Journal of Global Trends in Pharmaceutical Sciences
|
|
|
- Mervin Melton
- 5 years ago
- Views:
Transcription
1 Srujana G. L.V et al / J Global Trends Pharm Sci, 2016; 7( (2): An Elsevier Indexed Journal ISSN Journal of Global Trends in Pharmaceutical Sciences GASTRO RETENTIVE DRUG DELIVERY SYSTEM- A REVIEW G.L.V.Srujana*, M.Prasada Rao, Ramakotaiah.Mogili, Y.Sridevi, M.Usha, P.Vijaya raju,, R.Soundarya M.A.M College of Pharmacy, Kesanu Palli, Narasarao Pet, Guntur, Andhra Pradesh, India. ARTI CL E I NFO Key words: GRDDS Development and Evaluation AB ST RACT The purpose of writing this review on floating drug delivery systems (FDDS) was to compile the recent literature with spe- to achieve cial focus on the principal mechanism of floatation gastric retention. The recent developments of FDDS including the physiological and formulation variables affecting gastric retention, approaches to design single-unit and multiple-unit floating systems, and their classification and formulation as- the in pects are covered in detail. This review also summarizes vitro techniques, in vivo studies to evaluate the performance and application of floating systems, and applications of these sys- encountered tems. These systems are useful to several problems during the development of a pharmaceutical dosage form. INTRODUCTION: Considerable efforts have been made in the last decades to develop new pharmaceutically viable and therapeutically effective controlled drug delivery systems. Controlled drug delivery usually results in substantially constant blood levels of the active ingredient as compared to the uncontrolled fluctuations observed when multiple doses of quick releasing conventional dosage forms are administered to a patient. Controlled drug delivery results in optimum therapy, and not only reduces the frequency of dosing, but may also reduce the severity and frequency of side effects. An approach to oral controlled drug delivery: An orally administeredd controlled drug delivery system encounters a wide range of highly variable conditions, such as ph, agiof the ga- tation intensity, and composition strointestinal fluids as it passes down the G.I. tract. * Address for correspondence G.L.V.Srujana* M.A.M College of Pharmacy, Kesanu Palli, Narasarao Pet, Guntur, Andhra Pradesh, India srujana.2205@gmail.com Considerable efforts have been made to design oral controlled drug delivery systems that pro- bioavaila- duce more predictable and increased bility of drugs. However, the development process is precluded by several physiological difficulties, like inability to retain and localize the drug delivery systems within desired re- gions of the G.I. Tract and highly variable nature of gastric emptying process. An important factor, which may adversely affect the performance of an oral controlled drug delivery system, is the G.I. Transit time. The time for absorption in the 0.1. transit in humans, estimated to be 8-10 hrs from mouth to colon, is relatively brief with considerable fluctuation. G.I transit times vary widely between individuals, and depend up on the physical properties of the physiological conditions of the gut. This variability may lead to bioailabi1ity and times to achieve peak plasma levels. Determinants of G.I. transit is the residence time in the stomach. Most of the drugs are well absorbed from alll the regions of the G.I. tract, while some are absorbed only from specific areas, principally due to their low permeability or solubility in the intestinal tract, their chemical instability, the binding or the drug to the gut contents, as well as to the degradation of the drug by microorganisms present in the colon. Therefore, in instances 3065
2 where the drug is not absorbed uniformly over the G.I. tract, the rate of drug absorption may not be constant in spite of the drug delivery system delivering the drug at a constant rate into the G.I. fluids. More particularly, in instances where a drug has a clear cut absorption window, i.e., the drug is absorbed only from specific regions of the stomach or upper parts of the small intestine; it may not be completely absorbed when administered in the form of a typical oral controlled drug delivery system. This is due to the relatively brief gastric emptying time in humans, which normally averages 2-3 hrs through the major absorption zone. It may cause incomplete drug release from the dosage form at absorption sites leading to diminished efficacy of the administered dose. It is apparent that for a drug having such an absorption window, an effective oral controlled drug delivery system should be designed not only to deliver the drug at a controlled rate, but also to retain the drug in the stomach for a long period of time. For this drug, increased or more predictable availability would result if controlled release systems could be retained in the stomach for extended periods of time. It is suggested that compounding narrow absorption window drugs in a unique pharmaceutical dosage form with gastro retentive properties would enable an extended absorption phase of these drugs. After oral administration, such a dosage form would be retained in the stomach and release the drug there in a controlled and prolonged manner, so that the drug could be supplied continuously to its absorption sites in the upper gastro intestinal tract. This mode of administration would best achieve the known pharmacokinetic and pharmacodynamic advantages of controlled release dosage forms for these drugs. Gastric retention dosage forms would be particularly valuable for drugs ( i) That have an absorption window in the stomach or in the upper parts of small intestine (ii) That are locally active in the stomach (iii) That are unstable in the intestinal or colonic environment, and for (iv) Have low solubility at alkaline ph values. Gastric Emptying Process and Conditions: The process of gastric emptying occurs both during fasting and fed states: however, the pattern of motility differs markedly in the two states. In the fasted state, it is characterized by an inter digestive series of electrical events that cycle both through the stomach and small intestine every 2-3 hrs. This activity is called the inter digestive myoelectric cycle or migrating myoelectric complex (MMC), which is often divided into four consecutive phases. As described by Wilson and Washington. Phase I is a quiescent period lasting from 40 to 60 mm with rare contractions. Phase II is a period of similar duration consisting of intermittent action potentials and contractions that gradually increase in intensity and frequency as the phase progresses. Phase III is a short period of intense, large regular contractions lasting from 4 to 6 mm. It is this phase, which gives the cycle the term. Housekeeper wave, since it serves to sweep undigested materials out of the stomach and down the small intestine. As phase III of one cycle reaches the end of the small intestine, phase III of the next cycle begins in the duodenum. Phase IV is a brief transitional phase that occurs between phase Ill and phase I of two consecutive cycles. The motor activity in the fed state is induced 5-10 mm after ingestion of a meal and persists as long as food remains in the stomach, It consists of regular and frequent contractions. These contractions are not as severe as those in the third phase of fasted motility pattern. Requirements: To achieve gastro retention, a dosage form must satisfy the following requirements. One of the key issues is that the dosage form must be able to withstand the forces caused by peristaltic waves in the stomach and constant grinding and churning mechanism. It must resist premature gastric emptying and once the purpose has been served, it should be removed from the stomach with ease. FACTORS AFFECTING GASTRIC RESI- DENCE TIME OF FDDS a) Formulation Factors Size of Tablet: Retention of floating dosage forms in stomach depends on the size of tablets. Small tablets are emptied from the stomach during the digestive phase, but large ones are expelled during the house keeping waves. Floating and non floating capsules of 3 different sizes having a diameter of 4.8 mm (small units), 7.5 mm (medium units), and 9.9 mm (large units), were formulated and analyzed for their different properties. It was found that floating dosage units remained buoyant regardless of their sizes on the gastric contents 3066
3 Srujana G. L.V et al / J Global Trends Pharm Sci, 2016; 7(2): throughout their residence in the gastrointestinal tract, while the non floating dosage units sank and remained in the lower part of the stomach. Floating units away from the gastroduodenal junction were protected from the peristaltic waves during digestive phase while the non floating forms stayed close to the pylorus and were subjected to propelling and retropelling waves of the digestive phase. Density of Tablets :Density is the main factor affecting the gastric residence time of dosage form. A buoyant dosage form having a density less than that of the gastric fluids floats, since it is away from the pyloric sphincter, the dosage unit is retained in the stomach for a prolonged period. A density of less than 0.1mg/ml i.e. less than that of gastric contents has been reported. However, the floating force kinetics of such dosage form has shown that the bulk density of a dosage form is not the most appropriate parameter for describing its buoyancy capabilities. Shape of tablets: The shape of dosage form is one of the factors that affect its gastric residence time. Six shapes (ring tetrahedron, cloverleaf, string, pellet, and disk) were screened in-vivo for their gastric retention potential. The tetrahedron (each leg 2cm long) rings (3.6 cm in diameter) exhibited nearly 100% retention at 24 hr. Viscosity grade of polymer: Drug release and floating properties of FDDS are greatly affected by viscosity of polymers and their interaction. Low viscosity polymers (e.g., HPMC K100 LV) were found to be more beneficial than high viscosity polymers (e.g., HPMC K4M) in improving floating properties. In addition, a decrease in the release rate was observed with an increase in polymer viscosity. b) Idiosyncratic factors Gender: Women have slower gastric emptying time than do men. Mean ambulatory GRT in meals (3.4±0.4 hours) is less compared with their age and race-matched female counterparts (4.6± 1.2 hours), regardless of the weight, height and body surface. Age : Low gastric emptying time is observed in elderly than do in younger subjects. Intra subject and inter subject variations also are observed in gastric and intestinal transit