Response Characterstics of Spinothalamic Tract Neurons That Project to the Posterior Thalamus in Rats
|
|
|
- Amie Craig
- 5 years ago
- Views:
Transcription
1 J Neurophysiol 93: , 2005; doi: /jn Response Characterstics of Spinothalamic Tract Neurons That Project to the Posterior Thalamus in Rats Xijing Zhang and Glenn J. Giesler, Jr Department of Neuroscience, University of Minnesota, Minneapolis, Minnesota Submitted 6 December 2004; accepted in final form 21 December 2004 Zhang, Xijing and Glenn J. Giesler Jr. Response characterstics of spinothalamic tract neurons that project to the posterior thalamus in rats. J Neurophysiol 93: , 2005; doi: /jn A sizeable number of spinothalamic tract axons terminate in the posterior thalamus. The functional roles and precise areas of termination of these axons have been a subject of recent controversy. The goals of this study were to identify spinothalamic tract neurons (STT) within the cervical enlargement that project to this area, characterize their responses to mechanical and thermal stimulation of their receptive fields, and use microantidromic tracking methods to determine the nuclei in which their axons terminate. Forty-seven neurons were antidromically activated using low-amplitude ( 30 A) current pulses in the contralateral posterior thalamus. The 51 points at which antidromic activation thresholds were lowest were surrounded by ineffective tracks indicating that the surrounded axons terminated within the posterior thalamus. The areas of termination were located primarily in the posterior triangular, medial geniculate, posterior and posterior intralaminar, and suprageniculate nuclei. Recording points were located in the superficial and deep dorsal horn. The mean antidromic conduction velocity was 6.4 m/s, a conduction velocity slower than that of other projections to the thalamus or hypothalamus in rats. Cutaneous receptive fields appeared to be smaller than those of neurons projecting to other areas of the thalamus or to the hypothalamus. Each of the examined neurons responded exclusively or preferentially to noxious stimuli. These findings indicate that the STT carries nociceptive information to several target nuclei within the posterior thalamus. We discuss the evidence that this projection provides nociceptive information that plays an important role in fear conditioning. INTRODUCTION Several lines of evidence, dating back 40 yr, indicate that the posterior thalamus plays a prominent role in nociceptive processing. The seminal observation was made by Poggio and Mountcastle (1960), who found that a large number of neurons recorded in the posterior thalamus of cats were activated by noxious mechanical stimuli. In subsequent years, neurons within the posterior thalamus have been shown to respond to noxious mechanical, thermal, and electrical stimulation of the skin within frequently large, discontinuous bilateral receptive fields (Benedek et al. 1997; Bordi and LeDoux 1994; Brinkhaus et al. 1979; Calma 1965; Casey 1966; Craig et al. 1994; Curry 1972; Curry and Gordon 1972; Gauriau and Bernard 2004b; Guilbaud et al. 1977, 1980; Nyquist and Greenhoot 1974; Perl and Whitlock 1961; Whitlock and Perl 1961). Neurons in the posterior thalamus can also be activated by visceral noxious stimuli such as intra-arterial injection of bradykinin (Guilbaud et al. 1977) and noxious distention of visceral organs including the bladder, esophagus, duodenum, and colon (Carstens and Yokota 1980; Horn et al. 1999). Cells in the posterior thalamus are also activated by electrical stimulation of the pelvic nerve (Bruggeman et al. 1994) and tooth pulp (Matsumoto et al. 1988). Two studies noted that the percentage of nociceptive neurons that were encountered in the posterior thalamus appeared to be much greater than were present in the ventral posterior lateral thalamic nucleus (VPL) of cats (Guilbaud et al. 1977; Poggio and Mountcastle 1960). In humans, nociceptive units have also been recorded in areas posterior and inferior to the ventrocaudal thalamic nucleus (Lenz et al. 1993b, 1994). These neurons seem likely to be located within the posterior thalamus. Lenz and colleagues also found that small-amplitude stimulation pulses delivered through recording electrodes in this area elicit painful sensations in awake humans (Lenz et al. 1993a). There is currently considerable controversy surrounding the termination of the spinothalamic tract (STT) within the posterior thalamus. A number of early anatomical studies in which either degeneration or anterograde labeling techniques were used showed that many STT axons terminate within several nuclei within the posterior thalamus including the medial region of the medial geniculate (MGm), the suprageniculate nucleus (SG), and the posterior nucleus (Po) (Apkarian and Hodge 1989; Boivie 1979; Mehler 1969; Mehler et al. 1960; Ralston and Ralston 1992). However, Craig et al. (Craig 2004; Craig et al. 1994) suggested that many axons from marginal zone neurons projected specifically to a previously unrecognized nucleus that is rich in calbindin-immunoreactive fibers. They named this area the posterior portion of the ventral medial nucleus (VMpo). The evidence supporting the existence of VMpo was challenged in a review by Willis et al. (2002) and in an accompanying commentary by Jones (2002). In addition, Graziano and Jones (2004) recently re-examined the projections of the STT in cats. They concluded that the nociceptive, calbindin-rich zone described by Craig and colleagues was not, in fact, located within the posterior thalamus but was located within the medial portion of the ventral posterior medial nucleus. Graziano and Jones (2004) also found that fibers that were anterogradely labeled by injections into the marginal zone of trigeminal nucleus caudalis were distributed widely throughout the posterior thalamus, supporting the older anatomical studies. Graziano and Jones (2004) also demonstrated that none of the labeled trigeminothalamic fibers were immunoreactive for calbindin, refuting the suggestion by Craig et al. that Address for reprint requests and other correspondence: G. Giesler, Dept. of Neuroscience, Jackson Hall, University of Minnesota, Minneapolis, MN ( giesler@lenti.med.umn.edu). The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact /05 $8.00 Copyright 2005 The American Physiological Society
2 STT NEURONS PROJECTING TO POSTERIOR THALAMUS 2553 STT and trigeminothalamic fibers contained calbindin. Recently, Gauriau and Bernard (2004a) examined the projections of the STT in rats using injections of the anterograde tracer PHA-L into several specific regions of the spinal gray matter including the marginal zone. They found dense projections within the posterior thalamus in several nuclei including the posterior triangular nucleus (PoT), the posterior intralaminar nucleus (PIL), and Po. In several previous physiological studies, spino- or trigeminothalamic neurons have been antidromically activated from the posterior thalamus (Dostrovsky and Craig 1996; Hirata et al. 2000; Trevino et al. 1973). However, anatomical and other physiological studies have shown that many STT axons pass through the posterior thalamus in route to more rostral areas such as the ventral posterior lateral nucleus (VPL) and the hypothalamus (Apkarian and Hodge 1989; Applebaum et al. 1979; Boivie 1979; Dado et al. 1994a; Mehler 1969; Mehler et al. 1960; Zhang et al. 1995). Therefore it is not clear whether the axons that were antidromically activated in the posterior thalamus in previous studies passed through the area or terminated within it. In a previous study of spinohypothalamic tract axons in which microantidromic activation methods were used, we (Dado et al. 1994a) noted that several axons could not be followed into levels rostral to posterior thalamus. The primary goal of the present study was to pursue these initial observations and determine the functional characteristics of STT axons that terminate in the posterior thalamus in rats. We used the electrophysiological method of microantidromic activation techniques to identify the subnuclei within the posterior thalamus in which their axons terminate. METHODS Male Sprague-Dawley rats weighing g were anesthetized with urethan (1.3 g/kg ip), artificially ventilated, and paralyzed with a continuous infusion of flaxedil (20 mg/h). The external jugular vein was cannulated for drug administration. End-tidal CO 2 and core temperature were monitored and maintained at normal levels. The cervical enlargement of the spinal cord was exposed by laminectomy. The dura was retracted, and a pool of warm mineral oil was formed over the exposed area of the spinal cord. A craniotomy exposed the brain over the diencephalon and mesencephalon bilaterally to allow the insertion of stainless steel monopolar stimulating electrodes. Single units in the cervical enlargement of the spinal cord (C 7 C 8 ) were recorded using stainless steel (5 10 M ) or tungsten microelectrodes (2 5 M ). Cathodal current pulses (500 A, 200 s, 10 Hz) delivered through a stimulating electrode placed in the contralateral posterior thalamus served as search stimuli. The criteria used for antidromic activation included: responses occurred at a constant latency ( 0.2 ms variability), responses were able to follow highfrequency stimuli ( 333 Hz), and putative antidromic action potentials collided with orthodromic action potentials (Lipski 1981). To determine the area of termination for each axon, microantidromic mapping was done in the diencephalon as previously described (Dado et al. 1994a; Zhang et al. 1995). Briefly, the stimulating electrode was moved rostrally throughout the hypothalamus and thalamus in a series of electrode penetrations across the mediolateral extent of the brain. If the neuron was antidromically activated in the hypothalamus or in rostral levels of the thalamus, the unit was discarded. Antidromic tracking for each axon was begun at a level near the rostral pole of the diencephalon. After multiple tracks were made across the most rostral level, the stimulating electrode was moved mm caudally, and the procedure was repeated at the new level. Within an anterior-posterior plane, tracks were separated by m mediolaterally. Within each track the electrode was lowered from the dorsal to the ventral surface and antidromic thresholds was determined at 200- m intervals. A series of electrode tracks was made until a point was located at which the antidromic threshold was 30 A (low-threshold point). All examined units were antidromically activated using currents 30 A in the posterior thalamus; none could be activated at greater latencies at levels rostral to posterior thalamus. Current pulses 30 A have been shown to activate spinohypothalamic tract axons at a distance of 400 m from the stimulating electrode (Burstein et al. 1991; Dado et al. 1994a). To locate the apparent terminal areas of the axons, we surrounded penetrations containing the most rostral low threshold point dorsally, ventrally, rostrally, medially, and laterally with penetrations in which the axon could not be activated antidromically with 500 A. Failure to activate the axon antidromically with a 500- A pulse is interpreted as evidence that the axon did not pass through the stimulated areas (Zhang et al. 1995). The boundaries of cutaneous receptive fields and the response characteristics of recorded cells were determined using innocuous and noxious mechanical stimuli. Units were classified as low-threshold (LT), wide-dynamic-range (WDR), or high-threshold (HT) neurons according to their responses to graded mechanical stimulation of their receptive fields (see Dado et al. 1994b for details). Thermal stimuli were applied to the area of the cutaneous receptive fields in which mechanical stimuli produced the highest frequency responses. Thermal stimuli were delivered using a peltier-type stimulator with a contact area of 9 9 mm (Wilcox and Giesler 1984). Stimulus duration was 30 s, and the interstimulus interval was 180 s. The stimulating surface of the skin was maintained at 35 C during interstimulus intervals. The mean response frequency to each stimulus was defined as the mean frequency during stimulus minus the mean firing frequency in the 30 s before each stimulus. An ascending series of heat stimuli of 39, 41, 45, 50, 55, and 58 C were applied followed by a descending series of cooling stimuli of 20, 10, and 1 C. Skin temperature was increased at 10 C/s and decreased at 2 C/s. At the end of each experiment, electrolytic lesions were made at the tip of the stimulating electrode at low-threshold points in the brain (25 A DC for 40 s) and at the tip of the recording electrode (25 A DC for 15 s). Rats were perfused with 0.9% saline followed by 10% formalin containing 1% potassium ferrocyanide (Prussian blue reaction). The areas of the brain and spinal cord containing lesions were cut transversely at 50 m using a freezing microtome. Sections were counterstained with Neutral red and examined under a microscope. The locations of lesions were reconstructed with the aid of an attached camera-lucida drawing tube. Recording locations were categorized as being located within the superficial (i.e., marginal zone or substantia gelatinosa) or deep (i.e., nucleus proprius or lateral reticulated area) dorsal horn. The atlas of Paxinos and Watson (1986) was used to help identify the locations of lesions within the brain. RESULTS An example of a spinal cord neuron that was antidromically activated from the contralateral midbrain and posterior thalamus is illustrated in Fig. 1. The unit was initially antidromically activated from a low-threshold point within the anterior pretectal nucleus (B and C) at a latency of 2.4 ms (b1). The unit was antidromically activated at a second low-threshold point located in the PoT (A and C) at a latency of 2.7 ms. The antidromic threshold at this point was 10 A. Stimulus pulses of 10 A have been shown to activate axons a distance of not 170 m (Burstein et al. 1991; Dado et al. 1994a; Ranck 1975). The ability of the neuron to follow high-frequency (333 Hz) antidromic stimulus pulses and collision of antidromic with orthodromic action potentials are illustrated (a, 2 and 3).
