Lung Metastases Imaging
|
|
|
- Lionel Wade
- 6 years ago
- Views:
Transcription
1 Lung Metastases Imaging Updated: Oct 23, 2015 Author: Tanay Patel, MD; Chief Editor: Eugene C Lin, MD more... OVERVIEW Overview Pulmonary metastasis is seen in 20-54% of extrathoracic malignancies. [1] Lungs are the second most frequent site of metastases from extrathoracic malignancies. Twenty percent of metastatic disease is isolated to the lungs. [1] The development of pulmonary metastases in patients with known malignancies indicates disseminated disease and places the patient in stage IV in TNM (tumor, node metastasis) staging systems. This typically implies an adverse prognosis and alters the management plan. Imaging plays an important role in the screening and detection of pulmonary metastases. Imaging guidance is also used in histological confirmation of metastatic disease. In patients with poor cardiorespiratory function and comorbidities, imaging-guided thermal ablation procedures are an effective alternative to surgical resection to improve the survival. Chest radiography (CXR) is the initial imaging modality used in the detection of suspected pulmonary metastasis in patients with known malignancies. Chest CT scanning without contrast is more sensitive than CXR. For patients with bone or soft-tissue sarcoma, malignant melanoma, and head and neck carcinoma, CT scanning of the chest should be performed as an initial evaluation. In patients with primary renal or testicular cancer, chest CT scanning performed should be performed based on the presence of metastatic disease elsewhere. CT guidance is often required for obtaining samples from a suspected metastatic disease. Several thermal ablation options are available for treatment of pulmonary metastases, which is performed under CT guidance. See the images below.
2 Chest radiograph of a 58-year-old man with malignant melanoma (note surgical clips in right lower neck) shows multiple pulmonary nodules of varying sizes consistent with metastatic disease. There is also a small right basal effusion. Axial CT scan in a 58-year-old man with malignant melanoma shows multiple round nodules and masses of varying sizes in both lungs, consistent with metastases. There are also small bilateral pleural effusions. Volume-rendered 3-dimensional CT scan shows a metastatic mass in the trachea from squamous cell carcinoma of the lung.
3 Ultrasound guidance used for transthoracic aspiration of malignant effusion. See The Solitary Pulmonary Nodule: Is It Lung Cancer?, a Critical Images slideshow, for more information on benign and malignant etiologies of solitary pulmonary nodules. Pathophysiology Malignancies can reach the lung through 5 different pathways hematogenous through the pulmonary or bronchial artery, lymphatics, pleural space, airway, or direct invasion. [2] The most common path is the hematogenous spread, which occurs in tumors that have direct venous drainage to the lungs. This includes cancers of the head and neck, thyroid, adrenals, kidneys, and testes, as well as malignant melanoma and osteosarcoma. When the primary tumor invades the venous system, tumor cells embolize to the lungs through the pulmonary or bronchial arteries. Most of the tumor cells that reach the pulmonary capillary and arteriolar bed perish; however, some tumor cells pass through the vascular wall and develop parenchymal metastasis in the alveolar space or the interstitium. Lymphatic spread occurs to the lungs, pleura, or mediastinum. Lymphatic spread occurs either in an antegrade fashion by lymphatic invasion through the diaphragm and/or pleural surfaces or retrograde lymphatic spread from hilar lymph nodal metastasis. Lymphangitic spread refers to tumor growth in lymphatic channels, which are seen in the axial interstitium (peribronchovascular and centrilobular interstitium) and peripheral interstitium (interlobular septa and subpleural). The tumor initially spreads via a hematogenous route to the pulmonary arterioles and capillaries with retrograde spread from hilar nodal metastases or upper abdominal tumors, but subsequently extends through the vascular walls, invades the low resistant peribronchovascular lymphatics, and spreads along the lymphatics. The microscopic spread of metastasis through lymphatics and perilymphatic connective tissue is seen histopathologically in 56% of patients with pulmonary metastasis. [3] Lymphatic spread also occurs to the mediastinal lymph nodes through the thoracic duct, with subsequent retrograde
4 spread to the hilar lymph nodes and then the lungs. Spread within the pleural space can occur by pleural invasion by a local tumor, such as lung cancer or thymoma. Endobronchial spread of tumor cells occurs with airway tumors. It is more common in bronchoalveolar carcinoma, less common in other types of lung cancer, and even less common in tracheobronchial papillomatosis. Direct invasion of the lung occurs in tumors contiguous to the lung, including thyroid, esophageal, mediastinal, airway, and cardiovascular structures. Frequency The venous return containing lymphatic fluid from body tissues flows into the lungs through the pulmonary vascular system; thus, all tumors have the potential to involve the lungs. Pulmonary metastasis is seen in 20-54% of extrathoracic malignancies at autopsy. Breast, colorectal, lung, kidney, head and neck, and uterus cancers are the most common primary tumors with lung metastasis at autopsy. Choriocarcinoma, osteosarcoma, testicular tumors, malignant melanoma, Ewing sarcoma, and thyroid cancer frequently metastasize to lung, but the frequency of these tumors itself is low. Colorectal cancer, which accounts for 10% of all cancers, accounts for 15% of all cases of pulmonary metastases. [4] The Table illustrates the frequency of metastases in different primary malignancies. Table. Incidence of Pulmonary Metastases According to Site (Open Table in a new window) Primary Tumor Frequency at Presentation, % Frequency at Autopsy, % Choriocarcinoma Melanoma Testis, germ cell Osteosarcoma Thyroid 7 65 Kidney Head and neck Breast 4 60 Bronchus 30 40
5 Colorectal < Prostate Bladder Uterus < Cervix < Pancreas < Esophagus < Stomach < Ovary Hepatoma < Mortality & Morbidity The presence pulmonary metastasis usually indicates advanced disseminated disease. Occasionally, tumor spread can be an isolated event. The mortality depends on the primary tumor; for example, in pancreatic and bronchogenic carcinomas, the 5-year survival rate in patients with pulmonary metastases is less than 5%. [1] Early diagnosis is critical in planning effective therapy in patients who can be cured. Depending on several factors, metastasis can be resected, with 5-year survival rates up to 30-40%. [5] Clinical details While a large number of patients with pulmonary metastasis are asymptomatic at the time of diagnosis, some patients develop symptoms such as hemoptysis, cough, shortness of breath, chest pain, weakness, and weight loss. Particularly, patients with lymphangitic carcinomatosis present with respiratory dysfunction, including severe dyspnea. Other problems to consider The most common pattern of pulmonary metastasis is the presence of multiple, well-defined nodules. Differential diagnoses for multiple pulmonary nodules include infections (eg, histoplasmosis, coccidioidomycosis in endemic areas, cryptococcal and nocardial infections as opportunistic infections in immunocompromised patients, septic emboli, abscess, paragonimiasis, hydatid), granulomatous diseases (eg, tuberculosis, sarcoidosis), and vascular/collagen-vascular
6 diseases (eg, Wegener granulomatosis, rheumatoid arthritis). Differential diagnoses for other patterns are discussed in detail in Radiography and CT Scan. Intervention In specific circumstances, histopathological samples are required from the lung lesion. A few such scenarios include (1) atypical imaging findings; (2) the development of a solitary pulmonary nodule in a patient with known malignancy; (3) pulmonary metastasis without a known primary source; (4) assessment of response to therapy, particularly in nodules that are unchanged in size, but no positron emission tomography (PET) activity suggestive of sterilized metastasis. Tissue sampling can be performed by transthoracic needle aspiration, transthoracic needle biopsy, transbronchial needle aspiration and biopsy, or minimally invasive video-assisted surgical methods. Peripheral nodules are sampled using transthoracic aspiration/biopsy using CT guidance, provided they are not crossing major vascular structures or fissures. Central nodules and nodules involving airways are sampled using transbronchial needle aspiration and biopsy. Smaller nodules are now being sampled using state-of-the-art techniques such as electromagnetic-guided navigation bronchoscopy, usually with CT virtual bronchoscopic guidance. With an electromagnetic navigation system, a bronchoscopic probe sensor is placed within the electromagnetic field created around the chest. Real-time position is generated and superimposed on previously acquired thin CT images to navigate to the lesion. Biopsy or fine-needle aspiration (FNA) is typically performed under CT guidance. Full descriptions of the procedure and its complications are beyond the scope of the article. The definitive treatment for pulmonary metastases from extrathoracic malignancies is surgical resection (pulmonary metastasectomy). Surgery is performed if the primary tumor is controlled, if no extrathoracic lesions are present, if it is technically resectable, and if general and functional risks are tolerable. The 5-year overall survival rate for patients with pulmonary metastasectomy is 15-48%, compared with 13% for patients without the procedure. [5] The mean survival is months. Survival has been shown to better in patients with a fewer number of metastases. However, pulmonary metastasectomy can be performed only in 25-50% of patients, owing to the presence of multiple metastatic lesions or the presence of comorbid conditions, including poor respiratory function or refusal to have surgery. [6] Recurrence after pulmonary metastasectomy also limits further surgical options. In patients who are not in adequate physical condition to undergo pulmonary metastasectomy, alternative options available include stereotactic radiosurgery and thermal ablation procedures. Thermal ablation procedures induce coagulation necrosis of tumor cells and are typically performed with CT guidance. These include radiofrequency ablation (RFA), microwave ablation, laser ablation, and cryoablation. The primary goal of all these tumor ablation procedures is to eradicate all the malignant cells along with a margin of normal tissue, but cause minimal damage to normal lung disease. By doing this, adequate tumor control is achieved and survival is prolonged. The main advantage of thermal ablation procedures is selective and limited damage of lung tissue to minimally impact pulmonary function.
7 The ablation procedure can be repeated many times. In addition, ablation procedures can be performed regardless of previous therapy, even in patients who have adhesions from previous surgeries or radiation-induced pneumonitic changes. Because of this, ablation is often used as a salvage treatment for oligo-recurrence after surgery and radiation. Thermal ablation is also not an obstacle for performing concurrent or adjuvant chemotherapy or adjuvant radiation therapy. In fact, if the tumor size is downgraded by thermal ablation, the remaining tumor cells may become more sensitive to chemotherapy. As a result, the combination of thermal ablation, along with chemotherapy and other modalities, can increase the efficacy of thermal ablation through synergistic and even additive effects. Complications that can be seen during ablation procedure include pneumothorax, pulmonary hemorrhage, bronchopleural fistula, pulmonary artery pseudoaneurysm, systemic air embolism, injury of the brachial or phrenic nerve, pneumonia, needle-tract seeding of cancer, and deterioration of interstitial pneumonia. [7] Radiofrequency ablation RFA operates using alternating electrical current within the radiowave frequency ( khz). Using CT guidance, the RFA electrode is placed within the metastasis. Electrical current is concentrated near the noninsulated tip of the electrode, and the circuit is completed by returning to electrical ground pads in the patient s thighs. The electrical current causes agitation of ionic dipolar molecules in the surrounding tissue and fluids. The heat is radially distributed to surrounding tissues, usually in an ellipsoid shape with predictable distribution. RFA has been shown to improve survival in patients with pulmonary oligometastasis and oligorecurrence, which means one or a few metastatic or recurrent lesions, without and with controlled primary tumor, respectively. Several studies have been performed using RFA on several cancers. Generally, a disease free survival of 36 months or more is considered to indicate good response. [6] In colorectal cancers, Hiraki et al [7] demonstrated an overall survival rate of 96% at 1 year, 54% at 2 years, and 48% at 3 years. Yamakodo et al demonstrated 46% at 3 years and median survival of 60 months. [8] An absence of extrapulmonary metastasis, small tumor size (< 3 cm), single lung metastasis, and normal carcinoembryonic antigen (CEA) value were good prognostic indicators. In hepatocellular carcinoma, Hiraki et al [9] demonstrated an overall survival of 87% at 1 year and 57% at 2 and 3 years. Median and mean survival were 37.7 months and 43.2 months, respectively. Well-controlled primary cancer, an absence of intrahepatic recurrence, Child-Pugh class A, an absence of cirrhosis or hepatitis C infection, and an α-fetoprotein value of less than 10 ng/ml were good prognostic indicators. In renal tumors, [10] overall survival in curative and palliative ablation groups were shown to be 100% and 90%, respectively, at 1 year; 100% and 52% at 3 years; and 100% and 52% at 5 years. Maximum tumor diameter is an important factor. In bone and soft tissue sarcomas, 1- and 3-year survival rates were shown to be 92.2% and 65.2%, respectively. [11] Microwave ablation Microwave ablation is performed using microwave antennae and microwave generators with power
8 settings of W and an ablation time of 15 ±5 minutes under CT guidance. The efficacy of the treatment is determined by preablation tumor size and its location in relation to the hilum. The histopathologic nature of the primary tumor has no significant impact on the result of microwave ablation therapy. Tumors smaller than 3 cm and peripheral lesions (ie, >5 cm from the hilum) fared better than larger and more central lesions. With hilar lesions, the presence of large adjacent pulmonary arteries results in a current-sink effect, which diverts the heat current during ablation away from the core of the tumor, resulting in cooling of the tumor. Solutions to this issue include using prolonged current application and multiple simultaneous antennae, but these are associated with a higher risk of complications such as hemorrhage. Following microwave ablation, the initial CT scan may show increased tumor volume due to edema and an inflammatory response to heat energy. However, if the tumor size increases after 4-6 weeks, recurrence should be considered. Higher survival has been observed in patients with tumor-free states after successful ablation compared with patients with failed ablation. [12] Cryoablation Cryoablation is performed using a cryoprobe with high-pressure argon and helium gases for freezing and thawing on the basis of the Joule-Thomson principle. Three freeze-thaw cycles are performed to freeze a tumor cm in diameter. Initial freezing causes an ice ball with a diameter of only 1 cm, since air prevents conduction of low temperature and there is not enough water in the lung parenchyma. However, after the first thawing, the induced massive hemorrhage excludes air and results in the formation of a larger ice ball in subsequent freezing steps. During thawing, the probe reaches a temperature of 20 C. Cryoablation has been shown to result in 1- and 3-year progression-free intervals of 90.8% and 59%, respectively. The 3-year local progression-free interval of tumors smaller than 15 mm in diameter was 79.8% and of tumors larger than 15 mm was 18.6 %. One- and 3-year overall survival rates were 91% and 59.6%, respectively. [13] Laser ablation Laser ablation is performed with a miniaturized, internally cooled applicator system, which has an optical laser fiber with a flexible diffuser tip. Nd-YAG laser generators are typically used. Laser ablation is performed with single or multiple applicators under CT guidance. Wattage can be increased at 2 W/min, and a maximum energy of 14 W has been maintained for 15 minutes. The total amount of energy per tumor has ranged from W. Using laser ablation, definitive control of initial pulmonary disease has been achieved in 45% of patients, with 1-, 2-, 3-, 4-, and 5-year survival rates of 81%, 59%, 44%, 44%, and 27%, respectively. [14] The advantage of laser ablation is the use of laser light and its comparably well-studied conduction in lung tissue. Use of thin-caliber applicators and flexible fibers is a major advantage, and the procedure is more cost effective than other ablation techniques. Preferred examination Imaging modalities available for evaluation of pulmonary metastasis include CXR, CT scanning, MRI, scintigraphy, and PET scanning. The preferred imaging modality depends on the biological
9 behavior of the tumor, sensitivity and specificity of the imaging modality, radiation dose, and cost effectiveness. In a patient with known malignancy, CXR, with posteroanterior and lateral views, is usually the first imaging study performed to detect pulmonary metastases. Not uncommonly, metastases may be unexpectedly discovered on CXR performed for some other purpose. CXR performed with dual-energy subtraction has higher sensitivity in the detection of pulmonary metastasis by subtracting the overlying bones. Computer-aided detection (CAD) has been used in automatic detection of small pulmonary nodules. In a patient with known malignancy, if CXR demonstrates multiple pulmonary nodules, further imaging is usually not necessary, unless biopsy is planned or precise quantification of the metastatic burden is required prior to metastasectomy or as a baseline study to assess response following chemotherapy or radiation. CT scanning is the most sensitive modality in the detection of pulmonary metastasis, owing to its high spatial and contrast resolution and lack of superimposition with adjacent structures, such as bones and vessels. [72, 73, 74] Compared with CXR, CT scanning can detect a larger number of nodules and nodules smaller than 5 mm. CT scanning can detect 3 times as many noncalcified nodules as CXR. [15] In addition, it can detect additional findings such as lymphadenopathy; pleural, chest wall, airway, and vascular involvement; and upper abdominal and bony findings that may alter management. In a patient with known malignancy, chest CT scanning is performed if CXR shows a solitary nodule, equivocal nodule, negative findings but the extrathoracic malignancy has high risk of lung metastasis (eg, breast, kidney, colon, bladder), or multiple nodules (but biopsy or definitive treatment by mastectomy, chemotherapy, and radiation is planned). The radiation dose from frequent CT scanning can be reduced by using several dose-reduction techniques such as low kv, low mas, adaptive tube current modulation, and iterative reconstruction algorithms. The sensitivity of nodule detection can be increased by using postprocessing tools such as maximum-intensity projection (MIP) or volume rendering (VR). High-resolution CT (HRCT) scanning is used for detection of lymphangitic carcinomatosis. CT scan findings are not very specific, since nodules can be seen in a variety of benign conditions, including granulomas, hamartomas, and vascular abnormalities. The American College of Radiology (ACR) recommends that CXR should be the initial imaging modality used in the screening of pulmonary metastasis in patients with known extrathoracic malignancy. CT scanning without intravenous contrast is more sensitive than radiography in the detection of pulmonary metastasis. For patients with bone and soft-tissue sarcoma, malignant melanoma, and head and neck carcinoma, CT scanning of the chest should be performed as the primary imaging modality. In patients with primary kidney or testicular cancers, chest CT scanning should be performed based on the presence of metastatic disease elsewhere. [1] Detailed guidelines for few tumors are described below. The rest of the guidelines can be seen in the ACR article. [1] Bone and soft-tissue sarcomas CT scanning is the first and preferred imaging modality for screening metastases, since aggressive resection of pulmonary metastasis is recommended for survival. Patients with 3 or more pulmonary nodules, bilateral nodules, or large nodules are more likely to have metastasis. Routine chest radiographs and CT scans are recommended for the first 5 years, with radiography at each visit, chest CT scanning every 3 months for the first year, chest CT scanning every 4 months for the second year, chest CT scanning every 6 months for third year, and chest CT scanning once yearly thereafter.
