What Drives Mechanical Amplification in the Mammalian Cochlea?
|
|
|
- Dwight Dorsey
- 5 years ago
- Views:
Transcription
1 What Drives Mechanical Amplification in the Mammalian Cochlea? Robert H. Withnell, Lauren A. Shaffer, and David J. Lilly The recent report by Peter Dallos and colleagues of the gene and protein responsible for outer hair cell somatic motility (Zheng, Shen, He, Long, Madison, & Dallos, 2000), and the work of James Hudspeth and colleagues demonstrating that vestibular stereocilia are capable of providing power that may boost the vibration of structures within the inner ear (Martin & Hudspeth, 1999), presents the tantalizing possibility that we may not be far away from answering the question what drives mechanical amplification in the mammalian cochlea? This article reviews the evidence for and against each of somatic motility as the motor, and a motor in the hair cell bundle, producing cochlear mechanical amplification. We consider three models based on somatic motility as the motor and two based on a motor in the hair cell bundle. Available evidence supports a hair cell bundle motor in nonmammals but the upper frequency limit of mammalian hearing in general exceeds that of nonmammals, in many cases by an order of magnitude or more. Only time will tell whether an evolutionary dichotomy exists (Manley, Kirk, Köppl, & Yates, 2001). (Ear & Hearing 2002;23;49 57) Without doubt the most vexing problem facing auditory biophysicists interested in mammalian cochlear function is what drives mechanical amplification in the mammalian cochlea? The idea of a motor in the cochlea was first suggested by Thomas Gold in 1948, the existence of such a motor being necessary to overcome the viscous forces associated with the fluids in the cochlea and so provide the observed frequency selectivity seen in mammalian audition. Over time, evidence has accumulated to implicate outer hair cells (OHCs) as the origin of this cellular mechanical amplification (Dallos, 1992; Yates, Johnstone, Patuzzi, & Robertson, 1992); however, the mechanism by which these cells mechanically amplify basilar membrane vibration is not known. The cochlear amplifier (Davis, 1983) is a leveldependent, physiologically vulnerable process within the cochlea that amplifies basilar membrane Department of Speech and Hearing Sciences (R.H.W., L.A.S.), Indiana University, Bloomington, Indiana; and Veterans Affairs National Center for Rehabilitative Auditory Research (D.J.L.), Portland, Oregon. vibration. Inherent in the operation of the cochlear amplifier is a motor or active process* that imparts mechanical energy into the basilar membrane. Mechanical amplification improves hearing sensitivity, frequency selectivity, and increases dynamic range (Patuzzi, 1996). Enhancement of basilar membrane vibration associated with the operation of the cochlear amplifier is depicted schematically in Figure 1. A hypothetical basilar membrane traveling wave in response to a pure tone is shown for the passive and the active cases. The operation of the cochlear amplifier increases basilar membrane vibration (the active case), but only near to the place where the cochlea is tuned to that particular frequency. Remote from this characteristic frequency (CF) place, basilar membrane vibration is the same for both the active and passive cases. It is likely that the cochlear amplifier operates all along that part of the cochlear partition stimulated by the pure tone but that it is only effective near the CF place (Johnstone, Patuzzi, & Yates, 1986). Figure 2 (from Sellick, Patuzzi, & Johnstone, 1982) demonstrates the increased auditory sensitivity and frequency selectivity produced by cochlear mechanical amplification. Measurement of basilar membrane vibration was made at the 18 khz CF place in a cochlea in good condition, subsequently when the condition of the cochlea had deteriorated, and finally postmortem. With the cochlea in good condition, the stimulus level to evoke a threshold response was about 15 db SPL at the best or most sensitive response frequency (18 khz). With deterioration in cochlear condition, the cochlear amplifier had been affected, and it then took a 60 db SPL stimulus to evoke a threshold response at 18 khz. The tuning curve was also less sharp with cochlear damage, i.e., frequency selectivity was poorer. The *In this article, motor and active process are used synonymously. Strictly speaking, the motor is part of the active process, where the active process represents the electrical to mechanical transduction stage of the cochlear amplifier feedback loop. The increase in dynamic range is a result of the intensitydependence of this mechanical amplification, the amount of amplification being greatest at low stimulus levels. Basilar membrane measurements reflect only the cochlear mechanical response. Damage to IHCs presumably does not affect basilar membrane measurements. However, damage to OHCs, the generator of cochlear mechanical amplification, will affect the basilar membrane response. 0196/0202/02/ /0 Ear & Hearing Copyright 2002 by Lippincott Williams & Wilkins Printed in the U.S.A. 49
2 50 EAR &HEARING /FEBRUARY 2002 Figure 1. Schematic of hypothetical basilar membrane travelling wave in response to a pure tone in the active and the passive case, illustrating the increased amplification of the basilar membrane response near the CF place associated with the action of the cochlear amplifier. The horizontal line represents the basilar membrane at rest. Figure 2. Basilar membrane iso-response functions from measurements made in the first turn of a guinea pig cochlea. Filled circles represent the cochlea in good condition, unfilled circles when cochlear condition had deteriorated (from Sellick et al., 1982). Comparison of the stimulus level to generate a threshold response at 18 khz when the cochlea was in good condition versus when it had deteriorated reveals a 50 db change due to loss of cochlear mechanical amplification. (Reproduced from Figure 15(b) of Sellick et al. (1982), Journal of the Acoustical Society of America, 72, , with permission). cochlear amplifier provides up to ~60 db of gain (Patuzzi & Rajan, 1992; Rajan & Patuzzi, 1992) and provides for much finer resolution of frequency. It is also apparent from Figure 2 that the cochlear amplifier is effective only within a limited region near the CF place basilar membrane responses to stimuli with frequencies below 12 khz were unaffected by the loss of cochlear mechanical amplification. Associated with the action of the cochlear amplifier may be an alteration in the effective stiffness of the basilar membrane (Kolston, 2000), producing a change in basilar membrane tuning. This can readily be inferred from Figure 2: initially with the cochlea in good condition the basilar membrane was sharply tuned with the most sensitive response at 18 khz; with a deterioration in cochlear condition the basilar membrane was not as sharply tuned and the most sensitive response shifted to near 12 khz. The site of measurement on the basilar membrane, however, had not changed, i.e., the basilar membrane at the site of measurement was initially tuned to 18 khz with the cochlea in good condition and then to 12 khz with a deterioration in cochlear condition. This shift in tuning for the same place on the basilar membrane may demonstrate the effect of mechanical cochlear amplification on basilar membrane tuning the shift in tuning might be due to a change in effective stiffness of the basilar membrane produced by the operation of the cochlear amplifier, i.e., there is an alteration in the resonant place (resonance meaning that place where the reactive elements cancel, not the position of maximum basilar membrane vibration). It may also be that the operation of the cochlear amplifier does not alter the effective stiffness of the basilar membrane. Rather, the resonant place is unaltered and the change in frequency at which maximal vibration occurs is not due to a change in tuning but to a reduction in effectiveness of cochlear mechanical amplification (Shera, 2001). Both cases are illustrated in Figure 3. The difference between an amplifier that alters basilar membrane tuning and one that does not is not a trivial one it has significant implications for how mechanical amplification might be achieved. So, given that we do not know what drives mechanical amplification in the mammalian cochlea, do we have any good ideas? At present, the two best theories are 1) somatic motility or changes in cellbody length (this is perhaps the most well known), and 2) a motor in the hair cell bundle. Before considering both of these theories in detail it is provident to review first what we know about the cochlear amplifier and what is required for mechanical amplification in the cochlea to occur. The Cochlear Amplifier Positive Feedback Process Cochlear mechanical amplification is thought to be achieved by a positive feedback process (see Fig. 4). Positive feedback processes are prone to instability (hence spontaneous otoacoustic emissions) but have the virtue
3 EAR &HEARING, VOL. 23 NO Figure 4. The Cochlear Amplifier. A positive feedback process that enhances basilar membrane vibration on a cycle-bycycle basis. MET or mechanical to electrical transduction involves a modulation of the flow of ions through ion channels at the top of the stereocilia as a result of stereocilial deflection. EMT or electrical to mechanical transduction involves the conversion of an electrical event into a mechanical event. See text for further details. Figure 3. Schematic of basilar membrane travelling wave illustrating (i) a shift in basilar membrane stiffness producing a shift in the resonant place, and (ii) no change in basilar membrane stiffness with the resonant place unaltered, but the place of maximum vibration is shifted due to a reduction in effectiveness of cochlear mechanical amplification (Shera, 2001). In each case, the travelling wave is in response to the same low-level stimulus (no change in frequency), the darker line representing a cochlea in good condition, the lighter line when the same cochlea has deteriorated and the cochlear amplifier is less effective. The horizontal line represents the basilar membrane at rest to. Note: The resonant place is that place where the reactive elements cancel; this is not the place of maximal basilar membrane displacement but rather the place after peak displacement at which basilar membrane displacement is effectively zero (damping is thought to have dissipated all the energy in the travelling wave just before the resonant place is reached [Lighthill, 1991]). that existing conditions are amplified or boosted. The first step in the amplification process is the displacement of the basilar membrane produced by stapes vibration-induced volume displacement of fluids in the cochlea. This volume displacement produces a pressure gradient across the basilar membrane. Deformation of the basilar membrane produces a shearing action between the tectorial membrane and the reticular lamina, producing a deflection of the stereocilia of the receptor cells. Both inner and outer hair cells have fine (tip) links that mechanically join adjacent stereocilia. Deflection of the stereocilia is thought to produce a force on the transduction channels via these links, opening the channels (Pickles, Comis, & Osborne, 1984). Potassium ions flow through open transduction channels raising the intracellular potential and altering the potential difference across the basolateral wall, which in the inner hair cells (IHCs) results in a release of neurotransmitter at afferent synapses (see Fig. 5). The flow of ions through these transduction channels located at the top of the stereocilia as a result of stereocilial deflection is termed mechano-electrical transduction, or MET. Positive ion flow through transduction channels is occurring constantly. This ion flow is a product of the electromotive force or positive charge that is maintained in Scala Media by the active pumping of potassium ions through the Stria Vascularis, and the negative intracellular charge or cell resting membrane potential. This constant ion flow is termed a standing current to. Deflection of the stereocilia modulates this standing current so that current flow is increased when stereocilia are deflected away from the modiolus (excitatory direction), and current flow is decreased when stereocilia are deflected towards the modiolus (inhibitory direction). Mechanical amplification by OHCs is achieved when an electrical event (the current flow or a voltage difference) is converted into a mechanical event. This is called electromechanical transduction The opening of hair cell transduction channels is more complex than presented here, being probabilistic in nature. The probability of a channel being open or closed is described by Maxwell- Boltzmann statistics; deflection of stereocilia changes the likelihood of a channel being open or closed. These transduction channels are cation selective (Kros, 1996). Potassium, by virtue of it being the predominant cation in endolymph, provides most of the ion current through these channels.
