Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia
|
|
|
- Lorena Waters
- 5 years ago
- Views:
Transcription
1 Disease Models & Mechanisms 2, (2009) doi: /dmm Published by The Company of Biologists 2009 Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia M. Cecilia Ljungberg 1,2, *, C. Nicole Sunnen 1,3, *, Joaquin N. Lugo 1,2, Anne E. Anderson 1,2,3 and Gabriella D Arcangelo 4, SUMMARY Malformations of the cerebral cortex known as cortical dysplasia account for the majority of cases of intractable childhood epilepsy. With the exception of the tuberous sclerosis complex, the molecular basis of most types of cortical dysplasia is completely unknown. Currently, there are no good animal models available that recapitulate key features of the disease, such as structural cortical abnormalities and seizures, hindering progress in understanding and treating cortical dysplasia. At the neuroanatomical level, cortical abnormalities may include dyslamination and the presence of abnormal cell types, such as enlarged and misoriented neurons and neuroglial cells. Recent studies in resected human brain tissue suggested that a misregulation of the PI3K (phosphoinositide 3-kinase)-Akt-mTOR (mammalian target of rapamycin) signaling pathway might be responsible for the excessive growth of dysplastic cells in this disease. Here, we characterize neuronal subset (NS)-Pten mutant mice as an animal model of cortical dysplasia. In these mice, the Ptengene, which encodes a suppressor of the PI3K pathway, was selectively disrupted in a subset of neurons by using Cre-loxP technology. Our data indicate that these mutant mice, like cortical dysplasia patients, exhibit enlarged cortical neurons with increased mtor activity, and abnormal electroencephalographic activity with spontaneous seizures. We also demonstrate that a short-term treatment with the mtor inhibitor rapamycin strongly suppresses the severity and the duration of the seizure activity. These findings support the possibility that this drug may be developed as a novel antiepileptic treatment for patients with cortical dysplasia and similar disorders. INTRODUCTION Cortical dysplasia is a broad category of disorders that includes focal cortical dysplasia (FCD) and hemimegalencephaly (HMEG), which are developmental abnormalities of unknown etiology that are highly associated with intractable childhood epilepsy (Cepeda et al., 2006). Currently, no genetic animal models that recapitulate key traits of the disease are available to investigate molecular mechanisms and to develop new treatments. Because of the presence of abnormal brain structures (cortical tubers), tuberous sclerosis complex (TSC) can be considered a type of cortical dysplasia (Vinters et al., 1999). TSC is a congenital disorder resulting from mutations in the TSC1 or TSC2 genes (European Chromosome 16 Tuberous Sclerosis Consortium, 1993; van Slegtenhorst et al., 1997). A prominent neurological characteristic of TSC is epilepsy, which is often present in early childhood, but other manifestations such as subependymal giant cell astrocytomas, learning disabilities and autism are also present in varying degrees of comorbidity. Despite advances in understanding the molecular defects in TSC, the mechanisms of epileptogenesis are not well understood. Previous studies have implicated the PI3K (phosphoinositide 3-kinase)-Akt-mTOR (mammalian target of rapamycin) signaling pathway in cortical dysplasia, and demonstrated that mtor activity is dysregulated in the enlarged neurons and neuroglial cells that are found in TSC (Baybis et al., 2004; Miyata et al., 2004), as well as in FCD and HMEG patients (Ljungberg et al., 2006). 1 The Cain Foundation Laboratories, Texas Children s Hospital, 2 Department of Pediatrics and 3 Department of Neuroscience, Baylor College of Medicine, Houston, TX 77030, USA 4 Department of Cell Biology and Neuroscience, Rutgers, The State University of New Jersey, Piscataway, NJ 08854, USA *These authors contributed equally to this work Author for correspondence ( darcangelo@biology.rutgers.edu) The activity of this pathway is normally controlled by the product of the tumor suppressor gene PTEN (phosphatase and tensin homolog on chromosome ten) and by the products of the TSC1 (hamartin) and TSC2 (tuberin) genes. Hamartin and tuberin are upstream suppressors of mtor kinase. PTEN encodes a lipid and protein phosphatase that modulates cell growth by negatively regulating PI3K (Maehama and Dixon, 1998). The activation of PI3K results in the phosphorylation of Akt and mtor, which in turn stimulates protein synthesis through phosphorylation of ribosomal proteins and translation factors (Harris and Lawrence, 2003). This pathway is essential for control of normal cell growth, proliferation and survival in both invertebrates and vertebrates (Leevers et al., 1999). Inherited mutations in the human PTEN gene confer cancer predisposition, and sporadic loss-of-heterozygosity mutations result in a variety of cancers (Li et al., 1997; Chow and Baker, 2006). Germline mutations of the PTEN gene also produce distinct clinical syndromes with variable neurological manifestations (Eng, 2003). In the mouse, heterozygous Pten deletions resulted in the formation of tumors in multiple organs (Suzuki et al., 1998; Podsypanina et al., 1999). Brain neuronspecific Pten deletions resulted in ataxia, seizures and macrocephaly, which were associated with the phosphorylation of Akt and mtor in mutant neurons (Backman et al., 2001; Kwon et al., 2001; Kwon et al., 2006). In this study, we focused on mtor activation as a common mechanism of disease that is altered in several types of cortical dysplasia, and characterized mutant mice in which the Pten gene is selectively deleted in a subset of neurons (NS-Pten mutants). We report that these mutants display molecular, cellular and physiological traits of cortical dysplasia that can be effectively suppressed by a short-term treatment with the mtor inhibitor rapamycin. Disease Models & Mechanisms 389
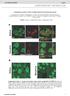
2 RESULTS Pten is deleted in a subset of cortical neurons in NS-Pten mutants We examined the brain anatomy and the cortical loss of Pten protein expression in homozygous mutants compared with their wild-type littermates. The mutant mice were described previously as Pten loxp/loxp ; Gfap-Cre mice (Backman et al., 2001; Kwon et al., 2001) and were reported to exhibit Pten deletion in a subset of neuronal cells, including the majority of granule cells in the dentate gyrus and cerebellum. To distinguish this mouse line from other lines where the Gfap promoter drives Cre expression and Pten deletion in astrocytes (Fraser et al., 2004), we refer to it here as neuronal subset-specific Pten (NS-Pten). Consistent with previous reports, we found that NS-Pten heterozygous mice appear normal, whereas homozygous mutants display ataxia and premature mortality. At the neuroanatomical level, mutants displayed macrocephaly, with apparent enlargement of cortical, hippocampal and cerebellar structures (Fig. 1A). The extent of the enlargement varied somewhat among individual mutants, but it was most readily and consistently observed in the hippocampus, a structure that also appeared mildly dysmorphic. The hippocampal and cerebellar abnormalities correlated strongly with loss of Pten gene expression in the majority of neurons in these structures (not shown), as reported previously (Backman et al., 2001; Kwon et al., 2001). When the neocortex of mutant mice was examined in detail by immunofluorescence, loss of Pten protein expression was noted in many cortical neurons in all cellular layers (Fig. 1B). Mutant neurons, identified by the expression of the neuronal marker NeuN, appeared enlarged compared with adjacent Pten-positive neurons, Fig. 1. Macrocephaly and Pten loss in the neocortex of NS- Pten mutant mice. (A) Hematoxylin and eosin (H&E) staining of sagittal brain sections derived from wild-type and NS-Pten mutant mice. The mutant brain is enlarged compared with the wild-type brain. The cerebral cortex (left panels) is affected mildly, whereas both the hippocampus (middle panels) and cerebellum (right panels) display obvious enlargement and structural abnormalities. (B) Pten (green) and NeuN (red) double immunofluorescence on paraffin-embedded sagittal sections (close to the midline) of the NS-Pten mutant cortex. The smaller panels on the right represent enlargements of the boxed areas in the Pten-stained and merged images. Many large pyramidalshaped neurons in layer V and some neurons in other cortical layers are Pten negative. (C) The top panels show a section of NS-Pten mutant neocortex that has been double labeled with Pten (green) and glutamate (red) antibodies. The lower panels show double immunofluorescence with Pten (green) and GABA (red) antibodies. Pten-negative neurons (arrows) always express glutamate but not GABA, whereas the large Pten-positive neurons (arrowheads) express glutamate and the small Ptenpositive neurons (arrowheads) express GABA. (D) Double labeling of cortical sections derived from E18.5 wild-type or NS- Pten mutant mice with antibodies against Cux1 (green, layer II- IV) and foxp2 (red, layer VI). The cortical layers appear normal in the mutant mice. 390 dmm.biologists.org
3 but retained proper orientation and morphology that are typical of their layer. For example, mutant neurons in layer V displayed a prominent apical dendrite, oriented radially towards the top of the cortex (Fig. 1B). This morphology is typical of large pyramidal neurons, which are excitatory, early-born principal neurons that originate in the ventricular zone of the neocortex. To determine whether Pten-negative neurons also retained their neurochemical characteristics, we conducted double immunolabeling with antibodies against Pten and either glutamate, a marker of excitatory neurons, or γ-aminobutyric acid (GABA), a marker of inhibitory neurons. We found that virtually all Pten-negative neurons were glutamatergic (Fig. 1C, top panels), whereas none were GABAergic (Fig. 1C, lower panels). Finally, we sought to determine whether loss of Pten results in abnormal radial migration and positioning of mutant neurons into cortical layers. Brain sections obtained from wild-type and homozygous NS-Pten mutant mice at embryonic day (E)18.5 were double labeled with antibodies against Cux1, a marker of upper cortical layers, and foxp2, a marker of deep cortical layers (Fig. 1D). These markers labeled the appropriate cellular layers in wild-type and mutant cortex, indicating that Pten loss does not affect neuronal migration. Together, our findings that neuronal morphology, neurotransmitter and layer-specific marker expression are not altered in NS-Pten mutant mice indicate that Pten loss in cortical neurons does not disrupt their cell-specific phenotype. Cortical neurons lacking Pten are hypertrophic Despite their overall normal phenotype, Pten mutant cortical neurons appeared enlarged compared with adjacent normal cells. To examine the size of mutant cortical neurons in more detail, we performed immunohistochemistry with Pten antibodies, and used hematoxylin counterstaining to distinguish and outline the cell bodies of Ptenpositive and -negative neurons in the neocortex of mutant mice (Fig. 2A,B). We used a similar approach to label Pten-positive neurons in the wild-type neocortex. Because the cell body size of cortical neurons varies greatly depending on the cellular layer where they reside, we limited our analysis to neurons located in layer