MQ Comparison of glucometers using whole blood
|
|
|
- Katherine Newton
- 5 years ago
- Views:
Transcription
1 MQ Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices are intended for patient s analysis of fresh capillary blood. Some of the devices are also suitable for the analysis of anticoagulated venous whole blood. Hemocue, StatStrip and AccuChek Inform 2 are intended exclusively for professional use. As part of our inter-laboratory comparisons (surveys) for external quality control, plasmabased control samples (sample K1: Clinical Chemistry) are sent to our participating laboratories. Because of the occurring matrix effects, an individual target value for each glucose meter must be determined. Unfortunately, these target values do not compare well, since the properties of plasma are different than those of fresh capillary blood. To nevertheless provide our participants with a target value that can be compared between instruments, we conduct additional comparative measurements with fresh blood in our laboratory. Approach The manufacturers provide equipment and test strips. All devices were tested using the manufacturer's control solutions and passed. We used heparin venous blood from the same donor for both samples. Sample A was used as is. In sample B, the glucose concentration was increased by addition of a 1 mol/l glucose solution. Both samples were taken approx. one hour prior to the measurements. Additional readings The oxygen content of both samples was monitored using istat from Axonlab. The glucose was measured with the istat, using a Glucoseoxidase (GOD) electrode. For measuring the samples with Cobas 8000, we used the plasma after centrifugation of the sample. The Cobas 8000 Glucose-reagent works according to the hexokinase method. The measurements with istat and Cobas 8000 are traceable to NIST 965 standard solution for glucose in plasma. Sample A Sample B Glucose, Cobas mmol/l 8.30 mmol/l Glucose, istat 4.47 mmol/l 8.13 mmol/l Group mmol/l 7.70 mmol/l Mean 4.46 mmol/l 8.04 mmol/l PO2, istat (normal: kpa) kpa 17.6 kpa Control samples The manufacturer s control solutions were measured ten times. MQ survey specimen K1 (2018-2) The plasma sample for the current survey was measured with instruments that were available to participants of the survey. Manufacturer information Not all devices are certified for analysis of venous blood. Please consult the list at the end of this report.. (c) Verein für medizinische Qualitätskontrolle ( Der Bericht darf nur vollständig verbreitet werden!
2 MQ Comparison of glucometers using whole blood Page 2/9 Precision To stay within the QUALAB tolerance range of 10%, the % CV for glucose concentration may not exceed 5%, and is ideally lower than 3.3%. 21 from 23 devices achieved CV values below 5% with sample A and 22 with sample B. 14 devices achieved the 3.3% limit with specimen A, and 19 units with specimen B. You can find the expected CV% - values according to the manufacturers in Table 6, in the columns "precision". Sample A corresponds to the concentration 2, Sample B corresponds to the concentration 4. With Sample A, 8 from 23 instruments reached the specifications of the manufacturer where as in Sample B, 11 from 23 instruments reached the specifications. Accuracy To check the accuracy we used the following criteria: - 3 measurements with istat (GOx Elektrode) - 3 measurements with Roche Cobas 8000 (Hexokinase) - Mean of all devices where measuring venous blood is part of their intended use (group 1). We distinguish two groups of instruments: Group 1 is approved for the analysis of venous blood according to the manufacturer's specifications Group 2 may be used with fresh capillary blood only. Ideal deviations are <4%. In the group 1, for the lower concentration 5 out of 13 instruments and for the higher concentration 7 out of 13 instruments achieved a deviation of less than 4%.
3 MQ Comparison of glucometers using whole blood Page 3/9 In Group 2, we did not assess the accuracy of measurements with venous blood. In addition, we compared the devices using fresh capillary blood. Green marked are the deviations (Dev%) of the measured values <10% of the mean value. With reference to ISO15197:2013, all devices were within the limits. Gluc Gluc Gluc GlucoCard Xmini plus 6,1 6,9 5,7 GlucoMen LX Plus 6,1 6 6,2 GlucoMen Ready 5,2 5,9 5,3 Glucomen areo 5,3 5,6 5,3 mylife Pura 5,7 5,9 5,4 Healthpro-X1 5,9 6,1 5,5 BG-Star 6,2 6,4 6,2 mean value 6,1 6,2 5,9 Table 1: Comparision of glucometers of group 2 with fresh capillary blood Total error Results within the QUALAB tolerance of ± 10% around the target value were highlighted in green. For blood glucose monitoring systems that are used by patients themselves, the ISO 15197:2013 standard applies as of May New is that 95% of the test results must be within the ± 15% range. For glucose concentrations below <5:55 mmol/l an absolute tolerance of ± 0.83 mmol/l must be observed. In Specimen A, this tolerance was 3.63 to 5.29 mmol/l, in Specimen B the tolerance was 6.84 to 9.25 mmol/l. All 13 devices of Group 1 conformed to the ISO 15197:2013 requirements. With the measured values of Group 2 with fresh capillary blood, 6 out of 7 devices meet the Qualab-requirements and all the ISO-requirements. Zürich, Dr. R. Fried
