Extracellular fluid expansion and autoregulation in nephrotoxic serum nephritis in rats
|
|
|
- Terence Lewis
- 6 years ago
- Views:
Transcription
1 Kidney International, Vol. 25 (1984), pp Extracellular fluid expansion and autoregulation in nephrotoxic serum nephritis in rats TENEI SAKAI, FRIEDRICHS H. HARRIS, JR., DONALD J. MARSH, CLEAVES M. BENNETT, and RICHARD J. GLASSOCK with the technical assistance of CHRISTINE MARTIN, BENNETT KAYSER, and CHARLES CHUNG Department of Physiology and Biophysics, University of Southern California School of Medicine, Los Angeles, and Department of Medicine, University of California Los Angeles School of Medicine, and Harbor-University of California Los Angeles Medical Center, Torrance, California Extracellular fluid expansion and autoregulation in nephrotoxic serum nephritis in rats. Previous studies of the early autologous phase of bilateral nephrotoxic serum nephritis (NSN) in rats showed that whole kidney and single nephron glomerular filtration rate (GFR) were maintained at normal levels despite a 60% reduction in the product of surface area and hydraulic permeability (Kf). Factors responsible for this compensation were an increase of net ultrafiltration pressure, due primarily to an increased glomerular capillary pressure (P0). This study was designed to investigate some possible causes of the compensation. Rats with bilateral NSN and normal GFR had an increased extracellular fluid volume (ECFV) 2 weeks after induction of NSN; control subjects did not change. To determine whether this ECFV expansion was responsible for triggering the compensation, we developed a unilateral NSN model with one diseased and one normal kidney. Unilaterally diseased rats did not experience an increase of ECFV. Values of Kf were ni sec' mm Hg' in control subjects, in bilateral NSN, and in unilateral NSN. The elevation in PGC was the same in unilateral NSN as in bilateral NSN subjects and the same was true for the hydrostatic pressure difference across glomerular capillaries (P). Furthermore, in paired measurements on both kidneys of rats with unilateral NSN, PGC was significantly higher in the unilaterally diseased kidney than in the nondiseased kidney; sham control subjects had no difference. These results are interpreted to indicate that the signal that causes elevation of net ultrafiltration pressure is not a consequence of a systemic effect of NSN, but arises within the diseased kidney itself. To determine whether that signal involved some change in the mechanisms mediating autoregulation measurements were made of the response of whole kidney GFR and RBF to acute changes in arterial BP. Control rats and rats with NSN autoregulated both GFR and blood flow equally well. Tubuloglomerular feedback was studied by microperfusing loops of Henle and measuring proximal stop-flow pressure and early proximal flow rate. Stop-flow pressure was 4.0mm Hg higher in rats with NSN at a loop perfusion rate of 10 ni/mm, approximately the same difference that was found by direct measurement of PGC, but the sensitivity of response to changes in perfusion rate was the same in NSN as in control subjects. Finally, end proximal tubule flow rate was higher in NSN than in control subjects reflecting decreased proximal reabsorption. Thus, a normal feedback mechanism receives a signal that should cause afferent arteriolar constriction in NSN rats. Afferent arteriolar constriction would lead to a fall in PGC, so the signal that causes the observed increase must arise elsewhere than in the mechanisms that mediate autoregulation, but the identity of the signal remains unknown. L'expansion volémique extracellulaire et l'autoregulation dans Ia néphrite sérique néphrotoxique chez des rats. Des etudes antérieures de Ia phase autologue précoce de la nephrite sérique nephrotoxique bilatérale (NSN) chez des rats ont montré que les debits de filtration glomerulaire (GFR) du rein entier et des néphrons individuels étaient maintenus a des niveaux normaux malgré une reduction de 60% du produit de Ia surface et de la perméabilité hydraulique (Kf). Les facteurs responsables de cette compensation étaient une augmentation de Ia pression d'ultrafil- tration nette due essentiellement a une augmentation de Ia pression capillaire glomerulaire (P0c). Ce travail a été entrepris pour étudier quelques causes possibles de cette compensation. Des rats avec une NSN bilatérale et une GFR normales avaient une augmentation do volume extra-cellulaire (ECFV) 2 semaines apres l'induction de Ia NSN: les contrôles n'avaient pas de modification. Afin de determiner si cette expansion de l'ecfv était responsable du déclenchement de Ia compensation, nous avons developpc un modèle de NSN unilatérale avec un rein normal et un rein malade. Les rats avec une atteinte unilatérale n'ont pas eu d'augmentation de I'ECFV. Les valeurs de Kf étaient de 0,069 0,012 ni sec_i mm Hg' chez les contróles, 0,037 0,005 chez les NSN bilatérales, et 0,043 0,006 chez les NSN unilatérales. L'élévation de PGC était identique chez les NSN unilatérales et les NSN bilatérales, et ceci était également vrai pour les differences depressions hydraustatiques a travers les capillaires gbmérulaires (P). En outre, lors de mesures appariées sur les deux reins des rats avec une NSN unilatérale, POC était significativement plus élevée dans Ic rein malade unilateral que dans le rein sam; les sujets contrôles n'avaient pas de difference. Ces résultats tendent a indiquer que Ic signal qui est a l'origine de I'élévation de Ia pression d'ultrafiltration nette n'est pas une consequence d'un effet systemique de Ia NSN, mais provient du rein malade lui méme. Afin de determiner si Ic signal mettait en jeu une modification des mécanismes médiant l'autoregulation, des mesures de Ia réponse de Ia GFR et du flux sanguin renal du rein entier ont éte effectuées lors de modifications aigues de Ia pression artérielle. Les rats contrôles et les rats atteints de NSN autoregulaient leur GFR et leur flux sanguin aussi bien. Le feedback tubuloglomerulaire a été étudié en microperfusant des anses de Henlé et en mesurant Ia pression d'occlusion proximale et le debit dans Ic proximal précoce. La pression d'occlusion était 4,0 mm Hg plus élevée chez les rats atteints de NSN pour une perfusion de l'anse de 10 ni/mm, approximativement Ia méme difference qui était trouvée par mesure directe de PGC' mais Ia sensibilité de Ia réponse aux modifications de vitesse de perfusion était Ia méme chez les NSN et les contrôles. Finalement, le debit liquidien dans Ia fin du tubule proximal était plus ClevC chez les NSN que chez les sujets contrôles, ce qui refléte une diminution de Ia reabsorption proximale. Ainsi, un mécanisme de feedback normal recoit un signal qui pourrait entrainer une constriction de l'artcriole afférente chez les rats NSN. La constriction artériolaire afférente pourrait entrainer une diminution de PGC, de sorte que le signal qui est a l'origine de l'augmentation observce doit se produire a un autre endroit que les mécanismes qui médient l'autorcgulation, mais l'identité du signal reste inconnue. An accelerated form of the autologous phase of nephrotoxic serum nephritis (NSN) can be induced in rats by the injection of Received for publication February 17, 1983 and in revised form August 3, by the International Society of Nephrology 619
2 620 Sakai et a! rabbit anti-rat glomerular basement membrane antibody in suitably sensitized animals [1, 2]. The severity of the lesion can be controlled, and it is routinely possible to observe normal glomerular filtration rate (GFR) despite histological evidence of glomerular injury. Maddox et al [3] used this model and found that nephritic animals with normal GFR have increased net ultrafiltration pressure due primarily to elevated glomerular capillary pressure (PGC). The surface area-filtration permeability product (Kf) in these nephritic rats was approximately onehalf of that found in normal rats. The GFR is the product of Kf and mean net ultrafiltration pressure. The normal GFR of NSN results from the fact that these two variables change in opposite directions. Some change in arteriolar resistances must cause the change in net ultrafiltration pressure. The change could be a decrease in afferent arteriolar resistance alone or in concert with an increase in efferent arteriolar resistance. Dilatation of the afferent arteriole would by itself increase PGC and increase renal blood flow (RBF). Maddox et al [3] reported a slight increase in RBF, but the increase was not significant. A decrease of about 20% in afferent arteriolar resistance would be necessary to account for the observed increase in PGC; a resistance change of this magnitude would increase RBF about 12%. If the decrease of arteriolar resistance were combined with an increase of efferent arteriolar resistance, the increase in GC would be reinforced, but the effect on total renal vascular resistance would be offset, and there would be little or no change in RBF. If the dilatation of the afferent arteriole is accompanied by a constriction of the efferent arteriole, the reduction of the afferent arteriolar resistance necessary to cause the observed elevation of POC would be less than the 20% reduction required if the afferent arteriole is the sole site of change. The methods available for the measurement of arteriolar resistances in the renal circulation can measure resistance changes of these magnitudes when intraanimal comparisons can be made, but they are less suitable when the circumstances dictate comparisons between groups of animals. Unfortunately, the change we wish to study develops over a 2-week period, and comparisons in single animals are not possible. We wish to determine the cause of the adaptation. Because we cannot expect to infer the cause from direct measurements of arteriolar resistances, we have sought to examine the operation of mechanisms that might affect the resistances. In this study we tested two possibilities: that expansion of the extracellular fluid volume (ECFV) generates an extrarenal signal or that a change of the operating characteristics of tubuloglomerular feedback is responsible. Maddox et al [3, 4] studied rats with NSN 10 to 14 days after the disease was induced. In preliminary studies we found that nephritic rats with disease of the same duration had an expanded ECFV compared to control litter mates [5]. There are several reports [6, 71 that ECFV expansion reduced the ability of feedback to modulate GFR when flow rate of tubular fluid past the macula densa was increased. A corollary to this result is that GFR could increase at normal macula densa flow rates and could restore GFR to normal