Mechanism of PHERES1 imprinting in Arabidopsis
|
|
|
- Edgar Hodge
- 5 years ago
- Views:
Transcription
1 906 Research Article Mechanism of PHERES1 imprinting in Arabidopsis Grigory Makarevich, Corina B. R. Villar, Aleksandra Erilova and Claudia Köhler* Institute of Plant Sciences and Zurich-Basel Plant Science Center, Swiss Federal Institute of Technology, ETH Centre, CH-8092 Zurich, Switzerland *Author for correspondence ( Accepted 8 January , Published by The Company of Biologists 2008 doi: /jcs Summary Genomic imprinting is a phenomenon where only one of the two alleles of a gene is expressed either the maternally or the paternally inherited allele. Imprinting of the plant gene PHERES1 requires the function of the FERTILIZATION INDEPENDENT SEED (FIS) Polycomb group (PcG) complex for repression of the maternal PHERES1 allele. In this study we investigated the mechanism of PHERES1 imprinting and found that PcG silencing is necessary but not sufficient for imprinting establishment of PHERES1. We provide evidence that silencing of the maternal PHERES1 allele depends on a distantly located region downstream of the PHERES1 locus. This region needs to be methylated to ensure PHERES1 Introduction A subset of genes in mammals and flowering plants is only expressed from one of the two homologous chromosomes, depending on whether they are maternally or paternally inherited. This process is called genomic imprinting and often affects genes with essential functions for normal development (Feil and Berger, 2007). In mammals, imprinted genes regulate placental development and fetal growth, and several human diseases are linked to mutations in imprinted genes (Reik, 2007). Mechanisms to distinguish maternal and paternal alleles have been extensively investigated in mammals. Most mammalian imprinted genes are clustered in the genome and are regulated by differentially methylated imprintingcontrol regions (ICRs) (Edwards and Ferguson-Smith, 2007). Different DNA methylation marks are applied during germ-line formation by de novo methyltransferases and are maintained in somatic tissues by maintenance methyltransferases. ICRs are also marked by different histone modifications and can either act as insulators preventing promoter-enhancer interactions or give rise to the formation of non-coding RNAs that attract chromatinmodifying complexes (Delaval and Feil, 2004). Some imprinted genes are regulated by Polycomb group (PcG) complexes that methylate histones and establish repressive chromatin domains (Delaval and Feil, 2004). In flowering plants, imprinting has only been detected in the endosperm, a terminal tissue that develops after fertilization of the central cell. In dicot species like Arabidopsis, the endosperm nourishes the embryo during its growth phase and is almost completely consumed during embryo development (Berger, 2003). Thus far, only four imprinted genes have been identified in Arabidopsis. Three of them [MEDEA (MEA), FWA and FERTILIZATION INDEPENDENT SEED 2 (FIS2)] are maternally expressed and paternally silenced (Vielle-Calzada et al., 1999; Kinoshita et al., 2004; Jullien et al., 2006b). The same paternally imprinted expression pattern applies to all imprinted genes that have expression but must not be methylated for PHERES1 repression. This mechanism is analogous to the regulation of several imprinted genes in mammals, suggesting the employment of similar evolutionary mechanisms for the regulation of imprinted genes in mammals and flowering plants. Supplementary material available online at Key words: Arabidopsis, Epigenetics, FERTILIZATION INDEPENDENT SEED genes, DNA methylation, Polycomb group proteins been identified in maize (Scott and Spielman, 2006). Paternal imprinting of MEA requires activity of the evolutionary conserved FIS-PcG complex, with MEA itself being a subunit of this complex. The FIS complex mediates trimethylation of histone H3 at the lysine residue at position 27 (H3K27me3) of the paternal MEA allele causing its repression (Baroux et al., 2006; Gehring et al., 2006; Jullien et al., 2006a). Activation of the maternal MEA allele in the female gametophyte requires the 5-methylcytosine excising activity of the DNA glycosylase DEMETER (DME). DME acts antagonistically to the maintenance methyltransferase MET1 that methylates MEA in the promoter and 3 regions of the gene (Xiao et al., 2003). DME activity is also necessary to activate expression of the paternally imprinted genes FWA and FIS2. Because MEA, FWA and FIS2 are only demethylated in the terminally differentiating endosperm, they remain heritably methylated throughout the life cycle of the plant (Xiao et al., 2003; Kinoshita et al., 2004; Jullien et al., 2006b). The promoter region of FWA and the 3 region of MEA contain tandem-repeat sequences that recruit de novo DNA methylation by the RNA-dependent DNA silencing pathway (Chan et al., 2006b). Whereas FWA tandem repeats are necessary to establish FWA silencing (Chan et al., 2006b), silencing of the paternal MEA allele is likely to be independent of the repeat sequences (Spillane et al., 2004). The type I MADS-box gene PHERES1 (PHE1) is the only plant gene known to be paternally expressed and maternally silenced. Maternal PHE1 silencing is caused by the repressing activity of the FIS complex (Köhler et al., 2005). The FIS complex is active in the female gametophyte and in the endosperm, prevents precocious PHE1 expression before fertilization and restricts PHE1 expression to the chalazal domain of the endosperm after fertilization. The FIS complex is directly associated with the PHE1 locus and FIS repressive activity is correlated with H3K27me3 modification at PHE1 (Köhler et al., 2003; Makarevich et al., 2006). Upregulation
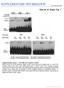
2 Imprinting of the PHERES1 locus 907 of PHE1 in mea mutants is in part responsible for the mea mutant phenotype that can be alleviated by reducing PHE1 expression (Köhler et al., 2003). In this study, we asked whether FIS-mediated repression is sufficient for PHE1 imprinting or whether additional mechanisms are involved to repress the maternal PHE1 allele. Our data clearly show that imprinting is not a direct consequence of FIS-mediated repression but necessitates the presence of additional elements. We identified elements within the PHE1 3 region that are necessary for PHE1 imprinting and predict a model that explains how the FIS complex together with the identified region confers stable silencing of the maternal PHE1 allele. Results The maternal PHE1 allele is not reactivated in mutants that are defective in DNA methylation We asked the question whether the FIS complex is sufficient to suppress the maternal PHE1 allele, or whether additional mechanisms cooperate with the FIS complex to silence the maternally derived PHE1 allele. Parental allele-specific DNA methylation has been found at most imprinted mammalian gene clusters and imprinted plant genes that have been examined (Köhler and Makarevich, 2006; Edwards and Ferguson-Smith, 2007). Therefore, we tested whether imprinting of PHE1 is also regulated by DNA methylation. Mutations in the maintenance MEHYLTRANSFERASE 1 (MET1) gene cause a drastic reduction of symmetric CG methylation (Kankel et al., 2003), whereas mutations in the CHROMOMETHYLASE 3 (CMT3) gene and the de novo methyltransferases DRM1 and DRM2 affect CNG and asymmetric methylation, respectively (Cao and Jacobsen, 2002). We pollinated met1, cmt3 and drm1/drm2 double mutants with pollen of C24 wildtype plants and analyzed allele-specific expression of PHE1. As shown in Fig. 1A and supplementary material Fig. S1A and Fig. S3, in none of the mutants a reactivation of the maternal PHE1 allele was detectable and the paternal PHE1 allele remained the predominantly expressed allele. In conclusion, DNA methylation is not responsible for repression of the maternal PHE1 allele. Lack of DNA methylation causes reduced expression of the paternal PHE1 allele In mammals, DNA demethylation is responsible for silencing of a large number of imprinted protein-coding genes (Sleutels and Barlow, 2002). Previously we observed strongly reduced PHE1 expression levels in the decrease in DNA methylation 1 (ddm1) mutant, which is impaired in the maintenance of DNA methylation (Köhler et al., 2003). This prompted us to test whether DNA methylation is required for expression of the paternal PHE1 allele. We did not detect substantial PHE1 expression in pollen (data not shown); however, fertilization of wild-type plants with hypomethylated pollen of met1 mutants caused much lower PHE1 transcript levels in developing seeds than fertilization with wildtype pollen (Fig. 2A), indicating reduced activity of the paternal PHE1 allele. This suggests that DNA methylation is important to maintain high levels of paternal PHE1 expression, but is not necessary to repress the maternal PHE1 allele. When we tested allele-specific PHE1 expression in seeds developing after pollination with met1 pollen, we clearly detected reduced expression of the paternal PHE1 allele (Fig. 2B), which supports our conclusion that DNA methylation is required for paternal PHE1 expression. However, we also detected expression of the maternal PHE1 allele in seeds that inherited a hypomethylated paternal PHE1 allele (Fig. Fig. 1. The maternal PHE1 allele is not reactivated in DNA-methylationdefective mutants. Allelic expression analysis of PHE1 in wild-type and met1, cmt3 and drm1/drm2 mutant plants after crosses of wild-type and mutant plants with the C24 accession. Analysis was performed before fertilization (0 DAP) and at indicated days after pollination (DAP). g, genomic DNA. 2B and supplementary material Fig. S1B), suggesting that relieve of repression of the maternal PHE1 allele depends on additional not-yet-known factors. Because DNA methylation appears to be important for PHE1 regulation, we asked the question which regions Fig. 2. DNA methylation is important for paternal PHE1 expression. (A) Relative PHE1 mrna levels in wild-type flowers before fertilization (0 DAP) and siliques after pollination with wild-type or met-mutant pollen harvested at different days after pollination (DAP). Because the maternal PHE1 allele is silent, the detected PHE1 expression represents mostly the activity of the paternal PHE1 allele. (B) Allelic expression analysis of PHE1 after pollination of C24 plants with wild-type or met1-mutant pollen. Analysis was performed at DAP1 to DAP4.
