Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts
|
|
|
- Ernest Price
- 5 years ago
- Views:
Transcription
1 LABORATORY INVESTIGATION MYOCARDIAL INFARCTION Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts PHYLLIS 1. GARDNER, M.D., PHILIP C. URSELL, M.D., JOHN J. FENOGLIO, JR., M.D., AND ANDREW L. WIT, PH.D. ABSTRACT The electrophysiologic and anatomic basis for fractionated electrograms were investigated in superfused epicardial preparations from infarcted canine hearts. Fractionated bipolar electrograms were frequently recorded in preparations from infarcts 2 weeks to 18 months old but only rarely in preparations from 5-day-old infarcts. The fractionated electrograms were not caused by movement artifacts. They were not associated with depressed transmembrane resting or action potentials (which were found in the 5-day-old infarcts), but rather transmembrane potentials recorded in the vicinity of the bipolar electrodes were normal. Despite the normal transmembrane potentials, activation time in regions where fractionated electrograms occurred was prolonged. However, prolonged activation time by itself did not cause fractionation, since fractionated electrograms were not recorded from normal preparations in which conduction was markedly slowed by a superfusate containing 16 mm potassium and epinephrine. Unipolar electrograms recorded with glass microelectrodes (tip size 1 to 5,m) showed that activation in regions where fractionated electrograms were recorded was inhomogeneous. Prepotentials were found preceding the upstrokes of some action potentials in regions where double potentials were recorded, suggesting the possibility of electrotonic transmission across high resistance or inexcitable gaps, but no electrotonic potentials were seen in regions with multicomponent fractionated electrograms. Fractionated electrograms were recorded in regions where infarct healing caused wide separation of individual myocardial fibers while distorting their orientation. The anatomic changes probably caused slow and inhomogeneous activation. Circulation 72, No. 3, , FRACTIONATED electrograms, 1-1 l consisting of either two discrete deflections separated by an isoelectric interval (double potentials or split deflections4' 7, 8. 12) or comprised of many components'6 7 have been recorded during mapping studies on patients with ischemic heart disease and a history of chronic ventricular tachycardia. These kinds of electrograms have been found during sinus rhythm2'6 7'9-1 as well as during tachycardia. 1-3 It is important to determine why fractionated electrograms occur and what they mean. Their occurrence during sinus rhythm might sometimes assist in identifying patients who can develop ventricular tachycardia.6' 8 The site at which these electrograms are record- From the Departments of Pharmacology and Pathology, College of Physicians and Surgeons, Columbia University, New York, NY. Supported by Program Project Grant HL and by a Cardiovascular Training Grant (HL-07271) from the National Heart, Lung, and Blood Institute. Also supported by BRSG SO7RR , NIH. Address for correspondence: Andrew L. Wit, Ph.D., Department of Pharmacology, College of Physicians and Surgeons, Columbia University, 630 W. 168th St., New York, NY Received Sept. 28, 1984; revision accepted June 13, ed either during sinus rhythm or during tachycardia may indicate the site where a reentrant circuit is located.2, 611 On the other hand, fractionated electrograms might sometimes be artifacts of recording techniques,5 caused by such things as electrode movement,'3 14 or distortions of the electrical signal by the filtering characteristics of the amplifiers. 14 Furthermore, since high amplification is often necessary to record fractionated activity in and around aneurysms, it is possible that the fractionated electrograms might sometimes represent activity originating in distant and possibly normal areas.5 13 The studies to determine the value of fractionated electrograms for indicating those patients at risk of having ventricular tachycardia or for locating reentrant circuits must be done in humans.9," However, it will be difficult to determine the exact physiologic causes for such electrograms in clinical studies. To understand why fractionated electrograms occur, we have done electrophysiologic and anatomic investigations on areas where such activity is present in isolated CIRCULATION
2 LABORATORY INVESTIGATION-MYOCARDIAL INFARCTION superfused preparations from canine infarcts. Preliminary reports of some of our results have been presented.'5' 16 Methods Surgical production of myocardial infarction. Myocardial infarction was caused in 50 mongrel dogs weighing 13 to 18 kg by a two-stage ligation of the left anterior descending coronary artery (LAD) 5 to 10 mm from its origin, with sterile techniques Forty-five dogs survived the surgical procedure. In this report we include only data from the 36 transmural infarcts. At various times after coronary occlusion the surviving dogs were reanesthetized with sodium pentobarbital (15 to 30 mg/kg iv) for the electrophysiologic and anatomic study. Ten animals were studied at 5 days, six at 15 days, seven at 2 months, six at 4 months, four at 8 months, two at 16 months, and one at 18 months. We chose these times because they represent periods during the subacute (5 days), healing (15 days), and healed (2 to 18 months) phases of myocardial infarction. We characterized the electrograms and the cellular electrophysiologic and microanatomic features of the muscle that survives on the epicardial surface of the transmural infarcts at each of these different stages. Five normal dogs that had not undergone coronary artery ligation served as controls. Electrophysiologic studies in vitro. After induction of anesthesia, the chest was reopened and the infarcted region was visually identified by a mottled or pale appearance. A piece of myocardium approximately 20 X 20 x 3 mm was then excised rapidly with a sharp scalpel blade from the epicardial aspect of the infarcted left ventricle in situ. Whenever possible this piece was taken from a region devoid of large epicardial coronary arteries. It was immediately pinned to the waxed base of a 25 ml superfusion chamber, epicardial surface upward, and superfused with oxygenated Tyrode's solution (composition previously described'9) at a rate of 50 ml/min. The solution was briskly bubbled with a 95% 02/5% CO2 gas mixture both in the reservoir and in the bath. The ph of the Tyrode's in the tissue bath under these conditions was 7.30 to The temperature was maintained constant at C. The preparations of epicardial muscle were stimulated through Teflon-coated, bipolar, silver-wire electrodes at basic stimulus cycle lengths of 800 to 1000 msec.'9 After an initial equilibration period of 60 min, extracellular electrograms and transmembrane action potentials were recorded from subepicardial fibers at multiple sites Extracellular electrograms were recorded through electrodes made from Teflon-coated silver wire. The diameter of the electrode surface in contact with the tissue was 0.3 mm. Bipolar electrodes had a distance between recording poles (measured from the center of one pole to the center of the other) of 0.5 to 1.0mm. When a unipolar electrode was used, the indifferent electrode was located in the bath approximately 30 mm from the recording electrode. Extracellular signals were amplified with a Tektronix 2A61 differential amplifier. The low and high bandpasses were 0.06 and 6000 Hz. We also used 3M KCI-filled glass capillary electrodes with tip diameters estimated to be about 1 to 5,um to record unipolar electrograms. The signal recorded with these electrodes was led into the high-input impedance negative capacitance amplifier that was used for the transmembrane potential recordings. Membrane potentials and electrograms were displayed on a Tektronix 565 oscilloscope and photographed with a Grass C-4 oscilloscopic camera. Action potential parameters were measured by methods described in other publications.ir 19 Histologic and morphometric analysis. We did not usually find fractionated electrograms in preparations from noninfarcted hearts or from 5-day infarcted hearts (see Results). Because electrograms in most regions had a similar configuration, it was not necessary to mark a specific recording site for anatomic study. Instead, the entire preparation of epicardium was fixed in 10% neutral buffered formaldehyde. After fixation, the tissue was sliced into five pieces and cut perpendicular to the LAD border and perpendicular to the plane of the epicardium. The slices were then processed for histologic study according to routine methods.20 Each histologic section (5 gm thick), stained with hematoxylin-phloxine-saffron, showed the full thickness of the preparation, from the epicardial surface to the surface where the preparation was cut from the ventricular wall. In the preparations from the healed infarcts, both fractionated and nonfractionated electrograms were found (see Results). Selected areas at which electrograms with different characteristics were recorded were marked with color-coded pins during the experiment, and then with India ink before fixation. These marked areas were then cut from the epicardial surface with a sharp razor blade and fixed in formaldehyde. The size of these pieces was approximately 2 x 4 mm and 1 to 2 mm thick. The remainder of each preparation was also fixed in the formaldehyde. The small, marked pieces were oriented in the paraffin embedding blocks so that the epicardial surface formed one margin of each section. These specimens were then serially sectioned (5,um thick) and each section stained with hematoxylin-phloxine-saffron. The number of muscle fibers surviving at the recording sites was determined from the serial sections. We also applied morphometric techniques to the histologic sections to determine the relative proportion of heart muscle to nonmuscular tissue in regions where different kinds of electrograms were recorded. Representative experiments from each age of infarct as well as noninfarcted hearts were selected for this analysis (two normal, four at 5 days, two at 15 days, two at 2 months, and four at 4 to 18 months). Morphometric analysis was done on blocks that were serially sectioned, yielding 50 to 150 5,um thick sections. The sections were stained and mounted on glass slides in the usual manner. Tissue sections without folds were selected randomly (eight to 10 sections per experiment) and photographed. Each negative was enlarged and printed on 8 x 10 inch photographic paper (final magnification x 130 to 500). Morphometric analysis was confined to the region designated as the epicardial border zone.'9 This area extends from the layer of muscle cells immediately subjacent to the epicardium to the deepest surviving muscle layer overlying the infarct. Within the region we determined the amount of muscular tissue relative to nonmuscular tissue (including blood vessels, connective tissue, and inflammatory cells) using the point-counting method.21 A grid composed of boxes no larger than the average muscle fiber diameter was superimposed on the photographs. If the tissue to be measured contained longitudinally oriented muscle fibers, the axis of the grid was placed at 19 degrees to the long axis of the fibers to counteract the bias introduced by anisotropy. 22 The appropriate density of points for each case was implied by the magnification of the photomicrograph.21 Each cross point on the grid was evaluated to determine whether it lay over heart muscle or nonmuscular tissue. A ratio of heart muscle "hits" to nonmuscular "hits" was made. The mean and standard deviation of the ratio from all determined. photographs from the experiments were Results Characteristics of extracellular electrograms Dependence on infarct age. Ventricular muscle survives on the epicardial surface of transmural canine infarcts. ' We determined the characteristics of the extracellular waveforms recorded from this subepicar- Vol. 72, No. 3, September
3 GARDNER et al. A B C D 7 5 mlec 005 my 10 "ms *0.5 V 10Osec /0 misc 0.2 mv E F G H I } V FJ0.2mV loasec louisec 0 msc J K L -J05my,.0sav, 0vJ.05m.05 m 10 misc 10 use 10meec 10luc FIGURE 1. Representative bipolar electrograms recorded from superfused subepicardial preparations. Preamplifier filters were set at 0.06 to 6000 Hz. The electrogram shown in A was recorded from a noninfarcted preparation, the electrograms in B and C from 5-day-old infarcts, the electrograms in D and G from 2-week-old infarcts, the electrograms in E, F, I, and J from 16-monthold infarcts, and the electrograms in H, K, and L from 2-month-old infarcts. dial muscle with bipolar electrodes and compared them with electrograms recorded from noninfarcted preparations. Bipolar recordings were used in much of this study because the clinical reports on fractionated electrograms have relied on this technique.' "Thirty to 60 electrograms were recorded at approximately 2 mm intervals over the entire surface of each preparation. Extracellular waveforms recorded from subepicardial muscle in preparations from noninfarcted hearts consisted of smooth contour, biphasic, or triphasic deflections with amplitudes of 2 to 4 mv and durations of less than 5 msec (figure 1, A). Most of the bipolar electrograms recorded from preparations from hearts with 5-day-old infarcts had similar configurations (figure 1, B). The main difference between electrograms recorded from these infarct preparations and normal preparations was a decrease in the amplitude of the electrograms recorded from the infarcts (range from 500,V to 2 mv) and a small increase in duration (up to 10 msec). Occasionally, electrograms were recorded from some preparations from 5-day-old infarcts, which had irregular waveforms, characterized by notching or multiple components (figure 1, C). The total duration of these electrograms was not longer than 10 msec. 598 Unlike the 5-day-old infarct preparations, fractionated electrograms were frequently recorded with bipolar electrodes from the 2-week- to 18-month-old infarcts (figure 1, D and G to L). In three of the preparations from healed infarcts (one 2 months old, one 16 months old, one 18 months old) all electrograms were fractionated. In the remaining preparations both fractionated electrograms and electrograms with normal contours were recorded (figure 1, E and F). Some of the fractionated electrograms had-two or more discrete spikes separated by isoelectric intervals (see figures 5 and 10). Other electrograms consisted of multiple spikes, separated either by slower waveforms or isoelectric segments (figure 1, G to L). The duration of the fractionated electrograms was highly variable and, in general, there was an increase with infarct age. The duration of electrograms was greater in 2- to 18- month-old infarcts (20 to 70 msec duration) (figure 1, H to L) than in 2-week-old infarcts (10 to 20 msec duration) (figure 1, D and G). We did not notice any marked differences in duration among the 2- to 18- month-old infarcts. The amplitude of the signals also decreased with infarct age so that at 2 weeks, amplitudes were consistently between 200,uV and 1 mv (figure 1, D and G) while at 2 months and more they CIRCULATION
