NEW/REVISED MATERIAL: Presented in italicized text EFFECTIVE DATE: January 1, 2008 GUIDELINES FOR REPORTING ADMINISTRATION OF EPOGEN MEDICARE
|
|
|
- Suzanna Hamilton
- 6 years ago
- Views:
Transcription
1 DISCLAIMER: Please be advised that while every effort has been made to ensure the accuracy of the information provided according to the most current LCD pertaining to the subject, periodic change to rules and coverage may occur. ICD-9-CM diagnosis codes are updated on the 1 st of October and HCPCS codes on the 1 st of January annually. NEW/REVISED MATERIAL: Presented in italicized text EFFECTIVE DATE: January 1, 2008 GUIDELINES FOR REPORTING ADMINISTRATION OF EPOGEN MEDICARE An erythropoietin stimulating agent (ESA) is an analog of erythropoietin. ESAs are biologically engineered hormones produced by recombinant DNA technology. Erythropoietin analogs contain the identical amino acid sequence as naturally occurring erythropoietin, and have the same biological effect. Primarily, the kidneys produce erythropoietin in response to hypoxia. Both erythropoietin and ESAs stimulate the bone marrow to form new red blood cells. They are used to treat anemia by elevating or maintaining the red blood cell level (as demonstrated by the hematocrit and/or hemoglobin levels), therefore decreasing anemia and the need for transfusions. In March 2007, the FDA issued new warnings against target HCT levels about 12 g/dl (36% hct) for all patients. The FDA also issued specific warnings against off-label use in cancer patients whose anemia is not directly linked to chemotherapy. The FDA also reminded physicians that the main endpoint in studies for on-label indications has been avoidance or reduction in transfusions. This updated copy contains descriptions of specific coverage guidelines and documentation that supports medical necessity for individual patients. INDICATIONS: Erythropoietin analogues are covered for the following indications: 1. Treatment of anemia associated with chronic renal failure, including August 2006 Page 1 of 38
2 patients on dialysis and patients not on dialysis; 2. Treatment of significant anemia in patients with non-myeloid malignancies where anemia is due to the effect of concomitantly administered chemotherapy; 3. Treatment of anemia induced by AZT and/or other*nucleoside Reverse Transcriptase Inhibitors (NRTI) used in treatment of HIV/AIDS; *Note: This update is effective 02/01/08 4. Treatment of selected patients with anemia related to myelodysplastic syndrome; 5. Perisurgical adjuvant therapy (epoetin alfa only); 6. Treatment of anemia of selected chronic diseases: rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel diseases, and hepatitis C undergoing treatment. The following causes of anemia should be considered, documented, and corrected (when possible) before starting erythropoietin analogue therapy for any of the covered indications: iron deficiency; underlying infection or inflammatory process; underlying hematological disease; hemolysis; vitamin deficiencies (e.g. folic acid or B12) blood loss; aluminum intoxication There are rare patients whose cardiac, pulmonary or other medical diseases warrant the use of ESAs to maintain a hemoglobin/hematocrit (Hb/HCT) higher than the target level. Documentation to support this practice must be available upon request. This does not apply to ESA therapy for anemia related to cancer chemotherapy, which follows the rules mandated by the National Coverage Decision. August 2006 Page 2 of 38
3 LIMITATIONS OF COVERAGE AND/OR MEDICAL NECESSITY During therapy treatment Epoetin alfa, virtually all patients will eventually require supplemental iron. Store of iron should be regularly monitored to ensure a transferrin saturation greater than 20% and/or serum ferritin levels greater than 100 mg/ml, in order to guide appropriate supplementation. Therapy Goal: 1. For patients receiving chemotherapy for non-myeloid malignancies, the goal of therapy is to maintain the Hb/HCT at 10/30. ESA therapy will not be reimbursed when the Hb/Hct is greater than 10/ For all other indications, the goal of therapy is to maintain a stable Hb/HCT, with a target of g/dl / 30-36%. Doses must be titrated according to the patient s response. Therapy need not be stopped completely simply due to the achievement of the target hgb/hct. However, judicious, appropriately timed dose adjustments are expected to prevent inappropriate increases in hgb/hct levels. (CMS PUB 100-2, Chapter 15, Paragraph and CMS Pub 100-4, chapter 8, paragraph 60.4) ADMINISTRATION ROUTE: 1. Intravenous, or 2. subcutaneous DOSAGE: The dosage may be dependent on several factors including the: 1. Availability of iron stores 2. The baseline hgb/hct, and the 3. Presence of concurrent medical problems. August 2006 Page 3 of 38
4 COVERAGE CRITERIA: A. For End Stage Renal Disease (ESRD) patients on dialysis: 1. Diagnosis of end stage renal disease; 2. Anemia of ESRD with a: Hb less than 10 gm/dl, or a HCT of less than 30% at initiation of therapy. B. For chronic kidney disease patients NOT on dialysis: 1. Anemia with Hb/HCT less than 10 / 30% at initiation of therapy. 2. Serum creatinine equal to or greater than 3, creatinine clearance less than 60 ml/min, or glomerular filtration rate (GFR) less than 60 ml/min/1.73 m2; C. For patients with non-myeloid malignancies where anemia is due to the effect of chemotherapy: 1. Anemia with Hb/HCT less than 10 / 30% prior to initiation of therapy. 2. The starting dose for ESA treatment is no more than 150 U/kg/3 times weekly for epoetin and 2.25 mcg/kg/1 time weekly for darpoetin alpha. Equivalent doses may be given over other FDA approved time periods. 3. The maintenance dose of ESA therapy is the same as the starting dose if the Hb/HCT level remains below 10/30 4 weeks after initiation of therapy AND the rise in Hb/HCT is >1/3. 4. If Hb/HCT rises <1/3 compared to pretreatment baseline after 4 weeks of therapy and Hb/HCT level remains <10/30, the above starting dose may be increased once by 25%. Continued use of the drug is not reasonable and August 2006 Page 4 of 38
5 necessary if the Hb/HCT rises <1/3 after 8 weeks of treatment. 5. Continued administration of the drug is not reasonable and necessary if there is a rapid rise in Hb/HCT >1/3 over 2 weeks of treatment unless the Hb/HCT remains below or subsequently falls to <10/30. Continuation and reinstitution of ESA therapy must include a dose reduction of 25% from previously administered dose. 6. The FDA labeling states that ESAs are indicated for treatment of anemia of malignancy when receiving concomitant chemotherapy, which means during an established course of planned chemotherapy. It will also cover ESAs for eight weeks following the final dose of myelosuppressive chemotherapy in a chemotherapy regimen. D. For patients with anemia related to AZT and/or other NRTI therapy for HIV/AIDS: 1. Anemia with Hb/HCT less than 10 / 30% at initiation of therapy. E. For patients with myelodysplastic syndrome: 1. Low risk myelodysplasia with less than 5% blasts. 2. Pretreatment erythropoietin levels of 100 IU/L or less. 3. Anemia with Hb/HCT less than 10 / 30% at initiation of therapy. If after two months of treatment, there is no significant increase in hgb/hct and/or a significant decrease in transfusion requirements, erythropoietin analogs therapy should be stopped. F. Perisurgical adjuvant therapy: epoetin alfa for patients who: 1. Are undergoing hip or knee surgery; 2. Have an anemia with a Hb between 10 and 13 gm/dl; 3. Are not a candidate for autologous blood transfusion; August 2006 Page 5 of 38
6 4. Are expected to lose more than two units of blood; 5. Have been evaluated to ensure that their anemia is due to chronic disease. G. For patients with anemia of chronic disease: 1. Anemia with Hb/HCT less than 10 / 30% at initiation of therapy. Currently there is evidence of patient benefit using ESA therapy to reduce transfusions for selected patients with significant refractory and symptomatic anemia who have: inflammatory diseases (rheumatoid arthritis, Crohn s disease, ulcerative colitis), and hepatitis C with anemia due to the medication therapy. Until further publications show clear benefit, ESAs for anemia of other chronic diseases other than those listed above will not be covered. Note: Use the lowest dose of an ESA that will gradually increase the Hb concentration to the lowest level sufficient to avoid the need for red blood cell transfusion. Other Coverage Comments: A claim that does not fulfill the coverage requirements described above and is not denied in the national coverage decision may be given individual consideration based on review of all pertinent medical information. Limitation of liability and refund requirements apply when denials are based on medical necessity. The provider/supplier must notify the beneficiary in writing, prior to rendering the service, if the provider/supplier is aware that the test, item or procedure may not be considered medically necessary by Medicare. The limitation of liability and refund requirements do not apply when the test, item or procedure is statutorily excluded, has no Medicare benefit category or is rendered for August 2006 Page 6 of 38
7 screening purposes. In these instances it is recommended, although not required, that the provider notify the beneficiary in writing with a Notice of Exclusion of Medicare Benefits (NEMB). DOCUMENTATION REQUIREMENTS: Medical record documentation must be legible and maintained in the patient s medical record, and meet the criteria as contained in these guidelines. Medical records such as physician s (or non-physician practioner s) order must be made available upon request of NGS. Documentation the provider is to maintain in the patient s medical record should include: 1. Patient s weight in kilograms. 2. EPO units administered per kilogram of body weight. 3. Medical justification for administration of EPO exceeding usual doses. Documentation supporting the indication for EPO must be made available upon the request of NGS. For all patients this includes: 1. hgb/hct, and 2. iron store levels. Regular reporting of Hgb/Hct is needed to show monitoring of ESA dose. Additional information requirements is determined by indication: A. Dialysis patients; Dialysis schedule Hgb/hct immediately prior to billing period. B. Chronic Renal Failure Non-dialysis patients: Serum creatinine Creatinine clearance, or GFR supporting a diagnosis of chronic renal failure C. Patients with myelodysplastic syndrome: bone marrow biopsy report, August 2006 Page 7 of 38
