F bladder function in patients with serious neuropathic
|
|
|
- Cuthbert Ellis
- 5 years ago
- Views:
Transcription
1 EEE TRANSACTONS ON BOMEDCAL ENGNEERNG, VOL. 41. NO. 5, MAY Selective Stimulation of Sacral Nerve Roots for Bladder Control: A Study by Computer Modeling Nico J. M. Rijkhoff, Jan Holsheimer, Evert L. Koldewijn, Johannes J. Struijk, Philip E. V. van Kerrebroeck, Frans M. J. Debruyne and Hessel Wijkstra AbstractThe aim of this study was to investigate theoretically the conditions for the activation of the detrusor muscle without activation of the urethral sphincter and afferent fibers, when stimulating the related sacral roots. Therefore, the sensitivity of excitation and blocking thresholds of nerve fibers within a sacral root to geometric and electrical parameters in tripolar stimulation using a cuff electrode, have been simulated by a computer model. A 3D rotationally symmetrical model, representing the geometry and electrical conductivity of a nerve root surrounded by cerebrospinal fluid and a cuff was used, in combination with a model representing the electrical properties of a myelinated nerve fiber. The electric behavior of nerve fibers having different diameters and positions in a sacral root was analyzed and the optimal geometric and electrical parameters to be used for sacral root stimulation were determined. The model predicts that an asymmetrical tripolar cuff can generate unidirectional action potentials in small nerve fibers while blocking the large fibers bidirectionally. This result shows that selective activation of the detrusor may be possible without activation of the urethral sphincter and the afferent fibers.. NTRODUCTON UNCTONAL electrical stimulation can be used to restore F bladder function in patients with serious neuropathic voiding disorders. There are several sites where stimulation can be applied to control the urinary bladder: the bladder wall (detrusor muscle) [3], 11.51, the pelvic nerves [16], sacral nerve roots [8] or the spinal cord [18]. Sacral root stimulation has been most successful in bladder evacuation and is commonly used in case of intact efferent innervation of the detrusor muscle. Sacral root stimulation can be performed by surface electrodes [38] or by implanted intradural [8], [ 191 or extradural [36] electrodes. The anatomy of the sacral roots allows their separation in ventral and dorsal branches. When implanted electrodes are used, stimulation can thus be restricted to the ventral roots (carrying most of the efferent fibers) and activation of dorsal root fibers (afferent pathways) can be avoided. However, a dorsal rhizotomy is usually performed in addition to reduce or abolish autonomic bladder reflex activity and to improve bladder responses [8], [36]. Attempts to empty the bladder by ventral sacral root stimulation have Manuscript received February 9, 1993: revised January 25, This research was supported by the Dutch Kidney Foundation C N. J. M. Rijkhoff, E. L. Koldewijn, P. E. V. van 'Kerrebroeck, F. M. J. Debruyne and H. Wijkstra are with the Department of Urology, University Hospital Nijmegen, The Netherlands. J. Holsheimer and J. J. Struijk are with the Biomedical Engineering Division, Department of Electrical Engineering, University of Twente, The Netherlands. EEE Log Number always been hampered by simultaneous contraction of the urethral sphincter. Unwanted movement of the lower limbs also occurs. This is due to the composition of the ventral sacral roots, which contain a mixed population of somatic fibers innervating some muscles of the lower limbs, the pelvic floor, the urethral and anal sphincter, and preganglionic parasympathetic efferents innervating the detrusor muscle. Since large fibers need a smaller stimulus for their excitation than small ones, activation of the small parasympathetic fibers (detrusor) is always accompanied by the activation of the larger somatic ones (urethral sphincter, lower limb muscles). Several attempts have been made to overcome the problem of simultaneous sphincter activation. The poststimulus voiding technique described by Jonas and Tanagho [ 181 takes advantage of differences in biomechanical characteristics of smooth and striated muscle. The relaxation times (90% to 10% pressure reduction) of the striated sphincter muscle and the smooth detrusor muscle are 0.4 s and 13 s respectively. When stimulating with pulse trains (36 s ON, 69 s OFF), voiding is achieved between the pulse trains due to the sustained high intravesical pressure. This principle is used in a commercially available system developed by Brindley et al. [7], [8]. However, this artificial micturition pattern often causes voiding in spurts and supranormal bladder pressures, with the risk of damaging the upper urinary tract. A more drastic method is described by Schmidt et al. [30]. They have shown that the increased urethral resistance due to sacral root stimulation can be largely reduced by pudendal nerve section or block. This technique has been applied to patients [35], [36] with variable success. The major drawback of this technique is the need for irreversible surgery. To avoid pudendal nerve section, Sweeney et al. [33], [341 introduced a technique to block reversibly the transmission of motor signals through the pudendal nerve to the urethral sphincter using the collision block principle. By stimulating the pudendal nerve with a special electrode, action potentials are generated which are propagated unidirectionally in a proximal direction. When those action potentials meet the distally propagating action potentials produced by simultaneous sacral root stimulation, the two signals collide and mutual annihilation occurs. n this way, the signals induced by sacral root stimulation cannot reach the sphincter muscle and the sphincter is thus prevented from contraction. A disadvantage is that distant stimulation sites are required. Furthermore, in both pudendal nerve section and blocking it is assumed that all efferent fibers innervating the human external urethral /94$ EEE
