The Role of the p53 Tumour Suppressor Protein in Relation to the Sensing of Ionizing Radiation-Induced DNA Double-Strand Breaks
|
|
|
- Christine Harrington
- 6 years ago
- Views:
Transcription
1 The Role of the p53 Tumour Suppressor Protein in Relation to the Sensing of Ionizing Radiation-Induced DNA Double-Strand Breaks by Shahnaz Tahihra Al Rashid A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy, in the Department of Medical Biophysics, University of Toronto Copyright by Shahnaz Tahihra Al Rashid 2010
2 The role of the p53 tumour suppressor protein in relation to the sensing of ionizing radiation-induced DNA double-strand breaks Shahnaz Tahihra Al Rashid Degree of Doctor of Philosophy Department of Medical Biophysics University of Toronto 2010 ABSTRACT Our cells are constantly dealing with DNA damage generated by endogenous cellular activity (e.g. DNA replication) and exogenous agents (e.g. ultraviolet and ionizing radiation (IR)). The cellular stress response to DNA damage requires strict co-ordination between cell cycle checkpoint control and DNA repair. In response to DNA double-strand breaks (DNA-dsbs), members of the phosphatidylinositol 3- kinase related kinase family (e.g. ATM and DNA-PKcs kinases) have been shown to redundantly phosphorylate substrates including the DNA-dsb marker, γ-h2ax, and the p53 tumour suppressor protein. The p53 protein is best known as the guardian of the genome through its transcriptional-dependent and -independent functions. Despite a clear link between ATM-dependent phosphorylation of p53 with cell cycle checkpoint control and various modes of DNA damage repair, the intracellular biology and sub-cellular localization of p53 and specifically its phosphoforms during DNA damage induction and repair remains poorly characterized. Using G0/G1 confluent primary human diploid fibroblast cultures, this thesis shows that endogenous p53, phosphorylated at serine 15 (p53 Ser15 ), accumulates as discrete, dose-dependent and chromatin-bound foci within 30 minutes following the induction of DNA breaks. This biologically distinct sub-pool of p53 Ser15 is ATM-dependent and resistant to 26S-proteasomal degradation. p53 Ser15 co-localizes and coimmunoprecipitates with γ-h2ax with kinetics similar to that of biochemical DNA-dsb rejoining. Sub-nuclear microbeam irradiation studies confirm that p53 Ser15 is recruited to sites of DNA damage containing γ-h2ax, ATM Ser1981 and DNA-PKcs Thr2609 in vivo. Furthermore, studies using isogenic human and murine cells, which express Ser15 ii
3 or Ser18 phosphomutant proteins, respectively, show defective nuclear foci formation, decreased induction of p21 WAF, decreased γ-h2ax-association and altered DNA-dsb kinetics following DNA damage. We further hypothesized that the non-specific DNA binding activity of the p53 carboxy-terminus mediates chromatin anchoring at sites of DNA damage. YFP-p53 fusion constructs expressing carboxy-terminus deletion mutants of p53 were transfected into p53-null H1299 cells to determine the role of the carboxy-terminus in chromatin-binding pre- and post-ir, independent of transcriptional activity. Within this isogenic human cell system, we observed exogenous YFP-p53 WT associated with ATM Ser1981 and 53BP1 within cellular chromatin in a dynamic manner. We confirmed that these associations also occurred between endogenous WTp53 with ATM Ser1981 and 53BP1 within the chromatin of primary human diploid fibroblasts. YFP-p fusion proteins, which lack transcriptional activity and the Ser15- residue, also associated within chromatin. Ser15-phosphorylation was found not to be essential for DNA damage-induced association of p53 with chromatin or with ATM Ser1981 and 53BP1. These data suggest a unique biology for p53 Ser15 phosphoforms in the initial steps of DNA damage signaling and implicates ATM-p53-53BP1 chromatin-based interactions as mediators of cell cycle checkpoint control and DNA repair. And we propose a model whereby a pre-existing pool of p53 that constantly scans the genome, responds immediately to radiation-induced DNA damage by virtue of its association with chromatin through its carboxy-terminus. The consequences for these p53-atm Ser BP1 complexes following DNA damage remains to be investigated: could residual complexes be associated with decreased DNA-dsb rejoining or error-prone repair, or could these complexes signal for cell survival or cell death? Since altered p53 function and biology is an important factor in cellular carcinogenesis and response to cancer therapy, this study provides a step towards a greater understanding of WTp53 and MTp53 biology in tumour development and therapeutic resistance, in the hopes to contribute towards predicting therapeutic response and/or improving p53-targeted therapies. iii
4 ACKNOWLEDGEMENTS First and foremost, I give my deepest gratitude to my family, mama, papa-in-spirit, Shahin, Shahir, Kwee, Doreen and Ian for their enduring support, advice and encouragement throughout my PhD studies. This PhD thesis would not have been possible without your inspiration and strength. I would like to thank my supervisor, Dr. Robert Bristow, for his immense patience, guidance and enthusiasm throughout my PhD journey, and for opening up the vast and intricate realm of cancer and radiation research to me, a naïve microbiology specialist who began this journey with solely the drive to understand carcinogenesis and therapy. I would also like to extend my warmest thanks and appreciation to Dr. Sam Benchimol, Dr. Cheryl Arrowsmith and Dr. Lothar Lilge for their constant feedback and encouragement throughout my PhD studies. And I would like to thank Dr. Gillian Wu, for all her help and advice during my initial PhD years. Throughout my PhD, my lab mates have been a constant source of support, help and laughter a million thanks and hugs to Ramya, Carla, Alice, Farid, Ananya, Gillian, Kumar, Norman, Cindy, Harshna, Graeme, Stephane, Marcia and of course, Christina D. and Carolynn M. for all your hard work in helping me to complete this thesis and getting me to cross the finish line. Last but definitely not least, I would like to thank my dearest friends, Sherry, Sharan, Celia, Margaret, Amy, Ruth, Anne H., Susan, Lisa K. and David N. who have stuck by me through my ups and downs, gently encouraging and relentlessly trying to get me out of the lab into other obsessions at least once a week! I probably have forgotten some names, so I want to again thank everyone (you know who you are!) who has been with me throughout this epic journey and undoubtedly influenced my thesis. iv
5 TABLE OF CONTENTS Abstract... ii Acknowledgements... iv Table of Contents... v List of Figures... x List of Abbreviations...xiv Chapter 1: Introduction Overview: the importance of sensing and repairing DNA double-strand breaks, in carcinogenesis and cancer treatment The p53 tumour suppressor protein and its role in mediating the G1/S cell cycle checkpoint and DNA-dsb repair The p53 family of proteins Structural and functional domains of the WTp53 protein Transcriptional and translational regulation of p DNA-dsb sensing and signaling to enact cell cycle checkpoints Molecular repair of DNA-dsbs The relationship between DNA-dsb repair and cellular radiosensitivity: cell death and survival assays Detection of DNA-dsbs: biochemical DNA-dsb rejoining assays Detection of DNA-dsbs: measuring DNA-dsbs in situ The ATM-p53 pathway and DNA-dsbs Interactions of ATM, MRN, and Artemis (NHEJ pathway) within chromatin...28 v
6 5.2 Interactions of ATM and CHK2, MDM2 and p21 WAF at the chromatin interface of DNA-dsb sensing and signal transduction Interactions of ATM, ATR and CHK p53 and the repair of DNA-dsbs Implications of p53 in carcinogenesis and cancer therapy Thesis Outline...36 Chapter 2: Evidence for the Direct Binding of Phosphorylated p53 to Sites of DNA Breaks In vivo Abstract Introduction Materials and Methods...53 Fibroblast strains and cell culture conditions...53 Irradiation and chemical induction of DNA damage...53 Confocal and wide-field immunofluorescence microscopy...55 Western blot, immunoprecipitation and cellular fractionation analyses...56 DNA-dsb rejoining assays...58 YFP-p53 phosphomutant constructs and transient transfection conditions Results...61 Ionizing radiation induces discrete dose-responsive p53 Ser15 nuclear foci from pre-existing p53 protien pool in an ATM-dependent manner...61 vi
7 p53 Ser15 is a unique chromatin-associated sub-pool of total p Dependency and association of p53 Ser15 foci on ATM, DNA-PKcs or MRN function...65 p53 Ser15 kinetics correlate with biochemical DNA-dsb rejoining...67 Lack of Ser15-phosphorylation leads to altered DNA rejoining, foci formation and γ-h2ax association following IR-induced DNA damage...68 Interaction between p53 Ser15, ATM Ser1981 and DNA-PKcs Thr2609 at focal DNA damage Discussion Chapter 3: The Carboxy-terminus of p53 and Chromatin-binding in Response to Radiation-induced DNA Damage Abstract Introduction Materials and Methods Cell lines, culture conditions and irradiation treatments p53 constructs and transient transfection conditions Wide-field and confocal immunofluorescence microscopy Real-time quantitative polymerase chain reaction (RT-qPCR) Biochemical fractionation, immunoprecipitation and western blot analyses Results vii
8 Sub-cellular expression and localization of exogenous YFP-p53 fusion proteins Phosphorylation status of the YFP-p53 fusion proteins within the cytoplasmic and nuclear compartments Transcriptional activity of the YFP-p53 fusion proteins Expression of YFP-p53 C-terminal deletion mutant fusion proteins does not alter DNA damage-induced phosphorylation of p Chromatin-bound levels of DNA damage-induced phosphoforms of p53 ATM Ser1981 and 53BP YFP-p53 fusion proteins interact with 53BP1 within the cytoplasm, soluble nuclear and chromatin-bound fractions, independent of DNA damageinduction YFP-p53 fusion proteins interact with ATM Ser1981 within soluble nuclear and chromatin-bound fractions, in response to DNA damage, but independent of p53 transcriptional activity DNA damage-induced chromatin interactions between p53, 53BP1 and ATM Ser Discussion Chapter 4: Discussion Summary and Discussion of Completed Work Irradiation-induced Ser15-phosphorylated p53 phosphoforms viii
9 4.1.2 Consequences of the loss of Ser15-phosphorylated p53 phosphoforms Outstanding Questions and Future Directions Is ATM a mediator of p53-directed DNA-dsb repair fidelity? p53 phosphoform interactions with other stress kinases Cancer Impact ix
10 LIST OF FIGURES Chapter 1: Figure 1.1 Introduction The central role of p53 in three cellular processes to maintain genomic stability: cell cycle checkpoint arrests, cell death and DNA repair Figure 1.2 Figure 1.3 The multiple roles of p53 in the response to radiotherapy Structural and functional domains of the full length WTp53 protein and its splice forms Figure 1.4 DNA damage-induced post-translational modifications of the full length WTp53 protein Figure 1.5 Figure 1.6 Figure 1.7 Figure 1.8 Downstream trans-activation targets of the full length WTp53 protein Ionizing radiation-induced G1-S-G2/M cell cycle checkpoint arrests Ionizing radiation-induced ATM-dependent pathway Summary of the two main DNA double-strand break repair pathways: non-homologous end joining and homologous recombination repair Figure 1.9 Clonogenic survival assays to determine the radiation sensitivity of cell strains and lines Figure 1.10 Biochemical assays (i.e. CFGE or COMET) to determine DNA-dsb rejoining kinetics and molecular assays (i.e. γ-h2ax radiation-induced foci) to quantitate DNA-dsbs Figure 1.11 Pathways involved in DNA-dsb sensing, signaling, and repair, and immunofluorescence microscopy methods to quantitate the number and kinetics of DNA-dsbs and repair protein interactions Figure 1.12 Thesis hypothesis and outline x
11 Chapter 2: Evidence for the Direct Binding of Phosphorylated p53 to Sites of DNA Breaks In Vivo Figure 2.1 p53 Ser15 nuclear foci form following genotoxic insult from a pre-existing p53 pool and form in response to DNA breaks and base damage Figure 2.2 IR-induced p53 Ser15 foci are a chromatin-bound sub-pool of total p53 protein Figure 2.3 p53 Ser15 foci dependency and association with ATM, DNA-PKcs, γ-h2ax and the MRN complex Figure 2.4 p53 Ser15 nuclear foci correlates with DNA-dsb rejoining and are resistant to proteolytic degradation Figure 2.5 Lack of Ser15-phosphorylation leads to altered DNA-dsb rejoining, foci formation and γ-h2ax association following IR-induced DNA damage Figure 2.6 Interaction in vivo between p53 Ser15, γ-h2ax, ATM Ser1981 and DNA-PKcs Thr2609 at DNA breaks Supplementary Figure 2.1 p53 Ser15 foci form in response to DNA breaks and base damage Supplementary Figure 2.2 p53 Ser15 foci are a transient sub-pool of total nuclear p53: qualitative and quantitative staining for total p53 and p53 Ser15 foci Supplementary Figure 2.3 p53 Ser15 foci formation is NBS1-independent, but do colocalize with NBS1 and RAD50 proteins (i.e. components of the MRN DNA repair complex); however, p53 Ser15 foci do not co-localize with the senescence-associated, PML protein. xi
12 Chapter 3: The Carboxy-terminus of p53 and Chromatin-binding in Response to Radiation-induced DNA Damage Figure 3.1 Characterization and sub-cellular expression of YFP-p53 fusion proteins Figure 3.2 Quantitative RT-PCR for p53-responsive genes at 16 hours post-transfection of the exogenous YFP-p53 fusion constructs Figure 3.3 Kinetics and dose-response of the YFP-p53 fusion proteins and the ATM-p53-γ-H2AX signaling pathway in transiently-transfected H1299 cells following irradiation Figure 3.4 Interactions between exogenous YFP-p53 fusion proteins with 53BP1-ATM Ser1981 within different sub-cellular compartments of transiently-transfected p53-null H1299 cells Figure 3.5 Reciprocal co-immunoprecipitation analyses using 53BP1-specific antibodies to determine the interactions between YFP-p53 fusion proteins with 53BP1 within different sub-cellular compartments of transiently-transfected p53-null H1299 cells Figure 3.6 Reciprocal co-immunoprecipitation analyses using p53- and ATM Ser1981 -specific antibodies to determine the interactions between 53BP1 and ATM Ser1981 within chromatin, in the presence and absence of WTp53 Supplementary Figure 3.1 Biochemical fractionation of YFP-alone transfected p53-null H1299 cells at 30 minutes post-5gy, displaying control proteins within each sub-cellular fraction xii
13 Chapter 4: Figure 4.1 Discussion Kinetics and residual p53 Ser15 foci in fibroblasts of varying cellular radiosensitivities Figure 4.2 Increased levels of soluble Ser15-phosphorylated YFP-p53 WT following micrococcal nuclease (MNase) digestion of insoluble/chromatin-bound cell fraction Figure 4.3 Levels of ATM-dependent phosphorylation following IR-induced DNA damage in G0-G1 synchronized primary human fibroblasts Figure 4.4 Cytoplasmic and soluble nuclear interactions of YFP-p53 mutant constructs with 53BP1 and ATM Ser1981, pre- and post-irradiation Figure 4.5 Final proposed model of dynamic interaction of p53-53bp1-atm following IR-induced DNA damage Figure 4.6 Immunofluorescence stained images of G0/G1-synhronized normal human fibroblasts, GM05757, pre- and post-irradiation: positive controls of both SC35 and PRP kinase co-localize following irradiation, and our novel observation that p53 Ser15 foci also highly co-localize with both SC35 and PRP kinase xiii
14 LIST OF ABBREVIATIONS 53BP1 p53 binding protein 1 α-mem alpha minimum essential medium AT Ataxia Telangiectasia ATLD AT-like disorder ATM ataxia-telangiectasia mutated ATM-i ATM inhibitor, KU55933 ATR ataxia-telangiectasia and Rad3 related BER base excision repair BRCA1 breast cancer 1, early onset BRCA2 breast cancer 2, early onset BS Bloom s syndrome BSA bovine serum albumin Cdc25A cell division cycle 25 homolog A Cdc45 cell division cycle 45 Cdk cyclin-dependent kinase CFGE constant field gel electrophoresis CHK1 checkpoint kinase 1 CHK2 checkpoint kinase 2 DAPI 4',6-diamidino-2-phenylindole DMSO dimethyl sulfoxide DNA deoxyribonucleic acid DNA-dsb DNA double-strand break DNA-PK DNA-dependent protein kinase DNA-PKcs DNA-dependent protein kinase catalytic subunit DNA-ssb DNA single-strand break EDTA ethylenediaminetetraacetic acid FAR fraction of activity released FCS fetal calf serum FDR fraction of DNA released FISH fluorescent in situ hybridization FLIP fluorescence loss in photobleaching FRAP fluorescence recovery after photobleaching FRET fluorescence resonance energy transfer γ-h2ax gamma H2AX (H2AX phosphorylated at serine 139) GADD45 growth arrest and DNA damage 45 Gy Gray H2A histone 2A H2AX histone H2AX, a variant of H2A H2B histone 2B H3 histone 3 H4 histone 4 HNPCC Hereditary Non-polyposis Colon Cancer HR homologous recombination IHC immunohistochemistry xiv
15 IF immunofluorescence IR ionizing radiation LFS Li-Fraumeni Syndrome LMA low melting point agarose LMDS local multiply damaged sites MDC1 mediator of DNA damage checkpoint 1 MDM2 mouse double minute 2 homolog MLH1 mutl homolog 1 MMC mitomycin c MMR mismatch repair MMS methyl methane sulfonate MRE11 meiotic recombination 11 MRN MRE11-RAD50-NBS1 complex mrna messenger ribonucleic acid MSH2 muts homolog 2 MTp53 mutant p53 NaCl sodium chloride NaOH sodium hydroxide NER nucleotide excision repair NBS Nijmegen Breakage Syndrome NBS1 Nijmegen breakage syndrome 1 protein NDF normal diploid fibroblast NHEJ non-homologous end-joining NIR non-irradiated NLS nuclear localization sequences NSDBD non-specific DNA binding domain NTM normalized tail moment OH hydroxyl radical p16 INK4a cyclin-dependent kinase inhibitor 2A p21 WAF1 cyclin-dependent kinase inhibitor 1A p53 tumour suppressor protein 53 PARP-1 poly (adenosine diphosphate-ribose) polymerase 1 PCR polymerase chain reaction PE plating efficiency PFGE pulse field gel electrophoresis PIKK phosphoinositide-3-kinase-related protein kinase PLDR potentially lethal damage repair PP2A protein phosphatase 2A RAD50 radiation 50 RAD51 radiation 51 RAD52 radiation 52 RAD54 radiation 54 RNA ribonucleic acid ROS reactive oxygen species RPA replication protein A SEM standard error of the means xv
16 SLDR sub-lethal damage repair SMC1 structural maintenance of chromosomes 1 hsmg-1 human homolog of SMG-1 (suppressor of morphogenetic effect on genitalia 1) ssdna single-stranded DNA SSDBD sequence-specific DNA binding domain TAD trans-activation domain TET tetramerization domain UV ultraviolet radiation WS Werner s syndrome WTp53 wild type p53 XLF XRCC4-like factor XP Xeroderma Pigmentosum XRCC4 X-ray repair cross complementing protein 4 YFP yellow fluorescent protein xvi
17 Chapter 1 Introduction 1
18 1.0 Overview: the importance of sensing and repairing DNA double-strand breaks, in carcinogenesis and cancer treatment Our cells are constantly dealing with DNA damage generated by endogenous cellular activity (e.g. respiratory metabolism which generates reactive oxygen species or DNA replication) and exogenous DNA damaging agents (e.g. ultraviolet (UV) and ionizing radiation (IR)). Cancer patients can be exposed to DNA damaging agents during radiotherapy and certain forms of chemotherapy. IR generates various types of DNA lesions and chromatin alterations including: DNA base damage, inter- and intra-strand DNA cross-links, DNA single-strand breaks (ssbs) and double-strand breaks (dsbs). Together, these lesions can occur at single dispersed sites or at clustered local multiply damaged sites (LMDS). Mammalian cells have evolved several DNA damage repair mechanisms depending on the DNA lesions, including DNA-ssb repair, base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR) and DNA-dsb repair; the latter is the most critical DNA repair pathway to prevent cell death following IR (1-3). There are two main DNA-dsb repair pathways: homologous recombination (HR) and nonhomologous end-joining (NHEJ). The p53 tumour suppressor protein has been shown to be involved in both HR and NHEJ DNA-dsb repair pathways. A DNA-dsb is a critical lesion and if left un- or mis-repaired, can be mutagenic and lead to genetic instability and cellular carcinogenesis (1). The cell senses DNA damage and promptly executes coordinated cellular responses, including cell cycle arrest, DNA repair and/or cell death (e.g. apoptosis, 2
19 DNA - dsb ATM MRN γ-h2ax MDC1/RNF8 53BP1 MRN CHK2 ATR BRCA1 (HR) RPA RAD51 p53 DNA repair Cell cycle checkpoints Cell death G1 arrest S arrest G2/M arrest Apoptosis Autophagy Necrosis Senescence Figure 1.1. Cell cycle checkpoints are activated in response to external and internal physiological stress and damage to allow for repair and maintain genomic stability. Cellular transformation is averted through repair or induction of cell death. This figure emphasizes the central role of p53 in all three cellular processes to maintain genomic stability. For example, ionizing radiation induces DNAdsbs that are sensed and results in activation of the MRN DNA repair complex (consisting of the MRE11, RAD50 and NBS1 proteins) and the ATM checkpoint kinase. The ATM kinase then activates downstream signaling cascades including the activation of p53 to mediate cell cycle checkpoint arrests as well as DNA-dsb repair, or cell death in the presence of irreparable DNA damage. 3
20 mitotic catastrophe, autophagy or permanent arrest) (Figure 1.1). These exact responses to DNA damage will be dependent on several factors, including histological cell type, cell cycle phase in which the DNA damage is incurred and the severity of DNA damage. Therefore, the determinants of mammalian cell IRsensitivity include the cell s capacity for DNA repair, cell cycle phase at the time of irradiation, oxygenation status during irradiation and the relative response to cell cycle checkpoint activation within the G1, S and G2/M phases of the cell cycle. In response to IR-induced DNA damage, cells predominantly undergo G1 and G2 cell cycle checkpoints to inhibit radiation-induced death or carcinogenesis (see Figure 1.1 for overview). The sensitivity of checkpoint activation has been shown in many studies. For example, Wahl and colleagues observed in human fibroblasts that one non-repaired DNA-dsb induced prior to the G1-S restriction point was sufficient to induce a permanent G1 cell cycle arrest mediated through the p53 protein (4). The critical importance of the integration of cell cycle checkpoint control and DNA repair to offset carcinogenesis is exemplified by the presence of several human diseases characterized by chromosomal instability in their tissues and an early onset of various types of cancer. These include: (1) the Li-Fraumeni syndrome (LFS: germline mutations in the p53 and CHK2 genes resulting in defective cell cycle checkpoint control); (2) Ataxia Telangiectasia (AT: mutation in the ATM kinase gene resulting in defective DNA damage sensing, cell cycle checkpoint control and DNAdsb repair); (3) the Nijmegen Breakage Syndrome (NBS: mutation in NBS1 resulting 4
21 in defective cell cycle checkpoint control and DNA-dsb repair); (4) hereditary nonpolyposis colon cancer (HNPCC: a manifestation of defective DNA mismatch repair); and (5) xeroderma pigmentosum (XP: a manifestation of defective nucleotide excision repair). Other syndromes include the AT-like Disorder (ATLD: defective function of MRE11), the Seckel Syndrome (mutation in ATR resulting in defective cell cycle checkpoint control), the Riddle Syndrome (defective RFN8 protein) and the DNA ligase IV syndrome (5). As will be discussed further in this thesis, the p53 tumour suppressor protein is a critical protein involved in mediating cross-talk between cell cycle checkpoints and DNA-dsb repair (see Figure 1.1). This thesis will focus on the ATM-p53 pathway that mediates the G1 checkpoint, and specifically on the p53 protein and its emerging role in directly associating with DNA-dsbs. The following sections and chapters in this thesis will develop the background information on the DNA-dsb response following IR and the activation of the ATM-p53 pathway. They will specifically present background and experimental studies on the localization and function of wild type and mutant p53 proteins as candidate proteins in partially determining DNA-dsb sensing and repair. They will present novel evidence that links p53 directly to DNA-dsb binding in a dose-responsive and phosphorylation-dependent manner to interact with other ATM-dependent proteins during DNA-dsb sensing and repair. These data may be useful in the interpretation and derivation of biomarkers relating to cancer risk and carcinogenesis or 5
22 Figure 1.2. The multiple roles of p53 in the response to radiotherapy. Obtained with permission from Cuddihy and Bristow (6). Creating DNA damage to tumour cells (e.g. using radiotherapy or chemotherapy) can be used to treat human cancer. The loss or alteration of p53 protein function within a tumour can affect many aspects of the patient s response to radiotherapy (i.e. 5 R s of radiotherapy : repair, radiosensitivity, repopulation, reoxygenation and redistribution). Therefore, various methods to improve eradication of the tumour are through treatments that target the p53- mediated radiation- and chemo-resistance. 6
23 biomarkers associated with the response of tumours within cancer patients undergoing radiotherapy or chemotherapy (Figure 1.2). 2.0 The p53 tumour suppressor protein and its role in mediating the G1/S cell cycle checkpoint and DNA-dsb repair The p53 protein has been shown to be critically involved in integrating signals of various genotoxic stresses and mediating cell-cycle arrest. This may allow for DNA damage repair or cell death to eliminate severely-damaged or mutant cells. The wild type p53 (WTp53) gene is located on chromosome 17p13.1. The gene has 11 exons which encode a 393 amino acid protein with a molecular weight of approximately 53 kilodaltons (kda). p53 has been best characterized for its function as a transcriptional trans-activator in mediating the G1 cell cycle checkpoint to prevent genomic instability and carcinogenesis (7). 2.1 The p53 family of proteins The p53 gene was initially discovered in 1979, followed by the identification of the two p53-related genes, p63 and p73. Together, these genes have sequence similarities and more prominently, conserved domain structure and conformation. Although highly similar, these genes have unique functions and are not entirely redundant. Whereas, the p53 protein has been well characterized as a tumour suppressor protein, the p63 protein has been found to play a role mostly in differentiation and during development (8). The p73 protein has been found to play a 7
24 role in apoptosis (in concert with p53 under certain conditions) and the DNA damage response (8). 2.2 Structural and functional domains of the WTp53 protein The crystal structure of the full-length p53 molecule was recently determined in solution and bound to DNA (9). This study was built on previous structural studies and suggested novel mechanisms for its structural flexibility and resolved conflicting data relating to its trans-activation and tumour suppressor functions. The WTp53 protein forms tetramers in solution and binds DNA as a tetramer (i.e. a dimer of dimers)(9, 10). Several conserved functional domains have been characterized within this protein (Figure 1.3). These include the amino-terminal trans-activation domain (TAD), central sequence-specific DNA binding domain (SSDBD), and the carboxy-terminal domain which encompasses the tetramerization domain (TET) and the basic regulatory non-sequence-specific DBD (NSDBD). Within the carboxy-terminus domain, there also exists several nuclear localization sequences (NLSs) and nuclear export signals (NESs). 2.3 Transcriptional and translational regulation of p53 The human p53 gene is encoded by 11 exons, from which transcription can be initiated at two promoters upstream of exon 1 and an internal promoter in intron 4. Based on studies by Bourdon and colleagues (11, 12), three classes of p53 8
25 NLS WTp53 TAD SSDBD TET NSDBD 53kDa p53β p53γ Np53 Np53β 331 TAD SSDBD DQTSFQKENC 331 TAD SSDBD MLLDLRWCYFLINSS 40 SSDBD TET 40 SSDBD 331 DQTSFQKENC 393 NSDBD 46kDa 46kDa 48kDa 41kDa Np53γ 40 SSDBD 331 MLLDLRWCYFLINSS 133p53 SSDBD TET NSDBD p53β 133 SSDBD 331 DQTSFQKENC kDa 35kDa 25kDa 133p53γ 133 SSDBD 331 MLLDLRWCYFLINSS 25kDa p53 TAD SSDBD TET NSDBD N/A Figure 1.3. Structural and functional domains of the full length WTp53 protein and its splice forms (Note: mrna and protein expression has been observed for all isoforms except p53, of which only its mrna expression has been detected; N/A: not applicable)(13). TAD: trans-activation domain; SSDBD: sequence-specific DNA-binding domain; NLS: nuclear localization sequences; TET: tetramerization domain; and NSDBD: non-specific DNA-binding domain. 9
26 S6 S9 S15 T18 S20 S33 S37 S46 T55 T81 NLS WTp53 1 TAD SSDBD TET NSDBD 393 S315 S371 S376 S378 S392 K320 K373 K382 K370 K372 K373 K372 K381 K382 K386 Phosphorylation Acetylation Ubiquitination Methylation Figure 1.4. DNA damage-induced post-translational modifications of the full length WTp53 protein. The sets of p53 modifications that occur depends on the type and amounts of stress that are incurred by the cell. 10
27 isoforms (altogether nine protein isoforms including the full length p53 protein) are generated through transcription from these promoters in combination with alternative splicing (Figure 1.3). In addition, another isoform has been identified by Rohaly and colleagues (14). While the function of p53 isoforms has remained largely unknown, Fujita and colleagues recently discovered that two isoforms may regulate cellular senescence (15). Transcriptional targets of p53 contain canonical p53-responsive elements (p53- REs), consisting of two decamer sequences with the consensus PuPuPuC(A/T)_(A/T)GPyPyPy (i.e. Pu: Purine; Py: pyrimidine)(7). The level of transcriptional activation and repression of p53 targets are controlled not only by the presence of the p53-res, but also the specific REs within a wide range of genes, the levels of p53 and the presence of cellular transcriptional co-factors. Non-canonical REs have also been identified, and while they are significantly different from the canonical p53-res, they have been shown to be activated by WTp53 and certain MTp53 proteins found in human cancer cells (16). Therefore, p53-mediated transcriptional regulation affects a large range of networks and can differ in normal and cancer cells (7). 11
28 Core regulation of p53 MDM4 MDM2 p53 p14/p19 ARF E2F-1 p21 WAF σ BAX NOXA PUMA PIDD RAD51 PCNA Cell cycle arrest/ Senescence Apoptosis DNA repair and damage prevention Figure 1.5. Downstream trans-activation targets of the full length WTp53 protein. Depending on the type and amount of stress and damage that is induced in a cell, the activation and stabilization of WTp53 results in either cell survival or cell death. p53-mediated checkpoint arrest allows for complete and accurate repair followed by cell survival. However, when the damage remains irreparable, cell death ensues through p53-mediated apoptosis, permanent cell cycle arrest or senescence. 12
29 In response to irradiation, multiple post-translational modifications are induced throughout the p53 protein, encompassing its trans-activation domain in the amino terminus and the carboxy-terminus (Figure 1.4). These modifications include phosphorylation, acetylation, ubiquitination and methylation, and have been shown to facilitate its stabilization and translocation/shuttling between the cytoplasm, nucleus and mitochondria (17, 18). These p53 modifications also regulate its degradation and mediate its multifaceted interactions and functions with DNA, RNA and proteins throughout the cell and during different phases of the cell cycle (Figure 1.5, adapted from Levine, et. al., 2006)(19). An important post-translational modification of the p53 protein is the phosphorylation of its Ser15 residue. This phosphorylation has been shown to precede multiple other post-translational modifications within its amino- and carboxy-termini, and the induction of its trans-activation activities following the formation of IR-induced DNA damage(20). Depending on the type and amount of cell stress, and cell cycle phase, different post-translational modifications of the p53 protein leads to its altered stabilization (e.g. MDM2-mediated degradation), localization and chromatin- and protein-interactions so as to trans-activate downstream target genes mediating critical events, including cell cycle arrest, DNA repair or cell death (Figure 1.5). 3.0 DNA-dsb sensing and signaling to enact cell cycle checkpoints The ATM (Ataxia Telangiectasia mutated), DNA-PKcs (DNA-dependent protein kinase catalytic subunit) and ATR (ATM- and Rad3-related) kinases are members of 13
30 the phosphatidylinositol 3-kinase related kinase (PI3KK) family that activate DNA damage-dependent signaling cascades in response to IR, UV radiation or replication arrest (21, 22). Recently, Kim and colleagues found that a sub-population of ATM is bound within chromatin in undamaged cells (23). The interaction between ATM and chromatin was observed to be modulated by histone modifications as well as by a nucleosomal-protein, HMGN1 (23). Prior to this study, several seminal papers reported that soluble nuclear ATM exists as an inactive dimer (24, 25). Upon sensing changes in global chromatin structure induced by DNA-dsbs, ATM undergoes autophosphorylation resulting in phosphorylation at Ser1981 (ATM Ser1981 ). ATM is then recruited to and tightly retained at sites of DNA-dsbs (23, 26, 27). Together, these studies show the integral role of ATM in initiating the cascade following DNAdamage sensing. ATM phosphorylates a number of substrates following activation during the G1 checkpoint including: p53; the DNA-dsb sensor histone protein, γ-h2ax; the repair proteins BRCA1 and NBS1; and the G1 checkpoint proteins CHK2, p21 WAF and MDM2 (28-34). Altogether, ATM-mediated phosphorylation of the p53 and CHK2 proteins, and subsequent trans-activation of p21 WAF and MDM2 enacts the G1 cell 14
