Comparative Study of Brain Morphology in Mecp2 Mutant Mouse Models of Rett Syndrome
|
|
|
- Amelia Griselda Nichols
- 5 years ago
- Views:
Transcription
1 THE JOURNAL OF COMPARATIVE NEUROLOGY 508: (2008) Comparative Study of Brain Morphology in Mecp2 Mutant Mouse Models of Rett Syndrome NADIA P. BELICHENKO, 1 PAVEL V. BELICHENKO, 2,3 * HONG HUA LI, 1 WILLIAM C. MOBLEY, 2,3 AND UTA FRANCKE 1 1 Department of Genetics, Stanford University, Stanford, California Department of Neurology & Neurological Sciences, Stanford University, Stanford, California Neuroscience Institute at Stanford University, Stanford, California ABSTRACT Rett syndrome (RTT) is caused by mutations in the X-linked gene MECP2. While patients with RTT show widespread changes in brain function, relatively few studies document changes in brain structure and none examine in detail whether mutations causing more severe clinical phenotypes are linked to more marked changes in brain structure. To study the influence of MeCP2-deficiency on the morphology of brain areas and axonal bundles, we carried out an extensive morphometric study of two Mecp2-mutant mouse models (Mecp2B and Mecp2J) of RTT. Compared to wildtype littermates, striking changes included reduced brain weight ( 13% and 9%) and the volumes of cortex ( 11% and 7%), hippocampus (both by 8%), and cerebellum ( 12% and 8%) in both mutant mice. At 3 weeks of age, most (24 of 47) morphological parameters were significantly altered in Mecp2B mice; fewer (18) were abnormal in Mecp2J mice. In Mecp2B mice, significantly lower values for cortical area were distributed along the rostrocaudal axis, and there was a reduced length of the olfactory bulb ( 10%) and periaqueductal gray matter ( 16%). In Mecp2J mice, while there was significant reduction in rostrocaudal length of cortex, this parameter was also abnormal in hippocampus ( 10%), periaqueductal gray matter ( 13%), fimbria ( 18%), and anterior commissure ( 10%). Our findings define patterns of Mecp2 mutation-induced changes in brain structure that are widespread and show that while some changes are present in both mutants, others are not. These observations provide the underpinning for studies to further define microarchitectural and physiological consequences of MECP2 deficiency. J. Comp. Neurol. 508: , Wiley-Liss, Inc. Indexing terms: Rett syndrome; MeCP2; brain; mouse models; morphometry; Cavalieri principle Rett syndrome (RTT), a neurologic disorder affecting mostly girls, is caused by heterozygous mutations in the X-linked MECP2 gene (Amir et al., 1999), and has a prevalence of 1 in 10,000 15,000 females (Hagberg, 1989, 1995). The clinical course has been well described (Rett, 1966; Hagberg et al., 1985; Kerr and Stephenson, 1986). The manifestations are primarily neurologic, with onset between 6 and 18 months of age and with developmental regression, loss of speech and purposeful hand use, stereotypic hand movements, seizures, autonomic dysfunction, and irregular breathing. Subtle behavioral changes may be detected even before 6 months of age (Einspieler et al., 2005). With the delineation of distinct mutations in MECP2 it has been possible to link the type of the mutation with clinical disease severity to some extent (Ham et al., 2005; Archer et al., 2007). This article includes Supplementary Material available via the Internet at Grant sponsor: International Rett Syndrome Association (to U.F.). *Correspondence to: Pavel V. Belichenko, Department of Neurology & Neurological Sciences, Stanford University Medical Center, 1201 Welch Rd., Room P220, Stanford, CA pavel_belichenko@yahoo.com Received 1 November 2007; Revised 21 December 2008; Accepted 10 January 2008 DOI /cne Published online in Wiley InterScience ( WILEY-LISS, INC.
2 STRUCTURAL PATHOLOGY IN Mecp2-MUTANT MICE Defining the relationships between MECP2 mutations and changes in brain morphology would enhance understanding of the pathogenesis of the developmental nervous system abnormalities. In fact, relatively few studies address brain morphology in RTT subjects. In most RTT subjects, head circumference was reduced (Dunn et al., 2002); brain volume was significantly reduced by 25% on average (Jellinger et al., 1988; Armstrong, 1992). MRI studies showed atrophy of cerebral cortex, basal ganglia, thalamus, and cerebellum. There was no sign of cortical neuronal microdysgenesis. Also detected were mild losses of cortical pyramidal neurons, more pronounced in layers II III than in layers V VII, as well as a reduction in areas occupied by synaptophysin immunoreactivity (Belichenko et al., 1997). Reduced neuronal cell size and an increased cell-packing density in cortical and subcortical areas were also reported (Bauman et al., 1995). Analysis of dendritic morphology showed a significant reduction in number and length of dendrites (Armstrong, 1992; Belichenko et al., 1994; Armstrong et al., 1995). In addition, dendritic spines were decreased in number and there was regional loss of spines. Afferent fibers to spines and the axonal bundles in which they traveled were disorganized in the cortex of RTT subjects (Belichenko et al., 1994). A recent detailed study of neuronal cell body and axonal fiber morphology showed involvement of the entorhinal cortex, hippocampal formation, and basal ganglia (Leontovich et al., 1999). Taking the intensity of cresyl violet staining in individual neurons as a marker of activity, both increases and decreases were detected (Leontovich et al., 1999). Corpus callosum widths were significantly reduced in RTT subjects (Murakami et al., 1992; Reiss et al., 1993). Although the findings are evidence of widespread brain changes, most studies were carried out before the identification of different mutations, leaving unanswered whether or not specific mutations are linked with specific anatomic abnormalities or with severity of involvement within affected regions. Studies in mice have significantly enhanced the understanding of the neurobiology of MeCP2 deficiency (Chen et al., 2001; Guy et al., 2001; Shahbazian et al., 2002; Shahbazian and Zoghbi, 2002). Consistent with the pervasive changes in brain function in RTT, in wildtype (WT) mice the MeCP2 protein is abundantly expressed, including olfactory bulb, cortex, hippocampus, striatum, thalamus, cerebellum as well as other brain regions (Shahbazian et al., 2002; Kishi and Macklis, 2004). Brain region-specific Mecp2 mrna expression levels are well documented (Lein et al., 2007). Regional and age-related differences in distribution of Mecp2 splice variants have been detected (Dragich et al., 2007). Two mouse models of RTT have been studied extensively. Mecp2-null mice with a large deletion of exons 3 and 4, here referred to as the B strain (Mecp2B), were generated by the Bird laboratory (Guy et al., 2001) and have been backcrossed onto C57BL/6 background. Mice with an in-frame deletion of Mecp2 exon 3, here referred to as the J strain (Mecp2J), were generated in the Jaenisch laboratory (Chen et al., 2001) and are bred on a mixed genetic background (129, C57BL/6 and BALB/ c). Male mice of both strains develop progressive neurological phenotypes that recapitulate many RTT-like neurologic symptoms. In male Mecp2B mice, where no mrna and MeCP2 protein were detected, early postnatal development was reported to be abnormal, with reduced performance in 185 postural reflex, negative geotaxis, and wire suspension tests before age 21 days (Santos et al., 2007). Body weight of males was significantly lower than that of WT littermates from 3 weeks onwards (Guy et al., 2001). Between 3 and 8 weeks of age, affected mice exhibited reduced spontaneous movement, stiff, uncoordinated gait, and occasional tremors. Later, most animals developed hindlimb clasping and irregular breathing. Progression of symptoms led to rapid weight loss and death at 54 days (Guy et al., 2001). Mecp2B hemizygous male and heterozygous female mice show different severity of phenotypes, with much milder expression in females due to X-inactivation mosaicism. Nevertheless, abnormal respiratory phenotypes in adult Mecp2B female mice were documented (Bissonnette and Knopp, 2006). MRI studies of 5 8-weekold male Mecp2B mice showed significant volumetric reductions of cortex plus cerebellum, and decreased ratios of the thickness of motor cortex to cerebrum and corpus callosum to cerebrum (Saywell et al., 2006). While cortical lamination was normal, the thickness of somatosensory and motor cortices was significantly reduced in comparison to WT males (Fukuda et al., 2005; Saywell et al., 2006; but see Metcalf et al., 2006). However, the number of neocortical neurons (NeuN-immunostained) was not changed in 7 9-week-old heterozygous and homozygous Mecp2B-mutants (Metcalf et al., 2006). Developmental increases in parvalbumin immunoreactivity were significantly delayed in the somatosensory cortex in 2-week-old male mutants (Fukuda et al., 2005). Abnormal dendrites and dendritic spines were reported in male Mecp2 mice (Kishi and Macklis, 2004; Fukuda et al., 2005); changes in induction of hippocampal LTP and in learning and memory have also been documented (Pelka et al., 2006; Guy et al., 2007). In Mecp2J mice, in contrast, the mrna corresponding to the mutant Mecp2 transcript is present in cerebellum at 8 weeks of age (Jordan et al., 2007); no MeCP2 protein was detected, but peptides of smaller sizes were revealed by Western blot (Chen et al., 2001). Mecp2J male mice appear to follow a milder clinical course than Mecp2B mice. They were apparently normal at birth. Recent studies, however, revealed significant behavioral alterations starting from postnatal day 4, including increases in ultrasonic vocalization in response to social isolation, as well as increased hindlimb placing and grasping (Picker et al., 2006). There was no difference in the running-wheel assay in mutant vs. WT at 4 weeks of age (Chang et al., 2006). Starting at 5 weeks of age, there was body trembling and occasional labored breathing; at 8 weeks of age 40% of the mutant mice were overweight. At the late stage of disease, mutants were hypoactive, trembled when handled, began to lose weight, and most died at 10 weeks (Chen et al., 2001). As for Mecp2B females, female Mecp2J mice showed a less severe phenotype than mutant males, as expected (Chen et al., 2001). In studies of brain structure and function, young (35 days old) Mecp2J males showed reductions in the volumes of whole brain, amygdala, hippocampus, and striatum (Stearns et al., 2007); in other studies brain weight was reported to be reduced at 8 weeks (Chen et al., 2001; Chang et al., 2006). By 9 weeks, neurons and neuronal nuclei in hippocampus (CA1/CA2 areas) (Chen et al., 2001; Chang et al., 2006), cerebral cortex (layer V), and cerebellum (folium II) were smaller than normal, but without evidence of neuronal degeneration (Chen et al., 2001). Spontaneous firing of layer V
