Urgent Field Safety Notice
|
|
|
- Marylou Martin
- 6 years ago
- Views:
Transcription
1 Urgent Field Safety Notice Urgent Field Safety Notice concerning CombiSets containing Medtronic Covidien Devon TM Light Glove according to attachment 1. May 12, 2015 Sender: PAUL HARTMANN AG Paul-Hartmann-Str Heidenheim Recipient: affiliate / authorized distributor Affected product: Complete list as per attachment 1 Description of the problem and Product Advisory Notice: We have been informed by our supplier Medtronic regarding their Covidien Devon Light Gloves about an Urgent Field Safety Notice as per attachment 2 (Urgent Field Safety Notice dated ). These Devon Light Gloves are used as a component in HARTMANN CombiSets. Medtronic informed that affected lots of Covidien Devon Light Gloves could contain splits or holes. Should the user be unaware that the Light Glove is torn/split, a transfer of microorganisms from the light handle into the patient wound is possible when the clinician touches the handle and then onto the sterile field. Surgical site infections can cause morbidity, prolonged hospitalization, and death. They pointed out, that no adverse events have been reported. So far, we have also not received any reports of incidents related to this issue in connection with our CombiSets. The Medtronic Light Gloves can be easily recognized among the kit components by their green colour and shape. The Devon Light Glove must be discarded. All the other CombiSet components are not affected and can be used. Advise on actions: Please take note and confirm receipt of this Urgent Field Safety Notice. We kindly ask you to complete and return attachment 3 Response form 1 Affiliate Reception & Transmission until Friday Users are advised to affix a copy of this advisory notice to the affected products to insure that the information communicated in this notice is followed. During preparation of the surgical procedure at the customer the affected component Devon Light Gloves should be identified and discarded. All the other set components are not affected and can be used. On demand we provide sterile packaged light gloves from an alternative supplier which you can offer to your customers as replacement. Customers should be advised to contact for their order their usual HARTMANN sales representative. For confirmation of closure please complete and return attachment 4 Response form 2 Closure until Friday Your contact for further information is Dr. Werner Casper, telephone: (+49)
2 Transmission of this Urgent Field Safety Notice: This notice needs to be passed on to all those who need to be aware within your organisation or to any organisation where the potentially affected products have been transferred to. Therefore please take adequate measures. A template for a customer letter is enclosed. Customers are requested to confirm receipt of the Urgent Field Safety Notice to the local HARTMANN affiliates / authorised distributor. We do require your written confirmation of implementation and closing of the actions above. This Urgent Field Safety Notice has been submitted to the Competent Authorities of the concerned countries within the European Economic Area (EEA). Heidenheim, May, PAUL HARTMANN AG Michael Brauner Dr. Werner Casper Attachments: 1. List of affected HARTMANN products 2. Urgent Field Safety Notice Medtronic Covidien 3. Response form 1 Affiliate Reception & Transmission Response form 2 Affiliate Closure
3 Anlage 1 / attachment 1: Artikelliste / product list DE REF (Artikelnr.) / REF (articel no.) Handelsname / Trade name LOT Nr. / Batch no CombiSet Bandscheiben-Set CombiSet Wirbelsäulenset CombiSet Gyn-Universal-Set I CombiSet Laparoskopie-Set CombiSet Hüft-Combiset CombiSet Uni-Set CombiSet Schrittmacher-Set CombiSet Sectio-Notfallset CombiSet Sectio-Notfallset CombiSet Standardset für große OP CombiSet Schlitztuch-Set CombiSet Extremitäten-Set CombiSet Standardset für kleine OP CombiSet Steinschnitt-Set für große OP CombiSet Schulterarthroskopie-Set CombiSet Hüft-/Schulter-Set FDS CombiSet Ventil-Set CombiSet CombiSet Sectio-Set
4 CombiSet Spongiosa Set CombiSet Plastisches Chirurgie Set CombiSet Sectio-Set CombiSet Sectio-Set CombiSet Bohrloch-Set CombiSet Rektum Set Frdst CombiSet Universal Set CombiSet TEP-Set CombiSet Bauch-Set CombiSet Bauch-Set CombiSet Extremitäten Set Frdst. CombiSet Schrittmacher-Port Set CombiSet Kreuzband-Set CombiSet SM-Port Set CombiSet OSG Fraktur-Set CombiSet OSG Fraktur-Set CombiSet Hand-Set CombiSet Knie-Arthroskopie-Set, FDS CombiSet Knie-TEP-Set FDS CombiSet Trauma-Set klein CombiSet ME - Set CombiSet Knie-TEP-Set FDS CombiSet Universal-Set Rückenlage MOD CombiSet Hand-Fuß-Set MOD ambulant
5 CombiSet Hand Set CombiSet PFNA+ DHS-Set CombiSet Sectio-Set CombiSet Vaginal-Set CombiSet Varizen-Set Hs. MOD CombiSet Uni-Set CombiSet Uni-Set CombiSet Varizen-Set Bretten CombiSet Sectio-Set CombiSet Spezial-Varizenset CombiSet Laparoskopie-Set CombiSet Varizen-Set Bretten CombiSet Basis-Hand-Tray "neu" CombiSet Basis-Hand-Tray "neu" CombiSet Universal-Set CombiSet Universal-Set CombiSet Basis-Set Diako, Augsburg CombiSet Abdominal-Set Bretten
6 CombiSet Gyn-Abdominal-Set CombiSet LSK-Set Bretten CombiSet Schlitztuch-Set FDS CombiSet LSK-Set Bretten CombiSet LSK-Set Bretten CombiSet Vertikaltuch-Set CombiSet Intertan /Gammanagel->Set CombiSet Hüft-TEP-Set CombiSet Knie-TEP-Set CombiSet Endoskopische Sigmaresektion CombiSet Endoskopische Sigmaresektion CombiSet ZMK-Set klein "neu" CombiSet ZMK-Set klein CombiSet Mamma OP Set CombiSet LAVH -Set Plus MIC-KL. CombiSet Schrittmacher-Set CombiSet Hand-Fuss-Set Diako CombiSet Hüft Set Bad Säckingen CombiSet Sectio- Set CombiSet Pelviskopie Set CombiSet Pelviskopie Set CombiSet vaginale HE Set
7 CombiSet Nieren Prostata Set CombiSet Schulter-Arthroskopie-Set DRK CombiSet Schulter-Arthroskopie-Set DR CombiSet Schulter-Arthroskopie-Set DR CombiSet Ambulanz Basis -Set groß CombiSet Schrittmacher-Port Set CombiSet Schrittmacher-Port Set CombiSet Knie-TEP-Set CombiSet Hand-Set CombiSet MKG-Set oral CombiSet MKG-Set oral CombiSet Mamma-Set CombiSet Mamma-Set CombiSet Knie-TEP-Set CombiSet vaginale HE Set CombiSet Laparotomie Set CombiSet Lap-Set klein
8 CombiSet Knie-TEP-Set CombiSet Knie-TEP-Set CombiSet Hüft-Set CombiSet Hüft-Set CombiSet Hüft-Set CombiSet Hüft-Set CombiSet Hüft-Set CombiSet Wirbelsäulen-Set CombiSet Wirbelsäulen-Set CombiSet Ambulanz Zirkumzisions-Set CombiSet Basis-Schlitztuch Set klein CombiSet Wirbelsäulen-Set CombiSet Schlitztuch Set klein CombiSet Laparoskopie-Set klein CombiSet Intraport ambulant Set CombiSet Intraport-Set CombiSet Hals-Set CombiSet Hals-Set CombiSet Hals-Set CombiSet Nasen-Ohren-Set CombiSet Nasen-Ohren-Set CombiSet Nasen-Ohren-Set CombiSet Nasen-Ohren-Set CombiSet Da Vinci-Uro-Set CombiSet Thorakotomie-Set CombiSet Thorakotomie-Set
