Activation of HIV-1 expression in latently infected CD4+ T cells by the small molecule PKC412
|
|
|
- Dominic Baldwin
- 6 years ago
- Views:
Transcription
1 Ao et al. Virology Journal (2016) 13:177 DOI /s RESEARCH Open Access Activation of HIV-1 expression in latently infected CD4+ T cells by the small molecule PKC412 Zhujun Ao 1,2, Rong Zhu 2, Xiaoli Tan 1, Lisa Liu 1, Liyu Chen 1, Shuiping Liu 1 and XiaoJian Yao 2* Abstract Background: HIV-1 latency is a major obstacle for HIV-1 eradication. Extensive efforts are being directed toward the reactivation of latent HIV reservoirs with the aim of eliminating latently infected cells via the host immune system and/or virus-mediated cell lysis. Results: We screened over 1,500 small molecules and kinase inhibitors and found that a small molecule, PKC412 (midostaurin, a broad-spectrum kinase inhibitor), can stimulate viral transcription and expression from the HIV-1 latently infected ACH2 cell line and primary resting CD4+ T cells. PKC412 reactivated HIV-1 expression in ACH2 cells in a dose- and time-dependent manner. Our results also suggest that the nuclear factor κb (NF-κB) signaling could be one of cellular pathways activated during PKC412-mediated activation of latent HIV-1 expression. Additionally, combining PKC412 with the HDAC inhibitor vorinostat (VOR) had an additive effect on HIV-1 reactivation in both ACH2 cells and infected resting CD4+ T cells. Conclusions: These studies provide evidence that PKC412 is a new compound with the potential for optimization as a latency-reactivator to eradicate HIV-1 infection. Keywords: HIV latency, PKC412, NF-κB signaling, ACH2 cells, Resting CD4+ T cells Background HIV latency has been defined as a reversibly nonproductive state of infection of individual cells that retain the capacity to produce infectious virus particles but allow the virus to evade the host immune response [1]. Several mechanisms can silence HIV gene expression and replication in HIV-1 latently infected resting CD4 + T cells, especially transcriptional interference and the chromatin environment. Other transcription factors in addition to Tat that are required for viral expression, such as nuclear factor κb (NF-κB), positive transcription elongation factor b (P-TEFb), nuclear factor of activated T cells (NFAT) and activator protein 1 (AP-1), are either insufficiently expressed or sequestered in an inactive form in resting cells [2 4]. The chromatin environment also influences * Correspondence: xiao-jian.yao@umanitoba.ca Equal contributors 2 Laboratory of Molecular Human Retrovirology, Department of Medical Microbiology, Rady Faculty of Medicine, University of Manitoba, Winnipeg, MB, Canada Full list of author information is available at the end of the article the establishment and maintenance of proviral quiescence [5 9]. Chromatin remodeling enzymes such as histone deacetylases (HDACs) maintain a hypoacetylated state of local histones, which diminishes the accessibility of the nucleosomal DNA to transcription factors. In contrast, transcriptional activators such as NF-κB and NFAT,recruit histone acetyltransferases (HATs) and this results in an open or accessible DNA conformation that is more amenable to the binding of additional transcriptional activators, initiation factors, and RNA polymerase II, and leads to an active transcription [10]. In addition to HDAC activity, DNA methylation is another latency mechanism that inhibits HIV transcription [11 14]. HIV latency is the major barrier to an HIV-1 cure. Although combination antiretroviral therapy (cart) has efficiently decreased the viral load in patients, the proviral latency established within the host genome remains largely unaffected by cart and can replenish systemic infection following interruption of therapy [15, 16]. Therefore, extensive research efforts have been focused on finding ways to reactivate HIV-1 latent reservoirs and The Author(s) Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( applies to the data made available in this article, unless otherwise stated.
2 Ao et al. Virology Journal (2016) 13:177 Page 2 of 14 force them to be exposed to the antiretroviral therapy and the host immune system. Therapies that interfere with HDAC1 or DNA methylation are promising candidates to reactivate the suppressed virus and purge the latent HIV-1 reservoir. HDAC inhibitors (HDACIs) have received the most attention as potential latencyreversing agents. A panel of HDACIs, including trichostatin A (TSA), valproic acid (VPA), vorinostat (VOR, or suberoylanilide hydroxamic acid (SAHA)), can reactivate HIV-1 expression in latently infected cell lines, latently infected primary T cells and resting CD4+ T cells isolated from HIV-1 infected patients [17 20]. VOR has entered clinical trials to evaluate whether it can induce virus production in HIV-1-infected patients on cart [18 20]. In a 2012 pilot study, 11 of 16 patients treated with cart showed potential susceptibility to VOR [21]. Eight of these patients were administered one dose of VOR and they showed a marked increase in their HIV RNA expression levels compared with baseline. However, another study did not observe the induction of HIV production, in response to HDACIs including VOR, from latent viral reservoirs in aviremic individuals [22]. Recently, two new HDACIs (romidepsin and panobinostat) were shown to increase HIV-1 transcription and plasma RNA levels in ART-suppressed participants [23, 24]. However, although romidepsin and panobinostat effectively disrupted HIV latency in vivo, they did not affect the number of latently infected cells, suggesting that HDACIs might need to be combined with other approaches to reduce the latent HIV reservoir. Agents associated with T cell activation through signal transduction pathways can also usually reactivate HIV latency in cell models. The protein kinase C (PKC) signaling pathway plays an important role in NFAT, NF-κB, and AP-1 activation; these steps are essential for T cell activation. PKC agonists such as phorbol esters, prostratin, bryostatin-1, and ingenol are effective at inducing viral expression from both latently infected cell lines and primary cells [25 30]. Disulfiram is a latency-reversing agent (LRA) that can reactivate latent HIV-1 in primary CD4+ T cell models without inducing global T cell activation. Disulfiram reactivates latent expression through NF-κB pathway activation [31, 32]. However, a pilot study showed that disulfiram administration to patients receiving ART did not significantly reduce the size of the latent reservoir [33, 34]. PKC412 is a derivative of the naturally occurring alkaloid staurosporine and has been shown to inhibit the conventional PKC isoforms (α, β I, β II, and γ). PKC412 targets a wide range of cell signaling pathways, including the FMS-like tyrosine kinase (FLT3) receptor, PKC, vascular endothelial growth factor receptor (VEGFR2), and c-kit (stem cell factor) receptor pathways [35, 36]. Because of its potent anti-proliferative activity, PKC412 has successfully passed a phase II clinical trial for the treatment of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) [37 39]. Sharkey et al. found that PKC412 activity was associated with ROS generation, up-regulation of phosphorylated c-jun, increased AP-1, and NF-κB transcription activity [40]. The present study demonstrates that PKC412 potently activates HIV-1 latency from the HIV-1 latently infected ACH2 cell line and primary resting CD4+ T cells by activating the NF-κB pathway. This study provides new insights into activators of HIV latency and it also provides compelling evidence for a novel HIV eradication strategy. Methods Reagents, antibodies, cells, and viruses Approximately 1,500 synthesized small molecules from ChemBridge,Inc.(SanDiego,USA),asdescribedpreviously [41] and a Screen-well Kinase inhibitor library (Cat No. BML , total 80 inhibitors), obtained from Enzo Life Sciences (Farmingdale, New York, USA), were used to screen the potential HIV-1 latency reactivator. PKC412 was supplied by LC Laboratories (Woburn, USA). VOR was obtained through the NIH AIDS Reagent Program, Division of AIDS. PMA, butyrate, TNF-α, 5-aza-2 deoxycytidine (5-azac) and aphidicolin were purchased from Sigma-Aldrich, Stemcell Technologies, Abcam and Fisher Scientific, respectively. Anti-HIV-1 gp120 was obtained through NIH AIDS Reagent Program, Division of AIDS. Anti-HIV-1 p24 and was described previously [42]. Anti-β-tubulin, anti-acetyl-histone H3 and antihistone H4, anti-nf-κb p65 (C-20) and anti-p-nf-κb p65(ser 311 ) antibodies were purchased from Santa Cruz Biotechnology (Dallas, USA). Horseradish peroxidaseconjugated donkey anti-rabbit IgG or sheep anti-mouse IgG (Amersham Biosciences, Piscataway, USA) was used as the secondary antibody. Human embryonic kidney 293 T cells, A3.01 cells and HIV-infected ACH2 cells were cultured in Dulbecco s modified Eagle s medium (DMEM) or RPMI 1640 medium supplemented with 1 % or 10 % fetal bovine serum (FBS). Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy adult volunteers by sedimentation in Ficoll-Hypaque (Sigma-Aldrich, St. Louis, USA). CD4+ T lymphocytes were isolated from PBMCs by negative selection with the EasySep Human CD4 + CD25+ T Cell Isolation Kit (Stemcell Technologies, Vancouver, Canada). The HIV-1 pnl4.3 virus stock was generated by transfecting 293 T cells with the corresponding HIV-1 proviral DNA, as previously described [42]. Forty-eight hours post-transfection, the supernatants were collected
3 Ao et al. Virology Journal (2016) 13:177 Page 3 of 14 and subjected to ultracentrifugation (35,000 rpm for 1.5 h at 4 C) to concentrate the viral particles. Stimulation of latently infected cell lines or primary infected resting CD4+ T cells Screening agents that can reactivate HIV-1 latency The synthesized small molecules and kinase inhibitors were individually dissolved in DMSO at a concentration of 5 mg/ml and stored at 20 C until they were used. To screen the latency-reversing activity of these small molecules, each compound was added to a single well of a 96-well plate at a final concentration of 2 μm. Then, ACH2 cells cultured in RPMI medium supplemented with 1 % FBS for 2 days were immediately added to the 96-well plates containing the synthesized molecules. Two days later, the supernatants were collected and the HIV-associated Gag p24 levels were measured by anti-hiv p24 ELISA. DMSO-treated ACH2 cells were included as the negative control and PMA-treated ACH2 cells were included as a positive control. The stimulatory effect of PKC412 ACH2 cells were cultured in RPMI medium containing 1 % FBS for 2 days and then treated with different concentrations of PKC412, VOR, or other agents for different time points. PMA (2 ng/ml) and TNF-α (10 ng/ml) were used as the positive controls and DMSO was used as the negative control. After 48 h, the cell culture supernatants were collected and HIV p24 production was measured. To examine HIV-1 expression after pulse treatment with PKC412, ACH2 cells were treated with PKC412 (1 μm) for 8, 12, 16, and 24 h and then washed. After 48 h, the cell culture supernatants were collected and HIV p24 production were measured. The cells were subjected to the immunofluorescence or Western blotting assay. HIV-1-infected primary CD4+ T cell model Unstimulated primary CD4+ T lymphocytes isolated from PBMCs were cultured in RPMI medium supplemented with 10 % FBS and IL-2 (10 U/ml) for 2 days. Cells ( ) were infected with 10 ng (P24 Gag) of HIV-1 pnl-4.3 via spinoculation at 1400 rpm for 2 h at room temperature. After spinoculation, the cells were washed and cultured in RPMI medium containing IL-2 for 4 days. Then, the cells were incubated with PKC412 and/or VOR at the indicated concentration for 48 h. Virus production was measured in the supernatant by detecting the levels of HIV-1 Gag p24. NF-κB and AP-1 activity luciferase reporter assay VSV-G pseudotyped lentiviral particles (Cignal Lenti vector) expressing the reporter firefly luciferase gene under the control of a minimal (m)cmv promoter and tandem repeats of the AP-1 or NF-κB transcriptional response element (TRE) (QIAGEN, Hilden, Germany) were used to monitor NF-κB and AP-1 signaling activity upon treatment of the different cell lines with the compounds. Briefly, target cells were transduced with the Cignal Lenti vector. Following transduction, the cells were cultured under puromycin selection to generate a homogenous population of transduced cells. Then, the cultures were treated with PKC412 or TNF-α for 12 to 24 h and subjected to the luciferase assay [43]. Western blotting and immunofluorescence assays To detect various viral and cellular protein expressions, ACH2 cells were treated with PKC412 for h, and cells were lysed with RIPA buffer and run on a 12 % SDS- PAGE gel, followed by immunoblotting with various antibodies, including anti-hiv-1 gp120, anti-hiv-1 p24, anti-tubulin, anti-acetyl-histone H3, anti-histone H3, anti- NF-κB p65, or anti-p-nf-κb p65 (Ser 311 ). The protein bands were visualized using an enhanced chemiluminescence kit (Perkin Elmer Life Science, Boston, USA). For subcellular protein fractionation and detection, a Thermo Scientific Subcellular Protein Fractionation Kit (Thermo Scientific, Waltham, USA) was used to prepare cytoplasmic, nuclear fractions from ACH2 cells, according to manufacturer s recommended procedures. Then, each subcellular fraction was subjected to Western Blotting to detect NF-κB p65, Histone-4, β-tubulin by using specific antibodies. For the indirect immunofluorescence assay, ACH2 cells were treated with PKC412 and then plated on a slide, fixed and permeabilized with methanol/acetone (1:1 ratio) for 30 min at room temperature. The cells were incubated with the mouse anti-p24 antibody (1:100) for 2 h at 37 C, followed by incubation with an anti-mouse IgG-488 antibody (1:500) for 1 h. The slide was mounted with Mowiol 4 88 and the cells were visualized under an Axiovert 200 microscope (Carl Zeiss, Oberkochen, Germany). Cytotoxicity assay and cell cycle profiles The trypan blue and WST-1 cell proliferation assay (Roche, Basel, switzerland) were used to determine the effect PKC412 on the cell viability and cytotoxicity, as previously described [41]. Briefly, ACH2 cells or primary CD4+ T cells were cultured at a density of cells/ well in a 96-well format and maintained at 37 C in the presence of various concentrations of PKC412. On day 2, cell viability was assessed using a TC20 Automated Cell Counter (Bio-Rad) or WST-1 method. The concentration of PKC412 that resulted in a 50 % decrease in cell proliferation was defined as the cell culture inhibition concentration (CCID 50 ) of the compound.