8 For patients on ESA therapy for MDS, initiated prior to 12 01/07, National Government Services requires that a physician s statement that the patient does have MDS be included in the medical record. For ESA therapy initiated on or after 12/01/07, a copy of the actual bone marrow report must be included in the medical record. MDS cannot be diagnosed definitively until a bone marrow biopsy is performed to confirm the diagnosis. date of initiation of erythropoietin analogue therapy, and response to erythropoietin analogue administration (change in Hb/HCT and/or transfusion requirements). CPT/HCPCS CODES FOR THE ADMINISTRATION OF THE MEDICATION: J0881 J0882 J0885 J0886 Q4081 Injection, darbepoetin alfa, 1 mcg (non-esrd use) Injection, darbepoetin alfa, 1 mcg ( for ESRD on dialysis) Injection, epoetin alfa, (for non-esrd use), 1000 units Injection, epoetin alfa, 1000 units (for ESRD on dialysis) Injection, epoetin alfa, 100 units ( for ESRD on dialysis) Therapeutic, prophylactic or diagnostic injection (specify substance), subcutaneous or intramuscular ICD-9 CODES THAT SUPPORT MEDICAL NECESSITY It is the responsibility of the provider to code to the highest level specified in the ICD-9-CM (e.g., to the fourth or fifth digit). The correct use of an ICD-9-CM code listed below does not assure coverage of a service. The service must be reasonable and necessary in the specific case and must meet the criteria specified in this determination. August 2006 Page 8 of 38
9 For patients on dialysis: Both diagnoses must be on claim. PRIMARY DX. CODE Anemia in chronic kidney disease SECONDARY DX. CODE End Stage Renal Disease For patients with Chronic Kidney Disease (not yet on dialysis) and Anemia: Must include and one other listed diagnosis. PRIMARY DX. CODE Anemia in chronic kidney disease SECONDARY DX. CODE * Hypertensive chronic kidney disease, malignant, with chronic kidney disease stage I through stage IV, or unspecified Hypertensive chronic kidney disease, malignant with chronic kidney disease stage V or end stage renal disease * Hypertensive chronic kidney disease, benign, with chronic kidney disease stage I through stage IV, or unspecified Hypertensive chronic kidney disease, benign, with chronic kidney disease stage V or end stage renal disease * Hypertensive chronic kidney disease, unspecified, with chronic kidney disease stage I through stage IV, or unspecified Hypertensive chronic kidney disease, unspecified, with chronic kidney disease stage V or end stage renal disease * Hypertensive heart and chronic kidney disease, malignant, without heart failure and with chronic kidney disease stage I through stage IV, or unspecified * Hypertensive heart and chronic kidney disease, malignant, with heart failure and with chronic kidney disease stage I through stage IV, or unspecified Hypertensive heart and chronic kidney disease, malignant, without heart failure and with chronic kidney disease stage V or end stage renal disease Hypertensive heart and chronic kidney disease, malignant, with heart failure and chronic kidney disease stage V or end stage renal disease August 2006 Page 9 of 38
10 404.10* Hypertensive heart and chronic kidney disease, benign, without heart failure and with chronic kidney disease stage I through stage IV, or unspecified * Hypertensive heart and chronic kidney disease, benign, with heart failure and with chronic kidney disease stage I through stage IV, or unspecified Hypertensive heart and chronic kidney disease, benign, without heart failure and with chronic kidney disease stage V or end stage renal disease Hypertensive heart and chronic kidney disease, benign, with heart failure and chronic kidney disease stage V or end stage renal disease * Hypertensive heart and chronic kidney disease, unspecified, without heart failure and with chronic kidney disease stage I through stage IV, or unspecified * Hypertensive heart and chronic kidney disease, unspecified, with heart failure and with chronic kidney disease stage I through stage IV, or unspecified Hypertensive heart and chronic kidney disease, unspecified, without heart failure and with chronic kidney disease stage V or end stage renal disease Hypertensive heart and chronic kidney disease, unspecified, with heart failure and chronic kidney disease stage V or end stage renal disease SECONDARY OR THIRD DX. CODE Chronic kidney disease, Stage III (moderate) Chronic kidney disease, Stage IV (severe) Chronic kidney disease, Stage V *Note: Patients with stage I and II do not meet the creatinine clearance or GFR requirements in Coverage Criteria B. For patients with anemia related to treatment with zidovudine (AZT) and/or other Nucleoside Reverse Transcriptase Inhibitors (NRTI) for HIV disease. Must have (aplastic anemia due to drugs) and either 042 or on claim. PRIMARY DX. August 2006 Page 10 of 38
11 CODE 042 Human immunodeficiency virus (HIV) disease SECONDARY DX. CODES Other specified aplastic anemia (due to drugs) E931.7 Antiviral drug causing adverse effects in therapeutic use OR PRIMARY DX. CODE Other specified aplastic anemia (due to drugs) SECONDARY DX. CODES E931.7 Antiviral drug causing adverse effects in therapeutic use Human immunodeficiency virus, type 2 (HIV-2) For patients with anemia related to chemotherapy: Must have (representing the aplastic anemia related to chemotherapy) plus E930.7 or (whichever is appropriate) plus the non-myeloid malignancy for which the chemotherapy was administered. (Note: , , are myeloid malignancies and are excluded from coverage.) PRIMARY DX. CODE Other specified aplastic anemia (due to drugs) Inc: Aplastic anemia due to chemotherapy and immunotherapy SECONDARY DX. CODE E933.1 Antineoplastic and immunosuppressive drugs causing adverse effects in therapeutic use Or E930.7 Antineoplastic antibiotic causing adverse effects in therapeutic use August 2006 Page 11 of 38
12 ADDITIONAL DIAGNOSIS CODE Appropriate code for the malignancy MALIGNANT NEOPLASM OF LIP Malignant neoplasm of; upper lip, vermilion border Lower lip, vermilion border Upper lip, inner aspect Lower lip, inner aspect Lip unspecified, inner aspect Commissure of lip Other sites of lip Lip, unspecified, vermilion border MALIGNANT NEOPLASM OF TONGUE Malignant neoplasm of; base of tongue Dorsal surface of tongue Tip and lateral border of tongue Ventral surface of tongue Anterior two-thirds of tongue part unspecified Junctional zone of tongue Lingual tonsil Other site of tongue Tongue unspecified MALIGNANT NEOPLASM OF MAJOR SALIVARY GLANDS Malignant neoplasm of; parotid gland Submandibular gland Sublingual gland Other major salivary glands Salivary gland unspecified MALIGNANT NEOPLASM OF GUM Malignant neoplasm of; upper gum Lower gum Other sites of gum Gum unspecified MALIGNANT NEOPLASM OF FLOOR OF MOUTH Malignant neoplasm of; anterior portion of floor of mouth Lateral portion of floor of mouth Other sites of floor of mouth August 2006 Page 12 of 38
13 144.9 Floor of mouth part unspecified MALIGNANT NEOPLASM OF OTHER AND UNSPECIFIED PARTS OF MOUTH Malignant neoplasm of; cheek mucosa Vestibule of mouth Hard palate Soft palate Uvula Palate unspecified Retromolar area Other specified parts of mouth Mouth unspecified MALIGNANT NEOPLASM OF OROPHARYNX Malignant neoplasm of; tonsil Tonsillar fossa Tonsillar pillars (anterior) (posterior) Vallecula epiglottica Anterior aspect of epiglottis Junctional region of oropharynx Lateral wall of oropharynx Posterior wall of oropharynx Other specified sites of oropharynx Oropharynx, unspecified site MALIGNANT NEOPLASM OF NASOPHARYNX Malignant neoplasm of; superior wall of nasopharynx Posterior wall of naspharynx Laterall wall of nasopharynx Anterior wall of nasopharynx Other specified sites of nasopharynx Nasopharynx unspecified site MALIGNANT NEOPLASM OF HYPOPHARYNX Malignant neoplasm of; postcricoid region of hyphopharynx Pyriform sinus Aryepiglottic fold hypoharyngeal aspect Posterior hypopharyngeal wall Other specified sites of hypopharynx Hypopharynx unspecified site MALIGNANT NEOPLASM OF OTHER AND ILL-DEFINED SITES WITHIN THE LIP, ORAL CAVITY, AND PHARYNX Pharynx, unspecified Waldeyer s ring August 2006 Page 13 of 38
14 149.8 Other sites within the lip and oral cavity Ill-defined sites within the lip and oral cavity MALIGNANT NEOPLASM OF ESOHAGUS Malignant neoplasm of; cervical esophagus Thoracic esophagus Abdominal esophagus Upper third of esophagus Middle third of esophagus Lower third of esophagus Other specified part of esophagus Esophagus unspecified site MALIGNANT NEOPLASM OF STOMACH Malignant neoplasm of; stomach cardia Stomach pylorus Pyloric antrum of stomach Fundus of stomach Body of stomach Lesser curvature of stomach unspecified Greater curvature of stomach Unspecified Other specified sites of stomach Stomach unspecified site MALIGNANT NEOPLASM OF SMALL INTESTINE, INCLUDING DUODENUM Malignant neoplasm of; duodenum Jejunum Ileum Meckel s diverticulum Other specified sites of small intestine Small intestine unspecified site MALIGNANT NEOPLASM OF COLON Malignant neoplasm of; hepatic flexure colon Transverse colon Descending colon Sigmoid colon Cecum Appendix vermiformis Ascending colon Splenic flexure Other specified sites of large intestine August 2006 Page 14 of 38
15 153.9 Colon, unspecified site MALIGNANT NEOPLASM OF RECTUM, RECTOSIGMOID JUNCTION, AND ANUS Malignant neoplasm of; rectosigmoid junction Rectum Anal canal Anus unspecified site Other sites of rectum, rectosigmoid Junction, and anus MALIGNANT NEOPLASM OF LIVER AND INTRAHEPATIC BILE DUCTS Malignant neoplasm of; liver primary Intrahepatic bile ducts Liver not specified as primary or Secondary MALIGNANT NEOPLASM OF GALLBLADDER AND EXTRAHEPATIC BILE DUCTS Malignant neoplasm of; gallbladder Extrahepatic bile ducts Ampulla of vater Other specified sites of gallbladder and extrahepatic bile ducts Biliary tract, part unspecified MALIGNANT NEOPLASM OF PANCREAS Malignant neoplasm of; head of pancreas Body of pancreas Tail of pancreas Pancreatic duct Islet of langerhans Other specified sites of pancreas Pancreas part, unspecified MALIGNANT NEOPLASM OF RETROPERITONEUM AND PERITONEUM Malignant neoplasm of; retroperitoneum Specified parts of peritoneum Peritoneum unspecified MALIGNANT NEOPLASM OF OTHER AND ILL-DEFINED SITES WITHIN THE DIGESTIVE ORGANS AND PERITONEUM Malignant neoplasm of; Intestinal tract, part unspecified Spleen, not elsewhere classified Other sites of digestive system and intra-abdominal organs August 2006 Page 15 of 38
16 159.9 Ill-defined sites within the digestive organs and peritoneum MALIGNANT NEOPLASM OF NASAL CAVITIES, MIDDLE EAR, AND ACCESSORY SINUSES Malignant neoplasm of; nasal cavities Auditory tube, middle ear, and mastoid air cells Maxillary sinus Ethmoidal sinus Frontal sinus Sphenoidal sinus Other accessory sinuses Accessory sinus unspecified MALIGNANT NEOPLASM OF LARYNX Malignant neoplasm of; Glottis Supraglottis Subglottis Laryngeal cartilages Other specified sites of larynx Larynx, unspecified MALIGNANT NEOPLASM OF TRACHEA, BRONCHUS, AND LUNG Malignant neoplasm of; trachea Main bronchus Upper lobe bronchus or lung Middle lobe bronchus or lung Lower lobe bronchus or lung Other parts of bronchus or lung Bronchus and lung unspecified MALIGNANT NEOPLASM OF PLEURA Malignant neoplasm of; parietal pleura Visceral pleura Other specified sites of pleura Pleura unspecified MALIGNANT NEOPLASM OF THYMUS, HEART, AND MEDIASTINUM Malignant neoplasm of; thymus Heart Anterior mediastinum Posterior mediastinum Other parts of mediastinum Mediastinum, part unspecified August 2006 Page 16 of 38