3 186 N.P. BELICHENKO ET AL. pyramidal neurons of the somatosensory cortex was significantly reduced in Mecp2J vs. WT at 4 weeks of age (Chang et al., 2006). In studies examining an unspecified mixture of B and J strain mice bred onto the C57Bl/6 background, synaptic plasticity was significantly impaired in CA1 at 6 10 weeks (i.e., in the symptomatic phase) but not at 3 5 weeks (the apparent presymptomatic phase) (Asaka et al., 2006). In spite of differences in neurological phenotypes in the two types of Mecp2-mutant mice, there has been little direct comparison of brain structures. To test the idea that measurable changes in brain structure underlie differences in neurological function, and to compare the mice directly, we used the MultiBrain technology, which allows one to embed many (n 24) brains in a single block prior to serial sectioning and staining. This tool reduces experimental variability, thereby increasing the ability to detect small changes in volume and shape. We studied male mice (21 days old) of either the Mecp2B strain (n 6) or Mecp2J stain (n 6), along with WT littermate control mice (n 6 for each allele). Our studies elucidated in detail the volume of seven brain regions and the shape of 14 brain regions. There were significant alterations in both parameters in both models, with increased severity in Mecp2B mice. We discuss the rationale for interpreting changes with respect to the type of mutation and the strain background, as well as possible links between the changes detected in mice with those that characterize RTT in humans. MATERIALS AND METHODS Mecp2-mutant mice The Mecp2 mouse colony was maintained at Stanford. All experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Animals and with an approved animal protocol from the Stanford University Institutional Animal Care and Use Committee. Female heterozygotes with an Mecp2 tm1.1bird -mutation (Mecp2B) (Guy et al., 2001) were purchased from the Jackson Laboratory (Bar Harbor, ME) on a C57BL/6J background and were maintained on this background by breeding heterozygous females to unrelated C57BL/6 males. Female heterozygotes with an Mecp2 tm1.1jae mutation (Mecp2J, (Chen et al., 2001) were obtained from Rudolf Jaenisch (MIT, Cambridge, MA) on a mixed 129/Sv, C57BL/6 and BALB/c background and maintained on this background by brother-sister mating and by breeding to unrelated BALB/c males for about 10 generations. To obtain the litters of WT and Mecp2J animals for this study, we mated two Mecp2 tm1.1jae /- female littermates to a single WT BALB/c male. To distinguish WT and Mecp2-mutant mice, we extracted genomic DNA from tail samples and genotyped them by polymerase chain reaction (PCR) for Mecp2 and Sry (for sexing) by using protocols supplied by the Jackson Laboratory and Sry primers previously described (Jordan et al., 2007). Each mouse was genotyped twice. We used only male mice to avoid the complexity of X-chromosome inactivation mosaicism in females that generates individual differences in the proportion of normal MeCP2-expressing cells. In addition, males manifest the most severe phenotype due to complete absence of the Mecp2B gene product in all neurons. Mice were studied at 21 days, the age of weaning, and at an early stage of the disease. Body and brain weight measurement WT and Mecp2-mutant mice were deeply anesthetized with sodium pentobarbital (200 mg/kg i.p.) (Abbott Laboratories, North Chicago, IL), weighed, and transcardially perfused for 1 minute with 0.9% sodium chloride (10 ml) and then for 10 minutes with ice-cold 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), ph 7.4 (100 ml). After perfusion the brain was immediately removed. The weight of the entire brain (including the olfactory bulbs, cortex, hippocampus, cerebellum, brainstem, and the cervical spinal cord through C1 C2) was recorded. The brain was then placed in the same fixative and shipped to NeuroScience Associates (Knoxville, TX) for the MultiBrain Technology procedure. Brain embedding and processing Twenty-four fixed brains (six each for Mecp2B and WT for Mecp2B, and six each for Mecp2J and WT for Mecp2J) were processed using the MultiBrain technology. Briefly, all 24 brains were encased in a single gelatin block and cut coronally in serial sections of 35 m. Beginning with the first section, every sixth section was stained with thionine and mounted on slides. The main advantage of this technique is that during cutting and staining, all sections from all brains are exposed to the same experimental conditions. The set of stained sections contained 61 individual slides, each containing tissue from all 24 brains. The sections started at the beginning of the olfactory bulb (rostral to bregma 5.00 mm level) and continued to bregma 9.00 mm, containing the cervical portion of the spinal cord. The location of each coronal section was designated based on its relative position to bregma in mm (Hof et al., 2000). Brain image collection Sections stained with thionine were examined using a Nikon digital camera DXM1200F attached to a Nikon Eclipse E600 microscope. The lens was either a 1 objective (Nikon; Plan UW, 1 /0.04) or a 2 objective (Nikon; Plan UW, 2 /0.06). Nikon ACT-1 v.2.70 software was used for digitizing images with pixel size and 150 pixel/inch resolution and maximum sensitivity. The images of over 1,500 individual sections were digitized using the 1 objective. More that 600 single brain section were digitized using the 2 objective. Each image was saved in TIFF format. Digital images were imported, enhanced for brightness and contrast, assembled, and labeled in Adobe Photoshop CS (San Jose, CA) and archived. Figures were printed with a Phaser 7300 color printer (Tektronix by Xerox, Norwalk, CT). Regional brain area and length measurements One investigator (N.P.B.), blinded to genotype, performed all measurements. The Image-Pro Plus program (Media Cybernetics, Silver Spring, MD) was used to outline each region in both hemispheres from each brain and to estimate the area of the structure. For each structure the beginning and the end of the structure was marked in an Excel file. The volume of each structure was then estimated by using the Cavalieri principle (Najafian et al., 2002) where the volume is equal to the sum of areas multiplied by the distance between the sections (6 35 m 210 m). The same method was used to define the
4 STRUCTURAL PATHOLOGY IN Mecp2-MUTANT MICE mean area occupied by axonal bundles, the corpus callosum, and the posterior commissure where these structures were clearly defined. Definition of the brain structures The brain atlas for the C57Bl/6 mouse was used to define the locations and borders of brain structures (Hof et al., 2000). Our sections included the entire brain, from the olfactory bulb to the cervical spinal cord through C1 C2. To select brain structures we used the following criteria: 1) participates in neurobiological processes relevant to RTT; 2) can be clearly outlined on thionine-stained sections; and 3) expresses Mecp2. As references for Mecp2 expression we used published data and the atlas of gene expression from the Allen Institute for Brain Science (Dragich et al., 2007; Lein et al., 2007). We examined 14 distinct structures, including olfactory bulb (OB), cortex, hippocampus, cerebellum, periaqueductal gray matter (PAG), nucleus ambiguus (NA), nucleus hypoglossus (nc. XII), fornix, fimbria, fasciculus retroflexus (FR), mammillothalamic tract (MTT), anterior (AC) and posterior (PC) commissures, and corpus callosum (CC). NA and nc. XII were outlined on 2 objective images; other brain structures were outlined on 1 objective images. The rostral to caudal extent of brain structures, as related to bregma levels, are displayed in Supplementary Table S1. For example, the OB was examined from brain sections at bregma 5.00 mm to bregma 2.40 mm (Fig. 1). For structures that are bilateral (i.e., OB, cortex, hippocampus, and NA), we measured both the right and left and calculated the average (Table 2). Thus, the measures recorded are for the structure as represented in one hemisphere. We measured the entire area of the cortex, including layers I through VI of the frontal, temporal, parietal, and caudal cortices. White matter and corpus callosum were excluded. The entire hippocampus was measured, including CA1-CA3, dentate gyrus, and subiculum. The cerebellum, including all lobes of cerebellum, flocculus, mid-cerebellar peduncle, and cerebellar commissure was outlined, but excluding deep cerebellar nuclei: fastigial, interpositus, and dentate. The entire PAG was outlined; special attention was paid to exclude the nucleus of Darkschewitsch, the oculomotor nucleus, dorsal nucleus of the raphe, tegmental nucleus, and aqueduct of Sylvius. The NA consists of darkly stained large cells in the ventrolateral part of the brainstem; it was measured by drawing a line around the most peripherally located cells. The nc. XII is located close to the midline of the medulla. Left and right nc. XII were outlined together from bregma 7.20 mm level through the next two sections. We were unable to trace this nucleus on our sections more caudally since this part of the brain was absent in some of the multibrain sections. Therefore, the volume of nc. XII was calculated only from three sections and thus represents only the rostral part of this nucleus. Several axonal bundles were outlined and for each the area was measured, yielding a mean area. For the PC, the area and the width at midline were recorded. For CC, only the width at midline was measured. Where the bundles was represented bilaterally (i.e., fornix, fimbria, AC, FR, MTT), the areas were measured on both the right and left sides and the data are the average. The rostral part of the fornix was recognized as axonal bundles in the dorsomedial part of the section that extended lateroventrally in more caudal sections. The fornix was measured on brain 187 TABLE 1. Body and Brain Weights in Wildtype and Mecp2 Mutant Mice 1 WT for Mecp2B Mecp2B WT for Mecp2J Mecp2J Body, g ** *** Brain, g * * 1 The number of mice used was as follows: WT for Mecp2B/Mecp2B/WT for Mecp2J/ Mecp2J 