2 414 EEE TRANSACTONS ON BOMEDCAL ENGNEERLNG, VOL. 41, NO. 5, MAY 1994 sphincter are in the pudendal nerve, which is not generally agreed upon 1231, [29]. Another technique takes advantage of the difference in excitation threshold between large fibers innervating the sphincter and small fibers innervating the detrusor muscle. Using a (high frequency) pulse train with an amplitude above the threshold for somatic fibers but below the threshold for parasympathetic fibers, the sphincter can be fatigued. This has been demonstrated in the baboon [7] and the dog [37]. After this fatiguing burst, a stronger stimulus must be given to activate the detrusor. A disadvantage of this method is that the sphincter is not relaxed to the prestimulus tone during detrusor activation, due to the presence of fatigue resistant slowtwitch muscle fibers [37]. Cocontraction of skeletal muscles in the lower limbs is not reduced by these methods. Moreover, sacral root stimulation can be applied only in patients with a complete spinal cord transection, or in patients with an incomplete lesion but without pelvic pain sensation. Other patients feel pain during stimulation which is probably due to the stimulation of afferent fibers present in the ventral roots [ 111, [27]. The abovementioned problems could be solved if the induced undesired activity in the concerned fiber groups could be arrested. This is possible with the anodal block technique [l], [6], [12], [17]. Near an anode the membranes of nerve fibers are hyperpolarized by the anodal current. f the nerve membrane is sufficiently hyperpolarized, action potentials carnot pass the hyperpolarized zone and are annihilated. As anodal current is increased, the larger fibers will be blocked before smaller ones; thus anodal blocking can be used to obtain a selective fiber blockade. This method would avoid activation of the urethral closure mechanism without the need for extra surgery or additional electrodes. Moreover, it may result in a more physiological evacuation of urine, including lower peak pressures than are obtained during poststimulus voiding. t may also eliminate cocontraction of skeletal muscles in the lower limbs during stimulation. By blocking most sensory fibers proximal to the cathode, pain sensation in patients with preserved pelvic pain sensitivity may be reduced. Thereby, the technique of sacral root stimulation could be applied to more patients suffering from neuropathic voiding disorders. The application of anodal block in sacral root stimulation has been described by Brindley et al. [6], [7], who used asymmetrical tripolar electrodes. Results have not been reported apart from the conclusion we have not yet succeeded in making the method well enough in patients for every day use. Tripolar cuff electrodes consisting of circular contacts embedded on the inner surface of a cylindrical insulating tube (Fig. (a)) can be used to achieve both excitation and anodal block. They can be wrapped around a nerve bundle and have been shown to be successful in various blocking experiments [12], [17], [32]. The design of a cuff electrode as a neural prosthesis, however, still mainly relies on rulesofthumb and improvements by animal or clinical testing. Few attempts have been made to analyze the behavior of nerve fibers in a cuff electrode and the influence of geometric and electrical parameters by modeling. Altman and Plonsey (21 x * p x cx selective block (b) complete block large fiber5 small fibers Fig.. (a) Asymmetrical tripolar cuff electrode. (b) Functional goals of the cuff. Complete block at proximal anode, selective block at distal anode. presented a simple model, but without a systematic evaluation of main parameters (e.g., electrode separation, cuff inner diameter). They assumed a uniform axial current density inside the cuff, as well as an isotropic and homogeneous medium. A more detailed approach of the volume conductor has been made by Ferguson ef al. in an initial study [ 131. We developed a model to calculate both the electric potential field generated by a tripolar cuff electrode with circular contacts and the responses of myelinated nerve fibers to this field. The effects of various geometric and electrical parameters on the behavior of the nerve fibers was simulated. n this way the parameters necessary to achieve maximum selectivity, minimum dimensions and minimum current were determined. The results of the modeling study on sacral root stimulation are presented in this paper. A. ntioduction 11. METHODS The cuff electrode will be placed intradurally around a sacral ventral root. The cuff should elicit solely bladder contraction: activation of the urethral sphincter should be avoided. n order to suppress reflex spasms and pain sensation, stimulation of afferents in the ventral root 1271 and antidromic stimulation of the parasympathetic fibers [22] should also be avoided. These effects can be accomplished by combining: A) excitation of all myelinated fibers near the cathode, B) abolition of induced action potentials in the somatic fibers, without affecting the parasympathetic fibers, by hyperpolarization near the anode placed distally to the cathode, and C) abolition of induced action potentials in all myelinated fibers by hyperpolarization near the anode placed proximally to the cathode The range of fiber diameters to be excited and blocked
