In vivo outer hair cell length changes expose the active process in the cochlea
|
|
|
- Kelly Carroll
- 6 years ago
- Views:
Transcription
1 In vivo outer hair cell length changes expose the active process in the cochlea Dingjun Zha, Fangyi Chen, Sripriya Ramamoorthy, Anders Fridberger, Niloy Choudhury, Steven L Jacques, Ruikang K Wang and Alfred L Nuttall Linköping University Post Print N.B.: When citing this work, cite the original article. Original Publication: Dingjun Zha, Fangyi Chen, Sripriya Ramamoorthy, Anders Fridberger, Niloy Choudhury, Steven L Jacques, Ruikang K Wang and Alfred L Nuttall, In vivo outer hair cell length changes expose the active process in the cochlea, 2012, PloS one, (7), 4, e Copyright: Public Library of Science Postprint available at: Linköping University Electronic Press
2 In Vivo Outer Hair Cell Length Changes Expose the Active Process in the Cochlea Dingjun Zha 1,2., Fangyi Chen 1 *., Sripriya Ramamoorthy 1, Anders Fridberger 6,1, Niloy Choudhury 7, Steven L. Jacques 4,3, Ruikang K. Wang 5, Alfred L. Nuttall 1,3,8 1 Oregon Hearing Research Center, Oregon Health and Science University, Portland, Oregon, United States of America, 2 Department of Otolaryngology/Head and Neck Surgery, Xijing Hospital, Fourth Military Medical University, Shaanxi, People s Republic of China, 3 Department of Biomedical Engineering, Oregon Health and Science University, Portland, Oregon, United States of America, 4 Department of Dermatology, Oregon Health and Science University, Portland, Oregon, United States of America, 5 Department of Bioengineering, University of Washington, Seattle, Washington, United States of America, 6 Karolinska Institutet, Center for Hearing and Communication Research, Department of Clinical Science, Intervention, and Technology, M1 Karolinska University Hospital, Stockholm, Sweden, 7 Department of Biomedical Engineering, Michigan Technological University, Houghton, Michigan, United States of America, 8 Kresge Hearing Research Institute, The University of Michigan, Ann Arbor, Michigan, United States of America Abstract Background: Mammalian hearing is refined by amplification of the sound-evoked vibration of the cochlear partition. This amplification is at least partly due to forces produced by protein motors residing in the cylindrical body of the outer hair cell. To transmit power to the cochlear partition, it is required that the outer hair cells dynamically change their length, in addition to generating force. These length changes, which have not previously been measured in vivo, must be correctly timed with the acoustic stimulus to produce amplification. Methodology/Principal Findings: Using in vivo optical coherence tomography, we demonstrate that outer hair cells in living guinea pigs have length changes with unexpected timing and magnitudes that depend on the stimulus level in the sensitive cochlea. Conclusions/Significance: The level-dependent length change is a necessary condition for directly validating that power is expended by the active process presumed to underlie normal hearing. Citation: Zha D, Chen F, Ramamoorthy S, Fridberger A, Choudhury N, et al. (2012) In Vivo Outer Hair Cell Length Changes Expose the Active Process in the Cochlea. PLoS ONE 7(4): e doi: /journal.pone Editor: Melissa J. Coleman, Claremont Colleges, United States of America Received November 23, 2011; Accepted January 30, 2012; Published April 9, 2012 Copyright: ß 2012 Zha et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: Supported by National Institute on Deafness and Other Communicable Disorders grants R01 DC (ALN) and DC (ALN), Swedish Research Counctil grant K X (AF), grants from the Tysta Skolan foundation and Hörselskadades Riksförbund (AF) and supported by Chinese National Nature Science Foundation, (DZ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Competing Interests: The authors have declared that no competing interests exist. * chenfa@ohsu.edu. These authors contributed equally to this work. Introduction The remarkable sensitivity and frequency resolution of mammalian hearing organs [1,2,3,4,5] stems from the fast motility of outer hair cells (OHCs) [6,7,8]. When the cochlea is functioning normally, this motility may boost basilar membrane (BM) vibrations by several tens of decibels [9,10], by adding energy into the sound-evoked motion. The central element of this process are the OHCs, which are capable of electrical-to-mechanical transduction, e.g. alterations in their membrane potential, induced by the gating of mechanically sensitive ion channels, leading to changes in cell length [6,11]. This process has been observed in vitro in isolated OHCs [6,12] and in situ in the low-frequency regions of cochlear explants [7] and recently published measurements suggest that length changes also occur in vivo [13].To be effective in augmenting sound-evoked vibrations in vivo, the length changes must be correctly synchronized to the acoustic stimulus. The most widely held hypothesis, as stated by Gummer et al. [14], suggests that amplification occurs if maximal OHC contraction coincides with maximal BM velocity in the direction of scala vestibuli. Other timing relationships are thought to cause smaller amplification or even attenuation. Indirect experimental evidence for OHC electromotility in vivo comes from the electrically driven salicylate-sensitive BM velocity measured up to 100 khz in guinea pigs [15]. However, direct characterization for the OHC length change in vivo is still lacking. Apart from their length change, isolated OHCs also demonstrate a voltage-dependent change in stiffness [16]. If the OHC stiffness change in vivo were larger than what is demonstrated in the isolated cells, such a phasic modulation could lead to amplified soundevoked movements in the absence of large length changes. To understand how the vibration of the cochlear partition is boosted in vivo, it is therefore important to determine amplitude and timing of OHC length changes relative to the sound stimulus. In this report, we focus on the measurement and direct characterization of the OHC length-change in vivo over a wide range of stimulus levels. PLoS ONE 1 April 2012 Volume 7 Issue 4 e32757
3 OHCs occupy a mechanically privileged place in the inner ear, connecting the reticular lamina (RL) with the BM through the body of the Deiters cells [17,18]. In this connection, the OHC is the only cell with fast motility and capacity for significant change of cell length [19,20,21]. Thus, should OHCs have sufficient strength to act on the impedances of the organ of Corti complex, they will move the RL and the BM independently in vivo. To directly measure OHCs length changes, we used an optical coherence tomography system [13,22,23] that allows imaging and vibration measurements from both the BM and the RL in living anaesthetized guinea pigs. We show that OHCs length changes indeed exist in the sensitive cochlea, that these length changes depend on the sound stimulus level, and that the polarity of the length change differs from accepted theory. These salient features of OHCs in vivo provide experimental evidence for mechanical power generation by OHCs and show that the current models of hearing organ function may need to be revised. Results Data collection and consistency of the data In this study of the dynamic length of OHCs, we first obtained an organ of Corti image with recognizable cellular morphology using the imaging mode of the optical coherence tomography system. From such images, the angle between the cell body of the OHCs and the optical axis can be determined (Fig. 1a, b, see Methods), which made it possible to determine the appropriate measurement locations for the RL and BM. Thirty-three animals were used in this study, but of the 33, 17 were excluded because surgical trauma caused more than 15 db loss of auditory sensitivity. Incomplete data were collected from an additional 10 cochleae, where the auditory sensitivity declined during data acquisition, leaving six preparations, where a complete data set was acquired. The present report is based on data from these six preparations. Difference of sound-induced vibration of the BM and RL In the sensitive cochlea, BM vibrations are highly frequencytuned (Fig. 2a). At low levels of stimulation, a distinct peak, near 20 khz in this example, is flanked by rapidly decaying segments on either side. As the stimulus level increases, the peak moves toward lower frequencies, but the response amplitude does not grow in proportion to the increase of the stimulus level. This nonlinearity characterizes the sensitive cochlea and has been described in many previous studies [3,4,24]. At the RL, displacements are also highly tuned, but they have a greater magnitude than those of the BM. At 30 db SPL, peak RL displacements are a factor two larger than those of the BM, a difference that becomes smaller as the stimulus level increases (Fig. 2b). This difference in magnitude is only seen in sensitive ears and disappears following the death of the animal (cf. Fig. 2c and d). Note that the level-dependent shift in the position of the peak and the nonlinear increase of the response magnitude are absent postmortem (Fig. 2c and d). To illustrate these differences, we plot displacement magnitudes as a function of stimulus intensity in Fig. 3a (mean and standard errors from the six sensitive preparations). At 30 db SPL and at the best frequency, RL vibrations are a factor larger than the BM (n = 6, p?0.05). When the stimulus level increases, the difference becomes smaller, but it remains statistically significant at 100 db SPL ( , p,0.05). The two structures also show a pronounced level-dependent alteration in response phase. At low levels, RL vibrations phase-lead the BM, but this difference in response timing becomes less pronounced as the stimulus Figure 1. The cochlea structure and function. a. The illustrated organ of Corti in cross-section. b. An OCT image of the organ of Corti in vivo. Circles mark the locations of vibration measurement. c. A cartoon of hair cell excitation without OHC length change. d. A cartoon showing that when depolarized, OHCs contract to become shorter in length. This will draw together the reticular lamina and basilar membrane; IHC, inner hair cell; OHC, outer hair cell; PCs, pillar cells. doi: /journal.pone g001 PLoS ONE 2 April 2012 Volume 7 Issue 4 e32757
