1 17 ACITRETIN 10MG CAP 20, ,000 14,000 4, ACITRETIN 25MG CAP 50, ,000 35,000 10,000
|
|
|
- Joshua Carpenter
- 5 years ago
- Views:
Transcription
1 ردیف کد ژنریک نام ژنریک مبلغ پوشش وزارت بهداشت مبلغ پوشش بیمه مبلغ پرداخت بیمار درصد پرداخت بیمار قیمت اعالمی وزارت بهداشت نام تجاری 1 17 ACITRETIN 10MG CAP 20, ,000 14,000 4, ACITRETIN 25MG CAP 50, ,000 35,000 10, ACTIVATED PROTHROMBIN COMPLEX CONC. PER IU VIAL 33, , ACTIVATED PROTHROMBIN COMPLEX CONC.500U VIAL - / / ACTIVATED PROTHROMBIN COMPLEX CONC. 1000U VIAL - / / ADALIMUMAB 40MG/0.8ML INJ 9,200, ,760,000-6,440, ALGLUCOSIDASE 50MG VIAL 24,000, ,000-23,520, AMPHOTERICIN-B 50MG/20ML VIAL 145, , ,500 29, AMPHOTERICIN-B LIPOSOMAL VIAL 2,600, ,000-1,820, ANAGRALIDE 0.5MG CAP 65, ,500-45, ANTIHEMOPHILIC FACTOR IX PER IU VIAL 6, , ANTIHEMOPHILIC FACTOR IX 500IU VIAL - / / ANTIHEMOPHILIC FACTOR IX 600IU VIAL - / / ANTIHEMOPHILIC FACTOR VIIa 1.2MG VIAL 10,900, ,900, ANTIHEMOPHILIC FACTOR VIII PER IU VIAL 6, , ANTIHEMOPHILIC FACTOR VIII 250IU VIAL - / / ANTIHEMOPHILIC FACTOR VIII 500IU VIAL - / / ANTIHEMOPHILIC FACTOR VIII VONWILLEBRAND 500/1200IU VIAL 16,000, ,806 15,174, ANTIHEMOPHILIC FACTOR VIII VONWILLEBRAND 500/1000IU VIAL 8,000, ,903 7,587, ANTIHEMOPHILIC FACTOR VIII(RECOMBINANT) PER IU VIAL 7, , ANTIHEMOPHILIC FACTOR VIII(RECOMBINANT) 500IU VIAL - / / ANTIHEMOPHILIC FACTOR XIII PER IU VIAL 22, , ANTIHEMOPHILIC FACTOR XIII 250IU VIAL - / / ANTITHYMOCYTE IMMUNOGLOBULIN 25MG/ML AMP 7,300, ,000 5,110,000 1,460, ANTITHYMOCYTE IMMUNOGLOBULIN 250MG/5ML AMP 9,200, ,000 6,440,000 1,840, APROTININ 100,000IU/10ML AMP 179, , ,650 35, ARSENIC TRIOXIDE 0.1% AMP 710, , , ASPARAGINASE 10,000U VIAL 1,250, , , , BCG INTRAVESICAL VIAL 1,000, , , , BERACTANT 100MG/4ML VIAL 6,250, ,000 4,375,000 1,250, BERACTANT 200MG/8ML INTRATRACHEAL INJ 7,500, ,000 5,250,000 1,500, BETAINE ANHYDROUS 100G ORAL POWDER 6,650, ,000-6,517, BEVACIZUMAB 100MG/4ML VIAL 12,000, ,600,000-8,400, BEVACIZUMAB 400MG/16ML VIAL 46,000, ,800,000-32,200, BICALUTAMIDE 50MG TAB 22, ,600-15, BICARBONATE IDEMSA POWDER 155, , BICARBONATE POWDER PACKAGE 75, , BLEOMYCIN SULFATE 15MG VIAL 550, , , , BORTEZOMIB 3.5MG INJ 15,000, ,500,000-10,500, BOSENTAN 62.5MG TAB 80, ,000 14,400 61, BOSENTAN 125MG TAB 120, ,000 9, , BOVACTANT 50MG/1.2ML AMP 8,500, ,000 5,950,000 1,700, BOVINE LIPID EXTRACT SURFACTANT 27MG/ML INJ 5,500, ,000 3,850,000 1,100,000
2 BUSERELIN ACETATE 5.5MG/5.5ML VIAL 450, , ,000 90, BUSULFAN 2MG TAB 4, , BUSULFAN 6MG/ML AMP 6,000, ,000 5,400, , CABERGULINE 0.5MG TAB 20, ,000-14, CABERGULINE 1MG TAB 33, ,110-23, CABERGULINE 2MG TAB 54, ,200-37, CALCITRIOL 0.25MCG CAP 8, ,600 1, CALCIUM FOLINATE 15MG TAB 8, ,600 1, CALCIUM FOLINATE 10MG/ML 5ML AMP 326, , ,550 65, CALCIUM FOLINATE 30MG/3ML AMP 57, ,730 40,110 11, CALCIUM FOLINATE 100MG VIAL 220, , ,000 44, CALCIUM FOLINATE 200MG/20ML VIAL 240, , ,000 48, CALCIUM FOLINATE 500MG/50ML VIAL 844, , , , CAPECITABINE 500MG TAB 62, ,200 43,400 12, CARBOPLATIN 50MG VIAL 390, , ,000 78, CARBOPLATIN 150MG VIAL 650, , , , CARBOPLATIN 450MG VIAL 1,750, ,000 1,225, , CASPOFUNGIN 50MG 10ML VIAL 6,400, ,000 5,760, CETRORELIX ACETATE 250MCG VIAL 730, , , CETUXIMAB 5MG/ML 20ML VIAL 6,850, ,000 6,165, CHLORAMBUCIL 2MG TAB 30, ,000 21,000 6, CHORIONIC GONADOTROPHIN (HUMAN) PER IU VIAL CHORIONIC GONADOTROPHIN (HUMAN) 500 U VIAL - / / CHORIONIC GONADOTROPHIN (HUMAN) 1500 U VIAL - / / CHORIONIC GONADOTROPHIN (HUMAN) 5000 U VIAL - / / CICLOSPORIN 0.05% OPH DROP 15, ,500 10,500 3, CICLOSPORIN 25MG CAP 6, , CICLOSPORIN 50MG CAP 13, , CICLOSPORIN 100MG CAP 26, , CICLOSPORIN 50MG/1ML AMP 185, , ,500 37, CICLOSPORIN 250MG/5ML AMP 412, , ,960 82, CICLOSPORIN I00MG/ML 50ML ORAL SOL 5,500, ,000 3,850,000 1,100, CINACALCET 30MG TAB 60, ,000 54, CISPLATIN 0.5MG/ML 20ML INF 140, ,000 98,000 28, CISPLATIN 0.5MG/ML 100ML INF 300, , ,000 60, CISPLATIN 10MG/10ML VIAL 140, ,000 98,000 28, CISPLATIN 50MG/50ML VIAL 320, , ,000 64, CLADRIBINE 10MG/5ML VIAL 11,800, ,180,000 8,260,000 2,360, CYCLOPHOSPHAMIDE 200MG VIAL 100, ,000 70,000 20, CYCLOPHOSPHAMIDE 500MG VIAL 219, , ,300 43, CYSTEAMINE BITARTRATE 50MG CAP 60, , , CYSTEAMINE BITARTRATE 150MG CAP 110, ,500 2, , CYTARABINE 100MG/5ML VIAL 145, , ,500 29, CYTARABINE 1G VIAL 610, , , , DACARBAZINE 100MG VIAL 180, , ,000 36,000
3 DACARBAZINE 200MG VIAL 235, , ,500 47, DACLATASVIR 60 MG 50, ,000-35, DACLATASVIR 60 MG+SOFOSBUVIR 400 MG 900, , , DACTINOMYCIN 0.5MG VIAL 1,850, ,000 1,295, , DAUNORUBICIN HCL 20MG VIAL 360, , ,000 72, DEFERASIROX 125MG TAB 8, , DEFERASIROEXJADE 125MG TAB 55, ,000 1,044 48, DEFERASIROX 250MG TAB 16, , DEFERASIROEXJADE 250MG TAB 110, ,000 2,088 97, DEFERASIROX 500MG TAB 32, , DEFERASIROEXJADE 500MG TAB 220, ,000 4, , DEFERIPRONE 500MG EC TAB 4, , DEFEROXAMINE MESYLATE 500MG VIAL 112, , DEFEROXAMDESFERAL 500MG VIAL 125, , , DOCETAXEL 20MG VIAL 1,500, ,000 1,050, , DOCETAXEL 80MG VIAL 6,000, ,000 4,200,000 1,200, DOCETAXEL 160MG/16ML VIAL 8,450, ,000 5,915,000 1,690, DOXORUBICIN HCL 10MG VIAL 145, , ,500 29, DOXORUBICIN HCL 50MG VIAL 445, , ,500 89, DOXORUBICIN LIPOSOMAL 20MG/10ML VIAL 7,000, ,000 6,300, ELSULFASE ALFA 5MG/5ML VIAL 30,000, ,200,000-28,800, EPIRUBICIN HCL 10MG VIAL 250, , ,000 50, EPIRUBICIN HCL 50MG VIAL 950, , , , EPTIFIBATIDE 2MG/ML INJ 1,050, , , , EPTIFIBATIDE 750MCG/ML INJ 3,250, ,000 2,275, , ERLOTINIB 100MG TAB 500, , , ERLOTINIB 150MG TAB 750, , , ERYTHROPOIETIN RECOMBINANT HU 2000 IU/PFS 92, ,200 64,400 18, ERYTHROPOIETIN RECOMBINANT HU 2000 IU/VIAL 92, ,200 64,400 18, ERYTHROPOIETIN RECOMBINANT HU 4000 IU/PFS 168, , ,600 33, ERYTHROPOIETIN RECOMBINANT HU 4000 IU/VIAL 168, , ,600 33, ERYTHROPOIETIN RECOMBINANT HU IU/PFS 462, , ,400 92, ERYTHROPOIETIN RECOMBINANT HU IU/VIAL 462, , ,400 92, ESTRAMUSTINE SODIUM PHOSPHATE 140MG CAP 34, ,400 23,800 6, ETANERCEPT PER MG PREFILLED SYRINGE 80, ,000 72, ETANERCEPT 25MGPREFILLED SYRINGE - / / ETANERCEPT 50MG PREFILLED SYRINGE - / / ETOPOSIDE 100MG/5ML AMP 180, , ,000 36, ETOPOSIDE 50MG CAP 95, ,500 66,500 19, EVEROLIMUS 0.25MG TAB 37, ,100-25, EVEROLIMUS 0.75MG TAB 90, ,000-63, EVEROLIMUS 5MG TAB 1,200, , , EVEROLIMUS 10MG TAB 2,200, ,000-1,540, EXEMESTANE 25MG TAB 30, ,000-21, FERRIC CARBOXYMALTOSE 500MG/10ML VIAL 2,640, ,000 1,848, ,000