4 Ao et al. Virology Journal (2016) 13:177 Page 4 of 14 The ACH2 cells were treated with PKC412 and/or aphidicolin for 24 h. The cell cycle profiles were measured by staining with propidium iodide (PI) and analyzed by flow cytometry. Chromatin immunoprecipitation (ChIP) assay Acetylation of Histone H3 at the HIV-1 LTR promoter ChIP assays were performed as previously described [44].Briefly, ACH2 cells were mock treated or treated with PKC412, VOR or butyrate for 48 h, and crossed-linked with 1.42 % formaldehyde. The reaction was stopped with 125 mm glycine. Cells were lysed and the cross-linked chromatin was sonicated to fragment base pairs using a Bioruptor standard sonicator. Immunoprecipitation reaction was performed with 5 μgof anti-acetyl-histone H3 (Ac-H3 catalogue #17 615, Millipore, Darmstadt, Germany) or rabbit pre-immune immunoglobulin G (negative control). PCR of immunoprecipitates was performed using primers targeting the Nuc1 region of HIV-1LTR. The primer sequences spanning +96 to +301 nucleotides within HIV-1 LTR were the following: forward 5-AGTAGTGTGTGCCCGTCTGT-3 and reverse 5-TTGGCGTACTCACCAGTCGC-3. ThecopyofHIV-1 promoter DNA was determined by comparing the cycle threshold values of each reaction to a standard curve generated from HIV-1 provirus DNA and is reported as fold change relative to negative control. The methylated DNA at HIV-1 LTR promoter and H19 imprinted control region (H19ICR) was detected using an EpiMark methylated DNA Enrichment kit (New England BioLabs, Ipswich, USA), according to manufacturer s protocol. Briefly, ACH2 cells were mock treated or treated with PKC412, 5-aza-2 deoxycytidine (5-azac) for 48 h. The DNA in the cells were fragmented and they bound to the methyl-cpg binding domain of human MBD2 protein fused to the Fc tail of human IgG1 (MBD2- Fc), which is coupled to protein A magnetic beads. Following the magnetic capture, the enriched DNA samples were eluted and the precipitated DNA was analyzed by PCR with primers for HIV-1 LTR U3 ( 300 to + 92; forward, 5 - GTTAGAGTGGAGGTTTGACAG-3 and reverse, 5 -AG ACCCAGTACAGGCAAAAAG-3 ) and H19ICR (forward, 5 -ATCCCCAGCCTTTTACTGAACT-3 and reverse, 5 - CAAACCTGCATTGAATGAG-3 ). For each reaction, 10 % of the recovered DNA was used as an import control. TheChIP-qPCRresultswerereportedastherelativefold of enrichment when comparing the ChIP fraction Ct values of input- and background- normalized experimental samples with that of the control sample. Measurement of intracellular HIV-1 RNA transcripts ACH2 cells were treated with PKC412 or/and VOR for 24 to 48 h and intracellular RNA was extracted using High Pure RNA isolation (Roche) and subjected to reverse transcription with MLV reverse transcriptase (Promega, Madison, USA). HIV transcription was measured by real time PCR using primers corresponding to the HIV 5 LTR and gag (R-gag) (forward, 5 -ATCAAGCAGCCATG CAAATG-3, and reverse, 5 -CTGAAGGGTACTAGTA GTTCC-3 ) and normalized to the GAPDH gene levels using following primers: 5-TGGGTGTGAACC ATGAGAAG-3; 5-ATGGACTGTGGTCATGAGTC-3. Statistical analysis Statistical analysis was performed using GraphPad Prism version 5.0 (GraphPad Software, La Jolla, USA). Results PKC412 reactivates HIV-1 expression in latently infected ACH2 cells The HIV-1 infected ACH2 cell line, which is a subclone of a chronically infected A3.01 T lymphocyte cell line that expresses the integrated HIV-1 genome at a very low level [45, 46], was used in this study to screen reactivating agents. To isolate the potential HIV-1 latency reactivator, a 1500-synthesized small molecule library that was previously described [41], and a kinase inhibitor library were screened at a final concentration of 2 μm. The HIV-1 expression stimulated by each molecule was measured with an HIV p24 ELISA. To induce a relative quiescent state in the in vitro cellular model, proliferating ACH2 cells were cultured in serum starvation medium containing only 1 % FBS starting 48 h before treatment [47]. As shown in Fig. 1a, among the screened compounds, PKC412 (also named as RHE-12) induced significant HIV-1 production in the ACH2 cells. PKC412, 4'-N-Benzoyl-staurosporine (Fig. 1b), is an orally available staurosporine derivative that inhibits protein kinase C. This effect of PKC412 on the activation of HIV-1 production was further evaluated by treating ACH2 cells with different concentrations of compound (ranging from 1 to 0.03 μm) (Fig. 1c). The DMSO (without PKC412)-treated cells were included as control. Result showed that PKC412 upregulated virus production in a dose-dependent manner. The effect of PKC412 on the activation of HIV-1 production in the serum starved ACH2 cells was more obvious than the effect in medium supplemented with 10 % FBS. Consistent with previous studies showing that PKC412 exhibited broad antiproliferative activity against various tumor and normal cell lines [48, 49], a proliferation inhibition effect of PKC412 was observed in proliferating ACH2 cells with a CCID 50 of 0.4 μm (Fig. 1d and data not shown). However, the cytotoxicity of PKC412 was relatively low in the serum-starved ACH2 cells and human resting CD4+ T cells (Fig. 1d). Therefore, the highest concentrations of PKC412 used in our study were 0.5 μm in the ACH2 cells and 1 μm in the human resting CD+ T cells.
5 Ao et al. Virology Journal (2016) 13:177 Page 5 of 14 a b A450 Value PKC synthesized small molecules and kinase inhibitors PKC412 Chemical structure c HIVp24 (pg/ml) %FBS 10%FBS The concentration of PKC412 (µm) 1 d Viable cells/well (x10 3 ) ACH2 cells (10% FBS) ACH2 cells (1% FBS) Resting CD4+ T cells The concentration of PKC412 (µm) Fig. 1 PKC412 stimulates HIV-1 expression in latently infected ACH2 cells. a A over 1,500 small molecules and kinase inhibitors were tested in HIV latently infected ACH2 cells in 96-well plates at a final concentration of 2 μm. After two days, the HIV-1 p24 level in each well was measured by ELISA. b PKC412 chemical structure. c ACH2 cells cultured in RPMI medium containing 1 % or 10 % FBS were treated with PKC412 at different concentrations for 48 h; then, HIV p24 production was measured in the cell culture supernatants. Error bars represent variations between duplicate samples and the data are representative of results obtained in three independent experiments. d Assessment of PKC412 cytotoxicity by the trypan blue dye exclusion assay. ACH2 cells in 1 % or 10 % FBS medium and human resting CD4+ T cells were treated with different PKC412 concentrations. After 48 h, the cells were assessed using the trypan blue dye exclusion assay and counted using a TC20 Automated Cell Counter. Error bars represent variation between duplicate samples and the data are representative of results obtained in three independent experiments We then examined whether PKC412-induced HIV-1 virus production occurred as a result of increased HIV-1 expression. A time course response experiment was performed in ACH2 cells treated with PKC412. Intracellular expression of the HIV-1 viral proteins was evaluated with anti-hiv p24 immunofluorescence and we found that the numbers of HIV Gag p24-positive cells increased in a time-dependent manner upon PKC412 treatment (Fig. 2a). The enhanced expression of HIV Gag p24, gp120, and gp160 in the ACH2 cells after PKC412 treatment was confirmed by Western blotting analysis (Fig. 2b). As expected, the increased viral protein expression levels in the cells treated with PKC412 corresponded with the augmented HIV-1 production detected in the culture supernatants (Fig. 2c), indicating that PKC412 stimulated viral protein expression. We analyzed whether PKC412 induced HIV-1 expression at the transcription level. Briefly, the quantitative PCR technique was used to monitor the HIV-1 mrna levels in ACH2 cells treated with or without PKC412 by measuring HIV-1 gag mrna expression using primers corresponding to the HIV 5 LTR and gag (R-gag). As shown in Fig. 2d, PKC412 increased the gag mrna levels by 1- to 6-fold at different concentrations, suggesting that PKC412 acted by inducing HIV transcription. Further evidence to support the effect of PKC412 on HIV-1 transcription was that PKC412 treatment increased HIV LTR-driven luciferase expression in the TZMb-1 cell line, which harbors an integrated HIV-1 LTR-driven luciferase gene (data not shown). No HIV proteins are expressed in TZMb-1 cells, suggesting that PKC412-mediated HIV LTR activation does not require the presence of viral proteins such as Tat. PKC412-induced cell cycle G2 arrest is not responsible for its HIV reactivation activity PKC412 inhibits multi-target tyrosine kinases and causes apoptosis and cell cycle arrest at the G1 or G2 phase [49 51]. Previous studies have shown that HIV-1 Vpr also is able to induce a cell cycle G2 arrest that provide
6 Ao et al. Virology Journal (2016) 13:177 Page 6 of 14 a 8h 16h 24h 48h 48h 48h PKC412 (0.5 µm ) PMA(2ng) DMSO b PKC412(0.5µM) DMSO PMA 8h 12h 16h 24h 48h 48h 48h -gp160 -gp120 -p24 - -tubulin c d HIVp24 (pg/ml) * * * Relative HIV-1 mrna * PKC412 (0.5 µm ) Time of treatemt PKC412 concentration (µm) Fig. 2 Pulse PKC412 treatment stimulates HIV-1 expression in ACH2 cells. ACH2 cells were pulse-treated with PKC412 (0.5 μm) for 8, 12, 16, 24, or 48 h. PMA (2 ng/ml) or DMSO-treated cells were used as the positive and negative controls, respectively. a After 24 h, the cells were fixed and labeled with an anti-hiv-1 p24 antibody/anti-mouse IgG-FITC antibody and visualized under the fluorescence microscope (10 magnification). b After 48 h, the cells were lysed and analyzed by SDS-PAGE followed by Western blotting with anti-hiv-1 gp120, anti-hiv-1 p24, and anti-tubulin antibodies. c The HIV p24 levels in supernatants were quantified using an HIV-1 p24 ELISA kit after 48 h. d ACH2 cells were incubated with RPMI medium (1 % FBS) containing different PKC412 concentrations. After 48 h, total RNA was extracted from the PKC412-treated or untreated ACH2 cells and HIV transcription was measured by real-time PCR using primers corresponding to the HIV 5 LTR and gag (R-gag). Transcription activity was calculated as the relative HIV-1 mrna level by setting the HIV-1 mrna level in the control ACH2 cells (without PKC412 treatment) to 1 arbitrary unit. Error bars represent variations between triplicate samples and the data are representative of results obtained in three independent experiments. The degree of significance for PKC412 treatment was relative to DMSO treatment. * p < 0.05, p <0.01 a favorable environment for stimulated HIV-1 LTRdriven transcription [52 54]. Therefore, we addressed whether G1 or G2 cell cycle arrest could contribute to the effect of PKC412 on the stimulation of HIV expression in ACH2 cells. As expected, PKC412 treatment caused G2 cell cycle arrest in the ACH2 cells (Fig. 3a). A significant enlargement in the cell size and cell death were observed 24 to 48 h post-pkc412
