Differential effect of angiotensin II on blood circulation in the renal medulla and cortex of anaesthetised rats
|
|
|
- Bryce Fitzgerald
- 5 years ago
- Views:
Transcription
1 Journal of Physiology (2002), 538.1, pp DOI: /jphysiol The Physiological Society Differential effect of angiotensin II on blood circulation in the renal medulla and cortex of anaesthetised rats Bożena Bądzyńska, Monika Grzelec-Mojzesowicz, Leszek Dobrowolski and Janusz Sadowski Laboratory of Renal and Body Fluid Physiology, Medical Research Centre of the Polish Academy of Sciences, Pawińskiego 5, Warsaw, Poland The renal medulla is sensitive to hypoxia, and a depression of medullary circulation, e.g. in response to angiotensin II (Ang II), could endanger the function of this zone. Earlier data on Ang II effects on medullary vasculature were contradictory. The effects of Ang II on total renal blood flow (RBF), and cortical and medullary blood flow (CBF and MBF: by laser-doppler flux) were studied in anaesthetised rats. Ang II infusion (30 ng kg _1 min _1 I.V.) decreased RBF 27 ± 2 % (mean ± S.E.M.), whereas MBF increased 12 ± 2 % (both P <0.001). Non-selective blockade of Ang II receptors with saralasin (3 µg kg _1 min _1 I.V.) increased RBF 12 ± 2 % and decreased MBF 8 ± 2 % (P <0.001). Blockade of AT 1 receptors with losartan (10 mg kg _1 ) increased CBF 10 ± 2 % (P <0.002) and did not change MBF. Losartan given during Ang II infusion significantly increased RBF (53 ± 7 %) and decreased MBF (27 ± 7 %). Blockade of AT 2 receptors with PD (50 µg kg _1 min _1 I.V.) did not change CBF or MBF. Intramedullary infusion of PD (10 µg min _1 ) superimposed on intravenous Ang II infusion did not change RBF, but slightly decreased MBF (4 ± 2 %, P <0.05). We conclude that in anaesthetised surgically prepared rats, exogenous or endogenous Ang II may not depress medullary circulation. In contrast to the usual vasoconstriction in the cortex, vasodilatation was observed, possibly related to secondary activation of vasodilator paracrine agents rather than to a direct action via AT 2 receptors. (Received 29 June 2001; accepted after revision 10 October 2001) Corresponding author J. Sadowski: Laboratory of Renal Physiology, Medical Research Centre, Polish Academy of Sciences, Pawińskiego 5, Warsaw, Poland. renal@cmdik.pan.pl An appropriate perfusion of the renal medulla with blood is necessary for the functional integrity of this zone and for the normal excretory and regulatory function of the whole kidney. This is of major physiological and pathophysiological importance, as the medulla is widely viewed as having a crucial role in maintaining body fluid homeostasis and in the control of arterial pressure (Cowley, 1997). The intrarenal vasculature can respond to neural and a variety of humoral stimuli with vasodilatation or vasoconstriction, resulting in increased or decreased perfusion of renal tissue, respectively. Such responses may have more serious functional consequences within the medulla than in the cortex. For instance, an increase in medullary blood flow (MBF) could result in a wash-out of solutes from the medullary interstitium, less efficient action of the countercurrent exchanger, and impaired ability of the kidney to concentrate urine (Thurau et al. 1962; Cupples & Sonnenberg, 1987). More recent studies have shown that this particular risk was exaggerated and that unrealistically large increases in MBF would be needed to significantly dissipate the medullary interstitial hypertonicity (Cupples & Sonnenberg, 1987; Sadowski et al. 1997). A decrease in blood and oxygen supply to the renal medulla could present a more serious danger as, even at normal perfusion, oxygen tension in the medullary tissue is low and the medulla appears to function at the edge of anoxia (Heyman et al. 1997). Thus, a depression of medullary circulation, e.g. following a release of renin and increased generation of angiotensin II (Ang II), could seriously endanger the medullary tissue. On the other hand, evidence has accumulated over the years to indicate that perfusion of the renal medulla is controlled separately, independent of the control of blood flow through the cortex, which represents the greater part of the renal mass. The present study was undertaken to examine whether changing activity of Ang II would affect the renal cortical and medullary circulation in a parallel fashion, and thus if high hormone activity would indeed compromise perfusion and, potentially, the function of the medulla. This subject has not yet been sufficiently elucidated, largely due to the doubtful value of early methods of measurement of the MBF or papillary blood flow (PBF). The progress in this area has accelerated following the introduction of laser-doppler (LD) methodology, and newer studies have suggested that, unlike the cortex, the medulla may not respond with vasoconstriction to
2 160 B. Ba dzyńska, M. Grzelec-Mojzesowicz, L. Dobrowolski and J. Sadowski J. Physiol exogenous Ang II (Nobes et al. 1991; Ortíz et al. 1998). To simplify interpretation, in the present work, effects of high and low Ang II level on MBF (LD flux) were examined while renal perfusion pressure (RPP) was artificially maintained constant. The study focused on the role of two Ang II receptor types, AT 1 and AT 2. We found that the medullary vasculature did not constrict with increasing or dilate with decreasing hormone concentration or action, as was simultaneously observed in the cortex. The MBF changed in the direction opposite to changes in cortical blood flow (CBF), a response that was probably related to the secondary activation of the synthesis of paracrine agents, such as prostaglandins, kinins and nitric oxide (NO). METHODS Male Wistar rats weighing g, maintained on a dry pellet diet and given free access to water, were anaesthetised with intraperitoneal thiopentabarbital (Thiopental, Biochemie GmbH, Vienna, Austria), 100 mg (kg body wt) _1. The experimental procedures were approved by the Ethical Committee of the Medical Research Centre, Polish Academy of Sciences. Throughout the surgical preparation and experimental procedures, body temperature was maintained at about 37 C by means of a heated pad. In order to compensate for fluid losses, during surgical preparation 3 % bovine albumin in Ringer solution was infused at 0.22 ml kg _1 min _1. Surgical preparations A cannula was placed in the trachea to ensure free airways. Femoral and jugular veins were cannulated for infusion of fluids and drugs. For measurement of mean arterial blood pressure, a Teflon catheter, 0.4 mm in outer diameter, was introduced into the right femoral artery. The left kidney was exposed by a subcostal flank incision and placed in a plastic holder similar to that used for micropuncture experiments; the inside of the holder was padded in such a way that the kidney s dorsal curvature (and not the side surface) was facing upwards. The ureter was cannulated to ensure drainage of the left renal pelvis. In order to keep RPP stable, a screw-controlled snare was placed on the aorta above the left renal artery. The snare was tightened slightly to lower blood pressure distal to the constriction site by 10 mmhg. Later during experiments, the snare could be loosened or tightened as needed, so that the mean blood pressure below the snare, equivalent to RPP, was maintained constant. Thereafter, a flow probe, 1 mm in diameter, was placed on the renal artery and connected with a Transonic flow meter (type T106, Transonic Systems Inc., Ithaca, NY, USA) for measurement of total renal blood flow (RBF). Subsequently, a needle laser-doppler (LD) probe (type PF 402, Perimed, Jarfalla, Sweden), for estimation of medullary blood flow (MBF), was inserted into the kidney from its dorsal surface along the cortico papillary axis. In one series of experiments, parallel to the LD probe, a stainless steel cannula (25 G) was inserted for drug infusion directly into the renal medulla. In some experimental series, total RBF was not measured. Instead, a second LD probe (type PF 407/415) was placed on the kidney surface for estimation of superficial cortical blood flow (CBF; LD flux); this measurement would not detect changes in perfusion of juxtamedullary glomeruli. To ensure a stable contact of this probe with the kidney surface, its tip was weighted and the probe was suspended on its fibre-optic lead, fastened to a horizontal rod positioned 20 cm above the kidney. The surface probe was applied exclusively in experiments in which RPP was maintained constant. In 20 preliminary experiments, the Transonic probe was placed on the renal artery and an LD probe was placed on the kidney surface for simultaneous measurement of total RBF and CBF. The percentage changes in both parameters, recorded after infusion of Ang II or saralasin, were highly correlated (r = 0.959, P <0.001). This indicated that measurement of total RBF provided a reliable estimate of the changes in perfusion of the superficial cortex. The LD probes were connected to a LD flow meter (Periflux 4001, Perimed). The system measures the LD flux (a product of the number of blood cells moving and of their mean velocity) within an area less than 1 mm 3 beneath the tip of the probes. Results are expressed in arbitrary perfusion units (PU). Thus, only relative changes are measured, but a calibration procedure enabled comparison of the results between animals. Each probe was calibrated using a motility standard, a colloidal suspension of latex particles (supplied by the manufacturer). The Brownian motion of the suspension provides the standard value of 250 PU. The biological zero flow was determined at the end of experiments after clamping the renal artery. In agreement with accepted usage, throughout this paper the cortical and medullary LD fluxes are termed flows (CBF and MBF, respectively). After experiments, the rats were killed with an overdose of the anaesthetic. The position of the LD probe in the inner medulla (close to the border with the outer medulla) and of the parallel cannula for intramedullary infusion were verified after the experiments in kidney cross-sections. Experimental procedures and protocols At the end of surgical preparations, bovine serum albumin infusion was replaced by isotonic saline solution at 0.22 ml kg _1 min _1. After placement of blood flow probes for measurement of MBF and RBF or CBF, about 1 h was allowed for Figure 1. Effect of I.V.Ang II infusion (30 ng kg _1 min _1 ) on total RBF and renal MBF (LD flux) The RPP was allowed to increase (A, n=8) or was maintained constant at 102 ± 1 mmhg (B, n =39 for RBF and n=31 for MBF). Data are presented as means ± S.D. as a percentage of the initial control value. C, control; R, post-ang II recovery. *P <0.05 or less versus control.
