Calcium-Induced Fragmentation of Skeletal Muscle Nebulin Filaments'
|
|
|
- Megan Stokes
- 6 years ago
- Views:
Transcription
1 J. Biochem. 112, (1992) Calcium-Induced Fragmentation of Skeletal Muscle Nebulin Filaments' Ryuichi Tatsumi and Koui Takahashi Meat Science Laboratory, Department of Animal Science, Faculty of Agriculture, Hokkaido University, Kita-ku, Sapporo, Hokkaido 060 Received for publication, August 6, 1992 When chicken breast muscle myofibrils were treated with a solution containing 0.1 mm CaCl2 and 30 fig of leupeptin/ml, nebulin filaments were fragmented into 200-, 180-, 40-, 33-, and 23-kDa subfragments. All the subfragments except the 180-kDa one were released from the myofibrils. The fragmentation of nebulin filaments seems to be induced by the binding of large amounts of calcium ions. Similar changes took place in nebulin filaments in post-mortem skeletal muscle. It has been proposed that nebulin co-exists with thin (actin) filaments and participates in stabilizing their organization [Wang, K. & Wright, J. (1988) J. Cell Biol. 107, ]. Thus, the above result suggests that Ca-induced fragmentation of nebulin filaments destabilizes the organization of thin filaments and is a key factor in meat tenderization during post-rigor aging. Nebulin is a giant structural protein, found by Wang and Williamson (1), existing in the sarcomeres of a wide range of skeletal muscle myofibrils (2, 3). In most vertebrates, nebulin accounts for 3-4% of the total myofibrillar protein (1), and its molecular mass varies from 600 to 900 kda in various muscle tissues (2, 3). Wang and Wright (4) have shown that nebulin constitutes a set of long inextensible longitudinal filaments that span the space between the Z-disk and the distal region of a thin (actin) filament, and proposed that nebulin might interact with and adhere to thin filaments, thereby stabilizing their organization. Wang described the decrease in nebulin during postmortem storage of rabbit skeletal muscle (5). On reexamination of post-mortem changes in nebulin, we confirmed that nebulin decreases during storage of skeletal muscle of various animals. We sought an essential factor which would induce post-mortem fragmentation of nebulin filaments using isolated myofibrils. This study shows that nebulin filaments are non-enzymatically fragmented by calcium ions. When chicken breast muscle myofibrils were treated with a solution containing 0.1 mm CaCl2 and 30ug of leupeptin/ml, 200-, 180-, 40-, 33-, and 23-kDa subfragments were produced; all the subfragments except the 180-kDa one were released from the myofibrils. There is a possibility that the fragmentation of nebulin filaments is induced by the binding of large amounts of calcium ions to specific regions of nebulin filaments. MATERIALS AND METHODS Preparation of Myofibrils-Breast muscle (Musculus pectoralis superficialis) of chicken, and back muscle (Musculus longissimus thoracis) of rabbit, pig, horse, and cow were used. If necessary, freshly dissected muscles were treated antiseptically by wrapping them with gauze containing 1 mm NaN3, and stored at 5'C. Myofibrils were ' This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan. prepared from fresh and stored muscles by the method of Perry and Grey (6), leupeptin (Peptide Institute, Osaka) being present at 30ug/ml throughout the preparation to inhibit proteases. Ca-Treatment of Myofibrils-As described in our previous paper (7), freshly prepared myofibrils (15 mg/ml) were treated with a solution containing 0.1 M KCI, 0.1 mm CaC12, 30,u g of leupeptin/ml, 1 mm dithiothreitol, 1 mm NaN,, and 10 mm Tris-maleate buffer, ph 7.0, at 5'C with gentle stirring. At appropriate times, the myofibril suspensions were centrifuged at 10,000 rpm for 30 min, and both the precipitates and supernatant solutions were analyzed by SDS-PAGE and immunoblotting. SDS-PAGE-According to the method of Fairbanks et al. (8), linear 2-12% polyacrylamide gradient gels were prepared, and 4-10,u l of the samples was applied. The gels were stained and destained by the method of Laemmli (9). Preparation of the samples for gel electrophoresis and densitometry of the stained gels were performed as described previously (7). Immunoblotting-According to the method of Matsuura et al. (10), the protein bands obtained on SDS-PAGE were transferred electrophoretically onto nitrocellulose membranes (Bio-Rad Laboratories, California), and the membranes were cut into strips and treated with antinebulin antiserum, which was kindly provided by Prof. K. Maruyama of the University of Chiba. RESULTS Figure 1 shows the post-mortem changes in nebulin of chicken breast muscle myofibrils. The band of nebulin decreased with post-mortem time and completely disappeared within 1 d of storage, in parallel with the disappearance of a-connectin. The disappearance of nebulin during post-mortem aging was a common phenomenon in all skeletal muscles of various animals, but the initial lag time and the rate of decrease in the amount of nebulin differed in the muscles of different species (Fig. 2). More detailed determination of the changes in nebulin was carried out by Vol_ 112. No
2 776 R. Tatsumi and K. Takahashi means of the immunoblotting technique using antinebulin antiserum (Fig. 3). In myofibrils prepared from stored muscle, immunologically stained polypeptides appeared under the band of nebulin, with a decrease in the amount of nebulin. However, these polypeptides disappeared, and a 180-kDa polypeptide was produced on prolonged storage. Because the molecular mass of the 180-kDa fragment accounted for only about 26% of the mass of the original nebulin (700 kda), the remaining portion of nebulin must have been released from the myofibrils during postmortem aging. Similar changes in nebulin could be induced by Catreatment of freshly prepared myofibrils (Fig. 4). After centrifugation of the Ca-treated myofibrils, both the precipitated myofibrils and supernatant solutions were sub- Fig. 1. Post-mortem changes in nebulin of chicken breast muscle. Myofibrils were prepared from chicken breast muscle stored at 5 C, dissolved in a SDS sample buffer solution containing 1% SDS, 5 mm EDTA, 5 mm Tris-HCl buffer, ph 8.0, 1% /3-mercaptoethanol, and 10% glycerol, and then boiled for 2 min. Samples were subjected to SDS-PAGE on linear 2-12% polyacrylamide gradient gels. Only the top portions of the stained gels are shown. Lane a, freshly prepared myofibrils; lanes b-f, myofibrils prepared from stored muscle: lane b, for 0.25 d; lane c, for 0.5 d; lane d, for 1 d; lane e, for 3 d; lane f, for 7 d. a, a-connectin;,q,q-connectin; N, nebulin; MHC, myosin heavy chain. Fig. 2. Post-mortem changes in the amount of nebulin in various skeletal muscles. Skeletal muscle specimens of various species were stored at 5 C. Myofibrils were prepared at appropriate times, treated with the SDS sample buffer solution, and then subjected to SDS-PAGE as shown in Fig. 1. The ratio of the nebulin band to the myosin heavy chain band was determined by densitometry of the stained gels, and expressed as a percentage of the ratio in fresh myofibrils. chicken; :, pig; -, horse; A, cow;, rabbit. Fig. 3. Detection of nebulin subfragments bound to myofibrils during post-mortem storage of muscle. Myofibrils were prepared from chicken breast muscle stored at 5 C, treated with the SDS Fig. 1. The separated proteins were transferred electrophoretically onto nitrocellulose membranes. Individual strips were blocked with a Tris buffered salt solution (0.5 M NaCl, 20 mm Tris-HC1 buffer, ph 7.5) containing 3% gelatin, and then incubated with antinebulin antiserum (diluted 500 times) in the Tris buffered salt solution containing 1% gelatin (lanes b-h). The binding of antibodies was detected by means of the horseradish peroxidase reaction using goat anti-rabbit IgG diluted 2,000 times with the Tris buffered salt solution containing 1% gelatin. Lane a, myofibrillar proteins stained with Coomassie Brilliant Blue R-250; lane b, fresh myofibrils; lanes c-h, myofibrils prepared from stored muscle: lane c, for 0.25 d; lane d, for 0.5 d; lane e, for 1 d; lane f, for 3 d; lane g, for 7 d; lane h, for 14 d. a, a-connectin; Q, 8-connectin; N, nebulin; MHC, myosin heavy chain; A, actin. Fig. 4. Changes in nebulin during Ca-treatment of myofibrils. Myofibrils (15 mg/ml) prepared from chicken breast muscle were treated with a solution containing 0.1 M KCI, 0.1 mm CaC12,30,Ug of leupeptin/ml, 1 mm dithiothreitol, 1 mm NaN,, and 10 mm Trismaleate buffer, ph 7.0, at 5 C. After the Ca-treatment, the myofibril suspensions were centrifuged. The precipitated myofibrils were treated with the SDS sample buffer solution and then subjected to SDS-PAGE as shown in Fig. 1. Lane a, fresh myofibrils; lanes b-f, myofibrils treated with 0.1 mm CaC12:lane b, for 1 d; lane c, for 3 d; lane d, for 4 d; lane e, for 5 d; lane f, for 7 d. a, a-connectin; Q, /3-connectin; N, nebulin; MHC, myosin heavy chain. J. Biochem.