17 MALIGNANT NEOPLASM OF OTHER AND ILL-DEFINED SITES WITHIN THE RESPIRATORY SYSTEM AND INTRATHORACIC ORGANS Malignant neoplasm of; upper respiratory tract, part unspecified Other sites within the respiratory system and intrathoracic organs Ill-defined sites within the respiratory system MALIGNANT NEOPLASM OF BONE AND ARTICULAR CARTILAGE Malignant neoplasm of; bones of skull and face except mandible Mandible Vertebral column excluding sacrum and Coccyx Ribs, sternum, and clavicle Scapula and long bones of upper limb Short bones of upper limb Pelvic bones, sacrum and coccyx Long bones of Short bones of Bone and articular cartilage, site unspecified MALIGNANT NEOPLASM OF CONNECTIVE TISSUE AND OTHER SOFT TISSUE Malignant neoplasm of; connective and other soft tissue of Head, face, and neck Connective and other soft tissue of upper limb, including shoulder Connective and other soft tissue of including hip Connective and other soft tissue of Thorax Connective and other soft tissue of Abdomen Connective and other soft tissue of Pelvis Connective and other soft tissue of Trunk, unspecified Other specified sites of connective and other soft tissue Connective and other soft tissue, site unspecified August 2006 Page 17 of 38
18 MALIGNANT MELANOMA OF SKIN Malignant melanoma of; skin of lip Skin of eyelid including canthus Skin of ear and external auditory canal Skin of other and unspecified parts of Face Skin of scalp and neck Skin of trunk except scrotum Skin of upper limb including shoulder Skin of including hip Other specified sites of skin Skin, site unspecified OTHER MALIGNANT NEOPLASM OF SKIN Other malignant neoplasm of; skin of lip Skin of eyelid including canthus Skin of ear and external auditory canal Skin of other and unspecified parts of face Scalp and skin of neck Skin of trunk except scrotum Skin of upper limb including Shoulder Skin of including hip Other specified sites of skin Skin, site unspecified MALIGNANT NEOPLASM OF FEMALE BREAST Malignant neoplasm of; nipple and areola of female breast Central portion of female breast Upper-inner quadrant of female breast Lower-inner quadrant of female breast Upper-outer quadrant of female breast Lower-outer quadrant of female breast Axillary tail of female breast Other specified sites of female breast Breast (female) unspecified site MALIGNANT NEOPLASM OF MALE BREAST Malignant neoplasm of; nipple and areola of male breast Other and unspecified sites of male Breast August 2006 Page 18 of 38
19 KAPOSIS S SARCOMA Kaposi s sarcoma; skin Soft tissue Palate Gastrointestinal sites Lung Lymph nodes Other specified sites Unspecified site MALIGNANT NEOPLASM OF GENITOURINARY ORGANS 179 Malignant neoplasm of uterus, part unspecified MALIGNANT NEOPLASM OF CERVIX UTERI Malignant neoplasm of; endocervix Exocervix Other specified sites of cervix Cervix uteri unspecified site MALIGNANT NEOPLASM OF PLACENTA 181 Malignant neoplasm of placenta MALIGNANT NEOPLASM OF BODY OF UTERUS Malignant neoplasm of; corpus uteri except isthmus Isthmus Other specified sites of body of uterus MALIGNANT NEOPLASM OF OVARY AND OTHER UTERINE ADNEXA Malignant neoplasm of; ovary Fallopian tube Broad ligament of uterus Parametrium Round ligaments of uterus Other specified sites of uterine adnexa Uterine adnexa uns, unspecified site MALIGNANT NEOPLASM OF OTHER AND UNSPECIFIED FEMALE GENITAL ORGANS Malignant neoplasm of; vagina Labia majora Labia minora Clitoris Vulva, unspecified site Other specified sites of female genital Organs Female genital organ, site unspecified August 2006 Page 19 of 38
20 MALIGNANT NEOPLASM OF PROSTATE 185 Malignant neoplasm of prostate MALIGNANT NEOPLASM OF TESTIS Malignant neoplasm of; undescended testis Other and unspecified testis MALIGNANT NEOPLASM OF PENIS AND OTHER MALE GENITAL ORGANS Malignant neoplasm of; prepuce Glans penis Body of penis Penis, part unspecified Epididymis Spermatic cord Scrotum Other specified site of male genital Organs Male genital organ, site unspecified MALIGNANT NEOPLASM BLADDER Malignant neoplasm of; trigone of urinary bladder Dome of urinary bladder Lateral wall urinary bladder Anterior wall of urinary bladder Posterior wall of urinary bladder Bladder neck Ureteric orifice Urachus Other specified sites of bladder Bladder, part unspecified MALIGNANT NEOPLASM OF KIDNEY AND OTHER AND UNSPECIFIED URINARY ORGANS Malignant neoplasm of;kidney except pelvis Renal pelvis Ureter Urethra Paraurethral glands Other specified sites of urinary organs Urinary organ, site unspecified MALIGNANT NEOPLASM OF EYE Malignant neoplasm of; eyeball except conjunctiva, cornea, retina, and choroid Orbit August 2006 Page 20 of 38
21 190.2 Lacrimal gland Conjunctiva Cornea Retina Choroid Lacrimal duct Other specified sites of eye Eye, part unspecified MALIGNANT NEOPLASM OF BRAIN Malignant neoplasm of; cerebrum except lobes and ventricles Frontal lobe Temporal lobe Parietal lobe Occipital lobe Ventricles Cerebellum NOS Brain stem Other parts of brain Brain, unspecified site MALIGNANT NEOPLASM OF OTHER AND UNSPECIFIED PARTS OF NERVOUS SYSTEM Malignant neoplasm of; cranial nerves Cerebral meninges Spinal cord Spinal meninges Other specified sites of nervous System Nervous system, part unspecified MALIGNANT NEOPLASM OF THYROID GLAND 193 Malignant neoplasm of thyroid gland MALIGNANT NEOPLASM OF OTHER ENDOCRINE GLANDS AND RELATED STRUCTURES Malignant neoplasm of; adrenal gland Parathyroid gland Pituitary gland and craniopharyngeal Duct Pineal gland Carotid body Aortic body and other paraganglia Other endocrine glands and related August 2006 Page 21 of 38
22 Structures Endocrine gland, site unspecified MALIGNANT NEOPLASM OF OTHER AND ILL-DEFINED SITES Malignant neoplasm of; head face and neck Thorax Abdomen Pelvis Upper limb Lower limb Other specified sites SECONDARY AND UNSPECIFIED MALIGNANT NEOPLASM OF LYMPH NODES Secondary and unspecified malignant neoplasm of lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper limb Lymph nodes of inguinal region and Intra-pelvic lymph nodes Lymph nodes of multiple sites Site unspecified SECONDARY MALIGNANT NEOPLASM OF RESPIRATORY AND DIGESTIVE SYSTEMS Secondary malignant neoplasm of; lung Mediastinum Pleura Other respiratory organs Small intestine including duodenum Large intestine and rectum Retroperitoneum and peritoneum Liver, specified as secondary Other digestive organs and spleen SECONDARY MALIGNANT NEOPLASM OF OTHER SPECIFIED SITES Secondary malignant neoplasm of; kidney Other urinary organs Skin Brain and spinal cord Other parts of nervous system Bone and bone marrow Ovary August 2006 Page 22 of 38
23 198.7 Adrenal gland Breast Genital organs Other specified sites MALIGNANT NEOPLASM WITHOUT SPECIFICATION OF SITE Disseminated malignant neoplasm Other malignant neoplasm of unspecified site LYMPHOSARCOMA AND RETICULOSARCOMA AND OTHER SPECIFIED MALIGNANT TUMORS OF LYMPHATIC TISSUE Reticulosarcoma unspecified site, extranodal and solid organ sites Reticulosarcoma involving lymph nodes of; head, face, and neck Intrathoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Lymphosarcoma unspecified site, extranodal and solid organ sites Lymphosarcoma involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Burkitt s tumor or lymphoma unspecified site, extranodal and solid organ sites Burkitt s tumor or lymphoma involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes August 2006 Page 23 of 38
24 Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Marginal zone lymphoma, unspecified site, extranodal and solid organ sites Marginal zone lymphoma involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Mantel cell lymphoma, unspecified site, extranodal and solid organ sites Mantel cell lymphoma, involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Primary central nervous system lymphoma, unspecified site, extranodal and solid organ sites Primary central nervous system lymphoma, involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes August 2006 Page 24 of 38
25 Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Anaplastic large cell lymphoma, unspecified site, extranodal and solid organ sites Anaplastic large cell lymphoma, involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Large cell lymphoma, unspecified site, extranodal and solid organ sites Large cell lymphoma, involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Other name variants of lymphosarcoma and reticulosarcoma, unspecified site, extranodal and solid organ sites Other name variants of lymphosarcoma and reticulosarcoma involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper August 2006 Page 25 of 38
26 Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites HODGKIN S DISEASE Hodgkin s paragranuloma unspecified site, extranodal and solid organ sites Hodgkin s paragranuloma, involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Hodgkin s granuloma, unspecified site, extranodal and solid organ sites Hodgkin s granuloma involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Hodgkin s sarcoma, unspecified site, extranodal and solid organ sites Hodgkin s sarcoma involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper August 2006 Page 26 of 38
27 Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Hodgkin s disease, lymphocitic-histiocytic predominance, unspecified site, extranodal and solid organ sites Hodgkin s disease, Lymphocytic-histiocytic predominance involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Hodgkin s disease, nodular sclerosis, unspecified site, extranodal and solid organ sites Hodgkin s disease, nodular sclerosis involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Hodgkin s disease, mixed cellularity, unspecified site, extranodal and solid organ sites Hodgkin s disease, mixed cellularity involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper August 2006 Page 27 of 38
28 Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Hodgkin s disease, lymphocytic depletion, unspecified site, extranodal and solid organ sites Hodgkin s disease, lymphocytic depletion, involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Hodgkin s disease, unspecified type, unspecified site, extranodal and solid organ sites Hodgkin s disease, unspecified type, involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites OTHER MALIGNANT NEOSPLASMS OF LYMPHOID AND HISTIOCYTIC TISSUE Nodular lymphoma, unspecified site, extranodal and solid organ sites Nodular lymphoma involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper August 2006 Page 28 of 38
29 Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Mycoses fungoides, unspecified site, extranodal and solid organ sites Mycoses fungoides involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Sezary s disease, unspecified site, extranodal and solid organ sites Sezary s disease involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Malignant histiocytosis, unspecified site, extranodal and solid organ sites Malignant histiocytosis involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and August 2006 Page 29 of 38
30 Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Leukemic reticuloendotheliosis, unspecified site, extranodal and solid organ sites Leukemic reticuloendotheliosis involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Letterer-Siwe disease, unspecified site, extranodal and solid organ sites Letterer-Siwe disease involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Malignant mast cell tumors, unspecified site, extranodal and solid organ sites Malignant mast cell tumors involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and August 2006 Page 30 of 38
