THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS?
|
|
|
- Phoebe Stafford
- 5 years ago
- Views:
Transcription
1 JOURNAL OF OTOLOGY THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS? LI Yi 1, He David Z 2 Abstract Cochlear outer hair cells (OHCs) are involved in a mechanical feedback loop in which the fast somatic motility of OHCs is required for cochlear amplification. Alternatively, amplification is thought to arise from active hair bundle movements observed in non-mammalian hair cells. We measured the voltage-evoked hair bundle motions in the gerbil cochlea to determine if such movements are also present in mammalian OHCs. The OHCs displayed a large hair bundle movement that was not based on mechanotransducer channels but based on somatic motility. Significantly, bundle movements were able to generate radial motion of the tectorial membrane in situ. This result implies that the motility-associated hair bundle motion may be part of the cochlear amplifier. Introduction It is generally believed that mechanical amplification by hair cells is necessary to enhance the sensitivity and frequency selectivity of hearing. In the mammalian cochlea OHCs function as the key elements in a mechanical feedback loop. This loop most likely includes OHCs, the tectorial membrane (TM), and inner hair cells (IHCs) with IHCs responding to the output of the feedback loop [1,2]. OHCs exhibit a voltage-dependent length change termed electromotility [3-5]. This somatic motility, when driven by the receptor potential in vivo, is thought to underlie cochlear amplification in mammals [1-6]. The alternative view is that the amplification arises from active hair bundle motion [7-9]. Active hair-bundle movements ranging between 5 and 80 nm have been observed in several non-mammalian species and can occur spontaneously with amplitudes in excess of that expected for Brownian motion [10-13]. Such movements have also been observed as reactions to hair bundle displacement with compliant probes [10,14-17], and in response to changes in membrane potential [10,11,16,18,19], the effects of which might be secondary to alteration of Ca 2 + influx [10,16,18]. The active hair bundle movements are intimately related to adaptation of the mechanotransducer channels [8,16]. Up to date, however, the bundle motion has not been thoroughly examined in mammalian OHCs. In order to determine if hair bundle movements are also present in mammalian OHCs and how it might be involved in cochlear amplification, we evaluated voltage-evoked hair bundle activity in the cochlea of gerbils and prestin knockout mice [6]. Materials and Methods Gerbils ranging in age between 4 and 30 days after birth and prestin knockout mice ranging in age between 4 and 7 weeks after birth were used. After the cochlear wall was removed, the basilar membrane-organ complex of Corti was unwrapped from the modiolus from the basal turn all the way to the apical turn, in the same way as described elsewhere [19,20]. The coil was digested in L-15 containing 0.06 mg/ml protease (type XXIV; Sigma, St. Affiliation: 1 Department of Otolaryngology-Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University, Beijing , P.R. China; 2 Department of Biomedical Sciences, Creighton University School of Medicine, Omaha, Nebraska, USA Corresponding authors: He David Z, hed@creighton.edu 64
2 Louis, MO). After the TM was lifted off to expose the hair bundles, the tissue was transferred to the experimental chamber. The coil was firmly attached to the bottom of the chamber by the weight of two thin platinum rods (0.5 mm in diameter), with one of their ends anchored in two small droplets of vacuum grease on the bottom of the chamber. The tissue was mounted with the hair bundle facing upward toward the water-immersion objective. Details for preparing gerbil hemicochlea are given elsewhere [21,22]. The hemicochlea (or coil preparation) was bathed in L-15 medium in an experimental chamber mounted on the stage of an upright microscope (Leica DMLFSA) with Burleigh platform (Giblator). Quality control of hemicochleae and coils was based on the physical appearance of the preparation [21-23]. In either preparation, OHCs became swollen in less than 25 minutes after the organ of Corti was exposed to the extracellular medium. Therefore, all the data presented were collected within 20 minutes. Experiments were done at room temperature (22±2 ). The coil preparation and the hemicochlea were both bathed in the L-15 medium (Invitrogen Corp.), which contained (in mm): 136 NaCl, 5.8 NaH 2PO 4, 5.4 KCl, 1.4 CaCl 2, 0.9 MgCl 2, 0.4 MgSO 4, 10 HEPES-NaOH, at ph 7.4, and 300 mmol kg -1. The pipette solution contained (in mm): 140 CsCl (or KCl), 0.1 CaCl 2, 3.5 Mg- Cl 2, 2.5 MgATP, 5 EGTA-KOH, 5 HEPES-KOH, at ph 7.4 and 300 mmol kg -1. In some experiments, artificial endolymph (150 mm KCl, 25 μm CaCl 2, 1 mm sodium pyruvate, 5 mm d-glucose, and 10 mm K 2HPO 4 at ph 7.35 and 300 mmol kg -1 ) was perfused to the ciliary area through a second pipette positioned approximately μm away from the hair bundle. Pipette resistances were 3-4 MΩ. Recordings were made in whole-cell voltage-clamp mode, using an Axopatch 200B patch-clamp amplifier (Axon Instruments). Series resistance was 8-14 MΩ, and ~75% of series resistance was compensated. The uncompensated voltage error was normally less than 4 mv at the largest voltage levels used since the membrane conductances were blocked. Currents were filtered at 1 khz, and digitized at 5 khz with a 16-bit A/ D converter (Digidata 1322A) and pclamp 9.0 software (Axon Instruments). The cells were held at -70 mv. To measure mechanotransducer currents in the coil preparation, a fluid-jet technique was used to deflect the hair bundle. The home-made fluid jet was under control by the Burleigh Driver/Amplifier (PZ-150M). Sinusoidal voltage commands (102 Hz) was used to drive the fluid-jet. The fluid-jet was positioned approximately μm away from the stereocilia. The preparation was obliquely illuminated by a 100-Watt lamp. The hair bundle was imaged using a 63x water-immersion objective and magnified by an additional 20x relay lens. The magnified image of the tip of the bundle was then split into two paths: one path projected onto the photodiode (Hamamatsu) through a slit and another projected onto a CCD camera so that the bundle could be viewed at all times on a television monitor. During measurements, the magnified image of the tip of the bundle was positioned near the edge of the slit. The output signal from the photodiode amplifier represented the motion of the tip of the hair bundle. The photodiode system had a cutoff (3-dB) frequency of 1,100 Hz. The signal was then amplified by a 60-dB fixed-gain dc-coupled amplifier. The amplified signal was then low-pass filtered (500 Hz) before being delivered to one of the A/D inputs of a Digidata (1322A, Axon Instruments) acquisition board in a Window-based PC. The measurement system was capable of measuring bundle tip motions down to ~5 nm with 100 averages. Calibration of bundle motion was obtained by moving the slit a known amount (0.5 μm) using a piezo driver attached to the slit. TM motion and somatic motility were measured and calibrated in the same way as described for the bundle motion. Results Voltage-Evoked Hair-Bundle Motion Sensory epithelia were dissected from the cochleae of adult gerbils and prestin knockout mice. The resulting coil preparation was bathed in artificial perilymph and mounted on the stage of a Leica upright microscope with a 63x water-immersion objective. The sensory epithelium was oriented with the hair bundles pointing up toward the objective. Under bright-field illumination at high magnification, the hair bundles behaved as light pipes [13] and appeared as bright V-shaped lines (Fig. 1A). To measure bundle motion, the magnified (1,260x) image of the edge of the hair bundle was projected onto a photodiode through a rectangular slit. The photodiode-based system, mounted on the photo-port of the Leica microscope, had a 3-dB cutoff frequency of 1,100 Hz and was capable of measuring motion down to ~5 nm with moderate averaging and low-pass filtering. Cells were selected if no obvious signs of deterioration in the soma and/or hair bundle were visible at high magnification. Before the voltage-evoked bundle motion was measured, we recorded mechanotransducer currents to verify that the mechanotransducer apparatus in the stereocilia was not damaged. Fig. 1B shows an example of such recording from an apical turn gerbil OHC. The hair bundle was deflected by a fluid jet (with pipette tip diameter of 10 µm) positioned µm away from the bundle. 65
