ALIGN Radial Head System INSTRUCTIONS FOR USE
|
|
|
- Lynne Moore
- 5 years ago
- Views:
Transcription
1 ALIGN Radial Head System INSTRUCTIONS FOR USE R x: For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may lead to patient injury. This package insert is designed to provide Instructions for Use of the ALIGN Radial Head System; it is not a reference to surgical techniques. Prior to use of the ALIGN Radial Head System the surgeon should become familiar with all information contained in this pamphlet. Description: The ALIGN Radial Head System is a radial head prosthesis and instrumentation platform that is designed to orient the Radial Head perpendicular to the axis of forearm rotation. The fluted plasma coated Radial Stem may assist in biological fixation, and is press fit into the medullary canal of the radius. Combined with its unique instrumentation, the ALIGN Radial Head offers the flexibility to adjust the orientation during implantation and restore motion at the Radial Head, then locks to form a monoblock prosthesis after the optimal implant positioning has been achieved. The ALIGN Radial Head System is comprised of: Multiple sized CoCr Radial Heads with Lock Screw Multiple sized titanium alloy Stems, titanium plasma spray coated System specific instrumentation Indications for Use: The ALIGN Radial Head System and accessories are designed specifically for: Replacement of the radial head for degenerative or post-traumatic disabilities presenting pain, crepitation, and decreased motion at the radio-humeral and/or proximal radio-ulnar joint with: o Joint destruction and/or subluxation o Resistance to conservative treatment Primary replacement after fracture of the radial head Symptomatic sequelae after radial head resection Revision following failed radial head arthroplasty The system is intended for press-fit use Page 1 of 6 IFU RAK Rev. March 2017
2 Contraindications: The ALIGN Radial Head System should not be used if any of the following are present: active or latent infection, sepsis, insufficient quantity or quality of bone and/or soft tissue, material sensitivity, or patients who are unwilling or incapable of following post operative care instructions. Warnings: Radial head prosthesis cannot be expected to withstand the activity levels and loads of normal healthy bone and joint tissue. Failure of the component can occur as a result of loss of fixation, strenuous activity, malalignment, trauma, non-union or excessive loads (estimated body weight equivalent of 350 lbs or greater). The Head Alignment Tool must be used during the procedure to correctly align the prosthetic head and to provide the necessary counter-torque when tightening the Lock Screw. The Lock Screw packaged with the Radial Head must be installed and fully tightened to fix the Radial Head to the Radial Stem. If the Lock Screw is not attached and/or fully secured, the Radial Head may loosen and/or disconnect from the Radial Stem, causing soft tissue irritation and/or device failure. Improper selection, placement, positioning, alignment or fixation of the implanted components may result in unusual stress conditions which may lead to subsequent reduction in the service life of the prosthetic components. Malalignment of the components or inaccurate implantation can lead to excessive wear and/or failure of the prosthesis or anatomical structures. The information in this document should be shared with the patient. The patient should be informed about the importance to follow the post operative rehabilitation to fully understand the possible limitations in normal activities of daily living. The patient must be warned that failure to follow postoperative care instructions may cause the prosthesis or treatment to fail. Potential ALIGN Radial Head System construct failures such as stress fractures of the bones, loosening of the construct and/or fixation, delayed fusion, non-fusion, or incomplete healing may occur as a result of non compliance to post operative rehabilitation, excessive