4 Figure 2. Vibration magnitude spectra of the basilar membrane (BM, -panels a, c) and reticular lamina (RL, -panels b, d) in a sensitive guinea pig cochlea and a postmortem guinea pig cochlea. Displacement magnitudes of vibration in nm are plotted as a function of stimulus frequency with sound level as the parameter. For these data, an auditory sensitivity loss of 12 db is caused by the surgical preparation. Numbers against each curve represent sound levels of db SPL at the eardrum used to stimulate the hearing organ vibration. In living animals (a, b), at 30 db SPL, the maximum amplitude of BM vibration is 0.05 nm, the maximum displacement amplitude RL of is 0.1 nm at the best frequency of 20 khz. In the postmortem animal(c, d), at 70 db SPL, the maximum amplitude of BM vibration is 0.52 nm, the maximum displacement amplitude of the RL is 0.51 nm. doi: /journal.pone g002 intensity increases (Fig. 3c). Postmortem, vibrations are much smaller, grow linearly with stimulus intensity, and different structures within the organ of Corti have nearly identical vibration amplitudes and phases (Fig. 3b and d, n = 4). Given the cochlear sensitivity to trauma and the well-known disappearance of the active mechanism following death, the most parsimonious explanation of these data is that motility of the OHCs is the primary cause of the displacement difference between RL and BM. Frequency dependence and nonlinearity of OHC length change The displacement data can be used to obtain a measure of OHC length changes, since the Deiters cells that connect the OHCs to the BM are very stiff and lack capacity for rapid movements [19,20,21]. The length change of the OHC depends on both stimulus intensity and frequency. At low levels, displacements peak near 20 khz; the peak moves to lower frequencies as the stimulus level increases (Fig. 4a). This behavior PLoS ONE 3 April 2012 Volume 7 Issue 4 e32757
5 Figure 3. Displacement magnitude difference (a, b) and the phase difference(c, d) as a function of the sound level between the basilar membrane (BM) and reticular lamina (RL). The mean 6 standard error of the BM vibration magnitude (solid lines) and the RL displacement (dashed lines) and the displacement difference between the RL and the BM as a function of sound level from live animals (a) and postmortem animals (b); The phase difference between the RL and the BM magnitude and the phase as a function of the sound level from live animals (c) and postmortem animals (d). In order to group the animals, the frequencies were normalized with respect to the best frequency, which varied among the animals at the chosen measurement locations. This compensates for the slight variations in the best frequencies in the different experiments (best frequency range, khz). BF P is around 15 khz at postmortem in the experiments. doi: /journal.pone g003 is similar to the one observed at the RL. However, when plotting OHC length change at the best frequency as a function of stimulus level, a nonlinearity stronger than either the RL or BM becomes apparent (Fig. 4b, see also Fig. 5a and b). At and above the CF, OHC length changes saturate at around 70 db SPL and then show a slight tendency to decline at the highest levels tested. The pronounced compression lends further support to the idea that OHC length change is a major factor in the nonlinearity of the cochlea [25]. Visualizing the timing of OHCs length change within the organ of Corti To illustrate the relationship between OHC length changes and other structures in the organ of Corti, the instantaneous values of RL, BM and OHC length changes are plotted as functions of time for BF tones at 30 db SPL (Fig. 6a), 40 db SPL (Fig. 6b), 90 db SPL (Fig. 6c) for the sensitive animal and at 70 db SPL for the postmortem animal (Fig. 6d). This information can also be equivalently represented by the relative phase (and magnitude) between the three structures at a given frequency. To distinguish the phase lead from the phase lag, it is important to note the wave j(v tzq) sign convention. We use e in our measurements and analysis, where positive Q is the phase lead. The RL and the BM displacements as a function of time over one cycle in Fig. 6 are generated with the equation a~a cos (2pftzQ) ð1þ The amplitude value A is given by the RL or BM value from one sensitive animal. For the RL, Q is the phase lead of RL displacement relative to the BM displacement (hence Q is zero for the BM; this analysis neglects possible radial motion components as these have previously been found to be small relative to the vertical motion [26]). OHC contraction is the difference between the BM and the RL displacements towards SV. The X-axis indicates one cycle. To be meaningful, data in this figure must be interpreted in the context of organ of Corti structure. The RL and the BM can be considered as approximately parallel beams (Fig. 1). As seen in Figs. 2 and 3, the RL displacements are larger than those of the BM. Consequently, the RL will approach the BM during motion directed at scala tympani, which implies OHC shortening (Fig. 6a, arrow pointed at the OHC length change curve, note that positive displacement in this figure means motion directed at the scala vestibuli for the BM and the RL and an elongation for the OHCs). The differences in phase mean that the OHCs are shortest when the BM moves with greatest velocity in the direction of the scala tympani (Fig. 6a). These phase relations depend on the stimulus level: phase differences decreases as the sound level increases, and at 90 db SPL, the phase difference between the OHC contraction and the BM displacement decreases to 32u (Fig. 6c). The length changes also depend on the physiological state of the animal. Postmortem, the OHC length changes are small, and both the RL and the BM moved in phase (Fig. 6d). These observations are inconsistent with the established theory and may therefore seem counter-intuitive. The geometric concept PLoS ONE 4 April 2012 Volume 7 Issue 4 e32757
6 the OHC length changes are very similar in magnitude to the RL vibration at low levels, but show more pronounced saturation than either of the two structures. This behavior is also apparent in the normalized data presented in Fig. 5c and d. The normalized OHC length change is the ratio between the OHC length change and the BM or the RL displacement. Fig. 5c demonstrates that the OHC contraction relative to the BM displacement decreases with the sound level. The relationship is responsible for the apparent decrease in cochlear amplification gain that occurs with increasing sound level. The maximum OHC contraction is about two times the displacement of the BM at 30 db SPL, decreasing to about 0.8 at 90 db SPL. At 40 db SPL, the maximum OHC contraction is 2.0 nm at 20 khz (CF). Fig. 5b and d demonstrates that the OHC contraction relative to the RL decreases from 0.9 at 30 db SPL to 0.4 at 90 db SPL. Figure 4. Outer Hair Cell length change (a) as a function of the frequency in a sensitive guinea pig cochlea (hearing loss,10 db), (b) as a function of the sound level from the six sensitive animals. doi: /journal.pone g004 of organ of Corti mechanics is that the RL motion directed toward the scala vestibule causes excitatory deflection of the hair cell stereocilia [27]. The resultant hair cell contractile force would tend to draw the BM upward. Thus, in a similar way, established theory posits that the hair cell expansive force must be timed with the motion of the BM toward the scala tympani [14]. To interpret the OHC length changes, it is necessary to understand that the OHC length changes originate from forces produced by and/or acting upon these cells, especially at low stimulus levels. The phase relationship between the force generation and the length change is unknown and influenced by the mechanical impedance of the surrounding structures. OHC contraction relative to BM, RL displacement To illustrate the relationship between the OHC length change and the displacement of the BM and the RL, we compare their absolute displacement magnitudes in Fig. 5a and b. Note that Phase difference between OHC contraction and BM, RL displacement To illustrate the timing relationship between the OHC contraction and the RL and the BM displacement, we plot the phase differences between the OHC length change and the RL and the BM displacement, as a function of sound pressure level from six sensitive animals. The OHC length changes do not occur in synchrony with the BM displacement but display a difference in phase that depends on the stimulus level (Fig. 5c, d). In sensitive animals, the phase difference between the OHC contraction and the BM displacement to the ST is 0.24 cycles; the OHC contraction precedes the BM displacement to the ST, resulting in a relative phase lead of about 90u (Fig. 6a and 5e). The phase systematically shifts as the sound level increases and at 90 db SPL, the phase difference between the OHC contraction and the BM displacement to the ST is 0.09 cycles, which means only 32u of phase lead between the OHC contraction and the BM displacement remains (Fig. 6c and 5e). The average phase difference between the OHC contraction and the BM displacement is 90610u at 30 db SPL, which is significantly different from zero, decreasing to 40611u at 100 db SPL (Fig. 5e). Postmortem, the phase and the amplitude of the two structures are the same (data not shown). The phase difference between the OHC contraction and the RL displacement to the ST is 0.09 cycle at 30 db SPL, the OHC contraction precedes the RL displacement, resulting in a relative phase lead of about 32u (Figs. 6a and 5f). At 90 db SPL, the phase difference between the OHC contraction and the RL displacement to the ST is 0.06 cycles, which means 22u of the phase lead between the OHC contraction and the RL displacement (Fig. 6c). The average phase difference between the OHC length change and the RL displacement was 2664u at 30 db SPL, and 2669u at 100 db SPL (Fig. 5f). Postmortem, the phase and the amplitude of the two structures are the same (data not shown). The 1:1 ratio between the OHC length change and the RL displacement suggests that the OHCs control the displacement of the RL at low stimulus levels and that the BM makes a major contribution to the mechanical impedance of the cochlear partition, providing a scaffold for the OHCs. As the stimulus level increases, the organ of Corti vibrations are increasingly determined by the stiffness, mass, and friction inherent to the organ of Corti and the OHC length changes have a smaller role. This is to say that the power produced by OHC force acting over the OHC length change, becomes a proportionally smaller part of that needed to move the organ. PLoS ONE 5 April 2012 Volume 7 Issue 4 e32757