4 LABORATORY INVESTIGATION-MYOCARDIAL INFARCTION were often less than 200 gv (figure 1, I to L). In these healed infarcts electrograms that were not fractionated often had low amplitudes as well (figure 1, F). Is fractionation caused by recording artifacts? It has been suggested that the fractionated nature of some electrograms might be artifactual, caused by contraction-induced electrode movement or by the filtering characteristics of the amplifier used to record them (see above). We recorded stable fractionated electrograms for 3 to 5 hr after the tissue was mounted in the superfusion chamber. Because these preparations -had only a narrow surface rim of viable muscle fibers (see histologic results) there was often no visible contraction or movement of the electrodes even when the preparation was observed under the high-power optics of the dissection scope over the tissue chamber. Therefore the fractionated nature of the electrograms could not be attributed to artifacts generated by gross movement at the electrode-tissue interface. Nor was fractionation an artifact introduced by filtering out low-frequency components of a single broad electrogram. The electrograms shown in figure 1 were recorded at a bandpass of 0.06 to 6000 Hz, which permits most slow and fast components of extracellular activity to be recorded except for direct current potentials. The total duration of fractionated electrograms and number of components was influenced by changing the bandpass but we did not study these effects in detail. In general, increasing the high bandpass filter setting from 0.06 to 60 Hz decreased the amplitude of the electrograms and attenuated the slow components (compare right panels A and B in figure 2). Effects of electrode configuration and direction of impulse propagation. The recording electrode configuration also influenced the characteristics of the fractionated electrograms. The electrograms shown in figure 1 were recorded with a bipolar electrode. Fractionated electrograms, however, were not caused by bipolar recording techniques per se. At all sites at which fractionated electrograms were recorded with bipolar electrodes we could also record fractionated activity with unipolar electrodes (figure 2, left). In experiments in which bipolar electrograms were first recorded and then unipolar electrograms recorded from each of the bipoles as shown in the figure, it appeared that the fractionated waveform of the bipolar electrogram resulted from summation of the fractionated unipolar waveforms (figure 2, left). The direction of impulse propagation with relation to the orientation of the bipoles also affected the characteristics of the fractionated electrograms. Usually when the site of stimulation was changed to alter the A 0.2r C BIPOLAR UNIPOLAR I UNIPOLAR 2 A Hz Hz >e~~~~~~~~ FIGURE 2. Left, Fractionated electrogram recorded with a bipolar electrode (panel A) and with each of the poles separately (panels B and C). Right, Fractionated electrogram recorded with different preamplifier filter settings. propagation direction the number of components either increased or decreased (figure 3). Electrophysiologic basis for fractionated electrograms Transmembrane potentials in regions offractionation. We have shown that fractionated electrograms appear during infarct healing. The question then arises, are there special electrophysiologic properties of the healing infarct that cause them? Spach et al.25 have indicated that complex polyphasic waveforms (fractionation) could arise from a peculiar shape of the transmembrane action potentials - from membrane currents associated with a complex or multicomponent phase of depolarization of individual cells. Fractionation might also be AB O.2mV 5 msec FIGURE 3. Effects of changing the direction of impulse propagation on a fractionated electrogram. Panels A and B show an electrogram recorded as the preparation was stimulated from two different sites. Vol. 72, No. 3, September
5 GARDNER et al. the result of superposition of extracellular currents from asynchronous depolarizations in a number of functionally different cells with normally shaped action potentials.25 We recorded transmembrane action potentials in regions from which fractionated electrograms were recorded and compared them with transmembrane potentials in regions where smooth, normally shaped electrograms were recorded to determine whether any particular characteristic could be correlated with the occurrence of fractionated activity. Action potentials were recorded from 10 to 30 sites within a 2 to 5 mm2 area around the extracellular electrode and then from the region from which this electrode was recording after it was removed from the surface of the tissue. In 5-day-old infarct preparations, resting potential (RMP) was - 77 ± 7 mv, action potential maximum upstroke velocity (Vmax) was 73 ± 9 V/sec, amplitude of phase 0 (APA) was 86 ± 8 mv, and action potential duration to 50% (APD50) and 90% (APDw) repolarization was 120 ± 10 and msec. These represent mean values + SE for 348 impalements at electrogram recording sites in 10 preparations. All values are significantly less (p <.05) than values for muscle fibers in noninfarcted preparations where normal electrograms were recorded (RMP -91 ± 4 mv, Vmax = V/sec, APA = 103 J 5 DAY INFARCT 6 mv, APD50 = 175 ± 8 msec, APD msec; 283 impalements in five preparations). A representative transmembrane potential recorded from a muscle fiber in a 5-day-old infarct is shown in the top left panel of figure 4. Similar results have been previously reported from other studies on transmembrane potentials of epicardial muscle surviving in infarcted regions at this period.'9' These alterations in the transmembrane potentials, however, were not associated with fractionated electrograms, which, as shown above, were not common in these preparations (figure 4, top left). In 2-week-old infarcts, resting potential (-88 ± 9 mv), Vmax ( v/sec), and action potential amplitude (101 ± 8 mv) were not significantly different than normal, although action potential duration (APD msec, APD ±+ 12 msec) was still short (p <.05; 169 impalements in six preparations). In the healed infarcts (2 months and older) where fractionated electrograms were most prominent, the transmembrane potentials of the muscle fibers were not significantly different from normal, including the time course for repolarization (RMP = mv, Vmax = 101 ± 15 V/sec, APA mv; APD50 = msec, APD ± 12 msec; mean values for 180 impalements in seven preparations). A representative action potential recorded at a site of fractionated activity in a healed infarct is 2 MONTH INFARCT I.Omm FIGURE 4. Activation maps of regions around bipolar electrodes in two different infarct preparations. The location and size of the electrodes are indicated by the stippled circle and the electrograms recorded in each experiment are shown above. The points at which action potentials were recorded in each panel are indicated by the dots. Representative action potentials are also shown. The arrows and isochrones show the direction of activation. The stimulus sites are not included in the maps. The distance scale for each panel is shown below; note that the scale is two times larger for the 5-day-old infarct preparation than for the 2-month-old infarct preparation. 600 CIRCULATION
6 LABORATORY INVESTIGATION-MYOCARDIAL INFARCTION shown in the top right panel of figure 4. In addition, in preparations from healed infarcts, transmembrane potentials recorded from regions where there were fractionated electrograms were no different from transmembrane potentials recorded from regions where electrograms had a normal contour. In the experiments in which action potentials were recorded from regions with fractionated electrograms containing a large number of spikes (such as electrograms K and L in figure 1), the upstrokes were characterized by a normal smooth S-shaped curve. Fractionation, therefore, could not be caused by a complex action potential depolarization phase. In experiments in which action potentials were recorded from regions where "double potentials" were found, we sometimes saw low-amplitude prepotentials before the action potential upstroke (figure 5, E and F). Action potentials with two component upstrokes were also found, each component corresponding with an electrogram deflection (figure 5, C and D). For reasons described later, we do not attribute these multiple components of the upstroke to different active membrane processes but rather the evidence suggests that they are caused by electrotonic interactions among adjacent, poorly coupled fibers. Our conclusion from the data presented in this section is that fractionated electrograms are not necessarily related to depressed resting potentials or action potential upstroke velocities. Complex upstrokes of action potentials of individual muscle cells do not appear to cause multiple component fractionated electrograms, although they were sometimes found to be associated with double potentials. Relationship offractionation to characteristics of local activation. It has been suggested that fractionated electrograms recorded in acute myocardial infarction are caused by slow activation.28 It was therefore surprising that the transmembrane action potentials at the sites where these electrograms were recorded in healed infarcts arose from normal resting potentials and had normal upstroke velocities, properties not usually associated with slow conduction.29 To evaluate conduction characteristics in regions where electrograms were recorded, we also mapped impulse propagation. Action potentials were recorded from 20 to 50 sites within a 10 to 20 mm2 area around the extracellular electrode. Activation maps were constructed for regions in which both normal and fractionated electrograms were recorded by using the beginning of the extracellular electrogram as a reference (where the potential first deviated from isoelectric) and determining the time of activation at each microelectrode recording site (measuring the time interval from the reference to the up- Vol. 72, No. 3, September 1985 stroke at 50% of its maximum amplitude). Normally shaped electrograms recorded from preparations from noninfarcted hearts and from some regions in preparations from infarcted hearts were found in areas where a broad wavefront propagated rapidly past the recording site (figure 4, left). The region around the bipolar recording electrode was activated in less than 5 msec. In the normal preparations and in these regions of preparations from healed infarcts 2 months and older, the apparent conduction velocity calculated in a direction normal to the isochrones and parallel to myocardial fiber orientation (verified by subsequent histologic study; see below) was 0.5 to 1.0 in/sec. In regions of 5- FIGURE 5. Action potentials in a region where a "double" potential was recorded. The two circles in the middle of the figure indicate the bipolar electrode. The x's show where action potentials were recorded. (The microelectrode was actually adjacent to the bipoles and not beneath them.) The thick dark lines are isochrones indicating activation times and the direction of propagation at this recording site. Panels A to G show simultaneous recordings of the action potentials and the extracellular electrogram. The solid arrows in each panel indicate the first component of the "double" potential. This component is preceded by a stimulus artifact. The upstroke of the action potentials in A and B occur simultaneously with the first electrogram deflection. The unfilled arrows indicate possible electrotonic influences on the plateau phase. The upstrokes of the action potentials in C and D have two components, one corresponding to the first electrogram deflection (unfilled arrow) and one corresponding to the second electrogram deflection. The upstrokes of the action potentials in E and F are simultaneous with the second electrogram deflection but are preceded by an electrotonic prepotential (unfilled arrows) that begins with the first electrogram deflection and spans the isoelectric segment between the two deflections. The upstroke of the action potential in G occurs just after the second electrogram deflection and is not preceded by a prepotential. In this experiment, no action potential upstrokes were found to occur in the isoelectric segment between electrogram deflections. Voltage calibration is for the transmembrane potential recording. 601
7 GARDNER et al. day-old infarcts where normally shaped electrograms were recorded, apparent conduction velocity calculated in the same manner was 0. 2 to 0.4 m/sec (figure 4, left). Activation of the regions in the vicinity of the electrodes recording fractionated electrograms took a much longer period of time. The prolonged activation time was particularly pronounced in the preparations from hearts with healed infarcts (2 to 18 months) despite the normal resting potentials and action potential upstroke velocities. In figure 4, right, the fractionated electrogram had a total duration of about 17 msec. In the region from which this electrogram was recorded it took more than 12 msec for the impulse to move a distance of about 0.5 mm between the electrode poles (indicated by the stippled circle) as compared with a time of 2 msec for the same distance in the activation map of a 5-day-old infarct shown in the left panel. If it is assumed that conduction occurred in a reasonably straight line past the electrode poles (and it may not have), conduction velocity would have been approximately 0.04 m/sec. In other experiments on healed infarct preparations, activation time in the vicinity of the electrodes with which fractionated activity was recorded was also similar to the total duration of the electrogram and therefore ranged from 10 to 70 msec (apparent conduction velocities of 0.05 to 0.01 m/sec). It is also possible that some of the fractionated activity might be accounted for by slow or late activation of myocardial fibers that are too deep from the endocardial surface to be impaled with the microelectrode. Activation may be three-dimensional rather than the two-dimensional activation shown on the map. Is the slow activation of regions where fractionated electrograms were recorded the primary cause of the fractionation? If it is, it would seem that slowing activation to a similar extent in regions where electrograms have a normal configuration should cause fractionation. We slowed conduction in these regions in both normal and 5-day-old infarct preparations by increasing the K + concentration to 16 mm, which caused inexcitability, and then by restoring very slow conduction by adding 1,g/ml epinephrine to the superfusate When this was done there was a marked decrease in electrogram amplitude, a slowing of its rate of depolarization, and an increase in its duration as shown in figure 6, A to C. At the high amplification used to display the electrogram, a second component was evident (figure 6, C), which always coincided with the repolarization phase of the action potential (figure 6, 1, 2, and 3) and was probably caused by repolarization. Similar double-component electrograms were recorded at all sites on the preparation where slow response action potentials were recorded. The upstroke of the action potential recorded near an extracellular electrode always coincided with the first electrogram component and repolarization always coincided with the second component, even when the microelectrode was moved to different sites around the extracellular recording electrode (see figure 6, 1, 2, and 3). In none of these experiments in which slow conduction (conduction velocities less than 0.05 m/sec) was caused by high K+ concentration and epinephrine did multicomponent fractionated electrograms occur. Therefore, slow conduction (alone) is not the cause of fractionated activity, although it does increase the total duration of the electrogram. We also elevated K concentration in experiments in which fractionated electrograms were recorded from healed infarcts. When the K+ concentration in the superfusate reached 12 to 14 mm, the resting potential and upstroke velocities of the fibers in the vicinity of the fractionated electrograms were significantly depressed and activation time of these regions prolonged. The total duration of the fractionated electrogram increased, its amplitude diminished, and the rate of change of the voltage of the individual spikes comprising the electrogram diminished. However, either the number of components comprising the fractionated electrogram did not change (figure 7) or actually decreased. These results suggest further that the activation time and the action potential characteristics are not the main determinants of the fractionated nature of these electrograms. Instead of simply slow activation, fractionated electrograms might be caused by slow activation in combination with inhomogeneities in activation around the recording electrode.28 Such inhomogeneities on a "micro" level would not be apparent in activation maps like the one shown in figure 4. To obtain more detailed information on characteristics of activation in the vicinity of the extracellular bipolar electrode we simultaneously recorded extracellular waveforms with glass capillary electrodes with tip diameters of about l to 5 gm. The small tip size enabled us to record extracellular activity from individual muscle fibers25' 32 at numerous sites within the 1 mm distance between the bipoles. Recordings are shown in figure 8 for a representative experiment in which the fractionated electrogram had three discrete components separated by short isoelectric segments. Within a 1 mm circumference around the positive electrode pole, a single biphasic deflection was recorded by the extracellular microelec- 602 CIRCULATION
