Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations
|
|
|
- Tamsin Burke
- 5 years ago
- Views:
Transcription
1 Development 128, (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations Margit Mahlapuu 1, Sven Enerbäck 2 and Peter Carlsson 1 1 Department of Molecular Biology, Göteborg University, The Lundberg Laboratory, Box 462, SE Göteborg, Sweden 2 Department of Medical Biochemistry, Göteborg University, SE Göteborg, Sweden *Author for correspondence ( peter.carlsson@molbio.gu.se) Accepted 10 April 2001 SUMMARY The murine Foxf1 gene, encoding a forkhead or winged helix transcription factor, is expressed in splanchnic mesenchyme during organogenesis. The concentration of expression to subepithelial mesenchyme suggested that Foxf1 is activated by paracrine signals from endodermal epithelia. Homozygous Foxf1-null mice die before embryonic day 10, owing to defects in extra-embryonic mesoderm, and do not provide any information about the role of Foxf1 in morphogenesis of endodermally derived organs. We show that, on CD1 genetic background, Foxf1 heterozygote perinatal mortality is around 90%. The haploinsufficiency causes a variable phenotype that includes lung immaturity and hypoplasia, fusion of right lung lobes, narrowing of esophagus and trachea, esophageal atresia and tracheo-esophageal fistula. Similar malformations are observed in mutants that are defective in the sonic hedgehog (Shh) signaling pathway, and we show that exogenous Shh activates transcription of Foxf1 in developing lung. Foxf1 mrna is absent in the lungs, foregut and sclerotomes of Shh / embryos, but persists in tissues where indian hedgehog (Ihh) is expressed. In lung organ cultures, activation of Foxf1 by Shh is counteracted by bone morphogenetic protein 4 (BMP4). Fibroblast growth factor (FGF) 10 and FGF7 both decrease Foxf1 expression and we speculate that this is mediated by transcriptional activation of epithelial Bmp4 (in the case of FGF10) and by inhibition of Shh expression for FGF7. Key words: Lung, Mouse, Foregut, Foxf1, forkhead INTRODUCTION Morphogenesis of the mammalian lungs and foregut is controlled by interactions between the endodermal epithelium and the surrounding mesenchyme. Grafting experiments have demonstrated the distinct inductive capacities of proximal and distal mesenchyme, derived from the trachea and the tips of the growing lungs, respectively (Wessells, 1970). In the last decade, several key components in the paracrine crosstalk between mesenchyme and epithelium have been identified. Hedgehog proteins are secreted signaling molecules that are involved in many morphogenetic processes. In Drosophila, Hedgehog signaling is received by the cell surface receptor Patched and transduced by Smoothened, which modulates the transcription factor Cubitus interruptus (Ci) through control of its proteolytic processing (reviewed by Aza-Blanc and Kornberg, 1999). The essentials of this pathway appear to be conserved in mammals, which have three different hedgehog proteins, indian (Ihh), desert (Dhh) and sonic hedgehog (Shh); two Patched homologs, patched 1 (Ptch) and patched 2 (Ptch2); and three homologs of Ci, Gli1, Gli2 and Gli3 (reviewed by McMahon, 2000). Shh is the most widely expressed of the mammalian hedgehogs and is the only one expressed in lung and foregut (Bitgood and McMahon, 1995). Shh mrna is found throughout the early respiratory epithelium, but high level expression is confined to the distal epithelium, near the tips of the growing buds (Bellusci et al., 1996; Urase et al., 1996). Gene targeting in mice, as well as transgenic overexpression, have demonstrated the importance of Shh for normal development and branching morphogenesis of the lungs (Bellusci et al., 1997a; Litingtung et al., 1998; Pepicelli et al., 1998). Inactivation of Shh or components in its signal transduction pathway, such as Gli2 and Gli3, gives rise to various degrees of lung and foregut malformations, with fusion of lung lobes, hypoplasia and esophageal atresia or stenosis, but the mechanism behind these morphogenetic defects is unknown (Litingtung et al., 1998; Motoyama et al., 1998; Park et al., 2000; Pepicelli et al., 1998). Mesenchymal genes, whose expression depends on hedgehog signaling have been identified. Several of these encode proteins that themselves are part of the hedgehog signaling pathway (such as Ptch, Gli1 and Gli3). Other target genes encode paracrine factors that are known to take part in morphogenesis and can either be activated, such as Wnt2, or repressed, like Fgf10, by Shh (Bellusci et al., 1997b; Lebeche et al., 1999; Litingtung et al., 1998; Pepicelli et al., 1998). However, in many cases it remains to be shown if the effect is direct or mediated by other secreted proteins. The forkhead genes Foxf1 (also known as FREAC1 or HFH8) and Foxf2 (also known as FREAC2 or LUN) encode
2 2398 M. Mahlapuu, S. Enerbäck and P. Carlsson two transcription factors that are closely related with regard to primary structure, DNA binding specificity and expression pattern, but with distinct activating properties (Clevidence et al., 1994; Hellqvist et al., 1998; Hellqvist et al., 1996; Mahlapuu et al., 1998; Pierrou et al., 1994). Both genes are expressed during organogenesis in mesenchymal tissue derived from splanchnic mesoderm and sclerotomes (Aitola et al., 2000; Mahlapuu et al., 2001; Mahlapuu et al., 1998; Peterson et al., 1997). In developing lung and in several other endodermally derived organs, Foxf1 and Foxf2 mrna is concentrated in subepithelial mesenchyme, which has prompted the speculation that their expression is induced by epithelial paracrine signaling (Aitola et al., 2000; Mahlapuu et al., 1998). We recently reported inactivation of Foxf1 by gene targeting (Mahlapuu et al., 2001). However, homozygous Foxf1-null mice degenerate at the early somite stage and die before organogenesis begins; hence, they do not provide any information on the role of Foxf1 in foregut or lung development. We show that Foxf1 is activated by Shh signaling and that, on a specific genetic background, Foxf1 haploinsufficiency gives rise to lung and foregut malformations similar to those observed in Shh and Gli mutants. MATERIALS AND METHODS Mouse mutants A targeting construct containing a total of 12 kb of the Foxf1 locus was made from 129/Sv genomic λ clones, in which the forkhead box of Foxf1 (from SstI to NotI) was replaced by a PGK-Neo cassette (Mahlapuu et al., 2001). The Foxf1 locus was targeted in two ES cell lines: RW4, derived from 129/SvJ, and E14, derived from 129/Ola. Germline transmission was obtained with clones derived from both cell lines and the targeted allele was maintained on mixed 129/Ola-C57Bl/6, 129/Ola-CD1, 129/Sv-CD1 and 129/Sv-C57Bl/6 backgrounds. Shh +/ mice (Chiang et al., 1996) were obtained from The Jackson Laboratory, Maine (Stock Shh<tm1>, #JR3318). In situ hybridization, immunohistochemistry, histology and skeletal preparations Pregnant CD1 mice were sacrificed at different stages of gestation. The embryonic lungs were dissected in phosphate-buffered saline containing 0.1% Tween-20. The unfixed tissue was photographed immediately. For in situ hybridization and immunohistochemistry embryonic lungs were fixed in 4% paraformaldehyde at 4 C. In situ hybridization of whole-mount embryos and cryosections (8 µm) or paraffin sections (3 µm) was performed as previously described (Blixt et al., 2000) using the following probes. The Foxf1 probe consists of a 400 bp NotI/KspI cdna fragment located immediately 3 of the forkhead box. Plasmids used to generate probes for uteroglobin (Utg; also known as Cc10 or Ccsp) and surfactant proteins A, B and C (Sftpa, Sftpb, Sftpc) were kindly provided by Dr B. Stripp; for patched (Ptch) by Dr M. P. Scott; for Foxa2 (HNF3β) and Bmp4 by Dr B. L. M. Hogan; and for sonic hedgehog (Shh) by Dr C. Betsholtz. The probe for vascular smooth muscle α-actin (Actvs; now known as Acta2 Mouse Genome Informatics) was generated from IMAGE cdna clone No Immunostaining was performed with antibodies to platelet/endothelial cell adhesion molecule (Pecam; Pharmingen, clone MEC 13.3). Antibody binding was detected with biotinylated secondary antibodies and HRPstreptavidine amplified by TSA TM Biotin System (NEN Life Science Products). Histological sections (3 µm, paraffin) were stained with Eosin and Hematoxylin. Bone and cartilage was visualized by staining with Alizarin Red and Alcian Blue (Hogan et al., 1994). Whole lung organ cultures Lung buds were dissected from E11.5 embryos and explants were placed on Millipore MF filters (Millipore), on top of stainless steel grids, in BGJb medium (Life Technologies) supplemented with 0.2 mg/ml ascorbic acid, 50 U/ml penicillin/streptomycin and 0.1% BSA. About 3 ml of medium was added to each 30 mm dish to establish an air-fluid interface at the level of the explants. The cultures were maintained in 5% CO 2, 100% humidity. Localized effects of fibroblast growth factors (FGFs) in lung explant cultures were tested by implanting heparin beads (Sigma) impregnated with human recombinant FGF10 (100 µg/ml, R & D) or FGF7 (100 µg/ml, R & D) into the mesenchyme of E11.5 lungs, as described (Lebeche et al., 1999). The effect of human recombinant transforming growth factor β1 (TGFβ1; 7.5 µg/ml, R & D), TGFβ2 (50 µg/ml, R & D), bone morphogenetic protein 4 (BMP4; 100 µg/ml, R & D) and epidermal growth factor (EGF; 100 µg/ml, Sigma) in lung cultures was tested by grafting Affi-gel blue beads (BioRad) soaked in recombinant protein. Explants were cultured for 24 hours and fixed for whole-mount in situ hybridization. Preparation of Shh-expressing cells COS-7 cells were transfected with either sense or antisense (control) Shh expression plasmids (kindly provided by Dr J. Ericson) using LipofectAMINE (Life Technologies). After 24 hours, transfected cells were trypsinized and plated in bacteriological grade Petri dishes to generate aggregates of cells. Cell aggregates were implanted into E11.5 lung explants in whole lung organ cultures. Explants were incubated for 24 hours and fixed for whole-mount in situ hybridization. RESULTS Survival of Foxf1 heterozygotes depends on genetic background A Foxf1-null mutant was created by homologous recombination in embryonic stem (ES) cells (Mahlapuu et al., 2001). Chimeric mice that passed the targeted allele through germline were generated with cell clones obtained from two different ES cell lines RW4 and E14 derived from different inbred strains of mice, 129/SvJ and 129/Ola. Offspring from matings between chimeric males and C57Bl/6 females had the expected allele frequencies, consistent with Mendelian inheritance of the Foxf1-null allele (48% heterozygotes, n=67; see also Table 1). This frequency remained stable (50%, n=117) when first generation heterozygous males were crossed with C57Bl/6 females. In contrast, when chimeras were mated with CD1 females the frequency of heterozygous offspring was only 14.6% (n=137), indicating that approximately 83% of heterozygotes died before or soon after birth. Crosses between Table 1. Genotype frequencies in offspring from crosses between Foxf1 heterozygous males and females with different genetic backgrounds Strain Generation Age +/+ +/ C57Bl/6 1 Born 35 (52%) 32 (48%) 2 Born 59 (50%) 58 (50%) CD1 1 Born 118 (86%) 19 (14%) 1 E (44%) 62 (56%) 2 Born 98 (93%) 7 (7%)