if macula densa flow rates fall. To test this possibility we developed and studied a unilateral model of NSN [8], in which ECFV is not increased because of the function of the normal kidney. The results with this model are the same as with bilateral NSN, that is, elevation of PGC. Even though ECFV expansion does not seem to be a necessary requirement for hemodynamic adaptations to NSN, other factors, known or unknown, could affect the operation of tubuloglomerular feedback. We therefore studied the known modalities of blood flow and GFR regulation by assessing whole kidney autoregulation and the response to perfusion of loops of Henle. No differences were found between nephritic and control rats. The cause of the dilatation must be sought elsewhere. Methods Animals. Male Sprague-Dawley and Wistar-Munich rats weighing 156 to 460 g were used throughout these studies. The Wistar-Munich rats were originally obtained from Drs. Barry Brenner and David Maddox of the University of California at San Francisco, and later from a breeding colony established in our laboratory. Except as otherwise specified, prior to the hemodynamic measurements the rats were maintained on standard laboratory chow containing sodium. Preparation and characterization of the heterologous anti-rat glomerular basement membrane antibody (anti-gbm Ab) New Zealand white rabbits were immunized repeatedly with ultrasonicated, insoluble rat GBM incorporated in complete Freund's adjuvant (CFA) as previously described [3]. Rabbit IgG (RaIgG) was isolated from normal, non-immune and immune rabbit sera by DEAE cellulose chromatography. Anti- GBM Ab content was determined by a paired-label method previously described [9]. Production of models of nephrotoxic serum nephritis (NSN) and controls Bilateral NSN. Rats were preimmunized once in each rear footpad with 2.0 mg of normal rabbit gamma globulin (Cohn Fraction II, Miles Laboratory-Research Products, Elkhart, Indiana) incorporated in 0.5 ml of CFA (9 ml of Baylol F, 1 ml Arlacel 83, and 6 mg Mycobacterium Tuberculosis, H37Ra, Difco Laboratories, Detroit, Michigan). Two days later the rats were infused via a tail vein with immune RaIgG calculated to contain 50 to 100 jsg of anti-gbm Ab. After the infusion the rats were permitted free access to fluids and food until the study was begun. Twenty-four-hour urinary protein excretion (Kingsbury- Clark method; normal values for rats, mg124 hr) was measured immediately prior to the hemodynamic studies. Formaim-fixed and snap-frozen specimens of the right and left kidney were obtained at sacrifice 5 to 15 days after infusion of anti-gbm Ab. Light microscopy (hemotoxylin and eosin and periodic acid-schiff stains) and immunofluorescent microscopy were performed on these specimens (see below). Unilateral NSN. The animals were preimmunized with RaIgG, as described above, and 2 days later were anesthetized with sodium pentobarbital (3 mg/100 g body weight); the left and right kidneys were exposed via a midline abdominal incision. Using a technique modified from that described by Hoyer, Mauer, and Michael [81, the left kidney was perfused with immune RaIgG calculated to contain 50 to 100 g of anti-gbm Ab. In brief, the left renal artery (RA) and vein (RV) and aorta, and right RA were mobilized. The spermatic and adrenal vessels were isolated and ligated. Loose ligatures were placed around the aorta proximal and distal to the left renal artery, around the right RA and left RV. At approximately the same
3 Glomerular hemodynamics in experimental nephritis 621 time, the ligatures around the aorta were tightened so as to occlude the vessels. Immediately thereafter, a 25-gauge needle was inserted into the aorta just proximal to the distal ligature and the tip was advanced to the level of the left RA. Heparinized physiological saline was infused via an infusion pump (Harvard, Harvard Apparatus, Edenridge, Kent, United Kingdom) at 0.8 mi/mm. The left renal vein was subsequently pierced with a 28-gauge needle. Perfusion was continued until the effluent from the renal vein was clear. The ligature on the right RA was then tightened, and using a three-way valve, RaIgG containing 50 to 100 sg of anti-gbm Ab in 0.4 ml of saline was infused manually over 30 sec into the left RA. The renal-venous effluent was blotted carefully during this and subsequent procedures. Three minutes after the conclusion of the infusion, the left kidney was again perfused with 2.0 ml of saline at a rate of 0.8 mllmin. The aortic perfusion needle was then removed and the aortic and renal venous puncture sites sealed with a small piece of sterile microcrystalline collagen hemostats (Avicon Inc., Puerto Rico). All ligatures on the aorta and left RV were then removed. Five minutes later the ligature on the right RA was removed. In no case was blood flow interrupted to either kidney for more than 15 mm. The abdominal incision was then closed. Rats were returned to metabolic cages and allowed free access to water and fluid as was the method used for bilateral NSN rats. At the time of sacrifice, 5 to 15 days after the infusion of anti-gbm Ab, specimens of the right and left kidney were obtained in formalin and snap-frozen state for light and immunofluorescence microscopy. Controls. Normal Wistar-Munich rats were preimmunized with normal rabbit gamma globulin as described above, but instead of receiving anti-gbm Ab, they were given a comparable amount of non-immune RaIgG intravenously (NSN control rats) or via the left renal artery as described above (unilateral sham control rats). At sacrifice, 5 to 15 days after infusion, specimens were also obtained for light and immunofluorescence microscopy as described below. In a further set of normal animals hemodynamic measurements were made following the acute infusion of homologous plasma (approximately 10% of the body weight) to calculate absolute values of Kf. In a separate set of observations animals with unilateral NSN and unilateral sham control rats underwent PGC measurements in both the left and right kidneys. Measurement of extra cellular fluid volume (ECFV) Twenty-six male Sprague-Dawley rats weighing 314 to 460 g were divided into three groups: group 1, animals with bilateral NSN (N = 10); group 2, unilateral NSN (N = 8); and group 3, normal controls (N = 8). Whole animal GFR and ECFV were measured before and 2 weeks after the induction of nephritis. GFR and ECFV were estimated by following the time course of tritiated inulin and sulfate-35, respectively, following single intracardiac injections. After simultaneous intracardiac injections of isotopes under ether anesthesia, blood was collected from the tail vein in previously weighed heparinized hematocrit tubes at 30, 60, 90, and 120 mm. The tubes were weighed again, the hematocrit was measured, and the weight of the plasma was calculated. The plasma water volume was assumed to be 0.93 times plasma weight. Urine samples were also collected by the use of metabolic cages after 30, 60, 90, and 120 mm. The collection funnel was washed with phosphate-buffered saline (PBS) solution and was included in each collection. The volume of isotope injected was determined by weighing the syringe before and after the injection. The amount of 3H and 35S administered averaged 1.4 and 0.7 Ci, respectively. The isotopes in the plasma, urine, and standard were measured by the use of a liquid scintillation counter (LS-333, Beckman Instruments, Inc., Fullerton, California). Appropriate corrections were made for dual labeling, quenching, and background, to calculate disintegration per minute. Calculations GFR was calculated as K1Q/A where K1 is the slope of inulin disappearance, Q is the total amount injected, and A is the Y intercept, that is, inulin concentration at zero time. ECFV was measured as the volume of distribution of S (VD) and was calculated from the equation: VD = (Total 35S injected Total urine 35S/plasma water 35S). VD was expressed as milliliters per 100 g of body weight and GFR as milliliters per minute. The VD was calculated for each time interval in which a urine collection was obtained. The time intervals in which no urine could be collected or in which there was a greater than 10% variation from the previous time interval were discarded. The remaining time intervals were then averaged for a final VD. At least two of the four time intervals were required. Recovery experiments that involved pouring rat urine with 35S into a metabolic cage and washing the collecting funnel with PBS resulted in an isotope recovery rate of 90% + 2. There was no difference in recovery rate as long as more than 10 ml of PBS were used. The urine count of 35 for each time interval was therefore divided by 0.9 for the total urine count. Micropuncture experiments Experiments were performed in adult Wistar-Munich rats allowed free access to food and water before study. Rats were anesthetized with mactin (100 mg/kg) and prepared for micropuncture as described previously [31. Sixty minutes before the micropuncture, rats received an intravenous infusion of isotonic sodium chloride containing 10% inulin at the rate of 0.02 mllmin. Mean femoral arterial pressure (AP) was monitored by means of an electronic transducer (Statham Instruments, Inc., Oxnard, California) connected to a direct-writing recorder. After a 60-mm equilibration period, exactly timed samples of fluid were collected from randomly selected proximal tubules for the determination of flow rate and inulin concentration and for calculation of SNGFR. Collections were made by holding an oil droplet in position distal to the puncture site. Coincident with these tubule fluid collections, femoral arterial blood samples (100 d) were obtained for the determination of hematocrit and plasma inulin concentration. Hydraulic pressures were measured in single glomerular capillaries of surface glomeruli using a continuous recording, servonulling micropipette transducer. Direct measurements of hydraulic pressure in single glomerular capillaries (PGC), proximal tubules (PT), efferent arterioles (PE), and second or thirdorder peritubular capillaries (Pc) were recorded in each rat. Mean values for each animal were derived from measurements in three to five individual structures of each type. To obtain estimates of colloid osmotic pressure (IT)of plasma entering and leaving glomerular capillaries, protein concentrations in femo-