9 CombiSet Thorakotomie-Set CombiSet Thorakotomie-Set CombiSet Struma-Set CombiSet Carotis-Set CombiSet Carotis-Set CombiSet Carotis-Set CombiSet Laparoskopie-Set groß CombiSet Laparoskopie-Set groß CombiSet Abdominal-Gyn-Set CombiSet Vaginal-Set CombiSet Vaginal-Set CombiSet BAA- Set CombiSet Kreuzband-Set CombiSet Fem-Pop-Set CombiSet Struma-Set (Komplett-Set) CombiSet Nephrektomie-Set CombiSet BAA-Komplett-Set CPT CombiSet Kreuzband-Set CombiSet Zystektomie- Set CombiSet Fem-Pop-Set CombiSet Basis-Schlitztuch-Set groß CombiSet Steinschnitt-Set CombiSet Lap-Gyn-Set CombiSet Lap-Gyn-Set CombiSet Hüft- und Knie-Set CombiSet Hüft- und Knie-Set CombiSet Kopf- Set CombiSet Vertikaltuch Set CombiSet Hand - Fuß Set Frdst CombiSet Sectio Set Standard
10 CombiSet Hand - Fuß Set Frdst CombiSet Universal Set Super Frdst CombiSet Varizen-Set CombiSet ASK-Set CombiSet ASK-Set CombiSet MIC-Set CombiSet MIC-Set CombiSet TEP-Set CombiSet Varizen-Set CombiSet Universal Set Super Frdst. CombiSet Hand Chirurgie Set CombiSet Vaginal- Set CombiSet Sectio Set Standard CombiSet MIC-Set CombiSet Struma-Set CombiSet Schulter-ASK-Set CombiSet ASK - Knie CombiSet Trauma-Set groß CombiSet Makrokopf-Set CombiSet Trauma-Set groß CombiSet PFNA+ DHS-Set CombiSet MIC-Universal-Set CombiSet MIC-Universal-Set CombiSet MIC-Universal-Set CombiSet Diskus-Set CombiSet Lid-OP-Set CombiSet MIC-Universal-Set CombiSet Uni-Set CombiSet Uni-Set CombiSet Laparotomie Set
11 CombiSet Hand-Fuß-Set MOD CombiSet Hand-Fuß-Set MOD ambulant CombiSet Laparoskopie-Set CombiSet Laparoskopie-Set CombiSet Hals-Set CombiSet Da Vinci-Uro-Set CombiSet Laparoskopie-Set groß CombiSet Schlitztuch-Set groß CombiSet Steinschnitt-Set CombiSet Steinschnitt-Set CombiSet Lap-Gyn-Set CombiSet Vertikaltuch Set CombiSet MIC-Hernien-Set CombiSet Schädel-Set CombiSet Schädel-Set CombiSet Schädel-Set CombiSet BSV-Set CombiSet Schulter-Ask-Set CombiSet Hüft-TEP-Set CombiSet Lap-Set klein CombiSet Hand-Fuß-Set Standard CombiSet Hand-Fuß-Set extra CombiSet Lid-OP-Set
URGENT FIELD SAFETY NOTICE: INSTRUCTIONS TO END USER
 URGENT FIELD SAFETY NOTICE: INSTRUCTIONS TO END USER Commercial Name: Unomedical Sterile Urine Drainage Bags LOT No: Please see attached sheet for reference numbers & affected LOT numbers FSCA Id: 2010/11
URGENT FIELD SAFETY NOTICE: INSTRUCTIONS TO END USER Commercial Name: Unomedical Sterile Urine Drainage Bags LOT No: Please see attached sheet for reference numbers & affected LOT numbers FSCA Id: 2010/11
Manometer Tray, Jamshidi Needle Biopsy, Illinois (TJ) Needle Aspiration, Jamshidi (TJ) Needle Bone Marrow, Thoracentesis/Paracentesis Kit
 URGENT FIELD SAFETY NOTICE Affected product: MANOMETER TRAY JAMSHIDI NEEDLE BIOPSY ILLINOIS (TJ) NEEDLE ASPIRATION JAMSHIDI (TJ) NEEDLE BONE MARROW THORACENTESIS/PARACENTESIS KIT FSCA reference number:
URGENT FIELD SAFETY NOTICE Affected product: MANOMETER TRAY JAMSHIDI NEEDLE BIOPSY ILLINOIS (TJ) NEEDLE ASPIRATION JAMSHIDI (TJ) NEEDLE BONE MARROW THORACENTESIS/PARACENTESIS KIT FSCA reference number:
Urgent field safety notice
 Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
Accu-Chek Aviva Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) potentially showing an increased number of strip errors prior to dosing or biased
URGENT: Important Safety Information
 October 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Crystallization, Cracked Needle Hubs and Particulate in Diazepam Injection, USP,
October 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Crystallization, Cracked Needle Hubs and Particulate in Diazepam Injection, USP,
Attachment 12 URGENT FIELD SAFETY REMOVAL FSCA3193. ETEST Ceftriaxone TXL32 (Ref , ) FOAM packaging
 16 January 2017 Attachment 12 URGENT FIELD SAFETY REMOVAL FSCA3193 ETEST Ceftriaxone TXL32 (Ref. 507058, 507018) FOAM packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 507058,
16 January 2017 Attachment 12 URGENT FIELD SAFETY REMOVAL FSCA3193 ETEST Ceftriaxone TXL32 (Ref. 507058, 507018) FOAM packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 507058,
URGENT: Important Safety Information
 July 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Cracked Needle Hubs and Particulate in Multiple Carpuject Luer Lock Glass Products-Corrected
July 2018 URGENT: Important Safety Information Subject: Notice of New Special Handling Instructions due to Potential for Cracked Needle Hubs and Particulate in Multiple Carpuject Luer Lock Glass Products-Corrected
PRODUCT RECALL NOTICE URGENT
 PRODUCT RECALL NOTICE URGENT Date: July 21, 2018 To: Re: Mondelēz Global LLC Customers Recall Notice Certain Ritz Cracker Sandwiches and Ritz Bits product in the U.S., including Puerto Rico & U.S. Virgin
PRODUCT RECALL NOTICE URGENT Date: July 21, 2018 To: Re: Mondelēz Global LLC Customers Recall Notice Certain Ritz Cracker Sandwiches and Ritz Bits product in the U.S., including Puerto Rico & U.S. Virgin
URGENT FIELD SAFETY NOTICE FSCA3193. Etest Ceftriaxone TXL32 (Ref , ) SPB Packaging
 16 January 2017 Attachment 3 URGENT FIELD SAFETY NOTICE FSCA3193 Etest Ceftriaxone TXL32 (Ref. 412302, 412303) SPB Packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 412302, 412303)
16 January 2017 Attachment 3 URGENT FIELD SAFETY NOTICE FSCA3193 Etest Ceftriaxone TXL32 (Ref. 412302, 412303) SPB Packaging Dear This letter is intended for all ETEST Ceftriaxone TXL32 (Ref. 412302, 412303)
Urgent Field Safety Notice
 Urgent Field Safety Notice Location, Date 2014 Important notice: Enhanced instructions for the proper handling of the Accu-Chek Mobile system to avoid the potential of falsely elevated
Urgent Field Safety Notice Location, Date 2014 Important notice: Enhanced instructions for the proper handling of the Accu-Chek Mobile system to avoid the potential of falsely elevated
Urgent field safety notice
 Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
Accu-Chek Aviva, Accu-Chek Performa Urgent field safety notice May 2018 Important information on selected lots of Accu-Chek Aviva (50s, 10s) and Accu-Chek Performa (10s) strips potentially showing an increased
NovoPen Echo URGENT MEDICAL DEVICE RECALL
 NovoPen Echo URGENT MEDICAL DEVICE RECALL ISSUE Novo Nordisk A/S has detected that the insulin cartridge holder used in a small number of batches may crack or break if exposed to certain chemicals, for