4 MQ Comparison of glucometers using whole blood Page 4/9 Group mean Bias VK% Hemocue ,6 4,2 4,3 4,2 4,5 4,3 4,0 4,4 4,2 4,2 4,3-3,89% 4,03 Hemocue 201RT 4,0 4,1 3,7 3,6 3,6 3,7 3,8 3,9 4,5 4,3 3,9-12,18% 7,77 StatStrip Express 4,2 3,9 4,1 3,8 4,1 4,3 4,1 4,1 3,9 3,9 4,04-9,49% 3,91 Accu-Chek Inform 2 4,3 4,4 4,2 4,3 4,3 4,2 4,3 4,2 4,4 4,2 4,28-4,11% 1,84 Accu-Chek Aviva 4,2 4,2 4,2 4,2 4,2 4,2 4,2 4,2 4,1 4,2 4,19-6,13% 0,75 Accu-Chek Guide 4,5 4,6 4,6 4,5 4,5 4,6 4,4 4,6 4,6 4,4 4,53 1,49% 1,82 Contour next one 4,5 4,3 4,3 4,4 4,3 4,5 4,5 4,4 4,3 4,3 4,38-1,87% 2,10 Freestyle lite 4 3,9 4,1 3,9 3,9 3, ,9 3,9 3,93-11,95% 2,70 Freestyle precision 4,4 4,4 4,4 4,4 4,4 4,4 4,4 4,3 4,2 4,1 4,34-2,77% 2,48 OneTouch Verio IQ 4,2 4,1 4,2 4,1 4,1 3,9 4,1 4,1 3,8 4 4,06-9,04% 3,12 mylife Unio 4,3 4,1 4,2 4,3 3,9 3,9 4,1 4,1 3,8 3,9 4,06-9,04% 4,38 FORA GD40a 4,6 4,9 4,7 4,8 4,7 4,8 4,6 4,7 4,8 4,7 4,73 5,97% 2,01 FORA GD50 3, ,8 3,7 3,8 3,9 3,7 3,8 3,85-13,75% 3,06 Group 2 (venous blood not approved) Accu-Chek Mobile 4,6 4,6 4,2 4,2 4,4 4,3 4,3 4,2 4,3 4,1 3,90 GlucoCard Xmini plus 3,9 4,6 4,1 3,9 4,1 3,9 3,8 3,9 3,7 3,8 6,40 GlucoMen LX Plus 5,1 5,1 5,2 4,9 5,1 5,1 4,8 5,1 4,9 5,1 Accuracy 2.69 GlucoMen Ready 3,7 3,7 3,5 3,4 3,2 3,5 3,5 3,5 3,4 3,5 see 3.23 Glucomen areo 4,6 4,3 4,4 4,6 4,3 4,3 4,2 4,3 4,2 4,4 Table mylife Pura 4,1 4,1 4,1 4,1 4,1 4 3,9 3,9 4,1 3,9 2,35 HealthproX1 4,1 3,9 3,9 3,8 3,8 3,8 3,9 3,7 3,6 3,8 3,49 MyStar 4,1 4,1 4 3,9 3,8 3,9 3,8 3,8 3,7 3,9 3,42 On Call Extra 3,5 3,7 3,5 3,7 3,6 3,6 3,6 3,4 3,6 3,6 2,57 On Call Vivid 3,7 3,8 3,7 3,7 3,6 3,6 3,7 3,6 3,7 3,8 2,00 Table 2: Sample A wholeblood, normal, postprandial. All Glucose values are in mmol/l, alle instruments are plasma calibrated. Values of group 1 within the Qualab-tolerance of 10% are green colored. Target value: 4.46 mmol/l, mean USZ (Cobas 8000): 4.67 mmol/ll, mean istat 4.47 mmol/l (PO2 = kpa), Hematocrit: 0.41 l/l
5 MQ Comparison of glucometers using whole blood Page 5/9 Group mean Bias VK% Hemocue ,3 8,3 8,3 8,2 8,3 8,2 8,2 8,1 8,1 7,8 8,2 1,69% 1,89 Hemocue 201RT 7,7 8,2 8,0 7,7 7,8 7,6 7,5 7,6 7,8 7,8 7,8-3,41% 2,65 Nova Statstrip 7 7,5 6,8 7,7 7,8 7,1 7,2 7,6 7,7 7,6 7,4-8,01% 4,68 Accu-Chek Inform 2 7,6 7,5 7,7 7,7 7,6 7,5 7,7 7,4 7,4 7,5 7,56-6,02% 1,55 Accu-Chek Aviva 7,4 7,4 7,3 7,5 7,3 7,2 7,4 7,4 7,2 7,4 7,35-8,63% 1,32 Accu-Chek Guide 8,1 8,2 7,9 7,9 8,4 8,4 8,2 8 8,4 8,2 8,17 1,56% 2,38 Contour XT 8 8,2 7,7 8 8,1 8,1 8 7,8 7,5 7,7 7,91-1,67% 2,82 Freestyle lite 7,3 7,5 7,4 7,5 7,2 7 6,8 7 6,9 7 7,16-10,99% 3,56 Freestyle precision 8,3 8,1 7,8 8 8,5 8,4 8,1 7,7 8,2 7,9 8,1 0,69% 3,19 OneTouch Verio IQ 7,6 7,4 7,3 7,4 7,4 7,3 7,3 7,2 7,3 7,2 7,34-8,75% 1,60 mylife Unio 7,6 7,2 7,3 7,3 7,2 7,3 7,2 7,2 7 7,3 7,26-9,75% 2,07 FORA GD40a 8,5 8,1 8 8,5 8,2 8,3 8 8,2 7,9 8,2 8,19 1,81% 2,47 FORA GD 50 6,6 6,6 6,7 6,6 6,5 6,7 6,7 6,8 6,6 6,5 6,63-17,58% 1,43 Group 2 (venous blood not approved) Accu-Chek Mobile 7,8 8 7,9 7,6 7,7 8,5 7,5 7,6 7,7 7,8 3,64 GlucoCard Xmini plus 7,4 7,3 7,1 7,6 7,1 7,6 7,3 7,4 7 7,7 3,22 GlucoMen LX Plus 7,8 7,9 7,7 8 7,5 7,9 7,9 7,9 7,8 7,2 Accuracy 3.57 GlucoMen Ready 6,1 6, ,9 5,9 5,8 5,7 5,7 6,1 see 2.93 Glucomen areo 7,6 6,6 7,4 7,3 7,1 7,3 7,1 6,9 6,5 6,6 Table mylife Pura 6,9 6,8 6,6 7,1 6,4 6,7 6,4 6,8 6,6 6,5 3,37 Healthpro Xl 6,5 6,5 6,2 6,3 6,4 6,1 6, ,20 My Star 7,2 6,8 6,9 6,9 6,7 6,6 6,6 6,7 6,8 6,5 2,96 On Call extra 6,8 6,5 6,8 6,7 6,3 6,7 6,4 6,6 6,3 6,3 3,16 On Call vivid 6,6 6,6 6,7 6,5 6,2 6,6 6,3 6,7 6,7 6,4 2,71 Table 3: Sample B, venous whole blood, normal with additional glucose. Target value: 8.04 mmol/l, mean USZ (Cobas 8000): 8.30 mmol/ll, mean istat 8.13 mmol/l (PO2 = kpa), hematocrit: 0.40 l/l
6 MQ Comparison of glucometers using whole blood Page 6/9 low high target mean Bias CV% Hemocue ,5 6,7 6,10 6,70 6,90 6,60 6,30 6,60 6,40 6,30 6,50 6,50 6,40 6,52 6,89 2,87 Hemocue ,9 10,9 9,90 10,90 10,80 11,10 10,90 10,90 10,80 11,10 11,00 11,90 10,80 11,02 11,31 2,99 Hemocue 201RT 5,0 6,1 5,50 6,00 4,10 4,30 5,90 6,00 6,20 6,20 6,10 6,20 6,10 5,71 3,82 14,07 Hemocue 201RT 8,6 10,5 9,50 10,60 10,70 10,60 10,60 10,60 10,50 10,70 10,50 10,80 11,00 10,66 12,21 1,41 Statstrip Express 14,5 17,7 16,10 14,80 15,40 16,50 16,20 15,00 16,00 15,20 16,30 15,30 14,50 15,52-3,60 4,43 AccuChek Inform 2 2,3 2,8 2,50 2,60 2,60 2,60 2,60 2,60 2,60 2,60 2,70 2,60 2,50 2,60 4,00 1,81 AccuChek Inform 2 15,3 18,8 17,05 14,80 15,40 16,50 16,20 15,00 16,00 15,20 16,30 15,30 14,50 15,52-8,97 4,43 AccuCheck Aviva 2,3 2,8 2,50 2,50 2,50 2,50 2,60 2,60 2,60 2,60 2,50 2,60 2,60 2,56 2,40 2,02 AccuCheck Aviva 