10 Renal cell cancer Pulmonary metastasis is seen in 25-30% of patients at the initial diagnosis and in 30-50% at later stages of renal cell carcinoma. Resection of pulmonary metastasis has been shown to improve survival. CXR is the recommended initial screening modality. Chest CT scanning is indicated only for (1) a solitary pulmonary nodule, (2) symptoms of endobronchial metastasis, (3) extensive regional disease, (4) the presence of other extrathoracic metastasis amenable to resection. CT scanning is not required if CXR shows typical multiple nodules or if CXR findings are normal in a patient with low-stage disease. Some authors advocate lifelong biannual CXR and CT scanning. [16] Testicular cancer CXR is the recommended primary imaging modality for patients with negative abdominal CT findings, and chest CT scanning is the recommended primary imaging modality for patients with an abnormal abdominal CT scan. This recommendation is based on studies that showed a direct correlation between abdominal CT and chest CT findings. For those with an abnormal abdominal CT scan, chest CT scanning detected 12.5% more nodules than seen on CXR. For those with negative abdominal CT findings, chest CT scanning did not increase the yield over CXR. In fact, the false-positive rate in such patients is 2.3%, which results in unnecessary increased morbidity. [17] Malignant melanoma The need for chest CT scanning depends on the stage of the primary tumor. Metastasectomy may be the only potentially curative treatment modality in stage IV disease, regardless of the number of lesions. Chest CT scanning is recommended to evaluate the number of nodules and other associated disease. [1] Head and neck carcinoma Distant metastasis is seen in 5.5% of patients with head and neck cancers. In addition, the risk of synchronous malignancies in head and neck cancers is 15-30%. Chest CT scanning is an important screening examination for determining metastatic disease. Chest CT scanning has been shown to identify malignant lesions in 25.8% of these individuals, of which 15% have been shown to be pulmonary metastases, 5.4% are lung cancer, and 1.1% are esophageal cancers. [18] Treatment response CT is also used in assessing response to treatment. Small changes in tumor volume can be detected using volumetric techniques. [19] Diagnostic studies Histopathological samples are often required for confirming the diagnosis of pulmonary metastasis and in select cases to identify the primary tumor. Samples can be obtained using CT-guided transthoracic biopsy or FNA cytology. The tissue fragments can be compared with those of the primary tumor. Immunohistochemistry is helpful in identifying the primary tumor. Transthoracic needle aspiration has a positive yield of 85-95% in the evaluation of pulmonary metastasis, but the yield is lower with
11 lymphangitic spread. Transbronchial biopsy or navigational bronchoscopic biopsy is performed in central lesions. Occasionally, thoracoscopic wedge resection may be essential for histological diagnosis. Extensive immunohistochemistry reveals a final diagnosis in 50% of patients. Additional information is provided by gene expression or reverse-transcription polymerase chain reaction (RT-PCR). [1] Sputum cytological analysis or bronchial brushings for malignant cells may be positive in 35-50% of patients with pulmonary metastases. Cytologic analysis of any pleural fluid of malignant origin may yield positive results in as many as 50% of patients. Such analysis usually does not distinguish between primary and secondary malignant lesions; however, this can be performed for renal and colonic primaries. Additional workup includes hematologic studies such as complete blood cell (CBC) count and a basic metabolic panel (BMP), which may identify abnormalities possibly related to a paraneoplastic syndrome. [1] Limitations of techniques CXR may not identify small metastatic lesions and may underestimate the tumor burden. Dual-energy subtracted radiographs are more sensitive than conventional radiographs, owing to subtraction of overlying bony tissue. CAD has also been used for automatic detection of pulmonary nodules. Chest tomosynthesis is another low-dose technique with higher sensitivity that is used in the detection of lung nodules. CT scanning is more sensitive, but it has high rates of false positivity. The limitations of each technique are discussed in detail in the sections below. Radiography The most common radiographic pattern of pulmonary metastasis is the presence of multiple nodules, ranging in size from 3 mm to 15 cm or more. The nodules are more common in the lung bases (owing to higher blood flow than upper lobes) and in the outer third of the lungs in the subpleural region. They are approximately spherical and of varying sizes. Nodules of same size are believed to originate at the same time, in a single shower of emboli from the primary tumor. Nodules that are smaller than 2 cm are usually round and have smooth margins. Larger nodules are lobulated and have irregular margins; they may become confluent with adjacent nodules, resulting in a conglomerate multinodular mass. Pulmonary metastatic disease has several atypical presentations. Nodules may calcify or cavitate. Spontaneous pneumothorax is a rare presentation. A solitary pulmonary nodule is a less common presentation of pulmonary metastasis. A miliary nodular pattern refers to the presence of innumerable 1- to 4-mm nodules in the lungs that resemble millet seeds (see the image below). An airspace pattern, presenting with areas of consolidation, is another atypical presentation of metastatic disease. Endobronchial metastasis is not directly visualized in a radiograph, but it should be in the differential list when a patient presents with postobstructive pneumonitis/atelectasis. Pleural metastatic disease is seen as pleural nodularities or thickening with or without pleural effusion. Isolated pleural effusion is another type of metastatic disease.
12 Chest radiograph in a patient with thyroid cancer shows multiple miliary metastases Lymphangitic spread is seen on plain films as reticular or reticulonodular interstitial markings with irregular contours, Kerley B lines (thickened interlobular septa), hilar adenopathy, and pleural disease. Lymphangitic spread is much less commonly seen on plain radiographs than it is at pathology. Chest radiographs have been reported as normal in 50 % of patients who had histopathologically proven lymphangitis. [4] In addition, the radiographic appearances of lymphangitis are very nonspecific and radiographs are accurate in only 24% of proven cases. [2] See the images below. Chest radiograph of a 58-year-old man with malignant melanoma (note surgical clips in right lower neck) shows multiple pulmonary nodules of varying sizes consistent with metastatic disease. There is also a small right basal effusion.
13 Chest radiograph in a 62-year-old woman with malignant ovarian tumor shows multiple predominantly peripheral metastatic nodules. There are also small bibasal pleural effusions. Chest radiograph in a 67-year-old man with a history of spindle cell sarcoma of the thigh shows 2 large masses in the right lower lobe and the left mid lung, consistent with cannonball metastases.
14 Chest radiograph in a 57-year-old woman with leiomyosarcoma of the uterus shows multiple masses in the lungs, a large one in the medial right upper lobe and right paratracheal region, and another one in the left suprahilar region. There is also left basal pleural effusion with atelectasis. Chest radiograph in a patient with chondrosarcoma shows calcified masses in the right and left mid lungs and the left apical region, consistent with calcified metastasis.
15 Chest radiograph in a 58-year-old man with squamous cell carcinoma of the tongue shows multiple bilateral metastatic nodules, which demonstrate cavitations, consistent with cavitating metastases. Chest radiograph in a patient with squamous cell carcinoma of the head and neck shows collapse of the left upper lobe seen as hazy veil-like opacification. In addition, there are multiple bilateral nodules, with cavitation of the nodules on the left, consistent with metastatic disease.
16 Chest radiograph in a patient with a history of angiosarcoma of the thigh shows sudden the development of large bilateral pneumothoraces. In addition, there is right pleural effusion. There are multiple bilateral cystic lesions identified in both the lungs. Posteroanterior chest radiograph in a patient with a history of bladder cancer and lung metastasis shows a large loculated hydropneumothorax.
17 Lateral chest radiograph in a patient with bladder cancer and lung metastasis shows the presence of air-fluid levels due to hydropneumothorax. Chest radiography in a patient with laryngeal cancer shows a solitary pulmonary nodule in the left upper lung. This was biopsy proven to be metastasis.
18 Chest radiograph in a 55-year-old patient with renal carcinoma shows collapse of the right-middle and lower lobes. Note silhouetting of the right heart border and the right dome of the diaphragm. Chest radiograph in a patient with breast cancer shows volume loss of the right lung with ipsilateral mediastinal shift. There is lobulated soft-tissue thickening of the pleura. Chest radiograph shows a large pleural effusion and pleural metastatic nodules in a patient with metastatic adenocarcinoma.
19 Chest radiograph in a patient with prostate cancer shows bilateral pleural effusions. There are also fine reticular changes in the lungs due to lymphangitic spread. Chest radiograph in a 62-year-old patient with breast cancer shows volume loss of the right lung. There is also diffuse reticular changes of the right lung. There are a moderately sized right pleural effusion with atelectasis. No focal lesion is seen in the left lung. The findings are consistent with lymphangitis carcinomatosis. Degree of confidence CXR is shown to have less sensitivity and specificity than CT scanning in detection of pulmonary nodules. [15, 20] Chest radiographs are known to have low sensitivity compared with CT scans in the detection of pulmonary metastatic nodules, especially in primary head and neck cancers. CXR
20 fails to depict pulmonary metastatic lesions smaller than 7 mm, particularly those at the lung apices, bases, central locations adjacent to heart and mediastinum, pleural surfaces, and under the ribs. Compared with CT scanning, CXR is limited by overlapping structures and low contrast of the nodule. Nodules may also be obscured by vascular markings or may be hidden in areas of atelectasis or consolidation. Of nodules smaller than 7 mm detected on CXR, 77% are calcified and are more likely to be granulomas. [20] One study demonstrated sensitivities of 67% versus 100%, respectively, for CXR and PET scanning in the detection of metastatic nodules. [21] In addition, CXR also demonstrates fewer nodules than are shown with CT scanning. Often, a solitary lesion seen with CXR is associated with multiple nodules on a CT scan. Other causes of failure of nodule detection include incomplete visual survey or interpretative failures. [22] Sensitivity may be increased by using dual-energy subtraction, with which the superimposed bony structures can be subtracted. This technique uses the differences in the degree to which body tissues attenuate high- and low-energy photons. The differences are used to generate tissueselective images. Bones have higher attenuation coefficient at lower photon and beam energy, so that structures containing calcium can be removed from image. Dual-energy systems can be single- or dual-exposure systems. With single-exposure system, one radiograph is obtained by exposing 2 storage phosphor plates separated by a copper filter. Since the front plate receives a whole-energy beam, a standard image is produced, whereas the copper filter filters out the lower-energy photons, so that the back plate receives higher-energy photons. One weighted subtraction produces a bone-selective image, whereas the different weighted subtraction produces a soft-tissue subtracted image. One disadvantage is a low signal-to-noise ratio. With dual-energy systems, 2 radiographs are obtained at 60 and 120 kv, with the higher kv exposure producing a standard image. The signal-to-noise ratio of a dual-exposure system is higher than a single-exposure system. Misregistration artifacts may be produced due to an approximately 200-millisecond delay between the 2 exposures, caused by cardiac, respiratory, and patient motion. Dual-energy subtraction improves the ability to detect calcified and noncalcified nodules by reducing anatomic noise from overlying bones. Detection of calcium improves the confidence in making a diagnosis of a benign nodule. [23] Temporal subtraction enables easier detection of areas that have changed between radiographs at different time points. [22] Chest tomosynthesis is a relatively novel technique that increases the sensitivity of radiographs. The tomosynthesis system involves an x-ray tube, flat panel detector, computer-controlled tube mover, and special reconstruction algorithms to produce an arbitrary number of a section of images of the chest from a single pass of the x-ray tube. Tomographic images are obtained at lesser radiation and cost than a CT scan. Compared with radiography, it improves detection of nodules by reducing visual clutter from the overlying normal anatomy. Visibility of normal structures, such as vessels and airways, is improved. [24] However, the depth resolution of tomosynthesis is lower than that of CT owing to the limited angle used and low radiation dose, but this is much better than with CXR. A typical tomosynthesis obtains 60 projection images, with radiation exposure of approximately 2 microsieverts (msv), with a total dose of 0.12 msv, which is 3 times higher than with CXR (0.04 msv) but much lesser than with CT (4 msv).
21 Studies have shown higher visualization of nodules with tomosynthesis than with radiography. For example, in the study by Vikgren et al, CXR detected only 7% of nodules sized 4-6 mm, while tomosynthesis detected 50% of nodules. [25] Sensitivity is increased, especially for nodules smaller than 9 mm. Increased detectability is associated with a modest increase in radiation dose. Computer-aided detection (CAD) can be used as a second reader to detect small nodules that may be overlooked. This is useful for radiologists in training and has been applied both for frontal and lateral radiographs. [22] Note that these technologies may not be routinely available at all institutions. False positives/negatives False-positive findings can be seen because of a variety of disease processes. This depends on the pattern of the disease. Multiple pulmonary nodules can be seen in several other entities. Solitary pulmonary nodules can be due to primary lung cancer, a granuloma, a hamartoma, a vascular lesion, infection, or focal fibrosis. Lymphangitis carcinomatosis may be mistaken for pulmonary edema and fibrosis. Pulmonary hypertension resulting from thromboembolic disease may mimic disease caused by intravascular emboli. False-negative findings are seen in small lesions or when nodules are obscured by adjacent bony or vascular structures or pathological processes, such as atelectasis or consolidation. As a result of these limitations, several authors have disputed the role of routine CXR in patients undergoing metastatic screening. A study showed that undiagnosed metastasis was shown in radiographs in only 0.93 % of patients with known breast cancer. [26] Another study showed that in localized cutaneous malignant melanoma, although 15% had abnormal findings on initial CXR, only 0.1 % had true-positive metastatic lesions in follow-up radiographs. [27] These false-positive findings have been shown to increase patient anxiety. These authors recommend that cost effectiveness may be increased by reducing the frequency of screening in the first 2 years and limiting screening to only the first 5-10 years after diagnosis. In patients with a higher chance of pulmonary metastasis, screening should be more frequent, and a more sensitive test, namely CT scanning, is preferred. [1] Computed Tomography CT scanning is the modality of choice for detection and follow-up of pulmonary metastasis, owing to its higher spatial, temporal, and contrast resolution and lack of superimposition of adjacent structures. It has been shown to have higher sensitivity than chest radiography (CXR) in the detection of pulmonary metastases. CT scanning is performed using a multislice technique, and no intravenous contrast is required for the detection of pulmonary metastases. Contrast may be useful when a nodule is located adjacent to the hilum and mediastinum. See the images below. [69, 70]
22 Axial CT scan in a 58-year-old man with malignant melanoma shows multiple round nodules and masses of varying sizes in both lungs, consistent with metastases. There are also small bilateral pleural effusions. Coronal CT scan in a 58-year-old man with malignant melanoma shows multiple, predominantly basal nodules of varying sizes, consistent with metastatic disease.
