Accuracy and Usability Evaluation of Six Commercially Available Blood Glucose Monitoring Systems
|
|
|
- Verity Hunt
- 5 years ago
- Views:
Transcription
1 Original Article Accuracy and Usability Evaluation of Six Commercially Available Blood Glucose Monitoring Systems DOI: /JCDR/2018/ Internal Medicine Section Anastasios Tentolouris 1, Ioanna Eleftheriadou 2, Pinelopi Grigoropoulou 3, Maria Nikoloudi 4, Evangelia Siami 5, Diamantis I. Tsilimigras 6, Nikolaos Tentolouris 7 ABSTRACT Introduction: Self-Monitoring of Blood Glucose (SMBG) is essential for achieving glycaemic control in individuals with diabetes mellitus. Aim: The aim of the study was to evaluate the accuracy and the usability of six different commercially available SMBG systems. Materials and Methods: A total of 120 individuals with both types of diabetes, aged between years, were recruited. Capillary Blood Glucose (BG) was measured on six SMBG systems, while the YSI 2300 STAT PLUS was used as a reference method. Accuracy performance was based on requirements resembling the ISO 15197:2013 (95% of the BG results within ±15 mg/dl of the reference method at BG concentrations <100 mg/dl and within ±15% of the reference method at BG concentration 100 mg/dl). Usability testing of the SMBG was performed using the Likert Scale Questionnaire. Results: Four out of six and five out of six glucometers demonstrated excellent performance in BG values <100 mg/dl (n=40) and in BG values 100 mg/dl (n=80), respectively. Across the overall BG range tested, five out of six SMBG systems had good accuracy performance. Among the three SMBG systems with the highest performance (>99%) in the overall BG range ( mg/dl) the one with an innovative strip insertion technique was less favoured by the participants. Conclusion: Most of the examined commercially available SMBG devices demonstrated adequate performance when they were tested in requirements resembling the ISO 15197:2013. Regarding usability, the Accu-Chek Aviva Plus and the Contour Next were the most convenient devices. Keywords: Blood glucose meters, Diabetes mellitus, ISO 15197:2013 requirements, Precision 10 INTRODUCTION Diabetes Mellitus (DM) is the most common metabolic disorder worldwide affecting 6-10% of the population [1]. Efficient management and appropriate treatment is required to achieve good diabetes control in order to reduce the risk of diabetic complications [1]. Glycemic control can be evaluated by measurement of glycated haemoglobin (HbA1c) and Self-Monitoring of Blood Glucose (SMBG) [2]. Both are equally important; HbA1c depicts the average Blood Glucose (BG) levels over the last three months, while SMBG values evaluate the individuals response to therapy and allow adjustment of antidiabetic treatment [2]. However, HbA1c measurement has several limitations. Firstly, a laboratory satisfying the established requirements for HbA1c measurement is required [3]. Moreover, conditions like chronic kidney disease, haemoglobinopathies and iron deficiency impact HbA1c reliability [3,4]. Furthermore, HbA1c does not provide a measure of glycaemic variability, which is an independent risk factor for diabetic complications [5]. The number of blood measurements depends on several parameters, such as type of diabetes, antidiabetic treatment and glycaemic targets. Patients who measure BG levels more frequently have lower HbA1c [6,7]. Regarding the importance of self-measurement, SMBG systems need to fulfill some minimum performance criteria in order to be accurate and safe for use [8]. Such requirements were established in 2003 and revised in 2013 (ISO 15197:2013) [9,10]. The revised criteria require that 95% of the individual BG results must be within ±15mg/dL of the comparison-method results at BG concentrations <100mg/dL and within ±15% of the comparison-method results at BG concentrations 100mg/dL. Moreover, consensus error grid analyses should be performed to determine the clinical relevance of the accuracy of BG measurements [11]. This was first established in 2000 and was included in the ISO guidelines [10,11]. GM700S (Bionime, Berneck, Switzerland), OneTouch Verio IQ (LifeScan, Chesterbrook, PA, USA), Freestyle Optium Neo (Abbott Diabetes Care, rth Chicago, IL, USA), Contour NEXT (Ascensia Diabetes Care, Parsippany, NJ, USA), Accu Chek Aviva Plus (Roche Diabetes Care Inc., Indianapolis, IN, USA) and Glucomen Areo (A. Menarini Diagnostics, FIorence, Italy) are SMBG systems that are widely available in Previous studies have shown that most of them fulfill the ISO requirements [12-17]. Nevertheless, apart from the ISO requirements; usability of glucometers is an important factor to be considered. However, data on the patient satisfaction of SMBG systems are scarce. The aim of the study was to evaluate the accuracy performance in requirements resembling the ISO 15197:2013 and the usability of six commonly used SMBG systems. MATERIALS AND METHODS i. Participants: This cross-sectional study took place for one week in July of 2016 in the Diabetes Center of Laiko General Hospital. A total of 120 patients with DM were recruited consecutively from the outpatient Diabetes Clinic since the ISO 15197:2013 require the evaluation of at least 100 different subjects [10]. Adults years of age with DM were included in the study. Diagnosis of DM was based on the criteria adopted from the American Diabetes Association [3]. Patients that were diagnosed with serious complications in the previous six months, such as myocardial infarction, serious trauma and major surgery, individuals with mental illness, severe anaemia (haematocrit lower than 30%) or pregnant women were excluded. The purpose of the study was clearly explained in written to all subjects, who then volunteered to participate. All patients gave their written informed consent before participation in the study, which was conducted according to the principles of the Declaration of Helsinki [18]. The ethics committee of our hospital approved the study.
2 ii. Laboratory Analyses: All procedures were carried out in the morning in an environment of stable temperature (23±5 o C) with a humidity range of the environment between 20% and 90% based on the manufacturers instruction for use. The finger skin was punctured using a disposable lancet and fresh capillary blood samples were collected into micro-tubes with lithium heparin anticoagulant by the study personnel (about 600 μl of fresh whole blood was collected) as described previously [12,14,19]. The sample was then applied on the six different glucometers that are frequently used in Greece (GM700S, OneTouch Verio IQ, Freestyle Optium Neo, Contour NEXT, Accu Chek Aviva Plus and Glucomen Areo), according to the manufacturers instructions. For each glucometer, two test strip lots were used in each sample. The order of testing was alternated among the SMBG devices and the YSI 2300 STAT PLUS (YSI Incorporated, Yellow Springs, Ohio, USA) in a predefined rotation. The rotation was performed to ensure that the test order would not affect the BG results. All strips had already been inserted in the different meters before blood drop was placed. The mean of BG measurements from each SMBG device were compared with the mean of duplicate results obtained from the YSI 2300 STAT PLUS. The mean time from sample collection to glucose measurements was less than 7 minutes, as described previously [14]. Participants were punctured for a second time at the finger and the blood was collected in a Micro Haematocrit capillary tube (Kimble Chase, Rockwood, TN, USA). Blood was then centrifuged and a ruler was used to measure the length of the column of the packed red cells, which was then divided by the length of the column of the whole blood and multiplied by 100% to obtain the haematocrit of the blood sample, as descried previously [12]. According to the recommendation of the ISO 15197:2013, a wide BG concentration needs to be tested for each SMBG system. More specifically, 5% of the tested glucose samples should have BG values 50mg/dL, 15% between 51-80mg/dL, 20% between mg/dL, 30% between mg/dL, 15% between mg/dL, 10% between mg/dL and 5% of the glucose samples should have BG values >400mg/dL [10]. If the collected samples did not provide a sufficient percentage of the required BG range, samples over 400mg/dL were artificially produced in the laboratory using glucose solution, while samples below 50mg/dL were produced by incubating blood samples at room temperature until BG concentration reached low levels because of glucose consumption by erythrocytes, as described previously [10,20]. The Partial Pressure of Oxygen (po 2 ) in adjusted blood samples was checked (OptiTM Check; OPTI Medical Systems Inc., Roswell, GA) in order to ensure a po 2 that is comparable to the po 2 in capillary blood, as described previously [19]. At the end of the visit patients had enough time to process and use the glucometers and perform BG measurement by themselves. Then, they were asked to complete a Likert Scale Questionnaire about the size of the glucometers, the