BRAINSTEM REGIONS WITH NEURONAL ACTIVITY PATTERNS CORRELATED WITH PRIMING OF LOCOMOTOR STEPPING IN THE ANESTHETIZED RAT
|
|
|
- Dominic Henderson
- 6 years ago
- Views:
Transcription
1 Pergamon Priming of locomotion Neuroscience Vol. 99, No. 1, pp. 77±91, q 2000 IBRO. Published by Elsevier Science Ltd Printed in Great Britain. All rights reserved PII: S (00) /00 $ BRAINSTEM REGIONS WITH NEURONAL ACTIVITY PATTERNS CORRELATED WITH PRIMING OF LOCOMOTOR STEPPING IN THE ANESTHETIZED RAT H. M. SINNAMON,* A. K. JASSEN and L. A. VITA Neuroscience & Behavior Program, Wesleyan University, Middletown, CT , USA AbstractÐLocomotor stimulation in the perifornical hypothalamus produces a transient facilitation of subsequent locomotion, a priming effect, such that stepping to a second train of stimulation occurs with a shorter latency of onset and increased amplitude. Neurons responsible for the initiation of this facilitated stepping presumably respond to locomotor stimulation with a similar priming effect, i.e. either a shorter latency or a larger change in activity rate. This study used anesthetized rats (urethane, 800 mg/ kg) to compare brainstem regions in terms of the relative rates of occurrence of single neurons that showed both speci c responses to locomotor stimulation and also priming effects. Speci c responses were characterized by a progressive increase in activity prior to the rst step (a Type I pattern). In that they co-varied in time with the increased probability of stepping onset, Type I responses were more speci c than Type II responses, which peaked early in the stimulation train several seconds before the onset of stepping. Regions with high proportions of neurons showing Type I responses and priming effects included the anterior dorsal tegmentum lateral to the central gray, the oral pontine reticular nucleus and the medial gigantocellular nucleus. Few Type I neurons showed a modulation of activity related to the step cycle. Type I primed neurons were uncommon in the cuneiform and the pedunculopontine regions, but neurons showing other patterns (decreases and antidromic responses) were relatively prevalent there. The ventral tegmental area was generally unresponsive. The results indicate that stepping elicited by perifornical stimulation in the anesthetized rat is mediated by circuits that differ at midbrain levels from the circuits implicated in other types of locomotion. Two regions, the anterior dorsal tegmentum and the oral pontine reticular nucleus, warrant further attention to determine their possible roles in the initiation of locomotion. q 2000 IBRO. Published by Elsevier Science Ltd. All rights reserved. Key words: movement initiation, midbrain, pons, medulla, hypothalamus. In the locomotor preparation developed by Shik et al., 71 electrical stimulation is applied to the cuneiform region of midbrain-transected cats to produce controlled locomotion. This model has important heuristic value in providing a method for the study of spinal and brainstem circuitry for stepping control. 16,35,55,59±61,65,68,70,72,91 It has also been in uential in the development of a conceptual framework for studying the circuitry involved in the initiation of locomotion. It has led to an emphasis on the midbrain locomotor area that includes the nucleus cuneiformis and the pedunculopontine area (here termed the peripeduncular/ midbrain locomotor area, PPA/MLA). This region is thought to provide a locomotor initiation signal to the medullary reticulospinal systems that in turn activate and modulate the spinal stepping generators. 12,22,24,25,27±29,35,54,68,91 Studies of midbrain locomotor mechanisms in the awake behaving rat have primarily concerned the control of open eld/exploratory locomotion by the limbic system. 9,33,49,86 This work indicates that the ventral tegmental area provides a locomotor facilitating signal to circuits involving the hippocampus, accumbens and ventral pallidum. 36,51 Some evidence indicates that the basal forebrain structures in turn provide a locomotor initiation signal to the PPA/MLA implicated by the work in the decerebrated cat. 7,9,47,48,50 However, other evidence indicates that additional brainstem regions can mediate this type of locomotion. 34,57,86 *To whom correspondence should be addressed. Tel.: ; fax: address: hsinnamon@wesleyan.edu (H. M. Sinnamon). Abbreviations: ADT, anterior dorsal tegmental region; CG, central gray; Gi, gigantocellular nucleus; PnC, caudal pontine reticular nucleus; PnO, oral pontine reticular nucleus; PPA/MLA, peripeduncular area/midbrain locomotor area. The anesthetized intact rat in which stepping is elicited by stimulation of the perifornical hypothalamus provides an alternative model for the study of midbrain locomotor circuitry. Consistent with early evidence for the independence of the ªsubthalamicº and ªmidbrainº locomotor regions in the cat, 58 elicited stepping in the anesthetized rat persists after electrolytic lesions or procaine inactivation of the cuneiformis region. 44,84 Electrolytic lesions and procaine inactivation of the ventral tegmental area block hypothalamic-elicited stepping, 44,82 but descending bers of passage from the hypothalamus appear to be involved here because injections of GABA, which operates on postsynaptic targets in this region, are not effective. 78 Neither the PPA/MLA nor the ventral tegmental area was found to have high occurrences of neuronal responses to hypothalamic locomotor stimulation. 18 By contrast, other midbrain regions have been implicated in hypothalamicelicited locomotion. In both the oral pontine reticular nucleus (PnO) and the anterior dorsal tegmentum (ADT), injections of both GABA 78 and procaine 44 block hypothalamic-induced stepping. Both regions show a relatively high occurrence of neurons with responses correlated with the onset of stepping elicited by hypothalamic stimulation. 18 In addition, GABA injections in the region of the median raphe have been shown to facilitate stepping in the anesthetized rat, 80 indicating that cells in this region have a locomotor suppressive function. 31,92 In summary, the picture of midbrain locomotor circuits derived from the anesthetized intact rat may differ signi cantly from that derived from work on either the midbrain-transected cat or the awake behaving rat. These differences may relate to the type of motivational systems activated in the different preparations. 76 This study surveyed brainstem regions for single neuron responses related to locomotor initiation in the anesthetized 77
2 78 H. M. Sinnamon et al. rat. The speci c goal was to comprehensively compare structures implicated in stepping of the anesthetized rat to structures implicated in stepping of other preparations. The focus of this study was the Type I pattern of response to locomotor stimulation found in a previous study. 18 In Type I responses, activity progressively increases to the onset of stepping. By contrast, in Type II responses, the activity peaks early during the train of locomotor stimulation, several seconds before the onset of stepping. Type I patterns, as a class, are more speci c than Type II patterns in that they more closely co-vary in time with the increased probability of stepping. Compared to the previous study, the approach used here was re ned in three ways. First, it increased both the range of regions studied and the number of neurons sampled in each region. In addition to the regions described above (the PPA/MLA, the ventral tegmental area, the ADT and the raphe region), other brainstem regions of locomotor signi cance were sampled. Second, neuronal activity was examined for modulation with the phase of the step cycle. Activity related to stepping, while not an essential characteristic of a neuron related to stepping initiation, should be found in neurons involved in control of stepping execution. Third and most important, conjunctive criteria were used to identify neurons possibly related to stepping initiation. To the Type I property was added a property related to the excitability of the locomotor circuitry, the priming effect. Priming of locomotor stimulation provides a means of modulating the excitability of locomotor initiation. In this method, 77,80,81,87 a pair of stimulation trains, Control and Test, separated by 20 s or less, is presented on each trial. Locomotor stimulation produces a residual excitation, a priming effect, such that the stepping occurs at a shorter latency or its amplitude is larger on the Test compared to the Control. A neuron with a response to locomotor stimulation that is earlier or larger on the Test than on the Control shows a priming effect. If a Type I response pattern were actually speci cally related to stepping onset, it would also have to show a priming effect. Although the set of Type I primed neurons may additionally include neurons not speci cally related to locomotion, it must include neurons that are responsible for stepping initiation. Any region showing a low occurrence of Type I primed neurons would have a lower likelihood of a role in the initiation of the elicited stepping compared to a region with a high rate of occurrence. EXPERIMENTAL PROCEDURES Subjects and surgery Male Sprague±Dawley rats (n ˆ 227), obtained from the Charles River Company and bred at Wesleyan University, weighing a mean of 367 g (S.D. 62 g) were used. Surgical and experimental procedures were designed to avoid discomfort of the animals, and were approved by the Wesleyan Institutional Animal Care and Use Committee. For initial anesthesia, the animal was placed in a small chamber, exposed to 2% halothane in oxygen for 5±10 min and given an intraperitoneal injection of either nembutal (25 mg/kg) or urethane (500 mg/kg). After an additional 5±10 min in the halothane chamber, the anesthetized rat was placed in a stereotaxic apparatus. The combination of halothane and nembutal or urethane at these doses maintained the rat at a surgical level of anesthesia for the 30±45 min required for the invasive procedures. A scalp incision was made and lidocaine was injected around its margins. Holes were drilled in the skull for the stimulation and recording electrodes, and for screws which served as connections in the ground and stimulation circuits. Following surgery, the rat remained in the stereotaxic apparatus and was maintained at the appropriate level of anesthesia by intraperitoneal injections of nembutal or urethane. Maintenance of anesthesia by continuous administration of halothane appears to be incompatible with the elicitation of stepping in this preparation. Injections were given whenever the rat showed increased respiration, increased vibrissae movement or any limb movement in the absence of brain stimulation. Earlier experiments were performed under nembutal (n ˆ 70), but when it was learned that apparently identical behavioral and neuronal responses were elicited under the more stable anesthesia afforded by urethane, the remainder of the experiments (n ˆ 157) used this. With nembutal, injections of 3.5±7.0 mg/kg were given at approximately 25±30-min intervals, or more frequently if needed throughout the course of the experiment. With urethane, the appropriate anesthetic level was achieved by additional injections of 50±100 mg/kg urethane over approximately the rst hour of the experiment. The average cumulative dose of urethane administered was 800 mg/kg. When the appropriate urethane level was reached, the rat would maintain an anesthetized state for several hours without a supplemental injection. Lidocaine injections were made into the incision at 3-h intervals. The level of anesthesia induced was deeper than that required to maintain the rat in a quiescent state, but not so deep that a exor response of the hindlimb to a strong pinch was abolished. Body temperature during surgery was maintained by means of a heat lamp. During testing, when stepping episodes were periodically elicited, the rat effectively autoregulated its temperature. CI CIC CnF DA Dk DLT DM DpGi DpMe DR DTg EICC f Gem GiA GiV IMLF InCo LH LPB MCPC Abbreviations used in the gures caudal interstitial nucleus of the medial longitudinal mfb medial forebrain bundle fasciculus ml medial lemniscus central nucleus of the inferior colliculus MM medial mammillary nucleus cuneiform nucleus MR median raphe nucleus dorsal hypothalamic area Mo5 motor trigeminal nucleus nucleus of Darkschewitsch Mt mammillothalamic tract dorsolateral tegmental region Mve medial vestibular nucleus dorsomedial hypothalamic nucleus pc posterior commissure dorsal paragigantocellular nucleus Pcom nucleus of the posterior commissure deep mesencephalic nucleus PeF perifornical nucleus dorsal raphe nucleus PH posterior hypothalamic area dorsal tegmental pontine nucleus PnV ventral pontine reticular nucleus inferior colliculus, external central PPTg pedunculopontine tegmental nucleus fornix PR prerubral area nucleus gemini R red nucleus gigantocellular nucleus, anterior Rli rostral linear nucleus gigantocellular nucleus, ventral RPC red nucleus, parvocellular interstitial nucleus of the medial longitudinal scp superior cerebellar peduncle fasciculus Sol solitary tract nucleus intercollicular nucleus SPTg subpeduncular tegmental nucleus lateral hypothalamic area SuM supramammillary nucleus lateral parabrachial nucleus VM ventromedial nucleus of the hypothalamus magnocellular nucleus of the posterior commissure VTA ventral tegmental area