V. This layer was chosen because it contained a large number of Ptennegative, as well as Pten-positive, pyramidal neurons that could be identified easily owing to their relatively large size. We then measured the cell body size of positive and negative neurons in layer V of the cortex in mutant mice, and the cell body size of positive layer V pyramidal neurons in wild-type animals. At postnatal day (P)12 there was a significant difference (~1.7-fold increase) in the soma size of neurons lacking Pten (393.1±10.8 μm 2 ) compared with that of Ptenexpressing neurons in both NS-Pten mutant (213.8±28 μm 2 ) and wild-type (227.6±30.6 μm 2 ) animals (Fig. 2C). Similar results were obtained when adult mice (9 weeks of age) were analyzed. The soma size of Pten-deficient neurons was significantly increased (381.9±22.4 μm 2 ) compared with that of Pten-positive neurons in the cortex from either mutant (234.9±15.9 μm 2 ) or wild-type mice (225.1±10.8 μm 2 ) (Fig. 2C). Thus, unlike the Pten-negative hippocampal and cerebellar granule neurons that were measured in previous studies (Backman et al., 2001; Kwon et al., 2001), the size of Pten-negative cortical pyramidal neurons did not increase progressively with age after the second postnatal week. There was no significant difference in soma size between the Pten-positive cells in mutant and wild-type mice at any age analyzed (Fig. 2C). Fig. 2. Cortical neurons lacking Pten are hypertrophic. Pten-positive and -negative cortical neurons were visualized by immunohistochemistry and hematoxylin counterstaining. (A) Sagittal section taken through the neocortex of a NS-Pten mutant mouse, illustrating the distribution of both Pten-positive (brown) and -negative cells. (B) Layer V neurons that lack Pten staining (red arrows) appear larger than adjacent Pten-positive neurons (black arrows) in the cortex of NS-Pten mutant mice. (C) Quantitative analysis of the cell body size of Pten-negative and -positive neurons in cortical layer V of mutant mice, and of the corresponding Pten-positive neurons in wild-type mice, at the indicated ages (n=3 for each age and genotype) is shown. Pten-negative neurons are significantly larger than Pten-positive neurons from either mutant or wild-type animals [*P<0.01 by analysis of variance (ANOVA) with Tukey s honestly significant difference (HSD) test]. Data is shown as the mean cell body area ± s.e.m. Increased activation of the mtor pathway in Pten-deficient cells Pten is a negative regulator of the PI3K pathway and its loss causes increased activation of the downstream Akt and mtor kinases (Backman et al., 2002). We investigated the activity of the PI3K pathway by immunohistochemistry and immunofluorescence using phospho-specific antibodies directed against Akt (Ser473) and downstream targets of mtor, such as the translation initiation factor eif4g (Ser1108) and the ribosomal protein S6 (Ser240/244) (Sarbassov et al., 2005). We have shown previously that the phosphorylation level of these proteins is increased in cytomegalic neurons in human cortical dysplasia (Ljungberg et al., 2006). Our present data revealed an increase in phosphorylated Akt, eif4g and ribosomal protein S6 in NS-Pten mutant animals (Fig. 3A). Consistent with earlier reports, the phospho-akt signal was particularly strong in the dentate gyrus and the hippocampus (Kwon et al., 2001), but it was also seen in the neocortex of NS-Pten mutant mice. The phospho-eif4g signal was weak in the normal brain but it appeared elevated in the dentate gyrus, the CA1 region of the hippocampus, and layer V of the neocortex in the NS-Pten mutant brain. The phospho-s6 signal, was generally strong in the forebrain of both wildtype and mutant animals, and it appeared to be increased in the cortical and hippocampal structures of NS-Pten mutant mice. Disease Models & Mechanisms 391
4 Fig. 3. The Akt-mTOR pathway is activated in the neocortex of NS-Pten mutant mice. (A) Sections of adult wild-type and mutant mice were processed for immunohistochemistry using antibodies against phospho-akt (pakt), phospho-eif4g (peif4g) and phospho-s6 (ps6). Elevated levels of these phosphoproteins can be seen in the neocortex and hippocampus of mutant animals. (B) Higher magnification images of cortical layer V from a mutant mouse section that has been double labeled by immunofluorescence show that many Pten-negative cells (arrows in green images) also exhibit elevated levels of pakt, peif4g or ps6 (arrows in all red images). (C) Quantification of the ps6 fluorescence signal in Pten-negative and -positive layer V neurons shows that the levels of S6 phosphorylation are statistically increased in mutant neurons compared with normal neurons in 9-week-old mutant mice (n=3, *P<0.05). To look in more detail at the activation of the PI3K pathway in the mutant neocortex, we performed double immunofluorescence using Pten and phospho-specific antibodies targeting the AktmTOR pathway (Fig. 3B). The majority of the Pten-negative neurons in the layer V cortex displayed strong phospho-akt, phospho-eif4g and phospho-s6 immunoreactivity in both the cytoplasm and the nucleus. Some Pten-positive cortical neurons were also positive for phospho-eif4g and phospho-s6, although, overall, the mutant neurons displayed stronger staining. Quantitative analysis confirmed that the phospho-s6 immunofluorescence was significantly increased in mutant neurons compared with Pten-positive neurons in NS-Pten mice (Fig. 3C). These data demonstrate that the PI3K-Akt-mTOR pathway is overactive in mutant Pten-deficient cortical neurons. NS-Pten mutant mice exhibit spontaneous seizures and abnormal electroencephalogram (EEG) activity Previous studies with NS-Pten mutants indicated that these mice have spontaneous seizures, characterized by forelimb clonus and tonic-clonic events (Backman et al., 2001; Kwon et al., 2001). We also observed tonic-clonic seizures in some of the mutant mice as they aged beyond 6 weeks. To examine the seizure phenotype in more detail, and to determine whether NS-Pten mutants have subclinical epileptiform activity that does not manifest in overt behavioral seizures, we conducted synchronized video-eeg recordings. Animals were implanted with cortical electrodes during postnatal week 3, and monitored for an average of 4.91 hours per week from postnatal week 4 to 9. By 6 weeks of age, all NS-Pten mutant mice (n=7) displayed subclinical electrographic seizures lasting for at least 10 seconds (Table 1), even though most of these mice (n=5/7) lacked any obvious behavioral correlate. However, at this age, two of the seven mice also exhibited behavioral changes that were characterized by tonic-clonic activity and, by 9 weeks of age, three of the seven mice exhibited these types of seizures. Moreover, we detected a number of different types of abnormal epileptiform activity, as well as abnormalities in the awake baseline EEG activity that were never seen in wild-type animals (n=6) (Table 1). In addition to subclinical seizures, mutant animals also exhibited isolated interictal spikes, short trains of Table 1. Number of animals exhibiting each type of epileptiform activity at 6 and 9 weeks of age Repetitive spikes Repetitive spike runs Seizures Genotype Treatment Age (wks) n Spikes (<5 seconds) (>5-<10 seconds) (>10 seconds) Wild type Naïve (0) 0 (0) 0 (0) 0 (0) Mutant Naïve (100) 7 (100) 7 (100) 7 (100) Mutant Vehicle (100) 9 (100) 9 (100) 9 (100) Mutant Rapamycin (100) 6 (85.7) 2 (28.6) 3 (42.9) Wild type Naïve (0) 0 (0) 0 (0) 0 (0) Mutant Naïve (100) 7 (100) 7 (100) 7 (100) Mutant Vehicle (100) 8 (100) 8 (100) 8 (100) Mutant Rapamycin (100) 5 (71.4) 2 (28.6) 1 (14.3) Percentage of animals is given in parentheses. 392 dmm.biologists.org
5 Fig. 4. NS-Pten mutant animals show abnormal epileptiform activity. Representative EEG traces from awake, adult mutant mice showing examples of different types of epileptiform activity. All of the mutant mice (n=7) analyzed at 6 or 9 weeks of age exhibited isolated interictal spikes, short trains of repetitive spikes lasting less than 5 seconds, repetitive spike runs lasting greater than 5 seconds, and subclinical seizures lasting at least 10 seconds. None of these activities was seen in wild-type animals (n=6). repetitive spikes lasting less than 5 seconds, and long runs of repetitive spikes lasting between 5 and 10 seconds (Fig. 4). Although individual animals varied with respect to the frequency and duration of these EEG patterns, all these types of epileptiform activity were observed in all of the mutants by 6 weeks of age. By 9 weeks of age, these abnormal EEG findings were more frequent than those observed at 6 weeks of age (Fig. 6A). Rapamycin treatment suppresses mtor activity and the hypertrophy of cortical Pten-deficient neurons in NS-Pten mice Since hypertrophic Pten mutant cortical neurons demonstrated elevated mtor activity, we investigated whether treatment with the specific mtor inhibitor rapamycin could rescue the cellular and molecular abnormalities of mutant mice. Mutant NS-Pten mice were treated for 2 weeks with rapamycin or with vehicle (control), and then sacrificed at the end of the treatment to examine mtor activity in brain sections. We conducted double immunofluorescence experiments using antibodies against phospho-s6, to measure mtor activity, and NeuN, the general neuronal marker. The levels of phospho-s6 appeared dramatically reduced in the cortical neurons of mutant mice treated with rapamycin compared with vehicle-treated controls, whereas levels of NeuN were similar (Fig. 5A). Quantitative analysis further confirmed that, relative to NeuN, the levels of phospho-s6 were reduced significantly in rapamycin-treated mutants that were examined either immediately after the end of the treatment (6 weeks of age) or 3 weeks later (at 9 weeks of age) (Fig. 5B). To determine whether rapamycin treatment rescued neuronal hypertrophy, we measured the soma size of cortical layer V pyramidal neurons in vehicle- and rapamycin-treated animals that were sacrificed either immediately after the 2-week treatment (6- week age group) or at 3 weeks after cessation of the treatment (9- week age group). In the animals examined immediately after treatment, we found a modest but significant decrease (~1.2-fold) in the neuronal cell body size in the rapamycin-treated animals (340.7±22.1 μm 2 ) compared with controls (410.3±5.2 μm 2 ). However, no significant difference was found in the 9-week old Fig. 5. Rapamycin treatment reduces the phospho-s6 immunoreactivity and hypertrophy of Pten mutant cortical neurons. Four-week-old mutant mice were treated with rapamycin or vehicle for 2 weeks and then processed for immunofluorescence, either immediately (6 weeks of age) or 3 weeks later (9 weeks of age; n=5 mice per treatment group). (A) Nine-week-old rapamycintreated animals (bottom panels) exhibit a marked reduction in phospho-s6 (ps6) immunofluorescence compared with vehicle-treated animals (top panels), whereas expression of the neuronal marker NeuN does not change. (B) Quantification of the fluorescence shows that the level of S6 phosphorylation is reduced in rapamycin-treated animals at 6 weeks of age (n=3 mice per treatment group) and that the reduction persists to 3 weeks after cessation of the treatment (9 weeks; n=5 mice per treatment group) (*P<0.05). (C) There is a significant decrease in the neuronal soma size in rapamycin-treated animals at 6 weeks of age (n=3 mice per treatment group), but not at 9 weeks of age (n=4 mice per treatment group) (*P<0.05). animals that had not been receiving treatment during the previous 3 weeks (rapamycin=414.4±31.5 μm 2 ; vehicle=448.5±49 μm 2 ) (Fig. 5C). Disease Models & Mechanisms 393