blood sample application on the test strip, the insertion of the test strip on the meter, the size of the indication on the screen and the removal of the test strip. iii. Reference Method: YSI 2300 STAT PLUS uses fresh venous or capillary whole blood. Measurements are performed using glucose oxidase as an assay method. For our study, quality control was performed for every five measurements, as described previously [12]. iv. Statistical Analysis: SMBG systems accuracy was assessed by comparison of the mean of the two BG measurements obtained with each SMBG device with the respective mean of the two glucose measurements obtained with the reference method. At BG concentrations <100mg/dL, the absolute and relative number Risk level (CEG zone) A Definition of risk level SMBG <20% deviation from true BG or both SMBG and BG <70mg/dL Risk to patient with diabetes effect on clinical B Deviation from true BG >20% Little or no effect on clinical C D E Overcorrection of acceptable BG levels Dangerous failure to detect and treat BG errors Treatment contradictory to that actually required Likely to affect the clinical Significant medical impact Dangerous Consequences [Table/Fig-1]: Definitions of the error grid zones. CEG: Consensus Error Grid,, BG: Blood Glucose of each glucometer results within ±15mg/dL, ±10mg/dL, and ±5mg/dL of the comparison measurement was calculated. At BG concentrations 100mg/dL, the absolute and relative number of each SMBG system results within ±15%, ±10%, and ±5% of the comparison measurement was calculated. The ment between each SMBG system measurement and the reference method result was plotted in a difference-plot as recommended in DIN EN ISO 15197:2013 [10]. Consensus error grid was used for evaluating the clinical relevance of the result between the BG measurement by SMBG device and the BG measurement by the reference method [10,11]. The five zones and their clinical importance are shown in [Table/Fig-1]. RESULTS i. Participants: A total of 120 participants were recruited. The characteristics of the study participants are shown in [Table/ Fig-2]. The haematocrit range was 30.8 to 52.9% and the glucose range was 38.7 to 475mg/dL. Regarding the distribution of BG concentrations of the blood samples collected, 6 (5.0%) had BG concentration <50mg/dL, 20 (16.7%) between 50 and 80mg/dL, 23 (19.2%) between 80 and 120mg/dL, 37 (30.8%) between 120 and 200mg/dL, 16 (13.3%) between 200 and 300mg/dL, 12 (10.0%) between 300 and 400mg/dL, and 6 (5.0%) had BG levels >400mg/ dl. ii. Accuracy: Above 95% of the results of GM700S, Contour NEXT, Accu-Chek Aviva Plus and OneTouch Verio IQ in both BG levels <100 mg/dl and 100 mg/dl were within the required limits. A total of 95.0% of the results of Glucomen Areo were within the required Gender Age (years) [Table/Fig-2]: Characteristics of the study participants. Type of diabetes Diabetes duration (years) SMBG use (years) Education level Male 67 (55.8) (1.7) Type 1 21 (17.5) (18.3) (20.8) Elementary school 17 (14.0) Female 53 (44.2) (4.2) Type 2 95 (79.2) (17.5) (16.6) Middle school 28 (23.0) (5.8) Unknown 4 (3.3) (7.5) (7.5) High school 41 (34.0) (14.2) (20.8) (20.0) Graduate school 33 (28.0) (20.0) (12.5) (11.7) College 0 (0) (32.5) (4.2) (5.0) answer 1 (1.0) (21.7) >25 21 (17.5) >25 20 (16.7) Unknown 2 (1.7) Unknown 2 (1.7) 11
3 [Table/Fig-3]: Self-monitoring blood glucose systems accuracy plots: a: GM700S, b: OneTouch Verio IQ, c: Freestyle Optium Neo, d: Contour NEXT, e: Accu-Chek Aviva Plus, f: Glucomen Areo. The black lines represent the ±15 mg/dl / ±15% limits recommended by the ISO 15197:2013 requirements. * The difference between each individual measured value from the SMBG system and the measured value from the reference method (YSI 2300 STAT PLUS). [Table/Fig-5]: Consensus error grid analysis for the results obtained with the 6 SMBG systems studied: a: GM700S, b: OneTouch Verio IQ, c: Freestyle Optium Neo, d: Contour NEXT, e: Accu-Chek Aviva Plus, f: Glucomen Areo For each plot the y-axis represents the blood glucose result obtained by the SMBG system and the x-axis depicts the blood glucose result obtained by the reference method. The zones within each plot (A to E) indicate the increasing clinical significance of an erroneous measurement. BG 100 mg/dl (n=80) BG < 100 mg/dl (n=40) All BG range (n=120) ±15 mg/dl ±10 mg/dl ±5 mg/dl ±15 % ±10 % ±5 mg/dl ±15 mg/dl or ±15 % ±10 mg/dl GM700S * 71/80 160/ / % 142/ / / % OneTouch Verio IQ 79/80 72/ % 53/ % 156/ % 131/ % 82/ % 235/ % 204/240 FreeStyle Optium Neo 74/ % 49/ % 17/ % 136/160 91/ % 32/ % 210/ % 140/ % Contour NEXT 78/ % 71/80 158/ / % 51/ % 238/ % 204/240 Accu-Chek Aviva Plus 79/80 58/ % 158/ / % 85/ % 238/ % 222/ % Glucomen Areo 73/ % 64/ % 38/ % 155/ % 127/ % 79/ % 228/ % 191/ % [Table/Fig-4]: SMBG systems accuracy according to ISO 15197:2013 and to tighter requirements. *Blood measurements were performed twice for each patient; SMBG: self-monitoring blood glucose, BG: blood glucose limits in all BG concentration, but only 91.3% of the results in BG levels <100 mg/dl were within the required limits. Less than 95% of the results of Freestyle Optium Neo were within the required limits in all BG concentration. The accuracy plots are shown in [Table/ Fig-3]. When more strict criteria where used, the glucometer with the highest percentage of results within the required limits was GM700S [Table/Fig-4]. iii. Clinical Relevance: Consensus error grid analyses were performed for each SMBG system independently and are depicted in [Table/Fig-5]. All (100%) results obtained were within zone A and zone B signifying that the measurement error had no or little effect on clinical. iv. Usability: The results of the Likert Scale Questionnaire are shown in detail in [Table/Fig-6]. The one with the most suitable size among 12 those tested was the One Touch Verio IQ followed by Contour NEXT. The SMBG device with the easiest blood sample application on the test strip was the Accu-Chek Aviva Plus, while the Contour Next was the second most convenient device. When patients were asked about the SMBG device with the clearest and most readable screen the FreeStyle Optium received the highest score. The SMBG device with the easiest strip insertion and the easier test strip removal was the Accu-Chek Aviva Plus [Table/Fig-6]. DISCUSSION In the present study, we demonstrated that the GM700S, the OneTouch Verio IQ, the Contour NEXT and the Accu-Chek Aviva Plus fulfilled the 2013 ISO-like requirements [10]. In addition, we found that Accu-Chek Aviva Plus was the SMBG device with the highest usability among the six devices studied.
4 Question How do you feel about the size of the glucose meters? to apply your blood sample to the test strip. to read the numbers/ icons displayed on the LCD Screen. to hold the strip and inserted the strip on the meter. to remove used strips from the meter without touch blood sample. Answer GM7-00S One Touch Verio IQ Percentages of responses Free- Style Optium Neo Contour NEXT Accu- Chek Aviva Plus GlucomenAreo Too large 0.8% 8.3% 35.0% 8.3% 21.7% 24.2% Suitable 80.8% 86.7% 63.3% 82.5% 74.2% 70.0% Too small 18.3% 5.0% 0.8% 8.3% 2.5% 5.8% 0.0% 0.0% 0.8% 0.8% 1.7% 0.0% 5.8% 10.8% 18.3% 25.8% 27.5% 10.8% Agree 60.8% 72.5% 75.8% 70.8% 64.2% 82.5% Dis 26.7% 15.0% 4.2% 3.3% 4.2% 5.8% dis 6.7% 1.7% 0.0% 0.0% 0.8% 0.0% 0.0% 0.0% 1.7% 0.0% 3.3% 0.8% 14.2% 24.2% 32.5% 28.3% 25.8% 16.7% Agree 77.5% 71.7% 64.2% 65.0% 67.5% 73.3% Dis 7.5% 3.3% 2.5% 5.0% 4.2% 10.0% dis 0.8% 0.0% 0.0% 0.8% 0.8% 0.0% 0.0% 0.8% 0.8% 0.8% 1.7% 0.0% 5.0% 9.2% 16.7% 23.3% 27.5% 11.7% Agree 53.3% 75.8% 76.7% 70.0% 65.0% 78.3% Dis 29.2% 13.3% 5.8% 5.8% 5.8% 9.2% dis 12.5% 1.7% 0.0% 0.8% 0.0% 0.0% 0.0% 0.0% 0.8% 0.0% 1.7% 0.8% 34.2% 16.7% 35.8% 17.5% 40.0% 23.3% Agree 44.2% 69.2% 59.2% 60.0% 52.5% 65.0% Dis 14.2% 9.2% 3.3% 15.0% 6.7% 11.7% dis 7.5% 5.0% 0.0% 6.7% 0.0% 0.0% 0.0% 0.0% 1.7% 0.8% 0.8% 0.0% [Table/Fig-6]: Participants Opinio about the Usability of the SMBG Systems according to the Likert Scale Questionnaire. Manufacturer details for each SMBG studied: GM700S (Bionime, Berneck, Switzerland), OneTouch Verio IQ (LifeScan, Chesterbrook, PA, USA), Freestyle Optium Neo (Abbott Diabetes Care, rth Chicago, IL, USA), Contour NEXT (Ascensia Diabetes Care, Parsippany, NJ, USA), Accu Chek Aviva Plus (Roche Diabetes Care Inc., Indianapolis, IN, USA) and Glucomen Areo (A. Menarini Diagnostics, FIorence, Italy). Previous studies have examined the accuracy of different SMBG systems according to the several ISO requirements over the years, but the reference method and the protocols used varied among them [12-17]. Brazg RL et al., examined the performance of 27 SMBG systems, including the Accu-Chek Aviva Plus in a population of 100 subjects [13]. This study was performed according to the ISO 15197:2003 requirements, but it was additionally tested for the ISO 15197:2013 criteria [13]. In the study by Brazg RL et al., perchloric acid hexokinase method was used as the reference method for measuring BG, while herein the YSI 2300 STAT PLUS was