min and washed in NP40 in TRIS-buffered saline. This was followed by N-glycanase F (80 U/ml in 0.1 M phosphate buffer, ph 7.4) in a moist chamber for 18 h at 37C. Afterwards, tissue sections were conditioned with PBS containing 1% BSA, 0.05% Triton X-100, and 0.05% Tween 20 for 20 min at ambient temperature and incubated with the lectins as described above. To enzymatically remove sialic acids, paraffin sections were treated with Vibrio cholerae neuraminidase (0.02 U/ml in 50 mm acetate buffer, ph 5.5) for 18 h at 37C in a moist chamber, washed, conditioned, and incubated with the lectins as described above. To assess the effect of the incubation buffers on the lectin staining, sections were incubated in the respective enzyme buffers only for 18 h at 37C before lectin labeling. Mild base treatment to de-o-acetylate sialic acids was performed by treating paraffin sections either with 0.5% KOH in 70% ethanol or with 0.1 N NaOH for 20 min at ambient temperature (Reid et al. 1978). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and lectin blotting Kidneys from embryonic day 20, postnatal days 2, 5, 7, 14, and 21 as well as adult animals were homogenized in PBS (ph 7.4) containing protease inhibitor tablets, and proteins were extracted with 1% Triton X-100 for 1 h at 4C. Extracts were also prepared from microscopically dissected kidney cortex and inner medulla. The homogenates were centrifuged at 14,000 g for 10 min at 4C and aliquots of the supernatants were used for SDS-PAGE. Lectin blotting was performed as described in detail previously (Zuber et al. 1998). In brief, proteins were denatured in Laemmli buffer and then separated by 3 15% gradient SDS-PAGE (100 mg/lane) and transferred to nitrocellulose membranes. Nitrocellulose membranes were incubated with digoxigenin-conjugated PSL (2.5 mg/ml PBS containing 0.05% Tween 20 and 1% BSA) or MAL (5 mg/ml 0.1 M TRIS-HCl, ph 7.5, 0.15 M NaCl containing 0.05% Tween 20 and 1% BSA) for 2 h, washed, and incubated with alkaline phosphataseconjugated anti-digoxigenin antibody (0.25 U/ml TRIS-buffered saline containing Tween 20) for 45 min followed by color reaction. Sialyltransferase assays Homogenates from whole kidneys from embryonic day 20, postnatal days 2, 5, 7, 14, and 21 as well as adult whole kidneys were assayed. Furthermore, homogenates from kidney cortex, outer and inner medulla obtained by microscopic dissection were assayed. The activity of the b-galactoside a2,6 sialyltransferase (ST6Gal-I) and b- galactoside a2,3 sialyltransferase (ST3Gal-III) was assessed in identical reaction mixtures with the exception of their specific acceptor substrates which were asialo-a 1 -acid glycoprotein and lacto-n-tetraose as described in detail previously (Weinstein et al. 1982). Results Distribution pattern of a2,6- and a2,3-linked sialic acid in embryonic kidneys Staining for both PSL and MAL indicative of the presence of oligosaccharides terminated in a2,6- and a2,3-linked sialic acid, respectively, was detectable in kidneys from embryonic days 16, 18, and 20. In general, the intensity of staining obtained with PSL was lower than that observed with MAL (Figs. 1, 2, 3). Staining patterns for SNL and PSL were identical irrespective of the used techniques. Although PSL staining was observed in all parts of the ureteric bud-derived collecting ducts (arrows in Figs. 1B, 2B, 3B), the staining with MAL was restricted to the terminal ampullae of the collecting ducts (arrows in Figs. 1D, 2E, 3D). This differential staining pattern was consistently observed in kidneys from embryonic days 16, 18, and 20. The condensed metanephrogenic mesenchyme and the loose mesenchymal stroma were largely unreactive with PSL (Figs. 1B asterisk, 2B asterisk, 3B). This was in striking contrast to the intense staining observed with MAL in the metanephrogenic mesenchyme of kidneys from embryonic days 16, 18, and 20 (Figs. 1C, D asterisk, 2D, E asterisk, 3C, D). Additional differences became obvious when the different developmental stages of the glomerulus were considered. Renal vesicles were unreactive with PSL in all embryonic stage kidneys examined (arrowhead in Fig. 1B), whereas strong reactivity with MAL was evident in the luminal cell surface and to a lesser degree in the basolateral cell surface of the vesicle epithelia (arrowhead in Fig. 1D). The S-shaped bodies showed some PSL reactivity which, however, was restricted to the cells of its proximal part facing the presumptive Bowman s space (double arrowheads in Figs. 1B, 2B). In contrast, the entire S-shaped body was stained with MAL (double arrowheads in Figs. 1D, 2E, 3D) and the weak staining observed at embryonic day 16 became strong in kidneys from embryonic days 18 and 20. Immature glomeruli which were observed in kidneys from embryonic days 18 and 20 were reactive with both PSL and MAL, in particular the presumptive podocytes (Gl in Figs. 2, 3). Likewise, immature proximal tubules observed in kidneys of these embryonic stages were positive for both lectins (Figs. 2C, F arrows, 3D open arrows). Endothelia of capillaries in the interstitium were faintly positive with PSL (En in Fig. 1B) and strongly stained with MAL (En in Fig. 1D). This difference in staining intensity was also observed in kidneys from embryonic days 18 and 20 (Figs. 2, 3).
4 146 Fig. 1A D Adjacent sagittal sections of paraffin-embedded rat kidney, embryonic day 16. Silver-intensified lectin gold staining in this and all other light microscopic illustrations appears in black. A, B Staining with Polyporus squamosus lectin (PSL) to detect a2,6- linked sialic acid is observed in ureteric bud-derived collecting ducts and their terminal ampulla (arrows). The condensed metanephrogenic mesenchyme (asterisk), stromal mesenchyme, and renal vesicle (arrowhead) appear unreactive, whereas the crevice of the proximal part of an S-shaped body, which is the first morphological sign of glomerulus formation, is stained (double arrowheads). C, D Staining with Maackia amurensis lectin (MAL) to detect a2,3-linked sialic acid. Of the ureteric bud-derived collecting ducts, only the terminal ampulla is stained (arrow). The condensed metanephrogenic mesenchyme (asterisk), stromal mesenchyme, renal vesicle (arrowhead), and entire S-shaped body (double arrowhead) are labeled. Both PSL and MAL stain endothelia (En in B and D). Bars 90 mm ina, B; 60mm inc, D In order to exclude that the differences in staining intensity and in distribution pattern were due to O- acetylation of sialic acids, tissue sections were subjected to saponification prior to lectin staining. However, no change in intensity and pattern of lectin staining could be observed indicating that the observed differences were not related to O-acetylation (not shown). Distribution pattern of a2,6- and a2,3-linked sialic acid in postnatal kidneys In rat kidney, nephrogenesis continues in the nephrogenic zone until postnatal day 5. We investigated kidneys from postnatal days 2, 5, 7, 14, and 21. The intensity of PSL staining in postnatal kidneys was stronger than that observed in kidneys from embryonic day 20 with the exception of the nephrogenic zone. At postnatal days 2 (Fig. 4A, B) and 5 (data not shown), the pattern of PSL staining corresponded to the one observed in embryonic day 20 kidneys. Likewise, the MAL staining in the nephrogenic zone was identical to that observed in embryonic kidneys (Fig. 4D). In the underlying cortical region, immature and mature glomeruli were intensely reactive with both PSL (Gl in Fig. 4B, see also E) and MAL (Gl in Fig. 4D, see also F). A most remarkable difference to embryonic kidneys existed with regard to the proximal tubules and this could be observed in all studied postnatal kidney stages (and in adult kidney, see below). All segments of the proximal tubules (S1, S2, S3) were equally and intensely stained with PSL (Fig. 4B, E), whereas the S1 and S2 parts of proximal tubules were
