Measurement of cochlear power gain in the sensitive gerbil ear
|
|
|
- Clyde Price
- 5 years ago
- Views:
Transcription
1 Received 4 Nov 2 Accepted 3 Feb 2 Published Mar 2 DOI:.38/ncomms226 Measurement of cochlear power gain in the sensitive gerbil ear Tianying Ren,2, Wenxuan He & Peter G. Gillespie,3 The extraordinary sensitivity of the mammalian ear is commonly attributed to the cochlear amplifier, a cellular process thought to locally boost responses of the cochlear partition to soft sounds. However, cochlear power gain has not been measured directly. Here we use a scanning laser interferometer to determine the volume displacement and volume velocity of the cochlear partition by measuring its transverse vibration along and across the partition. We show the transverse displacement at the peak-response location can be >, times greater than the displacement of the stapes, whereas the volume displacement of an area centred at this location is approximately tenfold greater than that of the stapes. Using the volume velocity and cochlear-fluid impedance, we discover that power at the peak-response area is > -fold greater than that at the stapes. These results demonstrate experimentally that the cochlea amplifies soft sounds, offering insight into the mechanism responsible for the cochlear sensitivity. Department of Otolaryngology and Head and Neck Surgery, Oregon Hearing Research Center, Oregon Health and Science University, Portland, Oregon 97239, USA. 2 Department of Physiology, Xi an Jiaotong University School of Medicine, Xi an, Shaanxi 76, China. 3 Vollum Institute, Oregon Health and Science University, Portland, Oregon 97239, USA. Correspondence and requests for materials should be addressed to T.R. ( rent@ohsu.edu). nature communications 2:26 DOI:.38/ncomms Macmillan Publishers Limited. All rights reserved.
2 nature communications DOI:.38/ncomms226 Excitation of auditory sensory hair cells within the inner ear requires a sequence of mechanical steps, which commence when external airborne sounds pass through the ear canal and vibrate the flexible eardrum. This vibration reaches the cochlea via the middleear ossicular chain, displacing cochlear fluid and consequently vibrating the cochlear partition (Fig. a). The cochlear partition includes the flexible acellular basilar membrane and the cellular organ of Corti, which houses the sensory hair cells. A travelling wave of excitation starts at the base of the cochlear partition and propagates towards the apex. As the wave travels along the basilar membrane, its magnitude increases and speed decreases. The wave reaches its maximal magnitude at the best-frequency location, then quickly dies away beyond that place (Fig. b,c). Hair cells at the best-frequency location encode time, frequency and magnitude information of sounds and send them to the brain. In living cochleae, the vibration at the best-frequency site saturates as stimulus level is increased and displays sharp tuning as frequency is modulated 2 7. Cochlear high sensitivity, sharp tuning and nonlinearity have been attributed to the cochlear amplifier, an active process derived from outer hair cells that has been proposed to amplify the basilar membrane response to soft sounds 8 6. The gain of cochlear amplification is often estimated by comparing the ratio of basilar membrane-to-stapes vibration magnitude at low sound levels to that at high sound levels 2 (typically > - fold or > 4 db). However, vibration magnitude at a single basilar membrane location does not provide information on the longitudinal and radial extent of the response nor indicates the energy of the vibration. Despite discovery of active force generation by outer hair cells through somatic motility and active bundle motion 7,8 and experimental studies of power flow in the cochlea 9 22, the expected cochlear-amplifier power gain has yet to be demonstrated experimentally. By measuring the volume displacement and volume velocity, here we show that the cochlea, indeed, can generate energy and amplify basilar membrane vibration in response to soft sounds. We thus directly demonstrate power amplification in mammalian cochleae, which support the theory that the cochlear amplifier is responsible for the remarkable hearing sensitivity. Out In Base Magnitude Phase Longitudinal pattern BM y t forward z (nm) d c Down Tympanic membrane BF /2 (µm) Up b a x BF BF BM width (µm) Stapes Round window Radial pattern Toward apex Results Consistency of the data. To quantify the energy gain of the basilar membrane vibration, we used a scanning laser interferometer to measure vibration not only at a single spot at the best-frequency location, but also along and across the basilar membrane. Because of the invasive surgery, extremely low reflectance of the cochlear partition 23, vulnerability of cochlear sensitivity and time-consuming data collection, the productivity of the scanning measurements was low. Among 28 animals used in this study, 2 were excluded because of > 8-dB hearing loss before data collection. No vibration data were collected from six animals because the poor transparency of the perilymph reduced the carrier signal and increased the noise floor. Incomplete longitudinal or radial data were collected from five cochleae due to deteriorating hearing during data acquisition. The sensitive scanning data presented here are thus from five cochleae, which had similar basilar membrane responses (Fig. 2). Basilar membrane vibration at a single location. A typical data set of the basilar membrane vibration measured at a single location ~2,5 µm from the base is presented in Figure 3. Magnitude transfer functions, the ratio of the basilar membrane-to-stapes vibration magnitude as a function of frequency, are plotted in Figure 3a. At low and intermediate sound levels (2 6 db SPL, wherein db SPL is 2 µpa), the basilar membrane vibrated maximally near 6 khz, that is, the best frequency of the measured location. At sound levels of 2 4 db SPL, the basilar membrane vibration at the best frequency was >,-fold greater than the stapes vibration. As the stimulus level increased from 2 to 9 db SPL, the ratio decreased by > 4 db, indicating a compressive nonlinear growth. As the sound Figure Measurement of the volume displacement. (a) When a tone is presented to the ear, vibrations are measured along and across the basilar membrane through the round window and at the stapes footplates. (b) Magnitude and phase longitudinal and radial patterns of basilar membrane vibration. (c) The relationship between the stapes vibration and the cochlear forward travelling wave. (d) Volume displacement of basilar membrane vibration, measured as the volume bounded by the instantaneous waveform with a maximum displacement centred at the best-frequency location and the in-phase vibrating area on the xy plane (area abcd). The length of this area is a half-wavelength and its width is approximately the basilar membrane width at the best-frequency location. BM, the basilar membrane; BF, best frequency; t forward, the forward delay; λ, the wavelength. Blue and red colours in panels c and d show the low and high magnitude of the basilar membrane vibration, respectively. level increased, the response peak at ~6 khz becomes broader and shifted towards low frequencies. The green curves in Figure 3 show the response at 4 db SPL, the level used for quantifying the volume displacement in five sensitive cochleae. The corresponding phase decreased progressively with frequency (Fig. 3b). The data in Figure 3 demonstrate that the sensitive cochlear preparations used in this study showed the same high sensitivity, nonlinearity and sharp tuning as reported previously 2. Longitudinal patterns of basilar membrane vibration. Scanning measurements of basilar membrane vibration at and around the best-frequency location are shown in Figure 4. The magnitudes of cochlear partition vibrations measured at longitudinal locations ~2,2 to ~2,8 µm from the cochlear base are shown in Figure 4a. nature communications 2:26 DOI:.38/ncomms Macmillan Publishers Limited. All rights reserved.