23 Axial CT scan in a 62-year-old woman with malignant ovarian tumor shows predominantly subpleural metastatic nodules of varying sizes. Coronal CT scan in a 62-year-old woman with malignant ovarian tumor shows predominantly peripheral subpleural metastatic nodules of varying sizes. Axial CT scan in a 67-year-old man with a history of spindle cell sarcoma of the thigh shows a heterogeneously enhancing mass in the right lower lobe that is extending to the mediastinum and into the chest wall. The radiation dose from frequent CT scannings can be reduced by using several dose-reduction techniques such as low kv, low mas, adaptive-tube current modulation, and iterative reconstruction algorithms. The sensitivity of nodule detection can be increased by using postprocessing tools such as maximum-intensity projection (MIP) or volume rendering (VR) or using cine viewing of data sets (see the images below). High-resolution CT (HRCT) scanning is
24 used for detection of lymphangitic carcinomatosis. In this technique, spatial resolution is maximized by narrow collimation (1-2 mm) and high-resolution reconstruction algorithm. Axial maximum-intensity projection image with lung window demonstrates the chondroid matrix, consistent with metastatic disease. Axial maximum-intensity projection CT scan (8 mm thick) shows a small nodule in the right apical region in a patient with colonic cancer metastasis. Detection of metastasis early in the course of cancer is essential for treatment.
25 Coronal maximum-intensity projection CT scan (8 mm thick) shows the metastatic nodule in the right apical region in a patient with colonic cancer metastasis. Axial maximum-intensity projection CT scan in another patient shows the presence of multiple small metastatic nodules. The most common CT pattern of pulmonary metastasis is the presence of multiple pulmonary nodules (see the images below). These nodules are in a random distribution and are of varying sizes, owing to multiple episodes of tumor embolization or different tumor growth rates. Nodules of the same size are believed to be due to a shower of emboli that occurred at the same time. The margins can be smooth or irregular and can be either well defined or ill defined. The nodule has soft-tissue attenuation and can have a prominent pulmonary vessel heading into it, which is called the feeding-vessel sign. They are more common in the lung bases, owing to higher vascular supply.
26 CT scan in a 58-year-old man with squamous cell carcinoma of the tongue shows multiple bilateral cavitating metastatic nodules. There is also a right pleural effusion. Coronal CT image in a patient with squamous cell carcinoma of the head and neck demonstrates multiple cavitating pulmonary metastases and left upper lobe collapse. When the nodules are numerous, they are distributed diffusely throughout the lungs in a random pattern without any specific anatomical distribution; when nodules are few, they are predominantly subpleural. Multiple pulmonary nodules in a patient with known malignancy are highly suggestive of metastasis. Of multiple pulmonary nodules detected with CT scanning, 73% are shown to be metastases. [28] While 80-90% of patients with multiple metastatic nodules have a history of malignancy, some do not have a malignancy at the time of diagnosis, and, in few rare cases, the primary may never be found. The margins of the pulmonary metastasis are well circumscribed since histologically the tumor cells invade perivascular interstitium and have clear, smooth margins. However, once the tumor grows out of the vessels into the adjacent interstitium and alveolar space and proliferates, the margins become irregular. A radiologic-pathologic correlation study showed that well-defined, smooth-marginated metastasis corresponded to an expanding alveolar space-filling type (eg, hepatocellular carcinoma); poorly defined, smooth-marginated metastasis corresponded to an alveolar cell type (adenocarcinoma); and poorly defined, irregular-marginated metastasis corresponded to an interstitial proliferating type (squamous carcinoma or metastases after
27 chemotherapy). Some correlation also exists between the histological type of primary tumor and CT appearance of a lesion margin. [4] Because of this nonspecificity of margins, it is difficult to distinguish metastases from other confounding lesions. For example, some metastases have smooth margins and, hence, cannot be distinguished from benign lesions based on margins. Since some metastases have irregular margins, the margins cannot be used as a factor to differentiate primary cancer in a solitary pulmonary nodule. The differential diagnosis for multiple pulmonary nodules includes infections (eg, histoplasmosis, coccidioidomycosis in endemic areas, cryptococcal and nocardial infections as opportunistic infections in immunocompromised patients, septic emboli, abscess, paragonimiasis, hydatid), granulomatous diseases (eg, tuberculosis, sarcoidosis), and vascular/collagen-vascular diseases (eg, Wegener granulomatosis, rheumatoid arthritis). Appropriate clinical history and a temporal radiographic pattern showing the evolution of ill-defined pulmonary opacities into organizing, more circumscribed nodules as part of the healing process is the key factor differentiating these from pulmonary metastatic disease. Follow-up CT scanning in 6 weeks to 3 months will show progression of metastatic nodules, while benign lesions show no growth, decrease in size, or undergo complete resolution. While these classic features are extremely helpful in narrowing the differential mainly down to metastases, it is the atypical features of lung metastases that are difficult to distinguish it from more benign lung pathology. Cannonball metastasis (see the image below) refers to the presence of few, large, well-circumscribed, and round metastatic masses. It is usually seen in metastasis from renal carcinoma, choriocarcinoma, colon cancer, prostate cancer, or endometrial carcinoma. Chest radiograph in a 67-year-old man with a history of spindle cell sarcoma of the thigh shows 2 large masses in the right lower lobe and the left mid lung, consistent with cannonball metastases.
28 Miliary nodules refer to numerous, small 1-4 mm, same-sized nodular opacities that resemble millet seeds. Miliary metastases are seen in cancers of the thyroid (medullary carcinoma), kidney, breast, and pancreas, as well as in malignant melanoma, osteosarcoma, and trophoblastic disease. They are believed to be caused from a single massive shower of tumor emboli. Miliary nodules are seen in a random distribution within the secondary pulmonary lobule and involve the subpleural regions. (Note that centrilobular nodules spare the subpleural regions, whereas perilymphatic nodules involve the subpleural regions and the peribronchovascular regions.) The differential diagnosis for miliary nodules includes granulomatous infections such as tuberculosis, histoplasmosis, healed varicella pneumonia, sarcoidosis, silicosis, coal worker s pneumonoconiosis, hypersensitivity pneumonitis, and Langerhans cell histiocytosis. Histological studies have described the distribution of a metastatic nodule within a secondary pulmonary nodule. The metastatic nodule initially proliferates from tumor emboli in the arteriole or capillary. Initially, a metastatic nodule is seen in a peripheral portion than the centrilobular structures. Only % were shown to be located in the central bronchovascular bundle, [4] with 60-68% between central and perilobular structures and 20-28% in perilobular structures. When the metastasis subsequently grows, it appears to be connected with the bronchovascular bundle, which is called the mass-vessel sign. Small, random metastatic nodules in secondary pulmonary lobules are randomly distributed with no uniform relationship to secondary pulmonary lobular structures. Cavitation is seen in 4% of metastases (vs 9% of lung primaries). [29] Tumor necrosis and discharge of necrotic material is thought to be the primary mechanism behind cavitating lung metastases (excavating metastasis). These tumors initially are solid and later become a cavitary lesion with thick and irregular walls. See the images below. Chest radiograph obtained 6 months later in the patient above shows the development of a cavitating lesion with air-fluid levels in the left lower lobe.
29 CT scan 6 months later than the initial CT scan (same patient as above) shows that the cavity has enlarged in size and has also cavitated, indicating the presence of excavated metastasis. Cavitation can also be caused by a check-valve mechanism of tumor infiltrating into bronchial structures. Head and neck cancers in males and genitalia cancers in females are the common causes. Squamous cell carcinomas are the most common (70%) primary tumor to cause cavitation, especially seen on radiographs, although adenocarcinomas (GI tract, breast), transitional cell tumors, and sarcomas are also known to cavitate on CT scans. On CT scans, 10% of squamous carcinoma metastases were shown to cavitate, while 9.5% of adenocarcinoma metastases were also shown to cavitate. [30] Cavitation can also be seen following chemotherapy of metastatic nodules. Cavitated metastasis usually has thick and irregular walls. Thin-walled cavities are seen in sarcomas, which are the ones that often result in pneumothorax. See the images below. Axial CT scan in a patient with history of angiosarcoma of the thigh shows a pneumothorax on the left. There are multiple thin-walled cystic metastatic lesions, some of which can be clearly seen extending up to the subpleural region. The pneumothorax is presumably caused by rupture of a cystic metastasis into the pleural space.
30 Coronal CT scan in a patient with history of angiosarcoma of the thigh shows a pneumothorax on the left. There are multiple thin-walled cystic metastatic lesions, some of which can be clearly seen extending up to the subpleural region. The pneumothorax is presumably caused by rupture of a cystic metastasis into the pleural space. The differential diagnosis for cavitating nodules includes septic emboli (eg, septic patients, intravenous drug abusers), lung abscess, tuberculosis, angiitis, Wegener granulomatosis, and rheumatoid nodules. Nine percent of primary lung tumors cavitate, most commonly squamous cell cancers. Spontaneous pneumothorax is an uncommon presentation of pulmonary metastasis. Osteosarcoma is the most common tumor known to produce pneumothorax. Pneumothorax has been shown in 5-7% of osteosarcoma metastasis. [31] Spontaneous pneumothorax in a patient with osteosarcoma should raise suspicion of pulmonary metastasis, which can be detected with CT scanning. Aggressive sarcomas and nonsarcomatous tumors can also produce pneumothorax. The proposed theory behind spontaneous pneumothorax is tumor necrosis in peripheral subpleural nodules resulting in a bronchopleural fistula with subsequent pneumothorax. The differential diagnosis for pneumothorax includes rupture of a subpleural bleb, trauma, mechanical ventilation complications, and underlying lung diseases (eg, cystic fibrosis, tuberculosis, fibrosis, sarcoidosis). Metastasis from teratoma of the testis may show complete fibrosis or necrosis after chemotherapy. Thin-walled air cysts, which contain no viable tumor, are present at the site of treated metastasis. Multiple thin-walled cystic metastases are also seen in metastasis from angiosarcoma. This is the second most common type of presentation for angiosarcoma metastasis after multiple pulmonary nodules. Rupture of subpleural cystic metastasis may result in pneumothorax. Proposed mechanisms for thin-walled cysts are (1) excavation of a solid nodular lesion through discharge of necrotic tumor material, (2) infiltration of tumor cells into walls of preexisting bulla, (3) a ball-valve
31 effect caused by circumferential growth of tumor around small bronchioles resulting in bronchiolar obstruction, and (4) proliferation of tumor cells forming blood-filled cystic spaces anastomosing the network of sinusoids. [32] Calcification of metastatic nodule is often seen only on CT scans. Calcification is seen in metastasis from osteosarcoma, chondrosarcoma, giant cell tumor of the bone, mucinous adenocarcinoma, and treated metastatic choriocarcinoma. Reasons for calcification vary but include bone formation in primary bone tumors (osteosarcoma, chondrosarcoma), dystrophic calcification (papillary thyroid cancers, giant cell tumor of bone, synovial sarcoma, treated metastasis), and mucoid calcification (mucinous adenocarcinomas of the GI tract, breast, thyroid, ovary). Punctate calcification may be seen following hemorrhagic necrosis in angiosarcoma. [32] Calcification can also be seen following chemotherapy and radiation therapy. In osteosarcomas, dense, eccentric calcification/ossification is seen. In rare instances, calcification may develop at the site of pulmonary metastasis (typically from a testicular primary site) that has vanished after chemotherapy. [30] The differential diagnosis for calcified nodule includes granulomatous diseases (tuberculosis, histoplasmosis), sarcoidosis, silicosis, coal worker s pneumoconiosis, and alveolar microlithiasis. CT scans cannot help differentiate calcifications or ossifications due to metastasis from calcifications or ossifications due to other lesions. Another atypical feature of metastatic disease is nodular density surrounded by a halo of ground-glass attenuation or ill-defined fuzzy margins (ie, the CT halo sign). This is seen in hypervascular primary tumors such as choriocarcinoma (parenchymal hemorrhage), angiosarcoma (fragility of neovascular tissue resulting in rupture of vessels), or renal carcinoma. A ruptured vessel secondary to fragile neovascular tissue leads to hemorrhage, causing the ground-glass halo on CT scans. The differential diagnosis for this appearance includes invasive aspergillosis, candidiasis, tuberculoma, Wegener granulomatosis, minimally invasive adenocarcinoma, pneumonia, eosinophilic pneumonia, abscess and lymphoma in immunocompromised patients (due to fibrin, less dense inflammatory reaction, edema, or less densely arranged malignant cells histopathologically), or post biopsy. An air-space pattern is another atypical presentation of metastasis. This can be due to lepidic growth of tumor along intact alveolar walls, which is seen in metastatic adenocarcinoma from GI tract, ovary, or breast. Imaging features include consolidation with air bronchography, ground-glass opacities, and air-space nodules. Another mechanism is pulmonary infarction due to tumor embolism, which is seen in tumors of the liver, breast, kidney, stomach, and prostate, as well as in choriocarcinoma. The differential diagnosis for this air-space pattern includes infections, edema, hemorrhage, organizing pneumonia, eosinophilic pneumonia, minimally invasive adenocarcinoma, lymphoma, and sarcoidosis, among other entities. Tumor embolism is a less common presentation of metastatic disease. Although most pulmonary metastases result from microscopic tumor embolization, only a few survive to proliferate as metastases. With tumor emboli, the tumor is confined to the vascular tree, without proliferation of metastasis into extravascular tissue. In an autopsy series, intravascular tumor emboli have been seen in % of patients with solid malignancy. [33] Tumor emboli is seen in metastasis from liver, breast, renal, gastric, and prostatic cancers, as well as in sarcomas and choriocarcinomas. Tumor emboli are seen in small or medium-sized arteries. Large tumor emboli within main, lobar, or segmental pulmonary arterial branches are only rarely seen.
32 Diagnosis may be difficult to make, even with HRCT scanning. On CT scans, multifocal dilatation and beading of the peripheral subsegmental arteries are seen due to smaller tumor emboli. Also seen are peripheral wedge-shaped areas of infarction. Perfusion defects can be identified with dual-source CT scanning. Occasionally, tumor emboli may be seen within the larger pulmonary vessels. The differential diagnosis of tumor emboli includes pulmonary thromboembolism and pulmonary artery sarcoma. Pulmonary artery enlargement (>2.9 cm, or larger than the ascending aorta) may be due to large tumor emboli or the development of pulmonary hypertension from large or numerous tumor emboli. Endobronchial metastasis is rare, seen in 2% of tumors. [30] Primaries that cause endobronchial metastases are renal, breast, colorectal, and pancreatic cancers. Endobronchial involvement occurs through 2 routes: (1) direct endobronchial deposition through aspiration, hematogenous, or lymphatic spread or (2) by airway invasion of tumor into adjacent lymph nodes/parenchyma. The endobronchial lesion, as well as the consequences (eg, lobar atelectasis or, less commonly, complete collapse of a unilateral lung) can be identified on CT scans. The differential diagnosis includes primary neoplasms such as bronchogenic carcinoma, carcinoid, granulomas such as in tuberculosis, histoplasmosis, foreign bodies, or broncholiths. See the images below. Axial CT scan of the same patient at a higher level shows an endobronchial mass within the bronchus intermedius, which is the cause of the collapse of the right upper and lower lobe.