31 IR - DNA damage ATM MRN DNA-PK ( Ku70/Ku80 DNA-PKcs ) CHK2 FAST ATR SLOW p53 p53 / Artemis CDC25A CHK1 p21 WAF CDC25A CDC25B CDC25C Wee1 GADD σ Cyclin D/E CDK2 Cyclin A/E CDK2 Cyclin B CDK1 prb G1 arrest S arrest G2/M arrest Figure 1.6. Ionizing radiation-induced G1-S-G2/M cell cycle checkpoint arrests. 15
32 cycle checkpoint following DNA damage (35). ATM facilitates both a rapid p53- independent and a slow p53-dependent induction of a G1 phase checkpoint through cdc25a and p21 WAF (36, 37). In addition to the G1 checkpoint, ATM is also responsible for the G2/M checkpoint, and together with the ATR kinase, mediates the S phase checkpoint (38-42)(Figure 1.6). The DNA-dsb repair proteins MRE11 and the KU70/80 heterodimer are thought to be the earliest sensors of DNA-dsbs (Figure 1.7)(43, 44). The MRN complex (consisting of the MRE11, RAD50 and NBS1 DNA-dsb repair proteins) is thought to sense DNA-dsbs and recruit the ATM kinase to these sites to facilitate activation of ATM (44-47). The Ku70/80 heterodimer recruits the catalytic subunit of the DNAdependent protein kinase (DNA-PKcs) to DNA ends, which results in its activation and auto-phosphorylation at threonine 2609, to initiate NHEJ-mediated DNA-dsb repair. The Ku70/80 heterodimer has also been shown to modulate the ATMdependent response to DNA-dsbs (48). There also may be co-dependence between these PI3 kinases as ATM is important for the phosphorylation and full activation of DNA-PKcs and NHEJ-mediated DNA-dsb repair (49). Similarly, DNA-PKcs activity has been shown to be important for ATM expression (50). Several protein phosphatases (e.g. PP2A, PP5 and PP6) regulate ATM and DNA-PKcs autophosphorylation and their kinase activity (51-54); although their relative expression and function between normal and malignant cells is not known. 16
33 The ATM, ATR and DNA-PK kinases all redundantly phosphorylate the histone variant H2AX at Ser139, γ-h2ax, within chromatin that extends through megabase regions around the IR-induced DNA-dsbs (73). These phosphorylations recruit MDC1 (mediator of checkpoint-1), which then acts to amplify the DNA damage signal by recruiting other mediator proteins such as RNF8-UBC13, an E3 ubiquitin ligase complex, and 53BP1, p53-binding protein 1 (55). Furthermore, ATM phosphorylates CHK2 at Thr68 within the chromatin, which is then released to act as effector, phosphorylating downstream targets, including p53, and initiating the checkpoint arrest (37). 4.0 Molecular repair of DNA-dsbs Figure 1.8 depicts a summary of the two main DNA-dsb repair pathways. NHEJ is active in all phases of the cell cycle (35, 43, 56). In contrast, the HR pathway carries out error-free DNA-dsb repair in the S and G2/M phases as it requires the presence of sister chromatids to provide an accurate template for base-pairing. There is crosstalk between NHEJ and HR, as Kim and colleagues have shown a sequential recruitment of NHEJ repair proteins followed by the HR repair proteins, in response to DNA damage during the G1-S border of the cell cycle (57). The roles that either 17
34 ATP-dependent chromatin remodelling PP2A ATM ATM Ser1981 MDC1 CHK2 ATM Ser BP1 MRN complex γ-h2ax RNF8 UBC13 ATM Ser1981 p53 Ser15 Release for downstream signal transduction enacting cell cycle arrest/repair/cell death Figure 1.7. Illustration of the ATM events following IR-induced DNA damage: (i) initial ATPdependent chromatin remodelling; (ii) autophosphorylation and activation of the ATM from a dimer to monomer; (iii) amplification of the DNA damage signal through widespread post-translational modification of chromatin-associated and DNA repair proteins; and subsequent activation of downstream signalling cascades to enact cell cycle arrest and repair, or cell death. 18
35 DNA - dsb NHEJ HR ATMSer1981 DNA-PKcs DNA end resection Ku70/80 MRN ATMSer1981 RPA MRN Artemis DNA end resection RAD51 RAD51 RAD51 DNA-PKcs BRCA2 RAD52 FA proteins DNA-PKcs Strand invasion DNA-PKcs Sister chromatid Ku70/80 RAD51 paralogs Cernunnos XRCC4 LigaseIV DNA Ligation Repair DNA synthesis Holiday junction formation Branch migration and resolution DNA-PKcs 19
36 Figure 1.8. Summary of the two main DNA-dsb repair pathways: NHEJ and HR (adapted from Iijima, 2008)(46). In NHEJ, the DNA-dsb is stabilized by both the Ku70/80 heterodimer and MRN complex. In HR, the MRN complex first localizes to the DNA-dsb. Both the MRN complex and Ku70/80 heterodimer tether and align the DNA ends and mediate DNA-end resection/processing. In NHEJ, the DNA-PKcs subunit is then recruited and activated, followed by the recruitment of Cernunnos, XRCC4 and Ligase IV which then mediates DNA ligation and completion of NHEJ repair. In HR, the DNA strand undergoing repair is marked in red and the homologous template used for repair is marked in green. Following alignment and resection by the MRN complex, RPA binds to the ssdna overhangs that have been generated. The RPA-coated ssdna is a substrate for the subsequent recruitment of the RAD51 protein which then forms a nucleo-protein filament. The formation of the RAD51 filament is enhanced by the further recruitment of the RAD52 protein family members (e.g. RAD52, RAD54, RAD55, RAD56 and RAD57), BRCA2 and FA proteins. The RAD51-filament then initiates homology search and strand invasion into the homologous template, thus resulting in repair DNA synthesis, Holliday junction formation, branch migration, resolution, ligation and completion of HR repair. 20
37 NHEJ or HR repair proteins play may also depend on the type and severity of DNA damage as well as cell type and context. 4.1 The relationship between DNA-dsb repair and cellular radiosensitivity: cell death and survival assays Radiation therapy, alone or in combination with chemotherapy, is a curative modality in certain head and neck, haemotopoietic, genitourinary, and gynaecologic cancers. External beam radiotherapy is typically given as a series of precision-guided highenergy x-rays in small (i.e Gy) daily fractions over six to eight weeks, up to total doses ranging from 60-80Gy in which the fractionated regimen maximizes tumour cell killing relative to normal cells (i.e. maximizes the therapeutic ratio ). Five factors (summarized as the 5 R s of radiotherapy ) contribute to final cell killing during fractionated radiotherapy and include: intrinsic radiosensitivity, redistribution of cells within the cell cycle, reoxygenation, repopulation and the repair capacity of normal tissues relative to tumour cells during the course of radiotherapy (58, 59). Several studies have shown that intrinsic cellular radiosensitivity is an important determinant of clinical radioresponse, indirectly suggesting that relative DNA-dsb repair capacity in tumour and normal cells may be a major determinant for radiotherapeutic curability (60-63). The presence of mutations in DNA-dsb damage repair or checkpoint genes leads to altered sensitivity of normal and tumour tissues 21
38 Figure 1.9. Clonogenic assays to determine the radiation sensitivity of cell strains and lines. (Left panel) The influence of (i) single dose and (ii) fractionated doses (e.g. low dose rate, LDR) treatments on the shape of cell survival curves (adapted from BSO). (Right panel) Survival curves of human fibroblast strains with altered ATM-p53 signalling and corresponding radiosensitivities: (i) AT-derived, (ii) NBS-derived, (iii) LFS-derived, and (iv) normal diploid fibroblasts (NDFs) (Bristow laboratory, unpublished data). Shaded area represents a range of radiosensitivities that normal diploid fibroblasts may display. 22
39 to radiotherapy or chemotherapy (e.g. increased radiosensitivity of normal tissues in AT patients and increased radioresistance of tumours in LFS patients; and a link between ATM/p53 status of tumours with therapeutic response)(64-66). There are inconsistencies in the literature with respect to this DNA repair capacity and tumour cell survival, potentially due to the use of different end-points for assaying relative radiosensitivity. As such, it is important to review cell death and survival assays that are used to inter-compare cellular radiosensitivity. To compare the relative radiosensitivity of normal and tumour tissues, and their capacity to repair IR-induced DNA damage, one needs to measure the radiosensitivity of clones within an irradiated population as a relative survival after a given IR dose (e.g. the relative survival of clonogens). The clonogenic radiation survival curve quantitates this response where clonogenic surviving fraction is calculated based on the plating efficiency of irradiated cell populations when compared to non-irradiated cell populations and plotted on a logarithmic scale as a function of radiation dose. This curve shows the radiosensitivity of clonogenic cells with unlimited proliferative potential (Figure 1.9). When normal cells are exposed to acute dose rate IR (ADR, ~1Gy/minute) the survival curve of many mammalian cell lines have a similar shape (Figure 1.9, left panel (i)). One can observe an initial shoulder region (i.e. at low doses of 2Gy irradiation which are similar to the daily treatments used in clinical radiotherapy regimens) which represents surviving cells that can repair sub-lethal DNA damage (i.e. SLDR) following doses ranging from 1-4Gy. The higher dose region represents doses that are thought to induce irreparable 23
40 and lethal DNA damage; this results in exponential cell killing. The width of the shoulder region is greater in radioresistant cells which have greater repair capacity. In sensitive cells with decreased repair, this shoulder can be non-existent (e.g. DNAdsb repair deficient cells; Figure 1.9, right panel (AT-derived fibroblasts)) as the cells have little capacity for SLDR. Fractionated IR doses allow a recovery period between each radiation dose that can maximize the process of SLDR (Figure 1.9, left panel (ii)). This can be studied to test the efficacy of clinically relevant doses and treatment schedules in vitro. Previous studies have suggested that MTp53- expressing cells have increased SLDR (67). However, the molecular mechanisms responsible for the concept of SLDR are currently unknown and most likely contributed by various factors including cell cycle checkpoint regulatory and DNAdsb repair processes. In addition to the clonogenic survival assay, other assays have been used to assess cell viability or death. These include viability stains (e.g. propidium iodide and trypan blue exclusion), analysis of apoptosis markers (e.g. by TUNEL, flow cytometry, DNA fragmentation, or morphology), analyses of permanent cell cycle arrest and senescence (e.g. by senescence-associated β-galactosidase staining) and assays of proliferation using metabolic dyes (e.g. the MTT and similar assays). However, these assays may not always correlate with the final long-term clonogenic kill measured by the clonogenic assay, and caution must be taken in over-interpreting these assays when presented without accompanying clonogenic survival data. 24
41 (i) 100% DNA-dsbs (FAR or NTM) DNA-PKcs -/- 10% residual dsbs ATM -/- NDFs 0% Time (hrs) (ii) Surviving fraction after 2 Gy MS571 SiHa SW756 U87 HeLa A549 TK6 HCC1937 WiDr CASKI C33A PC3 M059K 180BR MCF7 NH32 r = 0.66 M059J WIL2NS Residual γh2ax Figure Biochemical assays (i.e. CFGE or Comet) to determine DNA-dsb rejoining kinetics and molecular assays (i.e. γ-h2ax IR-induced foci) to quantitate DNA-dsbs. (i) Kinetics of DNA-dsb rejoining (i.e. FAR or NTM) in normal, ATM-null and DNA-PKcs-null fibroblasts. There is an initial fast component (t 1/2 = mins) followed by a slow component (t 1/2 = 2-3 hrs) of the curve ending with a final residual level of DNA-dsbs. These parameters can be used for comparing DNA-dsb rejoining capacities between cell strains and lines. (ii) The relationship between cellular radiosensitivity and residual DNA-dsbs as determined by plotting surviving fraction versus residual IR-induced γ-h2ax foci at 24 hours post-irradiation (68). 25
42 4.2 Detection of DNA-dsbs: biochemical DNA-dsb rejoining assays In a fibroblast model, residual DNA-dsbs have been shown to be the critical lesion correlated to cellular killing (69, 70). In addition to clonogenic survival assays, DNAdsb rejoining capacity can be assessed using biochemical rejoining assays or quantitating nuclear IR-induced foci at sites of DNA damage. Two examples of biochemical rejoining assays are the COMET assay and Pulsed- or Continuous- Field gel electrophoresis (PFGE or CFGE) assays. Both are designed to quantitate the amount of fragmented and un-fragmented DNA as a function of dose and time following irradiation. The COMET assay is based on analyzing irradiated cells following neutral lysis and electrophoresis to derive a parameter termed the normalized tail moment (NTM: a ratio of fragmented to un-fragmented DNA). In addition, cells in specific cell cycle phases and undergoing apoptosis can also be determined since it is a single-cell assay (71). In contrast, PFGE or CFGE is based on analyzing DNA derived from cell populations and obtaining the fraction of fragmented DNA released (FDR or FAR) into a gel-compression zone following electrophoresis, which represents DNA breaks. Plots of NTM or FAR as a function of time following irradiation can illustrate the DNA-dsb rejoining kinetics of cells with altered DNA rejoining capacities (Figure 1.10(i): comparing the relative kinetics and levels of residual DNA-dsbs between rejoining-proficient NDFs with DNA-dsb rejoining-defective ATM-null and DNA-PKcs-null cells). 26
43 4.3 Detection of DNA-dsbs: measuring DNA-dsbs in situ Advances in microscopic techniques have enabled intracellular interactions between DNA-dsb sensing, signaling and repair proteins to be tracked in situ using live cell or indirect immunofluorescence microscopy (Figure 1.11). These techniques allow for the visualization of discrete nuclear accumulations of proteins (as IR-induced foci) and protein-protein interactions (as co-localized foci) at sites of DNA damage following whole cell or sub-nuclear (e.g. UV microbeam) radiation (44, 72-75). Notably, biochemical and microscopic studies identified the γ-h2ax as a discrete biomarker of megabase domains containing DNA-dsbs (73). Furthermore, residual γ- H2AX foci (i.e. 24 hours post-irradiation) were found to correlate with relative radiation cell survival in vitro and in vivo (Figure 1.10(ii)) a correlation was found between residual DNA-dsbs and cellular radiosensitivity) (68, 76). Hence, γ-h2ax is currently used as the gold standard indicator of sites of DNA-dsbs in vivo (77, 78). 27
44 5.0 The ATM-p53 pathway and DNA-dsbs 5.1 Interactions of ATM, MRN, and Artemis (NHEJ pathway) within chromatin To-date, intracellular interactions between ATM and DNA-dsb sensing and repair proteins have been tracked in situ using immunofluorescence microscopy following whole cell or sub-cellular (e.g. UV microbeam) radiation at sites of DNA damage (44, 57, 72-75, 79). These have also been corroborated by biochemical chromatin immunoprecipitations (ChIPs) in which ATM forms complexes at sites of DNA-dsbs (27, 38, 39). Based on live-cell imaging of fluorescently-tagged proteins, ATM has been observed to phosphorylate its targets (e.g., NBS1) directly at the site of DNA damage. Defects in ATM lead to defects in DNA-dsb repair and increased radiosensitivity (65). While the radiosensitivity was attributed to the cell cycle checkpoint defects, the discovery of another component of the NHEJ pathway, Artemis (mutations which result in radiosensitive-severe combined immunodeficiency (RS-SCID) syndrome in humans) shed light on the ATMdependent repair defect (80). ATM phosphorylates and activates the Artemis nuclease function in response to IR-induced DNA-dsbs, and this ATM/Artemisdependent pathway is required for repairing the remaining DNA-dsbs at late times following irradiation (81, 82). 28
45 UBC13 DNA-dsb RNF8 MDC1 MRN ATM Ser BP1 DNA-PK γ-h2ax RAD51 MUS81 EME1 RPA BLM BRCA1 BRCA2 HR (S, G2) RAD51- B,C,D RAD54, RAD52 XRCC2 XRCC3 NHEJ (All phases) Ku80 Ku70 XLF DNA-PKcs Ligase IV Artemis XRCC4 DNA REPAIR FOCI ASSAYS Single Nuclei Single Nuclei - 3D (to quantitate DNA-dsbs and protein-protein co-localization) Figure Illustration of the pathways involved in DNA-dsb sensing, signaling and repair, and additional methods to quantitate the number and kinetics of DNA-dsbs and interactions of DNA repair proteins in situ using immunofluorescence microscopy (adapted with permission from Bristow, 2008 (63)). Green foci: γ-h2ax; Red foci: 53BP1; Blue: nuclear DNA. 29
46 5.2 Interactions of ATM and CHK2, MDM2 and p21 WAF at the chromatin interface of DNA-dsb sensing and signal transduction While CHK2 has been shown to be a tumour suppressor through its important role in mediating the G1 checkpoint, more recent studies have revealed an additional role in the sensing and amplification and signalling of DNA damage. Using live cell imaging techniques of fluorescence-loss-in-photobleaching (FLIP) and fluorescencerecovery-after-photobleaching (FRAP), Lukas and colleagues showed that dynamic local protein-chromatin interactions occur in which ATM phosphorylates CHK2 at residue Thr68 (CHK2 Thr68 ) at DNA-dsbs sites which rapidly releases CHK2 from the chromatin in order to transduce this signal to its downstream target proteins, including p53 (28). Subsequently, Li and Stern biochemically confirmed that this protein-chromatin interaction was transient and that upon phosphorylation, CHK2 Thr68 CHK2 Thr68 was released from chromatin (83). Studies have also shown that co-localizes with other sensing and repair proteins including γ-h2ax, 53BP1, NBS1 and BRCA1, as well as the mismatch repair factors, MSH2 and MLH1 at replication forks, implicating CHK2 in signaling in the HR pathway of DNA-dsb repair (84). Few data are available for phosphorylated p53 and ATM interactions at chromatin. The p53 protein directly trans-activates the p21 WAF gene in response to IR-induced DNA damage. The p21 WAF protein is an inhibitor of the cyclin E CDK2 complex and RB phosphorylation leading to maintenance of cell cycle arrested at the G1 to S transition. In addition to this role, Taucher-Scholz and colleagues have shown using 30
47 microbeam irradiation techniques that the p21 WAF protein can also localize rapidly at sites of DNA damage and co-localize with the MRE11 and γ-h2ax DNA damage sensing proteins in a p53-independent manner (85-87). This interaction has been suggested to inhibit DNA replication and progression into S phase, and it remains to be elucidated whether there is a direct role in DNA-dsb repair for p21 WAF in the G1 cell cycle phase in association with either ATM, p53, or HR or NHEJ repair proteins. While ATM-mediated p53 phosphorylation and stabilization also leads to transactivation and induction of another of its target genes, MDM2, ATM also phosphorylates MDM2. Both ATM phosphorylation sites on MDM2 abrogate the interaction between p53 and MDM2 (an E3 ubiquitin ligase), thus inhibiting ubiquitination of p53 and its degradation by the 26S proteasome (88, 89). Thus, this autoregulatory negative feedback loop accounts for MDM2 regulation of p53 activity and indirectly regulating DNA-dsb repair through p53-dependent mechanisms. White and colleagues also observed MDM2 regulation of p53 transactivation, thus indirectly regulating DNA-dsb repair (90). MDM2 is required for the association of p53 to chromatin and this chromatin-associated pool of MDM2-p53 was found at p53-specific transcriptional promoters (90). Upon induction of DNA damage, MDM2 was released to facilitate p53-transactivation. Evidence that MDM2 is more directly involved in DNA-dsb rejoining, in a p53-independent manner, was shown by Alt and colleagues, whereby MDM2 was found to interact with NBS1 and overexpression of MDM2 led to decreased DNA-dsb rejoining (33). How this MDM2-NBS1 complex 31
48 functions within the chromatin and directly in DNA-dsb repair remains to be elucidated. 5.3 Interactions of ATM, ATR and CHK1 In parallel with the ATM-CHK2 pathway, the ATR-CHK1 kinase pathway has also recently been shown to play a secondary role in DNA damage sensing and responses within the G1 phase of the cell cycle. While two groups showed ATR localization to sites of DNA damage (91, 92), Smits and colleagues showed the release of CHK1 from chromatin sites of DNA damage (93), similar to the release of CHK2 from chromatin (83). Following IR-induced DNA damage, both ATR and CHK1 activation are dependent on the activation of ATM and the MRN complex (38, 41). These studies emphasize the essential dynamic nature of these interactions to coordinate and amplify DNA damage sensing, signaling and cell cycle checkpoint enactment to maintain genomic stability. 6.0 p53 and the repair of DNA-dsbs The involvement of p53 in multiple DNA repair pathways is based on experimental data in which p53 binds at sites of DNA damage or affects the fidelity of DNA repair. p53 can bind to stalled replication forks during DNA replication and affect the capacity and fidelity of base excision repair (BER), nucleotide excision repair (NER), NHEJ and HR (94-98). 32
49 Initial studies suggesting a role for p53 in DNA-dsb repair include biochemical data in which p53 was shown to possess nuclease activity (DNA-processing) and DNAbinding ability to several types of DNA conformations (e.g. ds/ssdna (sequenceand non-sequence-specific), naked DNA as well as the chromatin fraction within nuclei), as well as various types of DNA lesions (e.g. DNA-dsbs, DNA-ssbs, irradiation-damaged DNA) (99-101). These observations complemented those obtained from in vivo mouse models, whereby loss of p53 led to increased HR rates, decreased DNA-dsb repair fidelity, genetic instability, and carcinogenesis, and resistance to ionizing radiation-induced DNA-damage (16, 99, 102, 103). The p53 protein was recently shown to be a co-factor in NHEJ or HR based on DNA-dsb repair assays in vitro and in vivo (6, 96, 99, ). The impact of post-translational modifications of p53 in DNA-dsb repair has also been explored. For example, it was found that WTp53 reduces error-prone NHEJ DNA-dsb repair (based on inter-chromosomal reporter substrates) in MEF cells and may protect against carcinogenesis (96). Unlike ATM-null mice, p53 Ser18Ala/Ser18Ala mice do not have increased rates of spontaneous tumorigenesis. And compared to ATM-null MEFs, the p53 Ser18Ala/Ser18Ala MEFs are not radiosensitive (108), which suggests that Ser15 phosphorylation and/or a deficient G1 checkpoint are not major factors of cell survival following DNA damage (99). However, what has been observed is that in combination with certain hot spot mutations of the p53 protein, phospho-site mutants do have increased rates of mutagen-induced tumorigenesis (16, 95, 109). Song and colleagues also found that common p53 cancer mutations 33
50 (e.g. R248W and R273H) resulted in gain-of-function MTp53 proteins that promoted tumorigenesis by binding to MRE11 and impairing ATM activation (110, 111). What remains to be determined is whether p53, specifically the post-translationally modified forms of p53, may be involved directly in DNA-dsb sensing, as well as DNA-dsb repair, within damaged chromatin in vivo. Two recent studies have also shown that p53 can downregulate the transcription of the RAD51 gene (112, 113). This raises the questions of the mechanisms resulting in the possible p53-dependent downregulation of RAD51 expression and related HR during the G1 phase, and a p53-dependent increased RAD51 expression observed in a variety of tumours due to the effects of polymorphisms in the RAD51 promoter or MTp53 expression. Could WTp53 have a decreased recognition of the polymorphic promoters? Or could MTp53 lacking its trans-activation activity and checkpoint function also have reduced recognition of the RAD51 promoter, as was shown by Arias-Lopez and colleagues (112, 113)? Together, both the p53-mediated transcription-independent and -dependent regulation of RAD51 and HR repair may cooperate to maintain genomic stability, and whether these mechanisms are operational during the G1 phase of the cell cycle and interact with the NHEJ repair pathway (as observed from the co-localizations of various HR and NHEJ repair proteins in G1 phase cells) remains to be determined (57). 34
51 7.0 Implications of p53 in carcinogenesis and cancer therapy A testament to its central role in maintaining genomic stability and preventing carcinogenesis, is that somatic loss or mutation of the WTp53 gene is observed in more than 50% of tumours. Futhermore, germline p53 mutations results in the cancer-prone Li-Fraumeni syndrome (LFS). MTp53 protein may interact with WTp53 or act independently resulting in either a loss-of-function, dominant negative or gainof-function phenotype leading to tumourigenesis through defective cell cycle control and possibly, defective DNA-dsb sensing and repair. Indeed, there has been a correlation between increased levels of ATM, p53 and γ-h2ax phosphorylation and activation with increased levels of genetic instability and later stages within the carcinogenesis process (114, 115). Fibroblasts that express MTp53 proteins (e.g. Li-Fraumeni Syndrome (LFS) fibroblasts) or that lack p53 function (p53 -/- fibroblasts) can acquire increased SLDR and survival (6). In contrast, radiosensitive ATM-deficient fibroblasts show little evidence of SLDR (116). This may be explained in part by a direct defect in the quantitative repair of DNA-dsbs in AT cells which lack end-joining activity compared to an indirect defect in the fidelity of DNA-dsbs due to G1-checkpoint deficiency in p53-defective cells (65, 96). Observations of G1-checkpoint deficient LFS cells or tumor cells lacking p53 or RBdeficient fibroblasts all with increased levels of DNA-dsbs exhibit a discordance between survival and DNA repair ( ). This reflects the complexity between 35
52 direct and indirect effects of G1 checkpoint-deficiency and the fact that in cells with MTp53, high levels of DNA-dsbs can be tolerated without activating cell death as a driving force for genetic instability (6). 8.0 Thesis Outline While previous indirect studies have suggested a role for p53 in regulating the frequency of HR and fidelity of NHEJ, none have specifically and quantitatively analyzed whether the p53 protein can bind to sites of DNA breaks in vivo. The central, sequence-specific domain has been well characterized as essential for p53 binding at its target trans-activated genes consensus sequences. The basic, carboxy-terminal domain has been suggested to mediate its DNA damage sensing functions, but there are few in vivo data to support this hypothesis. This thesis focuses at determining whether the p53 protein directly senses and binds to sites of DNA-dsbs in vivo, and whether the carboxy-terminus domain of the p53 protein mediates this function (as outlined in Figure 1.12). The overall global hypothesis of this thesis is that the p53 protein can directly bind to sites of DNA damage based on altered phosphorylation states. In Chapter 2, I present experimental data which addresses whether phosphorylated p53 form nuclear foci following IR-induced DNA damage and sense DNA-dsbs in vivo? I present data using immunofluorescence (IF) confocal microscopy and various p53-specific antibodies to determine the localization patterns and foci formation of endogenous total and phospho-forms of p53. Dose response and 36
53 kinetics of focal accumulation were determined and compared with those of γ-h2ax and biochemical DNA-dsb rejoining assays. As IR causes various types of lesions within LMDS, I also tested p53 foci formation following different types of DNA damaging agents were used to induce specific types of lesions (e.g. bleomycin, hydrogen peroxide, mitomycin C, methyl methane sulfonate, UV irradiation). Both IF confocal microscopy and co-ip analyses were used to determine whether the IRinduced foci of p53 co-localized with those of γ-h2ax, 53BP1, ATM, DNA-PKcs, RAD50, or MRE11, in vivo. In Chapter 3, I designed further experiments to test whether the p53 carboxyterminus domain is responsible for localization of p53 to sites of DNA breaks in vivo. Fusion proteins of YFP with full length WTp53, point mutants, and carboxy-terminal deletion mutants were transiently transfected into null p53 cells to determine the importance of different phospho-specific sites as well as carboxy-terminus domains in foci formation and interaction with DNA-dsb sensing, signaling and repair proteins. Co-immunoprecipitation analyses of cytoplasmic, soluble nuclear and insoluble chromatin-bound fractions were used to study the binding and interactions of the aforementioned YFP-p53 fusion proteins and endogenous WTp53 protein with ATM and 53BP1. Finally, in Chapter 4, I discuss the implications of my data in context of the world literature and discuss some future work integrating the p53 protein into DNA damage sensing networks. 37
54 Hypothesis: The p53 protein can directly bind to sites of DNA damage based on altered phosphorylation Chapter 2: (i) Determine if IR induce foci by p53 phospho-forms? (ii) Determine what DNA lesions induce p53 IRIF? (iii) Determine if p53 foci colocalize with other DNA-dsb sensing or repair proteins? Chapter 3: (i) Determine if the p53-nsdbd is responsible for sensing DNA-dsbs? (ii) Determine if p53 is required for ATM and 53BP1 chromatin-binding? Chapter 4: Implications in context of p53 in DNA-dsb sensing and repair and radiotherapy/chemotherapy Figure Thesis hypothesis and outline. 38
55 References 1. R. G. Bristow, H. Ozcelik, F. Jalali, N. Chan and D. Vesprini, Homologous recombination and prostate cancer: a model for novel DNA repair targets and therapies. Radiother Oncol 83, (2007). 2. A. Choudhury, A. Cuddihy and R. G. Bristow, Radiation and new molecular agents part I: targeting ATM-ATR checkpoints, DNA repair, and the proteasome. Semin Radiat Oncol 16, (2006). 3. T. Helleday, J. Lo, D. C. van Gent and B. P. Engelward, DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair (Amst) 6, (2007). 4. G. M. Wahl, S. P. Linke, T. G. Paulson and L. C. Huang, Maintaining genetic stability through TP53 mediated checkpoint control. Cancer Surv 29, (1997). 5. J. M. Pollard and R. A. Gatti, Clinical radiation sensitivity with DNA repair disorders: an overview. Int. J. Radiat. Oncol. Biol. Phys. 74, (2009). 6. A. R. Cuddihy and R. G. Bristow, The p53 protein family and radiation sensitivity: Yes or no? Cancer Metastasis Rev 23, (2004). 7. D. Menendez, A. Inga and M. A. Resnick, The expanding universe of p53 targets. Nat Rev Cancer 9, (2009). 8. S. Alsafadi, S. Tourpin, F. Andre, G. Vassal and J. C. Ahomadegbe, P53 Family: At the Crossroads in Cancer Therapy. Curr Med Chem (2009). 9. M. Wells, H. Tidow, T. J. Rutherford, P. Markwick, M. R. Jensen, E. Mylonas, D. I. Svergun, M. Blackledge and A. R. Fersht, Structure of tumor suppressor p53 and its intrinsically disordered N-terminal transactivation domain. Proc. Natl. Acad. Sci. USA 105, (2008). 10. K. A. Malecka, W. C. Ho and R. Marmorstein, Crystal structure of a p53 core tetramer bound to DNA. Oncogene 28, (2009). 11. A. A. Mills, p53: link to the past, bridge to the future. Genes Dev. 19, (2005). 12. J. C. Bourdon, K. Fernandes, F. Murray-Zmijewski, G. Liu, A. Diot, D. P. Xirodimas, M. K. Saville and D. P. Lane, p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 19, (2005). 13. V. Marcel and P. Hainaut, p53 isoforms - a conspiracy to kidnap p53 tumor suppressor activity? Cell Mol Life Sci 66, (2009). 14. G. Rohaly, J. Chemnitz, S. Dehde, A. M. Nunez, J. Heukeshoven, W. Deppert and I. Dornreiter, A novel human p53 isoform is an essential element of the ATRintra-S phase checkpoint. Cell 122, (2005). 15. K. Fujita, A. M. Mondal, I. Horikawa, G. H. Nguyen, K. Kumamoto, J. J. Sohn, E. D. Bowman, E. A. Mathe, A. J. Schetter, et al., p53 isoforms Delta133p53 and p53beta are endogenous regulators of replicative cellular senescence. Nat. Cell Biol. 11, (2009). 16. R. Brosh and V. Rotter, When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer 9, (2009). 17. C. Blattner, Regulation of p53: the next generation. Cell Cycle 7, (2008). 39
56 18. J. P. Kruse and W. Gu, Modes of p53 regulation. Cell 137, (2009). 19. A. J. Levine, W. Hu and Z. Feng, The P53 pathway: what questions remain to be explored? Cell Death Differ 13, (2006). 20. S. Saito, H. Yamaguchi, Y. Higashimoto, C. Chao, Y. Xu, A. J. Fornace, Jr., E. Appella and C. W. Anderson, Phosphorylation site interdependence of human p53 post-translational modifications in response to stress. J. Biol. Chem. 278, (2003). 21. A. Sancar, L. A. Lindsey-Boltz, K. Unsal-Kaccmaz and S. Linn, Molecular Mechanisms of Mammalian DNA Repair and the DNA Damage Checkpoints. Annu Rev Biochem 73, (2004). 22. J. Falck, J. Coates and S. P. Jackson, Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 434, (2005). 23. Y. C. Kim, G. Gerlitz, T. Furusawa, F. Catez, A. Nussenzweig, K. S. Oh, K. H. Kraemer, Y. Shiloh and M. Bustin, Activation of ATM depends on chromatin interactions occurring before induction of DNA damage. Nat. Cell Biol. 11, (2009). 24. C. J. Bakkenist and M. B. Kastan, DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, (2003). 25. Y. Shiloh, ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3, (2003). 26. E. Berkovich, R. J. Monnat, Jr. and M. B. Kastan, Roles of ATM and NBS1 in chromatin structure modulation and DNA double-strand break repair. Nat. Cell Biol. 9, (2007). 27. Y. Andegeko, L. Moyal, L. Mittelman, I. Tsarfaty, Y. Shiloh and G. Rotman, Nuclear retention of atm at sites of dna double strand breaks. J. Biol. Chem. 276, (2001). 28. C. Lukas, J. Falck, J. Bartkova, J. Bartek and J. Lukas, Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat. Cell Biol. 5, (2003). 29. M. Gatei, D. Young, K. M. Cerosaletti, A. Desai-Mehta, K. Spring, S. Kozlov, M. F. Lavin, R. A. Gatti, P. Concannon and K. Khanna, ATM-dependent phosphorylation of nibrin in response to radiation exposure. Nat. Genet. 25, (2000). 30. X. Wu, V. Ranganathan, D. S. Weisman, W. F. Heine, D. N. Ciccone, T. B. O'Neill, K. E. Crick, K. A. Pierce, W. S. Lane, et al., ATM phosphorylation of Nijmegen breakage syndrome protein is required in a DNA damage response. Nature 405, (2000). 31. T. Stiff, M. O'Driscoll, N. Rief, K. Iwabuchi, M. Lobrich and P. A. Jeggo, ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 64, (2004). 32. M. T. Jack, R. A. Woo, N. Motoyama, H. Takai and P. W. Lee, DNAdependent protein kinase and checkpoint kinase 2 synergistically activate a latent population of p53 upon DNA damage. J. Biol. Chem. 279, (2004). 40