6 Rapamycin treatment reduces the severity of electrographic abnormalities in NS-Pten mice Having determined that a short-term treatment with rapamycin suppresses, at least in part, the molecular and cellular abnormalities of NS-Pten mutant mice, we next determined whether this drug also suppresses the abnormal epileptiform activity observed in these animals. Mutant mice received EEG electrode placement at 3 weeks of age and were then treated with rapamycin or vehicle (control), or were untreated (naïve). Video-EEG recordings were analyzed immediately following treatment (6 weeks of age) and at 3 weeks after treatment was terminated (9 weeks of age). We found that, during the period of video-eeg recording at the 6 week time point, rapamycin-treated mutants spent significantly less time (median=2.9%) in repetitive spikes, repetitive spike runs, or in seizure activity as compared with vehicle-treated (38.4%) or naïve (39.6%) mutants (Fig. 6A). Interestingly, even at three weeks after cessation of treatment, the mutants that had received rapamycin spent significantly less time in epileptiform activity (median=2.8%) than either vehicle-treated (47.0%) or naïve (62.9%) controls. When different types of EEG abnormalities were analyzed separately, we also found that fewer rapamycin-treated mice displayed all forms of epileptiform activity (Table 1). Whereas 100% of naïve and vehicle-treated mutants exhibited all four types of epileptiform activity by 6 weeks of age, only two of seven (28.6%) rapamycin-treated animals exhibited every type. By 9 weeks, only one of seven rapamycin-treated mutants exhibited electrographic seizures in addition to both short and long trains of repetitive spikes. Isolated interictal spikes, however, were present in all mutant animals, regardless of age or treatment. Rapamycin treatment also noticeably improved the abnormal awake baseline EEG activity of mutant NS-Pten mice (Fig. 6B). Even at three weeks after cessation of treatment, the baseline EEG activity of rapamycin-treated mutants appeared similar to the baseline of wild-type mice. The baseline background activity of naïve and vehicle-treated mutants, however, was unchanged and remained abnormal. These findings may be related to the blockade of seizure activity or could be an effect of the rapamycin itself. However, it appears to be long lasting, even after termination of rapamycin treatment. DISCUSSION In this study we characterized NS-Pten mutant mice as the first reported genetic animal model for cortical dysplasia. This model does not recapitulate all morphological aspects of the human disease, for example we do not see the dyslamination that is typical of severe cortical dysplasia, or the loss of apical dendrites that occurs in some cytomegalic neurons. However, NS-Pten mutant mice recapitulate several key neuropathological features of cortical dysplasia: they exhibit enlarged cortical neurons in which the mtor kinase is overactive, and spontaneous epileptiform activity that can be detected by EEG. Furthermore, we have demonstrated that treatment with the mtor inhibitor rapamycin dramatically suppresses the epileptiform activity in NS-Pten mutant mice. This antiepileptic effect appears to be long lasting, persisting for at least 3 weeks after cessation of the treatment. Thus, we suggest that NS- Pten mice may be useful in the development of new antiepileptic treatments for patients with cortical dysplasia and other mtordependent abnormalities. 394 As general cortical dysplasia models, NS-Pten mutant mice complement the existing collection of genetically modified animals that more specifically model TSC. These latter models include the Eker rat (a heterozygous Tsc2 mutant) and several transgenic Cre mouse lines that drive conditional Tsc1 and Tsc2 knockout deletions in neurons, glial or progenitor cells (reviewed by Holmes and Stafstrom, 2007). Notably, Tsc1 GFAP knockout mice, in which the Tsc1 gene was deleted specifically in mature astrocytes, exhibit seizures similar to NS-Pten mice, which improve with rapamycin treatment (Zeng et al., 2008). Similar to our studies, these authors found that early treatment with rapamycin prevented seizures and improved interictal EEG activity, although they found no long-lasting anti-epileptic effect when rapamycin treatment was halted. Our study further supports a role for aberrant mtor signaling in the generation of seizures, and suggests that mtor pathway inhibitors are potential therapies for TSC and related disorders. One major finding of the present studies is that a short period of treatment with rapamycin suppresses the frequency and severity of electrographic seizures in NS-Pten mutant mice, indicating that aberrant regulation of the PI3K-mTOR pathway in these mice underlies the epilepsy phenotype. The dramatic effect of rapamycin on the epilepsy phenotype in the NS-Pten mutant mice raises the possibility that rapamycin could be developed as a therapy for epilepsy in FCD, HMEG and TSC patients, who are expected to have abnormal activation of PI3K-mTOR signaling in cortical neurons. Interestingly, a reduction of seizure frequency associated with astrocytoma tumor regression and behavioral improvement has been reported in a small group of TSC patients who were treated with the rapamycin analog RAD001 (everolimus) (Franz et al., 2006). Clearly, further clinical investigation of mtor signaling in epilepsy and an assessment of the efficacy of rapamycin derivatives in TSC, as well as in forms of cortical dysplasia, are necessary. In parallel, further studies in multiple animal models are needed to better assess the utility and the limitations of this intervention strategy. Although Cre expression in NS-Pten mice is driven by the Gfap promoter, no Pten mutant astrocytes have been found, indicating that Cre expression occurs only in some neuronal progenitors and not in glial precursors (Backman et al., 2001; Kwon et al., 2001). Thus, we hypothesize that the seizure phenotype observed in NS- Pten mutants results primarily from a neuronal dysfunction. However, further studies will be required to evaluate this possibility and to determine whether it originates in cortical, subcortical or hippocampal structures. Using the same conditional knockout line employed in the present studies, other investigators have reported previously that homozygous Pten mutant mice display macrocephaly, behavioral seizures and ataxia (Backman et al., 2001; Kwon et al., 2001). Both of these studies focused on the presence of enlarged neurons in the dentate gyrus and cerebellum, and proposed that Pten mutants are good models for Lhermitte-Duclos disease, a human genetic disorder caused by a PTEN germline mutation and characterized by cerebellar gangliocytomas. In the present study, we have shifted our attention to the abnormalities in the cerebral cortex, but readily observed the presence of hypertrophic granule neurons in the hippocampus and cerebellum, and the corresponding enlargement of these structures. We also observed the presence of cerebellardmm.biologists.org
7 Fig. 6. Rapamycin treatment reduces epileptiform activity in NS-Pten mutant mice. (A) The median percent of time that mutant animals spent in repetitive spikes, repetitive spike runs, and/or seizure activity at 6 and 9 weeks of age. Immediately following treatment (6 weeks), rapamycin-treated mutant (n=7) mice spent a significantly reduced amount of time in epileptiform activity compared with vehicle-treated (n=9) or naïve (n=7) animals. Three weeks after treatment termination (9 weeks), epileptiform activity was still significantly reduced for rapamycin-treated animals (n=7) compared with vehicle-treated (n=8) or naïve (n=7) animals. *P<0.05, **P<0.01, ***P< (B) Representative EEG traces taken during awake baseline activity at 9 weeks of age, illustrating the similarity of the background activity between wild type and rapamycin-treated mutant traces when compared with those seen in naïve and vehicle-treated mutants. derived ataxia in older mice, as well as increased mortality. We further explored the forebrain phenotype using video-eeg and identified abnormal baseline EEG and epileptiform activity. Thus, our observations are consistent with previous reports, but emphasize different behavioral, neurophysiological and anatomical features that have not previously been analyzed in detail. Using the neuron-specific enolase (Nse) promoter to drive Cre expression, other investigators have described the phenotype of mutant mice in which Pten was deleted in mature neuronal populations (Kwon et al., 2006). In addition to macrocephaly and neuronal hypertrophy, these authors reported a behavioral phenotype characterized by sporadic seizures, altered social interaction and inappropriate response to sensory stimuli. Based on these findings, they proposed that Nse-Pten mutants are valuable models for autism spectrum disorder (ASD), which is linked to the activation of the PI3K-mTOR pathway. Indeed, a modest degree of comorbidity of ASD with TSC and epilepsy has been reported (Gabis et al., 2005). Although patients with germline PTEN mutations do not exhibit seizures, it is interesting to note that a correlation exists between macrocephaly, PTEN mutations and autism (Zori et al., 1998; Goffin et al., 2001; Butler et al., 2005). Nse-Pten mice also exhibit altered circadian rhythms (Ogawa et al., 2007). These reports provide additional support for the concept that, through modulation of the PI3K-mTOR pathway, Pten activity critically regulates cortical excitability and cognitive function. We observed a transient reduction in the cell body size of the mutant cortical neurons following a 2-week treatment with rapamycin. These results are also consistent with a previous report (Kwon et al., 2003), in which treatment with the rapamycin analog CCI-779 reduced the cell size of Pten mutant neurons in the dentate gyrus and cerebellum. Unlike the effect on neuronal hypertrophy, we found that a short-term treatment with rapamycin produced a long-lasting effect on suppression of epileptiform activity on the EEG; this effect persisted even at 3 weeks after the cessation of treatment. This leads us to believe that the long-lasting epileptiform suppression by rapamycin treatment is not the result of soma size reduction, but perhaps the result of changes in subcellular structures and, possibly, in downstream molecular targets of mtor that are involved in synaptic plasticity and membrane excitability. Increasingly, both in vivo and in vitro studies are revealing links between increased mtor pathway activation and changes in both dendritic and axonal morphology (Jaworski et al., 2005; Kumar et al., 2005; Tavazoie et al., 2005; Kwon et al., 2006). Additionally, links have been made between this pathway and changes in certain ion channels, receptors and the expression of synaptic plasticity (Hou and Klann, 2004; Raab-Graham et al., 2006; Ruegg et al., 2007). Further studies will be required to determine the molecular mechanism underlying the regulation of neuronal network excitability by mtor. Together with previous studies in animal models, which documented prolonged survival and improved neurological function in association with mtor inhibition using either rapamycin or its analogues (Kwon et al., 2003; Zeng et al., 2008), our findings raise the hope that a limited modulation of mtor activity may be sufficient to achieve long-lasting seizure control in some cortical dysplasia and TSC patients, especially those who are currently neither helped by the available pharmacological treatments nor are suitable candidates for epilepsy surgery. METHODS Mice The generation of conditional neuron subset-specific Pten (NS- Pten) knockout mice (Gfap-Cre; Pten loxp/loxp ) has been described previously (Kwon et al., 2001). The mice were a gift from S. Baker (St Jude Children s Research Hospital, Memphis, TN). In this study we used NS-Pten loxp/+ (heterozygote) animals for breeding to generate NS-Pten +/+ (wild type) and NS-Pten loxp/loxp (mutants). Animal housing and use were in compliance with the NIH guidelines for the care and use of laboratory animals, and were approved by the institutional animal care committee at Baylor College of Medicine. Histology, immunohistochemistry and immunofluorescence Postnatal brains were fixed in 10% neutral buffered formalin (NBF, VWR), embedded in paraffin and 6 μm parasagittal sections were then cut on a microtome. One section from each animal was stained with H&E; adjacent sections were used for immunohistochemistry or immunofluorescence. For experiments using postnatal brain tissue, we performed antigen retrieval in 1 mm citric acid (ph 6) in a steamer for 30 minutes, plus 20 minutes cooling time, for all antibodies except NeuN, which required 1 mm EDTA (ph 8). Disease Models & Mechanisms 395