10/11/10/10. *P 0.05, significantly different from WT mice. **P 0.05, significantly different between WTs or between mutant mice. ***P 0.05, significantly different from WT and between mutant mice. sections caudal to the level where the AC (olfactory limb) crosses the midline, starting from bregma 0.20 mm level and extending to bregma 2.10 mm. The fimbria was measured starting from bregma 0.20 mm and extending to bregma 2.40 mm. The FR and MTT were measured as indicated (Table S1). The AC (olfactory limb) was measured from the rostral border to the point where it crosses the midline (Table S1). We measured the PC and the CC as defined in Table S1. Statistical analyses The data for body weight, brain weight, regional brain volume, area, and width were exported to Excel (Microsoft, Redmond, WA) and statistical comparisons were performed using two-way analysis of variance (ANOVA) and for two samples using two-tailed Student s t-tests. All results are expressed as mean SEM, and P values 0.05 were considered significant. RESULTS Body and brain weights To examine the effect of the Mecp2 gene mutations in Mecp2B and Mecp2J mice, we first measured body and brain weights. The body weight of Mecp2B mice was significantly reduced (by 24%) in comparison to controls, but there was no difference between WTs and Mecp2J mice (Table 1). There was a significant difference in body weight between WT mice of different strains (P 0.01); Mecp2B mice were significantly smaller ( 34%) than Mecp2J mice (P ). The brain weight of both Mecp2B and Mecp2J mice was smaller than WT littermate controls; Mecp2B was reduced by 13% while Mecp2J was smaller by 9% littermates (Table 1). There was no difference in brain weight between WT mice of different strains (P 0.10) or between Mecp2-mutant mice (P 0.75). These data provide evidence for an effect of strain background on body weight, but not brain weight, and for an effect of Mecp2 mutation on brain weight. Changes in volume and area of brain structures in Mecp2-mutant mice We detected no differences in gross brain anatomy. The morphology of cortical layers, hippocampus, OB, and cerebellum, as well as axonal bundles, was similar in mutants and WT mice. Next, we examined each of 14 brain regions or structures. Herein, we describe the results of studies of volume, rostrocaudal length, and shape in WT and mutants. Table 2 reports the results of volumetric studies in brain regions and, for axonal bundles, estimations of mean area and mean width at midline. In Table 3, we report data for the rostrocaudal length of the same structures, as defined by the number of coronal sections in
5 TABLE 2. Morphometry of Different Brain Regions in Wildtype and Mecp2 Mutant Mice 1 WT for Mecp2B Mecp2B WT for Mecp2J Mecp2J Volume of structure, 10 9 m 3 Olfactory bulb ** * Cortex ** * * Hippocampus * * Cerebellum * * Periaqueductal gray matter * Volume of structure, 10 6 m 3 Nucleus ambiguus *** Nucleus hypoglossus, rostral ** Mean area, 10 3 m 2 Fornix ** * Fimbria *** Fasciculus retroflexus * Mammillothalamic tract Anterior commissure, olfactory limb ** * Posterior commissure Mean width at midline, m Posterior commissure Corpus callosum 166 6** 162 5** The number of mice used for each genotype was as follows: WT for Mecp2B/Mecp2B/WT for Mecp2J/Mecp2J 6/6/6/6. *P 0.05, significantly different from WT mice. **P 0.05, significantly different between WTs or between mutant mice. ***P 0.05, significantly different from WT and between mutant mice. The Journal of Comparative Neurology 188 N.P. BELICHENKO ET AL. TABLE 3. Number of Sections in Brain Region was Contained in Wildtype and Mecp2 Mutant Mice 1,2 WT for Mecp2B Mecp2B WT for Mecp2J Mecp2J Olfactory bulb *** Cortex ** * Hippocampus ** ** * Cerebellum Periaqueductal gray matter * * Nucleus ambiguus Nucleus hypoglossus, rostral Fornix Fimbria ** * Fasciculus retroflexus Mammillothalamic tract Anterior commissure, olfactory limb ** * Posterior commissure Corpus callosum ** The number of mice used for each genotype was as follows: WT for Mecp2B/Mecp2B/WT for Mecp2J/Mecp2J 6/6/6/6. 2 The distance between sections was 210 m. *P 0.05, significantly different from WT mice. **P 0.05, significantly different between WTs or between mutant mice. ***P 0.05, significantly different from WT and between mutant mice. which the structure was present. In Figures 1 8, S1 S8, we report data on shape of the structure as defined by the area at cross section along the rostrocaudal axis. Regional structures Olfactory bulb (OB). As shown in Figure 1a, the area of OB was measured from the rostralmost part of the structure (bregma 5.00 mm) through the point at which cortex and OB are dorsally connected (bregma 2.40 mm) (see mouse atlas; Hof et al., 2000). Comparing OB volume in mutant and WT mice, there was a significant 18% decrease (P 0.001) in overall volume in Mecp2B mice (Table 2); in addition, there were two significant changes in shape, a decrease in the central area together with a decrease in the length of OB in the mutant (Fig. 1b). In Mecp2J mice, there was no change in overall volume (P 0.33). However, there was a significant change in shape, with an increase in area in the most caudal sections (Fig. 1c). Comparing the WT mice, OB volume was significantly greater in WT for Mecp2B than in WT for Mecp2J mice (P 0.001); the shape of the OB differed principally through increased area of sections located centrally (Fig. S1a in supplementary data) in the WT mice for Mecp2B (Fig. 1b,c). Comparing the two mutants, there was no difference in volume (P 0.10), but changes in shape were evident in area scores (Fig. S1b) and rostrocaudal length (P 0.049) (Table 3). Cortex. Relative to their WT controls, cortical volume was significantly reduced in both Mecp2B (P ) and Mecp2J (P 0.01) mice (Table 2). For Mecp2B mice, area scores were significantly decreased along virtually the entire rostrocaudal axis; the few exceptions were the frontal pole and caudal sections containing the primary visual, ectorhinal, and entorhinal cortices (Fig. 2a). In Mecp2J mice the following cortical areas were significantly reduced: 1) primary motor, cingulate, and rostral part of primary somatosensory cortices; 2) parietal association, visual, and auditory cortices; and 3) entorhinal and temporal association cortices (Fig. 2b). In contrast, frontal pole, barrel field, and secondary somatosensory cortex were not affected. Moreover, while there was no difference in the rostrocaudal length of the cortex between
6 STRUCTURAL PATHOLOGY IN Mecp2-MUTANT MICE 189 Fig. 1. Typical coronal plane images from olfactory bulb landmarks used for stereological analysis. a: Thionine-stained coronal sections of the olfactory bulb were measured, at different bregma levels, as outlined in each section. The level where olfactory bulb and cortex have a common border (section on the right) was not included in analysis. b: Quantitative analysis of the area of olfactory bulb in WT (open squares) and Mecp2B mice (solid squares) measured at different coronal plane images. c: Comparison of areas of olfactory bulb in WT (open diamonds) and Mecp2J mice (solid diamonds) at different coronal plane images. Area of olfactory bulb was measured between 5.00 mm and 2.40 mm bregma levels. Measurements were taken from six mice of each genotype: WT for Mecp2B, Mecp2B, WT for Mecp2J and Mecp2J. *P 0.05 indicates significant differences between WT and Mecp2-mutant mice. Scale bar 1 mm. WT and Mecp2B mice (P 0.44; Table 3; Fig. 2a), the cortex was shorter in Mecp2J mice than in WT (P 0.004; Table 3; Fig. 2b). The cortical volume in WT for Mecp2B was greater than in the WT for MecpJ (P 0.02; Table 2); no difference was present comparing the two mutants (P 0.44; Table 2). The rostrocaudal axis of the cortex showed no difference between the two WTs (P 0.17; Table 3; Fig. S2a); the cortex was significantly shorter in Mecp2J than in Mecp2B mice (P 0.005; Table 3; Fig. S2b). Hippocampus. The volume of hippocampus was significantly reduced in both Mecp2B (P 0.004, Table 2) and Mecp2J mice (P 0.004, Table 2). Lower values for hippocampal areas were equally distributed along the rostrocaudal axis in Mecp2B mice (Fig. 3a) and there was no difference in the rostrocaudal extension of hippocampus (P 0.17; Table 3; Fig. 3a). In Mecp2J mice the hippocampus was significantly shorter (P ; Table 3; Fig. 3b). Moreover, the shape of the hippocampus was quite different in comparison to the WT (Fig. 3b). No significant differences in hippocampal volumes between WTs (P 0.78) or between mutant mice (P 0.56) were found (Table 3). But there was a difference in shape in that the hippocampus in WT for Mecp2B mice was significantly shorter than the WT for Mecp2J (P 0.005; Table 3; Fig. S3a). In addition, in Mecp2J mice the hippocampus was significantly shorter than in Mecp2B mice (P 0.001; Table 3; Fig. S3b). Cerebellum. Cerebellar volumes were significantly reduced in both Mecp2B and Mecp2J mice (by 12% and 8%, respectively; Table 2). In Mecp2B mice the rostral part of the cerebellum was markedly affected (Fig. 3c), while in Mecp2J mice the caudal part was more affected (Fig. 3d), but due to variability these differences did not reach significance. There were no differences in cerebellar volume between WTs (P 0.21, Table 2) or between mutants (P 0.12, Table 2). The rostrocaudal axis was similar in all four groups of mice (Table 3; Fig. S3c,d). Periaqueductal gray matter (PAG). In comparison to WT mice, the PAG volume was reduced by 20% in Mecp2B mice (P 0.005), but was not altered in Mecp2J mice (P 0.23; Table 2). In both mutants the shape was changed, especially rostrally and in a decrease in the rostrocaudal axis (Mecp2B: P 0.01; Mecp2J: P 0.001;