3 Foxf1, and lung and foregut malformations 2399 first generation survivor heterozygous males and CD1 females resulted in an even higher heterozygote mortality, estimated to be 93% (6.7% heterozygous pups, n=105). When fetuses were genotyped the day before birth, at E18.5, heterozygote frequency was normal (44%, n=110), which, together with the heterozygous genotype of dead newborn pups, showed that the low heterozygote frequency is due to perinatal mortality and not early resorbtion. Knockout lines established from the RW4 and E14 cell lines gave similar heterozygote frequencies and passing the targeted allele several generations through C57Bl/6 before crossing with CD1 did not alter this. The elevated perinatal mortality of Foxf1 heterozygotes thus appears to reflect a sensitivity to Foxf1 gene dose in the CD1 strain. fraction of Foxf1 heterozygotes survive birth and reach maturity, we conclude that the mutants suffer from lung hypoplasia combined with a general and variable delay in lung maturation. The abnormal lobation indicates defects in lung branching morphogenesis. In normal mouse development, the primordial lung buds and tracheal groove form around E9.5 as an epithelial outpouching from the ventral foregut, surrounded by splanchnic mesenchyme. The tracheo-esophageal septum divides the tracheal lumen and the foregut at E10.5, and by E11.5 trachea and esophagus are completely separated. The esophagus of E18.5 Foxf1 +/ mutants has a narrow lumen (Fig. 2A), frequently merges with the trachea and sometimes ends before Foxf1 heterozygotes suffer from respiratory failure and foregut malformations Anatomical and histological analysis of E18.5 Foxf1 heterozygous fetuses from CD1 mothers revealed multiple malformations in the foregut and respiratory tract. The lungs are small and pale with hemorrhagic lesions along the edges (Fig. 1A). Instead of the four separate lobes of a normal right lung (Fig. 1B), various degrees of fusion between lobes are seen and one of the lobes the accessory lobe is consistently missing (Fig. 1A). Lung differentiation appears retarded; in some fetuses, formation of alveoli has not begun at E18.5, the lung mesenchyme is compact and looks undifferentiated (Fig. 1C,E). In others the histology looks normal, despite the smaller size and lobation defects of the lungs. In situ hybridization of E18.5 lung sections with probes for genes expressed in proximal (Utg) and distal (Sftpa, Sftpb, Sftpc) lung epithelium gave similar results in wild-type and Foxf1 heterozygotes, which indicates that proximo-distal epithelial differentiation proceeds normally in the mutant (Fig. 1G-J and data not shown). No major alterations in lung vascularization could be detected, as judged by the expression pattern of markers for endothelial cells (platelet endothelial cell adhesion molecule type 1; Pecam1; Fig. 1K,M) and vascular smooth muscle cells (Actvs; Fig. 1L,N). However, a lower level of Actvs expression suggests that vascular smooth muscle differentiation may be hampered; something that could explain the hemorragic lesions in mutant lungs. Based on these results, and the fact that a Fig. 1. Defects in lung lobation and maturation in Foxf1 +/ embryos. (A,B) Ventral views of lungs from E18.5 wild-type and Foxf1 +/ fetuses. The mutant lungs (A) are small, pale and have hemorrhagic lesions along the edges (arrowhead). The accessory lobe of the right lung is missing and the remaining lobes are partially fused. (C-F) Hematoxylin and Eosin staining of transversal sections from E18.5 Foxf1 +/ (C,E) and wild-type (D,F) lungs. Low magnification view (C,D) shows the smaller size and denser appearance of the mutant lung. Higher magnification (E,F) reveals that the lax alveolar structure of the wild-type lung is replaced by compact mesenchyme in the Foxf1 mutant. (G-N) Differentiation of epithelial and mesenchymal cells in E18.5 Foxf1 +/ (G,H,K,L) and wild-type (I,J,M,N) lungs. In situ hybridization with probes for Utg (G,I), a marker for Clara cells of the proximal, bronchiolar epithelium, and Sftpc (H,J) expressed in the distal respiratory epithelium. Immunostaining of an endothelial cell marker (K,M; Pecam) and detection of vascular smooth muscle cells by in situ hybridization with a probe for Actvs (L,N). Ac, accessory lobe; Ar, arteriole; Br, bronchiole; Ca, caudal lobe; Cr, cranial lobe; LL, left lung; Me, medial lobe; RL, right lung.
4 2400 M. Mahlapuu, S. Enerbäck and P. Carlsson Fig. 2. Foregut, respiratory tract and skeletal malformations in Foxf1 +/ embryos. (A-C) Hematoxylin and Eosin stained transversal sections showing trachea (Tr) and esophagus (Es) of E18.5 Foxf1 +/ (A,C) and wild-type (B) fetuses. The mutants have variable degrees of narrowing of trachea and esophagus with esophageal atresia and a fistula-like fusion between esophagus and trachea. (D,E) Ventral view of Alcian Blue-stained tracheas from E18.5 Foxf1 +/ (D) and wild-type (E) fetuses. The C-shaped cartilaginous rings, which are segmentally arranged along the trachea (E), are replaced by irregular, hypoplastic patches of cartilage in Foxf1 +/ embryos (D; compare also B with A,C). (F,G) Skeletal malformation in Foxf1 +/ mice shown by Alizarin Red and Alcian Blue staining of E18.5 fetuses. Attachment of the ribs to the sternum is asymmetrical in the mutant, and the ossification centra in the sternebrae (red staining parts of the sternum) are misaligned at the midline (F). reaching the pharynx (esophageal atresia; Fig. 2C). The tracheal cartilage of Foxf1 heterozygotes is hypoplastic (Fig. 2A,C,D) and does not form the ventral rings typical of a normal trachea (Fig. 2B,E). The gastrointestinal tract posterior of the esophagus did not exhibit any anatomical or histological abnormalities. Examination of the skeleton revealed that ribs are asymmetrically attached to the sternum in Foxf1 mutant embryos (Fig. 2F). As a consequence, the bilateral ossification centra in the sternebrae are not aligned at the midline. Based on the observed defects, we conclude that respiratory failure caused by lung immaturity and inability to feed, as a result of constriction or atresia of the esophagus, contributes to elevated perinatal mortality of Foxf1 heterozygotes. To investigate at what stage lung hypoplasia and lobation defects are first evident in Foxf1 heterozygotes, we examined bud branching at the early stages of lung development. Lung epithelium was visualized by whole-mount in situ hybridization with a Foxa2 probe. At E11.5, the left and right Fig. 3. (A-D) Foxa2 whole-mount in situ hybridization of E11.5 (A,B) and E12.5 (C,D) Foxf1 +/ (A,C) and wild-type (B,D) lungs. Primary epithelial branches of the right lung are formed normally at E11.5 in the mutant, although with a tendency of the buds to be wider proximally and less well defined (A). At E12.5, growth of the lungs and epithelial branching are retarded in mutant embryos (C) compared with wild type (D). The mesenchyme surrounding the accessory lobe epithelial bud of the mutant right lung remains fused with that of the caudal lobe (C; arrowhead), while a separate accessory lobe has already formed in wild-type embryos (D). Ac, accessory lobe; Ca, caudal lobe; Cr, cranial lobe; LL, left lung; Me, medial lobe. lungs are separated by the primitive bronchi, and primary epithelial buds have developed that will give rise to the four lobes of the right lung (Fig. 3B). At this stage the branching pattern in Foxf1 heterozygotes is similar to that in wild type, but with a tendency to form less well defined epithelial buds that are wider at the base (Fig. 3A). A day later, at E12.5, the respiratory tree has branched extensively in the wild type and the four lobes of the right lung are clearly distinguishable (Fig. 3D). Branching in the Foxf1 mutant is considerably less advanced and separation of right lung lobes is incomplete (Fig. 3C). The mesenchyme surrounding the accessory lobe bud remains fused with the caudal lobe, which explains why a separate accessory lobe does not form (arrowhead in Fig. 3C). Foxf1 is a target for Shh signaling Foxf1 is expressed in the lung mesenchyme with the highest mrna levels found adjacent to the epithelium (Mahlapuu et al., 1998). The expression pattern and the fact that Foxf1 heterozygosity affects airway morphogenesis indicate that Foxf1 may take part in epithelio-mesenchymal crosstalk. Several observations suggest a functional connection between Foxf1 and Shh. Shh is expressed throughout the epithelium (Bitgood and McMahon, 1995), but high level expression is restricted to the distal epithelium, in the growing buds (Fig. 4A; Bellusci et al., 1996; Urase et al., 1996). Target genes for Shh signaling, such as Ptch, show a complementary expression pattern, which is most prominent in the mesenchyme that surrounds the distal epithelium (Fig. 4B; Bellusci et al., 1997a). To investigate if Foxf1 expression matches the sites of Shh secretion, we localized Foxf1 mrna in E11.5 and E12.5 lungs with whole-mount in situ hybridization. The results show that