used [13]. The Accu-Check Aviva Plus fulfilled both ISO criteria and the percentages of the results within the required limits in all BG concentration were similar to the percentages found in our study. Moreover, Bedini JL et al., evaluated the accuracy of Contour NEXT USB, OneTouch Verio IQ and FreeStyle InsuLinx [14]. Tests were performed in 236 blood samples; unlike to our study, they used venous whole blood and not capillary blood and a hexokinase glucose analyser as the reference method. All glucometers used in that study fulfilled the ISO 15197:2013. Both Contour NEXT USB and OneTouch Verio IQ showed similar percentages of the results within the required limits, as in our study. Furthermore, Freckmann et al. tested the accuracy of Contour NEXT USB and OneTouch Verio IQ using the YSI 2300 STAT Plus as a reference method [17]. OneTouch Verio IQ failed to fulfil the ISO 15197:2013 ISO requirements, with only 84.6% of the results in BG levels <100 mg/dl and 91.9% of the results in BG levels 100 mg/dl within the required criteria. When tighter criteria were used (±10 mg/dl or ± 10% and ±5 mg/dl or ± 5%), the performance of OneTouch Verio IQ was worse than in our study, while similar to our findings the Contour NEXT USB had excellent performance in all BG range [17]. Regarding the GM700S there are not any previous studies that have examined the accuracy according to the ISO 15197:2013 requirements. However, Yu-Fei W et al., examined the performance of GM700, which is the previous model of GM700S; the reference method was the same, the YSI 2300 STAT PLUS [12]. GM700S satisfied the ISO 15197:2013 requirements. On the top of that, when GM700S was tested using tighter criteria (±10 mg/dl or ±10%) of the results in BG levels <100mg/dL were within the required limits in both studies by Yu-Fei W et al., and our study. Berti F et al., demonstrated that the Glucomen Areo satisfied the ISO 15197:2013 requirements [15]. The study by Berti F et al., was performed by A. Menarini Diagnostics, while the hexokinasebased method (Cobas Intergra 400 plus, Roche Instrument Center, Rotkreuz Switzerland) was used as reference method; in that study, more than 100 subjects participated and capillary blood samples were collected. In BG concentration <100mg/dL, of the results of the Glucomen Areo were within the required limits. However, only 91.3% of the results were within the required limits in our study. Nevertheless, in BG levels 100mg/dL similar percentages were found in the present study and in the study by Berti F et al., [15]. One previous study performed from the manufacturing company examined the accuracy of the FreeStyle Optium Neo device [16]. In that study, a total of 186 blood samples were collected from 165 patients and the YSI 2300 STAT PLUS was used as the reference method. FreeStyle Optium Neo fulfilled the ISO 15197:2013 criteria in that study, while we found that only 92.5% of the results in BG levels <100mg/dL and of the results in BG concentrations 100mg/dL were within the required limits. Although, most of the examined SMBG systems satisfied the ISO requirements, when tighter criteria were applied (±10 mg/dl or ±10%), the performance was strongly negatively affected. Hence, even though nowadays new systems, such as continuous glucose monitoring systems, are coming into the surface, the improvement of the classic SMBG systems should not be withheld, since they are still used by most people with diabetes mellitus. Consequently, they should be accurate and safe for use, especially when BG concentration is low and the threshold between hypoglycaemia and normoglycaemia is crucial. A new finding of our study was patients preference about the usability of the SMBG systems. Physicians often neglect the fact that patients are concerned not only about the accuracy, but find usability and design equally important, especially younger people with type 1 DM who constantly carry their SMBG devices. Additionally, other aspects, such as the resolution of the SMBG device screen or the size of the numbers on the screen are very important especially for older people. Interestingly, although the FreeStyle Optium Neo had the worst performance of all six SMBG systems examined, it was favoured by the users due to the easiness on reading the numbers on the screen. The same applies for the Glucomen Areo that had inadequate performance, but was considered by the participants as one of the devices with the easiest blood sample application on 13
5 the test strip. On the other hand, while the GM700S had the best performance in all BG levels according to both ISO 15197:2013 and to tighter criteria; it failed to gain patients preference. It is thus obvious that accuracy is not the only factor when choosing a SMBG device, but usability by the patients is also essential. One of the strengths of our study is that the population of 120 participants is larger than the population (100 subjects) needed according to the ISO 15197:2013 requirements. LIMITATION One limitation of the present study is that we did not perform the protocol exactly as it is defined in the ISO 15197:2013 requirements; we used two strip lots instead of three strip lots. In addition, the use of YSI 2300 STAT PLUS as a reference method, which uses glucose oxidase, could be a possible limitation, since several SMBG systems are calibrated against a hexokinase method and a systematic difference (bias) is reported between hexokinase and glucose oxidase methods. Furthermore, the protocols of several referenced studies for the tested devices vary from this study and therefore, it is incorrect to directly compare the numerical percentages and draw conclusions. Finally, another possible limitation is that the accuracy of these SMBG systems was assessed when they were used by trained Health Care professionals and not by the patients themselves. CONCLUSION In conclusion, most of the examined glucometers which are commonly used in Greece had adequate performance when they were tested in criteria resembling to the ISO 15197:2013. Besides accuracy, usability of glucose meters is an important factor to be considered by the manufacturers of the devices. Acknowledgements Glucose meters, glucose strips and reagents for the study were kindly provided by Bionime, Berneck, Switzerland. REFERENCES [1] [2] [3] [4] International Diabetes Federation. IDF Diabetes Atlas - 8th Edition [Internet] [cited 29 vember 2017]. Available from: American Diabetes Association. Glycaemic Targets. Diabetes care. 2017;40(Suppl 1):S48-S56. American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes care. 2017;40(Suppl 1):S11-S24. Florkowski C. HbA1c as a diagnostic test for diabetes mellitus - reviewing the evidence. The Clinical Biochemist Reviews. 2013;34(2): [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] Brownlee M, Hirsch IB. Glycaemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295(14): Karter AJ, Ackerson LM, Darbinian JA, D'Agostino RB, Jr., Ferrara A, Liu J, et al. Self-monitoring of blood glucose levels and glycemic control: the rthern California Kaiser Permanente Diabetes registry. The American Journal of Medicine. 2001;111(1):1-9. Miller KM, Beck RW, Bergenstal RM, Goland RS, Haller MJ, McGill JB, et al. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36(7): Hellman R. Glucose meter inaccuracy and the impact on the care of patients. Diabetes/Metabolism Research and Reviews. 2012;28(3): International Organization for Standardization. ISO 15197: 2003: In Vitro Diagnostic Test Systems Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. Geneva: International Organization for Standardization, International Organization for Standardization: ISO 15197: 2013: In Vitro Diagnostic Test Systems Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. Geneva: International Organization for Standardization, Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8): Yu-Fei W, Wei-Ping J, Ming-Hsun W, Miao OC, Ming-Chang H, Chi-Pin W, et al. Accuracy evaluation of 19 blood glucose monitoring systems manufactured in the Asia-Pacific Region: A multicenter study. Journal of Diabetes Science and Technology. 2017: Brazg RL, Klaff LJ, Parkin CG. Performance variability of seven commonly used self-monitoring of blood glucose systems: clinical considerations for patients and providers. Journal of Diabetes Science and Technology. 2013;7(1): Bedini JL, Wallace JF, Pardo S, Petruschke T. Performance evaluation of three blood glucose monitoring systems using ISO 15197:2013 accuracy criteria, consensus and surveillance error grid analyses, and insulin dosing error modeling in a hospital setting. Journal of Diabetes Science and Technology. 2015;10(1): Berti F, Scuffi C, Valgimigli F. Accuracy evaluation of two blood glucose monitoring systems following ISO 15197:2013. Journal of Diabetes Science and Technology. 