3 Transducer currents were recorded at the holding potential of -70 mv in the voltage-clamp mode. Large transducer current was observed (Fig. 1B). The size of the current is comparable to previous studies in mammalian OHCs [21,22, 24-26]. To determine bundle motions, sinusoidal voltage bursts (102 Hz) were applied to the OHCs through the patch electrode. The voltage command varied the membrane potential from -100 to -40 mv from a holding potential of -70 mv. Examples of the voltage-evoked hair bundle movements are shown in Fig. 1C. Bundle motion is asymmetrical with depolarization evoking larger bundle motions in the direction toward the tallest stereocilia (defined as positive bundle motion) than hyperpolarization does in the opposite direction (negative bundle motion). The direction of bundle motion during membrane potential change is consistent with that seen in turtle hair cells [8,13], but is of opposite polarity to that seen in bullfrog saccular hair cells [14,15]. Hair cells of several non-mammalian species display an active hair bundle motion in response to changes in membrane potential [8-15]. The active motion, intimately associated with mechanotransducer channels, is secondary to alternation of calcium influx in the stereocilia and is, therefore, dependent on the extracellular calcium concentration [8-15]. We sought to determine whether the bundle motion observed in OHCs also operates on a similar basis. Bundle motion was examined when the extracellular calcium concentration was altered. Fig. 1C shows an example when the extracellular calcium was reduced to 5 μm. Robust voltage-evoked bundle motion was still observed. We compared the bundle motion before and after the low-calcium medium was perfused to the ciliary bundle of 5 cells and no statistical significance was found. Streptomycin is known to block mechanotransducer channels [8] and eliminate active and spontaneous bundle motion [8,13]. We perfused 100 μm streptomycin to the ciliary area to see whether it blocked the voltage-evoked bundle motion in OHCs. As shown in Fig. 1D, the bundle motion was not affected by streptomycin. Collectively, these results suggest that the observed bundle motion in OHCs is different from the voltage-evoked bundle motion seen in non-mammalian hair cells. 66
4 Figure 1. A. Hair bundles of OHCs under high magnification (63x water-immersion objective) with bright field illumination. The bundles behaved as light pipes and appeared as bright V-shaped lines when focused at their tips. Double-headed arrow indicates the direction of bundle motion. Scale bar represents 10 µm. B. Mechanotransducer currents recorded from an apical turn gerbil OHC from the coil preparation. The bundle was deflected by an oscillating stream from a fluid jet positioned ~20-30 µm away from the bundle. The response shown is the average of 3 trials. The voltage command (102 Hz) to drive the water jet is presented at the bottom of the panel. Inward current is plotted downward. C. Voltage-evoked bundle motions of a gerbil OHC at two different extracellular calcium concentrations. The 102 Hz voltage command varied the membrane potential from -100 to-40 mv around the holding potential of -70 mv. Positive bundle motion (toward tall cilia) is plotted upward in this and all subsequent figures. D. Bundle motion before and after 100 µm streptomycin was perfused to the OHC stereociliary region. The responses in C and D were the averages of 100 trials. The scale bar in C also applies to the responses in D. As an alternative to motility derived from mechanotransducer processes, it is possible that bundle motion arises as some consequence of somatic motility. To demonstrate that the observed hair bundle motion is associated with somatic motility, we examined the voltage-evoked bundle motion in neonatal gerbils. Studies have shown that the onset of OHC motility occurs around 6-8 days after birth [27] while mechanotransducer channels are known to be mature at birth [24,26]. Voltage-evoked bundle motions of apical turn OHCs were measured from developing gerbils at 4, 8, and 12 days after birth (DAB). Fig. 2A shows some examples of the responses measured from those preparations. At 4 DAB when electromotility had not yet developed, no voltage-evoked bundle motion was detected (n=10), although large transducer currents could be measured (data not shown). At 8 DAB, we observed small bundle motion in 1 of 8 cells examined. At 12 DAB when all OHCs exhibit electromotility [27], voltage-evoked bundle motion was detected in all 7 cells studied, with a magnitude of approximately 72% of that of the adult OHCs with the same voltage stimulation. The fact that neonatal OHCs do not have voltage-evoked hair bundle motion before the onset of motility and that the development of bundle movements correlates with the development of somatic motility suggests that bundle motion in response to membrane potential change is related to somatic motility. OHC somatic motility is mediated by a voltage-sensitive membrane-bound motor protein, prestin [28]. To further demonstrate that hair bundle motion was related to the somatic motility of OHCs, we examined the voltage-evoked bundle motion in prestin knockout mice [5]. Such measurements are also important to determine whether there is any small transducer channel-based bundle motion that is overshadowed by the dominant motility-associated bundle motion. These mice have normal morphology of the hair bundles and normal mechanotransducer functions [5] with no OHC somatic motility. We measured transducer currents first to confirm that their mechanotransducer channels are functional. Fig. 2B shows an example of the transducer current recorded from an apical turn OHC of the prestin knockout mouse. As shown, large transducer current was observed. We measured the voltage-evoked bundle motion from the prestin-null OHCs. No voltage-evoked bundle motion was observed in any of the 11 cells examined (Fig. 2C). In contrast, when the voltage-evoked bundle motion was measured from OHCs of wild-type mice, large bundle motions were seen for all cells examined (data not shown). In order to rule out that the lack of bundle motion in the prestin-null OHCs was not due to perilymph solution bathing the stereocilia, we measured the voltage-evoked bundle motion with the ciliary area perfused with endolymph-like solution to mimic the in vivo chemical condition in 5 additional prestin-null OHCs. No voltage-evoked bundle motions were observed in any of the 5 cells examined. This confirms that the voltage-evoked bundle motion is indeed associated with somatic motility. 67
5 68 Figure 2. A. Voltage-evoked bundle motions measured from 4-, 8-, and 12-day-old gerbil OHCs. The stimulus waveform and voltage command is the same as shown in Fig. 1C. B. Mechanotransducer currents recorded from an apical turn OHC of prestin knockout mouse. C. Lack of voltage-evoked bundle motion from the prestin knockout mouse OHC. D. Bundle motions of an apical turn gerbil OHC as a function of voltage levels (from a holding potential of -70 mv). Steady-state response from D was fitted with second-order Boltzmann function (solid line) and plotted in the bottom panel. Slope function (dash line) was obtained as the derivative of the Boltzmann function. The series resistance was 75% compensated. Because membrane conductances were blocked, the uncompensated voltage error (not corrected in the plot) was less than 4 mv at the largest voltage levels. E. Motility-associated bundle motions evoked by a series of voltage bursts with different frequencies (frequency is shown on the top of the response waveforms). The peak-to-peak response was measured and plotted as a function of frequency in the bottom panel. We examined bundle motion as a function of membrane potential (input-output function). Fig. 2D shows an example of the response measured from an apical turn gerbil OHC when the membrane potential was stepped from the holding potential of -70 mv. The responses were asymmetrical and nonlinear with saturation in both directions, similar to that seen in OHC somatic motility [27]. We fitted the response with a second-order Boltzmann function, and the maximum sensitivity calculated from the derivative of this function was ~4.8 nm/mv (Fig. 2D). The maximum peak-to-peak response observed in the example was 567 nm. The largest peak-to-peak response observed among 6 cells examined was 832 nm. We also examined the frequency response of the volt-
6 age-evoked bundle motion between 50 and 1,000 Hz using sinusoidal voltage bursts. An example is shown in Fig. 2E. Apparently, large bundle motions were still present at 1,000 Hz. The frequency response of the bundle motion was similar to that of OHC somatic motility measured under whole-cell voltage-clamp condition [29-30]. Radial Motion of the Tectorial Membrane The TM is an important element of the mechanical feedback loop and its role in mechanoelectrical transduction, frequency tuning, and cochlear amplification has been demonstrated [31-33]. The most direct path for OHC bundle motion to influence input to IHCs is through TM. We questioned whether hair bundle motion could generate a radial TM motion. For this purpose, the gerbil hemicochlea [23] was used to examine TM radial motion driven by OHC bundle motion. Hemicochleae (Fig. 3A) were prepared from 25 to 30 day old gerbils. Whole-cell recordings were made from the upper basal turn where OHC mechanotransducer currents were previously recorded [21]. The cells were current-clamped to a level that would result in a membrane potential of approximately -70 mv. Current (100 Hz sinusoid, 100 to 300 μa) bursts generated by a modified battery-powered stimulator (Isostim A320, WPI) created a diverging electrical field between the stimulating pipette (tip diameter of 4 m) positioned 50-60μm away from the OHCs and the indifferent earth in the bath. This focal electrical stimulation depolarizes and hyperpolarizes OHCs near the electrode. Fig. 3C shows an example of simultaneous recordings of radial motion of the TM and the membrane-voltage response of an OHC. As shown, the focal electrical stimuli resulted in a net membrane potential change of 18 mv (peak-to-peak) and produced a radial TM motion of ~10 nm (peak-to-peak). Depolarization (hyperpolarization) resulted in TM motion toward the spiral ligament (modiolus). While the voltage response of the OHC was nearly symmetrical, TM motion was asymmetrical, with both ac and dc components. This asymmetry resembles that of the bundle motion considered above and may originate from the asymmetry of OHC somatic motility. To confirm that the TM motion was the result of bundle motion, we measured TM motion in preparations where the TM was detached from the hair bundles of OHCs. As expected, no TM motion was detected (Fig. 3D) in any of 15 preparations examined. We also observed radial TM motion of when 100 mv sinusoidal voltage (peak-to-peak) was applied to one OHC under the whole-cell voltage-clamp condition (data not shown). This again confirms that the TM motion was the result of bundle motion. 69