NovoPen Echo URGENT MEDICAL DEVICE RECALL ISSUE Novo Nordisk A/S has detected that the insulin cartridge holder used in a small number of batches may crack or break if exposed to certain chemicals, for
URGENT: VOLUNTARY DRUG RECALL 07/05/2016
 9075 Centre Pointe Drive, Suite 140 West Chester, OH 45069 Phone: 800.543.2111 URGENT: VOLUNTARY DRUG RECALL 07/05/2016 GLUCOSE TEST STRIPS, TRUETRACK, 50/BOX NDC# 56151-0850-50 Besse# 31330 TRUETRACK
9075 Centre Pointe Drive, Suite 140 West Chester, OH 45069 Phone: 800.543.2111 URGENT: VOLUNTARY DRUG RECALL 07/05/2016 GLUCOSE TEST STRIPS, TRUETRACK, 50/BOX NDC# 56151-0850-50 Besse# 31330 TRUETRACK
Urgent Safety Notice. Recall. concerning the. sterile Trauma Plate Systems
 aap Implantate AG Lorenzweg 5 D-12099 Berlln Germanv CUSTOMER NAME STREET No. ZIP-CODE, PLACE Urgent Safety Notice Recall concerning the sterile Trauma Plate Systems Berlin, May 5 1 h, 2017 Reference-No.:
aap Implantate AG Lorenzweg 5 D-12099 Berlln Germanv CUSTOMER NAME STREET No. ZIP-CODE, PLACE Urgent Safety Notice Recall concerning the sterile Trauma Plate Systems Berlin, May 5 1 h, 2017 Reference-No.:
URGENT FIELD SAFETY NOTICE - EXPANSION
 URGENT FIELD SAFETY NOTICE - EXPANSION August 18, 2017 Product Name Catalog Number BD MAX TM Vaginal Panel 443710 BD MAX UVE Specimen Collection Kit 443376 BD MAX CT/GC/TV 442970 BD MAX CT/GC 442969 Dear
URGENT FIELD SAFETY NOTICE - EXPANSION August 18, 2017 Product Name Catalog Number BD MAX TM Vaginal Panel 443710 BD MAX UVE Specimen Collection Kit 443376 BD MAX CT/GC/TV 442970 BD MAX CT/GC 442969 Dear
Single Use Curlew TM Multiple Biopsy Forceps
 Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
Single Use Curlew TM Multiple Biopsy Forceps 13 SPECIMEN WITH METAL STORAGE CYLINDER With In Situ Fixation, Batch or Single Specimen Collection US Patents 5,782,747; 5,980,468; 6,071,248; foreign patents
URGENT FIELD SAFETY NOTICE
 August X, 2018 Olympus reference: QIL 151-005 URGENT FIELD SAFETY NOTICE IMPORTANT UPDATED LABELING INFORMATION NEW REPROCESSING INSTRUCTIONS FOR THE OLYMPUS PJF-160 DUODENOSCOPE ATTENTION: Endoscopy Department,
August X, 2018 Olympus reference: QIL 151-005 URGENT FIELD SAFETY NOTICE IMPORTANT UPDATED LABELING INFORMATION NEW REPROCESSING INSTRUCTIONS FOR THE OLYMPUS PJF-160 DUODENOSCOPE ATTENTION: Endoscopy Department,
Unofficial translation /Ministry of Social Affairs and Health. No. 548/2008. Government Decree on Narcotics Control
 Unofficial translation /Ministry of Social Affairs and Health No. 548/2008 Government Decree on Narcotics Control Issued in Helsinki on 28 August 2008 Section 1 Scope of application This Decree lays down
Unofficial translation /Ministry of Social Affairs and Health No. 548/2008 Government Decree on Narcotics Control Issued in Helsinki on 28 August 2008 Section 1 Scope of application This Decree lays down
Winfried Meissner Dept. of Anesthesiology and Intensive Care Jena University Hospital. Main results from the PAIN OUT project
 Winfried Meissner Dept. of Anesthesiology and Intensive Care Jena University Hospital Main results from the PAIN OUT project Conflicts of interest Research: EU, Pfizer Speaker: Menarini, Grünenthal, Mundipharma,
Winfried Meissner Dept. of Anesthesiology and Intensive Care Jena University Hospital Main results from the PAIN OUT project Conflicts of interest Research: EU, Pfizer Speaker: Menarini, Grünenthal, Mundipharma,
Better Post-Op Pain Control Starts Here
 Better Post-Op Pain Control Starts Here POST-OP PAIN CONTROL PUMP It s Easy to Get Started About the ACCUFUSER Pump Thank you for considering the This brochure makes it easy for For complete information
Better Post-Op Pain Control Starts Here POST-OP PAIN CONTROL PUMP It s Easy to Get Started About the ACCUFUSER Pump Thank you for considering the This brochure makes it easy for For complete information
Urgent Notice September 16, 2014
 Urgent Notice September 16, 2014 Voluntary Recall Notification: EDS 3 CSF External Drainage System PLEASE DISTRIBUTE THIS INFORMATION TO CLINICIANS WHO USE THIS DEVICE Dear OR Manager, Materials Manager,
Urgent Notice September 16, 2014 Voluntary Recall Notification: EDS 3 CSF External Drainage System PLEASE DISTRIBUTE THIS INFORMATION TO CLINICIANS WHO USE THIS DEVICE Dear OR Manager, Materials Manager,
Uro-Scopic Trainer USER GUIDE
 USER GUIDE Uro-Scopic Trainer Designed and manufactured by Limbs & Things Limited, Sussex Street, St. Philips, Bristol, BS2 0RA, UK Telephone: +44 (0)117 311 0500 Fax: +44 (0)117 311 0501 E-mail: sales@limbsandthings.com
USER GUIDE Uro-Scopic Trainer Designed and manufactured by Limbs & Things Limited, Sussex Street, St. Philips, Bristol, BS2 0RA, UK Telephone: +44 (0)117 311 0500 Fax: +44 (0)117 311 0501 E-mail: sales@limbsandthings.com
Summary of Significant Changes. Policy. Purpose. Responsibilities
 This Management Process Description replaces NEW Copy Number Summary of Significant Changes N/A Effective 17/03/16 Policy All processes included within this document should be adopted by NHS Blood and
This Management Process Description replaces NEW Copy Number Summary of Significant Changes N/A Effective 17/03/16 Policy All processes included within this document should be adopted by NHS Blood and
Ident. Nr.: P Kidričeva 26, 3001 CELJE SLOVENIJA, +386 (0) (0)
 SAFETY DATA SHEET Page 1 of 5 Issued: 28.08.2008 Date of revision: Number of revision: 1. Product and company identification: 1.1. Ident. Nr.: P048704 Department: Metalurgija 1.2. Application: Metallurgical
SAFETY DATA SHEET Page 1 of 5 Issued: 28.08.2008 Date of revision: Number of revision: 1. Product and company identification: 1.1. Ident. Nr.: P048704 Department: Metalurgija 1.2. Application: Metallurgical
GOVERNMENT OF REPUBLIC OF TRINIDAD AND TOBAGO MINISTRY OF HEALTH CHEMIST- FOOD AND DRUGS DIVISION NEW DRUG SUBMISSION FORM
 GOVERNMENT OF REPUBLIC OF TRINIDAD AND TOBAGO MINISTRY OF HEALTH CHEMIST- FOOD AND DRUGS DIVISION NEW DRUG SUBMISSION FORM Guidelines: In order to expedite the processing of your New Drug Submissions,
GOVERNMENT OF REPUBLIC OF TRINIDAD AND TOBAGO MINISTRY OF HEALTH CHEMIST- FOOD AND DRUGS DIVISION NEW DRUG SUBMISSION FORM Guidelines: In order to expedite the processing of your New Drug Submissions,
Negative Pressure Wound Therapy
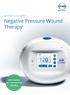 ATMOS S 042 NPWT Negative Pressure Wound Therapy USER FRIENDLY SELF-EXPLANATORY RELIABLE Negative Pressure Wound Therapy A therapy with new approaches For many indications in the areas of chronic and traumatic
ATMOS S 042 NPWT Negative Pressure Wound Therapy USER FRIENDLY SELF-EXPLANATORY RELIABLE Negative Pressure Wound Therapy A therapy with new approaches For many indications in the areas of chronic and traumatic