3 Priming of locomotion 79 Stimulation Brain stimulation, provided by a constant-current stimulator, was composed of 50-Hz biphasic pulses, each phase 0.5 ms, delivered in trains of 5 s. The stimulation electrode was a 125-mm-diameter tungsten wire insulated with Te on except at the tip. An uninsulated wire wrapped around screws in the skull completed the circuit to the stimulator. Measure of hindlimb stepping Descriptions and representative records of hindlimb stepping elicited by stimulation of the hypothalamus in this preparation have been given in previous reports. 76±78,80 The head of the rat was secured in the stereotaxic apparatus and the body was supported by an acrylic platform attached to the side rails. Below the platform was a wheel with a diameter of 30 cm and a surface width of 10 cm. At rest, the rat's hindlimbs hung in passive extension to make contact with the wheel. When the rat stepped, the extensor phase of the hindlimb movement caused the digits to engage the mesh surface of the wheel and rotated it. Moderate currents were selected to elicit stepping on the Control train at a latency of 3 s or more, and a priming effect on the Test train. At these currents, alternating steps of the hindlimbs, without stepping of the forelimbs, are elicited. In general, the two hindlimbs move to a similar extent, but it was common for the limb contralateral to the stimulation site to show a larger displacement amplitude. Higher currents, which elicited galloping gaits (synchronous exion and extension of the two hindlimbs), were avoided in order to maximize the priming effect and reduce body movements that caused loss of single-unit isolation. Hindlimb stepping movements were transduced by a pair of accelerometers, one attached to each metatarsus. The ampli ed output of the accelerometers was led to an analog/digital converter sampling at 1 khz and displayed on a computer monitor. Recording of neuronal activity Single-unit activity was recorded differentially through a pair of 25-mm-diameter stainless steel Te on-insulated wires cut to expose the cross-section of the tips. The wires protruded approximately 1 mm beyond the tip of a glass pipette, which was sealed by paraf n. Occasional dif culty in isolating single units with these large electrodes was offset by the bene t of their matched low impedance (,1MV) in reducing stimulation artifact. Typically, the amplitude of single units was 75±300 mv. Conventional ampli cation, ltering and spike discrimination procedures were used. The discriminator output (1.2-ms pulses) was suppressed for a variable amount of time with each stimulator pulse (1±3 ms) to prevent spurious discriminator pulses. Only well-isolated, stable single units were included in the analysis. There were two principal indications that axon spikes did not signi cantly contribute to the data pool. First, in recording tracks through ber bundles where somata are rare, the low-impedance electrodes that were used recorded virtually no isolated spikes. Second, the testing protocol required maintenance of isolation for several minutes during stepping and associated autonomic responses. Axon spikes show radical changes in amplitude or are easily lost under these conditions. The effectiveness of the single-unit discrimination during data acquisition was checked by monitoring the stability of the amplitude and waveform of the action potential, and the absence of action potentials during the refractory period. Later experiments occasionally used a customized, off-line, spike-sorting program to separate units that had similar amplitudes of the principal peak but different waveforms. The algorithms differentiated waveforms on the basis of the timing and amplitudes of the peaks that preceded or followed the principal peak. For analysis of unit responses, the discriminator pulses were passed to a computer-based A/D system operating at a sampling rate of 1 khz per channel and converted to a ratemeter display with a time bin of 20 ms. The ratemeter data were transformed to a spike density record by computing running averages over 100-ms intervals. The spike density record, the accelerometer signals and stimulation marker were stored for later analysis. Waveforms of the action potential were sampled at 25 khz. Locomotor stimulation The stimulation electrode was targeted at the perifornical hypothalamus. When bilateral hindlimb stepping was reliably elicited at an intensity of 50 ma or lower, the electrode was xed to the skull with dental acrylic. A stimulation regimen commenced in which a trial was presented every 70 s; it was composed of a pair of stimulation trains. A 10-s period separated the rst (Control) train and the second (Test) train; a 50-s period separated the offset of the Test train and the next Control train. The Control/Test stimulation sequence was maintained for the duration of the experiment, except for periods used for determination of baseline rates of unit activity. Testing brainstem units for locomotor responses The recording electrode was introduced into the brain ipsilateral to the stimulation electrode or within 0.5 mm of the midline. A standardized test was performed whenever a single unit was isolated or if no ongoing unit activity was apparent, at increments of 100 mm. At least one stimulation trial (composed of both Control and Test trains) that elicited stepping was given. If a single unit appeared to respond to either of the stimulation trains, or to change its rate of activity during stepping, it was tested with additional trials. If no response was apparent, the electrode was moved 100 mm deeper or to the next single unit. If a response was apparent, a testing sequence requiring 10±15 min was given. It included eight trials of stimulation, a 40-s sample of baseline activity after the stimulation was off for 2 min and a sample of the action potential waveform. Unit discrimination was frequently lost before all eight test trials were completed. If a neuron responded to individual stimulation pulses with a consistent xed latency, it was tested with three high-frequency pulses (typically an interpulse period of 3 ms). Unit responses that followed the high-frequency stimulation were classi ed as antidromic, and those that did not follow or that showed variable latencies were classi ed as synaptic. Collision tests were not used because most units had 0 base rates in the absence of stimulation. Histology A marking lesion was made at the end of the recording track by passing 15±30 ma anodal current through the recording wires for 30 s. The rat was given a lethal dose of nembutal and perfused transcardially with 10% formalin. The brain was placed in formalin and sectioned transversely every 100 mm with a Vibratome. The sections were viewed with a microscope and the locations of the sites were projected on to drawings adapted from an atlas. 64 Data analysis Response to stimulation. Sites were tested at least every 100 mm along a track, and the presence of a discriminated single unit was not a prerequisite for a test. However, only isolated single units were tested further, and if isolation was lost before suf cient testing was performed, the unit was not included in the data pool. We rst asked whether the unit responded to the Control stimulation. Particular attention was paid to the rst 3 s of stimulation because it preceded the onset of Control stepping. Neural correlates of stepping onset would have to be manifest in this pre-stepping period. The time around stimulation onset was divided into ve 1-s bins, two preceding the onset (pre-stimulation bins) and three following it (stimulation bins). The average rate of activity in each bin (spikes/s) was the data element for a repeated-measures analysis of variance with two factors, time-bin ( ve 1-s bins) and priming condition (Control and Test phases). Tukey's protected t-test (P, 0.05) was used for individual comparisons. A unit was classed as responsive to the Control stimulation if any one of the three stimulation bins was signi cantly different from the two pre-stimulation bins. Type I or Type II. The second question addressed was whether the activity of a responsive unit correlated in time with the onset of stepping. We classi ed neurons into one of two types based on the pattern of activity before the rst step to the Control train. Activity was averaged for each Control train that had a stepping latency of at least 3 s. The average was synchronized to the movement of the hindlimb that displayed the earliest stepping over most trials. Because the latency to step varied between trials, only the pre-stepping period common to all trials was used. Thus, the length of the pre-stepping average was no longer than the shortest stepping latency. Almost all averages were based on four or more pre-stepping periods of 3±4 s duration. Type I units showed an average response that increased up to the point of stepping onset; the increase was gradual in some cases and abrupt in others. To make this classi cation objectively, we used the
4 80 H. M. Sinnamon et al. criterion that Type I increases had to have a pre-step average with a positive slope and a linear correlation coef cient (r) of 0.67 or greater. Neurons that achieved this criterion were consistent on individual trials. Type II increases did not have a signi cant positive slope over time during the pre-step period. The average response pattern peaked early and changed little (or declined) in the 2±3-s period before the start of stepping. Priming effect. The next analysis compared Control and Test activity to determine whether the activity of a responsive unit showed a priming effect. The principal interest was in positive priming effects. Positive priming effects were declared for either of two cases. In stimulation priming, the Test response was greater (t-test, P, 0.05) than the Control. In pre-stimulation priming, the response to Control stimulation continued into the Test period, and caused the pre-test activity to differ (t-test, P, 0.05) from the pre-control activity. When responses were signi cantly smaller on the Test phase than on the Control, the priming effect was termed negative. Phase relations. The nal analysis looked for modulation of activity in phase with the step cycle. Each locomotor bout was examined for indications of phase relations and, where appropriate, averages of unit activity synchronized to normalized step cycles were computed. In summary, the analyses determined four independent properties of the activity of units that responded to locomotor stimulation: (i) whether the activity increased or decreased; (ii) whether the response pattern correlated with the onset of stepping (Type I or Type II); (iii) whether the response correlated with the facilitated stepping during the Test train (positive or negative priming effect or neither); and (iv) whether the activity pattern was modulated with respect to the phase of stepping (phase related or not). Regional analysis. The incidence rate of the response types was computed for each brainstem region. The regions varied in terms of the occurrence of the amount of neural activity occurring in the absence of locomotor stimulation (spontaneous activity). Most responsive units had 0 base rates and were found at the standard 100-mm steps along the electrode track. Therefore, it was not appropriate to use the number of tested units in a region as the base for the incidence rate because it would penalize regions with a higher incidence of spontaneously active neurons. For this reason, the number of standard tests at 100-mm steps through a region was used as the base, expressing the incidence rate of each response type as the occurrence per 100 sites tested. This measure was less biased in the sense of making regions with differing amounts of spontaneous activity and different sampling densities comparable. To evaluate the incidence rate for each region, it was compared to the remainder of the population by chi-square tests or, when expected frequencies were,5, by Fisher's Exact Probability Test. RESULTS Stimulation sites The locations of the stimulation sites used to elicit stepping are shown in Fig. 1. They were in the perifornical area and in the medial and lateral hypothalamus adjacent to it. The locations of effective sites are consistent with previous studies. 