31 Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Peripheral T-cell lymphoma, unspecified site, extranodal and solid organ sites Peripheral T-cell lymphoma involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Other malignant lymphomas, unspecified site, extranodal and solid organ sites Other malignant lymphomas involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites Other and unspecified malignant neoplasms of lymphoid and histiocytic tissue, unspecified site, extranodal and solid organ sites Other and unspecified malignant neoplasms of lymphoid and histiocytic tissue, involving lymph nodes of; head, face, and neck Intra-thoracic lymph nodes Intra-abdominal lymph nodes Lymph nodes of axilla and upper Lymph nodes of inguinal region and August 2006 Page 31 of 38
32 Intrapelvic lymph nodes Spleen Lymph nodes of multiple sites MULTIPLE MYELOMA AND IMMUNOPROLIFERATIVE NEOPLASMS Multiple myeloma; without mention of remission In remission Plasma cell leukemia; without remission In remission Other immunoproliferative neoplasms; without remission In remission LYMPHOID LEUKEMIA Lymphoid leukemia acute; without remission In remission Lymphoid leukemia chronic ; without remission In remission Lymphoid leukemia subacute; without remission In remission Other lymphoid leukemia; without remission In remission Unspecified lymphoid leukemia; without remission In remission CARCINOMA IN SITU Carcinoma in situ; unspecified female genital organ Vagina Vulva Other female genital organ NEOPLASM OF UNCERTAIN BEHAVIOR OF DIGESTIVE AND RESPIRATORY SYSTEMS Neoplasm of uncertain behavior of; major salivary glands Lip, oral cavity, and pharynx Stomach, intestines, and rectum Liver, and biliary passages Retroperitoneum, and peritoneum Other, and unspecified digestive Organs Larynx Trachea, bronchus, and lung Pleura, thymus, and mediastinum Other and unspecified respiratory August 2006 Page 32 of 38
33 organs NEOPLASM OF UNCERTAIN BEHAVIOR OF GENITOURINARY ORGANS Neoplasm of uncertain behavior of; uterus Placenta Ovary Other and unspecified female genital organs Testis Prostate Other and unspecified male genital organs Bladder Urinary organ unspecified Kidney and ureter Other and unspecified urinary Organs NEOPLASM OF UNCERTAIN BEHAVIOR OF ENDOCRINE GLANDS AND NERVOUS SYSTEM Neoplasm of uncertain behavior of; pituitary and craniopharyngeal duct Pineal gland Adrenal gland Paraganglia Other unspecified endocrine Glands Brain and spinal cord Meninges NEUROFIBROMATOSIS Neurofibromatosis, unspecified Neurofibromatosis, Type 1 (von recklinghausen s disease) Type 2 (acoustic neurofibromatosis) Neoplasm of uncertain behavior of other, and unspecified parts of nervous system NEOPLASM OF UNCERTAIN BEHAVIOR OF OTHER AND UNSPECIFIED SITES AND TISSUES Neoplasm of uncertain behavior of; bone and articular cartilage Connective and other soft tissue Skin Breast August 2006 Page 33 of 38
34 238.4 Polycythemia vera Neoplasm of uncertain behavior of; histiocytic and mast cells Plasma cells OTHER LYMPHATIC AND HEMATOPOIETIC TISSUES Essential thrombocytopenia Low grade myelodysplastic syndrome lesions Other lymphatic and hematopoietic tissues Neoplasm of uncertain behavior of; other specified sites Site unspecified NEOPLASM OF UNSPECIFIED NATURE Neoplasm of unspecified nature of; digestive system Respiratory system Bone, soft tissue, and skin Breast Bladder Other genitourinary organs Brain Endocrine glands and other parts of nervous system Other specified sites Site unspecified Macroglobulinemia V58.11 Encounter for antineoplastic chemotherapy V58.12 Encounter for immunotherapy for neoplastic condition For patients with anemia related to Myelodysplastic Syndrome: PRIMARY DX. CODE Low grade myelodysplastic syndrome lesions Inc. Refractory anemia (RA) Refractory anemia with ringed sideroblasts (RARS) Refractory cytopenia with multilineage dysplasia (RCMD) Refractory cytopenia with multilineage dysplasia and ringed siderobasts (RCMD-RS) August 2006 Page 34 of 38
35 For pre-operative use in specified patients: Preoperative use in patients undergoing hip or knee surgery. Must have a primary anemia diagnosis ( or 285.9), and other specified prophylactic measure diagnosis. PRIMARY DX. CODE Anemia of other chronic illness Or Anemia unspecified SECONDARY DX. CODE V07.8 Need for other specified prophylactic measure Note: Code also chronic disease causing anemia For patients with anemia of chronic inflammatory diseases and hepatitis C with anemia due medication therapy: Must include (anemia of other chronic disease) and one listed Hepatitis C (plus E931.7) or inflammatory diseases code. PRIMARY DX. CODE Anemia of other chronic illness SECONDARY DX. Appropriate code for the condition CODE VIRAL HEPATITIS C Acute hepatitis C with hepatic coma Chronic hepatitis C with hepatic coma Acute hepatitis C without mention of hepatic coma Chronic hepatitis C without mention of hepatic coma Unspecified viral hepatitis C without hepatic coma Unspecified viral hepatitis C with hepatic coma REGIONAL ENTERITIS Regional enteritis of; small intestine large intestine small intestine with large intestine Unspecified site ULCERATIVE COLITIS August 2006 Page 35 of 38
36 556.0 Ulcerative (chronic); enterocolitis Ileocolitis Proctitis Proctosigmoiditis Pseudopolyposis of colon Left-sided ulcerative (chronic) colitis Universal ulcerative (chronic) colitis Other ulcerative colitis Ulcerative colitis unspecified DIFFUSE DISEASE OF CONNECTIVE TISSUE Systemic lupus erythematosus RHEUMATOID ARTHRITIS Rheumatoid arthritis E Code E931.7 Antiviral drugs causing adverse effects in therapeutic use CODING TIP SEQUENCING: If a patient admission/encounter is: A. Solely for administration of chemotherapy, immunotherapy or radiation therapy assign: PRIMARY DX. CODE V58.11 Encounter for antineoplastic chemotherapy V58.12 Encounter for antineoplastic immunotherapy V58.0 Encounter for radiotherapy SECONDARY DX. Appropriate code for the malignancy CODE B. For management of an anemia associated with chemotherapy, immunotherapy and radiation therapy and the only treatment is for the anemia: PRIMARY DX. CODE Documented as aplastic anemia due to chemotherapy: Aplastic anemia Documented only as anemia due to chemotherapy: Unspecified anemia SECONDARY DX. August 2006 Page 36 of 38
37 CODE E933.1 Antineoplastic and immunosuppressive drugs causing adverse effects in therapeutic use ADDITIONAL DX. CODE Appropriate code for the malignancy C. For administration of chemotherapy, immunotherapy or radiation therapy assign and management of anemia of malignancy: PRIMARY DX. CODE V58.11 Encounter for antineoplastic chemotherapy V58.12 Encounter for antineoplastic immunotherapy V58.0 Encounter for radiotherapy SECONDARY DX. CODE Anemia in neoplastic disease ADDITIONAL DX. CODE Appropriate code for the malignancy HOSPITAL BILLING FOR EPOETIN ALFA (EPO) AND DARBEPOETIN ALFA (ARANESP) FOR NON-ESRD PATIENTS Subject: Implementation of new modifiers for Non-ESRD indications Reporting of Hgb/Hct levels on all Non-ESRD claims requesting payment for anti-anemia drugs. Effective Date: January 1, 2008 Implementation Date: April 7, Effective January 1, 2008, all non-esrd ESA claims billing HCPCS J0881 and J0885 must begin reporting one (and only one) of the following three modifiers on the same line as the ESA HCPCS: EA ESA, anemia, chemo-induced EB ESA, anemia, radio-induced, or EC ESA, anemia, non-chemo/radio Non-ESRD ESA hospital claims that do not report one of the above 3 modifiers along with HCPCS J0881 or J0885 will be returned to the provider. August 2006 Page 37 of 38
38 2. Effective January 1, 2008, all claims billing for the administration of an ESA (HCPCS J0881, J0882, J0885, J0886 and Q4081) must report the most recent hematocrit or hemoglobin reading. For hospital claims the hemoglobin reading is reported with a value code 48 and a hematocrit reading is reported with the value code 49. Claims not reporting a value code 48 or 49 will be returned to the provider. NOTE: For EPO and Aranesp billing instructions for beneficiaries with ESRD, see CMS, Claims Processing Manual, Chapter 8, sections60.4 and 60.7 References: Medicare Coverage Database LCD L25211, , NGS Medical Policy update, SIA (A44399) 01/01/08, 02/01/08 CMS, Medicare Claims Processing Manual, CR 5699, 01/11/08 Medlearn Matters No. MM5699, 01/11/08 PUB 100-2, Medicare Benefit Policy, Chapter 6, Hospital Services Covered Under Part B, paragraph 20.4,30 Chapter 11, ESRD, paragraph 90 Chapter 15, Covered Medical and Other Health Services, paragraph 50 PUB 100-4, Medicare Claims Processing Manual ICD-9-CM Official Guidelines for Coding and Reporting, ICD-9-CM Channel Publishing Coding Manual, Hospital Version, Current Procedural Terminology (CPT), AMA 2008HCPCS Level II, Ingenix For questions, please call: Lita Rubell BOA Bldg. 924 Westwood Blvd., Ste. 810 L.A., Ca Tel. No Fax No August 2006 Page 38 of 38
82330 CALCIUM; IONIZED. ICD-10 Codes that Support Medical Necessity. ICD-10 Code. Description. A15.0 Tuberculosis of lung
 82330 CALCIUM; IONIZED ICD-10 Codes that Support Medical Necessity ICD-10 Code Description A15.0 Tuberculosis of lung A15.4 Tuberculosis of intrathoracic lymph nodes A15.5 Tuberculosis of larynx, trachea
82330 CALCIUM; IONIZED ICD-10 Codes that Support Medical Necessity ICD-10 Code Description A15.0 Tuberculosis of lung A15.4 Tuberculosis of intrathoracic lymph nodes A15.5 Tuberculosis of larynx, trachea
Cancer Association of South Africa (CANSA)
 Cancer Association of South Africa (CANSA) Fact Sheet on ICD-10 Coding of Neoplasms Introduction The International Statistical Classification of Diseases and Related Health Problems, 10 th Revision (ICD-10)
Cancer Association of South Africa (CANSA) Fact Sheet on ICD-10 Coding of Neoplasms Introduction The International Statistical Classification of Diseases and Related Health Problems, 10 th Revision (ICD-10)
WLH Tumor Frequencies between cohort enrollment and 31-Dec Below the Women Lifestyle and Health tumor frequencies are tabulated according to:
 DESCRIPTION Below the Women Lifestyle and Health tumor frequencies are tabulated according to: Benign =171 (Cervix uteri) treated as not recorded =191 (non-melanoma skin cancer) treated as not recorded
DESCRIPTION Below the Women Lifestyle and Health tumor frequencies are tabulated according to: Benign =171 (Cervix uteri) treated as not recorded =191 (non-melanoma skin cancer) treated as not recorded
WLH Tumor Frequencies between cohort enrollment and 31-Dec Below the Women Lifestyle and Health tumor frequencies are tabulated according to:
 WLH Tumor Frequencies between cohort enrollment and 31-Dec 2012 DESCRIPTION Below the Women Lifestyle and Health tumor frequencies are tabulated according to: Benign =171 (Cervix uteri) treated as not
WLH Tumor Frequencies between cohort enrollment and 31-Dec 2012 DESCRIPTION Below the Women Lifestyle and Health tumor frequencies are tabulated according to: Benign =171 (Cervix uteri) treated as not