3 2554 X. ZHANG AND G. J. GIESLER FIG. 1. An example of a neuron in the C 7 spinal segment that projected to the contralateral posterior thalamus nucleus. Stimulating electrode penetrations were made initially in the contralateral posterior thalamus (A). The amplitude of current required to activate the neuron antidromically is indicated. The low threshold point (10 A) is circled and located in the contralateral nucleus triangularis of the posterior thalamus. The antidromic response had a stable latency (3 overlapping traces in a1), followed high-frequency (333 Hz) trains of pulses (a2), and collided with orthodromic action potentials (a3). The amplitudes of stimulus artifacts were reduced for clarity of presentation. The axon was activated at a caudal level within the anterior pretectal nucleus at a shorter latency, 2.4 ms (B and b1), but not at levels rostral to the posterior thalamus (C), indicating the axon terminated within the posterior thalamus. The neuron was recorded in C 7 (D) and had a relatively small cutaneous receptive field on the forepaw (E). The neuron responded only to noxious intensities of mechanical stimulation and was classified as a HT neuron. The neuron also responded to high-threshold heat stimuli applied to its cutaneous receptive fields (F). Mean firing frequencies (the mean background frequency was subtracted from each response) during each stimulus are indicated in parentheses. The unit was also antidromically activated from a low-threshold point in the upper cervical white matter, and antidromic action potentials from this point collided with those elicited in the midbrain or posterior thalamus (not illustrated) (see Dado et al. 1994a), demonstrating that the same unit was activated from both locations in the brain. This neuron could not be
4 STT NEURONS PROJECTING TO POSTERIOR THALAMUS 2555 antidromically activated using high-amplitude (500 A) stimulus pulses delivered throughout the dorsal-ventral extent of stimulation tracks located medial, rostral, and lateral to the most rostral low-threshold point (C). This unit was recorded in the deep dorsal horn (D), had a small receptive field on the lateral toes (E), and was activated only by noxious mechanical and heat stimuli (F). Figure 2 illustrates an example of an STT neuron in the marginal zone that projected to the posterior triangular nucleus of thalamus. A single lowest-threshold point for antidromic activation was seen in the posterior thalamus. The low-threshold point was surrounded medially, laterally, or rostrally by antidromic stimulation tracks. Within VPL, the area immediately rostral to the low threshold point (A, 4 mm from FIG. 2. An example of a neuron in the C 7 segment with an axon that projected to the contralateral nucleus triangularis of the posterior thalamus. A: cross-sections showing the multiple levels through which the stimulating electrode passed in the contralateral diencephalon. The low-threshold point is circled and located in contralateral posterior triangular nucleus. B: schematic representation of dorsal view of the brain indicating locations of stimulating tracks. Note that low-threshold point A was surrounded medially, laterally, and rostrally by tracks that 500- A current pulses did not activate antidromically, indicating that the axon terminated in the contralateral posterior thalamus. C: recording site in marginal zone of C 8. D: receptive field on ipsilateral forelimb. E: responses to cutaneous stimuli.
5 2556 X. ZHANG AND G. J. GIESLER bregma), stimulus pulses with amplitudes between 100 and 500 A activated the neuron at the same antidromic latency as that seen at the low-threshold point, indicating that activation from the rostral points resulted from spread of the high-amplitude pulses caudally to the low-threshold point. The unit could not be activated with high-amplitude pulses throughout any of the remaining stimulus tracks (A and B). The unit was recorded in the marginal zone (C), had a small receptive field on the forelimb (D), and was classified as a WDR neuron (E). Figure 3 depicts an STT neuron that was recorded in the marginal zone and projected to the posterior thalamus. The stimulating electrode was initially placed in the posterior thalamus level, 5 mm posterior to bregma (A). A single lowestthreshold point was found in a track that was 3.2 mm lateral to FIG. 3. An example of a neuron in C 8 with an axon that projects to the contralateral posterior thalamus. A: cross-sections showing the multiple levels through which the stimulating electrode passed in the contralateral diencephalon. The amplitude of current required to activate the neuron antidromically is indicated. The low-threshold point was in the contralateral posterior thalamus and was surrounded medially, laterally, and rostrally by ineffective stimulation tracks, indicating that the axon ended in the posterior thalamus. a1: 3 overlapping traces of antidromic responses of recorded neuron. The amplitudes of stimulus artifacts were reduced for clarity of presentation. B: representation of a dorsal view of the diencephalon. Locations of antidromic thresholds within stimulating tracks are indicated. The minimum antidromic threshold in each penetration is represented by a symbol (inset). C: responses of this neuron to cutaneous stimuli. D: recording site in marginal zone of C 8. E: receptive field on ipsilateral leg.
6 STT NEURONS PROJECTING TO POSTERIOR THALAMUS 2557 the midline. The antidromic threshold was 12 A and the latency was 6.5 ms (a1). The low-threshold point was located in the medial geniculate nucleus of the posterior thalamus (A). The low-threshold point was surrounded with stimulation tracks in which the unit could not be antidromically activated by high-amplitude current pulses (B), indicating that the axon terminated in the region surrounding the lowest-threshold point (A). The neuron was recorded in the marginal zone of C 8 (D). Its cutaneous receptive field was small and located on the ipsilateral forelimb (E). The unit responded with low-frequency discharges only to noxious stimulation of its cutaneous receptive field (C). Antidromic activation within the posterior thalamus Each of the 47 identified neurons were antidromically activated from low-threshold points ( 30 A) in the contralateral posterior thalamus that were surrounded medially, laterally, and rostrally by tracks in which current pulses of 500 A did not activate the neuron antidromically. Seventeen low-threshold points were located within the PoT, 13 were located in the medial geniculate nucleus (MG), 7 in Po, 6 in the PIL, 5 in SG, 2 in the optic tract (OT), and 1 in the lateral geniculate nucleus (Table 1, Fig. 4). In four cases, spinal neurons were antidromically activated at two distinct latencies from two low-threshold points within the posterior thalamus, suggesting that some spinal neurons send branches into more than one area of the posterior thalamus. In one case, low-threshold points were encountered in SG and Po, in another they were located in MG and PIL, in a third in PoT and MG, and in the fourth in PoT and OT. The mean current for antidromic activation from 51 low-threshold points in the contralateral posterior thalamus was (SE) A. Photomicrographs of lesions at the low threshold points within the PoT, MGm, PIL, SG, and Po are illustrated in Fig. 5C G. Recording sites The locations of lesions marking 43 of the recording sites of neurons are shown in Fig. 6. Four lesions were not recovered. Twenty-eight recording sites (65%) were located in the superficial dorsal horn (SDH). Fifteen recording sites were located in the deep dorsal horn (DDH). Within the DDH, 11 recording sites were located in the nucleus proprius and 4 in the lateral reticulated area. Twenty-eight neurons were recorded in C 7,19 FIG. 4. Summary of the locations of lesions in the posterior thalamus marking 51 low-threshold points for antidromic activation of 47 spinal neurons. The position of each symbol indicates the location of a lowest-threshold point. Lesions on the right side were located contralateral to the recording site. The distance from bregma of each plane is indicated to the left of each section. Note that many STT axons projected to the posterior triangular nucleus (PoT). in C 8. Photomicrographs of lesions at the recording site of a neuron in the SDH and another in the DDH are shown in Fig. 5, A and B. TABLE 1. Recording Location Areas of termination in posterior thalamus response categories and recording locations of recorded neurons Response Category PoT MG Po PIL SG OT LGN Totals SDH WDR HT NC Total DDH WDR HT NC Total Totals Recording locations are based on locations of lesions in all but four cases. In these, three were recorded at depths 100 m and are included as superficial dorsal horn (SDH) neurons; one was recorded at a depth of 400 m and is included in deep dorsal horn (DDH). PoT, nucleus triangular of the posterior thalamus; MG, medial geniculate nucleus; Po, posterior thalamus nucleus; PIL, posterior intralaminar thalamic nucleus; SG, suprageniculate thalamic nucleus; LGN, lateral geniculate nucleus; WDR, wide dynamic range; HT, high threshold; NC, not classified.