7 190 N.P. BELICHENKO ET AL. Fig. 2. Quantitative analysis of the areas of cortex along the rostrocaudal axis. a: Area of cortex in WT (open squares) and Mecp2B mice (solid squares) at different coronal plane images. b: Areas of cortex in WT (open diamonds) and Mecp2J mice (solid diamonds) at different coronal plane images. All values are expressed as mean. *P 0.05, significantly different between WT and Mecp2-mutant mice. Table 3; Fig. 4a,b). There were no differences in PAG volume or rostrocaudal axis length between the two WTs or between the two mutants (Tables 2, 3; Fig. S4a,b). Nucleus ambiguus (NA). The NA was significantly smaller in Mecp2B vs. WT (P 0.02), but not in Mecp2J vs. WT (P 0.65; Table 2). The NA volume also distinguished the mutants; it was much smaller in Mecp2B mice (P 0.02; Table 2). Significant local reductions of the NA areas were noted for both Mecp2B (Fig. 4c) and Mecp2J mice (Fig. 4d) in comparison to the respective WTs, but those for Mecp2B mice were much more marked. There were no differences in the length of the rostrocaudal axis between WTs or between mutant mice (Table 3; Fig. S4c,d). Nucleus hypoglossus (nc. XII). Nc. XII was unchanged in mutant mice compared to WTs (Tables 2, 3; Fig. S8). The only significant difference was in the volume of this nucleus between the WT mice (P ; Table 2). Axonal bundles Given the data for volume changes of brain structures in Mecp2-mutant mice, we expected to find changes in axonal pathways. We studied the influence of Mecp2 mutations by measuring the areas of fornix, fimbria, MTT, AC, and PC and the width of PC and CC. Table 2 shows the average values for these measures and Table 3 reports on their rostrocaudal length. Fornix. The area of fornix was 19% smaller in Mecp2B mice than in WT (P 0.03; Table 2); this difference was due mainly to decreases caudally (Fig. 5a). No changes from WT were seen in Mecp2J mice (P 0.25; Table 2; Fig. 5b). There were no differences in rostrocaudal length for either mutant in comparison to the WT Fig. 3. Quantitative analysis of the areas of hippocampus (a,b) and cerebellum (c,d) along the rostrocaudal axis. a,c: Comparison of the areas in WT (open squares) and Mecp2B mice (solid squares) at different coronal plane images. b,d: Comparison of the areas in WT (open diamonds) and Mecp2J mice (solid diamonds) at different coronal plane images. *P 0.05, significantly different between WT and Mecp2-mutant mice. (Table 3; Fig. 5a,b). However, the area of the fornix was significantly smaller (by 20%) in WT for Mecp2J than in WT for Mecp2B mice (P 0.004; Table 2; Fig. S5a). Fimbria. As for fornix, the fimbria was 19% smaller in Mecp2B than in WT (P 0.001; Table 2) and was registered mainly caudally (Fig. 5c). No change in fimbria area was present in Mecp2J mice (P 0.07; Table 2), but the rostrocaudal axis of the fimbria was significantly shorter (P 0.002; Table 3; Fig. 5d). The area of fimbria was smaller in Mecp2B vs. Mecp2J mice (P 0.001; Table 2; Fig. S5d), and the rostrocaudal length of fimbria was shorter in the WT for Mecp2B than in the WT for Mecp2J mice (P 0.03; Table 3; Fig. S5c). Fasciculus retroflexus (FR). The area of FR was 17% smaller in Mecp2B vs. WT (P 0.001; Table 2) and this difference was evident both rostrally and caudally (Fig. 6a). Although the overall area of FR was not significantly smaller in Mecp2J vs. WT (P 0.07; Table 2), a significant difference was present caudally (Fig. 6b). No other differences were detected in the FR (Tables 2, 3; Fig. S6a,b). Mammillothalamic tract (MTT). The area and rostrocaudal axis of MTT were not different in mutant vs. WTs mice (Tables 2, 3), but regional changes in area scores were evident in the rostrocaudal axis for both Mecp2-mutant mice (Fig. 6c,d). No change in MTT area was present in mutant mice (Fig. S6d). Anterior commissure, olfactory limb (AC). The area of AC was 17% smaller in Mecp2B vs. WT (P 0.001; Table 2) and this difference was evident in both rostral and middle sections (Fig. 7a). There was no difference in the average area in Mecp2J vs. WT (P 0.07; Table 2), but
8 STRUCTURAL PATHOLOGY IN Mecp2-MUTANT MICE 191 Fig. 4. Quantitative analysis of the areas of periaqueductal gray matter (a,b) and nucleus ambiguus (c,d) along the rostrocaudal axis. a,c: Comparison of the areas in WT (open squares) and Mecp2B mice (solid squares) at different coronal plane images. b,d: Comparison of the areas in WT (open diamonds) and Mecp2J mice (solid diamonds) at different coronal plane images. *P 0.05, significantly different between WT and Mecp2-mutant mice. Fig. 6. Quantitative analysis of the areas of fasciculus retroflexus (a,b) and mammillothalamic tract (c,d) along the rostrocaudal axis. a,c: Comparison of the areas in WT (open squares) and Mecp2B mice (solid squares) at different coronal plane images. b,d: Comparison of the areas in WT (open diamonds) and Mecp2J mice (solid diamonds) at different coronal plane images. *P 0.05, significantly different between WT and Mecp2-mutant mice. the rostral part of the AC showed slight regional differences (Fig. 7b). The average AC area in WT for Mecp2B was 24% larger than in WT for Mecp2J (P ; Table 2). The rostrocaudal length of the AC was shorter in Mecp2J vs. WT (P 0.001; Table 3; Fig. 7b) and in Mecp2J vs. Mecp2B (P 0.001; Table 3; Fig. S7b), but no differences were found in Mecp2B vs. WT (Fig. 7a) and between the two WT strains (Fig. S7a). Posterior commissure (PC). There were no differences in area or width of the PC between mutant and WT mice (P 0.05; Table 2, Table 3). Area and width examined throughout the rostrocaudal length of the PC revealed no differences (data not shown). Corpus callosum (CC). Significant differences in width ( 12%) and length ( 9%) were recorded for CC between WT strains (P 0.02) and between mutant mice (P 0.002), but no differences were found in WT vs. Mecp2B (P 0.57) or WT vs. Mecp2J (P 0.97) comparisons (Tables 2, 3; Figs. 7c,d, S7c,d). There were also essentially no differences in width along the rostrocaudal axis of the CC in Mecp2B vs. WT (Fig. 7c) and Mecp2J vs. WT (Fig. 7d). Fig. 5. Quantitative analysis of the areas of fornix (a,b) and fimbria (c,d) along the rostrocaudal axis. a,c: Comparison of the areas in WT (open squares) and Mecp2B mice (solid squares) at different coronal plane images. b,d: Comparison of the areas in WT (open diamonds) and Mecp2J mice (solid diamonds) at different coronal plane images. *P 0.05, significantly different between WT and Mecp2-mutant mice. DISCUSSION We describe here morphological changes in the brains of two MeCP2-deficient mouse models for RTT. By choosing a method that allows careful and quantitative comparisons of brain structures between mice of different genotypes, we examined a total of 47 phenotypes. Both large and small differences in the volume and shape of neuronal nuclei and axonal bundles in Mecp2-mutant mice have
9 192 N.P. BELICHENKO ET AL. been defined. The findings allow us to resolve the following: I) changes in brain regions or axonal bundles that are due to a deficiency in Mecp2 without evidence for difference attributable to type of mutation; II) changes that may be influenced in severity by the type of Mecp2 mutation; Fig. 7. Quantitative analysis of the areas of anterior commissure (a,b) and width of corpus callosum (c,d) along the rostrocaudal axis. a,c: Comparison of the areas/widths in WT (open squares) and Mecp2B mice (solid squares) at different coronal plane images. b,d: Comparison of the areas/widths in WT (open diamonds) and Mecp2J mice (solid diamonds) at different coronal plane images. *P 0.05, significantly different between WT and Mecp2-mutant mice. III) changes that further resist interpretation due to possible underlying influence of genetic strain difference; and IV) brain regions and axonal bundles apparently unaffected by MeCP2 deficiency. Tables 4 and S2 summarize the findings. Overall, 61.7% of observations describe changes due to mutations in Mecp2. Of these, 21.3% conform to category I, 17.0% to category II, and 23.4% to category III. Fully 38.3% of observations provided no evidence for an effect of MeCP2 deficiency. The background strain differences, specifically the action of strain-specific modifying genes, may also contribute to the variances observed. These data provide new insights into the effects of Mecp2 mutations and are evidence that detailed, quantitative evaluations of brain structure can be used to define more precisely the impact of such mutations. Changes in brain structure in Mecp2-mutant mice While at 21 postnatal days of age there were no gross anatomical abnormalities, brain region-specific changes were clearly defined. Indeed, significant differences were found in 24 out of 47 parameters in Mecp2B and 18 out of 47 parameters in Mecp2J mice as compared to their WT littermates (Table 4). Both mutations markedly affected brain weight, the volumes of cortex, hippocampus and cerebellum, the rostrocaudal length of PAG, and the shapes of OB, cortex, hippocampus, AC, FR, MTT, PAG, and NA (13 out of 47 parameters). Body weights at 21 days were significantly lower in Mecp2B mice than WT, and thus confirm data of Guy et al. (2001). Stearns et al. (2007) carried out brain region volume studies in 35-day-old Mecp2J mice, after the strain had been bred to C57BL/6 for more than eight generations. In agreement with our results, the authors reported significant reduction of whole brain weight/volume, hippocampal volume, and no changes in AC and CC. They also found reductions of the striatum and amygdala, structures that we did not study. Our results also agree with previously published data of TABLE 4. Summary of Parameters Changing in Wildtype and Mecp2 Mutant Mice No. Parameters Mecp2B Mecp2J Type 1 Body weight * n.s Brain weight * * 2 Volume, area or width Rostrocaudal length Shape Mecp2B Mecp2J Type Mecp2B Mecp2J Type Mecp2B Mecp2J Type 3 Olfactory bulb * n.s. 12 * n.s. 6 * * 9 4 Cortex * * 8 n.s. * 5 * * 8 5 Hippocampus * * 2 n.s. * 11 * * 3 6 Cerebellum * * 2 n.s. n.s. 1 n.s. n.s. 1 7 Corpus callosum n.s. n.s. 7 n.s. n.s. 7 n.s. * 11 8 Fornix * n.s. 12 n.s. n.s. 1 * n.s Fimbria * n.s. 6 n.s. * 11 * n.s Anterior commissure * n.s. 12 n.s. * 5 * * Posterior commissure (area) n.s. n.s. 1 n.s. n.s. 1 n.s. n.s Posterior commissure (width) n.s. n.s. 1 n.s. n.s. 1 n.s. n.s Fasciculus retroflexus * n.s. 6 n.s. n.s. 1 * * 2 14 Mammillothalamic tract n.s. n.s. 1 n.s. n.s. 1 * * 8 15 Periaqueductal gray matter * n.s. 6 * * 2 * * 2 16 Nucleus ambiguus * n.s. 6 n.s. n.s. 1 * * 2 17 Nucleus hypoglossus n.s. n.s. 7 n.s. n.s. 1 n.s. n.s. 1 *Significantly different; n.s., nonsignificant. Definitions of types were as follows: When WT for Mecp2B WT for Mecp2J and for Type 1 -WT Mecp2B, WT Mecp2J; Type 2-WT Mecp2B, WT Mecp2J, Mecp2B Mecp2J; Type 3-WT Mecp2B, WT Mecp2J, Mecp2B Mecp2J; Type 4-WT Mecp2B, WT Mecp2J, Mecp2B Mecp2J; Type 5-WT Mecp2B, WT Mecp2J; Type 6-WT Mecp2B, WT Mecp2J. When WT for Mecp2B WT for Mecp2J and for Type 7 -WT Mecp2B, WT Mecp2J; Type 8-WT Mecp2B, WT Mecp2J, Mecp2B Mecp2J; Type 9-WT Mecp2B, WT Mecp2J, Mecp2B Mecp2J; Type 10 - WT Mecp2B, WT Mecp2J, Mecp2B Mecp2J; Type 11 - WT Mecp2B, WT Mecp2J; Type 12 - WT Mecp2B, WT Mecp2J. Category I included types 2 and 8, category II - types 3-6, category III - types 9-12, and category IV - types 1 and 7.
