Coincident generation of pyramidal neurons and protoplasmic astrocytes in neocortical columns
|
|
|
- Austin Shelton
- 5 years ago
- Views:
Transcription
1 University of Massachusetts Medical School Neurobiology Publications and Presentations Neurobiology Coincident generation of pyramidal neurons and protoplasmic astrocytes in neocortical columns Sanjay Magavi Vertex Pharmaceuticals Drew Friedmann University of California Garrett Banks Columbia University See next page for additional authors Follow this and additional works at: Part of the Neuroscience and Neurobiology Commons Repository Citation Magavi, Sanjay; Friedmann, Drew; Banks, Garrett; Stolfi, Alberto; and Lois, Carlos, "Coincident generation of pyramidal neurons and protoplasmic astrocytes in neocortical columns" (2012). Neurobiology Publications and Presentations This material is brought to you by It has been accepted for inclusion in Neurobiology Publications and Presentations by an authorized administrator of For more information, please contact
2 Coincident generation of pyramidal neurons and protoplasmic astrocytes in neocortical columns Authors Sanjay Magavi, Drew Friedmann, Garrett Banks, Alberto Stolfi, and Carlos Lois Rights and Permissions Publisher PDF posted as allowed by the publisher's author rights policy at misc/ifa_policies.xhtml#copyright. This article is available at
3 4762 The Journal of Neuroscience, April 4, (14): Development/Plasticity/Repair Coincident Generation of Pyramidal Neurons and Protoplasmic Astrocytes in Neocortical Columns Sanjay Magavi, 1 Drew Friedmann, 2 Garrett Banks, 3 Alberto Stolfi, 2 and Carlos Lois 4 1 Department of Neurology, Vertex Pharmaceuticals, Cambridge, Massachusetts 02139, 2 Center for Integrative Genomics, Division of Genetics, Genomics and Development, Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, California 94720, 3 Columbia University, New York, New York 10027, and 4 Neurobiology Department, University of Massachusetts Medical School, Worcester, Massachusetts Astrocytes, one of the most common cell types in the brain, are essential for processes ranging from neural development through potassium homeostasis to synaptic plasticity. Surprisingly, the developmental origins of astrocytes in the neocortex are still controversial. To investigate the patterns of astrocyte development in the neocortex we examined cortical development in a transgenic mouse in which a random, sparse subset of neural progenitors undergoes CRE/lox recombination, permanently labeling their progeny. We demonstrate that neural progenitors in neocortex generate discrete columnar structures that contain both projection neurons and protoplasmic astrocytes. Ninety-five percent of developmental cortical columns labeled in our system contained both astrocytes and neurons. The astrocyte to neuron ratio of labeled cells in a developmental column was 1:7.4, similar to the overall ratio of 1:8.4 across the entire gray matter of the neocortex, indicating that column-associated astrocytes account for the majority of protoplasmic astrocytes in neocortex. Most of the labeled columns contained multiple clusters of several astrocytes. Dividing cells were found at the base of neuronal columns at the beginning of gliogenesis, and later within the cortical layers, suggesting a mechanism by which astrocytes could be distributed within a column. These data indicate that radial glia are the source of both neurons and astrocytes in the neocortex, and that these two cell types are generated in a spatially restricted manner during cortical development. Introduction Historically, astrocytes have been considered simple support cells with little functional specificity or regional identity. The current models regarding the generation of cortical astrocytes reflect this assumed uniformity. Several works suggest that the majority of cortical astrocytes are generated the subventricular zone (SVZ) (Levison et al., 1993; Levison and Goldman, 1993; Luskin and McDermott, 1994; Marshall and Goldman, 2002), from where they migrate and populate cortex (Zerlin et al., 1995; Kakita and Goldman, 1999; Kakita et al., 2003). This model indicates that the majority of astrocytes arise from precursors that disperse widely, without spatial specificity. In addition, radial glia are also thought to contribute a smaller fraction of the astrocyte population within the cortex. It has been reported that radial glia, after generating projecting neurons, retract their processes and differentiate into protoplasmic astrocytes (Schmechel and Rakic, 1979; Voigt, 1989; Culican et al., 1990; Gressens et al., 1992). Since these data suggested that individual radial glia transform into individual astrocytes, it was assumed that their contribution to the overall population of Received July 11, 2011; revised Jan. 30, 2012; accepted Feb. 9, Author contributions: S.M., A.S., and C.L. designed research; S.M., D.F., G.B., A.S., and C.L. performed research; S.M., G.B., A.S., and C.L. contributed unpublished reagents/analytic tools; S.M., D.F., G.B., A.S., and C.L. analyzed data; S.M. and C.L. wrote the paper. This work was supported by the David and Lucile Packard Foundation to C.L. Correspondence should be addressed to Carlos Lois, Neurobiology Department, University of Massachusetts Medical School, 364 Plantation Street, LRB 770Y, Worcester, MA Carlos.Lois@umassmed.edu. DOI: /JNEUROSCI Copyright 2012 the authors /12/ $15.00/0 cortical astrocytes was very small. However, other experiments examining neural development were not consistent with these findings. For instance, It has been reported that radial-glia derived columns in the cortex are composed exclusively of pyramidal neurons, without astrocytes (Luskin et al., 1988; Price and Thurlow, 1988; Parnavelas et al., 1991; Mione et al., 1994; Tan et al., 1998; McCarthy et al., 2001; Reid and Walsh, 2002; Wilkie et al., 2004). To investigate the development and distribution of astrocytes in the brain we performed fate mapping in the brains of transgenic mice that expresses Cre sparsely and produce labeling of a small and apparently random set of brain precursors and their progeny. We observe that cortical progenitors give rise to discrete columnar structures that contain both projection neurons and protoplasmic astrocytes. Most columns of neurons contained multiple clusters of astrocytes, and 98% of labeled astrocytes were found in or within 50 m of a labeled neuronal column. The astrocyte to neuron ratio of labeled cells in a developmental column was similar to the overall ratio across the entire neocortex, indicating that column-associated astrocytes account for the majority of protoplasmic astrocytes in the neocortex. These data suggest that cortical protoplasmic astrocytes are generated in a spatially restricted manner from the precursors that also give rise to developmental columns of pyramidal neurons. Materials and Methods Generation of transgenic mice We generated several lines of enhancer trap mice carrying an enhancer detector cassette expressing Cre recombinase under the transcriptional control of the minimal promoter (129 base pairs) of the mouse Thy-1.2
4 Magavi et al. Columnar Generation of Neurons and Astrocytes J. Neurosci., April 4, (14): gene. The transgenics were generated via lentiviral vectors, allowing us to rapidly generate large numbers of animals with random integration sites. The transgene integrated into different sites in each of the thy1.2-cre lines, giving rise to different patterns of recombination upon breeding with reporter mice. We crossed these thy1.2-cre mice to the reporters Z/EG or mch/lox/gfp mice (gift from S. Dymecki, Harvard Medical School, Cambridge, MA), which, upon Cre/loxP recombination, exhibit GFP expression. Immunohistochemistry All experiments were performed in accordance with the National Institutes of Health Guide and Use of Laboratory Animals, and approved by the Massachusetts Institute of Technology Committee on Animal Care. For the analysis of the progeny of thy1.2-cre lines we used animals of either sex, and did not observe any obvious difference between the distribution of GFP cells between males and females. Mice were killed following a lethal dosage of Avertin. Animals were perfused with PBS followed by 3% paraformaldehyde. Brains were postfixed in paraformaldehyde overnight at 4 C and sectioned at 50 m using a Leica VT1000 vibrating microtome. The sections were immunohistochemically stained with antibodies against BrdU (Accurate Chemical OBT0030G, 1:500), GFP (Aves Labs GFP-1020, 1:8000), NeuN (Millipore Bioscience Research Reagents MAB377, 1:400), GFAP (Sigma G9269, 1:100), S100B (Abcam ab868, 1:2000), MBP (Covance SMI94, 1:1000), Nestin (Abcam Ab6142, 1:500) and Zebrin (Santa Cruz Biotechnology sc-12065, 1:100). To expose the BrdU antigen, we washed sections in 2 M HCl for 2 h. at room temperature. We used species-appropriate Invitrogen highly crossadsorbed Alexa Fluor 488- and Alexa Fluor 546-conjugated secondary antibodies. Antibodies generated in different species were used in multilabeling experiments to minimize cross-reactivity. Primary and secondary antibodies were diluted in a blocking solution containing 0.3% Triton, 8% goat serum and 0.3% BSA diluted in PBS. Figures were assembled using Adobe Photoshop and Illustrator. Cell counting Quantification of cells was performed using an Olympus IX70 microscope, a Q-imaging Retiga 1300i camera, and Neurolucida software with the virtual slice extension module. Location of astrocytes relative to manually defined columns of neurons. We defined the boundaries of a column by drawing lines perpendicular to the pial surface of the cortex through the most lateral GFP neurons in the column. Columns were defined by GFP cells that had unambiguous pyramidal projection neuron morphologies and were located laterally within 50 m of another pyramidal neuron. We counted the numbers of GFP glia inside, near (within 50 m) and outside of these boundaries (Fig. 1D). Brains that displayed labeled interneurons in addition to labeled projections neurons in cortex were excluded from this analysis. Nearest neighbor analysis. Sections were imaged with the Neurolucida virtual slice software and imported into ImageJ (NIH). The labeled astrocytes and neurons were manually tagged in ImageJ, which recorded their locations. Custom Matlab software was written that detected the nearest neuronal neighbor to an astrocyte and computed its distance. Neuronal density as a function of distance. The dataset generated in the nearest neighbor analysis was analyzed using custom Matlab software that measured neuronal density as a function of distance. We compared the ratio of labeled cells to unlabeled cells within a column and the GFP astrocyte to neuron ratio with the overall astrocyte to neuron ratio in cortex using the following techniques. The boundaries of the column were defined as described above, by drawing lines perpendicular to the pial surface of cortex. Astrocytes were identified by S100B expression and neurons via NeuN expression. The somata of cells were counted using a modified version of the stereological optical dissector technique: cells intersecting the upper boundary were excluded from the counts, minimizing overcounting. The top and bottom 3 m of each section were excluded from analysis to reduce sectioning and vibratome chatter artifacts. The variable locations of the columns made assessing absolute numbers of neurons and astrocytes within columns in a stereologically correct manner technically challenging, thus we limited ourselves to assessing ratios of various cell types. Cluster analysis. Individual columns were imaged as described above and astrocytes were manually tagged using ImageJ. Custom Matlab software determined the number of clusters and the number of astrocytes within a cluster. A cluster was defined as a group of GFP astrocytes each within 25 m of another GFP astrocyte. Results Analysis of cell lineage in the cortex via recombination in transgenic mice We generated several lines of enhancer trap mice carrying the enhancer detector cassette thy1.2-cre that expresses Cre recombinase under the transcriptional control of the minimal promoter (129 base pairs) of the mouse Thy-1.2 gene. We crossed these thy1.2-cre mice to the reporters Z/EG (Novak et al., 2000) or mch/lox/gfp mice (gift from S. Dymecki), which, after Cre/ loxp recombination, permanently express GFP. The transgene integrated into different sites in each of the thy1.2-cre lines, giving rise to different patterns of GFP labeling in the brain. One of the thy1.2-cre lines, TFC.09, had a low rate of recombination in a sparse and apparently random subset of neural precursors during development. TFC.09 mice are able to breed, have normal lifespans, and their behavior and brain structure are grossly normal. In the TFC.09 line, a single copy of the provirus integrated into the seventh intron of the glypican-3 gene. The expression of Cre and the subsequent recombination events in the TFC.09 line showed no correlation with the expression pattern of the glypican-3 gene (unpublished results). While a low recombination efficiency is a common hindrance to the investigators using CRE/LOX systems, it was advantageous in our case, because labeling a small subset of cells in the brain allowed us to examine individual columns and cells, as described below. We examined 200 of the progeny of TFC.09 mice crossed to reporter mice. Approximately 40% of these mice showed some pattern of GFP labeling in their CNS. In 20% of the progeny of TFC.09 reporter mice, the loxp-recombination gave rise to discrete columnar structures of GFP cells in the neocortex (Fig. 1A C). The columns of GFP cells, which extended radially through layers 1 through 6, are similar to the columns of clonally related neurons that have been described in the literature (Fig. 1 A C) (Luskin et al., 1988; Price and Thurlow, 1988; Parnavelas et al., 1991; Mione et al., 1994; Tan et al., 1998; McCarthy et al., 2001; Reid and Walsh, 2002; Wilkie et al., 2004). The GFP neurons in these columns had morphologies consistent with projection neurons. In 5% of the progeny of TFC.09 lox reporter mice, labeled cells with the morphological characteristics of interneurons were found dispersed broadly throughout the striatum and cortex (Parnavelas et al., 1991; Luskin et al., 1993; Tan et al., 1998; Reid and Walsh, 2002) (Fig. 2A,B). Additionally, we found that some of the progeny of TFC.09 reporter mice had GFP labeled neurons in a number of extra-cortical regions, including the hippocampus, thalamus, olfactory bulb, cerebellum, septum and midbrain. These regional patterns of GFP cells were usually found only in one hemisphere; however, some mice had bilateral labeling, often with different patterns of labeling in each hemisphere. The above patterns of labeling were observed in both the Z/EG and mch/lox/gfp reporter mice, indicating that these results are independent of the particular reporter line used.
