Hypothyroidism impairs human stem cell-derived pancreatic progenitor cell maturation in mice
|
|
|
- Sydney Bridges
- 5 years ago
- Views:
Transcription
1 Page 1 of 8 Hypothyroidism impairs human stem cell-derived pancreatic progenitor cell maturation in mice Jennifer E Bruin 1, Nelly Saber 1, Shannon O Dwyer 1, Jessica K Fox 1, Majid Mojibian 1, Payal Arora 2, Alireza Rezania 2, Timothy J Kieffer 1,3 1 Laboratory of Molecular and Cellular Medicine, Department of Cellular & Physiological Sciences, Life Sciences Institute, University of British Columbia, Vancouver, BC, Canada 2 BetaLogics Venture, Janssen R & D LLC, Raritan, NJ, USA 3 Department of Surgery, University of British Columbia, Vancouver, BC, Canada Authors contributed equally to this work. Address correspondence to: Dr. Timothy Kieffer Rm Health Sciences Mall University of British Columbia Vancouver, BC V6T 1Z3 T: (6) F: (6) tim.kieffer@ubc.ca Abstract word count: 2 Main text word count: 87 1 Publish Ahead of Print, published online January 6, 216
2 Page 2 of 8 Abstract Pancreatic progenitors derived from human embryonic stem cells (hescs) are a potential source of transplantable cells for treating diabetes and are currently being tested in clinical trials. However, it remains unclear how the milieu of pancreatic progenitor cells, including exposure to different factors following transplant, may influence their maturation. Here, we examined the impact of thyroid dysregulation on the development of hesc-derived progenitor cells in vivo. Hypothyroidism was generated in SCID-beige mice using an iodine-deficient diet containing.15% propyl-2-thiouracil, and hyperthyroidism was generated by addition of L-thyroxine (T) to drinking water. All mice received macro-encapsulated hesc-derived progenitor cells and thyroid dysfunction was maintained either for the duration of the study ( chronic ) or for -weeks post-transplant ( acute ). Acute hyperthyroidism did not affect graft function, but acute hypothyroidism transiently impaired human C-peptide secretion at 16 weeks post-transplant. Chronic hypothyroidism resulted in severely blunted basal human C- peptide secretion, impaired glucose-stimulated insulin secretion, and elevated plasma glucagon levels. Grafts from chronic hypothyroid mice contained fewer β-cells, heterogenous MAFA expression, and increased glucagon+ and ghrelin+ cells. Taken together, these data suggest that long-term thyroid hormone deficiency may drive the differentiation of pancreatic progenitor cells towards α- and ε-cell lineages at the expense of β-cell formation. 2
3 Page 3 of 8 Introduction Transplantation of cadaveric human β-cells can restore insulin-independence in patients with type 1 diabetes (T1D (1, 2)), but is not widely available to most patients due to the inadequate supply of donor cells and burden of immunosuppression. Pluripotent stem cells are a highly scalable alternative cell source (3) and we have previously demonstrated that human stem cell-derived pancreatic progenitor cells can reverse hyperglycemia in mouse models of streptozotocin (STZ)-induced T1D (-6), and high fat diet-induced type 2 diabetes (7). However, glucose-responsive human insulin secretion was only achieved after a lengthy cell maturation period in vivo and it remains unclear how environmental factors within the host may impact this maturation process. Viacyte Inc. has initiated phase 1/2 clinical trials involving transplant of macroencapsulated human embyronic stem cell (hesc)-derived pancreatic progenitor cells into patients with T1D. Thus, it is important to understand how variability in the physiology of transplant recipients may impact the development of progenitor cells in vivo. Patients with diabetes have a significantly higher risk of developing thyroid disease than the general population (8). Up to one-third of patients with T1D also have thyroid dysfunction, which can exacerbate the impaired metabolic control and complications associated with diabetes, particularly when the thyroid disorder is undetected (8). Moreover, there is evidence to suggest that excessive or deficient levels of thyroid hormones may impact β-cell development and function. Maternal hypothyroidism caused impaired insulin secretion in neonatal rats, as well as glucose intolerance and β-cell dysfunction in adult offspring (9). Systemic knockout of Dio3 (the enzyme required for intracellular inactivation of thyroid hormones), caused significantly reduced islet area and pancreatic insulin content compared to 3
4 Page of 8 wild-type mice at birth, as well as glucose intolerance and impaired insulin secretion during adulthood (1). Triiodothyronine (T3) has also been shown to have pro-survival effects on adult β-cells by protecting mice from STZ-induced β-cell death and diabetes (11). Aguayo- Mazzucato and colleagues demonstrated that daily T3 injections from postnatal days 1-7 in rats increased expression and nuclear localization of Mafa, a transcription factor essential for β-cell maturation, whereas inhibition of postnatal thyroid hormone synthesis decreased Mafa levels in neonatal rat islets (12). Moreover, treatment of isolated neonatal rat islets with T3 in vitro induced glucose-stimulated insulin secretion, an effect that was blocked in the presence of dominant negative-mafa, suggesting that the effects of T3 on β-cell maturation are via Mafa regulation (12). Consistent with these findings, addition of T3 to differentiating hescs increased gene expression of INS and MAFA, and led to improved glucose-stimulared insulin secretion (13). Taken together, these studies suggest that thyroid hormone signaling may play an important role in β-cell development, maturation, survival, and maintenance of adult β-cell function. Here, we investigated the effects of thyroid hormone dysregulation on the maturation of encapsulated hesc-derived pancreatic progenitor cells in vivo. We hypothesized that hyperthyroidism may accelerate the development of hesc-derived pancreatic progenitor cells into mature insulin-secreting cells, whereas hypothyroidism may hinder the maturation process. Our findings indicate that chronic hypothyroidism impairs the development of hescderived β-cells in vivo, and instead promotes the formation of α- and ε-cells from pancreatic progenitor cells. Short-term exposure to hyperthyroidism for weeks post-transplant did not impact the development of glucose-dependent human insulin production from pancreatic progenitor cells in vivo.
5 Page 5 of 8 Research Design and Methods In Vitro Differentiation of hescs and Assessment of Pancreatic Progenitor Cells The H1 hesc line was obtained from WiCell Research Institute, Inc. (Madison, WI). All experiments at the University of British Columbia (UBC) with H1 cells were approved by the Canadian Stem Cell Oversight Committee and UBC Clinical Research Ethics Board. Pluripotent H1 cells were differentiated into pancreatic progenitor cells for transplantation studies according to a 1-day, -stage protocol as previously described (). Expression of key pancreatic progenitor cell markers was assessed prior to transplant using flow cytometry, as previously described (5); antibody information is provided in Supplementary Table 1. To determine the effect of T3 on hesc development in vitro, H1 cells were differentiated according to our recently published protocol (13), and T3 was added during Stage (S; 5 nm or 1 nm final concentration). Differentiated cells were assessed by qpcr on S, day 3 (SD3), S5D3 and S6D3, as described below. Animals Male 7-8 week old SCID-beige mice (C.B-Igh-1b/GbmsTac-Prkdc scid -Lyst bg N7; Taconic, Hudson, NY) were maintained on a 12h light/dark cycle throughout the study. The first cohort of mice (7-8 weeks of age) was used to characterize the different models of thyroid dysregulation: chronic hypothyroid (n=1), chronic hyperthyroid (n=1), and euthyroid (n=1); the treatment protocol is summarized in Fig. 1A and described in detail below. A subset of mice from this cohort was subsequently used for transplantation, as summarized in Fig. 2A. The chronic hypothyroid group was maintained on an iodine-deficient diet (n=8) and 5
6 Page 6 of 8 the euthyroid group was divided into two subgroups, euthyroid (A; n=5) and acute hypothyroid (n=). A second group of mice (8 weeks of age) was used for a new hyperthyroid cohort: acute hyperthyroid (n=8) versus euthyroid (B; n=8); see Fig. 2A. Euthyroid groups A and B were analyzed separately for blood glucose and body weight tracking, but subsequently combined for all further analysis. All experiments were approved by the UBC Animal Care Committee and carried out in accordance with the Canadian Council on Animal Care guidelines. Diets and L-Thyroxine Administration All mice received ad libitum access to a standard irradiated diet (Teklad Diet #2918; Harlan Laboratories, Madison, WI, USA) to allow for acclimatization following their arrival at UBC. At weeks old, mice from the first cohort were randomly selected to undergo one of the following treatments (summarized in Fig. 1A): 1) hypothyroid: iodine-deficient diet with.15% propylthiouracil (PTU; Cat # TD.8259; Harlan Laboratories, Madison, WI) and normal drinking water; 2) euthyroid: iodine control diet (Cat # TD.826; Harlan Laboratories) and normal drinking water; or 3) hyperthyroid: iodine control diet (Cat # TD.826; Harlan Laboratories) and drinking water containing various concentrations of L- thyroxine sodium salt pentahydrate (T, Cat # T251, Sigma Aldrich; a) 12 mg/l: 5% PBS, 5% ddh 2 O; b) 6 mg/l: 25% PBS, 75% ddh 2 O; c) 3 mg/l 12.5% PBS, 87.5% ddh 2 O). For the mice that received cell transplants, five treatment groups were followed for 18 days post-transplant (summarized in Fig. 2A). Euthyroid mice (A and B) and the acute hyperthyroid mice received the iodine control diet for the duration of the study. The acute 6
7 Page 7 of 8 hyperthyroid group received T drinking water (3 mg/l) for 1 week before and weeks following transplantation. The acute hypothyroid mice received the iodine-deficient diet with.15% PTU for weeks following transplantation and the chronic hypothyroid mice received the iodine-deficient diet for the duration of the study. Transplantation of hesc-derived Pancreatic Progenitor Cells Euthyroid (A), chronic and acute hypothyroid mice received hesc-derived pancreatic progenitor cell transplants at 2-21 weeks of age (after 8-9 weeks of treatment regimes; Fig. 2A). Euthyroid (B) and acute hyperthyroid mice received cell transplants at 1 weeks of age (Fig. 2A). All mice were anaesthetized with inhalable isoflurane and ~5x1 6 hesc-derived pancreatic progenitor cells were transplanted subcutaneously (s.c.) within a 2 µl Theracyte TM macroencapsulation device (TheraCyte Inc., Laguna Hills, CA) on the right flank, as previously described (). Metabolic Assessments All metabolic analyses were performed in conscious, restrained mice and blood samples were collected via saphenous vein at the indicated time points. Specific assays used to measure plasma analytes and detailed protocols for metabolic testing are described in the Supplementary Data. Quantitative RT-PCR Theracyte TM devices were harvested at 27 weeks post-transplant from all mice for qpcr analysis. Devices were cut longitudinally and one half was stored in RNAlater Stabilization 7
8 Page 8 of 8 Solution (Life Technologies, Carlsbad, CA) at -8 C until use (the other half was stored in % PFA, as described below). The procedure for isolating RNA from engrafted tissue within devices and qpcr analysis has been described in detail elsewhere (7). Data were analyzed using Expression Suite software (v1..3, Thermo Fisher Scientific/Life Technologies) and normalized to adult human islets (n=2 donors) using the Ct method. Gene expression in cultured hesc-derived cells was assessed as previously described (5) (n=2 biological replicates per condition). A list of primers is provided in Supplementary Table 2. Immunofluorescent staining and image quantification Engrafted hesc-derived cells (half of Theractye TM device), pancreas tissue, and thyroid glands were harvested at 27 weeks post-transplant, fixed in % PFA, and stored in 7% EtOH prior to paraffin-embedding. All paraffin sections (5 µm thickness) were prepared by Wax-it Histology Services (Vancouver, BC). Immunofluorescent staining and imaging was performed as previously described (1), and primary antibody information is provided in Supplementary Table 3. Refer to Supplementary Data for details about quantification of the endogenous pancreas and engrafted tissue. Statistical Analysis All statistics were performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA). Details about individual statistical tests are provided in the Supplementary Data. For all analyses, p<.5 was considered statistically significant. Data are presented as mean ± SEM with individual data points. 8
9 Page 9 of 8 Results Chronic hypothyroidism induced weight loss and hyperglycemia, whereas chronic hyperthyroidism induced hypoglycemia and hyperinsulinemia in SCID-beige mice Our first goal was to establish a protocol to induce hyperthyroidism or hypothyroidism in SCID-beige mice (outlined in Fig. 1A). Initially there were no differences in plasma T3 levels between groups, but T3 levels were significantly reduced following 1 days of feeding with an iodine-deficient diet compared to an iodine control diet (euthyroid) (Fig. 1B). In contrast, the addition of thyroxine (T) to drinking water produced dose-dependent increases in plasma T3 levels. A dose of 12 mg/l for 7 days, followed by 6 mg/l for 7 days produced plasma T3 levels in the hyperthyroid mice that were ~1-15-fold higher than the euthyroid group (Fig. 1B), but much higher than T3 levels reported in humans with clinical hyperthyroidism (euthyroid: 3.3 ±.7 ng/ml; hyperthyroid: 7.1 ± 1. ng/ml (15)). Therefore, the dose of T was further reduced to 3 mg/l to achieve clinically relevant plasma T3 levels (Fig. 1B) and in an effort to prevent the severe hypoglycemia observed with higher T doses (Fig. 1D,E). Mice displayed an initial reduction in body weight following administration of the iodine-deficient diet, which recovered and stabilized after 12 days of treatment, but remained significantly lower than euthyroid controls (Fig. 1C). There was no effect of hyperthyroidism on body weight (Fig. 1C). Hypothyroidism caused significantly elevated blood glucose levels both under fasting conditions (Fig. 1D) and following an oral glucose challenge (Fig. 1E), whereas hyperthyroidism resulted in significantly decreased blood glucose levels (Fig. 1D,E). Hyperthyroidism was also associated with significantly higher fasting plasma insulin levels 9
