REGENERATIVE MEDICINE
|
|
|
- Violet Hubbard
- 5 years ago
- Views:
Transcription
1 REGENERATIVE MEDICINE Enrichment of Human Embryonic Stem Cell-Derived NKX6.1- Expressing Pancreatic Progenitor Cells Accelerates the Maturation of Insulin-Secreting Cells In Vivo ALIREZA REZANIA, a JENNIFER E. BRUIN, b JEAN XU, a KAVITHA NARAYAN, a JESSICA K. FOX, b JOHN J. O NEIL, a TIMOTHY J. KIEFFER b,c a BetaLogics Venture, Janssen R & D LLC, Raritan, New Jersey, USA; b Laboratory of Molecular and Cellular Medicine, Department of Cellular and Physiological Sciences, Life Sciences Institute; c Department of Surgery, University of British Columbia, Vancouver, British Columbia, Canada Key words: Diabetes Beta cells Human embryonic stem cells Cell therapy Transplantation ABSTRACT Human embryonic stem cells (hescs) are considered a potential alternative to cadaveric islets as a source of transplantable cells for treating patients with diabetes. We previously described a differentiation protocol to generatepancreaticprogenitorcellsfromhescs,composed of mainly pancreatic endoderm (PDX1/NKX6.1-positive), endocrine precursors (NKX2.2/synaptophysin-positive, hormone/nkx6.1-negative), and polyhormonal cells (insulin/glucagon-positive, NKX6.1-negative). However, the relative contributions of NKX6.1-negative versus NKX6.1-positive cell fractions to the maturation of functional b-cells remained unclear. To address this question, we generated two distinct pancreatic progenitor cell populations using modified differentiation protocols. Prior to transplant, both populations contained a high proportion of PDX1-expressing cells (85% 90%) but were distinguished by their relatively high (80%) or low (25%) expression of NKX6.1. NKX6.1-high and NKX6.1-low progenitor populations were transplanted subcutaneously within macroencapsulation devices into diabetic mice. Disclosure of potential conflicts of interest is found at the end of this article. Mice transplanted with NKX6.1-low cells remained hyperglycemic throughout the 5-month post-transplant period whereas diabetes was reversed in NKX6.1-high recipients within 3 months. Fasting human C-peptide levels were similar between groups throughout the study, but only NKX6.1-high grafts displayed robust meal-, glucose- and arginine-responsive insulin secretion as early as 3 months post-transplant. NKX6.1-low recipients displayed elevated fasting glucagon levels. Theracyte devices from both groups contained almost exclusively pancreatic endocrine tissue, but NKX6.1-high grafts contained a greater proportion of insulin-positive and somatostatin-positive cells, whereas NKX6.1-low grafts contained mainly glucagon-expressing cells. Insulinpositive cells in NKX6.1-high, but not NKX6.1-low grafts expressed nuclear MAFA. Collectively, this study demonstrates that a pancreatic endoderm-enriched population can mature into highly functional b-cells with only a minor contribution from the endocrine subpopulation. STEM CELLS 2013;31: INTRODUCTION Diabetes is characterized by chronic high blood glucose levels resulting from deficient insulin-producing pancreatic b-cells. There are numerous experimental strategies aimed to restore endogenous regulated insulin production in patients with type 1 diabetes [1]. To date, islet cell transplantation is the most effective clinical therapy [2,3], but widespread application is severely limited by the shortage of cadaveric donor islets [4]. Human embryonic stem cells (hescs) may be a potential renewable source of insulin-producing cells to bridge the gap between cell supply and clinical demand [5]. Step-wise differentiation protocols designed to mimic pancreatic development have successfully generated insulin-producing cells from hescs in vitro, but these cells resemble fetal endocrine cells rather than adult pancreatic b-cells [6 17]. Mature, glucoseresponsive insulin-secreting cells have only been produced from hescs following transplantation of immature pancreatic progenitor cells; this was first demonstrated in healthy mice [12] and subsequently by our group in mice with diabetes [17,18]. We demonstrated that graft-derived human insulin levels gradually increased over time and after a 3-month maturation period, previously insulin-dependent diabetic mice achieved normal fasting blood glucose levels without exogenous insulin treatment [17]. After a lengthy maturation period Author contributions: A.R.: conception and design, collection and/or assembly of data, data analysis and interpretation; final approval of manuscript; J.E.B.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript; J.X.: conception and design, collection and/or assembly of data, and data analysis and interpretation; K.N., J.F., and J.J.O.: collection and/or assembly of data; T.J.K.: conception and design, data analysis and interpretation, and final approval of manuscript. A.R., J.E.B., and J.X. contributed equally to this article. Correspondence: Timothy Kieffer, Ph.D., Room Health Sciences Mall, University of British Columbia, Vancouver, British Columbia V6T 1Z3, Canada. Telephone: ; Fax: ; tim.kieffer@ubc.ca Received December 19, 2012; Revised June 9, 2013; accepted for publication July 1, 2013; first published online in STEM CELLS EXPRESS July 29, VC AlphaMed Press /2013/$30.00/0 doi: /stem.1489 STEM CELLS 2013;31:
2 Rezania, Bruin, Xu et al of approximately 30 weeks in vivo, human insulin secretion was regulated by various secretagogues, including meal, glucose, and arginine challenges, and a corresponding improvement in glucose tolerance was observed along with the appearance of insulin/v-maf musculoaponeurotic fibrosarcoma oncogene homolog A (MAFA) co-positive b-cells within kidney capsule grafts [17]. The pancreatic progenitor cells generated with our previous differentiation protocol were composed of mainly immature polyhormonal cells (insulin/glucagon-positive, NKX6.1-negative), endocrine precursor cells (NKX2.2/synaptophysin-positive, hormone/nkx6.1-negative), and pancreatic endoderm-like cells (NKX6.1/PDX1-positive, insulin-negative) [17]. The expression of NKX6.1 distinguishes these populations and is thought to mark the progenitor cells that arise during the second transition of pancreas development, as opposed to the polyhormonal cells that arise during the first transition [19]. Results from several recent studies suggest that polyhormonal cells may develop into pancreatic a-cells following transplantation [6,16,20]. In contrast, when hesc-derived pancreatic endoderm cells were purified by fluorescence-activated cell sorting (FACS) and transplanted into mice, functional islet-like clusters developed in vivo, although less efficiently than an unsorted progenitor cell population [6]. Thus, it remains unclear whether the NKX6.1-negative fraction contributes to maturation of the NKX6.1-positive cell fraction. Here, we examined the relative contributions of each pancreatic progenitor subpopulation to the development of mature insulinsecreting cells in vivo by transplanting cell populations that were enriched, but not FACS-purified, for either pancreatic endoderm or pan-endocrine cells. MATERIALS AND METHODS In Vitro Differentiation of hescs The H1 hesc line was obtained from WiCell Research Institute, Inc. (Madison, WI, All experiments at the University of British Columbia (UBC) with H1 cells were approved by the Canadian Stem Cell Oversight Committee and UBC Clinical Research Ethics Board. H1 cells were cultured on 1:30 diluted Matrigel (BD BioSciences, San Diego, com; Cat#356231) in mtesr-1 (Stem Cell Technologies, Vancouver, BC, Canada, Cat#05850). At approximately 70% 80% confluence, cultures were rinsed with 13 Dulbecco s phosphate-buffered saline (DPBS) without Mg 21 and Ca 21 (Invitrogen, Carlsbad, CA, Cat#14190) followed by incubation with Versene (EDTA), 0.02% (LONZA, Cat#17 711E) for 12 minutes at room temperature. Released single cells were rinsed with mtesr-1 and spun at 1,000 rpm for 5 minutes. The resulting cell pellet was resuspended in mtesr-1 medium supplemented with Y (10 mm; Sigma-Aldrich; St. Louis, MO, Cat#Y0503) and the single cell suspension was seeded at approximately cells per centimeter square. Cultures were fed every day and differentiation was initiated 48 hours following seeding, resulting in approximately 90% starting confluence. The following differentiation protocol variations were used to generate either NKX6.1-high or NKX6.1-low cell populations (summarized in Fig. 1A): Stage 1: Definitive Endoderm (3 Days). Undifferentiated H1 cells plated on 1:30 Matrigel-coated surfaces were exposed to RPMI 1640 medium (Invitrogen; Cat#22400) supplemented with 1.2 g/l sodium bicarbonate (Sigma, MO; Cat# S6297), 0.2% fetal bovine serum (FBS; Hyclone, Logan, UT, com; Cat# SH ), 100 ng/ml activin-a (AA; Pepro-tech, Rocky Hill, NJ, and 20 ng/ml of Wnt3A (R&D Systems, Minneapolis, for day 1 only. For the next 2 days, cells were cultured in RPMI with 0.5% FBS, 1.2 g/l sodium bicarbonate, and 100 ng/ml AA. Stage 2: Primitive Gut Tube (3 Days). Stage 1 cells were exposed to Dulbecco s modified Eagle s medium (DMEM)-F12 medium (Invitrogen) supplemented with 2 g/l sodium bicarbonate, 2% FBS, and 50 ng/ml of fibroblast growth factor 7 (Peprotech) for 3 days. Stage 3: Posterior Foregut (4 Days). Cultures were continued for 4 days in DMEM-HG (high-glucose) medium (Invitrogen) supplemented with 0.25 mm SANT-1 (Sigma-Aldrich), 2 mm retinoic acid (RA; Sigma-Aldrich), 100 ng/ml of Noggin (R&D Systems), and 1% (v/v) B27 (Invitrogen). At this stage, NKX6.1-low cells received 1 mm ALK5 inhibitor II (ALK5i; Axxora, San Diego, CA, ALK5i was not added to the NKX6.1-high cultures during stage 3. Stage 4: Pancreatic Endoderm/Endocrine Precursors (5 Days). Stage 3 cells were then cultured for 4 days in DMEM- HG medium supplemented with 100 ng/ml Noggin and 1% B27. For NKX6.1-high cells, 500 nm TPB (PKC activator; (2S,5S)- (E,E)-8-(5-(4-(trifluoromethyl)phenyl)-2,4-pentadienoylamino)benzolactam, EMD, Chemicals Inc, Gibbstown, NJ) was added to the stage 4 media. For NKX6.1- low cells, 1 mm ALK5i was added. For the last day of culture, all cells were treated with 5 mg/ml dispase for 5 minutes at 37 C, pipetted gently to break into cell clumps (<100 mm) and transferred into Spinner Flasks (Corning, Acton, MA, Cell clusters were spun at rpm overnight in suspension with DMEM-HG supplemented with 0.2 mm ALK5i, 100 nm LDN (LDN; bone morphogenetic protein (BMP) receptor inhibitor, Stemgent, CA, Cat# ) and 1% B27. Flow Cytometry Stage 4 hesc-derived cells were assessed before transplant to ensure that the starting population met the appropriate quality control standards. Cell clusters were released into a single-cell suspension and either stained directly for surface markers or fixed and stained for various intracellular markers, as described previously [17]. Refer to Supporting Information Table 1 for antibody details. Quantitative Reverse Transcriptase Polymerase Chain Reaction Gene expression was assessed in NKX6.1-high and NKX6.1-low progenitor cells using custom Taqman Arrays (Applied Biosystems, Foster City, CA, as previously described [17]. Data were analyzed using Sequence Detection Software (Applied Biosystems) and normalized to undifferentiated H1 cells using the DDCt method. Animal Studies All experiments were approved by the UBC Animal Care Committee. Male 8 10-week-old SCID-beige mice (C.B-Igh21b/GbmsTac- Prkdc scid -Lyst bg N7) were obtained from Taconic (Hudson, NY) and maintained on a 12-hour light/dark cycle with ad libitum access to a standard irradiated diet (Harlan Laboratories, Teklad Diet #2918; Madison, WI). Mice were rendered diabetic by a multiple low-dose streptozotocin (STZ) regimen of daily intraperitoneal STZ injections for 5 consecutive days (50 mg/kg per day). Mice were anesthetized with inhalable isoflurane and received approximately 5 million hesc-derived stage 4 cells (NKX6.1- high or NKX6.1-low) transplanted subcutaneously on the right flank within a single 20 ml Theracyte planar macroencapsulation device (TheraCyte Inc., Laguna Hills, CA; n 5 9 mice
3 2434 Treatment of Diabetes with Human Stem Cells Figure 1. Characterization of NKX6.1-high and NKX6.1-low populations prior to transplantation in macroencapsulation devices. (A): Schematic summary of the 15-day in vitro differentiation protocols used to generate NKX6.1-low (green) and NKX6.1-high (brown) cells. (B): FACS quantification of differentiated hesc-derived populations at stage 4, day 4 prior to transplant. NKX6.1-low (n 5 2) and NKX6.1-high (n 5 3) populations were defined primarily based on their respective NKX6.1-expressing cell fractions. NKX6.1-low cells contained a relatively high proportion of endocrine cells (synaptophysin-positive and NKX2.2-positive) compared to NKX6.1-high cells. Representative FACS plots from both groups are provided in panel (C). (D): qpcr for expression levels of various pancreatic endocrine genes (hormones and transcription factors) in NKX6.1-high cells (n 5 6) relative to NKX6.1-low cells (n 5 4). Dashed red line indicates equal expression in both groups. (E G): NKX6.1-low and NKX6.1-high cells were loaded into Theracyte devices pre-transplant and immunostained for insulin, glucagon and NKX6.1 (panel (E); scale bars mm), NKX2.2 (panel (F); scale bars 5 50 mm) and DAPI (panel (G); scale bars 5 50 mm). Abbreviations: DMEM-HG, Dulbecco s modified Eagle s medium-high glucose; DAPI, 4 0,6-diamidino-2-phenylindole; FACS, fluorescence activated cell sorting; FBS, fetal bovine serum; FGF, fibroblast growth factor; hesc, human embryonic stem cells; LDN, LDN ; qpcr, quantitative polymerase chain reaction; TPB, (2S,5S)-(E,E)-8-(5-(4-(trifluoromethyl)phenyl)-2,4-pentadienoylamino)benzolactam.
