Development of virtual patient model for simulation of solute and fluid transport during dialysis
|
|
|
- Shannon Johnston
- 5 years ago
- Views:
Transcription
1 BULLETIN OF THE POLISH ACADEMY OF SCIENCES TECHNICAL SCIENCES Vol. 53, No. 3, 2005 Development of virtual patient model for simulation of solute and fluid transport during dialysis M. GAŁACH and A. WERYŃSKI Institute of Biocybernetics and Biomedical Engineering, Polish Academy of Science 4 Ks. Trojdena Str., Warsaw, Poland Abstract. Optimization of dialysis needs methods for quantitative assessment of fluid and solutes transport in body compartments and solute and fluid exchange between body and dialysate. A mathematical model describing the dynamics of these quantities during dialysis is presented. This model is first and foremost based on the existing models, but also includes some new solutions. All parts were combined and extended by the detailed descriptions of selected aspects. The virtual patient model was applied to simulate and test different methods of treatment and their influence on the condition of the patient. The purpose of this model is to serve as a decision support system for selection of optimal treatment options for particular patient. Key words: mathematical model, dialysis, transport phenomena. 1. Introduction The mathematical models of the physiological systems are of paramount importance, because they can be used to improve our understanding of the behaviour of these complex systems. Important parts of them are models describing functions of the kidney or renal replacement therapies. Kidneys perform many functions. Healthy kidneys purify blood by removing excess fluid, minerals, metabolic waste products and toxins, help control blood pressure by releasing enzyme renin, participate in making red blood cells and help maintain the proper balance of acid-base and minerals. When kidneys fail, one of the renal replacement therapies can be a life-saving solution. The main type of this therapy, beside kidney transplantation, is dialysis. Dialysis removes excess of water and small molecular waste products of metabolism across a semipermeable membrane. Two main types of dialysis are: hemodialysis and peritoneal dialysis. Hemodialysis is the most common method used for treatment of end stage renal failure. Hemodialysis removes waste products, toxins and excess of fluid from the blood in an extracorporeal circuit in which the patient s blood is pumped to a purification system called dialyzer (Fig. 1). The dialyzer has two spaces separated by a membrane: one for blood and another one for dialysis fluid (a solution, which is intended to exchange solutes and fluid with blood during hemodialysis). Toxins and other excess substances move across the membrane from the blood into the dialysis fluid due to the concentration gradient (diffusion) and due to fluid flow carrying solutes (convection). Excess of fluid is removed due to the pressure (mainly hydrostatic) difference between blood and dialysate (ultrafiltration). The purified blood through tubes returns to the bloodstream. During peritoneal dialysis the blood is purified inside the body, solutes and water are transported across the peritoneum. Peritoneum is a thin membrane that lines the abdominal and pelvic cavities, and covers most abdominal viscera. There are two types of peritoneum: parietal peritoneum that lines the abdominal and pelvic cavities and visceral peritoneum that covers the external surfaces of most abdominal organs. Both types of peritoneum take part in peritoneal dialysis [1]. Dialysis fluid containing an osmotic agent, most frequently glucose, is infused into the peritoneal space, through a catheter, and remains for a period of time (usually 4 6 hours in continuous ambulatory peritoneal dialysis, CAPD). The peritoneum allows waste products of metabolism to pass from the blood into the dialysate (in the processes of diffusion and convection), and then dialysate is drained out (Fig. 2). Because osmotic agent creates high osmotic pressure in dialysis fluid exceeding substantially osmotic pressure in blood, water (and small solutes) is transported from blood removing excess of fluid. Fig. 1. Hemodialysis blood purification in the dialyzer magda@ibib.waw.pl 283
2 M. Gałach and A. Weryński Fig. 2. A scheme of peritoneal dialysis The model analyzed in this paper is first of all based on the partial models described mainly in [2 5] and is the most complete model of renal replacement therapies known to the authors. The model is composed of four parts. The first part deals with a fluid transport and kinetics of small solutes in the main compartments of the body. During hemodialysis, the blood volume decreases; therefore, the second part of the model describes the cardiovascular system. Additionally the control mechanisms which cause the compensatory reaction to blood volume loss are taken into account. The third part of model describes glucose-insulin interaction, and fourth part deals with description of dialysis (hemodialysis and peritoneal dialysis) treatments. The main objectives of the model are: to serve, in the future, as a decision support system in selection of optimal treatment options for selected patients, to help in teaching and to contribute to a better understanding of important processes, which take place in the patient during renal replacement therapies. Additionally, virtual patient model would allow to partly replace in vivo experiments with animals with new or modified therapies by in silico experiments with the computer. 2. Model A detailed description of different parts of the model and values of the parameters as well as detailed description of virtual patient model are presented in [2 7]. Because every mathematical model is a simplification of reality, the model cannot describe all details occurred in described processes. In virtual patient model, all simplification assumed in partial models [2 7] were adopted. The presented model consists of above 50 ordinary differential equations and about 200 algebraic equations and, unfortunately, there is no place in this paper to describe all these equations. Also, for this reason the values of the parameters (which are over 300) were omitted. In this paper only a general description and simulation results are presented. The scheme of the virtual patient model is presented in Fig. 3. Fig. 3. Scheme of the "virtual patient" model relationships between various part of the model; connection between different compartments, connection between extracellular and same other compartments 2.1. Fluid and solute transport. Fluid transport is described using a three-compartmental model of the exchange of the fluids (Fig. 4a) between the intracellular fluid, the interstitial fluid and blood plasma. Fig. 4. The compartmental model used for the description of body fluids exchange (a) and solute kinetics (b); V blood volume, V c fluid volume in compartment c (c = is interstitium, ic intracellular compartment, ex extracellular compartment, pl plasma), M s,c concentration of solute s in compartment c, C s,inf concentration of solute s in the replacement fluid, Q fluid flow rate caused by renal replacement treatment (hemodialysis or peritoneal dialysis), Q inf -infusion rate of fluid, R v venous reabsorption rate, F a arterial filtration rate, k f water exchange coefficient between the intraand extracellular compartments, J s solute transport caused by renal replacement treatment, k s mass transfer coefficient between intraand extracellular compartments for solute s, β s coefficient describing which kind of the solute s transport exists (active or passive) 284 Bull. Pol. Ac.: Tech. 53(3) 2005
3 Development of virtual patient model for simulation of solute and fluid transport during dialysis During dialysis, the total blood volume (V ) changes because of the fluid ultrafiltration flow across the hemodialyzer membrane or across peritoneal tissue to dialysate (Q fluid flow rate during dialysis), possible fluid infusion (Q inf fluid infusion rate), filtration at the arterial capillaries (F a filtration rate), reabsorption at the venous capillaries (R v reabsorption rate), residual kidney excretion (J k excretion rate), and delivery of the fluid i.e. drinking (J d rate of delivery of the fluid). Fluid exchange between the intracellular and extracellular compartments depends upon the osmotic pressure difference (V ic volume of intracellular fluid, k f water transport coefficient between the intra- and extracellular compartments, c ic, c is concentration of all osmotically effective solutes in the intracellular or interstitial fluids). Hence [2]: dv = F a + R v Q + Q inf J k + J d, (1) dv ic = k f (c is c ic ), (2) dv is = k f (c is c ic ) + F a R v. (3) The transport of solutes is governed by the two-compartment description (the intracellular fluid and the extracellular fluid) based on the assumption that the concentrations of small solutes in the blood plasma and in the interstitial fluid are approximately equal (Fig. 4b). In the model it is assumed that the total blood volume (V ) is sum of the plasma volume (V pl ) and the red cell volume (V rc ), therefore: V pl = V V rc, (4) V ex = V pl + V is, (5) where V ex denotes extracellular fluid volume. In the model, following substances were taken into account: potassium, sodium, urea, bicarbonate, chloride, glucose and insulin. However equations describing glucose-insulin system will be shown individually in the next section. In modelling peritoneal dialysis additionally eight fractions of icodextrin were analyzed (icodextrin is a mixture of polyglucose molecules with an average molecular weight D and is newly introduced osmotic agent in peritoneal dialysis). Substances can move into and out of cells across the cell membrane which is selectively permeable (allows selective movement of substances). There are two kinds of movement across the cell membrane: passive or active transport. Passive transport includes diffusion: molecules tend to move from an area (compartment) of high concentration to an area of low concentration