7 Figure 5. Outer hair cell length changes and phase differences between the OHCs length and the RL and the BM displacement as a function of the sound pressure level (db SPL). a. Normalized relative OHCs length changes vs. BM displacement as a function of the sound pressure level between 30 and 100 db SPL. b. Normalized relative OHCs length changes vs. the RL displacement as a function of the sound pressure level between 30 and 100 db SPL. c. Phase difference between the OHC length changes and the BM displacements as a function of the sound pressure level between 30 and 100 db SPL. d. Phase difference between the OHC length changes and the RL displacements as a function of the sound pressure level between 30 and 100 db SPL. Data were obtained from six sensitive animals. Note the changes in the ordinate scales. doi: /journal.pone g005 Discussion Experiments on isolated OHCs [12,28] have demonstrated that the OHC contracts in response to depolarization and elongates when hyper-polarized. In vivo, alternating potentials have been measured both intracellularly [29] and in the extracellular space adjacent to the OHCs during sound stimulation [30]. These potentials could serve as a driving stimulus for OHC motility. In vivo (and in situ), the OHC vibration is constrained by the impedances of the BM, the RL and the TM. The extent to which the OHC vibration is constrained causes force to be applied on these structures. The total force is given by [31]: The total force consists of the active force due to the OHC somatic electromotility [6,7,32], (second term in the right hand side of equation (2) and a passive force due to the OHC stiffness (first term in the right hand side of equation (2)). The force F OHC applied on the apical and basal ends of the OHC is contractile in equation (2) for a contractile displacement u OHC and depolarizing potential Q OHC. It is important to distinguish between the length changes in the isolated OHCs in vitro versus the length changes measured in vivo. An isolated OHC; there are no forces acting on the OHC, thus F OHC ~0. Therefore, from equation (2), the isolated OHC contraction is given by F OHC ~K OHC u OHC zeq OHC ð2þ u isolated OHC ~{ eq OHC K OHC ð3þ PLoS ONE 6 April 2012 Volume 7 Issue 4 e32757
8 Figure 6. The timing of the BM and the RL displacement and the OHC contractile length change. The timing of the OHCs contraction and the RL and the BM displacement at the sound level of 30 db (a), 40 db (b) and 90 db (c) from a sensitive animal (hearing loss,10 db) and at the sound level of 80 db from the postmortem animals. Positive direction is the direction to the SV for the RL and the BM displacements, and expansive for the OHC length-change. The arrow pointed at the OHC length change curve indicates the shortest position of the OHC, the arrow pointed at the RL vibration curve indicates the time (phase) when the RL has moved farthest into the ST, the arrow pointed at the BM vibration curve indicates the time (phase) when the BM has moved farthest into the ST. Note the phase difference between the OHC contractile length change and the RL and the BM displacement. doi: /journal.pone g006 where e is the piezoelectric coefficient and a negative quantity. This relationship is consistent with the OHC contraction for depolarizing transmembrane potential. Most researchers refer to the isolated OHC contraction u isolated OHC as the length change. The isolated cell length change is usually equated with the active force in vivo, and this force is given by F active OHC ~{K OHC u isolated OHC ~eq OHC ð4þ In other words, the active force in vivo is directly associated with the electromotile length-change in isolated cells. The in vivo length change, though likely arising from the same process, is given by the OHC contraction u OHC in equation (2). The in vivo OHC length change is an important aspect of the active process in the cochlea because the power transferred to the traveling wave, as proposed by cochlear models [33]. Our experimental data in this report demonstrates that the OHC length changes occur in vivo in the sensitive cochlea. The relative length changes decrease with the sound stimulus level and demonstrate compressive nonlinearity. These features are absent from the postmortem preparations, which lack amplification attributable to the OHCs. The maximum OHC contraction is about two times the displacement of the BM at 40 db SPL, decreasing to about 0.8 at 90 db SPL. The compression thus appears to be more pronounced in the OHC length-change compared to the BM and supports the hypothesis that the OHCs are the likely physiological site of the nonlinearity. Our experiments also show that the timing of length changes depends on the stimulus level. At low sound levels, the OHC begins to contract when the BM moves from scala vestibuli to the scala tympani; the OHC contraction phase leads the BM displacement to the scala tympani by about 90u. In contrast to the living organ of Corti, the RL and the BM within the organ of Corti,in the postmortem preparation, vibrate almost as a unit in response to the CF tones (Fig. 2c, 2d, Fig. 3b, 3d and Fig. 6d), and there is no phase and amplitude difference PLoS ONE 7 April 2012 Volume 7 Issue 4 e32757
9 between the RL and the BM. The disappearance of the amplitude and the phase difference between the BM and the RL postmortem certainly indicates a dependence on OHCs, as these cells are the only ones capable of changing cell length by generating force at the requisite speeds [8] and the effect of their activity is known to be highly dependent on the functional status of the cochlea [34] In studies of excised cochleae [7,35], electrical depolarization of the OHCs induces somatic contractions that cause the BM and the RL to be drawn together (Fig. 1c, 1d). These findings support [36] and form the basis of [37] the mechanical models of the BM tuning: with a phase lead of 90u between the OHCs contractile force and the displacement of the BM towards the SV, the OHCs would provide a properly timed force to enhance the BM s motion [5,37,38,39]. An erroneous timing would result in decreased rather than increased hearing sensitivity. It is often assumed that the transduction current is maximal when the RL reaches its uppermost point of displacement; each OHC, therefore, is being depolarized, so it continues to shorten [40,41]. Our data are not consistent with this idea, as the maximal OHC contraction occurs before the RL reaches its lowermost point (the OHC length changes have a phase lead over the displacement of the RL toward the scala tympani). There are two primary reasons for these differences. Firstly, the phase of the OHC length change is not the same as that of the active force. Their phase difference is expected to be influenced by the mechanical impedances of the surrounding structures in the cochlear partition [42]. Secondly, many in vitro studies simply assume that the hair bundle deflection precedes the OHC lengthchange. If we assume that the hair bundle deflection is a simple function of the RL displacement, this would mean that the RL displacement should phase-lead the OHC length change. These studies have based their assumption on the open-loop system or experiments on isolated cells, where stereocilia deflection causes change in trans-membrane potential leading to the OHC lengthchange. In vivo, however, in addition to the hair bundle deflection causing the OHC length change, the bundle deflections could also result from the OHC length-change (which implies that the hair bundle deflection would lag the OHC length-change). In other words, the deflections in vivo are the result of a closed-loop feedback system, along with the intricate effect of the tectorial membrane and the traveling-wave [14,43,44], which is lacking in vitro. The OHC electromotility is one of the two prime candidates for the active process leading to the amplification of the traveling wave in the cochlea. In isolated cells, the OHC electromotility process is known to cause length changes (contraction) in response to a change (depolarization) in the transmembrane potential. For the same process to transfer power to the traveling wave in vivo requires not only an active force, but also the affiliated active motion. For power addition to the traveling wave, it is usually said to require [14] that the active force have a component in-phase with the BM velocity. However, it is important to note that the power due to electromotility requires active level-dependent OHC length-changes in addition to the active OHC force. Although our analysis considers electromotility as the active process likely leading to the measured OHC length change in vivo, it remains open whether the length change we measured could also be influenced by the hair bundle motility [45,46]. In conclusion, we show in vivo the OHCs length changes in the sensitive cochlea and that the length changes depend on the stimulus level. The level-dependent OHC length changes provide direct evidence of the active process in vivo, which is important for mammalian hearing sensitivity and frequency selectivity. Unless the OHCs contract and elongate in vivo, the active electromotile force cannot transfer the electromechanical power to the cochlear partition. Thus, being a key element in the active power transfer to the traveling wave, the OHC length changes demonstrated in this article provide vital information on the active process in the cochlea. Methods Optical coherence tomography The vibration of the RL and the BM was measured with a low coherence interferometry (optical coherence tomography system) [22,23]. The sample arm of this interferometer system is a microscopic system. Using a wide bandwidth light source of nm, the system achieves optical axis resolution of,7 um, without using the focus power of the objective lens, which is limited by the achievable numerical aperture of the optical system, determined by the access hole opened on the cochlear bony wall. The infra-red light also has penetration capability so that the vibration inside the organ at the RL can be measured. Experimental procedures Albino guinea pigs ( g) with normal hearing were used. All procedures in this study were reviewed and approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University, approval date January 19, 2011 under Animal Welfare Assurance Number A After anesthesia, the animal was placed in a customized head-holder and the cochlea surgically exposed. To measure the in vivo OHC length changes with the OCT system, it is necessary to have a good image of the organ of Corti to ensure that the measured points on the RL and the BM are at the appropriate radial coordinates. To obtain the image of organ of Corti, a sufficiently large opening ( um diameter) was made in the otic capsule close to round window. The opening served to allow more focused light to fall on the organ and permitted observation across the entire width of the BM. CFs of the recording positions of the organ were khz and appropriate for the basal location of the opening near the round window. During the experiment, a cross-section image was firstly acquired (as shown in Fig. 1b), guiding the selection of the measurement site. After that, the light was directed by two scanning mirrors to the selected site (RL or BM), and vibration measurement conducted with this homodyne interferometer system. The RL and BM selected locations were determined from the image by noting the shape of the group of three OHCs that lie at an angle to the optical axis. The RL location was in the center of the group while the BM location was at the location of an imaginary line passing through the long axis of the group of OHCs and intersecting the BM. Calibration was performed by two lockin amplifiers (Stanford Research SR830) to decode the vibration amplitude and phase. Hearing sensitivity of the animal was monitored by recording the sound-induced auditory nerve response, via an electrode placed on the round window. Calculation of the OHC length change In our OCT measurements, positive displacements are in the vertical direction and towards the scala vestibuli (SV). Therefore, the increase in the length of the OHC-DC complex is given by DL~(u RL {u BM )sinh Here h is the angle made by the RL with vertical. Although the DC soma stiffness has not been directly measured, estimates based ð5þ PLoS ONE 8 April 2012 Volume 7 Issue 4 e32757