8 LABORATORY INVESTIGATION-MYOCARDIAL INFARCTION trode. This unipolar electrogram coincided in time with the initial deflection of the fractionated electrogram (figure 8, A). As the microelectrode was moved in steps of approximately 0.1 mm toward the negative pole, the amplitude of the waveform coinciding with the first fractionated component decreased and an extracellular potential coincident with the second deflection of the fractionated electrogram appeared (figure 8, B). This waveform became larger as the microelectrode was moved to another site (figure 8, C). Similarly, the amplitude of this waveform decreased as the extracellular electrode was moved to another site and a deflection coinciding with the third component of the fractionated electrogram appeared (figure 8, D) and increased in amplitude (figure 8, E) until only this deflection was found (figure 8, F). It is apparent from this experiment that an extracellular signal resulting from activation could not be recorded in the vicinity of the bipolar electrode during segments between the major components of the fractionated electrogram. The results of other experiments of this kind are similar. Spach et al.32 have shown that a uniform biphasic waveform such as those recorded with the extracellular microelectrode is characteristic of action potentials propagating at uniform velocity in a functionally single strand or bundle of cardiac muscle. Interpreted the same way, the results of the experiment shown in figure 8 indicate the presence of three functional strands or bundles of cardiac muscle, each giving rise to a biphasic waveform. The summation of the extracellular waveforms from each of these strands causes the fractionated electrogram recorded with the bipolar electrode.25 Furthermore, the results of this experiment show that activation in the area where the fractionated A B 10 msec J0.2 mv C J.05 mv FIGURE 6. Electrograms and action potentials recorded from a normal preparation superfused with high K + concentration Tyrode's solution containing epinephrine. At the top, panel A shows the control electrogram in normal Tyrode's solution, panel B shows the electrogram as the elevated K+ solution entered the chamber, and panel C shows the electrogram in the high-k+, catecholamine-containing solution. Note the two components to this electrogram. At the bottom center is shown a diagram of the preparation and the activation map in the high-k+, catecholamine solution. The dots and asterisks indicate sites at which extracellular electrograms were recorded with a roving electrode. They are 1 mm apart. Representative electrograms, recorded with this probe during mapping, are in the top trace of panels 4, 5, and 6. They all have two components (labeled 1 and 2). The electrogram in the bottom trace in each panel was recorded by a reference electrode near the stimulus (S). At the left, in the top traces of panels 1, 2, and 3, action potentials recorded in the vicinity of an extracellular bipolar electrode (indicated by circles) are shown. The bottom trace shows the electrogram. The depolarization phase of the action potential corresponds to the first electrogram component, and repolarization corresponds to the second component. Upstrokes coincident with the second component were not seen (see figures 5 and 10). Vol. 72, No. 3, September
9 GARDNER et al. 8 WEEK INFARCT 4mM KCL 12 mm KCL electrode occurred at all times throughout the duration of the broad bipolar electrogram and there were no time gaps without electrogram upstrokes. Propagation with the characteristics shown in figure 8 might occur for a number of different reasons, several of which are diagrammed in figure 9. The model shown in panel A assumes circuitous conduction (indicated by the arrows) in the vicinity of the bipolar electrode. Conduction velocity in each of three functionally separate strands of muscle is normal (action potentials are normal), yet activation time in the region of the bipolar electrode would appear slow because of the circuitous pathway of propagation. Isoelectric intervals occur during the time the impulse is out of the recording field of the bipolar electrode; components of the fractionated electrogram occur when the impulse returns in one of the muscle strands. (The term "functional strand" is not meant to indicate the actual number of muscle fibers. A functional strand might be composed of a single fiber or a number of closely coupled fibers in which there is uniform propagation.) The model shown in panel B assumes exaggerated discontinuous conduction33, 4 with large pauses be- lit ms 2o.lmv FIGURE 7. Effects of elevated K + concentration on a fractionated electrogram. The electrogram shown in the top panel was recorded in a preparation from a 2-month-old infarct, superfused with Tyrode's solution containing 4 mm K '. Some of the components of the electrogram, indicated by the arrows, are numbered. The electrogram recorded at the same site is shown in the bottom panel during superfusion of the preparation with Tyrode's solution containing 12 mm K. The elevated K' concentration depressed the transmembrane potentials and slowed conduction. The amplitude of the electrogram decreased and the total duration increased but the same fractionated components can be identified (arrows and numbers below the electrogram trace). electrogram was recorded occurred in steps or jumps, progressing from the 40 to 55 msec isochrones. No rapid biphasic deflections were detected at 42 to 46 msec or at 49 to 53 msec. In contrast, when similar experiments were done with extracellular microelectrodes during slow conduction caused by high K+ concentration and catecholamines, there was a smooth propagation of the waveform; upstrokes of biphasic extracellular electrograms recorded with the micro- 604 B C D EX F 8 ;';; FIGURE 8. In each panel the top trace is the unipolar electrogram recorded with an extracellular microelectrode and the bottom trace is the fractionated electrogram recorded with the bipolar electrode (indicated by the large circles). The dashed lines and the asterisks show the sites where the unipolar electrograms were recorded, relative to the poles of the bipolar electrode. The large black arrows indicate the direction of impulse propagation and the black lines are isochrones showing activation times. The voltage calibration above is 0.2 mv for the bipolar recording and 10 lxv for the unipolar recording CIRCULATION
10 LABORATORY INVESTIGATION-MYOCARDIAL INFARCTION A abc R cl b c electrotonic prepotentials occurring before the upstrokes of action potentials in regions where fractionated electrograms are recorded We did observe such prepotentials before the upstrokes of action potentials recorded in some areas where "double" potentials occurred. Examples are shown in figures 5 and 10. In the experiment illustrated in figure 10, D and E, action potentials with upstrokes coinciding either with the first or second component of the "double" potential were recorded in well-defined areas indicated on the diagram. No upstrokes occurred in the segment between the two electrogram components. The upstrokes coinciding with the second electrogram component were preceded by a low-amplitude prepotential (indicated by the open arrow with asterisks in figure 10, E), which began during the first electrogram component and spanned the isoelectric segment. The action potential recordings shown in figure 5 are c FIGURE 9. Two models that might explain the occurrence of the fractionated electrograms. In A, three cardiac fibers or bundles are indicated by the cylindrical structures. These fibers are separated by connective tissue as shown by the results of the anatomic studies and are not interconnected in the region shown by the diagram. However, interconnections do occur at a distant site. The large circles represent the location of the bipoles and the dark arrows show impulse propagation. In B, the three cardiac fibers are connected and impulse propagation occurs in a relatively straight line through these connections as indicated by the arrows. If it is assumed that the resistance at the connections is high, there might be a delay in conduction from one fiber to another. The model does not explain the different amplitudes of the electrogram components that may result from different diameters of the bundles or different distances of each bundle from the recording electrodes. tween activation of each functional muscle strand; unlike the model in panel A, the impulse is present at all times in the vicinity of the bipolar electrode. This might occur if the resistance at intercellular contacts (intercalated discs) between myocardial fibers is high, causing slow impulse transmission across high-resistance junctions or barriers.33"- Other possible causes for such exaggerated discontinuous conduction include (1) localized increases in other components of intracellular (cytoplasmic) resistance, (2) abrupt changes in cell size or surface-to-volume ratios, (3) marked differences in the number of cells connected across the high-resistance barrier, (4) localized increases in extracellular resistance, or (5) abrupt cell branching If abrupt increases in intercellular coupling resistance as shown in the second model (figure 9, B) accurately explain the multicomponent nature of the fractionated electrograms, we might expect to observe Vol. 72, No. 3, September 1985 A 50 mv D 20msec GI \ B EGoi 3 C 50mY 5 msec FIGURE 10. Action potentials at sites where fractionated electrograms were recorded in two different experiments, one shown in the left panels (A to C) and the other shown in the right panels (D and E). The large circles in the middle of the figure indicate the bipoles of the electrode. The electrograms in panels A, B, and C have three components (EG 1, 2, and 3). The upstrokes of the action potentials are indicated by AP,. Upstrokes were found corresponding to EG 1 (A), EG 2 (B), and EG 3 (C), but no upstrokes occurred in the segments between components. These action potentials were recorded in three areas indicated by the cross-hatching. No action potentials could be recorded from the stippled region. The electrograms in panels D and E have two components (EG1 and EG). The action potential upstrokes are indicated by AP0. Action potential upstrokes coincided either with the first component (D) or the second component (E) but never occurred in the isoelectric segment between components. Panel E also shows the electrotonic prepotential (indicated by asterisks) occurring before the action potential upstroke. Regions where action potentials could not be recorded were also found in this experiment (indicated schematically by shaded area)-. 605
11 GARDNER et al. more complex. Again, upstrokes were found that coincided either with the first or second electrogram component but not in the isoelectric segment between components. The action potentials with upstrokes occurring during the first electrogram deflection are shown in panels A and B. Also evident in these recordings are the probable electrotonic effects of distant activation on action potential repolarization: there is an exaggerated repolarization phase (unfilled arrows) before the plateau that coincides with the second electrogram deflection. As the microelectrode was moved toward sites C and D, two component upstrokes were seen, with each component coinciding with one of the electrogram deflections. At sites E and F there were only low-amplitude prepotentials before the upstrokes that coincided with the second electrogram deflection. At another site nearby (G), action potentials not preceded by electrotonic potentials were found. It is apparent from the recordings shown in both figures 5 and 10 that there are strong electrotonic interactions between myocardial fibers in regions where double potentials are found. However, the recordings do not completely solve the problem as to whether there is discontinuous electrotonic transmission, or circuitous conduction. Electrotonic interactions between adjacent fibers might occur even when an impulse is not conducting directly from one fiber to the other. We did not observe electrotonic prepotentials in.. most of our action potential recordings from the vicinity of fractionated electrograms with three or more components. Upstrokes of action potentials recorded near the bipolar electrode from the experiment illustrated in figure 8 are shown in figure 10, A to C. Upstrokes coincided with either the first (A), second (B), or third (C) component of the electrogram but the upstrokes are not preceded by prepotentials. This applies also to 20 other action potentials recorded in this region. In addition, action potential upstrokes were found only in well-defined regions (indicated schematically in the diagram by cross-hatching). No action potentials could be recorded in other regions (indicated by the shading). Furthermore, action potential upstrokes were not found to occur during the time be~ tween the major three components of the electrogram. The absence of electrotonic prepotentials, however, does not eliminate the possibility that slow conduction across high-resistance barriers occurred. High-resistance barriers might change the foot of the action potential upstroke without causing the marked prepotential shown in figures 5 and 10.33' 36 We did not perform the detailed analysis of the foot that would be necessary to determine whether it had been altered. Anatomic basis for fractionated electrograms. There were marked differences between the microanatomy of regions where normally shaped electrograms were recorded and regions where fractionated electrograms? B Aff FIGURE 11. Histologic appearance of regions where electrograms without tractionation were recorded in a 5-day-old infarct preparation (A) and in a 16-month-old infarct preparation (B). Just beneath the epicardiurn (E), the surviving muscle fibers are indicated by the arrows. Beneath this histologically normal muscle is the infarct (i). At 5 days the infarct consists of necrotic muscle fibers, whereas at 16 months the infarct zone in this field is composed largely of fat. (Hematoxylin-phloxine-saffron; A andb x 150.) 606t CIRC ULAl TION