5 Foxf1, and lung and foregut malformations 2401 Fig. 4. Foxf1 is a target for Sonic hedgehog signaling in lung. (A-C) Gene expression in E11.5 mouse lung examined by wholemount in situ hybridization. Shh (A) is expressed throughout the lung epithelium, but the highest expression level is restricted to distal epithelium in the bulbous ends of the buds. Ptch (B), encoding the Shh receptor, and Foxf1 (C) are both expressed in mesenchyme adjacent to lung epithelium. The expression patterns mirror the distribution of Shh, with low levels along the proximal tubular epithelium and higher levels distally. (D-G) Exogenous Shh activates Foxf1. COS-7 cells transfected to secrete Shh (D,F) or control COS-7 cells (E,G) were grafted into the mesenchyme (location of grafts indicated by white stars) of wild-type lung explants, which were then cultivated for 24 hours. The expression of Ptch (D,E) and Foxf1 (F,G) were examined by whole-mount in situ hybridization. The mesenchyme immediately adjacent to the Shh-producing cells express elevated levels of both Ptch (D) and Foxf1 (F), whereas no change is induced by control cells (E,G). although expression of Foxf1 is found in subepithelial mesenchyme throughout the airways, the highest level is confined to mesenchyme surrounding the distal epithelium of the buds (Fig. 4C). This pattern is similar to that of Ptch (Fig. 4B), which further supports the notion of a link between Shh signaling and Foxf1 expression. To determine if ectopic Shh secretion can activate Foxf1 in lung mesenchyme, we used in vitro cultures of wild-type lung explants. COS-7 cells, transfected to secrete Shh, were implanted into the mesenchyme and the effects on expression of Foxf1 was monitored by in situ hybridization. The expression of Ptch was also determined as an example of a mesenchymal target gene known to be activated by Shh. After 24 hours incubation, a zone of enhanced Ptch (Fig. 4D) and Foxf1 (Fig. 4F) expression was seen surrounding the Shhsecreting cells, whereas no change in expression of either gene could be detected when control COS-7 cells were used (Fig. 4E,G). This result shows that transcriptional activation of Foxf1 is a response of the lung mesenchyme to signaling by extracellular Shh. If Foxf1 transcription in lung mesenchyme is activated by Shh signaling, absence of Shh secretion from the endoderm should lead to downregulation of Foxf1. To test this, we investigated Foxf1 expression in Shh / embryos. This mutant suffers from holoprosencephaly, cyclopia and defects in hair follicle, floorplate and limb development (Fig. 5G; Chiang et al., 1996; Litingtung et al., 1998; Pepicelli et al., 1998; St- Jacques et al., 1998). Lung growth and branching are retarded and mesenchymal expression of the Shh responsive genes Ptch, Gli1, Gli3 and Wnt2 is reduced (Litingtung et al., 1998; Pepicelli et al., 1998). The mesenchymal coating of the epithelium is thinner than in wild-type lungs (Fig. 5H; Litingtung et al., 1998; Pepicelli et al., 1998), consistent with Shh being a mesenchymal mitogen (Bellusci et al., 1997a). However, the mutant lung mesenchyme has a more widespread expression of Fgf10 (Litingtung et al., 1998; Pepicelli et al., 1998), which is in agreement with other reports showing that Shh is a negative regulator of this gene (Bellusci et al., 1997b; Lebeche et al., 1999). Whole-mount in situ hybridization showed that in E10.5 and E12.5 Shh / embryos, Foxf1 mrna is absent from several of its normal sites of expression, including sclerotomes and mesenchyme of trachea, esophagus, oral cavity and lungs (Fig. 5). The midgut and hindgut, however, retain Foxf1 expression pattern in Shh mutants (Fig. 5F,G). The lack of Foxf1 expression in the lung mesenchyme and the splanchnic mesenchyme surrounding the foregut of E12.5 Shh / embryos, confirms that Shh signaling is required for activation of Foxf1 in these tissues. Foxf1 expression is downregulated by BMP4 A number of paracrine factors have been described to participate in the epithelio-mesenchymal crosstalk that controls morphogenesis and gene expression during early lung development (see Hogan, 1999; Hogan and Yingling, 1998). In order to identify other potential regulators of Foxf1, we investigated the effect of candidate growth factors on the Foxf1 expression pattern in in vitro cultures of lung explants. BMP4 is secreted by the distal epithelium of lung buds and inhibits epithelial branching and proliferation (Bellusci et al., 1996; Weaver et al., 1999). The distribution of Foxf1 mrna, which is lower adjacent to the Bmp4-expressing epithelium at the distal face of the bud than in a zone just behind the tip (compare Fig. 6B,D,H with Fig. 6F; see also Fig. 7B), suggests BMP4 as a potential negative regulator of Foxf1. To investigate the effect of BMP4 on Foxf1 expression, we implanted BMP4- saturated beads in the mesenchyme of lung explants. As shown in Fig. 6A, exogenous BMP4 from a bead decreases Foxf1 expression in the surrounding mesenchyme. Epithelial BMP4 is thus likely to act as a negative regulator of mesenchymal Foxf1 expression. No change in Foxf1 mrna level or distribution was induced by TGFβ1 or TGFβ2, two additional members of the TGFβ superfamily that have been implicated in lung morphogenesis (Lebeche et al., 1999; Zhao et al., 1996), or by EGF, which is also essential for normal lung development (Miettinen et al., 1997; data not shown). FGF10 and FGF7 are secreted by mesenchymal cells and signal through the FGFR2-IIIb receptor in the epithelium (Arman et al., 1999; De Moerlooze et al., 2000; Igarashi et al., 1998). As mesenchymal factors, FGFs are less likely to regulate Foxf1 directly, but may do so indirectly through effects on epithelial gene expression. When added exogenously, in implanted beads, both FGF10 and FGF7 stimulate epithelial
6 2402 M. Mahlapuu, S. Enerbäck and P. Carlsson Fig. 5. Foregut and sclerotome expression of Foxf1 depends on Shh. Foxf1 whole-mount in situ hybridization of wild-type (A-D) and Shh / (E- H) E10.5 (A,B,E,F) or E12.5 (C,G) embryos and E12.5 lungs (D,H). In Shh mutants, Foxf1 expression is missing in the anterior part of the gut, the oral cavity, in sclerotomes and lungs. Foxf1 mrna persists only in the posterior part of the gut, where Ihh is expressed. Embryos in A,E are in aqueous solution, to visualize the general morphology. Embryos in B,C,F,G are clarified in benzylalcohol:benzylacetate to show staining of internal structures. In, intestine; Li, liver; Lu, lung; OC, oral cavity; Sc, sclerotome. proliferation and bud dilation, whereas the expression of Foxf1 in subepithelial mesenchyme next to the bead decreases (Fig. 6C,G). FGF7 downregulates Foxf1 in a wide area around the bead, adjacent to both proximal and distal epithelium (Fig. 6G). FGF7 also reduces expression of Shh in the epithelium (Fig. 6K; Lebeche et al., 1999) and Ptch in the mesenchyme (Fig. 6I). In the area influenced by exogenous FGF10, Foxf1 is downregulated in the regions where it is normally expressed at high levels next to distal epithelium but the basal expression along the proximal, tubular epithelium is not affected (Fig. 6C). Distal epithelium also responds to exogenous FGF10 by activating Bmp4 (Fig. 6E; Lebeche et al., 1999; Weaver et al., 2000). DISCUSSION Foxf1 / embryos are deformed already at E8.5, never turn and die around E9.5, before organogenesis begins in the foregut (Mahlapuu et al., 2001). They cannot therefore be used to investigate the role of Foxf1 in lung and gut morphogenesis. However, the heterozygous phenotype described here gives some insight into the normal function of Foxf1 in this process. The fact that two independent targeted lines of mice, derived from ES cells of different genetic origin, produced the same phenotype and that the heterozygote frequency did not normalize during breeding, show that the observed defects are caused by the knockout. Foxf1 haploinsufficiency depends on genetic background, which suggests that either the expression level or the threshold concentration of Foxf1 required for normal development varies between the tested strains. Although the nature of this variability is not known, the observed defects demonstrate the importance of Foxf1 for normal foregut and lung development and also suggest links to upstream regulators. The malformations seen in Foxf1 heterozygotes bear a striking resemblance to those described for mutants in the Shh signaling pathway. Mice homozygous for null mutations in Shh have foregut defects with esophageal atresia or tracheoesophageal fistula; lung growth is retarded and branching morphogenesis is impaired, but proximo-distal differentiation of the airway epithelium is unaffected (Litingtung et al., 1998; Pepicelli et al., 1998). Gli transcription factors are expressed in the lung mesenchyme and are nuclear targets for hedgehog signaling transduced from the patched/smoothened receptor (Dominguez et al., 1996; Goodrich et al., 1996; Tabin and McMahon, 1997). Mutations in genes for Gli proteins give rise to various lung and foregut defects. The Gli2 knockout mice have narrowing of esophagus and trachea, tracheal cartilage dysplasia, small lungs with right lung lobe fusion and lack of accessory lobe (Motoyama et al., 1998). Heterozygosity for Gli3 in a Gli2 / background adds esophageal atresia with tracheo-esophageal fistula to the malformations and aggravates the lung hypoplasia (Motoyama et al., 1998). The similarities
7 Foxf1, and lung and foregut malformations 2403 Fig. 6. Foxf1 expression is downregulated by BMP4 and indirectly by FGF7 and FGF10. Beads soaked in BMP4 (A), FGF10 (C,E) or FGF7 (G,I,K) were implanted in the mesenchyme of wildtype lung explants, which were then cultivated for 24 hours. BSA-soaked beads served as a controls (B,D,F,H,J,L). Expression of Foxf1 (A-D,G,H), Bmp4 (E,F), Ptch (I,J) and Shh (K,L) was analyzed by whole-mount in situ hybridization. BMP4 reduces Foxf1 expression in mesenchyme adjacent to the bead (A). FGF10 attracts distal epithelium and stimulates its proliferation, which is seen as dilation of the bulbous part of buds close to the bead (C). The patches of elevated Foxf1 expression next to distal epithelium (D) are lost in the area influenced by exogenous FGF10 (C), whereas no effect is seen on the low level expression in mesenchyme along the proximal, tubular epithelium (C). A likely explanation for this inhibition is that it is mediated by BMP4, as Bmp4 is activated by FGF10 in distal, but not in proximal epithelium (E). FGF7 induces an even more pronounced epithelial proliferation and bud expansion (G,I,K). Foxf1 is generally downregulated in the area influenced by FGF7 (G), which may be mediated by a decreased Shh signaling, as epithelial Shh expression is reduced by FGF7 (K). This interpretation is supported by the similarity in the effect of FGF7 on Foxf1 and another Shh-responsive gene, Ptch (I). Fig. 7. Summary of paracrine interactions shaping the Foxf1 expression pattern in developing lung. (A) Activation of Foxf1 by Shh is based on both ectopic expression and loss-of-function experiments presented in this work. The inclusion of Gli2/3 in this pathway is inferred from their role in transducing the hedgehog signal and the similarity between Gli2/3 knockout phenotypes and the Foxf1 heterozygote phenotype. Inhibition by BMP4 refers to the effect of BMP4 beads on Foxf1 expression presented here. The role of FGF10 and FGF7 in regulation of Bmp4 and Shh, respectively, has been published by others (Lebeche et al., 1999) and has also been confirmed by us; the net effect of FGFs on Foxf1 expression was analyzed in this work. (B) High magnification view of lung explant hybridized with a Foxf1 probe. Foxf1 expression in subepithelial mesenchyme peaks at the transition between the proximal, tubular epithelium the distal, bulbous part of the bud. The circular object is a bead implanted in the mesenchyme as a negative control and has no relevance for the expression pattern discussed here. (C) Model of paracrine signaling in a lung bud that provides a possible explanation for the observed distribution of Foxf1 mrna. Proximal tubular epithelium secretes a low level of Shh (pink), which gives rise to a low level of Foxf1 expression (light blue) in the subepithelial mesenchyme. Shh secretion is higher in the distal epithelium (red), which activates high level expression of Foxf1 (dark blue). Fgf10 is expressed in mesenchyme at the distal tip of the bud (black). In response to FGF10 signaling, Bmp4 is activated in the most distal epithelium (green). Secretion of BMP4 inhibits Foxf1 expression, which therefore drops again towards the tip of the bud (light blue).