4 52 EAR &HEARING /FEBRUARY 2002 oppose friction. The force generated by the active process must be in phase with basilar membrane velocity. Viscous forces associated with cochlear fluids oppose basilar membrane vibration and a force in phase with this vibration or basilar membrane velocity will act to counter these viscous forces, i.e., will produce a negative damping. Figure 5. Schematic of a hair cell. The flow of potassium ions through open transduction channels depolarizes the hair cell, altering the trans-membrane voltage ( V). In inner hair cells, the alteration in this membrane potential opens voltage-gated calcium channels in the basolateral membrane, allowing calcium to flow in to the cell. Calcium mediates the release of neurotransmitter at the afferent synapse of the inner hair cell. (EMT) or the active process. The mechanical event (whatever it is) generates a force in phase with basilar membrane velocity that increases basilar membrane vibration. The increase in basilar membrane vibration further deflects the stereocilia at the top of the hair cells, increasing the modulated current that flows through the transduction channels, which in turn increases the power added by the active process. Accordingly, there is an inherent rise time for cochlear mechanical amplification to reach a stable level where the modulated current flowing through the transduction channels at the top of the stereocilia of OHCs is in balance with the resultant mechanical force on the basilar membrane. Requirements for the Active Process in the Cochlea to Work The active process, whatever it is, must possess the following properties (Yates, 1995): 1. It has to be fast. The upper limit to mammalian hearing is in excess of 100 khz, with some whales, for instance, having hearing that extends to 100 to 140 khz (Hemilä, Nummela, & Reuter, 2001). So the active process must operate on a time scale of microseconds (or faster) to influence basilar membrane vibration on a cycle-by-cycle basis. 2. The force generated by the active process must What is the Active Process? Somatic Motility In the mid 1980s, William Brownell and coworkers reported that mammalian OHCs in a dish (termed in vitro) demonstrated somatic motility, i.e., the cell bodies were observed to contract and elongate (Brownell, 1983; Brownell, Bader, Bertrand, de Ribaupierre, 1985). This somatic motility is voltage-dependent (Iwasa & Kachar, 1989; Santos-Sacchi & Dilger, 1988), being produced by changes in trans-membrane potential (Dallos, Evans, & Hallworth, 1991). The basis of this somatic motility was not known until recently, but was thought to be due to voltage-dependent conformational (shape) changes in proteins embedded in the cell membrane (Dallos et al., 1991). In 2000, Peter Dallos and coworkers identified the motor protein of the cochlear outer hair cell (Zheng et al., 2000, p. 149). This protein, called Prestin, is expressed in OHCs but not in IHCs, and is a trans-membrane protein; when Prestin is present in a cell, the cell can undergo voltageinduced changes in shape (Zheng et al., 2000). Somatic motility, if present in vivo, provides a possible cellular mechanism for cochlear mechanical amplification. But what is the source of the voltage drive in situ? Receptor Potential: One possibility is the voltage that develops across the basolateral membrane with the change in intracellular potential that follows modulation of the standing current with stereocilial deflection, i.e., the receptor potential. However, the cell membrane of an OHC (an IHC, and all other cells, in principle) possesses the property of not being able to develop a significant voltage at high frequencies due to its low-pass filtering characteristic # (Santos-Sacchi, 1992) (see Fig. 6). This means that as frequency increases, there will be a point above which there is insufficient receptor potential to provide a motile response that could amplify The cell membrane possesses resistance and capacitance; the resistance is quantified by the ion channels in the membrane, the capacitance by the ability of the membrane to separate charge. # Santos-Sacchi s (1992) measurements of the OHC cut-off frequency were qualified by the fact that the cut-off frequency he obtained was concordant with the voltage clamp amplifier he used.
5 EAR &HEARING, VOL. 23 NO Figure 6. The Receptor Potential. As frequency increases, a frequency may be reached above which the receptor potential cannot drive somatic motility due to low-pass filtering by the membrane capacitance. basilar membrane vibration. Dallos (1996) estimated this frequency to be approximately 6 khz. It has been observed though that without knowledge of the in vivo mechanical impedance of the OHC and its load, one cannot be certain that the electrically low-pass filtered receptor potential is insufficient to drive somatic motility** (Mountain & Hubbard, 1994). Extracellular Voltage-Drive: Dallos and Evans (1995) have suggested that an extracellular voltage source could overcome the frequency limitation set by membrane filtering. This extra-cellular voltage source is the voltage drop that is a stimulus-related consequence of current flow into the OHC. The electrical impedance of an OHC is treated as having two R-C (resistor-capacitor) components in series, where the apical cell membrane facing endolymph and the basolateral membrane facing perilymph are each comprised of a resistance and capacitance in parallel (see Fig. 7). At high frequencies the resistive elements can be ignored and the coupling between the two components is capacitive (a capacitive voltage divider). The extracellular voltage source is the weighted vector sum of contributions from OHCs basal to the CF place (Dallos, 1996). Such a mechanism can be independent of frequency if the time constants of the impedance elements of the OHC (the two R-C elements in series) are the same (Dallos & Evans, 1995). It has been observed that OHCs in vitro are capable of somatic motility up to at least 79 khz, **Mountain and Hubbard (1994) observed that at resonance the impedance could be quite low such that the receptor potential may be sufficient to drive somatic motility. However, extrapolating the in vitro findings of Frank et al. (1999) of constant force production suggests that the outer hair cell plus load impedance at resonance would have to reduce concurrently with receptor potential. Figure 7. The outer hair cell electrical impedance modeled as two R-C (resistor-capacitor) components in series (Dallos & Evans, 1995), where the apical cell membrane facing endolymph and the basolateral membrane facing perilymph are each comprised of a resistance and capacitance in parallel. At high frequencies, the coupling between the apical and basolateral membranes is capacitive, i.e., a capacitive voltage divider. with a force that is constant up to 50 khz (Frank, Hemmert, & Gummer, 1999). If such properties translate to in vivo, OHCs would be capable of a speed that goes some way towards that necessary to provide for cochlear mechanical amplification up to 100 to 140 khz (the upper limit for mammalian hearing). However, a constant force production may be insufficient the force generated by the active process must increase at 6 db/octave to overcome viscous forces (Yates, 1995). A capacitively coupled extra-cellular voltagedriven motor is perhaps at odds with some recent work by Yates and Kirk (1998). In this article, electrically evoked otoacoustic emissions (EEOAEs) were recorded that resulted from current injection into Scala Media. To examine whether the voltage that results from such a current source is capacitively coupled to the extracellular spaces within the organ of Corti, an acoustic low-frequency biasing tone was introduced that would bias the state of the OHC transduction channels. Yates and Kirk found that the EEOAE was modulated by the presence of the biasing tone (see Fig. 8), arguing for much of the electrical current to have passed through the transduction channels, as distinct from a capacitive coupling where the state of the transduction channels should be unimportant, i.e., the EEOAE would not be modulated by the biasing tone if the current did not flow through the transduction channels. To elucidate further the feasibility of the model of Dallos and Evans, appropriate measurements within the organ of Corti are needed. Electrical Energy as Drive Source: Santos-Sacchi, Kakehata, Kikuchi, Katori, and Takasaka (1998) have suggested that there is no frequency limitation due to membrane filtering, arguing that the impor-