in order to achieve a balance or to reach homeostasis. Active transport takes place when solute is transported up to its concentration gradient (from lower to higher concentration). This kind of transport involves the use of proteins that require the use of cellular energy (usually ATP). Therefore, the solute flow rate depends on the concentration gradient and on the mass transfer coefficients k s and β s [2]. β s describes which kind of the solute transport exists: β s = 1 when the solute does not exhibit active transport (i.e. urea), β s 1 when the solute is actively transported. Chloride has a special task in the model: the description of the chloride exchange across the cellular membrane is based on the assumption that the net flow of the charge is always equal to 0 [3]: dm s,ic = k s (c s,ic β s c s,ex ), (6) dm Cl,ic dm s,ex = dm K,ic + dm Na,ic dm HCO 3,ic, (7) = dm s,ic J s,t + Q inf c s,inf + G s,ex D s,k c s,ex, (8) where: M s,i mass of solute s in compartment i (i = ic, ex, s = K +, Na +, U, HCO 3 or Cl ), c s,i concentration of solute s in compartment i, c s,inf concentration of solute s in infusion fluid, J s,t total solute flow rate during renal replacement therapy: t = hd in the case of hemodialysis or t = pd in the case of peritoneal dialysis, G s,ex generation rate of solute s in the patient body, D s,k residual renal clearance Cardiovascular system. A simplified description of the heart and of the cardiovascular system has been included in the model. The cardiovascular system is described as a set of six compartments (Fig. 5). Fig. 5. The compartmental model used for the description of cardiovascular system (pv ( pulmonary veins, la left atrium, sa systemic arteries, pa pulmonary arteries, ra right atrium, sv systemic veins, is interstitium), P pressure, R resistance, C compliance, q l and q r cardiac outputs from the left and right ventricles, L a and L v the permeability coefficients at the arterial and venular capillary walls, R s1 resistance upstream of the arterial capillaries, R s2 resistance at the point of capillary circulation, R s3 resistance downstream of the venular capillaries A change of the pressure in each compartment depends on the compliance of the compartment (C c ), pressures in neighbouring compartments (or cardiac output (q l, q r ) and resistances (R c ) [2], i.e.: dp pv dp sa = 1 = 1 C pv C sa ( q l p ) sa p ac, (9) R s1 ( ppa p pv R pa p pv p la R pv ). (10) Bull. Pol. Ac.: Tech. 53(3)
4 M. Gałach and A. Weryński Blood vessels are elastic and therefore there is a relationship between hydraulic pressure and filling volume in these vessels. In the model it is assumed that this relationship is linear, thus: V c = V uc + C c p c, (11) where V c is the total blood volume in the compartment c ( sa systemic arteries, pa pulmonary arteries, pv pulmonary veins, ra right atrium, la left atrium), V uc is the volume of the compartment c at zero pressure (called unstressed volume), therefore: p c = V c V uc C c. (12) Additionally it is assumed that the blood volume in systemic veins is equal to the difference between the total blood volume (V ) and the blood volume in other five compartments, thus: V sv = V V sa V pa V pv V ra V la, (13) V usv = V u V usa V ups V upv V ura V ula. (14) Therefore, using Eq. (11): p sv = V sv V usv V sv = 1 C sv (V V u C sa p sa C pa p pa C pv p pv C ra p ra C la p la ). (15) Cardiac output in the left and right ventricles q l, q r (blood volume pumped by heart per a unit of time) is equal to the product of heart frequency (f) and stroke volume, S (blood volume ejected from the heart during systole). Frank-Starling mechanisms states that the more the heart is filled during diastole, the more will be the quantity of blood pumped into the aorta [8]. In other words, increasing left (or right) ventricular pressure increases stroke volume. According to this mechanism, the stroke volume depends on the diastolic volume, and, therefore, on the pressure in the left (or right) atrium (p la or p ra ) [2]: q s = S s f = S s T = k s(p c p c0 ) a s /T, (16) where s r (right ventricle) or l (left ventricle), p c p la (for s = l ) or p ra (for s = r ), k s (k l or k r ), p la0 and p ra0 describe the cardiac effectiveness, a s (a l or a r ) is the function describing the effect of the decrease of the stroke volume in response to an excessive increase of the arterial pressure or pressure in the pulmonary arteries: unstressed volume. This compensation depends upon the action of two groups of baroreceptors: the arterial and the cardiopulmonary receptors. If the systemic arteries pressure or the right atrium pressure decrease or increase above the normal value, then the signal is produced to modify the systemic resistance, heart period and systemic venous unstressed volume. When the blood volume falls below a critical level (the left atrial pressure falls below a specific threshold), the second response phase begins. This phase is triggered by a signal with very rapid dynamics. The signal is sent to the central nervous system, causing the inhibition of the control mechanism. The control mechanisms are described using equations and values of parameters as in the reference [2] Glucose-insulin interactions. Glucose-insulin system modelling is of special importance because kidney failure is a frequent late complication of diabetes. In Poland up to 20%, in Japan near 40% while in USA about 50% of all new patients on renal replacement therapies are diabetics [9]. The hormones which are the most important in glucose regulation are insulin and glucagon. When glucose concentration in the extracellular fluid increases (i.e. during eating) the rising concentration causes the pancreas to secrete increased quantities of insulin. The insulin, in turn, causes increased transport of glucose across the cell membranes to the interior of the cells in most parts of the body. The glucose is then used for energy. This returns the extracellular glucose concentration back toward the normal value. Changes in blood glucose concentration have exactly the opposite effect on glucagon secretion as on insulin secretion. That means, a decrease in blood glucose increases glucagon secretion. The most dramatic effect of glucagon is its ability to cause glycogenolysis in the liver, which in turn increases the blood glucose concentration within minutes. To describe glucose-insulin interactions model by Stolwijk-Hardy (1974) was used in the virtual patient [5,10]. In spite of being developed more than 30 years ago, the model is still referenced (e.g. in 2000 in [5]) and considered as suitable to describe regulation of glucose blood concentration. In this model, as well as in most of simple models describing glucose regulation, glucagon was not taken into account (Fig. 6). a s = { 1 pk p kn pk p kn p k p kn (17) where p k p sa (for s = l ) or p pa (for s = r ), p san and ppan describe the boundary conditions for this effect. Experimental studies have demonstrated the existence of a biphasic response to blood volume losses [2]. During the first phase, the blood pressure is maintained on the same level by changes of values of parameters of the cardiovascular system: the systemic resistance, heart period and systemic venous Fig. 6. Scheme of the model describing glucose-insulin interactions 286 Bull. Pol. Ac.: Tech. 53(3) 2005
5 Development of virtual patient model for simulation of solute and fluid transport during dialysis In the model it is assumed that the mass of glucose in extracellular fluid is influenced by glucose generation (G g,ex glucose generation rate), external source of glucose (S g ), renal loss R g renal loss rate, if residual renal function exists) and two types of glucose utilization: insulin dependent (U d ) and insulin independent (U id ), also impact of dialysis is taken into account J g,d glucose flow rate from or to dialysate). Thus [5,10]: dm g,ex = G g,ex + S g R g U d U id J g,d. (18) In the model it is assumed that when glucose concentration (C g,ex ) decreases below a certain threshold (θ), glucose is excreted by the kidneys (R g ) at a rate proportional to the gradient between C g,ex and θ (if residual renal function exists). Therefore [5]: { µ(cg,ex θ) C R g = g,ex > θ 0 C g,ex θ. (19) Glucose leaves the blood and enters to most cells by diffusion. In cases of insulin independent utilization, the rate of glucose utilization depends only on the extracellular-to-intracellular concentration gradient. The intracellular concentration is assumed equal to 0 (because in the cell glucose is changed to glycogen). Thus [5]: U id = λc g,ex. (20) In certain types of cells, such as those in muscle, insulin helps to stimulate diffusion process. Therefore, the rate at which glucose is taken up by these cells is proportional to C g,ex as well as to the blood insulin concentration C i,ex [5]: U d = γc g,ex C i,ex. (21) It is also assumed, that glucose generation rate (G g,ex ) can be constant (or depends on plasma glucose concentration). A similar mass balance equation is established for blood insulin. It is assumed that insulin mass changes due to insulin generation (G i,ex insulin generation rate), insulin destruction (DR i insulin destruction rate), external source of glucose (S i ) and transport during dialysis (J i,d insulin flow rate to dialysate). Therefore [5]: dm i,ex = G i,ex + S i DR J i,d. (22) Insulin is produced by the pancreas at a rate dependent on the plasma glucose level. However, if concentration of glucose falls below a certain threshold (ϕ), insulin production is interrupted. Thus [5]: { 0 Cg,ex ϕ G i,ex = β(c g,ex ϕ) C g,ex > ϕ. (23) Additionally, insulin is destroyed through a reaction involving the insulinase enzyme, at a rate proportional to its concentration in blood [5]: DR i = αc i,ex. (24) 2.4. Dialysis. In the model, several variables were introduced for the description of the influence of the renal replacement therapy on fluids and solutes in the body: Q described fluid ultrafiltration flow rate across the hemodialyzer membrane (during hemodialysis) or across peritoneal tissue to dialysate (during