wrist and forearm activities or construct overloading. DO NOT reuse any of the ALIGN Radial Head System implants or the T20 Driver. Reuse may compromise the structural integrity of the construct and/or lead to failure or infection which may result in patient injury. The Torque Handle included in the system requires calibration. DO NOT use if the calibration is overdue. Use of a Torque Handle out of calibration could result in device loosening or failure. Precautions: Patient must avoid placing excessive loads on the implant. The Radial Head with Lock Screw and Radial Stems are supplied sterile using gamma radiation sterilization. DO NOT use if sterile barrier is damaged or if the USE BY date has expired. Any implantable components used with an expired USE BY date will void the product warranty. The implantable components and the T20 Drivers are for single use only; DO NOT reuse, reprocess or resterilize. Reuse, reprocessing or re-sterilization of the implantable components may: o Compromise the structural integrity of the construct o Lead to failure resulting in patient injury o Create a risk of contamination of the device causing patient infection or cross-contamination o Lead to the transmission of infectious disease(s) from one patient to another Protect the implants against scratching or nicking as such stress concentration may lead to construct failure. Before using the ALIGN Radial Head System, inspect all prosthesis and instruments for wear, disfiguration and physical damage. If evidence of wear, disfiguration or physical damage is found, DO NOT use and contact your local Skeletal Dynamics representative or the Skeletal Dynamics Customer Care Department. The ALIGN Radial Head System has not been evaluated for safety and compatibility in the MR environment; nor has it been tested for heating or migration in the MR environment. To maintain product traceability, record the Lot number for each of the implanted system components. The Skeletal Dynamics ALIGN Radial Head System is to be used only with Skeletal Dynamics instruments, implants and accessories, including the Torque Handle (calibrated to 60in/lbs). Dispose of contaminated implants and instruments per established facility guidelines and protocols. Accuracy of Depth, Gap and Screw Gauges are within mm. Caution should be taken for interference to pacemakers during electrocautery or by uncertified drills. Seek medical help immediately if implant malfunctions. Potential Adverse Events: Page 2 of 6 IFU RAJ Rev. March 2017
3 The following potential risks or discomforts have been associated with radial head arthroplasty: Disassociation, loosening or migration of the prosthesis, infection, erosion of the capitellum, material sensitivity reaction, nerve injuries, undesirable shortening or lengthening of limb, stiffness of the elbow and/or forearm, dislocation or subluxation due to improper positioning, fretting and crevice corrosion can occur at interfaces between the components, wear and deformation of the articular surfaces, intraoperative and postoperative bone fracture and/or postoperative pain and infection. Directions for Use: The ALIGN Radial Head System should only be used by surgeons who have experience with the system. Each surgeon must evaluate the appropriateness for the use of the ALIGN Radial Head System during radial head arthroplasty procedures based on their experience with the ALIGN Radial Head System. Please refer to the ALIGN Radial Head Arthroplasty Surgical Technique Guide to review the surgical approach to radial head arthroplasty as described by Jorge L. Orbay, M.D. of the Miami Hand Institute located in Miami, Florida. Cleaning: The ALIGN Radial Head System instrumentation must be cleaned to achieve sterilization. The recommended manual cleaning instructions are set forth below. Other cleaning methods must be validated by the user. 1. Disassemble instrumentation, if applicable. 2. Rinse components thoroughly under running cool tap water. While rinsing, use a soft bristle brush to loosen and remove as much visible soil as possible from components. 