5 147 Fig. 2A F Consecutive sagittal sections of paraffin-embedded rat kidney, embryonic day 18. A C Ureteric bud-derived collecting ducts (arrows in B), proximal part of S-shaped bodies (double arrowheads), immature glomeruli (Gl), and immature proximal tubules (arrowhead and arrows in C) are positive for a2,6-linked sialic acid. Condensed metanephrogenic mesenchyme (asterisk) and stromal mesenchyme appear unreactive. D F Intense staining for a2,3-linked sialic acid is seen in condensed metanephrogenic mesenchyme (asterisk), stromal mesenchyme, entire S-shaped bodies (double arrowheads in E), immature glomeruli (Gl), and immature proximal tubules (arrows in F). Arrows in E point to collecting ducts and staining of the terminal ampullae. En Positive endothelium. Bars 90 mm ina, D; 25mm inb, C, E, F
6 148 Fig. 3A D Adjacent sagittal sections of paraffin-embedded rat kidney, embryonic day 20. A, B Staining pattern for a2,6-linked sialic acid corresponds to that of embryonic day 18. Terminal ampulla (arrowhead) of collecting ducts (arrows), glomeruli (Gl). C, D Staining pattern for a2,3-linked sialic acid corresponds to that of embryonic day 18. Positive terminal ampulla (arrowheads) of unreactive collecting ducts (arrows) are seen as well as positive S- shaped bodies (double arrowheads), glomeruli, and immature proximal tubules (open arrows). Bars 90 mm in A, B; 20 mm in C, D
7 149 Fig. 4A H Serial sections of paraffin-embedded rat kidney, postnatal day 2. The general distribution of a2,6-linked sialic acid in cortex (C), outer (OM), and inner (IM) medulla is shown in A. B is a detail from cortex with nephrogenic zone (asterisk). Glomeruli (Gl) and proximal tubules are positive. In C, the general distribution of a2,3-linked sialic acid in cortex (C), outer (OM) and inner (IM) medulla is shown. D represents a detail from cortex with positive nephrogenic zone (asterisk) and positive glomeruli (Gl); convoluted proximal tubules (PT) are unreactive. E and F show part of cortex with adjacent outer stripe of outer medulla. Although convoluted (PT in E) and straight (arrowheads in E) proximal tubules are positive for a2,6-linked sialic acid; reactivity for a2,3-linked sialic acid is undetectable in convoluted proximal tubules (PT in F) and only faint staining is detectable in straight proximal tubules (PT*). DT Positive distal tubule. G, H Details of inner medulla showing that all structures are reactive for a2,6- linked sialic acid (G) and a2,3-linked sialic acid (H) although the staining for the former is weak. Arrows in G and H point to positive large collecting ducts. Bars 280 mm ina, C; 60mm inb, D, G, H; 45 mm ine, F unreactive with MAL (PT in Fig. 4D). Only S3 of proximal tubules were faintly MAL positive (PT* in Fig. 4F). This differential PSL and MAL staining of proximal tubules is illustrated for postnatal day 7 in Fig. 5 and postnatal day 21 in Fig. 6. Thin loops of HenlØ and distal tubules as well as intertubular capillaries of the examined postnatal stages were positive with both PSL and MAL (Figs. 4, 5, 6). The stromal cells were largely
8 150 Fig. 5A F Serial sections of paraffin-embedded rat kidney, postnatal day 7. At low magnification, the distribution of a2,6-linked sialic acid (A) and of a2,3-linked sialic acid (C) in cortex (C), outer (OM) and inner (IM) medulla is seen. In the cortex, staining for a2,6-linked sialic acid (B) is present in glomeruli (Gl) and convoluted (PT) as well as straight (arrows) proximal tubules. Stromal mesenchyme (asterisks) appears unreactive. Although glomeruli (Gl), distal tubules (DT), and stromal mesenchyme (asterisks) are positive for a2,3-linked sialic acid (D), proximal tubules (PT) are unreactive. In inner medulla, all structures are weakly positive for a2,6-linked sialic acid (E) whereas all elements with the exception of large collecting ducts (arrows) were strongly positive for a2,3-linked sialic acid (F). TL Thin loops. Bars 200 mm in A, C; 45mm inb, D; 30mm ine, F unreactive with PSL (Figs. 4E, 5B) but stained by MAL (Figs. 4F, 5D). Likewise, the collecting ducts of cortex and outer as well as inner medulla were reactive for both PSL and MAL (Figs. 4, 5, 6). The de-o-acetylation of sialic acids by saponification of tissue sections had no influence on intensity and pattern of lectin staining (data not shown). Distribution pattern of a2,6- and a2,3-linked sialic acid in adult kidneys To achieve the highest spatial resolution and morphological comparability, consecutive serial semithin sections of Lowicryl K4 M-embedded kidney were investigated. Although the staining pattern of proximal tubules for PSL and MAL in adult kidney corresponded to that observed
9 151 Fig. 6A D Serial sections of paraffin-embedded rat kidney postnatal day 21. Low magnification showing staining for a2,6-linked sialic acid (A) and a2,3-linked sialic acid (B) in cortex (C) and adjacent outer medulla (OM). Details from cortex with glomeruli (Gl) and proximal tubules (arrowhead points to the origin of proximal tubule) positive for a2,6-linked sialic acid (C). Although glomeruli (Gl) and distal tubules (DT) are positive for a2,3-linked sialic acid (D), proximal tubules (PT) in cortex are unreactive. Bars 120 mm ina, B; 40mm inc, D from postnatal day 2 onward, subtle changes in the collecting ducts were noted. In the cortex, the glomeruli, parietal Bowman s capsule, proximal and distal tubules with the loops of HenlØ in between and including the macula densa, cortical collecting ducts, and intertubular capillaries as well as small arteries and veins were positive with PSL (Fig. 7A C). Proximal tubules stained stronger with PSL than distal tubules and cortical collecting ducts (Fig. 7B). Glomeruli were also positive for MAL but the staining was confined to the endothelia with the podocytes and mesangial cells being unreactive (compare Fig. 7F and C). As already observed in postnatal kidneys, the S1 and S2 segments of proximal tubules were unreactive with MAL (compare Fig. 7E, F and B, C). In contrast, the S3 of proximal tubules was weakly MAL positive (compare Fig. 8E and B). Thin loops were reactive with both lectins (Figs. 8, 10). Distal tubules and collecting ducts in cortex and outer medulla as well as capillaries and small arteries and veins were stained with MAL (Fig. 7E, F). Although the epithelia lining the collecting ducts in cortex and outer as well as inner medulla were reactive with PSL (Fig. 8B, C), a mosaic of MAL-positive and MALnegative epithelia in cortical and outer medulla collecting ducts was clearly visible (Figs. 8E, F, 9A C). By electron microscopy, the intercalated cells were identified as the MAL-positive cells (Fig. 9D). In Fig. 10, a detail of the inner medulla is presented. The large collecting duct principal cells were unreactive with MAL, whereas epithelia of thin loops and capillary endothelia were labeled.