peritoneal dialysis), J s,t described solute flow rate during treatment (in the hemodialyzer when t = hd or peritoneal dialysis when t = pd ). Hemodialysis. In hemodialysis, excess of fluid is removed due to transmembrane pressure gradient (which causes an ultrafiltration flow across the membrane), whereas solute removal is achieved by diffusion (driven by the difference of solute concentration between dialysate and blood) and convection (due to ultrafiltration). Transmembrane pressure gradient is set in automatic ultrafiltration control system (artificial kidney). Hemodialyzer efficiency of solute removal is described by dialysance defined as the amount of solute removed from the blood per unit time, divided by the solute concentration difference between blood and dialysate at the dialyzer inlet [11]: D s = Q BiC Bi Q Bo C Bo C Bi C Di (25) where: Q Bi, Q Bo blood flows at the dialyzer inlet and outlet, C Bi, C Bo substance concentrations in blood at the dialyzer inlet and outlet, C Di substance concentration in dialysate at the dialyzer inlet. When the solute concentration in dialysate is equal to 0 (e.g. urea), dialysance is called clearance. Ultrafiltration from blood to dialysate increases solute flow in the following way [6,12]: C s,ex D s = D 0,s + T rq f, (26) C s,ex C s,d where D s dialysance of solute s in the dialyzer (or clearance when C s,d = 0), D 0,s pure diffusive dialysance (clearance) when the ultrafiltration flow rate is equal to 0, T r transmittance coefficient, Q f ultrafiltration flow rate, C s,ex concentration of solute s in blood, C s,d concentration of solute s in dialysate. Diffusive dialysance (clearance) is usually provided by dialyzer manufacturer or can be determined experimentally and calculated for particular solute using Eq. (25) with Q Bi = Q Bo = Q B (Q f = 0). A simple linear description of T r can be obtained assuming that each kind of transport (diffusive and convective) occurs separately: first pure diffusion and then pure convection [6,12]: ( T r = S 1 D ) 0,s, (27) Q B where S sieving coefficient, Q B blood flow rate. Sieving coefficient describes the membrane property for a particular solute (sieving coefficient is between 0 and 1): sieving coefficient equal to 0 means that the membrane is impermeable for the solute, while value of 1 means that membrane is absolutely permeable for the substance [11]. In Eq. (8), variable J s,hd described the solute s flow rate in the dialyzer. Hence, from Eqs. (26) and (27) (assuming S = 1 Bull. Pol. Ac.: Tech. 53(3)
6 M. Gałach and A. Weryński for all small solutes): J s,hd = D s (C s,ex C s,d ( = (D 0,s 1 Q ) ) f + Q f c s,ex D 0.s c s,d, Q B (28) In virtual patient model fluid flow rate caused by hemodialysis (Q in Eq. (1)) is simply described as fluid ultrafiltration flow rate, thus: Q = Q f. (29) Peritoneal dialysis. Nowadays, peritoneal dialysis, in dependence on country, comprises of 5% to 45% of the population of renal failure patients [11]. Physiological mechanisms of water and solute transport across the peritoneum are rather complex. Recently, peritoneal microcirculation and transport across the peritoneum is an important subject of various studies [13,14]. In this article the new theoretical model, where interstitium was included as a separate compartment, is proposed. The model is partly based on the three-pore model [4] extended by the description of fluid uptake by absorption due to the Starling forces (i.e. forces that cause the exchange of fluids between the intravascular and interstitial space [8]) in addition to the absorption by lymphatics. The three-pore model of peritoneal transport has been used for description of the transport of fluid and solutes between interstitium and blood. It is assumed, that capillaries in the peritoneal tissue are heteroporous with three sizes of pores: large pores (radius 250 Ä), small pores (radius 43 Ä), and ultrasmall pores (radius 2 Ä). A large number of small pores causes that membrane is permeable to most small solutes, whereas low number of large pores allows the transport of macromolecules (proteins) from blood to peritoneal cavity. Ultrasmall pores (aquaporins) reject solute transport, and play an important role in osmotic water transport. The basic assumption of this model is the following idea: when the solute or fluid leaves the peritoneal cavity, it must first enter the interstitium and then be sieved by the capillary wall (Figs. 7a,b). In accordance with a scheme in Fig. 7a, the dialysate volume depends basically on the following factors: ultrafiltration flow rate from interstitium to dialysate (Q UI D ), fluid absorption rate due to hydrostatic pressure difference between dialysate and interstitial fluid (Q AD I ) and systemic lymphatic flow rate (L s ). Therefore: dv D = Q UI D Q AD I L s. (30) Change of interstitial volume results from ultrafiltration flow rate from interstitium to dialysate (Q UI D ), ultrafiltration flow rate from blood to interstitium (Q UB I ), fluid absorption rate due to hydrostatic pressure difference from dialysate to interstitum (Q AD I ), fluid absorption rate due to Starling forces from interstitium to blood (Q AI B ) and local lymphatic flow rate (L s ). Thus: dv I = Q AD I Q UI D + Q UB I Q AI B L L. (31) Mesothelial barrier between peritoneal cavity and interstitium is very permeable to substances [14,15], therefore it is assumed that ultrafiltration flow rate from blood to interstitium (Q UB I ) and ultrafiltration flow rate from interstitium to dialysate (Q UI D ) are equal. Q UB I depends on the hydrostatic pressure difference and crystalloid osmotic pressure difference between blood and interstitium. Therefore: Q UI D = Q UB I = Q Us + Q Ul + Q Uu (32) where Q Up is the ultrafiltration flow rate through the pore p (p: s small, l lagre, u ultrasmall) and: Q Up = α p L p S P σ s,p π s, (33) s P Fig. 7. Scheme of the compartmental model describing transport of fluids (a) and solutes (b) between dialysate and blood during peritoneal dialysis; V c volume of fluid in compartment c (c = ex extracellular fluid, I peritoneal interstitium, D dialysate), Q A fluid absorption rate (I B from interstitium to blood, D I from dialysate to interstitium), Q U fluid ultrafiltration rate (B I from blood to interstitium, I D from interstitium to dialysate), L s and L L systemic and local lymphatic flow rate, C s,c concentration of solute s in compartment c, K s permeability surface area coefficient for solute s, J s,p solute s flow through pores p (p = small or large ) where α p the fraction of L p S accounted for by the specific set of pore (α s +α l +α u = 1), L p S ultrafiltration coefficient, P hydrostatic pressure gradient between blood and interstitium ( P = P B P I, P C hydrostatic pressure in compartment C: B blood, I interstitium, D dialysate), σ s,p solute s osmotic reflection coefficient for pore p, π s osmotic pressure gradient between blood and interstitium for solute s ( π s = RT (C s,b C s,i ), RT the product of gas constant and the absolute temperature, C s,c concentration of solute s in compartment C (assuming C s,b = C s,ex ). Starling s hypothesis states that the fluid movement across the blood capillaries walls depends on the balance of the hydrostatic pressure gradient and the oncotic pressure gradient (oncotic pressure is a pressure due to the presence of large protein molecules) [8]. As mesothelial barrier between peritoneal cavity and interstitium is very permeable [14,15], the concentrations of the solutes (e.g. protein) in dialysate and interstitium are almost equal. Thus only hydrostatic pressure difference is important in fluid absorption from dialysate to interstitium: Q AD I = L p S 1 (P D P I ). (34) 288 Bull. Pol. Ac.: Tech. 53(3) 2005
7 Development of virtual patient model for simulation of solute and fluid transport during dialysis Whereas fluid absorption from interstitium to blood is described using the following equation: Q AI B = Q As + Q Al + Q Au, (35) where L p S 1 ultrafiltration coefficient, Q Ap fluid absorption through the pore p, and: Q Ap = α p L p S ( P σ Pr,p π Pr ) (36) where Pr protein. In virtual patient model fluid flow rate caused by peritoneal dialysis (Q in Eq. (1)) is described as: Q = Q UB I Q AI B L s L L. (37) The solute flow between dialysate and interstitium is caused by diffusion (driven by the difference of solute s concentrations in dialysate and interstitium), convection (due to ultrafiltration), fluid absorption due to Starling forces and fluid absorption due to lymphatics (Fig. 7 b). Thus, the mass balance equation for solute in dialysate can be written as follows: dm s,d = K s (C s,i C s,d ) Q AD I C s,d + S s Q UI D C s,i L s C s,d, (38) where: M s,c mass of solute s in compartment C, K s permeability surface area coefficient for solute s, S s sieving coefficient for solute s (as membrane between peritoneal cavity and interstitium is highly permeable, high value of K s and S s = 1 were assumed for all solutes). Change of the solute mass in the interstitium can be caused by solute flow between dialysate and interstitium and solute flow between interstitium and blood. Thus: dm s,i = Q AD I C s,d S s Q UI D C s,i K s (C s,i C s,d ) (39) L L C s,i + J Ss,s + J Ss,l, where: J Ss,p solute s flow through pores p (p: s small or l large) according to three-pore model [4] and: J Ss,p = J Vp (1 σ s,p ) C s,b C s,i e P e s,p 1 e P e, (40) s,p where P e s,p Peclet number given by P e s,p = J Vp (1 σ s,p ) /P S s,p (P S s,p is a permeability surface area for pore p and solute s) and J Vp the fluid flow through the pores yielded by, [4]: J Vp = Q Up Q Ap, (41) where Q Up is given by (33) and Q Ap is given by (36). In Eq. (6), variable J s,pd describes the solute s flow rate due to peritoneal dialysis. Therefore: J s,pd = J Ss,s + J Ss,l L L C s,i L s C s,d. (42) 3. Materials and methods The presented virtual patient model is described as a set of ordinary differential equations and algebraic equations with various types of nonlinearities: square root, exponential function, division by variable and rise to the power. This model has been implemented in computer using program Matlab. The system of ordinary differential equation was solved using function ode45. Function ode45 is based on an explicit Runge-Kutta (4,5) formula. To evaluate the model, fluid transport was studied in several groups of patients in various hospitals. A single six hour dwell study using standard glucose 3.86% dialysis fluid in 20 patients on continuous ambulatory peritoneal dialysis were carried on in Divisions of Baxter Novum and Renal Medicine, Department of Clinical Sciences, Karolinska University Hospital Huddinge, Stockholm, Sweden (Prof. B. Lindholm). Also, model was verified using data from the Department of Internal Medicine University Hospital Maastricht, the Nederlands (Prof. J. Kooman). These data (concerning 11 patients undergone hemodialysis) were from pilot study in which there was investigated the hemodynamic effects of different dialysis procedures during hemodialysis, mainly differing in their sodium handling. Additionally, the data from Department of Nephrology, Louis Pasteur District Hospital, Cherbourg, France (dr P. Freida) were used. During these investigations various dialysate compositions were tested and compared in 7 patients undergone a single fifteen hours peritoneal dialysis: dialysate with glucose 3.86%, dialysate with icodextrin 7.5%, and dialysate with mixture of glucose 2.73% and icodextrin 6.8%. 