3 J. Physiol Angiotensin and medullary blood flow 161 stabilisation, and RPP was adjusted to a value that was maintained constant throughout the experiment (with the exception of one experimental series, see below). We have shown that in this experimental preparation and at constant RPP, CBF and MBF are stable over at least 2 h (Dobrowolski et al. 1998; Kompanowska- Jezierska et al. 2001). The baseline values were first recorded for 30 min during isotonic saline infusion. Thereafter, records were obtained during infusion of drugs and, whenever possible, after cessation of drug infusion. Effects of exogenous Ang II on RBF and MBF. Infusion of Ang II (Hypertensin, Ciba-Geigy, Switzerland) at 30 ng kg _1 min _1 I.V. was performed in two groups of rats. In eight rats (Group 1), RPP was allowed to change freely during the experiment. After obtaining control records, Ang II was infused for about 30 min, until stabilisation of MBF and RBF. Thereafter, Ang II was replaced by saline vehicle for about 20 min to record recovery of the haemodynamic parameters measured. In a large group of animals (Group 2, n =39), Ang II was infused in the same dose as above while RPP was maintained constant. The usual baseline and Ang II recordings of RBF and MBF were performed. In these animals, Ang II infusion was later used as a background for administration of other drugs (see below). Therefore, postangiotensin recovery records could not be obtained. Effects of angiotensin converting enzyme (ACE) inhibition on CBF and MBF. The angiotensin converting enzyme (ACE) inhibitor captopril (E. R. Squibb & Sons Ltd, Hounslow, UK) was administered I.V. as a bolus of 1 mg kg _1 in 0.5 ml saline given over 5 min. Captopril was also added to the maintenance saline infusion to deliver 1 mg kg _1 h _1 over min until stabilisation of CBF and MBF (n = 17). Effects of non-selective blockade of Ang II receptors by saralasin on RBF and MBF. After baseline recordings, 1-Sar, 8-Ala Ang II (saralasin), a non-selective peptide inhibitor of Ang II receptors (Roehm Pharma GmbH, Darmstadt, Germany), was infused at 3µgkg _1 min _1 I.V. for 40 min while RPP was maintained constant (n = 13). In another group, the saralasin infusion was superimposed on Ang II infusion in the usual dose (n = 17). Effects of selective blockade of Ang II receptors by losartan or PD on MBF and RBF or CBF. (1) After baseline recordings, losartan, a selective, non-peptide AT 1 angiotensin receptor antagonist (Merck & Co., Rahway, NJ, USA), was injected I.V. as a bolus of 10 mg kg _1 in 0.5 ml saline (n = 10). In another group, losartan injection was made during Ang II infusion in the usual dose (n = 10). RBF or CBF and MBF were recorded for about 20 min. (2) PD , a selective, nonpeptide AT 2 angiotensin receptor antagonist (Research Biochemicals International, Natick, MA, USA), was infused I.V. at 50 µg kg _1 min _1 for 20 min (n = 8). In another group, the drug was infused directly into the medulla at 5 10 µg min _1 (infusion rate: 17 µl min _1 ) for 20 min, superimposed on I.V. infusion of Ang II in the usual dose (n = 12). Statistics The significance of changes within one group over time was first evaluated by repeat measurement analysis of variance (ANOVA), followed by Student s t test for dependent variables. Differences in mean values between groups were evaluated by Student s t test for independent variables. Absolute values were used for statistical calculations, whereas percentage changes are shown in graphs. S.E.M. was used throughout as a measure of data dispersion; however, when the values were too small to be visible on graphs, the S.D. is shown and the appropriate information is given in the legend. RESULTS The data on the effect of exogenous Ang II on RBF and MBF are given in Fig. 1. Figure 1A shows the influence of Ang II in experiments in which RPP was allowed to increase significantly, from a control value of 113 ± 3 mmhg to 123 ± 2 mmhg under Ang II. After cessation of infusion, RPP decreased to 116 ± 2 mmhg. Ang II induced a significant 31 % decrease in RBF (from a control value of 7.7 ± 0.4 ml min _1 ), contrasted by a significant 17 % increase in MBF from a control value of 186 ± 10 PU. The recovery after withdrawal of Ang II was almost complete; however, RBF remained 4 % below control. Figure 1B shows the data pooled from all experimental groups in which Ang II was infused while RPP was maintained constant at 102 ± 1 mmhg. After Ang II, RBF decreased significantly (27 %), from a control value of 8.6 ± 0.3 ml min _1, whereas MBF increased significantly (12 %), from a control value of 171 ± 8 PU. These changes were similar to those noted without control of RPP. Ang II infusion was continued in these experiments to provide elevated background activity before administration of other drugs and post-angiotensin recovery could not be recorded. Effects of inhibition of Ang II generation with captopril on CBF and MBF in a large group of rats (n = 17) are shown in Fig. 2. CBF increased significantly about 10 %, from a control value of 539 ± 17 PU, whereas MBF remained stable at about 120 PU. Sample records of RBF and MBF responses to saralasin, a non-selective inhibitor of Ang II receptors, observed without or with background infusion of Ang II, are shown in Fig. 3. The RBF first showed a clear decrease, which was more pronounced without background Ang II infusion. This was followed by an increase, which was slower under Figure 2. Effect of I.V.captopril administration on renal CBF and MBF (LD fluxes) Captopril was given I.V. as a bolus, 1 mg kg _1, followed by an infusion of 1 mg kg _1 h _1 I.V., at constant RPP (n = 17). Data are presented as means ± S.E.M., as a percentage of the initial control value. *P < versus control.