4 Rezania, Bruin, Xu et al transplanted per group). These devices support neovascularization via woven outer membranes, while containing the engrafted cells within cell impermeable inner membranes [18,21]. Although in our previous studies progenitor cells were transplanted under the kidney capsule [16,17], we found that macroencapsulated progenitor cells develop into human C-peptide secreting cells with similar efficiency to cells implanted under the kidney capsule (Supporting Information Fig. 1; [18]). All mice received a single s.c. injection of enrofloxacin, Baytril, at the time of transplantation (10 mg/kg; Bayer Animal Health; Shawnee Mission, KS). All metabolic analyses were performed in conscious, restrained mice on the indicated days. Blood glucose and body weight were monitored one to two times weekly following a 4 hour morning fast. For monthly meal challenges, blood glucose was measured and blood was collected after an overnight fast (16 hours) and then again after a 45 minute feeding period with normal chow. For the glucose tolerance test (GTT), mice received an oral bolus of glucose (2 g/kg, 30% solution; Vetoquinol, Lavaltrie, QC) and the arginine tolerance test (ArgTT), mice received i.p. arginine (2 g/kg, 40% solution; Sigma-Aldrich) following a 4 hour morning fast. For all tests, blood glucose was tested via saphenous vein using a handheld glucometer (Lifescan, Burnaby, BC), and saphenous blood samples were collected at the indicated time points using heparinized microhematocrit tubes. Plasma was stored at 230 C and later assayed using a human C-Peptide ELISA (Alpco Diagnostics, Salem, NH) or MSD Multi-Spot Assay for human glucagon-like peptide-1 (GLP-1), insulin, and glucagon (K15160C-2; Meso Scale Discovery, Gaithersburg, MD), according to manufacturer s instructions. Immunohistochemistry hesc-derived cells (pre-transplant and post-transplant) within Theracyte devices were fixed overnight in 4% PFA and stored in 70% ethanol prior to paraffin-embedding and sectioning (5 mm thickness; Wax-it Histology Services, Vancouver, Canada). Hematoxylin and eosin (H&E) and Masson s Trichrome staining were performed using standard protocols (Wax-it Histology Services), and all grafts were examined by an independent pathologist. H&E/Trichrome slides were scanned using the ScanScope CS system (Aperio, Vista, CA). Immunofluorescent staining was performed as previously described [16]; primary antibodies are detailed in Supporting Information Table 2. Images were captured using the ImageXpressMICRO Imaging System and analyzed using MetaXpress Software (Molecular Devices Corporation, Sunnyvale, CA, Endocrine cells were quantified within Theracyte devices harvested at weeks post-transplant (n 5 3 per group). Engrafted cells were coimmunostained for insulin, glucagon, and somatostatin, and whole slide fluorescence scanning was performed as described above. Individual images were stitched together to recreate the full length of each Theracyte device and the entire region between the inner membranes was quantified using MetaXpress software. The endocrine fraction was determined as the total number of insulin-, glucagon-, and/or somatostatin-positive cells relative to the total number of 4 0,6-diamidino-2-phenylindole (DAPI)-positive cells within the inner membranes. The endocrine population was further subdivided into the number of insulin-positive, glucagon-positive, or somatostatin-positive cells relative to the total number of endocrine cells (insulin, glucagon, and/or somatostatin-positive), regardless of the number of hormones present per cell. Finally, the number of single hormonal (insulin-only, glucagon-only, or somatostatinonly) and polyhormonal (insulin/glucagon, insulin/somatostatin, glucagon/somatostatin, or insulin/glucagon/somatostatin) cells were quantified as a proportion of the total number of endocrine cells (i.e., insulin, glucagon, and/or somatostatin-positive). Statistical Analysis All statistics were performed using GraphPad Prism software (GraphPad Software Inc., LA Jolla, CA). Specific statistical tests for each experiment are described in the figure legends. Student- Newman-Keuls post hoc test was used for comparisons between groups with all one-way ANOVAs. For all analyses, p <.05 was considered statistically significant RESULTS Characterization of NKX6.1-High and NKX6.1-Low Cells Pre-transplant Our revised in vitro differentiation protocols were designed to generate two different mixed populations of pancreatic progenitor cells (see Materials and Methods section for details and Fig. 1A for summary schematic). These two cell populations were thoroughly characterized by FACS (Fig. 1B, 1C), quantitative polymerase chain reaction (Fig. 1D), and immunofluorescent staining (Fig. 1E 1G) prior to transplant. The pancreatic endoderm-enriched population was designated as NKX6.1-high and the endocrine-enriched population as NKX6.1-low, since they contained approximately 80% versus 25% NKX6.1-positive cells, respectively (Fig. 1B, 1C). NKX6.1 expression was inversely proportional to the endocrine compartment; NKX6.1-high (pancreatic endodermenriched) and NKX6.1-low (endocrine-enriched) populations contained approximately 11% and 60% synaptophysin-positive cells, respectively (Fig. 1B, 1C). With the exception of NKX6.1 and PDX1, pancreatic endocrine transcription factors were generally upregulated in the NKX6.1-low cell population compared to the NKX6.1-high population (Fig. 1B, 1D). Both populations contained approximately 90% PDX1-positive cells (Fig. 1B) and PDX1 gene expression was similar (Fig. 1D). Immunofluorescent staining confirmed the profound difference in NKX6.1 expression between the two progenitor populations (Fig. 1E) and the enrichment of polyhormonal endocrine cells in the NKX6.1-low population (Fig. 1E 1G). Insulin/glucagon copositive cells were NKX6.1-negative (Fig. 1E) and NKX2.2- positive (Fig. 1F) prior to transplant. Metabolic Profile of NKX6.1-High and NKX6.1-Low Cells Following Transplant Macroencapsulated NKX6.1-high and NKX6.1-low cells were transplanted into mice with STZ-induced diabetes (average fasting blood glucose of mm at the time of transplant). Mice that received NKX6.1-high cells exhibited significantly lower fasting blood glucose levels compared to mice that received NKX6.1-low cells throughout the study beginning at 3 weeks post-transplant (Fig. 2A) and also during ArgTT and GTT at 17 and 20 weeks, respectively (Figs. 2C, 2F). By approximately 90 days post-transplant, NKX6.1-high cells had completely reversed STZ-induced hyperglycemia, whereas mice with NKX6.1-low cells remained moderately hyperglycemic until the endpoint of the study at 135 days (Fig. 2B). Blood glucose tracking data for individual animals are provided in Supporting Information Figure 2A and individual fasting glucose values pre-transplant (day 2) and post-transplant (day 113) are provided in Supporting Information Table 3. Overnight fasted human C-peptide levels were similar between groups at all ages, whereas only recipients of NKX6.1-high cells displayed meal-stimulated human C-peptide secretion (1.7- and 2.4-fold increase after feeding at 3 and 4 months, respectively; Fig. 2B). Refer to Supporting Information Figure 2B and Supporting Information Table 3 for individual human C-peptide tracking data during monthly meal challenges. NKX6.1-high cells secreted significantly higher levels of human insulin/c-peptide at minutes
5 2436 Treatment of Diabetes with Human Stem Cells Figure 2. In vivo metabolic tracking of NKX6.1-low and NKX6.1-high cell function following transplantation in macroencapsulation devices. (A): Fasting blood glucose levels (mm) in diabetic mice (low-dose STZ model) transplanted with macroencapsulated NKX6.1-low or NKX6.1- high cells. Blood glucose levels were significantly lower in mice with NKX6.1-high cells compared to NKX6.1-low cells after 21 days posttransplant (*, p <.05, t test NKX6.1-high vs. NKX6.1-low). (B): NKX6.1-low and NKX6.1-high cells secreted similar levels of human C- peptide after an overnight fast at all ages, but at 3 and 4 months post-transplant, NKX6.1-high cells secreted significantly higher levels of human C-peptide following a 45-minute meal challenge (*, p <.05, paired t test, fast vs. fed). (C E): Response to an ipargtt (2 g/kg) at 17 weeks post-transplant. (C): Blood glucose (*, p <.05, t test; NKX6.1-high vs. NKX6.1-low), (D) human insulin secretion (*, p <.05, paired t test; 0 vs. 15 minutes), and (E) glucagon secretion (*, p <.05, one-way ANOVA; fasting glucagon NKX6.1-high vs. NKX6.1-low, sham). Sham mice were nontransplanted, nondiabetic SCID-beige controls. (F): Blood glucose levels and (G) human C-peptide secretion in response to an oral GTT (2 g/kg) at 20 weeks post-transplant. AUC values are shown to the right (*, p <.05, t test; NKX6.1-high vs. NKX6.1-low). Abbreviations: AUC, area under the curve; ArgTT, arginine tolerance test; GTT, glucose tolerance test; ipargtt, intraperitoneal arginine tolerance test; STZ, streptozotocin. following arginine and glucose challenges, unlike NKX6.1- low cells, which were not responsive to either secretagogue in vivo (Fig. 2D, 2G). Fasting glucagon levels were significantly higher in NKX6.1-low versus NKX6.1-high mice; there was no difference in arginine-stimulated glucagon secretion between groups (Fig. 2E). Characterization of Grafts Derived from NKX6.1- High and NKX6.1-Low Cells At 5 months post-transplant, there were no obvious morphological differences in graft histology between NKX6.1-high and NKX6.1-low cells by H&E or Trichrome staining (Fig. 3; n 5 3 per group). An independent pathology analysis of H&Estained Theracyte devices indicated that grafts from NKX6.1- low and NKX6.1-high mice were largely composed of pancreatic endocrine cells (confirmed by quantification of % insulin, glucagon, and/or somatostatin-positive cells in Fig. 4B; NKX6.1-low: 67.3% 6 1.3%, NKX6.1-high: 73.5% 6 2.3%), as well as some loose mesenchymal-like cells (Fig. 3A, 3B, 3E, 3F). Based on Masson s trichrome staining, these regions of loose meschenchymal tissue (generally located adjacent to the inner membranes) were identified as collagen fibers (Fig. 3C, 3D, 3G, 3H). Notably, none of the grafts analyzed in this study contained mature bone or cartilage tissue, unlike grafts generated with our previous differentiation protocol [17]. Immunofluorescent staining of macroencapsulated cells revealed dramatic differences in the pancreatic endocrine subpopulations between groups (Fig. 4). NKX6.1-low cells
