Cerebral Microvessels
|
|
|
- Damian Rodgers
- 6 years ago
- Views:
Transcription
1 Cerebral Microvessels Miguel Marín-Padilla Contents Introduction... 3 The Meningeal Extracortical Vascular Compartments... 3 The Pial Anastomotic Capillary Plexus Compartment... 5 Pial Capillary Perforation and Entrance into the Cerebral Cortex... 7 Intracerebral Extrinsic Microvascular Compartment Intracerebral Intrinsic Microvascular Compartment Outlooks References Abstract It has always been customary to remove the meninges prior to examining the brain. Deprived of meninges, the brain surface appears smooth and without any apparent vascularity, a well-known fact to anyone interested in studying it. An apparently avascular surface is also applicable to the cerebellum, medulla, and spinal cord deprived of meninges (Fig. 1). This is intriguing considering that the first 2.5 mm below the brain surface has one richer cellular and functionally active region of the human body, namely, the cortex gray matter where most neurons reside. Brain oxygen consumption represents roughly 20 % of that consumed by the entire body although it only represents a small fraction of its volume (Hossmann 1994). Therefore, the cortex gray matter, where most neurons reside, must be richly vascularized, a fact that conflicts with its apparently avascular surface. By removing the meninges, the vascular elements connecting the meninges and brain are severed leaving its surface apparently avascular (Fig. 1). Recent studies have demonstrated that these connecting vessels originate in the pial M. Marín-Padilla (*) The Geisel School Medicine at Dartmouth, Hanover, New Hampshire, USA miguel.marin-padilla@dartmouth.edu # Springer Science+Business Media New York 2015 D.W. Pfaff, N.D. Volkow, (eds.), Neuroscience in the 21st Century, DOI / _
2 2 M. Marín-Padilla Fig. 1 Dorsal, ventral, and lateral views of the human brain deprived of the meninges, from a 30-week-old fetus, showing its smooth and apparently avascular surface. The meningeal removal carried out prior to any brain examination leaves its surface apparently avascular. By removing the meninges, the pial capillaries connecting meninges to the brain are all severed. An apparently avascular surface also applies to the cerebellum, pons, and spinal cord deprived of meninges. A good magnifying glass demonstrates the presence of small and equidistant orifices with a severed vessel inside each one, throughout the cortex entire surface. The vessels connecting the meninges and the brain originate in the pial anastomotic capillary plexus, an important meningeal vascular compartment often ignored. The pial capillaries are invisible to unaided (naked eye) observation and are invariably removed with the meninges anastomotic capillary plexus, an important vascular compartment of the meninges that has remained essentially ignored (Marín-Padilla 2012). The pial capillaries establish a short-linked anastomotic plexus that covers the cortex expanding surface. The capillary size ranges from 3 to 7 μm and is, therefore, invisible to unaided observation. The meninges removal inevitably will carry the pial capillaries that will be lost when it is discarded. Perhaps, the invisibility of pial capillaries as well as the customary meninges castoff could explain why both the connecting capillaries and the pial anastomotic capillary plexus have been seldom recognized. The smooth and apparently avascular surface of the mammalian brain, deprived of meninges, has become a well-known and undisputed fact (Fig. 1). The simple inspection of the cortex surface (deprived of meninges) with a good magnifying glass (or dissecting microscope) solves the dilemma (Marín- Padilla 2012). Throughout its entire surface, there are small openings with a
3 Cerebral Microvessels 3 severed small blood vessel on each one. The orifices represent the Virchow- Robin compartments, clearly distinguishable from the severed blood vessel inside them. Both the orifices and their inside vessels are invisible to unaided (naked eye) observation. Anyone with a good magnifying glass could corroborate these observations, which are also applicable to any mammalian brain deprived of meninges. Keywords External glia limiting membrane (EGLM) Human cerebral cortex microvascular system Intracerebral extrinsic microvascular compartment Intracerebral intrinsic microvascular compartment Meninges extracortical vascular compartments Pial capillary perforation and entrance Pial anastomotic capillary plexus (PACP) compartment Virchow-Robin Compartment (V-RC) Introduction The following events in the human cerebral cortex microvascularization will be explored herein: (a) the meningeal or extracortical vascular compartments, (b) the pial anastomotic capillary plexus and its role in the brain microvascularization, (c) the unique mode of entrance of pial capillaries into the brain and simultaneous establishment of a Virchow-Robin compartment (V-RC) around each perforating vessels, (d) the establishment of intracortical extrinsic and intrinsic microvascular compartments and their distinct functional roles, and (e) the cerebral cortex dual venous drainages (Table 1). The classic Golgi s staining procedure (Golgi 1873), an excellent tool to study CNS neurons (Cajal 1911; Marín-Padilla 2011), is also exceptional to study the development and structural organization of the brain blood vessels as well as the gray matter special protoplasmic astrocytes associated with them (Marín-Padilla 1995). Misunderstandings, technical queries, and qualifications concerning this classic staining procedure have been recently reexamined (The Golgi reaction: A personal quest) in a book (Marín-Padilla 2011). The material utilized in this chapter comes from the author collection of developing brains from hamsters, cats, and humans, and the methods include electron microscopy and the rapid variant of the Golgi reaction. The Meningeal Extracortical Vascular Compartments The central nervous system vascularization is an ascending process that accompanies the anatomical and functional maturations of its various ascending strata. It starts at the myelencephalon and ascends sequentially through the metencephalon, mesencephalon, diencephalon, and finally the telencephalon (Klosouskii 1963; Strong 1964;Bär and Wolff 1972; Gamble 1975; Wolff et al. 1975). The meningeal
4 4 M. Marín-Padilla Table 1 Human cerebral cortex vascular compartments A. Meningeal or extracerebral vascular compartment (a) Dural lamellae with the main venous sinuses (b) Arachnoidal lamellae with the main arteries and veins (c) Pial lamellae with the anastomotic capillary plexus, the source of all perforating vessels entering the cortex B. Dual intracerebral microvascular compartments (a) Extrinsic compartment represented by all perforating vessels throughout the cortex with their corresponding Virchow-Robin compartment* (b) Intrinsic compartment represented by the anastomotic capillary plexus establish among contiguous perforating vessels. It represents the cortex functional center of operations and the blood-brain barrier (BBB)* C. Dual venous drainage compartments (a) A rapid one for the gray matter, where neurons reside, through the arachnoidal veins (b) A slow one for the white matter vessels through the veins of Rosenthal and the brain ventral venous circle *Both the extrinsic (directly) and the intrinsic (indirectly) intracerebral microvascular compartments evolve from the pial anastomotic capillary plexus vascularization accompanies the ascending maturation of each of the CNS regions. Interrelationships between meningeal vessels and the perforating ones entering the CNS have been described in a variety of studies (Mall 1904; Strong 1964; Pape and Wigglesworth 1979; Hauw et al. 1975; Nabeshina et al. 1975; Krahn 1982; Krisch et al. 1982, 1983). The meningeal pial anastomotic capillary plexus and its role in the brain microvascularization have been recently explored (Marín-Padilla 2012). The very young embryo develops a primitive meninx around the developing CNS from both mesodermal and neural crest elements (Hamilton et al. 1972; Buitrago-Delgado et al. 2015). It has a denser external component and a looser internal one. The dura mater evolves from the outer denser component and both arachnoids and pia from the inner looser one. The meninges ongoing vascularization accompanies the ascending maturation of the CNS various regions. Three distinct vascular compartments are eventually established in the meninges: an outer or dural lamella that carries the venous sinuses, an intermediate or arachnoidal lamella that carries the main arteries and veins, and an inner pial lamella with the pial anastomotic capillary plexus (PACP) from where all perforating vessels that enter into the developing cortex originate (Table 1). Most of the available descriptions on the meningeal vascular composition refer almost exclusively to its dural and arachnoidal compartments without reference to its pial anastomotic capillary plexus (PACP). Moreover, the presence of a PACP has neither been described in most neuroanatomy and/or embryology textbooks (Hamilton et al. 1972; Larsen 1997; O Rahilly and M uller 1999). Possible reasons behind this oversight will be also explored herein. It is also important to emphasize that the cerebral cortex has dual venous drainages: a fast one for the gray matter, where most neurons reside, and a slower one for the white matter. The number of perforating vessels that enter into the gray
5 Cerebral Microvessels 5 matter is by far greater than those that eventually enter into the white matter. The blood circulates rapidly through the gray matter by entering arterial perforators and exiting venous ones. The gray matter oxygenation is both rapid and continuous. Moreover, blood flow through the gray matter is further controlled by the functional activity of local neurons. The cortex exiting venous perforators drain through the arachnoidal veins and into the dural sinuses. The slower venous drainage for the white matter fewer perforators is through a paraventricular venous plexus that interconnect all of them, the veins of Rosenthal, the meningeal ventral venous circle, and the ampulla of Galen into the dural venous sinuses (Larroche 1977; Marín-Padilla 2015). Streeter (1918) and Paget (1948, 1957) published the first detailed descriptions of the human brain s meningeal vascular compartments during embryonic development although the pial anastomotic capillary plexus is not described. One of the earlier descriptions of the meningeal vascularity is Streeter s beautiful drawing of a 50-day-old human embryo head (Fig. 2a). The drawing illustrates the dural and arachnoidal blood vessels, but the cortical surface is depicted without blood vessels. By this embryonic age, a pial anastomotic capillary plexus already covers the cortex surface (Fig. 2b d). The Pial Anastomotic Capillary Plexus Compartment The pial anastomotic capillary plexus (PACP) is an essential vascular compartment of the meninges. By the sixth week of gestation, the meningeal triple vascular compartments are already established (Fig. 2c). The early neuroectodermal, marginal zone and primordial plexiform developmental stages evolve within a still avascular cortex already covered by a PACP (Fig. 2b d). At this age, the still avascular neocortex is at the primordial plexiform (preplate) stage with a variety of neurons and fibers of extracortical origin between the pial and the matrix zone (Marín-Padilla 2011). The neural elements in close proximity to the pial capillaries permit adequate oxygen diffusion for their survival. Many red cells in the pial capillaries are still nucleated at this age (Fig. 2d). The PACP is composed of numerous and closely linked capillaries of various sizes that cover the developing cortex entire surface (Fig. 3a). Its growth parallels the cortex progressive expansion. Throughout the PACP, there are evidences of active angiogenesis, including very small capillaries, capillaries with growing internal and external endothelial cell filopodia, and scattered mitoses (Fig. 3a). The pial capillary diameter ranges from very small (3 μ) to larger ones (5 7 μ), and therefore, they are invisible to unaided (naked eye) observation. Among the capillaries, there are a variety of meningeal cells (pericytes, fibroblasts, and macrophages) as well as collagen fibers (Fig. 3a). Some pial cells will accompany the perforating vessels into the brain (Figs. 4a, b, and 5). Some of them are transformed into smooth muscles and become incorporated into the vessel wall (Jones 1970). Pial capillaries and pial cells are embedded within a loose trabecular tissue bade by
6 6 M. Marín-Padilla Fig. 2 Composite figure showing Stretter s original drawing of a 50-day-old human embryo (a), a schematic representation of the embryonic meningeal vascular composition (b), a photomicrograph of the meninges and cerebral cortex of a 42-day-old human embryo (c), and a photomicrograph of the a portion of the developing cerebral cortex of the same embryo (d). A. The cortex surface is depicted avascular in Streeter s drawing. At this age, the human meninges are fully vascularized (c); the dural sinuses, arachnoidal arteries, and veins as well as the pial anastomotic capillary plexus covering the cortex surface are all recognized (d). Photomicrograph of the cerebral cortex of a 42-day-old fetus showing a pial anastomotic capillary plexus covering the cortex surface. Many of its red cells are still nucleated. At this age, the human cortex (d) isatthe primordial plexiform (preplate) stages with a few scattered neurons (n) and fibers of extracortical origin below the pial. Horizontal Cajal-Retzius (C-R) cells are also recognized at this age cerebrospinal fluid (Fig. 2b, c). The PACP developmental expansion is carried out by the incorporation of additional arachnoidal capillaries as well as by local capillarogenesis. The human cerebral cortex begins its development around the sixth week of gestation, and a PACP already covers its surface (Fig. 2b d). At this age, the cortex is at the primordial plexiform stage with scattered neurons and fibers of extracortical origin. The cortex microvascularization by pial capillaries starts around the eighth week of gestation and coincides with the arrival of the first pyramidal neurons, from the ependyma, at the developing gray matter (Marín- Padilla 2011, 2014).