3 Ca-Induced Fragmentation of Nebulin Filaments 777 Fig. 5. Detection of the 180-kDa subfragment produced during Ca-treatment of myofibrils. Myofibrils (15 mg/ml) prepared from chicken breast muscle were treated with 0.1 mm CaC12 as shown in Fig. 4. After the Ca-treatment, the myofibril suspensions were centrifuged. The precipitated myofibrils were treated with the SDS Fig. 1. The separated proteins were transferred electrophoretically onto nitrocellulose membranes, and then individual strips were treated with antinebulin antiserum and with goat anti-rabbit IgG, as shown in Fig. 3 (lanes b-h). Lane a, myofibrils stained with Coomassie Brilliant Blue R-250; lane b, fresh myofibrils; lanes c-h, myofibrils treated with 0.1 mm CaC12: lane c, for 1 d; lane d, for 3 d; lane e, for 5 d; lane f, for 7 d; lane g, for 9 d; lane h, for 11 d. C, a- and fl-connectins; N, nebulin; MHC, myosin heavy chain; A, actin. Fig. 6. Detection of nebulin subfragments released from myofibrils during Ca-treatment of myofibrils. Myofibrils (15 mg/ ml) prepared from chicken breast muscle were treated with 0.1 mm CaCl2 as shown in Fig. 4. After the Ca-treatment, the myofibril suspensions were centrifuged. The supernatant solutions were treated with the SDS sample buffer solution and then subjected to SDS-PAGE as shown in Fig. 1. The separated proteins were transferred electrophoretically onto nitrocellulose membranes, and then individual strips were treated with antinebulin antiserum and with goat antirabbit IgG, as shown in Fig. 3. Lane a, myofibrils stained with Coomassie Brilliant Blue R-250. Lanes b-h, supernatant solutions of Ca-treated myofibrils: lane b, for 1 d; lane c, for 3 d; lane d, for 7 d; lane e, for 11 d; lane f, for 13 d; lane g, for 15 d; lane h, for 17 d. C, a- and Q-connectins; N, nebulin; MHC, myosin heavy chain; A, actin; TM, tropomyosin; Tn-C, troponin C. jected to immunoblotting. In this case, the changes in nebulin occurred more slowly and clearly than those in post-mortem muscle. While the 180-kDa fragment bound to myofibrils (Fig. 5), the 200-, 90-, 40-, 33-, and 23-kDa fragments were found in the supernatant solution (Fig. 6, lanes e and f), first the 90-kDa fragment and eventually the other four fragments (Fig. 6, lanes g and h). These results show that nebulin filaments are fragmented into 200-, 180-, 40-, 33-, and 23-kDa subfragments on Ca-treatment of myofibrils, regardless of the animal species or muscle type, and all the subfragments other than the 180-kDa one are released from the myofibrils. The fragmentation of nebulin filaments occurred uniquely in the presence of calcium ions (Fig. 7). When myofibrils were treated with a solution containing 0.1 mm CaCl2 in the absence of leupeptin, nebulin disappeared completely within 3 h, indicating that myofibrils are contaminated by some protease when they are prepared by the usual method. The changes in the amount of nebulin were completely inhibited in the presence of both 5 mm EGTA and 30 k g of leupeptin/ml. Leupeptin (11) was the most effective of the various protease inhibitors tested, i.e., antipain, E-64, pepstatin A, phenylmethanesulfonyl fluoride, and diisopropylfluorophosphate. Calcium-specific fragmentation of nebulin filaments was induced by the treatment of myofibrils with a solution containing 0.1 mm CaCl2 and 30 u g of leupeptin/ml. The addition of 15 k g each of calpain-inhibitors I and II (Nacalai Tesque, Kyoto)/ ml did not affect the rate of the Ca-specific fragmentation of nebulin filaments. Figure 8 shows the dependence of the calcium-specific fragmentation of nebulin filaments on the calcium ion concentration. The fragmentation occurred above 10 k M and was maximum at 0.1 mm. The addition of 2 mm MgC12 did not affect the Ca-dependence of the fragmentation of nebulin filaments. The calcium-specific fragmentation of nebulin filaments into five kinds of subfragments was affected by ph and temperature (Fig. 9). When myofibrils were treated at 5 C with a solution containing 0.1 mm CaC12, at various phs, and then analyzed by SDS-PAGE, the calcium-specific fragmentation of nebulin was found to be maximum around ph 7, and was suppressed at acidic phs. When myofibrils were treated with 0.1 mm CaCl2 at ph 7.0, the rate of fragmentation of nebulin increased almost linearly with increasing temperature until 25'C, and slowed down thereafter. DISCUSSION Fragmentation of nebulin filaments is induced by 0.1 mm Ca'. There is a possibility that nebulin filaments are Vol. 112, No. 6, 1992
4 778 R. Tatsumi and K. Takahashi Fig. 7. Calcium-specific fragmentation of nebulin filaments. Myofibrils (15 mg/ml) prepared from chicken breast muscle were treated witi a solution containing 0.1 M KCI, 30,ug of leupeptin/ml, 1 mm dithiothreitol, 1 mm NaN3, 10 mm Tris-maleate buffer, ph 7.0, and 0.1 mm CaC12 ( ) or 5 mm EGTA (0), at 5 C. At appropriate times during the treatment, the myofibril suspensions were centrifuged, and the precipitated myofibrils were treated with the SDS Fig. 1. The ratio of the nebulin band to the myosin heavy chain band was determined by densitometry of the stained gels and expressed as a percentage of the ratio in intact myofibrils. o, 0.1 mm CaC12 in the absence of leupeptin. Fig. 9. Temperature and ph dependence of the calcium-specific fragmentation of nebulin filaments. Myofibrils (15 mg/ml) prepared from rabbit back muscle were treated with a solution containing 0.1 M KCI, 0.1 mm CaC1,, 30 pg of leupeptin/ml, 1 mm dithiothreitol, 1 mm NaN,, and 10 mm Tris-maleate buffer, ph 7.0, at various temperatures for 3 h (!), or with a solution containing 0.1 M KCI, 0.1 mm CaCl,, 30y g of leupeptin/ml, 1 mm dithiothreitol, 1 mm NaN,, and 10 mm Tris-maleate buffer at various phs, at 5 C for 48 h (o). After the Ca-treatment, the myofibrils were precipitated, treated with the SDS sample buffer solution and then subjected to SDS-PAGE as shown in Fig. 1. The ratio of the nebulin band to the myosin heavy chain band was determined by densitometry of the stained gels and expressed as a percentage of the ratio in intact myofibrils. Fig. 8. Dependence of the calcium-specific fragmentation of nebulin filaments on the calcium ion concentration. Myofibrils (15 mg/ml) prepared from rabbit back muscle were treated with a solution containing 0.1 M KCI, various concentrations of CaCl2r 30,ug of leupeptin/ml, 1 mm dithiothreitol, 1 mm NaN,, and 10 mm Tris-maleate buffer, ph 7.0, for 48 hat 5 C. After centrifugation, the precipitated myofibrils were treated with the SDS sample buffer solution and then subjected to SDS-PAGE as shown in Fig. 1. The ratio of the nebulin band to the myosin heavy chain band was determined by densitometry of the stained gels and expressed as a percentage of the ratio in intact myofibrils. non-enzymatically fragmented on the binding of calcium ions, though we cannot completely exclude the possibility of proteolysis by an unknown protease which is active under the present experimental conditions. We will report in a following paper that nebulin is a calcium-binding protein, and that calcium ions bind to the 200-, 40-, and 23-kDa subfragments (12). The Ca-specific fragmentation of nebulin filaments is similar to the Ca-specific splitting of connectin filaments; connectin, which exists as an elastic filament connecting a thick filament with a Z-disk in a sarcomere (13, 14), is non-enzymatically split into f8- connectin and a 1,200-kDa subfragment on the binding of large amounts of calcium ions (0.1 mm) to the l-connectin portion of a-connectin (7). These high-molecular-weight proteins, nebulin and connectin, seem to show common characteristics in the presence of large amounts of calcium ions. Actually, the ph dependence and temperature dependence of the Ca-specific fragmentation of nebulin filaments shown in Fig. 9 are entirely the same as those of the Ca-specific splitting of connectin filaments (Fig. 8 of Ref. 7). At the physiological concentration of calcium ions, it is possible that the properties of nebulin filaments change during the contraction-relaxation cycle of skeletal muscle. Somerville and Wang reported (15, 16) that nebulin is phosphorylated in vivo. They also pointed out the possibility that phosphorylated nebulin is involved in regulatory functions. The phosphorylation of and calcium-binding on nebulin filaments might play roles in muscle contraction. In post-mortem muscles, the ultimate sarcoplasmic J. Biochem.