6 54 EAR &HEARING /FEBRUARY 2002 Figure 8. Amplitude-modulation of electrically evoked otoacoustic emissions (Yates & Kirk, 1998). Electrical current was injected into Scala Media, as illustrated in (a) and (b). The position of the electrode tip relative to the organ of Corti is illustrated in (c). Panel (d) shows the amplitude-modulated electrically evoked otoacoustic emissions obtained for an electrical current frequency of 12,015 Hz and a low-frequency acoustic biasing tone of 86 Hz at three different sound pressure levels (82, 94, 106 db). (Reproduced in adapted form from Figures 1 and 4, Yates and Kirk (1998), Journal of Neuroscience, 18(6), , with permission. Copyright 1998 by the Society for Neuroscience). tant quantity is the electrical energy (Q V) supplied to the OHC basolateral membrane. The lateral membrane motor protein density of an OHC is predicted to increase with decreasing cell length (cell length decreases as one goes from apex to base in the cochlea). So while V (receptor potential) is decreasing with increasing frequency, Q (the charge density, which is equivalent to the motor protein density) is increasing with frequency by the same amount, giving a constant electrical energy source. The end-product of such a model presumably would be an active process with constant force production along the cochlear partition, and as has been stated previously, a constant force production may not be adequate for cochlear mechanical amplification (Yates, 1995). However, if Q increased sufficiently rapidly with frequency, the force production could increase with frequency sufficient to overcome viscous forces. Increased motor-protein density with decreasing cell length is inconsistent with the findings of Frank et al. (1999). A Motor in the Hair Cell Bundle An active process powered by the hair cell bundle located at the top of the OHC was suggested as early as 1982 (Weiss, 1982). Indeed, in nonmammalian vertebrates such as birds and lizards, available evidence argues strongly for a mechanical motor driven by the stereocilia or hair cell bundle, nonmammals not having any cell in the cochlea that resembles the The lateral membrane is the outer layer of the lateral cortex or cell membrane. It is this layer that contains the proteins thought to undergo voltage-induced length changes (Holley, 1996). Figure 9. Myosin Motor Model. (i) Displacement of the hair cell bundle in the positive direction (away from the modiolus) triggers the contraction of myosin molecules associated with the tip-links. (ii) Contraction of myosin molecules alters tip-link tension, producing hair bundle movement in the negative direction. (Reproduced in adapted form from Figure 1, Current Opinion in Neurobiology, 7, Hudspeth, Mechanical amplification of stimuli by hair cells, , Copyright 1997, with permission from Elsevier Science). mammalian OHC (Manley et al., 2001; Manley & Köppl, 1998). But how could such a motor work? Hudspeth (1997) posited two possibilities: 1. A Myosin motor. Displacement of the hair-cell bundle away from the modiolus triggers the contraction of myosin molecules associated with the tip links (Hudspeth, 1997, p. 481), this contraction associated with an interaction between the myosin molecules and actin filaments within the stereocilium (Hudspeth & Gillespie, 1994). Actin-myosin interactions are more commonly associated with the sliding filament model of skeletal muscle contraction, a process that is ATP dependent. It is suggested that the interaction of myosin and actin in this model would alter tip-link tension, moving the hair-cell bundle in the opposite direction (see Fig. 9). A myosin-based motor, however, may be subject to a speed or frequency constraint: the rate at which ATP hydrolysis (breakdown of ATP) can occur (Martin, Mehta, & Hudspeth, 2000). It has been suggested that this speed constraint could be overcome if not every myosin molecule must contribute on every cycle of oscillation (Hudspeth & Gillespie, 1994; Manley & Gallo, 1997); however, it has been suggested that it is difficult to conceive how such a motor could operate at speeds of 100 khz and greater (Choe, Magnasco, & Hudspeth, 1998). 2. A MET channel motor. Displacement of the hair cell bundle away from the modiolus opens the MET channel, resulting in an increase in
7 EAR &HEARING, VOL. 23 NO thousands of hertz (Fettiplace, Ricci, & Hackney, 2001; Martin & Hudspeth, 1999; Ricci et al., 2000). Further, in vivo evidence of a motor in the hair cell bundle in a nonmammalian vertebrate operating at least on the order of a kilohertz has been reported recently by Manley et al. (2001). Figure 10. MET Channel Motor Model. (i) Displacement of the hair cell bundle in the positive direction (away from the modiolus) opens MET channels, allowing potassium and calcium to enter the cell. Calcium is hypothesized to bind to a site on the interior aspect of the channel, promoting channel reclosure. (ii) Channel reclosure alters tip-link tension, moving the hair cell bundle in the opposite direction. (Reproduced in adapted form from Figure 1, Current Opinion in Neurobiology, 7, Hudspeth, Mechanical amplification of stimuli by hair cells, , Copyright 1997, with permission from Elsevier Science). potassium and calcium entering the cell. Calcium, it has been hypothesized, binds to a site on or related to the channel, promoting channel re-closure. This channel re-closure produces a rise in tip-link tension [that] jerks the hair bundle back in the negative direction (Hudspeth, 1997, p. 481) (see Fig. 10). As with myosin, there may be a speed limitation, in this case the rate of binding and disassociation of calcium to and from the binding site (Choe et al., 1998). And as with myosin, this constraint could be overcome at higher frequencies if only some fraction of the total number of MET channels contributes on each cycle of oscillation. Both 1 and 2 suggest a force associated with channel re-closure. To be effective, this force must be in-phase with the inhibitory direction of stereocilia deflection. This requires a frequency-selective mechanism for force delivery (see Choe et al., 1998; Hudspeth, 1997), such tuning perhaps being inherent in the physical properties of the stereocilia, e.g., elastic properties of the hair bundle (Camalet, Duki, Jülicher, & Prost, 2000). Active hair-bundle movement, i.e., bundle movement larger than the stimulus, which represents a source of power that could drive cochlear mechanical amplification, has been observed in isolated hair cells from the sacculus of the bullfrog (Martin & Hudspeth, 1999) and the auditory papilla of the turtle (Ricci, Crawford, & Fettiplace, 2000). To date, such active hair-bundle movement has been observed on the order tens of hertz and perhaps Isolating the Active Process The active process or EMT is contained in a feedback loop that makes isolating it difficult, if not impossible. The operation of any element in a feedback loop is dependent on the operation of all other elements within the loop. Interfering with one or more elements affects all of the elements with the result that one cannot ascertain which element is the active process. Experiments have been performed with pharmacologic agents that in vitro have been found to damage the basolateral membrane of the OHC, perhaps the most widely investigated involving the perfusion of salicylate into Scala Tympani. Salicylate alters the properties of the OHC basolateral membrane (Shehata, Brownell, & Dieler, 1991; Stypulkowski, 1990) and interferes with somatic motility (Kakehata & Santos-Sacchi, 1996) while seemingly (in the short term) not affecting MET channels at the top of the stereocilia nor entering the cell through these MET channels (Fitzgerald, Robertson, & Johnstone, 1993). After salicylate perfusion in to Scala Tympani, it has been observed that: 1) cubic-distortion tone otoacoustic emissions (2f 1 -f 2 ) are reduced; 2) compound action potential thresholds are elevated; and 3) the cochlear microphonic measured at the round window in response to a 1 khz tone is increased in magnitude (endocochlear potential did not change) (Fitzgerald et al., 1993). Such findings are consistent with an alteration in the properties of the basolateral membrane and interference with an active process involving somatic motility; however, a shift in operating point associated with depolarization of the OHC would presumably also account for such findings. Indeed, Frank and Kossl (1996) observed changes in the cubic and quadratic distortion tone otoacoustic emis- Manley et al. (2001) measured electrically evoked otoacoustic emissions that were amplitude modulated by a low-frequency acoustical stimulus, the unique modulation pattern being traceable to a hair cell bundle motor (made possible by a unique hair cell bundle arrangement in the lizard in the high-frequency region where bundles are oppositely oriented). The operating point reflects the probability of a transduction channel being open versus closed in the absence of sound. In OHCs, the open channel probability is dependent on stereocilial displacement shortening of the cell body associated with depolarization of the OHC increases the open channel probability, i.e., shifts the operating point.