57 33. J. R. Alt, A. Bouska, M. R. Fernandez, R. L. Cerny, H. Xiao and C. M. Eischen, Mdm2 binds to Nbs1 at sites of DNA damage and regulates double strand break repair. J. Biol. Chem. 280, (2005). 34. J. Kang, D. Ferguson, H. Song, C. Bassing, M. Eckersdorff, F. W. Alt and Y. Xu, Functional interaction of H2AX, NBS1, and p53 in ATM-dependent DNA damage responses and tumor suppression. Mol. Cell. Biol. 25, (2005). 35. K. Rothkamm, I. Kruger, L. H. Thompson and M. Lobrich, Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol. Cell. Biol. 23, (2003). 36. J. Bartek and J. Lukas, Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol 13, (2001). 37. L. Li and L. Zou, Sensing, signaling, and responding to DNA damage: organization of the checkpoint pathways in mammalian cells. J Cell Biochem 94, (2005). 38. N. Rhind, Changing of the guard: how ATM hands off DNA double-strand break signaling to ATR. Mol. Cell 33, (2009). 39. B. Shiotani and L. Zou, Single-stranded DNA orchestrates an ATM-to-ATR switch at DNA breaks. Mol. Cell 33, (2009). 40. L. Geng, X. Zhang, S. Zheng and R. J. Legerski, Artemis links ATM to G2/M checkpoint recovery via regulation of Cdk1-cyclin B. Mol. Cell. Biol. 27, (2007). 41. A. Jazayeri, J. Falck, C. Lukas, J. Bartek, G. C. Smith, J. Lukas and S. P. Jackson, ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 8, (2006). 42. J. S. Myers and D. Cortez, Rapid activation of ATR by ionizing radiation requires ATM and Mre11. J. Biol. Chem. 281, (2006). 43. M. Lisby and R. Rothstein, Localization of checkpoint and repair proteins in eukaryotes. Biochimie 87, (2005). 44. S. Bekker-Jensen, C. Lukas, R. Kitagawa, F. Melander, M. B. Kastan, J. Bartek and J. Lukas, Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. J. Cell. Biol. 173, (2006). 45. K. P. Hopfner, DNA double-strand breaks come into focus. Cell 139, (2009). 46. K. Iijima, M. Ohara, R. Seki and H. Tauchi, Dancing on damaged chromatin: functions of ATM and the RAD50/MRE11/NBS1 complex in cellular responses to DNA damage. J Radiat Res (Tokyo) 49, (2008). 47. A. Dupre, L. Boyer-Chatenet and J. Gautier, Two-step activation of ATM by DNA and the Mre11-Rad50-Nbs1 complex. Nat Struct Mol Biol 13, (2006). 48. N. Tomimatsu, C. G. Tahimic, A. Otsuki, S. Burma, A. Fukuhara, K. Sato, G. Shiota, M. Oshimura, D. J. Chen and A. Kurimasa, Ku70/80 modulates ATM and ATR signaling pathways in response to DNA double strand breaks. J. Biol. Chem. 282, (2007). 49. B. P. Chen, N. Uematsu, J. Kobayashi, Y. Lerenthal, A. Krempler, H. Yajima, M. Lobrich, Y. Shiloh and D. J. Chen, Ataxia telangiectasia mutated (ATM) is essential for DNA-PKcs phosphorylations at the Thr-2609 cluster upon DNA double strand break. J. Biol. Chem. 282, (2007). 41
58 50. Y. Peng, R. G. Woods, H. Beamish, R. Ye, S. P. Lees-Miller, M. F. Lavin and J. S. Bedford, Deficiency in the catalytic subunit of DNA-dependent protein kinase causes down-regulation of ATM. Cancer Res. 65, (2005). 51. J. Mi, J. Dziegielewski, E. Bolesta, D. L. Brautigan and J. M. Larner, Activation of DNA-PK by ionizing radiation is mediated by protein phosphatase 6. PLoS One 4, e4395 (2009). 52. A. Ali, J. Zhang, S. Bao, I. Liu, D. Otterness, N. M. Dean, R. T. Abraham and X. F. Wang, Requirement of protein phosphatase 5 in DNA-damage-induced ATM activation. Genes Dev. 18, (2004). 53. A. A. Goodarzi, J. C. Jonnalagadda, P. Douglas, D. Young, R. Ye, G. B. Moorhead, S. P. Lees-Miller and K. K. Khanna, Autophosphorylation of ataxiatelangiectasia mutated is regulated by protein phosphatase 2A. Embo J. 23, (2004). 54. T. Wechsler, B. P. Chen, R. Harper, K. Morotomi-Yano, B. C. Huang, K. Meek, J. E. Cleaver, D. J. Chen and M. Wabl, DNA-PKcs function regulated specifically by protein phosphatase 5. Proc. Natl. Acad. Sci. USA 101, (2004). 55. B. Pardo, B. Gomez-Gonzalez and A. Aguilera, DNA repair in mammalian cells: DNA double-strand break repair: how to fix a broken relationship. Cell Mol Life Sci 66, (2009). 56. J. M. Hinz, N. A. Yamada, E. P. Salazar, R. S. Tebbs and L. H. Thompson, Influence of double-strand-break repair pathways on radiosensitivity throughout the cell cycle in CHO cells. DNA Repair (Amst) 4, (2005). 57. J. S. Kim, T. B. Krasieva, H. Kurumizaka, D. J. Chen, A. M. Taylor and K. Yokomori, Independent and sequential recruitment of NHEJ and HR factors to DNA damage sites in mammalian cells. J. Cell. Biol. 170, (2005). 58. R. Hill and R. Bristow, The Scientific Basis of Radiotherapy. In The Basic Science of Oncology (I. Tannock, R. Hill, R. Bristow and L. Harrington, Eds.), pp McGraw-Hill Ltd, New York, R. Bristow and R. Hill, Molecular and Cellular Basis of Radiotherapy. In The Basic Science of Oncology (I. Tannock, R. Hill, R. Bristow and L. Harrington, Eds.), pp McGraw-Hill Ltd, New York, S. K. Liu, P. L. Olive and R. G. Bristow, Biomarkers for DNA DSB inhibitors and radiotherapy clinical trials. Cancer Metastasis Rev 27, (2008). 61. D. Klokov, S. M. MacPhail, J. P. Banath, J. P. Byrne and P. L. Olive, Phosphorylated histone H2AX in relation to cell survival in tumor cells and xenografts exposed to single and fractionated doses of X-rays. Radiother Oncol 80, (2006). 62. C. E. Rube, S. Grudzenski, M. Kuhne, X. Dong, N. Rief, M. Lobrich and C. Rube, DNA double-strand break repair of blood lymphocytes and normal tissues analysed in a preclinical mouse model: implications for radiosensitivity testing. Clin Cancer Res 14, (2008). 63. R. G. Bristow and R. P. Hill, Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 8, (2008). 64. H. Jiang, H. C. Reinhardt, J. Bartkova, J. Tommiska, C. Blomqvist, H. Nevanlinna, J. Bartek, M. B. Yaffe and M. T. Hemann, The combined status of ATM 42
59 and p53 link tumor development with therapeutic response. Genes Dev. 23, (2009). 65. M. Kuhne, E. Riballo, N. Rief, K. Rothkamm, P. A. Jeggo and M. Lobrich, A double-strand break repair defect in ATM-deficient cells contributes to radiosensitivity. Cancer Res. 64, (2004). 66. R. Mirzayans, R. A. Aubin, W. Bosnich, W. A. Blattner and M. C. Paterson, Abnormal pattern of post-gamma-ray DNA replication in radioresistant fibroblast strains from affected members of a cancer-prone family with Li-Fraumeni syndrome. Br. J. Cancer 71, (1995). 67. R. G. Bristow, Q. Hu, A. Jang, S. Chung, J. Peacock, S. Benchimol and R. Hill, Radioresistant MTp53-expressing rat embryo cell transformants exhibit increased DNA-dsb rejoining during exposure to ionizing radiation. Oncogene 16, (1998). 68. P. L. Olive and J. P. Banath, Phosphorylation of histone H2AX as a measure of radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 58, (2004). 69. E. Dikomey, I. Brammer, J. Johansen, S. M. Bentzen and J. Overgaard, Relationship between DNA double-strand breaks, cell killing, and fibrosis studied in confluent skin fibroblasts derived from breast cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 46, (2000). 70. E. Dikomey and I. Brammer, Relationship between cellular radiosensitivity and non-repaired double-strand breaks studied for different growth states, dose rates and plating conditions in a normal human fibroblast line. Int. J. Radiat. Biol. 76, (2000). 71. T. S. Kumaravel, B. Vilhar, S. P. Faux and A. N. Jha, Comet Assay measurements: a perspective. Cell Biol Toxicol 25, (2009). 72. X. Kong, S. K. Mohanty, J. Stephens, J. T. Heale, V. Gomez-Godinez, L. Z. Shi, J. S. Kim, K. Yokomori and M. W. Berns, Comparative analysis of different laser systems to study cellular responses to DNA damage in mammalian cells. Nucleic Acids Res. 37, e68 (2009). 73. O. Fernandez-Capetillo, A. Lee, M. Nussenzweig and A. Nussenzweig, H2AX: the histone guardian of the genome. DNA Repair (Amst) 3, (2004). 74. J. S. Kim, T. B. Krasieva, V. LaMorte, A. M. Taylor and K. Yokomori, Specific recruitment of human cohesin to laser-induced DNA damage. J. Biol. Chem. 277, (2002). 75. B. E. Nelms, R. S. Maser, J. F. MacKay, M. G. Lagally and J. H. Petrini, In situ visualization of DNA double-strand break repair in human fibroblasts. Science 280, (1998). 76. N. Taneja, M. Davis, J. S. Choy, M. A. Beckett, R. Singh, S. J. Kron and R. R. Weichselbaum, Histone H2AX phosphorylation as a predictor of radiosensitivity and target for radiotherapy. J. Biol. Chem. 279, (2004). 77. J. S. Dickey, C. E. Redon, A. J. Nakamura, B. J. Baird, O. A. Sedelnikova and W. M. Bonner, H2AX: functional roles and potential applications. Chromosoma (2009). 78. Y. Liu, K. Zhou, H. Zhang, Y. Y. Shugart, L. Chen, Z. Xu, Y. Zhong, H. Liu, L. Jin, et al., Polymorphisms of LIG4 and XRCC4 involved in the NHEJ pathway interact to modify risk of glioma. Hum Mutat 29, (2008). 43
60 79. T. A. Mochan, M. Venere, R. A. DiTullio, Jr. and T. D. Halazonetis, 53BP1, an activator of ATM in response to DNA damage. DNA Repair (Amst) 3, (2004). 80. E. Riballo, M. Kuhne, N. Rief, A. Doherty, G. C. Smith, M. J. Recio, C. Reis, K. Dahm, A. Fricke, et al., A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-h2ax foci. Mol. Cell 16, (2004). 81. M. Lobrich and P. A. Jeggo, The two edges of the ATM sword: Co-operation between repair and checkpoint functions. Radiother Oncol 76, (2005). 82. M. Lobrich and P. A. Jeggo, Harmonising the response to DSBs: a new string in the ATM bow. DNA Repair (Amst) 4, (2005). 83. J. Li and D. F. Stern, DNA damage regulates Chk2 association with chromatin. J. Biol. Chem. 280, (2005). 84. J. Ahn, M. Urist and C. Prives, The Chk2 protein kinase. DNA Repair (Amst) 3, (2004). 85. B. Jakob, M. Scholz and G. Taucher-Scholz, Characterization of CDKN1A (p21) binding to sites of heavy-ion-induced damage: colocalization with proteins involved in DNA repair. Int. J. Radiat. Biol. 78, (2002). 86. M. Scholz, B. Jakob and G. Taucher-Scholz, Direct evidence for the spatial correlation between individual particle traversals and localized CDKN1A (p21) response induced by high-let radiation. Radiat. Res. 156, (2001). 87. B. Jakob, M. Scholz and G. Taucher-Scholz, Immediate localized CDKN1A (p21) radiation response after damage produced by heavy-ion tracks. Radiat. Res. 154, (2000). 88. R. Khosravi, R. Maya, T. Gottlieb, M. Oren, Y. Shiloh and D. Shkedy, Rapid ATM-dependent phosphorylation of MDM2 precedes p53 accumulation in response to DNA damage. Proc. Natl. Acad. Sci. USA 96, (1999). 89. R. Maya, M. Balass, S. T. Kim, D. Shkedy, J. F. Leal, O. Shifman, M. Moas, T. Buschmann, Z. Ronai, et al., ATM-dependent phosphorylation of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev. 15, (2001). 90. D. E. White, K. E. Talbott, N. C. Arva and J. Bargonetti, Mouse double minute 2 associates with chromatin in the presence of p53 and is released to facilitate activation of transcription. Cancer Res. 66, (2006). 91. K. E. Adams, A. L. Medhurst, D. A. Dart and N. D. Lakin, Recruitment of ATR to sites of ionising radiation-induced DNA damage requires ATM and components of the MRN protein complex. Oncogene 25, (2006). 92. M. Cuadrado, B. Martinez-Pastor, M. Murga, L. I. Toledo, P. Gutierrez- Martinez, E. Lopez and O. Fernandez-Capetillo, ATM regulates ATR chromatin loading in response to DNA double-strand breaks. J Exp Med 203, (2006). 93. V. A. Smits, P. M. Reaper and S. P. Jackson, Rapid PIKK-dependent release of Chk1 from chromatin promotes the DNA-damage checkpoint response. Curr. Biol. 16, (2006). 94. Y. C. Chang, C. B. Liao, P. Y. Hsieh, M. L. Liou and Y. C. Liu, Expression of tumor suppressor p53 facilitates DNA repair but not UV-induced G2/M arrest or apoptosis in Chinese hamster ovary CHO-K1 cells. J Cell Biochem 103, (2008). 44
61 95. A. Restle, M. Farber, C. Baumann, M. Bohringer, K. H. Scheidtmann, C. Muller-Tidow and L. Wiesmuller, Dissecting the role of p53 phosphorylation in homologous recombination provides new clues for gain-of-function mutants. Nucleic Acids Res. 36, (2008). 96. J. Dahm-Daphi, P. Hubbe, F. Horvath, R. A. El-Awady, K. E. Bouffard, S. N. Powell and H. Willers, Nonhomologous end-joining of site-specific but not of radiation-induced DNA double-strand breaks is reduced in the presence of wild-type p53. Oncogene 24, (2005). 97. Y. Lin, B. C. Waldman and A. S. Waldman, Suppression of high-fidelity double-strand break repair in mammalian chromosomes by pifithrin-alpha, a chemical inhibitor of p53. DNA Repair (Amst) 2, 1-11 (2003). 98. Y. R. Seo, M. L. Fishel, S. Amundson, M. R. Kelley and M. L. Smith, Implication of p53 in base excision DNA repair: in vivo evidence. Oncogene 21, (2002). 99. S. Sengupta and C. C. Harris, p53: traffic cop at the crossroads of DNA repair and recombination. Nat. Rev. Mol. Cell. Biol. 6, (2005) A. L. Okorokov, L. Warnock and J. Milner, Effect of wild-type, S15D and R175H p53 proteins on DNA end joining in vitro: potential mechanism of DNA double-strand break repair modulation. Carcinogenesis 23, (2002) C. P. Rubbi and J. Milner, p53--guardian of a genome's guardian? Cell Cycle 2, (2003) D. W. Meek, Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer 9, (2009) S. Rooney, J. Sekiguchi, S. Whitlow, M. Eckersdorff, J. P. Manis, C. Lee, D. O. Ferguson and F. W. Alt, Artemis and p53 cooperate to suppress oncogenic N- myc amplification in progenitor B cells. Proc. Natl. Acad. Sci. USA 101, (2004) M. Keimling and L. Wiesmuller, DNA double-strand break repair activities in mammary epithelial cells--influence of endogenous p53 variants. Carcinogenesis 30, (2009) S. A. Gatz and L. Wiesmuller, p53 in recombination and repair. Cell Death Differ 13, (2006) A. Restle, C. Janz and L. Wiesmuller, Differences in the association of p53 phosphorylated on serine 15 and key enzymes of homologous recombination. Oncogene 24, (2005) K. H. Shin, J. H. Ahn, M. K. Kang, P. K. Lim, F. K. Yip, M. A. Baluda and N. H. Park, HPV-16 E6 oncoprotein impairs the fidelity of DNA end-joining via p53- dependent and -independent pathways. Int J Oncol 28, (2006) C. Chao, M. Hergenhahn, M. D. Kaeser, Z. Wu, S. Saito, R. Iggo, M. Hollstein, E. Appella and Y. Xu, Cell type- and promoter-specific roles of Ser18 phosphorylation in regulating p53 responses. J. Biol. Chem. 278, (2003) D. B. Yap, J. K. Hsieh, S. Zhong, V. Heath, B. Gusterson, T. Crook and X. Lu, Ser392 Phosphorylation Regulates the Oncogenic Function of Mutant p53. Cancer Res. 64, (2004). 45
62 110. M. B. Kastan and E. Berkovich, p53: a two-faced cancer gene. Nat. Cell Biol. 9, (2007) H. Song, M. Hollstein and Y. Xu, p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nat. Cell Biol. 9, (2007) L. Hasselbach, S. Haase, D. Fischer, H. C. Kolberg and H. W. Sturzbecher, Characterisation of the promoter region of the human DNA-repair gene Rad51. Eur J Gynaecol Oncol 26, (2005) C. Arias-Lopez, I. Lazaro-Trueba, P. Kerr, C. J. Lord, T. Dexter, M. Iravani, A. Ashworth and A. Silva, p53 modulates homologous recombination by transcriptional regulation of the RAD51 gene. EMBO Rep 7, (2006) J. Bartkova, C. J. Bakkenist, E. Rajpert-De Meyts, N. E. Skakkebaek, M. Sehested, J. Lukas, M. B. Kastan and J. Bartek, ATM Activation in Normal Human Tissues and Testicular Cancer. Cell Cycle 4 (2005) J. Bartkova, Z. Horejsi, K. Koed, A. Kramer, F. Tort, K. Zieger, P. Guldberg, M. Sehested, J. M. Nesland, et al., DNA damage response as a candidate anticancer barrier in early human tumorigenesis. Nature 434, (2005) J. R. Williams, Y. Zhang, J. Russell, C. Koch and J. B. Little, Human tumor cells segregate into radiosensitivity groups that associate with ATM and TP53 status. Acta Oncol 46, (2007) J. L. Schwartz, R. Jordan, W. K. Kaufmann, J. Rasey, K. J. Russell and R. R. Weichselbaum, Evidence for the expression of radiation-induced potentially lethal damage being a p53-dependent process. Int. J. Radiat. Biol. 76, (2000) J. L. Schwartz, J. Rasey, L. Wiens, R. Jordan and K. J. Russell, Functional inactivation of p53 by HPV-E6 transformation is associated with a reduced expression of radiation-induced potentially lethal damage. Int. J. Radiat. Biol. 75, (1999) R. R. Weichselbaum, K. Tomkinson and J. B. Little, Repair of potentially lethal X-ray damage in fibroblasts derived from patients with hereditary and D-deletion retinoblastoma. Int J Radiat Biol Relat Stud Phys Chem Med 47, (1985). 46
63 Chapter 2 Evidence for the Direct Binding of Phosphorylated p53 to Sites of DNA Breaks In Vivo This chapter consists of the published work, Evidence for the direct binding of phosphorylated p53 to sites of DNA breaks in vivo in the journal, Cancer Research, 65, , by Shahnaz T Al Rashid, 1,2 Graham Dellaire, 3 Andrew Cuddihy, 1 Farid Jalali, 1 Mita Vaid, 4 Carla Coackley, 1 Melvyn Folkard, 4 Yang Xu, 5 Benjamin PC Chen, 6 David J Chen, 6 Lothar Lilge, 1,2 Kevin M Prise, 4 David P Bazett-Jones 3 and Robert G Bristow 1,2,7,8* 1 Ontario Cancer Institute-Princess Margaret Hospital (University Health Network), Toronto, Ontario, Canada M5G 2M9, 2 Department of Medical Biophysics, University of Toronto, Toronto, Ontario, Canada, 3 Programme in Cell Biology, The Hospital for Sick Children, Toronto, Canada, 4 Gray Cancer Institute, Mount Vernon Hospital, Northwood, Middlesex, UK HA6 2JR, 5 Division of Biological Sciences, University of California, San Diego, La Jolla, California , USA, 6 Department of Cell and Molecular Biology, Life Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA, 7 Department of Radiation Oncology, University of Toronto, Toronto, Ontario, Canada 47
64 2.1 Abstract Despite a clear link between ATM-dependent phosphorylation of p53 and cell cycle checkpoint control, the intracellular biology and sub-cellular localization of p53 phosphoforms during the initial sensing of DNA damage is poorly understood. Using G0/G1 confluent primary human diploid fibroblast cultures, we show that endogenous p53, phosphorylated at serine 15 (p53 Ser15 ), accumulates as discrete, dose-dependent and chromatin-bound foci within 30 minutes following induction of DNA breaks or DNA base damage. This biologically distinct sub-pool of p53 Ser15 is ATM-dependent and resistant to 26S-proteasomal degradation. p53 Ser15 co-localizes and co-immunoprecipitates with γ-h2ax with kinetics similar to that of biochemical DNA-dsb rejoining. Sub-nuclear microbeam irradiation studies confirm p53 Ser15 is recruited to sites of DNA damage containing γ-h2ax, ATM Ser1981 and DNA- PKcs Thr2609 in vivo. Furthermore, studies using isogenic human and murine cells, which express Ser15 or Ser18 phosphomutant proteins, respectively, show defective nuclear foci formation, decreased induction of p21 WAF, decreased γ-h2axassociation and altered DNA-dsb kinetics following DNA damage. Our results suggest a unique biology for this p53 phosphoform in the initial steps of DNA damage signaling and implicates ATM-p53 chromatin-based interactions as mediators of cell cycle checkpoint control and DNA repair. 48
65 2.2 Introduction The cellular stress response to DNA damage requires strict co-ordination between cell cycle checkpoint control and DNA repair. In response to DNA double-strand breaks, the ATM, DNA-PKcs and hsmg-1 kinases, all members of the phosphatidylinositol 3-kinase related kinase (PI3KK) family, have been shown to redundantly phosphorylate substrates including the DNA-dsb sensor histone protein, γ-h2ax, and the p53 tumor suppressor protein (1-4). The presence of DNA-dsbs activates an initial autophosphorylation of ATM, resulting in ATM monomers phosphorylated at Ser1981 (ATM Ser1981 ). This early activation of ATM is facilitated by the MRN (MRE11/RAD50/NBS1) complex and protein phosphatases 2A and 5 (PP2A and PP5) and hptip (5-8). Similarly, DNA-PKcs, involved in the nonhomologous end-joining (NHEJ) DNA-dsb repair pathway, is also activated by PP5 following DNA breaks and becomes autophosphorylated at its Thr2609 residue (DNA-PKcs Thr2609 ) (3, 9, 10). Specifically, the N-terminus protein interaction domain of ATM directly phosphorylates p53 at residue Ser15 and ATM indirectly phoshorylates CHK2 (11). The stabilization and activation of p53 induces transcription of p21 WAF, an inhibitor of the cyclin E CDK2 complex and RB phosphorylation leading to cell arrest at the G1 to S transition. ATM-mediated p53 phosphorylation also abrogates the interaction between p53 and MDM2 (an E3 ubiquitin ligase), thus inhibiting ubiquitination of p53 and its degradation by the 26S proteasome. Altogether, ATM-mediated phosphorylation of the p53 and CHK2 leads to a series of G1 and G2 cell cycle checkpoints that together act in preventing genomic instability following DNA damage (12). 49
66 An outstanding question still remains as to what function(s) are associated with p53 phosphoforms that are activated during the initial sensing and transduction of IRinduced DNA damage by ATM? Recent reports of clustered interdependence between select p53 phosphorylation sites suggest that certain p53 phosphoforms may have unique biology pertaining to DNA damage sensing or repair. For example, residue Ser15 phosphorylation occurs within 15 minutes of exposure to IR leading to a subsequent clustered phosphorylation of amino-terminus residues Thr18, Ser9 and Ser20 (11). These modifications are not solely linked to p53 protein stabilization (13, 14). Ljungman and colleagues have recently reported that Ser15- phosphorylation can be uncoupled from p53 nuclear accumulation, consistent with the concept that other possible biological activities may be associated with phosphorylation of the Ser15 residue (15). The timing, intracellular locale and exact residues of p53 phosphorylation, de-phosphorylation and acetylation may reflect the level and type of DNA damage following chromatin or nucleolar disruption (11, 13, 16). ATM can sense changes in global chromatin structure or resulting from DNA breaks and leading to pan-nuclear phosphorylation of its substrates, including that of p53 (17). Based on live-cell imaging of fluorescently-tagged proteins, ATM can also phosphorylate its targets (e.g., NBS1) directly at the site of DNA damage. This gives rise to dynamic local protein-chromatin interactions including CHK2 phosphorylation at the site of DNA damage (18). Individually, ATM and p53 have been shown to bind 50
67 dsdna, IR-induced DNA-dsb breaks and DNA base damage and be co-factors in NHEJ or HR (homologous recombination) based on DNA-dsb repair assays in vitro and in vivo (19-23). It is therefore plausible that IR-induced p53 or ATM phosphoforms may directly interact with sites of DNA damage and interact with DNA repair proteins during the initial hours of maximal DNA-dsb recognition and repair (23-25). Intracellular interactions between ATM-associated proteins and DNA repair proteins can now be tracked in situ using immunofluorescence microscopy in which discrete nuclear protein-protein interactions can be visualized following whole cell or subcellular (e.g. UV microbeam) radiation at sites of DNA damage (8, 17, 26-29). Biochemical and microscopic studies support the phosphorylation of H2AX at Ser139 (i.e. γ-h2ax) as a discrete biomarker of megabase domains containing DNAdsbs (27). Furthermore, residual γ-h2ax foci at late time points following irradiation (i.e. 24 hours) are thought to represent non-repaired sites of DNA damage which correlate with relative radiation cell survival in vitro and in vivo (29, 30). γ-h2ax was also found to be essential for the recruitment of 53BP1, BRCA1, MDC1, and the MRN complex to the site of DNA damage during ATM-mediated phosphorylation (27). Recently, Kang and colleagues found that although γ-h2ax was dispensable for the activation of ATM and p53 responses following DNA damage, and that NBS1, γ-h2ax and p53 can interact in parallel with ATM to maintain genetic stability (31). These results are supported by the recent work of Bartkova and colleagues in which increased endogenous activation of DNA damage signaling proteins (e.g. 51
68 ATM Ser1981, CHK2 Thr68, γ-h2ax, and p53 Ser15 ) was a biomarker of genetic instability and malignancy as a response to aberrant DNA replication in transformed cells (32). With the development of these phospho-specific antibodies, the phosphorylated p53 isoforms and their intracellular distribution following DNA damage can be studied in relation to total cellular p53 and other signaling and DNA repair proteins. We hypothesized that p53 Ser15 might participate within a larger genome surveillance complex within the first hour post-irradiation, similar to that reported for the ATM, DNA-PKcs, γ-h2ax/53bp1 and the MRN complex (8, 10, 28). To test this hypothesis, we utilized primary human fibroblast strains to determine the expression and subcellular localization of endogenous p53 species following DNA damage. Herein, we report that discrete p53 Ser15 nuclear foci are maximally induced by DNA breaks and base damage. These foci form within minutes from a pre-existing pool of p53. Using a variety of methodologies including whole cell and subnuclear irradiation techniques, we have determined that p53 Ser15 co-immunoprecipitates and colocalizes with γ-h2ax and interacting proteins at the site of DNA damage. Our data place the p53 Ser15 phosphoform at the site of DNA-dsbs during the initial surveillance of DNA damage and further exemplifies the unique biology of selected p53 phosphoforms during DNA damage signaling. 52
69 2.3 Materials and Methods Fibroblast Strains and Cell Culture Conditions Primary human fibroblast strains were obtained from Coriell (USA) and cultured as per supplier s instructions: normal diploid fibroblast strains NDF-GM03651 and NDF- GM05757; homozygous ATM -/- strains AT-GM05823 (also known as AT5B1) and AT-GM02052 (23); homozygous NBS -/- strain NBS-GM07166 (33); and the DNA Ligase IV-deficient strain GM (also known as 180BR) which shows a defective rate of DNA-dsb rejoining. Genomic DNA from all strains was isolated and sequenced for p53 to confirm that the NDF, AT and NBS fibroblast strains expressed two wild type p53 alleles. Isogenic p53 WT/WT and p53 -/- HCT116 colon carcinoma cell lines (a gift from Dr. B. Vogelstein) and the p53 WT/WT and p53 Ser18Ala/Ser18Ala murine embryonic fibroblasts (MEFs) have both been previously described (34). In order to preclude cell cycle bias in the initial quantification of p53 and γ-h2ax responses, a synchronization protocol was used to obtain density-inhibited, G0/G1- phase cultures as previously described by Little and co-workers (12, 33). Flow cytometric analyses confirmed 90-95% G1 content in NDF strains and 80-85% G1 content in AT, LFS and NBS strains prior to use. In selected experiments, asynchronous populations were also used. Irradiation and Chemical Induction of DNA Damage G0/G1-phase or asynchronous cultures were exposed to whole-cell ionizing radiation (doses ranging from 0 to 20 Gy) using a 137 Cs- irradiator (Nordion) at ~1 53
70 Gy/minute (room temperature; aerobic conditions) (35). UV-irradiation (20J/m 2 ) was carried out in PBS using a continuous UV-wavelength source (254 nm) as previously described (35). For subnuclear damage experiments, a high-linear energy transfer (LET) helium-3 ion microbeam (100keV/μm; irradiation of 1-, 3-, and 100-targeted helium ions) was used to target specific subnuclear locales within G0/G1-phase cells pre-stained with 1μM Hoechst33258 (Molecular Probes; method described in detail by Belyakov and colleagues)(36). In complementary subnuclear targeting experiments, a UV laser microbeam was used as previously described (27, 37). In this case, cells were irradiated with a 390nm laser using a LaserScissors TM Module 390/20 microscopic set-up (Cell Robotics, Inc.) at 75% power output (100% power output = 20μJ/pulse, 10 pulses/second, and maximum rate of 10 µm/s). For drug treatments, cells were also exposed to the PI3KK inhibitor, wortmannin (23μg/ml for 1 hour); DNA-dsb inducing agent, bleomycin (0.06U/ml for 3 hours); DNA-ssb inducing agent, H 2 O 2 (100μM for 1 hour); DNA base-damage inducing agent, MMS (methyl methane sulfonate; 0.01% (~1mM) for 1 hour); and DNA crosslinking agent, MMC (mitomycin C; 1μg/ml for 2 hours). Logarithmically-growing cultures were exposed to hydroxyurea (1mM for 16 hours), an inhibitor of DNA replication (20). G0/G1-phase NDF-GM05757 fibroblasts were also pre-treated with 30μg/ml cyclohexamide at 15 minutes prior to irradiation to inhibit protein synthesis or 10μM MG132 (for various times post-irradiation) to inhibit the activity of the 26S proteasome. Control cultures incubated with and without FBS, 0.1% DMSO, or ethanol carriers, served as negative controls for the latter experiments. 54
71 Confocal and Wide-Field Immunofluorescence Microscopy Intranuclear staining patterns of endogenous or exogenous protein expression were visualized using immunofluorescence microscopy. Cells cultured in 4-chamber slides (Lab-Tek, Nalgene) were fixed and permeabilized in 2% paraformaldehyde/0.2% Triton X-100 (ph 8.2) and 0.5% Nonidet P-40. Fixed cells were then incubated with diluted primary and fluorophore-conjugated secondary (rhodamine red X (RRX-red) or fluorescein isothiocyanate (FITC-green); Jackson Immunoresearch) antibodies in 3% BSA. Negative staining controls included cells incubated with 3% BSA alone or IgG in 3% BSA. Finally, cells were counter-stained for nuclear DNA using 0.1μg/ml DAPI (diamidinophenyl-indole) before mounting in Vectashield (Vector Labs) for microscopic analyses. Of note, fixation and permeabilization of cultures including using either 4% paraformaldehyde or methanol/acetone gave similar and consistent staining patterns. To determine whether p53 Ser15 foci were soluble or associated with nuclear matrix, RNA or DNA, the G0/G1-synchronized fibroblasts were permeabilized or permeabilized and pre-treated with either RNaseA or DnaseI, as previously described by Rubbi and Milner (38). Briefly, cells were washed with TBS (150mM NaCl, 10mM Tris, 5mM MgCl 2, ph7.4), then twice with TBS-G (TBS, 25% glycerol, 0.5mM EGTA), and then for three to four minutes in TBS-G-TX (TBS-G, 0.05% TritonX-100). The cells were then either incubated at room temperature for 1 hour in S buffer (soluble proteins are pre-extracted), or RNaseA in S buffer (200U/ml, Invitrogen), or DNaseI in TBS (1μg/μl, Invitrogen). Following this pre- 55
72 extraction/treatment, the cells were processed as indicated above for immunofluorescence staining and detection of the proteins of interest. Images were captured using a Zeiss LSM510 Confocal Microscope at a final magnification of 630x. The use of 1.8μm confocal sections allowed for a quantitative comparison of responses for doses up to 20 Gy. Nuclei with 3 or more foci were designated as foci-positive nuclei as previously described (39). For all experiments, at least 30 to 50 nuclei were scored after controlling for background staining based upon non-irradiated cultures. The final data is presented as the mean of 2 to 6 independent experiments with the associated standard error of the mean (SEM). Significant co-localization of nuclear foci was determined by visualization of merged red and green (resulting in yellow) images upon a DAPI background. Co-localization was confirmed by characterization of the fluorescence intensity profiles for given fluorochromes within a defined sub-nuclear region and confirmed by the calculation and plotting of the Pearson's correlation coefficient (r p ) (38). A lack of perfect alignment between channels monitoring different fluorophores results in the coefficient values oscillating around a constant background value. When channels monitoring different fluorophores correlate (or anti-correlate), the coefficients will depart positively (or negatively) from the background value. Western Blot, Immunoprecipitation and Cellular Fractionation Analyses Immunoprecipitation and western blot analyses were performed using standard protocols as previously described (40). Cytoplasmic, nuclear and chromatin-bound 56
73 cellular fractions were isolated using a modified Dignam method (41). Briefly, cells were lysed and incubated for 5 minutes on ice, in cytoplasmic buffer (25mM KCl, 5mM MgCl 2, 10mM Tris-HCl ph8.0, 0.5% Nonidet P-40, 1mM dithiothrietol, 1x protease inhibitors (Cθmplete EDTA-free, Roche), 1x phosphatase inhibitors (Cocktail Set II, Calbiochem)). Lysates were centrifuged at 3000 rpm for 5 minutes and the supernatant (cytoplasmic fraction) was separated. The nuclear pellet was rinsed three times with cytoplasmic buffer, then resuspended in nuclear buffer (10mM Tris-HCl ph8.0, 500mM NaCl, 0.1% Nonidet P-40, 5mM EDTA, 1x protease inhibitors (Cθmplete EDTA-free, Roche), 1x phosphatase inhibitors (Cocktail Set II, Calbiochem)). The nuclei were lysed by vigorous pipetting, vortex for 5 minutes and incubated on ice for 15 minutes. The nuclear fraction was centrifuged at rpm for 15 minutes to pellet the chromatin and the supernatant (nuclear fraction) was separated. The chromatin pellet was rinsed three times with nuclear buffer, then resuspended in nuclear buffer and subjected to 20 pulses of sonication (Branson Sonifier 450, 60Hz) to shear the DNA. All fractions were then analysed by Western blot as indicated above. Antibodies used in this study include: p53 Ser15 (Ab-3), Ab-7 (pantropic p53), DO-1 (amino-terminus-specific p53), Ab421 (carboxy-terminus-specific p53), p21 WAF (Ab- 1), RAD51(Ab-1), BRCA1(Ab-1) and α-tubulin (Ab-1) from Oncogene Research Products; Ab1801 (amino-terminus-specific p53) from Novocastra; polyclonal Ser 6-, 9-, 15-, 20-, 37-, 46- and 392-phosphorylated p53, and monoclonal Ser 15- phosphorylated p53, and Ab#9282 (pantropic p53) from Cell Signaling; polyclonal 57