3 (6) Fig. 3. The 3 region of PHE1 contains DNA-methylation marks. (A, left) Southern blot of DNA from wild-type and met1-mutant plants after digestion with the methylation-sensitive restriction enzyme ClaI. (Right) Overview of the PHE1 locus with large and small arrows corresponding to the PHE1 coding region and repeats, respectively. The expected fragment sizes and the region covered by the probe are indicated. The methylated ClaI site is indicated by a filled circle, the repeat regions by arrows. (B) Cytosine methylation profile of the PHE1 downstream region in different tissues analyzed by bisulfite sequencing. Cytosine positions relative to the translational stop codon and sequence contexts are indicated on the x-axis. The ClaI site corresponds to position The methylation status in the met1 mutant was analyzed in flowers. of the PHE1 locus contain DNA methylation. Using bisulfite sequencing we did not detect any DNA methylation within the promoter and coding regions of PHE1. Therefore, we extended our analysis to regions downstream of the PHE1 locus. We performed Southern blot analysis using the restriction enzyme ClaI, which is blocked by symmetric methylation at its target recognition site. Using this assay we detected a methylated site 2538 bps downstream of the PHE1 stop codon (Fig. 3A). No annotated genes are located in this region. Using Southern blot analysis and bisulfite sequencing we detected no substantial methylation at this site in the met1 mutant (Fig. 3A,B). Thus, reduced methylation at this site in met1 correlates with reduced PHE1 expression. Interestingly, this methylated ClaI site is located in close proximity to a tandem triple repeat, each repeat being 54 bps long. Bisulfite sequencing of the region surrounding the ClaI site revealed twelve consecutive methylated cysteine residues in a symmetric CG context and two methylated CNG sites (supplementary material Fig. S3). We performed tissuespecific analysis of DNA methylation in pollen, flowers and leaves. Consistent with our findings that in met1 mutants the paternal allele is less expressed, we detected high levels of DNA methylation in pollen at all sites tested (Fig. 3B). However, we also detected substantial DNA methylation in sporophytic tissues where only weak PHE1 expression is detectable, suggesting that DNA methylation alone is not sufficient to confer active PHE1 expression. Disruption of the PHE1 3 regions causes activation of the maternal PHE1 allele Our results suggest that DNA methylation in the identified PHE1 3 region is important for PHE1 imprinting. In order to test this hypothesis we addressed the question whether disruption of the PHE1 3 region disrupts PHE1 imprinting. We identified a T-DNA mutant containing a 4 kb T-DNA insertion 441 bps downstream of the PHE1 stop codon (referred to as phe1-3) (Fig. 4A). Using this line, we could test whether an insertion within the 3 region disrupts PHE1 imprinting. We tested allele-specific expression of PHE1 in phe1-3 by performing reciprocal crosses of phe1-3 with C24 plants. Whereas expression of the paternal PHE1 allele was not affected in phe1-3 (Fig. 4B, upper panel and supplementary material Fig. S1C), we observed a drastic effect on the expression of the maternal allele when phe1-3 was used as the maternal parent (Fig. 4B lower panel and supplementary material Fig. S1C). In phe1-3 mutants, PHE1 was not maternally silenced but, in contrast to wild-type, which has only a weak expression of the maternal PHE1 allele, this allele was strongly expressed (Fig. 4B, lower panel). Surprisingly, we detected a decrease in the expression of the paternal PHE1 allele when phe1-3 was used as the maternal parent (cross phe1-3 C24; Fig. 4B and supplementary material Fig. S1C). One possible explanation for this phenomenon might be the triploid nature of the endosperm consisting of two maternal versus one paternal genome copies. Therefore, if the two maternal PHE1 alleles become reactivated, they might outcompete expression of the single paternal PHE1 allele. We also considered the possibility that strong expression of the maternally derived PHE1 allele in phe1-3 is caused by de-repression of PHE1 in maternal sporophytic tissues of phe1-3 plants. Therefore, we tested expression of PHE1 in phe1-3 leaves. However, as shown in Fig. 4C, PHE1 remains as weakly expressed in phe1-3 leaves as in wild-type leaves. We further considered the possibility that transcripts derived from the T-DNA can influence PHE1 expression. To test this possibility, we performed northern blot analysis using probes flanking the insertion site. However, we did not obtain any expression signal with either of the probes, but detected a clear expression signal for the NEOMYCIN PHOSPHOTRANSFERASE II (NPTII) selection gene that is located within the T-DNA (Fig. 4D). Finally, we tested PHE1 imprinting in two additional transgenic lines containing a 4 kb T-DNA or a 6.6 kb Ds transposon insertion 1022 bps or 1168 bps after the PHE1 stop codon, respectively. The T-DNA in the insertion line (referred to as phe1-4) is inserted in the antisense orientation compared with the T-DNA of the phe1-3 allele, whereas insertion of the Ds element (referred to as phe1-5) is in the same orientation as phe1-3 (Fig. 4A). Consistent with the results obtained for the phe1-3 allele, we observed reactivation of the maternal PHE1 allele and a reduction of paternal PHE1 expression (Fig. 4E and supplementary material Fig. S1C), whereas we did not observe an effect on expression of the paternal PHE1 allele in those mutants (data not shown). Taken together, these results clearly demonstrate that the 3 region of PHE1 contains elements necessary for repression of the maternal PHE1 allele. The PHE1 downstream region is necessary for imprinting To obtain final proof that the identified region is necessary for PHE1 imprinting, we designed a construct containing 3 kb of the PHE1 promoter sequence, the PHE1 coding region fused to a GUS reporter gene and a 3.5 kb sequence downstream of the PHE1 stop codon (referred to as PHE ::GUS_3 ). We established transgenic plants containing this construct and tested imprinting of this
4 Imprinting of the PHERES1 locus 909 Fig. 4. Establishment of imprinting at the PHE1 locus is compromised in mutants whose PHE1 downstream region is disrupted. (A) Schematic overview of the location of the phe1-3-, phe1-4- and phe1-5- mutant alleles. Probes used for northern blot analysis are indicated. (B) Allelic expression analysis of PHE1 after reciprocal crosses of wild-type and phe1-3 mutant plants with the C24 accession. (C) Expression analysis of PHE1 in leaves of wild-type and phe1-3-mutant plants. (D) Northern blot analysis of RNA from leaves of wild-type and phe1-3-mutant plants with probes indicated in panel (A). (E) Allelic expression analysis of PHE1 in wild-type and phe1-4- and phe1-4-mutant plants after crosses of wild-type and mutant plants with the C24 accession. Lb, left border; Rb, right border; g, genomic DNA; ACT, ACTIN; wt, wild-type. construct by monitoring allele-specific GUS expression. As imprinting of the PHE1 transgene depends on de novo DNA methylation of the transgenic PHE1 3 region, we tested whether this region indeed becomes methylated when transformed into wildtype plants. Using bisulfite sequencing we analyzed a region that allowed us to distinguish between the transgene sequence and the endogenous PHE1 locus. As shown in Fig. 5, in this region we detected CG methylation at a level similar to that in wild type, as well as additional CNG methylation. Thus, the transgenic PHE1 3 region becomes methylated de novo when transformed into wildtype plants. We tested imprinting of this transgene by performing reciprocal crosses of three independent transgenic lines with wildtype plants. Indeed, as shown in Fig. 6A, PHE ::GUS_3 is exclusively expressed when inherited from the paternal parent; we did not detect expression when PHE ::GUS_3 became inherited from the maternal parent. By contrast, when we tested expression of the PHE ::GUS lacking the 3 PHE1 region, we always detected maternally derived PHE1::GUS expression (Köhler et al., 2005) (Fig. 6B). Our results suggest that DNA methylation of the paternal PHE1 allele is necessary for paternal PHE1 expression (Fig. 2). To substantiate these findings we transformed the PHE ::GUS_3 construct into a drm1/drm2 mutant background. DRM1 and DRM2 are necessary for de novo cytosine methylation in all known sequence contexts and are guided to their templates by small interfering (si) RNAs (Chan et al., 2004). It has previously been demonstrated that transgene sequences when transformed into a drm1/drm2 mutant background remain unmethylated owing to lack of de novo methyltransferase activity (Chan et al., 2004). Indeed, we did not detect significant levels of DNA methylation of the transgene in the drm1/drm2 mutant background (Fig. 5). Using this transformation assay allowed to directly address the question whether DNA methylation of the PHE ::GUS_3 transgene affects PHE1 expression and avoids secondary effects caused by global DNA demethylation. We tested allele-specific PHE1 expression by crossing wild-type plants with pollen from drm1/drm2; PHE ::GUS_3 transgenic lines. In none of the three tested independent transgenic lines did we detect expression of the paternally derived PHE ::GUS_3 construct (Fig. 6C). Thus, DNA methylation of the 3 region of the paternal PHE1 allele is necessary for PHE1 expression. Taken together, our data clearly demonstrate that the PHE1 downstream region contains important sequence elements necessary for imprinting of PHE1. Fig. 5. Cytosine methylation profile of the PHE ::GUS_3 transgene in wild-type and drm1/drm2-mutant background compared with the methylation profile of the endogenous PHE1 locus analyzed by bisulfite sequencing. Cytosine positions relative to the translational stop codon and sequence contexts (CG and CNG, CNN not indicated) are indicated on the x-axis.
5 (6) Fig. 6. The PHE ::GUS_3 transgene is maternally imprinted. (A,B) Expression analysis of a maternally or paternally derived (A) PHE ::GUS_3 or (B) PHE ::GUS transgene in seeds at DAP3. (C) Expression analysis of a paternally derived PHE ::GUS_3 transgene in a wild-type or drm1/drm2 mutant background in seeds at DAP3. Quantification of results are shown in the respective panels on the right. Scale bars, 50 μm. Discussion DNA methylation is necessary for PHE1 expression Our data demonstrate that FIS binding to the PHE1 promoter region and DNA demethylation of the 3 region of PHE1 are both necessary and sufficient for stable PHE1 imprinting. DNA methylation in intergenic regions is often associated with transposons and repeat sequences as well as noncoding RNAs (Zhang et al., 2006). De novo methylation of repeats depends on the de novo methyltransferase DRM2 that is guided by sirnas using the RNA-directed DNA methylation pathway (Chan et al., 2006b). Whereas DRM2-mediated de novo methylation occurs in all sequence contexts, DRM2-mediated maintenance methylation is restricted to CNG and asymmetric cytosine residues (Cao and Jacobsen, 2002). The identified direct repeats in the PHE1 3 region are preferentially methylated on symmetric CG residues, indicating that methylation of PHE1 repeats depends on MET1 maintenance methyltransferase activity. Thus, methylation of PHE1 repeats is likely to occur through similar mechanisms as methylation of repeats in the promoter of the imprinted FWA gene. Methylation of endogenous FWA repeats depends on MET1 activity, whereas methylation of transgenic FWA repeats depends on DRM2 activity (Kinoshita et al., 2004; Chan et al., 2006b). However, in contrast to the role of DNA methylation for silencing of the paternal FWA allele, we found DNA methylation being necessary for expression of the paternal PHE1 allele. This conclusion is supported by two findings: (1) endogenous paternal PHE1 expression is reduced in a met1 mutant and, (2) PHE ::GUS_3 is not paternally expressed when transformed into the de novo methyltransferase mutant drm1/drm2 that is deficient in de novo methylation of repeated transgene sequences during plant transformation (Chan et al., 2004; Chan et al., 2006b). As PHE1-repeat sequences are also methylated in tissues where PHE1 is not substantially expressed, we conclude that DNA methylation is necessary but not sufficient to determine PHE1 activity. It is possible that additional activating signals present only during seed development are necessary for PHE1 expression. Surprisingly, we detected expression of the maternal PHE1 alleles in seeds inheriting a hypomethylated paternal PHE1 allele. One possible explanation for this finding could involve recruitment of the FIS complex to the demethylated paternal PHE1 allele. If the number of FIS complexes is limited, additional FIS target genes could cause a reactivation of silenced maternal PHE1 alleles.