6 Rezania, Bruin, Xu et al Figure 3. Morphology of engrafted NKX6.1-high and NKX6.1-low cells within Theracyte devices at 5 months post-transplant. H&E staining (panels (A), (B), (E), (F)) and Masson s Trichrome staining (panels (C), (D), (G), (H)) of harvested Theracyte devices at 5 months post-transplant derived from: (A D) NKX6.1-low cells (enriched for polyhormonal endocrine cells) or (E H) NKX6.1-high cells (enriched for pancreatic endoderm cells). For H&E panels, low magnification images through the full length of devices are shown on top and magnified images below; corresponding scale bar dimensions are indicated on each image. Pancreatic endocrine and ductal cells are indicated on images. For trichrome staining, two magnified regions are shown to the right, illustrating varying degrees of collagen staining (blue) within the inner membranes. developed into mainly glucagon-positive cells (96.5% 6 0.4%), whereas NKX6.1-high cells became mainly insulin-positive cells (88.7% 6 5.7%); both groups contained a similar number of somatostatin-positive cells (28.6% 6 4.6% and 34.4% 6 5.4% in NKX6.1-low and NKX6.1-high grafts, respectively) (Fig. 4). Quantification of the insulin-only, glucagon-only, and somatostatin-only populations, as well as the various polyhormonal populations are provided in Figure 4B and 4C. Paradoxically, devices from NKX6.1-high mice, which secreted human insulin in response to various secretagogues (Fig. 2B, 2D, 2G), also contained approximately twice as many insulin-positive cells that coexpressed at least one other hormone (Fig. 4). Both groups contained a small ghrelin-positive population and rare cells expressing pancreatic polypeptide (Fig. 5). We next examined the transcription factor profiles of endocrine cells derived from NKX6.1-high and NKX6.1-low cells (Fig. 6). Insulin-positive cells in both groups expressed nuclear NKX6.1 (Fig. 6A, 6B) and PDX1 in the nucleus and cytoplasm (Fig. 6C). Insulin-positive cells that were negative for PDX and NKX6.1 generally coexpressed either glucagon or somatostatin (Fig. 6A 6C, double arrows). Notably, MAFA expression was distinctly nuclear in insulin-expressing cells from NKX6.1-high grafts versus strictly cytoplasmic in insulin-expressing cells from NKX6.1-low grafts (Fig. 6D). Otherwise, endocrine cells were similar between groups, with somatostatin-positive cells generally expressing nuclear Hhex1 (Fig. 6E) and glucagon-positive cells expressing nuclear ARX (Fig. 6F), regardless of insulin coexpression. Finally, we examined other markers found in mature pancreatic b-cells to determine if there were additional differences between insulin-positive cells derived from NKX6.1-high versus NKX6.1-low populations. In both groups, insulin-positive cells uniformly expressed glucose transporter 1 (GLUT1) and sporadically expressed amylin (Fig. 7A, 7B, respectively). Prohormone convertase (PC) 1/3 was robustly expressed in nearly all insulin-positive cells from NKX6.1-high grafts but only in a subset of insulinpositive cells from NKX6.1-low mice (Fig. 7C). Surprisingly, neither group appeared to express PC2 in the insulin-positive cell population, although this enzyme was highly expressed in glucagon-positive cells (Fig. 7D). The K ATP channel sulfonylurea receptor, SUR1, was uniformly expressed in insulin- and glucagon-positive cells from both groups, and if anything, at a higher level in insulin-positive cells from NKX6.1-low mice (Fig. 7E). SerpinB10 was recently reported to be exclusively expressed in mature b- cells within adult human pancreas [22]. We observed robust expression of serpinb10 in the majority of insulin-positive cells from both NKX6.1-high and NKX6.1-low grafts and never in glucagon-positive cells (Fig. 7F).
7 2438 Treatment of Diabetes with Human Stem Cells DISCUSSION Figure 4. Endocrine profiles of macroencapsulated NKX6.1-low and NKX6.1-high cells at 5 months post-transplant. (A): Harvested Theracyte devices were stained for insulin (red), glucagon (blue), somatostatin (green), and DAPI (white). Individual channels are shown for each magnified region in the bottom right of all panels. Two different animals are shown from each treatment group. Scale bars mm. (B): Quantification of the % endocrine cells (insulin-, glucagon-, and/or somatostatin-positive) relative to the total number of DAPI-positive nuclei within devices (n 5 3/group). Grafts from both groups contained a similar proportion of endocrine cells. (C): Quantification of individual endocrine subpopulations, expressed as a proportion of the total insulin-/glucagon-/somatostatin-positive population (n 5 3 per group). Grafts derived from NKX6.1-low cells contained significantly more glucagon-positive cells overall, as well as more glucagon-only and glucagon/somatostatin copositive cells compared to NKX6.1-high cells. In contrast, NKX6.1-high grafts contained significantly more insulin-positive and insulin-only cells compared to NKX6.1-low grafts. There was also a trend toward more polyhormonal insulin-expressing cells in NKX6.1-high cells compared to NKX6.1-low cells. *, p <.05, t test. Abbreviation: DAPI, diamidino-2-phenylindole. This study demonstrates that hesc-derived progenitor populations containing a relatively high proportion of NKX6.1- expressing precursor cells may be more suitable for transplantation into patients with diabetes than a population enriched for immature endocrine cells. Progenitor cells enriched for NKX6.1-positive cells matured more quickly in vivo into glucose-responsive insulin-secreting cells compared to an endocrine-enriched progenitor population. Moreover, the insulin secretion kinetics displayed by NKX6.1-high cells within only 3 months of transplantation are attractive from a clinical cell therapy perspective. Not only did appropriate secretagogues, including glucose, stimulate rapid secretion of human insulin but also circulating C-peptide levels returned to baseline within an hour of a glucose challenge, thus reducing the risk of hypoglycemia. Based on previous work by Kelly et al. [6], we predicted that the hesc-derived PDX1/NKX6.1 copositive subpopulation of pancreatic progenitor cells may have the developmental potential to become glucose-responsive b-cells following transplantation. We also suspected that hesc-derived polyhormonal cells likely contribute important developmental cues for endocrine cell maturation and thus should not be eliminated by FACS purification. Therefore, we generated two mixed pancreatic progenitor populations using modified in vitro differentiation protocols (summarized in Fig. 1A): (a) NKX6.1-high cells were enriched for pancreatic endoderm cells and contained a relatively minor endocrine component, whereas (b) NKX6.1-low cells were enriched for polyhormonal endocrine cells and expressed high levels of pancreatic endocrine transcription factors (except for NKX6.1). It is also possible that the presence of nonpancreatic cells may differ between progenitor populations and potentially influence the development of pancreatic endocrine cells post-transplant. Following transplant, NKX6.1-high and NKX6.1-low populations both developed into mainly pancreatic endocrine cells (70% insulin, glucagon, and/or somatostatin-positive), although the endocrine subpopulations differed dramatically between groups. Moreover, NKX6.1-high grafts not only contained a higher proportion of insulin-positive cells post-transplant but these cells also exhibited an advanced maturation state compared to insulin-positive cells from NKX6.1-low grafts. For instance, both groups secreted similar basal levels of human C-peptide, but NKX6.1-high cells secreted insulin in response to meal, glucose, and arginine challenges, and showed a remarkable capacity to treat diabetes. Notably, we observed meal responsiveness much earlier with NKX6.1-high cells than in our previous studies (at 12 vs. 30 weeks [17]) and the level of stimulation was considerably more robust (2.4-fold vs. 1.3-fold meal stimulus [17]). NKX6.1-high cells also displayed excellent insulin secretion kinetics, including rapid secretion within 15 minutes of a glucose challenge and return to baseline levels by 60 minutes. This is preferable over the lagging insulin secretion kinetics reported previously from hesc-derived cells, in which glucose-induced insulin secretion was observed by 60 minutes, but return to baseline insulin levels was not reported [6,12,17]. These findings begin to address important concerns about potential insulin overproduction by a surrogate b-cell lacking a dynamic off switch in response to falling glycemia [23]. Indeed, mice in the Kroon et al. [12] studies displayed significant hypoglycemia relative to controls (nonfasting levels of 55 mg/dl compared to 139 mg/dl). Hypoglycemia was never observed in mice engrafted with NKX6.1-high cells, either during a glucose challenge or weekly blood glucose tracking, likely due to the ability of engrafted cells to appropriately reduce insulin secretion.