10 152 Fig. 7A F Consecutive serial semithin sections from Lowicryl K4 M-embedded adult rat kidney. General staining pattern for a2,6- linked sialic acid (A) and of a2,3-linked sialic acid (D) in cortex and adjacent outer medulla. Glomeruli (Gl) as well as proximal (PT) and distal tubules are intensely stained for a2,6-linked sialic acid (B) whereas staining for a2,3-linked sialic acid (E) in glomeruli (Gl) and distal tubules (DT) is weak and absent in proximal tubules (PT). High power magnification reveals that podocytes, endothelia, and mesangial cells of glomeruli are positive for a2,6-linked sialic acid (C) whereas only the endothelia of glomeruli are positive for a2,3-linked sialic acid (F). The origin of the proximal tubule at the urinary pole (asterisk in C and F) and cross-sectioned profiles of the same segment S1 of proximal tubules (1, 2 in C and F) are reactive for a2,6-linked sialic acid (C) and not for a2,3-linked sialic acid (F). Arrowhead Parietal Bowman s capsule, arrow cross-sectioned intertubular capillaries. Bars 50 mm ina, D; 20mm inb, E; 5mm inc, F
11 153 Fig. 8A F Consecutive serial semithin sections from Lowicryl K4 M-embedded adult rat kidney. General staining pattern for a2,6- linked sialic acid (A) and of a2,3-linked sialic acid (D) in parts of outer and inner medulla. B, E Cross-sectioned profiles of the same tubules (1 3 in B and E) showing transition of S3 segment of proximal tubule (arrow in B and E) in descending thin loop (arrowhead in B and E) are positive for both a2,6-linked sialic acid (B) and a2,3-linked sialic acid (E). All epithelia of collecting ducts are positive for a2,6-linked sialic acid (asterisk in B and C) whereas the same collecting ducts exhibit a mosaic of cells positive and negative for a2,3-linked sialic acid (asterisk in E and F). Loops of HenlØ and capillaries are positive for both a2,6- and a2,3-linked sialic acid (C and F). Bars 50 mm ina, D; 5mm inb, C, E, F
12 154 Fig. 9A D Adult rat kidney, Lowicryl K4 M embedding, detection of a2,3-linked sialic acid. Detail from cortex and outer medulla with glomerulus (Gl) and unreactive S1 and S2 proximal tubules (PT) as well as weakly stained S3 proximal tubules (PT*). Collecting ducts (arrows in A) with a mosaic of positive (arrowheads in B and C) and negative cells are seen at higher magnification in B and C. The positive cells were identified as intercalated cells by electron microscopy and MAL gold labeling (D). Bars 15 mm ina; 10mm inb, C; 0.25 mm ind Sialyltransferase activities in embryonic, postnatal, and adult kidney A more than threefold difference in specific activity between b-galactoside a2,6 sialyltransferase (ST6Gal-I) and b-galactoside a2,3 sialyltransferase (ST3Gal-III) was measured in aliquots of pooled homogenates from embryonic day 20 kidneys (Fig. 11). Although activity levels of both sialyltransferases decreased postnatally, the one for b-galactoside a2,6 sialyltransferase was always higher and decreased steadily, whereas the activity levels for b-galactoside a2,3 sialyltransferase remained steady from postnatal day 5 onward. As compared to embryonic and postnatal kidneys, activity levels for both sialyltrans-
13 155 Fig. 10A E Details from inner medulla of adult rat, Lowicryl K4 M embedding, detection of a2,3-linked sialic acid. Principal cells of collecting ducts (asterisk in A) are unreactive and this was confirmed by electron microscopic MAL gold labeling (B, C), whereas gold particle labeling was detectable in thin loops (D) and endothelia of capillary (E). Bars 8 mm ina; 2mm inb; 0.25 mm in C; 0.3 mm ind, E
14 156 Fig. 11 Enzyme activity levels for b-galactoside a2,6 sialyltransferase (open squares) and b-galactoside a2,3 sialyltransferase (filled squares) in embryonic (E), postnatal (PN), and adult (Ad) kidneys. Enzyme activity levels are expressed as picomoles of [ 14 C] NeuAc transferred per milligram of acceptor substrate per hour Fig. 12 Enzyme activity levels for b-galactoside a2,6 sialyltransferase (open columns) and b-galactoside a2,3 sialyltransferase (filled columns) in whole adult kidney (K), dissected cortex (C), outer medulla (OM), and inner medulla (IM). Enzyme activity levels are expressed as picomoles of [ 14 C] NeuAc transferred per milligram of acceptor substrate per hour ferases were lowest in adult kidney. The activity for b- galactoside a2,6 sialyltransferase as compared to that of b-galactoside a2,3 sialyltransferase was almost fourfold higher in homogenates prepared from whole kidneys (Fig. 11). When microscopically dissected cortex, outer medulla, and inner medulla were assayed separately, activity for both sialyltransferases was lowest in cortex and highest in inner medulla (Fig. 12). Although activity for b-galactoside a2,6 sialyltransferase as compared to b- galactoside a2,3 sialyltransferase was higher in all three regions, this difference was most striking in inner medulla. Lectin blot analysis of embryonic, postnatal, and adult kidney The aim of this analysis was to establish solely the pattern of PSL- and MAL-reactive glycoproteins and not to identify specific glycoproteins. Although different from each other, the pattern of reactive bands for PSL and MAL was remarkably steady when homogenates of whole kidneys from embryonic day 20, postnatal days 2 21, and adult kidney were investigated (Fig. 13). Interestingly, only few major reactive bands were detected in the 3 15% gradient gels. A band with an approximate molecular mass of 200 kda was reactive with both PSL and MAL. Although its reactivity with MAL was observed in embryonic day 20 and all postnatal stages studied as well as in adult kidney, Fig. 13 Lectin blot analysis of glycoproteins bearing a2,6- linked sialic acid (a2,6 NANA) and a2,3-linked sialic acid (a2,3 NANA), kidney homogenates resolved in 3 15% SDSpolyacrylamide gels. Lanes 1 Embryonic day 20, lanes 2 postnatal day 2, lanes 3 postnatal day 5, lanes 4 postnatal day 7, lanes 5 postnatal day 14, lanes 6 postnatal day 21, lanes 7 whole adult kidney, lanes 8 adult kidney cortex, lanes 9 adult kidney inner medulla
15 157 Fig. 14A, B Schematic presentation of the distribution of a2,6- and a2,3-linked sialic acids in embryonic and adult rat kidney. A Embryonic kidney with condensed metanephrogenic mesenchyme (CMM), renal vesicle (RV), S-shaped body (SB), immature glomerulus (igl), immature proximal tubule (PT), collecting duct (CD), and ampulla of collecting duct (Amp). B Adult kidney with glomerulus (Gl), S1 S3 segment of proximal tubule (S1, S2, S3), loop of HenlØ (LH), straight distal tubule (SDT), convoluted distal tubule (CDT), and collecting duct (CD). Blue coloration in A and B indicates presence of a2,6-linked sialic acid as detected with PSL and SNL, whereas red represents a2,3-linked sialic acid as detected with MAL it became undetectable with PSL from postnatal day 7 onward. One of the high molecular mass bands seen in all samples (except for medulla) apparently represents megalin in cortex (Christensen et al. 1995; Farquhar et al. 1995; Kerjaschki and Farquhar 1982), which has previously been shown to carry both a2,6- and a2,3-linked sialic acids (Morelle et al. 2000; Ziak et al. 1999). When microscopically dissected cortex and inner medulla of adult kidney were probed with PSL and MAL, remarkable differences were observed between the lectins and the kidney regions investigated (Fig. 13 lanes 8, 9). Discussion In the present study, we showed that: (a) N-glycans terminated in a2,6- and a2,3-linked sialic acid as well as activity for the respective sialyltransferases are detectable in embryonic, postnatal, and adult kidney, (b) that a2,6- and a2,3-linked sialic acids are differently expressed in the two embryonic anlagen and in the mesenchymederived early stages of nephron morphogenesis (Fig. 14A), and (c) that regional and cell type-specific differences exist in adult kidney (Fig. 14B). Lectin blot analysis revealed steady patterns of bands reactive for a2,6- and a2,3-linked sialic acids from embryonic to postnatal and adult kidneys. The activity of both the b- galactoside a2,6 sialyltransferase and b-galactoside a2,3 sialyltransferase declined from embryonic over postnatal to adult kidney. The activity for b-galactoside a2,6 sialyltransferase was always three to fourfold higher than b-galactoside a2,3 sialyltransferase. The present lectin histochemical results indicate that distinct patterns of expression of a2,6- and a2,3-linked sialic acids exist during embryonic metanephron formation and postnatal kidney differentiation. One concerns the metanephrogenic mesenchyme which was reactive with MAL, but not with PSL and SNL, from embryonic day 16 until about postnatal day 5 after which nephrogenesis in rat kidney ceases. This indicates that the condensed metanephrogenic mesenchyme is rich in N- glycans bearing a2,3-linked sialic acids. This was also observed for the loose mesenchyme in embryonic, postnatal, and adult kidney. In contrast, a2,6-linked sialic acids were absent from this embryonic anlage or existed in amounts too low to be detectable with PSL and SNL. Likewise, the loose mesenchyme was unreactive with PSL and SNL in embryonic, postnatal, and adult kidney. In this regard, the only other studied sialoglycoconjugates were the polya2,8-neuraminic acid on NCAM (Lackie et al. 1990; Roth et al. 1987) and the oligo/poly a2,8-kdn of megalin (Ziak and Roth 1999) which exhibited a mutually exclusive distribution with the polya2,8-neuraminic acid being detectable in the metanephrogenic mesenchyme. A local mediator of signaling events during metanephrogenic mesenchyme induction is WT1 and together with Wnt-4 is representative of the very few genes expressed in the mesenchyme but not in the ureteric bud (Armstrong et al. 1993; Bard et al. 1994; Kispert et al. 1998; Kreidberg et al. 1993; Stark et al. 1994).
