Regulatory Proteins from Dorsal Muscle of the Carp
|
|
|
- Antonia Bennett
- 5 years ago
- Views:
Transcription
1 J. Biochent. 82, (1977) Regulatory Proteins from Dorsal Muscle of the Carp Kunihiko KONNO,* Ken-ichi ARAI,* and Shizuo WATANABE** *The Department of Food Science, Faculty of Fisheries, Hokkaido University, Hakodate, Hokkaido 041, and **the Department of Chemistry, Faculty of Science, Tokyo Institute of Technology, Ookayama, Meguro-ku, Tokyo 152 Received for publication, February 28, 1977 It was found that myosin B of carp dorsal muscle is identical with that of rabbit skeletal muscle as regards both calcium and strontium sensitivities. Carp dorsal myosin B is therefore dif ferent from bovine cardiac myosin B, the strontium sensitivity of which is known to be higher than that of rabbit skeletal myosin B. The sensitivities were estimated by measuring the Mg-ATPase activity in the presence of various concentrations of calcium or strontium ions. Half-maximal activation of the ATPase activity was obtained with either 1 ƒêm free calcium ions or 20 ƒêm free strontium ions. Relaxing protein (also called "native" tropomyosin) and troponin were prepared from carp dorsal muscle, and were shown to be as effective as those prepared from rabbit skeletal muscle in inhibiting Mg-ATPase of either rabbit or carp "desensitized" myosin B and in conferring calcium sensitivity on either of them. It was, however, observed that when desensitized myosin B is prepared from "frozen" instead of "fresh" rabbit skeletal muscles, Mg-ATPase of the desensitized myosin B of frozen muscle is only weakly inhibited by carp relaxing protein or carp troponin (plus rabbit tropo myosin), whereas it is fully inhibited by rabbit relaxing protein or rabbit troponin plus rabbit tropomyosin. It was then found that the desensitized myosin B prepared from frozen rabbit skeletal muscle can be made equally responsive to carp and rabbit relaxing proteins (or troponin plus tropomyosin) either by heating the desensitized myosin B in M KCl at 30 to 40 Ž for 10 to 30 min or by treating it with 1 to 1.5 M urea at 25 Ž for 20 min. It is thus suggested that freezing rabbit muscle caused a minor and reversible change in the actomyosin con formation. The observations described above were also reproduced using "reconstituted" actomyosin in place of "desensitized" myosin B. For example, it was observed that Mg-ATPase of rabbit actomyosin reconstituted from actin of frozen muscle and myosin of fresh muscle is only weakly inhibited by carp relaxing protein. It was, however, found that carp relaxing protein is as effective as rabbit relaxing protein if it is added to actin of frozen muscle before actin and myosin are mixed to reconstitute actomyosin. Abbreviations: EGTA, ethyleneglycol-bis(a-aminoethylether)-n, N Œ-tetraacetate; SDS, sodium dodecyl sulfate. Vol. 82, No. 4,
2 932 K. KONNO, K. ARAI, and S. WATANABE In a previous report (1, 2) from our laboratory, was shown that actin and myosin prepared from carp dorsal muscle were nearly identical, in bio chemical functions, with those of rabbit muscle. In the present report it is shown that relaxing protein ("native" tropomyosin) and troponin prepared from carp dorsal muscle are also essential ly identical, in biochemical functions, with those of rabbit skeletal muscle. However, it has been observed that Mg-ATPase of "desensitized" myosin B (3) prepared from "frozen" rabbit skeletal muscle is not inhibited by carp relaxing protein, whereas it is fully inhibited by rabbit relaxing protein. The reason for the effect of freezing muscle on the response of rabbit desensitized myosin B to carp relaxing protein is investigated in this work. EXPERIMENTAL All the muscular proteins we used were prepared from rabbit skeletal and carp dorsal muscles, fresh or stored at -20 Ž for a week. Myosin B was prepared from rabbit skeletal muscle by a routine method, extracting with Weber-Edsall solution, and also from carp dorsal muscle, extracting with 0.6 M KCl buffered with 40 mm sodium phosphate (ph 7.5) (4). "Desensitized" myosin B was obtained by the procedure described by Schaub et al. (3). Myosin was prepared by the method of Connell (5), extracting with 0.45 M KCl, 5 mm ATP-Mg, and 50 mm sodium phosphate (ph 6.4). Actin free from relaxing protein was prepared by the method of Spudich and Watt (6). Recon stituted actomyosin was prepared by mixing myosin and actin in a weight ratio of 3 : 1. Rabbit and carp relaxing proteins were obtained by the method originally reported for the preparation from rabbit skeletal muscle (7, 8). Troponin was also prepared from both carp dorsal and rabbit skeletal muscles by the method of Ebashi et al. (9). Tropomyosin was prepared only from rabbit skeletal muscle by the method of Bailey (10). ƒ - Actinin was prepared according to the method of Ebashi et al. (11). The Mg-ATPase reaction was carried out at 25 Ž in a medium containing 30 mm KCl, 20 mm Tris-maleate buffer (ph 7.5), 2 mm MgCl2, 2 mm ATP, and an appropriate concentration (0.1 mg/ml) of myosin B, "desensitized" myosin B or recon it stituted actomyosin. The reaction was stopped by adding perchioric acid (to 5%), and the in organic orthophosphate (Pi) liberated was de termined according to Fiske and Subbarow (12), though p-methylaminophenol was used in place of 1, 2, 4-aminonaphthol sulfonic acid as the reducing reagent. The initial rate of phosphate liberation in the steady state (ƒêmol Pi per min per mg of protein) was used to express the Mg-ATPase ac tivity. Ca sensitivity was defined by the following relationship: "Superprecipitation" of "desensitized" myosin B was also studied by measuring absorbance at 550 nm (13). The temperature was maintained at 20 Ž. SDS-gel electrophoresis was carried out ac cording to Weber and Osborn (14). A gel rod of 10 % polyacrylamide was used, and Coomassie Blue was used to stain the protein bands. The protein concentration was determined by the biuret method. RESULTS AND DISCUSSION The Calcium and Strontium Sensitivities of Myosin B-The Mg-ATPase activity of myosin B in a low salt medium was measured with various concentrations of calcium or strontium ions. The concentration of free ions was calculated by assum ing that the stability constants under the experi mental conditions employed are 3 x 105 M-1 for Ca-EGTA complex and 4 x 102 M-1 for Sr-EGTA complex (15). In Fig. 1, the ATPase activity is plotted as a function of pca or psr (negative logarithms of the free calcium or strontium concen trations). It is clear (Fig. 1) that carp dorsal myosin B is essentially identical with rabbit skeletal myosin B in both calcium and strontium sensitivi ties. The half-maximal activation of ATPase occurred with either I ƒêm free calcium ions or 20 ƒêm free strontium ions. It is known (15, 16) that bovine cardiac myosin B has a higher strontium sensitivity than rabbit skeletal myosin B. Carp myosin B is thus different in strontium sensitivity from bovine cardiac myosin B. Relaxing Protein (or "Native" Tropomyosin) "Desensitized" myosin B's and relaxing proteins were prepared from rabbit skeletal and carp dorsal J. Biochem.
3 REGULATORY PROTEINS FROM CARP MUSCLE 933 fresh muscles. SDS-gel electrophoretic patterns of the proteins thus obtained are shown in Fig. 2. The effect of relaxing protein on the Mg-ATPase activity of desensitized myosin B was studied, and the results obtained are summarized in Table I. Carp relaxing protein appears to be as effective as rabbit relaxing protein in inhibiting Mg-ATPase of either carp or rabbit desensitized myosin B and in conferring calcium sensitivity on desensitized myosin B. Rabbit Desensitized Myosin B of Frozen Muscle-It was, however, found (Fig. 3) that rabbit desensitized myosin B prepared from "frozen" muscle (a week at -20 Ž) behaves differently from that prepared from "fresh" muscle, whereas carp desensitized myosin B obtained from frozen muscle behaves in the same way as that prepared from fresh muscle. When rabbit de sensitized myosin B prepared from frozen muscle was used, its Mg-ATPase activity was not inhibited by carp relaxing protein, but it was strongly in hibited by rabbit relaxing protein. On the other Fig. 1. Activation effect of calcium and strontium ions on the Mg-ATPase activity of carp dorsal and rabbit skeletal myosin B's. (A): The EGTA concentration was kept at 0.5 mm, and the concentration of SrCl2 was varied. 143 ƒêg/ml of carp myosin B ( ü) or 110 ƒêg/ml of rabbit myosin B ( ) was ued. (B): The CaCl2 concentration was kept at I mm, and the concentration of EGTA was varied. 143 ƒêg/ml of carp myosin B ( ü) or 107 ƒêg/ml of rabbit myosin B ( ) was used. ATPase assay was carried out at 25 Ž in a medium containing 2 mm MgCl2, 2 mm ATP, 30 mm KCl, 20 mm Tris maleate (ph 7.0 in A, and ph 6.7 in B). hand, when carp desensitized myosin B prepared from frozen muscle was used, both carp and rabbit relaxing proteins were equally effective in inhibiting Mg-ATPase and accordingly in conferring calcium sensitivity. Table II shows that the strontium sensitivity was also low with a combination of carp relaxing protein and rabbit desensitized myosin B from frozen muscle. The difference between rabbit and carp relaxing proteins was also observed when superprecipitation instead of Mg-ATPase ac tivity was studied (Fig. 4). Superprecipitation of Fig. 2. SDS-gel electrophoresis of myosin, actin, desensitized myosin B, troponin, tropomyosin, and relaxing protein obtained from fresh and frozen muscles of carp and rabbit. SDS gels (10% polyacrylamide) of 10 cm length and 0.5 cm diameter were pre pared according to Weber and Osborn. Electrophoresis was carried out in 0.1 M sodium phosphate (ph 7.0) containing 0.1 % SDS at a constant current of 8 ma per gel for 4 h. Approximately 50 tug of myosin and desensitized myosin B (d-myosin B), 20ƒÊg of actin and relaxing protein (RP), and 10 ƒêg of troponin (TN) and tropomyosin (TM) were applied to each gel rod. The proteins were obtained from rabbit fresh skeletal (R), rabbit "frozen" skeletal (R Œ), carp fresh dorsal (C), and carp "frozen" dorsal (C Œ) muscles. Vol. 82, No. 4, 1977
4 934 K. KONNO, K. ARAI, and S. WATANABE TABLE I. Effect of relaxing protein on the Mg-ATPase activity of desensitized myosin B prepared from "fresh" muscles of carp and rabbit. (A) ƒêg/ml of rabbit desensitized myosin B and 37.0 ƒêg/ml of rabbit relaxing protein or 47.4 ƒêg/ml of carp relaxing protein were used. (B) ƒêg/ml of carp desensitized myosin B and 30.0 ƒê g/ml of rabbit relaxing protein or 29.0 ƒêg/ml of carp relaxing protein were used. The Mg-ATPase assay was carried out at 25 C in a medium containing 20 mm Tris-maleate (ph 7.5), 30 mm KCl, 2 mm MgCl2, and 0.1 mm CaCl2 (+Ca) or 0.5 mm EGTA (-Ca). Ca sensitivity was calculated as described in "EXPERIMENTAL." RP and d-myosin B denote relaxing protein and desensitized myosin B, respectively. The activity is expressed in ƒêmol Pi/min/mg of myosin B. arp and rabbit relaxing proteins prepared from frozen muscles were as effective those as prepared from fresh muscles in inhibitin Mg-ATPase and superprecipitation of desensitized myosin B of fresh muscles. We investigated the possibility that rabbit desensitized myosin B from frozen muscle is in capable of binding carp relaxing protein. De sensitized myosin B and relaxing protein were homogenized in 0.1 M KCl (ph 7.5), and centri fuged at 20,000 x g. The sediment was then analyzed by SDS-gel electrophoresis. The results Fig. 3. Effect of relaxing protein on the Mg-ATPase activity of desensitized myosin B obtained from "frozen" muscle of carp and rabbit. (A) 112 ƒêg/ml of rabbit desensitized myosin B, and carp ( œ, ü) or rabbit (, ) relaxing protein. (B) 97 ƒêg/ml of carp desensitized myosin B, and carp ( œ, ü) or rabbit (, ) relaxing protein. œ +Ca; ü, -Ca. Conditions for the ATPase assay were as in Table I. rabbit desensitized myosin B from "frozen" muscle is only weakly inhibited by carp relaxing protein, whereas it is strongly inhibited by rabbit relaxing protein (Fig. 4A and 4B). On the other hand, when carp desensitized myosin B prepared from frozen muscle was used, both carp and rabbit relaxing proteins were equally effective in inhibiting superprecipitation (Fig. 4C and 4D). It should also be mentioned (though the results are not pre (though not presented) indicated that rabbit de sensitized myosin B from frozen muscle can bind carp relaxing protein as strongly as it binds rabbit relaxing protein. Troponin-The observations described above suggest that relaxing protein can be replaced by troponin plus tropomyosin. We were able to prepare troponin from carp dorsal muscle using the same procedures reported for the preparation of rabbit skeletal troponin (9). The patterns obtained on SDS-gel electrophoresis of carp and rabbit tro ponins are shown in Fig. 2. In agreement with the results of other investigators (9), rabbit troponin showed three protein bands of 37,000, 24,000, and 19,000 daltons. Carp troponin also showed three protein bands, though they corresponded to 30,000, 21,000, and 19,000 daltons. The electrophoretic patterns of tropomyosin and relaxing proteins obtained are also shown in Fig. 2. J. Biochem.
