Pregnane X Receptor SUMOylation and De-SUMOylation
|
|
|
- Lilian Fisher
- 6 years ago
- Views:
Transcription
1 Pregnane X Receptor SUMOylation and De-SUMOylation By Chang Liu Submitted to the graduate degree program in Pharmacology and Toxicology and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Master of Science. Chairperson Dr. Jeff Staudinger Dr. Nancy Muma Dr. Alexander Moise
2 The Thesis Committee for Chang Liu certifies that this is the approved version of the following thesis: Pregnane X Receptor SUMOylation and De-SUMOylation Chairperson Dr. Jeff Staudinger Date approved: ii
3 Abstract The pregnane x receptor (PXR, NR1I2) is a member of the nuclear receptor (NR) superfamily of ligand-activated transcription factors. PXR is activated by numerous lipophilic compounds, a variety of drugs and drug metabolites in clinical use. It regulates xenobiotic-inducible cytochrome P450 (CYP) expression in the liver and intestine which are major organs for xenobiotic biotransformation. While PXR has been identified as the positive regulator of many drug metabolizing enzymes (DMEs) and membrane transporter proteins, the effect of certain PXR activators is to repress the inflammatory response. Although it has been known for 40 years now that the PXR activator rifampicin inhibits immunological responses in liver cells, the mechanisms remain poorly understood. Previous results indicate that modification of PXR by small ubiquitin-related modifier (SUMO) likely contributes to this phenomenon. We hypothesize that PXR is SUMOylated to transrepress the inflammatory response genes in liver. Here, this thesis examines PXR SUMOylation/de-SUMOylation reaction mechanisms using in vitro and cell-based methods. Bacterial expression and purification systems were used in conjunction with in vitro SUMOylation reactions to further analyze PXR SUMOylation by both SUMO-1 and SUMO-3. The data reveal that the E3 SUMO-protein ligase protein inhibitor of activated STAT protein y (PIASy) potentiated SUMOylation of the PXR ligand binding domain (PXR-LBD) in vitro. Cell-based methods were used to characterize the de-sumoylation of PXR using expression vectors encoding six different sentrin protease (SENP) enzymes including SENP1, SENP2, SENP3, SENP5, SENP6, and SENP7. Both SENP1 and SENP2 effectively iii
4 de-conjugated both SUMO-1 and SUMO-3 from PXR, whereas SENP6 inhibited the formation of SUMO-3 chains on PXR. Collectively, the data suggest that (1) PXR can serve as a substrate for either SUMO-1 or SUMO-3 in vitro and in cell-based assays, (2) both SENP1 and SENP2 are able to remove SUMO moieties in a cellular environment, and (3) that SENP6 removes or prevents the formation of SUMO-chains on PXR. Taken together, the work presented in this thesis contributes to understanding the interface between PXR activators, the SUMOylation pathway and PXR activity. Future efforts should seek to determine the extent to which the biochemical mechanisms described here function in human liver and intestine in patients undergoing therapy with PXR activators. These studies are deemed critical for safe pharmacological strategies for addressing adverse drug reactions and provide a new paradigm for exploring novel approaches to repress inflammatory signaling in liver and intestine. iv
5 Acknowledgement First and foremost, I would like to thank my advisor Dr. Jeff Staudinger for his advice and guidance in the completion of this thesis. Not only is he an excellent scientist, but his creativity and passion for science have been an inspiration to me throughout my graduate career. In addition, I would like to thank all Staudinger lab members, specifically Ms. Wenqi Cui, for their patience, instruction and guidance. I would also like to thank the faculty of the Department of Pharmacology and Toxicology for their role in my education and development at a scientist. I am very grateful to Dr. Honglian Shi and Dr. Alex Moise for serving on my committee and for providing me with valuable suggestions, criticisms and guidance. Finally, I would like to thank my family, specifically my fiancéxu Wang, for their unwavering support in this pursuit. I am thankful for the financial support that I have received. The research presented in this dissertation was funded by the National Institute of General Medical Sciences (P20 GM103418) and the National Institute of Digestive Diabetic and Kidney Diseases (R01DK090558) from the National Institutes of Health. v
6 List of Abbreviations AF-1: Activation Function 1 AF-2: Activation Function 2 AR: Androgen Receptor CAR: Constitutive Androstane Receptor CBP: CREB Binding Protein CCRP: Cytoplasmic CAR Retention Protein COX2: Cyclooxygenase 2 CREB: Cyclic AMP Response Element Binding Protein CYP: Cytochrome P450 DBD: DNA-binding Domain DME: Drug Metabolizing Enzyme ER: Estrogen Receptor FOXO1/A2: Forkhead Box Transcription Factor O1/A2 FXR: Farnesoid X Receptor G6Pase: Glucose 6 Phosphatase GR: Glucocorticoid Receptor GST: Glutathione S Transferase vi
7 HAT: Histone Acetyl Transferase HDAC: Histone Deacetylase HMGCS2: 3-Hydroxy-3-Methylglutarate-CoA Synthase 2 HNF4α: Hepatocyte Nuclear Factor 4 Alpha HSP90: Heat Shock Protein 90 IBD: Inflammatory Bowel Disease LBD: Ligand-binding Domain ICAM-1: Intercellular Adhesion Molecule 1 IFN : Interferon Gamma IL: Interleukin LBD: Ligand-binding Domain LCA: Lithocholic Acid LPS: Lipopolysaccharide LXR: Liver X Receptor MDR1: Multi-drug Resistance 1 MRP2/3: Multi-drug Resistance Associated Protein 2/3 NCoR: Nuclear Receptor Co-repressor NF-κB: Nuclear Transcription Factor Kappa B vii
8 NLS: Nuclear Localization Signal NR: Nuclear Receptor OATP2: Organic Ion Transporting Protein 2 PCN: Pregnenolone 16α-carbonitrile PEPCK: Phosphoenolpyruvate Carboxykinase PGC-1α: Peroxisome Proliferator Activated Receptor Gamma Co-activator 1 Alpha PI3K: Phosphatidylinositol 3-Kinase PKA: Cyclic-AMP-dependent Protein Kinase PPAR: Perioxisome Proliferator Activated Receptor PXR: Pregnane X Receptor PXRE: PXR Response Elements RAR: Retinoic Acid Receptor RIF: Rifampicin RXR: Retinoid X Receptor SF-1: Steroidogenic Factor 1 SMRT: Silencing-mediator for Retinoid and Thyroid Hormone Receptors SRC: Steroid Receptor Co-activator viii
9 SREBP: Sterol Regulatory Element Binding Protein SULT: Sulfotransferase TNFα: Tumor Necrosis Factor Alpha VDR: Vitamin D Receptor XREM: Xenobiotic Responsive Enhancer Module ix
10 Table of Contents Acceptance Page... ii Abstract... iii Acknowledgements...v List of Abbreviations... vi Table of Contents... x List of Table... xiii List of Figures... xiv Chapter 1: Introduction 1. General Introduction to NR Superfamily 1 2. NR Co-regulators.3 3. Pregnane X Receptor Physiological Functions of PXR PXR is a Positive Regulator of Genes Encoding Enzymes that Participate in Biotransformation Xenobiotic Responses Bile Acid Homeostasis Negative Physiological Functions...13 x
11 4.2.1 Glucose Homeostasis Lipid Metabolism Inflammatory Response Mechanisms of PXR Transcriptional Regulation PXR SUMOylation 24 Chapter 2: Materials and Methods 1. Cell Culture and Treatment of Hepa1-6 Cells RNA Isolation and q-pcr Analysis Bacterial Expression and Purification of GST-tagged Fusion Proteins In Vitro SUMOylation Assay Cell-Based SUMOylation Assay...35 Chapter 3: Results 1. Expression of PXR Transrepresses the Induction of Endogenous Inflammatory Response Genes in Hepa1-6 cells The PXR Protein Is SUMOylated in vitro PXR is Preferentially SUMOylated in Hepa1-6 Cells by SUMO3 and PIASy The Human PXR-K108 Residue Is A SUMOylation Site.50 xi
12 5. Inflammatory Mediator LPS Induces SUMOylation of PXR and human PXR K108R Mutant Sentrin Proteases Differentially De-SUMOylate PXR-SUMO Conjugates in Hepa1-6 Cells SENP6 Together with Human PXR Mutants Provide Insights into PXR SUMOylation Sites Prediction..59 Chapter 4: Discussion 1. PXR SUMOylation/De-SUMOylation Mechanism SUMO-1 versus SUMO E3 Ligases De-SUMOylation PXR SUMOylation Sites PXR Transrepression Model Clinical Significance Conclusion References..79 xii
13 List of Table Table 1. PXR SUMOylation Pattern 73 xiii
14 List of Figures Figure 1. Domain Structure of NRs.2 Figure 2. Regulation of Bile Acid Homeostasis by PXR...12 Figure 3. Mutual Transrepression between PXR and NF- B Signaling Pathway...20 Figure 4. PXR Activity Is Regulated by Different Co-factors Interactions...23 Figure 5. PXR Reversible SUMOylation Pathway...26 Figure 6. The Sentrin Proteases...29 Figure 7. Model of PXR-mediated Transrepression of Inflammatory Response Pathway...32 Figure 8. Expression of PXR Transrepresses the Induction of Endogenous Inflammatory Response Genes in Hepa1-6 Cells...39 Figure 9-1. Structure and Post-translational Modification of Human PXR Lysine Residues...42 Figure 9-2. Purification Scheme for GST-tagged Proteins...42 Figure 9-3. SDS-PAGE Analysis of Purified Proteins...43 Figure 9-4. In Vitro SUMOylation of GST-human PXR LBD by SUMO-1 and SUMO-3 is enhanced by PIASy Figure 10. Detection of SUMOylated Human PXR Protein in Hepa1-6 Cells. 47 xiv
15 Figure 11. The Lysine Residue 108 Mutation Down-regulates PXR SUMOylation...52 Figure Inflammatory Stimulus and Ligand Modulate Wide Type Human PXR Protein SUMO-1 Modification in Hepa1-6 Cells Figure K108 Is Not the Primary LPS-inducible SUMOylation Residue Figure Cell-based Analysis of Human PXR SUMOylation/De-SUMOylation by SUMO-1 and Sentrin Proteases in Hepa1-6 Cells Figure Cell-based Analysis of Human PXR SUMOylation/De-SUMOylation by SUMO3 and Sentrin Proteases in Hepa1-6 Cells Figure Detection Wide Type Human PXR and Human PXR-K108R Mutant SUMO-3 Modification in Hepa1-6 Cells Figure Detection Wide Type Human PXR and Human PXR-K108R Mutant SUMO-1 Modification in Hepa1-6 Cells Figure PXR is Mainly SUMOylated in the Hinge Domain by SUMO Figure PXR is Mainly SUMOylated in the Hinge Domain by SUMO xv
16 Introduction 1. General Introduction to NR Superfamily Historically, the lipophilic hormones, including steroids, retinoids and thyroids, are known as regulators of differential control of gene expression in development, cell differentiation, metamorphosis and organ physiology (1). The steroid receptors were first identified in the mid-1980s with the help of purified hormones and antibodies (2-5). The cloning of cdnas encoding the glucocorticoid (GR) and estrogen (ER) receptors has led to the discovery of the receptors for numerous fat-soluble hormones (6). During this time, the receptors were found to exhibit high sequence similarity; hence the concept of the existence of a NR superfamily emerged. By 1990, there were a total of 15 members in the NR superfamily. To date, there are 48 functional NR family members encoded in the human genome. A group of receptors, called orphan receptors, were first found in metazoan species based on low stringency hybridization screening technique and PCR-based cloning strategies (1). However, at the time of their cloning, their physiological ligands were not known. Effort to identify the physiological ligands of orphan receptors enabled receptor adoption after their ligands were found. This search has led to the discovery of novel metabolic gene regulation networks and endocrine signaling pathways in animal biology (7). Members of adopted NRs include receptors for fatty acids (peroxisome proliferator-activated receptors (PPARs)), bile acids (farnesoid x receptor (FXR)), oxysterols (liver x receptors (LXRs)) and xenobiotics (PXR). 1
17 All members of NR superfamily share several conserved domains that are essential for receptor function. At the N-terminal region of NRs, the activation function 1 (AF-1) domain can act to activate transcription of target genes in a ligand-independent manner (8). It contains consensus phosphorylation sites and is responsive to several kinase signaling pathways (9). The DNA binding domain (DBD) contains two helices and two zinc fingers that provide DNA binding specificity. The DBD of NRs binds to well-defined hormone response elements (HREs), and also imparts NR dimerization characteristics (10). Most NRs function as dimers, while some NRs are active as monomers, such as steroidogenic factor 1 (SF-1) (11). Most steroid NRs function as homodimers and bind to DNA binding sites organized as inverted repeats (12). Several important liver-enriched NRs function as heterodimers with the retinoid X receptor (RXR) including LXR, FXR, constitutive androgen receptor (CAR) and PXR. The C-terminal region of NRs includes the activation function-2 (AF-2) domain and the ligand binding domain (LBD). The LBD is connected to DBD by a flexible hinge domain which contains a nuclear localization signal (NLS). The AF-2 domain is predicted to undergo a conformational change upon ligand binding, and also serves as a binding surface for co-activator proteins necessary for transactivation (13). (Figure 1) 2
18 Figure 1. Domain Structures of Nuclear Receptors. Most members of the NR superfamily have a common domain structure consisting of an N-terminal AF-1, a central DBD and a C-terminal LBD. 2. NR Co-regulators Expression cloning and functional characterization of a larger number of proteins which interact with NRs in either a ligand-dependent or independent manner have gained increasing attention (14). NR co-regulator proteins are critical components of large multi-protein complexes, including co-activators and co-repressors, which do not bind to DNA directly but have pivotal roles in regulation of target gene expression (15). Generally, genes are maintained in an off-state by recruitment of co-repressor complexes. Such complexes include nuclear receptor corepressor (NCoR) and silencing mediator for retinoic acid and thyroid hormone receptors (SMRT) (16). NCoR and SMRT do not harbor intrinsic enzymatic activity; instead they serve as the scaffold to interact with other protein co-factors which contain intrinsic histone deactylase (HDAC) activity (17). Non-liganded NRs recruit HADCs to remove acetyl groups from histone tails and induce chromatin compaction that prevents association of basal transcriptional machinery with enhancer regions of genes (18). This leads to NRs-mediated active repression of target gene expression in the non-liganded condition. The binding of ligand to NRs results in the dissociation of the co-repressor complexes and subsequent recruitment of co-activator proteins to initiate target 3
19 gene transcription, which is called transactivation (19). Most co-activators proteins interact with NRs in a ligand-dependent manner through the C-terminal AF-2 domain via an -LXXLL- motif, where L is leucine and X is any amino acid (20). The NR-associated co-activator multi-protein complexes contain both intrinsic and extrinsic histone acetyltransferase (HAT) activity (21), a chromatin remodeling function (22), as well as mrna elongation and splicing activity (23). Steroid receptor co-activator (SRC) proteins are co-activators for PXR transactivation. The SRC family contains intrinsic HAT activity and it also interacts with another HAT and p300/cbp-associated factor (PCAF) to form the co-activator complexes (24). Besides ligand-induced co-regulator exchange, the activation of cell signaling cascades and phosphorylation events by protein kinase directly regulate the strength of interaction between the NRs and co-regulator multi-protein complexes (25-27). Understanding the role of cell signaling pathways that modulate NRs protein-protein interactions, DNA binding and transcriptional activity is necessary for functional implication of these signaling events. Based on the enzymatic functions of co-regulator proteins, NRs can likely regulate gene transcription by different mechanisms, including ligand-dependent transcriptional activation, ligand-independent active repression and transrepression (28). Several important questions regarding the molecular mechanisms of NRs-mediated gene transrepression will be addressed in the next few sections of this thesis. 4
20 3. Pregnane X Receptor (PXR) The mouse pregnane x receptor (PXR, NR1I2) was first identified in 1998 based on its sequence homology to the LBD of a number of NRs in the motif search of sequence tag databases (7). The receptor was named as PXR based on its activation by several synthetic pregnanes (7). The tissue-specific expression profiles of PXR were examined and characterized. Northern blot analysis showed that abundant level of PXR mrna is observed in liver and at a moderate level in the intestine (29). Since the promoter which drives Pxr gene expression has not been well characterized, the molecular mechanisms underlying this tissue-specific expression of PXR are currently unknown. Previous studies suggest that the Pxr gene might be regulated by liver-enriched transcription factor hepatic nuclear factor 4 (HNF-4 and/or steroid hormones (31,32). PXR functions as a heterodimer with RXR and is activated by numerous prescription drugs, xenobiotics, steroids and toxic bile acids (33). In fact, elucidation of the crystal structure of the LBD of human PXR revealed that it has a relatively large and spherical ligand-binding pocket which allows it to interact with a vast variety of hydrophobic chemicals (33,34). However, marked differences in the activation profiles of PXR across species exist. The rodent-specific PXR ligand pregnenalone 16 -carbonitrile (PCN) and several other naturally occurring 21-carbon steroids have little or no effect on PXR activity in human and rabbit hepatocytes. On the other hand, rifampicin (Rif), the human PXR activator, fails to induce the transcription of Cyp3a11 which is 5