76 It was usually necessary to raise the current over the course of an experiment to maintain stepping. The mean initial current was 37 ma(s.d.ˆ9.6) and the mean increment was 10 ma (S.D. ˆ 9.2). Response types A total of 1159 single units was tentatively classed as responsive to locomotor stimulation. Analysis showed 82% (n ˆ 959) of them to have statistically signi cant responses during the rst 3 s of Control stimulation. The 52 units that failed to reach the response criterion indicate that the testing procedure was not biased toward units with large responses. The response type of main interest, the Type I increase, was shown by 25% of the responsive units, 57% of which showed statistically signi cant positive priming effects. Neurons with activity patterns modulated with the phase of the locomotor step cycle were rare (n ˆ 47, 4% of units tested). They were similarly rare (5.4%) among the units with Type I increases. The most common response pattern was the Type II increase (43%), of which 30% showed positive priming effects. Negative priming effects, smaller responses in the Test phase, occurred in 13% of responses. Negative priming effects were common (43%) for an unanticipated subtype of Type II increase units that showed a decline before stepping onset (II 1 decline). They were rare (4%) for the more common type of Type II increase pattern, which was termed constant II1. Other response types included decreases (26%) and antidromically activated (5%). Type I primed increases. As shown in Fig. 2, Type I increases had progressive increases up to the time of the rst step in the locomotor episode. Note the priming effects on stepping as re ected in the shorter latencies and/or greater amplitudes of Test stepping. Unit priming effects are re ected in the differences between the Test trace (heavy line) and the Control trace (thin line). The units in Fig. 2B±E showed both pre-stimulation and stimulation priming effects. The effect of the priming stimulation was to advance or amplify the increase in activity during the Test train. Although all Type I responses had the characteristic positive slope in the pre-step period, there were several indications that the class was not homogeneous. First, in 34% of the Type I units, an increase in activity appeared at the offset of the stimulation train. The magnitude of the offset increase varied, and often it was small, appearing only on stimulation averages. Second, for most units, the increase in activity started in the rst second of Control stimulation, but for others (16%) the rise began later. An example of a unit with both a large offset increase and a late-onset pattern is shown in Fig. 2E. Third, 6% of Type I patterns showed an initial decrease in activity that preceded the upward ramp. It was often undetectable on single trial records and appeared on the stimulation averages. This pattern can be seen in stimulation averages for the Test in Fig. 2B and for the Control in Fig. 2D. Type II increases. Type II increases, the most common response type, had early peaks that preceded stepping by 1 or more seconds. In the more common constant II 1 pattern, the rate increased to an early peak and maintained a constant high level. Examples are shown in Fig. 3A±C. In an unanticipated variation that was seen in 29% of the Type II increases, termed declining II1, the peak was early, but activity declined before the onset of stepping. The decline usually did not reach a level lower than the pre-stimulation rate. An example is shown in Fig. 3D. Note the negative stimulation priming effect, i.e. less increase in activity on the Test than the Control. Antidromic responses. Antidromic responses appeared with the rst pulse of the stimulation and continued at a virtually constant rate throughout the train. The latencies of 50 antidromic responses ranged from 0.7 to 7.0 ms, with a median of 1.9 ms. There was no indication of increased latencies for sites at greater distances from the site of stimulation. An example of an antidromically activated unit showing an entrained response to the 50-Hz hypothalamic stimulation and a post-stimulation depression in rate is shown in Fig. 4.
5 Priming of locomotion 81 locomotor stimulation are illustrated in Figs 6±8. Type I increases are indicated on the left side, and Type II increases and antidromic responses on the right side. The frequency of responses should be viewed in the context of the sampling density of the recording tracks, which are represented by grey lines. The lled circles represent the Type I and II units that showed positive priming effects. The grey squares indicate the II 1 decline units that showed negative priming effects. For simplicity, the locations of units that showed decreased patterns are not illustrated. Generally, they occurred proportionally among units showing increased patterns. Table 1 compares the regions of interest in terms of the rate of incidence of selected response patterns. The population value in Table 1 shows that the overall incidence rate of all responses to locomotor stimulation was 10.3 per 100 sites. The total number of recording sites in the regions of interest constituted approximately 41% of the population sites, and no one region accounted for more than 9% of the population. Signi cant differences relative to the population values are indicated with asterisks. Three regions, the ADT, the caudal pontine reticular nucleus (PnC) and the gigantocellular nucleus (Gi), were particularly responsive (.20%) to locomotor stimulation, compared to the 10.3% incidence rate for the population. Two other regions, the PnO and the PPA/MLA, were less responsive but also had incidence rates greater than the population. The ventral tegmental area was signi cantly less responsive than the population. Fig. 1. Schematic representation of the locations of the stimulation sites used to elicit locomotion. The anterior±posterior (AP) numbers on the left of each panel indicate the distance in mm posterior to bregma. A total of 238 sites is represented; 12 rats had two sites. One site located at AP 2.3 is not represented. Sites on both sides were used but all are plotted on the left. Drawings adapted from the atlas of Paxinos and Watson. 64 Decrease responses. The typical (n ˆ 217) pattern of decrease in activity in response to locomotor stimulation is shown in Fig. 5A. Post-stimulation increases in the rate of activity were common (33%) among these units. An example of a unit that decreased in rate during Control and Test stimulation with a progressive decrease in activity that was inverse to the Type I increases is given in Fig. 5A. Units of this type were rare, and few showed positive priming effects. Regional comparison of response types The locations of units showing increases in activity to Anterior midbrain. In Fig. 6, the grey rectangles show the ADT. The boundaries are based on the ndings of previous studies 18,78 and encompass the magnocellular nucleus of the posterior commissure, the interstitial nucleus of the medial longitudinal fasciculus and the adjacent tegmental area. As shown in Table 1, the ADT had an incidence of Type I primed and constant II 1 primed increases that was more than three times the population value. Type I primed units were localized to the ADT compared to the rest of the anterior midbrain (Fig. 6). The ADT was the only region with a high incidence of both Type I and II 1 primed units. Constant II 1 primed units were actually more common than the Type I primed units in the ADT, but they were more widely distributed. Decrease responses showed a similar pattern. Declining Type II increases were rare in the ADT region and in the anterior midbrain generally. Responses to locomotor stimulation in general, and primed responses in particular, were rare in several well-sampled regions of the anterior midbrain. Responses of all types, except for decreases, had a low incidence in the ventral tegmental area. Other low-response regions included the lateral tegmentum at AP 4.8 and 5.3, the medial central gray (CG), the red nucleus and the rostral linear nucleus. Antidromic responses were found only in the nucleus of the posterior commissure. Posterior midbrain. The locations of units in the posterior midbrain showing the various response types are represented in Fig. 7. The dorsolateral tegmentum region includes structures in the posterior midbrain dorsal to the peduncle and cuneiform nucleus, usually not considered part of the midbrain locomotor area. The region includes the lateral CG, the intercollicular nucleus and the tegmentun lateral to the CG. As shown in Table 1, this well-sampled region
6 82 H. M. Sinnamon et al. Fig. 2. Examples of Type I primed patterns. Each panel shows a representative trial, an average of unit activity synchronized to the rst step, and averages of the unit activity synchronized to the start of the Control and Test trains. (A) Stimulation priming effect. (B±E) Pre-stimulation and stimulation priming effects. The left column shows the spike density and accelerometer traces from single trials consisting of a Control train followed in 10 s by a Test train. The stimulation marker at the bottom shows a break, which indicates a 5-s gap between Control and Test records. The upper trace in each panel shows the spike density record. The vertical calibration marker to the left of the trace represents ve spikes/100 ms; all spike density calibration markers in subsequent illustrations have this value. The bottom of the calibration marker locates the 0 value. The trace below each spike density trace represents the output of an accelerometer attached to the metatarsus of the hindlimb that most frequently led the stepping sequence. Flexion produced an upward de ection; extension produced downward de ection. The middle column, Step Average, shows the average of the spike density and accelerometer traces synchronized to the start of stepping during the Control train. The length of the average trace to the left of the rst step re ects the trial with the shortest latency of stepping; thus, the average is of activity occurring between the stimulation onset and stepping onset on all trials. The right column, Stimulation Averages, shows the average spike density records synchronized to the start of stimulation. The Control (thin trace) and Test (heavy trace) records are overlapped to highlight unit priming effects. Locations of units: (A, B) magnocellular nucleus of the posterior commissure; (C) PnO; (D, E) Gi. showed a general responsiveness and a distribution of responses that paralleled the general population. The exception was the high incidence of antidromic responses (1.5% of sites). They were particularly common in the ventrolateral posterior CG and adjacent tegmentum at AP 7.8 (Fig. 7). The boundaries of the PPA/MLA were based on data for the rat. 7±9,12,22,23,45,50,63,67,78,79,85 The region includes the areas adjacent to the superior cerebellar peduncle at AP 7.3, 7.8 and 8.3 (chie y the pedunculopontine tegmental nucleus, the subpeduncular tegmental nucleus and the cuneiform nucleus at AP 8.3 and 8.8). The superior cerebellar peduncle (which contains few cell bodies) occupies a signi cant proportion of the region, and to ensure comparability of incidence rates, the estimated number of recording sites in the PPA/MLA was adjusted by subtracting the number in the superior cerebellar peduncle. The PPA/MLA was densely sampled, particularly at AP 8.3. As shown in Table 1, it showed a general responsiveness moderately larger than the general population that was attributable to a relatively high incidence of decreased (4.5%) and antidromic responses (1.3%). There was no indication in the region of a high incidence of primed Type I or Type II responses. The PnO was well sampled, particularly at AP 8.3. Although the region showed a general responsiveness similar to the PPA/MLA, it showed a large incidence of Type I responses with priming effects. As shown in Fig. 7, they appeared to be present throughout the region. The median raphe region included the median raphe nucleus at AP 7.3±8.3 and the paramedian raphe nucleus. At AP 8.3, the dorsal boundary extended up to the ventral aspect of the dorsal raphe. The general responsiveness and the distribution of response types were similar to the general population, with the exception of a relatively high incidence of declining II 1 responses with negative priming effects.
