Blood glucose test strips (BGTS) evaluation protocol and results. Update April Version 4.1. Note:
|
|
|
- Cameron Crawford
- 6 years ago
- Views:
Transcription
1 Blood glucose test strips (BGTS) evaluation protocol and results Update April 2016 Version 4.1 Note: December 2017: Prescribing data indicates overall compliance with the GMMMG recommended BGTS of 86%. This number is increasing and indicates both a high level of prescribing of preferred strips and satisfaction with the choices already available. Therefore, while it is acknowledged some strips may now meet ISO15197, GM CCGs have agreed that there is sufficient choice of strips and further reviews are postponed indefinitely. Whilst this document may be printed, the electronic version maintained on the Greater Manchester Medicines Management Group (GMMMG) website is the controlled copy and should be used in its entirety. Any printed copies of this document are not controlled. It is the responsibility of every individual to ensure that they access the most current version of this document.
2 DOCUMENT CONTROL Document Location Copies of this document can be obtained from: Name: Address: Medicines Optimisation Team North West CSU St James s House Pendleton Way Salford M6 5FW Telephone: Revision History The latest and master version of this document is held on the Medicines Management SharePoint: REVISION ACTIONED SUMMARY OF CHANGES DATE BY 12/11/2015 J Cheung Following Sept/October 2015 re-review and changes to the evaluation scoring. Updated report. 07/12/2015 J Cheung Updated report following NICE type 2 diabetes guidance publication and response to changes to evaluation scoring. 01/04/2016 J Cheung Evaluation and summary sheet updated following new independent and published evidence - Contour TS and Contour testing strips. Summary sheet amended as per communication with the following manufacturers/distributors: TRUEyou - Haematocrit range. Corrected and reflected in scoring FreeStyle Lite and Optium - Blood glucose reading range. Corrected and reflected in scoring Footnote added on Summary sheet beside criterion - Haematocrit range between 30-60% (or more). Section 5 and 6 amended as per changes above. VERSION 05/10/2016 A Pracz Front page and section 7 updated with information regarding next review /12/2017 A Martin Front page and section 7 further updated with information cancelling review until further notice. Approvals This document must be approved by the following before distribution: NAME TITLE DATE OF ISSUE VERSION GMMMG Blood Glucose Test Strip (BGTS) evaluation protocol and results 13/11/ GMMMG Blood Glucose Test Strip (BGTS) evaluation protocol and results 17/12/ H. Burgess Chair of GMMMG 08/04/ Distribution This document has been distributed to: NAME TITLE DATE OF ISSUE VERSION CCG Leads Blood Glucose Test Strip (BGTS) evaluation protocol 1.0 GMMMG members GMMMG members DATE OF REVIEW Blood Glucose Test Strip (BGTS) evaluation protocol and results 11/05/ June 2016 Blood Glucose Test Strip (BGTS) evaluation protocol and results Dec June 2016 H. Burgess Chair of GMMMG 08/04/ GMMMG members Blood Glucose Test Strip (BGTS) evaluation protocol and results Apr June 2016 Updated BGTS evaluation report and results. October2016. V4.1 Page 2 of 15
3 1 Background 1.1 The Greater Manchester Medicines Management Group (GMMMG) aims to identify and champion the appropriate use of medicines across Greater Manchester taking into account cost effectiveness, quality, equity and patient safety. The group consists of General Practitioners (GP), pharmacists and other key healthcare professionals and is formally accountable to the Greater Manchester collaboration of 12 clinical commissioning groups (CCG), NHS England Area Team and local NHS providers. The GMMMG work plan is facilitated and supported by the Regional Drug & Therapeutics Centre in Newcastle and the Greater Manchester Shared Services (GMSS). In addition to medicines management, the GMMMG s role has recently been broadened to monitor the use and prescribing of specific medical devices. 1.2 The aim of this decision aid is to provide a description of the process, methodology and scoring mechanism to select a preferred blood glucose testing strip or strips (BGTS) for Greater Manchester. This intends to support GMMMG to recommend which BGTS available on the UK market offer comprehensive and high level accuracy monitoring whilst being cost effective to the health economy. 2 Introduction 2.1 It is recognised that self-monitoring of blood glucose (SMBG) is an integral part of the management of diabetes for some individuals especially those individuals with type 1 diabetes and those with type 2 diabetes treated with insulin. It can allow individuals to see what impact particular behaviours, such as dietary habits or exercise, can have on their glycaemic control, thus allowing them to understand results and adjust their behaviour in a beneficial way. 2.2 NICE guideline recommends that SMBG is indicated for all individuals with type 1 diabetes 1, 2, 3 and to only adults with type 2 diabetes 4 if one or more of the following applies: the person is on insulin or there is evidence/ suspected hypoglycaemic episodes or the person is on oral medication that may increase their risk of hypoglycaemia while driving or operating machinery or the person is pregnant, or is planning to become pregnant or the person is starting with oral or intravenous corticosteroids. There has already been work undertaken to implement NICE guidance across Greater Manchester and as such this guidance will concentrate on the selection of ISO (international standards) compliant SMBG systems. 2.3 In 2015 there are over 156,000 patients with diabetes in Greater Manchester according to the latest QOF figures 5 and this number has been increasing every year. Individuals with diabetes monitor their blood glucose to educate themselves, maintain better blood glucose control and to minimise the risks of hypoglycaemia. 2.4 In 2015, the total spend across Greater Manchester on BGTS was in excess of 8.5m, an increase of over 500K from As of October 2015 there are 62 varieties of BGTS funded within the NHS 7 with prices ranging from for 50 strips. The wide range of BGTS and meters enables individuals with diabetes to select a system that best meets their needs, albeit whilst adding complexity for healthcare professionals. 2.5 BGTS and meters are medical devices, not medicines. As such the process to market is different and less robust. For a medicine, randomised controlled trials (RCT) and a product licence are required. To obtain a drug tariff listing in England for a BGTS the process is to complete a DT1 form 8. This form requires information regarding the manufacturer, the product and the supporting Updated BGTS evaluation report and results. October2016. V4.1 Page 3 of 15
4 material regarding accuracy and the Conformité Européenne (CE) mark (as opposed to RCT data for a medicine). 2.6 The European Association for the Study of Diabetes (EASD) issued a position statement in March questioning the robustness of the procedure by which medical devices in diabetes, including BGTS and meters get to market and are evaluated post marketing. As these devices are potentially used to alter the dose of an administered medication i.e. insulin, it is vital that blood glucose meters and strips give accurate results when used to avoid any serious consequences. Considering the position statement from the EASD this document has also considered data beyond the drug tariff listing and includes a review of available published accuracy data for BGTS and meter to international accuracy standards. 2.7 BGTS and meters have an international standard that they should be manufactured to - ISO The standard from 2003 was recently updated in The new standard has implications not only for the manufacturers of currently available and future devices but also for the end-users. The manufacturers have 3 years from the date of the new standard update to meet the new requirements before compliance becomes mandatory from June The ISO standard requires a complex series of tests and requirements to be completed internally with the results assessed by a regulatory notifying body. It is clear that there has been concern at the lack of consistent performance of many BGTS after regulatory clearance and as a result the new standard and tighter accuracy will be an important criterion for consideration 10. The ISO 15197: 2013 requirements for BGTS and meters differ from the previous 2003 version on the following points in terms of enhanced accuracy requirements 7 (Figure 1). Figure 1: ISO 15197: 2003 ISO 15197:2013 Higher level accuracy >4.2mmol/l +/- 20% >5.5mmol/l +/- 15% Lower level accuracy <4.2mmol/l +/- 0.83mmol/l <5.5mmol/l +/- 0.83mmol/l Number of lots 1 3 Results in zone A/B of Clarke Error Grid n/a 99% Note: There are many other differences published by the international standard but these are the key accuracy differences. For a BGTS and meter to surpass the accuracy requirements for ISO 15197:2013 it is required to have the above high and low level accuracy across 3 lots (or batches) of test strips, with all results in Zone A/B of a Clarke Error Grid 10. Updated BGTS evaluation report and results. October2016. V4.1 Page 4 of 15