21 the prototypical PXR-target gene in mouse and rat hepatocytes (29,35). The recent availability of high-resolution PXR LBD crystal structure has provided critical clues that elucidate the basis for the observed species-specific differences in PXR activation profiles (33,36,37). The comparison of the residues in the LBD pocket of PXR across species showed that only four polar residues are different between mouse and human PXR. Recent targeted site-directed mutagenesis studies of the four key residues effectively humanized mouse PXR and conferred mouse PXR a human-like response in a cellular environment (33,38). Upon biding with the ligand, the PXR-RXR heterodimer binds to the PXR response elements (PXREs) that are located in the CYP promoter region and activates CYP gene expression in liver (39). In addition, PXR regulates the expression of numerous other genes involved in the metabolism and transport of xenobiotic compound, including phase I oxidation enzymes carboxylesterases (CESs) (40), phase II conjugation enzymes glutathione S-transferases (GSTs) (41) and sulfotransferases (SULTs) (42), and the genes encoding phase III drug transporters organic anion transporting polypeptide 2 (Oatp2) (43) and multi-drug resistance-associated protein family (Mrp2 and Mrp3) (44) in the entero-hepatic system. 4. Physiological Functions of PXR 4.1 PXR is a Positive Regulator of Genes Encoding Enzymes that Participate in Biotransformation Xenobiotic Responses 6
22 PXR is activated by a myriad of lipophilic compounds, including certain natural and synthetic steroids, bile acids, and a variety of drugs (7). It is a master-regulator of drug-inducible CYP3A gene expression in drug metabolism and detoxification and elimination of xeno-chemicals prevalent in the environment (45). When specific xenobiotic compounds enter the liver, they activate PXR and up-regulate gene transcription for gene products responsible for xenobiotic biotransformation reactions. The catalytic action of the CYP3A family of enzymes, as well as other drug metabolizing enzymes (DMEs) in the liver, increases the water solubility of substrates mainly through oxidative metabolic reactions that result in the addition of hydrophilic groups to polar substrates. Additional PXR-target genes encode key drug transporter proteins that promote the uptake and excretion of xenobiotics to urine and bile. In this manner, PXR activation represents an adaptive response to protect the body against toxic assaults through accelerating the clearance of xenobiotics by inducing drug metabolism and drug transport (46). PXR activation and up-regulation of CYP3A genes also form the molecular basis for an important class of drug-drug, herb-drug, and food-drug interactions in patients on combination therapy (47). Many of the xenobiotics that induce PXR are widely used prescription drugs, such as the antibiotic Rif (29), the glucocorticoid dexamethasone (32), and the antimycotic clotrimazole (48). The human CYP3A4 enzyme is involved in the oxidative metabolism of approximately 50-60% of all clinically prescribed drugs (49). Therefore, PXR-mediated activation of high levels of CYP3A in the liver would be 7
23 expected to interfere with the pharmacokinetics to increase metabolism of other co-administered drugs which are CYP3A substrates. Since many patients are likely to take more than one medication simultaneously (50), this phenomenon can be a serious problem during combination therapy, especially in patients with underlying co-morbidities that alter drug metabolic pathways. The major clinical consequence is therapeutic failure because of the undesirable lowering of the plasma level of the affected drug to non-therapeutic range (51,52). The active compounds in natural herbal remedies contribute to inadvertent activation of PXR and thereby represent the molecular basis for a significant portion of herb-drug interactions (53-55). An example is the compound hyperforin, the active component of St John s wort (Hypericum perforatum L.), which binds to PXR with high affinity and activates CYP gene expression (56,57) to enhance the oxidative metabolism of various prescription drugs, including combination oral contraceptives, cyclosporin, and indinavir (58-63). Food constituents also have the ability to activate PXR and are responsible for food-drug interactions (55,64). Food consisting of phytochemical mixtures, such as fruits, vegetables (65), beverages, and teas possess the ability to alter the activity of CYP3A through PXR activation (66), and modulate the metabolism of more than 50% of clinical pharmaceuticals by CYP3A (67). Taken together, PXR activation in the liver and intestine could lead to undesirable life-threatening drug-drug and supplement-drug interactions in patients using combination therapy. Therefore, the drug development industry is employing high throughput in vitro and in vivo PXR activation and binding 8
24 assays to test novel drug candidates for their ability to induce CYP3A gene expression (68). In order to reduce adverse drug effect, the ideal drug candidate should be neutral with respect to their effects on CYP gene expression (69). The compounds might be removed from the drug development process if they are potent PXR activators (34). For example, the anti-diabetic drug troglitazone, a potent PPAR activator (70), was withdrawn from the market due to hepatotoxicity (71). It activates PXR at the therapeutic dose (35) and its metabolites quinones catalyzed by CYP 2C8 and CYP3A4 are active intermediates in drug-induced hepatic toxicities (72-74). In the contrast, the related drugs rosiglitazone and pioglitazone, which are potent PPAR activators and remedies for diabetes (75), do not activate PXR and form reactive quinones metabolites (76). Thus they have not shown any evidence of hepatotoxicity in patients (72,77,78) PXR Regulates Bile Acid Homeostasis In addition to a myriad of foreign chemicals, animals confront numerous endogenous toxicants whose efficient detoxification is important to animal survival (79). Bile acids are synthesized from cholesterol in the liver and are among the first natural products isolated in pure form (80). They are present in dried extracts of bear bile, which have been used for their medicinal properties by Asian cultures for thousands of years (81,82). Bile acid synthesis provides a direct means of converting a cholesterol molecule, which is the insoluble and hydrophobic membrane constituent, to a bile acid molecule that, when ionized, is 9
25 the membrane-dissolving, water-soluble and readily-excreted detergent (83,84). The physiological roles of bile acids involve elimination of cholesterol, absorption of dietary lipids, hepatic bile formation and regulation of hepatic cholesterol synthesis (85). The primary bile acids, including cholic acid and chenodeoxycholic acid, are synthesized in the liver through cholesterol oxidative catabolism (86). The secondary bile acids deoxycholic acid and lithocholic acid (LCA) are formed in the intestine from primary bile acids (87). The synthesis of a full complement of bile acids is a complex process, requiring 17 enzymes (84,88) and the multi-step conversion of cholesterol to bile acids confers the detergent-like properties to the bile acids that are essential for their physiological functions (89). The expression of selected enzymes in the bile acids synthesis pathway is tightly regulated by NRs and other transcription factors, which ensure a constant supply of bile acids and the maintenance of hepatic cholesterol catabolism (83). While FXR (NR1H4) and LXR (NR1H3) play a fundamental role in regulating bile acid and cholesterol levels in the liver (79,90), PXR is also involved in the regulation of biosynthesis, transport and metabolism of bile acids that are extremely toxic at excessive concentrations occurring during hypercholesterolemia and cholestasis (79,91). The rate limiting step in bile acid formation is the 7-hydroxylation of cholesterol by CYP7A1 enzyme (92). PCN treatment negatively regulates Cyp7a1 expression in mice, which suggests that PXR activation blocks bile acids synthesis (93). Among the genes that are up-regulated by PCN treatment are 10
26 Oatp2 and CYP3a11 (43,45). The CYP3A11 enzyme catalyzes the hydroxylation of bile acids, and OatP2 transports bile acids across the sinusoidal and canalicular membranes of hepatocytes (84) (Figure 2). Since PXR regulates key enzymes in bile acid PXR biosynthesis, transport and metabolism, it provides a fundamental molecular basis for Rif treatment in patients suffering from chronic cholestatic liver disease (43,94). Cholestasis, a pathogenic state characterized by cessation or impairment of bile flow and the accumulation of toxins normally excreted in bile, can cause nutritional imbalance and irreversible liver damage (95). LCA is a highly toxic secondary bile acid that causes cholestasis when accumulated in the body (43,79). The harmful effects of LCA and other bile acids are attenuated by two mechanisms, namely hydroxylation and conjugation, for elimination and detoxification of bile acids (96). LCA at the pathophysiological level binds to PXR, whose activation down-regulates Cyp7a1 to block bile acids synthesis, accelerates metabolism catalyzed by CYP3A and promotes uptake of bile acids from the blood by induction of OatP2 (43,97). These reactions make LCA more hydrophilic and facilitate the excretion of bile acids in the urine or feces. Therefore, PXR is critical as an endogenous bile acid receptor and regulates bile acid homeostasis in bile acid elimination and detoxification. Potent PXR ligands may be efficacious in the treatment of cholestasis and other hepatic diseases (98). 11
27 Figure 2 Figure 2. Regulation of Bile Acid Homeostasis by PXR. PXR is activated by bile acids and regulates genes involved in the biosynthesis, transport and metabolism of cholesterol and bile acids. Genes up-regulated by PXR are indicated by the green arrows. Genes down-regulated by PXR are indicated by the red line. PXRE, PXR response element; MRP2, Multidrug resistance associated protein 2. 12
28 4.2 Negative Physiological Functions of PXR Besides the involvement in the regulation of xenobiotics/endobiotics metabolism and transport, PXR also governs ligand-dependent repressive effects upon gluconeogenesis, lipid -oxidation and subsequent ketogenesis, and the inflammatory response, likely through crosstalk with other signaling pathways controlling these essential biological functions Glucose Homeostasis Hepatic glucose production is tightly regulated by insulin and glucagon signaling pathways which play a major role in animal survival during fasting and starvation. Genes that are involved in gluconeogenesis include glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (Pepck), which are the rate-limiting enzymes that control the serum level of glucose (99). In the liver, gluconeogenesis is positively regulated by glucagon, camp and glucocorticoid, which increase glucose production by induction the transcription of G6Pase and Pepck, whereas insulin and glucose suppress the expression of these genes (100). Ligand-mediated activation of PXR interferes with the regulatory gene networks that control glucose homeostasis. For example, PCN decreases blood glucose levels in fasting wild-type mice but not in PXR-null mice (101). Pepck1 and G6Pase genes are reduced in the transgenic mice expressing constitutively activated PXR in the liver and intestine (102). These observations are in consistent with the result that PXR activation down-regulates the transcriptional activity of FoxO1, a positive regulator of gluconeogenic genes (103). 13
29 FoxO1 is a member of the fork head family of transcription factors which are characterized by the fork head domain (104). The de-phosphorylated form of FoxO1 positively regulates the transcription of genes involved in gluconeogenesis. Insulin exerts a repressive action on FoxO1 by activating the phosphatidylinostiol-3-kinase (PI3K)-Akt pathway to increase phosphorylation of FoxO1 (105). The phosphorylated FoxO1 protein is then sequestered in the cytoplasm to remain transcriptionally inactive (106). Additional studies reveal crosstalk between FoxO1 and PXR (103,107). Ligand-dependent activation of PXR prevents FoxO1 binding to its insulin response sequences in target genes such as G6Pase and Pepck, thereby inactivating FoxO1 transcriptional activity (100,103). Glucose production is also mediated by liver-enriched hepatic nuclear factor 4α (HNF4α) transactivation through its recruitment of co-activator protein, PPARγ coactivator-1α (PGC-1α) (108). Ligand-activated PXR interferes with the interaction of HNF4 with PGC-1α to suppress HNF4α transactivation (109). This represents another mechanism by which PXR ligand-dependent activation might suppress hepatic gluconeogenesis. Finally, it has been proposed that PXR could interfere with the camp-response element binding protein (CREB) signaling pathway (101). CREB is phosphorylated by camp-dependent protein kinase (PKA) in response to glucagon secretion during fasting state. CREB binds to camp-responsive elements (CRE) to activate the transcription of genes encoding gluconeogenic enzymes G6Pase and PEPCK1 (110). PXR binds to CREB to prevent the 14
30 interaction between CREB and CRE on the promoter regions of these genes, thus repressing CREB-mediated gene activation programs. In this manner, the crosstalk between the signaling pathways that control glucose homeostasis and drug metabolism demonstrate that PXR directly interacts with other transcription factors and accessory proteins, such as FOXO1, CREB, and PGC-1α, all of which are critical for efficient gluconeogenic gene transcriptional regulatory circuits (111) Lipid Metabolism As described above, liver is the major site for drug metabolism. Liver is also the organ that metabolizes lipids to produce energy in the form of ATP from fat during the fasting and starvation response. Hepatic lipid homeostasis involves balanced lipid synthesis (lipogenesis), fatty acid catabolism (β-oxidation), lipid uptake and secretion (102). When blood glucose is low, liver provides the energy sources in the form of ketone bodies (acetoacetate and 3-hydroxybutylate) to the extra-hepatic tissues via β-oxidation of fat and ketogenesis from the Krebs cycle (112). Recent clinical observations reveal that PXR activation affects lipid metabolism in patients treated with PXR activators. For example, treatment with the antibiotic and PXR activator Rif induces hepatic steatosis in tuberculosis patients (113). Transgenic mice expressing constitutively active PXR exhibit marked triglyceride accumulation (114). The role of PXR in the development of hepatic steatosis is linked to expression of transcription factors and enzymes that function in lipogenesis, such as stearoyl-coa desaturase 1 (SCD1), a key enzyme in the synthesis of 15
31 unsaturated fatty acids (115). Insulin increases the transcription of SCD1 by activating the lipogenic transcription factor sterol regulatory element-binding protein (SREBP) (116). But, PXR activation-induced hepatic lipid accumulation in transgenic mice is independent of SREBP, and is linked to increased expression of the free fatty acid transporter Cd36 and accessory lipogenic enzymes such as SCD1 and long-chain free fatty acid elongase (102,117). Cd36 has been shown to be regulated by oxidized low density lipoprotein and long chain fatty acids (118). Cd36 is a previously indentified PPARγ target gene (119), and this study showed that PXR may increase the expression of Cd36 by directly binding to the Cd36 promoter. In fact, there is a direct repeat spaced by three nucleotides spacer (DR-3) PXRE in the mouse Cd36 promoter, which suggests that Cd36 serves as a direct transcription target of PXR and it can be activated by both PXR and PPARγ to regulate lipid homeostasis (102). PXR activation also provokes suppression of several genes whose gene products function in β-oxidation. Treatment of mice with PCN inhibits lipid oxidation by down-regulating the expression of carnitine palmitoyltransferase 1α (CPT1α) and mitochondrial 3-hydroxy-3-methylglutarate-CoA synthase 2 (HMGCS2), two key enzymes involved in β-oxidation and ketogenesis. Importantly, PXR-mediated down-regulation of these two genes is present in wide type but not PXR-null mice. It has been shown that activated PXR can physically interact with Forkhead box A2 (FoxA2) and prevent FoxA2 binding to the Cpt1a and Hmgcs2 gene promoters, thereby rendering FoxA2 transcriptionally inactive. 16