7 2558 X. ZHANG AND G. J. GIESLER fields that were restricted to the ipsilateral forepaw, forelimb, or shoulder. (Fig. 7). The receptive fields of seven (18%) neurons could not be located despite repeated applications of innocuous and noxious mechanical, thermal and electrical stimuli over much of the limbs and trunk. With the exception of neurons in the lateral spinal nucleus (unpublished observations), we have encountered very few projection neurons that were unresponsive to all forms of stimulation in previous studies in rats (Burstein et al. 1991; Dado et al. 1994b; Kostarczyk et al. 1997; Zhang et al. 1995). FIG. 5. Photomicrographs of lesions. A: lesion marking recording site in the superficial dorsal horn of the spinal cord cervical enlargement. B: lesion at recording site in deep dorsal horn. C G: lesions marking low-threshold points for antidromic activation in the contralateral nucleus posterior triangular nucleus (C), medial geniculate nucleus (D), posterior intralaminar thalamic nucleus (E), suprageniculate nucleus (F), and posterior nucleus (G). Scale bar for A and B equals 0.5 mm and is located in B. Scale bar for C G is in G and equals 1.0 mm. Cutaneous receptive fields Attempts were made to locate the receptive fields of 40 of the examined neurons. Thirty-three had cutaneous receptive Classification by responses to cutaneous mechanical stimuli Twenty-five units (76%) responded only to noxious stimuli and were classified as HT neurons (Table 1, Figs. 1 and 3). Sixteen HT neurons were recorded in the SDH and nine in the DDH. Eight (24%) of 33 tested units responded to innocuous mechanical stimulation but responded at higher frequencies to increasingly intense noxious stimulation. These units were classified as WDR neurons (Fig. 2). Five WDR neurons were recorded in the SDH, three in the DDH. The mean responses of all examined neurons to the pinch and squeeze stimuli were and spikes/s. The mean responses of SDH neurons to these stimuli were and spikes/s; the mean responses of DDH neurons were and spikes/s. Responses to cutaneous thermal stimuli Thirteen neurons were tested for their responses to an ascending series of heat stimuli. Nine examined neurons (69%) responded to heat stimuli applied to their cutaneous receptive fields. The individual and overall mean responses ( SE) are shown in Fig. 8. Eight heat-response neurons were classified HT and one as WDR. Four neurons did not respond to heat stimuli; three were classified as HT and one as WDR. These 13 neurons were also tested with a series of innocuous cooling and noxious cold stimuli, and only 1 neuron responded to noxious cold stimuli (10 and 1 C). This neuron projected to Po. Among nine cells that responded to heat stimuli, four projected to PoT, FIG. 6. Locations of lesions marking recording sites of 43 neurons in the cervical enlargement (C 7 8 ). The position of each symbol indicates the location of a recording site. Each drawing of the spinal cord illustrates the location and functional classification of spinal neurons that terminated within various regions of the posterior thalamus. The name of the area and number of STT neurons found to project to that region are indicated near the bottom of each drawing. Note that lesions were located in 2 groups, 1 in the marginal zone, the other in the deep dorsal horn.
8 STT NEURONS PROJECTING TO POSTERIOR THALAMUS 2559 In these experiments, microantidromic stimulation methods were used to identify STT neurons that project to the posterior thalamus and to determine the nuclei to which they project. We also determined the responses of these neurons to innocuous and noxious mechanical and thermal cutaneous stimuli. Each unit examined in this study was antidromically activated from the posterior thalamus using stimulus pulses of 30 (mean 21 A). Current pulses 30 A have been shown to activate spinal axons in the brain at a distance of 400 m from the stimulating electrode (Burstein et al. 1991; Dado et al. 1994a). We surrounded lowest-threshold points medially and laterally with stimulating tracks within which the axon could not be activated antidromically with high-amplitude, 500- A stimulus pulses. Current pulses of this amplitude have been shown to activate axons of spinal cord neurons within a radius of 600 1, 600 m (Burstein et al. 1991; Dado et al. 1994a). Penetrations with stimulating electrodes in our experiments were separated by only m, indicating that it is unlikely that examined axons passed through these areas without detection. In addition, at least two or three rows of stimulating tracks were made anterior to all low-threshold points in posterior thalamus using identical high-amplitude pulses; STT axons projecting to the posterior thalamus could not be activated from any point within these penetrations. Low-threshold points were also surrounded dorsally and ventrally with many points from which high-amplitude pulses failed to activate the neuron. Therefore we are confident that the examined axons terminated FIG. 7. Cutaneous receptive fields of neurons that project to the posterior thalamus. two to MG, and two to Po. One neuron projected to both PoT and MG. The mean stimulus-response function for neurons that responded to heat stimuli is plotted on the double-natural logarithmic scale in Fig. 9. The function was fit with a line that had a slope of 2.3 (r , P 0.02). The approximate mean response threshold (48.1 C) to heat stimuli was extrapolated from the stimulus-response function. Conduction velocities The mean antidromic latency from the low-threshold points in the contralateral posterior thalamus to the recording sites in the cervical enlargement for 47 examined neurons was (SE) ms (range, ms). Assuming that the axons crossed in the spinal cord and ascended in a straight path to the thalamus, the estimated mean conduction velocity was m/s (range, m/s; Fig. 10). The mean conduction velocity of neurons located in the SDH ( m/s) was significantly slower than that of cells in the DDH ( m/s; P 0.001, t-test). Two STT neurons had conduction velocities within the range of unmyelinated fibers; these conducted at 0.9 and 1.0 m/s. DISCUSSION FIG. 8. Responses of posterior thalamus projecting neurons to a series of innocuous and noxious heat stimuli. A: responses of individual neurons that projected to the posterior thalamus. B: mean SE stimulus-response functions.
9 2560 X. ZHANG AND G. J. GIESLER FIG. 9. Stimulus-response function for posterior thalamus projecting neurons that responded to a series of innocuous and noxious heat stimuli plotted on double-natural logarithmic scales. Regression analysis of the stimulus response function yielded a curve with a slope of 2.3 (r ). Response threshold was defined as a mean firing frequency 1.5 spikes/s above background. 2, the calculated response threshold for heat stimuli. within the posterior thalamus and did not simply course through the area in route to other targets. The method of antidromic activation has been used to identify and characterize axons within a number of ascending and descending projection systems including the spinohypothalamic, spinomesencephalic, spinothalamic, reticulospinal, rubrospinal, vestibulospinal, and corticospinal tracts (Applebaum et al. 1979; Burstein et al. 1991; Dado et al. 1994; Katter et al. 1996; McMahon and Wall 1985; Shinoda et al. 1976, 1977; Zhang et al. 1995). Axons of several of these systems have been examined subsequently using intra-axonal dye injections (Dick et al. 1988; Shinoda et al. 1982a,b; Yen et al. 1991). These studies have shown that microantidromic activation methods can provide an accurate, but less detailed, description of the path and termination of axons within the CNS. Applebaum et al. (1979) also used microantidromic activation methods to examine the projections of functionally identified primate STT axons. The bulk of the examined axons were found to terminate in VPL. However, a single HT axon from a neuron recorded in the marginal zone was followed to its termination medial to the MG. The authors describe the termination point as near the posterior thalamus, although based on their Fig. 6A, it is possible that it was located within the mesencephalic reticular formation. In our earlier study in rats, we (Dado et al. 1994a) antidromically activated four axons from low-threshold points within and adjacent to the PoT. None of these could be activated from rostral levels within the diencephalons, indicating that they terminated within the PoT. In the present study, more STT axons were found to terminate within PoT (36%) than within any other area of the posterior thalamus. A recent anatomical anterograde tracing study (Gauriau and Bernard 2004a) also indicated that a large percentage of STT fibers projected to the PoT in rats. Our findings suggest that the overwhelming majority of axons that form this large projection to PoT carry information regarding nociceptive mechanical and heat stimuli. STT neurons that project to PoT were located within both the marginal zone and DDH. Gauriau and Bernard (2004a) found that injections of PHA-L that involved the marginal zone produced rich labeling of fibers and terminals within PoT, and injections that were restricted to the DDH produced only sparse labeling within PoT. In the present study, neurons that were found to project to PoT were found almost equally within the marginal zone and deep dorsal horn, suggesting that the contribution from the DDH might be larger than originally believed. In addition to PoT, the SG, MGm, Po, and PIL were each found to receive direct nociceptive STT projections. These findings confirm previous anatomical results (Boivie 1979; Cliffer et al. 1991; Dado and Giesler 1990; Gauriau and Bernard 2004a; Graziano and Jones 2004; Jones and Burton FIG. 10. Histogram of conduction velocities of examined axons between recording sites and low-threshold points in the contralateral posterior thalamus. The mean conduction velocity of axons of SDH (1) neurons was significantly slower than that of DDH neurons ( ).