7 Priming of locomotion 83 Fig. 3. Four units showing Type II increases, level (or declining) spike density traces for several seconds prior to the onset of stepping. (A±C) Constant II1 patterns; A shows a stimulation priming effect, B shows stimulation and pre-stimulation effects, and C shows no priming effect. (D) Declining II1 pattern showing negative priming effect. Unit locations: (A) anteromedial Gi; (B) medial to the red nucleus; (C) magnocellular nucleus of the posterior commissure; (D) ventral margin of the medial longitudinal fasciculus at AP 78. Rate calibration: ve spikes/100 ms. Pons and medulla. Sites tested in the pons and medulla are shown in Fig. 8. The medial aspects of the PnC are shown in the transverse drawing for AP 9.3 in the upper left and in the sagittal drawing for ML 0.1. The medial aspects of the Gi are illustrated in the transverse drawing for AP 12.3 and in both of the sagittal representations. As may be seen in Table 1, these two regions were the most responsive of any tested region. The Gi had a higher incidence of Type I primed responses than any other region. It also had high incidences of decrease responses and of declining II 1 responses that showed negative priming. The responsiveness of the PnC was attributable to high incidences of constant II 1 primed responses, declining II 1 responses with negative priming, decrease responses and antidromic responses. Lateral aspects of the Gi were sampled at posterior levels, shown at AP 12.3 in Fig. 8. The area appeared to be as responsive as the medial regions but, contrary to the pattern in other regions, most of the Type I responses did not show priming effects. The ventrolateral aspects of the Gi also showed high incidences of decrease responses. The regions dorsal to the PnC and Gi were notable for the rarity of Type I responses. Dorsally, the unresponsive regions included the caudal interstitial nucleus of the medial longitudinal fasciculus and the dorsal paragigantocellular nucleus. At AP 12.3, the caudal interstitial nucleus of the medial longitudinal fasciculus contained a few Type I units and, at more anterior levels, it contained a variety of other response types. Type I increases were virtually absent in the dorsal regions associated with sensory functions, including the inferior colliculus (central and caudal), the parabrachial region (lateral), the vestibular complex (medial) and the nucleus of the solitary tract. The responsiveness of the PnC and the Gi likewise did not appear to extend ventrally to the magnocellular nuclei. Note, in all panels of Fig. 8, the general decline in responses in the ventral regions, which include the ventral pontine reticular, and the anterior and ventral Gi. DISCUSSION The results show the regional distribution of neurons with activity patterns correlated with both the onset of stepping (Type I increase) and the facilitated stepping produced by priming stimulation. Neurons showing the conjunction of these independent properties should include, but not necessarily be restricted to, neurons with a role in locomotion initiation. Neurons with similar Type I primed patterns rarely showed activity related to the phase of stepping and were found in widespread regions of the brainstem. They were prevalent as expected in the Gi of the medulla, a region previously implicated in locomotion, and they were also prevalent in regions not generally considered to be locomotor regions, the ADT and PnO. Conversely, they were relatively sparse in other regions, such as the ventral tegmental area and ªmidbrain locomotor areaº, which have been implicated in locomotion by studies using other preparations. Interpretation of response types Multiple processes are activated during the initiation of stepping. They include postural changes to provide antigravity support, 52 the arrest of antagonistic behaviors, orientation of the head and anterior torso, 81 shifting the center of mass
8 84 H. M. Sinnamon et al. Fig. 4. Response patterns of an antidromically activated unit in the medial PnC during trains of locomotor stimulation of the hypothalamus. Note the nearly veridical response of the neuron to the 50-Hz stimulation and the post-stimulation decrease in activity rate relative to baseline. The inset shows the unit response following each of three pulses at an interpulse period of 3 ms. Rate calibration: ve spikes/100 ms. forward 32,83 and cardiopulmonary adjustments appropriate for exercise. 17,90 In addition, hypothalamic locomotor stimulation, even at minimal currents, activates non-locomotor processes. Therefore, neurons that respond to locomotor stimulation will vary in their degree of relatedness to stepping onset. Some will be critical, some associated and some extraneous. The group of Type I primed neurons must include neurons that are critical to the onset of stepping. Extraneous neurons are no doubt included as well, and there were variations in the Type I primed patterns that indicate that future studies will need to use additional criteria to improve speci city. One interesting variation was a pattern appearing in one-third of the cases, in which an increase in activity appeared at the offset of the stimulation train. Although this rise was usually brief and small, often appearing only on the averaged records, its occurrence at the offset of stimulation when stepping was declining is dif cult to reconcile with a simple role in locomotor initiation. Other variations in the typical ramp-toasymptote Type I pattern were a delayed rise in activity until just prior to the start of stepping and a downward in ection early in the upward ramp that appeared in the averaged records. The variations in the Type I primed pattern suggest caution in attributing to the neurons displaying them a de nite role in locomotor initiation. For the purposes of this study, however, the speci city of the Type I primed pattern was less critical than its relative rate of occurrence in different regions. High occurrence rates of Type I primed patterns are at least consistent with a role for a region in the initiation of hypothalamic stepping. However, it is dif cult to conceive of a mechanism by which a region showing a low rate of occurrence of Type I primed responses would importantly contribute to the onset of stepping. The focus here on Type I primed response patterns does not minimize the potential importance of other response patterns for the initiation of locomotion. For example, the early rise to an asymptote of constant Type II responses is consistent with neuronal patterns involved in the establishment of postural and exercise-related states, which precede locomotion. Type II responses had a regional distribution that was similar to, but wider than, Type I responses. The earlier responding Type II neurons with positive priming are feasible candidates for providing excitatory drive for neurons showing Type I patterns. Feasible proposals for functions in locomotor initiation can also be made for the other response patterns but, lacking the behavioral correlations that support the Type I and II primed neurons, they would be unduly speculative. The mechanisms underlying the priming effects on locomotor stepping and on the neuronal responses studied here need further study. The appearance of primed responses throughout the extent of the brainstem is consistent with the evidence that priming re ects widespread processes. Stimulation at sites from the posterior hypothalamus to the preoptic area produces apparently similar locomotor priming effects. Stimulation at one locomotor site will prime stepping elicited by a second train at that same site, and at ipsilateral and contralateral sites as well. 87 At longer Control±Test intervals than used here, suppressive effects of priming stimulation in the hypothalamus can also appear for some sites. 77 The amplitude and frequency of hippocampal rhythmic slowwave activity in the 3±6-Hz band generally co-vary with the facilitation of stepping in the priming situation. 78 In cases where the priming effect shows a monotonic decline, the correlation is positive, but in cases where priming effects are mixed, the correlation can be absent or negative. 77 In the awake and freely moving rat, the priming effect is associated with a reduction in pre-locomotor head scanning movements. 81 Although there is no direct evidence, it would seem likely that priming effects would also be correlated with facilitated exercise-related, cardiopulmonary processes. 17,90 Fig. 5. Two units showing decreases in activity during locomotor stimulation. (A) Most common pattern, low or zero activity rate for several seconds preceding the rst step. (B) Progressive decrease in activity precedes the rst step. Locations of units: (A) dorsal raphe nucleus; (B) dorsal Gi. Rate calibration: ve spikes/100 ms.
9 Priming of locomotion 85 Table 1. Responses to locomotor stimulation: regional comparison of the incidence rate of selected response types and priming effects All responses Incidence (per 100 sites) of response types Region Recording sites Frequency Incidence Type I1 primed Constant II1 primed Declining II1 neg. prime Decreased Antidromic ADT * 4.6* 6.2* * 0.0 Ventral tegmental area * Dorsolateral tegmentum * PPA/MLA * * 1.3* PnO * 3.8* Median raphe * PnC * * 4.0* 8.7* 1.3* Gi * 5.2* * 7.3* 0.3 Population *Signi cant (P, 0.05) difference from remainder of population by chi-square or Fisher tests. Regional differences in response patterns Neurons in the ADT are known to show Type I patterns 18 and this study demonstrates that most of these neurons also show priming effects. These patterns are consistent with functional and anatomical evidence that indicates a role for the ADT in processes related to locomotor initiation. Reversible inactivation of neurons in the region using GABA injections blocked stepping produced by hypothalamic stimulation. 78 Stimulation in the region produces stepping in both intact-anesthetized and brain-transected rats. 5 In sh, electrical or glutamate activation of the region elicits swimming Fig. 6. Schematic representation of the locations of the units in the anterior midbrain showing increases in activity during locomotor stimulation. The grey lines represent the recording tracks and indicate the sampling density. All units were ipsilateral to the side of stimulation or on the midline, but Type I units are represented on the left, and Type II and antidromic (stars) units on the right. Two Type II variants are represented. The smaller circles represent the constant II 1 (e.g. Fig. 3A±C) and the squares indicate the declining II 1 (e.g. Fig. 3D). Filled circles indicate positive priming effects; open circles indicate no priming effects or negative priming effects. Stippled squares indicate negative priming effects for the declining II 1 units. The grey rectangle indicates the boundaries of the ADT. The numbers to the left of each drawing indicate the distance in mm posterior to bregma.