4 FIBRINOGEN 1G VIAL 12,800, , ,000 12,244, FILGRASTIM(GCSF) 300MCG/ML AMP 560, , , , FILGRASTIM(GCSF) 300MCG/ML SYRINGE 560, , , , FINGOLIMOD 0.5MG CAP 450, , , FLUDARABINE PHOSPHATE 50MG VIAL 1,600, ,000 1,120, , FLUMAZENIL 0.5MG/5 ML AMP 200, , ,000 40, FLUOROURACIL 250MG/5ML AMP 100, ,000 70,000 20, FLUOROURACIL 1G/20ML AMP 270, , ,000 54, FLUOROURACIL 500MG/10ML AMP 165, , ,500 33, FLUOROURACIL 5% 20GR CREAM 350, , ,000 70, FLUTAMIDE 250MG TAB 10, ,000 7,000 2, FLUTICASONE 50MCG/DOSE SPRAY 150, , ,000 30, FLUTICASONE 50MCG/DOSE 100DOSE NASAL SPRAY 200, , ,000 40, FLUTICASONE 50MCG/DOSE 120DOSE 15ML NASAL SPRAY 120, ,000 84,000 24, FLUTICASONE 50MCG/DOSE 200DOSE 20ML NASAL SPRAY 125, ,500 87,500 25, FLUTICASONE 125MCG/DOSE 120DOSE SPRAY 170, , ,000 34, FLUTICASONE 125MCG/DOSE 60DOSE SPRAY 150, , ,000 30, FLUTICASONE 250MCG/DOSE 120DOSE SPRAY 220, , ,000 44, FLUTICASONE 250MCG/DOSE 60DOSE SPRAY 200, , ,000 40, FOLITROPIN 75IU INJ 368, , , FOLITROPIN 150IU PEN 1,300, , , FOLITROPIN 300IU PEN 2,560, ,000-1,792, FOLITROPIN 450IU PEN 3,920, ,176,000-2,744, FOLITROPIN 900IU PEN 7,000, ,100,000-4,900, FORMOTEROL 12MCG CAP 14, ,200-9, FORMOTEROL 12MCG/DOSE SOL FOR INHALATION 120, ,000-84, GALSULFASE 5MG/5ML VIAL 50,000, ,500,000-47,500, GANCICLOVIR 500MG VIAL 1,000, , , , GANIRELIX 0.25MG INJ 1,582, ,600-1,107, GEMCITABINE HCl 200MG VIAL 380, , ,000 76, GEMCITABINE HCl 1 G VIAL 1,700, ,000 1,190, , GLATIRAMER ACETATE 20MG/ML PREFILLED SYRINGE 600, , , GOSERELIN ACETATE 3.6MG AMPMG SYRINGE 2,250, ,000-1,575, GOSERELIN ACETATE 10.8MG SYRINGE 7,000, ,100,000-4,900, GRANISETRON 1MG/1ML AMP 17, ,750 12,250 3, GRANISETRON HCL 3MG/3ML VIAL 49, ,900 34,300 9, GRANISETRON HCL 1MG TAB 8, ,600 1, HEMODIALYSIS PER 5LIT CONC SOLUTION 126, , , HEMODIALYSIS ACIDIC II 5LIT CONC SOLUTION - / / HEMODIALYSIS I 5LIT CONC SOLUTION - / / HEMODIALYSIS II 5LIT CONC SOLUTION - / / HEPATITIS B IMMUNE GLOBULIN PER IU VIAL 6, , HEPATITIS B IMMUNE GLOBULIN 100IU/2ML VIAL - / / HEPATITIS B IMMUNE GLOBULIN 180IU/1ML VIAL - / / HEPATITIS B IMMUNE GLOBULIN 200IU/1ML VIAL - / / - - -
5 18064 HEPATITIS B IMMUNE GLOBULIN 500IU/10ML VIAL - / / HEPATITIS B IMMUNE GLOBULIN 540IU/3ML VIAL - / / HEPATITIS B IMMUNE GLOBULIN 1000IU/5ML VIAL - / / HEPATITIS B IMMUNE GLOBULIN 2000 IU/40ML VIAL - / / HEPATITIS B IMMUNE GLOBULIN 2500IU/50ML VIAL - / / HYDROCORTISONE 100MG/60ML RETENTION ENEMA 430, , ,000 86, HYDROXYPROGESTERONE CAPROATE 250MG/ML AMP 25, ,500-17, HYDROXYPROGESTERONE CAPROATE 500MG/2ML AMP 45, ,500-31, HYDROXYUREA 500MG CAP 5, ,780 1, ICODEXTRIN 7.5% 2L INJECTION 490, , IDARUBICIN HCl 5MG VIAL 1,150, , , , IDARUBICIN HCl 10MG VIAL 2,300, ,000 1,610, , IFOSFAMIDE 1 G VIAL 900, , , , IFOSFAMIDE 2 G VIAL 1,750, ,000 1,225, , IMATINIB MESYLATE 100MG CAP 75, ,500 52,500 15, IMATINIB MESYLATE 100MG TAB 75, ,500 52,500 15, IMIGLUCERASE PER IU VIAL 47, , IMIGLUCERASE 200IU VIAL - / / IMIGLUCERASE 400IU VIAL - / / IMMUNE GLOBULIN PER G (IV ) VIAL 1,450, ,000 1,015, , IMMUNE GLOBULIN 1G (IV ) VIAL - / / IMMUNE GLOBULIN 2.5G INJECTION POWDER (IV) VIAL - / / IMMUNE GLOBULIN 5G INJECTION POWDER (IV) VIAL - / / INDOCYANINE GREEN 25MG VIAL 3,150, ,150-2,205, INFLIXIMAB 100MG VIAL 8,500, ,000 7,650, INSULIN ASPART 100IU/ML 3ML FOR INJ 295, , , INSULIN ASPART RAPID 100IU/ML 3ML FOR INJ 295, , , INSULIN BIPHASIC ISOPHANE (70+30)U/ML 10ML VIAL 140, , ,000 28, INSULIN DETEMIR 300IU/3ML PREFILLED PEN 280, , , INSULIN GLARGINE 300IU/3ML PREFILLED PEN 295, , , INSULIN GLULISINE 300IU/3ML PREFILLED PEN 235, , , INSULIN ISOPHAN (NPH) HUMAN 1000U/10ML VIAL 140, , ,000 28, INSULIN REGULAR HUMAN 1000U/10ML VIAL 140, , ,000 28, INTERFERON B 1A 30 MCG VIAL 1,375, ,750 1,237,500 68, INTERFERO AVONEX 30MCG VIAL 5,040, ,369,500 1,237,500 1,433, INTERFERON B 1A 44MCG/0.5ML SYRINGE 900, , ,000 45, INTERFERO REBIF 44MCG/0.5ML SYRINGE 1,500, , ,000 95, INTERFERON B 1B 8MIU VIAL 660, , ,000 33, INTERFERO EXTAVIA POWDER INJ 1,200, , , , INTERFERO BETAFERON 8MIU VIAL 1,200, , , , INTERFERON GAMMA 100MCG 2,300, ,000 2,070, IRINOTECAN HCL 40MG/2ML POWDER VIAL 850, , , , IRINOTECAN HCL 100MG/5ML POWDER VIAL 1,100, , , , IRINOTECAN HCL 300MG/15ML SOLUTION FOR INFUSION 4,200, ,000 2,940, , LARONIDASE 100IU/ML 5ML VIAL 21,700, ,000 10,500 21,255,500
6 LEDIPASVIR 90 MG+SOFOSBUVIR 400 MG 900, , , LEUPRORELIN ACETATE 3.75MG VIAL 1,650, ,000 1,155, , LEUPRORELIN ACETATE 7.5MG SYRINGE 2,600, ,000 1,820, , LIPID INFUSION 10% (SOYA OIL) INF 250, , ,000 50, LIPID INFUSION 20% (SOYA OIL) INF 350, , ,000 70, LIRAGLUTIDE 6MG/ML SOLUTION FOR INJECTION 2,600, ,000-1,820, LOMUSTINE 40MG CAP 280, , ,000 56, LUTROPIN ALFA 75IU VIAL 750, , , MELPHALAN 2MG TAB 32, ,200 22,400 6, MELPHALAN 50MG VIAL 1,500, ,000 1,050, , MENOTROPINS 75 IU FSH+75IU LH AMP 250, , , MERCAPTOPURINE 50MG TAB 14, ,400 9,800 2, MESALAZINE 400MG TAB 2, , MESALAZINE 500MG TAB 3, , MESALAZINE 800MG TAB 5, ,500 1, MESALAZINE 500MG SUPP 8, ,740 1, MESALAZINE 1000MG SUPP 73, ,300 51,100 14, MESALAZINE 4G ENEMA 250, , ,000 50, METHOTREXATE SODIUM 2.5MG TAB 2, , METHOTREXATE SODIUM 5MG/ML AMP 51, ,100 35,700 10, METHOTREXATE SODIUM 5MG/2ML VIAL 51, ,100 35,700 10, METHOTREXATE SODIUM 7.5MG/0.75ML INJ 385, , ,500 77, METHOTREXATE SODIUM 10MG/ML INJ 490, , ,000 98, METHOTREXATE SODIUM 20MG/2ML INJ 700, , , , METHOTREXATE SODIUM 15MG/1.5ML INJ 675, , , , METHOTREXATE SODIUM 