4. Results The virtual patient model was applied to simulate the behaviour of pressures, fluids and solutes during hemodialysis and peritoneal dialysis. Parameters describing typical patient were taken from literature [1 4,8,14,16] and used in simulations. Peritoneal dialysis patient treated with 3.86% glucose dialysis solution is exposed to the high glucose concentrations, which can lead to metabolic problem such as hyperglycemia, hyperinsulinemia or obesity, also glycation of peritoneal membrane proteins may be responsible for membrane failure. To avoid this situation a combination fluid with a small glucose concentration and icodextrin was evaluated [17]. Therefore, simulations were carried out to predict the behavior of the dialysate volume and the concentration of the dialysate glucose during the peritoneal dialysis, using various dialysis fluid compositions (3.86% glucose, 7.5% icodextrin and 2.73% glucose/6.8% icodextrin, Figs. 8 and 9). It turned out that the combination fluid (Fig. 9) removes larger volumes of water than 3.86% glucose fluid (Fig. 8). Fluid and solute transport was also studied in a single six hour dwell study using standard glucose 3.86% dialysis fluid in 20 patients on continuous ambulatory peritoneal dialysis (data from Stockholm, Sweden). As shown in Fig. 10, there was a good agreement between simulations of the model and experimental data. The model allows for differentiation of the nature of the fluid uptake: lymphatic absorption or absorption of the fluid due to Starling forces. In three-pore model [4] the fluid uptake is described inadequately by assuming that fluid absorption is by systemic lymphatics only. Physiological investigations show that a rate of fluid absorption via lymphatics is about 0.3 Bull. Pol. Ac.: Tech. 53(3)
8 M. Gałach and A. Weryński Fig. 8. Dialysate volume (a) and concentration of dialysate glucose (b) during peritoneal dialysis (data from Cherbourg, France) with glucose dialysis fluid; model simulation, experimental data Fig. 9. Dialysate volume (a) and concentration of dialysate glucose (b) during peritoneal dialysis (data from Cherbourg, France) with bimodal solution with glucose 2.7% and icodextrin 6.8%; model simulation, experimental data Fig. 10. Simulation of the dialysate volume (a) and glucose concentration (b) during peritoneal dialysis (data from Stockholm, Sweden): simulation results, X experimental data (mean value with standard deviation) 290 Bull. Pol. Ac.: Tech. 53(3) 2005
9 Development of virtual patient model for simulation of solute and fluid transport during dialysis Fig. 11. Relative blood volume, ( ) (a) and mean arterial pressure, (mmhg) (b) during standard hemodialysis (data from Maastricht, the Nederlands) experimental data, simulation result Fig. 12. Relative blood volume, ( ) (a) and mean arterial pressure, (mmhg) (b) during hemodialysis with sodium profile (data from Maastricht, the Nederlands) experimental data, simulation result ml/min, [13,18]. However various studies with volume marker have shown that the fluid absorption rate is above 1 ml/min, [19 21]. This also is confirmed by the simulations of the model. Usually, hemodialysis sessions are carried out in the cycle with the intervals between the sessions lasting 2 3 days. One session usually takes 4 5 hour (the relative risk of death is lowest at treatment times of 4.5 to 5 hours, [22]). Control of extracellular volume is one of the primary targets of dialysis therapy. Inadequate sodium and fluid removal by dialysis may result in extracellular volume overload, hypertension and increased cardiovascular morbidity and mortality. Virtual patient model could be used for simulation of various hemodialysis procedures. In Figs. 11 and 12 two procedures were shown: standard dialysis (with sodium concentration 140 mmol/l) and dialysis with sodium profiling (with sodium concentration decrease from 148 mmol/l to 136 mmol/l). Figures 11 and 12 show the behaviour of the blood volume and mean arterial pressure during dialysis. Again, the model reproduced the clinical data rather correctly. 5. Conclusions The presented virtual patient is a computer model of basic, short-term physiological phenomena. It is important for understanding the body s response to hemodialysis and/or peritoneal dialysis. The model was used for simulations of solutes concentrations and fluids volumes changes during the peritoneal dialysis with different dialysis solutions and during various procedures of hemodialysis. The developed model seems to provide efficient description of the fluid and solute transport during dialysis treatments (hemodialysis ir peritoneal dialysis). However, this model still requires verification and confirmation. REFERENCES [1] J.T. Daugirdas, P.G. Blake, and S. Todd, Handbook of Dialysis, Lippincot, Williams and Wilkins, [2] M. Ursino and M. Innocenti, Modeling arterial hypotension during hemodialysis, Artif. Organs 21(8), (1997). [3] L. Coli, M. Ursino, A. De Pascalis, C. Brighenti, V. Dalmastri, G. La Manna, E. Isola, G. Cianciolo, D. Patrono, P. Boni, and Bull. Pol. Ac.: Tech. 53(3)
10 M. Gałach and A. Weryński S. Stefoni, Evaluation of intradialytic solute and fluid kinetics. Setting up a predictive mathematical model, Blood Purif. 18(1), (2000). [4] B. Rippe, and L. Levin, Computer simulations of ultrafiltration profiles for an icodextrin-based peritoneal fluid in CAPD, Kidney Int. 57(6), (2000). [5] M.C.K. Khoo, Physiological Control Systems; Analysis, Simulation, and Estimation, New York, IEEE Press, [6] M. Galach, A. Ciechanowska, S. Sabalinska, J. Waniewski, J. Wojcicki, and A. Werynski, Impact of convective transport on dialyzer clearance, J. Artif. Organs 6(1), (2003). [7] M. Galach and A. Werynski, Mathematical modeling of renal replacement therapies, Biocybernetics and Biomedical Engineering 24(4), 3 18 (2004). [8] A.C. Guyton, Textbook of Medical Physiology, W.B. Saunders Company, [9] W. Grzeszczak, Diabetes mellitus serious nefrological problem in XXI century, Progresses of Medical Sciences XIV(1 2), (2003), (in Polish). [10] V.B. Mountcastle, Medical Physilogy, St. Louis, C.V. Mosby, [11] M. Darowski, T. Orłowski, A. Weryński, and J.M. Wójcicki, Artificial organs, Biocybernetics and Biomedical Engineering , 523 (2001), (in Polish). [12] A. Werynski, Evaluation of the impact of ultrafiltration on dialyzer clearance, Artif. Organs 3(2), (1979). [13] B. Rippe, B.I. Rosengren, and D. Venturoli, The peritoneal microcirculation in peritoneal dialysis, Microcirculation 8(5), (2001). [14] D. Venturoli and B. Rippe, Transport asymmetry in peritoneal dialysis: application of a serial heteroporous peritoneal membrane model, Am. J. Physiol. Renal Physiol. 280(4), F (2001). [15] M.F. Flessner, The transport barrier in intraperitoneal therapy, Am. J. Physiol. Renal Physiol. 288(3), F (2005). [16] D.G. Struijk, G.C. Koomen, R.T. Krediet, and L. Arisz, Indirect measurement of lymphatic absorption in CAPD patients is not influenced by trapping, Kidney Int. 41(6), (1992). [17] S.B. Jenkins and M.E. Wilkie, An exploratory study of a novel peritoneal combination dialysate (1.36% glucose/7.5% icodextrin), demonstrating improved ultrafiltration compared to either component studied alone, Perit. Dial. Int. 23(5), (2003). [18] J.T. Daugirdas, T.S. Ing, V.C. Gandhi, J.E. Hano, W.T. Chen, and L. Yuan, Kinetics of peritoneal fluid absorption in patients with chronic renal failure, J. Lab. Clin. Med. 95(3), (1980). [19] J. Waniewski, O. Heimburger, A. Werynski, and B. Lindholm, Simple models for fluid transport during peritoneal dialysis, Int. J. Artif. Organs 19(8), (1996). [20] M. Flessner, Effective lymphatic absorption rate is not a useful or accurate term to use in the physiology of peritoneal dialysis, Perit. Dial. Int. 24(4), , discussion (2004). [21] R.T. Krediet, The effective lymphatic absorption rate is an accurate and useful concept in the physiology of peritoneal dialysis, Perit. Dial. Int. 24(4), , discussion (2004). [22] I. Uhlenbush-Korwer, E. Bonnie-Schorn, A. Grassmann, and J. Vienken, Understanding Membranes and Dialysers, Lengerich: Pabst Science Publishers, Bull. Pol. Ac.: Tech. 53(3) 2005
Physiology of Blood Purification: Dialysis & Apheresis. Outline. Solute Removal Mechanisms in RRT
 Physiology of Blood Purification: Dialysis & Apheresis Jordan M. Symons, MD University of Washington School of Medicine Seattle Children s Hospital Outline Physical principles of mass transfer Hemodialysis
Physiology of Blood Purification: Dialysis & Apheresis Jordan M. Symons, MD University of Washington School of Medicine Seattle Children s Hospital Outline Physical principles of mass transfer Hemodialysis
How to evaluate the peritoneal membrane?