8 56 EAR &HEARING /FEBRUARY 2002 sions initially after salicylate perfusion in to Scala Tympani that are consistent with an operating point shift. DISCUSSION Nonmammalian vertebrates would seem to have a motor in the hair cell bundle that provides cochlear mechanical amplification (Manley et al., 2001). OHCs appear to be unique to mammals, demonstrating a somatic motility not seen in nonmammalian vertebrate hair cells. So if mammals have a hair cell bundle motor as it appears do nonmammalian vertebrates, what is the purpose of somatic motility an automatic gain control via shifts in operating point, or does indeed an evolutionary dichotomy exist? The determination of what mechanism is responsible for the active process in the mammalian cochlea presupposes that there is only one mechanism. Indeed, this article has considered cochlear mechanical amplification in mammals due to a motor in the hair cell bundle versus a motor based on somatic motility. It may be that the active process in the mammalian cochlea consists of more than one mechanism, i.e., cochlear mechanical amplification in mammals may be achieved by a motor in the hair cell bundle and a motor based on somatic motility (Manley, 2001), or indeed, the motor may be spatially dependent, e.g., a different mechanism may operate in the base versus the apex of the cochlea. Of course, such a dual motor system introduces the additional complication of timing differences in the operation of each of the motors. With the discovery by Dallos and coworkers of Prestin, the protein in OHCs that provides for somatic motility, the next step presumably in the quest for isolating the active process is the development of a knock-out mouse, a mouse lacking the gene for Prestin. Of course, concomitant with a hair cell deficient in this protein may be changes in cochlear function that preclude a simple answer to the question of the location of the motor in the mammalian OHC. The determination of what is the active process involves understanding how electrical energy is converted into mechanical energy with the generation of a force that amplifies basilar membrane vibration. This can only be achieved by being able to answer the following questions: 1. what is the molecular motor? 2. how does this motor utilize electrical energy to produce mechanical energy? 3. how is the force produced by this motor coupled in to the organ of Corti with a phase that opposes viscous forces so as to enhance basilar membrane vibration? Only by being able to answer all of the above questions can we be certain that the active process has truly been found (and in the process of course satisfying the constraint of speed, i.e., operating on a time scale of microseconds or faster). For nonmammalian vertebrates a general consensus has been reached that the motor is in the hair cell bundle. It remains to be seen whether mammals have evolved a different solution for mechanical amplification in the cochlea. ACKNOWLEDGMENTS: This work was supported in part by an NIH-NIDCD T32 DC00012 Training Grant (L.A.S.), and in part by a grant to the National Center for Rehabilitative Auditory Research (RCTR S ) and a Merit-Review Award from the Department of Veterans Affairs Rehabilitation Research and Development Service (C2225R) (D.J.L.). Portions of this paper were presented at the conference on Perceptual Consequences of Cochlear Nonlinearity, Hanse Institute for Advanced Study, Delmenhorst, Germany, August, We wish to express our sincerest gratitude to Dr. Christopher Shera, Professor Geoffrey Manley, and Professor Joseph Santos-Sacchi for the valuable advice they provided that improved this paper. Our thanks also to Dr. Carrick Talmadge for providing the mathematical equations to produce the traveling wave schematics in Figures 1 and 3. Address for correspondence: Robert H. Withnell, Ph.D., Department of Speech and Hearing Sciences, Indiana University, 200 South Jordan Avenue, Bloomington, IN Received April 24, 2001; accepted September 28, 2001 REFERENCES Brownell, W. E. (1983). Observations on a motile response in isolated outer hair cells. In W. R. Webster & L. M. Aitkin (Eds.), Mechanisms of Hearing (pp. 5 10). Clayton, Victoria, Australia: Monash University Press. Brownell, W. E., Bader, C. R., Bertrand, D., & de Ribaupierre, Y. (1985). Evoked mechanical responses of isolated cochlear outer hair cells. Science, 227, Camalet, S., Duke, T., Jülicher, F., & Prost, J. (2000). Auditory sensitivity provided by self-tuned critical oscillations of hair cells. Proceedings of the National Academy of Sciences, 97, Choe, Y., Magnasco, M. O., & Hudspeth, A. J. (1998). A model for amplification of hair-bundle motion by cyclical binding of Ca2 to mechanoelectrical-transduction channels. Proceedings of the National Academy of Sciences, 95, Dallos, P. (1992). The active cochlea. Journal of Neuroscience, 12, Dallos, P. (1996). Overview: Cochlear neurobiology. In P. Dallos, A. N. Popper, & R. R. Fay (Eds.), The Cochlea (pp. 1 43). New York: Springer. Dallos, P., & Evans, B. N. (1995). High-frequency motility of outer hair cells and the cochlear amplifier. Science, 31, 267, Dallos, P., Evans, B. N., & Hallworth, R. (1991). Nature of the motor element in electrokinetic shape changes of cochlear outer hair cells. Nature, 14, 350,
9 EAR &HEARING, VOL. 23 NO Davis, H. (1983). An active process in cochlear mechanics. Hearing Research, 9, Fettiplace, R., Ricci, A. J., & Hackney, C. M. (2001). Clues to the cochlear amplifier from the turtle ear. Trends in Neuroscience, 24, Fitzgerald, J. J., Robertson, D., & Johnstone, B. M. (1993). Effects of intra-cochlear perfusion of salicylates on cochlear microphonic and other auditory responses in the guinea pig. Hearing Research, 67, Frank, G., Hemmert, W., & Gummer, A. W. (1999). Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proceedings of the National Academy of Sciences, 13, 96, Frank, G., & Kossl, M. (1996). The acoustic two-tone distortions 2f1-f2 and f2-f1 and their possible relation to changes in the operating point of the cochlear amplifier. Hearing Research, 98, Gold, T. (1948). Hearing II. The physical basis of the action of the cochlea. Proceedings of the Royal Society of London [Biol], 135, Hemilä, S., Nummela, S., & Reuter, T. (2001). Modelling whale audiograms: Effects of bone mass on high-frequency hearing. Hearing Research, 151, Holley, M. C. (1996). Outer hair cell motility. In P. Dallos, A. N. Popper, & R. R. Fay (Eds.), The Cochlea (pp ). New York: Springer. Hudspeth, A. J. (1997). Mechanical amplification of stimuli by hair cells. Current Opinions in Neurobiology, 7, Hudspeth, A. J., & Gillespie, P. G. (1994). Pulling springs to tune transduction: adaptation by hair cells. Neuron, 12, 1 9. Iwasa, K. H., & Kachar, B. (1989). Fast in vitro movement of outer hair cells in an external electric field: Effect of digitonin, a membrane permeabilizing agent. Hearing Research, 40, Johnstone, B. M., Patuzzi, R. B., & Yates, G. K. (1986). Basilar membrane measurements and the travelling wave. Hearing Research, 22, Kakehata, S., & Santos-Sacchi, J. (1996). Effects of salicylate and lanthanides on outer hair cell motility and associated gating charge. Journal of Neuroscience, 16, Kolston, P. J. (2000). The importance of phase data and model dimensionality to cochlear mechanics. Hearing Research, 145, Kros, C. J. (1996). Physiology of mammalian cochlear hair cells. In P. Dallos, A. N. Popper, & R. R. Fay (Eds.), The Cochlea (pp ). New York: Springer. Lighthill, J. (1991). Biomechanics of hearing sensitivity. Journal of Vibration and Acoustics, 113, Manley, G. A. (2001). Evidence for an active process and a cochlear amplifier in non-mammals. Journal of Neurophysiology, 86, Manley, G. A. & Gallo, L. (1997). Otoacoustic emmisions, hair cells, and myosin motors. Journal of the Acoustical Society of America, 102, Manley, G. A., Kirk, D. L., Köppl, C., & Yates, G. K. (2001). In vivo evidence for a cochlear amplifier in the hair-cell bundle of lizards. Proceedings of the National Academy of Sciences, 98, Manley, G. A., & Köppl, C. (1998). Phylogenetic development of the cochlea and its innervation. Current Opinions in Neurobiology, 8, Martin, P., & Hudspeth, A. J. (1999). Active hair-bundle movements can amplify a hair cell s response to oscillatory mechanical stimuli. Proceedings of the National Academy of Sciences, 7, Martin, P., Mehta, A. D., & Hudspeth, A. J. (2000). Negative hair-bundle stiffness betrays a mechanism for mechanical amplification by the hair cell. Proceedings of the National Academy of Sciences, 97, Mountain, D. C., & Hubbard, A. E. (1994). A piezoelectric model of outer hair cell function. Journal of the Acoustical Society of America, 95, Patuzzi, R. (1996). Cochlear micromechanics and macromechanics. In P. Dallos, A. N. Popper, & R. R. Fay (Eds.), The Cochlea (pp ). New York: Springer. Patuzzi, R. B., & Rajan, R. (1992). Additivity of threshold elevations produced by disruption of outer hair cell function. Hearing Research, 60, Pickles, J. O., Comis, S. D., & Osborne, M. P. (1984). Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hearing Research, 15, Rajan, R., & Patuzzi, R. B. (1992). Additivity of threshold losses produced by acute acoustic trauma. Hearing Research, 60, Ricci, A. J., Crawford, A. C., & Fettiplace, R. (2000). Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. Journal of Neuroscience, 20, Santos-Sacchi, J. (1992). On the frequency limit and phase of outer hair cell motility: Effects of the membrane filter. Journal of Neuroscience, 12, Santos-Sacchi, J., & Dilger, J. P. (1988). Whole cell currents and mechanical responses of isolated outer hair cells. Hearing Research, 35, Santos-Sacchi, J., Kakehata, S., Kikuchi, T., Katori, Y., & Takasaka, T. (1998). Density of motility-related charge in the outer hair cell of the guinea pig is inversely related to best frequency. Neuroscience Letters, 13, Sellick, P. M., Patuzzi, R. B., & Johnstone, B. M. (1982). Measurement of basilar membrane motion in the guinea pig using the Mossbauer technique. Journal of the Acoustical Society of America, 72, Shehata, W. E., Brownell, W. E., & Dieler, R. (1991). Effects of salicylate on shape, electromotility and membrane characteristics of isolated outer hair cells from guinea pig cochlea. Acta Otolaryngologica, 111, Shera, C. A. (2001). Intensity-invariance of fine time structure in basilar-membrane click responses: Implications for cochlear mechanics. Journal of the Acoustical Society of America, 110, Stypulkowski, P. H. (1990). Mechanisms of salicylate ototoxicity. Hearing Research, 46, Weiss, T. F. (1982). Bidirectional transduction in vertebrate hair cells: a mechanism for coupling mechanical and electrical processes. Hearing Research, 7, Yates, G. K. (1995). Cochlear structure and function. In B. Moore (Ed.), Hearing (pp ). San Diego: Academic Press. Yates, G. K., Johnstone, B., Patuzzi, R., & Robertson, D. (1992). Mechanical preprocessing in the mammalian cochlea. Trends in Neuroscience, 15, Yates, G. K., & Kirk, D. L. (1998). Cochlear electrically evoked emissions modulated by mechanical transduction channels. Journal of Neuroscience, 18, Zheng, J. Shen, W., He, D. Z., Long, K. B., Madison, L. B., & Dallos, P. (2000). Prestin is the motor protein of cochlear outer hair cells. Nature, 11, 405,
Chapter 3: Anatomy and physiology of the sensory auditory mechanism
 Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA
 451 THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA DÁIBHID Ó MAOILÉIDIGH, FRANK JÜLICHER Max Planck Institute für Physik komplexer Systeme, Nöthnitzerstr. 38, 01187
451 THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA DÁIBHID Ó MAOILÉIDIGH, FRANK JÜLICHER Max Planck Institute für Physik komplexer Systeme, Nöthnitzerstr. 38, 01187
Auditory System Feedback
 Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier
 HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier 1 Cochlear Mechanics: Measures of Basilar Membrane Motion 2 Cochlear Mechanics: Measures of Basilar Membrane Motion Bekesy s experiments
HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier 1 Cochlear Mechanics: Measures of Basilar Membrane Motion 2 Cochlear Mechanics: Measures of Basilar Membrane Motion Bekesy s experiments
Cochlear anatomy, function and pathology I. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology I Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of these lectures Introduction to gross anatomy of the cochlea Focus (1) on
Cochlear anatomy, function and pathology I Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of these lectures Introduction to gross anatomy of the cochlea Focus (1) on
The magnificent outer hair cell of Corti's organ
 The magnificent outer hair cell of Corti's organ Yale University J. Santos-Sacchi School of Medicine, New Haver, CT, USA Abstract The outer hair cell is one of two receptor cell types in the organ of Corti.