7 Figure 3. A. Hemicochlea from a 30-day-old gerbil. The square represents the area where the TM motion was measured using a photodiode-based technique. The white double-headed arrows indicate directions of the TM motion measured. Small black arrow indicates the Hensen s stripe on the underside of the TM. B. Hensen s stripe and IHC bundle at high magnification. The Hensen s stripe is on the medial side of the IHC hair bundle. Bars in A and B represent 10 µm. C. Simultaneous recordings of TM radial motion and membrane potential changes of an OHC from upper basal turn of a 28-day-old gerbil hemicochlea. Current (100 Hz sinusoid, bottom trace in B) was injected through another pipette positioned ~50 µm away the cell under recording to depolarize and hyperpolarize it. Upward deflection in the trace represents the movement of the TM toward the spiral ligament. D.TM motion and membrane voltage response measured from another hemicochlea when the TM was detached from the hair bundles. No TM motion was observed, even though the receptor potential was almost twice the size of that shown in C. The responses were the averages of 200 trials. Discussion This work demonstrates significant ciliary rotation evoked by OHC electromotility. Yet, in the absence of electromotility, ciliary rotation, presumably related to the mechanotransducer channels, is below the resolution limit of our system. The motility-associated bundle motion is large (over 800 nm), approximately ten times (20 db) larger than the transducer-channel based bundle motions observed in non-mammalian hair cells [8,12,13]. Voltage-evoked bundle motion of IHCs was reported in a recent study using a two-chamber preparation [18]. While stated otherwise, the possibility that such motion may have been mediated by OHC motility can not be completely ruled out. The motility-associated response possibly overshadows transducer channel-based mechanisms in OHCs. It is not fully established how OHC length changes result in bundle motion. However, tilting of the cuticular plate during motility has been said to occur at high frequencies (up to 15 khz) in coil preparations [34]. Rotation of the reticular lamina as a result of OHC motility was also seen in situ [35]. It is, therefore, likely that rotation of the reticular lamina along its fulcrum at the pillar heads and possibly tilting of the cuticular plate within the reticular lamina during OHC length change that can produce bundle motions. OHC somatic motility has been proposed to be responsible for cochlear amplification in mammals. These theories, which assume that force generated by OHC somatic motility amplifies the motion of the basilar membrane - organ of Corti complex, are supported by several observations. Among these are the demonstration of somatic motility upon ciliary deflection [36], electromotility-driven movements of the reticular lamina and basilarmembrane insitu [34,35], and experiments using prestin-knockout mice [5]. However, it is yet to be fully determined how active somatic movements of OHCs excite IHCs. Obviously, coupling OHC motility to basilar membrane and reticular lamina movements in an appropriate phase would boost their displacements. In addition, the bundle motion associated with OHC somatic motility provides another possibility for OHC motility to boost the input to IHCs. Because movement of the bundle is able to produce radial motion of the TM, this motion could amplify mechanical input to the IHC by increasing fluid motion in the TM-reticular lamina gap. It is such fluid flow that stimulates the freestanding IHC cilia [37]. It is conceivable that fluid-pumping by Hensen's stripe (see Fig. 3B) onto the closely apposed IHC cilia is the excitatory mechanism. The V or W shaped staircase structure of the OHC stereocilia is well suited for promoting mechanical coupling between the tectorial membrane and reticular lamina, which can transfer the motility-driven hair bundle motion into the radial motion of the tectorial membrane. It is, therefore, conceivable that the motility-associated hair bundle motion may be part of cochlear amplification in mammals. Under this scheme, OHC somatic motility not only boosts basilar membrane vibration but also drives the hair bundle motion, which is able to produce the radial motion of TM. In addition, OHC hair bundle can also produce force due to the transducer channel-based mechanisms [19]. This will further enhance the radial motion of the TM. Since the bundle and TM motions are both associated with somatic motility of OHCs, this scheme is in line with studies using prestin-knockout mice [5], which support motility-based amplification as the dominant mechanism in the mammalian cochlea. 70
8 The principal argument against somatic motility as the amplifier is that the low-pass filter characteristics of OHC membrane attenuate receptor potentials at high frequencies. Therefore, the receptor potentials would be too small to drive somatic motility and the bundle motion [5]. However, it has been proposed that extracellular potential changes within the organ of Corti could drive OHC motility at high frequencies [38]. These extracellular potentials are not filtered by the membrane. Recent measurements of basilar membrane vibration and extracellular potentials in the guinea pig cochlea at high frequencies provide evidence that those extracellular potentials can indeed drive OHC motors at high frequencies [39]. Furthermore, theoretical modeling also indicates that the piezoelectric property of OHCs can significantly increase the frequency response of OHCs [40]. In summary, we demonstrate that mammalian OHCs display a large hair-bundle motion that is dependent on OHC somatic motility. Such bundle motion may boost the mechanical input to the IHC stereocilia through the radial motion of the TM. While the forward-transduction-based hair-bundle motion may underlie the cochlear amplification in non-mammals, mammals have evolved OHC somatic motility to boost basilar membrane vibration as well as drive the bundle motion, thereby providing mechanical amplification of low-level signals in the mammalian inner ear. References [1] Dallos P. The active cochlea. J Neurosci. 1992; 12: [2] Santos-Sacchi J. New tunes from Corti's organ: the outer hair cell boogie rules. Curr Opin Neurobiol. 2003; 13: [3] Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses in isolated cochlear outer hair cells. Science 1985; 227: [4] Kachar B, Brownell WE, Altschuler R, Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature 1986; 322: [5] Liberman MC et al. Prestin is required for outer hair cell electromotility and the cochlear amplifier. Nature 2002; 419: [6] Hudspeth AJ. Mechanical amplification of stimuli by hair cells. Curr Opin Neurobiol. 1997; 7: [7] Fettiplace R, Ricci AJ, Hackney CM. Clues to the cochlear amplifier from the turtle ear. Trends Neurosci. 2001; 24: [8] Crawford AC, Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol. 1985; 364: [9] Rüsch A, Thurm U. Spontaneous and electrically induced movements of ampullary kinocilia and stereovilli. Hear Res. 1990; 48: [10] Martin P, Hudspeth AJ. Active hair-bundle movements can amplify a hair cell's response to oscillatory mechanical stimuli. Proc Natl Acad Sci USA. 1999; 96: [11] Howard J, Hudspeth AJ. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by 71 the bullfrog s saccular hair cell Proc Natl Acad Sci USA. 1987; 84: [12] Benser ME, Marquis RE, Hudspeth AJ. Rapid, active hair bundle movements in hair cells from the bullfrog's sacculus. J Neurosci. 1996; 16: [13] Ricci AL, Crawford AC, Fettiplace R. Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. J Neurosci. 2000; 20: [14] Assad JA, Hacohen N, Corey DP. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc Natl Acad Sci USA. 1989; 86: [15] Bozovic D, Hudspeth AJ. Hair-bundle movements elicited by transepithelial electrical stimulation of hair cells in the sacculus of the bullfrog. Proc Natl Acad Sci USA. 2003; 100: [16] Gillespie PG, Corey DP. Myosin and adaptation by hair cells. Neuron 1997; 19: [17] Holt JR et al. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 2002; 108: [18] Chan DK, Hudspeth AJ. Ca(2+) current-driven nonlinear amplification by the mammalian cochlea in vitro. Nat Neurosci. 2005; 8: [19] Kennedy HJ, Crawford AC, Fettiplace R. Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature 2005; 433: [20] He DZ. Relationship between the development of outer hair cell electromotility and efferent innervation: a study in cultured organ of Corti of neonatal gerbils. J Neurosci. 1997; 17: [21] He DZ, Jia SP, Dallos P. Mechanoelectrical transduction of outer hair cells studied in a gerbil hemicochlea. Nature 2004; 429: [22] Jia SP, Dallos P, He DZ. Mechanoelectric transduction of adult inner hair cells. J Neurosci. 2007; 27: [23] Hu XT, Evans BN, Dallos P. Direct visualization of organ of Corti kinematics in a hemicochlea. J Neurophysiol. 1999; 82: [24] Kros CJ, Rüsch A, Richardson GP. Mechano-electrical transducer currents in hair cells of the cultured neonatal mouse cochlea. Proc R Soc Lond B Biol Sci. 1992; 249: [25] Kennedy HJ, Evans MG, Crawford AC, Fettiplace R. Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nature Neurosci. 2003; 6: [26] Géléoc GSG, Holt JF. Developmental acquisition of sensory transduction in hair cells of the mouse inner ear. Nature Neurosci. 2003; 6: [27] He DZ, Evans BN, Dallos P. First appearance and development of electromotility in neonatal gerbil outer hair cells. Hear Res. 1994; 78: [28] Zheng J. et al. Prestin is the motor protein of cochlear outer hair cells. Nature 2000; 405: [29] Santos-Sacchi J. Asymmetry in voltage-dependent movements of isolated outer hair cells from the organ of Corti. J Neurosci. 1989; 9: [30] Santos-Sacchi J. On the frequency limit and phase of outer hair cell motility: effects of the membrane filter. J Neurosci. 1992; 12: [31] Legan PK. et al. A targeted deletion in alpha-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron 2000; 28: [32] Lukashkin AN. et al. Role of the tectorial membrane revealed