4 162 B. Ba dzyńska, M. Grzelec-Mojzesowicz, L. Dobrowolski and J. Sadowski J. Physiol (8 %) than control, at 117 ± 8 PU, throughout saralasin infusion. When saralasin was given on a background of Ang II infusion (Fig. 4B), the initial 8 % decrease in RBF, from a control value of 6.2 ± 0.5 ml min _1, was significant but significantly smaller than in the absence of exogenous Ang II. In the second phase, RBF increased to 8.3 ± 0.5 ml min _1, significantly above control, a change greater than that seen without Ang II infusion. MBF decreased in the first phase by about 17 %, from a control value of 180 ± 7 PU, but then tended to recover, and at the end of saralasin infusion was not significantly different from control. Figure 3. Sample records of total RBF (A) and MBF (B) responses to I.V. saralasin (3 µg kg _1 min _1 ) in two experiments In one experiment, Ang II was continuously infused at 30 ng kg _1 min _1 I.V. In the other, Ang II vehicle was given. PU, LD perfusion units. Ang II, but the final level achieved was higher than that seen without hormone infusion. In the absence of exogenous Ang II, RBF stabilised at an elevated level beginning from about the sixth minute of saralasin infusion. MBF decreased after saralasin, similarly in the presence or absence of exogenous Ang II; however, the recovery that followed was only partial, and MBF remained low throughout saralasin infusion. All the data on the effects of saralasin infusion are collected in Fig. 4. In the absence of exogenous Ang II (Fig. 4A), RBF decreased about 15 %, from a control value of 8.0 ± 0.9 ml min _1, a change similar to that seen for MBF. Subsequently, RBF increased to a value significantly higher than control (8.7 ± 1.0 ml min _1 ), whereas MBF recovered only slightly and remained significantly lower Selective inhibition of angiotensin AT 1 receptors with losartan moderately but significantly increased CBF, by 10 % from a control value of 440 ± 23 PU, without affecting MBF (Fig. 5A). Losartan was also administered during infusion of Ang II; in this series, total RBF was measured instead of CBF (Fig. 5B). After losartan, RBF increased 53 %, from a control value of 6.4 ± 0.6 ml min _1, significantly more than did CBF in the absence of exogenous Ang II. In this series, MBF decreased significantly (21 %), from a control value of 163 ± 13 PU. PD , a relatively selective inhibitor of angiotensin AT 2 receptors, did not change RBF or CBF, either with I.V. PD infusion or with the intramedullary infusion superimposed on I.V. Ang II infusion (data not shown). After I.V. PD , MBF tended to decrease (not significant; Fig. 6). After intramedullary infusion of the drug, a slight (4 %) decrease in MBF, from 187 ± 17 to 179 ± 16 PU, proved significant (P =0.03; Fig. 6). DISCUSSION The present study extensively documents increased perfusion of the renal medulla in response to a moderate pressor dose of Ang II, in contrast to the well established Figure 4. Biphasic effect of I.V.saralasin (S, 3 µg kg _1 min _1 ) on total RBF and MBF A, data recorded with Ang II vehicle (n = 13). B, data recorded with background Ang II infusion, 30 ng kg _1 min _1 I.V. (n = 17). The RPP was maintained constant. S-1, S-2, early and final effects of saralasin, respectively. Means ± S.D. for values expressed as a percentage of the pre-saralasin control value (C). *P < 0.05 or less versus pre-saralasin control. P < or less versus the change in the corresponding value measured without background Ang II infusion (A).
5 J. Physiol Angiotensin and medullary blood flow 163 Figure 5. Effects of i.v. losartan on renal haemodynamics A, effects of losartan, 10 mg kg _1 I.V. (n = 10), on renal CBF and MBF. B, total RBF and MBF after losartan given in the same dose during Ang II infusion, 30 ng kg _1 min _1 I.V. (n = 10). Data are presented as means ± S.D., as a percentage of the pre-losartan control value. *P < 0.05 or less versus pre-losartan control. P <0.001 versus the CBF value measured after losartan alone (as shown in A). vasoconstrictor action of the hormone within the cortex. Both when RPP was allowed to increase, and when it was maintained constant, the total RBF, representing largely perfusion of the cortex, decreased after Ang II, whereas the MBF showed a significant increase. Previous data on the response of the intrarenal circulation to angiotensin were not uniform. In agreement with our results, all studies reported a dose-dependent decrease in total RBF or CBF. When perfusion of the medulla was measured separately, a number of workers reported no change in MBF or PBF (Huang et al. 1991; Mattson et al. 1991; Nobes et al. 1991; Parekh & Zou 1996). A medullary vasodilatation in response to Ang II was also seen. Nobes et al. (1991), who positioned an LD probe over the exposed papilla of young rats, noted an increase in PBF, albeit only with the highest dose used (300 ng kg _1 min _1 ); no change was seen at 100 ng kg _1 min _1. Using similar methodology (an external LD probe over the exposed papilla) in adult rats, Ortiz et al. (1998) were able to demonstrate a dosedependent increase in PBF both at 100 and 300 ng kg _1 min _1. It is apparent that in studies involving exposure of the papilla, variability of the results may relate to the use of rats of different size (age) or strain. As in the two studies mentioned above (Nobes et al. 1991; Ortiz et al. 1998), we measured regional renal perfusion by the LD technique. However, we used an internal probe placed in the inner (white) medulla, close to the border with the outer medullary zone. This approach does not require exposure of the papilla, a manoeuvre that can alter papillary circulation and its responsiveness to any stimuli (Pallone et al. 1990). Perhaps due to a relatively high sensitivity of our experimental preparation, we were able to demonstrate a definite increase in MBF with an Ang II dose as low as 30 ng kg _1 min _1. The absence of a depression of medullary circulation after Ang II, as documented in the above quoted and our own whole kidney studies, does not accord with evidence from work using a variety of other experimental preparations, such as split hydronephrotic kidney, isolated perfused juxtaglomerular nephron, isolated renal arterial vessels of different calibre, as well as from measurements of blood cell velocity in visualised vasa recta of an exposed papilla; virtually all of these studies showed constriction of medullary vessels after Ang II (reviewed in Pallone et al. 1990; Navar et al. 1996; Arendshorst et al. 1999). This indicates that the medullary vasculature has the potential to respond to Ang II in the same way as is seen in the cortex; however, vasoconstriction does not manifest itself in many whole animal studies, with the kidney functioning in the natural milieu of the organism. The majority of data indicate that inhibition of ACE results in dilatation of both the cortical and medullary vasculature (reviewed in Pallone et al. 1990). This is at variance with Figure 6. The renal MBF responses to PD (PD) PD was given I.V., 50 µg kg _1 min _1 (PD; n =8), or infused directly into the medullary interstitium, 5 10 µg min _1, during background infusion of Ang II (30 ng kg _1 min _1 I.V.) (Ang II + PD; n=12). The RPP was maintained constant in both groups. Data are presented as means ± S.E.M. as a percentage of the pre-pd control value. *P <0.05 versus pre-pd control.
6 164 B. Ba dzyńska, M. Grzelec-Mojzesowicz, L. Dobrowolski and J. Sadowski J. Physiol our present finding of no change of MBF after captopril, as opposed to a clear increase in CBF. However, since ACE is also a kininase, the concentration of vasodilator kinins would increase after captopril administration. Indeed, there is long-standing evidence for interaction of Ang II and kinins in the control of PBF (Roman et al. 1988). Since in our experience, exogenous Ang II dilated medullary vasculature, elimination of the hormone after captopril could be expected to cause vasoconstriction. Possibly, such an action was, indeed, induced but the effect was offset by an accumulation of the vasodilator bradykinin, and the final result was no change in MBF. In a recent study in the dog, a blockade of bradykinin B 2 receptors abolished the effect of ACE inhibition on MBF (Omoro et al. 2000). In order to further explore the role of endogenous Ang II in the control of regional renal circulation, we examined effects of non-selective blockade of both AT 1 and AT 2 angiotensin receptors with saralasin. It is remarkable that an expected increase of RBF after saralasin was preceded by a significant decrease. This probably reflected the known agonistic properties of saralasin and other similar peptides (Hollenberg, 1979; Harris et al. 1986; Walker et al. 1986); the agonistic action was reported to be more pronounced when endogenous Ang II levels were low and to subside within about 15 min. These observations agree with our data showing that the initial decrease in RBF was less pronounced when saralasin was given during Ang II infusion and was, indeed, transient (Fig. 4). It is remarkable that the biphasic, first agonistic and later antagonistic, action of saralasin did not become apparent in the medulla, an observation that confirms the view of different