SCCA REFERENCE MANUAL ICD-10
 SCCA REFERENCE MANUAL ICD-10 NORTHWEST HOSPITAL 1 BREAST CANCER BREAST (INC. PAGET S DISEASE) 0 - Nipple and areola 1 - Central portion 2 - Upper-inner quadrant 3 - Lower-inner quadrant 4 - Upper-outer
SCCA REFERENCE MANUAL ICD-10 NORTHWEST HOSPITAL 1 BREAST CANCER BREAST (INC. PAGET S DISEASE) 0 - Nipple and areola 1 - Central portion 2 - Upper-inner quadrant 3 - Lower-inner quadrant 4 - Upper-outer
Colony Stimulating Factors: Neupogen (filgrastim), Neulasta (pegfilgrastim), Leukine (sargramostim), Granix (tbo-filgrastim)
 Neupogen (filgrastim), Neulasta (sargramostim), Granix (tbo-filgrastim) Date of Origin: 10/17/2008 Dates Reviewed: 6/17/2009, 12/22/2009, 06/15/2010/ 7/20/2010, 09/2010, 12/2010, 03/2011, 06/2011, 09/2011,
Neupogen (filgrastim), Neulasta (sargramostim), Granix (tbo-filgrastim) Date of Origin: 10/17/2008 Dates Reviewed: 6/17/2009, 12/22/2009, 06/15/2010/ 7/20/2010, 09/2010, 12/2010, 03/2011, 06/2011, 09/2011,
Model Policy. Coverage of Proton Therapy
 Model Policy Coverage of Proton Therapy Last Revised - February 2019 INTRODUCTION Proton therapy is a technologically advanced method to deliver curative radiation doses to cancerous tumors. The unique
Model Policy Coverage of Proton Therapy Last Revised - February 2019 INTRODUCTION Proton therapy is a technologically advanced method to deliver curative radiation doses to cancerous tumors. The unique
CLINICAL MEDICATION POLICY
 Policy Name: Policy Number: Approved By: CLINICAL MEDICATION POLICY Keytruda (pembrolizumab) MP-014-MD-DE Medical Management; Clinical Pharmacy Provider Notice Date: 01/15/2018; 08/01/2017; 06/01/2016
Policy Name: Policy Number: Approved By: CLINICAL MEDICATION POLICY Keytruda (pembrolizumab) MP-014-MD-DE Medical Management; Clinical Pharmacy Provider Notice Date: 01/15/2018; 08/01/2017; 06/01/2016
Serum Iron Studies
 190.18 - Serum Iron Studies Serum iron studies are useful in the evaluation of disorders of iron metabolism, particularly iron deficiency and iron excess. Iron studies are best performed when the patient
190.18 - Serum Iron Studies Serum iron studies are useful in the evaluation of disorders of iron metabolism, particularly iron deficiency and iron excess. Iron studies are best performed when the patient
CLINICAL MEDICATION POLICY
 CLINICAL MEDICATION POLICY Policy Name: Opdivo (nivolumab) injection Policy Number: Approved By: Medical Management, Clinical Pharmacy Products: Highmark Health Options Application: All participating hospitals
CLINICAL MEDICATION POLICY Policy Name: Opdivo (nivolumab) injection Policy Number: Approved By: Medical Management, Clinical Pharmacy Products: Highmark Health Options Application: All participating hospitals
155.2 Malignant neoplasm of liver not specified as primary or secondary. C22.9 Malignant neoplasm of liver, not specified as primary or secondary
 ICD-9 TO ICD-10 Reference ICD-9 150.9 Malignant neoplasm of esophagus unspecified site C15.9 Malignant neoplasm of esophagus, unspecified 151.9 Malignant neoplasm of stomach unspecified site C16.9 Malignant
ICD-9 TO ICD-10 Reference ICD-9 150.9 Malignant neoplasm of esophagus unspecified site C15.9 Malignant neoplasm of esophagus, unspecified 151.9 Malignant neoplasm of stomach unspecified site C16.9 Malignant
Contractor Information. LCD Information. Local Coverage Determination (LCD): Computerized Axial Tomography of the Chest/Thorax (L34416)
 Local Coverage Determination (LCD): Computerized Axial Tomography of the Chest/Thorax (L34416) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
Local Coverage Determination (LCD): Computerized Axial Tomography of the Chest/Thorax (L34416) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor
CLINICAL MEDICATION POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICATION POLICY Granulocyte Colony Stimulating Factors (G-CSFs) MP-016-MD-DE Medical Management; Clinical Pharmacy Provider Notice Date:
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICATION POLICY Granulocyte Colony Stimulating Factors (G-CSFs) MP-016-MD-DE Medical Management; Clinical Pharmacy Provider Notice Date:
Cancer in Estonia 2014
 Cancer in Estonia 2014 Estonian Cancer Registry (ECR) is a population-based registry that collects data on all cancer cases in Estonia. More information about ECR is available at the webpage of National
Cancer in Estonia 2014 Estonian Cancer Registry (ECR) is a population-based registry that collects data on all cancer cases in Estonia. More information about ECR is available at the webpage of National
Clinical Coding for CRS Standards
 Clinical Coding for CRS Standards The following appendices set out the amended PRIMARY DIAGNOSIS coding structure to be used for the monitoring of cancer waiting times following the implementation of 4th
Clinical Coding for CRS Standards The following appendices set out the amended PRIMARY DIAGNOSIS coding structure to be used for the monitoring of cancer waiting times following the implementation of 4th
DATA REQUEST RESPONSE- XRT AND BRACHYTHERAPY
 Date: 10 th April 2018 DATA REQUEST RESPONSE- XRT AND BRACHYTHERAPY Request: 1. Utilization Data of Overseas Beam Therapy and Brachytherapy 2. Diagnoses Data of Overseas Claims for Beam Therapy and Brachytherapy
Date: 10 th April 2018 DATA REQUEST RESPONSE- XRT AND BRACHYTHERAPY Request: 1. Utilization Data of Overseas Beam Therapy and Brachytherapy 2. Diagnoses Data of Overseas Claims for Beam Therapy and Brachytherapy
CEA (CARCINOEMBRYONIC ANTIGEN)
 (CARCINOEMBRYONIC ANTIGEN) 428 C15.3 Malignant neoplasm of upper third of esophagus C15.4 Malignant neoplasm of middle third of esophagus C15.5 Malignant neoplasm of lower third of esophagus C15.8 Malignant
(CARCINOEMBRYONIC ANTIGEN) 428 C15.3 Malignant neoplasm of upper third of esophagus C15.4 Malignant neoplasm of middle third of esophagus C15.5 Malignant neoplasm of lower third of esophagus C15.8 Malignant
ICD-10 and Radiation Oncology
 ICD-10 and Radiation Oncology Steven M. Verno, CEMCS ICD-10 and Radiation Oncology Steven M. Verno, CEMCS September 23, 2008 Note: ICD-9-CM and ICD-10 are owned and copyrighted by the World Health Organization.
ICD-10 and Radiation Oncology Steven M. Verno, CEMCS ICD-10 and Radiation Oncology Steven M. Verno, CEMCS September 23, 2008 Note: ICD-9-CM and ICD-10 are owned and copyrighted by the World Health Organization.
MALIGNANT NEOPLASMS OF THE BREAST MALIGNANT NEOPLASMS OF FEMALE GENITAL ORGANS
 MALIGNANT NEOPLASMS OF THE (INC. PAGET S DISEASE) 0 - Nipple and areola 1 - Central portion 2 - Upper-inner quadrant 3 - Lower-inner quadrant 4 - Upper-outer quadrant 5 - Lower-outer quadrant 6 - Axillary
MALIGNANT NEOPLASMS OF THE (INC. PAGET S DISEASE) 0 - Nipple and areola 1 - Central portion 2 - Upper-inner quadrant 3 - Lower-inner quadrant 4 - Upper-outer quadrant 5 - Lower-outer quadrant 6 - Axillary
Intensity Modulated Radiation Therapy (IMRT)
 Intensity Modulated Radiation Therapy (IMRT) [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas Circulares.]
Intensity Modulated Radiation Therapy (IMRT) [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas Circulares.]
Pembrolizumab (Keytruda )
 Last Review Date: March 14, 2017 Number: MG.MM.PH.10f Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: March 14, 2017 Number: MG.MM.PH.10f Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Peripheral Nerve Blocks
 Last Review Date: April 21, 2017 Number: MG.MM.ME.64v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: April 21, 2017 Number: MG.MM.ME.64v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
LCD for Interferon (L29202)
 LCD for Interferon (L29202) Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B Contractor Information LCD ID Number L29202 LCD Information LCD Title
LCD for Interferon (L29202) Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B Contractor Information LCD ID Number L29202 LCD Information LCD Title
S2 File. Clinical Classifications Software (CCS). The CCS is a
 S2 File. Clinical Classifications Software (CCS). The CCS is a diagnosis categorization scheme based on the ICD-9-CM that aggregates all diagnosis codes into 262 mutually exclusive, clinically homogeneous
S2 File. Clinical Classifications Software (CCS). The CCS is a diagnosis categorization scheme based on the ICD-9-CM that aggregates all diagnosis codes into 262 mutually exclusive, clinically homogeneous
Anatomical Considerations for Lab Practical II
 Anatomical Considerations for Lab Practical II For each of the following please be prepared to provide: Identification System Organ(s) or ducts to Function(s) location which it is attached Use your lecture
Anatomical Considerations for Lab Practical II For each of the following please be prepared to provide: Identification System Organ(s) or ducts to Function(s) location which it is attached Use your lecture
Neoplasms/Lymphoma/Leukemia
 Neoplasms/Lymphoma/Leukemia Session Guidelines This is a 15 minute webinar session for CNC physicians and staff CNC holds webinars monthly to address topics related to risk adjustment documentation and
Neoplasms/Lymphoma/Leukemia Session Guidelines This is a 15 minute webinar session for CNC physicians and staff CNC holds webinars monthly to address topics related to risk adjustment documentation and
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Opdivo (nivolumab) MP-004-MC-PA Medical Management; Clinical Pharmacy Provider Notice Date: 09/01/2018; 06/15/2018; 04/01/2017
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Opdivo (nivolumab) MP-004-MC-PA Medical Management; Clinical Pharmacy Provider Notice Date: 09/01/2018; 06/15/2018; 04/01/2017
SITES (ALPHABETICAL) HPV CS SITE SPECIFIC FACTOR
 SITES (ALPHABETICAL) HPV CS SITE SPECIFIC FACTOR Anus: Anal Canal; Anus, NOS; Other Parts of Rectum C21.0-C21.2, C21.8 C21.0 Anus, NOS (excludes skin of anus and perianal skin C44.5) C21.1 Anal canal C21.2
SITES (ALPHABETICAL) HPV CS SITE SPECIFIC FACTOR Anus: Anal Canal; Anus, NOS; Other Parts of Rectum C21.0-C21.2, C21.8 C21.0 Anus, NOS (excludes skin of anus and perianal skin C44.5) C21.1 Anal canal C21.2
Table of Contents. Last updated by CLO: 5/8/2013 1
 Table of Contents Drug-induced liver injury algorithm - Cases... 2 Drug-induced liver injury algorithm - Controls... 3 1. Summary of drug-induced liver injury algorithm... 4 2. Terminology... 4 3. Threshold
Table of Contents Drug-induced liver injury algorithm - Cases... 2 Drug-induced liver injury algorithm - Controls... 3 1. Summary of drug-induced liver injury algorithm... 4 2. Terminology... 4 3. Threshold
ANNUAL CANCER REGISTRY REPORT-2005
 ANNUAL CANCER REGISTRY REPORT-25 CANCER STATISTICS Distribution of neoplasms Of a total of 3,115 new neoplasms diagnosed or treated at the Hospital from January 25 to December, 25, 1,473 were seen in males
ANNUAL CANCER REGISTRY REPORT-25 CANCER STATISTICS Distribution of neoplasms Of a total of 3,115 new neoplasms diagnosed or treated at the Hospital from January 25 to December, 25, 1,473 were seen in males
Annual Report. Cape Cod Hospital and Falmouth Hospital Regional Cancer Network Expert physicians. Quality hospitals. Superior care.