10 on the presence of the microtubules suggest a stiffness at least 1000 times higher than the OHC [19,20,21]. Based on this estimate, the increase in the length of the OHC is well approximated by the increase in the length of the OHC-DC complex given by DL. The contractile displacement of the OHC is therefore given by u OHC &{DL~(u BM {u RL )sinh: This OHC displacement is derived from the directly measured RL and BM displacements in sensitive guinea pigs in vivo. The angle h is approximately 65u, as measured from our OCT images (Fig. 1b). References 1. Cooper NP, Rhode WS (1997) Mechanical responses to two-tone distortion products in the apical and basal turns of the mammalian cochlea. J Neurophysiol 78: Khanna SM, Leonard DG (1982) Basilar membrane tuning in the cat cochlea. Science 215: Nuttall AL, Dolan DF, Avinash G (1991) Laser Doppler velocimetry of basilar membrane vibration. Hear Res 51: Rhode WS (1971) Observations of the vibration of the basilar membrane in squirrel monkeys using the Mossbauer technique. J Acoust Soc Am 49: Suppl 2: Robles L, Ruggero MA, Rich NC (1986) Basilar membrane mechanics at the base of the chinchilla cochlea. I. Input-output functions, tuning curves, and response phases. J Acoust Soc Am 80: Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y (1985) Evoked mechanical responses of isolated cochlear outer hair cells. Science 227: Mammano F, Ashmore JF (1993) Reverse transduction measured in the isolated cochlea by laser Michelson interferometry. Nature 365: Nilsen KE, Russell IJ (1999) Timing of cochlear feedback: spatial and temporal representation of a tone across the basilar membrane. Nat Neurosci 2: Davis H (1983) An active process in cochlear mechanics. Hear Res 9: de Boer E, Nuttall AL (2001) Power gain of the cochlear amplifier. In: Breebaart DJ, Houtsma AJM, Kohlrausch A, Prijs VV, Schoonhoven R, eds. Physiological and psychological bases of auditory function Shaker, Maastricht. pp Zenner HP, Zimmermann U, Schmitt U (1985) Reversible contraction of isolated mammalian cochlear hair cells. Hear Res 18: Ashmore JF (1987) A fast motile response in guinea pig outer hair cells: The cellular basis of the cochlear amplifier. J Physiol 388: Chen F, Zha D-J, Fridberger A, Zheng J, Choudhury N, et al. (2011) A differentially amplified motion in the ear for near-threshold sound detection. Nat Neurosci 14: Gummer AW, Hemmert W, Zenner HP (1996) Resonant tectorial membrane motion in the inner ear: its crucial role in frequency tuning. Proc Natl Acad Sci U S A 93: Grosh K, Nuttall AL, Zheng J, de Boer E (2004) High frequency electromotile response to the cochlea. Journal of the Accoustical Society of America 115: He D, Dallos P (1999) Somatic stiffness of cochlear outer hair cells is voltagedependent. Proc Natl Acad Sci U S A 96: Neely ST (1993) A model of cochlear mechanics with outer hair cell motility. J Acoust Soc Am 94: Nobili R, Mammano F (1996) Biophysics of the cochlea. II: Stationary nonlinear phenomenology. J Acoust Soc Am 99: Angelborg C, Engstrom H (1972) Supporting elements in the organ of Corti. I. Fibrillar structures in the supporting cells of the organ of Corti of mammals. Acta Otolaryngol Suppl 301: Slepecky N, Chamberlain SC (1983) Distribution and polarity of actin in inner ear supporting cells. Hear Res 10: Tolomeo JA, Holley MC (1997) Mechanics of microtubule bundles in pillar cells from the inner ear. Biophys J 73: Chen F, Choudhury N, Zheng J, Matthews SK, Nuttall AL, et al. (2007) In vivo imaging and low-coherence interferometry of organ of Corti vibration. J Biomed Opt 12: Acknowledgments We gratefully acknowledge the helpful comments of Egbert de Boer on earlier versions of the manuscript. Author Contributions Conceived and designed the experiments: DZ FC ALN. Performed the experiments: DZ FC. Analyzed the data: DZ FC SR AF ALN. Contributed reagents/materials/analysis tools: DZ FC SR AF ALN. Wrote the paper: DZ FC SR AF ALN. Developed OCT System: NC SLJ RKW. 23. Choudhury N, Chen F, Matthews SK, Tschinkel T, Zheng J, et al. (2006) Low coherence interferometry of the cochlear partition. Hear Res 220(1 2): de Boer E, Nuttall AL (2000) The mechanical waveform of the basilar membrane. III. Intensity effects. J Acoust Soc Am 107: Brownell WE (1990) Outer hair cell electromotility and otoacoustic emissions. Ear Hear 11: Tomo I, Boutet de Monvel J, Fridberger A (2007) Sound-Evoked Radial Strain in the Hearing Organ. Biophys J 93: ter Kuile E (1900) Die ubertragung der energie von der grundmembran auf die haarzellen. Pflugers Arch 79: Santos-Sacchi J, Dilger JP (1988) Whole cell currents and mechanical responses of isolated outer hair cells. Hear Res 35: Cody AR, Russell IJ (1987) The responses of hair cells in the basal turn of the guinea-pig cochlea to tones. J Physiol (Lond) 383: Fridberger A, de Monvel JB, Zheng J, Hu N, Zou Y, et al. (2004) Organ of Corti potentials and the motion of the basilar membrane. J Neurosci 24: Ramamoorthy S, Deo N, Grosh K (2007) A mechano-electro-acoustical model for the cochlea: Response to acoustic stimuli. J Acoust Soc Am 121: Zheng J, Shen W, He DZ, Long KB, Madison LD, et al. (2000) Prestin is the motor protein of cochlear outer hair cells. Nature 405: de Boer E, Nuttall AL (2000) The mechanical waveform of the basilar membrane. II. From data to models - and back. J Accoust Soc Am 107: Ruggero MA, Rich NC (1991) Furosemide alters organ of corti mechanics: evidence for feedback of outer hair cells upon the basilar membrane. J Neurosci 11: Mammano F, Kros CJ, Ashmore JF (1995) Patch clamped responses from outer hair cells in the intact adult organ of Corti. Pflugers Arch 430: Geisler CD (1986) A model of the effect of outer hair cell motility on cochlear vibrations. Hear Res 24: Markin VS, Hudspeth AJ (1995) Modeling the active process of the cochlea: phase relations, amplification, and spontaneous oscillation. Biophys J 69: Geisler CD, Sang C (1995) A cochlear model using feed-forward outer-hair-cell forces. Hear Res 86: Hallworth R (1995) Passive compliance and active force generation in the guinea pig outer hair cell. J Neurophysiol 74: Dallos P, Evans BN, Hallworth R (1991) Nature of the motor element in electrokinetic shape changes of cochlear outer hair cells. Nature 350: Santos-Sacchi J (1992) On the frequency limit and phase of outer hair cell motility: effects of the membrane filter. J Neurosci 12: Jacob S, Pienkowski M, Fridberger A (2011) The endocochlear potential alters cochlear micromechanics. Biophys J 100: Allen JB (1980) Cochlear micromechanics - A physical model of tranduction. J Acoust Soc Am 68: Zwislocki JJ, Kletsky EJ (1979) Tectorial membrane: a possible effect on frequency analysis in the cochlea. Science 204: Chan DK, Hudspeth AJ (2005) Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro. Nat Neurosci 8: Kennedy HJ, Crawford AC, Fettiplace R (2005) Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature 433: PLoS ONE 9 April 2012 Volume 7 Issue 4 e32757
Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier
 Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier Sripriya Ramamoorthy 1 *, Alfred L. Nuttall 1,2 1 Oregon Hearing Research Center, Department of Otolaryngology, Oregon
Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier Sripriya Ramamoorthy 1 *, Alfred L. Nuttall 1,2 1 Oregon Hearing Research Center, Department of Otolaryngology, Oregon
Chapter 3: Anatomy and physiology of the sensory auditory mechanism
 Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Reticular lamina and basilar membrane vibrations in living mouse cochleae
 Reticular lamina and basilar membrane vibrations in living mouse cochleae Tianying Ren a,1, Wenxuan He a, and David Kemp b a Oregon Hearing Research Center, Department of Otolaryngology, Oregon Health
Reticular lamina and basilar membrane vibrations in living mouse cochleae Tianying Ren a,1, Wenxuan He a, and David Kemp b a Oregon Hearing Research Center, Department of Otolaryngology, Oregon Health
HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier
 HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier 1 Cochlear Mechanics: Measures of Basilar Membrane Motion 2 Cochlear Mechanics: Measures of Basilar Membrane Motion Bekesy s experiments
HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier 1 Cochlear Mechanics: Measures of Basilar Membrane Motion 2 Cochlear Mechanics: Measures of Basilar Membrane Motion Bekesy s experiments
Processing of sounds in the inner ear
 Processing of sounds in the inner ear Sripriya Ramamoorthy Associate Professor, IIT Bombay WiSSAP 2018 Cochlea converts sound into electrical signals [Picture courtesy of Northwestern University] von Bekesy
Processing of sounds in the inner ear Sripriya Ramamoorthy Associate Professor, IIT Bombay WiSSAP 2018 Cochlea converts sound into electrical signals [Picture courtesy of Northwestern University] von Bekesy
Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics
 Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3676 3681, March 1999 Biophysics Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics PAUL J.
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3676 3681, March 1999 Biophysics Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics PAUL J.
THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA
 451 THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA DÁIBHID Ó MAOILÉIDIGH, FRANK JÜLICHER Max Planck Institute für Physik komplexer Systeme, Nöthnitzerstr. 38, 01187
451 THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA DÁIBHID Ó MAOILÉIDIGH, FRANK JÜLICHER Max Planck Institute für Physik komplexer Systeme, Nöthnitzerstr. 38, 01187
Mechanical Properties of the Cochlea. Reading: Yost Ch. 7
 Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression
 1398 Biophysical Journal Volume 106 March 2014 1398 1405 Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression Julien Meaud * and Karl Grosh Department
1398 Biophysical Journal Volume 106 March 2014 1398 1405 Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression Julien Meaud * and Karl Grosh Department
Measurement of cochlear power gain in the sensitive gerbil ear
 Received 4 Nov 2 Accepted 3 Feb 2 Published Mar 2 DOI:.38/ncomms226 Measurement of cochlear power gain in the sensitive gerbil ear Tianying Ren,2, Wenxuan He & Peter G. Gillespie,3 The extraordinary sensitivity
Received 4 Nov 2 Accepted 3 Feb 2 Published Mar 2 DOI:.38/ncomms226 Measurement of cochlear power gain in the sensitive gerbil ear Tianying Ren,2, Wenxuan He & Peter G. Gillespie,3 The extraordinary sensitivity
Cochlear anatomy, function and pathology II. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
Auditory System Feedback
 Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
Structure, Energy Transmission and Function. Gross Anatomy. Structure, Function & Process. External Auditory Meatus or Canal (EAM, EAC) Outer Ear
 Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea
 Article Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea Wei Dong and Elizabeth S. Olson 2, * VA Loma Linda Health Care System and Otolaryngology/Head & Neck Surgery,
Article Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea Wei Dong and Elizabeth S. Olson 2, * VA Loma Linda Health Care System and Otolaryngology/Head & Neck Surgery,
An active cochlear model showing sharp tuning and high sensitivity
 Hearing Research, 9 (1983) 123-13 Elsevier Biomedical Press 123 An active cochlear model showing sharp tuning and high sensitivity Stephen T. Neely * and D.O. Kim Box 811, Washington Unitlersit}', St.
Hearing Research, 9 (1983) 123-13 Elsevier Biomedical Press 123 An active cochlear model showing sharp tuning and high sensitivity Stephen T. Neely * and D.O. Kim Box 811, Washington Unitlersit}', St.
A truly remarkable aspect of human hearing is the vast
 AUDITORY COMPRESSION AND HEARING LOSS Sid P. Bacon Psychoacoustics Laboratory, Department of Speech and Hearing Science, Arizona State University Tempe, Arizona 85287 A truly remarkable aspect of human
AUDITORY COMPRESSION AND HEARING LOSS Sid P. Bacon Psychoacoustics Laboratory, Department of Speech and Hearing Science, Arizona State University Tempe, Arizona 85287 A truly remarkable aspect of human
The magnificent outer hair cell of Corti's organ
 The magnificent outer hair cell of Corti's organ Yale University J. Santos-Sacchi School of Medicine, New Haver, CT, USA Abstract The outer hair cell is one of two receptor cell types in the organ of Corti.
The magnificent outer hair cell of Corti's organ Yale University J. Santos-Sacchi School of Medicine, New Haver, CT, USA Abstract The outer hair cell is one of two receptor cell types in the organ of Corti.
FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS. Hans P. Zenner, Ulrike Zimmermann and Alfred H. Gitter
 Vol. 149,No. 1, 1987 November 30, 1987 BlOCHEMlCALANDBlOPHYSlCALRESEARCHCOMMUNlCATlONS Pages 304-308 FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS Hans P. Zenner, Ulrike Zimmermann and Alfred
Vol. 149,No. 1, 1987 November 30, 1987 BlOCHEMlCALANDBlOPHYSlCALRESEARCHCOMMUNlCATlONS Pages 304-308 FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS Hans P. Zenner, Ulrike Zimmermann and Alfred
Detection of Cochlear Amplification and Its Activation
 Biophysical Journal Volume 5 August 23 67 78 67 Detection of Cochlear Amplification and Its Activation Wei Dong and Elizabeth S. Olson * Otolaryngology, Head and Neck Surgery and Biomedical Engineering,
Biophysical Journal Volume 5 August 23 67 78 67 Detection of Cochlear Amplification and Its Activation Wei Dong and Elizabeth S. Olson * Otolaryngology, Head and Neck Surgery and Biomedical Engineering,
Auditory System. Barb Rohrer (SEI )
 Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
Acoustics Research Institute
 Austrian Academy of Sciences Acoustics Research Institute Modeling Modelingof ofauditory AuditoryPerception Perception Bernhard BernhardLaback Labackand andpiotr PiotrMajdak Majdak http://www.kfs.oeaw.ac.at
Austrian Academy of Sciences Acoustics Research Institute Modeling Modelingof ofauditory AuditoryPerception Perception Bernhard BernhardLaback Labackand andpiotr PiotrMajdak Majdak http://www.kfs.oeaw.ac.at
Cochlear anatomy, function and pathology I. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology I Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of these lectures Introduction to gross anatomy of the cochlea Focus (1) on
Cochlear anatomy, function and pathology I Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of these lectures Introduction to gross anatomy of the cochlea Focus (1) on
Backward Propagation of Otoacoustic Emissions
 40 Review Backward Propagation of Otoacoustic Emissions HE Wenxuan, 1, 2 REN Tianying, 1, 2 1. Oregon Hearing Research Center, Department of Otolaryngology and Head & Neck Surgery, Oregon Health & Science
40 Review Backward Propagation of Otoacoustic Emissions HE Wenxuan, 1, 2 REN Tianying, 1, 2 1. Oregon Hearing Research Center, Department of Otolaryngology and Head & Neck Surgery, Oregon Health & Science
Improving the diagnostic power of otoacoustic emissions. Arturo Moleti Physics Department University of Roma Tor Vergata
 Improving the diagnostic power of otoacoustic emissions Arturo Moleti Physics Department University of Roma Tor Vergata The human ear Ear canal: resonant cavity Middle ear: impedance adapter and pressure
Improving the diagnostic power of otoacoustic emissions Arturo Moleti Physics Department University of Roma Tor Vergata The human ear Ear canal: resonant cavity Middle ear: impedance adapter and pressure
Signals, systems, acoustics and the ear. Week 5. The peripheral auditory system: The ear as a signal processor
 Signals, systems, acoustics and the ear Week 5 The peripheral auditory system: The ear as a signal processor Think of this set of organs 2 as a collection of systems, transforming sounds to be sent to
Signals, systems, acoustics and the ear Week 5 The peripheral auditory system: The ear as a signal processor Think of this set of organs 2 as a collection of systems, transforming sounds to be sent to
The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992)
 The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992) by Pedro da Fonseca (pedrofon@mail.telepac.pt) Neuroscience course Presented in 17.12.99 to professor STEPHEN
The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992) by Pedro da Fonseca (pedrofon@mail.telepac.pt) Neuroscience course Presented in 17.12.99 to professor STEPHEN
Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs
 Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs The Auditory Laboratory, Physiology University of Western Australia Greg O Beirne Dept. of Communication
Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs The Auditory Laboratory, Physiology University of Western Australia Greg O Beirne Dept. of Communication
Force Transmission in the Organ of Corti Micromachine
 Biophysical Journal Volume 98 June 2010 2813 2821 2813 Force Transmission in the Organ of Corti Micromachine Jong-Hoon Nam and Robert Fettiplace* Department of Physiology, University of Wisconsin Medical
Biophysical Journal Volume 98 June 2010 2813 2821 2813 Force Transmission in the Organ of Corti Micromachine Jong-Hoon Nam and Robert Fettiplace* Department of Physiology, University of Wisconsin Medical
Effects of Remaining Hair Cells on Cochlear Implant Function
 Effects of Remaining Hair Cells on Cochlear Implant Function N1-DC-2-15QPR1 Neural Prosthesis Program N. Hu, P.J. Abbas, C.A. Miller, B.K. Robinson, K.V. Nourski, F. Jeng, B.A. Abkes, J.M. Nichols Department
Effects of Remaining Hair Cells on Cochlear Implant Function N1-DC-2-15QPR1 Neural Prosthesis Program N. Hu, P.J. Abbas, C.A. Miller, B.K. Robinson, K.V. Nourski, F. Jeng, B.A. Abkes, J.M. Nichols Department
HST 721 Efferent Control Lecture October 2004
 HST 721 Efferent Control Lecture October 2004 1 Stapedius Muscle Central Circuitry 2 Hypotheses for MEM Function A. Stapedius 1. Extend Dynamic Range - a gain control system 2. Protect the Inner Ear from
HST 721 Efferent Control Lecture October 2004 1 Stapedius Muscle Central Circuitry 2 Hypotheses for MEM Function A. Stapedius 1. Extend Dynamic Range - a gain control system 2. Protect the Inner Ear from
Chapter 11: Sound, The Auditory System, and Pitch Perception
 Chapter 11: Sound, The Auditory System, and Pitch Perception Overview of Questions What is it that makes sounds high pitched or low pitched? How do sound vibrations inside the ear lead to the perception
Chapter 11: Sound, The Auditory System, and Pitch Perception Overview of Questions What is it that makes sounds high pitched or low pitched? How do sound vibrations inside the ear lead to the perception
Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea
 Keywords: Cochlea, Basilar membrane, Hair cell 7006 Journal of Physiology (1998), 509.1, pp. 277 288 277 Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea Nigel P.
Keywords: Cochlea, Basilar membrane, Hair cell 7006 Journal of Physiology (1998), 509.1, pp. 277 288 277 Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea Nigel P.
SOLUTIONS Homework #3. Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03
 SOLUTIONS Homework #3 Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03 Problem 1: a) Where in the cochlea would you say the process of "fourier decomposition" of the incoming
SOLUTIONS Homework #3 Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03 Problem 1: a) Where in the cochlea would you say the process of "fourier decomposition" of the incoming
Systems Neuroscience Oct. 16, Auditory system. http:
 Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
Representation of sound in the auditory nerve
 Representation of sound in the auditory nerve Eric D. Young Department of Biomedical Engineering Johns Hopkins University Young, ED. Neural representation of spectral and temporal information in speech.