3. Soak components in a neutral enzymatic cleaner for a minimum of ten (10) minutes. Components must be fully immersed in the cleaner. Follow the cleaner manufacturer s instructions for cleaner preparation and exposure time. 4. Thoroughly rinse the components with cool water. While rinsing, use soft bristle brushes, pipettes or a water jet to clean out lumens, holes, and other challenging features. 5. Manually scrub the components thoroughly in newly made, clean, neutral ph enzymatic cleaner using soft bristle brushes or pipettes. All lumens, holes, hinged components, mating surfaces, and crevices, and challenging components should be thoroughly scrubbed. Actuate all moveable features and expose all areas to cleaner and to the brush or pipette. Note: When scrubbing rasps or planers, a stiff bristle brush will be required. 6. Rinse components thoroughly with deionized or purified water; using pipettes or a water jet to clean out lumens, holes, and other hard to reach or challenging features. Actuate all movable features to fully irrigate all areas. 7. Visually inspect components for soil. Repeat the cleaning procedure until no visible soil remains on the components. 8. Perform a final rinse on the components using deionized water or purified water. 9. Dry the clean components using compressed air or a soft, lint free, clean cloth. Functional Checks should be performed where possible: 1. Mating devices should be checked for proper assembly. 2. Reusable devices with moving parts should be operated to check correct operation (medical grade lubricant suitable for steam sterilization can be applied as required). 3. Rotating instruments (e.g. drill bits, reamers) should be checked for straightness. This can be achieved by rolling the instrument on a flat surface. Note: The useful life of these devices is dependent on many factors including, but not limited to the method and duration of each use and the handling of the devices between uses. Routine and careful inspection and functional testing of the device is the best method of determining the serviceable life span for the medical device. Sterilization: The ALIGN Radial Head System implantable components have been sealed then sterilized by gamma radiation. The implants are provided in an undamaged package. If any of the implants or the package appears damaged, expiration date has been exceeded, or if sterility is questionable, the implant should not be used. DO NOT re-sterilize the implantable components. Trial components are available in the system to avoid opening the sterile package prior to prosthesis implantation. The implants should be removed from their sterile package only after the implant site has been prepared and properly sized. The Skeletal Dynamics ALIGN Radial Head System is provided non sterile. This system is intended for steam sterilization at the healthcare facility. 1. Place all components and accessories into the designated areas of the sterilization tray 2. Steam sterilization may be accomplished using one of the cycles shown below: Page 3 of 6 IFU RAJ Rev. March 2017
4 Cycle Type Temperature Duration Drying Time Pre-Vacuum Autoclave 270 F (132 C) 4 minutes (wrapped) 20 minutes Gravity Autoclave 270 F (132 C) 15 minutes (wrapped) 20 minutes Follow ANSI/AAMI ST79: Comprehensive guide to steam sterilization and sterility assurance in health care facilities. Flash sterilization is not recommended, but if used, should only be performed according to the requirements of ANSI/AAMI ST79: Comprehensive guide to steam sterilization and sterility assurance in health care facilities. Usage of an FDA cleared wrap is recommended to ensure the accessories and instruments are sterile prior to use. Subsequent instrument sterilization needs to be performed in the caddy provided. For reuse and sterilization, instruments should be arranged within the caddy in the manner supplied by the company. Calibration: The Torque Handle included in the