8 Rezania, Bruin, Xu et al Figure 5. Ghrelin and pancreatic polypeptide expression in Theracyte devices at 5 months post-transplant. Macroencapsulated human embryonic stem cell-derived pancreatic endocrine cells contained a small ghrelin-positive population and rare cells that expressed pancreatic polypeptide (white arrows). Cells derived from NKX6.1-low cells are shown on the left and NKX6.1-high cells on the right. Scale bars mm. Abbreviation: DAPI, diamidino-2-phenylindole. We next sought to understand what distinguishes insulinexpressing cells derived from NKX6.1-high versus NKX6.1-low progenitor populations, since the increased number of insulinsecreting cells observed post-transplant in NKX6.1-high grafts could not explain the differences in insulin secretion kinetics. GLUT1 (the predominant glucose transporter in human b-cells [24,25]) and SUR1 (regulatory subunit of the K ATP channel) were each similarly expressed in insulin-positive cells from both groups and did not correspond with differences in insulin secretion kinetics. This supports recent findings that gene expression for glucose transporter and ATP-sensitive K1 channel subunits was similar between immature neonatal and glucose-responsive adult rat b-cells [26]. We also examined amylin and PC1/3 expression, as our previous study demonstrated that these b-cell specific proteins were absent from immature hesc-derived insulin-positive cells in the early post-transplant period [17]. Here, we observed a similar pattern of expression for amylin between groups, excluding this as an important maturation factor in our system. Interestingly, PC1/3 expression was more uniform in insulin-positive cells from NKX6.1-high grafts compared to NKX6.1-low grafts, whereas PC2 expression was relatively low in b-cells of both groups. Loss of PC1/3 expression has been reported to cause more severe defects in proinsulin processing than PC2 deficiency [27]. Considering that PC1/3 expression is required and sufficient for processing of proinsulin [27], the lack of PC1/3 expression in many insulin-positive cells from NKX6.1-low grafts may lead to proinsulin processing defects but still would not explain the immature insulin secretion kinetics in these mice. Finally, we examined a relatively new putative marker of mature b-cells, SerpinB10 [22], and confirmed that it was specifically localized to insulin-expressing cells but was not differentially expressed between NKX6.1-low and NKX6.1-high grafts. We believe that impaired MAFA nuclear translocation may be a key defect in insulin-secreting cells derived from NKX6.1-low progenitor cells. MAFA is a known regulator of glucose-stimulated insulin secretion and key gene required for mature b-cell function [28 30]. Indeed, immature neonatal rat b-cells lacking glucose-stimulated insulin secretion not only express low levels of MAFA relative to adult b-cells but also expression is almost exclusively restricted to the cytoplasm of b-cells until postnatal day 15 [30], thus resembling the insulin-positive cells in NKX6.1-low grafts. Furthermore, overexpression of nuclear MAFA in neonatal (postnatal day 2) rodent b-cells promoted glucose-induced insulin secretion, suggesting that MAFA nuclear localization is a key regulator of b-cell maturity during normal pancreas development [30]. Altered MAFA localization may also account for a loss of b- cell function during the pathogenesis of diabetes. Cytoplasmic localization of MAFA was observed in b-cells from hyperglycemic db/db mice [31] and humans with type 2 diabetes [32]. Moreover, overexpression of an endogenous antioxidant enzyme was able to preserve intranuclear MAFA expression as well as ameliorate hyperglycemia in the db/db mouse model [31]. Although the physiological mechanisms that control MAFA localization are still unknown, the abundant nuclear MAFA expression could be a key factor driving the improved insulin secretion kinetics in b-cells derived from NKX6.1-high pancreatic progenitor cells. Previous evidence in mice suggested that the timing of NGN3 induction may control the competency for pancreatic progenitors to generate specific endocrine cell types [33]. Therefore, we predicted that the timing of NGN3 induction during hesc differentiation would also influence the production of insulin-producing cells in vivo. Indeed, our findings suggest that the manner and timing of NGN3 induction in
9 2440 Treatment of Diabetes with Human Stem Cells Figure 6. Transcription factor expression in endocrine cells from macroencapsulated NKX6.1-low and NKX6.1-high cells at 5 months posttransplant. (A, B): NKX6.1 was expressed in insulin-positive cells from both groups (single arrowheads) but was generally not expressed in cells coexpressing either insulin/glucagon (A) or insulin/somatostatin (B), as indicated by double arrowheads. (C): Insulin-positive cells in both groups expressed PDX1 in the cytoplasmic and nuclear compartments (single arrowheads); insulin/glucagon co-positive cells did not express PDX1 (double arrowheads). (D): Insulin-positive cells in grafts derived from NKX6.1-low cells expressed MAFA in the cytoplasm, but not the nucleus, whereas NKX6.1-high grafts contained insulin-positive cells with nuclear MAFA expression (single arrowheads). MAFA was not expressed in polyhormonal insulin-positive cells (double arrowheads). (E, F): In both groups somatostatin-positive cells were copositive for Hhex1 (E) and glucagon-positive cells for ARX (F), even when insulin was also expressed in the same cell (double arrowheads). Unihormonal insulin-positive cells were negative for both Hhex1 (E) and ARX (F), as indicated by single arrowheads. For all panels, magnified regions illustrate three channels on top, blue/green/dapi channels in the middle and red/green/dapi channels on the bottom. Scale bars 5 50 mm. Abbreviation: DAPI, diamidino-2-phenylindole. developing hescs does control the relative proportions of pancreatic endocrine lineages. NGN3 timing was manipulated in our studies by adjusting exposure to ALK5i and PKC activator. We had previously reported that combined inhibition of transforming growth factor beta (TGF)b/BMP signaling with ALK5i/Noggin during stage 4 caused dramatic induction of pancreatic endocrine markers, including NGN3 [16,17], whereas addition of a PKC activator reduced NGN3 while increasing NKX6.1 expression [17]. Here, the addition of ALK5i during stages 3 4 and absence of the PKC activator during stage 4 in the NKX6.1-low protocol not only reduced NKX6.1 expression at stage 4 but also caused a dramatic induction of NGN3 during stages 3 4 (20-fold induction compared to NKX6.1-high cells). In contrast, induction of NGN3 was delayed in NKX6.1-high cells due to the absence of ALK5i during stage 3; PKC activation also reduced NGN3 and increased NKX6.1 expression. Similar to mice in which early induction of NGN3 favored a-cell development [33], early induction of NGN3 during stage 3 in the NKX6.1-low population resulted in the development of mainly mature a- cells in vivo, as indicated by both circulating glucagon levels and the overwhelming number of glucagon/arx co-positive cells within the harvested devices. Our observations also support previous studies [6,16,20], which collectively indicate that polyhormonal cells may be lineage committed toward an a-cell fate and challenge a long-accepted lineage tracing study, which concluded that fetal insulin/glucagon copositive cells did not contribute to adult a- or b-cell populations [34]. We also observed an unexpected population of cells expressing either insulin/glucagon or insulin/somatostatin derived from NKX6.1- high cells at 5 months post-transplant, particularly around the edges of encapsulation devices. The insulin/somatostatin copositive population is unusual, as this cell type is not typically observed during human fetal pancreas development [35] and
10 Rezania, Bruin, Xu et al Figure 7. Characteristics of insulin-expressing cells in NKX6.1-low and NKX6.1-high cells post-transplant. NKX6.1-low and NKX6.1-high grafts at 5 months post-transplant were immunostained for insulin, glucagon, DAPI, and various markers of mature pancreatic b-cells, including: (A) GLUT1, (B) amylin, (C) PC 1/3, (D) PC2, (E) SUR1, and (F) serpin peptidase inhibitor (serpin) B10. Insulin-positive cells from both groups expressed similar levels of GLUT1 (A), amylin (B), SUR1 (E) and serpinb10 (F) but were generally negative for PC2 (D). PC1/3 was more uniformly expressed in insulinpositive cells from NKX-high grafts than in NKX6.1-low grafts, where many PC1/3-negative insulin-expressing cells were observed (C). Scale bars mm. Abbreviations: DAPI, diamidino-2-phenylindole; GLUT1, glucose transporter 1; PC, prohormone convertase; SUR1, sulfonylurea receptor. appeared to resemble mature d-cells rather than b-cells (hexpositive, NKX6.1-negative). The potential contribution of these polyhormonal cells to the function of surrounding unihormonal insulin-secreting cells remains to be determined. CONCLUSION Taken together, these studies indicate that when enriched (but not purified), hesc-derived pancreatic endoderm cells may be a suitable cell therapy product for treating patients with diabetes. Within a relatively short time frame (3 months), the NKX6.1-enriched population developed into insulin-secreting cells that responded to various physiological secretagogues and were capable of reducing insulin secretion when necessary. Moreover, these cells expressed key markers of mature pancreatic b-cells, including robust nuclear MAFA expression. In contrast, the hesc-derived population enriched for polyhormonal endocrine cells developed into mostly mature a-cells following transplant and produced immature pancreatic b-cells, that did not secrete insulin in a glucose-responsive manner or express nuclear MAFA. These data support the concept that hescs may be a feasible alternative to cadaveric islets for transplantation within macroencapsulation devices into patients with type 1 diabetes.
11 2442 Treatment of Diabetes with Human Stem Cells ACKNOWLEDGMENTS T.J.K. was supported by a senior scholarship from the Michael Smith Foundation for Health Research. J.E.B. was funded by a JDRF postdoctoral fellowship, CIHR postdoctoral fellowship, and the CIHR Transplantation Training Program. J.E.B. also received a L Oreal Canada for Women in Science Research Excellence Fellowship. We thank Mr. Ali Asadi for his technical expertise with immunofluorescent staining and Stem Cell Technologies for their financial support; Dr. Clifford Bogue from the Yale University School of Medicine for kindly providing the hex antibody. This work was funded by the Canadian Institutes of Health Research (CIHR) Regenerative Medicine and Nanomedicine Initiative, the Stem Cell Network (SCN) and the Juvenile Diabetes Research Foundation (JDRF). DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST A.R., J.X., K.N., and J.J.O. are employees of Janssen R&D, LLC; T.J.K. received financial support from Janssen R&D, LLC, for the research described in this article. No other potential conflicts of interest relevant to this article were reported. REFERENCES 1 Robertson RP. Update on transplanting beta cells for reversing type 1 diabetes. Endocrinol Metab Clin North Am 2010;39: Ryan EA, Lakey JR, Rajotte RV et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes 2001;50: Shapiro AM, Lakey JR, Ryan EA et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343: Shapiro AM. State of the art of clinical islet transplantation and novel protocols of immunosuppression. Curr Diab Rep 2011;11: Bruin JE, Kieffer TJ. Differentiation of human embryonic stem cells into pancreatic endocrine cells. In: Hayat MA, ed. Stem Cells and Cancer Stem Cells: Therapeutic Applications in Disease and Injury. New York: Springer, 2012: Kelly OG, Chan MY, Martinson LA et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol 2011;29: Jiang J, Au M, Lu K et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 2007;25: Jiang W, Shi Y, Zhao D et al. In vitro derivation of functional insulinproducing cells from human embryonic stem cells. Cell Res 2007;17: Zhang D, Jiang W, Liu M et al. Highly efficient differentiation of human ES cells and ips cells into mature pancreatic insulin-producing cells. Cell Res 2009;19: Nostro MC, Sarangi F, Ogawa S et al. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development 2011; 138: D Amour KA, Bang AG, Eliazer S et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006;24: Kroon E, Martinson LA, Kadoya K et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26: Mfopou JK, Chen B, Mateizel I et al. Noggin, retinoids, and fibroblast growth factor regulate hepatic or pancreatic fate of human embryonic stem cells. Gastroenterology 2010;138: , 2245 e Shim JH, Kim SE, Woo DH et al. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia 2007;50: Cai J, Yu C, Liu Y et al. Generation of homogeneous PDX1(1) pancreatic progenitors from human ES cell-derived endoderm cells. J Mol Cell Biol 2010;2: Rezania A, Riedel MJ, Wideman RD et al. Production of functional glucagon-secreting alpha-cells from human embryonic stem cells. Diabetes 2011;60: Rezania A, Bruin JE, Riedel MJ et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 2012;61: Bruin JE, Rezania A, Xu J et al. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia 2013;56: Rieck S, Bankaitis ED, Wright CV. Lineage determinants in early endocrine development. Semin Cell Dev Biol 2012;23: Basford CL, Prentice KJ, Hardy AB et al. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia 2012;55: Brauker JH, Carr-Brendel VE, Martinson LA et al. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res 1995;29: Lindskog C, Korsgren O, Ponten F et al. Novel pancreatic beta cellspecific proteins: Antibody-based proteomics for identification of new biomarker candidates. J Proteomics 2012;75: Halban PA, German MS, Kahn SE et al. Current status of islet cell replacement and regeneration therapy. J Clin Endocrinol Metab 2010; 95: Coppieters KT, Wiberg A, Amirian N et al. Persistent glucose transporter expression on pancreatic beta cells from longstanding type 1 diabetic individuals. Diabetes Metab Res Rev 2011;27: De Vos A, Heimberg H, Quartier E et al. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest 1995;96: Jermendy A, Toschi E, Aye T et al. Rat neonatal beta cells lack the specialised metabolic phenotype of mature beta cells. Diabetologia 2011;54: Zhu X, Orci L, Carroll R et al. Severe block in processing of proinsulin to insulin accompanied by elevation of des-64,65 proinsulin intermediates in islets of mice lacking prohormone convertase 1/3. Proc Natl Acad Sci USA 2002;99: Wang H, Brun T, Kataoka K et al. MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia 2007;50: Zhang C, Moriguchi T, Kajihara M et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol 2005;25: Aguayo-Mazzucato C, Koh A, El Khattabi I et al. Mafa expression enhances glucose-responsive insulin secretion in neonatal rat beta cells. Diabetologia 2011;54: Harmon JS, Bogdani M, Parazzoli SD et al. beta-cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology 2009;150: Butler AE, Robertson RP, Hernandez R et al. Beta cell nuclear musculoaponeurotic fibrosarcoma oncogene family A (MafA) is deficient in type 2 diabetes. Diabetologia 2012;55: Johansson KA, Dursun U, Jordan N et al. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell 2007;12: Herrera PL, Nepote V, Delacour A. Pancreatic cell lineage analyses in mice. Endocrine 2002;19: Riedel MJ, Asadi A, Wang R et al. Immunohistochemical characterisation of cells co-producing insulin and glucagon in the developing human pancreas. Diabetologia 2012;55: See for supporting information available online.