3 nature communications DOI:.38/ncomms226 ARTICLE Phase (radians) Mean ±s.e. 2,2 2,4 2,6 2,8 Distance from base (µm) Distance from OSL (µm) Figure 2 Grouped magnitude and phase data of basilar membrane vibration. (a) The longitudinal magnitude pattern of the basilar membrane response to a 4-dB SPL 6-kHz tone, presented by means (blue solid line) and range of the standard error (red dotted lines) from five cochleae. (b) Corresponding longitudinal phase data. (c) Displacement magnitude as a function of the radial location. Standard errors near the osseous spiral lamina (OSL) are smaller than those in the region between 8 to 24 µm due to the relatively high reflectivity. (d) Corresponding radial phase data show little change across the basilar membrane. For a 6-kHz tone at intensities below 5 db SPL, the best-frequency location was ~2,5 µm from the base. As the stimulus magnitude increased,-fold from to 9 db SPL, the displacement magnitude at the best-frequency location only increased about -fold, from ~. to ~ nm, indicating nonlinear compression of the response. At intensities below 5 db SPL, the displacementlongitudinal location curves showed an approximately symmetrical peak centred at the best-frequency location. As the sound level increased, the response increased more in the basal direction than towards the apex, shifting the peak towards the base 24,25. The phase of the basilar membrane response decreased with distance from the cochlear base (Fig. 4b), which indicated that waves travelled in the apical direction, that is, they formed a forward travelling wave,26. At the basal side of the best-frequency location, the rate of phase decrease was small, indicating that the wave travelled rapidly through this region. As the observed location was moved through the best-frequency location towards the apex, the phase progressively decreased, indicating that the wave slowed. As sound level increased, the phase longitudinal distance relation flattened modestly, indicating that wave travelled faster at high sound levels. Radial patterns of basilar membrane vibration. Radial measurements of basilar membrane vibrations are shown in Figure 4c d. As the sound level increased, the basilar membrane response increased in magnitude (Fig. 4c) but changed little in phase along the radial direction (Fig. 4d), consistent with previous reports 25,27,28. The radial pattern of basilar membrane vibration magnitude was asymmetric across all sound levels (Fig. 4c); locations near the osseous spiral lamina vibrated at significantly higher magnitudes than those near the spiral ligament. At low levels ( < 3 db SPL), basilar membrane vibration was mainly located over the region of ~25 75 µm, where a Ratio (BM/stapes), 2 db SPL 9 db SPL BF 2 Frequency (Hz) >4 db 2 3 the hair cells are located. With increased sound level, the vibration spread both centrally and laterally. Spatial patterns of basilar membrane vibration. To determine the volume displacement of the area of the cochlear partition centred at the best-frequency location (area abcd in Fig. d), we measured the spatial patterns of basilar membrane vibration. Figure 4e shows the spatial envelope of the basilar membrane response to a 4-dB SPL 6-kHz tone; we calculated the instantaneous waveform (Fig. 4f) using magnitude and phase data (see Methods). The basilar membrane volume displacement is the volume bounded by the instantaneous waveform and the in-phase vibrating area centred at the bestfrequency location (Figs d and 4f). The half-wavelength centred at the best-frequency location across sound levels was only 83 µm (Fig. 4b), which is ~.5% of the basilar membrane length (Fig. 4g). The size of this area suggests that fewer than outer hair cells work in-phase for a 6-kHz tone at the best-frequency location. Volume displacement and power gain. By measuring basilar membrane vibration at different sound pressure levels, we found that the volume displacement (solid line, Fig. 5a) showed nonlinear compressive growth with stimulus level, whereas the growth of the stapes volume displacement (dotted line, Fig. 5a) was linear. At db SPL, the cochlear volume displacement was approximately ten times greater than that of the stapes vibration; this ratio decreased as the sound level increased, and was unity at ~8 db SPL. The energy associated with basilar membrane vibration at the best-frequency location and near the stapes was calculated from the volume velocity and characteristic impedance of cochlear fluids (see Methods) and is displayed in Figure 5b. Although the shape of the energy input output function of the basilar membrane at the bestfrequency area (solid blue line, Fig. 5b) was similar to that of the volume displacement (solid red line, Fig. 5a), because energy depends on the square of the volume velocity, energy increased much more than the volume displacement when the sound level increased. As shown in Figure 5c, at low sound levels, the single-point basilar membrane vibration magnitude (solid line) was ~,-fold greater than that of the stapes vibration (dotted line). Although the ratio decreased significantly at high sound levels, the basilar b Phase (radians) Frequency (Hz) Figure 3 Single-point basilar membrane vibration. The magnitude and phase of the basilar membrane vibration were measured at a single longitudinal location as a function of frequency. The ratio of basilar membrane-to-stapes vibration magnitude (BM/stapes) and phase difference between the stapes and basilar membrane are presented in a and b. (a) At 2 6 db SPL, the magnitude increases with frequency and reaches the maximum at ~6 khz. The magnitude and sharpness of the peak decreased with the sound level and the peak shifted to low frequencies from ~6 to ~ khz (horizontal dotted arrowed line). The magnitude at the peak frequency decreased > 4 db as the sound level increased from 2 to 9 db SPL (indicated by vertical dotted arrowed lines). (b) The corresponding phase decreased progressively with frequency. The green curves show the responses at 4 db SPL, the level used for quantifying the volume displacement in five sensitive cochleae. nature communications 2:26 DOI:.38/ncomms Macmillan Publishers Limited. All rights reserved.
4 nature communications DOI:.38/ncomms226.. db SPL 9 db SPL Volume (µm 3 ) 2. Energy (joule) Phase (radians) db SPL 5 9 db SPL 5 /2=83 µm 2 2,2 2,4 2,6 2, Distance from base (µm) Distance from OSL (µm) Gains, Sound level (db SPL) Sound level (db SPL) Figure 5 Power gain of basilar membrane vibration. (a) Volume displacements of the basilar membrane (solid line) and stapes (dotted line) vibration as a function of the sound level. (b) Energy input and output functions of the basilar membrane (solid line) and stapes (dotted line) vibration. (c) Point displacements of the basilar membrane (solid line) and stapes (dotted line) vibration. (d) Energy (blue), volume- (red) and point- (black) displacement gains as a function of the sound level. Green circles show energy gains from five sensitive cochleae (mean = 6, s.e. =, n = 5) , 2 2,5 2, Base /2=83 µm Distance from base (µm) Distance from OSL (µm) Apex Distance from base (µm) Figure 4 Spatial patterns of basilar membrane vibration. Magnitude and phase of the basilar membrane vibration were measured as functions of the longitudinal and radial locations (a d). The spatial pattern (e) and volume displacement (f) were calculated from the longitudinal and radial data. (a) At low sound levels from to 4 db SPL, the vibration increases with the distance from the cochlear base and forms a peak at ~2,5 µm. As the stimulus increased ~,-fold from to 9 db SPL, the response peak increased only ~-fold, from ~. to ~ nm and shifted towards the base (left). (b) Phase decreased with longitudinal location. The phase slope became flatter at high sound levels. (c) Magnitude as a function of radial location, indicated by the distance from the osseous spiral lamina (OSL). The displacement magnitude varied significantly radially. (d) Phase show no significant change across the basilar membrane. Cartoon inset indicates the cross-section of the cochlear partition, with one inner hair cell (left) and three outer hair cells in red. (e) Magnitude spatial pattern of basilar membrane response to a 4-dB SPL 6-kHz tone. (f) Instantaneous waveform of the basilar membrane vibration. Blue and red colours in e and f show the low and high magnitude of the basilar membrane vibration, respectively. (g) Spatial relationship of the quantified half wavelength and the basilar membrane length. membrane vibration remained > times greater than the stapes vibration. The single-point data in Figure 5c is consistent with that in Figure 3a; both show 3 4 db compression. Ratios of the basilar membrane single-point displacement (black), volume displacement (red) or energy (blue) to the corresponding measures at the stapes (gains) are presented as a function of the sound level in Figure 5d. Green circles show energy gains from five sensitive cochleae (mean = 6, s.e. =, n = 5). As the sound level increased from to 9 db SPL, single-point and volume displacement gains decreased by ~3 db, whereas the energy gain decreased as much as 6 db; the energy gain of the cochlear partition vibration in response to low-level tones can be >, yet dropped below at high sound levels. Discussion Cochlear amplification was originally predicted by Gold 29 and was found to be possible by the ground-breaking discovery of otoacoustic emissions by Kemp 3. In vitro experiments have indicated that electrical motility of outer hair cells 6,7,3 37 and active hair-bundle movement 6,7,3 37 can generate mechanical energy, whereas modelling studies 38 and accumulated experimental results 2 have supported the existence of cochlear amplification. Nevertheless, the expected cochlear power gain has not been demonstrated experimentally. Here, we measured the volume displacement and velocity of basilar membrane vibration, which, along with the cochlear-fluid impedance, allowed us to determine the associated energy. We found a power gain of ~ from cochlear base to best-frequency location, which provides the first direct experimental evidence of power amplification in the sensitive living cochlea. The principle for measuring the volume displacement or energy gain is the same as that for the conventional method for determining the single-point displacement gain 2. In either case, the gain is quantified by the ratio of basilar membrane vibration magnitude at the best-frequency location to that of the stapes; the current and conventional methods are distinguished by different units, one of volume or energy and the other of length. Figure b,c shows the temporal and spatial relationships between the stapes and basilar membrane nature communications 2:26 DOI:.38/ncomms Macmillan Publishers Limited. All rights reserved.