The magnificent outer hair cell of Corti's organ Yale University J. Santos-Sacchi School of Medicine, New Haver, CT, USA Abstract The outer hair cell is one of two receptor cell types in the organ of Corti.
Cochlear anatomy, function and pathology II. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
Structure, Energy Transmission and Function. Gross Anatomy. Structure, Function & Process. External Auditory Meatus or Canal (EAM, EAC) Outer Ear
 Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
Mechanical Properties of the Cochlea. Reading: Yost Ch. 7
 Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs
 Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs The Auditory Laboratory, Physiology University of Western Australia Greg O Beirne Dept. of Communication
Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs The Auditory Laboratory, Physiology University of Western Australia Greg O Beirne Dept. of Communication
What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components
 1 2 3 4 What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components as a function of time here during the production
1 2 3 4 What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components as a function of time here during the production
Processing of sounds in the inner ear
 Processing of sounds in the inner ear Sripriya Ramamoorthy Associate Professor, IIT Bombay WiSSAP 2018 Cochlea converts sound into electrical signals [Picture courtesy of Northwestern University] von Bekesy
Processing of sounds in the inner ear Sripriya Ramamoorthy Associate Professor, IIT Bombay WiSSAP 2018 Cochlea converts sound into electrical signals [Picture courtesy of Northwestern University] von Bekesy
What is the effect on the hair cell if the stereocilia are bent away from the kinocilium?
 CASE 44 A 53-year-old man presents to his primary care physician with complaints of feeling like the room is spinning, dizziness, decreased hearing, ringing in the ears, and fullness in both ears. He states
CASE 44 A 53-year-old man presents to his primary care physician with complaints of feeling like the room is spinning, dizziness, decreased hearing, ringing in the ears, and fullness in both ears. He states
SOLUTIONS Homework #3. Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03
 SOLUTIONS Homework #3 Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03 Problem 1: a) Where in the cochlea would you say the process of "fourier decomposition" of the incoming
SOLUTIONS Homework #3 Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03 Problem 1: a) Where in the cochlea would you say the process of "fourier decomposition" of the incoming
A truly remarkable aspect of human hearing is the vast
 AUDITORY COMPRESSION AND HEARING LOSS Sid P. Bacon Psychoacoustics Laboratory, Department of Speech and Hearing Science, Arizona State University Tempe, Arizona 85287 A truly remarkable aspect of human
AUDITORY COMPRESSION AND HEARING LOSS Sid P. Bacon Psychoacoustics Laboratory, Department of Speech and Hearing Science, Arizona State University Tempe, Arizona 85287 A truly remarkable aspect of human
Unit VIII Problem 9 Physiology: Hearing
 Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
Lecture 6 Hearing 1. Raghav Rajan Bio 354 Neurobiology 2 January 28th All lecture material from the following links unless otherwise mentioned:
 Lecture 6 Hearing 1 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
Lecture 6 Hearing 1 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA. Invited Paper LINEAR RESPONSE OF THE COCHLEA
 FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA Invited Paper LINEAR RESPONSE OF THE COCHLEA David Alan Bies Visiting Research Fellow Department of Mechanical
FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA Invited Paper LINEAR RESPONSE OF THE COCHLEA David Alan Bies Visiting Research Fellow Department of Mechanical
The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons.
 1 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
1 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
HST 721 Efferent Control Lecture October 2004
 HST 721 Efferent Control Lecture October 2004 1 Stapedius Muscle Central Circuitry 2 Hypotheses for MEM Function A. Stapedius 1. Extend Dynamic Range - a gain control system 2. Protect the Inner Ear from
HST 721 Efferent Control Lecture October 2004 1 Stapedius Muscle Central Circuitry 2 Hypotheses for MEM Function A. Stapedius 1. Extend Dynamic Range - a gain control system 2. Protect the Inner Ear from
Auditory Physiology Richard M. Costanzo, Ph.D.
 Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
9.01 Introduction to Neuroscience Fall 2007
 MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
Improving the diagnostic power of otoacoustic emissions. Arturo Moleti Physics Department University of Roma Tor Vergata
 Improving the diagnostic power of otoacoustic emissions Arturo Moleti Physics Department University of Roma Tor Vergata The human ear Ear canal: resonant cavity Middle ear: impedance adapter and pressure
Improving the diagnostic power of otoacoustic emissions Arturo Moleti Physics Department University of Roma Tor Vergata The human ear Ear canal: resonant cavity Middle ear: impedance adapter and pressure
Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression
 1398 Biophysical Journal Volume 106 March 2014 1398 1405 Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression Julien Meaud * and Karl Grosh Department
1398 Biophysical Journal Volume 106 March 2014 1398 1405 Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression Julien Meaud * and Karl Grosh Department
Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics
 Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3676 3681, March 1999 Biophysics Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics PAUL J.
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3676 3681, March 1999 Biophysics Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics PAUL J.
An active cochlear model showing sharp tuning and high sensitivity
 Hearing Research, 9 (1983) 123-13 Elsevier Biomedical Press 123 An active cochlear model showing sharp tuning and high sensitivity Stephen T. Neely * and D.O. Kim Box 811, Washington Unitlersit}', St.
Hearing Research, 9 (1983) 123-13 Elsevier Biomedical Press 123 An active cochlear model showing sharp tuning and high sensitivity Stephen T. Neely * and D.O. Kim Box 811, Washington Unitlersit}', St.
Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells
 Biophysical Journal Volume 84 February 2003 739 749 739 Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells Mark Ospeck, Xiao-xia Dong, and Kuni H. Iwasa Biophysics
Biophysical Journal Volume 84 February 2003 739 749 739 Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells Mark Ospeck, Xiao-xia Dong, and Kuni H. Iwasa Biophysics
ENT 318 Artificial Organs Physiology of Ear
 ENT 318 Artificial Organs Physiology of Ear Lecturer: Ahmad Nasrul Norali The Ear The Ear Components of hearing mechanism - Outer Ear - Middle Ear - Inner Ear - Central Auditory Nervous System Major Divisions
ENT 318 Artificial Organs Physiology of Ear Lecturer: Ahmad Nasrul Norali The Ear The Ear Components of hearing mechanism - Outer Ear - Middle Ear - Inner Ear - Central Auditory Nervous System Major Divisions
Required Slide. Session Objectives
 Auditory Physiology Required Slide Session Objectives Auditory System: At the end of this session, students will be able to: 1. Characterize the range of normal human hearing. 2. Understand the components
Auditory Physiology Required Slide Session Objectives Auditory System: At the end of this session, students will be able to: 1. Characterize the range of normal human hearing. 2. Understand the components
Auditory System. Barb Rohrer (SEI )
 Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
Deafness and hearing impairment
 Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium!
 Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! Chapter 17 Part 2 Special Senses! The Special Senses! Hearing and Equilibrium!
 Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Auditory Periphery! external middle inner. stapes movement initiates a pressure wave in cochlear fluid
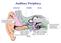 Auditory Periphery! external middle inner sound causes air pressure to increase at eardrum stapes movement initiates a pressure wave in cochlear fluid VIIIth nerve conveys neural signal to cochlear nucleus
Auditory Periphery! external middle inner sound causes air pressure to increase at eardrum stapes movement initiates a pressure wave in cochlear fluid VIIIth nerve conveys neural signal to cochlear nucleus
THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS?