 Annual Report Cape Cod Hospital and Falmouth Hospital Regional Cancer Network 2013 Expert physicians. Quality hospitals. Superior care. Cape Cod Hospital s Davenport- Mugar Hematology/Oncology Center and
Annual Report Cape Cod Hospital and Falmouth Hospital Regional Cancer Network 2013 Expert physicians. Quality hospitals. Superior care. Cape Cod Hospital s Davenport- Mugar Hematology/Oncology Center and
Carcinoembryonic Antigen
 Other Names/Abbreviations CEA 190.26 - Carcinoembryonic Antigen Carcinoembryonic antigen (CEA) is a protein polysaccharide found in some carcinomas. It is effective as a biochemical marker for monitoring
Other Names/Abbreviations CEA 190.26 - Carcinoembryonic Antigen Carcinoembryonic antigen (CEA) is a protein polysaccharide found in some carcinomas. It is effective as a biochemical marker for monitoring
Genetic Testing for Cancer Susceptibility
 Last Review Date: March 10, 2017 Number: MG.MM.AD.17v3 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: March 10, 2017 Number: MG.MM.AD.17v3 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Contractor Information. LCD Information. Local Coverage Determination (LCD): Computerized Axial Tomography (CT), Thorax (L33459) Document Information
 Local Coverage Determination (LCD): Computerized Axial Tomography (CT), Thorax (L33459) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
Local Coverage Determination (LCD): Computerized Axial Tomography (CT), Thorax (L33459) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information
Contractor Information. LCD Information. Local Coverage Determination (LCD): CT of the Abdomen and Pelvis (L34415) Document Information
 Local Coverage Determination (LCD): CT of the Abdomen and Pelvis (L34415) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): CT of the Abdomen and Pelvis (L34415) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Crosswalk File of ICD9 Diagnosis Codes to Risk Group Assignment 1-Apr-15
 1 1500 MALIGNANT NEOPLASM OF CERVICAL ESOPHAGUS 1 1501 MALIGNANT NEOPLASM OF THORACIC ESOPHAGUS 1 1502 MALIGNANT NEOPLASM OF ABDOMINAL ESOPHAGUS 1 1503 MALIGNANT NEOPLASM OF UPPER THIRD OF ESOPHAGUS 1
1 1500 MALIGNANT NEOPLASM OF CERVICAL ESOPHAGUS 1 1501 MALIGNANT NEOPLASM OF THORACIC ESOPHAGUS 1 1502 MALIGNANT NEOPLASM OF ABDOMINAL ESOPHAGUS 1 1503 MALIGNANT NEOPLASM OF UPPER THIRD OF ESOPHAGUS 1
Florida Cancer Data System STAT File Documentation Version 2019
 Florida Cancer Data System STAT File Documentation Version 2019 Field Description NAACCR Item Recoded Patient ID Number 20 Addr at DX - State 80 X County at DX 90 Addr at DX Country 102 X Marital Status
Florida Cancer Data System STAT File Documentation Version 2019 Field Description NAACCR Item Recoded Patient ID Number 20 Addr at DX - State 80 X County at DX 90 Addr at DX Country 102 X Marital Status
2011 to 2015 New Cancer Incidence Truman Medical Center - Hospital Hill
 Number of New Cancers Truman Medical Center Hospital Hill Cancer Registry 2015 Statistical Summary Incidence In 2015, Truman Medical Center diagnosed and/or treated 406 new cancer cases. Four patients
Number of New Cancers Truman Medical Center Hospital Hill Cancer Registry 2015 Statistical Summary Incidence In 2015, Truman Medical Center diagnosed and/or treated 406 new cancer cases. Four patients
Group B: Organ systems (digestive, respiratory, urinary, genital system, heart, glands and skin) green
 Group B: Organ systems (digestive, respiratory, urinary, genital system, heart, glands and skin) green Digestive system 1. Teeth Main points: external and internal structure of a tooth, fixation of a tooth
Group B: Organ systems (digestive, respiratory, urinary, genital system, heart, glands and skin) green Digestive system 1. Teeth Main points: external and internal structure of a tooth, fixation of a tooth
Contractor Information. LCD Information. Local Coverage Determination (LCD): CT of the Abdomen and Pelvis (L34415) Document Information
 Local Coverage Determination (LCD): CT of the Abdomen and Pelvis (L34415) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): CT of the Abdomen and Pelvis (L34415) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Khapzory (levoleucovorin) (Intravenous)
 Khapzory (levoleucovorin) (Intravenous) Last Review Date: 12/04/2018 Date of Origin: 12/04/2018 Dates Reviewed: 12/2018 Document Number: IC-0408 I. Length of Authorization Coverage will be provided for
Khapzory (levoleucovorin) (Intravenous) Last Review Date: 12/04/2018 Date of Origin: 12/04/2018 Dates Reviewed: 12/2018 Document Number: IC-0408 I. Length of Authorization Coverage will be provided for
LCD L Flow CytometryPrint
 LCD L32562 - Flow CytometryPrint Contractor Information Contractor Name: Novitas Solutions, Inc. Contractor Number(s): 12501, 12101, 12102, 12201, 12202, 12301, 12302, 12401, 12402, 12901, 12502 Contractor
LCD L32562 - Flow CytometryPrint Contractor Information Contractor Name: Novitas Solutions, Inc. Contractor Number(s): 12501, 12101, 12102, 12201, 12202, 12301, 12302, 12401, 12402, 12901, 12502 Contractor
BRAF Mutation Analysis
 Last Review Date: October 13, 2017 Number: MG.MM.LA.38aC Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: October 13, 2017 Number: MG.MM.LA.38aC Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Fusilev (levoleucovorin) Document Number: IC-0183
 Fusilev (levoleucovorin) Document Number: IC-0183 Last Review Date: 2/1/2018 Date of Origin: 01/02/2014 Dates Reviewed: 08/2014, 03/2015, 05/2015, 08/2015, 11/2015, 02/2016, 05/2016, 08/2016, 11/2016,
Fusilev (levoleucovorin) Document Number: IC-0183 Last Review Date: 2/1/2018 Date of Origin: 01/02/2014 Dates Reviewed: 08/2014, 03/2015, 05/2015, 08/2015, 11/2015, 02/2016, 05/2016, 08/2016, 11/2016,
Truman Medical Center-Hospital Hill Cancer Registry 2014 Statistical Summary Incidence
 Truman Medical Center-Hospital Hill Cancer Registry 2014 Statistical Summary Incidence In 2014, there were 452 new cancer cases diagnosed and or treated at Truman Medical Center- Hospital Hill and an additional
Truman Medical Center-Hospital Hill Cancer Registry 2014 Statistical Summary Incidence In 2014, there were 452 new cancer cases diagnosed and or treated at Truman Medical Center- Hospital Hill and an additional
Fentora (Fentanyl Buccal)
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Fentora (Fentanyl Buccal) Clinical Edit Information Included in this Document Fentora 100mcg Drugs requiring prior authorization:
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Fentora (Fentanyl Buccal) Clinical Edit Information Included in this Document Fentora 100mcg Drugs requiring prior authorization:
Anatomy. Contents Brain (Questions)
 Anatomy 12 Contents 12.1 Brain (Questions).................................................... 683 12.2 Head and Neck (Questions)............................................. 685 12.3 Thorax (Questions)...................................................
Anatomy 12 Contents 12.1 Brain (Questions).................................................... 683 12.2 Head and Neck (Questions)............................................. 685 12.3 Thorax (Questions)...................................................
REPORTABLE CASES MISSISSIPPI For cases diagnosed 10/1/2015 and after
 REPORTABLE CASES MISSISSIPPI For cases diagnosed 10/1/2015 and after The following lists are intended to assist you, as the reporter, in identifying the reportable neoplasms for your facility. Any reportable
REPORTABLE CASES MISSISSIPPI For cases diagnosed 10/1/2015 and after The following lists are intended to assist you, as the reporter, in identifying the reportable neoplasms for your facility. Any reportable
Gamma Glutamyl Transferase
 Other Names/Abbreviations GGT 190.32 - Gamma Glutamyl Transferase Gamma glutamyl transferase (GGT) is an intracellular enzyme that appears in blood following leakage from cells. Renal tubules, liver, and
Other Names/Abbreviations GGT 190.32 - Gamma Glutamyl Transferase Gamma glutamyl transferase (GGT) is an intracellular enzyme that appears in blood following leakage from cells. Renal tubules, liver, and
CODING PRIMARY SITE. Nadya Dimitrova
 CODING PRIMARY SITE Nadya Dimitrova OUTLINE What is coding and why do we need it? ICD-10 and ICD-O ICD-O-3 Topography coding rules ICD-O-3 online WHAT IS CODING AND WHY DO WE NEED IT? Coding: to assign
CODING PRIMARY SITE Nadya Dimitrova OUTLINE What is coding and why do we need it? ICD-10 and ICD-O ICD-O-3 Topography coding rules ICD-O-3 online WHAT IS CODING AND WHY DO WE NEED IT? Coding: to assign
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Avastin (bevacizumab) Policy Number: MP-030-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
CLINICAL MEDICAL POLICY Policy Name: Avastin (bevacizumab) Policy Number: MP-030-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
Introduction to ICD-O-3 coding rules
 Introduction to ICD-O-3 coding rules Weena Laddachayaporn, MD National Cancer Institute, Bangkok, Thailand ICD-O-3 The International Classification of Diseases for Oncology Is a coding system for primary
Introduction to ICD-O-3 coding rules Weena Laddachayaporn, MD National Cancer Institute, Bangkok, Thailand ICD-O-3 The International Classification of Diseases for Oncology Is a coding system for primary
MEDICAL POLICY Gene Expression Profiling for Cancers of Unknown Primary Site
 POLICY: PG0364 ORIGINAL EFFECTIVE: 04/22/16 LAST REVIEW: 07/26/18 MEDICAL POLICY Gene Expression Profiling for Cancers of Unknown Primary Site GUIDELINES This policy does not certify benefits or authorization
POLICY: PG0364 ORIGINAL EFFECTIVE: 04/22/16 LAST REVIEW: 07/26/18 MEDICAL POLICY Gene Expression Profiling for Cancers of Unknown Primary Site GUIDELINES This policy does not certify benefits or authorization
END-SEMESTER EXAM 2018 ANATOMY, HISTOLOGY AND EMBRYOLOGY FACULTY OF MEDICINE, 2 ND SEMESTER
 University of Szeged, Faculty of Medicine Department of Anatomy, Histology and Embryology Chairman: Prof. Antal Nógrádi MD, PhD, DSc Kossuth L. sgt. 40., H-6724 Szeged, Hungary Tel.: +36-62-545-665 P.
University of Szeged, Faculty of Medicine Department of Anatomy, Histology and Embryology Chairman: Prof. Antal Nógrádi MD, PhD, DSc Kossuth L. sgt. 40., H-6724 Szeged, Hungary Tel.: +36-62-545-665 P.