5 nature communications DOI:.38/ncomms226 ARTICLE vibration. For a sinusoidal vibration, the volume displacement by the stapes footplates (V s ) varies with time t and frequency f and is given by V s = V s + cos(ωt), where ω = 2πf. V s + is the absolute value of V s at t = nπ/ω (n =,, 2, ), which is determined by multiplying the area of the stapes footplate with its point displacement. As the vibration of the cochlear partition travels from the cochlear base to its best-frequency location, the magnitude may be reduced by damping or increased by the cochlear amplifier 8,9, 4,6,39. The volume displacement at the best-frequency location (V bm ) is described by V bm = V bm + cos[ω(t + t forward )], where t forward is the forward delay of the basilar membrane vibration from the base to the best-frequency location (Fig. b,c). Similar to V s +, V bm + is the absolute value of V bm at t forward + nπ/ω (n =,, 2, ), which is defined by the instantaneous waveform of basilar membrane vibration with the maximal volume displacement centred at the best-frequency location. The longitudinal extent of V bm is defined by the half-wavelength distance because the cochlear partition displaces in the same direction over this region, and the radial extent by the basilar membrane width (see Fig. d and Methods). This volume displacement differs from the net volume displacement over the basilar membrane length, which occurs simultaneously with the stapes vibration. The former is a measure of the travelling wave, whereas the later is related to the fast longitudinal wave 4. At low sound levels, cochlear vibration is mostly restricted to the outer hair cell region (Fig. 4c) at the best-frequency area (Fig. 4a). Steep longitudinal phase slopes (Fig. 4b) indicate shorter wavelengths and smaller in-phase-vibration areas at low sound levels. For a given volume displacement or velocity, a smaller in-phasevibration area results in a larger vibration in the transverse direction. Thus, in addition to level-dependent power amplification, the cochlea can increase the transverse vibration at the best-frequency location by focusing energy on the spatially restricted best-frequency region 4. In turn, maximal transverse vibration optimally stimulates auditory sensory cells. Reflective of this focusing mechanism, the point-displacement gain (black line in Fig. 5d) is about -fold greater than that of the volume displacement gain (red line in Fig. 5d) at the low and intermediate sound levels. Because sharp cochlear tuning profits from a limited longitudinal extent of basilar membrane vibration 24, the narrow longitudinal restriction of vibration contributes to cochlear sharp tuning. One may wonder whether the interferometer signal is from the basilar membrane or from deeper structures. Reflected light from a very reflective surface out of the focus might contribute to or even dominate the interferometer signal due to the large coherent length of lasers However, because of the difference in refraction index of the perilymph and the basilar membrane, incident light is reflected on the perilymph-basilar membrane interface in the current experiment. As a consequence of this reflection, the surface of the basilar membrane is visible through the microscope under the white light illumination. Because of the diffusion of the tissue, the structures on the scala-vestibuli side of the basilar membrane are not readily visible. Because the interferometer laser light is in the visible range of the spectrum, it too is subject to the same reflectivity as white light. Indeed, the carrier signal is optimized when the object beam is focused on the surface of the basilar membrane. Thus, the focal plane of the object beam was indicated by the optimal carrier signal and the sharpest image of the surface of the basilar membrane at the scala-tympani side. This was confirmed when we measured sound-induced vibration of the cochlear partition as a function of the transverse position using a low coherence interferometer with a visible light source. We found that the carrier signal was largest when the object beam was focused on the basilar membrane. In most classical cochlear mathematical models 26,39,4, the cochlear travelling wave is presented by a series of independent sections along the longitudinal direction, and acoustic energy is transmitted dominantly through the cochlear fluid. According to these theories, the energy of the cochlear partition vibration at the best-frequency location can be quantified by measuring the power in the cochlear fluid surrounding the partition. Here, the fluid energy was quantified from the volume velocity and characteristic impendence of the cochlear fluid (see Methods). Because cochlear fluids may move radially and longitudinally, this method may underestimate the energy; our measured power gain of ~ should therefore be considered to be a lower bound. When the membrane potential of an outer hair cell changes as a result of sound-induced hair-bundle deflection, the cell body changes length and produces force due to a voltage-dependent conformational change in the membrane protein prestin 6,7,3 37. Hair bundles of mammalian outer hair cells can also produce mechanical force 4,5,8, In either case, outer hair cell-generated forces can enhance basilar membrane responses to soft sounds when the timing of force generation is appropriate 48. The energy gain we observed in the living cochlea likely resulted from energy generated by somatic motility and/or active bundle motion of outer hair cells. Methods Materials and general methods. Twenty-eight young healthy Mongolian gerbils (4 8 g) were used in this study. Anaesthesia was induced by intraperitoneal injection of ketamine (3 mg kg ) followed by intramuscular xylazine (5 mg kg ) 24. The animal use protocol was approved by the Oregon Health and Science University Institutional Animal Care and Use Committee. The method for measuring the basilar membrane vibration at a single longitudinal location was the same as previously 49. Cochlear sensitivity was measured by recording the compound action potential and estimated by the nonlinear compression of basilar membrane responses. Scanning measurement of basilar membrane vibration. The sensitivity of the laser interferometer was improved by increasing optical efficiency, which was achieved by removing the original collimating and focusing lenses and by precisely aligning different optical components 24,5. The alignment result of each optical component was monitored using a compact laser power meter, and the optical sensitivity of the system was measured by the carrier signal levels when the object beam was focused on a low reflective (~.%) surface. The minimized noise floor of the instrument was achieved by maximizing the carrier signal level. For scanning measurements of the basilar membrane vibration, the transparency of the perilymph in the optical path significantly affected the carrier signal level and the noise floor. Blood cells suspended in the perilymph often prevented the interferometer from detecting the basilar membrane vibration. Great care was taken to minimize bleeding and to avoid blood cells entering the cochlea. Approximately mm of basilar membrane in the first turn was exposed through the round window. The object beam of a scanning interferometer was focused on the basilar membrane through a glass coverslip and the perilymph. The scanning paths were determined by to 2 reference points using a three-dimensional positioning system. The longitudinal scanning path was approximately underneath the second row of outer hair cells; the radial scanning path was at the best-frequency location. As the longitudinal position of the laser focus spot was changed at 5. µm s, magnitudes and phases of the basilar membrane vibration velocity in response to a continuous best-frequency tone were collected at two samples per second, giving.4 sample per µm. The scanning rate along the radial direction was 2. µm s, giving one sample per µm. The displacement magnitude (D) in nm and phase (φ) in radians at each location was calculated from the velocity magnitude (V) and phase (θ) according to D = V/2πf and φ = θ π/2, where f is the stimulus frequency in Hz. Volume displacement measurement. Because radial phase patterns were approximately constant over a half-wavelength region along the longitudinal direction, we quantified the volume displacement of the basilar membrane vibration (V bm ) as follows. The measured magnitude-radial location function was normalized with the maximum magnitude as.; the radial magnitude pattern at each longitudinal location was obtained by multiplying the normalized radial magnitude pattern by the measured displacement at the given longitudinal location. The surface plot consisting of all displacement values over the in phase vibrating area shows the vibration magnitude in the space (Fig. 4e). The phase value at each location inside the in-phase vibrating area was obtained from the longitudinal and radial phase. To calculate the amplitude of the maximum volume displacement of the basilar membrane V bm +, the phase value at the best-frequency location was shifted to nπ (n =,, 2 ) by adding a constant to all phase values over the in-phase vibrating area. The instantaneous waveform (Fig. 4f) consists of the real value (R) at each location, which was derived from magnitude (M p ) and phase (φ p ) using R = M p *cos(φ p ). V bm + was quantified by integrating R over the in-phase vibration area, which was defined by the basilar membrane width in the radial direction and a half-wavelength distance in the longitudinal direction (area abcd in Fig. d). nature communications 2:26 DOI:.38/ncomms Macmillan Publishers Limited. All rights reserved.
6 nature communications DOI:.38/ncomms226 V s +, the amplitude of the volume displacement of the stapes vibration was obtained from the product of D ps, the single-point displacement amplitude and A s, the area of the stapes footplate. D ps was taken approximately from the centre of the stapes footplate. Although complex stapes vibration has been reported 5, our pilot experiments showed that vibrations from four distributed locations on the stapes footplate were in phase and that their mean was very close to the measured singlepoint displacement from the centre. In addition, the single-point vibration of the stapes has been commonly used for calculating the basilar membrane vibration transfer function 2. To quantify A s, the stapes were collected and mounted using bone wax, with the perilymphatic surface of the stapes footplate in an approximately horizontal plane. The image of the stapes footplate was captured using a digital camera through a stereomicroscope. The edge of the stapes footplate was detected and A s was calculated by multiplying the number of pixels of stapes footplate image with the area of each pixel. The volume displacement gain of the basilar membrane vibration (G vol ) was obtained according to G vol = V bm + /V s + at different intensities. The displacement gain measured from a single location (G p ) was calculated using G p = D pbm /D ps, where D pbm and D ps are displacements at the best-frequency location and at the stapes. Measurement of energy and energy gain. Despite a recent study 52, most classical cochlear mathematical models 26,39,4 present the cochlear travelling wave using a series of independent sections along the longitudinal direction, and neglect longitudinal coupling. According to these theories, the relevant energy of the cochlear partition vibration can be quantified by measuring power in the cochlear fluid surrounding the partition. As energy in fluid can be quantified based on the volume velocity and fluid characteristic impedance 53, the sound energy passing through the in-phase vibrating area centred at the best-frequency site in s (I bf ) was quantified according to the equation I bf = (V bfv ) 2 ρ c, where V bfv is the volume velocity measured centred at the best-frequency location (V bfv = 2 π f V bm +, where f is frequency and V bm + is the volume displacement), ρ is the density of the cochlear fluid (ρ, kg m 3 ) and c is the speed of sound in water (c,5 m s ). Similarly, the energy in the cochlear fluid near the stapes (I s ) was quantified according to equation I s = (V sv ) 2 ρ c, where V sv is the volume velocity of the stapes vibration (V sv = 2πf V s +, where f is frequency and V s + is volume displacement of the stapes vibration. The energy gain (G e ) was obtained from equation G e = I bf /I s. According to the above relationship between energy and volume displacement, G e = G vol2, where G vol is the volume displacement gain. References. von Békésy, G. Experiments in Hearing (McGraw-Hill, 96). 