10 86 H. M. Sinnamon et al. Fig. 7. Schematic representation of the locations of the units in the posterior midbrain showing Type I and Type II increases and antidromic responses. Format similar to Fig. 6. One track located at AP 6.7 is not represented. The grey polygons indicate the boundaries of the dorsolateral tegmentum, the PPA/MLA, the PnO and the median raphe nucleus. movements. 6,88 In the cat, neural activity phase-related to stepping was found in the interstitial nucleus of Cajal. 29 The ADT has been implicated in the control of vertical eye and head movements in cats and monkeys, 20,21 and in the awake rat, vertical head movements and related postural adjustments are a prominent component of locomotor initiation. 81,83 The presence of Type I primed neurons in this region suggests a possible role in the interaction between locomotion and either neck posture or head scanning. In the ventral tegmental area, the most common response was a decrease, but overall few responses were seen. Recordings there contrasted with those immediately dorsal in the red nucleus, which was similarly unresponsive but showed more baseline activity. Stimulation in the ventral tegmental area elicits stepping in the awake 63 and anesthetized rat. 74,79 Lesions 82 and procaine injections 44 there block stepping elicited by perifornical stimulation, possibly by interrupting bers of passage. The locomotor effects of drugs applied to this region in the awake rat appear to depend on the ascending projections of dopamine neurons. 36,51 In the anesthetized rat, large lesions in the anterior hypothalamus, which would interrupt these bers, do not block locomotion elicited by stimulation in the perifornical hypothalamus. 75 Neither the present data nor previous ndings provide evidence that the neurons in the ventral tegmental area or their ascending axons directly contribute to the locomotion studied here. The PPA/MLA, which includes both the pedunculopontine tegmental and the cuneiform nuclei, showed few Type I
GABA injected into the anterior dorsal tegmentum ADT of the midbrain blocks stepping initiated by stimulation of the hypothalamus
 Ž. Brain Research 766 1997 271 275 Short communication Ž. GABA injected into the anterior dorsal tegmentum ADT of the midbrain blocks stepping initiated by stimulation of the hypothalamus H.M. Sinnamon
Ž. Brain Research 766 1997 271 275 Short communication Ž. GABA injected into the anterior dorsal tegmentum ADT of the midbrain blocks stepping initiated by stimulation of the hypothalamus H.M. Sinnamon
PRIMING PATTERN DETERMINES THE CORRELATION BETWEEN HIPPOCAMPAL THETA ACTIVITY AND LOCOMOTOR STEPPING ELICITED BY STIMULATION IN ANESTHETIZED RATS
 Pergamon www.elsevier.com/locate/neuroscience Theta and locomotion Neuroscience Vol. 98, No. 3, pp. 459 470, 2000 459 2000 IBRO. Published by Elsevier Science Ltd Printed in Great Britain. All rights reserved
Pergamon www.elsevier.com/locate/neuroscience Theta and locomotion Neuroscience Vol. 98, No. 3, pp. 459 470, 2000 459 2000 IBRO. Published by Elsevier Science Ltd Printed in Great Britain. All rights reserved
 Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy
 By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific
By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific
Spinal Interneurons. Control of Movement
 Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
Page 1 L 58. The University of Connecticut Schools of Medicine and Dental Medicine Humans Systems: Organ Systems /2013 RETICULAR FORMATION
 Page 1 L 58 Douglas L. Oliver, Ph.D. The University of Connecticut Schools of Medicine and Dental Medicine Humans Systems: Organ Systems 1 2012/2013 RETICULAR FORMATION Lecture Lecture: Douglas Oliver
Page 1 L 58 Douglas L. Oliver, Ph.D. The University of Connecticut Schools of Medicine and Dental Medicine Humans Systems: Organ Systems 1 2012/2013 RETICULAR FORMATION Lecture Lecture: Douglas Oliver
Internal Organisation of the Brainstem
 Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
Brainstem. Amadi O. Ihunwo, PhD School of Anatomical Sciences
 Brainstem Amadi O. Ihunwo, PhD School of Anatomical Sciences Lecture Outline Constituents Basic general internal features of brainstem External and Internal features of Midbrain Pons Medulla Constituents
Brainstem Amadi O. Ihunwo, PhD School of Anatomical Sciences Lecture Outline Constituents Basic general internal features of brainstem External and Internal features of Midbrain Pons Medulla Constituents
DEVELOPMENT OF BRAIN
 Ahmed Fathalla OBJECTIVES At the end of the lecture, students should: List the components of brain stem. Describe the site of brain stem. Describe the relations between components of brain stem & their
Ahmed Fathalla OBJECTIVES At the end of the lecture, students should: List the components of brain stem. Describe the site of brain stem. Describe the relations between components of brain stem & their
Hypothalamus. To learn how the brain regulates neuroendocrine secretions NTA Ch 14, pgs Key Figs: 14-3; 14-4,
 Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
SENSORY (ASCENDING) SPINAL TRACTS
 SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
Brainstem. Steven McLoon Department of Neuroscience University of Minnesota
 Brainstem Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Change in Lab Sequence Week of Oct 2 Lab 5 Week of Oct 9 Lab 4 2 Goal Today Know the regions of the brainstem. Know
Brainstem Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Change in Lab Sequence Week of Oct 2 Lab 5 Week of Oct 9 Lab 4 2 Goal Today Know the regions of the brainstem. Know
Lecture 4 The BRAINSTEM Medulla Oblongata
 Lecture 4 The BRAINSTEM Medulla Oblongata Introduction to brainstem 1- Medulla oblongata 2- Pons 3- Midbrain - - - occupies the posterior cranial fossa of the skull. connects the narrow spinal cord
Lecture 4 The BRAINSTEM Medulla Oblongata Introduction to brainstem 1- Medulla oblongata 2- Pons 3- Midbrain - - - occupies the posterior cranial fossa of the skull. connects the narrow spinal cord
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Reticular Formation George R. Leichnetz, Ph.D.
 Reticular Formation George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the anatomical and functional organization of the brainstem reticular formation into three general regions: median (raphe), medial
Reticular Formation George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the anatomical and functional organization of the brainstem reticular formation into three general regions: median (raphe), medial
The Nervous System: Sensory and Motor Tracts of the Spinal Cord
 15 The Nervous System: Sensory and Motor Tracts of the Spinal Cord PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction Millions of sensory
15 The Nervous System: Sensory and Motor Tracts of the Spinal Cord PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction Millions of sensory
Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota
 Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Tuesday (Sept 11) 10:00-11:00am Friday (Sept 14) 8:30-9:30am Surdyk s
Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Tuesday (Sept 11) 10:00-11:00am Friday (Sept 14) 8:30-9:30am Surdyk s
Auditory and Vestibular Systems
 Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Located below tentorium cerebelli within posterior cranial fossa. Formed of 2 hemispheres connected by the vermis in midline.
 The Cerebellum Cerebellum Located below tentorium cerebelli within posterior cranial fossa. Formed of 2 hemispheres connected by the vermis in midline. Gray matter is external. White matter is internal,
The Cerebellum Cerebellum Located below tentorium cerebelli within posterior cranial fossa. Formed of 2 hemispheres connected by the vermis in midline. Gray matter is external. White matter is internal,
Brainstem: Medulla oblongata and pons
 Brainstem: Medulla oblongata and pons 1. Overview of the brainstem subdivisions 2. Embryonic development of the brainstem 3. Medulla oblongata external features 4. Internal structure of the medulla oblongata
Brainstem: Medulla oblongata and pons 1. Overview of the brainstem subdivisions 2. Embryonic development of the brainstem 3. Medulla oblongata external features 4. Internal structure of the medulla oblongata
Cerebellum. Steven McLoon Department of Neuroscience University of Minnesota
 Cerebellum Steven McLoon Department of Neuroscience University of Minnesota 1 Anatomy of the Cerebellum The cerebellum has approximately half of all the neurons in the central nervous system. The cerebellum
Cerebellum Steven McLoon Department of Neuroscience University of Minnesota 1 Anatomy of the Cerebellum The cerebellum has approximately half of all the neurons in the central nervous system. The cerebellum
Systems Neuroscience Dan Kiper. Today: Wolfger von der Behrens
 Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
Developmental sequence of brain
 Cerebellum Developmental sequence of brain Fourth week Fifth week Location of cerebellum Lies above and behind the medullar and pons and occupies posterior cranial fossa Location of cerebellum External
Cerebellum Developmental sequence of brain Fourth week Fifth week Location of cerebellum Lies above and behind the medullar and pons and occupies posterior cranial fossa Location of cerebellum External
LESIONS OF THE MESOLIMBIC DOPAMINE SYSTEM DISRUPT SIGNALLED ESCAPE RESPONSES IN THE RAT
 ACTA NEUROBIOL: EXP. 1988, 48: 117-121 Short communication LESIONS OF THE MESOLIMBIC DOPAMINE SYSTEM DISRUPT SIGNALLED ESCAPE RESPONSES IN THE RAT W. Jeffrey WILSON and Jennifer C. HALL Department of Psychological
ACTA NEUROBIOL: EXP. 1988, 48: 117-121 Short communication LESIONS OF THE MESOLIMBIC DOPAMINE SYSTEM DISRUPT SIGNALLED ESCAPE RESPONSES IN THE RAT W. Jeffrey WILSON and Jennifer C. HALL Department of Psychological
NEURAL MECHANISMS OF SLEEP (p.1) (Rev. 3/21/07)
 NEURAL MECHANISMS OF SLEEP (p.1) (Rev. 3/21/07) 1. Revisitation of Bremer s 1936 Isolated Brain Studies Transected the brain: a. Cut between the medulla and the spinal cord ( encephale isole ) Note: recall
NEURAL MECHANISMS OF SLEEP (p.1) (Rev. 3/21/07) 1. Revisitation of Bremer s 1936 Isolated Brain Studies Transected the brain: a. Cut between the medulla and the spinal cord ( encephale isole ) Note: recall
The Cerebellum. The Little Brain. Neuroscience Lecture. PhD Candidate Dr. Laura Georgescu
 The Cerebellum The Little Brain Neuroscience Lecture PhD Candidate Dr. Laura Georgescu Learning Objectives 1. Describe functional anatomy of the cerebellum - its lobes, their input and output connections
The Cerebellum The Little Brain Neuroscience Lecture PhD Candidate Dr. Laura Georgescu Learning Objectives 1. Describe functional anatomy of the cerebellum - its lobes, their input and output connections
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Brainstem. By Dr. Bhushan R. Kavimandan
 Brainstem By Dr. Bhushan R. Kavimandan Development Ventricles in brainstem Mesencephalon cerebral aqueduct Metencephalon 4 th ventricle Mylencephalon 4 th ventricle Corpus callosum Posterior commissure
Brainstem By Dr. Bhushan R. Kavimandan Development Ventricles in brainstem Mesencephalon cerebral aqueduct Metencephalon 4 th ventricle Mylencephalon 4 th ventricle Corpus callosum Posterior commissure
9.14 Class 32 Review. Limbic system
 9.14 Class 32 Review Limbic system 1 Lateral view Medial view Brainstem, sagittal section Sensory- Perceptual Motor Behavior Major functional modules of the CNS Motivation Courtesy of MIT Press. Used with
9.14 Class 32 Review Limbic system 1 Lateral view Medial view Brainstem, sagittal section Sensory- Perceptual Motor Behavior Major functional modules of the CNS Motivation Courtesy of MIT Press. Used with
Brainstem: Midbrain. 1. Midbrain gross external anatomy 2. Internal structure of the midbrain:
 Brainstem: Midbrain 1. Midbrain gross external anatomy 2. Internal structure of the midbrain: cerebral peduncles tegmentum tectum (guadrigeminal plate) Midbrain Midbrain general features location between
Brainstem: Midbrain 1. Midbrain gross external anatomy 2. Internal structure of the midbrain: cerebral peduncles tegmentum tectum (guadrigeminal plate) Midbrain Midbrain general features location between
Voluntary Movement. Ch. 14: Supplemental Images
 Voluntary Movement Ch. 14: Supplemental Images Skeletal Motor Unit: The basics Upper motor neuron: Neurons that supply input to lower motor neurons. Lower motor neuron: neuron that innervates muscles,
Voluntary Movement Ch. 14: Supplemental Images Skeletal Motor Unit: The basics Upper motor neuron: Neurons that supply input to lower motor neurons. Lower motor neuron: neuron that innervates muscles,
Brain Stem and cortical control of motor function. Dr Z Akbari
 Brain Stem and cortical control of motor function Dr Z Akbari Brain stem control of movement BS nuclear groups give rise to descending motor tracts that influence motor neurons and their associated interneurons
Brain Stem and cortical control of motor function Dr Z Akbari Brain stem control of movement BS nuclear groups give rise to descending motor tracts that influence motor neurons and their associated interneurons
Brain anatomy and artificial intelligence. L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia
 Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D.
 SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements
 Y. Isomura et al. 1 Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements Yoshikazu Isomura, Rie Harukuni, Takashi Takekawa, Hidenori Aizawa & Tomoki Fukai
Y. Isomura et al. 1 Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements Yoshikazu Isomura, Rie Harukuni, Takashi Takekawa, Hidenori Aizawa & Tomoki Fukai
THE BACK. Dr. Ali Mohsin. Spinal Cord
 Spinal Cord THE BACK Dr. Ali Mohsin The spinal cord is the elongated caudal part of the CNS. It starts as the inferior continuation of the medulla oblongata at the level of foramen magnum, & ends as an
Spinal Cord THE BACK Dr. Ali Mohsin The spinal cord is the elongated caudal part of the CNS. It starts as the inferior continuation of the medulla oblongata at the level of foramen magnum, & ends as an
Pain and Temperature Objectives
 Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
Organization of the nervous system 2
 Organization of the nervous system 2 Raghav Rajan Bio 334 Neurobiology I August 22nd 2013 1 Orienting within the brain absolute axes and relative axes SUPERIOR (above) ANTERIOR (in front) Anterior/Posterior,
Organization of the nervous system 2 Raghav Rajan Bio 334 Neurobiology I August 22nd 2013 1 Orienting within the brain absolute axes and relative axes SUPERIOR (above) ANTERIOR (in front) Anterior/Posterior,
Chapter 3. Structure and Function of the Nervous System. Copyright (c) Allyn and Bacon 2004
 Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Unit VIII Problem 5 Physiology: Cerebellum
 Unit VIII Problem 5 Physiology: Cerebellum - The word cerebellum means: the small brain. Note that the cerebellum is not completely separated into 2 hemispheres (they are not clearly demarcated) the vermis
Unit VIII Problem 5 Physiology: Cerebellum - The word cerebellum means: the small brain. Note that the cerebellum is not completely separated into 2 hemispheres (they are not clearly demarcated) the vermis
Nsci 2100: Human Neuroanatomy 2017 Examination 3
 Name KEY Lab Section Nsci 2100: Human Neuroanatomy 2017 Examination 3 On this page, write your name and lab section. On your bubble answer sheet, enter your name (last name, space, first name), internet
Name KEY Lab Section Nsci 2100: Human Neuroanatomy 2017 Examination 3 On this page, write your name and lab section. On your bubble answer sheet, enter your name (last name, space, first name), internet
Reflexes. Dr. Baizer
 Reflexes Dr. Baizer 1 Learning objectives: reflexes Students will be able to describe: 1. The clinical importance of testing reflexes. 2. The essential components of spinal reflexes. 3.The stretch reflex.
Reflexes Dr. Baizer 1 Learning objectives: reflexes Students will be able to describe: 1. The clinical importance of testing reflexes. 2. The essential components of spinal reflexes. 3.The stretch reflex.
Neural Basis of Motor Control
 Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
General Sensory Pathways of the Trunk and Limbs
 General Sensory Pathways of the Trunk and Limbs Lecture Objectives Describe gracile and cuneate tracts and pathways for conscious proprioception, touch, pressure and vibration from the limbs and trunk.
General Sensory Pathways of the Trunk and Limbs Lecture Objectives Describe gracile and cuneate tracts and pathways for conscious proprioception, touch, pressure and vibration from the limbs and trunk.
Dopamine in Ube3a m-/p+ mice. Online Supplemental Material
 Online Supplemental Material S1 Supplemental Figure 1. Schematic of rate-dependent intracranial self-stimulation (ICSS) (A) Mice implanted with monopolar stimulating electrodes to the medial forebrain
Online Supplemental Material S1 Supplemental Figure 1. Schematic of rate-dependent intracranial self-stimulation (ICSS) (A) Mice implanted with monopolar stimulating electrodes to the medial forebrain
Nature Methods: doi: /nmeth Supplementary Figure 1. Activity in turtle dorsal cortex is sparse.
 Supplementary Figure 1 Activity in turtle dorsal cortex is sparse. a. Probability distribution of firing rates across the population (notice log scale) in our data. The range of firing rates is wide but
Supplementary Figure 1 Activity in turtle dorsal cortex is sparse. a. Probability distribution of firing rates across the population (notice log scale) in our data. The range of firing rates is wide but
Nature Neuroscience doi: /nn Supplementary Figure 1. Characterization of viral injections.
 Supplementary Figure 1 Characterization of viral injections. (a) Dorsal view of a mouse brain (dashed white outline) after receiving a large, unilateral thalamic injection (~100 nl); demonstrating that
Supplementary Figure 1 Characterization of viral injections. (a) Dorsal view of a mouse brain (dashed white outline) after receiving a large, unilateral thalamic injection (~100 nl); demonstrating that
Neural Recording Methods
 Neural Recording Methods Types of neural recording 1. evoked potentials 2. extracellular, one neuron at a time 3. extracellular, many neurons at a time 4. intracellular (sharp or patch), one neuron at
Neural Recording Methods Types of neural recording 1. evoked potentials 2. extracellular, one neuron at a time 3. extracellular, many neurons at a time 4. intracellular (sharp or patch), one neuron at
Supplemental Information. Dorsal Raphe Dual Serotonin-Glutamate Neurons. Drive Reward by Establishing Excitatory Synapses
 Cell Reports, Volume 26 Supplemental Information Dorsal Raphe Dual Serotonin-Glutamate Neurons Drive Reward by Establishing Excitatory Synapses on VTA Mesoaccumbens Dopamine Neurons Hui-Ling Wang, Shiliang
Cell Reports, Volume 26 Supplemental Information Dorsal Raphe Dual Serotonin-Glutamate Neurons Drive Reward by Establishing Excitatory Synapses on VTA Mesoaccumbens Dopamine Neurons Hui-Ling Wang, Shiliang
Experience-dependent recovery of vision following chronic deprivation amblyopia. Hai-Yan He, Baisali Ray, Katie Dennis and Elizabeth M.
 Experience-dependent recovery of vision following chronic deprivation amblyopia Hai-Yan He, Baisali Ray, Katie Dennis and Elizabeth M. Quinlan a 3. 2.5 2. 1.5.5 Deprived eye Non-deprived VCtx * * b 8.
Experience-dependent recovery of vision following chronic deprivation amblyopia Hai-Yan He, Baisali Ray, Katie Dennis and Elizabeth M. Quinlan a 3. 2.5 2. 1.5.5 Deprived eye Non-deprived VCtx * * b 8.
EEG Sleep Circadian rhythms Learning Objectives: 121, 122
 EEG Sleep Circadian rhythms Learning Objectives: 121, 122 Zoltán Lelkes Electroencenphalography Hans Berger pen time amplifier electrodes 1 The waves of the EEG gamma > 30 Hz beta: 13-30 Hz Mental activity:
EEG Sleep Circadian rhythms Learning Objectives: 121, 122 Zoltán Lelkes Electroencenphalography Hans Berger pen time amplifier electrodes 1 The waves of the EEG gamma > 30 Hz beta: 13-30 Hz Mental activity:
Theme 2: Cellular mechanisms in the Cochlear Nucleus
 Theme 2: Cellular mechanisms in the Cochlear Nucleus The Cochlear Nucleus (CN) presents a unique opportunity for quantitatively studying input-output transformations by neurons because it gives rise to
Theme 2: Cellular mechanisms in the Cochlear Nucleus The Cochlear Nucleus (CN) presents a unique opportunity for quantitatively studying input-output transformations by neurons because it gives rise to
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal:
 Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
Detailed protocol Only dissected human brain samples are stored. The microdissection is performed on frozen brains and the samples are kept on -70 C.