responsiveness of this zone to Ang II. The ultimate effect of non-selective blockade of Ang II receptors with saralasin vasodilatation within the cortex and a tendency towards, or overt, vasoconstriction within the medulla fits the overall pattern of dissociated responses of the cortical versus the medullary vasculature, which emerged from our studies with exogenous Ang II and with captopril. Collectively, the data provide coherent evidence that the net vasoconstrictor action of both exogenous and endogenous hormone is confined to the cortical vasculature. The mechanisms underlying the unusual influence of Ang II on medullary vasculature are not clear. One possibility is the hormone s action via AT 2 receptors. Whereas earlier literature emphasised their role in growth processes in the fetus, there is now increasing evidence that AT 2 receptors can mediate systemic vasodilatation and lower arterial blood pressure (de Gasparo & Siragy, 1999). However, a direct angiotensin-dependent vasodilatation in the medulla might become apparent only if the action mediated by AT 2 receptors prevailed over vasoconstriction dependent on stimulation of AT 1 receptors. This is unlikely, as the density of AT 2 receptors in the kidney seems to be much lower than that of AT 1. Thus, AT 2 receptor-dependent vasodilatation could probably be demonstrated only when AT 1 receptors have been blocked (Csikós et al. 1998; Matsubara, 1998; Arendshorst et al. 1999). Ang II-induced vasodilatation, as seen in the medulla, could also have been indirect, dependent on stimulation by this hormone of the biosynthesis of vasodilator agents, such as prostaglandins, kinins or NO. It has been established that the rate of synthesis and tissue concentration of prostaglandins are much higher in the medulla compared with the cortex, and Ang II stimulates prostaglandin synthesis via AT 1 receptors (Siragy & Carey, 1996). A potential vasodilator role of kinins is suggested by the observation that an increase in PBF in response to Ang II was prevented by inhibition of kallikrein (Nobes et al. 1991). Still another possible mediator of vasodilatation could be NO. First, exogenous Ang II was reported to increase NO concentration in renal tissue more effectively in the medulla (Zou et al. 1997). Furthermore, inhibition of NO synthesis prevented an increase in perfusion of the medulla after Ang II (Zou et al. 1997; Ortíz et al. 1998). It was proposed that stimulation of NO synthesis by Ang II is mediated by AT 2 receptors (Siragy & Carey, 1997); however, there is also evidence that AT 1 and other illdefined Ang II or angiotensin (1 7) receptors may also be involved (Ferrario et al. 1998; Thorup et al. 1999). In order to further investigate the mechanism of medullary vasodilatation after angiotensin, we examined renal regional circulatory responses to losartan, a selective antagonist of AT 1 receptors, and to PD , a relatively selective AT 2 antagonist. As expected, losartan increased perfusion of the cortex and, as observed with saralasin, the increase was greater in the presence of high Ang II concentration induced by I.V. infusion (Figs 4 and 5). In this study, both saralasin and losartan caused a decrease in medullary perfusion, an effect compatible with the increase seen in the present studies after exogenous Ang II. Obviously, the decrease in MBF after losartan cannot be explained by abolition of a direct action of Ang II on AT 1 receptors in the medullary vasculature, and indirect mechanisms discussed above must be invoked. The blockade of these receptors would also eliminate stimulation by Ang II of the biosynthesis of prostaglandin E 2 (PGE 2 ) and remove its tonic vasodilator influence on medullary vasculature, leading ultimately to vasoconstriction. Indeed, losartan was found to decrease renal interstitial fluid PGE 2 concentration (Siragy et al. 1996). The effect would be more pronounced at high baseline Ang II concentration, as was, indeed, observed when losartan was administered during Ang II infusion (Fig. 5). There is ample evidence for a tonic vasodilator influence of prostaglandins on the medullary circulation (Knox & Granger, 1992); in our acute experiments with
7 J. Physiol Angiotensin and medullary blood flow 165 anaesthetised rats, indomethacin always reduced MBF (Sadowski et al. 1997; Kompanowska-Jezierska et al. 1999). On the other hand, Nobes et al. (1991) showed that, at least in young rats with exposed papilla, Ang II-induced increase in PBF could not be prevented by indomethacin. Since the stimulation of kinins by Ang II seems to be mediated by non-at 1, possibly by AT 2, receptors (Siragy et al. 1996; Siragy, 2000), the decrease of MBF after losartan cannot be explained by the elimination of a tonic vasodilator action of kinins. It is not clear why losartan decreased MBF distinctly more than did saralasin; obviously, a confounding factor was that the latter displayed a transition in time from AT 1 agonistic to antagonistic properties as described above. Effects of blockade of angiotensin AT 2 receptors on regional renal circulation detected in this study were nonexistent to modest. After I.V. infusion of PD , a selective AT 2 antagonist, neither RBF nor MBF changed. In order to provide a fairly high concentration of the drug in the medulla, without provoking possible extrarenal effects resulting from a high concentration in systemic blood, PD was infused directly into the medulla. Moreover, to enhance any potential effect of receptor blockade, the baseline plasma concentration of Ang II was first raised by its I.V. infusion. Under such conditions, a slight decrease in MBF (but not in RBF) was seen and proved significant. These results suggest that in acute experiments with anaesthetised surgically prepared rats, the vasodilator activity of AT 2 receptors, whether direct or mediated by NO and/or kinins, is minor. Nevertheless, this is the first demonstration of an effect of AT 2 antagonism on regional RBF in normal rats. A tendency (insignificant) to a decrease in RBF after an I.V. dose of PD sixfold higher than we used was reported in the anaesthetised dog (Keiser et al. 1992). It should be noted that in this study, a dose comparable to ours appeared biologically active; for instance, it significantly reduced urine osmolality. This result is not compatible with our observation of a decrease in MBF: such a change could possibly enhance urine concentration due to a more effective action of the countercurrent exchanger and an increase in medullary hypertonicity. In summary, our results document opposite vascular effects of exogenous and endogenous Ang II on the circulation in the renal cortex and medulla. The focus of the study was on the role of AT 1 and AT 2 receptors; it was shown that differential regional responses cannot simply be explained by constriction or relaxation of the vascular smooth muscle as a direct consequence of the different activation status of two angiotensin receptor types. The present data suggest very strongly that activation of the renin angiotensin system, as observed under conditions of arterial hypotension, hypovolaemia or sodium deficit or following a variety of stressful stimuli, would probably not compromise perfusion of the renal medulla with blood. This is of major importance, as even under normal conditions, the medullary tissue has only a small reserve of oxygen and, indeed, appears to function on the verge of hypoxia (Heyman et al. 1997). REFERENCES ARENDSHORST, W. J., BRÄNNSTRÖM, K. & RUAN, X. (1999). Action of angiotensin II on the renal microvasculature. Journal of the American Society of Nephrology 10, S COWLEY, A. W. (1997). Role of the renal medulla in volume and arterial blood pressure regulation. American Journal of Physiology 273, R1 15. CSIKÓS, T., CHUNG, O. & UNGER, TH. (1998). Receptors and their classification: Focus on angiotensin II and the AT2 receptor. Journal of Human Hypertension 12, CUPPLES, W. A. & SONNENBERG, H. (1987). Renal medullary plasma flow rate and reabsorption of salt and water from inner medullary collecting duct. Canadian Journal of Physiology and Pharmacology 65, DE GASPARO, M. & SIRAGY, H. M. (1999). The AT2 receptor: fact, fancy and fantasy. Regulatory Peptides 81, DOBROWOLSKI, L., BĄDZYŃSKA, B., WALKOWSKA, A. & SADOWSKI, J. (1998). Osmotic hypertonicity of the renal medulla during changes in renal perfusion pressure in the rat. Journal of Physiology 508, FERRARIO, C. M., CHAPPEL, M. C., DEAN, R. H. & IYER, S. N. (1998). Novel angiotensin peptides regulate blood pressure, endothelial function, and natriuresis. Journal of the American Society of Nephrology 9, HARRIS, P. J., MITCHELL, K. D. & MUNRO, J. O. (1986). Sar 1-leu 8- angiotensin II reverses the effect of captopril on renal function in rats. Clinical and Experimental Pharmacology and Physiology 13, HEYMAN, S. N., ROSEN, S. & BREZIS, M. (1997). The renal medulla: Life at the edge of anoxia. Blood Purification 15, HOLLENBERG, N. K. (1979). Pharmacologic interruption of the renin-angiotensin system. Annual Review of Pharmacology and Toxicology 19, HUANG, C., DAVIS, G. & JOHNS, E. J. (1991). A study of the action of angiotensin II on perfusion through the cortex and papilla of the rat kidney. Experimental Physiology 76, KEISER, J. A., BJOERK, F. A., HODGES, J. C. & TAYLOR, D. G. JR (1992). Renal hemodynamic and excretory responses to PD and losartan, nonpeptide AT1 and AT2 subtype-specific angiotensin II ligands. Journal of Pharmacology and Experimental Therapeutics 262, KNOX, F. G. & GRANGER, J. P. (1992). Control of sodium excretion: An integrative approach. In Renal Physiology, ed. WINDHAGER, E. E., pp Oxford University Press, New York and Oxford. KOMPANOWSKA-JEZIERSKA, E., WALKOWSKA, A., JOHNS, E. J. & SADOWSKI, J. (2001). Early effects of renal denervation in the anaesthetised rat: Natriuresis and increased cortical blood flow. Journal of Physiology 531, KOMPANOWSKA-JEZIERSKA, E., WALKOWSKA, A. & SADOWSKI, J. (1999). Exaggerated volume expansion natriuresis in rats preloaded with hypertonic saline: A paradoxical enhancement by inhibition of prostaglandin synthesis. Acta Physiologica Scandinavica 167,