5 Ca-Induced Fragmentation of Nebulin Filaments 779 calcium ion concentration is increased to about 0.2 mm (Ji, J.R. & Takahashi, K., manuscript in preparation), due to the disappearance of the Call -accumulating ability of sarcoplasmic reticular and mitochondrial membranes (17, 18). This concentration of calcium ions is approximately 2,000 times higher than that in resting muscle. The Ca-specific fragmentation of nebulin filaments takes place under these conditions. The Ca-specific fragmentation of nebulin filaments must destabilize the organization of thin filaments, and thus contribute to the tenderization of meat during post-rigor aging. In addition to the weakening of Z-disks (19), the weakening of rigor linkages formed between actin and myosin (20, 21), and the splitting of connectin filaments (7), all of which we have already reported to be important factors in the tenderization of meat during post-rigor aging, the fragmentation of nebulin filaments appears to be a fourth factor. All of these forms of structural weakening of myofibrils occur non-enzymatically at 0.1 mm Ca". The results presented in this paper support the "calcium theory of meat tenderization" proposed by us (7, 22). We wish to express our gratitude to Prof. K. Maruyama, Dr. S. Kimura, and Mr. T. Matsuura of the Faculty of Science, the University of Chiba, for providing the antinebulin antiserum, and for the technical guidance concerning the SDS-PAGE and immunoblotting techniques. REFERENCES 1. Wang, K. & Williamson, C.L. (1980) Proc. Natl. Acad. Sci. USA 77, Hu, D.H., Kimura, S., & Maruyama, K. (1986) J. Biochem. 99, Locker, R.H. & Wild, D.J.C. (1986) J. Biochem. 99, Wang, K. & Wright, J. (1988) J. Cell Biol. 107, Wang, K. (1985) in Cell and Muscle Motility (Shay, J.W., ed.) Vol. 6, pp , Plenum Press, New York and London 6. Perry, S.V. & Grey, T.C. (1956) Biochem. J. 64, Takahashi, K., Hattori, A., Tatsumi, R., & Takai, K. (1992) J. Biochem. 111, Fairbanks, G., Steck, T.L., & Wallach, D.F.H. (1971) Biochemistry 10, Laemmli, U.K. (1970) Nature 227, Matsuura, T., Kimura, S., Ohtsuka, S., & Maruyama, K. (1991) J. Biochern. 110, Aoyagi, T., Miyata, S., Nanbo, M., Kojima, F., Matsuzaki, M., Ishizuka, M., Takeuchi, T., & Umezawa, H. (1969) J. Antibiot. 22, Tatsumi, R., Hattori, A., & Takahashi, K. (1992) J. Biochem. 112, Maruyama, K., Yoshioka, T., Higuchi, H., Ohashi, K., Kimura, S., & Natori, R. (1985) J. Cell Biol. 101, FUrst, D.O., Osborn, M., Nave, R., & Weber, K. (1988) J. Cell Biol. 106, Somerville, L.L. & Wang, K. (1987) Biochem. Biophys. Res. Commun. 147, Somerville, L.L. & Wang, K. (1988) Arch. Biochem. Biophys. 262, Greaser, M.L., Cassens, R.G., Hoekstra, W.G., & Briskey, E.J. (1969) J. Food Sci. 34, Buege, D.R. & Marsh, B.B. (1975) Biochem. Biophys. Res. Commun. 65, Takahashi, K., Kim, O.H., & Yano, K. (1987) J. Biochem. 101, Takahashi, K., Yamanoue, M., Murakami, T., Nishimura, T., & Yoshikawa, R. (1987) J. Biochem. 102, Yamanoue, M. & Takahashi, K. (1988) J. Biochem. 103, Takahashi, K. (1992) Biochimie 74, Vol. 112, No. 6, 1992
Mode of IMP and Pyrophosphate Enhancement of Myosin and Actin Extraction from Porcine Meat
 Biosci. Biotechnol. Biochem., 77 (6), 1214 1218, 2013 Mode of IMP and Pyrophosphate Enhancement of Myosin and Actin Extraction from Porcine Meat Yukinobu NAKAMURA, 1;y Koshiro MIGITA, 2 Akihiro OKITANI,
Biosci. Biotechnol. Biochem., 77 (6), 1214 1218, 2013 Mode of IMP and Pyrophosphate Enhancement of Myosin and Actin Extraction from Porcine Meat Yukinobu NAKAMURA, 1;y Koshiro MIGITA, 2 Akihiro OKITANI,
Title. Author(s)Takahashi, Koui; Ji, JR. CitationMeat Science, 73(3): Issue Date Doc URL. Rights. Type.
 Title Changes in concentration of sarcoplasmic free calciu Author(s)Takahashi, Koui; Ji, JR CitationMeat Science, 73(3): 395-403 Issue Date 2006-07-01 Doc URL http://hdl.handle.net/2115/14511 Rights Copyright
Title Changes in concentration of sarcoplasmic free calciu Author(s)Takahashi, Koui; Ji, JR CitationMeat Science, 73(3): 395-403 Issue Date 2006-07-01 Doc URL http://hdl.handle.net/2115/14511 Rights Copyright
Postmortem Proteolysis of Breast and Leg Muscles from Taiwan Colored Chickens and Silkie Bantams
 739 Postmortem Proteolysis of Breast and Leg Muscles from Taiwan Colored Chickens and Silkie Bantams Shih-Fen Tsai, Chia-Ying Lin, Jin-Jenn Lu and Rong-Ghi R. Chou* Department of Animal Science, National
739 Postmortem Proteolysis of Breast and Leg Muscles from Taiwan Colored Chickens and Silkie Bantams Shih-Fen Tsai, Chia-Ying Lin, Jin-Jenn Lu and Rong-Ghi R. Chou* Department of Animal Science, National
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
Elisabeth Huff Lonergan, PhD Steven M. Lonergan, PhD Mark J. Anderson, PhD
 Elisabeth Huff Lonergan, PhD Steven M. Lonergan, PhD Mark J. Anderson, PhD The complexity of tenderness. Differences in tenderness due to postmortem aging is difficult to predict and manage Structure is
Elisabeth Huff Lonergan, PhD Steven M. Lonergan, PhD Mark J. Anderson, PhD The complexity of tenderness. Differences in tenderness due to postmortem aging is difficult to predict and manage Structure is
OF LIGHT CHAINS OF CARDIAC MYOSIN ISOZYMES: ATRIAL AND VENTRICULAR MYOSINS
 CROSS-HYBRIDIZATION OF LIGHT CHAINS OF CARDIAC MYOSIN ISOZYMES: ATRIAL AND VENTRICULAR MYOSINS Gabor HOLLGSI*, Sudhir SRIVASTAVA** and Joan WIKMAN-COFFELT University of California, San Francisco Cardiovascular
CROSS-HYBRIDIZATION OF LIGHT CHAINS OF CARDIAC MYOSIN ISOZYMES: ATRIAL AND VENTRICULAR MYOSINS Gabor HOLLGSI*, Sudhir SRIVASTAVA** and Joan WIKMAN-COFFELT University of California, San Francisco Cardiovascular
Muscle Cells & Muscle Fiber Contractions. Packet #8
 Muscle Cells & Muscle Fiber Contractions Packet #8 Skeletal muscle is attached to bones and is responsible for movement. Introduction Introduction II Skeletal muscle is composed of bundles of muscle fibers
Muscle Cells & Muscle Fiber Contractions Packet #8 Skeletal muscle is attached to bones and is responsible for movement. Introduction Introduction II Skeletal muscle is composed of bundles of muscle fibers
Supplementary material: Materials and suppliers
 Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
MYOFIBRILLAR STRUCTURAL CHANGES CAUSED BY MARINATION WITH CALCIUM PHOSPHATE OR CALCIUM CHLORIDE AND SODIUM PYROPHOSPHATE
 Cattlemen s Day 2002 MYOFIBRILLAR STRUCTURAL CHANGES CAUSED BY MARINATION WITH CALCIUM PHOSPHATE OR CALCIUM CHLORIDE AND SODIUM PYROPHOSPHATE T. E. Lawrence, A. T. Waylan, and C. L. Kastner Summary Ultrastructural
Cattlemen s Day 2002 MYOFIBRILLAR STRUCTURAL CHANGES CAUSED BY MARINATION WITH CALCIUM PHOSPHATE OR CALCIUM CHLORIDE AND SODIUM PYROPHOSPHATE T. E. Lawrence, A. T. Waylan, and C. L. Kastner Summary Ultrastructural
Regulatory Proteins from Dorsal Muscle of the Carp
 J. Biochent. 82, 931-938 (1977) Regulatory Proteins from Dorsal Muscle of the Carp Kunihiko KONNO,* Ken-ichi ARAI,* and Shizuo WATANABE** *The Department of Food Science, Faculty of Fisheries, Hokkaido
J. Biochent. 82, 931-938 (1977) Regulatory Proteins from Dorsal Muscle of the Carp Kunihiko KONNO,* Ken-ichi ARAI,* and Shizuo WATANABE** *The Department of Food Science, Faculty of Fisheries, Hokkaido
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
Skeletal Muscle Contraction and ATP Demand
 Skeletal Muscle Contraction and ATP Demand Anatomy & Structure Contraction Cycling Calcium Regulation Types of Contractions Force, Power, and Contraction Velocity Epimysium - separates fascia and muscle
Skeletal Muscle Contraction and ATP Demand Anatomy & Structure Contraction Cycling Calcium Regulation Types of Contractions Force, Power, and Contraction Velocity Epimysium - separates fascia and muscle
Western Blot Analysis of Rat Pituitar Recognized by Human Antipituitary. y Antigens A. antibodies