8 Endogenous peroxidase activity was blocked with 1.8% H 2 O 2 [in PBS with 0.1% Triton X-100] and the sections were incubated with 10% normal goat or donkey serum (Jackson ImmunoResearch Laboratories) in PBS with 0.1% Triton X-100. For the analysis of cortical layers, embryonic brains were submersion fixed overnight in 4% paraformaldehyde (v/w), cryoprotected in 30% sucrose (v/w) overnight, and 20 μm sections were then cut on a cryostat. Primary antibodies were: Pten (rabbit polyclonal, 1:50, Cell Signaling Technologies, or mouse monoclonal, 1:1600, Cascade Bioscience), NeuN (mouse monoclonal, 1:50, Chemicon), glutamate (rabbit polyclonal, 1:1600, Sigma), phospho-ser473-akt (rabbit polyclonal, 1:50, Cell Signaling), GABA (rabbit polyclonal, 1:400, Chemicon), phospho-ser1108-eif4g (rabbit polyclonal, 1:50, Cell Signaling), phospho-ser240/244-s6 (rabbit monoclonal, 1:100, Cell Signaling), Cux1 (rabbit polyclonal, 1:200, Santa Cruz) and foxp2 (goat polyclonal, 1:200, Santa Cruz). All primary antibodies were diluted in PBS with 0.1% Triton X-100 and antibody binding was performed overnight at 4 C. For immunohistochemistry, we used biotinlabeled secondary antibodies raised in goat (1:200, Jackson ImmunoResearch Laboratories) followed by peroxidase-conjugated avidin (Vectastain Elite ABC kit, Vector Laboratories), which was detected by 3,3 -diaminobenzidine (DAB) substrate (Vector Laboratories). Slides were counterstained with Mayer s hematoxylin (VWR) and mounted in Cytoseal 60 (VWR). For immunofluorescence, we used donkey anti-rabbit, anti-mouse or anti-goat secondary antibodies conjugated with Alexa Fluor 488 or 594 (1:1000, Invitrogen). Slides were mounted in Vectashield with DAPI (Vector Laboratories). Fluorescence microscopy was performed using an Olympus BX61 microscope equipped with epifluorescence filters and a Hamamatsu monochrome digital camera, and bright field microscopy was performed using an Olympus BX51 microscope equipped with an Olympus DP70 digital color camera. Rapamycin treatment Rapamycin (LC Laboratories) was dissolved in a vehicle solution containing 4% ethanol, 5% polyethylene glycol 400 (Sigma) and 5% Tween 80 (Sigma), essentially as described previously (Eshleman et al., 2002). During the fourth and fifth weeks of life, animals received daily 10 mg/kg intraperitoneal injections, five times per week, of either rapamycin or vehicle. Prior to the 2-week treatment, mice were implanted during postnatal week 3 with EEG electrodes. Video-EEG recordings were collected throughout the 2-week treatment and during the 3-week period following treatment. The mice were then euthanized and the brains were processed as described above. Electrode implantation Cortical EEG electrodes were implanted in wild-type and mutant NS-Pten mice during postnatal week 3; then, starting at postnatal week 4, the mice were monitored using video-eeg until week 9 when they were euthanized and their brains were processed as described above. Animals were anaesthetized with a ketaminexylazine-acepromazine mixture (obtained from the Baylor College of Medicine Center for Comparative Medicine) and placed in a stereotaxic frame fitted with a mouse adaptor. Four mm diameter stainless steel electrodes (Plastics One) were placed bilaterally over the cortex, two at the anterior and two at the 396 posterior, and a reference was placed anterior to the bregma and a ground was placed in the cervical paraspinous area. Animals were allowed to recover for 4-7 days before video-eeg recording. EEG acquisition and analysis After recovery from surgery, a digital video-eeg system (Stellate) was used to record synchronized video-eegs at an average of twice per week, for 2 to 4 hours per recording. In a subset of animals, recordings lasted for 8 hours per session. Two investigators (A.E.A. and C.N.S.) retrospectively reviewed the traces obtained from the recording sessions. Except for a 20-minute acclimation period, entire video-eeg traces were scored for the presence of isolated spikes, repetitive spike trains, long runs of repetitive spikes, and seizures. Isolated spikes were defined as fast (<200 milliseconds) single events of high amplitude (5 baseline), whereas repetitive spikes included trains of fast spikes and/or spike and slow wave combinations lasting less than 5 seconds. Any repetitive spike and wave activity lasting greater than 5 seconds but less than 10 seconds was classified as a repetitive spike run. Seizures were defined as repetitive spiking activity, or a combination of high-frequency and -amplitude trains of activity, or low frequency, high-amplitude spike and wave discharges that lasted for at least 10 seconds. The associated behavior of the animal was noted during these electrographic events. We also analyzed the effect of a 2-week vehicle or rapamycin treatment on the EEG activity in mutant animals and compared it to that of naïve mutant animals at 6 and 9 weeks of age. For this part of the study, the video-eegs were collected and analyzed as described above. In addition, we selected 30-minute samples of EEG traces after 1-hour acclimation in the recording chamber and quantified the amount of time (in seconds) spent in repetitive spikes, repetitive spike runs or seizure activity, and reported this data as the percentage of the total 30-minute (1800-second) observation time. Any activity lasting less than half a second was not counted as epileptiform, hence isolated interictal spikes were eliminated. Investigators who were blinded to the treatment performed this analysis. Data were not of equal variance, and so were analyzed with the Kruskal-Wallis test and the median values were plotted. Post-hoc analysis was accomplished using Dunn s method. These 30-minute epochs were found to be representative of entire recordings when scores from a subset of 9-week EEG recordings (two per treatment group, n=6) were compared with a second 30-minute epoch taken 2-3 hours into the same recording (paired t-test, P=0.37). Neuronal soma size measurement For naïve animals, we measured the soma size of pyramidal neurons in cortical layer V in P12 and adult brains from both wildtype and mutant animals (n=3 for each age and genotype). For our measurements, we selected a 1 mm 2 area that was immediately dorsal to the hippocampus on parasagittal sections. We used Pten immunohistochemistry with a hematoxylin counterstain to differentiate Pten-positive pyramidal neurons from Pten-negative neurons in mutant mice, and we measured the Pten-positive pyramidal neurons in wild-type animals using the ImageJ software (NIH). Only cells with a pyramidal-shaped soma and a clear nucleolus were measured. We measured 37±19 (mean±s.d.) Ptennegative neurons and 63±18 Pten-positive neurons in each mutant mouse, and 81±20 Pten-positive neurons in each wild-type mouse. dmm.biologists.org
9 TRANSLATIONAL IMPACT Clinical issue Most cases of intractable childhood epilepsy are caused by malformations of the cerebral cortex known as cortical dysplasia. Currently, progress in understanding and treating cortical dysplasia is hindered by a lack of animal models that recapitulate key features of the disease, such as the presence of abnormal cell types, structural cortical abnormalities and seizures. The molecular basis of most types of cortical dysplasia is unknown. Recent studies using human brain tissue suggest that a misregulation of the PI3K (phosphoinositide 3-kinase)-Akt-mTOR (mammalian target of rapamycin) signaling pathway might be responsible for the excessive growth of dysplastic cells in this disease. Other studies support the notion that it is the dysplastic cells, or their faulty networks, that cause the seizures. This raises the possibility of using the mtor inhibitor rapamycin as an antiepileptic treatment for patients with cortical dysplasia. Results In this report, the authors characterize a conditional Pten knockout mouse as an animal model of cortical dysplasia. The Pten gene, which encodes a suppressor of the PI3K-mTOR pathway, was selectively disrupted in a subset of neurons. These mutant mice have several key features seen in cortical dysplasia, such as enlarged cortical neurons with increased mtor activity, and abnormal electrographic activity with spontaneous seizures. Similar to patients with focal cortical dysplasia (FCD), the time of onset and the severity of the seizures varied. However, by 6 weeks of age, all mutant mice exhibited seizures and benefited from short-term treatment with the mtor inhibitor rapamycin. This drug reduced the mtor activity in the enlarged neurons, transiently reduced the size of the enlarged neurons, and strongly suppressed the severity and the duration of the epileptiform activity. Implications and future directions This paper presents a conditional Pten knockout mouse that is a novel model of cortical dysplasia. Furthermore, treatment of these mice with the drug rapamycin suppresses seizures. Previous studies in other seizure-prone mouse models have also demonstrated that treatment using either rapamycin or its analogues results in prolonged survival and improved neurological function. Together, these findings suggest that a limited modulation of mtor activity may be sufficient to achieve long-lasting seizure control in some cortical dysplasia patients, especially those who are currently neither helped by available pharmacological treatments nor are suitable candidates for epilepsy surgery. doi: /dmm Data are presented as soma size in μm 2 ±s.e.m. and are analyzed using ANOVA and Tukey s HSD. For rapamycin- and vehicle-treated animals, the soma size of Pten-negative pyramidal neurons in the layer V cortex was measured, as described above, in animals that were either euthanized directly after the 2-week treatment (n=3, vehicle or rapamycin) or in the group that was followed by EEG until 9 weeks of age (n=4, vehicle or rapamycin). We measured 36±13 (mean±s.d.) Pten-negative neurons in each animal and the data were analyzed using the Student s t-test. Analysis of fluorescence intensity We performed Pten and phospho-s6 double immunofluorescence and compared the