14,9 18,3 16,60 17,50 17,30 17,50 17,20 17,50 17,50 17,20 17,50 17,30 17,30 17,30 4,22 0,76 AccuChek Guide 2,3 2,8 2,5 2,40 2,50 2,50 2,60 2,60 2,60 2,50 2,40 2,60 2,40 2,51 0,40 3,49 AccuChek Guide 14,9 18,2 16,5 16,70 16,90 16,80 17,10 17,10 16,90 16,90 17,00 16,80 16,80 16,90 2,42 0,79 AcuCheck Mobile 2,9 3,6 3,25 3,40 3,50 3,40 3,60 3,60 3,40 3,50 3,20 3,50 3,40 3,45 6,15 3,42 AcuCheck Mobile 8,1 10,0 9,05 9,70 9,40 9,90 9,50 9,90 9,90 9,80 9,70 9,60 10,30 9,77 7,96 2,60 Contour next one 6,3 7,7 7,00 7,00 7,20 7,20 7,00 7,50 7,40 7,30 7,30 7,30 7,40 7,26 3,71 2,27 Freestyle lite 2,3 2,8 2,50 2,60 2,60 2,50 2,50 2,60 2,50 2,40 2,50 2,40 2,60 2,52 0,80 3,13 Freestyle lite 15,5 19,0 17,25 17,50 16,80 17,70 16,90 17,60 17,70 17,30 16,80 16,30 17,70 17,23-0,12 2,88 Freestyle precision 2,3 2,8 2,50 1,90 2,00 2,10 2,30 2,10 2,00 2,20 2,00 2,10 1,90 2,06-17,60 6,14 Freestyle precision 14,5 17,7 16,10 17,40 14,70 17,20 14,30 17,20 14,20 13,90 14,30 17,70 14,80 15,57-3,29 10,14 OneTouch Veriopro 6,0 7,4 6,70 6,80 6,70 7,00 6,50 6,80 6,80 6,80 6,70 6,70 6,80 6,76 0,90 1,87 GlucoCard Xmini plus 7,2 8,8 8,00 8,90 8,90 8,90 8,60 9,90 9,10 8,80 8,50 8,60 9,20 8,94 11,75 4,51 GlucoMen LX Plus 5,9 7,2 6,55 6,60 6,70 6,60 6,60 6,70 6,70 6,60 6,50 6,70 6,50 6,62 1,07 1,19 GlucoMen LX Plus 16,2 19,9 18,05 17,40 17,50 17,70 17,60 18,00 18,10 16,90 17,70 17,50 17,80 17,62-2,38 1,91 ML Pura 2,0 2,5 2,25 2,30 2,20 2,40 2,20 2,20 2,20 2,20 2,30 2,30 2,20 2,25 0,00 3,14 ML Pura 12,7 15,5 14,10 12,80 12,80 13,40 12,40 13,10 13,20 13,20 12,90 13,10 13,50 13,04-7,52 2,48 Table 4a: Control solution of manufacturer
7 MQ Comparison of glucometers using whole blood Page 7/9 low high target mean Bias CV% ML Unio 6,2 7,6 6,90 6,10 6,20 6,00 6,10 5,90 5,80 5,80 5,80 5,90 5,80 5,94-13,91 2,53 ML Unio 13,6 16,7 15,15 15,50 15,60 15,20 15,60 15,30 15,60 15,50 14,80 15,20 15,30 15,36 1,39 1,66 GlucoMen areo 5,7 6,9 6,30 5,90 5,90 6,10 6,80 5,90 6,10 6,00 6,10 5,90 6,00 6,07-3,65 4,46 GlucoMen areo 10,4 12,8 11,60 11,60 11,60 11,70 10,80 10,80 11,40 11,60 11,00 10,40 11,90 11,28-2,76 4,38 FORA GD40a 7,2 8,7 7,95 7,80 7,70 7,60 7,70 7,60 7,90 7,70 7,70 7,80 7,70 7,72-2,89 1,19 FORA G50 6,4 7,9 7,15 7,20 7,20 7,10 7,10 7,20 7,10 7,30 7,00 7,20 7,50 7,19 0,56 1,91 Healthpro-X1 4,2 5,2 4,70 4,80 4,90 4,70 4,80 4,90 4,90 5,00 5,00 4,90 5,10 4,90 4,26 2,36 MyStar 6,8 8,3 7,55 7,40 7,90 8,10 9,00 7,80 8,00 7,90 7,70 7,80 8,10 7,97 5,56 5,23 GlucoMen Ready 7,0 8,5 7,75 7,90 8,10 8,20 8,20 8,20 8,00 8,20 8,20 8,30 8,20 8,15 5,16 1,45 On Call vivid 6,1 7,4 6,75 6,80 6,80 7,20 6,90 7,00 7,00 6,90 7,00 7,30 7,00 6,99 3,56 2,28 ML Unio 6,2 7,6 6,90 6,10 6,20 6,00 6,10 5,90 5,80 5,80 5,80 5,90 5,80 5,94-13,91 2,53 ML Unio 13,6 16,7 15,15 15,50 15,60 15,20 15,60 15,30 15,60 15,50 14,80 15,20 15,30 15,36 1,39 1,66 Table 4b: Control solution of manufacturer
8 MQ Comparison of glucometers using whole blood Page 8/9 target mean CV% Bias % Hemocue ,3 6,3 6,6 6,8 6,7 6,7 7,1 6,5 6,6 6,9 6,5 6,73 3,05 6,75 Hemocue 201RT 6,3 6,3 6,2 6,2 6,3 6,2 6,3 6,5 6,6 6,5 6,5 6,39 2,43 1,39 Nova Statstrip 5,2 5,1 5,2 4,8 4,9 4,7 4,7 5,3 4,7 5,3 4,95 5,51 AccuChek Inform 2 5,5 5,4 5,4 5,4 5,4 5,6 5,4 5,6 5,6 5,4 5,6 5,50 1,94 0,00 AccuChek Aviva 5,5 5,1 5 5,1 5,2 5,2 5,3 5,2 5,3 5,2 5,2 5,21 1,23-5,23 AC Guide 4,6 4,6 4,6 4,6 4,7 4,6 4,7 4,6 4,7 4,7 4,6 4,65 1,08 1,09 Contour next one 5,2 4,7 4,6 4,7 4,7 4,7 4,8 4,7 4,6 4,6 4,7 4,69 1,37-9,86 Freestyle lite 4,9 4,9 4,9 5,1 5,1 4,9 5,1 4,9 5 5,1 4,8 5,00 2,39 2,04 Freestyle precision 5,1 4,9 4,8 5, ,9 4,9 4,8 4,9 4,8 4,93 2,10-3,43 OneTouch VerioIQ 4,7 4,8 4,9 4,9 5 4,9 4,9 4,9 4,9 4,9 4,9 4,91 0,72 4,52 mylife Unio 5,5 5,2 5,4 5,1 5,2 5,1 5,4 5,4 5,3 5,3 5,3 5,26 2,26-4,32 FORA GD40a 3, ,6 4,2 4,1 4,1 3,8 4,1 3,8 3,96 4,88 FORA GD50 6,2 6,1 6,1 6,1 6, ,1 6,1 6,1 6,08 0,71 AccuChek Mobile 5,4 5,4 5,4 5,4 5,6 5,4 5,6 5,6 5,4 5,6 5,50 1,82 GlucoCard Xmini plus 5,7 6,4 6,4 6,3 6,3 6,4 6,2 6,1 6,1 6,2 6,1 6,25 1,71 9,65 GlucoMen Ready 7,4 7,6 7,5 7,6 7,6 7,9 8 7,8 7,8 7,7 7,74 2,04 GlucoMen areo 5,7 5,4 5,4 5,7 5,7 5,5 5,9 7,9 5,6 5,7 5,93 13,70 mylife Pura 4,8 4,8 4,8 5,1 5,1 4,9 4,7 4,7 4,6 4,7 4,7 4,81 3,81 0,26 Healthpro Xl 7,8 7,5 7,4 7,7 7,8 7,5 7,9 7,8 7,8 7,8 8,2 7,81 2,35 0,16 MY Star 6,2 6,1 6,3 6,2 6,2 6,2 5,9 6,2 6,1 6,1 6,15 1,82 On Call Extra 6,9 7,4 7,4 7,8 7,3 7,2 7,1 7,2 8,2 6,5 7,34 6,39 GlucomenLX 5,5 5,6 5,5 5,6 5,8 5,6 5,7 5,5 5,7 5,8 5,65 1,98 Table 5: Survey Sample MQ K1 (Plasma-sample). The target value for the hexokinase method on Cobas instruments was 4.9 mmol/l. None of the listed instruments is approved for the analysis of plasma glucose. The systems respond quite differently to plasma, depending on the type of electrode and hematocrit compensation. Therefore, a separate target value is calculated in the survey for each system. * Not enough participants at the MQ-surveys for this instrument. It was not possible to calculate a consensus value as target.