6 Imprinting of the PHERES1 locus 911 Is there a difference in DNA methylation between maternal and paternal alleles? We failed to solve this question for technical reasons, as it requires DNA isolation of either female gametophytes or pure endosperm tissues. However, given the presence of active DNA-demethylating enzymes in Arabidopsis, with DME having an assigned function of demethylation in the central cell, it is possible that PHE1 becomes demethylated in the central cell of the female gametophyte. DME is necessary for MEA and FIS2 expression, and lack of DME function causes seeds to abort with a fis-like phenotype. As MEA is necessary for repression of the maternal PHE1 allele, a direct function of DME for PHE1 regulation cannot be unequivocally addressed. Fig. 7. Model for the establishment of PHE1 imprinting. In the central cell of the female gametophyte the FIS-PcG complex binds to a polycomb response element (PRE) in the promoter region of PHE1. The differentially methylated region (DMR) is unmethylated in the central cell of the female gametophyte and blocks transcription together with the FIS complex, resulting in stable PHE1 repression in the endosperm. The FIS complex is absent in pollen und the methylated DMR prevents silencing activity, causing the paternal allele to be active in the endosperm. In met1-mutant pollen, the DMR is demethylated and can block PHE1 expression. Whether this involves PcG complexes is currently not known. Maternal alleles are shown in red, paternal alleles in blue. Model for PHE1 imprinting establishment We suggest a model for PHE1 imprinting establishment in male and female gametes (Fig. 7). We propose that the 3 region of PHE1 is differentially methylated, with the maternal allele being specifically demethylated in the central cell of the female gametophyte. If unmethylated, this region together with the FIS complex can block transcription in the endosperm either by forming a repressive chromatin loop or by facilitating the long range action of silencer regions. By contrast, in pollen, this region is methylated and either inhibits the formation of repressive chromatin loops or blocks the action of silencer elements by adopting insulator function. Both models are currently being tested. In met1-mutant pollen, this region is unmethylated and blocks transcription. Whether this involves the action of PcG complexes, is currently unknown. However, based on the finding that the maternal PHE1 alleles become reactivated in seeds inheriting a hypomethylated paternal PHE1 allele (Fig. 2B), we currently favor this hypothesis. After fertilization, the maternal PHE1 alleles remain unmethylated in the endosperm and targeted by the FIS complex, whereas the methylated paternal PHE1 allele is not targeted by FIS and active. This model implies that there is no reset of the PHE1 DNA methylation imprint, because demethylation is restricted to the terminally differentiated endosperm. This suggests that maternal imprinting of PHE1 relies on similar molecular mechanisms as paternal imprinting of MEA, FWA and FIS2 (Xiao et al., 2003; Kinoshita et al., 2004; Jullien et al., 2006b). Our model provides an explanation for the seemingly contradictory findings that demethylation of the paternal PHE1 allele causes paternal PHE1 repression, whereas insertions of transgene sequences do not impair PHE1 expression. We propose that the demethylated paternal PHE1 allele becomes repressed, either by formation of repressive chromatin loops or the action of silencing elements. By contrast, insertions of long transgene sequences in the 3 PHE1 region will negatively interfere with loop formation or inhibit the long range action of silencing elements, resulting in active PHE1 expression. We failed to detect antisense transcripts generated within the 3 PHE1 region in wild-type and met1-mutant plants (data not shown), suggesting that long antisense RNAs are not involved in PHE1 imprinting. Similar to the situation for PHE1, several imprinted genes in mammals depend on DNA demethylation for silencing, whereas DNA methylation is necessary for expression (Sleutels and Barlow, 2002). Similar to the model proposed by Sleutels and Barlow (Sleutels and Barlow, 2002), we assume that imprinting of PHE1 evolved in two steps. First, PHE1 expression became silenced by insertion of a repetitive sequence into a regulatory region located in the 3 region of the gene. In a second step this repetitive sequence became methylated, thereby causing restoration of PHE1 expression. Owing to a female-gamete-specific demethylation activity, the maternal PHE1 allele is silenced, whereas the paternal allele is methylated and active. This model makes two predictions, (1) removal of the insertion mutation that causes the silencing effect should reactivate the silenced allele and, (2) loss of DNA methylation should result in silencing. Indeed, both predictions are supported by the results presented in this study. We could demonstrate that removal of the PHE1 3 region causes derepression of the maternal PHE1 allele and loss of DNA methylation causes silencing of the paternal PHE1 allele. The effect of DNA methylation to suppress the silencing effect of transposons has long been appreciated in plants (Martienssen, 1998) and was probably used as a mechanism to achieve active PHE1 expression in male gametes.
7 (6) Materials and Methods Plant material and growth conditions The mea mutant used in this study is the mea-1 allele (Ler accession) described by Grossniklaus et al. (Grossniklaus et al., 1998). The met1 mutant used in this study corresponds to the met1-3 allele (Col accession) described by Saze et al. (Saze et al., 2003). For all experiments with met1 mutants, only first-generation homozygous plants were used. The drm1/drm2 double mutant (Wassilewskija accession) was obtained from the Nottingham Arabidopsis Stock Centre (CS6366). The cmt3-11 allele corresponds to SALK_ (Col accession) (Chan et al., 2006a). The mutant alleles phe1-3, phe1-4 and phe1-5 correspond to SALK_ (Col accession), SALK_ (Col accession) and GT_5_ (Ler accession), respectively. The PHE ::GUS line (Ler accession) have been described by Köhler et al. (Köhler et al., 2003). Plants were grown in a greenhouse at 70 % humidity and daily cycles of 16 hours light at 21 C and 8 hours darkness at 18 C. Developed gynoecia were emasculated and hand pollinated one day after emasculation. For RNA expression analysis, three gynoecia or siliques were harvested at the indicated time points. Plasmid constructs and generation of transgenic plants To generate the PHE ::GUS_3 construct, a 3000 bp sequence upstream of the PHE1 translational start and the PHE1-coding region were amplified by PCR, introducing EcoRI and XmaI restriction sites. The fragment was ligated into pcambia1381xc, creating an in-frame fusion with the GUS gene. Using EcoRI and BstEII sites, a fragment containing the PHE1 promoter, PHE1 coding region and the GUS gene was removed and introduced together with a fragment containing 3540 bps of PHE1 3 region flanked by BstEII and PstI sites into pcambia The PHE ::GUS_3 construct was introduced into homozygous drm1/drm2 plants and the corresponding Wassilewskija accession. RNA extraction and qpcr analysis RNA extraction and generation of cdnas were performed as described previously (Köhler et al., 2005). Quantitative PCR was done on an ABI Prism 7700 Sequence Detection System (Applied Biosystems) using SYBR Green PCR master mix (Applied Biosystems) according to the supplier s recommendations. The mean value of three replicates was normalized using ACTIN11 as control. The primers used in this study are specified in the supplementary material (Table S1). Allele-specific expression analysis Allele-specific PHE1 expression was analyzed using the assay described by (Köhler et al., 2005). The amplified products were digested with HphI and analyzed on a 2.5% agarose gel. All primers are specified in the supplementary material (Table S1). GUS expression analysis Staining of seeds to detect GUS activity was done as described previously (Köhler et al., 2003). Northern and Southern blot analysis Northern and Southern blot analyses were performed as described previously (Köhler et al., 2003). Primers used to generate probes are specified in supplementary material Table S1. Bisulfite sequencing Bisulfite sequencing was performed following the protocol of (Jacobsen et al., 2000). Used primers are specified in supplementary material Table S1. We sequenced six clones covering the regions of bp (primers Fwd1 and Rev1) and bp (primers Fwd2 and Rev2) and ten clones of the region bp (primers Fwd3 and Rev3). We sequenced 12 clones covering the region 2099 bp-2470 bp (primers Fwd2 and Rev2) of transgenic lines containing the PHE ::GUS_3 construct in wild-type and drm1/drm2-mutant background. We thank O. Mittelsten Scheid and L. Hennig for helpful comments on the manuscript. We thank J. Paszkowski for providing the met1-3 allele. We are grateful to U. Grossniklaus for sharing greenhouse facilities and W. Gruissem for sharing laboratory facilities. This research was supported by the Swiss National Science Foundation to C.K. (PP00A /1), and (3100A ). C.K. is supported by an EMBO Young Investigator Award. References Baroux, C., Gagliardini, V., Page, D. R. and Grossniklaus, U. (2006). Dynamic regulatory interactions of Polycomb group genes, MEDEA autoregulation is required for imprinted gene expression in Arabidopsis. Genes Dev. 20, Berger, F. (2003). Endosperm, the crossroad of seed development. Curr. Opin. Plant Biol. 6, Cao, X. and Jacobsen, S. E. (2002). Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proc. Natl. Acad. Sci. USA 99 Suppl. 4, Chan, S. W., Zilberman, D., Xie, Z., Johansen, L. K., Carrington, J. C. and Jacobsen, S. E. (2004). RNA silencing genes control de novo DNA methylation. Science 303, Chan, S. W., Henderson, I. R., Zhang, X., Shah, G., Chien, J. S. and Jacobsen, S. E. (2006a). RNAi, DRD1, and histone methylation actively target developmentally important non-cg DNA methylation in Arabidopsis. PLoS Genet. 2, e83. Chan, S. W., Zhang, X., Bernatavichute, Y. V. and Jacobsen, S. E. (2006b). Twostep recruitment of RNA-directed DNA methylation to tandem repeats. PLoS Biol. 4, e363. Delaval, K. and Feil, R. (2004). Epigenetic regulation of mammalian genomic imprinting. Curr. Opin. Genet. Dev. 14, Edwards, C. A. and Ferguson-Smith, A. C. (2007). Mechanisms regulating imprinted genes in clusters. Curr. Opin. Cell Biol. 19, Feil, R. and Berger, F. (2007). Convergent evolution of genomic imprinting in plants and mammals. Trends Genet. 23, Gehring, M., Huh, J. H., Hsieh, T. F., Penterman, J., Choi, Y., Harada, J. J., Goldberg, R. B. and Fischer, R. L. (2006). DEMETER DNA glycosylase establishes MEDEA Polycomb gene self-imprinting by allele-specific demethylation. Cell 124, Grossniklaus, U., Vielle-Calzada, J. P., Hoeppner, M. A. and Gagliano, W. B. (1998). Maternal control of embryogenesis by MEDEA a Polycomb group gene in Arabidopsis. Science 280, Jacobsen, S. E., Sakai, H., Finnegan, E. J., Cao, X. and Meyerowitz, E. M. (2000). Ectopic hypermethylation of flower-specific genes in Arabidopsis. Curr. Biol. 10, Jullien, P. E., Katz, A., Oliva, M., Ohad, N. and Berger, F. (2006a). Polycomb group complexes self-regulate imprinting of the Polycomb group gene MEDEA in Arabidopsis. Curr. Biol. 16, Jullien, P. E., Kinoshita, T., Ohad, N. and Berger, F. (2006b). Maintenance of DNA methylation during the Arabidopsis life cycle is essential for parental imprinting. Plant Cell 18, Kankel, M. W., Ramsey, D. E., Stokes, T. L., Flowers, S. K., Haag, J. R., Jeddeloh, J. A., Riddle, N. C., Verbsky, M. L. and Richards, E. J. (2003). Arabidopsis MET1 cytosine methyltransferase mutants. Genetics 163, Kinoshita, T., Miura, A., Choi, Y., Kinoshita, Y., Cao, X., Jacobsen, S. E., Fischer, R. L. and Kakutani, T. (2004). One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science 303, Köhler, C. and Makarevich, G. (2006). Epigenetic mechanisms governing seed development in plants. EMBO Rep. 7, Köhler, C., Hennig, L., Spillane, C., Pien, S., Gruissem, W. and Grossniklaus, U. (2003). The Polycomb-group protein MEDEA regulates seed development by controlling expression of the MADS-box gene PHERES1. Genes Dev. 17, Köhler, C., Page, D. R., Gagliardini, V. and Grossniklaus, U. (2005). The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat. Genet. 37, Makarevich, G., Leroy, O., Akinci, U., Schubert, D., Clarenz, O., Goodrich, J., Grossniklaus, U. and Köhler, C. (2006). Different Polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep. 7, Martienssen, R. (1998). Transposons, DNA methylation and gene control. Trends Genet. 14, Reik, W. (2007). Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 447, Saze, H., Scheid, O. M. and Paszkowski, J. (2003). Maintenance of CpG methylation is essential for epigenetic inheritance during plant gametogenesis. Nat. Genet. 34, Scott, R. J. and Spielman, M. (2006). Deeper into the maize, new insights into genomic imprinting in plants. BioEssays 28, Sleutels, F. and Barlow, D. P. (2002). The origins of genomic imprinting in mammals. Adv. Genet. 46, Spillane, C., Baroux, C., Escobar-Restrepo, J. M., Page, D. R., Laoueille, S. and Grossniklaus, U. (2004). Transposons and tandem repeats are not involved in the control of genomic imprinting at the MEDEA locus in Arabidopsis. Cold Spring Harb. Symp. Quant. Biol. 69, Vielle-Calzada, J. P., Thomas, J., Spillane, C., Coluccio, A., Hoeppner, M. A. and Grossniklaus, U. (1999). Maintenance of genomic imprinting at the Arabidopsis MEDEA locus requires zygotic DDM1 activity. Genes Dev. 13, Xiao, W., Gehring, M., Choi, Y., Margossian, L., Pu, H., Harada, J. J., Goldberg, R. B., Pennell, R. I. and Fischer, R. L. (2003). Imprinting of the MEA Polycomb gene is controlled by antagonism between MET1 methyltransferase and DME glycosylase. Dev. Cell 5, Zhang, X., Yazaki, J., Sundaresan, A., Cokus, S., Chan, S. W., Chen, H., Henderson, I. R., Shinn, P., Pellegrini, M., Jacobsen, S. E. et al. (2006). Genome-wide highresolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell 126,
Epigenetic regulation of endogenous genes and developmental processes
 Epigenetic regulation of endogenous genes and developmental processes Epigenetic programming in plants helps control developmental transitions Embryonic to vegetative transition Vegetative to reproductive
Epigenetic regulation of endogenous genes and developmental processes Epigenetic programming in plants helps control developmental transitions Embryonic to vegetative transition Vegetative to reproductive
NIH Public Access Author Manuscript Curr Opin Genet Dev. Author manuscript; available in PMC 2008 December 1.
 NIH Public Access Author Manuscript Published in final edited form as: Curr Opin Genet Dev. 2007 December ; 17(6): 480 485. Endosperm Gene Imprinting and Seed Development Jin Hoe Huh, Matthew J. Bauer,
NIH Public Access Author Manuscript Published in final edited form as: Curr Opin Genet Dev. 2007 December ; 17(6): 480 485. Endosperm Gene Imprinting and Seed Development Jin Hoe Huh, Matthew J. Bauer,
Report. Epigenetic Resetting of a Gene Imprinted in Plant Embryos
 Current Biology 19, 1677 1681, October 13, 2009 ª2009 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2009.08.053 Epigenetic Resetting of a Gene Imprinted in Plant Embryos Report Stephanie Jahnke 1
Current Biology 19, 1677 1681, October 13, 2009 ª2009 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2009.08.053 Epigenetic Resetting of a Gene Imprinted in Plant Embryos Report Stephanie Jahnke 1
Epigenetic and Genetic Control of Imprinting at the Mez1 Locus in Maize
 Epigenetic and Genetic Control of Imprinting at the Mez1 Locus in Maize A THESIS SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY William John Haun IN PARTIAL FULFILLMENT
Epigenetic and Genetic Control of Imprinting at the Mez1 Locus in Maize A THESIS SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY William John Haun IN PARTIAL FULFILLMENT
DNA methylation & demethylation
 DNA methylation & demethylation Lars Schomacher (Group Christof Niehrs) What is Epigenetics? Epigenetics is the study of heritable changes in gene expression (active versus inactive genes) that do not
DNA methylation & demethylation Lars Schomacher (Group Christof Niehrs) What is Epigenetics? Epigenetics is the study of heritable changes in gene expression (active versus inactive genes) that do not
Regulation and Flexibility of Genomic Imprinting during Seed Development W
 The Plant Cell, Vol. 23: 16 26, January 2011, www.plantcell.org ã 2011 American Society of Plant Biologists REVIEW Regulation and Flexibility of Genomic Imprinting during Seed Development W Michael T.
The Plant Cell, Vol. 23: 16 26, January 2011, www.plantcell.org ã 2011 American Society of Plant Biologists REVIEW Regulation and Flexibility of Genomic Imprinting during Seed Development W Michael T.
Report. DNA Methylation Dynamics during Sexual Reproduction in Arabidopsis thaliana
 Current Biology 22, 1825 1830, October 9, 2012 ª2012 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2012.07.061 DNA Methylation Dynamics during Sexual Reproduction in Arabidopsis thaliana
Current Biology 22, 1825 1830, October 9, 2012 ª2012 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2012.07.061 DNA Methylation Dynamics during Sexual Reproduction in Arabidopsis thaliana
Today. Genomic Imprinting & X-Inactivation
 Today 1. Quiz (~12 min) 2. Genomic imprinting in mammals 3. X-chromosome inactivation in mammals Note that readings on Dosage Compensation and Genomic Imprinting in Mammals are on our web site. Genomic
Today 1. Quiz (~12 min) 2. Genomic imprinting in mammals 3. X-chromosome inactivation in mammals Note that readings on Dosage Compensation and Genomic Imprinting in Mammals are on our web site. Genomic
Genetics and Genomics in Medicine Chapter 6 Questions
 Genetics and Genomics in Medicine Chapter 6 Questions Multiple Choice Questions Question 6.1 With respect to the interconversion between open and condensed chromatin shown below: Which of the directions
Genetics and Genomics in Medicine Chapter 6 Questions Multiple Choice Questions Question 6.1 With respect to the interconversion between open and condensed chromatin shown below: Which of the directions
Genomic Imprinting: Comparative analysis Between Plants and Mammals
 Plant Tissue Cult. & Biotech. 26(2): 159-174, 2016 (December) - Review article PTC&B Genomic Imprinting: Comparative analysis Between Plants and Mammals Oluwaseun Ogunwuyi 1, Ankur Upadhyay, Simeon K.
Plant Tissue Cult. & Biotech. 26(2): 159-174, 2016 (December) - Review article PTC&B Genomic Imprinting: Comparative analysis Between Plants and Mammals Oluwaseun Ogunwuyi 1, Ankur Upadhyay, Simeon K.