phospho-s6 signal intensity in the cortical layer V Pten-positive neurons with the Pten-negative neurons in sections obtained from 9-week mutant mice (n=3) using the ImageJ software (NIH). The phospho-s6 pixel values in Pten-negative neurons were expressed in each animal as the fold change compared with the phospho-s6 pixel values in Pten-positive neurons. Data were analyzed using one sample t-test. Using a modified protocol, we also analyzed the phospho-s6 immunofluorescence intensity in vehicle- and rapamycin-treated animals, directly after the 2-week treatment protocol (n=3 for each of the vehicle- or rapamycin-treated animals) or at 9 weeks of age (n=5 for each treatment group), using NeuN and DAPI immunofluorescence to define the neuronal cytosol. For each animal, three non-overlapping adjacent fields of the neocortex dorsal to the hippocampus were selected, including layers I-VI, and three images (phospho-s6 in green, NeuN in red and DAPI in blue) were collected using the same camera settings. Using a Matlab script developed by T. J. Vadakkan, we created a threshold for all of the NeuN images to include only positive neurons. Since the localization of phospho-s6 is cytoplasmic, we excluded the DAPI-stained nuclei from the analysis. We then compared the phospho-s6 signal with the NeuN signal (control) in the cytoplasm. Data were analyzed using two-way ANOVA. ACKNOWLEDGEMENTS We thank S. Baker for the gift of NS-Pten mice; B. A. Antalffy, D. Parghi and the Mental Retardation and Developmental Disabilities Research Center at Baylor College of Medicine for help with tissue processing; S. Willis and F. Vanegas-Roman for assistance with EEG electrode implants; T. J. Vadakkan for programming the fluorescence analysis; R. Azevedo for the use of the Olympus BX-61 microscope and advice on statistical analysis; and J. Swann for critical reading of the manuscript. This work was supported by a 2004 Research Award and a 2007 Challenge Award from the Citizens United for Research in Epilepsy (CURE) (to G.D.), NIH/NINDS R01 NS (to G.D.), a pre-doctoral fellowship from the American Epilepsy Foundation (to C.N.S.), a postdoctoral fellowship from the American Epilepsy Foundation (to J.N.L.), NIH/NINDS F32 NS (to J.N.L.) and NIH/NINDS R01 NS (to A.E.A.). Deposited in PMC for release after 12 months. COMPETING INTERESTS The authors declare no competing financial interests. AUTHOR CONTRIBUTIONS M.C.L. and C.N.S. contributed equally to this work. M.C.L. and C.N.S. designed some of the experiments, performed the experiments, analyzed the data, wrote parts of the manuscript and edited the manuscript. J.N.L. performed some experiments and edited the manuscript. A.E.A. designed some of the experiments, analyzed the data and edited the manuscript. G.D. conceived the study, designed some of the experiments and wrote the manuscript. Received 4 December 2008; Accepted 9 March REFERENCES Backman, S. A., Stambolic, V., Suzuki, A., Haight, J., Elia, A., Pretorius, J., Tsao, M. S., Shannon, P., Bolon, B., Ivy, G. O. et al. (2001). Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat. Genet. 29, Backman, S., Stambolic, V. and Mak, T. (2002). PTEN function in mammalian cell size regulation. Curr. Opin. Neurobiol. 12, Baybis, M., Yu, J., Lee, A., Golden, J. A., Weiner, H., McKhann, G., 2nd, Aronica, E. and Crino, P. B. (2004). mtor cascade activation distinguishes tubers from focal cortical dysplasia. Ann. Neurol. 56, Butler, M. G., Dasouki, M. J., Zhou, X. P., Talebizadeh, Z., Brown, M., Takahashi, T. N., Miles, J. H., Wang, C. H., Stratton, R., Pilarski, R. et al. (2005). Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J. Med. Genet. 42, Cepeda, C., Andre, V. M., Levine, M. S., Salamon, N., Miyata, H., Vinters, H. V. and Mathern, G. W. (2006). Epileptogenesis in pediatric cortical dysplasia: the dysmature cerebral developmental hypothesis. Epilepsy Behav. 9, Chow, L. M. and Baker, S. J. (2006). PTEN function in normal and neoplastic growth. Cancer Lett. 241, Eng, C. (2003). PTEN: one gene, many syndromes. Hum. Mutat. 22, Eshleman, J. S., Carlson, B. L., Mladek, A. C., Kastner, B. D., Shide, K. L. and Sarkaria, J. N. (2002). Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res. 62, Disease Models & Mechanisms 397
Differential localisation of hamartin and tuberin and increased S6 phosphorylation in a tuber
 Differential localisation of hamartin and tuberin in a tuber 10 Differential localisation of hamartin and tuberin and increased S6 phosphorylation in a tuber Floor Jansen*, Robbert Notenboom*, Mark Nellist*,
Differential localisation of hamartin and tuberin in a tuber 10 Differential localisation of hamartin and tuberin and increased S6 phosphorylation in a tuber Floor Jansen*, Robbert Notenboom*, Mark Nellist*,
CONTRACTING ORGANIZATION: Rutgers, the State University of New Jersey New Brunswick, NJ 08901
 AWARD NUMBER: W81XWH-16-1-0415 TITLE: Cell Type-Specific Contributions to the TSC Neuropathology PRINCIPAL INVESTIGATOR: Gabriella D Arcangelo CONTRACTING ORGANIZATION: Rutgers, the State University of
AWARD NUMBER: W81XWH-16-1-0415 TITLE: Cell Type-Specific Contributions to the TSC Neuropathology PRINCIPAL INVESTIGATOR: Gabriella D Arcangelo CONTRACTING ORGANIZATION: Rutgers, the State University of
TITLE: Altered Astrocyte-Neuron Interactions and Epileptogenesis in Tuberous Sclerosis Complex Disorder
 AWARD NUMBER: W81XWH-12-1-0196 TITLE: Altered Astrocyte-Neuron Interactions and Epileptogenesis in Tuberous Sclerosis Complex Disorder PRINCIPAL INVESTIGATOR: Dr. David Sulzer, Ph.D. CONTRACTING ORGANIZATION:
AWARD NUMBER: W81XWH-12-1-0196 TITLE: Altered Astrocyte-Neuron Interactions and Epileptogenesis in Tuberous Sclerosis Complex Disorder PRINCIPAL INVESTIGATOR: Dr. David Sulzer, Ph.D. CONTRACTING ORGANIZATION:
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Behavioural effects of ketamine in non-stressed and stressed mice. Naive C57BL/6 adult male mice (n=10/group) were given a single dose of saline vehicle or ketamine (3.0 mg/kg,
Supplementary Figure 1. Behavioural effects of ketamine in non-stressed and stressed mice. Naive C57BL/6 adult male mice (n=10/group) were given a single dose of saline vehicle or ketamine (3.0 mg/kg,
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AWARD NUMBER: W81XWH-15-1-0112 TITLE: Molecular Mechanisms Underlying the Epileptogenesis and Seizure Progression in Tuberous Sclerosis Complex 1 Deficient Mouse Models PRINCIPAL INVESTIGATOR: James E.
AWARD NUMBER: W81XWH-15-1-0112 TITLE: Molecular Mechanisms Underlying the Epileptogenesis and Seizure Progression in Tuberous Sclerosis Complex 1 Deficient Mouse Models PRINCIPAL INVESTIGATOR: James E.
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1.
 Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
The Role of Brain Inflammation in Epileptogenesis in TSC. CONTRACTING ORGANIZATION: Washington University School of Medicine St Louis, M
 AD Award Number: W81XWH-12-1-0190 TITLE: The Role of Brain Inflammation in Epileptogenesis in TSC PRINCIPAL INVESTIGATOR: Michael Wong CONTRACTING ORGANIZATION: Washington University School of Medicine
AD Award Number: W81XWH-12-1-0190 TITLE: The Role of Brain Inflammation in Epileptogenesis in TSC PRINCIPAL INVESTIGATOR: Michael Wong CONTRACTING ORGANIZATION: Washington University School of Medicine
Molecular alterations in epilepsy-associated malformations of cortical development Boer, K.
 UvA-DARE (Digital Academic Repository) Molecular alterations in epilepsy-associated malformations of cortical development Boer, K. Link to publication Citation for published version (APA): Boer, K. (009).
UvA-DARE (Digital Academic Repository) Molecular alterations in epilepsy-associated malformations of cortical development Boer, K. Link to publication Citation for published version (APA): Boer, K. (009).
Potential Treatment and Current Research in Phelan-McDermid Syndrome. 11/16/2016 Frambu Center for Rare Disorders
 Potential Treatment and Current Research in Phelan-McDermid Syndrome 11/16/2016 Frambu Center for Rare Disorders Genetics is Complicated! Deletion 22q13: Therapies Under Investigation Intranasal insulin
Potential Treatment and Current Research in Phelan-McDermid Syndrome 11/16/2016 Frambu Center for Rare Disorders Genetics is Complicated! Deletion 22q13: Therapies Under Investigation Intranasal insulin
Zhu et al, page 1. Supplementary Figures
 Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse
 Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Molecular alterations in epilepsy-associated malformations of cortical development Boer, K.
 UvA-DARE (Digital Academic Repository) Boer, K. Link to publication Citation for published version (APA): Boer, K. (2009).. General rights It is not permitted to download or to forward/distribute the text
UvA-DARE (Digital Academic Repository) Boer, K. Link to publication Citation for published version (APA): Boer, K. (2009).. General rights It is not permitted to download or to forward/distribute the text
Intracranial Studies Of Human Epilepsy In A Surgical Setting
 Intracranial Studies Of Human Epilepsy In A Surgical Setting Department of Neurology David Geffen School of Medicine at UCLA Presentation Goals Epilepsy and seizures Basics of the electroencephalogram
Intracranial Studies Of Human Epilepsy In A Surgical Setting Department of Neurology David Geffen School of Medicine at UCLA Presentation Goals Epilepsy and seizures Basics of the electroencephalogram
Manuscript Draft. Title: mtor pathway inhibition as a new therapeutic strategy in epilepsy and epileptogenesis.
 Pharmacological Research Elsevier Editorial System(tm) for Manuscript Draft Manuscript Number: YPHRS-D-16-00011R2 Title: mtor pathway inhibition as a new therapeutic strategy in epilepsy and epileptogenesis.
Pharmacological Research Elsevier Editorial System(tm) for Manuscript Draft Manuscript Number: YPHRS-D-16-00011R2 Title: mtor pathway inhibition as a new therapeutic strategy in epilepsy and epileptogenesis.
Challenges and opportunities for understanding epilepsy and cognitive and behavioral comorbidities. Eleonora Aronica
 Biobanks and Databases -Basis for Translational Research Challenges and opportunities for understanding epilepsy and cognitive and behavioral comorbidities Eleonora Aronica Department of (Neuro)Pathology,
Biobanks and Databases -Basis for Translational Research Challenges and opportunities for understanding epilepsy and cognitive and behavioral comorbidities Eleonora Aronica Department of (Neuro)Pathology,
Supplementary Figure S1: Histological analysis of kainate-treated animals
 Supplementary Figure S1: Histological analysis of kainate-treated animals Nissl stained coronal or horizontal sections were made from kainate injected (right) and saline injected (left) animals at different
Supplementary Figure S1: Histological analysis of kainate-treated animals Nissl stained coronal or horizontal sections were made from kainate injected (right) and saline injected (left) animals at different
Supplementary Figure 1
 Supplementary Figure 1 The average sigmoid parametric curves of capillary dilation time courses and average time to 50% peak capillary diameter dilation computed from individual capillary responses averaged
Supplementary Figure 1 The average sigmoid parametric curves of capillary dilation time courses and average time to 50% peak capillary diameter dilation computed from individual capillary responses averaged
Supplementary Figure 1: Kv7 currents in neonatal CA1 neurons measured with the classic M- current voltage-clamp protocol.