 Endocrine Journal 1995, 42(1), 115-119 NOTE Western Blot Analysis of Rat Pituitar Recognized by Human Antipituitary y Antigens A ntibodies SHIGEKI YABE, MASAMI MURAKAMI*, KAYOKO MARUYAMA, HIDEKO MIWA,
Endocrine Journal 1995, 42(1), 115-119 NOTE Western Blot Analysis of Rat Pituitar Recognized by Human Antipituitary y Antigens A ntibodies SHIGEKI YABE, MASAMI MURAKAMI*, KAYOKO MARUYAMA, HIDEKO MIWA,
Ch 12 can be done in one lecture
 Ch 12 can be done in one lecture Developed by John Gallagher, MS, DVM Chapter 12: Muscles Review muscle anatomy (esp. microanatomy of skeletal muscle) Terminology: sarcolemma t-tubules sarcoplasmic reticulum
Ch 12 can be done in one lecture Developed by John Gallagher, MS, DVM Chapter 12: Muscles Review muscle anatomy (esp. microanatomy of skeletal muscle) Terminology: sarcolemma t-tubules sarcoplasmic reticulum
Western Immunoblotting Preparation of Samples:
 Western Immunoblotting Preparation of Samples: Total Protein Extraction from Culture Cells: Take off the medium Wash culture with 1 x PBS 1 ml hot Cell-lysis Solution into T75 flask Scrap out the cells
Western Immunoblotting Preparation of Samples: Total Protein Extraction from Culture Cells: Take off the medium Wash culture with 1 x PBS 1 ml hot Cell-lysis Solution into T75 flask Scrap out the cells
Some Properties of Glycerinated Skeletal Muscle Fibres Containing Phosphorylated Myosin
 Gen. Physiol. Biophys. (1989), 8, 569-578 569 Some Properties of Glycerinated Skeletal Muscle Fibres Containing Phosphorylated Myosin M. WROTEK', YU. S. BOROVIKOV 2, N. N. LEBEDEVA 2 and I. K^KOĽ 1 Department
Gen. Physiol. Biophys. (1989), 8, 569-578 569 Some Properties of Glycerinated Skeletal Muscle Fibres Containing Phosphorylated Myosin M. WROTEK', YU. S. BOROVIKOV 2, N. N. LEBEDEVA 2 and I. K^KOĽ 1 Department
Skeletal muscle in the light of its structure
 Mechanism of contraction of Skeletal muscle in the light of its structure By Dr. Mudassar Ali Roomi (MBBS, M. Phil) Muscle Tissue Skeletal Muscle Cardiac Muscle Smooth Muscle Skeletal Muscle Long cylindrical
Mechanism of contraction of Skeletal muscle in the light of its structure By Dr. Mudassar Ali Roomi (MBBS, M. Phil) Muscle Tissue Skeletal Muscle Cardiac Muscle Smooth Muscle Skeletal Muscle Long cylindrical
The organization of skeletal muscles. Excitation contraction coupling. Whole Skeletal Muscles contractions. Muscle Energetics
 Muscle and Movement The organization of skeletal muscles Excitation contraction coupling Whole Skeletal Muscles contractions Muscle Energetics The molecular bases of movement Muscular cells use molecular
Muscle and Movement The organization of skeletal muscles Excitation contraction coupling Whole Skeletal Muscles contractions Muscle Energetics The molecular bases of movement Muscular cells use molecular
Ch 12: Muscles sarcolemma, t-tubules, sarcoplasmic reticulum, myofibrils, myofilaments, sarcomere...
 Ch 12: Muscles Review micro-anatomy of muscle tissue Terminology examples: sarcolemma, t-tubules, sarcoplasmic reticulum, myofibrils, myofilaments, sarcomere... SLOs Differentiate levels of muscle structure:
Ch 12: Muscles Review micro-anatomy of muscle tissue Terminology examples: sarcolemma, t-tubules, sarcoplasmic reticulum, myofibrils, myofilaments, sarcomere... SLOs Differentiate levels of muscle structure:
Antibodies to the calmodulin-binding Ca2+-transport ATPase from smooth muscle
 Antibodies to the calmodulin-binding Ca2+-transport ATPase from smooth muscle Frank Wuytack, Greet De Schutter, Jan Verbist and Rik Casteels Laboratorium voor Fysiologie, Katholieke Universiteit Leuven,
Antibodies to the calmodulin-binding Ca2+-transport ATPase from smooth muscle Frank Wuytack, Greet De Schutter, Jan Verbist and Rik Casteels Laboratorium voor Fysiologie, Katholieke Universiteit Leuven,
Relative contribution of calpain and cathepsins to protein degradation in muscle of sea bass (Dicentrarchus labrax L.)
 Please note that this is an author-produced PDF of an article accepted for publication following peer review. The definitive publisher-authenticated version is available on the publisher Web site Food
Please note that this is an author-produced PDF of an article accepted for publication following peer review. The definitive publisher-authenticated version is available on the publisher Web site Food
EXCITATION- CONTRACTION COUPLING IN SKELETAL MUSCLES 1
 EXCITATION- CONTRACTION COUPLING IN SKELETAL MUSCLES 1 Summary: The sequence of events from the movement of an AP moving down a neuron to the completion of a contraction is examined. These events are referred
EXCITATION- CONTRACTION COUPLING IN SKELETAL MUSCLES 1 Summary: The sequence of events from the movement of an AP moving down a neuron to the completion of a contraction is examined. These events are referred
About This Chapter. Skeletal muscle Mechanics of body movement Smooth muscle Cardiac muscle Pearson Education, Inc.
 About This Chapter Skeletal muscle Mechanics of body movement Smooth muscle Cardiac muscle Skeletal Muscle Usually attached to bones by tendons Origin: closest to the trunk or to more stationary bone Insertion:
About This Chapter Skeletal muscle Mechanics of body movement Smooth muscle Cardiac muscle Skeletal Muscle Usually attached to bones by tendons Origin: closest to the trunk or to more stationary bone Insertion:
ANSC/FSTC 607 Biochemistry and Physiology of Muscle as a Food PRIMARY, SECONDARY, AND TERTIARY MYOTUBES
 ANSC/FSTC 607 Biochemistry and Physiology of Muscle as a Food PRIMARY, SECONDARY, AND TERTIARY MYOTUBES I. Satellite Cells A. Proliferative, myoblastic cells that lie in invaginations in the sarcolemma
ANSC/FSTC 607 Biochemistry and Physiology of Muscle as a Food PRIMARY, SECONDARY, AND TERTIARY MYOTUBES I. Satellite Cells A. Proliferative, myoblastic cells that lie in invaginations in the sarcolemma
Title. Author(s)Thavaroj, Wichulada; Sakamoto, Mari; Konno, Yoshiko; CitationFisheries science, 82(5): Issue Date Doc URL.
 Title Preceding actin denaturation accelerates myosin dena Author(s)Thavaroj, Wichulada; Sakamoto, Mari; Konno, Yoshiko; CitationFisheries science, 82(5): 843-850 Issue Date 2016-09 Doc URL http://hdl.handle.net/2115/67081
Title Preceding actin denaturation accelerates myosin dena Author(s)Thavaroj, Wichulada; Sakamoto, Mari; Konno, Yoshiko; CitationFisheries science, 82(5): 843-850 Issue Date 2016-09 Doc URL http://hdl.handle.net/2115/67081
Chiung-Ying Ho*, Marvin H. Stromer*,3, and Richard M. Robson*, Introduction
 Effect of Electrical Stimulation on Postmortem Titin, Nebulin, Desmin, and Troponin-T Degradation and Ultrastructural Changes in Bovine Longissimus Muscle 1,2 Chiung-Ying Ho*, Marvin H. Stromer*,3, and
Effect of Electrical Stimulation on Postmortem Titin, Nebulin, Desmin, and Troponin-T Degradation and Ultrastructural Changes in Bovine Longissimus Muscle 1,2 Chiung-Ying Ho*, Marvin H. Stromer*,3, and
Structure of the striated muscle general properties
 Structure of the striated muscle general properties Structure of the striated muscle membrane systems 1. Myofibrillum (contractile proteins) 2. Sarcoplasmic reticulum (SR) longitudinal tubule 3. SR terminal
Structure of the striated muscle general properties Structure of the striated muscle membrane systems 1. Myofibrillum (contractile proteins) 2. Sarcoplasmic reticulum (SR) longitudinal tubule 3. SR terminal
Muscle Dr. Ted Milner (KIN 416)
 Muscle Dr. Ted Milner (KIN 416) Muscles are biological motors which actively generate force and produce movement through the process of contraction. The molecular mechanism responsible for muscle contraction
Muscle Dr. Ted Milner (KIN 416) Muscles are biological motors which actively generate force and produce movement through the process of contraction. The molecular mechanism responsible for muscle contraction