Important information on the Accu-Chek Insight insulin pump system: Update of the handling instructions for changing an insulin cartridge
 Consumer Letter (Accu-Chek Insight PFC) Urgent field safety notice Location, Date Important information on the Accu-Chek Insight insulin pump system: Update of the handling instructions
Consumer Letter (Accu-Chek Insight PFC) Urgent field safety notice Location, Date Important information on the Accu-Chek Insight insulin pump system: Update of the handling instructions
Urinary Catheter Passport SAMPLE COPY. A guide to looking after a urinary catheter. (for service users and healthcare workers) 2nd Edition
 Urinary Catheter Passport A guide to looking after a urinary catheter (for service users and healthcare workers) 2nd Edition Contact details Urinary Catheter Passport Service user Name Address Postcode
Urinary Catheter Passport A guide to looking after a urinary catheter (for service users and healthcare workers) 2nd Edition Contact details Urinary Catheter Passport Service user Name Address Postcode
URGENT FIELD SAFETY NOTICE
 AMO Ireland Block B, Liffey Valley Office Campus, Quarryvale, Dublin D22 XOY3 Ireland URGENT FIELD SAFETY NOTICE April X, 2017 Dear AMO Customer: RE: Voluntary Recall of Select Lots of AMO OVDs Abbott
AMO Ireland Block B, Liffey Valley Office Campus, Quarryvale, Dublin D22 XOY3 Ireland URGENT FIELD SAFETY NOTICE April X, 2017 Dear AMO Customer: RE: Voluntary Recall of Select Lots of AMO OVDs Abbott
Guidelines for Product Recall or Withdrawal
 REPUBLIC OF KENYA PHARMACY AND POISONS BOARD Guidelines for Product Recall or Withdrawal Edition 1 Date of release for publication June 2006 Date of implementation June 2006 This document has been prepared
REPUBLIC OF KENYA PHARMACY AND POISONS BOARD Guidelines for Product Recall or Withdrawal Edition 1 Date of release for publication June 2006 Date of implementation June 2006 This document has been prepared
URGENT: FIELD SAFETY NOTICE MSS A-FA / MSS B-FA. Medical Device Safety Advisory Notice
 URGENT: FIELD SAFETY NOTICE MSS-16-837A-FA / MSS-16-837B-FA Medical Device Safety Advisory Notice Date: Friday 13th January BD Eclipse Injection Needle, BD Eclipse Injection Needle with BD Luer-Lok Syringe
URGENT: FIELD SAFETY NOTICE MSS-16-837A-FA / MSS-16-837B-FA Medical Device Safety Advisory Notice Date: Friday 13th January BD Eclipse Injection Needle, BD Eclipse Injection Needle with BD Luer-Lok Syringe
SiOCX TF prosthetic sockets
 SiOCX TF prosthetic sockets Greater flexibility for you and your patients Information for technicians Complete sockets SiOCX TF SiOCX TF Pro Inner sockets NEW SiOCX TF inner socket SiOCX TF Basic inner
SiOCX TF prosthetic sockets Greater flexibility for you and your patients Information for technicians Complete sockets SiOCX TF SiOCX TF Pro Inner sockets NEW SiOCX TF inner socket SiOCX TF Basic inner
Altus Product description / Instructions for Use IFU 6 Rev. 1
 Content: 1 Introduction... 2 1.1 Legend... 2 1.2 Indication... 2 1.3 Contraindication... 2 1.4 Field of Application... 2 1.5 Sterilization... 2 1.6 Storage... 2 1.7 Allergies... 2 2 SYSTEM DESCRIPTION...
Content: 1 Introduction... 2 1.1 Legend... 2 1.2 Indication... 2 1.3 Contraindication... 2 1.4 Field of Application... 2 1.5 Sterilization... 2 1.6 Storage... 2 1.7 Allergies... 2 2 SYSTEM DESCRIPTION...
ADVANCED LEARNING SCHOLARSHIP. Including the. JOHN and BETTY ROSE SCHOLARSHIP APPLICATION. All applications to be posted to:
 ADVANCED LEARNING SCHOLARSHIP Including the JOHN and BETTY ROSE SCHOLARSHIP APPLICATION All applications to be posted to: The Secretary New Zealand Federation for Deaf Children Inc Johnsonville Wellington
ADVANCED LEARNING SCHOLARSHIP Including the JOHN and BETTY ROSE SCHOLARSHIP APPLICATION All applications to be posted to: The Secretary New Zealand Federation for Deaf Children Inc Johnsonville Wellington
NEPHROCHECK Liquid Control Kit Package Insert
 NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
Food Fortification Regulations, 2016 (Gazetted on 24 October, 2016) ARRANGEMENT OF SECTIONS PART I PRELIMINARY
 Statutory Instrument 120 of 2016 Food Fortification Regulations, 2016 (Gazetted on 24 October, 2016) [Cap 15:05 Section 1 Title 2 Interpretation ARRANGEMENT OF SECTIONS PART I PRELIMINARY 3 Inspection
Statutory Instrument 120 of 2016 Food Fortification Regulations, 2016 (Gazetted on 24 October, 2016) [Cap 15:05 Section 1 Title 2 Interpretation ARRANGEMENT OF SECTIONS PART I PRELIMINARY 3 Inspection
STANDARDIZED PROCEDURE REPROGRAMMING AND REFILLING INTRATHECAL BACLOFEN PUMPS and ACCESSING THE CATHETER ACCESS PORT (Adult,Peds)
 I. Definition The purpose of this procedure is to allow the Advanced Health Practitioner (AHP) to reprogram and refill intrathecal Baclofen pumps, as well as access the catheter access port for those AHPs
I. Definition The purpose of this procedure is to allow the Advanced Health Practitioner (AHP) to reprogram and refill intrathecal Baclofen pumps, as well as access the catheter access port for those AHPs
Suspected Defective Product Report
 Defective Product Report Form European Medicines Agency Inspections Suspected Defective Product Report London, 9 February 2007 Doc. Ref. EMEA/INS/GMP/75464/2007 1. Origin of Report Shaded areas to be completed
Defective Product Report Form European Medicines Agency Inspections Suspected Defective Product Report London, 9 February 2007 Doc. Ref. EMEA/INS/GMP/75464/2007 1. Origin of Report Shaded areas to be completed
URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan
 URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan FSCA identifier: CRM-SAL-2017-002 Affected Devices: Platinium Implantable Cardiac Defibrillators (ICDs) and Cardiac
URGENT FIELD SAFETY NOTICE Overconsumption following ElectroStatic Discharge or MRI scan FSCA identifier: CRM-SAL-2017-002 Affected Devices: Platinium Implantable Cardiac Defibrillators (ICDs) and Cardiac
Victorian Certificate of Education 2001 FOOD AND TECHNOLOGY. Written examination. Monday 19 November 2001
 SUPERVISOR TO ATTACH PROCESSING LABEL HERE Figures Words STUDENT NUMBER Letter Victorian Certificate of Education 2001 FOOD AND TECHNOLOGY Written examination Monday 19 November 2001 Reading time: 11.45
SUPERVISOR TO ATTACH PROCESSING LABEL HERE Figures Words STUDENT NUMBER Letter Victorian Certificate of Education 2001 FOOD AND TECHNOLOGY Written examination Monday 19 November 2001 Reading time: 11.45
3.5 mm LCP Tibial Plateau Sets. Configuration options.