32 In addition, ligand-activated PXR interferes with HNF4α by targeting the PGC-1α co-activator protein to function in lipid homeostasis. HNF4α and PGC-1α jointly regulate the expression of CPT1α (120). Besides the interaction with FoxA2, PXR interferes with HNF4α in the regulation of CPT1α as well. Therefore, PGC-1α represents a coordinate point of crosstalk between PXR and other signaling pathways (100) Inflammatory Response Exposure to xenobiotic chemicals can impair immune function. Treatment of patients with Rif suppresses humoral and cellular immune responses in liver cells (121). Rif s immunosuppressive role has been well described in humans since 1970s ( ). It had been suggested that the immunosuppressive effects were mediated by Rif acting as a ligand and activator for the GR (126). But the result was not supported by other groups who showed that Rif is not a biologically significant activator for GR (126,127). After PXR (NR1I2) was characterized and cloned in 1998 (29), Rif is identified as a potent ligand and activator of human PXR (7). Considering that human PXR activates CYP3A, these findings help to explain the prior reported studies that CYP3A is the major CYP gene induced by Rif in human primary hepatocytes (128). But the mechanism through which Rif exerts immunosuppressive effects remains unknown. Hepatic P450 activity is down-regulated by various infectious and inflammatory stimuli, primarily due to inhibition of P450 gene transcription (129). Since PXR is a mater-regulator of CYP3A gene, it has been hypothesized that inflammation could suppress PXR 17
33 transcriptional activity. Recent reports have described mutually repressive and negative crosstalk between the PXR and NF-κB signaling pathway, thus providing a potential molecular mechanism that links xenobiotic response and inflammation ( ) (Figure 3). There are five members in the NF-κB family, namely p65 or Rel A, Rel B, c-rel, p50, and p52 (133). NF-κB is a key regulator of inflammation and the innate and adaptive immune responses. Under basal condition, p65 is normally sequestered in the cytoplasm by the inhibitory protein inhibitor of NF-κB (IκB). (133). In response to activation signals, such as inflammatory stimuli, pro-inflammatory cytokines, reactive oxygen species and viral products, downstream signaling events induce phosphorylation, ubiquitination, and proteosome-dependent degradation of IκB and release NF-κB to translocate to the nucleus where it regulates the expression of inflammatory genes. (134) Activation of PXR by Rif suppresses the expression of typical NF-κB target genes, such as cyclooxygenase 2 (COX-2), tumor necrosis factor alpha (TNF ), intercellular adhesion molecule 1 (ICAM-1 or CD54), and several interleukins. (130) Conversely, NF-κB target gene expression is elevated in hepatocytes derived from the PXR-null mice compared to that from the wild type animals (132). The PXR-null mice also exhibit a prominent and increased small bowel inflammatory infiltrate. (130) These observations indicate that PXR is involved in suppressing the NF-κB-regulated gene expression and deregulation of PXR expression or activity may make the gastrointestinal tract susceptible to inflammatory injuries (100). 18
34 In turn, inflammatory stimuli decrease xenobiotic metabolism capacity in human and experimental animals. One example is the bacterial toxin lipopolysaccharide (LPS), which activates NF-κB through a sequential TLR4 intracellular signaling cascade. (135). Another example is the inflammatory cytokine TNFα. It binds to its receptor (TNFR) and recruits TNFR1-associated death domain protein (TRADD), TNF receptor-associated factor protein (TRAF2) and other signaling transducer proteins, which lead to translocation of NF-κB into the nucleus (136). LPS and TNFα-induced NF-κB activation have been shown to suppress CYP3a4 gene expression. NF-κB p65 subunit directly interacts with the DBD of RXR and inhibits its binding to the consensus DNA sequences. Because PXR functions as a heterodimer with RXR the transactivation by the PXR-RXR complex is down-regulated in response to NF-κB signaling pathway (131). 19
35 Figure 3 Figure 3. Mutual transrepression between PXR and NF-κB signaling pathway. The PXR and NF-κB mutual suppression may link xenobiotic response and inflammation in the hepato-intestinal axis. COX-2, Cyclooxygenase 2; ICAM, intercellular adhesion molecule; LPS, lipopolysaccharide; NF-κB, nuclear factor kappa B; PXR, pregnane X receptor; PXRE, PXR response element; TNF, tumor necrosis factor. 20
36 5. Mechanisms of PXR Transcription Regulation Considering the distinct regulatory roles of PXR in several physiological conditions, we next discuss its different mechanisms of transcription regulation, including both transactivation and transrepression. The most prevalent activation of CYP3A by PXR is mediated by a linear series of events. It was previously believed that human PXR resided exclusively to the nucleus even in the absence of ligand (137). However, other studies have detected cytoplasmic localization of PXR and ligand-dependent translocation of PXR from the cytoplasm to the nucleus after binding with a ligand (138,139). Further evidences demonstrate the interaction of PXR with heat shock protein 90 (HSP90) and cytoplasmic CAR retention protein (CCRP) in the cytoplasm of liver cells (139). Upon ligand binding, PXR dissociates from the CCRP-HSP90 complex and translocates to the nucleus where it binds as a heterodimer with RXR to the DNA response element composed of two copies of the consensus NR binding motif AG (G/T) TCA (34,140). The response elements are organized as direct repeats with 3 to 5 nucleotides separating the DBD binding sites (DR-3, DR-4, and DR-5 elements), as well as everted repeats separated by 6 or 8 nucleotides (ER-6 and ER-8 elements) (29). The full functional activity of PXR is linked to the recruitment of co-regulator proteins that have pronounced effect on gene expression through protein-protein interaction instead of binding to DNA directly. Generally, non-liganded PXR interacts with co-repressor complexes including NCoR/ SMRT and HDACs (141,142). The co-repressor complexes exert inhibitory 21
37 effect on transcriptional activity and maintain chromatin structure in the compact state, thus reducing the accessibility of enhancer region of target gene to the necessary basal transcriptional machinery. For example, un-liganded PXR interacts with SMRT to repress the basal transcription of CYP3A gene which is reversed by PXR ligand paclitaxel (143). In the presence of ligand, the C-terminal AF-2 domain undergoes a conformational change that favors binding with co-activator proteins via the LXXLL- motif. The co-repressor protein complexes are dissociated and multi-protein co-activator complexes including HAT are recruited subsequently (Figure 4). In this process, TBL1/TBLR1, the component of NCoR co-repressor complexes (144), serves as the NR co-repressor/co-activator exchange factor (N-CoEx) (145). It functions as the adaptor factor to recruit the ubiquitin conjugating/19s proteasome complex that targets the co-repressor complexes for degradation after ligand binding (145). In the contrast to transcriptional activation, which usually involves the binding of PXR to its HRE in the promoter region of CYP3A gene, the inhibition of NF- B activity does not require PXR to directly bind to typical response elements. Transrepression is widely involved in negative regulation of gene expression. Previous results suggest that PXR may negatively regulate inflammatory response through protein-protein interaction in a SUMOylation-dependent pathway. But this SUMOylation-dependent transrepression mechanism remains to be further identified. 22
38 Figure 4 Figure 4. PXR activity is regulated by different co-factor interactions. PXR interacts with co-repressor complexes which recruits HDACs. This inhibits transcriptional activity through promoting chromatin compaction and reducing accessibility of genes to basal transcriptional machinery. Ligand binding induces a conformational change and dissociates the co-repressor complexes. The co-activator complexes with HAT activity are recruited to promote histone acetylation and enhance gene transcription. There are several models of NRs-mediated transrepression: first, NRs regulate key components in the NF- B and AP-1 signal-transduction pathways ( ); second, NRs compete with NF- B and AP1 for limiting amount of co-activators (149,150); third, NRs alter NF- B and AP1 co-regulator complex composition (151,152); and last, NRs modify basal transcription machinery (153,154). Several recent reports indicate a general molecular strategy for SUMOylation of a subset of NRs that transrepress immunity in a signal and gene-dependent manner. For example, a SUMOylation-dependent pathway has been identified in which PPAR mediates the transrepression of inflammatory 23
39 response genes in mouse macrophages. Ligand induced SUMO modification of PPAR- and then the SUMOylated ligand-bound PPAR- inhibits gene transcription by preventing signal-dependent recruitment of ubiquitin-19s proteasome complex required for NCoR clearance from inflammatory gene promoter/enhancer region. Subsequent research in LXRs indicates that SUMOylation is required for the repression of interferon- (IFN- ) response genes in brain astrocytes. SUMOylated LXRs form complexes with SUMO E3 ligases and signal transducer and activator of transcription 1 (STAT1), thus preventing STAT1 binding to DNA in response to IFN- stimulation. Importantly, previous results from our lab have demonstrated that PXR is SUMOylated in response to inflammatory stimulation and represses the NF- B target gene expression (132). These findings raise our interest in further studying PXR SUMOylation/de-SUMOylation mechanisms to unravel the importance of this post-translational modification in inflammatory response. 6. PXR SUMOylation The SUMOylation pathway results in the covalent attachment of SUMO to substrate proteins. SUMO is a small polypeptide, ~10kD in size. The first SUMO protein found was the Saccharomyces cerevisiae suppressor of mif two 3 homolog 1 (SMT3) (155). Based on two-hybrid interaction screening and cloning, SUMO proteins have been found to be present in plant, yeast, fly and other species. Within mouse and human, there are four types of SUMO proteins, named as SUMO-1, -2, -3 and -4. However the functionality of SUMO-4 remains currently unclear, so that SUMO-4 will not be discussed in this thesis. SUMO-1, 24
40 -2 and -3 are all highly conserved during the evolutionary process (156). SUMO-2 and SUMO-3 share 97% sequence identity so that they are always termed as SUMO-2/3, while SUMO-1 shares only 50% sequence identity with SUMO2/3 (Figure 5A). The SUMO proteins have C-terminal extension of 2-11 amino acids. They are expressed in the immature forms initially and need C-terminal extension removed by SUMO-specific proteases (SENPs) to expose the invariant glycine-glycine motif for maturation. After the maturation process, the mature forms could enter the three-step SUMOylation pathway. The first step is the activation of the mature SUMO protein by the SUMO-specific heterodimeric E1 activating enzyme, AOS1-UBA2 complex, which has both adenylation and thioesterifiation functions (157,158). The ATP-dependent reaction forms a thioester bond between the C-terminal carboxyl group of glycine and a catalytic cysteine residue in E1 (159). The second step involves transferring the SUMO protein from the E1 activating enzyme to the E2 conjugating enzyme, Ubc9, which leads to a new thioester bond between the C-terminal carboxyl group of SUMO and a catalytic cysteine residue in E2 (160). Efficient modification by SUMO protein, especially in vivo, further requires the action of specific SUMO E3 ligases, which catalyze the shift of SUMO to the target and the formation of an isopeptide bond between the glycine residue of SUMO and the lysine residue of the target (161) (Figure 5B). Several E3 ligases have been identified. The first E3 ligase found in yeast was Siz1(162) and the mammalian PIAS proteins have been found to increase the rate of SUMO conjugation in vivo subsequently (163). There are at least four 25
41 subtypes of PIAS proteins, namely 1, 3, x and y (164). The PIAS family has a central RING-FINGER-like motif that binds to targets and Ubc9 directly and interacts with SUMO through a non-covalent SUMO-interaction/binding motif (SIM) (165). The nucleoporin Ran-binding protein 2 (RanBP2) represents another type of E3 ligase (166). It increases the rate by positioning the thioester bond between SUMO and Ubc9 to an optimal orientation for attack by substrate (167) instead of directly interacting with substrate. In addition, the human Polycomb group member Pc2 (168) and histone deacetylase HDAC4 (169) have also been recently found to function as E3 ligases. The acceptor sites on SUMO targets follow a consensus sequence. It is shown to be -K-x-D/E, where is a hydrophobic amino acid, K is the lysine residue, x is any amino acid, and D/E is an acidic residue. Many proteins carry the consensus SUMOylation sequence, even the SUMO proteins themselves. The N-terminal extension of SUMO-2/3 has the SUMO acceptor site and is targeted by E1, E2 and E3 to form polymeric chains. This feature is not shared by SUMO1, since it does not contain the consensus SUMOylation sequence on itself. The distinct chain formation capacity may suggest in part different roles and functional consequences of modification by SUMO-2/3 versus SUMO-1 which will be discussed in the following sections. 26
42 Figure 5 A) B) Figure 5. PXR Reversible SUMOylation Pathway. A) The sequences of human SUMO-1, -2, and -3 are compared. Similarities are indicated by red asterisk. The consensus SUMOylation site (K11) on SUMO-2/3 is shown with green box. Sentrin proteases cut C-terminal extensions to expose the glycine-glycine motif. B) The mature SUMO protein is incorporated into 27
43 its specific substrate by a cascade of enzymes termed as E1, E2, and E3 to produce SUMO-modified substrates in a signal-dependent manner. Specific sentrin protease enzymes then cleave the SUMO moiety from substrates and allow the SUMO moiety to recycle. The SUMOylation pathway is highly dynamic and reversible. De-SUMOylation is potentially catalyzed by SENPs, which belong to the family of cysteine proteases with conserved C-terminal catalytic domains. Mammalian genomes encode up to seven SENPs, designated SENP1-3 and SENP 5-8 (Figure 6A). Although SENP8 shares the conserved catalytic domain, it has been shown to be a NEDD8-specific protease and lack the ability to process or de-conjugate SUMO substrates (170). Other six SENPs contain both endopeptidase and isopeptidase activity for de-conjugating SUMO from substrates (Figure 6B). Based on their functional activities and preferences towards targets, mammalian SENPs can be classified into three groups. SENP1 and SENP2 are able to target all SUMO isoforms and function both in their processing and de-conjugation pathways. SENP3 and SENP5 regulate the modification of monomeric SUMO-2/3 and to a less extent SUMO-1 conjugates. SENP6 and SENP7 also regulate preferentially on SUMO-2/3. Neither SENP6 nor SENP7 act on maturation of SUMO protein precursors and they show no activity in the de-conjugation of monomeric SUMO-2/3 from substrates. Rather, the main role of SENP6 and SENP7 is to de-conjugate poly-sumo-2/3 chains. SENPs can also be distinguished based on their different sub-cellular localizations. SENP1 resides in the nucleus, excluding the nucleolus (171), whereas SENP2 localizes to the nuclear envelope (172). SENP3 and SENP5 localize to the nucleolus 28
44 (173,174), whereas SENP6 localizes to the cytosol (175). And SENP7 localizes to the nucleoplasm (176). Figure6 A) Figure 6A). The Sentrin Protease Family. Each SENP family member contains a C-terminal catalytic domain. Both SENP6 and SENP7 contain additional sequences within their C-terminal domain. The N-terminus of each SENP family member contains sequences that impart both sub-cellular localization and substrate specificity. 29
45 B) Figure 6B). The SUMO-specific Proteases Dual Functions. Certain SUMO-specific proteases (SENPs) are responsible for SUMO precursor maturation and cleavage at the C-terminal extension of SUMO. They also cleave at the isopeptide bond between the terminal glycine of mature SUMO and the lysine of a substrate. The sites of cleavage by SUMO proteases are highlighted in pink and the scissile bonds are indicated by the arrow. 30
