Paracetamol. Hi I m Molly Cool. A common painkiller used to treat headaches and relieve fever in cold and flu
|
|
|
- Margaret Haynes
- 5 years ago
- Views:
Transcription
1 Paracetamol A common painkiller used to treat headaches and relieve fever in cold and flu 8 C Carbon (black) 2 xygen (red) 1 N Nitrogen (dark blue) 9 H Hydrogen (white) 7 single bonds (straight) 8 double bonds (bendy)
2 Salbutamol Also called Ventolin used in asthma inhalers 13 C Carbon (black) 3 xygen (red) 1 N Nitrogen (light blue) 21 H Hydrogen (white) 14 single bonds (straight) 6 double bonds (bendy)
3 Aspirin A common pain killer, also used to reduce fever 9 C Carbon (black) 4 xygen (red) 8 H Hydrogen (white) 8 single bonds (straight) 10 double bonds (bendy)
4 Prozac An anti-depressant drug 17 C Carbon (black) 1 xygen (red) 1 N Nitrogen (light blue) 3 F Fluorine (green) 18 H Hydrogen (white) 17 single bonds (straight) 12 double bonds (bendy)
5 Tamoxifen This drug is used to treat breast cancer C Carbon (black) 1 xygen (red) 1 N Nitrogen (light blue) 29 H Hydrogen (white) 20 single bonds (straight) 20 double bonds (bendy)
6 Penicillin G An antibiotic drug used to treat bacterial infections 16 C Carbon (black) 4 xygen (red) 2 N Nitrogen (light blue) 1 S Sulfur (yellow, 2 holes) 18 H Hydrogen (white) 19 single bonds (straight) 12 double bonds (bendy)
7 Thalidomide A drug to treat morning sickness in pregnancy that caused serious birth defects in the 1960 s 13 C Carbon (black) 4 xygen (red) 2 N Nitrogen (dark blue) 10 H Hydrogen (white) 14 single bonds (straight) 14 double bonds (bendy)
8 Leucine ne of 20 amino acids that make up protein 6 C Carbon (black) 2 xygen (red) 1 N Nitrogen (light blue) 13 H Hydrogen (white) 7 single bonds (straight) 2 double bonds (bendy)
9 Glucose A type of sugar. Glucose tastes sweet and is the basic building block of carbohydrate 6 C Carbon (black) 6 xygen (red) 12 H Hydrogen (white) 12 single bonds (straight)
10 α-linolenic Acid An omega-3 fatty acid found in vegetable oils, especially rapeseed oil C Carbon (black) 2 xygen (red) 30 H Hydrogen (white) 15 single bonds (straight) 8 double bonds (bendy)
11 Vitamin A A vitamin important for eyesight, found in carrots 20 C Carbon (black) 1 xygen (red) 30 H Hydrogen (white) 15 single bonds (straight) 10 double bonds (bendy)
12 Vitamin C Found in fresh fruit and vegetables, a deficiency of this vitamin causes scurvy in humans 6 C Carbon (black) 6 xygen (red) 8 H Hydrogen (white) 10 single bonds (straight) 4 double bonds (bendy)
13 Adrenaline Chemical messenger released in excitement or danger results in an adrenaline rush 9 C Carbon (black) 3 xygen (red) 1 N Nitrogen (light blue) 13 H Hydrogen (white) 10 single bonds (straight) 6 double bonds (bendy)
14 Serotonin A chemical messenger which contributes to the feeling of happiness 10 C Carbon (black) 1 xygen (red) 1 N Nitrogen (dark blue) 1 N Nitrogen (light blue) 12 H Hydrogen (white) 10 single bonds (straight) 8 double bonds (bendy)
15 Histamine A chemical messenger which triggers an immune response causing swelling and itching 5 C Carbon (black) 1 N Nitrogen (dark blue) 2 N Nitrogen (light blue) 9 H Hydrogen (white) 6 single bonds (straight) 4 double bonds (bendy)
16 Adenine A DNA and RNA base 5 C Carbon (black) 3 N Nitrogen (light blue) 2 N Nitrogen (dark blue) 5 H Hydrogen (white) 7 single bonds (straight) 8 double bonds (bendy)
17 Deoxyribose & Phosphate The backbone of DNA 5 C Carbon (black) 7 xygen (red) 1 P Phosphorus (purple) 11 H Hydrogen (white) 12 single bonds (straight) 2 double bonds (bendy)
18 Caffeine Stimulant found in tea & coffee 8 C Carbon (black) 2 xygen (red) 3 N Nitrogen (dark blue) 1 N Nitrogen (light blue) 10 H Hydrogen (white) 11 single bonds (straight) 8 double bonds (bendy)
19 MSG Monosodium glutamate a flavour enhancer 5 C Carbon (black) 4 xygen (red) 1 N Nitrogen (light blue) 1 Na Sodium (grey) 8 H Hydrogen (white) 8 single bonds (straight) 4 double bonds (bendy)
20 Sucrose The most commonly used form of sugar in cooking. Also used to sweeten tea & coffee 12 C Carbon (black) 11 xygen (red) 22 H Hydrogen (white) 24 single bonds (straight)
21 Aspartame An artificial sweetener 200 times sweeter than sugar 14 C Carbon (black) 5 xygen (red) 1 N Nitrogen (dark blue) 1 N Nitrogen (light blue) 18 H Hydrogen (white) 15 single bonds (straight) 12 double bonds (bendy)
22 Citric acid The acid found in oranges and lemons 6 C Carbon (black) 7 xygen (red) 8 H Hydrogen (white) 9 single bonds (straight) 6 double bonds (bendy)
23 Acetic Acid The acid in vinegar 2 C Carbon (black) 2 xygen (red) 4 H Hydrogen (white) 2 single bonds (straight) 2 double bonds (bendy)
24 Ethanol The alcohol in alcoholic drinks such as beer, wine and spirits 2 C Carbon (black) 1 xygen (red) 6 H Hydrogen (white) 2 single bonds (straight)
25 Sodium Laureth Sulphate A detergent found in soaps and shampoos 17 C Carbon (black) 7 xygen (red) 1 Na Sodium (grey) 1 S Sulfur (yellow, 6 holes) 35 H Hydrogen (white) 23 single bonds (straight) 4 double bonds (bendy)
26 Dettol A liquid antiseptic used in a range of cleaning products 8 C Carbon (black) 1 xygen (red) 1 Cl Chlorine (green) 9 H Hydrogen (white) 7 single bonds (straight) 6 double bonds (bendy)
27 Menthol A soothing, minty tasting molecule used in sweets and cough medicines 10 C Carbon (black) 1 xygen (red) 20 H Hydrogen (white) 11 single bonds (straight)
28 Nicotine The addictive mood-altering molecule in tobacco that makes smoking so hard to give up 10 C Carbon (black) 2 N Nitrogen (light blue) 14 H Hydrogen (white) 10 single bonds (straight) 6 double bonds (bendy)
29 Acetone A useful solvent commonly found in paint thinner and nail polish remover 3 C Carbon (black) 1 xygen (red) 6 H Hydrogen (white) 2 single bonds (straight) 2 double bonds (bendy)
30 Antifreeze Mixes with water and reduces its freezing point to stop it turning to ice on car windscreens 2 C Carbon (black) 2 xygen (red) 6 H Hydrogen (white) 3 single bonds (straight)
31 Lawsone A red-orange dye extracted from the leaves of the Henna plant, used as a hair/skin pigment 10 C Carbon (black) 3 xygen (red) 6 H Hydrogen (white) 8 single bonds (straight) 12 double bonds (bendy)