 3.5 mm LCP Tibial Plateau Sets. Configuration options. Multiple trays Multiple levels Multiple options 3.5 mm LCP Tibial Plateau Sets The tibial plateau trays allow the hospital to build a custom - ized
3.5 mm LCP Tibial Plateau Sets. Configuration options. Multiple trays Multiple levels Multiple options 3.5 mm LCP Tibial Plateau Sets The tibial plateau trays allow the hospital to build a custom - ized
Food Safety for Restaurants: How to Prevent Foodborne Illness, Food Contamination & Lawsuits
 Food Safety for Restaurants: How to Prevent Foodborne Illness, Food Contamination & Lawsuits Foodborne illness causes an estimated 7.8 million sicknesses and about 3,000 deaths in the U.S. annually. In
Food Safety for Restaurants: How to Prevent Foodborne Illness, Food Contamination & Lawsuits Foodborne illness causes an estimated 7.8 million sicknesses and about 3,000 deaths in the U.S. annually. In
URGENT CUSTOMER NOTIFICATION CHANGE TO TRIGGER SPRAY 500ML AND 1L PRODUCTS
 August 2008 URGENT CUSTOMER NOTIFICATION CHANGE TO TRIGGER SPRAY 500ML AND 1L PRODUCTS Dear Customer, We have recently received the news that the supplier of our current trigger spray head has gone into
August 2008 URGENT CUSTOMER NOTIFICATION CHANGE TO TRIGGER SPRAY 500ML AND 1L PRODUCTS Dear Customer, We have recently received the news that the supplier of our current trigger spray head has gone into
Initial placement 24FR Pull PEG kit REORDER NO:
 Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
Initial placement 24FR Pull PEG kit REORDER NO: 00710805 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 24FR Pull PEG kit is
NEPHROCHECK Liquid Control Kit Package Insert
 NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
NEPHROCHECK Liquid Control Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Liquid Controls are used for
SUSPECT ADVERSE REACTION REPORT
 CIOMS FORM SUSPECT ADVERSE REACTION REPORT DE-BFARM-17099788 I. REACTION INFORMATION 1. PATIENT INITIALS 1a. COUNTRY 2. DATE OF BIRTH 2a. AGE 3. SEX 4-6 REACTION ONSET privacy DA MO YR DA MO YR Female
CIOMS FORM SUSPECT ADVERSE REACTION REPORT DE-BFARM-17099788 I. REACTION INFORMATION 1. PATIENT INITIALS 1a. COUNTRY 2. DATE OF BIRTH 2a. AGE 3. SEX 4-6 REACTION ONSET privacy DA MO YR DA MO YR Female
NEPHROCHECK Calibration Verification Kit Package Insert
 NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
NEPHROCHECK Calibration Verification Kit Package Insert Manufactured for Astute Medical, Inc. 3550 General Atomics Ct. Building 2 San Diego, CA 92121 USA Intended Use The NEPHROCHECK Calibration Verification
SUSPECT ADVERSE REACTION REPORT
 CIOMS FORM SUSPECT ADVERSE REACTION REPORT DE-BFARM-17144825 I. REACTION INFORMATION 1. PATIENT INITIALS 1a. COUNTRY 2. DATE OF BIRTH 2a. AGE 3. SEX 4-6 REACTION ONSET privacy (10010774): Constipation
CIOMS FORM SUSPECT ADVERSE REACTION REPORT DE-BFARM-17144825 I. REACTION INFORMATION 1. PATIENT INITIALS 1a. COUNTRY 2. DATE OF BIRTH 2a. AGE 3. SEX 4-6 REACTION ONSET privacy (10010774): Constipation
THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST
 THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST THE LOCATOR OVERDENTURE IMPLANT SYSTEM Each package includes your choice of a 2.4mm or 2.9mm narrow diameter LOCATOR Overdenture
THE LOCATOR OVERDENTURE IMPLANT SYSTEM PRODUCT ORDERING INFORMATION AND PRICE LIST THE LOCATOR OVERDENTURE IMPLANT SYSTEM Each package includes your choice of a 2.4mm or 2.9mm narrow diameter LOCATOR Overdenture
URGENT MEDICAL DEVICE CORRECTION. Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps
 URGENT MEDICAL DEVICE CORRECTION May 26, 2015 Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps Dear Healthcare Professional: At Animas, we hold our products to the highest standards
URGENT MEDICAL DEVICE CORRECTION May 26, 2015 Important Information about Animas 2020, IR 1250 or IR 1200 Insulin Pumps Dear Healthcare Professional: At Animas, we hold our products to the highest standards
TOP GLOVE (ZHANGJIAGANG) CO. LTD. PRODUCT SAFETY DATA SHEET VINYL EXAM POWDERED AND POWDER-FREE
 SECTION I : PRODUCT DESCRIPTION Product Name Chemical Family : Vinyl Examination Glove [Powdered and Powder-Free] : PVC SECTION II : COMPOSITION / MAIN RAW MATERIAL Corn Starch, Polyurethane, PVC Paste
SECTION I : PRODUCT DESCRIPTION Product Name Chemical Family : Vinyl Examination Glove [Powdered and Powder-Free] : PVC SECTION II : COMPOSITION / MAIN RAW MATERIAL Corn Starch, Polyurethane, PVC Paste
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
SENSATION and CS300 Smaller meets faster. IABP System
 SENSATION and CS300 Smaller meets faster IABP System Cardiac Assist SENSATION and CS300 3 Cardiac Assist SENSATION and CS300 5 Smaller The SENSATION 7Fr. Fiber-Optic IAB Catheter Faster The CS300 with
SENSATION and CS300 Smaller meets faster IABP System Cardiac Assist SENSATION and CS300 3 Cardiac Assist SENSATION and CS300 5 Smaller The SENSATION 7Fr. Fiber-Optic IAB Catheter Faster The CS300 with
AST Standards of Practice for Monitoring Sterility
 AST Standards of Practice for Monitoring Sterility Introduction The following Standards of Practice were researched and authored by the AST Education and Professional Standards Committee and have been
AST Standards of Practice for Monitoring Sterility Introduction The following Standards of Practice were researched and authored by the AST Education and Professional Standards Committee and have been
CytoCoreinc. 414 North Orleans St. Suite 502 Chicago, IL p: cytocoreinc.com. core solutions for innovative care.
 CytoCore, Inc. is a biomolecular diagnostics company engaged in the design, development and commercialization of cost-effective screening systems for the early detection of cancer and cancer-related diseases.
CytoCore, Inc. is a biomolecular diagnostics company engaged in the design, development and commercialization of cost-effective screening systems for the early detection of cancer and cancer-related diseases.