 How to evaluate the peritoneal membrane? B. Bammens Brussels, May 12 2016 BELGIUM How to evaluate a hemodialyzer? How to evaluate a hemodialyzer? How to evaluate a hemodialyzer? From: Robert W. Schrier
How to evaluate the peritoneal membrane? B. Bammens Brussels, May 12 2016 BELGIUM How to evaluate a hemodialyzer? How to evaluate a hemodialyzer? How to evaluate a hemodialyzer? From: Robert W. Schrier
Free water transport, small pore transport and the osmotic pressure gradient
 Nephrol Dial Transplant (2008) 23: 2350 2355 doi: 10.1093/ndt/gfm768 Advance Access publication 5 November 2007 Original Article Free water transport, small pore transport and the osmotic pressure gradient
Nephrol Dial Transplant (2008) 23: 2350 2355 doi: 10.1093/ndt/gfm768 Advance Access publication 5 November 2007 Original Article Free water transport, small pore transport and the osmotic pressure gradient
Sequential peritoneal equilibration test: a new method for assessment and modelling of peritoneal transport
 Nephrol Dial Transplant (2013) 28: 447 454 doi: 10.1093/ndt/gfs592 Sequential peritoneal equilibration test: a new method for assessment and modelling of peritoneal transport Magda Galach 1, Stefan Antosiewicz
Nephrol Dial Transplant (2013) 28: 447 454 doi: 10.1093/ndt/gfs592 Sequential peritoneal equilibration test: a new method for assessment and modelling of peritoneal transport Magda Galach 1, Stefan Antosiewicz
Objectives. Peritoneal Dialysis vs. Hemodialysis 02/27/2018. Peritoneal Dialysis Prescription and Adequacy Monitoring
 Peritoneal Dialysis Prescription and Adequacy Monitoring Christine B. Sethna, MD, EdM Division Director, Pediatric Nephrology Cohen Children s Medical Center Associate Professor Hofstra Northwell School
Peritoneal Dialysis Prescription and Adequacy Monitoring Christine B. Sethna, MD, EdM Division Director, Pediatric Nephrology Cohen Children s Medical Center Associate Professor Hofstra Northwell School
Ana Paula Bernardo. CHP Hospital de Santo António ICBAS/ Universidade do Porto
 Ana Paula Bernardo CHP Hospital de Santo António ICBAS/ Universidade do Porto Clinical relevance of hyperphosphatemia Phosphate handling in dialysis patients Phosphate kinetics in PD peritoneal phosphate
Ana Paula Bernardo CHP Hospital de Santo António ICBAS/ Universidade do Porto Clinical relevance of hyperphosphatemia Phosphate handling in dialysis patients Phosphate kinetics in PD peritoneal phosphate
Drug Use in Dialysis
 (Last Updated: 08/22/2018) Created by: Socco, Samantha Drug Use in Dialysis Drambarean, B. (2017). Drug Use in Dialysis. Lecture presented at PHAR 503 Lecture in UIC College of Pharmacy, Chicago. DIALYSIS
(Last Updated: 08/22/2018) Created by: Socco, Samantha Drug Use in Dialysis Drambarean, B. (2017). Drug Use in Dialysis. Lecture presented at PHAR 503 Lecture in UIC College of Pharmacy, Chicago. DIALYSIS
Chapter 12. Excretion and the Interaction of Systems
 Chapter 12 Excretion and the Interaction of Systems 1 2 Goals for This Chapter 1. Identify the main structures and functions of the human excretory system 2. Explain the function of the nephron 3. Describe
Chapter 12 Excretion and the Interaction of Systems 1 2 Goals for This Chapter 1. Identify the main structures and functions of the human excretory system 2. Explain the function of the nephron 3. Describe
What is a PET? Although there are many types of pets, we will be discussing the Peritoneal Equilibration Test
 1 2 3 What is a PET? Although there are many types of pets, we will be discussing the Peritoneal Equilibration Test 4 Background information about the PET 1983 Dr. Twardowski and colleagues began measuring
1 2 3 What is a PET? Although there are many types of pets, we will be discussing the Peritoneal Equilibration Test 4 Background information about the PET 1983 Dr. Twardowski and colleagues began measuring
Hyaluronan Influence on Diffusive Permeability of the Peritoneum In Vitro
 Advances in Peritoneal Dialysis, Vol. 24, 2008 Teresa Grzelak, Beata Szary, Krystyna Czyzewska Hyaluronan Influence on Diffusive Permeability of the Peritoneum In Vitro Hyaluronan (HA), an essential component
Advances in Peritoneal Dialysis, Vol. 24, 2008 Teresa Grzelak, Beata Szary, Krystyna Czyzewska Hyaluronan Influence on Diffusive Permeability of the Peritoneum In Vitro Hyaluronan (HA), an essential component
Hemodialysis today has evolved
 Lessons in Dialysis, Dialyzers, and Dialysate Robert Hootkins, MD, PhD The author is Chief of Nephrology and Hypertension at The Austin Diagnostic Clinic, Austin, Texas. He is also a member of D&T s editorial
Lessons in Dialysis, Dialyzers, and Dialysate Robert Hootkins, MD, PhD The author is Chief of Nephrology and Hypertension at The Austin Diagnostic Clinic, Austin, Texas. He is also a member of D&T s editorial
Body Fluids and Fluid Compartments
 Body Fluids and Fluid Compartments Bởi: OpenStaxCollege The chemical reactions of life take place in aqueous solutions. The dissolved substances in a solution are called solutes. In the human body, solutes
Body Fluids and Fluid Compartments Bởi: OpenStaxCollege The chemical reactions of life take place in aqueous solutions. The dissolved substances in a solution are called solutes. In the human body, solutes
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Physiology Unit 3 CARDIOVASCULAR PHYSIOLOGY: THE VASCULAR SYSTEM
 Physiology Unit 3 CARDIOVASCULAR PHYSIOLOGY: THE VASCULAR SYSTEM In Physiology Today Hemodynamics F = ΔP/R Blood flow (F) High to low pressure Rate = L/min Pressure (P) Hydrostatic pressure Pressure exerted
Physiology Unit 3 CARDIOVASCULAR PHYSIOLOGY: THE VASCULAR SYSTEM In Physiology Today Hemodynamics F = ΔP/R Blood flow (F) High to low pressure Rate = L/min Pressure (P) Hydrostatic pressure Pressure exerted
Renal Disease and PK/PD. Anjay Rastogi MD PhD Division of Nephrology
 Renal Disease and PK/PD Anjay Rastogi MD PhD Division of Nephrology Drugs and Kidneys Kidney is one of the major organ of drug elimination from the human body Renal disease and dialysis alters the pharmacokinetics
Renal Disease and PK/PD Anjay Rastogi MD PhD Division of Nephrology Drugs and Kidneys Kidney is one of the major organ of drug elimination from the human body Renal disease and dialysis alters the pharmacokinetics
Tala Saleh. Riham Abu Arrah, Abdallah AlQawasmeh. Yanal Shafagoj
 27 Tala Saleh Riham Abu Arrah, Abdallah AlQawasmeh Yanal Shafagoj Cardiovascular system Think of the following situation: 5 Cancerous cells (for example: Lymphoma cells) are placed in a proper medium with
27 Tala Saleh Riham Abu Arrah, Abdallah AlQawasmeh Yanal Shafagoj Cardiovascular system Think of the following situation: 5 Cancerous cells (for example: Lymphoma cells) are placed in a proper medium with
Microcirculation and Edema. Faisal I. Mohammed MD, PhD.
 Microcirculation and Edema Faisal I. Mohammed MD, PhD. Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchang in capillaries. Outline what
Microcirculation and Edema Faisal I. Mohammed MD, PhD. Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchang in capillaries. Outline what
UNDERSTANDING THE CRRT MACHINE
 UNDERSTANDING THE CRRT MACHINE Helen Dickie Renal Sister Critical Care Unit Guy s and St.Thomas NHS Foundation Trust 18.10.14 RRT options - IHD vs CRRT (1) Intermittent HaemoDialysis e.g. 4hrs daily or
UNDERSTANDING THE CRRT MACHINE Helen Dickie Renal Sister Critical Care Unit Guy s and St.Thomas NHS Foundation Trust 18.10.14 RRT options - IHD vs CRRT (1) Intermittent HaemoDialysis e.g. 4hrs daily or
Physiology of Circulation
 Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
Chapter 14 Blood Vessels, Blood Flow and Pressure Exam Study Questions
 Chapter 14 Blood Vessels, Blood Flow and Pressure Exam Study Questions 14.1 Physical Law Governing Blood Flow and Blood Pressure 1. How do you calculate flow rate? 2. What is the driving force of blood
Chapter 14 Blood Vessels, Blood Flow and Pressure Exam Study Questions 14.1 Physical Law Governing Blood Flow and Blood Pressure 1. How do you calculate flow rate? 2. What is the driving force of blood
Peritoneal Dialysis Prescriptions: A Primer for Nurses
 Peritoneal Dialysis Prescriptions: A Primer for Nurses A Primer ABCs of PD R x Betty Kelman RN-EC MEd CNeph (C) Toronto General Hospital University Health Network Toronto, Ontario, Canada A moment to remember
Peritoneal Dialysis Prescriptions: A Primer for Nurses A Primer ABCs of PD R x Betty Kelman RN-EC MEd CNeph (C) Toronto General Hospital University Health Network Toronto, Ontario, Canada A moment to remember
Ch 17 Physiology of the Kidneys
 Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Renal Replacement Therapies
 Renal Replacement Therapies M I H Á L Y T A P O L Y A I, M D, F A S N, F A C P A s s o c i a t e P r o f e s s o r D e p a r t m e n t o f N e p h r o l o g y L o u i s i a n a S t a t e U n i v e r s
Renal Replacement Therapies M I H Á L Y T A P O L Y A I, M D, F A S N, F A C P A s s o c i a t e P r o f e s s o r D e p a r t m e n t o f N e p h r o l o g y L o u i s i a n a S t a t e U n i v e r s
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Jacek Waniewski, 1 Stefan Antosiewicz, 2 Daniel Baczynski, 2 Jan Poleszczuk, 1 Mauro Pietribiasi, 1 Bengt Lindholm, 3 and Zofia Wankowicz 2
 Computational and Mathematical Methods in Medicine Volume 216, Article ID 824294, 1 pages http://dx.doi.org/1.1155/216/824294 Research Article Peritoneal Fluid Transport rather than Peritoneal Solute Transport
Computational and Mathematical Methods in Medicine Volume 216, Article ID 824294, 1 pages http://dx.doi.org/1.1155/216/824294 Research Article Peritoneal Fluid Transport rather than Peritoneal Solute Transport
5. Maintaining the internal environment. Homeostasis
 5. Maintaining the internal environment Homeostasis Blood and tissue fluid derived from blood, flow around or close to all cells in the body. Blood and tissue fluid form the internal environment of the
5. Maintaining the internal environment Homeostasis Blood and tissue fluid derived from blood, flow around or close to all cells in the body. Blood and tissue fluid form the internal environment of the
MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER
 MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER The glomerular filtration barrier consists of three elements: (1) endothelial
MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER The glomerular filtration barrier consists of three elements: (1) endothelial
Toward Optimal Adaptive Control of Hemodialysis
 Toward Optimal Adaptive Control of Hemodialysis by Nat Hemasilpin B.S.E.E., Faculty of Engineering, Chulalongkorn University, Thailand, 1975 M.S., Department of Electrical and Computer Engineering, University
Toward Optimal Adaptive Control of Hemodialysis by Nat Hemasilpin B.S.E.E., Faculty of Engineering, Chulalongkorn University, Thailand, 1975 M.S., Department of Electrical and Computer Engineering, University
Analysis of fluid transport pathways and their determinants in peritoneal dialysis patients with ultrafiltration failure
 original article http://www.kidney-international.org & 26 International Society of Nephrology Analysis of fluid transport pathways and their determinants in peritoneal dialysis patients with ultrafiltration
original article http://www.kidney-international.org & 26 International Society of Nephrology Analysis of fluid transport pathways and their determinants in peritoneal dialysis patients with ultrafiltration
Ultrafiltration failure (UFF) is an important cause of
 Peritoneal Dialysis International, Vol. 32, pp. 537 544 doi: 10.3747/pdi.2011.00175 0896-8608/12 $3.00 +.00 Copyright 2012 International Society for Peritoneal Dialysis TWO-IN-ONE PROTOCOL: SIMULTANEOUS
Peritoneal Dialysis International, Vol. 32, pp. 537 544 doi: 10.3747/pdi.2011.00175 0896-8608/12 $3.00 +.00 Copyright 2012 International Society for Peritoneal Dialysis TWO-IN-ONE PROTOCOL: SIMULTANEOUS
Microcirculation and Edema- L1 L2
 Microcirculation and Edema- L1 L2 Faisal I. Mohammed MD, PhD. University of Jordan 1 Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchanged
Microcirculation and Edema- L1 L2 Faisal I. Mohammed MD, PhD. University of Jordan 1 Objectives: Point out the structure and function of the microcirculation. Describe how solutes and fluids are exchanged
CVS Hemodynamics. Faisal I. Mohammed, MD,PhD.