 Supplementary Figures 1-11 Supplementary Figure 1: Kv7 currents in neonatal CA1 neurons measured with the classic M- current voltage-clamp protocol. (a), Voltage-clamp recordings from CA1 pyramidal neurons
Supplementary Figures 1-11 Supplementary Figure 1: Kv7 currents in neonatal CA1 neurons measured with the classic M- current voltage-clamp protocol. (a), Voltage-clamp recordings from CA1 pyramidal neurons
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) 108 (b-c) (d) (e) (f) (g)
 Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Supplementary Figure 1 Information on transgenic mouse models and their recording and optogenetic equipment. (a) In four mice, cre-dependent expression of the hyperpolarizing opsin Arch in pyramidal cells
Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale
 Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale (b) show stronger immunolabeling for CB 1 than those in
Supplementary Figure 1. SDS-FRL localization of CB 1 in the distal CA3 area of the rat hippocampus. (a-d) Axon terminals (t) in stratum pyramidale (b) show stronger immunolabeling for CB 1 than those in
Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary Materials and Methods
 Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
Sleep-Wake Cycle I Brain Rhythms. Reading: BCP Chapter 19
 Sleep-Wake Cycle I Brain Rhythms Reading: BCP Chapter 19 Brain Rhythms and Sleep Earth has a rhythmic environment. For example, day and night cycle back and forth, tides ebb and flow and temperature varies
Sleep-Wake Cycle I Brain Rhythms Reading: BCP Chapter 19 Brain Rhythms and Sleep Earth has a rhythmic environment. For example, day and night cycle back and forth, tides ebb and flow and temperature varies
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Supplementary Figure 1
 Supplementary Figure 1 Miniature microdrive, spike sorting and sleep stage detection. a, A movable recording probe with 8-tetrodes (32-channels). It weighs ~1g. b, A mouse implanted with 8 tetrodes in
Supplementary Figure 1 Miniature microdrive, spike sorting and sleep stage detection. a, A movable recording probe with 8-tetrodes (32-channels). It weighs ~1g. b, A mouse implanted with 8 tetrodes in
Nature Neuroscience: doi: /nn Supplementary Figure 1. Large-scale calcium imaging in vivo.
 Supplementary Figure 1 Large-scale calcium imaging in vivo. (a) Schematic illustration of the in vivo camera imaging set-up for large-scale calcium imaging. (b) High-magnification two-photon image from
Supplementary Figure 1 Large-scale calcium imaging in vivo. (a) Schematic illustration of the in vivo camera imaging set-up for large-scale calcium imaging. (b) High-magnification two-photon image from
NNZ-2566 in Rett Syndrome and Autism Spectrum Disorders Role and Update
 NNZ-2566 in Rett Syndrome and Autism Spectrum Disorders Role and Update 1 Overview The natural growth factor IGF-1 is broken down in the body to IGF-1[1-3] NNZ-2566 is an analogue of IGF-1[1-3] developed
NNZ-2566 in Rett Syndrome and Autism Spectrum Disorders Role and Update 1 Overview The natural growth factor IGF-1 is broken down in the body to IGF-1[1-3] NNZ-2566 is an analogue of IGF-1[1-3] developed
Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex
 Supplementary Information Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex Luc Gentet, Yves Kremer, Hiroki Taniguchi, Josh Huang, Jochen Staiger and Carl
Supplementary Information Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex Luc Gentet, Yves Kremer, Hiroki Taniguchi, Josh Huang, Jochen Staiger and Carl
Epilepsy and Brain Tumor. Apasri Lusawat M.D. Pediatric Neurology Prasat Neurological Institute
 Epilepsy and Brain Tumor Apasri Lusawat M.D. Pediatric Neurology Prasat Neurological Institute Outline Overview Pathogenesis : Tumor-> epilepsy Etiology of Epilepsy, age EPILEPSY AND BRAIN TUMORS CNS neoplasms
Epilepsy and Brain Tumor Apasri Lusawat M.D. Pediatric Neurology Prasat Neurological Institute Outline Overview Pathogenesis : Tumor-> epilepsy Etiology of Epilepsy, age EPILEPSY AND BRAIN TUMORS CNS neoplasms
Pharmacological Inhibition of mtorc1 Suppresses Anatomical, Cellular, and Behavioral Abnormalities in Neural-Specific Pten Knock-Out Mice
 The Journal of Neuroscience, February 11, 2009 29(6):1773 1783 1773 Cellular/Molecular Pharmacological Inhibition of mtorc1 Suppresses Anatomical, Cellular, and Behavioral Abnormalities in Neural-Specific
The Journal of Neuroscience, February 11, 2009 29(6):1773 1783 1773 Cellular/Molecular Pharmacological Inhibition of mtorc1 Suppresses Anatomical, Cellular, and Behavioral Abnormalities in Neural-Specific
TITLE: Altered Astrocyte-Neuron Interactions and Epileptogenesis in Tuberous Sclerosis Complex Disorder
 AD Award Number: W81XW -12-1-0196 TITLE: Altered Astrocyte-Neuron Interactions and Epileptogenesis in Tuberous Sclerosis Complex Disorder PRINCIPAL INVESTIGATOR: Dr. David Sulzer, Ph.D CONTRACTING ORGANIZATION:
AD Award Number: W81XW -12-1-0196 TITLE: Altered Astrocyte-Neuron Interactions and Epileptogenesis in Tuberous Sclerosis Complex Disorder PRINCIPAL INVESTIGATOR: Dr. David Sulzer, Ph.D CONTRACTING ORGANIZATION:
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature06994 A phosphatase cascade by which rewarding stimuli control nucleosomal response A. Stipanovich*, E. Valjent*, M. Matamales*, A. Nishi, J.H. Ahn, M. Maroteaux, J. Bertran-Gonzalez,
doi: 10.1038/nature06994 A phosphatase cascade by which rewarding stimuli control nucleosomal response A. Stipanovich*, E. Valjent*, M. Matamales*, A. Nishi, J.H. Ahn, M. Maroteaux, J. Bertran-Gonzalez,
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
How Nicotinic Signaling Shapes Neural Networks
 How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
How Nicotinic Signaling Shapes Neural Networks Darwin K. Berg Division of Biological Sciences University of California, San Diego Nicotinic Cholinergic Signaling Uses the transmitter ACh to activate cation-selective
Nature Neuroscience: doi: /nn Supplementary Figure 1. Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons
 Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Supplementary Figure 1 Diverse anorexigenic signals induce c-fos expression in CEl PKC-δ + neurons a-c. Quantification of CEl c-fos expression in mice intraperitoneal injected with anorexigenic drugs (a),
Supplementary Materials for
 www.sciencetranslationalmedicine.org/cgi/content/full/4/117/117ra8/dc1 Supplementary Materials for Notch4 Normalization Reduces Blood Vessel Size in Arteriovenous Malformations Patrick A. Murphy, Tyson
www.sciencetranslationalmedicine.org/cgi/content/full/4/117/117ra8/dc1 Supplementary Materials for Notch4 Normalization Reduces Blood Vessel Size in Arteriovenous Malformations Patrick A. Murphy, Tyson
Embryonic MGE Cells as a Treatment for Epilepsy December 1, 2012
 Embryonic MGE Cells as a Treatment for Epilepsy December 1, 2012 Scott C. Baraban, PhD University of California, San Francisco American Epilepsy Society Annual Meeting Disclosure Name of Commercial Interest
Embryonic MGE Cells as a Treatment for Epilepsy December 1, 2012 Scott C. Baraban, PhD University of California, San Francisco American Epilepsy Society Annual Meeting Disclosure Name of Commercial Interest
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Part 11: Mechanisms of Learning
 Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
Neurophysiology and Information: Theory of Brain Function Christopher Fiorillo BiS 527, Spring 2012 042 350 4326, fiorillo@kaist.ac.kr Part 11: Mechanisms of Learning Reading: Bear, Connors, and Paradiso,
You submitted this quiz on Sun 19 May :32 PM IST (UTC +0530). You got a score of out of
 Feedback Ex6 You submitted this quiz on Sun 19 May 2013 9:32 PM IST (UTC +0530). You got a score of 10.00 out of 10.00. Question 1 What is common to Parkinson, Alzheimer and Autism? Electrical (deep brain)
Feedback Ex6 You submitted this quiz on Sun 19 May 2013 9:32 PM IST (UTC +0530). You got a score of 10.00 out of 10.00. Question 1 What is common to Parkinson, Alzheimer and Autism? Electrical (deep brain)
Nature Neuroscience: doi: /nn Supplementary Figure 1. MADM labeling of thalamic clones.
 Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated,
 1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated, embedded in matrigel and exposed
1 2 3 4 5 6 7 8 9 10 Supplementary Figure 1: Neuregulin 1 increases the growth of mammary organoids compared to EGF. (a) Mammary epithelial cells were freshly isolated, embedded in matrigel and exposed
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10353 Supplementary Figure 1. Mutations of UBQLN2 in patients with ALS and ALS/dementia. (a) A mutation, c.1489c>t (p.p497s), was identified in F#9975. The pedigree is shown on the left
doi:10.1038/nature10353 Supplementary Figure 1. Mutations of UBQLN2 in patients with ALS and ALS/dementia. (a) A mutation, c.1489c>t (p.p497s), was identified in F#9975. The pedigree is shown on the left
Postn MCM Smad2 fl/fl Postn MCM Smad3 fl/fl Postn MCM Smad2/3 fl/fl. Postn MCM. Tgfbr1/2 fl/fl TAC
 A Smad2 fl/fl Smad3 fl/fl Smad2/3 fl/fl Tgfbr1/2 fl/fl 1. mm B Tcf21 MCM Tcf21 MCM Smad3 fl/fl Tcf21 MCM Smad2/3 fl/fl Tcf21 MCM Tgfbr1/2 fl/fl αmhc MCM C 1. mm 1. mm D Smad2 fl/fl Smad3 fl/fl Smad2/3
A Smad2 fl/fl Smad3 fl/fl Smad2/3 fl/fl Tgfbr1/2 fl/fl 1. mm B Tcf21 MCM Tcf21 MCM Smad3 fl/fl Tcf21 MCM Smad2/3 fl/fl Tcf21 MCM Tgfbr1/2 fl/fl αmhc MCM C 1. mm 1. mm D Smad2 fl/fl Smad3 fl/fl Smad2/3
Mass Histology Service
 Mass Histology Service A complete anatomical pathology laboratory www.masshistology.com Telephone: (877) 286-6004 Report on Pathology A Time Course Study of the Local Effects of Intramuscular XXXXXXX Injection
Mass Histology Service A complete anatomical pathology laboratory www.masshistology.com Telephone: (877) 286-6004 Report on Pathology A Time Course Study of the Local Effects of Intramuscular XXXXXXX Injection
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AWARD NUMBER: W81XWH-13-1-0463 TITLE: The Ketogenic Diet and Potassium Channel Function PRINCIPAL INVESTIGATOR: Dr. Geoffrey Murphy CONTRACTING ORGANIZATION: University of Michigan Ann Arbor, MI 48109
AWARD NUMBER: W81XWH-13-1-0463 TITLE: The Ketogenic Diet and Potassium Channel Function PRINCIPAL INVESTIGATOR: Dr. Geoffrey Murphy CONTRACTING ORGANIZATION: University of Michigan Ann Arbor, MI 48109
Supplementary Materials
 Supplementary Materials Fig. S1. Weights of full-dose treatment groups comparing 1 st, 2 nd, and 3 rd generation gene replacement therapy. Mice were treated at p1 with 4x10 11 GC of the three different
Supplementary Materials Fig. S1. Weights of full-dose treatment groups comparing 1 st, 2 nd, and 3 rd generation gene replacement therapy. Mice were treated at p1 with 4x10 11 GC of the three different
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons
 Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Modeling Depolarization Induced Suppression of Inhibition in Pyramidal Neurons Peter Osseward, Uri Magaram Department of Neuroscience University of California, San Diego La Jolla, CA 92092 possewar@ucsd.edu
Terminology. Terminology. Terminology. Terminology. Terminology. Bromodeoxyuridine
 Kateřina Náměstková, Zuzana Šimonová, Eva Syková Behavioural Brain Research Bromodeoxyuridine : Doublecortin : DCX Glial Fibrillary Acidic Protein : GFAP Trace eye blink conditioning 1 Volume 163 : pp.