2015;9(6): Evaluation of the FreeStyle Optium NeoBlood Glucose and Ketone Monitoring System [Internet]. [cited 29 vember 2017]. Available from: freestylediabetes.co.uk/images/uploads/documents/white_paper Clinical_ FreeStyle_Optium_Neo.pdf Freckmann G, Link M, Schmid C, Pleus S, Baumstark A, Haug C. System accuracy evaluation of different blood glucose monitoring systems following ISO 15197:2013 by using two different comparison methods. Diabetes Technology & Therapeutics. 2015;17(9): World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20): Freckmann G, Baumstark A, Schmid C, Pleus S, Link M, Haug C. Evaluation of 12 blood glucose monitoring systems for self-testing: system accuracy and measurement reproducibility. Diabetes Technology & Therapeutics. 2014;16(2): Pfutzner A, Hengesbach C, Demircik F, Schipper C, Forst T, Musholt PB. Performance of blood glucose meters in compliance with current and future clinical ISO15197 accuracy criteria. Curr Med Res Opin. 2014;30(2): PARTICULARS OF CONTRIBUTORS: 1. Diabetes Center, Department of Propaedeutic Internal Medicine, Medical School, National and Kapodistrian University of Athens, Laiko General Hospital, Athens, Attiki, 2. Diabetes Center, Department of Propaedeutic Internal Medicine, Medical School, National and Kapodistrian University of Athens, Laiko General Hospital, Athens, Attiki, 3. Diabetes Center, Department of Propaedeutic Internal Medicine, Medical School, National and Kapodistrian University of Athens, Laiko General Hospital, Athens, Attiki, 4. Diabetes Center, Department of Propaedeutic Internal Medicine, Medical School, National and Kapodistrian University of Athens, Laiko General Hospital, Athens, Attiki, 5. Diabetes Center, Department of Propaedeutic Internal Medicine, Medical School, National and Kapodistrian University of Athens, Laiko General Hospital, Athens, Attiki, 6. Medical School, National and Kapodistrian University of Athens, Athens, Attiki, 7. Diabetes Center, Department of Propaedeutic Internal Medicine, Medical School, National and Kapodistrian University of Athens, Laiko General Hospital, Athens, Attiki, NAME, ADDRESS, ID OF THE CORRESPONDING AUTHOR: Dr. Nikolaos Tentolouris, 17 Agiou Thoma St, 11527, Athens, Attiki, ntentol@med.uoa.gr Financial OR OTHER COMPETING INTERESTS: ne. Date of Submission: Oct 02, 2017 Date of Peer Review: v 22, 2017 Date of Acceptance: Jan 24, 2018 Date of Publishing: Jun 01,
System accuracy evaluation of FORA Test N Go Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013
 System accuracy evaluation of FORA Test N Go Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013 Abstract Objective: The goal of the study was to assess
System accuracy evaluation of FORA Test N Go Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013 Abstract Objective: The goal of the study was to assess
System accuracy evaluation of FORA Test N Go Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013
 System Accuracy Evaluation of Project number: Fora092614-01 System accuracy evaluation of Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013 Date: 13 th
System Accuracy Evaluation of Project number: Fora092614-01 System accuracy evaluation of Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013 Date: 13 th
Equivalent Accuracy Evaluation of FORA Premium V10 Blood Glucose Monitoring System as Compared to Fora V30 Blood Glucose Monitoring System
 Equivalent Accuracy Evaluation of FORA Premium V10 Blood Glucose Monitoring System as Compared to Fora V30 Blood Glucose Monitoring System Abstract Objective: Both Fora Premium V10 and V30 blood glucose
Equivalent Accuracy Evaluation of FORA Premium V10 Blood Glucose Monitoring System as Compared to Fora V30 Blood Glucose Monitoring System Abstract Objective: Both Fora Premium V10 and V30 blood glucose
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
MQ Comparison of glucometers using whole blood
 MQ 2017-1 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
MQ 2017-1 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
Report. Evaluation of system accuracy of the blood glucose monitoring system VivaChek Ino according to DIN EN ISO 15197: 2013
 Institute of Diabetes Gerhardt Katsch Karlsburg (IDK) Report Evaluation of system accuracy of the blood glucose monitoring system VivaChek Ino according to DIN EN ISO 15197: 2013 Sponsor: The study was
Institute of Diabetes Gerhardt Katsch Karlsburg (IDK) Report Evaluation of system accuracy of the blood glucose monitoring system VivaChek Ino according to DIN EN ISO 15197: 2013 Sponsor: The study was
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
FreeStyle Lite A Blood Glucose Meter That Requires No Coding
 Journal of Diabetes Science and Technology Volume 2, Issue 4, July 2008 Diabetes Technology Society SYMPOSIUM Shridhara, Ph.D. Abstract Background: Abbott Diabetes Care introduced the FreeStyle Lite blood
Journal of Diabetes Science and Technology Volume 2, Issue 4, July 2008 Diabetes Technology Society SYMPOSIUM Shridhara, Ph.D. Abstract Background: Abbott Diabetes Care introduced the FreeStyle Lite blood
GlucoMen LX PLUS blood glucose and blood ketone meter. Accuracy Evaluation to New ISO 15197:2013, with Technical and Specification Data
 GlucoMen LX PLUS: Accuracy Evaluations to ISO 15197:2013 with Technical and Specification Data GlucoMen LX PLUS blood glucose and blood ketone meter Accuracy Evaluation to New ISO 15197:2013, with Technical
GlucoMen LX PLUS: Accuracy Evaluations to ISO 15197:2013 with Technical and Specification Data GlucoMen LX PLUS blood glucose and blood ketone meter Accuracy Evaluation to New ISO 15197:2013, with Technical
Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System
 Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX AIR Blood Glucose Monitoring System, from Trividia
Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX AIR Blood Glucose Monitoring System, from Trividia
MQ Comparison of glucometers using whole blood
 MQ 2018-2 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
MQ 2018-2 Comparison of glucometers using whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing devices
NICO /14 Nipro Diagnostics, Inc.
 Clinical Performance of the TRUEresult twist Blood Glucose Monitoring System () Using ISO 15197:13 Criteria for Accuracy Utilizing GDH-FAD Enzyme NICO-8 1/1 Nipro Diagnostics, Inc. NICO_8_mmol.indd CLINICAL
Clinical Performance of the TRUEresult twist Blood Glucose Monitoring System () Using ISO 15197:13 Criteria for Accuracy Utilizing GDH-FAD Enzyme NICO-8 1/1 Nipro Diagnostics, Inc. NICO_8_mmol.indd CLINICAL
ABSTRACT ORIGINAL RESEARCH. Guido Freckmann. Nina Jendrike. Annette Baumstark. Stefan Pleus. Christina Liebing. Cornelia Haug
 Diabetes Ther (2018) 9:683 697 https://doi.org/10.1007/s13300-018-0392-6 ORIGINAL RESEARCH User Performance Evaluation of Four Blood Glucose Monitoring Systems Applying ISO 15197:2013 Accuracy Criteria
Diabetes Ther (2018) 9:683 697 https://doi.org/10.1007/s13300-018-0392-6 ORIGINAL RESEARCH User Performance Evaluation of Four Blood Glucose Monitoring Systems Applying ISO 15197:2013 Accuracy Criteria
Bern Harrison, B.A., Cheryl Leazenby, B.S., and Solveig Halldorsdottir, Ph.D.
 Journal of Diabetes Science and Technology Volume 5, Issue 4, July 2011 Diabetes Technology Society TECHNOLOGY REPORTS Accuracy of the CONTOUR Blood Glucose Monitoring System Bern, B.A., Cheryl Leazenby,
Journal of Diabetes Science and Technology Volume 5, Issue 4, July 2011 Diabetes Technology Society TECHNOLOGY REPORTS Accuracy of the CONTOUR Blood Glucose Monitoring System Bern, B.A., Cheryl Leazenby,
Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System
 Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX Blood Glucose Monitoring System, from Trividia Health,
Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX Blood Glucose Monitoring System, from Trividia Health,
Smart & Simple Mobile Solutions.
 Higher Measuring Accuracy Smart & Simple Mobile Solutions. Test Simply. Live Better. FORA Diamond lets you connect all your Bluetooth devices. *Also available with a Micro USB. ifora Diabetes Manager All
Higher Measuring Accuracy Smart & Simple Mobile Solutions. Test Simply. Live Better. FORA Diamond lets you connect all your Bluetooth devices. *Also available with a Micro USB. ifora Diabetes Manager All
Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System
 Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System Summary Objectives: To demonstrate that the TRUE METRIX Self-Monitoring Blood Glucose System*, from Trividia
Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System Summary Objectives: To demonstrate that the TRUE METRIX Self-Monitoring Blood Glucose System*, from Trividia
GD50. Simple. Accurate. Reliable. Test Simply. Live Better. All FORA Diamond Series Blood Glucose Monitoring Systems use FORA Diamond Test Strips.
 GD50 Higher Measuring Accuracy Simple. Accurate. Reliable. Test Simply. Live Better. All FORA Diamond Series Blood Glucose Monitoring Systems use FORA Diamond Test Strips. GD50 Simple. Accurate. Reliable.
GD50 Higher Measuring Accuracy Simple. Accurate. Reliable. Test Simply. Live Better. All FORA Diamond Series Blood Glucose Monitoring Systems use FORA Diamond Test Strips. GD50 Simple. Accurate. Reliable.