74 and monoclonal γ-h2ax from Upstate Biotechnology; ATM Ser1981 from Rockland Immunochemicals; RAD50 (2C6) from Novus Biologicals; MRE11 from Genetex; actin from Sigma; PML (1B9) from MBL; 53BP1, FL393, BP53-12 (pantropic p53) and nucleolin/c23 from Santa Cruz; GFP (also recognizes YFP-variant) from BD Biosciences; and DNA-PKcs Thr2609 as previously described (10). The specificity of p53 antibodies was confirmed using p53 -/- cells (SAOS-2, PC3 and HCT116 p53 -/- ) in which no detectable p53 and p53 Ser15 protein expression was observed following western blot, immunoprecipitation and immunostaining analyses. Two polyclonal (rabbit; Oncogene and Cell Signalling) and one monoclonal (mouse; Cell Signalling) p53 Ser15 -specific antibodies all revealed similar staining patterns preand post-irradiation. Thus, all subsequent experiments were done using the polyclonal rabbit Oncogene p53 Ser15 -specific antibody. DNA-dsb Rejoining Assays Biochemical DNA-dsb rejoining kinetics for human and murine fibroblasts were determined using the continuous-field gel electrophoresis (CFGE) assay and neutral Comet assay as previously described (35, 40). CFGE assays have been used to accurately quantify DNA-dsb rejoining kinetics (12). Briefly, cells were grown in 60- mm dishes and either irradiated or mock-irradiated on ice and then incubated at 37C in fresh media until lysis at various times of 0-24 hours post-irradiation (100Gy and 20Gy for the CFGE and COMET assays, respectively). For the CFGE assay, samples were loaded into the wells of a 0.8% agarose-0.5x TBE gel prior to 58
75 electrophoresis at room temperature for 40 hours at 0.6 V cm -1 in 0.5X TBE buffer. After electrophoresis, gels were stained with 1 μg ml -1 ethidium bromide, de-stained in deionized water and imaged using an UV imaging system equipped with a CCD camera and imaging software (LabWorks, UVP Inc.). For murine embryonic fibroblasts (MEFs) in which total cell numbers were limiting, the single-cell neutral Comet assay was utilized. Single cell suspensions were mixed with 75 μl of 0.5% low melting agarose at 37ºC and spread on a 1% agarose precoated slide. Slides were then incubated in Proteinase-K solution for 60 minutes at 37ºC, followed by incubation in ice-cold lysis buffer (2.5 M NaCl, 100mM EDTA, 10 mm Trizma base, 10% DMSO, 1% Triton-X) overnight. After lysis, the slides were placed in horizontal electrophoresis tanks filled with electrophoresis buffer (1x TBE, ph 8.0) for 20 minutes, and then subjected to electrophoresis at 25V/30-45mA for a further 20 minutes. After electrophoresis, the slides were washed (0.4 M Tris HCl, ph 7.5) three times, air-dried and stained with ethidium bromide (2µg ml -1 ) prior to scoring. The relative amount of fragmented DNA contained within the Comet s tail, compared to the non-fragmented DNA within the Comet head, was determined by fluorescent image analysis (Northern Eclipse software) to determine the normalized tail moment as a measure of residual DNA breaks over time following irradiation. YFP-p53 Phosphomutant Constructs and Transient Transfection Conditions The role of the Ser15 residue in mediating foci formation was tested by expressing exogenous yellow fluorescent proteins (YFP) fused to p53 WT or p53 Ser15Ala (incapable 59
76 of Ser15 phosphorylation) proteins to track sub-cellular protein patterns following DNA damage. Briefly, the p2516 plasmid containing the human full-length p53 WT/WT cdna fragment cloned into the pcdna3.1 backbone vector was used as a template for generating the p53 fusion and mutant constructs (42). The full length p53 fragment was amplified from p2516 plasmid DNA using the forward and reverse oligonucleotide primers, 5 -TTT TAA GCT TCG ATG GAG GAG CCG CAG TCA GA- 3 and 5 -TTT TGG ATC CTC AGT CTG AGT CAG GCC C-3, respectively. The polymerase chain reactions (PCRs) were optimized with the Platinum Pfx DNA polymerase (Gibco BRL) in a Peltier Thermal Cycler (MJ Research). The amplified fragment was subjected to BamH1 and HindIII digestion (New England Biolabs), and ligated in-frame, fused to the carboxy-terminus of the enhanced yellow fluorescent gene (YFP) in the peyfp-c1 vector (CLONTECH Laboratories, Inc.). Subsequently, the Ser15 site of this clone, YFP-p53, was specifically mutated using the QuikChange site-directed mutagenesis kit (Stratagene) to generate the YFPp53 Ser15Ala clone using the following primers: Ser15Ala-forward 5 -AGC GTC GAG CCC CCT CTG GCT CAG GAA ACA TTT TCA GAC-3 and Ser15Ala-reverse 5 - GTC TGA AAA TGT TTC CTG AGC CAG AGG GGG CTC GAC GCT-3. All plasmid clones were purified using the CONCERT High Purity Maxiprep System (Gibco BRL) and sequenced on both strands to confirm wild type and site-specific mutated status. Metafectene (Biontex) was used to transfect the HCT116 p53 WT/WT and HCT116 p53 -/- cells with YFP-p53 vectors. All reactions were carried out according to the manufacturer s instructions. Following transfection, cells were fixed and imaged at regular intervals following irradiation using a Zeiss LSM510 Confocal Microscope. 60
77 Results Ionizing radiation induces discrete dose-responsive p53 Ser15 nuclear foci from a pre-existing p53 protein pool in an ATM-dependent manner To our knowledge, there are no systematic microscopic studies of relative intracellular localization of p53 phosphoforms following DNA damage. Therefore, a panel of p53 pan- and phospho-specific antibodies was initially tested to determine relative p53 phosphorylation and sub-nuclear localization following whole cell irradiation (Figure 2.1A to 2.1D). These included pantropic p53 antibodies that recognized the amino-terminus (Ab1801, DO-1); carboxy-terminus (Ab421); fulllength p53 (BP53-12, Ab-7, FL393); and specific p53-phosphoforms (e.g. phosphorylated serine residues 6, 9, 15, 20, 37, 46, and 392). To prevent cell cycle bias relating to p53 staining patterns during DNA replication (20, 43), we conducted our initial experiments using G0/G1-synchronized cells (33). We reasoned that if p53 phosphoforms were involved in DNA damage sensing or recognition, discrete intranuclear foci representing protein aggregates at sites of DNA damage should be observed following irradiation similar to that reported for γ- H2AX (27). Staining with antibodies specific to the Ser15-phosphorylated residue of p53 detected discrete p53 Ser15 foci similar in size and morphology to the DNA-dsbassociated foci of γ-h2ax, ATM Ser1981, DNA-PKcs Thr2609 and BRCA1 (Figure 2.1C). In contrast, cell staining with antibodies to pantropic p53 (Ab1801 and DO-1) revealed an accumulation of p53 in a homogenous, non-punctate nuclear pattern (Figure 61
78 2.1C), similar to that observed with antibodies directed against p21 WAF and pantropic p53 (e.g. Ab421, BP53-12 and Ab-7; data not shown). Only antibodies recognizing p53 Ser15 detected a non-nucleolar and dose-responsive accumulation of p53 foci within 10 minutes following whole cell irradiation (Figure 2.1C, 2.1E and 2.1F). While the other phospho-specific p53 antibodies were able to specifically immunoprecipitate their respective pools of IR-activated p53 protein (Figure 2.1A), they did not detect increased p53 protein or p53 nuclear foci postirradiation. As such, p53 Ser15 was tracked thereafter in subsequent DNA damage experiments. Both p53 Ser15 and γ-h2ax expression levels, and foci formation, were dose-dependent and detected within minutes post-irradiation (Figure 2.1E and 2.1F)(27), in contrast to the induction of p21 WAF which was maximally induced 3-6 hours post-irradiation (Figure 2.1E). Neither the staining intensity nor induction of p53 Ser15 foci was affected by cyclohexamide, an inhibitor of de novo protein synthesis, prior to irradiation (Figure 2.1D). This is consistent with the p53 Ser15 signal representing a rapid phosphorylation of a pre-existing pool of p53 protein (2). As ionizing radiation causes several types of DNA lesions within clustered local multiply damaged sites, we used a variety of DNA damaging agents to determine the lesion specificity for p53 Ser15 foci (Figure 2.1G). In addition to IR, p53 Ser15 foci also formed following treatment of G0/G1-synchronized cells with bleomycin, H 2 O 2 and methyl methane sulfonate (MMS) (Figure 2.1G). In asynchronously growing NDFs pre-treated with hydroxyurea or UV, both agents which can lead to DNA breaks 62
79 within stalled DNA replication forks, p53 Ser15 foci also formed (data not shown)(20). Altogether, these results support that p53 Ser15 foci formation are maximally induced by DNA strand breaks. Conversely, in G0/G1-synchronized NDF cells, minimal or no p53 Ser15 foci were observed in the first 3 hours following mitomycin C (MMC; DNA cross-linking agent) or UV-induced damage (pyrimidine dimers and photoproducts) (Figure 2.1G). In parallel-treated cultures, cytoplasmic to nuclear translocation and accumulation of total p53 protein was observed following both UV and IR (as detected by a pantropic p53 antibody, Ab1801; see Supplementary Figure 2.1A). Western blot analyses confirmed delayed and reduced p53 Ser15 phosphorylation following UV-irradiation of G0/G1-synchronized NDF cells in agreement with the lack of p53 Ser15 foci following UV in the absence of stalled replication forks (see Supplementary Figure 2.1A). We conclude that the induction of p53 Ser15 intranuclear foci is maximally responsive to DNA breaks and DNA base damage within G0/G1- phase cells; a role potentially distinct from that reported for p53 during DNA replication and homologous recombination in S-phase cells (20). p53 Ser15 is a unique chromatin-associated sub-pool of total p53 To confirm that nuclear p53 Ser15 foci are chromatin-associated, we treated cells with DNaseI or RNaseA following the pre-extraction of soluble cellular proteins. Permeabilization removed most of the nucleoplasmic signal of total nuclear p53 protein detected by Ab1801 (Figure 2.2A, bottom panel). In contrast, p53 Ser15 was chromatin-bound following DNaseI digestion (Figure 2.2A, top panel). The chromatin-associated p53 sub-pool was similar to the pattern of γ-h2ax staining 63
80 (Figure 2.2A, top panel). RNaseA treatment also removed only a small proportion of p53 Ser15 foci, which may relate to previously described RNA-associated p53 species (14). The NDFs lysates were also biochemically fractionated into cytoplasmic, nuclear, and chromatin-bound fractions (see Materials and Methods). As shown in Figure 2.2B, and in agreement with our immunofluorescence data, the p53 Ser15 subpool is found within the chromatin-bound fraction similar to the chromatin-bound γ- H2AX. While total p53 protein can be found in all fractions and increased post-ir, this is also consistent with our hypothesis that p53 Ser15 phosphoforms are a sub-pool of total p53 protein that is chromatin-bound. Despite differential staining patterns within the first three hours post-10gy of irradiation (Figure 2.1C), at 24 hours we observed some foci formation by pantropic p53 antibodies (as detected by either Ab1801 or FL393; see Supplementary Figure 2.2A). This Ab1801 sub-population significantly co-localized with residual p53 Ser15 foci (see Supplementary Figure 2.1B and 2.2), consistent with Ab1801 detecting residual p53 Ser15 foci and with p53 Ser15 being a sub-pool of total p53. Further analyses by immunoprecipitation and immunodepletion of cell extracts confirmed this finding (Figure 2.2C). The relative levels of total p53, p53 Ser15 and remaining p53 following p53 Ser15 -depletion were determined and were consistent with a ratio of 0.25 for p53 Ser15 protein to total p53 protein. This ratio increased to 0.5 at 24 hours post- IR (Figure 2.2C). Taken together, these results support p53 Ser15 protein as a distinct chromatin-associated sub-pool of total p53 during the DNA damage response. 64
81 Dependency and association of p53 Ser15 foci on ATM, DNA-PKcs or MRN function Initial microscopic control experiments were undertaken as a means to document the formation of p53 Ser15 as a function of upstream kinases. As predicted, p53 Ser15 foci formation was attenuated or delayed in irradiated AT-GM05823, AT-GM02052 fibroblasts and NDF-GM05757 fibroblasts pre-treated with Wortmannin at concentrations that inhibit the ATM kinase activity (Figures 2.1B, 2.3A and 2.3B). p53 Ser15 foci formation was observed in the DNA-PKcs-deficient MO59J glioblastoma cell line, consistent with a primary role for ATM as the PI3-kinase responsible for the phosphorylation of p53 Ser15 (data not shown)(23, 27). We observed delayed p53 Ser15 foci formation in the NBS-GM07166 fibroblast strain (Figures 2.3A and 2.3B, right panel) most probably reflecting the need for intact MRN signaling upstream of ATMmediated phosphorylation (5, 31). Both p53 Ser15 protein levels and foci were elevated pre- and post-irradiation in the NHEJ-defective 180BR fibroblast strain, consistent with residual DNA breaks being associated with residual p53 Ser15 foci at 24 hours following DNA damage. In addition, we also observed co-localized p53 Ser15 and γ- H2AX foci within acentric chromosome fragments contained within micronuclei in irradiated 180BR cells. This observation is consistent with residual foci being a manifestation of non-repaired breaks (Figures 2.3A and 2.3B, right panels)(44). We next determined whether p53 Ser15 foci would co-localize with γ-h2ax/53bp1, MRN, and PML-associated complexes following whole-cell irradiation (2, 27, 45). Although 53BP1 has been shown to bind p53 in yeast 2-hybrid, and crystallization 65
82 studies (8), there are few data reporting p53 and 53BP1 co-localization in non-s phase cells in vivo (20). Using microscopic and statistical analyses, we observed a significant induction of co-localization of approximately 30-60% of p53 Ser15 foci with γ-h2ax, 53BP1, RAD50 and MRE11 foci following whole cell irradiation, in a timeand dose-dependent manner (Figure 2.3C to 2.3G). For comparison, in the same experiment, there was almost a 1:1 co-localization between 53BP1 and γ-h2ax at 3 hours post-10gy (Figure 2.3C and 2.3E) and co-localization between RAD50 and NBS1 was observed at 6 hours post-10gy (data not shown; Supplementary Figure 2.3B). Co-localized foci were specifically chosen to highlight the coincident peaks of the respective fluorescent signals based on the line scan plots beneath the corresponding images in Figure 2.3C and 2.3D. In separate analyses based on focal staining patterns within the entire nucleus, Pearson correlation coefficient calculations (see Materials and Methods, as described by Rubbi and Milner)(38) confirmed significant, non-random co-localizations between p53 Ser15 foci and γ- H2AX, 53BP1, RAD50 and MRE11 foci. In addition, Z-stacks of the intra-nuclear confocal sections were also collected and used to reconstruct the nuclei with foci in three dimensional space, which also confirmed that the foci were co-localized in all three nuclear planes (Figure 2.3E). While there can be almost a 1:1 co-localization observed between γ-h2ax and 53BP1 and γ-h2ax and DNA-PKcs Thr2609 (46), the co-localization of p53 Ser15 and any of γ-h2ax, 53BP1, RAD50 and MRE11 is less than this. This may reflect differences in the kinetics of p53 Ser15 binding over time (e.g. transient interactions) with chromatin-associated sites of DNA damage. In our study, p53 Ser15 and PML foci co-localized minimally (Supplementary Figure 2.3E), 66
83 suggesting that these p53 Ser15 foci are a separate sub-pool to that described as interacting with PML during cellular senescence (47). We were able to biochemically confirm a direct p53 Ser15 /γ-h2ax interaction by coimmunoprecipitating endogenous p53 Ser15 and γ-h2ax in vivo from both G0/G1- synchronized NDF-GM05757 fibroblasts and asynchronously-growing HCT116 p53 +/+ colorectal cancer cells following DNA damage (Figure 2.3H). Maximal amounts of p53 Ser15 and γ-h2ax were co-immunoprecipitated at 3 hours and then decreased at 24 hours post-irradiation (Figure 2.3H); these data are consistent with observed kinetics of p53 Ser15 foci formation and resolution. p53 Ser15 kinetics correlate with biochemical DNA-dsb rejoining Quantitative microscopy confirmed that p53 Ser15 and γ-h2ax foci had similar kinetics of induction and resolution over the first and subsequent hours following irradiation (Figure 2.4A and 2.4B). These data are consistent with the Western blot analyses in Figure 2.1E. The residual number of γ-h2ax foci at 24 hours was similar to that of the residual number of p53 Ser15 foci after 2 and 10Gy (Figure 2.4A and 2.4B). To ascertain relative for formation following both low (2Gy) and high (10Gy) doses, we used confocal microscopy. Typical nuclear depths for G0/G1 NDF-GM05757 cells are approximately 5μm which is equivalent to two to three 1.8μm confocal sections through the nucleus. Therefore, the total number of p53 Ser15 and γ-h2ax residual foci at 24 hours following 2 or 10Gy is estimated at 3 to 5 or 15 to 25 foci per nucleus. This approximates the predicted 2.5 to 5 DNA-dsbs per Gy at 24 hours following 67
84 irradiation (based on 5-10% residual DNA breaks remaining at 24 hours as observed by continuous field gel electrophoresis; see Figures 2.4C). In the absence of DNA damage, the short half-life of endogenous p53 protein is regulated by the MDM2 protein, an E3 ubiquitin ligase, that targets p53 for 26Sproteosomal degradation (48). If p53 Ser15 foci were associated with DNA damage sensing or repair, then the observed kinetics of this IR-phosphorylated nuclear p53 pool should be resistant to proteasomal-mediated degradation and also distinct from nucleolar p53 sub-pools (Figure 2.1C)(38). We therefore investigated whether p53 Ser15 foci formation is altered in NDF cells treated with MG132, an inhibitor of the 26S-proteosome. Consistent with previous data (49), neither DMSO nor MG132 induced p53 Ser15 in the absence of DNA damage. We observed in both the DMSO control- and MG132-treated cells, an increase at 3 hours followed by a decrease in p53 Ser15 foci and protein levels over 24 hours (Figure 2.4D). We conclude that p53 Ser15 foci induction and resolution represents a dose-responsive nuclear p53 subpool that is resistant to proteasomal degradation. Lack of Ser15-phosphorylation leads to altered DNA rejoining, foci formation and γ-h2ax association following IR-induced DNA damage To determine whether Ser15 phosphorylation and G1 checkpoint control could functionally impact on DNA-dsb sensing and/or repair, we utilized murine embryo fibroblasts (MEFs) that expressed a knock-in p53 mutation at murine Ser18 (p53 Ser18Ala/Ser18Ala ) whereby the mouse Serine 18 residue is homologous and 68
85 functionally equivalent to human Serine 15 residue. This knock-in phosphorylationdeficient mutation does not affect p53 stabilization or DNA binding (34). Using the COMET assay to afford single cell analysis, we observed increased residual DNAdsbs following doses in excess of 20Gy in the p53 Ser18Ala/Ser18Ala MEFs as compared to p53 WT/WT MEFs (Figure 2.5A), suggesting that local multiply damaged sites following irradiation may be less effectively sensed in the mutant cells. High levels of non-specific p53-staining in MEF cells precluded correlative microscopy of p53 Ser15 and γ-h2ax in these experiments. To determine whether foci formation is directly affected by p53 Ser15 phosphorylation, transient transfection studies of HCT116 p53 -/- human carcinoma cells with human YFP-p53 phosphomutant constructs were performed using DNA sequences encoding wild-type p53 or p53 mutated from serine to alanine at residue 15. Control YFP-alone and YFP-p53 Ser15Ala protein expression did not lead to foci formation in the absence or presence of DNA damage induction. This is in contrast to YFP-p53 WT expression in which foci are induced following DNA damage (Figure 2.5B). Due to toxicity concerns using p53 WT constructs in null-p53 cells, we also used HCT116 p53 +/+ human carcinoma cells to transiently-transfect p53 fusion constructs and determine their association with γ-h2ax following DNA damage. YFP-p53 WT levels were stabilized at 3 hours post-10gy with an associated induction of p21 WAF and γ- H2AX (Figure 2.5C). In contrast, YFP-p53 Ser15Ala phosphomutant levels remained unchanged and resulted in relatively reduced levels of p21 WAF protein levels following irradiation. γ-h2ax induction was similar in both YFP-p53 WT -and 69
86 p53 Ser15Ala -expressing cells (Figure 2.5C). Finally, in agreement with our endogenous data using NDF cells, we observed an increased biochemical association of γ-h2ax and YFP-p53 WT at 3 hours post-ir, but not for YFP-p53 Ser15Ala (Figure 2.5D). Taken together, our data is consistent with a role for p53 Ser15-phosphorylation as a mediator of chromatin binding to γ-h2ax megabase domains during DNA-dsb sensing and repair. Interaction between p53 Ser15, ATM Ser1981 and DNA-PKcs Thr2609 at focal DNA damage Given the morphologic similarity between p53 Ser15 foci and that of γ-h2ax, ATM Ser1981 and DNA-PKcs Thr2609 foci, we next determined whether p53 Ser15 could directly co-localize at discrete sites of DNA-dsbs in vivo within the first hour during DNA-dsb signaling. We initially used a high-let (linear energy transfer) helium-3 ion microbeam to irradiate discrete subnuclear areas of less than 5μm diameter with minimal scatter (detailed by Belyakov and colleagues) (36). Using this microbeam technique, between 4 and 6 DNA-dsbs per helium ion are created within the dense ionization cluster of the irradiated cylindrical nuclear volume. Within targeted G0/G1 NDF-GM05757 cells, at 30 minutes following a dose of 1 to 100 helium-3 ions, a discrete 3-dimensional γ-h2ax focus was observed and co-localized with 53BP1 (Figure 2.6A). In similar-targeted cells, p53 Ser15 and ATM were also observed to colocalize within the discrete irradiated cylindrical volume of nuclear damage (Figure 2.6A). 70
87 A similar discrete recruitment of p53 Ser15 was observed within sub-nuclear tracks of DNA-dsbs created with a UV laser microbeam technique (Figure 2.6C and 2.6D)(27). Within 10 minutes of recovery, we consistently observed γ-h2ax signal accumulating along TUNEL-positive tracks (Figure 2.6B). DNA-PKcs Thr2609 and ATM Ser1981 also co-localized within 10 minutes with γ-h2ax and TUNEL tracks (Figure 2.6B), supporting recent data in which ATM and DNA-PKcs were shown to redundantly phosphorylate γ-h2ax and p53 Ser15 and that ATM is recruited quickly to DNA-dsbs (1, 2, 28). Subsequently, using the γ-h2ax and DNA-PKcs Thr2609 signals as indicators of UV laser-induced DNA-dsbs, we observed p53 Ser15 accumulation within these tracks as early as 10 minutes following irradiation (Figure 2.6C) with maximal recruitment of p53 Ser15 occurring at 30 to 60 minutes post-irradiation (Figure 2.6D). We conclude that p53 Ser15 binds directly to damaged chromatin domains containing γ-h2ax, 53BP1, ATM Ser1981 and DNA-PKcs Thr2609 in the first hour during the sensing and repair of DNA-dsbs. 2.4 Discussion The p53 tumor suppressor protein achieves a broad realm of cellular functions through a series of discrete and well-timed post-translational modifications. These initiate its well-characterized function as a transcriptional trans-activator and a mediator of cell cycle checkpoint control and cell death following DNA damage (13). Our study is unique in that it documents temporal data regarding specific p53- phosphoforms in response to DNA damage in vivo under physiologic conditions in primary human fibroblasts. The data herein indicates that p53 Ser15 phosphoforms are 71
88 a unique sub-pool of total cellular p53 (38) and are chromatin-associated at sites of DNA breaks and γ-h2ax megabase domains. However, this p53 Ser15 sub-pool most probably possesses other post-translational modifications given the interdependency of phosphorylation and acetylation (11) and these will require further study. Our kinetic, co-localization and co-immunoprecipitation data indicate that the preexisting latent sub-pool of nuclear p53 is rapidly phosphorylated in vivo at γ-h2ax domains and this is dependent on ATM and MRN, but not DNA-PKcs. The recruitment of latent p53 that is phosphorylated within minutes following DNA damage is consistent with the high affinity of latent p53 to bind DNA in a non-specific manner, which may relate to additional chromatin binding at DNA breaks rather than solely at p53 consensus sequences within downstream target genes(figure 2.2A)(50). Nonetheless, in response to genotoxic stress, p53 has been recently localized at transcription sites including that of p21 WAF and is phosphorylated by hsmg-1 during RNA processing (4, 13, 14). Our observation that there are qualitatively less p53 Ser15 foci following RNaseA treatment (Figure 2.2A) could be consistent with a proportion of p53 Ser15 at sites of transcription or associated with RNA processing. Further experiments are required to clarify the relative extent and dynamics of chromatin-bound versus RNA-bound p53 phosphoforms following genotoxic insult. The delayed kinetics of p53 Ser15 foci formation when compared to γ- H2AX foci formation reflects the requirement of initial ATM phosphorylation, but may also reflect a secondary recruitment of p53 to clustered damage within local multiple 72
89 damaged sites or DNA breaks created during DNA-dsb, DNA-ssb or BER lesion processing (24). We speculate that the number, site and nature of p53 Ser15 interactions may serve as a counting mechanism for cells to assess the quantity, type and severity of damage in order to mediate cell cycle checkpoint control or cell death in a timely manner to prevent cellular carcinogenesis. We observed less than 1:1 co-localization of p53 Ser15 with γ-h2ax in contrast to other chromatin-associated proteins, such as 53BP1 (Figure 2.3C and 2.3E). This may reflect a transient p53 Ser15 /γ-h2ax interaction, similar to that reported for CHK2, in which initial direct recognition of DNA damage by MRN and ATM is subsequently followed by phosphorylation of p53 and CHK2 at damaged chromatin prior to initiation of downstream signaling events throughout the nucleus (18). Based on our data, we propose a transient interaction model in which p53 Ser15 initially localizes at sites of γ-h2ax-associated megabase domains (Figure 2.6E); this interaction could be mediated by the p53 non-specific DNA binding domain in the carboxy-terminus and involve linear protein-chromatin diffusion (Figure 2.6E)(20, 50, 51). While the requirement of N-terminal phosphoserine residues for all of the transcriptional transactivation by p53 remains controversial, phosphorylation of the Ser15 and other residues may be required for nuclear retention and/or immediate binding to damaged chromatin in addition to downstream gene activation (e.g. p21 WAF )(20, 50, 51). As DNA repair ensues, chromatin-bound activated p53 Ser15 phosphoform may be de-phosphorylated similar to the de-phosphorylation of γ-h2ax and DNA-PKcs by PP1 and PP5, respectively (52) or be released into the nucleoplasm to transactivate 73
90 downstream p53-target genes at a distance from the original binding site of DNA damage (14, 18). The observed ATM-p53 interactions at DNA breaks supports previous chromatin immunoprecipitations (ChIPs) that determined murine ATM and p53 Ser18 form a complex at sites of DNA-dsbs during V(D)J recombination and microscopy studies in which ATM can co-localize to γ-h2ax domains (25, 28, 53). Our observation of colocalized ATM Ser1981 and DNA-PKcs Thr2609 at sites of DNA breaks is also corroborated by the recent finding that both kinases redundantly phosphorylate γ- H2AX (1). Our finding that p53 Ser15 and DNA-PKcs Thr2609 co-localize at sites of DNA breaks in situ in G1 cells is consistent with the concept that phosphorylated p53 species may act as a mediator between checkpoint control and NHEJ during the G1 phase of the cell cycle. This is also consistent with DNA-PKcs and CHK2 synergistically activating pre-existing p53 following DNA damage (3, 9). Additionally, the importance of the Ser15 residue in our YFP-p53 transfection assays is strengthened by previous data in which NHEJ is stimulated in vitro by the addition of recombinant p53 Asp15 (which mimics a constitutively phosphorylated p53 Ser15 ), but not by recombinant phosphorylation-inhibited p53 Ala15 (54). Taken together with reports of a role for MDM2 in DNA-dsb repair and the observation of p21 WAF foci at sites of sub-nuclear damage (55, 56), localized chromatin-bound proteins associated with the ATM-p53 signaling axis may interact at an exquisite local level near DNA 74
91 damaged sites. These interactions may thus amplify the signaling for presence of DNA breaks that initiates the G1 checkpoint to protect genomic stability. The fact that unlike ATM -/- MEFs, the p53 Ser18Ala/Ser18Ala MEFs (34) are not radiosensitive, allows us to conclude that Ser15 phosphorylation and/or a deficient G1 checkpoint are not major factors of cell survival following DNA damage (20). Indeed, the consequences of DNA damage sensing by p53 Ser15 may relate more to the fidelity, rather than the overall level, of DNA repair as a control against genetic instability. This role was recently supported by the finding that wild type p53 reduces error-prone NHEJ DNA-dsb repair (based on inter-chromosomal reporter substrates) in MEF cells and may protect against carcinogenesis(21, 34). Furthermore, abrogated Ser15 phosphorylation, observed in many mutant p53 proteins (19), may affect mutagen/carcinogen-induced rates of transformation/tumorformation, especially in combination with p53 hotspot mutations (16, 57, 58). Cells defective in Ser392 and Ser389 phosphorylation of p53, in combination with either p53 Arg175His or p53 Arg248Trp hotspot mutations, acquired increased cellular transformation in vitro (16, 57, 58). The fact that ATM-dependent p53 localization to centrosomes during the post-mitotic checkpoint also requires Ser15 phosphorylation attests to a broad role for p53 modifications in preventing aneuploidy and protecting against genetic instability through multiple mechanisms (59). 75
92 Discordance between DNA-dsb sensing/repair and cell-cycle checkpoint control, in the absence of cell death signals, may be one factor in the selection of mutant clones during the process of cellular carcinogenesis. Our studies may partially explain the relative negative prognosis for mutant p53-expressing tumors, which would exhibit defects in p53 Ser15 signaling and DNA-specific binding, and lead to the selection of clones that exhibit therapeutic resistance (19). 76
93 References 1. T. Stiff, M. O'Driscoll, N. Rief, K. Iwabuchi, M. Lobrich and P. A. Jeggo, ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res 64, (2004). 2. R. A. Woo, M. T. Jack, Y. Xu, S. Burma, D. J. Chen and P. W. Lee, DNA damage-induced apoptosis requires the DNA-dependent protein kinase, and is mediated by the latent population of p53. Embo J 21, (2002). 3. M. T. Jack, R. A. Woo, N. Motoyama, H. Takai and P. W. Lee, DNA-dependent protein kinase and checkpoint kinase 2 synergistically activate a latent population of p53 upon DNA damage. J Biol Chem 279, (2004). 4. K. M. Brumbaugh, D. M. Otterness, C. Geisen, V. Oliveira, J. Brognard, X. Li, F. Lejeune, R. S. Tibbetts, L. E. Maquat and R. T. Abraham, The mrna surveillance protein hsmg-1 functions in genotoxic stress response pathways in mammalian cells. Mol Cell 14, (2004). 5. J. H. Lee and T. T. Paull, Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 304, (2004). 6. A. Ali, J. Zhang, S. Bao, I. Liu, D. Otterness, N. M. Dean, R. T. Abraham and X. F. Wang, Requirement of protein phosphatase 5 in DNA-damage-induced ATM activation. Genes Dev 18, (2004). 7. A. A. Goodarzi, J. C. Jonnalagadda, P. Douglas, D. Young, R. Ye, G. B. Moorhead, S. P. Lees-Miller and K. K. Khanna, Autophosphorylation of ataxiatelangiectasia mutated is regulated by protein phosphatase 2A. Embo J 23, (2004). 8. T. A. Mochan, M. Venere, R. A. DiTullio, Jr. and T. D. Halazonetis, 53BP1, an activator of ATM in response to DNA damage. DNA Repair (Amst) 3, (2004). 9. S. Burma and D. J. Chen, Role of DNA-PK in the cellular response to DNA double-strand breaks. DNA Repair (Amst) 3, (2004). 10. B. P. Chen, D. W. Chan, J. Kobayashi, S. Burma, A. Asaithamby, K. Morotomi- Yano, E. Botvinick, J. Qin and D. J. Chen, Cell cycle dependence of DNAdependent protein kinase phosphorylation in response to DNA double strand breaks. J Biol Chem 280, (2005). 11. S. Saito, H. Yamaguchi, Y. Higashimoto, C. Chao, Y. Xu, A. J. Fornace, Jr., E. Appella and C. W. Anderson, Phosphorylation site interdependence of human p53 post-translational modifications in response to stress. J Biol Chem 278, (2003). 12. K. Rothkamm, I. Kruger, L. H. Thompson and M. Lobrich, Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol 23, (2003). 13. P. Fei and W. S. El-Deiry, P53 and radiation responses. Oncogene 22, (2003). 14. J. M. Espinosa, Verdun, R. E., and Emerson, B. M., p53 functions through stressand promoter-specific recruitment of transcription initiation components before and after DNA damage. Molecular Cell 12, (2003). 77