33 Axial positron emission tomography scan of the same patient shows high uptake in the endobronchial mass, which was proven to be endobronchial metastasis. Solitary pulmonary nodule (see the image below) is a less common presentation of metastatic disease. Solitary pulmonary nodule is a round opacity that is at least moderately well marginated and less than 3 cm in maximum diameter. [22] Solitary pulmonary metastasis is frequent in melanoma; sarcoma; and cancers of colon, breast, kidney, bladder, and testicle. Carcinoma of the colon, especially from the rectosigmoid area, accounts for a third of cases with solitary pulmonary metastasis. Metastasis accounts for 2-10% of solitary nodules. [34] The differential diagnosis is extensive, but the most common lesions are primary lung neoplasms, granuloma, hamartoma, and arteriovenous malformation. Granulomas (tuberculosis, histoplasmosis) show calcification, which is usually central, diffuse, or laminated. Hamartoma may have popcorn calcification, fat attenuation, or a combination. Arteriovenous malformation has a feeding vessel. Axial CT scan in a patient with laryngeal cancer shows a solitary pulmonary nodule in the apicoposterior segment of the left upper lobe. This was biopsy proven to be metastasis. In a patient with known malignancy, development of a solitary pulmonary nodule is a challenge. Distinguishing between a primary and secondary neoplasm is important, since it has prognostic and therapeutic implications. At surgery, 0.4-9% of solitary pulmonary nodules are likely to be metastasis in a patient with known extrathoracic malignancy. [34] Solitary pulmonary nodules seen on radiographs have a 25% chance of being metastasis, [30] while those seen on chest CT scans have a 46% chance of being metastasis in a patient with known extrathoracic malignancy. [30] It should be noted that CT scans may show additional lesions compared with a radiograph, in which case the diagnosis becomes easier. More than one additional nodule was seen on a CT scan in 32% of patients with suspected solitary pulmonary nodule based on radiographs. [35] The likelihood of a solitary pulmonary nodule being metastasis depends on the histopathology of
34 the primary tumor and age of the patient. [30] The incidence of a second primary lung malignancy is higher than a solitary metastasis in patients with cancers of the head and neck (8:1 ratio), bladder, breast, cervix, bile ducts, esophagus, ovary, prostate, or stomach (3:1 ratio for all these). This likelihood also exists for tumors of the salivary gland, adrenals, colon, kidney, thyroid, thymus, or uterus. There is higher chance of metastasis (2.5:1) with melanoma, sarcoma, and testicular cancer. [36] In patients with melanoma or sarcoma, solitary lung metastasis is more common than a second primary lung cancer. No reliable imaging features help to distinguish a solitary pulmonary metastatic nodule from primary pulmonary neoplasms. Metastatic nodules may be round or oval, or they may have lobulated margins. Initially, it was thought that metastatic solitary pulmonary nodules have smooth margins compared with primary lung cancers, which can have speculated or lobulated margins. However, it is now known that margins are not helpful in distinguishing primary and secondary tumors, since metastasis can also have irregular, speculated margins due to a desmoplastic reaction or tumor infiltration into the adjacent lymphatics or bronchovascular structures. Smooth borders and lobulated margins can also be seen in benign lesions such as hamartomas. See the images below. Coronal contrast CT scan in a patient with breast cancer shows volume loss of the right lung with multiple lobulated pleural metastatic nodules.
35 Axial CT scan in the same patient shows volume loss of the right lung and lobulated pleural metastasis extending to the mediastinal pleural surface Laminated, central, diffuse, and popcorn calcification are benign patterns, while stippled and eccentric calcifications are considered malignant. Solitary metastases are more common in lower lobes, while primary lung cancer is more common in upper lobes. Attenuation measurements may be of some value. In a recent study, [37] the mean attenuation value of pulmonary metastasis from renal cancer was found to be higher than that of primary lung cancer nodules. The interval between appearance of initial tumor and solitary pulmonary nodule may be useful. An interval of more than 5 years in patients with osteosarcoma more likely represents a new primary tumor. However, in patients with carcinoma of the breast or kidney, pulmonary metastases may occur many years after the primary tumor is diagnosed. Biopsy is often required for histopathological diagnosis in this scenario. Lymphangitic carcinomatosis refers to spread of a neoplasm through the lymphatics (see the images below). It is most commonly seen in adenocarcinomas, particularly primary tumors of breast, lung, stomach, pancreas, uterus, rectum, or prostate. It is seen in 35% of autopsies of patients with solid tumors. [2] It occurs from hematogenous spread to the lungs, with subsequent lymphatic invasion or direct lymphatic spread from mediastinal and hilar lymph nodes. Axial high-resolution CT scan in another patient with renal cancer shows smooth interlobar septal thickening in the upper lobes, right greater than left, and bilateral pleural effusions. This was caused by lymphangitic spread. The differential diagnosis includes edema and infection.
36 Coronal CT scan in the same patient with breast cancer shows smooth interlobar septal thickening in the upper lobes, right greater than left, and bilateral pleural effusions. This was caused by lymphangitic spread. The differential diagnosis includes edema and infection. Microscopically, malignant cells are seen in lymphatic cells and interlobular septa. Edema or a desmoplastic reaction can contribute to interstitial thickening. Associated pleural involvement is common. The imaging appearance is due to direct tumor growth in pulmonary capillaries and lymphatics within septal interstitium. The typical CT pattern consists of smooth, beaded, or nodular thickening of the interlobular septa. Smooth subpleural interstitial thickening is seen as thickening of fissures. There is also thickening of the axial interstitium surrounding the vessels and bronchi in parahilar regions, resulting in peribronchial cuffing. Nodules are seen in a perilymphatic distribution in the peribronchovascular and subpleural regions. However, the lung architecture is preserved at the lobular level. A nodular component from intraparenchymal extension may be associated with lymphangitic carcinomatosis. A polygonal structure with a central dot may be seen due to thickened interlobular septa and thickened intralobular axial interstitium by tumor growth. The above-mentioned findings can be bilateral and symmetrical or asymmetrical, or diffuse or focal. Lymphangitic spread is more common in the lower lobes. In patients with malignancies and dyspnea, diagnosis is confirmed on imaging findings, and no further examination is indicated. Hilar lymphadenopathy and mediastinal lymphadenopathy are present in 20-40% of patients, which may be symmetrical or asymmetrical, and pleural effusions are present in 30-50% of patients with lymphangitic carcinomatosis. [2] Early diagnosis of lymphangitic carcinomatosis based on CXR findings can be difficult, as they may be normal in 30-50% of proven cases. [2] Lymphangitic spread can also be caused by primary tumors of the lungs, especially small cell carcinoma and adenocarcinoma. The differential diagnosis of perilymphatic nodules includes sarcoidosis, silicosis, coal worker s pneumoconiosis, and amyloidosis. When the septal thickening is smooth, the differential diagnosis includes edema, infection, and fibrosis. An absence, distortion, or destruction of normal lung architecture at the lobular level distinguishes lymphangitis from pulmonary fibrosis, which is associated with architectural distortion. Although sarcoidosis has a similar appearance, with perilymphatic nodules and nodular or beaded interstitial thickening, the degree of septal thickening is less than that of lymphangitic spread. Pleural metastases (see the first image below) originate from hematogenous spread to the pleura, but occasionally they may be caused by lymphangitic spread or by direct infiltration of chest wall, abdomen, and mediastinum or from established hepatic metastases. Tumors that spread to the pleura are lung, breast, pancreas, and stomach. Pleural metastases are seen as nodularities, a plaquelike formation on the pleural surface with or without associated pleural effusion. Pleural metastases in contact with the fissures and the diaphragm may be easily detected using CT
37 scanning (see the second and third images below). The differential diagnosis includes malignant mesothelioma, invasive thymoma, and lymphoma. Axial CT scan in the same patient shows volume loss of the right lung and lobulated pleural metastasis extending to the mediastinal pleural surface Axial CT scan of a patient with thyroid cancer shows multiple solid masses along the right major fissure, which are metastatic. There is also right pleural effusion.
38 Coronal CT scan of a patient with thyroid cancer shows multiple solid masses along the right major fissure, which are metastatic. There is also right pleural effusion. Malignant pleural effusion is seen in the lung, breast, and ovaries, as well as in lymphoma. It is seen in up to 42% of cases. There may be associated pleural thickening and contrast enhancement. [30] See the images below. Axial CT scan in a patient with breast cancer shows malignant effusion on the right with multiple contrastenhancing metastatic nodules.
39 Coronal CT scan in a patient with breast cancer shows malignant effusion on the right with multiple contrastenhancing metastatic nodules. Dilated, tortuous, and enhancing vessels are occasionally seen within metastasis. This is seen in very vascular primaries, particularly sarcomas such as alveolar soft-tissue sarcoma and leiomyosarcoma. Tracheal metastasis (see the images below) is seen either from local invasion of tumors such as thyroid, larynx, esophagus, or lung tumors, or by hematogenous spread of tumors, most commonly colorectal, breast, renal, sarcoma, melanoma, and hematological malignancies such as plasmacytoma and chloroma. Typically, tracheal metastasis is discovered 4 years after the primary tumor. The clinical presentation is hemoptysis and coughing. CT scans shows solitary or multiple masses within the trachea. The imaging appearances are nonspecific and can be seen in infections (tuberculosis, histoplasmosis), inflammatory conditions (sarcoidosis), and neoplastic conditions (squamous cell carcinoma, mucoepidermoid carcinoma, adenoid cystic carcinoma). A history of extratracheal malignancy is helpful. The diagnosis can be confirmed by biopsy. Volumerendered images of the airway are helpful in planning bronchoscopic biopsy. Axial CT scan shows a large metastatic mass in the trachea from squamous cell carcinoma of the lung.
40 Coronal CT scan shows a large metastatic mass in the trachea from squamous cell carcinoma of the lung. Occasionally, a metastatic nodule that is seen on a CT scan may histologically contain only necrotic nodule viable tumor cells, with or without fibrosis. This is called sterilized metastases. This is seen in choriocarcinoma or testicular carcinoma after chemotherapy. Serial CT scans show no change in the size of the nodules. Fluorodeoxyglucose (FDG) positron emission tomograph (PET) scanning may be useful in such a scenario, since a previously active tumor may now have no FDG uptake. Biological markers such as β-human chorionic gonadotrophin and α-fetoprotein may be negative. Biopsy is often required for confirmation and for excluding the presence of a viable tumor. Occasionally, a sterilized and viable tumor may coexist. [30] Another interesting scenario is when a malignant germ cell tumor converts into a benign mature teratoma, resulting in a persistent or sometimes even enlarging mass. Again, in this scenario, the tumor markers may be negative. Diagnosis can be confirmed by biopsy. [30] Even rarer is the development of thin-walled cavities, namely pulmonary lacunae that develop at sites of germ cell tumors treated with chemotherapy. These may persist for many years and are not malignant. Occasionally, benign tumors may metastasize to the lung. This is seen in leiomyoma of the uterus, hydatidiform mole of the uterus, giant cell tumor of bone, chondroblastoma, meningioma, and pleomorphic adenoma of the salivary gland. The radiological findings of these lesions are indistinguishable from those of metastatic malignant lesions, with the only difference being that they show slow or no growth. Maximum-intensity projection/volume rendering While significant progress has been made with technological advancements resulting in today s multidetector CT scanning techniques, human perception errors continue to be a significant hurdle in the detection of small intrapulmonary nodules. To that end, there are commercially available techniques such as MIP and VR that allow displaying a subvolume of the 3-dimensional data set. In MIP, only voxels with the maximum intensity are displayed along a projection line from the viewer s eye through the 3-dimensional volume of interest. On the other hand, the VR technique incorporates the assignment of opacity values to CT numbers, meaning high opacity values produce an appearance similar to surface rendering and low opacity values allow the user to see
41 through structures. A publication from 2007 directly compared MIP with VR in the detection of pulmonary nodules. VR performed significantly better than MIP for lung nodules smaller than 11 mm in diameter (P <.001) and was equivalent to MIP (P =.061) for larger nodules. Additionally, VR was better in the detection of perihilar nodules. [38] A study by Jankowski et al [39] showed that the sensitivity of nodule detection was higher with MIP than with 1-mm axial images or computer-aided detection (CAD) for all nodules (F-values=0.046). For nodules larger than 3 mm, sensitivities were higher with 1-mm images or MIP than with CAD (P <.0001). In addition, MIP is the least time-consuming technique, and CAD was the most time-consuming technique. MIP and CAD reduced the number of overlooked small nodules. Using MIP reduces the number of overlooked small pulmonary nodules, especially in the central lung and in junior-reviewer detection of pulmonary nodules. [40] Kawel et al [41] showed that the sensitivity of nodule detection was superior for 8-mm MIP than for 11-mm MIP and all thicknesses of volume-rendered images, independent of nodule localization and size. Computer-aided detection CAD has been shown to assist radiologists in the detection of pulmonary nodules. Modern multislice CT systems are associated with huge data sets, in which small lesions may be easily missed. A CAD system recognizes opaque lesions surrounded by lung parenchymal attenuation as nodules. The sensitivity of a CAD system varies from 38-95% in various studies, [42] owing to varying algorithms, CT input, and varying populations. Nodules not surrounded by lung parenchyma are likely to be missed, particularly in the subpleural areas, fissural areas, and costophrenic angle areas. Some lung parenchymal nodules may also be messed. In a study by Song et al, CAD detected an additional 5% of nodules compared with human readers. [42] However, a high false-positive rate is a major limitation, which hinders its widespread application. The main use of CAD is as a second reader to improve the sensitivity of the human reader. CAD identifies nodules overlooked by radiologists. A study by Armato et al showed that CAD detected 84% of 38 cancers missed by radiologists. [43] CAD can also be used to determine the probabilities of malignancy. The potential exists for integrating CT and PET data to improve characterization. [44] CAD can also measure tumor size, tumor volume, attenuation, and enhancement characteristics. It can also assess temporal changes in the characteristics of nodules based on CT scans obtained at different time points. By automatically identifying corresponding CT sections, it decreases interpreter time. Textural characteristics of the tumor may also be assessed to determine the presence of solid components, which have a higher likelihood of transformation into malignant nodules. [22] A limitation is of CAD is the decreased efficiency in the detection of ground-glass nodules. Indications CT scanning is not required if CXR shows multiple nodules in a patient with a known primary malignancy. In a patient with known malignancy, chest CT scanning is performed if (1) CXR shows
42 a solitary nodule; (2) CXR shows an equivocal nodule; (3) CXR findings are negative but the extrathoracic malignancy has a high risk of lung metastasis (eg, breast, kidney, colon, bladder); or (4) CXR shows multiple nodules, but biopsy or definitive treatment by mastectomy, chemotherapy, and radiation is planned. In patients with high-risk tumors such as bone and soft-tissue sarcomas, testicular tumors, and choriocarcinomas, CT scanning is recommended every 3-6 months for 2 years. [1] Degree of confidence CT scanning has much higher sensitivity than CXR in the detection of pulmonary metastases. However, studies including that by McCormack et al showed that CT scanning underestimated the pulmonary metastatic involvement discovered in surgery by up to 25%. [45] Another study showed that surgery discovered 22% more malignant nodules than those detected by helical CT scanning. [1] Hence, manual palpation is recommended during surgery to detect subtle lesions. CAD is also available for chest CT scanning, most often as a second look after the radiologist has reviewed the study. It has been shown to detect 82.4% of known pulmonary nodules. [46] Currently, these techniques are still at the developmental stage and are used only if the image quality is good; additionally, breathing artifact is limited with stable lung expansion. False positives/negatives Although CT scanning is very sensitive, the finding of a nodule is not specific. False-positive results may be caused by hamartomas, granulomas (eg, tuberculosis, histoplasmosis, Wegener granulomatosis), sarcoidosis, silicosis, small infarcts, small areas of fibrosis, and intrapulmonary lymph nodes. The specificity of CT scanning depends on the following: Propensity of the primary malignancy to metastasize to the lung Stage of primary malignancy Age Smoking history History of prior treatment History of granulomatous disease Prevalence of benign nodules in the population Features that are more favorable of malignancy are as follows: Noncalcified nodule Spherical or ovoid shape rather than linear or irregular shape Close relationship to adjacent vessel Lesion with decreased attenuation distally Lesion with reticular changes Doubling time of metastases from 2-10 months Size is not useful in distinguishing benign and malignant nodules. Although a direct correlation exists between nodule size and malignancy, the size should be evaluated in the context of a known malignancy. In patients with known malignancy, it should be noted that nodules smaller than 1 cm can also be metastasis. A study showed that in patients with known malignancy, video-assisted thoracic surgery showed