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
BLOOD GLUCOSE METERS ACCURACY REQUIREMENTS
 BLOOD GLUCOSE METERS ACCURACY REQUIREMENTS International Standard ISO 15197:2013 (Second Edition 15/05/2013) In vitro diagnostic test systems Requirements for blood-glucose monitoring systems for self-testing
BLOOD GLUCOSE METERS ACCURACY REQUIREMENTS International Standard ISO 15197:2013 (Second Edition 15/05/2013) In vitro diagnostic test systems Requirements for blood-glucose monitoring systems for self-testing
MQ Comparison of glucometers using heparin whole blood
 MQ 2014-1 Comparison of glucometers using heparin whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing
MQ 2014-1 Comparison of glucometers using heparin whole blood Note: The instrument comparison is structured as a survey. This is a sample test, not a complete evaluation. Introduction Glucose self-testing
Clinical Performance of the TRUE2go Blood Glucose Monitoring System Exceeds Minimum Criteria for Accuracy Using ISO 15197:2013
 linical Performance of the TRUE2go lood Glucose Monitoring System Exceeds Minimum riteria for Accuracy Using ISO 197:213 LINIAL STUY SUMMARY EVALUATION OJETIVES This clinical evaluation: 1. Evaluates the
linical Performance of the TRUE2go lood Glucose Monitoring System Exceeds Minimum riteria for Accuracy Using ISO 197:213 LINIAL STUY SUMMARY EVALUATION OJETIVES This clinical evaluation: 1. Evaluates the
Walsall CCG Supported Blood Glucose Meters April 2018
 Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
A New, Wireless-enabled Blood Glucose Monitoring System That Links to a Smart Mobile Device: Accuracy and User Performance Evaluation
 691301DSTXXX10.1177/1932296817691301Journal of Diabetes Science and TechnologyChristiansen et al research-article2017 Original Article A New, Wireless-enabled Blood Glucose Monitoring System That Links
691301DSTXXX10.1177/1932296817691301Journal of Diabetes Science and TechnologyChristiansen et al research-article2017 Original Article A New, Wireless-enabled Blood Glucose Monitoring System That Links
Accuracy Evaluation of Five Blood Glucose Monitoring Systems: The North American Comparator Trial
 Journal of Diabetes Science and Technology Volume 7, Issue 5, September 2013 Diabetes Technology Society ORIGINAL ARTICLE Accuracy Evaluation of Five Blood Glucose Monitoring Systems: The North American
Journal of Diabetes Science and Technology Volume 7, Issue 5, September 2013 Diabetes Technology Society ORIGINAL ARTICLE Accuracy Evaluation of Five Blood Glucose Monitoring Systems: The North American
EVALUATION OF THE PERFORMANCE OF THE TRUETRACK BLOOD GLUCOSE MONITORING SYSTEM USING THE ISO 15197:2003 ACCURACY AND USER EVALUATION REQUIREMENTS
 EVALUATION OF THE PERFORMANCE OF THE TRUETRACK BLOOD GLUCOSE MONITORING SYSTEM USING THE ISO 15197:23 ACCURACY AND USER EVALUATION REQUIREMENTS Douglas E. Bell, PhD Teri A. Sasse, RN, MS BACKGROUND: The
EVALUATION OF THE PERFORMANCE OF THE TRUETRACK BLOOD GLUCOSE MONITORING SYSTEM USING THE ISO 15197:23 ACCURACY AND USER EVALUATION REQUIREMENTS Douglas E. Bell, PhD Teri A. Sasse, RN, MS BACKGROUND: The
White Paper, Evaluation of WaveSense JAZZ Blood Glucose Monitoring System Analytical Performance to EN ISO 15197:2015 Standard
 White Paper, Evaluation of WaveSense JAZZ Blood Glucose Monitoring System Analytical Performance to EN ISO 15197:2015 Standard 7500-10032 Rev F Table of Contents Executive Summary 3 Changes introduced
White Paper, Evaluation of WaveSense JAZZ Blood Glucose Monitoring System Analytical Performance to EN ISO 15197:2015 Standard 7500-10032 Rev F Table of Contents Executive Summary 3 Changes introduced
Aina Blood Monitoring System
 Aina Blood Monitoring System Analytical Performance Summary The Aina Blood Monitoring System is a high quality and versatile multi-parameter diagnostic platform that is CE-marked and approved for sale
Aina Blood Monitoring System Analytical Performance Summary The Aina Blood Monitoring System is a high quality and versatile multi-parameter diagnostic platform that is CE-marked and approved for sale
Effects of Simulated Altitude on Blood Glucose Meter Performance: Implications for In-Flight Blood Glucose Monitoring
 Journal of Diabetes Science and Technology Volume 6, Issue 4, July 2012 Diabetes Technology Society ORIGINAL ARTICLE Effects of Simulated Altitude on Blood Glucose Meter Performance: Tolu, M.B.B.S., M.R.C.P.,
Journal of Diabetes Science and Technology Volume 6, Issue 4, July 2012 Diabetes Technology Society ORIGINAL ARTICLE Effects of Simulated Altitude on Blood Glucose Meter Performance: Tolu, M.B.B.S., M.R.C.P.,
Clinical Evaluation for. Embrace No Code Blood Glucose Monitoring System
 Embrace No Code Report Clinical Evaluation for Embrace No Code Blood Glucose Monitoring System Applicant: Apex Biotechnology Corp. Test Date: 2007/8/28~2007/9/21 Clinical Site: SCHMIDT Group Practice Clinic
Embrace No Code Report Clinical Evaluation for Embrace No Code Blood Glucose Monitoring System Applicant: Apex Biotechnology Corp. Test Date: 2007/8/28~2007/9/21 Clinical Site: SCHMIDT Group Practice Clinic
Determination of Hematocrit Interference in Blood Samples Derived from Patients with Different Blood Glucose Concentrations
 Journal of Diabetes Science and Technology Volume 7, Issue 1, January 2013 Diabetes Technology Society ORIGINAL ARTICLE Determination of Hematocrit Interference in Blood Samples Andreas, M.D., Ph.D., 1
Journal of Diabetes Science and Technology Volume 7, Issue 1, January 2013 Diabetes Technology Society ORIGINAL ARTICLE Determination of Hematocrit Interference in Blood Samples Andreas, M.D., Ph.D., 1
Validity and Reliability of a Glucometer Against Industry Reference Standards
 514315DSTXXX10.1177/1932296813514315Journal of Diabetes Science and TechnologySalacinski et al research-article2014 Original Article Validity and Reliability of a Glucometer Against Industry Reference
514315DSTXXX10.1177/1932296813514315Journal of Diabetes Science and TechnologySalacinski et al research-article2014 Original Article Validity and Reliability of a Glucometer Against Industry Reference
Glucometers Gastrostomy Tubes Nasogastric Tubes Insulin pumps Continuous Glucose monitoring system Closed Loop Smart phones/tablets
 Devices Glucometers Gastrostomy Tubes Nasogastric Tubes Insulin pumps Continuous Glucose monitoring system Closed Loop Smart phones/tablets Accuracy Glucometers The ISO (International Organization for
Devices Glucometers Gastrostomy Tubes Nasogastric Tubes Insulin pumps Continuous Glucose monitoring system Closed Loop Smart phones/tablets Accuracy Glucometers The ISO (International Organization for
NHS Greater Glasgow and Clyde Adult Blood Glucose Meter and Test Strip Formulary
 NHS Greater Glasgow and Clyde Adult Blood Glucose and Test Strip Formulary 2016-17 Primary Care and Acute Joint Formulary Blood Glucose formulary developed by the Diabetes MCN Blood Glucose Formulary Review
NHS Greater Glasgow and Clyde Adult Blood Glucose and Test Strip Formulary 2016-17 Primary Care and Acute Joint Formulary Blood Glucose formulary developed by the Diabetes MCN Blood Glucose Formulary Review
GUIDELINE FOR SELF MONITORING OF BLOOD GLUCOSE IN ADULTS WITH DIABETES
 It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa
 MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
Improving Self-Monitoring of Blood Glucose among Adults with Type 1 Diabetes: Results of the Mobile TM Study
 Diabetes Ther (2014) 5:557 565 DOI 10.1007/s13300-014-0092-9 ORIGINAL RESEARCH Improving Self-Monitoring of Blood Glucose among Adults with Type 1 Diabetes: Results of the Mobile TM Study Jane Overland
Diabetes Ther (2014) 5:557 565 DOI 10.1007/s13300-014-0092-9 ORIGINAL RESEARCH Improving Self-Monitoring of Blood Glucose among Adults with Type 1 Diabetes: Results of the Mobile TM Study Jane Overland
Glucose Analytical Comparability Evaluation of the YSI 2300 STAT Plus and YSI 2900D Biochemistry Analyzers
 Glucose Analytical Comparability Evaluation of the YSI 2300 STAT Plus and YSI 2900D iochemistry Analyzers Life Sciences Data for Life. TM YSI Life Sciences White Paper 91 Authors: Kevin Schlueter, PhD,
Glucose Analytical Comparability Evaluation of the YSI 2300 STAT Plus and YSI 2900D iochemistry Analyzers Life Sciences Data for Life. TM YSI Life Sciences White Paper 91 Authors: Kevin Schlueter, PhD,
Performance Evaluation of B. Braun Omnitest 5 Blood Glucose Monitoring System for Self-Monitoring of Blood Glucose
 ORIGINL RTILE https://doi.org/10.15263/jlmqa.2016.38.4.234 Performance Evaluation of. raun Omnitest 5 lood Glucose Monitoring System for Self-Monitoring of lood Glucose Kyungso Jeon 1 and Miseon Shin 2
ORIGINL RTILE https://doi.org/10.15263/jlmqa.2016.38.4.234 Performance Evaluation of. raun Omnitest 5 lood Glucose Monitoring System for Self-Monitoring of lood Glucose Kyungso Jeon 1 and Miseon Shin 2
DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER
 DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER 2017-404 Question/s to be Answered Can you please provide the following