We get your personal data from the following sources (examples detailed below are not exhaustive):
 Novo Nordisk Limited processes (e.g. collects, uses, stores, and shares) personal data for different reasons (purpose) and uses a number of legal bases to process that personal data. This Notice explains
Novo Nordisk Limited processes (e.g. collects, uses, stores, and shares) personal data for different reasons (purpose) and uses a number of legal bases to process that personal data. This Notice explains
INFORMATION FOR FILLING OUT APPLICATION FORM FOR AN EXEMPTION TO USE A CONTROLLED SUBSTANCE FOR SCIENTIFIC PURPOSES (a Health Canada document)
 INFORMATION FOR FILLING OUT APPLICATION FORM FOR AN EXEMPTION TO USE A CONTROLLED SUBSTANCE FOR SCIENTIFIC PURPOSES (a Health Canada document) Quote from Health Canada website: http://www.hc-sc.gc.ca/hc-ps/substancontrol/exemptions/index-eng.php
INFORMATION FOR FILLING OUT APPLICATION FORM FOR AN EXEMPTION TO USE A CONTROLLED SUBSTANCE FOR SCIENTIFIC PURPOSES (a Health Canada document) Quote from Health Canada website: http://www.hc-sc.gc.ca/hc-ps/substancontrol/exemptions/index-eng.php
GRANGE PARK SURGERY LOCAL PATIENT PARTICIPATION REPORT
 GRANGE PARK SURGERY LOCAL PATIENT PARTICIPATION REPORT Date: February 2012 1. Introduction The Patient Participation Group (PPG) at Grange Park Surgery was established in 2009 with the first open meeting
GRANGE PARK SURGERY LOCAL PATIENT PARTICIPATION REPORT Date: February 2012 1. Introduction The Patient Participation Group (PPG) at Grange Park Surgery was established in 2009 with the first open meeting
Human Influenza A (Swine Flu) Rapid test
 Human Influenza A (Swine Flu) Rapid test Cat.No: DTSXY-Z9 Lot. No. (See product label) Size 20T Intended use The Influenza A (Swine Flu) test is a rapid chromatographic immunoassay for the qualitative
Human Influenza A (Swine Flu) Rapid test Cat.No: DTSXY-Z9 Lot. No. (See product label) Size 20T Intended use The Influenza A (Swine Flu) test is a rapid chromatographic immunoassay for the qualitative
Subject: Temporary importation of Hartmann s (Compound Sodium Lactate) 1L from Spain to address manufacturing disruption in UK
 5 th September 2018 Subject: Temporary importation of Hartmann s (Compound Sodium Lactate) 1L from Spain to address manufacturing disruption in UK Dear Customer, In order to address shortages of critical
5 th September 2018 Subject: Temporary importation of Hartmann s (Compound Sodium Lactate) 1L from Spain to address manufacturing disruption in UK Dear Customer, In order to address shortages of critical
Permit to Import Quarantine Material
 Quarantine Act 1908 Section 13(2AA) Permit to Import Quarantine Material Phone: 02 6272 4578 Fax: 02 6249 1798 File Ref: Permit: IP14011730 Valid From: 9 Jul 2014 Valid To: 9 Jul 2016 Page 1 of 7 Importer
Quarantine Act 1908 Section 13(2AA) Permit to Import Quarantine Material Phone: 02 6272 4578 Fax: 02 6249 1798 File Ref: Permit: IP14011730 Valid From: 9 Jul 2014 Valid To: 9 Jul 2016 Page 1 of 7 Importer
CultureWell Culture Systems and CultureWell Coverslips
 CultureWell Culture Systems and CultureWell Coverslips Table 1. CultureWell product specifications. Product Type Well Depth Volume per Well Quantity per Package CultureWell MultiWell cell culture system
CultureWell Culture Systems and CultureWell Coverslips Table 1. CultureWell product specifications. Product Type Well Depth Volume per Well Quantity per Package CultureWell MultiWell cell culture system
URGENT FIELD SAFETY NOTICE MEDICAL DEVICE FIELD CORRECTION Maquet/Datascope CARDIOSAVE Intra-Aortic Balloon Pump (IABP)
 May 4, 2018 via [INSERT METHOD] URGENT FIELD SAFETY NOTICE MEDICAL DEVICE FIELD CORRECTION Maquet/Datascope CARDIOSAVE Intra-Aortic Balloon Pump (IABP) AFFECTED PRODUCT PART NUMBER DISTRIBUTION DATE Cardiosave
May 4, 2018 via [INSERT METHOD] URGENT FIELD SAFETY NOTICE MEDICAL DEVICE FIELD CORRECTION Maquet/Datascope CARDIOSAVE Intra-Aortic Balloon Pump (IABP) AFFECTED PRODUCT PART NUMBER DISTRIBUTION DATE Cardiosave
SHARPS PROCEDURE PROCEDURE FOR THE SAFE USE & DISPOSAL OF SHARPS AND THE MANAGEMENT OF SHARPS INJURIES AND BLOOD EXPOSURE INCIDENTS.
 SHARPS PROCEDURE PROCEDURE FOR THE SAFE USE & DISPOSAL OF SHARPS AND THE MANAGEMENT OF SHARPS INJURIES AND BLOOD EXPOSURE INCIDENTS February 2012 Version 1.0 1 CONTENTS Section Page 1.0 Introduction 3
SHARPS PROCEDURE PROCEDURE FOR THE SAFE USE & DISPOSAL OF SHARPS AND THE MANAGEMENT OF SHARPS INJURIES AND BLOOD EXPOSURE INCIDENTS February 2012 Version 1.0 1 CONTENTS Section Page 1.0 Introduction 3
with Blom-Singer Gel Cap Insertion System
 with Blom-Singer Gel Cap Insertion System MEDICAL PROFESSIONAL Instructions For Use R2 37269-02G 37269-02G Effective January 2016 Blom-Singer and InHealth Technologies are registered trademarks of Freudenberg
with Blom-Singer Gel Cap Insertion System MEDICAL PROFESSIONAL Instructions For Use R2 37269-02G 37269-02G Effective January 2016 Blom-Singer and InHealth Technologies are registered trademarks of Freudenberg
Surgical Technique. Distal Humerus Locking Plate
 Surgical Technique Distal Humerus Locking Plate PERI-LOC Locked Plating System Distal Humerus Locking Plate Surgical Technique Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
Surgical Technique Distal Humerus Locking Plate PERI-LOC Locked Plating System Distal Humerus Locking Plate Surgical Technique Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
CATHETER PASSPORT. Looking after your Urinary Catheter. The Catheter Passport should be given to all patients with a urinary catheter.
 CATHETER PASSPORT Looking after your Urinary Catheter The Catheter Passport should be given to all patients with a urinary catheter. Health care staff should ensure that page 10 is completed. This document
CATHETER PASSPORT Looking after your Urinary Catheter The Catheter Passport should be given to all patients with a urinary catheter. Health care staff should ensure that page 10 is completed. This document
CHOOSING AND OBTAINING A VACUUM PUMP
 CHOOSING AND OBTAINING A VACUUM PUMP After my radical prostatectomy in January 2013 I decided that I would use a vacuum pump, which is a device recommended for penile rehabilitation following a radical
CHOOSING AND OBTAINING A VACUUM PUMP After my radical prostatectomy in January 2013 I decided that I would use a vacuum pump, which is a device recommended for penile rehabilitation following a radical
Evidenzbasiertes, perioperatives Analgesie- Konzept in der Fast Track Chirurgie. Christoph Konrad Luzern
 Evidenzbasiertes, perioperatives Analgesie- Konzept in der Fast Track Chirurgie Christoph Konrad Luzern Prävalenz Prävalenz 40.0 Hüft TEP Leistenhernie Knie TEP Thorakotomie 30.0 20.0 Br J Anaesth. 2010
Evidenzbasiertes, perioperatives Analgesie- Konzept in der Fast Track Chirurgie Christoph Konrad Luzern Prävalenz Prävalenz 40.0 Hüft TEP Leistenhernie Knie TEP Thorakotomie 30.0 20.0 Br J Anaesth. 2010
MagniSort Human CD4 T cell Enrichment Kit Catalog Number: RUO: For Research Use Only. Not for use in diagnostic procedures.