43 that 84% of nodules smaller than 1 cm were malignant, of which 54% were metastasis, 29% were new primaries, and only 18% were benign. [47] Another study by Ginsberg et al showed that in oncologic patients undergoing video-assisted thoracic surgery, nodules smaller than 5 mm were malignant in 42% of patients with cancer. [48] Lesions smaller than 7 mm cannot be characterized on CT scans, because they are not amenable to biopsy and are not palpated at surgery. [1] Temporal assessment of nodules is also useful in determining malignancy. However, nodule measurements have significant interobserver and intraobserver variability, which can be reduced by automated or semiautomated measurements. Three-dimensional volume measurement may be more accurate and reproducible, but it is also prone to precision errors. [22] Doubling times less than days are suggestive of infections or rapidly growing metastasis. Doubling times greater than 400 days are benign lesions. Nodules being stable for at least 2 years is an indicator of benignity (with the exception of subsolid nodules). Nodule enhancement can be also used to distinguish benign and malignant nodules. Thin-section CT images are obtained through the nodules before and after 1, 2, 3, and 4 minutes following administration of contrast at 2 ml/second. Enhancement of 15 HU or less is suggestive of benignity, while higher degrees of enhancement are suggestive of malignancy or inflammatory. This technique has 98% sensitivity for malignancy and 58% specificity for benignity. This technique is suitable for nodules between 7 mm and 3 cm and for noncalcified nodules. Peak attenuation of nodules correlates positively with microvessel density and vascular endothelial growth factor staining on pathology. (Malignant lesions have higher vascular endothelial growth factor expression. [49] ) With the advent of dual-source CT scanning, simultaneous 80 kv and 140 kv images can be obtained, which helps in identifying areas of fat, bone, soft tissue, and iodinated contrast. Virtual unenhanced images can be generated, which then can be subtracted from contrast enhanced scans to evaluate areas of enhancement, yielding an estimate of tumor perfusion. [22] CT may miss nodules that are centrally located (either within bronchi or adjacent to vessels), are small, have faint attenuation, are at a lower lobe location, or are adjacent to or within parenchymal abnormalities. Sensitivity may be improved by using MIP, VR, or cine viewing of datasets. Magnetic Resonance Imaging In the lungs, MRI is typically used in the evaluation of involvement of the mediastinum and the chest wall. MRI has the advantages of no radiation or iodinated contrast media exposure and higher soft-tissue contrast resolution, which makes it useful patients requiring frequent follow up, especially in young and female patients. However, MRI is not used in the evaluation of pulmonary nodules, including metastasis, owing to several limitations and challenges. These include the following: Lower spatial resolution Inability to detect calcification Motion artifacts from breathing and cardiac pulsation on sequences with lower temporal resolution Low proton density and very short T2* value of lung Higher susceptibility differences between air spaces and pulmonary interstitium
44 Inhomogeneity of magnetic field However, novel sequences have been developed and adapted for the lungs. Findings MRI sequences that have been evaluated in the evaluation of pulmonary nodules include the following [50] : T2-weighted half-fourier acquisition single-shot turbo spin-echo (HASTE) Three-dimensional gradient-related echo (eg, volume-interpolated breath-hold [VIBE]) T1-weighed T2-weighted Short-tau inversion recovery (STIR) sequences [51] Diffusion-weighted imaging (DWI) Dynamic contrast-enhanced (DCE) sequences Metastatic nodules have low- or intermediate-signal intensities on T1-weighted images and slightly higher intensity on T2-weighted spin-echo or turbo spin-echo sequences. T1-weighted, T2-weighted, and STIR sequences can help distinguish neoplasms from other lesions, such as tuberculoma, bronchocele, mucin-containing tumors, hamartoma, and aspergilloma, but differentiating a benign from malignant lesion is not easy with MRI. [51] HASTE sequence is favored owing to higher T2 relaxivity and a higher signal for neoplastic lesions relative to the air-filled low signal of lungs. Vessels are seen as flow voids. The sensitivity of HASTE for nodules of 6-10 mm has been shown to be 95.7%, [52] while the sensitivity for nodules smaller than 3 mm is 73%. [53] Although HASTE has the lowest motion artifact, breath-hold T2-weighted turbo spin-echo sequences have been shown to detect more lesions than HASTE. [54] Three-dimensional gradient echo sequences such as VIBE MRI sequences are also good in detecting pulmonary nodules. Although multidetector CT scanning is better than MRI at detecting 1- to 3-mm nodules, one can argue that these nodules are not significant in a low-risk population, while MRI is a good alternative to multidetector CT scanning for the detection of nodules larger than 5 mm. [54] DCE MRI and parameters such as maximal enhancement ratio and slope of contrast uptake with three-dimensional gradient-echo sequences can be used to distinguish malignant from benign nodules with a sensitivity, specificity, and accuracy of 100%, 70%, and 95%, respectively. [55] However, it is not possible to distinguish malignancy and active infection, a problem similar to positron emission tomography (PET) scanning. Kono et al [56] showed an early peak pattern of enhancement in malignancy and active infection. Higher signal is seen on DWI sequences and low signal on apparent diffusion coefficient (ADC), owing to increased cellularity, high tissue disorganization, and extracellular space tortuosity. However, higher signal is also occasionally seen in granulomas, inflammatory nodules, and fibrous nodules. DCE MRI has the capability of distinguishing benign and malignant pulmonary nodules based on the presence of tumor angiogenesis, tumor interstitial spaces, fibrosis, scarring, and necrosis.
45 Malignant pulmonary nodules have homogeneous contrast enhancement, but at different levels of T1-weighted images after contrast media, compared with benign nodules. Degree of confidence MRI has lower sensitivity than CT in the detection of metastatic pulmonary nodules. Using turbo spin-echo, the sensitivity of MRI was 84% compared with CT scanning and only 36% for nodules smaller than 5 mm. [57] With STIR, the sensitivity was 72% for nodules larger than 5 mm. [54] False positives/negatives False-positive findings may be seen with MRI owing to diaphragmatic motion, especially in the lower lobes. Small nodules near the diaphragm may be missed because of respiratory motion, resulting in false-negative findings. Lesions may also be missed because of lower spatial resolution and motion artifacts, either from breathing or cardiac pulsation. Ultrasonography Ultrasound has only a very limited role in the evaluation of pulmonary metastases. Ultrasound may be used in aspiration of pleural effusions to detect malignant cells and to obtain a biopsy specimen from pleural nodules (see the image below). Parenchymal lesions in subpleural regions may undergo biopsy using ultrasound guidance. Endoscopic ultrasound with bronchoscopy is used in the evaluation and biopsy of pulmonary nodules and mediastinal and hilar lymph nodes. Ultrasound guidance used for transthoracic aspiration of malignant effusion. Nuclear Imaging
46 The primary scintigraphic modality used in the evaluation of pulmonary metastasis is fluorine fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET). FDG is a glucose analogue. One of the important biochemical alterations in a cancer cell is the increased rate of glycolysis that results in increased cellular glucose uptake. This principle is used in the detection of neoplastic lesions. See the images below. CT scan in a patient with squamous carcinoma of the tonsil shows a 2.5-cm lesion in the left lower lobe. Positron emission tomography in the same patient (with squamous carcinoma of the tonsil) shows high fluorodeoxyglucose uptake in the nodule, which was shown by biopsy to be metastatic.
47 Axial positron emission tomography scan of the same patient shows high uptake in the endobronchial mass, which was proven to be endobronchial metastasis. FDG-PET increases the specificity of nodules based on their metabolic activity. It works well with extrathoracic primaries such as bone and soft-tissue sarcomas, malignant melanomas, and head and neck cancers. However, it is not the study of choice when the primary tumor is renal cell carcinoma or testicular cancer, for which it receives an American College of Radiology (ACR) appropriateness criteria of 1 and 3, respectively, equating to usually not appropriate. [1] This is related to the poor FDG avidity of these tumors. Another application of FDG-PET is in differentiating benign and malignant nodules, especially in solitary pulmonary nodules. PET scanning has been shown to have a sensitivity of 96% and specificity of 88% in diagnosing a nodule as malignant. [58] The positive predictive value is lower owing to false positives caused by infection/inflammation. The negative predictive value and sensitivity are lower owing to lower spatial resolution. It is also important to note that PET scanning has very poor sensitivity in the detection of nodules smaller than 1 cm. Hence, a negative FDG-PET scan does not exclude metastatic disease, owing to an absence of uptake in lesions smaller than 1 cm and in non FDG-avid primaries. Specificity is also affected secondary to false-positive results from non-neoplastic inflammatory processes. In a study on head and neck cancers, PET-positive lesions were seen in 27% of patients; however, 83% of these lesions were shown to be benign, indicating a low specificity. [59] With improved technology, evaluation of nodules as small as 7 mm is possible. New developments, such as the development novel radiotracers and delayed imaging, can further refine the role of FDG-PET scanning in the workup of lung nodules and cancer. PET scanning versus CT scanning alone [60, 61, 62, 63] Several studies have evaluated the benefits of PET versus standard CT in the screening of pulmonary metastases. For nodules larger than 1 cm, in head and neck cancers, there was no statistical difference between PET and CT. [64] However for nodules smaller than 1 cm, high-resolution CT (HRCT) is more sensitive in evaluating pulmonary metastasis than PET. One study by Krug et al showed that using PET may help in avoiding 20% of futile surgeries in patients who were thought to be free of metastasis. [65] However, this study is limited because it was based
48 on data published from other studies. Other isotopes Several other isotopes have potential applications in the evaluation of pulmonary metastasis. Technetium (Tc) 99m methoxyisobutylisonitrile (Tc-MIBI) scintigraphy has been shown to detect 92% of metastatic lesions in patients with melanoma. [66] Indium (In)-111 labelled monoclonal antibody (CCR 086) has been shown to detect colorectal metastasis as small as 1 cm. [67] Bone scintigraphy with 99 Tc methylene diphosphonate (Tc-MDP) can be used to detect osteosarcoma metastasis. [67] Fluorobenzamide (FBZA) coupled with FDG can be used in the detection of melanin-producing tumors. [68] Degree of confidence Most false-negative FDG-PET results are caused by micrometastases and lesions smaller than 10 mm. In addition, some pulmonary lesions are not FDG avid, such as renal and testicular cancers. CT scanning is equivalent to or more sensitive than FDG-PET scanning for detecting small pulmonary lesions. False positives/negatives Physiologic variants, benign tumors, and inflammatory diseases may all cause increased uptake of FDG and mimic malignant disease. Angiography Angiography is not extensively used in the evaluation of pulmonary metastasis. In tumor embolism, pulmonary angiography may show delayed filling of segmental arteries, pruning and tortuosity of third- to fifth-order vessels, and subsegmental filling defects. [30] See the image below.
49 Coronal MR angiography in a patient with renal carcinoma shows tumor emboli in segmental branches of pulmonary arteries. Angiographic embolization may be used in metastatic tumors presenting with massive hemoptysis, for which bronchial artery embolization may stop bleeding. References Mohammed TL, Chowdhry A, Reddy GP, et al. ACR Appropriateness Criteria screening for pulmonary metastases. J Thorac Imaging Feb. 26(1):W1-3. [Medline]. Hirakata K, Nakata H, Nakagawa T. CT of pulmonary metastases with pathological correlation. Semin Ultrasound CT MR Oct. 16(5): [Medline]. Fraser RG, Pare JAP, Pare PD, Fraser RS, Genereaux GP. Diagnosis of diseases of the chest. 3rd ed. Saunders: Philadelphia; Hirakata K, Nakata H, Haratake J. Appearance of pulmonary metastases on high-resolution CT scans: comparison with histopathologic findings from autopsy specimens. AJR Am J Roentgenol Jul. 161(1): [Medline]. Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg Jul. 84(1): [Medline]. Hiraki T, Kanazawa S. Lung radiofrequency ablation: potential as a therapy to oligometastasis and oligorecurrence. Pulm Med : [Medline]. [Full Text]. Hiraki T, Gobara H, Iishi T, Sano Y, Iguchi T, Fujiwara H. Percutaneous radiofrequency ablation for pulmonary metastases from colorectal cancer: midterm results in 27 patients. J Vasc Interv Radiol Oct. 18(10): [Medline]. Yamakado K, Hase S, Matsuoka T, Tanigawa N, Nakatsuka A, Takaki H. Radiofrequency ablation for the treatment of unresectable lung metastases in patients with colorectal cancer: a multicenter study in Japan. J Vasc Interv Radiol Mar. 18(3): [Medline]. Hiraki T, Yamakado K, Ikeda O, Matsuoka T, Kaminou T, Yamagami T. Percutaneous radiofrequency ablation for pulmonary metastases from hepatocellular carcinoma: results of a multicenter study in Japan. J Vasc Interv Radiol Jun. 22(6): [Medline]. Soga N, Yamakado K, Gohara H, Takaki H, Hiraki T, Yamada T. Percutaneous radiofrequency ablation for unresectable pulmonary metastases from renal cell carcinoma. BJU Int Sep. 104(6): [Medline]. 11. La Quaglia MP. Osteosarcoma. Specific tumor management and results. Chest Surg Clin N Am Feb. 8(1): [Medline].
How to Analyse Difficult Chest CT
 How to Analyse Difficult Chest CT Complex diseases are:- - Large lesion - Unusual or atypical pattern - Multiple discordant findings Diffuse diseases are:- - Numerous findings in both sides 3 basic steps
How to Analyse Difficult Chest CT Complex diseases are:- - Large lesion - Unusual or atypical pattern - Multiple discordant findings Diffuse diseases are:- - Numerous findings in both sides 3 basic steps
HRCT in Diffuse Interstitial Lung Disease Steps in High Resolution CT Diagnosis. Where are the lymphatics? Anatomic distribution
 Steps in High Resolution CT Diagnosis Pattern of abnormality Distribution of disease Associated findings Clinical history Tomás Franquet MD What is the diagnosis? Hospital de Sant Pau. Barcelona Secondary
Steps in High Resolution CT Diagnosis Pattern of abnormality Distribution of disease Associated findings Clinical history Tomás Franquet MD What is the diagnosis? Hospital de Sant Pau. Barcelona Secondary
TB Radiology for Nurses Garold O. Minns, MD
 TB Nurse Case Management Salina, Kansas March 31-April 1, 2010 TB Radiology for Nurses Garold O. Minns, MD April 1, 2010 TB Radiology for Nurses Highway Patrol Training Center Salina, KS April 1, 2010
TB Nurse Case Management Salina, Kansas March 31-April 1, 2010 TB Radiology for Nurses Garold O. Minns, MD April 1, 2010 TB Radiology for Nurses Highway Patrol Training Center Salina, KS April 1, 2010
An Introduction to Radiology for TB Nurses
 An Introduction to Radiology for TB Nurses Garold O. Minns, MD September 14, 2017 TB Nurse Case Management September 12 14, 2017 EXCELLENCE EXPERTISE INNOVATION Garold O. Minns, MD has the following disclosures
An Introduction to Radiology for TB Nurses Garold O. Minns, MD September 14, 2017 TB Nurse Case Management September 12 14, 2017 EXCELLENCE EXPERTISE INNOVATION Garold O. Minns, MD has the following disclosures
Diagnosis of TB: Radiology David Finlay, MD
 TB Intensive Tyler, Texas June 2-4, 2010 Diagnosis of TB: Radiology David Finlay, MD June 3, 2010 2stages stages- Tuberculosis 1. primary infection 2. reactivation, or post primary disease 2 1 Primary
TB Intensive Tyler, Texas June 2-4, 2010 Diagnosis of TB: Radiology David Finlay, MD June 3, 2010 2stages stages- Tuberculosis 1. primary infection 2. reactivation, or post primary disease 2 1 Primary
Bronchogenic Carcinoma
 A 55-year-old construction worker has smoked 2 packs of ciggarettes daily for the past 25 years. He notes swelling in his upper extremity & face, along with dilated veins in this region. What is the most
A 55-year-old construction worker has smoked 2 packs of ciggarettes daily for the past 25 years. He notes swelling in his upper extremity & face, along with dilated veins in this region. What is the most
PULMONARY TUBERCULOSIS RADIOLOGY
 PULMONARY TUBERCULOSIS RADIOLOGY RADIOLOGICAL MODALITIES Medical radiophotography Radiography Fluoroscopy Linear (conventional) tomography Computed tomography Pulmonary angiography, bronchography Ultrasonography,
PULMONARY TUBERCULOSIS RADIOLOGY RADIOLOGICAL MODALITIES Medical radiophotography Radiography Fluoroscopy Linear (conventional) tomography Computed tomography Pulmonary angiography, bronchography Ultrasonography,
10/17/2016. Nuts and Bolts of Thoracic Radiology. Objectives. Techniques
 Nuts and Bolts of Thoracic Radiology October 20, 2016 Carleen Risaliti Objectives Understand the basics of chest radiograph Develop a system for interpreting chest radiographs Correctly identify thoracic
Nuts and Bolts of Thoracic Radiology October 20, 2016 Carleen Risaliti Objectives Understand the basics of chest radiograph Develop a system for interpreting chest radiographs Correctly identify thoracic
TB Intensive Houston, Texas
 TB Intensive Houston, Texas October 15-17, 17 2013 Diagnosis of TB: Radiology Rosa M Estrada-Y-Martin, MD MSc FCCP October 16, 2013 Rosa M Estrada-Y-Martin, MD MSc FCCP, has the following disclosures to
TB Intensive Houston, Texas October 15-17, 17 2013 Diagnosis of TB: Radiology Rosa M Estrada-Y-Martin, MD MSc FCCP October 16, 2013 Rosa M Estrada-Y-Martin, MD MSc FCCP, has the following disclosures to
Radiology Pathology Conference
 Radiology Pathology Conference Sharlin Johnykutty,, MD, Cytopathology Fellow Sara Majewski, MD, Radiology Resident Friday, August 28, 2009 Presentation material is for education purposes only. All rights
Radiology Pathology Conference Sharlin Johnykutty,, MD, Cytopathology Fellow Sara Majewski, MD, Radiology Resident Friday, August 28, 2009 Presentation material is for education purposes only. All rights
Case 1 : Question. 1.1 What is the intralobular distribution? 1. Centrilobular 2. Perilymphatic 3. Random
 Interesting case Case 1 Case 1 : Question 1.1 What is the intralobular distribution? 1. Centrilobular 2. Perilymphatic 3. Random Case 1: Answer 1.1 What is the intralobular distribution? 1. Centrilobular
Interesting case Case 1 Case 1 : Question 1.1 What is the intralobular distribution? 1. Centrilobular 2. Perilymphatic 3. Random Case 1: Answer 1.1 What is the intralobular distribution? 1. Centrilobular
Case Scenario 1. The patient agreed to a CT guided biopsy of the left upper lobe mass. This was performed and confirmed non-small cell carcinoma.