DATE RECEIVED 09/10/2017 SUBJECT Formulary PASSED TO Mary DATE PASSED 09/10/2017 RESPOND BY 27/10/2017 CATEGORY Business FoI NUMBER 2017-404 Question/s to be Answered Can you please provide the following
Accuracy and Precision Performance of the Accu-Chek Mobile System. Introduction. Method
 Accuracy and Precision Performance of the Accu-Chek Mobile System I. ACCURACY The accuracy of the system was assessed according to ISO 15197. Introduction The purpose of this study was to determine the
Accuracy and Precision Performance of the Accu-Chek Mobile System I. ACCURACY The accuracy of the system was assessed according to ISO 15197. Introduction The purpose of this study was to determine the
An Evaluation of the Barriers to Patient use of Glucometer Control Solutions: A Survey of Patients, Pharmacists, and Providers
 AADE14 ANNUAL MEETING & EXHIBITION AUGUST 6-9, 2014 ORLANDO, FL An Evaluation of the Barriers to Patient use of Glucometer s: A Survey of Patients, Pharmacists, and Providers Katherine S. O Neal, Pharm.D.,
AADE14 ANNUAL MEETING & EXHIBITION AUGUST 6-9, 2014 ORLANDO, FL An Evaluation of the Barriers to Patient use of Glucometer s: A Survey of Patients, Pharmacists, and Providers Katherine S. O Neal, Pharm.D.,
A model for managing and monitoring the quality of glucometers used in a high-volume clinical setting
 Original papers A model for managing and monitoring the quality of glucometers used in a high-volume clinical setting Güzin Aykal*, Ayşenur Yegin, Özgür Tekeli, Necat Yilmaz Central Laboratories, Antalya
Original papers A model for managing and monitoring the quality of glucometers used in a high-volume clinical setting Güzin Aykal*, Ayşenur Yegin, Özgür Tekeli, Necat Yilmaz Central Laboratories, Antalya
Description of the technology
 Advice Note 2017/ 001 What is the clinical effectiveness, safety and budget impact of the Freestyle Libre System compared with current glucose monitoring methods for people aged 4 years and over with diabetes
Advice Note 2017/ 001 What is the clinical effectiveness, safety and budget impact of the Freestyle Libre System compared with current glucose monitoring methods for people aged 4 years and over with diabetes
PREVENTION OF NOCTURNAL HYPOGLYCEMIA USING PREDICTIVE LOW GLUCOSE SUSPEND (PLGS)
 PREVENTION OF NOCTURNAL HYPOGLYCEMIA USING PREDICTIVE LOW GLUCOSE SUSPEND (PLGS) Pathways for Future Treatment and Management of Diabetes H. Peter Chase, MD Carousel of Hope Symposium Beverly Hilton, Beverly
PREVENTION OF NOCTURNAL HYPOGLYCEMIA USING PREDICTIVE LOW GLUCOSE SUSPEND (PLGS) Pathways for Future Treatment and Management of Diabetes H. Peter Chase, MD Carousel of Hope Symposium Beverly Hilton, Beverly
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE
 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k103542 B. Purpose for Submission: Alternative blood glucose test strips for use with LifeScan OneTouch
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE A. 510(k) Number: k103542 B. Purpose for Submission: Alternative blood glucose test strips for use with LifeScan OneTouch
Guidance for the choice of blood glucose testing meters, strips and lancets
 Guidance for the choice of blood glucose testing meters, strips and lancets This guidance is intended to assist healthcare professionals in their selection of appropriate blood glucose meters and testing
Guidance for the choice of blood glucose testing meters, strips and lancets This guidance is intended to assist healthcare professionals in their selection of appropriate blood glucose meters and testing
Ultra Blue. Test Strips UNISTRIP. Charlotte, NC V2-10/25/2018 TECHNOLOGIES,LLC
 VS UNISTRIP TECHNOLOGIES,LLC Charlotte, NC 28269 1-866-889-6229 V2-10/25/2018 UniStrip Generic Blood Glucose VS OneTouch Ultra Blue 1. Purpose This study is intended to evaluate the difference in values
VS UNISTRIP TECHNOLOGIES,LLC Charlotte, NC 28269 1-866-889-6229 V2-10/25/2018 UniStrip Generic Blood Glucose VS OneTouch Ultra Blue 1. Purpose This study is intended to evaluate the difference in values
I know my value CoaguChek XS Pro system
 I know my value CoaguChek XS Pro system The smart way to manage your anticoagulation clinic CoaguChek XS Pro system A barcode scanner can make a difference There has been an extraordinary increase in the
I know my value CoaguChek XS Pro system The smart way to manage your anticoagulation clinic CoaguChek XS Pro system A barcode scanner can make a difference There has been an extraordinary increase in the
Blood glucose test strips (BGTS) evaluation protocol and results. Update April Version 4.1. Note:
 Blood glucose test strips (BGTS) evaluation protocol and results Update April 2016 Version 4.1 Note: December 2017: Prescribing data indicates overall compliance with the GMMMG recommended BGTS of 86%.
Blood glucose test strips (BGTS) evaluation protocol and results Update April 2016 Version 4.1 Note: December 2017: Prescribing data indicates overall compliance with the GMMMG recommended BGTS of 86%.
Type 2 Diabetes Recommended SMBG
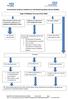 Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Smart Diagnostics for Point-of-Care!
 Smart Diagnostics for Point-of-Care! The one special multi-parameter Point-of-Care (POC) instrument that covers the constantly expanding range of applications with the highest precision. Designed for HbA1c
Smart Diagnostics for Point-of-Care! The one special multi-parameter Point-of-Care (POC) instrument that covers the constantly expanding range of applications with the highest precision. Designed for HbA1c
Comparison of Lancing Devices for Self-Monitoring of Blood Glucose Regarding Lancing Pain
 Journal of Diabetes Science and Technology Volume 3, Issue 5, September 2009 Diabetes Technology Society ORIGINAL ARTICLES Comparison of Lancing Devices for Self-Monitoring of Blood Glucose Regarding Lancing
Journal of Diabetes Science and Technology Volume 3, Issue 5, September 2009 Diabetes Technology Society ORIGINAL ARTICLES Comparison of Lancing Devices for Self-Monitoring of Blood Glucose Regarding Lancing
For more comprehensive information, please refer to the t:connect Application User Guide available online at: Getting Started Guide.
 Congratulations on the purchase of your new insulin pump from Tandem Diabetes Care. Your decision to use insulin pump therapy is a sign of your commitment to actively manage your diabetes. This guide provides
Congratulations on the purchase of your new insulin pump from Tandem Diabetes Care. Your decision to use insulin pump therapy is a sign of your commitment to actively manage your diabetes. This guide provides
OneTouch Blood Glucose Monitoring Systems Impact of New Technologies on the Efficacy of Self-monitoring Blood Glucose
 Blood Glucose Monitoring Systems Impact of New Technologies on the Efficacy of Self-monitoring Blood Glucose Laurence B Katz, Mike Grady, Steven J Setford, and Brian L Levy US Endocrinology SUPPLEMENT
Blood Glucose Monitoring Systems Impact of New Technologies on the Efficacy of Self-monitoring Blood Glucose Laurence B Katz, Mike Grady, Steven J Setford, and Brian L Levy US Endocrinology SUPPLEMENT
A meter designed for glucose self-measurement manufactured by LifeScan, Johnson & Johnson. Report from an evaluation organised by SKUP
 SKUP Scandinavian evaluation of laboratory equipment for primary health care ONETOUCH Ultra A meter designed for glucose self-measurement manufactured by LifeScan, Johnson & Johnson Report from an evaluation
SKUP Scandinavian evaluation of laboratory equipment for primary health care ONETOUCH Ultra A meter designed for glucose self-measurement manufactured by LifeScan, Johnson & Johnson Report from an evaluation
How to Access A Free Blood Glucose Meter
 How to Access A Free Abbott FreeStyle Lite FreeStyle Lite FreeStyle Freedom Lite FreeStyle InsuLinx How to Access A Free contacting their health care professionals at diabetes clinics; or calling the FreeStyle
How to Access A Free Abbott FreeStyle Lite FreeStyle Lite FreeStyle Freedom Lite FreeStyle InsuLinx How to Access A Free contacting their health care professionals at diabetes clinics; or calling the FreeStyle
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE
 A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa
 MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa CARE OF PATIENT POLICY & PROCEDURE Policy Number: 4:10 Subject: Policy: Glucose Monitoring (Accuchek) Nursing department staff and laboratory staff
Computing the Surveillance Error Grid Analysis: Procedure and Examples
 5959DSTXXX.77/99685959Journal of Diabetes Science and TechnologyKovatchev et al research-article Original Article Computing the Surveillance Error Grid Analysis: Procedure and Examples Journal of Diabetes
5959DSTXXX.77/99685959Journal of Diabetes Science and TechnologyKovatchev et al research-article Original Article Computing the Surveillance Error Grid Analysis: Procedure and Examples Journal of Diabetes
Advances Towards the Bionic Pancreas.