10 STRUCTURAL PATHOLOGY IN Mecp2-MUTANT MICE significant reduction of cortical thickness in 4-week-old Mecp2B (Fukuda et al., 2005) and 8-week-old Mecp2J mice (Kishi and Macklis, 2004). Stearns et al. (2007) did not observe significantly reduced volume of the rostral part of cerebellum in Mecp2J mice at 35 days of age, but the cerebellar volume was reduced in Mecp2B mice (Saywell et al., 2006). The significant reduction in total cerebellar volume for both mutant mice that we found may reflect more severe changes in volume for caudal then for rostral parts for Mecp2J, in agreement between our (see Fig. 3d) and Stearns et al. (2007) data. It is also interesting to note that although significant differences in morphology of cortex, NA, FR, and MTT were found in WT vs. mutant mice, the shapes of these structures were more similar in Mecp2B vs. Mecp2J mice (Figs. S2b, S4d, S6b,d). This may suggest that the mutations reshaped the tissue in such a way as to obviate strain difference. As examples of differentiated effects on rostrocaudal length of structure, we point out that both mutation and strain contribute to the effect on rostrocaudal length of hippocampus. For the PAG, only mutations affect rostrocaudal length, and for the CC, only strain background affects rostrocaudal length. In only two (PC and nc. XII) out of 14 brain structures were we unable to detect any significant changes in Mecp2-mutant mice (Table 4). Differences in severity in brain morphology of Mecp2B and Mecp2J mice A consistent finding to emerge from our comparative studies is that the volume, area, and shape abnormalities detected in the Mecp2J mice are present at increased severity in Mecp2B mice. Indeed, 11 measures were significantly worse in Mecp2B than Mecp2J mice and only five were significantly worse in Mecp2J than Mecp2B (Table 4). When significant changes were noted for both mutant mice the severity of such changes was still more pronounced in Mecp2B: the cortical volume was reduced in Mecp2B on average by 11%, and in Mecp2J mice 7%; the cerebellar volume was reduced by 12% and 8%; the rostrocaudal axis of the PAG was significantly shorter by 14% and 11%, respectively (Table 2). In contrast, the volume of the hippocampus was reduced by 8% in both mutants. In addition, we found the cortical areas were significantly reduced in a region-specific manner in both mutant mice (see Fig. 2). Consistent with previous reports (Chen et al., 2001; Guy et al., 2001; Chang et al., 2006; Santos et al., 2007), we observed that the onset of symptoms and time of death were on average somewhat later in the Mecp2J mice (H.H.L and U.F., pers. obs.). The reason why the mutations should differ is uncertain. Although both mutations are generally considered null, the possibility that the Mecp2J-mutant gene product may retain some function, at the RNA or protein level, must be considered. In addition, one must carefully consider strain-specific modifying genes that would not have been revealed as changes in the structure of the region in WT mice. We were careful to limit assignment to category II those observations in which there was no difference in comparing WT mice of different strains. For this reason, our assignment of an observation to category II i.e., as revealing a difference in severity of the mutants must be seen as provisional until direct comparisons can be made on an identical strain background. 193 Our findings provide evidence for the Mecp2 mutations involvement in volume defects and suggest that many areas may be affected by such defects. Furthermore, the Mecp2J mutation had no effect on axonal bundles, while the Mecp2B mutation significantly influenced this parameter in four of the seven structures studied that represent axonal bundles (fornix, fimbria, FR, and AC). In contrast, measurements of CC, PC, and MTT showed reductions in the mutants that were not statistically significant. Taken together, these data and previously published results on human RTT brain studies (Belichenko et al., 1994; Leontovich et al., 1999) point to heavy involvement of axonal connections in the pathogenesis of RTT. Similarity in the alterations of brain morphology in RTT subjects and Mecp2- mutant mice and their possible pathophysiological relevance Neuropathological and MRI studies on RTT brains revealed global hypoplasia and significant reduction of gray matter throughout the cortex (Subramaniam et al., 1997; Gotoh et al., 2001; reviewed by Armstrong, 2002). The prefrontal, posterior frontal, and anterior temporal regions were more severely reduced than other cortical areas (Subramaniam et al., 1997). The volume reduction was seen postmortem in both the brains of heterozygous female RTT and hemizygous MECP2 mutant males with congenital encephalopathy. The male mice studied herein are a genetic model for the male phenotype, but their clinical course is protracted and resembles more that of female RTT (Chen et al., 2001; Guy et al., 2001). Detailed neuropathological studies of congenital encephalopathy brains are currently lacking. Most structures that were reported to be abnormal in female RTT brains are not easily defined in mouse brain and were not the subject of our studies. Progressive atrophy of cerebellum was described in one MRI study (Murakami et al., 1992), and reduction of Purkinje neurons was reported twice (Oldfors et al., 1990; Bauman et al., 1995). Histological changes in hippocampal formation, entorhinal cortex and basal ganglia systems in RTT (Leontovich et al., 1999) correlate with our data on significant reduction of hippocampal and cortical volumes in both Mecp2- mutant mice, with reduced areas of fornix and fimbria in Mecp2B mice, and with published data on striatum (Stearns et al., 2007). Significant changes in hippocampal volume may reflect a dramatic reduction in LTP, seen in both Mecp2-mutant mice (Asaka et al., 2006) and changes in behavior (Stearns et al., 2007). Synaptic plasticity was significantly altered in the CA1 area of the hippocampus in both Mecp2B and Mecp2J mice at 6 10 weeks of age, but not at 3 5 weeks (Asaka et al., 2006). Deficiencies of learning and memory has been documented in the Mecp2- mutant mice (Stearns et al., 2007). The body of evidence is consistent with significant deficits in hippocampal function in the Mecp2-mutants. Similarly, the reduction in the volume of NA in Mecp2B may reflect the known abnormal respiratory abnormalities reported in RTT subjects (Julu et al., 1997) and in these mice (Bissonnette and Knopp, 2006). There is a possibility that Mecp2J brains could show more severe phenotypes if studied at a later age, and be more similar to the Mecp2B mutants at postnatal day 21.
Ch 13: Central Nervous System Part 1: The Brain p 374
 Ch 13: Central Nervous System Part 1: The Brain p 374 Discuss the organization of the brain, including the major structures and how they relate to one another! Review the meninges of the spinal cord and
Ch 13: Central Nervous System Part 1: The Brain p 374 Discuss the organization of the brain, including the major structures and how they relate to one another! Review the meninges of the spinal cord and
Introduction to the Central Nervous System: Internal Structure
 Introduction to the Central Nervous System: Internal Structure Objective To understand, in general terms, the internal organization of the brain and spinal cord. To understand the 3-dimensional organization
Introduction to the Central Nervous System: Internal Structure Objective To understand, in general terms, the internal organization of the brain and spinal cord. To understand the 3-dimensional organization
Chapter 3. Structure and Function of the Nervous System. Copyright (c) Allyn and Bacon 2004
 Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
PROPERTY OF ELSEVIER SAMPLE CONTENT - NOT FINAL. Gross Anatomy and General Organization of the Central Nervous System
 3 Gross Anatomy and General Organization of the Central Nervous System C h a p t e r O u t l i n e The Long Axis of the CNS Bends at the Cephalic Flexure Hemisecting a Brain Reveals Parts of the Diencephalon,
3 Gross Anatomy and General Organization of the Central Nervous System C h a p t e r O u t l i n e The Long Axis of the CNS Bends at the Cephalic Flexure Hemisecting a Brain Reveals Parts of the Diencephalon,
Regional and Lobe Parcellation Rhesus Monkey Brain Atlas. Manual Tracing for Parcellation Template
 Regional and Lobe Parcellation Rhesus Monkey Brain Atlas Manual Tracing for Parcellation Template Overview of Tracing Guidelines A) Traces are performed in a systematic order they, allowing the more easily
Regional and Lobe Parcellation Rhesus Monkey Brain Atlas Manual Tracing for Parcellation Template Overview of Tracing Guidelines A) Traces are performed in a systematic order they, allowing the more easily
Cerebral Cortex 1. Sarah Heilbronner
 Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
Cerebral Cortex 1 Sarah Heilbronner heilb028@umn.edu Want to meet? Coffee hour 10-11am Tuesday 11/27 Surdyk s Overview and organization of the cerebral cortex What is the cerebral cortex? Where is each
LIMBIC SYSTEM. Dr. Amani A. Elfaki Associate Professor Department of Anatomy
 LIMBIC SYSTEM Dr. Amani A. Elfaki Associate Professor Department of Anatomy Learning Objectives Define the limbic system Identify the parts of the limbic system Describe the circulation of the limbic system
LIMBIC SYSTEM Dr. Amani A. Elfaki Associate Professor Department of Anatomy Learning Objectives Define the limbic system Identify the parts of the limbic system Describe the circulation of the limbic system
Anatomy and Physiology (Bio 220) The Brain Chapter 14 and select portions of Chapter 16
 Anatomy and Physiology (Bio 220) The Brain Chapter 14 and select portions of Chapter 16 I. Introduction A. Appearance 1. physical 2. weight 3. relative weight B. Major parts of the brain 1. cerebrum 2.
Anatomy and Physiology (Bio 220) The Brain Chapter 14 and select portions of Chapter 16 I. Introduction A. Appearance 1. physical 2. weight 3. relative weight B. Major parts of the brain 1. cerebrum 2.
Strick Lecture 3 March 22, 2017 Page 1
 Strick Lecture 3 March 22, 2017 Page 1 Cerebellum OUTLINE I. External structure- Inputs and Outputs Cerebellum - (summary diagram) 2 components (cortex and deep nuclei)- (diagram) 3 Sagittal zones (vermal,
Strick Lecture 3 March 22, 2017 Page 1 Cerebellum OUTLINE I. External structure- Inputs and Outputs Cerebellum - (summary diagram) 2 components (cortex and deep nuclei)- (diagram) 3 Sagittal zones (vermal,
9.14 Class 32 Review. Limbic system
 9.14 Class 32 Review Limbic system 1 Lateral view Medial view Brainstem, sagittal section Sensory- Perceptual Motor Behavior Major functional modules of the CNS Motivation Courtesy of MIT Press. Used with
9.14 Class 32 Review Limbic system 1 Lateral view Medial view Brainstem, sagittal section Sensory- Perceptual Motor Behavior Major functional modules of the CNS Motivation Courtesy of MIT Press. Used with
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Cerebellum. Steven McLoon Department of Neuroscience University of Minnesota
 Cerebellum Steven McLoon Department of Neuroscience University of Minnesota 1 Anatomy of the Cerebellum The cerebellum has approximately half of all the neurons in the central nervous system. The cerebellum
Cerebellum Steven McLoon Department of Neuroscience University of Minnesota 1 Anatomy of the Cerebellum The cerebellum has approximately half of all the neurons in the central nervous system. The cerebellum
The Cerebellum. Outline. Lu Chen, Ph.D. MCB, UC Berkeley. Overview Structure Micro-circuitry of the cerebellum The cerebellum and motor learning
 The Cerebellum Lu Chen, Ph.D. MCB, UC Berkeley 1 Outline Overview Structure Micro-circuitry of the cerebellum The cerebellum and motor learning 2 Overview Little brain 10% of the total volume of the brain,
The Cerebellum Lu Chen, Ph.D. MCB, UC Berkeley 1 Outline Overview Structure Micro-circuitry of the cerebellum The cerebellum and motor learning 2 Overview Little brain 10% of the total volume of the brain,
The Central Nervous System I. Chapter 12
 The Central Nervous System I Chapter 12 The Central Nervous System The Brain and Spinal Cord Contained within the Axial Skeleton Brain Regions and Organization Medical Scheme (4 regions) 1. Cerebral Hemispheres
The Central Nervous System I Chapter 12 The Central Nervous System The Brain and Spinal Cord Contained within the Axial Skeleton Brain Regions and Organization Medical Scheme (4 regions) 1. Cerebral Hemispheres
For more information about how to cite these materials visit
 Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Prof. Saeed Abuel Makarem & Dr.Sanaa Alshaarawy
 Prof. Saeed Abuel Makarem & Dr.Sanaa Alshaarawy 1 Objectives By the end of the lecture, you should be able to: Describe the anatomy and main functions of the thalamus. Name and identify different nuclei
Prof. Saeed Abuel Makarem & Dr.Sanaa Alshaarawy 1 Objectives By the end of the lecture, you should be able to: Describe the anatomy and main functions of the thalamus. Name and identify different nuclei
Is Intrinsic Hyperexcitability in CA3 the Culprit for Seizures in Rett Syndrome?