 Page 1 of 2 MagniSort Human CD4 T cell Enrichment Kit RUO: For Research Use Only. Not for use in diagnostic procedures. Normal human peripheral blood mononuclear cells were unsorted (left) or sorted with
Page 1 of 2 MagniSort Human CD4 T cell Enrichment Kit RUO: For Research Use Only. Not for use in diagnostic procedures. Normal human peripheral blood mononuclear cells were unsorted (left) or sorted with
SAFE HANDLING OF VACCINES
 STANDARD OPERATING PROCEDURE SAFE HANDLING OF VACCINES Issue History Issue Version One Purpose of Issue/Description of Change Planned Review Date To ensure vaccines are stored in accordance with manufacturers
STANDARD OPERATING PROCEDURE SAFE HANDLING OF VACCINES Issue History Issue Version One Purpose of Issue/Description of Change Planned Review Date To ensure vaccines are stored in accordance with manufacturers
New England Compounding Center 04-Dec-06
 New England Compounding Center 04-Dec-06 Department of Health and Human Services Public Health Service Food and Drug Administration New England District One Montvale Avenue Stoneham, Massachusetts 02180
New England Compounding Center 04-Dec-06 Department of Health and Human Services Public Health Service Food and Drug Administration New England District One Montvale Avenue Stoneham, Massachusetts 02180
EFSA PRE-SUBMISSION GUIDANCE FOR APPLICANTS INTENDING TO SUBMIT APPLICATIONS FOR AUTHORISATION OF HEALTH CLAIMS MADE ON FOODS
 EFSA PRE-SUBMISSION GUIDANCE FOR APPLICANTS INTENDING TO SUBMIT APPLICATIONS FOR AUTHORISATION OF HEALTH CLAIMS MADE ON FOODS Last updated (Rev.): 21 December 2007 Publication Date: 14 March 2007 NOTES
EFSA PRE-SUBMISSION GUIDANCE FOR APPLICANTS INTENDING TO SUBMIT APPLICATIONS FOR AUTHORISATION OF HEALTH CLAIMS MADE ON FOODS Last updated (Rev.): 21 December 2007 Publication Date: 14 March 2007 NOTES
limbsandthings.com Advanced Female Catheterisation Trainer User Guide For more skills training products visit Limbs & Things Ltd.
 Advanced Female Catheterisation Trainer Product No: 60155 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
Advanced Female Catheterisation Trainer Product No: 60155 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
ExiPrep TM Viral DNA/ RNA Kit
 Ver. 2.0 ExiPrep TM Viral DNA Kit (K-3515) ExiPrep TM Viral RNA Kit (K-3525) (K-3535) Safety Warnings and Precautions is developed and sold for research purposes only. It is not recommended for human or
Ver. 2.0 ExiPrep TM Viral DNA Kit (K-3515) ExiPrep TM Viral RNA Kit (K-3525) (K-3535) Safety Warnings and Precautions is developed and sold for research purposes only. It is not recommended for human or
Correction System. Surgical Technique
 Nextra Hammertoe Correction System Surgical Technique Maximized Bone Purchase* Stable and Secure Phalanx Optimized Screw Design Adjustable Bone-to-Bone Apposition Progressive Ratchet Tightening Mechanism
Nextra Hammertoe Correction System Surgical Technique Maximized Bone Purchase* Stable and Secure Phalanx Optimized Screw Design Adjustable Bone-to-Bone Apposition Progressive Ratchet Tightening Mechanism
ANTIBODY SCREENING by Uni-Gold Recombigen HIV
 ANTI-HIV SPECIMEN 1 REQUIREMENTS ANTIBODY SCREENING by Uni-Gold Recombigen HIV PRINCIPLE: The Uni-Gold Recombigen HIV was designed as a rapid immunoassay and is intended to detect antibodies to HIV- 1
ANTI-HIV SPECIMEN 1 REQUIREMENTS ANTIBODY SCREENING by Uni-Gold Recombigen HIV PRINCIPLE: The Uni-Gold Recombigen HIV was designed as a rapid immunoassay and is intended to detect antibodies to HIV- 1
UK Council on Deafness Access to Work Group
 UK Council on Deafness Access to Work Group Meeting 28 October 2014 Minutes In attendance Christine Archer, ANP Dan Sumners, Signature (minutes) David Buxton, BDA (chair) Frances Dobson, ALS Gail Dixon,
UK Council on Deafness Access to Work Group Meeting 28 October 2014 Minutes In attendance Christine Archer, ANP Dan Sumners, Signature (minutes) David Buxton, BDA (chair) Frances Dobson, ALS Gail Dixon,
low ProfIle neuro PlaTIng system
 low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
low ProfIle neuro PlaTIng system surgical TeChnIque Table of Contents Introduction Low Profile Neuro Cranial Plating System 2 Surgical Technique Technique 5 Product Information Low Profile Neuro Plates
Guideline on influenza vaccines submission and procedural requirements
 1 2 3 October 2014 EMA/56793/2014 Human Medicines Research and Development Support 4 5 6 Guideline on influenza vaccines submission and procedural requirements Regulatory and procedural requirements module
1 2 3 October 2014 EMA/56793/2014 Human Medicines Research and Development Support 4 5 6 Guideline on influenza vaccines submission and procedural requirements Regulatory and procedural requirements module
URGENT FIELD SAFETY NOTICE IMMEDIATE ACTION REQUIRED
 Ref No: Date: 22.08.2018 Type of Action: PFSN18 Deviations of high (>4.5) CoaguChek INR values due to calibration with WHO reference standard rtf/16 SBN-CPS-2018-014 Field Safety Corrective Action (FSCA)
Ref No: Date: 22.08.2018 Type of Action: PFSN18 Deviations of high (>4.5) CoaguChek INR values due to calibration with WHO reference standard rtf/16 SBN-CPS-2018-014 Field Safety Corrective Action (FSCA)
Initial placement 20FR Guidewire PEG kit REORDER NO:
 Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Initial placement 20FR Guidewire PEG kit REORDER NO: 00710802 INSTRUCTIONS FOR USE 1 of 5 These products have been manufactured not to include latex. Intended Use: The Initial placement 20FR Guidewire
Integra Flowable Wound Matrix
 Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Integra Flowable Wound Matrix Table of Contents How Supplied...2 Storage...2 Product Information Disclosure...2 Symbols Used On Labeling...2 Instructions for Use... 3 Instructions for Use (Continued)...
Document Details Patient Group Direction Hepatitis A vaccine (Havrix Monodose ) Trust Ref No Local Ref (optional) Main points the
 Document Details Title Patient Group Direction Hepatitis A vaccine (Havrix Monodose ) Trust Ref No 1506-41174 Local Ref (optional) Main points the Active immunisation against infection caused by Hepatitis
Document Details Title Patient Group Direction Hepatitis A vaccine (Havrix Monodose ) Trust Ref No 1506-41174 Local Ref (optional) Main points the Active immunisation against infection caused by Hepatitis
Chronic Care. Coloplast Capital Market Day Jan Rolin Frederiksen, President Coloplast US. Coloplast Capital Market Day 2009
 Coloplast Capital Market Day 2009 Jan Rolin Frederiksen, President Coloplast US Page 1 Agenda Product Portfolio Ostomy Care Continence Care Wound and Skin Care Page 2 Portfolio covers four product-business
Coloplast Capital Market Day 2009 Jan Rolin Frederiksen, President Coloplast US Page 1 Agenda Product Portfolio Ostomy Care Continence Care Wound and Skin Care Page 2 Portfolio covers four product-business
MagniSort Mouse CD4 T cell Enrichment Kit Catalog Number: RUO: For Research Use Only. Not for use in diagnostic procedures.