9 MQ Comparison of glucometers using whole blood Page 9/9 Precision Instrument Company Samp. Ac Enzyme Meas Cal. Hc% Hemocue 201+ Hemocue KVAN HEF GDH-NAD AF ID-GCMS Hemocue 201RT Hemocue KVAN HEF GDH-NAD AF ID-GCMS StatStrip Express Menarini KVAN H GOx A YSI Accu-Chek Inform 2 Roche KVAN HEF mgdh-pqq A HK Accu-Chek Aviva Roche KVAN HE mgdh-pqq A HK Accu-Chek Guide Roche KVAN HE GDH-FAD A HK Contour next one Ascensia KVN H GDH-FAD A YSI Freestyle Precision Abbott KVAN HE GDH-NAD A YSI Freestyle Freedom Lite Abbott KV H GDH C YSI OneTouch Verio pro Lifescan KV HEC FAD-GDH A YSI mylife Unio Ypsomed KV HE GDH-FAD A HK FORA GD40a FORA KV H GDH-FAD A YSI Accu-Chek Mobile Roche K mgdh-pqq RF HK GlucoCard Xmini plus Axonlab K GDH A YSI GlucoMen LX Plus Menarini K H GOx A YSI GlucoMen Ready Menarini K GOx A YSI GlucoMen areo Menarini K GOx A YSI mylife Pura Ypsomed K GOx A HK Healthpro-X1 Axapharm K E GOx A HK MyStar Extra Sanofi K GOx A YSI On Call Vivid ACON K GOx A YSI On Call Extra ACON K GOx A YSI Table 6: Manufacturer informations on the instruments Sample: K=capillary blood, V=venous blood, A=arteriel blood, N=neonatal blood Anticoagulans (Ac): H=Heparin, E=EDTA, C=Citrate, F=Fluoride Enzyme: GDH=Glukosedehydrogenase, GOx=Glukoseoxidase Method: A=Amperometry, C=Coulometry, RF=Reflection-photometry, AF=Absorption-photometry Calibration: HK=wet chemistry, with Hexokinase-Method, YSI=instrument with Glucoseoxidase-Electrode HK: Hämatocrit-range Precision after ISO15197 (concentrations: 1: mmol/l; 2: mmol/l; 3: mmol/l; 4: mmol/l; 5: mmol/l) Alle data in table 5 are from the package inserts of the teststrips or from additional documents of the manufactures.. Version 1.0 vom
MQ Comparison of glucometers using whole blood
 MQ 2017-1 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
MQ 2017-1 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
MQ Comparison of glucometers using heparin whole blood
 MQ 2014-1 Comparison of glucometers using heparin whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing
MQ 2014-1 Comparison of glucometers using heparin whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing
GlucoMen LX PLUS blood glucose and blood ketone meter. Accuracy Evaluation to New ISO 15197:2013, with Technical and Specification Data
 GlucoMen LX PLUS: Accuracy Evaluations to ISO 15197:2013 with Technical and Specification Data GlucoMen LX PLUS blood glucose and blood ketone meter Accuracy Evaluation to New ISO 15197:2013, with Technical
GlucoMen LX PLUS: Accuracy Evaluations to ISO 15197:2013 with Technical and Specification Data GlucoMen LX PLUS blood glucose and blood ketone meter Accuracy Evaluation to New ISO 15197:2013, with Technical
Accuracy and Precision Performance of the Accu-Chek Mobile System. Introduction. Method
 Accuracy and Precision Performance of the Accu-Chek Mobile System I. ACCURACY The accuracy of the system was assessed according to ISO 15197. Introduction The purpose of this study was to determine the
Accuracy and Precision Performance of the Accu-Chek Mobile System I. ACCURACY The accuracy of the system was assessed according to ISO 15197. Introduction The purpose of this study was to determine the
" EXTERNAL QUALITY EVALUATION PROGRAM FOR POCT GLUCOSE TESTING years of experience
 Commissie voor Klinische Biologie Commission de Biologie Clinique " EXTERNAL QUALITY EVALUATION PROGRAM FOR POCT GLUCOSE TESTING 8 3 years of experience CM Van Campenhout JC Libeer WIV/ISP/IPH Clinical
Commissie voor Klinische Biologie Commission de Biologie Clinique " EXTERNAL QUALITY EVALUATION PROGRAM FOR POCT GLUCOSE TESTING 8 3 years of experience CM Van Campenhout JC Libeer WIV/ISP/IPH Clinical
System accuracy evaluation of FORA Test N Go Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013
 System Accuracy Evaluation of Project number: Fora092614-01 System accuracy evaluation of Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013 Date: 13 th
System Accuracy Evaluation of Project number: Fora092614-01 System accuracy evaluation of Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013 Date: 13 th
Blood Glucose Test Strip Guidelines
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Country-Specific Glucose Monitor List
 Country-Specific Glucose Monitor List Country Name: United States Important Information This is a non-comprehensive list, current as of December 2017. Absence of a specific glucose monitor or test strips
Country-Specific Glucose Monitor List Country Name: United States Important Information This is a non-comprehensive list, current as of December 2017. Absence of a specific glucose monitor or test strips
Determination of Hematocrit Interference in Blood Samples Derived from Patients with Different Blood Glucose Concentrations
 Journal of Diabetes Science and Technology Volume 7, Issue 1, January 2013 Diabetes Technology Society ORIGINAL ARTICLE Determination of Hematocrit Interference in Blood Samples Andreas, M.D., Ph.D., 1
Journal of Diabetes Science and Technology Volume 7, Issue 1, January 2013 Diabetes Technology Society ORIGINAL ARTICLE Determination of Hematocrit Interference in Blood Samples Andreas, M.D., Ph.D., 1
Accuracy and Usability Evaluation of Six Commercially Available Blood Glucose Monitoring Systems
 Original Article Accuracy and Usability Evaluation of Six Commercially Available Blood Glucose Monitoring Systems DOI: 10.7860/JCDR/2018/34055.11671 Internal Medicine Section Anastasios Tentolouris 1,
Original Article Accuracy and Usability Evaluation of Six Commercially Available Blood Glucose Monitoring Systems DOI: 10.7860/JCDR/2018/34055.11671 Internal Medicine Section Anastasios Tentolouris 1,
SUMMARY... 2 PLANNING OF THE EVALUATION... 4 ANALYTICAL QUALITY SPECIFICATIONS... 5
 USER-EVALUATION ASCENSIA CONTOUR Blood Glucose Meter System Table of contents SUMMARY... 2 PLANNING OF THE EVALUATION... 4 ANALYTICAL QUALITY SPECIFICATIONS... 5 MATERIALS AND METHODS... 6 ASCENSIA CONTOUR...
USER-EVALUATION ASCENSIA CONTOUR Blood Glucose Meter System Table of contents SUMMARY... 2 PLANNING OF THE EVALUATION... 4 ANALYTICAL QUALITY SPECIFICATIONS... 5 MATERIALS AND METHODS... 6 ASCENSIA CONTOUR...
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
System accuracy evaluation of FORA Test N Go Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013
 System accuracy evaluation of FORA Test N Go Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013 Abstract Objective: The goal of the study was to assess
System accuracy evaluation of FORA Test N Go Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013 Abstract Objective: The goal of the study was to assess
FreeStyle Lite A Blood Glucose Meter That Requires No Coding
 Journal of Diabetes Science and Technology Volume 2, Issue 4, July 2008 Diabetes Technology Society SYMPOSIUM Shridhara, Ph.D. Abstract Background: Abbott Diabetes Care introduced the FreeStyle Lite blood
Journal of Diabetes Science and Technology Volume 2, Issue 4, July 2008 Diabetes Technology Society SYMPOSIUM Shridhara, Ph.D. Abstract Background: Abbott Diabetes Care introduced the FreeStyle Lite blood
Aina Blood Monitoring System
 Aina Blood Monitoring System Analytical Performance Summary The Aina Blood Monitoring System is a high quality and versatile multi-parameter diagnostic platform that is CE-marked and approved for sale
Aina Blood Monitoring System Analytical Performance Summary The Aina Blood Monitoring System is a high quality and versatile multi-parameter diagnostic platform that is CE-marked and approved for sale
A meter designed for glucose self-measurement manufactured by Roche Diagnostics. Report from an evaluation organised by SKUP
 SKUP Scandinavian evaluation of laboratory equipment for primary health care ACCU-CHEK Compact Plus A meter designed for glucose self-measurement manufactured by Roche Diagnostics Report from an evaluation
SKUP Scandinavian evaluation of laboratory equipment for primary health care ACCU-CHEK Compact Plus A meter designed for glucose self-measurement manufactured by Roche Diagnostics Report from an evaluation
A meter designed for glucose self-measurement manufactured by LifeScan, Johnson & Johnson. Report from an evaluation organised by SKUP
 SKUP Scandinavian evaluation of laboratory equipment for primary health care ONETOUCH Ultra A meter designed for glucose self-measurement manufactured by LifeScan, Johnson & Johnson Report from an evaluation
SKUP Scandinavian evaluation of laboratory equipment for primary health care ONETOUCH Ultra A meter designed for glucose self-measurement manufactured by LifeScan, Johnson & Johnson Report from an evaluation
CHEMICAL REAGENTS 478 CHEMICAL REAGENTS. This document is dated 1 st October 2017
 This document is dated 1 st October 2017 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
This document is dated 1 st October 2017 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
Supported devices/compatibility list
 Supported devices/compatibility list DIABASS / DIABASS PRO Version:18.11.0.2 / Release date: 29.11.2018 A101-05102017 Blood glucose meters The following blood glucose meters are compatible with DIABASS/DIABASS
Supported devices/compatibility list DIABASS / DIABASS PRO Version:18.11.0.2 / Release date: 29.11.2018 A101-05102017 Blood glucose meters The following blood glucose meters are compatible with DIABASS/DIABASS
CHEMICAL REAGENTS 478 CHEMICAL REAGENTS. This document is dated 1 st March 2018
 This document is dated 1 st March 2018 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
This document is dated 1 st March 2018 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE
 A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
HYDROXYBUTERATE) Gluco Rx Evolve KetoneTest Strips
 Scottish Drug Tariff Part 9 This document is dated 1 st November 2018 Page REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR BLOOD GLUCOSE FOR KETONES (BETA- HYDROXYBUTERATE) 478 DETECTION
Scottish Drug Tariff Part 9 This document is dated 1 st November 2018 Page REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR BLOOD GLUCOSE FOR KETONES (BETA- HYDROXYBUTERATE) 478 DETECTION
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE
 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k103542 B. Purpose for Submission: Alternative blood glucose test strips for use with LifeScan OneTouch
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k103542 B. Purpose for Submission: Alternative blood glucose test strips for use with LifeScan OneTouch
CHEMICAL REAGENTS 478 PART 9 CHEMICAL REAGENTS. This document is dated 1 st July 2015.