 Current Literature In Basic Science Is Intrinsic Hyperexcitability in CA3 the Culprit for Seizures in Rett Syndrome? Network Hyperexcitability in Hippocampal Slices From Mecp2 Mutant Mice Revealed by Voltage-Sensitive
Current Literature In Basic Science Is Intrinsic Hyperexcitability in CA3 the Culprit for Seizures in Rett Syndrome? Network Hyperexcitability in Hippocampal Slices From Mecp2 Mutant Mice Revealed by Voltage-Sensitive
Fig.1: A, Sagittal 110x110 mm subimage close to the midline, passing through the cingulum. Note that the fibers of the corpus callosum run at a
 Fig.1 E Fig.1:, Sagittal 110x110 mm subimage close to the midline, passing through the cingulum. Note that the fibers of the corpus callosum run at a slight angle are through the plane (blue dots with
Fig.1 E Fig.1:, Sagittal 110x110 mm subimage close to the midline, passing through the cingulum. Note that the fibers of the corpus callosum run at a slight angle are through the plane (blue dots with
Outline of the next three lectures
 Outline of the next three lectures Lecture 35 Anatomy of the human cerebral cortex gross and microscopic cell types connections Vascular supply of the cerebral cortex Disorders involving the cerebral cortex
Outline of the next three lectures Lecture 35 Anatomy of the human cerebral cortex gross and microscopic cell types connections Vascular supply of the cerebral cortex Disorders involving the cerebral cortex
M555 Medical Neuroscience Lab 1: Gross Anatomy of Brain, Crainal Nerves and Cerebral Blood Vessels
 M555 Medical Neuroscience Lab 1: Gross Anatomy of Brain, Crainal Nerves and Cerebral Blood Vessels Anatomical Directions Terms like dorsal, ventral, and posterior provide a means of locating structures
M555 Medical Neuroscience Lab 1: Gross Anatomy of Brain, Crainal Nerves and Cerebral Blood Vessels Anatomical Directions Terms like dorsal, ventral, and posterior provide a means of locating structures
Lecture - Chapter 13: Central Nervous System
 Lecture - Chapter 13: Central Nervous System 1. Describe the following structures of the brain, what is the general function of each: a. Cerebrum b. Diencephalon c. Brain Stem d. Cerebellum 2. What structures
Lecture - Chapter 13: Central Nervous System 1. Describe the following structures of the brain, what is the general function of each: a. Cerebrum b. Diencephalon c. Brain Stem d. Cerebellum 2. What structures
Human Brain and Senses October 13, 2008 Page 1. Examination of the Human Brain
 Human Brain and Senses October 13, 2008 Page 1 Examination of the Human Brain With only a few hours today we can only begin to scratch the surface of a complex subject like neuroanatomy. The purpose of
Human Brain and Senses October 13, 2008 Page 1 Examination of the Human Brain With only a few hours today we can only begin to scratch the surface of a complex subject like neuroanatomy. The purpose of
By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy
 By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific
By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific
Brain and Cranial Nerves (Ch. 15) Human Anatomy lecture. caudal = toward the spinal cord)
 Insight: Some cranial nerve disorders Brain and Cranial Nerves (Ch. 15) Human Anatomy lecture I. Overview (Directional terms: rostral = toward the forehead caudal = toward the spinal cord) A. 3 Major parts
Insight: Some cranial nerve disorders Brain and Cranial Nerves (Ch. 15) Human Anatomy lecture I. Overview (Directional terms: rostral = toward the forehead caudal = toward the spinal cord) A. 3 Major parts
Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota
 Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Tuesday (Sept 11) 10:00-11:00am Friday (Sept 14) 8:30-9:30am Surdyk s
Overview of the Nervous System (some basic concepts) Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Tuesday (Sept 11) 10:00-11:00am Friday (Sept 14) 8:30-9:30am Surdyk s
Located below tentorium cerebelli within posterior cranial fossa. Formed of 2 hemispheres connected by the vermis in midline.
 The Cerebellum Cerebellum Located below tentorium cerebelli within posterior cranial fossa. Formed of 2 hemispheres connected by the vermis in midline. Gray matter is external. White matter is internal,
The Cerebellum Cerebellum Located below tentorium cerebelli within posterior cranial fossa. Formed of 2 hemispheres connected by the vermis in midline. Gray matter is external. White matter is internal,
Nature Neuroscience: doi: /nn.2275
 Supplementary Figure S1. The presence of MeCP2 in enriched primary glial cultures from rat or mouse brains is not neuronal. Western blot analysis of protein extracts from (a) rat glial and neuronal cultures.
Supplementary Figure S1. The presence of MeCP2 in enriched primary glial cultures from rat or mouse brains is not neuronal. Western blot analysis of protein extracts from (a) rat glial and neuronal cultures.
Student Lab #: Date. Lab: Gross Anatomy of Brain Sheep Brain Dissection Organ System: Nervous Subdivision: CNS (Central Nervous System)
 Lab: Gross Anatomy of Brain Sheep Brain Dissection Organ System: Nervous Subdivision: CNS (Central Nervous System) Student Lab #: Date 1 Objectives: 1. Learn the main components making up a motor neuron.
Lab: Gross Anatomy of Brain Sheep Brain Dissection Organ System: Nervous Subdivision: CNS (Central Nervous System) Student Lab #: Date 1 Objectives: 1. Learn the main components making up a motor neuron.
Telencephalon (Cerebral Hemisphere)
 Telencephalon (Cerebral Hemisphere) OUTLINE The Cortex - Lobes, Sulci & Gyri - Functional Subdivisions - Limbic Lobe & Limbic System The Subcortex - Basal Ganglia - White Matter (Internal Capsule) - Relations
Telencephalon (Cerebral Hemisphere) OUTLINE The Cortex - Lobes, Sulci & Gyri - Functional Subdivisions - Limbic Lobe & Limbic System The Subcortex - Basal Ganglia - White Matter (Internal Capsule) - Relations
P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center. Wednesday, 16 March 2009, 1:00p.m. 2:00p.m.
 Normal CNS, Special Senses, Head and Neck TOPIC: CEREBRAL HEMISPHERES FACULTY: LECTURE: READING: P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center Wednesday, 16 March
Normal CNS, Special Senses, Head and Neck TOPIC: CEREBRAL HEMISPHERES FACULTY: LECTURE: READING: P. Hitchcock, Ph.D. Department of Cell and Developmental Biology Kellogg Eye Center Wednesday, 16 March
Auditory and Vestibular Systems
 Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Systems Neuroscience Dan Kiper. Today: Wolfger von der Behrens
 Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
Systems Neuroscience Dan Kiper Today: Wolfger von der Behrens wolfger@ini.ethz.ch 18.9.2018 Neurons Pyramidal neuron by Santiago Ramón y Cajal (1852-1934, Nobel prize with Camillo Golgi in 1906) Neurons
Brain anatomy and artificial intelligence. L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia
 Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
Brain anatomy and artificial intelligence L. Andrew Coward Australian National University, Canberra, ACT 0200, Australia The Fourth Conference on Artificial General Intelligence August 2011 Architectures
Nsci 2100: Human Neuroanatomy 2017 Examination 3
 Name KEY Lab Section Nsci 2100: Human Neuroanatomy 2017 Examination 3 On this page, write your name and lab section. On your bubble answer sheet, enter your name (last name, space, first name), internet
Name KEY Lab Section Nsci 2100: Human Neuroanatomy 2017 Examination 3 On this page, write your name and lab section. On your bubble answer sheet, enter your name (last name, space, first name), internet
CASE 48. What part of the cerebellum is responsible for planning and initiation of movement?
 CASE 48 A 34-year-old woman with a long-standing history of seizure disorder presents to her neurologist with difficulty walking and coordination. She has been on phenytoin for several days after having
CASE 48 A 34-year-old woman with a long-standing history of seizure disorder presents to her neurologist with difficulty walking and coordination. She has been on phenytoin for several days after having
CEREBRUM & CEREBRAL CORTEX
 CEREBRUM & CEREBRAL CORTEX Seonghan Kim Dept. of Anatomy Inje University, College of Medicine THE BRAIN ANATOMICAL REGIONS A. Cerebrum B. Diencephalon Thalamus Hypothalamus C. Brain Stem Midbrain Pons
CEREBRUM & CEREBRAL CORTEX Seonghan Kim Dept. of Anatomy Inje University, College of Medicine THE BRAIN ANATOMICAL REGIONS A. Cerebrum B. Diencephalon Thalamus Hypothalamus C. Brain Stem Midbrain Pons
1. The basic anatomy of the Central Nervous System (CNS)
 Psyc 311A, fall 2008 Conference week 1 Sept 9 th to 11 th TA: Jürgen Germann; e-mail: jurgen.germann@mcgill.ca Overview: 1. The basic anatomy of the Central Nervous System (CNS) 2. Cells of the CNS 3.
Psyc 311A, fall 2008 Conference week 1 Sept 9 th to 11 th TA: Jürgen Germann; e-mail: jurgen.germann@mcgill.ca Overview: 1. The basic anatomy of the Central Nervous System (CNS) 2. Cells of the CNS 3.
SUPPLEMENTARY INFORMATION. Rett Syndrome Mutation MeCP2 T158A Disrupts DNA Binding, Protein Stability and ERP Responses
 SUPPLEMENTARY INFORMATION Rett Syndrome Mutation T158A Disrupts DNA Binding, Protein Stability and ERP Responses Darren Goffin, Megan Allen, Le Zhang, Maria Amorim, I-Ting Judy Wang, Arith-Ruth S. Reyes,
SUPPLEMENTARY INFORMATION Rett Syndrome Mutation T158A Disrupts DNA Binding, Protein Stability and ERP Responses Darren Goffin, Megan Allen, Le Zhang, Maria Amorim, I-Ting Judy Wang, Arith-Ruth S. Reyes,
THE CENTRAL NERVOUS SYSTE M
 THE CENTRAL NERVOUS SYSTE M Structure and Functio n THIRD EDITIO N PER BRODAL A Brief Survey, x i Studying the Structures and Function of the Nervous System, xii i Animal Experiments Crucial for Progress,
THE CENTRAL NERVOUS SYSTE M Structure and Functio n THIRD EDITIO N PER BRODAL A Brief Survey, x i Studying the Structures and Function of the Nervous System, xii i Animal Experiments Crucial for Progress,
Brainstem. Steven McLoon Department of Neuroscience University of Minnesota
 Brainstem Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Change in Lab Sequence Week of Oct 2 Lab 5 Week of Oct 9 Lab 4 2 Goal Today Know the regions of the brainstem. Know
Brainstem Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Change in Lab Sequence Week of Oct 2 Lab 5 Week of Oct 9 Lab 4 2 Goal Today Know the regions of the brainstem. Know
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1.
 Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Genesis of cerebellar interneurons and the prevention of neural DNA damage require XRCC1. Youngsoo Lee, Sachin Katyal, Yang Li, Sherif F. El-Khamisy, Helen R. Russell, Keith W. Caldecott and Peter J. McKinnon.