10 Page 1 of 8 compared to euthyroid controls (Fig. 1F). Unfortunately, after 2 days of T treatment, four of ten hyperthyroid mice died and the remaining mice were consequently switched to normal drinking water, resulting in return to normoglycemia within 2 weeks (Fig. 1D). Thyroid hormone deficiency hinders the development of hesc-derived progenitor cells into mature insulin-secreting cells in vivo We next assessed the effects of either excessive or deficient thyroid hormone levels on the development of hesc-derived pancreatic progenitor cells in vivo. Following the 1-day differentiation in vitro, hesc-derived cells were ~99.5% PDX1-positive and 7% NKX6.1- positive prior to transplant (Supplementary Fig. 1), consistent with previous studies (5-7, 13). The progenitor population also contained ~1% endocrine cells, which co-expressed NKX2.2, but were largely NKX6.1-negative (Supplementary Fig. 1). These pancreatic progenitor cells were transplanted subcutaneously within Theracyte TM devices into mice with thyroid hormone dysregulation (as outlined in Fig. 2A). Our experimental groups enabled us to examine the impact of acute exposure to deficient or excessive thryoid hormone during the first 3 days post-transplant and chronic thyroid hormone deficency for the duration of the study. We chose not to include a chronic hyperthyroid treatment group because even the lowest dose of T tested (3 mg/l) caused dangerous hypoglycemia and fatality (Fig. 1D,E). To validate the efficacy of treatments, plasma T3 levels were measured at 28 days post-transplant (during the acute intervention) and were confirmed to be significantly lower in the acute hypothyroid mice and significantly higher in the acute hyperthyroid group compared to euthyroid controls (Fig. 2B). On day 2 (12 days after the cessation of the acute thyroid interventions), T3 levels were normal in the acute hyper/hypothyroid mice, but remained significantly lower in the chronic 1
11 Page 11 of 8 hypothyroid mice (reflecting their ongoing treatment with iodine-deficient diet; Fig. 2B). As further validation of the model, the thyroid gland was examined at the end of the study. Thyroid weight (normalized to body weight) was significantly increased in both the chronic and acute hypothyroid groups compared to euthyroid, although this was most pronounced in the chronic group (Supplementary Fig. 2A). Moreover, we observed severe thyroid follicular atrophy and absence of colloid in the thyroid gland of mice with chronic hypothyroidism (Supplementary Fig. 2B). Consistent with the first study (Fig. 1), acute hypothyroidism resulted in a transient decrease in body weight, which quickly recovered once the mice were taken off the iodinedeficient diet at 3 days post-transplant (Fig. 2C). The initial weight loss in the chronic hypothyroid group plateaued after ~8 days and body weight remained stable thereafter (Fig. 2C). The reduction in body weight was associated with a significant decrease in fat pad weight (relative to body weight) in the chronic hypothyroid mice at 27 weeks post-transplant (Supplementary Fig. 3). Chronic hypothyroidism also led to elevated fasting blood glucose levels whereas acute hypothyroid mice remained normoglycemic throughout the study (Fig. 2C). Acute hyperthyroidism had no effect body weight, but caused a transient decrease in blood glucose levels during the T treatment period (Fig. 2D), consistent with the previous cohort of mice (Fig. 1D). From this point forward all data from the two euthyroid groups were pooled, as their body weight and blood glucose levels were not significantly different. To assess the development of the hesc-derived grafts under conditions of thyroid dysregulation, human C-peptide and blood glucose levels were measured in response to various secretagogues following transplant. Chronic hypothyroid mice exhibited elevated blood glucose levels during an oral mixed-meal challenge at all ages examined (Fig. 3A,C), as 11
12 Page 12 of 8 well as during i.p. glucose (Fig. 3D) and arginine (Fig. 3G) tolerance tests at 22 and 2 weeks post-transplant, respectively. Mice with acute hyperthyroidism had decreased blood glucose levels following the meal challenge at weeks post-transplant (during their T treatment period; Fig. 3A), but mild hyperglycemia during the meal and glucose challenges at 8 and 22 weeks post-transplant, respectively (Fig. 3C,D). There was no effect of acute hypothyroidism on glycemia during any metabolic challenge (Fig. 3A,C,D,G). Beginning at 8 weeks post-transplant and persisting throughout the study, the chronic hypothyroid mice had significantly decreased human C-peptide levels compared to euthyroid control mice, both under fasting conditions (data not shown) and at minutes following the oral mixed-meal (Fig. 3B). Additionally, mice with acute hypothyroidism had a transient decrease in meal-stimulated human C-peptide levels compared to euthyroid mice at 16 weeks post-transplant, which was recovered by 25 weeks (Fig. 3B). At 22 weeks post-transplant chronic hypothyroid mice exhibited severely blunted human C-peptide secretion during an i.p. glucose challenge (Fig. 3E), and also displayed altered C-peptide secretion kinetics compared to euthyroid mice (Fig. 3F). While peak human C-peptide levels were observed at 6 minutes post-glucose administration in the chronic hypothyroid mice (~2.5-times higher than basal), the other groups had reached similar peak C-peptide levels at 3 minutes and were approaching basal levels by 6 minutes (Fig. 3F). The acute hyperthyroid group also had significantly reduced human C-peptide levels at 3 minutes following the glucose injection (Fig. 3E), but unlike the chronic hypothyroid group they possessed similar human C-peptide secretion kinetics to euthyroid control mice (Fig. 3F). Consistent with the meal and glucose challenges, the chronic hypothyroid group also displayed significantly reduced human insulin levels both at fasting and 15 minutes post-arginine injection compared to euthyroid mice at 2 12
13 Page 13 of 8 weeks post-transplant (Fig. 3H). Interestingly, these mice also had higher plasma glucagon levels post-arginine (Fig. 3I), as well as elevated GLP-1 levels both at fasting and postarginine (Fig. 3J) compared to euthyroid mice. Chronic hypothyroidism affects the endocrine composition of hesc-derived grafts, but not the endogenous pancreas Theracyte devices were harvested at 27 weeks post-transplant for qpcr and histology analysis. Although the chronic hypothyroid group displayed decreased plasma human insulin, and increased plasma glucagon levels (Fig. 3H,I), INS and GCG mrna levels in these hesc-derived grafts were not significantly different from the euthyroid group (Fig. ). However, chronic hypothyroidism resulted in significantly increased levels of SST, GHRL, and ISL1, as well as a pronounced reduction in IAPP and G6PC2 mrna in hesc-derived grafts compared to grafts from euthyroid controls (Fig. ). Acute hyperthyroidism also caused a mild, but significant reduction in graft IAPP and G6PC2, and elevated GCG mrna levels (Fig. ). Given that the most pronounced effects on graft function and gene expresssion were a result of chronic hypothyroidism (Figs 3 and ), we focussed our detailed characterization of graft composition on the chronic hypothyroid versus euthyroid mice. Thyroid hormone deficiency did not impact the overall formation of endocrine (synaptophysin+) or ductal (CK19+) cells from hescs (Fig. 5A), but appeared to promote differentiation towards an α- cell lineage at the expense of β-cells (Fig. 5B,C). Indeed, grafts from chronic hypothyroid mice contained a significantly lower fraction of insulin+ cells and more than twice as many glucagon+ cells compared to euthyroid grafts (Fig. 6A). Thus there was a significantly higher 13
14 Page 1 of 8 ratio of glucagon:insulin immunoreactive cells in hesc-derived grafts from chronic hypothyroid compared to euthyroid mice, whereas the glucagon:insulin ratio in the endogenous pancreas was not affected by chronic hypothyroidism (Fig. 6B). Moreover, thyroid hormone deficiency did not affect the β-cell area per islet in the endogenous pancreas (Fig. 6C). At 27 weeks post-transplant proliferating (PCNA+) endocrine cells were rare and there were no obvious differences in the number of proliferating insulin+ or glucagon+ cells between treatment groups (Supplementary Fig. ). Despite the significant increase in SST transcript levels in chronic hypothyroid grafts (Fig. ), there was no significant difference in the fraction of somatostatin+ cells between groups (quantification not shown; Supplementary Fig. 5). Pancreatic polypeptide (PP) immunoreactivity was rare in grafts and did not appear to differ between groups, although this was not quantified (Supplementary Fig. 5). Interestingly, chronic hypothyroid grafts had an ~8-fold increase in GHRL transcript levels (Fig. ) and a significantly higher proportion of ghrelin+ cells (relative to DAPI+ cells; Figs 5C and 6A) compared to euthyroid grafts. Moreover, while ghrelin+ cells were abundant in the hesc-derived engrafted tissue, they were only rarely detected in the endogenous pancreas (Fig. 6D). Chronic hypothyroid grafts contained approximately equal proportions of ghrelin+ and insulin+ cells, whereas euthyroid grafts had ~ insulin+ cells for every 1 ghrelin+ cell (Fig. 6E). In the pancreas the ratio of ghrelin:insulin immunoreactive cells was not affected by exposure to chronic hypothyroidism (Fig. 6E). There was no significant difference in circulating acylated (active) or unacylated (inactive) ghrelin levels between groups, but the ratio of unacylated:acylated ghrelin was ~2-times higher in the chronic hypothyroid mice compared to euthyroid mice (Fig. 6F). 1
15 Page 15 of 8 Thyroid hormone deficiency affects β-cell maturation in hesc-derived grafts, but not the endogenous pancreas Chronic hypothyroidism resulted in a reduced number of hesc-derived β-cells (Figs 5 and 6), which may explain the decreased absolute human C-peptide levels compared to euthyroid mice (Figs 3B,E,H). However, the disrupted human insulin secretion kinetics in chronic hypothyroid mice (Fig. 3D), suggested that the maturation status of individual hescderived β-cells may also be altered by thyroid hormone deficiency. Based on evidence that islet MAFA expression was regulated by thyroid hormones in neonatal rats (12), we examined expression of MAFA in grafts and the endogenous mouse pancreas. Although MAFA transcript levels were not affected in whole grafts (Fig. ), we observed substantial heterogeneity in nuclear MAFA immunoreactivity within the hesc-derived insulin+ population from chronic hypothyroid mice, but no difference in MAFA expression within the endogenous pancreas of hypothyroid versus euthyroid mice (Fig. 7A). Similarly, hypothyroidism also caused decreased NKX2.2 immunoreactivity in the grafts, but no differences were observed between groups in the endogenous pancreas (Fig. 7B). Additionally there was less amylin immunoreactivity within the insulin+ cell population in the hypothyroid compared to euthyroid grafts (Fig. 7C), confirming the significant reduction in overall gene expression of IAPP in hypothyroid grafts (Fig. ); amylin immunoreactivity was not affected in the β-cells from the endogenous pancreas (Fig. 7C). HESC-derived pancreatic progenitor cells were also treated in vitro with T3 during Stage to determine if the effects on graft maturation in vivo might be a result of direct action by thyroid hormones. Consistent with our in vivo study, we observed reduced GHRL, GCG, and ARX mrna during Stage 5 following treatment with either 5 nm or 1 nm T3 15
16 Page 16 of 8 during Stage (Supplementary Fig. 6). T3 treatment also increased mrna levels of INS and mature β-cell markers, G6PC2, IAPP, and MAFA during Stage 6 (Supplementary Fig. 6). Discussion The high incidence of thyroid disease in patients with T1D (8) means that hescderived pancreatic progenitor cells transplanted into these patients may be exposed to abnormal thyroid hormone levels in vivo. Notably, we found that chronic thyroid hormone deficiency had a detrimental impact on hesc-derived β-cell development and appeared to promote the differentiation of pancreatic progenitor cells towards α- and ε-cell lineages at the expense of β-cell formation. Beginning at 8 weeks post-transplant (and persisting throughout the study), grafts from chronic hypothyroid mice secreted less than half as much human C- peptide than grafts from euthyroid mice. Blunted human C-peptide secretion was also observed in mice acutely exposed to hypothyroidism, but this effect was transient and fully recovered at 25 weeks post-transplant. Chronic hypothyroid mice also displayed impaired glucose-stimulated insulin secretion kinetics, suggesting that the maturation status of β-cells was affected by thyroid hormone deficiency. The elevated plasma glucagon and GLP-1 levels in chronic hypothyroid mice pointed to preferential formation of α-cells in hesc-derived grafts exposed to thyroid hormone deficiency. Indeed, grafts harvested from chronic hypothyroid mice contained approximately three-times fewer insulin+ cells and more than twice as many glucagon+ cells as grafts from euthyroid mice. It is unclear from these studies whether thyroid hormone deficiency altered the lineage commitment of differentiating endocrine cells, thus resulting in a fate-switch towards α-cells at the expense of β-cells, or if 16