50MG/2ML AMP 100, ,000 70,000 20, METHOTREXATE SODIUM 50MG/5ML AMP 280, , ,000 56, METHOTREXATE SODIUM 1000MG/10ML VIAL 970, , , , METHYLPREDNISOLONE 1000MG POWDER FOR INJ 376, , ,550 75, METHYLPREDNISOLONE SODIUM SUCCINATE 500MG VIAL 112, ,200 78,400 22, MIGLUSTAT 100MG CAP 2,100, ,000-2,058, MILRINONE LACTATE 10MG/10ML INJ 500, , , , MITOTANE 500MG TAB 280, , ,000 56, MITOXANTRONE HCL 20MG/10ML VIAL 1,400, , , , MYCOPHENOLATE MOFETIL 250MG CAP 8, ,204 3, MYCOPHEN CELLCEPT 250MG TAB 17, ,500 5,882 8, MYCOPHENOLATE MOFETIL 500MG TAB 19, ,999 7, MYCOPHEN CELLCEPT 500MG TAB 34, ,000 11,765 17, MYCOPHENOLATE SODIUM 360MG TAB 34, ,000 23,800 5, NILOTINIB 200MG CAP 800, , , NITISINONE 2MG CAP 66, ,980-64, NITISINONE 5MG CAP 165, , , NITISINONE 10MG CAP 330, , , OCTREOTIDE 0.05MG AMP 25, ,560 17,920 5, OCTREOTIDE 20MG FOR INJ 22,000, ,200,000 15,400,000 4,400,000
7 OXALIPLATIN 50MG VIAL 1,180, , , , OXALIPLATIN 100MG VIAL 2,360, ,000 1,652, , PACLITAXEL PER MG VIAL 20, ,000 14,000 4, PACLITAXEL 30MG/5ML VIAL - / / PACLITAXEL 100MG/16.7ML VIAL - / / PACLITAXEL 150MG/25ML VIAL - / / PACLITAXEL 300MG/50ML VIAL - / / PAMIDRONATE DISODIUM 90MG/10ML AMP 325, , ,500 65, PEGASPARGASE 3750IU/5ML VIAL 34,000, ,200,000-23,800, PEGFILGRASTIM 6MG/0.6ML INJ 5,200, ,000 3,640,000 1,040, PEGINTERFERON 180MCG/1ML INJ + RIBAVIRIN 200MG CAP 1,500, ,000 1,050, , PEMETREXED 100MG VIAL 4,000, ,200,000-2,800, PEMETREXED 500MG VIAL 13,500, ,050,000-9,450, PERITONEAL DIALYSIS PER 2LIT SOL 290, , PERITONEAL DIALYSIS I 1.5% 2LIT SOL 290,000 / / PERITONEAL DIALYSIS II 2.5% 2LIT SOL 290,000 / / PERITONEAL DIALYSIS III 4.25% 2LIT SOL 290,000 / / PORACTANT 240/3ML AMP 9,750, ,000 6,825,000 1,950, PRIMIDONE 125MG/5ML 250ML SUSP 370, , ,000 74, PROCARBAZINE HCL 50MG CAP 170, , ,000 34, PROTHROMBIN COMPLEX CONCENTRATED PER IU VIAL 9, , PROTHROMBIN COMPLEX CONCENTRATED 500 IU VIAL - / / RASBURICASE 1.5MG VIAL 3,000, ,000-2,100, RIBAVIRIN 200MG CAP 3, , RILUZOLE 50MG TAB 31, ,100 27, RITUXIMAB 100MG/10ML VIAL 4,350, ,000 3,915, RITUXIMAB 500MG/50ML VIAL 21,500, ,150,000 19,350, ROMIPLOSTIM 250MCG VIAL 31,000, ,300,000-21,700, SAPROPTERIN 100MG TAB 1,050, ,300-1,022, SEVELAMER 800MG TAB 16, ,650 11,550 3, SIROLIMUS 1MG TAB 50, ,000 5, SIROLIMUS RAPAMUNE 1MG TAB 110, ,000 18,000 82, SODIUM BENZOATE 2G/10ML AMP 227, , , SODIUM BENZOATE 500MG TAB 7, ,280-5, SODIUM GLYCEROPHOSPHATE INJ 65, ,500-45, SODIUM PHENYL BUTYRATE 500MG TAB 90, ,500-85, SODIUM PHENYL BUTYRATE 2G/10ML AMP 557, , , SOFOSBUVIR 400 MG 500, , , SORAFENIB 200MG TAB 1,050, , , STREPTOKINASE 1,500,000IU VIAL 3,430, ,029,000 2,401, SUCCIMER 200MG CAP 450, , , SUNITINIB MALATE 12.5MG CAP 1,100, , , , SUNITINIB MALATE 25MG CAP 2,200, ,000 1,540, , SUNITINIB MALATE 50MG CAP 4,400, ,000 3,080, , TACROLIMUS 0.5MG CAP 17, ,300 1,700
8 5804 TACROLIMUPROGRAF 0.5MG CAP 34, ,500 4,500 26, TACROLIMUS 1MG CAP 31, ,900 3, TACROLIMUPROGRAF 1MG CAP 41, ,000 9,000 26, TACROLIMUS 5MG/ML FOR INFU 2,400, ,000 1,680, , TEMOZOLAMIDE 5MG CAP 60, ,000 54, TEMOZOLAMIDE 20MG CAP 210, , , TEMOZOLAMIDE 100MG CAP 750, , , TEMOZOLAMIDE 140MG CAP 1,050, , , , TEMOZOLAMIDE 250MG CAP 1,680, ,000 1,512, TENOFOVIR 300MG TAB 14, ,450 13, TERIPARATIDE 750MCG/3ML VIAL 4,800, ,000 3,360, , THALIDOMIDE 100MG CAP 100, ,000 70,000 20, THALIDOMIDE 100MG TAB 108, ,800 75,600 21, THIOGUANINE 40MG TAB 240, , , THYROTROPIN ALFA 1.1MG VIAL 21,000, ,300,000-14,700, TIOTROPIUM 0.018MG INH CAP 19, ,900 13,300 3, TOBRAMYCIN 28 MG INH CAP 480, , , TRASTUZUMAB 150MG VIAL 15,000, ,500,000 13,500, TRASTUZUMAB 440MG VIAL 40,000, ,000,000 36,000, TRETINOIN 10MG CAP 150, , , TRIENTINE 300MG CAP 550, , , TRIPTORELIN ACETATE 0.1MG/1ML SYRINGE 186, , ,550 37, TRIPTORELIN ACETATE 3.75MG SYRINGE 1,500, ,000 1,050, , TRIPTORELIN ACETATE 3.75MG VIAL 1,600, ,000 1,120, , TRIPTORELIN PAMOATE 11.25MG VIAL 4,800, ,000 4,320, UROFOLLITROPIN 75 I U FSH AMP 200, , , VALGANCICLOVIR 450MG TAB 250, , , VALPROATE SODIUM 100MG/ML 5ML INJ 250, , ,000 50, VALPROATE SODIUM 200MG/5ML 300ML SYRUP 480, , ,000 96, VALPROATE SODIUM 300MG/5ML 250ML SYRUP 270, , ,000 54, VINBLASTINE SULFATE 10MG VIAL 400, , ,000 80, VINCRISTINE SULFATE 1MG VIAL 220, , ,000 44, VINORELBINE 20MG CAP 1,450, ,000 1,015, , VINORELBINE 30MG CAP 2,270, ,000 1,589, , VINORELBINE 10MG/1ML VIAL 412, , ,400 82, VINORELBINE 50MG/5ML VIAL 1,660, ,000 1,162, , VORICONAZOLE 50MG TAB 230, , , VORICONAZOLE 200MG TAB 750, , , VORICONAZOLE 200MG VIAL 3,000, ,000 2,700, ZOLEDRONIC ACID 4MG VIAL 1,112, ,250 1,001,250 - قیمت واحد
Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites.
 WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
WARDS/DEPARTMENTS By: 0 01/09/019 1 of 3 Objective: To provide a standard procedure for the recycling of unused medication and the disposal of medicines across all BCPFT Hospital sites. Scope: All Black
COST CONSIDERATIONS Union for International Cancer Control 2014 Review of Cancer Medicines on the WHO List of Essential Medicines!!!!!!!!!
 UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
UICCEMLCostingScenarios BackoftheEnvelope Calculations PreparedforWorkingGroupSession:19621November2014,Geneva MethodsSummary We have chosen a conservative approach, calculating cost per vial. We have
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced
 ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
ICON Formulary - October 2018 Legend - ICON Protocols Essential (previously Standard), Core, Enhanced Core, Enhanced Enhanced Class Medicine Name Nappi Strength Form Size Route Abiraterone Acetate ZYTIGA
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting
 West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
West of Scotland Cancer Network Guideline for Managing Chemotherapy Induced Nausea and Vomiting Definitions Acute nausea and vomiting Delayed nausea and vomiting Anticipatory nausea and vomiting Initial