10 STT NEURONS PROJECTING TO POSTERIOR THALAMUS ; Jones and Powell 1971; Kerr 1975; LeDoux et al. 1987; Lund and Webster 1967; Mehler 1969; Ralston and Ralston 1992; Rockel et al. 1972) and demonstrate that all neurons that provide input to these regions in rats appear to be nociceptive. Electrophysiological studies have shown that many neurons in the MG, Po, SG, and PIL of the posterior thalamus respond to noxious inputs (Benedek et al. 1997; Brinkhus et al. 1979; Carstens and Yokota 1980; Guilbaud et al. 1977, 1980; Matsumoto et al. 1988; Peschanski et al. 1981). Our results indicate that STT axons contribute prominently to the responses of neurons in this region. Our findings indicate that a far larger percentage of the STT projection to the posterior thalamus originates from neurons in the marginal zone than is the case for projections to other regions of the diencephalon in rats. Nearly two-thirds of the neurons (65%) that project to the posterior thalamus were located within the superficial dorsal horn (SDH). In contrast, only 16 32% of rat spinohypothalamic tract neurons were located in the SDH (Dado et al. 1994b; Kostarczyk et al. 1997; Zhang et al. 1995). Giesler et al. (1979) reported that injections of HRP into the medial thalamus of rats failed to label any neurons in the SDH. Our results also suggest that neurons that project to the posterior thalamus have smaller-diameter, more slowly conducting axons than do other spinal projection neurons in rats. The mean conduction velocity of posterior thalamus projecting axons was found to be 6.4 m/s. The majority of axons in this study had conduction velocities between 2 and 8 m/s, suggesting that they had thinly myelinated axons. This conduction velocity is only about one-third of that for spinohypothalamic tract neurons also recorded in the cervical enlargements of rats (Dado et al. 1994b; Kostarczyk et al. 1997; Zhang et al. 1995). Giesler et al. (1976) recorded from STT neurons in the lumbar enlargement of rats. These neurons, backfired from the area of the medial lemniscus and VPL, had conduction velocities between 14 and 26 m/s. Two axons in this study had conduction velocities of 1 m/s. Craig and colleagues (Andrew and Craig 2001a,b; Craig and Dostrovsky 1991, 2001) have shown that many STT neurons in the marginal zone of cats have unmyelinated axons. To our knowledge, this is the first report of any type of spinal cord projection neurons in rats with unmyelinated axons. None have been demonstrated in monkeys. The receptive fields of neurons projecting to the posterior thalamus had small or medium sized receptive fields that were located in the ipsilateral forepaw, forelimb, or shoulder. In contrast, Dado et al. (1994b) reported that almost half of spinohypothalamic tract neurons recorded in the cervical enlargement of rats have large or complex receptive fields. Based on the sizes of the receptive fields of neurons in this study, it appears that STT neurons that project to the posterior thalamus are capable of providing information on the location of nociceptive stimuli. Because nociceptive neurons in the posterior thalamus generally have large complex, discontinuous bilateral receptive fields (Guilbaud et al. 1980; Poggio and Mountcastle 1960), it is likely that a considerable convergence of information occurs on posterior thalamic neurons. An additional distinctive feature of many STT neurons that project to the posterior thalamus is their low-frequency discharges to noxious stimuli. We found that the mean responses for all examined neurons (recorded in SDH and DDH) to pinch and squeeze 10 spikes/s. We re-examined responses to these same stimuli from previous studies of spinohypothalamic tract neurons in our lab (Dado et al. 1994; Kostarczyk et al. 1997; Zhang et al. 1995). The mean responses of spinohypothalamic tract neurons to pinch ( spikes/s) and squeeze ( ) were roughly four times greater than those of STT neurons that project to the posterior thalamus and differed significantly (P 0.001, t-test). We and others have also seen similar higher-frequency responses to comparable stimuli by primate STT neurons projecting to VPL (Dougherty et al. 1992; Owens et al. 1992; Palecek et al. 1994a,b; Zhang et al. 2000). The low-frequency responses to noxious stimuli by STT neurons that project to the posterior thalamus are reminiscent of those seen in nociceptors (Willis and Coggeshall 2004). There are several possible reasons why STT neurons that project to posterior thalamus appear to respond to noxious cutaneous stimuli with lower-frequency discharges. It is conceivable that such neurons are more susceptible to suppressive influences of the urethan anesthesia than are spinohypothalamic tract neurons. It is also possible that the inherent cellular properties (e.g., density of potassium channels, etc.) of posterior thalamic projecting cells limit the frequency of their responses. Each of these possibilities could be examined in future studies. Another possibility is that posterior thalamusprojecting neurons receive and convey input from a comparatively small number of nociceptors. The fact that these neurons have smaller receptive fields supports this idea. Almost 70% of STT neurons that project to the posterior thalamus responded to noxious heat stimuli. These thermally responsive cells terminated in the PoT, MG, and Po. The examined neurons had accelerating responses to increasingly intense noxious heat. They are capable of producing clear increases in their firing rates in response to small changes in the intensity of noxious heat stimulus. The mean response was described by a power function with a slope of 2.3. Human psychophysical studies indicate that the stimulus-response functions that describe the human sensory experience of noxious heat have slopes of (Price 1988). The calculated threshold for examined neurons that responded to heating was 48.1 C, a temperature that is considerably higher than the threshold for pain in humans and other animals (44 45 C) (Hardy et al. 1950). It appears that these neurons receive a specific input from high-threshold thermal afferent receptors (e.g., A mechanoheat nociceptors) (Willis and Coggesshall 2004) and mainly transmit information related to intense heat stimuli to posterior thalamic targets. Nociceptive and fear-producing stimuli alter blood pressure and heart rate and produce freezing behavior in rats (Iwata et al. 1986). The repeated pairing of a neutral auditory or visual stimulus [conditioned stimulus (CS)] with noxious foot shock [unconditioned stimulus (US)] causes presentation of the CS alone to produce autonomic responses (Jarrel et al. 1986; LeDoux et al. 1986a, 1986b, 1990a). This form of learning is called fear or emotional conditioning. Considerable evidence indicates that the posterior thalamus and the amygdala play important roles in the production of fear conditioning (Iwata et al. 1986; Jarrell et al. 1986; LeDoux et al. 1990a). Lesions of the central and lateral nuclei of the amygdala (LeDoux et al. 1986; 1990a; Shi and Davis 1999) prevent rats from successfully acquiring fear conditioning (LeDoux 1990b). Neurons in these nuclei that have been implicated in fear conditioning
11 2562 X. ZHANG AND G. J. GIESLER have been shown to respond to nociceptive as well as auditory and visual stimuli (Romanski et al. 1993). Previous findings indicate that neurons within the central and lateral nuclei of the amygdala receive a direct nociceptive input from posterior thalamic nuclei including the PoT, SG, PIL, and Po (Bordi and LeDoux 1994; Burton and Jones 1976; LeDoux et al. 1985; 1990b; Ottersen and Ben-Ari 1979). These nuclei of the posterior thalamus also send large projections to a number of cortical areas including perirhinal, primary auditory, visceral, ventral temporal association, second and third somatic sensory, and insular and frontal cortices (Burton and Jones 1976; Friedman and Murray 1986; Gauriau and Bernard 2004b; Graybiel 1972; Heath and Jones 1971: Jones and Leavitt 1973; Kurokawa et al. 1990; Ledoux et al. 1985; Linke 1999; Linke and Schwegler 2000). Many of these cortical regions in turn project to the amygdala, suggesting that, in addition to direct inputs, the amygdala receives input from the posterior thalamus via thalamo-cortico-amygdaloid projections (Linke and Schwegler 2000). Lesions in posterior thalamus can also block the acquisition of fear conditioning (LeDoux et al. 1986a,b; Shi and Davis 1999). Additional evidence for an important role of posterior thalamic nuclei in fear conditioning was reported by Cruikshank et al. (1992), who showed that conditioned fear responses can be produced using low-amplitude stimulation within the PIL nucleus as a US instead of foot shock, indicating that activation within the posterior thalamus produces sufficiently aversive emotional responses that can be used as the US in fear-conditioning paradigms. Our findings indicate that the STT sends a large nociceptive projection to several nuclei within the posterior thalamus. It is reasonable to conclude therefore that at least a portion of the nociceptive information that is necessary for fear conditioning is provided by this STT projection. In addition, the STT projection to the posterior thalamus is capable of providing nociceptive input to a sizeable number of cortical regions via the widespread projections of neurons within the posterior thalamus. ACKNOWLEDGMENTS We thank H. Truong for valuable technical assistance, Drs. C. N. Honda and D. A. Simone for critically reading an early version of the manuscript, and S. Davidson for valuable assistance analyzing the data and critically reading the manuscript. GRANTS This work was supported by National Institute of Neurological Disorders and Stroke Grants NS and NS REFERENCES Andrew D and Craig AD. Spinothalamic lamina I neurones selectively responsive to cutaneous warming in cats. J Physiol 537: , 2001a. Andrew D and Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci 4: 72 77, 2001b. Apkarian AV and Hodge CJ. Primate spinothalamic pathways. III. Thalamic termination of the dorsolateral and ventral spinothalamic pathways. J Comp Neurol 288: , Applebaum AE, Leonard RB, Kenshalo DR Jr, Martin RF, and Willis WD. Nuclei in which functionally identified spinothalamic tract neurons terminate. J Comp Neurol 188: , Benedek G, Pereny J, Kovacs G, Fischer-Szatmari L, and Katoh YY. Visual, somatosensory, auditory and nociceptive modality properties in the feline suprageniculate nucleus. Neuroscience 78: , Boivie J. An anatomical reinvestigation of the termination of the spinothalamic tract in the monkey. J Comp Neurol 186: , Bordi F and LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. II. Cells receiving convergent auditory and somatosensory inputs and cells antidromically activated by amygdala stimulation. Exp Brain Res 98: , Brinkhus HB, Carstens E, and Zimmermann M. Encoding of graded noxious skin heating by neurons in posterior thalamus and adjacent areas in the cat. Neurosci Lett 15: 37 42, Bruggemann J, Vahle-Hinz C, and Kniffki KD. Projections from the pelvic nerve to the periphery of the cat s thalamic ventral posterior lateral nucleus and adjacent regions of the posterior complex. J Neurophysiol 72: , Burstein R, Dado RJ, Cliffer KD, and GJ Giesler Jr. Physiological characterization of spinohypothalamic tract neurons in the lumbar enlargement of rats. J Neurophysiol 66: , Burton H and Jones EG. The posterior thalamic region and its cortical projection in new world and old world monkeys. J Comp Neurol 168: , Calma N. The activity of the posterior group of thalamic nuclei in the cat. J Physiol 180: , Carstens E and Yokota T. Viscerosomatic convergence and responses to intestinal distension of neurons at the junction of midbrain and posterior thalamus in the cat. Exp Neurol 70: , Casey KL. Unit analysis of nociceptive mechanisms in the thalamus of the awake squirrel monkey. J Neurophysiol 29: , Cliffer KD, Burstein R, and Giesler GJ Jr. Distributions of spinothalamic, spinohypothalamic and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci 11: , Craig AD. Distribution of trigeminothalamic and spinothalamic lamina I terminations in the Macaque monkey. J Comp Neurol 477: , Craig AD, Bushnell MC, Zhang ET, and Blomqvist A. A thalamic nucleus specific for pain and temperature sensation. Nature 372: , Craig AD and Dostrovsky JO. Thermoreceptive lamina I trigeminothalamic neurons project to the nucleus submedius in the cat. Exp Brain Res 85: , Craig AD and Dostrovsky JO. Differential projections of thermoreceptive and nociceptive lamina I trigeminothalamic and spinothalamic neurons in the cat. J Neurophysiol 86: , Cruikshank SJ, Edeline J-M, and Weinberger NM. Stimulation at a site of auditory-somatosensory convergence in the medial geniculate nucleus is an effective unconditioned stimulus for fear conditioning. Behav Neurosci 106: , Curry MJ. The exteroceptive properties of neurons in the somatic part of the posterior group (PO). Brain Res 44: , Curry MJ and Gordon G. The spinal input to the posterior group in the cat: an electrophysiological investigation. Brain Res 44: , Dado RJ and Giesler GJ Jr. Afferent input to nucleus submedius in rats: retrograde labeling of neurons in the spinal cord and caudal medulla. J Neurosci 10: , Dado RJ, Katter JT, and Giesler GJ Jr. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. I. Locations of antidromically identified axons in the thalamus and hypothalamus. J Neurophysiol 71: , 1994a. Dado RJ, Katter JT, and Giesler GJ Jr. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. II. Responses to innocuous and noxious mechanical and thermal stimuli. J Neurophysiol 71: , 1994b. Dick TE, Jodkowski JS, Viana F, and Berger AJ. Projections and terminations of single respiratory axons in the cervical spinal cord of cat. Brain Res 449: , Dostrovsky JO and Craig AD. Cooling-specific spinothalamic neurons in the monkey. J Neurophysiol 76: , Dougherty PM, Palecek J, Paleckova V, Sorkin LS, and Willis WD. The role of NMDA and non-nmda excitatory amino acid receptors in the excitation of primate spinothalamic tract neurons by mechanical, chemical, thermal, and electrical stimuli. J Neurosci 12: , Friedman DP and Murray EA. Thalamic connectivity of the second somatosensory area and neighboring somatosensory fields of the lateral sulcus of the macaque. J Comp Neurol 252: , Gauriau C and Bernard J-F. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain J Comp Neurol 468: 24 56, 2004a. Gauriau C and Bernard J-F. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci 24: , 2004b.