5 3 Aims 3.1 In line with the principles of GMMMG, the aim of the protocol was: To provide better support for patients in the effective utilisation of BGTS To improve the cost effective use of BGTS in Greater Manchester To support CCGs and NHS providers in the delivery of an evidence based rationale on selection of a preferred brand of BGTS from the large variety available 3.2 The intentions of this protocol were NOT to: There are widely available reports of individuals with diabetes being denied access to BGTS 11. It is therefore important to state that this protocol was not intended to deny access to BGTS nor was it an exercise in cutting cost. However it is possible that cost savings may be a consequence of the recommendations following the protocol findings. This protocol was an evaluation tool to enable clear and transparent assessment of available data in relation to BGTS provisions to the CCG s of Greater Manchester looking to optimise expenditure and support for individual patients requiring SMBG. It was not a tender process, as no contract award will be made as a consequence of this protocol since all devices are freely available via NHS Drug Tariff listing 4 Review Process 4.1 Initially, a review of existing evaluations using the internet was undertaken to identify guidance available on BGTS. This stage did not result in scoring for a BGTS but helped with the selection of the type of guideline categories used in other areas of the country. Guidelines can be broadly separated into two cohorts. Those that have focused on appropriate use of BGTS and those that have focused on acquisition cost. A significant number of guidelines recommend using meters with strips that cost less than 10 although there appear to be minimal or no reviews of available evidence associated with these. Other guidelines appear to focus on the appropriate use of BGTS and separate individual users into existing treatments and define as to whether a patient should be testing. 4.2 In Greater Manchester, a pass or fail and scoring process was undertaken to evaluate the preferred BGTS and meter. If any BGTS received a fail at Stage 1 (see overleaf) then they were excluded from any further scoring within the process. 4.3 The following representatives were involved in the project group in 2014/15: Specialist nursing and clinicians with interest in SMBG Patient representation through specialist clinicians Commissioners and; Medicines optimisation leads/pharmacists The project group reserved the right to select meters based upon characteristics for unique categories of patients. Any categories identified at this stage were scored within the questions to suppliers as part of the evaluation. 4.4 The BGTS currently included within the NHS Drug Tariff (October 2015) were all assessed and scored according the following review process overleaf. Updated BGTS evaluation report and results. October2016. V4.1 Page 5 of 15
6 Stage 1: A review of the currently available manufacturers and independent accuracy evidence of blood glucose meters and strips A detailed review of the manufacturer s evidence and its source, plus an independent comprehensive literature search was undertaken to identify BGTS and meters that met the following essential criteria. GMSS Medicines Optimisation team ensured all submitted and searched data were reviewed, without bias and confirmed accuracy to standards claimed. This stage was undertaken in advance of stages 2, 3 and 4. Any blood glucose systems not meeting this essential criterion below did not proceed. Essential Criteria: Pass or Fail Group 1 (1st choice): Manufacturers provide independent and published evidence of attainment of ISO 15197: 2013 accuracy standards as set out in Figure 1. AND Group 2: Manufacturers provide Independent evidence only of attainment of ISO 15197: 2013 accuracy standards as set out in Figure 1. Note: As this protocol will be in place for at least 1 year it is vital that ISO 15197:2013 is considered as this will be enforceable by June Stage 2: A review of desirable features that is offered to users The previous GMMMG BGTS evaluation process (archived - version 2.0 May 2015) required a review of the BGTS essential features (agreed by the project group) with a strict pass/fail response only. A fail at this stage of the evaluation meant that the BGTS was excluded completely from the review. Following comments from NHS colleagues and specialist in the field, the essential criteria could be seen as subjective and excluding potentially quality assured options could be considered biased and unfair for local decisions to be made. As a result, the essential criteria has been renamed as desirable features and scored accordingly i.e. 2 points per desirable criteria met. The manufacturers and suppliers that fulfilled mandatory requirements of Stage 1 were subsequently required to provide additional information on the following below points. Desirable criteria: Scored 0 or 2 Free meters to NHS locations and service users (minimum UK current stock 10,000 meters). Essential to mitigate against the risk of significant change in use within a locality the size of Greater Manchester. Free replacement batteries, log books, lancing pens. Technical support provided via freephone number (not answering machine). Free support material and meter training for all healthcare professionals. Free internal control solution. Measures only in mmol/l units and cannot be changed. Provides plasma-calibrated meter readings. Hematocrit range between 30-60% (or more). Measurement range between 1.1 to 33.3mmol/L (or more). Unable to delete readings from memory. No calibration or coding required. Expiry date of BGTS minimum 6 months from opening. Updated BGTS evaluation report and results. October2016. V4.1 Page 6 of 15
7 Stage 3: A review of the acquisition cost of BGTS Weighted scored 1-10 The acquisition cost of all BGTS was taken from the NHS Drug Tariff at the time of the assessment. The GMSS Medicines Optimisation team ranked BGTS deemed to offer cost effectiveness to the health economy by calculating a weighted score (WS). The WS was evaluated against cost of available BGTS by the following steps: WS = (Lowest cost BGTS per 50 strips divided by current BGTS price per 50 strips) multiplied by the weighted score i.e. 10. This will allow each strip to achieve a WS, calculated to one decimal point, out of the weighting for the priced element of the evaluation. The strip with the lowest price will be awarded a score of 10 i.e. 100% of the weighting. The remaining strips will be allocated a pro rata weighted score using the formula above. Stage 4: A review of added-value features and support offered to users Stage 4 required all partaking manufacturers/ distributors to review the non-essential but addedvalue features and support offered. Each criterion (agreed by the project group) met were awarded one point. Added-value features (based on project group decisions): Scored 0 or 1 Guarantee stability of pricing and available BGTS and meters. Starter meter pack available which includes BGTS and lancets. Sample under-fill detection. Able to apply more blood to the same test strip; if under-fill. Capillary fill function. Small sample size required ( 0.5µl). Measurement time ( 5 seconds). Sufficient memory capacity as per project group expectations. Meter set up is not required (e.g. date and time). However minor adjustment maybe required in BST/battery changes. The manufacturer can provide material and deliver training to patients and carers free of charge Manufacturers to provide records and evaluation of all training to all recipients and highlight learning outcomes achieved and any areas of concerns. The manufacturer supports any promotion of local guidelines for SMBG. Allow electronic download to personal computers and clinical systems. The manufacturer has alternative meters that may support other patient cohorts e.g. measures ketones, supports visually impaired, dexterity issues, gestational diabetes, paediatrics, insulin pump users. Manufacturer provides information of their MHRA product recall process and actions to be taken. Free independent external quality assurance for healthcare professionals in GP practices and insulin users who self-monitors blood glucose. Manufacturers were also given the opportunity to provide other additional features which may be considered by the GMSS Medicines Optimisation team. Note: Any evaluations carried out must be done in such circumstances to allow unbiased comparison of like for like features and consequently all stages of the review criteria were applied in an objective manner. Whilst we acknowledge there is always an element of subjectivity in any scoring system there is a transparent and auditable process to minimise the risk of a challenge should this occur at a later stage. Updated BGTS evaluation report and results. October2016. V4.1 Page 7 of 15
8 5 Results In summary, of the 62 BGTS available on the NHS (October 2015) the results were as follows: Figure 2: BGTS results overview Group 1 (First choice BGTS) submitted independent and published evidence of meeting ISO 15197: 2013 accuracy standards as per protocol Group 2 (Alternative choice) submitted independent evidence of meeting ISO 15197: 2013 accuracy standards as per protocol Manufacturers unable to submit the required evidence as per protocol and excluded from further evaluation Number of BGTS Excluded - No longer promoted and declined to partake 15 Non-responders to evaluation and excluded 9 Excluded - New BGTS added to Drug Tariff post GMMMG re-evaluation start date Figure 3 below presents the partaking manufacturers and their BGTS that were able to provide independent and published evidence demonstrating ISO 15197: 2013 accuracy standards i.e. Group 1. A summary of the available evidence for Group 1 BGTS can be found in Appendix 1. Six BGTS (Figure 4) were able to provide independently assessed but non-published evidence of conformity to ISO 15197: 2013 accuracy standards i.e. Group 2. Although such BGTS could not provide published evidence, it was felt the submitted independent evidence could be taken into consideration by Greater Manchester providers or commissioners. Figure 3: Group 1 (First choice) - BGTS and evaluation results Manufacturer Blood Glucose Test Strips Cost per 50 strips Evaluation process score (out of 50) Abbott FreeStyle Lite FreeStyle Optium Contour Next Bayer Contour TS Contour GlucoRx GlucoRx Nexus LifeScan OneTouch Verio OneTouch Select Plus Menarini Diagnostics GlucoMen Areo GlucoMen LX Neon Diagnostics Element GluNeo Nipro Diagnostics TRUEyou Aviva Roche Active Performa Mobile Sanofi BGStar Spirit Healthcare CareSens N TEE Updated BGTS evaluation report and results. October2016. V4.1 Page 8 of 15