2. Robles, L. & Ruggero, M. A. Mechanics of the mammalian cochlea. Physiol. Rev. 8, (2). 3. Rhode, W. S. Observations of the vibration of the basilar membrane in squirrel monkeys using the Mossbauer technique. J. Acoust. Soc. Am. 49, (97). 4. Ruggero, M. A. & Rich, N. C. Application of a commercially-manufactured Doppler-shift laser velocimeter to the measurement of basilar-membrane vibration. Hear. Res. 5, (99). 5. Nuttall, A. L., Dolan, D. F. & Avinash, G. Laser Doppler velocimetry of basilar membrane vibration. Hear. Res. 5, (99). 6. Cooper, N. P. & Rhode, W. S. Basilar membrane mechanics in the hook region of cat and guinea-pig cochleae: sharp tuning and nonlinearity in the absence of baseline position shifts. Hear. Res. 63, 63 9 (992). 7. Russell, I. J. & Nilsen, K. E. The location of the cochlear amplifier: spatial representation of a single tone on the guinea pig basilar membrane. Proc. Natl Acad. Sci. USA 94, (997). 8. de Boer, E. Power amplification in an active model of the cochlea--short-wave case. J. Acoust. Soc. Am. 73, (983). 9. Davis, H. An active process in cochlear mechanics. Hear. Res. 9, 79 9 (983).. Neely, S. T. & Kim, D. O. A model for active elements in cochlear biomechanics. J. Acoust. Soc. Am. 79, (986).. Dallos, P. The active cochlea. J Neurosci 2, (992). 2. Hudspeth, A. Mechanical amplification of stimuli by hair cells. Curr. Opin. Neurobiol. 7, (997). 3. Fettiplace, R. Active hair bundle movements in auditory hair cells. J. Physiol. 576, (26). 4. Ricci, A. J., Crawford, A. C. & Fettiplace, R. Mechanisms of active hair bundle motion in auditory hair cells. J. Neurosci. 22, (22). 5. Hudspeth, A. J. Making an effort to listen: mechanical amplification in the ear. Neuron 59, (28). 6. Ashmore, J. Cochlear outer hair cell motility. Physiol. Rev. 88, 73 2 (28). 7. Brownell, W. E., Bader, C. R., Bertrand, D. & de Ribaupierre, Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science 227, (985). 8. Martin, P. & Hudspeth, A. J. Active hair-bundle movements can amplify a hair cell s response to oscillatory mechanical stimuli. Proc. Natl Acad. Sci. USA 96, (999). 9. Allen, J. B. & Fahey, P. F. Using acoustic distortion products to measure the cochlear amplifier gain on the basilar membrane. J. Acoust. Soc. Am. 92, (992). 2. Olson, E. S. Intracochlear pressure measurements related to cochlear tuning. J. Acoust. Soc. Am., (2). 2. de Boer, E., Nuttall, A. L., Hu, N., Zou, Y. & Zheng, J. The Allen-Fahey experiment extended. J. Acoust. Soc. Am. 7, (25). 22. Lukashkin, A. N., Walling, M. N. & Russell, I. J. Power amplification in the mammalian cochlea. Curr. Biol. 7, (27). 23. Khanna, S. M., Willemin, J. F. & Ulfendahl, M. Measurement of optical reflectivity in cells of the inner ear. Acta Otolaryngol. Suppl. 467, (989). 24. Ren, T. Longitudinal pattern of basilar membrane vibration in the sensitive cochlea. Proc. Natl Acad. Sci. USA 99, 7 76 (22). 25. Rhode, W. S. & Recio, A. Study of mechanical motions in the basal region of the chinchilla cochlea. J. Acoust. Soc. Am. 7, (2). 26. Zwislocki, J. J. Wave motion in the cochlea caused by bone conduction. J. Acoust. Soc. Am. 25, (953). 27. Cooper, N. P. in Recent Developments in Auditory Mechanics (eds H. Wada, T. Takasaka, K. Ikeda, K. Ohyama & T. Koike) 9 5 (World Scientific, 2). 28. Ren, T. et al. in Biophysics of the Cochlea from Molecules to Models (ed. A.W. Gummer) 2 29 (World Scientific, 23). 29. Gold, T. Hearing. II. The physical basis of the action of the cochlea. Proc. R. Soc. Lond. Ser. B Biol. Sci. 35, (948). 3. Kemp, D. T. Stimulated acoustic emissions from within the human auditory system. J. Acoust. Soc. Am. 64, (978). 3. Dallos, P., Zheng, J. & Cheatham, M. A. Prestin and the cochlear amplifier. J. Physiol. 576, (26). 32. Zheng, J., Madison, L. D., Oliver, D., Fakler, B. & Dallos, P. Prestin, the motor protein of outer hair cells. Audiol. Neurootol. 7, 9 2 (22). 33. He, D. Z., Jia, S. & Dallos, P. Prestin and the dynamic stiffness of cochlear outer hair cells. J. Neurosci. 23, (23). 34. Liberman, M. C. et al. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 49, 3 34 (22). 35. Gao, J. et al. Prestin-based outer hair cell electromotility in knockin mice does not appear to adjust the operating point of a cilia-based amplifier. Proc. Natl Acad. Sci. USA 4, (27). 36. Santos-Sacchi, J. Asymmetry in voltage-dependent movements of isolated outer hair cells from the organ of Corti. J. Neurosci. 9, (989). 37. Mellado Lagarde, M. M., Drexl, M., Lukashkina, V. A., Lukashkin, A. N. & Russell, I. J. Outer hair cell somatic, not hair bundle, motility is the basis of the cochlear amplifier. Nat. Neurosci., (28). 38. de Boer, E. & Nuttall, A. L. The mechanical waveform of the basilar membrane. III. Intensity effects. J. Acoust. Soc. Am. 7, (2). 39. Neely, S. T. & Kim, D. O. An active cochlear model showing sharp tuning and high sensitivity. Hear. Res. 9, 23 3 (983). 4. Peterson, L. C. & Bogert, B. P. A dynamic theory of the cochlea. J. Acoust. Soc. Am. 22, (95). 4. Ren, T. & Gillespie, P. G. A mechanism for active hearing. Curr. Opin. Neurobiol. 7, (27). 42. Ren, T. & Nuttall, A. L. Recording depth of the heterodyne laser interferometer for cochlear vibration measurement. J. Acoust. Soc. Am. 9, (2). 43. Dalhoff, E., Gartner, R., Zenner, H. P., Tiziani, H. J. & Gummer, A. W. Remarks about the depth resolution of heterodyne interferometers in cochlear investigations. J. Acoust. Soc. Am., (2). 44. de La Rochefoucauld, O., Khanna, S. M. & Olson, E. S. Recording depth and signal competition in heterodyne interferometry. J. Acoust. Soc. Am. 7, (25). 45. Hudspeth, A. J., Choe, Y., Mehta, A. D. & Martin, P. Putting ion channels to work: mechanoelectrical transduction, adaptation, and amplification by hair cells. Proc. Natl Acad. Sci. USA 97, (2). 46. Kennedy, H. J., Evans, M. G., Crawford, A. C. & Fettiplace, R. Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat. Neurosci. 6, (23). 47. Fettiplace, R. & Hackney, C. M. The sensory and motor roles of auditory hair cells. Nat. Rev. Neurosci. 7, 9 29 (26). 48. Nilsen, K. E. & Russell, I. J. Timing of cochlear feedback: spatial and temporal representation of a tone across the basilar membrane. Nat. Neurosci. 2, (999). 49. He, W., Fridberger, A., Porsov, E., Grosh, K. & Ren, T. Reverse wave propagation in the cochlea. Proc. Natl Acad. Sci. USA 5, (28). 5. Ren, T. Reverse propagation of sound in the gerbil cochlea. Nat. Neurosci. 7, (24). nature communications 2:26 DOI:.38/ncomms Macmillan Publishers Limited. All rights reserved.
7 nature communications DOI:.38/ncomms226 ARTICLE 5. Decraemer, W. F., Khanna, S. M. & Funnell, W. R. A method for determining three-dimensional vibration in the ear. Hear. Res. 77, 9 37 (994). 52. Meaud, J. & Grosh, K. The effect of tectorial membrane and basilar membrane longitudinal coupling in cochlear mechanics. J. Acoust. Soc. Am. 27, 4 42 (2). 53. Crocker, M. J. Handbook of Acoustics (John Wiley & Sons, 998). Acknowledgments We thank E. Porsov, S. Matthews and Y. Zou for technical help; and A.L. Nuttall, S. Neely, C.R. Steele, and A. Fridberger for valuable discussion on an early version of the manuscript. This work was supported by the National Institute of Deafness and Other Communication Disorders (R DC4554 to T.R. and R DC2368 to P.G.). Author contributions T.R. designed and performed the experiments; T.R., W.H. and P.G. analysed the data; T.R. and P.G. wrote the paper. Additional information Competing financial interests The authors declare no competing financial interests. Reprints and permission information is available online at reprintsandpermissions/ How to cite this article: Ren, T. et al. Measurement of cochlear power gain in the sensitive gerbil ear. Nat. Commun. 2:26 doi:.38/ncomms226 (2). nature communications 2:26 DOI:.38/ncomms Macmillan Publishers Limited. All rights reserved.
Reticular lamina and basilar membrane vibrations in living mouse cochleae
 Reticular lamina and basilar membrane vibrations in living mouse cochleae Tianying Ren a,1, Wenxuan He a, and David Kemp b a Oregon Hearing Research Center, Department of Otolaryngology, Oregon Health
Reticular lamina and basilar membrane vibrations in living mouse cochleae Tianying Ren a,1, Wenxuan He a, and David Kemp b a Oregon Hearing Research Center, Department of Otolaryngology, Oregon Health
THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA
 451 THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA DÁIBHID Ó MAOILÉIDIGH, FRANK JÜLICHER Max Planck Institute für Physik komplexer Systeme, Nöthnitzerstr. 38, 01187
451 THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA DÁIBHID Ó MAOILÉIDIGH, FRANK JÜLICHER Max Planck Institute für Physik komplexer Systeme, Nöthnitzerstr. 38, 01187
Backward Propagation of Otoacoustic Emissions
 40 Review Backward Propagation of Otoacoustic Emissions HE Wenxuan, 1, 2 REN Tianying, 1, 2 1. Oregon Hearing Research Center, Department of Otolaryngology and Head & Neck Surgery, Oregon Health & Science
40 Review Backward Propagation of Otoacoustic Emissions HE Wenxuan, 1, 2 REN Tianying, 1, 2 1. Oregon Hearing Research Center, Department of Otolaryngology and Head & Neck Surgery, Oregon Health & Science
Chapter 3: Anatomy and physiology of the sensory auditory mechanism
 Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Mechanical Properties of the Cochlea. Reading: Yost Ch. 7
 Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
Structure, Energy Transmission and Function. Gross Anatomy. Structure, Function & Process. External Auditory Meatus or Canal (EAM, EAC) Outer Ear
 Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
Processing of sounds in the inner ear
 Processing of sounds in the inner ear Sripriya Ramamoorthy Associate Professor, IIT Bombay WiSSAP 2018 Cochlea converts sound into electrical signals [Picture courtesy of Northwestern University] von Bekesy
Processing of sounds in the inner ear Sripriya Ramamoorthy Associate Professor, IIT Bombay WiSSAP 2018 Cochlea converts sound into electrical signals [Picture courtesy of Northwestern University] von Bekesy
Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier
 Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier Sripriya Ramamoorthy 1 *, Alfred L. Nuttall 1,2 1 Oregon Hearing Research Center, Department of Otolaryngology, Oregon
Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier Sripriya Ramamoorthy 1 *, Alfred L. Nuttall 1,2 1 Oregon Hearing Research Center, Department of Otolaryngology, Oregon
Auditory System Feedback
 Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics
 Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3676 3681, March 1999 Biophysics Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics PAUL J.