9 by otoacoustic emissions recorded from wild-type and transgenic Tecta (deltaent/deltaent) mice. J Neurophysiol. 2004; 91: [33] Gummer AW, Hemmert W, Zenner HP. Resonant tectorial membrane motion in the inner ear: its crucial role in frequency tuning. Proc Natl Acad Sci USA 1996; 93: [34] Reuter G, Gitter AH, Thürm U, Zenner HP. High frequency radial movements of the reticular lamina induced by outer hair cell motility. Hear Res. 1992; 60: [35] Mammano F, Ashmore JF. Reverse transduction measured in the isolated cochlea by laser Michelson interferometry. Nature 1994; 365: [36] Evans BN, Dallos P. Stereocilia displacement induced somatic motility of cochlear outer hair cells. Proc Natl Acad Sci USA. 1993; 90: [37] Dallos P, Billone MC, Durrant JD, Wang C, Raynor S. Cochlear inner and outer hair cells: functional differences. Science 1972; 177: [38] Dallos P, Evans BN. High-frequency motility of outer hair cells and the cochlear amplifier. Science 1995; 267: [39] Fridberger A. et al. Organ of Corti potentials and the motion of the basilar membrane. J Neurosci. 2004; 24: [40] Spector AA, Brownell WE, Popel AS. Effect of outer hair cell piezoelectricity on high-frequency receptor potentials. J Acoust Soc Am. 2003;113: (Received July 21, 2014 ) 72
THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA
 451 THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA DÁIBHID Ó MAOILÉIDIGH, FRANK JÜLICHER Max Planck Institute für Physik komplexer Systeme, Nöthnitzerstr. 38, 01187
451 THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA DÁIBHID Ó MAOILÉIDIGH, FRANK JÜLICHER Max Planck Institute für Physik komplexer Systeme, Nöthnitzerstr. 38, 01187
Mechanoelectric Transduction of Adult Inner Hair Cells
 1006 The Journal of Neuroscience, January 31, 2007 27(5):1006 1014 Cellular/Molecular Mechanoelectric Transduction of Adult Inner Hair Cells Shuping Jia, 1 Peter Dallos, 2 and David Z. Z. He 1 1 Hair Cell
1006 The Journal of Neuroscience, January 31, 2007 27(5):1006 1014 Cellular/Molecular Mechanoelectric Transduction of Adult Inner Hair Cells Shuping Jia, 1 Peter Dallos, 2 and David Z. Z. He 1 1 Hair Cell
Chapter 3: Anatomy and physiology of the sensory auditory mechanism
 Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Auditory System Feedback
 Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS
 SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS Katherine Knisely, D. Bozovic University of California at Los Angeles It has long been observed that there is some form of amplification
SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS Katherine Knisely, D. Bozovic University of California at Los Angeles It has long been observed that there is some form of amplification
HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier
 HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier 1 Cochlear Mechanics: Measures of Basilar Membrane Motion 2 Cochlear Mechanics: Measures of Basilar Membrane Motion Bekesy s experiments
HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier 1 Cochlear Mechanics: Measures of Basilar Membrane Motion 2 Cochlear Mechanics: Measures of Basilar Membrane Motion Bekesy s experiments
Mechanical Properties of the Cochlea. Reading: Yost Ch. 7
 Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons.
 1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
Cochlear anatomy, function and pathology I. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology I Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of these lectures Introduction to gross anatomy of the cochlea Focus (1) on
Cochlear anatomy, function and pathology I Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of these lectures Introduction to gross anatomy of the cochlea Focus (1) on
Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression
 1398 Biophysical Journal Volume 106 March 2014 1398 1405 Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression Julien Meaud * and Karl Grosh Department
1398 Biophysical Journal Volume 106 March 2014 1398 1405 Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression Julien Meaud * and Karl Grosh Department
Structure, Energy Transmission and Function. Gross Anatomy. Structure, Function & Process. External Auditory Meatus or Canal (EAM, EAC) Outer Ear
 Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components
 1 2 3 4 What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components as a function of time here during the production
1 2 3 4 What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components as a function of time here during the production
HST 721 Efferent Control Lecture October 2004
 HST 721 Efferent Control Lecture October 2004 1 Stapedius Muscle Central Circuitry 2 Hypotheses for MEM Function A. Stapedius 1. Extend Dynamic Range - a gain control system 2. Protect the Inner Ear from
HST 721 Efferent Control Lecture October 2004 1 Stapedius Muscle Central Circuitry 2 Hypotheses for MEM Function A. Stapedius 1. Extend Dynamic Range - a gain control system 2. Protect the Inner Ear from
The magnificent outer hair cell of Corti's organ
 The magnificent outer hair cell of Corti's organ Yale University J. Santos-Sacchi School of Medicine, New Haver, CT, USA Abstract The outer hair cell is one of two receptor cell types in the organ of Corti.
The magnificent outer hair cell of Corti's organ Yale University J. Santos-Sacchi School of Medicine, New Haver, CT, USA Abstract The outer hair cell is one of two receptor cell types in the organ of Corti.
Prestin-Driven Cochlear Amplification Is Not Limited by the Outer Hair Cell Membrane Time Constant
 Article Prestin-Driven Cochlear Amplification Is Not Limited by the Outer Hair Cell Membrane Time Constant Stuart L. Johnson, 1 Maryline Beurg, 2 Walter Marcotti, 1, * and Robert Fettiplace 3, * 1 Department
Article Prestin-Driven Cochlear Amplification Is Not Limited by the Outer Hair Cell Membrane Time Constant Stuart L. Johnson, 1 Maryline Beurg, 2 Walter Marcotti, 1, * and Robert Fettiplace 3, * 1 Department
Cochlear anatomy, function and pathology II. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
Reticular lamina and basilar membrane vibrations in living mouse cochleae
 Reticular lamina and basilar membrane vibrations in living mouse cochleae Tianying Ren a,1, Wenxuan He a, and David Kemp b a Oregon Hearing Research Center, Department of Otolaryngology, Oregon Health
Reticular lamina and basilar membrane vibrations in living mouse cochleae Tianying Ren a,1, Wenxuan He a, and David Kemp b a Oregon Hearing Research Center, Department of Otolaryngology, Oregon Health
What Drives Mechanical Amplification in the Mammalian Cochlea?
 What Drives Mechanical Amplification in the Mammalian Cochlea? Robert H. Withnell, Lauren A. Shaffer, and David J. Lilly The recent report by Peter Dallos and colleagues of the gene and protein responsible
What Drives Mechanical Amplification in the Mammalian Cochlea? Robert H. Withnell, Lauren A. Shaffer, and David J. Lilly The recent report by Peter Dallos and colleagues of the gene and protein responsible
Processing of sounds in the inner ear
 Processing of sounds in the inner ear Sripriya Ramamoorthy Associate Professor, IIT Bombay WiSSAP 2018 Cochlea converts sound into electrical signals [Picture courtesy of Northwestern University] von Bekesy
Processing of sounds in the inner ear Sripriya Ramamoorthy Associate Professor, IIT Bombay WiSSAP 2018 Cochlea converts sound into electrical signals [Picture courtesy of Northwestern University] von Bekesy
The transformation of sound stimuli into electrical signals
 The transformation of sound stimuli into electrical signals Robert Fettiplace 2 1 Introduction Our sense of hearing depends on the correct performance of about 15 000 hair cells in each cochlea that serve
The transformation of sound stimuli into electrical signals Robert Fettiplace 2 1 Introduction Our sense of hearing depends on the correct performance of about 15 000 hair cells in each cochlea that serve
Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics
 Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3676 3681, March 1999 Biophysics Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics PAUL J.