32 Cochineal A bright red dye used in foods and cosmetics, extracted from a South American insect 22 C Carbon (black) 13 xygen (red) 20 H Hydrogen (white) 7 single bonds (straight) 8 double bonds (bendy)
33 Fake Tan Dihydroxyacetone is found in fake tan. It reacts with the proteins on the surface of your skin and turns them brown. 3 C Carbon (black) 3 xygen (red) 6 H Hydrogen (white) 4 single bonds (straight) 2 double bonds (bendy)
34 Indigo An ancient dye obtained from woad. Now used to make the blue dye to colour jeans 16 C Carbon (black) 2 xygen (red) 2 N Nitrogen (dark blue) 10 H Hydrogen (white) 14 single bonds (straight) 18 double bonds (bendy)
35 Sudan 1 A red dye that made the news in 2005 when it was improperly used to colour chilli powder 16 C Carbon (black) 1 xygen (red) 2 N Nitrogen (light blue) 12 H Hydrogen (white) 12 single bonds (straight) 18 double bonds (bendy)
36 Vanillin The smell of vanilla. Extracted from the vanilla pod and used to flavour ice-cream and other foods 8 C Carbon (black) 3 xygen (red) 8 H Hydrogen (white) 7 single bonds (straight) 8 double bonds (bendy)
37 Skatole The smell of poo. Produced naturally by digestion of the amino acid tryptophan 9 C Carbon (black) 1 N Nitrogen (dark blue) 9 H Hydrogen (white) 7 single bonds (straight) 8 double bonds (bendy)
38 Leaf alcohol Correctly called cis-3-hexen-1-ol, this molecule smells of freshly cut green grass 6 C Carbon (black) 1 xygen (red) 12 H Hydrogen (white) 5 single bonds (straight) 2 double bonds (bendy)
39 Musk A smelly molecule used in perfumes. It was originally extracted from Musk deer 16 C Carbon (black) 1 xygen (red) 30 H Hydrogen (white) 16 single bonds (straight) 2 double bonds (bendy)
40 Spearmint Extracted from leaves of the spearmint plant and used to flavour toothpaste and chewing gum 10 C Carbon (black) 1 xygen (red) 14 H Hydrogen (white) 8 single bonds (straight) 6 double bonds (bendy)
41 Cubane This highly strained molecule was first made in It is not very stable! 8 C Carbon (black) 8 H Hydrogen (white) 12 single bonds (straight)
42 Twistane A funny shaped molecule first made in the 1970 s 10 C Carbon (black) 16 H Hydrogen (white) 12 single bonds (straight)
43 Buckyball Also called C 60 or Buckminster Fullerene Add 10 bonds Add 20 carbons & 20 bonds 20 carbons 20 bonds Join 2 and 3 together 2 3 Tips: Every pentagon is surrounded by 5 hexagons Every carbon is joined to three other carbons 60 C Carbon (black) 90 single bonds (straight)
44 TNT Trinitrotoluene a common explosive 7 C Carbon (black) 6 xygen (red) 3 N Nitrogen (dark blue) 5 H Hydrogen (white) 13 single bonds (straight) 6 double bonds (bendy)
45 Teflon A non-stick coating used in saucepans and frying pans 8 C Carbon (black) 16 F Fluorine (green) 23 single bonds (straight)
46 Biodiesel Methyl linoleate is a common diesel fuel formed from soya bean oil and methanol 19 C Carbon (black) 2 xygen (red) 34 H Hydrogen (white) 17 single bonds (straight) 6 double bonds (bendy)
47 Sea-Nine 211 An environmentally friendly antifoulant used to remove sea creatures from the hulls of ships 11 C Carbon (black) 1 xygen (red) 1 N Nitrogen (dark blue) 1 S Sulfur (yellow, 2 holes) 2 Cl Chlorine (green) 17 H Hydrogen (white) 14 single bonds (straight) 4 double bonds (bendy)
48 Tebufenozide A rapidly biodegradable insecticide with low toxicity to humans 22 C Carbon (black) 2 xygen (red) 2 N Nitrogen (dark blue) 28 H Hydrogen (white) 19 single bonds (straight) 16 double bonds (bendy)
49 C 2 surfactants Polymers of these molecules allow C 2 to be used for dry cleaning instead of ozone depleting CFCs 19 C Carbon (black) 2 xygen (red) 15 F Fluorine (green) 13 H Hydrogen (white) 34 single bonds (straight) 8 double bonds (bendy)
50 Iron-TAML An environmentally friendly way to bleach paper uses this catalyst and hydrogen peroxide 19 C Carbon (black) 4 xygen (red) 4 N Nitrogen (dark blue) 1 Fe Iron (brown) 22 H Hydrogen (white) 25 single bonds (straight) 14 double bonds (bendy)
Lawsone. Hi I m Molly Cool. A red-orange dye extracted from the leaves of the Henna plant, used as a hair/skin pigment
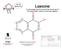 Lawsone A red-orange dye extracted from the leaves of the Henna plant, used as a hair/skin pigment 10 C Carbon (black) 3 xygen (red) 6 H Hydrogen (white) 8 single bonds (straight) 12 double bonds (bendy)
Lawsone A red-orange dye extracted from the leaves of the Henna plant, used as a hair/skin pigment 10 C Carbon (black) 3 xygen (red) 6 H Hydrogen (white) 8 single bonds (straight) 12 double bonds (bendy)
Salicylic acid. Hi I m Molly Cool. Extracted from the bark of the willow tree and used to ease aches and pains and reduce fever -
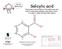 Salicylic acid Extracted from the bark of the willow tree and used to ease aches and pains and reduce fever - it is used to make the pain-killer aspirin and makeitmolecular designed by 7 C Carbon (black)
Salicylic acid Extracted from the bark of the willow tree and used to ease aches and pains and reduce fever - it is used to make the pain-killer aspirin and makeitmolecular designed by 7 C Carbon (black)
Summary Consumer Products
 Summary Consumer Products National 4 Carbohydrates are naturally occurring compounds which contain the elements Carbon, Hydrogen and Oxygen, with the Hydrogen and Oxygen in the ratio of two to one. Plants
Summary Consumer Products National 4 Carbohydrates are naturally occurring compounds which contain the elements Carbon, Hydrogen and Oxygen, with the Hydrogen and Oxygen in the ratio of two to one. Plants
1.3.1 Function of Food. Why do we need food?
 1.3.1 Function of Food Why do we need food? Need to know The Function of Food Three reasons for requiring food 2 Food is needed for: 1.Energy 2.Growth of new cells and Repair of existing cells, tissues,
1.3.1 Function of Food Why do we need food? Need to know The Function of Food Three reasons for requiring food 2 Food is needed for: 1.Energy 2.Growth of new cells and Repair of existing cells, tissues,
2. Compared to normal corn syrup, high fructose corn syrup is A) sweeter. B) less sweet. C) the same sweetness. D) higher in calories.