5 REGULATORY PROTEINS FROM CARP MUSCLE 935 TABLE II. Effect of relaxing protein (RP) on the Mg-ATPase activity of desensitized myosin B from "frozen" muscles of carp and rabbit: strontium sensitivity. (A) 75.2 ƒêg/ml of rabbit desensitized myosin B and 37.9 ƒêg/ml of rabbit relaxing protein or 44.8 beg/ml of carp relaxing protein were used. (B) ƒêg/ml of carp desensitized myosin B and 37.9 ƒêg/ml of rabbit relaxing protein or 44.5 ƒêg/ml of carp relaxing protein were used. The Mg ATPase assay was carried out at 25 Ž in a medium containing 20 mm Tris-maleate (ph 7.5), 30 mm KCl, 2 mm ATP, 12 mm MgCl2, and 0.5 mm EGTA plus 5 x 10-" m SrCl2 (+Sr) or 0.5 mm EGTA (-Sr). The activity was expressed in ƒêmol P, min/mg of myosin B. Sr sensitivity is calculated using the relationship: When desensitized myosin B obtained from frozen rabbit muscle was used (Fig. 5A), its Mg ATPase was only weakly inhibited by carp troponin (plus rabbit tropomyosin) but was strongly inhibited by rabbit troponin (plus rabbit tropomyosin). When desensitized myosin B obtained from frozen carp muscle was employed (Fig. 5B), its Mg- ATPase was strongly inhibited by carp troponin as well as by rabbit troponin. Rabbit desensitized Fig. 4. Effect of relaxing protein on superprecipitation of desensitized myosin B obtained from frozen muscles of carp and rabbit. Superprecipitation of 308 ƒêg/ml of rabbit (A, B) or 320 beg/ml of carp (C, D) desensitized myosin B was followed by measuring the absorbance at 550 nm. The reaction was carried out at 20 Ž in a medium containing 2 mm MgCl2, 0.5 mm ATP, 60 mm KCl, 20 mm Tris-maleate (ph 7.0), and 0.5 mm EGTA (open symbols) or 0.1 mm CaCl2 (filled symbols). The arrow on the curve with open symbols indicates addition of 1.7 mm CaCl2. Circles, carp relaxing protein; squares rabbit relaxing protein; triangles, control (with no relaxing protein). Fig. 5. Effect of troponin (plus tropomyosin) on the Mg-ATPase activity of desensitized myosin B obtained from frozen muscle of carp and rabbit. (A) 91.9 ƒêg/ml of rabbit desensitized myosin B. (B) 120 ƒêg/ml of carp desensitized myosin B. Circles ( œ, ü), carp troponin; squares (, ), rabbit troponin. Rabbit skeletal tropo myosin was always added at a concentration equal to the concentration of troponin added. Conditions for the ATPase assay were as in Table I. Vol. 82, No. 4, 1977
6 936 K. KONNO, K. ARAI, and S. WATANABE myosin B obtained from frozen muscle was thus different from that obtained from fresh muscle as regards response to carp relaxing protein or carp troponin, although they showed no difference in shows that the effect of heat treatment requires KCl at a concentration higher than 0.4 M. Effects very similar to those of heat treatment were observed when rabbit desensitized myosin B SDS-gel electrophoretic pattern (Fig. 2). Treatment with Heat or Urea-It was found, however, that rabbit desensitized myosin B prepared from frozen muscle can be made as responsive to carp relaxing protein as that prepared from fresh muscle by treating the desensitized myosin B with heat or urea. Figure 6 shows that the Mg-ATPase activities in the presence and absence of calcium ions are both reduced by heat treatment, but that the activity is more strongly reduced in the absence of calcium ions than in its presence, thus resulting in an increase in the calcium ions than in its pres ence, thus resulting in an increase in the calcium sensitivity of the heat-treated desensitized myosin B (30 to 40 Ž). The effect of heat treatment at 35 Ž was studied as a function of time. Figure 7 shows that rabbit desensitized myosin B of frozen muscle acquires calcium sensitivity in 10 to 15 min, while that obtained from fresh muscle is not affected in any way by the same heat treatment. Figure 8 Fig. 7. Heat treatment of desensitized myosin B's of rabbit fresh and frozen muscles, and resensitization to Cal+ by carp relaxing protein. (A) ƒêg/ml of desensitized myosin B of fresh rabbit muscle and 44.2 ƒê g/ml of carp relaxing protein. (B) leg/ml of desensitized myosin B of frozen rabbit muscle and 44.6 ƒêg/ml of carp relaxing protein. Desensitized myo sion B was incubated at 35 Ž for various times. Con ditions for the ATPase assay were as in Table I. The symbols used are the same as in Fig. 6. Fig. 6. Heat treatment of desensitized myosin B of rabbit frozen muscle, and resensitization to Cal+ by carp relaxing protein. Rabbit desensitized myosin B (4 mg/ml) in 0.6 M KCl-20 mm Tris-maleate, ph 7.5, was incubated at various temperatures for 30 min. After cooling to 0 Ž, the Mg-ATPase activity was assayed with 98.4 ƒêg/ml of rabbit desensitized myosin B and 46.5 leg/ml of carp relaxing protein ( œ -I-Ca; ü, -Ca). The Ca sensitivity ( ) was calculated as described in " EXPERIMENTAL." The Mg-ATPase activity of desensitized myosin B alone was also assayed (x). Conditions for the ATPase assay were as in Table T. Fig. 8. KCl concentration and heat treatment of rabbit desensitized myosin B of rabbit frozen muscle. Rabbit desensitized myosin B (4 mg/ml) was treated at 35 Ž for 30 min at various KCl concentrations. 105 leg/ml of rabbit desensitized myosin B and 50.3 leg/ml of carp relaxing protein were used for the Mg-ATPase assay. Conditions for the ATPase assay were as in Table I. The symbols used are the same as in Fig. 6. J. Biochem.