7 Ao et al. Virology Journal (2016) 13:177 Page 7 of 14 b a Mock PKC412, 24h PKC412(µM) PKC412, 48h Cell Count h Propidium iodide G1=22.00% G1=16.41% G2=20.62% G2=65.91% G2=83.59% S=46.26% S=12.10% S=0.00% Mock G1=27.55% G2=13.88% S=41.05% Aphidicolin + PKC412 G1=11.86% G2=6.68% S=81.46% 48h d 1 HIVp24 (pg/ml) c G1=33.12% P=0.03 * ns Fig. 3 The effect of PKC412 on HIV-1 re-activation is independent of PKC412-induced cell cycle G2 arrest. a ACH2 cells were treated with PKC412 (0.5 μm) for 24 h and the cell cycle profiles were measured by staining with propidium iodide (PI) and analyzed with flow cytometry. b After 24 or 48 h, the cells were fixed and visualized under a microscope (20 magnification). c ACH2 cells were treated with aphidicolin (2 μm) for 12 h and then PKC412 (0.5 μm) for another 24 h. The cell cycle profiles of the ACH2 cells were analyzed with flow cytometry. The plot shows cells in G0/G1 phase (Green), S phase (yellow) and G2/M phase (blue). d ACH2 cells were treated with aphidicolin (2 μm) and PKC412 (0.5 μm) alone or in combination. After 48 h, the HIV-1 p24 level in each well was measured by HIV-1 p24 ELISA. Error bars represent variations between duplicate samples and the data are representative of results obtained in three independent experiments. The results were significant (* p < 0.05 and p < 0.01) compared to the mock treated cells treatment (Fig. 3b). To elucidate the functional relationship between PKC412-mediated HIV reactivation and its G2 cell cycle arrest activity, we treated the ACH2 cells with aphidicolin for 12 h to arrest the cells at the G1/S border [55], and PKC412 was then added for another 24 h. Cell cycle profile analysis showed that aphidicolin treatment arrested the ACH2 cells in the G1/S phase (Fig. 3c) and modestly decreased viral production (Fig. 3d, bar 1). However, PKC412 treatment with aphidicolin still increased HIV-1 p24 expression after the cells were in G1/S phase (Fig. 3d, bar 4). It should be noticed that the HIV-1 p24 level increased in the presence of both aphidicolin and PKC412 was less than that of PKC412 treated alone (Fig. 3d, bar 4 vs. bar 2). This may be partially because of a negative effect of aphidicolin on viral expression (Fig. 3d, bar 3 vs. bar 1). Nevertheless, the results indicated that the ability of PKC412 to reactivate HIV was not dependent on the cell cycle G2 arrest.
8 Ao et al. Virology Journal (2016) 13:177 Page 8 of 14 DNA modifications are not associated with PKC412 s HIV reactivation activity Previous studies showed that treatment of latently HIV- 1-infected cell lines with HDACIs induced viral transcription [18 20, 56]. To test the possibility that PKC412 reactivated HIV by affecting histone acetylation, we examined the histone acetylation of ACH2 cells following PKC412 treatment by Western blotting using anti-acetyl-histone H3 and anti-histone H3 antibodies. The HDACIs VOR and butyrate were used as the positive controls. The results revealed that PKC412 did not have an effect on histone H3 acetylation (Fig. 4a line 2), whereas butyrate and VOR significantly increased the level of acetylated histone H3 (Fig. 4a, Lines 3 and 4). This result was confirmed using ChIP with anti-acetylhistone H3 followed by real-time PCR with primers located within the nucleosome-1 (nuc-1) of the HIV-1 LTR promoter (Fig. 4b). Because DNA methylation has been proposed as a vital transcription restriction factor that contributes to the maintenance of HIV latency and the promoter region in the 5 LTR of HIV-1 is epigenetically regulated by DNA methylation [12, 14, 57], we investigated the level of CpG methylation in the HIV-1 5 LTR after PKC412 a Mock PKC412 Butyrate VOR b Nuc-0 U3 R U5 GAG Nuc-1 6 hours -Ac-H3 -Histone H3 PCR 12 hours 24 hours Ac-H3 -Histone H3 -Ac-H3 -Histone H3 HIV-LTR Ac-H3 fold change ns c Relative fold of enrichement in MeDIP * * d HIV-1 P24 (pg/ml) d * * H19ICR HIV U3 Fig. 4 PKC412 reactivation is not associated with chromatin modulation. a Western blotting detection of acetylated histone H3 levels in latently infected cells. ACH2 cells were mock treated or treated with PKC412 (0.5 μm), butyrate (5 mm) or VOR (330 nm) and the cells were collected at different time points. Western blotting analysis was performed with anti-acetyl-histone H3 and anti-histone H3 antibodies. b The diagram shows the positions of the nucleosomes bound to the HIV-1 LTR and the location of the primers used for the real-time PCR in the ChIP assay (upper panel). Cells treated with or without PKC412 were assayed by ChIP with an anti-acetyl-histone H3 antibody. Immunoprecipitation with IgG served as the negative control. Cells treated with butyrate or VOR were used as the positive control. Real-time quantitation of the fold change relative to the negative control is shown (lower panel). c, d ACH2 cells were treated with PKC412 (0.5 μm) or the methyltransferase inhibitor 5-Azac (5 μm) for 24 h and the methylation levels of the H19 imprinted control region (ICR) and the HIV-1 LTR U3 region were detected by real-time PCR using specific primers following MeDIP ChIP with anti-5-methlcytosine (c). HIV-1 productions were measured with an HIV-1 p24 ELISA kit (d). Error bars represent variations between duplicate samples and the data are representative of results obtained in two independent experiments. The results were significant (* p < 0.05 and p < 0.01) compared to the mock treated cells
9 Ao et al. Virology Journal (2016) 13:177 Page 9 of 14 treatment using the ChIP method using anti-methylated cytosine followed by real-time PCR with primers located in the imprinting control region (ICR) of the H19 gene and U3 of the HIV-1 5 LTR. Cells treated with the methylation inhibitor 5-azac were used as the positive control. The results showed that although 5-azac decreased the DNA methylation of H19 ICR and the HIV- 1 LTR by approximately %, PKC412 only reduced DNA methylation by approximately 10 % (Fig. 4c), which was inconsistent with the level of p24 production detected in the culture supernatant (Fig. 4d). Taken together, these observations indicate that DNA acetylation and methylation events are not responsible for the HIV LTR activating effect of PKC412. PKC412 induces the HIV-1 LTR and promotes gene transcription by increasing NF-κB activity in ACH2 cells PKC412 was previously reported to upregulate c-jun phosphorylation and NF-κB and AP-1 transcription activity in human multiple myeloma cells [40]. Thus, we tested whether PKC412 could activate the NF-κB or AP- 1 signaling pathways by using NF-κB- or AP-1-Cignal Lenti luciferase report assay. After transducing 293 T and A3.01 cells (the parental CD4+ T cell line of ACH2 cells) with NF-κB-Cignal Lenti particles, the effect of the various treatments on the activity of the NF-κB or AP-1 signaling pathways can be easily detected by measuring the luc activity in transduced cells, as described in the Materials and Methods. The TNF-α treated cells were included as positive control. The results revealed that PKC412 treatment significantly activated the NF-κB activity in a dose-dependent manner in both the 293 T and A3.01 cells (Fig. 5a and b), whereas both VOR and 5- azac only showed a modest stimulating effect on the NF-κB pathway (Fig. 5a and data not shown). In contrast, PKC412 treatment did not show any stimulating effect the AP-1 signal pathway in, A3.01 cells, the parental cell lines of ACH2 (data not shown). We further tested the NF-κB-Cignal Lenti luciferase report assay in human resting CD4 + T cells, and the results showed that PKC412 induced approximately 2 3-fold higher luc activity than the mock transduced cells, whereas TNF α treatment induced 10-fold higher luc activity (Fig. 5c). These data suggest that NF-κB signing may be involved in the HIV reactivating activity of PKC412. To further assess how PKC-412 is able to activate NF-κB signaling, we first investigated the NK-κB nuclear translocation by detecting nucleus- and Fig. 5 PKC-412 activates the NF-kappa B signaling pathway. The 293 T cells (a), A3.01 cells (b), and resting PBMCs (d) were transduced by Cignal Lenti particles encoding a luciferase (luc) gene under the control of a minimal (m)cmv promoter (Promo) and tandem repeats of the NF-κB transcriptional response element (TRE). After being transduced, cells were treated with different concentrations of PKC412 and TNF-α for 12 h and activation of NF-κB signaling was detected by measuring the luc activity. d ACH2 cells were treated with various concentrations of PKC412 and TNF-α for 12 h, and then cells were fractionated into nuclear and cytoplasmic fractions. The nucleus- and cytoplasm-associated p65, histone-4 and β-tubulin were detected by WB with corresponding antibodies. e ACH2 cells were treated with various concentrations of PKC412 and TNF-α for 12 h. Then the cells were lysed, and Ser 311 phosphorylated p65 was detected by WB with corresponding antibody. Error bars represent variations between triplicate samples and the data are representative of results obtained in three independent experiments. The degree of significance for the PKC412 or TNF-α treatment was reported relative to the mock treatment. p < 0.01, * p < 0.001, and p <
10 Ao et al. Virology Journal (2016) 13:177 Page 10 of 14 cytoplasm-associated p65 levels after ACH2 cells being treated by PKC-412. PKC-412 treatment only resulted in a modest increase of nucleus-associated NFκB p65, compared to no treatment (Fig. 5d, lanes 3 4 to lane 1). Since it is known that NF-κB phosphorylation at position of Ser311 plays an important role in enhancing NF-κB-mediated gene transcription [58, 59], we also assessed the NF-κB Ser311 phosphorylation level at PKC-412 treated ACH2 cells. Interestingly, there was a significant increase in Ser311 phosphorylation of NF-κB p65 in ACH2 cells following PKC-412 treatment (Fig. 5e, compare lanes 3 5 to lane 1), indicating that PKC-412 treatment facilitates Ser311 phosphorylation of NF-κB, which may contribute to its increased transcription signaling in ACH2 cells. Additive effect of PKC412 and the HDACI VOR on the activation of HIV-1 expression in ACH2 cells and primary resting HIV-infected CD4+ T cells Multiple mechanisms contribute to the maintenance of proviral latency. Therefore, the use of combination latency reversal strategies might optimize the reactivation of silenced proviral DNA. The results described above indicate that PKC412 induced NF-κB signaling but not DNA modifications, whereas VOR primarily affected the level of acetylated histones. Therefore, we tested whether PKC412 acted synergistically with VOR on HIV expression. As expected, we observed a dose-dependent proviral response following increased exposure of up to 0.5 μm VOR or PKC412 in the ACH2 cells. Higher levels of virus production were observed when PKC412 and VOR were simultaneously added to the cell cultures (Fig. 6a). The HIV-1 transcript levels in the PKC412- and VOR-treated ACH2 cells were consistently increased, with the PKC412/VOR co-treated cells showing the highest level of HIV-1 transcription (Fig. 6b). Overall, the above results