8 2404 M. Mahlapuu, S. Enerbäck and P. Carlsson of the mutant phenotypes suggest that Shh, Gli and Foxf1 are parts of the same signaling pathway. The mesenchymal expression of Foxf1, its activation by ectopic Shh secretion and loss of expression in Shh / lung bud mesenchyme place Foxf1 downstream of Shh. Ci, the Drosophila homolog of mammalian Gli genes, encodes a protein whose activity as transcription factor is regulated directly by hedgehog signaling (Dominguez et al., 1996). It is therefore likely that Foxf1 is downstream of Gli in mammals and perhaps directly transcriptionally activated by Gli proteins (Fig. 7A). Shh is expressed in axial structures, floorplate and notochord (Echelard et al., 1993), where it is involved in somite patterning and formation of sclerotomes from cells of the ventromedial part of the somites (Fan et al., 1995; Fan and Tessier-Lavigne, 1994; Johnson et al., 1994). In Shh / embryos that develop to term, most sclerotomal derivatives, including the entire vertebral column, are absent (Chiang et al., 1996). However, sclerotomal differentiation in Shh mutant embryos is initiated normally, as judged by expression of the sclerotomal marker Pax1 (Chiang et al., 1996). Based on this, Shh is postulated to be essential for the maintenance, but not the initiation, of sclerotome differentiation (Chiang et al., 1996; Marcelle et al., 1999). Foxf1 is not expressed in somites, but is turned on in sclerotomal cells as these separate from the dermomyotome (Mahlapuu et al., 2001). As development of the axial skeleton proceeds, Foxf1 is turned off in the condensed, chondrogenic mesenchyme of the future vertebra and remains expressed in the gradually narrowing intervertebral discs (Fig. 5B,C; Aitola et al., 2000; Mahlapuu et al., 2001; Mahlapuu et al., 1998). In Shh / embryos, no sclerotomal expression of Foxf1 is observed, which is consistent with our conclusion that Foxf1 is a target for Shh signaling. This also shows that although Pax1 is induced normally in the Shh mutant and apparently can be activated by a Shh-independent pathway, other sclerotomal markers such as Foxf1 are not. Thus, Shh is necessary for specific aspects of sclerotome induction and not just for maintenance. The absence of Foxf1 expression in sclerotomes and in mesenchyme surrounding the foregut epithelium and lung buds of Shh / embryos, indicates that transcriptional activation of Foxf1 is a response to Shh signaling in these tissues. In contrast, the midgut and hindgut expression of Foxf1 is normal in the Shh mutant. The expression range of Foxf1 in gut mesenchyme of Shh / embryos exactly matches the expression of another member of the hedgehog family, Ihh. Ihh signals via the same receptor as Shh and is expressed in gut epithelium from the hindstomach to the rectum (Bitgood and McMahon, 1995). It is thus possible that activation by hedgehog signaling is a general requirement for Foxf1 expression. Indeed, throughout embryonic development, expression of Foxf1 correlates with sites of hedgehog secretion. In addition to the gut, lungs and sclerotomes, Foxf1 is also expressed in the subepithelial mesenchyme of the oral cavity, the tongue and dental follicles (Aitola et al., 2000; Mahlapuu et al., 2001; Mahlapuu et al., 1998), all of which are proximal to sites of epithelial Shh production (Bitgood and McMahon, 1995). At the early somite stage, Foxf1 is expressed in extra-embryonic mesoderm, including the yolk sac blood islands (Mahlapuu et al., 2001; Peterson et al., 1997); an expression pattern that it shares with Ptch (Maye et al., 2000) and which matches the secretion of Ihh from the yolk sac endoderm (Farrington et al., 1997; Maye et al., 2000). In the patterning of primitive mesoderm, Foxf1 expression characterizes the lateral plate mesoderm, which is adjacent to regions of visceral endoderm that produce Shh and Ihh (Echelard et al., 1993). Asymmetry of rib attachment and in ossification of the sternum is a common malformation in Foxf1 heterozygotes. This represents an exaggerated version of the crankshaft sternum (Theiler, 1989), which can also be caused by inactivation of engrailed 1 (Wurst et al., 1994) and by experimental amniotic sac puncture (Chang et al., 1996). The connection between Foxf1 gene dose and sternal morphogenesis is not clear, as Foxf1 is expressed neither in the sternal, nor the costal, primordia. As discussed above, Foxf1 is expressed in sclerotomal mesenchyme and is turned off during chondroblast differentiation (Aitola et al., 2000; Mahlapuu et al., 2001; Mahlapuu et al., 1998). It is possible that a reduced expression level of Foxf1 could induce a subtle vertebral asymmetry that causes the left and right costal processes to grow at slightly different angles. Another possibility is that the composition of the amniotic fluid is altered in response to a lowered Foxf1 expression. Foxf1 is expressed in amnion (Peterson et al., 1997) and is essential for its development and function; in homozygous Foxf1 knockout embryos the mesodermal component of the amnion has severe defects in differentiation and cell adhesion (Mahlapuu et al., 2001). Finally, the skeletal defects may be secondary to the altered chest morphology that is a consequence of a considerable reduction in lung volume. Bmp4 is expressed in the distal face of the epithelial buds of the developing lung and is induced by FGF10 signaling from the adjacent mesenchyme (Lebeche et al., 1999; Weaver et al., 2000). The stimulation of epithelial cell proliferation, caused by FGF10, is counteracted by BMP4 (Weaver et al., 2000). Locally applied BMP4 has an inhibitory effect on mesenchymal Foxf1 expression in lung explants. Lowering the Foxf1 expression by reducing its gene dose, as in Foxf1 heterozygotes, may therefore have similar effects as an increase in Bmp4 expression. In fact, epithelial overexpression of Bmp4 in transgenic mice results in lung hypoplasia, inhibition of branching, and pulmonary immaturity at birth (Bellusci et al., 1996). FGF10 is produced by groups of mesenchymal cells located distally of the epithelial buds (Bellusci et al., 1997b) and activates Bmp4 expression in the adjacent epithelium. It functions as a mitogen and a chemoattractant for epithelial cells and induce bud dilation when provided exogenously (Bellusci et al., 1997b; Park et al., 1998). Fgf10 is essential for lung bud formation and branching; Fgf10 / embryos develop trachea, but no lungs (Min et al., 1998; Sekine et al., 1999). In response to implantation of a bead containing FGF10, mesenchymal cells adjacent to bud epithelium express less Foxf1. A likely mechanism behind the decrease in Foxf1 expression in response to FGF10 is enhanced inhibition by BMP4, as Bmp4 expression increases in bud epithelium next to the bead (Fig. 6A). This interpretation is supported by the observation that Foxf1 expression is only downregulated in the sites of high expression, next to the bud epithelium. Along the proximal, tubular epithelium of the future bronchi, Foxf1 expression is normal. This is exactly the pattern that would be expected if the effect was mediated by BMP4, as, unless the mesenchyme is removed experimentally, FGF10 activates
9 Foxf1, and lung and foregut malformations 2405 Bmp4 only in bud epithelium, not in proximal tubular epithelium (Lebeche et al., 1999; Weaver et al., 2000). A second FGF family member, FGF7, is also produced by the lung mesenchyme. The effect on epithelium induced by FGF7 is distinct from that of FGF10, although both factors signal through the same receptor, FGFR2-IIIb (Arman et al., 1999; De Moerlooze et al., 2000; Igarashi et al., 1998). Shh expression is inhibited by FGF7, but not by FGF10 (Lebeche et al., 1999). Locally applied exogenous FGF7 decreases Foxf1 expression. As Shh activates Foxf1, decreased activation by Shh signaling is a plausible mechanism behind the reduced expression of Foxf1 seen in response to exogenous FGF7. A reduction in Ptch expression similar to that observed for Foxf1 in the area influenced by FGF7 is consistent with this interpretation (Fig. 6I; Lebeche et al., 1999). The paracrine interactions thought to influence Foxf1 expression in the lung are summarized in Fig. 7A. A low level of Foxf1 mrna is found in a zone of mesenchyme that lines the proximal, tubular epithelium of the developing lung (Fig. 7B); an expression pattern that matches the basal level of Shh expression in this part of the epithelial tree. In the buds, the expression level of Shh is significantly higher, which will upregulate Foxf1 in the surrounding mesenchyme. At the same time, BMP4 secreted from the distal epithelium of the bud will inhibit Foxf1 expression. However, whereas Bmp4 expression is confined to the most distal part of the bud, Shh expression is elevated in the entire bulbous part and thus extends further proximally. These interactions may explain the observed distribution of Foxf1 mrna, which peaks at the transition between the distal, bulbous and the proximal, tubular epithelium and then falls back to a lower level along the outer, distal face of the bud (Fig. 7B,C). An important question that remains to be answered is what target genes are regulated by Foxf1 and the role that it plays in formation of the lung. The expression pattern suggests that Foxf1 could be involved in reshaping the growing bud into a narrower, tubular shape (Fig. 7B). Heterozygous lungs exhibit a general growth retardation, which suggests that Foxf1 promotes proliferation. Shh is a mesenchymal mitogen (Bellusci et al., 1997a) and this function may be mediated by Foxf1. The notion that Foxf1 can be involved in growth control is supported by the observation that proliferation of primitive streak mesoderm is reduced in Foxf1 / embryos (Mahlapuu et al., 2001). Their phenotypical similarity with Foxf1 heterozygotes suggests that Shh, Gli2 and Gli3 mutants owe many of their malformations to a reduction in Foxf1 expression, thus implicating Foxf1 as a key target of hedgehog signaling in lung and foregut morphogenesis. Although the phenotype depends on genetic background, it shows that Foxf1 gene dose can be crucial for developmental processes. In humans, foregut malformations including esophageal atresia, tracheo-esophageal fistula and lung anomalies occur in 1 in live births (Skandalakis and Gray, 1994). The human FOXF1 gene, located at 16q24 (Larsson et al., 1995), should be considered a candidate locus in familial cases of these malformations. We would like to thank Drs B. L. M. Hogan, B. Stripp, M. P. Scott, J. Ericson and C. Betsholtz for plasmid clones and Amel Linde for technical advice on organ culture. This work was supported by a grant to P. C. from the Swedish Cancer Foundation. REFERENCES Aitola, M., Carlsson, P., Mahlapuu, M., Enerbäck, S. and Pelto-Huikko, M. (2000). Forkhead transcription factor FoxF2 is expressed in mesodermal tissues involved in epithelio-mesenchymal interactions. Dev. Dyn. 18, Arman, E., Haffner-Krausz, R., Gorivodsky, M. and Lonai, P. (1999). Fgfr2 is required for limb outgrowth and lung-branching morphogenesis. Proc. Natl. Acad. Sci. USA 96, Aza-Blanc, P. and Kornberg, T. B. (1999). Ci: a complex transducer of the hedgehog signal. Trends Genet. 15, Bellusci, S., Henderson, R., Winnier, G., Oikawa, T. and Hogan, B. L. (1996). Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development 122, Bellusci, S., Furuta, Y., Rush, M. G., Henderson, R., Winnier, G. and Hogan, B. L. (1997a). Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development 124, Bellusci, S., Grindley, J., Emoto, H., Itoh, N. and Hogan, B. L. (1997b). Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124, Bitgood, M. J. and McMahon, A. P. (1995). Hedgehog and Bmp genes are coexpressed at many diverse sites of cell- cell interaction in the mouse embryo. Dev. Biol. 172, Blixt, Å., Mahlapuu, M., Aitola, M., Pelto-Huikko, M., Enerbäck, S. and Carlsson, P. (2000). A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 14, Chang, H. H., Schwartz, Z. and Kaufman, M. H. (1996). Limb and other postcranial skeletal defects induced by amniotic sac puncture in the mouse. J. Anat. 189, Chiang, C., Litingtung, Y., Lee, E., Young, K. E., Corden, J. L., Westphal, H. and Beachy, P. A. (1996). Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, Clevidence, D. E., Overdier, D. G., Peterson, R. S., Porcella, A., Ye, H., Paulson, K. E. and Costa, R. H. (1994). Members of the HNF-3/forkhead family of transcription factors exhibit distinct cellular expression patterns in lung and regulate the surfactant protein B promoter. Dev. Biol. 166, De Moerlooze, L., Spencer-Dene, B., Revest, J., Hajihosseini, M., Rosewell, I. and Dickson, C. (2000). An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 127, Dominguez, M., Brunner, M., Hafen, E. and Basler, K. (1996). Sending and receiving the hedgehog signal: control by the Drosophila Gli protein Cubitus interruptus. Science 272, Echelard, Y., Epstein, D. J., St-Jacques, B., Shen, L., Mohler, J., McMahon, J. A. and McMahon, A. P. (1993). Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, Fan, C. M., Porter, J. A., Chiang, C., Chang, D. T., Beachy, P. A. and Tessier-Lavigne, M. (1995). Long-range sclerotome induction by sonic hedgehog: direct role of the amino-terminal cleavage product and modulation by the cyclic AMP signaling pathway. Cell 81, Fan, C. M. and Tessier-Lavigne, M. (1994). Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell 79, Farrington, S. M., Belaoussoff, M. and Baron, M. H. (1997). Winged-helix, Hedgehog and Bmp genes are differentially expressed in distinct cell layers of the murine yolk sac. Mech. Dev. 62, Goodrich, L. V., Johnson, R. L., Milenkovic, L., McMahon, J. A. and Scott, M. P. (1996). Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev. 10, Hellqvist, M., Mahlapuu, M., Samuelsson, L., Enerbäck, S. and Carlsson, P. (1996). Differential activation of lung-specific genes by two forkhead proteins, FREAC-1 and FREAC-2. J. Biol. Chem. 271, Hellqvist, M., Mahlapuu, M., Blixt, Å., Enerbäck, S. and Carlsson, P. (1998). The human forkhead protein FREAC-2 contains two functionally redundant activation doamins and interacts with TBP and TFIIB. J. Biol. Chem. 273, Hogan, B., Beddington, R., Costantini, F. and Lacy, E. (1994). Manipulating the Mouse Embryo: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press. Hogan, B. L. (1999). Morphogenesis. Cell 96,