time. Elderly people, especially those over 70 years have a significantly longer GRT. Posture: i) Upright position : An upright position protects floating forms against postprandial emptying because the floating form remains above the gastric contents irrespective of its size l4. Floating dosage forms show prolonged and more reproducible GRTs while the conventional dosage form sink to the lower part of the distal stomach from where they are expelled through the pylorus by antral peristaltic movements. ii) Supine position : This position offers no reliable protection against early and erratic emptying. In supine subjects large dosage forms (both conventional and floating) experience prolonged retention. The gastric retention of floating forms appear to remain buoyant anywhere between the lesser and greater curvature of the stomach. On moving distally, these units may be swept away by the peristaltic movements that propel the gastric contents towards the pylorus, leading to significant reduction in ORT compared with upright subjects. Concomitant intake of drugs: Drugs such as prokinetic agents (e.g.. metoclopramide and cisapride). Anti Cholinergics (e.g., atropine or propantheline), opiates (e.g., codeine) may affect the performance of FDDS. The coadministration of G.I motility decreasing drugs can increase gastric emptying time. Feeding regimen : Gastric residence time increases in the presence of food, leading to increased drug dissolution of the dosage form at the most favorable site of absorption. A GRT of 4-10 hrs has been reported after a meal of fats and proteins. Absorption window: The G.I. tract offers a varied environment capable of affecting the absorption of per orally administered drugs. Anatomical features, physiological phenomenon, and nature of gastrointestinal milieu contribute these changes. This can lead to the variations in intestinal permeability of drug molecules, resulting in the phenomenon of absorption window as shown fig. no. 1, where in the drug is preferentially absorbed only from a particular region of the G.I. tract. Physicochemical and/or physiological factors are responsible for the formation of absorption window for certain classes of drugs. Various Factors for absorption window: Physico-chemical factors: 3067
4 ph-dependent solubility and stability: A drug experiences a Ph range of 1-8 across the G.I. tract, and needs to be in solubilised and stable form to successfully cross the biological membrane. Most of the drugs are passively absorbed, in their un-ionized form and the extent of ionization at different ph values in different regions of G.I tract can significantly alter the absorption profile. ph dependent solubility, stability and ionization by changing the physical properties of the drug in different portions of the G.I. tract, can lead to regional variability in absorption of drugs. Physiological factors: (a). Mechanism of absorption: Per orally administered drugs are absorbed both by passive diffusion as well as by non-passive means of absorption. Drugs absorbed by active and facilitated transport mechanisms show higher regional specificity due to the prevalence of these mechanisms only in a particular region of G.I. tract. (b). Metabolic enzymes: Presence of certain enzymes in a particular region of G.I. tract can also lead to regional variability in absorption of drugs that are substrates to those enzymes; Intestinal metabolic enzymes principally, phase one metabolizes like cytochrome P-450 are abundantly present in the intestinal epithelium. Their activity decreases longitudinally along the small intestine, with the levels rising slightly from the duodenum to the jejunum and then declining in the ileum and colon. This nonuniform distribution of cytochrome P-450 causes regional variability in the absorption of drugs that are substrate to these enzymes. Gastric Retention System: Gastric Retention System is a device, which resides in the confines of the stomach over a prolonged period of time (prolonging the residence time) for the purpose of Providing.a platform for controlled release of biologically active agents. The system releases the active agent to be absorbed or released from the stomach to be absorbed in the Upper parts of the small intestine. In particular it allows for less frequent dosing of active agent than with immediate release formulations or sustained release formulations that are not gastric retention dosage forms. In other applications the frequency of dosing may be the same, but the gastric retention dosage forms will beneficially alter the absorption profile of the active agent from that available with immediate release formulations. This may result in increased bioavailability of the active agent with reduced side effects. Over the last three decades, various approaches have been pursued to prolong the residence time of an oral dosage form in the stomach. These methods include floating systems, Swelling and expanding systems, Bio adhesive systems, Modified-shape system, Highdensity systems, Other gastric emptying devices. Floating Drug Delivery Systems or Hydrodynamically Balanced Systems (HBS): These systems have a bulk density lower than gastric fluids and thus remain buoyant in the stomach without affecting the gastric emptying rate for a prolonged period of time. While the system is floating on the gastric contents, the drug is released slowly at a desired rate from the system. After the release of drug, the residual system is emptied from the stomach. This results in an increase in the gastric retention lime and a better control of fluctuations in plasma drug concentrations. HBS system containing a homogeneous mixture of drug and the hydrocolloid in a capsule, which upon contact with gastric fluid acquires and maintains a bulk density of less than I and there by being buoyant on the gastric contents of stomach until the entire drug was released. Hydro dynamically balanced sustained release tablets containing drug and hydrophilic hydrocolloids, which on contact with gastric fluids at body temperature forms a soft waterimpermeable colloid gel barrier on their surface. The drug is slowly released from the surface of the gelatinous mass that remained buoyant on gastric fluids. Hydro dynamically balanced systems are designed to prolong the stay of the dosage form in the gastro intestinal tract and aid in enhancing the absorption. Such systems are best suited for drugs having a better solubility in acidic environment and also for the drugs having specific site of absorption in the upper part of the small intestine. It should stay in the stomach, maintain its structural integrity, and release drug constantly from the dosage form. The success of FIBS capsule as a better system is best exemplified with chlordiazeopoxide hydrochloride. The drug is a classical example of a solubility problem where in it exhibits a 4000 fold difference in solubility going from pᴴ 3 to 6 (the solubility of chlordiazepoxide hydrochloride is 150 mg/ml and is mg/ml at neutral ph). Swelling and Expanding Systems: One way to retain a dosage form in the stomach is by increasing its size. The stomach discharges its content through the pylorus into intestine. If the dosage form can attain a size larger than that of the pylorus, it can be retained in the Stomach for a long time. Swelling type dosage forms as shown in 3068
5 Srujana G. L.V et al / J Global Trends Pharm Sci, 2016; 7(2): fig. nol.4 are such Systems that after swallowing swell to an extent that prevents their exit from the stomach trough the pylorus. As a result, the dosage form is retained in the stomach for a long period of time. These systems may be referred to as plug type systems since they exhibit a tendency to remain lodged at the pyloric sphincter. Bio adhesive Systems: Bio-adhesive systems are used to localize a delivery device within the lumen and cavity of the body to enhance the drug absorption process in a site specific manner. This approach involves the use of bioadhesive polymers that can adhere to the epithelial surface of the G.I, tract. The proposed mechanism of bioadhesion is the formation of hydrogen and electrostatic bonding at the mucus-polymer boundary. Rapid hydration in contact with the muco-epithelial surface appears to favour adhesion, particularly if water can be excluded at the reactive surfaces. These bioadhesive systems do not seem to be a very feasible solution as this bond formation is prevented by the acidic environment and thick mucus present in the stomach. High turnover of mucus adds to the difficulties in retaining a hioadhesive system at the site. The commonly used mucoadhesive polymers are carboxymethyl cellulose. carhopol. polycarbophil, tragacanth, sodium alginate, gelatin and pectin etc. Modified Shape Systems: Modified-shape systems are non-disintegrating geometric shapes molded from silastic elastomer or extruded from polyethylene blends, which extend the gastric retention time depending on size, shape and flexural modulus of the drug delivery device. High Density System: High-density formulations include coated pellets, which have a density greater than that of the stomach contents (~l,004 g/cm3). This is accomplished by coating the drug with a heavy inert material such as barium sulfate, zinc oxide, titanium dioxide, N iron powder etc. Other delayed gastric emptying approaches of interest include sham feeding of indigestible polymers or fatty acid salts that change the motility pattern of the stomach to a fed state, thereby decreasing the gastric emptying rate and permitting considerable prolongation of drug release. Of these abovementioned approaches, floating drug delivery or hydro dynamically balanced drug delivery systems are given much importance, because of their ease of preparation and reliable and reproducible gastric retentive action. Gastric Floating Drug Delivery Systems (GFDDS): The various buoyant preparations include tablets, pills, granules, powders, capsules, hollow microspheres (micro balloons) and laminated films. Based on the mechanism of buoyancy, two distinctly different technologies i.e., non effervescent and effervescent systems have been utilized in the development of GFDDS. Non Effervescent GFDDS:The approach involved in the formulation of these floating dosage fhrrns is intimate mixing of drug with a gelforming hydrocolloid, which swells in con act with gastric fluid after oral administration and maintains a relative integrity of shape and a bulk density of less than unity within the outer gelatinous barrier. The air trapped by the swollen polymer confers buoyancy to these