7 Cerebral Microvessels 7 Pial Capillary Perforation and Entrance into the Cerebral Cortex The cerebral cortex (and entire CNS) is separated from surrounding tissues by an external glial limiting membrane (EGLM) composed of radial glial endfeet united by junction and covered by basal lamina material manufactured by them (Fig. 3a c). It represents a physical barrier that guarantees the brain anatomical and functional integrities. During the cortex prenatal and postnatal maturations, the expanding Fig. 3 Composite figure of photomicrographs showing various developmental events in the perforation of the cerebral cortex by pial capillaries, from electron microscopic studies of the developing cortex of 12-day-old hamster embryos. (a) General view of the pial anastomotic plexus with capillaries (*) of different calibers, pial cells, and the cortex EGLM formed by joined glial endfeet (G) covered by a basal lamina manufactured by them. One large capillary with an adjoining endothelial cell and internal and external filopodia has established contact with the brain EGLM with fusion of vascular and CNS basal laminae. Some of its external filopodia have penetrated into the brain (thick arrow) that has a few scattered neurons (N). (b) Detail of two filopodia from a pial capillary making contacts with the cortex EGLM with fusions of vascular and glial basal laminae. (c) High-power electron micrograph of two endothelial cell filopodia penetrating into the brain (thick arrow) with fusion of vascular and CNS basal laminae, formation of funnels (thin arrows) around the fused laminae, and destruction of the local glial endfeet, from the cortex of a 12-day-old mouse embryo. (d) Detail of filopodia (PF) from a pial capillary (*) that have entered into the cortex accompanied by the funnel (white arrow) formed by the fussed vascular and CNS basal laminae
8 8 M. Marín-Padilla EGLM is maintained by the uninterrupted incorporation of additional glial endfeet. During early prenatal development, radial glial fibers provided all the necessary endfeet. Later in prenatal development and postnatally, first lamina special astrocytes will provide the additional glial endfeet needed for its maintenance and progressive expansion (Marín-Padilla 1995). The cortex EGLM is frequently damaged in perinatal hemorrhagic, ischemic, and/or traumatic brain injuries resulting in local ruptures (Marín-Padilla 1996). EGLM pathological ruptures are invariable associated with the formation of leptomeningeal heterotopias that contain displaced neural, vascular, and glial elements and are often associated with epilepsy (Marín- Padilla 1997, 1999). The leptomeningeal heterotopias size varies depending on that of the original EGLM rupture and ranges from microscopic to rather extensive ones (Marín-Padilla 1997). Pial capillaries although capable of perforating through the EGLM to enter into the brain are incapable of exiting from it. Circulatory dynamics and functional demands will determine which ones become entering arterial perforators and which ones exiting venous. During cortical development, the ratio between arterial and venous perforators changes continuously responding to gray matter neurons changing functional requirements. In other brain regions, the EGLM can also be perforated by entering as well as by exiting nerves (Andres 1976a, b). During the cortex microvascularization, a remarkable event occurs repeatedly: two different tissues covered by their distinct basal laminae establish anatomical and functional interrelationships. Pial capillaries of mesodermal origin, covered by their own basal lamina, establish contact with and penetrate into the brain of neuroectodermal origin also covered by its own basal lamina. This remarkable event that occurs many thousands of times during the cortex prenatal and postnatal maturations has been seldom studied and/or described. Pial capillary perforations and formation of corresponding V-RCs occur continuously during the cortex prenatal and postnatal maturations, and their number increases exponentially. For example, the possible number of equidistant perforating vessels already present in the newborn cerebral cortex with surface area around 7000 mm 2 might be around 63,000. In the adult brain, with a surface area of 16,350 mm 2, the possible number of perforating vessels could be around 147,000 (Blinkov and Glezer 1968). Although these numbers are mere approximations, they provide a general idea of the large number of microvascular perforations that occur during the human brain maturation. Despite the large number of capillary perforations and their functional relevance, the mammalian brain microvascularization has remained, until recently, essentially unexplored (Marín-Padilla 2012). Three sequential stages have been observed in the perforation and entrance of pial capillaries into the developing cortex: (1) pial capillary approach and contact with the cortex EGLM with fusion of both vascular and CNS basal laminae (Fig. 3b d), (2) endothelial cell filopodia perforation and entrance into the nervous tissue through the fused basal laminae with preservation of the cortex anatomical integrity (Fig. 3a d), and (3) whole pial capillary penetration into the nervous tissue through the fused laminae and concomitant establishment of a perivascular Virchow-Robin compartment (V-RC) around each perforating vessel (Figs. 4a d, and 5a, b).
9 Cerebral Microvessels 9 Fig. 4 Composite figure of electron photomicrographs (a, b, c) showing different views of a pial perforating capillary that has entered the cortex, from EM studies of 12-day-old hamster embryos and a photomicrograph (d) of a section of the motor cortex stained with glial fibrillary acidic protein (GFAP) procedure from a newborn infant. (a) General view of a perforating pial capillary entrance into the cerebral cortex of 12-day-old hamster embryo, through its external glial limiting membrane (EGLM, arrows), with an accompanying pial cell (curve arrow) and formation of a perivascular Virchow-Robin compartment (V-RC) around it. (b) Detail of the perforating vessel leading head composed of several sliding endothelial cells separated by narrow spaces (white arrows). Its main lumen (*) is occupied by a nucleated red cell. (c) Detail of the pial capillary (*) entrance into the cortex showing the formation of the perivascular Virchow-Robin compartment (V-RC, arrows) between the pial capillary and the glial wall (G, arrows) composed of joined glial endfeet that represent an extension of the cortex superficial EGLM. A pial cell (P) has penetrated into the V-RC accompanying the perforating vessel into the brain. A junction between adjacent glial endfeet (curve arrow) is illustrated. (d) Glial fibrillary acidic protein (GFAP) preparation of the motor cortex of a newborn infant showing the entrance of a pial perforating capillary (arrow) within the V-RC perivascular space and hence outside (extrinsic) to the nervous tissue. The V-RC outer wall is formed by joined glial endfeet covered by basal lamina that is an extension of the cortex EGLM, and its inner wall is the perforating capillary basal lamina (Compare Figs. 4d and 5b) Pial capillaries that have already established contact with the cortex EGLM show considerable activity (Fig. 3a). They may carry additional sliding endothelial cells and have numerous internal as well as external filopodia (Fig. 3a). Some of the external filopodia penetrate into the nervous tissue through the fused basal laminae (Fig. 3a, d). During the early stages, individual endothelial cell filopodia establish
10 10 M. Marín-Padilla Fig. 5 Composite figure showing schematic drawings of the three basic developmental stages (1, 2, 3) of the perforation of the cerebral cortex by pial capillary and a dilated V-RC (b) from a 5-year-old child who suffered perinatal brain damage. (a) The schematic drawings illustrating the three developmental stages of the cerebral cortex perforation by pial capillaries are selfexplanatory. (b) The glial fibrillary acidic protein stained preparation of the motor cortex of a 5-year-old child that has suffered perinatal brain damage shows an enlarged V-RC containing several macrophages filled with glial cell products and abundant fluid. Several macrophages filled with glial cell products are recognized crossing the V-RC outer glial wall and entering into it (Compare Fig. 4d with Fig. 5b) direct contacts with the cortex EGLM with fusion of both basal laminae (Fig. 3b). Glial endfeet perforated by capillary filopodia show signs of vacuolar (cystic) degeneration (Fig. 3c, d). At the filopodia entrance, the fused vascular and brain
11 Cerebral Microvessels 11 basal laminae form a funnel that accompany the filopodia (and eventually the entire perforating vessel) into the nervous tissue while remaining open to the meningeal interstitium (Figs. 3c, d, and 4a, b, d). It is important to emphasize that although pial capillaries are capable of entering into the cortex by perforating through its EGLM, they are incapable of exiting from it. Circulatory dynamics and increasing functional demands determine the perforating vessel arterial and/or venous nature as well as its size and caliber. In the adult cortex, functional demands continue to modify the perforating vessels nature, size, and caliber as well as the ratio among entering arterial perforator versus exiting venous ones. As the whole capillary penetrates into the nervous tissue, a perivascular space is established around it (Figs. 4a d, and 5a, b). This perivascular space, known as the Virchow-Robin compartment (V-RC), remains open to the meningeal interstitium for life. The V-RC is formed by the funnel of fused vascular and CNS basal laminae that accompanies the perforating vessel into the nervous tissue, throughout its entire length, keeping it extrinsic (outside) of the nervous tissue (Figs. 4d, and 5b). The V-RC is lined internally by the perforating vessels basal laminae and externally by joined glial cell endfeet covered by basal lamina, which are an extension of the cortex superficial EGLM (Figs. 4d and 5b). All V-RCs remain open to the meningeal interstitium, are bade by cerebrospinal fluid, permit the exchange of fluids and cells between brain and meninges, and will operate as the brain sole drainage (perilymphatic) system (Marín-Padilla and Knopman 2011). The V-RC accompanying the perforating vessel into the brain grows by the incorporation of additional glial endfeet. Similarly, the cortex EGLM expands, through life, by the continued incorporation of additional endfeet from first lamina special astrocytes. During late prenatal maturation and postnatal life, first lamina special astrocytes (comet cells) number increases by cell divisions contributing to the cortex EGLM progressive expansion and maintenance (Marín-Padilla 1995). Because the mammalian brain lacks a lymphatic system, the V-RC is its only drainage system (Marín-Padilla and Knopman 2011; Jeffrey et al. 2012). Substances injected into the brain can be detected in the neck lymph nodes (Casley- Smith et al. 1976; Pile-Spelman et al. 1984). Liquids as well as cells entering into the V-RCs reach the meningeal interstitium and are cleared through the perivascular lymphatics of arachnoidal vessels. Under physiological (normal) conditions, the V-RC drainage capability remains adequate through life. However, it becomes inadequate in pathological conditions characterized by diffuse and/or repetitive neuronal damage, such as Alzheimer, post-traumatic, and other degenerative encephalopathies (Marín-Padilla and Knopman 2011). In diffuse and/or recurrent brain damage, macrophages carrying necrotic debris eventually obliterate local V-RCs impairing their drainage capabilities (compare Figs. 4d, and 5b). Macrophages unable to reach the local and already filled V-RCs will die (limited life-span) in situ liberating their debris and proteolysis enzymes and causing additional brain damage. This process, repeated through the brain, could explain the innumerable and increasing number of lesions found throughout the cerebral cortex in degenerative encephalopathies, a common and well-known phenomenon not yet adequately explained (Marín-Padilla and Knopman 2011).