J1556 INJECTION, IMMUNE GLOBULIN (BIVIGAM) 500 MG $ J1559 INJECTION, IMMUNE GLOBULIN (HIZENTRA) 100 MG $14.364
 G0333 INITIAL 30-DAY SUPPLY AS A BENEFICIARY $57.000 J0133 INJECTION, ACYCLOVIR 5 MG $0.470 J0285 INJECTION, AMPHOTERICIN B 50 MG $10.280 J0287 INJECTION, AMPHOTERICIN B LIPID COMPLEX 10 MG $21.850 J0288
G0333 INITIAL 30-DAY SUPPLY AS A BENEFICIARY $57.000 J0133 INJECTION, ACYCLOVIR 5 MG $0.470 J0285 INJECTION, AMPHOTERICIN B 50 MG $10.280 J0287 INJECTION, AMPHOTERICIN B LIPID COMPLEX 10 MG $21.850 J0288
MEDICAL NECESSITY GUIDELINE
 PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PAGE: 1 of 10 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
Guidelines on Chemotherapy-induced Nausea and Vomiting in Pediatric Cancer Patients
 Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
Guidelines on Chemotherapy-induced Nausea Vomiting in Pediatric Cancer Patients COG Supportive Care Endorsed Guidelines Click here to see all the COG Supportive Care Endorsed Guidelines. DISCLAIMER For
 Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Exhibit B United States Patent Application 20020012663 Kind Code A1 Waksal, Harlan W. January 31, 2002 Treatment of refractory human tumors with epidermal growth factor receptor antagonists Abstract A
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19
 Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
Vivida Health Specialty Pharmacy Drugs (Injectable) Prior-Authorization Requirements Effective 1/1/19 All Non-Par Provider Requests Requires Authorization Regardless of Service J0178 J0180 J0202 J0205
DME MAC Jurisdiction C Drug Fees, Pharmacy Dispensing Fees and Pharmacy Supply Fees Effective 01/01/2018 through 03/31/2018
 G0333 PHARMACY DISPENSING FEE FOR INHALATION DRUG(S); INITIAL 30-DAY SUPPLY AS A BENEFICIARY $57.000 J0133 INJECTION, ACYCLOVIR 5 MG $0.068 J0285 INJECTION, AMPHOTERICIN B 50 MG $32.987 J0287 INJECTION,
G0333 PHARMACY DISPENSING FEE FOR INHALATION DRUG(S); INITIAL 30-DAY SUPPLY AS A BENEFICIARY $57.000 J0133 INJECTION, ACYCLOVIR 5 MG $0.068 J0285 INJECTION, AMPHOTERICIN B 50 MG $32.987 J0287 INJECTION,
DME MAC Jurisdiction B Drug Fees, Pharmacy Dispensing Fees and Pharmacy Supply Fees Effective 01/01/2019 through 03/31/2019
 G0333 PHARMACY DISPENSING FEE FOR INHALATION DRUG(S); INITIAL 30-DAY SUPPLY AS A BENEFICIARY $57.000 J0133 INJECTION, ACYCLOVIR 5 MG $0.048 J0285 INJECTION, AMPHOTERICIN B 50 MG $31.668 J0287 INJECTION,
G0333 PHARMACY DISPENSING FEE FOR INHALATION DRUG(S); INITIAL 30-DAY SUPPLY AS A BENEFICIARY $57.000 J0133 INJECTION, ACYCLOVIR 5 MG $0.048 J0285 INJECTION, AMPHOTERICIN B 50 MG $31.668 J0287 INJECTION,
The following are J Code requirements
 The following are J Code requirements J Codes 20610 Arthrocentesis, aspiration and/or injection; major joint or bursa (eg, shoulder, hip, knee joint, subacromial bursa) A9579 Injection, gadolinium based
The following are J Code requirements J Codes 20610 Arthrocentesis, aspiration and/or injection; major joint or bursa (eg, shoulder, hip, knee joint, subacromial bursa) A9579 Injection, gadolinium based
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY
 ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
ELECTRONIC HEALTH RECORD (EHR) ENHANCEMENTS FOR MARCH 15, 2016 SUMMARY Problem Opening PACS Images on ipads or ibooks has Been Fixed Changes have been made in PROD to enable user credentials to be passed
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY CLASSIFICATION
 GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
GUIDELINES FOR ANTIEMETIC USE IN ONCOLOGY SUMMARY More than half of all cancer patients experience nausea or vomiting during the course of their treatment. If nausea or vomiting becomes severe enough,
DOXOrubicin, Cyclophosphamide (AC 60/600) 21 day followed by weekly PACLitaxel (80) Therapy (AC-T) 261 CARBOplatin (AUC4-6) Monotherapy-21 days
 Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
Last updated Oct 17, 2018 Tumour Group Protocol Number Protocol Name on NCCP website Breast 200 Trastuzumab (IV) Monotherapy 21 days 201 Trastuzumab (IV) Monotherapy 7 days 202 DOCEtaxel Monotherapy 100mg/m2
SCI. SickKids-Caribbean Initiative Enhancing Capacity for Care in Paediatric Cancer and Blood Disorders
 1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
1.0 Introduction The (SCI) is a not-for-profit collaboration between the Hospital for Sick Children (SickKids), Toronto, Canada, and seven Caribbean health care institutions across six countries that strive
MASCC Guidelines for Antiemetic control: An update
 MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
MASCC / ISOO 17 th International Symposium Supportive Care in Cancer June 30 July 2, 2005 / Geneva, Switzerland MASCC Guidelines for Antiemetic control: An update Sussanne Börjeson, RN, PhD Linköping University,
DRUG PROPERTIES YOU NEED TO KNOW
 Dr. Janet Fitzakerley Summer 2013 Med 6541 Hematopoiesis and Host Defences jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 11 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class
Dr. Janet Fitzakerley Summer 2013 Med 6541 Hematopoiesis and Host Defences jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 11 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class
Primary malignant neoplasms, not lymphatic or hematopoietic. Secondary malignant neoplasms (i.e.metastatic) Malignant neoplasm, unknown site
 Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
Supplementary Table 1. ICD-9-CM codes used to define cancer ICD-9 Diagnosis code 140.xx-172.xx 174.xx-195.xx 196.xx 198.xx 199.xx 200.xx-208.xx Description Primary malignant neoplasms, not lymphatic or
Haematology, Oncology and Palliative Care Directorate.
 Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Anticancer Treatment for Administration on the Somerset Mobile Chemotherapy Unit The table below details the suitability of different types of anticancer treatment for administration on the Somerset Mobile
Table III: 2019 Medicare Drug Fee Schedule* CY st Quarter Average Sales Price (ASP) Data Plus 6 Percent
 Table III: 2019 Medicare Drug Fee Schedule* CY 2019 1st Quarter Average Sales Price (ASP) Data Plus 6 Percent *The Medicare payments allowance limits are effective Jan. 1 thru March. 31, 2019. CY 2019
Table III: 2019 Medicare Drug Fee Schedule* CY 2019 1st Quarter Average Sales Price (ASP) Data Plus 6 Percent *The Medicare payments allowance limits are effective Jan. 1 thru March. 31, 2019. CY 2019
Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and
 35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
35 Adverse effects of anticancer drugs (Antimetabolites agents, Alkylating agents, Antimicrotubule agents, Miscellaneous agents, Immune therapies and Biologically directed therapies ) 1 1- Nausea and vomiting
Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A
 Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Supportive Care in Cancer Use of Prophylactic Growth Factors and Antimicrobials in Elderly Patients with Cancer: A Systematic Review of the Medicare Database Romina Sosa, Shuling Li, Julia T. Molony, Jiannong
Guidelines for the Use of Anti-Emetics with Chemotherapy
 Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Guidelines for the Use of Anti-Emetics with The purpose of this document is to provide guidance on the rational use of anti-emetics for prevention and treatment of chemotherapy-induced nausea and vomiting
Protocol Number Tumour Group Protocol Name on NCCP website 22/02/ Lung Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy
 Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Last Updated 22-Feb-18 Date of last update Protocol Number Tumour Group Protocol Name on NCCP website 22/02/2018 221 Afatinib Monotherapy 244 Gastrointestinal Regorafenib Monotherapy 249 Gynaecology Intrathecal
Release of the 2017/18 Invitation to Tender
 2 November 2017 Release of the 2017/18 Invitation to Tender The 2017/18 Invitation to Tender (2017/18 ITT) has been distributed today. If you have already registered your e-mail address with PHARMAC s
2 November 2017 Release of the 2017/18 Invitation to Tender The 2017/18 Invitation to Tender (2017/18 ITT) has been distributed today. If you have already registered your e-mail address with PHARMAC s
OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY)
 European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref. OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY) Table 1: Organisations
European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref. OVERVIEW OF COMMENTS RECEIVED ON LIST OF PAEDIATRIC NEEDS ONCOLOGY I (CYTOTOXIC THERAPY) Table 1: Organisations
1 The Cancer Programs Regulation (AR 242/98) is amended by this Regulation.
 Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
Alberta Regulation 18/2005 Cancer Programs Act AMENDMENT REGULATION Filed: February 22, 2005 For information only: Made by the Minister of Health and Wellness (M.O. 9/2005) on February 17, 2005 pursuant
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer
 APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
APPHON/ROPPHA Guideline for the Prevention and Management of Chemotherapy Induced Nausea and Vomiting in Children with Cancer 5850/5980 University Avenue, PO Box 9700, Halifax, N.S. B3K 6R8 PEDIATRIC HEMATOLOGY/ONCOLOGY
An introduction to the Essential Medicines concept: balancing innovation with public health priorities
 An introduction to the Essential Medicines concept: balancing innovation with public health priorities WHO HQ, Geneva 11 October 2017 Nicola Magrini Secretary, WHO Expert Committee on the Selection and
An introduction to the Essential Medicines concept: balancing innovation with public health priorities WHO HQ, Geneva 11 October 2017 Nicola Magrini Secretary, WHO Expert Committee on the Selection and
Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249
 Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Last updated Feb 9, 2018 Revision due Protocol Name on NCCP website Tumour Group Protocol Number Intrathecal Methotrexate for CNS 01/02/2018 Prophylaxis in GTN Gynaecology 249 Two Day Etoposide CISplatin
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Drug Name. Code Artesunate Injection 60 mg/ml Chloroquine Phosphate Injection 40 mg/ml
 Sr.No New Drug Code Drug Name 1 26.1.275.2 Artesunate Injection 60 mg/ml 2 26.1.277.2 Chloroquine Phosphate Injection 40 mg/ml 3 26.1.277.4 Chloroquine Phosphate Tablet 250 mg, equivalent to 155 mg chloroquine
Sr.No New Drug Code Drug Name 1 26.1.275.2 Artesunate Injection 60 mg/ml 2 26.1.277.2 Chloroquine Phosphate Injection 40 mg/ml 3 26.1.277.4 Chloroquine Phosphate Tablet 250 mg, equivalent to 155 mg chloroquine
Formulary Chemotherapy Agents: (Current as of 6/2018) Therapeutic Class
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY Cancer LAST REVIEW 9/11/18 THERAPEUTIC CLASS Oncology REVIEW HISTORY 5/17, 5/16 LOB AFFECTED Medi-Cal (MONTH/YEAR) This policy
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY Cancer LAST REVIEW 9/11/18 THERAPEUTIC CLASS Oncology REVIEW HISTORY 5/17, 5/16 LOB AFFECTED Medi-Cal (MONTH/YEAR) This policy
Description The following are synthetic cannabinoids requiring prior authorization: dronabinol (Marinol, Syndros ), nabilone (Cesamet )
 Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Clinical Policy: Nabilone (Cesamet), Dronabinol (Marinol, Syndros) Reference Number: CP.CPA.242 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important
Emetogenicity level 1. Emetogenicity level 2
 Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Emetogenicity level 1 15 mins Pre-Chemo Maxalon 10mg po During chemo and Post Chemo 3 days Maxalon10mg po 8 hourly Increase Maxalon 20mg po 8 hourly Change to Cyclizine 50mg po 8 hourly 3 days If nausea
Job title: Consultant Pharmacist/Advanced Practice Pharmacist
 Title : Guidelines for the Use of Antiemetics Purpose: To provide trust-wide guidance on the safe and effective use of antiemetics for the prevention and treatment of chemotherapy and radiotherapy induced
Title : Guidelines for the Use of Antiemetics Purpose: To provide trust-wide guidance on the safe and effective use of antiemetics for the prevention and treatment of chemotherapy and radiotherapy induced
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients
 Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic Induced Nausea and Vomiting Guideline Development Panel: L.