SEER Advanced Topic 2018 Presentation. EOD 2018 and SS2018 Jennifer Ruhl
 SEER Advanced Topic 2018 Presentation EOD 2018 and SS2018 Jennifer Ruhl May 25, 2018 Outline General overview of EOD Schemas Basic review of what is needed to collect Primary Tumor, Regional Nodes and
SEER Advanced Topic 2018 Presentation EOD 2018 and SS2018 Jennifer Ruhl May 25, 2018 Outline General overview of EOD Schemas Basic review of what is needed to collect Primary Tumor, Regional Nodes and
ACTIVITY 11: RESPIRATORY AND DIGESTIVE SYSTEMS RESPIRATORY SYSTEM
 ACTIVITY 11: RESPIRATORY AND DIGESTIVE SYSTEMS OBJECTIVES: 1) How to get ready: Read Chapters 25 and 26, McKinley et al., Human Anatomy, 4e. All text references are for this textbook. 2) Identify structures
ACTIVITY 11: RESPIRATORY AND DIGESTIVE SYSTEMS OBJECTIVES: 1) How to get ready: Read Chapters 25 and 26, McKinley et al., Human Anatomy, 4e. All text references are for this textbook. 2) Identify structures
APPENDIX ONE: ICD CODES
 APPENDIX ONE: ICD CODES ICD-10-AM ICD-9-CM Malignant neoplasms C00 C97 140 208, 238.6, 273.3 Lip, oral cavity and pharynx C00 C14 140 149 Digestive organs C15 C26 150 157, 159 Oesophagus 4 C15 150 excluding
APPENDIX ONE: ICD CODES ICD-10-AM ICD-9-CM Malignant neoplasms C00 C97 140 208, 238.6, 273.3 Lip, oral cavity and pharynx C00 C14 140 149 Digestive organs C15 C26 150 157, 159 Oesophagus 4 C15 150 excluding
RESPIRATORY SYSTEM. described: pp. 744,746 fig. 25.1, described: p. 746 fig described: p. 776 fig. 26.3
 ACTIVITY 11: RESPIRATORY AND DIGESTIVE SYSTEMS OBJECTIVES: 1) How to get ready: Read Chapters 25 and 26, McKinley et al., Human Anatomy, 5e. All text references are for this textbook. 2) Identify structures
ACTIVITY 11: RESPIRATORY AND DIGESTIVE SYSTEMS OBJECTIVES: 1) How to get ready: Read Chapters 25 and 26, McKinley et al., Human Anatomy, 5e. All text references are for this textbook. 2) Identify structures
California Cancer Registry Production Automation and Quality Control Unit Data Alert - Registrar
 Production Automation and Quality Control Unit Data Alert - Registrar 2015-061 REVISED Casefinding List FY2016 Please see additions and revisions noted below in red text. ICD-10-CM Codes have been adopted
Production Automation and Quality Control Unit Data Alert - Registrar 2015-061 REVISED Casefinding List FY2016 Please see additions and revisions noted below in red text. ICD-10-CM Codes have been adopted
List of Codes Used to Identify Measures Reported in the Dialysis Facility Report for FY 2019
 List of Codes Used to Identify Measures Reported in the Dialysis Facility Report for FY 2019 Table of Contents Table 4: Hospitalization Summary for Medicare Dialysis Patients... 4 SEPTICEMIA 4 MYOCARDIAL
List of Codes Used to Identify Measures Reported in the Dialysis Facility Report for FY 2019 Table of Contents Table 4: Hospitalization Summary for Medicare Dialysis Patients... 4 SEPTICEMIA 4 MYOCARDIAL
2017 ICD-10-CM Casefinding List (Abbreviated listing from SEER website) COMPREHENSIVE ICD-10-CM Casefinding Code List for Reportable Tumors
 2017 ICD-10-CM Casefinding List (Abbreviated listing from SEER website) COMPREHENSIVE ICD-10-CM Casefinding Code List for Reportable Tumors ICD-10 Code Explanation of Code Malignant neoplasms (excluding
2017 ICD-10-CM Casefinding List (Abbreviated listing from SEER website) COMPREHENSIVE ICD-10-CM Casefinding Code List for Reportable Tumors ICD-10 Code Explanation of Code Malignant neoplasms (excluding
2016 Cancer Registry Annual Report
 2016 Cancer Registry Annual Report Cancer Committee Chairman s Report The Cancer Committee at Cancer Treatment Centers of America (CTCA) at Eastern Regional Medical Center (Eastern), established in 2006,
2016 Cancer Registry Annual Report Cancer Committee Chairman s Report The Cancer Committee at Cancer Treatment Centers of America (CTCA) at Eastern Regional Medical Center (Eastern), established in 2006,
This lab activity is aligned with Visible Body s Human Anatomy Atlas app. Learn more at visiblebody.com/professors
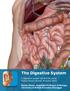 1 This lab activity is aligned with Visible Body s Human Anatomy Atlas app. Learn more at visiblebody.com/professors 2 A. Digestive System Overview To Start: Go to the Views menu and scroll down to the
1 This lab activity is aligned with Visible Body s Human Anatomy Atlas app. Learn more at visiblebody.com/professors 2 A. Digestive System Overview To Start: Go to the Views menu and scroll down to the
General Anatomy p. 1 Organization of the Human Body p. 1 Skeleton of the Human Body p. 4 Ossification of the Bones p. 6 Bone Structure p. 8 Joints p.
 General Anatomy p. 1 Organization of the Human Body p. 1 Skeleton of the Human Body p. 4 Ossification of the Bones p. 6 Bone Structure p. 8 Joints p. 10 Principal Joints (Immovable) p. 12 Synovial Joints
General Anatomy p. 1 Organization of the Human Body p. 1 Skeleton of the Human Body p. 4 Ossification of the Bones p. 6 Bone Structure p. 8 Joints p. 10 Principal Joints (Immovable) p. 12 Synovial Joints
Table E1. Standardized Mortality Ratios for Total and Specific Causes of Death Parameter Radiologists Psychiatrists No. of Deaths
 RSNA, 2016 10.1148/radiol.2016152472 Table E1. Standardized Mortality Ratios for Total and Specific Causes of Death Parameter Radiologists Psychiatrists No. of Deaths Observed/Expected No. of Deaths Observed/Expected
RSNA, 2016 10.1148/radiol.2016152472 Table E1. Standardized Mortality Ratios for Total and Specific Causes of Death Parameter Radiologists Psychiatrists No. of Deaths Observed/Expected No. of Deaths Observed/Expected
Globally Optimal Statistical Classification Models, I: Binary Class Variable, One Ordered Attribute
 Globally Optimal Statistical Classification Models, I: Binary Class Variable, One Ordered Attribute Paul R. Yarnold, Ph.D. and Robert C. Soltysik, M.S. Optimal Data Analysis, LLC Imagine a random sample
Globally Optimal Statistical Classification Models, I: Binary Class Variable, One Ordered Attribute Paul R. Yarnold, Ph.D. and Robert C. Soltysik, M.S. Optimal Data Analysis, LLC Imagine a random sample
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.138.MH Oral Maxillofacial Prosthesis This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP MedStar CareFirst
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.138.MH Oral Maxillofacial Prosthesis This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP MedStar CareFirst
Descriptive Histology
 Atlas of Descriptive Histology Michael H. Ross University of Florida College of Medicine Gainesville, Florida Wojciech Pawlina Mayo Medical School College of Medicine, Mayo Clinic Rochester, Minnesota
Atlas of Descriptive Histology Michael H. Ross University of Florida College of Medicine Gainesville, Florida Wojciech Pawlina Mayo Medical School College of Medicine, Mayo Clinic Rochester, Minnesota
John R. Marsh Cancer Center
 John R. Marsh Cancer Center Lung Program Overview: 2014-2015 Initiatives Lung CT Screening Dr. Gregory Zimmerman In cooperation with The Lung Cancer Steering Committee, Diagnostic Imaging Services at the
John R. Marsh Cancer Center Lung Program Overview: 2014-2015 Initiatives Lung CT Screening Dr. Gregory Zimmerman In cooperation with The Lung Cancer Steering Committee, Diagnostic Imaging Services at the
Contractor Information. LCD Information. Local Coverage Determination (LCD): Flow Cytometry (L34513) Document Information
 Local Coverage Determination (LCD): Flow Cytometry (L34513) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor Name Contract
Local Coverage Determination (LCD): Flow Cytometry (L34513) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor Name Contract
2012 Cancer Report 2011 Registry Data
 2012 Cancer Report 2011 Registry Data Contents Goals and Objectives 1 2012 Cancer Committee Members 2 Total Cancer Cases 1981-2011 3 Cancer Registry Frequency Report 1981-2011 4-5 Cancer Registry Frequency
2012 Cancer Report 2011 Registry Data Contents Goals and Objectives 1 2012 Cancer Committee Members 2 Total Cancer Cases 1981-2011 3 Cancer Registry Frequency Report 1981-2011 4-5 Cancer Registry Frequency
incidence rate x 100,000/year
 Tier R=rare C=common Cancer Entity European crude and age adjusted incidence by cancer, years of diagnosis 2000 and 2007 Analisys based on 83 population-based cancer registries * applying the European
Tier R=rare C=common Cancer Entity European crude and age adjusted incidence by cancer, years of diagnosis 2000 and 2007 Analisys based on 83 population-based cancer registries * applying the European
Medical Nutrition Services
 Medical Nutrition Services Chapter.1 Enrollment..................................................................... -2.2 Vitamins and Minerals...........................................................
Medical Nutrition Services Chapter.1 Enrollment..................................................................... -2.2 Vitamins and Minerals...........................................................
Keytruda (pembrolizumab) (Intravenous)
 Keytruda (pembrolizumab) (Intravenous) Last Review Date: 02/06/2018 Date of Origin: 09/30/2014 Document Number: IC-0209 Dates Reviewed: 9/2014, 3/2015, 5/2015, 8/2015, 10/2015, 11/2015, 2/2016, 5/2016,
Keytruda (pembrolizumab) (Intravenous) Last Review Date: 02/06/2018 Date of Origin: 09/30/2014 Document Number: IC-0209 Dates Reviewed: 9/2014, 3/2015, 5/2015, 8/2015, 10/2015, 11/2015, 2/2016, 5/2016,
AMBULATORY SURGERY CENTER CODING AND PAYMENT GUIDE
 Targeted Drug Delivery AMBULATORY SURGERY CENTER CODING AND PAYMENT GUIDE 2018 Flowonix Medical has compiled this coding information for your convenience. This information is gathered from third party
Targeted Drug Delivery AMBULATORY SURGERY CENTER CODING AND PAYMENT GUIDE 2018 Flowonix Medical has compiled this coding information for your convenience. This information is gathered from third party
Jhia Anjela D. Rivera 1 1. BS Biology, Department of Biology, College of Science, Polytechnic University of the Philippines
 DIGESTIVE SYSTEM Jhia Anjela D. Rivera 1 1 BS Biology, Department of Biology, College of Science, Polytechnic University of the Philippines DIGESTIVE SYSTEM Consists of the digestive tract (gastrointestinal
DIGESTIVE SYSTEM Jhia Anjela D. Rivera 1 1 BS Biology, Department of Biology, College of Science, Polytechnic University of the Philippines DIGESTIVE SYSTEM Consists of the digestive tract (gastrointestinal
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
 Local Coverage Determination (LCD): Flow Cytometry (L34215) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor Name Contract
Local Coverage Determination (LCD): Flow Cytometry (L34215) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor Name Contract
Morphine Equivalent Dosing
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class This criteria was recommended for review by the Texas Medicaid Vendor Drug Program to ensure appropriate and safe utilization. Clinical
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class This criteria was recommended for review by the Texas Medicaid Vendor Drug Program to ensure appropriate and safe utilization. Clinical
Lungs a. d. b. c. e.