dosage forms. In addition, the gel structure acts as a reservoir for sustained drug release since the drug is slowly released by a controlled diffusion through the gelatinous barrier. The most commonly used excipients in this type of GFDDS are gel forming or highly sellable cellulose type hydrocolloids, polysaccharides and matrix forming polymers such as polycarbonate. polyacrylate, polymethacrylate and polystyrene. Effervescent GFDDS: These floating drug delivery systems utilize matrices prepared with swellable polymers such as methocel or polysaccharides and effervescent components or matrices containing chambers of liquid that gasify at body temperature. The matrices are fabricated so that upon arrival in the stomach, carbon dioxide is liberated by the acidity of gastric contents and is entrapped in the gellified hydrocolloid. This produces an upward motion of the dosage form and maintains its buoyancy. A decrease in the specific gravity causes the dosage form to float on the gastric fluids. The carbon dioxide generating components may be intimately mixed within the tablet matrix, in which case a single-layered tablet is produced or a bilayered tablet may be compressed which contains the gas generating mechanism in one hydrocolloid containing layer and the drug in the other layer formulated for a sustained release effect. This concept has also been exploited for floating capsule systems as shown in fig. no1.5 Development and evaluation of GFDD Formulation development: For the optimum design of a GFDDS, the key step is to understand the principles of G.I. dynamics such as gastric emptying, small intestinal transit, colonic transit43, etc. Acquiring knowledge about the rate and extent of drug absorption from different sites of G.I. tract, and factors that can alter or limit the absorption further aid in designing the type of dosage form that is needed for a particular drug. For instance, with drugs such as sulpiride, furosemide, theophylline and albuterol, which are predominantly absorbed from the upper pan of the G.I. tract, designing a gastric retention dosage form 3069
6 is a logical strategy for improving and extending their limited oral bioavailability. For the formulation of a hydro dynamically balanced dosage form, three major conditions must be met: (i) It must have sufficient structure to form a cohesive gel barrier; (ii) It must maintain an overall density lower than that of gastric contents (reported as g/ cc) & (iii) It should dissolve slowly, enough to serve as a reservoir for the delivery system. The task of designing a dosage form to achieve a consistent and controlled residence in the stomach begins with selection of potential excipients that allow the formulation of matrices having sustained delivery characteristics and a bulk density of less than unity. As far as the ideal floating dosage form is concerned, it should have high buoyancy, adequate mechanical strength, excellent acid resistance and a high drug releasing capacity in the stomach. Ideally water-soluble cellulose derivatives are best suited for such purposes. In Vitro and In vivo evaluation: The various parameters that need to be evaluated for their effects on gastric retention time of buoyant formulations can mainly be categorized into following different classes. * Galenic parameters: diametral size ( cut-off size ), flexibility and density of matrices. *Control parameters: floating time, dissolution, content uniformity, hardness and &inability. *Geometric parameters: shape. *Physiological parameters: age. Sex, posture. Food and bio adhesion. The test for buoyancy and in vivo drug release studies are usually carried out in simulated gastric fluid maintained at 37 C. The in vivo gastric retentivity of a floating dosage form is usually determined by y-scintigraphy or roentgenography. Studies are done both under fasted and fed conditions using floating and non floating (control) dosage forms. It is also important that both dosage forms are non-disintegrating units, and human subjects are young and healthy. Advantages of GFDDS: Sustained drug delivery: Sustained drug absorption from oral controlled release dosage forms is often limited due to short gastric retention time. However, GFDDS remain in the stomach for more hours due to their increased GRT. It has been suggested that due to their low density than the gastric contents and relatively large size they do not pass through the pylorus that has an opening of approximately cm53. It has been observed that major portion of drug releases in the colon rather than stomach in case of modified release capsule. However, prolongation in the GRT may sustain the drug-release behavior. Site specific drug delivery: Drugs having absorption sites in the upper small intestine like furosemide and riboflavin are typically formulated in floating dosage form. It has been reported that absorption of furosemide takes place mainly through stomach followed by duodenum55. This characteristics of furosemide prompted scientists to develop a monolithic floating system, which could prolong the ORT and thereby increase the bioavailability. A bilayer floating capsule has been developed for local delivery of misoprostol to the gastric mucosa for prevention of gastric ulcers caused by non-steroidal anti-inflammatory drugs (NSAID s). Mechanistically, the drug replenishes the GI-protective prostaglandins that are depleted by NSAID s. Therefore, sustained and controlled delivery of misoprostol to the stomach provides sufficient local therapeutic levels vis-a-vis exposure to the drug. This in turn reduces the side effects caused by the presence of the drug in systemic circulation (uterotonic activity) and also retards diarrhea, which is result of combination of intestinal and systemic exposure of drug. Moreover, the prolonged gastric availability of the misoprostol from FDDS also reduces the dosing frequency 5- fluorouracil bearing floating tablets have been successfully evaluated in four patients with stomach neoplasms. Limitations: 1. The major disadvantage of floating systems is requirement of a sufficiently high level of fluids in the stomach for the drug delivery. However, this limitation can be overcome by coating the dosage form with the help of bio-adhesive polymers that easily adhere to the mucosal lining of the stomach. 2. The dosage form should be administered with a minimum of glass full of water ( ml). 3. Floating system is not feasible for those drugs that have solubility or stability problems in gastric fluids. 4. The drugs, which are absorbed throughout gastrointestinal tract. which undergo significant first pass metabolism, are not desirable candidates. 5. Some drugs present in the floating system causes irritation to gastric mucosa. 3070
7 Srujana G. L.V et al / J Global Trends Pharm Sci, 2016; 7(2): Gastro retentive Drug Delivery System (GRDDS) Floating System Non- Floating System Bio adhesive Swelling High density Expandable Effervescent System Non- Effervescent System Hydro Dynamically Balanced System Micro balloon Voltage liquid Matrix Gas generating Containing Tablets System Floating Capsules Intra gastric floating GRDDS Pills, Ion exchange Resins Alginate Beads Inflatable gastrointestinal DDS Layered Tablets Intra gastric osmotic ally DDS Single layer Tablets Bi-Layer tablets Ideal Properties of GRDDS Effective Retention in The stomach Sufficient Drug Loading capacity Controlled Drug release profile Full degradation & evacuation after drug release No effect on gastric motility including emptying pattern No other local effects S.no Dosage Form Examples of Drugs 1 Microspheres Aspirin, Grisiofulvin, Pnitroanilline, Ibuprofen, Terfinadine, Tranilast 2 Granules Diclofenac sodium, Indomethacin, Prednisolone 3 Films Cinnarizine 4 Powders Several basic drugs 5 Capsules Chlordiazepoxide HCl, Diazepam, Furosemide, 1-dopa, Benserazide,Misoprostol, Propranolol HCl, Ursodeoxycholic acid 6 Tablets/pills Acetaminophen, Acetyl salicylic acid, Amoxicillin, Trihydrate, mpicillin, Atenolol, Isosorbide 3071
8 REFERENCES: 1. Vishal Bhardwaj, Effervescent Floating Drug Delivery System: A Review Pharmacophore, Pharmacophore,2013,Vol. 4 (1), Akhlak Ahmad, Narendra Kr. Floating Drug Delivery System: An Overview, Global Journal of pharmacology 8 (4): , Anupama Sarawade, Floating Drug Delivery System: A better approach, Research and Development in Pharmacy and Life Sciences, http// ISSN: Abhishek Chandel, Chandel et al,gastro Retentive Drug Delivery System, International current Pharmaceutical journal 2012, 1(5): A.Badoni, Stomach Specific Floating Drug Delivery System: A Review The Pharma Innovativation, ISSN: , CODEN CODE:PIHNBQ, ZDB NO: , IC Journal No: International Journal of Pharm Tech Research,, Coden(usa):ijprif, issn: , vol.1, No.3, pp Manju Maria Mathew, Review Article on Floating Drug Delivery System, International Journal of Pharmaceutical and Chemical Sciences, ISSN: Deepak Sharma et al, Review on Multi particular Floating Drug Delivery System, International Journal of Pharmaceutical and Chemical and Biological Sciences, ISSN: , 9. Srujana Katta, Overview On Floating Drug Delivery System American Journal of Pharmtech Research, ISSN: , Chaturvedi Shashank, Approches to Increase the Gastric Residence Time : Floating Drug Delivery System- AReview, Asian Journal of Pharmaceutical and Clinical Research, ISSN: Azhar Danish Khan, Floating Drug Delivery System: An Overview, International Journal of Pharm Tech Research, ISSN: , CODEN (USA): IJPRIF, 12. Neha Narang, An Updated Review On : Floating Drug Delivery System (FDDS), International Journal of Applied Pharmaceutics, Vol 3, issue1, 2011, ISSN : , 13. Mandeep Sharma, Review Of Floating Drug Delivery System,Asian Journal of Biological nd Pharmaceutical Sciences, 3(24) 2013,1-6, ISSN : X. 14. Ayarivan Puratchikody, Enhancement of Drug Bio-availability by Floating Drug Delivery System A Review,International Journal of Drug Delivery 3(2011) , ISSN: , ndex 15. Shukla Shruti, A Review On : Recent Advancement of Stomach Specific Floating Drug Delivery System,International Journal of Pharmaceutical and Biological Archives 2011:2(6): , ISSN: , 16. Kadam Shashikant M et al / IJRAP 2011, 2 (6) , ISSN: ,, Review On Floating Drug Delivery System : An Approch to Oral Controlled Drug Delivery Via Gastric Retention, Bharkatiya et al.,, Floating Drug Delivery System : A Review Journal of Drug Delivery & Therapeutics; 2014,4(2), , ISSN: , 18. Rizwana Khan, Gastro retentive Drug Delivery System : A Review,International Journal of Pharma and Bio Sciences, ISSN: , Shweta Arora, Floating Drug Delivery System : A Review,AAPS PharmSci- Tech 2005; 6(3) 20. A.Arunachalam, Floating Drug Delivery System : A Review, ISSN: , Hetangi Rathod, Floating Drug Delivery System : Innovative Approch of Gastro Retention, volume-4, Issue 3, ISSN X, Pharmar et al, Floating Drug Delivery System : A Novel Approch to Prolong Gastric Retention, Paresh D. Parmar, World Journal of Pharmacy and Pharmaceutical Sciences, Volume 3, Issue 4, , ISSN: , Purnima Tripathi, Floating Drug Delivery System, International Journal of Research and Development in Pharmacy and Life Sciences,