46 Previously, the PXR protein has been shown to be modified by SUMO-2/3 in response to TNF signaling in hepatocytes. The SUMOylated PXR protein incorporated SUMO-2/3 chains and the feedback repressed the immune response in hepatocytes (132). Therefore, SUMOylation of PXR represents the likely molecular basis underlying the inhibition of hepatic immune response in Rif-treated patients. Here, we further study the mechanism of the PXR SUMOylation and the transrepression mechanism of inflammation. By integrating our previous data, the working model has been proposed (Figure 7). PXR is SUMOylated by SUMO-1 and/or SUMO-2/3 in response to an inflammatory stimulus in a ligand-dependent and/or ligand-independent manner. The SUMOylated PXR prevents co-repressor complex dissociation from NF- B through protein-protein interactions. In order to test the hypothesis, NF- B target gene expression was analyzed in Hepa1-6 cell line, including COX-2, IL-6 and IL-1rn. We also used cell-based methods to compare SUMO-1 modification capacity with SUMO-3 and an in vitro SUMOylation assay to determine the PXR SUMOylation reaction mechanisms. Furthermore, we described the substrate preferences of six SENPs and indentified possible SUMOylation site(s) based on site-directed mutagenesis. Taken together, the work presented in this thesis contributes to understanding the interface between PXR-mediated gene activation, the SUMOylation pathway and inflammation. 31
47 Figure 7 Figure 7. Model of PXR-mediated Transrepression of Inflammatory Response Pathway. Co-stimulation of cells with PXR activators and inflammatory stimuli leads to the formation of trans-repressive multi-protein complexes on the promoters of specific inflammatory-response genes. 32
48 Materials and Methods Cell Culture and Treatment of Hepa1-6 Cells. The Hepa1-6 cell line is a readily available immortalized hepatocyte cell line isolated from mouse hepatocytes by Darlington et al., in 1980 (177). Studies in our laboratory have confirmed that induction of Cyp3a11 and expression of PXR is absent in this cell line (data not shown). The Hepa1-6 cell line has been used successfully in a variety of studies such as cell hybridization, biochemical analysis of tissue-specific gene products, and the modulation of expression of genes governing differentiated phenotypes. Hepa1-6 cells were maintained in DMEM supplemented with 10% FBS supplemented with pen-strep. Sub-culturing was performed at ~70% confluence using a 1:5 dilution. Cells were transfected with the indicated plasmids using lipofectamine 2000 per manufacturer s instructions (Invitrogen). Cells were were with treated with 10 micromolar pregnenalone 16a-carbonitrile (PCN), a well-known mouse PXR agonist, for 24 hours. Treatment of cells was continued with lipopolysaccharide (LPS, 10 micrograms/ml media) or tumor necrosis factor alpha (TNFa, 10 nanograms/ml media) in the presence and absence of PCN for an additional 12 hours. RNA Isolation and q-pcr Analysis Total RNA was isolated from cell culture using a commercially available kit (Rneasy, Qiagen) according to the manufacturer s instructions. RNA was visualized by electrophoresis to ensure its integrity. Isolated RNA was DNase 33
49 treated (Sigma), reverse transcribed (Promega), and quantitative PCR was performed using an Applied Biosystems StepOne Plus real-time PCR system to detect mrna expression specific for indicated genes. Fold induction was calculated using beta-actin mrna as a normalization control. Bacterial Expression and Purification of GST-tagged Fusion Proteins GST fusion protein expression vectors were transformed into BL21DE3 cells (Novagen # ), which contain the T7 polymerase stably intergrated under the control of an IPTG inducible promoter. After plated the plasmid on LB-amp (100ug/mL) plates, a single colony was used to inoculate a 10 ml LB liquid culture containing 100ug/mL ampicillin. This culture was grown at 37 C shaking for 6 hours. A 15% glycerol stock was prepared from the 10mL culture in a final volume of 0.5 ml. Transformants were inoculated and grown as before for 10 hours. A 50 ml culture was then inoculated and grown as before for 2-3 hours. The culture was induced to express the fusion protein with 0.5 mm IPTG (0.24g/L) for 4-6 hours. The cells were pelleted and resuspended in 5.0mls NETN (NETN=100mM NaCl, 20 mm tris ph=8.0, 1 mm EDTA, 0.5% NP-40). The cells were sonicated 3 times, on a medium setting, for 1 minute each on ice. The sonicated cell lysates were centrifuged at 12,000 rpm for ten minutes at 4 C. The supernatant was made 10% in glycerol and frozen at - 80 C for future use. 25 µl of glutathione agarose beads (Pharmacia # ) was added to the 300 µl supernatant of the -80 C frozen supernatant after thawing and incubated with shaking at 4 C for 30minutes in a 1.5 ml eppendorf tube. The beads were pelleted for 1 minute and then 34
50 washed three times in a 1mL NETN. In Vitro SUMOylation Assay Each SUMOylation reaction (Enzo Life Sciences Inc) contained 1 μm recombinant purified PXR in total 20-μl volume in the presence or absence of Mg2+-ATP. The assay components including SUMO proteins, E1, E2 and E3 enzymes were mixed in a microcentrifuge tube and incubated at 30 C for 60 min, and the reaction was quenched by the addition of 20 μl of 2 SDS-PAGE gel loading buffer. To detect the SUMOylated proteins, a 5-μl sample of each reaction was resolved by using 10% SDS-PAGE, and the immunoblot analysis was conducted by using anti-pxr antibodies (H-11, Santa Cruz). Cell-Based SUMOylation Assay Plasmids pcdna3-6x-his-sumo1, and pcdna3-6his-sumo3 were kind gifts from Dr. Ronald T. Hay (University of Dundee, Dundee, United Kingdom). For transfection assays, Hepa1-6 cells were grown in six-well dishes for 24 h until 80% confluence. Cells were transfected with the expression plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Forty-eight hours after transfection, cells were washed twice with phosphate-buffered saline and harvested in 200 μl of lysis buffer (6 M guanidinium-hcl, 10 mm Tris, 100 mm sodium phosphate buffer, ph 8.0). After sonication the cell lysates were cleared by centrifugation at 3,000 x g for 15 min. The cleared cell lysates were mixed with 25 μl of Cobalt-linked agarose (QIAGEN) that had been prewashed three times in cell lysis buffer. The mixture was incubated for 2 h on a rotator at room temperature and centrifuged for 2 min at 1,000 rpm to gather the beads. The beads were washed once in wash buffer I (8 M urea, 10 mm Tris, and
51 mm sodium phosphate buffer, ph 8.0), three times in wash buffer II (8 M urea, 10 mm Tris, 100 mm sodium phosphate buffer, ph 6.3, 0.1% Triton X-100, and 5 mm β-mercaptoethanol, and once in wash buffer III (150 mm NaCl, 10 mm imidazole, and 50 mm sodium phosphate buffer, ph 6.75). The beads were resuspended in 40 μl of 2 SDS-PAGE gel loading buffer and boiled for 5 min, and 20-μl samples were resolved by using 10% SDS-PAGE. The gel was transferred to polyvinylidene difluoride membrane using standard methods, and immunoblot analysis was performed to detect the SUMOylated form of PXR using the H-11 monoclonal anti-pxr antibody. 36
52 Results 1. Expression of PXR Transrepresses the Induction of Endogenous Inflammatory Response Genes in Hepa1-6 Cells. The NF-κB transcription factor is a critical regulator of the host immune and inflammatory response in various disease states. We analyzed the NF-κB target gene mrna levels by RT-qPCR in Hepa1-6 cell line, which is a derivative of the BW7756 mouse hepatoma arose in a C57/L mouse. As there is no endogenous PXR protein expressed in Hepa1-6 cell line, we transfected the cells with mouse PXR or mock plasmid as indicated and treated with mouse PXR activator PCN for 24 hours. Cells were then treated for an additional 12 hours with LPS in the presence and absence of PCN as indicated. We isolated total RNA and examined the relative expression levels of several known NF-κB target genes (Figure 8). Previous study of the expression levels of genes encoding TNFα, IL-6, IL-1α, and IL-1β showed that these genes are significantly induced when PXR is ablated in PXR-KO mice (132). To confirm the involvement of PXR in anti-inflammation process, we first tested inflammatory response genes in Hepa1-6 cells in the absence of PXR. Among the four non-transefected groups (Veh, PCN, LPS, PCN+LPS), analysis of the expression levels of genes encoding Cox-2, IL-6 and IL-1rn using RT-qPCR showed that their levels were significantly increased in LPS-treated group. These results reveal an active role for 12h LPS treatment in inducing the expression of genes that encode key inflammatory cytokines. Also, in the 37
53 absence of mouse PXR, there was no difference between LPS and PCN+LPS co-treatment groups, which proved that Hepa1-6 cell line is an ideal cell model to study PXR-dependent effect. In order to investigate the repressive role of PXR on NF-κB-mediated inflammatory signaling pathway, we analyzed the gene expression levels in mouse PXR-transfected groups. When mouse PXR was activated by PCN, the endogenous inflammatory cytokines were repressed about one fold. When cells were challenged with LPS stimulation, mouse PXR-transfected cells were highly resistant to LPS-induced cytokine up-regulation (~10 fold). These data provide supporting evidence for establishing the existence of transcription factor crosstalk between the PXR and NF-κB in liver cells. We next determined ligand-dependent or ligand-independent effect of mouse PXR on its activity. When mouse PXR was activated by PCN, the ligand-activated mouse PXR repressed LPS-induced gene expression at the comparable level to that of non-liganded mouse PXR. The results suggested that mouse PXR repressed NF- B signaling pathway in a ligand-independent manner in Hepa1-6 cells. The underlying molecular mechanism(s) responsible for the PXR repressive effect on inflammatory genes has been proposed. In response to LPS activation, PXR is SUMOylated by endogenous SUMO proteins at low stoichiometry. The SUMOylated PXR is highly dominant efficient to repress NF- B transcriptional activity through protein-protein interactions, instead of direct DNA binding. 38
54 Figure 8 Figure 8. Expression of PXR Transrepresses the Induction of Endogenous Inflammatory Response Genes in Hepa1-6 Cells. Cells were transfected with mouse PXR or mock-transfected as indicated and treated with 10 mm PCN for 24 hours. Cells were treated for an additional 12 hours with LPS in the presence and absence of PCN as indicated. Gene expression levels of indicated endogenous inflammatory response genes were determined using real-time PCR as described in Materials and Methods. Data were expressed as the fold induction over the vehicle and were reported as the mean +/- SEM of three independent reactions. 39
55 2. The PXR Protein Is SUMOylated in vitro To demonstrate that PXR is a substrate of SUMOylation, we first analyzed the PXR SUMOylation mechanism in vitro. There are 28 lysine residues on human PXR protein (Figure 9-1). Previously, the bioinformatic approach has been used to scan the amino acid sequence of human PXR for the presence of a consensus SUMOylation site (132). Using this strategy we identified four potential sites. Since the four sites are all located in the C-terminal hinge domain and LBD, the PXR protein was cut into two parts for the in vitro SUMOylation assay. The first part included AF-1 and DBD domains and was named as human PXR-DBD (1-105 amino acids). The second part included hinge domain, LBD and AF-2 domains and was referred as human PXR-LBD ( amino acids). The strategy shown in Figure 9-2 was followed to express, isolate and purify GST-tagged human PXR DBD and GST-tagged human PXR LBD proteins. Purified components were collected, separated on SDS-PAGE gels, and visualized by staining with Coomassie blue (Figure 9-3). As shown, the bands at 26kD in GST-human PXR DBD and LBD proteins lanes are de-associated GST proteins. The GST-human PXR LBD (64 kd) migrated slower than the GST-human PXR DBD (37kD) protein on the gel and we noticed that the higher molecular-weight protein was more degraded and less soluble in buffers. We next used in vitro methods to determine the extent to which purified recombinant GST-human PXR LBD served as a substrate in the 40
56 SUMO-conjugation pathway. We incubated GST-tagged purified recombinant human PXR LBD in vitro together with E1, E2, E3, and SUMO-1 or SUMO-3 proteins in the presence or absence of the required magnesium and ATP co-factors. The extent of SUMOylation after the incubation was determined by SDS-PAGE and Western blot analysis with anti-hpxr monoclonal antibodies (Figure 9-4). This type of analysis revealed that the human PXR LBD protein can serve as an effective substrate for both SUMO-1 and SUMO-3 in the SUMO-conjugation pathway in vitro. And the in vitro SUMOylation of GST-human PXR LBD was strongly enhanced by E3 ligase PIASy. There is one primary SUMOylation band (~110kD) when either SUMO-1 or -3 was used in the reaction, which indicated human PXR LBD could be SUMOylated at one primary site in vitro. When we compared the molecular mass shift of the SUMO-1 modified band with SUMO-3 modified band on the blot, SUMO-1 conjugated PXR appeared at a higher molecular weight. And this is in consistent with the fact that SUMO-1 protein is larger in size than SUMO-3 protein. It is noteworthy that addition of PIASy dramatically enhanced SUMO modifications in vitro. In order to figure out whether GST-human PXR LBD could be SUMOylated without E3 in vitro, a blot with longer exposure time was shown in Figure 9-4 lower panel. The results suggested that GST-human PXR LBD was able to be SUMOylated to less extent by both SUMO-1 and SUMO-3 in the absence of E3 in vitro. The data presented indicated that SUMO-1 and SUMO-3 could modify GST-human PXR LBD in vitro, and SUMOylation was enhanced by PIASy. Although the in vitro assay is highly suggestive of potential PXR 41
57 SUMO modification, it is still necessary to examine PXR SUMO conjugation in cultured cell lines or primary cells. Figure 9-1 Figure 9-1. Structure and Post-translational Modification of Human PXR Lysine Residues. A) All the lysine residues on human PXR were determined and there are 28 lysine residues. The human PXR protein was analyzed for the presence of the consensus SUMOylation sequences as defined by an online SUMO Plot server ( This type of bioinformatic analysis identifies four potential sites for SUMOylation, one of which is predicted as a high probability SUMOylation site and three others that are predicted as low probability SUMOylation sites. Figure 9-2 Figure 9-2. Purification Scheme for GST-tagged Proteins. 42
58 Figure 9-3 A) B) * * * GST GST-human PXR DBD GST-human PXR LBD Figure 9-3. SDS-PAGE Analysis of Purified Proteins. A) GST and GST-human PXR DBD proteins were separated on a 12.5% gel. B) GST-human PXR LBD protein was separated on a 10% gel. Proteins were visualized by staining with Coomassie blue. The purified proteins are indicated with asterisks. 43
59 Figure 9-4 Figure 9-4. In Vitro SUMOylation of GST-human PXR LBD by SUMO-1 and SUMO-3 is enhanced by PIASy. The recombinant purified GST-human PXR LBD fusion protein was incubated together with E1, E2 in the presence and absence of Mg 2+ -ATP as indicated. The recombinant purified E3 ligase (PIASy) was included as indicated. Input protein Coomassie stained gel is shown in the inset at the right (Input). Five microliter aliquots of the in vitro SUMOylation reaction were resolved using 10% SDS-PAGE and western blotting was performed using anti-human PXR monoclonal antibodies to detect SUMO-modification. The long-exposure blot is shown at the lower panel. 44
60 3. PXR is Preferentially SUMOylated in Hepa1-6 Cells by SUMO-3 and PIASy. We initiated a series of studies using an over-expression and transfection approach in Hepa1-6 and HeLa cells (132). Co-transfection of Hepa1-6 cells with 1) the human PXR expression vector, 2) the 6 His-SUMO-1 or 6 His-SUMO-3 expression vector and 3) the PIAS1 or PIASy E3 enzyme expression vector allows the rapid and selective purification of SUMOylated forms of PXR using cobalt-linked agarose beads and a strong denaturing buffer containing high levels of guanidine-hcl. The reason for choosing cobalt beads instead of nickel beads is that cobalt beads exert stronger and more specific binding to SUMOylated human PXR (data not shown). But the human PXR might still non-specifically bind to cobalt beads through zinc fingers. Hepa1-6 cells have endogenous E1 and E2 enzymes which can support PXR SUMO modification efficiently without over-expression of E1 and E2. The endogenous SENPs that would probably cleave SUMOylated forms of PXR during cell lysis are rapidly de-activated under denaturing conditions. Based on this experimental approach we detected SUMOylated PXR using a Western blot analysis with the α-human PXR monoclonal antibody (Figure 10-1). When human PXR alone was exogenously expressed, the un-conjugated human PXR (~52kD) was pulled down by cobalt-linked beads which represented the non-specific binding. When His-tagged SUMO plasmid and E3 ligase PIAS were co-transfected, the un-conjugated human PXR was detected along with a 45