10 2406 M. Mahlapuu, S. Enerbäck and P. Carlsson Hogan, B. L. and Yingling, J. M. (1998). Epithelial/mesenchymal interactions and branching morphogenesis of the lung. Curr. Opin. Genet. Dev. 8, Igarashi, M., Finch, P. W. and Aaronson, S. A. (1998). Characterization of recombinant human fibroblast growth factor (FGF)-10 reveals functional similarities with keratinocyte growth factor (FGF-7). J. Biol. Chem. 273, Johnson, R. L., Laufer, E., Riddle, R. D. and Tabin, C. (1994). Ectopic expression of Sonic hedgehog alters dorsal-ventral patterning of somites. Cell 79, Larsson, C., Hellqvist, M., Pierrou, S., White, I., Enerbäck, S. and Carlsson, P. (1995). Chromosomal localization of six human forkhead genes, freac-1, -3, -4, -5, -6 and -8. Genomics 30, Lebeche, D., Malpel, S. and Cardoso, W. V. (1999). Fibroblast growth factor interactions in the developing lung. Mech. Dev. 86, Litingtung, Y., Lei, L., Westphal, H. and Chiang, C. (1998). Sonic hedgehog is essential to foregut development. Nat. Genet. 20, Mahlapuu, M., Pelto-Huikko, M., Aitola, M., Enerbäck, S. and Carlsson, P. (1998). FREAC-1 contains a cell type-specific transcriptional activation domain and is expressed in epithelial-mesenchymal interfaces. Dev. Biol. 202, Mahlapuu, M., Ormestad, M., Enerbäck, S. and Carlsson, P. (2001). The forkhead transcription factor Foxf1 is required for differentiation of extraembryonic and lateral plate mesoderm. Development 128, Marcelle, C., Ahlgren, S. and Bronner-Fraser, M. (1999). In vivo regulation of somite differentiation and proliferation by Sonic Hedgehog. Dev. Biol. 214, Maye, P., Becker, S., Kasameyer, E., Byrd, N. and Grabel, L. (2000). Indian hedgehog signaling in extraembryonic endoderm and ectoderm differentiation in ES embryoid bodies. Mech. Dev. 94, McMahon, A. P. (2000). More surprises in the Hedgehog signaling pathway. Cell 100, Miettinen, P. J., Warburton, D., Bu, D., Zhao, J. S., Berger, J. E., Minoo, P., Koivisto, T., Allen, L., Dobbs, L., Werb, Z. et al. (1997). Impaired lung branching morphogenesis in the absence of functional EGF receptor. Dev. Biol. 186, Min, H., Danilenko, D. M., Scully, S. A., Bolon, B., Ring, B. D., Tarpley, J. E., DeRose, M. and Simonet, W. S. (1998). Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 12, Motoyama, J., Liu, J., Mo, R., Ding, Q., Post, M. and Hui, C. C. (1998). Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat. Genet. 20, Park, W. Y., Miranda, B., Lebeche, D., Hashimoto, G. and Cardoso, W. V. (1998). FGF-10 is a chemotactic factor for distal epithelial buds during lung development. Dev. Biol. 201, Park, H. L., Bai, C., Platt, K. A., Matise, M. P., Beeghly, A., Hui, C. C., Nakashima, M. and Joyner, A. L. (2000). Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 127, Pepicelli, C. V., Lewis, P. M. and McMahon, A. P. (1998). Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr. Biol. 8, Peterson, R. S., Lim, L., Ye, H., Zhou, H., Overdier, D. G. and Costa, R. H. (1997). The winged helix transcriptional activator HFH-8 is expressed in the mesoderm of the primitive streak stage of mouse embryos and its cellular derivatives. Mech. Dev. 69, Pierrou, S., Hellqvist, M., Samuelsson, L., Enerbäck, S. and Carlsson, P. (1994). Cloning and characterization of seven human forkhead proteins: binding site specificity and DNA bending. EMBO J. 13, Sekine, K., Ohuchi, H., Fujiwara, M., Yamasaki, M., Yoshizawa, T., Sato, T., Yagishita, N., Matsui, D., Koga, Y., Itoh, N. et al. (1999). Fgf10 is essential for limb and lung formation. Nat. Genet. 21, Skandalakis, J. E. and Gray, S. W. (1994). Embryology for Surgeons. Baltimore, MD: Williams & Wilkins. St-Jacques, B., Dassule, H. R., Karavanova, I., Botchkarev, V. A., Li, J., Danielian, P. S., McMahon, J. A., Lewis, P. M., Paus, R. and McMahon, A. P. (1998). Sonic hedgehog signaling is essential for hair development. Curr. Biol. 8, Tabin, C. J. and McMahon, A. P. (1997). Recent advances in Hedgehog signaling. Trends Cell Biol. 7, Theiler, K. (1989). The House Mouse: Atlas of Embryonic Development. New York: Springer-Verlag. Urase, K., Mukasa, T., Igarashi, H., Ishii, Y., Yasugi, S., Momoi, M. Y. and Momoi, T. (1996). Spatial expression of Sonic hedgehog in the lung epithelium during branching morphogenesis. Biochem. Biophys. Res. Commun. 225, Weaver, M., Dunn, N. R. and Hogan, B. L. (2000). Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development 127, Weaver, M., Yingling, J. M., Dunn, N. R., Bellusci, S. and Hogan, B. L. (1999). Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development 126, Wessells, N. K. (1970). Mammalian lung development: interactions in formation and morphogenesis of tracheal buds. J. Exp. Zool. 175, Wurst, W., Auerbach, A. B. and Joyner, A. L. (1994). Multiple developmental defects in Engrailed-1 mutant mice: an early mid-hindbrain deletion and patterning defects in forelimbs and sternum. Development 120, Zhao, J., Bu, D., Lee, M., Slavkin, H. C., Hall, F. L. and Warburton, D. (1996). Abrogation of transforming growth factor-beta type II receptor stimulates embryonic mouse lung branching morphogenesis in culture. Dev. Biol. 180,
Review From fruitflies to mammals: mechanisms of signalling via the Sonic hedgehog pathway in lung development Minke van Tuyl* and Martin Post*
 Review From fruitflies to mammals: mechanisms of signalling via the Sonic hedgehog pathway in lung development Minke van Tuyl* and Martin Post* *Hospital for Sick Children Research Institute, Toronto,
Review From fruitflies to mammals: mechanisms of signalling via the Sonic hedgehog pathway in lung development Minke van Tuyl* and Martin Post* *Hospital for Sick Children Research Institute, Toronto,
Branching morphogenesis of the lung: new molecular insights into an old problem
 86 Review TRENDS in Cell Biology Vol.13 No.2 February 2003 Branching morphogenesis of the lung: new molecular insights into an old problem Pao-Tien Chuang 1 and Andrew P. McMahon 2 1 Cardiovascular Research
86 Review TRENDS in Cell Biology Vol.13 No.2 February 2003 Branching morphogenesis of the lung: new molecular insights into an old problem Pao-Tien Chuang 1 and Andrew P. McMahon 2 1 Cardiovascular Research
Development of Respiratory System. Dr. Sanaa Alshaarawy& Dr. Saeed Vohra
 Development of Respiratory System Dr. Sanaa Alshaarawy& Dr. Saeed Vohra OBJECTIVES At the end of the lecture the students should be able to: Identify the development of the laryngeotracheal (respiratory)
Development of Respiratory System Dr. Sanaa Alshaarawy& Dr. Saeed Vohra OBJECTIVES At the end of the lecture the students should be able to: Identify the development of the laryngeotracheal (respiratory)
Histology and development of the respiratory system
 Histology and development of the respiratory system Árpád Dobolyi Semmelweis University, Department of Anatomy, Histology and Embryology Outline of the lecture 1. Structure of the trachea 2. Histology
Histology and development of the respiratory system Árpád Dobolyi Semmelweis University, Department of Anatomy, Histology and Embryology Outline of the lecture 1. Structure of the trachea 2. Histology
Vertebrate Limb Patterning
 Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Vertebrate Limb Patterning What makes limb patterning an interesting/useful developmental system How limbs develop Key events in limb development positioning and specification initiation of outgrowth establishment
Lecture 21Development of respiratory system Dr. Rehan Asad At the end of session students should able to Describe formation of lung buds Describe
 Lecture 21Development of respiratory system Dr. Rehan Asad At the end of session students should able to Describe formation of lung buds Describe development of larynx, trachea and bronchi. Describe the
Lecture 21Development of respiratory system Dr. Rehan Asad At the end of session students should able to Describe formation of lung buds Describe development of larynx, trachea and bronchi. Describe the
Week 14. Development of the Musculoskeletal System
 Week 14 Development of the Musculoskeletal System Skeletal System Derived from: paraxial mesoderm somites and somitomeres sclerotome sclerotome differentiation induced by SHH from notochord and floor plate
Week 14 Development of the Musculoskeletal System Skeletal System Derived from: paraxial mesoderm somites and somitomeres sclerotome sclerotome differentiation induced by SHH from notochord and floor plate
Tetrapod Limb Development
 IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
IBS 8102 Cell, Molecular and Developmental Biology Tetrapod Limb Development February 11, 2008 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Patterning
Development of the Axial Skeleton and Limbs. Professor Alfred Cuschieri Department of Anatomy University of Malta
 Development of the Axial Skeleton and Limbs Professor Alfred Cuschieri Department of Anatomy University of Malta During the Fourth Week the Embryo Is Segmented. Each segment consists of: a segment of neural
Development of the Axial Skeleton and Limbs Professor Alfred Cuschieri Department of Anatomy University of Malta During the Fourth Week the Embryo Is Segmented. Each segment consists of: a segment of neural
SONIC HEDGEHOG (Shh) is a secreted glycoprotein
 Role of Sonic Hedgehog in the Development of the Trachea and Oesophagus By Adonis S. Ioannides, Deborah J. Henderson, Lewis Spitz, and Andrew J. Copp London, England and Newcastle, England Backround/Purpose:
Role of Sonic Hedgehog in the Development of the Trachea and Oesophagus By Adonis S. Ioannides, Deborah J. Henderson, Lewis Spitz, and Andrew J. Copp London, England and Newcastle, England Backround/Purpose:
Midgut. Over its entire length the midgut is supplied by the superior mesenteric artery
 Gi Embryology 3 Midgut the midgut is suspended from the dorsal abdominal wall by a short mesentery and communicates with the yolk sac by way of the vitelline duct or yolk stalk Over its entire length the
Gi Embryology 3 Midgut the midgut is suspended from the dorsal abdominal wall by a short mesentery and communicates with the yolk sac by way of the vitelline duct or yolk stalk Over its entire length the
Skeletal System. Prof. Dr. Malak A. Al-yawer Department of Anatomy/Embryology Section
 Skeletal System Prof. Dr. Malak A. Al-yawer Department of Anatomy/Embryology Section Learning objectives At the end of this lecture, the medical student will be able to: State the embryonic origin of skeletal
Skeletal System Prof. Dr. Malak A. Al-yawer Department of Anatomy/Embryology Section Learning objectives At the end of this lecture, the medical student will be able to: State the embryonic origin of skeletal
Developing Molecularly Targeted Therapies for Basal Cell Carcinoma. Ivor Caro, MD, FAAD
 Developing Molecularly Targeted Therapies for Basal Cell Carcinoma Ivor Caro, MD, FAAD Disclosures Genentech, Inc Medical Director, Dermatology (employee) Stock holder Hedgehog Signaling Pathway Fundamental
Developing Molecularly Targeted Therapies for Basal Cell Carcinoma Ivor Caro, MD, FAAD Disclosures Genentech, Inc Medical Director, Dermatology (employee) Stock holder Hedgehog Signaling Pathway Fundamental
Defects in Pulmonary Vasculature and Perinatal Lung Hemorrhage in Mice Heterozygous Null for the Forkhead Box f1 Transcription Factor
 Developmental Biology 235, 489 507 (2001) doi:10.1006/dbio.2001.0322, available online at http://www.idealibrary.com on Defects in Pulmonary Vasculature and Perinatal Lung Hemorrhage in Mice Heterozygous
Developmental Biology 235, 489 507 (2001) doi:10.1006/dbio.2001.0322, available online at http://www.idealibrary.com on Defects in Pulmonary Vasculature and Perinatal Lung Hemorrhage in Mice Heterozygous
Tetrapod Limb Development
 Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Tetrapod Limb Development
 Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Biology 4361 Developmental Biology Tetrapod Limb Development July 29, 2009 Tetrapod Limbs Merlin D. Tuttle Vicki Lockard and Paul Barry Father Alejandro Sanchez Anne Fischer Limb Development - Overview
Fig. S1. Weight of embryos during development. Embryos are collected at different time points (E12.5, E14.5, E16.5 and E18.5) from matings between
 Fig. S1. Weight of embryos during development. Embryos are collected at different time points (E12.5, E14.5, E16.5 and E18.5) from matings between Myod +/ or Myod / females and Myod +/ ;Igf2 +/ males and
Fig. S1. Weight of embryos during development. Embryos are collected at different time points (E12.5, E14.5, E16.5 and E18.5) from matings between Myod +/ or Myod / females and Myod +/ ;Igf2 +/ males and
Bronchioles. Alveoli. Type I alveolar cells are very thin simple squamous epithelial cells and form most of the lining of an alveolus.