5 4764 J. Neurosci., April 4, (14): Magavi et al. Columnar Generation of Neurons and Astrocytes Figure 1. Developmentally generated columns of cells in cortex contain both neurons and astrocytes. A, B, Adult TFC.09 Z/EG mice exhibit radially arrayed columns of cells in cortex apparent both along the sagittal (A) and coronal (B) planes. These columns of cells display the characteristicmorphologiesofdevelopmentalcolumnsgeneratedinpreviouscorticalclonal(figurelegendcontinues.)
6 Magavi et al. Columnar Generation of Neurons and Astrocytes J. Neurosci., April 4, (14): Figure 2. Interneurons in cortex are generated independently of cortical astrocytes. Approximately 5% of adult TFC.09 Z/EG mice exhibited widely distributed GFP cells within cortex that were not arranged in columns (A). In these mice, GFP cells were distributed sparsely in layers 2 through 6 of neocortex through the entire anteroposterior and mediolateral axes (A, B). The GFP cells in cortex of these mice displayed the characteristic morphologies of interneurons, with fine local processes (C). The cortices of these mice did not contain labeled astrocytes (C). TFC.09 Z/EG micewithinterneuronsincortexalwayshadgfp cellsbroadlydistributedthroughtheiripsilateralstriatum(a,b,d).inmicewithgfp interneuronsincortex,thestriatumcontainedbothgfp neurons and astrocytes (D). In the striata of these mice, GFP neurons and astrocytes had the characteristic morphologies of medium spiny interneurons and protoplasmic astrocytes, respectively (D). C and D are high-magnification views of the insets labeled C and D in B. B is a higher-magnification view of the inset in A. Scale bars: A, 500 m; B, 250 m; C, D,50 m. Coincident generation of protoplasmic astrocytes and pyramidal neurons within developmental cortical columns Prior experiments involving retroviral labeling of precursor cells in embryonic animals indicated that few or none of the labeled cortical columns contained both glia and neurons (Luskin et al., 1988; Price and Thurlow, 1988; Parnavelas et al., 1991; Mione et al., 1994; Tan et al., 1998; McCarthy et al., 2001; Reid and Walsh, 2002; Wilkie et al., 2004). However, the columns of labeled cells we observed appeared to contain both neurons and glia (Fig. 1A C). The GFP glial cells had typical morphologies of protoplasmic astrocytes and their processes appeared to occupy nonoverlapping domains, as has been previously described (Bushong et al., 2002; Wilhelmsson et al., 2006; Halassa et al., 2007). A total of %. GFP cells with astrocytic morphologies (of 603 cells examined in n 3 mice) expressed S100B (Fig. 3A C), a protein expressed almost exclusively by astrocytes in cortex. A total of % of GFP cells with astrocytic morphologies (611 cells examined in n 3 mice) expressed GFAP (Fig. 3D F), 4 (Figure legend continued.) labeling experiments using other methods. C, These columns of GFP labeled cells (white) contained both neurons and astrocytes, which have been pseudocolored blue for the purposes of visualization. C, D, F, G, We measured whether GFP astrocytes were in (blue lines), near (within 50 m as indicated by the red lines), or outside of GFP columns. Columns were defined as discreet, elongated structures in the cortex that contained GFP cells with unambiguous pyramidal neuron morphologies spanning from the ventricular to the pial surface. F, G, We measured the distance between astrocytes (blue) and their nearest neighboring neuron (green) to assess their spatial relationship. The average distance between an astrocyte and its nearest neighbor was m. H, I, We assessed neuronal density as a function of distance, measuring the density of neurons within 100 m of an astrocyte, 200 m, 300 m, etc. Astrocytes were found in regions of high neuronal density, as would be expected of astrocytes located within neuronal columns. J, Astrocytes were found associated with neuronal columns even when the columns were curved, as they are commonly in the occipital cortex, suggesting their association is not merely accidental. K, In piriform cortex, with its three-layered structure that is distinct from neocortex, neither layer 2 interneurons nor astrocytes exhibited columnar organization, demonstrating that the columnar structures we foundinneocortexareunlikelytobeartifactual.scalebars:a,b,m,n,500 m;d,j,k,100 m; C, F, H,50 m. a protein expressed in activated astrocytes. GFP cells with astrocytic morphologies did not express NeuN, a marker of mature neurons (Fig. 3G I), or MBP, a marker of oligodendrocytes. Thus, both the morphology of these cells and their immunochemical profile indicate that they are astrocytes. GFP astrocytes exhibited numerous processes that enveloped NeuN neurons within the same columnar structures. The neurons encompassed by GFP astrocytes were often GFP themselves, suggesting a possible functional relationship between labeled cells (Fig. 3G I). To measure the spatial relationship between GFP astrocytes and columns of labeled neurons we used three metrics. First, we defined the boundaries of a column by drawing lines perpendicular to the pial surface of the cortex through the most lateral GFP neurons in a column and counted the numbers of GFP glia inside, near (within 50 m) and outside of these boundaries (Fig. 1 D). Columns were defined as discrete, elongated structures in the cortex that contained GFP cells with unambiguous pyramidal neuron morphologies spanning from layer 6 to the pial surface. We measured the position of 3509 GFP astrocytes relative to 343 GFP columns in the brains of 9 adult mice and found that % of astrocytes were found within the boundaries of a column, % were found within 50 m outside of these boundaries and % were found further away (Fig. 1E). These results suggest that GFP astrocytes are predominantly located within columns delineated by labeled pyramidal neurons. Second, to avoid biases due to the manual selection of column boundaries we measured the distance between individual GFP astrocytes and the nearest GFP neuron (Fig. 1F). We analyzed a total of 1878 astrocytes and 10,944 neurons, marking the position of every GFP astrocyte and every GFP neuron in cortex in every 12th brain section from 5 adult mice. The average distance between a GFP astrocyte and the nearest GFP neuron was m (Fig. 1G). A total of 95% of GFP astrocytes had their nearest GFP pyramidal neuronal neighbor within 80 m.