MABCAMPATH mg 3 INJ GENZYME BIOPHARMACEUTICALS. MABCAMPBATH mg 3 INJ GENZYME BIOPHARMACEUTICALS
 ICON Formulary - November 2016 Class Medicine Name Nappi Strength Form Size Route Manufacturer Abiraterone Acetate ZYTIGA 250 720690001 250 mg 120 ORAL JANSSEN PHARMACEUTICALS Aldesleukin CHIRON IL-2 18
ICON Formulary - November 2016 Class Medicine Name Nappi Strength Form Size Route Manufacturer Abiraterone Acetate ZYTIGA 250 720690001 250 mg 120 ORAL JANSSEN PHARMACEUTICALS Aldesleukin CHIRON IL-2 18
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting
 Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Prevention and Management of chemo-and radiotherapy-induced nausea and vomiting Focusing on the updated MASCC/ESMO guidelines Karin Jordan Department of Hematology and Oncology, University of Heidelberg
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care
 Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
Part B payment for drugs in Medicare: Phase 1 of CMS s proposed pilot and its impact on oncology care Raina H. Jain, Stephen M. Schleicher, Coral L. Atoria, Peter B. Bach Executive Summary The recent pilot
LIST OF DRUGS / MEDICINES ITEMS FOR THE YEAR (Non-Prequalified Items) A: Injection Antimicrobials Sr. No.
 LIST OF DRUGS / MEDICINES ITEMS FOR THE YEAR 2017-2018 (Non-Prequalified Items) A: Injection Antimicrobials Sr. 1 Amphotericin B vial of 50mg with wfi individually packed in carton with 2 Artemether 80mg/ml
LIST OF DRUGS / MEDICINES ITEMS FOR THE YEAR 2017-2018 (Non-Prequalified Items) A: Injection Antimicrobials Sr. 1 Amphotericin B vial of 50mg with wfi individually packed in carton with 2 Artemether 80mg/ml
List of Designated High-Cost Drugs
 List of Designated High-Cost Drugs UPDATED APRIL 25, 2018 For details on the High-Cost Drug policy, see Section 5.8 of the PharmaCare Policy Manual. Recent updates appear in red. Deletions are listed at
List of Designated High-Cost Drugs UPDATED APRIL 25, 2018 For details on the High-Cost Drug policy, see Section 5.8 of the PharmaCare Policy Manual. Recent updates appear in red. Deletions are listed at
DRUG PROPERTIES YOU NEED TO KNOW
 jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 8 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class b. resistance 2. Pharmacokinetics 3. Therapeutic uses 4. Major side effects/toxicities
jfitzake@d.umn.edu www.d.umn.edu/~jfitzake Page 1 of 8 DRUG PROPERTIES YOU NEED TO KNOW 1. Mechanism of action a. chemical class b. resistance 2. Pharmacokinetics 3. Therapeutic uses 4. Major side effects/toxicities
Hazardous Medication List
 Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
Hazardous Medication List REDUCING OCCUPATIONAL EXPOSURE TO HAZARDOUS MEDICATION FOR ALL STAFF Table of Contents Hazardous Medication List Key Points... 1 Hazardous Medication List... 2 Special Handling
Drug Name Tier Drug Name Tier
 Drug Name Tier Drug Name Tier ABELCET 100 MG/20 ML VIAL 4 ACETYLCYSTEINE 10% VIAL 2 ACETYLCYSTEINE 20% VIAL 2 ACYCLOVIR 1,000 MG/20 ML VIAL 2 ACYCLOVIR 500 MG/10 ML VIAL 2 ADRUCIL 500 MG/10 ML VIAL 2 ALBUTEROL
Drug Name Tier Drug Name Tier ABELCET 100 MG/20 ML VIAL 4 ACETYLCYSTEINE 10% VIAL 2 ACETYLCYSTEINE 20% VIAL 2 ACYCLOVIR 1,000 MG/20 ML VIAL 2 ACYCLOVIR 500 MG/10 ML VIAL 2 ADRUCIL 500 MG/10 ML VIAL 2 ALBUTEROL
Part B payment for drugs in Medicare 0
 Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Part B payment for drugs in Medicare 0 Introduction: The recent pilot proposal 1 on Part B drug payment from the Center for Medicare and Medicaid Innovation of CMS has met strong resistance 2 from the
Adult Intravenous Systemic Anticancer Therapy (SACT) Section A. SUMMARY of SCHEME QIPP Reference
 CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
CA2 Nationally standardised Dose banding for Adult Intravenous Anticancer Therapy (SACT) Scheme Name CA2: Nationally Standardised Dose Banding for Adult Intravenous Systemic Anticancer Therapy (SACT) Section
VMG Pharmaceuticals Pvt. Ltd.
 S. No. Product Name S trength Dos age form Oncology 1 A nastrazole 1 mg Tablets 2 Bicalutamide 50 mg Tablets 3 Bleomycin 15units,30units Injection 4 Bortezomib 3.5 mg Injection 5 Capecitabine 500 mg Tablets
S. No. Product Name S trength Dos age form Oncology 1 A nastrazole 1 mg Tablets 2 Bicalutamide 50 mg Tablets 3 Bleomycin 15units,30units Injection 4 Bortezomib 3.5 mg Injection 5 Capecitabine 500 mg Tablets
1. Purpose Documentation Description.1 4. References 8 5. Cross-References.8 6. Development of the guideline.8
 CLINICAL GUIDELINE For use in (clinical areas): For use by (staff groups): For use for (patients): Document owner: Status: Oncology/Haematology Unit (excluding Paediatrics) Oncologists, Haematologists,
CLINICAL GUIDELINE For use in (clinical areas): For use by (staff groups): For use for (patients): Document owner: Status: Oncology/Haematology Unit (excluding Paediatrics) Oncologists, Haematologists,
COMMERCIAL APIs. S. No Molecule Name Therapeutic Category USDMF EDMF CEP IH
 1 Abacavir Sulphate Antiretroviral - * - 2 Abiraterone Acetate Oncology - - - 3 Albendazole Anti-infective - - - 4 Albuterol Sulphate Respiratory - - 5 Alendronate Sodium Trihydrate Metabolic Disorder
1 Abacavir Sulphate Antiretroviral - * - 2 Abiraterone Acetate Oncology - - - 3 Albendazole Anti-infective - - - 4 Albuterol Sulphate Respiratory - - 5 Alendronate Sodium Trihydrate Metabolic Disorder
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Reference Number: CP.CPA.223 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important regulatory
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Etiologies:
 VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Acute Nausea and Vomiting (N&V) Incidence: The incidence of acute and delayed N&V was investigated in highly and moderately emetogenic
HCPCS Code/ generic (Brand) Name J7506. J8520 capecitabine (Xeloda) 1. J8521 capecitabine. J8530 cyclophosphamide. (Cytoxan) 1
 drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
drug and administration compendia Current Price () J7506 Prednisone, oral, per 5 mg 12/1/07 $0.07 $0.19 N/A prednisone 2 J8520 capecitabine (Xeloda) 1 Capecitabine, oral, 150 mg 8/1/07 $5.79 $4.59 N/A
Working Formulary January 2013 Oncology Chemotherapy Regimens
 Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
Working Formulary January 2013 Oncology Chemotherapy Regimens In the currently changing commissioning landscape, this document is intended to represent the up to date list of non clinical trial chemotherapy
LIST OF DRUGS THAT MAY BE COVERED UNDER YOUR MEDICAL BENEFIT
 LIST OF DRUGS THAT MAY BE COVERED UNDER YOUR MEDICAL BENEFIT The following medications may be covered under your medical benefit if they are provided to you in your doctor s office or outpatient infusion
LIST OF DRUGS THAT MAY BE COVERED UNDER YOUR MEDICAL BENEFIT The following medications may be covered under your medical benefit if they are provided to you in your doctor s office or outpatient infusion
Managements of Chemotherpay Induded Nausea and Vomiting
 REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
REVIEW ARTICLE Managements of Chemotherpay Induded Nausea and Vomiting Department of Surgery, The Catholic University of Korea Sung Geun Kim 23 24 Sung Geun Kim Korean Journal of Clinical Oncology Summer
Product Name Strength Dosage Form Therapeutic Area Dossier Status. Aceclofenac 100mg Film coated Tablets Analgesia-inflammation Approved
 Product Name Strength Dosage Form Therapeutic Area Dossier Status Aceclofenac 100mg Film coated Tablets Analgesia-inflammation Approved Alendronate Sodium 10/70mg Tablets Osteoporosis Approved Anastrozole
Product Name Strength Dosage Form Therapeutic Area Dossier Status Aceclofenac 100mg Film coated Tablets Analgesia-inflammation Approved Alendronate Sodium 10/70mg Tablets Osteoporosis Approved Anastrozole
The AngCN Antiemetic Guidelines
 The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
The AngCN Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy AngCN Document Reference: AngCN-CCG-C31 CONTENTS 1.0 Introduction
Supplementary Figures
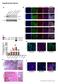 Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