 CVS Hemodynamics Faisal I. Mohammed, MD,PhD. Objectives point out the physical characteristics of the circulation: distribution of blood volume total cross sectional area velocity blood pressure List the
CVS Hemodynamics Faisal I. Mohammed, MD,PhD. Objectives point out the physical characteristics of the circulation: distribution of blood volume total cross sectional area velocity blood pressure List the
EXCRETION QUESTIONS. Use the following information to answer the next two questions.
 EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
BIOL 219 Spring Chapters 14&15 Cardiovascular System
 1 BIOL 219 Spring 2013 Chapters 14&15 Cardiovascular System Outline: Components of the CV system Heart anatomy Layers of the heart wall Pericardium Heart chambers, valves, blood vessels, septum Atrioventricular
1 BIOL 219 Spring 2013 Chapters 14&15 Cardiovascular System Outline: Components of the CV system Heart anatomy Layers of the heart wall Pericardium Heart chambers, valves, blood vessels, septum Atrioventricular
November 30, 2016 & URINE FORMATION
 & URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
& URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
The relationship between effluent potassium due to cellular release, free water transport and CA125 in peritoneal dialysis patients
 NDT Plus (2008) 1 [Suppl 4]: iv41 iv45 doi: 10.1093/ndtplus/sfn123 The relationship between effluent potassium due to cellular release, free water transport and CA125 in peritoneal dialysis patients Annemieke
NDT Plus (2008) 1 [Suppl 4]: iv41 iv45 doi: 10.1093/ndtplus/sfn123 The relationship between effluent potassium due to cellular release, free water transport and CA125 in peritoneal dialysis patients Annemieke
Implementing therapy-delivery, dose adjustments and fluid balance. Eileen Lischer MA, BSN, RN, CNN University of California San Diego March 6, 2018
 Implementing therapy-delivery, dose adjustments and fluid balance. Eileen Lischer MA, BSN, RN, CNN University of California San Diego March 6, 2018 Objectives By the end of this session the learner will
Implementing therapy-delivery, dose adjustments and fluid balance. Eileen Lischer MA, BSN, RN, CNN University of California San Diego March 6, 2018 Objectives By the end of this session the learner will
Managing Acid Base and Electrolyte Disturbances with RRT
 Managing Acid Base and Electrolyte Disturbances with RRT John R Prowle MA MSc MD MRCP FFICM Consultant in Intensive Care & Renal Medicine RRT for Regulation of Acid-base and Electrolyte Acid base load
Managing Acid Base and Electrolyte Disturbances with RRT John R Prowle MA MSc MD MRCP FFICM Consultant in Intensive Care & Renal Medicine RRT for Regulation of Acid-base and Electrolyte Acid base load
Fluid and electrolyte balance, imbalance
 Fluid and electrolyte balance, imbalance Body fluid The fluids are distributed throughout the body in various compartments. Body fluid is composed primarily of water Water is the solvent in which all solutes
Fluid and electrolyte balance, imbalance Body fluid The fluids are distributed throughout the body in various compartments. Body fluid is composed primarily of water Water is the solvent in which all solutes
Excretory System Workbook
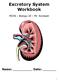 Excretory System Workbook MCHS Biology 20 Mr. Korotash Name: Date: 1 Study the diagram above. Name the structures and indicate their functions by completing the following table: Structure 1. Function 2.
Excretory System Workbook MCHS Biology 20 Mr. Korotash Name: Date: 1 Study the diagram above. Name the structures and indicate their functions by completing the following table: Structure 1. Function 2.
P215 SPRING 2019: CIRCULATORY SYSTEM Chaps 13, 14 & 15: pp , , , I. Major Functions of the Circulatory System
 P215 SPRING 2019: CIRCULATORY SYSTEM Chaps 13, 14 & 15: pp 360-390, 395-404, 410-428 433-438, 441-445 I. Major Functions of the Circulatory System 1. 2. 3. 4. II. Structure of the Heart 1. atria 2. ventricles
P215 SPRING 2019: CIRCULATORY SYSTEM Chaps 13, 14 & 15: pp 360-390, 395-404, 410-428 433-438, 441-445 I. Major Functions of the Circulatory System 1. 2. 3. 4. II. Structure of the Heart 1. atria 2. ventricles
ACUTE KIDNEY INJURY AND RENAL REPLACEMENT THERAPY IN CHILDREN. Bashir Admani KPA Precongress 24/4/2018
 ACUTE KIDNEY INJURY AND RENAL REPLACEMENT THERAPY IN CHILDREN Bashir Admani KPA Precongress 24/4/2018 Case presentation SP 11month old Presenting complaint: bloody diarrhea, lethargy On exam: dehydration,
ACUTE KIDNEY INJURY AND RENAL REPLACEMENT THERAPY IN CHILDREN Bashir Admani KPA Precongress 24/4/2018 Case presentation SP 11month old Presenting complaint: bloody diarrhea, lethargy On exam: dehydration,
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Free water transport: Clinical implications. Sodium sieving during short very hypertonic dialysis exchanges
 Free water transport: Clinical implications Raymond T Krediet, MD,PhD University of Amsterdam Sodium sieving during short very hypertonic dialysis exchanges Nolph KD et al. Ann Int Med 1969;70:931-947
Free water transport: Clinical implications Raymond T Krediet, MD,PhD University of Amsterdam Sodium sieving during short very hypertonic dialysis exchanges Nolph KD et al. Ann Int Med 1969;70:931-947
3/21/2017. Solute Clearance and Adequacy Targets in Peritoneal Dialysis. Peritoneal Membrane. Peritoneal Membrane
 3/21/2017 Solute Clearance and Adequacy Targets in Peritoneal Dialysis Steven Guest MD Director, Medical Consulting Services Baxter Healthcare Corporation Deerfield, IL, USA Peritoneal Membrane Image courtesy
3/21/2017 Solute Clearance and Adequacy Targets in Peritoneal Dialysis Steven Guest MD Director, Medical Consulting Services Baxter Healthcare Corporation Deerfield, IL, USA Peritoneal Membrane Image courtesy
Cardiovascular system: Blood vessels, blood flow. Latha Rajendra Kumar, MD
 Cardiovascular system: Blood vessels, blood flow Latha Rajendra Kumar, MD Outline 1- Physical laws governing blood flow and blood pressure 2- Overview of vasculature 3- Arteries 4. Capillaries and venules
Cardiovascular system: Blood vessels, blood flow Latha Rajendra Kumar, MD Outline 1- Physical laws governing blood flow and blood pressure 2- Overview of vasculature 3- Arteries 4. Capillaries and venules
Rq : Serum = plasma w/ fibrinogen and other other proteins involved in clotting removed.
 Functions of the blood Transport Nutritive Respiratory Excretory Hormone transport Temperature regulation Acid base balance ph (7.30 7.45) Protective (immunology) Rq : It comprises both ECF (plasma) &
Functions of the blood Transport Nutritive Respiratory Excretory Hormone transport Temperature regulation Acid base balance ph (7.30 7.45) Protective (immunology) Rq : It comprises both ECF (plasma) &
Failure to obtain adequate rates of ultrafiltration (UF) is
 Page 1 of 6 Peritoneal Dialysis International Peritoneal Dialysis International, inpress www.pdiconnect.com 0896-8608/16 $3.00 +.00 Copyright 2016 International Society for Peritoneal Dialysis ANALYSIS
Page 1 of 6 Peritoneal Dialysis International Peritoneal Dialysis International, inpress www.pdiconnect.com 0896-8608/16 $3.00 +.00 Copyright 2016 International Society for Peritoneal Dialysis ANALYSIS
Nephrology Dialysis Transplantation
 Nephrol Dial Transplant (1996) 11 [Suppl 8]: 10-15 Nephrology Dialysis Transplantation Urea, sodium, and water changes in profiling dialysis H. Mann and S. Stiller ntroduction Control of osmolarity, as
Nephrol Dial Transplant (1996) 11 [Suppl 8]: 10-15 Nephrology Dialysis Transplantation Urea, sodium, and water changes in profiling dialysis H. Mann and S. Stiller ntroduction Control of osmolarity, as
Ch 19: The Kidneys. Functional unit of kidneys:?? Developed by John Gallagher, MS, DVM
 Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
The functions of the kidney:
 The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
Potassium secretion. E k = -61 log ([k] inside / [k] outside).