Representation of sound in the auditory nerve Eric D. Young Department of Biomedical Engineering Johns Hopkins University Young, ED. Neural representation of spectral and temporal information in speech.
What Drives Mechanical Amplification in the Mammalian Cochlea?
 What Drives Mechanical Amplification in the Mammalian Cochlea? Robert H. Withnell, Lauren A. Shaffer, and David J. Lilly The recent report by Peter Dallos and colleagues of the gene and protein responsible
What Drives Mechanical Amplification in the Mammalian Cochlea? Robert H. Withnell, Lauren A. Shaffer, and David J. Lilly The recent report by Peter Dallos and colleagues of the gene and protein responsible
Acoustics, signals & systems for audiology. Psychoacoustics of hearing impairment
 Acoustics, signals & systems for audiology Psychoacoustics of hearing impairment Three main types of hearing impairment Conductive Sound is not properly transmitted from the outer to the inner ear Sensorineural
Acoustics, signals & systems for audiology Psychoacoustics of hearing impairment Three main types of hearing impairment Conductive Sound is not properly transmitted from the outer to the inner ear Sensorineural
Optimal Electrical Properties of Outer Hair Cells Ensure Cochlear Amplification
 Optimal Electrical Properties of Outer Hair Cells Ensure Cochlear Amplification Jong-Hoon Nam 1,2 *, Robert Fettiplace 3 1 Department of Mechanical Engineering, Department of Biomedical Engineering, University
Optimal Electrical Properties of Outer Hair Cells Ensure Cochlear Amplification Jong-Hoon Nam 1,2 *, Robert Fettiplace 3 1 Department of Mechanical Engineering, Department of Biomedical Engineering, University
What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components
 1 2 3 4 What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components as a function of time here during the production
1 2 3 4 What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components as a function of time here during the production
Microstructures in the Organ of Corti Help Outer Hair Cells Form Traveling Waves along the Cochlear Coil
 2426 Biophysical Journal Volume 106 June 2014 2426 2433 Microstructures in the Organ of Corti Help Outer Hair Cells Form Traveling Waves along the Cochlear Coil Jong-Hoon Nam* Department of Mechanical
2426 Biophysical Journal Volume 106 June 2014 2426 2433 Microstructures in the Organ of Corti Help Outer Hair Cells Form Traveling Waves along the Cochlear Coil Jong-Hoon Nam* Department of Mechanical
A cochlear AGC model, proposing a new type of cochlear non-linearity
 TEL-AVIV UNIVERSITY The Iby and Aladar Fleischman faculty of engineering The Zandman-Slaner school of Graduate studies A cochlear AGC model, proposing a new type of cochlear non-linearity A thesis submitted
TEL-AVIV UNIVERSITY The Iby and Aladar Fleischman faculty of engineering The Zandman-Slaner school of Graduate studies A cochlear AGC model, proposing a new type of cochlear non-linearity A thesis submitted
THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS?
 JOURNAL OF OTOLOGY THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS? LI Yi 1, He David Z 2 Abstract Cochlear outer hair cells (OHCs) are involved in a mechanical feedback loop in which
JOURNAL OF OTOLOGY THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS? LI Yi 1, He David Z 2 Abstract Cochlear outer hair cells (OHCs) are involved in a mechanical feedback loop in which
Efferent-mediated control of basilar membrane motion
 J Physiol 576. (2006) pp 49 54 49 Topical Review Efferent-mediated control of basilar membrane motion N. P. Cooper and J. J. Guinan Jr 2 School of Life Sciences, Keele University, Keele, Staffordshire
J Physiol 576. (2006) pp 49 54 49 Topical Review Efferent-mediated control of basilar membrane motion N. P. Cooper and J. J. Guinan Jr 2 School of Life Sciences, Keele University, Keele, Staffordshire
FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA. Invited Paper LINEAR RESPONSE OF THE COCHLEA
 FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA Invited Paper LINEAR RESPONSE OF THE COCHLEA David Alan Bies Visiting Research Fellow Department of Mechanical
FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA Invited Paper LINEAR RESPONSE OF THE COCHLEA David Alan Bies Visiting Research Fellow Department of Mechanical
Auditory Periphery! external middle inner. stapes movement initiates a pressure wave in cochlear fluid
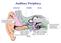 Auditory Periphery! external middle inner sound causes air pressure to increase at eardrum stapes movement initiates a pressure wave in cochlear fluid VIIIth nerve conveys neural signal to cochlear nucleus
Auditory Periphery! external middle inner sound causes air pressure to increase at eardrum stapes movement initiates a pressure wave in cochlear fluid VIIIth nerve conveys neural signal to cochlear nucleus
The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons.
 1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
Hearing Sound. The Human Auditory System. The Outer Ear. Music 170: The Ear
 Hearing Sound Music 170: The Ear Tamara Smyth, trsmyth@ucsd.edu Department of Music, University of California, San Diego (UCSD) November 17, 2016 Sound interpretation in the auditory system is done by
Hearing Sound Music 170: The Ear Tamara Smyth, trsmyth@ucsd.edu Department of Music, University of California, San Diego (UCSD) November 17, 2016 Sound interpretation in the auditory system is done by
Music 170: The Ear. Tamara Smyth, Department of Music, University of California, San Diego (UCSD) November 17, 2016
 Music 170: The Ear Tamara Smyth, trsmyth@ucsd.edu Department of Music, University of California, San Diego (UCSD) November 17, 2016 1 Hearing Sound Sound interpretation in the auditory system is done by
Music 170: The Ear Tamara Smyth, trsmyth@ucsd.edu Department of Music, University of California, San Diego (UCSD) November 17, 2016 1 Hearing Sound Sound interpretation in the auditory system is done by
FOUR COUNTER-ARGUMENTS FOR SLOW-WAVE OAEs
 FOUR COUNTER-ARGUMENTS FOR SLOW-WAVE OAEs CHRISTOPHER A. SHERA Eaton-Peabody Laboratory, Boston, MA 02114, USA email: shera@epl.meei.harvard.edu ARNOLD TUBIS Institute for Nonlinear Science, La Jolla,
FOUR COUNTER-ARGUMENTS FOR SLOW-WAVE OAEs CHRISTOPHER A. SHERA Eaton-Peabody Laboratory, Boston, MA 02114, USA email: shera@epl.meei.harvard.edu ARNOLD TUBIS Institute for Nonlinear Science, La Jolla,
NIH Public Access Author Manuscript Nat Commun. Author manuscript; available in PMC 2013 March 12.
 NIH Public Access Author Manuscript Published in final edited form as: Nat Commun. 2012 ; 3: 1094. doi:10.1038/ncomms2100. Sound-induced length changes in outer hair cell stereocilia Pierre Hakizimana
NIH Public Access Author Manuscript Published in final edited form as: Nat Commun. 2012 ; 3: 1094. doi:10.1038/ncomms2100. Sound-induced length changes in outer hair cell stereocilia Pierre Hakizimana
Modelling the micromechanics of the cochlea in Femlab
 Modelling the micromechanics of the cochlea in Femlab R.R.J.J. van Doorn DCT 27.7 Traineeship report Coach(es): Supervisor: Prof. S.J. Elliott Prof. P. Gardonio Prof. H. Nijmeijer Technische Universiteit
Modelling the micromechanics of the cochlea in Femlab R.R.J.J. van Doorn DCT 27.7 Traineeship report Coach(es): Supervisor: Prof. S.J. Elliott Prof. P. Gardonio Prof. H. Nijmeijer Technische Universiteit
sensitivity (db) frequency (khz) I: MONGOLIAN GERBIL FREQUENCY HEARING CHARACTERISTICS
 Supplemental materials to Frequency sensitivity in mammalian hearing from a fundamental nonlinear physics model of the inner ear, by Kanders, K., Lorimer, T., Gomez, F. & Stoop, R. -6-5 sensitivity (db)
Supplemental materials to Frequency sensitivity in mammalian hearing from a fundamental nonlinear physics model of the inner ear, by Kanders, K., Lorimer, T., Gomez, F. & Stoop, R. -6-5 sensitivity (db)
ENT 318 Artificial Organs Physiology of Ear
 ENT 318 Artificial Organs Physiology of Ear Lecturer: Ahmad Nasrul Norali The Ear The Ear Components of hearing mechanism - Outer Ear - Middle Ear - Inner Ear - Central Auditory Nervous System Major Divisions
ENT 318 Artificial Organs Physiology of Ear Lecturer: Ahmad Nasrul Norali The Ear The Ear Components of hearing mechanism - Outer Ear - Middle Ear - Inner Ear - Central Auditory Nervous System Major Divisions
Educational Module Tympanometry. Germany D Germering
 Educational Module anometry PATH medical Germany D-82110 Germering Our educational modules 1 are made for providing information on how the hearing organ works and which test procedures are used to test
Educational Module anometry PATH medical Germany D-82110 Germering Our educational modules 1 are made for providing information on how the hearing organ works and which test procedures are used to test
Auditory Physiology Richard M. Costanzo, Ph.D.
 Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
Lecture 6 Hearing 1. Raghav Rajan Bio 354 Neurobiology 2 January 28th All lecture material from the following links unless otherwise mentioned:
 Lecture 6 Hearing 1 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
Lecture 6 Hearing 1 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
Frequency refers to how often something happens. Period refers to the time it takes something to happen.
 Lecture 2 Properties of Waves Frequency and period are distinctly different, yet related, quantities. Frequency refers to how often something happens. Period refers to the time it takes something to happen.