9 Ypsomed MyLife Pura MyLife Unio Figure 4: Group 2 (Alternative choice) BGTS and evaluation results Manufacturer Blood Glucose Test Strips Cost per 50 strips Evaluation process score (out of 50) Agamatrix WaveSense Jazz (and Duo) Apollo Medical SuperCheck Plus SuperCheck B. Braun Omnitest HomeHealth UK SD Codefree Group 1 and 2 BGTS were further assessed and scored against a set of project group approved criteria based on cost, desirable functions or services, and added-value features. The maximum score any BGTS could achieve was 50 and full results of the assessment can be found in the Excel document link below Figure 5. Figure 5: Group 1 and 2 BGTS evaluation results summary BGTS Evaluation Summary April 2016 v Note: GMMMG accepts that due to potential subjective responses in the evaluation and a changing dynamic market; local decision makers may not select their preferred testing device based on the results of the above evaluation score. The above evaluation score provides guidance and support for local commissioners to consider when selecting preferred blood glucose monitoring devices. Updated BGTS evaluation report and results. October2016. V4.1 Page 9 of 15
10 6 Conclusion Inaccurate SMBG readings can potentially adversely impact clinical decision making and outcomes. The current application process of a CE mark on a SMBG system is a one-time procedure and it is generally assumed that these systems are equal in providing accurate test results. Unfortunately, regular and independent quality controls are not mandatory after market approval and SMBG systems may not all consistently meet the requirements outlined in ISO criteria. The recently revised and more stringent ISO 15197: 2013 standard should enhance patient safety by improving accuracy of SMBG systems but the adherence to the updated ISO standard remains based on manufacturers only submitting internal data on file (often non-published) to their certifying bodies demonstrating compliance. At the point of writing this conclusion, the evaluation identified 22 BGTS (Group 1) could provide robust independent and published evidence demonstrating compliance to the updated ISO 15197: 2013 accuracy standards. It is acknowledged that potential limitations of this evaluation include: - Manufacturers have until 2016 to demonstrate ISO 15197: 2013 compliance and are not mandated to provide independent published data demonstrating conformity - Even the highest quality published research can be vulnerable to publication bias - Although there is no official requirement of further regulatory proof in accuracy performance after the marketing process; many companies ensure the quality of their products through comprehensive, internal quality assurance proficiency testing. This is an essential and on-going step for companies to mitigate their liability risk as a device and strip manufacturer. The evaluation acknowledges the limitations and although internal data is considered adequate for BGTS market approval processes and even Drug Tariff listing, the validity and quality of an unpublished and nonpeer reviewed evidence is unknown. In addition, it may be argued that since there are no mandatory reviews after market approval of BGTS (including beyond the 2016 mandate); there remains a gap in the on-going policing and compliance to ISO standards. In summary, a high accuracy blood glucose monitoring system is an obvious requirement in ensuring patient safety and treatment quality in daily routine. During the last few decades and in line with the development of more sophisticated glucose meter technologies, the accuracy performance requirements have become more and more strict. Although several variables (e.g. user technique) are known to affect the accuracy of SMBG results, clinicians can reduce controllable variables by prescribing accurate and evidence based reproducible SMBG systems with minimal lot-to-lot variations. This evaluation process requests both independent and published evidence and consequently it is felt that Group 1 - BGTS provided greater and more robust evidence confirming compliance to ISO 15197: 2013 accuracy standards. In addition to the findings, GMMMG has recommended that those BGTS that did not fall into either Group 1 or 2 shall also be considered for the Greater Manchester Do Not Prescribe list. This is due to their decision not to partake in the evaluation and/or their inability to demonstrate compliance with the standards requested in this process (see Appendix 2). Updated BGTS evaluation report and results. October2016. V4.1 Page 10 of 15
11 7 Future Evaluation Review Prescribing data indicates overall compliance with the GMMMG recommended BGTS of 86%. This number is increasing and indicates both a high level of prescribing of preferred strips and satisfaction with the choices already available. Therefore, while some strips may now meet ISO15197, GM CCGs have agreed that there is sufficient choice of strips and further reviews are postponed indefinitely. Any new BGTS listed in the Drug Tariff will only be evaluated by the GMSS if it is considered by GMMMG sub-groups to have a significant impact on Greater Manchester health economy. GMSS Medicines Optimisation team will not accept any new information or evidence for any BGTS unless directed by the GMMMG subgroups for review. 8 References 1. National Institute for Health and Care Excellence (NICE) Clinical guideline NG17 Type 1 diabetes in adults: diagnosis and management. August Accessed August National Institute for Health and Care Excellence (NICE) Clinical guideline NG3 Diabetes in pregnancy: management from preconception to the postnatal period. February Accessed October National Institute for Health and Care Excellence (NICE) Clinical guideline NG18 Diabetes (type 1and type 2) in children and young people: diagnosis and management. August Accessed October National Institute for Health and Care Excellence (NICE) Clinical guideline NG28 Type 2 diabetes in adults: management. December Accessed December Accessed 1 st November Electronic Prescribing Analysis and Cost data (epact). Accessed 1 st November Accessed 1st October Accessed 31st January EASD Report on medical device accuracy. Accessed 19th January pdf. Accessed 19th January ISO website: Accessed. Accessed 19th January 2014 Updated and reviewed by: J Cheung (Medicines Optimisation Pharmacist) Updated BGTS evaluation report and results. October2016. V4.1 Page 11 of 15
12 9 Appendices Appendix 1: Published evidence of ISO 15197: 2013 accuracy standards Blood Glucose Testing Strip Aviva BGStar CareSens N Contour Next Contour TS Contour Element FreeStyle Lite FreeStyle Optium GlucoMen areo Sensor GlucoMen LX Sensor GlucoRx Nexus Strips GluNEO Mylife Pura Mylife Unio Manufacturer/ Distributor Roche Sanofi Spirit Healthcare Bayer Bayer Bayer Neon Diagnostics Abbott Abbott Menarini Menarini GlucoRx Neon Diagnostics Ypsomed Ypsomed Published evidence of meeting ISO 15197: 2013 accuracy standards (1) Pleus et al (2014) Accuracy Assessment of Two Novel Systems for Self- Monitoring of Blood Glucose ISO 15197: J Diabetes Sci Technol; DOI: / May 2014 (2) Baumstark et al (2012) Lot-to-Lot Variability of Test Strips and Accuracy Assessment of Ssystems for Self-Monitoring of Blood Glucose according to ISO J Diabetes Sci Technol; 6(5): Freckmann G et al (2015) System accuracy evaluation of different blood glucose monitoring systems following ISO 151:2013 by using two different comparison methods. J Diabetes Sci Technol; Vol 17 (9), DOI: 1089/dia Link M et al (2014) Accuracy Evaluation of Three Systems for Self-monitoring of Blood Glucose With Three Different Test Strip Lots Following ISO J Diabetes Sci Technol; 8(2): Different Test Strip Lots Following ISO (1) Bernstein et al (2013) A New Test Strip Technology Platform for Self- Monitoring of Blood Glucose. J Diabetes Sci Technol; 7(5): (2) Freckmann G et al (2015) System accuracy evaluation of different blood glucose monitoring systems following ISO 151:2013 by using two different comparison methods. J Diabetes Sci Technol; Vol 17 (9), DOI: 1089/dia Pleus et al (2016) Performance of two updated blood glucose monitoring systems: an evaluation following ISO 15197:2013. Current Medical Research and Opinion, DOI: / Feb 2016 Pleus et al (2016) Performance of two updated blood glucose monitoring systems: an evaluation following ISO 15197:2013. Current Medical Research and Opinion, DOI: / Feb 2016 Jeanny G and Hope P (2015) Surveillance of the system accuracy of two systems for self-monitoring of blood glucose after market approval. J Diabetes Sci Technol; DOI: / Sept Freckmann G et al (2015) System accuracy evaluation of different blood glucose monitoring systems following ISO 151:2013 by using two different comparison methods. J Diabetes Sci Technol; Vol 17 (9), DOI: 1089/dia [FreeStyle Lite test strips and FreeStyle InsuLinx meter] Brannan C (2015) Evaluation of the FreeStyle Precision Neo blood glucose and ketone monitoring system.perfusion 2015; 28: [FreeStyle Optium test strips and FreeStyle Precision Neo meter (alternatively named FreeStyle Optium Neo meter in the UK)] Berti F et al (2015) Accuracy evaluation of two blood glucose monitoring systems following ISO 15197:2013. J Diabetes Sci Technol; DOI: / July (1) Pfutzner et al. Evaluation of system accuracy of the GlucoMen LX Plus blood glucose monitoring system with reference to ISO 15197: J Diabetes Sci Technol; DOI: / th November 2015 Salzsieder E and Berg S (2015) Accuracy evaluation of a CE-marked system for self monitoring of blood glucose with three reagent system lts following ISO15197: J Diabetes Sci Technol; DOI: / September 2015 Jeanny G and Hope P (2015) Surveillance of the system accuracy of two systems for self-monitoring of blood glucose after market approval. J Diabetes Sci Technol; DOI: / Sept Freckmann G et al (2015) System accuracy evaluation of different blood glucose monitoring systems following ISO 151:2013 by using two different comparison methods. J Diabetes Sci Technol; Vol 17 (9), DOI: 1089/dia Huang Ta-you et al: Evaluation of accuracy of FAD-GDH and mutant Q-GDH based blood glucose monitors in multi-patient populations. Clinica Chimica Acta. 2014, 433: Updated BGTS evaluation report and results. October2016. V4.1 Page 12 of 15