![Potassium secretion. E k = -61 log ([k] inside / [k] outside). Potassium secretion. E k = -61 log ([k] inside / [k] outside).](/thumbs/80/80478709.jpg) 1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
1 Potassium secretion In this sheet, we will continue talking about ultrafiltration in kidney but with different substance which is K+. Here are some informations that you should know about potassium;
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Filtration and Reabsorption Amount Filter/d
 Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Functional morphology of kidneys Clearance
 Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
Free-water transport in fast transport status: A comparison between CAPD peritonitis and long-term PD
 Kidney International, Vol. 65 (2004), pp. 298 303 Free-water transport in fast transport status: A comparison between CAPD peritonitis and long-term PD WATSKE SMIT, NICOLE VAN DEN BERG, NATALIE SCHOUTEN,
Kidney International, Vol. 65 (2004), pp. 298 303 Free-water transport in fast transport status: A comparison between CAPD peritonitis and long-term PD WATSKE SMIT, NICOLE VAN DEN BERG, NATALIE SCHOUTEN,
Blood Flow, Blood Pressure, Cardiac Output. Blood Vessels
 Blood Flow, Blood Pressure, Cardiac Output Blood Vessels Blood Vessels Made of smooth muscle, elastic and fibrous connective tissue Cells are not electrically coupled Blood Vessels Arteries arterioles
Blood Flow, Blood Pressure, Cardiac Output Blood Vessels Blood Vessels Made of smooth muscle, elastic and fibrous connective tissue Cells are not electrically coupled Blood Vessels Arteries arterioles
Homeostatic Regulation
 Homeostatic Regulation A hormone is :a Water-soluble hormones: Composed of amino acids and bind a receptor protein on the of the target cell. This starts a signal cascade inside the cell and the signal
Homeostatic Regulation A hormone is :a Water-soluble hormones: Composed of amino acids and bind a receptor protein on the of the target cell. This starts a signal cascade inside the cell and the signal
STUDIES ON ULTRAFILTRATION IN PERITONEAL DIALYSIS: INFLUENCE OF PLASMA PROTEINS AND CAPILLARY BLOOD FLOW
 STUDIES ON ULTRAFILTRATION IN PERITONEAL DIALYSIS: INFLUENCE OF PLASMA PROTEINS AND CAPILLARY BLOOD FLOW ABSTRACT Claudio Ronco Alessandra Brendolan Luisa Bragantini Stefano Chiaramonte Mariano Feriani
STUDIES ON ULTRAFILTRATION IN PERITONEAL DIALYSIS: INFLUENCE OF PLASMA PROTEINS AND CAPILLARY BLOOD FLOW ABSTRACT Claudio Ronco Alessandra Brendolan Luisa Bragantini Stefano Chiaramonte Mariano Feriani
2016 Annual Dialysis Conference Michelle Hofmann RN, BSN, CNN Renal Clinical Educator - Home
 Fluid Management 2016 Annual Dialysis Conference Michelle Hofmann RN, BSN, CNN Renal Clinical Educator - Home Objectives Define euvolemia Determine factors which contribute to fluid imbalance Discuss strategies
Fluid Management 2016 Annual Dialysis Conference Michelle Hofmann RN, BSN, CNN Renal Clinical Educator - Home Objectives Define euvolemia Determine factors which contribute to fluid imbalance Discuss strategies
Chapter 15 Fluid and Acid-Base Balance
 Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
Chapter 15 Fluid and Acid-Base Balance by Dr. Jay M. Templin Brooks/Cole - Thomson Learning Fluid Balance Water constitutes ~60% of body weight. All cells and tissues are surrounded by an aqueous environment.
Physiology Lecture 2. What controls GFR?
 Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
Any of these questions could be asked as open question or lab question, thus study them well
 Any of these questions could be asked as open question or lab question, thus study them well describe the factors which regulate cardiac output describe the sympathetic and parasympathetic control of heart
Any of these questions could be asked as open question or lab question, thus study them well describe the factors which regulate cardiac output describe the sympathetic and parasympathetic control of heart
Transport through membranes
 Transport through membranes Membrane transport refers to solute and solvent transfer across both cell membranes, epithelial and capillary membranes. Biological membranes are composed of phospholipids stabilised
Transport through membranes Membrane transport refers to solute and solvent transfer across both cell membranes, epithelial and capillary membranes. Biological membranes are composed of phospholipids stabilised
Balance point characterization of interstitial fluid volume regulation
 Am J Physiol Regul Integr Comp Physiol 297: R6 R16, 2009. First published May 6, 2009; doi:10.1152/ajpregu.00097.2009. Balance point characterization of interstitial fluid volume regulation R. M. Dongaonkar,
Am J Physiol Regul Integr Comp Physiol 297: R6 R16, 2009. First published May 6, 2009; doi:10.1152/ajpregu.00097.2009. Balance point characterization of interstitial fluid volume regulation R. M. Dongaonkar,
(D) (E) (F) 6. The extrasystolic beat would produce (A) increased pulse pressure because contractility. is increased. increased
 Review Test 1. A 53-year-old woman is found, by arteriography, to have 5% narrowing of her left renal artery. What is the expected change in blood flow through the stenotic artery? Decrease to 1 2 Decrease
Review Test 1. A 53-year-old woman is found, by arteriography, to have 5% narrowing of her left renal artery. What is the expected change in blood flow through the stenotic artery? Decrease to 1 2 Decrease
Microcirculation. Lecture Block 11 (contributions from Brett Burton)
 Lecture Block 11 (contributions from Brett Burton) Elements of Arterioles, capillaries, venules Structure and function: transport Fluid balance Lymph system Vessels of the Circulatory System Diameter Aorta
Lecture Block 11 (contributions from Brett Burton) Elements of Arterioles, capillaries, venules Structure and function: transport Fluid balance Lymph system Vessels of the Circulatory System Diameter Aorta
OT Exam 3, August 19, 2002 Page 1 of 6. Occupational Therapy Physiology, Summer Examination 3. August 19, 2002
 Page 1 of 6 Occupational Therapy Physiology, Summer 2002 Examination 3 August 19, 2002 There are 20 questions and each question is worth 5 points for a total of 100 points. Dr. Heckman's section is questions
Page 1 of 6 Occupational Therapy Physiology, Summer 2002 Examination 3 August 19, 2002 There are 20 questions and each question is worth 5 points for a total of 100 points. Dr. Heckman's section is questions
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
CAPILLARY FLUID EXCHANGE
 CAPILLARY FLUID EXCHANGE Aubrey E. Taylor and Timothy M. Moore Department of Physiology, University of South Alabama, College of Medicine, Mobile, Alabama 36688-0002 AM. J. PHYSIOL. 277 (ADV. PHYSIOL.
CAPILLARY FLUID EXCHANGE Aubrey E. Taylor and Timothy M. Moore Department of Physiology, University of South Alabama, College of Medicine, Mobile, Alabama 36688-0002 AM. J. PHYSIOL. 277 (ADV. PHYSIOL.