system requires calibration. DO NOT use if calibration is overdue. Contact the Skeletal Dynamics Customer Care Department to arrange for the Torque Handle to be recalibrated. Handling and Storage: When not in use, store the clean and disinfected ALIGN Radial Head System within the Sterilization Tray. Prior to use, inspect the instrumentation for serviceability and the sterile packaged implants for signs of tampering or water contamination. Disclaimer of Warranty and Limited Remedies: Skeletal Dynamics, LLC makes no express or implied warranty, including any implied warranty of merchantability or fitness for a particular purpose, on the product(s) described in this publication. Skeletal Dynamics, LLC shall not be liable under any circumstances for any direct, incidental or consequential damages other than as expressly provided by specific law. No person has authority to bind Skeletal Dynamics, LLC to any representation or warranty except as specifically set forth in this publication. Descriptions or specifications provided by Skeletal Dynamics, LLC in any publication are only included to generally describe the product when manufactured and do not constitute any express warranties. ALIGN Radial Head System Ordering Information: ALN-RHS-SYS Catalog # Nomenclature Implants ALN-RHI-180 ALN-RHI-200 ALN-RHI-220 ALN-RHI-240 ALN-RHI-260 ALN-RST-0700 ALN-RST-0702 ALN-RST-0704 ALN-RST-0706 ALN-RST-0708 ALN-RST-0800 ALN-RST-0802 ALN-RST-0804 ALN-RST-0806 ALN-RST-0808 ALN-RST-0900 ALN-RST-0902 ALIGN Radial Head Implant & Lockscrew, 18mm, CoCr ALIGN Radial Head Implant & Lockscrew, 20mm, CoCr ALIGN Radial Head Implant & Lockscrew, 22mm, CoCr ALIGN Radial Head Implant & Lockscrew, 24mm, CoCr ALIGN Radial Head Implant & Lockscrew, 26mm, CoCr ALIGN Radial Stem Implant, 7mm x 0mm, Ti ALIGN Radial Stem Implant, 7mm x 2mm, Ti ALIGN Radial Stem Implant, 7mm x 4mm, Ti ALIGN Radial Stem Implant, 7mm x 6mm, Ti ALIGN Radial Stem Implant, 7mm x 8mm, Ti ALIGN Radial Stem Implant, 8mm x 0mm, Ti ALIGN Radial Stem Implant, 8mm x 2mm, Ti ALIGN Radial Stem Implant, 8mm x 4mm, Ti ALIGN Radial Stem Implant, 8mm x 6mm, Ti ALIGN Radial Stem Implant, 8mm x 8mm, Ti ALIGN Radial Stem Implant, 9mm x 0mm, Ti ALIGN Radial Stem Implant, 9mm x 2mm, Ti Page 4 of 6 IFU RAJ Rev. March 2017
5 ALN-RST-0904 ALIGN Radial Stem Implant, 9mm x 4mm, Ti ALN-RST-0906 ALIGN Radial Stem Implant, 9mm x 6mm, Ti ALN-RST-0908 ALIGN Radial Stem Implant, 9mm x 8mm, Ti ALN-RST-1000 ALIGN Radial Stem Implant, 10mm x 0mm, Ti ALN-RST-1002 ALIGN Radial Stem Implant, 10mm x 2mm, Ti ALN-RST-1004 ALIGN Radial Stem Implant, 10mm x 4mm, Ti ALN-RST-1006 ALIGN Radial Stem Implant, 10mm x 6mm, Ti ALN-RST-1008 ALIGN Radial Stem Implant, 10mm x 8mm, Ti ALN-RST-1100 ALIGN Radial Stem Implant, 11mm x 0mm, Ti ALN-RST-1102 ALIGN Radial Stem Implant, 11mm x 2mm, Ti ALN-RST-1104 ALIGN Radial Stem Implant, 11mm x 4mm, Ti ALN-RST-1106 ALIGN Radial Stem Implant, 11mm x 6mm, Ti ALN-RST-1108 ALIGN Radial Stem Implant, 11mm x 8mm, Ti Trial Heads ALN-RHT-180 ALIGN Radial Head Trial, 18mm ALN-RHT-200 ALIGN Radial Head Trial, 20mm ALN-RHT-220 ALIGN Radial Head Trial, 22mm ALN-RHT-240 ALIGN Radial Head Trial, 24mm ALN-RHT-260 ALIGN Radial Head Trial, 26mm Trial Necks ALN-RNT-000 ALIGN Radial Neck Trial, 0.0mm ALN-RNT-020 ALIGN Radial Neck Trial, 2.0mm ALN-RNT-040 ALIGN Radial Neck Trial, 4.0mm ALN-RNT-060 ALIGN Radial Neck Trial, 6.0mm ALN-RNT-080 ALIGN Radial Neck Trial, 8.0mm Trial Stems ALN-STT-070 ALIGN Radial Stem Trial, 7.0mm ALN-STT-080 ALIGN Radial Stem Trial, 8.0mm ALN-STT-090 ALIGN Radial Stem Trial, 9.0mm ALN-STT-100 ALIGN Radial Stem Trial, 10.0mm ALN-STT-110 ALIGN Radial Stem Trial, 11.0mm System Instrumentation ALN-RHG-PFA ALIGN Percutaneous Forearm Axis Jig, Radial Head Guide ALN-RHG-CRL ALIGN Captive Rail, Radial Head Guide ALN-RHG-BHF ALIGN Bone Holding Forceps, Radial Head Guide ALN-RHG-HAT ALIGN Head Alignment Tool, Radial Head Guide ALN-RRA-070 ALIGN Radial Rasp, 7.0mm ALN-RRA-080 ALIGN Radial Rasp, 8.0mm ALN-RRA-090 ALIGN Radial Rasp, 9.0mm ALN-RRA-100 ALIGN Radial Rasp, 10.0mm ALN-RRA-110 ALIGN Radial Rasp, 11.0mm ALN-RPL-070 ALIGN Radial Planer, 7.0mm ALN-RPL-080 ALIGN Radial Planer, 8.0mm ALN-RPL-090 ALIGN Radial Planer, 9.0mm Page 5 of 6 IFU RAJ Rev. March 2017