Making Mature Human Islet Cells from Stem Cells to Model Disease and Treat Diabetes
 University of British Columbia Departments of Surgery and Cellular & Physiological Sciences Making Mature Human Islet Cells from Stem Cells to Model Disease and Treat Diabetes 2016 International Conference
University of British Columbia Departments of Surgery and Cellular & Physiological Sciences Making Mature Human Islet Cells from Stem Cells to Model Disease and Treat Diabetes 2016 International Conference
Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro.
 Manuscript EMBO-2015-91058 Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. Holger A Russ, Audrey V Parent, Jennifer J Ringler, Thomas G Hennings, Gopika
Manuscript EMBO-2015-91058 Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. Holger A Russ, Audrey V Parent, Jennifer J Ringler, Thomas G Hennings, Gopika
Stem Cell Reports Ar ticle
 Stem Cell Reports Ar ticle Treating Diet-Induced Diabetes and Obesity with Human Embryonic Stem Cell-Derived Pancreatic Progenitor Cells and Antidiabetic Drugs Jennifer E. Bruin, 1 Nelly Saber, 1 Natalie
Stem Cell Reports Ar ticle Treating Diet-Induced Diabetes and Obesity with Human Embryonic Stem Cell-Derived Pancreatic Progenitor Cells and Antidiabetic Drugs Jennifer E. Bruin, 1 Nelly Saber, 1 Natalie
Cell Therapy for Diabetes: Generating Functional Islets from Human Exocrine Tissue
 Cell Therapy for Diabetes: Generating Functional Islets from Human Exocrine Tissue Kevin Docherty University of Aberdeen ELRIG Drug Discovery 2016 ACC Liverpool 14 th October 2016 Autoimmune destruction
Cell Therapy for Diabetes: Generating Functional Islets from Human Exocrine Tissue Kevin Docherty University of Aberdeen ELRIG Drug Discovery 2016 ACC Liverpool 14 th October 2016 Autoimmune destruction
Hypothyroidism impairs human stem cell-derived pancreatic progenitor cell maturation in mice
 Page 1 of 8 Hypothyroidism impairs human stem cell-derived pancreatic progenitor cell maturation in mice Jennifer E Bruin 1, Nelly Saber 1, Shannon O Dwyer 1, Jessica K Fox 1, Majid Mojibian 1, Payal Arora
Page 1 of 8 Hypothyroidism impairs human stem cell-derived pancreatic progenitor cell maturation in mice Jennifer E Bruin 1, Nelly Saber 1, Shannon O Dwyer 1, Jessica K Fox 1, Majid Mojibian 1, Payal Arora
Supplemental Experimental Procedures
 Cell Stem Cell, Volume 2 Supplemental Data A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis Andrew S. Brack, Irina M. Conboy, Michael J. Conboy,
Cell Stem Cell, Volume 2 Supplemental Data A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis Andrew S. Brack, Irina M. Conboy, Michael J. Conboy,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature11463 %Sox17(+) 9 8 7 6 5 4 3 2 1 %Sox17(+) #Sox17(+) d2 d4 d6 d8 d1 d12 d14 d18 25 2 15 1 5 Number of Sox17(+) cells X 1 Supplementary Figure 1: Expression of
SUPPLEMENTARY INFORMATION doi:1.138/nature11463 %Sox17(+) 9 8 7 6 5 4 3 2 1 %Sox17(+) #Sox17(+) d2 d4 d6 d8 d1 d12 d14 d18 25 2 15 1 5 Number of Sox17(+) cells X 1 Supplementary Figure 1: Expression of
Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Introduction Kit Components Cat. # # of vials Reagent Quantity Storage
 Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Catalog #5901 Introduction Human pluripotent stem cells (hpsc), including embryonic stem cells (ESC) and induced pluripotent stem
Human Pluripotent Stem Cell Cardiomyocyte Differentiation Kit (PSCCDK) Catalog #5901 Introduction Human pluripotent stem cells (hpsc), including embryonic stem cells (ESC) and induced pluripotent stem
SUPPLEMENTARY DATA. Supplementary Table 2. Antibodies used for Immunofluoresence. Supplementary Table 3. Real-time PCR primer sequences.
 Supplementary Table 2. Antibodies used for Immunofluoresence. Antibody Dilution Source Goat anti-pdx1 1:100 R&D Systems Rabbit anti-hnf6 1:100 Santa Cruz Biotechnology Mouse anti-nkx6.1 1:200 Developmental
Supplementary Table 2. Antibodies used for Immunofluoresence. Antibody Dilution Source Goat anti-pdx1 1:100 R&D Systems Rabbit anti-hnf6 1:100 Santa Cruz Biotechnology Mouse anti-nkx6.1 1:200 Developmental
MPB333:Molecular Endocrinology of Obesity and Diabetes
 MPB333:Molecular Endocrinology of Obesity and Diabetes The Use of Stem Cells as a Cure for Type 1 Diabetes January 15, 2010 Trish Labosky 9415C MRBIV trish.labosky@vanderbilt.edu In theory. 2. ~Easy and
MPB333:Molecular Endocrinology of Obesity and Diabetes The Use of Stem Cells as a Cure for Type 1 Diabetes January 15, 2010 Trish Labosky 9415C MRBIV trish.labosky@vanderbilt.edu In theory. 2. ~Easy and
Transplantation of Human Pancreatic Endoderm Cells Reverses Diabetes. Post Transplantation in a Prevascularized Subcutaneous Site
 Stem Cell Reports, Volume 8 Supplemental Information Transplantation of Human Pancreatic Endoderm Cells Reverses Diabetes Post Transplantation in a Prevascularized Subcutaneous Site Andrew R. Pepper, Rena
Stem Cell Reports, Volume 8 Supplemental Information Transplantation of Human Pancreatic Endoderm Cells Reverses Diabetes Post Transplantation in a Prevascularized Subcutaneous Site Andrew R. Pepper, Rena
Stage 5: Immature endocrine Stage 4 cells were cultured for seven days in DM-F12 medium supplemented with 1% B27 and 1 µm ALK5 inhibitor.
 Supplemental Data Research Design and Methods Differentiation of Human ES Cells Stage 1: Mesoendoderm 60-70% confluent adherent cultures of undifferentiated H1 cells plated on 1:30 Matrigel coated surfaces
Supplemental Data Research Design and Methods Differentiation of Human ES Cells Stage 1: Mesoendoderm 60-70% confluent adherent cultures of undifferentiated H1 cells plated on 1:30 Matrigel coated surfaces
Supplemental Information. b Cell Aging Markers Have Heterogeneous. Distribution and Are Induced by Insulin Resistance
 Cell Metabolism, Volume 25 Supplemental Information b Cell Aging Markers Have Heterogeneous Distribution and Are Induced by Insulin Resistance Cristina Aguayo-Mazzucato, Mark van Haaren, Magdalena Mruk,
Cell Metabolism, Volume 25 Supplemental Information b Cell Aging Markers Have Heterogeneous Distribution and Are Induced by Insulin Resistance Cristina Aguayo-Mazzucato, Mark van Haaren, Magdalena Mruk,
In vitro differentiation optimization Flow Cytometry
 In vitro differentiation optimization The effect of various basal media formulations were examined during stages 3 4 of the differentiation protocol. For these studies, conditions were as described for
In vitro differentiation optimization The effect of various basal media formulations were examined during stages 3 4 of the differentiation protocol. For these studies, conditions were as described for
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
NIH Public Access Author Manuscript Diabetologia. Author manuscript; available in PMC 2014 February 01.
 NIH Public Access Author Manuscript Published in final edited form as: Diabetologia. 2013 February ; 56(2): 231 233. doi:10.1007/s00125-012-2788-6. Lipotoxicity impairs incretin signalling V. Poitout 1,2
NIH Public Access Author Manuscript Published in final edited form as: Diabetologia. 2013 February ; 56(2): 231 233. doi:10.1007/s00125-012-2788-6. Lipotoxicity impairs incretin signalling V. Poitout 1,2
Journal Club WS 2012/13 Stefanie Nickl
 Journal Club WS 2012/13 Stefanie Nickl Background Mesenchymal Stem Cells First isolation from bone marrow 30 ys ago Isolation from: spleen, heart, skeletal muscle, synovium, amniotic fluid, dental pulp,
Journal Club WS 2012/13 Stefanie Nickl Background Mesenchymal Stem Cells First isolation from bone marrow 30 ys ago Isolation from: spleen, heart, skeletal muscle, synovium, amniotic fluid, dental pulp,
Figure legends Supplemental Fig.1. Glucose-induced insulin secretion and insulin content of islets. Supplemental Fig. 2.
 Figure legends Supplemental Fig.. Glucose-induced insulin secretion and insulin content of islets. Insulin secretory responses to.,., and. mm glucose (A) (n = 7-), and the insulin content in the islets
Figure legends Supplemental Fig.. Glucose-induced insulin secretion and insulin content of islets. Insulin secretory responses to.,., and. mm glucose (A) (n = 7-), and the insulin content in the islets
Discussion & Conclusion
 Discussion & Conclusion 7. Discussion DPP-4 inhibitors augment the effects of incretin hormones by prolonging their half-life and represent a new therapeutic approach for the treatment of type 2 diabetes
Discussion & Conclusion 7. Discussion DPP-4 inhibitors augment the effects of incretin hormones by prolonging their half-life and represent a new therapeutic approach for the treatment of type 2 diabetes
Protocol for A-549 VIM RFP (ATCC CCL-185EMT) TGFβ1 EMT Induction and Drug Screening
 Protocol for A-549 VIM RFP (ATCC CCL-185EMT) TGFβ1 EMT Induction and Drug Screening Introduction: Vimentin (VIM) intermediate filament (IF) proteins are associated with EMT in lung cancer and its metastatic
Protocol for A-549 VIM RFP (ATCC CCL-185EMT) TGFβ1 EMT Induction and Drug Screening Introduction: Vimentin (VIM) intermediate filament (IF) proteins are associated with EMT in lung cancer and its metastatic
SUPPLEMENTAL INFORMATION FOR. PAX7 expression defines germline stem cells in the adult testis
 SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
Product Use HPSC-CC are for research use only. It is not approved for human or animal use, or for application in in vitro diagnostic procedures.