94 15. H. M. O'Hagan and M. Ljungman, Phosphorylation and nuclear accumulation are distinct events contributing to the activation of p53. Mutat Res 546, 7-15 (2004). 16. D. MacPherson, J. Kim, T. Kim, B. K. Rhee, C. T. Van Oostrom, R. A. DiTullio, M. Venere, T. D. Halazonetis, R. Bronson, et al., Defective apoptosis and B-cell lymphomas in mice with p53 point mutation at Ser 23. Embo J 23, (2004). 17. C. J. Bakkenist and M. B. Kastan, DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, (2003). 18. C. Lukas, J. Falck, J. Bartkova, J. Bartek and J. Lukas, Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat Cell Biol 5, (2003). 19. A. R. Cuddihy and R. G. Bristow, The p53 protein family and radiation sensitivity: Yes or no? Cancer Metastasis Rev 23, (2004). 20. S. Sengupta and C. C. Harris, p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol 6, (2005). 21. J. Dahm-Daphi, P. Hubbe, F. Horvath, R. A. El-Awady, K. E. Bouffard, S. N. Powell and H. Willers, Nonhomologous end-joining of site-specific but not of radiation-induced DNA double-strand breaks is reduced in the presence of wildtype p53. Oncogene 24, (2005). 22. A. Restle, C. Janz and L. Wiesmuller, Differences in the association of p53 phosphorylated on serine 15 and key enzymes of homologous recombination. Oncogene 24, (2005). 23. E. Riballo, M. Kuhne, N. Rief, A. Doherty, G. C. Smith, M. J. Recio, C. Reis, K. Dahm, A. Fricke, et al., A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-h2ax foci. Mol Cell 16, (2004). 24. M. Gulston, C. de Lara, T. Jenner, E. Davis and P. O'Neill, Processing of clustered DNA damage generates additional double-strand breaks in mammalian cells post-irradiation. Nucleic Acids Res 32, (2004). 25. Y. Andegeko, L. Moyal, L. Mittelman, I. Tsarfaty, Y. Shiloh and G. Rotman, Nuclear retention of atm at sites of dna double strand breaks. J Biol Chem 276, (2001). 26. B. E. Nelms, R. S. Maser, J. F. MacKay, M. G. Lagally and J. H. Petrini, In situ visualization of DNA double-strand break repair in human fibroblasts. Science 280, (1998). 27. O. Fernandez-Capetillo, A. Lee, M. Nussenzweig and A. Nussenzweig, H2AX: the histone guardian of the genome. DNA Repair (Amst) 3, (2004). 28. P. S. Bradshaw, D. J. Stavropoulos and M. S. Meyn, Human telomeric protein TRF2 associates with genomic double-strand breaks as an early response to DNA damage. Nat Genet 37, (2005). 29. J. P. Banath and P. L. Olive, Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks. Cancer Res 63, (2003). 78
95 30. N. Taneja, M. Davis, J. S. Choy, M. A. Beckett, R. Singh, S. J. Kron and R. R. Weichselbaum, Histone H2AX phosphorylation as a predictor of radiosensitivity and target for radiotherapy. J Biol Chem 279, (2004). 31. J. Kang, D. Ferguson, H. Song, C. Bassing, M. Eckersdorff, F. W. Alt and Y. Xu, Functional interaction of H2AX, NBS1, and p53 in ATM-dependent DNA damage responses and tumor suppression. Mol Cell Biol 25, (2005). 32. J. Bartkova, Z. Horejsi, K. Koed, A. Kramer, F. Tort, K. Zieger, P. Guldberg, M. Sehested, J. M. Nesland, et al., DNA damage response as a candidate anticancer barrier in early human tumorigenesis. Nature 434, (2005). 33. J. B. Little, H. Nagasawa, W. K. Dahlberg, M. Z. Zdzienicka, S. Burma and D. J. Chen, Differing responses of Nijmegen breakage syndrome and ataxia telangiectasia cells to ionizing radiation. Radiat Res 158, (2002). 34. C. Chao, M. Hergenhahn, M. D. Kaeser, Z. Wu, S. Saito, R. Iggo, M. Hollstein, E. Appella and Y. Xu, Cell type- and promoter-specific roles of Ser18 phosphorylation in regulating p53 responses. J Biol Chem 278, (2003). 35. R. G. Bristow, J. Peacock, A. Jang, J. Kim, R. P. Hill and S. Benchimol, Resistance to DNA-damaging agents is discordant from experimental metastatic capacity in MEF ras-transformants-expressing gain of function MTp53. Oncogene 22, (2003). 36. O. V. Belyakov, A. M. Malcolmson, M. Folkard, K. M. Prise and B. D. Michael, Direct evidence for a bystander effect of ionizing radiation in primary human fibroblasts. Br J Cancer 84, (2001). 37. C. L. Limoli and J. F. Ward, A new method for introducing double-strand breaks into cellular DNA. Radiat Res 134, (1993). 38. C. P. Rubbi and J. Milner, Non-activated p53 co-localizes with sites of transcription within both the nucleoplasm and the nucleolus. Oncogene 19, (2000). 39. T. L. Tan, J. Essers, E. Citterio, S. M. Swagemakers, J. de Wit, F. E. Benson, J. H. Hoeijmakers and R. Kanaar, Mouse Rad54 affects DNA conformation and DNA-damage-induced Rad51 foci formation. Curr Biol 9, (1999). 40. S. Gangopadhyay, F. Jalali, D. Reda, J. Peacock, R. G. Bristow and S. Benchimol, Expression of different mutant p53 transgenes in neuroblastoma cells leads to different cellular responses to genotoxic agents. Exp Cell Res 275, (2002). 41. J. D. Dignam, R. M. Lebovitz and R. G. Roeder, Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11, (1983). 42. G. Matlashewski, P. Lamb, D. Pim, J. Peacock, L. Crawford and S. Benchimol, Isolation and characterization of a human p53 cdna clone: expression of the human p53 gene. Embo J 3, (1984). 43. E. A. Komarova, C. R. Zelnick, D. Chin, M. Zeremski, A. S. Gleiberman, S. S. Bacus and A. V. Gudkov, Intracellular localization of p53 tumor suppressor protein in gamma-irradiated cells is cell cycle regulated and determined by the nucleus. Cancer Res 57, (1997). 79
96 44. L. E. Smith, S. Nagar, G. J. Kim and W. F. Morgan, Radiation-induced genomic instability: radiation quality and dose response. Health Phys 85, (2003). 45. R. Bernardi and P. P. Pandolfi, Role of PML and the PML-nuclear body in the control of programmed cell death. Oncogene 22, (2003). 46. D. W. Chan, B. P. Chen, S. Prithivirajsingh, A. Kurimasa, M. D. Story, J. Qin and D. J. Chen, Autophosphorylation of the DNA-dependent protein kinase catalytic subunit is required for rejoining of DNA double-strand breaks. Genes Dev 16, (2002). 47. R. Carbone, M. Pearson, S. Minucci and P. G. Pelicci, PML NBs associate with the hmre11 complex and p53 at sites of irradiation induced DNA damage. Oncogene 21, (2002). 48. N. H. Chehab, A. Malikzay, E. S. Stavridi and T. D. Halazonetis, Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Natl Acad Sci U S A 96, (1999). 49. S. A. Klibanov, H. M. O'Hagan and M. Ljungman, Accumulation of soluble and nucleolar-associated p53 proteins following cellular stress. J Cell Sci 114, (2001). 50. J. Wolcke, M. Reimann, M. Klumpp, T. Gohler, E. Kim and W. Deppert, Analysis of p53 "latency" and "activation" by fluorescence correlation spectroscopy. Evidence for different modes of high affinity DNA binding. J Biol Chem 278, (2003). 51. K. McKinney, M. Mattia, V. Gottifredi and C. Prives, p53 Linear Diffusion along DNA Requires Its C Terminus. Mol Cell 16, (2004). 52. I. B. Nazarov, A. N. Smirnova, R. I. Krutilina, M. P. Svetlova, L. V. Solovjeva, A. A. Nikiforov, S. L. Oei, I. A. Zalenskaya, P. M. Yau, et al., Dephosphorylation of histone gamma-h2ax during repair of DNA double-strand breaks in mammalian cells and its inhibition by calyculin A. Radiat Res 160, (2003). 53. E. J. Perkins, A. Nair, D. O. Cowley, T. Van Dyke, Y. Chang and D. A. Ramsden, Sensing of intermediates in V(D)J recombination by ATM. Genes Dev 16, (2002). 54. A. L. Okorokov, L. Warnock and J. Milner, Effect of wild-type, S15D and R175H p53 proteins on DNA end joining in vitro: potential mechanism of DNA doublestrand break repair modulation. Carcinogenesis 23, (2002). 55. J. R. Alt, A. Bouska, M. R. Fernandez, R. L. Cerny, H. Xiao and C. M. Eischen, Mdm2 binds to Nbs1 at sites of DNA damage and regulates double strand break repair. J Biol Chem 280, (2005). 56. B. Jakob, M. Scholz and G. Taucher-Scholz, Characterization of CDKN1A (p21) binding to sites of heavy-ion-induced damage: colocalization with proteins involved in DNA repair. Int J Radiat Biol 78, (2002). 57. D. B. Yap, J. K. Hsieh, S. Zhong, V. Heath, B. Gusterson, T. Crook and X. Lu, Ser392 Phosphorylation Regulates the Oncogenic Function of Mutant p53. Cancer Res 64, (2004). 58. W. Bruins, E. Zwart, L. D. Attardi, T. Iwakuma, E. M. Hoogervorst, R. B. Beems, B. Miranda, C. T. Van Oostrom, J. Van Den Berg, et al., Increased Sensitivity to UV Radiation in Mice with a p53 Point Mutation at Ser389. Mol Cell Biol 24, (2004). 80
97 59. A. Tritarelli, E. Oricchio, M. Ciciarello, R. Mangiacasale, A. Palena, P. Lavia, S. Soddu and E. Cundari, p53 localization at centrosomes during mitosis and postmitotic checkpoint are ATM-dependent and require serine 15 phosphorylation. Mol Biol Cell 15, (2004). 81
98 Figure Legends Figure 2.1. p53 Ser15 nuclear foci form following genotoxic insult from a preexisting p53 pool and form in response to DNA breaks and base damage. (A) G0/G1-phase NDF-GM05757 lysates from non-irradiated (NIR) and irradiated (IR; 3 hours post-10gy) cultures immunoprecipitated with phospho-specific p53 antibodies and followed by Western blotting using a pantropic p53 (Ab-7) antibody. All p53 bands were distinct from the IgG control. (B) Western blot analysis of p53 Ser15 induction within G0/G1-phase NDF-GM05757 but not in AT-GM05823 fibroblasts. (C) Representative confocal images of G0/G1-phase NDF-GM05757 cells stained at 3 hours post-10gy for: pantropic p53 (Ab1801 and DO-1; both red), p53 Ser15 (green) and nucleolin (red), and p53 Ser15, γ- H2AX, ATM Ser1981, DNA-PKcs Thr2609 and BRCA1 (all green). All cells were counterstained with DAPI for nuclear DNA (blue). Bar indicates 5μm. (D) p53 Ser15 foci formation in G0/G1-phase NDF-GM05757 fibroblasts at 30 minutes post-10gy is similar in the presence of cycloheximide (30μg/ml) as compared to vehicle-alone (ethanol) treated cells. Bar indicates 20μm. (E) Time- and dose-dependency of protein expression of p53 Ser15, p21 WAF, γ-h2ax and actin (loading control) in irradiated G0/G1-phase NDF-GM05757 fibroblasts using Western blot analyses. (F) The percent of G0/G1-phase NDF-GM05757 cells positive for p53 Ser15 or γ-h2ax foci at 3 hours as a function of radiation dose. 82
99 (G) Summary of data in which cells were treated with a variety of DNA damaging agents and then observed at periods from 30 to 360 minutes following treatment for evidence of p53 Ser15 foci. The data are consistent with p53 Ser15 foci being maximally induced by DNA breaks and DNA base damage (i.e. bleomycin, H 2 O 2, hydroxyurea and MMS)(20) in an ATM-dependent manner (top panel). Chart Legend: +++ dim-to-bright, intermediate-to-large sized foci in more than 80% of cells; ++ dim-to-bright, intermediate-to-large sized foci in 50% - 80% of cells; + dim-to-bright, intermediate-to-large sized foci in 10% - 50% of cells; +/ dim-to-bright, intermediate-to-large sized foci in less than 10% of cells; no p53 Ser15 foci, comparable to untreated cells. Representative confocal images of p53 Ser15 foci in G0/G1-phase NDF-GM05757 at 3 hours following bleomycin (0.06U/ml; 3 hours) and UV (25J/m 2 ). Bar indicates 10μm (bottom panel). Figure 2.2. IR-induced p53 Ser15 foci are a chromatin-bound sub-pool of total p53 protein. (A) G0/G1-phase NDF-GM05757 cells stained for p53 Ser15 at 3 hours post-10gy with or without pre-extraction of soluble proteins or treatment with RNaseA or DNaseI as denoted. Top panel: cells are co-stained for p53 Ser15 (green) and γ-h2ax (red). Bottom panel: total p53 protein (red) detected using Ab1801 antibodies. The extent 83
100 of each cell nucleus is outlined based on other images of the same cell stained for DAPI (DNA). Bar indicates 10μm. (B) Western blot analyses of the cytoplasmic, nuclear and chromatin-bound cellular fractions of asynchronously-growing NDF-GM05757 cells pre- (NIR) and 2 hours post-10gy (IR) of irradiation. p84 protein was used as a positive control for nuclear protein and γ-h2ax as a positive control for chromatin-bound protein following irradiation. (C) Immunodepletion analyses of G0/G1-phase NDF-GM05757 lysates pre- and post-irradiation (20Gy). From these two lysates, total p53 (Total p53) and p53 Ser15 (Depleted p53 Ser15 ) were immunoprecipitated with a cocktail of anti-p53 antibodies or p53 Ser15 antibody, respectively. Following p53 Ser15 -immunoprecipitation, the supernatant was subjected to a subsequent immunoprecipitation with the p53 antibody cocktail to determine the amount of remaining total p53 (Remaining p53) after p53 Ser15 -depletion. Immunoprecipitated protein was detected by Western blot using pantropic p53 (Ab-7). Figure 2.3. p53 Ser15 foci dependency and association with ATM, DNA-PKcs, γ- H2AX and the MRN complex. (A) Representative confocal images of p53 Ser15 (green) and γ-h2ax (red) foci formation in G0/G1-phase NDF-GM05757, NBS-GM07166 and 180BR (DNA Ligase IV-deficient) fibroblasts at 3 hours following 10Gy. Micronuclei containing colocalized p53 Ser15 and γ-h2ax (yellow) are denoted by arrows in inset panel. No p53 Ser15 foci are observed following pre-treatment of NDF-GM05757 cells with the 84
101 PI3K-inhibitor Wortmannin or in irradiated AT-GM05823 fibroblasts. For NDF- GM05757 strains, nuclei are also counter-stained with DAPI to delineate nuclear DNA. Bar indicates 20μm. (B) The percentage of G0/G1-phase NDF-GM05757, AT-GM05823, NBS-GM07166 and 180BR cells with more than two p53 Ser15 foci over time post-irradiation showing a dose-response in the NDF cells. Right panel shows deficient or delayed foci formation in AT or NBS cells and increased endogenous and late residual foci formation in DNA Ligase IV-deficient 180BR cells. (C) Representative confocal images of G0/G1-phase NDF-GM05757 nuclei costained with p53 Ser15, γ-h2ax and 53BP1at 3 hours following 10Gy. (D) Co-staining of p53 Ser15, RAD50 and MRE11 was conducted at 6 hours following 10Gy. Co-localization between foci in merged images appears as yellow foci. The dotted line outlines the cells nuclei based on DAPI-DNA staining. Bar indicates 5μm. Co-localization was confirmed with (i) fluorescent intensity profiles (see coincident red-green intensity profiles within nuclear regions traversed by white line) (middle panel) and (ii) separate calculations of Pearson correlation coefficients for colocalization (rp) based on the whole nucleus (bottom panel). Control data for the colocalization of the MRN complex is shown in Supplementary Figure 2.3. (E) G0/G1-phase NDF-GM05757 nuclei co-stained with either γ-h2ax and DNA- PKcs Thr2609 (1hr following 1Gy) or γ-h2ax and p53 Ser15 (3 hrs following 2Gy). Left three panels are single confocal sections. Right single panels are three-dimensional 85
102 rendered reconstructed from Z-stacks of confocal sections. Yellow voxels indicate volumes within foci that are co-localized. Bar indicates 2μm. (F) The percentage of 53BP1 foci co-localized with γ-h2ax and p53 Ser15 foci in G0/G1-phase NDF-GM05757 nuclei at 3 hours following 10Gy. (G) The percentage of cells with more than two co-localized p53 Ser15 /RAD50 foci as a function of time and dose in G0/G1-phase NDF-GM05757 nuclei. (H) Confirmation of biochemical interaction between p53 Ser15 and γ-h2ax using coimmunoprecipitation-western blot analyses in G0/G1-phase NDF-GM05757 or HCT116-p53 +/+ or HCT116-p53 -/- cells (left and middle panels). All bands detected were specific and distinct from the IgG and beads-only controls. Western blot of p53 Ser15 and γ-h2ax expression in HCT116 p53 +/+ and HCT116 p53 -/- cell lysates are also shown (right panel). Figure 2.4. p53 Ser15 nuclear foci correlates with DNA-dsb rejoining and are resistant to proteolytic degradation. (A) The mean number of γ-h2ax foci per 1.8μm confocal section in G0/G1-phase NDF-GM05757 cells as a function of time and dose (2 or 10 Gy). (B) The mean number of p53 Ser15 foci per 1.8μm confocal section in G0/G1-phase NDF-GM05757 cells as a function of time and dose (2 or 10 Gy). (C) Biochemical DNA-dsb rejoining kinetics in G0/G1-phase NDF-GM05757 fibroblasts using continuous field gel electrophoresis, plotted as the fraction of DNAdsbs remaining (FDR) versus time. Note: ~5-10% of initial DNA-dsbs remaining at 24 hours post-irradiation. 86
103 (D) Quantitation of the mean number of p53 Ser15 nuclear foci in G0/G1-phase NDF- GM05757 cells over 24 hours in the presence of the 26S proteasome inhibitor, MG132, as compared to vehicle-alone control (DMSO)-treated cells. Figure 2.5. Lack of Ser15-phosphorylation leads to altered DNA-dsb rejoining, foci formation and γ-h2ax association following IR-induced DNA damage. (A) Biochemical DNA-dsb rejoining in asynchronous isogenic p53 WT/WT or p53 Ser18Ala/ Ser18Ala MEFs as determined from the COMET assay expressed as the fraction of DNA-dsbs released (FDR) over time following 20Gy. (B) Representative confocal images of HCT116-p53 -/- cells transiently transfected with YFP alone (control), YFP-p53 WT and YFP-p53 Ser15Ala fusion constructs pre- and post-irradiation (3 hours following 10Gy). Bar indicates 5μm. (C) Western blot analyses of lysates pre- and post-irradiation (3 hours following 5Gy) from HCT116-p53 +/+ cells transiently transfected with YFP-p53WT and YFPp53Ser15Ala. (D) Co-immunoprecipitation analyses using YFP and γ-h2ax antibodies to detect YFP-p53 fusion proteins in complex with γ-h2ax pre- and post- 5 Gy (3hrs). Negative controls of YFP-vector expressing cells, and IgG alone immunoprecipitations, showed no interaction with γ-h2ax (data not shown). 87
104 Figure 2.6. Interaction in vivo between p53 Ser15, γ-h2ax, ATM Ser1981 and DNA- PKcs Thr2609 at DNA breaks. (A) G0/G1-phase NDF-GM05757 nuclei after 30 minutes of recovery following subnuclear microbeam irradiation with 100-targeted helium ions. Confocal images in both X-Y and Y-Z planes confirmed focal co-localizations within targeted nuclear volumes between γ-h2ax/53bp1 and p53 Ser15 /ATM. Bar indicates 5μm. Wide field immunofluorescent microscope images of asynchronous NDF-GM05757 nuclei at various times following UV laser microbeam irradiation showing the colocalization between: (B) γ-h2ax, DNA-PKcs Thr2609 and ATM Ser1981 at sites of DNA-dsbs, as detected by TUNEL; (C) DNA-PKcs Thr2609 with ATM Ser1981, and p53 Ser15 with DNA-PKcs Thr2609 ; and (D) p53 Ser15 with γ-h2ax and ATM Ser1981. The arrows indicate the traversal of the UV laser beam. In similar experiments, both ATM Ser1981 and p53 Ser15 staining was absent in AT-GM05823 (data not shown). Bar indicates 10μm. (E) Proposed model for p53 Ser15 in IR-induced DNA damage sensing Within minutes following cellular irradiation, DNA damage sensing and repair proteins are recruited to sites of DNA damage. Both ATM and DNA-PKcs undergo auto-phosphorylation in response to DNA-breaks leading to phosphorylation of H2AX. Upon binding to damaged chromatin, ATM directly phosphorylates p53 Ser15, 53BP1 and CHK2 Thr68 close to or within chromatin sites containing γ-h2ax for varying time intervals. Subsequent p53-mediated transactivation can occur in a pannuclear fashion at a distance from damaged DNA. Phosphorylation of Ser15 on p53 88
105 also blocks MDM2-mediated p53 degradation and nuclear export allowing for increased nuclear p53 Ser15 concentration to facilitate optimal DNA damage signal transduction within the nucleus. Cells that are defective in DNA-dsb rejoining or have residual DNA-dsb 24 hours following irradiation show residual p53 Ser15 foci as an indicator of persistent DNA damage and genetic instability. 89
106 Supplementary Figure Legends Suppl. Figure 2.1. p53 Ser15 foci form in response to DNA breaks and base damage (A) Top panel: Representative confocal images of p53 Ser15 foci and total p53 (as detected byab1801 pantropic p53 antibody) induction in G0/G1-phase NDF- GM05757 fibroblasts at 3 hours following exposure to IR (10Gy) and UV (25J/m 2 ). Bar indicates 10μm. Bottom panel: Western blot analyses of G0/G1-phase NDF-GM05757 cells at various times following UV-irradiation (20J/m 2 ). In comparison to ionizing irradiation, p53 Ser15 and total p53 accumulation is delayed in UV-irradiated G0/G1 fibroblasts (e.g. maximal at 16 hours). (B) Analysis of theoretical versus experimental values of total p53 (Ab1801) and p53 Ser15 foci co-localization, in G0/G1 NDF-GM05757 fibroblasts. The individual number of p53 Ser15 or Ab1801 foci per nuclear area (μm 2 ) within a confocal section was initially determined in 10 to 15 cells at either 3 or 24 hours following 10Gy. This represented the probability of a focus being observed within the nucleus for a given time. The theoretical probability that p53 Ser15 and Ab1801 foci colocalize randomly is the product of these two individual values. Subsequently, the actual number of p53 Ser15 and Ab1801 foci that were observed to be co-localized per nuclear area (μm 2 ), was determined as the experimental co-localization. If colocalization is not random, the experimental number of co-localized foci should be much greater than the calculated theoretical probability. The experimental probability (2x10-4 ) of co-localization was indeed two orders magnitude greater than the 90
107 calculated theoretical probability (6.9x10-6 or 1.1x10-6 ). These calculations support the conclusion that p53 Ser15 and Ab1801 foci co-localization is not random. Suppl. Figure 2.2. To study p53 Ser15 as a transient sub-pool of total nuclear p53, qualitative and quantitative staining for total p53 and p53 Ser15 foci was determined following 10Gy irradiation. (A) Left panel: a representative confocal image of a NDF-GM05757 nucleus stained for total p53 (Ab1801-red) and p53 Ser15 (green) at 24 hours post-10gy. Bar indicates 5μm. Middle panel: a representative focus with p53 Ser15 co-localizing with Ab1801 (observed as yellow foci) and is associated with coincident peaks of fluorescent intensity over a 3μm distance (encircled in white within the nucleus). The DNA-signal (blue) remains invariant. Right panel: a separate analyses of a representative plot of the Pearson correlation coefficient calculated for the whole nucleus shown in the left panel confirming a positive correlation, thus significant p53 Ser15 and Ab1801 foci co-localization (see Methods). (B) A significant and non-random co-localization was also observed by determining that between 20-25% of p53 Ser15 foci with Ab1801- or FL393-detected p53 foci at 24 hours post-10gy as opposed to 0% in non-irradiated cultures. 91
108 Suppl. Figure 2.3. (A) Western blot analyses of asynchronously growing 180BR (DNA Ligase IVdeficient cells with a defect in DNA-dsb rejoining) fibroblasts at various times following 10Gy of irradiation. Similar to p53 Ser15 foci, high levels of initial and sustained residual p53 Ser15 protein levels were observed pre- and post-irradiation. (B) Representative confocal images of positive co-localization of RAD50 and NBS1 in G0/G1-phase NDF-GM p53 Ser15 foci can form in the absence of an intact MRN complex in G0/G1-phase NBS-GM07166 fibroblasts as shown by p53 Ser15 and RAD50 (lack of foci formation due to absence of NBS1 expression) staining at 6 hours following 10Gy. Bar indicates 5μm. Also shown are the two separate analyses of the corresponding fluorescent intensity profiles and the Pearson correlation coefficient plots. (C) Western blots of RAD50, NBS1 and α-tubulin (loading control) expression in G0/G1-phase NDF-GM05757 and NBS-GM07166 fibroblasts confirm the lack of NBS1 expression in the NBS-GM07166 strain (5). (D) RAD50 foci formation in G0/G1-phase NDF-GM05757 cells is dose-responsive at 6 hours post-irradiation. RAD51 foci are not dose-responsive in similar-treated cells (12). (E) p53 Ser15 and PML kinetics in irradiated G0/G1-phase NDF cells. Left panel: quantitative confocal microscopy revealed the presence of 2 or more endogenous PML foci in % of G0/G1-phase NDF-GM05757 cells pre- or postirradiation. The number of PML foci per confocal section increased minimally over 24 hours post-irradiation. 92
109 Middle panel: a representative confocal image and a low Pearson s correlation coefficient analysis both confirming that p53 Ser15 and PML foci co-localize minimally. Bar indicates 5μm. Right panel: the percentage of p53 Ser15 and PML co-localized foci is consistent with minimal co-localization as a function of time following irradiation. These data are in stark contrast to the dose and time dependent kinetics of γ-h2ax and p53 Ser15 foci over a period of 24 hours (refer to Figures 2.1E, and 2.4A and 2.4B). 93
110 Figure A Ser6 Ser9 NIR IR NIR IR Ser15 Ser20 Ser46 NIR IR NIR IR NIR IR B Ser392 IgG GM05757 GM05823 NIR IR IR NIR IR NIR IR C D Ab1801 DO-1 p53 Ser15 p53 Ser15 + nucleolin Ethanol-control γ-h2ax ATM Ser1981 DNA-PKcs Thr26 09 BRCA1 Cycloheximide E G p53 Ser15 p21 γ-h2ax Actin NIR 10 mins 10Gy 30 mins 3 hrs 24 hrs NIR 3hrs 2 Gy 10 Gy F Foci positive nuclei (%) p53 Ser15 γ-h2ax Dose (Gy) Agent IR UV Bleomycin MMS MMC H 2 O 2 Hydroxyurea DMSO GM / GM /- +/- - ND - ND ND - UV Bleomycin p53 Ser15 +DAPI p53 Ser15 +DAPI 94
111 Figure 2.22 A Non-extracted Pre-extracted Pre-extracted + RNase treated Pre-extracted + DNase treated B C CYT NUC CHROM NIR-24hrs 20Gy-24hrs Total p53 p53 Ser15 γ-h2ax p84 NIR IR NIR IR NIR IR 20 Total p53 Depleted p53 Ser15 Remaining p53 Total p53 Depleted p53 Ser15 Remaining p53 Densitometry
112 Figure A GM GM GM GM05823 GM BR NIR IR IR+Wortmannin IR IR IR B C Foci positive nuclei (%) GM05823, 10Gy GM05757, 2Gy GM07166, 10Gy GM05757, 10Gy 180BR, 10Gy Time after irradiation (hrs) D 24 γ-h2ax p53 Ser15 p53 Ser15 p53 Ser15 p53 Ser15 53BP1 γ-h2ax 53BP1 RAD50 MRE11 Merge Merge Merge Merge Merge r p X axis shift (μ m) 96
113 Figure 42.4 A Mean # of foci per confocal section γ-h2ax, 2Gy p53 Ser15, 2Gy γ-h2ax, 10Gy p53 Ser15, 10Gy Time after irradiation (hrs) B C D Fraction of DNA -dsbs released (FDR) Time after irradiation (hrs) Mean # of foci per confocal section DMSO MG Time post-10gy (hrs) 97
114 Figure A B Fraction of DNA -dsbs remaining (FDR) p53 WT p53 Ser18Ala/S er18ala Time after irradiation (hrs) YFP YFP-p53 WT YFP-p53 S15A NIR IR C D Transfection: YFP-p53 YFP-S15A NIR IR NIR IR Transfection: IP: YFP YFP-p53 YFP-S15A IP: γ-h2ax YFP-p53 YFP-S15A YFP γ-h2ax WB: YFP NIR IR NIR IR NIR IR NIR IR p21 WAF 98
115 Figure A B γ-h2ax X 53BP1 Merge Z Merge γ-h2ax TUNEL γ-h2ax DAPI Y 10mins TUNEL ATM Ser1981 DAPI p53 Ser15 ATM Merge Merge Y 10mins γ-h2ax DNA- PKcs Thr2609 DAPI 10mins C DNA- PKcs Thr2609 ATM Ser1981 DAPI D p53 Ser15 γ-h2ax DAPI 10mins p53 Ser15 DNA- PKcs Thr2609 DAPI 1hr p53 Ser 15 DAPI ATM Ser mins 1hr E CHK2 p53 53BP1 ATM Ser1981 γ-h2ax PP2A ATM Thr26 09 DNA-PKcs Ku70 Ku80 MRN DNA break induction (e.g. IR) Downstream target signalling (e.g. p21 WAF ) and DNA repair CHK2 Thr68 p53 Ser15 53BP1 ATM S er1981 γ-h2ax DNA-PKcs Thr2609 Ku70 Ku80 MRN 99
116 Suppl. Figure A Untreated IR Untreated IR UV p53 Ser15 p53 Ser15 Ab1801 Ab1801 Ab1801 Merge Merge Merge Merge Merge G0/G1 GM J/m 2 NIR 0.5hr 1hr 3hrs 6hrs 16hrs 24hrs p53 Ser15 Actin B Analysis of theoretical versus experimental values of total p53 (Ab1801) and p53 Ser15 foci co-localization NIR-3hr 10Gy-3hr 10Gy-24hr Number of foci per nuclear area (μm 2 ) Experimental Theoretical p53 Ser15 foci 1801 foci Co-localized foci Co-localized foci X X
117 Suppl. Figure 2.22 A p53 Ser15 +Ab1801 Intensity p53 Ser15 Ab1801 DAPI Distance (μm) r p X axis shift (μm) B p53 Ser15 foci co -localization (%) NIR With 1801 foci With FL393 foci 10Gy-24hrs 101
118 Suppl. Figure A 10Gy NIR 0.5hr 1hr 3hrs 6hrs 16hrs 24hrs p53 Ser15 p21 Actin B GM05757 GM07166 NBS1 p53 Ser15 RAD50 RAD50 Merge Merge 0.6 r p X axis shift (μm) C RAD50 NBS1 α-tubulin GM Gy NIR 2hrs 6hrs GM Gy NIR 2hrs 6hrs D Foci positive nuclei (%) RAD50 RAD Dose (Gy) 102
119 Suppl. Figure 32.3 E 100 Mean # of foci per confocal section Time post-10gy (hrs) r p p53 Ser15 +PML X axis shift (μm) p53 Ser15 /PML foci co-localization (%) Time post-10gy (hrs) 103
120 Chapter 3 The Carboxy-terminus of p53 and Chromatin-binding in Response to Radiation-induced DNA Damage This chapter consists of the work, The carboxy-terminus of p53 and chromatinbinding in response to radiation-induced DNA damage submitted and under review in the journal, Radiation Research 2009, by Shahnaz T Al Rashid, 1,3,4 Cindy Law, 1 Carla Coackley, 3 and Robert G Bristow 1,2,3 Departments of Medical Biophysics 1 and Radiation Oncology 2, University of Toronto, Division of Applied Molecular Oncology 3, Ontario Cancer Institute/Princess Margaret Hospital (University Health Network) 3, Toronto, Ontario, Canada M5G 2M9, and Radiation Biology Group 4, Centre for Cancer Research and Cell Biology, Queen s University Belfast, 97 Lisburn Road, Belfast, County Antrim, Northern Ireland BT9 7BL. 104
121 3.1 Abstract We have previously shown that the Ser15-phosphorylated p53 phosphoform, p53 Ser15, localizes at sites of ionizing radiation (IR)-induced DNA damage. In this study, we hypothesized that the non-specific DNA binding activity of the p53 carboxy-terminus mediates chromatin anchoring at sites of DNA damage. YFP-p53 fusion constructs expressing carboxy-terminus deletion mutants of p53 were transfected into p53-null H1299 cells to determine the role of the C-terminus in chromatin-binding pre- and post-ir, independent of transcriptional trans-activation. We observed exogenous YFP-p53 WT associated with ATM Ser1981 and 53BP1 within cellular chromatin in a dynamic manner. We confirmed that these associations also occurred between endogenous WTp53 with ATM Ser1981 and 53BP1 within chromatin. YFP-p53 Δ1-299 fusion proteins, which lack transcriptional trans-activation and the Ser15-residue, also associated within chromatin. Ser15-phosphorylation was found not to be essential for DNA damage-induced association of p53 with chromatin or with ATM Ser1981 and 53BP1. Based on these observations, we propose a model whereby a pre-existing pool of p53 responds immediately to radiation-induced DNA damage by virtue of its association with chromatin through its carboxy-terminus. 105
122 3.2 Introduction In response to IR-induced DNA damage, the ATM kinase becomes activated through auto-phosphorylation of its Ser1981 residue (ATM Ser1981 ). Optimal activation of ATM Ser1981 requires the MRN complex (consisting of the MRE11, RAD50 and NBS1 proteins) which is a critical sensor of DNA-dsbs (1, 2). Furthermore, a recent study also showed that a fraction of ATM is associated with chromatin prior to irradiation, and this interaction and ATM activation are both modulated by the nucleosome-binding protein, HMGN1 via its ability to modify chromatin (i.e. increasing levels of histone H3 acetylation)(3). Once activated, ATM Ser1981 phosphorylates the Ser15 and Ser20 residues of the tumour suppressor protein p53 resulting in the latter s stabilization and activation (4, 5) of downstream signal transduction pathways leading to DNA-dsb repair and cell cycle checkpoint enactment (1, 2). The p53 protein initiates cell cycle arrest, senescence or apoptosis, mainly through trans-activation of its downstream target genes (6). p53 trans-activation-independent mechanisms have also been described, for example in initiating mitochondrial-based apoptosis (7, 8) or during homologous recombination (HR) or non-homologous end-joining (NHEJ) (9-12). Wild type p53 (WTp53) has been shown to suppress the level of inter- and intra-chromosomal HR both in vitro and in vivo (13). In vitro enzymatic properties of WTp53 include DNA and RNA renaturation, DNA strand transfer and 3-5 exonuclease activity, and recognition of various forms of damaged DNA (14-17). Furthermore, WTp53 in vivo has been shown to interact with RAD50, MRE11, RAD51 and topoisomerase I, all of which are involved in DNA-dsb sensing, repair and HR (18-20). Song and colleagues recently 106
123 showed that MTp53 (i.e. p53 R248W and p53 R275H ) could abrogate MRN signaling by preventing MRN binding to DNA-dsbs and subsequently impairing the activation of ATM Ser1981 and downstream pathways (21). A transcription-independent function for p53 in DNA-dsb repair would require its association with chromatin during DNA damage induction and repair. Studies have shown a functional role of the p53-binding protein, 53BP1, in DNA-dsb repair and cell cycle checkpoints (as reviewed by Fitzgerald, et. al., 2009(22)). In response to IR-induced DNA damage, 53BP1 is phosphorylated by ATM and was shown to interact with the DNA-dsb marker, serine 139-phosphorylated histone H2AX (γ-h2ax, also phosphorylated by ATM) (22-24). The carboxy-terminal BRCT motifs of 53BP1 have been mapped to interact with the central domain of p53. However this in vivo interaction and its role remain to be determined. Current models propose that 53BP1 acts both upstream and downstream of ATM: as an upstream activator, 53BP1 facilitates ATM Ser1981 activation, while it is a downstream target of ATM phosphorylation (23-25). These models place p53 downstream of ATM and 53BP1 as a DNA damage signal transducer within the nucleoplasm to mediate cell cycle checkpoints or cell death (1). In our previous study, we showed that ATM-mediated phosphorylation of p53 Ser15 and p53 Ser15 -phosphoforms were found to be important for localization to IR-induced foci and sites of DNA damage within chromatin, respectively (26). This p53 Ser15 subpool was found to interact and co-localize with γ-h2ax, 53BP1, RAD50 and MRE11 107
124 proteins within the first hour of DNA-dsb induction following broad beam γ-irradiation and co-localize with γ-h2ax, ATM Ser1981 and DNA-PKcs Thr2609 (a critical component of the NHEJ repair complex) at sites of microbeam-induced sub-nuclear DNA breaks. Altogether, these results presented strong evidence of a role for a p53 Ser15 sub-pool in DNA damage localization and suggested new biology for p53 Ser15 phosphoforms at sites of DNA-dsbs in vivo. And yet conflicting evidence remains with respect to p53 subspecies being recruited to DNA breaks and the importance of the p53 Ser15 -phosphorylation in DNA damage localization and cell cycle checkpoint induction. Based on UV laser-hoechst-induced DNA-dsbs, Bekker-Jensen and colleagues proposed that ATM, 53BP1 and the MRN complex associate with γ-h2ax at sites flanking DNA-dsbs within chromatin, while the NHEJ repair complex of DNA-PKcs/KU70/KU86 exist pre-assembled on chromatin and were modified following DNA damage (25). WTp53 was not found at sites of DNA-dsbs, in contrast to our previous study (26). Based on live cell DNA repair studies of endonuclease-induced DNA-dsbs, Berkovich and colleagues proposed that the phosphorylated ATM Ser1981 and NBS1 proteins are at the DNA-dsb site, while γ-h2ax and ATM Ser1981 flanked the site of damage (2). In this latter study, Ser15-phosphorylation of p53 was evaluated as a target of ATM Ser1981 activity, but its localization within chromatin and role in damage sensing was not examined. The carboxy-terminus of p53 includes domains involved in tetramerization, regulation of sequence-specific DNA binding activity and non-specific DNA binding 108