8 166 B. Ba dzyńska, M. Grzelec-Mojzesowicz, L. Dobrowolski and J. Sadowski J. Physiol MATSUBARA, H. (1998). Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circulation Research 83, MATTSON, D. L., RAFF, H. & ROMAN, R. J. (1991). Influence of angiotensin II on pressure natriuresis and renal hemodynamics in volume-expanded rats. American Journal of Physiology 260, R NAVAR, L. G., INSCHO, E. W., MAJID, D. S. A., IMIG, J. D., HARRISON- BERNARD, L. M. & MITCHELL, K. D. (1996). Paracrine regulation of the renal microcirculation. Physiological Reviews 76, NOBES, M. S., HARRIS, P. J., YAMADA, H. & MENDELSOHN, F. A. O. (1991). Effects of angiotensin on renal cortical and papillary blood flows measured by laser-doppler flowmetry. American Journal of Physiology 261, F OMORO, S. A., MAJID, D. S., EL DAHR, S. S. & NAVAR, L. G. (2000). Roles of Ang II and bradykinin in the renal regional blood flow responses to ACE inhibition in sodium-depleted dogs. American Journal of Physiology Renal Physiology 279, F ORTÍZ, M. C., FORTEPIANI, L. A., RUIZ-MARCOS, F. M., ATUCHA, N. M. & GARCÍA-ESTAŃ, J. (1998). Role of AT1 receptors in the renal papillary effects of acute and chronic nitric oxide inhibition. American Journal of Physiology 274, R PALLONE, T. L., ROBERTSON, C. R. & JAMISON, R. L. (1990). Renal medullary microcirculation. Physiological Reviews 70, PAREKH, N. & ZOU, A. P. (1996). Role of prostaglandins in renal medullary circulation: Responses to different vasoconstrictors. American Journal of Physiology 271, F ROMAN, R. J., KALDUNSKI, M. L., SCICLI, A. G. & CARRETERO, O. A. (1988). Influence of kinins and angiotensin II on the regulation of papillary blood flow. American Journal of Physiology 255, F SADOWSKI, J., KOMPANOWSKA-JEZIERSKA, E., DOBROWOLSKI, L., WALKOWSKA, A. & BĄDZYŃSKA, B. (1997). Simultaneous recording of tissue ion content and blood flow in rat renal medulla: Evidence on interdependence. American Journal of Physiology 273, F SIRAGY, H. M. (2000). AT 1 and AT 2 receptors in the kidney; role in disease and treatment. American Journal of Kidney Diseases 6, S4 S9. SIRAGY, H. M. & CAREY, R. M. (1996). The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3,5- monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. Journal of Clinical Investigation 97, SIRAGY, H. M. & CAREY, R. M. (1997). The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. Journal of Clinical Investigation 100, SIRAGY, H. M., JAFFA, A. A., MARGOLIUS, H. S. & CAREY, R. M. (1996). Renin-angiotensin system modulates renal bradykinin production. American Journal of Physiology 271, R THORUP, C., KORNFELD, M., GOLIGORSKY, M. S. & MOORE, L. C. (1999). AT1 receptor inhibition blunts angiotensin II-stimulated nitric oxide release in renal arteries. Journal of the American Society of Nephrology 10, S THURAU, K., DEETJEN, P. & GÜNZLER, H. (1962). Die Diurese bei arteriellen Drucksteigerungen. Bedeutung der Haemodynamik des Nierenmarks für die Harnkonzentrierung. Pflügers Archiv für die Gesamte Physiologie des Menschen und Tiere 274, WALKER, L. A., GELLAI, M. & VALTIN, H. (1986). Renal response to pentobarbital anesthesia in rats: Effect of interrupting the reninangiotensin system. Journal of Pharmacology and Experimental Therapeutics 236, ZOU, A., WU, F. & COWLEY, A. W. JR. (1997). Protective effect of angiotensin II-induced increase in nitric oxide in the renal medullary circulation. Hypertension 31, Acknowledgements Losartan was kindly supplied by Dr Ronald D. Smith of Merck Research Laboratories, Rahway, NJ, USA.
The effects of angiotensin II on blood perfusion in the rat renal papilla
 9325 Journal of Physiology (1999), 519.1, pp. 273 278 273 The effects of angiotensin II on blood perfusion in the rat renal papilla L. L. Walker, A. A. J. Rajaratne, J. R. Blair-West and P. J. Harris Department
9325 Journal of Physiology (1999), 519.1, pp. 273 278 273 The effects of angiotensin II on blood perfusion in the rat renal papilla L. L. Walker, A. A. J. Rajaratne, J. R. Blair-West and P. J. Harris Department
A STUDY OF THE ACTION OF ANGIOTENSIN II ON PERFUSION THROUGH THE CORTEX AND PAPILLA OF THE RAT KIDNEY
 Experimental Physiology (1991) 76, 787-798 Printed in Great Britain A STUDY OF THE ACTION OF ANGIOTENSIN II ON PERFUSION THROUGH THE CORTEX AND PAPILLA OF THE RAT KIDNEY CHUNLONG HUANG*, GERARD DAVIS AND
Experimental Physiology (1991) 76, 787-798 Printed in Great Britain A STUDY OF THE ACTION OF ANGIOTENSIN II ON PERFUSION THROUGH THE CORTEX AND PAPILLA OF THE RAT KIDNEY CHUNLONG HUANG*, GERARD DAVIS AND
The ability of the kidneys to regulate extracellular fluid volume by altering sodium
 REGULATION OF EXTRACELLULAR FLUID VOLUME BY INTEGRATED CONTROL OF SODIUM EXCRETION Joey P. Granger Department of Physiology and Biophysics, University of Mississippi Medical Center, Jackson, Mississippi
REGULATION OF EXTRACELLULAR FLUID VOLUME BY INTEGRATED CONTROL OF SODIUM EXCRETION Joey P. Granger Department of Physiology and Biophysics, University of Mississippi Medical Center, Jackson, Mississippi
modulating the tubuloglomerular feed-back mechanism in the canine kidney; Sciences Center, 4200 East Ninth Avenue, Denver, CO 80262, U.S.A.
 J. Physiol. (1986), 380, pp. 35-43 35 With 3 text-figures Printed in Great Britain RENAL VASOCONSTRICTOR RESPONSE TO HYPERTONIC SALINE IN THE DOG: EFFECTS OF PROSTAGLANDINS, INDOMETHACIN AND THEOPHYLLINE
J. Physiol. (1986), 380, pp. 35-43 35 With 3 text-figures Printed in Great Britain RENAL VASOCONSTRICTOR RESPONSE TO HYPERTONIC SALINE IN THE DOG: EFFECTS OF PROSTAGLANDINS, INDOMETHACIN AND THEOPHYLLINE
Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats
 Am J Physiol Renal Physiol 279: F319 F325, 2000. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats CHI-TARNG WANG, SO YEON CHIN, AND L. GABRIEL NAVAR Department
Am J Physiol Renal Physiol 279: F319 F325, 2000. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats CHI-TARNG WANG, SO YEON CHIN, AND L. GABRIEL NAVAR Department
It is well recognized that the kidney plays a fundamental
 337 Renal Medullary Captopril Delivery Lowers Blood Pressure in Spontaneously Hypertensive Rats Shanhong Lu, David L. Mattson, Allen W. Cowley, Jr Abstract We examined the contribution of renal medullary
337 Renal Medullary Captopril Delivery Lowers Blood Pressure in Spontaneously Hypertensive Rats Shanhong Lu, David L. Mattson, Allen W. Cowley, Jr Abstract We examined the contribution of renal medullary
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
Studies on the Mechanism of Sodium Excretion during Drug-induced Vasodilatation in the Dog
 Studies on the Mechanism of Sodium Excretion during Drug-induced Vasodilatation in the Dog STEPHEN Z. FADEM, GUILLERMO HERNANDEZ-LLAMAS, RAM V. PATAK, STEVEN G. ROSENBLATT, MEYER D. LIFSCHITZ, and JAY
Studies on the Mechanism of Sodium Excretion during Drug-induced Vasodilatation in the Dog STEPHEN Z. FADEM, GUILLERMO HERNANDEZ-LLAMAS, RAM V. PATAK, STEVEN G. ROSENBLATT, MEYER D. LIFSCHITZ, and JAY
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY
 RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
RENAL PHYSIOLOGY DR.CHARUSHILA RUKADIKAR ASSISTANT PROFESSOR PHYSIOLOGY GROSS ANATOMY Location *Bean-shaped *Retroperitoneal *At level of T12 L1 vertebrae. *The right kidney lies slightly inferior to left
Chapter 25 The Urinary System
 Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
Chapter 25 The Urinary System 10/30/2013 MDufilho 1 Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph Gluconeogenesis
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
Basic Functions of the Kidneys
 Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Excretory Lecture Test Questions Set 1
 Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1
 BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
Angiotensin II and prostaglandins in control of vasa recta blood flow
 Angiotensin II and prostaglandins in control of vasa recta blood flow W. A. CUPPLES, T. SAKAI, AND D. J. MARSH Department of Physiology and Biophysics, University of Southern California, Los Angeles, California
Angiotensin II and prostaglandins in control of vasa recta blood flow W. A. CUPPLES, T. SAKAI, AND D. J. MARSH Department of Physiology and Biophysics, University of Southern California, Los Angeles, California
Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla.