 PART 9 This document is dated 1 st July 2015. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
PART 9 This document is dated 1 st July 2015. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
Blood glucose monitoring and compatibility equipment checker
 Introduction This resource has been created to assist pharmacists, and other appropriately trained members of the pharmacy team, to ensure compatibility of insulin products/devices and blood glucose monitoring
Introduction This resource has been created to assist pharmacists, and other appropriately trained members of the pharmacy team, to ensure compatibility of insulin products/devices and blood glucose monitoring
GUIDELINE FOR SELF MONITORING OF BLOOD GLUCOSE IN ADULTS WITH DIABETES
 It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
Relationship between glucose meter error and glycemic control efficacy
 Relationship between glucose meter error and glycemic control efficacy Brad S. Karon, M.D., Ph.D. Professor of Laboratory Medicine and Pathology Department of Laboratory Medicine and Pathology Mayo Clinic
Relationship between glucose meter error and glycemic control efficacy Brad S. Karon, M.D., Ph.D. Professor of Laboratory Medicine and Pathology Department of Laboratory Medicine and Pathology Mayo Clinic
CHEMICAL REAGENTS 478 PART 9 CHEMICAL REAGENTS. This document is dated 1 st March 2016.
 PART 9 This document is dated 1 st March 2016. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
PART 9 This document is dated 1 st March 2016. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
In humans, self-monitoring of blood glucose is crucial
 J Vet Intern Med 2014;28:1405 1413 ISO-Based Assessment of Accuracy and Precision of Glucose Meters in Dogs Y. Brito-Casillas, P. Figueirinhas, J.C. Wiebe, L. Lopez-Rıos, D. Perez-Barreto, C. Melian, and
J Vet Intern Med 2014;28:1405 1413 ISO-Based Assessment of Accuracy and Precision of Glucose Meters in Dogs Y. Brito-Casillas, P. Figueirinhas, J.C. Wiebe, L. Lopez-Rıos, D. Perez-Barreto, C. Melian, and
Blood glucose test strips (BGTS) evaluation protocol and results. Update April Version 4.1. Note:
 Blood glucose test strips (BGTS) evaluation protocol and results Update April 2016 Version 4.1 Note: December 2017: Prescribing data indicates overall compliance with the GMMMG recommended BGTS of 86%.
Blood glucose test strips (BGTS) evaluation protocol and results Update April 2016 Version 4.1 Note: December 2017: Prescribing data indicates overall compliance with the GMMMG recommended BGTS of 86%.
Meter and test strips designed for glucose self-measurement manufactured by Arkray, Inc. Report from an evaluation organised by SKUP
 SKUP Scandinavian evaluation of laboratory equipment for primary health care GLUCOCARD X-METER GLUCOCARD X-SENSOR Meter and test strips designed for glucose self-measurement manufactured by Arkray, Inc.
SKUP Scandinavian evaluation of laboratory equipment for primary health care GLUCOCARD X-METER GLUCOCARD X-SENSOR Meter and test strips designed for glucose self-measurement manufactured by Arkray, Inc.
Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System
 Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX Blood Glucose Monitoring System, from Trividia Health,
Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX Blood Glucose Monitoring System, from Trividia Health,
Equivalent Accuracy Evaluation of FORA Premium V10 Blood Glucose Monitoring System as Compared to Fora V30 Blood Glucose Monitoring System
 Equivalent Accuracy Evaluation of FORA Premium V10 Blood Glucose Monitoring System as Compared to Fora V30 Blood Glucose Monitoring System Abstract Objective: Both Fora Premium V10 and V30 blood glucose
Equivalent Accuracy Evaluation of FORA Premium V10 Blood Glucose Monitoring System as Compared to Fora V30 Blood Glucose Monitoring System Abstract Objective: Both Fora Premium V10 and V30 blood glucose
WHITE PAPER LOOKING PAST THE WINDOW DRESSING: UNDERSTANDING BLOOD GLUCOSE TESTING STRIPS IN A FORMULARY ENVIRONMENT
 WHITE PAPER LOOKING PAST THE WINDOW DRESSING: UNDERSTANDING BLOOD GLUCOSE TESTING STRIPS IN A FORMULARY ENVIRONMENT PURPOSE This paper explores the science behind glucose test strips and proposes the format
WHITE PAPER LOOKING PAST THE WINDOW DRESSING: UNDERSTANDING BLOOD GLUCOSE TESTING STRIPS IN A FORMULARY ENVIRONMENT PURPOSE This paper explores the science behind glucose test strips and proposes the format
(a) y = 1.0x + 0.0; r = ; N = 60 (b) y = 1.0x + 0.0; r = ; N = Lot 1, Li-heparin whole blood, HbA1c (%)
 cobas b system - performance evaluation Study report from a multicenter evaluation of the new cobas b system for the measurement of HbAc and lipid panel Introduction The new cobas b system provides a point-of-care
cobas b system - performance evaluation Study report from a multicenter evaluation of the new cobas b system for the measurement of HbAc and lipid panel Introduction The new cobas b system provides a point-of-care
White Paper, Evaluation of WaveSense JAZZ Blood Glucose Monitoring System Analytical Performance to EN ISO 15197:2015 Standard
 White Paper, Evaluation of WaveSense JAZZ Blood Glucose Monitoring System Analytical Performance to EN ISO 15197:2015 Standard 7500-10032 Rev F Table of Contents Executive Summary 3 Changes introduced
White Paper, Evaluation of WaveSense JAZZ Blood Glucose Monitoring System Analytical Performance to EN ISO 15197:2015 Standard 7500-10032 Rev F Table of Contents Executive Summary 3 Changes introduced
SKUP Scandinavian evaluation of laboratory equipment for primary health care
 SKUP Scandinavian evaluation of laboratory equipment for primary health care Accu-Chek Mobile Meter and test cassettes designed for glucose self-testing manufactured by Roche Diagnostics GmbH Report from
SKUP Scandinavian evaluation of laboratory equipment for primary health care Accu-Chek Mobile Meter and test cassettes designed for glucose self-testing manufactured by Roche Diagnostics GmbH Report from
Multi User Model. Product Overview
 Multi User Model Product Overview Features and Benefits NO CODING EASY TO USE o o o Large LCD display. Large buttons with simple functions Easy Setup. Pre-Setup before shipping. Easy strip insertion. SMALL
Multi User Model Product Overview Features and Benefits NO CODING EASY TO USE o o o Large LCD display. Large buttons with simple functions Easy Setup. Pre-Setup before shipping. Easy strip insertion. SMALL
bedside glucose testing systems
 42 CAP TODAY JANUARY 2017 Part 1 of 4 Abbott Diabetes Care Abbott Diabetes Care Arkray 1420 Harbor Bay Parkway 1420 Harbor Bay Parkway 5198 W. 76th St. Alameda, CA 94502 Alameda, CA 94502 Edina, MN 55439
42 CAP TODAY JANUARY 2017 Part 1 of 4 Abbott Diabetes Care Abbott Diabetes Care Arkray 1420 Harbor Bay Parkway 1420 Harbor Bay Parkway 5198 W. 76th St. Alameda, CA 94502 Alameda, CA 94502 Edina, MN 55439
Accuracy Evaluation of Five Blood Glucose Monitoring Systems: The North American Comparator Trial
 Journal of Diabetes Science and Technology Volume 7, Issue 5, September 2013 Diabetes Technology Society ORIGINAL ARTICLE Accuracy Evaluation of Five Blood Glucose Monitoring Systems: The North American
Journal of Diabetes Science and Technology Volume 7, Issue 5, September 2013 Diabetes Technology Society ORIGINAL ARTICLE Accuracy Evaluation of Five Blood Glucose Monitoring Systems: The North American
Walsall CCG Supported Blood Glucose Meters April 2018
 Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
Bern Harrison, B.A., Cheryl Leazenby, B.S., and Solveig Halldorsdottir, Ph.D.