 2008 Detailed protocol Only dissected human brain samples are stored. The microdissection is performed on frozen brains and the samples are kept on -70 C. BrainNet Europe II Project Co-ordinator: Prof.
2008 Detailed protocol Only dissected human brain samples are stored. The microdissection is performed on frozen brains and the samples are kept on -70 C. BrainNet Europe II Project Co-ordinator: Prof.
Medical Neuroscience Tutorial Notes
 Medical Neuroscience Tutorial Notes Blood Supply to the Brain MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning
Medical Neuroscience Tutorial Notes Blood Supply to the Brain MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning
Brain Mechanisms of Emotion 1 of 6
 Brain Mechanisms of Emotion 1 of 6 I. WHAT IS AN EMOTION? A. Three components (Oately & Jenkins, 1996) 1. caused by conscious or unconscious evaluation of an event as relevant to a goal that is important
Brain Mechanisms of Emotion 1 of 6 I. WHAT IS AN EMOTION? A. Three components (Oately & Jenkins, 1996) 1. caused by conscious or unconscious evaluation of an event as relevant to a goal that is important
Introduction to the Central Nervous System: Internal Structure
 Introduction to the Central Nervous System: Internal Structure Objective To understand, in general terms, the internal organization of the brain and spinal cord. To understand the 3-dimensional organization
Introduction to the Central Nervous System: Internal Structure Objective To understand, in general terms, the internal organization of the brain and spinal cord. To understand the 3-dimensional organization
Supplementary materials for: Executive control processes underlying multi- item working memory
 Supplementary materials for: Executive control processes underlying multi- item working memory Antonio H. Lara & Jonathan D. Wallis Supplementary Figure 1 Supplementary Figure 1. Behavioral measures of
Supplementary materials for: Executive control processes underlying multi- item working memory Antonio H. Lara & Jonathan D. Wallis Supplementary Figure 1 Supplementary Figure 1. Behavioral measures of
Basal nuclei, cerebellum and movement
 Basal nuclei, cerebellum and movement MSTN121 - Neurophysiology Session 9 Department of Myotherapy Basal Nuclei (Ganglia) Basal Nuclei (Ganglia) Role: Predict the effects of various actions, then make
Basal nuclei, cerebellum and movement MSTN121 - Neurophysiology Session 9 Department of Myotherapy Basal Nuclei (Ganglia) Basal Nuclei (Ganglia) Role: Predict the effects of various actions, then make
Medial View of Cerebellum
 Meds 5371 System Neuroscience D. L. Oliver CEREBELLUM Anterior lobe (spinal) Posterior lobe (cerebral) Flocculonodular lobe (vestibular) Medial View of Cerebellum 1 Ventral View of Cerebellum Flocculus
Meds 5371 System Neuroscience D. L. Oliver CEREBELLUM Anterior lobe (spinal) Posterior lobe (cerebral) Flocculonodular lobe (vestibular) Medial View of Cerebellum 1 Ventral View of Cerebellum Flocculus
Babak Tamizi Far MD. Assistant professor of internal medicine Al-zahra hospital, Isfahan university of medical sciences
 Babak Tamizi Far MD. Assistant professor of internal medicine Al-zahra hospital, Isfahan university of medical sciences ٢ Level of consciousness is depressed Stuporous patients respond only to repeated
Babak Tamizi Far MD. Assistant professor of internal medicine Al-zahra hospital, Isfahan university of medical sciences ٢ Level of consciousness is depressed Stuporous patients respond only to repeated
b. The groove between the two crests is called 2. The neural folds move toward each other & the fuse to create a
 Chapter 13: Brain and Cranial Nerves I. Development of the CNS A. The CNS begins as a flat plate called the B. The process proceeds as: 1. The lateral sides of the become elevated as waves called a. The
Chapter 13: Brain and Cranial Nerves I. Development of the CNS A. The CNS begins as a flat plate called the B. The process proceeds as: 1. The lateral sides of the become elevated as waves called a. The
Done by : Areej Al-Hadidi
 Brainstem &diencephalon Done by : Areej Al-Hadidi Brainstem Functions Ascending and descending tracts Reflex centers Cardiovascular and respiratory centers Coughing, sneezing, swallowing Nuclei of the
Brainstem &diencephalon Done by : Areej Al-Hadidi Brainstem Functions Ascending and descending tracts Reflex centers Cardiovascular and respiratory centers Coughing, sneezing, swallowing Nuclei of the
I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts.
 Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Spectro-temporal response fields in the inferior colliculus of awake monkey
 3.6.QH Spectro-temporal response fields in the inferior colliculus of awake monkey Versnel, Huib; Zwiers, Marcel; Van Opstal, John Department of Biophysics University of Nijmegen Geert Grooteplein 655
3.6.QH Spectro-temporal response fields in the inferior colliculus of awake monkey Versnel, Huib; Zwiers, Marcel; Van Opstal, John Department of Biophysics University of Nijmegen Geert Grooteplein 655
The Cerebellum. Little Brain. Neuroscience Lecture. Dr. Laura Georgescu
 The Cerebellum Little Brain Neuroscience Lecture Dr. Laura Georgescu Learning Objectives 1. Describe functional anatomy of the cerebellum- its lobes, their input and output connections and their functions.
The Cerebellum Little Brain Neuroscience Lecture Dr. Laura Georgescu Learning Objectives 1. Describe functional anatomy of the cerebellum- its lobes, their input and output connections and their functions.
Supplementary Figure 1. Example of an amygdala neuron whose activity reflects value during the visual stimulus interval. This cell responded more
 1 Supplementary Figure 1. Example of an amygdala neuron whose activity reflects value during the visual stimulus interval. This cell responded more strongly when an image was negative than when the same
1 Supplementary Figure 1. Example of an amygdala neuron whose activity reflects value during the visual stimulus interval. This cell responded more strongly when an image was negative than when the same
Nervous System C H A P T E R 2
 Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Role of brainstem in somatomotor (postural) functions
 Role of brainstem in somatomotor (postural) functions (vestibular apparatus) The muscle tone and its regulation VESTIBULAR SYSTEM (Equilibrium) Receptors: Otolith organs Semicircular canals Sensation (information):
Role of brainstem in somatomotor (postural) functions (vestibular apparatus) The muscle tone and its regulation VESTIBULAR SYSTEM (Equilibrium) Receptors: Otolith organs Semicircular canals Sensation (information):
Department of Neurology/Division of Anatomical Sciences
 Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Lecturer. Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014
 Lecturer Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014 Dorsal root: The dorsal root carries both myelinated and unmyelinated afferent fibers to the spinal cord. Posterior gray column: Long
Lecturer Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014 Dorsal root: The dorsal root carries both myelinated and unmyelinated afferent fibers to the spinal cord. Posterior gray column: Long
Introduction and Basic structural organization of the nervous system
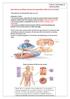 Introduction and Basic structural organization of the nervous system **the slides are in bold and the book is in red Done by : razan krishan & marah marahleh INTRODUCTION The nervous system, along with
Introduction and Basic structural organization of the nervous system **the slides are in bold and the book is in red Done by : razan krishan & marah marahleh INTRODUCTION The nervous system, along with
Note: Waxman is very sketchy on today s pathways and nonexistent on the Trigeminal.