7 REGULATORY PROTEINS FROM CARP MUSCLE 937 of frozen muscle was incubated with urea at 25 Ž for 20 min. The Mg-ATPase activity was then measured in the presence of carp relaxing protein. Figure 9 shows that 1 to 1.5 M urea can make the desensitized myosin B responsive to the addition of carp relaxing protein. Since heat and urea treatments are generally thought to cause some change in the protein conformation, it is suggested that rabbit desensitized myosin B obtained from frozen muscle is different in conformation from that obtained from fresh muscle. On the other hand, it was reported by Fukazawa et al. (17) that freezing of rabbit skeletal or chicken breast muscle makes the Z-line structure more fragile. It is therefore possible that rabbit desensitized myosin B from frozen muscle is contaminated by a-actinin (11), one of the constituents of the Z-line structure - However, (though the result is not shown) addition of ƒ -actinin obtained from rabbit skeletal muscle to desensitized myosin B obtained from fresh rabbit muscle did not affect the Mg-ATPase activity of the latter. "Reconstituted" Actomyosin -We next in vestigated whether actin or myosin in the de Fig. 9. Urea treatment of desensitized myosin B of frozen muscle. Rabbit desensitized myosin B (2 mg/ml) in 0.6 M KCl-20 mm Tris-maleate (ph 7.5) was treated with various concentrations of urea at 25 Ž for 20 min. The treatment was halted by adding eight volumes of distilled water containing other components for the ATPase assay ƒêg/ml of rabbit desensitized myosin B and 50.3 ƒêg/ml of carp relaxing protein were used for the ATPase assay. Conditions for the ATPase assay were as in Table I. The symbols used are the same as Fig. 6. sensitized myosin B is changed, probably in conformation, upon freezing rabbit skeletal muscle. Myosins and actins were prepared from both frozen and fresh muscles of rabbit and carp. In the SDSgel electrophoretic patterns (Fig. 2), actins and myosins prepared from frozen muscles were not distinguishable from those obtained from fresh muscles. However, when the Mg-ATPase activity of "reconstituted" actomyosin was measured (sixteen different combinations), it was found that TABLE III. Resensitization effect of relaxing protein on the Mg-ATPase activity of "reconstituted" actomyosin. Myosins were always prepared from fresh muscles of carp (C) and rabbit (R). Actins were prepared from both fresh and frozen muscles. Relaxing protein (40% relative to actomyosin) was added to reconstituted actomyosin (1 part of actin and 3 parts of myosin by weight). Conditions for the ATPase assay were as described in Table I. The activity is expressed in ƒêmol Pi/min/mg of actomyosin. Vol. 82, No. 4, 1977
8 938 K. KONNO, K. ARAI, and S. WATANABE rabbit actin of frozen muscle behaves differently from the other three actins. Table III summarizes the results obtained for eight combinations with fresh myosins. Although omitted from the table, essentially the same results were obtained for eight other combinations with frozen muscle myosins. In other words, freezing of muscles caused no detectable change in carp and rabbit myosins. On the other hand, as shown in Table III, whenever rabbit actin of frozen muscle was used to reconsti tute actomyosin, the calcium sensitivity (or the relaxing protein-induced inhibition) was low. Freezing rabbit muscle must, therefore, have caused a definite change in rabbit actin, whereas it did not seem to cause any appreciable change in carp actin. It should, however, be noted that the effect of freezing rabbit skeletal muscle on the properties of the actin preparation does not fully account for that on the properties of desensitized myosin B preparation. The effect (for example, the inhibi tory effect) of carp relaxing protein observed here with actomyosin reconstituted by using rabbit actin of frozen muscle is stronger (50 or 55 in Table III) than that observed with rabbit desensitized myosin B of frozen muscle (10 or 16 in Table II). It was, moreover, observed (though not shown in the table) that even after heat or urea treatment of rabbit actin of frozen muscle, the inhibitory effect of carp relaxing protein on the reconstituted actomyosin was not enhanced: 59 before and 65 after urea treatment, and 59 before and 60 after heat treat ment. On the other hand, it was found that when rabbit actomyosin reconstituted from rabbit actin and myosin of fresh muscle was kept frozen at -20 Ž for 7-10 days, it behaved exactly like rabbit desensitized myosin B of frozen muscle. In other words, Mg-ATPase of the reconstituted actomyosin was only weakly inhibited (10-15 instead of 50-59) by carp troponin (plus tropomyosin), and was fully inhibited by carp troponin (plus tropomyosin) after urea or heat treatment. It may be reasonable to assume that rabbit actin (in the filament form) of frozen muscle assumes an irregular conformation, relative to a regular conformation assumed by rabbit actin obtained from fresh muscle, and also that actin in the irregular conformation binds irregularly with myosin. If so, the observations described above suggest that (a) Carp troponin is different from combined with tropomyosin) can normalize the irregular binding between actin and myosin. (b) The irregular binding in reconstituted acto myosin is different from that in desensitized myosin B of frozen rabbit muscle. In view of the following findings, the differences seem to be of a subtle nature. When carp relaxing protein was added to rabbit actin of frozen muscle prior to reconstituting actomyosin, it was found to be as effective as rabbit relaxing protein in inhibiting Mg-ATPase of the reconstituted actomyosin. In all other experiments presented in this report, relaxing protein was added after actin and myosin had been mixed. It is therefore suggested that the binding of relaxing protein to rabbit actin of frozen muscle restored the conformation of the latter to the regular one, which is in turn capable of binding in the regular way with the subsequently added myosin. REFERENCES 1. Takashi, R. (1973) Bull. Japanese Soc. Sci. Fisheries (Nihon Suisan Gakkai Shi) (Abstract in English) 39, Seki, N., Kitao, M., Konno, K., & Arai, K. (1973) Bull. Japanese Soc. Sci. Fisheries (Nihon Suisan Gakkai Shi) (Abstract in English) 39, Schaub, M.C., Hartshorne, D.J., & Perry, S.V. (1964) Biochem. J. 104, Takashi, R., Arai, K., & Saito, T. (1970) Bull. Japanese Soc. Sci. Fisheries (Nihon Suisan Gakkai Shi) (Abstract in English) 36, Connell, J.J. (1954) Biochem. J. 58, Spudich, J.A. & Watt, S. (1971) J. Biol. Chem. 246, Wilkinson, T.M., Perry, S.V., Cole, H.A., & Trayer, I.P. (1972) Biochem. J. 127, Arai, K. & Watanabe, S. (1968) J. Biochem. 64, Ebashi, S., Wakabayashi, T., & Ebashi, F. (1971) J. Biochem. 69, Bailey, K. (1948) Biochem. J. 43, Ebashi, S. & Ebashi, F. (1965) J. Biochem. 58, Fiske, C.H. & Subbarow, Y. (1925) J. Biol. Chem. 66, Yasui, T. & Watanabe, S. (1965) J. Biochern. 240, Weber, K. & Osborn, M. (1969) J. Biol. Chem. 244, Ebashi, S., Kodama, A., & Ebashi, F. (1968) J. Biochem. 64, Staprans, I., Takahashi, H., Russell, M.P., & Watanabe, S. (1972) J. Biochem. 72, Fukazawa, T. Hashimoto, Y., & Tonomura, Y. (1963) Biochim. Biophys. Ada 75, rabbit troponin in that only the latter (when J. Biochem.
Calcium Regulation in Squid Mantle and Scallop Adductor Muscles 1
 . Biochem. 89, 581-589 (1981) Calcium Regulation in Squid Mantle and Scallop Adductor Muscles 1 Kunihiko KONNO,* Ken-ichi ARM,* Mikiharu YOSHIDA,** and Shizuo WATANABE*** Department of Food Science, Faculty
. Biochem. 89, 581-589 (1981) Calcium Regulation in Squid Mantle and Scallop Adductor Muscles 1 Kunihiko KONNO,* Ken-ichi ARM,* Mikiharu YOSHIDA,** and Shizuo WATANABE*** Department of Food Science, Faculty
The Major Component of Metin from Rabbit Skeletal and Bovine Cardiac Muscle*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 24, No. 1, October 1965 Printed in U.S.A. The Major Component of Metin from Rabbit Skeletal and Bovine Cardiac Muscle* NAOMI AzUMAt AND SHIZUO WATANABE~ From the
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 24, No. 1, October 1965 Printed in U.S.A. The Major Component of Metin from Rabbit Skeletal and Bovine Cardiac Muscle* NAOMI AzUMAt AND SHIZUO WATANABE~ From the
Effect of Ouabain on the ATPase of Cardiac Myosin B at High Ionic Strength
 Effect of Ouabain on the ATPase of Cardiac Myosin B at High Ionic Strength By Ada L. Jacobson, Ph.D. ABSTRACT The effect of ouabain (0" 8 to (H M) on the hydrolysis of ATP by beef cardiac myosin B was
Effect of Ouabain on the ATPase of Cardiac Myosin B at High Ionic Strength By Ada L. Jacobson, Ph.D. ABSTRACT The effect of ouabain (0" 8 to (H M) on the hydrolysis of ATP by beef cardiac myosin B was
Title. Author(s)Thavaroj, Wichulada; Sakamoto, Mari; Konno, Yoshiko; CitationFisheries science, 82(5): Issue Date Doc URL.