5. What component of the sarcomere is not attached to the Z line?
 Model 2: Anatomy of a Sarcomere 1. Label the thick filament and the thin filament in Model 2. 2. How many sarcomeres are shown in Model 2? 3. Using Model 2, based on the locations of thick and thin filaments,
Model 2: Anatomy of a Sarcomere 1. Label the thick filament and the thin filament in Model 2. 2. How many sarcomeres are shown in Model 2? 3. Using Model 2, based on the locations of thick and thin filaments,
Muscle Tissue. Muscle Development and Repair. Development: fusion of myoblasts. Repair: Satellite cells (S) 3 Types of Muscle
 ANNOUNCEMENTS Review Session Every Friday at 12:20 Muscle Tissue 3 Types of Muscle Function: Force generation Lab Practical Coming up! October 26 th, 27 th Muscle Tissue Striated Nonstriated Skeletal Smooth
ANNOUNCEMENTS Review Session Every Friday at 12:20 Muscle Tissue 3 Types of Muscle Function: Force generation Lab Practical Coming up! October 26 th, 27 th Muscle Tissue Striated Nonstriated Skeletal Smooth
Concept 50.5: The physical interaction of protein filaments is required for muscle function
 Concept 50.5: The physical interaction of protein filaments is required for muscle function Muscle activity is a response to input from the nervous system The action of a muscle is always to contract Vertebrate
Concept 50.5: The physical interaction of protein filaments is required for muscle function Muscle activity is a response to input from the nervous system The action of a muscle is always to contract Vertebrate
Skeletal Muscle and the Molecular Basis of Contraction. Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry
 Skeletal Muscle and the Molecular Basis of Contraction Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Like neurons, all muscle cells can be excited chemically, electrically, and
Skeletal Muscle and the Molecular Basis of Contraction Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Like neurons, all muscle cells can be excited chemically, electrically, and
Protein MultiColor Stable, Low Range
 Product Name: DynaMarker Protein MultiColor Stable, Low Range Code No: DM670L Lot No: ******* Size: 200 μl x 3 (DM670 x 3) (120 mini-gel lanes) Storage: 4 C Stability: 12 months at 4 C Storage Buffer:
Product Name: DynaMarker Protein MultiColor Stable, Low Range Code No: DM670L Lot No: ******* Size: 200 μl x 3 (DM670 x 3) (120 mini-gel lanes) Storage: 4 C Stability: 12 months at 4 C Storage Buffer:
ANSC (FSTC) 607 Physiology and Biochemistry of Muscle as a Food MUSCLE CONTRACTION
 I. Basic model of muscle contraction A. Overall ANSC (FSTC) 607 Physiology and Biochemistry of Muscle as a Food MUSCLE CONTRACTION 1. Calcium is released from sarcoplasmic reticulum. 2. Myosin globular
I. Basic model of muscle contraction A. Overall ANSC (FSTC) 607 Physiology and Biochemistry of Muscle as a Food MUSCLE CONTRACTION 1. Calcium is released from sarcoplasmic reticulum. 2. Myosin globular
Muscle Tissue- 3 Types
 AN INTRODUCTION TO MUSCLE TISSUE Muscle Tissue- 3 Types Skeletal muscle (focus on these) Cardiac muscle Smooth muscle FUNCTIONS OF SKELETAL MUSCLES Produce movement of the skeleton Maintain posture and
AN INTRODUCTION TO MUSCLE TISSUE Muscle Tissue- 3 Types Skeletal muscle (focus on these) Cardiac muscle Smooth muscle FUNCTIONS OF SKELETAL MUSCLES Produce movement of the skeleton Maintain posture and
Calcium Regulation in Squid Mantle and Scallop Adductor Muscles 1
 . Biochem. 89, 581-589 (1981) Calcium Regulation in Squid Mantle and Scallop Adductor Muscles 1 Kunihiko KONNO,* Ken-ichi ARM,* Mikiharu YOSHIDA,** and Shizuo WATANABE*** Department of Food Science, Faculty
. Biochem. 89, 581-589 (1981) Calcium Regulation in Squid Mantle and Scallop Adductor Muscles 1 Kunihiko KONNO,* Ken-ichi ARM,* Mikiharu YOSHIDA,** and Shizuo WATANABE*** Department of Food Science, Faculty
Muscle Contraction A BIOCHEMICAL PERSPECTIVE
 Muscle Contraction A BIOCHEMICAL PERSPECTIVE This presentation was last edited Oct 2016, by Koni Stone, Professor of Chemistry California State University, Stanislaus Muscle cells! Myoblasts are large
Muscle Contraction A BIOCHEMICAL PERSPECTIVE This presentation was last edited Oct 2016, by Koni Stone, Professor of Chemistry California State University, Stanislaus Muscle cells! Myoblasts are large
Vol. 46, No. 6, December.1998 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 11 o1-1108
 Vol. 46, No. 6, December.1998 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 11 o1-1108 THE SHORTENING OF THE N-TERMINUS OF MYOSIN ESSENTIAL LIGHT CHAIN A1 INFLUENCES THE INTERACTION OF HEAVY MEROMYOSIN
Vol. 46, No. 6, December.1998 BIOCHEMISTRY and MOLECULAR BIOLOGY INTERNATIONAL Pages 11 o1-1108 THE SHORTENING OF THE N-TERMINUS OF MYOSIN ESSENTIAL LIGHT CHAIN A1 INFLUENCES THE INTERACTION OF HEAVY MEROMYOSIN
AP Biology. Animal Locomotion. Muscles & Motor Locomotion. Why Do We Need All That ATP? Lots of ways to get around. Muscle
 Muscles & Motor Locomotion Animal Locomotion What are the advantages of locomotion? sessile motile Why Do We Need All That? 2006-2007 Lots of ways to get around Lots of ways to get around mollusk mammal
Muscles & Motor Locomotion Animal Locomotion What are the advantages of locomotion? sessile motile Why Do We Need All That? 2006-2007 Lots of ways to get around Lots of ways to get around mollusk mammal
Class XI Chapter 20 Locomotion and Movement Biology
 Question 1: Draw the diagram of a sarcomere of skeletal muscle showing different regions. The diagrammatic representation of a sarcomere is as follows: Question 2: Define sliding filament theory of muscle
Question 1: Draw the diagram of a sarcomere of skeletal muscle showing different regions. The diagrammatic representation of a sarcomere is as follows: Question 2: Define sliding filament theory of muscle
CHAPTER 6 2/9/2016. Learning Objectives List the four traits that all muscle types have in common.
 Learning Objectives List the four traits that all muscle types have in common. CHAPTER 6 The Muscular System Demonstrate and explain the use of antagonistic muscle pairs. Describe the attachment of muscle
Learning Objectives List the four traits that all muscle types have in common. CHAPTER 6 The Muscular System Demonstrate and explain the use of antagonistic muscle pairs. Describe the attachment of muscle
Muscles & Motor Locomotion Why Do We Need All That ATP?
 Muscles & Motor Locomotion Why Do We Need All That ATP? 2006-2007 Animal Locomotion What are the advantages of locomotion? sessile motile Lots of ways to get around Lots of ways to get around mollusk mammal
Muscles & Motor Locomotion Why Do We Need All That ATP? 2006-2007 Animal Locomotion What are the advantages of locomotion? sessile motile Lots of ways to get around Lots of ways to get around mollusk mammal
Lecture 9A. Muscle structure. Outline
 Lecture 9A Muscle structure Outline Smooth, skeletal, and cardiac muscle tissues Structure and function of skeletal muscle cells. Sarcomeres structure and contraction Actin-myosin interaction and sliding
Lecture 9A Muscle structure Outline Smooth, skeletal, and cardiac muscle tissues Structure and function of skeletal muscle cells. Sarcomeres structure and contraction Actin-myosin interaction and sliding
Anatomy and Physiology 1 Chapter 10 self quiz Pro, Dima Darwish,MD.