 CHEM 100 MODULE 10 PRETEST 1. Blood sugar is A) fructose. B) glucose. C) lactose. D) sucrose. 2. Compared to normal corn syrup, high fructose corn syrup is A) sweeter. B) less sweet. C) the same sweetness.
CHEM 100 MODULE 10 PRETEST 1. Blood sugar is A) fructose. B) glucose. C) lactose. D) sucrose. 2. Compared to normal corn syrup, high fructose corn syrup is A) sweeter. B) less sweet. C) the same sweetness.
The four levels of protein structure are: primary structure, secondary structure, tertiary structure, and quaternary structure.
 Proteins Proteins are organic complex nitrogenous compounds of high molecular weight, formed of C, H, O and N. They are formed of a number of amino acids linked together by peptide linkage [-CO-NH-]. Proteins
Proteins Proteins are organic complex nitrogenous compounds of high molecular weight, formed of C, H, O and N. They are formed of a number of amino acids linked together by peptide linkage [-CO-NH-]. Proteins
Unit 2: Nature s Chemistry Topic 2 Consumer Products Summary Notes
 St Ninian s High School Chemistry Department National 5 Chemistry Unit 2: Nature s Chemistry Topic 2 Consumer Products Summary Notes Name Learning Outcomes After completing this topic you should be able
St Ninian s High School Chemistry Department National 5 Chemistry Unit 2: Nature s Chemistry Topic 2 Consumer Products Summary Notes Name Learning Outcomes After completing this topic you should be able
FOOD. Why do we need food? What's in our food? There are 3 trace elements, Iron (Fe), Copper (Cu) and Zinc (Zn).
 Why do we need food? FOOD 1. As a source of energy keeps our cells and us alive. 2. To make chemicals for our metabolic reactions. 3. As raw materials for growth and repair of our cells and body. What's
Why do we need food? FOOD 1. As a source of energy keeps our cells and us alive. 2. To make chemicals for our metabolic reactions. 3. As raw materials for growth and repair of our cells and body. What's
Biomolecules. 1) Carbohydrate Facts. Types of Biomolecules. Carbohydrate Facts. What are Biomolecules? 12/25/13. Two Types of Carbohydrates
 Biomolecules What are Biomolecules? Organic compounds made by living things Also called biochemicals Some are very large There are thousands of different biomolecules, but are separated into 4 categories
Biomolecules What are Biomolecules? Organic compounds made by living things Also called biochemicals Some are very large There are thousands of different biomolecules, but are separated into 4 categories
There are four classes of biological macromolecules: Proteins, lipids, carbohydrates and nucleic acids
 There are four classes of biological macromolecules: Proteins, lipids, carbohydrates and nucleic acids Before you can understand the topics in this unit there are some key vocabulary terms you need to
There are four classes of biological macromolecules: Proteins, lipids, carbohydrates and nucleic acids Before you can understand the topics in this unit there are some key vocabulary terms you need to
AGRE Chemical Sensitivities
 Page 1 of 7 AGRE Chemical Sensitivities Section A: Chemical Sensitivities 1. Do you consider yourself especially sensitive to chemicals or odor? Don t Know The following items ask about YOUR responses
Page 1 of 7 AGRE Chemical Sensitivities Section A: Chemical Sensitivities 1. Do you consider yourself especially sensitive to chemicals or odor? Don t Know The following items ask about YOUR responses
Biomolecule: Carbohydrate
 Biomolecule: Carbohydrate This biomolecule is composed of three basic elements (carbon, hydrogen, and oxygen) in a 1:2:1 ratio. The most basic carbohydrates are simple sugars, or monosaccharides. Simple
Biomolecule: Carbohydrate This biomolecule is composed of three basic elements (carbon, hydrogen, and oxygen) in a 1:2:1 ratio. The most basic carbohydrates are simple sugars, or monosaccharides. Simple
Access 3 1 Unit 3 Chemistry and Life. Plants make their own food by a chemical reaction called photosynthesis: Carbon dioxide
 Access 3 1 Unit 3 Chemistry and Life Unit 3 Chemistry and Life Photosynthesis Plants make their own food by a chemical reaction called photosynthesis: 1. Carbon dioxide from the air is absorbed through
Access 3 1 Unit 3 Chemistry and Life Unit 3 Chemistry and Life Photosynthesis Plants make their own food by a chemical reaction called photosynthesis: 1. Carbon dioxide from the air is absorbed through
What is an atom? An atom is the smallest component of all living and nonliving materials.
 What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
Macromolecules are large molecules. Macromolecules are large structures made of many smaller structures linked together.
 Biomolecules Macromolecules are large molecules. Macromolecules are large structures made of many smaller structures linked together. The small single structure is a monomer (mono=one). The larger structure
Biomolecules Macromolecules are large molecules. Macromolecules are large structures made of many smaller structures linked together. The small single structure is a monomer (mono=one). The larger structure
Downloaded from
 Question 16.1: Why do we need to classify drugs in different ways? The classification of drugs and the reasons for classification are as follows: (i) On the basis of pharmacological effect: This classification
Question 16.1: Why do we need to classify drugs in different ways? The classification of drugs and the reasons for classification are as follows: (i) On the basis of pharmacological effect: This classification
2.3: Carbon- Based Molecules
 2.3: Carbon- Based Molecules Carbon-based molecules are the foundation of life. Bonding Properties of Carbon Carbon forms covalent bonds with up to four other atoms, including other carbon atoms. 1 3,
2.3: Carbon- Based Molecules Carbon-based molecules are the foundation of life. Bonding Properties of Carbon Carbon forms covalent bonds with up to four other atoms, including other carbon atoms. 1 3,
Macromolecules. SC.912.L.18.1 Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules.
 Macromolecules SC.912.L.18.1 Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules. FOUR MAJOR BIOLOGICAL MACROMOLECULES 1.Carbohydrates
Macromolecules SC.912.L.18.1 Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules. FOUR MAJOR BIOLOGICAL MACROMOLECULES 1.Carbohydrates
The Atoms of Life. What are other elements would you expect to be on this list? Carbon Hydrogen Nitrogen Oxygen Phosphorous Sulfur (sometimes)
 Macromolecules The Atoms of Life The most frequently found atoms in the body are Carbon Hydrogen Nitrogen Oxygen Phosphorous Sulfur (sometimes) What are other elements would you expect to be on this list?