17 Page 17 of 8 perhaps thyroid hormone is required for survival and/or expansion of newly differentiated β- cells. T3-treated neonatal rats had increased β-cell proliferation but no measureable change in beta cell apoptosis (12). While proliferation of hesc-derived endocrine cells was not affected by thyroid hormone deficiency in our study after 27 weeks, it is possible that impaired β-cell replication and/or increased β-cell apoptosis may have occurred in hypothyroid grafts at an earlier time point. Thyroid hormone deficiency resulted in heterogeneous protein expression of nuclear MAFA as well as reduced NKX2.2 and amylin levels, important markers of mature β-cells. IAPP (amylin) and G6PC2 mrna levels were also significantly reduced in grafts from hypothyroid mice compared to euthyroid controls, whereas the observed differences in insulin+, glucagon+, and MAFA+ cells were not reflected at the level of gene expression. Since mrna levels represent the whole population of hesc-derived cells (including all endocrine cell types, ductal cells etc.), examining protein expression in individual insulin+ cells by immunofluorescent staining is a more accurate assessment of the hesc-derived β-cell phenotype. The various lines of evidence pointing to a β-cell deficiency in hypothyroid mice is consistent with our previous observations in nude rats implanted with hesc-derived progenitor cells (16). Interestingly, nude rats had significantly higher circulating T3 levels than SCID-beige mice, and also had improved glucose-stimulated human insulin secretion, a higher proportion of insulin:glucagon in grafts and more consistent nuclear MAFA expression in hesc-derived β-cells than SCID-beige mice (16). Neonatal rats with hypothyroidism also exhibited decreased islet MAFA expression (12), which is consistent with our findings and suggests a potential role for thyroid hormone in regulating β-cell maturation. This is further supported by evidence that thyroid hormones bind directly to the thyroid hormone response 17
18 Page 18 of 8 elements in the Mafa promoter to induce Mafa expression and promote glucose-stimulated insulin secretion in immature neonatal rat islets (12). Ghrelin production by hesc-derived grafts also proved to be interesting in this study. Grafts from euthyroid mice contained substantially higher (2X) ghrelin mrna levels compared to human islets and a :1 ratio of insulin:ghrelin immunoreactive cells, whereas ghrelin+ cells were exceedingly rare (<.5%) in the adult human and mouse pancreas. This disprepancy was even further amplified in the chronic hypothyroid grafts, which contained ~125X more ghrelin mrna than human islets and approximately a 1:1 ratio of insulin:ghrelin immunoreactive cells. In human fetal pancreas ghrelin+ cells constitute a relatively high proportion (~1%) of endocrine cells (17). Therefore the high ghrelin levels in chronic hypothyroid grafts could reflect graft immaturity or may simply indicate a shift in lineage commitment during development. Grafts from chronic hypothyroid mice also contained less NKX2.2 immunoreactivity, which was previously shown to be required for specification and maintenance of β-cell fate (18). Moreover, the absence of NKX2.2 in mice resulted in a dramatic expansion of ghrelin-producing cells at the expense of β-cells (18). Interestingly, patients with hypothyroidism were reported to have elevated serum ghrelin levels (acylated and unacylated), which was normalized by thyroxine treatment (19). Although the chronic hypothyroid mice in our study did not produce elevated circulating ghrelin levels, there was clearly an effect of thyroid hormone deficiency to induce ghrelin locally within hesc-derived grafts. Pancreatic ghrelin serves as a local regulator of insulin release even though it may not contribute to the circulating ghrelin levels (2). Therefore, ghrelin may be acting in a paracrine manner to impair insulin secretion in the hesc-derived grafts from chronic hypothyroid mice. 18
19 Page 19 of 8 Although thyroid hormone deficiency caused clear effects on the transplanted human pancreatic progenitor cells, the endogenous pancreas appeared to be largely unaffected in our study. It is possible that developing endocrine cells are more susceptible to thyroid hormone dysregulation than adult islet cells or that human pancreatic cells are more susceptible than mouse cells, although we suspect the former is more likely. It is also possible that the effects on differentiation of hesc-derived cells are due to the profoundly disrupted metabolic control in the mice with hyper- or hypothyroidism. However, we have previously observed efficient graft maturation and robust human C-peptide production in the setting of chronic hyperglycemia (), and thus feel that the impaired glycemic control in hypothyroid mice is unlikely to be the underlying cause of impaired maturation in the current study. Thyroid hormones are also known to profoundly affect the stress response in vivo (12), which could also indirectly mediate the observed effects of hypothyroidism on β-cell maturation. Interestingly, dexamethasone treatment of neonatal rat islets blocked the T3-mediated induction of Mafa mrna and glucose-stimulated insulin secretion ex vivo (12). Our previous discovery that T3 treatment at late stages of hesc differentiation enhanced β-cell maturation in culture (13) supports the notion that the hesc-derived grafts may be directly affected by thyroid hormone levels. Moreover, when Stage pancreatic progenitor cells were treated with T3 in vitro, we observed a transient decrease in GHRL, GCG, and ARX mrna at Stage 5, and elevated INS, G6PC2, IAPP, and MAFA mrna levels at Stage 6 of in vitro differentiation. These data are consistent with our in vivo study, in which reduced thyroid hormone levels caused increased α- and ε-cell formation along with impaired β-cell maturation in hescderived grafts (including reduced G6PC2 and IAPP mrna). Further studies are required to determine whether thyroid hormones are acting directly on developing human β-cells or if the 19
20 Page 2 of 8 indirect effects of thyroid hormones on metabolism and/or the hypothalamic-pituitary-adrenal (HPA) axis may be mediating the observed changes in β-cell development in vivo. Clinically, hyperthyroidism can lead to elevated blood glucose levels, possibly due to enhanced gluconeogenesis and increased absorption of sugars from the intestine (21), whereas the opposite typically occurs with hypothyroidism. Interestingly, we observed contradictory effects of thyroid hormones on blood glucose homeostasis in SCID-beige mice. Hyperthyroidism led to hypoglycemia whereas hypothyroidism caused hyperglycemia compared to euthyroid controls. Hypothyroidism also caused decreased body weight in mice whereas in humans hypothyroidism generally leads to weight gain. Although our data differs from the clinical situation, these findings are consistent with previous studies examining hyperthyroidism and hypothyroidism in rodents. For example, rodents that received subcutaneous T3 injections had lowered fasting glucose levels compared to controls (12, 22), and hypothyroidism in rats (induced via thyroidectomy and PTU or methimazole treatment) caused decreased body weight (12, 23) and increased serum glucose levels (23). The differences between human and rodent thyroid physiology that account for these opposing phenotypes are currently unknown. Stem cell-derived pancreatic progenitor cells are currently being transplanted into patients with T1D in a phase 1/2 clinical trial with Viacyte. Our findings raise the possibility that the maturation and ultimate function of the transplanted cells can be influenced by the hormononal and metabolic milieu of the cells. Specifically, we recommend that eligible patients are screened for thyroid dysfunction and treated accordingly to reduce the risk of offtarget cell differentiation leading to compromised graft function. Alternatively, hescs could be differentiated to a more mature stage of development in vitro as we have recently described 2
21 Page 21 of 8 (13), to minimize the maturation period following transplantation. It is possible that more mature hesc-derived cells will be less susceptible to altered levels of thyroid hormones because they are closer to adult cells, although this remains to be examined. Author Contributions J.E.B. and N.S. wrote the manuscript. J.E.B., A.R. and T.J.K. contributed to conception and design of experiments. J.E.B., N.S., S.O., M.M., J.K.F., P.A., A.R., and T.J.K. were responsible for acquisition, analysis and interpretation of data. All authors contributed to manuscript revisions and approved the final version of the manuscript. Acknowledgements This work was funded by the Canadian Institutes of Health Research (CIHR) Regenerative Medicine and Nanomedicine Initiative, Stem Cell Network (SCN) and JDRF. J.E.B. was funded by a JDRF postdoctoral fellowship, a Canadian Association (CDA) postdoctoral fellowship, the CIHR Transplantation Training Program, and a L Oréal Canada for Women in Science Research Excellence Fellowship. N.S. received funding from the Stem Cell Network. We would like to thank Diana Rosman-Balzer (Janssen R&D, LLC) for her technical assistance with qpcr experiments and Ali Asadi (UBC Department of Cellular & Physiological Sciences ) for his assistance with histology. A.R. and P.A. are employees of Janssen R&D, LLC, and A.R. is also a shareholder. T.J.K. received financial support from Janssen R&D, LLC for the research described in this article. There are no other potential conflicts of interest relevant to this article. T.J.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data 21
22 Page 22 of 8 and the accuracy of the data analysis. References 1. Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, Kneteman NM, Warnock GL, Larsen I, and Shapiro AM. 21. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. 5: Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, and Rajotte RV. 2. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 33: Bruin JE, Rezania A, and Kieffer TJ Replacing and safeguarding pancreatic β cells for diabetes. Science Translational Medicine 7:316ps323.. Bruin JE, Rezania A, Xu J, Narayan K, Fox JK, O'Neil JJ, and Kieffer TJ Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia 56: Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O'Neil JJ, Ao Z, Warnock GL, and Kieffer TJ Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. 61: Rezania A, Bruin JE, Xu J, Narayan K, Fox JK, O'Neil JJ, and Kieffer TJ Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells 31: Bruin JE, Saber N, Braun N, Fox JK, Mojibian M, Asadi A, Drohan C, O Dwyer S, Rosman-Balzer DS, Swiss VA, Rezania A, and Kieffer TJ Treating diet-induced diabetes and obesity with human embryonic stem cell-derived pancreatic progenitor cells and antidiabetic drugs. Stem Cell Reports : Kadiyala R, Peter R, and Okosieme OE. 21. Thyroid dysfunction in patients with diabetes: clinical implications and screening strategies. International journal of clinical practice 6: Karbalaei N, Ghasemi A, Hedayati M, Godini A, and Zahediasl S. 21. The possible mechanisms by which maternal hypothyroidism impairs insulin secretion in adult male offspring in rats. Experimental physiology 99:
23 Page 23 of 8 1. Medina MC, Molina J, Gadea Y, Fachado A, Murillo M, Simovic G, Pileggi A, Hernandez A, Edlund H, and Bianco AC The thyroid hormone-inactivating type III deiodinase is expressed in mouse and human beta-cells and its targeted inactivation impairs insulin secretion. Endocrinology 152: Verga Falzacappa C, Mangialardo C, Madaro L, Ranieri D, Lupoi L, Stigliano A, Torrisi MR, Bouche M, Toscano V, and Misiti S Thyroid hormone T3 counteracts STZ induced diabetes in mouse. PLoS One 6:e Aguayo-Mazzucato C, Zavacki AM, Marinelarena A, Hollister-Lock J, El Khattabi I, Marsili A, Weir GC, Sharma A, Larsen PR, and Bonner-Weir S Thyroid hormone promotes postnatal rat pancreatic beta-cell development and glucoseresponsive insulin secretion through MAFA. 62: Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O'Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, and Kieffer TJ. 21. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 32: Asadi A, Bruin JE, and Kieffer TJ Characterization of Antibodies to Products of Proinsulin Processing Using Immunofluorescence Staining of Pancreas in Multiple Species. J Histochem Cytochem 63: Nauman JA, Nauman A, and Werner SC Total and free triiodothyronine in human serum. J Clin Invest 6: Bruin JE, Asadi A, Fox JK, Erener S, Rezania A, and Kieffer TJ Accelerated maturation of human stem cell-derived pancreatic progenitor cells into insulinsecreting cells in immunodeficient rats relative to mice. Stem Cell Reports In Press, published online November Wierup N, Svensson H, Mulder H, and Sundler F. 22. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regulatory peptides 17: Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, and Sussel L. 2. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci U S A 11: Gjedde S, Vestergaard ET, Gormsen LC, Riis AL, Rungby J, Moller N, Weeke J, and Jorgensen JO. 28. Serum ghrelin levels are increased in hypothyroid patients and become normalized by L-thyroxine treatment. J Clin Endocrinol Metab 93: Yada T, Damdindorj B, Rita RS, Kurashina T, Ando A, Taguchi M, Koizumi M, Sone H, Nakata M, Kakei M, and Dezaki K. 21. Ghrelin signalling in beta-cells regulates insulin secretion and blood glucose. Obes Metab 16 Suppl 1:
24 Page 2 of Potenza M, Via MA, and Yanagisawa RT. 29. Excess thyroid hormone and carbohydrate metabolism. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 15: Lin Y, and Sun Z Thyroid hormone potentiates insulin signaling and attenuates hyperglycemia and insulin resistance in a mouse model of type 2 diabetes. British journal of pharmacology 162: Godini A, Ghasemi A, Karbalaei N, and Zahediasl S. 21. The effect of thyroidectomy and propylthiouracil-induced hypothyroidism on insulin secretion in male rats. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 6: Figure Legends Figure 1: Characterization of hyperthyroid and hypothyroid models in immunodeficient SCID-beige mice. A) Schematic of study timeline and treatment groups to assess the effects of hyper- and hypothyroidism in SCID-beige mice. B) Random fed plasma triiodothyronine (T3) levels in mice prior to administration of treatments (day ), mid-hypothyroid treatment (day 1), and mid-hyperthyroid treatment (using T in drinking water at a dose of 12 mg/l (day 7), 6 mg/l (day 1), or 3 mg/l (day 2)). Dashed line indicates the lower limit of detection for the T3 assay. p<.5, t-test (vs euthyroid). C) Body weight and D) blood glucose levels following a -hour morning fast. Red (hypothyroid) and blue (hyperthyroid) bars at bottom of graphs indicate timing and dosage for treatment regimens. p<.5, twoway repeated measures ANOVA with Dunnet post-hoc test (vs euthyroid). E) Blood glucose levels during an oral glucose tolerance test (2 g/kg glucose) at day 22. p<.5, two-way repeated measures ANOVA (vs euthyroid). For area under curve, p<.5, one-way ANOVA (vs euthyroid). F) Mouse insulin levels following a 6-hour morning fast; p<.5, one-way ANOVA. Data are presented as mean ± SEM plus individual biological replicates. 2
25 Page 25 of 8 Figure 2: Metabolic characterization of transplant recipients with thyroid dysfunction. A) Schematic of study timeline and treatment groups used to assess the effects of acute hyperthyroidism and acute or chronic hypothyroidism on the maturation of hesc-derived pancreatic progenitor cells in SCID-beige mice. B) Random fed plasma triiodothyronine (T3) levels in mice at 28 days post-transplant (during acute thyroid treatments) and at 2 days posttransplant (after cessation of acute treatments). Dashed line indicates the lower limit of detection for the T3 assay. p<.5, unpaired two-tailed t-tests (vs euthyroid). C) Tracking of body weight change (relative to day -56, prior to administration of chronic hypothyroid treatment) and blood glucose for euthyroid (group A), and chronic/acute hypothyroid mice before and after transplant. D) Tracking of body weight change (relative to day, prior to administration of acute hyperthyroid treatment) and blood glucose for euthyroid (group B), and acute hyperthyroid mice before and after transplant. C-D) p<.5, two-way repeated measures ANOVA with Dunnet post-hoc for panel C, Bonferonni for panel D (vs euthyroid). Data are presented as mean ± SEM plus individual biological replicates. Figure 3: Maturation and function of hesc-derived pancreatic progenitor cells in mice with thyroid dysfunction. A-B) Blood glucose (panel A) and plasma human C-peptide (panel B) levels minutes following an oral mixed-meal challenge at, 8, 12, 16, and 25 weeks post-transplant. p<.5, one-way ANOVA (vs euthyroid). C-D) Blood glucose levels during an oral mixed-meal challenge at 8 weeks post-transplant (panel C) and an intraperitoneal glucose tolerance test (ipgtt) at 22 weeks post-transplant (panel D). E-F) Plasma human C-peptide levels during the ipgtt at 22 weeks, expressed in ng/ml (panel E) 25
26 Page 26 of 8 or relative to basal levels at time (panel F). C-F) Line graphs: p<.5, two-way repeated measures ANOVA (vs euthyroid). Bar graphs: p<.5, one-way ANOVA (vs euthyroid). G- J) Blood glucose levels (G) and plasma levels of human insulin (H), glucagon (I), and GLP-1 (J) following a -hour morning fast and 15 minutes following an ip arginine challenge at 2 weeks post-transplant. # p<.5, paired t-test ( vs 15 min); p<.5 one-way ANOVA (vs euthyroid). Data are presented as mean ± SEM plus individual biological replicates. Figure : qpcr analysis of hesc-derived grafts from mice with thyroid dysfunction. Theracyte TM devices were harvested at 27 weeks post-transplant for qpcr analysis of key β- cell markers. Gene expression is expressed relative to levels in adult human islets (indicated by dashed red line and orange bars; n = 2 donors). p<.5, p<.1; one-way ANOVA (vs euthyroid for hesc-derived engrafted cells only). Data are presented as mean ± SEM plus individual biological replicates. Figure 5: Immunofluorescent staining for pancreatic endocrine hormones in hescderived grafts and endogenous pancreas from euthyroid or chronic hypothyroid mice at 27 weeks post-transplant. A-B) Low-magnification images of the hesc-derived grafts from mice with euthyroid or chronic hypothyroid treatments showing immunofluorescent staining for: A) endocrine (synaptophysin; red) and ductal (CK19; green) populations, and B) insulin (red) and glucagon (green) cell populations. C) Representative high magnification images of hesc-derived grafts from euthyroid or chronic hypothyroid mice stained for insulin (ins; red), glucagon (gcg; green), or ghrelin (green). D) Images of representative islets in the 26
27 Page 27 of 8 endogenous pancreas stained for insulin (red) and glucagon (green) or ghrelin (green). DAPI nuclear staining is shown in grey for all images. All scale bars = 5 µm. Figure 6: Quantification of the pancreatic endocrine populations in hesc-derived grafts and endogenous pancreas from euthyroid versus chronic hypothyroid mice at 27 weeks post-transplant. A) The number of cells that were immunoreactive (IR) for insulin (ins), glucagon (gcg), or ghrelin, expressed relative to the toal number of DAPI+ cells in the hescderived grafts from euthyroid versus chronic hypothyroid mice. B) The ratio of glucagon+ cells to insulin+ cells in hesc-derived grafts and the endogenous pancreas. C) Average β-cell (i.e. insulin+) area per islet in the endogenous pancreas. D) The total number of cells immunoreactive for insulin or ghrelin that were counted in the hesc-derived grafts versus the endogenous pancreas (every visible ghrelin+ or insulin+ cell was counted within each section). E) The ratio of ghrelin+ to insulin+ cells in the hesc-derived grafts and the endogenous pancreas. F) Plasma levels of active (acylated) and inactive (unacylated) ghrelin, as well as the ratio of unacylated:acylated ghrelin in mice from all treatment groups. A-E) p<.5, two-tailed unpaired t-tests. F) p<.5, one-way ANOVA(vs euthyroid). Data are presented as mean ± SEM plus individual biological replicates. Figure 7: Characterization of β-cells in hesc-derived grafts compared to the endogenous pancreas of euthryoid versus chronic hypothyroid mice at 27 weeks posttransplant. Representative immunofluorescent staining images of Theracyte TM devices containing hesc-derived grafts and islets from the endogenous pancreas for insulin (red) with: A) MAFA (green; insets highlight the heterogeneity of MAFA immunoreactivity in 27
28 Page 28 of 8 chronic hypothyroid group); B) NKX2.2 (green); or C) amylin (green). Images are shown with DAPI nuclear staining (grey) on left or without DAPI on right. All scale bars = 5 µm. 28
29 A Page 29 of 8 B Plasma T3 (ng/ml) Pre-treatment (d) Post-hypothyroid (d1) Plasma T3 (ng/ml) Plasma T3 (ng/ml) Post-hyperthyroid (d7-2) Euthyroid Hypothyroid Hyperthyroid C Body Weight (g) E Blood Glucose (mm) Euthyroid Hypothyroid Hyperthyroid [T]: Iodine-deficient diet: 12 mg/l 6 mg/l 3 mg/l Time (days) Euthyroid Hypothyroid Hyperthyroid Time (mins) Area Under Curve D Blood Glucose (mm) T Dose (mg/l) 12 mg/l 6 mg/l 3 mg/l [T]: Iodine-deficient diet: Time (days) F Mouse Insulin (ng/ml)
30 B 6 Euthyroid Page 3 of 8 Chronic Hypo 5 Acute Hypo Acute Hyper Plasma T3 (ng/ml) Day 28 Day 2 Change in Body Weight (g) Transplant Time (days) Blood Glucose (mm) Transplant Time (days) Euthyroid Chronic Hypo Acute Hypo Change in Body Weight (g) Transplant Time (days) Blood Glucose (mm) Transplant Euthyroid Acute Hyper Time (days)
31 A Page 1531 of 8 C Blood Glucose (mm) Blood Glucose (mm) Weeks Post-Transplant Area Under Curve B D Human C-Peptide (ng/ml) Blood Glucose (mm) Euthyroid Chronic Hypo Acute Hypo Acute Hyper Weeks Post-Transplant Area Under Curve E G Human C-Peptide (ng/ml) Blood Glucose (mm) Time (Minutes) # 3 6 Time (Minutes) p= Time (Minutes) # p=.55 H Human Insulin (ng/ml) Area Under Curve # 6 2 # Time (Minutes) # # I Glucagon (pg/ml) F Human C-Peptide (relative to basal) # # # 3 6 Time (Minutes) 3 6 Time (Minutes) Time (Minutes) # J GLP-1 (pg/ml) # # # Euthyroid Chronic Hypo Acute Hypo Acute Hyper Time (Minutes) # Euthyroid Chronic Hypo Acute Hypo Acute Hyper
32 INS GCG SST GHRL Page 32 of ISL MAFA NKX PAX6 Gene expression (relative to human islets) IAPP ABCC PCSK1 G6PC2 5 PCSK2 15 MNX SLC3A8 8 UCN Euthyroid Chronic Hypothyroid Acute Hypothyroid Acute Hyperthyroid Human Islets
33 Euthyroid Page 33 of 8 A Synaptophysin CK19 DAPI Hypothyroid Euthyroid Hypothyroid Euthyroid Hypothyroid B Insulin Glucagon DAPI C hesc-derived Grafts D Endogenous Pancreas Ins Ghrl DAPI Ins Gcg DAPI Ghrelin DAPI Gcg DAPI Ins DAPI
34 A.6 B 2 C D F # IR Cells / Total DAPI+ Cells # IR Cells Counted Acylated Ghrelin (pg/ml) Ins Gcg Ghrelin Insulin Graft Ghrelin Glucagon:Insulin # IR Cells Counted Unacylated Ghrelin (pg/ml) Graft Insulin Pancreas Pancreas Ghrelin E Ghrelin:Insulin Average Beta Cell Area Per Islet (µm 2, x1) Unacyl:Acyl Ghrelin (pg/ml) Graft Page 3 of 8 Euthyroid Chronic Hypothyroid Pancreas Euthyroid Chronic Hypothyroid Acute Hypothyroid Acute Hyperthyroid
35 Endogenous Pancreas hesc-derived Graft Insulin Amylin DAPI Endogenous Pancreas hesc-derived Graft Insulin NKX2.2 DAPI Endogenous Pancreas hesc-derived Graft Insulin MAFA DAPI Page 35 of 8 Euthyroid Hypothyroid
36 Page 36 of 8 Supplementary data for: Hypothyroidism impairs human stem cell-derived pancreatic progenitor cell maturation in mice Jennifer E Bruin 1, Nelly Saber 1, Shannon O Dwyer 1, Jessica K Fox 1, Majid Mojibian 1, Payal Arora 2, Alireza Rezania 2, Timothy J Kieffer 1,3 1 Department of Cellular and Physiological Sciences, Life Sciences Institute, University of British Columbia, Vancouver, British Columbia, Canada. 2 BetaLogics Venture, Janssen R & D LLC, Raritan, NJ, USA 3 Department of Surgery, University of British Columbia, Vancouver, British Columbia, Canada. Authors contributed equally to this work.
37 Page 37 of 8 Metabolic Assessments Blood glucose levels were measured using a handheld glucometer (Lifescan; Burnaby, Canada). Circulating hormone levels were assessed in plasma using the following ELISA kits: human total triiodothyronine (T3, 2.5-fold dilution of plasma samples; #17; Alpha Diagnostic International, San Antonio, TX, USA), mouse insulin (#8- INSMSU-E1; ALPCO, Salem, NH, USA), human C-peptide (2-fold dilution of plasma samples; #8-CPTHU-E1.1; ALPCO), human unacylated ghrelin (# ; ALPCO), and human acylated ghrelin (#32-516; ALCO). Human insulin, glucagon and GLP-1 levels were measured with a multiplex assay (-fold dilution of plasma samples; #K1516C-1; Meso Scale Discovery, Gaithersburg, MD). Body weight and blood glucose levels were assessed weekly or bi-weekly throughout the study following a -hour morning fast. Glycemic control was assessed in cohort 1 with an oral glucose tolerance test (GTT) following a 6-hour morning fast and administration of glucose by oral gavage (2 g glucose/kg BW, 3% solution; Vétoquinol, Lavaltrie, QC). An intraperitoneal (i.p.) GTT (2 g/kg) was performed in cohort 2 at 22 weeks posttransplant to assess glycemic control and graft function at 3 and 6 minutes post-glucose following an overnight fast. For monthly mixed-meal challenges, mice received an oral gavage of Ensure (8 μl/g body weight; Abbott Laboratories, Abbott Park, Illinois, USA) following a 6-hour morning fast. For arginine tolerance tests (ArgTT), mice received an i.p. injection of arginine (2 g/kg, % solution; Sigma-Aldrich) following a -hour morning fast.
38 Page 38 of 8 Quantification of Immunofluorescent Images Endogenous beta cell area was measured in pancreas tissue (n=7-8 animals per group) by immunostaining for insulin and quantifying the average insulin-positive cell area per islet (considering only islets with >1 beta cells). To quantify the endocrine composition within Theracyte TM devices, the total number of DAPI+ nuclei, as well as the number of cells that were immunoreactive for insulin, glucagon, somatostatin, or ghrelin were counted using the MetaXpress Multi Wavelength Cell Scoring module (Molecular Devices Corporation, Sunnyvale, CA). The total number of immunoreactive cells was expressed relative to the total number DAPI+ cells per device. The number of cells immunoreactive for either glucagon or ghrelin was also expressed relative to the number of insulin immunoreactive cells for both the endogenous pancreas and engrafted cells. Statistical Analysis Two-way repeated measure ANOVAs were performed with either: a) a Dunnet post-hoc test to compare multiple groups to euthyroid controls, or b) a Bonferonni post-hoc test to compare between two groups of mice (Fig. 2D) at different time points. One-way ANOVAs were performed with a Dunnett post-hoc test for multiple comparisons to euthryoid control mice. Plasma T3 levels were assessed by comparing each group to euthyroid mice with an unpaired two-tailed t-test. Paired two-tailed t-tests were used when comparing samples pre- and post-administration of arginine and unpaired twotailed t-tests were used to compare graft and pancreas quantification between chronic