 Journal of Diabetes Science and Technology Volume 5, Issue 4, July 2011 Diabetes Technology Society TECHNOLOGY REPORTS Accuracy of the CONTOUR Blood Glucose Monitoring System Bern, B.A., Cheryl Leazenby,
Journal of Diabetes Science and Technology Volume 5, Issue 4, July 2011 Diabetes Technology Society TECHNOLOGY REPORTS Accuracy of the CONTOUR Blood Glucose Monitoring System Bern, B.A., Cheryl Leazenby,
Polymer Technology Systems, Inc. CardioChek PA Comparison Study
 Polymer Technology Systems, Inc. CardioChek PA Comparison Study Evaluation Protocol: Accuracy Precision Clinical Correlation PTS Panels Lipid Panel Test Strips For Use in Comparisons to a Reference Laboratory
Polymer Technology Systems, Inc. CardioChek PA Comparison Study Evaluation Protocol: Accuracy Precision Clinical Correlation PTS Panels Lipid Panel Test Strips For Use in Comparisons to a Reference Laboratory
COBAS, ACCU-CHECK INFORM, MODULAR and LIFE NEEDS ANSWERS are trademarks of Roche Roche
 Accu-Chek Inform II test strip Results of the evaluation study on newborns, conducted by the Flevo Hospital (FVZH) Almere and the Maxima Medical Center (MMC) Veldhoven in the Netherlands COBAS, ACCU-CHECK
Accu-Chek Inform II test strip Results of the evaluation study on newborns, conducted by the Flevo Hospital (FVZH) Almere and the Maxima Medical Center (MMC) Veldhoven in the Netherlands COBAS, ACCU-CHECK
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa
 MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System
 Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX AIR Blood Glucose Monitoring System, from Trividia
Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX AIR Blood Glucose Monitoring System, from Trividia
Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System
 Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System Summary Objectives: To demonstrate that the TRUE METRIX Self-Monitoring Blood Glucose System*, from Trividia
Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System Summary Objectives: To demonstrate that the TRUE METRIX Self-Monitoring Blood Glucose System*, from Trividia
DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER
 DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER 2017-404 Question/s to be Answered Can you please provide the following
DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER 2017-404 Question/s to be Answered Can you please provide the following
Mission Hb accurately detects both
 Mission Digital Hb-Testing System: Save Cost by testing 2- Parameters with Single Strip real time ie: i.e: Hemoglobin & Hematocrit-[PCV]. Presented By : Remedy Healthcare Private Limited, New Delhi, [INDIA].
Mission Digital Hb-Testing System: Save Cost by testing 2- Parameters with Single Strip real time ie: i.e: Hemoglobin & Hematocrit-[PCV]. Presented By : Remedy Healthcare Private Limited, New Delhi, [INDIA].
How to Access A Free Blood Glucose Meter
 How to Access A Free Abbott FreeStyle Lite FreeStyle Lite FreeStyle Freedom Lite FreeStyle InsuLinx How to Access A Free contacting their health care professionals at diabetes clinics; or calling the FreeStyle
How to Access A Free Abbott FreeStyle Lite FreeStyle Lite FreeStyle Freedom Lite FreeStyle InsuLinx How to Access A Free contacting their health care professionals at diabetes clinics; or calling the FreeStyle
GD50. Simple. Accurate. Reliable. Test Simply. Live Better. All FORA Diamond Series Blood Glucose Monitoring Systems use FORA Diamond Test Strips.
 GD50 Higher Measuring Accuracy Simple. Accurate. Reliable. Test Simply. Live Better. All FORA Diamond Series Blood Glucose Monitoring Systems use FORA Diamond Test Strips. GD50 Simple. Accurate. Reliable.
GD50 Higher Measuring Accuracy Simple. Accurate. Reliable. Test Simply. Live Better. All FORA Diamond Series Blood Glucose Monitoring Systems use FORA Diamond Test Strips. GD50 Simple. Accurate. Reliable.
Ultra Blue. Test Strips UNISTRIP. Charlotte, NC V2-10/25/2018 TECHNOLOGIES,LLC
 VS UNISTRIP TECHNOLOGIES,LLC Charlotte, NC 28269 1-866-889-6229 V2-10/25/2018 UniStrip Generic Blood Glucose VS OneTouch Ultra Blue 1. Purpose This study is intended to evaluate the difference in values
VS UNISTRIP TECHNOLOGIES,LLC Charlotte, NC 28269 1-866-889-6229 V2-10/25/2018 UniStrip Generic Blood Glucose VS OneTouch Ultra Blue 1. Purpose This study is intended to evaluate the difference in values
For more comprehensive information, please refer to the t:connect Application User Guide available online at: Getting Started Guide.
 Congratulations on the purchase of your new insulin pump from Tandem Diabetes Care. Your decision to use insulin pump therapy is a sign of your commitment to actively manage your diabetes. This guide provides
Congratulations on the purchase of your new insulin pump from Tandem Diabetes Care. Your decision to use insulin pump therapy is a sign of your commitment to actively manage your diabetes. This guide provides
NHS Greater Glasgow and Clyde Adult Blood Glucose Meter and Test Strip Formulary
 NHS Greater Glasgow and Clyde Adult Blood Glucose and Test Strip Formulary 2016-17 Primary Care and Acute Joint Formulary Blood Glucose formulary developed by the Diabetes MCN Blood Glucose Formulary Review
NHS Greater Glasgow and Clyde Adult Blood Glucose and Test Strip Formulary 2016-17 Primary Care and Acute Joint Formulary Blood Glucose formulary developed by the Diabetes MCN Blood Glucose Formulary Review
ISO 15197:2013 / EN ISO 15197:2015 Clinical Validation Study List for Blood Glucose Monitoring System
 List No. STUDY TITLE DATE / PLACE SCIENTIST BRIEF RESULTS TYPE OF STUDY 01 Intermeiate measurement precision Jan 2018 The FORA 6 Connect fulfille the requirements of evaluation of FORA 6 Connect Multi-functional
List No. STUDY TITLE DATE / PLACE SCIENTIST BRIEF RESULTS TYPE OF STUDY 01 Intermeiate measurement precision Jan 2018 The FORA 6 Connect fulfille the requirements of evaluation of FORA 6 Connect Multi-functional
Effects of Simulated Altitude on Blood Glucose Meter Performance: Implications for In-Flight Blood Glucose Monitoring
 Journal of Diabetes Science and Technology Volume 6, Issue 4, July 2012 Diabetes Technology Society ORIGINAL ARTICLE Effects of Simulated Altitude on Blood Glucose Meter Performance: Tolu, M.B.B.S., M.R.C.P.,
Journal of Diabetes Science and Technology Volume 6, Issue 4, July 2012 Diabetes Technology Society ORIGINAL ARTICLE Effects of Simulated Altitude on Blood Glucose Meter Performance: Tolu, M.B.B.S., M.R.C.P.,
Glucometers Gastrostomy Tubes Nasogastric Tubes Insulin pumps Continuous Glucose monitoring system Closed Loop Smart phones/tablets
 Devices Glucometers Gastrostomy Tubes Nasogastric Tubes Insulin pumps Continuous Glucose monitoring system Closed Loop Smart phones/tablets Accuracy Glucometers The ISO (International Organization for
Devices Glucometers Gastrostomy Tubes Nasogastric Tubes Insulin pumps Continuous Glucose monitoring system Closed Loop Smart phones/tablets Accuracy Glucometers The ISO (International Organization for
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa
 MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
SKUP Scandinavian evaluation of laboratory equipment for primary health care
 S K U P i n D e n m a r k, D E K S, R i g s h o s p i t a l e t - G l o s t r u p, DK- 2 6 0 0 G l o s t r u p, P h o n e + 4 5 3 8 6 3 4 4 0 0, w w w. s k u p. n u S K U P i n N o r w a y, N o k l u s,
S K U P i n D e n m a r k, D E K S, R i g s h o s p i t a l e t - G l o s t r u p, DK- 2 6 0 0 G l o s t r u p, P h o n e + 4 5 3 8 6 3 4 4 0 0, w w w. s k u p. n u S K U P i n N o r w a y, N o k l u s,
Evaluation Report: Eurolyser CRP test (ST0100 and ST0102) on. CUBE analyser (CA0100)
 Evaluation Report: Eurolyser CRP test (ST0100 and ST0102) on CUBE analyser (CA0100) Location Location: Eurolyser Diagnostica GmbH Operators: Simone Wieser; Franz Helminger; Michael Gruber Date: July-November
Evaluation Report: Eurolyser CRP test (ST0100 and ST0102) on CUBE analyser (CA0100) Location Location: Eurolyser Diagnostica GmbH Operators: Simone Wieser; Franz Helminger; Michael Gruber Date: July-November
Type 2 Diabetes Recommended SMBG
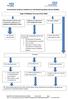 Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary
 NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary 2018-20 Primary Care and Acute Joint Formulary Blood Glucose Meter formulary developed by the Diabetes
NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary 2018-20 Primary Care and Acute Joint Formulary Blood Glucose Meter formulary developed by the Diabetes
GB/T Translated English of Chinese Standard: GB/T
 Translated English of Chinese Standard: GB/T 19634-2005 www.chinesestandard.net Sales@ChineseStandard.net GB ICS 11.100 C 44 National Standard of the People s Republic of China GB/T 19634-2005 In vitro
Translated English of Chinese Standard: GB/T 19634-2005 www.chinesestandard.net Sales@ChineseStandard.net GB ICS 11.100 C 44 National Standard of the People s Republic of China GB/T 19634-2005 In vitro
Smart & Simple Mobile Solutions.