Macromolecules The Atoms of Life The most frequently found atoms in the body are Carbon Hydrogen Nitrogen Oxygen Phosphorous Sulfur (sometimes) What are other elements would you expect to be on this list?
Elements & Macromolecules in Organisms
 Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds can be
Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds can be
2.1 Matter and Organic Compounds
 2.1 Matter and Organic Compounds Lesson Objectives Define elements and compounds. Explain why carbon is essential to life on Earth. Describe the structure and function of the four major types of organic
2.1 Matter and Organic Compounds Lesson Objectives Define elements and compounds. Explain why carbon is essential to life on Earth. Describe the structure and function of the four major types of organic
B i o c h e m i s t r y N o t e s
 14 P a g e Carbon Hydrogen Nitrogen Oxygen Phosphorus Sulfur ~Major ~Found in all ~Found in most ~Found in all component of all organic organic molecules. molecules. ~Major structural atom in all organic
14 P a g e Carbon Hydrogen Nitrogen Oxygen Phosphorus Sulfur ~Major ~Found in all ~Found in most ~Found in all component of all organic organic molecules. molecules. ~Major structural atom in all organic
Molecules of Life. Carbohydrates Lipids Proteins Nucleic Acids
 Molecules of Life Carbohydrates Lipids Proteins Nucleic Acids Molecules of Life All living things are composed of the following basic elements: Carbon Hydrogen Oxygen Nitrogen Phosphorous Sulfur Remember
Molecules of Life Carbohydrates Lipids Proteins Nucleic Acids Molecules of Life All living things are composed of the following basic elements: Carbon Hydrogen Oxygen Nitrogen Phosphorous Sulfur Remember
1 Small molecules are used as the basic units in the synthesis of large food molecules. Which statement is correct? A
 1 Small molecules are used as the basic units in the synthesis of large food molecules. Which statement is correct? mino acids are basic units of carbohydrates. Fatty acids are basic units of glycogen.
1 Small molecules are used as the basic units in the synthesis of large food molecules. Which statement is correct? mino acids are basic units of carbohydrates. Fatty acids are basic units of glycogen.
Biology Unit 2 Elements & Macromolecules in Organisms Date/Hour
 Biology Unit 2 Name Elements & Macromolecules in rganisms Date/our Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body
Biology Unit 2 Name Elements & Macromolecules in rganisms Date/our Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body
Carbohydrates, Lipids, Proteins, and Nucleic Acids
 Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
Carbohydrates, Lipids, Proteins, and Nucleic Acids Is it made of carbohydrates? Organic compounds composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. A carbohydrate with 6 carbon atoms would have
Carbon. Has four valence electrons Can bond with many elements. Can bond to other carbon atoms. Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
 Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
Organic Compounds Carbon Has four valence electrons Can bond with many elements Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen Can bond to other carbon atoms Gives carbon the ability to form chains
You Are What You Eat
 An Investigation of Macromolecules Student Materials Introduction....2 Pre-Lab Questions.5 Lab Protocol..6 Post-Lab Questions and Analysis 9 Last updated: September 26 th, 2017 1 Introduction When deciding
An Investigation of Macromolecules Student Materials Introduction....2 Pre-Lab Questions.5 Lab Protocol..6 Post-Lab Questions and Analysis 9 Last updated: September 26 th, 2017 1 Introduction When deciding
There are four classes of biological macromolecules: Proteins, lipids, carbohydrates and nucleic acids
 There are four classes of biological macromolecules: Proteins, lipids, carbohydrates and nucleic acids Before you can understand the topics in this unit there are some key vocabulary terms you need to
There are four classes of biological macromolecules: Proteins, lipids, carbohydrates and nucleic acids Before you can understand the topics in this unit there are some key vocabulary terms you need to
Essential Components of Food
 Essential Components of Food The elements of life living things are mostly (98%) made of 6 elements: C carbon H hydrogen O oxygen P phosphorus N nitrogen S sulphur -each element makes a specific number
Essential Components of Food The elements of life living things are mostly (98%) made of 6 elements: C carbon H hydrogen O oxygen P phosphorus N nitrogen S sulphur -each element makes a specific number
CHEMISTRY OF LIFE 30 JANUARY 2013
 CHEMISTRY OF LIFE 30 JANUARY 2013 Lesson Description In this lesson, we will: Investigate the structure and function of molecules that are essential for life. Key Concepts Terminology A molecule is any
CHEMISTRY OF LIFE 30 JANUARY 2013 Lesson Description In this lesson, we will: Investigate the structure and function of molecules that are essential for life. Key Concepts Terminology A molecule is any
2 3 Carbon Compounds Slide 1 of 37
 1 of 37 The Chemistry of Carbon The Chemistry of Carbon Organic chemistry is the study of all compounds that contain bonds between carbon atoms. Carbon atoms have four valence electrons that can join with
1 of 37 The Chemistry of Carbon The Chemistry of Carbon Organic chemistry is the study of all compounds that contain bonds between carbon atoms. Carbon atoms have four valence electrons that can join with
The Structure and Function of Biomolecules
 The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
The Structure and Function of Biomolecules The student is expected to: 9A compare the structures and functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic
Lesson 2. Biological Molecules. Introduction to Life Processes - SCI 102 1
 Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Lesson 2 Biological Molecules Introduction to Life Processes - SCI 102 1 Carbon in Biological Molecules Organic molecules contain carbon (C) and hydrogen (H) Example: glucose (C 6 H 12 O 6 ) Inorganic
Elements & Macromolecules in Organisms
 Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds can be
Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds can be
2-2 Properties of Water
 2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
2-2 Properties of Water 1 A. The Water Molecule o o o Water is polar Hydrogen bonds form between water molecules Properties of Water: cohesion adhesion capillary action high specific heat ice floats good
What is an atom? An atom is the smallest component of all living and nonliving materials.