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3676 3681, March 1999 Biophysics Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics PAUL J.
Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression
 1398 Biophysical Journal Volume 106 March 2014 1398 1405 Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression Julien Meaud * and Karl Grosh Department
1398 Biophysical Journal Volume 106 March 2014 1398 1405 Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression Julien Meaud * and Karl Grosh Department
Cochlear anatomy, function and pathology II. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
A truly remarkable aspect of human hearing is the vast
 AUDITORY COMPRESSION AND HEARING LOSS Sid P. Bacon Psychoacoustics Laboratory, Department of Speech and Hearing Science, Arizona State University Tempe, Arizona 85287 A truly remarkable aspect of human
AUDITORY COMPRESSION AND HEARING LOSS Sid P. Bacon Psychoacoustics Laboratory, Department of Speech and Hearing Science, Arizona State University Tempe, Arizona 85287 A truly remarkable aspect of human
Signals, systems, acoustics and the ear. Week 5. The peripheral auditory system: The ear as a signal processor
 Signals, systems, acoustics and the ear Week 5 The peripheral auditory system: The ear as a signal processor Think of this set of organs 2 as a collection of systems, transforming sounds to be sent to
Signals, systems, acoustics and the ear Week 5 The peripheral auditory system: The ear as a signal processor Think of this set of organs 2 as a collection of systems, transforming sounds to be sent to
Auditory Physiology Richard M. Costanzo, Ph.D.
 Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
FOUR COUNTER-ARGUMENTS FOR SLOW-WAVE OAEs
 FOUR COUNTER-ARGUMENTS FOR SLOW-WAVE OAEs CHRISTOPHER A. SHERA Eaton-Peabody Laboratory, Boston, MA 02114, USA email: shera@epl.meei.harvard.edu ARNOLD TUBIS Institute for Nonlinear Science, La Jolla,
FOUR COUNTER-ARGUMENTS FOR SLOW-WAVE OAEs CHRISTOPHER A. SHERA Eaton-Peabody Laboratory, Boston, MA 02114, USA email: shera@epl.meei.harvard.edu ARNOLD TUBIS Institute for Nonlinear Science, La Jolla,
Chapter 11: Sound, The Auditory System, and Pitch Perception
 Chapter 11: Sound, The Auditory System, and Pitch Perception Overview of Questions What is it that makes sounds high pitched or low pitched? How do sound vibrations inside the ear lead to the perception
Chapter 11: Sound, The Auditory System, and Pitch Perception Overview of Questions What is it that makes sounds high pitched or low pitched? How do sound vibrations inside the ear lead to the perception
An active cochlear model showing sharp tuning and high sensitivity
 Hearing Research, 9 (1983) 123-13 Elsevier Biomedical Press 123 An active cochlear model showing sharp tuning and high sensitivity Stephen T. Neely * and D.O. Kim Box 811, Washington Unitlersit}', St.
Hearing Research, 9 (1983) 123-13 Elsevier Biomedical Press 123 An active cochlear model showing sharp tuning and high sensitivity Stephen T. Neely * and D.O. Kim Box 811, Washington Unitlersit}', St.
Deafness and hearing impairment
 Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
Hearing Sound. The Human Auditory System. The Outer Ear. Music 170: The Ear
 Hearing Sound Music 170: The Ear Tamara Smyth, trsmyth@ucsd.edu Department of Music, University of California, San Diego (UCSD) November 17, 2016 Sound interpretation in the auditory system is done by
Hearing Sound Music 170: The Ear Tamara Smyth, trsmyth@ucsd.edu Department of Music, University of California, San Diego (UCSD) November 17, 2016 Sound interpretation in the auditory system is done by
Music 170: The Ear. Tamara Smyth, Department of Music, University of California, San Diego (UCSD) November 17, 2016
 Music 170: The Ear Tamara Smyth, trsmyth@ucsd.edu Department of Music, University of California, San Diego (UCSD) November 17, 2016 1 Hearing Sound Sound interpretation in the auditory system is done by
Music 170: The Ear Tamara Smyth, trsmyth@ucsd.edu Department of Music, University of California, San Diego (UCSD) November 17, 2016 1 Hearing Sound Sound interpretation in the auditory system is done by
HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier
 HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier 1 Cochlear Mechanics: Measures of Basilar Membrane Motion 2 Cochlear Mechanics: Measures of Basilar Membrane Motion Bekesy s experiments
HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier 1 Cochlear Mechanics: Measures of Basilar Membrane Motion 2 Cochlear Mechanics: Measures of Basilar Membrane Motion Bekesy s experiments
Required Slide. Session Objectives
 Auditory Physiology Required Slide Session Objectives Auditory System: At the end of this session, students will be able to: 1. Characterize the range of normal human hearing. 2. Understand the components
Auditory Physiology Required Slide Session Objectives Auditory System: At the end of this session, students will be able to: 1. Characterize the range of normal human hearing. 2. Understand the components
SOLUTIONS Homework #3. Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03
 SOLUTIONS Homework #3 Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03 Problem 1: a) Where in the cochlea would you say the process of "fourier decomposition" of the incoming
SOLUTIONS Homework #3 Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03 Problem 1: a) Where in the cochlea would you say the process of "fourier decomposition" of the incoming
In vivo outer hair cell length changes expose the active process in the cochlea
 In vivo outer hair cell length changes expose the active process in the cochlea Dingjun Zha, Fangyi Chen, Sripriya Ramamoorthy, Anders Fridberger, Niloy Choudhury, Steven L Jacques, Ruikang K Wang and
In vivo outer hair cell length changes expose the active process in the cochlea Dingjun Zha, Fangyi Chen, Sripriya Ramamoorthy, Anders Fridberger, Niloy Choudhury, Steven L Jacques, Ruikang K Wang and
Auditory System. Barb Rohrer (SEI )
 Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
Auditory Physiology PSY 310 Greg Francis. Lecture 30. Organ of Corti
 Auditory Physiology PSY 310 Greg Francis Lecture 30 Waves, waves, waves. Organ of Corti Tectorial membrane Sits on top Inner hair cells Outer hair cells The microphone for the brain 1 Hearing Perceptually,
Auditory Physiology PSY 310 Greg Francis Lecture 30 Waves, waves, waves. Organ of Corti Tectorial membrane Sits on top Inner hair cells Outer hair cells The microphone for the brain 1 Hearing Perceptually,
Sound and its characteristics. The decibel scale. Structure and function of the ear. Békésy s theory. Molecular basis of hair cell function.
 Hearing Sound and its characteristics. The decibel scale. Structure and function of the ear. Békésy s theory. Molecular basis of hair cell function. 19/11/2014 Sound A type of longitudinal mass wave that
Hearing Sound and its characteristics. The decibel scale. Structure and function of the ear. Békésy s theory. Molecular basis of hair cell function. 19/11/2014 Sound A type of longitudinal mass wave that
ENT 318 Artificial Organs Physiology of Ear
 ENT 318 Artificial Organs Physiology of Ear Lecturer: Ahmad Nasrul Norali The Ear The Ear Components of hearing mechanism - Outer Ear - Middle Ear - Inner Ear - Central Auditory Nervous System Major Divisions
ENT 318 Artificial Organs Physiology of Ear Lecturer: Ahmad Nasrul Norali The Ear The Ear Components of hearing mechanism - Outer Ear - Middle Ear - Inner Ear - Central Auditory Nervous System Major Divisions
HST 721 Efferent Control Lecture October 2004
 HST 721 Efferent Control Lecture October 2004 1 Stapedius Muscle Central Circuitry 2 Hypotheses for MEM Function A. Stapedius 1. Extend Dynamic Range - a gain control system 2. Protect the Inner Ear from
HST 721 Efferent Control Lecture October 2004 1 Stapedius Muscle Central Circuitry 2 Hypotheses for MEM Function A. Stapedius 1. Extend Dynamic Range - a gain control system 2. Protect the Inner Ear from
Acoustics Research Institute
 Austrian Academy of Sciences Acoustics Research Institute Modeling Modelingof ofauditory AuditoryPerception Perception Bernhard BernhardLaback Labackand andpiotr PiotrMajdak Majdak http://www.kfs.oeaw.ac.at
Austrian Academy of Sciences Acoustics Research Institute Modeling Modelingof ofauditory AuditoryPerception Perception Bernhard BernhardLaback Labackand andpiotr PiotrMajdak Majdak http://www.kfs.oeaw.ac.at
Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea
 Keywords: Cochlea, Basilar membrane, Hair cell 7006 Journal of Physiology (1998), 509.1, pp. 277 288 277 Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea Nigel P.
Keywords: Cochlea, Basilar membrane, Hair cell 7006 Journal of Physiology (1998), 509.1, pp. 277 288 277 Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea Nigel P.
Systems Neuroscience Oct. 16, Auditory system. http:
 Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea
 Article Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea Wei Dong and Elizabeth S. Olson 2, * VA Loma Linda Health Care System and Otolaryngology/Head & Neck Surgery,
Article Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea Wei Dong and Elizabeth S. Olson 2, * VA Loma Linda Health Care System and Otolaryngology/Head & Neck Surgery,
Representation of sound in the auditory nerve
 Representation of sound in the auditory nerve Eric D. Young Department of Biomedical Engineering Johns Hopkins University Young, ED. Neural representation of spectral and temporal information in speech.