12 STT NEURONS PROJECTING TO POSTERIOR THALAMUS 2563 Giesler GJ Jr, Menetrey D, Basbaum AI. Differential origins of spinothalamic tract projections to medial and lateral thalamus in the rat. J Comp Neurol 184: , Giesler GJ Jr, Menetrey D, Guilbaud G, and Besson J-M. Lumbar cord neurons at the origin of the spinothalamic tract in the rat. Brain Res 118: , Graziano A and Jones EG. Widespread thalamic terminations of fibers arising in the superficial meduallary dorsal horn of monkeys and their relation to calbindin immunoreactivity. J Neurosci 24: , Graybiel AM. Some fiber pathways related to the posterior thalamic region in the cat. Brain Behav Evol 6: , Guilbaud G, Caille D, Besson JM, Benelli G. Single unit activities in ventral posterior and posterior group thalamic nuclei during nociceptive and nonnociceptive stimulations in the cat. Arch Ital Biol 115: 35 56, Guilbaud G, Peschanski M, Gautron M, Binder D. Neurons responding to noxious stimulation in VB complex and caudal adjacent regions in the thalamus of the rat. Pain 8: , Hardy JD, Goodell H, and Wolff HG. The influence of skin temperature upon the pain threshold as evoked by thermal radiation. Science 114: , Heath CJ and Jones EG. Anexperimental study of ascending connections from the posterior group of thalamic nuclei in the cat. J Comp Neurol 141: , Hirata H, Takeshita S, Hu JW, and Bereiter DA. Cornea-responsive medullary dorsal horn neurons: modulation by local opioids and projections to thalamus and brain stem. J Neurophysiol 84: , Horn AC, Vahle-Hinz C, Bruggemann J, Petersen M, and Kniffki KD. Responses of neurons in the lateral thalamus of the cat to stimulation of urinary bladder, colon, esophagus, and skin. Brain Res 851: , Iwata J, LeDoux JE, Meeley MP, Arneric S, and Reis DJ. Intrinsic neurons in the amygdaloid field projected to by the medial geniculate body mediate emotional responses conditioned to acoustic stimuli. Brain Res 383: , Jarrell TW, Gentile CG, McCabe PM, and Schneiderman N. The role of the medial geniculate region in differential Pavlovian conditioning of bradycardia in rabbits. Brain Res 374: , Jones EG. A pain in the thalamus. J Pain 3: , Jones EG and Burton H. Cytoarchitecture and somatic sensory connectivity of thalamic nuclei other than the ventrobasal complex in the cat. J Comp Neurol 154: , Jones EG and Leavitt RY. Demonstration of thalamo-cortical connectivity in the cat somato-sensory system by retrograde axonal transport of horseradish peroxidase. Brain Res 63: , Jones EG and Powell TP. An analysis of the posterior group of thalamic nuclei on the basis of its afferent connections. J Comp Neurol 143: , Katter JT, Dado RJ, Kostarczyk E, and Giesler GJ Jr. Spinothalamic and spinohypothalamic tract neurons in the sacral spinal cord of rats. I. Locations of antidromically identified axons in the cervical cord and diencephalon. J Neurophysiol 75: , Kerr FWL. The ventral spinothalamic tract and other ascending systems of the ventral funiculus of the spinal cord. J Comp Neurol 159: , Kostarczyk E, Zhang X, and Giesler GJ Jr. Spinohypothalamic tract neurons in the cervical enlargement of rats: locations of antidromically identified ascending axons and their collateral branches in the contralateral brain. J Neurophysiol 77: , Kurokawa T, Yoshida K, Yamamoto T, and Oka H. Frontal cortical projections from the suprageniculate nucleus in the rat, as demonstrated with the PHA-L method. Neurosci Lett 120: , LeDoux JE, Cicchette P, Xagoraris A, and Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdale in fear conditioning. J Neurosci 10: , 1990a. LeDoux JE, Farb C, and Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci 10: , 1990b. LeDoux JE, Iwata J, Pearl D, and Reis DJ. Disruption of auditory but not visual learning by destruction of intrinsic neurons in the rat medial geniculate. Brain Res 371: , 1986a. LeDoux JE, Ruggiero DA, Forest R, Stornetta R, and Reis DJ. Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol 264: , LeDoux JE, Ruggiero DA, and Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol 242: , LeDoux JE, Sakaguchi A, Iwata J, and Reis DJ. Interruption of projections from the medial geniculate body to an archi-neostriatal field disrupts the classical conditioning of emotional responses to acoustic stimuli. Neurosci 17: , 1986b. Lenz F, Gracely RH, Rowland LH, and Dougherty PW. A population of cells in the human principal sensory nucleus respond to painful mechanical stimuli. Neurosci Lett 180: 46 50, Lenz F, Seike M, Lin YC, Baker FH, Richarson RT, and Gracely RH. Thermal and pain sensation evoked by microstimulation in the area of the human ventrocaudal nucleus (Vc). J Neurophysiol 70: , 1993a. Lenz F, Seike M, Lin TC, Baker FH, Rowland LH, Gracely RH, and Richardson RT. Neurons in the area of human thalamic nucleus ventralis caudalis respond to painful heat stimuli. Brain Res 623: , 1993b. Linke R. Organization of projections to temporal cortex originating in the thalamic posterior intralaminar nucleus of the rat. Exp Brain Res 127: , Linke R and Schwegler H. Convergent and complementary projections of the caudal paralaminar thalamic nuclei to rat temporal and insular cortex. Cereb Cort 10: , Lipski J. Antidromic activation of neurons as an analytic tool in the study of the central nervous system. J Neurosci Methods 4: 1 32, Lund RD and Webster KE. Thalamic afferents from the spinal cord and trigeminal nuclei. An experimental anatomical study in the rat. J Comp Neurol 130: , Matsumoto N, Sato T, Sawano H, Tochinai A, and Suzuki TA. Characteristics of tooth pulp-driven neurons in the posterior group of the cat thalamus. Neurosci Lett 93: , McMahon SB and Wall PD. The distribution and central termination of single cutaneous and muscle unmyelinated fibres in rat spinal cord. Brain Res 359: 39 48, Mehler WR. Some neurological species differences a posteriori. Ann NY Acad Sci 167: , Mehler WR, Feferman ME, and Nauta WJH. Ascending axon degeneration following anterolateral cordotomy. An experimental study in the monkey. Brain 83: , Nyquist JK and Greenhoot JH. U nit analysis of nonspecific thalamic responses to high-intensity cutaneous input in the cat. Exp Neurol 42: , Ottersen OP and Ben-Ari Y. Afferent connections to the amygdaloid complex of the rat and cat: I. Projections from the thalamus. J Comp Neurol 187: , Owens CM, Zhang D, and Willis WD. Changes in the response states of primate spinothalamic tract cells caused by mechanical damage of the skin or activation of descending controls. J Neurophysiol 67: , Palecek J, Paleckova V, Dougherty PM, and Willis WD. The effect of phorbol esters on the responses of primate spinothalamic neurons to mechanical and thermal stimuli. J Neurophysiol 71: , 1994a. Palecek J, Paleckova V, Dougherty PM, and Willis WD. The effect of trans-acpd, a metabotropic excitatory amino acid receptor agonist, on the responses of primate spinothalamic tract neurons. Pain 56: , 1994b. Paxinos G and Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, Perl ER and Whitlock DG. Somatic stimuli exciting spinothalamic projection to thalamic neurons in cat and monkey. Exp Neurol 3: , Peschanski M, Guilbaud G, and Gautron M. Posterior intralaminar region in rat: neuronal responses to noxious and nonnoxious cutaneous stimuli. Exp Neurol 72: , Poggio GF and Mountcastle VB. A study of the functional contributions of the lemniscal and spinothalamic systems to somatic sensibility. Johns Hopkins Hosp Bull 106: , Price DD. Psychological and Neural Mechanisms of Pain. New York, NY: Raven Press, Ralston HJ III and Ralston DD. The primate dorsal spinothalamic tract: evidence for a specific termination in the posterior nuclei (Po/SG) of the thalamus. Pain 48 : , Ranck JB. Which elements are excited by electrical stimulation of mammalian central nervous system: a review. Brain Res 98: , 1975.