 2011-2-21 Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla. Oxygen delivery = Blood flow CaO 2 Where Blood flow determined by (arterial
2011-2-21 Outline the functional anatomy, and the physiological factors, that determine oxygen delivery to the renal medulla. Oxygen delivery = Blood flow CaO 2 Where Blood flow determined by (arterial
Urinary System kidneys, ureters, bladder & urethra
 Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate
 Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Renal physiology The kidneys Allow us to live on dry land. Body fluid volume is small (~5L (blood + serum)) Composition can change rapidly e.g. due to increase in metabolic rate Kidneys maintain composition
Acute Kidney Injury. APSN JSN CME for Nephrology Trainees May Professor Robert Walker
 Acute Kidney Injury APSN JSN CME for Nephrology Trainees May 2017 Professor Robert Walker Kidney International (2017) 91, 1033 1046; http://dx.doi.org/10.1016/ j.kint.2016.09.051 Case for discussion 55year
Acute Kidney Injury APSN JSN CME for Nephrology Trainees May 2017 Professor Robert Walker Kidney International (2017) 91, 1033 1046; http://dx.doi.org/10.1016/ j.kint.2016.09.051 Case for discussion 55year
Questions? Homework due in lab 6. PreLab #6 HW 15 & 16 (follow directions, 6 points!)
 Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Questions? Homework due in lab 6 PreLab #6 HW 15 & 16 (follow directions, 6 points!) Part 3 Variations in Urine Formation Composition varies Fluid volume Solute concentration Variations in Urine Formation
Functions of the kidney
 Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
What would be the response of the sympathetic system to this patient s decrease in arterial pressure?
 CASE 51 A 62-year-old man undergoes surgery to correct a herniated disc in his spine. The patient is thought to have an uncomplicated surgery until he complains of extreme abdominal distention and pain
CASE 51 A 62-year-old man undergoes surgery to correct a herniated disc in his spine. The patient is thought to have an uncomplicated surgery until he complains of extreme abdominal distention and pain
BIOL2030 Human A & P II -- Exam 6
 BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
BIOL2030 Human A & P II -- Exam 6 Name: 1. The kidney functions in A. preventing blood loss. C. synthesis of vitamin E. E. making ADH. B. white blood cell production. D. excretion of metabolic wastes.
Physiology Lecture 2. What controls GFR?
 Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Physiology Lecture 2 Too much blood is received by the glomerular capillaries, this blood contains plasma, once this plasma enters the glomerular capillaries it will be filtered to bowman s space. The
Blood Pressure Fox Chapter 14 part 2
 Vert Phys PCB3743 Blood Pressure Fox Chapter 14 part 2 T. Houpt, Ph.D. 1 Cardiac Output and Blood Pressure How to Measure Blood Pressure Contribution of vascular resistance to blood pressure Cardiovascular
Vert Phys PCB3743 Blood Pressure Fox Chapter 14 part 2 T. Houpt, Ph.D. 1 Cardiac Output and Blood Pressure How to Measure Blood Pressure Contribution of vascular resistance to blood pressure Cardiovascular
describe the location of the kidneys relative to the vertebral column:
 Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System kidneys, ureters, bladder & urethra
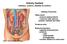 Urinary System kidneys, ureters, bladder & urethra Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Kidney Function Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Kidney Function Endocrine functions regulates
Other Factors Affecting GFR. Chapter 25. After Filtration. Reabsorption and Secretion. 5 Functions of the PCT
 Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Other Factors Affecting GFR Chapter 25 Part 2. Renal Physiology Nitric oxide vasodilator produced by the vascular endothelium Adenosine vasoconstrictor of renal vasculature Endothelin a powerful vasoconstrictor
Renal Blood flow; Renal Clearance. Dr Sitelbanat
 Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
Renal Blood flow; Renal Clearance Dr Sitelbanat Objectives At the end of this lecture student should be able to describe: Renal blood flow Autoregulation of GFR and RBF Regulation of GFR The Calcuation
Nephron Anatomy Nephron Anatomy
 Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Kidney Functions: (Eckert 14-17) Mammalian Kidney -Paired -1% body mass -20% blood flow (Eckert 14-17) -Osmoregulation -Blood volume regulation -Maintain proper ion concentrations -Dispose of metabolic
Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D.
 Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Why body sodium content determines ECF volume and the relationships
Regulation of Body Fluids: Na + and Water Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. Why body sodium content determines ECF volume and the relationships
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
Urinary System. consists of the kidneys, ureters, urinary bladder and urethra
 Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Chapter 19 The Urinary System Fluid and Electrolyte Balance
 Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Chapter 19 The Urinary System Fluid and Electrolyte Balance Chapter Outline The Concept of Balance Water Balance Sodium Balance Potassium Balance Calcium Balance Interactions between Fluid and Electrolyte
Kidney and urine formation
 Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
Kidney and urine formation Renal structure & function Urine formation Urinary y concentration and dilution Regulation of urine formation 1 Kidney and urine formation 1.Renal structure & function 1)General
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
BIOL 2402 Fluid/Electrolyte Regulation
 Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
Dr. Chris Doumen Collin County Community College BIOL 2402 Fluid/Electrolyte Regulation 1 Body Water Content On average, we are 50-60 % water For a 70 kg male = 40 liters water This water is divided into
MAJOR FUNCTIONS OF THE KIDNEY
 MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
Chapter 23. The Nephron. (functional unit of the kidney
 Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Blood Pressure Regulation 2. Faisal I. Mohammed, MD,PhD
 Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Blood Pressure Regulation 2. Faisal I. Mohammed, MD,PhD
 Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Blood Pressure Regulation 2 Faisal I. Mohammed, MD,PhD 1 Objectives Outline the intermediate term and long term regulators of ABP. Describe the role of Epinephrine, Antidiuretic hormone (ADH), Renin-Angiotensin-Aldosterone
Osmoregulation and Renal Function
 1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
1 Bio 236 Lab: Osmoregulation and Renal Function Fig. 1: Kidney Anatomy Fig. 2: Renal Nephron The kidneys are paired structures that lie within the posterior abdominal cavity close to the spine. Each kidney
RENAL PHYSIOLOGY. Zekeriyya ALANOGLU, MD, DESA Ahmet Onat Bermede, MD, Ankara University School of Medicine Dept. Anesthesiology and ICM
 RENAL PHYSIOLOGY Zekeriyya ALANOGLU, MD, DESA Ahmet Onat Bermede, MD, Ankara University School of Medicine Dept. Anesthesiology and ICM Kidneys Stabilize the composition of the ECF (electrolyte, H
RENAL PHYSIOLOGY Zekeriyya ALANOGLU, MD, DESA Ahmet Onat Bermede, MD, Ankara University School of Medicine Dept. Anesthesiology and ICM Kidneys Stabilize the composition of the ECF (electrolyte, H
Counter-Current System Regulation of Renal Functions
 Counter-Current System Regulation of Renal Functions Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most
Counter-Current System Regulation of Renal Functions Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most
Kidney Structure. Renal Lobe = renal pyramid & overlying cortex. Renal Lobule = medullary ray & surrounding cortical labryinth.