7 4766 J. Neurosci., April 4, (14): Magavi et al. Columnar Generation of Neurons and Astrocytes Third, using the dataset from the second analysis, we constructed histograms to assess the density of GFP pyramidal neurons within 100, 200, 300 m, etc. of GFP astrocytes (Fig. 1H). The density of labeled pyramidal neurons was highest near labeled astrocytes, and dropped rapidly with distance (Fig. 1I). These data show that GFP astrocytes are closely associated with regions with high density of GFP projection neurons, as in the labeled columns we observed. Together, these measurements indicate that labeled progenitors form columns of cells in cortex containing both pyramidal neurons and protoplasmic astrocytes. Furthermore, GFP astrocytes were found within columns of GFP neurons even when these columns were not straight, such as the curved column in posterior cortex shown in Figure 1J. The colocalization of astrocytes and neurons in curved columns suggests that their proximity is not merely artifactual or accidental. Generation of astrocytes outside of the neocortex While we detected columns comprised of GFP pyramidal neurons and astrocytes in the neocortex, we did not find columns of labeled cells in regions of the brain in which columnar patterning has not been described. For example, the piriform cortex has a 3-layered architecture in which layer 2 consists almost exclusively of densely packed interneurons in a pattern reminiscent of granular neurons. In TFC.09 lox reporter mice both the labeled layer 2 piriform cortex neurons and the labeled astrocytes were broadly distributed, and showed no columnar organization (Fig. 1 K). We also found mice (n 11) that contained GFP cells in their neocortex with characteristic interneuron morphologies. In these mice, GFP interneurons were found widely distributed throughout both the cortex and the ipsilateral striatum, without any apparent columnar organization (Fig. 2A). These GFP neurons were distributed in a pattern similar to that of cells derived from the ganglionic eminences, which generate both striatal and cortical interneurons (Parnavelas et al., 1991; Luskin et al., 1993; Tan et al., 1998; Reid and Walsh, 2002). Whereas all 11 mice had both interneurons and astrocytes labeled in their striata (Fig. 2B,D), only interneurons were labeled in the cortex (Fig. 2C). GFP astrocytes were only present in the striatum when GFP interneurons were also present in the striatum and cortex. These data suggest that interneurons and striatal astrocytes share a common precursor. Furthermore, these data indicate that the coincident, columnar labeling of GFP projection neurons and astrocytes in the neocortex of these transgenic mice accurately represents the distribution of the progeny from neuronal and astrocyte progenitors in the brain. Lineage relationship between neurons and protoplasmic astrocytes within neocortical columns To examine whether the GFP neurons and astrocytes we observed within cortical columns shared a common precursor, we Figure 3. Non-neuronal GFP cells displayed characteristics of protoplasmic astrocytes. A C, Non-neuronal GFP cells in developmental columns in adult mice exhibited characteristic protoplasmic astrocyte morphologies (arrows). D F, A total of % (603 cells examined in n 3 mice) of non-neuronal GFP cells expressed S100B, an astrocytic marker. A total of % of non-neuronal GFP cells (611 cells examined in n 3 mice) expressed GFAP, a protein expressed in reactive and fibrous astrocytes in cortex. G I, Labeled cells with neuronal morphologies expressed neither S100B nor GFAP (arrowheads). Conversely, GFP cells with astrocytic morphologies did not express NeuN, a mature neuronal marker. The processes of GFP astrocytes often encompassed GFP neurons. Scale bars: A F,10 m; G I,25 m. examined the frequency with which the GFP pyramidal neurons and GFP astrocytes overlapped. We found that 95% (325 of 343 columns, n 9 mice) of GFP columns contained at least one astrocyte. Sixteen columns were found to contain only neurons, and in two instances a columnar structure consisting of only astrocytes was found. The few columns we observed that contained only neurons or only astrocytes had very few GFP cells, suggesting that, due to the plane of section, we may have been only sampling the edge of the column. Although it is theoretically possible that GFP glial columns and GFP neuronal columns arose independently from each other in the same locations, this is an unlikely scenario. There are neurons in the neocortex of the adult mouse (Schüz and Palm, 1989). Approximately 70% of cortical neurons are projection neurons (Jones, 1993; Wonders and Anderson, 2005). Thus there are projection neurons in neocortex. Tan et al., report that cortical columns arise in multiples of 600, with their largest column containing 5000 neurons, and the average column containing 1800 neurons (Tan et al., 1998). Dividing by 1800 indicates that there are 3900 columns in the cortex of the adult mouse. According to these estimates, in the 9 mice we examined, there were a total of 35,000 columns (9 3900) in their cortices. Of these estimated 35,000 columns, 343 contained GFP neurons; thus, the probability of finding a column of GFP neurons (P N ) is A total of 327 of 35,000 columns contained GFP astrocytes; the probability of finding a column of GFP astrocytes
8 Magavi et al. Columnar Generation of Neurons and Astrocytes J. Neurosci., April 4, (14): (P A ) is Thus, the probability that independent events would generate a column containing both GFP neurons and astrocytes is P N P A, or The likelihood that a given GFP column of neurons would contain independently generated GFP astrocytes is (P N P A )/P N, 0.01 or 1%. The most parsimonious explanation for the 95% overlap we saw between GFP neurons and GFP astrocytes is that the neurons and astrocytes in these columns are generated from a shared precursor cell. We examined the ratio of labeled cells to unlabeled cells within a column using the optical fractionator method. GFP astrocytes comprised % of all S100B astrocytes within a column (10 columns from each of n 3 mice). GFP neurons accounted for % of all NeuN neurons within a column (10 columns from each of n 3 mice). Thus, the precursor that generates a developmental column makes a similar contribution to both populations of cells; it generates 10% of both neurons and astrocytes within a given cortical column. We compared the GFP astrocyte to GFP neuron ratio with the overall astrocyte to neuron ratio in cortex. We found the ratio of GFP astrocytes to GFP pyramidal neurons was 1: (50 randomly selected columns sampled from n 5 mice). The overall ratio of S100B astrocytes to NeuN neurons was 1: (50 randomly selected columns sampled from n 5 mice). NeuN cells include both pyramidal neurons and interneurons in cortex. Our astrocyte to neuron ratios are similar to the 1:6 astrocyte to neuron ratio in motor cortex and the 1:8 ratio in sensory cortex previously reported (Irintchev et al., 2005). Since pyramidal projection neurons generated in columns account for the 70% of the neurons in cortex, these results suggest that most cortical astrocytes are derived from developmental columns as well. Figure 4. Astrocytes develop within cortical columns and maintain their spatial relationship through adulthood. A, B, At E18, columns of radially arrayed projection neurons were visible in cortex. C, D, By P1, GFP cells with morphologies resembling immatureastrocytes(pseudocoloredinred) werevisiblewithinthecolumns. E, F, AtP10, matureastrocytes(pseudocoloredinblue in F) were found within columns distributed through the depth of cortex, much like they are in adult mice. G, H, Columns in 2-month-old adult mice contained pyramidal neurons and multiple clusters of multiple astrocytes. GFP astrocytes were still found closely arrayed with columns of GFP cortical neurons in 10-month-old mice, indicating that astrocyte addition and/or turnoverintheadultmousedoes notappeartoresultinthewidespreaddispersionofastrocytes.scale bars:a C,E,G J, 100 m; D,F;25 m.matureastrocytesarepseudocoloredinblueine,h,andj.animmatureastrocyteispseudocoloredinredind.arrows in A, C, E, G, and I point toward the pial surface of the neocortex. Dynamics of astrocyte generation in neocortical columns Prior in vivo experiments suggested that radial glia transform directly into individual astrocytes as neurogenesis comes to an end (Schmechel and Rakic, 1979; Voigt, 1989; Culican et al., 1990; Gressens et al., 1992). Such data would neatly explain the colocalization of a single astrocyte with each developmentally generated column of neurons in the neocortex. However, most labeled developmental cortical columns contained multiple clusters, each comprised of multiple astrocytes (Figs.
9 4768 J. Neurosci., April 4, (14): Magavi et al. Columnar Generation of Neurons and Astrocytes Figure 5. GFP cells express radial glia markers zebrin and nestin during development. Between E16 and E18, GFP cells form columns of extending from the ventricles to the pia. GFP cells (green)neartheventriclesinthesecolumnsexpressednestin(a D,red)orZebrin(E H,red),proteinsexpressedbyradialglia.ByP0,zebrin /GFP cellswerefoundintheparenchymaofcortex, away from the ventricular wall, presenting morphologies resembling a shortened radial glia. A D, Arrows indicate GFP /Nestin cells. E L, Arrows indicate GFP /zebrin processes and cells. A, E, F, Asterisks indicate the neocortical ventricular wall. Zebrin /GFP cells were absent by P4, a time by which most radial glia have disappeared from the brain. Scale bars, 50 m. 1C,D,J,3A,G,4E,G,J). There were an average of clusters of GFP astrocytes per column (114 columns examined from n 5 mice). A cluster was defined as a group of GFP astrocytes each within 25 m of another GFP astrocyte. Each cluster contained between 1 and 15 astrocytes, with an average of astrocytes per cluster (139 clusters counted from 50 columns in n 5 mice). These counts are likely underestimates, because they are based on counting cells in a single 50 m thick section through each column, and columns generally extended through multiple sections. Nonetheless, the distribution of labeled astrocytes we observe is unlikely to be explained by the transformation of single radial glia into an individual astrocyte. We followed the development of cells present in the GFP columns in the cortex by examining mice at embryonic day 18 (E18), postnatal day 0 (P0), P1, P2, P4, P6, P8, P10, young adult mice (2 months old), and older adult mice (10 months old). At E18 and P1, GFP columns in cortex contained exclusively pyramidal neurons, and we did not detect any GFP cells with mature astrocytic morphologies in cortex (Fig. 4A D). This observation is consistent with previous works indicating that cortical gliogenesis occurs predominantly in the postnatal period. In contrast, subcortical gliogenesis was apparent by P1, a time at which we could already detect labeled cells displaying the morphologies of mature protoplasmic astrocytes in the striatum. At P1, we detected columns in the neocortex that contained, in addition to GFP pyramidal neurons, GFP cells that resembled the immature astrocytes previously described in cortex (Fig. 4 D) (Zerlin and Goldman, 1997). By P4, we found that columns contained both GFP pyramidal neurons and GFP cells with mature astrocytic morphologies. Thus, the astrocytes observed in these transgenic mice developed along the well established timeframe of astrocyte generation. Columns in P10 mice appeared to contain more astrocytes than in younger mice (Fig. 4E,F) and closely resembled the columns we observed in 2-month-old adult mice, which included GFP pyramidal neurons and multiple clusters of GFP astrocytes (Fig. 4G,H). GFP astrocytes in more mature adults (10 months old) were still found almost exclusively within developmental cortical columns consisting of both GFP pyramidal neurons and astrocytes (Fig. 4I,J). Thus, astrocyte addition and/or turnover in the adult brain does not lead to their dispersion outside of the developmental columns in which they originate, as they maintained their spatial relationships well into adulthood. To examine the relationship between radial glia and the GFP developmental columns, we examined the expression of nestin and zebrin, two proteins commonly used to identify radial glia. At E16 and E17, toward the end of cortical neurogenesis, we found columns of cells extending from the ventricle (Fig. 5A), through the corpus callosum, to the pial surface of cortex (Figs. 4A, 5E). This organization reflects the well characterized organization of cortical columns previously described (Tan et al., 1998). During the late embryonic period (E16 E18), a subset of GFP cells near