12 LABORATORY INVESTIGATION-MYOCARDIAL INFARCTION were recorded in the infarct preparations. These differences may explain the occurrence of slow, inhomogeneous conduction and fractionated extracellular waveforms. Figure 11, A, shows the histologic appearance of the 5-day-old infarct preparation from which the normally shaped electrogram in figure 4 (left) was recorded. These histologic features are representative of areas in 5-day-old infarcts where smooth, biphasic electrograms were found. In these regions a thin rim of five to 30 intact ventricular muscle fibers survived the coronary occlusion and overlay the infarct (a mean value of 12 fibers in figure 11 determined from serial sections). These muscle fibers had a normal appearance and were characterized by intact cell membranes, myofibrils exhibiting cross-striations and ovoid nuclei with evenly dispersed chromatin. The muscle fibers were arranged parallel to each other, in closely packed fascicles, separated by edema and normal amounts of connective tissue. The separation of the muscle fibers is indicated by the results of the morphometric analysis. The ratio of muscular to nonmuscular elements (M/NM) at electrogram recording sites in noninfarcted subepicardium was and 12.9 ± 1.9 in the two experiments in which it was determined (average 14.4, which is equivalent to 93.5% muscular tissue and 6.5% nonmuscular tissue). In the experiment illustrated in figure 1 1, A, the M/NM ratio was or 13.5% nonmuscular tissue. The mean value for the four experiments on 5-day-old infarcts that were analyzed was (19.2% nonmuscular tissue). The increase in the nonmuscular component of the epicardial border zone was mainly caused by the edema. In the 2- to 16-month-old infarcts where normally shaped electrograms such as the ones shown in figure 1 (E and F) were recorded, closely packed, paralleloriented subepicardial muscle fibers were also found overlying the fibrotic, healed region (figure 11, B). In these areas, there were at least 10 surviving cell layers and sometimes as many as 100. In figure 11, B, showing the site at which the electrogram in figure 1, E, was recorded, there are 14 to 18 layers of muscle cells determined from serial sections. The MINM ratio was in this experiment; nonmuscular tissue composed 17.9% of the epicardial border zone. This is similar to the results of the analysis of the 5-day-old infarcts. The fibrous tissue from the healed infarct did not penetrate into the subepicardial aspect of this rim of muscle. In contrast, in regions where fractionated electrograms were recorded, large amounts of fibrous tissue from the adjacent healed infarct had invaded the surviving subepicardial muscle layers (figure 12). This expansive growth of fibrous tissue separated the surviving muscle fibers from each other along their length to such an extent that often side-to-side contacts between bundles could not be seen. Moreover, the myocardial fibers were no longer arranged in parallel to one another. In the six experiments in which morphometric analysis was done, the M/NM ratio was (42.6% nonmuscular tissue in the epicardial border zone, significantly different from 5-day-old infarcts (p <.05). An exact correlation between nistologic ana eiectrophysiologic results was accomplished from the serial sections of regions where electrograms were recorded. For example, from the data presented in figure 8 it was postulated that there were three functional strands of muscle giving rise to the fractionated electrogram. Figure 12, A and B, shows the histologic appearance of the area under the bipolar electrode in that experiment. Between three and 10 muscle fibers were found in each of the 120 serial sections that were examined. This epicardial border zone was 41% nonmuscular tissue. In all of the regions analyzed at which multiple component fractionated electrograms were found, the number of individual muscle fibers was usually greater than the number of electrogram components. However, as we indicated before, a functional strand might contain more than one fiber and the number of functional strands could not be determined by the anatomic study. Figure 12, C, shows the histologic appearance of the region where the "double" potential of figure 10, D and E, was recorded. In fact, only two muscle fibers were found throughout the serial sections. However, six to eight muscle fibers were found in the serial sections of another experiment in which double potentials were recorded. Discussion Importance of fractionated electrograms. Fractionated electrograms were first recorded with bipolar electrodes from the canine heart during experimental studies on acute ischemia and on healed myocardial infarcts.28'4>" Similar electrograms have also been recorded from patients with ischemic heart disease with catheter electrodes and intraoperative probes, during both sinus rhythm and ventricular tachycardia.'1- Fractionated electrograms, recorded during sinus rhythm, have been found to be more numerous in the border zones of aneurysms in patients with healed infarcts and a history of tachycardias than in patients with similar cardiac pathologic status but without tachycardia.6 In addition, the fractionated electro- Vol. 72, No. 3, September
13 GARDNER et al. grams in patients with tachycardias had longer durations.6 The sites from which fractionated electrograms are recorded during sinus rhythm may therefore delineate regions where reentry occurs, causing tachycar- dia.t8 Fractionated electrograms, however, might also occur in regions other than those in which tachycardia originates.9', 1, 14, 45 Fractionated electrograms may not always correlate FIGURE 12. Surviving muscle fibers in healed infarcts from which fractionated electrograms were recorded. Beneath the epicardium (E) a few layers of viable muscle, indicated by the arrows, remain at 16 months (A). Directly subjacent is the healed infarct (i). Black ink at the upper right marks the position of the microelectrode used to record extracellular potentials in figure 8. At high magnification (B) the entrapped muscle fibers can be readily seen to be separated and distorted by connective tissue. In panel C, two isolated muscle fibers, in a different preparation, course between the thickened epicardium and the fat of the subjacent healed infarct. (Hematoxylin-phloxine-saffron; original magnification A x 150, B x 350, C x400.) 608 CIRCULATION
14 LABORATORY INVESTIGATION-MYOCARDIAL INFARCTION with the occurrence of arrhythmias in canine preparations of ventricular tachycardia. After complete occlusion of the left anterior descending coronary artery, ventricular tachycardia is often inducible during the first week (at a time fractionated electrograms were not usually found in the isolated preparations) but may become more difficult to induce later on (when fractionated electrograms were often found in isolated preparations).23' We do not have any data explaining this discrepancy. One possibility is that during infarct healing in the canine heart, the area of infarction shrinks markedly. If reentrant tachycardia at 3 to 5 days in this canine preparation requires relatively large circuits,47'48 the infarcted area after healing may be too small for reentry despite the persistent slow conduction. Characteristics of fractionated electrograms. The electrograms recorded in our experiments are not entirely comparable to those in the clinical studies. The bipolar electrograms that we recorded from normal muscle had durations of 5 msec or less and amplitudes of 2 to 4 mv, whereas fractionated electrograms had prolonged durations of 10 to 70 msec and reduced amplitudes that were often less than 1 mv. The durations of both normal and fractionated electrograms were shorter than those of electrograms recorded with a catheter electrode, recently reported by Cassidy et al."'49 (mean duration of 54 msec for normal electrograms and durations of more than 133 msec for fractionated electrograms) or the duration of electrograms recorded intraoperatively by Weiner et al.6 (mean duration of fractionated electrograms was msec). These differences in the absolute values of normal and abnormal electrogram durations may be related to the size of the recording electrodes and the distance between poles. The catheter electrodes used in the studies of Cassidy et al."'49 had a 10 mm distance between bipoles, whereas the electrodes themselves consisted of relatively wide rings. They are therefore expected to record electrical activity from a larger area than our electrodes, which had tip diameters of 0.3 mm and interelectrode-pole distances of 0.5 to 1.0 mm. The bipolar electrodes generally used for intraoperative mapping are also larger than the ones we used.6 Our data are comparable to the clinical data if electrogram characteristics are expressed as the ratio of amplitude to duration, as suggested by Cassidy et al. " Because of the reduced amplitudes and increased durations, fractionated electrograms have significantly decreased ratios compared with normal in both the clinical studies and our experimental study. Electrophysiologic and anatomic causes of fractionated Vol. 72, No. 3, September 1985 electrograms. Fractionated electrograms became more apparent as the infarcts healed. Healing was associated with a return in resting potential and action potential characteristics to normal. A similar improvement in action potentials with infarct healing has also been shown in the studies of Myerburg et al.50 These data show that fractionated electrograms in the healed infarcts are not caused by abnormal or depressed action potentials (although they might be caused by depressed transmembrane potentials in the instances where fractionation has been recorded during acute ischemia) Despite the normal transmembrane potentials in the healed infarcts, activation in the vicinity of the extracellular recording electrodes was much slower than that in the subacute (5-day-old) infarcts; it sometimes took as long as 70 msec for the wavefront to pass by the electrode. We were also sometimes able to show marked inhomogeneities in activation with closely adjacent areas around the electrode activated at very different times. Different components of the fractionated electrograms coincided with activation of the different adjacent regions. It is therefore apparent that the slow and inhomogeneous conduction and the summation of extracellular currents occurring at disparate times cause fractionation. This possibility had been proposed in earlier studies demonstrating fractionated electrograms.28 Why is conduction slow and inhomogeneous in healed infarcts when transmembrane action potentials are normal? The answer to this question may be found in the structural changes that occur during infarct healing. Connective tissue from the necrotic region invades the bordering (subepicardial) areas where there is surviving muscle. These muscle fibers become widely separated and their orientation distorted. These structural changes might lead to an increase in the effective axial resistance, a term used by Spach et al.33 to describe the resistance to current flow in the direction of propagation. The effective axial resistance depends on the intracellular and extracellular resistivities, the size and shape of the cells, cellular packing, and the resistance, extent, and distribution of cell-tocell couplings.33 Distortion of the cells by the connective tissue with a resulting decrease in intercellular connections might increase resistance to current flow and thereby slow conduction.34 A reduced space constant has been found in regions of chronically infarcted canine myocardium by Spear et al.5' and supports this suggestion. It is possible that slow electrotonic transmission across high-resistance or inexcitable gaps sometimes caused the marked conduction delays. Models of this kind of propagation 609
15 GARDNER et al. have been studied by Wennemark et al.,52 who used direct current to cause the area of conduction block, and by Antzelevitch et al.39 and Jalife et al.,' who used a sucrose gap. In our study, intervals between deflections comprising the fractionated electrograms ranged from 5 to 20 msec, similar to the delays that can be caused by either a high intracellular resistance53 or electrotonic transmission across inexcitable areas.52 However, we never observed delays on the order of 100 or 200 msec that sometimes occurred in the sucrose gap experiments."0 Action potentials in regions where we recorded double potentials sometimes showed prepotentials before the upstroke, similar to the prepotentials recorded from regions just distal to an area of block in other experiments in which slow, electrotonic transmission has been demonstrated.3"40' 52 On occasion we also saw two distinct components during the depolarization phase of the action potential in these regions (figure 5, A to D), previously shown during electrotonic transmission by Wennemark et al.52 There is also the possibility that a change in extracellular resistance related to the increased connective tissue alters current flow and conduction velocity. Additional experiments are required to determine whether effective axial resistance is increased or whether electrotonic transmission across inexcitable gaps is occurring in the healed infarcts. The slowed and inhomogeneous activation we observed might also be caused by conduction at normal velocities over circuitous pathways caused by the structural changes (diagrammed in figure 9). Relevance of experimental data to clinical observations. The pathologic anatomy of endocardial regions where fractionated electrograms are recorded in humans is very similar to that of the epicardial regions of healed infarcts that we studied. Bundles of muscle fibers are widely separated during infarct healing by the ingrowth of large amounts of connective tissue.54 This anatomic phenomenon and the action potential characteristics we found in our experimental study may explain some of the features of fractionated electrograms. First, fractionated electrograms are sometimes comprised of rapid spikes (no quantification of rapidity is available) called "high frequency" components,1' although they may also consist of slowly rising deflections. Electrograms with rapid components would not be expected if action potential upstrokes were depressed, since slow upstrokes cause slow rates of extracellular voltage change.32 On the other hand, rapid spikes might be generated by action potentials with rapid upstrokes. Second, fractionated electrograms have very low amplitudes. Our data suggest that such low amplitudes are not caused by a reduction in the rate of rise or amplitude of the action potential32 but may be a result of the sparsity of viable muscle fibers surviving in the ischemic region. This would be expected to decrease the density of extracellular current. Low-amplitude electrograms may also be caused by propagation in nonuniformly anisotropic tissue.34 Third, the very nature of fractionated electrograms asynchronous or repetitive spikes appears to be caused by asynchronous excitation of different poorly interconnected muscle bundles or by delay in conduction from one region of a fiber to another region of the same fiber across high-resistance intercellular connections. If, in fact, these areas of fractionated electrograms delineate reentrant circuits in healed infarcts, the role of slow conduction caused by changes in passive properties of cardiac fibers must be given consideration as a cause of reentrant tachycardia. References 1. Josephson ME, Horowitz LN, Farshidi A, Spear JF, Kastor JA, Moore EN: Recurrent sustained ventricular tachycardia. II. Endocardial mapping. Circulation 57: 440, Josephson ME, Horowitz LN, Farshidi A: Continuous local electrical activity: a mechanism of recurrent ventricular tachycardia. Circulation 57: 659, Horowitz LN, Josephson ME, Harken AH: Epicardial and endocardial activation during sustained ventricular tachycardia in man. Circulation 61: 1227, Waldo AL, Arciniegas JG, Klein H: Surgical treatment of life threatening ventricular arrhythmias: the role of intraoperative mapping and consideration of the presently available surgical techniques. Prog Cardiovasc Dis 23: 247, Gallagher JJ, Kasell JH, Cox JL, Smith WM, Ideker RE, Smith WM: Techniques of intraoperative electrophysiologic mapping. Am J Cardiol 49: 221, Weiner 1, Mindich B, Pitchon R: Determinants of ventricular tachycardia in patients with ventricular aneurysms: results of intraoperative epicardial and endocardial mapping. Circulation 65: 856, Klein H, Karp RB, Kouchoukos NT, Zorn GL, James TN, Waldo AL: Intraoperative electrophysiologic mapping of the ventricles during sinus rhythm in patients with a previous myocardial infarction: identification of the electrophysiologic substrate of ventricular arrhythmias. Circulation 66: 847, Josephson ME, Horowitz LN, Spielman SR, Waxman HL, Greenspan AM: Role of catheter mapping in the preoperative evaluation of ventricular tachycardia. Am J Cardiol 49: 207, Kienzle MG, Falcone RA, Kempf FC, Miller JM, Harken AH, Josephson ME: Intraoperative endocardial mapping: relation of fractionated electrograms in sinus rhythm to endocardial activation in ventricular tachycardia surgical implications. J Am Coll Cardiol 1: 582, Weiner I, Mindich B, Pitchon R: Fragmented endocardial electrical activity in patients with ventricular tachycardia: a new guide to surgical therapy. Am Heart J 107: 86, Cassidy DM, Vassallo JA, Buxton AE, Doherty JU, Marchlinski FE, Josephson ME: The value of catheter mapping during sinus rhythm to localize site of origin of ventricular tachycardia. Circulation 69: 1103, Fontaine G, Guiraudon G, Frank R: Double epicardial potentials in idiopathic ventricular tachycardia. Circulation 54(suppl II): 11-78, Ideker RE, Lofland GK, Bardy GH, Smith WM, Worley SJ, Wallace AG, Cox JL, Gallagher JJ: Late fractionated potentials and continuous electrical activity caused by electrode motion. PACE 6: 908, CIRCULATION