 What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
What is an atom? An atom is the smallest component of all living and nonliving materials. It is composed of protons (+), neutrons (0), and electrons (-). The Periodic Table Elements are composed of all
Biochemistry. Biome. & Compound. Macromolecules
 Biochemistry Biome Macromolecules & Compound 1 ATOMS the smallest unit of an element. Ex: Carbon- C MOLECULE A molecule is formed when two or more atoms join together chemically. EX: O 2 (Oxygen Gas) 2
Biochemistry Biome Macromolecules & Compound 1 ATOMS the smallest unit of an element. Ex: Carbon- C MOLECULE A molecule is formed when two or more atoms join together chemically. EX: O 2 (Oxygen Gas) 2
Biology 20 Laboratory Life s Macromolecules OBJECTIVE INTRODUCTION
 Biology 20 Laboratory Life s Macromolecules OBJECTIVE To observe and record reactions between three classes of macromolecules in the presence of simple chemical indictors. To be able to distinguish positive
Biology 20 Laboratory Life s Macromolecules OBJECTIVE To observe and record reactions between three classes of macromolecules in the presence of simple chemical indictors. To be able to distinguish positive
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
Biology Chapter 2 Review
 Biology Chapter 2 Review Vocabulary: Define the following words on a separate piece of paper. Element Compound Ion Ionic Bond Covalent Bond Molecule Hydrogen Bon Cohesion Adhesion Solution Solute Solvent
Biology Chapter 2 Review Vocabulary: Define the following words on a separate piece of paper. Element Compound Ion Ionic Bond Covalent Bond Molecule Hydrogen Bon Cohesion Adhesion Solution Solute Solvent
½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = X X= 325 g
 BIOCHEMISTRY ½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = 13 100 X X= 325 g These spinach imposters contain less than 2 percent of
BIOCHEMISTRY ½ cup of CHEX MIX contains 13 g of carbs = 4% daily value. How much more can you have the rest of the day??? _4_ = 13 100 X X= 325 g These spinach imposters contain less than 2 percent of
Biomolecules. Organic compounds of life
 Biomolecules Organic compounds of life TEKS 9A: Students will Compare the structure and function of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids 9D: Students
Biomolecules Organic compounds of life TEKS 9A: Students will Compare the structure and function of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids 9D: Students
Carbon. p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms
 Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
Organic Compounds Carbon p Has four valence electrons p Can bond with many elements p Can bond to other carbon atoms n Gives carbon the ability to form chains that are almost unlimited in length. p Organic
6 The chemistry of living organisms
 Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
Living organisms are composed of about 22 different chemical elements. These are combined to form a great variety of compounds. Six major elements make up almost 99% of the mass of the human body, as shown
Sip Smart! BC Bubble Tea - Front
 Sip Smart! BC Bubble Tea - Front Nutrition Facts Per 500 ml Amount % Daily Value Calories 540 Fat 26 g 42 % Saturated 16 g + Trans 0 g 85 % Cholesterol 104 mg Sodium 115 mg 5 % Carbohydrate 105 g 35 %
Sip Smart! BC Bubble Tea - Front Nutrition Facts Per 500 ml Amount % Daily Value Calories 540 Fat 26 g 42 % Saturated 16 g + Trans 0 g 85 % Cholesterol 104 mg Sodium 115 mg 5 % Carbohydrate 105 g 35 %
Honors Biology Chapter 3: The Molecules of Cells Name Amatuzzi Carbohydrates pp Homework
 Honors Biology Chapter 3: The Molecules of Cells Name Amatuzzi Carbohydrates pp. 37-39 1. Which elements make up carbohydrates? a. In which ratio? 2. How do living things use most of their carbohydrates?
Honors Biology Chapter 3: The Molecules of Cells Name Amatuzzi Carbohydrates pp. 37-39 1. Which elements make up carbohydrates? a. In which ratio? 2. How do living things use most of their carbohydrates?
CP Biology Chapter 2: Molecules of Life Name Amatuzzi #1: Carbohydrates pp Period Homework
 Amatuzzi #1: Carbohydrates pp. 46-47 Period 1. Which elements make up carbohydrates? a. In which ratio? 2. How do living things use most of their carbohydrates? 3. How do cells get energy from carbs? a.
Amatuzzi #1: Carbohydrates pp. 46-47 Period 1. Which elements make up carbohydrates? a. In which ratio? 2. How do living things use most of their carbohydrates? 3. How do cells get energy from carbs? a.
the nature and importance of biomacromolecules in the chemistry of the cell: synthesis of biomacromolecules through the condensation reaction lipids
 the nature and importance of biomacromolecules in the chemistry of the cell: synthesis of biomacromolecules through the condensation reaction lipids and their sub-units; the role of lipids in the plasma
the nature and importance of biomacromolecules in the chemistry of the cell: synthesis of biomacromolecules through the condensation reaction lipids and their sub-units; the role of lipids in the plasma
6/9/2015. Unit 15: Organic Chemistry Lesson 15.2: Substituted Hydrocarbons & Functional Groups
 1-chloropropane 2-methylpropane 1-iodobutane Ethanoic Acid Unit 15: Organic Chemistry Lesson 15.2: Substituted Hydrocarbons & Functional Groups 43 It Ain t Just Hydrocarbons There are all sorts of organic
1-chloropropane 2-methylpropane 1-iodobutane Ethanoic Acid Unit 15: Organic Chemistry Lesson 15.2: Substituted Hydrocarbons & Functional Groups 43 It Ain t Just Hydrocarbons There are all sorts of organic
Macromolecules. Macromolecules. What are the macromolecules? Organic molecules. The human body uses complex organic molecules known as macromolecules.
 Macromolecules Macromolecules Biochemistry The human body uses complex organic molecules known as macromolecules. Macro - long or large It is a large molecule that is made up of smaller units joined together.
Macromolecules Macromolecules Biochemistry The human body uses complex organic molecules known as macromolecules. Macro - long or large It is a large molecule that is made up of smaller units joined together.