9 Srujana G. L.V et al / J Global Trends Pharm Sci, 2016; 7(2): Pooja Gupta, Floating Drug Delivery System : A Review International Journal of Pharma Research and Review, ISSN : Bhalla Neetika, Floating Drug Delivery System, International Journal of Pharmaceutical Research & Allied Sciences, ISSN: AGeetha, Review on Floating Drug Delivery System, A International Journal of Pharmaceutical Research & Biomedical Analysis, ISSN: , 27. S.Gopalakrisknan et al, Review on Floating Drug Delivery System, Journal of Pharmaceutical Science and Technology vol 3(2), Floating Drug Delivery System : A Review ISSN: , 28. Natasha Sharma,, A Comprehensive Review on Floating Drug Delivery System International Journal of Research in Pharmaceutical and Biomedical Sciences, ISSN Ajay Vijayakumar, Review on Floating Drug Delivery System, International Journal of Pharmacy and Pharmaceutical Sciences, ISSN: P.S.Dongare, Review on Floating Drug Delivery System,International Journal of Pharmacy and Biomedical Sciences, Raghunath Guptha, Dwarakanadha Reddy,Vijayaratna, Purusthoman, Gastrao retentive drug delivery systems, Journal of Pharmacy and Chemistry, How to cite this article: G.L.V.Srujana*, M.Prasada Rao, Ramakotaiah.Mogili, Y.Sridevi, M.Usha, P.Vijaya raju, R.Soundarya, Gastro retentive drug delivery system (GRDDS) - A Review, 7 (2): (2016) All 2010 are reserved by Journal of Global Trends in Pharmaceutical Sciences. 3073
Gastro Retentive Drug Delivery System
 Review Article *Hemali Soni 1, V. A. Patel 2 1 Department of Pharmaceutics, A. R. College of Pharmacy, V. V. Nagar, Gujarat, India. 2 Associate professor, Department of Pharmaceutics, A. R. College of
Review Article *Hemali Soni 1, V. A. Patel 2 1 Department of Pharmaceutics, A. R. College of Pharmacy, V. V. Nagar, Gujarat, India. 2 Associate professor, Department of Pharmaceutics, A. R. College of
Int. J. Pharm. Sci. Rev. Res., 27(2), July August 2014; Article No. 15, Pages: Review on Gastro Retentive Drug Delivery System (GRDDS)
 Review Article Review on Gastro Retentive Drug Delivery System (GRDDS) Sachin A. Nitave*, Vishin A. Patil, Amrita A. Kagalkar Anil Alias Pintu Magdum Memorial Pharmacy College, Dharangutti, Shirol, Kolhapur,
Review Article Review on Gastro Retentive Drug Delivery System (GRDDS) Sachin A. Nitave*, Vishin A. Patil, Amrita A. Kagalkar Anil Alias Pintu Magdum Memorial Pharmacy College, Dharangutti, Shirol, Kolhapur,
SHRI JAGDISHPRASAD JHABARMAL TIBREWALA UNIVERSITY 1
 INTRODUCTION Oral delivery of drugs is by far the most preferred route of drug delivery due to ease of administration, patient compliance and flexibility in formulation 1. Conventional oral dosage forms
INTRODUCTION Oral delivery of drugs is by far the most preferred route of drug delivery due to ease of administration, patient compliance and flexibility in formulation 1. Conventional oral dosage forms
CHAPTER 1 INTRODUCTION
 1 CHAPTER 1 INTRODUCTION 1.1 ORAL SUSTAINED RELEASE DRUG DELIVERY SYSTEMS The oral route is the most promising and convenient route of drug administration. Conventional immediate release system achieves
1 CHAPTER 1 INTRODUCTION 1.1 ORAL SUSTAINED RELEASE DRUG DELIVERY SYSTEMS The oral route is the most promising and convenient route of drug administration. Conventional immediate release system achieves
DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE
 1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
1. Introduction: DESIGN AND DEVELOPMENT OF COLON TARGETED DRUG DELIVERY SYSTEM OF 5 FLUORURACIL & METRONIDAZOLE Oral controlled - release formulations for the small intestine and colon have received considerable
DISSOLUTION RATE LIMITED ABSORPTION:
 INTRODUCTION Oral drug delivery is the most widely utilized route of administration among all the routes that have been explored for systemic delivery of drugs via pharmaceutical products of different
INTRODUCTION Oral drug delivery is the most widely utilized route of administration among all the routes that have been explored for systemic delivery of drugs via pharmaceutical products of different
1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small
 Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
MODIFIED DISSOLUTION APPARATUS FOR FLOATING DRUG DELIVERY SYSTEM: A REVIEW
 MODIFIED DISSOLUTION APPARATUS FOR FLOATING DRUG DELIVERY SYSTEM: A REVIEW * Patel N. 1, Raiyani D. 1, Patel A. 1, Patel N. 1, Jain H. 1 and Upadhyay U. 1 Department of Pharmaceutics, Sigma Institute of
MODIFIED DISSOLUTION APPARATUS FOR FLOATING DRUG DELIVERY SYSTEM: A REVIEW * Patel N. 1, Raiyani D. 1, Patel A. 1, Patel N. 1, Jain H. 1 and Upadhyay U. 1 Department of Pharmaceutics, Sigma Institute of
ph Dependent Drug Delivery System: Review
 ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
ph Dependent Drug Delivery System: Review Korake.S.P. SVERI s College of Pharmacy (Poly.), Pandharpur The ph-dependent CTDDS exploit the generally accepted view that ph of the human GIT increases progressively
INTERNATIONAL JOURNAL OF PHARMACEUTICAL RESEARCH AND BIO-SCIENCE
 INTERNATIONAL JOURNAL OF PHARMACEUTICAL RESEARCH AND BIO-SCIENCE FLOATING DRUG DELIVERY SYSTEM AS A NOVEL VITAL CONCEPT BABITA SHARMA, NITAN BHARTI GUPTA Sri Sai College of Pharmacy, Pathankot, India.
INTERNATIONAL JOURNAL OF PHARMACEUTICAL RESEARCH AND BIO-SCIENCE FLOATING DRUG DELIVERY SYSTEM AS A NOVEL VITAL CONCEPT BABITA SHARMA, NITAN BHARTI GUPTA Sri Sai College of Pharmacy, Pathankot, India.
CONTROLLED-RELEASE & SUSTAINED-RELEASE DOSAGE FORMS. Pharmaceutical Manufacturing-4
 CONTROLLED-RELEASE & SUSTAINED-RELEASE DOSAGE FORMS Pharmaceutical Manufacturing-4 The improvement in drug therapy is a consequence of not only the development of new chemical entities but also the combination
CONTROLLED-RELEASE & SUSTAINED-RELEASE DOSAGE FORMS Pharmaceutical Manufacturing-4 The improvement in drug therapy is a consequence of not only the development of new chemical entities but also the combination
FLOATING DRUG DELIVERY SYSTEM (FDDS): A REVIEW
 25 P a g e International Standard Serial Number (ISSN): 2319-8141 International Journal of Universal Pharmacy and Bio Sciences 5(1): January-February 2016 INTERNATIONAL JOURNAL OF UNIVERSAL PHARMACY AND
25 P a g e International Standard Serial Number (ISSN): 2319-8141 International Journal of Universal Pharmacy and Bio Sciences 5(1): January-February 2016 INTERNATIONAL JOURNAL OF UNIVERSAL PHARMACY AND
EVALUATION OF EFFERVESCENT FLOATING TABLETS. 6.7 Mathematical model fitting of obtained drug release data
 EVALUATION OF EFFERVESCENT FLOATING TABLETS 6.1 Technological characteristics of floating tablets 6.2 Fourier transform infrared spectroscopy (FT-IR) 6.3 Differential scanning calorimetry (DSC) 6.4 In
EVALUATION OF EFFERVESCENT FLOATING TABLETS 6.1 Technological characteristics of floating tablets 6.2 Fourier transform infrared spectroscopy (FT-IR) 6.3 Differential scanning calorimetry (DSC) 6.4 In
International Journal of Medicine and Pharmaceutical Research
 International Journal of Medicine and Pharmaceutical Research Journal Home Page: www.pharmaresearchlibrary.com/ijmpr Research Article Open Access Formulation and Evaluation of Gastroretentive Floating
International Journal of Medicine and Pharmaceutical Research Journal Home Page: www.pharmaresearchlibrary.com/ijmpr Research Article Open Access Formulation and Evaluation of Gastroretentive Floating
Define the terms biopharmaceutics and bioavailability.
 Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Pharmaceutics Reading Notes Define the terms biopharmaceutics and bioavailability. Biopharmaceutics: the area of study concerning the relationship between the physical, chemical, and biological sciences
Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p.
 Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p. 3 Dynamic behaviour of membranes p. 4 Modulation of membrane fluidity
Cell Membranes, Epithelial Barriers and Drug Absorption p. 1 Introduction p. 2 The Plasma Membrane p. 2 The phospholipid bilayer p. 3 Dynamic behaviour of membranes p. 4 Modulation of membrane fluidity
Available Online through Research Article
 ISSN: 0975-766X Available Online through Research Article www.ijptonline.com DESIGN AND EVALUATION OF GASTRORETENTIVE TABLETS FOR CONTROLLED DELIVERY OF NORFLOXOCIN Ganesh Kumar Gudas*, Subal Debnath,
ISSN: 0975-766X Available Online through Research Article www.ijptonline.com DESIGN AND EVALUATION OF GASTRORETENTIVE TABLETS FOR CONTROLLED DELIVERY OF NORFLOXOCIN Ganesh Kumar Gudas*, Subal Debnath,
FLOATING DRUG DELIVERY OF RANITIDINE HYDROCHLORIDE
 Int. J. LifeSc. Bt & Pharm. Res. 2012 Varsha Deva and Manoj Kr Gautam, 2012 Research Paper ISSN 2250-3137 www.ijlbpr.com Vol. 1, No. 3, July 2012 2012 IJLBPR. All Rights Reserved FLOATING DRUG DELIVERY
Int. J. LifeSc. Bt & Pharm. Res. 2012 Varsha Deva and Manoj Kr Gautam, 2012 Research Paper ISSN 2250-3137 www.ijlbpr.com Vol. 1, No. 3, July 2012 2012 IJLBPR. All Rights Reserved FLOATING DRUG DELIVERY
Preparation and Evaluation of Gastro Retentive Floating Tablets of Atorvastatin Calcium
 Preparation and Evaluation of Gastro Retentive Floating Tablets of Atorvastatin Calcium Florida Sharmin 1, Md. Abdullah Al Masum 1, S. M. Ashraful Islam 1 and Md. Selim Reza 2 1 Department of Pharmacy,
Preparation and Evaluation of Gastro Retentive Floating Tablets of Atorvastatin Calcium Florida Sharmin 1, Md. Abdullah Al Masum 1, S. M. Ashraful Islam 1 and Md. Selim Reza 2 1 Department of Pharmacy,
DEVELOPMENT AND IN VITRO EVALUATION OF SUSTAINED RELEASE FLOATING MATRIX TABLETS OF METFORMIN HYDROCHLORIDE
 ISSN: 0975-8232 IJPSR (2010), Vol. 1, Issue 8 (Research article) Received 11 April, 2010; received in revised form 17 June, 2010; accepted 13 July, 2010 DEVELOPMENT AND IN VITRO EVALUATION OF SUSTAINED
ISSN: 0975-8232 IJPSR (2010), Vol. 1, Issue 8 (Research article) Received 11 April, 2010; received in revised form 17 June, 2010; accepted 13 July, 2010 DEVELOPMENT AND IN VITRO EVALUATION OF SUSTAINED
2- Minimum toxic concentration (MTC): The drug concentration needed to just produce a toxic effect.
 BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
What is Nanotechnology?
 Use of Nanotechnology to Optimize Delivery of Probiotics and Prebiotics to Target Sites Ian G Tucker Professor of Pharmaceutical Sciences University of What is Nanotechnology? Some people say they have
Use of Nanotechnology to Optimize Delivery of Probiotics and Prebiotics to Target Sites Ian G Tucker Professor of Pharmaceutical Sciences University of What is Nanotechnology? Some people say they have
Design and Evaluation of Atenolol Floating Drug Delivery System
 Available online on www.ijcpr.com International Journal of Current Pharmaceutical Review and Research, 4(1), 1-26 Research Article ISSN: 976-822X Design and Evaluation of Atenolol Floating Drug Delivery
Available online on www.ijcpr.com International Journal of Current Pharmaceutical Review and Research, 4(1), 1-26 Research Article ISSN: 976-822X Design and Evaluation of Atenolol Floating Drug Delivery
Floating Drug Delivery System Novel Tool for Delivering H 2 Antagonist
 Human Journals Review Article January 2017 Vol.:8, Issue:2 All rights are reserved by Dharmendra Solanki et al. Floating Drug Delivery System Novel Tool for Delivering H 2 Antagonist Keywords: Floating
Human Journals Review Article January 2017 Vol.:8, Issue:2 All rights are reserved by Dharmendra Solanki et al. Floating Drug Delivery System Novel Tool for Delivering H 2 Antagonist Keywords: Floating
FORMULATION AND EVALUATION OF GASTRORETENTIVE FLOATING TABLETS OF FAMOTIDINE
 FORMULATION AND EVALUATION OF GASTRORETENTIVE FLOATING TABLETS OF FAMOTIDINE Chandira. Margret R. *, Sahu Chandra Mohan and Jayakar B. Vinayaka Mission s College of Pharmacy Vinayaka Missions University,
FORMULATION AND EVALUATION OF GASTRORETENTIVE FLOATING TABLETS OF FAMOTIDINE Chandira. Margret R. *, Sahu Chandra Mohan and Jayakar B. Vinayaka Mission s College of Pharmacy Vinayaka Missions University,
Swelling System: A Novel Approach Towards Gastroretentive Drug Delivery System
 Swelling System: A Novel Approach Towards Gastroretentive Drug Delivery System Santosh Shep *, Sham Dodiya, Sandeep Lahoti, Rahul Mayee Dr. Vedprakash Patil Pharmacy College, Aurangabad, India INDO GLOBAL
Swelling System: A Novel Approach Towards Gastroretentive Drug Delivery System Santosh Shep *, Sham Dodiya, Sandeep Lahoti, Rahul Mayee Dr. Vedprakash Patil Pharmacy College, Aurangabad, India INDO GLOBAL
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5
 Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Biopharmaceutics Dosage form factors influencing bioavailability Lec:5 Ali Y Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School of Pharmacy University of Sulaimani 09/01/2019
Chapter 2 Rationale and Objective
 Chapter 2 SPP School of Pharmacy & Technology Management, SVKM s NMIMS, Mumbai 44 2.0 Rationale Need for extended release drug delivery systems Over the past 50 years or so, substantial research in the
Chapter 2 SPP School of Pharmacy & Technology Management, SVKM s NMIMS, Mumbai 44 2.0 Rationale Need for extended release drug delivery systems Over the past 50 years or so, substantial research in the
Scholars Research Library
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2011, 3(1): 121-137 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2011, 3(1): 121-137 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Effect of Polymer Concentration and Viscosity Grade on Atenolol Release from Gastric Floating Drug Delivery Systems
 Effect of Polymer Concentration and Viscosity Grade on Atenolol Release from Gastric Floating Drug Delivery Systems 1 1 1 2 U.V. Bhosale*, V. Kusum Devi, Nimisha Jain and P.V. Swamy 1 Al-Ameen College
Effect of Polymer Concentration and Viscosity Grade on Atenolol Release from Gastric Floating Drug Delivery Systems 1 1 1 2 U.V. Bhosale*, V. Kusum Devi, Nimisha Jain and P.V. Swamy 1 Al-Ameen College
Design and In-vitro Evaluation of Silymarin Bilayer Tablets
 CODEN (USA)-IJPRUR, e-issn: 2348-6465 International Journal of Pharma Research and Health Sciences Available online at www.pharmahealthsciences.net Original Article Design and In-vitro Evaluation of Silymarin
CODEN (USA)-IJPRUR, e-issn: 2348-6465 International Journal of Pharma Research and Health Sciences Available online at www.pharmahealthsciences.net Original Article Design and In-vitro Evaluation of Silymarin
BUCCAL DRUG DELIVERY SYSTEM
 BUCCAL DRUG DELIVERY SYSTEM Mr. Kambagiri Swamy M.Pharm.,(Ph.D) KRISHNA TEJA PHARMACY COLLEGE Introduction Novel drug delivery systems(ndds) are the system where the man searches for now method of entry
BUCCAL DRUG DELIVERY SYSTEM Mr. Kambagiri Swamy M.Pharm.,(Ph.D) KRISHNA TEJA PHARMACY COLLEGE Introduction Novel drug delivery systems(ndds) are the system where the man searches for now method of entry
Volume 8, Issue 2, May June 2011; Article-029
 Review Article A REVIEW ON GASTRORETENTIVE DRUG DELIVERY SYSTEM: AN EMERGING APPROACH TO IMPROVE THE GASTRIC RESIDENCE TIME OF SOLID DOSAGE FORMS Manmohan *, Shukla T P, Mathur A, Upadhyay N, Sharma S
Review Article A REVIEW ON GASTRORETENTIVE DRUG DELIVERY SYSTEM: AN EMERGING APPROACH TO IMPROVE THE GASTRIC RESIDENCE TIME OF SOLID DOSAGE FORMS Manmohan *, Shukla T P, Mathur A, Upadhyay N, Sharma S
OPTIMIZATION OF CONTROLLED RELEASE GASTRORETENTIVE BUOYANT TABLET WITH XANTHAN GUM AND POLYOX WSR 1105
 Digest Journal of Nanomaterials and Biostructures Vol. 9, No. 3, July September 2014, p. 1077-1084 OPTIMIZATION OF CONTROLLED RELEASE GASTRORETENTIVE BUOYANT TABLET WITH XANTHAN GUM AND POLYOX WSR 1105
Digest Journal of Nanomaterials and Biostructures Vol. 9, No. 3, July September 2014, p. 1077-1084 OPTIMIZATION OF CONTROLLED RELEASE GASTRORETENTIVE BUOYANT TABLET WITH XANTHAN GUM AND POLYOX WSR 1105
SUMMARY AND CONCLUSION
 SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
SUMMARY AND CONCLUSION 8 SUMMARY AND CONCLUSIONS In spite of the many challenges faced by researchers while designing an effective, reproducible and stable dosage form, oral dosage forms continued to maintain
D9G : Oro-Mucosal Dosage Forms Development Background Paper
 D9G : Oro-Mucosal Dosage Forms Development Background Paper Introduction This background paper is intended to provide a basic rationale for initial formulation efforts, and define some of the terminology
D9G : Oro-Mucosal Dosage Forms Development Background Paper Introduction This background paper is intended to provide a basic rationale for initial formulation efforts, and define some of the terminology
LIST OF TABLES. Page No. No List of US Patents for FDDS Gastroretentive products available in the market
 LIST OF TABLES Sl. Table Title of the table 1 3.01 List of US Patents for FDDS 27 2 3.02 List of drugs explored for various floating dosage forms 35 3 3.03 Gastroretentive products available in the market
LIST OF TABLES Sl. Table Title of the table 1 3.01 List of US Patents for FDDS 27 2 3.02 List of drugs explored for various floating dosage forms 35 3 3.03 Gastroretentive products available in the market
Formulation and Evaluation of Gastroretentive Dosage form of Ciprofloxacin Hydrochloride.