39 Page 39 of 8 hypothyroid versus euthyroid controls. Area under the curve was calculated with y = as the baseline.
40 Page of 8 Supplementary Table 1: Antibody information for FACS. Conjugated antibodies Source / Catalog # Dilution PE Mouse IgG1,k, Isotype Control BD cat # : Alexa Fluor 67 IgG1, Isotype control BD cat# : PE mouse anti-pdx1 BD cat# : Alexa Fluor 67 Ki67 BD cat# :2 PE mouse anti-human Pax6 BD cat# :2 PE mouse anti-nkx6.1 BD cat# : PE mouse anti-human HNF3b/FOXA2 BD cat# :8 PE mouse anti-islet1 BD cat# : PE mouse anti-neurod BD cat#5631 1: Rb Insulin, AF67 Cell Signaling cat# 98S 1:8 Unconjugated antibodies Source / Catalog # Dilution Rb Chromogranin A DAKO Ref# IS52 1:1 Purified Rabbit IgG, k Isotype BD cat# :1 Rb Insulin Cell Signaling cat#31s 1:1 Rb C-peptide Cell Signaling cat# 593S 1:1 Mouse NKX2.2 Developmental Studies Hybridoma Bank University of Iowa 1:1 Purified Mouse IgG, k Isotype BD cat# :5 Mouse NKX6.1 Developmental Studies Hybridoma Bank University of Iowa 1:5 Mouse Glucagon Sigma 1:25
41 Page 1 of 8 Supplementary Table 2: List of primers used for the Applied Biosystems qpcr array. GENE NAME ABCC8 ARX CHGA GCG GHRL G6PC2 IAPP INS ISL1 MAFA NGN3 MNX1 NKX2.2 NKX6.1 PAX6 PCSK1 PCSK2 PDX1 SLC3A8 SST UCN3 PRIMER REFERENCE / SEQUENCE Hs165861_m1 Hs29265_m1 Hs151_m1 Hs17967_m1 Hs17582_m1 Hs159773_m1 Hs16995_m1 Hs355773_m1 Hs158126_m1 Hs165125_s1 Hs367_g1 Hs232128_m1 Hs159616_m1 Hs232355_m1 Hs2871_m1 Hs175619_m1 Hs13737_m1 Hs23683_m1 Hs55183_m1 Hs3561_m1 Hs8699_s1
42 Page 2 of 8 Supplementary Table 3: Antibody information for immunofluorescent staining. ANTIGEN SPECIES SOURCE, CATALOGUE NUMBER DILUTION Amylin Rabbit Abcam, Ab :5 CK19 Mouse Dako, Denmark, M 888 1:2 Glucagon Mouse Sigma-Aldrich, G 265 1:1 Glucagon (D16G1) Rabbit Cell Signaling Technology, 8233S 1:5 Insulin (C27C9) Rabbit Cell Signaling Technology, 31S 1:2 Insulin (Mab1) Mouse Millipore, :2 Insulin (L6B1) Mouse mab Mouse Cell Signaling Technology, 8138S 1:25 Insulin Guinea Pig Thermo Scientific, PA :1 MAFA Rabbit Betalogics (Janssen R&D), LP9872 1:1 NKX2.2 Mouse Developmental Studies Hybridoma Bank; University of Iowa, 7.5A5 1:1 Somatostatin Mouse Beta Cell Biology Consortium, AB1985 1:5 Somatostatin Rabbit Sigma-Aldrich, HPA1972 1:5 Synaptophysin Rabbit Novus Biologicals, NB :5 Trypsin Sheep R&D Systems, AF3586 1:1 Ghrelin Rabbit BioVision, :2 Ghrelin Chicken Abcam, ab :5 Pancreatic polypeptide Goat R&D Systems, AF6297 1:2 Proliferating cell nuclear antigen (PCNA) Mouse BD Biosciences, :1
43 Page 3 of 8 NKX6.1 PDX Synaptophysin NKX FOXA2 PDX1 Chromogranin KI67 PAX PDX OCT3/ ISLET1 Supplementary Figure 1: FACS characterization of hesc-derived pancreatic progenitor cells. After the 1 day in vitro differentiation, the population of hesc-derived cells was characterized by flow cytometry prior to transplantation.
44 A Thyroid Weight (g) / BW (g) B.15.1 Euthyroid Chronic Hypo Acute Hypo Acute Hyper.5. Euthyroid Chronic Hypothyroid Acute Hypothyroid Acute Hyperthyroid Supplementary Figure 2: Thyroid gland characterization. A) Thyroid gland weight (g) normalized to body weight (g) at 27 weeks post-transplant in mice from euthyroid (grey), chronic hypothyroid (red), acute hypothyroid (green), and acute hyperthyroid (blue) treatment groups. p<.5, one-way ANOVA vs euthyroid. B) Hematoxylin & eosin (H&E) staining of thyroid gland sections shows atrophy of thyroid follicles in the chronic hypothyroid group. Data are presented as mean ± SEM plus individual biological replicates. Page of 8
45 Page 5 of 8 Body Weight (g) Fat Pad Weight (g) / BW (g) Euthyroid Chronic Hypothyroid Acute Hypothyroid Acute Hyperthyroid Supplementary Figure 3: Body composition at 27 weeks post-transplant. Body weight (g) and fat pad weight (mesenteric + epididymal + perirenal fat pad weight normalized to body weight; g/g) in mice from euthyroid (grey), chronic hypothyroid (red), acute hypothyroid (green), and acute hyperthyroid (blue) treatment groups. p<.5, one-way ANOVA vs euthyroid. Data are presented as mean ± SEM plus individual biological replicates.
46 Page 6 of 8 A Euthyroid Hypothyroid Glucagon PCNA DAPI Insulin PCNA DAPI B Supplementary Figure : Immunofluorescent staining for proliferating cells in hescderived grafts from euthyroid or chronic hypothyroid mice at 27 weeks post-transplant. A- B) Representative images of the hesc-derived grafts from euthyroid and chronic hypothyroid mice for insulin (panel A; red) or glucagon (panel B; red) and proliferating cell nuclear antigen (PCNA; green). For each graft, the red channel and green channels are shown separately, along with an overlay image. DAPI nuclear staining is shown in grey for all images. All scale bars = 5 μm.
47 Page 7 of 8 A Euthyroid Hypothyroid B Euthyroid Hypothyroid Somatostatin PP DAPI hesc-derived Grafts C Endogenous Pancreas PP DAPI Somatostatin DAPI PP DAPI Somatostatin DAPI Supplementary Figure 5: Immunofluorescent staining for pancreatic endocrine hormones in hesc-derived grafts and endogenous pancreas from euthyroid or chronic hypothyroid mice at 27 weeks post-transplant. A-B) Representative low-magnification (panel A; overlay) and high magnification (panel B; separate channels) images of the hesc-derived grafts from euthyroid and chronic hypothyroid mice for somatostatin (red) and/or pancreatic polypeptide (PP; green). C) Representative images of islets from the endogenous pancreas of euthyroid or chronic hypothyroid mice with immunostaining for either somatostatin (red) or PP (green). DAPI nuclear staining is shown in grey for all images. All scale bars = 5 μm.
Making Mature Human Islet Cells from Stem Cells to Model Disease and Treat Diabetes
 University of British Columbia Departments of Surgery and Cellular & Physiological Sciences Making Mature Human Islet Cells from Stem Cells to Model Disease and Treat Diabetes 2016 International Conference
University of British Columbia Departments of Surgery and Cellular & Physiological Sciences Making Mature Human Islet Cells from Stem Cells to Model Disease and Treat Diabetes 2016 International Conference
Stem Cell Reports Ar ticle
 Stem Cell Reports Ar ticle Treating Diet-Induced Diabetes and Obesity with Human Embryonic Stem Cell-Derived Pancreatic Progenitor Cells and Antidiabetic Drugs Jennifer E. Bruin, 1 Nelly Saber, 1 Natalie
Stem Cell Reports Ar ticle Treating Diet-Induced Diabetes and Obesity with Human Embryonic Stem Cell-Derived Pancreatic Progenitor Cells and Antidiabetic Drugs Jennifer E. Bruin, 1 Nelly Saber, 1 Natalie
Abbreviations: P- paraffin-embedded section; C, cryosection; Bio-SA, biotin-streptavidin-conjugated fluorescein amplification.
 Supplementary Table 1. Sequence of primers for real time PCR. Gene Forward primer Reverse primer S25 5 -GTG GTC CAC ACT ACT CTC TGA GTT TC-3 5 - GAC TTT CCG GCA TCC TTC TTC-3 Mafa cds 5 -CTT CAG CAA GGA
Supplementary Table 1. Sequence of primers for real time PCR. Gene Forward primer Reverse primer S25 5 -GTG GTC CAC ACT ACT CTC TGA GTT TC-3 5 - GAC TTT CCG GCA TCC TTC TTC-3 Mafa cds 5 -CTT CAG CAA GGA
Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro.
 Manuscript EMBO-2015-91058 Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. Holger A Russ, Audrey V Parent, Jennifer J Ringler, Thomas G Hennings, Gopika
Manuscript EMBO-2015-91058 Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. Holger A Russ, Audrey V Parent, Jennifer J Ringler, Thomas G Hennings, Gopika
Supplemental Information. b Cell Aging Markers Have Heterogeneous. Distribution and Are Induced by Insulin Resistance
 Cell Metabolism, Volume 25 Supplemental Information b Cell Aging Markers Have Heterogeneous Distribution and Are Induced by Insulin Resistance Cristina Aguayo-Mazzucato, Mark van Haaren, Magdalena Mruk,
Cell Metabolism, Volume 25 Supplemental Information b Cell Aging Markers Have Heterogeneous Distribution and Are Induced by Insulin Resistance Cristina Aguayo-Mazzucato, Mark van Haaren, Magdalena Mruk,
REGENERATIVE MEDICINE
 REGENERATIVE MEDICINE Enrichment of Human Embryonic Stem Cell-Derived NKX6.1- Expressing Pancreatic Progenitor Cells Accelerates the Maturation of Insulin-Secreting Cells In Vivo ALIREZA REZANIA, a JENNIFER
REGENERATIVE MEDICINE Enrichment of Human Embryonic Stem Cell-Derived NKX6.1- Expressing Pancreatic Progenitor Cells Accelerates the Maturation of Insulin-Secreting Cells In Vivo ALIREZA REZANIA, a JENNIFER
Supplementary Information. Induction of human pancreatic beta cell replication by inhibitors of dual specificity tyrosine regulated kinase
 Journal: Nature Medicine Supplementary Information Induction of human pancreatic beta cell replication by inhibitors of dual specificity tyrosine regulated kinase 1,2 Peng Wang PhD, 1,2 Juan-Carlos Alvarez-Perez
Journal: Nature Medicine Supplementary Information Induction of human pancreatic beta cell replication by inhibitors of dual specificity tyrosine regulated kinase 1,2 Peng Wang PhD, 1,2 Juan-Carlos Alvarez-Perez
NIH Public Access Author Manuscript Diabetologia. Author manuscript; available in PMC 2014 February 01.
 NIH Public Access Author Manuscript Published in final edited form as: Diabetologia. 2013 February ; 56(2): 231 233. doi:10.1007/s00125-012-2788-6. Lipotoxicity impairs incretin signalling V. Poitout 1,2
NIH Public Access Author Manuscript Published in final edited form as: Diabetologia. 2013 February ; 56(2): 231 233. doi:10.1007/s00125-012-2788-6. Lipotoxicity impairs incretin signalling V. Poitout 1,2
Supplementary Table 1. The primers used for quantitative RT-PCR. Gene name Forward (5 > 3 ) Reverse (5 > 3 )
 770 771 Supplementary Table 1. The primers used for quantitative RT-PCR. Gene name Forward (5 > 3 ) Reverse (5 > 3 ) Human CXCL1 GCGCCCAAACCGAAGTCATA ATGGGGGATGCAGGATTGAG PF4 CCCCACTGCCCAACTGATAG TTCTTGTACAGCGGGGCTTG
770 771 Supplementary Table 1. The primers used for quantitative RT-PCR. Gene name Forward (5 > 3 ) Reverse (5 > 3 ) Human CXCL1 GCGCCCAAACCGAAGTCATA ATGGGGGATGCAGGATTGAG PF4 CCCCACTGCCCAACTGATAG TTCTTGTACAGCGGGGCTTG
Cell Therapy for Diabetes: Generating Functional Islets from Human Exocrine Tissue
 Cell Therapy for Diabetes: Generating Functional Islets from Human Exocrine Tissue Kevin Docherty University of Aberdeen ELRIG Drug Discovery 2016 ACC Liverpool 14 th October 2016 Autoimmune destruction
Cell Therapy for Diabetes: Generating Functional Islets from Human Exocrine Tissue Kevin Docherty University of Aberdeen ELRIG Drug Discovery 2016 ACC Liverpool 14 th October 2016 Autoimmune destruction
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature11463 %Sox17(+) 9 8 7 6 5 4 3 2 1 %Sox17(+) #Sox17(+) d2 d4 d6 d8 d1 d12 d14 d18 25 2 15 1 5 Number of Sox17(+) cells X 1 Supplementary Figure 1: Expression of
SUPPLEMENTARY INFORMATION doi:1.138/nature11463 %Sox17(+) 9 8 7 6 5 4 3 2 1 %Sox17(+) #Sox17(+) d2 d4 d6 d8 d1 d12 d14 d18 25 2 15 1 5 Number of Sox17(+) cells X 1 Supplementary Figure 1: Expression of
islets scored 1 week month months
 Supplementary Table 1. Sampling parameters for the morphometrical analyses Time (post- DT) Control mice (age-matched) α-cells mice pancreatic surface (mm 2 ) scored DT-treated mice islets scored mice pancreatic
Supplementary Table 1. Sampling parameters for the morphometrical analyses Time (post- DT) Control mice (age-matched) α-cells mice pancreatic surface (mm 2 ) scored DT-treated mice islets scored mice pancreatic
SUPPLEMENTARY DATA. Supplementary Table 2. Antibodies used for Immunofluoresence. Supplementary Table 3. Real-time PCR primer sequences.