Lecture 2 Properties of Waves Frequency and period are distinctly different, yet related, quantities. Frequency refers to how often something happens. Period refers to the time it takes something to happen.
Comment by Delgutte and Anna. A. Dreyer (Eaton-Peabody Laboratory, Massachusetts Eye and Ear Infirmary, Boston, MA)
 Comments Comment by Delgutte and Anna. A. Dreyer (Eaton-Peabody Laboratory, Massachusetts Eye and Ear Infirmary, Boston, MA) Is phase locking to transposed stimuli as good as phase locking to low-frequency
Comments Comment by Delgutte and Anna. A. Dreyer (Eaton-Peabody Laboratory, Massachusetts Eye and Ear Infirmary, Boston, MA) Is phase locking to transposed stimuli as good as phase locking to low-frequency
Required Slide. Session Objectives
 Auditory Physiology Required Slide Session Objectives Auditory System: At the end of this session, students will be able to: 1. Characterize the range of normal human hearing. 2. Understand the components
Auditory Physiology Required Slide Session Objectives Auditory System: At the end of this session, students will be able to: 1. Characterize the range of normal human hearing. 2. Understand the components
Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells
 Biophysical Journal Volume 84 February 2003 739 749 739 Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells Mark Ospeck, Xiao-xia Dong, and Kuni H. Iwasa Biophysics
Biophysical Journal Volume 84 February 2003 739 749 739 Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells Mark Ospeck, Xiao-xia Dong, and Kuni H. Iwasa Biophysics
Deafness and hearing impairment
 Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
The Structure and Function of the Auditory Nerve
 The Structure and Function of the Auditory Nerve Brad May Structure and Function of the Auditory and Vestibular Systems (BME 580.626) September 21, 2010 1 Objectives Anatomy Basic response patterns Frequency
The Structure and Function of the Auditory Nerve Brad May Structure and Function of the Auditory and Vestibular Systems (BME 580.626) September 21, 2010 1 Objectives Anatomy Basic response patterns Frequency
Study the Effect of the Quality Factor of the Transient Evoked Oto-acoustic Emissions (TEOAE)
 Research in Otolaryngology 217, 6(4): 47-4 DOI:.923/j.otolaryn.21764.1 Study the Effect of the Quality Factor of the Transient Evoked Oto-acoustic Emissions (TEOAE) Adnan AL-Maamury *, Dhifaf Ahmed Al-Mustansiriyah
Research in Otolaryngology 217, 6(4): 47-4 DOI:.923/j.otolaryn.21764.1 Study the Effect of the Quality Factor of the Transient Evoked Oto-acoustic Emissions (TEOAE) Adnan AL-Maamury *, Dhifaf Ahmed Al-Mustansiriyah
to vibrate the fluid. The ossicles amplify the pressure. The surface area of the oval window is
 Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
In Vivo Impedance of the Gerbil Cochlear Partition at Auditory Frequencies
 Biophysical Journal Volume 97 September 2009 1233 1243 1233 In Vivo Impedance of the Gerbil Cochlear Partition at Auditory Frequencies Wei Dong * and Elizabeth S. Olson Department of Otolaryngology, Head
Biophysical Journal Volume 97 September 2009 1233 1243 1233 In Vivo Impedance of the Gerbil Cochlear Partition at Auditory Frequencies Wei Dong * and Elizabeth S. Olson Department of Otolaryngology, Head
Auditory nerve model for predicting performance limits of normal and impaired listeners
 Heinz et al.: Acoustics Research Letters Online [DOI 1.1121/1.1387155] Published Online 12 June 21 Auditory nerve model for predicting performance limits of normal and impaired listeners Michael G. Heinz
Heinz et al.: Acoustics Research Letters Online [DOI 1.1121/1.1387155] Published Online 12 June 21 Auditory nerve model for predicting performance limits of normal and impaired listeners Michael G. Heinz
Issues faced by people with a Sensorineural Hearing Loss
 Issues faced by people with a Sensorineural Hearing Loss Issues faced by people with a Sensorineural Hearing Loss 1. Decreased Audibility 2. Decreased Dynamic Range 3. Decreased Frequency Resolution 4.
Issues faced by people with a Sensorineural Hearing Loss Issues faced by people with a Sensorineural Hearing Loss 1. Decreased Audibility 2. Decreased Dynamic Range 3. Decreased Frequency Resolution 4.
external middle inner
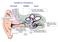 Auditory Periphery external middle inner 3. movement of stapes initiates a pressure wave in cochlear fluid. 1. sound causes air pressure to increase at eardrum 5. auditory nerve conveys neural signal to
Auditory Periphery external middle inner 3. movement of stapes initiates a pressure wave in cochlear fluid. 1. sound causes air pressure to increase at eardrum 5. auditory nerve conveys neural signal to
Vibration of the organ of Corti within the cochlear apex in mice
 J Neurophysiol 2: 92 2, 2. First published June, 2; doi:.2/jn.3.2. Vibration of the organ of Corti within the cochlear apex in mice Simon S. Gao,,2 Rosalie Wang, Patrick D. Raphael, Yalda Moayedi, 3 ndrew
J Neurophysiol 2: 92 2, 2. First published June, 2; doi:.2/jn.3.2. Vibration of the organ of Corti within the cochlear apex in mice Simon S. Gao,,2 Rosalie Wang, Patrick D. Raphael, Yalda Moayedi, 3 ndrew
So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to
 So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to emphasize the intricate and well-protected structure of the
So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to emphasize the intricate and well-protected structure of the
Consequences of Location-Dependent Organ of Corti Micro-Mechanics
 RESEARCH ARTICLE Consequences of Location-Dependent Organ of Corti Micro-Mechanics Yanju Liu 1, Sheryl M. Gracewski 1,2, Jong-Hoon Nam 1,2 * 1 Department of Mechanical Engineering, University of Rochester,
RESEARCH ARTICLE Consequences of Location-Dependent Organ of Corti Micro-Mechanics Yanju Liu 1, Sheryl M. Gracewski 1,2, Jong-Hoon Nam 1,2 * 1 Department of Mechanical Engineering, University of Rochester,
Chapter 1: Introduction to digital audio
 Chapter 1: Introduction to digital audio Applications: audio players (e.g. MP3), DVD-audio, digital audio broadcast, music synthesizer, digital amplifier and equalizer, 3D sound synthesis 1 Properties
Chapter 1: Introduction to digital audio Applications: audio players (e.g. MP3), DVD-audio, digital audio broadcast, music synthesizer, digital amplifier and equalizer, 3D sound synthesis 1 Properties
A computer model of medial efferent suppression in the mammalian auditory system
 A computer model of medial efferent suppression in the mammalian auditory system Robert T. Ferry a and Ray Meddis Department of Psychology, University of Essex, Colchester, CO4 3SQ, United Kingdom Received
A computer model of medial efferent suppression in the mammalian auditory system Robert T. Ferry a and Ray Meddis Department of Psychology, University of Essex, Colchester, CO4 3SQ, United Kingdom Received
Outer Hair Cells in the Cochlea
 Discrete Dynamics in Nature and Society, Vol. 2, pp. 161-165 Reprints available directly from the publisher Photocopying permitted by license only (C) 1998 OPA (Overseas Publishers Association) N.V. Published
Discrete Dynamics in Nature and Society, Vol. 2, pp. 161-165 Reprints available directly from the publisher Photocopying permitted by license only (C) 1998 OPA (Overseas Publishers Association) N.V. Published
HEARING AND PSYCHOACOUSTICS
 CHAPTER 2 HEARING AND PSYCHOACOUSTICS WITH LIDIA LEE I would like to lead off the specific audio discussions with a description of the audio receptor the ear. I believe it is always a good idea to understand
CHAPTER 2 HEARING AND PSYCHOACOUSTICS WITH LIDIA LEE I would like to lead off the specific audio discussions with a description of the audio receptor the ear. I believe it is always a good idea to understand
Unit VIII Problem 9 Physiology: Hearing
 Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
Feed-Forward and Feed-Backward Amplification Model from Cochlear Cytoarchitecture: An Interspecies Comparison
 Biophysical Journal Volume 100 January 2011 1 10 1 Feed-Forward and Feed-Backward Amplification Model from Cochlear Cytoarchitecture: An Interspecies Comparison Yong-Jin Yoon, Charles R. Steele, and Sunil
Biophysical Journal Volume 100 January 2011 1 10 1 Feed-Forward and Feed-Backward Amplification Model from Cochlear Cytoarchitecture: An Interspecies Comparison Yong-Jin Yoon, Charles R. Steele, and Sunil
Advanced otoacoustic emission detection techniques and clinical diagnostics applications
 Advanced otoacoustic emission detection techniques and clinical diagnostics applications Arturo Moleti Physics Department, University of Roma Tor Vergata, Roma, ITALY Towards objective diagnostics of human
Advanced otoacoustic emission detection techniques and clinical diagnostics applications Arturo Moleti Physics Department, University of Roma Tor Vergata, Roma, ITALY Towards objective diagnostics of human
Biological Basis of Hearing-Aid Design
 Annals of Biomedical Engineering, Vol. 30, pp. 157 168, 2002 Printed in the USA. All rights reserved. 0090-6964/2002/30 2 /157/12/$15.00 Copyright 2002 Biomedical Engineering Society Biological Basis of
Annals of Biomedical Engineering, Vol. 30, pp. 157 168, 2002 Printed in the USA. All rights reserved. 0090-6964/2002/30 2 /157/12/$15.00 Copyright 2002 Biomedical Engineering Society Biological Basis of
OtoAcoustic Emissions (OAE s)