13 OneTouch Select Plus OneTouch Verio Performa TEE2 TRUEyou Active Mobile Lifescan Lifescan Roche Spirit Healthcare Nipro Diagnostics Roche Roche Evaluation of the performance of the OneTouch Select Plus blood glucose test system against ISO15197: Expert review of medical device journal. 21st October Vol 6. DOI: / Katz L et al (2015) A comprehensive evaluation of strip performance in multiple blood glucose monitoring systems. Expert Review of Medical Devices Early online, 1 9 (2015) Please note that this study includes VerioPro, VerioVue and OmniPod (1) Pleus S et al (2014) Accuracy Assessment of Two Novel Systems for Self- Monitoring of Blood Glucose ISO 15197:2013. J Diabetes Sci Technol; DOI: / May (2) Freckmann G et al (2015) System accuracy evaluation of different blood glucose monitoring systems following ISO 151:2013 by using two different comparison methods. J Diabetes Sci Technol; Vol 17 (9), DOI: 1089/dia Link M et al (2014) Accuracy Evaluation of Three Systems for Self-monitoring of Blood Glucose With Three Different Test Strip Lots Following ISO J Diabetes Sci Technol; 8(2): Different Test Strip Lots Following ISO Jendrike N et al. ISO 15197:2013 Accuracy Evaluation of Two CE-Marked Systems for Self-Monitoring of Blood Glucose. Journal of Diabetes Science and Technology. DOI: / Baumstark et al. Accuracy Assessment of an Advanced Blood Glucose Monitoring System for Self Testing with three reagent system lots following ISO 15197: 2013 Baumstark A et al (2015) Accuracy evluation of an integrated blood glucose monitoring system with improved test cassesttes following ISO15197:2013. J Diabetes Sci Technol; DOI: / May Updated BGTS evaluation report and results. October2016. V4.1 Page 13 of 15
14 Appendix 2: GMMMG Do Not Prescribe List (Updated: April 2016) GMMMG has recommended that those BGTS that did not fall into either Group 1 or 2 will also be considered for the Greater Manchester Do Not Prescribe list. Healthcare professionals are advised to review and consider discontinuing BGTS listed below and if required consider an alternative approved SMBG device: Blood Glucose Test Strip Manufacturer/ Distributor Reasons for exclusion Advantage Plus Roche No longer promoted by manufacturer Advocate Redi-Code+ Pharma Supply Inc standards as per evaluation AutoSense Elektronika Kft Excluded No response Betachek C50 Betachek G5 National Diagnostic Products Pty Ltd National Diagnostic Products Pty Ltd Excluded No response Excluded No response Breeze 2 Bayer No longer promoted by manufacturer Compact Roche No longer promoted by manufacturer CozyLab S7 Health Integrated Technologies Ltd Excluded No response Dario Farla Medical Ltd Excluded No response FineTouch testing tips Terumo UK Ltd Excluded No response FreeStyle Abbott Laboratories Ltd No longer promoted by manufacturer GlucoDock Medisana No longer promoted by manufacturer GlucoLab Neon Diagnostics standards as per evaluation. In October 2015 Neon diagnostics have confirmed with the GMSS medicines optimisation team that independent and published evidence will be available confirming the system meets updated international standards. No further information had been provided within the evaluation period. GlucoMen GM Menarini Diagnostics No longer promoted by manufacturer GlucoMen Sensor Menarini Diagnostics No longer promoted by manufacturer GlucoMen Visio Menarini Diagnostics No longer promoted by manufacturer GlucoRx Original GlucoRx No longer promoted by manufacturer icare Advanced icare Advanced Solo IME-DC icare Medical UK Ltd icare Medical UK Ltd Arctic Medical standards as per evaluation standards as per evaluation standards as per evaluation MediSense Softsense Abbott Laboratories Ltd No longer promoted by manufacturer MediTouch Medisana No longer promoted by manufacturer Mendor Discreet Merck Sorono Excluded No response Microdot + Cambridge Sensors Excluded No response MyGlucoHealth Entra Health standards as per evaluation Updated BGTS evaluation report and results. October2016. V4.1 Page 14 of 15
15 On-Call Advanced Point Of Care Testing Ltd standards as per evaluation OneTouch Ultra LifeScan No longer promoted by manufacturer OneTouch Vita LifeScan No longer promoted by manufacturer Sensocard Elektronika Kft Excluded No response SURESIGN Resure Ciga Healthcare standards as per evaluation TRUEone Nipro Diagnostics No longer promoted by manufacturer TRUEresult Nipro Diagnostics No longer promoted by manufacturer TrueTrack System Nipro Diagnostics No longer promoted by manufacturer Note: The following BGTS - MODZ and ihealth; were NOT evaluated. The BGTS were added to Drug Tariff September and October 2015 respectively, post re-evaluation start date. Both BGTS will be invited for the next planned review in Updated BGTS evaluation report and results. October2016. V4.1 Page 15 of 15
CHEMICAL REAGENTS 478 PART 9 CHEMICAL REAGENTS. This document is dated 1 st March 2016.
 PART 9 This document is dated 1 st March 2016. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
PART 9 This document is dated 1 st March 2016. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
CHEMICAL REAGENTS 478 CHEMICAL REAGENTS. This document is dated 1 st October 2017
 This document is dated 1 st October 2017 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
This document is dated 1 st October 2017 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
CHEMICAL REAGENTS 478 PART 9 CHEMICAL REAGENTS. This document is dated 1 st July 2015.
 PART 9 This document is dated 1 st July 2015. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
PART 9 This document is dated 1 st July 2015. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
CHEMICAL REAGENTS 478 CHEMICAL REAGENTS. This document is dated 1 st March 2018
 This document is dated 1 st March 2018 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
This document is dated 1 st March 2018 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
HYDROXYBUTERATE) Gluco Rx Evolve KetoneTest Strips
 Scottish Drug Tariff Part 9 This document is dated 1 st November 2018 Page REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR BLOOD GLUCOSE FOR KETONES (BETA- HYDROXYBUTERATE) 478 DETECTION
Scottish Drug Tariff Part 9 This document is dated 1 st November 2018 Page REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR BLOOD GLUCOSE FOR KETONES (BETA- HYDROXYBUTERATE) 478 DETECTION
Blood Glucose Test Strip Guidelines
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
Guidance for the choice of blood glucose testing meters, strips and lancets
 Guidance for the choice of blood glucose testing meters, strips and lancets This guidance is intended to assist healthcare professionals in their selection of appropriate blood glucose meters and testing
Guidance for the choice of blood glucose testing meters, strips and lancets This guidance is intended to assist healthcare professionals in their selection of appropriate blood glucose meters and testing
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
Type 2 Diabetes Recommended SMBG
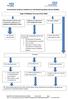 Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
GUIDELINE FOR SELF MONITORING OF BLOOD GLUCOSE IN ADULTS WITH DIABETES
 It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
Blood glucose monitoring and compatibility equipment checker
 Introduction This resource has been created to assist pharmacists, and other appropriately trained members of the pharmacy team, to ensure compatibility of insulin products/devices and blood glucose monitoring
Introduction This resource has been created to assist pharmacists, and other appropriately trained members of the pharmacy team, to ensure compatibility of insulin products/devices and blood glucose monitoring
NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary
 NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary 2018-20 Primary Care and Acute Joint Formulary Blood Glucose Meter formulary developed by the Diabetes
NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary 2018-20 Primary Care and Acute Joint Formulary Blood Glucose Meter formulary developed by the Diabetes
Walsall CCG Supported Blood Glucose Meters April 2018
 Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
NHS Greater Glasgow and Clyde Adult Blood Glucose Meter and Test Strip Formulary
 NHS Greater Glasgow and Clyde Adult Blood Glucose and Test Strip Formulary 2016-17 Primary Care and Acute Joint Formulary Blood Glucose formulary developed by the Diabetes MCN Blood Glucose Formulary Review
NHS Greater Glasgow and Clyde Adult Blood Glucose and Test Strip Formulary 2016-17 Primary Care and Acute Joint Formulary Blood Glucose formulary developed by the Diabetes MCN Blood Glucose Formulary Review
medicines_management/correspondence/pathway-for-the-managed- Access-of-FreeStyle-Libre.