 276 Bronchioles Bronchioles continue on to form bronchi. The primary identifying feature is the loss of hyaline cartilage. The epithelium has become simple ciliated columnar, and there is a complete ring
276 Bronchioles Bronchioles continue on to form bronchi. The primary identifying feature is the loss of hyaline cartilage. The epithelium has become simple ciliated columnar, and there is a complete ring
Urogenital Development
 2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
2-5-03 Urogenital Development Greg Dressler Assoc. Professor Dept. of Pathology x46490 Dressler@umich.edu The Origin of the Kidney In the vertebrate embryo, the first stage of kidney development occurs
Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation
 Development 127, 1593-1605 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV4314 1593 Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2
Development 127, 1593-1605 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV4314 1593 Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2
Development of Spinal Cord & Vertebral Column. Dr. Sanaa Alshaarawi & Prof. Ahmed Fathalla
 Development of Spinal Cord & Vertebral Column Dr. Sanaa Alshaarawi & Prof. Ahmed Fathalla OBJECTIVES At the end of the lecture, students should be able to: q Describe the development of the spinal cord
Development of Spinal Cord & Vertebral Column Dr. Sanaa Alshaarawi & Prof. Ahmed Fathalla OBJECTIVES At the end of the lecture, students should be able to: q Describe the development of the spinal cord
Gli2 is required for induction of floor plate and adjacent cells, but not most
 Development 125, 2759-2770 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV9597 2759 Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons
Development 125, 2759-2770 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV9597 2759 Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons
Function of Breathing. Jeanine D Armiento, M.D., Ph.D. Respiratory Portion. Conducting Portion. Critical to the Development of the Lung
 Function of Breathing Jeanine D Armiento, M.D., Ph.D. Associate Professor Department of Medicine P&S 9-449 5-3745 jmd12@columbia.edu Air Sacs (alveoli) Ventilation-air conduction Moving gas in and out
Function of Breathing Jeanine D Armiento, M.D., Ph.D. Associate Professor Department of Medicine P&S 9-449 5-3745 jmd12@columbia.edu Air Sacs (alveoli) Ventilation-air conduction Moving gas in and out
When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval
 When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval disc 2 Why the embryo needs the vascular system? When
When you see this diagram, remember that you are looking at the embryo from above, through the amniotic cavity, where the epiblast appears as an oval disc 2 Why the embryo needs the vascular system? When
Skeletal Development Multiple Cellular Origins. Intramembranous Bone. Endochondrial Bone. Cartilage template of the limb in the Chick wing
 Skeletal Development Multiple Cellular Origins 1 - Paraxial Mesoderm Somite, Sclerotome Axial Skeleton (e.g. vertebra) 2 - Lateral Plate Mesoderm Appendicular Skeleton (e.g. limb) 3 - Neural Crest Head
Skeletal Development Multiple Cellular Origins 1 - Paraxial Mesoderm Somite, Sclerotome Axial Skeleton (e.g. vertebra) 2 - Lateral Plate Mesoderm Appendicular Skeleton (e.g. limb) 3 - Neural Crest Head
a) They are the most common cause of pediatric kidney failure. b) They are always symptomatic. c) They can be asymmetric.
 Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
Practice questions: 1. The paraxial mesoderm gives rise to somites. The structure of the somite a) is a loose mesenchymal sheet that will migrate toward the notochord. b) is an epithelial rosette with
Hepatogenesis I Liver development
 Hepatogenesis I Liver development HB 308 George Yeoh Room 2.59 MCS Building yeoh@cyllene.uwa.edu.au Topics Early liver development Tissue interaction - role of morphogens and cytokines Liver enriched transcription
Hepatogenesis I Liver development HB 308 George Yeoh Room 2.59 MCS Building yeoh@cyllene.uwa.edu.au Topics Early liver development Tissue interaction - role of morphogens and cytokines Liver enriched transcription
Regulation of retinoic acid signaling during lung morphogenesis
 Development 127, 3057-3067 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV4324 3057 Regulation of retinoic acid signaling during lung morphogenesis Sarah Malpel 1, Cathy Mendelsohn
Development 127, 3057-3067 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV4324 3057 Regulation of retinoic acid signaling during lung morphogenesis Sarah Malpel 1, Cathy Mendelsohn
Regionalization of the nervous system. Paul Garrity 7.68J/9.013J February 25, 2004
 Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
Regionalization of the nervous system Paul Garrity 7.68J/9.013J February 25, 2004 Patterning along: Rostral/Caudal (AP) axis Dorsal/Ventral (DV) axis Start with DV axial patterning in Spinal Cord Dorsal/Ventral
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Gli1 can rescue the in vivo function of Gli2
 Development 128, 5161-5172 (2001) Printed in Great ritain The Company of iologists Limited 2001 DEV4578 5161 Gli1 can rescue the in vivo function of Gli2 Chunyang rian ai 1 and Alexandra L. Joyner 1,2,
Development 128, 5161-5172 (2001) Printed in Great ritain The Company of iologists Limited 2001 DEV4578 5161 Gli1 can rescue the in vivo function of Gli2 Chunyang rian ai 1 and Alexandra L. Joyner 1,2,
Fareed Khdair, MD Assistant Professor Chief, Section of Pediatric Gastroenterology, Hepatology, and Nutrition University of Jordan School of Medicine
 Fareed Khdair, MD Assistant Professor Chief, Section of Pediatric Gastroenterology, Hepatology, and Nutrition University of Jordan School of Medicine Outline Lecture one : Gut formation Foregut: esophagus,
Fareed Khdair, MD Assistant Professor Chief, Section of Pediatric Gastroenterology, Hepatology, and Nutrition University of Jordan School of Medicine Outline Lecture one : Gut formation Foregut: esophagus,
Chapter 2. Genetic interaction between Gli3 and Alx4 during limb and craniofacial development CH Utrecht, The Netherlands
 Genetic interaction between Gli3 and Alx4 during limb and craniofacial development Lia Panman 1, Thijs Drenth 1, Pascal te Welscher 1,3, Aimee Zuniga 1,2, Rolf Zeller 1,2 1 Department of Developmental
Genetic interaction between Gli3 and Alx4 during limb and craniofacial development Lia Panman 1, Thijs Drenth 1, Pascal te Welscher 1,3, Aimee Zuniga 1,2, Rolf Zeller 1,2 1 Department of Developmental
Development of pancreas and Small Intestine. ANATOMY DEPARTMENT DR.SANAA AL-AlSHAARAWY DR.ESSAM Eldin Salama
 Development of pancreas and Small Intestine ANATOMY DEPARTMENT DR.SANAA AL-AlSHAARAWY DR.ESSAM Eldin Salama OBJECTIVES At the end of the lecture, the students should be able to : Describe the development
Development of pancreas and Small Intestine ANATOMY DEPARTMENT DR.SANAA AL-AlSHAARAWY DR.ESSAM Eldin Salama OBJECTIVES At the end of the lecture, the students should be able to : Describe the development
The embryonic endoderm initially is widely connected with the yolk sac. As a consequence of cephalocaudal and lateral folding, a portion of the
 DIGESTIVE SYSTEM The embryonic endoderm initially is widely connected with the yolk sac. As a consequence of cephalocaudal and lateral folding, a portion of the endoderm-lined yolk sac cavity is incorporated
DIGESTIVE SYSTEM The embryonic endoderm initially is widely connected with the yolk sac. As a consequence of cephalocaudal and lateral folding, a portion of the endoderm-lined yolk sac cavity is incorporated
Transduction of graded Hedgehog signaling by a combination of Gli2 and Gli3 activator functions in the developing spinal cord
 Research article 3593 Transduction of graded Hedgehog signaling by a combination of Gli2 and Gli3 activator functions in the developing spinal cord Qiubo Lei, Alice K. Zelman, Ed Kuang, Shike Li and Michael
Research article 3593 Transduction of graded Hedgehog signaling by a combination of Gli2 and Gli3 activator functions in the developing spinal cord Qiubo Lei, Alice K. Zelman, Ed Kuang, Shike Li and Michael
Development of the Digestive System. W.S. O School of Biomedical Sciences, University of Hong Kong.
 Development of the Digestive System W.S. O School of Biomedical Sciences, University of Hong Kong. Organization of the GI tract: Foregut (abdominal part) supplied by coeliac trunk; derivatives include
Development of the Digestive System W.S. O School of Biomedical Sciences, University of Hong Kong. Organization of the GI tract: Foregut (abdominal part) supplied by coeliac trunk; derivatives include
Developmental Biology
 Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Developmental Biology 362 (2012) 141 153 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Dual function of suppressor of
Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification
 Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification Marcus K. Gustafsson, 1,3,4 Hua Pan, 1,3,4 Deborah F. Pinney, 1,3 Yongliang Liu, 1,3 Anna Lewandowski, 1,3
Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification Marcus K. Gustafsson, 1,3,4 Hua Pan, 1,3,4 Deborah F. Pinney, 1,3 Yongliang Liu, 1,3 Anna Lewandowski, 1,3
The Foregut. At first the esophagus is short. but with descent of the heart and lungs it lengthens rapidly
 GI embryology 2 The Foregut At first the esophagus is short but with descent of the heart and lungs it lengthens rapidly The muscular coat, which is formed by surrounding splanchnic mesenchyme, is striated
GI embryology 2 The Foregut At first the esophagus is short but with descent of the heart and lungs it lengthens rapidly The muscular coat, which is formed by surrounding splanchnic mesenchyme, is striated
Polarity and Segmentation. Chapter Two
 Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
Polarity and Segmentation Chapter Two Polarization Entire body plan is polarized One end is different than the other Head vs. Tail Anterior vs. Posterior Front vs. Back Ventral vs. Dorsal Majority of neural
The Evolution and Development of the Gut. Dr Mike Wride School of Natural Sciences Zoology Department
 The Evolution and Development of the Gut Dr Mike Wride School of Natural Sciences Zoology Department email: wridem@tcd.ie The gut? Gut Function and Regulation (Dr. Alan Tuffery) Absorption of nutrients
The Evolution and Development of the Gut Dr Mike Wride School of Natural Sciences Zoology Department email: wridem@tcd.ie The gut? Gut Function and Regulation (Dr. Alan Tuffery) Absorption of nutrients
Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate
 Research article Related Commentary, page 1676 Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate Ritva Rice, 1,2 Bradley Spencer-Dene, 3 Elaine C. Connor, 1
Research article Related Commentary, page 1676 Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate Ritva Rice, 1,2 Bradley Spencer-Dene, 3 Elaine C. Connor, 1
Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme
 Development 128, 2095-2106 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV4537 2095 Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator
Development 128, 2095-2106 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV4537 2095 Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator
Patterning the Embryo
 Patterning the Embryo Anteroposterior axis Regional Identity in the Vertebrate Neural Tube Fig. 2.2 1 Brain and Segmental Ganglia in Drosophila Fig. 2.1 Genes that create positional and segment identity
Patterning the Embryo Anteroposterior axis Regional Identity in the Vertebrate Neural Tube Fig. 2.2 1 Brain and Segmental Ganglia in Drosophila Fig. 2.1 Genes that create positional and segment identity
CNS Developmental. Anke van Eekelen, PhD. Telethon Institute for Child Health Research
 CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