2009 LANDES BIOSCIENCE. DO NOT DISTRIBUTE.
 [Epigenetics 4:2, 1-6; 16 February 2009]; 2009 Landes Bioscience Research Paper Determining the conservation of DNA methylation in Arabidopsis This manuscript has been published online, prior to printing.once
[Epigenetics 4:2, 1-6; 16 February 2009]; 2009 Landes Bioscience Research Paper Determining the conservation of DNA methylation in Arabidopsis This manuscript has been published online, prior to printing.once
Parent-of-origin effects on seed development in Arabidopsis thaliana require DNA methylation
 Development 127, 2493-2502 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV0291 2493 Parent-of-origin effects on seed development in Arabidopsis thaliana require DNA methylation
Development 127, 2493-2502 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV0291 2493 Parent-of-origin effects on seed development in Arabidopsis thaliana require DNA methylation
Lecture 27. Epigenetic regulation of gene expression during development
 Lecture 27 Epigenetic regulation of gene expression during development Development of a multicellular organism is not only determined by the DNA sequence but also epigenetically through DNA methylation
Lecture 27 Epigenetic regulation of gene expression during development Development of a multicellular organism is not only determined by the DNA sequence but also epigenetically through DNA methylation
Genomic Analysis of Parent-of-Origin Allelic Expression in Arabidopsis thaliana Seeds
 Genomic Analysis of Parent-of-Origin Allelic Expression in Arabidopsis thaliana Seeds Mary Gehring 1,2,3,4 *, Victor Missirian 2,5, Steven Henikoff 1,2 1 Howard Hughes Medical Institute, Seattle, Washington,
Genomic Analysis of Parent-of-Origin Allelic Expression in Arabidopsis thaliana Seeds Mary Gehring 1,2,3,4 *, Victor Missirian 2,5, Steven Henikoff 1,2 1 Howard Hughes Medical Institute, Seattle, Washington,
Epigenetics: Basic Principals and role in health and disease
 Epigenetics: Basic Principals and role in health and disease Cambridge Masterclass Workshop on Epigenetics in GI Health and Disease 3 rd September 2013 Matt Zilbauer Overview Basic principals of Epigenetics
Epigenetics: Basic Principals and role in health and disease Cambridge Masterclass Workshop on Epigenetics in GI Health and Disease 3 rd September 2013 Matt Zilbauer Overview Basic principals of Epigenetics
Review Article Epigenetic Mechanisms of Genomic Imprinting: Common Themes in the Regulation of Imprinted Regions in Mammals, Plants, and Insects
 Genetics Research International Volume 2012, Article ID 585024, 17 pages doi:10.1155/2012/585024 Review Article Epigenetic echanisms of Genomic Imprinting: Common Themes in the Regulation of Imprinted
Genetics Research International Volume 2012, Article ID 585024, 17 pages doi:10.1155/2012/585024 Review Article Epigenetic echanisms of Genomic Imprinting: Common Themes in the Regulation of Imprinted
Regulation of Imprinted Gene and Small RNA Expression in the Arabidopsis Endosperm
 Regulation of Imprinted Gene and Small RNA Expression in the Arabidopsis Endosperm By Juhyun Shin A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy
Regulation of Imprinted Gene and Small RNA Expression in the Arabidopsis Endosperm By Juhyun Shin A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy
Imprinting. Joyce Ohm Cancer Genetics and Genomics CGP-L2-319 x8821
 Imprinting Joyce Ohm Cancer Genetics and Genomics CGP-L2-319 x8821 Learning Objectives 1. To understand the basic concepts of genomic imprinting Genomic imprinting is an epigenetic phenomenon that causes
Imprinting Joyce Ohm Cancer Genetics and Genomics CGP-L2-319 x8821 Learning Objectives 1. To understand the basic concepts of genomic imprinting Genomic imprinting is an epigenetic phenomenon that causes
The MADS Domain Protein DIANA Acts Together with AGAMOUS-LIKE80 to Specify the Central Cell in Arabidopsis Ovules W
 The Plant Cell, Vol. 20: 2088 2101, August 2008, www.plantcell.org ã 2008 American Society of Plant Biologists The MADS Domain Protein DIANA Acts Together with AGAMOUS-LIKE80 to Specify the Central Cell
The Plant Cell, Vol. 20: 2088 2101, August 2008, www.plantcell.org ã 2008 American Society of Plant Biologists The MADS Domain Protein DIANA Acts Together with AGAMOUS-LIKE80 to Specify the Central Cell
Evolution and function of genomic imprinting in plants
 REVIEW Evolution and function of genomic imprinting in plants Jessica A. Rodrigues and Daniel Zilberman Department of Plant and Microbial Biology, University of California at Berkeley, Berkeley, California
REVIEW Evolution and function of genomic imprinting in plants Jessica A. Rodrigues and Daniel Zilberman Department of Plant and Microbial Biology, University of California at Berkeley, Berkeley, California
An Unexpected Function of the Prader-Willi Syndrome Imprinting Center in Maternal Imprinting in Mice
 An Unexpected Function of the Prader-Willi Syndrome Imprinting Center in Maternal Imprinting in Mice Mei-Yi Wu 1 *, Ming Jiang 1, Xiaodong Zhai 2, Arthur L. Beaudet 2, Ray-Chang Wu 1 * 1 Department of
An Unexpected Function of the Prader-Willi Syndrome Imprinting Center in Maternal Imprinting in Mice Mei-Yi Wu 1 *, Ming Jiang 1, Xiaodong Zhai 2, Arthur L. Beaudet 2, Ray-Chang Wu 1 * 1 Department of
Transcriptional repression of Xi
 Transcriptional repression of Xi Xist Transcription of Xist Xist RNA Spreading of Xist Recruitment of repression factors. Stable repression Translocated Xic cannot efficiently silence autosome regions.
Transcriptional repression of Xi Xist Transcription of Xist Xist RNA Spreading of Xist Recruitment of repression factors. Stable repression Translocated Xic cannot efficiently silence autosome regions.
Jayanti Tokas 1, Puneet Tokas 2, Shailini Jain 3 and Hariom Yadav 3
 Jayanti Tokas 1, Puneet Tokas 2, Shailini Jain 3 and Hariom Yadav 3 1 Department of Biotechnology, JMIT, Radaur, Haryana, India 2 KITM, Kurukshetra, Haryana, India 3 NIDDK, National Institute of Health,
Jayanti Tokas 1, Puneet Tokas 2, Shailini Jain 3 and Hariom Yadav 3 1 Department of Biotechnology, JMIT, Radaur, Haryana, India 2 KITM, Kurukshetra, Haryana, India 3 NIDDK, National Institute of Health,
Epigenetics and Chromatin Remodeling
 Epigenetics and Chromatin Remodeling Bradford Coffee, PhD, FACMG Emory University Atlanta, GA Speaker Disclosure Information Grant/Research Support: none Salary/Consultant Fees: none Board/Committee/Advisory
Epigenetics and Chromatin Remodeling Bradford Coffee, PhD, FACMG Emory University Atlanta, GA Speaker Disclosure Information Grant/Research Support: none Salary/Consultant Fees: none Board/Committee/Advisory
Repressive Transcription
 Repressive Transcription The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Guenther, M. G., and R. A.
Repressive Transcription The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Guenther, M. G., and R. A.
Joanna Hillman Michael Higgins Lab Oncology for Scientists I 10/29/2015
 Joanna Hillman Michael Higgins Lab Oncology for Scientists I 10/29/2015 ! Define Epigenetics & Genomic Imprinting! Discovery! What is the imprint! Lifecycle of an Imprint DMRs and ICEs! 2 main mechanisms
Joanna Hillman Michael Higgins Lab Oncology for Scientists I 10/29/2015 ! Define Epigenetics & Genomic Imprinting! Discovery! What is the imprint! Lifecycle of an Imprint DMRs and ICEs! 2 main mechanisms
Not IN Our Genes - A Different Kind of Inheritance.! Christopher Phiel, Ph.D. University of Colorado Denver Mini-STEM School February 4, 2014
 Not IN Our Genes - A Different Kind of Inheritance! Christopher Phiel, Ph.D. University of Colorado Denver Mini-STEM School February 4, 2014 Epigenetics in Mainstream Media Epigenetics *Current definition:
Not IN Our Genes - A Different Kind of Inheritance! Christopher Phiel, Ph.D. University of Colorado Denver Mini-STEM School February 4, 2014 Epigenetics in Mainstream Media Epigenetics *Current definition:
Supplemental Figure 1. Genes showing ectopic H3K9 dimethylation in this study are DNA hypermethylated in Lister et al. study.
 mc mc mc mc SUP mc mc Supplemental Figure. Genes showing ectopic HK9 dimethylation in this study are DNA hypermethylated in Lister et al. study. Representative views of genes that gain HK9m marks in their
mc mc mc mc SUP mc mc Supplemental Figure. Genes showing ectopic HK9 dimethylation in this study are DNA hypermethylated in Lister et al. study. Representative views of genes that gain HK9m marks in their
Epigenetic memory in plants
 Review Focus: Molecular Memory Epigenetic memory in plants Mayumi Iwasaki * & Jerzy Paszkowski ** Abstract Epigenetics refers to heritable changes in patterns of gene expression that occur without alterations
Review Focus: Molecular Memory Epigenetic memory in plants Mayumi Iwasaki * & Jerzy Paszkowski ** Abstract Epigenetics refers to heritable changes in patterns of gene expression that occur without alterations
Cell-FateSwitchofSynergidtoEggCellinArabidopsis eostre Mutant Embryo Sacs Arises from Misexpression of the BEL1-Like Homeodomain Gene BLH1 W
 The Plant Cell, Vol. 19: 3578 3592, November 2007, www.plantcell.org ª 2007 American Society of Plant Biologists Cell-FateSwitchofSynergidtoEggCellinArabidopsis eostre Mutant Embryo Sacs Arises from Misexpression
The Plant Cell, Vol. 19: 3578 3592, November 2007, www.plantcell.org ª 2007 American Society of Plant Biologists Cell-FateSwitchofSynergidtoEggCellinArabidopsis eostre Mutant Embryo Sacs Arises from Misexpression
R. Piazza (MD, PhD), Dept. of Medicine and Surgery, University of Milano-Bicocca EPIGENETICS
 R. Piazza (MD, PhD), Dept. of Medicine and Surgery, University of Milano-Bicocca EPIGENETICS EPIGENETICS THE STUDY OF CHANGES IN GENE EXPRESSION THAT ARE POTENTIALLY HERITABLE AND THAT DO NOT ENTAIL A
R. Piazza (MD, PhD), Dept. of Medicine and Surgery, University of Milano-Bicocca EPIGENETICS EPIGENETICS THE STUDY OF CHANGES IN GENE EXPRESSION THAT ARE POTENTIALLY HERITABLE AND THAT DO NOT ENTAIL A
Fragile X Syndrome. Genetics, Epigenetics & the Role of Unprogrammed Events in the expression of a Phenotype
 Fragile X Syndrome Genetics, Epigenetics & the Role of Unprogrammed Events in the expression of a Phenotype A loss of function of the FMR-1 gene results in severe learning problems, intellectual disability
Fragile X Syndrome Genetics, Epigenetics & the Role of Unprogrammed Events in the expression of a Phenotype A loss of function of the FMR-1 gene results in severe learning problems, intellectual disability
Epigenetics: A historical overview Dr. Robin Holliday
 Epigenetics 1 Rival hypotheses Epigenisis - The embryo is initially undifferentiated. As development proceeds, increasing levels of complexity emerge giving rise to the larval stage or to the adult organism.