 Dental Neuroanatomy Thursday, February 3, 2011 Suzanne Stensaas, PhD Note: Waxman is very sketchy on today s pathways and nonexistent on the Trigeminal. Resources: Pathway Quiz for HyperBrain Ch. 5 and
Dental Neuroanatomy Thursday, February 3, 2011 Suzanne Stensaas, PhD Note: Waxman is very sketchy on today s pathways and nonexistent on the Trigeminal. Resources: Pathway Quiz for HyperBrain Ch. 5 and
DIRECT SURGERY FOR INTRA-AXIAL
 Kitakanto Med. J. (S1) : 23 `28, 1998 23 DIRECT SURGERY FOR INTRA-AXIAL BRAINSTEM LESIONS Kazuhiko Kyoshima, Susumu Oikawa, Shigeaki Kobayashi Department of Neurosurgery, Shinshu University School of Medicine,
Kitakanto Med. J. (S1) : 23 `28, 1998 23 DIRECT SURGERY FOR INTRA-AXIAL BRAINSTEM LESIONS Kazuhiko Kyoshima, Susumu Oikawa, Shigeaki Kobayashi Department of Neurosurgery, Shinshu University School of Medicine,
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Atlas representations of the midcingulate (MCC) region targeted in this study compared against the anterior cingulate (ACC) region commonly reported. Coronal sections are shown on
Supplementary Figure 1 Atlas representations of the midcingulate (MCC) region targeted in this study compared against the anterior cingulate (ACC) region commonly reported. Coronal sections are shown on
Arterial Blood Supply
 Arterial Blood Supply Brain is supplied by pairs of internal carotid artery and vertebral artery. The four arteries lie within the subarachnoid space Their branches anastomose on the inferior surface of
Arterial Blood Supply Brain is supplied by pairs of internal carotid artery and vertebral artery. The four arteries lie within the subarachnoid space Their branches anastomose on the inferior surface of
Central nervous system (CNS): brain and spinal cord Collections of cell body and dendrites (grey matter) are called nuclei/nucleus Nucleus can also
 Chapter 3 Part 1 Orientation Directions in the nervous system are described relatively to the neuraxis An imaginary line drawn through the center of the length of the central nervous system, from the bottom
Chapter 3 Part 1 Orientation Directions in the nervous system are described relatively to the neuraxis An imaginary line drawn through the center of the length of the central nervous system, from the bottom
Unit VIII Problem 3 Neuroanatomy: Brain Stem, Cranial Nerves and Scalp
 Unit VIII Problem 3 Neuroanatomy: Brain Stem, Cranial Nerves and Scalp - Brain stem: It is connected to the cerebellum and cerebral hemispheres. Rostral end of brain stem: diencephalon is the area which
Unit VIII Problem 3 Neuroanatomy: Brain Stem, Cranial Nerves and Scalp - Brain stem: It is connected to the cerebellum and cerebral hemispheres. Rostral end of brain stem: diencephalon is the area which
The Cerebellum. Outline. Overview Structure (external & internal) Micro-circuitry of the cerebellum Cerebellum and motor learning
 The Cerebellum P.T Ji Jun Cheol Rehabilitation Center 1 HansarangAsan Hospital. Outline Overview Structure (external & internal) Micro-circuitry of the cerebellum Cerebellum and motor learning 2 1 Cerebellum
The Cerebellum P.T Ji Jun Cheol Rehabilitation Center 1 HansarangAsan Hospital. Outline Overview Structure (external & internal) Micro-circuitry of the cerebellum Cerebellum and motor learning 2 1 Cerebellum
Nature Neuroscience: doi: /nn.4335
 Supplementary Figure 1 Cholinergic neurons projecting to the VTA are concentrated in the caudal mesopontine region. (a) Schematic showing the sites of retrograde tracer injections in the VTA: cholera toxin
Supplementary Figure 1 Cholinergic neurons projecting to the VTA are concentrated in the caudal mesopontine region. (a) Schematic showing the sites of retrograde tracer injections in the VTA: cholera toxin
DISSECTION OF THE SHEEP'S BRAIN
 Sheep Brain Dissection Guide Page 1 DISSECTION OF THE SHEEP'S BRAIN Introduction The purpose of the sheep brain dissection is to familiarize you with the threedimensional structure of the brain and teach
Sheep Brain Dissection Guide Page 1 DISSECTION OF THE SHEEP'S BRAIN Introduction The purpose of the sheep brain dissection is to familiarize you with the threedimensional structure of the brain and teach
1. The cerebellum coordinates fine movement through interactions with the following motor-associated areas:
 DENT/OBHS 131 2009 Take-home test 4 Week 6: Take-home test (2/11/09 close 2/18/09) 1. The cerebellum coordinates fine movement through interactions with the following motor-associated areas: Hypothalamus
DENT/OBHS 131 2009 Take-home test 4 Week 6: Take-home test (2/11/09 close 2/18/09) 1. The cerebellum coordinates fine movement through interactions with the following motor-associated areas: Hypothalamus
Spinal Cord Protection. Chapter 13 The Spinal Cord & Spinal Nerves. External Anatomy of Spinal Cord. Structures Covering the Spinal Cord
 Spinal Cord Protection Chapter 13 The Spinal Cord & Spinal Nerves We are only going to cover Pages 420-434 and 447 Together with brain forms the CNS Functions spinal cord reflexes integration (summation
Spinal Cord Protection Chapter 13 The Spinal Cord & Spinal Nerves We are only going to cover Pages 420-434 and 447 Together with brain forms the CNS Functions spinal cord reflexes integration (summation
TWO TYPES OF PATTERN OF HIPPOCAMPAL ELECTRICAL ACTIVITY INDUCED BY STIMULATION OF HYPOTHALAMUS AND SURROUNDING PARTS OF RABBIT'S BRAIN
 TWO TYPES OF PATTERN OF HIPPOCAMPAL ELECTRICAL ACTIVITY INDUCED BY STIMULATION OF HYPOTHALAMUS AND SURROUNDING PARTS OF RABBIT'S BRAIN Shizuo TORII* Department of Physiology, School of Medicine, Toho University,
TWO TYPES OF PATTERN OF HIPPOCAMPAL ELECTRICAL ACTIVITY INDUCED BY STIMULATION OF HYPOTHALAMUS AND SURROUNDING PARTS OF RABBIT'S BRAIN Shizuo TORII* Department of Physiology, School of Medicine, Toho University,
PHYSIOLOHY OF BRAIN STEM
 PHYSIOLOHY OF BRAIN STEM Learning Objectives The brain stem is the lower part of the brain. It is adjoining and structurally continuous with the spinal cord. 1 Mid Brain 2 Pons 3 Medulla Oblongata The
PHYSIOLOHY OF BRAIN STEM Learning Objectives The brain stem is the lower part of the brain. It is adjoining and structurally continuous with the spinal cord. 1 Mid Brain 2 Pons 3 Medulla Oblongata The
Hippocampal theta activity related to elicitation and inhibition of approach locomotion
 Behavioural Brain Research 160 (2005) 236 249 Research report Hippocampal theta activity related to elicitation and inhibition of approach locomotion Harry M. Sinnamon Neuroscience and Behavior Program,
Behavioural Brain Research 160 (2005) 236 249 Research report Hippocampal theta activity related to elicitation and inhibition of approach locomotion Harry M. Sinnamon Neuroscience and Behavior Program,
M555 Medical Neuroscience Lab 1: Gross Anatomy of Brain, Crainal Nerves and Cerebral Blood Vessels
 M555 Medical Neuroscience Lab 1: Gross Anatomy of Brain, Crainal Nerves and Cerebral Blood Vessels Anatomical Directions Terms like dorsal, ventral, and posterior provide a means of locating structures
M555 Medical Neuroscience Lab 1: Gross Anatomy of Brain, Crainal Nerves and Cerebral Blood Vessels Anatomical Directions Terms like dorsal, ventral, and posterior provide a means of locating structures
nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
 Nucleus accumbens From Wikipedia, the free encyclopedia Brain: Nucleus accumbens Nucleus accumbens visible in red. Latin NeuroNames MeSH NeuroLex ID nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
Nucleus accumbens From Wikipedia, the free encyclopedia Brain: Nucleus accumbens Nucleus accumbens visible in red. Latin NeuroNames MeSH NeuroLex ID nucleus accumbens septi hier-259 Nucleus+Accumbens birnlex_727
Gross Organization I The Brain. Reading: BCP Chapter 7
 Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Distribution of GlyT2::eGFP fibers in the mouse thalamus at three different coronal levels. Note the innervation centered in the rostral (CL, PC) and caudal (PF) nuclear groups of
Supplementary Figure 1 Distribution of GlyT2::eGFP fibers in the mouse thalamus at three different coronal levels. Note the innervation centered in the rostral (CL, PC) and caudal (PF) nuclear groups of
The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible:
 NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
Sound waves from the auditory environment all combine in the ear canal to form a complex waveform. This waveform is deconstructed by the cochlea with
 1 Sound waves from the auditory environment all combine in the ear canal to form a complex waveform. This waveform is deconstructed by the cochlea with respect to time, loudness, and frequency and neural
1 Sound waves from the auditory environment all combine in the ear canal to form a complex waveform. This waveform is deconstructed by the cochlea with respect to time, loudness, and frequency and neural
Medical Neuroscience Tutorial Notes
 Medical Neuroscience Tutorial Notes Cranial Nerve Nuclei MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials,
Medical Neuroscience Tutorial Notes Cranial Nerve Nuclei MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials,
Nature Neuroscience: doi: /nn Supplementary Figure 1. Trial structure for go/no-go behavior
 Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
Supplementary Figure 1 Trial structure for go/no-go behavior a, Overall timeline of experiments. Day 1: A1 mapping, injection of AAV1-SYN-GCAMP6s, cranial window and headpost implantation. Water restriction
VENTRAL TEGMENTAL AREA LESIONS DIFFERENTIALLY AFFECT RESPONSES CONTROLLED BY CS-US CONTIGUITY AND RESPONSE-REINFORCER CONTINGENCY IN THE RAT
 ACTA NEUROBIOL. EXP. 1987, 47: 83-91 VENTRAL TEGMENTAL AREA LESIONS DIFFERENTIALLY AFFECT RESPONSES CONTROLLED BY CS-US CONTIGUITY AND RESPONSE-REINFORCER CONTINGENCY IN THE RAT W. Jeffrey WILSON and Ericka
ACTA NEUROBIOL. EXP. 1987, 47: 83-91 VENTRAL TEGMENTAL AREA LESIONS DIFFERENTIALLY AFFECT RESPONSES CONTROLLED BY CS-US CONTIGUITY AND RESPONSE-REINFORCER CONTINGENCY IN THE RAT W. Jeffrey WILSON and Ericka
Supplemental Information. A Visual-Cue-Dependent Memory Circuit. for Place Navigation
 Neuron, Volume 99 Supplemental Information A Visual-Cue-Dependent Memory Circuit for Place Navigation Han Qin, Ling Fu, Bo Hu, Xiang Liao, Jian Lu, Wenjing He, Shanshan Liang, Kuan Zhang, Ruijie Li, Jiwei
Neuron, Volume 99 Supplemental Information A Visual-Cue-Dependent Memory Circuit for Place Navigation Han Qin, Ling Fu, Bo Hu, Xiang Liao, Jian Lu, Wenjing He, Shanshan Liang, Kuan Zhang, Ruijie Li, Jiwei
Gross Morphology of Spinal Cord
 Gross Morphology of Spinal Cord Lecture Objectives Describe the gross anatomical features of the spinal cord. Describe the level of the different spinal segments compared to the level of their respective
Gross Morphology of Spinal Cord Lecture Objectives Describe the gross anatomical features of the spinal cord. Describe the level of the different spinal segments compared to the level of their respective
Supplementary Figure 1. GABA depolarizes the majority of immature neurons in the
 Supplementary Figure 1. GABA depolarizes the majority of immature neurons in the upper cortical layers at P3 4 in vivo. (a b) Cell-attached current-clamp recordings illustrate responses to puff-applied
Supplementary Figure 1. GABA depolarizes the majority of immature neurons in the upper cortical layers at P3 4 in vivo. (a b) Cell-attached current-clamp recordings illustrate responses to puff-applied
Cranial Nerve VIII (The Vestibulo-Cochlear Nerve)
 Cranial Nerve VIII (The Vestibulo-Cochlear Nerve) Please view our Editing File before studying this lecture to check for any changes. Color Code Important Doctors Notes Notes/Extra explanation Objectives
Cranial Nerve VIII (The Vestibulo-Cochlear Nerve) Please view our Editing File before studying this lecture to check for any changes. Color Code Important Doctors Notes Notes/Extra explanation Objectives