 Anatomy and Physiology 1 Chapter 10 self quiz Pro, Dima Darwish,MD. 1) Which of the following is a recognized function of skeletal muscle? A) produce movement B) maintain posture C) maintain body temperature
Anatomy and Physiology 1 Chapter 10 self quiz Pro, Dima Darwish,MD. 1) Which of the following is a recognized function of skeletal muscle? A) produce movement B) maintain posture C) maintain body temperature
#1 20. physiology. Muscle tissue 30/9/2015. Ahmad Adel Sallal. Mohammad Qudah
 # 20 physiology Muscle tissue Ahmad Adel Sallal 30/9/205 Mohammad Qudah MUSCLES PHYSIOLOGY Awn, welcome to the first physiology lecture in the MSS, I wish you a perfect exams with high grades, and never
# 20 physiology Muscle tissue Ahmad Adel Sallal 30/9/205 Mohammad Qudah MUSCLES PHYSIOLOGY Awn, welcome to the first physiology lecture in the MSS, I wish you a perfect exams with high grades, and never
Isolation and Molecular Characterization of Local Goat Milk Casein for Nutraceutical Value
 Isolation and Molecular Characterization of Local Goat Milk Casein for Nutraceutical Value Mohd Akmal Azhar 1,*, and Norshafiqa Salim 2 1 Faculty of Engineering Technology, UMP Gambang, Lebuhraya Tun Razak,
Isolation and Molecular Characterization of Local Goat Milk Casein for Nutraceutical Value Mohd Akmal Azhar 1,*, and Norshafiqa Salim 2 1 Faculty of Engineering Technology, UMP Gambang, Lebuhraya Tun Razak,
Journal of Coastal Development ISSN: CHEMICAL ANALYSIS DURING THE PROCESSING OF DRIED SALTED ANCHOVY
 CHEMICAL ANALYSIS DURING THE PROCESSING OF DRIED SALTED ANCHOVY Eko Nurcahya Dewi *) Faculty of Fisheries and Marine Sciences University of Diponegoro, Jl. Hayam Wuruk 4A Semarang, Indonesia Received:
CHEMICAL ANALYSIS DURING THE PROCESSING OF DRIED SALTED ANCHOVY Eko Nurcahya Dewi *) Faculty of Fisheries and Marine Sciences University of Diponegoro, Jl. Hayam Wuruk 4A Semarang, Indonesia Received:
Inhibitory antibodies to plasmalemmal Ca2+-transporting ATPases
 Biochem. J. (1985) 231, 737-742 (Printed in Great Britain) Inhibitory antibodies to plasmalemmal Ca2+-transporting ATPases Their use in subcellular localization of (Ca2+ +Mg2+)-dependent ATPase activity
Biochem. J. (1985) 231, 737-742 (Printed in Great Britain) Inhibitory antibodies to plasmalemmal Ca2+-transporting ATPases Their use in subcellular localization of (Ca2+ +Mg2+)-dependent ATPase activity
Conformational changes in subdomain- of G-actin upon polymerization into F-actin and upon binding myosin subfragment-l
 Volume 316, number 2, 186190 FEBS 12036 0 1993 Federation of European Biochemical Societies 00145793/93/$6.00 January 1993 Conformational changes in subdomain- of G-actin upon polymerization into F-actin
Volume 316, number 2, 186190 FEBS 12036 0 1993 Federation of European Biochemical Societies 00145793/93/$6.00 January 1993 Conformational changes in subdomain- of G-actin upon polymerization into F-actin
Properties of Tropomyosin from Tilapia, Tilapia nilotica, Dorsal Muscle 2
 Properties of Tropomyosin from Tilapia,Tilapia nilotica, Dorsal Muscle. 2 Properties of Tropomyosin from Tilapia, Tilapia nilotica, Dorsal Muscle 2 Comparison of Tilapia Tropomyosin with Rabbit Skeletal
Properties of Tropomyosin from Tilapia,Tilapia nilotica, Dorsal Muscle. 2 Properties of Tropomyosin from Tilapia, Tilapia nilotica, Dorsal Muscle 2 Comparison of Tilapia Tropomyosin with Rabbit Skeletal
Mechanism of Muscular Contraction
 ~ Sorin2:er Jack A. Rail Mechanism of Muscular Contraction Contents 1 Setting the Stage: Myosin, Actin, Actomyosin and ATP... 1.1 Introduction... 1 1.2 Muscle Structure as Observed by Nineteenth Century
~ Sorin2:er Jack A. Rail Mechanism of Muscular Contraction Contents 1 Setting the Stage: Myosin, Actin, Actomyosin and ATP... 1.1 Introduction... 1 1.2 Muscle Structure as Observed by Nineteenth Century
BIOLOGY - CLUTCH CH.49 - MUSCLE SYSTEMS.
 !! www.clutchprep.com BIOLOGY - CLUTCH Muscle system organ system that includes skeletal, cardiac, and smooth muscle Muscle tissue capable of contracting through the interaction of actin and myosin proteins
!! www.clutchprep.com BIOLOGY - CLUTCH Muscle system organ system that includes skeletal, cardiac, and smooth muscle Muscle tissue capable of contracting through the interaction of actin and myosin proteins
AP Biology
 Chapter 49. Animal Locomotion What are the advantages of locomotion? sessile motile Muscles & Motor Locomotion Muscle voluntary, striated involuntary, striated auto-rhythmic involuntary, non-striated 1
Chapter 49. Animal Locomotion What are the advantages of locomotion? sessile motile Muscles & Motor Locomotion Muscle voluntary, striated involuntary, striated auto-rhythmic involuntary, non-striated 1
Chapter 49. Muscles & Motor Locomotion. AP Biology
 Chapter 49. Muscles & Motor Locomotion Animal Locomotion What are the advantages of locomotion? sessile motile Muscle voluntary, striated involuntary, striated auto-rhythmic involuntary, non-striated
Chapter 49. Muscles & Motor Locomotion Animal Locomotion What are the advantages of locomotion? sessile motile Muscle voluntary, striated involuntary, striated auto-rhythmic involuntary, non-striated
The Sliding Filament Theory
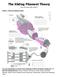 The Sliding Filament Theory Model 1: Muscle Histology Review How do muscle cells contract? Use your knowledge of muscle tissue histology to fill in the blanks numbered 1-11 with the following terms: Fasicle,
The Sliding Filament Theory Model 1: Muscle Histology Review How do muscle cells contract? Use your knowledge of muscle tissue histology to fill in the blanks numbered 1-11 with the following terms: Fasicle,
Brush border myosin heavy chain phosphorylation regulated by calcium and calmodulin
 Volume 212, number 1, 154-158 FEB 04421 February 1987 Brush border myosin heavy chain phosphorylation regulated by calcium and calmodulin is James P. Rieker, Helena Swanljung-Collins, Judith Montibeller
Volume 212, number 1, 154-158 FEB 04421 February 1987 Brush border myosin heavy chain phosphorylation regulated by calcium and calmodulin is James P. Rieker, Helena Swanljung-Collins, Judith Montibeller
Recombinant Trypsin, Animal Origin Free
 Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
Antigenic Change of Native and Heat-Denatured Ovalbumin Digested with Pepsin, Trypsin or Chymotrypsin
 J. Home Econ. Jpn. Vol. 48 No. 8 717 ` 722 (1997) Note Antigenic Change of Native and Heat-Denatured Ovalbumin Digested with Pepsin, Trypsin or Chymotrypsin Sumiko ODANI, Hiroko AWATUHARA* and Yukie KATO**
J. Home Econ. Jpn. Vol. 48 No. 8 717 ` 722 (1997) Note Antigenic Change of Native and Heat-Denatured Ovalbumin Digested with Pepsin, Trypsin or Chymotrypsin Sumiko ODANI, Hiroko AWATUHARA* and Yukie KATO**
Contraction, Locomotion (Ch 16), Shelden
 Contraction, Locomotion (Ch 16), Shelden 1 Molec Biol Cell 5 th ed. Supplemental Videos Actin-dependent ATP hydrolysis by myosin generates motile force The Myosin ATPase activity cycle 2 ATP binding releases
Contraction, Locomotion (Ch 16), Shelden 1 Molec Biol Cell 5 th ed. Supplemental Videos Actin-dependent ATP hydrolysis by myosin generates motile force The Myosin ATPase activity cycle 2 ATP binding releases
Muscle Tissue. Muscle Tissue (skeletal muscle) Some soluble proteins Connective Tissue Adipose Tissue (fat)
 Muscle Tissue (skeletal muscle) Some soluble proteins Connective Tissue Adipose Tissue (fat) Between muscles, it is called seam fat (usually trimmed) Within the muscles, it is called marbling (often prized)
Muscle Tissue (skeletal muscle) Some soluble proteins Connective Tissue Adipose Tissue (fat) Between muscles, it is called seam fat (usually trimmed) Within the muscles, it is called marbling (often prized)
Organismic Biology Bio 207. Lecture 6. Muscle and movement; sliding filaments; E-C coupling; length-tension relationships; biomechanics. Prof.
 Organismic Biology Bio 207 Lecture 6 Muscle and movement; sliding filaments; E-C coupling; length-tension relationships; biomechanics Prof. Simchon Today s Agenda Skeletal muscle Neuro Muscular Junction
Organismic Biology Bio 207 Lecture 6 Muscle and movement; sliding filaments; E-C coupling; length-tension relationships; biomechanics Prof. Simchon Today s Agenda Skeletal muscle Neuro Muscular Junction
MUSCLE TISSUE (MUSCLE PHYSIOLOGY) PART I: MUSCLE STRUCTURE
 PART I: MUSCLE STRUCTURE Muscle Tissue A primary tissue type, divided into: skeletal muscle cardiac muscle smooth muscle Functions of Skeletal Muscles Produce skeletal movement Maintain body position Support
PART I: MUSCLE STRUCTURE Muscle Tissue A primary tissue type, divided into: skeletal muscle cardiac muscle smooth muscle Functions of Skeletal Muscles Produce skeletal movement Maintain body position Support
3 muscle function_scr.notebook April 20, 2015
 the key to muscle function is an excitable membrane sarcolemma proteins on the sarcolemma allow muscle cells to communicate with other cells and the environment specific to muscle function is communication
the key to muscle function is an excitable membrane sarcolemma proteins on the sarcolemma allow muscle cells to communicate with other cells and the environment specific to muscle function is communication
Ch. 6: Contraction of Skeletal Muscle Physiological Anatomy of Skeletal Muscle
 Ch. 6: Contraction of Skeletal Muscle 40% skeletal muscle + 10% smooth and cardiac muscle Ch. 7: Excitation of Skeletal Muscle Ch. 9: Contraction and Excitation of Smooth Muscle Physiological Anatomy of
Ch. 6: Contraction of Skeletal Muscle 40% skeletal muscle + 10% smooth and cardiac muscle Ch. 7: Excitation of Skeletal Muscle Ch. 9: Contraction and Excitation of Smooth Muscle Physiological Anatomy of
Chapter Skeletal Muscle Structure and Function
 Chapter 10.2 Skeletal Muscle Structure and Function Introduction to Muscle Physiology Movement is a fundamental characteristic of all living things All muscle cells (skeletal, cardiac, and smooth) are
Chapter 10.2 Skeletal Muscle Structure and Function Introduction to Muscle Physiology Movement is a fundamental characteristic of all living things All muscle cells (skeletal, cardiac, and smooth) are
Chapter 50. You re on your own for: Sensory Reception Mechanoreceptors Gravity, Hearing and Equilibrium. Chemoreception taste and smell
 1 Sensory and Motor Mechanisms 2 Chapter 50 You re on your own for: Sensory Reception Mechanoreceptors Gravity, Hearing and Equilibrium Chemoreception taste and smell Photoreceptors vision It s interesting.