 JOURNAL OF OTOLOGY THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS? LI Yi 1, He David Z 2 Abstract Cochlear outer hair cells (OHCs) are involved in a mechanical feedback loop in which
JOURNAL OF OTOLOGY THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS? LI Yi 1, He David Z 2 Abstract Cochlear outer hair cells (OHCs) are involved in a mechanical feedback loop in which
The transformation of sound stimuli into electrical signals
 The transformation of sound stimuli into electrical signals Robert Fettiplace 2 1 Introduction Our sense of hearing depends on the correct performance of about 15 000 hair cells in each cochlea that serve
The transformation of sound stimuli into electrical signals Robert Fettiplace 2 1 Introduction Our sense of hearing depends on the correct performance of about 15 000 hair cells in each cochlea that serve
The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992)
 The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992) by Pedro da Fonseca (pedrofon@mail.telepac.pt) Neuroscience course Presented in 17.12.99 to professor STEPHEN
The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992) by Pedro da Fonseca (pedrofon@mail.telepac.pt) Neuroscience course Presented in 17.12.99 to professor STEPHEN
Systems Neuroscience Oct. 16, Auditory system. http:
 Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS. Hans P. Zenner, Ulrike Zimmermann and Alfred H. Gitter
 Vol. 149,No. 1, 1987 November 30, 1987 BlOCHEMlCALANDBlOPHYSlCALRESEARCHCOMMUNlCATlONS Pages 304-308 FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS Hans P. Zenner, Ulrike Zimmermann and Alfred
Vol. 149,No. 1, 1987 November 30, 1987 BlOCHEMlCALANDBlOPHYSlCALRESEARCHCOMMUNlCATlONS Pages 304-308 FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS Hans P. Zenner, Ulrike Zimmermann and Alfred
Intro to Audition & Hearing
 Intro to Audition & Hearing Lecture 16 Chapter 9, part II Jonathan Pillow Sensation & Perception (PSY 345 / NEU 325) Fall 2017 1 Sine wave: one of the simplest kinds of sounds: sound for which pressure
Intro to Audition & Hearing Lecture 16 Chapter 9, part II Jonathan Pillow Sensation & Perception (PSY 345 / NEU 325) Fall 2017 1 Sine wave: one of the simplest kinds of sounds: sound for which pressure
Reticular lamina and basilar membrane vibrations in living mouse cochleae
 Reticular lamina and basilar membrane vibrations in living mouse cochleae Tianying Ren a,1, Wenxuan He a, and David Kemp b a Oregon Hearing Research Center, Department of Otolaryngology, Oregon Health
Reticular lamina and basilar membrane vibrations in living mouse cochleae Tianying Ren a,1, Wenxuan He a, and David Kemp b a Oregon Hearing Research Center, Department of Otolaryngology, Oregon Health
So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to
 So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to emphasize the intricate and well-protected structure of the
So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to emphasize the intricate and well-protected structure of the
SPECIAL SENSES: THE AUDITORY SYSTEM
 SPECIAL SENSES: THE AUDITORY SYSTEM REVISION OF PHYSICS: WAVES A wave is an oscillation of power, sound waves have two main characteristics: amplitude, which is the maximum displacement or the power of
SPECIAL SENSES: THE AUDITORY SYSTEM REVISION OF PHYSICS: WAVES A wave is an oscillation of power, sound waves have two main characteristics: amplitude, which is the maximum displacement or the power of
Chapter 11: Sound, The Auditory System, and Pitch Perception
 Chapter 11: Sound, The Auditory System, and Pitch Perception Overview of Questions What is it that makes sounds high pitched or low pitched? How do sound vibrations inside the ear lead to the perception
Chapter 11: Sound, The Auditory System, and Pitch Perception Overview of Questions What is it that makes sounds high pitched or low pitched? How do sound vibrations inside the ear lead to the perception
SPHSC 462 HEARING DEVELOPMENT. Overview Review of Hearing Science Introduction
 SPHSC 462 HEARING DEVELOPMENT Overview Review of Hearing Science Introduction 1 Overview of course and requirements Lecture/discussion; lecture notes on website http://faculty.washington.edu/lawerner/sphsc462/
SPHSC 462 HEARING DEVELOPMENT Overview Review of Hearing Science Introduction 1 Overview of course and requirements Lecture/discussion; lecture notes on website http://faculty.washington.edu/lawerner/sphsc462/
Hearing Research 273 (2011) 109e122. Contents lists available at ScienceDirect. Hearing Research. journal homepage:
 Hearing Research 273 (2011) 109e122 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares Somatic motility and hair bundle mechanics, are both necessary
Hearing Research 273 (2011) 109e122 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares Somatic motility and hair bundle mechanics, are both necessary
The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons.
 1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
OtoAcoustic Emissions (OAE s)
 OtoAcoustic Emissions (OAE s) Phenomenon and applications in audiological diagnostics Measurement procedures TEOAE and DPOAE Physiological backgound, functional models Acknowledgment: several illustrations
OtoAcoustic Emissions (OAE s) Phenomenon and applications in audiological diagnostics Measurement procedures TEOAE and DPOAE Physiological backgound, functional models Acknowledgment: several illustrations
Modelling the micromechanics of the cochlea in Femlab
 Modelling the micromechanics of the cochlea in Femlab R.R.J.J. van Doorn DCT 27.7 Traineeship report Coach(es): Supervisor: Prof. S.J. Elliott Prof. P. Gardonio Prof. H. Nijmeijer Technische Universiteit
Modelling the micromechanics of the cochlea in Femlab R.R.J.J. van Doorn DCT 27.7 Traineeship report Coach(es): Supervisor: Prof. S.J. Elliott Prof. P. Gardonio Prof. H. Nijmeijer Technische Universiteit
In vivo outer hair cell length changes expose the active process in the cochlea
 In vivo outer hair cell length changes expose the active process in the cochlea Dingjun Zha, Fangyi Chen, Sripriya Ramamoorthy, Anders Fridberger, Niloy Choudhury, Steven L Jacques, Ruikang K Wang and
In vivo outer hair cell length changes expose the active process in the cochlea Dingjun Zha, Fangyi Chen, Sripriya Ramamoorthy, Anders Fridberger, Niloy Choudhury, Steven L Jacques, Ruikang K Wang and
Educational Module Tympanometry. Germany D Germering
 Educational Module anometry PATH medical Germany D-82110 Germering Our educational modules 1 are made for providing information on how the hearing organ works and which test procedures are used to test
Educational Module anometry PATH medical Germany D-82110 Germering Our educational modules 1 are made for providing information on how the hearing organ works and which test procedures are used to test
The Structure and Function of the Auditory Nerve
 The Structure and Function of the Auditory Nerve Brad May Structure and Function of the Auditory and Vestibular Systems (BME 580.626) September 21, 2010 1 Objectives Anatomy Basic response patterns Frequency
The Structure and Function of the Auditory Nerve Brad May Structure and Function of the Auditory and Vestibular Systems (BME 580.626) September 21, 2010 1 Objectives Anatomy Basic response patterns Frequency
Evidence for an Active Process and a Cochlear Amplifier in Nonmammals
 INVITED REVIEW Evidence for an Active Process and a Cochlear Amplifier in Nonmammals GEOFFREY A. MANLEY Lehrstuhl für Zoologie, Technische Universität München, 85747 Garching, Germany Received 26 January
INVITED REVIEW Evidence for an Active Process and a Cochlear Amplifier in Nonmammals GEOFFREY A. MANLEY Lehrstuhl für Zoologie, Technische Universität München, 85747 Garching, Germany Received 26 January
Auditory Physiology PSY 310 Greg Francis. Lecture 30. Organ of Corti
 Auditory Physiology PSY 310 Greg Francis Lecture 30 Waves, waves, waves. Organ of Corti Tectorial membrane Sits on top Inner hair cells Outer hair cells The microphone for the brain 1 Hearing Perceptually,
Auditory Physiology PSY 310 Greg Francis Lecture 30 Waves, waves, waves. Organ of Corti Tectorial membrane Sits on top Inner hair cells Outer hair cells The microphone for the brain 1 Hearing Perceptually,
Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier
 Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier Sripriya Ramamoorthy 1 *, Alfred L. Nuttall 1,2 1 Oregon Hearing Research Center, Department of Otolaryngology, Oregon
Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier Sripriya Ramamoorthy 1 *, Alfred L. Nuttall 1,2 1 Oregon Hearing Research Center, Department of Otolaryngology, Oregon
Detection of Cochlear Amplification and Its Activation
 Biophysical Journal Volume 5 August 23 67 78 67 Detection of Cochlear Amplification and Its Activation Wei Dong and Elizabeth S. Olson * Otolaryngology, Head and Neck Surgery and Biomedical Engineering,
Biophysical Journal Volume 5 August 23 67 78 67 Detection of Cochlear Amplification and Its Activation Wei Dong and Elizabeth S. Olson * Otolaryngology, Head and Neck Surgery and Biomedical Engineering,
SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS
 SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS Katherine Knisely, D. Bozovic University of California at Los Angeles It has long been observed that there is some form of amplification
SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS Katherine Knisely, D. Bozovic University of California at Los Angeles It has long been observed that there is some form of amplification
Measurement of cochlear power gain in the sensitive gerbil ear
 Received 4 Nov 2 Accepted 3 Feb 2 Published Mar 2 DOI:.38/ncomms226 Measurement of cochlear power gain in the sensitive gerbil ear Tianying Ren,2, Wenxuan He & Peter G. Gillespie,3 The extraordinary sensitivity
Received 4 Nov 2 Accepted 3 Feb 2 Published Mar 2 DOI:.38/ncomms226 Measurement of cochlear power gain in the sensitive gerbil ear Tianying Ren,2, Wenxuan He & Peter G. Gillespie,3 The extraordinary sensitivity
Signals, systems, acoustics and the ear. Week 5. The peripheral auditory system: The ear as a signal processor
 Signals, systems, acoustics and the ear Week 5 The peripheral auditory system: The ear as a signal processor Think of this set of organs 2 as a collection of systems, transforming sounds to be sent to
Signals, systems, acoustics and the ear Week 5 The peripheral auditory system: The ear as a signal processor Think of this set of organs 2 as a collection of systems, transforming sounds to be sent to
Acoustics, signals & systems for audiology. Psychoacoustics of hearing impairment
 Acoustics, signals & systems for audiology Psychoacoustics of hearing impairment Three main types of hearing impairment Conductive Sound is not properly transmitted from the outer to the inner ear Sensorineural
Acoustics, signals & systems for audiology Psychoacoustics of hearing impairment Three main types of hearing impairment Conductive Sound is not properly transmitted from the outer to the inner ear Sensorineural
P T P V U V I. INTRODUCTION. II. MODEL FORMULATION A. Cochlear fluid dynamics.