6 ALN-RPL-100 ALN-RPL-110 ALN-RHS-SZR ALN-NGI-000 ALN-NGI-020 ALN-NGI-040 ALN-NGI-060 ALN-NGI-080 ALN-RST-IMP ALN-RHA-TQH General Instrumentation ALIGN Radial Planer, 10.0mm ALIGN Radial Planer, 11.0mm ALIGN Sizer, Radial Head Implant ALIGN Neck Gauge & Head Inserter, 0mm ALIGN Neck Gauge & Head Inserter, 2mm ALIGN Neck Gauge & Head Inserter, 4mm ALIGN Neck Gauge & Head Inserter, 6mm ALIGN Neck Gauge & Head Inserter, 8mm ALIGN Impactor, Radial Stem ALIGN Torque Handle, Radial Head DRVR-UQC-T20 Driver, Universal QC, T-20 HNDL-UQC-FXD Handle, Universal Quick Connect, Fixed HNDL-UQC-DXT Driver Extraction Tool Sterilization Trays ALN-RHA-TRAY ALIGN Sterilization Tray System Customer Care Center: Skeletal Dynamics, LLC / 8905 SW 87 th Ave. / Suite 201 / Miami, FL / Emergo Europe, Molenstraat 15, 2513 BH The Hague, The Netherlands Page 6 of 6 IFU RAJ Rev. March 2017
7 Quantity Radial Head, 18mm ALN-RHI-180 (01) Radial Head, 20mm ALN-RHI-200 (01) Radial Head, 22mm ALN-RHI-220 (01) ALIGN Radial Head System Inventory Control Sheet Radial Heads Radial Head, 24mm ALN-RHI-240 (01) Radial Head, 26mm ALN-RHI-260 (01) Stem, 7mm x 0mm ALN-RST-0700 (01) Stem, 7mm x 2mm ALN-RST-0702 (01) Stem, 7mm x 4mm ALN-RST-0704 (01) Stem, 7mm x 6mm ALN-RST-0706 (01) Stem, 7mm x 8mm ALN-RST-0708 (01) Stem, 8mm x 0mm ALN-RST-0800 (01) Stem, 8mm x 2mm ALN-RST-0802 (01) Stem, 8mm x 4mm ALN-RST-0804 (01) Stem, 8mm x 6mm ALN-RST-0806 (01) Stem, 8mm x 8mm ALN-RST-0808 (01) Stem, 9mm x 0mm ALN-RST-0900 (01) Stem, 9mm x 2mm ALN-RST-0902 (01) Stem, 9mm x 4mm ALN-RST-0904 (01) Driver, Universal QC, T-20 DRVR-UQC-T20 (01) Radial Stems Instruments Stem, 9mm x 6mm ALN-RST-0906 (01) Stem, 9mm x 8mm ALN-RST-0908 (01) Stem, 10mm x 0mm ALN-RST-1000 (01) Stem, 10mm x 2mm ALN-RST-1002 (01) Stem, 10mm x 4mm ALN-RST-1004 (01) Stem, 10mm x 6mm ALN-RST-1006 (01) Stem, 10mm x 8mm ALN-RST-1008 (01) Stem, 11mm x 0mm ALN-RST-1100 (01) Stem, 11mm x 2mm ALN-RST Stem, 11mm x 4mm ALN-RST-1104 (01) Stem, 11mm x 6mm ALN-RST-1106 (01) Stem, 11mm x 8mm ALN-RST-1108 (01)
8 Handle, Universal Handle QC, Fixed HNDL-UQC-FXD (01) Torque Handle, Radial Head ALN-RHA-TQH (01) Stem Impactor ALN-RST-IMP (01) Radial Head Sizing Tray ALN-RHS-SZR (01) Neck Gauge, 0mm Offset ALN-NGI-000 (01) Neck Gauge, 2mm Offset ALN-NGI-020 (01) Neck Gauge, 4mm Offset ALN-NGI-040 (01) Neck Gauge, 6mm Offset ALN-NGI-060 (01) Neck Gauge, 8mm Offset ALN-NGI-080 (01) Planer Tool Assembly, 7mm ALN-RPL-070 (01) Planer Tool Assembly, 8mm ALN-RPL-080 (01) ALIGN, Trial, Radial Head, 18mm ALN-RHT-180 (01) ALIGN, Trial, Radial Head, 20mm ALN-RHT-200 (01) ALIGN, Trial, Radial Head, 22mm ALN-RHT-220 (01) ALIGN, Trial, Radial Head, 24mm ALN-RHT-240 (01) Reusable Instruments Planer Tool Assembly, 9mm ALN-RPL-090 (01) Planer Tool Assembly, 10mm ALN-RPL-100 (01) Planer Tool Assembly, 11mm ALN-RPL-110 (01) Rasp, 7mm ALN-RRA-070 (01) Rasp, 8mm ALN-RRA-080 (01) Rasp, 9mm ALN-RRA-090 (01) Rasp, 10mm ALN-RRA-100 (01) Rasp, 11mm ALN-RRA-110 (01) Head Alignment Tool Assembly ALN-RHG-HAT (01) Bone Holding Forceps ALN-RHG-BHF (01) Captive Rail & Axis Jig ALN-RHG-CRL (01) Trial Heads and Necks ALIGN, Trial, Radial Head, 26mm ALN-RHT-260 (01) ALIGN, Trial, Radial Neck, 0.0mm ALN-RNT-000 (01) ALIGN, Trial, Radial Neck, 2.0mm ALN-RNT-020 (01) ALIGN, Trial, Radial Neck, 4.0mm ALN-RNT-040 (01)
SURGICAL TECHNIQUE GUIDE
 SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand Institute Miami, Florida. System Overview The ALIGN Radial Head System is designed to restore the kinematics of the native radial
SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand Institute Miami, Florida. System Overview The ALIGN Radial Head System is designed to restore the kinematics of the native radial
Failure to follow instructions may lead to patient injury.