61 series of higher molecular weight proteins that represented SUMO-conjugated human PXR. The over-expressed SUMO-3 protein formed chains on the human PXR protein, which were present at a much higher level in cells transfected with PIASy compared with those transfected with PIAS1(Figure 10-1 lane 4 and 5). We also used SUMO-2/3 antibody to detect global SUMO-2/3 modification (Figure 10-2). PIASy preferentially increased global SUMO modification by SUMO-2/3 to a higher level compared with PIAS1. The whole cell lysates of Hepa1-6 cells were subjected to detection of endogenous SUMO-2/3 proteins. Consistent with the prevailing theory that SUMO-2/3 remains in a free or un-conjugated form under normal condition (178), the Hepa1-6 whole cell lysates showed endogenous free and un-conjugated SUMO-2/3 proteins (Figure 10-3 lower panel). PXR could also be SUMOylated by SUMO-1 in our cell-based assay, although we found that human PXR was preferentially modified by SUMO-3. SUMO-1 cannot form chains on PXR since its self did not possess consensus SUMO sequences. PIASy preferentially promoted SUMO-1 modification both on human PXR and global proteins as shown in Figure When the blot was over-exposed, the free His-tagged SUMO-1 was shown to be present (Figure 10-4 middle panel). Besides endogenous SUMO-2/3, the Hepa1-6 cells contained endogenous SUMO-1 protein as well (Figure 10-1-C lower panel). 46
62 Figure 10-1 A) 47
63 B) 48
64 C) Figure Detection of SUMOylated Human PXR Protein in Hepa1-6 Cells. The human PXR protein was co-expressed in Hepa1-6 cells with 6X-His-tagged SUMO-1 or SUMO-3, and E3 enzyme (PIAS1 or PIASy). After 48h transfection, cells were lysed using denaturing buffer containing guanidinium hydrochloride to inactivate de-sumoylation enzymes. SUMOylated proteins were purified by cobalt-linked agarose beads. Purified His-tagged proteins and Hepa1-6 whole cell lysates were resolved using SDS-PAGE and subjected to Western blot analysis using antibodies that recognize A) human PXR or B) SUMO-2/3 or C) SUMO-1 as indicated. 49
65 4. The hpxr-k108 Residue Is A SUMOylation Site. It was previously reported that human PXR contains multiple SUMO consensus sequences and that one site, K108, is of high probability (Figure 9-1). To directly assess K108-SUMO conjugation capacity, the PXR protein containing site108 lysine-to-arginine mutation was employed. We used cell-based assay to detect the extent of human PXR-K108R SUMO-1 and SUMO-3 modification (Figure 11). The K108R mutation down-regulated, but did not blocked the SUMOylation of PXR. The result indicated that K108 was involved in both SUMO-1 and SUMO-3 conjugation pathway. The monomeric SUMO modified-pxr still existed which suggested there were other potential SUMOylation sites besides K108, which calls for further analysis. Another thing to note is that endogenous SUMO-2/3 proteins may contribute to unexpected migrating form of human PXR detected in SUMO-1 over-expression groups because the endogenous free-state SUMO-2/3 in Hepa1-6 cells is likely to be conjugated to human PXR when PIASy is over-expressed. 5. Inflammatory Mediator LPS Induces SUMOylation of Human PXR Wide Type and K108R Mutant. We next sought to determine if inflammation could influence SUMOylation of PXR in Hepa1-6 cells. Previous results performed in human hepatocytes reported that treatment of cells with TNFα alone or TNFα together with Rif produced an increased level of SUMO-2/3 modified PXR (132). The identification of PXR SUMO(1)ylation raised the question of whether LPS also induced SUMO-1 conjugation to PXR. 50
66 The Hepa1-6 cells were transfected with plasmids that express human PXR, His-tagged SUMO-1 and E3 PIASy. Transfected cultures were subjected to vehicle treatment (Figure 12-1, lane1) or LPS treatment for 12 hours (Figure 12-1, lane 3). SUMO-modified proteins were purified after drug treatment using cobalt bound beads and PXR protein was examined on Western blots. As expected, LPS-treated cells significantly induced SUMO(1)ylation of human PXR at 12 hrs (Figure 12-1, lane 3). Some NR ligands enhance SUMOylation of target NR family members (e.g., LXRα and PPARγ), but this has not been detected for PXR. Transfected Hepa1-6 cells were treated with either vehicle (DMSO) (Figure 12-1, lane 1) or the human PXR ligand Rif (Figure 12-1, lane 2) for 36 hours before harvest. Rif treatment significantly decreased SUMO(1)ylation of human PXR compared with vehicle (Figure 12-1, lane 1). Next we compared ligand treatment group with co-treatment group comprising of both ligand pre-activation and inflammatory stimulation (Figure 12-1, lane 4). LPS co-treatment ameliorated ligand-induced down-regulation of PXR SUMO1 modification. These results indicated that inflammatory signaling induced both non-liganded and ligand-bound PXR SUMO-1 modification. Since K108 is one of the potential SUMO acceptor sites, we next analyzed human PXR K108R SUMO-1 conjugation levels in response to LPS stimulation. As shown in Figure 12-2 left panel, LPS induced SUMO-1 modification on human PXR K108R mutant at a comparable level to that of wide type human PXR. The global SUMO-1 conjugations in response to LPS did not distinguish between over-expressed wide type and mutated human PXR groups. The data 51
67 revealed a tentative result that K108 was not required for LPS-inducible PXR SUMOylation. Figure 11 Figure 11. The Lysine Residue 108 Mutation Decreases PXR SUMOylation. Hepa1-6 cells were transfected with His-tagged SUMO1 or SUMO3 and either human PXR or human PXR-K108R. The ~75kDa bands were SUMOylated PXR and were reduced in human PXR-K108R SUMO-1 and SUMO-3 conjugation groups. 52
68 Figure 12-1 Figure Inflammatory Stimulus and Ligand Modulate Wide Type Human PXR Protein SUMO-1 Modification in Hepa1-6 Cells. Hepa1-6 cells were transfected with human PXR, PIASy and His-tagged SUMO1, or SUMO-3. Cells were treated for 36 hours with vehicle or Rif (10 μm) or LPS (10 mg/ml) for 12 hours, or Rif (10 μm) pre-treatment together with LPS (10 mg/ml) 12 hours co-treatment. The cells were harvested 48hrs post-transfection and purified proteins were immunoblotted with anti-human PXR. 53
69 Figure 12-2 Figure K108 Is Not the Primary LPS-inducible SUMOylation residue. Hepa1-6 cells were transfected with SUMO-1 and either human PXR or human PXR-K108R and treated with vehicle or LPS (10 mg/ml) for 12hrs before harvest. The purified protein extracts were analyzed by immunoblotting with PXR monoclonal antibody and anti-sumo1 antibody. 54
70 6. Sentrin Proteases Differentially De-SUMOylate PXR-SUMO Conjugates in Hepa1-6 Cells. Because SUMOylation is a reversible pathway, we next studied the reversibility of PXR SUMOylation reaction and how SUMOylated PXR may be selectively processed by six distinct mammalian SUMO proteases. We first examined the capability of SENP1, -2, -3, -5, -6 and -7 to de-conjugate SUMO-1 from PXR. All SENPs were expressed as N-terminal Flag fusions, and Hepa1-6 cells were transfected with expression plasmids encoding His-tagged SUMO-1 and PIASy. Because His-tagged SUMO-1 was expressed as the C-terminally processed Gly-Gly form, our de-conjugation assays in Hepa1-6 cells compared merely the isopeptidase activity of different SENPs. As shown in Fig. 13-1, SENP1 and SENP2 were potent in removing SUMO-1 from human PXR protein. The isopeptidase function of SENP3 was very weak on SUMO-1-modified human PXR. SENP5, 6 and 7 showed no activity on SUMO-1 conjugates (Figure 13-1). We next compared the isopeptidase activity of the SENPs toward human PXR-SUMO-3 conjugations in Hepa1-6 cells. In line with the above results for human PXR-SUMO-1 conjugates de-sumoylation, both SENP1 and SENP2 were efficient in de-conjugating SUMO-3 from human PXR in Hepa1-6 cells. Interestingly, SENP3 and 5 were also capable of de-conjugating SUMO-3 to some extent. Thus, SENP3 and SENP5 appeared to show preference for SUMO-3 conjugates. SENP6, which showed no activity on human PXR-SUMO1 modifications in Hepa1-6 cells, was efficient toward human 55
71 PXR-SUMO3 conjugates. It completely removed SUMO3 chain formation. SENP7 was characterized as a SUMO-2/3-specific protease that was likely to regulate poly-sumo-2/3chains rather than SUMO-1 conjugation (176). However, SENP7 had no effect on removing SUMO-3 from the hpxr SUMO3 conjugates (Figure 13-2). Mutation of the catalytic cysteine in the C-terminal of SENPs to serine has been shown to inactivate their catalytic function (179). We used the mutants SENP1C603S, SENP2C466S, SENP3C532S, SENP5C713S, and SENP6C1030S as controls in Hepa1-6 cells de-sumoylation assays. In keeping with the importance of the conserved cysteine residue for the fully catalytic function of SENPs, the mutated SENPs were totally inactive in de-conjugating SUMOs from cellular proteins (Fig and 13-2). Taken together, these results identified SENP1 and SENP2 as strong candidates for SUMO-specific proteases reversing PXR SUMOylation by both SUMO-1 and SUMO-3 in cells. SENP3 and 5 showed substrate preferences towards SUMO-3 conjugates. SENP6 effectively removed SUMO-3 poly-chain formation. SENP7 had no effect on PXR SUMOylation. 56
72 Figure 13-1 Figure Cell-based Analysis of Human PXR SUMOylation/De-SUMOylation by SUMO1 and Sentrin Proteases in Hepa1-6 Cells. Indicated mammalian expression vectors are transfected using lipofectamine per manufacturer s instructions. Forty-eight hrs post-transfection cells were lysed as described in Materials and Methods. Proteins were resolved using 10% SDS-PAGE and western blotting was performed using anti-human PXR monoclonal antibody. m1:senp1c603s, m2: SENP2C466S, m3: SENP3C532S, m5: SENP5C713S, m6: SENP6C1030S 57
73 Figure 13-2 Figure Cell-based Analysis of Human PXR SUMOylation/De-SUMOylation by SUMO-3 and Sentrin Proteases in Hepa1-6 Cells. Indicated mammalian expression vectors are transfected as described. Proteins were resolved using 10% SDS-PAGE and western blotting was performed using anti-human PXR monoclonal antibody. 58
74 7. SENP6 together with Human PXR Mutants Provide Insights into PXR SUMOylation Sites Prediction Taken together, the results shown above suggest that 1) The ~75kD band is the primary human PXR SUMOylation band, 2) K108 is one of the potential SUMOylation sites, 3) The ~110 kd band indicates that there are two SUMO proteins conjugated to PXR. We hypothesized that there were two major SUMOylation sites. As discussed above, Hepa1-6 cells have high amount of endogenous SUMO-2/3 proteins which may lead to chain formation. In order to further map PXR SUMOylation sites, human PXR-K108R was transfected together with SENP6 to remove all possible SUMO chain formation. As shown in Figure 14-1, human PXR-K108R mutation together with SENP6 isopeptidase left two monomeric bands (~75 kd and ~110kD). The data suggest that there could be at least three SUMO-3 modifiable sites on human PXR (Figure 14-1). When we looked at SUMO-1 modification, we found that SENP6 cleaved the higher molecular bands on hpxr-k108r and left one primary monomeric SUMO-1 site (~75kD). We speculated that two major sites were modified by SUMO-1 (Figure 14-2). The double mutants and triple mutants were used to further analyze the potential SUMOylation sites. The systematic site-directed mutagenesis approach revealed two major sites of SUMOylation at positions K108 and K128 on the human PXR protein. The K108R, K128R double mutant could not be modified 59
75 by SUMO-1 (Fig 14-3 lane 7); whereas it could still form monomeric conjugates by SUMO-3 (Fig 14-4 lane7) (~75kD). Figure 14-1 Figure Detection Wide Type Human PXR and Human PXR-K108R Mutant SUMO-3 Modification in Hepa1-6 Cells. The human PXR protein or hpxr-k108r was co-expressed in Hepa1-6 cells together with His-tagged SUMO3 proteins, E3 enzyme PIASy and SENP6. After 48h transfection, cells were lysed and SUMOylated proteins were purified by using cobalt-linked agarose beads. Purified proteins were resolved using SDS-PAGE and subjected to Western blot analysis using antibodies that recognize human PXR as indicated. 60
76 Figure 14-2 Figure Detection Wide Type Human PXR and Human PXR-K108R Mutant SUMO-1 Modification in Hepa1-6 Cells. The human PXR protein or hpxr-k108r was co-expressed in Hepa1-6 cells together with His-tagged SUMO1 proteins, E3 enzyme PIASy and SENP6. After 48h transfection, cells were lysed and SUMOylated proteins were purified by using cobalt-linked agarose beads. Purified proteins were resolved using SDS-PAGE and subjected to Western blot analysis using antibodies that recognize human PXR as indicated. 61
77 Figure 14-3 Figure PXR is Mainly SUMOylated in the Hinge Domain by SUMO-1. The human PXR protein and seven human PXR mutants were expressed in Hepa1-6 cells together with His-tagged SUMO1 proteins and E3 enzyme PIASy. After 48h transfection, cells were lysed and SUMOylated proteins were purified by using cobalt-linked agarose beads. Purified proteins were resolved using SDS-PAGE and subjected to Western blot analysis using antibodies that recognize human PXR as indicated. 62
78 Figure 14-4 Figure PXR is Mainly SUMOylated in the Hinge Domain by SUMO-3. The human PXR protein and seven human PXR mutants were expressed in Hepa1-6 cells together with His-tagged SUMO3 proteins and E3 enzyme PIASy. After 48h transfection, cells were lysed and SUMOylated proteins were purified by using cobalt-linked agarose beads. Purified proteins were resolved using SDS-PAGE and subjected to Western blot analysis using antibodies that recognize human PXR as indicated. 63
79 Discussion 1. PXR SUMOylation/De-SUMOylation Mechanism 1.1 SUMO-1 versus SUMO-2/3 The PXR protein belongs to a family of ligand-activated transcription factors that regulate xenobiotic response and drug metabolism in liver and intestine. Recent studies have characterized PXR as a regulator of hepatic innate and adaptive immune response. PXR agonist Rif exerts immunological effect (122) and PXR is required for normal immunity to bacterial infection (180). In addition, PXR activation inhibits LPS induction of a number of pro-inflammatory genes (132). Repression of inflammatory mediator by PXR agonists is abolished in hepatocytes lacking PXR indicating that PXR mediates this anti-inflammatory response (181). Although recent results have provided a link between xenobiotic metabolism and inflammation, relatively few mechanisms have been established for the transrepression pathway regarding the post-translational modification of the human PXR protein. Our lab has previously identified that PXR SUMOylation is implicated to repress inflammatory response in hepatocytes (132). Here we provided direct evidence of PXR modification by both SUMO-1 and SUMO-2/3. Previous study showed the existence of endogenous un-conjugated form of SUMO-2/3 in great abundance and the free state SUMO-1 to a lesser extent in COS-7 cells (178). In our mouse hepatoma cell 64
80 model, both SUMO-1 and SUMO-2/3 are endogenously expressed in the un-conjugated form with the potential capacity to modify cellular proteins. Over-expression of SUMO proteins and E3 ligases markedly increased the level of human PXR modification by both SUMO-1 and SUMO-3. Furthermore, compared with SUMO-1, PXR was preferentially targeted by SUMO-3 which formed poly-sumo chains in vivo. In vitro SUMOylation assays showed that PXR LBD was a substrate for both SUMO-1 and SUMO-3. PXR LBD was preferentially targeted by SUMO-1 rather than SUMO-3 in vitro which contradictory to the cell-based result. However, it is questionable whether the in vitro SUMOylation assays could faithfully reproduce physiological substrate selection mechanisms. The endogenous free and un-conjugated SUMO-2/3 is readily available for conjugation reactions when a series of stress-responsive kinase cascades is activated in response to cellular stresses such as heat shock (178), hypoxia/ischemia (182) and oxidative stress (183), whereas SUMO-1 modification level appears to be unaffected (184). The increase of incorporation of SUMO-2/3 into PXR and formation of SUMO-2/3 chains were found in response to TNF stimulation and the feedback mediated active repression of NF- B activity in hepatocytes (132). Here, we found that SUMO-1 modification of PXR was also induced in LPS-mediated inflammatory response in Hepa1-6 cells. On the contrary, ligand activation was observed to lead to a reduction in the amount of SUMO-1 conjugation to PXR. Such changes in modification have been observed by other NRs as well, for example PPAR 2 65