12 12 M. Marín-Padilla Fig. 6 Photomicrographs (a, b) from rapid Golgi preparations of the motor cortex of a 2.5-monthold showing the gray matter extrinsic and intrinsic microvascular compartments. (a) Photomicrograph from a rapid Golgi preparation of an infant motor cortex showing the composition and interrelationships of the gray matter extrinsic (E) and intrinsic (I) microvascular compartments. (a) The rectangular section of the motor cortex illustrated herein measures roughly 9 5 mm. The distance among perforating vessels and hence the width of the intrinsic capillary plexuses, between them, remain essentially unchanged through the cerebral cortex prenatal and postnatal maturations and are considered to represent a biological constant needed for the functional activity of gray matter (GM) neurons. Fewer perforators penetrate into the white matter (WM) and their separation is greater as well as variable. (b) Detail, at a higher magnification, of the intrinsic capillary plexus established between contiguous arterial (A) and venous (V) perforators with few scattered neurons (N). Gray matter intrinsic capillaries although capable of emerging from perforators are incapable
13 Cerebral Microvessels 13 Intracerebral Extrinsic Microvascular Compartment All perforating vessels within their corresponding V-RCs constitute the cortex extrinsic microvascular compartment (Fig. 6a, b). Golgi preparation thickness ( mμ) is ideal for visualizing tridimensionally the cortex extrinsic as well as the intrinsic microvascular compartments (Figs. 6, 7, and 8). Similar images of the brain perforating vessels have been described in intravascular injections of colorant as well as in intravascular castings (Pape and Wigglesworth 1979; Duvernoy et al. 1981). All perforating vessels recognized in the adult brain (arterioles, venules, small arteries, and small veins) evolve originally from pial capillaries. Circulatory dynamics and increasing functional demands will determine the perforating vessel arterial or venous nature as well as its size and caliber well into postnatal life. In the newborn cortex, each exiting venous perforator is surrounded by 8 10 entering arterial ones. This ratio will continue to change during the cortex postnatal maturation. During cortical development, pial perforating vessels enter into the cortex at constant intervals that range between 400 and 600 μm. The equidistant distance between the perforating vessels throughout the cortex is considered to represent a biological constant needed for the functional activity of gray matter neurons (Marín-Padilla 2012). Moreover, the intervascular distance between gray matter perforators is comparable to the diameter (500 μm) of Mountcastle (1978) functional columns. Only a fraction of gray matter perforating vessels penetrates into the white matter, and their intervascular distances are greater as well as variable (Fig. 8). Perforating vessels (arterial and/or venous) cross vertically the cortex entire thickness, reach the paraventricular region, and establish an anastomotic plexus among them through the region (Figs. 6a, b, and 8a). This periventricular anastomotic plexus permits the circulation of blood through them (Fig. 8a). During cortical maturation, additional anastomotic capillary plexuses between contiguous perforators are subsequently established throughout the white matter and the subplate zone and eventually through the developing gray matter (Fig. 8a). The gray matter vascularization starts after the completion of the pyramidal cell plate formation that occurs around the 15th 16th week of gestation (Fig. 8a). At this age, an anastomotic capillary plexus, between contiguous perforators, is established throughout the gray matter deepest, older, and larger pyramidal cell stratum that is starting its functional maturation (Fig. 8a). The gray matter ascending ä Fig. 6 (continued) of reentering them. Circulatory dynamics and functional demands will determine their arterial and/or venous nature. During the cerebral cortex prenatal and postnatal maturations, the ratio among arterial versus venous perforators changes continuously in response to the evolving functional activities of gray matter neurons
14 14 M. Marín-Padilla Fig. 7 Higher power views (a, b) of the gray matter intrinsic microvascular compartment from the motor cortex of a 2.5-month-old child showing its three-dimensional organization with scattered neurons, protoplasmic astrocytes (G), and a basket cell (BC). The basket cell (BC) axon terminals (at) extend throughout the entire region. The BC axon terminals form pericellular nests of axosomatic synapses around pyramidal cell bodies. Basket cell is one of the main inhibitory neurons of the mammalian cerebral cortex. The gray matter intrinsic capillaries, neurons, and protoplasmic astrocytes interexchange information that will determine the blood flow of the region in response to local neurons functional demands. The intrinsic microvascular compartment represents the gray matter center of functional operations as well as its blood-brain
15 Cerebral Microvessels 15 microvascularization parallels the ascending and stratified maturation of its various pyramidal cell strata well into postnatal life (Marín-Padilla 2011, 2014). In degenerative encephalopathies and in post-traumatic brain injuries, local V-RCs are invariably filled with inflammatory cells, and their drainage capabilities are seriously impaired. The inflammatory cells include those exiting from the perforating vessel as well as those reentering the V-RCs from the injured brain site. The cortex perforating vessels are the only one capable of responding to inflammatory insults. Inflammatory cells exiting the perforating vessels cross the V-RC and enter into the brain tissue by perforating through its outer glial wall. Inflammatory cells are also capable of reentering local V-RCs but incapable of reentering the perforating vessel. Fluids and inflammatory cells reentering the V-RCs must reach the meningeal interstitium to be eventually eliminated through the arachnoidal vessels perivascular lymphatics. Since V-RCs lack valves, the only mechanism that helps in moving fluids and inflammatory cells toward the meningeal interstitium is the perforating vessel muscular activity, which is not a very efficient method. The relatively short distance ( mμ) between perforators and hence of the V-RCs reflects, in my opinion, the distance that macrophages could travel to and from an injured site. The number of gray matter perforating vessels (where neurons reside) is far greater than those reaching the white matter. Only a fraction of gray matter perforators penetrate into the white matter. The white matter, with fewer neurons, needs fewer perforating vessels, and the distance among them is greater as well as irregular. Therefore, the white matter, with fewer perforating vessels, is more vulnerable to perinatal brain damage that is the gray matter (Marín-Padilla 1997). Intracerebral Intrinsic Microvascular Compartment During the cortex functional maturation, capillaries emerge from contiguous perforating vessels, puncture through the V-RC outer glial wall, enter into the nervous tissue, and establish a three-dimensional anastomotic capillary plexus between them (Figs. 6a, b, 7a, b, and 8a c). The anastomotic capillary plexuses among perforating vessels represent the cortex intrinsic microvascular compartments, the ä Fig. 7 (continued) barrier (BBB). (a) The illustrated rectangular portion of the motor cortex gray matter measures roughly μm and has scattered pyramidal cells (Py), a basket cell (BC), and protoplasmic astrocyte (G). The local neurons, glial cells, dendrites, and axonic terminals occupied the intercapillary spaces. (b) Another higher power view of the gray matter intrinsic microvascular compartment capillary plexus illustrating its three-dimensional organization and composition. The inhibitory basket cell (BC) axon terminals (a) extend throughout the entire region. It should be pointed out that if all neurons, glial cells, dendrites, and axonic terminal were stained, the section will be totally black and useless. A secret in using the rapid Golgi procedure is to make as many preparations as possible and stain them slightly. The brain complex neuronal, vascular, and glial structures will be simplified and easier to study and comprehend
16 16 M. Marín-Padilla Fig. 8 Composite figure with three developmental stages of the human brain microvascularization, including the cerebral cortices of a 15-wg fetus (a), of a newborn infant (b), and of an adult brain (c). (a) Montage of camera lucida drawings, from rapid Golgi preparations of the developing cortex of a 15-wg fetus, representing a 2-mm-thick section of the cortex from pia to ependymal surfaces. The perforating vessels cross the cortex entire thickness and establish an anastomotic plexus between them throughout the paraventricular zone. At this age, anastomotic capillary plexuses are recognized (arrow heads) through the white matter, the subplate zone, and the pyramidal cell plate lower region where the deeper, older, and larger pyramidal neurons are starting their functional maturation. Horizontal bundles of axonic fibers and ascending glial cell progenitors are recognized throughout the white matter as well as a variety of neurons throughout the subplate zone. Radial glial fibers (RG) cross the cortex entire thickness and contribute glial endfeet for the maintenance of its EGLM. A bundle of horizontal fibers, of unknown nature, is recognized through the paraventricular zone. (b) Newborn motor cortex rapid Golgi preparation showing the extrinsic and intrinsic microvascular compartments through the gray (GM) and white matters (WM). The white matter has fewer capillaries and larger intercapillary spaces than the gray matter and is more vulnerable to perinatal brain damage. (c) View of an adult cerebral cortex microvascular system from intravascular casting studies (Duvernoy et al. 1981). Both the newborn (b) and the adult cerebral cortex (c) gray matters have more intrinsic capillaries than the white matter. The similarities of the microvascular system between the newborn and the adult cerebral cortices are remarkable despite their considerable size and volume differences. Moreover, during brain maturation, the separation among perforating vessels and, hence, the width of the intrinsic microvascular compartment between them remain essentially unchanged regardless of the increases in volume and age. The similar width of the intrinsic microvascular compartment is considered to represent a biological constant needed for the functional activity of gray matter neurons regardless of size and/or age