Lesson Overview. Carbon Compounds. Lesson Overview. 2.3 Carbon Compounds
 Lesson Overview 2.3 The Chemistry of Carbon What elements does carbon bond with to make up life s molecules? Carbon can bond with many elements, including Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
Lesson Overview 2.3 The Chemistry of Carbon What elements does carbon bond with to make up life s molecules? Carbon can bond with many elements, including Hydrogen, Oxygen, Phosphorus, Sulfur, and Nitrogen
Carbon Compounds (2.3) (Part 1 - Carbohydrates)
 Carbon Compounds (2.3) (Part 1 - Carbohydrates) The Chemistry of Carbon (Organic Chemistry) Organic Chemistry: The study of compounds that contain bonds between carbon atoms. Carbon can bond with many
Carbon Compounds (2.3) (Part 1 - Carbohydrates) The Chemistry of Carbon (Organic Chemistry) Organic Chemistry: The study of compounds that contain bonds between carbon atoms. Carbon can bond with many
Products Detailed Tests/Clause No Standards/Tests Method Sample Preparation LABORATORY: CONSUMER PRODUCTS LABORATORY Beehoon
 Beehoon MS 955 : 2011 (Rice Vermicelli) 2. Total Ash 3. Total Protein 4. Crude Fibre 5. Cooking Test : Total Solid in Gruel Butter/Recombined Butter 1. Milk Fat MS 242 : 1988 8 X 250 g 3. Salt 4. Non-Fat
Beehoon MS 955 : 2011 (Rice Vermicelli) 2. Total Ash 3. Total Protein 4. Crude Fibre 5. Cooking Test : Total Solid in Gruel Butter/Recombined Butter 1. Milk Fat MS 242 : 1988 8 X 250 g 3. Salt 4. Non-Fat
How safe is the food we eat? - May 2010
 How safe is the food we eat? - May 2010 Inside this months issue Research findings Food additives can be divided into several groups If, like many people today, you buy convenient, packaged or processed
How safe is the food we eat? - May 2010 Inside this months issue Research findings Food additives can be divided into several groups If, like many people today, you buy convenient, packaged or processed
BIOMOLECULES. (AKA MACROMOLECULES) Name: Block:
 BIOMOLECULES (AKA MACROMOLECULES) Name: Block: BIOMOLECULES POGIL All living things share the same chemical building blocks and depend on chemical processes for survival. Life without carbon (C) would
BIOMOLECULES (AKA MACROMOLECULES) Name: Block: BIOMOLECULES POGIL All living things share the same chemical building blocks and depend on chemical processes for survival. Life without carbon (C) would
I. Polymers & Macromolecules Figure 1: Polymers. Polymer: Macromolecule: Figure 2: Polymerization via Dehydration Synthesis
 I. Polymers & Macromolecules Figure 1: Polymers Polymer: Macromolecule: Figure 2: Polymerization via Dehydration Synthesis 1 Dehydration Synthesis: Figure 3: Depolymerization via Hydrolysis Hydrolysis:
I. Polymers & Macromolecules Figure 1: Polymers Polymer: Macromolecule: Figure 2: Polymerization via Dehydration Synthesis 1 Dehydration Synthesis: Figure 3: Depolymerization via Hydrolysis Hydrolysis:
Chemistry in Everyday life
 Therapeutic Action of drugs Chemistry in Everyday life Question 1 What are competitive inhibitors? Drugs inhibit the attachment of substrate on active site of enzymes by specific ways. When the drugs compete
Therapeutic Action of drugs Chemistry in Everyday life Question 1 What are competitive inhibitors? Drugs inhibit the attachment of substrate on active site of enzymes by specific ways. When the drugs compete
Molecules of Life. Chapter 22. Great Idea: A cell s major parts are constructed from a few simple molecular building blocks 1
 Molecules of Life Chapter 22 Great Idea: A cell s major parts are constructed from a few simple molecular building blocks 1 Chapter Outline Organic Molecules Organic Chemistry Proteins: The Workhorses
Molecules of Life Chapter 22 Great Idea: A cell s major parts are constructed from a few simple molecular building blocks 1 Chapter Outline Organic Molecules Organic Chemistry Proteins: The Workhorses
Carbon-based molecule showcase:
 Carbon-based molecule showcase: Beignet (pronounced ben-yay)-celtic word bigne meaning "to raise." It is also French for "fritter." Beignets, a New Orleans specialty, are fried, raised pieces of yeast
Carbon-based molecule showcase: Beignet (pronounced ben-yay)-celtic word bigne meaning "to raise." It is also French for "fritter." Beignets, a New Orleans specialty, are fried, raised pieces of yeast
Chapter 7-2 Hydrocarbons
 Chapter 7-1 Carbon C atom - atomic # is 6; it has 6 protons and therefore 6 electrons - is in group 14; it has 4 valence electrons - atomic mass is 12; it has 6 neutrons - shares electrons when forming
Chapter 7-1 Carbon C atom - atomic # is 6; it has 6 protons and therefore 6 electrons - is in group 14; it has 4 valence electrons - atomic mass is 12; it has 6 neutrons - shares electrons when forming
2-3 Carbon Compounds 10/22/2013. The Chemistry of Carbon. More Carbon. Chemistry (cont) More Macromolecules. Macromolecules
 The Chemistry of Carbon 2-3 Carbon Compounds Because of carbons 4 valence electrons it can form covalent bonds with many other elements (octet rule) 2 Chemistry (cont) Plus, it can bond with itself More
The Chemistry of Carbon 2-3 Carbon Compounds Because of carbons 4 valence electrons it can form covalent bonds with many other elements (octet rule) 2 Chemistry (cont) Plus, it can bond with itself More
4 Biological Molecules
 For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ iological Molecules Question Paper Level Subject Exam oard Unit ooklet IGSE iology ambridge International Examinations 4 iological
For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ iological Molecules Question Paper Level Subject Exam oard Unit ooklet IGSE iology ambridge International Examinations 4 iological
There are four classes of organic molecules: Proteins, lipids, carbohydrates and nucleic acids
 There are four classes of organic molecules: Proteins, lipids, carbohydrates and nucleic acids Monomer Polymer The big four organic molecules are POLYMERS. Each is made up of smaller units called MONOMERS
There are four classes of organic molecules: Proteins, lipids, carbohydrates and nucleic acids Monomer Polymer The big four organic molecules are POLYMERS. Each is made up of smaller units called MONOMERS
Product Nutrition Information
 Product Nutrition Information for OTE Sports Nutrition Products OTE ph Neutral Energy Drink Maltodextrin (purified and produced by the partial hydrolysis of a special starch), Fructose, Electrolytes (Sodium
Product Nutrition Information for OTE Sports Nutrition Products OTE ph Neutral Energy Drink Maltodextrin (purified and produced by the partial hydrolysis of a special starch), Fructose, Electrolytes (Sodium
In any solution, a scientist can talk about the concentration of the atoms that are dissolved in the solvent.
 Acids and Bases Acids and Bases In any solution, a scientist can talk about the concentration of the atoms that are dissolved in the solvent. i.e. Salt water is an example of Na + and Cl - in a solution
Acids and Bases Acids and Bases In any solution, a scientist can talk about the concentration of the atoms that are dissolved in the solvent. i.e. Salt water is an example of Na + and Cl - in a solution
Elements & Macromolecules in Organisms
 Name: Period: Date: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Period: Date: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
2 3 Carbon Compounds (Macromolecules)
 2 3 Carbon Compounds (Macromolecules) Slide 1 of 37 Organic Chemistry Organic chemistry is the study of all compounds that contain bonds between carbon atoms. Slide 2 of 37 Carbon Living organisms are
2 3 Carbon Compounds (Macromolecules) Slide 1 of 37 Organic Chemistry Organic chemistry is the study of all compounds that contain bonds between carbon atoms. Slide 2 of 37 Carbon Living organisms are
Organic Chemistry. Organic chemistry is the chemistry of carbon compounds. Biochemistry is the study of carbon compounds that crawl.