10 Magavi et al. Columnar Generation of Neurons and Astrocytes J. Neurosci., April 4, (14): the bases of these columns expressed nestin and zebrin. The example included in Figure 5, A D, shows a GFP cell with nestinpositive proximal processes at E16. The example GFP /zebrin cell in Figure 5, E H, was found lining the ventricle at E17 and exhibited zebrin-positive perinuclear staining. By P0, GFP /zebrin cells were found in the parenchyma of cortex away from the ventricular wall (Fig. 5J L). Previous studies have described cells similar to these ones we observed, with morphologies resembling shortened radial glia (Marshall and Goldman, 2002). It has been suggested that these cells may be in the process of differentiating from radial glia into protoplasmic astrocytes (Marshall and Goldman, 2002). These results suggest that some, if not all, the GFP cells located in the columns we observe are descendants of GFP radial glia. To identify the location of dividing cells during the period of gliogenesis, we treated mice with BrdU, a thymidine analog that integrates into the DNA of dividing cells, at P0, P2, P4, P6, or P8 and killed them 4 h later. At P0 (n 5 mice), we found 13 GFP /BrdU cells at the bases of columns and 2 GFP /BrdU cells within the cortical layers (Fig. 6A D). At P2 (n 3 mice) and P4 (n 5 mice) we found 21 and 8 GFP /BrdU cells within the cortical layers, respectively. At P2 and P4, we did not find GFP /BrdU cells at the bases of columns. The example illustrated in Figure 6, E G, contains GFP /BrdU cells in both layers 2 and 6 of P2 cortex. By P6 (n 3 mice), GFP /BrdU cells in developmental columns were infrequent or absent. Dividing GFP cells were found near the ventricle early in development and within the cortical layers later. In the proliferation experiments, we found two P0 mice and one P4 mouse that exclusively had GFP cells with interneuron morphologies distributed widely throughout cortex. While mice with GFP columns had both GFP pyramidal neurons and astrocytes in their cortices, mice with GFP interneurons did not exhibit GFP astrocytes in their cortices. Mice with selective labeling of interneurons had no GFP /BrdU cells in their cortices, suggesting that the postnatal GFP /BrdU cells are in the astrocyte lineage. To analyze the fate of GFP dividing cells during the postnatal period, we treated P0 mice with BrdU and killed them at P4 (Fig. 6H K). We found GFP /BrdU cells that had adopted astrocytic morphologies throughout the depth of cortex by P4 (n 5). These GFP /BrdU cells were often found in pairs, suggesting that they underwent their most recent division near their final location. In summary, dividing cells were found near the bases of columns during early postnatal gliogenesis and within the cortical layers thereafter, suggesting a pattern of cell proliferation and radial migration that could account for the regionally specific distribution of astrocytes in the mature cortex. Discussion We examined the development of cell lineages in neocortex using a transgenic mouse that undergoes apparently random recombination in a small subset of cells during embryogenesis, yielding GFP cells that can be followed from embryonic development through adulthood. We found developmental columns of GFP cells in neocortex consisting of both projection neurons and protoplasmic astrocytes. Ninety-five percent of labeled columns, as defined by the distribution of GFP pyramidal neurons in a radially oriented structure spanning from the ventricular to the pial surface, also contained GFP protoplasmic astrocytes. In addition, almost all of the labeled astrocytes in the neocortex were found in or near developmental columns of labeled neurons. The ratio of GFP astrocytes to GFP neurons in columns was 1: 7.4, which is similar to the overall ratio of astrocytes to neurons in cortex, 1:8.4, and suggests that most cortical astrocytes originate in developmental columns. After birth, some GFP cells incorporated BrdU during the period of gliogenesis in a pattern suggestive of a precursor that underwent an initial division in the germinal zone and subsequent divisions within more superficial cortical layers. Most of the resulting columns contained multiple clusters of several astrocytes. Our results indicate that a significant population of cortical astrocytes is generated in a spatially restricted manner, a process that may contribute to the diversity and regional specification of astrocytes. We observed columns of labeled cells in the neocortex that are similar to those previously described (Luskin et al., 1988; Tan and Breen, 1993; Mione et al., 1994; Tan et al., 1998; McCarthy et al., 2001; Reid and Walsh, 2002; Wilkie et al., 2004). However, in contrast to previous experiments, we found that our columns consisted of both astrocytes and neurons. We examined the spatial relationship between astrocytes and neurons and found that 98% of astrocytes were in or within 50 m of a column (as defined by the presence of radially oriented clusters of GFP pyramidal neurons) and, on average, GFP astrocytes were within 33 m of their nearest GFP neuron. Previous research may have been unable to detect astrocytes within developmental columns due to technical limitations and/or the comparatively small number of mice and columns examined. For instance, the promoters used in previous efforts may have driven robust gene expression only in neurons. In addition, older labeling techniques, such as lacz labeling, do not fill the cytoplasm entirely, making identification of cell types more challenging. Several pieces of evidence indicate that the colocalization of labeled neurons and astrocytes in developmental columns accurately represents the physiological pattern of development of neurons and astrocytes in the cortex. First, GFP neurons and astrocytes were found together in developmental columns even when the columns are curved, reducing the likelihood that the labeled cells are colocalized merely due to chance. Second, regions of the brain in which columnar patterning has not been described do not show colocalization of GFP neurons and astrocytes in discrete patterns. For instance, piriform cortex contained both widely distributed GFP layer 2 interneurons and GFP astrocytes, without any obvious pattern. Third, a subset of mice exhibited GFP interneurons but no developmental columns of GFP projection neurons in cortex. In these mice, GFP interneurons were always found broadly distributed both in the striatum and throughout neocortex, reproducing previous findings about neurons derived from the ganglionic eminences (Parnavelas et al., 1991; Luskin et al., 1993; Tan et al., 1998; Reid and Walsh, 2002). However, even though these mice exhibited GFP astrocytes in their striata and GFP interneurons in their cortices, their cortices did not contain any GFP astrocytes. These results show that TFC.09 mice recapitulate well established developmental patterns of brain development and indicate that astrocytes in cortex are generated by spatially restricted progenitors, whose progeny display little tangential dispersion. Radial glia simultaneously function both as a scaffold supporting the migration of projection neurons, and as progenitor cells generating new neurons in the neocortex (Noctor et al., 2001; Tamamaki et al., 2001; Malatesta et al., 2003; Anthony et al., 2004). Each radial glia generates neurons that migrate along their radial process into cortex, forming a developmental column of projection neurons, and previous work indicates that clonally related pyramidal neurons in the cortex are arranged in develop-
11 4770 J. Neurosci., April 4, (14): Magavi et al. Columnar Generation of Neurons and Astrocytes Figure6. Astrocyteprecursorsproliferatewithincorticalcolumns.Toidentifyproliferatingcells,miceweretreatedwithBrdUandkilled4hlater.A D,AtP0,dividing,BrdU (red),gfp (green)cellswere most frequently found at the bases of cortical columns, nearest the ventricle. E G, By P2, the majority of dividing GFP cells were distributed within cortex and few were found near the bases of columns. To examinethefateofdividinggfp cells,wetreatedmicewithbrduatp0andkilledthematp4.gfp cellslabeledwithbrduatp0werefoundthroughthedepthofcortex.h K,GFP /BRDU cellswerealso oftenfoundinpairs, suggestingthattheydifferentiatednearthesiteoftheirfinaldivision. Theseresultssuggestthefollowingmodelofcorticalastrogenesis(L): Radialgliafirstgeneratecolumnsofprojection neuronsincortex. Subsequently, theradialgliagenerateseveralastrocyticprecursorsthatdistributeradiallyalongthecolumnofneurons. Theseprecursorsundergotheirfinaldivisionsinthedifferentlayersof the cortex, forming multiple clusters of multiple astrocytes within a developmental cortical column. Scale bars:a,e,h, 100 m; B D,F,G,I K,10 m. mental columns (Luskin et al., 1988; Parnavelas et al., 1991; Mione et al., 1994; Tan et al., 1998; McCarthy et al., 2001). We found that 95% of GFP columns, as defined by the distribution of radial clusters of GFP pyramidal neurons, also contained GFP astrocytes. If labeled neuronal columns and astrocytic columns arose completely independently, from separate progenitors, their spatial overlap would be expected to be much lower, 1%, as discussed above. Our data indicate that the appearance
12 Magavi et al. Columnar Generation of Neurons and Astrocytes J. Neurosci., April 4, (14): of labeled neuronal and astrocytic columns are not independent events. The observed overlap is best explained by a progenitor that gives rise to both neurons and astrocytes within a developmental column. The origin of cortical astrocytes is not well understood. It has been postulated that both radial glia and the subventricular zone (SVZ) may give rise to astrocytes in the neocortex. Prior in vivo experiments suggested that radial glia retract their processes and differentiate into protoplasmic astrocytes after neurogenesis came to an end. Individual radial glia were thought to transform into individual astrocytes (Schmechel and Rakic, 1979; Voigt, 1989; Culican et al., 1990). In contrast, in vitro experiments suggested that radial glia can proliferate after neurogenesis and generate multiple astrocytes (Malatesta et al., 2000). In contrast to both these findings, experiments tracing the generation of developmental columns of cortical projection neurons found no associated astrocytes (Luskin et al., 1988; Price and Thurlow, 1988; Parnavelas et al., 1991; Mione et al., 1994; Tan et al., 1998; McCarthy et al., 2001; Reid and Walsh, 2002; Wilkie et al., 2004). The apparent inconsistencies in these data concerning the radial glial contribution to astrocyte development were difficult to reconcile. In addition to the reported small number of astrocytes derived from radial glia, several works suggested that the majority of astrocytes were derived from SVZ precursors that dispersed widely to populate cortex. As neurogenesis comes to an end, cells from the SVZ were thought to proliferate, generating oligodendrocyte and astrocyte precursors (Levison et al., 1993; Levison and Goldman, 1993; Luskin and McDermott, 1994; Marshall and Goldman, 2002) that migrated from the SVZ and dispersed widely through cortex (Zerlin et al., 1995; Kakita and Goldman, 1999; Kakita et al., 2003). These experiments were performed by injecting retroviruses into the SVZ of early postnatal mice and assessing the locations and phenotypes of labeled cells. Injecting viruses into the brain induces injury, which could perturb the migration patterns and fate of cells labeled. Gliosis is a common response to injury and could potentially increase the number of astrocytes labeled in the cortex after a retroviral injection into the SVZ. In addition, the timing of these injections, after neurogenesis has been completed, likely explains why these experiments labeled glial cells, but no neurons. Our data indicate that precursors originating in developmental columns generate the majority of cortical astrocytes. Within a developmental column, there is 1 GFP astrocyte for every 7.4 GFP pyramidal neurons. In the cortical gray matter overall, there is 1 astrocyte for every 8.4 neurons. Since pyramidal neurons, which in mice are exclusively generated in developmental columns, comprise 70% of the neurons in cortex (Wonders and Anderson, 2005), these data suggest that protoplasmic astrocytes associated with columns comprise the majority of protoplasmic astrocytes in cortex. Our experiments indicate a pattern of neocortical gliogenesis in which proliferation of astrocyte precursors occurs initially at the base of columns and later occurs within the cortical layers. At P0, dividing GFP cells are found at the base of columns near the ventricle. At P2 and P4, dividing GFP cells are found within the cortical layers. At P2, some columns contained multiple dividing GFP cells in different layers of cortex. Precursors labeled with BrdU at P0 frequently formed closely located pairs of astrocytes within cortex by P4, supporting the hypothesis they underwent their final division in cortex. Consistent with these findings, columns in adult mice frequently contained multiple clusters each consisting of multiple astrocytes. Together our data suggest the following model (Fig. 6L): radial glia give rise to both projection neurons and protoplasmic astrocytes in the mammalian neocortex. After generating a column of neurons, the radial glia generates multiple astrocyte precursors. These astrocyte precursors distribute through the depth of cortex, migrating radially within the neuronal column. They undergo their final divisions in the layers of the cortex, differentiate into protoplasmic astrocytes and form clusters of cells that respect the boundaries of the neuronal column. Our findings suggest a developmental process that could confer positional identity upon astrocytes along the axes of the neocortex. Cortical astrocytes serve essential roles in the development of synapses and in modifying neurotransmission (Pfrieger and Barres, 1997; Allen and Barres, 2005; Volterra and Meldolesi, 2005; Zhang and Haydon, 2005; Stevens, 2008). Thus it has been hypothesized that cortical astrocytes are likely to be regionally specified like cortical neurons (Reisin and Colombo, 2002; Wilhelmsson et al., 2006; Hochstim et al., 2008; Allen and Barres, 2009). Consistent with this hypothesis, it has been shown that spinal cord astrocytes are regionally specified in the spinal cord (Muroyama et al., 2005). Establishing whether astrocytes in the neocortex exhibit region-specific differences and examining the potential relationship between these astrocytes and their neighboring neurons within columns will be of particular interest in the future. References Allen NJ, Barres BA (2005) Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol 15: Allen NJ, Barres BA (2009) Neuroscience: Glia more than just brain glue. Nature 457: Anthony TE, Klein C, Fishell G, Heintz N (2004) Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron 41: Bushong EA, Martone ME, Jones YZ, Ellisman MH (2002) Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22: Costa MR, Bucholz O, Schroeder T, Götz M (2009) Late origin of gliarestricted progenitors in the developing mouse cerebral cortex. Cereb Cortex 19 [Suppl 1]:i135 i143. Culican SM, Baumrind NL, Yamamoto M, Pearlman AL (1990) Cortical radial glia: identification in tissue culture and evidence for their transformation to astrocytes. J Neurosci 10: Gressens P, Richelme C, Kadhim HJ, Gadisseux JF, Evrard P (1992) The germinative zone produces the most cortical astrocytes after neuronal migration in the developing mammalian brain. Biol Neonate 61:4 24. Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG (2007) Synaptic islands defined by the territory of a single astrocyte. J Neurosci 27: Hochstim C, Deneen B, Lukaszewicz A, Zhou Q, Anderson DJ (2008) Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 133: Irintchev A, Rollenhagen A, Troncoso E, Kiss JZ, Schachner M (2005) Structural and functional aberrations in the cerebral cortex of tenascin-c deficient mice. Cereb Cortex 15: Jones EG (1993) GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex 3: Kakita A, Goldman JE (1999) Patterns and dynamics of SVZ cell migration in the postnatal forebrain: monitoring living progenitors in slice preparations. Neuron 23: Kakita A, Zerlin M, Takahashi H, Goldman JE (2003) Some glial progenitors in the neonatal subventricular zone migrate through the corpus callosum to the contralateral cerebral hemisphere. J Comp Neurol 458: Levison SW, Goldman JE (1993) Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron 10: Levison SW, Chuang C, Abramson BJ, Goldman JE (1993) The migrational
Nature Neuroscience: doi: /nn Supplementary Figure 1. MADM labeling of thalamic clones.
 Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Supplementary Figure 1 MADM labeling of thalamic clones. (a) Confocal images of an E12 Nestin-CreERT2;Ai9-tdTomato brain treated with TM at E10 and stained for BLBP (green), a radial glial progenitor-specific
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Investigating the Diversity of Radial Glia Fates in the Rat Neocortex
 University of Connecticut DigitalCommons@UConn Honors Scholar Theses Honors Scholar Program Spring 5-9-2010 Investigating the Diversity of Radial Glia Fates in the Rat Neocortex Abraham William Aron University
University of Connecticut DigitalCommons@UConn Honors Scholar Theses Honors Scholar Program Spring 5-9-2010 Investigating the Diversity of Radial Glia Fates in the Rat Neocortex Abraham William Aron University
Gene co-expression networks in the mouse, monkey, and human brain July 16, Jeremy Miller Scientist I
 Gene co-expression networks in the mouse, monkey, and human brain July 16, 2013 Jeremy Miller Scientist I jeremym@alleninstitute.org Outline 1. Brief introduction to previous WGCNA studies in brain 2.
Gene co-expression networks in the mouse, monkey, and human brain July 16, 2013 Jeremy Miller Scientist I jeremym@alleninstitute.org Outline 1. Brief introduction to previous WGCNA studies in brain 2.
Plasticity of Cerebral Cortex in Development
 Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Plasticity of Cerebral Cortex in Development Jessica R. Newton and Mriganka Sur Department of Brain & Cognitive Sciences Picower Center for Learning & Memory Massachusetts Institute of Technology Cambridge,
Tangential migration of neurons in the developing cerebral cortex
 Development 121, 2165-2176 (1995) Printed in Great Britain The Company of Biologists Limited 1995 2165 Tangential migration of neurons in the developing cerebral cortex Nancy A. O Rourke*, Daniel P. Sullivan,
Development 121, 2165-2176 (1995) Printed in Great Britain The Company of Biologists Limited 1995 2165 Tangential migration of neurons in the developing cerebral cortex Nancy A. O Rourke*, Daniel P. Sullivan,
Prss56, a novel marker of adult neurogenesis in the mouse brain. - Supplemental Figures 1 to 5- Brain Structure and Function
 Prss56, a novel marker of adult neurogenesis in the mouse brain - Supplemental Figures 1 to 5- Brain Structure and Function Alexandre Jourdon 1,2, Aurélie Gresset 1, Nathalie Spassky 1, Patrick Charnay
Prss56, a novel marker of adult neurogenesis in the mouse brain - Supplemental Figures 1 to 5- Brain Structure and Function Alexandre Jourdon 1,2, Aurélie Gresset 1, Nathalie Spassky 1, Patrick Charnay
Supplementary Information
 Supplementary Information Astrocytes regulate adult hippocampal neurogenesis through ephrin-b signaling Randolph S. Ashton, Anthony Conway, Chinmay Pangarkar, Jamie Bergen, Kwang-Il Lim, Priya Shah, Mina
Supplementary Information Astrocytes regulate adult hippocampal neurogenesis through ephrin-b signaling Randolph S. Ashton, Anthony Conway, Chinmay Pangarkar, Jamie Bergen, Kwang-Il Lim, Priya Shah, Mina
Cell Migration II: CNS Cell Migration. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Hey! The major concepts discussed relative to neural crest cell migration apply to cell migration
Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Hey! The major concepts discussed relative to neural crest cell migration apply to cell migration
Cell Birth and Death. Chapter Three
 Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Birth and Death Chapter Three Neurogenesis All neurons and glial cells begin in the neural tube Differentiated into neurons rather than ectoderm based on factors we have already discussed If these
Cell Migration II: CNS Cell Migration. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Oct 18) 9:00-10:00am Surdyk s Café in Northrop Auditorium Stop
Cell Migration II: CNS Cell Migration Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Oct 18) 9:00-10:00am Surdyk s Café in Northrop Auditorium Stop
ErbB4 migrazione I parte. 3- ErbB4- NRG1
 ErbB4 migrazione I parte 3- ErbB4- NRG1 1 In rodent brains postnatal neuronal migration is evident in three main areas: the cerebellum (CB), the hippocampus (Hipp) and the rostral migratory stream (RMS).
ErbB4 migrazione I parte 3- ErbB4- NRG1 1 In rodent brains postnatal neuronal migration is evident in three main areas: the cerebellum (CB), the hippocampus (Hipp) and the rostral migratory stream (RMS).
Are Both Embryonic Migratory Pathways Preserved in the Adult Brain Cerebral Cortex?
 Prague Medical Report / Vol. 107 (2006) No. 1, p. 71 80 71) Are Both Embryonic Migratory Pathways Preserved in the Adult Brain Cerebral Cortex? Šimonová Z., Dutt J. Department of Neuroscience of the Institute
Prague Medical Report / Vol. 107 (2006) No. 1, p. 71 80 71) Are Both Embryonic Migratory Pathways Preserved in the Adult Brain Cerebral Cortex? Šimonová Z., Dutt J. Department of Neuroscience of the Institute
Supplementary Figure 1
 Supplementary Figure 1 The average sigmoid parametric curves of capillary dilation time courses and average time to 50% peak capillary diameter dilation computed from individual capillary responses averaged
Supplementary Figure 1 The average sigmoid parametric curves of capillary dilation time courses and average time to 50% peak capillary diameter dilation computed from individual capillary responses averaged
Introduction. Materials and Methods
 4240 The Journal of Neuroscience, May 15, 2003 23(10):4240 4250 Multiple Cell Populations in the Early Postnatal Subventricular Zone Take Distinct Migratory Pathways: A Dynamic Study of Glial and Neuronal
4240 The Journal of Neuroscience, May 15, 2003 23(10):4240 4250 Multiple Cell Populations in the Early Postnatal Subventricular Zone Take Distinct Migratory Pathways: A Dynamic Study of Glial and Neuronal
Supplementary Figure 1. Nature Neuroscience: doi: /nn.4547
 Supplementary Figure 1 Characterization of the Microfetti mouse model. (a) Gating strategy for 8-color flow analysis of peripheral Ly-6C + monocytes from Microfetti mice 5-7 days after TAM treatment. Living
Supplementary Figure 1 Characterization of the Microfetti mouse model. (a) Gating strategy for 8-color flow analysis of peripheral Ly-6C + monocytes from Microfetti mice 5-7 days after TAM treatment. Living
ErbB4 migrazione II parte
 ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
ErbB4 migrazione II parte Control SVZ cells prefer to migrate on the NRG1 type III substrate the substrate preference of the neuroblasts migrating out of the SVZ explant was evaluated SVZ cells had a strong
Supplementary Figure S1: Tanycytes are restricted to the central/posterior hypothalamus
 Supplementary Figure S1: Tanycytes are restricted to the central/posterior hypothalamus a: Expression of Vimentin, GFAP, Sox2 and Nestin in anterior, central and posterior hypothalamus. In the anterior
Supplementary Figure S1: Tanycytes are restricted to the central/posterior hypothalamus a: Expression of Vimentin, GFAP, Sox2 and Nestin in anterior, central and posterior hypothalamus. In the anterior
Neural stem cells and the neurobiology of ageing. Chen Siyun 1, Dawe G.S. 2
 ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
ABSTRACT Neural stem cells and the neurobiology of ageing Chen Siyun 1, Dawe G.S. 2 Department of Physics, Faculty of Science, National University of Singapore 10 Kent Ridge Road, Singapore 117546 The
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References
 File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
File name: Supplementary Information Description: Supplementary Figures, Supplementary Table and Supplementary References File name: Supplementary Data 1 Description: Summary datasheets showing the spatial
Nature Neuroscience doi: /nn Supplementary Figure 1. Characterization of viral injections.
 Supplementary Figure 1 Characterization of viral injections. (a) Dorsal view of a mouse brain (dashed white outline) after receiving a large, unilateral thalamic injection (~100 nl); demonstrating that
Supplementary Figure 1 Characterization of viral injections. (a) Dorsal view of a mouse brain (dashed white outline) after receiving a large, unilateral thalamic injection (~100 nl); demonstrating that
In vivo reprogramming reactive glia into ipscs to produce new neurons in the
 In vivo reprogramming reactive glia into ipscs to produce new neurons in the cortex following traumatic brain injury Xiang Gao 1, Xiaoting Wang 1, Wenhui Xiong 1, Jinhui Chen 1, * 1 Spinal Cord and Brain
In vivo reprogramming reactive glia into ipscs to produce new neurons in the cortex following traumatic brain injury Xiang Gao 1, Xiaoting Wang 1, Wenhui Xiong 1, Jinhui Chen 1, * 1 Spinal Cord and Brain
Citation for published version (APA): Martina-Mamber, C. E. (2014). GFAP as an understudy in adult neurogenesis. 's-hertogenbosch: Boxpress.