16 158 Another difference in expression of a2,6- and a2,3- linked sialic acids concerns the mesenchyme epithelium transition and glomerulus morphogenesis. All stages of nephron formation were reactive with MAL, as shown previously with the sialic acid specific Limax flavus lectin (Wagner and Roth 1988) and for polya2,8-neuraminic acid on NCAM (Lackie et al. 1990; Roth et al. 1987). In contrast, the renal vesicle, which represents the first epithelial differentiation, was unreactive with PSL and SNA. The S-shaped body which consists of a proximal and a distal portion gives raise to the glomerulus and tubular part of the nephron, respectively, exhibited PSL and SNL staining only in the presumptive visceral (podocytes) and parietal Bowman s epithelium which coincides with transient labeling by GalNAc-reactive lectins (Laitinen et al. 1987) and the expression of WT-1 (Horster et al. 1999). However, immature proximal tubules occurring with the capillary loop stage were then reactive with both PSL and SNL. This indicates that a2,6- linked sialic acids occur rather late in early nephrogenesis and initially are cell type restricted. They are, however, maintained during maturation of the tubule epithelium and persist in adult kidney. This contrasts with the MAL reactivity in proximal tubules which is transient during embryonic development and no longer observed in S1 and S2 segments of mature proximal tubules from postnatal day 2 kidneys onward. A similar transient expression of polya2,8-neuraminic acid on NCAM was observed in chicken and rat kidney (Lackie et al. 1990; Thiery et al. 1982). With regard to the mechanism of remodeling of the S-shaped body which results in the formation of a double slit, changes in cell cell contact and adhesiveness have been postulated to be involved (Hilfer and Hilfer 1983; Kiremidjian and Kopac 1972). One might speculate that negatively charged sialic acids are involved in this process (Lackie et al. 1990; Roth et al. 1987; Ziak and Roth 1999; present study). The ureteric bud and its ramification give rise to the collecting duct system. Both, PSL and SNL staining indicative of the presence of a2,6-linked sialic acids were detectable in the entire collecting duct system of embryonic kidneys in contrast to the metanephrogenic mesenchyme. Staining persisted in postnatal kidneys and was observed in collecting ducts of adult kidneys as well. However, MAL staining indicative of a2,3-linked sialic acids was detectable only in the terminal ampullae (tips) of the collecting ducts during embryonic days 16, 18, and 20. This resembled the reported restricted expression of polya2,8-neuraminic acid on NCAM (Lackie et al. 1990) and coincides with the expression of Wnt-11 (Horster et al. 1999). The observed changing pattern of a2,6- and a2,3- linked sialic acids as detected by lectin histochemistry during induction, nephrogenesis, and differentiation in embryonic and postnatal rat kidneys revealed a high degree of cell type-specific expression during these processes. Their functional importance remains to be investigated. Due to the terminal position of sialic acids in glycans at the cell surface, it has been suggested that they are involved in cell cell interactions during embryonic differentiation and functional maturation in adult organs. The regulated expression of a Gal b1,3 GalNAc a2,3 sialyltransferase in developing thymocytes and during T lymphocyte activation (Gillespie et al. 1993; Priatel et al. 2000) and the control of lymphocyte activation by the Gal b1,4 GlcNAc a2,6 sialyltransferase provide appropriate examples (Hennet et al. 1998). However, in Gal b1,4 GlcNAc a2,6 sialyltransferase knockout mice, biological defects in addition to those of B and T lymphocytes are unknown at present (Martin et al. 2002). In adult kidney, all cells of the nephron and the collecting duct system expressed a2,6-linked sialic acids and this correlates well with previously published data of Gal b1,4 GlcNAc a2,6 sialyltransferase immunohistochemistry (Burger et al. 1998; Kaneko et al. 1995) and SNL staining (Kaneko et al. 1995). In contrast, expression of a2,3-linked sialic acid of N-glycans in kidney cortex was much more limited. We previously reported an even more restricted expression of a2,3-linked sialic acid of O- glycans in cortex and parts of medulla (Toma et al. 1999). Based on the intensity of staining and abundance of positive structures, kidney cortex contains a2,6-linked sialic acid of N-glycans in higher amounts than a2,3- linked sialic acid which is in contrast to most other organs. Based on the same histochemical criteria, the kidney medulla appears to contain similar amounts of a2,6- and a2,3-linked sialic acids of N-glycans. At the cellular level, other remarkable differences existed. Proximal tubular epithelia in S1 and S2 segments were unreactive with MAL, whereas those in S3 segment were positive for a2,3-linked sialic acid. To our knowledge, the only other example of such a drastic difference in glycocalyx composition in a single cell type depending on its position in the nephron are the intercalated cells of collecting ducts with regard to reactivity toward the Helix pomatia lectin (Brown et al. 1985). Although the principal and intercalated cells of collecting ducts were positive with PSL, SNL, and MAL in all studied embryonic and postnatal stages, in adult kidney, principal cells were reactive with PSL and SNL but not with MAL. Thus, our study revealed that regional and cell type-related expression patterns for a2,6- and a2,3-linked sialic acids of N- glycans are characteristic features of rat kidney. By immunocytochemistry, Gal b1,4 GlcNAc a2,3 sialyltransferase immunoreactivity was detected in S1 and S2 segments of proximal tubules and in principal cells of collecting ducts (Burger et al. 1998). We were unable to detect the product of this sialyltransferase in these regions. The most plausible explanation could be that this is the result of competition between the b-galactoside a2,6 sialyltransferase and b-galactoside a2,3 sialyltransferase for the common donor substrate. In this regard, we found higher activity levels for Gal b1,4 GlcNAc a2,6 sialyltransferase in adult kidney and also in embryonic and postnatal kidneys. However, it may not be appropriate to relate results of the localization at the cellular level to those obtained from homogenates of the entire organ. We consider it unlikely that the undetectability of a2,3-linked
17 sialic acids in S1 and S2 segments of proximal tubules and principal cells of collecting ducts is due to a shift from sialylation to fucosylation as reported for postnatal small intestine (Taatjes and Roth 1990), because fucose residues in adult kidney are detectable only in endothelia (Holthöfer et al. 1981; Roth 1983). Based on the lectin blot analysis, we also consider it unlikely that lack of acceptor substrate for the Gal b1,4 GlcNAc a2,3 sialyltransferase, which has been shown in another system to be the rate limiting step (Baum et al. 1996), is the cause although this would require the application of techniques providing higher resolution. In summary, we found that commonly occurring a2,6- and a2,3-linked sialic acids are differently expressed in the two embryonic anlagen of kidney, exhibit a dynamic expression pattern during nephron morphogenesis and differentiation, and show regional as well as cell typespecific expression in adult kidney. Acknowledgements We are grateful to U. Schaub for skilled technical assistance, N. Wey for help in preparing the photographs, S. Sulzer for compiling Fig. 14, and A. Schumacher for preparing the manuscript. We would like to thank Dr. B. Kaissling (Institute of Anatomy, University of Zurich) for helpful comments and discussion. This work was supported by the Canton of Zurich and NIH grant GM29470 (to I.J.G.). References Al-Awqati Q (1996) Plasticity in epithelial polarity of renal intercalated cells: targeting of the H+-ATPase and band 3. Am J Physiol Cell Physiol 39:C1571 C1580 Al-Awqati Q (2003) Terminal differentiation of intercalated cells: the role of hensin. Annu Rev Physiol 65: Armstrong JF, Pritchard J-K, Bickmore WA, Hastie ND, Bard JB (1993) The expression of the Wilms tumour gene, WT1, in the developing mammalian embryo. Mech Dev 40:85 97 Bard JB, McConnell JE, Davies JA (1994) Towards a genetic basis