 Pathway for the Managed Access of FreeStyle Libre (Flash Glucose monitoring) for Adults and Children in the care of Trust Specialist Diabetes Clinics in Northern Ireland www.hscboard.hscni.net/download/publications/pharmacy_and_
Pathway for the Managed Access of FreeStyle Libre (Flash Glucose monitoring) for Adults and Children in the care of Trust Specialist Diabetes Clinics in Northern Ireland www.hscboard.hscni.net/download/publications/pharmacy_and_
Commissioning Statement
 Commissioning Statement Treatment/ device For the treatment of Commissioning position Flash Glucose Monitoring Systems (including Freestyle Libre ) Monitoring glucose levels in adults and children over
Commissioning Statement Treatment/ device For the treatment of Commissioning position Flash Glucose Monitoring Systems (including Freestyle Libre ) Monitoring glucose levels in adults and children over
How to Access A Free Blood Glucose Meter
 How to Access A Free Abbott FreeStyle Lite FreeStyle Lite FreeStyle Freedom Lite FreeStyle InsuLinx How to Access A Free contacting their health care professionals at diabetes clinics; or calling the FreeStyle
How to Access A Free Abbott FreeStyle Lite FreeStyle Lite FreeStyle Freedom Lite FreeStyle InsuLinx How to Access A Free contacting their health care professionals at diabetes clinics; or calling the FreeStyle
DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER
 DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER 2017-404 Question/s to be Answered Can you please provide the following
DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER 2017-404 Question/s to be Answered Can you please provide the following
Commissioning Statement. Flash Glucose Monitoring system (FreeStyle Libre ) March 2018
 Technology Commissioning Statement Flash Glucose Monitoring system (FreeStyle Libre ) March 2018 FreeStyle Libre (Abbott) Flash Glucose Monitoring System for use in adults, young people and children. Recommendation
Technology Commissioning Statement Flash Glucose Monitoring system (FreeStyle Libre ) March 2018 FreeStyle Libre (Abbott) Flash Glucose Monitoring System for use in adults, young people and children. Recommendation
NHS Leeds CCG. Policy for the Funding of Flash Glucose Monitoring (FlashGM) in Paediatrics and Adults
 NHS Leeds CCG Policy for the Funding of Flash Glucose Monitoring (FlashGM) in Paediatrics and Adults Produced by: Jo Alldred, Medicines Effectiveness Lead, NHS Leeds CCG Dr Bryan Power, Long Term Conditions
NHS Leeds CCG Policy for the Funding of Flash Glucose Monitoring (FlashGM) in Paediatrics and Adults Produced by: Jo Alldred, Medicines Effectiveness Lead, NHS Leeds CCG Dr Bryan Power, Long Term Conditions
In keeping with the Scottish Diabetes Group criteria, use should be restricted to those who:
 Advice Statement 009-18 July 2018 Advice Statement What is the clinical and cost effectiveness of Freestyle Libre flash glucose monitoring for patients with diabetes mellitus treated with intensive insulin
Advice Statement 009-18 July 2018 Advice Statement What is the clinical and cost effectiveness of Freestyle Libre flash glucose monitoring for patients with diabetes mellitus treated with intensive insulin
DIABETES PATIENT STRATIFICATION AND TESTING SAVING MONEY, SAVING LIVES
 DIABETES PATIENT STRATIFICATION AND TESTING SAVING MONEY, SAVING LIVES Contents 2 The Rising Cost of Diabetes 5 Implementation 3 P atient Stratification is the Key to Saving Lives and Money 6 M eeting
DIABETES PATIENT STRATIFICATION AND TESTING SAVING MONEY, SAVING LIVES Contents 2 The Rising Cost of Diabetes 5 Implementation 3 P atient Stratification is the Key to Saving Lives and Money 6 M eeting
Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes
 Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
NHS GG&C Introduction of Freestyle Libre flash glucose monitoring system
 NHS GG&C Introduction of Freestyle Libre flash glucose monitoring system The Freestyle Libre flash glucose monitoring system is a sensor based, factory-calibrated system that measures interstitial fluid
NHS GG&C Introduction of Freestyle Libre flash glucose monitoring system The Freestyle Libre flash glucose monitoring system is a sensor based, factory-calibrated system that measures interstitial fluid
MQ Comparison of glucometers using whole blood
 MQ 2017-1 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
MQ 2017-1 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
Approval of Multiple Diabetes Management Products and Mesalazine proposals
 8 June Approval of Multiple Diabetes Management Products and Mesalazine proposals PHARMAC is pleased to announce the approval of five agreements in relation to funding of blood glucose, blood glucose diagnostic
8 June Approval of Multiple Diabetes Management Products and Mesalazine proposals PHARMAC is pleased to announce the approval of five agreements in relation to funding of blood glucose, blood glucose diagnostic
Implementation of Freestyle Libre prescribing guidance across the NHS in London
 Implementation of FreeStyle Libre prescribing guidance across the NHS in London Contents Section 1 Background to the document (pages 2-5) Section 2 Implementation guidance pathways 1. Recommended implementation
Implementation of FreeStyle Libre prescribing guidance across the NHS in London Contents Section 1 Background to the document (pages 2-5) Section 2 Implementation guidance pathways 1. Recommended implementation
Flash Glucose Monitoring (Flash GM) Frequently asked questions (November 2018)
 Flash Glucose Monitoring (Flash GM) Frequently asked questions (November 2018) What is Flash Glucose Monitoring (Flash GM)? Flash glucose monitoring is a small sensor that you wear on your skin (usually
Flash Glucose Monitoring (Flash GM) Frequently asked questions (November 2018) What is Flash Glucose Monitoring (Flash GM)? Flash glucose monitoring is a small sensor that you wear on your skin (usually
THE SHEFFIELD AREA PRESCRIBING GROUP. Position Statement for Prescribing of Freestyle Libre In Type 1 Diabetes. Date: March 2018.