Biological Bases of Behavior. 8: Control of Movement
 Biological Bases of Behavior 8: Control of Movement m d Skeletal Muscle Movements of our body are accomplished by contraction of the skeletal muscles Flexion: contraction of a flexor muscle draws in a
Biological Bases of Behavior 8: Control of Movement m d Skeletal Muscle Movements of our body are accomplished by contraction of the skeletal muscles Flexion: contraction of a flexor muscle draws in a
DISSECTION OF THE SHEEP'S BRAIN
 Sheep Brain Dissection Guide Page 1 DISSECTION OF THE SHEEP'S BRAIN Introduction The purpose of the sheep brain dissection is to familiarize you with the threedimensional structure of the brain and teach
Sheep Brain Dissection Guide Page 1 DISSECTION OF THE SHEEP'S BRAIN Introduction The purpose of the sheep brain dissection is to familiarize you with the threedimensional structure of the brain and teach
Note: Waxman is very sketchy on today s pathways and nonexistent on the Trigeminal.
 Dental Neuroanatomy Thursday, February 3, 2011 Suzanne Stensaas, PhD Note: Waxman is very sketchy on today s pathways and nonexistent on the Trigeminal. Resources: Pathway Quiz for HyperBrain Ch. 5 and
Dental Neuroanatomy Thursday, February 3, 2011 Suzanne Stensaas, PhD Note: Waxman is very sketchy on today s pathways and nonexistent on the Trigeminal. Resources: Pathway Quiz for HyperBrain Ch. 5 and
Hypothalamus. To learn how the brain regulates neuroendocrine secretions NTA Ch 14, pgs Key Figs: 14-3; 14-4,
 Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
Hypothalamus Objectives To learn the general organization of the hypothalamus and the functions of the major nuclei NTA Ch 14, pgs. 419-422 Key Figs: 14-2, 14-3 To learn how the brain regulates neuroendocrine
1. Processes nutrients and provides energy for the neuron to function; contains the cell's nucleus; also called the soma.
 1. Base of brainstem; controls heartbeat and breathing 2. tissue destruction; a brain lesion is a naturally or experimentally caused destruction of brain tissue 3. A thick band of axons that connects the
1. Base of brainstem; controls heartbeat and breathing 2. tissue destruction; a brain lesion is a naturally or experimentally caused destruction of brain tissue 3. A thick band of axons that connects the
Gross Organization I The Brain. Reading: BCP Chapter 7
 Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
Gross Organization I The Brain Reading: BCP Chapter 7 Layout of the Nervous System Central Nervous System (CNS) Located inside of bone Includes the brain (in the skull) and the spinal cord (in the backbone)
A sketch of the central nervous system and its origins. MIT 9.14 Classes 31
 A sketch of the central nervous system and its origins G. E. Schneider 2014 Part 9: Hypothalamus & Limbic System MIT 9.14 Classes 31 The vertebrate medial pallium; in mammals: the hippocampal formation
A sketch of the central nervous system and its origins G. E. Schneider 2014 Part 9: Hypothalamus & Limbic System MIT 9.14 Classes 31 The vertebrate medial pallium; in mammals: the hippocampal formation
The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible:
 NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
NERVOUS SYSTEM The neurvous system senses, interprets, and responds to changes in the environment. Two types of cells makes this possible: the neuron and the supporting cells ("glial cells"). Neuron Neurons
Unit VIII Problem 5 Physiology: Cerebellum
 Unit VIII Problem 5 Physiology: Cerebellum - The word cerebellum means: the small brain. Note that the cerebellum is not completely separated into 2 hemispheres (they are not clearly demarcated) the vermis
Unit VIII Problem 5 Physiology: Cerebellum - The word cerebellum means: the small brain. Note that the cerebellum is not completely separated into 2 hemispheres (they are not clearly demarcated) the vermis
Zhu et al, page 1. Supplementary Figures
 Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Zhu et al, page 1 Supplementary Figures Supplementary Figure 1: Visual behavior and avoidance behavioral response in EPM trials. (a) Measures of visual behavior that performed the light avoidance behavior
Cerebellum: little brain. Cerebellum. gross divisions
 Cerebellum The anatomy of the cerebellum and its gross divisions Its principal input and output pathways The organization of the cerebellar cortex Role of climbing vs. mossy fibre input The parallel-fibre/
Cerebellum The anatomy of the cerebellum and its gross divisions Its principal input and output pathways The organization of the cerebellar cortex Role of climbing vs. mossy fibre input The parallel-fibre/
SENSORY (ASCENDING) SPINAL TRACTS
 SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
CEREBRUM Dr. Jamila Elmedany Dr. Essam Eldin Salama
 CEREBRUM Dr. Jamila Elmedany Dr. Essam Eldin Salama Objectives At the end of the lecture, the student should be able to: List the parts of the cerebral hemisphere (cortex, medulla, basal nuclei, lateral
CEREBRUM Dr. Jamila Elmedany Dr. Essam Eldin Salama Objectives At the end of the lecture, the student should be able to: List the parts of the cerebral hemisphere (cortex, medulla, basal nuclei, lateral
Model 3-50B or 3-88 III VIII. Olfactory Nerve. Optic Nerve. Oculomotor Nerve. Trochlear Nerve. Trigeminal Nerve. Abducens Nerve.
 Model 3-50B or 3-88 I Olfactory Nerve II Optic Nerve Oculomotor Nerve III IV Trochlear Nerve Trigeminal Nerve V VI Abducens Nerve Glossopharyngeal Nerve IX VII Facial Nerve VIII Vestibocochlear Nerve or
Model 3-50B or 3-88 I Olfactory Nerve II Optic Nerve Oculomotor Nerve III IV Trochlear Nerve Trigeminal Nerve V VI Abducens Nerve Glossopharyngeal Nerve IX VII Facial Nerve VIII Vestibocochlear Nerve or
Nature Neuroscience doi: /nn Supplementary Figure 1. Characterization of viral injections.
 Supplementary Figure 1 Characterization of viral injections. (a) Dorsal view of a mouse brain (dashed white outline) after receiving a large, unilateral thalamic injection (~100 nl); demonstrating that
Supplementary Figure 1 Characterization of viral injections. (a) Dorsal view of a mouse brain (dashed white outline) after receiving a large, unilateral thalamic injection (~100 nl); demonstrating that
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Parts of the Brain. Hindbrain. Controls autonomic functions Breathing, Heartbeat, Blood pressure, Swallowing, Vomiting, etc. Upper part of hindbrain
 Parts of the Brain The human brain is made up of three main parts: 1) Hindbrain (or brainstem) Which is made up of: Myelencephalon Metencephalon 2) Midbrain Which is made up of: Mesencephalon 3) Forebrain
Parts of the Brain The human brain is made up of three main parts: 1) Hindbrain (or brainstem) Which is made up of: Myelencephalon Metencephalon 2) Midbrain Which is made up of: Mesencephalon 3) Forebrain
Histology of the CNS
 Histology of the CNS Lecture Objectives Describe the histology of the cerebral cortex layers. Describe the histological features of the cerebellum; layers and cells of cerebellar cortex. Describe the elements
Histology of the CNS Lecture Objectives Describe the histology of the cerebral cortex layers. Describe the histological features of the cerebellum; layers and cells of cerebellar cortex. Describe the elements
 Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Brainstem. By Dr. Bhushan R. Kavimandan
 Brainstem By Dr. Bhushan R. Kavimandan Development Ventricles in brainstem Mesencephalon cerebral aqueduct Metencephalon 4 th ventricle Mylencephalon 4 th ventricle Corpus callosum Posterior commissure
Brainstem By Dr. Bhushan R. Kavimandan Development Ventricles in brainstem Mesencephalon cerebral aqueduct Metencephalon 4 th ventricle Mylencephalon 4 th ventricle Corpus callosum Posterior commissure
Announcement. Danny to schedule a time if you are interested.
 Announcement If you need more experiments to participate in, contact Danny Sanchez (dsanchez@ucsd.edu) make sure to tell him that you are from LIGN171, so he will let me know about your credit (1 point).
Announcement If you need more experiments to participate in, contact Danny Sanchez (dsanchez@ucsd.edu) make sure to tell him that you are from LIGN171, so he will let me know about your credit (1 point).
The human brain weighs roughly 1.5 kg and has an average volume of 1130 cm 3. A sheep s brain weighs in however at kg.
 Sheep Brain Dissection Objectives: 1. List and describe the principal structures of the sheep brain 2. Identify important parts of the sheep brain in a preserved specimen Materials: Dissection tools, lab
Sheep Brain Dissection Objectives: 1. List and describe the principal structures of the sheep brain 2. Identify important parts of the sheep brain in a preserved specimen Materials: Dissection tools, lab
The Neuroscience of Music in Therapy
 Course Objectives The Neuroscience of Music in Therapy Unit I. Learn Basic Brain Information Unit II. Music in the Brain; Why Music Works Unit III. Considerations for Populations a. Rehabilitation b. Habilitation
Course Objectives The Neuroscience of Music in Therapy Unit I. Learn Basic Brain Information Unit II. Music in the Brain; Why Music Works Unit III. Considerations for Populations a. Rehabilitation b. Habilitation
For more information about how to cite these materials visit
 Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa3.0/
Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa3.0/
Chapter 14, Part 2! Chapter 14 Part 2 Brain/Cranial Nerves! The Cerebrum and Cranial Nerves! pp !
 Chapter 14, Part 2! The Cerebrum and Cranial pp. 482 505! SECTION 14-9! The cerebrum, the largest region of the brain, contains motor, sensory, and association areas! 2! White Matter of the Cerebrum! 1.
Chapter 14, Part 2! The Cerebrum and Cranial pp. 482 505! SECTION 14-9! The cerebrum, the largest region of the brain, contains motor, sensory, and association areas! 2! White Matter of the Cerebrum! 1.
3/20/13. :: Slide 1 :: :: Slide 39 :: How Is the Nervous System Organized? Central Nervous System Peripheral Nervous System and Endocrine System
 :: Slide 1 :: :: Slide 39 :: How Is the Nervous System Organized? Central Nervous System Peripheral Nervous System and Endocrine System The nervous system is organized into several major branches, each
:: Slide 1 :: :: Slide 39 :: How Is the Nervous System Organized? Central Nervous System Peripheral Nervous System and Endocrine System The nervous system is organized into several major branches, each
Organization of The Nervous System PROF. SAEED ABUEL MAKAREM
 Organization of The Nervous System PROF. SAEED ABUEL MAKAREM Objectives By the end of the lecture, you should be able to: List the parts of the nervous system. List the function of the nervous system.
Organization of The Nervous System PROF. SAEED ABUEL MAKAREM Objectives By the end of the lecture, you should be able to: List the parts of the nervous system. List the function of the nervous system.