indicate that the combined use of PKC412 and VOR additively activates HIV-1 production in the ACH2 cells. To investigate whether PKC412 can activate virus expression from naturally HIV-1-infected resting CD4+ T cells, we established an HIV-1-infected primary resting CD4+ T cell model, as shown in Fig. 6c. The resting CD4+ T cells from the blood of 6 healthy donors was infected with HIV-1 for 4 days and then exposed to PKC412 (1 μm), VOR (1 μm), or PKC412 (1 μm) + VOR (1 μm) for 48 h. The supernatants were collected to measure the HIV p24 levels. The results showed that both VOR and PKC412 alone resulted in higher levels of viral expression than non-treated HIV-1-infected resting primary cell (p <0.01; (Fig. 6d). PKC412 plus VOR treatment induced the highest levels (3.6-fold) of HIV p24 antigen production compared to the non-treated cells (p < ; Fig. 6d). These results indicate that PKC412 is able to stimulate HIV expression in HIV-1- infected resting CD4+ T cells. Additionally, the PKC412 + VOR combined treatment can additively activate virus expression compared with PKC412 or VOR treatment alone (p < 0.01). Discussion Recently, many studies have focused on the development of molecules that can reactivate HIV-1 latent reservoirs to render them susceptible to antiretroviral therapy, viral cytopathic effects, and host immune responses. To date, the candidate molecules under evaluation include HDAC inhibitors (HDACIs), PKC agonists, agents that induce the JNK or Akt pathway, and DNA methylation inhibitors [18, 28, 31, 32, 56, 60 64]. Because multiple mechanisms contribute to the maintenance of proviral latency, a combined therapy targeting multiple pathways would be the most effective for the activation of HIV latency. Thus, it is important to identify new agents that can activate HIV latency through different pathways. In this report, we found that the PKC412, a broadspectrum kinase inhibitor with multiple known and unknown cellular targets, was able to reactivate viral transcription from an HIV-1 latently infected ACH2 cell line and HIV-infected primary resting CD4+ T cells. Our data also suggested that PKC412 mediated the activation of HIV-1 through its effect on the NF-κB Ser311 phosphorylation and signaling. Moreover, an additive effect on HIV-1 reactivation was observed when PKC412 was combined with the HDAC inhibitor VOR in both the ACH2 cells and primary resting CD4+ T cells. Previous studies indicated that some transcription factors, including NF-κB, P-TEFb, NFAT, and AP-1, were either insufficiently expressed or sequestered in an inactive form in HIV-1-infected resting CD4 + T cells [2 4]. It is conceivable that activation of the NF-κB or AP-1 signaling pathway could lead to activation of HIV expression even when the cells are in a quiescent non-dividing state. Because PKC412 was previously reported to up-regulate phosphorylation of c-jun, increased AP-1 and NF-κB transcription activities in human multiple myeloma cells [40], we tested the possibility that PKC412 stimulates HIV transcription in HIV latent infected cells through activating NF-κB or AP-1 signal pathways. Our analysis showed that PKC412 was able to up-regulate NF-κB signaling, but not AP-1, and activated HIV-1 production in latently infected ACH2 cells and human primary resting CD4+ T cells. It is known that NF-κB plays a major role in HIV-1 transcription by binding to κb siteswithinthe HIV-1 long terminal repeat (LTR) enhancer region [65]. Generally, NF-κB is found in the cytoplasm in resting human CD4+ T cells associated with its inhibitor
11 Ao et al. Virology Journal (2016) 13:177 Page 11 of 14 a HIVp24 (pg/ml) PKC412 VOR PKC412+VOR b Relative HIV-1 mrna P=0.016 P=0.01 * Concentration of PKC412 and VOR (µm) c Resting CD4 T cell Spinoculate with HIV-1 2 hours Culture cells with Activators Check HIV-1 p24 in supernatant Culture cells for 4 Days 2 Days Day -2 Day 0 Day 5 d Relative p24 values ratio P= P= Infected PKC412 VOR PKC412+VOR untreated Fig. 6 Additive effect of PKC412 and VOR on the activation of latent HIV-1 expression. a ACH2 cells were treated with different concentrations of PKC412 and/or VOR. After 48 h, the activating effect of PKC412 and/or VOR on HIV expression was determined by measuring the HIV p24 level in the supernatants. b HIV transcript levels in ACH2 cells treated with PKC412 and/or VOR were measured with real-time PCR using primers corresponding to the HIV 5 LTR and gag (R-gag) and calculated as relative HIV-1 mrna levels to non-treated ACH2 cells. c The schematic diagram of HIV infection in resting CD4+ T cells and the effects of PKC412 and/or VOR. d The infected resting CD4+ T cells from different donors were treated with PKC412 and/or VOR or untreated for 2 days. Then, the HIV Gag p24 levels from the supernatants were monitored by ELISA and calculated as relative P24 levels compared to the infected-untreated cells. The experiments were performed as triplicate repeats per assay for each donor. The mean values from each donor are shown with thestandarderrorofthemean(sem).thedegreeofsignificanceforpkc412 or VOR treatment was relative to the infected-untreated cells. * p <0.05,p < 0.01, * p < 0.001, and p < (IκBα), which masks the NF-κB nuclear localization signal. Some cellular stimuli trigger the phosphorylation of specific serine residues of the IκBα protein and result in the degradation of IκBα and the release of NF-κB to translocate into the nucleus and induce the transcription of many genes by binding to specific consensus sequences in their promoter regions. In addition, it is known that release from IκB may not be insufficient to allow full activation of NF-κB. Several posttranslational modifications, including phosphorylation of Ser 311,inNF-κB afteriκb degradation appear to regulate DNA binding and transcriptional transactivation [66, 67]. Our data reveals that PKC412 can significantly increase Ser 311 phosphorylation level of NF-κB (Fig. 5e). Several studies have indicated that Ser 311
12 Ao et al. Virology Journal (2016) 13:177 Page 12 of 14 phosphorylation can enhance NF-κB-mediated gene transcription without affecting nuclear localization [58]. Previous studies showed that an amino acid Lys310 in NFκB could either be acetylated or methylated, which in turn up- or down-regulates NF-κB s transactivation activity [68, 69]. Because Lys310 is in close proximity to Ser311, it has been speculated that the Ser 311 phosphorylation may play a regulatory role for either acetylation or methylation of Lys310, which in turn optimizes or suppresses NF-κB transactivation activity [59]. PKC412-induced increased Ser 311 phosphorylation may be one of the mechanisms that activates this effect on HIV transcription. In addition, a previous report also showed that PKC412 treatment leads to a significant elevation of HSP90 [40], which has been shown to play an important role in controlling HIV-1 reactivation from latency [70, 71]. More detailed studies will be required to elucidate the functional association of PKC-412-mediated NF-κB Ser 311 phosphorylation and increased HSP90 expression and their roles in HIV activation. In the ACH2 cell line, both VOR and PKC412 could increase the p24 level by approximately 4.5-fold, (Fig. 6a). However, in human resting CD4+ T cells, the capacity of PKC412 to induce viral gene expression was a little lower than that of VOR (Fig. 6d). VOR is an extensively studied HDAC inhibitor that induces HIV transcription in latently infected cell lines and resting CD4+ T cells from HIV-infected patients on suppressive cart [18, 21]. However, a recent study found that the levels of HIV production stimulated from resting CD4+ T cells from aviremic donors by VOR were not significantly different from those of control treated cells [22]. A previous study demonstrated a strong synergistic activation of HIV-1 production by clinically used HDACIs including VOR combined with the nontumor-promoting NF-κB inducer prostratin in latently infected cell lines and resting CD4+ T cells isolated from cart-treated patients [72]. Therefore, we tested the effect of combining VOR and PKC412 on viral transcription and expression and detected an additive effect in ACH2 cells (Fig. 6a and b). We also extended our studies to resting CD4+ T cells, which are the primary reservoir of HIV-1, and documented that PKC412 or VOR enhanced HIV p24 production by fold, whereas the PKC412 + VOR combined treatment resulted in an approximate increase of p24 production of 3.6-fold. Our study did not observe a stimulating effect of PKC412 on HIV production in proliferating CD4+ T cells (data not shown), suggesting a possible specific effect on HIV expression activation in resting T cells. More studies are needed to investigate PKC412 s latent-reactivating effects alone or in combination with VOR or other latent reactive agents in resting CD4+ T cells isolated from HIV-1 infected individuals on ART and to evaluate the feasibility of developing this agent for HIV eradication. Conclusions In this study, we demonstrated that the small molecule PKC412 (a broad-spectrum kinase inhibitor), induced viral transcription from latently HIV-1-infected ACH2 cells and primary resting CD4+ T cells by increasing NFκB signaling. The combination of PKC412 with the HDAC inhibitor VOR had an additive effect on HIV-1 reactivation, suggesting that PKC412 has the potential for optimization as a latency-reactivator for the eradication of HIV-1 infection. Abbreviations NF-κB: Nuclear factor κb; VOR: Vorinostat; cart: Combination antiretroviral therapy; HDACs: Histone deacetylases; HDACIs: Histone deacetylases inhibitor; PKC: Protein kinase C; P-TEFb: Positive transcription elongation factor b; NFAT: Nuclear factor of activated T cells; AP-1: Activator protein 1; ChIP: Chromatin immunoprecipitation; 5-azac: 5-aza-2 deoxycytidine Acknowledgments We would like to thank Mr. J. Huang and Ms. T. Tian for technique support. We also thank Dr. Jodi Smith for proofreading the manuscript. The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: ACH-2 (Cat# 349) from Dr. Thomas Folks; and SAHA (Vorinostat) (Cat# 12130). X-J. Yao is a recipient of the Manitoba Research Chair Award. Funding This work was financially supported by grants from Canadian Institute of Health Research (CIHR) (HBF )/Canadian HIV Vaccine Initiative (CHVI) Bridge Funding (201309OCB), CIHR/MHRC RPP grant (RPA ), the Canadian Foundation for AIDS Research (CANFAR grant# ), and National Natural Science Foundation of China (NNSFC ). Authors contributions ZA and XY conceived and designed the experiments; ZA, RZ, XT, LL, LC and SL performed the experiments; and ZA and XY analyzed the data and wrote the paper. All authors read and approved the final manuscript. Competing interests The authors declare that they have no competing interests. Author details 1 Department of Microbiology, School of Basic Medical Sciences, Central South University, Changsha, Hunan , People s Republic of China. 2 Laboratory of Molecular Human Retrovirology, Department of Medical Microbiology, Rady Faculty of Medicine, University of Manitoba, Winnipeg, MB, Canada. Received: 20 May 2016 Accepted: 13 October 2016 References 1. Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012;37: Williams SA, Kwon H, Chen LF, Greene WC. Sustained induction of NF-kappa B is required for efficient expression of latent human immunodeficiency virus type 1. J Virol. 2007;81: Kilareski EM, Shah S, Nonnemacher MR, Wigdahl B. Regulation of HIV-1 transcription in cells of the monocyte-macrophage lineage. Retrovirology. 2009;6: Cary DC, Fujinaga K, Peterlin BM. Molecular mechanisms of HIV latency. J Clin Invest. 2016;126: Van Lint C. Role of chromatin in HIV-1 transcriptional regulation. Adv Pharmacol. 2000;48:
13 Ao et al. Virology Journal (2016) 13:177 Page 13 of Quivy V, Van Lint C. Diversity of acetylation targets and roles in transcriptional regulation: the human immunodeficiency virus type 1 promoter as a model system. Biochem Pharmacol. 2002;64: Jordan A, Defechereux P, Verdin E. The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J. 2001;20: Lusic M, Marcello A, Cereseto A, Giacca M. Regulation of HIV-1 gene expression by histone acetylation and factor recruitment at the LTR promoter. EMBO J. 2003;22: Pearson R, Kim YK, Hokello J, Lassen K, Friedman J, Tyagi M, Karn J. Epigenetic silencing of human immunodeficiency virus (HIV) transcription by formation of restrictive chromatin structures at the viral long terminal repeat drives the progressive entry of HIV into latency. J Virol. 2008;82: Schiralli Lester GM, Henderson AJ. Mechanisms of HIV Transcriptional Regulation and Their Contribution to Latency. Mol Biol Int. 2012;2012: Bednarik DP, Cook JA, Pitha PM. Inactivation of the HIV LTR by DNA CpG methylation: evidence for a role in latency. EMBO J. 1990;9: Kauder SE, Bosque A, Lindqvist A, Planelles V, Verdin E. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathog. 2009;5:e Schulze-Forster K, Gotz F, Wagner H, Kroger H, Simon D. Transcription of HIV1 is inhibited by DNA methylation. Biochem Biophys Res Commun. 1990;168: Blazkova J, Trejbalova K, Gondois-Rey F, Halfon P, Philibert P, Guiguen A, Verdin E, Olive D, Van Lint C, Hejnar J, Hirsch I. CpG methylation controls reactivation of HIV from latency. PLoS Pathog. 2009;5:e Harrigan PR, Whaley M, Montaner JS. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. Aids. 1999;13:F Zhang L, Chung C, Hu BS, He T, Guo Y, Kim AJ, Skulsky E, Jin X, Hurley A, Ramratnam B, et al. Genetic characterization of rebounding HIV-1 after cessation of highly active antiretroviral therapy. J Clin Invest. 2000;106: ArchinNM,EspesethA,ParkerD,CheemaM,HazudaD,MargolisDM.Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses. 2009;25: Contreras X, Schweneker M, Chen CS, McCune JM, Deeks SG, Martin J, Peterlin BM. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284: Edelstein LC, Micheva-Viteva S, Phelan BD, Dougherty JP. Short communication: activation of latent HIV type 1 gene expression by suberoylanilide hydroxamic acid (SAHA), an HDAC inhibitor approved for use to treat cutaneous T cell lymphoma. AIDS Res Hum Retroviruses. 2009;25: Lehrman G, Hogue IB, Palmer S, Jennings C, Spina CA, Wiegand A, Landay AL, Coombs RW, Richman DD, Mellors JW, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366: Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487: Blazkova J, Chun TW, Belay BW, Murray D, Justement JS, Funk EK, Nelson A, Hallahan CW, Moir S, Wender PA, Fauci AS. Effect of histone deacetylase inhibitors on HIV production in latently infected, resting CD4(+) T cells from infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2012;206: Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV. 2014;1:e Sogaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, et al. The Depsipeptide Romidepsin Reverses HIV-1 Latency In Vivo. PLoS Pathog. 2015;11:e Warrilow D, Gardner J, Darnell GA, Suhrbier A, Harrich D. HIV type 1 inhibition by protein kinase C modulatory compounds. AIDS Res Hum Retroviruses. 2006;22: Marquez N, Calzado MA, Sanchez-Duffhues G, Perez M, Minassi A, Pagani A, Appendino G, Diaz L, Munoz-Fernandez MA, Munoz E. Differential effects of phorbol-13-monoesters on human immunodeficiency virus reactivation. Biochem Pharmacol. 2008;75: Bedoya LM, Marquez N, Martinez N, Gutierrez-Eisman S, Alvarez A, Calzado MA, Rojas JM, Appendino G, Munoz E, Alcami J. SJ23B, a jatrophane diterpene activates classical PKCs and displays strong activity against HIV in vitro. Biochem Pharmacol. 2009;77: Kulkosky J, Culnan DM, Roman J, Dornadula G, Schnell M, Boyd MR, Pomerantz RJ. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood. 2001;98: Kulkosky J, Sullivan J, Xu Y, Souder E, Hamer DH, Pomerantz RJ. Expression of latent HAART-persistent HIV type 1 induced by novel cellular activating agents. AIDS Res Hum Retroviruses. 2004;20: Abreu CM, Price SL, Shirk EN, Cunha RD, Pianowski LF, Clements JE, Tanuri A, Gama L. Dual role of novel ingenol derivatives from Euphorbia tirucalli in HIV replication: inhibition of de novo infection and activation of viral LTR. PLoS One. 2014;9:e Doyon G, Zerbato J, Mellors JW, Sluis-Cremer N. Disulfiram reactivates latent HIV-1 expression through depletion of the phosphatase and tensin homolog. AIDS. 2013;27:F7 F Xing S, Bullen CK, Shroff NS, Shan L, Yang HC, Manucci JL, Bhat S, Zhang H, Margolick JB, Quinn TC, et al. Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J Virol. 2011;85: Spivak AM, Andrade A, Eisele E, Hoh R, Bacchetti P, Bumpus NN, Emad F, Buckheit 3rd R, McCance-Katz EF, Lai J, et al. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin Infect Dis. 2014;58: Elliott JH, McMahon JH, Chang CC, Lee SA, Hartogensis W, Bumpus N, Savic R, Roney J, Hoh R, Solomon A, et al. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV. 2015;2:e Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, Gilliland DG, Griffin JD. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1: Fabbro D, Ruetz S, Bodis S, Pruschy M, Csermak K, Man A, Campochiaro P, Wood J, O'Reilly T, Meyer T. PKC412 a protein kinase inhibitor with a broad therapeutic potential. Anticancer Drug Des. 2000;15: Monnerat C, Henriksson R, Le Chevalier T, Novello S, Berthaud P, Faivre S, Raymond E. Phase I study of PKC412 (N-benzoyl-staurosporine), a novel oral protein kinase C inhibitor, combined with gemcitabine and cisplatin in patients with non-small-cell lung cancer. Ann Oncol. 2004;15: Fischer T, Stone RM, Deangelo DJ, Galinsky I, Estey E, Lanza C, Fox E, Ehninger G, Feldman EJ, Schiller GJ, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28: Stone RM, De Angelo J, Galinsky I, Estey E, Klimek V, Grandin W, Lebwohl D, Yap A, Cohen P, Fox E, et al. PKC 412 FLT3 inhibitor therapy in AML: results of a phase II trial. Ann Hematol. 2004;83 Suppl 1:S Sharkey J, Khong T, Spencer A. PKC412 demonstrates JNK-dependent activity against human multiple myeloma cells. Blood. 2007;109: Chen L, Ao Z, Jayappa KD, Kobinger G, Liu S, Wu G, Wainberg MA, Yao X. Characterization of antiviral activity of benzamide derivative AH0109 against HIV-1 infection. Antimicrob Agents Chemother. 2013;57: Ao Z, Yu Z, Wang L, Zheng Y, Yao X. Vpr14-88-Apobec3G fusion protein is efficiently incorporated into Vif-positive HIV-1 particles and inhibits viral infection. PLoS ONE. 2008;3:e Ao Z, Danappa Jayappa K, Wang B, Zheng Y, Kung S, Rassart E, Depping R, Kohler M, Cohen EA, Yao X. Importin alpha3 interacts with HIV-1 integrase and contributes to HIV-1 nuclear import and replication. J Virol. 2010;84: Nelson JD, Denisenko O, Bomsztyk K. Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat Protoc. 2006;1: Pomerantz RJ, Trono D, Feinberg MB, Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990;61: Emiliani S, Van Lint C, Fischle W, Paras Jr P, Ott M, Brady J, Verdin E. A point mutation in the HIV-1 Tat responsive element is associated with postintegration latency. Proc Natl Acad Sci U S A. 1996;93: Larsson O, Zetterberg A, Engstrom W. Cell-cycle-specific induction of quiescence achieved by limited inhibition of protein synthesis: counteractive effect of addition of purified growth factors. J Cell Sci. 1985;73:
Development of 5 LTR DNA methylation of latent HIV-1 provirus in cell line models and in long-term-infected individuals
 Trejbalová et al. Clinical Epigenetics (2016) 8:19 DOI 10.1186/s13148-016-0185-6 RESEARCH Development of 5 LTR DNA methylation of latent HIV-1 provirus in cell line models and in long-term-infected individuals
Trejbalová et al. Clinical Epigenetics (2016) 8:19 DOI 10.1186/s13148-016-0185-6 RESEARCH Development of 5 LTR DNA methylation of latent HIV-1 provirus in cell line models and in long-term-infected individuals
Supporting Information
 Supporting Information Palmisano et al. 10.1073/pnas.1202174109 Fig. S1. Expression of different transgenes, driven by either viral or human promoters, is up-regulated by amino acid starvation. (A) Quantification
Supporting Information Palmisano et al. 10.1073/pnas.1202174109 Fig. S1. Expression of different transgenes, driven by either viral or human promoters, is up-regulated by amino acid starvation. (A) Quantification
Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression
 Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Supplementary Figure 1 Supplementary Figure 1 Role of Raf-1 in TLR2-Dectin-1-mediated cytokine expression. Quantitative real-time PCR of indicated mrnas in DCs stimulated with TLR2-Dectin-1 agonist zymosan
Targeting latent HIV infection: on the road towards an HIV Cure
 Towards an HIV Cure Pre-Conference Symposium 20 & 21 July 2012 Targeting latent HIV infection: on the road towards an HIV Cure David M. Margolis, MD Professor of Medicine Treatment Cure Prevention Prevention
Towards an HIV Cure Pre-Conference Symposium 20 & 21 July 2012 Targeting latent HIV infection: on the road towards an HIV Cure David M. Margolis, MD Professor of Medicine Treatment Cure Prevention Prevention
Towards an HIV Cure. Steven G. Deeks Professor of Medicine University of California, San Francisco
 Towards an HIV Cure Steven G. Deeks Professor of Medicine University of California, San Francisco Why are we now talking about a cure? Emerging recognition that HAART does not fully restore health and/or
Towards an HIV Cure Steven G. Deeks Professor of Medicine University of California, San Francisco Why are we now talking about a cure? Emerging recognition that HAART does not fully restore health and/or
Human Immunodeficiency Virus
 Human Immunodeficiency Virus Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Viruses and hosts Lentivirus from Latin lentis (slow), for slow progression of disease
Human Immunodeficiency Virus Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Viruses and hosts Lentivirus from Latin lentis (slow), for slow progression of disease
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Tel: ; Fax: ;
 Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
Can we eradicate HIV?