81 (185) and VDR (186). Ligand binding might induce PXR conformation changes which results in reduced accessibility of acceptor sites to SUMO modification. Since we only utilized Hepa1-6 cell line in our studies, future work on detecting SUMO-1 modification of endogenous human PXR in primary cultures in response to inflammatory stimulation is necessary. If inflammation actively induced endogenous PXR SUMO-1 conjugation in human hepatocytes, we could further focus on a comparison of functional consequences between SUMO-1 conjugation and SUMO-3 conjugation. It is possible that SUMO-1- and SUMO-3-dependent transrepression pathways converge on the regulation of NCoR clearance from inflammatory response genes. It will be of interest to study the signal-specific and gene-specific usage of the two parallel pathways by PXR. Also characterization of proteins specifically modified by SUMO-2/3 or SUMO-1 in a stress-responsive manner and the fate of such SUMO-modified proteins is an interesting question to study. It has been shown that SUMO-2/3 possesses the capacity to form polymeric chains via lysine 11. Interestingly, SUMO-1 has been found to be conjugated to lysine 11 on SUMO-2/3, suggesting that SUMO-1 may act as a SUMO-2/3 chain terminator in vivo (187). The physiological significance of these polymeric forms of SUMO-2/3 requires further discussion. Intriguingly, it has been reported that ubiquitination was induced in response to a variety of cellular damages and environmental stresses such as heat shock, oxidative stress, viral infection, or mutation (188,189), which results in rapid degradation of damaged cellular proteins by 26S proteasomes. Because many proteins are substrates for both SUMO and ubiquitin, it has been suggested that SUMO might compete 66
82 with ubiquitin for the same lysine residue and stabilize the target protein by preventing ubiquitin-mediated proteasomal degradation. For example, SUMO-1 modified I B lysine 21 conferred the resistance to TNF -induced ubiquitination and degradation of I B (190). However, it is now clear that SUMO-2/3 chains can act as signals for the recruitment of E3 ubiquitin ligase RING finger protein 4 (RNF4) that ubiquitylates SUMO chains and degrades the proteins modified with SUMO chains (191). For example, promyelocytic leukemia (PML) has been identified as the first substrate in the SUMO chain formation dependent ubiquitination and degradation pathway in response to arsenic ( ). Further studies are needed to study PXR ubiquitination and SUMOylation crosstalk and determine the mutual effects between SUMO-2/3 chain and poly-ubiquitin chain formation. Moreover, the relative reaction kinetics of SUMO-1 and SUMO-2/3 conjugation to PXR has not been studied. Based on current findings, we hypothesize that SUMO-2/3 conjugation is rapidly induced, whereas SUMO-1 modification acts later on. The rapid kinetics of SUMO-2/3 reaction may reveal highly dynamic regulation of the SUMO-2/3 modification in the context of inflammatory stress. 1.2 E3 Ligases Initially not clear whether E3 ligases are required in the SUMOylation process because SUMO conjugation could take place in two enzymatic steps in vitro, including E1 and E2 (194). However, the majority of SUMOylation reactions in yeast are E3-dependent (162,195). Later, E3 ligases have been 67
83 shown to enhance SUMO attachment to many substrates both in vitro (163,196,197) and in vivo (164,198,199). Our in vitro SUMOylation assays revealed that PXR could be SUMOylated in two enzymatic steps in vitro, whereas PIASy enhanced the reaction capacity greatly. We also suggest that both PIAS1 and PIASy could function as E3 ligases in cell-based assays. PIASy showed stronger E3 ligase functional activity on targeting both PXR and overall cellular substrates than that of PIAS1 in vivo. However, some previous studies indicated that although cells were over-expressed with the same amount of PIAS protein expression plasmids, the protein expression levels of each PIAS protein were different (200). Future work on analysis of PIAS1 and PIASy protein levels after transient transfection is required before we make the absolute conclusion that PIASy is a stronger E3 ligase to enhance PXR SUMOylation than PIAS1 in vivo. The PIAS proteins have also shown preferences in selection of SUMO isoforms to be attached to PXR. We showed that PIAS1 and PIASy preferentially enhanced PXR modification by SUMO-3 rather than by SUMO-1. Another group has found that the transcription factor GATA-2 was SUMOylated and that PIASy could increase the extent of this modification, especially in the case of the SUMO-2 isoform (201). Therefore, it is tempting to speculate that PIAS proteins favor SUMO-2/3 over SUMO-1 in the SUMOylation reactions of transcription factors. Besides the E3 ligating enzymatic activity, PIAS proteins have also been identified to regulate gene transcription through other mechanisms. PIASy has 68
84 been found to repress the transcriptional activity of AR independent of SUMOylation. It exerted repressive effects through the distinct repression domains and the recruitment of HDACs to the promoter region of AR target genes (202). In another case, PIAS1, but not other PIAS proteins, blocked the DNA binding activity of activated Stat1 and inhibited Stat1-mediated gene activation in response to interferon signaling (203). PIAS proteins-mediated subnuclear sequestration of transcription factors might represent another mechanism. For example, PIASy could target lymphoid enhancer factor 1 (LEF1) to nuclear bodies through the RING domain, which leads to the repression of LEF1 activity (163). It is unknown as to whether or not PIAS proteins could repress PXR trans-activation independent of SUMOylation through reducing PXR DNA binding activity, enhancing co-repressor recruitment, inhibiting the interaction with co-activator or subnuclear sequestration of PXR to nuclear bodies. 1.3 De-SUMOylation In keeping with previous findings, our results support that the SENPs are functionally different in their ability to de-conjugate SUMOylated PXR. In particular, we found out that SENP3 and SENP5 prefer SUMO-3 monomeric conjugates. This is unlike SENP1 and SENP2, which can de-conjugate both SUMO-1 and SUMO-3. SENP2 removed SUMO conjugates completely; otherwise SENP6 efficiently removed SUMO-3 poly-chains. The SENPs were shown to localize differently in cells. We need to further analyze the localization of SENPs in Hepa1-6 cells when cells are co-transfected 69
85 with PXR and SUMO plasmids. We postulate that in the absence of PXR, SENP1 shows primarily a nuclear localization. But SENP5 may reside mainly in nucleoli. By analyzing the co-localization of SUMOylated PXR and SENPs, we are able to distinguish the mechanism of their substrates specificity. If SENP1 was co-localized with SUMO-1-conjugated PXR and SENP5 was completely sequestered from SUMO-1-modified PXR, we might relate the different sub-cellular localization to the alternative isopepetidase activities of the SENPs. Since SENPs are functionally different towards targets, the overall conjugation state of SUMO-1- and SUMO-2/3 modified proteins may in part be regulated at the level of de-conjugation rather than conjugation (204). The activity of SENPs was regulated by cellular conditions through control of their stability. For example, SENP3 was rapidly degraded under basal condition. In an oxidative state, the ubiquitin proteasome pathway mediated degradation of SENP3 was inhibited. SENP3 re-distributed from the nucleolus to the nucleoplasm where it participated in the removal of SUMO-2/3 from a number of proteins under mild oxidative stress. Since SENP3 did not de-conjugate SUMO-1, the SUMO-1 conjugated protein level was not affected by oxidative stress stimulation (205). From this point of view, the different activity or stability of substrate-specific SENPs may account for the disparate SUMO conjugation patterns. Previously, we found that the co-expression of SUMO-3 and Ubc9 in CV-1 cells had no effect on a PXR-response element luciferase reporter gene (XREM-LUC) (132). While SUMOylation of PXR did not inhibit its 70
86 trans-activation, little is known regarding the effect of de-sumoylation of PXR by SENPs on transcriptional activation. Several results revealed that de-sumoylation by SENPs increased NRs transcriptional activity through different mechanisms. The studies discussed above (205) showed that the increased SENP3 activity under oxidative state de-sumoylated p300, which is a co-activator of HIF1. HIF1 is a master-regulator of stress-responsive genes (206) and SUMOylation of the p300 protein inhibited HIF1 transcription activity (207). Therefore, by down-regulating co-activator p300 SUMOylation level, SENP3 promoted HIF1 trans-activation of stress-responsive genes under oxidative stress (205). Another study on SENP1 and AR showed that SENP1 could increase AR transcriptional activation. It was not mediated by de-conjugation of SUMO from AR but through de-sumoylation of HDAC1, a component of co-repressor complexes (208). HDAC1 is modified by SUMO-1 in vivo, which is essential for its repressive function (209). SENP1 inhibited the transcriptional repressive effect of HDAC1 through de-conjugating HDAC1 SUMO-1 modification, which in turn enhanced AR transcription activity (208). The effect of SENPs on PXR-mediated transcription activity has not been uncovered. Whether SENPs modulate PXR activity through de-conjugating SUMOylation of PXR itself or of its co-regulators is currently unknown. Therefore, it is of interest to study the potent regulatory activity of SENPs that modulate transcription systems in order to further understand the biological functions of SUMOylation/de-SUMOylation in a big picture. 71
87 1.4 PXR SUMOylation Sites The bioinformatic approach to scan the sequence of PXR for the presence of a consensus SUMO acceptor site identified four potential sites within human PXR. Since the four sites are all located in the C-terminal hinge domain and LBD domain, we tested the SUMOylation capability of the recombinant protein composed of human PXR hinge and LBD parts in vitro. The hinge and LBD domains were SUMOylated by both SUMO-1 and SUMO-3 in vitro which is consistent with our hypothesis that SUMOylation sites reside in the C-terminal. However, it is likely that SUMO may be attached to non-consensus lysine residue, thus the total 28 lysine residues on PXR were analyzed systematically based on site-directed mutagenesis. The single, double, triple or quadruple PXR mutant was co-transfected with SUMO and PIAS plasmids in Hepa1-6 cells (data not shown). We revealed one potential site of human PXR SUMOylation at K108. By mutating K108 to arginine, the human PXR SUMO-1 and SUMO-3 conjugation levels were significantly down-regulated. After double mutation of K108 and K128, the SUMO-1 modification was completely lost, whereas the double mutant could still form monomeric conjugates by SUMO-3. The other SUMO-3 site(s) besides K108 and K128 has not yet been identified. It has been suggested that mutation both the lysine and an adjacent acidic amino acid might be required to inactivate some SUMO consensus sites (210,211). Further experimental design of site-directed mutagenesis based on this idea may help to solve the puzzle. According to our current data, the possible SUMOylation pattern on human PXR is summarized in the Table 1. 72
88 Table 1. Table 1. PXR SUMOylation Pattern. The PXR protein is targeted by SUMO-1 at two major sites, K108 and K128. SUMO-1 modification leads to monomeric and dimeric conjugates. The SUMO-3 modification pattern is different from SUMO-1. There are at least three potential sites, including K108 and K128. The lysine residues could support mono-, multi-mono-, and poly-sumo-3 conjugations of PXR. The identification of PXR SUMOylation sites is of great value. It will facilitate our understanding of PXR SUMOylation biological functions and physiological roles. For example, it could provide a direct and strong basis to 73
INTERACTION DRUG BODY
 INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
INTERACTION DRUG BODY What the drug does to the body What the body does to the drug Receptors - intracellular receptors - membrane receptors - Channel receptors - G protein-coupled receptors - Tyrosine-kinase
Biochemistry 673 Lecture 2 Jason Kahn, UMCP Introduction to steroid hormone receptor (nuclear receptor) signalling
 Biochemistry 673 Lecture 2 Jason Kahn, UMCP Introduction to steroid hormone receptor (nuclear receptor) signalling Resources: Latchman Lodish chapter 10, 20 Helmreich, chapter 11 http://www.nursa.org,
Biochemistry 673 Lecture 2 Jason Kahn, UMCP Introduction to steroid hormone receptor (nuclear receptor) signalling Resources: Latchman Lodish chapter 10, 20 Helmreich, chapter 11 http://www.nursa.org,
Principles of Genetics and Molecular Biology
 Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Cell signaling Dr. Diala Abu-Hassan, DDS, PhD School of Medicine Dr.abuhassand@gmail.com Principles of Genetics and Molecular Biology www.cs.montana.edu Modes of cell signaling Direct interaction of a
Post-translational modifications of proteins in gene regulation under hypoxic conditions
 203 Review Article Post-translational modifications of proteins in gene regulation under hypoxic conditions 1, 2) Olga S. Safronova 1) Department of Cellular Physiological Chemistry, Tokyo Medical and
203 Review Article Post-translational modifications of proteins in gene regulation under hypoxic conditions 1, 2) Olga S. Safronova 1) Department of Cellular Physiological Chemistry, Tokyo Medical and
G-Protein Signaling. Introduction to intracellular signaling. Dr. SARRAY Sameh, Ph.D
 G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
G-Protein Signaling Introduction to intracellular signaling Dr. SARRAY Sameh, Ph.D Cell signaling Cells communicate via extracellular signaling molecules (Hormones, growth factors and neurotransmitters
cholesterol structure Cholesterol FAQs Cholesterol promotes the liquid-ordered phase of membranes Friday, October 15, 2010
 cholesterol structure most plasma cholesterol is in the esterified form (not found in cells or membranes) cholesterol functions in all membranes (drives formation of lipid microdomains) cholesterol is
cholesterol structure most plasma cholesterol is in the esterified form (not found in cells or membranes) cholesterol functions in all membranes (drives formation of lipid microdomains) cholesterol is
Lecture #27 Lecturer A. N. Koval
 Lecture #27 Lecturer A. N. Koval Hormones Transduce Signals to Affect Homeostatic Mechanisms Koval A. (C), 2011 2 Lipophilic hormones Classifying hormones into hydrophilic and lipophilic molecules indicates
Lecture #27 Lecturer A. N. Koval Hormones Transduce Signals to Affect Homeostatic Mechanisms Koval A. (C), 2011 2 Lipophilic hormones Classifying hormones into hydrophilic and lipophilic molecules indicates
Oxidation of Long Chain Fatty Acids
 Oxidation of Long Chain Fatty Acids Dr NC Bird Oxidation of long chain fatty acids is the primary source of energy supply in man and animals. Hibernating animals utilise fat stores to maintain body heat,
Oxidation of Long Chain Fatty Acids Dr NC Bird Oxidation of long chain fatty acids is the primary source of energy supply in man and animals. Hibernating animals utilise fat stores to maintain body heat,
Lecture 15. Signal Transduction Pathways - Introduction
 Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
Lecture 15 Signal Transduction Pathways - Introduction So far.. Regulation of mrna synthesis Regulation of rrna synthesis Regulation of trna & 5S rrna synthesis Regulation of gene expression by signals
B. Incorrect! Compounds are made more polar, to increase their excretion.
 Pharmacology - Problem Drill 04: Biotransformation Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. What is biotransformation?
Pharmacology - Problem Drill 04: Biotransformation Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as 1. What is biotransformation?
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system
 Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Cell Biology Lecture 9 Notes Basic Principles of cell signaling and GPCR system Basic Elements of cell signaling: Signal or signaling molecule (ligand, first messenger) o Small molecules (epinephrine,
Chapter 15: Signal transduction
 Chapter 15: Signal transduction Know the terminology: Enzyme-linked receptor, G-protein linked receptor, nuclear hormone receptor, G-protein, adaptor protein, scaffolding protein, SH2 domain, MAPK, Ras,
Chapter 15: Signal transduction Know the terminology: Enzyme-linked receptor, G-protein linked receptor, nuclear hormone receptor, G-protein, adaptor protein, scaffolding protein, SH2 domain, MAPK, Ras,
Constitutive Regulation of P450s by Endocrine Factors
 References: Constitutive Regulation of P450s by Endocrine Factors Meyer UA. Endo-xenobiotic crosstalk and the regulation of cytochromes P450. Drug Metab Rev 39:639-46, 2007. Waxman DJ and O Connor C. Growth
References: Constitutive Regulation of P450s by Endocrine Factors Meyer UA. Endo-xenobiotic crosstalk and the regulation of cytochromes P450. Drug Metab Rev 39:639-46, 2007. Waxman DJ and O Connor C. Growth
Cell Quality Control. Peter Takizawa Department of Cell Biology
 Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
The Orphan Nuclear Receptors: Unrecognized Targets of Botanical Medicines?
 The Orphan Nuclear Receptors: Unrecognized Targets of Botanical Medicines? Kevin Spelman, PhD, MCPP These notes are designed as a primer for the lecture and do not necessarily represent lecture content.
The Orphan Nuclear Receptors: Unrecognized Targets of Botanical Medicines? Kevin Spelman, PhD, MCPP These notes are designed as a primer for the lecture and do not necessarily represent lecture content.
GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1
 GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1 1. The endocrine system consists of glands that secrete chemical signals, called hormones, into the blood. In addition, other organs and cells
GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1 1. The endocrine system consists of glands that secrete chemical signals, called hormones, into the blood. In addition, other organs and cells
REGULATORY MECHANISMS OF TRANSCRIPTION FACTOR FUNCTION
 Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) RG Clerc 04.04.2012 REGULATORY MECHANISMS OF TRANSCRIPTION FACTOR FUNCTION Protein synthesized Protein phosphorylated
Transcription Regulation And Gene Expression in Eukaryotes Cycle G2 (lecture 13709) RG Clerc 04.04.2012 REGULATORY MECHANISMS OF TRANSCRIPTION FACTOR FUNCTION Protein synthesized Protein phosphorylated
Cell Signaling part 2
 15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
15 Cell Signaling part 2 Functions of Cell Surface Receptors Other cell surface receptors are directly linked to intracellular enzymes. The largest family of these is the receptor protein tyrosine kinases,
Chapter 26 Biochemistry 5th edition. phospholipids. Sphingolipids. Cholesterol. db=books&itool=toolbar
 http://www.ncbi.nlm.nih.gov/sites/entrez? db=books&itool=toolbar 1 The surface of a soap bubble is a bilayer formed by detergent molecules 2 Chapter 26 Biochemistry 5th edition phospholipids Sphingolipids
http://www.ncbi.nlm.nih.gov/sites/entrez? db=books&itool=toolbar 1 The surface of a soap bubble is a bilayer formed by detergent molecules 2 Chapter 26 Biochemistry 5th edition phospholipids Sphingolipids
Receptor mediated Signal Transduction
 Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
Receptor mediated Signal Transduction G-protein-linked receptors adenylyl cyclase camp PKA Organization of receptor protein-tyrosine kinases From G.M. Cooper, The Cell. A molecular approach, 2004, third
Cell Signaling (part 1)
 15 Cell Signaling (part 1) Introduction Bacteria and unicellular eukaryotes respond to environmental signals and to signaling molecules secreted by other cells for mating and other communication. In multicellular
15 Cell Signaling (part 1) Introduction Bacteria and unicellular eukaryotes respond to environmental signals and to signaling molecules secreted by other cells for mating and other communication. In multicellular
MOLECULAR CELL BIOLOGY
 1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
1 Lodish Berk Kaiser Krieger scott Bretscher Ploegh Matsudaira MOLECULAR CELL BIOLOGY SEVENTH EDITION CHAPTER 13 Moving Proteins into Membranes and Organelles Copyright 2013 by W. H. Freeman and Company
Receptors Functions and Signal Transduction- L4- L5
 Receptors Functions and Signal Transduction- L4- L5 Faisal I. Mohammed, MD, PhD University of Jordan 1 PKC Phosphorylates many substrates, can activate kinase pathway, gene regulation PLC- signaling pathway
Receptors Functions and Signal Transduction- L4- L5 Faisal I. Mohammed, MD, PhD University of Jordan 1 PKC Phosphorylates many substrates, can activate kinase pathway, gene regulation PLC- signaling pathway
MODULATION OF THE ACTIVITY OF A KEY METABOLIC REGULATOR SMALL HETERODIMER PARTNER BY POST-TRANSLATIONAL MODIFICATIONS
 MODULATION OF THE ACTIVITY OF A KEY METABOLIC REGULATOR SMALL HETERODIMER PARTNER BY POST-TRANSLATIONAL MODIFICATIONS BY DEEPTHI KANAMALURU DISSERTATION Submitted in partial fulfillment of the requirements
MODULATION OF THE ACTIVITY OF A KEY METABOLIC REGULATOR SMALL HETERODIMER PARTNER BY POST-TRANSLATIONAL MODIFICATIONS BY DEEPTHI KANAMALURU DISSERTATION Submitted in partial fulfillment of the requirements
Mechanistic Toxicology
 SECOND EDITION Mechanistic Toxicology The Molecular Basis of How Chemicals Disrupt Biological Targets URS A. BOELSTERLI CRC Press Tavlor & France Croup CRC Press is an imp^t o* :H Taylor H Francn C'r,,jpi
SECOND EDITION Mechanistic Toxicology The Molecular Basis of How Chemicals Disrupt Biological Targets URS A. BOELSTERLI CRC Press Tavlor & France Croup CRC Press is an imp^t o* :H Taylor H Francn C'r,,jpi
KEY CONCEPT QUESTIONS IN SIGNAL TRANSDUCTION
 Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Signal Transduction - Part 2 Key Concepts - Receptor tyrosine kinases control cell metabolism and proliferation Growth factor signaling through Ras Mutated cell signaling genes in cancer cells are called
Hepatic Transporter Proteins involved in Bile Formation
 Bile salt synthesis Hepatic Transporter Proteins involved in Bile Formation Basolateral membrane transporter proteins fx: NTCP uptake of bile salts OATP bulky organic anions Canalicular membrane transporter
Bile salt synthesis Hepatic Transporter Proteins involved in Bile Formation Basolateral membrane transporter proteins fx: NTCP uptake of bile salts OATP bulky organic anions Canalicular membrane transporter
Eukaryotic transcription (III)
 Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
Eukaryotic transcription (III) 1. Chromosome and chromatin structure Chromatin, chromatid, and chromosome chromatin Genomes exist as chromatins before or after cell division (interphase) but as chromatids
BIOMARKERS AND TOXICITY MECHANISMS 07 Mechanisms Metabolism & Detoxification. Luděk Bláha, PřF MU, RECETOX
 BIOMARKERS AND TOXICITY MECHANISMS 07 Mechanisms Metabolism & Detoxification Luděk Bláha, PřF MU, RECETOX www.recetox.cz Metabolism and detoxification Chemicals enter body... mostly via food Pass directly
BIOMARKERS AND TOXICITY MECHANISMS 07 Mechanisms Metabolism & Detoxification Luděk Bláha, PřF MU, RECETOX www.recetox.cz Metabolism and detoxification Chemicals enter body... mostly via food Pass directly
Signal Transduction Cascades
 Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
March 19 th Batool Aqel
 March 19 th - 2013 6 Batool Aqel Hormones That Bind to Nuclear Receptor Proteins Hormones bind to their receptors.whether the receptor is found in the nucleus or the cytoplasm, at the end they are translocated
March 19 th - 2013 6 Batool Aqel Hormones That Bind to Nuclear Receptor Proteins Hormones bind to their receptors.whether the receptor is found in the nucleus or the cytoplasm, at the end they are translocated
Chapter 9. Cellular Signaling
 Chapter 9 Cellular Signaling Cellular Messaging Page 215 Cells can signal to each other and interpret the signals they receive from other cells and the environment Signals are most often chemicals The
Chapter 9 Cellular Signaling Cellular Messaging Page 215 Cells can signal to each other and interpret the signals they receive from other cells and the environment Signals are most often chemicals The
Insulin Resistance. Biol 405 Molecular Medicine
 Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Insulin Resistance Biol 405 Molecular Medicine Insulin resistance: a subnormal biological response to insulin. Defects of either insulin secretion or insulin action can cause diabetes mellitus. Insulin-dependent
Role of metabolism in Drug-Induced Liver Injury (DILI) Drug Metab Rev. 2007;39(1):
 Role of metabolism in Drug-Induced Liver Injury (DILI) Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 A schematic representation of the most relevant
Role of metabolism in Drug-Induced Liver Injury (DILI) Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 Drug Metab Rev. 2007;39(1):159-234 A schematic representation of the most relevant
Model Answer. M.Sc. Zoology (First Semester) Examination Paper LZT 103 (Endocrinology)
 Model Answer M.Sc. Zoology (First Semester) Examination-2013 Paper LZT 103 (Endocrinology) Section A 1. (i) d (ii) b (iii) b (iv) c (v) c (vi) a (vii) c (viii) a (ix) d (x) b Section B Q.2 Answer Hormonal
Model Answer M.Sc. Zoology (First Semester) Examination-2013 Paper LZT 103 (Endocrinology) Section A 1. (i) d (ii) b (iii) b (iv) c (v) c (vi) a (vii) c (viii) a (ix) d (x) b Section B Q.2 Answer Hormonal
Molecular Cell Biology - Problem Drill 19: Cell Signaling Pathways and Gene Expression
 Molecular Cell Biology - Problem Drill 19: Cell Signaling Pathways and Gene Expression Question No. 1 of 10 1. Which statement about cell signaling is correct? Question #1 (A) Cell signaling involves receiving
Molecular Cell Biology - Problem Drill 19: Cell Signaling Pathways and Gene Expression Question No. 1 of 10 1. Which statement about cell signaling is correct? Question #1 (A) Cell signaling involves receiving
HORMONES (Biomedical Importance)
 hormones HORMONES (Biomedical Importance) Hormones are the chemical messengers of the body. They are defined as organic substances secreted into blood stream to control the metabolic and biological activities.
hormones HORMONES (Biomedical Importance) Hormones are the chemical messengers of the body. They are defined as organic substances secreted into blood stream to control the metabolic and biological activities.
Computational Biology I LSM5191
 Computational Biology I LSM5191 Aylwin Ng, D.Phil Lecture 6 Notes: Control Systems in Gene Expression Pulling it all together: coordinated control of transcriptional regulatory molecules Simple Control:
Computational Biology I LSM5191 Aylwin Ng, D.Phil Lecture 6 Notes: Control Systems in Gene Expression Pulling it all together: coordinated control of transcriptional regulatory molecules Simple Control:
Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes
 Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes The major site of acetoacetate and 3-hydorxybutyrate production is in the liver. 3-hydorxybutyrate is the
Companion to Biosynthesis of Ketones & Cholesterols, Regulation of Lipid Metabolism Lecture Notes The major site of acetoacetate and 3-hydorxybutyrate production is in the liver. 3-hydorxybutyrate is the
Cellular Signaling Pathways. Signaling Overview
 Cellular Signaling Pathways Signaling Overview Signaling steps Synthesis and release of signaling molecules (ligands) by the signaling cell. Transport of the signal to the target cell Detection of the
Cellular Signaling Pathways Signaling Overview Signaling steps Synthesis and release of signaling molecules (ligands) by the signaling cell. Transport of the signal to the target cell Detection of the
Integration Of Metabolism
 Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Integration Of Metabolism Metabolism Consist of Highly Interconnected Pathways The basic strategy of catabolic metabolism is to form ATP, NADPH, and building blocks for biosyntheses. 1. ATP is the universal
Signaling Through Immune System Receptors (Ch. 7)
 Signaling Through Immune System Receptors (Ch. 7) 1. General principles of signal transduction and propagation. 2. Antigen receptor signaling and lymphocyte activation. 3. Other receptors and signaling
Signaling Through Immune System Receptors (Ch. 7) 1. General principles of signal transduction and propagation. 2. Antigen receptor signaling and lymphocyte activation. 3. Other receptors and signaling
Week 3 The Pancreas: Pancreatic ph buffering:
 Week 3 The Pancreas: A gland with both endocrine (secretion of substances into the bloodstream) & exocrine (secretion of substances to the outside of the body or another surface within the body) functions
Week 3 The Pancreas: A gland with both endocrine (secretion of substances into the bloodstream) & exocrine (secretion of substances to the outside of the body or another surface within the body) functions
6.5 Enzymes. Enzyme Active Site and Substrate Specificity
 180 Chapter 6 Metabolism 6.5 Enzymes By the end of this section, you will be able to: Describe the role of enzymes in metabolic pathways Explain how enzymes function as molecular catalysts Discuss enzyme
180 Chapter 6 Metabolism 6.5 Enzymes By the end of this section, you will be able to: Describe the role of enzymes in metabolic pathways Explain how enzymes function as molecular catalysts Discuss enzyme
23.1 Lipid Metabolism in Animals. Chapter 23. Micelles Lipid Metabolism in. Animals. Overview of Digestion Lipid Metabolism in
 Denniston Topping Caret Copyright! The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 23 Fatty Acid Metabolism Triglycerides (Tgl) are emulsified into fat droplets
Denniston Topping Caret Copyright! The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 23 Fatty Acid Metabolism Triglycerides (Tgl) are emulsified into fat droplets
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION
 CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
CYTOKINE RECEPTORS AND SIGNAL TRANSDUCTION What is Cytokine? Secreted popypeptide (protein) involved in cell-to-cell signaling. Acts in paracrine or autocrine fashion through specific cellular receptors.
Cholesterol and its transport. Alice Skoumalová
 Cholesterol and its transport Alice Skoumalová 27 carbons Cholesterol - structure Cholesterol importance A stabilizing component of cell membranes A precursor of bile salts A precursor of steroid hormones
Cholesterol and its transport Alice Skoumalová 27 carbons Cholesterol - structure Cholesterol importance A stabilizing component of cell membranes A precursor of bile salts A precursor of steroid hormones
A. Incorrect! It s not correct. Synergism of cytokines refers to two or more cytokines acting together.
 Immunology - Problem Drill 11: Cytokine and Cytokine Receptors Question No. 1 of 10 1. A single cytokine can act on several different cell types, which is known as. Question #1 (A) Synergism (B) Pleiotropism
Immunology - Problem Drill 11: Cytokine and Cytokine Receptors Question No. 1 of 10 1. A single cytokine can act on several different cell types, which is known as. Question #1 (A) Synergism (B) Pleiotropism
Vets 111/Biov 111 Cell Signalling-2. Secondary messengers the cyclic AMP intracellular signalling system
 Vets 111/Biov 111 Cell Signalling-2 Secondary messengers the cyclic AMP intracellular signalling system The classical secondary messenger model of intracellular signalling A cell surface receptor binds
Vets 111/Biov 111 Cell Signalling-2 Secondary messengers the cyclic AMP intracellular signalling system The classical secondary messenger model of intracellular signalling A cell surface receptor binds
Published on Second Faculty of Medicine, Charles University (http://www.lf2.cuni.cz )
 Published on Second Faculty of Medicine, Charles University (http://www.lf2.cuni.cz ) Biochemistry Submitted by Marie Havlová on 8. February 2012-0:00 Syllabus of Biochemistry Mechanisms of enzyme catalysis.
Published on Second Faculty of Medicine, Charles University (http://www.lf2.cuni.cz ) Biochemistry Submitted by Marie Havlová on 8. February 2012-0:00 Syllabus of Biochemistry Mechanisms of enzyme catalysis.
REGULATION OF ENZYME ACTIVITY. Medical Biochemistry, Lecture 25
 REGULATION OF ENZYME ACTIVITY Medical Biochemistry, Lecture 25 Lecture 25, Outline General properties of enzyme regulation Regulation of enzyme concentrations Allosteric enzymes and feedback inhibition
REGULATION OF ENZYME ACTIVITY Medical Biochemistry, Lecture 25 Lecture 25, Outline General properties of enzyme regulation Regulation of enzyme concentrations Allosteric enzymes and feedback inhibition
Chapter 11: Enzyme Catalysis
 Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
Chapter 11: Enzyme Catalysis Matching A) high B) deprotonated C) protonated D) least resistance E) motion F) rate-determining G) leaving group H) short peptides I) amino acid J) low K) coenzymes L) concerted
MODULE No.26: Drug Metabolism
 SUBJECT Paper No. and Title Module No. and Title Module Tag PAPER No. 9: Drugs of Abuse MODULE No. 26: Drug Metabolism FSC_P9_M26 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Sites of Drug
SUBJECT Paper No. and Title Module No. and Title Module Tag PAPER No. 9: Drugs of Abuse MODULE No. 26: Drug Metabolism FSC_P9_M26 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Sites of Drug
Bile acid receptor FXR: metabolic regulator in the gut. Sungsoon Fang
 Bile acid receptor FXR: metabolic regulator in the gut Sungsoon Fang Nuclear hormone receptor 1905: Ernest Starling coined hormone 1929: Estrogen structure 1958: Estrogen receptor by Elwood Jensen 1985:
Bile acid receptor FXR: metabolic regulator in the gut Sungsoon Fang Nuclear hormone receptor 1905: Ernest Starling coined hormone 1929: Estrogen structure 1958: Estrogen receptor by Elwood Jensen 1985:
Principles of cell signaling Lecture 4
 Principles of cell signaling Lecture 4 Johan Lennartsson Molecular Cell Biology (1BG320), 2014 Johan.Lennartsson@licr.uu.se 1 Receptor tyrosine kinase-induced signal transduction Erk MAP kinase pathway
Principles of cell signaling Lecture 4 Johan Lennartsson Molecular Cell Biology (1BG320), 2014 Johan.Lennartsson@licr.uu.se 1 Receptor tyrosine kinase-induced signal transduction Erk MAP kinase pathway
Lipids and Membranes
 Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
Lipids and Membranes Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Membrane transport D. Endocytosis and Exocytosis
Chapter 10. Regulatory Strategy
 Chapter 10 Regulatory Strategy Regulation of enzymatic activity: 1. Allosteric Control. Allosteric proteins have a regulatory site(s) and multiple functional sites Activity of proteins is regulated by
Chapter 10 Regulatory Strategy Regulation of enzymatic activity: 1. Allosteric Control. Allosteric proteins have a regulatory site(s) and multiple functional sites Activity of proteins is regulated by
Lipid Chemistry. Presented By. Ayman Elsamanoudy Salwa Abo El-khair
 Lipid Chemistry Presented By Ayman Elsamanoudy Salwa Abo El-khair 6 1. By the end of this chapter the student should be able to: define lipids. describe the biological importance of lipids. point out basic
Lipid Chemistry Presented By Ayman Elsamanoudy Salwa Abo El-khair 6 1. By the end of this chapter the student should be able to: define lipids. describe the biological importance of lipids. point out basic
Fatty acid breakdown
 Fatty acids contain a long hydrocarbon chain and a terminal carboxylate group. Most contain between 14 and 24 carbon atoms. The chains may be saturated or contain double bonds. The complete oxidation of
Fatty acids contain a long hydrocarbon chain and a terminal carboxylate group. Most contain between 14 and 24 carbon atoms. The chains may be saturated or contain double bonds. The complete oxidation of
number Done by Corrected by Doctor Nayef Karadsheh
 number 13 Done by Asma Karameh Corrected by Saad hayek Doctor Nayef Karadsheh Gluconeogenesis This lecture covers gluconeogenesis with aspects of: 1) Introduction to glucose distribution through tissues.
number 13 Done by Asma Karameh Corrected by Saad hayek Doctor Nayef Karadsheh Gluconeogenesis This lecture covers gluconeogenesis with aspects of: 1) Introduction to glucose distribution through tissues.
Summary of fatty acid synthesis
 Lipid Metabolism, part 2 1 Summary of fatty acid synthesis 8 acetyl CoA + 14 NADPH + 14 H+ + 7 ATP palmitic acid (16:0) + 8 CoA + 14 NADP + + 7 ADP + 7 Pi + 7 H20 1. The major suppliers of NADPH for fatty
Lipid Metabolism, part 2 1 Summary of fatty acid synthesis 8 acetyl CoA + 14 NADPH + 14 H+ + 7 ATP palmitic acid (16:0) + 8 CoA + 14 NADP + + 7 ADP + 7 Pi + 7 H20 1. The major suppliers of NADPH for fatty
NFκB What is it and What s the deal with radicals?