 Title Preceding actin denaturation accelerates myosin dena Author(s)Thavaroj, Wichulada; Sakamoto, Mari; Konno, Yoshiko; CitationFisheries science, 82(5): 843-850 Issue Date 2016-09 Doc URL http://hdl.handle.net/2115/67081
Title Preceding actin denaturation accelerates myosin dena Author(s)Thavaroj, Wichulada; Sakamoto, Mari; Konno, Yoshiko; CitationFisheries science, 82(5): 843-850 Issue Date 2016-09 Doc URL http://hdl.handle.net/2115/67081
Mode of IMP and Pyrophosphate Enhancement of Myosin and Actin Extraction from Porcine Meat
 Biosci. Biotechnol. Biochem., 77 (6), 1214 1218, 2013 Mode of IMP and Pyrophosphate Enhancement of Myosin and Actin Extraction from Porcine Meat Yukinobu NAKAMURA, 1;y Koshiro MIGITA, 2 Akihiro OKITANI,
Biosci. Biotechnol. Biochem., 77 (6), 1214 1218, 2013 Mode of IMP and Pyrophosphate Enhancement of Myosin and Actin Extraction from Porcine Meat Yukinobu NAKAMURA, 1;y Koshiro MIGITA, 2 Akihiro OKITANI,
The Action of Thiamine Phosphates on the Contraction of Glycerinated Psoas Muscle
 THE JOURNAL OF VITAMINOLOGY 14, 101-105 (1968) The Action of Thiamine Phosphates on the Contraction of Glycerinated Psoas Muscle ATSUSHI MURAI AND EISUKE KATSURA2 Nutrition Laboratory, Kyoto University
THE JOURNAL OF VITAMINOLOGY 14, 101-105 (1968) The Action of Thiamine Phosphates on the Contraction of Glycerinated Psoas Muscle ATSUSHI MURAI AND EISUKE KATSURA2 Nutrition Laboratory, Kyoto University
Calcium-Induced Fragmentation of Skeletal Muscle Nebulin Filaments'
 J. Biochem. 112, 775-779 (1992) Calcium-Induced Fragmentation of Skeletal Muscle Nebulin Filaments' Ryuichi Tatsumi and Koui Takahashi Meat Science Laboratory, Department of Animal Science, Faculty of
J. Biochem. 112, 775-779 (1992) Calcium-Induced Fragmentation of Skeletal Muscle Nebulin Filaments' Ryuichi Tatsumi and Koui Takahashi Meat Science Laboratory, Department of Animal Science, Faculty of
Some Properties of Glycerinated Skeletal Muscle Fibres Containing Phosphorylated Myosin
 Gen. Physiol. Biophys. (1989), 8, 569-578 569 Some Properties of Glycerinated Skeletal Muscle Fibres Containing Phosphorylated Myosin M. WROTEK', YU. S. BOROVIKOV 2, N. N. LEBEDEVA 2 and I. K^KOĽ 1 Department
Gen. Physiol. Biophys. (1989), 8, 569-578 569 Some Properties of Glycerinated Skeletal Muscle Fibres Containing Phosphorylated Myosin M. WROTEK', YU. S. BOROVIKOV 2, N. N. LEBEDEVA 2 and I. K^KOĽ 1 Department
Properties of Tropomyosin from Tilapia, Tilapia nilotica, Dorsal Muscle 2
 Properties of Tropomyosin from Tilapia,Tilapia nilotica, Dorsal Muscle. 2 Properties of Tropomyosin from Tilapia, Tilapia nilotica, Dorsal Muscle 2 Comparison of Tilapia Tropomyosin with Rabbit Skeletal
Properties of Tropomyosin from Tilapia,Tilapia nilotica, Dorsal Muscle. 2 Properties of Tropomyosin from Tilapia, Tilapia nilotica, Dorsal Muscle 2 Comparison of Tilapia Tropomyosin with Rabbit Skeletal
actin-troponin-tropomyosin complex (muscle relaxation/cooperativity/regulated actin)
 Proc. Nati. Acad. Sci. USA Vol. 77, No. 5, pp. 2616-2620, May 1980 Biochemistry Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex (muscle relaxation/cooperativity/regulated
Proc. Nati. Acad. Sci. USA Vol. 77, No. 5, pp. 2616-2620, May 1980 Biochemistry Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex (muscle relaxation/cooperativity/regulated
Journal of FisheriesSciences.com
 11(2): 001-008(2017) Journal of FisheriesSciences.com E-ISSN 1307-234X 2017 www.fisheriessciences.com Research Article The Comparative Study of Myofibrillar Proteins of Skeletal Muscles of Some Deep-Sea
11(2): 001-008(2017) Journal of FisheriesSciences.com E-ISSN 1307-234X 2017 www.fisheriessciences.com Research Article The Comparative Study of Myofibrillar Proteins of Skeletal Muscles of Some Deep-Sea
YASUSHI OHIZUMI, KIMIHIRO MATSUNAGA, KEIGO NAKATANI and JUN ICHI KOBAYASHI
 0022-3565/98/2852-0695$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 2 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/98/2852-0695$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 285, No. 2 Copyright 1998 by The American Society for Pharmacology and Experimental Therapeutics Printed in
Muscle Cells & Muscle Fiber Contractions. Packet #8
 Muscle Cells & Muscle Fiber Contractions Packet #8 Skeletal muscle is attached to bones and is responsible for movement. Introduction Introduction II Skeletal muscle is composed of bundles of muscle fibers
Muscle Cells & Muscle Fiber Contractions Packet #8 Skeletal muscle is attached to bones and is responsible for movement. Introduction Introduction II Skeletal muscle is composed of bundles of muscle fibers
DIDS INHIBITION OF SARCOPLASMIC RETICULUM ANION EFFLUX AND CALCIUM TRANSPORT
 DIDS INHIBITION OF SARCOPLASMIC RETICULUM ANION EFFLUX AND CALCIUM TRANSPORT Kevin P. Campbell and David H. MacLennan Reprinted from ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Volume 358 Pages 328-331
DIDS INHIBITION OF SARCOPLASMIC RETICULUM ANION EFFLUX AND CALCIUM TRANSPORT Kevin P. Campbell and David H. MacLennan Reprinted from ANNALS OF THE NEW YORK ACADEMY OF SCIENCES Volume 358 Pages 328-331
Contribution of the polymerization of protein by disulfide bonding to increased gel strength of walleye pollack surimi gel with preheating time
 FISHERIES SCIENCE 2001; 67: 710 717 Original Article Contribution of the polymerization of protein by disulfide bonding to increased gel strength of walleye pollack surimi gel with preheating time Mohammed
FISHERIES SCIENCE 2001; 67: 710 717 Original Article Contribution of the polymerization of protein by disulfide bonding to increased gel strength of walleye pollack surimi gel with preheating time Mohammed
Ch. 6: Contraction of Skeletal Muscle Physiological Anatomy of Skeletal Muscle
 Ch. 6: Contraction of Skeletal Muscle 40% skeletal muscle + 10% smooth and cardiac muscle Ch. 7: Excitation of Skeletal Muscle Ch. 9: Contraction and Excitation of Smooth Muscle Physiological Anatomy of
Ch. 6: Contraction of Skeletal Muscle 40% skeletal muscle + 10% smooth and cardiac muscle Ch. 7: Excitation of Skeletal Muscle Ch. 9: Contraction and Excitation of Smooth Muscle Physiological Anatomy of
Biochemical Studies on the Mineral Components in Sake Yeast. Part V. The Relationship of the Mineral Composition of Yeast to Fermentation
 [Agr, Biol. Chem. Vol. 30, No. 9, p. 925 `930, 1966] Biochemical Studies on the Mineral Components in Sake Yeast Part V. The Relationship of the Mineral Composition of Yeast to Fermentation By Tsuyoshi
[Agr, Biol. Chem. Vol. 30, No. 9, p. 925 `930, 1966] Biochemical Studies on the Mineral Components in Sake Yeast Part V. The Relationship of the Mineral Composition of Yeast to Fermentation By Tsuyoshi
OF LIGHT CHAINS OF CARDIAC MYOSIN ISOZYMES: ATRIAL AND VENTRICULAR MYOSINS
 CROSS-HYBRIDIZATION OF LIGHT CHAINS OF CARDIAC MYOSIN ISOZYMES: ATRIAL AND VENTRICULAR MYOSINS Gabor HOLLGSI*, Sudhir SRIVASTAVA** and Joan WIKMAN-COFFELT University of California, San Francisco Cardiovascular
CROSS-HYBRIDIZATION OF LIGHT CHAINS OF CARDIAC MYOSIN ISOZYMES: ATRIAL AND VENTRICULAR MYOSINS Gabor HOLLGSI*, Sudhir SRIVASTAVA** and Joan WIKMAN-COFFELT University of California, San Francisco Cardiovascular
ROLE OF CALCIUM IN DRUG ACTION ON SMOOTH MUSCLE 1, 2 NORIKO YUKISADA AND FUMIKO EBASHI
 Jap. J. Pharmacol. 11, 46-53 (1961) ROLE OF CALCIUM IN DRUG ACTION ON SMOOTH MUSCLE 1, 2 NORIKO YUKISADA AND FUMIKO EBASHI Department of Pharmacology, Faculty of Medicine, University of Tokyo, Tokyo Received
Jap. J. Pharmacol. 11, 46-53 (1961) ROLE OF CALCIUM IN DRUG ACTION ON SMOOTH MUSCLE 1, 2 NORIKO YUKISADA AND FUMIKO EBASHI Department of Pharmacology, Faculty of Medicine, University of Tokyo, Tokyo Received
S. B. RAO. b) Settling beyond 10min and upto 60 min recorded as 10 + c) Partial and incomplete settling was reported as + ve.
 s S. B. RAO Grant ~Medical College, Bombay - 400 008 The preventive effect of fumarate, maleate, tartrate and oxalate on the denaturation of frozen rohu actomyosin at-20oc in 0. 7 M KC1 for 7 weeks was
s S. B. RAO Grant ~Medical College, Bombay - 400 008 The preventive effect of fumarate, maleate, tartrate and oxalate on the denaturation of frozen rohu actomyosin at-20oc in 0. 7 M KC1 for 7 weeks was
B. 50 mm Calcium Chloride Solution (CaCl 2 ) (Prepare 25 ml in Reagent A using Calcium Chloride, Dihydrate, Sigma Prod. No. C-3881.
 SIGMA QUALITY CONTROL TEST PROCEDURE ProductInformation Enzymatic Assay of PHOSPHOLIPASE C PRINCIPLE: L-α-Lecithin + H 2 O Phospholipase C > 1,2-Diglyceride + Choline Phosphate Choline phosphate + H 2
SIGMA QUALITY CONTROL TEST PROCEDURE ProductInformation Enzymatic Assay of PHOSPHOLIPASE C PRINCIPLE: L-α-Lecithin + H 2 O Phospholipase C > 1,2-Diglyceride + Choline Phosphate Choline phosphate + H 2
The Sliding Filament Theory
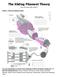 The Sliding Filament Theory Model 1: Muscle Histology Review How do muscle cells contract? Use your knowledge of muscle tissue histology to fill in the blanks numbered 1-11 with the following terms: Fasicle,
The Sliding Filament Theory Model 1: Muscle Histology Review How do muscle cells contract? Use your knowledge of muscle tissue histology to fill in the blanks numbered 1-11 with the following terms: Fasicle,
The rabbit femoral artery was prepared and each arterial ring was permeabilized
 Online Supplement Nakmura et al. cgmp-dependent relaxation of smooth muscle Materials and Methods Measurement of tension The rabbit femoral artery was prepared and each arterial ring was permeabilized
Online Supplement Nakmura et al. cgmp-dependent relaxation of smooth muscle Materials and Methods Measurement of tension The rabbit femoral artery was prepared and each arterial ring was permeabilized
About This Chapter. Skeletal muscle Mechanics of body movement Smooth muscle Cardiac muscle Pearson Education, Inc.