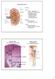 Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
RENAL PHYSIOLOGY. Zekeriyya ALANOGLU, MD, DESA. Ahmet Onat Bermede, MD. Ankara University School of Medicine Dept. Anesthesiology and ICM
 RENAL PHYSIOLOGY Zekeriyya ALANOGLU, MD, DESA. Ahmet Onat Bermede, MD. Ankara University School of Medicine Dept. Anesthesiology and ICM Kidneys Stabilize the composition of the ECF (electrolyte,
RENAL PHYSIOLOGY Zekeriyya ALANOGLU, MD, DESA. Ahmet Onat Bermede, MD. Ankara University School of Medicine Dept. Anesthesiology and ICM Kidneys Stabilize the composition of the ECF (electrolyte,
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Beginning the renal physiology section with a detailed consideration of renal
 REGULATION OF RENAL HEMODYNAMICS L. Gabriel Navar Department of Physiology, Tulane University School of Medicine, New Orleans, Louisiana 70112 Beginning the renal physiology section with a detailed consideration
REGULATION OF RENAL HEMODYNAMICS L. Gabriel Navar Department of Physiology, Tulane University School of Medicine, New Orleans, Louisiana 70112 Beginning the renal physiology section with a detailed consideration
URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY
 URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY Dilute and concentrated urine 1-Dilute urine : Nephron function continuous reabsorption. Solutes while failing to reabsorbe water in distal tubule and
URINE CONCENTRATION AND REGULATION OF ECF OSMOLARITY Dilute and concentrated urine 1-Dilute urine : Nephron function continuous reabsorption. Solutes while failing to reabsorbe water in distal tubule and
(Received 22 July 1957) It is now generally accepted that the unequal distribution of ions between cells
 190 J. Physiol. (I958) I40, I90-200 THE EFFECT OF ALTERATIONS OF PLASMA SODIUM ON THE SODIUM AND POTASSIUM CONTENT OF MUSCLE IN THE RAT By F. 0. DOSEKUN AND D. MENDEL From the Department of Physiology,
190 J. Physiol. (I958) I40, I90-200 THE EFFECT OF ALTERATIONS OF PLASMA SODIUM ON THE SODIUM AND POTASSIUM CONTENT OF MUSCLE IN THE RAT By F. 0. DOSEKUN AND D. MENDEL From the Department of Physiology,
Sunday, July 17, 2011 URINARY SYSTEM
 URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
URINARY SYSTEM URINARY SYSTEM Let s take a look at the anatomy first! KIDNEYS: are complex reprocessing centers where blood is filtered through and waste products are removed. Wastes and extra water become
Interactions between neural and hormonal mediators of renal vascular tone in anaesthetized rabbits
 Interactions between neural and hormonal mediators of renal vascular tone in anaesthetized rabbits Sarah-Jane Guild *, Carolyn J. Barrett, Roger G. Evans and Simon C. Malpas Circulatory Control Laboratory,
Interactions between neural and hormonal mediators of renal vascular tone in anaesthetized rabbits Sarah-Jane Guild *, Carolyn J. Barrett, Roger G. Evans and Simon C. Malpas Circulatory Control Laboratory,
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Diagram of the inner portions of the kidney
 Excretory and Endocrine functions of the kidney The kidneys are the main excretory organs which eliminate in the urine, most metabolites primarily those containing nitrogen such as ammonia, urea and creatinine.
Excretory and Endocrine functions of the kidney The kidneys are the main excretory organs which eliminate in the urine, most metabolites primarily those containing nitrogen such as ammonia, urea and creatinine.
Kidney Physiology. Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed
 Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Physiology of Circulation. Dr. Hiwa Shafiq 16/12/2018
 Physiology of Circulation Dr. Hiwa Shafiq 16/12/2018 Overview of the circulation The function of the circulation is to: 1. transport nutrients to the body tissues 2. transport waste products away 3. conduct
Physiology of Circulation Dr. Hiwa Shafiq 16/12/2018 Overview of the circulation The function of the circulation is to: 1. transport nutrients to the body tissues 2. transport waste products away 3. conduct
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
BIOLOGY - CLUTCH CH.44 - OSMOREGULATION AND EXCRETION.
 !! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
!! www.clutchprep.com Osmoregulation regulation of solute balance and water loss to maintain homeostasis of water content Excretion process of eliminating waste from the body, like nitrogenous waste Kidney
Regulation of fluid and electrolytes balance
 Regulation of fluid and electrolytes balance Three Compartment Fluid Compartments Intracellular = Cytoplasmic (inside cells) Extracellular compartment is subdivided into Interstitial = Intercellular +
Regulation of fluid and electrolytes balance Three Compartment Fluid Compartments Intracellular = Cytoplasmic (inside cells) Extracellular compartment is subdivided into Interstitial = Intercellular +
Relation Between Sodium Intake, Renal Function, and the Regulation of Arterial Pressure. Jeffrey L. Osborn
 1-91 Relation Between Sodium Intake, Renal Function, and the Regulation of Arterial Pressure Jeffrey L. Osborn The long-term regulation of arterial pressure requires the maintenance of a balance between
1-91 Relation Between Sodium Intake, Renal Function, and the Regulation of Arterial Pressure Jeffrey L. Osborn The long-term regulation of arterial pressure requires the maintenance of a balance between
Salt Sensitivity: Mechanisms, Diagnosis, and Clinical Relevance
 Salt Sensitivity: Mechanisms, Diagnosis, and Clinical Relevance Matthew R. Weir, MD Professor and Director Division of Nephrology University of Maryland School of Medicine Overview Introduction Mechanisms
Salt Sensitivity: Mechanisms, Diagnosis, and Clinical Relevance Matthew R. Weir, MD Professor and Director Division of Nephrology University of Maryland School of Medicine Overview Introduction Mechanisms
Helmy M. Siragy and Robert M. Carey Department of Medicine, University of Virginia Health Sciences Center, Charlottesville, Virginia 22908
 Rapid Publication The Subtype 2 (AT 2 ) Angiotensin Receptor Mediates Renal Production of Nitric Oxide in Conscious Rats Helmy M. Siragy and Robert M. Carey Department of Medicine, University of Virginia
Rapid Publication The Subtype 2 (AT 2 ) Angiotensin Receptor Mediates Renal Production of Nitric Oxide in Conscious Rats Helmy M. Siragy and Robert M. Carey Department of Medicine, University of Virginia
Nitric oxide blunts myogenic autoregulation in rat renal but not skeletal muscle circulation via tubuloglomerular feedback
 J Physiol 569.3 (25) pp 959 974 959 Nitric oxide blunts myogenic autoregulation in rat renal but not skeletal muscle circulation via tubuloglomerular feedback Armin Just and William J. Arendshorst Department
J Physiol 569.3 (25) pp 959 974 959 Nitric oxide blunts myogenic autoregulation in rat renal but not skeletal muscle circulation via tubuloglomerular feedback Armin Just and William J. Arendshorst Department
PHARMACEUTICAL INFORMATION AZILSARTAN
 AZEARLY Tablets Each Tablet Contains Azilsartan 20/40/80 mg PHARMACEUTICAL INFORMATION AZILSARTAN Generic name: Azilsartan Chemical name: 2-Ethoxy-1-{[2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)-4-biphenylyl]methyl}-
AZEARLY Tablets Each Tablet Contains Azilsartan 20/40/80 mg PHARMACEUTICAL INFORMATION AZILSARTAN Generic name: Azilsartan Chemical name: 2-Ethoxy-1-{[2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)-4-biphenylyl]methyl}-
Class XI Chapter 19 Excretory Products and their Elimination Biology
 Class XI Chapter 19 Excretory Products and their Elimination Biology Question 1: Define Glomerular Filtration Rate (GFR) Glomerular filtration rate is the amount of glomerular filtrate formed in all the
Class XI Chapter 19 Excretory Products and their Elimination Biology Question 1: Define Glomerular Filtration Rate (GFR) Glomerular filtration rate is the amount of glomerular filtrate formed in all the
URINARY SYSTEM ANATOMY PART
 URINARY SYSTEM ANATOMY PART 1 DANIL HAMMOUDI.MD Urinary System Composed of kidneys, ureters, urinary bladder, and urethra Eliminates nitrogenous wastes from the body Regulates water, electrolyte, and ph
URINARY SYSTEM ANATOMY PART 1 DANIL HAMMOUDI.MD Urinary System Composed of kidneys, ureters, urinary bladder, and urethra Eliminates nitrogenous wastes from the body Regulates water, electrolyte, and ph
Homeostasis. Thermoregulation. Osmoregulation. Excretion. how organisms regulate their body temperature
 Homeostasis the steady-state physiological condition of the body Ability to regulate the internal environment important for proper functioning of cells Thermoregulation Homeostasis how organisms regulate
Homeostasis the steady-state physiological condition of the body Ability to regulate the internal environment important for proper functioning of cells Thermoregulation Homeostasis how organisms regulate
Role of Angiotensin II in the Renal Effects Induced by Nitric Oxide and Prostaglandin Synthesis Inhibition
 Role of Angiotensin II in the Renal Effects Induced by Nitric Oxide and Prostaglandin Synthesis Inhibition MARIA T. LLINAS, JUAN D. GONZALEZ, EDUARDO NAVA, and F. JAVIER SALAZAR Departamento de Fisiologla,
Role of Angiotensin II in the Renal Effects Induced by Nitric Oxide and Prostaglandin Synthesis Inhibition MARIA T. LLINAS, JUAN D. GONZALEZ, EDUARDO NAVA, and F. JAVIER SALAZAR Departamento de Fisiologla,
Physiological role for P2X 1 receptors in renal microvascular autoregulatory behavior
 Physiological role for P2X 1 receptors in renal microvascular autoregulatory behavior Edward W. Inscho, 1,2 Anthony K. Cook, 1,2 John D. Imig, 1,2,3 Catherine Vial, 4 and Richard J. Evans 4 1 Department
Physiological role for P2X 1 receptors in renal microvascular autoregulatory behavior Edward W. Inscho, 1,2 Anthony K. Cook, 1,2 John D. Imig, 1,2,3 Catherine Vial, 4 and Richard J. Evans 4 1 Department
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D.
 Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
Regulation of Arterial Blood Pressure 2 George D. Ford, Ph.D. OBJECTIVES: 1. Describe the Central Nervous System Ischemic Response. 2. Describe chemical sensitivities of arterial and cardiopulmonary chemoreceptors,
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX
 One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
One Minute Movies: Molecular Action at the Nephron Joy Killough / Westwood High School / Austin,TX To prepare your nephron model: ( A nephron is a tubule and the glomerulus. There are about a million of
19. RENAL PHYSIOLOGY ROLE OF THE URINARY SYSTEM THE URINARY SYSTEM. Components and function. V BS 122 Physiology II 151 Class of 2011
 19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
Urinary System and Excretion. Bio105 Lecture 20 Chapter 16
 Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION
 3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
3 Chapter 1 RENAL HAEMODYNAMICS AND GLOMERULAR FILTRATION David Shirley, Giovambattista Capasso and Robert Unwin The kidney has three homeostatic functions that can broadly be described as excretory, regulatory
3.4.6 The Excretory System in the Human
 3.4.6 The Excretory System in the Human Objectives What you will need to know from this section Explain the role of the excretory system in homeostasis -- the ability and necessity to maintain constancy
3.4.6 The Excretory System in the Human Objectives What you will need to know from this section Explain the role of the excretory system in homeostasis -- the ability and necessity to maintain constancy
Cardiovascular System B L O O D V E S S E L S 2
 Cardiovascular System B L O O D V E S S E L S 2 Blood Pressure Main factors influencing blood pressure: Cardiac output (CO) Peripheral resistance (PR) Blood volume Peripheral resistance is a major factor
Cardiovascular System B L O O D V E S S E L S 2 Blood Pressure Main factors influencing blood pressure: Cardiac output (CO) Peripheral resistance (PR) Blood volume Peripheral resistance is a major factor
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Physiology of Circulation
 Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
Physiology of Circulation Dr. Ali Ebneshahidi Blood vessels Arteries: Blood vessels that carry blood away from the heart to the lungs and tissues. Arterioles are small arteries that deliver blood to the
Original Contribution. R. Mileva 1, A. Tolekova 2, G. Ilieva 2, D. Popov 1, P. Markova 1* 50 Trakia Journal of Sciences, Vol.
 Trakia Journal of Sciences, No 1, pp 50-54, 2013 Copyright 2013 Trakia University Available online at: http://www.uni-sz.bg ISSN 1313-7050 (print) ISSN 1313-3551 (online) Original Contribution PLASMA RENIN
Trakia Journal of Sciences, No 1, pp 50-54, 2013 Copyright 2013 Trakia University Available online at: http://www.uni-sz.bg ISSN 1313-7050 (print) ISSN 1313-3551 (online) Original Contribution PLASMA RENIN
Salt and Water Balance and Nitrogen Excretion
 Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
Announcements Exam is in class on WEDNESDAY. Bring a #2 pencil and your UFID. You must come to your registered class section (except those with DRC accommodations). Office hours Mon 1-3 pm. Teaching evals:
URINARY SYSTEM. Primary functions. Major organs & structures
 URINARY SYSTEM Primary functions Excretion of metabolic wastes Regulation of water and ion balances Regulation of blood pressure Vitamin D activation Regulation of rbc s (erythropoietin) Gluconeogenesis
URINARY SYSTEM Primary functions Excretion of metabolic wastes Regulation of water and ion balances Regulation of blood pressure Vitamin D activation Regulation of rbc s (erythropoietin) Gluconeogenesis
RENAL PHYSIOLOGY. Physiology Unit 4
 RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
RENAL PHYSIOLOGY Physiology Unit 4 Renal Functions Primary Function is to regulate the chemistry of plasma through urine formation Additional Functions Regulate concentration of waste products Regulate
Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note
![Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note Running head: NEPHRON 1. The nephron the functional unit of the kidney. [Student Name] [Name of Institute] Author Note](/thumbs/77/75431210.jpg) Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
Running head: NEPHRON 1 The nephron the functional unit of the kidney [Student Name] [Name of Institute] Author Note NEPHRON 2 The nephron the functional unit of the kidney The kidney is an important excretory
Chapter 17: Urinary System
 Introduction Chapter 17: Urinary System Organs of the Urinary System REFERENCE FIGURE 17.1 2 kidneys filters the blood 2 ureters transport urine from the kidneys to the urinary bladder Urinary bladder
Introduction Chapter 17: Urinary System Organs of the Urinary System REFERENCE FIGURE 17.1 2 kidneys filters the blood 2 ureters transport urine from the kidneys to the urinary bladder Urinary bladder
Cornerstone: A Collection of Scholarly and Creative Works for Minnesota State University, Mankato. Minnesota State University, Mankato
 Minnesota State University, Mankato Cornerstone: A Collection of Scholarly and Creative Works for Minnesota State University, Mankato Theses, Dissertations, and Other Capstone Projects 2009 The Increase
Minnesota State University, Mankato Cornerstone: A Collection of Scholarly and Creative Works for Minnesota State University, Mankato Theses, Dissertations, and Other Capstone Projects 2009 The Increase
Clinical Science (2010) 118, (Printed in Great Britain) doi: /cs
 www.clinsci.org Clinical Science (2010) 118, 617 632 (Printed in Great Britain) doi:10.1042/cs20090459 617 Combined inhibition of 20-hydroxyeicosatetraenoic acid formation and of epoxyeicosatrienoic acids
www.clinsci.org Clinical Science (2010) 118, 617 632 (Printed in Great Britain) doi:10.1042/cs20090459 617 Combined inhibition of 20-hydroxyeicosatetraenoic acid formation and of epoxyeicosatrienoic acids
The functions of the kidney:
 The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
Influence of Captopril on Red Cell Velocity in the Vasa Recta of the Renal Medulla
 Upsala Journal of Medical Sciences ISSN: 0300-9734 (Print) 2000-1967 (Online) Journal homepage: http://www.tandfonline.com/loi/iups20 Influence of Captopril on Red Cell Velocity in the Vasa Recta of the
Upsala Journal of Medical Sciences ISSN: 0300-9734 (Print) 2000-1967 (Online) Journal homepage: http://www.tandfonline.com/loi/iups20 Influence of Captopril on Red Cell Velocity in the Vasa Recta of the
A. Correct! Flushing acids from the system will assist in re-establishing the acid-base equilibrium in the blood.
 OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