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3676 3681, March 1999 Biophysics Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics PAUL J.
FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS. Hans P. Zenner, Ulrike Zimmermann and Alfred H. Gitter
 Vol. 149,No. 1, 1987 November 30, 1987 BlOCHEMlCALANDBlOPHYSlCALRESEARCHCOMMUNlCATlONS Pages 304-308 FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS Hans P. Zenner, Ulrike Zimmermann and Alfred
Vol. 149,No. 1, 1987 November 30, 1987 BlOCHEMlCALANDBlOPHYSlCALRESEARCHCOMMUNlCATlONS Pages 304-308 FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS Hans P. Zenner, Ulrike Zimmermann and Alfred
What is the effect on the hair cell if the stereocilia are bent away from the kinocilium?
 CASE 44 A 53-year-old man presents to his primary care physician with complaints of feeling like the room is spinning, dizziness, decreased hearing, ringing in the ears, and fullness in both ears. He states
CASE 44 A 53-year-old man presents to his primary care physician with complaints of feeling like the room is spinning, dizziness, decreased hearing, ringing in the ears, and fullness in both ears. He states
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/317/5841/183/dc1 Supporting Online Material for Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses Gertrudis Perea and Alfonso Araque* *To whom
www.sciencemag.org/cgi/content/full/317/5841/183/dc1 Supporting Online Material for Astrocytes Potentiate Transmitter Release at Single Hippocampal Synapses Gertrudis Perea and Alfonso Araque* *To whom
So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to
 So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to emphasize the intricate and well-protected structure of the
So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to emphasize the intricate and well-protected structure of the
Deafness and hearing impairment
 Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
Force Transmission in the Organ of Corti Micromachine
 Biophysical Journal Volume 98 June 2010 2813 2821 2813 Force Transmission in the Organ of Corti Micromachine Jong-Hoon Nam and Robert Fettiplace* Department of Physiology, University of Wisconsin Medical
Biophysical Journal Volume 98 June 2010 2813 2821 2813 Force Transmission in the Organ of Corti Micromachine Jong-Hoon Nam and Robert Fettiplace* Department of Physiology, University of Wisconsin Medical
Voltage-clamp errors cause anomalous interaction between independent ion channels
 AUDITORYAND VESTIBULAR SYSTEMS Voltage-clamp errors cause anomalous interaction between independent ion channels Hamilton E. Farris CA and Anthony J. Ricci Neuroscience Center and Kresge Hearing Labs,
AUDITORYAND VESTIBULAR SYSTEMS Voltage-clamp errors cause anomalous interaction between independent ion channels Hamilton E. Farris CA and Anthony J. Ricci Neuroscience Center and Kresge Hearing Labs,
Is action potential threshold lowest in the axon?
 Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
Required Slide. Session Objectives
 Auditory Physiology Required Slide Session Objectives Auditory System: At the end of this session, students will be able to: 1. Characterize the range of normal human hearing. 2. Understand the components
Auditory Physiology Required Slide Session Objectives Auditory System: At the end of this session, students will be able to: 1. Characterize the range of normal human hearing. 2. Understand the components
Supplementary Information
 Hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K + channels Meena S. George, L.F. Abbott, Steven A. Siegelbaum Supplementary Information Part 1: Supplementary Figures
Hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K + channels Meena S. George, L.F. Abbott, Steven A. Siegelbaum Supplementary Information Part 1: Supplementary Figures
The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons.
 1 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
1 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
Systems Neuroscience Oct. 16, Auditory system. http:
 Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
Lecture 6 Hearing 1. Raghav Rajan Bio 354 Neurobiology 2 January 28th All lecture material from the following links unless otherwise mentioned:
 Lecture 6 Hearing 1 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
Lecture 6 Hearing 1 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
Fate of Mammalian Cochlear Hair Cells and Stereocilia after Loss of the Stereocilia
 The Journal of Neuroscience, December 2, 2009 29(48):15277 15285 15277 Development/Plasticity/Repair Fate of Mammalian Cochlear Hair Cells and Stereocilia after Loss of the Stereocilia Shuping Jia, 1 Shiming
The Journal of Neuroscience, December 2, 2009 29(48):15277 15285 15277 Development/Plasticity/Repair Fate of Mammalian Cochlear Hair Cells and Stereocilia after Loss of the Stereocilia Shuping Jia, 1 Shiming
Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier
 Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier Sripriya Ramamoorthy 1 *, Alfred L. Nuttall 1,2 1 Oregon Hearing Research Center, Department of Otolaryngology, Oregon
Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier Sripriya Ramamoorthy 1 *, Alfred L. Nuttall 1,2 1 Oregon Hearing Research Center, Department of Otolaryngology, Oregon
Measurement of cochlear power gain in the sensitive gerbil ear
 Received 4 Nov 2 Accepted 3 Feb 2 Published Mar 2 DOI:.38/ncomms226 Measurement of cochlear power gain in the sensitive gerbil ear Tianying Ren,2, Wenxuan He & Peter G. Gillespie,3 The extraordinary sensitivity
Received 4 Nov 2 Accepted 3 Feb 2 Published Mar 2 DOI:.38/ncomms226 Measurement of cochlear power gain in the sensitive gerbil ear Tianying Ren,2, Wenxuan He & Peter G. Gillespie,3 The extraordinary sensitivity
Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs
 Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs The Auditory Laboratory, Physiology University of Western Australia Greg O Beirne Dept. of Communication
Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs The Auditory Laboratory, Physiology University of Western Australia Greg O Beirne Dept. of Communication
A truly remarkable aspect of human hearing is the vast
 AUDITORY COMPRESSION AND HEARING LOSS Sid P. Bacon Psychoacoustics Laboratory, Department of Speech and Hearing Science, Arizona State University Tempe, Arizona 85287 A truly remarkable aspect of human
AUDITORY COMPRESSION AND HEARING LOSS Sid P. Bacon Psychoacoustics Laboratory, Department of Speech and Hearing Science, Arizona State University Tempe, Arizona 85287 A truly remarkable aspect of human
NIH Public Access Author Manuscript Nat Commun. Author manuscript; available in PMC 2013 March 12.
 NIH Public Access Author Manuscript Published in final edited form as: Nat Commun. 2012 ; 3: 1094. doi:10.1038/ncomms2100. Sound-induced length changes in outer hair cell stereocilia Pierre Hakizimana
NIH Public Access Author Manuscript Published in final edited form as: Nat Commun. 2012 ; 3: 1094. doi:10.1038/ncomms2100. Sound-induced length changes in outer hair cell stereocilia Pierre Hakizimana
Adaptation in auditory hair cells Robert Fettiplace and Anthony J Ricci y
 446 Adaptation in auditory hair cells Robert Fettiplace and Anthony J Ricci y The narrow stimulus limits of hair cell transduction, equivalent to a total excursion of about 100 nm at the tip of the hair
446 Adaptation in auditory hair cells Robert Fettiplace and Anthony J Ricci y The narrow stimulus limits of hair cell transduction, equivalent to a total excursion of about 100 nm at the tip of the hair
Hearing Research 273 (2011) 109e122. Contents lists available at ScienceDirect. Hearing Research. journal homepage:
 Hearing Research 273 (2011) 109e122 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares Somatic motility and hair bundle mechanics, are both necessary
Hearing Research 273 (2011) 109e122 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares Somatic motility and hair bundle mechanics, are both necessary
Tectorial Membrane Stiffness Gradients
 Biophysical Journal Volume 93 September 2007 2265 2276 2265 Tectorial Membrane Stiffness Gradients Claus-Peter Richter,* y Gulam Emadi, z Geoffrey Getnick, y Alicia Quesnel, y and Peter Dallos* yz *Auditory
Biophysical Journal Volume 93 September 2007 2265 2276 2265 Tectorial Membrane Stiffness Gradients Claus-Peter Richter,* y Gulam Emadi, z Geoffrey Getnick, y Alicia Quesnel, y and Peter Dallos* yz *Auditory
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Analysis of hair bundle morphology in Ush1c c.216g>a mice at P18 by SEM.