1 Sensory and Motor Mechanisms 2 Chapter 50 You re on your own for: Sensory Reception Mechanoreceptors Gravity, Hearing and Equilibrium Chemoreception taste and smell Photoreceptors vision It s interesting.
Muscle Contraction and (Microscopic) Anatomy. Some copyright issues but I won t tell if you
 Muscle Contraction and (Microscopic) Anatomy Some copyright issues but I won t tell if you don t What is a contraction? It is so important to visualise the fact that muscles work their magic by simply
Muscle Contraction and (Microscopic) Anatomy Some copyright issues but I won t tell if you don t What is a contraction? It is so important to visualise the fact that muscles work their magic by simply
Muscle and Neuromuscular Junction. Peter Takizawa Department of Cell Biology
 Muscle and Neuromuscular Junction Peter Takizawa Department of Cell Biology Types and structure of muscle cells Structural basis of contraction Triggering muscle contraction Skeletal muscle consists of
Muscle and Neuromuscular Junction Peter Takizawa Department of Cell Biology Types and structure of muscle cells Structural basis of contraction Triggering muscle contraction Skeletal muscle consists of
RayBio KinaseSTAR TM Akt Activity Assay Kit
 Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
Activity Assay Kit User Manual Version 1.0 March 13, 2015 RayBio KinaseSTAR TM Akt Activity Kit Protocol (Cat#: 68AT-Akt-S40) RayBiotech, Inc. We Provide You With Excellent Support And Service Tel:(Toll
Muscles Muscles are effectors which enable movement to be carried out
 Muscles 13.8 Muscles are effectors which enable movement to be carried out Muscle Is responsible for almost all the movements in animals 3 types Cardiac muscle Smo oth muscle Skeletal mus cle (aka striped
Muscles 13.8 Muscles are effectors which enable movement to be carried out Muscle Is responsible for almost all the movements in animals 3 types Cardiac muscle Smo oth muscle Skeletal mus cle (aka striped
Skeletal muscle. General features :
 Muscular tissues In the first embryonic life the muscular tissues arise from mesoderm, The function of movement in multicellular organisms is usually assumed by specialized cells called muscle fibers which
Muscular tissues In the first embryonic life the muscular tissues arise from mesoderm, The function of movement in multicellular organisms is usually assumed by specialized cells called muscle fibers which
BIOL 347L Laboratory Three
 Introduction BIOL 347L Laboratory Three Osmosis in potato and carrot samples Osmosis is the movement of water molecules through a selectively permeable membrane into a region of higher solute concentration,
Introduction BIOL 347L Laboratory Three Osmosis in potato and carrot samples Osmosis is the movement of water molecules through a selectively permeable membrane into a region of higher solute concentration,
Lab Recipes. Destain 50% MeOH 40% milli-q water 10% Acetic Acid For 5 L total 2.5 liters Methanol 2 liters milli-q 0.5 liters Acetic Acid
 1% Blotto 1 liter TTBS (aka TBST) 50 g nonfat dry milk 10 g BSA (Albumin, Bovine) Spike with 2 of 5% Sodium Azide (in hood) Shake it up and put it in the fridge Coomassie Stain 50% MeOH 10% Acetic Acid
1% Blotto 1 liter TTBS (aka TBST) 50 g nonfat dry milk 10 g BSA (Albumin, Bovine) Spike with 2 of 5% Sodium Azide (in hood) Shake it up and put it in the fridge Coomassie Stain 50% MeOH 10% Acetic Acid
Muscles and Animal Movement
 Muscles and Animal Movement Evolution of Muscle and Movement Animals are the only multicellular organisms that actively move. Movement is due to muscle cells (motor proteins) Muscle proteins have homologues
Muscles and Animal Movement Evolution of Muscle and Movement Animals are the only multicellular organisms that actively move. Movement is due to muscle cells (motor proteins) Muscle proteins have homologues
BIL 256 Cell and Molecular Biology Lab Spring, Tissue-Specific Isoenzymes
 BIL 256 Cell and Molecular Biology Lab Spring, 2007 Background Information Tissue-Specific Isoenzymes A. BIOCHEMISTRY The basic pattern of glucose oxidation is outlined in Figure 3-1. Glucose is split
BIL 256 Cell and Molecular Biology Lab Spring, 2007 Background Information Tissue-Specific Isoenzymes A. BIOCHEMISTRY The basic pattern of glucose oxidation is outlined in Figure 3-1. Glucose is split
Item Catalog Number Manufacturer 1,4-Dithioerythritol (1 g) D9680 Sigma-Aldrich
 SOP: Nuclei isolation from fresh mouse tissues and DNaseI treatment Date modified: 01/12/2011 Modified by: E. Giste/ T. Canfield (UW) The following protocol describes the isolation of nuclei and subsequent
SOP: Nuclei isolation from fresh mouse tissues and DNaseI treatment Date modified: 01/12/2011 Modified by: E. Giste/ T. Canfield (UW) The following protocol describes the isolation of nuclei and subsequent
Medical Biology. Dr. Khalida Ibrahim
 Dr. Khalida Ibrahim Medical Biology MUSCLE TISSUE 1. Muscle tissue is characterized by its well-developed properties of contraction. 2. Muscle is responsible for the movements of the body and the various
Dr. Khalida Ibrahim Medical Biology MUSCLE TISSUE 1. Muscle tissue is characterized by its well-developed properties of contraction. 2. Muscle is responsible for the movements of the body and the various
Chapter 10 Muscle Tissue Lecture Outline
 Chapter 10 Muscle Tissue Lecture Outline Muscle tissue types 1. Skeletal muscle = voluntary striated 2. Cardiac muscle = involuntary striated 3. Smooth muscle = involuntary nonstriated Characteristics
Chapter 10 Muscle Tissue Lecture Outline Muscle tissue types 1. Skeletal muscle = voluntary striated 2. Cardiac muscle = involuntary striated 3. Smooth muscle = involuntary nonstriated Characteristics
Chapter PURIFICATION OF ALKALINE PROTEASES
 Chapter PURIFICATION OF ALKALINE PROTEASES E /xtracellular alkaline proteases produced by Bacillus sp. K 25 and bacillus pumilus K 242, were purified and the homogeneity was examined by electrophoresis.
Chapter PURIFICATION OF ALKALINE PROTEASES E /xtracellular alkaline proteases produced by Bacillus sp. K 25 and bacillus pumilus K 242, were purified and the homogeneity was examined by electrophoresis.
CHAPTER 9 PARTIAL PURIFICATION AND CHARACTERIZATION OF CYSTEINE PROTEINASE INHIBITOR FROM CHICKEN PLASMA. 9.1 Abstract. 9.
 154 CHAPTER 9 PARTIAL PURIFICATION AND CHARACTERIZATION OF CYSTEINE PROTEINASE INHIBITOR FROM CHICKEN PLASMA 9.1 Abstract A high-molecular-weight cysteine proteinase inhibitor (CPI) was purified from chicken
154 CHAPTER 9 PARTIAL PURIFICATION AND CHARACTERIZATION OF CYSTEINE PROTEINASE INHIBITOR FROM CHICKEN PLASMA 9.1 Abstract A high-molecular-weight cysteine proteinase inhibitor (CPI) was purified from chicken
Movement of Scallop Myosin on Nitella Actin Filaments: Regulation by Calcium. Ronald D. Vale, Andrew G. Szent-Gyorgyi, and Michael P.