 UvA-DARE (Digital Academic Repository) GFAP as an understudy in adult neurogenesis Mamber, C.E. Link to publication Citation for published version (APA): Martina-Mamber, C. E. (2014). GFAP as an understudy
UvA-DARE (Digital Academic Repository) GFAP as an understudy in adult neurogenesis Mamber, C.E. Link to publication Citation for published version (APA): Martina-Mamber, C. E. (2014). GFAP as an understudy
NG2 + CNS Glial Progenitors Remain Committed to the Oligodendrocyte Lineage in Postnatal Life and following Neurodegeneration
 Article NG2 + CNS Glial Progenitors Remain Committed to the Oligodendrocyte Lineage in Postnatal Life and following Neurodegeneration Shin H. Kang, 1 Masahiro Fukaya, 2,4 Jason K. Yang, 1 Jeffrey D. Rothstein,
Article NG2 + CNS Glial Progenitors Remain Committed to the Oligodendrocyte Lineage in Postnatal Life and following Neurodegeneration Shin H. Kang, 1 Masahiro Fukaya, 2,4 Jason K. Yang, 1 Jeffrey D. Rothstein,
Department of Cognitive Science UCSD
 Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Neurons vs. glia. Traditionally, glia have been viewed as passive cells that help to maintain the function of neurons.
 GLIA Neurons vs. glia The defining characteristic of a neuron is its ability to transmit rapid electrical signals in the form of action potentials. All other neural cells that lack this property are broadly
GLIA Neurons vs. glia The defining characteristic of a neuron is its ability to transmit rapid electrical signals in the form of action potentials. All other neural cells that lack this property are broadly
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1.
 Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Cognitive Neuroscience Structure and Function
 Phylogeny of the cortex Cognitive Neuroscience Structure and Function The neocortex of mammals developed out of the primordial neopallium, which, like that of certain present-day amphibians, consisted
Phylogeny of the cortex Cognitive Neuroscience Structure and Function The neocortex of mammals developed out of the primordial neopallium, which, like that of certain present-day amphibians, consisted
SUPPLEMENTARY FIG. S2. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of dnscs.
 Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
Supplementary Data SUPPLEMENTARY FIG. S1. Representative counting fields used in quantification of the in vitro neural differentiation of pattern of anpcs. A panel of lineage-specific markers were used
Announcement. Danny to schedule a time if you are interested.
 Announcement If you need more experiments to participate in, contact Danny Sanchez (dsanchez@ucsd.edu) make sure to tell him that you are from LIGN171, so he will let me know about your credit (1 point).
Announcement If you need more experiments to participate in, contact Danny Sanchez (dsanchez@ucsd.edu) make sure to tell him that you are from LIGN171, so he will let me know about your credit (1 point).
P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center. Wednesday, 16 March 2009, 1:00p.m. 2:00p.m.
 Normal CNS, Special Senses, Head and Neck TOPIC: CEREBRAL HEMISPHERES FACULTY: LECTURE: READING: P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center Wednesday, 16 March
Normal CNS, Special Senses, Head and Neck TOPIC: CEREBRAL HEMISPHERES FACULTY: LECTURE: READING: P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center Wednesday, 16 March
Evidence of Common Progenitors and Patterns of Dispersion in Rat Striatum and Cerebral Cortex
 The Journal of Neuroscience, May 15, 2002, 22(10):4002 4014 Evidence of Common Progenitors and Patterns of Dispersion in Rat Striatum and Cerebral Cortex Christopher B. Reid 1,2 and Christopher A. Walsh
The Journal of Neuroscience, May 15, 2002, 22(10):4002 4014 Evidence of Common Progenitors and Patterns of Dispersion in Rat Striatum and Cerebral Cortex Christopher B. Reid 1,2 and Christopher A. Walsh
Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70%
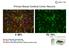 Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70% Pictures: 9 days after electroporation Red: MAP2 Blue: GFAP Green: GFP The cells were from Embryonic Day 14 Mouse Cerebral Cortex Primary Mouse Hippocampal
Primary Mouse Cerebral Cortex Neurons V: 80% TE: 70% Pictures: 9 days after electroporation Red: MAP2 Blue: GFAP Green: GFP The cells were from Embryonic Day 14 Mouse Cerebral Cortex Primary Mouse Hippocampal
Neocortex. Cortical Structures in the Brain. Neocortex Facts. Laminar Organization. Bark-like (cortical) structures: Shepherd (2004) Chapter 12
 Neocortex Shepherd (2004) Chapter 12 Rodney Douglas, Henry Markram, and Kevan Martin Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Cortical Structures in the Brain Bark-like (cortical) structures:
Neocortex Shepherd (2004) Chapter 12 Rodney Douglas, Henry Markram, and Kevan Martin Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Cortical Structures in the Brain Bark-like (cortical) structures:
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
SUPPLEMENTARY FIGURES 1 Supplementary Figure 1, Adult hippocampal QNPs and TAPs uniformly express REST a-b) Confocal images of adult hippocampal mouse sections showing GFAP (green), Sox2 (red), and REST
Supplementary Figure 1
 Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
Supplementary Figure 1 Kif1a RNAi effect on basal progenitor differentiation Related to Figure 2. Representative confocal images of the VZ and SVZ of rat cortices transfected at E16 with scrambled or Kif1a
MIDTERM EXAM 1 COGNITIVE SCIENCE 107A
 MIDTERM EXAM 1 COGNITIVE SCIENCE 107A FALL 2011 Name: Points: / 100 PID: I. SHORT ANSWERS (6 points each for a total of 30 points) 1. Describe two contributions made by Ramon y Cajal (1852-1934) in terms
MIDTERM EXAM 1 COGNITIVE SCIENCE 107A FALL 2011 Name: Points: / 100 PID: I. SHORT ANSWERS (6 points each for a total of 30 points) 1. Describe two contributions made by Ramon y Cajal (1852-1934) in terms
BIOL Dissection of the Sheep and Human Brain
 BIOL 2401 Dissection of the Sheep and Human Brain Laboratory Objectives After completing this lab, you should be able to: Identify the main structures in the sheep brain and to compare them with those
BIOL 2401 Dissection of the Sheep and Human Brain Laboratory Objectives After completing this lab, you should be able to: Identify the main structures in the sheep brain and to compare them with those
mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons
 Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Supplemental Material mir-7a regulation of Pax6 in neural stem cells controls the spatial origin of forebrain dopaminergic neurons Antoine de Chevigny, Nathalie Coré, Philipp Follert, Marion Gaudin, Pascal
Computational Approaches to Transcriptome Signatures in the Human Brain
 Computational Approaches to Transcriptome Signatures in the Human Brain Agilent Technologies eseminar February 18, 2016 Mike Hawrylycz, Ph.D. ALLEN Adult Human Atlas Online tools An Anatomic Transcriptional
Computational Approaches to Transcriptome Signatures in the Human Brain Agilent Technologies eseminar February 18, 2016 Mike Hawrylycz, Ph.D. ALLEN Adult Human Atlas Online tools An Anatomic Transcriptional
Broad Integration of Expression Maps and Co-Expression Networks Compassing Novel Gene Functions in the Brain
 Supplementary Information Broad Integration of Expression Maps and Co-Expression Networks Compassing Novel Gene Functions in the Brain Yuko Okamura-Oho a, b, *, Kazuro Shimokawa c, Masaomi Nishimura b,
Supplementary Information Broad Integration of Expression Maps and Co-Expression Networks Compassing Novel Gene Functions in the Brain Yuko Okamura-Oho a, b, *, Kazuro Shimokawa c, Masaomi Nishimura b,
CISC 3250 Systems Neuroscience
 CISC 3250 Systems Neuroscience Levels of organization Central Nervous System 1m 10 11 neurons Neural systems and neuroanatomy Systems 10cm Networks 1mm Neurons 100μm 10 8 neurons Professor Daniel Leeds
CISC 3250 Systems Neuroscience Levels of organization Central Nervous System 1m 10 11 neurons Neural systems and neuroanatomy Systems 10cm Networks 1mm Neurons 100μm 10 8 neurons Professor Daniel Leeds
Development of the Nervous System 1 st month
 Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
The neuron. Biocytin labeled pyramidal neuron recorded in piriform cortex
 The neuron Biocytin labeled pyramidal neuron recorded in piriform cortex Discovery of the neuron (A) Reticularist Doctrine (B) Neuron Doctrine Exception.GAP JUNCTIONS between neurons Neuronal shape Dendrites
The neuron Biocytin labeled pyramidal neuron recorded in piriform cortex Discovery of the neuron (A) Reticularist Doctrine (B) Neuron Doctrine Exception.GAP JUNCTIONS between neurons Neuronal shape Dendrites
Neocortex Zbtb20 / NFIA / Sox9
 Neocortex / NFIA / Sox9 Supplementary Figure 1. Expression of, NFIA, and Sox9 in the mouse neocortex at. The lower panels are higher magnification views of the oxed area. Arrowheads indicate triple-positive
Neocortex / NFIA / Sox9 Supplementary Figure 1. Expression of, NFIA, and Sox9 in the mouse neocortex at. The lower panels are higher magnification views of the oxed area. Arrowheads indicate triple-positive
Supplementary information. Nkx2.1 regulates the generation of telencephalic astrocytes during embryonic
 Supplementary information Nkx2.1 regulates the generation of telencephalic astrocytes during embryonic development Shilpi Minocha 1*, Delphine Valloton 1*, Yvan Arsenijevic 2, Jean-René Cardinaux 3, Raffaella
Supplementary information Nkx2.1 regulates the generation of telencephalic astrocytes during embryonic development Shilpi Minocha 1*, Delphine Valloton 1*, Yvan Arsenijevic 2, Jean-René Cardinaux 3, Raffaella
Supplementary Information
 Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
Supplementary Information Title Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis Authors Shin H. Kang, Ying Li, Masahiro Fukaya, Ileana Lorenzini,
Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing
 SUPPLEMENTARY INFORMATION Resource https://doi.org/10.1038/s41593-017-0056-2 In the format provided by the authors and unedited. Conserved properties of dentate gyrus neurogenesis across postnatal development
SUPPLEMENTARY INFORMATION Resource https://doi.org/10.1038/s41593-017-0056-2 In the format provided by the authors and unedited. Conserved properties of dentate gyrus neurogenesis across postnatal development
Cellular components of CNS
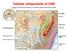 Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
Gross Organization I The Brain. Reading: BCP Chapter 7
 Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
Early Postnatal Astroglial Cells Produce Multilineage Precursors and Neural Stem Cells In Vivo
 The Journal of Neuroscience, August 16, 2006 26(33):8609 8621 8609 Development/Plasticity/Repair Early Postnatal Astroglial Cells Produce Multilineage Precursors and Neural Stem Cells In Vivo Yosif M.