 Supplementary Figure 1 Analysis of hair bundle morphology in Ush1c c.216g>a mice at P18 by SEM. (a-c) Heterozygous c.216ga mice displayed normal hair bundle morphology at P18. (d-i) Disorganized hair bundles
Supplementary Figure 1 Analysis of hair bundle morphology in Ush1c c.216g>a mice at P18 by SEM. (a-c) Heterozygous c.216ga mice displayed normal hair bundle morphology at P18. (d-i) Disorganized hair bundles
Academic and Research Staff Professor Dennis M. Freeman, Dr. Alexander Aranyosi, Dr. Stanley Hong
 Cochlear Mechanics Academic and Research Staff Professor Dennis M. Freeman, Dr. Alexander Aranyosi, Dr. Stanley Hong Graduate Students Chris Bergevin, Roozbeh Ghaffari, Wendy Gu, Kinuko Masaki, Scott Page
Cochlear Mechanics Academic and Research Staff Professor Dennis M. Freeman, Dr. Alexander Aranyosi, Dr. Stanley Hong Graduate Students Chris Bergevin, Roozbeh Ghaffari, Wendy Gu, Kinuko Masaki, Scott Page
Evidence and synthesis
 CHAPTER D 9 (Discussion continued) Evidence and synthesis 9.1 Synthesis 9.1/a Outer hair cells as dual detectors Pressure detection and transduction currents The silent current 9.1/b Cancellation effects
CHAPTER D 9 (Discussion continued) Evidence and synthesis 9.1 Synthesis 9.1/a Outer hair cells as dual detectors Pressure detection and transduction currents The silent current 9.1/b Cancellation effects
Functional motor microdomains of the outer hair cell lateral membrane
 Pflugers Arch - Eur J Physiol (2002) 445:331 336 DOI 10.1007/s00424-002-0928-4 CELL AND MOLECULAR PHYSIOLOGY Joseph Santos-Sacchi Functional motor microdomains of the outer hair cell lateral membrane Received:
Pflugers Arch - Eur J Physiol (2002) 445:331 336 DOI 10.1007/s00424-002-0928-4 CELL AND MOLECULAR PHYSIOLOGY Joseph Santos-Sacchi Functional motor microdomains of the outer hair cell lateral membrane Received:
to vibrate the fluid. The ossicles amplify the pressure. The surface area of the oval window is
 Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
Auditory System. Barb Rohrer (SEI )
 Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
Sensory Transduction and Adaptation in Inner and Outer Hair Cells of the Mouse Auditory System
 J Neurophysiol 98: 3360 3369, 2007. First published October 17, 2007; doi:10.1152/jn.00914.2007. Sensory Transduction and Adaptation in Inner and Outer Hair Cells of the Mouse Auditory System Eric A. Stauffer
J Neurophysiol 98: 3360 3369, 2007. First published October 17, 2007; doi:10.1152/jn.00914.2007. Sensory Transduction and Adaptation in Inner and Outer Hair Cells of the Mouse Auditory System Eric A. Stauffer
In vivo outer hair cell length changes expose the active process in the cochlea
 In vivo outer hair cell length changes expose the active process in the cochlea Dingjun Zha, Fangyi Chen, Sripriya Ramamoorthy, Anders Fridberger, Niloy Choudhury, Steven L Jacques, Ruikang K Wang and
In vivo outer hair cell length changes expose the active process in the cochlea Dingjun Zha, Fangyi Chen, Sripriya Ramamoorthy, Anders Fridberger, Niloy Choudhury, Steven L Jacques, Ruikang K Wang and
Unit VIII Problem 9 Physiology: Hearing
 Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
The cochlea: auditory sense. The cochlea: auditory sense
 Inner ear apparatus 1- Vestibule macula and sacculus sensing acceleration of the head and direction of gravity 2- Semicircular canals mainly for sensing direction of rotation of the head 1 3- cochlea in
Inner ear apparatus 1- Vestibule macula and sacculus sensing acceleration of the head and direction of gravity 2- Semicircular canals mainly for sensing direction of rotation of the head 1 3- cochlea in
Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells
 Biophysical Journal Volume 84 February 2003 739 749 739 Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells Mark Ospeck, Xiao-xia Dong, and Kuni H. Iwasa Biophysics
Biophysical Journal Volume 84 February 2003 739 749 739 Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells Mark Ospeck, Xiao-xia Dong, and Kuni H. Iwasa Biophysics
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium!
 Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! Chapter 17 Part 2 Special Senses! The Special Senses! Hearing and Equilibrium!
 Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Chapter 17, Part 2! The Special Senses! Hearing and Equilibrium! SECTION 17-5! Equilibrium sensations originate within the inner ear, while hearing involves the detection and interpretation of sound waves!
Trajectory of the Aging Cochlea
 Trajectory of the Aging Cochlea Sumitrajit (Sumit) Dhar Professor & Chair Roxelyn & Richard Pepper Department of Communication Sciences and Disorders Fellow, Hugh Knowles Center for Hearing Science Northwestern
Trajectory of the Aging Cochlea Sumitrajit (Sumit) Dhar Professor & Chair Roxelyn & Richard Pepper Department of Communication Sciences and Disorders Fellow, Hugh Knowles Center for Hearing Science Northwestern
Before we talk about the auditory system we will talk about the sound and waves
 The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
Signals, systems, acoustics and the ear. Week 5. The peripheral auditory system: The ear as a signal processor
 Signals, systems, acoustics and the ear Week 5 The peripheral auditory system: The ear as a signal processor Think of this set of organs 2 as a collection of systems, transforming sounds to be sent to
Signals, systems, acoustics and the ear Week 5 The peripheral auditory system: The ear as a signal processor Think of this set of organs 2 as a collection of systems, transforming sounds to be sent to
Auditory mechanotransduction in the absence of functional myosin-xva
 J Physiol 576.3 (2006) pp 801 808 801 RAPID REPORT Auditory mechanotransduction in the absence of functional myosin-xva Ruben Stepanyan 1, Inna A. Belyantseva 2, Andrew J. Griffith 3, Thomas B. Friedman
J Physiol 576.3 (2006) pp 801 808 801 RAPID REPORT Auditory mechanotransduction in the absence of functional myosin-xva Ruben Stepanyan 1, Inna A. Belyantseva 2, Andrew J. Griffith 3, Thomas B. Friedman
Cochlear function in Prestin knockout mice
 J Physiol 560.3 (2004) pp 821 830 821 Cochlear function in Prestin knockout mice M. A. Cheatham 1,K.H.Huynh 1,J.Gao 2,J.Zuo 2 and P. Dallos 1,3 1 Departments of Communication Sciences and Disorders and
J Physiol 560.3 (2004) pp 821 830 821 Cochlear function in Prestin knockout mice M. A. Cheatham 1,K.H.Huynh 1,J.Gao 2,J.Zuo 2 and P. Dallos 1,3 1 Departments of Communication Sciences and Disorders and
Efferent-mediated control of basilar membrane motion
 J Physiol 576. (2006) pp 49 54 49 Topical Review Efferent-mediated control of basilar membrane motion N. P. Cooper and J. J. Guinan Jr 2 School of Life Sciences, Keele University, Keele, Staffordshire
J Physiol 576. (2006) pp 49 54 49 Topical Review Efferent-mediated control of basilar membrane motion N. P. Cooper and J. J. Guinan Jr 2 School of Life Sciences, Keele University, Keele, Staffordshire
Active Hair Bundle Movements and the Cochlear Amplifier
 Active Hair Bundle Movements and the Cochlear Amplifier Anthony Ricci* Abstract The active process is a term used to describe amplification and filtering processes that are essential for obtaining the
Active Hair Bundle Movements and the Cochlear Amplifier Anthony Ricci* Abstract The active process is a term used to describe amplification and filtering processes that are essential for obtaining the