 About This Chapter Skeletal muscle Mechanics of body movement Smooth muscle Cardiac muscle Skeletal Muscle Usually attached to bones by tendons Origin: closest to the trunk or to more stationary bone Insertion:
About This Chapter Skeletal muscle Mechanics of body movement Smooth muscle Cardiac muscle Skeletal Muscle Usually attached to bones by tendons Origin: closest to the trunk or to more stationary bone Insertion:
Improved Thermal Stability and Emulsifying Properties of Carp Myofibrillar Proteins by Conjugation with Dextran
 J. Agric. Food Chem. 1998, 46, 1257 1261 1257 Improved Thermal Stability and Emulsifying Properties of Carp Myofibrillar Proteins by Conjugation with Dextran Kiyoshi Fujiwara, Toshishige Oosawa, and Hiroki
J. Agric. Food Chem. 1998, 46, 1257 1261 1257 Improved Thermal Stability and Emulsifying Properties of Carp Myofibrillar Proteins by Conjugation with Dextran Kiyoshi Fujiwara, Toshishige Oosawa, and Hiroki
Muscle Dr. Ted Milner (KIN 416)
 Muscle Dr. Ted Milner (KIN 416) Muscles are biological motors which actively generate force and produce movement through the process of contraction. The molecular mechanism responsible for muscle contraction
Muscle Dr. Ted Milner (KIN 416) Muscles are biological motors which actively generate force and produce movement through the process of contraction. The molecular mechanism responsible for muscle contraction
Microbial Production of L-Threonine. Part III. Production by Methionine and Lysine Auxotrophs. Derived from ƒ -Amino-ƒÀ-hydroxyvaleric Acid Resistant
 I [Agr. Biol. Chem., Vol. 36, No. 7, p. 12091216, 1972] Microbial Production of L-Threonine Part III. Production by Methionine and Lysine Auxotrophs Derived from ƒ -Amino-ƒÀ-hydroxyvaleric Acid Resistant
I [Agr. Biol. Chem., Vol. 36, No. 7, p. 12091216, 1972] Microbial Production of L-Threonine Part III. Production by Methionine and Lysine Auxotrophs Derived from ƒ -Amino-ƒÀ-hydroxyvaleric Acid Resistant
COMPARISON OF PHYSICOCHEMICAL PROPERTIES OF MUSCLE PROTEINS IN CULTURED AND WILD PRAWNS (PENAEUS (FENNEROPENAEUS) ORIENTALIS KISHINOUYE, 1918)
 Pakistan Journal of Marine Sciences, Vol.5(1), 25-29, 1996. COMPARISON OF PHYSICOCHEMICAL PROPERTIES OF MUSCLE PROTEINS IN CULTURED AND WILD PRAWNS (PENAEUS (FENNEROPENAEUS) ORIENTALIS KISHINOUYE, 1918)
Pakistan Journal of Marine Sciences, Vol.5(1), 25-29, 1996. COMPARISON OF PHYSICOCHEMICAL PROPERTIES OF MUSCLE PROTEINS IN CULTURED AND WILD PRAWNS (PENAEUS (FENNEROPENAEUS) ORIENTALIS KISHINOUYE, 1918)
PhosFree TM Phosphate Assay Biochem Kit
 PhosFree TM Phosphate Assay Biochem Kit (Cat. # BK050) ORDERING INFORMATION To order by phone: (303) - 322-2254 To order by Fax: (303) - 322-2257 To order by e-mail: cservice@cytoskeleton.com Technical
PhosFree TM Phosphate Assay Biochem Kit (Cat. # BK050) ORDERING INFORMATION To order by phone: (303) - 322-2254 To order by Fax: (303) - 322-2257 To order by e-mail: cservice@cytoskeleton.com Technical
EXCITATION- CONTRACTION COUPLING IN SKELETAL MUSCLES 1
 EXCITATION- CONTRACTION COUPLING IN SKELETAL MUSCLES 1 Summary: The sequence of events from the movement of an AP moving down a neuron to the completion of a contraction is examined. These events are referred
EXCITATION- CONTRACTION COUPLING IN SKELETAL MUSCLES 1 Summary: The sequence of events from the movement of an AP moving down a neuron to the completion of a contraction is examined. These events are referred
MRP2 TR ATPase Assay Protocol CAT. NO. SBAT03
 MRP2 TR ATPase CAT. NO. SBAT03 Page 1 of 18 Determination of the interaction of drugs with the human MRP2 (ABCC2) transporter using the ATPase Assay For the following membrane products: SB-MRP2-Sf9-ATPase
MRP2 TR ATPase CAT. NO. SBAT03 Page 1 of 18 Determination of the interaction of drugs with the human MRP2 (ABCC2) transporter using the ATPase Assay For the following membrane products: SB-MRP2-Sf9-ATPase
Identification of Three Major Components in Fish Sarcoplasmic Proteins
 Nippon Suisan Gakkaishi 54(6), 999-1004 (1988) Identification of Three Major Components in Fish Sarcoplasmic Proteins Takayuki Nakagawa,*1 Shugo Watabe,*2 and Kanehisa Hashimoto*2 (Received November 6,
Nippon Suisan Gakkaishi 54(6), 999-1004 (1988) Identification of Three Major Components in Fish Sarcoplasmic Proteins Takayuki Nakagawa,*1 Shugo Watabe,*2 and Kanehisa Hashimoto*2 (Received November 6,
A Component of Wheat Flour Globulin Polymerized at Alkaline Sides and Depolymerized by Reduction Reversibly
 Agric. Biol. Chem., 42 (7), 1397 `1402, 1978 A Component of Wheat Flour Globulin Polymerized at Alkaline Sides and Depolymerized by Reduction Reversibly Masaki TERADA, Junichi MINAMI and Takehiko YAMAMOTO*'
Agric. Biol. Chem., 42 (7), 1397 `1402, 1978 A Component of Wheat Flour Globulin Polymerized at Alkaline Sides and Depolymerized by Reduction Reversibly Masaki TERADA, Junichi MINAMI and Takehiko YAMAMOTO*'
#1 20. physiology. Muscle tissue 30/9/2015. Ahmad Adel Sallal. Mohammad Qudah
 # 20 physiology Muscle tissue Ahmad Adel Sallal 30/9/205 Mohammad Qudah MUSCLES PHYSIOLOGY Awn, welcome to the first physiology lecture in the MSS, I wish you a perfect exams with high grades, and never
# 20 physiology Muscle tissue Ahmad Adel Sallal 30/9/205 Mohammad Qudah MUSCLES PHYSIOLOGY Awn, welcome to the first physiology lecture in the MSS, I wish you a perfect exams with high grades, and never
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
Vol. 46, No. 6, December.1998 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 11 o1-1108
 Vol. 46, No. 6, December.1998 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 11 o1-1108 THE SHORTENING OF THE N-TERMINUS OF MYOSIN ESSENTIAL LIGHT CHAIN A1 INFLUENCES THE INTERACTION OF HEAVY MEROMYOSIN
Vol. 46, No. 6, December.1998 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 11 o1-1108 THE SHORTENING OF THE N-TERMINUS OF MYOSIN ESSENTIAL LIGHT CHAIN A1 INFLUENCES THE INTERACTION OF HEAVY MEROMYOSIN
Masashi KOBAYASHI, Makoto IWASAKI, and Yukio SHIGETA. The Third Department of Medicine, Shiga University of Medical Science, Ohtsu, Shiga
 J. Biochem. 88, 39-44 (1980) Receptor Mediated Insulin Degradation Decreased by Chloroquine in Isolated Rat Adipocytes1 Masashi KOBAYASHI, Makoto IWASAKI, and Yukio SHIGETA The Third Department of Medicine,
J. Biochem. 88, 39-44 (1980) Receptor Mediated Insulin Degradation Decreased by Chloroquine in Isolated Rat Adipocytes1 Masashi KOBAYASHI, Makoto IWASAKI, and Yukio SHIGETA The Third Department of Medicine,
Chapter 10 Muscle Tissue Lecture Outline
 Chapter 10 Muscle Tissue Lecture Outline Muscle tissue types 1. Skeletal muscle = voluntary striated 2. Cardiac muscle = involuntary striated 3. Smooth muscle = involuntary nonstriated Characteristics
Chapter 10 Muscle Tissue Lecture Outline Muscle tissue types 1. Skeletal muscle = voluntary striated 2. Cardiac muscle = involuntary striated 3. Smooth muscle = involuntary nonstriated Characteristics
Naoki YAMANAKA, Toshio IMANARI,* Zenzo TAMURA,*
 J. Biochem., 73, 993-998 (1973) Uncoupling of Oxidative Phosphorylation of Rat Liver Mitochondria by Chinoform Naoki YAMANAKA, Toshio IMANARI,* Zenzo TAMURA,* and Kunio YAGI Institute of Biochemistry,
J. Biochem., 73, 993-998 (1973) Uncoupling of Oxidative Phosphorylation of Rat Liver Mitochondria by Chinoform Naoki YAMANAKA, Toshio IMANARI,* Zenzo TAMURA,* and Kunio YAGI Institute of Biochemistry,
Organismic Biology Bio 207. Lecture 6. Muscle and movement; sliding filaments; E-C coupling; length-tension relationships; biomechanics. Prof.
 Organismic Biology Bio 207 Lecture 6 Muscle and movement; sliding filaments; E-C coupling; length-tension relationships; biomechanics Prof. Simchon Today s Agenda Skeletal muscle Neuro Muscular Junction
Organismic Biology Bio 207 Lecture 6 Muscle and movement; sliding filaments; E-C coupling; length-tension relationships; biomechanics Prof. Simchon Today s Agenda Skeletal muscle Neuro Muscular Junction
Session 3-Part 2: Skeletal Muscle
 Session 3-Part 2: Skeletal Muscle Course: Introduction to Exercise Science-Level 2 (Exercise Physiology) Presentation Created by Ken Baldwin, M.ED, ACSM-H/FI Copyright EFS Inc. All Rights Reserved. Skeletal
Session 3-Part 2: Skeletal Muscle Course: Introduction to Exercise Science-Level 2 (Exercise Physiology) Presentation Created by Ken Baldwin, M.ED, ACSM-H/FI Copyright EFS Inc. All Rights Reserved. Skeletal
Ch 12: Muscles sarcolemma, t-tubules, sarcoplasmic reticulum, myofibrils, myofilaments, sarcomere...
 Ch 12: Muscles Review micro-anatomy of muscle tissue Terminology examples: sarcolemma, t-tubules, sarcoplasmic reticulum, myofibrils, myofilaments, sarcomere... SLOs Differentiate levels of muscle structure:
Ch 12: Muscles Review micro-anatomy of muscle tissue Terminology examples: sarcolemma, t-tubules, sarcoplasmic reticulum, myofibrils, myofilaments, sarcomere... SLOs Differentiate levels of muscle structure:
PSK4U THE NEUROMUSCULAR SYSTEM
 PSK4U THE NEUROMUSCULAR SYSTEM REVIEW Review of muscle so we can see how the neuromuscular system works This is not on today's note Skeletal Muscle Cell: Cellular System A) Excitation System Electrical
PSK4U THE NEUROMUSCULAR SYSTEM REVIEW Review of muscle so we can see how the neuromuscular system works This is not on today's note Skeletal Muscle Cell: Cellular System A) Excitation System Electrical
Movement of Scallop Myosin on Nitella Actin Filaments: Regulation by Calcium. Ronald D. Vale, Andrew G. Szent-Gyorgyi, and Michael P.