Supplementary Figures Supplementary Figure 1. Characterization of the generated hipsc lines a) Western blot analysis indicating the efficient downregulation of p53 upon knockdown in three hipsc lines (
UP Health System Marquette Medication Guideline High Alert Drugs
 1 ALTEPLASE SOLR 100 MG ACTIVASE 305010 50242008527 TRUE ALTEPLASE SOLR 50 MG ACTIVASE 305008 50242004413 TRUE AMIODARONE INJ 50 MG/ML CORDARONE IV 305035 63323061609 TRUE AMIODARONE INJ 50 MG/ML CORDARONE
1 ALTEPLASE SOLR 100 MG ACTIVASE 305010 50242008527 TRUE ALTEPLASE SOLR 50 MG ACTIVASE 305008 50242004413 TRUE AMIODARONE INJ 50 MG/ML CORDARONE IV 305035 63323061609 TRUE AMIODARONE INJ 50 MG/ML CORDARONE
PHARMACY PEARLS CHANIN WRIGHT, PHARMD
 PHARMACY PEARLS CHANIN WRIGHT, PHARMD OBJECTIVES From the pearls heard, discuss with someone near you What is something you can apply to your setting Traumatic brain injury in children Goals Correct and
PHARMACY PEARLS CHANIN WRIGHT, PHARMD OBJECTIVES From the pearls heard, discuss with someone near you What is something you can apply to your setting Traumatic brain injury in children Goals Correct and
DRUGS YOU NEED TO KNOW
 jfitzake@d.umn.edu Page 1 of 7 DRUGS YOU NEED TO KNOW ALKYLATING AGENTS (blue cards) BUSULFAN CARMUSTINE (BCNU) CYCLOPHOSPHAMIDE DACARBAZINE LOMUSTINE (CCNU) MECHLORETHAMINE MELPHALAN THIOTEPA NATURAL
jfitzake@d.umn.edu Page 1 of 7 DRUGS YOU NEED TO KNOW ALKYLATING AGENTS (blue cards) BUSULFAN CARMUSTINE (BCNU) CYCLOPHOSPHAMIDE DACARBAZINE LOMUSTINE (CCNU) MECHLORETHAMINE MELPHALAN THIOTEPA NATURAL
An assessment of the price of oncology drugs in Ukraine 2015
 An assessment of the price of oncology drugs in Ukraine 2015 Prashant Yadav University of Michigan October 2016 The views and opinions expressed in this report are those of the author and should not be
An assessment of the price of oncology drugs in Ukraine 2015 Prashant Yadav University of Michigan October 2016 The views and opinions expressed in this report are those of the author and should not be
ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER
 European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref.: EMEA/384641/2006 ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER The Paediatric
European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref.: EMEA/384641/2006 ASSESSMENT OF THE PAEDIATRIC NEEDS CHEMOTHERAPY PRODUCTS (PART I) DISCLAIMER The Paediatric
8. Malignant disease and immunosuppression
 1 8. Malignant disease and immunosuppression An ever increasing number of cytotoxic drugs and biological therapies - now referred to as Systemic anticancer therapy (SACT) - are used in the management of
1 8. Malignant disease and immunosuppression An ever increasing number of cytotoxic drugs and biological therapies - now referred to as Systemic anticancer therapy (SACT) - are used in the management of
Guideline Update on Antiemetics
 Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Guideline Update on Antiemetics Clinical Practice Guideline Special Announcements Please check www.asco.org/guidelines/antiemetics for current FDA alert(s) and safety announcement(s) on antiemetics 2 Introduction
Committee Approval Date: December 12, 2014 Next Review Date: July 2015
 Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
Medication Policy Manual Policy No: dru378 Topic: Akynzeo, netupitant/palonosetron Date of Origin: December 12, 2014 Committee Approval Date: December 12, 2014 Next Review Date: July 2015 Effective Date:
List of changes in Out-Patients Formulary
 List of changes in Out-Patients Formulary Change in prescriber criteria Alpha Tocopheryl (Vitamin E) suspension 100mg/mL, tablets 50-150mg, tablets 670mg Atorvastatin tablets Bezafibrate tablets 400mg
List of changes in Out-Patients Formulary Change in prescriber criteria Alpha Tocopheryl (Vitamin E) suspension 100mg/mL, tablets 50-150mg, tablets 670mg Atorvastatin tablets Bezafibrate tablets 400mg
Subject: Palonosetron Hydrochloride (Aloxi )
 09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J0000-87 Original Effective Date: 02/15/09 Reviewed: 07/09/14 Revised: 03/15/18 Subject: Palonosetron Hydrochloride (Aloxi ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
Acute Lymphocytic Leukemia
 Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
Acute Lymphocytic Leukemia Splenectomy (Removal of Spleen) 6-Mercaptopurine (Purinethol, 6-MP) Alemtuzumab (Campath ) Arsenic Trioxide (Trisenox ) Bendamustine (Treanda ) Bexarotene (Targretin ) Bleomycin
MDwise Self-Administered Codes for Medical
 The following codes are associated with medications that can be self-administered by the patient or a caregiver. As a result, MDwise will transfer coverage of these self-administered medications exclusively
The following codes are associated with medications that can be self-administered by the patient or a caregiver. As a result, MDwise will transfer coverage of these self-administered medications exclusively
AD regimen, 576 adrenal steroid inhibitor. aminoglutethimide, 23 25
 A 640 Index Index abiraterone acetate, 5 7 Abraxane. See albuminbound paclitaxel ABVD regimen, 57, 540 ABV regimen, 519 520 AC docetaxel regimen, 476, 478 acetaminophen busulfan and, 71 AC regimen, 475,
A 640 Index Index abiraterone acetate, 5 7 Abraxane. See albuminbound paclitaxel ABVD regimen, 57, 540 ABV regimen, 519 520 AC docetaxel regimen, 476, 478 acetaminophen busulfan and, 71 AC regimen, 475,
1 ( concentrated electrolytes ) ก
 ก ก HAD 1 ( concentrated electrolytes ) ก ก ก ( ) ก ก ก ก 2 ก ก ก (Patient care unit) ก ก ก ก ( Hemodialysis ) ก ก ก ก ก ก stock card ก ก ( High Alert Drug (HAD)) ก ก ก ก (Pharmacy and Therapeutic Committee
ก ก HAD 1 ( concentrated electrolytes ) ก ก ก ( ) ก ก ก ก 2 ก ก ก (Patient care unit) ก ก ก ก ( Hemodialysis ) ก ก ก ก ก ก stock card ก ก ( High Alert Drug (HAD)) ก ก ก ก (Pharmacy and Therapeutic Committee
NICE TA Adherence Check List
 NICE TA Adherence Check List KEY NICE TA PAS Not Terminated Indication Technology Appraisal carried out by the National Institute of Clinical Excellence - It is the process by which new and existing drugs
NICE TA Adherence Check List KEY NICE TA PAS Not Terminated Indication Technology Appraisal carried out by the National Institute of Clinical Excellence - It is the process by which new and existing drugs
Blue Cross and Blue Shield of Minnesota GenRx Formulary Updates
 Blue Cross and Blue Shield of Minnesota GenRx Formulary Updates July 2018 TRADE NAME (generic name) or generic name ADVAIR DISKUS (fluticasone-salmeterol aer powder ba 100-50 mcg/dose) Brand Addition ADVAIR
Blue Cross and Blue Shield of Minnesota GenRx Formulary Updates July 2018 TRADE NAME (generic name) or generic name ADVAIR DISKUS (fluticasone-salmeterol aer powder ba 100-50 mcg/dose) Brand Addition ADVAIR
8. Malignant disease and immunosuppression
 1 8. Malignant disease and immunosuppression An ever increasing number of cytotoxic drugs and biological therapies - now referred to as Systemic anticancer therapy (SACT) - are used in the management of
1 8. Malignant disease and immunosuppression An ever increasing number of cytotoxic drugs and biological therapies - now referred to as Systemic anticancer therapy (SACT) - are used in the management of
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Objectives
 Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Current and Emerging Therapeutic Options in the Management of Chemotherapy-Induced Nausea and Vomiting (CINV) Susan Urba, M.D. University of Michigan Comprehensive Cancer Center Objectives Mechanisms of
Miracalus Pharma Pvt Limited
 Dealers in Vaccines, TPN, Blood products and All Anti-Cancer Drugs Miracalus Pharma Pvt Limited ANTI CANCER - Injections ING CISPLATIN 10MG CISTEEN 1 VIAL ING CISPLATIN 50MG CISTEEN 1 VIAL INJ 5 FLURO
Dealers in Vaccines, TPN, Blood products and All Anti-Cancer Drugs Miracalus Pharma Pvt Limited ANTI CANCER - Injections ING CISPLATIN 10MG CISTEEN 1 VIAL ING CISPLATIN 50MG CISTEEN 1 VIAL INJ 5 FLURO
Clinical Tools and Resources for Self-Study and Patient Education
 Clinical Tools and Resources for Self-Study and Patient Education CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING CLINICIAN'S RESOURCE GUIDE The clinical tools and resources contained herein are provided as educational