125 activity (27). The mechanism of how the carboxy-terminus regulates the sequencespecific DNA binding activity, through either interference of sequence-specific with non-specific binding or conformational affects, remains poorly understood (27, 28). It has been suggested that the p53 continuously scans the genome by sliding on DNA (29). This non-specific DNA binding activity of p53 has been modeled according to diffusion kinetics and was found to be independent of p53 sequence-specific DNAbinding capacity, suggesting that this may be mediated through the non-specific DNA binding activity of the carboxy-terminus(30). In vitro studies have shown that the carboxy-terminus domain can bind a variety of DNA structures including ss- and ds-dna, irradiated DNA, Holliday junctions, and insertion/deletions, thus suggesting that this domain may mediate DNA damage recognition and/or repair (17, 27, 31-33). However, these studies have yet to show the role of the carboxy-terminus in directly mediating p53 localization within chromatin at sites of DNA damage in vivo versus indirect chromatin association through interactions with other chromatinbinding proteins. Whether p53 localization to chromatin is mediated through the carboxy-terminus domain to allow 53BP1 and ATM Ser1981 interactions remains to be determined. In this report, we studied exogenous p53 in a cell model system of transientlytransfected asynchronously-growing transformed p53-null H1299 human lung carcinoma cells. We present novel in vivo p53-atm-53bp1 dynamic associations within the chromatin complex pre- and post-irradiation. The carboxy-terminus is important for association of p53 with chromatin, and p53-chromatin-atm 109
126 associations are not mediated through 53BP1. Through biochemical fractionation, immunoprecipitation and immunofluorescence microscopy analyses, we show that there is a dynamic exchange of pools of p53 Ser15, ATM Ser1981 and 53BP1 interacting within chromatin prior to and following IR-induced DNA damage. This study thus reveals further nuances in the role of p53 and its carboxy-terminus in the early sensing and signaling of DNA damage. 3.3 Materials and Methods Cell Lines, Culture Conditions and Irradiation Treatments The primary human fibroblast strain NDF-GM05757 (normal diploid fibroblasts) was obtained from Coriell (USA) and cultured in α-mem media supplemented with antibiotics and 15% fetal bovine serum. G0/G1-phase NDF-GM05757 fibroblasts were synchronized using contact inhibition as previously described (26). Human lung carcinoma p53-null H1299 cells were obtained from Dr. Simon Powell (34). These cells were cultured in DMEM media supplemented with antibiotics, 20 mm HEPES, and 10% fetal bovine serum. Cultures were exposed to broad beam ionizing radiation (doses ranging from 0 to 20 Gy) using a 137 Cs- irradiator (Nordion) at ~1 Gy/minute (room temperature; aerobic conditions)(35). p53 Constructs and Transient Transfection Conditions p53 protein sub-cellular localization patterns following DNA damage were studied using immunofluorescence microscopy as previously described (26). To specifically determine whether the p53 carboxy-terminus mediates its localization to sites of 110
127 DNA damage in vivo, we expressed exogenous yellow fluorescent protein (YFP) fused to either full length p53 WT, or deletion mutants of p53 that lack various aminoor carboxy-terminal domains. Briefly, the p2516 plasmid containing the human fulllength p53 WT cdna fragment cloned into the pcdna3.1 backbone vector was used as a template for generating the p53 fusion and mutant constructs (36). The full length WTp53 fragment was amplified from p2516 plasmid DNA using the forward WT and reverse WT oligonucleotide primers, 5 -TTT TAA GCT TCG ATG GAG GAG CCG CAG TCA GA-3 and 5 -TTT TGG ATC CTC AGT CTG AGT CAG GCC C-3, respectively. To generate the amino-terminal deleted, carboxy-terminal peptide p53 Δ1-299, the forward primer 5 - TTT TAA GCT TCG CCT CAC CAC GAG CTG CC- 3 was used together with the reverse WT primer. To generate the carboxy-terminal deleted constructs, the forward WT primer was used together with the corresponding reverse primers: for p53 Δ , 5 - TTT TGG ATC CTC AGG GCA GCT CGT GGT GAG GC -3 and for p53 Δ , 5 - TTT TGG ATC CTC AGT GAG CCC TGC TCC CCC C -3. Polymerase chain reactions (PCRs) were optimized with the Platinum Pfx DNA polymerase (Gibco BRL) in a Peltier Thermal Cycler (MJ Research). The amplified fragment was ligated in-frame, fused to the carboxy-terminus of the enhanced yellow fluorescent gene (YFP) in the peyfp-c1 vector (CLONTECH Laboratories, Inc.). All plasmid clones were purified using the CONCERT High Purity Maxiprep System (Gibco BRL) and sequenced on both strands to confirm wild type and site-specific mutated status. Metafectene (Biontex) was used to transfect the H1299 p53 -/- cells 111
128 with YFP-p53 vectors as previously described (26). Transiently-transfected cells were imaged and subjected to various treatments at 16 hours post-transfection. This time was chosen for optimal expression of YFP-p53 fusion proteins and prior to any signs of transfection- and construct-mediated cytotoxicity (e.g. preclude apoptosis). Transfection efficiency for each construct was determined based on transfected cells. Wide-Field and Confocal Immunofluorescence Microscopy Intranuclear staining patterns of exogenous protein expression were visualized using wide field fluorescence microscopy. Cells were imaged using a Zeiss Axioskop inverted microscope, filter set for fluorescein isothiocyanate and phase contrast, a Retiga CCD camera (QIMAGING, Canada), and Northern Eclipse software (Empix, Canada) to acquire 8-bit images. Immunofluorescence microscopy was carried out on endogenous proteins as previously described (26). Immunofluorescent images of 1.8μm confocal sections were captured using a Zeiss LSM510 confocal microscope at a final magnification of x630. All images were prepared using the corresponding LSM510 software and exported using Adobe Photoshop software. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Total cellular RNA was isolated from cells using the Qiagen RNeasy isolation kit according to the manufacturer s recommendations, after which a Nanodrop ND-100 spectrophotometer (Peqlab Biotechnology, USA) was used to quantitate the concentration and purity of the RNA samples. The quality of RNA was also 112
129 determined by examining the integrity of the 28S and 18S rrna bands resolved in a 1% agarose gel. Experimental RNA samples and human reference RNA (Stratagene, USA) were pre-treated with DNase I (Roche, USA), to eliminate genomic DNA cross-contamination of the subsequent PCRs. The human reference RNA is a pool of RNA obtained from 10 different cell lines representing a variety of tissue types. Reverse transcription was performed using random hexamers and the TaqMan Reverse Transcription kit (Applied Biosystems, USA) as previously described (37). The following genes were assayed: 18S (endogenous control), p53, p21 WAF1, BAX, and PIDD. Each gene-specific PCR was set up in triplicate and performed in an ABI 7900 RT-qPCR machine (ABI Biosystems, USA) under the control of SDS software (ABI Biosystems, USA). Analysis was performed using the relative standard curve quantification method, as previously described (37). Values were normalized to the experimental control sample for each gene and then normalized to the values for the housekeeping gene, 18S, for each sample. This method was used to obtain a logarithmic fold change in target gene expression between the control and experimental samples when normalized to a housekeeping gene whose RNA levels do not vary significantly under all experimental conditions. All p53-responsive genes were compared at similar levels of each construct transcribed and normalized to the housekeeping 18S rrna gene transcription (endogenous control). Biochemical Fractionation, Immunoprecipitation and Western Blot Analyses Cytoplasmic, nuclear and chromatin-bound cellular fractions were isolated using a modified Dignam method (38). Briefly, cells were lysed and incubated for 5 minutes 113
130 on ice, in cytoplasmic buffer (25mM KCl, 5mM MgCl 2, 10mM Tris-HCl ph8.0, 0.5% Nonidet P-40, 1mM dithiothrietol, 1x protease inhibitors (Cθmplete EDTA-free, Roche), 1x phosphatase inhibitors (Cocktail Set II, Calbiochem)). Lysates were centrifuged at 3000 rpm for 5 minutes and the supernatant (cytoplasmic fraction) was separated. The nuclear pellet was rinsed three times with cytoplasmic buffer, then resuspended in nuclear buffer (10mM Tris-HCl ph8.0, 500mM NaCl, 0.1% Nonidet P-40, 5mM EDTA, 1x protease inhibitors (Cθmplete EDTA-free, Roche), 1x phosphatase inhibitors (Cocktail Set II, Calbiochem)). The nuclei were lysed by vigorous pipetting, vortexing for 5 minutes and incubated on ice for 15 minutes. The nuclear fraction was centrifuged at rpm for 15 minutes to pellet the chromatin (insoluble nuclear fraction) from the supernatant (soluble nuclear fraction). The chromatin pellet was rinsed three times with nuclear buffer then resuspended and subjected to 20 pulses of sonication (Branson Sonifier 450, 60Hz) to shear the DNA and solubilize the chromatin-bound fraction. Immunoprecipitation followed by western blot analyses were performed as previously described (26). Briefly, all lysates in nuclear buffer were pre-cleared with Ig isotype antibody for 1 hour, incubated with primary antibody (1μg of antibody per 1mg of protein lysate) overnight, and incubated with Protein G-beads for 1 hour, all kept at 4 0 C. Finally, beads were washed four times with nuclear buffer and prepared for electrophoresis and western blot analyses. For western blot analyses, NOVEX 4-12% Bis-Tris PAGE (Invitrogen) and LI-COR imaging systems were used according to the manufacturer s specifications (Odyssey Infrared Imaging System). Actin was 114
131 used as a loading control for whole cell lysates. H2AX was used as a positive and loading control for chromatin-bound fractions (Supplementary Figure 3.1). Levels of total exogenous p53 were detected using either the YFP-specific antibody or a pool of p53-specific antibodies (Total p53: pool of DO-1, Ab421, Ab1801, BP53-12 and p53 Ser15 ; data not shown). The specificity of p53 antibodies to detect total and phospho-specific endogenous and exogenous p53 species within p53 WT - and p53- null cells, as well as ATM-null fibroblasts (which lacked early p53 Ser15 - phosphorylation), was confirmed in our previous study (26). Antibodies used in this study include: DO-1 (amino-terminus-specific p53), Ab421 (carboxy-terminus-specific p53), and p21 WAF (Ab-1) from Calbiochem; Ab1801 (amino-terminus-specific p53) from Novocastra; polyclonal and monoclonal phospho-specific p53 Ser15 from Cell Signaling; polyclonal H2AX, and polyclonal and monoclonal γ-h2ax from Upstate Biotechnology; monoclonal phospho-specific ATM Ser1981 from Rockland Immunochemicals; BP53-12 (pantropic p53) from Santa Cruz; polyclonal actin from Sigma; monoclonal 53BP1 from Chemicon; polyclonal 53BP1 from Alexis; monoclonal GFP (recognizes YFP-variant) from BD Biosciences; and rabbit and mouse IgG control antibodies from Jackson Immunoresearch Labs. 3.4 Results Sub-cellular expression and localization of exogenous YFP-p53 fusion proteins The p53-null H1299 human lung carcinoma cell line was utilized to study exogenously-expressed carboxy-terminus deletion constructs of the human full 115
132 length p53 protein, which were fused to the carboxy-terminal end of the yellow fluorescent protein, YFP (Figure 3.1a and 3.1b). At 16 hours post-transfection, we characterized the cellular distribution of the YFP-alone or YFP-p53 fusion proteins using wide-field fluorescence microscopy as summarized in Figure 3.1b. Transfection efficiency ranged from 22% - 50% for all YFP and YFP-fusion constructs (inset of Figure 3.1b). The YFP-p53 WT transfection efficiency was lowest (~22%) presumably due to induction of cell death in the p53-null H1299 cells as we have previously reported (39). The control YFP-alone protein was observed to be diffuse throughout the cell (Figure 3.1b). There was nuclear accumulation of the YFP-p53 Δ1-299 rendered by the carboxy-terminus domain harboring its major nuclear localization signals (NLSs)(Figure 3.1b). Both nuclear and cytoplasmic localization of the YFP-p53 Δ fusion proteins were observed (Figure 3.1b). Nuclear accumulation of YFP-p53 WT was observed (Figure 3.1b) consistent with its stabilization in response to the cellular stress of transfection as previously described (40). We conclude that cellular distribution of the YFP-p53 fusion proteins are unaffected by the YFP moiety and reflect the activity and are directed by the p53 protein moiety. Phosphorylation status of the YFP-p53 fusion proteins within the cytoplasmic and nuclear compartments It is unknown whether Ser15-phosphorylation depends on the presence or absence of conserved domains or occurs in specific sub-cellular compartments (e.g. the basic carboxy-terminus domain, nuclear localization, tetramerization, and non-specific 116
133 DNA binding). Thus, cellular fractions were isolated and confirmed with cytoplasmic YFP marker and insoluble nuclear/chromatin-bound fraction H2AX marker (Supplementary Figure 3.1). We found that all exogenously-expressed YFP-p53 fusion proteins were Ser15-phosphorylated (with the exception of the YFP-p53 Δ1-299 which lacked the Ser15 residue), and Ser15-phosphoforms localized throughout the cytoplasmic, soluble nuclear and chromatin-bound fractions of the cell (Figure 3.1c). The largest proportion of Ser15-phosphorylated YFP-p53 WT was found within the soluble nuclear fraction. As compared to the fraction of chromatin-bound Ser15- phosphorylated YFP-p53 WT, higher levels of Ser15-phosphorylated YFP-p53 Δ (that lacks the carboxy-terminus non-specific DNA binding domain, NSDBD) were observed to be chromatin-bound (Figure 3.1c). Transcriptional activity of the YFP-p53 fusion proteins We next determined the transcriptional activity of the YFP-p53 fusion proteins using quantitative RT-PCR at 16 hours following transfection (Figure 3.2). Expression of YFP-p53 WT led to increased transcription of p53-downstream target genes, including p21 WAF, BAX and PIDD. This was not observed in cells expressing the YFP alone and the carboxy-terminus peptide YFP-p53 Δ1-299 (Figure 3.2). Interestingly, expression of the YFP-p53 Δ fusion protein, which lacked the non-specific DNA binding domain, was able to trans-activate one of the downstream target genes, p21 WAF, albeit to a lesser degree than YFP-p53 WT (Figure 3.2). Therefore, the p53 transcriptional activity of the YFP-p53 WT fusion protein is functional, while it is partially or fully deficient in the p53 Δ and p53 Δ1-299 fusion proteins, respectively. 117
134 Expression of YFP-p53 C-terminal deletion mutant fusion proteins does not alter DNA damage-induced phosphorylation of p53 We examined the IR-induced DNA damage activation of the ATM-p53 phosphorylation cascade by Western blot analyses of whole cell lysates collected at various times post-ir following 16 hours post-transfection (Figure 3.3a). Irradiation did not induce increased YFP expression in the control YFP-alone transfected cells (Figure 3.3a). YFP-p53 WT protein was stabilized and levels were increased by 6 hours post-ir. The levels of YFP-p53 Δ1-299 and YFP-p53 Δ remained relatively unchanged following irradiation. Ser15-phosphorylation of both YFP-p53 WT and YFP-p53 Δ was induced within 2 hours and remained unchanged up to 6 hours post-ir (Figure 3.3a). Chromatin-bound levels of DNA damage-induced phosphoforms of p53, ATM Ser1981 and 53BP1 We next assessed chromatin-bound levels of total p53, p53 Ser15 -phosphoforms, ATM Ser1981 and γ-h2ax proteins, following DNA damage (Figure 3.3b and 3.3c). Figure 3.3b displays the kinetics of total p53, p53 Ser15, ATM Ser1981 and γ-h2ax protein levels within chromatin pre-and post-irradiation. In response to irradiation, increased Ser15-phosphoform levels of YFP-p53 WT and YFP-p53 Δ were observed within 30 minutes and total p53 levels were stabilized within 6 hours. Of note, levels of YFP-p53 Δ1-299 protein were observed to increase within 30 minutes 118
135 post-ir. This suggests that the carboxy-terminus can mediate chromatin-binding of the p53 protein in response to IR-induced DNA damage. Interestingly, YFP-p53 WT transfectants expressed significantly higher levels of ATM Ser1981 and γ-h2ax than in YFP-p53 Δ1-299 and YFP-p53 Δ transfectants (Figure 3.3b). This suggests that the chromatin-association of these constructs may affect the ATM Ser1981 -γ-h2ax DNA damage sensing and signaling pathway. The dose-dependency of chromatin-bound γ-h2ax (consistent with DNA-dsb damage) was determined at 30 minutes post-ir and was observed in all transfectants (Figure 3.3c). Consistently higher levels of γ-h2ax induction was also observed in the YFP-p53 WT transfectants compared to those in the YFP-p53 Δ1-299 and YFP-p53 Δ transfectants (Figure 3.3c). YFP-p53 fusion proteins interact with 53BP1 within the cytoplasm, soluble nuclear and chromatin-bound fractions, independently of DNA damageinduction Sequential co-immunoprecipitation analyses were used to determine the interactions of YFP-p53 fusion proteins with the activated ATM Ser1981 kinase and 53BP1 proteins within cytoplasmic, soluble nuclear and insoluble nuclear (chromatin-bound) fractions. A schematic of the cell treatments, biochemical fractionation and use of the sub-cellular fractions is shown in Figure 3.4a. 119
136 Consistent with previous findings (41), the YFP-p53 Δ1-299 fusion protein did not coimmunoprecipitate with 53BP1 in any of the sub-cellular fractions since it lacks the 53BP1-interaction domain which maps within the p53 core region (Figure 3.4b, 3.4c and 3.4d). This indicates that p53 chromatin-binding pre- and post-ir does not require 53BP1, and that the carboxy-terminus of p53 (containing the non-specific DNA-binding domain) can mediate direct binding of p53 within chromatin. Based on reciprocal co-immunoprecipitations, both the YFP-p53 WT and YFP-p53 Δ fusion proteins interact with endogenous 53BP1, within all sub-cellular fractions, pre- and post-irradiation (Figure 3.4b, 3.4c, 3.4d, 3.5 and 3.6a). Thus, p53-53bp1 interactions do not require IR-induced DNA damage and are not mediated through DNA. Furthermore, since 53BP1 is found within the chromatin in YFP control fractions, p53 is not required for 53BP1 chromatin-binding (Figure 3.5c). These results suggest that there are individual and interacting pools of p53 and 53BP1 within the chromatin. For example, there are sites within chromatin that are bound by p53 (which is mediated through its carboxy-terminus) in the absence of 53BP1 that have separate functions than the sites with p53-53bp1 interacting within chromatin (e.g. 53BP1-related and -unrelated functions of p53). YFP-p53 fusion proteins interact with ATM Ser1981 within soluble nuclear and chromatin-bound fractions, in response to DNA damage, but independent of p53 transcriptional activity We next determined whether the WTp53 and carboxy-terminus deletion mutant YFP-p53 fusion proteins interact with endogenous ATM Ser1981 in the cytoplasmic, 120
137 soluble nuclear and chromatin fractions. None of the YFP-p53 fusion proteins were observed to interact with ATM Ser1981 within cytoplasmic fractions (Figure 3.4b). Thus, p53-atm Ser1981 interactions were observed only within the nucleus (Figure 3.4b and 3.4c). YFP-p53 WT and YFP-p53 Δ fusion proteins, but not YFP-p53 Δ1-299, were observed to interact with ATM Ser1981 within soluble nuclear fractions post-irradiation (Figure 3.4c). And all three YFP-p53 WT, YFP-p53 Δ1-299 and YFP-p53 Δ fusion proteins interact with ATM Ser1981 within chromatin following IR-induced DNA damage (Figure 3.4d and 3.6b). This suggests that the interaction of chromatin-bound YFPp53 Δ1-299 with ATM Ser1981 may be mediated through chromatin since this interaction was not observed within soluble nuclear fractions (lacking chromatin). All YFP-p53 fusion proteins interactions with ATM Ser1981 occurred in an IR-inducible manner (Figure 3.4c and 3.4d). Thus, this suggests that these interactions occur within sites of IR-induced damaged chromatin. Furthermore, based on our previous results whereby p53 Ser15 could form foci at later times post-ir in ATM-null fibroblasts, it suggests that p53 can bind chromatin following DNA damage in the absence of ATM and this is mediated through the p53 carboxy-terminus(26). Altogether, we conclude that the interaction of p53 within chromatin following IR-induced DNA damage may be mediated through its carboxyterminal NSDBD, that the chromatin-associated p53 interactions with ATM Ser1981 induced following DNA damage is independent of p53-transcriptional activity (i.e. YFP-p53 Δ1-299 ) and may be mediated through both protein-protein and proteinchromatin-protein interactions. 121
138 DNA damage-induced chromatin interactions between p53, 53BP1 and ATM Ser1981 Figures 3.4 and 3.6 demonstrate that p53, 53BP1 and ATM Ser1981 form a triple complex within the chromatin, pre- and post-irradiation. Based on these coimmunoprecipitation analyses, we observed increased interaction between p53-53bp1-atm Ser1981 within irradiation-induced DNA-damaged chromatin, which is independent of p53 transcriptional activities and may be mediated through the p53 carboxy-terminal NSDBD and chromatin (Figure 3.4d). These results suggest that this p53-53bp1-atm Ser1981 interaction occurs in a DNA damage-recruited manner. In addition, since the IR-induced increased interaction between chromatin-bound ATM Ser1981 and 53BP1 is observed in YFP-alone transfected cells, this indicates that the ATM Ser BP1 interaction does not require p53 (Figure 3.6a and 3.6b). We also observed and confirmed endogenous p53-53bp1-atm Ser1981 interactions within chromatin-bound fractions of G0/G1-synchronized NDF-GM05757 fibroblasts (Figure 3.6a and 3.6b). These interactions were present at low levels prior to irradiation and increased within 30 minutes post-ir, confirming an early response of p53 in association with 53BP1 and ATM Ser1981 to IR-induced DNA damage. We also confirmed these co-immunoprecipitation results using immunofluorescence microscopy and observed co-localization of ATM Ser1981 foci with both γ-h2ax and 53BP1 foci in irradiated G0/G1-synchronized NDF-GM05757 fibroblasts within 2 hours post-irradiation (Figure 3.6c). 122
139 3.5 Discussion In this study, we show novel in vivo data for the presence of the p53 protein within undamaged and IR-induced DNA-damaged chromatin, interacting dynamically with ATM Ser1981 and 53BP1. Our current results characterizing total p53 and its phosphoforms interacting with ATM Ser1981 and 53BP1 within sub-nuclear fractions is the first in vivo demonstration of these interactions and adds to our earlier study of p53 Ser15 at sites of DNA damage within chromatin (26). The results of this study differ from that of Bekker-Jensen and colleagues as they did not detect WTp53 at or around sites of UV laser-hoechst-induced DNA-dsbs (25). However, this may be due to the spectrum of lesions created by the different sources used (i.e. UV laser- Hoechst-treatment versus γ-irradiation) to induce DNA damage and the extent to which NHEJ or HR DNA repair pathway is engaged (42, 43). Our in vivo observations confirm previous in vitro studies that the NSDBD within the carboxy-terminus of p53 mediates binding to IR-damaged DNA. This study presents evidence of the role of p53 and its carboxy-terminus in IR-induced DNA-damage association and early signaling by showing its IR-induced chromatin associations together with 53BP1 and ATM Ser1981 mediated through chromatin. It also presents cellular biochemical data in agreement with current models of ATM- and p53- mediated genome surveillance and DNA damage signaling within chromatin (3, 30). 123
140 We show novel evidence that complexes of p53 and 53BP1 exist within the cytoplasm, soluble nucleoplasm and on chromatin under non-irradiated conditions in vivo. Since H1299 are asynchronously growing cells, the p53 and 53BP1 complexes are possibly at sites of stalled replication forks as has been observed by Sengupta and colleagues (44). Sengupta et. al. showed that the p53 and 53BP1 interaction at stalled DNA replication forks was facilitated by the BLM helicase. In addition to stalled replication forks, the p53 protein may also be at other sites of DNA damage. We conclude this because we observe the YFP-p53 Δ1-299 fusion protein binds chromatin, despite lacking the core SSDBD, trans-activation activity, and 53BP1- binding domain. This shows that some proportion of WTp53 protein localizes within chromatin through the NSDBD independent of 53BP1, Ser15-phosphorylation of p53 and p53 transcriptional activity. This does not preclude our previous findings of p53 Ser15 foci formation at sites of DNA damage within chromatin since there may be separate pools of chromatin-bound p53 Ser15 : tethered through the core-ssdbd and carboxy-terminal NSDBD which mediates transcription and DNA damage-binding. The latter is supported by our observation that the interaction between YFP-p53 Δ1-299 and ATM Ser1981 may be mediated through chromatin and in response to IR-induced DNA damage (Figure 4d). We also show for the first time, the altered levels and kinetics of chromatin-bound p53, 53BP1 and ATM Ser1981, and their interactions pre- and post-ir which suggest a dynamic movement and exchange of these factors during which DNA damage sensing, signaling and repair occur. We propose that nuclear chromatin-bound p53, 124
141 alone or in complex with 53BP1 and/or ATM, constantly scans the genome prior to DNA damage or stress. Chromatin-bound p53 is thus readily available to be activated at the site of DNA damage. Our finding that ATM Ser1981 is present within undamaged chromatin is in agreement with recent findings by Kim and colleagues who also detected a small fraction of ATM within undamaged chromatin(3). While levels of ATM Ser1981 were difficult to detect, further investigation of total ATM within these chromatin-bound fractions will confirm these observations of Kim and colleagues. Soluble p53, alone or in complex with 53BP1 and/or ATM Ser1981, is available to be activated and translocated to promoter or damage sites to transactivate downstream target genes or associate with DNA repair or apoptotic machinery. Furthermore, we observed higher levels of ATM Ser1981 and γ-h2ax within IR-induced DNA-damaged chromatin following over-expression of the YFP-p53 WT compared to YFP-p53 Δ and YFP-p53 Δ1-299 fusion proteins (Figure 3b and 3c). As previously mentioned, analyses of total ATM levels are also required to determine ATM expression levels in response to transfection of these constructs as well as irradiation-induced DNA damage. And additional studies will explore the mechanisms and impact of these observations. This study expands the intricate role for p53 in the DNA damage-induced sensing and signaling pathway in addition to the study by Song and colleagues (21). They showed that WTp53 did not interact with MRE11, while core MTp53 proteins (i.e. gain-of-function mutants, p53 R248W and p53 R273H, that lack transcriptional activity) interact with MRE11 of the MRN complex 125
142 and diminishes its binding to DNA damage, leading to impaired ATM recruitment and activation, and subsequent downstream signaling (21). While our study did not analyze the association of p53 with the MRN complex, we did study p53 chromatinbinding which may be controlled by its monomer- versus tetramer status (i.e. we observed a lower ratio of soluble nuclear to chromatin-bound Ser15-phosphorylated YFP-p53 Δ compared to YFP-p53 Δ , data not shown; which requires further study as well), or other chromatin or nuclear factors (i.e. ATM and 53BP1). These phenomena can be further investigated using more precise and higher resolution methods (e.g. live cell imaging and electron microscopy). Altogether, our study reveals subtle and integral details in the regulatory role that p53 may play in DNA damage sensing and signaling, which are mediated through its association directly within the chromatin with ATM Ser1981 and 53BP1, in addition to its association with the MRN complex. 126
143 References 1. M. B. Kastan and E. Berkovich, p53: a two-faced cancer gene. Nat Cell Biol 9, (2007). 2. E. Berkovich, R. J. Monnat, Jr. and M. B. Kastan, Roles of ATM and NBS1 in chromatin structure modulation and DNA double-strand break repair. Nat Cell Biol 9, (2007). 3. Y. C. Kim, G. Gerlitz, T. Furusawa, F. Catez, A. Nussenzweig, K. S. Oh, K. H. Kraemer, Y. Shiloh and M. Bustin, Activation of ATM depends on chromatin interactions occurring before induction of DNA damage. Nat Cell Biol 11, (2009). 4. S. W. Lee, E. J. Kim and S. J. Um, Transcriptional regulation of the p73 gene, a member of the p53 family, by early growth response-1 (Egr-1). Biochem Biophys Res Commun 362, (2007). 5. M. F. Lavin and N. Gueven, The complexity of p53 stabilization and activation. Cell Death Differ 13, (2006). 6. A. J. Levine, W. Hu and Z. Feng, The P53 pathway: what questions remain to be explored? Cell Death Differ 13, (2006). 7. U. M. Moll, S. Wolff, D. Speidel and W. Deppert, Transcription-independent proapoptotic functions of p53. Curr Opin Cell Biol 17, (2005). 8. J. E. Chipuk, T. Kuwana, L. Bouchier-Hayes, N. M. Droin, D. D. Newmeyer, M. Schuler and D. R. Green, Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303, (2004). 9. A. Restle, M. Farber, C. Baumann, M. Bohringer, K. H. Scheidtmann, C. Muller- Tidow and L. Wiesmuller, Dissecting the role of p53 phosphorylation in homologous recombination provides new clues for gain-of-function mutants. Nucleic Acids Res 36, (2008). 10. S. A. Gatz and L. Wiesmuller, p53 in recombination and repair. Cell Death Differ 13, (2006). 11. J. Dahm-Daphi, P. Hubbe, F. Horvath, R. A. El-Awady, K. E. Bouffard, S. N. Powell and H. Willers, Nonhomologous end-joining of site-specific but not of radiation-induced DNA double-strand breaks is reduced in the presence of wildtype p53. Oncogene 24, (2005). 12. R. G. Bristow, Q. Hu, A. Jang, S. Chung, J. Peacock, S. Benchimol and R. Hill, Radioresistant MTp53-expressing rat embryo cell transformants exhibit increased DNA-dsb rejoining during exposure to ionizing radiation. Oncogene 16, (1998). 13. P. Bertrand, Y. Saintigny and B. S. Lopez, p53's double life: transactivationindependent repression of homologous recombination. Trends Genet 20, (2004). 14. E. Kim and W. Deppert, The complex interactions of p53 with target DNA: we learn as we go. Biochem Cell Biol 81, (2003). 15. M. M. Sugrue, D. Y. Shin, S. W. Lee and S. A. Aaronson, Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc Natl Acad Sci U S A 94, (1997). 127
144 16. F. Janus, N. Albrechtsen, I. Dornreiter, L. Wiesmuller, F. Grosse and W. Deppert, The dual role model for p53 in maintaining genomic integrity. Cell Mol Life Sci 55, (1999). 17. R. Wolkowicz and V. Rotter, The DNA binding regulatory domain of p53: see the C. Pathol Biol (Paris) 45, (1997). 18. N. A. Lanson, Jr., D. B. Egeland, B. A. Royals and W. C. Claycomb, The MRE11- NBS1-RAD50 pathway is perturbed in SV40 large T antigen-immortalized AT-1, AT-2 and HL-1 cardiomyocytes. Nucleic Acids Res 28, (2000). 19. S. P. Linke, S. Sengupta, N. Khabie, B. A. Jeffries, S. Buchhop, S. Miska, W. Henning, R. Pedeux, X. W. Wang, et al., p53 interacts with hrad51 and hrad54, and directly modulates homologous recombination. Cancer Res 63, (2003). 20. A. Albor, S. Kaku and M. Kulesz-Martin, Wild-type and mutant forms of p53 activate human topoisomerase I: a possible mechanism for gain of function in mutants. Cancer Res 58, (1998). 21. H. Song, M. Hollstein and Y. Xu, p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nat Cell Biol 9, (2007). 22. J. E. FitzGerald, M. Grenon and N. F. Lowndes, 53BP1: function and mechanisms of focal recruitment. Biochem Soc Trans 37, (2009). 23. K. A. Wilson and D. F. Stern, NFBD1/MDC1, 53BP1 and BRCA1 have both redundant and unique roles in the ATM pathway. Cell Cycle 7, (2008). 24. H. Lee, H. J. Kwak, I. T. Cho, S. H. Park and C. H. Lee, S1219 residue of 53BP1 is phosphorylated by ATM kinase upon DNA damage and required for proper execution of DNA damage response. Biochem Biophys Res Commun 378, (2009). 25. S. Bekker-Jensen, C. Lukas, R. Kitagawa, F. Melander, M. B. Kastan, J. Bartek and J. Lukas, Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. J Cell Biol 173, (2006). 26. S. T. Al Rashid, G. Dellaire, A. Cuddihy, F. Jalali, M. Vaid, C. Coackley, M. Folkard, Y. Xu, B. P. Chen, et al., Evidence for the direct binding of phosphorylated p53 to sites of DNA breaks in vivo. Cancer Res 65, (2005). 27. M. Sauer, A. C. Bretz, R. Beinoraviciute-Kellner, M. Beitzinger, C. Burek, A. Rosenwald, G. S. Harms and T. Stiewe, C-terminal diversity within the p53 family accounts for differences in DNA binding and transcriptional activity. Nucleic Acids Res 36, (2008). 28. A. Ayed, F. A. Mulder, G. S. Yi, Y. Lu, L. E. Kay and C. H. Arrowsmith, Latent and active p53 are identical in conformation. Nat Struct Biol 8, (2001). 29. A. Tafvizi, F. Huang, J. S. Leith, A. R. Fersht, L. A. Mirny and A. M. van Oijen, Tumor suppressor p53 slides on DNA with low friction and high stability. Biophys J 95, L01-03 (2008). 30. P. Hinow, C. E. Rogers, C. E. Barbieri, J. A. Pietenpol, A. K. Kenworthy and E. DiBenedetto, The DNA binding activity of p53 displays reaction-diffusion kinetics. Biophys J 91, (2006). 128