 Case Scenario 1 An 89 year old male patient presented with a progressive cough for approximately six weeks for which he received approximately three rounds of antibiotic therapy without response. A chest
Case Scenario 1 An 89 year old male patient presented with a progressive cough for approximately six weeks for which he received approximately three rounds of antibiotic therapy without response. A chest
RF Ablation: indication, technique and imaging follow-up
 RF Ablation: indication, technique and imaging follow-up Trongtum Tongdee, M.D. Radiology Department, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand Objective Basic knowledge
RF Ablation: indication, technique and imaging follow-up Trongtum Tongdee, M.D. Radiology Department, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand Objective Basic knowledge
Chief Complain. For chemotherapy
 Chief Complain For chemotherapy Present Illness 93.12 Progressive weakness of R t arm for 1 year X-ray: peneative lesion over right proximal humorous Bone scan: multiple increased intake Biopsy of distal
Chief Complain For chemotherapy Present Illness 93.12 Progressive weakness of R t arm for 1 year X-ray: peneative lesion over right proximal humorous Bone scan: multiple increased intake Biopsy of distal
Chest XRay interpretation INTERPRETATIONS Identifications: Name & Date Technical evaluation Basic Interpretations
 Chest XRay interpretation INTERPRETATIONS Identifications: Name & Date Technical evaluation Basic Interpretations TECHNICAL EVALUATION 1. Projection: AP/PA view To differentiate between AP & PA films,
Chest XRay interpretation INTERPRETATIONS Identifications: Name & Date Technical evaluation Basic Interpretations TECHNICAL EVALUATION 1. Projection: AP/PA view To differentiate between AP & PA films,
Chest Radiology Interpretation: Findings of Tuberculosis
 Chest Radiology Interpretation: Findings of Tuberculosis Get out your laptops, smart phones or other devices pollev.com/chestradiology Case #1 1 Plombage Pneumonia Cancer 2 Reading the TB CXR Be systematic!
Chest Radiology Interpretation: Findings of Tuberculosis Get out your laptops, smart phones or other devices pollev.com/chestradiology Case #1 1 Plombage Pneumonia Cancer 2 Reading the TB CXR Be systematic!
Resident Case Review CHEST. Daria Manos CAR 2016
 Resident Case Review CHEST CAR 2016 Daria Manos Disclosure Speakers bureau, Roche CAR 2016 Daria Manos 1. Recognize common and critical chest radiograph and computed tomography signs and use these clues
Resident Case Review CHEST CAR 2016 Daria Manos Disclosure Speakers bureau, Roche CAR 2016 Daria Manos 1. Recognize common and critical chest radiograph and computed tomography signs and use these clues
Interstitial syndrome
 Interstitial syndrome Ground-glass attenuation Miliary and nodular images linear images Etienne Leroy Terquem Pierre L Her SPI / ISP Soutien Pneumologique International / International Support for Pulmonology
Interstitial syndrome Ground-glass attenuation Miliary and nodular images linear images Etienne Leroy Terquem Pierre L Her SPI / ISP Soutien Pneumologique International / International Support for Pulmonology
September 2014 Imaging Case of the Month. Michael B. Gotway, MD. Department of Radiology Mayo Clinic Arizona Scottsdale, AZ
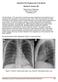 September 2014 Imaging Case of the Month Michael B. Gotway, MD Department of Radiology Mayo Clinic Arizona Scottsdale, AZ Clinical History: A 57-year-old non-smoking woman presented to her physician as
September 2014 Imaging Case of the Month Michael B. Gotway, MD Department of Radiology Mayo Clinic Arizona Scottsdale, AZ Clinical History: A 57-year-old non-smoking woman presented to her physician as
David E. Griffith, MD has the following disclosures to make:
 Diagnosis of TB: Radiology David E. Griffith, MD March 13, 2015 TB for Pulmonologist March 13, 2015 Phoenix, AZ EXCELLENCE EXPERTISE INNOVATION David E. Griffith, MD has the following disclosures to make:
Diagnosis of TB: Radiology David E. Griffith, MD March 13, 2015 TB for Pulmonologist March 13, 2015 Phoenix, AZ EXCELLENCE EXPERTISE INNOVATION David E. Griffith, MD has the following disclosures to make:
Case 1: Question. 1.1 What is the main pattern of this HRCT? 1. Intralobular line 2. Groundglass opacity 3. Perilymphatic nodule
 HRCT WORK SHOP Case 1 Case 1: Question 1.1 What is the main pattern of this HRCT? 1. Intralobular line 2. Groundglass opacity 3. Perilymphatic nodule Case 1: Question 1.2 What is the diagnosis? 1. Hypersensitivity
HRCT WORK SHOP Case 1 Case 1: Question 1.1 What is the main pattern of this HRCT? 1. Intralobular line 2. Groundglass opacity 3. Perilymphatic nodule Case 1: Question 1.2 What is the diagnosis? 1. Hypersensitivity
Acute and Chronic Lung Disease
 KATHOLIEKE UNIVERSITEIT LEUVEN Faculty of Medicine Acute and Chronic Lung Disease W De Wever, JA Verschakelen Department of Radiology, University Hospitals Leuven, Belgium Clinical utility of HRCT To detect
KATHOLIEKE UNIVERSITEIT LEUVEN Faculty of Medicine Acute and Chronic Lung Disease W De Wever, JA Verschakelen Department of Radiology, University Hospitals Leuven, Belgium Clinical utility of HRCT To detect
Thoracic Sarcoidosis Imaging Updated: Jul 19, 2013
 Thoracic Sarcoidosis Imaging Updated: Jul 19, 2013 Overview Radiography Computed Tomography Magnetic Resonance Imaging Nuclear Imaging Show All Multimedia Library References Overview For patients with
Thoracic Sarcoidosis Imaging Updated: Jul 19, 2013 Overview Radiography Computed Tomography Magnetic Resonance Imaging Nuclear Imaging Show All Multimedia Library References Overview For patients with
An Image Repository for Chest CT
 An Image Repository for Chest CT Francesco Frajoli for the Chest CT in Antibody Deficiency Group An Image Repository for Chest CT he Chest CT in Antibody Deficiency Group is an international and interdisciplinary
An Image Repository for Chest CT Francesco Frajoli for the Chest CT in Antibody Deficiency Group An Image Repository for Chest CT he Chest CT in Antibody Deficiency Group is an international and interdisciplinary
Lung Cancer-a primer. Sai Yendamuri, MD Professor and Chair, Dept of Thoracic Surgery,RPCI,Buffalo
 Lung Cancer-a primer Sai Yendamuri, MD Professor and Chair, Dept of Thoracic Surgery,RPCI,Buffalo CLINICAL CATEGORIES THE SOLITARY PULMONARY NODULE MULTIPLE PULMONARY NODULES Differential Diagnosis Malignant
Lung Cancer-a primer Sai Yendamuri, MD Professor and Chair, Dept of Thoracic Surgery,RPCI,Buffalo CLINICAL CATEGORIES THE SOLITARY PULMONARY NODULE MULTIPLE PULMONARY NODULES Differential Diagnosis Malignant
Radiology Pathology Conference
 Radiology Pathology Conference Nadia F. Yusaf, M.D. PGY-3 1/29/2010 Presentation material is for education purposes only. All rights reserved. 2010 URMC Radiology Page 1 of 90 Case 1 60 year- old man presents
Radiology Pathology Conference Nadia F. Yusaf, M.D. PGY-3 1/29/2010 Presentation material is for education purposes only. All rights reserved. 2010 URMC Radiology Page 1 of 90 Case 1 60 year- old man presents
performed to help sway the clinician in what the appropriate diagnosis is, which can substantially alter the treatment of management.
 Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
Interesting Cases. Pulmonary
 Interesting Cases Pulmonary 54M with prior history of COPD, hep B/C, and possible history of TB presented with acute on chronic dyspnea, and productive cough Hazy opacity overlying the left hemithorax
Interesting Cases Pulmonary 54M with prior history of COPD, hep B/C, and possible history of TB presented with acute on chronic dyspnea, and productive cough Hazy opacity overlying the left hemithorax
Interstitial Syndrome Ground glass attenuation miliary and nodular images Linear images
 Interstitial Syndrome Ground glass attenuation miliary and nodular images Linear images Dr Etienne Leroy-Terquem Centre hospitalier de Meulan les Mureaux. France French-cambodian association for pneumology
Interstitial Syndrome Ground glass attenuation miliary and nodular images Linear images Dr Etienne Leroy-Terquem Centre hospitalier de Meulan les Mureaux. France French-cambodian association for pneumology
Comparison of High-resolution CT Findings between Miliary Metastases and Miliary Tuberculosis 1
 Comparison of High-resolution CT Findings between Miliary Metastases and Miliary Tuberculosis 1 Chan Sung Kim, M.D., Ki-Nam Lee, M.D., Jin Hwa Lee, M.D. Purpose: To compare the findings of high-resolution
Comparison of High-resolution CT Findings between Miliary Metastases and Miliary Tuberculosis 1 Chan Sung Kim, M.D., Ki-Nam Lee, M.D., Jin Hwa Lee, M.D. Purpose: To compare the findings of high-resolution
Imaging in gastric cancer
 Imaging in gastric cancer Gastric cancer remains a deadly disease because of late diagnosis. Adenocarcinoma represents 90% of malignant tumors. Diagnosis is based on endoscopic examination with biopsies.
Imaging in gastric cancer Gastric cancer remains a deadly disease because of late diagnosis. Adenocarcinoma represents 90% of malignant tumors. Diagnosis is based on endoscopic examination with biopsies.
Lung. 10/24/13 Chest X-ray: 2.9 cm mass like density in the inferior lingular segment worrisome for neoplasm. Malignancy cannot be excluded.
 Lung Case Scenario 1 A 54 year white male presents with a recent abnormal CT of the chest. The patient has a history of melanoma, kidney, and prostate cancers. 10/24/13 Chest X-ray: 2.9 cm mass like density
Lung Case Scenario 1 A 54 year white male presents with a recent abnormal CT of the chest. The patient has a history of melanoma, kidney, and prostate cancers. 10/24/13 Chest X-ray: 2.9 cm mass like density
objectives Pitfalls and Pearls in PET/CT imaging Kevin Robinson, DO Assistant Professor Department of Radiology Michigan State University
 objectives Pitfalls and Pearls in PET/CT imaging Kevin Robinson, DO Assistant Professor Department of Radiology Michigan State University To determine the regions of physiologic activity To understand
objectives Pitfalls and Pearls in PET/CT imaging Kevin Robinson, DO Assistant Professor Department of Radiology Michigan State University To determine the regions of physiologic activity To understand
Undergraduate Teaching
 Prof. James F Meaney Undergraduate Teaching Chest X-Ray Understanding the normal anatomical by reference to cross sectional imaging Radiology? It s FUN! Cryptic puzzle Sudoku (Minecraft?) It s completely
Prof. James F Meaney Undergraduate Teaching Chest X-Ray Understanding the normal anatomical by reference to cross sectional imaging Radiology? It s FUN! Cryptic puzzle Sudoku (Minecraft?) It s completely
Systemic lupus erythematosus (SLE): Pleuropulmonary Manifestations
 08/30/10 09/26/10 Systemic lupus erythematosus (SLE): Pleuropulmonary Manifestations Camila Downey S. Universidad de Chile, School of Medicine, Year VII Harvard University, School of Medicine Sept 17,
08/30/10 09/26/10 Systemic lupus erythematosus (SLE): Pleuropulmonary Manifestations Camila Downey S. Universidad de Chile, School of Medicine, Year VII Harvard University, School of Medicine Sept 17,
Exercise 15: CSv2 Data Item Coding Instructions ANSWERS
 Exercise 15: CSv2 Data Item Coding Instructions ANSWERS CS Tumor Size Tumor size is the diameter of the tumor, not the depth or thickness of the tumor. Chest x-ray shows 3.5 cm mass; the pathology report
Exercise 15: CSv2 Data Item Coding Instructions ANSWERS CS Tumor Size Tumor size is the diameter of the tumor, not the depth or thickness of the tumor. Chest x-ray shows 3.5 cm mass; the pathology report
Tuberculosis: The Essentials
 Tuberculosis: The Essentials Kendra L. Fisher, MD, PhD THORACIC TUBERCULOSIS: THE BARE ESSENTIALS Kendra Fisher MD, FRCP (C) Department of Radiology Loma Linda University Medical Center TUBERCULOSIS ()
Tuberculosis: The Essentials Kendra L. Fisher, MD, PhD THORACIC TUBERCULOSIS: THE BARE ESSENTIALS Kendra Fisher MD, FRCP (C) Department of Radiology Loma Linda University Medical Center TUBERCULOSIS ()
Radiological staging of lung cancer. Shukri Loutfi,MD,FRCR Consultant Thoracic Radiologist KAMC-Riyadh
 Radiological staging of lung cancer Shukri Loutfi,MD,FRCR Consultant Thoracic Radiologist KAMC-Riyadh Bronchogenic Carcinoma Accounts for 14% of new cancer diagnoses in 2012. Estimated to kill ~150,000
Radiological staging of lung cancer Shukri Loutfi,MD,FRCR Consultant Thoracic Radiologist KAMC-Riyadh Bronchogenic Carcinoma Accounts for 14% of new cancer diagnoses in 2012. Estimated to kill ~150,000
Excavated pulmonary nodule: steps to diagnosis?