 HPSC-derived Cardiomyocyte Cells (HPSC-CC) Catalog #6240 Cell Specification Human primary cardiomyocytes and cardiac tissue are superior modeling systems for heart disease studies, drug discovery and toxicity
HPSC-derived Cardiomyocyte Cells (HPSC-CC) Catalog #6240 Cell Specification Human primary cardiomyocytes and cardiac tissue are superior modeling systems for heart disease studies, drug discovery and toxicity
Pair-fed % inkt cells 0.5. EtOH 0.0
 MATERIALS AND METHODS Histopathological analysis Liver tissue was collected 9 h post-gavage, and the tissue samples were fixed in 1% formalin and paraffin-embedded following a standard procedure. The embedded
MATERIALS AND METHODS Histopathological analysis Liver tissue was collected 9 h post-gavage, and the tissue samples were fixed in 1% formalin and paraffin-embedded following a standard procedure. The embedded
Glucose Homeostasis. Liver. Glucose. Muscle, Fat. Pancreatic Islet. Glucose utilization. Glucose production, storage Insulin Glucagon
 Glucose Homeostasis Liver Glucose Glucose utilization Glucose production, storage Insulin Glucagon Muscle, Fat Pancreatic Islet Classification of Diabetes Type 1 diabetes Type 2 diabetes Other types of
Glucose Homeostasis Liver Glucose Glucose utilization Glucose production, storage Insulin Glucagon Muscle, Fat Pancreatic Islet Classification of Diabetes Type 1 diabetes Type 2 diabetes Other types of
Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signalling pathway
 Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signalling pathway Jieyuan Zhang, Xiaolin Liu, Haiyan Li, Chunyuan Chen, Bin Hu, Xin Niu, Qing
Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signalling pathway Jieyuan Zhang, Xiaolin Liu, Haiyan Li, Chunyuan Chen, Bin Hu, Xin Niu, Qing
Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h)
 Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h) after feeding. A small slice (~5-1 mm 3 ) was taken
Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h) after feeding. A small slice (~5-1 mm 3 ) was taken
3D differentiation enhances the efficiency of differentiation of human induced pluripotent stem cells to insulin producing cells
 University of Iowa Iowa Research Online Theses and Dissertations Fall 2014 3D differentiation enhances the efficiency of differentiation of human induced pluripotent stem cells to insulin producing cells
University of Iowa Iowa Research Online Theses and Dissertations Fall 2014 3D differentiation enhances the efficiency of differentiation of human induced pluripotent stem cells to insulin producing cells
Abbreviations: P- paraffin-embedded section; C, cryosection; Bio-SA, biotin-streptavidin-conjugated fluorescein amplification.
 Supplementary Table 1. Sequence of primers for real time PCR. Gene Forward primer Reverse primer S25 5 -GTG GTC CAC ACT ACT CTC TGA GTT TC-3 5 - GAC TTT CCG GCA TCC TTC TTC-3 Mafa cds 5 -CTT CAG CAA GGA
Supplementary Table 1. Sequence of primers for real time PCR. Gene Forward primer Reverse primer S25 5 -GTG GTC CAC ACT ACT CTC TGA GTT TC-3 5 - GAC TTT CCG GCA TCC TTC TTC-3 Mafa cds 5 -CTT CAG CAA GGA
Exploring the Use of Human Pluripotent Stem Cells to Create Functional Pancreatic \(\beta\) Cells
 Exploring the Use of Human Pluripotent Stem Cells to Create Functional Pancreatic \(\beta\) Cells The Harvard community has made this article openly available. Please share how this access benefits you.
Exploring the Use of Human Pluripotent Stem Cells to Create Functional Pancreatic \(\beta\) Cells The Harvard community has made this article openly available. Please share how this access benefits you.
Generation of pancreatic β cells for treatment of diabetes: advances and challenges
 Shahjalal et al. Stem Cell Research & Therapy (2018) 9:355 https://doi.org/10.1186/s13287-018-1099-3 REVIEW Generation of pancreatic β cells for treatment of diabetes: advances and challenges Hussain Md.
Shahjalal et al. Stem Cell Research & Therapy (2018) 9:355 https://doi.org/10.1186/s13287-018-1099-3 REVIEW Generation of pancreatic β cells for treatment of diabetes: advances and challenges Hussain Md.
Notch Signaling Pathway Notch CSL Reporter HEK293 Cell line Catalog #: 60652
 Notch Signaling Pathway Notch CSL Reporter HEK293 Cell line Catalog #: 60652 Background The Notch signaling pathway controls cell fate decisions in vertebrate and invertebrate tissues. Notch signaling
Notch Signaling Pathway Notch CSL Reporter HEK293 Cell line Catalog #: 60652 Background The Notch signaling pathway controls cell fate decisions in vertebrate and invertebrate tissues. Notch signaling
SUPPLEMENTARY FIG. S2. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of dnscs.
 Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
α-cell role in β-cell generation and regeneration
 Islets ISSN: 1938-2014 (Print) 1938-2022 (Online) Journal homepage: http://www.tandfonline.com/loi/kisl20 α-cell role in β-cell generation and regeneration Joel F. Habener & Violeta Stanojevic To cite
Islets ISSN: 1938-2014 (Print) 1938-2022 (Online) Journal homepage: http://www.tandfonline.com/loi/kisl20 α-cell role in β-cell generation and regeneration Joel F. Habener & Violeta Stanojevic To cite
Cell therapeutics for the Insulin-Dependent Diabetes Mellitus
 Cell therapeutics for the Insulin-Dependent Diabetes Mellitus Haekwon Kim Dept. of Biotechnology Seoul Women s University Introduction Type I diabetes is caused by the autoimmune destruction of pancreatic
Cell therapeutics for the Insulin-Dependent Diabetes Mellitus Haekwon Kim Dept. of Biotechnology Seoul Women s University Introduction Type I diabetes is caused by the autoimmune destruction of pancreatic
Supporting Information
 Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
B16-F10 (Mus musculus skin melanoma), NCI-H460 (human non-small cell lung cancer
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Experimental Methods Cell culture B16-F10 (Mus musculus skin melanoma), NCI-H460 (human non-small
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Experimental Methods Cell culture B16-F10 (Mus musculus skin melanoma), NCI-H460 (human non-small
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Advances in pancreatic islet monolayer culture on glass surfaces enable superresolution microscopy and insights into beta cell ciliogenesis and proliferation Edward A. Phelps,
SUPPLEMENTARY INFORMATION Advances in pancreatic islet monolayer culture on glass surfaces enable superresolution microscopy and insights into beta cell ciliogenesis and proliferation Edward A. Phelps,
In vitro bactericidal assay Fig. S8 Gentamicin protection assay Phagocytosis assay
 In vitro bactericidal assay Mouse bone marrow was isolated from the femur and the tibia. Cells were suspended in phosphate buffered saline containing.5% BSA and 2 mm EDTA and filtered through a cell strainer.
In vitro bactericidal assay Mouse bone marrow was isolated from the femur and the tibia. Cells were suspended in phosphate buffered saline containing.5% BSA and 2 mm EDTA and filtered through a cell strainer.
Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells.
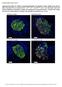 Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells. Pancreata from 16-weeks-old 6J +/+, 6J db/db, KS +/+ and KS db/db mice were harvested, fixed with
Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells. Pancreata from 16-weeks-old 6J +/+, 6J db/db, KS +/+ and KS db/db mice were harvested, fixed with
ENGINEERING THREE DIMENSIONAL CARDIOSPHERES FROM PLURIPOTENT STEM CELLS
 ENGINEERING THREE DIMENSIONAL CARDIOSPHERES FROM PLURIPOTENT STEM CELLS A Thesis Presented to The Academic Faculty by Nicole Votaw In Partial Fulfillment of the Requirements for the Degree B.S. in Biomedical
ENGINEERING THREE DIMENSIONAL CARDIOSPHERES FROM PLURIPOTENT STEM CELLS A Thesis Presented to The Academic Faculty by Nicole Votaw In Partial Fulfillment of the Requirements for the Degree B.S. in Biomedical
β-cell Preservation and Regeneration After Islet Transplantation
 β-cell Preservation and Regeneration After Islet Transplantation Jyuhn-Huarng Juang, MD Division of Endocrinology and Metabolism, Department of Internal Medicine, Chang Gung University and Memorial Hospital,
β-cell Preservation and Regeneration After Islet Transplantation Jyuhn-Huarng Juang, MD Division of Endocrinology and Metabolism, Department of Internal Medicine, Chang Gung University and Memorial Hospital,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2697 Figure S1 Cytokeratin 5 is a specific marker for basal and intermediate cells in all mouse prostate lobes. (a) Immunofluorescence staining showing co-localization of YFP with p63 in
DOI: 10.1038/ncb2697 Figure S1 Cytokeratin 5 is a specific marker for basal and intermediate cells in all mouse prostate lobes. (a) Immunofluorescence staining showing co-localization of YFP with p63 in
Generation of Insulin-Producing Islet-Like Clusters from Human Embryonic Stem Cells
 EMBRYONIC STEM CELLS Generation of Insulin-Producing Islet-Like Clusters from Human Embryonic Stem Cells JIANJIE JIANG, a MELINDA AU, a KUANGHUI LU, a ALANA ESHPETER, b GREGORY KORBUTT, b GREG FISK, a
EMBRYONIC STEM CELLS Generation of Insulin-Producing Islet-Like Clusters from Human Embryonic Stem Cells JIANJIE JIANG, a MELINDA AU, a KUANGHUI LU, a ALANA ESHPETER, b GREGORY KORBUTT, b GREG FISK, a
Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell and patient-derived tumor organoids
 Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell and patient-derived tumor organoids Ling Huang 1, Audrey Holtzinger 1,2,11, Ishaan Jagan 1,11, Michael BeGora 1, Ines
Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell and patient-derived tumor organoids Ling Huang 1, Audrey Holtzinger 1,2,11, Ishaan Jagan 1,11, Michael BeGora 1, Ines
SUPPLEMENTARY DATA. Supplementary Table 1. Primers used for PCR and qpcr Primer Name
 Supplementary Table. Primers used for PCR and qpcr Primer Name ccession Number Fwd Rev Type of PCR Cre NC_8 GGCGTCTTCCGC GTGCCCCTCGTTTG Standard PCR LoUcp CCGGGCTGTCTCCGCGG GGCTGTTCGCCCGGCC Standard PCR
Supplementary Table. Primers used for PCR and qpcr Primer Name ccession Number Fwd Rev Type of PCR Cre NC_8 GGCGTCTTCCGC GTGCCCCTCGTTTG Standard PCR LoUcp CCGGGCTGTCTCCGCGG GGCTGTTCGCCCGGCC Standard PCR
The regenerative therapy of type 1 diabetes mellitus 21April 2017 Girne, Northern Cyprus 53rd Turkish National Diabetes Congress
 The regenerative therapy of type 1 diabetes mellitus 21April 2017 Girne, Northern Cyprus 53rd Turkish National Diabetes Congress Thomas Linn Clinical Research Unit Centre of Internal Medicine Justus Liebig
The regenerative therapy of type 1 diabetes mellitus 21April 2017 Girne, Northern Cyprus 53rd Turkish National Diabetes Congress Thomas Linn Clinical Research Unit Centre of Internal Medicine Justus Liebig
Supplemental Table 1. Primer sequences for transcript analysis
 Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Pathways to Protecting from Autoimmunity the Resurrected/Regenerated Beta Cells. Jeffrey Bluestone, PhD Diabetes Center at UCSF
 Pathways to Protecting from Autoimmunity the Resurrected/Regenerated Beta Cells Jeffrey Bluestone, PhD Diabetes Center at UCSF Number of Transplants Is islet replacement a viable option? 2000 1800 1600
Pathways to Protecting from Autoimmunity the Resurrected/Regenerated Beta Cells Jeffrey Bluestone, PhD Diabetes Center at UCSF Number of Transplants Is islet replacement a viable option? 2000 1800 1600
GLP-2 ELISA. For the quantitative determination of GLP-2 in human serum and plasma samples.