 Integration of outer hair cell activity in a one-dimensional cochlear model Azaria Cohen and Miriam Furst a) School of Electrical Engineering, Faculty of Engineering, Tel-Aviv University, Tel-Aviv 69978,
Integration of outer hair cell activity in a one-dimensional cochlear model Azaria Cohen and Miriam Furst a) School of Electrical Engineering, Faculty of Engineering, Tel-Aviv University, Tel-Aviv 69978,
Efferent-mediated control of basilar membrane motion
 J Physiol 576. (2006) pp 49 54 49 Topical Review Efferent-mediated control of basilar membrane motion N. P. Cooper and J. J. Guinan Jr 2 School of Life Sciences, Keele University, Keele, Staffordshire
J Physiol 576. (2006) pp 49 54 49 Topical Review Efferent-mediated control of basilar membrane motion N. P. Cooper and J. J. Guinan Jr 2 School of Life Sciences, Keele University, Keele, Staffordshire
Cochlear anatomy, function and pathology III. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology III Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (3) on the cochlear lateral wall and Reissner s membrane:
Cochlear anatomy, function and pathology III Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (3) on the cochlear lateral wall and Reissner s membrane:
EMANATIONS FROM RESIDUUM OSCILLATIONS IN HUMAN AUDITORY SYSTEM
 EMANATIONS FROM RESIDUUM OSCILLATIONS IN HUMAN AUDITORY SYSTEM V.S. Balaji, N.R.Raajan, S. Rakesh Kumar, Har Narayan Upadhyay School of Electrical & Electronics Engineering, SASTRA University Thanjavur,
EMANATIONS FROM RESIDUUM OSCILLATIONS IN HUMAN AUDITORY SYSTEM V.S. Balaji, N.R.Raajan, S. Rakesh Kumar, Har Narayan Upadhyay School of Electrical & Electronics Engineering, SASTRA University Thanjavur,
Receptors / physiology
 Hearing: physiology Receptors / physiology Energy transduction First goal of a sensory/perceptual system? Transduce environmental energy into neural energy (or energy that can be interpreted by perceptual
Hearing: physiology Receptors / physiology Energy transduction First goal of a sensory/perceptual system? Transduce environmental energy into neural energy (or energy that can be interpreted by perceptual
Hearing: Physiology and Psychoacoustics
 9 Hearing: Physiology and Psychoacoustics Click Chapter to edit 9 Hearing: Master title Physiology style and Psychoacoustics The Function of Hearing What Is Sound? Basic Structure of the Mammalian Auditory
9 Hearing: Physiology and Psychoacoustics Click Chapter to edit 9 Hearing: Master title Physiology style and Psychoacoustics The Function of Hearing What Is Sound? Basic Structure of the Mammalian Auditory
Persistent Hair Cell Malfunction Contributes to Hidden Hearing Loss. The Auditory Laboratory, School of Human Sciences, The University of Western
 1 Persistent Hair Cell Malfunction Contributes to Hidden Hearing Loss 2 3 4 5 Wilhelmina H.A.M. Mulders 1,2, Ian L. Chin 1, Donald Robertson 1 6 7 8 1 The Auditory Laboratory, School of Human Sciences,
1 Persistent Hair Cell Malfunction Contributes to Hidden Hearing Loss 2 3 4 5 Wilhelmina H.A.M. Mulders 1,2, Ian L. Chin 1, Donald Robertson 1 6 7 8 1 The Auditory Laboratory, School of Human Sciences,
Before we talk about the auditory system we will talk about the sound and waves
 The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
Acoustics Research Institute
 Austrian Academy of Sciences Acoustics Research Institute Modeling Modelingof ofauditory AuditoryPerception Perception Bernhard BernhardLaback Labackand andpiotr PiotrMajdak Majdak http://www.kfs.oeaw.ac.at
Austrian Academy of Sciences Acoustics Research Institute Modeling Modelingof ofauditory AuditoryPerception Perception Bernhard BernhardLaback Labackand andpiotr PiotrMajdak Majdak http://www.kfs.oeaw.ac.at
Trajectory of the Aging Cochlea
 Trajectory of the Aging Cochlea Sumitrajit (Sumit) Dhar Professor & Chair Roxelyn & Richard Pepper Department of Communication Sciences and Disorders Fellow, Hugh Knowles Center for Hearing Science Northwestern
Trajectory of the Aging Cochlea Sumitrajit (Sumit) Dhar Professor & Chair Roxelyn & Richard Pepper Department of Communication Sciences and Disorders Fellow, Hugh Knowles Center for Hearing Science Northwestern
Integrating the active process of hair cells with cochlear function
 Integrating the active process of hair cells with cochlear function A. J. Hudspeth Abstract Uniquely among human senses, hearing is not simply a passive response to stimulation. Our auditory system is
Integrating the active process of hair cells with cochlear function A. J. Hudspeth Abstract Uniquely among human senses, hearing is not simply a passive response to stimulation. Our auditory system is
Can You Hear Me Now?
 An Introduction to the Mathematics of Hearing Department of Applied Mathematics University of Washington April 26, 2007 Some Questions How does hearing work? What are the important structures and mechanisms
An Introduction to the Mathematics of Hearing Department of Applied Mathematics University of Washington April 26, 2007 Some Questions How does hearing work? What are the important structures and mechanisms
Νευροφυσιολογία και Αισθήσεις
 Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 11 Ακουστικό και Αιθουσιαίο Σύστημα (Auditory and Vestibular Systems) Introduction Sensory Systems Sense of
Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 11 Ακουστικό και Αιθουσιαίο Σύστημα (Auditory and Vestibular Systems) Introduction Sensory Systems Sense of
College of Medicine Dept. of Medical physics Physics of ear and hearing /CH
 College of Medicine Dept. of Medical physics Physics of ear and hearing /CH 13 2017-2018 ***************************************************************** o Introduction : The ear is the organ that detects
College of Medicine Dept. of Medical physics Physics of ear and hearing /CH 13 2017-2018 ***************************************************************** o Introduction : The ear is the organ that detects
Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea
 Article Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea Wei Dong and Elizabeth S. Olson 2, * VA Loma Linda Health Care System and Otolaryngology/Head & Neck Surgery,
Article Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea Wei Dong and Elizabeth S. Olson 2, * VA Loma Linda Health Care System and Otolaryngology/Head & Neck Surgery,
MECHANISM OF HEARING
 MECHANISM OF HEARING Sound: Sound is a vibration that propagates as an audible wave of pressure, through a transmission medium such as gas, liquid or solid. Sound is produced from alternate compression
MECHANISM OF HEARING Sound: Sound is a vibration that propagates as an audible wave of pressure, through a transmission medium such as gas, liquid or solid. Sound is produced from alternate compression
Neurobiology Biomed 509 Sensory transduction References: Luo , ( ), , M4.1, M6.2
 Neurobiology Biomed 509 Sensory transduction References: Luo 4.1 4.8, (4.9 4.23), 6.22 6.24, M4.1, M6.2 I. Transduction The role of sensory systems is to convert external energy into electrical signals
Neurobiology Biomed 509 Sensory transduction References: Luo 4.1 4.8, (4.9 4.23), 6.22 6.24, M4.1, M6.2 I. Transduction The role of sensory systems is to convert external energy into electrical signals
Active Hair Bundle Movements and the Cochlear Amplifier
 Active Hair Bundle Movements and the Cochlear Amplifier Anthony Ricci* Abstract The active process is a term used to describe amplification and filtering processes that are essential for obtaining the
Active Hair Bundle Movements and the Cochlear Amplifier Anthony Ricci* Abstract The active process is a term used to describe amplification and filtering processes that are essential for obtaining the
Ear. Utricle & saccule in the vestibule Connected to each other and to the endolymphatic sac by a utriculosaccular duct
 Rahaf Jreisat *You don t have to go back to the slides. Ear Inner Ear Membranous Labyrinth It is a reflection of bony labyrinth but inside. Membranous labyrinth = set of membranous tubes containing sensory
Rahaf Jreisat *You don t have to go back to the slides. Ear Inner Ear Membranous Labyrinth It is a reflection of bony labyrinth but inside. Membranous labyrinth = set of membranous tubes containing sensory
to vibrate the fluid. The ossicles amplify the pressure. The surface area of the oval window is
 Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
NIH Public Access Author Manuscript Nat Commun. Author manuscript; available in PMC 2013 March 12.