Representation of sound in the auditory nerve Eric D. Young Department of Biomedical Engineering Johns Hopkins University Young, ED. Neural representation of spectral and temporal information in speech.
Improving the diagnostic power of otoacoustic emissions. Arturo Moleti Physics Department University of Roma Tor Vergata
 Improving the diagnostic power of otoacoustic emissions Arturo Moleti Physics Department University of Roma Tor Vergata The human ear Ear canal: resonant cavity Middle ear: impedance adapter and pressure
Improving the diagnostic power of otoacoustic emissions Arturo Moleti Physics Department University of Roma Tor Vergata The human ear Ear canal: resonant cavity Middle ear: impedance adapter and pressure
Cochlear anatomy, function and pathology I. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology I Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of these lectures Introduction to gross anatomy of the cochlea Focus (1) on
Cochlear anatomy, function and pathology I Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of these lectures Introduction to gross anatomy of the cochlea Focus (1) on
FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA. Invited Paper LINEAR RESPONSE OF THE COCHLEA
 FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA Invited Paper LINEAR RESPONSE OF THE COCHLEA David Alan Bies Visiting Research Fellow Department of Mechanical
FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA Invited Paper LINEAR RESPONSE OF THE COCHLEA David Alan Bies Visiting Research Fellow Department of Mechanical
Modelling the micromechanics of the cochlea in Femlab
 Modelling the micromechanics of the cochlea in Femlab R.R.J.J. van Doorn DCT 27.7 Traineeship report Coach(es): Supervisor: Prof. S.J. Elliott Prof. P. Gardonio Prof. H. Nijmeijer Technische Universiteit
Modelling the micromechanics of the cochlea in Femlab R.R.J.J. van Doorn DCT 27.7 Traineeship report Coach(es): Supervisor: Prof. S.J. Elliott Prof. P. Gardonio Prof. H. Nijmeijer Technische Universiteit
Advanced otoacoustic emission detection techniques and clinical diagnostics applications
 Advanced otoacoustic emission detection techniques and clinical diagnostics applications Arturo Moleti Physics Department, University of Roma Tor Vergata, Roma, ITALY Towards objective diagnostics of human
Advanced otoacoustic emission detection techniques and clinical diagnostics applications Arturo Moleti Physics Department, University of Roma Tor Vergata, Roma, ITALY Towards objective diagnostics of human
Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs
 Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs The Auditory Laboratory, Physiology University of Western Australia Greg O Beirne Dept. of Communication
Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs The Auditory Laboratory, Physiology University of Western Australia Greg O Beirne Dept. of Communication
Educational Module Tympanometry. Germany D Germering
 Educational Module anometry PATH medical Germany D-82110 Germering Our educational modules 1 are made for providing information on how the hearing organ works and which test procedures are used to test
Educational Module anometry PATH medical Germany D-82110 Germering Our educational modules 1 are made for providing information on how the hearing organ works and which test procedures are used to test
SPHSC 462 HEARING DEVELOPMENT. Overview Review of Hearing Science Introduction
 SPHSC 462 HEARING DEVELOPMENT Overview Review of Hearing Science Introduction 1 Overview of course and requirements Lecture/discussion; lecture notes on website http://faculty.washington.edu/lawerner/sphsc462/
SPHSC 462 HEARING DEVELOPMENT Overview Review of Hearing Science Introduction 1 Overview of course and requirements Lecture/discussion; lecture notes on website http://faculty.washington.edu/lawerner/sphsc462/
Before we talk about the auditory system we will talk about the sound and waves
 The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
sensitivity (db) frequency (khz) I: MONGOLIAN GERBIL FREQUENCY HEARING CHARACTERISTICS
 Supplemental materials to Frequency sensitivity in mammalian hearing from a fundamental nonlinear physics model of the inner ear, by Kanders, K., Lorimer, T., Gomez, F. & Stoop, R. -6-5 sensitivity (db)
Supplemental materials to Frequency sensitivity in mammalian hearing from a fundamental nonlinear physics model of the inner ear, by Kanders, K., Lorimer, T., Gomez, F. & Stoop, R. -6-5 sensitivity (db)
Intro to Audition & Hearing
 Intro to Audition & Hearing Lecture 16 Chapter 9, part II Jonathan Pillow Sensation & Perception (PSY 345 / NEU 325) Fall 2017 1 Sine wave: one of the simplest kinds of sounds: sound for which pressure
Intro to Audition & Hearing Lecture 16 Chapter 9, part II Jonathan Pillow Sensation & Perception (PSY 345 / NEU 325) Fall 2017 1 Sine wave: one of the simplest kinds of sounds: sound for which pressure
to vibrate the fluid. The ossicles amplify the pressure. The surface area of the oval window is
 Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
Frequency refers to how often something happens. Period refers to the time it takes something to happen.
 Lecture 2 Properties of Waves Frequency and period are distinctly different, yet related, quantities. Frequency refers to how often something happens. Period refers to the time it takes something to happen.
Lecture 2 Properties of Waves Frequency and period are distinctly different, yet related, quantities. Frequency refers to how often something happens. Period refers to the time it takes something to happen.
The Structure and Function of the Auditory Nerve
 The Structure and Function of the Auditory Nerve Brad May Structure and Function of the Auditory and Vestibular Systems (BME 580.626) September 21, 2010 1 Objectives Anatomy Basic response patterns Frequency
The Structure and Function of the Auditory Nerve Brad May Structure and Function of the Auditory and Vestibular Systems (BME 580.626) September 21, 2010 1 Objectives Anatomy Basic response patterns Frequency
Detection of Cochlear Amplification and Its Activation
 Biophysical Journal Volume 5 August 23 67 78 67 Detection of Cochlear Amplification and Its Activation Wei Dong and Elizabeth S. Olson * Otolaryngology, Head and Neck Surgery and Biomedical Engineering,
Biophysical Journal Volume 5 August 23 67 78 67 Detection of Cochlear Amplification and Its Activation Wei Dong and Elizabeth S. Olson * Otolaryngology, Head and Neck Surgery and Biomedical Engineering,
Lecture 6 Hearing 1. Raghav Rajan Bio 354 Neurobiology 2 January 28th All lecture material from the following links unless otherwise mentioned:
 Lecture 6 Hearing 1 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
Lecture 6 Hearing 1 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
In Vivo Impedance of the Gerbil Cochlear Partition at Auditory Frequencies
 Biophysical Journal Volume 97 September 2009 1233 1243 1233 In Vivo Impedance of the Gerbil Cochlear Partition at Auditory Frequencies Wei Dong * and Elizabeth S. Olson Department of Otolaryngology, Head
Biophysical Journal Volume 97 September 2009 1233 1243 1233 In Vivo Impedance of the Gerbil Cochlear Partition at Auditory Frequencies Wei Dong * and Elizabeth S. Olson Department of Otolaryngology, Head
A cochlear AGC model, proposing a new type of cochlear non-linearity
 TEL-AVIV UNIVERSITY The Iby and Aladar Fleischman faculty of engineering The Zandman-Slaner school of Graduate studies A cochlear AGC model, proposing a new type of cochlear non-linearity A thesis submitted
TEL-AVIV UNIVERSITY The Iby and Aladar Fleischman faculty of engineering The Zandman-Slaner school of Graduate studies A cochlear AGC model, proposing a new type of cochlear non-linearity A thesis submitted
Academic and Research Staff Professor Dennis M. Freeman, Dr. Alexander Aranyosi, Dr. Stanley Hong
 Cochlear Mechanics Academic and Research Staff Professor Dennis M. Freeman, Dr. Alexander Aranyosi, Dr. Stanley Hong Graduate Students Chris Bergevin, Roozbeh Ghaffari, Wendy Gu, Kinuko Masaki, Scott Page
Cochlear Mechanics Academic and Research Staff Professor Dennis M. Freeman, Dr. Alexander Aranyosi, Dr. Stanley Hong Graduate Students Chris Bergevin, Roozbeh Ghaffari, Wendy Gu, Kinuko Masaki, Scott Page
Acoustics, signals & systems for audiology. Psychoacoustics of hearing impairment
 Acoustics, signals & systems for audiology Psychoacoustics of hearing impairment Three main types of hearing impairment Conductive Sound is not properly transmitted from the outer to the inner ear Sensorineural
Acoustics, signals & systems for audiology Psychoacoustics of hearing impairment Three main types of hearing impairment Conductive Sound is not properly transmitted from the outer to the inner ear Sensorineural
Auditory Periphery! external middle inner. stapes movement initiates a pressure wave in cochlear fluid
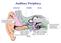 Auditory Periphery! external middle inner sound causes air pressure to increase at eardrum stapes movement initiates a pressure wave in cochlear fluid VIIIth nerve conveys neural signal to cochlear nucleus
Auditory Periphery! external middle inner sound causes air pressure to increase at eardrum stapes movement initiates a pressure wave in cochlear fluid VIIIth nerve conveys neural signal to cochlear nucleus
Can You Hear Me Now?