145 31. H. Pivonkova, M. Brazdova, J. Kasparkova, V. Brabec and M. Fojta, Recognition of cisplatin-damaged DNA by p53 protein: critical role of the p53 C-terminal domain. Biochem Biophys Res Commun 339, (2006). 32. W. Tang, H. Willers and S. N. Powell, p53 directly enhances rejoining of DNA double-strand breaks with cohesive ends in gamma-irradiated mouse fibroblasts. Cancer Res 59, (1999). 33. M. Reed, B. Woelker, P. Wang, Y. Wang, M. E. Anderson and P. Tegtmeyer, The C-terminal domain of p53 recognizes DNA damaged by ionizing radiation. Proc Natl Acad Sci U S A 92, (1995). 34. L. Y. Romanova, H. Willers, M. V. Blagosklonny and S. N. Powell, The interaction of p53 with replication protein A mediates suppression of homologous recombination. Oncogene 23, (2004). 35. R. G. Bristow, J. Peacock, A. Jang, J. Kim, R. P. Hill and S. Benchimol, Resistance to DNA-damaging agents is discordant from experimental metastatic capacity in MEF ras-transformants-expressing gain of function MTp53. Oncogene 22, (2003). 36. G. Matlashewski, P. Lamb, D. Pim, J. Peacock, L. Crawford and S. Benchimol, Isolation and characterization of a human p53 cdna clone: expression of the human p53 gene. Embo J 3, (1984). 37. N. Chan, M. Koritzinsky, H. Zhao, R. Bindra, P. M. Glazer, S. Powell, A. Belmaaza, B. Wouters and R. G. Bristow, Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res 68, (2008). 38. J. D. Dignam, R. M. Lebovitz and R. G. Roeder, Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11, (1983). 39. A. R. Cuddihy, F. Jalali, C. Coackley and R. G. Bristow, WTp53 induction does not override MTp53 chemoresistance and radioresistance due to gain-of-function in lung cancer cells. Mol Cancer Ther 7, (2008). 40. P. S. Norris and M. Haas, A fluorescent p53gfp fusion protein facilitates its detection in mammalian cells while retaining the properties of wild-type p53. Oncogene 15, (1997). 41. D. J. Derbyshire, B. P. Basu, L. C. Serpell, W. S. Joo, T. Date, K. Iwabuchi and A. J. Doherty, Crystal structure of human 53BP1 BRCT domains bound to p53 tumour suppressor. Embo J 21, (2002). 42. C. Dinant, M. de Jager, J. Essers, W. A. van Cappellen, R. Kanaar, A. B. Houtsmuller and W. Vermeulen, Activation of multiple DNA repair pathways by sub-nuclear damage induction methods. J Cell Sci 120, (2007). 43. M. J. Kruhlak, A. Celeste, G. Dellaire, O. Fernandez-Capetillo, W. G. Muller, J. G. McNally, D. P. Bazett-Jones and A. Nussenzweig, Changes in chromatin structure and mobility in living cells at sites of DNA double-strand breaks. J Cell Biol 172, (2006). 44. S. Sengupta, A. I. Robles, S. P. Linke, N. I. Sinogeeva, R. Zhang, R. Pedeux, I. M. Ward, A. Celeste, A. Nussenzweig, et al., Functional interaction between BLM helicase and 53BP1 in a Chk1-mediated pathway during S-phase arrest. J Cell Biol 166, (2004). 129
146 Figure Legends Figure 3.1. Characterization and sub-cellular expression of YFP-p53 fusion proteins. Figure 3.1a. Schematic diagram of transient transfections of asynchronouslygrowing p53-null H1299 cells, followed by the analyses of fusion construct expression and sub-cellular localization. This flow chart displays how the exogenous system was used to study the functional p53 domains involved in sub-cellular localization and interactions. Figure 3.1b. Summary of YFP-p53 fusion constructs and sub-cellular distribution based on fluorescence-microscopy analyses. Representative fluorescence microscopy images of p53-null H1299 cells at 16 hours post-transfection expressing YFP alone or YFP-p53 fusion proteins. Legend: Yellow fluorescent protein (YFP), trans-activation domain (TAD), nuclear localization signal (NLS), tetramerization domain (TET), sequence-specific DNAbinding domain (SSDBD), and non-specific DNA-binding domain (NSDBD). YFP signals are in green and light microscopy was used to image the monolayer of cells. Inset: Transfection efficiency (%). Figure 3.1c. Biochemical cellular fractionation followed by Western blot analysis to determine the sub-cellular distribution of Ser15-phosphorylated species of exogenously expressed YFP-p53 fusion proteins. Figure 3.2. Quantitative RT-PCR for p53-responsive genes at 16 hours posttransfection of the exogenous YFP-p53 fusion constructs. 130
147 Figure 3.3: Kinetics and dose-response of the YFP-p53 fusion proteins and the ATM-p53-γ-H2AX signaling pathway in transiently-transfected H1299 cells following irradiation. Figure 3.3a. Western blot analyses of whole cell lysates at various times pre- and post-10gy of irradiation. Total p53 was detected using a YFP-specific antibody. Figure 3.3b. Kinetic expression of proteins within insoluble nuclear (chromatinbound) fractions: IR-induced expression levels of YFP-p53 fusion proteins, phosphorylated p53 Ser15, ATM Ser1981 and γ-h2ax DNA damage sensing and signaling proteins post-10gy of irradiation. Figure 3.3c. Dose-response induction of γ-h2ax within chromatin was observed at 30 minutes following irradiation in the transiently-transfected H1299 cells. For figures 3b and 3c, total p53 was detected using a pool of p53-specific antibodies: DO-1, Ab421, Ab1801, BP53-12 and p53 Ser15. Figure 3.4. Interactions between exogenous YFP-p53 fusion proteins with 53BP1-ATM Ser1981 within different sub-cellular compartments of transientlytransfected p53-null H1299 cells, pre- and 2 hours post-10gy of irradiation Figure 3.4a. Flow chart outlining the treatment, sub-cellular fraction isolation and coimmunoprecipitation analyses conducted in the exogenous isogenic system. Figure 3.4b. p53-specific co-immunoprecipitation analyses of cytoplasmic fractions Figure 3.4c. p53-specific co-immunoprecipitation analyses of soluble nuclear fractions Figure 3.4d. p53-specific co-immunoprecipitation analyses of chromatin-bound fractions 131
148 Total p53 was co-immunoprecipitated and detected by Western blot analyses using a pool of p53-specific antibodies: DO-1, Ab421, Ab1801, BP53-12 and p53 Ser15. Figure 3.5. Reciprocal co-immunoprecipitation analyses using 53BP1-specific antibodies to determine the interactions between YFP-p53 fusion proteins with 53BP1 within different sub-cellular compartments of transiently-transfected p53-null H1299 cells, pre- and 2 hours post-10gy of irradiation. Figure 3.5a. 53BP1-specific co-immunoprecipitation analyses of cytoplasmic fractions Figure 3.5b. 53BP1-specific co-immunoprecipitation analyses of soluble nuclear fractions Figure 3.5c. 53BP1-specific co-immunoprecipitation analyses of chromatin-bound fractions Figure 3.6. Reciprocal co-immunoprecipitation analyses using p53- and ATM Ser1981 -specific antibodies to determine the interactions between 53BP1 and ATM Ser1981 within chromatin, in the presence and absence of WTp53. Figure 3.6a. Co-immunoprecipitation analyses using p53-specific antibodies: chromatin-bound fractions from transiently-transfected p53-null H1299 cells (left panel) and G0/G1-synchronized normal diploid fibroblasts (right panel). Figure 3.6b. Co-immunoprecipitation analyses using ATM Ser1981 -specific antibodies: chromatin-bound fractions from transiently-transfected p53-null H1299 cells (left panel) and G0/G1-synchronized normal diploid fibroblasts (right panel). 132
149 Figure 3.6c. Representative immunofluorescence microscopy images of co-localized endogenous γ-h2ax/53bp1, γ-h2ax/atm Ser1981 and ATM Ser1981 /53BP1 IR-induced foci analysed in G0/G1-synchronized normal diploid fibroblasts. Supplementary Figure Legend Supplementary Figure 3.1. Biochemical fractionation of YFP-alone transfected p53-null H1299 cells at 30 minutes post-5gy, displaying control proteins within each sub-cellular fractions: YFP alone, mainly found within the cytoplasmic fraction; H2AX, positive and loading control for the chromatin-bound fraction; γ-h2ax, positive control for irradiation-induced response and DNA damage signal. 133
150 Figure 1 Figure 3.1 (a) YFP-p53 constructs transfect Asynchronous 16h p53-null H1299 cells ±IR Fluorescence microscopy Sub-cellular localization Cell fractionation WB RNA QRT-PCR Transcription Protein WB (b) YFP YFP-p53 WT YFP-p53 Δ1-299 YFP-p53 Δ ~53% ~22% ~35% ~40% ~31% YFP-p53 WT (CONTROL) YFP vector (CONTROL) CONSTRUCTS YFP YFP NLS TAD SSDBD TET NSDBD NLS 20nm LOCALE CYTO NUC YFP- p53?1-299 YFP 300 TET NSDBD 393 NLS YFP- p53? YFP TAD 1 SSDBD TET
151 Figure 1 Figure 3.1 (c) WB: p53 Ser15 YFP YFPp53 WT YFPp53 Δ1-299 YFPp53 Δ Cytoplasmic Soluble nuclear Chromatin-bound 10Gy:
152 Figure 2 Figure YFP YFP- p53 WT YFP- p53 Δ1-299 YFP- p53 Δ Fold Change in mrna S p53 p21 WAF BAX Gene PIDD 136
153 Figure 3 Figure 3.3 (a) Whole cell lysates: Time (h): YFP p53 Ser15 YFP NIR 2 6 YFP-p53 WT YFP-p53 Δ1-299 YFP-p53 Δ NIR 2 6 NIR 2 6 NIR 2 6 Actin (b) Insoluble/chromatin-bound fractions: YFP-p53 WT YFP-p53 Δ1-299 YFP-p53 Δ Time (h): NIR NIR NIR ATM Ser1981 p53 Ser15 Total p53 γ-h2ax Total H2AX (c) Insoluble/chromatin-bound fractions: Dose (Gy): YFP-p53 WT YFP-p53 Δ1-299 YFP-p53 Δ γ-h2ax Total H2AX 137
154 Figure 3.4 (a) YFP-p53 constructs transfect 16h Asynchronous p53-null H1299 cells ±IR CYTO SOL NUC INSOL NUC / CB WB: IP: Total p53 Total p53 IP: 53BP1 p53 Ser15 53BP1 IP: ATM Ser1981 ATM Ser1981 (b) IP from cytoplasmic fraction IP: Total p53 10Gy: WB: Total p53 YFP YFPp53 WT YFPp53 Δ1-299 YFPp53 Δ BP1 ATM Ser
155 Figure 4 Figure 3.4 (c) IP from soluble nuclear fraction IP: Total p53 WB: Total p53 10Gy: YFP YFPp53 WT YFP - p53 Δ1-299 YFPp53 Δ BP1 ATM Ser1981 (d) IP from insoluble/chromatin-bound fraction IP: Total p53 YFPp53 WT YFP - p53 Δ1-299 YFPp53 Δ WB: Total p53 10Gy: BP1 ATM Ser
156 Figure 5 Figure 3.5 (a) IP from cytoplasmic fraction IP: 53BP1 10Gy: WB: 53BP1 YFP YFP - p53 WT YFPp53 Δ1-299 YFP - p53 Δ Total p53 (b) IP from soluble nuclear fraction IP: 53BP1 10Gy: WB: 53BP1 YFP YFP - p53 WT YFPp53 Δ1-299 YFP - p53 Δ Total p53 (c) IP from insoluble/chromatin-bound fraction IP: 53BP1 10Gy: WB: 53BP1 YFP YFPp53 WT YFPp53 Δ1-299 YFP - p53 Δ Total p53 140
157 Figure 6 Figure 3.6 (a) IP from insoluble/chromatin-bound fraction H1299 IP: 53BP1 YFP YFP-p53 WT 10Gy: - +2h h +2h 53BP1 NDFs GM h +2h ATM Ser1981 p53 Ser15 p53 (b) IP from insoluble/chromatin-bound fraction IP: ATM Ser1981 YFP H1299 YFP-p53 WT NDFs GM05757 ATM Ser BP1 p53 Ser15 p53 10Gy: - +2h h +2h h +2h (c) NDFs GM05757 γ-h2ax + 53BP1 NIR ATM Ser γ-h2ax NIR ATM Ser BP1 NIR 2Gy-2h 2Gy-2h 2Gy-2h 141
158 Supplementary figure 1 Supplementary Figure 3.1 WB: YFP H2AX γ-h2ax YFP- 5 Gy CYT NUC CB 142
159 Chapter 4 Discussion 143
160 4.1 Summary and Discussion of Completed Work In response to DNA damage, cells maintain their genomic stability by enacting cell cycle checkpoints to allow for DNA repair or induction of cell death. This eliminates the perpetuation of incorrectly repaired or unrepaired DNA damage. The tumour suppressor protein, p53, plays a key role in maintaining cell cycle checkpoints as well as mediating cell death and repair in response to genotoxic stress. A direct role for p53 in DNA-dsb sensing and repair has been previously suggested solely based on biochemical evidence. This required further study to understand how and the consequences of p53 and its post-translationally modified species interact at the level of chromatin with DNA-dsb sensing proteins. The data presented in Chapter 2 was the first at showing in situ p53 localization, and specifically a sub-pool of p53 phosphoforms, at sites of DNA-dsbs induced by various DNA damaging agents. Both Chapters 2 and 3 documented p53 phosphoforms in different sub-cellular compartments following IR-induced DNA damage. This thesis contains one of the first direct demonstrations of phosphorylated p53 and ATM at the site of DNA-dsbs in vivo. It proposes that phosphoforms of p53 have a unique biology in mediating cell cycle checkpoint control and DNA repair which may relate to cellular carcinogenesis as well as the role of WTp53 and MTp53 proteins as determinants of therapeutic radiation- and chemo-resistance. 144
161 4.1.1 Irradiation-induced Ser15-phosphorylated p53 phosphoforms In Chapter 2, immunofluorescence microscopic and immunodepletion analyses showed that a specific subset of p53 existed as p53 Ser15 foci. Using whole cell and microbeam (helium ion and UV laser) irradiation techniques, I showed that within thirty minutes, p53 Ser15 phosphoforms are recruited to sites of DNA-dsbs and colocalize with γ-h2ax, 53BP1, RAD50, serine 1981-phosphorylated ATM (ATM Ser1981 ), and threonine 2609-phosphorylated DNA-PKcs (DNA-PKcs Thr2609 ). IRinduced in vivo interaction between p53 Ser15 and γ-h2ax was affirmed by coimmunoprecipitation analyses. Analyses of the induction and resolution of p53 Ser15 foci revealed that they temporally correlated with the kinetics of γ-h2ax foci and biochemical DNA-dsb rejoining. While these multiple immunofluorescence colocalization analyses suggested a close interaction between p53 Ser15 and the DNAdsb sensing and repair proteins, recent studies have suggested they are limited by the resolution of light microscopy (0.5 λ wavelength of light used) and higher resolution could be achieved by electron microscopy analyses which would further define chromatin-protein interactions (1, 2). While levels of γ-h2ax, 53BP1, CHK2 Thr68 and p53 Ser15 foci decreased over time, levels of RAD50, BRCA1, RAD51 and RPA34 foci remained high even at 24 hours post-ir (Chapter 2 and unpublished data). Of note, the foci were quantitated in response to a high dose of 10Gy in which more than 99.9% of the cells are nonclonogenic and the type of foci were large. Subsequent to these experiments, different sizes of foci (which were not quantitated in this unpublished data) were 145
162 documented in the literature and suggested to reflect either sites of single-stranded DNA in the periphery of DNA-dsbs, on-going repair, or irreparable damage (1-3). In these studies, it was noted that there was a distribution of smaller (formed immediately post-dna damage) and larger (formed late post-dna damage) foci that accumulated in response to DNA-dsbs. Bartek and colleagues observed the presence of p53 at sites of single-stranded DNA at later times post-dna damage induction and suggested p53 to be involved in DNA repair as opposed to DNA damage sensing (3). My results of p53 Ser15 co-localizing together with γ-h2ax, 53BP1, ATM Ser1981 and DNA-PKcs Thr2609 within one hour and also remaining at late times following DNA damage induction consolidates these studies. These results suggest that p53 can also be present at these sites of DNA damage in relation to the sensing of DNA-dsbs. The exact role and cellular consequences of the different size of foci with respect to the cell cycle phase and type of chromatin damage and its repair remains to be determined for p53 Ser15 and other p53 phosphoforms Consequences of the loss of Ser15-phosphorylated p53 phosphoforms In Chapter 2, the functional consequence of p53 Ser15 loss on the G1 checkpoint control and DNA repair was determined by comparing isogenic p53 WT/WT and p53 Ser18Ala/Ser18Ala murine embryonic fibroblasts, and isogenic human cells expressing human WTp53 and p53 phospho-specific mutants. In the latter, isogenic human colorectal carcinoma cells, HCT116-p53 WT/WT and HCT116-p53 -/- were transiently transfected with fusion constructs of the yellow fluorescent protein (YFP) with full length WTp53, p53 Ser15Ala (precluding phosphorylation) and p53 Ser15Asp (mimicking a 146
163 permanently phosphorylated species). Lack of Ser15-phosphorylation resulted in defective nuclear foci formation, decreased induction of p21 WAF, decreased p53/γ- H2AX association and altered DNA-dsb rejoining kinetics following DNA damage. The implication of the level and phosphorylation status of WTp53 during nonhomologous end-joining in G1 phase cells may impact on the fidelity or rate of DNAdsb repair (4). Furthermore, whether this is maintained by the MTp53 forms associated with the Li-Fraumeni syndrome or tumours harbouring common p53 mutations remains to be determined. There is conflicting data on whether MTp53 proteins can be post-translationally modified by ATM following DNA damage and would warrant further study given that MTp53 may be downstream of the MRE11 DNA-dsb signaling pathway (5). I did study the levels and kinetics of p53 Ser15 foci in AT-, NBS-, and LFS-derived fibroblasts (see Figures 2.3 and 4.1a). These were analyzed to determine whether there could be a correlation between p53 Ser15 foci levels and cellular radiosensitivity or genomic instability. While a small trend was observed in which increased levels of p53 Ser15 foci correlated with increased radioresistance, these were rough estimates based on foci-positive nuclei and surviving fraction at 2Gy (Figure 4.1b). Long-term studies, such as the impact of phosphomutants on cellular radiosensitivity using clonogenic assays (requiring 2 weeks tissue culture) or DNA-dsb rejoining and repair for periods of up to 72 hours, could not be further explored due to the toxicity and cell death induced by over-expression of p53 in p53-null human cells. To overcome this toxicity, a single genomic insertion vector system and expression from the 147
164 (a) Foci positive nuclei (%) (b) 100 NIR IR Time after irradiation (hrs) LFS-2800T Higher radioresistance Higher residual p53 Ser15 foci 2Gy surviving fraction (SF2: % clonogenic survival) p53 Ser15 foci-positive nuclei at 24hrs post-10gy (%) NDF- NBS- AT- GM05757 GM07166 GM05823 Figure 4.1. Kinetics and residual p53 Ser15 foci in fibroblasts of varying radiosensitivities: NDF-GM05757, LFS-2800T, NBS-GM07166 and AT-GM (a) Ionizing radiation-induced p53 Ser15 foci kinetics following 10Gy in LFS-derived fibroblasts, LFS2800T. The inset images are representative of p53 Ser15 foci within unirradiated (NIR) and 3 hours post-10gy irradiated (IR) LFS-2800T fibroblasts. (b) A trend is observed when comparing residual p53 Ser15 foci at 24 hours post-10gy with cellular radiosensitivity (i.e. SF2: surviving fraction following 2Gy of irradiation). Higher residual foci tend to correlate with higher radioresistance. 148
165 endogenous WTp53 promoter (e.g. FLIP-In vector system, Clontech, U.S.A.) can be used in the future (such as those used by Song and colleagues)(6). The cell models I derived were robust for the study of p53 phosphorylation and biology, as characterized by fluorescence microscopy, Western blot and quantitative reverse-transcriptase polymerase chain reaction (RT-PCR), to understand endogenous and exogenous p53 sub-cellular localization, expression and transcriptional function. It was determined that the YFP moiety did not interfere with the function of full length WTp53 given that expression of YFP-WTp53 resulted in localization throughout the cytoplasm and nucleus, was stabilized following IRinduce DNA damage, induced p21 WAF transcription and protein expression. The localization of other constructs and transcriptional activity were characteristic of the presence of respective sequences and functional domains, consistent with previously published data and supports the potential use of my biochemical and cell biological approach in the study of other DNA damage sensing proteins. Subsequently, to delineate the domains that mediate binding to DNA breaks within chromatin and interaction with DNA-dsb sensing, signaling and repair proteins, normal diploid human fibroblasts and the transiently-transfected cells were subjected to cellular biochemical fractionation analyses. All constructs were found to be bound within chromatin in vivo even prior to irradiation, confirming previous in vitro studies that supported that latent p53 can bind DNA (7, 8). Indeed, in Figure 4.2, I show an additional experiment that was designed to determine if the YFP-WTp53 fusion 149
166 Soluble nuclear MNase treatedsoluble nuclear YFP YFP-p53 WT YFP YFP-p53 WT NIR IR NIR IR NIR IR NIR IR Histones WB: ORC2 p53 Ser15 Figure 4.2. Increased levels of soluble Ser15-posphorylated YFP-p53WT pre- and 2 hours post-10gy were observed following micrococcal nuclease (Mnase) digestion of insoluble/chromatin cell fraction. Insoluble positive control proteins were the histones and ORC2 (origin of replication core protein 2) for the chromatin fraction, which were also released and subsequently detected in the MNase-treated solublenuclear fraction. 150
167 protein was bound to chromatin following DNA damage (similar to endogenous WTp53). The figure shows the release of YFP-p53 proteins from the chromatin fraction following micrococcal nucease (MNase) digestion. Similar data has been shown for the release of CHK2 Thr68 from chromatin after DNA damage (9). To-date, studies have shown that ATM is the main kinase that mediates p53 Ser15 phosphorylation, and this phosphorylation occurs with similar kinetics in DNA-PKcsdeficient cells. In Chapter 3, I characterized the ATM-dependency of p53-atm- 53BP1 associations within chromatin following IR. In comparing the levels of chromatin-bound ATM Ser1981, p53 Ser15 and 53BP1 post-ir, these were all decreased following ATM inhibition. In comparison, total WTp53 levels remained similar in untreated and DMSO-controls (Figure 4.3). Based on the YFP-p53 constructs results and ATM-inhibition results, neither Ser15- phosphorylation, the non-specific DNA binding domain in the p53 carboxy-terminus, or 53BP1 are required for chromatin association of total p53. However, this does not exclude the possibility that other p53 isoforms (e.g. p47), or WTp53 subpools/phosphoforms may be required (Figure 4.3: levels of p47 isoforms are induced following irradiation and unaffected by ATM-inhibition). The fact that the YFPp53 Ala138Val mutant protein had a similar dose, kinetics and interaction profile with the YFP-p53 WT protein confirms that the ability to bind sites of DNA damage within chromatin does not require its transcriptional trans-activation function. 151
168 WB: ATM Ser1981 G0/G1 NDFs (GM05757): Untreated Vehicle-DMSO KU Gy 10Gy 10Gy NIR 0.5 2H NIR 0.5 2H NIR 0.5 2H p53 Ser15 p53 p47 (p53 isoform) Actin Figure 4.3. Levels of ATM-dependent phosphorylation following IR-induced DNA damage in G0-G1 synchronized primary human fibroblasts. 152
169 In addition to the chromatin fraction, levels of p53, ATM Ser1981 and 53BP1 were also analyzed within the cytoplasmic and soluble nuclear fractions of G0-G1 synchronized primary fibroblasts and in H1299 transiently-transfected cells (see Figure 4.4). In these experiments, ATM Ser1981 was not detected in cytoplasmic fractions while it was found in soluble nuclear fractions. 53BP1 was present in both cytoplasmic and soluble nuclear fractions. Minimal interactions were observed between p53 Ser15 -ATM Ser BP1 in cytoplasmic fractions. Since mitochondria are present within the cytoplasm and the biochemical fractionation process does not definitively separate these cellular compartments, the interactions detected within this fraction may be occurring within mitochondrial DNA. This may have cellular implications since oxidative damage can induce increased phosphorylation and trigger the ATM-p53 signaling cascade during which p53 has been associated with mitochondrial compartments following this oxidative-induced DNA damage (10). Previous studies have suggested that the carboxy-terminus domain of the p53 protein can mediate DNA damage binding in vitro, however whether this function occurs in vivo has not been well-characterized. The in vitro studies utilized naked DNA oligonucleotide subtrates and cell-free assays. In Chapter 3, I also tested the hypothesis that the carboxy-terminus of p53 mediates its function in DNA-dsb binding and repair within chromatin by studying chromatin-bound exogenous YFPp53 wild type and mutant proteins and endogenous wild type p53 proteins. In my studies of endogenous and exogenous p53 in vivo, the ability of the carboxy- 153
170 Figure 4.4. Cytoplasmic and soluble nuclear interactions of endogenous and exogenous Ser15-posphorylated p53 phosphoforms with 53BP1 and ATM Ser1981, pre- and 2 hours post-10gy. 154
171 terminus YFP-p53 Δ1-299 protein to bind chromatin (i.e. lacking the consensussequence specific DNA binding, trans-activation and 53BP1 interaction domains) suggests that the p53 protein can localize at sites of damaged chromatin via its carboxy-terminus. This localization within chromatin occurs with ATM Ser1981, but is not mediated through 53BP1. Thus, I also concluded that the interaction of p53 with 53BP1 and ATM Ser1981 does not require Ser15-phosphorylation. At present, it is unclear as to whether both the core and carboxy-terminus (or only carboxy-terminus) is needed for DNA damage surveillance. This would require the creation of double mutants to test this hypothesis in functional assays (i.e. Milner, et.al., Weismuller, et.al., Harris, et.al., and Jasin, et.al., Powell, et.al., and Kiltie, et.al.)(4, 11-15). In summary, there is altered association kinetics of chromatin-bound ATM Ser1981 -p53-53bp1 complexes within 30 minutes to 2 hours post-irradiation. The data are consistent with a dynamic sub-cellular trafficking and exchange of these factors during rapid DNA damage sensing and repair (see proposed model in Figure 4.5). 155
172 Nucleoplasm NIR ATM p53 53BP1 p53 53BP1 IR ATM p53 53BP1 p53 Ser15 53BP1 ATM Ser1981 p53 Ser15 ATM Ser1981 p53 Ser15 53BP1 Chromatin NIR 53BP1 p53 p53 53BP1 IR MRN complex γ-h2ax ATM Ser1981 p53 Ser15 ATM Ser1981 p53 Ser15 53BP1 MRN complex γ-h2ax p53 53BP1 ATM Ser1981 Recruitment of ATM Ser1981, p53 Ser15 -phosphorylation and release for downstream signal transduction Recruitment of repair proteins (e.g. RAD51) p53 Ser15 ATM Ser1981 may or may not be phosphorylated; NSDBD-tethered p53 53BP1 ATM Ser1981 MRN complex γ-h2ax p53 ATM Ser1981 Figure 4.5. Final proposed model of the dynamic interactions between p53-53bp1- ATM within the nucleoplasm and chromatin following IR-induced DNA damage. 156
Tumour growth environment modulates Chk1 signalling pathways and sensitivity to Chk1 inhibition
 Tumour growth environment modulates Chk1 signalling pathways and sensitivity to Chk1 inhibition Andrew J Massey Supplementary Information Supplementary Figure S1. Related to Fig. 1. (a) HT29 or U2OS cells
Tumour growth environment modulates Chk1 signalling pathways and sensitivity to Chk1 inhibition Andrew J Massey Supplementary Information Supplementary Figure S1. Related to Fig. 1. (a) HT29 or U2OS cells
Removal of Shelterin Reveals the Telomere End-Protection Problem
 Removal of Shelterin Reveals the Telomere End-Protection Problem DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci DSB e CROMATINA Higher-order chromatin packaging is a barrier to
Removal of Shelterin Reveals the Telomere End-Protection Problem DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci DSB e CROMATINA Higher-order chromatin packaging is a barrier to
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
DSB. Double-Strand Breaks causate da radiazioni stress ossidativo farmaci
 DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci METODI DDR foci formation in irradiated (2 Gy) cells fixed 2 h later IRIF IRradiation Induced Focus Laser micro-irradiation DDR
DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci METODI DDR foci formation in irradiated (2 Gy) cells fixed 2 h later IRIF IRradiation Induced Focus Laser micro-irradiation DDR
Brian T Burgess, DO, PhD, GYN Oncology Fellow Rachel W. Miller, MD, GYN Oncology
 Brian T Burgess, DO, PhD, GYN Oncology Fellow Rachel W. Miller, MD, GYN Oncology Epithelial Ovarian Cancer - Standard Current Treatment: Surgery with De-bulking + Platinum-Taxane based Chemotherapy - No
Brian T Burgess, DO, PhD, GYN Oncology Fellow Rachel W. Miller, MD, GYN Oncology Epithelial Ovarian Cancer - Standard Current Treatment: Surgery with De-bulking + Platinum-Taxane based Chemotherapy - No
Chapter 2. Aims & Objectives
 2.1. Statement of the problem: Earlier reports have shown ambiguous alteration of histone marks in response to DNA damage in asynchronized population of cells. These histone marks not only undergo dynamic
2.1. Statement of the problem: Earlier reports have shown ambiguous alteration of histone marks in response to DNA damage in asynchronized population of cells. These histone marks not only undergo dynamic
Removal of Shelterin Reveals the Telomere End-Protection Problem
 Removal of Shelterin Reveals the Telomere End-Protection Problem DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci DSB e CROMATINA Higher-order chromatin packaging is a barrier to
Removal of Shelterin Reveals the Telomere End-Protection Problem DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci DSB e CROMATINA Higher-order chromatin packaging is a barrier to
DSB. Double-Strand Breaks causate da radiazioni stress ossidativo farmaci
 DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci DSB e CROMATINA Higher-order chromatin packaging is a barrier to the detection and repair of DNA damage DSBs induce a local decrease
DSB Double-Strand Breaks causate da radiazioni stress ossidativo farmaci DSB e CROMATINA Higher-order chromatin packaging is a barrier to the detection and repair of DNA damage DSBs induce a local decrease
SUPPLEMENTARY INFORMATION
 Supplementary Discussion The cell cycle machinery and the DNA damage response network are highly interconnected and co-regulated in assuring faithful duplication and partition of genetic materials into
Supplementary Discussion The cell cycle machinery and the DNA damage response network are highly interconnected and co-regulated in assuring faithful duplication and partition of genetic materials into
DNA double strand break repair: a radiation perspective
 DNA double strand break repair: a radiation perspective Kavanagh, J. N., Redmond, K. M., Schettino, G., & Prise, K. M. (2013). DNA double strand break repair: a radiation perspective. Antioxidants & Redox
DNA double strand break repair: a radiation perspective Kavanagh, J. N., Redmond, K. M., Schettino, G., & Prise, K. M. (2013). DNA double strand break repair: a radiation perspective. Antioxidants & Redox
XPC DNA REPAIR PROTEIN REGULATION IN THE CONTEXT OF THE G1/S CELL CYCLE CHECKPOINT
 XPC DNA REPAIR PROTEIN REGULATION IN THE CONTEXT OF THE G1/S CELL CYCLE CHECKPOINT Tabitha M. Hardy Submitted to the faculty of the University Graduate School in partial fulfillment of the requirements
XPC DNA REPAIR PROTEIN REGULATION IN THE CONTEXT OF THE G1/S CELL CYCLE CHECKPOINT Tabitha M. Hardy Submitted to the faculty of the University Graduate School in partial fulfillment of the requirements
Prognostic value of DNA repair based stratification of hepatocellular carcinoma
 Prognostic value of DNA repair based stratification of hepatocellular carcinoma Zhuo Lin 1, 2 #, Shi-Hao Xu 3 #, Hai-Qing Wang 4, Yi-Jing Cai 1, 2, Li Ying 3, Mei Song 1, 2, Yu-Qun Wang 1, 2, Shan-Jie
Prognostic value of DNA repair based stratification of hepatocellular carcinoma Zhuo Lin 1, 2 #, Shi-Hao Xu 3 #, Hai-Qing Wang 4, Yi-Jing Cai 1, 2, Li Ying 3, Mei Song 1, 2, Yu-Qun Wang 1, 2, Shan-Jie
Radiation response in human cells
 Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1157 Radiation response in human cells DNA damage formation, repair and signaling ANN-SOFIE GUSTAFSSON ACTA UNIVERSITATIS
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1157 Radiation response in human cells DNA damage formation, repair and signaling ANN-SOFIE GUSTAFSSON ACTA UNIVERSITATIS
Multistep nature of cancer development. Cancer genes
 Multistep nature of cancer development Phenotypic progression loss of control over cell growth/death (neoplasm) invasiveness (carcinoma) distal spread (metastatic tumor) Genetic progression multiple genetic
Multistep nature of cancer development Phenotypic progression loss of control over cell growth/death (neoplasm) invasiveness (carcinoma) distal spread (metastatic tumor) Genetic progression multiple genetic
DNA damage and DNA repair
 DNA damage and DNA repair Susan P. Lees-Miller, PhD, Professor, Departments of Biochemistry & Molecular Biology and Oncology, Southern Alberta Cancer Research Institute, University of Calgary, Calgary,
DNA damage and DNA repair Susan P. Lees-Miller, PhD, Professor, Departments of Biochemistry & Molecular Biology and Oncology, Southern Alberta Cancer Research Institute, University of Calgary, Calgary,
Basics of Radiation Biology
 Basics of Radiation Biology Sally A. Amundson Columbia University Center for Radiological Research http://www.cmcr.columbia.edu/ Overview Radiation damage to cells DNA Effects of radiation damage on cells
Basics of Radiation Biology Sally A. Amundson Columbia University Center for Radiological Research http://www.cmcr.columbia.edu/ Overview Radiation damage to cells DNA Effects of radiation damage on cells
Basics of Radiation Biology
 Basics of Radiation Biology Sally A. Amundson Columbia University Center for Radiological Research http://www.cmcr.columbia.edu/ Overview Radiation damage to cells DNA Effects of radiation damage on cells
Basics of Radiation Biology Sally A. Amundson Columbia University Center for Radiological Research http://www.cmcr.columbia.edu/ Overview Radiation damage to cells DNA Effects of radiation damage on cells
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
BIO360 Fall 2013 Quiz 1
 BIO360 Fall 2013 Quiz 1 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function mutation.
BIO360 Fall 2013 Quiz 1 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function mutation.