 Movement of Scallop Myosin on Nitella Actin Filaments: Regulation by Calcium Ronald D. Vale, Andrew G. Szent-Gyorgyi, and Michael P. Sheetz PNAS 1984;81;6775-6778 doi:10.1073/pnas.81.21.6775 This information
Movement of Scallop Myosin on Nitella Actin Filaments: Regulation by Calcium Ronald D. Vale, Andrew G. Szent-Gyorgyi, and Michael P. Sheetz PNAS 1984;81;6775-6778 doi:10.1073/pnas.81.21.6775 This information
Muscle Tissue. Xie Fenfen. Department of Histology and Embryology School of Basic Medicine Anhui Medical University
 Muscle Tissue Xie Fenfen Email:xff2005024@126.com Department of Histology and Embryology School of Basic Medicine Key points The structural differences (LM) of 3 types of muscle fibers Molecular structure
Muscle Tissue Xie Fenfen Email:xff2005024@126.com Department of Histology and Embryology School of Basic Medicine Key points The structural differences (LM) of 3 types of muscle fibers Molecular structure
Enzymatic Assay of PROTEASE (EC )
 Enzymatic Assay of PROTEASE PRINCIPLE: Hemoglobin + H 2 O Protease > Amino Acids CONDITIONS: T = 37 C, ph = 2.8, A 660nm, Light path = 1 cm METHOD: Colorimetric REAGENTS: A. 50 mm Potassium Phthalate Buffer,
Enzymatic Assay of PROTEASE PRINCIPLE: Hemoglobin + H 2 O Protease > Amino Acids CONDITIONS: T = 37 C, ph = 2.8, A 660nm, Light path = 1 cm METHOD: Colorimetric REAGENTS: A. 50 mm Potassium Phthalate Buffer,
MW.SDS.70L and MW-SDS.200 Kits
 ~'A'.'.A'k'~ ~ ~ ':if';"7'~~'!11;~\ C HEM IC A I CQ P.O. ~X 14508,$T,LQV1S,MQ;, ~17,;U$A SDS MOLECULAR WEIGHT MARKERS IN A DISCONTINUOUS BUFFER July 1988 Technical Bulletin No. MWS-877L ORDER DIRECT: USA/Canada
~'A'.'.A'k'~ ~ ~ ':if';"7'~~'!11;~\ C HEM IC A I CQ P.O. ~X 14508,$T,LQV1S,MQ;, ~17,;U$A SDS MOLECULAR WEIGHT MARKERS IN A DISCONTINUOUS BUFFER July 1988 Technical Bulletin No. MWS-877L ORDER DIRECT: USA/Canada
Muscle Tissue- 3 Types
 AN INTRODUCTION TO MUSCLE TISSUE Muscle Tissue- 3 Types Skeletal muscle (focus on these) Cardiac muscle Smooth muscle FUNCTIONS OF SKELETAL MUSCLES Produce movement of the skeleton Maintain posture and
AN INTRODUCTION TO MUSCLE TISSUE Muscle Tissue- 3 Types Skeletal muscle (focus on these) Cardiac muscle Smooth muscle FUNCTIONS OF SKELETAL MUSCLES Produce movement of the skeleton Maintain posture and
Skeletal Muscle. Connective tissue: Binding, support and insulation. Blood vessels
 Chapter 12 Muscle Physiology Outline o Skeletal Muscle Structure o The mechanism of Force Generation in Muscle o The mechanics of Skeletal Muscle Contraction o Skeletal Muscle Metabolism o Control of Skeletal
Chapter 12 Muscle Physiology Outline o Skeletal Muscle Structure o The mechanism of Force Generation in Muscle o The mechanics of Skeletal Muscle Contraction o Skeletal Muscle Metabolism o Control of Skeletal
Role of the Carbohydrate Moiety in Phospholipase B from Torulaspora delbrueckii
 Agric. Bioi Chern., 54 (3), 599-603, 1990 599 Role of the Carbohydrate Moiety in Phospholipase B from Torulaspora delbrueckii Masafumi Maruyama, Hideki Kadowaki, Yasuo Watanabe and Youichi Tamai Department
Agric. Bioi Chern., 54 (3), 599-603, 1990 599 Role of the Carbohydrate Moiety in Phospholipase B from Torulaspora delbrueckii Masafumi Maruyama, Hideki Kadowaki, Yasuo Watanabe and Youichi Tamai Department
Mechanism of Muscular Contraction
 ~ Sorin2:er Jack A. Rail Mechanism of Muscular Contraction Contents 1 Setting the Stage: Myosin, Actin, Actomyosin and ATP... 1.1 Introduction... 1 1.2 Muscle Structure as Observed by Nineteenth Century
~ Sorin2:er Jack A. Rail Mechanism of Muscular Contraction Contents 1 Setting the Stage: Myosin, Actin, Actomyosin and ATP... 1.1 Introduction... 1 1.2 Muscle Structure as Observed by Nineteenth Century
Glucose Oxidase Pellets
 BIOTECHNOLOGY AND BIOENGINEERING VOL. XIX (1977) Glucose Oxidase Pellets INTRODUCTION Considerable world-wide interest has arisen in the use of immobilized enzymes as catalysts in industrial process and
BIOTECHNOLOGY AND BIOENGINEERING VOL. XIX (1977) Glucose Oxidase Pellets INTRODUCTION Considerable world-wide interest has arisen in the use of immobilized enzymes as catalysts in industrial process and
A New Mass Screening Method of Detecting UDP-Galactose-4-Epimerase
 Tohoku J. exp. Med., 1980, 131, 15-22 A New Mass Screening Method of Detecting UDP-Galactose-4-Epimerase Deficiency YUSHIN FUJIMURA, MASAHIKO KAWAMURA* and HIROSHI NARUSE t Department of Microbiology,
Tohoku J. exp. Med., 1980, 131, 15-22 A New Mass Screening Method of Detecting UDP-Galactose-4-Epimerase Deficiency YUSHIN FUJIMURA, MASAHIKO KAWAMURA* and HIROSHI NARUSE t Department of Microbiology,
Supplementary Figures
 Supplementary Figures Supplementary Figure 1 Minicircle topoisomer generation. a, Generation of supercoiled topoisomers. Minicircles were nicked with the sequence-specific nicking endonuclease, Nb.BbvCI.
Supplementary Figures Supplementary Figure 1 Minicircle topoisomer generation. a, Generation of supercoiled topoisomers. Minicircles were nicked with the sequence-specific nicking endonuclease, Nb.BbvCI.
2, 6, 3, 7, 8, 9, 10, 5
 A novel regulatory element in cardiac muscle troponin T. Striated muscle contraction is regulated by the position of tropomyosin on actin. That position, in turn, is regulated by the troponin complex (troponin
A novel regulatory element in cardiac muscle troponin T. Striated muscle contraction is regulated by the position of tropomyosin on actin. That position, in turn, is regulated by the troponin complex (troponin
Interaction of Flavonoid Compounds with Contractile Proteins of Skeletal Muscle
 Gen. Physiol. Biophys. (1988). 7,165 175 165 Interaction of Flavonoid Compounds with Contractile Proteins of Skeletal Muscle V. L. ZYMA, N. S. MIROSHNICHENKO, V. M. DANILOVA and E. En GIN Department of
Gen. Physiol. Biophys. (1988). 7,165 175 165 Interaction of Flavonoid Compounds with Contractile Proteins of Skeletal Muscle V. L. ZYMA, N. S. MIROSHNICHENKO, V. M. DANILOVA and E. En GIN Department of
(Received 6 November 1974)
 J. Phyiiol. (1975), 249, pp. 497-517 497 With 9 text-figure Printed in Great Britain EFFECTS OF MAGNESIUM ON CONTRACTILE ACTIVATION OF SKINNED CARDIAC CELLS BY ALEXANDRE FABIATO AND FRANOISE FABIATO From
J. Phyiiol. (1975), 249, pp. 497-517 497 With 9 text-figure Printed in Great Britain EFFECTS OF MAGNESIUM ON CONTRACTILE ACTIVATION OF SKINNED CARDIAC CELLS BY ALEXANDRE FABIATO AND FRANOISE FABIATO From
Ch.10 Muscle Tissue. Copyright 2009, John Wiley & Sons, Inc.