 OtoAcoustic Emissions (OAE s) Phenomenon and applications in audiological diagnostics Measurement procedures TEOAE and DPOAE Physiological backgound, functional models Acknowledgment: several illustrations
OtoAcoustic Emissions (OAE s) Phenomenon and applications in audiological diagnostics Measurement procedures TEOAE and DPOAE Physiological backgound, functional models Acknowledgment: several illustrations
Resonant tectorial membrane motion in the inner ear: Its crucial
 Proc. Natl. Acad. Sci. USA Vol. 93, pp. 8727-8732, August 1996 Neurobiology Resonant tectorial membrane motion in the inner ear: Its crucial role in frequency tuning (cochlear amplifier/outer hair cell/basilar
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 8727-8732, August 1996 Neurobiology Resonant tectorial membrane motion in the inner ear: Its crucial role in frequency tuning (cochlear amplifier/outer hair cell/basilar
Trajectory of the Aging Cochlea
 Trajectory of the Aging Cochlea Sumitrajit (Sumit) Dhar Professor & Chair Roxelyn & Richard Pepper Department of Communication Sciences and Disorders Fellow, Hugh Knowles Center for Hearing Science Northwestern
Trajectory of the Aging Cochlea Sumitrajit (Sumit) Dhar Professor & Chair Roxelyn & Richard Pepper Department of Communication Sciences and Disorders Fellow, Hugh Knowles Center for Hearing Science Northwestern
Estimates of compression at low and high frequencies using masking additivity in normal and impaired ears
 Estimates of compression at low and high frequencies using masking additivity in normal and impaired ears Christopher J. Plack a Department of Psychology, Lancaster University, Lancaster LA1 4YF, United
Estimates of compression at low and high frequencies using masking additivity in normal and impaired ears Christopher J. Plack a Department of Psychology, Lancaster University, Lancaster LA1 4YF, United
Academic and Research Staff Professor Dennis M. Freeman, Dr. Alexander Aranyosi, Dr. Stanley Hong
 Cochlear Mechanics Academic and Research Staff Professor Dennis M. Freeman, Dr. Alexander Aranyosi, Dr. Stanley Hong Graduate Students Chris Bergevin, Roozbeh Ghaffari, Wendy Gu, Kinuko Masaki, Scott Page
Cochlear Mechanics Academic and Research Staff Professor Dennis M. Freeman, Dr. Alexander Aranyosi, Dr. Stanley Hong Graduate Students Chris Bergevin, Roozbeh Ghaffari, Wendy Gu, Kinuko Masaki, Scott Page
Pitfalls in behavioral estimates of basilar-membrane compression in humans a)
 Pitfalls in behavioral estimates of basilar-membrane compression in humans a) Magdalena Wojtczak b and Andrew J. Oxenham Department of Psychology, University of Minnesota, 75 East River Road, Minneapolis,
Pitfalls in behavioral estimates of basilar-membrane compression in humans a) Magdalena Wojtczak b and Andrew J. Oxenham Department of Psychology, University of Minnesota, 75 East River Road, Minneapolis,
SPHSC 462 HEARING DEVELOPMENT. Overview Review of Hearing Science Introduction
 SPHSC 462 HEARING DEVELOPMENT Overview Review of Hearing Science Introduction 1 Overview of course and requirements Lecture/discussion; lecture notes on website http://faculty.washington.edu/lawerner/sphsc462/
SPHSC 462 HEARING DEVELOPMENT Overview Review of Hearing Science Introduction 1 Overview of course and requirements Lecture/discussion; lecture notes on website http://faculty.washington.edu/lawerner/sphsc462/
The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons.
 1 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
1 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
Evidence and synthesis
 CHAPTER D 9 (Discussion continued) Evidence and synthesis 9.1 Synthesis 9.1/a Outer hair cells as dual detectors Pressure detection and transduction currents The silent current 9.1/b Cancellation effects
CHAPTER D 9 (Discussion continued) Evidence and synthesis 9.1 Synthesis 9.1/a Outer hair cells as dual detectors Pressure detection and transduction currents The silent current 9.1/b Cancellation effects
Effects of Medial Olivocochlear Auditory Reflex Activation on Cochlear Vibration
 University of Iowa Honors Theses University of Iowa Honors Program Spring 2017 Effects of Medial Olivocochlear Auditory Reflex Activation on Cochlear Vibration Alexandra Redfern Shawn Goodman University
University of Iowa Honors Theses University of Iowa Honors Program Spring 2017 Effects of Medial Olivocochlear Auditory Reflex Activation on Cochlear Vibration Alexandra Redfern Shawn Goodman University
SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS
 SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS Katherine Knisely, D. Bozovic University of California at Los Angeles It has long been observed that there is some form of amplification
SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS Katherine Knisely, D. Bozovic University of California at Los Angeles It has long been observed that there is some form of amplification
Possible mechanisms of cochlear two-tone suppression represented by vector subtraction within a model
 Acoust. Sci. & Tech. 39, 1 (018) PAPER #018 The Acoustical Society of Japan Possible mechanisms of cochlear two-tone suppression represented by vector subtraction within a model Yasuki Murakami 1; and
Acoust. Sci. & Tech. 39, 1 (018) PAPER #018 The Acoustical Society of Japan Possible mechanisms of cochlear two-tone suppression represented by vector subtraction within a model Yasuki Murakami 1; and
Sound and its characteristics. The decibel scale. Structure and function of the ear. Békésy s theory. Molecular basis of hair cell function.
 Hearing Sound and its characteristics. The decibel scale. Structure and function of the ear. Békésy s theory. Molecular basis of hair cell function. 19/11/2014 Sound A type of longitudinal mass wave that
Hearing Sound and its characteristics. The decibel scale. Structure and function of the ear. Békésy s theory. Molecular basis of hair cell function. 19/11/2014 Sound A type of longitudinal mass wave that
9.01 Introduction to Neuroscience Fall 2007
 MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
COCHLEAR MODEL FOR NORMAL AND DAMAGED EARS
 TEL AVIV UNIVERSITY THE IBY AND ALADAR FLEISCHMAN FACULTY OF ENGINEERING The Zandman-Slaner Graduate School of Engineering COCHLEAR MODEL FOR NORMAL AND DAMAGED EARS By Azaria Cohen THESIS SUBMITTED FOR
TEL AVIV UNIVERSITY THE IBY AND ALADAR FLEISCHMAN FACULTY OF ENGINEERING The Zandman-Slaner Graduate School of Engineering COCHLEAR MODEL FOR NORMAL AND DAMAGED EARS By Azaria Cohen THESIS SUBMITTED FOR
Sound and Hearing. Decibels. Frequency Coding & Localization 1. Everything is vibration. The universe is made of waves.
 Frequency Coding & Localization 1 Sound and Hearing Everything is vibration The universe is made of waves db = 2log(P1/Po) P1 = amplitude of the sound wave Po = reference pressure =.2 dynes/cm 2 Decibels
Frequency Coding & Localization 1 Sound and Hearing Everything is vibration The universe is made of waves db = 2log(P1/Po) P1 = amplitude of the sound wave Po = reference pressure =.2 dynes/cm 2 Decibels
P T P V U V I. INTRODUCTION. II. MODEL FORMULATION A. Cochlear fluid dynamics.
 Integration of outer hair cell activity in a one-dimensional cochlear model Azaria Cohen and Miriam Furst a) School of Electrical Engineering, Faculty of Engineering, Tel-Aviv University, Tel-Aviv 69978,
Integration of outer hair cell activity in a one-dimensional cochlear model Azaria Cohen and Miriam Furst a) School of Electrical Engineering, Faculty of Engineering, Tel-Aviv University, Tel-Aviv 69978,
MECHANISM OF HEARING
 MECHANISM OF HEARING Sound: Sound is a vibration that propagates as an audible wave of pressure, through a transmission medium such as gas, liquid or solid. Sound is produced from alternate compression
MECHANISM OF HEARING Sound: Sound is a vibration that propagates as an audible wave of pressure, through a transmission medium such as gas, liquid or solid. Sound is produced from alternate compression
Implementation of Spectral Maxima Sound processing for cochlear. implants by using Bark scale Frequency band partition
 Implementation of Spectral Maxima Sound processing for cochlear implants by using Bark scale Frequency band partition Han xianhua 1 Nie Kaibao 1 1 Department of Information Science and Engineering, Shandong
Implementation of Spectral Maxima Sound processing for cochlear implants by using Bark scale Frequency band partition Han xianhua 1 Nie Kaibao 1 1 Department of Information Science and Engineering, Shandong
I. INTRODUCTION.
 Inner hair cell response patterns: Implications for low-frequency hearing M. A. Cheatham a) and P. Dallos Audiology and Hearing Sciences, Communication Sciences and Disorders, The Hugh Knowles Center,
Inner hair cell response patterns: Implications for low-frequency hearing M. A. Cheatham a) and P. Dallos Audiology and Hearing Sciences, Communication Sciences and Disorders, The Hugh Knowles Center,
I. INTRODUCTION. J. Acoust. Soc. Am. 111 (1), Pt. 1, Jan /2002/111(1)/271/14/$ Acoustical Society of America
 The use of distortion product otoacoustic emission suppression as an estimate of response growth Michael P. Gorga, a) Stephen T. Neely, Patricia A. Dorn, and Dawn Konrad-Martin Boys Town National Research
The use of distortion product otoacoustic emission suppression as an estimate of response growth Michael P. Gorga, a) Stephen T. Neely, Patricia A. Dorn, and Dawn Konrad-Martin Boys Town National Research