17 Cerebral Microvessels 17 center of its functional activities, and the cortex blood-brain barrier (Dorovini-Zis 2015; Marin-Padilla 2015). The thickness of rapid Golgi preparations is unique to appreciate the tridimensional interrelationships of the intrinsic microvascular compartment s neurons, capillaries, and protoplasmic astrocytes (Figs. 6, 7, and 8). The intrinsic capillaries emerge from perforators, cross V-RC, and perforate through its outer glial wall to enter into the nervous tissue (Fig. 6b). The intrinsic capillary perforation through the V-RC outer glial wall is a process similar to the original perforation of the brain EGLM by pial capillaries. The V-RC outer glial wall actually represents an extension of the cortex EGLM that accompanies the perforating vessel into the brain throughout its entire length. Intrinsic capillaries are incapable of reentering V-RCs. Similarly, the original pial capillaries are also incapable of exiting the brain by perforating through its EGLM. Local circulatory dynamics and functional demands will determine which intrinsic capillaries become arterial and which one becomes venous. During the cortex prenatal and postnatal maturations, the ratio between arterial and venous intrinsic capillaries changes continuously in response to the gray matter neurons changing and increasing functional demands. Intrinsic capillaries entering into the nervous tissue carry a single basal lamina and become immediately surrounded by endfeet from the gray matter protoplasmic astrocytes. Local gray matter neurons, protoplasmic astrocytes, dendrites, and axonic terminals occupy the intercapillary spaces (Fig. 7a, b). The gray matter intrinsic microvascular compartment represents the cortex functional center of operations as well as the blood-brain barrier (Marín-Padilla 2012). Additional information concerning the blood-brain barrier functional role in health and in disease could be found in a recent book (Dorovini-Zis 2015). The intrinsic microvascular compartment s neurons, capillaries, and protoplasmic astrocytes interexchange information that will determine the local blood flow in response to the neurons functional activities (Hossmann 1994; Marín-Padilla 2012; Ussui et al. 2015). Functional magnetic resonance image studies are based on this property. During brain maturation, the number of gray matter perforators and of intrinsic microvascular compartments among them increases progressively. However, the composition and the width of the resulting intrinsic capillary plexuses remain essentially unchanged through the brain entire maturation. Although the adult brain is considerably larger than the newborn one, there are no appreciable differences in their microvasculature (Fig. 8b, c). The preservation of the gray matter intrinsic microvascular compartment overall dimension, composition, and organization reflects its unique role as a biological constant needed for the functional activities of gray matter neurons regardless of brain size and/or age (Marín-Padilla 2012). Moreover, the similar width of the intrinsic microvascular compartments, between perforating vessels, throughout the brain gray matter reflects the optimal size for the oxygen diffusion needed for their functional activity and represents a biological constant. During the cortex prenatal and postnatal maturations, the intrinsic capillaries grow by local angiogenesis in response to the neurons functional needs. Both
18 18 M. Marín-Padilla Fig. 9 Composite figure of photomicrographs (a f) showing sprouting capillaries, from preexisting ones, with searching radiating filopodia, from the cerebral cortex of 12-day-old hamster embryos. A polyp-like head composed of sliding endothelial cells with radiating filopodia characterizes a sprouting capillary. The searching filopodia, measure between 3 and 7 μm, will determine the direction of the growing capillary. In my opinion, they also participate in searching for and in interconnecting approaching capillaries, in reestablishing a common lumen between them, and in the building of a three-dimensional anastomotic capillary plexus between contiguous perforating vessels (From Marín-Padilla 1988) capillary angiogenesis and reabsorption are found throughout the developing gray matter. Although sprouting brain capillaries have been known for some time (Klosouskii 1963), they have received little attention in the literature (Puelles et al. 1976; Marín-Padilla 1988). Moreover, Cajal recognized these capillary excrescences, in Golgi preparations, but considered them to represent glial processes (Cajal 1911). In Golgi preparations, endothelial cell sprouts emerge from existing intrinsic capillaries and form a polyp-like structure composed of sliding endothelial cells with numerous fine filopodia that radiate in all directions (Fig. 9a e). The filopodia measure between 3 and 7 μm in length. These growing intrinsic capillaries interconnect as well as disconnect responding to local gray matter neurons functional
19 Cerebral Microvessels 19 needs. They establish an ever-changing three-dimensional anastomotic capillary plexus in the gray matter between perforating vessels (Figs. 6, 7, and 9). They also establish vascular loops between the different levels of the intrinsic microvascular compartment. A short and thin excrescence still attached to the capillary wall characterizes a reabsorbing capillary. How growing intrinsic capillaries encounter each other, interconnect, and reestablish a common lumen remains poorly understood. However, intercapillary connections among intrinsic capillaries occur continuously during the gray matter maturation. In my opinion, the leading endothelial cell filopodia of growing capillaries play important roles in searching for approaching capillaries, in interconnecting them, and in reestablishing a common lumen between them (Marín-Padilla 1988). Perhaps supporting this idea is the presence, in the cortex of 12-day-old hamster embryos, of small complex capillaries as the one shown in Fig. 10. These small capillaries have a small and irregular main lumen and additional peripheral smaller ones, all containing electron dense (basal lamina) material (Fig. 10). Tight junctions are recognized between the endothelial cells as well as between some peripheral filopodia (Fig. 10, arrows). Peripheral filopodia have established spaces between them also filled with electron dense (basal lamina) material (Fig. 10). These complex capillaries could represent a stage in the process of interconnecting approaching ones by their leading filopodia (Marín-Padilla 1988). Golgi Fig. 10 Electron photomicrograph of a small and complex capillary with a central lumen (white start) and several peripheral ones (*) all containing electron dense (basal lamina) material, from a 12-day-old hamster embryo cortex. The capillary seems to be the result of the confluence of approaching capillary endothelial cell filopodia that have already established a small central lumen and several peripheral smaller ones. Tight junctions separate the capillary endothelial cells as well as some of the peripheral filopodia (arrows). This capillary could represent one stage in the process of the interconnecting approaching ones, establishing of a common lumen and several smaller ones and with still unresolved peripheral endothelial cell filopodia (From Marín-Padilla 1988)
20 20 M. Marín-Padilla preparations are inadequate to further resolve this problem. Perhaps, tridimensional electron microscopic studies could help in solving it. The number of gray matter perforators and hence of intrinsic capillary plexuses among them is by far the greater throughout the brain. The abundance of intrinsic capillaries and their direct connections to pial capillaries protect the gray matter neurons from hypoxic, hemorrhagic, and/or traumatic perinatal brain damage (Marín-Padilla 1996, 1997, 1999). In perinatal brain damage, the less vascularized white matter is by far more frequently damaged (infarcted) than the overlying gray matter. The gray matter overlying white matter infarcts survives, often for life, because its microvascular system remains essentially intact as well as directly connected to pial capillaries. Local arterial and venous perforators, of the overlying gray matter, establish an interconnecting venous plexus bordering the damaged white matter that permits the circulation of blood (Marín-Padilla 1997). Although blood continues to circulate through the gray matter overlying white matter infarcts, its neurons are incapable of receiving and/or sending information through the infarcted regions. The functionally isolated gray matter neurons continue to exchange information, but only among them, and undergo morphological and functional transformations. In my opinion, morphologically and functionally transformed gray matter neurons overlying white matter infarcts play a significant role in the pathogenesis of ensuing neurological sequelae, including epilepsy (Marín-Padilla 1996, 1997, 1999, 2000; Marín-Padilla et al. 2002). Outlooks The little attention that the cerebral cortex microvascularization normal and/or altered has received in the literature despite its importance and clinical relevance is indeed surprising. The cortex gray matter intrinsic microvascular compartment through ongoing angiogenesis, reabsorption, and remodeling may be among the most active microvascular systems of the human body. During the brain s entire functional maturation, the intrinsic microvascular compartment overall dimension, width, and composition remain essentially unchanged regardless of its gradual increase in both size and age. During the brain maturation, the formation and maintenance of the gray matter intrinsic anastomotic capillary plexus between contiguous perforators imply a series of events which are not yet clearly understood. Why are these capillaries capable of perforating through the V-RC outer glial wall to enter into the nervous tissue but incapable of reentering them? Why are intrinsic capillaries capable of exiting the perforating vessels but incapable of reentering them? What is the nature of the single basal lamina that surrounds the gray matter intrinsic capillaries? How do these growing intrinsic capillaries encounter each other, become interconnected, and establish a common lumen between them? What mechanisms regulate the interexchange of information among local neurons, protoplasmic astrocytes, and intrinsic capillaries for controlling the local blood flow in response to neurons functional demands? What is the renewal capability of gray matter intrinsic capillaries throughout the individuals life?