4 622 Sakai et a! ral arterial and efferent arteriolar blood plasma were measured as previously described, Colloid osmotic pressures (ir) for control and experimental rats were calculated from these measured values by using the equation ir = 1.63 c c2 (c = total protein concentration in g!dl). For plasma with albumin! globulin ratios of less than 0.8, the equation IT = 2.24 c c2 was utilized [10]. These estimates of pre- and postglomerular protein concentration permit calculation of single nephron filtration fraction (SNFF) and initial glomerular plasma flow (GPF). From direct measurements of the decline in pressure along single afferent and efferent arterioles and the estimates of blood flow through these vessels, vascular resistances to blood flow were calculated (see below). Analytical. The volume of tubule fluid collected from individual nephrons was estimated from the length of the fluid column in a constant-bore capillary tube of known internal diameter. Concentration of inulin in tubule fluid was measured in duplicate by the microfluorescence method of Vurek and Pegram [11]. Inulin concentration and arterial plasma was determined by the macroanathrone method [12]. Protein concentration in efferent arteriolar and femoral arterial blood plasmas was determined in duplicate with an ultramicro-colorimetric method using the micro-adaptation of the method of Lowry et al [13]. Calculations Single nephron glomerular filtration rate: SNGFR = (TF!P)1 X VTF where (TF!P)1 and VTF are referred to transtubular inulin concentration ratio and tubular fluid flow rate, respectively. Single nephron filtration fraction: SNFF = 1 CA/CE (2) where CA and CE denote afferent and efferent arteriolar protein concentrations, respectively. Afferent arteriolar glomerular plasma flow rates: GPF = SNGFRJSNFF (3) Afferent arteriolar blood flow rate: Single efferent arteriole resistance: RE = X l0' (Poc P)!EGBF (7) Total arteriolar resistance: RT=RA+RE (8) Net ultrafiltration pressure at the afferent end of the glomerular capillary: PUFA = PGC ira (9) Net ultrafiltration pressure at the efi'erent end of the glomerular capillary: PUFE = PGC ire (10) Mean transglomerular hydraulic pressure difference: = PGC PT (11) Whole kidney GFR autoregulation study (Sprague-Dawley rats, bilateral NSN) After tracheostomy, a jugular vein was catheterized and a 10% inulin solution in isotonic saline was infused. A priming dose of about 0.5 ml was given over 10 mm and thereafter a steady infusion (1.2 ml!hr) was maintained for at least 45 mm before measurements were made. A left flank incision was made and the left kidney was placed in a cup (Lucite ). The surface of the kidney was covered with transparent wrap (Saran-wrap ) and the ureter was cannulated with PE 10 tubing. A section of the aorta between the two renal arteries was gently dissected free with care taken to avoid rupturing the thoracic duct. An aortic clamp was placed cephalad to the left renal artery to vary the arterial perfusion pressure of the left kidney. The systemic arterial pressure was elevated by bilateral occlusion of the carotid arteries. Urine samples were collected with glass capillary tubes over 3- to 5-mm periods. Triplicate or quadruplicate collections were made at both high arterial pressure of around 140 mm Hg (carotid occlusion) and at a low pressure of 105 mm Hg (aortic constriction). Urine flow rates were determined gravimetrically. Blood samples were obtained before and after each period of urine collections. GBF = GPF!(1 Hct) where Hct is the arterial hematocrit. Efferent arteriolar blood flow rate: EGBF = GBF - SNGFR Single afferent arteriole resistance: Ra = x l0' (A PGC)/GBF where the factor x 10I provides resistance in units of dynes x seconds x centimeter/s 5 where AP and GC are expressed in millimeters of mercury and GBF in nanoliters per minute. (4) Renal blood flow autoregulation (Sprague-Dawley rats, bilateral NSN) A separate group of animals was used for this study. A left flank incision was made and the left renal artery was gently freed from its surrounding tissue. Whole kidney RBF measure- (5) ments were made by fitting the renal artery with a small lumen size (2.0 mm circumference) flow transducer (Model EP 102, Carolina Medical Electronic Inc.). The flow transducer was connected to an electromagnetic flow meter (Model BL-610, (6) Biotronex Lab. Inc.). Calibration of the flow meter was done by pumping heparinized blood from donor rats at known rates through an excised piece of carotid artery. After the surgical preparations, the RBF and arterial pressure were allowed to stabilize for at least 20 mm. In each experimental run, arterial pressure was first elevated by bilaterial carotid artery occlusion
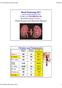
5 and then lowered in discrete steps of 10 to 20 mm Hg from a starting pressure near 140 mm Hg down to 90 mm Hg while RBF changes were noted on a recorder (Gould). Arterial pressure was held at each level for around 3 mm. The systemic arterial pressure was elevated by bilateral occlusion of the carotid arteries. Zero baseline for RBF measurement was obtained by occluding the renal artery distal to the flow transducer after the completion of the flow measurements. These experiments were conducted in Sprague-Dawley rats; the nephritic animals had bilateral disease. Tubuloglomerular feedback (Sprague-Dawley rats, bilateral NSN) A group of animals separate from those in the whole kidney studies was used. The left kidney was isolated by a flank incision and was placed in a cup (Lucite ). The microperfusion techniques for characterizing the tubuloglomerular feedback response were essentially the same as those described previously by Schnermann, Persson, and Agerup [141. Late proximal tubular segments were perfused with an artificial solution composed of 136 mrvi NaC1, 4 m'vi NaHCO3, 4 mivi KCI, 2 mm CaCl 2, 7.5 m urea, and 0.1% lissamine green. An 8-/x tip pipette filled with the perfusate was attached to a microperfusion pump (Hampel, Frankfurt, Germany) and with the perfusion rate set at 10 nl/min, a nephron with several surface loops was identified. The perfusion pipette was then withdrawn and reinserted into the last accessible proximal loop. Either vaseline stained with Sudan black (for stop-flow pressure measurement) or bone wax (for early proximal flow rate measurement) was injected into the middle surface proximal loop with a 10 to 15 j tip pipette. Stop-flow pressure measurements were obtained in segments proximal to the vaseline block using continuous recording servo-nulling micropipette transducer techniques. Micropipettes with 1 to 3 x tips filled with 1.5 M NaC1 were used. The late proximal perfusion rate was alternately set at 15, 20, 30, or 40 nl/min in a random fashion with a reference perfusion rate at 10 nllmin while stop-flow pressures in early proximal tubules were recorded at each setting. In a separate group of animals, exactly timed tubular fluid collections were made in segments proximal to the bone wax block using 8- pipettes filled with stained paraffin oil in conjunction with alternating late proximal perfusion rate of 0, 10, 25, or 40 ni/mm in a random fashion. A 3-mm equilibration period was allowed between pump rate changes before starting either stop-flow pressure measurement or timed collection of tubular fluid. These experiments were performed on Sprague-Dawley rats; the nephritic animals had bilateral disease. Late proximal flow rate (Sprague-Dawley rats, bilateral NSN) A separate group of animals served for this study. A jugular vein was catheterized for the injection of filtered 1% FDC and Green dye in isotonic saline. Surgical preparation was made as previously described for tubuloglomerular feedback study. A bolus of 0.05 ml of FDC and Green solution was injected and the passage of the dye through surface tubules was observed. The last accessible superficial proximal loops were identified, and timed tubular fluid collections were made in the last segment using 8- pipettes filled with stained paraffin oil. These Glomerular hemodynamics in experimental nephritis 623 Table 1. Changes in extracellular fluid volume (ECFV) and whole kidney GFR in bilateral NSN, unilateral NSN, and control rats before (B) and after (A) immunization; values are expressed as mean SEM Control rats Bilateral NSN Unilateral NSN a p < 0.02 N B ECFV ml/100 g A a 0.7 B GFR mi/mm A experiments were performed on Sprague-Dawley rats; the nephritic animals had bilateral disease. Pathologic observations Light microscopy. Mid-coronal slices of both kidneys were fixed in 10% neutral buffered formalin and sectioned at 4 to 6 after being embedded in paraffin. Hematoxylin- and eosinstained sections were evaluated after slides were coded and randomized. The severity of acute proliferative glomerular lesions were graded 0 to 4+ on an arbitrary semi-quantitative scale. Rats receiving intravenous anti-gbm Ab and revealing lesions less than or equal to 1 + in either kidney were excluded from analysis, as were rats in the unilateral NSN group which revealed less than or equal to 1 + lesions in the left (perfused) kidney or greater than or equal to I + lesions in the right (nonperfused) kidney. Immunofluorescence. A mid-coronal section of each kidney was snap-frozen in isopentane, precooled in liquid nitrogen, and 4 to 6 x frozen sections were obtained using cryomicrotome (Mills Tissue Tech II, Miles Scientific, Naperville, Illinois). After fixation in ether-alcohol the sections were reacted with fluorescein conjugated goat anti-rabbit IgG, rabbit anti-rat IgG, and rabbit anti-rat C3 (Cappel Laboratories, Cochranville, Pennsylvania). Slides were interpreted without knowledge of the experimental group. Rats receiving intravenous anti-rat GBM Ab which revealed negative reactions for rabbit or rat IgG along GBM were excluded from further analysis. Rats in the unilateral-nsn group which revealed positive reactions for rabbit or rat IgG along GBM of the right (nonperfused) kidney or negative reactions for rabbit or rat IgG along GBM of the left (perfused) kidney were also excluded from further analysis. Results Ext racellular fluid volume The results of measurements of whole kidney GFR and ECFV are presented in Table 1. GFR did not change significantly in nephritic rats with either unilateral or bilateral disease, or in sham control rats. GFR values obtained in this set of experiments with a single injection technique were about 60% of those obtained with conventional inulin clearance methods. We found a consistent correlation between the two methods in both normal and diseased animals (Harris and Glassock, unpublished observations). We used the single injection method here because it permits serial determinations on the same animal. The results are presented to show that GFR did not differ among the groups. Measurements of ECFV indicate that animals with bilateral NSN had a consistent increase, compared to sham control rats.