Auditory Periphery! external middle inner. stapes movement initiates a pressure wave in cochlear fluid
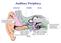 Auditory Periphery! external middle inner sound causes air pressure to increase at eardrum stapes movement initiates a pressure wave in cochlear fluid VIIIth nerve conveys neural signal to cochlear nucleus
Auditory Periphery! external middle inner sound causes air pressure to increase at eardrum stapes movement initiates a pressure wave in cochlear fluid VIIIth nerve conveys neural signal to cochlear nucleus
Neurons of the Bed Nucleus of the Stria Terminalis (BNST)
 Neurons of the Bed Nucleus of the Stria Terminalis (BNST) Electrophysiological Properties and Their Response to Serotonin DONALD G. RAINNIE a Harvard Medical School and Department of Psychiatry, Brockton
Neurons of the Bed Nucleus of the Stria Terminalis (BNST) Electrophysiological Properties and Their Response to Serotonin DONALD G. RAINNIE a Harvard Medical School and Department of Psychiatry, Brockton
Neuroscience 201A Problem Set #1, 27 September 2016
 Neuroscience 201A Problem Set #1, 27 September 2016 1. The figure above was obtained from a paper on calcium channels expressed by dentate granule cells. The whole-cell Ca 2+ currents in (A) were measured
Neuroscience 201A Problem Set #1, 27 September 2016 1. The figure above was obtained from a paper on calcium channels expressed by dentate granule cells. The whole-cell Ca 2+ currents in (A) were measured
Detection of Cochlear Amplification and Its Activation
 Biophysical Journal Volume 5 August 23 67 78 67 Detection of Cochlear Amplification and Its Activation Wei Dong and Elizabeth S. Olson * Otolaryngology, Head and Neck Surgery and Biomedical Engineering,
Biophysical Journal Volume 5 August 23 67 78 67 Detection of Cochlear Amplification and Its Activation Wei Dong and Elizabeth S. Olson * Otolaryngology, Head and Neck Surgery and Biomedical Engineering,
Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea
 Article Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea Wei Dong and Elizabeth S. Olson 2, * VA Loma Linda Health Care System and Otolaryngology/Head & Neck Surgery,
Article Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea Wei Dong and Elizabeth S. Olson 2, * VA Loma Linda Health Care System and Otolaryngology/Head & Neck Surgery,
Supplemental Information. Octopamine Neurons Mediate Flight-Induced Modulation of Visual Processing in Drosophila. Supplemental Inventory
 1 Current Biology, Volume 22 Supplemental Information Octopamine Neurons Mediate Flight-Induced Modulation of Visual Processing in Drosophila Marie P. Suver, Akira Mamiya, and Michael H. Dickinson Supplemental
1 Current Biology, Volume 22 Supplemental Information Octopamine Neurons Mediate Flight-Induced Modulation of Visual Processing in Drosophila Marie P. Suver, Akira Mamiya, and Michael H. Dickinson Supplemental
ENT 318 Artificial Organs Physiology of Ear
 ENT 318 Artificial Organs Physiology of Ear Lecturer: Ahmad Nasrul Norali The Ear The Ear Components of hearing mechanism - Outer Ear - Middle Ear - Inner Ear - Central Auditory Nervous System Major Divisions
ENT 318 Artificial Organs Physiology of Ear Lecturer: Ahmad Nasrul Norali The Ear The Ear Components of hearing mechanism - Outer Ear - Middle Ear - Inner Ear - Central Auditory Nervous System Major Divisions
Correlation between Membrane Potential Responses and Tentacle Movement in the Dinoflagellate Noctiluca miliaris
 ZOOLOGICAL SCIENCE 21: 131 138 (2004) 2004 Zoological Society of Japan Correlation between Membrane Potential Responses and Tentacle Movement in the Dinoflagellate Noctiluca miliaris Kazunori Oami* Institute
ZOOLOGICAL SCIENCE 21: 131 138 (2004) 2004 Zoological Society of Japan Correlation between Membrane Potential Responses and Tentacle Movement in the Dinoflagellate Noctiluca miliaris Kazunori Oami* Institute
The cochlear amplifier is a surface acoustic wave resonator
 The cochlear amplifier is a surface acoustic wave resonator by Andrew Bell P.O. Box A348 Australian National University Canberra, ACT 2601 Australia Phone: 61 2 6258 7276 Fax: 61 2 6258 0014 Email: bellring@smartchat.net.au
The cochlear amplifier is a surface acoustic wave resonator by Andrew Bell P.O. Box A348 Australian National University Canberra, ACT 2601 Australia Phone: 61 2 6258 7276 Fax: 61 2 6258 0014 Email: bellring@smartchat.net.au
Auditory Physiology Richard M. Costanzo, Ph.D.
 Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
SMELL 2
 SENSORY SYSTEMS 1 SMELL 2 TASTE 3 HEARING 4 TOUCH EQUILIBRIUM 5 PAIN 6 OTHER SENSES 7 HOW DO SENSORY CELLS CONVERT STIMULI INTO ACTION POTENTIALS? HOW DO SENSORY SYSTEMS DETECT CHEMICAL STIMULI? HOW DO
SENSORY SYSTEMS 1 SMELL 2 TASTE 3 HEARING 4 TOUCH EQUILIBRIUM 5 PAIN 6 OTHER SENSES 7 HOW DO SENSORY CELLS CONVERT STIMULI INTO ACTION POTENTIALS? HOW DO SENSORY SYSTEMS DETECT CHEMICAL STIMULI? HOW DO
P T P V U V I. INTRODUCTION. II. MODEL FORMULATION A. Cochlear fluid dynamics.
 Integration of outer hair cell activity in a one-dimensional cochlear model Azaria Cohen and Miriam Furst a) School of Electrical Engineering, Faculty of Engineering, Tel-Aviv University, Tel-Aviv 69978,
Integration of outer hair cell activity in a one-dimensional cochlear model Azaria Cohen and Miriam Furst a) School of Electrical Engineering, Faculty of Engineering, Tel-Aviv University, Tel-Aviv 69978,
Chapter 18 Senses SENSORY RECEPTION 10/21/2011. Sensory Receptors and Sensations. Sensory Receptors and Sensations. Sensory Receptors and Sensations
 SENSORY RECEPTION Chapter 18 Senses s convert stimulus energy to action potentials s 1. Are specialized cells, or 2. Specialized endings that detect stimuli All stimuli are forms of energy s in eyes detect
SENSORY RECEPTION Chapter 18 Senses s convert stimulus energy to action potentials s 1. Are specialized cells, or 2. Specialized endings that detect stimuli All stimuli are forms of energy s in eyes detect
Ear. Utricle & saccule in the vestibule Connected to each other and to the endolymphatic sac by a utriculosaccular duct
 Rahaf Jreisat *You don t have to go back to the slides. Ear Inner Ear Membranous Labyrinth It is a reflection of bony labyrinth but inside. Membranous labyrinth = set of membranous tubes containing sensory
Rahaf Jreisat *You don t have to go back to the slides. Ear Inner Ear Membranous Labyrinth It is a reflection of bony labyrinth but inside. Membranous labyrinth = set of membranous tubes containing sensory
Biophysical Journal Volume 108 February
 Biophysical Journal Volume 18 February 215 479 488 479 Article Power Dissipation in the Subtectorial Space of the Mammalian Cochlea Is Modulated by Inner Hair Cell Stereocilia Srdjan Prodanovic, 1 Sheryl
Biophysical Journal Volume 18 February 215 479 488 479 Article Power Dissipation in the Subtectorial Space of the Mammalian Cochlea Is Modulated by Inner Hair Cell Stereocilia Srdjan Prodanovic, 1 Sheryl
Tmc1 is necessary for normal functional maturation and survival of inner and outer hair cells in the mouse cochlea
 J Physiol 574.3 (2006) pp 677 698 677 Tmc1 is necessary for normal functional maturation and survival of inner and outer hair cells in the mouse cochlea Walter Marcotti 1, Alexandra Erven 2, Stuart L.
J Physiol 574.3 (2006) pp 677 698 677 Tmc1 is necessary for normal functional maturation and survival of inner and outer hair cells in the mouse cochlea Walter Marcotti 1, Alexandra Erven 2, Stuart L.