 Can we eradicate HIV? New therapeutic strategies towards a cure Laurence COLIN Laboratory of Molecular Virology University of Brussels Belgium The success story of HAART encountered solid barriers to virus
Can we eradicate HIV? New therapeutic strategies towards a cure Laurence COLIN Laboratory of Molecular Virology University of Brussels Belgium The success story of HAART encountered solid barriers to virus
BRIEF REPORT METHODS. Twenty-seven HIV-infected individuals receiving ART for a median of 2 years were included in this study (Table 1).
 BRIEF REPORT Effect of Histone Deacetylase Inhibitors on HIV Production in Latently Infected, Resting CD4 + T Cells From Infected Individuals Receiving Effective Antiretroviral Therapy Jana Blazkova, 1,a
BRIEF REPORT Effect of Histone Deacetylase Inhibitors on HIV Production in Latently Infected, Resting CD4 + T Cells From Infected Individuals Receiving Effective Antiretroviral Therapy Jana Blazkova, 1,a
HIV transcription, Tat transactivation mrna processing and latency
 HIV transcription, Tat transactivation mrna processing and latency Tat transactivation Roles of CTD kinases in HIV replication Latency and reservoir Strategies to eliminate the reservoir of HIV in the
HIV transcription, Tat transactivation mrna processing and latency Tat transactivation Roles of CTD kinases in HIV replication Latency and reservoir Strategies to eliminate the reservoir of HIV in the
Supplementary information. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins
 Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Doctoral Degree Program in Marine Biotechnology, College of Marine Sciences, Doctoral Degree Program in Marine Biotechnology, Academia Sinica, Taipei,
 Cyclooxygenase 2 facilitates dengue virus replication and serves as a potential target for developing antiviral agents Chun-Kuang Lin 1,2, Chin-Kai Tseng 3,4, Yu-Hsuan Wu 3,4, Chih-Chuang Liaw 1,5, Chun-
Cyclooxygenase 2 facilitates dengue virus replication and serves as a potential target for developing antiviral agents Chun-Kuang Lin 1,2, Chin-Kai Tseng 3,4, Yu-Hsuan Wu 3,4, Chih-Chuang Liaw 1,5, Chun-
Nature Medicine: doi: /nm.2109
 HIV 1 Infects Multipotent Progenitor Cells Causing Cell Death and Establishing Latent Cellular Reservoirs Christoph C. Carter, Adewunmi Onafuwa Nuga, Lucy A. M c Namara, James Riddell IV, Dale Bixby, Michael
HIV 1 Infects Multipotent Progenitor Cells Causing Cell Death and Establishing Latent Cellular Reservoirs Christoph C. Carter, Adewunmi Onafuwa Nuga, Lucy A. M c Namara, James Riddell IV, Dale Bixby, Michael
HIV Cure Update. Christine Durand, MD 14 de abril de 2016, XIII Conferência Brasil Johns Hopkins University em HIV/AIDS
 HIV Cure Update Christine Durand, MD 14 de abril de 2016, XIII Conferência Brasil Johns Hopkins University em HIV/AIDS Financial Disclosures Research grants paid to my institution: Gilead Sciences, Bristol
HIV Cure Update Christine Durand, MD 14 de abril de 2016, XIII Conferência Brasil Johns Hopkins University em HIV/AIDS Financial Disclosures Research grants paid to my institution: Gilead Sciences, Bristol
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Supplementary Data Table of Contents:
 Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Compounds producing an effective combinatorial regimen for disruption of HIV-1 latency
 Research Article Compounds producing an effective combinatorial regimen for disruption of HIV-1 latency Pargol Hashemi 1, Kris Barreto 1, Wendy Bernhard 1, Adam Lomness 1, Nicolette Honson 2, Tom A Pfeifer
Research Article Compounds producing an effective combinatorial regimen for disruption of HIV-1 latency Pargol Hashemi 1, Kris Barreto 1, Wendy Bernhard 1, Adam Lomness 1, Nicolette Honson 2, Tom A Pfeifer
HIV Remission: Compound screening and optimization. Michael D. Miller
 HIV Remission: Compound screening and optimization Michael D. Miller Can we drive HIV into remission in large numbers of patients? "George Bernard Shaw, speaking as an Irishman, summed up an approach to
HIV Remission: Compound screening and optimization Michael D. Miller Can we drive HIV into remission in large numbers of patients? "George Bernard Shaw, speaking as an Irishman, summed up an approach to
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Data Sheet. NFAT Reporter (Luc) Jurkat Cell line Catalog #: 60621
 Data Sheet NFAT Reporter (Luc) Jurkat Cell line Catalog #: 60621 Background The nuclear factor of activator T cells (NFAT) family of transcription factors plays an important role in immune response. T
Data Sheet NFAT Reporter (Luc) Jurkat Cell line Catalog #: 60621 Background The nuclear factor of activator T cells (NFAT) family of transcription factors plays an important role in immune response. T
MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands)
 Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Disulfiram Reactivates Latent HIV-1 in a Bcl-2-Transduced Primary CD4 T Cell Model without Inducing Global T Cell Activation
 JOURNAL OF VIROLOGY, June 2011, p. 6060 6064 Vol. 85, No. 12 0022-538X/11/$12.00 doi:10.1128/jvi.02033-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Disulfiram Reactivates
JOURNAL OF VIROLOGY, June 2011, p. 6060 6064 Vol. 85, No. 12 0022-538X/11/$12.00 doi:10.1128/jvi.02033-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Disulfiram Reactivates
Recombinant Protein Expression Retroviral system
 Recombinant Protein Expression Retroviral system Viruses Contains genome DNA or RNA Genome encased in a protein coat or capsid. Some viruses have membrane covering protein coat enveloped virus Ø Essential
Recombinant Protein Expression Retroviral system Viruses Contains genome DNA or RNA Genome encased in a protein coat or capsid. Some viruses have membrane covering protein coat enveloped virus Ø Essential
SUPPLEMENTARY INFORMATION
 Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
HIV Eradication: Combinatorial Approaches to Activate Latent Viruses
 Viruses 2014, 6, 4581-4608; doi:10.3390/v6114581 Review OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses HIV Eradication: Combinatorial Approaches to Activate Latent Viruses Elisa De Crignis
Viruses 2014, 6, 4581-4608; doi:10.3390/v6114581 Review OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses HIV Eradication: Combinatorial Approaches to Activate Latent Viruses Elisa De Crignis
IAS 2013 Towards an HIV Cure Symposium
 Entinostat is a histone deacetylase (HDAC) inhibitor selective for class 1 HDACs and activates HIV production from latently infected primary T-cells Fiona Wightman Monash University, Melbourne, Australia
Entinostat is a histone deacetylase (HDAC) inhibitor selective for class 1 HDACs and activates HIV production from latently infected primary T-cells Fiona Wightman Monash University, Melbourne, Australia
Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice
 Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 EGFR inhibition activates signaling pathways (a-b) EGFR inhibition activates signaling pathways (a) U251EGFR cells were treated with erlotinib (1µM) for the indicated times followed
Supplementary Figure 1 EGFR inhibition activates signaling pathways (a-b) EGFR inhibition activates signaling pathways (a) U251EGFR cells were treated with erlotinib (1µM) for the indicated times followed
VIROLOGY. Engineering Viral Genomes: Retrovirus Vectors
 VIROLOGY Engineering Viral Genomes: Retrovirus Vectors Viral vectors Retrovirus replicative cycle Most mammalian retroviruses use trna PRO, trna Lys3, trna Lys1,2 The partially unfolded trna is annealed
VIROLOGY Engineering Viral Genomes: Retrovirus Vectors Viral vectors Retrovirus replicative cycle Most mammalian retroviruses use trna PRO, trna Lys3, trna Lys1,2 The partially unfolded trna is annealed
Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation
 Technical advance Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation Hung-Chih Yang, 1 Sifei Xing, 1,2 Liang
Technical advance Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation Hung-Chih Yang, 1 Sifei Xing, 1,2 Liang
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
Supplementary Information. Supplementary Figure 1
 Supplementary Information Supplementary Figure 1 1 Supplementary Figure 1. Functional assay of the hcas9-2a-mcherry construct (a) Gene correction of a mutant EGFP reporter cell line mediated by hcas9 or
Supplementary Information Supplementary Figure 1 1 Supplementary Figure 1. Functional assay of the hcas9-2a-mcherry construct (a) Gene correction of a mutant EGFP reporter cell line mediated by hcas9 or
Pre-made Reporter Lentivirus for NF-κB Signal Pathway
 Pre-made Reporter for NF-κB Signal Pathway Cat# Product Name Amounts LVP965-P or: LVP965-P-PBS NFKB-GFP (Puro) LVP966-P or: LVP966-P-PBS NFKB-RFP (Puro) LVP967-P or: LVP967-P-PBS NFKB-Luc (Puro) LVP968-P
Pre-made Reporter for NF-κB Signal Pathway Cat# Product Name Amounts LVP965-P or: LVP965-P-PBS NFKB-GFP (Puro) LVP966-P or: LVP966-P-PBS NFKB-RFP (Puro) LVP967-P or: LVP967-P-PBS NFKB-Luc (Puro) LVP968-P
Oncolytic Viruses as a Potential Approach to Eliminate the HIV Reservoir
 Oncolytic Viruses as a Potential Approach to Eliminate the HIV Reservoir Costiniuk CT, Côté SC, Al-Ghazawi FM, Carrasco-Medina L,Young CD, & Angel JB University of Ottawa, November 12 th, 2012 HIV Reservoirs
Oncolytic Viruses as a Potential Approach to Eliminate the HIV Reservoir Costiniuk CT, Côté SC, Al-Ghazawi FM, Carrasco-Medina L,Young CD, & Angel JB University of Ottawa, November 12 th, 2012 HIV Reservoirs
Impact factor: Reporter:4A1H0019 Chen Zi Hao 4A1H0023 Huang Wan ting 4A1H0039 Sue Yi Zhu 4A1H0070 Lin Guan cheng 4A1H0077 Chen Bo xuan
 Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway Wei Yu,1,2 Wenliang Zha,1 Zhiqiang Ke,1 Qing Min,2 Cairong Li,1 Huirong Sun,3 and Chao
Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway Wei Yu,1,2 Wenliang Zha,1 Zhiqiang Ke,1 Qing Min,2 Cairong Li,1 Huirong Sun,3 and Chao
Supplemental Information
 Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Supplemental Information Tobacco-specific Carcinogen Induces DNA Methyltransferases 1 Accumulation through AKT/GSK3β/βTrCP/hnRNP-U in Mice and Lung Cancer patients Ruo-Kai Lin, 1 Yi-Shuan Hsieh, 2 Pinpin
Targeted Derepression of the Human Immunodeficiency Virus Type 1 Long Terminal Repeat by Pyrrole-Imidazole Polyamides
 JOURNAL OF VIROLOGY, Dec. 2002, p. 12349 12354 Vol. 76, No. 23 0022-538X/02/$04.00 0 DOI: 10.1128/JVI.76.23.12349 12354.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Targeted
JOURNAL OF VIROLOGY, Dec. 2002, p. 12349 12354 Vol. 76, No. 23 0022-538X/02/$04.00 0 DOI: 10.1128/JVI.76.23.12349 12354.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Targeted
Quantitative Evaluation and Optimization of Co-drugging to Improve Anti-HIV Latency Therapy
 Cellular and Molecular Bioengineering (Ó 21) DOI: 1.7/s12195-1-336-9 Quantitative Evaluation and Optimization of Co-drugging to Improve Anti-HIV Latency Therapy VICTOR C. WONG, 1 LINDA E. FONG, 2 NICHOLAS
Cellular and Molecular Bioengineering (Ó 21) DOI: 1.7/s12195-1-336-9 Quantitative Evaluation and Optimization of Co-drugging to Improve Anti-HIV Latency Therapy VICTOR C. WONG, 1 LINDA E. FONG, 2 NICHOLAS
Determinants of the Establishment of Human Immunodeficiency Virus Type 1 Latency
 JOURNAL OF VIROLOGY, Apr. 2009, p. 3078 3093 Vol. 83, No. 7 0022-538X/09/$08.00 0 doi:10.1128/jvi.02058-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Determinants of the Establishment
JOURNAL OF VIROLOGY, Apr. 2009, p. 3078 3093 Vol. 83, No. 7 0022-538X/09/$08.00 0 doi:10.1128/jvi.02058-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Determinants of the Establishment
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538
 Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
Data Sheet TIGIT / NFAT Reporter - Jurkat Cell Line Catalog #60538 Background: TIGIT is a co-inhibitory receptor that is highly expressed in Natural Killer (NK) cells, activated CD4+, CD8+ and regulatory
Journal of Infectious Diseases Advance Access published July 11, Effect of Antiretroviral Therapy on HIV Reservoirs in Elite Controllers
 Journal of Infectious Diseases Advance Access published July 11, 2013 1 Effect of Antiretroviral Therapy on HIV Reservoirs in Elite Controllers Tae-Wook Chun 1,6, J. Shawn Justement 1, Danielle Murray
Journal of Infectious Diseases Advance Access published July 11, 2013 1 Effect of Antiretroviral Therapy on HIV Reservoirs in Elite Controllers Tae-Wook Chun 1,6, J. Shawn Justement 1, Danielle Murray
The relation between HIV- 1 integration and latency
 The relation between HIV- 1 integration and latency Linos Vandekerckhove HIV translational research, Ghent Department of General Internal Medicine, Infectious Diseases and Psychosomatic Medicine University
The relation between HIV- 1 integration and latency Linos Vandekerckhove HIV translational research, Ghent Department of General Internal Medicine, Infectious Diseases and Psychosomatic Medicine University
EpiQuik Circulating Acetyl Histone H3K18 ELISA Kit (Colorimetric)
 EpiQuik Circulating Acetyl Histone H3K18 ELISA Kit (Colorimetric) Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE Uses: The EpiQuik Circulating Acetyl Histone H3K18 ELISA Kit (Colorimetric)
EpiQuik Circulating Acetyl Histone H3K18 ELISA Kit (Colorimetric) Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE Uses: The EpiQuik Circulating Acetyl Histone H3K18 ELISA Kit (Colorimetric)
DATA SHEET. Provided: 500 µl of 5.6 mm Tris HCl, 4.4 mm Tris base, 0.05% sodium azide 0.1 mm EDTA, 5 mg/liter calf thymus DNA.