 An Introduction to the Mathematics of Hearing Department of Applied Mathematics University of Washington April 26, 2007 Some Questions How does hearing work? What are the important structures and mechanisms
An Introduction to the Mathematics of Hearing Department of Applied Mathematics University of Washington April 26, 2007 Some Questions How does hearing work? What are the important structures and mechanisms
MECHANISM OF HEARING
 MECHANISM OF HEARING Sound: Sound is a vibration that propagates as an audible wave of pressure, through a transmission medium such as gas, liquid or solid. Sound is produced from alternate compression
MECHANISM OF HEARING Sound: Sound is a vibration that propagates as an audible wave of pressure, through a transmission medium such as gas, liquid or solid. Sound is produced from alternate compression
SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS
 SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS Katherine Knisely, D. Bozovic University of California at Los Angeles It has long been observed that there is some form of amplification
SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS Katherine Knisely, D. Bozovic University of California at Los Angeles It has long been observed that there is some form of amplification
College of Medicine Dept. of Medical physics Physics of ear and hearing /CH
 College of Medicine Dept. of Medical physics Physics of ear and hearing /CH 13 2017-2018 ***************************************************************** o Introduction : The ear is the organ that detects
College of Medicine Dept. of Medical physics Physics of ear and hearing /CH 13 2017-2018 ***************************************************************** o Introduction : The ear is the organ that detects
Receptors / physiology
 Hearing: physiology Receptors / physiology Energy transduction First goal of a sensory/perceptual system? Transduce environmental energy into neural energy (or energy that can be interpreted by perceptual
Hearing: physiology Receptors / physiology Energy transduction First goal of a sensory/perceptual system? Transduce environmental energy into neural energy (or energy that can be interpreted by perceptual
Auditory Physiology PSY 310 Greg Francis. Lecture 29. Hearing
 Auditory Physiology PSY 310 Greg Francis Lecture 29 A dangerous device. Hearing The sound stimulus is changes in pressure The simplest sounds vary in: Frequency: Hertz, cycles per second. How fast the
Auditory Physiology PSY 310 Greg Francis Lecture 29 A dangerous device. Hearing The sound stimulus is changes in pressure The simplest sounds vary in: Frequency: Hertz, cycles per second. How fast the
PSY 310: Sensory and Perceptual Processes 1
 Auditory Physiology PSY 310 Greg Francis Lecture 29 A dangerous device. Hearing The sound stimulus is changes in pressure The simplest sounds vary in: Frequency: Hertz, cycles per second. How fast the
Auditory Physiology PSY 310 Greg Francis Lecture 29 A dangerous device. Hearing The sound stimulus is changes in pressure The simplest sounds vary in: Frequency: Hertz, cycles per second. How fast the
Hearing. istockphoto/thinkstock
 Hearing istockphoto/thinkstock Audition The sense or act of hearing The Stimulus Input: Sound Waves Sound waves are composed of changes in air pressure unfolding over time. Acoustical transduction: Conversion
Hearing istockphoto/thinkstock Audition The sense or act of hearing The Stimulus Input: Sound Waves Sound waves are composed of changes in air pressure unfolding over time. Acoustical transduction: Conversion
Week 2 Systems (& a bit more about db)
 AUDL Signals & Systems for Speech & Hearing Reminder: signals as waveforms A graph of the instantaneousvalue of amplitude over time x-axis is always time (s, ms, µs) y-axis always a linear instantaneousamplitude
AUDL Signals & Systems for Speech & Hearing Reminder: signals as waveforms A graph of the instantaneousvalue of amplitude over time x-axis is always time (s, ms, µs) y-axis always a linear instantaneousamplitude
SPECIAL SENSES: THE AUDITORY SYSTEM
 SPECIAL SENSES: THE AUDITORY SYSTEM REVISION OF PHYSICS: WAVES A wave is an oscillation of power, sound waves have two main characteristics: amplitude, which is the maximum displacement or the power of
SPECIAL SENSES: THE AUDITORY SYSTEM REVISION OF PHYSICS: WAVES A wave is an oscillation of power, sound waves have two main characteristics: amplitude, which is the maximum displacement or the power of
Chapter 3. Sounds, Signals, and Studio Acoustics
 Chapter 3 Sounds, Signals, and Studio Acoustics Sound Waves Compression/Rarefaction: speaker cone Sound travels 1130 feet per second Sound waves hit receiver Sound waves tend to spread out as they travel
Chapter 3 Sounds, Signals, and Studio Acoustics Sound Waves Compression/Rarefaction: speaker cone Sound travels 1130 feet per second Sound waves hit receiver Sound waves tend to spread out as they travel
THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS?
 JOURNAL OF OTOLOGY THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS? LI Yi 1, He David Z 2 Abstract Cochlear outer hair cells (OHCs) are involved in a mechanical feedback loop in which
JOURNAL OF OTOLOGY THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS? LI Yi 1, He David Z 2 Abstract Cochlear outer hair cells (OHCs) are involved in a mechanical feedback loop in which
9.01 Introduction to Neuroscience Fall 2007
 MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
Issues faced by people with a Sensorineural Hearing Loss
 Issues faced by people with a Sensorineural Hearing Loss Issues faced by people with a Sensorineural Hearing Loss 1. Decreased Audibility 2. Decreased Dynamic Range 3. Decreased Frequency Resolution 4.
Issues faced by people with a Sensorineural Hearing Loss Issues faced by people with a Sensorineural Hearing Loss 1. Decreased Audibility 2. Decreased Dynamic Range 3. Decreased Frequency Resolution 4.
Microstructures in the Organ of Corti Help Outer Hair Cells Form Traveling Waves along the Cochlear Coil
 2426 Biophysical Journal Volume 106 June 2014 2426 2433 Microstructures in the Organ of Corti Help Outer Hair Cells Form Traveling Waves along the Cochlear Coil Jong-Hoon Nam* Department of Mechanical
2426 Biophysical Journal Volume 106 June 2014 2426 2433 Microstructures in the Organ of Corti Help Outer Hair Cells Form Traveling Waves along the Cochlear Coil Jong-Hoon Nam* Department of Mechanical
Study the Effect of the Quality Factor of the Transient Evoked Oto-acoustic Emissions (TEOAE)
 Research in Otolaryngology 217, 6(4): 47-4 DOI:.923/j.otolaryn.21764.1 Study the Effect of the Quality Factor of the Transient Evoked Oto-acoustic Emissions (TEOAE) Adnan AL-Maamury *, Dhifaf Ahmed Al-Mustansiriyah
Research in Otolaryngology 217, 6(4): 47-4 DOI:.923/j.otolaryn.21764.1 Study the Effect of the Quality Factor of the Transient Evoked Oto-acoustic Emissions (TEOAE) Adnan AL-Maamury *, Dhifaf Ahmed Al-Mustansiriyah
Chapter 13 Physics of the Ear and Hearing
 Hearing 100 times greater dynamic range than vision Wide frequency range (20 ~ 20,000 Hz) Sense of hearing Mechanical system that stimulates the hair cells in the cochlea Sensors that produce action potentials
Hearing 100 times greater dynamic range than vision Wide frequency range (20 ~ 20,000 Hz) Sense of hearing Mechanical system that stimulates the hair cells in the cochlea Sensors that produce action potentials
Trajectory of the Aging Cochlea
 Trajectory of the Aging Cochlea Sumitrajit (Sumit) Dhar Professor & Chair Roxelyn & Richard Pepper Department of Communication Sciences and Disorders Fellow, Hugh Knowles Center for Hearing Science Northwestern
Trajectory of the Aging Cochlea Sumitrajit (Sumit) Dhar Professor & Chair Roxelyn & Richard Pepper Department of Communication Sciences and Disorders Fellow, Hugh Knowles Center for Hearing Science Northwestern
EMANATIONS FROM RESIDUUM OSCILLATIONS IN HUMAN AUDITORY SYSTEM
 EMANATIONS FROM RESIDUUM OSCILLATIONS IN HUMAN AUDITORY SYSTEM V.S. Balaji, N.R.Raajan, S. Rakesh Kumar, Har Narayan Upadhyay School of Electrical & Electronics Engineering, SASTRA University Thanjavur,
EMANATIONS FROM RESIDUUM OSCILLATIONS IN HUMAN AUDITORY SYSTEM V.S. Balaji, N.R.Raajan, S. Rakesh Kumar, Har Narayan Upadhyay School of Electrical & Electronics Engineering, SASTRA University Thanjavur,
HEARING AND PSYCHOACOUSTICS
 CHAPTER 2 HEARING AND PSYCHOACOUSTICS WITH LIDIA LEE I would like to lead off the specific audio discussions with a description of the audio receptor the ear. I believe it is always a good idea to understand
CHAPTER 2 HEARING AND PSYCHOACOUSTICS WITH LIDIA LEE I would like to lead off the specific audio discussions with a description of the audio receptor the ear. I believe it is always a good idea to understand
PSY 215 Lecture 10 Topic: Hearing Chapter 7, pages
 PSY 215 Lecture 10 Topic: Hearing Chapter 7, pages 189-197 Corrections: NTC 09-1, page 3, the Superior Colliculus is in the midbrain (Mesencephalon). Announcements: Movie next Monday: Case of the frozen
PSY 215 Lecture 10 Topic: Hearing Chapter 7, pages 189-197 Corrections: NTC 09-1, page 3, the Superior Colliculus is in the midbrain (Mesencephalon). Announcements: Movie next Monday: Case of the frozen
Sound. Audition. Physics of Sound. Properties of sound. Perception of sound works the same way as light.
 Sound Audition Perception of sound works the same way as light. Have receptors to convert a physical stimulus to action potentials Action potentials are organized in brain structures You apply some meaning
Sound Audition Perception of sound works the same way as light. Have receptors to convert a physical stimulus to action potentials Action potentials are organized in brain structures You apply some meaning
Audition. Sound. Physics of Sound. Perception of sound works the same way as light.