 Ch.10 Muscle Tissue Preview Chapter 10 In groups we will define the following terms 1. Skeletal muscle 2. Smooth muscle 3. Cardiac muscle 4. Sarcomere 5. Myofibril 6. Myofilament 7. Sarcoplasmic reticulum
Ch.10 Muscle Tissue Preview Chapter 10 In groups we will define the following terms 1. Skeletal muscle 2. Smooth muscle 3. Cardiac muscle 4. Sarcomere 5. Myofibril 6. Myofilament 7. Sarcoplasmic reticulum
Skeletal muscle in the light of its structure
 Mechanism of contraction of Skeletal muscle in the light of its structure By Dr. Mudassar Ali Roomi (MBBS, M. Phil) Muscle Tissue Skeletal Muscle Cardiac Muscle Smooth Muscle Skeletal Muscle Long cylindrical
Mechanism of contraction of Skeletal muscle in the light of its structure By Dr. Mudassar Ali Roomi (MBBS, M. Phil) Muscle Tissue Skeletal Muscle Cardiac Muscle Smooth Muscle Skeletal Muscle Long cylindrical
The organization of skeletal muscles. Excitation contraction coupling. Whole Skeletal Muscles contractions. Muscle Energetics
 Muscle and Movement The organization of skeletal muscles Excitation contraction coupling Whole Skeletal Muscles contractions Muscle Energetics The molecular bases of movement Muscular cells use molecular
Muscle and Movement The organization of skeletal muscles Excitation contraction coupling Whole Skeletal Muscles contractions Muscle Energetics The molecular bases of movement Muscular cells use molecular
Concept 50.5: The physical interaction of protein filaments is required for muscle function
 Concept 50.5: The physical interaction of protein filaments is required for muscle function Muscle activity is a response to input from the nervous system The action of a muscle is always to contract Vertebrate
Concept 50.5: The physical interaction of protein filaments is required for muscle function Muscle activity is a response to input from the nervous system The action of a muscle is always to contract Vertebrate
QS S Assist KINASE_ADP-Glo TM Kit
 QS S Assist KINASE_ADP-Glo TM Kit Description KINASE ADP-Glo TM kit is designed for use in biochemical kinase assays based on a Luminescent ADP Detection Assay (ADP-Glo TM ). The kit includes human kinase,
QS S Assist KINASE_ADP-Glo TM Kit Description KINASE ADP-Glo TM kit is designed for use in biochemical kinase assays based on a Luminescent ADP Detection Assay (ADP-Glo TM ). The kit includes human kinase,
Product Information. Instruction for Use. Troponin I ELISA kit Catalog Number: EA Storage Temperature: 2 8 C
 9620 Medical Center Dr., Suite 200, Rockville, MD 20850 Phone: 1.888.267.4436 Fax: 301-340-9254 Email: techsupport@origene.com Web: www.origene.com Product Information Troponin I ELISA kit Catalog Number:
9620 Medical Center Dr., Suite 200, Rockville, MD 20850 Phone: 1.888.267.4436 Fax: 301-340-9254 Email: techsupport@origene.com Web: www.origene.com Product Information Troponin I ELISA kit Catalog Number:
Communication MULTIPLE FORMS OF ACID PHOSPHATASE PRODUCED BY ASPERGJLL US OR YZAE YONEKICHI SAKURAI AND HIDEO SHIOTA
 Short Communication J. Gen. Appl. Microbiol., 16, 335-339 (1970) MULTIPLE FORMS OF ACID PHOSPHATASE PRODUCED BY ASPERGJLL US OR YZAE YONEKICHI SAKURAI AND HIDEO SHIOTA Faculty of Agriculture, Iwate University,
Short Communication J. Gen. Appl. Microbiol., 16, 335-339 (1970) MULTIPLE FORMS OF ACID PHOSPHATASE PRODUCED BY ASPERGJLL US OR YZAE YONEKICHI SAKURAI AND HIDEO SHIOTA Faculty of Agriculture, Iwate University,
Roti -Quant universal
 Roti -Quant universal Colorimetric protein concentration analysis 0120.1 A. The kit contains Roti -Quant universal Reagenz 1 (0118): 500 ml (0120.1) / 200 ml (0120.2) Danger H318-H315 P280- P305+P351+P338-P310
Roti -Quant universal Colorimetric protein concentration analysis 0120.1 A. The kit contains Roti -Quant universal Reagenz 1 (0118): 500 ml (0120.1) / 200 ml (0120.2) Danger H318-H315 P280- P305+P351+P338-P310
Step 4. Step 4. Choose MW markers. Choose MW markers. Gel Electrophoresis of Proteins
 Gel Electrophoresis of Proteins Choose MW markers 16 Step 4 Gel Electrophoresis of Proteins A Practical Approach, Third Edition An overview of basic techniques to benefit any life scientist using electrophoretic
Gel Electrophoresis of Proteins Choose MW markers 16 Step 4 Gel Electrophoresis of Proteins A Practical Approach, Third Edition An overview of basic techniques to benefit any life scientist using electrophoretic
Synopsis. Received March 2, adrenaline. Mosinger and Kujalova (1964) reported that adrenaline-induced lipolysis
 Studies on Reduction of Lipolysis in Adipose Tissue on Freezing and Thawing YASUSHI SAITO1, NoBUO MATSUOKA1, AKIRA KUMAGAI1, HIROMICHI OKUDA2, AND SETSURO FUJII3 Chiba University, Chiba 280, Japan, 2Department
Studies on Reduction of Lipolysis in Adipose Tissue on Freezing and Thawing YASUSHI SAITO1, NoBUO MATSUOKA1, AKIRA KUMAGAI1, HIROMICHI OKUDA2, AND SETSURO FUJII3 Chiba University, Chiba 280, Japan, 2Department
MUSCLE TISSUE (MUSCLE PHYSIOLOGY) PART I: MUSCLE STRUCTURE
 PART I: MUSCLE STRUCTURE Muscle Tissue A primary tissue type, divided into: skeletal muscle cardiac muscle smooth muscle Functions of Skeletal Muscles Produce skeletal movement Maintain body position Support
PART I: MUSCLE STRUCTURE Muscle Tissue A primary tissue type, divided into: skeletal muscle cardiac muscle smooth muscle Functions of Skeletal Muscles Produce skeletal movement Maintain body position Support
Ch 12 can be done in one lecture
 Ch 12 can be done in one lecture Developed by John Gallagher, MS, DVM Chapter 12: Muscles Review muscle anatomy (esp. microanatomy of skeletal muscle) Terminology: sarcolemma t-tubules sarcoplasmic reticulum
Ch 12 can be done in one lecture Developed by John Gallagher, MS, DVM Chapter 12: Muscles Review muscle anatomy (esp. microanatomy of skeletal muscle) Terminology: sarcolemma t-tubules sarcoplasmic reticulum
Minute TM Plasma Membrane Protein Isolation and Cell Fractionation Kit User Manual (v5)
 Minute TM Plasma Membrane Protein Isolation and Cell Fractionation Kit Catalog number: SM-005 Description Minute TM plasma membrane (PM) protein isolation kit is a novel and patented native PM protein
Minute TM Plasma Membrane Protein Isolation and Cell Fractionation Kit Catalog number: SM-005 Description Minute TM plasma membrane (PM) protein isolation kit is a novel and patented native PM protein
B. 15 mm Ouabain Solution (Ouabain) (Prepare 10 ml in Reagent A using Ouabain Octahydrate, Sigma Prod. No. O3125.)
 SIGMA QUALITY CONTROL TEST PROCEDURE Sigma Prod. No. A7510 PRINCIPLE: ATP + H 2 O ATPase > ADP + P i Abbreviations used: ATPase = Adenosine 5'-Triphosphatase ATP = Adenosine 5'-Triphosphate ADP = Adenosine
SIGMA QUALITY CONTROL TEST PROCEDURE Sigma Prod. No. A7510 PRINCIPLE: ATP + H 2 O ATPase > ADP + P i Abbreviations used: ATPase = Adenosine 5'-Triphosphatase ATP = Adenosine 5'-Triphosphate ADP = Adenosine
Exercise Science (Muscle Anatomy and Physiology) PPL10 Date: May 11 th, 2015
 Exercise Science (Muscle Anatomy and Physiology) PPL10 Date: May 11 th, 2015 Examples of Strength and Physique Athletes The Principle of Muscles The most important principle for muscles is the use it or
Exercise Science (Muscle Anatomy and Physiology) PPL10 Date: May 11 th, 2015 Examples of Strength and Physique Athletes The Principle of Muscles The most important principle for muscles is the use it or
Structure of the striated muscle general properties
 Structure of the striated muscle general properties Structure of the striated muscle membrane systems 1. Myofibrillum (contractile proteins) 2. Sarcoplasmic reticulum (SR) longitudinal tubule 3. SR terminal
Structure of the striated muscle general properties Structure of the striated muscle membrane systems 1. Myofibrillum (contractile proteins) 2. Sarcoplasmic reticulum (SR) longitudinal tubule 3. SR terminal
Animal Skeletons. Earthworm peristaltic movement. Hydrostatic Skeletons
 Animal Skeletons The Musculo-Skeletal System Functions: Support Protection Movement all movement results from: muscle working against a skeleton 3 Types of skeletons hydrostatic exoskeleton endoskeleton
Animal Skeletons The Musculo-Skeletal System Functions: Support Protection Movement all movement results from: muscle working against a skeleton 3 Types of skeletons hydrostatic exoskeleton endoskeleton
THE SEPARATION OF THE SOLUBILIZED PROTEINS OF THE SARCOPLASMIC RETICULUM ON DEAE-CELLULOSE AND ITS MODIFICATION. W. HASSELBACH and A.
 THE SEPARATION OF THE SOLUBILIZED PROTEINS OF THE SARCOPLASMIC RETICULUM ON DEAE-CELLULOSE AND ITS MODIFICATION W. HASSELBACH and A. MIGALA Max-Planck-Institut fiir medizinische Forschung, Abt. Physiologic,
THE SEPARATION OF THE SOLUBILIZED PROTEINS OF THE SARCOPLASMIC RETICULUM ON DEAE-CELLULOSE AND ITS MODIFICATION W. HASSELBACH and A. MIGALA Max-Planck-Institut fiir medizinische Forschung, Abt. Physiologic,
Chapter 8: Skeletal Muscle: Structure and Function
 Chapter 8: Skeletal Muscle: Structure and Function Objectives Draw & label the microstructure of skeletal muscle Outline the steps leading to muscle shortening Define the concentric and isometric Discuss:
Chapter 8: Skeletal Muscle: Structure and Function Objectives Draw & label the microstructure of skeletal muscle Outline the steps leading to muscle shortening Define the concentric and isometric Discuss:
Excitation-Contraction Coupling & Reflexes, Proprioception and Movement. PSK 4U Unit 4, Day 4
 Excitation-Contraction Coupling & Reflexes, Proprioception and Movement PSK 4U Unit 4, Day 4 Excitation-Contraction Coupling Muscles work by converting electrical and chemical energy into mechanical energy!
Excitation-Contraction Coupling & Reflexes, Proprioception and Movement PSK 4U Unit 4, Day 4 Excitation-Contraction Coupling Muscles work by converting electrical and chemical energy into mechanical energy!
Chapter 10: Muscles. Vocabulary: aponeurosis, fatigue
 Chapter 10: Muscles 37. Describe the structural components of skeletal muscle tissue from the molecular to the organ level. 38. Describe the structure, function, and importance of sarcomeres. 39. Identify
Chapter 10: Muscles 37. Describe the structural components of skeletal muscle tissue from the molecular to the organ level. 38. Describe the structure, function, and importance of sarcomeres. 39. Identify
A protocol for enhancement of the AAV-mediated expression of transgenes
 A protocol for enhancement of the AAV-mediated expression of transgenes Hiroaki Mizukami, Takeharu Kanazawa, Takashi Okada, and Keiya Ozawa Division of Genetic Therapeutics, Center for Molecular Medicine,
A protocol for enhancement of the AAV-mediated expression of transgenes Hiroaki Mizukami, Takeharu Kanazawa, Takashi Okada, and Keiya Ozawa Division of Genetic Therapeutics, Center for Molecular Medicine,
Nerve Muscle Relationship and Neural Muscular Junction Quiz. Remember, you need to know the structure and the function!