 Available online on www.ijcpr.com International Journal of Current Pharmaceutical Review and Research, 3(4), 105-109 Research Article ISSN: 0976-822X Formulation and Evaluation of Gastroretentive Dosage
Available online on www.ijcpr.com International Journal of Current Pharmaceutical Review and Research, 3(4), 105-109 Research Article ISSN: 0976-822X Formulation and Evaluation of Gastroretentive Dosage
Gastroretentive Drug Delivery Systems: A Review
 J Chronother Drug Deliv Vol 5 Issue 1 2014: 1-7 Authors Journal of Chronotherapy and Drug Delivery REVIEW ARTICLE Gastroretentive Drug Delivery Systems: A Review Rupa Gupta 1 *, PK Sharma 1, Ashish Arora
J Chronother Drug Deliv Vol 5 Issue 1 2014: 1-7 Authors Journal of Chronotherapy and Drug Delivery REVIEW ARTICLE Gastroretentive Drug Delivery Systems: A Review Rupa Gupta 1 *, PK Sharma 1, Ashish Arora
A Review on Gastroretentive Drug Delivery System
 A Review on Gastroretentive Drug Delivery System Pranav Joshi*, Priyank Patel,Hiren Modi, Dr.M.R. Patel, Dr.K.R.Patel, Dr.N.M.Patel Department of pharmaceutics, Shri B.M.Shah College of Pharmaceutical
A Review on Gastroretentive Drug Delivery System Pranav Joshi*, Priyank Patel,Hiren Modi, Dr.M.R. Patel, Dr.K.R.Patel, Dr.N.M.Patel Department of pharmaceutics, Shri B.M.Shah College of Pharmaceutical
Figure 1.1: (a) Drug release mechanism and (b) Floating drug delivery systems
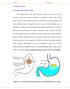 1 1. INTRODUCTION 1.1. Floating drug delivery system Oral administration is the most convenient mode of drug delivery and is associated with superior patient compliance as compared to other modes of drug
1 1. INTRODUCTION 1.1. Floating drug delivery system Oral administration is the most convenient mode of drug delivery and is associated with superior patient compliance as compared to other modes of drug
Suppository Chapter Content
 10 min SUPPOSITORY Suppository Chapter Content 1. Suppositories and Factors Affecting Drug Absorption 2. Ideal Suppository and Different Types of Bases 3. Methods of Suppository Manufacturing Suppository
10 min SUPPOSITORY Suppository Chapter Content 1. Suppositories and Factors Affecting Drug Absorption 2. Ideal Suppository and Different Types of Bases 3. Methods of Suppository Manufacturing Suppository
A REVIEW ON GASTRO RETENTIVE DRUG DELIVERY SYSTEM (GRDDS) WITH SPECIAL EMPHASIS ON FORMULATION AND DEVELOPMENT OF FLOATING MICROSPHERES
 Review Article A REVIEW ON GASTRO RETENTIVE DRUG DELIVERY SYSTEM (GRDDS) WITH SPECIAL EMPHASIS ON FORMULATION AND DEVELOPMENT OF FLOATING MICROSPHERES Kamini Vasava, Rajesh Ks, Lalit Lata Jha * Department
Review Article A REVIEW ON GASTRO RETENTIVE DRUG DELIVERY SYSTEM (GRDDS) WITH SPECIAL EMPHASIS ON FORMULATION AND DEVELOPMENT OF FLOATING MICROSPHERES Kamini Vasava, Rajesh Ks, Lalit Lata Jha * Department
Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, ,
 IDP Biological Systems Gastrointestinal System Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, 504-568-2669, jbresl@lsuhsc.edu Overall Learning Objectives 1. Characterize
IDP Biological Systems Gastrointestinal System Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, 504-568-2669, jbresl@lsuhsc.edu Overall Learning Objectives 1. Characterize
Fundamentals of Pharmacology for Veterinary Technicians Chapter 4
 (A) (B) Figure 4-1 A, B (C) FIGURE 4-1C The active transport process moves particles against the concentration gradient from a region of low concentration to a region of high concentration. Active transport
(A) (B) Figure 4-1 A, B (C) FIGURE 4-1C The active transport process moves particles against the concentration gradient from a region of low concentration to a region of high concentration. Active transport
Drug Absorption in the Gastrointestinal Tract Drugs may be absorbed by passive diffusion from all parts of the alimentary canal including sublingual,
 Drug Absorption in the Gastrointestinal Tract Drugs may be absorbed by passive diffusion from all parts of the alimentary canal including sublingual, buccal, GI, and rectal absorption. For most drugs,
Drug Absorption in the Gastrointestinal Tract Drugs may be absorbed by passive diffusion from all parts of the alimentary canal including sublingual, buccal, GI, and rectal absorption. For most drugs,
Chapter 3 Drug Absorption and Bioavailability
 Chapter 3 Drug Absorption and Bioavailability Debra Si Mui Sim Abstract Most drugs are prescribed as oral preparations or extravascular injections (other than intravenous injections) for the treatment
Chapter 3 Drug Absorption and Bioavailability Debra Si Mui Sim Abstract Most drugs are prescribed as oral preparations or extravascular injections (other than intravenous injections) for the treatment
DESIGN AND EVALUATION OF CONTROLLED RELEASE MATRIX TABLETS OF FLURBIPROFEN
 Int. J. Chem. Sci.: 10(4), 2012, 2199-2208 ISSN 0972-768X www.sadgurupublications.com DESIGN AND EVALUATION OF CONTROLLED RELEASE MATRIX TABLETS OF FLURBIPROFEN K. V. R. N. S. RAMESH *, B. HEMA KIRNAMAYI
Int. J. Chem. Sci.: 10(4), 2012, 2199-2208 ISSN 0972-768X www.sadgurupublications.com DESIGN AND EVALUATION OF CONTROLLED RELEASE MATRIX TABLETS OF FLURBIPROFEN K. V. R. N. S. RAMESH *, B. HEMA KIRNAMAYI
Digestive System 7/15/2015. Outline Digestive System. Digestive System
 Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS
 International Journal of PharmTech Research CODEN( USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 634-638, July-Sept 2009 FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS K. Reeta Vijaya
International Journal of PharmTech Research CODEN( USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 634-638, July-Sept 2009 FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS K. Reeta Vijaya
Conferencia Inaugural
 Conferencia Inaugural UNDERSTANDING GASTRIC DIGESTION TO DEVELOP NEW FOODS FOR HEALTH R. Paul Singh Distinguished Professor of Food Engineering Department of Biological and Agricultural Engineering University
Conferencia Inaugural UNDERSTANDING GASTRIC DIGESTION TO DEVELOP NEW FOODS FOR HEALTH R. Paul Singh Distinguished Professor of Food Engineering Department of Biological and Agricultural Engineering University
Topic 6: Human Physiology
 Topic 6: Human Physiology 6.1 Digestion and Absorption D.1 Human Nutrition D.2 Digestion Essential Understandings: The structure of the digestive system allows it to move, digest, and absorb food. A balanced
Topic 6: Human Physiology 6.1 Digestion and Absorption D.1 Human Nutrition D.2 Digestion Essential Understandings: The structure of the digestive system allows it to move, digest, and absorb food. A balanced
Digestive System Module 4: The Stomach *
 OpenStax-CNX module: m49286 1 Digestive System Module 4: The * Donna Browne Based on The by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0
OpenStax-CNX module: m49286 1 Digestive System Module 4: The * Donna Browne Based on The by OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0
Includes mouth, pharynx, esophagus, stomach, small intestine, large intestine, rectum, anus. Salivary glands, liver, gallbladder, pancreas
 Chapter 14 The Digestive System and Nutrition Digestive System Brings Nutrients Into the Body The digestive system includes Gastrointestinal (GI) tract (hollow tube) Lumen: space within this tube Includes
Chapter 14 The Digestive System and Nutrition Digestive System Brings Nutrients Into the Body The digestive system includes Gastrointestinal (GI) tract (hollow tube) Lumen: space within this tube Includes
FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels
 Seite 1 von 8 Share this story: Issue: April 2015, Posted Date: 3/30/2015 FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels INTRODUCTION Soft gelatin capsules (softgels) continue
Seite 1 von 8 Share this story: Issue: April 2015, Posted Date: 3/30/2015 FORMULATION DEVELOPMENT - A QbD Approach to Develop Extended Release Softgels INTRODUCTION Soft gelatin capsules (softgels) continue
This PDF is available for free download
 Research Paper Formulation Strategy for Low Absorption Window Antihypertensive Agent M. J. QURESHI, J. ALI*, ALKA AHUJA AND SANJULA BABOOTA Department of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard,
Research Paper Formulation Strategy for Low Absorption Window Antihypertensive Agent M. J. QURESHI, J. ALI*, ALKA AHUJA AND SANJULA BABOOTA Department of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard,
DESIGN AND CHARACTERIZATION OF FLOATING TABLETS OF ANTI-DIABETIC DRUG
 INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article DESIGN AND CHARACTERIZATION OF FLOATING TABLETS OF ANTI-DIABETIC DRUG M Seth *, DS Goswami,
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article DESIGN AND CHARACTERIZATION OF FLOATING TABLETS OF ANTI-DIABETIC DRUG M Seth *, DS Goswami,
A review on Gastroretentive Drug Delivery Systems
 29 A review on Gastroretentive Drug Delivery Systems Hemendrasinh J Rathod*, Dhruti P. Mehta, Jitendra Singh Yadav Department of Pharmaceutics, Vidyabharti Trust College of Pharmacy, Umrakh, Gujarat, India.
29 A review on Gastroretentive Drug Delivery Systems Hemendrasinh J Rathod*, Dhruti P. Mehta, Jitendra Singh Yadav Department of Pharmaceutics, Vidyabharti Trust College of Pharmacy, Umrakh, Gujarat, India.
ORGANS OF THE DIGESTIVE SYSTEM
 ORGANS OF THE DIGESTIVE SYSTEM OBJECTIVES: 1. List and describe the major activities of the digestive system. 2. Identify and give the functions of the organs in and along the digestive tract. MAJOR ACTIVITIES
ORGANS OF THE DIGESTIVE SYSTEM OBJECTIVES: 1. List and describe the major activities of the digestive system. 2. Identify and give the functions of the organs in and along the digestive tract. MAJOR ACTIVITIES
Two main groups Alimentary canal continuous coiled hollow tube Accessory digestive organs
 Digestion Breakdown of ingested food Absorption of nutrients into the blood Metabolism Production of cellular energy (ATP) Constructive and degradative cellular activities Two main groups Alimentary canal
Digestion Breakdown of ingested food Absorption of nutrients into the blood Metabolism Production of cellular energy (ATP) Constructive and degradative cellular activities Two main groups Alimentary canal
Topical Preparations
 Topical Preparations One of the functions of the skin is to protect the internal body components against the external environment and thus to control the passage of chemicals into and out of the body.
Topical Preparations One of the functions of the skin is to protect the internal body components against the external environment and thus to control the passage of chemicals into and out of the body.