16 LABORATORY INVESTIGATION-MYOCARDIAL INFARCTION 14. Waxman HL, Sung RJ: Significance of fragmented ventricular electrogram observed using intracardiac recording techniques in man. Circulation 62: 1349, Gardner PI, Ursell PC, Fenoglio JJ, Wit AL: Anatomical and electrophysiological basis for electrograms showing fractionated activity. Circulation 66(suppl II): 11-78, Wit AL, Josephson ME: Fractionated electrograms and continuous electrical activity: fact or artifact. In Zipes DP, Jalife J, editors: Cardiac electrophysiology and arrhythmias. New York, 1985, Grune & Stratton, p Harris AS: Delayed development of ventricular ectopic rhythm following experimental coronary occlusion. Circulation 1: 1318, Friedman PL, Stewart JR, Fenoglio JJ Jr, Wit AL: Survival of subendocardial Purkinje fibers after extensive myocardial infarction in dogs: in vitro and in vivo correlations. Circ Res 33: 597, Ursell PC, Gardner PI, Albala A, Fenoglio JJ Jr, Wit AL: Structural and electrophysiological changes in the epicardial border zone of canine infarcts during infarct healing. Circ Res 56: 436, Friedman PL, Fenoglio JJ Jr, Wit AL: Time course for reversal of electrophysiological and ultrastructural abnormalities in subendocardial Purkinje fibers surviving extensive myocardial infarction in dogs. Circ Res 36: 127, Weibel ER: Stereological methods, vol 1. London, 1979, Academic Press, p Eisenberg BR, Kuda AM, Peter JB: Stereological analysis of mammalian skeletal muscle. I. Soleus muscle of the adult guinea pig. J Cell Biol 60: 732, El-Sherif N, Scherlag BJ, Lazzara R, Hope RR: Reentrant arrhythmias in the late myocardial infarction period. I. Conduction characteristics in the infarct zone. Circulation 55: 686, Gardner PI, Ursell PC, Pham TD, Fenoglio JJ Jr, Wit AL: Experimental chronic ventricular tachycardia: anatomic and electrophysiologic substrates. In Josephson ME, Wellens HJJ, editors: Tachycardias: mechanisms, diagnosis and treatment. Philadelphia, 1984, Lea and Febiger, p Spach MS, Barr RC, Johnson EA, Kootsey JM: Cardiac extracellular potentials: analysis of complex wave forms about the Purkinje networks in dogs. Circ Res 33: 465, Lazzara R, Scherlag BJ: Role of the slow current in the generation of arrhythmias in ischemic myocardium. In Zipes DP, Bailey JC, Elharrar V, editors: The slow inward current and cardiac arrhythmia. The Hague, 1980, Martinus Nijhoff, p Spear JF, Michelson EL, Moore EN: Cellular electrophysiologic characteristics of chronically infarcted myocardium in dogs susceptible to sustained ventricular tachyarrhythmias. J Am Coll Cardiol 1: 1099, Boineau JP, Cox JL: Slow ventricular activation in acute myocardial infarction: a source of reentrant premature ventricular contractions. Circulation 43: 702, Cranefield PF, Wit AL, Hoffman BF: Conduction of the cardiac impulse. III. Characteristics of very slow conduction. J Gen Physiol 59: 227, Carmeliet E, Vereecke J: Adrenaline and the plateau phase of the cardiac action potential: importance of Ca, Na and K conductance. Pfluegers Arch 313: 300, Wit AL, Hoffman BF, Cranefield PF: Slow conduction and reentry in the ventricular conducting system. I. Return extrasystole in canine Purkinje fibers. Circ Res 30: 1, Spach MS, Barr RC, Serwer GA, Kootsey JM, Johnson EA: Extracellular potentials related to intracellular action potentials in the dog Purkinje system. Circ Res 30: 505, Spach MS, Miller WT, Geselowitz DB, Barr RC, Kootsey JM, Johnson EA: The discontinuous nature of propagation in normal canine cardiac muscle: evidence for recurrent discontinuities of intracellular resistance that affect the membrane currents. Circ Res 48: 39, Spach MS, Miller WT, Dolber P, Kootsey JM, Sommer JR, Mosher CE Jr: The functional role of structural complexities in the propagation of depolarization in the atrium of the dog: cardiac conduction disturbances due to discontinuous effective axial resistivity. Circ Res 50: 175, Heppner DB, Plonsey R: Simulation of electrical interaction of cardiac cells. Biophys J 10: 1057, Joyner RW: Effects of the discrete pattern of electrical coupling on propagation through an electrical syncytium. Circ Res 50: 192, Joyner RW, Veenstra R, Rawling D, Chorro A: Propagation through electrically coupled cells: effects of a resistive barrier. Biophys J 45: 1017, Bandura JP: The role of electrotonus in slow potential development and conduction in canine Purkinje tissue. In Zipes DP, Bailey JC, Elharrar V, editors: The slow inward current and cardiac arrhythmia. The Hague, 1980, Martinus Nijhoff, p Antzelevitch C, Jalife J, Moe GK: Characteristics of reflection as a mechanism of reentrant arrhythmias and its relationship to parasystole. Circulation 61: 182, Jalife J, Moe GK: Excitation, conduction and reflection of impulses in isolated bovine and canine cardiac Purkinje fibers. Circ Res 49: 233, Durrer D, Formijne P, van Dam RTH, Buller J, van Lier AAW, Meijler FL: Electrocardiogram in normal and some abnormal conditions: in revived human fetal heart and in acute and chronic coronary occlusion. Am Heart J 61: 303, Durrer D, van Lier AAW, Buller J: Epicardial and intramural excitation in chronic myocardial infarction. Am Heart J 68: 765, Daniel TM, Boineau JP, Sabiston DC: Comparison of human ventricular activation with a canine model in chronic myocardial infarction. Circulation 44: 74, Waldo AL, Kaiser GA: A study of ventricular arrhythmias associated with acute myocardial infarction in the canine heart. Circulation 42: 1222, Brugada P, Abdollah H, Wellens HJJ: Significance of continuous electrical activity during ventricular tachycardia. Circulation 68(suppl III): II-422, 1983 (abst) 46. Karagueuzian HS, Fenoglio JJ Jr, Weiss MB, Wit AL: Protracted ventricular tachycardia induced by premature stimulation of the canine heart after coronary artery occlusion and reperfusion. Circ Res 44: 833, El-Sherif N, Smith A, Evans K: Canine ventricular arrhythmias in the late myocardial infarction period: epicardial mapping of reentrant circuits. Circ Res 49: 255, Wit AL, Allessie MA, Bonke FIM, Lammers W, Smeets J, Fenoglio JJ Jr: Electrophysiologic mapping to determine the mechanism of experimental ventricular tachycardia initiated by premature impulses. Am J Cardiol 49: 166, Cassidy DM, Vassallo JA, Marchlinski FE, Buxton AE, Untereker WJ, Josephson ME: Endocardial mapping in humans in sinus rhythm with normal left ventricles: activation patterns and characteristics of electrograms. Circulation 70: 37, Myerburg RJ, Gelband H, Nilsson K, Sung RJ, Thurer RJ, Morales AR, Bassett AL: Long term electrophysiologic abnormalities resulting from experimental myocardial infarction in cats. Circ Res 41: 73, Spear JF, Michelson EL, Moore EN: Reduced space constant in slowly conducting regions of chronically infarcted canine myocardium. Circ Res 53: 176, Wennemark JR, Ruesta VJ, Brody DA: Microelectrode study of delayed conduction in the canine right bundle branch. Circ Res 23: 753, Lieberman M, Kootsey JM, Johnson EA, Sawanobori T: Slow conduction in cardiac muscle: a biophysical model. Biophys J 13: 37, Fenoglio JJ Jr, Pham TD, Harken AH, Horowitz LN, Josephson ME, Wit AL: Recurrent sustained ventricular tachycardia: structure and ultrastructure of subendocardial regions where tachycardia originates. Circulation 68: 518, 1983 Vol. 72, No. 3, September
Influences of Anisotropic Tissue Structure on Reentrant Circuits in the Epicardial Border Zone of Subacute Canine Infarcts
 182 Influences of Anisotropic Tissue Structure on Reentrant Circuits in the Epicardial Border Zone of Subacute Canine Infarcts Stephen M. Dillon, Maurits A. Allessie, Philip C. Ursell, and Andrew L. Wit
182 Influences of Anisotropic Tissue Structure on Reentrant Circuits in the Epicardial Border Zone of Subacute Canine Infarcts Stephen M. Dillon, Maurits A. Allessie, Philip C. Ursell, and Andrew L. Wit
Microelectrode Study of Alternating Responses to Repetitive Premature Excitation in Canine Purkinje Fibers
 Microelectrode Study of Alternating Responses to Repetitive Premature Excitation in Canine Purkinje Fibers By Jack P. Bandura and Daniel A. Brody ABSTRACT Microelectrode studies were performed to produce
Microelectrode Study of Alternating Responses to Repetitive Premature Excitation in Canine Purkinje Fibers By Jack P. Bandura and Daniel A. Brody ABSTRACT Microelectrode studies were performed to produce
Microstructural Basis of Conduction II Introduction to Experimental Studies
 Bioeng 6460 Electrophysiology and Bioelectricity Microstructural Basis of Conduction II Introduction to Experimental Studies Frank B. Sachse fs@cvrti.utah.edu Overview Microstructural Basis of Conduction
Bioeng 6460 Electrophysiology and Bioelectricity Microstructural Basis of Conduction II Introduction to Experimental Studies Frank B. Sachse fs@cvrti.utah.edu Overview Microstructural Basis of Conduction
The Normal Electrocardiogram
 C H A P T E R 1 1 The Normal Electrocardiogram When the cardiac impulse passes through the heart, electrical current also spreads from the heart into the adjacent tissues surrounding the heart. A small
C H A P T E R 1 1 The Normal Electrocardiogram When the cardiac impulse passes through the heart, electrical current also spreads from the heart into the adjacent tissues surrounding the heart. A small
This presentation will deal with the basics of ECG description as well as the physiological basics of
 Snímka 1 Electrocardiography basics This presentation will deal with the basics of ECG description as well as the physiological basics of Snímka 2 Lecture overview 1. Cardiac conduction system functional
Snímka 1 Electrocardiography basics This presentation will deal with the basics of ECG description as well as the physiological basics of Snímka 2 Lecture overview 1. Cardiac conduction system functional
Electrophysiological Properties of Canine Purkinje Cells in One-Day-Old Myocardial Infarction
 Electrophysiological Properties of Canine Purkinje Cells in One-Day-Old Myocardial Infarction By Ralph Lazzara, Nabil El-Sherif, and Benjamin J. Scherlag ABSTRACT Electrophysiological mechanisms which
Electrophysiological Properties of Canine Purkinje Cells in One-Day-Old Myocardial Infarction By Ralph Lazzara, Nabil El-Sherif, and Benjamin J. Scherlag ABSTRACT Electrophysiological mechanisms which
Mapping Cardiac Pacemaker Circuits: Methodological puzzles of SAN optical mapping
 Mapping Cardiac Pacemaker Circuits: Methodological puzzles of SAN optical mapping Igor R. Efimov, Vadim V. Fedorov, Boyoung Joung, and Shien-Fong Lin Journal of American College of Cardiology, 66(18):1976-86,
Mapping Cardiac Pacemaker Circuits: Methodological puzzles of SAN optical mapping Igor R. Efimov, Vadim V. Fedorov, Boyoung Joung, and Shien-Fong Lin Journal of American College of Cardiology, 66(18):1976-86,
ECG CONVENTIONS AND INTERVALS
 1 ECG Waveforms and Intervals ECG waveforms labeled alphabetically P wave== represents atrial depolarization QRS complex=ventricular depolarization ST-T-U complex (ST segment, T wave, and U wave)== V repolarization.
1 ECG Waveforms and Intervals ECG waveforms labeled alphabetically P wave== represents atrial depolarization QRS complex=ventricular depolarization ST-T-U complex (ST segment, T wave, and U wave)== V repolarization.
ECG. Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13
 ECG Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13 The Concept When the cardiac impulse passes through the heart, electrical current
ECG Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13 The Concept When the cardiac impulse passes through the heart, electrical current
Myocardial Infarction Period
 131 Ventricular Arrhythmias in the Subacute Myocardial Infarction Period High-Resolution Activation and Refractory Patterns of Reentrant Rhythms Mark Restivo, William B. Gough, and Nabil El-Sherif Patterns
131 Ventricular Arrhythmias in the Subacute Myocardial Infarction Period High-Resolution Activation and Refractory Patterns of Reentrant Rhythms Mark Restivo, William B. Gough, and Nabil El-Sherif Patterns
Full file at
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What electrical event must occur for atrial kick to occur? 1) A) Atrial repolarization B) Ventricular
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What electrical event must occur for atrial kick to occur? 1) A) Atrial repolarization B) Ventricular
Figure 2. Normal ECG tracing. Table 1.
 Figure 2. Normal ECG tracing that navigates through the left ventricle. Following these bundle branches the impulse finally passes to the terminal points called Purkinje fibers. These Purkinje fibers are
Figure 2. Normal ECG tracing that navigates through the left ventricle. Following these bundle branches the impulse finally passes to the terminal points called Purkinje fibers. These Purkinje fibers are
Premature Ventricular Depolarization
 Premature Ventricular Depolarization By CESAR A. CACERES, M.D. A DIFFERENCE has been observed in certain instances between the onset of QRS in intracavitary and surface limb leads. Simultaneous tracings
Premature Ventricular Depolarization By CESAR A. CACERES, M.D. A DIFFERENCE has been observed in certain instances between the onset of QRS in intracavitary and surface limb leads. Simultaneous tracings
Lab Activity 24 EKG. Portland Community College BI 232
 Lab Activity 24 EKG Reference: Dubin, Dale. Rapid Interpretation of EKG s. 6 th edition. Tampa: Cover Publishing Company, 2000. Portland Community College BI 232 Graph Paper 1 second equals 25 little boxes
Lab Activity 24 EKG Reference: Dubin, Dale. Rapid Interpretation of EKG s. 6 th edition. Tampa: Cover Publishing Company, 2000. Portland Community College BI 232 Graph Paper 1 second equals 25 little boxes
ELECTROCARDIOGRAPHY (ECG)
 ELECTROCARDIOGRAPHY (ECG) The heart is a muscular organ, which pumps blood through the blood vessels of the circulatory system. Blood provides the body with oxygen and nutrients, as well as assists in
ELECTROCARDIOGRAPHY (ECG) The heart is a muscular organ, which pumps blood through the blood vessels of the circulatory system. Blood provides the body with oxygen and nutrients, as well as assists in
CASE 10. What would the ST segment of this ECG look like? On which leads would you see this ST segment change? What does the T wave represent?
 CASE 10 A 57-year-old man presents to the emergency center with complaints of chest pain with radiation to the left arm and jaw. He reports feeling anxious, diaphoretic, and short of breath. His past history
CASE 10 A 57-year-old man presents to the emergency center with complaints of chest pain with radiation to the left arm and jaw. He reports feeling anxious, diaphoretic, and short of breath. His past history
This study was supported by the National Institutes of Health, Grant HE Received for publication December 6,
 Mechanisms of Ventricular Fibrillation Yoshio WATANABE, M.D. and Leonard S. DREIFUS, M.D. T was pointed out more than two decades ago, by Wegria and Wiggers, that any satisfactory theory of fibrillation
Mechanisms of Ventricular Fibrillation Yoshio WATANABE, M.D. and Leonard S. DREIFUS, M.D. T was pointed out more than two decades ago, by Wegria and Wiggers, that any satisfactory theory of fibrillation
Muscle Tissue. General concepts. Classification of muscle. I. Functional classification is based on the type of neural control.