21 Cerebral Microvessels 21 In my opinion, the similar distance among perforators and hence the similar width of the intrinsic microvascular compartments throughout the cerebral cortex gray matter represent a biological constant needed for the functional activities of its neurons regardless of age and/or size. The optimal oxygen diffusion through gray matter neurons may become determined by the constant width of the intrinsic capillary plexus through the brain entire developmental maturation. The distance macrophages could travel to and from contiguous V-RCs and may also become determined by the similar and constant separation between perforating vessels. An interesting physiological possibility is that the similar intervascular distance between perforators and, hence, the width of the intrinsic microvascular compartment predicts the functional activities of gray matter neurons as well as the width (about 500 μm) of Mountcastle s functional columns (1987). Further inquires and investigations will be needed to explore the above propositions. None of the propositions is applicable to the white matter with fewer perforating vessels and larger as well as variable intervascular distances. References Andres KH (1976a) Überdie Feinstrukture der Arachnoidea und Dura mater von Mammalia. Zeitschrift Zellsforschung Microskoscopic Anat 82: Andres KH (1976b) Zur Feinstruktur der Arachnoidal zotten bei Mammalia. Zeitschrift Zellsforchung Mikroskoscopic Anat 82: Bär T, Wolff JR (1972) The formation of capillary basement membranes during internal vascularization of the rat s cerebral cortex. Zellsforchung Microskopic Anat 133: Blinkov SM, Glezer II (1968) The human brain in figures and tables. Plenum Press/Basic Books, New York Buitrago-Delgado E, Nordin K, Rao A, Geary L, LaBonne C (2015) Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells. Science 384: Cajal S y R (1911) Histologie du Systéme Nerveux de L homme et des Vertebrés. Maloine, Paris. (Reprinted by CSIC, Madrid 1952) Casley-Smith AB, Földi-Börcsök E, Földi M (1976) The prelymphatic pathways of the brain as revealed by cervical lymphatic obstruction and the passage of particles. Br J Exp Pathol 57: Dorovini-Zis K (2015) In: Dorovini-Zis K (ed) The blood-brain barrier in health and disease, vol 1, Morphology, biology, and immune function. CRC Press/Taylor & Francis Group, Boca Raton/New York Duvernoy HM, Delon S, Vannson JL (1981) Cortical blood vessels of the human brain. Brain Res Bull 7: Gamble HJ (1975) Preliminary observations on the vascularization of the human embryonic brain. J Anat 118: Golgi C (1873) Sulla sostanza grigia del cervello. Gaz Med Ital Lombardia 6: Hamilton WJ, Boyd JD, Mossman HW (1972) Human embryology. Heffer, Cambridge, UK Hauw JJ, Berger JR, Escourolle R (1975) Electron microscopic study of the developing capillaries of the human brain. Acta Neuropathol 31: Hossmann KA (1994) Viability thresholds and the penumbra of focal ischemia. Ann Neurol 36: Jeffrey JI, Wang M, Liao J, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Golman SA, Nagelhus BA, Nedergard M (2012) Paravascular pathway facilitates CSF flow
Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture
 Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture 2 The prenatal developmental of the mammalian cerebral cortex, including that of humans, is characterized by two sequential and interrelated
Mammalian Cerebral Cortex: Embryonic Development and Cytoarchitecture 2 The prenatal developmental of the mammalian cerebral cortex, including that of humans, is characterized by two sequential and interrelated
Developmental Aspects of the Intracerebral Microvasculature and Perivascular Spaces: Insights into Brain Response to Late-Life Diseases
 J Neuropathol Exp Neurol Copyright Ó 2011 by the American Association of Neuropathologists, Inc. Vol. 70, No. 12 December 2011 pp. 1060Y1069 REVIEW ARTICLE Developmental Aspects of the Intracerebral Microvasculature
J Neuropathol Exp Neurol Copyright Ó 2011 by the American Association of Neuropathologists, Inc. Vol. 70, No. 12 December 2011 pp. 1060Y1069 REVIEW ARTICLE Developmental Aspects of the Intracerebral Microvasculature
Department of Cognitive Science UCSD
 Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Department of Cognitive Science UCSD Verse 1: Neocortex, frontal lobe, Brain stem, brain stem, Hippocampus, neural node, Right hemisphere, Pons and cortex visual, Brain stem, brain stem, Sylvian fissure,
Nervous system. Dr. Rawaa Salim Hameed
 Nervous system Dr. Rawaa Salim Hameed Central nervous system (CNS) CNS consists of the brain (cerebrum, cerebellum, and brainstem) and spinal cord CNS is covered by connective tissue layers, the meninges
Nervous system Dr. Rawaa Salim Hameed Central nervous system (CNS) CNS consists of the brain (cerebrum, cerebellum, and brainstem) and spinal cord CNS is covered by connective tissue layers, the meninges
Histology of the CNS
 Histology of the CNS Lecture Objectives Describe the histology of the cerebral cortex layers. Describe the histological features of the cerebellum; layers and cells of cerebellar cortex. Describe the elements
Histology of the CNS Lecture Objectives Describe the histology of the cerebral cortex layers. Describe the histological features of the cerebellum; layers and cells of cerebellar cortex. Describe the elements
Central Nervous System - Brain & Cranial Nerves. Chapter 14 Part A
 Central Nervous System - Brain & Cranial Nerves Chapter 14 Part A Central Nervous System Central nervous system (CNS) is responsible for: Receiving impulses from receptors Integrating information Sending
Central Nervous System - Brain & Cranial Nerves Chapter 14 Part A Central Nervous System Central nervous system (CNS) is responsible for: Receiving impulses from receptors Integrating information Sending
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves
 Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Organization of The Nervous System PROF. SAEED ABUEL MAKAREM
 Organization of The Nervous System PROF. SAEED ABUEL MAKAREM Objectives By the end of the lecture, you should be able to: List the parts of the nervous system. List the function of the nervous system.
Organization of The Nervous System PROF. SAEED ABUEL MAKAREM Objectives By the end of the lecture, you should be able to: List the parts of the nervous system. List the function of the nervous system.
Biological Bases of Behavior. 3: Structure of the Nervous System
 Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Biological Bases of Behavior 3: Structure of the Nervous System Neuroanatomy Terms The neuraxis is an imaginary line drawn through the spinal cord up to the front of the brain Anatomical directions are
Organization of The Nervous System PROF. MOUSAED ALFAYEZ & DR. SANAA ALSHAARAWY
 Organization of The Nervous System PROF. MOUSAED ALFAYEZ & DR. SANAA ALSHAARAWY Objectives At the end of the lecture, the students should be able to: List the parts of the nervous system. List the function
Organization of The Nervous System PROF. MOUSAED ALFAYEZ & DR. SANAA ALSHAARAWY Objectives At the end of the lecture, the students should be able to: List the parts of the nervous system. List the function
The Human Brain. Prenatal Development and Structure. Bearbeitet von Miguel Marín-Padilla
 The Human Brain Prenatal Development and Structure Bearbeitet von Miguel Marín-Padilla 1st Edition. 2010. Buch. xii, 145 S. Hardcover ISBN 978 3 642 14723 4 Format (B x L): 19,3 x 26 cm Gewicht: 563 g
The Human Brain Prenatal Development and Structure Bearbeitet von Miguel Marín-Padilla 1st Edition. 2010. Buch. xii, 145 S. Hardcover ISBN 978 3 642 14723 4 Format (B x L): 19,3 x 26 cm Gewicht: 563 g
The CNS Part II pg
 The CNS Part II pg. 455-474 Protection of the Brain Objectives Describe how the meninges, cerebrospinal fluid, and the blood brain barrier protect the CNS. Explain how Cerebrospinal fluid is formed, and
The CNS Part II pg. 455-474 Protection of the Brain Objectives Describe how the meninges, cerebrospinal fluid, and the blood brain barrier protect the CNS. Explain how Cerebrospinal fluid is formed, and
Ventricles, CSF & Meninges. Steven McLoon Department of Neuroscience University of Minnesota
 Ventricles, CSF & Meninges Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Thursday (Sept 14) 8:30-9:30am Surdyk s Café in Northrop Auditorium Stop by for a minute or an
Ventricles, CSF & Meninges Steven McLoon Department of Neuroscience University of Minnesota 1 Coffee Hour Thursday (Sept 14) 8:30-9:30am Surdyk s Café in Northrop Auditorium Stop by for a minute or an
Development of the Nervous System 1 st month
 Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Development of the Nervous System 1 st month day 1 - fertilization of egg day 6 - uterine implantation day 18 - trilaminar (3-layered) disc (blastoderm, embryo) ectoderm (dorsal) - nervous system and skin
Development of the Nervous System. Leah Militello, class of 2018
 Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
Development of the Nervous System Leah Militello, class of 2018 Learning Objectives 1. Describe the formation and fate of the neural tube and neural crest including timing and germ layer involved. 2. Describe
Central nervous system (CNS): brain and spinal cord Collections of cell body and dendrites (grey matter) are called nuclei/nucleus Nucleus can also
 Chapter 3 Part 1 Orientation Directions in the nervous system are described relatively to the neuraxis An imaginary line drawn through the center of the length of the central nervous system, from the bottom
Chapter 3 Part 1 Orientation Directions in the nervous system are described relatively to the neuraxis An imaginary line drawn through the center of the length of the central nervous system, from the bottom
Human Motor Cortex Excitatory Inhibitory Neuronal Systems: Development and Cytoarchitecture
 Human Motor Cortex Excitatory Inhibitory Neuronal Systems: Development and Cytoarchitecture 6 An essential feature of the mammalian cerebral cortex is the sequential establishment of excitatory inhibitory
Human Motor Cortex Excitatory Inhibitory Neuronal Systems: Development and Cytoarchitecture 6 An essential feature of the mammalian cerebral cortex is the sequential establishment of excitatory inhibitory
The 7 th lecture. Anatomy and Physiology For the. 1 st Class. By Dr. Ala a Hassan Mirza
 The 7 th lecture In Anatomy and Physiology For the 1 st Class By Dr. Ala a Hassan Mirza Nervous System (part I) The Nerve Tissue and the Nervous System The Tissues of the Body There are 4 types of tissues
The 7 th lecture In Anatomy and Physiology For the 1 st Class By Dr. Ala a Hassan Mirza Nervous System (part I) The Nerve Tissue and the Nervous System The Tissues of the Body There are 4 types of tissues
Human Histology The Nervous System. Dr. Rawaa Salim Hameed
 Human Histology The Nervous System Dr. Rawaa Salim Hameed The organization of the nervous system Anatomically, the nervous system is divided into:- Neurohistology Structurally, nerve tissue consists of
Human Histology The Nervous System Dr. Rawaa Salim Hameed The organization of the nervous system Anatomically, the nervous system is divided into:- Neurohistology Structurally, nerve tissue consists of
Neurodevelopment II Structure Formation. Reading: BCP Chapter 23
 Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
Neurodevelopment II Structure Formation Reading: BCP Chapter 23 Phases of Development Ovum + Sperm = Zygote Cell division (multiplication) Neurogenesis Induction of the neural plate Neural proliferation
The Nervous System. Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes =
 The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
The Nervous System Functions of the Nervous System input gathering To monitor occurring inside and outside the body Changes = To process and sensory input and decide if is needed output A response to integrated
V. CENTRAL NERVOUS SYSTEM TRAUMA
 V. CENTRAL NERVOUS SYSTEM TRAUMA I. Concussion - Is a clinical syndrome of altered consiousness secondary to head injury - Brought by a change in the momentum of the head when a moving head suddenly arrested
V. CENTRAL NERVOUS SYSTEM TRAUMA I. Concussion - Is a clinical syndrome of altered consiousness secondary to head injury - Brought by a change in the momentum of the head when a moving head suddenly arrested
Neuroanatomy. Assistant Professor of Anatomy Faculty of Medicine The University of Jordan Dr Maha ELBeltagy
 Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Development of the Central Nervous System Development of the nervous system Development
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Development of the Central Nervous System Development of the nervous system Development
Neurons vs. glia. Traditionally, glia have been viewed as passive cells that help to maintain the function of neurons.