 Lungs d. e. Lungs Right superior lobe Right middle lobe Right inferior lobe d. Left superior lobe e. Left inferior lobe Sinuses d. Nasal Cavity & Sinuses g. g. i. Nasal Cavity & Sinuses g. h. d. f. e.
Lungs d. e. Lungs Right superior lobe Right middle lobe Right inferior lobe d. Left superior lobe e. Left inferior lobe Sinuses d. Nasal Cavity & Sinuses g. g. i. Nasal Cavity & Sinuses g. h. d. f. e.
OVARIES URETER FALLOPIAN TUBES BLADDER UROGENITAL OPENINGS (BOTH SEXES) PENIS VAGINA UTERUS
 URETER OVARIES FALLOPIAN TUBES BLADDER UROGENITAL OPENINGS (BOTH SEXES) PENIS VAGINA UTERUS REPRODUCTIVE PRODUCE FEMALE HORMONES EXCRETORY FROM KIDNEY TO BLADDER EXCRETORY STORES URINE REPRODUCTIVE TRANSPORTS
URETER OVARIES FALLOPIAN TUBES BLADDER UROGENITAL OPENINGS (BOTH SEXES) PENIS VAGINA UTERUS REPRODUCTIVE PRODUCE FEMALE HORMONES EXCRETORY FROM KIDNEY TO BLADDER EXCRETORY STORES URINE REPRODUCTIVE TRANSPORTS
Jurisdiction South Carolina. Retirement Date N/A
 Local Coverage Determination (LCD): Ionized Calcium (L31580) Contractor Information Contractor Name Palmetto GBA opens in Back to Top LCD Information Document Information Contract Number 11201 Contract
Local Coverage Determination (LCD): Ionized Calcium (L31580) Contractor Information Contractor Name Palmetto GBA opens in Back to Top LCD Information Document Information Contract Number 11201 Contract
Human Anatomy & Physiology. Introduction (Ch. 1)
 Human Anatomy & Physiology Introduction (Ch. 1) Overview of Anatomy and Physiology Anatomy the study of the structure of body parts and their relationships to one another Gross or macroscopic Microscopic
Human Anatomy & Physiology Introduction (Ch. 1) Overview of Anatomy and Physiology Anatomy the study of the structure of body parts and their relationships to one another Gross or macroscopic Microscopic
Body Regions Review. Anatomical Position. Anatomical Planes. Supine versus Prone 9/9/2009
 Body Regions Review The fundamental divisions of the human body Christine Sparks Anatomy / Physiology I Sept. 9, 2009 Anatomical Position Universal terms are used to describe the body accurately and result
Body Regions Review The fundamental divisions of the human body Christine Sparks Anatomy / Physiology I Sept. 9, 2009 Anatomical Position Universal terms are used to describe the body accurately and result
Fig. A.1. Frontal. plane. Transverse. plane. Sagittal plane. Copyright McGraw-Hill Education. Permission required for reproduction or display.
 Fig. A.1 Frontal plane Transverse plane Sagittal plane McGraw-Hill Education/Joe DeGrandis Fig. A.2 (a) Sagittal section (b) Frontal section (c) Transverse section Table A.1 Fig. A.3 Cephalic r. (head)
Fig. A.1 Frontal plane Transverse plane Sagittal plane McGraw-Hill Education/Joe DeGrandis Fig. A.2 (a) Sagittal section (b) Frontal section (c) Transverse section Table A.1 Fig. A.3 Cephalic r. (head)
California Cancer Registry Production Automation and Quality Control Unit Data Alert - Registrar
 Production Automation and Quality Control Unit Data Alert - Registrar 2015-063 REVISED - Clarification Casefinding List for FY2016 Updated Please see additional codes inadvertently excluded from the previous
Production Automation and Quality Control Unit Data Alert - Registrar 2015-063 REVISED - Clarification Casefinding List for FY2016 Updated Please see additional codes inadvertently excluded from the previous
RAMATHIBODI CANCER REPORT
 RAMATHIBODI CANCER REPORT 2016 Ramathibodi Cancer Registry : A subsidiary of Ramathibodi Comprehensive Cancer Center Faculty of Medicine, Ramathibodi Hospital Mahidol University Table of content INTRODUCTION...III
RAMATHIBODI CANCER REPORT 2016 Ramathibodi Cancer Registry : A subsidiary of Ramathibodi Comprehensive Cancer Center Faculty of Medicine, Ramathibodi Hospital Mahidol University Table of content INTRODUCTION...III
All Discovered Death Outcome Detail (Form 124/120)
 This file includes all reported deaths regardless of consent. ID WHI Common ID Col#1 DEATHALL All Discovered Death Col#2 Any report of death, regardless of consent status. 0 No 106,931 66.1 1 Yes 54,877
This file includes all reported deaths regardless of consent. ID WHI Common ID Col#1 DEATHALL All Discovered Death Col#2 Any report of death, regardless of consent status. 0 No 106,931 66.1 1 Yes 54,877
Medications currently available to treat Multiple Myeloma include: Current Code Price (AWP) Effective Date. Code Price (AWP)
 drug and administration compendia Compendia information developed and maintained by With each publication Managed Care Oncology s Drug & Compendia will highlight a single medication or a group of medications
drug and administration compendia Compendia information developed and maintained by With each publication Managed Care Oncology s Drug & Compendia will highlight a single medication or a group of medications
Interventions for non-metastatic squamous cell carcinoma of the skin: a systematic review and pooled analysis of observational studies
 Web appendix 2: SEARCH STRATEGIES Interventions for non-metastatic squamous cell carcinoma of the skin: a systematic review and pooled analysis of observational studies MEDLINE 1. exp epidemiologic studies/
Web appendix 2: SEARCH STRATEGIES Interventions for non-metastatic squamous cell carcinoma of the skin: a systematic review and pooled analysis of observational studies MEDLINE 1. exp epidemiologic studies/
Lab 5 Digestion and Hormones of Digestion. 7/16/2015 MDufilho 1
 Lab 5 Digestion and Hormones of Digestion 1 Figure 23.1 Alimentary canal and related accessory digestive organs. Mouth (oral cavity) Tongue* Parotid gland Sublingual gland Submandibular gland Salivary
Lab 5 Digestion and Hormones of Digestion 1 Figure 23.1 Alimentary canal and related accessory digestive organs. Mouth (oral cavity) Tongue* Parotid gland Sublingual gland Submandibular gland Salivary
*
 Introduction Cancer is complex, can have many possible causes, and is increasingly common. For the U.S. population, 1 in 2 males and 1 in 3 females is at risk of developing cancer in their lifetime. The
Introduction Cancer is complex, can have many possible causes, and is increasingly common. For the U.S. population, 1 in 2 males and 1 in 3 females is at risk of developing cancer in their lifetime. The
OP-10: ABDOMEN CT USE OF CONTRAST MATERIAL
 Description of Measure OP-10: ABDOMEN CT USE OF CONTRAST MATERIAL This measure calculates the percentage of abdomen studies that are performed with and without contrast out of all abdomen studies performed
Description of Measure OP-10: ABDOMEN CT USE OF CONTRAST MATERIAL This measure calculates the percentage of abdomen studies that are performed with and without contrast out of all abdomen studies performed
A Time- and Resource-Efficient Method for Annually Auditing All Reporting Hospitals in Your State: the Inpatient & Outpatient Hospital Discharge Files
 A Time- and Resource-Efficient Method for Annually Auditing All Reporting Hospitals in Your State: the Inpatient & Outpatient Hospital Discharge Files By Dr. Martin A. Whiteside Director, Office of Cancer
A Time- and Resource-Efficient Method for Annually Auditing All Reporting Hospitals in Your State: the Inpatient & Outpatient Hospital Discharge Files By Dr. Martin A. Whiteside Director, Office of Cancer
Cancer Program Report 2014
 Cancer Program Report 2014 Queen of the Valley Hospital St Joseph Health Queen of the Valley Hospital - 2014 Site Table Site Total Class Sex Group Cases Analytic NonAn M F 0 I II ALL SITES 661 494 167
Cancer Program Report 2014 Queen of the Valley Hospital St Joseph Health Queen of the Valley Hospital - 2014 Site Table Site Total Class Sex Group Cases Analytic NonAn M F 0 I II ALL SITES 661 494 167
REPORTABLE CASES MISSISSIPPI For cases diagnosed 10/1/2017 and after
 REPORTABLE CASES MISSISSIPPI For cases diagnosed 10/1/2017 and after The following lists are intended to assist you, as the reporter, in identifying the reportable neoplasms for your facility. Any reportable
REPORTABLE CASES MISSISSIPPI For cases diagnosed 10/1/2017 and after The following lists are intended to assist you, as the reporter, in identifying the reportable neoplasms for your facility. Any reportable
SAMPLE. Laboratory Services. An essential coding, billing, and reimbursement resource for laboratory and pathology services ICD-10
 Coding and Payment Guide www.optumcoding.com Laboratory Services An essential coding, billing, and reimbursement resource for laboratory and pathology services 2017 ICD-10 A full suite of resources including
Coding and Payment Guide www.optumcoding.com Laboratory Services An essential coding, billing, and reimbursement resource for laboratory and pathology services 2017 ICD-10 A full suite of resources including
3 Circulatory Pathways
 40 Chapter 3 Circulatory Pathways Systemic Arteries -Arteries carry blood away from the heart to the various organs of the body. -The aorta is the longest artery in the body; it branches to give rise to
40 Chapter 3 Circulatory Pathways Systemic Arteries -Arteries carry blood away from the heart to the various organs of the body. -The aorta is the longest artery in the body; it branches to give rise to
Respiratory & Digestive Organs of the Head and Neck, Human;
 Name Date Lab Exercise 5: Lab Exercise 6: Lab Exercise 7: Lab Exercise 8: Respiratory & Digestive Organs of the Head and Neck, Human; Histology of the Respiratory System Digestive System Models, Human
Name Date Lab Exercise 5: Lab Exercise 6: Lab Exercise 7: Lab Exercise 8: Respiratory & Digestive Organs of the Head and Neck, Human; Histology of the Respiratory System Digestive System Models, Human
Chapter 2 Neoplasms C00-D49 November 11, Chapter 2: Neoplasms Category: C00-D49 Official Coding Guidelines: Section 1.C.2, p.
 Chapter 2: Neoplasms Category: C00-D49 Official Coding Guidelines: Section 1.C.2, p. 22 1 C00 C75 Malignant neoplasms, stated or presumed to be primary, of specified sites, except of lymphoid, hematopoietic
Chapter 2: Neoplasms Category: C00-D49 Official Coding Guidelines: Section 1.C.2, p. 22 1 C00 C75 Malignant neoplasms, stated or presumed to be primary, of specified sites, except of lymphoid, hematopoietic
Digestive System. In one end and out the other.
 Digestive System In one end and out the other. Overview Every cell in the body needs nourishment, yet most cells cannot leave their position in the body and travel to a food source, so the food must be
Digestive System In one end and out the other. Overview Every cell in the body needs nourishment, yet most cells cannot leave their position in the body and travel to a food source, so the food must be