A NOVEL APPROACH FOR FLOATING DRUG DELIVERY SYSTEM
 ISSN: 2230-7346 Suresh Rewar et al. / JGTPS / 6(1)-(2015) 2417 2422 (Review Article) Journal of Global Trends in Pharmaceutical Sciences Journal home page: www.jgtps.com A NOVEL APPROACH FOR FLOATING DRUG
ISSN: 2230-7346 Suresh Rewar et al. / JGTPS / 6(1)-(2015) 2417 2422 (Review Article) Journal of Global Trends in Pharmaceutical Sciences Journal home page: www.jgtps.com A NOVEL APPROACH FOR FLOATING DRUG
The Digestive System. Prepares food for use by all body cells.
 The Digestive System Prepares food for use by all body cells. Digestion The chemical breakdown of complex biological molecules into their component parts. Lipids to fatty acids Proteins to individual amino
The Digestive System Prepares food for use by all body cells. Digestion The chemical breakdown of complex biological molecules into their component parts. Lipids to fatty acids Proteins to individual amino
Scholars Research Library. Formulation and evaluation of buccoadhesive tablet of Atenolol
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2011, 3(2): 34-38 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2011, 3(2): 34-38 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4
Asian Journal of Biochemical and Pharmaceutical Research
 ISSN: 2231-2560 Research Article Asian Journal of Biochemical and Pharmaceutical Research Design and Evaluation of Gastroretentive Floating Tablets of Dipyridamole: Influence of Formulation Variables A.
ISSN: 2231-2560 Research Article Asian Journal of Biochemical and Pharmaceutical Research Design and Evaluation of Gastroretentive Floating Tablets of Dipyridamole: Influence of Formulation Variables A.
Chapter 20 The Digestive System Exam Study Questions
 Chapter 20 The Digestive System Exam Study Questions 20.1 Overview of GI Processes 1. Describe the functions of digestive system. 2. List and define the four GI Processes: 20.2 Functional Anatomy of the
Chapter 20 The Digestive System Exam Study Questions 20.1 Overview of GI Processes 1. Describe the functions of digestive system. 2. List and define the four GI Processes: 20.2 Functional Anatomy of the
Advantages and Limitations of In Vivo Predictive Dissolution (IPD) Systems
 Advantages and Limitations of In Vivo Predictive Dissolution (IPD) Systems November 16 th Prof. Gregory E. Amidon - University of Michigan What is an In vivo Predictive Dissolution (IPD) System? An IPD
Advantages and Limitations of In Vivo Predictive Dissolution (IPD) Systems November 16 th Prof. Gregory E. Amidon - University of Michigan What is an In vivo Predictive Dissolution (IPD) System? An IPD
An introduction to Liposomal Encapsulation Technology
 An introduction to Liposomal Encapsulation Technology Mother Nature has the innate ability to solve problems through the most efficient and effective route possible. The problem of how to make an oil-soluble
An introduction to Liposomal Encapsulation Technology Mother Nature has the innate ability to solve problems through the most efficient and effective route possible. The problem of how to make an oil-soluble
Formulation and Development of Sustained Release Tablets of Valsartan Sodium
 INTERNATIONAL JOURNAL OF ADVANCES IN PHARMACY, BIOLOGY AND CHEMISTRY Research Article Formulation and Development of Sustained Release Tablets of Valsartan Sodium G. Sandeep * and A. Navya Department of
INTERNATIONAL JOURNAL OF ADVANCES IN PHARMACY, BIOLOGY AND CHEMISTRY Research Article Formulation and Development of Sustained Release Tablets of Valsartan Sodium G. Sandeep * and A. Navya Department of
Small-Bowel and colon Transit. Mahsa Sh.Nezami October 2016
 Small-Bowel and colon Transit Mahsa Sh.Nezami October 2016 Dyspeptic symptoms related to dysmotility originating from the small bowel or colon usually include : Abdominal pain Diarrhea Constipation However,
Small-Bowel and colon Transit Mahsa Sh.Nezami October 2016 Dyspeptic symptoms related to dysmotility originating from the small bowel or colon usually include : Abdominal pain Diarrhea Constipation However,
Indian J.Pharm.Biol.Res. 2018; 6(1):22-29 CODEN (USA): IJPB07 ISSN: Journal homepage:
 Indian J.Pharm.Biol.Res. 2018; 6(1):22-29 CODEN (USA): IJPB07 ISSN: 2320-9267 Indian Journal of Pharmaceutical and Biological Research (IJPBR) Journal homepage: www.ijpbr.in Review Article A REVIEW ON
Indian J.Pharm.Biol.Res. 2018; 6(1):22-29 CODEN (USA): IJPB07 ISSN: 2320-9267 Indian Journal of Pharmaceutical and Biological Research (IJPBR) Journal homepage: www.ijpbr.in Review Article A REVIEW ON
EUDRATEC GRS. Your floating solution for an improved absorption in the stomach.
 EUDRATEC GRS Your floating solution for an improved absorption in the stomach. L+L 16-01-375 Flyer EUDRATEC GRS, 6 Seiten, DIN-A5.indd 02. Dec 2016; 11:44 Uhr EUDRATEC GRS unique gastro-retentive system
EUDRATEC GRS Your floating solution for an improved absorption in the stomach. L+L 16-01-375 Flyer EUDRATEC GRS, 6 Seiten, DIN-A5.indd 02. Dec 2016; 11:44 Uhr EUDRATEC GRS unique gastro-retentive system
Development and evaluation of controlled release mucoadhesive tablets of Tramadol Hydrochloride
 Development and evaluation of controlled release mucoadhesive tablets of Tramadol Hydrochloride R. Margret Chandira*, C.M. Sahu and B. Jayakar Department of Pharmaceutics Vinayaka Mission s College of
Development and evaluation of controlled release mucoadhesive tablets of Tramadol Hydrochloride R. Margret Chandira*, C.M. Sahu and B. Jayakar Department of Pharmaceutics Vinayaka Mission s College of
Advantages and Limitations of In Vivo Predictive Dissolution (IPD) Systems
 Advantages and Limitations of In Vivo Predictive Dissolution (IPD) Systems November 16 th Prof. Gregory E. Amidon - University of Michigan What is an In vivo Predictive Dissolution (IPD) System? An IPD
Advantages and Limitations of In Vivo Predictive Dissolution (IPD) Systems November 16 th Prof. Gregory E. Amidon - University of Michigan What is an In vivo Predictive Dissolution (IPD) System? An IPD
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 211, 3(2):786-791 Development and optimization of colon targeted
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 211, 3(2):786-791 Development and optimization of colon targeted
CHAPTER-I DRUG CHARACTERIZATION & DOSAGE FORMS
 CHAPTER-I DRUG CHARACTERIZATION & DOSAGE FORMS by: j. jayasutha lecturer department of pharmacy practice Srm college of pharmacy srm university DRUG CHARACTERIZATION: Pre-formulation studies will attempt
CHAPTER-I DRUG CHARACTERIZATION & DOSAGE FORMS by: j. jayasutha lecturer department of pharmacy practice Srm college of pharmacy srm university DRUG CHARACTERIZATION: Pre-formulation studies will attempt
The Digestive System
 The Digestive System Key words Pharynx oesophagus stomach intestine epiglottis gall bladder Pancreas peristalsis liver enzyme rectum sphincter Pyloric duodenum jejunum ileum bile lipase Amylase trypsin
The Digestive System Key words Pharynx oesophagus stomach intestine epiglottis gall bladder Pancreas peristalsis liver enzyme rectum sphincter Pyloric duodenum jejunum ileum bile lipase Amylase trypsin
Formulation and Evaluation of Atenolol Floating Tablets Using Different Polymers: Guargum, Sodium Alginate, Hpmc100cps and Carbopol940
 ISSN 0976 3333 Available Online at www.ijpba.info International Journal of Pharmaceutical & Biological Archives 2011; 2(4):1146-1151 ORIGINAL RESEARCH ARTICLE Formulation and Evaluation of Atenolol Floating
ISSN 0976 3333 Available Online at www.ijpba.info International Journal of Pharmaceutical & Biological Archives 2011; 2(4):1146-1151 ORIGINAL RESEARCH ARTICLE Formulation and Evaluation of Atenolol Floating
Ingestion Digestion- Absorption- Elimination
 DIGESTIVE SYSTEM 1 FUNCTIONS Organization GI tract==mouth anus Accessory organs Salivary glands, liver, pancreas, gallbladder Major Functions: Ingestion-mouth, teeth, tongue Digestion- chemical and mechanical
DIGESTIVE SYSTEM 1 FUNCTIONS Organization GI tract==mouth anus Accessory organs Salivary glands, liver, pancreas, gallbladder Major Functions: Ingestion-mouth, teeth, tongue Digestion- chemical and mechanical
Chapter 1 Introduction
 Chapter 1 SPP School of Pharmacy and Technology Management, SVKM s NMIMS, Mumbai 1.0 Extended release dosage form In the past few decades, significant advances have been made in the area of drug delivery
Chapter 1 SPP School of Pharmacy and Technology Management, SVKM s NMIMS, Mumbai 1.0 Extended release dosage form In the past few decades, significant advances have been made in the area of drug delivery
Development of Nutrient Delivery Systems: Ingredients & Challenges
 Development of Nutrient Delivery Systems David Julian McClements and Hang Xiao Department of Food Science University of Massachusetts Development of Nutrient Delivery Systems: Ingredients & Challenges
Development of Nutrient Delivery Systems David Julian McClements and Hang Xiao Department of Food Science University of Massachusetts Development of Nutrient Delivery Systems: Ingredients & Challenges