 GLIA Neurons vs. glia The defining characteristic of a neuron is its ability to transmit rapid electrical signals in the form of action potentials. All other neural cells that lack this property are broadly
GLIA Neurons vs. glia The defining characteristic of a neuron is its ability to transmit rapid electrical signals in the form of action potentials. All other neural cells that lack this property are broadly
What Cell Make Up the Brain and Spinal Cord
 What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
What Cell Make Up the Brain and Spinal Cord Jennifer LaVail, Ph.D. (http://anatomy.ucsf.edu/pages/lavaillab/index.html) What kinds of cells are these?" Neuron?" Epithelial cell?" Glial cell?" What makes
HST-583 Cerebrovascular anatomy and neural regulation of CNS blood flow
 HST.583: Functional Magnetic Resonance Imaging: Data Acquisition and Analysis Harvard-MIT Division of Health Sciences and Technology Dr. Randy Gollub HST-583 Cerebrovascular anatomy and neural regulation
HST.583: Functional Magnetic Resonance Imaging: Data Acquisition and Analysis Harvard-MIT Division of Health Sciences and Technology Dr. Randy Gollub HST-583 Cerebrovascular anatomy and neural regulation
SOME BASIC TERMINOLOGY CNS: Central Nervous System: Brain + Spinal Cord
 SOME BASIC TERMINOLOGY CNS: Central Nervous System: Brain + Spinal Cord CEREBROSPINAL FLUID (CSF): The fluid filling the ventricles, cerebral aqueduct, central canal, and subarachnoid space. It is a filtrate
SOME BASIC TERMINOLOGY CNS: Central Nervous System: Brain + Spinal Cord CEREBROSPINAL FLUID (CSF): The fluid filling the ventricles, cerebral aqueduct, central canal, and subarachnoid space. It is a filtrate
The Nervous System An overview
 Nervous System The Nervous System An overview Includes Nerve tissue Sense organs Functions to Sense environment Process information it receives Respond to information 1 Copyright 2009 Pearson Education,
Nervous System The Nervous System An overview Includes Nerve tissue Sense organs Functions to Sense environment Process information it receives Respond to information 1 Copyright 2009 Pearson Education,
Chapter 7 Nerve tissue 1 Liu Jiamei
 Chapter 7 Nerve tissue 1 Liu Jiamei General description: nerve tissue nerve cells (neurons): show numerous long processes receive the stimulation make contact with each other, conduct the nerve impulse
Chapter 7 Nerve tissue 1 Liu Jiamei General description: nerve tissue nerve cells (neurons): show numerous long processes receive the stimulation make contact with each other, conduct the nerve impulse
Sinusoids and venous sinuses
 LYMPHOID SYSTEM General aspects Consists of organs that are made of lymphoid tissue; Immune defense Breakdown of red blood cells. 1 Sinusoids In place of capillaries Endothelium; often fenestrated More
LYMPHOID SYSTEM General aspects Consists of organs that are made of lymphoid tissue; Immune defense Breakdown of red blood cells. 1 Sinusoids In place of capillaries Endothelium; often fenestrated More
Anatomy of the Nervous System. Brain Components
 Anatomy of the Nervous System Brain Components NERVOUS SYSTEM INTRODUCTION Is the master system of human body, controlling the functions of rest of the body systems Nervous System CLASSIFICATION A. Anatomical
Anatomy of the Nervous System Brain Components NERVOUS SYSTEM INTRODUCTION Is the master system of human body, controlling the functions of rest of the body systems Nervous System CLASSIFICATION A. Anatomical
b. The groove between the two crests is called 2. The neural folds move toward each other & the fuse to create a
 Chapter 13: Brain and Cranial Nerves I. Development of the CNS A. The CNS begins as a flat plate called the B. The process proceeds as: 1. The lateral sides of the become elevated as waves called a. The
Chapter 13: Brain and Cranial Nerves I. Development of the CNS A. The CNS begins as a flat plate called the B. The process proceeds as: 1. The lateral sides of the become elevated as waves called a. The
Medical Neuroscience Tutorial Notes
 Medical Neuroscience Tutorial Notes Blood Supply to the Brain MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning
Medical Neuroscience Tutorial Notes Blood Supply to the Brain MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning
Lymphoid Organs. Dr. Sami Zaqout. Dr. Sami Zaqout IUG Faculty of Medicine
 Lymphoid Organs Dr. Sami Zaqout Cells of the Immune System Lymphocytes Plasma cells Mast cells Neutrophils Eosinophils Cells of the mononuclear phagocyte system Distribution of cells of the immune system
Lymphoid Organs Dr. Sami Zaqout Cells of the Immune System Lymphocytes Plasma cells Mast cells Neutrophils Eosinophils Cells of the mononuclear phagocyte system Distribution of cells of the immune system
Chapter 3. Structure and Function of the Nervous System. Copyright (c) Allyn and Bacon 2004
 Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Chapter 3 Structure and Function of the Nervous System 1 Basic Features of the Nervous System Neuraxis: An imaginary line drawn through the center of the length of the central nervous system, from the
Nervous system is the most complex system in our body. It is formed by a network of more than 100 million nerve cells (neurons) assisted by many more
 Nervous system Nervous system is the most complex system in our body. It is formed by a network of more than 100 million nerve cells (neurons) assisted by many more glial cells. Devoid from connective
Nervous system Nervous system is the most complex system in our body. It is formed by a network of more than 100 million nerve cells (neurons) assisted by many more glial cells. Devoid from connective
Human Anatomy and Physiology I Laboratory
 Human Anatomy and Physiology I Laboratory Histology of Nervous Tissue and The Spinal Cord This lab involves two laboratory exercises: 1) Histology of Nervous Tissue, and 2) Spinal Cord, Spinal Nerves,
Human Anatomy and Physiology I Laboratory Histology of Nervous Tissue and The Spinal Cord This lab involves two laboratory exercises: 1) Histology of Nervous Tissue, and 2) Spinal Cord, Spinal Nerves,
Central Nervous System: Part 2
 Central Nervous System: Part 2 1. Meninges 2. CSF 3. Spinal Cord and Spinal Nerves Explain spinal cord anatomy, including gray and white matter and meninges (give the general functions of this organ).
Central Nervous System: Part 2 1. Meninges 2. CSF 3. Spinal Cord and Spinal Nerves Explain spinal cord anatomy, including gray and white matter and meninges (give the general functions of this organ).
CSF. Cerebrospinal Fluid(CSF) System
 Cerebrospinal Fluid(CSF) System By the end of the lecture, students must be able to describe Physiological Anatomy of CSF Compartments Composition Formation Circulation Reabsorption CSF Pressure Functions
Cerebrospinal Fluid(CSF) System By the end of the lecture, students must be able to describe Physiological Anatomy of CSF Compartments Composition Formation Circulation Reabsorption CSF Pressure Functions
Brain Meninges, Ventricles and CSF
 Brain Meninges, Ventricles and CSF Lecture Objectives Describe the arrangement of the meninges and their relationship to brain and spinal cord. Explain the occurrence of epidural, subdural and subarachnoid
Brain Meninges, Ventricles and CSF Lecture Objectives Describe the arrangement of the meninges and their relationship to brain and spinal cord. Explain the occurrence of epidural, subdural and subarachnoid
Nerve tissue & the Nervous System
 Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
Nerve tissue & the Nervous System The human nervous system, by far the most complex system in the body, is formed by a network of many billion nerve cells (neurons), all assisted by many more supporting
Development of Spinal Cord & Vertebral Column. Dr. Sanaa Alshaarawi & Prof. Ahmed Fathalla
 Development of Spinal Cord & Vertebral Column Dr. Sanaa Alshaarawi & Prof. Ahmed Fathalla OBJECTIVES At the end of the lecture, students should be able to: q Describe the development of the spinal cord
Development of Spinal Cord & Vertebral Column Dr. Sanaa Alshaarawi & Prof. Ahmed Fathalla OBJECTIVES At the end of the lecture, students should be able to: q Describe the development of the spinal cord
Nervous system part 1. Danil Hammoudi.MD
 Nervous system part 1 Danil Hammoudi.MD The central nervous system (CNS) is formed by : the brain spinal cord. These elements are enclosed within the skull and spinal vertebral canal. They are covered
Nervous system part 1 Danil Hammoudi.MD The central nervous system (CNS) is formed by : the brain spinal cord. These elements are enclosed within the skull and spinal vertebral canal. They are covered
Basic Histology. By Mrs. Bailey
 Basic Histology By Mrs. Bailey Primary Tissues 1. Epithelial Tissue 2. Connective Tissue 3. Muscle Tissue 4. Nervous Tissue Very cellular Supported by underlying connective tissue Epithelial & connective
Basic Histology By Mrs. Bailey Primary Tissues 1. Epithelial Tissue 2. Connective Tissue 3. Muscle Tissue 4. Nervous Tissue Very cellular Supported by underlying connective tissue Epithelial & connective
Nervous Systems: Diversity & Functional Organization
 Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Duus' Topical Diagnosis in Neurology
 Duus' Topical Diagnosis in Neurology Anatomy - Physiology - Signs - Symptoms Bearbeitet von Michael Frotscher 1. Auflage 2005. Taschenbuch. 532 S. Paperback ISBN 978 3 13 612804 6 Format (B x L): 19 x
Duus' Topical Diagnosis in Neurology Anatomy - Physiology - Signs - Symptoms Bearbeitet von Michael Frotscher 1. Auflage 2005. Taschenbuch. 532 S. Paperback ISBN 978 3 13 612804 6 Format (B x L): 19 x
2. Subarachnoid Hemorrhage
 Causes: 2. Subarachnoid Hemorrhage A. Saccular (berry) aneurysm - Is the most frequent cause of clinically significant subarachnoid hemorrhage is rupture of a saccular (berry) aneurysm. B. Vascular malformation
Causes: 2. Subarachnoid Hemorrhage A. Saccular (berry) aneurysm - Is the most frequent cause of clinically significant subarachnoid hemorrhage is rupture of a saccular (berry) aneurysm. B. Vascular malformation
Review of Nervous System Anatomy
 For the real amazement, if you wish to be amazed, is this process. You start out as a single cell derived from the coupling of a sperm and an egg; this divides in two, then four, then eight, and so on,
For the real amazement, if you wish to be amazed, is this process. You start out as a single cell derived from the coupling of a sperm and an egg; this divides in two, then four, then eight, and so on,
Cellular components of CNS
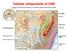 Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
Cellular components of CNS Cellular components of CNS Neurons Glial cells: Astrocytes (including radial glia), oligodendrocytes, microglia, ependymal cells Epithelial cells of choroid plexus Endothelial
Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System. Assoc.Prof. E.Elif Güzel, M.D.
 Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System Assoc.Prof. E.Elif Güzel, M.D. Third week of Embryogenesis Primitive streak/pit appears on the epiblast (day
Early Development of Neural Tube Development of Medulla Spinalis and Peripheral Nervous System Assoc.Prof. E.Elif Güzel, M.D. Third week of Embryogenesis Primitive streak/pit appears on the epiblast (day
The Nervous System. PowerPoint Lecture Slides C H A P T E R 7. Prepared by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slides Prepared by Patty Bostwick-Taylor, Florence-Darlington Technical College C H A P T E R 7 The Nervous System NERVOUS SYSTEM OVERVIEW Essential Question: What are the primary functions
PowerPoint Lecture Slides Prepared by Patty Bostwick-Taylor, Florence-Darlington Technical College C H A P T E R 7 The Nervous System NERVOUS SYSTEM OVERVIEW Essential Question: What are the primary functions
Chapter 12b. Overview
 Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
The cells of the nervous system
 The cells of the nervous system LESSON N.9 - PSYCHOBIOLOGY because of the location and volume as compared to our body, the brain has always been a matter of conjecture about its fundamental role in the
The cells of the nervous system LESSON N.9 - PSYCHOBIOLOGY because of the location and volume as compared to our body, the brain has always been a matter of conjecture about its fundamental role in the
NERVOUS TISSUE. 1. Functional units of the nervous system; receive, process, store and transmit information to other neurons, muscle cells or glands.
 NERVOUS TISSUE LEARNING OBJECTIVES 1. Characterize and contrast the structure of neuronal cell bodies, dendrites and axons 2. List the classification of synapses and identify the basic structures of a
NERVOUS TISSUE LEARNING OBJECTIVES 1. Characterize and contrast the structure of neuronal cell bodies, dendrites and axons 2. List the classification of synapses and identify the basic structures of a
Nervous System: An Introduction. HAP Susan Chabot Lemon Bay High School
 Nervous System: An Introduction HAP Susan Chabot Lemon Bay High School Function of the Nervous System 3 overlapping functions SENSORY INPUT - Monitor changes inside and outside of the body; these changes
Nervous System: An Introduction HAP Susan Chabot Lemon Bay High School Function of the Nervous System 3 overlapping functions SENSORY INPUT - Monitor changes inside and outside of the body; these changes
8.2. Types of Neurons
 Chapter 8 Nervous Tissue The neuron is the functional and the structural unit of the nervous system. It displays two highly developed physiological traits: 1. Irritability - the capacity to generate a
Chapter 8 Nervous Tissue The neuron is the functional and the structural unit of the nervous system. It displays two highly developed physiological traits: 1. Irritability - the capacity to generate a
Nervous System. Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI
 Nervous System Overview.I Histology.II Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI Repairs.VII Pathology.VIII.IV 1 Controls and integrates all body activities
Nervous System Overview.I Histology.II Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI Repairs.VII Pathology.VIII.IV 1 Controls and integrates all body activities
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon
 Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Dendrites Receive impulse from the axon of other neurons through synaptic connection. Conduct impulse towards the cell body Axon Page 22 of 237 Conduct impulses away from cell body Impulses arise from
Nervous system. The main regulation mechanism of organism's functions
 Nervous system The main regulation mechanism of organism's functions Questions Neuron The reflex arc The nervous centers Properties of the nervous centers The general principles of coordination Inhibition
Nervous system The main regulation mechanism of organism's functions Questions Neuron The reflex arc The nervous centers Properties of the nervous centers The general principles of coordination Inhibition
8: Lymphatic vessels and lymphoid tissue. nur
 8: Lymphatic vessels and lymphoid tissue nur Lymphatic vascular system Functions return to the blood extracellular fluid (Lymph) from connective tissue spaces. ensures the return of water, electrolytes
8: Lymphatic vessels and lymphoid tissue nur Lymphatic vascular system Functions return to the blood extracellular fluid (Lymph) from connective tissue spaces. ensures the return of water, electrolytes
action potential afferent neuron Weblike; specifically, the weblike middle layer of the three meninges. arachnoid astrocytes autonomic nervous system
 action potential A large transient depolarization event, including polarity reversal, that is conducted along the membrane of a muscle cell or a nerve fiber. afferent neuron Nerve cell that carries impulses
action potential A large transient depolarization event, including polarity reversal, that is conducted along the membrane of a muscle cell or a nerve fiber. afferent neuron Nerve cell that carries impulses
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
Lymphatic System and Immunity. Lymphatic System
 Lymphatic System and Immunity Lymphatic System Lymphatic System High hydrostatic pressure in the arterioles and capillaries at the arterial part of the circulation leads to move plasma fluid from the capillaries
Lymphatic System and Immunity Lymphatic System Lymphatic System High hydrostatic pressure in the arterioles and capillaries at the arterial part of the circulation leads to move plasma fluid from the capillaries
The peripheral (secondary) lymphoid tissues
 The peripheral (secondary) lymphoid tissues The peripheral (secondary) lymphoid tissues : are the lymph nodes, spleen, Mucosal associated lymphoid tissue (MALT). All secondary lymphoid organs have one
The peripheral (secondary) lymphoid tissues The peripheral (secondary) lymphoid tissues : are the lymph nodes, spleen, Mucosal associated lymphoid tissue (MALT). All secondary lymphoid organs have one
Histology of the Eye
 Histology of the Eye Objectives By the end of this lecture, the student should be able to describe: The general structure of the eye. The microscopic structure of:»cornea.»retina. EYE BULB Three coats
Histology of the Eye Objectives By the end of this lecture, the student should be able to describe: The general structure of the eye. The microscopic structure of:»cornea.»retina. EYE BULB Three coats
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 14 The Brain and Cranial Nerves Introduction The purpose of the chapter is to: 1. Understand how the brain is organized, protected, and supplied
Principles of Anatomy and Physiology 14 th Edition CHAPTER 14 The Brain and Cranial Nerves Introduction The purpose of the chapter is to: 1. Understand how the brain is organized, protected, and supplied
Chapter 9. Nervous System
 Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 9 Nervous System Central Nervous System (CNS) vs. Peripheral Nervous System(PNS) CNS Brain Spinal cord PNS Peripheral nerves connecting CNS to the body Cranial nerves Spinal nerves Neurons transmit
Chapter 8 Nervous System
 Chapter 8 Nervous System Two message centers: Functions of these systems: 1. * 2. * Overview of the Nervous System Parts: General Functions: Functions Sensory input: Sensation via nerves Integration: interpretation
Chapter 8 Nervous System Two message centers: Functions of these systems: 1. * 2. * Overview of the Nervous System Parts: General Functions: Functions Sensory input: Sensation via nerves Integration: interpretation
Urinary system. Urinary system
 INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
INTRODUCTION. Several organs system Produce urine and excrete it from the body Maintenance of homeostasis. Components. two kidneys, produce urine; two ureters, carry urine to single urinary bladder for
Neocortex. Cortical Structures in the Brain. Neocortex Facts. Laminar Organization. Bark-like (cortical) structures: Shepherd (2004) Chapter 12
 Neocortex Shepherd (2004) Chapter 12 Rodney Douglas, Henry Markram, and Kevan Martin Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Cortical Structures in the Brain Bark-like (cortical) structures:
Neocortex Shepherd (2004) Chapter 12 Rodney Douglas, Henry Markram, and Kevan Martin Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Cortical Structures in the Brain Bark-like (cortical) structures:
Lecture 9. General Medicine_3rd semester
 Lecture 9 General Medicine_3rd semester MICROSCOPIC STRUCTURE AND DEVELOPMENT OF THE CENTRAL AND PERIPHERAL NERVOUS SYSTEM Structure of gray matters in the CNS: Iso- and allocortex, cerebellar cortex,
Lecture 9 General Medicine_3rd semester MICROSCOPIC STRUCTURE AND DEVELOPMENT OF THE CENTRAL AND PERIPHERAL NERVOUS SYSTEM Structure of gray matters in the CNS: Iso- and allocortex, cerebellar cortex,
Lesson 33. Objectives: References: Chapter 16: Reading for Next Lesson: Chapter 16:
 Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Vascular Pattern in Tumours
 Acta Radiologica ISSN: 0001-6926 (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/iaro20 Vascular Pattern in Tumours To cite this article: (1957) Vascular Pattern in Tumours, Acta Radiologica,
Acta Radiologica ISSN: 0001-6926 (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/iaro20 Vascular Pattern in Tumours To cite this article: (1957) Vascular Pattern in Tumours, Acta Radiologica,
Fig The C.S. of the Spinal Cord A ganglion is a term for a collection of perikarya located outside of the CNS. In certain regions of the spinal
 Chapter 9 Nervous System The nervous system is divided into two components: The CNS - the brain and spinal cord and the PNS - the nerves emanating from the spinal cord and brain that distribute to other
Chapter 9 Nervous System The nervous system is divided into two components: The CNS - the brain and spinal cord and the PNS - the nerves emanating from the spinal cord and brain that distribute to other
Brain Stem. Nervous System (Part A-3) Module 8 -Chapter 14
 Nervous System (Part A-3) Module 8 -Chapter 14 Overview Susie Turner, M.D. 1/9/13 Cellular structure of the nervous system Neurons Neuroglia Nervous System Divisions Central nervous system Peripheral nervous
Nervous System (Part A-3) Module 8 -Chapter 14 Overview Susie Turner, M.D. 1/9/13 Cellular structure of the nervous system Neurons Neuroglia Nervous System Divisions Central nervous system Peripheral nervous
The Nervous System PART C. PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Nervous System 7 PART C Protection of the Central Nervous System Scalp and skin Skull and vertebral