 Viral Load DNA >> Standard PCR standard 0 Copies Catalog Number: 1122 Lot Number: 150298 Release Category: A Provided: 500 µl of 5.6 mm Tris HCl, 4.4 mm Tris base, 0.05% sodium azide 0.1 mm EDTA, 5 mg/liter
Viral Load DNA >> Standard PCR standard 0 Copies Catalog Number: 1122 Lot Number: 150298 Release Category: A Provided: 500 µl of 5.6 mm Tris HCl, 4.4 mm Tris base, 0.05% sodium azide 0.1 mm EDTA, 5 mg/liter
Received 18 March 2002/Accepted 17 July 2002
 JOURNAL OF VIROLOGY, Nov. 2002, p. 11091 11103 Vol. 76, No. 21 0022-538X/02/$04.00 0 DOI: 10.1128/JVI.76.21.11091 11103.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Synergistic
JOURNAL OF VIROLOGY, Nov. 2002, p. 11091 11103 Vol. 76, No. 21 0022-538X/02/$04.00 0 DOI: 10.1128/JVI.76.21.11091 11103.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Synergistic
Comparative effect of Various HDAC-inhibitors in-vitro on T- Cell Lymphoma cell lines alone and in combination with conventional anti-cancer drugs
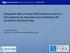 Comparative effect of Various HDAC-inhibitors in-vitro on T- Cell Lymphoma cell lines alone and in combination with conventional anti-cancer drugs Arshad H. Banday Mentor:Dr. Francisco Hernandez-Illizaliturri
Comparative effect of Various HDAC-inhibitors in-vitro on T- Cell Lymphoma cell lines alone and in combination with conventional anti-cancer drugs Arshad H. Banday Mentor:Dr. Francisco Hernandez-Illizaliturri
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Eukaryotic transcription (III)
 Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
Fayth K. Yoshimura, Ph.D. September 7, of 7 HIV - BASIC PROPERTIES
 1 of 7 I. Viral Origin. A. Retrovirus - animal lentiviruses. HIV - BASIC PROPERTIES 1. HIV is a member of the Retrovirus family and more specifically it is a member of the Lentivirus genus of this family.
1 of 7 I. Viral Origin. A. Retrovirus - animal lentiviruses. HIV - BASIC PROPERTIES 1. HIV is a member of the Retrovirus family and more specifically it is a member of the Lentivirus genus of this family.
CD14 + S100A9 + Monocytic Myeloid-Derived Suppressor Cells and Their Clinical Relevance in Non-Small Cell Lung Cancer
 CD14 + S1A9 + Monocytic Myeloid-Derived Suppressor Cells and Their Clinical Relevance in Non-Small Cell Lung Cancer Po-Hao, Feng M.D., Kang-Yun, Lee, M.D. Ph.D., Ya-Ling Chang, Yao-Fei Chan, Lu- Wei, Kuo,Ting-Yu
CD14 + S1A9 + Monocytic Myeloid-Derived Suppressor Cells and Their Clinical Relevance in Non-Small Cell Lung Cancer Po-Hao, Feng M.D., Kang-Yun, Lee, M.D. Ph.D., Ya-Ling Chang, Yao-Fei Chan, Lu- Wei, Kuo,Ting-Yu
cure research HIV & AIDS
 Glossary of terms HIV & AIDS cure research Antiretroviral Therapy (ART) ART involves the use of several (usually a cocktail of three or more) antiretroviral drugs to halt HIV replication. ART drugs may
Glossary of terms HIV & AIDS cure research Antiretroviral Therapy (ART) ART involves the use of several (usually a cocktail of three or more) antiretroviral drugs to halt HIV replication. ART drugs may
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
Total Histone H3 Acetylation Detection Fast Kit (Colorimetric)
 Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Catalog Number KA1538 48 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use...
Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Catalog Number KA1538 48 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use...
Li et al. Journal of Experimental & Clinical Cancer Research (2018) 37:108
 Li et al. Journal of Experimental & Clinical Cancer Research (2018) 37:108 https://doi.org/10.1186/s13046-018-0774-7 CORRECTION Correction to: Novel smac mimetic APG- 1387 elicits ovarian cancer cell killing
Li et al. Journal of Experimental & Clinical Cancer Research (2018) 37:108 https://doi.org/10.1186/s13046-018-0774-7 CORRECTION Correction to: Novel smac mimetic APG- 1387 elicits ovarian cancer cell killing
Mohammad Husain Department of Biotechnology, Jamia Millia Islamia New Delhi
 Role of Vitamin D receptor (VDR) in HIV induced tubular injury Mohammad Husain Department of Biotechnology, Jamia Millia Islamia New Delhi 07/10/2015 INTRODUCTION Vitamin D is technically not a Vitamin;
Role of Vitamin D receptor (VDR) in HIV induced tubular injury Mohammad Husain Department of Biotechnology, Jamia Millia Islamia New Delhi 07/10/2015 INTRODUCTION Vitamin D is technically not a Vitamin;
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Stewart et al. CD36 ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer
 NFκB (fold induction) Stewart et al. ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer a. mrna (fold induction) 5 4 3 2 1 LDL oxldl Gro1a MIP-2 RANTES mrna (fold induction)
NFκB (fold induction) Stewart et al. ligands promote sterile inflammation through assembly of a TLR 4 and 6 heterodimer a. mrna (fold induction) 5 4 3 2 1 LDL oxldl Gro1a MIP-2 RANTES mrna (fold induction)
Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection
 Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection Melissa Mihelidakis May 6, 2004 7.340 Research Proposal Introduction Apoptosis, or programmed cell
Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection Melissa Mihelidakis May 6, 2004 7.340 Research Proposal Introduction Apoptosis, or programmed cell
Not IN Our Genes - A Different Kind of Inheritance.! Christopher Phiel, Ph.D. University of Colorado Denver Mini-STEM School February 4, 2014
 Not IN Our Genes - A Different Kind of Inheritance! Christopher Phiel, Ph.D. University of Colorado Denver Mini-STEM School February 4, 2014 Epigenetics in Mainstream Media Epigenetics *Current definition:
Not IN Our Genes - A Different Kind of Inheritance! Christopher Phiel, Ph.D. University of Colorado Denver Mini-STEM School February 4, 2014 Epigenetics in Mainstream Media Epigenetics *Current definition:
Induction and Clearance of Latent HIV Infection:
 214 Towards an HIV Cure symposium Melbourne Induction and Clearance of Latent HIV Infection: an Ex-Vivo Assessment of Immune Effectors using Cells from ART-treated Patients DM Margolis 1, C Garrido 1,
214 Towards an HIV Cure symposium Melbourne Induction and Clearance of Latent HIV Infection: an Ex-Vivo Assessment of Immune Effectors using Cells from ART-treated Patients DM Margolis 1, C Garrido 1,
~Lentivirus production~
 ~Lentivirus production~ May 30, 2008 RNAi core R&D group member Lentivirus Production Session Lentivirus!!! Is it health threatening to lab technician? What s so good about this RNAi library? How to produce
~Lentivirus production~ May 30, 2008 RNAi core R&D group member Lentivirus Production Session Lentivirus!!! Is it health threatening to lab technician? What s so good about this RNAi library? How to produce
Supplementary Material
 Supplementary Material Nuclear import of purified HIV-1 Integrase. Integrase remains associated to the RTC throughout the infection process until provirus integration occurs and is therefore one likely
Supplementary Material Nuclear import of purified HIV-1 Integrase. Integrase remains associated to the RTC throughout the infection process until provirus integration occurs and is therefore one likely
York criteria, 6 RA patients and 10 age- and gender-matched healthy controls (HCs).
 MATERIALS AND METHODS Study population Blood samples were obtained from 15 patients with AS fulfilling the modified New York criteria, 6 RA patients and 10 age- and gender-matched healthy controls (HCs).
MATERIALS AND METHODS Study population Blood samples were obtained from 15 patients with AS fulfilling the modified New York criteria, 6 RA patients and 10 age- and gender-matched healthy controls (HCs).