 STABLYX CMC Arthroplasty System INSTRUCTIONS FOR USE R x : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions
STABLYX CMC Arthroplasty System INSTRUCTIONS FOR USE R x : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions
REDUCT Headless Compression Screw System
 REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE
 English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
SURGICAL TECHNIQUE GUIDE
 SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida. 1 ELBOW LANDMARKS With the elbow flexed 90 0, palpate and mark the lateral epicondyle.
SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida. 1 ELBOW LANDMARKS With the elbow flexed 90 0, palpate and mark the lateral epicondyle.
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
SURGICAL TECHNIQUE GUIDE
 SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida. 1 EXPOSURE Make an 8cm to 10cm longitudinal incision centered over Lister s tubercle
SURGICAL TECHNIQUE GUIDE As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida. 1 EXPOSURE Make an 8cm to 10cm longitudinal incision centered over Lister s tubercle
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
WristMotion Wrist Hemiarthroplasty System Instructions for Use
 WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
WristMotion Wrist Hemiarthroplasty System Instructions for Use Description The Arthrosurface WristMotion Wrist Hemiarthroplasty System consists of a contoured capitate articular implant designed to articulate
Customized Cranial and Craniofacial Implants. Instructions For Use. 7000revD_Instructions_For_Use. Page 1 of 8
 Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON
 ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Duraloc CONSTRAINED LINER
 SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
Velocity Orthopedics Instrument Cleaning and Sterilization
 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
SURGICAL TECHNIQUE GUIDE PROTEAN. radial head plate. As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida
 SURGICAL TECHNIQUE GUIDE PROTEAN radial head plate As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida PROTEAN radial head plate Indications for Use The PROTEAN
SURGICAL TECHNIQUE GUIDE PROTEAN radial head plate As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida PROTEAN radial head plate Indications for Use The PROTEAN
OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
SURGICAL TECHNIQUE GUIDE PROTEAN. radial head plate. As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida
 SURGICAL TECHNIQUE GUIDE PROTEAN radial head plate As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida PROTEAN radial head plate Indications for Use The PROTEAN
SURGICAL TECHNIQUE GUIDE PROTEAN radial head plate As described by: Jorge L. Orbay, M.D. Miami Hand & Upper Extremity Institute Miami, Florida PROTEAN radial head plate Indications for Use The PROTEAN
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide Wrist Hemiarthroplasty System
 Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
Technique Guide Wrist Hemiarthroplasty System The WristMotion TM Wrist Hemiarthroplasty System restores mobility and maintains native biomechanics using a dual curvature HemiCAPITATE implant that locks
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Technique Guide Lapidus Arthrodesis System
 Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Positioning Sleeve Surgical Technique
 Positioning Sleeve Surgical Technique Surgical Technique The Comprehensive Fracture System offers a unique tool to evaluate and re-establish humeral height, which can be critical to restoring proper biomechanics
Positioning Sleeve Surgical Technique Surgical Technique The Comprehensive Fracture System offers a unique tool to evaluate and re-establish humeral height, which can be critical to restoring proper biomechanics
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
Preparation Instructions for the CAMLOG /CONELOG Implant System
 J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
J8000.0032 Rev.6 EN 06/2015 Preparation Instructions for the CAMLOG /CONELOG Implant System ENGLISH The following descriptions contain detailed instructions on cleaning, disinfection and sterilization
Hip Resurfacing System
 Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM
 METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