 Muscle Tissue LEARNING OBJECTIVES 1. Identify the three types of muscle tissue at the light microscopic level. 2. List and compare the structural and functional features of each of the three muscle fiber
Muscle Tissue LEARNING OBJECTIVES 1. Identify the three types of muscle tissue at the light microscopic level. 2. List and compare the structural and functional features of each of the three muscle fiber
Cardiac physiology. b. myocardium -- cardiac muscle and fibrous skeleton of heart
 I. Heart anatomy -- general gross. A. Size/orientation - base/apex B. Coverings D. Chambers 1. parietal pericardium 2. visceral pericardium 3. Layers of heart wall a. epicardium Cardiac physiology b. myocardium
I. Heart anatomy -- general gross. A. Size/orientation - base/apex B. Coverings D. Chambers 1. parietal pericardium 2. visceral pericardium 3. Layers of heart wall a. epicardium Cardiac physiology b. myocardium
Ventricular Activation Patterns of Spontaneous and Induced Ventricular Rhythms in Canine One-Day-Old Myocardial Infarction
 152 Ventricular Activation Patterns of Spontaneous and Induced Ventricular Rhythms in Canine One-Day-Old Myocardial Infarction Evidence for Focal and Reentrant Mechanisms Nabil El-Sherif, Rahul Mehra,
152 Ventricular Activation Patterns of Spontaneous and Induced Ventricular Rhythms in Canine One-Day-Old Myocardial Infarction Evidence for Focal and Reentrant Mechanisms Nabil El-Sherif, Rahul Mehra,
Transverse Spread and Longitudinal Dissociation
 Transverse Spread and Longitudinal Dissociation in the Distal A-V Conducting System ROBERT J. MYERBURG, KRISTINA NILSSON, BENJAMIN BEFELER, AGuSTIN CASTELLANOS, JR., and HENRY GELBAND From the Medical
Transverse Spread and Longitudinal Dissociation in the Distal A-V Conducting System ROBERT J. MYERBURG, KRISTINA NILSSON, BENJAMIN BEFELER, AGuSTIN CASTELLANOS, JR., and HENRY GELBAND From the Medical
Mechanisms of Digitalis Toxicity
 Effects Mechanisms of Digitalis Toxicity of Ouabain on Phase Four of Canine Purkinje Fiber Transmembrane Potentials By MICHAEL R. ROSEN, M.D., HENRY GELBAND, M.D., CHARLES MERKER, M.D., AND BRIAN F. HOFFMAN,
Effects Mechanisms of Digitalis Toxicity of Ouabain on Phase Four of Canine Purkinje Fiber Transmembrane Potentials By MICHAEL R. ROSEN, M.D., HENRY GELBAND, M.D., CHARLES MERKER, M.D., AND BRIAN F. HOFFMAN,
ELECTROCARDIOGRAPHY (III) THE ANALYSIS OF THE ELECTROCARDIOGRAM
 ELECTROCARDIOGRAPHY (III) THE ANALYSIS OF THE ELECTROCARDIOGRAM Scridon Alina, Șerban Răzvan Constantin Recording and analysis of the 12-lead ECG is part of the basic medical assessment performed for every
ELECTROCARDIOGRAPHY (III) THE ANALYSIS OF THE ELECTROCARDIOGRAM Scridon Alina, Șerban Răzvan Constantin Recording and analysis of the 12-lead ECG is part of the basic medical assessment performed for every
12 Lead ECG Interpretation: Color Coding for MI s
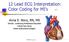 12 Lead ECG Interpretation: Color Coding for MI s Anna E. Story, RN, MS Director, Continuing Professional Education Critical Care Nurse Online Instructional Designer 2004 Anna Story 1 Objectives review
12 Lead ECG Interpretation: Color Coding for MI s Anna E. Story, RN, MS Director, Continuing Professional Education Critical Care Nurse Online Instructional Designer 2004 Anna Story 1 Objectives review
Systolic and Diastolic Currents of Injury
 Systolic and Diastolic Currents of Injury Figure 1 Action Potentials of Normal and Ischemic Tissue In Figure 1 above, the action potential of normal myocardium is represented by the solid lines and the
Systolic and Diastolic Currents of Injury Figure 1 Action Potentials of Normal and Ischemic Tissue In Figure 1 above, the action potential of normal myocardium is represented by the solid lines and the
Chapter 12: Cardiovascular Physiology System Overview
 Chapter 12: Cardiovascular Physiology System Overview Components of the cardiovascular system: Heart Vascular system Blood Figure 12-1 Plasma includes water, ions, proteins, nutrients, hormones, wastes,
Chapter 12: Cardiovascular Physiology System Overview Components of the cardiovascular system: Heart Vascular system Blood Figure 12-1 Plasma includes water, ions, proteins, nutrients, hormones, wastes,
Introduction to ECG Gary Martin, M.D.
 Brief review of basic concepts Introduction to ECG Gary Martin, M.D. The electrical activity of the heart is caused by a sequence of rapid ionic movements across cell membranes resulting first in depolarization
Brief review of basic concepts Introduction to ECG Gary Martin, M.D. The electrical activity of the heart is caused by a sequence of rapid ionic movements across cell membranes resulting first in depolarization
Nonuniform Epicardial Activation and Repolarization Properties of in Vivo Canine Pulmonary Conus
 Nonuniform Epicardial Activation and Repolarization Properties of in Vivo Canine Pulmonary Conus 233 Mary Jo Burgess, Bruce M. Steinhaus, Kenneth W. Spitzer, and Philip R. Ershler The relation between
Nonuniform Epicardial Activation and Repolarization Properties of in Vivo Canine Pulmonary Conus 233 Mary Jo Burgess, Bruce M. Steinhaus, Kenneth W. Spitzer, and Philip R. Ershler The relation between
Cardiac Muscle Tissue. Cardiac Muscle Tissue
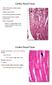 Walls of the heart (cardia: heart); myocardium. Cardiac muscle fibers not as densely packed as skeletal cardiac muscle tissue is highly vascularized Other components; dense C.T. septa, larger blood vessels,
Walls of the heart (cardia: heart); myocardium. Cardiac muscle fibers not as densely packed as skeletal cardiac muscle tissue is highly vascularized Other components; dense C.T. septa, larger blood vessels,
Sheet 5 physiology Electrocardiography-
 *questions asked by some students Sheet 5 physiology Electrocardiography- -why the ventricles lacking parasympathetic supply? if you cut both sympathetic and parasympathetic supply of the heart the heart
*questions asked by some students Sheet 5 physiology Electrocardiography- -why the ventricles lacking parasympathetic supply? if you cut both sympathetic and parasympathetic supply of the heart the heart
The Effects of Extracellular Calcium Removal on Sino-atrial Node Cells Treated with Potassium-depleted Solutions
 Short Communication Japanese Journal of Physiology, 36, 403-409, 1986 The Effects of Extracellular Calcium Removal on Sino-atrial Node Cells Treated with Potassium-depleted Solutions Shun-ichi MIYAMAE
Short Communication Japanese Journal of Physiology, 36, 403-409, 1986 The Effects of Extracellular Calcium Removal on Sino-atrial Node Cells Treated with Potassium-depleted Solutions Shun-ichi MIYAMAE
Matters of the Heart: Comprehensive Cardiology SARAH BEANLANDS RN BSCN MSC
 Matters of the Heart: Comprehensive Cardiology SARAH BEANLANDS RN BSCN MSC Who am I? Class Outline Gross anatomy of the heart Trip around the heart Micro anatomy: cellular and tissue level Introduction
Matters of the Heart: Comprehensive Cardiology SARAH BEANLANDS RN BSCN MSC Who am I? Class Outline Gross anatomy of the heart Trip around the heart Micro anatomy: cellular and tissue level Introduction
Ventricular Reentry Around a Fixed Barrier
 267 Ventricular Reentry Around a Fixed Barrier Resetting With Advancement in an In Vitro Model Robert C. Bernstein, MD, and Lawrence H. Frame, MD We studied an in vitro model of reentrant tachycardia in
267 Ventricular Reentry Around a Fixed Barrier Resetting With Advancement in an In Vitro Model Robert C. Bernstein, MD, and Lawrence H. Frame, MD We studied an in vitro model of reentrant tachycardia in
A STUDY OF SUBSTANCES WHICH ALTER INTRAVENTRICULAR CONDUCTION IN THE ISOLATED DOG HEART. Received for publication December 7, 1956
 A STUDY OF SUBSTANCES WHICH ALTER INTRAVENTRICULAR CONDUCTION IN THE ISOLATED DOG HEART HENRY H. SWAIN AND CHARLES L. WEIDNER2 Department of Pharmacology, University of Michigan Medical School, Ann A rbor
A STUDY OF SUBSTANCES WHICH ALTER INTRAVENTRICULAR CONDUCTION IN THE ISOLATED DOG HEART HENRY H. SWAIN AND CHARLES L. WEIDNER2 Department of Pharmacology, University of Michigan Medical School, Ann A rbor
Outline. Electrical Activity of the Human Heart. What is the Heart? The Heart as a Pump. Anatomy of the Heart. The Hard Work
 Electrical Activity of the Human Heart Oguz Poroy, PhD Assistant Professor Department of Biomedical Engineering The University of Iowa Outline Basic Facts about the Heart Heart Chambers and Heart s The
Electrical Activity of the Human Heart Oguz Poroy, PhD Assistant Professor Department of Biomedical Engineering The University of Iowa Outline Basic Facts about the Heart Heart Chambers and Heart s The
PART I. Disorders of the Heart Rhythm: Basic Principles
 PART I Disorders of the Heart Rhythm: Basic Principles FET01.indd 1 1/11/06 9:53:05 AM FET01.indd 2 1/11/06 9:53:06 AM CHAPTER 1 The Cardiac Electrical System The heart spontaneously generates electrical
PART I Disorders of the Heart Rhythm: Basic Principles FET01.indd 1 1/11/06 9:53:05 AM FET01.indd 2 1/11/06 9:53:06 AM CHAPTER 1 The Cardiac Electrical System The heart spontaneously generates electrical
THE ACTIVATION of the surface of
 Spread of Electrical Activity Through the Wall of the Ventricle By ALLEN M. SCHER, ALLAN C. YOUNG, ARTHUR L. MALMGHEN AND RICHARD It. PATON Multipolar recording technics have been employed to record the
Spread of Electrical Activity Through the Wall of the Ventricle By ALLEN M. SCHER, ALLAN C. YOUNG, ARTHUR L. MALMGHEN AND RICHARD It. PATON Multipolar recording technics have been employed to record the
Electrocardiography for Healthcare Professionals. Chapter 14 Basic 12-Lead ECG Interpretation
 Electrocardiography for Healthcare Professionals Chapter 14 Basic 12-Lead ECG Interpretation 2012 The Companies, Inc. All rights reserved. Learning Outcomes 14.1 Discuss the anatomic views seen on a 12-lead
Electrocardiography for Healthcare Professionals Chapter 14 Basic 12-Lead ECG Interpretation 2012 The Companies, Inc. All rights reserved. Learning Outcomes 14.1 Discuss the anatomic views seen on a 12-lead
By the end of this lecture, you will be able to: Understand the 12 lead ECG in relation to the coronary circulation and myocardium Perform an ECG
 By the end of this lecture, you will be able to: Understand the 12 lead ECG in relation to the coronary circulation and myocardium Perform an ECG recording Identify the ECG changes that occur in the presence
By the end of this lecture, you will be able to: Understand the 12 lead ECG in relation to the coronary circulation and myocardium Perform an ECG recording Identify the ECG changes that occur in the presence
FURTHER STUDIES OF THE CONDUCTING SYSTEM OF THE BIRD'S HEART
 FURTHER STUDIES OF THE CONDUCTING SYSTEM OF THE BIRD'S HEART By FRANCIS DAVIES, M.D. (LONDON) Anatomy Department, University College, London INTRODUCTION T1HE histological investigation of the conducting
FURTHER STUDIES OF THE CONDUCTING SYSTEM OF THE BIRD'S HEART By FRANCIS DAVIES, M.D. (LONDON) Anatomy Department, University College, London INTRODUCTION T1HE histological investigation of the conducting
Phase 2 Early Afterdepolarization as a Trigger of Polymorphic Ventricular Tachycardia in Acquired Long-QT Syndrome
 Phase 2 Early Afterdepolarization as a Trigger of Polymorphic Ventricular Tachycardia in Acquired Long-QT Syndrome Direct Evidence From Intracellular Recordings in the Intact Left Ventricular Wall Gan-Xin
Phase 2 Early Afterdepolarization as a Trigger of Polymorphic Ventricular Tachycardia in Acquired Long-QT Syndrome Direct Evidence From Intracellular Recordings in the Intact Left Ventricular Wall Gan-Xin
Introduction to Electrocardiography
 Introduction to Electrocardiography Class Objectives: Introduction to ECG monitoring Discuss principles of interpretation Identify the components and measurements of the ECG ECG analysis ECG Monitoring
Introduction to Electrocardiography Class Objectives: Introduction to ECG monitoring Discuss principles of interpretation Identify the components and measurements of the ECG ECG analysis ECG Monitoring
Principles and Applications of Electrical Circuits and Signal Theories in EP
 Principles and Applications of Electrical Circuits and Signal Theories in EP Graydon Beatty, VP Therapy Development Atrial Fibrillation Division, St. Jude Medical, Inc. CardioRhythm 2009 Background Biophysics
Principles and Applications of Electrical Circuits and Signal Theories in EP Graydon Beatty, VP Therapy Development Atrial Fibrillation Division, St. Jude Medical, Inc. CardioRhythm 2009 Background Biophysics
Figure 1: Schematic of basic principles underlying DSI-tractography. Panel A: Schematic of a voxel with
 Figure 1 Figure 1: Schematic of basic principles underlying DSI-tractography. Panel A: Schematic of a voxel with 2 fiber populations. Water diffuses primarily in the direction of fiber orientation (arrows).
Figure 1 Figure 1: Schematic of basic principles underlying DSI-tractography. Panel A: Schematic of a voxel with 2 fiber populations. Water diffuses primarily in the direction of fiber orientation (arrows).