Brd4 Coactivates Transcriptional Activation of NF- B via Specific Binding to Acetylated RelA
 MOLECULAR AND CELLULAR BIOLOGY, Mar. 2009, p. 1375 1387 Vol. 29, No. 5 0270-7306/09/$08.00 0 doi:10.1128/mcb.01365-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Brd4 Coactivates
MOLECULAR AND CELLULAR BIOLOGY, Mar. 2009, p. 1375 1387 Vol. 29, No. 5 0270-7306/09/$08.00 0 doi:10.1128/mcb.01365-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Brd4 Coactivates
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Approaching a Cure Daniel R. Kuritzkes, MD
 Approaching a Cure Daniel R. Kuritzkes, MD Division of Infectious Diseases Brigham and Women s Hospital Harvard Medical School Disclosures The speaker is a consultant and/or has received speaking honoraria
Approaching a Cure Daniel R. Kuritzkes, MD Division of Infectious Diseases Brigham and Women s Hospital Harvard Medical School Disclosures The speaker is a consultant and/or has received speaking honoraria
Tat inhibition by didehydro Cortistatin A promotes heterochromatin formation at the HIV 1 long terminal repeat
 https://doi.org/10.1186/s13072-019-0267-8 Epigenetics & Chromatin RESEARCH Open Access Tat inhibition by didehydro Cortistatin A promotes heterochromatin formation at the HIV 1 long terminal repeat Chuan
https://doi.org/10.1186/s13072-019-0267-8 Epigenetics & Chromatin RESEARCH Open Access Tat inhibition by didehydro Cortistatin A promotes heterochromatin formation at the HIV 1 long terminal repeat Chuan
Recent Insights into HIV Pathogenesis and Treatment: Towards a Cure
 Recent Insights into HIV Pathogenesis and Treatment: Towards a Cure Deborah Persaud, M.D. Associate Professor Department of Pediatrics Division of Infectious Diseases Johns Hopkins University School of
Recent Insights into HIV Pathogenesis and Treatment: Towards a Cure Deborah Persaud, M.D. Associate Professor Department of Pediatrics Division of Infectious Diseases Johns Hopkins University School of
With over 20 drugs and several viable regimens, the mo6vated pa6ent with life- long access to therapy can control HIV indefinitely, elimina6ng the
 Towards an HIV Cure Steven G. Deeks, MD Professor of Medicine in Residence HIV/AIDS Division University of California, San Francisco (UCSF) WorldMedSchool; July 22, 2013 1 With over 20 drugs and several
Towards an HIV Cure Steven G. Deeks, MD Professor of Medicine in Residence HIV/AIDS Division University of California, San Francisco (UCSF) WorldMedSchool; July 22, 2013 1 With over 20 drugs and several
Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) FS 2014 P Matthias & RG Clerc
 Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) FS 2014 P Matthias & RG Clerc P. Matthias, 16 April 2014 DNA methylation basics Acetylation Acetyltransferases/Deacetylases
Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) FS 2014 P Matthias & RG Clerc P. Matthias, 16 April 2014 DNA methylation basics Acetylation Acetyltransferases/Deacetylases
Clinical HIV-1 eradication studies
 Clinical HIV-1 eradication studies Mathias Lichterfeld, M. D., Ph. D. Massachusetts General Hospital Harvard Medical School No disclosures HIV-1 persistence despite HAART Blood Tissue HIV RNA (cps/ml)
Clinical HIV-1 eradication studies Mathias Lichterfeld, M. D., Ph. D. Massachusetts General Hospital Harvard Medical School No disclosures HIV-1 persistence despite HAART Blood Tissue HIV RNA (cps/ml)
RAS Genes. The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes.
 ۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
Supplementary Figure 1. mrna targets were found in exosomes and absent in free-floating supernatant. Serum exosomes and exosome-free supernatant were
 Supplementary Figure 1. mrna targets were found in exosomes and absent in free-floating supernatant. Serum exosomes and exosome-free supernatant were separated via ultracentrifugation and lysed to analyze
Supplementary Figure 1. mrna targets were found in exosomes and absent in free-floating supernatant. Serum exosomes and exosome-free supernatant were separated via ultracentrifugation and lysed to analyze
Under the Radar Screen: How Bugs Trick Our Immune Defenses
 Under the Radar Screen: How Bugs Trick Our Immune Defenses Session 7: Cytokines Marie-Eve Paquet and Gijsbert Grotenbreg Whitehead Institute for Biomedical Research HHV-8 Discovered in the 1980 s at the
Under the Radar Screen: How Bugs Trick Our Immune Defenses Session 7: Cytokines Marie-Eve Paquet and Gijsbert Grotenbreg Whitehead Institute for Biomedical Research HHV-8 Discovered in the 1980 s at the
HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation
 SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
- 1 - Cell types Monocytes THP-1 cells Macrophages. LPS Treatment time (Hour) IL-6 level (pg/ml)
 Supplementary Table ST1: The dynamic effect of LPS on IL-6 production in monocytes and THP-1 cells after GdA treatment. Monocytes, THP-1 cells and macrophages (5x10 5 ) were incubated with 10 μg/ml of
Supplementary Table ST1: The dynamic effect of LPS on IL-6 production in monocytes and THP-1 cells after GdA treatment. Monocytes, THP-1 cells and macrophages (5x10 5 ) were incubated with 10 μg/ml of
Egr-1 regulates RTA transcription through a cooperative involvement of transcriptional regulators
 /, 2017, Vol. 8, (No. 53), pp: 91425-91444 Egr-1 regulates RTA transcription through a cooperative involvement of transcriptional regulators Roni Sarkar 1 and Subhash C. Verma 1 1 Department of Microbiology
/, 2017, Vol. 8, (No. 53), pp: 91425-91444 Egr-1 regulates RTA transcription through a cooperative involvement of transcriptional regulators Roni Sarkar 1 and Subhash C. Verma 1 1 Department of Microbiology
Viral Genetics. BIT 220 Chapter 16
 Viral Genetics BIT 220 Chapter 16 Details of the Virus Classified According to a. DNA or RNA b. Enveloped or Non-Enveloped c. Single-stranded or double-stranded Viruses contain only a few genes Reverse
Viral Genetics BIT 220 Chapter 16 Details of the Virus Classified According to a. DNA or RNA b. Enveloped or Non-Enveloped c. Single-stranded or double-stranded Viruses contain only a few genes Reverse
Transcriptional Interference Antagonizes Proviral Gene Expression to Promote HIV Latency
 Article Transcriptional Interference Antagonizes Proviral Gene Expression to Promote HIV Latency Tina Lenasi, 1, * Xavier Contreras, 1 and B. Matija Peterlin 1, * 1 Departments of Medicine, Microbiology,
Article Transcriptional Interference Antagonizes Proviral Gene Expression to Promote HIV Latency Tina Lenasi, 1, * Xavier Contreras, 1 and B. Matija Peterlin 1, * 1 Departments of Medicine, Microbiology,
Transcriptional Regulation of Latent Feline Immunodeficiency Virus in Peripheral CD4+ T-lymphocytes
 Viruses 2012, 4, 878-888; doi:10.3390/v4050878 Brief Report OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Transcriptional Regulation of Latent Feline Immunodeficiency Virus in Peripheral
Viruses 2012, 4, 878-888; doi:10.3390/v4050878 Brief Report OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Transcriptional Regulation of Latent Feline Immunodeficiency Virus in Peripheral
Beyond HAART: Outline. HIV-1 Time Line. Outline. Approaches to HIV Eradication 8/15/2013
 8/5/203 Beyond HAART: Approaches to HIV Eradication I have no financial conflicts of interest to disclose 8 August 203 Adam Spivak MD Visiting Instructor, Division of Infectious Diseases University of
8/5/203 Beyond HAART: Approaches to HIV Eradication I have no financial conflicts of interest to disclose 8 August 203 Adam Spivak MD Visiting Instructor, Division of Infectious Diseases University of
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/366/ra25/dc1 Supplementary Materials for Viral entry route determines how human plasmacytoid dendritic cells produce type I interferons Daniela Bruni, Maxime
www.sciencesignaling.org/cgi/content/full/8/366/ra25/dc1 Supplementary Materials for Viral entry route determines how human plasmacytoid dendritic cells produce type I interferons Daniela Bruni, Maxime
Supporting Information
 Supporting Information Horwitz et al. 73/pnas.35295 A Copies ml - C 3NC7 7 697 698 7 7 73 76-2 2 Days Gp2 residue G458D G459D T278A 7/36 N28 K D 28 459 A28T ID# 697 ID# 698 ID# 7 ID# 7 ID# 73 ID# 76 ID#
Supporting Information Horwitz et al. 73/pnas.35295 A Copies ml - C 3NC7 7 697 698 7 7 73 76-2 2 Days Gp2 residue G458D G459D T278A 7/36 N28 K D 28 459 A28T ID# 697 ID# 698 ID# 7 ID# 7 ID# 73 ID# 76 ID#
Procaspase-3. Cleaved caspase-3. actin. Cytochrome C (10 M) Z-VAD-fmk. Procaspase-3. Cleaved caspase-3. actin. Z-VAD-fmk
 A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
A HeLa actin - + + - - + Cytochrome C (1 M) Z-VAD-fmk PMN - + + - - + actin Cytochrome C (1 M) Z-VAD-fmk Figure S1. (A) Pan-caspase inhibitor z-vad-fmk inhibits cytochrome c- mediated procaspase-3 cleavage.
Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to
 Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
Pathogenesis Update Robert F. Siliciano, MD, PhD
 Pathogenesis Update Robert F. Siliciano, MD, PhD Professor of Medicine and Molecular Biology and Genetics Johns Hopkins University School of Medicine Investigator, Howard Hughes Medical Institute HIV-1
Pathogenesis Update Robert F. Siliciano, MD, PhD Professor of Medicine and Molecular Biology and Genetics Johns Hopkins University School of Medicine Investigator, Howard Hughes Medical Institute HIV-1
Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival
 Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
Supplementary Information for Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival Tatsuro Kawamura 1, Makoto Kawatani 1, Makoto Muroi, Yasumitsu Kondoh,
EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric)
 EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric)
EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric) Base Catalog # PLEASE READ THIS ENTIRE USER GUIDE BEFORE USE The EpiQuik Total Histone H3 Acetylation Detection Fast Kit (Colorimetric)
Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the
 Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the genome-wide methylation microarray data. Mean ± s.d.; Student
Supplementary Figure 1. HOPX is hypermethylated in NPC. (a) Methylation levels of HOPX in Normal (n = 24) and NPC (n = 24) tissues from the genome-wide methylation microarray data. Mean ± s.d.; Student
Ex vivo analysis identifies effective HIV-1 latency reversing drug combinations
 The Journal of Clinical Investigation Research article Ex vivo analysis identifies effective HIV-1 latency reversing drug combinations Gregory M. Laird, 1 C. Korin Bullen, 1 Daniel I.S. Rosenbloom, 2 Alyssa
The Journal of Clinical Investigation Research article Ex vivo analysis identifies effective HIV-1 latency reversing drug combinations Gregory M. Laird, 1 C. Korin Bullen, 1 Daniel I.S. Rosenbloom, 2 Alyssa
MANUSCRIPT TITLE: Protein kinase C δ signaling is required for dietary prebiotic-induced strengthening of intestinal epithelial barrier function
 MANUSCRIPT TITLE: Protein kinase C δ signaling is required for dietary prebiotic-induced strengthening of intestinal epithelial barrier function Authors: Richard Y. Wu 1,2, Majd Abdullah 1, Pekka Määttänen
MANUSCRIPT TITLE: Protein kinase C δ signaling is required for dietary prebiotic-induced strengthening of intestinal epithelial barrier function Authors: Richard Y. Wu 1,2, Majd Abdullah 1, Pekka Määttänen
Retroviruses. ---The name retrovirus comes from the enzyme, reverse transcriptase.
 Retroviruses ---The name retrovirus comes from the enzyme, reverse transcriptase. ---Reverse transcriptase (RT) converts the RNA genome present in the virus particle into DNA. ---RT discovered in 1970.
Retroviruses ---The name retrovirus comes from the enzyme, reverse transcriptase. ---Reverse transcriptase (RT) converts the RNA genome present in the virus particle into DNA. ---RT discovered in 1970.
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein
 Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein Lei Wang 1, Tian-Peng Zhang 1, Yuan Zhang 2, Hai-Lian
Essential Medium, containing 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin. Huvec were cultured in
 Supplemental data Methods Cell culture media formulations A-431 and U-87 MG cells were maintained in Dulbecco s Modified Eagle s Medium. FaDu cells were cultured in Eagle's Minimum Essential Medium, containing
Supplemental data Methods Cell culture media formulations A-431 and U-87 MG cells were maintained in Dulbecco s Modified Eagle s Medium. FaDu cells were cultured in Eagle's Minimum Essential Medium, containing