 Supplementary Table 2. Antibodies used for Immunofluoresence. Antibody Dilution Source Goat anti-pdx1 1:100 R&D Systems Rabbit anti-hnf6 1:100 Santa Cruz Biotechnology Mouse anti-nkx6.1 1:200 Developmental
Supplementary Table 2. Antibodies used for Immunofluoresence. Antibody Dilution Source Goat anti-pdx1 1:100 R&D Systems Rabbit anti-hnf6 1:100 Santa Cruz Biotechnology Mouse anti-nkx6.1 1:200 Developmental
Transplantation of Human Pancreatic Endoderm Cells Reverses Diabetes. Post Transplantation in a Prevascularized Subcutaneous Site
 Stem Cell Reports, Volume 8 Supplemental Information Transplantation of Human Pancreatic Endoderm Cells Reverses Diabetes Post Transplantation in a Prevascularized Subcutaneous Site Andrew R. Pepper, Rena
Stem Cell Reports, Volume 8 Supplemental Information Transplantation of Human Pancreatic Endoderm Cells Reverses Diabetes Post Transplantation in a Prevascularized Subcutaneous Site Andrew R. Pepper, Rena
Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h)
 Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h) after feeding. A small slice (~5-1 mm 3 ) was taken
Online Appendix Material and Methods: Pancreatic RNA isolation and quantitative real-time (q)rt-pcr. Mice were fasted overnight and killed 1 hour (h) after feeding. A small slice (~5-1 mm 3 ) was taken
Neurotrophic factor GDNF and camp suppress glucocorticoid-inducible PNMT expression in a mouse pheochromocytoma model.
 161 Neurotrophic factor GDNF and camp suppress glucocorticoid-inducible PNMT expression in a mouse pheochromocytoma model. Marian J. Evinger a, James F. Powers b and Arthur S. Tischler b a. Department
161 Neurotrophic factor GDNF and camp suppress glucocorticoid-inducible PNMT expression in a mouse pheochromocytoma model. Marian J. Evinger a, James F. Powers b and Arthur S. Tischler b a. Department
Glucose Homeostasis. Liver. Glucose. Muscle, Fat. Pancreatic Islet. Glucose utilization. Glucose production, storage Insulin Glucagon
 Glucose Homeostasis Liver Glucose Glucose utilization Glucose production, storage Insulin Glucagon Muscle, Fat Pancreatic Islet Classification of Diabetes Type 1 diabetes Type 2 diabetes Other types of
Glucose Homeostasis Liver Glucose Glucose utilization Glucose production, storage Insulin Glucagon Muscle, Fat Pancreatic Islet Classification of Diabetes Type 1 diabetes Type 2 diabetes Other types of
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
doi:10.1038/nature12215 Supplementary Figure 1. The effects of full and dissociated GR agonists in supporting BFU-E self-renewal divisions. BFU-Es were cultured in self-renewal medium with indicated GR
Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents
 Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents Jarrad M Scarlett 1,,1, Jennifer M Rojas 1,1, Miles E Matsen 1, Karl J Kaiyala 3, Darko
Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents Jarrad M Scarlett 1,,1, Jennifer M Rojas 1,1, Miles E Matsen 1, Karl J Kaiyala 3, Darko
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
Inflammation & Type 2 Diabetes Prof. Marc Y. Donath
 Inflammation & Type 2 Diabetes 1, MD Chief Endocrinology, Diabetes & Metabolism University Hospital Basel Petersgraben 4 CH-431 Basel, Switzerland MDonath@uhbs.ch Innate immunity as a sensor of metabolic
Inflammation & Type 2 Diabetes 1, MD Chief Endocrinology, Diabetes & Metabolism University Hospital Basel Petersgraben 4 CH-431 Basel, Switzerland MDonath@uhbs.ch Innate immunity as a sensor of metabolic
Discussion & Conclusion
 Discussion & Conclusion 7. Discussion DPP-4 inhibitors augment the effects of incretin hormones by prolonging their half-life and represent a new therapeutic approach for the treatment of type 2 diabetes
Discussion & Conclusion 7. Discussion DPP-4 inhibitors augment the effects of incretin hormones by prolonging their half-life and represent a new therapeutic approach for the treatment of type 2 diabetes
Journal Club WS 2012/13 Stefanie Nickl
 Journal Club WS 2012/13 Stefanie Nickl Background Mesenchymal Stem Cells First isolation from bone marrow 30 ys ago Isolation from: spleen, heart, skeletal muscle, synovium, amniotic fluid, dental pulp,
Journal Club WS 2012/13 Stefanie Nickl Background Mesenchymal Stem Cells First isolation from bone marrow 30 ys ago Isolation from: spleen, heart, skeletal muscle, synovium, amniotic fluid, dental pulp,
Hormonal Regulations Of Glucose Metabolism & DM
 Hormonal Regulations Of Glucose Metabolism & DM What Hormones Regulate Metabolism? What Hormones Regulate Metabolism? Insulin Glucagon Thyroid hormones Cortisol Epinephrine Most regulation occurs in order
Hormonal Regulations Of Glucose Metabolism & DM What Hormones Regulate Metabolism? What Hormones Regulate Metabolism? Insulin Glucagon Thyroid hormones Cortisol Epinephrine Most regulation occurs in order
Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell and patient-derived tumor organoids
 Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell and patient-derived tumor organoids Ling Huang 1, Audrey Holtzinger 1,2,11, Ishaan Jagan 1,11, Michael BeGora 1, Ines
Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell and patient-derived tumor organoids Ling Huang 1, Audrey Holtzinger 1,2,11, Ishaan Jagan 1,11, Michael BeGora 1, Ines
Figure legends Supplemental Fig.1. Glucose-induced insulin secretion and insulin content of islets. Supplemental Fig. 2.
 Figure legends Supplemental Fig.. Glucose-induced insulin secretion and insulin content of islets. Insulin secretory responses to.,., and. mm glucose (A) (n = 7-), and the insulin content in the islets
Figure legends Supplemental Fig.. Glucose-induced insulin secretion and insulin content of islets. Insulin secretory responses to.,., and. mm glucose (A) (n = 7-), and the insulin content in the islets
A novel role for vitamin D: modulation of expression and function of the local renin angiotensin system in mouse pancreatic islets
 Diabetologia () 5:77 DOI.7/s5--- SHORT COMMUNICATION A novel role for vitamin D: modulation of expression and function of the local renin angiotensin system in mouse pancreatic islets Q. Cheng & Y. C.
Diabetologia () 5:77 DOI.7/s5--- SHORT COMMUNICATION A novel role for vitamin D: modulation of expression and function of the local renin angiotensin system in mouse pancreatic islets Q. Cheng & Y. C.
Diabetes: What is the scope of the problem?
 Diabetes: What is the scope of the problem? Elizabeth R. Seaquist MD Division of Endocrinology and Diabetes Department of Medicine Director, General Clinical Research Center Pennock Family Chair in Diabetes
Diabetes: What is the scope of the problem? Elizabeth R. Seaquist MD Division of Endocrinology and Diabetes Department of Medicine Director, General Clinical Research Center Pennock Family Chair in Diabetes
Transplantation in Nagasaki
 NAOSITE: Nagasaki University's Ac Title Author(s) Citation Evaluation of the Presumable Incide Transplantation in Nagasaki Tanaka, Takayuki; Kuroki, Tamotsu; Tatsuya; Ono, Shinichiro; Abiru, No Susumu
NAOSITE: Nagasaki University's Ac Title Author(s) Citation Evaluation of the Presumable Incide Transplantation in Nagasaki Tanaka, Takayuki; Kuroki, Tamotsu; Tatsuya; Ono, Shinichiro; Abiru, No Susumu
Inhibition of DYRK1A stimulates human beta-cell proliferation
 Inhibition of DYRK1A stimulates human beta-cell proliferation Ercument Dirice 1,, Deepika Walpita 2,, Amedeo Vetere 2, Bennett C. Meier 2,5, Sevim Kahraman 1, Jiang Hu 1, Vlado Dančík 2, Sean M. Burns
Inhibition of DYRK1A stimulates human beta-cell proliferation Ercument Dirice 1,, Deepika Walpita 2,, Amedeo Vetere 2, Bennett C. Meier 2,5, Sevim Kahraman 1, Jiang Hu 1, Vlado Dančík 2, Sean M. Burns
Module 2 Endocrine System
 Module 2 Endocrine System Student Name: 1 Lesson 1 Lesson 2 Lesson 3 Lesson 4 Lesson 5 Total Marks Total Possible Marks 10 8 14 21 16 69 Your Mark Teacher Comments: 2 (10 marks) Lesson 1: Structure and
Module 2 Endocrine System Student Name: 1 Lesson 1 Lesson 2 Lesson 3 Lesson 4 Lesson 5 Total Marks Total Possible Marks 10 8 14 21 16 69 Your Mark Teacher Comments: 2 (10 marks) Lesson 1: Structure and
β-cell Preservation and Regeneration After Islet Transplantation
 β-cell Preservation and Regeneration After Islet Transplantation Jyuhn-Huarng Juang, MD Division of Endocrinology and Metabolism, Department of Internal Medicine, Chang Gung University and Memorial Hospital,
β-cell Preservation and Regeneration After Islet Transplantation Jyuhn-Huarng Juang, MD Division of Endocrinology and Metabolism, Department of Internal Medicine, Chang Gung University and Memorial Hospital,
The Realities of Technology in Type 1 Diabetes
 The Realities of Technology in Type 1 Diabetes May 6, 2017 Rosanna Fiallo-scharer, MD Margaret Frederick, RN Disclosures I have no conflicts of interest to disclose I will discuss some unapproved treatments
The Realities of Technology in Type 1 Diabetes May 6, 2017 Rosanna Fiallo-scharer, MD Margaret Frederick, RN Disclosures I have no conflicts of interest to disclose I will discuss some unapproved treatments
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11464 Supplemental Figure S1. The expression of Vegfb is increased in obese and diabetic mice as compared to lean mice. a-b, Body weight and postprandial blood
SUPPLEMENTARY INFORMATION doi:10.1038/nature11464 Supplemental Figure S1. The expression of Vegfb is increased in obese and diabetic mice as compared to lean mice. a-b, Body weight and postprandial blood
Supplementary Figure 1: Steviol and stevioside potentiate TRPM5 in a cell-free environment. (a) TRPM5 currents are activated in inside-out patches
 Supplementary Figure 1: Steviol and stevioside potentiate TRPM5 in a cell-free environment. (a) TRPM5 currents are activated in inside-out patches during application of 500 µm Ca 2+ at the intracellular
Supplementary Figure 1: Steviol and stevioside potentiate TRPM5 in a cell-free environment. (a) TRPM5 currents are activated in inside-out patches during application of 500 µm Ca 2+ at the intracellular
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Baidal DA, Ricordi C, Berman DM, et al. Bioengineering of an
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Baidal DA, Ricordi C, Berman DM, et al. Bioengineering of an
Supplementary Figure 1.
 Supplementary Figure 1. Female Pro-ins2 -/- mice at 5-6 weeks of age were either inoculated i.p. with a single dose of CVB4 (1x10 5 PFU/mouse) or PBS and treated with αgalcer or control vehicle. On day
Supplementary Figure 1. Female Pro-ins2 -/- mice at 5-6 weeks of age were either inoculated i.p. with a single dose of CVB4 (1x10 5 PFU/mouse) or PBS and treated with αgalcer or control vehicle. On day
MPB333:Molecular Endocrinology of Obesity and Diabetes
 MPB333:Molecular Endocrinology of Obesity and Diabetes The Use of Stem Cells as a Cure for Type 1 Diabetes January 15, 2010 Trish Labosky 9415C MRBIV trish.labosky@vanderbilt.edu In theory. 2. ~Easy and
MPB333:Molecular Endocrinology of Obesity and Diabetes The Use of Stem Cells as a Cure for Type 1 Diabetes January 15, 2010 Trish Labosky 9415C MRBIV trish.labosky@vanderbilt.edu In theory. 2. ~Easy and
9.3 Stress Response and Blood Sugar
 9.3 Stress Response and Blood Sugar Regulate Stress Response Regulate Blood Sugar Stress Response Involves hormone pathways that regulate metabolism, heart, rate and breathing The Adrenal Glands a pair
9.3 Stress Response and Blood Sugar Regulate Stress Response Regulate Blood Sugar Stress Response Involves hormone pathways that regulate metabolism, heart, rate and breathing The Adrenal Glands a pair
Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells.