Lect.6 Electrical axis and cardiac vector Cardiac vector: net result Vector that occurs during depolarization of the ventricles Figure:
 Lect.6 Electrical axis and cardiac vector Objectives: 1. State the relationship between the direction of cardiac vector with the direction (-ve, +ve) and amplitude of an ECG waves. 2. Draw diagram indicting
Lect.6 Electrical axis and cardiac vector Objectives: 1. State the relationship between the direction of cardiac vector with the direction (-ve, +ve) and amplitude of an ECG waves. 2. Draw diagram indicting
Prolongation of conduction time during premature stimulation in the human atrium is primarily caused by local stimulus response latency
 European Heart Journal (1995) 16, 1920-1924 Prolongation of conduction time during premature stimulation in the human atrium is primarily caused by local stimulus response latency B. S. KOLLER, P. E. KARASIK,
European Heart Journal (1995) 16, 1920-1924 Prolongation of conduction time during premature stimulation in the human atrium is primarily caused by local stimulus response latency B. S. KOLLER, P. E. KARASIK,
Long Term Monitoring of the Intrinsic Ventricular Monophasic Action Potential with an Implantable DDD Pacemaker
 May 1998 79 Long Term Monitoring of the Intrinsic Ventricular Monophasic Action Potential with an Implantable DDD Pacemaker T. LAWO, J. BARMEYER Abteilung für Kardiologie, Universitätsklinik Bergmannsheil,
May 1998 79 Long Term Monitoring of the Intrinsic Ventricular Monophasic Action Potential with an Implantable DDD Pacemaker T. LAWO, J. BARMEYER Abteilung für Kardiologie, Universitätsklinik Bergmannsheil,
Chapter 20: Cardiovascular System: The Heart
 Chapter 20: Cardiovascular System: The Heart I. Functions of the Heart A. List and describe the four functions of the heart: 1. 2. 3. 4. II. Size, Shape, and Location of the Heart A. Size and Shape 1.
Chapter 20: Cardiovascular System: The Heart I. Functions of the Heart A. List and describe the four functions of the heart: 1. 2. 3. 4. II. Size, Shape, and Location of the Heart A. Size and Shape 1.
DR QAZI IMTIAZ RASOOL OBJECTIVES
 PRACTICAL ELECTROCARDIOGRAPHY DR QAZI IMTIAZ RASOOL OBJECTIVES Recording of electrical events in heart Established electrode pattern results in specific tracing pattern Health of heart i. e. Anatomical
PRACTICAL ELECTROCARDIOGRAPHY DR QAZI IMTIAZ RASOOL OBJECTIVES Recording of electrical events in heart Established electrode pattern results in specific tracing pattern Health of heart i. e. Anatomical
Department of medical physiology 7 th week and 8 th week
 Department of medical physiology 7 th week and 8 th week Semester: winter Study program: Dental medicine Lecture: RNDr. Soňa Grešová, PhD. Department of medical physiology Faculty of Medicine PJŠU Cardiovascular
Department of medical physiology 7 th week and 8 th week Semester: winter Study program: Dental medicine Lecture: RNDr. Soňa Grešová, PhD. Department of medical physiology Faculty of Medicine PJŠU Cardiovascular
Transient Ventricular Conduction Disturbances Produced by Intra-atrial Injection of Single Doses of KCI
 Transient Ventricular Conduction Disturbances Produced by Intra-atrial Injection of Single Doses of KCI By Jaok Han, M.D., Ph.D., Anna Mae Malozzi, B.S., and Gordon K. Moe, Ph.D. ABSTRACT In dogs under
Transient Ventricular Conduction Disturbances Produced by Intra-atrial Injection of Single Doses of KCI By Jaok Han, M.D., Ph.D., Anna Mae Malozzi, B.S., and Gordon K. Moe, Ph.D. ABSTRACT In dogs under
Conventional Mapping. Introduction
 Conventional Mapping Haitham Badran Ain Shams University it Introduction The mapping approach used to guide ablation depends on the type of arrhythmia being assessed. Simple fluoroscopic anatomy is essential
Conventional Mapping Haitham Badran Ain Shams University it Introduction The mapping approach used to guide ablation depends on the type of arrhythmia being assessed. Simple fluoroscopic anatomy is essential
An Official Journal of the American Heart Association
 Circulation Research JANUARY VOL. XXX An Official Journal of the American Heart Association 1972 NO. 1 Slow Conduction and Reentry in the Ventricular Conducting System I. RETURN EXTRASYSTOLE IN CANINE
Circulation Research JANUARY VOL. XXX An Official Journal of the American Heart Association 1972 NO. 1 Slow Conduction and Reentry in the Ventricular Conducting System I. RETURN EXTRASYSTOLE IN CANINE
Effect of Extrasystoles on Idioventricular Rhythm
 Effect of Extrasystoles on Idioventricular Rhythm By Herman O. Klein, Paul F. Cranefield, and Brian F. Hoffman ABSTRACT Ventricular extrasystoles exert a variable effect on idioventricular rhythm. The
Effect of Extrasystoles on Idioventricular Rhythm By Herman O. Klein, Paul F. Cranefield, and Brian F. Hoffman ABSTRACT Ventricular extrasystoles exert a variable effect on idioventricular rhythm. The
Ask Mish. EKG INTERPRETATION part i
 EKG INTERPRETATION part i What is EKG? EKG or ECG= electrocardiogram(~graphy) means the recording of the heart electrical activity from Greek kardio= heart, graphein= to write cardiac cell physiology Cardiac
EKG INTERPRETATION part i What is EKG? EKG or ECG= electrocardiogram(~graphy) means the recording of the heart electrical activity from Greek kardio= heart, graphein= to write cardiac cell physiology Cardiac
Interpreting Electrocardiograms (ECG) Physiology Name: Per:
 Interpreting Electrocardiograms (ECG) Physiology Name: Per: Introduction The heart has its own system in place to create nerve impulses and does not actually require the brain to make it beat. This electrical
Interpreting Electrocardiograms (ECG) Physiology Name: Per: Introduction The heart has its own system in place to create nerve impulses and does not actually require the brain to make it beat. This electrical
Electrocardiogram ECG. Hilal Al Saffar FRCP FACC College of medicine,baghdad University
 Electrocardiogram ECG Hilal Al Saffar FRCP FACC College of medicine,baghdad University Tuesday 29 October 2013 ECG introduction Wednesday 30 October 2013 Abnormal ECG ( ischemia, chamber hypertrophy, heart
Electrocardiogram ECG Hilal Al Saffar FRCP FACC College of medicine,baghdad University Tuesday 29 October 2013 ECG introduction Wednesday 30 October 2013 Abnormal ECG ( ischemia, chamber hypertrophy, heart
11/18/13 ECG SIGNAL ACQUISITION HARDWARE DESIGN. Origin of Bioelectric Signals
 ECG SIGNAL ACQUISITION HARDWARE DESIGN Origin of Bioelectric Signals 1 Cell membrane, channel proteins Electrical and chemical gradients at the semi-permeable cell membrane As a result, we get a membrane
ECG SIGNAL ACQUISITION HARDWARE DESIGN Origin of Bioelectric Signals 1 Cell membrane, channel proteins Electrical and chemical gradients at the semi-permeable cell membrane As a result, we get a membrane
(D) (E) (F) 6. The extrasystolic beat would produce (A) increased pulse pressure because contractility. is increased. increased
 Review Test 1. A 53-year-old woman is found, by arteriography, to have 5% narrowing of her left renal artery. What is the expected change in blood flow through the stenotic artery? Decrease to 1 2 Decrease
Review Test 1. A 53-year-old woman is found, by arteriography, to have 5% narrowing of her left renal artery. What is the expected change in blood flow through the stenotic artery? Decrease to 1 2 Decrease
Conduction of the Cardiac Impulse III. Characteristics of very slow conduction
 Published Online: 1 February, 1972 Supp Info: http://doi.org/10.1085/jgp.59.2.227 Downloaded from jgp.rupress.org on October 21, 2018 Conduction of the Cardiac Impulse III. Characteristics of very slow
Published Online: 1 February, 1972 Supp Info: http://doi.org/10.1085/jgp.59.2.227 Downloaded from jgp.rupress.org on October 21, 2018 Conduction of the Cardiac Impulse III. Characteristics of very slow
ECG Interpretation Cat Williams, DVM DACVIM (Cardiology)
 ECG Interpretation Cat Williams, DVM DACVIM (Cardiology) Providing the best quality care and service for the patient, the client, and the referring veterinarian. GOAL: Reduce Anxiety about ECGs Back to
ECG Interpretation Cat Williams, DVM DACVIM (Cardiology) Providing the best quality care and service for the patient, the client, and the referring veterinarian. GOAL: Reduce Anxiety about ECGs Back to
Cardiovascular system
 BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
- why the T wave is deflected upwards although it's a repolarization wave?
 Cardiac Electrograph: - why the T wave is deflected upwards although it's a repolarization wave? After depolarization the ventricle contracts but since the heart is a volume conductor (3D not 2D), when
Cardiac Electrograph: - why the T wave is deflected upwards although it's a repolarization wave? After depolarization the ventricle contracts but since the heart is a volume conductor (3D not 2D), when
Effects of myocardial infarction on catheter defibrillation threshold
 Purdue University Purdue e-pubs Weldon School of Biomedical Engineering Faculty Publications Weldon School of Biomedical Engineering 1983 Effects of myocardial infarction on catheter defibrillation threshold
Purdue University Purdue e-pubs Weldon School of Biomedical Engineering Faculty Publications Weldon School of Biomedical Engineering 1983 Effects of myocardial infarction on catheter defibrillation threshold
myocardial infarction period: prevention of reentry by dual stimulation during basic rhythm
 LABORATORY INVESTIGATION ARRHYTMA Reentrant ventricular rhythms in the late myocardial infarction period: prevention of reentry by dual stimulation during basic rhythm MARK RESTIVO, PH.D., WILLIAM B. GOUGH,
LABORATORY INVESTIGATION ARRHYTMA Reentrant ventricular rhythms in the late myocardial infarction period: prevention of reentry by dual stimulation during basic rhythm MARK RESTIVO, PH.D., WILLIAM B. GOUGH,
Biology 212: Anatomy and Physiology II. Lab #5: Physiology of the Cardiovascular System For Labs Associated With Dr. Thompson s Lectures
 Biology 212: Anatomy and Physiology II Lab #5: Physiology of the Cardiovascular System For Labs Associated With Dr. Thompson s Lectures References: Saladin, KS: Anatomy and Physiology, The Unity of Form
Biology 212: Anatomy and Physiology II Lab #5: Physiology of the Cardiovascular System For Labs Associated With Dr. Thompson s Lectures References: Saladin, KS: Anatomy and Physiology, The Unity of Form
for Heart-Health Scanning
 Magnetocardiography for Heart-Health Scanning CardioMag Imaging, Inc. 1 Basic Principles of Magnetocardiography (MCG) The cardiac electric activity that produces a voltage difference on the body surface
Magnetocardiography for Heart-Health Scanning CardioMag Imaging, Inc. 1 Basic Principles of Magnetocardiography (MCG) The cardiac electric activity that produces a voltage difference on the body surface
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists
 PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
PERMANENT PACEMAKERS AND IMPLANTABLE DEFIBRILLATORS Considerations for intensivists Craig A. McPherson, MD, FACC Associate Professor of Medicine Constantine Manthous, MD, FACP, FCCP Associate Clinical
Skeletal muscle. General features :
 Muscular tissues In the first embryonic life the muscular tissues arise from mesoderm, The function of movement in multicellular organisms is usually assumed by specialized cells called muscle fibers which
Muscular tissues In the first embryonic life the muscular tissues arise from mesoderm, The function of movement in multicellular organisms is usually assumed by specialized cells called muscle fibers which
Effect of Lidocaine on the Electrophysiological Properties of Ventricular Muscle and Purkinje Fibers
 Effect of Lidocaine on the Electrophysiological Properties of Ventricular Muscle and Purkinje Fibers J. THOMAS BicGER, JR., and WLLAM J. MANDEL From the Departments of Pharmacology and Medicine, College
Effect of Lidocaine on the Electrophysiological Properties of Ventricular Muscle and Purkinje Fibers J. THOMAS BicGER, JR., and WLLAM J. MANDEL From the Departments of Pharmacology and Medicine, College
also aid the clinician in recognizing both the obvious and subtle abnormalities that may help guide therapy.