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Anatomical Substrates of Somatic Sensation
 Anatomical Substrates of Somatic Sensation John H. Martin, Ph.D. Center for Neurobiology & Behavior Columbia University CPS The 2 principal somatic sensory systems: 1) Dorsal column-medial lemniscal system
Anatomical Substrates of Somatic Sensation John H. Martin, Ph.D. Center for Neurobiology & Behavior Columbia University CPS The 2 principal somatic sensory systems: 1) Dorsal column-medial lemniscal system
Sensory coding and somatosensory system
 Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D.
 SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
Thalamo-Cortical Pain Mechanisms Prof. Fred A. Lenz
 Thalamo-Cortical Pain Mechanisms Hopkins Hospital, Baltimore, USA F.H. Baker, D.S. Borett, T. Chase, D. Chau, H. Chen, R. Coghill, N.E. Crone, R.G Cutler, M.R. DeLong, P.M. Dougherty, J. Dostrovsky, I.M.
Thalamo-Cortical Pain Mechanisms Hopkins Hospital, Baltimore, USA F.H. Baker, D.S. Borett, T. Chase, D. Chau, H. Chen, R. Coghill, N.E. Crone, R.G Cutler, M.R. DeLong, P.M. Dougherty, J. Dostrovsky, I.M.
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Pain and Temperature Objectives
 Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
Department of Neurology/Division of Anatomical Sciences
 Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE
 Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Auditory and Vestibular Systems
 Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Posterior White Column-Medial Lemniscal Pathway
 Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
The Pain System The Neural Basis of Nociceptive Transmission in the Mammalian Nervous System
 The Pain System The Neural Basis of Nociceptive Transmission in the Mammalian Nervous System Pain and Headache Vol. 8 Series Editor Philip L. Gildenberg, Houston, Tex. KARGER S.Karger Basel Miinchen: Paris
The Pain System The Neural Basis of Nociceptive Transmission in the Mammalian Nervous System Pain and Headache Vol. 8 Series Editor Philip L. Gildenberg, Houston, Tex. KARGER S.Karger Basel Miinchen: Paris
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007)
 Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
Afferent Input to Nucleus Submedius in Rats: Retrograde Labeling of Neurons in the Spinal Cord and Caudal Medulla
 The Journal of Neuroscience, August 1990, 70(E): 2672-2666 Afferent Input to Nucleus Submedius in Rats: Retrograde Labeling of Neurons in the Spinal Cord and Caudal Medulla Robert J. Dado and Glenn J.
The Journal of Neuroscience, August 1990, 70(E): 2672-2666 Afferent Input to Nucleus Submedius in Rats: Retrograde Labeling of Neurons in the Spinal Cord and Caudal Medulla Robert J. Dado and Glenn J.
CHAPTER 10 THE SOMATOSENSORY SYSTEM
 CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
Pain classifications slow and fast
 Pain classifications slow and fast Fast Pain Slow Pain Sharp, pricking (Aδ) fiber Short latency Well localized Short duration Dull, burning (C) fiber Slower onset Diffuse Long duration Less emotional Emotional,
Pain classifications slow and fast Fast Pain Slow Pain Sharp, pricking (Aδ) fiber Short latency Well localized Short duration Dull, burning (C) fiber Slower onset Diffuse Long duration Less emotional Emotional,
The Nervous System: Sensory and Motor Tracts of the Spinal Cord
 15 The Nervous System: Sensory and Motor Tracts of the Spinal Cord PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction Millions of sensory
15 The Nervous System: Sensory and Motor Tracts of the Spinal Cord PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction Millions of sensory
Enhanced Responses of Spinal Dorsal Horn Neurons to Heat and Cold Stimuli Following Mild Freeze Injury to the Skin
 Enhanced Responses of Spinal Dorsal Horn Neurons to Heat and Cold Stimuli Following Mild Freeze Injury to the Skin SERGEY G. KHASABOV, 1 DAVID M. CAIN, 2 DINH THONG, 3 PATRICK W. MANTYH, 1 AND DONALD A.
Enhanced Responses of Spinal Dorsal Horn Neurons to Heat and Cold Stimuli Following Mild Freeze Injury to the Skin SERGEY G. KHASABOV, 1 DAVID M. CAIN, 2 DINH THONG, 3 PATRICK W. MANTYH, 1 AND DONALD A.
Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit. Supplementary Information. Adam W. Hantman and Thomas M.
 Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit Supplementary Information Adam W. Hantman and Thomas M. Jessell Supplementary Results Characterizing the origin of primary
Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit Supplementary Information Adam W. Hantman and Thomas M. Jessell Supplementary Results Characterizing the origin of primary
Rewiring of hindlimb corticospinal neurons after spinal cord injury
 Rewiring of hindlimb corticospinal neurons after spinal cord injury Arko Ghosh, Florent Haiss, Esther Sydekum, Regula Schneider, Miriam Gullo, Matthias T. Wyss, Thomas Mueggler, Christof Baltes, Markus
Rewiring of hindlimb corticospinal neurons after spinal cord injury Arko Ghosh, Florent Haiss, Esther Sydekum, Regula Schneider, Miriam Gullo, Matthias T. Wyss, Thomas Mueggler, Christof Baltes, Markus
How strong is it? What is it? Where is it? What must sensory systems encode? 9/8/2010. Spatial Coding: Receptive Fields and Tactile Discrimination
 Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination
 Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
Spatial Coding: Receptive Fields and Tactile Discrimination What must sensory systems encode? How strong is it? What is it? Where is it? When the brain wants to keep certain types of information distinct,
SOMATOSENSORY SYSTEMS
 SOMATOSENSORY SYSTEMS Schematic diagram illustrating the neural pathways that convey somatosensory information to the cortex and, subsequently, to the motor system. Double arrows show reciprocal connections.
SOMATOSENSORY SYSTEMS Schematic diagram illustrating the neural pathways that convey somatosensory information to the cortex and, subsequently, to the motor system. Double arrows show reciprocal connections.
 Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Physiological Society Symposium Nociceptors as Homeostatic Afferents: Central Processing
 Physiological Society Symposium Nociceptors as Homeostatic Afferents: Central Processing Distinct central representations of inescapable and escapable pain: observations and speculation Kevin A. Keay *
Physiological Society Symposium Nociceptors as Homeostatic Afferents: Central Processing Distinct central representations of inescapable and escapable pain: observations and speculation Kevin A. Keay *
Spinothalamocortical inputs nonpreferentially innervate the superficial and deep cortical layers of SI
 Neuroseienee Letters, 160 (1993) 209 213 209 1993 Elsevier Scientific Publishers Ireland Ltd. All fights reser'/ed 0304-3940/93/$ 06.00 - NSL 09823 Spinothalamocortical inputs nonpreferentially innervate
Neuroseienee Letters, 160 (1993) 209 213 209 1993 Elsevier Scientific Publishers Ireland Ltd. All fights reser'/ed 0304-3940/93/$ 06.00 - NSL 09823 Spinothalamocortical inputs nonpreferentially innervate
211MDS Pain theories
 211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
ANAT2010. Concepts of Neuroanatomy (II) S2 2018
 ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
SENSORY (ASCENDING) SPINAL TRACTS
 SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
Thalamus and Sensory Functions of Cerebral Cortex
 Thalamus and Sensory Functions of Cerebral Cortex I: To describe the functional divisions of thalamus. II: To state the functions of thalamus and the thalamic syndrome. III: To define the somatic sensory
Thalamus and Sensory Functions of Cerebral Cortex I: To describe the functional divisions of thalamus. II: To state the functions of thalamus and the thalamic syndrome. III: To define the somatic sensory
Internal Organisation of the Brainstem
 Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
Receptors and Neurotransmitters: It Sounds Greek to Me. Agenda. What We Know About Pain 9/7/2012
 Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Chapter 16. Sense of Pain
 Chapter 16 Sense of Pain Pain Discomfort caused by tissue injury or noxious stimulation, and typically leading to evasive action important /// helps to protect us lost of pain in diabetes mellitus = diabetic
Chapter 16 Sense of Pain Pain Discomfort caused by tissue injury or noxious stimulation, and typically leading to evasive action important /// helps to protect us lost of pain in diabetes mellitus = diabetic
Brainstem. Steven McLoon Department of Neuroscience University of Minnesota
 Brainstem Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Change in Lab Sequence Week of Oct 2 Lab 5 Week of Oct 9 Lab 4 2 Goal Today Know the regions of the brainstem. Know
Brainstem Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Change in Lab Sequence Week of Oct 2 Lab 5 Week of Oct 9 Lab 4 2 Goal Today Know the regions of the brainstem. Know
Somatosensory System. Steven McLoon Department of Neuroscience University of Minnesota
 Somatosensory System Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Dr. Riedl s review session this week: Tuesday (Oct 10) 4-5pm in MCB 3-146B 2 Sensory Systems Sensory
Somatosensory System Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Dr. Riedl s review session this week: Tuesday (Oct 10) 4-5pm in MCB 3-146B 2 Sensory Systems Sensory
Somatic Sensory System I. Background
 Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
General Sensory Pathways of the Trunk and Limbs
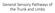 General Sensory Pathways of the Trunk and Limbs Lecture Objectives Describe gracile and cuneate tracts and pathways for conscious proprioception, touch, pressure and vibration from the limbs and trunk.
General Sensory Pathways of the Trunk and Limbs Lecture Objectives Describe gracile and cuneate tracts and pathways for conscious proprioception, touch, pressure and vibration from the limbs and trunk.