The Journal of Neuroscience, August 16, 2006 26(33):8609 8621 8609 Development/Plasticity/Repair Early Postnatal Astroglial Cells Produce Multilineage Precursors and Neural Stem Cells In Vivo Yosif M.
Supplemental Information. Otic Mesenchyme Cells Regulate. Spiral Ganglion Axon Fasciculation. through a Pou3f4/EphA4 Signaling Pathway
 Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Neuron, Volume 73 Supplemental Information Otic Mesenchyme Cells Regulate Spiral Ganglion Axon Fasciculation through a Pou3f4/EphA4 Signaling Pathway Thomas M. Coate, Steven Raft, Xiumei Zhao, Aimee K.
Hanna Stevens, MD, PhD
 The Amazing Brain Webinar Series: Select Topics in Neuroscience and Child Development for the Clinician Part VI What a Baby Can and Can t Do: Early Brain Development Hanna Stevens, MD, PhD Jointly sponsored
The Amazing Brain Webinar Series: Select Topics in Neuroscience and Child Development for the Clinician Part VI What a Baby Can and Can t Do: Early Brain Development Hanna Stevens, MD, PhD Jointly sponsored
Neuronal or Glial Progeny: Regional Differences in Radial Glia Fate
 Neuron, Vol. 37, 751 764, March 6, 2003, Copyright 2003 by Cell Press Neuronal or Glial Progeny: Regional Differences in Radial Glia Fate Paolo Malatesta, 1 Michael A. Hack, 1 Eva Hartfuss, 1 Helmut Kettenmann,
Neuron, Vol. 37, 751 764, March 6, 2003, Copyright 2003 by Cell Press Neuronal or Glial Progeny: Regional Differences in Radial Glia Fate Paolo Malatesta, 1 Michael A. Hack, 1 Eva Hartfuss, 1 Helmut Kettenmann,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
SUPPLEMENTARY INFORMATION doi:10.1038/nature11306 Supplementary Figures Supplementary Figure 1. Basic characterization of GFP+ RGLs in the dentate gyrus of adult nestin-gfp mice. a, Sample confocal images
During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches
 During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches Progenitors are Contained within Unique Domains and Tangentially Fixed. EMBRYO ADULT Migratory Behavior
During Brain Development Final Destinations for Neurons and Glia Get Separated from Germinal Niches Progenitors are Contained within Unique Domains and Tangentially Fixed. EMBRYO ADULT Migratory Behavior
Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota
 Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Tuesday (Sept 11) 10:00-11:00am Friday (Sept 14) 8:30-9:30am Surdyk s
Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Tuesday (Sept 11) 10:00-11:00am Friday (Sept 14) 8:30-9:30am Surdyk s
Cortical Organization. Functionally, cortex is classically divided into 3 general types: 1. Primary cortex:. - receptive field:.
 Cortical Organization Functionally, cortex is classically divided into 3 general types: 1. Primary cortex:. - receptive field:. 2. Secondary cortex: located immediately adjacent to primary cortical areas,
Cortical Organization Functionally, cortex is classically divided into 3 general types: 1. Primary cortex:. - receptive field:. 2. Secondary cortex: located immediately adjacent to primary cortical areas,
Semmelweis University of Budapest. Department of Anatomy, Histology and Embryology. Béla Ajtai, M.D. COMPARATIVE MORPHOLOGY OF REACTIVE GLIOSIS
 Semmelweis University of Budapest Department of Anatomy, Histology and Embryology Béla Ajtai, M.D. COMPARATIVE MORPHOLOGY OF REACTIVE GLIOSIS Theses for PhD degree (short form) Tutor: Dr Mihály Kálmán,
Semmelweis University of Budapest Department of Anatomy, Histology and Embryology Béla Ajtai, M.D. COMPARATIVE MORPHOLOGY OF REACTIVE GLIOSIS Theses for PhD degree (short form) Tutor: Dr Mihály Kálmán,
Cerebral Cortex 1. Sarah Heilbronner
 Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
Dissection of the Sheep Brain
 Dissection of the Sheep Brain Laboratory Objectives After completing this lab, you should be able to: 1. Identify the main structures in the sheep brain and to compare them with those of the human brain.
Dissection of the Sheep Brain Laboratory Objectives After completing this lab, you should be able to: 1. Identify the main structures in the sheep brain and to compare them with those of the human brain.
Zhu et al, page 1. Supplementary Figures
 Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
The neuron. Biocytin labeled pyramidal neuron recorded in piriform cortex
 The neuron Biocytin labeled pyramidal neuron recorded in piriform cortex Discovery of the neuron (A) Reticularist Doctrine (B) Neuron Doctrine Exception.GAP JUNCTIONS between neurons Neuronal shape Dendrites
The neuron Biocytin labeled pyramidal neuron recorded in piriform cortex Discovery of the neuron (A) Reticularist Doctrine (B) Neuron Doctrine Exception.GAP JUNCTIONS between neurons Neuronal shape Dendrites
Terminology. Terminology. Terminology. Terminology. Terminology. Bromodeoxyuridine
 Kateřina Náměstková, Zuzana Šimonová, Eva Syková Behavioural Brain Research Bromodeoxyuridine : Doublecortin : DCX Glial Fibrillary Acidic Protein : GFAP Trace eye blink conditioning 1 Volume 163 : pp.
Kateřina Náměstková, Zuzana Šimonová, Eva Syková Behavioural Brain Research Bromodeoxyuridine : Doublecortin : DCX Glial Fibrillary Acidic Protein : GFAP Trace eye blink conditioning 1 Volume 163 : pp.
CNS Developmental. Anke van Eekelen, PhD. Telethon Institute for Child Health Research
 CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture
 Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture 2 The prenatal developmental of the mammalian cerebral cortex, including that of humans, is characterized by two sequential and interrelated
Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture 2 The prenatal developmental of the mammalian cerebral cortex, including that of humans, is characterized by two sequential and interrelated
Hypoxic-Ischemic Injury Stimulates Subventricular Zone Proliferation and Neurogenesis in the Neonatal Rat
 0031-3998/05/5803-0600 PEDIATRIC RESEARCH Vol. 58, No. 3, 2005 Copyright 2005 International Pediatric Research Foundation, Inc. Printed in U.S.A. Hypoxic-Ischemic Injury Stimulates Subventricular Zone
0031-3998/05/5803-0600 PEDIATRIC RESEARCH Vol. 58, No. 3, 2005 Copyright 2005 International Pediatric Research Foundation, Inc. Printed in U.S.A. Hypoxic-Ischemic Injury Stimulates Subventricular Zone
The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible:
 NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex
 Supplementary Information Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex Luc Gentet, Yves Kremer, Hiroki Taniguchi, Josh Huang, Jochen Staiger and Carl
Supplementary Information Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex Luc Gentet, Yves Kremer, Hiroki Taniguchi, Josh Huang, Jochen Staiger and Carl
Central nervous system (CNS): brain and spinal cord Collections of cell body and dendrites (grey matter) are called nuclei/nucleus Nucleus can also
 Chapter 3 Part 1 Orientation Directions in the nervous system are described relatively to the neuraxis An imaginary line drawn through the center of the length of the central nervous system, from the bottom
Chapter 3 Part 1 Orientation Directions in the nervous system are described relatively to the neuraxis An imaginary line drawn through the center of the length of the central nervous system, from the bottom
Contact: Course outline: Contact for other times.
 Contact: kdelaney@uvic.ca Course outline: http://web.uvic.ca/~kdelaney/b367 Scheduled office hours: 1:00-3:00, M&Th Cunn. 259A Contact kdelaney@uvic.ca for other times. Quiz (0.5 hrs) midterm (1.4 hrs)
Contact: kdelaney@uvic.ca Course outline: http://web.uvic.ca/~kdelaney/b367 Scheduled office hours: 1:00-3:00, M&Th Cunn. 259A Contact kdelaney@uvic.ca for other times. Quiz (0.5 hrs) midterm (1.4 hrs)
UNIT 5 REVIEW GUIDE - NERVOUS SYSTEM 1) State the 3 functions of the nervous system. 1) 2) 3)
 UNIT 5 REVIEW GUIDE - NERVOUS SYSTEM State the 3 functions of the nervous system. Briefly describe the general function(s) of each of the following neuron types: a) SENSORY NEURONS: b) INTERNEURONS: c)
UNIT 5 REVIEW GUIDE - NERVOUS SYSTEM State the 3 functions of the nervous system. Briefly describe the general function(s) of each of the following neuron types: a) SENSORY NEURONS: b) INTERNEURONS: c)
Milestones of neuronal development in the adult hippocampus
 Milestones of neuronal development in the adult hippocampus Gerd Kempermann 1,2, Sebastian Jessberger 2, Barbara Steiner 2 and Golo Kronenberg 1,3 1 Max Delbrück Center for Molecular Medicine (MDC) Berlin-Buch,
Milestones of neuronal development in the adult hippocampus Gerd Kempermann 1,2, Sebastian Jessberger 2, Barbara Steiner 2 and Golo Kronenberg 1,3 1 Max Delbrück Center for Molecular Medicine (MDC) Berlin-Buch,
Olfactory ensheathing glia
 Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Olfactory ensheathing glia From Wikipedia, the free encyclopedia Neuroglia of the brain shown by Golgi's method. Olfactory ensheathing glia (OEG), also known as olfactory ensheathing cells (OECs) or olfactory
Supplementary Figure 1
 Supplementary Figure 1 Genetic labeling of microglia Male and female 2-3 month-old CreERT2;R26-tdTomato mice or CreERT2;R26-tdTomato;Iba1-eGFP transgenic mice were treated with 1x, 2x (48 h apart), or
Supplementary Figure 1 Genetic labeling of microglia Male and female 2-3 month-old CreERT2;R26-tdTomato mice or CreERT2;R26-tdTomato;Iba1-eGFP transgenic mice were treated with 1x, 2x (48 h apart), or
marker. DAPI labels nuclei. Flies were 20 days old. Scale bar is 5 µm. Ctrl is
 Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Supplementary Figure 1. (a) Nos is detected in glial cells in both control and GFAP R79H transgenic flies (arrows), but not in deletion mutant Nos Δ15 animals. Repo is a glial cell marker. DAPI labels
Title: Chapter 5 Recorded Lecture. Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu
 Title: Chapter 5 Recorded Lecture Speaker: Title: What Anthony is the title Berger/Angela of this lecture? Williams Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu Chapter