 Advances Towards the Bionic Pancreas. Ron Brazg MD, FACE Rainier Clinical Research Center Renton, WA DISCLOSURES Rainier Clinical Research Center is an independent research facility not directly affiliated
Advances Towards the Bionic Pancreas. Ron Brazg MD, FACE Rainier Clinical Research Center Renton, WA DISCLOSURES Rainier Clinical Research Center is an independent research facility not directly affiliated
Relationship between glucose meter error and glycemic control efficacy
 Relationship between glucose meter error and glycemic control efficacy Brad S. Karon, M.D., Ph.D. Professor of Laboratory Medicine and Pathology Department of Laboratory Medicine and Pathology Mayo Clinic
Relationship between glucose meter error and glycemic control efficacy Brad S. Karon, M.D., Ph.D. Professor of Laboratory Medicine and Pathology Department of Laboratory Medicine and Pathology Mayo Clinic
1 PROTOCOL. Comparison Study Summary. Springs Memorial Hospital. Springs Memorial Hospital 800 W. Meeting St. Lancaster, SC (803)
 Comparison Study Summary Springs Memorial Hospital 800 W. Meeting St. Lancaster, SC 29720 (803) 286-1480 February 10, 2016 1 PROTOCOL This evaluation was conducted on February 10, 2016 at Springs Memorial
Comparison Study Summary Springs Memorial Hospital 800 W. Meeting St. Lancaster, SC 29720 (803) 286-1480 February 10, 2016 1 PROTOCOL This evaluation was conducted on February 10, 2016 at Springs Memorial
Omnitest : 2013
 ISO 15197: 2013 Omnitest 3 Blood Glucose Monitoring System Omnitest 3 Could it be easier? After strip insertion, Omnitest 3 is ready for the measurement. The code is set automatically. Only 0.3 µl sample
ISO 15197: 2013 Omnitest 3 Blood Glucose Monitoring System Omnitest 3 Could it be easier? After strip insertion, Omnitest 3 is ready for the measurement. The code is set automatically. Only 0.3 µl sample
FreeStyle Mini Blood Glucose Results Are Accurate and Suitable for Use in Glycemic Clamp Protocols
 Journal of Diabetes Science and Technology Volume 2, Issue 5, September 2008 Diabetes Technology Society CLINICAL APPLICATIONS FreeStyle Mini Blood Glucose Results Are Accurate and Suitable for Use in
Journal of Diabetes Science and Technology Volume 2, Issue 5, September 2008 Diabetes Technology Society CLINICAL APPLICATIONS FreeStyle Mini Blood Glucose Results Are Accurate and Suitable for Use in
Role of SMBG in Non-Insulin Treated Subjects with T2DM Richard M. Bergenstal, MD
 Role of SMBG in Non-Insulin Treated Subjects with T2DM Richard M. Bergenstal, MD International Diabetes Center and Health Services University of Minnesota Minneapolis, MN richard.bergenstal@parknicollet.com
Role of SMBG in Non-Insulin Treated Subjects with T2DM Richard M. Bergenstal, MD International Diabetes Center and Health Services University of Minnesota Minneapolis, MN richard.bergenstal@parknicollet.com
Performance of three portable blood glucose monitoring devices used in a veterinary application
 Performance of three portable blood glucose monitoring devices used in a veterinary application Prepared by Michael I. Lindinger, PhD, Vice-President of Research, The Nutraceutical Alliance, Campbellville,
Performance of three portable blood glucose monitoring devices used in a veterinary application Prepared by Michael I. Lindinger, PhD, Vice-President of Research, The Nutraceutical Alliance, Campbellville,
Software Version 2.0. User s Guide
 Software Version 2.0 User s Guide Table of Contents Contents Contents Important Information About Your FreeStyle Auto-Assist Software...1 Intended Use...1 System Requirements...1 Connecting to your Abbott
Software Version 2.0 User s Guide Table of Contents Contents Contents Important Information About Your FreeStyle Auto-Assist Software...1 Intended Use...1 System Requirements...1 Connecting to your Abbott
A Study of Patient Experience Using a Blood Glucose Meter With an In-Built Insulin Dose Calculator
 532489DSTXXX10.1177/1932296814532489Journal of Diabetes Science and TechnologyRamtoola et al research-article2014 Original Article A Study of Patient Experience Using a Blood Glucose Meter With an In-Built
532489DSTXXX10.1177/1932296814532489Journal of Diabetes Science and TechnologyRamtoola et al research-article2014 Original Article A Study of Patient Experience Using a Blood Glucose Meter With an In-Built
Plug-and-play goes online
 The next-generation Accu-Chek T Smart Pix software Plug-and-play goes online Proven tools for efficient and effective diabetes management 1 even for patients who are not in your office How many of these
The next-generation Accu-Chek T Smart Pix software Plug-and-play goes online Proven tools for efficient and effective diabetes management 1 even for patients who are not in your office How many of these
SUMMARY... 2 PLANNING OF THE EVALUATION... 4 ANALYTICAL QUALITY SPECIFICATIONS... 5
 USER-EVALUATION ASCENSIA CONTOUR Blood Glucose Meter System Table of contents SUMMARY... 2 PLANNING OF THE EVALUATION... 4 ANALYTICAL QUALITY SPECIFICATIONS... 5 MATERIALS AND METHODS... 6 ASCENSIA CONTOUR...
USER-EVALUATION ASCENSIA CONTOUR Blood Glucose Meter System Table of contents SUMMARY... 2 PLANNING OF THE EVALUATION... 4 ANALYTICAL QUALITY SPECIFICATIONS... 5 MATERIALS AND METHODS... 6 ASCENSIA CONTOUR...
Blood Glucose Test Strip Guidelines
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Quality Control of Self-Monitoring of Blood Glucose: Why and How?
 Journal of Diabetes Science and Technology Volume 1, Issue 2, March 2007 Diabetes Technology Society SYMPOSIUM Quality Control of Self-Monitoring of Blood Glucose: Why and How? Bogdan, M.D., Ph.D. and
Journal of Diabetes Science and Technology Volume 1, Issue 2, March 2007 Diabetes Technology Society SYMPOSIUM Quality Control of Self-Monitoring of Blood Glucose: Why and How? Bogdan, M.D., Ph.D. and
Accuracy of Blood Glucose Meters for Self-Monitoring Affects Glucose Control and Hypoglycemia Rate in Children and Adolescents with Type 1 Diabetes
 DIABETES TECHNOLOGY & THERAPEUTICS Volume 17, Number 4, 2015 ª Mary Ann Liebert, Inc. DOI: 10.1089/dia.2014.0262 ORIGINAL ARTICLE Accuracy of Blood Glucose Meters for Self-Monitoring Affects Glucose Control
DIABETES TECHNOLOGY & THERAPEUTICS Volume 17, Number 4, 2015 ª Mary Ann Liebert, Inc. DOI: 10.1089/dia.2014.0262 ORIGINAL ARTICLE Accuracy of Blood Glucose Meters for Self-Monitoring Affects Glucose Control
CHEMICAL REAGENTS 478 PART 9 CHEMICAL REAGENTS. This document is dated 1 st July 2015.
 PART 9 This document is dated 1 st July 2015. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
PART 9 This document is dated 1 st July 2015. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
Subjects and Methods 2017, 64 (8),
 2017, 64 (8), 827-832 Careful readings for a flash glucose monitoring system in nondiabetic Japanese subjects: individual differences and discrepancy in glucose concentrarion after glucose loading Keiko
2017, 64 (8), 827-832 Careful readings for a flash glucose monitoring system in nondiabetic Japanese subjects: individual differences and discrepancy in glucose concentrarion after glucose loading Keiko
Management of Central Venous Access Devices. Blood Glucose Monitoring
 Management of Central Venous Access Devices Blood Glucose Monitoring Purpose To provide education on the standard of care regarding the use and monitoring of the Accu- Chek Blood glucose machine, including
Management of Central Venous Access Devices Blood Glucose Monitoring Purpose To provide education on the standard of care regarding the use and monitoring of the Accu- Chek Blood glucose machine, including
COBAS, ACCU-CHECK INFORM, MODULAR and LIFE NEEDS ANSWERS are trademarks of Roche Roche
 Accu-Chek Inform II test strip Results of the evaluation study on newborns, conducted by the Flevo Hospital (FVZH) Almere and the Maxima Medical Center (MMC) Veldhoven in the Netherlands COBAS, ACCU-CHECK
Accu-Chek Inform II test strip Results of the evaluation study on newborns, conducted by the Flevo Hospital (FVZH) Almere and the Maxima Medical Center (MMC) Veldhoven in the Netherlands COBAS, ACCU-CHECK
Summary of Significant Changes at this Revision. Items Required
 Update Approver/checker Summary of Significant Changes at this Revision Purpose and Scope Items Required 1. The Abbott FreeStyle Optium meter is a battery-powered device designed for the measurement of
Update Approver/checker Summary of Significant Changes at this Revision Purpose and Scope Items Required 1. The Abbott FreeStyle Optium meter is a battery-powered device designed for the measurement of
A meter designed for glucose self-measurement manufactured by Roche Diagnostics. Report from an evaluation organised by SKUP
 SKUP Scandinavian evaluation of laboratory equipment for primary health care ACCU-CHEK Compact Plus A meter designed for glucose self-measurement manufactured by Roche Diagnostics Report from an evaluation
SKUP Scandinavian evaluation of laboratory equipment for primary health care ACCU-CHEK Compact Plus A meter designed for glucose self-measurement manufactured by Roche Diagnostics Report from an evaluation
Performance-powered. The OneTouch. Ping insulin pump and meter-remote.