 Page 1 of 2 MagniSort Mouse CD4 T cell Enrichment Kit RUO: For Research Use Only. Not for use in diagnostic procedures. Mouse splenocytes were unsorted (left) or sorted with the MagniSort Mouse CD4 T cell
Page 1 of 2 MagniSort Mouse CD4 T cell Enrichment Kit RUO: For Research Use Only. Not for use in diagnostic procedures. Mouse splenocytes were unsorted (left) or sorted with the MagniSort Mouse CD4 T cell
Q.E.D. Saliva Alcohol Test
 Q.E.D. Saliva Alcohol Test A Quantitative Test for the Determination of Equivalent Blood Alcohol Content (BAC) Using a Saliva Sample. Approved by the Federal Department of Transportation (DOT) for Commercial
Q.E.D. Saliva Alcohol Test A Quantitative Test for the Determination of Equivalent Blood Alcohol Content (BAC) Using a Saliva Sample. Approved by the Federal Department of Transportation (DOT) for Commercial
SECTION 13.0 SPECIMENS FOR LABORATORY EXAMINATION
 SECTION 13.0 SPECIMENS FOR LABORATORY EXAMINATION Introduction Collection of Specimens for Microbiological Examination: Safe Handling of Specimens Packaging & Transportation of Specimens Specimen Rejection
SECTION 13.0 SPECIMENS FOR LABORATORY EXAMINATION Introduction Collection of Specimens for Microbiological Examination: Safe Handling of Specimens Packaging & Transportation of Specimens Specimen Rejection
Stoma Care Policy and Procedures
 Stoma Care Policy and Procedures Policy number 19.09 Approved by :CEO Version 1 Scheduled review date 28/3/2018 Created on 28/3/2017 POLICY STATEMENT Clients requiring management with the stoma will be
Stoma Care Policy and Procedures Policy number 19.09 Approved by :CEO Version 1 Scheduled review date 28/3/2018 Created on 28/3/2017 POLICY STATEMENT Clients requiring management with the stoma will be
Patient Reimbursement Guide. Brainsway Deep Transcranial Magnetic Stimulation (TMS) Treatment. Obtain Coverage - the Right Way
 Patient Reimbursement Guide Brainsway Deep Transcranial Magnetic Stimulation (TMS) Treatment Obtain Coverage - the Right Way Ever since Brainsway Deep TMS was approved by the FDA in 2013 for the treatment
Patient Reimbursement Guide Brainsway Deep Transcranial Magnetic Stimulation (TMS) Treatment Obtain Coverage - the Right Way Ever since Brainsway Deep TMS was approved by the FDA in 2013 for the treatment
West Community Treatment Team Houghton Day Unit
 West Community Treatment Team Houghton Day Unit Patient information leaflet Introduction This leaflet provides you with information that you may find useful during your time with our service. If you are
West Community Treatment Team Houghton Day Unit Patient information leaflet Introduction This leaflet provides you with information that you may find useful during your time with our service. If you are
Surgical Technique. Clavicle Locking Plate
 Surgical Technique Clavicle Locking Plate PERI-LOC Locked Plating System Clavicle Locking Plate Surgical Technique Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient Positioning...4
Surgical Technique Clavicle Locking Plate PERI-LOC Locked Plating System Clavicle Locking Plate Surgical Technique Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient Positioning...4
Distal Radius Plate 2.4/2.7 dorsal and volar
 Distal Radius Plate 2.4/2.7 dorsal and volar Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Distal Radius Plate
Distal Radius Plate 2.4/2.7 dorsal and volar Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation. Distal Radius Plate
Document Details. Patient Group Direction
 Document Details Title Patient Group Direction (PGD) Hepatitis B Vaccine (Engerix ) Trust Ref No 1505-41182 Local Ref (optional) Main points the Immunisation against Hepatitis B document covers Who is
Document Details Title Patient Group Direction (PGD) Hepatitis B Vaccine (Engerix ) Trust Ref No 1505-41182 Local Ref (optional) Main points the Immunisation against Hepatitis B document covers Who is
Manchester Cytology Centre Synovial Fluid Analysis Service User Manual 2017/2018
 Page 1 of 12 Author: Sarah Ferris Directorate of Laboratory Medicine Manchester Cytology Centre Synovial Fluid Analysis Service User Manual 2017/2018 Page 2 of 12 Author: Sarah Ferris Table of contents
Page 1 of 12 Author: Sarah Ferris Directorate of Laboratory Medicine Manchester Cytology Centre Synovial Fluid Analysis Service User Manual 2017/2018 Page 2 of 12 Author: Sarah Ferris Table of contents
Please read this User s Manual completely before use to ensure full and proper function of your newly purchased SmokeStik. Please keep this manual in
 Please read this User s Manual completely before use to ensure full and proper function of your newly purchased SmokeStik. Please keep this manual in good condition and readily accessible for future reference.
Please read this User s Manual completely before use to ensure full and proper function of your newly purchased SmokeStik. Please keep this manual in good condition and readily accessible for future reference.
Surgical Technique. Olecranon Locking Plate
 Surgical Technique Olecranon Locking Plate PERI-LOC Locked Plating System Olecranon Locking Plate Surgical Techniquealog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
Surgical Technique Olecranon Locking Plate PERI-LOC Locked Plating System Olecranon Locking Plate Surgical Techniquealog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
MagniSort Human CD4 Memory T cell Enrichment Kit Catalog Number: RUO: For Research Use Only. Not for use in diagnostic procedures.
 Page 1 of 2 MagniSort Human CD4 Memory T cell Enrichment Kit RUO: For Research Use Only. Not for use in diagnostic procedures. Normal human peripheral blood mononuclear cells were unsorted (left) or sorted
Page 1 of 2 MagniSort Human CD4 Memory T cell Enrichment Kit RUO: For Research Use Only. Not for use in diagnostic procedures. Normal human peripheral blood mononuclear cells were unsorted (left) or sorted
KASM Knee Articulating Spacer Mold. Surgical Summary
 KASM Knee Articulating Spacer Mold Surgical Summary KASM Designing Surgeons: Michael H. Bourne, M.D. Salt Lake Orthopedic Clinic E. Marc Mariani, M.D. Salt Lake Orthopedic Clinic U.S. Patent 6,942,475
KASM Knee Articulating Spacer Mold Surgical Summary KASM Designing Surgeons: Michael H. Bourne, M.D. Salt Lake Orthopedic Clinic E. Marc Mariani, M.D. Salt Lake Orthopedic Clinic U.S. Patent 6,942,475
Guidance on Bulk Prescribing for Care Home Patients
 Guidance on Bulk Prescribing for Care Home Patients Introduction Many patients in care homes taking medicines when required (PRN) can inevitably present problems for the prescriber in determining the quantity
Guidance on Bulk Prescribing for Care Home Patients Introduction Many patients in care homes taking medicines when required (PRN) can inevitably present problems for the prescriber in determining the quantity
CLICK SEAL SYSTEM. Thermo Scientific Samco Clicktainer Vial. Unique click seal system providing sample protection and user safety
 LEAKPROOF 95KPA TESTED CE MARK CLICK SEAL SYSTEM Thermo Scientific Samco Clicktainer Vial Unique click seal system providing sample protection and user safety Thermo Scientific Samco Clicktainer Vial Providing
LEAKPROOF 95KPA TESTED CE MARK CLICK SEAL SYSTEM Thermo Scientific Samco Clicktainer Vial Unique click seal system providing sample protection and user safety Thermo Scientific Samco Clicktainer Vial Providing