 THE SHEFFIELD AREA PRESCRIBING GROUP Position Statement for Prescribing of Freestyle Libre In Type 1 Diabetes Date: March 2018 Overview Freestyle Libre is a flash glucose sensor device that measures *interstitial
THE SHEFFIELD AREA PRESCRIBING GROUP Position Statement for Prescribing of Freestyle Libre In Type 1 Diabetes Date: March 2018 Overview Freestyle Libre is a flash glucose sensor device that measures *interstitial
Placename CCG. Policies for the Commissioning of Healthcare
 Placename CCG Policies for the Commissioning of Healthcare Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus. This document is part
Placename CCG Policies for the Commissioning of Healthcare Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus. This document is part
Policy for Continuous Glucose Monitoring for Type 1 Diabetic Paediatric Patients (<18 years of age)
 Policy for Continuous Glucose Monitoring for Type 1 Diabetic Paediatric Patients (
Policy for Continuous Glucose Monitoring for Type 1 Diabetic Paediatric Patients (
Case scenarios: Patient Group Directions
 Putting NICE guidance into practice Case scenarios: Patient Group Directions Implementing the NICE guidance on Patient Group Directions (MPG2) Published: March 2014 [updated March 2017] These case scenarios
Putting NICE guidance into practice Case scenarios: Patient Group Directions Implementing the NICE guidance on Patient Group Directions (MPG2) Published: March 2014 [updated March 2017] These case scenarios
MQ Comparison of glucometers using whole blood
 MQ 2018-2 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
MQ 2018-2 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
Insulin Pumps and Glucose Monitors in Adults Policy
 Insulin Pumps and Glucose Monitors in Adults Policy Version: 2016-19 Ratified by: NHS Leeds West CCG Assurance Committee on; 16 November 2016 NHS Leeds North CCG Governance on Performance and Risk Committee
Insulin Pumps and Glucose Monitors in Adults Policy Version: 2016-19 Ratified by: NHS Leeds West CCG Assurance Committee on; 16 November 2016 NHS Leeds North CCG Governance on Performance and Risk Committee
Description of the technology
 Advice Note 2017/ 001 What is the clinical effectiveness, safety and budget impact of the Freestyle Libre System compared with current glucose monitoring methods for people aged 4 years and over with diabetes
Advice Note 2017/ 001 What is the clinical effectiveness, safety and budget impact of the Freestyle Libre System compared with current glucose monitoring methods for people aged 4 years and over with diabetes
Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes
 Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
Introduction Guidance on the Self-Monitoring of Blood Glucose in Adults with Diabetes This guideline is designed to offer guidance for primary and secondary care on the use of selfmonitoring of blood glucose
MQ Comparison of glucometers using heparin whole blood
 MQ 2014-1 Comparison of glucometers using heparin whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing
MQ 2014-1 Comparison of glucometers using heparin whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE)
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA151 Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus This guidance was issued in
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA151 Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus This guidance was issued in
FreeStyle Libre for glucose monitoring: Interim Position Statement for GPs & Patient FAQ
 North Central London Joint Formulary Committee FreeStyle Libre for glucose monitoring: Interim Position Statement for GPs & Patient FAQ GPs should not prescribe FreeStyle Libre sensors on the NHS until
North Central London Joint Formulary Committee FreeStyle Libre for glucose monitoring: Interim Position Statement for GPs & Patient FAQ GPs should not prescribe FreeStyle Libre sensors on the NHS until
Guideline on the Choice of Blood Glucose Meters, Ketone Meters and Test Strips for children and young people with. TYPE 1 DIABETES (November 2018)
 Guideline on the Choice of Blood Glucose Meters, Ketone Meters and Test Strips for children and young people with TYPE 1 DIABETES (November 2018) Introduction This guideline aims to assist healthcare professionals
Guideline on the Choice of Blood Glucose Meters, Ketone Meters and Test Strips for children and young people with TYPE 1 DIABETES (November 2018) Introduction This guideline aims to assist healthcare professionals
Placename CCG. Policies for the Commissioning of Healthcare
 Placename CCG Policies for the Commissioning of Healthcare Policy for the funding of insulin pumps and continuous glucose monitoring devices for patients with diabetes 1 Introduction 1.1 This document
Placename CCG Policies for the Commissioning of Healthcare Policy for the funding of insulin pumps and continuous glucose monitoring devices for patients with diabetes 1 Introduction 1.1 This document
Insulin Pumps and Glucose Monitors in Adults, Children and Young People Policy
 Insulin Pumps and Glucose Monitors in Adults, Children and Young People Policy Version: 2017-20 Ratified by: NHS Leeds West CCG Assurance Committee on; 16 November 2016 NHS Leeds North CCG Governance on
Insulin Pumps and Glucose Monitors in Adults, Children and Young People Policy Version: 2017-20 Ratified by: NHS Leeds West CCG Assurance Committee on; 16 November 2016 NHS Leeds North CCG Governance on
BRISTOL-MYERS SQUIBB and ASTRAZENECA v SANOFI
 CASE AUTH/2638/9/13 and AUTH/2639/9/13 BRISTOL-MYERS SQUIBB and ASTRAZENECA v SANOFI Promotion of Lyxumia Bristol-Myers Squibb and AstraZeneca jointly complained about cost comparison claims in a Lyxumia
CASE AUTH/2638/9/13 and AUTH/2639/9/13 BRISTOL-MYERS SQUIBB and ASTRAZENECA v SANOFI Promotion of Lyxumia Bristol-Myers Squibb and AstraZeneca jointly complained about cost comparison claims in a Lyxumia
Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181)
 Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
TABLE OF CONTENTS. HLC102A - Continuous Glucose Monitoring (CGM): Technologies and Global Markets
 TABLE OF CONTENTS CHAPTER 1 INTRODUCTION 1 STUDY GOALS AND OBJECTIVES 1 REASONS FOR DOING THIS STUDY 1 CONTRIBUTIONS TO THE STUDY AND INTENDED AUDIENCE 1 SCOPE AND FORMAT 1 METHODOLOGY AND INFORMATION
TABLE OF CONTENTS CHAPTER 1 INTRODUCTION 1 STUDY GOALS AND OBJECTIVES 1 REASONS FOR DOING THIS STUDY 1 CONTRIBUTIONS TO THE STUDY AND INTENDED AUDIENCE 1 SCOPE AND FORMAT 1 METHODOLOGY AND INFORMATION
Urgent field safety notice
 Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
Guideline on the Choice of Glucometers and Ketometers for ADULTS with TYPE 1 DIABETES (December 2018)
 Guideline on the Choice of Glucometers and Ketometers for ADULTS with TYPE 1 DIABETES (December 2018) This guideline aims to assist healthcare professionals (HCPs) in secondary and primary care (if applicable)
Guideline on the Choice of Glucometers and Ketometers for ADULTS with TYPE 1 DIABETES (December 2018) This guideline aims to assist healthcare professionals (HCPs) in secondary and primary care (if applicable)
Supported devices/compatibility list
 Supported devices/compatibility list DIABASS / DIABASS PRO Version:18.11.0.2 / Release date: 29.11.2018 A101-05102017 Blood glucose meters The following blood glucose meters are compatible with DIABASS/DIABASS
Supported devices/compatibility list DIABASS / DIABASS PRO Version:18.11.0.2 / Release date: 29.11.2018 A101-05102017 Blood glucose meters The following blood glucose meters are compatible with DIABASS/DIABASS
Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus
 Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus Version No. Changes Made Version of 05.10.2018 V1 Policy ratified by Healthier
Policy for the Provision of Continuous Glucose Monitoring and Flash Glucose Monitoring to patients with Diabetes Mellitus Version No. Changes Made Version of 05.10.2018 V1 Policy ratified by Healthier
Policy for the Provision of Insulin Pumps for Patients with Diabetes Mellitus
 Policy for the Provision of Insulin Pumps for Patients with Diabetes Mellitus Version No. Changes Made Version of July 2018 V0.5 Changes made to the policy following patient engagement including: - the
Policy for the Provision of Insulin Pumps for Patients with Diabetes Mellitus Version No. Changes Made Version of July 2018 V0.5 Changes made to the policy following patient engagement including: - the
Dear Colleague. DL (2017) June Additional Funding for CGMs and Adult Insulin Pumps Summary
 The Scottish Government Healthcare Quality & Improvement Directorate DG Health & Social Care Dear Colleague Additional Funding for CGMs and Adult Insulin Pumps 2017-18 Summary On 7 December 2016, the First
The Scottish Government Healthcare Quality & Improvement Directorate DG Health & Social Care Dear Colleague Additional Funding for CGMs and Adult Insulin Pumps 2017-18 Summary On 7 December 2016, the First
Commissioning Policy
 Commissioning Policy Abbott FreeStyle Libre Flash Glucose Monitoring System Individual Funding Request Date Adopted: 09 September 2018 Version: 1819.1.02 Title of document: Authors job title(s): Document
Commissioning Policy Abbott FreeStyle Libre Flash Glucose Monitoring System Individual Funding Request Date Adopted: 09 September 2018 Version: 1819.1.02 Title of document: Authors job title(s): Document
System accuracy evaluation of FORA Test N Go Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013
 System Accuracy Evaluation of Project number: Fora092614-01 System accuracy evaluation of Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013 Date: 13 th
System Accuracy Evaluation of Project number: Fora092614-01 System accuracy evaluation of Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013 Date: 13 th