MARCH 2001 VOLUME 14, NUMBER 3. Copyright 2001 by the American Chemical Society. p53 Signaling and Cell Cycle Checkpoints
 MARCH 2001 VOLUME 14, NUMBER 3 Copyright 2001 by the American Chemical Society Invited Review p53 Signaling and Cell Cycle Checkpoints Zoe A. Stewart and Jennifer A. Pietenpol* Department of Biochemistry,
MARCH 2001 VOLUME 14, NUMBER 3 Copyright 2001 by the American Chemical Society Invited Review p53 Signaling and Cell Cycle Checkpoints Zoe A. Stewart and Jennifer A. Pietenpol* Department of Biochemistry,
Genomic instability. Amin Mahpour
 Genomic instability Amin Mahpour 1 Some questions to ponder What is Genomic instability? What factors contribute to the genomic integrity? How we identify these aberrations? 2 PART I: MOLECULAR BIOLOGY
Genomic instability Amin Mahpour 1 Some questions to ponder What is Genomic instability? What factors contribute to the genomic integrity? How we identify these aberrations? 2 PART I: MOLECULAR BIOLOGY
Chapt 15: Molecular Genetics of Cell Cycle and Cancer
 Chapt 15: Molecular Genetics of Cell Cycle and Cancer Student Learning Outcomes: Describe the cell cycle: steps taken by a cell to duplicate itself = cell division; Interphase (G1, S and G2), Mitosis.
Chapt 15: Molecular Genetics of Cell Cycle and Cancer Student Learning Outcomes: Describe the cell cycle: steps taken by a cell to duplicate itself = cell division; Interphase (G1, S and G2), Mitosis.
Lecture 10. G1/S Regulation and Cell Cycle Checkpoints. G1/S regulation and growth control G2 repair checkpoint Spindle assembly or mitotic checkpoint
 Lecture 10 G1/S Regulation and Cell Cycle Checkpoints Outline: G1/S regulation and growth control G2 repair checkpoint Spindle assembly or mitotic checkpoint Paper: The roles of Fzy/Cdc20 and Fzr/Cdh1
Lecture 10 G1/S Regulation and Cell Cycle Checkpoints Outline: G1/S regulation and growth control G2 repair checkpoint Spindle assembly or mitotic checkpoint Paper: The roles of Fzy/Cdc20 and Fzr/Cdh1
Tumor cell reassortment within the cell cycle (including checkpoints and cell-cycle arrest)
 Tumor cell reassortment within the cell cycle (including checkpoints and cell-cycle arrest) Carsten Herskind Dept. of Radiation Oncology. Universitätsmedizin Mannheim Medical Faculty Mannheim, Heidelberg
Tumor cell reassortment within the cell cycle (including checkpoints and cell-cycle arrest) Carsten Herskind Dept. of Radiation Oncology. Universitätsmedizin Mannheim Medical Faculty Mannheim, Heidelberg
Cell cycle and Apoptosis. Chalermchai Mitrpant
 Cell cycle and Apoptosis 2556 Chalermchai Mitrpant Overview of the cell cycle Outline Regulatory mechanisms controlling cell cycle Progression of the cell cycle Checkpoint of the cell cycle Phases of the
Cell cycle and Apoptosis 2556 Chalermchai Mitrpant Overview of the cell cycle Outline Regulatory mechanisms controlling cell cycle Progression of the cell cycle Checkpoint of the cell cycle Phases of the
C H A R A C T E R I Z A T I O N O F T H E N O V E L D O M A I N W I T H N O N A M E G E N E I N C O L O N C A N C E R
 C H A R A C T E R I Z A T I O N O F T H E N O V E L D O M A I N W I T H N O N A M E G E N E I N C O L O N C A N C E R Charleen Rupnarain A dissertation submitted to the Faculty of Science, University of
C H A R A C T E R I Z A T I O N O F T H E N O V E L D O M A I N W I T H N O N A M E G E N E I N C O L O N C A N C E R Charleen Rupnarain A dissertation submitted to the Faculty of Science, University of
Introduction to Cancer Biology
 Introduction to Cancer Biology Robin Hesketh Multiple choice questions (choose the one correct answer from the five choices) Which ONE of the following is a tumour suppressor? a. AKT b. APC c. BCL2 d.
Introduction to Cancer Biology Robin Hesketh Multiple choice questions (choose the one correct answer from the five choices) Which ONE of the following is a tumour suppressor? a. AKT b. APC c. BCL2 d.
Molecular biology :- Cancer genetics lecture 11
 Molecular biology :- Cancer genetics lecture 11 -We have talked about 2 group of genes that is involved in cellular transformation : proto-oncogenes and tumour suppressor genes, and it isn t enough to
Molecular biology :- Cancer genetics lecture 11 -We have talked about 2 group of genes that is involved in cellular transformation : proto-oncogenes and tumour suppressor genes, and it isn t enough to
RAS Genes. The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes.
 ۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
Open Access Book Chapter
 ERC-OAPEN-2015, Deposit cover page Open Access Book Chapter Published version Chapter title Signalling DNA Damage Title in English Author(s) Andres Joaquin Lopez-Contreras and Oscar Fernandez-Capetillo
ERC-OAPEN-2015, Deposit cover page Open Access Book Chapter Published version Chapter title Signalling DNA Damage Title in English Author(s) Andres Joaquin Lopez-Contreras and Oscar Fernandez-Capetillo
Accumulation of DNA double strand breaks. in normal tissues after fractionated low dose irradiation
 Aus der Klinik für Strahlentherapie und Radioonkologie Direktor: Prof. Dr. Ch. Rübe UniversitätskliniKum des Saarlandes Accumulation of DNA double strand breaks in normal tissues after fractionated low
Aus der Klinik für Strahlentherapie und Radioonkologie Direktor: Prof. Dr. Ch. Rübe UniversitätskliniKum des Saarlandes Accumulation of DNA double strand breaks in normal tissues after fractionated low
Cell cycle, signaling to cell cycle, and molecular basis of oncogenesis
 Cell cycle, signaling to cell cycle, and molecular basis of oncogenesis MUDr. Jiří Vachtenheim, CSc. CELL CYCLE - SUMMARY Basic terminology: Cyclins conserved proteins with homologous regions; their cellular
Cell cycle, signaling to cell cycle, and molecular basis of oncogenesis MUDr. Jiří Vachtenheim, CSc. CELL CYCLE - SUMMARY Basic terminology: Cyclins conserved proteins with homologous regions; their cellular
C-Phycocyanin (C-PC) is a n«sjfc&c- waefc-jduble phycobiliprotein. pigment isolated from Spirulina platensis. This water- soluble protein pigment is
 ' ^Summary C-Phycocyanin (C-PC) is a n«sjfc&c- waefc-jduble phycobiliprotein pigment isolated from Spirulina platensis. This water- soluble protein pigment is of greater importance because of its various
' ^Summary C-Phycocyanin (C-PC) is a n«sjfc&c- waefc-jduble phycobiliprotein pigment isolated from Spirulina platensis. This water- soluble protein pigment is of greater importance because of its various
Oncolytic virus strategy
 Oncolytic viruses Oncolytic virus strategy normal tumor NO replication replication survival lysis Oncolytic virus strategy Mechanisms of tumor selectivity of several, some of them naturally, oncolytic
Oncolytic viruses Oncolytic virus strategy normal tumor NO replication replication survival lysis Oncolytic virus strategy Mechanisms of tumor selectivity of several, some of them naturally, oncolytic
Radiation Oncology. Initial Certification Qualifying (Computer-based) Examination: Study Guide for Radiation and Cancer Biology
 Radiation Oncology Initial Certification Qualifying (Computer-based) Examination: Study Guide for Radiation and Cancer Biology This exam tests your knowledge of the principles of cancer and radiation biology
Radiation Oncology Initial Certification Qualifying (Computer-based) Examination: Study Guide for Radiation and Cancer Biology This exam tests your knowledge of the principles of cancer and radiation biology
BCHM3972 Human Molecular Cell Biology (Advanced) 2013 Course University of Sydney
 BCHM3972 Human Molecular Cell Biology (Advanced) 2013 Course University of Sydney Page 2: Immune Mechanisms & Molecular Biology of Host Defence (Prof Campbell) Page 45: Infection and Implications for Cell
BCHM3972 Human Molecular Cell Biology (Advanced) 2013 Course University of Sydney Page 2: Immune Mechanisms & Molecular Biology of Host Defence (Prof Campbell) Page 45: Infection and Implications for Cell
BIO360 Fall 2013 Quiz 1
 BIO360 Fall 2013 Quiz 1 Name: Key 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function
BIO360 Fall 2013 Quiz 1 Name: Key 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function
Introduction. Cancer Biology. Tumor-suppressor genes. Proto-oncogenes. DNA stability genes. Mechanisms of carcinogenesis.
 Cancer Biology Chapter 18 Eric J. Hall., Amato Giaccia, Radiobiology for the Radiologist Introduction Tissue homeostasis depends on the regulated cell division and self-elimination (programmed cell death)
Cancer Biology Chapter 18 Eric J. Hall., Amato Giaccia, Radiobiology for the Radiologist Introduction Tissue homeostasis depends on the regulated cell division and self-elimination (programmed cell death)
p53 and Apoptosis: Master Guardian and Executioner Part 2
 p53 and Apoptosis: Master Guardian and Executioner Part 2 p14arf in human cells is a antagonist of Mdm2. The expression of ARF causes a rapid increase in p53 levels, so what would you suggest?.. The enemy
p53 and Apoptosis: Master Guardian and Executioner Part 2 p14arf in human cells is a antagonist of Mdm2. The expression of ARF causes a rapid increase in p53 levels, so what would you suggest?.. The enemy
nuclear science and technology
 EUROPEAN COMMISSION nuclear science and technology The role of intercellular communication and DNA double-strand breaks in the induction of bystander effects (INTERSTANDER) Contract N o FIGH-CT2002-00218
EUROPEAN COMMISSION nuclear science and technology The role of intercellular communication and DNA double-strand breaks in the induction of bystander effects (INTERSTANDER) Contract N o FIGH-CT2002-00218
Cancer Biology How a cell responds to DNA Damage
 1 Cancer Biology How a cell responds to DNA Damage Jann Sarkaria Department of Oncology Mayo Clinic 2 EDUCATIONAL GOALS How proteins can transmit signals to each other. The definition of a tumor suppressor
1 Cancer Biology How a cell responds to DNA Damage Jann Sarkaria Department of Oncology Mayo Clinic 2 EDUCATIONAL GOALS How proteins can transmit signals to each other. The definition of a tumor suppressor
Cancer. Questions about cancer. What is cancer? What causes unregulated cell growth? What regulates cell growth? What causes DNA damage?
 Questions about cancer What is cancer? Cancer Gil McVean, Department of Statistics, Oxford What causes unregulated cell growth? What regulates cell growth? What causes DNA damage? What are the steps in
Questions about cancer What is cancer? Cancer Gil McVean, Department of Statistics, Oxford What causes unregulated cell growth? What regulates cell growth? What causes DNA damage? What are the steps in
Radiobiology of fractionated treatments: the classical approach and the 4 Rs. Vischioni Barbara MD, PhD Centro Nazionale Adroterapia Oncologica
 Radiobiology of fractionated treatments: the classical approach and the 4 Rs Vischioni Barbara MD, PhD Centro Nazionale Adroterapia Oncologica Radiobiology It is fundamental in radiation oncology Radiobiology
Radiobiology of fractionated treatments: the classical approach and the 4 Rs Vischioni Barbara MD, PhD Centro Nazionale Adroterapia Oncologica Radiobiology It is fundamental in radiation oncology Radiobiology
Importance of ATM in Radiotherapy
 Importance of ATM in Radiotherapy Radiology and Physical Medicine María Trinidad Rueda Cáceres Radiotherapy Importance of ATM in Radiotherapy One of the standard treatment options for various malignant
Importance of ATM in Radiotherapy Radiology and Physical Medicine María Trinidad Rueda Cáceres Radiotherapy Importance of ATM in Radiotherapy One of the standard treatment options for various malignant
Part-4. Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death
 Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Molecular Cell Biology (Bio 5068) Cell Cycle I. Ron Bose, MD PhD November 14, 2017
 Molecular Cell Biology (Bio 5068) Cell Cycle I Ron Bose, MD PhD November 14, 2017 CELL DIVISION CYCLE M G2 S G1 DISCOVERY AND NAMING OF CYCLINS A protein (called cyclin ) was observed to increase as cells
Molecular Cell Biology (Bio 5068) Cell Cycle I Ron Bose, MD PhD November 14, 2017 CELL DIVISION CYCLE M G2 S G1 DISCOVERY AND NAMING OF CYCLINS A protein (called cyclin ) was observed to increase as cells
Cell Cycle, Mitosis, and Microtubules. LS1A Final Exam Review Friday 1/12/07. Processes occurring during cell cycle
 Cell Cycle, Mitosis, and Microtubules LS1A Final Exam Review Friday 1/12/07 Processes occurring during cell cycle Replicate chromosomes Segregate chromosomes Cell divides Cell grows Cell Growth 1 The standard
Cell Cycle, Mitosis, and Microtubules LS1A Final Exam Review Friday 1/12/07 Processes occurring during cell cycle Replicate chromosomes Segregate chromosomes Cell divides Cell grows Cell Growth 1 The standard
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Biochemistry 201: DNA repair January 24, 26, 2000 Gilbert Chu
 1) Why study DNA repair? Biochemistry 201: DNA repair January 24, 26, 2000 Gilbert Chu The genome is assaulted by a myriad of different agents that cause DNA damage. Sources of damage can be both exogenous
1) Why study DNA repair? Biochemistry 201: DNA repair January 24, 26, 2000 Gilbert Chu The genome is assaulted by a myriad of different agents that cause DNA damage. Sources of damage can be both exogenous
Regulators of Cell Cycle Progression
 Regulators of Cell Cycle Progression Studies of Cdk s and cyclins in genetically modified mice reveal a high level of plasticity, allowing different cyclins and Cdk s to compensate for the loss of one
Regulators of Cell Cycle Progression Studies of Cdk s and cyclins in genetically modified mice reveal a high level of plasticity, allowing different cyclins and Cdk s to compensate for the loss of one
CELL CYCLE MOLECULAR BASIS OF ONCOGENESIS
 CELL CYCLE MOLECULAR BASIS OF ONCOGENESIS Summary of the regulation of cyclin/cdk complexes during celll cycle Cell cycle phase Cyclin-cdk complex inhibitor activation Substrate(s) G1 Cyclin D/cdk 4,6
CELL CYCLE MOLECULAR BASIS OF ONCOGENESIS Summary of the regulation of cyclin/cdk complexes during celll cycle Cell cycle phase Cyclin-cdk complex inhibitor activation Substrate(s) G1 Cyclin D/cdk 4,6
Regulation of the Cell Cycle
 Regulation of the Cell Cycle 21 I. OVERVIEW Quiescent differentiated cell / can be induced to re-enter the active cell cycle. urvival Cell division Apoptosis 1 Daughter cells Apoptic cell enescent cell
Regulation of the Cell Cycle 21 I. OVERVIEW Quiescent differentiated cell / can be induced to re-enter the active cell cycle. urvival Cell division Apoptosis 1 Daughter cells Apoptic cell enescent cell
Supplementary Figure 1
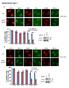 Supplementary Figure 1 a γ-h2ax MDC1 RNF8 FK2 BRCA1 U2OS Cells sgrna-1 ** 60 sgrna 40 20 0 % positive Cells (>5 foci per cell) b ** 80 sgrna sgrna γ-h2ax MDC1 γ-h2ax RNF8 FK2 MDC1 BRCA1 RNF8 FK2 BRCA1
Supplementary Figure 1 a γ-h2ax MDC1 RNF8 FK2 BRCA1 U2OS Cells sgrna-1 ** 60 sgrna 40 20 0 % positive Cells (>5 foci per cell) b ** 80 sgrna sgrna γ-h2ax MDC1 γ-h2ax RNF8 FK2 MDC1 BRCA1 RNF8 FK2 BRCA1
SUPPLEMENTAL FIGURE LEGENDS
 SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
Cell cycle control (mammalian)
 Apr. 21, 2005 Cell cycle control (mammalian) Basic mechanisms & protein components Checkpoints Chap. 21, by Lodish et al., 5 th ed. 2004 Chap. 17, by Alberts et al., 4 th ed. 2002 鍾明怡 mychung@vghtpe.gov.tw
Apr. 21, 2005 Cell cycle control (mammalian) Basic mechanisms & protein components Checkpoints Chap. 21, by Lodish et al., 5 th ed. 2004 Chap. 17, by Alberts et al., 4 th ed. 2002 鍾明怡 mychung@vghtpe.gov.tw
Factors determining DNA double strand break repair pathway choice in G2 phase.
 Supplementary Data Factors determining DN double strand break repair pathway choice in G2 phase. tsushi Shibata 1, Sandro Conrad 2, Julie irraux 1, Verena Geuting 2, Olivia arton 2, mani Ismail 1, ndreas
Supplementary Data Factors determining DN double strand break repair pathway choice in G2 phase. tsushi Shibata 1, Sandro Conrad 2, Julie irraux 1, Verena Geuting 2, Olivia arton 2, mani Ismail 1, ndreas
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,900 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,900 116,000 120M Open access books available International authors and editors Downloads Our
Chapter 9. Cells Grow and Reproduce
 Chapter 9 Cells Grow and Reproduce DNA Replication DNA polymerase Addition of a nucleotide to the 3 end of a growing strand Use dntps as substrate Release of pyrophosphate Initiation of Replication Replication
Chapter 9 Cells Grow and Reproduce DNA Replication DNA polymerase Addition of a nucleotide to the 3 end of a growing strand Use dntps as substrate Release of pyrophosphate Initiation of Replication Replication
Karyotype analysis reveals transloction of chromosome 22 to 9 in CML chronic myelogenous leukemia has fusion protein Bcr-Abl
 Chapt. 18 Cancer Molecular Biology of Cancer Student Learning Outcomes: Describe cancer diseases in which cells no longer respond Describe how cancers come from genomic mutations (inherited or somatic)
Chapt. 18 Cancer Molecular Biology of Cancer Student Learning Outcomes: Describe cancer diseases in which cells no longer respond Describe how cancers come from genomic mutations (inherited or somatic)
Mitosis and the Cell Cycle
 Mitosis and the Cell Cycle Chapter 12 The Cell Cycle: Cell Growth & Cell Division Where it all began You started as a cell smaller than a period at the end of a sentence Getting from there to here Cell
Mitosis and the Cell Cycle Chapter 12 The Cell Cycle: Cell Growth & Cell Division Where it all began You started as a cell smaller than a period at the end of a sentence Getting from there to here Cell
Assessing the role of Ku70 vwa domain phosphorylation in the inhibition of Aurora B and activation of the DNA damage response
 Western University Scholarship@Western Electronic Thesis and Dissertation Repository April 2017 Assessing the role of Ku70 vwa domain phosphorylation in the inhibition of Aurora B and activation of the
Western University Scholarship@Western Electronic Thesis and Dissertation Repository April 2017 Assessing the role of Ku70 vwa domain phosphorylation in the inhibition of Aurora B and activation of the
Shuttling towards a predictive assay for radiotherapy
 Editorial Shuttling towards a predictive assay for radiotherapy Faissal Ouenzar, Michael J. Hendzel, Michael Weinfeld Department of Oncology, University of Alberta, Edmonton, Canada Correspondence to:
Editorial Shuttling towards a predictive assay for radiotherapy Faissal Ouenzar, Michael J. Hendzel, Michael Weinfeld Department of Oncology, University of Alberta, Edmonton, Canada Correspondence to:
IDENTIFICATION OF NF-KAPPAB AND DNA-DEPENDENT PROTEIN KINASE (DNA-PK) AS NEW PLAYERS IN THE REGULATION AND SIGNALING OF THE ONCOGENIC PHOSPHATASE
 IDENTIFICATION OF NF-KAPPAB AND DNA-DEPENDENT PROTEIN KINASE (DNA-PK) AS NEW PLAYERS IN THE REGULATION AND SIGNALING OF THE ONCOGENIC PHOSPHATASE WIP1 A Dissertation submitted to the Faculty of the Graduate
IDENTIFICATION OF NF-KAPPAB AND DNA-DEPENDENT PROTEIN KINASE (DNA-PK) AS NEW PLAYERS IN THE REGULATION AND SIGNALING OF THE ONCOGENIC PHOSPHATASE WIP1 A Dissertation submitted to the Faculty of the Graduate
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Cell Quality Control. Peter Takizawa Department of Cell Biology
 Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Legends for Supplemental Figures.
 1 Legends for Supplemental Figures. Supplemental Figure 1. Proteasome Inhibition does not abrogate the expression of FA core complex proteins or formation of the FA core complex. (Full-length blots are
1 Legends for Supplemental Figures. Supplemental Figure 1. Proteasome Inhibition does not abrogate the expression of FA core complex proteins or formation of the FA core complex. (Full-length blots are
UNC-Duke Biology Course for Residents Fall
 UNC-Duke Biology Course for Residents Fall 2018 1 UNC-Duke Biology Course for Residents Fall 2018 2 UNC-Duke Biology Course for Residents Fall 2018 3 UNC-Duke Biology Course for Residents Fall 2018 4 UNC-Duke
UNC-Duke Biology Course for Residents Fall 2018 1 UNC-Duke Biology Course for Residents Fall 2018 2 UNC-Duke Biology Course for Residents Fall 2018 3 UNC-Duke Biology Course for Residents Fall 2018 4 UNC-Duke
Supplementary Information for. A cancer-associated BRCA2 mutation reveals masked nuclear. export signals controlling localization
 Supplementary Information for A cancer-associated BRCA2 mutation reveals masked nuclear export signals controlling localization Anand D Jeyasekharan 1, Yang Liu 1, Hiroyoshi Hattori 1,3, Venkat Pisupati
Supplementary Information for A cancer-associated BRCA2 mutation reveals masked nuclear export signals controlling localization Anand D Jeyasekharan 1, Yang Liu 1, Hiroyoshi Hattori 1,3, Venkat Pisupati
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
1. Identify and characterize interesting phenomena! 2. Characterization should stimulate some questions/models! 3. Combine biochemistry and genetics
 1. Identify and characterize interesting phenomena! 2. Characterization should stimulate some questions/models! 3. Combine biochemistry and genetics to gain mechanistic insight! 4. Return to step 2, as
1. Identify and characterize interesting phenomena! 2. Characterization should stimulate some questions/models! 3. Combine biochemistry and genetics to gain mechanistic insight! 4. Return to step 2, as
Prof. R. V. Skibbens. Cell Cycle, Cell Division and Cancer (Part 2)
 Prof. R. V. Skibbens November 22, 2010 BIOS 10: BioScience in the 21 st Century Cell Cycle, Cell Division and Cancer (Part 2) Directionality - clocks go in only one direction G1 doesn t have replication-inducing
Prof. R. V. Skibbens November 22, 2010 BIOS 10: BioScience in the 21 st Century Cell Cycle, Cell Division and Cancer (Part 2) Directionality - clocks go in only one direction G1 doesn t have replication-inducing
nuclear science and technology
 EUROPEAN COMMISSION nuclear science and technology Radiation-specific DNA non-double strand break lesions: repair mechanisms and biological effects (Non-DSB Lesions) Contract N o FIGH-CT2002-00207 Final
EUROPEAN COMMISSION nuclear science and technology Radiation-specific DNA non-double strand break lesions: repair mechanisms and biological effects (Non-DSB Lesions) Contract N o FIGH-CT2002-00207 Final
TUMOR-SUPPRESSOR GENES. Molecular Oncology Michael Lea
 TUMOR-SUPPRESSOR GENES Molecular Oncology 2011 Michael Lea TUMOR-SUPPRESSOR GENES - Lecture Outline 1. Summary of tumor suppressor genes 2. P53 3. Rb 4. BRCA1 and 2 5. APC and DCC 6. PTEN and PPA2 7. LKB1
TUMOR-SUPPRESSOR GENES Molecular Oncology 2011 Michael Lea TUMOR-SUPPRESSOR GENES - Lecture Outline 1. Summary of tumor suppressor genes 2. P53 3. Rb 4. BRCA1 and 2 5. APC and DCC 6. PTEN and PPA2 7. LKB1
Cellular Stress Response Induced by Low Dose Gamma Radiation
 Proceedings of the Pakistan Academy of Sciences 51 (2): 139 144 (2014) Pakistan Academy of Sciences Copyright Pakistan Academy of Sciences ISSN: 0377-2969 (print), 2306-1448 (online) Research Article Cellular
Proceedings of the Pakistan Academy of Sciences 51 (2): 139 144 (2014) Pakistan Academy of Sciences Copyright Pakistan Academy of Sciences ISSN: 0377-2969 (print), 2306-1448 (online) Research Article Cellular
Selective targeting of cancer cells through inhibition of Checkpoint kinase 1
 Selective targeting of cancer cells through inhibition of Checkpoint kinase 1 Grete Hasvold Department of Radiation Biology Institute for Cancer Research The Norwegian Radium Hospital Oslo University Hospital
Selective targeting of cancer cells through inhibition of Checkpoint kinase 1 Grete Hasvold Department of Radiation Biology Institute for Cancer Research The Norwegian Radium Hospital Oslo University Hospital
Course Title Form Hours subject
 Course Title Form Hours subject Types, and structure of chromosomes L 1 Histology Karyotyping and staining of human chromosomes L 2 Histology Chromosomal anomalies L 2 Histology Sex chromosomes L 1 Histology
Course Title Form Hours subject Types, and structure of chromosomes L 1 Histology Karyotyping and staining of human chromosomes L 2 Histology Chromosomal anomalies L 2 Histology Sex chromosomes L 1 Histology
ATM in focus: A damage sensor and cancer target
 BioDiscovery MONOGRAPH ATM in focus: A damage sensor and cancer target Hilal S Khalil, Hemanth Tummala, Nikolai Zhelev * School of Contemporary Sciences, University of Abertay Dundee, Dundee DD1 1HG, Scotland,
BioDiscovery MONOGRAPH ATM in focus: A damage sensor and cancer target Hilal S Khalil, Hemanth Tummala, Nikolai Zhelev * School of Contemporary Sciences, University of Abertay Dundee, Dundee DD1 1HG, Scotland,
PARP AND PROSTATE CANCER
 PARP AND PROSTATE CANCER Terrence P McGarty White Paper No 143 February 2017 DNA breaks often lead to Cancer. Double strand breaks are handled by BRCA gene products and Single strand breaks by PARP products.
PARP AND PROSTATE CANCER Terrence P McGarty White Paper No 143 February 2017 DNA breaks often lead to Cancer. Double strand breaks are handled by BRCA gene products and Single strand breaks by PARP products.
Targeting the ATR Kinase in Cancer Therapy
 Targeting the ATR Kinase in Cancer Therapy 2017 Chabner Colloquium October 30, 2017 Lee Zou MGH Cancer Center Harvard Medical School Disclosure Consultant/advisory role: Loxo Oncology DNA Damage and Replication
Targeting the ATR Kinase in Cancer Therapy 2017 Chabner Colloquium October 30, 2017 Lee Zou MGH Cancer Center Harvard Medical School Disclosure Consultant/advisory role: Loxo Oncology DNA Damage and Replication
609G: Concepts of Cancer Genetics and Treatments (3 credits)
 Master of Chemical and Life Sciences Program College of Computer, Mathematical, and Natural Sciences 609G: Concepts of Cancer Genetics and Treatments (3 credits) Text books: Principles of Cancer Genetics,
Master of Chemical and Life Sciences Program College of Computer, Mathematical, and Natural Sciences 609G: Concepts of Cancer Genetics and Treatments (3 credits) Text books: Principles of Cancer Genetics,
CELL CYCLE REGULATION AND CANCER. Cellular Reproduction II
 CELL CYCLE REGULATION AND CANCER Cellular Reproduction II THE CELL CYCLE Interphase G1- gap phase 1- cell grows and develops S- DNA synthesis phase- cell replicates each chromosome G2- gap phase 2- cell
CELL CYCLE REGULATION AND CANCER Cellular Reproduction II THE CELL CYCLE Interphase G1- gap phase 1- cell grows and develops S- DNA synthesis phase- cell replicates each chromosome G2- gap phase 2- cell
Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy
 Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
Kavya Puchhalapalli CALS Honors Project Report Spring 2017 Studying The Role Of DNA Mismatch Repair In Brain Cancer Malignancy Abstract Malignant brain tumors including medulloblastomas and primitive neuroectodermal
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells
 Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Supplementary Figure S1: Defective heterochromatin repair in HGPS progeroid cells Immunofluorescence staining of H3K9me3 and 53BP1 in PH and HGADFN003 (HG003) cells at 24 h after γ-irradiation. Scale bar,
Regulation of Cell Division (Ch. 12)
 Regulation of Cell Division (Ch. 12) Coordination of cell division A multicellular organism needs to coordinate cell division across different tissues & organs critical for normal growth, development &
Regulation of Cell Division (Ch. 12) Coordination of cell division A multicellular organism needs to coordinate cell division across different tissues & organs critical for normal growth, development &
Complexity DNA. Genome RNA. Transcriptome. Protein. Proteome. Metabolites. Metabolome
 DNA Genome Complexity RNA Transcriptome Systems Biology Linking all the components of a cell in a quantitative and temporal manner Protein Proteome Metabolites Metabolome Where are the functional elements?
DNA Genome Complexity RNA Transcriptome Systems Biology Linking all the components of a cell in a quantitative and temporal manner Protein Proteome Metabolites Metabolome Where are the functional elements?
Chapter 12. Regulation of Cell Division. AP Biology
 Chapter 12. Regulation of Cell Division Coordination of cell division! Multicellular organism " need to coordinate across different parts of organism! timing of cell division! rates of cell division "
Chapter 12. Regulation of Cell Division Coordination of cell division! Multicellular organism " need to coordinate across different parts of organism! timing of cell division! rates of cell division "
Modelling of Biological Processes
 Modelling of Biological Processes WHAT HAPPENS AFTER EARLY MOLECULAR DAMAGE? Stephen McMahon Queen s University, Belfast, Northern Ireland 3 rd August 2016 1 Do we need biology? The Linear-quadratic relationship
Modelling of Biological Processes WHAT HAPPENS AFTER EARLY MOLECULAR DAMAGE? Stephen McMahon Queen s University, Belfast, Northern Ireland 3 rd August 2016 1 Do we need biology? The Linear-quadratic relationship
Chemistry 107 Exam 4 Study Guide
 Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
CELL CYCLE,CHECK POINTS. Prof S.N.Senapati, A.H.REGIONAL CANCER CENTRE, MANGALABAG, CUTTACK
 CELL CYCLE,CHECK POINTS Prof S.N.Senapati, A.H.REGIONAL CANCER CENTRE, MANGALABAG, CUTTACK E-Mail:-snsenapati2007@gmail.com IN RADIATION ONCOLOGY TUMOR CELL DEATH CELL DEATH AFTER IRRADIATION OCCURS MOSTLY
CELL CYCLE,CHECK POINTS Prof S.N.Senapati, A.H.REGIONAL CANCER CENTRE, MANGALABAG, CUTTACK E-Mail:-snsenapati2007@gmail.com IN RADIATION ONCOLOGY TUMOR CELL DEATH CELL DEATH AFTER IRRADIATION OCCURS MOSTLY
DNA repair and synthetic lethality
 Washington University School of Medicine Digital Commons@Becker Open Access Publications 2011 DNA repair and synthetic lethality Gong-she Guo Shandong University Feng-mei Zhang Shandong University Rui-jie
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2011 DNA repair and synthetic lethality Gong-she Guo Shandong University Feng-mei Zhang Shandong University Rui-jie
Regulation of Cell Division. AP Biology
 Regulation of Cell Division 2006-2007 Coordination of cell division A multicellular organism needs to coordinate cell division across different tissues & organs critical for normal growth, development
Regulation of Cell Division 2006-2007 Coordination of cell division A multicellular organism needs to coordinate cell division across different tissues & organs critical for normal growth, development
Supplementary Figure 1: High-throughput profiling of survival after exposure to - radiation. (a) Cells were plated in at least 7 wells in a 384-well
 Supplementary Figure 1: High-throughput profiling of survival after exposure to - radiation. (a) Cells were plated in at least 7 wells in a 384-well plate at cell densities ranging from 25-225 cells in
Supplementary Figure 1: High-throughput profiling of survival after exposure to - radiation. (a) Cells were plated in at least 7 wells in a 384-well plate at cell densities ranging from 25-225 cells in
Radiobiological Effects of Alpha-Particles from Astatine-211
 Radiobiological Effects of Alpha-Particles from Astatine-211 From DNA Damage to Cell Death Kristina Claesson Department of Oncology Institute of Clinical Sciences The Sahlgrenska Academy at University
Radiobiological Effects of Alpha-Particles from Astatine-211 From DNA Damage to Cell Death Kristina Claesson Department of Oncology Institute of Clinical Sciences The Sahlgrenska Academy at University
Eukaryotic transcription (III)
 Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
Evidence for variation in human radiosensitivity: potential impact on radiological protection
 Evidence for variation in human radiosensitivity: potential impact on radiological protection Simon Bouffler 22 October 2015 ICRP Symposium 2015, Seoul, South Korea Current system of protection Avoid tissue
Evidence for variation in human radiosensitivity: potential impact on radiological protection Simon Bouffler 22 October 2015 ICRP Symposium 2015, Seoul, South Korea Current system of protection Avoid tissue
Ex vivo functional assays for Homologous Recombination deficiency in breast cancer. Dik C. van Gent
 Ex vivo functional assays for Homologous Recombination deficiency in breast cancer Dik C. van Gent Breast cancer types treatments ER/PR: anti-hormonal therapy HER2: Herceptin Triple negative (TNBC): no
Ex vivo functional assays for Homologous Recombination deficiency in breast cancer Dik C. van Gent Breast cancer types treatments ER/PR: anti-hormonal therapy HER2: Herceptin Triple negative (TNBC): no
Regulation of DNA double-strand break repair pathway choice
 Regulation of DSB repair pathway choice 134 REVIEW Cell Research (2008) 18:134-147. 2008 IBCB, SIBS, CAS All rights reserved 1001-0602/08 $ 30.00 www.nature.com/cr Regulation of DNA double-strand break
Regulation of DSB repair pathway choice 134 REVIEW Cell Research (2008) 18:134-147. 2008 IBCB, SIBS, CAS All rights reserved 1001-0602/08 $ 30.00 www.nature.com/cr Regulation of DNA double-strand break
Spring 2015 Module 2 System Engineering and Protein Founda<ons
 20.109 Spring 2015 Module 2 System Engineering and Protein Founda
20.109 Spring 2015 Module 2 System Engineering and Protein Founda
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system
 Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Bio 111 Study Guide Chapter 17 From Gene to Protein
 Bio 111 Study Guide Chapter 17 From Gene to Protein BEFORE CLASS: Reading: Read the introduction on p. 333, skip the beginning of Concept 17.1 from p. 334 to the bottom of the first column on p. 336, and
Bio 111 Study Guide Chapter 17 From Gene to Protein BEFORE CLASS: Reading: Read the introduction on p. 333, skip the beginning of Concept 17.1 from p. 334 to the bottom of the first column on p. 336, and