Kateřina Náměstková, Zuzana Šimonová, Eva Syková Behavioural Brain Research Bromodeoxyuridine : Doublecortin : DCX Glial Fibrillary Acidic Protein : GFAP Trace eye blink conditioning 1 Volume 163 : pp.
Interictal High Frequency Oscillations as Neurophysiologic Biomarkers of Epileptogenicity
 Interictal High Frequency Oscillations as Neurophysiologic Biomarkers of Epileptogenicity December 10, 2013 Joyce Y. Wu, MD Associate Professor Division of Pediatric Neurology David Geffen School of Medicine
Interictal High Frequency Oscillations as Neurophysiologic Biomarkers of Epileptogenicity December 10, 2013 Joyce Y. Wu, MD Associate Professor Division of Pediatric Neurology David Geffen School of Medicine
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
ANIMAL MODELS OF EPILEPSY Index
 INDEX A Action potentials BK channel effect on... 89 90 See also Synaptic transmission Adeno-associated virus (AAV) vector gene therapy... 234 inhibitory GABA receptors and... 238 240 neuroactive peptides
INDEX A Action potentials BK channel effect on... 89 90 See also Synaptic transmission Adeno-associated virus (AAV) vector gene therapy... 234 inhibitory GABA receptors and... 238 240 neuroactive peptides
Supplementary Figure 1. GABA depolarizes the majority of immature neurons in the
 Supplementary Figure 1. GABA depolarizes the majority of immature neurons in the upper cortical layers at P3 4 in vivo. (a b) Cell-attached current-clamp recordings illustrate responses to puff-applied
Supplementary Figure 1. GABA depolarizes the majority of immature neurons in the upper cortical layers at P3 4 in vivo. (a b) Cell-attached current-clamp recordings illustrate responses to puff-applied
Supplementary Information
 Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
Supplementary Figure 1
 Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Supplementary Information
 1 Supplementary Information A role for primary cilia in glutamatergic synaptic integration of adult-orn neurons Natsuko Kumamoto 1,4,5, Yan Gu 1,4, Jia Wang 1,4, Stephen Janoschka 1,2, Ken-Ichi Takemaru
1 Supplementary Information A role for primary cilia in glutamatergic synaptic integration of adult-orn neurons Natsuko Kumamoto 1,4,5, Yan Gu 1,4, Jia Wang 1,4, Stephen Janoschka 1,2, Ken-Ichi Takemaru
Excessive Activation of mtor in Postnatally Generated Granule Cells Is Sufficient to Cause Epilepsy
 Article Excessive Activation of mtor in Postnatally Generated Granule Cells Is Sufficient to Cause Epilepsy Raymund Y.K. Pun, 1 Isaiah J. Rolle, 4 Candi L. LaSarge, 1 Bethany E. Hosford, 4 Jules M. Rosen,
Article Excessive Activation of mtor in Postnatally Generated Granule Cells Is Sufficient to Cause Epilepsy Raymund Y.K. Pun, 1 Isaiah J. Rolle, 4 Candi L. LaSarge, 1 Bethany E. Hosford, 4 Jules M. Rosen,
Advanced multimodal imaging in malformations of cortical development
 Advanced multimodal imaging in malformations of cortical development Seok Jun Hong (sjhong@bic.mni.mcgill.ca) NOEL Neuroimaging of Epilepsy Lab MICA Multimodal Imaging and Connectome Analysis Lab w4 w5
Advanced multimodal imaging in malformations of cortical development Seok Jun Hong (sjhong@bic.mni.mcgill.ca) NOEL Neuroimaging of Epilepsy Lab MICA Multimodal Imaging and Connectome Analysis Lab w4 w5
Charlie Taylor, PhD CpTaylor Consulting Chelsea, MI, USA
 Contribution of Calcium Channel α 2 δ Binding Sites to the Pharmacology of Gabapentin and Pregabalin Charlie Taylor, PhD CpTaylor Consulting Chelsea, MI, USA Disclosure Information Charlie Taylor, PhD
Contribution of Calcium Channel α 2 δ Binding Sites to the Pharmacology of Gabapentin and Pregabalin Charlie Taylor, PhD CpTaylor Consulting Chelsea, MI, USA Disclosure Information Charlie Taylor, PhD
Tuberous sclerosis complex (TSC) is a developmental disorder
 Articles https://doi.org/1.138/s41591-18-139-y Genetically engineered human cortical spheroid models of tuberous sclerosis John D. Blair 1, Dirk Hockemeyer 1 and Helen S. Bateup 1,2 Tuberous sclerosis
Articles https://doi.org/1.138/s41591-18-139-y Genetically engineered human cortical spheroid models of tuberous sclerosis John D. Blair 1, Dirk Hockemeyer 1 and Helen S. Bateup 1,2 Tuberous sclerosis
Supplementary Table I Blood pressure and heart rate measurements pre- and post-stroke
 SUPPLEMENTARY INFORMATION doi:10.1038/nature09511 Supplementary Table I Blood pressure and heart rate measurements pre- and post-stroke Pre Post 7-days Systolic Diastolic BPM Systolic Diastolic BPM Systolic
SUPPLEMENTARY INFORMATION doi:10.1038/nature09511 Supplementary Table I Blood pressure and heart rate measurements pre- and post-stroke Pre Post 7-days Systolic Diastolic BPM Systolic Diastolic BPM Systolic
Nature Neuroscience: doi: /nn Supplementary Figure 1. Trial structure for go/no-go behavior
 Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
Serotonergic Control of the Developing Cerebellum M. Oostland
 Serotonergic Control of the Developing Cerebellum M. Oostland Summary Brain development is a precise and crucial process, dependent on many factors. The neurotransmitter serotonin is one of the factors
Serotonergic Control of the Developing Cerebellum M. Oostland Summary Brain development is a precise and crucial process, dependent on many factors. The neurotransmitter serotonin is one of the factors
Chapter 1 Introduction
 Chapter 1 Introduction Men think epilepsy divine, merely because they do not understand it. But if they called everything divine which they do not understand, why, there would be no end to divine things.
Chapter 1 Introduction Men think epilepsy divine, merely because they do not understand it. But if they called everything divine which they do not understand, why, there would be no end to divine things.
(A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and a
 Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Tuning properties of individual circuit components and stimulus-specificity of experience-driven changes.
 Supplementary Figure 1 Tuning properties of individual circuit components and stimulus-specificity of experience-driven changes. (a) Left, circuit schematic with the imaged component (L2/3 excitatory neurons)
Supplementary Figure 1 Tuning properties of individual circuit components and stimulus-specificity of experience-driven changes. (a) Left, circuit schematic with the imaged component (L2/3 excitatory neurons)
CASE 49. What type of memory is available for conscious retrieval? Which part of the brain stores semantic (factual) memories?
 CASE 49 A 43-year-old woman is brought to her primary care physician by her family because of concerns about her forgetfulness. The patient has a history of Down syndrome but no other medical problems.
CASE 49 A 43-year-old woman is brought to her primary care physician by her family because of concerns about her forgetfulness. The patient has a history of Down syndrome but no other medical problems.
Germline disruption of Pten localization causes enhanced sex-dependent social motivation and increased glial production
 Human Molecular Genetics, 2014, Vol. 23, No. 12 3212 3227 doi:10.1093/hmg/ddu031 Advance Access published on January 26, 2014 Germline disruption of Pten localization causes enhanced sex-dependent social
Human Molecular Genetics, 2014, Vol. 23, No. 12 3212 3227 doi:10.1093/hmg/ddu031 Advance Access published on January 26, 2014 Germline disruption of Pten localization causes enhanced sex-dependent social
EEG workshop. Epileptiform abnormalities. Definitions. Dr. Suthida Yenjun
 EEG workshop Epileptiform abnormalities Paroxysmal EEG activities ( focal or generalized) are often termed epileptiform activities EEG hallmark of epilepsy Dr. Suthida Yenjun Epileptiform abnormalities
EEG workshop Epileptiform abnormalities Paroxysmal EEG activities ( focal or generalized) are often termed epileptiform activities EEG hallmark of epilepsy Dr. Suthida Yenjun Epileptiform abnormalities
Supplementary Figure 1. ACE robotic platform. A. Overview of the rig setup showing major hardware components of ACE (Automatic single Cell
 2 Supplementary Figure 1. ACE robotic platform. A. Overview of the rig setup showing major hardware components of ACE (Automatic single Cell Experimenter) including the MultiClamp 700B, Digidata 1440A,
2 Supplementary Figure 1. ACE robotic platform. A. Overview of the rig setup showing major hardware components of ACE (Automatic single Cell Experimenter) including the MultiClamp 700B, Digidata 1440A,
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1. Normal AMPAR-mediated fepsp input-output curve in CA3-Psen cdko mice. Input-output curves, which are plotted initial slopes of the evoked fepsp as function of the amplitude of the
Supplementary Figure 1
 Supplementary Figure 1 AAV-GFP injection in the MEC of the mouse brain C57Bl/6 mice at 4 months of age were injected with AAV-GFP into the MEC and sacrificed at 7 days post injection (dpi). (a) Brains
Supplementary Figure 1 AAV-GFP injection in the MEC of the mouse brain C57Bl/6 mice at 4 months of age were injected with AAV-GFP into the MEC and sacrificed at 7 days post injection (dpi). (a) Brains
Introduction to EEG del Campo. Introduction to EEG. J.C. Martin del Campo, MD, FRCP University Health Network Toronto, Canada
 Introduction to EEG J.C. Martin, MD, FRCP University Health Network Toronto, Canada What is EEG? A graphic representation of the difference in voltage between two different cerebral locations plotted over
Introduction to EEG J.C. Martin, MD, FRCP University Health Network Toronto, Canada What is EEG? A graphic representation of the difference in voltage between two different cerebral locations plotted over
Nature Neuroscience: doi: /nn.2275
 Supplementary Figure S1. The presence of MeCP2 in enriched primary glial cultures from rat or mouse brains is not neuronal. Western blot analysis of protein extracts from (a) rat glial and neuronal cultures.