 NIH Public Access Author Manuscript Published in final edited form as: Nat Commun. 2012 ; 3: 1094. doi:10.1038/ncomms2100. Sound-induced length changes in outer hair cell stereocilia Pierre Hakizimana
NIH Public Access Author Manuscript Published in final edited form as: Nat Commun. 2012 ; 3: 1094. doi:10.1038/ncomms2100. Sound-induced length changes in outer hair cell stereocilia Pierre Hakizimana
Advanced otoacoustic emission detection techniques and clinical diagnostics applications
 Advanced otoacoustic emission detection techniques and clinical diagnostics applications Arturo Moleti Physics Department, University of Roma Tor Vergata, Roma, ITALY Towards objective diagnostics of human
Advanced otoacoustic emission detection techniques and clinical diagnostics applications Arturo Moleti Physics Department, University of Roma Tor Vergata, Roma, ITALY Towards objective diagnostics of human
Carlson (7e) PowerPoint Lecture Outline Chapter 7: Audition, the Body Senses, and the Chemical Senses
 Carlson (7e) PowerPoint Lecture Outline Chapter 7: Audition, the Body Senses, and the Chemical Senses This multimedia product and its contents are protected under copyright law. The following are prohibited
Carlson (7e) PowerPoint Lecture Outline Chapter 7: Audition, the Body Senses, and the Chemical Senses This multimedia product and its contents are protected under copyright law. The following are prohibited
Backward Propagation of Otoacoustic Emissions
 40 Review Backward Propagation of Otoacoustic Emissions HE Wenxuan, 1, 2 REN Tianying, 1, 2 1. Oregon Hearing Research Center, Department of Otolaryngology and Head & Neck Surgery, Oregon Health & Science
40 Review Backward Propagation of Otoacoustic Emissions HE Wenxuan, 1, 2 REN Tianying, 1, 2 1. Oregon Hearing Research Center, Department of Otolaryngology and Head & Neck Surgery, Oregon Health & Science
A Critique of the Critical Cochlea: Hopf a
 A Critique of the Critical Cochlea: Hopf a Bifurcation Is Better Than None A. J. Hudspeth, Frank Jülicher and Pascal Martin J Neurophysiol 104:1219-1229, 2010. First published 10 June 2010; doi:10.1152/jn.00437.2010
A Critique of the Critical Cochlea: Hopf a Bifurcation Is Better Than None A. J. Hudspeth, Frank Jülicher and Pascal Martin J Neurophysiol 104:1219-1229, 2010. First published 10 June 2010; doi:10.1152/jn.00437.2010
Hearing and Balance 1
 Hearing and Balance 1 Slide 3 Sound is produced by vibration of an object which produces alternating waves of pressure and rarefaction, for example this tuning fork. Slide 4 Two characteristics of sound
Hearing and Balance 1 Slide 3 Sound is produced by vibration of an object which produces alternating waves of pressure and rarefaction, for example this tuning fork. Slide 4 Two characteristics of sound
COCHLEAR MODEL FOR NORMAL AND DAMAGED EARS
 TEL AVIV UNIVERSITY THE IBY AND ALADAR FLEISCHMAN FACULTY OF ENGINEERING The Zandman-Slaner Graduate School of Engineering COCHLEAR MODEL FOR NORMAL AND DAMAGED EARS By Azaria Cohen THESIS SUBMITTED FOR
TEL AVIV UNIVERSITY THE IBY AND ALADAR FLEISCHMAN FACULTY OF ENGINEERING The Zandman-Slaner Graduate School of Engineering COCHLEAR MODEL FOR NORMAL AND DAMAGED EARS By Azaria Cohen THESIS SUBMITTED FOR
The origin of short-latency transient-evoked otoacoustic emissions
 University of Iowa Iowa Research Online Theses and Dissertations Fall 2013 The origin of short-latency transient-evoked otoacoustic emissions James Douglas Lewis University of Iowa Copyright 2013 James
University of Iowa Iowa Research Online Theses and Dissertations Fall 2013 The origin of short-latency transient-evoked otoacoustic emissions James Douglas Lewis University of Iowa Copyright 2013 James
Evidence and synthesis
 CHAPTER D 9 (Discussion continued) Evidence and synthesis 9.1 Synthesis 9.1/a Outer hair cells as dual detectors Pressure detection and transduction currents The silent current 9.1/b Cancellation effects
CHAPTER D 9 (Discussion continued) Evidence and synthesis 9.1 Synthesis 9.1/a Outer hair cells as dual detectors Pressure detection and transduction currents The silent current 9.1/b Cancellation effects
Effects of Remaining Hair Cells on Cochlear Implant Function
 Effects of Remaining Hair Cells on Cochlear Implant Function N1-DC-2-15QPR1 Neural Prosthesis Program N. Hu, P.J. Abbas, C.A. Miller, B.K. Robinson, K.V. Nourski, F. Jeng, B.A. Abkes, J.M. Nichols Department
Effects of Remaining Hair Cells on Cochlear Implant Function N1-DC-2-15QPR1 Neural Prosthesis Program N. Hu, P.J. Abbas, C.A. Miller, B.K. Robinson, K.V. Nourski, F. Jeng, B.A. Abkes, J.M. Nichols Department
Auditory Physiology PSY 310 Greg Francis. Lecture 29. Hearing
 Auditory Physiology PSY 310 Greg Francis Lecture 29 A dangerous device. Hearing The sound stimulus is changes in pressure The simplest sounds vary in: Frequency: Hertz, cycles per second. How fast the
Auditory Physiology PSY 310 Greg Francis Lecture 29 A dangerous device. Hearing The sound stimulus is changes in pressure The simplest sounds vary in: Frequency: Hertz, cycles per second. How fast the
PSY 310: Sensory and Perceptual Processes 1
 Auditory Physiology PSY 310 Greg Francis Lecture 29 A dangerous device. Hearing The sound stimulus is changes in pressure The simplest sounds vary in: Frequency: Hertz, cycles per second. How fast the
Auditory Physiology PSY 310 Greg Francis Lecture 29 A dangerous device. Hearing The sound stimulus is changes in pressure The simplest sounds vary in: Frequency: Hertz, cycles per second. How fast the
Academic and Research Staff Professor Dennis M. Freeman, Dr. Alexander Aranyosi, Dr. Stanley Hong
 Cochlear Mechanics Academic and Research Staff Professor Dennis M. Freeman, Dr. Alexander Aranyosi, Dr. Stanley Hong Graduate Students Chris Bergevin, Roozbeh Ghaffari, Wendy Gu, Kinuko Masaki, Scott Page
Cochlear Mechanics Academic and Research Staff Professor Dennis M. Freeman, Dr. Alexander Aranyosi, Dr. Stanley Hong Graduate Students Chris Bergevin, Roozbeh Ghaffari, Wendy Gu, Kinuko Masaki, Scott Page
Optimal Electrical Properties of Outer Hair Cells Ensure Cochlear Amplification
 Optimal Electrical Properties of Outer Hair Cells Ensure Cochlear Amplification Jong-Hoon Nam 1,2 *, Robert Fettiplace 3 1 Department of Mechanical Engineering, Department of Biomedical Engineering, University
Optimal Electrical Properties of Outer Hair Cells Ensure Cochlear Amplification Jong-Hoon Nam 1,2 *, Robert Fettiplace 3 1 Department of Mechanical Engineering, Department of Biomedical Engineering, University
Hearing Sound. The Human Auditory System. The Outer Ear. Music 170: The Ear
 Hearing Sound Music 170: The Ear Tamara Smyth, trsmyth@ucsd.edu Department of Music, University of California, San Diego (UCSD) November 17, 2016 Sound interpretation in the auditory system is done by
Hearing Sound Music 170: The Ear Tamara Smyth, trsmyth@ucsd.edu Department of Music, University of California, San Diego (UCSD) November 17, 2016 Sound interpretation in the auditory system is done by
Music 170: The Ear. Tamara Smyth, Department of Music, University of California, San Diego (UCSD) November 17, 2016
 Music 170: The Ear Tamara Smyth, trsmyth@ucsd.edu Department of Music, University of California, San Diego (UCSD) November 17, 2016 1 Hearing Sound Sound interpretation in the auditory system is done by
Music 170: The Ear Tamara Smyth, trsmyth@ucsd.edu Department of Music, University of California, San Diego (UCSD) November 17, 2016 1 Hearing Sound Sound interpretation in the auditory system is done by
Lecture 7 Hearing 2. Raghav Rajan Bio 354 Neurobiology 2 February 04th All lecture material from the following links unless otherwise mentioned:
 Lecture 7 Hearing 2 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
Lecture 7 Hearing 2 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
A cochlear AGC model, proposing a new type of cochlear non-linearity
 TEL-AVIV UNIVERSITY The Iby and Aladar Fleischman faculty of engineering The Zandman-Slaner school of Graduate studies A cochlear AGC model, proposing a new type of cochlear non-linearity A thesis submitted
TEL-AVIV UNIVERSITY The Iby and Aladar Fleischman faculty of engineering The Zandman-Slaner school of Graduate studies A cochlear AGC model, proposing a new type of cochlear non-linearity A thesis submitted
Repeatability of medial olivocochlear efferent effects on transient-evoked otoacoustic emissions in normal-hearing adults
 University of Iowa Iowa Research Online Theses and Dissertations Summer 2014 Repeatability of medial olivocochlear efferent effects on transient-evoked otoacoustic emissions in normal-hearing adults Ian
University of Iowa Iowa Research Online Theses and Dissertations Summer 2014 Repeatability of medial olivocochlear efferent effects on transient-evoked otoacoustic emissions in normal-hearing adults Ian
Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea
 Keywords: Cochlea, Basilar membrane, Hair cell 7006 Journal of Physiology (1998), 509.1, pp. 277 288 277 Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea Nigel P.
Keywords: Cochlea, Basilar membrane, Hair cell 7006 Journal of Physiology (1998), 509.1, pp. 277 288 277 Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea Nigel P.