 Audition Sound Perception of sound works the same way as light. Have receptors to convert a physical stimulus to action potentials Action potentials are organized in brain structures You apply some meaning
Audition Sound Perception of sound works the same way as light. Have receptors to convert a physical stimulus to action potentials Action potentials are organized in brain structures You apply some meaning
What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components
 1 2 3 4 What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components as a function of time here during the production
1 2 3 4 What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components as a function of time here during the production
OtoAcoustic Emissions (OAE s)
 OtoAcoustic Emissions (OAE s) Phenomenon and applications in audiological diagnostics Measurement procedures TEOAE and DPOAE Physiological backgound, functional models Acknowledgment: several illustrations
OtoAcoustic Emissions (OAE s) Phenomenon and applications in audiological diagnostics Measurement procedures TEOAE and DPOAE Physiological backgound, functional models Acknowledgment: several illustrations
Chapter 1: Introduction to digital audio
 Chapter 1: Introduction to digital audio Applications: audio players (e.g. MP3), DVD-audio, digital audio broadcast, music synthesizer, digital amplifier and equalizer, 3D sound synthesis 1 Properties
Chapter 1: Introduction to digital audio Applications: audio players (e.g. MP3), DVD-audio, digital audio broadcast, music synthesizer, digital amplifier and equalizer, 3D sound synthesis 1 Properties
What Drives Mechanical Amplification in the Mammalian Cochlea?
 What Drives Mechanical Amplification in the Mammalian Cochlea? Robert H. Withnell, Lauren A. Shaffer, and David J. Lilly The recent report by Peter Dallos and colleagues of the gene and protein responsible
What Drives Mechanical Amplification in the Mammalian Cochlea? Robert H. Withnell, Lauren A. Shaffer, and David J. Lilly The recent report by Peter Dallos and colleagues of the gene and protein responsible
Printable version - Hearing - OpenLearn - The Open University
 Skip to content Accessibility Sign in Contact Search the OU The Open University Study at the OU Research at the OU OU Community About the OU Hearing Printable page generated Saturday, 12 November 2011,
Skip to content Accessibility Sign in Contact Search the OU The Open University Study at the OU Research at the OU OU Community About the OU Hearing Printable page generated Saturday, 12 November 2011,
Sound and Hearing. Decibels. Frequency Coding & Localization 1. Everything is vibration. The universe is made of waves.
 Frequency Coding & Localization 1 Sound and Hearing Everything is vibration The universe is made of waves db = 2log(P1/Po) P1 = amplitude of the sound wave Po = reference pressure =.2 dynes/cm 2 Decibels
Frequency Coding & Localization 1 Sound and Hearing Everything is vibration The universe is made of waves db = 2log(P1/Po) P1 = amplitude of the sound wave Po = reference pressure =.2 dynes/cm 2 Decibels
Comment by Delgutte and Anna. A. Dreyer (Eaton-Peabody Laboratory, Massachusetts Eye and Ear Infirmary, Boston, MA)
 Comments Comment by Delgutte and Anna. A. Dreyer (Eaton-Peabody Laboratory, Massachusetts Eye and Ear Infirmary, Boston, MA) Is phase locking to transposed stimuli as good as phase locking to low-frequency
Comments Comment by Delgutte and Anna. A. Dreyer (Eaton-Peabody Laboratory, Massachusetts Eye and Ear Infirmary, Boston, MA) Is phase locking to transposed stimuli as good as phase locking to low-frequency
FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS. Hans P. Zenner, Ulrike Zimmermann and Alfred H. Gitter
 Vol. 149,No. 1, 1987 November 30, 1987 BlOCHEMlCALANDBlOPHYSlCALRESEARCHCOMMUNlCATlONS Pages 304-308 FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS Hans P. Zenner, Ulrike Zimmermann and Alfred
Vol. 149,No. 1, 1987 November 30, 1987 BlOCHEMlCALANDBlOPHYSlCALRESEARCHCOMMUNlCATlONS Pages 304-308 FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS Hans P. Zenner, Ulrike Zimmermann and Alfred
HEARING. Structure and Function
 HEARING Structure and Function Rory Attwood MBChB,FRCS Division of Otorhinolaryngology Faculty of Health Sciences Tygerberg Campus, University of Stellenbosch Analyse Function of auditory system Discriminate
HEARING Structure and Function Rory Attwood MBChB,FRCS Division of Otorhinolaryngology Faculty of Health Sciences Tygerberg Campus, University of Stellenbosch Analyse Function of auditory system Discriminate
Effects of Remaining Hair Cells on Cochlear Implant Function
 Effects of Remaining Hair Cells on Cochlear Implant Function N1-DC-2-15QPR1 Neural Prosthesis Program N. Hu, P.J. Abbas, C.A. Miller, B.K. Robinson, K.V. Nourski, F. Jeng, B.A. Abkes, J.M. Nichols Department
Effects of Remaining Hair Cells on Cochlear Implant Function N1-DC-2-15QPR1 Neural Prosthesis Program N. Hu, P.J. Abbas, C.A. Miller, B.K. Robinson, K.V. Nourski, F. Jeng, B.A. Abkes, J.M. Nichols Department
The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992)
 The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992) by Pedro da Fonseca (pedrofon@mail.telepac.pt) Neuroscience course Presented in 17.12.99 to professor STEPHEN
The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992) by Pedro da Fonseca (pedrofon@mail.telepac.pt) Neuroscience course Presented in 17.12.99 to professor STEPHEN
Nonlinear Cochlear Signal Processing
 Nonlinear Cochlear Signal Processing Jont B. Allen Florham Park, NJ March 13, 2001 Contents 1 Macromechanics 5 1.1 The early history of cochlear modeling...................... 6 1.2 The 1 model of the
Nonlinear Cochlear Signal Processing Jont B. Allen Florham Park, NJ March 13, 2001 Contents 1 Macromechanics 5 1.1 The early history of cochlear modeling...................... 6 1.2 The 1 model of the
Physiological basis of sound design. Prof. Dr. med. Eckhard Hoffmann Dipl.-Ing. (FH) Steffen Kreikemeier Aalen University of Applied Sciences
 Physiological basis of sound design Prof. Dr. med. Eckhard Hoffmann Dipl.-Ing. (FH) Steffen Kreikemeier Aalen University of Applied Sciences Index of contents Physiological basis of the inner ear Organ
Physiological basis of sound design Prof. Dr. med. Eckhard Hoffmann Dipl.-Ing. (FH) Steffen Kreikemeier Aalen University of Applied Sciences Index of contents Physiological basis of the inner ear Organ
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium!
 Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! Chapter 17 Part 2 Special Senses! The Special Senses! Hearing and Equilibrium!
 Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Ganglion Cells Blind Spot Cornea Pupil Visual Area of the Bipolar Cells Thalamus Rods and Cones Lens Visual cortex of the occipital lobe
 How We See How We See Cornea Ganglion Cells whose axons form the optic nerve Blind Spot the exit point at the back of the retina Pupil which is controlled by the iris Bipolar Cells Visual Area of the Thalamus
How We See How We See Cornea Ganglion Cells whose axons form the optic nerve Blind Spot the exit point at the back of the retina Pupil which is controlled by the iris Bipolar Cells Visual Area of the Thalamus
Chapter Fourteen. The Hearing Mechanism. 1. Introduction.
 Chapter Fourteen The Hearing Mechanism 1. Introduction. 2. Hearing. 3. The Ear. 4. The External Ear. 5. The Inner Ear. 6. Frequency Discrimination. 7. The Organ of Corti. 8. Tests and Exrecises. 9. References.
Chapter Fourteen The Hearing Mechanism 1. Introduction. 2. Hearing. 3. The Ear. 4. The External Ear. 5. The Inner Ear. 6. Frequency Discrimination. 7. The Organ of Corti. 8. Tests and Exrecises. 9. References.
Effects of Medial Olivocochlear Auditory Reflex Activation on Cochlear Vibration
 University of Iowa Honors Theses University of Iowa Honors Program Spring 2017 Effects of Medial Olivocochlear Auditory Reflex Activation on Cochlear Vibration Alexandra Redfern Shawn Goodman University
University of Iowa Honors Theses University of Iowa Honors Program Spring 2017 Effects of Medial Olivocochlear Auditory Reflex Activation on Cochlear Vibration Alexandra Redfern Shawn Goodman University
Vision and Audition. This section concerns the anatomy of two important sensory systems, the visual and the auditory systems.
 Vision and Audition Vision and Audition This section concerns the anatomy of two important sensory systems, the visual and the auditory systems. The description of the organization of each begins with
Vision and Audition Vision and Audition This section concerns the anatomy of two important sensory systems, the visual and the auditory systems. The description of the organization of each begins with
Feed-Forward and Feed-Backward Amplification Model from Cochlear Cytoarchitecture: An Interspecies Comparison
 Biophysical Journal Volume 100 January 2011 1 10 1 Feed-Forward and Feed-Backward Amplification Model from Cochlear Cytoarchitecture: An Interspecies Comparison Yong-Jin Yoon, Charles R. Steele, and Sunil
Biophysical Journal Volume 100 January 2011 1 10 1 Feed-Forward and Feed-Backward Amplification Model from Cochlear Cytoarchitecture: An Interspecies Comparison Yong-Jin Yoon, Charles R. Steele, and Sunil
Efferent-mediated control of basilar membrane motion
 J Physiol 576. (2006) pp 49 54 49 Topical Review Efferent-mediated control of basilar membrane motion N. P. Cooper and J. J. Guinan Jr 2 School of Life Sciences, Keele University, Keele, Staffordshire
J Physiol 576. (2006) pp 49 54 49 Topical Review Efferent-mediated control of basilar membrane motion N. P. Cooper and J. J. Guinan Jr 2 School of Life Sciences, Keele University, Keele, Staffordshire