 Organic Chemistry Organic chemistry is the chemistry of carbon compounds. Biochemistry is the study of carbon compounds that crawl. Organic Compounds - have carbon bonded to other atoms and determine structure/function
Organic Chemistry Organic chemistry is the chemistry of carbon compounds. Biochemistry is the study of carbon compounds that crawl. Organic Compounds - have carbon bonded to other atoms and determine structure/function
Organic compounds. Lipids, Carbohydrates, Proteins, and Nucleic Acids
 Organic compounds Lipids, Carbohydrates, Proteins, and Nucleic Acids Essential for life Organic compounds: Contain carbon Most are covalently bonded Example: C 6 H 12 O 6 (Glucose) Inorganic Compounds:
Organic compounds Lipids, Carbohydrates, Proteins, and Nucleic Acids Essential for life Organic compounds: Contain carbon Most are covalently bonded Example: C 6 H 12 O 6 (Glucose) Inorganic Compounds:
Biological Molecules. Carbohydrates, Proteins, Lipids, and Nucleic Acids
 Biological Molecules Carbohydrates, Proteins, Lipids, and Nucleic Acids Organic Molecules Always contain Carbon (C) and Hydrogen (H) Carbon is missing four electrons Capable of forming 4 covalent bonds
Biological Molecules Carbohydrates, Proteins, Lipids, and Nucleic Acids Organic Molecules Always contain Carbon (C) and Hydrogen (H) Carbon is missing four electrons Capable of forming 4 covalent bonds
Chapter 2 The Chemistry of Life Part 2
 Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
Chapter 2 The Chemistry of Life Part 2 Carbohydrates are Polymers of Monosaccharides Three different ways to represent a monosaccharide Carbohydrates Carbohydrates are sugars and starches and provide
Name: Class: Honors Biology Period: Question: What is the molecular formula of this molecule?
 Chapter 3: The Chemistry of Organic Molecules Exercise 1 Diversity of Carbon-Based Molecules (3.1) The great variety of organic compounds results from the ability of carbon atoms to bond with four other
Chapter 3: The Chemistry of Organic Molecules Exercise 1 Diversity of Carbon-Based Molecules (3.1) The great variety of organic compounds results from the ability of carbon atoms to bond with four other
Biomolecules. The chemistry of life
 Biomolecules The chemistry of life Knowing Word Parts can help you remember big words in Biochem Mono one, single Di two, double Poly many, much Hydro water (think: hydrate) Bio related to life (think:
Biomolecules The chemistry of life Knowing Word Parts can help you remember big words in Biochem Mono one, single Di two, double Poly many, much Hydro water (think: hydrate) Bio related to life (think:
FROZEN DRINKS DATA Marylou's News, Inc.
 FROZEN RED BULL INFUSION Original Red Bull FROZEN RED BULL INFUSION Sugar Free Red Bull SCREAMER VANILLA FROSTYLOU Carbonated Water, Sucrose, Glucose, Citric Acid, Taurine, Sodium Citrate, Magnesium Carbonate,
FROZEN RED BULL INFUSION Original Red Bull FROZEN RED BULL INFUSION Sugar Free Red Bull SCREAMER VANILLA FROSTYLOU Carbonated Water, Sucrose, Glucose, Citric Acid, Taurine, Sodium Citrate, Magnesium Carbonate,
Macromolecules. SC.912.L.18.1 Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules.
 Macromolecules SC.912.L.18.1 Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules. FOUR MAJOR BIOLOGICAL MACROMOLECULES 1.Carbohydrates
Macromolecules SC.912.L.18.1 Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules. FOUR MAJOR BIOLOGICAL MACROMOLECULES 1.Carbohydrates
National 5 Unit Two : Nature s Chemistry
 National 5 Unit Two : Nature s Chemistry Fuels A fuel is a chemical which burns, giving off energy. Combustion is a reaction of a substance with oxygen giving off energy. The test for oxygen is it relights
National 5 Unit Two : Nature s Chemistry Fuels A fuel is a chemical which burns, giving off energy. Combustion is a reaction of a substance with oxygen giving off energy. The test for oxygen is it relights
small molecules that make up larger molecules organic compound made up of sugar molecules sugar that contains one sugar unit
 organic molecule carbon based compound inorganic molecule hydrocarbon functional group hydrophilic NON-carbon based compound organic molecule made of only carbon and hydrogen group of atoms bonded to a
organic molecule carbon based compound inorganic molecule hydrocarbon functional group hydrophilic NON-carbon based compound organic molecule made of only carbon and hydrogen group of atoms bonded to a
Macromolecules Cut & Paste
 Macromolecules Cut & Paste Adapted from http://mrswords.weebly.com/uploads/1/5/2/4/15244382/ch_6-3_life_molecules_cut-out_lab.pdf INTRODUCTION Many of the molecules in living cells are so large that they
Macromolecules Cut & Paste Adapted from http://mrswords.weebly.com/uploads/1/5/2/4/15244382/ch_6-3_life_molecules_cut-out_lab.pdf INTRODUCTION Many of the molecules in living cells are so large that they
2.3: Carbon-Based Molecules Notes
 2.3: Carbon-Based Molecules Notes Carbon-based molecules are the of life. Bonding Properties of Carbon Carbon forms bonds with up to other atoms, including other carbon atoms. QUESTION: What types of elements
2.3: Carbon-Based Molecules Notes Carbon-based molecules are the of life. Bonding Properties of Carbon Carbon forms bonds with up to other atoms, including other carbon atoms. QUESTION: What types of elements
Carbon Compounds. Lesson Overview. Lesson Overview. 2.3 Carbon Compounds
 Lesson Overview Carbon Compounds Lesson Overview 2.3 THINK ABOUT IT In the early 1800s, many chemists called the compounds created by organisms organic, believing they were fundamentally different from
Lesson Overview Carbon Compounds Lesson Overview 2.3 THINK ABOUT IT In the early 1800s, many chemists called the compounds created by organisms organic, believing they were fundamentally different from
Chemotherapy effects. your PR.i.VATES.
 your PR.i.VATES Testicular cancer awareness, advice and support for men in the UK affected by testicular cancer. Chemotherapy effects www.yourprivates.org.uk Chemotherapy effects While the chemotherapy
your PR.i.VATES Testicular cancer awareness, advice and support for men in the UK affected by testicular cancer. Chemotherapy effects www.yourprivates.org.uk Chemotherapy effects While the chemotherapy
NUTRITION 101 & CLEAN EATING
 NUTRITION 101 & CLEAN EATING Macro-Nutrients vs Micro-Nutrients NUTRIENTS Carbohydrates 4 Calories/gram Fiber Fats 9 Calories/gram Proteins 4 Calories/gram Alcohol: 7 calories/gram NUTRITION LABEL Required
NUTRITION 101 & CLEAN EATING Macro-Nutrients vs Micro-Nutrients NUTRIENTS Carbohydrates 4 Calories/gram Fiber Fats 9 Calories/gram Proteins 4 Calories/gram Alcohol: 7 calories/gram NUTRITION LABEL Required
Lect 2- Organic Chem Biomolecules student copy
 3 pts ec Lect 2- Organic Chem Biomolecules student copy printing Other organic compounds Take a cheeseburger... hamburger, covered with American (yellow) cheese on a hamburger bun... yummy! Now, if you
3 pts ec Lect 2- Organic Chem Biomolecules student copy printing Other organic compounds Take a cheeseburger... hamburger, covered with American (yellow) cheese on a hamburger bun... yummy! Now, if you
Restaurant Allergy Information
 Brussels Pâté May Contain Celery May Contain Cereals Containing Gluten May Contain Eggs Contains Milk May Contain Mustard May Contain Nuts May Contain Soya Free From Artificial Colours Pork Liver (31%),
Brussels Pâté May Contain Celery May Contain Cereals Containing Gluten May Contain Eggs Contains Milk May Contain Mustard May Contain Nuts May Contain Soya Free From Artificial Colours Pork Liver (31%),
4. CARBON AND ITS COMPOUND
 . ARBN AND ITS MPUND rganic hemistry- The study of carbon compounds. rganic ompounds- The compounds of carbon (except the oxides of carbon, carbonates, hydro carbonates and carbides) are called organic
. ARBN AND ITS MPUND rganic hemistry- The study of carbon compounds. rganic ompounds- The compounds of carbon (except the oxides of carbon, carbonates, hydro carbonates and carbides) are called organic
30 Days to Healthy Living and Beyond
 30 Days to Healthy Living and Beyond Arbonne's mission is to create Pure, Safe, Benficial products Key Points of Difference Essential nutrients help provide a foundation for optimal health An easy way
30 Days to Healthy Living and Beyond Arbonne's mission is to create Pure, Safe, Benficial products Key Points of Difference Essential nutrients help provide a foundation for optimal health An easy way
ALL OF THESE DRINKS: WHAT DO YOU THINK? HOW MUCH SUGAR IS IN YOUR DRINK?