for kidney development. Mech Dev 48:3 11 Baum LG, Derbin K, Perillo NL, Wu T, Pang M, Uittenbogaart C (1996) Characterization of terminal sialic acid linkages on human thymocytes: correlation between lectin-binding phenotype and sialyltransferase expression. J Biol Chem 271: Birchmeier C, Birchmeier W (1993) Molecular aspects of mesenchymal epithelial interactions. Annu Rev Cell Biol 9: Brown D, Roth J, Orci L (1985) Lectin-gold cytochemistry reveals intercalated cell heterogeneity along rat kidney collecting ducts. Am J Physiol 248:C348 C356 Burger PC, Lotscher M, Streiff M, Kleene R, Kaissling B, Berger EG (1998) Immunocytochemical localization of alpha 2,3(N)- sialyltransferase (ST3Gal III) in cell lines and rat kidney tissue sections: evidence for Golgi and post-golgi localization. Glycobiology 8: Charest PM, Roth J (1985) Localization of sialic acid in kidney glomeruli: regionalization in the podocyte plasma membrane and loss in experimental nephrosis. Proc Natl Acad Sci U S A 82: Christensen E, Nielsen S, Moestrup S, Borre C, Maunsbach A, Deheer E, Ronco P, Hammond T, Verroust P (1995) Segmental distribution of the endocytosis receptor gp330 in renal proximal tubules. Eur J Cell Biol 66: Farquhar MG, Saito A, Kerjaschki D, Orlando RA (1995) The Heymann nephritis antigenic complex: megalin (gp330) and RAP. J Am Soc Nephrol 6: Gillespie W, Paulson JC, Kelm S, Pang M, Baum LG (1993) Regulation of alpha 2,3-sialyltransferase expression correlates with conversion of peanut agglutinin (PNA)+ to PNAŸ phenotype in developing thymocytes. J Biol Chem 268: Hammerman M, Rogers S, Ryan G (1992) Growth factors and metanephrogenesis. Am J Physiol 262:F523 F532 Hennet T, Chui D, Paulson JC, Marth JD (1998) Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci U S A 95: Hilfer SR, Hilfer ES (1983) Computer simulation of organogenesis: an approach to the analysis of shape changes in epithelial organs. Dev Biol 97: Holthöfer H, Virtanen I (1987) Glycosylation of developing human glomeruli: lectin binding sites during cell induction and maturation. J Histochem Cytochem 35:33 37 Holthöfer H, Virtaanen I, Pettersson E, Törnroth T, Alfthan O, Linder E, Miettinen A (1981) Lectins as fluorescence microscopic markers for saccharides in human kidney. Lab Invest 45: Horster M, Braun G, Huber S (1999) Embryonic renal epithelia: induction, nephrogenesis, and cell differentiation. Physiol Rev 79: Kaissling B, Dorup J (1995) Functional anatomy of the kidney. In: Greger RF, Knauf H, Mutschler E (eds) Diuretics. Springer, Berlin Heidelberg New York, pp 1 66 Kaneko Y, Yamamoto H, Colley KJ, Moskal JR (1995) Expression of Ga1 beta 1,4GlcNAc alpha 2,6-sialyltransferase and alpha 2,6-linked sialoglycoconjugates in normal human and rat tissues. J Histochem Cytochem 43: Kelm S, Schauer R (1997) Sialic acids in molecular and cellular interactions. In: Jeon KW (ed) International review of cytology. Academic, San Diego, pp Kerjaschki D, Farquhar MG (1982) The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A 79: Kerjaschki D, Sharky DJ, Farquhar MG (1984) Identification and characterization of podocalyxin: the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol 98: Kiremidjian L, Kopac MJ (1972) Changes in cell adhesiveness associated with the development of Rana pipiens pronephros. Dev Biol 27: Kispert A, Vainio S, McMahon AP (1998) Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125: Knibbs RN, Goldstein IJ, Ratcliffe RM, Shibuya N (1991) Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis: comparison with other sialic acid-specific lectins. J Biol Chem 266:83 88 Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R (1993) WT-1 is required for early kidney development. Cell 74: Kriz A, Kaissling B (2000) Structural organization of the mammalian kidney. In: Seldin D, Giebisch G (eds) The kidney: physiology and pathophysiology. Raven, New York, pp Kumar A, Wallner EI, Carone FA, Scarpelli DG, Kanwar YS (1997) Relevance of proto-oncogenes as growth modulators in organogenesis of the mammalian embryonic kidney. Int J Dev Biol 41: Kunz A, Brown D, Orci L (1984) Appearance of Helix pomatia lectin-binding sites at podocyte plasma membrane during glomerular differentiation: a quantitative analysis using the lectin-gold technique. Lab Invest 51: Lackie PM, Zuber C, Roth J (1990) Polysialic acid and N-CAM expression in embryonic rat kidney: mesenchymal and epithelial elements show different patterns of expression. Development 110: Laitinen L, Virtanen I, SaxØn L (1987) Changes in the glycosylation pattern during embryonic development of mouse kidney
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Histology Urinary system
 Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Urinary system. Urinary system
 INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
LABORATORY EXERCISES FOR THE URINARY SYSTEM
 LABORATORY EXERCISES FOR THE URINARY SYSTEM cortex Medulla DEMO SLIDE BOX 172 (450-E001-H-76). Kidney, horse. the inner medulla medullary rays, Uriniferous tubules expand both the cortex and medulla corticomedullary
LABORATORY EXERCISES FOR THE URINARY SYSTEM cortex Medulla DEMO SLIDE BOX 172 (450-E001-H-76). Kidney, horse. the inner medulla medullary rays, Uriniferous tubules expand both the cortex and medulla corticomedullary
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The topic of normal vascular and glomerular anatomy is introduced
 Normal Vascular and Glomerular Anatomy Arthur H. Cohen Richard J. Glassock The topic of normal vascular and glomerular anatomy is introduced here to serve as a reference point for later illustrations of
Normal Vascular and Glomerular Anatomy Arthur H. Cohen Richard J. Glassock The topic of normal vascular and glomerular anatomy is introduced here to serve as a reference point for later illustrations of
Chapter 23. The Nephron. (functional unit of the kidney
 Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Introduction ORIGINAL PAPER. Valeriu Toma Christian Zuber Harry C. Winter Irwin J. Goldstein Jürgen Roth
 Histochem Cell Biol (2001) 116:183 193 DOI 10.1007/s004180100304 ORIGINAL PAPER Valeriu Toma Christian Zuber Harry C. Winter Irwin J. Goldstein Jürgen Roth Application of a lectin from the mushroom Polysporus
Histochem Cell Biol (2001) 116:183 193 DOI 10.1007/s004180100304 ORIGINAL PAPER Valeriu Toma Christian Zuber Harry C. Winter Irwin J. Goldstein Jürgen Roth Application of a lectin from the mushroom Polysporus
PHGY210 Renal Physiology
 PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
Figure 26.1 An Introduction to the Urinary System
 Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Urinary bladder provides a temporary storage reservoir for urine
 Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY
 RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Construction of Nephron by Fusion of Adult Glomeruli to Ureteric Buds with Type V Collagen. Yusuke Murasawa, Pi-chao Wang
 Construction of Nephron by Fusion of Adult Glomeruli to Ureteric Buds with Type V Collagen Yusuke Murasawa, Pi-chao Wang Abstract Although tissue engineering of artificial organs such as skin or cartilage
Construction of Nephron by Fusion of Adult Glomeruli to Ureteric Buds with Type V Collagen Yusuke Murasawa, Pi-chao Wang Abstract Although tissue engineering of artificial organs such as skin or cartilage
Urinary System kidneys, ureters, bladder & urethra
 Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
INVESTIGATION OF THE ULTRAFINE STRUCTURE OF THE KIDNEY BY MEANS OF SCANNING ELECTRON MICROSCOPE
 THE KURUME MEDICAL JOURNAL 1975 Vol.22, No.3, P.135-141 INVESTIGATION OF THE ULTRAFINE STRUCTURE OF THE KIDNEY BY MEANS OF SCANNING ELECTRON MICROSCOPE I. THE GLOMERULUS SHINSHI NODA Department of Urology,
THE KURUME MEDICAL JOURNAL 1975 Vol.22, No.3, P.135-141 INVESTIGATION OF THE ULTRAFINE STRUCTURE OF THE KIDNEY BY MEANS OF SCANNING ELECTRON MICROSCOPE I. THE GLOMERULUS SHINSHI NODA Department of Urology,
Urinary System kidneys, ureters, bladder & urethra
 Urinary System kidneys, ureters, bladder & urethra Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Kidney Function Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Kidney Function Endocrine functions regulates
Kidney Structure. Renal Lobe = renal pyramid & overlying cortex. Renal Lobule = medullary ray & surrounding cortical labryinth.
 Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Lab Activity 31. Anatomy of the Urinary System. Portland Community College BI 233
 Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Histology / First stage The Urinary System: Introduction. Kidneys
 The Urinary System: Introduction The urinary system consists of the paired kidneys and ureters, the bladder, and the urethra. This system helps maintain homeostasis by a complex combination of processes
The Urinary System: Introduction The urinary system consists of the paired kidneys and ureters, the bladder, and the urethra. This system helps maintain homeostasis by a complex combination of processes
Urinary System. Dr. Ahmed Maher Dr. Ahmed Manhal
 Urinary System Dr. Ahmed Maher Dr. Ahmed Manhal Presentation Map Kidney (cortex & medulla). Nephron. Duct system. Juxtaglomerular apparatus. Ureter, bladder & urethra. Definition & General Structure The
Urinary System Dr. Ahmed Maher Dr. Ahmed Manhal Presentation Map Kidney (cortex & medulla). Nephron. Duct system. Juxtaglomerular apparatus. Ureter, bladder & urethra. Definition & General Structure The
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Lithium-induced Tubular Dysfunction. Jun Ki Park 11/30/10
 Lithium-induced Tubular Dysfunction Jun Ki Park 11/30/10 Use of Lithium Mid 19 th century: treatment of gout Late 19 th century: used for psychiatric disorders Early 20 th century: sodium substitute to
Lithium-induced Tubular Dysfunction Jun Ki Park 11/30/10 Use of Lithium Mid 19 th century: treatment of gout Late 19 th century: used for psychiatric disorders Early 20 th century: sodium substitute to
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
Frontal section of human kidney. (Scheme).
 15 Urinary system 15-001 Kidney Cortex Medulla Papilla renalis Medial Lateral Medullary ray Labyrinth Columna renalis Pelvis renalis Calix renalis Ureter Capsula fibrosa Lobus renalis Columna renalis 15-01.
15 Urinary system 15-001 Kidney Cortex Medulla Papilla renalis Medial Lateral Medullary ray Labyrinth Columna renalis Pelvis renalis Calix renalis Ureter Capsula fibrosa Lobus renalis Columna renalis 15-01.
H I S T O L O G Y O F T H E U R I N A R Y S Y S T E M
 SCPA 602- Anatomical Basis For Pathological Study H I S T O L O G Y O F T H E U R I N A R Y S Y S T E M S O M P H O N G N A R K P I N I T, M. D. D E P A R T M E N T O F P A T H O B I O L O G Y F A C U
SCPA 602- Anatomical Basis For Pathological Study H I S T O L O G Y O F T H E U R I N A R Y S Y S T E M S O M P H O N G N A R K P I N I T, M. D. D E P A R T M E N T O F P A T H O B I O L O G Y F A C U
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 16 Urinary System 1 The Kidneys Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Functions of Urinary system o The Kidneys:
BIOH122 Human Biological Science 2 Session 16 Urinary System 1 The Kidneys Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Functions of Urinary system o The Kidneys:
The Ebf1 knockout mouse and glomerular maturation
 Repository of the Max Delbrück Center for Molecular Medicine (MDC) Berlin (Germany) http://edoc.mdc-berlin.de/14009/ The Ebf1 knockout mouse and glomerular maturation Schmidt-Ott, K.M. Published in final
Repository of the Max Delbrück Center for Molecular Medicine (MDC) Berlin (Germany) http://edoc.mdc-berlin.de/14009/ The Ebf1 knockout mouse and glomerular maturation Schmidt-Ott, K.M. Published in final
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Basic Urinary Tract Anatomy and Histology
 Basic Urinary Tract Anatomy and Histology The two kidneys are located in the retroperitoneum on either side of the vertebral bladder and the contraction of the detrusor muscle. Any mechanical barrier,
Basic Urinary Tract Anatomy and Histology The two kidneys are located in the retroperitoneum on either side of the vertebral bladder and the contraction of the detrusor muscle. Any mechanical barrier,
URINARY SYSTEM. These organs lie posterior or inferior to the. (membrane).
 URINARY SYSTEM I. INTRODUCTION Each kidney is made up of about a million tiny tubules called nephrons. Each nephron individually filters the blood and makes urine and it does the job completely, from start
URINARY SYSTEM I. INTRODUCTION Each kidney is made up of about a million tiny tubules called nephrons. Each nephron individually filters the blood and makes urine and it does the job completely, from start
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1: immunoprecipitation with anti-casr antibody The Casr protein was expressed in transiently transfected HEK cells. Cell lysates from HEK cells were subjected
SUPPLEMENTARY FIGURES Supplementary Figure 1: immunoprecipitation with anti-casr antibody The Casr protein was expressed in transiently transfected HEK cells. Cell lysates from HEK cells were subjected
Question 1: Solution 1: Question 2: Question 3: Question 4: Class X The Excretory System Biology
 A. MULTIPLE CHOICE TYPE: (select the most appropriate option in each case) Book Name: Selina Concise Question 1: Excretion primarily involves (a) removal of all byproducts during catabolism (b) removal
A. MULTIPLE CHOICE TYPE: (select the most appropriate option in each case) Book Name: Selina Concise Question 1: Excretion primarily involves (a) removal of all byproducts during catabolism (b) removal
The functional anatomy of the urinary system. Human Anatomy Department Dr. Anastasia Bendelic
 The functional anatomy of the urinary system Human Anatomy Department Dr. Anastasia Bendelic Plan Development of the kidneys and their abnormalities Development of the urinary ways and their abnormalities
The functional anatomy of the urinary system Human Anatomy Department Dr. Anastasia Bendelic Plan Development of the kidneys and their abnormalities Development of the urinary ways and their abnormalities
The functions of the kidney:
 The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Not all renal stem cell niches are the same: anatomy of an evolution
 eissn: 2281-0692 Journal of Pediatric and Neonatal Individualized Medicine 2016;5(2):e050225 doi: 10.7363/050225 Received: 2015 Sept 11; revised: 2015 Dec 21; accepted: 2016 Mar 01; published online: 2016
eissn: 2281-0692 Journal of Pediatric and Neonatal Individualized Medicine 2016;5(2):e050225 doi: 10.7363/050225 Received: 2015 Sept 11; revised: 2015 Dec 21; accepted: 2016 Mar 01; published online: 2016
Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note
![Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note](/thumbs/77/75431210.jpg) Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