Technique Guide Hip Resurfacing System
 Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
Technique Guide Hip Resurfacing System Approximately 5%-18% of all hip arthroplasties are completed on patients with a primary diagnosis of osteonecrosis. Patients are generally younger adults age 35 years
Document No Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE
 Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Document No. 0400-0205 Rev. B Pg. 1 of 5 Approvals / Date Title: INSTRUCTIONS FOR USE DJO SURGICAL MODULAR REVISION HIP SYSTEM AND ACETABULAR CAGE Revision ECO Date ECO Summary of Changes Deann Rector
Salto Talaris Total Ankle Prosthesis
 Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
SI-BONE ifuse Implant System Instruments Hospital Cleaning and Sterilization Instructions 0344
 HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Description Materials Indications Patient selection factors to be considered include: Contraindications Absolute contraindications include:
 Description The Patello-Femoral Wave Arthroplasty Systems incorporate a distal femoral trochlear surface articular component that mates to a taper post via a taper interlock, and an all-polyethylene patella
Description The Patello-Femoral Wave Arthroplasty Systems incorporate a distal femoral trochlear surface articular component that mates to a taper post via a taper interlock, and an all-polyethylene patella
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Headless Compession Screw 2.5 / 3.0
 SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw 4.5 / 6.5 SURGICAL TECHNIQUE. Titanium or Stainless Steel. Cannulated Headless Design. Multiple Thread Options.
 Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
ATEC Porous Ti System
 ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
Endorectal Balloon (ERB) Endorectal Balloon (ERB) Instructions for Use
 Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
Endorectal Balloon (ERB) Instructions for Use GRF-0004-00 Rev. 08 Page 1 of 9 Contents Instructions for Use... 3 Indications for Use... 3 Contraindications... 3 Storage... 4 Warnings... 4 Accessories Provided...
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Integra Total Foot System 2
 Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
JRI Thompson Hemiarthroplasty
 JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
JRI ORTHOPAEDICS LTD 18 Churchill Way, 35A Business Park, Chapeltown, Sheffield, S35 2PY, UK Instructions for Use JRI Thompson Hemiarthroplasty Page 1 of 6 English 3 Page 2 of 6 Important Information Please
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
Flexible Fragment Fixation. Surgical Technique
 Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP
 RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
RAPIDSORB RAPID ReSORBABLe CRANIAL CLAMP Resorbable fixation of cranial bone flaps SURGICAL TeCHNIQUe RAPIDSORB Rapid Resorbable CRANIAL CLAMP Indication DePuy Synthes Companies of Johnson & Johnson RAPIDSORB
Battalion TM Universal Spacer System
 Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
Battalion TM Universal Spacer System GENERAL INFORMATION: The Battalion Universal Spacer System (Battalion System) is an intervertebral body fusion device with implants of various lengths, widths, heights,
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
Peanut Growth Control Plating System
 Surgical Technique Peanut Growth Control Plating System An Innovative Approach To Hemi-Epiphysiodesis Provides controlled growth of healthy epiphysis, restoring proper anatomic alignment Advanced instrumentation
Surgical Technique Peanut Growth Control Plating System An Innovative Approach To Hemi-Epiphysiodesis Provides controlled growth of healthy epiphysis, restoring proper anatomic alignment Advanced instrumentation
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
Surgical Technique. Hip System
 Surgical Technique Hip System INDICATIONS FOR USE The TaperSet Hip System is designed for total or partial hip arthroplasty and is intended to be used with compatible components of the Consensus Hip System.
Surgical Technique Hip System INDICATIONS FOR USE The TaperSet Hip System is designed for total or partial hip arthroplasty and is intended to be used with compatible components of the Consensus Hip System.