 Higher Measuring Accuracy Smart & Simple Mobile Solutions. Test Simply. Live Better. FORA Diamond lets you connect all your Bluetooth devices. *Also available with a Micro USB. ifora Diabetes Manager All
Higher Measuring Accuracy Smart & Simple Mobile Solutions. Test Simply. Live Better. FORA Diamond lets you connect all your Bluetooth devices. *Also available with a Micro USB. ifora Diabetes Manager All
Guidance for the choice of blood glucose testing meters, strips and lancets
 Guidance for the choice of blood glucose testing meters, strips and lancets This guidance is intended to assist healthcare professionals in their selection of appropriate blood glucose meters and testing
Guidance for the choice of blood glucose testing meters, strips and lancets This guidance is intended to assist healthcare professionals in their selection of appropriate blood glucose meters and testing
BLOOD GLUCOSE METERS ACCURACY REQUIREMENTS
 BLOOD GLUCOSE METERS ACCURACY REQUIREMENTS International Standard ISO 15197:2013 (Second Edition 15/05/2013) In vitro diagnostic test systems Requirements for blood-glucose monitoring systems for self-testing
BLOOD GLUCOSE METERS ACCURACY REQUIREMENTS International Standard ISO 15197:2013 (Second Edition 15/05/2013) In vitro diagnostic test systems Requirements for blood-glucose monitoring systems for self-testing
ABSTRACT ORIGINAL RESEARCH. Guido Freckmann. Nina Jendrike. Annette Baumstark. Stefan Pleus. Christina Liebing. Cornelia Haug
 Diabetes Ther (2018) 9:683 697 https://doi.org/10.1007/s13300-018-0392-6 ORIGINAL RESEARCH User Performance Evaluation of Four Blood Glucose Monitoring Systems Applying ISO 15197:2013 Accuracy Criteria
Diabetes Ther (2018) 9:683 697 https://doi.org/10.1007/s13300-018-0392-6 ORIGINAL RESEARCH User Performance Evaluation of Four Blood Glucose Monitoring Systems Applying ISO 15197:2013 Accuracy Criteria
3. System Accuracy Evaluation LUX Hgb test 1. Samples
 3. System Accuracy Evaluation LUX Hgb test 1. Samples - Type of sample: Capillary Blood System accuracy evaluation was performed with 200 fresh capillary blood samples, each with sufficient volume to be
3. System Accuracy Evaluation LUX Hgb test 1. Samples - Type of sample: Capillary Blood System accuracy evaluation was performed with 200 fresh capillary blood samples, each with sufficient volume to be
Set-up and special features
 Set-up and special features Set Time 2 : 45 Set Date 23 Apr 202 Choose to keep your Verio IQ simple or customise it to your desire with its various features Alerts On Of f Please set with care. In the
Set-up and special features Set Time 2 : 45 Set Date 23 Apr 202 Choose to keep your Verio IQ simple or customise it to your desire with its various features Alerts On Of f Please set with care. In the
Worksheet. Worksheet. Worksheet. Worksheet. Student Performance Guide. Student Performance Guide
 LESSON 6-3 Laboratory Reagent Preparation and Calculations Worksheet LESSON 6-4 Chemistry Instrumentation in the Physician Office Laboratory Worksheet LESSON 6-6 Measuring Blood Glucose Worksheet LESSON
LESSON 6-3 Laboratory Reagent Preparation and Calculations Worksheet LESSON 6-4 Chemistry Instrumentation in the Physician Office Laboratory Worksheet LESSON 6-6 Measuring Blood Glucose Worksheet LESSON
NICO /14 Nipro Diagnostics, Inc.
 Clinical Performance of the TRUEresult twist Blood Glucose Monitoring System () Using ISO 15197:13 Criteria for Accuracy Utilizing GDH-FAD Enzyme NICO-8 1/1 Nipro Diagnostics, Inc. NICO_8_mmol.indd CLINICAL
Clinical Performance of the TRUEresult twist Blood Glucose Monitoring System () Using ISO 15197:13 Criteria for Accuracy Utilizing GDH-FAD Enzyme NICO-8 1/1 Nipro Diagnostics, Inc. NICO_8_mmol.indd CLINICAL
Performance of three portable blood glucose monitoring devices used in a veterinary application
 Performance of three portable blood glucose monitoring devices used in a veterinary application Prepared by Michael I. Lindinger, PhD, Vice-President of Research, The Nutraceutical Alliance, Campbellville,
Performance of three portable blood glucose monitoring devices used in a veterinary application Prepared by Michael I. Lindinger, PhD, Vice-President of Research, The Nutraceutical Alliance, Campbellville,
Prescription Refill List Insulin and Related Supplies
 Insulin and Related Supplies Name Date Local Pharamcy Name/Address : 30 day/ 5 refills 90 day/ 3 refills Mail Away Pharamacy Name/Address: 30 day/ 5 refills 90day / 3 refills *Check only those items that
Insulin and Related Supplies Name Date Local Pharamcy Name/Address : 30 day/ 5 refills 90 day/ 3 refills Mail Away Pharamacy Name/Address: 30 day/ 5 refills 90day / 3 refills *Check only those items that
Meter and test strips designed for glucose self-measurement manufactured by A. Menarini Diagnostics. Report from an evaluation organised by SKUP
 SKUP Scandinavian evaluation of laboratory equipment for primary health care GlucoMen LX Meter and test strips designed for glucose self-measurement manufactured by A. Menarini Diagnostics Report from
SKUP Scandinavian evaluation of laboratory equipment for primary health care GlucoMen LX Meter and test strips designed for glucose self-measurement manufactured by A. Menarini Diagnostics Report from
College of American Pathologists (CAP) GH2 Survey Data: (updated 12/09) 2009 GH2-B (fresh pooled samples) * = NGSP certified at the time of the survey
 College of American Pathologists (CAP) GH2 Survey Data: (updated 12/09) The American Diabetes Association (ADA) recommends that laboratories use only HbA1c assay methods that have been NGSP certified and
College of American Pathologists (CAP) GH2 Survey Data: (updated 12/09) The American Diabetes Association (ADA) recommends that laboratories use only HbA1c assay methods that have been NGSP certified and
Why do we need SD LipidoCare?
 SD Biosensor, Inc. Innovative Global Leader of In Vitro Diagnostics Total supplier of Blood Glucose Monitoring System and Lipid Analyzer System in vitro diagnostics test kits for Point of Care Testing
SD Biosensor, Inc. Innovative Global Leader of In Vitro Diagnostics Total supplier of Blood Glucose Monitoring System and Lipid Analyzer System in vitro diagnostics test kits for Point of Care Testing
Title/Description: Point of Care Glucose Testing-Waived Department: Organization Wide Personnel: All Effective Date: November 29, 2016 Revised:
 Title/Description: Point of Care Glucose Testing-Waived Department: Organization Wide Personnel: All Effective Date: November 29, 2016 Revised: PURPOSE The Abbott FreeStyle Precision Pro (FSPP) Blood Glucose
Title/Description: Point of Care Glucose Testing-Waived Department: Organization Wide Personnel: All Effective Date: November 29, 2016 Revised: PURPOSE The Abbott FreeStyle Precision Pro (FSPP) Blood Glucose
Self-monitoring of Blood Glucose Historical perspective
 Self-monitoring of Blood Glucose Historical perspective DROKeystoneSMBG2009 Monitoring Glycaemic Control : Early History Methodology: Urinalysis for glucose In 1941 Clinitest effervescent urine sugar testing
Self-monitoring of Blood Glucose Historical perspective DROKeystoneSMBG2009 Monitoring Glycaemic Control : Early History Methodology: Urinalysis for glucose In 1941 Clinitest effervescent urine sugar testing
ACCU-CHEK AVIVA TEST STRIPS ACCU-CHEK COMPACT TEST STRIP ACCU-CHEK COMPACT TEST STRIP ACCU-CHEK FASTCLIX 6 LANCET DRUM (102S)
 Appendix H Product Identification Number (PIN) DIN Code Brand Name Canadian Product Name 00999997 99 EXTEMPOR. CPDS - NFLD- 00977062 ACCU-CHEK ADVANTAGE TEST ACCU-CHEK ADVANTAGE TEST 00977124 ACCU-CHEK
Appendix H Product Identification Number (PIN) DIN Code Brand Name Canadian Product Name 00999997 99 EXTEMPOR. CPDS - NFLD- 00977062 ACCU-CHEK ADVANTAGE TEST ACCU-CHEK ADVANTAGE TEST 00977124 ACCU-CHEK
Quality Control of Self-Monitoring of Blood Glucose: Why and How?