Clinical Tools and Resources for Self-Study and Patient Education CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING CLINICIAN'S RESOURCE GUIDE The clinical tools and resources contained herein are provided as educational
CANCER PREVENTION AND ACCESS TO MEDICINES. Gracemarie Bricalli ESMO Head of International Affairs
 CANCER PREVENTION AND ACCESS TO MEDICINES Gracemarie Bricalli ESMO Head of International Affairs ESMO s 2020 Vision: Access to cancer care ESMO s 2020 vision statement recognises that progress in the management
CANCER PREVENTION AND ACCESS TO MEDICINES Gracemarie Bricalli ESMO Head of International Affairs ESMO s 2020 Vision: Access to cancer care ESMO s 2020 vision statement recognises that progress in the management
Antiemetic Guidelines. Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy
 Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy For approvals and version control see Document Management Record
Antiemetic Guidelines Guidelines for the Management of Nausea and Vomiting in Adult Patients Receiving Chemotherapy and/or Radiotherapy For approvals and version control see Document Management Record
Systemic Anti-cancer Therapy Care Pathway Guidelines for the management of SACT induced nausea and vomiting in adult patients
 Systemic Anti-cancer Therapy Care Pathway Guidelines for the management of SACT induced nausea and vomiting in adult patients Pathway of Care Kent & Medway Cancer Collaborative Publication date June 2018
Systemic Anti-cancer Therapy Care Pathway Guidelines for the management of SACT induced nausea and vomiting in adult patients Pathway of Care Kent & Medway Cancer Collaborative Publication date June 2018
Northern Cancer Alliance
 Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Adult Oncology & Haematology Document Control Document Title: Antiemetic Guidelines for CINV NESCN v2.2
Northern Cancer Alliance Anti-emetic Guidelines for Chemotherapy Induced Nausea and Vomiting (CINV) Adult Oncology & Haematology Document Control Document Title: Antiemetic Guidelines for CINV NESCN v2.2
Drug Use Evaluation: Physician Administered Drugs (PADs)
 Drug Use Research & Management Program OHA Division of Medical Assistance Programs 500 Summer Street NE, E35; Salem, OR 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Drug Use Evaluation: Physician Administered
Drug Use Research & Management Program OHA Division of Medical Assistance Programs 500 Summer Street NE, E35; Salem, OR 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Drug Use Evaluation: Physician Administered
Medication Review. Cancer Chemotherapy Drugs. Pharmacy Technician Training Systems Passassured, LLC
 Medication Review Cancer Chemotherapy Drugs Pharmacy Technician Training Systems Passassured, LLC Medication Review, Cancer Chemotherapy Drugs PassAssured's Pharmacy Technician Training Program Medication
Medication Review Cancer Chemotherapy Drugs Pharmacy Technician Training Systems Passassured, LLC Medication Review, Cancer Chemotherapy Drugs PassAssured's Pharmacy Technician Training Program Medication
To help doctors give their patients the best possible care, the American
 Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
Patient Information Resources from ASCO What to Know ASCO s Guideline on Preventing Vomiting Caused by Cancer Treatment SEPTEMBER 2011 KEY MESSAGES The risk of nausea and vomiting depends on the specific
Trust Guideline for Prevention and Control of Chemotherapy and Radiotherapy Induced Nausea and Vomiting in Adults
 A Clinical Guideline For Use in: Organisation-wide By: For: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author s Line Manager:
A Clinical Guideline For Use in: Organisation-wide By: For: Key words: Name of document author: Job title of document author: Name of document author s Line Manager: Job title of author s Line Manager:
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
Clinical Policy: (Zofran, Zuplenz) Reference Number: CP.CPA.173 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this
Guidelines for the Management of Extravasation (Version 5 May 2012)
 Guidelines for the Management of Extravasation (Version 5 May 2012) Quality and safety for every patient every time Document Control Prepared By Chemo Nurse Group Chemo Nurse Group/ South Tees FT NECDAG
Guidelines for the Management of Extravasation (Version 5 May 2012) Quality and safety for every patient every time Document Control Prepared By Chemo Nurse Group Chemo Nurse Group/ South Tees FT NECDAG
Vasco OP Underglove Sterile Surgical and Protective Gloves
 data sheet EN 374:2003 EN 374:2003 AQL 0.65 B. Braun Melsungen AG confirms that Vasco OP Underglove gloves comply with the following standards and directives: EC CERTIFICATES AND Applied standards Medical
data sheet EN 374:2003 EN 374:2003 AQL 0.65 B. Braun Melsungen AG confirms that Vasco OP Underglove gloves comply with the following standards and directives: EC CERTIFICATES AND Applied standards Medical
Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines
 North of England Cancer Network Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines Adult Oncology & Haematology Quality and safety for every patient every time For more information
North of England Cancer Network Chemotherapy Induced Nausea and Vomiting (CINV) Anti-emetic Guidelines Adult Oncology & Haematology Quality and safety for every patient every time For more information
Subject: Fosnetupitant-Palonosetron (Akynzeo) IV
 09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
09-J3000-01 Original Effective Date: 06/15/18 Reviewed: 05/09/18 Revised: 01/01/19 Subject: Fosnetupitant-Palonosetron (Akynzeo) IV THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION,
Title Developed By. Implementation Contact person(s) Review Date Group Responsible. NICaN Policy: Management of Chemotherapy Extravasation; Version 3
 Title Developed By Management of Chemotherapy Extravasation NICaN Regional Pharmacy Group Version History Version 3: 2009 (See appendix 6) Version 2: January 2006 Version 1: June 2005 Consultation Period
Title Developed By Management of Chemotherapy Extravasation NICaN Regional Pharmacy Group Version History Version 3: 2009 (See appendix 6) Version 2: January 2006 Version 1: June 2005 Consultation Period
Standard Regimens for Haematology
 Regimens for Haematology ChlVPP Chlorambucil 6mg/m 2 PO D1 to 14 Vinblastine 6mg/m 2 (max 10mg) IV on D1 & 8 Procarbazine 100mg/m 2 PO on D1 to 14 Prednisolone 40mg PO D1 to 14 ABVD Doxorubicin 25mg/m
Regimens for Haematology ChlVPP Chlorambucil 6mg/m 2 PO D1 to 14 Vinblastine 6mg/m 2 (max 10mg) IV on D1 & 8 Procarbazine 100mg/m 2 PO on D1 to 14 Prednisolone 40mg PO D1 to 14 ABVD Doxorubicin 25mg/m
Vasco OP Free Sterile Surgical and Protective Gloves
 DATA SheeT EN 374:2003 EN 374:2003 AQl 0.65 B. Braun Melsungen AG confirms that Vasco OP Free gloves comply with the following standards and directives: ec certificates AnD APPlieD STAnDArDS Medical device
DATA SheeT EN 374:2003 EN 374:2003 AQl 0.65 B. Braun Melsungen AG confirms that Vasco OP Free gloves comply with the following standards and directives: ec certificates AnD APPlieD STAnDArDS Medical device
Cytostatics Definition, Terminology
 Dept. of Pharmacology, Faculty of Medicine, Masaryk University in Brno Cytostatics Notes for Pharmacology II Practicals This study material is exclusively for students of general medicine and dentistry
Dept. of Pharmacology, Faculty of Medicine, Masaryk University in Brno Cytostatics Notes for Pharmacology II Practicals This study material is exclusively for students of general medicine and dentistry
ESCMID Online Lecture Library. by author
 Hepatitis B virus and solid organ transplantation Prof. Hakan Leblebicioglu Department of Clinical Microbiology and Infectious Diseases Ondokuz Mayis University, Samsun, Turkey Conflict of interest Outline
Hepatitis B virus and solid organ transplantation Prof. Hakan Leblebicioglu Department of Clinical Microbiology and Infectious Diseases Ondokuz Mayis University, Samsun, Turkey Conflict of interest Outline