CNS Developmental Anke van Eekelen, PhD Telethon Institute for Child Health Research (Some slides are modified versions of Prof. Alan Harvey s Neuroscience lecture at ANHB and Dr. Joanne Britto s Dev Neuroscience
WNT2 and WNT7B Cooperative Signaling in Lung Development
 University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 1-1-2012 WNT2 and WNT7B Cooperative Signaling in Lung Development Mayumi Miller University of Pennsylvania, mayumimiller@gmail.com
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 1-1-2012 WNT2 and WNT7B Cooperative Signaling in Lung Development Mayumi Miller University of Pennsylvania, mayumimiller@gmail.com
Tooth development in the 'crooked' mouse
 /. Embryo!, exp. Morph. Vol. 41, pp. 279-287, 1977 279 Printed in Great Britain Company of Biologists Limited 1977 Tooth development in the 'crooked' mouse By J. A. SOFAER 1 From the University of Edinburgh,
/. Embryo!, exp. Morph. Vol. 41, pp. 279-287, 1977 279 Printed in Great Britain Company of Biologists Limited 1977 Tooth development in the 'crooked' mouse By J. A. SOFAER 1 From the University of Edinburgh,
Supporting Information
 Supporting Information Creuzet 10.1073/pnas.0906072106 Fig. S1. Migrating CNC cells express Bmp inhibitors. (A) HNK1 immunolabeling of migrating CNC cells at 7 ss. (B) At this stage, Fgf8 is strongly expressed
Supporting Information Creuzet 10.1073/pnas.0906072106 Fig. S1. Migrating CNC cells express Bmp inhibitors. (A) HNK1 immunolabeling of migrating CNC cells at 7 ss. (B) At this stage, Fgf8 is strongly expressed
Development of the Digestive System. W.S. O The University of Hong Kong
 Development of the Digestive System W.S. O The University of Hong Kong Plan for the GI system Then GI system in the abdomen first develops as a tube suspended by dorsal and ventral mesenteries. Blood
Development of the Digestive System W.S. O The University of Hong Kong Plan for the GI system Then GI system in the abdomen first develops as a tube suspended by dorsal and ventral mesenteries. Blood
Lung Physiology. Jamie Havrilak, PhD Postdoctoral Research Associate Layden Lab October 26th, 2018
 Lung Physiology Jamie Havrilak, PhD Postdoctoral Research Associate Layden Lab October 26th, 2018 Nkx2.1- Lung epithelium Endomucin- Vasculature Alveoli/Capillaries: Site of Gas Exchange in the Lung
Lung Physiology Jamie Havrilak, PhD Postdoctoral Research Associate Layden Lab October 26th, 2018 Nkx2.1- Lung epithelium Endomucin- Vasculature Alveoli/Capillaries: Site of Gas Exchange in the Lung
- Tamara Wahbeh. - Fareed Khdair. 0 P a g e
 -1 - Tamara Wahbeh - - Fareed Khdair 0 P a g e GI Embryology Note: I included everything in the records and slides; anything in the slide not included in this sheet was not mentioned by the doctor during
-1 - Tamara Wahbeh - - Fareed Khdair 0 P a g e GI Embryology Note: I included everything in the records and slides; anything in the slide not included in this sheet was not mentioned by the doctor during
Respiratory System Embryology
 Respiratory System Embryology Development of the nose and Palate Development of the nose At the end of the fourth week, facial prominences consisting primarily of neural crest-derived mesenchyme and formed
Respiratory System Embryology Development of the nose and Palate Development of the nose At the end of the fourth week, facial prominences consisting primarily of neural crest-derived mesenchyme and formed
Differential requirement for Gli2 and Gli3 in ventral neural cell fate specification
 Available online at www.sciencedirect.com R Developmental Biology 259 (2003) 150 161 www.elsevier.com/locate/ydbio Differential requirement for Gli2 and Gli3 in ventral neural cell fate specification Jun
Available online at www.sciencedirect.com R Developmental Biology 259 (2003) 150 161 www.elsevier.com/locate/ydbio Differential requirement for Gli2 and Gli3 in ventral neural cell fate specification Jun
Effect of the T-mutation on histogenesis of the mouse embryo under the testis capsule
 /. Embryol. exp. Morph. Vol. 5, pp. -3, 79 Printed in Great Britain Company of Biologists Limited 79 Effect of the T-mutation on histogenesis of the mouse embryo under the testis capsule By H. FUJIMOTO
/. Embryol. exp. Morph. Vol. 5, pp. -3, 79 Printed in Great Britain Company of Biologists Limited 79 Effect of the T-mutation on histogenesis of the mouse embryo under the testis capsule By H. FUJIMOTO
Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System. Assoc.Prof. E.Elif Güzel, M.D.
 Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System Assoc.Prof. E.Elif Güzel, M.D. Third week of Embryogenesis Primitive streak/pit appears on the epiblast (day
Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System Assoc.Prof. E.Elif Güzel, M.D. Third week of Embryogenesis Primitive streak/pit appears on the epiblast (day
Problem Set 5 KEY
 2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
2006 7.012 Problem Set 5 KEY ** Due before 5 PM on THURSDAY, November 9, 2006. ** Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. You are studying the development
FGF-18 INFLUENCES PROXIMAL PROGRAMING DURING LUNG MORPHOGENESIS
 JBC Papers in Press. Published on April 1, 2002 as Manuscript M202253200 FGF-18 INFLUENCES PROXIMAL PROGRAMING DURING LUNG MORPHOGENESIS Jeffrey A. Whitsett 1 Jean C. Clark 1 Lara Picard 1 Jay W. Tichelaar
JBC Papers in Press. Published on April 1, 2002 as Manuscript M202253200 FGF-18 INFLUENCES PROXIMAL PROGRAMING DURING LUNG MORPHOGENESIS Jeffrey A. Whitsett 1 Jean C. Clark 1 Lara Picard 1 Jay W. Tichelaar
BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE. Thomas Marino, Ph.D.
 BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE Thomas Marino, Ph.D. Development of the Brain I. Competencies: Upon completion of this section of the course, the student must be able to: 1. Understand
BRAIN DEVELOPMENT I: ESTABLISHMENT OF BASIC ARCHITECTURE Thomas Marino, Ph.D. Development of the Brain I. Competencies: Upon completion of this section of the course, the student must be able to: 1. Understand
Supplemental Data. Wnt/β-Catenin Signaling in Mesenchymal Progenitors. Controls Osteoblast and Chondrocyte
 Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
Supplemental Data Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis Timothy F. Day, Xizhi Guo, Lisa Garrett-Beal, and
NEUROCRANIUM VISCEROCRANIUM VISCEROCRANIUM VISCEROCRANIUM
 LECTURE 4 SKULL NEUROCRANIUM VISCEROCRANIUM VISCEROCRANIUM VISCEROCRANIUM CRANIUM NEUROCRANIUM (protective case around brain) VISCEROCRANIUM (skeleton of face) NASOMAXILLARY COMPLEX MANDIBLE (DESMOCRANIUM)
LECTURE 4 SKULL NEUROCRANIUM VISCEROCRANIUM VISCEROCRANIUM VISCEROCRANIUM CRANIUM NEUROCRANIUM (protective case around brain) VISCEROCRANIUM (skeleton of face) NASOMAXILLARY COMPLEX MANDIBLE (DESMOCRANIUM)
Distinct Roles Of CCN1 And CCN2 In Limb Development
 Distinct Roles Of CCN1 And CCN2 In Limb Development Jie Jiang, PhD 1, Jessica Ong, BS 1, Faith Hall-Glenn, PhD 2, Teni Anbarchian, BS 1, Karen M. Lyons, PhD 1. 1 University of California, Los Angeles,
Distinct Roles Of CCN1 And CCN2 In Limb Development Jie Jiang, PhD 1, Jessica Ong, BS 1, Faith Hall-Glenn, PhD 2, Teni Anbarchian, BS 1, Karen M. Lyons, PhD 1. 1 University of California, Los Angeles,
Embryo#1. Mohammad Hisham Al-Mohtaseb باشق جهاد. 0 P a g e
 Embryo#1 Mohammad Hisham Al-Mohtaseb باشق جهاد 0 P a g e Before you start, it is important to link what you learn in gross anatomy with developmental stages discussed in embryology. Cells that form organs
Embryo#1 Mohammad Hisham Al-Mohtaseb باشق جهاد 0 P a g e Before you start, it is important to link what you learn in gross anatomy with developmental stages discussed in embryology. Cells that form organs
The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis
 Development 126, 5771-5783 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV9673 5771 The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis
Development 126, 5771-5783 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV9673 5771 The basic-helix-loop-helix protein Pod1 is critically important for kidney and lung organogenesis
Regenerative medicine of the gastrointestinal tract
 Regenerative medicine of the gastrointestinal tract Assoc. Prof. Prasit Suwannalert, Ph.D. (Email: prasit.suw@mahidol.ac.th) Department of Pathobiology Faculty of Science, Mahidol University (SCBM301-
Regenerative medicine of the gastrointestinal tract Assoc. Prof. Prasit Suwannalert, Ph.D. (Email: prasit.suw@mahidol.ac.th) Department of Pathobiology Faculty of Science, Mahidol University (SCBM301-
Neuroepithelial Cells and Neural Differentiation
 Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Neuroepithelial Cells and Neural Differentiation Neurulation The cells of the neural tube are NEUROEPITHELIAL CELLS Neural crest cells migrate out of neural tube Neuroepithelial cells are embryonic stem
Combined activities of hedgehog signaling inhibitors regulate pancreas development
 Research article 4871 Combined activities of hedgehog signaling inhibitors regulate pancreas development Hiroshi Kawahira 1, Nancy H. Ma 1, Emmanouhl S. Tzanakakis 1, Andrew P. McMahon 2, Pao-Tien Chuang
Research article 4871 Combined activities of hedgehog signaling inhibitors regulate pancreas development Hiroshi Kawahira 1, Nancy H. Ma 1, Emmanouhl S. Tzanakakis 1, Andrew P. McMahon 2, Pao-Tien Chuang
A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines
 Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Supplemental Data A Cxcl12-Cxcr4 Chemokine Signaling Pathway Defines the Initial Trajectory of Mammalian Motor Axons Ivo Lieberam, Dritan Agalliu, Takashi Nagasawa, Johan Ericson, and Thomas M. Jessell
Children's Hospital of Pittsburgh Annual Progress Report: 2008 Formula Grant
 Children's Hospital of Pittsburgh Annual Progress Report: 2008 Formula Grant Reporting Period July 1, 2011 June 30, 2012 Formula Grant Overview The Children's Hospital of Pittsburgh received $958,038 in
Children's Hospital of Pittsburgh Annual Progress Report: 2008 Formula Grant Reporting Period July 1, 2011 June 30, 2012 Formula Grant Overview The Children's Hospital of Pittsburgh received $958,038 in
Biology Developmental Biology Spring Quarter Midterm 1 Version A
 Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Development of the nasal cavity :
 Development of the nasal cavity : several processes contribute to the development of the nose, the nose consists of 2 cavities separated by a septum, and the nasal cavity is separated from the oral cavity
Development of the nasal cavity : several processes contribute to the development of the nose, the nose consists of 2 cavities separated by a septum, and the nasal cavity is separated from the oral cavity
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Supplementary Figure 1. Generation of a conditional allele of the Kindlin-2 gene. (A) A restriction map of the relevant genomic region of Kindlin-2 (top), the targeting construct
SUPPLEMENTARY INFORMATION Supplementary Figure 1. Generation of a conditional allele of the Kindlin-2 gene. (A) A restriction map of the relevant genomic region of Kindlin-2 (top), the targeting construct
The site of action of the ichthyosis locus (ic) in the mouse, as determined by dermal-epidermal recombinations
 /. Embryol. exp. Morph. Vol. 32, 3, pp. 715-721, 1974 715 Printed in Great Britain The site of action of the ichthyosis locus (ic) in the mouse, as determined by dermal-epidermal recombinations BY MARGARET
/. Embryol. exp. Morph. Vol. 32, 3, pp. 715-721, 1974 715 Printed in Great Britain The site of action of the ichthyosis locus (ic) in the mouse, as determined by dermal-epidermal recombinations BY MARGARET
The Beauty of the Skin
 The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
The Beauty of the Skin Rose-Anne Romano, Ph.D Assistant Professor Department of Oral Biology School of Dental Medicine State University of New York at Buffalo The Big Question How do approximately 50 trillion