USE OF UNLICENSED MEDICINES AND OFF-LABEL MEDICINES WHERE A LICENSED MEDICINE IS AVAILABLE
 NHS Scotland Directors of Pharmacy and Scottish Association of Medical Directors USE OF UNLICENSED MEDICINES AND OFF-LABEL MEDICINES WHERE A LICENSED MEDICINE IS AVAILABLE CONSENSUS STATEMENT This consensus
NHS Scotland Directors of Pharmacy and Scottish Association of Medical Directors USE OF UNLICENSED MEDICINES AND OFF-LABEL MEDICINES WHERE A LICENSED MEDICINE IS AVAILABLE CONSENSUS STATEMENT This consensus
Standard Operating Procedure: Early Intervention in Psychosis Access Times
 Corporate Standard Operating Procedure: Early Intervention in Psychosis Access Times Document Control Summary Status: New Version: V1.0 Date: Author/Owner: Rob Abell, Senior Performance Development Manager
Corporate Standard Operating Procedure: Early Intervention in Psychosis Access Times Document Control Summary Status: New Version: V1.0 Date: Author/Owner: Rob Abell, Senior Performance Development Manager
Switch protocol: Insulin pen needles to cost effective brand
 Switch protocol: Insulin pen needles to cost effective brand Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team hosted by
Switch protocol: Insulin pen needles to cost effective brand Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team hosted by
PROCEDURE FOR BLOOD GLUCOSE MONITORING
 PROCEDURE FOR BLOOD GLUCOSE MONITORING First Issued Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote safe and effective blood glucose monitoring using Trust equipment
PROCEDURE FOR BLOOD GLUCOSE MONITORING First Issued Issue Version Two Purpose of Issue/Description of Change Planned Review Date To promote safe and effective blood glucose monitoring using Trust equipment
Local Improvement Scheme (LIS) 2016/17 Local Service for Dementia Care in East Lancashire GP Practices
 Local Improvement Scheme (LIS) 2016/17 Local Service for Dementia Care in East Lancashire GP Practices CONTENT Page number 1. Introduction 2 2. 5 key elements of the Dementia LIS 3 Practice Awareness Practice
Local Improvement Scheme (LIS) 2016/17 Local Service for Dementia Care in East Lancashire GP Practices CONTENT Page number 1. Introduction 2 2. 5 key elements of the Dementia LIS 3 Practice Awareness Practice
GOVERNING BOARD. Assisted Conception (IVF): Review of access criteria. Date of Meeting 21 January 2015 Agenda Item No 13. Title
 GOVERNING BOARD Date of Meeting 21 January 2015 Agenda Item No 13 Title Assisted Conception (IVF): Review of access criteria Purpose of Paper The SHIP (Southampton, Hampshire, Isle of Wight and Portsmouth)
GOVERNING BOARD Date of Meeting 21 January 2015 Agenda Item No 13 Title Assisted Conception (IVF): Review of access criteria Purpose of Paper The SHIP (Southampton, Hampshire, Isle of Wight and Portsmouth)
How many spoonfuls of sugar? A Bert s-eye view of prescribing to manage blood sugar
 How many spoonfuls of sugar? A Bert s-eye view of prescribing to manage blood sugar What would Mary Poppins think? Please discuss Some Sums Annual cost of diabetes (1 &2) in England = c. 24 billion Around
How many spoonfuls of sugar? A Bert s-eye view of prescribing to manage blood sugar What would Mary Poppins think? Please discuss Some Sums Annual cost of diabetes (1 &2) in England = c. 24 billion Around
This is a licensed product of Ken Research and should not be copied
 1 TABLE OF CONTENTS 1. The US Diabetes Care Devices Market Introduction 1.1. What is Diabetes and it s Types? 2. The US Diabetes Care Devices Market Size, 2007-2013 3. The US Diabetes Care Devices Market
1 TABLE OF CONTENTS 1. The US Diabetes Care Devices Market Introduction 1.1. What is Diabetes and it s Types? 2. The US Diabetes Care Devices Market Size, 2007-2013 3. The US Diabetes Care Devices Market
SPECIALIST FERTILITY SERVICES CLINICAL CRITERIA & CONTRACT AWARD
 AGENDA ITEM 8 GOVERNING BODY MEETING IN PUBLIC ON 25 TH SEPTEMBER 2014 SPECIALIST FERTILITY SERVICES CLINICAL CRITERIA & CONTRACT AWARD Date of the meeting 25 th September 2014 Author Sponsoring Board
AGENDA ITEM 8 GOVERNING BODY MEETING IN PUBLIC ON 25 TH SEPTEMBER 2014 SPECIALIST FERTILITY SERVICES CLINICAL CRITERIA & CONTRACT AWARD Date of the meeting 25 th September 2014 Author Sponsoring Board
Putting NICE guidance into practice. Resource impact report: Hearing loss in adults: assessment and management (NG98)
 Putting NICE guidance into practice Resource impact report: Hearing loss in adults: assessment and management (NG98) Published: June 2018 Summary This report focuses on the recommendation from NICE s guideline
Putting NICE guidance into practice Resource impact report: Hearing loss in adults: assessment and management (NG98) Published: June 2018 Summary This report focuses on the recommendation from NICE s guideline
Urgent field safety notice
 Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 Gestational diabetes: risk assessment, testing, diagnosis and management bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They
Gestational diabetes: risk assessment, testing, diagnosis and management bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They
Clinical Commissioning Policy: Chemotherapy Algorithms for Adults and Children. January 2013 Reference: NHS England XXX/X/X.
 Clinical Commissioning Policy: Chemotherapy Algorithms for Adults and Children January 2013 Reference: NHS England XXX/X/X England 1 NHS England Clinical Commissioning Policy: Chemotherapy Algorithms for
Clinical Commissioning Policy: Chemotherapy Algorithms for Adults and Children January 2013 Reference: NHS England XXX/X/X England 1 NHS England Clinical Commissioning Policy: Chemotherapy Algorithms for
National Diabetes Treatment and Care Programme
 National Diabetes Treatment and Care Programme Introduction to and supporting documentation for VALUE BASED TRANSFORMATION FUNDING SITE SELECTION December 2016 1 Introduction and Contents The Planning
National Diabetes Treatment and Care Programme Introduction to and supporting documentation for VALUE BASED TRANSFORMATION FUNDING SITE SELECTION December 2016 1 Introduction and Contents The Planning
requesting information regarding prescribing incentive schemes in Canterbury and Coastal Clinical Commissioning Group
 requesting information regarding prescribing incentive schemes in Canterbury and Coastal Clinical Commissioning Group Canterbury and Coastal Clinical Consortium Group Medicine Management plans 2013/14
requesting information regarding prescribing incentive schemes in Canterbury and Coastal Clinical Commissioning Group Canterbury and Coastal Clinical Consortium Group Medicine Management plans 2013/14
Policy and Procedure for the Development, Approval and Implementation of Patient Group Directions in NHS Haringey Clinical Commissioning Group
 BEFORE USING THIS POLICY ALWAYS ENSURE YOU ARE USING THE MOST UP TO DATE VERSION Policy and Procedure for the Development, Approval and Implementation of Patient Group Directions in NHS Haringey Clinical
BEFORE USING THIS POLICY ALWAYS ENSURE YOU ARE USING THE MOST UP TO DATE VERSION Policy and Procedure for the Development, Approval and Implementation of Patient Group Directions in NHS Haringey Clinical
GlucoMen LX PLUS blood glucose and blood ketone meter. Accuracy Evaluation to New ISO 15197:2013, with Technical and Specification Data
 GlucoMen LX PLUS: Accuracy Evaluations to ISO 15197:2013 with Technical and Specification Data GlucoMen LX PLUS blood glucose and blood ketone meter Accuracy Evaluation to New ISO 15197:2013, with Technical
GlucoMen LX PLUS: Accuracy Evaluations to ISO 15197:2013 with Technical and Specification Data GlucoMen LX PLUS blood glucose and blood ketone meter Accuracy Evaluation to New ISO 15197:2013, with Technical
Home Glucose Monitoring
 Home Glucose Monitoring Blood Glucose Monitors Name (Manufacturer/Distributor) Size (inches) Weight (ounces) Active (Roche) 4.6 X 1.07 X 0.9 2.01 without Advantage (Roche) 3.3 X 2.8 X 0.8 1.8 without Test
Home Glucose Monitoring Blood Glucose Monitors Name (Manufacturer/Distributor) Size (inches) Weight (ounces) Active (Roche) 4.6 X 1.07 X 0.9 2.01 without Advantage (Roche) 3.3 X 2.8 X 0.8 1.8 without Test
Public consultation: Seeking your views on IVF
 Public consultation: Seeking your views on IVF Introduction We (NHS Bury Clinical Commissioning Group (CCG)) are seeking views from patients registered with a Bury GP practice, Bury health care professionals
Public consultation: Seeking your views on IVF Introduction We (NHS Bury Clinical Commissioning Group (CCG)) are seeking views from patients registered with a Bury GP practice, Bury health care professionals
A Suite of Enhanced Services for. Prudent Structured Care for Adults with Type 2 Diabetes
 An Enhanced Service for Prudent Structured Care for Adults with Type 2 Diabetes Page 1 A Suite of Enhanced Services for Prudent Structured Care for Adults with Type 2 Diabetes 1. Introduction All practices
An Enhanced Service for Prudent Structured Care for Adults with Type 2 Diabetes Page 1 A Suite of Enhanced Services for Prudent Structured Care for Adults with Type 2 Diabetes 1. Introduction All practices
Your Ref: VY March Dear. Your Request for Information
 Your Ref: VY 207 31 March 2014 NHS Vale of York CCG c/o North Yorkshire and Humber CSU Health House Grange Park Lane Willerby East Yorkshire HU10 6DT Telephone: (01482) 672191 Dear E-mail: VoYCCG.FOI@nhs.net
Your Ref: VY 207 31 March 2014 NHS Vale of York CCG c/o North Yorkshire and Humber CSU Health House Grange Park Lane Willerby East Yorkshire HU10 6DT Telephone: (01482) 672191 Dear E-mail: VoYCCG.FOI@nhs.net
Sandwell & West Birmingham integrated community care diabetes model (DICE) the future of diabetes services?