Epigenetics 1 Rival hypotheses Epigenisis - The embryo is initially undifferentiated. As development proceeds, increasing levels of complexity emerge giving rise to the larval stage or to the adult organism.
Epigenetic Inheritance
 (2) The role of Epigenetic Inheritance Lamarck Revisited Lamarck was incorrect in thinking that the inheritance of acquired characters is the main mechanism of evolution (Natural Selection more common)
(2) The role of Epigenetic Inheritance Lamarck Revisited Lamarck was incorrect in thinking that the inheritance of acquired characters is the main mechanism of evolution (Natural Selection more common)
sirna count per 50 kb small RNAs matching the direct strand Repeat length (bp) per 50 kb repeats in the chromosome
 Qi et al. 26-3-2564C Qi et al., Figure S1 sirna count per 5 kb small RNAs matching the direct strand sirna count per 5 kb small RNAs matching the complementary strand Repeat length (bp) per 5 kb repeats
Qi et al. 26-3-2564C Qi et al., Figure S1 sirna count per 5 kb small RNAs matching the direct strand sirna count per 5 kb small RNAs matching the complementary strand Repeat length (bp) per 5 kb repeats
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
DNA Methylation Is Critical for Arabidopsis Embryogenesis and Seed Viability
 The Plant Cell, Vol. 18, 805 814, April 2006, www.plantcell.org ª 2006 American Society of Plant Biologists RESEARCH ARTICLES DNA Methylation Is Critical for Arabidopsis Embryogenesis and Seed Viability
The Plant Cell, Vol. 18, 805 814, April 2006, www.plantcell.org ª 2006 American Society of Plant Biologists RESEARCH ARTICLES DNA Methylation Is Critical for Arabidopsis Embryogenesis and Seed Viability
Chromatin-Based Regulation of Gene Expression
 Chromatin-Based Regulation of Gene Expression.George J. Quellhorst, Jr., PhD.Associate Director, R&D.Biological Content Development Topics to be Discussed Importance of Chromatin-Based Regulation Mechanism
Chromatin-Based Regulation of Gene Expression.George J. Quellhorst, Jr., PhD.Associate Director, R&D.Biological Content Development Topics to be Discussed Importance of Chromatin-Based Regulation Mechanism
Allelic reprogramming of the histone modification H3K4me3 in early mammalian development
 Allelic reprogramming of the histone modification H3K4me3 in early mammalian development 张戈 Method and material STAR ChIP seq (small-scale TELP-assisted rapid ChIP seq) 200 mouse embryonic stem cells PWK/PhJ
Allelic reprogramming of the histone modification H3K4me3 in early mammalian development 张戈 Method and material STAR ChIP seq (small-scale TELP-assisted rapid ChIP seq) 200 mouse embryonic stem cells PWK/PhJ
Histones modifications and variants
 Histones modifications and variants Dr. Institute of Molecular Biology, Johannes Gutenberg University, Mainz www.imb.de Lecture Objectives 1. Chromatin structure and function Chromatin and cell state Nucleosome
Histones modifications and variants Dr. Institute of Molecular Biology, Johannes Gutenberg University, Mainz www.imb.de Lecture Objectives 1. Chromatin structure and function Chromatin and cell state Nucleosome
Epigenetics DNA methylation. Biosciences 741: Genomics Fall, 2013 Week 13. DNA Methylation
 Epigenetics DNA methylation Biosciences 741: Genomics Fall, 2013 Week 13 DNA Methylation Most methylated cytosines are found in the dinucleotide sequence CG, denoted mcpg. The restriction enzyme HpaII
Epigenetics DNA methylation Biosciences 741: Genomics Fall, 2013 Week 13 DNA Methylation Most methylated cytosines are found in the dinucleotide sequence CG, denoted mcpg. The restriction enzyme HpaII
Figure S1 Expression of AHL gene family members in diploid (Ler Col) and triploid (Ler
 Supplemental material Supplemental figure legends Figure S Expression of AHL gene family members in diploid (Ler ) and triploid (Ler osd) seeds. AHLs from clade B are labelled with (I), and AHLs from clade
Supplemental material Supplemental figure legends Figure S Expression of AHL gene family members in diploid (Ler ) and triploid (Ler osd) seeds. AHLs from clade B are labelled with (I), and AHLs from clade
Session 2: Biomarkers of epigenetic changes and their applicability to genetic toxicology
 Session 2: Biomarkers of epigenetic changes and their applicability to genetic toxicology Bhaskar Gollapudi, Ph.D The Dow Chemical Company Workshop: Genetic Toxicology: Opportunities to Integrate New Approaches
Session 2: Biomarkers of epigenetic changes and their applicability to genetic toxicology Bhaskar Gollapudi, Ph.D The Dow Chemical Company Workshop: Genetic Toxicology: Opportunities to Integrate New Approaches
Understanding the interaction between RNA-directed DNA methylation and DNA demethylases and its role in Fusarium oxysporum
 Understanding the interaction between RNA-directed DNA methylation and DNA demethylases and its role in Fusarium oxysporum disease response in Arabidopsis thaliana Joanne R.M. Lee, B. Biotech (Hons.) A
Understanding the interaction between RNA-directed DNA methylation and DNA demethylases and its role in Fusarium oxysporum disease response in Arabidopsis thaliana Joanne R.M. Lee, B. Biotech (Hons.) A
Antagonism between DNA and H3K27 Methylation at the Imprinted Rasgrf1 Locus
 Antagonism between DNA and H3K27 Methylation at the Imprinted Rasgrf1 Locus Anders M. Lindroth 1., Yoon Jung Park 1., Chelsea M. McLean 1, Gregoriy A. Dokshin 1, Jenna M. Persson 1, Herry Herman 1,2, Diego
Antagonism between DNA and H3K27 Methylation at the Imprinted Rasgrf1 Locus Anders M. Lindroth 1., Yoon Jung Park 1., Chelsea M. McLean 1, Gregoriy A. Dokshin 1, Jenna M. Persson 1, Herry Herman 1,2, Diego
Gene Expression DNA RNA. Protein. Metabolites, stress, environment
 Gene Expression DNA RNA Protein Metabolites, stress, environment 1 EPIGENETICS The study of alterations in gene function that cannot be explained by changes in DNA sequence. Epigenetic gene regulatory
Gene Expression DNA RNA Protein Metabolites, stress, environment 1 EPIGENETICS The study of alterations in gene function that cannot be explained by changes in DNA sequence. Epigenetic gene regulatory
Sexual and Apomictic Seed Formation in Hieracium Requires the Plant Polycomb-Group Gene FERTILIZATION INDEPENDENT ENDOSPERM W
 The Plant Cell, Vol. 20: 2372 2386, September 2008, www.plantcell.org ã 2008 American Society of Plant Biologists Sexual and Apomictic Seed Formation in Hieracium Requires the Plant Polycomb-Group Gene
The Plant Cell, Vol. 20: 2372 2386, September 2008, www.plantcell.org ã 2008 American Society of Plant Biologists Sexual and Apomictic Seed Formation in Hieracium Requires the Plant Polycomb-Group Gene
Methylation reprogramming dynamics and defects in gametogenesis and embryogenesis: implications for reproductive medicine
 Univ.-Prof. Dr. Thomas Haaf Methylation reprogramming dynamics and defects in gametogenesis and embryogenesis: implications for reproductive medicine Epigenetics and DNA methylation Heritable change of
Univ.-Prof. Dr. Thomas Haaf Methylation reprogramming dynamics and defects in gametogenesis and embryogenesis: implications for reproductive medicine Epigenetics and DNA methylation Heritable change of
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10495 WWW.NATURE.COM/NATURE 1 2 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 3 4 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 5 6 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 7 8 WWW.NATURE.COM/NATURE
doi:10.1038/nature10495 WWW.NATURE.COM/NATURE 1 2 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 3 4 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 5 6 WWW.NATURE.COM/NATURE WWW.NATURE.COM/NATURE 7 8 WWW.NATURE.COM/NATURE
Stem Cell Epigenetics
 Stem Cell Epigenetics Philippe Collas University of Oslo Institute of Basic Medical Sciences Norwegian Center for Stem Cell Research www.collaslab.com Source of stem cells in the body Somatic ( adult )
Stem Cell Epigenetics Philippe Collas University of Oslo Institute of Basic Medical Sciences Norwegian Center for Stem Cell Research www.collaslab.com Source of stem cells in the body Somatic ( adult )
Epigenetics: The Future of Psychology & Neuroscience. Richard E. Brown Psychology Department Dalhousie University Halifax, NS, B3H 4J1
 Epigenetics: The Future of Psychology & Neuroscience Richard E. Brown Psychology Department Dalhousie University Halifax, NS, B3H 4J1 Nature versus Nurture Despite the belief that the Nature vs. Nurture
Epigenetics: The Future of Psychology & Neuroscience Richard E. Brown Psychology Department Dalhousie University Halifax, NS, B3H 4J1 Nature versus Nurture Despite the belief that the Nature vs. Nurture
GCATCCATCTTGGGGCGTCCCAATTGCTGAGTAACAAATGAGACGC TGTGGCCAAACTCAGTCATAACTAATGACATTTCTAGACAAAGTGAC TTCAGATTTTCAAAGCGTACCCTGTTTACATCATTTTGCCAATTTCG
 Lecture 6 GCATCCATCTTGGGGCGTCCCAATTGCTGAGTAACAAATGAGACGC TGTGGCCAAACTCAGTCATAACTAATGACATTTCTAGACAAAGTGAC TTCAGATTTTCAAAGCGTACCCTGTTTACATCATTTTGCCAATTTCG CGTACTGCAACCGGCGGGCCACGCCCCCGTGAAAAGAAGGTTGTT TTCTCCACATTTCGGGGTTCTGGACGTTTCCCGGCTGCGGGGCGG
Lecture 6 GCATCCATCTTGGGGCGTCCCAATTGCTGAGTAACAAATGAGACGC TGTGGCCAAACTCAGTCATAACTAATGACATTTCTAGACAAAGTGAC TTCAGATTTTCAAAGCGTACCCTGTTTACATCATTTTGCCAATTTCG CGTACTGCAACCGGCGGGCCACGCCCCCGTGAAAAGAAGGTTGTT TTCTCCACATTTCGGGGTTCTGGACGTTTCCCGGCTGCGGGGCGG
Epigenetics. Lyle Armstrong. UJ Taylor & Francis Group. f'ci Garland Science NEW YORK AND LONDON
 ... Epigenetics Lyle Armstrong f'ci Garland Science UJ Taylor & Francis Group NEW YORK AND LONDON Contents CHAPTER 1 INTRODUCTION TO 3.2 CHROMATIN ARCHITECTURE 21 THE STUDY OF EPIGENETICS 1.1 THE CORE