1. The Fibrous Capsule covers the outside of the kidney. It is made of fat and fibers.
 Slide 2 The kidney has a number of functions. First is the excretion of toxic metabolic waste through urine production. The kidneys filter blood plasma and as a result of filtering blood, the kidneys help
Slide 2 The kidney has a number of functions. First is the excretion of toxic metabolic waste through urine production. The kidneys filter blood plasma and as a result of filtering blood, the kidneys help
19. RENAL PHYSIOLOGY ROLE OF THE URINARY SYSTEM THE URINARY SYSTEM. Components and function. V BS 122 Physiology II 151 Class of 2011
 19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats
 Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
Analysis on the mechanism of reduced nephron number and the pathological progression of chronic renal failure in Astrin deficient rats Summary of Doctoral Thesis Hidenori Yasuda Graduate School of Veterinary
Bio 322 Human Anatomy Objectives for the laboratory exercise Urinary System Filtration Reabsorption Secretion Concentration
 Bio 322 Human Anatomy Objectives for the laboratory exercise Urinary System Required reading before beginning this lab: Saladin, KS: Human Anatomy 5 th ed (2017) Chapter 25 For this lab you will use parts
Bio 322 Human Anatomy Objectives for the laboratory exercise Urinary System Required reading before beginning this lab: Saladin, KS: Human Anatomy 5 th ed (2017) Chapter 25 For this lab you will use parts
BIOSYNTHESIS OF CANCER-RELATED CARBOHYDRATE ANTIGENS. Fabio Dall Olio Department of Experimental Pathology University of Bologna, Italy
 BIOSYNTHESIS OF CANCER-RELATED CARBOHYDRATE ANTIGENS Fabio Dall Olio Department of Experimental Pathology University of Bologna, Italy TOPICS OF THE LECTURE 1. Structure and function of some representative
BIOSYNTHESIS OF CANCER-RELATED CARBOHYDRATE ANTIGENS Fabio Dall Olio Department of Experimental Pathology University of Bologna, Italy TOPICS OF THE LECTURE 1. Structure and function of some representative
URINARY SYSTEM. Urinary System
 URINARY SYSTEM Urinary System Kidney Functions Excretion Regulation of blood volume and pressure Regulation of electrolyte and ph levels Kidney Structure Gross Anatomy Fibrous Capsule Renal Cortex Renal
URINARY SYSTEM Urinary System Kidney Functions Excretion Regulation of blood volume and pressure Regulation of electrolyte and ph levels Kidney Structure Gross Anatomy Fibrous Capsule Renal Cortex Renal
HRP cytochemistry. Division of Radiooncology, Deutsches Krebsforschungszentrum, Heidelberg, Germany
 HRP cytochemistry WOLF D. KUHLMANN, M.D. Division of Radiooncology, Deutsches Krebsforschungszentrum, 69120 Heidelberg, Germany A range of substrates is available for the cytochemical staining of peroxidase
HRP cytochemistry WOLF D. KUHLMANN, M.D. Division of Radiooncology, Deutsches Krebsforschungszentrum, 69120 Heidelberg, Germany A range of substrates is available for the cytochemical staining of peroxidase
Excretory System 1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- X- Y- Z- b) Which of the following is not a function of the organ shown? A. to produce
sfigure 1: Detection of L-fucose in normal mouse renal cortex using the plant lectin LTL
 sfigure 1: Detection of L-fucose in normal mouse renal cortex using the plant lectin LTL LTL staining Negative control Fluorescence microscopy of normal (CL-11 +/+ ) mouse renal tissue after staining with
sfigure 1: Detection of L-fucose in normal mouse renal cortex using the plant lectin LTL LTL staining Negative control Fluorescence microscopy of normal (CL-11 +/+ ) mouse renal tissue after staining with
AP2, Lab 7 - THE URINARY SYSTEM
 AP2, Lab 7 - THE URINARY SYSTEM I. SYSTEM COMPONENTS (Figs. 25.1 25.4) KIDNEYS Each kidney contains approx. 1,000,000 tubular NEPHRONS which produce FILTRATE from the plasma and then add to or take from
AP2, Lab 7 - THE URINARY SYSTEM I. SYSTEM COMPONENTS (Figs. 25.1 25.4) KIDNEYS Each kidney contains approx. 1,000,000 tubular NEPHRONS which produce FILTRATE from the plasma and then add to or take from
Formation of Urine: Formation of Urine
 The Urinary outflow tract: monitors and regulates extra-cellular fluids excretes harmful substances in urine, including nitrogenous wastes (urea) returns useful substances to bloodstream maintain balance
The Urinary outflow tract: monitors and regulates extra-cellular fluids excretes harmful substances in urine, including nitrogenous wastes (urea) returns useful substances to bloodstream maintain balance
organs of the urinary system
 organs of the urinary system Kidneys (2) bean-shaped, fist-sized organ where urine is formed. Lie on either sides of the vertebral column, in a depression beneath peritoneum and protected by lower ribs
organs of the urinary system Kidneys (2) bean-shaped, fist-sized organ where urine is formed. Lie on either sides of the vertebral column, in a depression beneath peritoneum and protected by lower ribs
CHAPTER 25 URINARY. Urinary system. Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1. functions
 CHAPTER 25 URINARY Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1 fluid waste elimination secretion of wastes control blood volume and BP control blood ph electrolyte levels RBC levels hormone production
CHAPTER 25 URINARY Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1 fluid waste elimination secretion of wastes control blood volume and BP control blood ph electrolyte levels RBC levels hormone production
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
The Urinary S. (Chp. 10) & Excretion. What are the functions of the urinary system? Maintenance of water-salt and acidbase
 10.1 Urinary system The Urinary S. (Chp. 10) & Excretion 10.1 Urinary system What are the functions of the urinary system? 1. Excretion of metabolic wastes (urea, uric acid & creatinine) 1. Maintenance
10.1 Urinary system The Urinary S. (Chp. 10) & Excretion 10.1 Urinary system What are the functions of the urinary system? 1. Excretion of metabolic wastes (urea, uric acid & creatinine) 1. Maintenance
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Urinary Anatomy. Lab 40. Kidneys. Nephrons. Renal Corpuscle
 Urinary Anatomy Lab 40. Urinary Anatomy and Kidney Dissection Kidneys: filters blood, produces urine Ureters: convey urine to bladder Bladder: holding tank Urethra: carries urine to the outside for elimination
Urinary Anatomy Lab 40. Urinary Anatomy and Kidney Dissection Kidneys: filters blood, produces urine Ureters: convey urine to bladder Bladder: holding tank Urethra: carries urine to the outside for elimination
Cell Proliferation in the Loop of Henle in the Developing Rat Kidney
 J Am Soc Nephrol 12: 1410 1421, 2001 Cell Proliferation in the Loop of Henle in the Developing Rat Kidney JUNG-HO CHA,* YOUNG-HEE KIM,* JU-YOUNG JUNG,* KI-HWAN HAN,* KIRSTEN M. MADSEN, and JIN KIM* *Department
J Am Soc Nephrol 12: 1410 1421, 2001 Cell Proliferation in the Loop of Henle in the Developing Rat Kidney JUNG-HO CHA,* YOUNG-HEE KIM,* JU-YOUNG JUNG,* KI-HWAN HAN,* KIRSTEN M. MADSEN, and JIN KIM* *Department
28/04/2013 LEARNING OUTCOME C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS STUDENT ACHIEVEMENT INDICATORS URINARY SYSTEM & EXCRETION
 LEARNING OUTCOME C13 Analyse the functional interrelationships of the structures of the urinary system Learning Outcome C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS Students who have fully met this
LEARNING OUTCOME C13 Analyse the functional interrelationships of the structures of the urinary system Learning Outcome C13 URINARY SYSTEM STUDENT ACHIEVEMENT INDICATORS Students who have fully met this
Excretion Chapter 29. The Mammalian Excretory System consists of. The Kidney. The Nephron: the basic unit of the kidney.
 Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
1. Anatomy / Vascularisation. 2. Urine concentration. 3. Axial heterogeneity of some segments
 Lise BANKIR 1. Anatomy / Vascularisation 2. Urine concentration 3. Axial heterogeneity of some segments Rat kidney. Arterial filling with Microfil silicone rubber Alcian Blue staining Filling of arterial
Lise BANKIR 1. Anatomy / Vascularisation 2. Urine concentration 3. Axial heterogeneity of some segments Rat kidney. Arterial filling with Microfil silicone rubber Alcian Blue staining Filling of arterial
Chapter 25: Urinary System
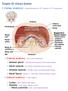 Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
Chapter 25: Urinary System I. Kidney anatomy: retroperitoneal from 12 th thoracic to 3 rd lumbar area A. External anatomy: hilus is the indentation 1. Adrenal gland: in the fat at the superior end of each
Nephron Function and Urine Formation. Ms. Kula December 1, 2014 Biology 30S
 Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Nature Biotechnology: doi: /nbt Supplementary Figure 1. RNAseq expression profiling of selected glycosyltransferase genes in CHO.
 Supplementary Figure 1 RNAseq expression profiling of selected glycosyltransferase genes in CHO. RNAseq analysis was performed on two common CHO lines (CHO-K1, CHO-GS) and two independent CHO-GS triple
Supplementary Figure 1 RNAseq expression profiling of selected glycosyltransferase genes in CHO. RNAseq analysis was performed on two common CHO lines (CHO-K1, CHO-GS) and two independent CHO-GS triple
After studying this lecture, you should be able to...
 Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
Reabsorption of Salt and Water After studying this lecture, you should be able to... 1. Define the obligatory water loss. 2. Describe the mechanism of Na ++ reabsorption in the distal tubule and explain
THE RENAL / URINARY SYSTEM
 1 THE RENAL / URINARY SYSTEM Definition/Description: The Renal/Urinary system is the body system, which plays a vital role in the maintenance of homeostasis by the following processes: 1. Production of
1 THE RENAL / URINARY SYSTEM Definition/Description: The Renal/Urinary system is the body system, which plays a vital role in the maintenance of homeostasis by the following processes: 1. Production of
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Urinary System and Fluid Balance. Urine Production
 Urinary System and Fluid Balance Name Pd Date Urine Production The three processes critical to the formation of urine are filtration, reabsorption, and secretion. Match these terms with the correct statement
Urinary System and Fluid Balance Name Pd Date Urine Production The three processes critical to the formation of urine are filtration, reabsorption, and secretion. Match these terms with the correct statement
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
DOI: 10.1038/ncb2988 Supplementary Figure 1 Kif7 L130P encodes a stable protein that does not localize to cilia tips. (a) Immunoblot with KIF7 antibody in cell lysates of wild-type, Kif7 L130P and Kif7
LECTURE ON THE URINARY SYSTEM
 LECTURE ON THE URINARY SYSTEM (Uropoetic System) AN OVERVIEW Dr HAMIADJI THE URINARY SYSTEM URINARY SYSTEM The kidneys are responsible for removing wastes from the body, regulating electrolyte balance
LECTURE ON THE URINARY SYSTEM (Uropoetic System) AN OVERVIEW Dr HAMIADJI THE URINARY SYSTEM URINARY SYSTEM The kidneys are responsible for removing wastes from the body, regulating electrolyte balance
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
Urinary System. BSC 2086 A & P 2 Professor Tcherina Duncombe Palm Beach State College
 Urinary System BSC 2086 A & P 2 Professor Tcherina Duncombe Palm Beach State College Filter plasma, separate and eliminate wastes Functions Regulate blood volume and pressure Regulate osmolarity of body
Urinary System BSC 2086 A & P 2 Professor Tcherina Duncombe Palm Beach State College Filter plasma, separate and eliminate wastes Functions Regulate blood volume and pressure Regulate osmolarity of body
Basic Functions of the Kidneys
 Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Biology Slide 1 of 36
 Biology 1 of 36 38 3 The Excretory System 2 of 36 Functions of the Excretory System 1.Function: process which eliminates metabolic wastes 3 of 36 Functions of the Excretory System (The skin excretes excess
Biology 1 of 36 38 3 The Excretory System 2 of 36 Functions of the Excretory System 1.Function: process which eliminates metabolic wastes 3 of 36 Functions of the Excretory System (The skin excretes excess
Chapter 13 The Urinary System
 Biology 12 Name: Urinary System Per: Date: Chapter 13 The Urinary System Complete using BC Biology 12, page 408-435 13.1 The Urinary System pages 412-413 1. As the kidneys produce urine, they carry out
Biology 12 Name: Urinary System Per: Date: Chapter 13 The Urinary System Complete using BC Biology 12, page 408-435 13.1 The Urinary System pages 412-413 1. As the kidneys produce urine, they carry out
Renal Physiology II Tubular functions
 Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Renal Physiology II Tubular functions LO. 42, 43 Dr. Kékesi Gabriella Basic points of renal physiology 1. Glomerular filtration (GF) a) Ultrafiltration 2. Tubular functions active and passive a) Reabsorption
Urinary system. Lab-7
 Urinary system Lab-7 Excretion: processes that remove wastes and excess materials from the body Urinary system (kidneys): excretes nitrogenous wastes, excess solutes, and water The Kidneys Regulate Water
Urinary system Lab-7 Excretion: processes that remove wastes and excess materials from the body Urinary system (kidneys): excretes nitrogenous wastes, excess solutes, and water The Kidneys Regulate Water
osmoregulation mechanisms in gills, salt glands, and kidneys
 Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
Ionic & Osmotic Homeostasis osmoregulation mechanisms in gills, salt glands, and kidneys extracellular intracellular 22 23 Salt Secretion: recycle Figure in Box 26.2 Hill et al. 2004 active Down electrochemical
Sunday, July 17, 2011 URINARY SYSTEM
 URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
Early nephron formation in the developing mouse kidney
 J. Anat. (2001) 199, pp. 385 392, with 7 figures Printed in the United Kingdom 385 Early nephron formation in the developing mouse kidney JONATHAN B. L. BARD 1, ADELE GORDON, LINDA SHARP AND WILLIAM I.
J. Anat. (2001) 199, pp. 385 392, with 7 figures Printed in the United Kingdom 385 Early nephron formation in the developing mouse kidney JONATHAN B. L. BARD 1, ADELE GORDON, LINDA SHARP AND WILLIAM I.
Chapter 11 Lecture Outline
 Chapter 11 Lecture Outline See separate PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright 2016 McGraw-Hill Education. Permission required for reproduction
Chapter 11 Lecture Outline See separate PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright 2016 McGraw-Hill Education. Permission required for reproduction
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University
 Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
A. Correct! Flushing acids from the system will assist in re-establishing the acid-base equilibrium in the blood.
 OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
Lecture 16: The Nephron
 Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
Renal System Physiology
 M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
PARTS OF THE URINARY SYSTEM
 EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
Mammalian-type Glycosylation l in LEXSY
 Mammalian-type Glycosylation l in LEXSY Case Study: Recombinant hu Erythropoietin Jena Bioscience GmbH Loebstedter Str. 80 07749 Jena, Germany Tel.: +49-3641-628-5000 Fax: +49-3641-628-5100 628 e-mail:
Mammalian-type Glycosylation l in LEXSY Case Study: Recombinant hu Erythropoietin Jena Bioscience GmbH Loebstedter Str. 80 07749 Jena, Germany Tel.: +49-3641-628-5000 Fax: +49-3641-628-5100 628 e-mail:
The addition of sugar moiety determines the blood group
 The addition of sugar moiety determines the blood group Sugars attached to glycoproteins and glycolipids on the surfaces of red blood cells determine the blood group termed A, B, and O. The A and B antigens
The addition of sugar moiety determines the blood group Sugars attached to glycoproteins and glycolipids on the surfaces of red blood cells determine the blood group termed A, B, and O. The A and B antigens