 Journal of Diabetes Science and Technology Volume 1, Issue 2, March 2007 Diabetes Technology Society SYMPOSIUM Quality Control of Self-Monitoring of Blood Glucose: Why and How? Bogdan, M.D., Ph.D. and
Journal of Diabetes Science and Technology Volume 1, Issue 2, March 2007 Diabetes Technology Society SYMPOSIUM Quality Control of Self-Monitoring of Blood Glucose: Why and How? Bogdan, M.D., Ph.D. and
PROCEDURE NO. POC LBH. Printed copies are for reference only. Please refer to the electronic copy for the latest version.
 Department Of Pathology POC.514.06- Blood Glucose Monitoring Accu-Chek Inform II Procedure-LBH Version# 6 Department PROCEDURE NO. PAGE NO. Point-of-Care Testing POC.514.05 LBH 1 OF 7 Printed copies are
Department Of Pathology POC.514.06- Blood Glucose Monitoring Accu-Chek Inform II Procedure-LBH Version# 6 Department PROCEDURE NO. PAGE NO. Point-of-Care Testing POC.514.05 LBH 1 OF 7 Printed copies are
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
Electrochemical Glucose Biosensors for Diabetes Care
 BIOREV DOI: 10.1007/11663_2016_3 Springer International Publishing Switzerland 2016 Electrochemical Glucose Biosensors for Diabetes Care Gregor Ocvirk, Harvey Buck, and Stacy Hunt DuVall Abstract Blood
BIOREV DOI: 10.1007/11663_2016_3 Springer International Publishing Switzerland 2016 Electrochemical Glucose Biosensors for Diabetes Care Gregor Ocvirk, Harvey Buck, and Stacy Hunt DuVall Abstract Blood
Advances in Diabetes Technology Part II: Analysis of Diabetes Self Management Devices and Support Tools
 Advances in Diabetes Technology Part II: Analysis of Diabetes Self Management Devices and Support Tools Jennifer Trujillo, PharmD, FCCP, BCPS, CDE, BC ADM Associate Professor University of Colorado School
Advances in Diabetes Technology Part II: Analysis of Diabetes Self Management Devices and Support Tools Jennifer Trujillo, PharmD, FCCP, BCPS, CDE, BC ADM Associate Professor University of Colorado School
Home Glucose Monitoring
 Home Glucose Monitoring Blood Glucose Monitors Name (Manufacturer/Distributor) Size (inches) Weight (ounces) Active (Roche) 4.6 X 1.07 X 0.9 2.01 without Advantage (Roche) 3.3 X 2.8 X 0.8 1.8 without Test
Home Glucose Monitoring Blood Glucose Monitors Name (Manufacturer/Distributor) Size (inches) Weight (ounces) Active (Roche) 4.6 X 1.07 X 0.9 2.01 without Advantage (Roche) 3.3 X 2.8 X 0.8 1.8 without Test
SKUP Scandinavian evaluation of laboratory equipment for primary health care. FreeStyle Lite
 SKUP Scandinavian evaluation of laboratory equipment for primary health care FreeStyle Lite Meter and test strips designed for glucose self-measurement and measurements by health care professionals Manufactured
SKUP Scandinavian evaluation of laboratory equipment for primary health care FreeStyle Lite Meter and test strips designed for glucose self-measurement and measurements by health care professionals Manufactured
I know my value CoaguChek XS Pro system
 I know my value CoaguChek XS Pro system The smart way to manage your anticoagulation clinic CoaguChek XS Pro system A barcode scanner can make a difference There has been an extraordinary increase in the
I know my value CoaguChek XS Pro system The smart way to manage your anticoagulation clinic CoaguChek XS Pro system A barcode scanner can make a difference There has been an extraordinary increase in the
1 PROTOCOL. Comparison Study Summary. Springs Memorial Hospital. Springs Memorial Hospital 800 W. Meeting St. Lancaster, SC (803)
 Comparison Study Summary Springs Memorial Hospital 800 W. Meeting St. Lancaster, SC 29720 (803) 286-1480 February 10, 2016 1 PROTOCOL This evaluation was conducted on February 10, 2016 at Springs Memorial
Comparison Study Summary Springs Memorial Hospital 800 W. Meeting St. Lancaster, SC 29720 (803) 286-1480 February 10, 2016 1 PROTOCOL This evaluation was conducted on February 10, 2016 at Springs Memorial
CONTOUR NEXT and CONTOUR XT
 SKUP Scandinavian evaluation of laboratory equipment for primary health care CONTOUR NEXT and CONTOUR XT Test strips and meter designed for self-monitoring of blood glucose Report from the evaluation The
SKUP Scandinavian evaluation of laboratory equipment for primary health care CONTOUR NEXT and CONTOUR XT Test strips and meter designed for self-monitoring of blood glucose Report from the evaluation The
Analytical error of home glucose monitors: a comparison of 18 systems
 Original Article Ann Clin Biochem 1999; 36: 72-79 Analytical error of home glucose monitors: a comparison of 18 systems Roger N Johnson and John R Baker From the Department of Clinical Biochemistry, Green
Original Article Ann Clin Biochem 1999; 36: 72-79 Analytical error of home glucose monitors: a comparison of 18 systems Roger N Johnson and John R Baker From the Department of Clinical Biochemistry, Green
Monitoring of Blood Glucose Level
 University of Zagreb Faculty of Electrical Engineering and Computing Biomedical instrumentation Monitoring of Blood Glucose Level Biomedical instrumentation 1/17 Outline Introduction Principles of Blood
University of Zagreb Faculty of Electrical Engineering and Computing Biomedical instrumentation Monitoring of Blood Glucose Level Biomedical instrumentation 1/17 Outline Introduction Principles of Blood
Report. Evaluation of system accuracy of the blood glucose monitoring system VivaChek Ino according to DIN EN ISO 15197: 2013
 Institute of Diabetes Gerhardt Katsch Karlsburg (IDK) Report Evaluation of system accuracy of the blood glucose monitoring system VivaChek Ino according to DIN EN ISO 15197: 2013 Sponsor: The study was
Institute of Diabetes Gerhardt Katsch Karlsburg (IDK) Report Evaluation of system accuracy of the blood glucose monitoring system VivaChek Ino according to DIN EN ISO 15197: 2013 Sponsor: The study was
OneTouch Reveal web app Report Reference Guide
 OneTouch Reveal web app Report Reference Guide Your step-by-step guide to setting up and using the OneTouch Reveal web app OneTouch Verio meter OneTouch Verio Flex meter OneTouch Verio IQ meter OneTouch
OneTouch Reveal web app Report Reference Guide Your step-by-step guide to setting up and using the OneTouch Reveal web app OneTouch Verio meter OneTouch Verio Flex meter OneTouch Verio IQ meter OneTouch
Urgent field safety notice
 Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
A model for managing and monitoring the quality of glucometers used in a high-volume clinical setting
 Original papers A model for managing and monitoring the quality of glucometers used in a high-volume clinical setting Güzin Aykal*, Ayşenur Yegin, Özgür Tekeli, Necat Yilmaz Central Laboratories, Antalya
Original papers A model for managing and monitoring the quality of glucometers used in a high-volume clinical setting Güzin Aykal*, Ayşenur Yegin, Özgür Tekeli, Necat Yilmaz Central Laboratories, Antalya