... Epigenetics Lyle Armstrong f'ci Garland Science UJ Taylor & Francis Group NEW YORK AND LONDON Contents CHAPTER 1 INTRODUCTION TO 3.2 CHROMATIN ARCHITECTURE 21 THE STUDY OF EPIGENETICS 1.1 THE CORE
Epigenetics and Chromatin
 Progress in Molecular and Subcellular Biology 38 Epigenetics and Chromatin Bearbeitet von Philippe Jeanteur 1. Auflage 2008. Taschenbuch. xiii, 266 S. Paperback ISBN 978 3 540 85236 0 Format (B x L): 15,5
Progress in Molecular and Subcellular Biology 38 Epigenetics and Chromatin Bearbeitet von Philippe Jeanteur 1. Auflage 2008. Taschenbuch. xiii, 266 S. Paperback ISBN 978 3 540 85236 0 Format (B x L): 15,5
Supplemental Information. Figures. Figure S1
 Supplemental Information Figures Figure S1 Identification of JAGGER T-DNA insertions. A. Positions of T-DNA and Ds insertions in JAGGER are indicated by inverted triangles, the grey box represents the
Supplemental Information Figures Figure S1 Identification of JAGGER T-DNA insertions. A. Positions of T-DNA and Ds insertions in JAGGER are indicated by inverted triangles, the grey box represents the
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
www.sciencemag.org/cgi/content/full/1171320/dc1 Supporting Online Material for A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance Long Yang, David S. Garbe, Greg J. Bashaw*
DNA methylation in an intron of the IBM1 histone demethylase gene stabilizes chromatin modification patterns
 The EMBO Journal (2012) 31, 2981 2993 & 2012 European Molecular Biology Organization All Rights Reserved 0261-4189/12 www.embojournal.org DNA methylation in an intron of the histone demethylase gene stabilizes
The EMBO Journal (2012) 31, 2981 2993 & 2012 European Molecular Biology Organization All Rights Reserved 0261-4189/12 www.embojournal.org DNA methylation in an intron of the histone demethylase gene stabilizes
Epigenetic Regulation of Health and Disease Nutritional and environmental effects on epigenetic regulation
 Epigenetic Regulation of Health and Disease Nutritional and environmental effects on epigenetic regulation Robert FEIL Director of Research CNRS & University of Montpellier, Montpellier, France. E-mail:
Epigenetic Regulation of Health and Disease Nutritional and environmental effects on epigenetic regulation Robert FEIL Director of Research CNRS & University of Montpellier, Montpellier, France. E-mail:
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Supplementary Figures
 Supplementary Figures a miel1-2 (SALK_41369).1kb miel1-1 (SALK_978) b TUB MIEL1 Supplementary Figure 1. MIEL1 expression in miel1 mutant and S:MIEL1-MYC transgenic plants. (a) Mapping of the T-DNA insertion
Supplementary Figures a miel1-2 (SALK_41369).1kb miel1-1 (SALK_978) b TUB MIEL1 Supplementary Figure 1. MIEL1 expression in miel1 mutant and S:MIEL1-MYC transgenic plants. (a) Mapping of the T-DNA insertion
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. Confirmation of Dnmt1 conditional knockout out mice. a, Representative images of sorted stem (Lin - CD49f high CD24 + ), luminal (Lin - CD49f low CD24 + )
Supplementary Figures Supplementary Figure 1. Confirmation of Dnmt1 conditional knockout out mice. a, Representative images of sorted stem (Lin - CD49f high CD24 + ), luminal (Lin - CD49f low CD24 + )
Department of Molecular, Cellular, and Developmental Biology, Yale University, New Haven, Connecticut c
 This article is a Plant Cell Advance Online Publication. The date of its first appearance online is the official date of publication. The article has been edited and the authors have corrected proofs,
This article is a Plant Cell Advance Online Publication. The date of its first appearance online is the official date of publication. The article has been edited and the authors have corrected proofs,
Nature Genetics: doi: /ng Supplementary Figure 1. Assessment of sample purity and quality.
 Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
Supplementary Figure 1 Assessment of sample purity and quality. (a) Hematoxylin and eosin staining of formaldehyde-fixed, paraffin-embedded sections from a human testis biopsy collected concurrently with
Epigenetic regulation and inheritance of. autonomous seed development in apomictic. Hieracium
 Epigenetic regulation and inheritance of autonomous seed development in apomictic Hieracium Julio Carlyle M Rodrigues Thesis submitted for the degree of Doctor of Philosophy University of Adelaide, School
Epigenetic regulation and inheritance of autonomous seed development in apomictic Hieracium Julio Carlyle M Rodrigues Thesis submitted for the degree of Doctor of Philosophy University of Adelaide, School
Supplementary Figure 1 Transcription assay of nine ABA-responsive PP2C. Transcription assay of nine ABA-responsive PP2C genes. Total RNA was isolated
 Supplementary Figure 1 Transcription assay of nine ABA-responsive PP2C genes. Transcription assay of nine ABA-responsive PP2C genes. Total RNA was isolated from 7 day-old seedlings treated with or without
Supplementary Figure 1 Transcription assay of nine ABA-responsive PP2C genes. Transcription assay of nine ABA-responsive PP2C genes. Total RNA was isolated from 7 day-old seedlings treated with or without
he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003)
 MicroRNAs: Genomics, Biogenesis, Mechanism, and Function (D. Bartel Cell 2004) he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003) Vertebrate MicroRNA Genes (Lim et al. Science
MicroRNAs: Genomics, Biogenesis, Mechanism, and Function (D. Bartel Cell 2004) he micrornas of Caenorhabditis elegans (Lim et al. Genes & Development 2003) Vertebrate MicroRNA Genes (Lim et al. Science
Biology Developmental Biology Spring Quarter Midterm 1 Version A
 Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Bi 8 Lecture 17. interference. Ellen Rothenberg 1 March 2016
 Bi 8 Lecture 17 REGulation by RNA interference Ellen Rothenberg 1 March 2016 Protein is not the only regulatory molecule affecting gene expression: RNA itself can be negative regulator RNA does not need
Bi 8 Lecture 17 REGulation by RNA interference Ellen Rothenberg 1 March 2016 Protein is not the only regulatory molecule affecting gene expression: RNA itself can be negative regulator RNA does not need
Phenomena first observed in petunia
 Vectors for RNAi Phenomena first observed in petunia Attempted to overexpress chalone synthase (anthrocyanin pigment gene) in petunia. (trying to darken flower color) Caused the loss of pigment. Bill Douherty
Vectors for RNAi Phenomena first observed in petunia Attempted to overexpress chalone synthase (anthrocyanin pigment gene) in petunia. (trying to darken flower color) Caused the loss of pigment. Bill Douherty
Eukaryotic Gene Regulation
 Eukaryotic Gene Regulation Chapter 19: Control of Eukaryotic Genome The BIG Questions How are genes turned on & off in eukaryotes? How do cells with the same genes differentiate to perform completely different,
Eukaryotic Gene Regulation Chapter 19: Control of Eukaryotic Genome The BIG Questions How are genes turned on & off in eukaryotes? How do cells with the same genes differentiate to perform completely different,
The Biology and Genetics of Cells and Organisms The Biology of Cancer
 The Biology and Genetics of Cells and Organisms The Biology of Cancer Mendel and Genetics How many distinct genes are present in the genomes of mammals? - 21,000 for human. - Genetic information is carried
The Biology and Genetics of Cells and Organisms The Biology of Cancer Mendel and Genetics How many distinct genes are present in the genomes of mammals? - 21,000 for human. - Genetic information is carried
Nature Genetics: doi: /ng Supplementary Figure 1. Immunofluorescence (IF) confirms absence of H3K9me in met-2 set-25 worms.
 Supplementary Figure 1 Immunofluorescence (IF) confirms absence of H3K9me in met-2 set-25 worms. IF images of wild-type (wt) and met-2 set-25 worms showing the loss of H3K9me2/me3 at the indicated developmental
Supplementary Figure 1 Immunofluorescence (IF) confirms absence of H3K9me in met-2 set-25 worms. IF images of wild-type (wt) and met-2 set-25 worms showing the loss of H3K9me2/me3 at the indicated developmental
Cross-Talk Between Sporophyte and Gametophyte Generations Is Promoted by CHD3 Chromatin Remodelers in Arabidopsis thaliana
 HIGHLIGHTED ARTICLE INVESTIGATION Cross-Talk Between Sporophyte and Gametophyte Generations Is Promoted by CHD3 Chromatin Remodelers in Arabidopsis thaliana Benjamin Carter,* James T. Henderson,* Elisabeth
HIGHLIGHTED ARTICLE INVESTIGATION Cross-Talk Between Sporophyte and Gametophyte Generations Is Promoted by CHD3 Chromatin Remodelers in Arabidopsis thaliana Benjamin Carter,* James T. Henderson,* Elisabeth
Biochemical Determinants Governing Redox Regulated Changes in Gene Expression and Chromatin Structure
 Biochemical Determinants Governing Redox Regulated Changes in Gene Expression and Chromatin Structure Frederick E. Domann, Ph.D. Associate Professor of Radiation Oncology The University of Iowa Iowa City,
Biochemical Determinants Governing Redox Regulated Changes in Gene Expression and Chromatin Structure Frederick E. Domann, Ph.D. Associate Professor of Radiation Oncology The University of Iowa Iowa City,
Chromosome-Wide Analysis of Parental Allele-Specific Chromatin and DNA Methylation
 MOLECULAR AND CELLULAR BIOLOGY, Apr. 2011, p. 1757 1770 Vol. 31, No. 8 0270-7306/11/$12.00 doi:10.1128/mcb.00961-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Chromosome-Wide
MOLECULAR AND CELLULAR BIOLOGY, Apr. 2011, p. 1757 1770 Vol. 31, No. 8 0270-7306/11/$12.00 doi:10.1128/mcb.00961-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Chromosome-Wide
Nature Immunology: doi: /ni Supplementary Figure 1. DNA-methylation machinery is essential for silencing of Cd4 in cytotoxic T cells.