 Sandwell & West Birmingham integrated community care diabetes model (DICE) the future of diabetes services? Dr PARIJAT DE DUK Clinical Champion Clinical Lead for Diabetes & Endocrinology, Sandwell & West
Sandwell & West Birmingham integrated community care diabetes model (DICE) the future of diabetes services? Dr PARIJAT DE DUK Clinical Champion Clinical Lead for Diabetes & Endocrinology, Sandwell & West
Surveillance report Published: 28 March 2018 nice.org.uk
 Surveillance report 2018 Type 2 diabetes prevention: ention: population and community-level el interventions entions (2011) NICE guideline PH35 and Type 2 diabetes: prevention ention in people at high
Surveillance report 2018 Type 2 diabetes prevention: ention: population and community-level el interventions entions (2011) NICE guideline PH35 and Type 2 diabetes: prevention ention in people at high
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Continuous subcutaneous insulin infusion for the treatment of diabetes (review) Final scope Appraisal objective To review
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Continuous subcutaneous insulin infusion for the treatment of diabetes (review) Final scope Appraisal objective To review
Alzheimer s Society. Consultation response. Our NHS care objectives: A draft mandate to the NHS Commissioning Board.
 Alzheimer s Society Our NHS care objectives: A draft mandate to the NHS Commissioning Board 26 September 2012 Delivering Dignity Securing dignity in care for older people in hospitals and care homes: A
Alzheimer s Society Our NHS care objectives: A draft mandate to the NHS Commissioning Board 26 September 2012 Delivering Dignity Securing dignity in care for older people in hospitals and care homes: A
Leeds West CCG Paediatric asthma project. January 2015-January 2017
 Leeds West CCG Paediatric asthma project. January 2015-January 2017 Aims to raise asthma awareness improve care reduce emergency attendances and unplanned admissions to secondary care for children with
Leeds West CCG Paediatric asthma project. January 2015-January 2017 Aims to raise asthma awareness improve care reduce emergency attendances and unplanned admissions to secondary care for children with
A. Service Specification
 A. Service Specification Service Specification No: 1767 Service Adult Highly Specialist Pain Management Services Commissioner Lead For local completion Lead For local completion 1. Scope 1.1 Prescribed
A. Service Specification Service Specification No: 1767 Service Adult Highly Specialist Pain Management Services Commissioner Lead For local completion Lead For local completion 1. Scope 1.1 Prescribed
Cataract Surgery Criteria Based Access Protocol Supporting people in Dorset to lead healthier lives
 NHS Dorset Clinical Commissioning Group Cataract Surgery Criteria Based Access Protocol Supporting people in Dorset to lead healthier lives 1. INTRODUCTION AND SCOPE NHS DORSET CLINICAL COMMISSIONING GROUP
NHS Dorset Clinical Commissioning Group Cataract Surgery Criteria Based Access Protocol Supporting people in Dorset to lead healthier lives 1. INTRODUCTION AND SCOPE NHS DORSET CLINICAL COMMISSIONING GROUP
Software Version 2.0. User s Guide
 Software Version 2.0 User s Guide Table of Contents Contents Contents Important Information About Your FreeStyle Auto-Assist Software...1 Intended Use...1 System Requirements...1 Connecting to your Abbott
Software Version 2.0 User s Guide Table of Contents Contents Contents Important Information About Your FreeStyle Auto-Assist Software...1 Intended Use...1 System Requirements...1 Connecting to your Abbott
Summary of Significant Changes at this Revision. Items Required
 Update Approver/checker Summary of Significant Changes at this Revision Purpose and Scope Items Required 1. The Abbott FreeStyle Optium meter is a battery-powered device designed for the measurement of
Update Approver/checker Summary of Significant Changes at this Revision Purpose and Scope Items Required 1. The Abbott FreeStyle Optium meter is a battery-powered device designed for the measurement of
Switch protocol: Insulin pen needles to cost effective brand
 Switch protocol: Insulin pen needles to cost effective brand Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team hosted by
Switch protocol: Insulin pen needles to cost effective brand Applies to HaRD CCG employed Pharmacists and Medicines Optimisation Technicians. These protocols are produced by the NY&AWC MM team hosted by
GENERAL PRACTICTIONER AND GP PRESCRIBING LEAD v TAKEDA
 CASE AUTH/2367/10/10 GENERAL PRACTICTIONER AND GP PRESCRIBING LEAD v TAKEDA Use of inverted black triangle A general practitioner and GP prescribing lead, complained about a two page advertisement for
CASE AUTH/2367/10/10 GENERAL PRACTICTIONER AND GP PRESCRIBING LEAD v TAKEDA Use of inverted black triangle A general practitioner and GP prescribing lead, complained about a two page advertisement for
Accuracy and Usability Evaluation of Six Commercially Available Blood Glucose Monitoring Systems
 Original Article Accuracy and Usability Evaluation of Six Commercially Available Blood Glucose Monitoring Systems DOI: 10.7860/JCDR/2018/34055.11671 Internal Medicine Section Anastasios Tentolouris 1,
Original Article Accuracy and Usability Evaluation of Six Commercially Available Blood Glucose Monitoring Systems DOI: 10.7860/JCDR/2018/34055.11671 Internal Medicine Section Anastasios Tentolouris 1,
CONSULTANT PHYSICIAN v SANOFI
 CASE AUTH/2477/2/12 CONSULTANT PHYSICIAN v SANOFI Conduct of representative A consultant physician alleged that at a hospital diabetes meeting a Sanofi representative had been unprofessional in that she
CASE AUTH/2477/2/12 CONSULTANT PHYSICIAN v SANOFI Conduct of representative A consultant physician alleged that at a hospital diabetes meeting a Sanofi representative had been unprofessional in that she
Polypharmacy and Deprescribing. A special report on views from the PrescQIPP landscape review
 Polypharmacy and Deprescribing A special report on views from the PrescQIPP landscape review Introduction and background In recent years, we have seen the emergence of an international discussion around
Polypharmacy and Deprescribing A special report on views from the PrescQIPP landscape review Introduction and background In recent years, we have seen the emergence of an international discussion around
Enhanced Service Specification. Childhood seasonal influenza vaccination programme 2017/18
 Enhanced Service Specification Childhood seasonal influenza vaccination programme 2017/18 2 Enhanced Service Specification Childhood seasonal influenza vaccination programme Version number: 1 First published:
Enhanced Service Specification Childhood seasonal influenza vaccination programme 2017/18 2 Enhanced Service Specification Childhood seasonal influenza vaccination programme Version number: 1 First published:
PRIMARY CARE CO-COMMISSIONING COMMITTEE 18 March 2016
 Part 1 Part 2 PRIMARY CARE CO-COMMISSIONING COMMITTEE 18 March 2016 Title of Report Supporting deaf patients to access primary care services Purpose of the Report The report is to provide the co-commissioning
Part 1 Part 2 PRIMARY CARE CO-COMMISSIONING COMMITTEE 18 March 2016 Title of Report Supporting deaf patients to access primary care services Purpose of the Report The report is to provide the co-commissioning
Third party prescription request for Continence and Stoma products. August 2013
 Third party prescription request for Continence and Stoma products August 2013 DOCUMENT CONTROL Document Location Copies of this document can be obtained from: Name: Address: Medicines Management Team
Third party prescription request for Continence and Stoma products August 2013 DOCUMENT CONTROL Document Location Copies of this document can be obtained from: Name: Address: Medicines Management Team