Cochlear anatomy, function and pathology III. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology III Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (3) on the cochlear lateral wall and Reissner s membrane:
Cochlear anatomy, function and pathology III Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (3) on the cochlear lateral wall and Reissner s membrane:
Outer Hair Cells in the Cochlea
 Discrete Dynamics in Nature and Society, Vol. 2, pp. 161-165 Reprints available directly from the publisher Photocopying permitted by license only (C) 1998 OPA (Overseas Publishers Association) N.V. Published
Discrete Dynamics in Nature and Society, Vol. 2, pp. 161-165 Reprints available directly from the publisher Photocopying permitted by license only (C) 1998 OPA (Overseas Publishers Association) N.V. Published
Optimal Electrical Properties of Outer Hair Cells Ensure Cochlear Amplification
 Optimal Electrical Properties of Outer Hair Cells Ensure Cochlear Amplification Jong-Hoon Nam 1,2 *, Robert Fettiplace 3 1 Department of Mechanical Engineering, Department of Biomedical Engineering, University
Optimal Electrical Properties of Outer Hair Cells Ensure Cochlear Amplification Jong-Hoon Nam 1,2 *, Robert Fettiplace 3 1 Department of Mechanical Engineering, Department of Biomedical Engineering, University
9.01 Introduction to Neuroscience Fall 2007
 MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
MIT OpenCourseWare http://ocw.mit.edu 9.01 Introduction to Neuroscience Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 9.01 Recitation (R02)
Cochlear Outer Hair Cell Motility
 Physiol Rev 88: 173 210, 2008; doi:10.1152/physrev.00044.2006. Cochlear Outer Hair Cell Motility JONATHAN ASHMORE Department of Physiology and UCL Ear Institute, University College London, London, United
Physiol Rev 88: 173 210, 2008; doi:10.1152/physrev.00044.2006. Cochlear Outer Hair Cell Motility JONATHAN ASHMORE Department of Physiology and UCL Ear Institute, University College London, London, United
Effects of Medial Olivocochlear Auditory Reflex Activation on Cochlear Vibration
 University of Iowa Honors Theses University of Iowa Honors Program Spring 2017 Effects of Medial Olivocochlear Auditory Reflex Activation on Cochlear Vibration Alexandra Redfern Shawn Goodman University
University of Iowa Honors Theses University of Iowa Honors Program Spring 2017 Effects of Medial Olivocochlear Auditory Reflex Activation on Cochlear Vibration Alexandra Redfern Shawn Goodman University
Otoconia: Calcium carbonate crystals Gelatinous mass. Cilia. Hair cells. Vestibular nerve. Vestibular ganglion
 VESTIBULAR SYSTEM (Balance/Equilibrium) The vestibular stimulus is provided by Earth s, and. Located in the of the inner ear, in two components: 1. Vestibular sacs - gravity & head direction 2. Semicircular
VESTIBULAR SYSTEM (Balance/Equilibrium) The vestibular stimulus is provided by Earth s, and. Located in the of the inner ear, in two components: 1. Vestibular sacs - gravity & head direction 2. Semicircular
The lagena a presumptive vestibular epithelium found at the low frequency end of the auditory end organ, is absent from mammals.
 1 Structure and Function of the Auditory and Vestibular System 1 Increasing elaboration of the auditory end organ during evolution. Increasing length of the cochlea associated with higher frequency hearing.
1 Structure and Function of the Auditory and Vestibular System 1 Increasing elaboration of the auditory end organ during evolution. Increasing length of the cochlea associated with higher frequency hearing.
SOLUTIONS Homework #3. Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03
 SOLUTIONS Homework #3 Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03 Problem 1: a) Where in the cochlea would you say the process of "fourier decomposition" of the incoming
SOLUTIONS Homework #3 Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03 Problem 1: a) Where in the cochlea would you say the process of "fourier decomposition" of the incoming
January 22 nd to 26 th, 2008
 Advanced theoretical and practical training course on Hearing in Mammals January 22 nd to 26 th, 2008 F ro m M o l e c u l e s t o A u d i t o r y P h y s i o l o g y ORGANIZERS Tobias Moser FACULTY Frank,
Advanced theoretical and practical training course on Hearing in Mammals January 22 nd to 26 th, 2008 F ro m M o l e c u l e s t o A u d i t o r y P h y s i o l o g y ORGANIZERS Tobias Moser FACULTY Frank,
Acoustics, signals & systems for audiology. Psychoacoustics of hearing impairment
 Acoustics, signals & systems for audiology Psychoacoustics of hearing impairment Three main types of hearing impairment Conductive Sound is not properly transmitted from the outer to the inner ear Sensorineural
Acoustics, signals & systems for audiology Psychoacoustics of hearing impairment Three main types of hearing impairment Conductive Sound is not properly transmitted from the outer to the inner ear Sensorineural
Effects of Remaining Hair Cells on Cochlear Implant Function
 Effects of Remaining Hair Cells on Cochlear Implant Function N1-DC-2-15QPR1 Neural Prosthesis Program N. Hu, P.J. Abbas, C.A. Miller, B.K. Robinson, K.V. Nourski, F. Jeng, B.A. Abkes, J.M. Nichols Department
Effects of Remaining Hair Cells on Cochlear Implant Function N1-DC-2-15QPR1 Neural Prosthesis Program N. Hu, P.J. Abbas, C.A. Miller, B.K. Robinson, K.V. Nourski, F. Jeng, B.A. Abkes, J.M. Nichols Department
FOUR COUNTER-ARGUMENTS FOR SLOW-WAVE OAEs
 FOUR COUNTER-ARGUMENTS FOR SLOW-WAVE OAEs CHRISTOPHER A. SHERA Eaton-Peabody Laboratory, Boston, MA 02114, USA email: shera@epl.meei.harvard.edu ARNOLD TUBIS Institute for Nonlinear Science, La Jolla,
FOUR COUNTER-ARGUMENTS FOR SLOW-WAVE OAEs CHRISTOPHER A. SHERA Eaton-Peabody Laboratory, Boston, MA 02114, USA email: shera@epl.meei.harvard.edu ARNOLD TUBIS Institute for Nonlinear Science, La Jolla,
Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea
 Keywords: Cochlea, Basilar membrane, Hair cell 7006 Journal of Physiology (1998), 509.1, pp. 277 288 277 Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea Nigel P.
Keywords: Cochlea, Basilar membrane, Hair cell 7006 Journal of Physiology (1998), 509.1, pp. 277 288 277 Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea Nigel P.
Acoustics Research Institute
 Austrian Academy of Sciences Acoustics Research Institute Modeling Modelingof ofauditory AuditoryPerception Perception Bernhard BernhardLaback Labackand andpiotr PiotrMajdak Majdak http://www.kfs.oeaw.ac.at
Austrian Academy of Sciences Acoustics Research Institute Modeling Modelingof ofauditory AuditoryPerception Perception Bernhard BernhardLaback Labackand andpiotr PiotrMajdak Majdak http://www.kfs.oeaw.ac.at
Νευροφυσιολογία και Αισθήσεις
 Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 11 Ακουστικό και Αιθουσιαίο Σύστημα (Auditory and Vestibular Systems) Introduction Sensory Systems Sense of
Biomedical Imaging & Applied Optics University of Cyprus Νευροφυσιολογία και Αισθήσεις Διάλεξη 11 Ακουστικό και Αιθουσιαίο Σύστημα (Auditory and Vestibular Systems) Introduction Sensory Systems Sense of
Intro to Audition & Hearing
 Intro to Audition & Hearing Lecture 16 Chapter 9, part II Jonathan Pillow Sensation & Perception (PSY 345 / NEU 325) Fall 2017 1 Sine wave: one of the simplest kinds of sounds: sound for which pressure
Intro to Audition & Hearing Lecture 16 Chapter 9, part II Jonathan Pillow Sensation & Perception (PSY 345 / NEU 325) Fall 2017 1 Sine wave: one of the simplest kinds of sounds: sound for which pressure
Vestibular physiology
 Vestibular physiology 2017 Utricle A flat epithelium: horizontal in the upright head Utricle Hair cells: no axons hair cells Utricle Hair cells synapse onto 8th nerve afferents. 8th nerve afferents Hair
Vestibular physiology 2017 Utricle A flat epithelium: horizontal in the upright head Utricle Hair cells: no axons hair cells Utricle Hair cells synapse onto 8th nerve afferents. 8th nerve afferents Hair
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/312/5779/1533/dc1 Supporting Online Material for Long-Term Potentiation of Neuron-Glia Synapses Mediated by Ca 2+ - Permeable AMPA Receptors Woo-Ping Ge, Xiu-Juan Yang,
www.sciencemag.org/cgi/content/full/312/5779/1533/dc1 Supporting Online Material for Long-Term Potentiation of Neuron-Glia Synapses Mediated by Ca 2+ - Permeable AMPA Receptors Woo-Ping Ge, Xiu-Juan Yang,
Innervation of the Cochlea. Reading: Yost Ch. 8
 Innervation of the Cochlea Reading: Yost Ch. 8 Fine Structure of the Organ of Corti Auditory Nerve Auditory nerve (AN) is a branch of the VIII th cranial nerve (other branch is vestibular). AN is composed
Innervation of the Cochlea Reading: Yost Ch. 8 Fine Structure of the Organ of Corti Auditory Nerve Auditory nerve (AN) is a branch of the VIII th cranial nerve (other branch is vestibular). AN is composed
Hearing Research 250 (2009) Contents lists available at ScienceDirect. Hearing Research. journal homepage:
 Hearing Research 250 (2009) 63 75 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares Research paper Displacements of the organ of Corti by gel injections
Hearing Research 250 (2009) 63 75 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares Research paper Displacements of the organ of Corti by gel injections