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Nervous System 7 PART C Protection of the Central Nervous System Scalp and skin Skull and vertebral
Spinal Cord and Properties of Cerebrospinal Fluid: Options for Drug Delivery. SMA Foundation New York
 Spinal Cord and Properties of Cerebrospinal Fluid: Options for Drug Delivery New York Why Do We Need to Know about the Spinal Cord Anatomy and Properties of Cerebrospinal Fluid? SMA therapeutics need to
Spinal Cord and Properties of Cerebrospinal Fluid: Options for Drug Delivery New York Why Do We Need to Know about the Spinal Cord Anatomy and Properties of Cerebrospinal Fluid? SMA therapeutics need to
Meninges and Ventricles
 Meninges and Ventricles Irene Yu, class of 2019 LEARNING OBJECTIVES Describe the meningeal layers, the dural infolds, and the spaces they create. Name the contents of the subarachnoid space. Describe the
Meninges and Ventricles Irene Yu, class of 2019 LEARNING OBJECTIVES Describe the meningeal layers, the dural infolds, and the spaces they create. Name the contents of the subarachnoid space. Describe the
Characteristic features of CNS pathology. By: Shifaa AlQa qa
 Characteristic features of CNS pathology By: Shifaa AlQa qa Normal brain: - The neocortex (gray matter): six layers: outer plexiform, outer granular, outer pyramidal, inner granular, inner pyramidal, polymorphous
Characteristic features of CNS pathology By: Shifaa AlQa qa Normal brain: - The neocortex (gray matter): six layers: outer plexiform, outer granular, outer pyramidal, inner granular, inner pyramidal, polymorphous
Introduction and Basic structural organization of the nervous system
 Introduction and Basic structural organization of the nervous system **the slides are in bold and the book is in red Done by : razan krishan & marah marahleh INTRODUCTION The nervous system, along with
Introduction and Basic structural organization of the nervous system **the slides are in bold and the book is in red Done by : razan krishan & marah marahleh INTRODUCTION The nervous system, along with
Biology Dr. Khalida Ibrahim Nervous system The nervous system is responsible for communication between different regions of the body, it is divided
 Biology Dr. Khalida Ibrahim Nervous system The nervous system is responsible for communication between different regions of the body, it is divided into: CNS (central nervous system) = brain + spinal cord
Biology Dr. Khalida Ibrahim Nervous system The nervous system is responsible for communication between different regions of the body, it is divided into: CNS (central nervous system) = brain + spinal cord
SPINAL CORD AND PROPERTIES OF CEREBROSPINAL FLUID: OPTIONS FOR DRUG DELIVERY
 SPINAL CORD AND PROPERTIES OF CEREBROSPINAL FLUID: OPTIONS FOR DRUG DELIVERY WHY DO WE NEED TO KNOW ABOUT THE SPINAL CORD ANATOMY AND PROPERTIES OF CEREBROSPINAL FLUID? SMA therapeutics need to reach cells
SPINAL CORD AND PROPERTIES OF CEREBROSPINAL FLUID: OPTIONS FOR DRUG DELIVERY WHY DO WE NEED TO KNOW ABOUT THE SPINAL CORD ANATOMY AND PROPERTIES OF CEREBROSPINAL FLUID? SMA therapeutics need to reach cells
The Circulatory System
 The Circulatory System Dr. Sami Zaqout The circulatory system Circulatory system Blood vascular systems Lymphatic vascular systems Blood vascular systems Blood vascular systems The circulatory system Circulatory
The Circulatory System Dr. Sami Zaqout The circulatory system Circulatory system Blood vascular systems Lymphatic vascular systems Blood vascular systems Blood vascular systems The circulatory system Circulatory
Human Anatomy. Spinal Cord and Spinal Nerves
 Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Nervous System. Lecture 4
 Nervous System Lecture 4 Neurons Functional unit of the nervous system Also called the nerve cell Soma or body Axon Dendrites Neuroglial cells support cells Schwann cells produce myelin in PNS Oligodendrocytes
Nervous System Lecture 4 Neurons Functional unit of the nervous system Also called the nerve cell Soma or body Axon Dendrites Neuroglial cells support cells Schwann cells produce myelin in PNS Oligodendrocytes
Nervous System: Part IV The Central Nervous System The Brain
 Nervous System: Part IV The Central Nervous System The Brain Can you survive when part of your brain is destroyed? 2 Essential Knowledge 3.D.2 2. Cells communicate with each other through direct contact
Nervous System: Part IV The Central Nervous System The Brain Can you survive when part of your brain is destroyed? 2 Essential Knowledge 3.D.2 2. Cells communicate with each other through direct contact
Nervous System: An Introduction. HAP Susan Chabot Lemon Bay High School
 Nervous System: An Introduction HAP Susan Chabot Lemon Bay High School Function of the Nervous System 3 overlapping functions SENSORY INPUT - Monitor changes inside and outside of the body; these changes
Nervous System: An Introduction HAP Susan Chabot Lemon Bay High School Function of the Nervous System 3 overlapping functions SENSORY INPUT - Monitor changes inside and outside of the body; these changes
Biology 218 Human Anatomy
 Chapter 17 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Overview of the Nervous System (p. 537) 1. The nervous system and the endocrine system are the body s major control and integrating centers.
Chapter 17 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Overview of the Nervous System (p. 537) 1. The nervous system and the endocrine system are the body s major control and integrating centers.
The Nervous System: Neural Tissue Pearson Education, Inc.
 13 The Nervous System: Neural Tissue Introduction Nervous System Characteristics Controls and adjust the activity of the body Provides swift but brief responses The nervous system includes: Central Nervous
13 The Nervous System: Neural Tissue Introduction Nervous System Characteristics Controls and adjust the activity of the body Provides swift but brief responses The nervous system includes: Central Nervous
CEREBROVASCULAR DISEASES. By: Shifaa AlQa qa
 CEREBROVASCULAR DISEASES By: Shifaa AlQa qa Cerebrovascular diseases Brain disorders caused by pathologic processes involving blood vessels 3 pathogenic mechanisms (1) thrombotic occlusion, (2) embolic
CEREBROVASCULAR DISEASES By: Shifaa AlQa qa Cerebrovascular diseases Brain disorders caused by pathologic processes involving blood vessels 3 pathogenic mechanisms (1) thrombotic occlusion, (2) embolic
Central Nervous System (CNS) -> brain and spinal cord. Major Divisions of the nervous system:
 Central Nervous System (CNS) -> brain and spinal cord Major Divisions of the nervous system: Afferent (sensory input) -> cell bodies outside of the central nervous system (CNS), carry info into the CNS
Central Nervous System (CNS) -> brain and spinal cord Major Divisions of the nervous system: Afferent (sensory input) -> cell bodies outside of the central nervous system (CNS), carry info into the CNS
Vascular Malformations of the Brain. William A. Cox, M.D. Forensic Pathologist/Neuropathologist. September 8, 2014
 Vascular Malformations of the Brain William A. Cox, M.D. Forensic Pathologist/Neuropathologist September 8, 2014 Vascular malformations of the brain are classified into four principal groups: arteriovenous
Vascular Malformations of the Brain William A. Cox, M.D. Forensic Pathologist/Neuropathologist September 8, 2014 Vascular malformations of the brain are classified into four principal groups: arteriovenous
Development of Brain Stem, Cerebellum and Cerebrum
 Development of Brain Stem, Cerebellum and Cerebrum The neural tube cranial to the 4th pair of somites develop into the brain. 3 dilatations and 2 flexures form at the cephalic end of the neural tube during
Development of Brain Stem, Cerebellum and Cerebrum The neural tube cranial to the 4th pair of somites develop into the brain. 3 dilatations and 2 flexures form at the cephalic end of the neural tube during
Embryology of the Nervous System. Steven McLoon Department of Neuroscience University of Minnesota
 Embryology of the Nervous System Steven McLoon Department of Neuroscience University of Minnesota In the blastula stage embryo, the embryonic disk has two layers. During gastrulation, epiblast cells migrate
Embryology of the Nervous System Steven McLoon Department of Neuroscience University of Minnesota In the blastula stage embryo, the embryonic disk has two layers. During gastrulation, epiblast cells migrate
The subarachnoid space develops early in the human embryonic period
 O R I G I N A L A R T I C L E Folia Morphol. Vol. 64, No. 3, pp. 212 216 Copyright 2005 Via Medica ISSN 0015 5659 www.fm.viamedica.pl The subarachnoid space develops early in the human embryonic period
O R I G I N A L A R T I C L E Folia Morphol. Vol. 64, No. 3, pp. 212 216 Copyright 2005 Via Medica ISSN 0015 5659 www.fm.viamedica.pl The subarachnoid space develops early in the human embryonic period
Histology Urinary system
 Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Announcement. Danny to schedule a time if you are interested.
 Announcement If you need more experiments to participate in, contact Danny Sanchez (dsanchez@ucsd.edu) make sure to tell him that you are from LIGN171, so he will let me know about your credit (1 point).
Announcement If you need more experiments to participate in, contact Danny Sanchez (dsanchez@ucsd.edu) make sure to tell him that you are from LIGN171, so he will let me know about your credit (1 point).
LYMPH GLAND. By : Group 1
 LYMPH GLAND By : Group 1 ANATOMY LYMPH NODE Lymphatic Organs Red bone marrow Thymus gland Lymph nodes Lymph nodules Spleen Primary organs Secondary organs Lymph Nodes Firm, smooth-surfaced, bean-shaped
LYMPH GLAND By : Group 1 ANATOMY LYMPH NODE Lymphatic Organs Red bone marrow Thymus gland Lymph nodes Lymph nodules Spleen Primary organs Secondary organs Lymph Nodes Firm, smooth-surfaced, bean-shaped
POST-INJURY INTERVALS 1
 POST-INJURY INTERVALS 1 Introduction 1 Contusion dating 2 Skin 2 Brain 5 Hypoxic/ischemic injury and increased intracranial pressure 18 Brain incidentals (non-injurious) 21 Sexual violence 27 INTRODUCTION
POST-INJURY INTERVALS 1 Introduction 1 Contusion dating 2 Skin 2 Brain 5 Hypoxic/ischemic injury and increased intracranial pressure 18 Brain incidentals (non-injurious) 21 Sexual violence 27 INTRODUCTION
NOTES: CH 40 Introduction to Human Anatomy & Physiology
 NOTES: CH 40 Introduction to Human Anatomy & Physiology THE HUMAN BODY Anatomy Physiology (= structures) (= functions or processes) Characteristics of LIFE: 1) Made up of 1 or more CELLS. 2) Obtain and
NOTES: CH 40 Introduction to Human Anatomy & Physiology THE HUMAN BODY Anatomy Physiology (= structures) (= functions or processes) Characteristics of LIFE: 1) Made up of 1 or more CELLS. 2) Obtain and