Supplementary Figure S1. The presence of MeCP2 in enriched primary glial cultures from rat or mouse brains is not neuronal. Western blot analysis of protein extracts from (a) rat glial and neuronal cultures.
Kaul 1. Kaul et al _Inventory of Supplemental Materials. Supplemental Figures and Figure Legends. Figure S1, related to Figure 1
 Kaul 1 Kaul et al _Inventory of Supplemental Materials Supplemental Figures and Figure Legends Figure S1, related to Figure 1 Figure S2, related to Figure 2 Figure S3, related to Figure 3 Figure S4, related
Kaul 1 Kaul et al _Inventory of Supplemental Materials Supplemental Figures and Figure Legends Figure S1, related to Figure 1 Figure S2, related to Figure 2 Figure S3, related to Figure 3 Figure S4, related
Nature Neuroscience: doi: /nn Supplementary Figure 1. Iliopsoas and quadratus lumborum motor neurons in the L2 spinal segment.
 Supplementary Figure 1 Iliopsoas and quadratus lumborum motor neurons in the L2 spinal segment. (A) IL and QL motor neurons were labeled after CTb-488 (green) muscle injections at birth. At P4, the L2
Supplementary Figure 1 Iliopsoas and quadratus lumborum motor neurons in the L2 spinal segment. (A) IL and QL motor neurons were labeled after CTb-488 (green) muscle injections at birth. At P4, the L2
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature05772 SUPPLEMENTARY INFORMATION Supplemental figure 1. Enrichment facilitates learning. a. Images showing a home cage and a cage used for environmental enrichment (EE). For EE up to
doi: 10.1038/nature05772 SUPPLEMENTARY INFORMATION Supplemental figure 1. Enrichment facilitates learning. a. Images showing a home cage and a cage used for environmental enrichment (EE). For EE up to
MOLECULAR AND CELLULAR NEUROSCIENCE
 MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
MOLECULAR AND CELLULAR NEUROSCIENCE BMP-218 November 4, 2014 DIVISIONS OF THE NERVOUS SYSTEM The nervous system is composed of two primary divisions: 1. CNS - Central Nervous System (Brain + Spinal Cord)
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy
 Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
When cells are already maximally potentiated LTP is occluded.
 When cells are already maximally potentiated LTP is occluded. Stein, V et al., (2003) J Neurosci, 23:5503-6606. Also found in Rat Barrel Cortex Ehrlich & Malinow (2004) J. Neurosci. 24:916-927 Over-expression
When cells are already maximally potentiated LTP is occluded. Stein, V et al., (2003) J Neurosci, 23:5503-6606. Also found in Rat Barrel Cortex Ehrlich & Malinow (2004) J. Neurosci. 24:916-927 Over-expression
Focal fast rhythmic epileptiform discharges on scalp EEG in a patient with cortical dysplasia
 Seizure 2002; 11: 330 334 doi:10.1053/seiz.2001.0610, available online at http://www.idealibrary.com on CASE REPORT Focal fast rhythmic epileptiform discharges on scalp EEG in a patient with cortical dysplasia
Seizure 2002; 11: 330 334 doi:10.1053/seiz.2001.0610, available online at http://www.idealibrary.com on CASE REPORT Focal fast rhythmic epileptiform discharges on scalp EEG in a patient with cortical dysplasia
Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) (B) (C) (D) (E)
 Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) Confocal images of septal olfactory epithelium of an adult Gγ8-sypGFP-tdTom mouse showing colocalization
Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) Confocal images of septal olfactory epithelium of an adult Gγ8-sypGFP-tdTom mouse showing colocalization
The Amazing Brain Webinar Series: Select Topics in Neuroscience and Child Development for the Clinician
 The Amazing Brain Webinar Series: Select Topics in Neuroscience and Child Development for the Clinician Part VII Recent Advances in the Genetics of Autism Spectrum Disorders Abha R. Gupta, MD, PhD Jointly
The Amazing Brain Webinar Series: Select Topics in Neuroscience and Child Development for the Clinician Part VII Recent Advances in the Genetics of Autism Spectrum Disorders Abha R. Gupta, MD, PhD Jointly
GABA A Receptors and GABAergic Interneurons in Chronic Temporal Lobe Epilepsy
 GABA A Receptors and GABAergic Interneurons in Chronic Temporal Lobe Epilepsy December 4, 2010 Carolyn R. Houser Professor, David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare
GABA A Receptors and GABAergic Interneurons in Chronic Temporal Lobe Epilepsy December 4, 2010 Carolyn R. Houser Professor, David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare
Dissecting the phenotypes of Dravet syndrome by gene deletion
 doi:10.1093/brain/awv142 BRAIN 2015: 138; 2219 2233 2219 Dissecting the phenotypes of Dravet syndrome by gene deletion Moran Rubinstein, Sung Han, Chao Tai, Ruth E. Westenbroek, Avery Hunker, Todd Scheuer
doi:10.1093/brain/awv142 BRAIN 2015: 138; 2219 2233 2219 Dissecting the phenotypes of Dravet syndrome by gene deletion Moran Rubinstein, Sung Han, Chao Tai, Ruth E. Westenbroek, Avery Hunker, Todd Scheuer
Is Intrinsic Hyperexcitability in CA3 the Culprit for Seizures in Rett Syndrome?
 Current Literature In Basic Science Is Intrinsic Hyperexcitability in CA3 the Culprit for Seizures in Rett Syndrome? Network Hyperexcitability in Hippocampal Slices From Mecp2 Mutant Mice Revealed by Voltage-Sensitive
Current Literature In Basic Science Is Intrinsic Hyperexcitability in CA3 the Culprit for Seizures in Rett Syndrome? Network Hyperexcitability in Hippocampal Slices From Mecp2 Mutant Mice Revealed by Voltage-Sensitive
Dorsal Cochlear Nucleus. Amanda M. Lauer, Ph.D. Dept. of Otolaryngology-HNS
 Dorsal Cochlear Nucleus Amanda M. Lauer, Ph.D. Dept. of Otolaryngology-HNS May 30, 2016 Overview Structure Response properties Hypothesized roles in hearing Review of VCN-DCN circuits and projections Structure
Dorsal Cochlear Nucleus Amanda M. Lauer, Ph.D. Dept. of Otolaryngology-HNS May 30, 2016 Overview Structure Response properties Hypothesized roles in hearing Review of VCN-DCN circuits and projections Structure
Case reports functional imaging in epilepsy
 Seizure 2001; 10: 157 161 doi:10.1053/seiz.2001.0552, available online at http://www.idealibrary.com on Case reports functional imaging in epilepsy MARK P. RICHARDSON Medical Research Council Fellow, Institute
Seizure 2001; 10: 157 161 doi:10.1053/seiz.2001.0552, available online at http://www.idealibrary.com on Case reports functional imaging in epilepsy MARK P. RICHARDSON Medical Research Council Fellow, Institute
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
AP VP DLP H&E. p-akt DLP
 A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
Supplemental Information. Induction of Expansion and Folding. in Human Cerebral Organoids
 Cell Stem Cell, Volume 20 Supplemental Information Induction of Expansion and Folding in Human Cerebral Organoids Yun Li, Julien Muffat, Attya Omer, Irene Bosch, Madeline A. Lancaster, Mriganka Sur, Lee
Cell Stem Cell, Volume 20 Supplemental Information Induction of Expansion and Folding in Human Cerebral Organoids Yun Li, Julien Muffat, Attya Omer, Irene Bosch, Madeline A. Lancaster, Mriganka Sur, Lee
Functional Development of Neuronal Networks in Culture -An in vitro Assay System of Developing Brain for Endocrine Disruptors
 Functional Development of Neuronal Networks in Culture -An in vitro Assay System of Developing Brain for Endocrine Disruptors Masahiro Kawahara and Yoichiro Kuroda Tokyo Metropolitan Institute for Neuroscience
Functional Development of Neuronal Networks in Culture -An in vitro Assay System of Developing Brain for Endocrine Disruptors Masahiro Kawahara and Yoichiro Kuroda Tokyo Metropolitan Institute for Neuroscience
A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy se
 A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy serves as a defence mechanism that prevents or retards
A particular set of insults induces apoptosis (part 1), which, if inhibited, can switch to autophagy. At least in some cellular settings, autophagy serves as a defence mechanism that prevents or retards
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Atlas representations of the midcingulate (MCC) region targeted in this study compared against the anterior cingulate (ACC) region commonly reported. Coronal sections are shown on
Supplementary Figure 1 Atlas representations of the midcingulate (MCC) region targeted in this study compared against the anterior cingulate (ACC) region commonly reported. Coronal sections are shown on
Reversing the Effects of Fragile X Syndrome
 CLINICAL IMPLICATIONS OF BASIC RESEARCH Paul J. Lombroso, M.D., Marilee P. Ogren, Ph.D. Assistant Editors Reversing the Effects of Fragile X Syndrome MARILEE P. OGREN, PH.D., AND PAUL J. LOMBROSO, M.D.
CLINICAL IMPLICATIONS OF BASIC RESEARCH Paul J. Lombroso, M.D., Marilee P. Ogren, Ph.D. Assistant Editors Reversing the Effects of Fragile X Syndrome MARILEE P. OGREN, PH.D., AND PAUL J. LOMBROSO, M.D.
Supplementary Figure 1. Nature Neuroscience: doi: /nn.4547
 Supplementary Figure 1 Characterization of the Microfetti mouse model. (a) Gating strategy for 8-color flow analysis of peripheral Ly-6C + monocytes from Microfetti mice 5-7 days after TAM treatment. Living
Supplementary Figure 1 Characterization of the Microfetti mouse model. (a) Gating strategy for 8-color flow analysis of peripheral Ly-6C + monocytes from Microfetti mice 5-7 days after TAM treatment. Living
CYTOARCHITECTURE OF CEREBRAL CORTEX
 BASICS OF NEUROBIOLOGY CYTOARCHITECTURE OF CEREBRAL CORTEX ZSOLT LIPOSITS 1 CELLULAR COMPOSITION OF THE CEREBRAL CORTEX THE CEREBRAL CORTEX CONSISTS OF THE ARCHICORTEX (HIPPOCAMPAL FORMA- TION), PALEOCORTEX
BASICS OF NEUROBIOLOGY CYTOARCHITECTURE OF CEREBRAL CORTEX ZSOLT LIPOSITS 1 CELLULAR COMPOSITION OF THE CEREBRAL CORTEX THE CEREBRAL CORTEX CONSISTS OF THE ARCHICORTEX (HIPPOCAMPAL FORMA- TION), PALEOCORTEX
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
DOI: 10.1038/ncb2566 Figure S1 CDKL5 protein expression pattern and localization in mouse brain. (a) Multiple-tissue western blot from a postnatal day (P) 21 mouse probed with an antibody against CDKL5.