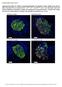 Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells. Pancreata from 16-weeks-old 6J +/+, 6J db/db, KS +/+ and KS db/db mice were harvested, fixed with
Supplementary Figure S1. Distinct compartmentalization of proinsulin in obese db/db mouse islet ß- cells. Pancreata from 16-weeks-old 6J +/+, 6J db/db, KS +/+ and KS db/db mice were harvested, fixed with
B220 CD4 CD8. Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN
 B220 CD4 CD8 Natarajan et al., unpublished data Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN showing B cell follicles and T cell areas. 20 µm thick. Image of magnification
B220 CD4 CD8 Natarajan et al., unpublished data Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN showing B cell follicles and T cell areas. 20 µm thick. Image of magnification
Endocrine System Notes
 Endocrine System Notes is the tendency to maintain a stable internal environment. - parts of the body that secrete hormones directly into the body. - parts of the body that make secretions which travel
Endocrine System Notes is the tendency to maintain a stable internal environment. - parts of the body that secrete hormones directly into the body. - parts of the body that make secretions which travel
Potentiation of Glucose-stimulated Insulin Secretion by the GPR40 PLC TRPC
 Supplementary information Potentiation of Glucose-stimulated Insulin Secretion by the GPR40 PLC TRPC Pathway in Pancreatic -Cells Authors: Hodaka Yamada 1,*, Masashi Yoshida 1,*, Kiyonori Ito 1, Katsuya
Supplementary information Potentiation of Glucose-stimulated Insulin Secretion by the GPR40 PLC TRPC Pathway in Pancreatic -Cells Authors: Hodaka Yamada 1,*, Masashi Yoshida 1,*, Kiyonori Ito 1, Katsuya
ENERGY FROM INGESTED NUTREINTS MAY BE USED IMMEDIATELY OR STORED
 QUIZ/TEST REVIEW NOTES SECTION 1 SHORT TERM METABOLISM [METABOLISM] Learning Objectives: Identify primary energy stores of the body Differentiate the metabolic processes of the fed and fasted states Explain
QUIZ/TEST REVIEW NOTES SECTION 1 SHORT TERM METABOLISM [METABOLISM] Learning Objectives: Identify primary energy stores of the body Differentiate the metabolic processes of the fed and fasted states Explain
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
IPS Modern management of childhood diabetes mellitus
 Modern management of childhood diabetes mellitus Professor Philippe LYSY, MD PhD Pediatric endocrinology and diabetology Institut de Recherche Expérimentale et Clinique Université Catholique de Louvain
Modern management of childhood diabetes mellitus Professor Philippe LYSY, MD PhD Pediatric endocrinology and diabetology Institut de Recherche Expérimentale et Clinique Université Catholique de Louvain
Islet Cell Allo-Transplantation. Disclosure. Objectives
 Islet Cell Allo-Transplantation Gregory P. Forlenza, MD MCR Assistant Professor Barbara Davis Center University of Colorado Denver Special thanks to Melena Bellin, MD at The University of Minnesota who
Islet Cell Allo-Transplantation Gregory P. Forlenza, MD MCR Assistant Professor Barbara Davis Center University of Colorado Denver Special thanks to Melena Bellin, MD at The University of Minnesota who
The regenerative therapy of type 1 diabetes mellitus 21April 2017 Girne, Northern Cyprus 53rd Turkish National Diabetes Congress
 The regenerative therapy of type 1 diabetes mellitus 21April 2017 Girne, Northern Cyprus 53rd Turkish National Diabetes Congress Thomas Linn Clinical Research Unit Centre of Internal Medicine Justus Liebig
The regenerative therapy of type 1 diabetes mellitus 21April 2017 Girne, Northern Cyprus 53rd Turkish National Diabetes Congress Thomas Linn Clinical Research Unit Centre of Internal Medicine Justus Liebig
18. PANCREATIC FUNCTION AND METABOLISM. Pancreatic secretions ISLETS OF LANGERHANS. Insulin
 18. PANCREATIC FUNCTION AND METABOLISM ISLETS OF LANGERHANS Some pancreatic functions have already been discussed in the digestion section. In this one, the emphasis will be placed on the endocrine function
18. PANCREATIC FUNCTION AND METABOLISM ISLETS OF LANGERHANS Some pancreatic functions have already been discussed in the digestion section. In this one, the emphasis will be placed on the endocrine function
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle.
 Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated,
 1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated, embedded in matrigel and exposed
1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated, embedded in matrigel and exposed
SUPPLEMENTARY DATA. Supplementary Table 1. Primers used for PCR and qpcr Primer Name
 Supplementary Table. Primers used for PCR and qpcr Primer Name ccession Number Fwd Rev Type of PCR Cre NC_8 GGCGTCTTCCGC GTGCCCCTCGTTTG Standard PCR LoUcp CCGGGCTGTCTCCGCGG GGCTGTTCGCCCGGCC Standard PCR
Supplementary Table. Primers used for PCR and qpcr Primer Name ccession Number Fwd Rev Type of PCR Cre NC_8 GGCGTCTTCCGC GTGCCCCTCGTTTG Standard PCR LoUcp CCGGGCTGTCTCCGCGG GGCTGTTCGCCCGGCC Standard PCR
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Confirmation of Dnmt1 conditional knockout out mice. a, Representative images of sorted stem (Lin - CD49f high CD24 + ), luminal (Lin - CD49f low CD24 + )
Supplementary Figures Supplementary Figure 1. Confirmation of Dnmt1 conditional knockout out mice. a, Representative images of sorted stem (Lin - CD49f high CD24 + ), luminal (Lin - CD49f low CD24 + )
Diabetic pdx1-mutant zebrafish show conserved responses to nutrient overload and anti-glycemic treatment
 Supplementary Information Diabetic pdx1-mutant zebrafish show conserved responses to nutrient overload and anti-glycemic treatment Robin A. Kimmel, Stefan Dobler, Nicole Schmitner, Tanja Walsen, Julia
Supplementary Information Diabetic pdx1-mutant zebrafish show conserved responses to nutrient overload and anti-glycemic treatment Robin A. Kimmel, Stefan Dobler, Nicole Schmitner, Tanja Walsen, Julia
Pancreas. Endocrine pancreas - Islets of Langerhans A or alpha cells glucagon B or beta cells insulin Delta cells somatostatin
 Endocrine System Pancreas Endocrine pancreas - Islets of Langerhans A or alpha cells glucagon B or beta cells insulin Delta cells somatostatin Glucagon & Metabolism Produced by beta cells of Islets Primary
Endocrine System Pancreas Endocrine pancreas - Islets of Langerhans A or alpha cells glucagon B or beta cells insulin Delta cells somatostatin Glucagon & Metabolism Produced by beta cells of Islets Primary
PRESENTED TO Name Line One Name Line Two (optional - Delete this line if not needed)
 Encapsulation JDRF Encapsulation Research Progress Report 2015 PRESENTED TO Name Line One Name Line Two (optional - Delete this line if not needed) MARCH 12, 2015 Back to top IN THIS ISSUE INTRODUCTION
Encapsulation JDRF Encapsulation Research Progress Report 2015 PRESENTED TO Name Line One Name Line Two (optional - Delete this line if not needed) MARCH 12, 2015 Back to top IN THIS ISSUE INTRODUCTION
Table 1. Oligonucleotides and RT-PCR conditions Supplementary Material and Methods Fig. 1
 Table 1. Oligonucleotides and RT-PCR conditions. Overview of PCR templates, gene accession number of sequences used as template, product size, annealing temperatures and optimal cycles, cdna and MgCl 2
Table 1. Oligonucleotides and RT-PCR conditions. Overview of PCR templates, gene accession number of sequences used as template, product size, annealing temperatures and optimal cycles, cdna and MgCl 2
Inhibition of Cdk5 Promotes β-cell Differentiation from Ductal Progenitors
 Inhibition of Cdk5 Promotes β-cell Differentiation from Ductal Progenitors Ka-Cheuk Liu, Gunter Leuckx, Daisuke Sakano, Philip A. Seymour, Charlotte L. Mattsson, Linn Rautio, Willem Staels, Yannick Verdonck,
Inhibition of Cdk5 Promotes β-cell Differentiation from Ductal Progenitors Ka-Cheuk Liu, Gunter Leuckx, Daisuke Sakano, Philip A. Seymour, Charlotte L. Mattsson, Linn Rautio, Willem Staels, Yannick Verdonck,
Supplemental Figure 1. Western blot analysis indicated that MIF was detected in the fractions of
 Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supplemental Figure Legends Supplemental Figure 1. Western blot analysis indicated that was detected in the fractions of plasma membrane and cytosol but not in nuclear fraction isolated from Pkd1 null
Supporting Information
 Supporting Information Franco et al. 10.1073/pnas.1015557108 SI Materials and Methods Drug Administration. PD352901 was dissolved in 0.5% (wt/vol) hydroxyl-propyl-methylcellulose, 0.2% (vol/vol) Tween
Supporting Information Franco et al. 10.1073/pnas.1015557108 SI Materials and Methods Drug Administration. PD352901 was dissolved in 0.5% (wt/vol) hydroxyl-propyl-methylcellulose, 0.2% (vol/vol) Tween
Control of Glucose Metabolism
 Glucose Metabolism Control of Glucose Metabolism The pancreas is both an exocrine and endocrine gland. It secretes digestive enzymes into the duodenum (exocrine) and 3 specific hormones into the bloodstream
Glucose Metabolism Control of Glucose Metabolism The pancreas is both an exocrine and endocrine gland. It secretes digestive enzymes into the duodenum (exocrine) and 3 specific hormones into the bloodstream
Therapeutic strategy to reduce Glucagon secretion
 Clinical focus on glucagon: α-cell as a companion of β-cell Therapeutic strategy to reduce Glucagon secretion Sunghwan Suh Dong-A University Conflict of interest disclosure None Committee of Scientific
Clinical focus on glucagon: α-cell as a companion of β-cell Therapeutic strategy to reduce Glucagon secretion Sunghwan Suh Dong-A University Conflict of interest disclosure None Committee of Scientific
Report on Pathology. Study: The effect of Compound X on pancreatic islets in rhesus macaques
 Report on Pathology Study: The effect of Compound X on pancreatic islets in rhesus macaques Prepared for: Client Name Client Address January 1, 2013 Prepared by: Charter Preclinical Services 21 Main St.,
Report on Pathology Study: The effect of Compound X on pancreatic islets in rhesus macaques Prepared for: Client Name Client Address January 1, 2013 Prepared by: Charter Preclinical Services 21 Main St.,
Gamma-aminobutyric acid (GABA) treatment blocks inflammatory pathways and promotes survival and proliferation of pancreatic beta cells
 Gamma-aminobutyric acid (GABA) treatment blocks inflammatory pathways and promotes survival and proliferation of pancreatic beta cells Gérald J. Prud homme, MD, FRCPC Keenan Research Centre for Biomedical
Gamma-aminobutyric acid (GABA) treatment blocks inflammatory pathways and promotes survival and proliferation of pancreatic beta cells Gérald J. Prud homme, MD, FRCPC Keenan Research Centre for Biomedical
CHAPTER 50 Endocrine Systems. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 CHAPTER 50 Endocrine Systems Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Endocrine system All the endocrine glands and other organs with hormonesecreting
CHAPTER 50 Endocrine Systems Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Endocrine system All the endocrine glands and other organs with hormonesecreting
Supplemental Figure 1
 Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplemental Information. CD4 + CD25 + Foxp3 + Regulatory T Cells Promote. Th17 Cells In Vitro and Enhance Host Resistance
 Immunity, Volume 34 Supplemental Information D4 + D25 + + Regulatory T ells Promote Th17 ells In Vitro and Enhance Host Resistance in Mouse andida albicans Th17 ell Infection Model Pushpa Pandiyan, Heather
Immunity, Volume 34 Supplemental Information D4 + D25 + + Regulatory T ells Promote Th17 ells In Vitro and Enhance Host Resistance in Mouse andida albicans Th17 ell Infection Model Pushpa Pandiyan, Heather
Supplemental Figure S1. RANK expression on human lung cancer cells.
 Supplemental Figure S1. RANK expression on human lung cancer cells. (A) Incidence and H-Scores of RANK expression determined from IHC in the indicated primary lung cancer subgroups. The overall expression
Supplemental Figure S1. RANK expression on human lung cancer cells. (A) Incidence and H-Scores of RANK expression determined from IHC in the indicated primary lung cancer subgroups. The overall expression
Supplementary Figure 1: Expression of NFAT proteins in Nfat2-deleted B cells (a+b) Protein expression of NFAT2 (a) and NFAT1 (b) in isolated splenic
 Supplementary Figure 1: Expression of NFAT proteins in Nfat2-deleted B cells (a+b) Protein expression of NFAT2 (a) and NFAT1 (b) in isolated splenic B cells from WT Nfat2 +/+, TCL1 Nfat2 +/+ and TCL1 Nfat2
Supplementary Figure 1: Expression of NFAT proteins in Nfat2-deleted B cells (a+b) Protein expression of NFAT2 (a) and NFAT1 (b) in isolated splenic B cells from WT Nfat2 +/+, TCL1 Nfat2 +/+ and TCL1 Nfat2
SUPPLEMENTARY INFORMATION
 doi: 1.138/nature7221 Brown fat selective genes 12 1 Control Q-RT-PCR (% of Control) 8 6 4 2 Ntrk3 Cox7a1 Cox8b Cox5b ATPase b2 ATPase f1a1 Sirt3 ERRα Elovl3/Cig3 PPARα Zic1 Supplementary Figure S1. stimulates
doi: 1.138/nature7221 Brown fat selective genes 12 1 Control Q-RT-PCR (% of Control) 8 6 4 2 Ntrk3 Cox7a1 Cox8b Cox5b ATPase b2 ATPase f1a1 Sirt3 ERRα Elovl3/Cig3 PPARα Zic1 Supplementary Figure S1. stimulates
Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells.
 SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure S1. Expression of Cirbp mrna in mouse tissues and NIH3T3 cells. A) Cirbp mrna expression levels in various mouse tissues collected around the clock
Figure S1A. Blood glucose levels in mice after glucose injection
 ## Figure S1A. Blood glucose levels in mice after glucose injection Blood glucose (mm/l) 25 2 15 1 5 # 15 3 6 3+3 Time after glucose injection (min) # Figure S1B. α-kg levels in mouse livers after glucose
## Figure S1A. Blood glucose levels in mice after glucose injection Blood glucose (mm/l) 25 2 15 1 5 # 15 3 6 3+3 Time after glucose injection (min) # Figure S1B. α-kg levels in mouse livers after glucose
Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes
 Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes T Jourdan, G Godlewski, R Cinar, A Bertola, G Szanda, J Liu, J Tam, T Han, B Mukhopadhyay,
Endocannabinoid-activated Nlrp3 inflammasome in infiltrating macrophages mediates β- cell loss in type 2 diabetes T Jourdan, G Godlewski, R Cinar, A Bertola, G Szanda, J Liu, J Tam, T Han, B Mukhopadhyay,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
DOI: 10.1038/ncb3461 In the format provided by the authors and unedited. Supplementary Figure 1 (associated to Figure 1). Cpeb4 gene-targeted mice develop liver steatosis. a, Immunoblot displaying CPEB4
BIOLOGY - CLUTCH CH.45 - ENDOCRINE SYSTEM.