6 624 Sakai et al Normal, hydropenia N A PGC " ±4.1 ±1.0 Table 2. Summary of glomerular measurement" PT 11A '1E 12.9 ±0.4 SNGFR mmhg 'TE'P ni/mm SNFF 29.6b lSb ±0.8 ±0.5 ±0.6 ±0.6 ±0.05 ±0.7 ±0.01 Bilateral NSN, hydropenia ±3.6 ± ± ± ±0.3 ±0.8 ±1.8 ± ±0.02 Unilateral NSN, hydropenia ±4.9 ± " ± ± b ±0.3 ±0.4 ±0.6 ± ±2.6 ±0.01 ANOVAd NS P < P < P < P < NS NS P < NS NS a Values listed are means ± SCM. b The value indicated differs significantly from the other two, as judged by a Newman-Keuls multiple range test. In no instance did all three means differ from each other. The Kf value for the normal group was taken from the plasma-loaded normal group. All other measurements in the row labelled "Normal" are from hydropenic animals. d Probabilities listed in the row labelled ANOVA are the probabilities that all means in a given column are the same, as tested by one-way analysis of variance. This increase is the abnormality the effect of which we wish to examine. The results show no increase in ECFV in unilateral disease. Thus, the unilateral model is one with nephritis but without expansion of ECFV. We have used a comparison of the two models to determine whether the measured ECFV expansion of bilateral NSN is required for the hemodynamic adaptation to the disease. Glomerular hemodynamics in normal animals Hydropenic normal rats. Because this study involved some nephritic rats that were in a state of volume expansion caused by the disease, no attempt was made to correct the extravascular shift in extracellular fluid volume known to be caused by laparotomy [15]. The results reflect this effect. Glomerular capillary hydraulic pressure, SNGFR, SNGPF, and SNGBF all tended to be at the lower end of the range of values published for normal animals [16] while hematocrit values were high. Since filtration pressure equilibrium prevailed, exact values for Kf could not be estimated, but the calculated minimum values averaged ± ni x sec' x mm Hg, a value not different from that reported for normal Wistar-Munich rats [16]. Plasma volume-expanded normal rats. The purpose of these measurements was to provide a reference value of Kf against which the value for nephritic animals could be compared (Table 2). The mean value ± nl/sec' mm Hg' does not differ from the value of ± nl/sec' mm Hg' reported by Deen et al [161 for this strain of Wistar Munich rats. Glomerular hemodynamics in nephrotoxic serum nephritis (NSN) Bilateral NSN. Compared to hydropenic control rats, these animals had significant elevations of PGC and AP and were not at filtration pressure equilibrium (Table 2). Kf was significantly reduced from normal for this population. Since SNGFR remained unchanged from normal, our results indicate that an elevation of net ultrafiltration pressure compensates for the reduction in Kf to maintain normal SNGFR. Similar results and conclusions have been presented earlier [3]. Glomerular blood flow rate was increased in this group, hut Al5 was unchanged; total renal vascular resistance therefore decreased. On the assumption that renal vascular resistance resides primarily in the afferent and efferent arterioles, and given that POC increased while overall vascular resistance decreased, it follows that there must have been a reduction of afferent arteriolar resistance. The results of measurements of arteriolar resistances tend to bear out this analysis, but the variances are large and the differences do not achieve statistical significance. Unilateral sham control rats. These groups were designed to serve as a control for the surgical procedure imposed on the unilateral nephritic group. These animals had normal PGC (42.2 ± 0.2 mm Hg) and P (31.9 ± 0.1 mm Hg), and all were at filtration pressure equilibrium (1rE/zP = 1.15 ± 0.6). None of these values were significantly different from those found in hydropenic control rats. Unilateral NSN. In all measures except one, these animals exhibited mean values that were not significantly different from those for the bilateral NSN group (Table 2). The single exception was a difference in PT of 1.9 mm Hg; the unilateral group was lower. This slight difference was too small to cause a significant difference in, however. The unilateral NSN animals had an elevation of PGC,, a reduction in Kf, and unchanged SNGFR. Just as in the bilateral NSN group, the elevation of net ultrafiltration pressure compensated for the reduction in Kf to maintain normal SNGFR. This compensation occurred despite the presence of a normally functioning right kidney and normal ECFV (see Results). This result suggests that the stimulus for dilatation is largely intrarenal and does not necessarily involve an extrarenal pathway. Unilateral NSN two kidney comparisons As a further test of the conclusion that the signal mediating the compensation is intrarenal, we measured PGC in both kidneys of unilaterally diseased animals. If there is an extrarenal signal, it should affect both kidneys. Moreover, this comparison avoids interanimal variation as a source of uncertainty and is therefore a more rigorous test of the existence of a difference in PGC than can be obtained from comparisons of one
7 Glomerular hemodyna,nics in experimental nephriiis 625 SNGPFA ni/mm Table 2. (Continued) RA 10' dyn sec cm5 R KF nl sec' mm Hg' b,c NS NS NS P < group of animals with another. As can be seen in Table 3, the diseased kidneys had significantly higher values of PGC than the nonperfused kidney, and PGC was not different in the two kidneys of a sham-perfused group. Thus, the elevation of PGC in the diseased kidney appears to result primarily from a response generated within the organ itself, and not to a systemic signal. Whole kidney autoregulation in NSN Figure 1 shows the BP-GFR autoregulation curve in control and bilateral NSN rats. Although GFR was lower in NSN than in control at both high and low ranges of perfusion pressure ( in NSN vs ml'min/g of kidney weight in control at high BP, P < 0.001; vs at low BP, P < 0.01), the mean slopes of the regression lines were not statistically different from each other, indicating NSN rats autoregulate GFR as well as control rats. As shown in Figure 2 RBF is as well autoregulated in rats with NSN as in control rats over a range of renal perfusion pressures from 133 to 93 mm Hg. The mean slopes of the regression lines were not different from each other. RBF averaged mllmin!g of kidney weight in control animals and mllmin/g of kidney weight in rats with NSN. The difference was not significant. Tubuloglomerular feedback in NSN Stop-flow pressure (SFP) in early proximal tubules was higher in NSN than in control rats by 4.0 mm Hg, at a perfusion rate of 10 ni/mm, and remained higher at higher perfusion rates (Fig. 3). This difference corresponds to the difference found by direct micropuncture in Wistar Munich rats (Table 2). The data points from each group of animals were fit to a curve of the form y = a exp(bx). The values of the coefficient b were: control, 5.8 x l0-2.2 x l0; NSN, 8.4 X l0 2.1 X i0. There was no significant difference between the calculated values of the two coefficients. Thus, apart from the higher absolute value of SFP, these results suggest that the sensitivity of SFP to the loop of Henle perfusion rate is the same in NSN as in control rats. Early proximal flow rate, an approximation of SNGFR responded to changes in the loop of Henle perfusion rate in rats with NSN as it did in control rats (Fig. 4). Finally, we measured the late proximal flow rate to determine whether the signal to tubuloglomerular feedback might be altered in NSN. Late proximal flow rate was significantly higher in rats with NSN than in control rats, indicating that the Table 3. Comparison of glomerular capillary pressures (P0) in right and left kidneys of unilaterally diseased and unilaterally shamoperated rats; operative procedures were performed on the left kidneys PGC, mm Hg Paired difference, mm Hg Sham (N = 6) NSN (N = 8) Left Right Left Right <P< P<0.001 macula densa receives a signal that should cause a lower level of GFR than in control rats (Table 4). Pathology and immunopathology Bilateral NSN. The left and right kidneys of rats in the bilateral NSN group displayed mild to moderate (2 to 3+) acute proliferative glomerulonephritic lesions consisting of infiltration with one to five polymorphonuclear leucocytes and/or monocytes. The glomeruli were hypercellular and the capillary lumens were segmentally obliterated. Necrosis and crescent formation were not observed. The tubules, extraglomerular vasculature and interstitium, were normal. The lesions were uniform throughout the superficial and deep cortex. Intense diffuse linear deposits of rabbit IgG were seen in all glomeruli, but no deposits were seen in tubular basement membranes or extraglomerular vessels. Diffuse glomerular deposits of rat IgG and C3 were seen in all rats, although the pattern tended to be less linear and more segmental. These findings are similar to those reported previously [3]. Unilateral NSN. Mild to moderate proliferative lesions (2+ to 3+) similar in character to that observed in bilateral NSN were seen in the left (perfused) kidney of unilateral NSN group, whereas the right (nonperfused) kidneys could not be distinguished from normal (Fig. 5). Diffuse linear deposits of rabbit IgG and to a lesser extent rat IgG in C3 were found in the left (perfused) kidney, while the right kidneys showed only scattered, trace deposits of rabbit IgG or rat IgG/C3 (Fig. 6). Control rats. The glomeruli in the normal hydropenic, plasma-loaded, unilateral-sham control groups were uniformly normal by light microscopy and negative by immunofluorescence for deposits of rabbit IgG, rat IgG, or rat C3. Discussion The results of this study confirm earlier findings in the early autologous phase of NSN [3] in that a significant reduction of Kf can be tolerated without a fall in SNGFR because of a compensating increase in net ultrafiltration pressure (PUF). Of the three components of UF, the principal change is an elevation of PGC. The compensation appears to be quite precise, because SNGFR does not decline despite a substantial reduction of Kf. This precision suggests that a rather vigorous control mechanism is at work. The identification of this mechanism is the goal of this study. Although SNGFR can be maintained at normal levels despite a reduction in Kf to 30 to 50% of normal and an increase in end proximal flow rate (present results and [4]), we found that ECFV is increased in bilateral NSN. The response of the 1.8