 Nerve Muscle Relationship and Neural Muscular Junction Quiz Remember, you need to know the structure and the function! What is this called? What is this? Schwann cell What is this called? Basal lamina
Nerve Muscle Relationship and Neural Muscular Junction Quiz Remember, you need to know the structure and the function! What is this called? What is this? Schwann cell What is this called? Basal lamina
Preventive effects of amino acids and glutathione on the formaldehyde-induced denaturation of myofibrillar proteins
 Blackwell Science, LtdOxford, UK FISFisheries Science0919-92682003 Blackwell Science Asia Pty Ltd 693June 2003 663 Formaldehyde-induced denaturation E Abe et al. 10.1046/j.0919-9268.2003.00663.x Original
Blackwell Science, LtdOxford, UK FISFisheries Science0919-92682003 Blackwell Science Asia Pty Ltd 693June 2003 663 Formaldehyde-induced denaturation E Abe et al. 10.1046/j.0919-9268.2003.00663.x Original
Recombinant Trypsin, Animal Origin Free
 Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
(12) Patent Application Publication (10) Pub. No.: US 2010/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/01 19655A1 Kuwahara et al. US 201001 19655A1 (43) Pub. Date: May 13, 2010 (54) (75) (73) (21) (22) (86) MUSCULAR PROTEIN DENATURATION
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/01 19655A1 Kuwahara et al. US 201001 19655A1 (43) Pub. Date: May 13, 2010 (54) (75) (73) (21) (22) (86) MUSCULAR PROTEIN DENATURATION
5. What component of the sarcomere is not attached to the Z line?
 Model 2: Anatomy of a Sarcomere 1. Label the thick filament and the thin filament in Model 2. 2. How many sarcomeres are shown in Model 2? 3. Using Model 2, based on the locations of thick and thin filaments,
Model 2: Anatomy of a Sarcomere 1. Label the thick filament and the thin filament in Model 2. 2. How many sarcomeres are shown in Model 2? 3. Using Model 2, based on the locations of thick and thin filaments,
[GANN, 59, ; October, 1968] CHANGES IN ALDOLASE ISOZYME PATTERNS OF HUMAN CANCEROUS TISSUES
![[GANN, 59, ; October, 1968] CHANGES IN ALDOLASE ISOZYME PATTERNS OF HUMAN CANCEROUS TISSUES [GANN, 59, ; October, 1968] CHANGES IN ALDOLASE ISOZYME PATTERNS OF HUMAN CANCEROUS TISSUES](/thumbs/87/96205960.jpg) [GANN, 59, 415-419; October, 1968] UDC 616-006-092.18 CHANGES IN ALDOLASE ISOZYME PATTERNS OF HUMAN CANCEROUS TISSUES Kiyoshi TSUNEMATSU, Shin-ichi YOKOTA, and Tadao SHIRAISHI (Third Department of Internal
[GANN, 59, 415-419; October, 1968] UDC 616-006-092.18 CHANGES IN ALDOLASE ISOZYME PATTERNS OF HUMAN CANCEROUS TISSUES Kiyoshi TSUNEMATSU, Shin-ichi YOKOTA, and Tadao SHIRAISHI (Third Department of Internal
Activation of Sea Urchin Sperm Flagellar Dynein ATPase Activity by Salt-Extracted Axonemes
 /. Blochem. 102, 31-41 (1987) Activation of Sea Urchin Sperm Flagellar Dynein ATPase Activity by Salt-Extracted Axonemes Etsuo YOKOTA,***' 1 Issei MABUCHI,*'*** and Hidemi SATO** Department of Cell Biology,
/. Blochem. 102, 31-41 (1987) Activation of Sea Urchin Sperm Flagellar Dynein ATPase Activity by Salt-Extracted Axonemes Etsuo YOKOTA,***' 1 Issei MABUCHI,*'*** and Hidemi SATO** Department of Cell Biology,
SCS MOLECULAR WEIGHT MARKERS 2,500-17,000 Caltons
 LECTROPHORES/S Revised November 1992 SCS MOLECULAR WEIGHT MARKERS 2,500-17,000 Caltons I NTRODUCTION Electrophoresis in polyacrylamide gels in the presence of sodium dodecyl sulfate (SDS), an anionic detergent,
LECTROPHORES/S Revised November 1992 SCS MOLECULAR WEIGHT MARKERS 2,500-17,000 Caltons I NTRODUCTION Electrophoresis in polyacrylamide gels in the presence of sodium dodecyl sulfate (SDS), an anionic detergent,
Evaluation of the Possibility That Free Fatty Acids Cause False-Positive Result in Diarrhetic Shellfish Poisoning Mouse Bioassay in Actual Use
 194 22 11 11,1,2 1 1 2 Evaluation of the Possibility That Free Fatty Acids Cause False-Positive Result in Diarrhetic Shellfish Poisoning Mouse Bioassay in Actual Use Satoshi H6H=>BDID,1,2, Kazuhiko N>H=>BJG6
194 22 11 11,1,2 1 1 2 Evaluation of the Possibility That Free Fatty Acids Cause False-Positive Result in Diarrhetic Shellfish Poisoning Mouse Bioassay in Actual Use Satoshi H6H=>BDID,1,2, Kazuhiko N>H=>BJG6
Effect of Washing on Quality Improvement of Mechanically Deboned Chicken Meat
 Effect of Washing on Quality Improvement of Mechanically Deboned Chicken Meat Cho Cho Thein 1* and Kanithaporn Vangnai 2 ABSTRACT Mechanically deboned chicken meat (MDCM) is obtained from the skeletal
Effect of Washing on Quality Improvement of Mechanically Deboned Chicken Meat Cho Cho Thein 1* and Kanithaporn Vangnai 2 ABSTRACT Mechanically deboned chicken meat (MDCM) is obtained from the skeletal
Skeletal Muscle and the Molecular Basis of Contraction. Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry
 Skeletal Muscle and the Molecular Basis of Contraction Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Like neurons, all muscle cells can be excited chemically, electrically, and
Skeletal Muscle and the Molecular Basis of Contraction Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Like neurons, all muscle cells can be excited chemically, electrically, and
N- and C-terminal amino acids of [alpha]-actinin and studies on the effects of [alpha]-actinin on F- actin
![N- and C-terminal amino acids of [alpha]-actinin and studies on the effects of [alpha]-actinin on F- actin N- and C-terminal amino acids of [alpha]-actinin and studies on the effects of [alpha]-actinin on F- actin](/thumbs/75/72030944.jpg) Retrospective Theses and Dissertations 1974 N- and C-terminal amino acids of [alpha]-actinin and studies on the effects of [alpha]-actinin on F- actin Inderjit Singh Iowa State University Follow this and
Retrospective Theses and Dissertations 1974 N- and C-terminal amino acids of [alpha]-actinin and studies on the effects of [alpha]-actinin on F- actin Inderjit Singh Iowa State University Follow this and
Journal of Coastal Development ISSN: CHEMICAL ANALYSIS DURING THE PROCESSING OF DRIED SALTED ANCHOVY
 CHEMICAL ANALYSIS DURING THE PROCESSING OF DRIED SALTED ANCHOVY Eko Nurcahya Dewi *) Faculty of Fisheries and Marine Sciences University of Diponegoro, Jl. Hayam Wuruk 4A Semarang, Indonesia Received:
CHEMICAL ANALYSIS DURING THE PROCESSING OF DRIED SALTED ANCHOVY Eko Nurcahya Dewi *) Faculty of Fisheries and Marine Sciences University of Diponegoro, Jl. Hayam Wuruk 4A Semarang, Indonesia Received:
Components in Soybean Seeds Affecting the Consistency of Tofu
 JARQ 43 (4), 295 300 (2009) http://www.jircas.affrc.go.jp Components in Soybean Seeds Affecting the Consistency of Tofu Kyoko TODA, Yoshiyuki NAKAMURA, Koji TAKAHASHI and Katsumi KOMAKI National Institute
JARQ 43 (4), 295 300 (2009) http://www.jircas.affrc.go.jp Components in Soybean Seeds Affecting the Consistency of Tofu Kyoko TODA, Yoshiyuki NAKAMURA, Koji TAKAHASHI and Katsumi KOMAKI National Institute
The Muscular System. Muscle tissue is one of the 4 tissue types in vertebrates Muscle
 The Muscular System The Muscular System Muscle tissue is one of the 4 tissue types in vertebrates Muscle The Muscular System Muscle tissue is one of the 4 tissue types in vertebrates Muscle Nervous The
The Muscular System The Muscular System Muscle tissue is one of the 4 tissue types in vertebrates Muscle The Muscular System Muscle tissue is one of the 4 tissue types in vertebrates Muscle Nervous The
BIOCHEMICAL EXAMINATION OF ACUTE MYOCARDIAL INFARCTION. Written by Lenka Fialová, translated by Jan Pláteník
 BIOCHEMICAL EXAMINATION OF ACUTE MYOCARDIAL INFARCTION 1 Structure of heart muscle Written by Lenka Fialová, translated by Jan Pláteník Heart muscle (myocardium) is a particular form of striated muscle,
BIOCHEMICAL EXAMINATION OF ACUTE MYOCARDIAL INFARCTION 1 Structure of heart muscle Written by Lenka Fialová, translated by Jan Pláteník Heart muscle (myocardium) is a particular form of striated muscle,
MYOFIBRILLAR STRUCTURAL CHANGES CAUSED BY MARINATION WITH CALCIUM PHOSPHATE OR CALCIUM CHLORIDE AND SODIUM PYROPHOSPHATE
 Cattlemen s Day 2002 MYOFIBRILLAR STRUCTURAL CHANGES CAUSED BY MARINATION WITH CALCIUM PHOSPHATE OR CALCIUM CHLORIDE AND SODIUM PYROPHOSPHATE T. E. Lawrence, A. T. Waylan, and C. L. Kastner Summary Ultrastructural
Cattlemen s Day 2002 MYOFIBRILLAR STRUCTURAL CHANGES CAUSED BY MARINATION WITH CALCIUM PHOSPHATE OR CALCIUM CHLORIDE AND SODIUM PYROPHOSPHATE T. E. Lawrence, A. T. Waylan, and C. L. Kastner Summary Ultrastructural
SKELETAL MUSCLE CHARACTERISTICS
 THE MUSCULAR SYSTEM SKELETAL MUSCLE CHARACTERISTICS Most are attached by tendons to bones Cells are multinucleate Striated have visible banding Voluntary subject to conscious control Cells are surrounded
THE MUSCULAR SYSTEM SKELETAL MUSCLE CHARACTERISTICS Most are attached by tendons to bones Cells are multinucleate Striated have visible banding Voluntary subject to conscious control Cells are surrounded
Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples:
 Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
AN INTRODUCTION TO INVOLUNTARY (ESPECIALLY SMOOTH) MUSCLES 1
 AN INTRODUCTION TO INVOLUNTARY (ESPECIALLY SMOOTH) MUSCLES 1 Summary: This section is an introduction to a fascinating and extremely important group of tissue, the smooth muscles. As you will see, their
AN INTRODUCTION TO INVOLUNTARY (ESPECIALLY SMOOTH) MUSCLES 1 Summary: This section is an introduction to a fascinating and extremely important group of tissue, the smooth muscles. As you will see, their
ATP Hydrolysis. Presented by: Rami Amro Instructor: Dr. Neiman
 ATP Hydrolysis Presented by: Rami Amro Instructor: Dr. Neiman Ch. 13: Direct movement of the motors along the filament thought to be a result of conformational changes while it is attached. Ch.14: How
ATP Hydrolysis Presented by: Rami Amro Instructor: Dr. Neiman Ch. 13: Direct movement of the motors along the filament thought to be a result of conformational changes while it is attached. Ch.14: How