MODULATION OF GASTROINTESTINAL TRANSIT TIME OF SALBUTAMOL SULPHATE BY FLOATING APPROCHES
 Pooja et al Journal of Drug Delivery & Therapeutics; 213, 3(3), 37-41 37 Available online at http://jddtonline.info RESEARCH ARTICLE MODULATION OF GASTROINTESTINAL TRANSIT TIME OF SALBUTAMOL SULPHATE BY
Pooja et al Journal of Drug Delivery & Therapeutics; 213, 3(3), 37-41 37 Available online at http://jddtonline.info RESEARCH ARTICLE MODULATION OF GASTROINTESTINAL TRANSIT TIME OF SALBUTAMOL SULPHATE BY
KRISHNA TEJA PHARMACY COLLEGE HUMAN ANATOMY AND PHYSIOLOGY. DIGESTIVE SYSTEM Dr.B.Jyothi
 KRISHNA TEJA PHARMACY COLLEGE HUMAN ANATOMY AND PHYSIOLOGY DIGESTIVE SYSTEM Dr.B.Jyothi Prof, Dept. Of Pharmacology KTPC The Digestive System Food undergoes six major processes: 1. Ingestion : process
KRISHNA TEJA PHARMACY COLLEGE HUMAN ANATOMY AND PHYSIOLOGY DIGESTIVE SYSTEM Dr.B.Jyothi Prof, Dept. Of Pharmacology KTPC The Digestive System Food undergoes six major processes: 1. Ingestion : process
Pharmacokinetics I. Dr. M.Mothilal Assistant professor
 Pharmacokinetics I Dr. M.Mothilal Assistant professor DRUG TRANSPORT For a drug to produce a therapeutic effect, it must reach to its target and it must accumulate at that site to reach to the minimum
Pharmacokinetics I Dr. M.Mothilal Assistant professor DRUG TRANSPORT For a drug to produce a therapeutic effect, it must reach to its target and it must accumulate at that site to reach to the minimum
Innovations in Design: NIA-West. Missy Lowery, MSc Head of Integrated Marketing Capsugel, now a Lonza company 11/13/2017
 Innovations in Design: NIA-West Missy Lowery, MSc Head of Integrated Marketing Capsugel, now a Lonza company 11/13/2017 1 Innovations in Design: How Do You Stand Out? If all other competitors are the same
Innovations in Design: NIA-West Missy Lowery, MSc Head of Integrated Marketing Capsugel, now a Lonza company 11/13/2017 1 Innovations in Design: How Do You Stand Out? If all other competitors are the same
Biopharmaceutics. Lec: 4
 64 Biopharmaceutics Physicochemical Properties of Drugs Affecting Bioavailability Lec: 4 1 Assist. Lecturer Ali Yaseen Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School
64 Biopharmaceutics Physicochemical Properties of Drugs Affecting Bioavailability Lec: 4 1 Assist. Lecturer Ali Yaseen Ali BSc Pharmacy MSc Industrial Pharmaceutical Sciences Dept. of Pharmaceutics School
Formulation and evaluation of gastro retentive floating tablets of Terbutaline sulphate
 Formulation and evaluation of gastro retentive floating tablets of Terbutaline sulphate Lokesh Kumar*, Virendra Singh, Rakesh Kumar Meel Department of Pharmaceutics, Goenka College of Pharmacy, NH-11,
Formulation and evaluation of gastro retentive floating tablets of Terbutaline sulphate Lokesh Kumar*, Virendra Singh, Rakesh Kumar Meel Department of Pharmaceutics, Goenka College of Pharmacy, NH-11,
Long-Term Care Updates
 Long-Term Care Updates May 2016 By Katee Miller, PharmD Candidate, Mohamed Jalloh, PharmD, & Zara Risoldi Cochrane, PharmD, MS, FASCP Introduction Enteral nutrition (EN) through a tube is the preferred
Long-Term Care Updates May 2016 By Katee Miller, PharmD Candidate, Mohamed Jalloh, PharmD, & Zara Risoldi Cochrane, PharmD, MS, FASCP Introduction Enteral nutrition (EN) through a tube is the preferred
Asma Karameh. -Shatha Al-Jaberi محمد خطاطبة -
 -2 Asma Karameh -Shatha Al-Jaberi محمد خطاطبة - 1 P a g e Gastrointestinal motilities Chewing: once you introduce the first bolus to the mouth you started what we call chewing reflex appears by muscle
-2 Asma Karameh -Shatha Al-Jaberi محمد خطاطبة - 1 P a g e Gastrointestinal motilities Chewing: once you introduce the first bolus to the mouth you started what we call chewing reflex appears by muscle
FORMULATION AND EVALUATION OF FLOATING TABLETS OF NORFLOXACIN
 FORMULATION AND EVALUATION OF FLOATING TABLETS OF NORFLOXACIN Ms. Jyoti Rathore 1*, Mr. Hitesh Kumar Parmar 1 Ujjain Institute of Pharmaceutical Sciences, Ujjain. Email- hkparmar7@rediffmail.com ABSTRACT
FORMULATION AND EVALUATION OF FLOATING TABLETS OF NORFLOXACIN Ms. Jyoti Rathore 1*, Mr. Hitesh Kumar Parmar 1 Ujjain Institute of Pharmaceutical Sciences, Ujjain. Email- hkparmar7@rediffmail.com ABSTRACT
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY ISSN Review Article
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Review Article AN OVERVIEW ON VARIOUS APPROACHES TO ORAL CONTROLLED DRUG DELIVERY SYSTEM VIA GASTRORETENTIVE DRUG DELIVERY SYSTEM
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Review Article AN OVERVIEW ON VARIOUS APPROACHES TO ORAL CONTROLLED DRUG DELIVERY SYSTEM VIA GASTRORETENTIVE DRUG DELIVERY SYSTEM
Journal of Pharmaceutical and Scientific Innovation Review Article
 Journal of Pharmaceutical and Scientific Innovation www.jpsionline.com Review Article FLOATING DRUG DELIVERY SYSTEM: A NOVEL APPROACH Mahale G. S.* and Derle Nikita D. Department of Pharmaceutics, M.V.P.s
Journal of Pharmaceutical and Scientific Innovation www.jpsionline.com Review Article FLOATING DRUG DELIVERY SYSTEM: A NOVEL APPROACH Mahale G. S.* and Derle Nikita D. Department of Pharmaceutics, M.V.P.s
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY ISSN Research Article
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article FORMULATION AND EVALUATION OF BILAYERED FLOATING TABLETS OF METFORMIN HYDROCHLORIDE R.Margret Chandira*, P.Palanisamy,
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Research Article FORMULATION AND EVALUATION OF BILAYERED FLOATING TABLETS OF METFORMIN HYDROCHLORIDE R.Margret Chandira*, P.Palanisamy,
1 INTRODUCTION. 1.1 Oral Drug Delivery System: 1. Introduction
 1 INTRODUCTION 1.1 Oral Drug Delivery System: The most widely utilized and preferred route of administration amongst all the routes that have been explored till date, is the oral route for the systemic
1 INTRODUCTION 1.1 Oral Drug Delivery System: The most widely utilized and preferred route of administration amongst all the routes that have been explored till date, is the oral route for the systemic
* Produces various chemicals to break. down the food. * Filters out harmful substances * Gets rid of solid wastes
 * * Produces various chemicals to break down the food * Filters out harmful substances * Gets rid of solid wastes * *Mouth *Pharynx *Oesophagus *Stomach *Small and large intestines * *Changes the physical
* * Produces various chemicals to break down the food * Filters out harmful substances * Gets rid of solid wastes * *Mouth *Pharynx *Oesophagus *Stomach *Small and large intestines * *Changes the physical
University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester
 University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester 5/21/2017 Pharmaceutical Compounding, Dr. rer. nat. Rebaz Ali 1 Outlines Why rectal or vaginal route? Suppository
University of Sulaimani School of Pharmacy Dept. of Pharmaceutics Third level - Second semester 5/21/2017 Pharmaceutical Compounding, Dr. rer. nat. Rebaz Ali 1 Outlines Why rectal or vaginal route? Suppository
The Digestive System. Basic process of digestion. Mouth and Teeth 10/30/2016
 The Digestive System Basic process of digestion 1. Ingestion: animal eats food. 2. Digestion: animal body breaks food down. Mechanical digestion: chewing (mastication). Chemical digestion: enzymes and
The Digestive System Basic process of digestion 1. Ingestion: animal eats food. 2. Digestion: animal body breaks food down. Mechanical digestion: chewing (mastication). Chemical digestion: enzymes and
The Relevance of USP Methodology in the Development of a Verapamil Hydrochloride (240 mg) Extended Release Formulation
 METHOCEL Premium Cellulose Ethers Application Data The Relevance of USP Methodology in the Development of a Verapamil Hydrochloride (240 mg) Extended Release Formulation INTRODUCTION Hydrophilic matrices
METHOCEL Premium Cellulose Ethers Application Data The Relevance of USP Methodology in the Development of a Verapamil Hydrochloride (240 mg) Extended Release Formulation INTRODUCTION Hydrophilic matrices
EUROPEAN JOURNAL OF PHARMACEUTICAL AND MEDICAL RESEARCH
 ejpmr, 2015,2(4), 185-203 EUROPEAN JOURNAL OF PHARMACEUTICAL AND MEDICAL RESEARCH www.ejpmr.com Vishvesh et al. SJIF Impact Factor 2.026 Review Article ISSN 3294-3211 EJPMR RAFT FORMING TABLETS: A NOVEL
ejpmr, 2015,2(4), 185-203 EUROPEAN JOURNAL OF PHARMACEUTICAL AND MEDICAL RESEARCH www.ejpmr.com Vishvesh et al. SJIF Impact Factor 2.026 Review Article ISSN 3294-3211 EJPMR RAFT FORMING TABLETS: A NOVEL
Digestive System. - Food is ingested
 11 V. Digestive Processes in the Mouth - Food is ingested - Mechanical digestion begins (chewing) - Salivary amylase begins chemical breakdown of starch - Propulsion is initiated by Deglutition (Swallowing)
11 V. Digestive Processes in the Mouth - Food is ingested - Mechanical digestion begins (chewing) - Salivary amylase begins chemical breakdown of starch - Propulsion is initiated by Deglutition (Swallowing)