 GLP-2 ELISA For the quantitative determination of GLP-2 in human serum and plasma samples. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 48-GP2HU-E01.1 Size: 96 wells Version:
GLP-2 ELISA For the quantitative determination of GLP-2 in human serum and plasma samples. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 48-GP2HU-E01.1 Size: 96 wells Version:
Human Total GIP Kit v1-2016Apr 1
 Human Total GIP Kit 1-Plate Kit 5-Plate Kit 25-Plate Kit K151RPD-1 K151RPD-2 K151RPD-4 18067-v1-2016Apr 1 MSD Metabolic Assays Human Total GIP Kit This package insert must be read in its entirety before
Human Total GIP Kit 1-Plate Kit 5-Plate Kit 25-Plate Kit K151RPD-1 K151RPD-2 K151RPD-4 18067-v1-2016Apr 1 MSD Metabolic Assays Human Total GIP Kit This package insert must be read in its entirety before
Inflammation & Type 2 Diabetes Prof. Marc Y. Donath
 Inflammation & Type 2 Diabetes 1, MD Chief Endocrinology, Diabetes & Metabolism University Hospital Basel Petersgraben 4 CH-431 Basel, Switzerland MDonath@uhbs.ch Innate immunity as a sensor of metabolic
Inflammation & Type 2 Diabetes 1, MD Chief Endocrinology, Diabetes & Metabolism University Hospital Basel Petersgraben 4 CH-431 Basel, Switzerland MDonath@uhbs.ch Innate immunity as a sensor of metabolic
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Future Therapies in the Treatment of Diabetes: Islet Transplantation
 Future Therapies in the Treatment of Diabetes: Islet Transplantation Michael R. Rickels, MD, MS Associate Professor of Medicine University of Pennsylvania Perelman School of Medicine Medical Director,
Future Therapies in the Treatment of Diabetes: Islet Transplantation Michael R. Rickels, MD, MS Associate Professor of Medicine University of Pennsylvania Perelman School of Medicine Medical Director,
2017 American Diabetes Association. Published online at
 Patients and cell lines 1018-NT-ES cell line was derived from dermal fibroblasts of a female type 1 diabetes patient via somatic cell nuclear transfer to a human oocyte specifically donated to research
Patients and cell lines 1018-NT-ES cell line was derived from dermal fibroblasts of a female type 1 diabetes patient via somatic cell nuclear transfer to a human oocyte specifically donated to research
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Metabolic Disease drug discovery at Evotec
 Metabolic Disease drug discovery at Evotec Evotec, Metabolic Disease Drug Discovery, 2016 Evotec, an ideal partner in metabolic disease drug discovery The different ways to work with us On your specific
Metabolic Disease drug discovery at Evotec Evotec, Metabolic Disease Drug Discovery, 2016 Evotec, an ideal partner in metabolic disease drug discovery The different ways to work with us On your specific
9.3 Stress Response and Blood Sugar
 9.3 Stress Response and Blood Sugar Regulate Stress Response Regulate Blood Sugar Stress Response Involves hormone pathways that regulate metabolism, heart, rate and breathing The Adrenal Glands a pair
9.3 Stress Response and Blood Sugar Regulate Stress Response Regulate Blood Sugar Stress Response Involves hormone pathways that regulate metabolism, heart, rate and breathing The Adrenal Glands a pair
Canberra, Australia). CD11c-DTR-OVA-GFP (B6.CD11c-OVA), B6.luc + and. Cancer Research Center, Germany). B6 or BALB/c.FoxP3-DTR-GFP mice were
 Supplemental Materials and Methods Mice Female C57BL/6 (B6, I-E null, H-2 b ), BALB/c (H-2 d ) + ), FVB/N (H-2 q, I-E null, CD45.1 + ), and B6D2F1 (H-2 b/d ) mice were purchased from the Animal Resources
Supplemental Materials and Methods Mice Female C57BL/6 (B6, I-E null, H-2 b ), BALB/c (H-2 d ) + ), FVB/N (H-2 q, I-E null, CD45.1 + ), and B6D2F1 (H-2 b/d ) mice were purchased from the Animal Resources
Mass Histology Service
 Mass Histology Service A complete anatomical pathology laboratory www.masshistology.com Telephone: (877) 286-6004 Report on Pathology A Time Course Study of the Local Effects of Intramuscular XXXXXXX Injection
Mass Histology Service A complete anatomical pathology laboratory www.masshistology.com Telephone: (877) 286-6004 Report on Pathology A Time Course Study of the Local Effects of Intramuscular XXXXXXX Injection
Human pluripotent stem cell differentiation to functional pancreatic cells for diabetes therapies: Innovations, challenges and future directions
 Jacobson and Tzanakakis Journal of Biological Engineering (2017) 11:21 DOI 10.1186/s13036-017-0066-3 REVIEW Human pluripotent stem cell differentiation to functional pancreatic cells for diabetes therapies:
Jacobson and Tzanakakis Journal of Biological Engineering (2017) 11:21 DOI 10.1186/s13036-017-0066-3 REVIEW Human pluripotent stem cell differentiation to functional pancreatic cells for diabetes therapies:
The Egyptian Journal of Hospital Medicine (April 2018) Vol. 71 (6), Page
 The Egyptian Journal of Hospital Medicine (April 2018) Vol. 71 (6), Page 3308-3313 Knowledge, Attitude and Practice of Doctors and Medical Students towards Stem Cell Use in The Management of Diabetes Mellitus
The Egyptian Journal of Hospital Medicine (April 2018) Vol. 71 (6), Page 3308-3313 Knowledge, Attitude and Practice of Doctors and Medical Students towards Stem Cell Use in The Management of Diabetes Mellitus
islets scored 1 week month months
 Supplementary Table 1. Sampling parameters for the morphometrical analyses Time (post- DT) Control mice (age-matched) α-cells mice pancreatic surface (mm 2 ) scored DT-treated mice islets scored mice pancreatic
Supplementary Table 1. Sampling parameters for the morphometrical analyses Time (post- DT) Control mice (age-matched) α-cells mice pancreatic surface (mm 2 ) scored DT-treated mice islets scored mice pancreatic
Stem Cells and The Endometrium. Director, Division of Reproductive Endocrinology and infertility
 Stem Cells and The Endometrium Hugh S. Taylor, M.D. Director, Division of Reproductive Endocrinology and infertility Nothing to disclose Stem Cells Cells that are capable of both self-renewal and have
Stem Cells and The Endometrium Hugh S. Taylor, M.D. Director, Division of Reproductive Endocrinology and infertility Nothing to disclose Stem Cells Cells that are capable of both self-renewal and have
Islet Cell Allo-Transplantation. Disclosure. Objectives
 Islet Cell Allo-Transplantation Gregory P. Forlenza, MD MCR Assistant Professor Barbara Davis Center University of Colorado Denver Special thanks to Melena Bellin, MD at The University of Minnesota who
Islet Cell Allo-Transplantation Gregory P. Forlenza, MD MCR Assistant Professor Barbara Davis Center University of Colorado Denver Special thanks to Melena Bellin, MD at The University of Minnesota who
What systems are involved in homeostatic regulation (give an example)?
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
Mouse GLP-2 EIA FOR LABORATORY USE ONLY
 YK142 Mouse GLP-2 EIA FOR LABORATORY USE ONLY Kasumigaseki place, 3-6-7, Kasumigaseki, Chiyoda-ku, Tokyo 100-0013 Japan http://www.sceti.co.jp/english/export e-mail exp-pet@sceti.co.jp
YK142 Mouse GLP-2 EIA FOR LABORATORY USE ONLY Kasumigaseki place, 3-6-7, Kasumigaseki, Chiyoda-ku, Tokyo 100-0013 Japan http://www.sceti.co.jp/english/export e-mail exp-pet@sceti.co.jp
Santulli G. et al. A microrna-based strategy to suppress restenosis while preserving endothelial function
 ONLINE DATA SUPPLEMENTS Santulli G. et al. A microrna-based strategy to suppress restenosis while preserving endothelial function Supplementary Figures Figure S1 Effect of Ad-p27-126TS on the expression
ONLINE DATA SUPPLEMENTS Santulli G. et al. A microrna-based strategy to suppress restenosis while preserving endothelial function Supplementary Figures Figure S1 Effect of Ad-p27-126TS on the expression
In Vitro Differentiation and Expansion of Human Pluripotent Stem Cell-Derived Pancreatic Progenitors
 Reprint from The Review of DIABETIC STUDIES Vol 11 No 1 2014 Special Edition Stem Cells and Pancreas Regeneration The Review of DIABETIC STUDIES REVIEW In Vitro Differentiation and Expansion of Human Pluripotent
Reprint from The Review of DIABETIC STUDIES Vol 11 No 1 2014 Special Edition Stem Cells and Pancreas Regeneration The Review of DIABETIC STUDIES REVIEW In Vitro Differentiation and Expansion of Human Pluripotent
Postn MCM Smad2 fl/fl Postn MCM Smad3 fl/fl Postn MCM Smad2/3 fl/fl. Postn MCM. Tgfbr1/2 fl/fl TAC
 A Smad2 fl/fl Smad3 fl/fl Smad2/3 fl/fl Tgfbr1/2 fl/fl 1. mm B Tcf21 MCM Tcf21 MCM Smad3 fl/fl Tcf21 MCM Smad2/3 fl/fl Tcf21 MCM Tgfbr1/2 fl/fl αmhc MCM C 1. mm 1. mm D Smad2 fl/fl Smad3 fl/fl Smad2/3
A Smad2 fl/fl Smad3 fl/fl Smad2/3 fl/fl Tgfbr1/2 fl/fl 1. mm B Tcf21 MCM Tcf21 MCM Smad3 fl/fl Tcf21 MCM Smad2/3 fl/fl Tcf21 MCM Tgfbr1/2 fl/fl αmhc MCM C 1. mm 1. mm D Smad2 fl/fl Smad3 fl/fl Smad2/3
Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival
 Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Determination of serum insulin level by ELISA
 Practical course: Basic biochemical methods and ischemic heart models Determination of serum insulin level by ELISA A practical manual Tamas Csont, MD, PhD Supported by: HURO/0901/069/2.3.1 1 BACKGROUND
Practical course: Basic biochemical methods and ischemic heart models Determination of serum insulin level by ELISA A practical manual Tamas Csont, MD, PhD Supported by: HURO/0901/069/2.3.1 1 BACKGROUND
Supplemental figure 1. PDGFRα is expressed dominantly by stromal cells surrounding mammary ducts and alveoli. A) IHC staining of PDGFRα in
 Supplemental figure 1. PDGFRα is expressed dominantly by stromal cells surrounding mammary ducts and alveoli. A) IHC staining of PDGFRα in nulliparous (left panel) and InvD6 mouse mammary glands (right
Supplemental figure 1. PDGFRα is expressed dominantly by stromal cells surrounding mammary ducts and alveoli. A) IHC staining of PDGFRα in nulliparous (left panel) and InvD6 mouse mammary glands (right
Neurotrophic factor GDNF and camp suppress glucocorticoid-inducible PNMT expression in a mouse pheochromocytoma model.