 Movement of Scallop Myosin on Nitella Actin Filaments: Regulation by Calcium Ronald D. Vale, Andrew G. Szent-Gyorgyi, and Michael P. Sheetz PNAS 1984;81;6775-6778 doi:10.1073/pnas.81.21.6775 This information
Movement of Scallop Myosin on Nitella Actin Filaments: Regulation by Calcium Ronald D. Vale, Andrew G. Szent-Gyorgyi, and Michael P. Sheetz PNAS 1984;81;6775-6778 doi:10.1073/pnas.81.21.6775 This information
Both skeletal and cardiac muscles are striated muscles,
 REGULAR PAPER J. Physiol. Sci. Vol. 56, No. 2; Apr. 2006; pp. 145 151 Online Apr. 5, 2006; doi:10.2170/physiolsci.rp003205 Transverse Stiffness of Myofibrils of Skeletal and Cardiac Muscles Studied by
REGULAR PAPER J. Physiol. Sci. Vol. 56, No. 2; Apr. 2006; pp. 145 151 Online Apr. 5, 2006; doi:10.2170/physiolsci.rp003205 Transverse Stiffness of Myofibrils of Skeletal and Cardiac Muscles Studied by
Contribution of the polymerization of protein by disulfide bonding to increased gel strength of walleye pollack surimi gel with preheating time
 FISHERIES SCIENCE 2001; 67: 710 717 Original Article Contribution of the polymerization of protein by disulfide bonding to increased gel strength of walleye pollack surimi gel with preheating time Mohammed
FISHERIES SCIENCE 2001; 67: 710 717 Original Article Contribution of the polymerization of protein by disulfide bonding to increased gel strength of walleye pollack surimi gel with preheating time Mohammed
2D protein gel analysis tool for studying the protein phosphorylation changes associated with contraction and relaxation of vascular smooth muscle
 2D protein gel analysis tool for studying the protein phosphorylation changes associated with contraction and relaxation of vascular smooth muscle Internship details Where: Department of Bioengineering
2D protein gel analysis tool for studying the protein phosphorylation changes associated with contraction and relaxation of vascular smooth muscle Internship details Where: Department of Bioengineering
Effect of Taurine on Acinar Cell Apoptosis and Pancreatic Fibrosis in Dibutyltin Dichloride-induced Chronic Pancreatitis
 212 66 4 329334 Effect of Taurine on Acinar Cell Apoptosis and Pancreatic Fibrosis in Dibutyltin Dichloride-induced Chronic Pancreatitis a,c a* b b a a b a a b c 33 66 4 ʼ 6 6 6 28 6 5 5 5 28 45 ʼ ʼ ʼ
212 66 4 329334 Effect of Taurine on Acinar Cell Apoptosis and Pancreatic Fibrosis in Dibutyltin Dichloride-induced Chronic Pancreatitis a,c a* b b a a b a a b c 33 66 4 ʼ 6 6 6 28 6 5 5 5 28 45 ʼ ʼ ʼ
Chapter 8: Skeletal Muscle: Structure and Function
 Chapter 8: Skeletal Muscle: Structure and Function Objectives Draw & label the microstructure of skeletal muscle Outline the steps leading to muscle shortening Define the concentric and isometric Discuss:
Chapter 8: Skeletal Muscle: Structure and Function Objectives Draw & label the microstructure of skeletal muscle Outline the steps leading to muscle shortening Define the concentric and isometric Discuss:
Protocol for protein SDS PAGE and Transfer
 Protocol for protein SDS PAGE and Transfer According to Laemmli, (1970) Alaa El -Din Hamid Sayed, Alaa_h254@yahoo.com Serum Selection of a protein source cell cultures (bacteria, yeast, mammalian, etc.)
Protocol for protein SDS PAGE and Transfer According to Laemmli, (1970) Alaa El -Din Hamid Sayed, Alaa_h254@yahoo.com Serum Selection of a protein source cell cultures (bacteria, yeast, mammalian, etc.)
Total Histone H3 Acetylation Detection Fast Kit (Colorimetric)
 Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Catalog Number KA1538 48 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use...
Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Catalog Number KA1538 48 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use...
The effect of calcium activated protease (CAF) and cathepsin D on bovine muscle myofibrils under varying conditions of ph and temperature
 Retrospective Theses and Dissertations Iowa State University Capstones, Theses and Dissertations 1984 The effect of calcium activated protease (CAF) and cathepsin D on bovine muscle myofibrils under varying
Retrospective Theses and Dissertations Iowa State University Capstones, Theses and Dissertations 1984 The effect of calcium activated protease (CAF) and cathepsin D on bovine muscle myofibrils under varying
ASSAY OF SPHINGOMYELINASE ACTIVITY
 ASSAY OF SPHINGOMYELINASE ACTIVITY Protocol for Protein Extraction Stock Solution 1. Leupeptin/hydrochloride (FW 463.0,
ASSAY OF SPHINGOMYELINASE ACTIVITY Protocol for Protein Extraction Stock Solution 1. Leupeptin/hydrochloride (FW 463.0,
Chapter 9 Muscle. Types of muscle Skeletal muscle Cardiac muscle Smooth muscle. Striated muscle
 Chapter 9 Muscle Types of muscle Skeletal muscle Cardiac muscle Smooth muscle Striated muscle Chapter 9 Muscle (cont.) The sliding filament mechanism, in which myosin filaments bind to and move actin
Chapter 9 Muscle Types of muscle Skeletal muscle Cardiac muscle Smooth muscle Striated muscle Chapter 9 Muscle (cont.) The sliding filament mechanism, in which myosin filaments bind to and move actin
Connective tissue MUSCLE TISSUE
 Connective tissue MUSCLE TISSUE Part 1 General features of MT Develop from mesoderm Many cells, less intercellular matrix Function contraction (shortening) Skeletal (striated, voluntary) Types of MT Cardiac
Connective tissue MUSCLE TISSUE Part 1 General features of MT Develop from mesoderm Many cells, less intercellular matrix Function contraction (shortening) Skeletal (striated, voluntary) Types of MT Cardiac
Localization of talin in skeletal and cardiac muscles
 Volume 200, number 1 FEBS 3591 May 1986 Localization of talin in skeletal and cardiac muscles A.M. Belkin, N.I. Zhidkova and V.E. Koteliansky* Laboratory of Molecular and Cellular Cardiology, Institute
Volume 200, number 1 FEBS 3591 May 1986 Localization of talin in skeletal and cardiac muscles A.M. Belkin, N.I. Zhidkova and V.E. Koteliansky* Laboratory of Molecular and Cellular Cardiology, Institute
FROM MOVEMENT to meat production, the muscular
 Structures and Functions of the Muscular System FROM MOVEMENT to meat production, the muscular system provides benefits to animals and producers. Like other body systems, the muscular system is a fantastic
Structures and Functions of the Muscular System FROM MOVEMENT to meat production, the muscular system provides benefits to animals and producers. Like other body systems, the muscular system is a fantastic
PNGase F Instruction Manual
 PNGase F Instruction Manual Catalog Number 170-6883 Bio-Rad Laboratories, 2000 Alfred Nobel Dr., Hercules, CA 94547 4006094 Rev A Table of Contents Section 1 Introduction...1 Section 2 Kit Components and
PNGase F Instruction Manual Catalog Number 170-6883 Bio-Rad Laboratories, 2000 Alfred Nobel Dr., Hercules, CA 94547 4006094 Rev A Table of Contents Section 1 Introduction...1 Section 2 Kit Components and
10 mm KCl in a Ti-15 zonal rotor at 35,000 rpm for 16 hr at
 Proc. Nat. Acad. SCi. USA Vol. 68, No. 11, pp. 2752-2756, November 1971 Translation of Exogenous Messenger RNA for Hemoglobin on Reticulocyte and Liver Ribosomes (initiation factors/9s RNA/liver factors/reticulocyte
Proc. Nat. Acad. SCi. USA Vol. 68, No. 11, pp. 2752-2756, November 1971 Translation of Exogenous Messenger RNA for Hemoglobin on Reticulocyte and Liver Ribosomes (initiation factors/9s RNA/liver factors/reticulocyte
N-Glycosidase F Deglycosylation Kit
 For life science research only. Not for use in diagnostic procedures. FOR IN VITRO USE ONLY. N-Glycosidase F Deglycosylation Kit Kit for the deglycosylation of asparagine-linked glycan chains on glycoproteins.
For life science research only. Not for use in diagnostic procedures. FOR IN VITRO USE ONLY. N-Glycosidase F Deglycosylation Kit Kit for the deglycosylation of asparagine-linked glycan chains on glycoproteins.
The following protocol describes the isolation of nuclei from tissue. Item. Catalog No Manufacturer
 SOP: Nuclei isolation from tissue and DNaseI treatment Date modified: 090923 Modified by: P. Sabo. (UW) The following protocol describes the isolation of nuclei from tissue. Ordering Information Item.
SOP: Nuclei isolation from tissue and DNaseI treatment Date modified: 090923 Modified by: P. Sabo. (UW) The following protocol describes the isolation of nuclei from tissue. Ordering Information Item.
2, 6, 3, 7, 8, 9, 10, 5
 A novel regulatory element in cardiac muscle troponin T. Striated muscle contraction is regulated by the position of tropomyosin on actin. That position, in turn, is regulated by the troponin complex (troponin
A novel regulatory element in cardiac muscle troponin T. Striated muscle contraction is regulated by the position of tropomyosin on actin. That position, in turn, is regulated by the troponin complex (troponin