Title: Chapter 5 Recorded Lecture Speaker: Title: What Anthony is the title Berger/Angela of this lecture? Williams Speaker: Amit Dhingra Created by: (remove if same as speaker) online.wsu.edu Chapter
Astroglia induce neurogenesis from adult neural stem cells
 Astroglia induce neurogenesis from adult neural stem cells Hongjun Song*, Charles F. Stevens* & Fred H. Gage * Molecular Neurobiology Laboratory, Howard Hughes Medical Institute at the Salk Institute,
Astroglia induce neurogenesis from adult neural stem cells Hongjun Song*, Charles F. Stevens* & Fred H. Gage * Molecular Neurobiology Laboratory, Howard Hughes Medical Institute at the Salk Institute,
Chapter 5. Summary and Future directions
 95 Chapter 5 Summary and Future directions 96 Much of our knowledge about glial development in the vertebrate CNS comes from studies of purified oligodendrocyte precursor cells (Raff 1989; Pfeiffer et
95 Chapter 5 Summary and Future directions 96 Much of our knowledge about glial development in the vertebrate CNS comes from studies of purified oligodendrocyte precursor cells (Raff 1989; Pfeiffer et
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
doi:10.1038/nature10188 Supplementary Figure 1. Embryonic epicardial genes are down-regulated from midgestation stages and barely detectable post-natally. Real time qrt-pcr revealed a significant down-regulation
COGNITIVE SCIENCE 107A MIDTERM EXAM 1 - FALL Name: PID: Total Pts: /100pts
 COGNITIVE SCIENCE 107A MIDTERM EXAM 1 - FALL 2009 Name: PID: Total Pts: /100pts I. SHORT ANSWERS (5 points each for a total of 30 points) 1. Label the three meningeal layers in the following diagram. Describe
COGNITIVE SCIENCE 107A MIDTERM EXAM 1 - FALL 2009 Name: PID: Total Pts: /100pts I. SHORT ANSWERS (5 points each for a total of 30 points) 1. Label the three meningeal layers in the following diagram. Describe
Structural basis for the role of inhibition in facilitating adult brain plasticity
 Structural basis for the role of inhibition in facilitating adult brain plasticity Jerry L. Chen, Walter C. Lin, Jae Won Cha, Peter T. So, Yoshiyuki Kubota & Elly Nedivi SUPPLEMENTARY FIGURES 1-6 a b M
Structural basis for the role of inhibition in facilitating adult brain plasticity Jerry L. Chen, Walter C. Lin, Jae Won Cha, Peter T. So, Yoshiyuki Kubota & Elly Nedivi SUPPLEMENTARY FIGURES 1-6 a b M
SOME BASIC TERMINOLOGY CNS: Central Nervous System: Brain + Spinal Cord
 SOME BASIC TERMINOLOGY CNS: Central Nervous System: Brain + Spinal Cord CEREBROSPINAL FLUID (CSF): The fluid filling the ventricles, cerebral aqueduct, central canal, and subarachnoid space. It is a filtrate
SOME BASIC TERMINOLOGY CNS: Central Nervous System: Brain + Spinal Cord CEREBROSPINAL FLUID (CSF): The fluid filling the ventricles, cerebral aqueduct, central canal, and subarachnoid space. It is a filtrate
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) (B) (C) (D) (E)
 Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) Confocal images of septal olfactory epithelium of an adult Gγ8-sypGFP-tdTom mouse showing colocalization
Supplementary Figure 1: Validation of labeling specificity of immature OSNs and presynaptic terminals. (A) Confocal images of septal olfactory epithelium of an adult Gγ8-sypGFP-tdTom mouse showing colocalization
Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse
 Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Supplemental figure legends Supplemental Figure 1. Intracranial transduction of a modified ptomo lentiviral vector in the mouse hippocampus targets GFAP-positive but not NeuN-positive cells. (A) Stereotaxic
Nervous system. Dr. Rawaa Salim Hameed
 Nervous system Dr. Rawaa Salim Hameed Central nervous system (CNS) CNS consists of the brain (cerebrum, cerebellum, and brainstem) and spinal cord CNS is covered by connective tissue layers, the meninges
Nervous system Dr. Rawaa Salim Hameed Central nervous system (CNS) CNS consists of the brain (cerebrum, cerebellum, and brainstem) and spinal cord CNS is covered by connective tissue layers, the meninges
Advanced multimodal imaging in malformations of cortical development
 Advanced multimodal imaging in malformations of cortical development Seok Jun Hong (sjhong@bic.mni.mcgill.ca) NOEL Neuroimaging of Epilepsy Lab MICA Multimodal Imaging and Connectome Analysis Lab w4 w5
Advanced multimodal imaging in malformations of cortical development Seok Jun Hong (sjhong@bic.mni.mcgill.ca) NOEL Neuroimaging of Epilepsy Lab MICA Multimodal Imaging and Connectome Analysis Lab w4 w5
Option A: Neurobiology & Behavior HL BIOLOGY 2 ND EDITION DAMON, MCGONEGAL, TOSTO, AND
 Option A: Neurobiology & Behavior A1: NEURAL DEVELOPMENT USE THE INFO IN THE PRESENTATION TO COMPLETE A1 NOTES GUIDE INFORMATION TAKEN FROM: HL BIOLOGY 2 ND EDITION DAMON, MCGONEGAL, TOSTO, AND WARD BIOLOGY
Option A: Neurobiology & Behavior A1: NEURAL DEVELOPMENT USE THE INFO IN THE PRESENTATION TO COMPLETE A1 NOTES GUIDE INFORMATION TAKEN FROM: HL BIOLOGY 2 ND EDITION DAMON, MCGONEGAL, TOSTO, AND WARD BIOLOGY
Cellular Neurobiology course December/January calendar and deadlines (updated )
 Cellular Neurobiology course 2016-17 December/January calendar and deadlines (updated 20-12-2016) Thu December 22 Scadenza ricerca articoli per il Topic# 3 (prorogato a 28 dicembre) Wed December 28 Scadenza
Cellular Neurobiology course 2016-17 December/January calendar and deadlines (updated 20-12-2016) Thu December 22 Scadenza ricerca articoli per il Topic# 3 (prorogato a 28 dicembre) Wed December 28 Scadenza
Visual system invades the endbrain: pathways to striatum and cortex (continued) Why this happened in evolution
 Visual system invades the endbrain: pathways to striatum and cortex (continued) Why this happened in evolution What were the adaptive advantages? Visual information reaching the striatum directly: Advantages
Visual system invades the endbrain: pathways to striatum and cortex (continued) Why this happened in evolution What were the adaptive advantages? Visual information reaching the striatum directly: Advantages
Supplementary Figure 1. ACE robotic platform. A. Overview of the rig setup showing major hardware components of ACE (Automatic single Cell
 2 Supplementary Figure 1. ACE robotic platform. A. Overview of the rig setup showing major hardware components of ACE (Automatic single Cell Experimenter) including the MultiClamp 700B, Digidata 1440A,
2 Supplementary Figure 1. ACE robotic platform. A. Overview of the rig setup showing major hardware components of ACE (Automatic single Cell Experimenter) including the MultiClamp 700B, Digidata 1440A,
1. The basic anatomy of the Central Nervous System (CNS)
 Psyc 311A, fall 2008 Conference week 1 Sept 9 th to 11 th TA: Jürgen Germann; e-mail: jurgen.germann@mcgill.ca Overview: 1. The basic anatomy of the Central Nervous System (CNS) 2. Cells of the CNS 3.
Psyc 311A, fall 2008 Conference week 1 Sept 9 th to 11 th TA: Jürgen Germann; e-mail: jurgen.germann@mcgill.ca Overview: 1. The basic anatomy of the Central Nervous System (CNS) 2. Cells of the CNS 3.
label the basement membrane). Different fixation methods of EB-perfused P8 mice to optimize the combination
 Supplementary Figure 1 Optimization of the tissue fixation protocol to combine EB perfusion and IB4 endothelial tip cell staining in the postnatal mouse brain. a-l Labeling of EB-perfused P8 mice with
Supplementary Figure 1 Optimization of the tissue fixation protocol to combine EB perfusion and IB4 endothelial tip cell staining in the postnatal mouse brain. a-l Labeling of EB-perfused P8 mice with
Healthy Brain Development: Protective and Risk Factors
 Healthy Brain Development: Protective and Risk Factors Bryce Geeraert PhD Candidate Developmental Neuroimaging Lab January 31, 2018 About me BSc Psychology, Biomedical Engineering PhD candidate Interest
Healthy Brain Development: Protective and Risk Factors Bryce Geeraert PhD Candidate Developmental Neuroimaging Lab January 31, 2018 About me BSc Psychology, Biomedical Engineering PhD candidate Interest
Nature Neuroscience: doi: /nn.2275
 Supplementary Figure S1. The presence of MeCP2 in enriched primary glial cultures from rat or mouse brains is not neuronal. Western blot analysis of protein extracts from (a) rat glial and neuronal cultures.
Supplementary Figure S1. The presence of MeCP2 in enriched primary glial cultures from rat or mouse brains is not neuronal. Western blot analysis of protein extracts from (a) rat glial and neuronal cultures.
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
Supplementary Materials
 Supplementary Materials Fig. S1. Weights of full-dose treatment groups comparing 1 st, 2 nd, and 3 rd generation gene replacement therapy. Mice were treated at p1 with 4x10 11 GC of the three different
Supplementary Materials Fig. S1. Weights of full-dose treatment groups comparing 1 st, 2 nd, and 3 rd generation gene replacement therapy. Mice were treated at p1 with 4x10 11 GC of the three different
( neural progeni2 tor cell), ,,,, , (neural crest) (radial glial cell) mrna [2 ]
![( neural progeni2 tor cell), ,,,, , (neural crest) (radial glial cell) mrna [2 ] ( neural progeni2 tor cell), ,,,, , (neural crest) (radial glial cell) mrna [2 ]](/thumbs/92/110608454.jpg) 246, 1999 6, 51 (3), 246 252 Acta Physiologica Sinica 3 P19 3 3 (, 200031 ;, 200031) (nestin),, RA P19,, (neural precursor cell) BMP4, (NF160), ( neural progeni2 tor cell),, : ; P19 ; BMP4 ; : Q71,,,,
246, 1999 6, 51 (3), 246 252 Acta Physiologica Sinica 3 P19 3 3 (, 200031 ;, 200031) (nestin),, RA P19,, (neural precursor cell) BMP4, (NF160), ( neural progeni2 tor cell),, : ; P19 ; BMP4 ; : Q71,,,,
Sox9 is critical for suppression of neurogenesis but not initiation of gliogenesis in the cerebellum. Vong et al.
 Sox9 is critical for suppression of neurogenesis but not initiation of gliogenesis in the cerebellum Vong et al. Vong et al. Molecular Brain (2015) 8:25 DOI 10.1186/s13041-015-0115-0 Vong et al. Molecular
Sox9 is critical for suppression of neurogenesis but not initiation of gliogenesis in the cerebellum Vong et al. Vong et al. Molecular Brain (2015) 8:25 DOI 10.1186/s13041-015-0115-0 Vong et al. Molecular