 Supplementary Figure 1 DNA-methylation machinery is essential for silencing of Cd4 in cytotoxic T cells. (a) Scheme for the retroviral shrna screen. (b) Histogram showing CD4 expression (MFI) in WT cytotoxic
Supplementary Figure 1 DNA-methylation machinery is essential for silencing of Cd4 in cytotoxic T cells. (a) Scheme for the retroviral shrna screen. (b) Histogram showing CD4 expression (MFI) in WT cytotoxic
Ch. 18 Regulation of Gene Expression
 Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
Nature Immunology: doi: /ni Supplementary Figure 1. Characteristics of SEs in T reg and T conv cells.
 Supplementary Figure 1 Characteristics of SEs in T reg and T conv cells. (a) Patterns of indicated transcription factor-binding at SEs and surrounding regions in T reg and T conv cells. Average normalized
Supplementary Figure 1 Characteristics of SEs in T reg and T conv cells. (a) Patterns of indicated transcription factor-binding at SEs and surrounding regions in T reg and T conv cells. Average normalized
Epigenetics Armstrong_Prelims.indd 1 04/11/2013 3:28 pm
 Epigenetics Epigenetics Lyle Armstrong vi Online resources Accessible from www.garlandscience.com, the Student and Instructor Resource Websites provide learning and teaching tools created for Epigenetics.
Epigenetics Epigenetics Lyle Armstrong vi Online resources Accessible from www.garlandscience.com, the Student and Instructor Resource Websites provide learning and teaching tools created for Epigenetics.
Session 6: Integration of epigenetic data. Peter J Park Department of Biomedical Informatics Harvard Medical School July 18-19, 2016
 Session 6: Integration of epigenetic data Peter J Park Department of Biomedical Informatics Harvard Medical School July 18-19, 2016 Utilizing complimentary datasets Frequent mutations in chromatin regulators
Session 6: Integration of epigenetic data Peter J Park Department of Biomedical Informatics Harvard Medical School July 18-19, 2016 Utilizing complimentary datasets Frequent mutations in chromatin regulators
ddm1a (PFG_3A-51065) ATG ddm1b (PFG_2B-60109) ATG osdrm2 (PFG_3A-04110) osdrm2 osdrm2 osdrm2
 Relative expression.6.5.4.3.2.1 OsDDM1a OsDDM1b OsDRM2 TG TG TG ddm1a (PFG_3-5165) P1 P3 ddm1b (PFG_2-619) P2 531bp 5233bp P1 P4 P3 P2 F1 R1 F1 TG TG TG F1 R1 C ddm1a -/- -/- ddm1b -/- +/- +/- -/- D (PFG_3-411)
Relative expression.6.5.4.3.2.1 OsDDM1a OsDDM1b OsDRM2 TG TG TG ddm1a (PFG_3-5165) P1 P3 ddm1b (PFG_2-619) P2 531bp 5233bp P1 P4 P3 P2 F1 R1 F1 TG TG TG F1 R1 C ddm1a -/- -/- ddm1b -/- +/- +/- -/- D (PFG_3-411)
Parent-of-Origin Effects on Gene Expression and DNA Methylation in the Maize Endosperm W
 The Plant Cell, Vol. 23: 4221 4233, December 2011, www.plantcell.org ã 2011 American Society of Plant Biologists. All rights reserved. LARGE-SCALE BIOLOGY ARTICLE Parent-of-Origin Effects on Gene Expression
The Plant Cell, Vol. 23: 4221 4233, December 2011, www.plantcell.org ã 2011 American Society of Plant Biologists. All rights reserved. LARGE-SCALE BIOLOGY ARTICLE Parent-of-Origin Effects on Gene Expression
A role for LORELEI, a putative glycosylphosphatidylinositolanchored protein, in Arabidopsis thaliana double fertilization and early seed development
 The Plant Journal (2010) 62, 571 588 doi: 10.1111/j.1365-313X.2010.04177.x A role for LORELEI, a putative glycosylphosphatidylinositolanchored protein, in Arabidopsis thaliana double fertilization and
The Plant Journal (2010) 62, 571 588 doi: 10.1111/j.1365-313X.2010.04177.x A role for LORELEI, a putative glycosylphosphatidylinositolanchored protein, in Arabidopsis thaliana double fertilization and
Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy
 Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
I) Development: tissue differentiation and timing II) Whole Chromosome Regulation
 Epigenesis: Gene Regulation Epigenesis : Gene Regulation I) Development: tissue differentiation and timing II) Whole Chromosome Regulation (X chromosome inactivation or Lyonization) III) Regulation during
Epigenesis: Gene Regulation Epigenesis : Gene Regulation I) Development: tissue differentiation and timing II) Whole Chromosome Regulation (X chromosome inactivation or Lyonization) III) Regulation during
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
Supplemental Data. Müller-Xing et al. (2014). Plant Cell /tpc
 Supplemental Figure 1. Phenotypes of iclf (clf-28 swn-7 CLF pro :CLF-GR) plants. A, Late rescue of iclf plants by renewed DEX treatment; senescent inflorescence with elongated siliques (arrow; 90 DAG,
Supplemental Figure 1. Phenotypes of iclf (clf-28 swn-7 CLF pro :CLF-GR) plants. A, Late rescue of iclf plants by renewed DEX treatment; senescent inflorescence with elongated siliques (arrow; 90 DAG,
Supplemental Figure S1. Tertiles of FKBP5 promoter methylation and internal regulatory region
 Supplemental Figure S1. Tertiles of FKBP5 promoter methylation and internal regulatory region methylation in relation to PSS and fetal coupling. A, PSS values for participants whose placentas showed low,
Supplemental Figure S1. Tertiles of FKBP5 promoter methylation and internal regulatory region methylation in relation to PSS and fetal coupling. A, PSS values for participants whose placentas showed low,
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
Lecture 3-4. Identification of Positive Regulators downstream of SA
 Lecture 3-4 Identification of Positive Regulators downstream of SA Positive regulators between SA and resistance/pr Systemic resistance PR gene expression? SA Local infection Necrosis SA What could they
Lecture 3-4 Identification of Positive Regulators downstream of SA Positive regulators between SA and resistance/pr Systemic resistance PR gene expression? SA Local infection Necrosis SA What could they
An epigenetic approach to understanding (and predicting?) environmental effects on gene expression
 www.collaslab.com An epigenetic approach to understanding (and predicting?) environmental effects on gene expression Philippe Collas University of Oslo Institute of Basic Medical Sciences Stem Cell Epigenetics
www.collaslab.com An epigenetic approach to understanding (and predicting?) environmental effects on gene expression Philippe Collas University of Oslo Institute of Basic Medical Sciences Stem Cell Epigenetics
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature23267 Discussion Our findings reveal unique roles for the methylation states of histone H3K9 in RNAi-dependent and - independent heterochromatin formation. Clr4 is the sole S. pombe enzyme
doi:10.1038/nature23267 Discussion Our findings reveal unique roles for the methylation states of histone H3K9 in RNAi-dependent and - independent heterochromatin formation. Clr4 is the sole S. pombe enzyme
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/8/e1701143/dc1 Supplementary Materials for Impaired DNA replication derepresses chromatin and generates a transgenerationally inherited epigenetic memory Adam
advances.sciencemag.org/cgi/content/full/3/8/e1701143/dc1 Supplementary Materials for Impaired DNA replication derepresses chromatin and generates a transgenerationally inherited epigenetic memory Adam
Genome-editing via Oviductal Nucleic Acids Delivery (GONAD) system: a novel microinjection-independent genome engineering method in mice
 Supplementary Information Genome-editing via Oviductal Nucleic Acids Delivery (GONAD) system: a novel microinjection-independent genome engineering method in mice Gou Takahashi, Channabasavaiah B Gurumurthy,
Supplementary Information Genome-editing via Oviductal Nucleic Acids Delivery (GONAD) system: a novel microinjection-independent genome engineering method in mice Gou Takahashi, Channabasavaiah B Gurumurthy,
LACHESIS-dependent egg-cell signaling regulates the development of female gametophytic cells
 498 RESEARCH REPORT Development 139, 498-502 (2012) doi:10.1242/dev.075234 2012. Published by The Company of Biologists Ltd LACHESIS-dependent egg-cell signaling regulates the development of female gametophytic
498 RESEARCH REPORT Development 139, 498-502 (2012) doi:10.1242/dev.075234 2012. Published by The Company of Biologists Ltd LACHESIS-dependent egg-cell signaling regulates the development of female gametophytic
Open Flower. Juvenile leaf Flowerbud. Carpel 35 NA NA NA NA 61 NA 95 NA NA 15 NA 41 3 NA
 PaxDB Root Juvenile leaf Flowerbud Open Flower Carpel Mature Pollen Silique Seed Sec3a Sec3b Sec5a Sec5b Sec6 Sec8 Sec10a/b Sec15a Sec15b Exo84a Exo84b Exo84c Exo70A1 Exo70A2 Exo70A3 49 47 8 75 104 79
PaxDB Root Juvenile leaf Flowerbud Open Flower Carpel Mature Pollen Silique Seed Sec3a Sec3b Sec5a Sec5b Sec6 Sec8 Sec10a/b Sec15a Sec15b Exo84a Exo84b Exo84c Exo70A1 Exo70A2 Exo70A3 49 47 8 75 104 79
Circular RNAs (circrnas) act a stable mirna sponges
 Circular RNAs (circrnas) act a stable mirna sponges cernas compete for mirnas Ancestal mrna (+3 UTR) Pseudogene RNA (+3 UTR homolgy region) The model holds true for all RNAs that share a mirna binding
Circular RNAs (circrnas) act a stable mirna sponges cernas compete for mirnas Ancestal mrna (+3 UTR) Pseudogene RNA (+3 UTR homolgy region) The model holds true for all RNAs that share a mirna binding
p53 cooperates with DNA methylation and a suicidal interferon response to maintain epigenetic silencing of repeats and noncoding RNAs
 p53 cooperates with DNA methylation and a suicidal interferon response to maintain epigenetic silencing of repeats and noncoding RNAs 2013, Katerina I. Leonova et al. Kolmogorov Mikhail Noncoding DNA Mammalian
p53 cooperates with DNA methylation and a suicidal interferon response to maintain epigenetic silencing of repeats and noncoding RNAs 2013, Katerina I. Leonova et al. Kolmogorov Mikhail Noncoding DNA Mammalian