 Karen Lieberman, MS, CRNP f the many diagnostic tools used to screen for and evaluate cardiac abnormalities, the 12-lead electrocardiogram (ECG) is among the most basic. This inexpensive and noninvasive
Karen Lieberman, MS, CRNP f the many diagnostic tools used to screen for and evaluate cardiac abnormalities, the 12-lead electrocardiogram (ECG) is among the most basic. This inexpensive and noninvasive
1. General characteristics of muscle tissues: 2. A. Skeletal muscle tissue ("striated muscle tissue")
 1. General characteristics of muscle tissues: Muscle fibers, AKA, muscle cells Vascularized. Other tissues dense and loose C.T. nerves and nerve fibers Muscle fibers (muscle cells) close together. From
1. General characteristics of muscle tissues: Muscle fibers, AKA, muscle cells Vascularized. Other tissues dense and loose C.T. nerves and nerve fibers Muscle fibers (muscle cells) close together. From
The Circulatory System. The Heart, Blood Vessels, Blood Types
 The Circulatory System The Heart, Blood Vessels, Blood Types The Closed Circulatory System Humans have a closed circulatory system, typical of all vertebrates, in which blood is confined to vessels and
The Circulatory System The Heart, Blood Vessels, Blood Types The Closed Circulatory System Humans have a closed circulatory system, typical of all vertebrates, in which blood is confined to vessels and
Localized Reentry Mechanism of Induced Sustained Ventricular Tachycardia in Canine Model of Recent Myocardial Infarction
 Localized Reentry Mechanism of Induced Sustained Ventricular Tachycardia in Canine Model of Recent Myocardial Infarction Hasan Garan and Jeremy N. Ruskin Cardiac Unit, Massachusetts General Hospital, Boston,
Localized Reentry Mechanism of Induced Sustained Ventricular Tachycardia in Canine Model of Recent Myocardial Infarction Hasan Garan and Jeremy N. Ruskin Cardiac Unit, Massachusetts General Hospital, Boston,
12 Lead ECG. Presented by Rebecca Sevigny BSN, RN Professional Practice & Development Dept.
 12 Lead ECG Presented by Rebecca Sevigny BSN, RN Professional Practice & Development Dept. Two Main Coronary Arteries RCA LCA which branches into Left Anterior Descending Circumflex Artery Two Main Coronary
12 Lead ECG Presented by Rebecca Sevigny BSN, RN Professional Practice & Development Dept. Two Main Coronary Arteries RCA LCA which branches into Left Anterior Descending Circumflex Artery Two Main Coronary
Chapter 3 Biological measurement 3.1 Nerve conduction
 Chapter 3 Biological measurement 3.1 Nerve conduction Learning objectives: What is in a nerve fibre? How does a nerve fibre transmit an electrical impulse? What do we mean by action potential? Nerve cells
Chapter 3 Biological measurement 3.1 Nerve conduction Learning objectives: What is in a nerve fibre? How does a nerve fibre transmit an electrical impulse? What do we mean by action potential? Nerve cells
Collin County Community College. ! BIOL Anatomy & Physiology! WEEK 5. The Heart
 Collin County Community College! BIOL. 2402 Anatomy & Physiology! WEEK 5 The Heart 1 (1578-1657) A groundbreaking work in the history of medicine, English physician William Harvey s Anatomical Essay on
Collin County Community College! BIOL. 2402 Anatomy & Physiology! WEEK 5 The Heart 1 (1578-1657) A groundbreaking work in the history of medicine, English physician William Harvey s Anatomical Essay on
Nature Methods: doi: /nmeth Supplementary Figure 1. Activity in turtle dorsal cortex is sparse.
 Supplementary Figure 1 Activity in turtle dorsal cortex is sparse. a. Probability distribution of firing rates across the population (notice log scale) in our data. The range of firing rates is wide but
Supplementary Figure 1 Activity in turtle dorsal cortex is sparse. a. Probability distribution of firing rates across the population (notice log scale) in our data. The range of firing rates is wide but
V. TACHYCARDIAS Rapid rhythm abnormalities
 V. TACHYCARDIAS Rapid rhythm abnormalities Tachyarrhythmias currently account for up to 350,000 deaths annually in the US. In addition to these clearly dangerous rhythm disturbances, other forms of more
V. TACHYCARDIAS Rapid rhythm abnormalities Tachyarrhythmias currently account for up to 350,000 deaths annually in the US. In addition to these clearly dangerous rhythm disturbances, other forms of more
THE HEART. A. The Pericardium - a double sac of serous membrane surrounding the heart
 THE HEART I. Size and Location: A. Fist-size weighing less than a pound (250 to 350 grams). B. Located in the mediastinum between the 2 nd rib and the 5 th intercostal space. 1. Tipped to the left, resting
THE HEART I. Size and Location: A. Fist-size weighing less than a pound (250 to 350 grams). B. Located in the mediastinum between the 2 nd rib and the 5 th intercostal space. 1. Tipped to the left, resting
EEG, ECG, EMG. Mitesh Shrestha
 EEG, ECG, EMG Mitesh Shrestha What is Signal? A signal is defined as a fluctuating quantity or impulse whose variations represent information. The amplitude or frequency of voltage, current, electric field
EEG, ECG, EMG Mitesh Shrestha What is Signal? A signal is defined as a fluctuating quantity or impulse whose variations represent information. The amplitude or frequency of voltage, current, electric field
11/10/2014. Muscular pump Two atria Two ventricles. In mediastinum of thoracic cavity 2/3 of heart's mass lies left of midline of sternum
 It beats over 100,000 times a day to pump over 1,800 gallons of blood per day through over 60,000 miles of blood vessels. During the average lifetime, the heart pumps nearly 3 billion times, delivering
It beats over 100,000 times a day to pump over 1,800 gallons of blood per day through over 60,000 miles of blood vessels. During the average lifetime, the heart pumps nearly 3 billion times, delivering
238. Picrotoxin: A Potentiator of Muscle Contraction
 No. 101 Proc. Japan Acad., 46 (1970) 1051 238. Picrotoxin: A Potentiator of Muscle Contraction By Kimihisa TAKEDA and Yutaka OOMURA Department of Physiology, Faculty of Medicine Kanazawa University, Kanazawa
No. 101 Proc. Japan Acad., 46 (1970) 1051 238. Picrotoxin: A Potentiator of Muscle Contraction By Kimihisa TAKEDA and Yutaka OOMURA Department of Physiology, Faculty of Medicine Kanazawa University, Kanazawa
Electrocardiography Biomedical Engineering Kaj-Åge Henneberg
 Electrocardiography 31650 Biomedical Engineering Kaj-Åge Henneberg Electrocardiography Plan Function of cardiovascular system Electrical activation of the heart Recording the ECG Arrhythmia Heart Rate
Electrocardiography 31650 Biomedical Engineering Kaj-Åge Henneberg Electrocardiography Plan Function of cardiovascular system Electrical activation of the heart Recording the ECG Arrhythmia Heart Rate
5- The normal electrocardiogram (ECG)
 5- The (ECG) Introduction Electrocardiography is a process of recording electrical activities of heart muscle at skin surface. The electrical current spreads into the tissues surrounding the heart, a small
5- The (ECG) Introduction Electrocardiography is a process of recording electrical activities of heart muscle at skin surface. The electrical current spreads into the tissues surrounding the heart, a small
12 Lead ECG Skills: Building Confidence for Clinical Practice. Presented By: Cynthia Webner, BSN, RN, CCRN-CMC. Karen Marzlin, BSN, RN,CCRN-CMC
 12 Lead ECG Skills: Building Confidence for Clinical Practice NTI 2009 Preconference Session 803 Presented By: Karen Marzlin, BSN, RN,CCRN-CMC 1 12 Lead ECG Fundamentals: The Starting Place for Linking
12 Lead ECG Skills: Building Confidence for Clinical Practice NTI 2009 Preconference Session 803 Presented By: Karen Marzlin, BSN, RN,CCRN-CMC 1 12 Lead ECG Fundamentals: The Starting Place for Linking
Farah Khreisat. Raghad Abu Jebbeh. Faisal Mohammad. 1 P a g e
 5 Farah Khreisat Raghad Abu Jebbeh Faisal Mohammad 1 P a g e بسم هللا الرحمن الرحيم Hello guys, hope you're doing well, as you've seen in the previous lecture, the Dr started with an extremely important
5 Farah Khreisat Raghad Abu Jebbeh Faisal Mohammad 1 P a g e بسم هللا الرحمن الرحيم Hello guys, hope you're doing well, as you've seen in the previous lecture, the Dr started with an extremely important
affect contractions in cardiac tissue (Koch-Weser & Blinks, 1963), and in
 J. Physiol. (1965), 18, pp. 225-238 225 With 12 text-figures Printed in Great Britain THE RELATION BETWEEN RESPONSE AND THE INTERVAL BETWEEN STIMULI OF THE ISOLATED GUINEA-PIG URETER BY A. W. CUTHBERT
J. Physiol. (1965), 18, pp. 225-238 225 With 12 text-figures Printed in Great Britain THE RELATION BETWEEN RESPONSE AND THE INTERVAL BETWEEN STIMULI OF THE ISOLATED GUINEA-PIG URETER BY A. W. CUTHBERT
******************************************************************************************************* MUSCLE CYTOLOGY AND HISTOLOGY
 BIOLOGY 211: HUMAN ANATOMY & PHYSIOLOGY ******************************************************************************************************* MUSCLE CYTOLOGY AND HISTOLOGY *******************************************************************************************************
BIOLOGY 211: HUMAN ANATOMY & PHYSIOLOGY ******************************************************************************************************* MUSCLE CYTOLOGY AND HISTOLOGY *******************************************************************************************************
INTRODUCTION TO ECG. Dr. Tamara Alqudah
 INTRODUCTION TO ECG Dr. Tamara Alqudah Excitatory & conductive system of the heart + - The ECG The electrocardiogram, or ECG, is a simple & noninvasive diagnostic test which records the electrical
INTRODUCTION TO ECG Dr. Tamara Alqudah Excitatory & conductive system of the heart + - The ECG The electrocardiogram, or ECG, is a simple & noninvasive diagnostic test which records the electrical
The Fundamentals of 12 Lead EKG. ECG Recording. J Point. Reviewing the Cardiac Conductive System. Dr. E. Joe Sasin, MD Rusty Powers, NRP
 The Fundamentals of 12 Lead EKG Dr. E. Joe Sasin, MD Rusty Powers, NRP SA Node Intranodal Pathways AV Junction AV Fibers Bundle of His Septum Bundle Branches Purkinje System Reviewing the Cardiac Conductive
The Fundamentals of 12 Lead EKG Dr. E. Joe Sasin, MD Rusty Powers, NRP SA Node Intranodal Pathways AV Junction AV Fibers Bundle of His Septum Bundle Branches Purkinje System Reviewing the Cardiac Conductive
Where are the normal pacemaker and the backup pacemakers of the heart located?
 CASE 9 A 68-year-old woman presents to the emergency center with shortness of breath, light-headedness, and chest pain described as being like an elephant sitting on her chest. She is diagnosed with a
CASE 9 A 68-year-old woman presents to the emergency center with shortness of breath, light-headedness, and chest pain described as being like an elephant sitting on her chest. She is diagnosed with a
12 LEAD EKG BASICS. By: Steven Jones, NREMT P CLEMC
 12 LEAD EKG BASICS By: Steven Jones, NREMT P CLEMC ECG Review Waves and Intervals P wave: the sequential activation (depolarization) of the right and left atria QRS complex: right and left ventricular
12 LEAD EKG BASICS By: Steven Jones, NREMT P CLEMC ECG Review Waves and Intervals P wave: the sequential activation (depolarization) of the right and left atria QRS complex: right and left ventricular
Electrophysiological Effects of Procainamide in Acute and Healed Experimental Ischemic Injury of Cat Myocardium
 386 Electrophysiological Effects of Procainamide in Acute and Healed Experimental Ischemic Injury of Cat Myocardium Robert J. Myerburg, Arthur L. Bassett, Kristina Epstein, Marion S. Gaide, Patricia Kozlovskis,
386 Electrophysiological Effects of Procainamide in Acute and Healed Experimental Ischemic Injury of Cat Myocardium Robert J. Myerburg, Arthur L. Bassett, Kristina Epstein, Marion S. Gaide, Patricia Kozlovskis,
Chapter 13 The Cardiovascular System: Cardiac Function
 Chapter 13 The Cardiovascular System: Cardiac Function Overview of the Cardiovascular System The Path of Blood Flow through the Heart and Vasculature Anatomy of the Heart Electrical Activity of the Heart
Chapter 13 The Cardiovascular System: Cardiac Function Overview of the Cardiovascular System The Path of Blood Flow through the Heart and Vasculature Anatomy of the Heart Electrical Activity of the Heart
Atlantic Health System
 Atlantic Health System Morristown Medical Center Newton Medical Center Overlook Medical Center Basic Dysrhythmia Course Day 1 1 2 Chapter 1 Anatomy and Physiology Learning Objectives 1) Identify electrophysiology
Atlantic Health System Morristown Medical Center Newton Medical Center Overlook Medical Center Basic Dysrhythmia Course Day 1 1 2 Chapter 1 Anatomy and Physiology Learning Objectives 1) Identify electrophysiology
Electrocardiography negative zero LA/VL RA/VR LL/VF recording electrode exploring electrode Wilson right arm right arm, left arm left arm
 Electrocardiography In the previous lecture, we were talking about the unipolar limb leads. We said that to make the unipolar lead, you have to make the negative electrode as zero electrode, this is done
Electrocardiography In the previous lecture, we were talking about the unipolar limb leads. We said that to make the unipolar lead, you have to make the negative electrode as zero electrode, this is done
Electrical disconnection of pulmonary vein (PV) myocardium
 Left Atrial Appendage Activity Masquerading as Pulmonary Vein Potentials Dipen Shah, MD; Michel Haissaguerre, MD; Pierre Jais, MD; Meleze Hocini, MD; Teiichi Yamane, MD; Laurent Macle, MD; Kee Joon Choi,
Left Atrial Appendage Activity Masquerading as Pulmonary Vein Potentials Dipen Shah, MD; Michel Haissaguerre, MD; Pierre Jais, MD; Meleze Hocini, MD; Teiichi Yamane, MD; Laurent Macle, MD; Kee Joon Choi,
Electrocardiography Abnormalities (Arrhythmias) 7. Faisal I. Mohammed, MD, PhD
 Electrocardiography Abnormalities (Arrhythmias) 7 Faisal I. Mohammed, MD, PhD 1 Causes of Cardiac Arrythmias Abnormal rhythmicity of the pacemaker Shift of pacemaker from sinus node Blocks at different
Electrocardiography Abnormalities (Arrhythmias) 7 Faisal I. Mohammed, MD, PhD 1 Causes of Cardiac Arrythmias Abnormal rhythmicity of the pacemaker Shift of pacemaker from sinus node Blocks at different
Characteristics of Extracellular Potentials Recorded from the Sinoatrial Pacemaker of the Rabbit
 292 Characteristics of Extracellular Potentials Recorded from the Sinoatrial Pacemaker of the Rabbit MARVIN CRAMER, MICHAEL SIEGAL, J. THOMAS BIGGER, JR., AND BRIAN F. HOFFMAN excitation by nerve impulses.
292 Characteristics of Extracellular Potentials Recorded from the Sinoatrial Pacemaker of the Rabbit MARVIN CRAMER, MICHAEL SIEGAL, J. THOMAS BIGGER, JR., AND BRIAN F. HOFFMAN excitation by nerve impulses.