Lecturer. Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014
 Lecturer Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014 Dorsal root: The dorsal root carries both myelinated and unmyelinated afferent fibers to the spinal cord. Posterior gray column: Long
Lecturer Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014 Dorsal root: The dorsal root carries both myelinated and unmyelinated afferent fibers to the spinal cord. Posterior gray column: Long
Chapter 8. Esther-Marije Klop, Leonora J. Mouton, Rogier Hulsebosch, José Boers and Gert Holstege. Neuroscience; 134(1): (2005)
 In cat four times as many lamina I neurons project to the parabrachial nuclei and twice as many to the periaqueductal gray as to the thalamus Esther-Marije Klop, Leonora J. Mouton, Rogier Hulsebosch, José
In cat four times as many lamina I neurons project to the parabrachial nuclei and twice as many to the periaqueductal gray as to the thalamus Esther-Marije Klop, Leonora J. Mouton, Rogier Hulsebosch, José
Neural Integration I: Sensory Pathways and the Somatic Nervous System
 15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
Chapter 3. Structure and Function of the Nervous System. Copyright (c) Allyn and Bacon 2004
 Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
SOMATOSENSORY SYSTEMS: Conscious and Non-Conscious Proprioception Kimberle Jacobs, Ph.D.
 SOMATOSENSORY SYSTEMS: Conscious and Non-Conscious Proprioception Kimberle Jacobs, Ph.D. Divisions of Somatosensory Systems The pathways that convey sensory modalities from the body to consciousness are
SOMATOSENSORY SYSTEMS: Conscious and Non-Conscious Proprioception Kimberle Jacobs, Ph.D. Divisions of Somatosensory Systems The pathways that convey sensory modalities from the body to consciousness are
Pain. Pain. Pain: One definition. Pain: One definition. Pain: One definition. Pain: One definition. Psyc 2906: Sensation--Introduction 9/27/2006
 Pain Pain Pain: One Definition Classic Paths A new Theory Pain and Drugs According to the international Association for the Study (Merskey & Bogduk, 1994), Pain is an unpleasant sensory and emotional experience
Pain Pain Pain: One Definition Classic Paths A new Theory Pain and Drugs According to the international Association for the Study (Merskey & Bogduk, 1994), Pain is an unpleasant sensory and emotional experience
Fig Cervical spinal nerves. Cervical enlargement C7. Dural sheath. Subarachnoid space. Thoracic. Spinal cord Vertebra (cut) spinal nerves
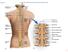 Fig. 13.1 C1 Cervical enlargement C7 Cervical spinal nerves Dural sheath Subarachnoid space Thoracic spinal nerves Spinal cord Vertebra (cut) Lumbar enlargement Medullary cone T12 Spinal nerve Spinal nerve
Fig. 13.1 C1 Cervical enlargement C7 Cervical spinal nerves Dural sheath Subarachnoid space Thoracic spinal nerves Spinal cord Vertebra (cut) Lumbar enlargement Medullary cone T12 Spinal nerve Spinal nerve
Spinal Cord Organization. January 12, 2011
 Spinal Cord Organization January 12, 2011 Spinal Cord 31 segments terminates at L1-L2 special components - conus medullaris - cauda equina no input from the face Spinal Cord, Roots & Nerves Dorsal root
Spinal Cord Organization January 12, 2011 Spinal Cord 31 segments terminates at L1-L2 special components - conus medullaris - cauda equina no input from the face Spinal Cord, Roots & Nerves Dorsal root
*Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord.
 *somatic sensations : PAIN *Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord. *This pathway carries a variety of sensory modalities:
*somatic sensations : PAIN *Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord. *This pathway carries a variety of sensory modalities:
Primate Spinothalamic Pathways: III. Thalamic Terminations of the Dorsolateral and Ventral Spinothalamic Pathways
 THE JOURNAL OF COMPARATIVE NEUROLOGY 288493-511 (1989) Primate Spinothalamic Pathways: III. Thalamic Terminations of the Dorsolateral and Ventral Spinothalamic Pathways A. VANIA APKARIAN AND CHARLES J.
THE JOURNAL OF COMPARATIVE NEUROLOGY 288493-511 (1989) Primate Spinothalamic Pathways: III. Thalamic Terminations of the Dorsolateral and Ventral Spinothalamic Pathways A. VANIA APKARIAN AND CHARLES J.
Non-cranial nerve nuclei
 Brainstem Non-cranial nerve nuclei Nucleus Gracile nucleus Cuneate nucleus Infeiro olivary nucleus Pontine nucleus inferior colliculus superior colliculus Red nucleus Substantia nigra Pretectal area Site
Brainstem Non-cranial nerve nuclei Nucleus Gracile nucleus Cuneate nucleus Infeiro olivary nucleus Pontine nucleus inferior colliculus superior colliculus Red nucleus Substantia nigra Pretectal area Site
Systems Neuroscience Dan Kiper. Today: Wolfger von der Behrens
 Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
Chapter 9. Nervous System
 Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. Pain is always subjective
 Pain & Acupuncture What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. NOCICEPTION( the neural processes of encoding and processing noxious stimuli.)
Pain & Acupuncture What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. NOCICEPTION( the neural processes of encoding and processing noxious stimuli.)
By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy
 By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific
By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific
For more information about how to cite these materials visit
 Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa3.0/
Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa3.0/
SOMATOSENSORY SYSTEMS AND PAIN
 SOMATOSENSORY SYSTEMS AND PAIN A 21 year old man presented with a stab wound of the right side of the neck (Panel A). Neurological examination revealed right hemiplegia and complete right-sided loss of
SOMATOSENSORY SYSTEMS AND PAIN A 21 year old man presented with a stab wound of the right side of the neck (Panel A). Neurological examination revealed right hemiplegia and complete right-sided loss of
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM
 STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
STRUCTURAL ORGANIZATION OF THE NERVOUS SYSTEM STRUCTURAL ORGANIZATION OF THE BRAIN The central nervous system (CNS), consisting of the brain and spinal cord, receives input from sensory neurons and directs
San Francisco Chronicle, June 2001
 PAIN San Francisco Chronicle, June 2001 CONGENITAL INSENSITIVITY TO PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional)
PAIN San Francisco Chronicle, June 2001 CONGENITAL INSENSITIVITY TO PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional)
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
Exploring the pulvinar path to visual cortex
 C. Kennard & R.J. Leigh (Eds.) Progress in Brain Research, Vol. 171 ISSN 0079-6123 Copyright r 2008 Elsevier B.V. All rights reserved CHAPTER 5.14 Exploring the pulvinar path to visual cortex Rebecca A.
C. Kennard & R.J. Leigh (Eds.) Progress in Brain Research, Vol. 171 ISSN 0079-6123 Copyright r 2008 Elsevier B.V. All rights reserved CHAPTER 5.14 Exploring the pulvinar path to visual cortex Rebecca A.
Medical Neuroscience Tutorial Notes
 Medical Neuroscience Tutorial Notes Blood Supply to the Brain MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning
Medical Neuroscience Tutorial Notes Blood Supply to the Brain MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning
Laurie L. Wellman Ph.D.
 Laurie L. Wellman Ph.D. Theodore Tzavaras MD2015 Eastern Virginia Medical School Dr. Craig Goodmurphy Anatomy Guy 1. Discuss -- what is pain? 2. Outline the Anterolateral Quadrant (ALQ) pathway 3. Locate
Laurie L. Wellman Ph.D. Theodore Tzavaras MD2015 Eastern Virginia Medical School Dr. Craig Goodmurphy Anatomy Guy 1. Discuss -- what is pain? 2. Outline the Anterolateral Quadrant (ALQ) pathway 3. Locate
Ascending projections from spinal cord and brainstem to periaqueductal gray and thalamus Klop, Esther
 University of Groningen Ascending projections from spinal cord and brainstem to periaqueductal gray and thalamus Klop, Esther IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Ascending projections from spinal cord and brainstem to periaqueductal gray and thalamus Klop, Esther IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
Sensory information processing, somato-sensory systems
 mm? Sensory information processing, somato-sensory systems Recommended literature 1. Kandel ER, Schwartz JH, Jessel TM (2000) Principles of Neural Science, McGraw-Hill, Ch. xx. 2. Berne EM, Levy MN, Koeppen
mm? Sensory information processing, somato-sensory systems Recommended literature 1. Kandel ER, Schwartz JH, Jessel TM (2000) Principles of Neural Science, McGraw-Hill, Ch. xx. 2. Berne EM, Levy MN, Koeppen
Chapter 6. Gathering information; the sensory systems
 Chapter 6 Gathering information; the sensory systems Gathering information the sensory systems The parts of the nervous system that receive and process information are termed sensory systems. There are
Chapter 6 Gathering information; the sensory systems Gathering information the sensory systems The parts of the nervous system that receive and process information are termed sensory systems. There are
Chapter 13. The Spinal Cord & Spinal Nerves. Spinal Cord. Spinal Cord Protection. Meninges. Together with brain forms the CNS Functions
 Spinal Cord Chapter 13 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward
Spinal Cord Chapter 13 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward
Unit VIII Problem 3 Neuroanatomy: Brain Stem, Cranial Nerves and Scalp
 Unit VIII Problem 3 Neuroanatomy: Brain Stem, Cranial Nerves and Scalp - Brain stem: It is connected to the cerebellum and cerebral hemispheres. Rostral end of brain stem: diencephalon is the area which
Unit VIII Problem 3 Neuroanatomy: Brain Stem, Cranial Nerves and Scalp - Brain stem: It is connected to the cerebellum and cerebral hemispheres. Rostral end of brain stem: diencephalon is the area which
Nigral Projections to the Inferior and the Superior Colliculus in the Rat: A Horseradish Peroxidase Study
 Okajimas Foils Anat. Jpn., 56(5) : 289-296, December 1979 Nigral Projections to the Inferior and the Superior Colliculus in the Rat: A Horseradish Peroxidase Study By KAZUO WATANABE and ETSURO KAWANA Department
Okajimas Foils Anat. Jpn., 56(5) : 289-296, December 1979 Nigral Projections to the Inferior and the Superior Colliculus in the Rat: A Horseradish Peroxidase Study By KAZUO WATANABE and ETSURO KAWANA Department
Skin types: hairy and glabrous (e.g. back vs. palm of hand)
 Lecture 19 revised 03/10 The Somatic Sensory System Skin- the largest sensory organ we have Also protects from evaporation, infection. Skin types: hairy and glabrous (e.g. back vs. palm of hand) 2 major
Lecture 19 revised 03/10 The Somatic Sensory System Skin- the largest sensory organ we have Also protects from evaporation, infection. Skin types: hairy and glabrous (e.g. back vs. palm of hand) 2 major
Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D.
 Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture the student should be familiar with: 1. The relationship between nociception
Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture the student should be familiar with: 1. The relationship between nociception
OVERVIEW. Today. Sensory and Motor Neurons. Thursday. Parkinsons Disease. Administra7on. Exam One Bonus Points Slides Online
 OVERVIEW Today Sensory and Motor Neurons Thursday Parkinsons Disease Administra7on Exam One Bonus Points Slides Online 7 major descending motor control pathways from Cerebral Cortex or Brainstem
OVERVIEW Today Sensory and Motor Neurons Thursday Parkinsons Disease Administra7on Exam One Bonus Points Slides Online 7 major descending motor control pathways from Cerebral Cortex or Brainstem
Nervous System C H A P T E R 2
 Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves
 Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Lesson 33. Objectives: References: Chapter 16: Reading for Next Lesson: Chapter 16:
 Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Ascending projections from spinal cord and brainstem to periaqueductal gray and thalamus Klop, Esther
 University of Groningen Ascending projections from spinal cord and brainstem to periaqueductal gray and thalamus Klop, Esther IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Ascending projections from spinal cord and brainstem to periaqueductal gray and thalamus Klop, Esther IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
Gross Morphology of Spinal Cord
 Gross Morphology of Spinal Cord Lecture Objectives Describe the gross anatomical features of the spinal cord. Describe the level of the different spinal segments compared to the level of their respective
Gross Morphology of Spinal Cord Lecture Objectives Describe the gross anatomical features of the spinal cord. Describe the level of the different spinal segments compared to the level of their respective
THE SOMATOSENSORY SYSTEM
 Daniel J. Simons, Ph.D. I. Peripheral Nervous System THE SOMATOSENSORY SYSTEM The somatosensory system consists of those components of the peripheral and central nervous systems that mediate sensations
Daniel J. Simons, Ph.D. I. Peripheral Nervous System THE SOMATOSENSORY SYSTEM The somatosensory system consists of those components of the peripheral and central nervous systems that mediate sensations
Unit VIII Problem 1 Physiology: Sensory Pathway
 Unit VIII Problem 1 Physiology: Sensory Pathway - Process of sensation: Sensory receptors: they are specialized cells considered as biologic signal transducers which can detect stimuli and convert them
Unit VIII Problem 1 Physiology: Sensory Pathway - Process of sensation: Sensory receptors: they are specialized cells considered as biologic signal transducers which can detect stimuli and convert them
Spinal Cord Protection. Chapter 13 The Spinal Cord & Spinal Nerves. External Anatomy of Spinal Cord. Structures Covering the Spinal Cord
 Spinal Cord Protection Chapter 13 The Spinal Cord & Spinal Nerves We are only going to cover Pages 420-434 and 447 Together with brain forms the CNS Functions spinal cord reflexes integration (summation
Spinal Cord Protection Chapter 13 The Spinal Cord & Spinal Nerves We are only going to cover Pages 420-434 and 447 Together with brain forms the CNS Functions spinal cord reflexes integration (summation
Arterial Blood Supply
 Arterial Blood Supply Brain is supplied by pairs of internal carotid artery and vertebral artery. The four arteries lie within the subarachnoid space Their branches anastomose on the inferior surface of
Arterial Blood Supply Brain is supplied by pairs of internal carotid artery and vertebral artery. The four arteries lie within the subarachnoid space Their branches anastomose on the inferior surface of
Brain Stem and cortical control of motor function. Dr Z Akbari
 Brain Stem and cortical control of motor function Dr Z Akbari Brain stem control of movement BS nuclear groups give rise to descending motor tracts that influence motor neurons and their associated interneurons
Brain Stem and cortical control of motor function Dr Z Akbari Brain stem control of movement BS nuclear groups give rise to descending motor tracts that influence motor neurons and their associated interneurons
Human Anatomy. Spinal Cord and Spinal Nerves
 Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts.
 Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Clinical and experimental studies show serial interactions between the intensity of pain
 Central Neural Mechanisms that Interrelate Clinical and experimental studies show serial interactions between the intensity of pain sensation, pain unpleasantness, and secondary emotions associated with
Central Neural Mechanisms that Interrelate Clinical and experimental studies show serial interactions between the intensity of pain sensation, pain unpleasantness, and secondary emotions associated with
Introduction to the Central Nervous System: Internal Structure
 Introduction to the Central Nervous System: Internal Structure Objective To understand, in general terms, the internal organization of the brain and spinal cord. To understand the 3-dimensional organization
Introduction to the Central Nervous System: Internal Structure Objective To understand, in general terms, the internal organization of the brain and spinal cord. To understand the 3-dimensional organization
ANAT2010. Concepts of Neuroanatomy (II) S2 2018
 ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
Gross Morphology of Spinal Cord
 Gross Morphology of Spinal Cord Done By : Rahmeh Alsukkar ** I did my best and sorry for any mistake ** the sheet does not contain pictures, tables and some slides so please be careful and go back to slides
Gross Morphology of Spinal Cord Done By : Rahmeh Alsukkar ** I did my best and sorry for any mistake ** the sheet does not contain pictures, tables and some slides so please be careful and go back to slides
 BRAIN AND ITS VITAL FUNCTIONS 1 Brain and Its Vital Functions Student s Name Institution Name Professor s Name Course Title BRAIN AND ITS VITAL FUNCTIONS 2 The brain is the integral organism and all its
BRAIN AND ITS VITAL FUNCTIONS 1 Brain and Its Vital Functions Student s Name Institution Name Professor s Name Course Title BRAIN AND ITS VITAL FUNCTIONS 2 The brain is the integral organism and all its
Spinal cord. We have extension of the pia mater below L1-L2 called filum terminale
 Spinal cord Part of the CNS extend from foramen magnum to the level of L1-L2 (it is shorter than the vertebral column) it is covered by spinal meninges. It is cylindrical in shape. It s lower end become
Spinal cord Part of the CNS extend from foramen magnum to the level of L1-L2 (it is shorter than the vertebral column) it is covered by spinal meninges. It is cylindrical in shape. It s lower end become
PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus. MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive
 PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive MEASUREMENT OF PAIN: A BIG PROBLEM Worst pain ever
PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive MEASUREMENT OF PAIN: A BIG PROBLEM Worst pain ever
Spinal Interneurons. Control of Movement
 Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
Structure and Function of the Auditory and Vestibular Systems (Fall 2014) Auditory Cortex (1) Prof. Xiaoqin Wang
 580.626 Structure and Function of the Auditory and Vestibular Systems (Fall 2014) Auditory Cortex (1) Prof. Xiaoqin Wang Laboratory of Auditory Neurophysiology Department of Biomedical Engineering Johns
580.626 Structure and Function of the Auditory and Vestibular Systems (Fall 2014) Auditory Cortex (1) Prof. Xiaoqin Wang Laboratory of Auditory Neurophysiology Department of Biomedical Engineering Johns
Psychophysical laws. Legge di Fechner: I=K*log(S/S 0 )
 Psychophysical laws Legge di Weber: ΔS=K*S Legge di Fechner: I=K*log(S/S 0 ) Sensory receptors Vision Smell Taste Touch Thermal senses Pain Hearing Balance Proprioception Sensory receptors Table 21-1 Classification
Psychophysical laws Legge di Weber: ΔS=K*S Legge di Fechner: I=K*log(S/S 0 ) Sensory receptors Vision Smell Taste Touch Thermal senses Pain Hearing Balance Proprioception Sensory receptors Table 21-1 Classification
PETER PAZMANY CATHOLIC UNIVERSITY Consortium members SEMMELWEIS UNIVERSITY, DIALOG CAMPUS PUBLISHER
 PETER PAZMANY CATHOLIC UNIVERSITY SEMMELWEIS UNIVERSITY Development of Complex Curricula for Molecular Bionics and Infobionics Programs within a consortial* framework** Consortium leader PETER PAZMANY
PETER PAZMANY CATHOLIC UNIVERSITY SEMMELWEIS UNIVERSITY Development of Complex Curricula for Molecular Bionics and Infobionics Programs within a consortial* framework** Consortium leader PETER PAZMANY
Lecture VIII. The Spinal Cord, Reflexes and Brain Pathways!
 Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Neurophysiology of systems
 Neurophysiology of systems Motor cortex (voluntary movements) Dana Cohen, Room 410, tel: 7138 danacoh@gmail.com Voluntary movements vs. reflexes Same stimulus yields a different movement depending on context
Neurophysiology of systems Motor cortex (voluntary movements) Dana Cohen, Room 410, tel: 7138 danacoh@gmail.com Voluntary movements vs. reflexes Same stimulus yields a different movement depending on context
Anatomy of the Spinal Cord
 Spinal Cord Anatomy of the Spinal Cord Anatomy of the Spinal Cord Posterior spinal arteries Lateral corticospinal tract Dorsal column Spinothalamic tract Anterior spinal artery Anterior white commissure
Spinal Cord Anatomy of the Spinal Cord Anatomy of the Spinal Cord Posterior spinal arteries Lateral corticospinal tract Dorsal column Spinothalamic tract Anterior spinal artery Anterior white commissure
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal:
 Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014
 UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition 2011 Eman Al-Khateeb,
UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition 2011 Eman Al-Khateeb,
Circuits & Behavior. Daniel Huber
 Circuits & Behavior Daniel Huber How to study circuits? Anatomy (boundaries, tracers, viral tools) Inactivations (lesions, optogenetic, pharma, accidents) Activations (electrodes, magnets, optogenetic)
Circuits & Behavior Daniel Huber How to study circuits? Anatomy (boundaries, tracers, viral tools) Inactivations (lesions, optogenetic, pharma, accidents) Activations (electrodes, magnets, optogenetic)
Lateral view of human brain! Cortical processing of touch!
 Lateral view of human brain! Cortical processing of touch! How do we perceive objects held in the hand?! Touch receptors deconstruct objects to detect local features! Information is transmitted in parallel
Lateral view of human brain! Cortical processing of touch! How do we perceive objects held in the hand?! Touch receptors deconstruct objects to detect local features! Information is transmitted in parallel