 161 Neurotrophic factor GDNF and camp suppress glucocorticoid-inducible PNMT expression in a mouse pheochromocytoma model. Marian J. Evinger a, James F. Powers b and Arthur S. Tischler b a. Department
161 Neurotrophic factor GDNF and camp suppress glucocorticoid-inducible PNMT expression in a mouse pheochromocytoma model. Marian J. Evinger a, James F. Powers b and Arthur S. Tischler b a. Department
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb3399 a b c d FSP DAPI 5mm mm 5mm 5mm e Correspond to melanoma in-situ Figure a DCT FSP- f MITF mm mm MlanaA melanoma in-situ DCT 5mm FSP- mm mm mm mm mm g melanoma in-situ MITF MlanaA mm mm
DOI:.38/ncb3399 a b c d FSP DAPI 5mm mm 5mm 5mm e Correspond to melanoma in-situ Figure a DCT FSP- f MITF mm mm MlanaA melanoma in-situ DCT 5mm FSP- mm mm mm mm mm g melanoma in-situ MITF MlanaA mm mm
(Stem) cells to treat type 1 diabetes
 (Stem) cells to treat type 1 diabetes Prof. Robert Hilbrands Diabetologist - Nephrologist Diabeteskliniek UZBrussel Type 1 diabetes Insulitis = inflammatory infiltrate involving Ilsets of Langerhans *Willy
(Stem) cells to treat type 1 diabetes Prof. Robert Hilbrands Diabetologist - Nephrologist Diabeteskliniek UZBrussel Type 1 diabetes Insulitis = inflammatory infiltrate involving Ilsets of Langerhans *Willy
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization
 Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Cell Reports, Volume 9 Supplemental Information VEGFR2-Mediated Vascular Dilation as a Mechanism of VEGF-Induced Anemia and Bone Marrow Cell Mobilization Sharon Lim, Yin Zhang, Danfang Zhang, Fang Chen,
Fructose Upregulates FGF23 Expression In MC3T3 Pre-osteoblasts
 Fructose Upregulates FGF23 Expression In MC3T3 Pre-osteoblasts Edek A. Williams, B.S.E., Veronique Douard, Ph.D., Joseph M. Lomuti, B.S., Ronaldo Ferraris, Ph.D., J. C. Fritton, Ph.D.. Rutgers University,
Fructose Upregulates FGF23 Expression In MC3T3 Pre-osteoblasts Edek A. Williams, B.S.E., Veronique Douard, Ph.D., Joseph M. Lomuti, B.S., Ronaldo Ferraris, Ph.D., J. C. Fritton, Ph.D.. Rutgers University,
Hepatogenesis I Liver development
 Hepatogenesis I Liver development HB 308 George Yeoh Room 2.59 MCS Building yeoh@cyllene.uwa.edu.au Topics Early liver development Tissue interaction - role of morphogens and cytokines Liver enriched transcription
Hepatogenesis I Liver development HB 308 George Yeoh Room 2.59 MCS Building yeoh@cyllene.uwa.edu.au Topics Early liver development Tissue interaction - role of morphogens and cytokines Liver enriched transcription
SUPPLEMENTARY INFORMATION
 Suppl. Fig. 1 in vivo expression of ISL1 in the human fetal heart. a, Hematoxylin eosin staining showing structures of left atrium and left atrium appendage (*) of a human fetal heart at 11 weeks of gestation.
Suppl. Fig. 1 in vivo expression of ISL1 in the human fetal heart. a, Hematoxylin eosin staining showing structures of left atrium and left atrium appendage (*) of a human fetal heart at 11 weeks of gestation.
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2638 Figure S1 Morphological characteristics of fetal testes and ovaries from 6.5-20 developmental weeks. Representative images of Hematoxylin and Eosin staining of testes and ovaries over
DOI: 10.1038/ncb2638 Figure S1 Morphological characteristics of fetal testes and ovaries from 6.5-20 developmental weeks. Representative images of Hematoxylin and Eosin staining of testes and ovaries over
Report on Pathology. Study: The effect of Compound X on pancreatic islets in rhesus macaques
 Report on Pathology Study: The effect of Compound X on pancreatic islets in rhesus macaques Prepared for: Client Name Client Address January 1, 2013 Prepared by: Charter Preclinical Services 21 Main St.,
Report on Pathology Study: The effect of Compound X on pancreatic islets in rhesus macaques Prepared for: Client Name Client Address January 1, 2013 Prepared by: Charter Preclinical Services 21 Main St.,
ENERGY FROM INGESTED NUTREINTS MAY BE USED IMMEDIATELY OR STORED
 QUIZ/TEST REVIEW NOTES SECTION 1 SHORT TERM METABOLISM [METABOLISM] Learning Objectives: Identify primary energy stores of the body Differentiate the metabolic processes of the fed and fasted states Explain
QUIZ/TEST REVIEW NOTES SECTION 1 SHORT TERM METABOLISM [METABOLISM] Learning Objectives: Identify primary energy stores of the body Differentiate the metabolic processes of the fed and fasted states Explain
GLP-2 (Rat) ELISA. For the quantitative determination of glucagon-like peptide 2 (GLP-2) in rat serum and plasma
 GLP-2 (Rat) ELISA For the quantitative determination of glucagon-like peptide 2 (GLP-2) in rat serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 48-GP2RT-E01
GLP-2 (Rat) ELISA For the quantitative determination of glucagon-like peptide 2 (GLP-2) in rat serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 48-GP2RT-E01
Data Sheet. NFAT Reporter (Luc) Jurkat Cell line Catalog #: 60621
 Data Sheet NFAT Reporter (Luc) Jurkat Cell line Catalog #: 60621 Background The nuclear factor of activator T cells (NFAT) family of transcription factors plays an important role in immune response. T
Data Sheet NFAT Reporter (Luc) Jurkat Cell line Catalog #: 60621 Background The nuclear factor of activator T cells (NFAT) family of transcription factors plays an important role in immune response. T
Magnetic Resonance Imaging of Transplanted Mouse Islets Labeled With Chitosan-Coated Superparamagnetic Iron Oxide Nanoparticles
 Magnetic Resonance Imaging of Transplanted Mouse Islets Labeled With Chitosan-Coated Superparamagnetic Iron Oxide Nanoparticles J.-H. Juang, J.-J. Wang, C.-R. Shen, C.-H. Kuo, Y.-W. Chien, H.-Y. Kuo, Z.-T.
Magnetic Resonance Imaging of Transplanted Mouse Islets Labeled With Chitosan-Coated Superparamagnetic Iron Oxide Nanoparticles J.-H. Juang, J.-J. Wang, C.-R. Shen, C.-H. Kuo, Y.-W. Chien, H.-Y. Kuo, Z.-T.
Regenerative Medicine for Cardiomyocytes
 Regenerative Medicine Regenerative Medicine for JMAJ 47(7): 328 332, 2004 Keiichi FUKUDA Assistant Professor, Institute for Advanced Cardiac Therapeutics, Keio University School of Medicine Abstract: Heart
Regenerative Medicine Regenerative Medicine for JMAJ 47(7): 328 332, 2004 Keiichi FUKUDA Assistant Professor, Institute for Advanced Cardiac Therapeutics, Keio University School of Medicine Abstract: Heart
Gladstone Institutes, University of California (UCSF), San Francisco, USA
 Fluorescence-linked Antigen Quantification (FLAQ) Assay for Fast Quantification of HIV-1 p24 Gag Marianne Gesner, Mekhala Maiti, Robert Grant and Marielle Cavrois * Gladstone Institutes, University of
Fluorescence-linked Antigen Quantification (FLAQ) Assay for Fast Quantification of HIV-1 p24 Gag Marianne Gesner, Mekhala Maiti, Robert Grant and Marielle Cavrois * Gladstone Institutes, University of
Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO
 Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO Mice. WT mice and KHK-A/C KO mice were provided drinking water containing 10% glucose or tap water with normal chow ad
Supplementary Figure S1. Effect of Glucose on Energy Balance in WT and KHK A/C KO Mice. WT mice and KHK-A/C KO mice were provided drinking water containing 10% glucose or tap water with normal chow ad
The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells
 1 SUPPLEMENTARY INFORMATION The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells Karin Loser 1,2,6, Thomas Vogl 2,3, Maik Voskort 1, Aloys
1 SUPPLEMENTARY INFORMATION The toll-like receptor 4 ligands Mrp8 and Mrp14 play a critical role in the development of autoreactive CD8 + T cells Karin Loser 1,2,6, Thomas Vogl 2,3, Maik Voskort 1, Aloys
Chief of Endocrinology East Orange General Hospital
 Targeting the Incretins System: Can it Improve Our Ability to Treat Type 2 Diabetes? Darshi Sunderam, MD Darshi Sunderam, MD Chief of Endocrinology East Orange General Hospital Age-adjusted Percentage
Targeting the Incretins System: Can it Improve Our Ability to Treat Type 2 Diabetes? Darshi Sunderam, MD Darshi Sunderam, MD Chief of Endocrinology East Orange General Hospital Age-adjusted Percentage
Anti-ceramide Antibody Prevents the Radiation GI Syndrome in Mice
 Anti-ceramide Antibody Prevents the Radiation GI Syndrome in Mice Jimmy A. Rotolo 1, Branka Stancevic 1, Jianjun Zhang 1, Guoqiang Hua 1, John Fuller 1, Xianglei Yin 1, Adriana Haimovitz-Friedman 2, Kisu
Anti-ceramide Antibody Prevents the Radiation GI Syndrome in Mice Jimmy A. Rotolo 1, Branka Stancevic 1, Jianjun Zhang 1, Guoqiang Hua 1, John Fuller 1, Xianglei Yin 1, Adriana Haimovitz-Friedman 2, Kisu
The current state-of-the-art for achieving longterm. Leptin Administration Enhances Islet Transplant Performance in Diabetic Mice
 ORIGINAL ARTICLE Leptin Administration Enhances Islet Transplant Performance in Diabetic Mice Heather C. Denroche, 1 Whitney L. Quong, 1 Jennifer E. Bruin, 1 Eva Tudurí, 1 Ali Asadi, 1 Maria M. Glavas,
ORIGINAL ARTICLE Leptin Administration Enhances Islet Transplant Performance in Diabetic Mice Heather C. Denroche, 1 Whitney L. Quong, 1 Jennifer E. Bruin, 1 Eva Tudurí, 1 Ali Asadi, 1 Maria M. Glavas,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
The Effects of Exenatide on the Autoimmune Development of Diabetes. A Senior Honors Thesis
 The Effects of Exenatide on the Autoimmune Development of Diabetes A Senior Honors Thesis Presented in Partial Fulfillment of the Requirements for Graduation with Distinction in Microbiology in the Undergraduate
The Effects of Exenatide on the Autoimmune Development of Diabetes A Senior Honors Thesis Presented in Partial Fulfillment of the Requirements for Graduation with Distinction in Microbiology in the Undergraduate
Chapter 15 Pancreatic Differentiation from Human Pluripotent Stem Cells
 Chapter 15 Pancreatic Differentiation from Human Pluripotent Stem Cells Nicholas Vinckier, Jinzhao Wang, and Maike Sander Abstract The ability to produce human pancreatic cells in vitro would open new
Chapter 15 Pancreatic Differentiation from Human Pluripotent Stem Cells Nicholas Vinckier, Jinzhao Wang, and Maike Sander Abstract The ability to produce human pancreatic cells in vitro would open new
Supplementary Figure 1: Steviol and stevioside potentiate TRPM5 in a cell-free environment. (a) TRPM5 currents are activated in inside-out patches
 Supplementary Figure 1: Steviol and stevioside potentiate TRPM5 in a cell-free environment. (a) TRPM5 currents are activated in inside-out patches during application of 500 µm Ca 2+ at the intracellular
Supplementary Figure 1: Steviol and stevioside potentiate TRPM5 in a cell-free environment. (a) TRPM5 currents are activated in inside-out patches during application of 500 µm Ca 2+ at the intracellular
Suppl Video: Tumor cells (green) and monocytes (white) are seeded on a confluent endothelial
 Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
ENABLING TECHNOLOGIES FOR CELL-BASED CLINICAL TRANSLATION
 Enabling Technologies for Cell-Based Clinical Translation ENABLING TECHNOLOGIES FOR CELL-BASED CLINICAL TRANSLATION Concise Review: Markers for Assessing Human Stem Cell-Derived Implants as b-cell Replacement
Enabling Technologies for Cell-Based Clinical Translation ENABLING TECHNOLOGIES FOR CELL-BASED CLINICAL TRANSLATION Concise Review: Markers for Assessing Human Stem Cell-Derived Implants as b-cell Replacement
Studying pancreas development and diabetes using human pluripotent stem cells
 Editorial Studying pancreas development and diabetes using human pluripotent stem cells David W. Scoville, Anton M. Jetten Cell Biology Group, Immunity, Inflammation, and Disease Laboratory, National Institute
Editorial Studying pancreas development and diabetes using human pluripotent stem cells David W. Scoville, Anton M. Jetten Cell Biology Group, Immunity, Inflammation, and Disease Laboratory, National Institute