8 626 Sakai et al 1.4 Loop of Henle perfusion rate, ni/rn/n ) E II + 1' I I I I I I I Arterial pressure, rnrn Hg Fig. 1. Whole kidney GFR as a function of arterial pressure. Symbols are: , rats with nephrotoxic serum nephritis;, control rats U- I 1.0 Fig. 3. The change in proximal stop-flow pressure as a function of ioop of Henle perfusion rate. Symbols are the same as those used in Figure ) E z,,,i I I I E L /,I 90 I I I Arterial pressure, mm Hg I 140 Fig. 2. Whole kidney bloodflow as afunction of arterial pressure in rats with and without nephrotoxic serum nephritis. Blood flows were normalized by the flow at the ambient arterial pressure before the pressure was raised by carotid occlusion. Symbols are the same as those used in Figure 1. systems mediating blood flow and GFR autoregulation are known to be altered by changes in extracellular fluid volume [5, 6]. To identify the mechanism responsible for the adaptation of NSN, it becomes important to decide whether the volume expansion is a major factor in the response. The unilateral NSN model was designed to address this question. With unilateral disease, both the normal and the diseased kidney are exposed to the same signal arising from a given perturbation of extracellular fluid volume. If both kidneys experience the same elevation of PUF, volume expansion might be a major factor; if the difference between the two kidneys remains, an intrarenal mechanism will have to be identified. The results of Kf measurements show that with the unilateral model of NSN a lesion of comparable severity to that induced with the more routine bilateral approach was obtained. Direct comparisons of PGC in the same animal revealed a difference in each animal as great as was found between the bilaterally diseased and normal animals, although ECFV was normal. This comparison was made in 10 0 Loop of Henle perfusion rate, ni/mm Fig. 4. Early proximal flow rate as a function of loop of Henle perfusion rate. Symbols are the same as those used in Figure 1. Table 4. Comparison of late proximal tubule flow rate in rats with and without bilateral NSN Control rats NSN Number of animals 6 7 Number of tubules Flow rate, ni/mm P < 0.01 anesthetized animals subjected to animal surgery, while the ECF volume measurements were made in conscious animals. If the results of the direct POC are applicable to conscious animals, it would follow that an intrarenal mechanism plays a major role in the renal adaptation to moderate NSN. We next assessed several measures of autoregulation in 1.0
9 Glomerular hemodynamics in experimental nephritis 627 Left kidney (perfused) Right kidney (non-perfused) Fig. 5. A comparison of the light microscopic changes in the left (perfused) and right (nonperfused) kidneys of a rat with unilateral nephrotoxic serum nephritis. (14 days postinjection of anti-gbm antibody, hematoxylin and eosin, x250), S a. a a Loft kidney (perfused) Anti-rabbit IgG Right kidney (non-perfused) Fig. 6. A comparison of the deposition of rabbit IgG anti-gbm antibody in the left (perfused) and right (nonperfused) kidneys of a rat with unilateral NSN. (14 days, postinjection of anti-gbm antibody, immunofluorescence with fluorescein-labeled goat anti-rabbit IgG, x200) NSN. The two mechanisms thought to be of principal importance are a myogenic mechanism and tubuloglomerular feedback mediated via the macula densa. Stop-flow pressure is a measure of GC, but differs from it because the suppression of filtration that is a necessary part of the determination increases efferent arteriolar plasma flow and GC [17]. Changes in stopflow pressure probably mirror changes in GC With the loop of Henle perfusion set at 10 nl/min, stop-flow pressure was higher in rats with NSN than in control rats, by an amount comparable to the difference in GC between NSN and normal rats. The response of stop-flow pressure and early proximal flow rate to changes in loop of Henle perfusion rate was not detectably different than the response of control animals. We conclude that immune glomerular injury does not alter the performance of tubuloglomerular feedback in a way that could explain the afferent arteriolar dilatation that is presumed to be a major adaptive response to NSN. Since our results show that the disease causes a mild expansion of ECFV, the fact that the feedback response has not been attenuated suggests either that the expansion is too mild to produce a measurable effect on the measures of feedback we have used, or that the feedback mechanism has developed an increased sensitivity that compensates for the effect of expansion. We have not attempted to determine which of these explanations is correct. If the response of this control mechanism to isolated testing is not abnormal, feedback could cause afferent arteriolar dilatation only if the macula densa received a reduced flow signal. Instead as we and Maddox et al [4] have shown, the flow rate of tubular fluid into the loop of Henle is increased because of reduced fluid reabsorption in the proximal tubule. Finally, with tubuloglomerular feedback in a normal functional state, and with whole kidney autoregulation also normal, it follows that there can be no major abnormality in the myogenic mechanism. Our studies suggest that some possible causes of the adaptation are unlikely but have not further identified the mechanism. As we pointed out previously [18], mechanisms like the myogenic response and tubuloglomerular feedback have time constants of response that are measured in seconds and tenths of seconds. In retrospect it is perhaps not surprising that the response to a slowly developing lesion should involve mechanisms different from these fast reacting controllers. Our current working hypothesis is that the stimulus to adaptation induces slow changes in the afferent arteriole, possibly structural, and that mechanisms responsible for autoregulation adapt sufficiently and continue to maintain blood flow and GFR constant in response to higher frequency perturbations. The glomerular hemodynamic response to other forms of experimental injury does not reveal a consistent pattern of response and so does not help to identify possible adaptive mechanisms operating in NSN. Bohrer et al [19] have analyzed glomerular hemodynamics in acute (8-day) aminonucleoside of puromycin-induced nephrosis in rats. Both GPF and Kf were reduced compared to control rats. Interestingly, zp was unchanged in the nephrotic as compared to the control rats. These
10 628 Sakai et a! findings would be most compatible with an increase in renal vascular resistance shared equally by the afferent and efferent arterioles. Baylis, Rennke, and Brenner [201 examined the response to acute (11-day) gentamicin nephrotoxicity in rats. The pattern of disturbance in glomerular hemodynamics observed in this model was quite similar to that seen in the aminonucleoside of puromycin model. Thus, these two experimental models of glomerular injury, both of which result in reductions in Kf comparable to that observed in NSN are not accompanied by a reduction in renal vascular resistance but rather are accompanied by an increase in both RA and RE. On the other hand, Ichikawa et al [21] recently examined two experimental models of a nonprolilerative immune complex mediated membranous glomerulopathy; namely, heterologous and autologous immune complex nephritis. In these models Kf was reduced to an equal extent and was not different from that seen in the model of NSN utilized in the present study. ip was increased in both models, partially offsetting the effect of reduced Kf on SNGFR; however, GPF was either unchanged or decreased indicating a degree of increased efferent resistance with a minor change in afferent resistance. Taken together, these findings indicate that the hemodynamic response to acute glomerular injury depends on the method of induction of injury, rather than being a generalized response to a reduction in Kf. The failure of zp to rise in conjunction with the reduced Kf in aminonucleoside of puromycin nephrosis and gentamicin nephrotoxicity contributes to the observed fall in SNGFR. The increase in P seen in heterologous and autologous immune complex glomerulonephritis and nephrotoxic serum nephritis offsets the reduction in Kf and permits maintenance of a normal or near normal SNGFR. The failure of a single pattern of response to emerge means that each model will have to be pursued independently to determine the cause of adaptation. Acknowledgments This work was supported by National Institutes of Health grants AM16565 and AM15968 and by Investigator Group Award 5361G from the American Heart Association, Greater Los Angeles Affiliate. Dr. F. H. Harris, Jr., was supported by a Fellowship Award also from the American Heart Association, Greater Los Angeles Affiliate. Reprint requests to Dr. R. J. Glassock, Department of Medicine, Harbor-UCLA Medical Center, 1000 W. Carson St., Torrance, Ca1fornia 90509, USA References 1. ROCHA A, MARCONDES M, MALNIC A: Micropuncture study in rats with experimental glomerulonephritis. Kidney mt 3:356, ALLISON MEM, WILSON CB, GOTTSCHALK CW: Pathophysiology of experimental glomerulonephritis in rats. J C/in Invest 53:1402, MADDOX DA, BENNETT CM, DEEN WM, GLASSOCK RJ, KNUTSON D, DAUGHARTY TM, BRENNER BM: Determinants of glomerular filtration in experimental glomerulonephritis in the rat. J Clin Invest 55:305, MADDOX DA, BENNETT CM, DEEN WM, GLASSOCK Ri, BRENNER BM: Control of proximal tubule fluid reabsorption in experimental glomerulonephritis. J Clin Invest 55:1315, BENNETT CM, GLASSOCK RI: Exchangeable sodium in acute nephrotoxic serum nephntis in rats, in VII In! Cong Nephrol, 1978, abstracts, K-IS 6. SCHNERMANN J, HERMLE M, SCHMIDMEIER E, DAHLHEIM H: Impaired potency for feedback regulation of glomerular filtration rate in DOCA escaped rats. Pfluegers Arch 358: , PLOTH DW, RUDOLPH J, THOMAS C, NAVAR LG: Renal and tubuloglomerular feedback responses to plasma expansion in the rat. Am J Physiol 235:F156 Fl62, HOYER JH, MAUER SM, MICHAEL AF: Unilateral renal disease in the rat. I. Clinical, morphologic and glomerular mesangial functional features of the experimental model produced by renal perfusion with aminonucleoside. J Lab C/in Med 85: , LERNER RA, DIXON FJ: Transfer of ovine experimental allergic glomerulonephritis (EAG) with serum. J Exp Med 124: , LANDIS EM, PAPPENHEIMER JR: Exchange of substances through capillary walls. Handbook Physiol 2: , VUREK GG, PEG1M SE: Flurometric method for the determination of nanogram quantities of insulin. Anal Biochem 16: , FUHR J, KACZMARCZYK J, KRUTTGEN CD: Eine einfache colorimetrische methode zur Inulin Bestimung fur Nieren-Clearanceuntersuchungen bei Stoffwechselgesunden und diabetikern. K/in Wochenschr 33: , LOWRY AH, ROSEBROUGH NF, FARR AL, RANDALL RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 193: , SCHNERMANN J, Pnssor. AEG, AGERUP B: Tubuloglomerular feedback: nonlinear relation between glomerular hydrostatic pressure and 1oop of Henle perfusion rate. J Clin Invest 52: , MADDOX DA, PRICE DC, RECTOR FC JR: Effects of surgery on plasma volume and salt and water excretion in rats. Am J Physiol 233: F600 F606, DEEN WM, TROY JL, ROBERTSON CR, BRENNER BM: Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J C/in Invest 52: , ICHIKAWA I: Evidence for altered glomemlar hemodynamics during acute nephron obstruction. Am J Physiol 242:F580 F585, MARSH DJ: Frequency response of autoregulation. Kidney mt 22:S165 S172, BOHRER MP, BAYLIS C, ROBERTSON CR, BRENNER BM: Mechanism of the puromycin-induced defects in the transglomerular passage of water and macromolecules. J C/in Invest 60: , BAYLIS C, RENNKE HR, BRENNER BW: Mechanism of the defect in glomerular ultrafiltration associated with gentamicin administration. Kidney In! 12: , ICHIKAWA I, HOYER JR, SElLER MW, BRENNER BM: Mechanism of glomerulotubular balance in the setting of heterogenous glomerular injury-preservation of a close functional linkage between individual nephrons and surrounding microvasculature. J C/in Invest 69: , 1982
MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER
 MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER The glomerular filtration barrier consists of three elements: (1) endothelial
MOLECULAR SIZE, ELECTRICAL CHARGE, AND SHAPE DETERMINE THE FILTERABILITY OF SOLUTES ACROSS THE GLOMERULAR FILTRATION BARRIER The glomerular filtration barrier consists of three elements: (1) endothelial
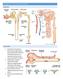
MAJOR FUNCTIONS OF THE KIDNEY
 MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
Determinants of Glomerular Filtration in Experimental Glomerulonephritis in the Rat
 Determinants of Glomerular Filtration in Experimental Glomerulonephritis in the Rat D. A. MADDOx, C. M. BENNETr, W. M. DEEN, R. J. GLASSOCK, D. KNUTSON, T. M. DAUGHARTY, and B. M. BRENNER From the Departments
Determinants of Glomerular Filtration in Experimental Glomerulonephritis in the Rat D. A. MADDOx, C. M. BENNETr, W. M. DEEN, R. J. GLASSOCK, D. KNUTSON, T. M. DAUGHARTY, and B. M. BRENNER From the Departments
Preparation of Animals for Live Animal Imaging
 Preparation of Animals for Live Animal Imaging George A. Tanner, Ph.D. Department of Cellular and Integrative Physiology Indiana University School of Medicine Ideal Condition of Rats during Experiments:
Preparation of Animals for Live Animal Imaging George A. Tanner, Ph.D. Department of Cellular and Integrative Physiology Indiana University School of Medicine Ideal Condition of Rats during Experiments:
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Autoregulation of Single Nephron Filtration Rate in the Presence and the Absence of Flow to the Macula Densa
 Autoregulation of Single Nephron Filtration Rate in the Presence and the Absence of Flow to the Macula Densa By Franklyn G. Knox, Cobern Ott, Jean-Louis Cuche, Josianne Gasser, and John Haas ABSTRACT Flow
Autoregulation of Single Nephron Filtration Rate in the Presence and the Absence of Flow to the Macula Densa By Franklyn G. Knox, Cobern Ott, Jean-Louis Cuche, Josianne Gasser, and John Haas ABSTRACT Flow
Glomerular Capillary Blood Pressure
 Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
Glomerular Capillary Blood Pressure Fluid pressure exerted by blood within glomerular capillaries Depends on Contraction of the heart Resistance to blood flow offered by afferent and efferent arterioles
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences. Body fluids and.
 By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
By: Dr. Foadoddini Department of Physiology & Pharmacology Birjand University of Medical Sciences Body fluids and Renal physiology 25 Volume and Osmolality of Extracellular and Intracellular Fluids
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION
 LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
LECTURE 25: FILTRATION AND CLEARANCE NEPHRON FILTRATION 1. Everything in the plasma is filtered except large proteins and red blood cells. The filtrate in Bowman s capsule is an isosmotic fluid that is
Hydrostatic pressures in proximal tubules and peritubule capillaries in the dog
 Kidney International, Vol. 2 (1972), p. 11 16 Hydrostatic pressures in proximal tubules and peritubule capillaries in the dog FRANKLYN U. KNOX, L. R. WILLIS, JACK W. STRANDHOY and EDWARD U. SCHNEIDER Nephrology
Kidney International, Vol. 2 (1972), p. 11 16 Hydrostatic pressures in proximal tubules and peritubule capillaries in the dog FRANKLYN U. KNOX, L. R. WILLIS, JACK W. STRANDHOY and EDWARD U. SCHNEIDER Nephrology
Introduction to the kidney: regulation of sodium & glucose. Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health
 Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
Introduction to the kidney: regulation of sodium & glucose Dr Nick Ashton Senior Lecturer in Renal Physiology Faculty of Biology, Medicine & Health Objectives Overview of kidney structure & function Glomerular
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Physio 12 -Summer 02 - Renal Physiology - Page 1
 Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Physiology 12 Kidney and Fluid regulation Guyton Ch 20, 21,22,23 Roles of the Kidney Regulation of body fluid osmolarity and electrolytes Regulation of acid-base balance (ph) Excretion of natural wastes
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Renal Blood flow; Renal Clearance. Dr Sitelbanat
 Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
Ch 17 Physiology of the Kidneys
 Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Ch 17 Physiology of the Kidneys Review Anatomy on your own SLOs List and describe the 4 major functions of the kidneys. List and explain the 4 processes of the urinary system. Diagram the filtration barriers
Physiology Lecture 2. What controls GFR?
 Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
Glomerular filtration rate (GFR)
 LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
LECTURE NO (2) Renal Physiology Glomerular filtration rate (GFR) Faculty Of Medicine Dept.Of Physiology The glomerulus Is a tuft of capillaries enclosed within a Bowman capsule. It is supplied by an afferent
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
Basic Functions of the Kidneys
 Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Filtration and Reabsorption Amount Filter/d
 Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD Contact me at lharris@lsuhsc.edu Renal Physiology Lecture 3 Renal Clearance and Glomerular Filtration Filtration and Reabsorption Amount Filter/d Amount
Vittorio E. Andreucci,, Floyd C. Rector Jr., Donald W. Seldin
 Effective glomerular filtration pressure and single nephron filtration rate during hydropenia, elevated ureteral pressure, and acute volume expansion with isotonic saline Vittorio E. Andreucci,, Floyd
Effective glomerular filtration pressure and single nephron filtration rate during hydropenia, elevated ureteral pressure, and acute volume expansion with isotonic saline Vittorio E. Andreucci,, Floyd
Lecture 16: The Nephron
 Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
Lecture 16: The Nephron Reading: OpenStax A&P Text Chapter 25 Primary functions of the kidneys 1. Regulating osmolarity (blood concentration!) A. Regulating blood pressure B. Maintaining ion balance C.
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Urinary System and Fluid Balance. Urine Production
 Urinary System and Fluid Balance Name Pd Date Urine Production The three processes critical to the formation of urine are filtration, reabsorption, and secretion. Match these terms with the correct statement
Urinary System and Fluid Balance Name Pd Date Urine Production The three processes critical to the formation of urine are filtration, reabsorption, and secretion. Match these terms with the correct statement
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1
 BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
Ch 19: The Kidneys. Functional unit of kidneys:?? Developed by John Gallagher, MS, DVM
 Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
Ch 19: The Kidneys Homeostatic regulation of ECF volume and BP Osmolarity 290 mosm Ion balance Na+ and K+, etc. ph (acid-base balance Excretion of wastes & foreign substances Hormone production EPO Renin
the Impaired Salt Excretion of Experimental Nephrotic Syndrome
 Role for Intrarenal Mechanisms in the Impaired Salt Excretion of Experimental Nephrotic Syndrome I. ICHIKAWA, H. G. RENNKE, J. R. HOYER, K. F. BADR, N. SCHOR, J. L. TROY, C. P. LECHENE, and B. M. BRENNER,
Role for Intrarenal Mechanisms in the Impaired Salt Excretion of Experimental Nephrotic Syndrome I. ICHIKAWA, H. G. RENNKE, J. R. HOYER, K. F. BADR, N. SCHOR, J. L. TROY, C. P. LECHENE, and B. M. BRENNER,
Urinary System. consists of the kidneys, ureters, urinary bladder and urethra
 Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Effects of pregnancy on glomerular dynamics: Micropuncture study in the rat
 Kidney international, Vol. 22 (1982), pp. 608 612 Effects of pregnancy on glomerular dynamics: Micropuncture study in the rat ANTONIO DAL CANTON, GIUSEPPE CONTE, CIR0 ESPOSITO, GI0RGI0 FUIANO, RAFFAELE
Kidney international, Vol. 22 (1982), pp. 608 612 Effects of pregnancy on glomerular dynamics: Micropuncture study in the rat ANTONIO DAL CANTON, GIUSEPPE CONTE, CIR0 ESPOSITO, GI0RGI0 FUIANO, RAFFAELE
** Accordingly GFR can be estimated by using one urine sample and do creatinine testing.
 This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
This sheet includes the lecture and last year s exam. When a patient goes to a clinic, we order 2 tests: 1) kidney function test: in which we measure UREA and CREATININE levels, and electrolytes (Na+,
Effect of leukocyte depletion on glomerular dynamics during acute glomerular immune injury
 Kidney International, Vol. 28 (1985), PP. 28 35 Effect of leukocyte depletion on glomerular dynamics during acute glomerular immune injury BRYAN J. TUCKER, LESLIE C. GUSHWA, CURTIS B. WILSON, and ROLAND
Kidney International, Vol. 28 (1985), PP. 28 35 Effect of leukocyte depletion on glomerular dynamics during acute glomerular immune injury BRYAN J. TUCKER, LESLIE C. GUSHWA, CURTIS B. WILSON, and ROLAND
EXCRETION QUESTIONS. Use the following information to answer the next two questions.
 EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
Dietary protein restriction and glomerular permselectivity in nephrotoxic serum nephritis
 Kidney International, Vol. 40 (1991), pp. 57 61 Dietary protein restriction and glomerular permselectivity in nephrotoxic serum nephritis JOEL NEUGARTEN, ARTHUR K0zIN, ROBERT GAYNER, ROBERT G. SCHACHT,
Kidney International, Vol. 40 (1991), pp. 57 61 Dietary protein restriction and glomerular permselectivity in nephrotoxic serum nephritis JOEL NEUGARTEN, ARTHUR K0zIN, ROBERT GAYNER, ROBERT G. SCHACHT,
Mechanisms of Action of Histamine and Histamine Antagonists on the Glomerular Microcirculation in the Rat
 737 Mechanisms of Action of Histamine and Histamine Antagonists on the Glomerular Microcirculation in the Rat IEKUNI ICHIKAWA AND BARRY M. BRENNER SUMMARY We made direct measurements of pregiomerular,
737 Mechanisms of Action of Histamine and Histamine Antagonists on the Glomerular Microcirculation in the Rat IEKUNI ICHIKAWA AND BARRY M. BRENNER SUMMARY We made direct measurements of pregiomerular,
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION
 3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Renal physiology II. Basic renal processes. Dr Alida Koorts BMS
 Renal physiology II Basic renal processes Dr Alida Koorts BMS 7-12 012 319 2921 akoorts@medic.up.ac.za Basic renal processes 1. filtration 2. reabsorption 3. secretion Glomerular filtration The filtration
Renal physiology II Basic renal processes Dr Alida Koorts BMS 7-12 012 319 2921 akoorts@medic.up.ac.za Basic renal processes 1. filtration 2. reabsorption 3. secretion Glomerular filtration The filtration
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
MATHEMATICAL MODELING OF THE TUBULOGLOMERULAR FEEDBACK MECHANISM IN THE KIDNEY
 MATHEMATICAL MODELING OF THE TUBULOGLOMERULAR FEEDBACK MECHANISM IN THE KIDNEY Susanne Ditlevsen Department of Mathematical Sciences, University of Copenhagen Donald Marsh Department of Molecular Pharmacology,
MATHEMATICAL MODELING OF THE TUBULOGLOMERULAR FEEDBACK MECHANISM IN THE KIDNEY Susanne Ditlevsen Department of Mathematical Sciences, University of Copenhagen Donald Marsh Department of Molecular Pharmacology,
Physiology (4) 2/4/2018. Wael abu-anzeh
 Physiology (4) 2/4/2018 Wael abu-anzeh In the previous lectures we have discussed the filtration and the reabsorption processes but in this lecture we will talk about the factor that will regulate or control
Physiology (4) 2/4/2018 Wael abu-anzeh In the previous lectures we have discussed the filtration and the reabsorption processes but in this lecture we will talk about the factor that will regulate or control
Glomerular Filtration Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.
 Glomerular Filtration Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Formation of urine by the kidney involves
Glomerular Filtration Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Formation of urine by the kidney involves
An in vitro approach to the study of macula densa-mediated
 Kidney International, Vol. 38 (1990), pp. 1206 1210 TECHNICAL NOTE An in vitro approach to the study of macula densa-mediated glomerular hemodynamics SADAYOSHI ITO and OSCAR A. CARRETERO Hypertension Research
Kidney International, Vol. 38 (1990), pp. 1206 1210 TECHNICAL NOTE An in vitro approach to the study of macula densa-mediated glomerular hemodynamics SADAYOSHI ITO and OSCAR A. CARRETERO Hypertension Research
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 25, 2013 Total POINTS: % of grade in class
 NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 25, 2013 Total POINTS: 100 20% of grade in class 1) During exercise, plasma levels of Renin increase moderately. Why should Renin levels be elevated during
NROSCI/BIOSC 1070 and MSNBIO 2070 Exam # 2 October 25, 2013 Total POINTS: 100 20% of grade in class 1) During exercise, plasma levels of Renin increase moderately. Why should Renin levels be elevated during
Renal Disease and PK/PD. Anjay Rastogi MD PhD Division of Nephrology
 Renal Disease and PK/PD Anjay Rastogi MD PhD Division of Nephrology Drugs and Kidneys Kidney is one of the major organ of drug elimination from the human body Renal disease and dialysis alters the pharmacokinetics
Renal Disease and PK/PD Anjay Rastogi MD PhD Division of Nephrology Drugs and Kidneys Kidney is one of the major organ of drug elimination from the human body Renal disease and dialysis alters the pharmacokinetics
** TMP mean page 340 in 12 th edition. Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2:
 QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
QUESTION Questions 1 and 2 Use the following clinical laboratory test results for questions 1 and 2: Urine flow rate = 1 ml/min Urine inulin concentration = 100 mg/ml Plasma inulin concentration = 2 mg/ml
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
The topic of normal vascular and glomerular anatomy is introduced
 Normal Vascular and Glomerular Anatomy Arthur H. Cohen Richard J. Glassock The topic of normal vascular and glomerular anatomy is introduced here to serve as a reference point for later illustrations of
Normal Vascular and Glomerular Anatomy Arthur H. Cohen Richard J. Glassock The topic of normal vascular and glomerular anatomy is introduced here to serve as a reference point for later illustrations of
Renal Physiology - Lectures
 Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD lharris@lsuhsc.edu Renal Physiology - Lectures Physiology of Body Fluids 2. Structure & Function of the Kidneys 3. Renal Clearance & Glomerular Filtration
Renal Physiology 2011 Lisa M. Harrison-Bernard, PhD lharris@lsuhsc.edu Renal Physiology - Lectures Physiology of Body Fluids 2. Structure & Function of the Kidneys 3. Renal Clearance & Glomerular Filtration
1. Urinary System, General
 S T U D Y G U I D E 16 1. Urinary System, General a. Label the figure by placing the numbers of the structures in the spaces by the correct labels. 7 Aorta 6 Kidney 8 Ureter 2 Inferior vena cava 4 Renal
S T U D Y G U I D E 16 1. Urinary System, General a. Label the figure by placing the numbers of the structures in the spaces by the correct labels. 7 Aorta 6 Kidney 8 Ureter 2 Inferior vena cava 4 Renal
Collin County Community College RENAL PHYSIOLOGY
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 12 Urinary System 1 RENAL PHYSIOLOGY Glomerular Filtration Filtration process that occurs in Bowman s Capsule Blood is filtered and
Role of peritubular capillary forces in the renal action of carbonic anhydrase inhibitor
 Kidney International, Vol. 30 (1986), pp. 828 835 Role of peritubular capillary forces in the renal action of carbonic anhydrase inhibitor IEKUNI ICHIKAWA and VALENTINA KON Laboratory of Renal Physiology,
Kidney International, Vol. 30 (1986), pp. 828 835 Role of peritubular capillary forces in the renal action of carbonic anhydrase inhibitor IEKUNI ICHIKAWA and VALENTINA KON Laboratory of Renal Physiology,
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
41B. Metabolism produces wastes that must be eliminated from the body. This. Renal System Physiology: Computer Simulation
 41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
Nephron Function and Urine Formation. Ms. Kula December 1, 2014 Biology 30S
 Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19
![QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19](/thumbs/77/74887026.jpg) QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion,
QUIZ/TEST REVIEW NOTES SECTION 1 RENAL PHYSIOLOGY FILTRATION [THE KIDNEYS/URINARY SYSTEM] CHAPTER 19 CHAPTER 19 Learning Objectives: Differentiate the following processes: filtration, reabsorption, secretion,
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 17 Urinary System 2 Glomerular Filtration Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Overview of Renal Physiology
BIOH122 Human Biological Science 2 Session 17 Urinary System 2 Glomerular Filtration Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Overview of Renal Physiology
Functional morphology of kidneys Clearance
 Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1)
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
Relation of distal tubular NaCl delivery and glomerular
 Kidney Internationa4 Vol. 2 (1972), p. 22 32 Relation of distal tubular NaCl delivery and glomerular hydrostatic pressure ROLAND C. BLANTZ, ARNOLD H. JSRAELIT, FLOYD C. RECTOR, JR. and DONALD W. SELDIN
Kidney Internationa4 Vol. 2 (1972), p. 22 32 Relation of distal tubular NaCl delivery and glomerular hydrostatic pressure ROLAND C. BLANTZ, ARNOLD H. JSRAELIT, FLOYD C. RECTOR, JR. and DONALD W. SELDIN
Tubule to Decreased and Increased Renal Perfusion Pressure
 1184 Response of Superficial Proximal Convoluted Tubule to Decreased and Increased Renal Perfusion Pressure In Vivo Microperfusion Study in Rats Yoshikazu Kinoshita and Franklyn G. Knox Although urinary
1184 Response of Superficial Proximal Convoluted Tubule to Decreased and Increased Renal Perfusion Pressure In Vivo Microperfusion Study in Rats Yoshikazu Kinoshita and Franklyn G. Knox Although urinary