 Excavated pulmonary nodule: steps to diagnosis? Poster No.: C-1044 Congress: ECR 2014 Type: Authors: Keywords: DOI: Educational Exhibit W. Mnari, M. MAATOUK, A. Zrig, B. Hmida, M. GOLLI; Monastir/ TN Metastases,
Excavated pulmonary nodule: steps to diagnosis? Poster No.: C-1044 Congress: ECR 2014 Type: Authors: Keywords: DOI: Educational Exhibit W. Mnari, M. MAATOUK, A. Zrig, B. Hmida, M. GOLLI; Monastir/ TN Metastases,
Do you want to be an excellent Radiologist? - Focus on the thoracic aorta on lateral chest image!!!
 The lateral chest radiograph: Challenging area around the thoracic aorta!!! Do you want to be an excellent Radiologist? - Focus on the thoracic aorta on lateral chest image!!! Dong Yoon Han 1, So Youn
The lateral chest radiograph: Challenging area around the thoracic aorta!!! Do you want to be an excellent Radiologist? - Focus on the thoracic aorta on lateral chest image!!! Dong Yoon Han 1, So Youn
Learning Objectives. 1. Identify which patients meet criteria for annual lung cancer screening
 Disclosure I, Taylor Rowlett, DO NOT have a financial interest /arrangement or affiliation with one or more organizations that could be perceived as a real or apparent conflict of interest in the context
Disclosure I, Taylor Rowlett, DO NOT have a financial interest /arrangement or affiliation with one or more organizations that could be perceived as a real or apparent conflict of interest in the context
October 2012 Imaging Case of the Month. Michael B. Gotway, MD Associate Editor Imaging. Department of Radiology Mayo Clinic Arizona Scottsdale, AZ
 October 2012 Imaging Case of the Month Michael B. Gotway, MD Associate Editor Imaging Department of Radiology Mayo Clinic Arizona Scottsdale, AZ Clinical History: A 65-year-old non-smoking woman presented
October 2012 Imaging Case of the Month Michael B. Gotway, MD Associate Editor Imaging Department of Radiology Mayo Clinic Arizona Scottsdale, AZ Clinical History: A 65-year-old non-smoking woman presented
Thoracic Surgery; An Overview
 Thoracic Surgery What we see Thoracic Surgery; An Overview James P. Locher, Jr, MD Methodist Cardiovascular and Thoracic Surgery Lung cancer Mets Fungus and TB Lung abcess and empyema Pleural based disease
Thoracic Surgery What we see Thoracic Surgery; An Overview James P. Locher, Jr, MD Methodist Cardiovascular and Thoracic Surgery Lung cancer Mets Fungus and TB Lung abcess and empyema Pleural based disease
Pictorial essay of unusual radiologic manifestations of pulmonary and airway metastasis at initial presentation of lung cancer
 Pictorial essay of unusual radiologic manifestations of pulmonary and airway metastasis at initial presentation of lung cancer Poster No.: C-2297 Congress: ECR 2012 Type: Educational Exhibit Authors: Y.
Pictorial essay of unusual radiologic manifestations of pulmonary and airway metastasis at initial presentation of lung cancer Poster No.: C-2297 Congress: ECR 2012 Type: Educational Exhibit Authors: Y.
Imaging of the Lung. István Battyány
 Imaging of the Lung István Battyány Anatomy of airways: (asszimetric dichotomy) trachea Main bronchus Lobar bronchus }lung, lobe 1. segmental bronchus subsegmental bronchus bronchus lobularis bronchiolus
Imaging of the Lung István Battyány Anatomy of airways: (asszimetric dichotomy) trachea Main bronchus Lobar bronchus }lung, lobe 1. segmental bronchus subsegmental bronchus bronchus lobularis bronchiolus
Lung Cancer Imaging. Terence Z. Wong, MD,PhD. Department of Radiology Duke University Medical Center Durham, NC 9/9/09
 Lung Cancer Imaging Terence Z. Wong, MD,PhD Department of Radiology Duke University Medical Center Durham, NC 9/9/09 Acknowledgements Edward F. Patz, Jr., MD Jenny Hoang, MD Ellen L. Jones, MD, PhD Lung
Lung Cancer Imaging Terence Z. Wong, MD,PhD Department of Radiology Duke University Medical Center Durham, NC 9/9/09 Acknowledgements Edward F. Patz, Jr., MD Jenny Hoang, MD Ellen L. Jones, MD, PhD Lung
Detection of Soft Tissue Tumors on Bone Scintigraphy: Report of Four Cases
 Detection of Soft Tissue Tumors on Bone Scintigraphy: Report of Four Cases Fariba Akhzari 1 MD and Mahrokh Daemi MD 2 1 Nuclear Medicine Department, 2 General Surgery Department, Sina Hospital, Faculty
Detection of Soft Tissue Tumors on Bone Scintigraphy: Report of Four Cases Fariba Akhzari 1 MD and Mahrokh Daemi MD 2 1 Nuclear Medicine Department, 2 General Surgery Department, Sina Hospital, Faculty
Pulmonary Sarcoidosis - Radiological Evaluation
 Original Research Article Pulmonary Sarcoidosis - Radiological Evaluation Jayesh Shah 1, Darshan Shah 2*, C. Raychaudhuri 3 1 Associate Professor, 2 1 st Year Resident, 3 Professor and HOD Radiology Department,
Original Research Article Pulmonary Sarcoidosis - Radiological Evaluation Jayesh Shah 1, Darshan Shah 2*, C. Raychaudhuri 3 1 Associate Professor, 2 1 st Year Resident, 3 Professor and HOD Radiology Department,
Stereotactic Body Radiation Therapy and Radiofrequency Ablation 2014 Masters of Minimally Invasive Surgery
 Stereotactic Body Radiation Therapy and Radiofrequency Ablation 2014 Masters of Minimally Invasive Surgery Matthew Hartwig, M.D. Duke Cancer Institute Case Presentation I: Patient ER 74 y/o male with A1A
Stereotactic Body Radiation Therapy and Radiofrequency Ablation 2014 Masters of Minimally Invasive Surgery Matthew Hartwig, M.D. Duke Cancer Institute Case Presentation I: Patient ER 74 y/o male with A1A
Pneumocystis jirovecci pneumonia: from mild disease to a real disaster. A pictorial review of the different radiologic patterns in acute settings
 Pneumocystis jirovecci pneumonia: from mild disease to a real disaster. A pictorial review of the different radiologic patterns in acute settings Poster No.: C-1425 Congress: ECR 2017 Type: Educational
Pneumocystis jirovecci pneumonia: from mild disease to a real disaster. A pictorial review of the different radiologic patterns in acute settings Poster No.: C-1425 Congress: ECR 2017 Type: Educational
PET IMAGING (POSITRON EMISSION TOMOGRAPY) FACT SHEET
 Positron Emission Tomography (PET) When calling Anthem (1-800-533-1120) or using the Point of Care authorization system for a Health Service Review, the following clinical information may be needed to
Positron Emission Tomography (PET) When calling Anthem (1-800-533-1120) or using the Point of Care authorization system for a Health Service Review, the following clinical information may be needed to
Financial disclosure COMMON DIAGNOSES IN HRCT. High Res Chest HRCT. HRCT Pre test. I have no financial relationships to disclose. Anatomy Nomenclature
 Financial disclosure I have no financial relationships to disclose. Douglas Johnson D.O. Cardiothoracic Imaging Gaston Radiology COMMON DIAGNOSES IN HRCT High Res Chest Anatomy Nomenclature HRCT Sampling
Financial disclosure I have no financial relationships to disclose. Douglas Johnson D.O. Cardiothoracic Imaging Gaston Radiology COMMON DIAGNOSES IN HRCT High Res Chest Anatomy Nomenclature HRCT Sampling
GUIDELINES FOR CANCER IMAGING Lung Cancer
 GUIDELINES FOR CANCER IMAGING Lung Cancer Greater Manchester and Cheshire Cancer Network Cancer Imaging Cross-Cutting Group April 2010 1 INTRODUCTION This document is intended as a ready reference for
GUIDELINES FOR CANCER IMAGING Lung Cancer Greater Manchester and Cheshire Cancer Network Cancer Imaging Cross-Cutting Group April 2010 1 INTRODUCTION This document is intended as a ready reference for
RADIOFREQUENCY ABLATION
 RADIOFREQUENCY ABLATION ELIZABETH DAVID M D FRCPC VASCULAR A ND INTERVENTIONAL RADIOLOGIST SUNNYBROOK HEALTH SCIENCES CENTRE GIST GASTROINTESTINAL STROMAL TUMORS Stromal or mesenchymal neoplasms affecting
RADIOFREQUENCY ABLATION ELIZABETH DAVID M D FRCPC VASCULAR A ND INTERVENTIONAL RADIOLOGIST SUNNYBROOK HEALTH SCIENCES CENTRE GIST GASTROINTESTINAL STROMAL TUMORS Stromal or mesenchymal neoplasms affecting
RFA of Tumors of the Lung: How and Why. Radiofrequency Ablation. Radiofrequency Ablation. RFA of pulmonary metastases. Radiofrequency Ablation of Lung
 RFA of Tumors of the Lung: How and Why Radiofrequency Ablation of Lung Ernest Scalzetti MD SUNY Upstate Medical University Syracuse NY FDA WARNING: Off-label use of a medical device Radiofrequency Ablation
RFA of Tumors of the Lung: How and Why Radiofrequency Ablation of Lung Ernest Scalzetti MD SUNY Upstate Medical University Syracuse NY FDA WARNING: Off-label use of a medical device Radiofrequency Ablation
Thoracic Imaging: A Case of Metastatic Adenocarcinoma of Unknown Primary
 January 28, 2009 Thoracic Imaging: A Case of Metastatic Adenocarcinoma of Unknown Primary Kristina Mirabeau-Beale, Harvard Medical School Year III Gillian Lieberman, MD Agenda Introduce Patient RS Discuss
January 28, 2009 Thoracic Imaging: A Case of Metastatic Adenocarcinoma of Unknown Primary Kristina Mirabeau-Beale, Harvard Medical School Year III Gillian Lieberman, MD Agenda Introduce Patient RS Discuss
Charles Mulligan, MD, FACS, FCCP 26 March 2015
 Charles Mulligan, MD, FACS, FCCP 26 March 2015 Review lung cancer statistics Review the risk factors Discuss presentation and staging Discuss treatment options and outcomes Discuss the status of screening
Charles Mulligan, MD, FACS, FCCP 26 March 2015 Review lung cancer statistics Review the risk factors Discuss presentation and staging Discuss treatment options and outcomes Discuss the status of screening
Index. Surg Oncol Clin N Am 16 (2007) Note: Page numbers of article titles are in boldface type.
 Surg Oncol Clin N Am 16 (2007) 465 469 Index Note: Page numbers of article titles are in boldface type. A Adjuvant therapy, preoperative for gastric cancer, staging and, 339 B Breast cancer, metabolic
Surg Oncol Clin N Am 16 (2007) 465 469 Index Note: Page numbers of article titles are in boldface type. A Adjuvant therapy, preoperative for gastric cancer, staging and, 339 B Breast cancer, metabolic
August 2018 Imaging Case of the Month: Dyspnea in a 55-Year-Old Smoker. Michael B. Gotway, MD
 August 2018 Imaging Case of the Month: Dyspnea in a 55-Year-Old Smoker Michael B. Gotway, MD Department of Radiology Mayo Clinic Arizona Scottsdale, AZ USA Clinical History: A 55 year old woman presented
August 2018 Imaging Case of the Month: Dyspnea in a 55-Year-Old Smoker Michael B. Gotway, MD Department of Radiology Mayo Clinic Arizona Scottsdale, AZ USA Clinical History: A 55 year old woman presented
Adam J. Hansen, MD UHC Thoracic Surgery
 Adam J. Hansen, MD UHC Thoracic Surgery Sometimes seen on Chest X-ray (CXR) Common incidental findings on computed tomography (CT) chest and abdomen done for other reasons Most lung cancers discovered
Adam J. Hansen, MD UHC Thoracic Surgery Sometimes seen on Chest X-ray (CXR) Common incidental findings on computed tomography (CT) chest and abdomen done for other reasons Most lung cancers discovered
OBJECTIVES. Solitary Solid Spiculated Nodule. What would you do next? Case Based Discussion: State of the Art Management of Lung Nodules.
 Organ Imaging : September 25 2015 OBJECTIVES Case Based Discussion: State of the Art Management of Lung Nodules Dr. Elsie T. Nguyen Dr. Kazuhiro Yasufuku 1. To review guidelines for follow up and management
Organ Imaging : September 25 2015 OBJECTIVES Case Based Discussion: State of the Art Management of Lung Nodules Dr. Elsie T. Nguyen Dr. Kazuhiro Yasufuku 1. To review guidelines for follow up and management
CT findings of high-attenuation pulmonary abnormalities
 Insights Imaging (2010) 1:287 292 DOI 10.1007/s13244-010-0039-2 PICTORIAL REVIEW CT findings of high-attenuation pulmonary abnormalities Naim Ceylan & Selen Bayraktaroglu & Recep Savaş & Hudaver Alper
Insights Imaging (2010) 1:287 292 DOI 10.1007/s13244-010-0039-2 PICTORIAL REVIEW CT findings of high-attenuation pulmonary abnormalities Naim Ceylan & Selen Bayraktaroglu & Recep Savaş & Hudaver Alper
Take Home Quiz 1 Please complete the quiz below prior to the session. Use the Multiple Primary and Histology Rules
 Take Home Quiz 1 Please complete the quiz below prior to the session. Use the Multiple Primary and Histology Rules Case 1 72 year old white female presents with a nodular thyroid. This was biopsied in
Take Home Quiz 1 Please complete the quiz below prior to the session. Use the Multiple Primary and Histology Rules Case 1 72 year old white female presents with a nodular thyroid. This was biopsied in
FDG PET/CT in Lung Cancer Read with the experts. Homer A. Macapinlac, M.D.
 FDG PET/CT in Lung Cancer Read with the experts Homer A. Macapinlac, M.D. Patient with suspected lung cancer presents with left sided chest pain T3 What is the T stage of this patient? A) T2a B) T2b C)
FDG PET/CT in Lung Cancer Read with the experts Homer A. Macapinlac, M.D. Patient with suspected lung cancer presents with left sided chest pain T3 What is the T stage of this patient? A) T2a B) T2b C)
OFF THE BEATEN TRACK: A Pictorial Review of Atypical Features of Pulmonary Metastases
 OFF THE BEATEN TRACK: A Pictorial Review of Atypical Features of Pulmonary Metastases Megan Hora, MD Chi Wan Koo, MD Christian Cox, MD Department of Diagnostic Radiology Thoracic Imaging Section Mayo Clinic
OFF THE BEATEN TRACK: A Pictorial Review of Atypical Features of Pulmonary Metastases Megan Hora, MD Chi Wan Koo, MD Christian Cox, MD Department of Diagnostic Radiology Thoracic Imaging Section Mayo Clinic
Chest imaging III: Nodular pulmonary disease. Ádám Domonkos Tárnoki, MD, PhD Assistant professor Department of Radiology, Semmelweis University 1
 Chest imaging III: Nodular pulmonary disease Ádám Domonkos Tárnoki, MD, PhD Assistant professor Department of Radiology, Semmelweis University 1 Pattern 2 Nodular pattern Several round opacity, typically
Chest imaging III: Nodular pulmonary disease Ádám Domonkos Tárnoki, MD, PhD Assistant professor Department of Radiology, Semmelweis University 1 Pattern 2 Nodular pattern Several round opacity, typically
Slide 1. Slide 2. Slide 3. Investigation and management of lung cancer Robert Rintoul. Epidemiology. Risk factors/aetiology
 Slide 1 Investigation and management of lung cancer Robert Rintoul Department of Thoracic Oncology Papworth Hospital Slide 2 Epidemiology Second most common cancer in the UK (after breast). 38 000 new
Slide 1 Investigation and management of lung cancer Robert Rintoul Department of Thoracic Oncology Papworth Hospital Slide 2 Epidemiology Second most common cancer in the UK (after breast). 38 000 new
is time consuming and expensive. An intra-operative assessment is not going to be helpful if there is no more tissue that can be taken to improve the
 My name is Barry Feig. I am a Professor of Surgical Oncology at The University of Texas MD Anderson Cancer Center in Houston, Texas. I am going to talk to you today about the role for surgery in the treatment
My name is Barry Feig. I am a Professor of Surgical Oncology at The University of Texas MD Anderson Cancer Center in Houston, Texas. I am going to talk to you today about the role for surgery in the treatment
100 Chest X Rays for Study Group. by Dr. Suneet Khurana
 100 Chest X Rays for Study Group by Dr. Suneet Khurana Approach to - Chest X Ray (shadow of the viscera on a photographic plate) Gas appears Black Fat appears Dark Grey Water Appears as Light Grey Bone
100 Chest X Rays for Study Group by Dr. Suneet Khurana Approach to - Chest X Ray (shadow of the viscera on a photographic plate) Gas appears Black Fat appears Dark Grey Water Appears as Light Grey Bone
Role of CT imaging to evaluate solitary pulmonary nodule with extrapulmonary neoplasms
 Original Research Article Role of CT imaging to evaluate solitary pulmonary nodule with extrapulmonary neoplasms Anand Vachhani 1, Shashvat Modia 1*, Varun Garasia 1, Deepak Bhimani 1, C. Raychaudhuri
Original Research Article Role of CT imaging to evaluate solitary pulmonary nodule with extrapulmonary neoplasms Anand Vachhani 1, Shashvat Modia 1*, Varun Garasia 1, Deepak Bhimani 1, C. Raychaudhuri
Tuberculosis - clinical forms. Dr. A.Torossian,, M.D., Ph. D. Department of Respiratory Diseases
 Tuberculosis - clinical forms Dr. A.Torossian,, M.D., Ph. D. Department of Respiratory Diseases 1 TB DISEASE Primary Post-primary (Secondary) Common primary forms Primary complex Tuberculosis of the intrathoracic
Tuberculosis - clinical forms Dr. A.Torossian,, M.D., Ph. D. Department of Respiratory Diseases 1 TB DISEASE Primary Post-primary (Secondary) Common primary forms Primary complex Tuberculosis of the intrathoracic
A Case of Pediatric Plasma Cell Granuloma
 August 2001 A Case of Pediatric Plasma Cell Granuloma Nii Tetteh, Harvard Medical School Year IV Our Patient 8 year old male with history of recurrent left lower lobe and lingular pneumonias since 1994.