 LESSON 3: WINNING THE BALANCE GAME - FATS AND SUGARS, EVALUATING FOOD ADS, AND IDENTIFYING PORTION SIZES WHAT DO YOU THINK? HOW MUCH SUGAR IS IN YOUR DRINK? USE THE SUGAR CUBES ON THE TABLE TO CREATE A
LESSON 3: WINNING THE BALANCE GAME - FATS AND SUGARS, EVALUATING FOOD ADS, AND IDENTIFYING PORTION SIZES WHAT DO YOU THINK? HOW MUCH SUGAR IS IN YOUR DRINK? USE THE SUGAR CUBES ON THE TABLE TO CREATE A
For example, monosaccharides such as glucose are polar and soluble in water, whereas lipids are nonpolar and insoluble in water.
 Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
Biology 4A Laboratory Biologically Important Molecules Objectives Perform tests to detect the presence of carbohydrates, lipids, proteins, and nucleic acids Recognize the importance of a control in a biochemical
Calderglen High School CfE Higher Chemistry. Nature s Chemistry. Soaps, Detergents and Emulsions
 Calderglen High School CfE Higher Chemistry Nature s Chemistry Soaps, Detergents and Emulsions Page 1 of 12 No. Learning Outcome Understanding? 1 Soaps are produced by the alkaline hydrolysis of the ester
Calderglen High School CfE Higher Chemistry Nature s Chemistry Soaps, Detergents and Emulsions Page 1 of 12 No. Learning Outcome Understanding? 1 Soaps are produced by the alkaline hydrolysis of the ester
Lesson Overview. Carbon Compounds. Lesson Overview. 2.3 Carbon Compounds
 Lesson Overview 2.3 THINK ABOUT IT In the early 1800s, many chemists called the compounds created by organisms organic, believing they were fundamentally different from compounds in nonliving things. We
Lesson Overview 2.3 THINK ABOUT IT In the early 1800s, many chemists called the compounds created by organisms organic, believing they were fundamentally different from compounds in nonliving things. We
Most life processes are a series of chemical reactions influenced by environmental and genetic factors.
 Biochemistry II Most life processes are a series of chemical reactions influenced by environmental and genetic factors. Metabolism the sum of all biochemical processes 2 Metabolic Processes Anabolism-
Biochemistry II Most life processes are a series of chemical reactions influenced by environmental and genetic factors. Metabolism the sum of all biochemical processes 2 Metabolic Processes Anabolism-
CHAPTER 3: EXCRETION
 CHAPTER 3: EXCRETION Excretion Human excretion Urinary system Excretion in plants Excretory organs Excretion Kidney and other parts of urinary system Shape of kidney Ways of excretion Excretory products
CHAPTER 3: EXCRETION Excretion Human excretion Urinary system Excretion in plants Excretory organs Excretion Kidney and other parts of urinary system Shape of kidney Ways of excretion Excretory products
Guided Inquiry Skills Lab. Additional Lab 1 Making Models of Macromolecules. Problem. Introduction. Skills Focus. Materials.
 Additional Lab 1 Making Models of Macromolecules Guided Inquiry Skills Lab Problem How do monomers join together to form polymers? Introduction A small number of elements make up most of the mass of your
Additional Lab 1 Making Models of Macromolecules Guided Inquiry Skills Lab Problem How do monomers join together to form polymers? Introduction A small number of elements make up most of the mass of your
The building blocks of life.
 The building blocks of life. The 4 Major Organic Biomolecules The large molecules (biomolecules OR polymers) are formed when smaller building blocks (monomers) bond covalently. via anabolism Small molecules
The building blocks of life. The 4 Major Organic Biomolecules The large molecules (biomolecules OR polymers) are formed when smaller building blocks (monomers) bond covalently. via anabolism Small molecules
Chapter 3. The Molecules of Life
 Chapter 3 The Molecules of Life Biology and Society: Got Lactose? Lactose is the main sugar found in milk. Lactose intolerance is the inability to properly digest lactose. Instead of lactose being broken
Chapter 3 The Molecules of Life Biology and Society: Got Lactose? Lactose is the main sugar found in milk. Lactose intolerance is the inability to properly digest lactose. Instead of lactose being broken
Activity 4. Count the Cubes! (15-20 mins)
 Source: Composite Example Activity 4. Count the Cubes! (15-20 mins) Key Messages Knowing what is in drinks helps us to make healthy choices. Sugar is a major ingredient in many popular drinks. Objectives
Source: Composite Example Activity 4. Count the Cubes! (15-20 mins) Key Messages Knowing what is in drinks helps us to make healthy choices. Sugar is a major ingredient in many popular drinks. Objectives
When people don t eat enough complex carbohydrates they don t have enough energy and feel tired and less alert. They also may not get enough fiber.
 Carbohydrates Carbohydrates are compounds that come from plants and contain carbon, hydrogen, and oxygen. These nutrients supply energy, which all living things need. Carbohydrates are the body s most
Carbohydrates Carbohydrates are compounds that come from plants and contain carbon, hydrogen, and oxygen. These nutrients supply energy, which all living things need. Carbohydrates are the body s most
Calderglen High School CfE Higher Chemistry. Nature s Chemistry Esters, Fats and Oils. Page 1 of 11
 Calderglen High School CfE Higher Chemistry Nature s Chemistry Esters, Fats and Oils Page 1 of 11 No. Learning Outcome Understanding? 1 An ester can be identified from the name containing the -yl-oate
Calderglen High School CfE Higher Chemistry Nature s Chemistry Esters, Fats and Oils Page 1 of 11 No. Learning Outcome Understanding? 1 An ester can be identified from the name containing the -yl-oate