A. Correct! Flushing acids from the system will assist in re-establishing the acid-base equilibrium in the blood.
 OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
Functions of the kidney
 Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
19. RENAL PHYSIOLOGY ROLE OF THE URINARY SYSTEM THE URINARY SYSTEM. Components and function. V BS 122 Physiology II 151 Class of 2011
 19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
Answers and Explanations
 Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
Answers and Explanations 1. The answer is D [V B 4 b]. Distal K + secretion is decreased by factors that decrease the driving force for passive diffusion of K + across the luminal membrane. Because spironolactone
Glomerular filtration during furosemide diuresis in the dog
 Kidney International, Vol. 16 (1979), pp. 672-680 Glomerular filtration during furosemide diuresis in the dog THOMAS J. BURKE and KENNETH L. DUCHIN Department of Physiology, University of Colorado Medical
Kidney International, Vol. 16 (1979), pp. 672-680 Glomerular filtration during furosemide diuresis in the dog THOMAS J. BURKE and KENNETH L. DUCHIN Department of Physiology, University of Colorado Medical
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary system. Urinary system
 INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
Urinary bladder provides a temporary storage reservoir for urine
 Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Bladder Schistosomes. Normally, urine is sterile. Presence of blood may indicate an infection.
 Bladder Schistosomes Normally, urine is sterile. Presence of blood may indicate an infection. 17.1 Introduction -Cells produce waste that can become toxic if they accumulate Functions the urinary system
Bladder Schistosomes Normally, urine is sterile. Presence of blood may indicate an infection. 17.1 Introduction -Cells produce waste that can become toxic if they accumulate Functions the urinary system
Dynamics of Glomerular Ultrafiltration in the Rat
 Dynamics of Glomerular Ultrafiltration in the Rat VIM. EFFECTS OF HEMATOCRIT By Bryan D. Myers, William M. Deen, Channing R. Robertson, and Barry M. Brenner ABSTRACT This study was undertaken in an effort
Dynamics of Glomerular Ultrafiltration in the Rat VIM. EFFECTS OF HEMATOCRIT By Bryan D. Myers, William M. Deen, Channing R. Robertson, and Barry M. Brenner ABSTRACT This study was undertaken in an effort
The functions of the kidney:
 The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
Kidney and urine formation
 Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Renal System Physiology
 M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
M58_MARI0000_00_SE_EX09.qxd 7/18/11 2:37 PM Page 399 E X E R C I S E 9 Renal System Physiology Advance Preparation/Comments 1. Prior to the lab, suggest to the students that they become familiar with the
WHILE it is generally agreed that elevation
 The Derivation of Coronary Sinus Flow During Elevation of Right Ventricular Pressure By HERMAN M. GELLER, B.S., M.D., MARTIN BRANDFONBRENEU, M.D., AND CARL J. WIGGERS, M.D., The derivation of coronary
The Derivation of Coronary Sinus Flow During Elevation of Right Ventricular Pressure By HERMAN M. GELLER, B.S., M.D., MARTIN BRANDFONBRENEU, M.D., AND CARL J. WIGGERS, M.D., The derivation of coronary
Effect of Saline Infusions on Intrarenal Distribution of Glomerular Filtrate and Proximal Reabsorption in the Dog
 Effect of Saline Infusions on Intrarenal Distribution of Glomerular Filtrate and Proximal Reabsorption in the Dog HENRY MANDIN, ARNOLD H ISRAELIT, FLOYD C REcroR, JR, and DONALD W SELDIN From the Department
Effect of Saline Infusions on Intrarenal Distribution of Glomerular Filtrate and Proximal Reabsorption in the Dog HENRY MANDIN, ARNOLD H ISRAELIT, FLOYD C REcroR, JR, and DONALD W SELDIN From the Department
Actualités néphrologiques Jean Hamburger 23 Avril Marie Courbebaisse, Service de Physiologie Hôpital Européen Georges Pompidou, Paris
 Single nephron GFR Actualités néphrologiques Jean Hamburger 23 Avril 2018 Marie Courbebaisse, Service de Physiologie Hôpital Européen Georges Pompidou, Paris Introdution Glomerular filtration and SNGFR
Single nephron GFR Actualités néphrologiques Jean Hamburger 23 Avril 2018 Marie Courbebaisse, Service de Physiologie Hôpital Européen Georges Pompidou, Paris Introdution Glomerular filtration and SNGFR
modulating the tubuloglomerular feed-back mechanism in the canine kidney; Sciences Center, 4200 East Ninth Avenue, Denver, CO 80262, U.S.A.
 J. Physiol. (1986), 380, pp. 35-43 35 With 3 text-figures Printed in Great Britain RENAL VASOCONSTRICTOR RESPONSE TO HYPERTONIC SALINE IN THE DOG: EFFECTS OF PROSTAGLANDINS, INDOMETHACIN AND THEOPHYLLINE
J. Physiol. (1986), 380, pp. 35-43 35 With 3 text-figures Printed in Great Britain RENAL VASOCONSTRICTOR RESPONSE TO HYPERTONIC SALINE IN THE DOG: EFFECTS OF PROSTAGLANDINS, INDOMETHACIN AND THEOPHYLLINE
Atrial natriuretic peptide and dopamine in a rat model of ischemic acute renal failure
 Kidney International, Vol. 35 (1989), pp. 1126 1132 Atrial natriuretic peptide and dopamine in a rat model of ischemic acute renal failure JOHN D. CONGER, SANDOR A. FALK, BRAD H. YUAN, and ROBERT W. SCHRIER
Kidney International, Vol. 35 (1989), pp. 1126 1132 Atrial natriuretic peptide and dopamine in a rat model of ischemic acute renal failure JOHN D. CONGER, SANDOR A. FALK, BRAD H. YUAN, and ROBERT W. SCHRIER
Pressure Diuresis 9 Sample Student Essays
 Pressure Diuresis 9 Sample Student Essays Below please find assembled consecutively in one document the brief analyses submitted by nine students in Mammalian Physiology 08 to the Teach Yourself Pressure
Pressure Diuresis 9 Sample Student Essays Below please find assembled consecutively in one document the brief analyses submitted by nine students in Mammalian Physiology 08 to the Teach Yourself Pressure
mid ihsan (Physiology ) GFR is increased when A -Renal blood flow is increased B -Sym. Ganglion activity is reduced C-A and B **
 (Physiology ) mid ihsan GFR is increased when A -Renal blood flow is increased B -Sym. Ganglion activity is reduced C-A and B ** Colloid pressure in the efferent arteriole is: A- More than that leaving
(Physiology ) mid ihsan GFR is increased when A -Renal blood flow is increased B -Sym. Ganglion activity is reduced C-A and B ** Colloid pressure in the efferent arteriole is: A- More than that leaving
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
Urine Formation by the Kidneys: I. Glomerular Filtration, Renal Blood Flow and Their Control.
 Urine Formation by the Kidneys: I. Glomerular Filtration, Renal Blood Flow and Their Control. Chapter 26 Yanal A Shafagoj. MD. PhD Lecture-1 Introduction 31/3/2015 1 University of Jordan Faculty of Medicine
Urine Formation by the Kidneys: I. Glomerular Filtration, Renal Blood Flow and Their Control. Chapter 26 Yanal A Shafagoj. MD. PhD Lecture-1 Introduction 31/3/2015 1 University of Jordan Faculty of Medicine
Lund, 1948), the effect of which was to produce glomerular lesions without. relationship between increased protein loads and the tubular reabsorption
 544 J. Phy8iol. (1961), 156, pp. 544-554 With 5 text-ftgure8 Printed in Great Britain TUBULAR REABSORPTION OF PROTEIN IN RATS WITH EXPERIMENTAL PROTEINURIA BY D. MENDEL* From the Department of Physiology,
544 J. Phy8iol. (1961), 156, pp. 544-554 With 5 text-ftgure8 Printed in Great Britain TUBULAR REABSORPTION OF PROTEIN IN RATS WITH EXPERIMENTAL PROTEINURIA BY D. MENDEL* From the Department of Physiology,
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
The Acute Effects of Antiglomerular Basement Membrane Antibody upon Glomerular Filtration in the Rat
 The Acute Effects of Antiglomerular Basement Membrane Antibody upon Glomerular Filtration in the Rat THE INFLUENCE OF DOSE AND COMPLEMENT DEPLETION ROLAND C. BLANTZ, Department of Medicine, University
The Acute Effects of Antiglomerular Basement Membrane Antibody upon Glomerular Filtration in the Rat THE INFLUENCE OF DOSE AND COMPLEMENT DEPLETION ROLAND C. BLANTZ, Department of Medicine, University
Physiology (6) 2/4/2018. Rahmeh Alsukkar
 Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