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 14 The Brain and Cranial Nerves Introduction The purpose of the chapter is to: 1. Understand how the brain is organized, protected, and supplied
Principles of Anatomy and Physiology 14 th Edition CHAPTER 14 The Brain and Cranial Nerves Introduction The purpose of the chapter is to: 1. Understand how the brain is organized, protected, and supplied
Name: Period: Chapter 2 Reading Guide The Biology of Mind
 Name: Period: Chapter 2 Reading Guide The Biology of Mind The Nervous System (pp. 55-58) 1. What are nerves? 2. Complete the diagram below with definitions of each part of the nervous system. Nervous System
Name: Period: Chapter 2 Reading Guide The Biology of Mind The Nervous System (pp. 55-58) 1. What are nerves? 2. Complete the diagram below with definitions of each part of the nervous system. Nervous System
Central Nervous System. January 7, 2016
 Central Nervous System January 7, 2016 Anatomy of a neuron Cell Body (soma) Receives information from the soma s extensions (dendrites) Passes on information away from the soma towards extensions (axons)
Central Nervous System January 7, 2016 Anatomy of a neuron Cell Body (soma) Receives information from the soma s extensions (dendrites) Passes on information away from the soma towards extensions (axons)
Central Nervous System
 Central Nervous System January 7, 2016 Anatomy of a neuron Cell Body (soma) Receives information from the soma s extensions (dendrites) Passes on information away from the soma towards extensions (axons)
Central Nervous System January 7, 2016 Anatomy of a neuron Cell Body (soma) Receives information from the soma s extensions (dendrites) Passes on information away from the soma towards extensions (axons)
Internal Organisation of the Brainstem
 Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
Cerebellum 1/20/2016. Outcomes you need to be able to demonstrate. MHD Neuroanatomy Module
 This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the
This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the
Chapter 14, Part 2! The Cerebrum and Cranial Nerves! pp !
 Chapter 14, Part 2! The Cerebrum and Cranial pp. 482 505! SECTION 14-9! The cerebrum, the largest region of the brain, contains motor, sensory, and association areas! 2! 1! ! Chapter 14 Part 2 Brain/Cranial
Chapter 14, Part 2! The Cerebrum and Cranial pp. 482 505! SECTION 14-9! The cerebrum, the largest region of the brain, contains motor, sensory, and association areas! 2! 1! ! Chapter 14 Part 2 Brain/Cranial
Nervous System. Student Learning Objectives:
 Nervous System Student Learning Objectives: Identify the primary parts of the neuron Identify the major structures of the central nervous system Identify the major structures of the peripheral nervous
Nervous System Student Learning Objectives: Identify the primary parts of the neuron Identify the major structures of the central nervous system Identify the major structures of the peripheral nervous
BIOL Dissection of the Sheep and Human Brain
 BIOL 2401 Dissection of the Sheep and Human Brain Laboratory Objectives After completing this lab, you should be able to: Identify the main structures in the sheep brain and to compare them with those
BIOL 2401 Dissection of the Sheep and Human Brain Laboratory Objectives After completing this lab, you should be able to: Identify the main structures in the sheep brain and to compare them with those
Neocortex. Cortical Structures in the Brain. Neocortex Facts. Laminar Organization. Bark-like (cortical) structures: Shepherd (2004) Chapter 12
 Neocortex Shepherd (2004) Chapter 12 Rodney Douglas, Henry Markram, and Kevan Martin Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Cortical Structures in the Brain Bark-like (cortical) structures:
Neocortex Shepherd (2004) Chapter 12 Rodney Douglas, Henry Markram, and Kevan Martin Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Cortical Structures in the Brain Bark-like (cortical) structures:
CISC 3250 Systems Neuroscience
 CISC 3250 Systems Neuroscience Levels of organization Central Nervous System 1m 10 11 neurons Neural systems and neuroanatomy Systems 10cm Networks 1mm Neurons 100μm 10 8 neurons Professor Daniel Leeds
CISC 3250 Systems Neuroscience Levels of organization Central Nervous System 1m 10 11 neurons Neural systems and neuroanatomy Systems 10cm Networks 1mm Neurons 100μm 10 8 neurons Professor Daniel Leeds
CEREBRUM. Dr. Jamila EL Medany
 CEREBRUM Dr. Jamila EL Medany Objectives At the end of the lecture, the student should be able to: List the parts of the cerebral hemisphere (cortex, medulla, basal nuclei, lateral ventricle). Describe
CEREBRUM Dr. Jamila EL Medany Objectives At the end of the lecture, the student should be able to: List the parts of the cerebral hemisphere (cortex, medulla, basal nuclei, lateral ventricle). Describe
Pediatric MS MRI Study Methodology
 General Pediatric MS MRI Study Methodology SCAN PREPARATION axial T2-weighted scans and/or axial FLAIR scans were obtained for all subjects when available, both T2 and FLAIR scans were scored. In order
General Pediatric MS MRI Study Methodology SCAN PREPARATION axial T2-weighted scans and/or axial FLAIR scans were obtained for all subjects when available, both T2 and FLAIR scans were scored. In order
Dissection of the Sheep Brain
 Dissection of the Sheep Brain Laboratory Objectives After completing this lab, you should be able to: 1. Identify the main structures in the sheep brain and to compare them with those of the human brain.
Dissection of the Sheep Brain Laboratory Objectives After completing this lab, you should be able to: 1. Identify the main structures in the sheep brain and to compare them with those of the human brain.
Central Nervous System
 Anatomy of a neuron Cell Body (soma) Receives information from the soma s extensions (dendrites) Central Nervous System January 7, 2016 Passes on information away from the soma towards extensions (axons)
Anatomy of a neuron Cell Body (soma) Receives information from the soma s extensions (dendrites) Central Nervous System January 7, 2016 Passes on information away from the soma towards extensions (axons)
Motor Functions of Cerebral Cortex
 Motor Functions of Cerebral Cortex I: To list the functions of different cortical laminae II: To describe the four motor areas of the cerebral cortex. III: To discuss the functions and dysfunctions of
Motor Functions of Cerebral Cortex I: To list the functions of different cortical laminae II: To describe the four motor areas of the cerebral cortex. III: To discuss the functions and dysfunctions of
Biology 3201 Nervous System #2- Anatomy. Components of a Nervous System
 Biology 3201 Nervous System #2- Anatomy Components of a Nervous System In any nervous system, there are 4 main components: (1) sensors: gather information from the external environment (sense organs) (2)
Biology 3201 Nervous System #2- Anatomy Components of a Nervous System In any nervous system, there are 4 main components: (1) sensors: gather information from the external environment (sense organs) (2)
I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts.
 Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Cerebellum John T. Povlishock, Ph.D.
 Cerebellum John T. Povlishock, Ph.D. OBJECTIVES 1. To identify the major sources of afferent inputs to the cerebellum 2. To define the pre-cerebellar nuclei from which the mossy and climbing fiber systems
Cerebellum John T. Povlishock, Ph.D. OBJECTIVES 1. To identify the major sources of afferent inputs to the cerebellum 2. To define the pre-cerebellar nuclei from which the mossy and climbing fiber systems
Cerebellum: little brain. Cerebellum. gross divisions
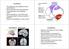 Cerebellum The anatomy of the cerebellum and its gross divisions Its principal input and output pathways The organization of the cerebellar cortex Role of climbing vs. mossy fibre input The parallel-fibre/
Cerebellum The anatomy of the cerebellum and its gross divisions Its principal input and output pathways The organization of the cerebellar cortex Role of climbing vs. mossy fibre input The parallel-fibre/
Department of Cognitive Science UCSD
 Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
The Nervous System PART B
 7 The Nervous System PART B PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reflex Arc Reflex
7 The Nervous System PART B PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB The Reflex Arc Reflex
Nervous System, Neuroanatomy, Neurotransmitters
 Nervous System, Neuroanatomy, Neurotransmitters Neurons Structure of neurons Soma Dendrites Spines Axon Myelin Nodes of Ranvier Neurons Structure of neurons Axon collaterals 1 Neurons Structure of neurons
Nervous System, Neuroanatomy, Neurotransmitters Neurons Structure of neurons Soma Dendrites Spines Axon Myelin Nodes of Ranvier Neurons Structure of neurons Axon collaterals 1 Neurons Structure of neurons
Brain dissection protocol for amyotrophic lateral sclerosis/motor neurone disease
 Brain dissection protocol for amyotrophic lateral sclerosis/motor neurone disease Prepared by Approved by Approved by Revised by Name Signature Date Sampling and biomarker OPtimization and Harmonization
Brain dissection protocol for amyotrophic lateral sclerosis/motor neurone disease Prepared by Approved by Approved by Revised by Name Signature Date Sampling and biomarker OPtimization and Harmonization
10/3/2016. T1 Anatomical structures are clearly identified, white matter (which has a high fat content) appears bright.
 H2O -2 atoms of Hydrogen, 1 of Oxygen Hydrogen just has one single proton and orbited by one single electron Proton has a magnetic moment similar to the earths magnetic pole Also similar to earth in that
H2O -2 atoms of Hydrogen, 1 of Oxygen Hydrogen just has one single proton and orbited by one single electron Proton has a magnetic moment similar to the earths magnetic pole Also similar to earth in that
Organization of The Nervous System PROF. MOUSAED ALFAYEZ & DR. SANAA ALSHAARAWY
 Organization of The Nervous System PROF. MOUSAED ALFAYEZ & DR. SANAA ALSHAARAWY Objectives At the end of the lecture, the students should be able to: List the parts of the nervous system. List the function
Organization of The Nervous System PROF. MOUSAED ALFAYEZ & DR. SANAA ALSHAARAWY Objectives At the end of the lecture, the students should be able to: List the parts of the nervous system. List the function
ANATOMY & PHYSIOLOGY DISSECTION OF THE SHEEP BRAIN LAB GROUP:
 ANATOMY & PHYSIOLOGY DISSECTION OF THE SHEEP BRAIN LAB GROUP: Introduction The purpose of the sheep brain dissection is to familiarize you with the three dimensional structure of the brain and teach you
ANATOMY & PHYSIOLOGY DISSECTION OF THE SHEEP BRAIN LAB GROUP: Introduction The purpose of the sheep brain dissection is to familiarize you with the three dimensional structure of the brain and teach you
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
TOXIC AND NUTRITIONAL DISORDER MODULE
 TOXIC AND NUTRITIONAL DISORDER MODULE Objectives: For each of the following entities the student should be able to: 1. Describe the etiology/pathogenesis and/or pathophysiology, gross and microscopic morphology
TOXIC AND NUTRITIONAL DISORDER MODULE Objectives: For each of the following entities the student should be able to: 1. Describe the etiology/pathogenesis and/or pathophysiology, gross and microscopic morphology
Organization of the nervous system 2
 Organization of the nervous system 2 Raghav Rajan Bio 334 Neurobiology I August 22nd 2013 1 Orienting within the brain absolute axes and relative axes SUPERIOR (above) ANTERIOR (in front) Anterior/Posterior,
Organization of the nervous system 2 Raghav Rajan Bio 334 Neurobiology I August 22nd 2013 1 Orienting within the brain absolute axes and relative axes SUPERIOR (above) ANTERIOR (in front) Anterior/Posterior,
b. The groove between the two crests is called 2. The neural folds move toward each other & the fuse to create a
 Chapter 13: Brain and Cranial Nerves I. Development of the CNS A. The CNS begins as a flat plate called the B. The process proceeds as: 1. The lateral sides of the become elevated as waves called a. The
Chapter 13: Brain and Cranial Nerves I. Development of the CNS A. The CNS begins as a flat plate called the B. The process proceeds as: 1. The lateral sides of the become elevated as waves called a. The