ESC. Enhanced Stability Liners. Design Rationale & Surgical Technique
 ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
Progeny Hip Stem. Surgical Protocol and Product Specifications
 Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
SYMBOLOGY. = Manufacturer. = Manufacturing Serial Number. = Manufacturing Lot Number = Catalog Number LOT REF. = Do not resterilize Single Use Only
 damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
damage or expenses directly or indirectly arising from use of this product. This warranty is in lieu of and excludes all other warranties not expressly set forth herein, whether express or implied by operation
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
Gallery Laminoplasty Spine System
 Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
3.5 mm LCP Extra-articular Distal Humerus Plate
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Extra-articular Distal Humerus Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Extra-articular Distal Humerus
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Extra-articular Distal Humerus Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Extra-articular Distal Humerus
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
METAL HEMI SYSTEM
 METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
3.5 mm LCP Olecranon Plates
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
Distal Radius Plate Instrument and Implant Set. Discontinued December 2017 DSUS/TRM/0916/1063(1)
 Distal Radius Plate Instrument and Implant Set Surgical Technique Discontinued December 2017 DSUS/TRM/0916/1063(1) The Distal Radius Plates Indications For fixation of fractures and osteotomies, including
Distal Radius Plate Instrument and Implant Set Surgical Technique Discontinued December 2017 DSUS/TRM/0916/1063(1) The Distal Radius Plates Indications For fixation of fractures and osteotomies, including
Olecranon Locking Plate II
 INDEX Indications Patient Position Fracture Reduction and Fixation Surgical Technique Step 1 Surgical Approach Step 2 Implantation Step 3 Proximal Locking Screw Insertion Step 4 Distal Screw Insertion
INDEX Indications Patient Position Fracture Reduction and Fixation Surgical Technique Step 1 Surgical Approach Step 2 Implantation Step 3 Proximal Locking Screw Insertion Step 4 Distal Screw Insertion
Integra. Modular Radial Head System SURGICAL TECHNIQUE
 Integra Modular Radial Head System SURGICAL TECHNIQUE Table of Contents System Overview...2 Indications and Contraindications... 3 Modular Radial Head Implant Technique...4 Component Dimensions...8 Implant
Integra Modular Radial Head System SURGICAL TECHNIQUE Table of Contents System Overview...2 Indications and Contraindications... 3 Modular Radial Head Implant Technique...4 Component Dimensions...8 Implant
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
A U X I L I A R Y C O N N E C T O R S Surgical Technique
 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
Zavation Z-Link Cervical
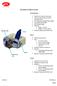 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Doc. No.: IU/8 for. Rev. No.: 07 ResTOR Prosthesis
 0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
0434 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION PURPOSE: The ResTOR system is designed to restore structural skeletal stability and enable functional joint
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
ACETABULAR CUP SURGICAL TECHNIQUE
 ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
U2 PSA. Revision Knee. Surgical Protocol
 U2 PSA TM Revision Knee Surgical Protocol Table of Contents 1 Component Removal... 1 2 Tibial Preparation... 1 2.1 Tibial Canal Preparation... 1 2.2 Proximal Tibial Resection... 2 2.3 Non Offset Tibial
U2 PSA TM Revision Knee Surgical Protocol Table of Contents 1 Component Removal... 1 2 Tibial Preparation... 1 2.1 Tibial Canal Preparation... 1 2.2 Proximal Tibial Resection... 2 2.3 Non Offset Tibial
TMJ Fossa-Eminence Prosthesis System. Instructions for Use. For partial reconstruction (hemiarthroplasty) of the temporomandibular joint
 TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
SMV Scientific Bone Plate and Screw System Surgical Technique
 SMV Scientific Bone Plate and Screw System Surgical Technique Description: The SMV Scientific Bone Plate and Screw System consists of non-locking plates and bone screw fasteners in a variety of lengths,
SMV Scientific Bone Plate and Screw System Surgical Technique Description: The SMV Scientific Bone Plate and Screw System consists of non-locking plates and bone screw fasteners in a variety of lengths,
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
Encina Taper Stem. Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA
 Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA 94065 info@stinsonortho.com www.stinsonortho.com Table of Contents Introduction 3 Features 4 Surgical Technique 5 Preoperative
Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA 94065 info@stinsonortho.com www.stinsonortho.com Table of Contents Introduction 3 Features 4 Surgical Technique 5 Preoperative
CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM
 CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
CONSENSUS ORTHOPEDICS INC. UNISYN HIP SYSTEM Instructions for Use (IFU) IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR
Distal Ulnar Locking Plate
 INDEX Indications Patient Position Surgical Technique - Step 1 Approach - Step 2 Plate Contouring - Step 3 Fracture Reduction - Step 4 Distal Plate Fixation - Step 5 Confirm Proper Reconstruction - Step
INDEX Indications Patient Position Surgical Technique - Step 1 Approach - Step 2 Plate Contouring - Step 3 Fracture Reduction - Step 4 Distal Plate Fixation - Step 5 Confirm Proper Reconstruction - Step