Restriction of sonic hedgehog signalling during early tooth development
 Research article 2875 Restriction of sonic hedgehog signalling during early tooth development Martyn T. Cobourne 1, Isabelle Miletich 2 and Paul T. Sharpe 2, * 1 Department of Craniofacial Development
Research article 2875 Restriction of sonic hedgehog signalling during early tooth development Martyn T. Cobourne 1, Isabelle Miletich 2 and Paul T. Sharpe 2, * 1 Department of Craniofacial Development
2/2/2011. Primitive Gut Tube Proctodeum and Stomodeum Stomach Duodenum Pancreas Liver and Biliary Apparatus Spleen Midgut
 DEVELOPMENT OF THE DIGESTIVE SYSTEM Development of Endodermal Organs Primitive Gut Tube Proctodeum and Stomodeum Stomach Duodenum Pancreas Liver and Biliary Apparatus Spleen Midgut Wednesday, February
DEVELOPMENT OF THE DIGESTIVE SYSTEM Development of Endodermal Organs Primitive Gut Tube Proctodeum and Stomodeum Stomach Duodenum Pancreas Liver and Biliary Apparatus Spleen Midgut Wednesday, February
Supplementary Figure S1 Enlarged coronary artery branches in Edn1-knockout mice. a-d, Coronary angiography by ink injection in wild-type (a, b) and
 Supplementary Figure S1 Enlarged coronary artery branches in Edn1-knockout mice. a-d, Coronary angiography by ink injection in wild-type (a, b) and Edn1-knockout (Edn1-KO) (c, d) hearts. The boxed areas
Supplementary Figure S1 Enlarged coronary artery branches in Edn1-knockout mice. a-d, Coronary angiography by ink injection in wild-type (a, b) and Edn1-knockout (Edn1-KO) (c, d) hearts. The boxed areas
Programmed Cell Death (apoptosis)
 Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
Programmed Cell Death (apoptosis) Stereotypic death process includes: membrane blebbing nuclear fragmentation chromatin condensation and DNA framentation loss of mitochondrial integrity and release of
Remember from the first year embryology Trilaminar disc has 3 layers: ectoderm, mesoderm, and endoderm
 Development of face Remember from the first year embryology Trilaminar disc has 3 layers: ectoderm, mesoderm, and endoderm The ectoderm forms the neural groove, then tube The neural tube lies in the mesoderm
Development of face Remember from the first year embryology Trilaminar disc has 3 layers: ectoderm, mesoderm, and endoderm The ectoderm forms the neural groove, then tube The neural tube lies in the mesoderm
Sonic hedgehog regulates growth and morphogenesis of the tooth
 Development 127, 4775-4785 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV3251 4775 Sonic hedgehog regulates growth and morphogenesis of the tooth Hélène R. Dassule 1, Paula
Development 127, 4775-4785 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV3251 4775 Sonic hedgehog regulates growth and morphogenesis of the tooth Hélène R. Dassule 1, Paula
Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear
 RESEARCH ARTICLE 1713 Development 134, 1713-1722 (2007) doi:10.1242/dev.000760 Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear Jinwoong
RESEARCH ARTICLE 1713 Development 134, 1713-1722 (2007) doi:10.1242/dev.000760 Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear Jinwoong
-Tamara Wahbeh. -Razan Abu Rumman. Dr. Mohammed Al-Muhtaseb
 -2 -Tamara Wahbeh -Razan Abu Rumman Dr. Mohammed Al-Muhtaseb I tried to include everything the doctor mentioned in both the lecture and his slides in the simplest way possible, so hopefully there would
-2 -Tamara Wahbeh -Razan Abu Rumman Dr. Mohammed Al-Muhtaseb I tried to include everything the doctor mentioned in both the lecture and his slides in the simplest way possible, so hopefully there would
Development of the Liver and Pancreas
 Development of the Liver and Pancreas Professor Alfred Cuschieri Department of Anatomy University of Malta Three glandular buds arise from the distal end of the foregut during the fourth week Day 22 -The
Development of the Liver and Pancreas Professor Alfred Cuschieri Department of Anatomy University of Malta Three glandular buds arise from the distal end of the foregut during the fourth week Day 22 -The
Today. Genomic Imprinting & X-Inactivation
 Today 1. Quiz (~12 min) 2. Genomic imprinting in mammals 3. X-chromosome inactivation in mammals Note that readings on Dosage Compensation and Genomic Imprinting in Mammals are on our web site. Genomic
Today 1. Quiz (~12 min) 2. Genomic imprinting in mammals 3. X-chromosome inactivation in mammals Note that readings on Dosage Compensation and Genomic Imprinting in Mammals are on our web site. Genomic
Formation defects Scoliosis Deformities I 07 1
 What is congenital scoliosis? Congenital scoliosis is a spinal deformity with lateral deviation and rotation of the spinal column, where congenital dysfunctions in embryonal vertebra development cause
What is congenital scoliosis? Congenital scoliosis is a spinal deformity with lateral deviation and rotation of the spinal column, where congenital dysfunctions in embryonal vertebra development cause
Organs Histology D. Sahar AL-Sharqi. Respiratory system
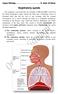 Respiratory system The respiratory system provides for exchange of O2 and CO2 to and from the blood. Respiratory organs include the lungs and a branching system of bronchial tubes that link the sites of
Respiratory system The respiratory system provides for exchange of O2 and CO2 to and from the blood. Respiratory organs include the lungs and a branching system of bronchial tubes that link the sites of
Organogenesis Part 2. V. Lateral Plate Mesoderm VI. Endoderm VII. Development of the Tetrapod Limb VIII. Sex Determination. V. Lateral Plate Mesoderm
 Organogenesis Part 2 V. Lateral Plate Mesoderm VI. Endoderm VII. Development of the Tetrapod Limb VIII. Sex Determination V. Lateral Plate Mesoderm chordamesoderm paraxial mesoderm intermediate mesoderm
Organogenesis Part 2 V. Lateral Plate Mesoderm VI. Endoderm VII. Development of the Tetrapod Limb VIII. Sex Determination V. Lateral Plate Mesoderm chordamesoderm paraxial mesoderm intermediate mesoderm
Douglas J. Epstein 1, *, Andrew P. McMahon 2 and Alexandra L. Joyner 1,3, SUMMARY
 Development 126, 281-292 (1999) Printed in Great Britain The Company of Biologists Limited 1998 DEV4093 281 Regionalization of Sonic hedgehog transcription along the anteroposterior axis of the mouse central
Development 126, 281-292 (1999) Printed in Great Britain The Company of Biologists Limited 1998 DEV4093 281 Regionalization of Sonic hedgehog transcription along the anteroposterior axis of the mouse central
MCB140: Second Midterm Spring 2010
 MCB140: Second Midterm Spring 2010 Before you start, print your name and student identification number (S.I.D) at the top of each page. There are 11 pages including this page. You will have 150 minutes
MCB140: Second Midterm Spring 2010 Before you start, print your name and student identification number (S.I.D) at the top of each page. There are 11 pages including this page. You will have 150 minutes
The role of mechanical stimuli induced by prenatal movements in skeletal development
 The role of mechanical stimuli induced by prenatal movements in skeletal development Niamh Nowlan, PhD Department of Bioengineering, Imperial College London Mechanobiology of Bone Mechanoregulation of
The role of mechanical stimuli induced by prenatal movements in skeletal development Niamh Nowlan, PhD Department of Bioengineering, Imperial College London Mechanobiology of Bone Mechanoregulation of
strain), were tested by growing explants of neural fold from the posterior Woronzowa,2' 3 implanting pituitaries in postlarval stages, observed
 VOL. 35, 1949 GENETICS: H. C. DALTON 277 DE VELOPMENTAL ANAL YSIS OF GENETIC DIFFERENCES IN PIGMENTATION IN THE AXOLOTL By H. CLARK DALTON DEPARTMENT OF GENETICS, CARNEGIE INSTITUTION OF WASHINGTON, COLD
VOL. 35, 1949 GENETICS: H. C. DALTON 277 DE VELOPMENTAL ANAL YSIS OF GENETIC DIFFERENCES IN PIGMENTATION IN THE AXOLOTL By H. CLARK DALTON DEPARTMENT OF GENETICS, CARNEGIE INSTITUTION OF WASHINGTON, COLD
T analysis of purely genetical problems. Students of other biological sciences
 THE MORPHOLOGICAL MANIFESTATIONS OF A DOMINANT MUTATION IN MICE AFFECTING TAIL AND UROGENITAL SYSTEM S. GLUECKSOHN-SCHOENHEIMER' Columbia University Received March 24, 1943 HE study of hereditary variations
THE MORPHOLOGICAL MANIFESTATIONS OF A DOMINANT MUTATION IN MICE AFFECTING TAIL AND UROGENITAL SYSTEM S. GLUECKSOHN-SCHOENHEIMER' Columbia University Received March 24, 1943 HE study of hereditary variations
Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/-
 Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Supplemental Material Results. Expression of acid base transporters in the kidney collecting duct in Slc2a7 -/- and Slc2a7 -/- mice. The expression of AE1 in the kidney was examined in Slc26a7 KO mice.
Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
 Wright et al. BMC Developmental Biology (2015) 15:33 DOI 10.1186/s12861-015-0083-8 RESEARCH ARTICLE Open Access Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
Wright et al. BMC Developmental Biology (2015) 15:33 DOI 10.1186/s12861-015-0083-8 RESEARCH ARTICLE Open Access Cooperative and independent functions of FGF and Wnt signaling during early inner ear development
Lecture 1: Embryology of the Limbs (Development of Skeletal and Muscular Systems)
 Lecture 1: Embryology of the Limbs (Development of Skeletal and Muscular Systems) Musculoskeletal Block Editing file Colour Index: Red: Important Gray: Notes Green: Dr. s notes After this lecture you should
Lecture 1: Embryology of the Limbs (Development of Skeletal and Muscular Systems) Musculoskeletal Block Editing file Colour Index: Red: Important Gray: Notes Green: Dr. s notes After this lecture you should
7.013 Problem Set 5 FRIDAY April 2nd, 2004
 MIT Department of Biology 7.013: Introductory Biology - Spring 2004 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Problem Set 5 FRIDAY April 2nd, 2004 Problem sets
MIT Department of Biology 7.013: Introductory Biology - Spring 2004 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Problem Set 5 FRIDAY April 2nd, 2004 Problem sets
The Epithelial-Mesenchymal Interaction Plays a Role in the Maintenance of the Stem Cell Niche of Mouse Incisors via Fgf10 and Fgf9 Signaling
 The Open Biotechnology Journal, 2008, 2, 111-115 111 The Epithelial-Mesenchymal Interaction Plays a Role in the Maintenance of the Stem Cell Niche of Mouse Incisors via Fgf10 and Fgf9 Signaling Tamaki
The Open Biotechnology Journal, 2008, 2, 111-115 111 The Epithelial-Mesenchymal Interaction Plays a Role in the Maintenance of the Stem Cell Niche of Mouse Incisors via Fgf10 and Fgf9 Signaling Tamaki
Biology 340 Comparative Embryology Lecture 10 Dr. Stuart Sumida. Further Development of the Mesoderm (and Endoderm)
 Biology 340 Comparative Embryology Lecture 10 Dr. Stuart Sumida Further Development of the Mesoderm (and Endoderm) Further Development: Digestive System Foregut, Midgut, Hindgut Heart and Aortic Arches
Biology 340 Comparative Embryology Lecture 10 Dr. Stuart Sumida Further Development of the Mesoderm (and Endoderm) Further Development: Digestive System Foregut, Midgut, Hindgut Heart and Aortic Arches
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons.
 Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Supplementary Fig. 1 Blocking shh function at the protein level confirms its role as a guidance cue for postcommissural axons. As an alternative method to demonstrate the role of shh as a guidance cue
Development of the Pharyngeal Arches
 Development of the Pharyngeal Arches Thomas A. Marino, Ph.D. Temple University School of Medicine Competencies: Upon completion of this section of the course, the student must be able to: 1. Recall the
Development of the Pharyngeal Arches Thomas A. Marino, Ph.D. Temple University School of Medicine Competencies: Upon completion of this section of the course, the student must be able to: 1. Recall the