 The Virtual Free Radical School NFκB What is it and What s the deal with radicals? Emily Ho, Ph.D Linus Pauling Institute Scientist Department of Nutrition and Food Management Oregon State University 117
The Virtual Free Radical School NFκB What is it and What s the deal with radicals? Emily Ho, Ph.D Linus Pauling Institute Scientist Department of Nutrition and Food Management Oregon State University 117
Biosynthesis of Fatty Acids. By Dr.QUTAIBA A. QASIM
 Biosynthesis of Fatty Acids By Dr.QUTAIBA A. QASIM Fatty Acids Definition Fatty acids are comprised of hydrocarbon chains terminating with carboxylic acid groups. Fatty acids and their associated derivatives
Biosynthesis of Fatty Acids By Dr.QUTAIBA A. QASIM Fatty Acids Definition Fatty acids are comprised of hydrocarbon chains terminating with carboxylic acid groups. Fatty acids and their associated derivatives
PCEUT 527 Enzyme Induction: Biochemical Mechanisms 2/13/15
 PCEUT 527 Enzyme Induction: Biochemical Mechanisms 2/13/15 1. General Principles 2. Increase in enzyme content through transcription 3. Protein degradation 4. Protein stabilization Why Does Induction Occur?
PCEUT 527 Enzyme Induction: Biochemical Mechanisms 2/13/15 1. General Principles 2. Increase in enzyme content through transcription 3. Protein degradation 4. Protein stabilization Why Does Induction Occur?
The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism
 Supplementary Information The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism Address correspondence to Yong Li (yongli@xmu.edu.cn, Tel: 86-592-218151) GW464 CDCA Supplementary
Supplementary Information The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism Address correspondence to Yong Li (yongli@xmu.edu.cn, Tel: 86-592-218151) GW464 CDCA Supplementary
BIOL 4374/BCHS 4313 Cell Biology Exam #1 February 13, 2001
 BIOL 4374/BCHS 4313 Cell Biology Exam #1 February 13, 2001 SS# Name This exam is worth a total of 100 points. The number of points each question is worth is shown in parentheses. Good luck! 1. (2) The
BIOL 4374/BCHS 4313 Cell Biology Exam #1 February 13, 2001 SS# Name This exam is worth a total of 100 points. The number of points each question is worth is shown in parentheses. Good luck! 1. (2) The
Chemistry 107 Exam 4 Study Guide
 Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
Chemistry 107 Exam 4 Study Guide Chapter 10 10.1 Recognize that enzyme catalyze reactions by lowering activation energies. Know the definition of a catalyst. Differentiate between absolute, relative and
Effects of Second Messengers
 Effects of Second Messengers Inositol trisphosphate Diacylglycerol Opens Calcium Channels Binding to IP 3 -gated Channel Cooperative binding Activates Protein Kinase C is required Phosphorylation of many
Effects of Second Messengers Inositol trisphosphate Diacylglycerol Opens Calcium Channels Binding to IP 3 -gated Channel Cooperative binding Activates Protein Kinase C is required Phosphorylation of many
number Done by Corrected by Doctor Faisal Al-Khatibe
 number 24 Done by Mohammed tarabieh Corrected by Doctor Faisal Al-Khatibe 1 P a g e *Please look over the previous sheet about fatty acid synthesis **Oxidation(degradation) of fatty acids, occurs in the
number 24 Done by Mohammed tarabieh Corrected by Doctor Faisal Al-Khatibe 1 P a g e *Please look over the previous sheet about fatty acid synthesis **Oxidation(degradation) of fatty acids, occurs in the
Lecture: CHAPTER 13 Signal Transduction Pathways
 Lecture: 10 17 2016 CHAPTER 13 Signal Transduction Pathways Chapter 13 Outline Signal transduction cascades have many components in common: 1. Release of a primary message as a response to a physiological
Lecture: 10 17 2016 CHAPTER 13 Signal Transduction Pathways Chapter 13 Outline Signal transduction cascades have many components in common: 1. Release of a primary message as a response to a physiological
Close to site of release (at synapse); binds to receptors in
 Chapter 18: The Endocrine System Chemical Messengers 1. Neural 2. Endocrine 3. Neuroendocrine 4. Paracrine 5. Autocrine Endocrine System --Endocrine and nervous systems work together --Endocrine vs. Nervous
Chapter 18: The Endocrine System Chemical Messengers 1. Neural 2. Endocrine 3. Neuroendocrine 4. Paracrine 5. Autocrine Endocrine System --Endocrine and nervous systems work together --Endocrine vs. Nervous
Introduction. Biochemistry: It is the chemistry of living things (matters).
 Introduction Biochemistry: It is the chemistry of living things (matters). Biochemistry provides fundamental understanding of the molecular basis for the function and malfunction of living things. Biochemistry
Introduction Biochemistry: It is the chemistry of living things (matters). Biochemistry provides fundamental understanding of the molecular basis for the function and malfunction of living things. Biochemistry
Ayman Mesleh & Leen Alnemrawi. Bayan Abusheikha. Faisal
 24 Ayman Mesleh & Leen Alnemrawi Bayan Abusheikha Faisal We were talking last time about receptors for lipid soluble hormones.the general mechanism of receptors for lipid soluble hormones: 1. Receptors
24 Ayman Mesleh & Leen Alnemrawi Bayan Abusheikha Faisal We were talking last time about receptors for lipid soluble hormones.the general mechanism of receptors for lipid soluble hormones: 1. Receptors
Biol220 Cell Signalling Cyclic AMP the classical secondary messenger
 Biol220 Cell Signalling Cyclic AMP the classical secondary messenger The classical secondary messenger model of intracellular signalling A cell surface receptor binds the signal molecule (the primary
Biol220 Cell Signalling Cyclic AMP the classical secondary messenger The classical secondary messenger model of intracellular signalling A cell surface receptor binds the signal molecule (the primary
Chapter 11 CYTOKINES
 Chapter 11 CYTOKINES group of low molecular weight regulatory proteins secreted by leukocytes as well as a variety of other cells in the body (8~30kD) regulate the intensity and duration of the immune
Chapter 11 CYTOKINES group of low molecular weight regulatory proteins secreted by leukocytes as well as a variety of other cells in the body (8~30kD) regulate the intensity and duration of the immune
Enzymes Part III: regulation II. Dr. Mamoun Ahram Summer, 2017
 Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Enzymes Part III: regulation II Dr. Mamoun Ahram Summer, 2017 Advantage This is a major mechanism for rapid and transient regulation of enzyme activity. A most common mechanism is enzyme phosphorylation
Glycolysis Part 2. BCH 340 lecture 4
 Glycolysis Part 2 BCH 340 lecture 4 Regulation of Glycolysis There are three steps in glycolysis that have enzymes which regulate the flux of glycolysis These enzymes catalyzes irreversible reactions of
Glycolysis Part 2 BCH 340 lecture 4 Regulation of Glycolysis There are three steps in glycolysis that have enzymes which regulate the flux of glycolysis These enzymes catalyzes irreversible reactions of
Supplementary Figure 1.
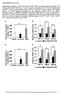 Supplementary Figure 1. FGF21 does not exert direct effects on hepatic glucose production. The liver explants from C57BL/6J mice (A, B) or primary rat hepatocytes (C, D) were incubated with rmfgf21 (2
Supplementary Figure 1. FGF21 does not exert direct effects on hepatic glucose production. The liver explants from C57BL/6J mice (A, B) or primary rat hepatocytes (C, D) were incubated with rmfgf21 (2
RAS Genes. The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes.
 ۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
Module C CHEMISTRY & CELL BIOLOGY REVIEW
 Module C CHEMISTRY & CELL BIOLOGY REVIEW Note: This module is provided for A&P courses that do not have a prerequisite class which includes chemistry and cell biology. Content covered by required prerequisite
Module C CHEMISTRY & CELL BIOLOGY REVIEW Note: This module is provided for A&P courses that do not have a prerequisite class which includes chemistry and cell biology. Content covered by required prerequisite
2013 W. H. Freeman and Company. 12 Signal Transduction
 2013 W. H. Freeman and Company 12 Signal Transduction CHAPTER 12 Signal Transduction Key topics: General features of signal transduction Structure and function of G protein coupled receptors Structure
2013 W. H. Freeman and Company 12 Signal Transduction CHAPTER 12 Signal Transduction Key topics: General features of signal transduction Structure and function of G protein coupled receptors Structure
Chapter 4. Drug Biotransformation
 Chapter 4 Drug Biotransformation Drug Biotransformation 1 Why is drug biotransformation necessary 2 The role of biotransformation in drug disposition 3 Where do drug biotransformation occur 4 The enzymes
Chapter 4 Drug Biotransformation Drug Biotransformation 1 Why is drug biotransformation necessary 2 The role of biotransformation in drug disposition 3 Where do drug biotransformation occur 4 The enzymes
Supplemental Table 1 Primer sequences (mouse) used for real-time qrt-pcr studies
 Supplemental Table 1 Primer sequences (mouse) used for real-time qrt-pcr studies Gene symbol Forward primer Reverse primer ACC1 5'-TGAGGAGGACCGCATTTATC 5'-GCATGGAATGGCAGTAAGGT ACLY 5'-GACACCATCTGTGATCTTG
Supplemental Table 1 Primer sequences (mouse) used for real-time qrt-pcr studies Gene symbol Forward primer Reverse primer ACC1 5'-TGAGGAGGACCGCATTTATC 5'-GCATGGAATGGCAGTAAGGT ACLY 5'-GACACCATCTGTGATCTTG
Nuclear Receptor Monoclonal Antibodies
 High affinity and specificity 研究用 Research www.funakoshi.co.jp Use Only Nuclear Receptor Monoclonal Antibodies All antibodies are validated by reactivity with proteins from full length cdna expressed in
High affinity and specificity 研究用 Research www.funakoshi.co.jp Use Only Nuclear Receptor Monoclonal Antibodies All antibodies are validated by reactivity with proteins from full length cdna expressed in
Signal Transduction Pathway Smorgasbord
 Molecular Cell Biology Lecture. Oct 28, 2014 Signal Transduction Pathway Smorgasbord Ron Bose, MD PhD Biochemistry and Molecular Cell Biology Programs Washington University School of Medicine Outline 1.
Molecular Cell Biology Lecture. Oct 28, 2014 Signal Transduction Pathway Smorgasbord Ron Bose, MD PhD Biochemistry and Molecular Cell Biology Programs Washington University School of Medicine Outline 1.
4. Lysosomes, Smooth Endoplasmic Reticulum, Mitochondria, and Inclusions
 4. Lysosomes, Smooth Endoplasmic Reticulum, Mitochondria, and Inclusions Undergraduate Graduate Histology Lecture Series Larry Johnson, Professor Veterinary Integrative Biosciences Texas A&M University
4. Lysosomes, Smooth Endoplasmic Reticulum, Mitochondria, and Inclusions Undergraduate Graduate Histology Lecture Series Larry Johnson, Professor Veterinary Integrative Biosciences Texas A&M University
BCM 221 LECTURES OJEMEKELE O.
 BCM 221 LECTURES BY OJEMEKELE O. OUTLINE INTRODUCTION TO LIPID CHEMISTRY STORAGE OF ENERGY IN ADIPOCYTES MOBILIZATION OF ENERGY STORES IN ADIPOCYTES KETONE BODIES AND KETOSIS PYRUVATE DEHYDROGENASE COMPLEX
BCM 221 LECTURES BY OJEMEKELE O. OUTLINE INTRODUCTION TO LIPID CHEMISTRY STORAGE OF ENERGY IN ADIPOCYTES MOBILIZATION OF ENERGY STORES IN ADIPOCYTES KETONE BODIES AND KETOSIS PYRUVATE DEHYDROGENASE COMPLEX
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment
 Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Overview: Conducting the Genetic Orchestra Prokaryotes and eukaryotes alter gene expression in response to their changing environment In multicellular eukaryotes, gene expression regulates development
Bile acid metabolism. doc. Ing. Zenóbia Chavková, CSc.
 Bile acid metabolism doc. Ing. Zenóbia Chavková, CSc. Bile acid metabolism Importance: Availability for fat & cholesterol absorption Regulates total body pool of cholesterol Factors that synthesis promote
Bile acid metabolism doc. Ing. Zenóbia Chavková, CSc. Bile acid metabolism Importance: Availability for fat & cholesterol absorption Regulates total body pool of cholesterol Factors that synthesis promote
Tala Saleh. Ahmad Attari. Mamoun Ahram
 23 Tala Saleh Ahmad Attari Minna Mushtaha Mamoun Ahram In the previous lecture, we discussed the mechanisms of regulating enzymes through inhibitors. Now, we will start this lecture by discussing regulation
23 Tala Saleh Ahmad Attari Minna Mushtaha Mamoun Ahram In the previous lecture, we discussed the mechanisms of regulating enzymes through inhibitors. Now, we will start this lecture by discussing regulation
Enzyme-coupled Receptors. Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors
 Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Toll-like Receptor Signaling
 Toll-like Receptor Signaling 1 Professor of Medicine University of Massachusetts Medical School, Worcester, MA, USA Why do we need innate immunity? Pathogens multiply very fast We literally swim in viruses
Toll-like Receptor Signaling 1 Professor of Medicine University of Massachusetts Medical School, Worcester, MA, USA Why do we need innate immunity? Pathogens multiply very fast We literally swim in viruses
CELLS. Cells. Basic unit of life (except virus)
 Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Basic unit of life (except virus) CELLS Prokaryotic, w/o nucleus, bacteria Eukaryotic, w/ nucleus Various cell types specialized for particular function. Differentiation. Over 200 human cell types 56%
Regulation of P450. b. competitive-inhibitor but not substrate. c. non-competitive inhibition. 2. Covalent binding to heme or protein
 10. Inhibition of P450 Regulation of P450 a. competitive-2 chemicals metabolized by same isoform b. competitive-inhibitor but not substrate c. non-competitive inhibition 11. Induction of P450 1. Non-covalent
10. Inhibition of P450 Regulation of P450 a. competitive-2 chemicals metabolized by same isoform b. competitive-inhibitor but not substrate c. non-competitive inhibition 11. Induction of P450 1. Non-covalent
Ch. 18 Regulation of Gene Expression
 Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
Ch. 18 Regulation of Gene Expression 1 Human genome has around 23,688 genes (Scientific American 2/2006) Essential Questions: How is transcription regulated? How are genes expressed? 2 Bacteria regulate
(de novo synthesis of glucose)
 Gluconeogenesis (de novo synthesis of glucose) Gluconeogenesis Gluconeogenesis is the biosynthesis of new glucose. The main purpose of gluconeogenesis is to maintain the constant blood Glc concentration.
Gluconeogenesis (de novo synthesis of glucose) Gluconeogenesis Gluconeogenesis is the biosynthesis of new glucose. The main purpose of gluconeogenesis is to maintain the constant blood Glc concentration.
Metabolic integration and Regulation
 Metabolic integration and Regulation 109700: Graduate Biochemistry Trimester 2/2016 Assistant Prof. Dr. Panida Khunkaewla kpanida@sut.ac.th School of Chemistry Suranaree University of Technology 1 Overview
Metabolic integration and Regulation 109700: Graduate Biochemistry Trimester 2/2016 Assistant Prof. Dr. Panida Khunkaewla kpanida@sut.ac.th School of Chemistry Suranaree University of Technology 1 Overview
REVIEW ARTICLE Regulation of cytochrome P450 (CYP) genes by nuclear receptors
 Biochem. J. (2000) 347, 321 337 (Printed in Great Britain) 321 REVIEW ARTICLE Regulation of cytochrome P450 (CYP) genes by nuclear receptors Paavo HONKAKOSKI* 1 and Masahiko NEGISHI *Department of Pharmaceutics,
Biochem. J. (2000) 347, 321 337 (Printed in Great Britain) 321 REVIEW ARTICLE Regulation of cytochrome P450 (CYP) genes by nuclear receptors Paavo HONKAKOSKI* 1 and Masahiko NEGISHI *Department of Pharmaceutics,
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM. Triacylglycerol and Fatty Acid Metabolism
 ANSC/NUTR 618 LIPIDS & LIPID METABOLISM II. Triacylglycerol synthesis A. Overall pathway Glycerol-3-phosphate + 3 Fatty acyl-coa à Triacylglycerol + 3 CoASH B. Enzymes 1. Acyl-CoA synthase 2. Glycerol-phosphate
ANSC/NUTR 618 LIPIDS & LIPID METABOLISM II. Triacylglycerol synthesis A. Overall pathway Glycerol-3-phosphate + 3 Fatty acyl-coa à Triacylglycerol + 3 CoASH B. Enzymes 1. Acyl-CoA synthase 2. Glycerol-phosphate
Propagation of the Signal
 OpenStax-CNX module: m44452 1 Propagation of the Signal OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
OpenStax-CNX module: m44452 1 Propagation of the Signal OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section,