 Performance-powered. The OneTouch Ping insulin pump and meter-remote. I We don t just deliver insulin. We deliver outstanding clinical performance. P36337_OTP_DetAid_OmniPodUpdate_r12.indd 1 OneTouch Ping.
Performance-powered. The OneTouch Ping insulin pump and meter-remote. I We don t just deliver insulin. We deliver outstanding clinical performance. P36337_OTP_DetAid_OmniPodUpdate_r12.indd 1 OneTouch Ping.
CHEMICAL REAGENTS 478 CHEMICAL REAGENTS. This document is dated 1 st March 2018
 This document is dated 1 st March 2018 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
This document is dated 1 st March 2018 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
Polymer Technology Systems, Inc. CardioChek PA Comparison Study
 Polymer Technology Systems, Inc. CardioChek PA Comparison Study Evaluation Protocol: Accuracy Precision Clinical Correlation PTS Panels Lipid Panel Test Strips For Use in Comparisons to a Reference Laboratory
Polymer Technology Systems, Inc. CardioChek PA Comparison Study Evaluation Protocol: Accuracy Precision Clinical Correlation PTS Panels Lipid Panel Test Strips For Use in Comparisons to a Reference Laboratory
OGCARE is a blood glucose monitoring device for checking glucose concentration in whole blood from fingertip pricking.
 ITALIAN PRODUCT S E L F T E S T I N G Personal medical devices for metabolic disorders MONITORING and PREVENTION, taking advantage of reflectometric and electrochemical technologies. Blood glucose monitoring
ITALIAN PRODUCT S E L F T E S T I N G Personal medical devices for metabolic disorders MONITORING and PREVENTION, taking advantage of reflectometric and electrochemical technologies. Blood glucose monitoring
When Will CGM Replace SMBG? Roy W. Beck, MD, PhD. JAEB Center for Health Research Tampa, Florida
 When Will CGM Replace SMBG? Roy W. Beck, MD, PhD JAEB Center for Health Research Tampa, Florida Financial Disclosures Dr. Beck does not have any personal conflicts of interest His employer, the JAEB Center
When Will CGM Replace SMBG? Roy W. Beck, MD, PhD JAEB Center for Health Research Tampa, Florida Financial Disclosures Dr. Beck does not have any personal conflicts of interest His employer, the JAEB Center
Sample Pages. Glycated Haemoglobin (HbA1c) Testing Market,
 Sample Pages Glycated Haemoglobin (HbA1c) Testing Market, 2014-2024 This page is intentionally left blank Copyright 2014 Roots Analysis Private Ltd. All rights reserved. No part of this report should be
Sample Pages Glycated Haemoglobin (HbA1c) Testing Market, 2014-2024 This page is intentionally left blank Copyright 2014 Roots Analysis Private Ltd. All rights reserved. No part of this report should be
Closing the loop, squaring the circle: Studies on insulin delivery, glucose monitoring and the artificial pancreas Luijf, Y.M.
 UvA-DARE (Digital Academic Repository) Closing the loop, squaring the circle: Studies on insulin delivery, glucose monitoring and the artificial pancreas Luijf, Y.M. Link to publication Citation for published
UvA-DARE (Digital Academic Repository) Closing the loop, squaring the circle: Studies on insulin delivery, glucose monitoring and the artificial pancreas Luijf, Y.M. Link to publication Citation for published
HYDROXYBUTERATE) Gluco Rx Evolve KetoneTest Strips
 Scottish Drug Tariff Part 9 This document is dated 1 st November 2018 Page REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR BLOOD GLUCOSE FOR KETONES (BETA- HYDROXYBUTERATE) 478 DETECTION
Scottish Drug Tariff Part 9 This document is dated 1 st November 2018 Page REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR BLOOD GLUCOSE FOR KETONES (BETA- HYDROXYBUTERATE) 478 DETECTION
TheraSense FreeStyle blood glucose meter
 May 2002 Evaluation Report MDA 02049 TheraSense FreeStyle blood glucose meter Crown Copyright 60 WHAT YOU CAN EXPECT FROM MDA EVALUATION REPORTS The Device Evaluation Service (DES) aims to provide independent
May 2002 Evaluation Report MDA 02049 TheraSense FreeStyle blood glucose meter Crown Copyright 60 WHAT YOU CAN EXPECT FROM MDA EVALUATION REPORTS The Device Evaluation Service (DES) aims to provide independent
Title: Abbott Optium Xceed Glucose Meter Effective date: 16/05/2011. Summary of Significant Changes at this Revision. SOP updated.
 COPY Summary of Significant Changes at this Revision SOP updated Purpose and Scope Items Required 1. The Abbott Optium Xceed meter is a battery-powered device designed for the measurement of blood glucose
COPY Summary of Significant Changes at this Revision SOP updated Purpose and Scope Items Required 1. The Abbott Optium Xceed meter is a battery-powered device designed for the measurement of blood glucose
CHEMICAL REAGENTS 478 CHEMICAL REAGENTS. This document is dated 1 st October 2017
 This document is dated 1 st October 2017 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
This document is dated 1 st October 2017 Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS FOR
Advances in Diabetes Care Technologies
 1979 Advances in Diabetes Care Technologies 2015 Introduction Insulin pump use: ~ 20% - 30% of patients with T1DM < 1% of insulin-treated patients with T2DM 2007 FDA estimates ~375,000 insulin pumps for
1979 Advances in Diabetes Care Technologies 2015 Introduction Insulin pump use: ~ 20% - 30% of patients with T1DM < 1% of insulin-treated patients with T2DM 2007 FDA estimates ~375,000 insulin pumps for
Mission Hb accurately detects both
 Mission Digital Hb-Testing System: Save Cost by testing 2- Parameters with Single Strip real time ie: i.e: Hemoglobin & Hematocrit-[PCV]. Presented By : Remedy Healthcare Private Limited, New Delhi, [INDIA].
Mission Digital Hb-Testing System: Save Cost by testing 2- Parameters with Single Strip real time ie: i.e: Hemoglobin & Hematocrit-[PCV]. Presented By : Remedy Healthcare Private Limited, New Delhi, [INDIA].
Auto HbA1C ANALYZER HbA1LC HPLC 3660
 Turnkey Laboratories Solutions Auto HbA1C ANALYZER HbA1LC HPLC 3660 Viewable Accuracy Real Time Summarized Chromatogram Authentic Results EPC / PRODUCTS / APPLICATION / SOFTWARE / ACCESSORIES / CONSUMABLES
Turnkey Laboratories Solutions Auto HbA1C ANALYZER HbA1LC HPLC 3660 Viewable Accuracy Real Time Summarized Chromatogram Authentic Results EPC / PRODUCTS / APPLICATION / SOFTWARE / ACCESSORIES / CONSUMABLES
NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary
 NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary 2018-20 Primary Care and Acute Joint Formulary Blood Glucose Meter formulary developed by the Diabetes
NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary 2018-20 Primary Care and Acute Joint Formulary Blood Glucose Meter formulary developed by the Diabetes
CHEMICAL REAGENTS 478 PART 9 CHEMICAL REAGENTS. This document is dated 1 st March 2016.
 PART 9 This document is dated 1 st March 2016. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
PART 9 This document is dated 1 st March 2016. Page 478 REAGENT SOLUTIONS 478 URINE TESTING REAGENTS 478 DETECTION STRIPS FOR GLYCOSURIA 478 DETECTION STRIPS FOR KETONURIA (ACETONURIA) 478 DETECTION STRIPS
Artificial Pancreas Device Systems. Populations Interventions Comparators Outcomes. pump. pump
 Protocol Artificial Pancreas Device Systems (10130) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/19 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18 Preauthorization is required.
Protocol Artificial Pancreas Device Systems (10130) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/19 Preauthorization Yes Review Dates: 03/15, 03/16, 03/17, 01/18 Preauthorization is required.