Body fluids. Lecture 13:
 Lecture 13: Body fluids Body fluids are distributed in compartments: A. Intracellular compartment: inside the cells of the body (two thirds) B. Extracellular compartment: (one third) it is divided into
Lecture 13: Body fluids Body fluids are distributed in compartments: A. Intracellular compartment: inside the cells of the body (two thirds) B. Extracellular compartment: (one third) it is divided into
Capillary vessel. A) permeability which can vary between tissues, within tissues at different times and along the capillary
 I. Capillary bed structure Single layer of endothelium supports diffusion MedSoc Teaching CRH Session 2 Capillary circualtion Chanel Tobinska Arteriole Capillary vessel Venules BLOOD Blood flow velocity
I. Capillary bed structure Single layer of endothelium supports diffusion MedSoc Teaching CRH Session 2 Capillary circualtion Chanel Tobinska Arteriole Capillary vessel Venules BLOOD Blood flow velocity
Peritoneal transport testing
 THOROUGH CRITICAL APPRAISAL www.sin-italy.org/jnonline www.jnephrol.com Peritoneal transport testing Vincenzo La Milia Nephrology and Dialysis Department, A. Manzoni Hospital, Lecco - Italy Ab s t r a
THOROUGH CRITICAL APPRAISAL www.sin-italy.org/jnonline www.jnephrol.com Peritoneal transport testing Vincenzo La Milia Nephrology and Dialysis Department, A. Manzoni Hospital, Lecco - Italy Ab s t r a
An event-based point of view on the control of insulin-dependent diabetes
 An event-based point of view on the control of insulin-dependent diabetes Brigitte Bidégaray-Fesquet Laboratoire Jean Kuntzmann Univ. Grenoble Alpes, France Réunion e-baccuss September 4th 20 Réunion e-baccuss,
An event-based point of view on the control of insulin-dependent diabetes Brigitte Bidégaray-Fesquet Laboratoire Jean Kuntzmann Univ. Grenoble Alpes, France Réunion e-baccuss September 4th 20 Réunion e-baccuss,
ONLINE HEMODIALYSIS TRAINING SESSION 1
 ONLINE HEMODIALYSIS TRAINING SESSION 1 This document is a supplement to the Online Training. Do not reproduce. Copyright Dialysis4Career. All Rights Reserved. The Renal System - A highly sophisticated
ONLINE HEMODIALYSIS TRAINING SESSION 1 This document is a supplement to the Online Training. Do not reproduce. Copyright Dialysis4Career. All Rights Reserved. The Renal System - A highly sophisticated
Blood Pressure. a change in any of these could cause a corresponding change in blood pressure
 Blood Pressure measured as mmhg Main factors affecting blood pressure: 1. cardiac output 2. peripheral resistance 3. blood volume a change in any of these could cause a corresponding change in blood pressure
Blood Pressure measured as mmhg Main factors affecting blood pressure: 1. cardiac output 2. peripheral resistance 3. blood volume a change in any of these could cause a corresponding change in blood pressure
Chapter 9, Part 2. Cardiocirculatory Adjustments to Exercise
 Chapter 9, Part 2 Cardiocirculatory Adjustments to Exercise Electrical Activity of the Heart Contraction of the heart depends on electrical stimulation of the myocardium Impulse is initiated in the right
Chapter 9, Part 2 Cardiocirculatory Adjustments to Exercise Electrical Activity of the Heart Contraction of the heart depends on electrical stimulation of the myocardium Impulse is initiated in the right
Renal Physiology Intro to CRRT Concepts. Catherine Jones September 2017
 Renal Physiology Intro to CRRT Concepts Catherine Jones September 2017 Learning Outcomes To revise anatomy & physiology of kidney in health: To understand basic principles of continuous renal replacement
Renal Physiology Intro to CRRT Concepts Catherine Jones September 2017 Learning Outcomes To revise anatomy & physiology of kidney in health: To understand basic principles of continuous renal replacement
Unit 1 Matter & Energy for Life
 Unit 1 Matter & Energy for Life Chapter 2 Interaction of Cell Structure Biology 2201 Primary Membrane Function: Homeostasis Conditions in the cell must remain more or less constant under many different
Unit 1 Matter & Energy for Life Chapter 2 Interaction of Cell Structure Biology 2201 Primary Membrane Function: Homeostasis Conditions in the cell must remain more or less constant under many different
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
Volume Management 2/25/2017. Disclosures statement: Objectives. To discuss evaluation of hypervolemia in peritoneal dialysis patients
 Volume Management Sagar Nigwekar MD, MMSc Massachusetts General Hospital E-mail: snigwekar@mgh.harvard.edu March 14, 2017 Disclosures statement: Consultant: Allena, Becker Professional Education Grant
Volume Management Sagar Nigwekar MD, MMSc Massachusetts General Hospital E-mail: snigwekar@mgh.harvard.edu March 14, 2017 Disclosures statement: Consultant: Allena, Becker Professional Education Grant
Outline. Model Development GLUCOSIM. Conventional Feedback and Model-Based Control of Blood Glucose Level in Type-I Diabetes Mellitus
 Conventional Feedback and Model-Based Control of Blood Glucose Level in Type-I Diabetes Mellitus Barış Ağar, Gülnur Birol*, Ali Çınar Department of Chemical and Environmental Engineering Illinois Institute
Conventional Feedback and Model-Based Control of Blood Glucose Level in Type-I Diabetes Mellitus Barış Ağar, Gülnur Birol*, Ali Çınar Department of Chemical and Environmental Engineering Illinois Institute
PERITONEAL EQUILIBRATION TEST. AR. Merrikhi. MD. Isfahan University of Medical Sciences
 PERITONEAL EQUILIBRATION TEST AR. Merrikhi. MD. Isfahan University of Medical Sciences INTRODUCTION The peritoneal equilibration test (PET) is a semiquantitative assessment of peritoneal membrane transport
PERITONEAL EQUILIBRATION TEST AR. Merrikhi. MD. Isfahan University of Medical Sciences INTRODUCTION The peritoneal equilibration test (PET) is a semiquantitative assessment of peritoneal membrane transport
Cardiovascular Physiology III.
 Cardiovascular Physiology III. 43. The microcirculation: capillary solute exchange and fluid dynamics. 44. The microcirculation: lymphatic circulation and edema formation. 45. The characteristics of the
Cardiovascular Physiology III. 43. The microcirculation: capillary solute exchange and fluid dynamics. 44. The microcirculation: lymphatic circulation and edema formation. 45. The characteristics of the
Dr. Mohamed S. Daoud Biochemistry Department College of Science, KSU. Dr. Mohamed Saad Daoud
 Dr. Mohamed S. Daoud Biochemistry Department College of Science, KSU 1 Course symbol: BCH 472 Course Title: Biochemistry of biological fluids Credit hours: 3(2+1) 2 Clinical Biochemistry is one of the
Dr. Mohamed S. Daoud Biochemistry Department College of Science, KSU 1 Course symbol: BCH 472 Course Title: Biochemistry of biological fluids Credit hours: 3(2+1) 2 Clinical Biochemistry is one of the
A&P 2 CANALE T H E U R I N A R Y S Y S T E M
 A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
Lecture 10. Circulatory systems; flow dynamics, flow regulation in response to environmental and internal conditions.
 Lecture 10 Circulatory systems; flow dynamics, flow regulation in response to environmental and internal conditions Professor Simchon Influence of P O2 on Hemoglobin Saturation Hemoglobin saturation plotted
Lecture 10 Circulatory systems; flow dynamics, flow regulation in response to environmental and internal conditions Professor Simchon Influence of P O2 on Hemoglobin Saturation Hemoglobin saturation plotted
Geriatric Nutritional Risk Index, home hemodialysis outcomes 131
 Subject Index Aksys PHD system 113 Anemia, home outcomes 111, 172, 173 Automated peritoneal dialysis dialysis comparison 17, 18 selection factors 18, 19 telemedicine system 19 21 Blood pressure -peritoneal
Subject Index Aksys PHD system 113 Anemia, home outcomes 111, 172, 173 Automated peritoneal dialysis dialysis comparison 17, 18 selection factors 18, 19 telemedicine system 19 21 Blood pressure -peritoneal
 IN THE NAME OF GOD Uremic toxins I. Small (< 500 D); water soluble Surrogate marker urea or sodium (ionic dialysance) Rapidly produced in intracellular fluid compartment Large variability in intra-patient
IN THE NAME OF GOD Uremic toxins I. Small (< 500 D); water soluble Surrogate marker urea or sodium (ionic dialysance) Rapidly produced in intracellular fluid compartment Large variability in intra-patient
There are no shortcuts to Dialysis
 There are no shortcuts to Dialysis 1 Outcomes John Sweeny Wednesday, March 21 st, 2018 (3:10 pm 4:10 pm) 2 Quality in Hemodialysis Quality Health Care is the degree to which health services increases the
There are no shortcuts to Dialysis 1 Outcomes John Sweeny Wednesday, March 21 st, 2018 (3:10 pm 4:10 pm) 2 Quality in Hemodialysis Quality Health Care is the degree to which health services increases the
PHYSIOEX 3.0 EXERCISE 33B: CARDIOVASCULAR DYNAMICS
 PHYSIOEX 3.0 EXERCISE 33B: CARDIOVASCULAR DYNAMICS Objectives 1. To define the following: blood flow; viscosity; peripheral resistance; systole; diastole; end diastolic volume; end systolic volume; stroke
PHYSIOEX 3.0 EXERCISE 33B: CARDIOVASCULAR DYNAMICS Objectives 1. To define the following: blood flow; viscosity; peripheral resistance; systole; diastole; end diastolic volume; end systolic volume; stroke
** Accordingly GFR can be estimated by using one urine sample and do creatinine testing.
 This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
Cardiac Output 1 Fox Chapter 14 part 1
 Vert Phys PCB3743 Cardiac Output 1 Fox Chapter 14 part 1 T. Houpt, Ph.D. Regulation of Heart & Blood Pressure Keep Blood Pressure constant if too low, not enough blood (oxygen, glucose) reaches tissues
Vert Phys PCB3743 Cardiac Output 1 Fox Chapter 14 part 1 T. Houpt, Ph.D. Regulation of Heart & Blood Pressure Keep Blood Pressure constant if too low, not enough blood (oxygen, glucose) reaches tissues
Please remember that based on your personalized learning plan and your past experience you may not be required to complete all four modules.
 Course Overview Welcome to the Team PD pre-work participant guide. Team PD is a new education program offered by Baxter that utilizes individual learning maps so nurses receive a personalized approach
Course Overview Welcome to the Team PD pre-work participant guide. Team PD is a new education program offered by Baxter that utilizes individual learning maps so nurses receive a personalized approach
LLL Session - Nutritional support in renal disease
 ESPEN Congress Leipzig 2013 LLL Session - Nutritional support in renal disease Peritoneal dialysis D. Teta (CH) Nutrition Support in Patients undergoing Peritoneal Dialysis (PD) Congress ESPEN, Leipzig
ESPEN Congress Leipzig 2013 LLL Session - Nutritional support in renal disease Peritoneal dialysis D. Teta (CH) Nutrition Support in Patients undergoing Peritoneal Dialysis (PD) Congress ESPEN, Leipzig
Complete the table below to compare the processes of excretion and secretion.
 1. Excretion and secretion are two processes that take place in the body of a mammal. Complete the table below to compare the processes of excretion and secretion. excretion secretion one difference one
1. Excretion and secretion are two processes that take place in the body of a mammal. Complete the table below to compare the processes of excretion and secretion. excretion secretion one difference one