August 2001 A Case of Pediatric Plasma Cell Granuloma Nii Tetteh, Harvard Medical School Year IV Our Patient 8 year old male with history of recurrent left lower lobe and lingular pneumonias since 1994.
Chest X-ray Interpretation
 Chest X-ray Interpretation Introduction Routinely obtained Pulmonary specialist consultation Inherent physical exam limitations Chest x-ray limitations Physical exam and chest x-ray provide compliment
Chest X-ray Interpretation Introduction Routinely obtained Pulmonary specialist consultation Inherent physical exam limitations Chest x-ray limitations Physical exam and chest x-ray provide compliment
UERMMMC Department of Radiology. Basic Chest Radiology
 UERMMMC Department of Radiology Basic Chest Radiology PHYSICS DENSITIES BONE SOFT TISSUES WATER FAT AIR TELEROENTGENOGRAM Criteria for an Ideal Chest Radiograph 1. Upright 2. Posteroanterior View 3. Full
UERMMMC Department of Radiology Basic Chest Radiology PHYSICS DENSITIES BONE SOFT TISSUES WATER FAT AIR TELEROENTGENOGRAM Criteria for an Ideal Chest Radiograph 1. Upright 2. Posteroanterior View 3. Full
American College of Radiology ACR Appropriateness Criteria
 American College of Radiology ACR Criteria Radiologic Management of Thoracic Nodules and Masses Variant 1: Middle-aged patient (35 60 years old) with an incidental 1.5-cm lung nodule. The lesion was smooth.
American College of Radiology ACR Criteria Radiologic Management of Thoracic Nodules and Masses Variant 1: Middle-aged patient (35 60 years old) with an incidental 1.5-cm lung nodule. The lesion was smooth.
Collaborative Stage. Site-Specific Instructions - LUNG
 Slide 1 Collaborative Stage Site-Specific Instructions - LUNG In this presentation, we are going to review the AJCC Cancer Staging criteria for the lung primary site. Slide 2 Reading Assignments As each
Slide 1 Collaborative Stage Site-Specific Instructions - LUNG In this presentation, we are going to review the AJCC Cancer Staging criteria for the lung primary site. Slide 2 Reading Assignments As each
Atlas of the Vasculitic Syndromes
 CHAPTER e40 Atlas of the Vasculitic Syndromes Carol A. Langford Anthony S. Fauci Diagnosis of the vasculitic syndromes is usually based upon characteristic histologic or arteriographic findings in a patient
CHAPTER e40 Atlas of the Vasculitic Syndromes Carol A. Langford Anthony S. Fauci Diagnosis of the vasculitic syndromes is usually based upon characteristic histologic or arteriographic findings in a patient
Sectional Anatomy Quiz II
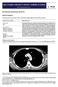 Sectional Anatomy II Rashid Hashmi Rural Clinical School, University of New South Wales, Wagga Wagga, New South Wales, Australia A R T I C L E I N F O Article type: Article history: Received: 3 Aug 2017
Sectional Anatomy II Rashid Hashmi Rural Clinical School, University of New South Wales, Wagga Wagga, New South Wales, Australia A R T I C L E I N F O Article type: Article history: Received: 3 Aug 2017
Pulmonary Nodules & Masses
 Pulmonary Nodules & Masses A Diagnostic Approach Heber MacMahon The University of Chicago Department of Radiology Disclosure Information Consultant for Riverain Technology Minor equity in Hologic Royalties
Pulmonary Nodules & Masses A Diagnostic Approach Heber MacMahon The University of Chicago Department of Radiology Disclosure Information Consultant for Riverain Technology Minor equity in Hologic Royalties
Abstracting Upper GI Cancer Incidence and Treatment Data Quiz 1 Multiple Primary and Histologies Case 1 Final Pathology:
 Abstracting Upper GI Cancer Incidence and Treatment Data Quiz 1 Multiple Primary and Histologies Case 1 A 74 year old male with a history of GERD presents complaining of dysphagia. An esophagogastroduodenoscopy
Abstracting Upper GI Cancer Incidence and Treatment Data Quiz 1 Multiple Primary and Histologies Case 1 A 74 year old male with a history of GERD presents complaining of dysphagia. An esophagogastroduodenoscopy
Case of the Day Chest
 Case of the Day Chest Darin White MDCM FRCPC Department of Radiology, Mayo Clinic 76 th Annual Scientific Meeting Canadian Association of Radiologists Montreal, QC April 26, 2013 2013 MFMER slide-1 Disclosures
Case of the Day Chest Darin White MDCM FRCPC Department of Radiology, Mayo Clinic 76 th Annual Scientific Meeting Canadian Association of Radiologists Montreal, QC April 26, 2013 2013 MFMER slide-1 Disclosures
PET CT for Staging Lung Cancer
 PET CT for Staging Lung Cancer Rohit Kochhar Consultant Radiologist Disclosures Neither I nor my immediate family members have financial relationships with commercial organizations that may have a direct
PET CT for Staging Lung Cancer Rohit Kochhar Consultant Radiologist Disclosures Neither I nor my immediate family members have financial relationships with commercial organizations that may have a direct
Lung cancer in patients with chronic empyema
 Lung cancer in patients with chronic empyema Poster No.: P-0025 Congress: ESTI 2015 Type: Scientific Poster Authors: Y. Lee, C.-K. Park; Guri/KR Keywords: Neoplasia, Biopsy, PET-CT, CT, Thorax, Lung DOI:
Lung cancer in patients with chronic empyema Poster No.: P-0025 Congress: ESTI 2015 Type: Scientific Poster Authors: Y. Lee, C.-K. Park; Guri/KR Keywords: Neoplasia, Biopsy, PET-CT, CT, Thorax, Lung DOI:
GROUP 1: Peripheral tumour with normal hilar and mediastinum on staging CT with no disant metastases. Including: Excluding:
 GROUP 1: Including: Excluding: Peripheral tumour with normal hilar and mediastinum on staging CT with no disant metastases Solid pulmonary nodules 8mm diameter / 300mm3 volume and BROCK risk of malignancy
GROUP 1: Including: Excluding: Peripheral tumour with normal hilar and mediastinum on staging CT with no disant metastases Solid pulmonary nodules 8mm diameter / 300mm3 volume and BROCK risk of malignancy
Usual Interstitial pneumonia and Nonspecific Interstitial Pneumonia. Nitra and the Gangs.
 Usual Interstitial pneumonia and Nonspecific Interstitial Pneumonia Nitra and the Gangs. บทน ำและบทท ๓, ๑๐, ๑๒, ๑๓, ๑๔, ๑๕, ๑๗ Usual Interstitial Pneumonia (UIP) Most common & basic pathologic pattern
Usual Interstitial pneumonia and Nonspecific Interstitial Pneumonia Nitra and the Gangs. บทน ำและบทท ๓, ๑๐, ๑๒, ๑๓, ๑๔, ๑๕, ๑๗ Usual Interstitial Pneumonia (UIP) Most common & basic pathologic pattern
ONCOLOGIC PERCUTANEOUS INTERVENTION: 2015 UPDATE HANH VU NGHIEM, MD OAKLAND UNIVERSITY WILLIAM BEAUMONT SCHOOL OF MEDICINE
 ONCOLOGIC PERCUTANEOUS INTERVENTION: 2015 UPDATE HANH VU NGHIEM, MD OAKLAND UNIVERSITY WILLIAM BEAUMONT SCHOOL OF MEDICINE ONCOLOGIC PERCUTANEOUS IMAGE GUIDED TUMOR ABLATION Evolving, growing and increasingly
ONCOLOGIC PERCUTANEOUS INTERVENTION: 2015 UPDATE HANH VU NGHIEM, MD OAKLAND UNIVERSITY WILLIAM BEAUMONT SCHOOL OF MEDICINE ONCOLOGIC PERCUTANEOUS IMAGE GUIDED TUMOR ABLATION Evolving, growing and increasingly
Lung Cancer - Suspected
 Lung Cancer - Suspected Shared Decision Making Lung Cancer: http://www.enhertsccg.nhs.uk/ Patient presents with abnormal CXR Lung cancer - clinical presentation History and Examination Incidental finding
Lung Cancer - Suspected Shared Decision Making Lung Cancer: http://www.enhertsccg.nhs.uk/ Patient presents with abnormal CXR Lung cancer - clinical presentation History and Examination Incidental finding
Imaging in breast cancer. Mammography and Ultrasound Donya Farrokh.MD Radiologist Mashhad University of Medical Since
 Imaging in breast cancer Mammography and Ultrasound Donya Farrokh.MD Radiologist Mashhad University of Medical Since A mammogram report is a key component of the breast cancer diagnostic process. A mammogram
Imaging in breast cancer Mammography and Ultrasound Donya Farrokh.MD Radiologist Mashhad University of Medical Since A mammogram report is a key component of the breast cancer diagnostic process. A mammogram
UCLA General Surgery Residency Program Rotation Educational Policy Goals and Objectives
 UPDATED: July 2009 ROTATION: THORACIC SURGERY UCLA General Surgery Residency Program ROTATION DIRECTOR: Mary Maish, M.D. CHIEF OF CARDIAC SURGERY: Robert Cameron, M.D. SITES: UCLA Medical Center - Westwood
UPDATED: July 2009 ROTATION: THORACIC SURGERY UCLA General Surgery Residency Program ROTATION DIRECTOR: Mary Maish, M.D. CHIEF OF CARDIAC SURGERY: Robert Cameron, M.D. SITES: UCLA Medical Center - Westwood
Low-dose CT Lung Cancer Screening Guidelines for Pulmonary Nodules Management Version 2
 Low-dose CT Lung Cancer Screening Guidelines for Pulmonary Nodules Management Version 2 The Committee for Management of CT-screening-detected Pulmonary Nodules 2009-2011 The Japanese Society of CT Screening
Low-dose CT Lung Cancer Screening Guidelines for Pulmonary Nodules Management Version 2 The Committee for Management of CT-screening-detected Pulmonary Nodules 2009-2011 The Japanese Society of CT Screening
Case Scenario 1. Pathology report Specimen from mediastinoscopy Final Diagnosis : Metastatic small cell carcinoma with residual lymphatic tissue
 Case Scenario 1 Oncology Consult: Patient is a 51-year-old male with history of T4N3 squamous cell carcinoma of tonsil status post concurrent chemoradiation finished in October two years ago. He was hospitalized
Case Scenario 1 Oncology Consult: Patient is a 51-year-old male with history of T4N3 squamous cell carcinoma of tonsil status post concurrent chemoradiation finished in October two years ago. He was hospitalized
Cystic Lung Disease. Cristopher A. Meyer, MD
 Cystic Lung Disease Cristopher A. Meyer, MD Air filled structure with definable wall typically less than 1 mm thick Cris A. Meyer, M.D. Professor of Radiology University of Wisconsin School of Medicine
Cystic Lung Disease Cristopher A. Meyer, MD Air filled structure with definable wall typically less than 1 mm thick Cris A. Meyer, M.D. Professor of Radiology University of Wisconsin School of Medicine
Case Scenario 1: Thyroid
 Case Scenario 1: Thyroid History and Physical Patient is an otherwise healthy 80 year old female with the complaint of a neck mass first noticed two weeks ago. The mass has increased in size and is palpable.
Case Scenario 1: Thyroid History and Physical Patient is an otherwise healthy 80 year old female with the complaint of a neck mass first noticed two weeks ago. The mass has increased in size and is palpable.
pulmonary metastasis 80EE4727C6037E7F69A9981B7E55A238 Pulmonary Metastasis 1 / 6
 Pulmonary Metastasis 1 / 6 2 / 6 3 / 6 Pulmonary Metastasis Metastatic tumors in the lungs are cancers that developed at other places in the body (or other parts of the lungs). They then spread through
Pulmonary Metastasis 1 / 6 2 / 6 3 / 6 Pulmonary Metastasis Metastatic tumors in the lungs are cancers that developed at other places in the body (or other parts of the lungs). They then spread through
Radiation Exposure in Pregnancy. John R. Mayo UNIVERSITY OF BRITISH COLUMBIA
 Radiation Exposure in Pregnancy John R. Mayo UNIVERSITY OF BRITISH COLUMBIA Illustrative Clinical Scenario 32 year old female 34 weeks pregnant with recent onset shortness of breath and central chest pain
Radiation Exposure in Pregnancy John R. Mayo UNIVERSITY OF BRITISH COLUMBIA Illustrative Clinical Scenario 32 year old female 34 weeks pregnant with recent onset shortness of breath and central chest pain
Bronchial carcinosarcoma
 Bronchial carcinosarcoma Carolina Carcano 1*, Edward Savage 2, Maria Julia Diacovo 3, Jacobo Kirsch 1 1. Division of Radiology, Cleveland Clinic Florida, Weston, Fl, USA 2. Department of Thoracic and Cardiovascular
Bronchial carcinosarcoma Carolina Carcano 1*, Edward Savage 2, Maria Julia Diacovo 3, Jacobo Kirsch 1 1. Division of Radiology, Cleveland Clinic Florida, Weston, Fl, USA 2. Department of Thoracic and Cardiovascular
Use of Integrated PET CT in the Clinical Staging of Non Small Cell Lung Cancer
 November 2010 Use of Integrated PET CT in the Clinical Staging of Non Small Cell Lung Cancer Laura Myers, Harvard Medical School, Year III Clinical Presentation 79yo woman with cough productive of green
November 2010 Use of Integrated PET CT in the Clinical Staging of Non Small Cell Lung Cancer Laura Myers, Harvard Medical School, Year III Clinical Presentation 79yo woman with cough productive of green
Chest X-ray (CXR) Interpretation Brent Burbridge, MD, FRCPC
 Chest X-ray (CXR) Interpretation Brent Burbridge, MD, FRCPC An approach to reviewing a chest x-ray will create a foundation that will facilitate the detection of abnormalities. You should create your own
Chest X-ray (CXR) Interpretation Brent Burbridge, MD, FRCPC An approach to reviewing a chest x-ray will create a foundation that will facilitate the detection of abnormalities. You should create your own