 !! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
!! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
Mango Modulates Body Fat and Plasma Glucose and Lipids in Mice Fed High Fat Diet
 Title of Study: Principal Investigator: Co-Investigators: Mango Modulates Body Fat and Plasma Glucose and Lipids in Mice Fed High Fat Diet Dr. Edralin A. Lucas Nutritional Sciences Department Oklahoma
Title of Study: Principal Investigator: Co-Investigators: Mango Modulates Body Fat and Plasma Glucose and Lipids in Mice Fed High Fat Diet Dr. Edralin A. Lucas Nutritional Sciences Department Oklahoma
Jonathan RT Lakey, PhD Associate Professor, Director of Surgical Research Director, Clinical Islet Program
 Jonathan RT Lakey, PhD Associate Professor, Director of Surgical Research Director, Clinical Islet Program University of California, Irvine Irvine, CA Challenges and Emerging Opportunities DONOR Donor
Jonathan RT Lakey, PhD Associate Professor, Director of Surgical Research Director, Clinical Islet Program University of California, Irvine Irvine, CA Challenges and Emerging Opportunities DONOR Donor
Generation of pancreatic β cells for treatment of diabetes: advances and challenges
 Shahjalal et al. Stem Cell Research & Therapy (2018) 9:355 https://doi.org/10.1186/s13287-018-1099-3 REVIEW Generation of pancreatic β cells for treatment of diabetes: advances and challenges Hussain Md.
Shahjalal et al. Stem Cell Research & Therapy (2018) 9:355 https://doi.org/10.1186/s13287-018-1099-3 REVIEW Generation of pancreatic β cells for treatment of diabetes: advances and challenges Hussain Md.
Week 3 The Pancreas: Pancreatic ph buffering:
 Week 3 The Pancreas: A gland with both endocrine (secretion of substances into the bloodstream) & exocrine (secretion of substances to the outside of the body or another surface within the body) functions
Week 3 The Pancreas: A gland with both endocrine (secretion of substances into the bloodstream) & exocrine (secretion of substances to the outside of the body or another surface within the body) functions
Effects of growth hormone secretagogue receptor agonist and antagonist in nonobese type 2 diabetic MKR mice
 Effects of growth hormone secretagogue receptor agonist and antagonist in nonobese type 2 diabetic MKR mice Rasha Mosa (MBCHC, M.D, PhD candidate) School of Biomedical Sciences University of Queensland
Effects of growth hormone secretagogue receptor agonist and antagonist in nonobese type 2 diabetic MKR mice Rasha Mosa (MBCHC, M.D, PhD candidate) School of Biomedical Sciences University of Queensland
control kda ATGL ATGLi HSL 82 GAPDH * ** *** WT/cTg WT/cTg ATGLi AKO/cTg AKO/cTg ATGLi WT/cTg WT/cTg ATGLi AKO/cTg AKO/cTg ATGLi iwat gwat ibat
 body weight (g) tissue weights (mg) ATGL protein expression (relative to GAPDH) HSL protein expression (relative to GAPDH) ### # # kda ATGL 55 HSL 82 GAPDH 37 2.5 2. 1.5 1..5 2. 1.5 1..5.. Supplementary
body weight (g) tissue weights (mg) ATGL protein expression (relative to GAPDH) HSL protein expression (relative to GAPDH) ### # # kda ATGL 55 HSL 82 GAPDH 37 2.5 2. 1.5 1..5 2. 1.5 1..5.. Supplementary
Thyrotoxicosis in Pregnancy: Diagnose and Management
 Thyrotoxicosis in Pregnancy: Diagnose and Management Yuanita Asri Langi email: meralday@yahoo.co.id Endocrinology & Metabolic Division, Internal Medicine Department, Prof.dr.R.D. Kandou Hospital/ Sam Ratulangi
Thyrotoxicosis in Pregnancy: Diagnose and Management Yuanita Asri Langi email: meralday@yahoo.co.id Endocrinology & Metabolic Division, Internal Medicine Department, Prof.dr.R.D. Kandou Hospital/ Sam Ratulangi
28 Regulation of Fasting and Post-
 28 Regulation of Fasting and Post- Prandial Glucose Metabolism Keywords: Type 2 Diabetes, endogenous glucose production, splanchnic glucose uptake, gluconeo-genesis, glycogenolysis, glucose effectiveness.
28 Regulation of Fasting and Post- Prandial Glucose Metabolism Keywords: Type 2 Diabetes, endogenous glucose production, splanchnic glucose uptake, gluconeo-genesis, glycogenolysis, glucose effectiveness.
Type 2 DM in Adolescents: Use of GLP-1 RA. Objectives. Scope of Problem: Obesity. Background. Pathophysiology of T2DM
 Type 2 DM in Adolescents: Use of GLP-1 RA Objectives Identify patients in the pediatric population with T2DM that would potentially benefit from the use of GLP-1 RA Discuss changes in glycemic outcomes
Type 2 DM in Adolescents: Use of GLP-1 RA Objectives Identify patients in the pediatric population with T2DM that would potentially benefit from the use of GLP-1 RA Discuss changes in glycemic outcomes
Defective Hepatic Autophagy in Obesity Promotes ER Stress and Causes Insulin Resistance
 Cell Metabolism, Volume 11 Supplemental Information Defective Hepatic Autophagy in Obesity Promotes ER Stress and Causes Insulin Resistance Ling Yang, Ping Li, Suneng Fu, Ediz S. Calay, and Gökhan S. Hotamisligil
Cell Metabolism, Volume 11 Supplemental Information Defective Hepatic Autophagy in Obesity Promotes ER Stress and Causes Insulin Resistance Ling Yang, Ping Li, Suneng Fu, Ediz S. Calay, and Gökhan S. Hotamisligil
Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets
 Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets Gene 5 Forward 3 5 Reverse 3.T. Product (bp) ( C) mnox1 GTTCTTGGGCTGCCTTGG GCTGGGGCGGCGG 60 300 mnoxa1 GCTTTGCCGCGTGC GGTTCGGGTCCTTTGTGC
Supplementary Table 1. Primer sequences for conventional RT-PCR on mouse islets Gene 5 Forward 3 5 Reverse 3.T. Product (bp) ( C) mnox1 GTTCTTGGGCTGCCTTGG GCTGGGGCGGCGG 60 300 mnoxa1 GCTTTGCCGCGTGC GGTTCGGGTCCTTTGTGC
well for 2 h at rt. Each dot represents an individual mouse and bar is the mean ±
 Supplementary data: Control DC Blimp-1 ko DC 8 6 4 2-2 IL-1β p=.5 medium 8 6 4 2 IL-2 Medium p=.16 8 6 4 2 IL-6 medium p=.3 5 4 3 2 1-1 medium IL-1 n.s. 25 2 15 1 5 IL-12(p7) p=.15 5 IFNγ p=.65 4 3 2 1
Supplementary data: Control DC Blimp-1 ko DC 8 6 4 2-2 IL-1β p=.5 medium 8 6 4 2 IL-2 Medium p=.16 8 6 4 2 IL-6 medium p=.3 5 4 3 2 1-1 medium IL-1 n.s. 25 2 15 1 5 IL-12(p7) p=.15 5 IFNγ p=.65 4 3 2 1
The Effects of Exenatide on the Autoimmune Development of Diabetes. A Senior Honors Thesis
 The Effects of Exenatide on the Autoimmune Development of Diabetes A Senior Honors Thesis Presented in Partial Fulfillment of the Requirements for Graduation with Distinction in Microbiology in the Undergraduate
The Effects of Exenatide on the Autoimmune Development of Diabetes A Senior Honors Thesis Presented in Partial Fulfillment of the Requirements for Graduation with Distinction in Microbiology in the Undergraduate
Biology 30. Morinville Community High School. Unit 2: Endocrine System. Name:
 Biology 30 Morinville Community High School Unit 2: Endocrine System Name: 2 Endocrine System Unit Outline Chapter 13 text p. 434-471 Key Concept A: The endocrine system and nervous system both mediate
Biology 30 Morinville Community High School Unit 2: Endocrine System Name: 2 Endocrine System Unit Outline Chapter 13 text p. 434-471 Key Concept A: The endocrine system and nervous system both mediate
Chapter 45-Hormones and the Endocrine System. Simple Hormone Pathways
 Chapter 45-Hormones and the Endocrine System Simple Hormone s Low ph in duodenum Hormones are released from an endocrine, travel through the bloodstream, and interact with the receptor or a target to cause
Chapter 45-Hormones and the Endocrine System Simple Hormone s Low ph in duodenum Hormones are released from an endocrine, travel through the bloodstream, and interact with the receptor or a target to cause
The endocrine system is complex and sometimes poorly understood.
 1 CE Credit Testing the Endocrine System for Adrenal Disorders and Diabetes Mellitus: It Is All About Signaling Hormones! David Liss, BA, RVT, VTS (ECC) Platt College Alhambra, California For more information,
1 CE Credit Testing the Endocrine System for Adrenal Disorders and Diabetes Mellitus: It Is All About Signaling Hormones! David Liss, BA, RVT, VTS (ECC) Platt College Alhambra, California For more information,
Scope. History. History. Incretins. Incretin-based Therapy and DPP-4 Inhibitors
 Plasma Glucose (mg/dl) Plasma Insulin (pmol/l) Incretin-based Therapy and Inhibitors Scope Mechanism of action ผศ.ดร.นพ.ว ระเดช พ ศประเสร ฐ สาขาว ชาโภชนว ทยาคล น ก ภาคว ชาอาย รศาสตร คณะแพทยศาสตร มหาว ทยาล
Plasma Glucose (mg/dl) Plasma Insulin (pmol/l) Incretin-based Therapy and Inhibitors Scope Mechanism of action ผศ.ดร.นพ.ว ระเดช พ ศประเสร ฐ สาขาว ชาโภชนว ทยาคล น ก ภาคว ชาอาย รศาสตร คณะแพทยศาสตร มหาว ทยาล
The Endocrine System
 The Endocrine System Endocrine Glands Glands that secrete their products (HORMONES) into extracellular spaces around cells. The hormones then enter into the bloodstream by diffusing into the capillaries
The Endocrine System Endocrine Glands Glands that secrete their products (HORMONES) into extracellular spaces around cells. The hormones then enter into the bloodstream by diffusing into the capillaries
Nature Immunology: doi: /ni Supplementary Figure 1. Huwe1 has high expression in HSCs and is necessary for quiescence.
 Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Supplementary Figure 1 Huwe1 has high expression in HSCs and is necessary for quiescence. (a) Heat map visualizing expression of genes with a known function in ubiquitin-mediated proteolysis (KEGG: Ubiquitin
Improving the Quality of Life for Patients with Chronic Disease
 TSX-V: SVA OTCQB: SEOVF - 2017 Presentation June, 2017 Improving the Quality of Life for Patients with Chronic Disease Forward Looking Statements 2 This presentation may contain forward looking statements.
TSX-V: SVA OTCQB: SEOVF - 2017 Presentation June, 2017 Improving the Quality of Life for Patients with Chronic Disease Forward Looking Statements 2 This presentation may contain forward looking statements.
Αναγκαιότητα και τρόπος ρύθμισης του διαβήτη στους νοσηλευόμενους ασθενείς
 Αναγκαιότητα και τρόπος ρύθμισης του διαβήτη στους νοσηλευόμενους ασθενείς Αναστασία Θανοπούλου Επίκουρη Καθηγήτρια Β Παθολογικής Κλινικής Πανεπιστημίου Αθηνών Διαβητολογικό Κέντρο, Ιπποκράτειο Νοσοκομείο
Αναγκαιότητα και τρόπος ρύθμισης του διαβήτη στους νοσηλευόμενους ασθενείς Αναστασία Θανοπούλου Επίκουρη Καθηγήτρια Β Παθολογικής Κλινικής Πανεπιστημίου Αθηνών Διαβητολογικό Κέντρο, Ιπποκράτειο Νοσοκομείο
Endocrine System Physiology
 M53_MARI0000_00_SE_EX04.qxd 7/15/11 4:32 PM Page 369 4 E X E R C I S E Endocrine System Physiology Advance Preparation/Comments Consider covering the following topics to prepare students for the simulation:
M53_MARI0000_00_SE_EX04.qxd 7/15/11 4:32 PM Page 369 4 E X E R C I S E Endocrine System Physiology Advance Preparation/Comments Consider covering the following topics to prepare students for the simulation:
Supplemental Table 1. Primer sequences for transcript analysis
 Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
Supplemental Table 1. Primer sequences for transcript analysis Primer Sequence (5 3 ) Primer Sequence (5 3 ) Mmp2 Forward CCCGTGTGGCCCTC Mmp15 Forward CGGGGCTGGCT Reverse GCTCTCCCGGTTTC Reverse CCTGGTGTGCCTGCTC
DRUGS. 4- Two molecules of DIT combine within the thyroglobulinto form L-thyroxine (T4)' One molecule of MIT & one molecule of DIT combine to form T3
 THYROID HORMONEs & ANTITHYROID The thyroid secretes 2 types of hormones: DRUGS 1- Iodine containing amino acids (are important for growth, development and metabolism) and these are: triodothyronine, tetraiodothyronine,(
THYROID HORMONEs & ANTITHYROID The thyroid secretes 2 types of hormones: DRUGS 1- Iodine containing amino acids (are important for growth, development and metabolism) and these are: triodothyronine, tetraiodothyronine,(