3 RJKHOFF et al.: SELECTVE STMULATON OF SACRAL NERVE ROOTS FOR BLADDER CONTROL 415 proximally should thus include the parasympathetic fibers and the somatic fibers. While only the large fibers must be blocked at the distal anode, at the proximal anode all fibers must be blocked. Since the stimulus amplitude needed for blocking will increase as the fiber diameter decreases [12], the proximal anode should inject more current into the medium than the distal one. When equallyspaced contacts (symmetrical configuration) are used, unequal anodal currents will lead to different electric potentials at the two outer contacts (anodes) and flow of electrical current not only inside, but also outside the cuff. An external current leak can have two disadvantageous effects. First. a virtual cathode [17] will be formed at the end of the cuff where the current leaves the nerve root. f the current is sufficiently high, the virtual cathode will excite (large) fibers and thus undo the blockage. Secondly. other nerve roots in the vicinity of the cuff can be stimulated. n order to confine the stimulus current to the inside of the cuff, an asymmetrical tripole with two synchronized current sources should be used. f the conductivity inside the cuff and the cuff inner diameter are uniform along the length of the cuff, the anodal curient ratio should be the reciprocal of the intercontact separation ratio in order to obtain the same potentials at the two anodes and no leakage of current outside the cuff. The calculation of the effects of electrical stimulation on myelinated fibers in a sacral root consisted of two steps. n the first step, the potential field generated by a cuff electrode was determined using a volume conduction model which incorporated inhomogeneities and anisotropy. n the next step, the response of a myelinated nerve fiber model. at a specific position in the nerve bundle, to the stimulus induced potential field was calculated. B. Volume Conduction Model The electric potential field can be calculated by solving Poisson's equation with appropriate boundary conditions. The volume conductor is rotationally symmetrical. the axis of the cuff electrode and nerve being the axis of symmetry of the model. Therefore, Poisson's equation can be expressed in cylindrical coordinates and the 3D field problem is reduced to a 2D problem (see Appendix). At the axis of symmetry (i = 0) the Neumann boundary condition has been applied. while the other 3 boundaries have been considered as Dirichlet boundaries. n our volume conduction model a cylindrical nerve root with a diameter of 1.4 mm [261 is assumed (compartment 1 in Fig. 2). Compartment 2 represents the insulating part of the cuff electrode, which has a thickness of 0.6 mm. Compartment 3 represents the metal contacts (thickness: 0.2 mm). Medium 4, surrounding cuff and nerve, represents the cerebrospinal fluid (CSF, intradural space). Compartment 5 at the border of the model is a compressed representation of the tissues at a large distance from the nerve (thickness: mm). Compartments 25 are isotropic while compartment 1 (nerve root) is anisotropic with conductivity U? in a direction parallel to the bundle axis and U,. perpendicular to this axis. The conductivities of nerve and CSF were obtained from 5 L. Fig. 2. The 2D volume conductor model. : nerve, 2: cuff. 3: metal contact, 4: cerebrospinal fluid, 5: boundary layer. lsopotential lines plotted in the volume conductor model. Contact separation: 3 mm and 6 mm, inner diameter: 2.4 mm, ta,: 5.4 ma,,,,, am: 3.6 ma, clib, :,, <, 1.8 ma. sopotential range: mv. TABLE COhDLCTlVlTlES OF THE MEDM U THE COMPARTMENTS OF HE VOLUME CONDUCTOR MODEL Model Conductivity [S/m] compartment Tissue 0, 0; 1 nerve cuff contact csf boundary Geddes and Baker With respect to these values, the conductivities of cuff and contacts have been given a low and a high value, respectively. All conductivities are summarized in Table. The current sources have been modeled as infinitely small circles. When modeling an electrode contact with finite width, the applied current is distributed evenly over all grid points of that contact in the 2D model. The high conductivity of the contacts allows for a nonuniform current density over the boundary between the contact and CSF. A circular contact consisting of one grid point is called a contact with zero width. An example of the potential distribution is shown in Fig. 2. At the lower boundary of the model (the axis) the isopotential lines are normal to this boundary, which means that no current crosses the symmetry axis of the model (Neumann boundary condition). The spacing of successive isopotential lines between the cathode and proximal anode is nearly constant. The spacing of isopotential lines between the cathode and the distal anode is also nearly constant but larger, indicating a lower current density between these contacts. A small amount of current will leak away to the three Dirichlet boundaries (V = 0) because the impedance between anode and boundary is not infinitely high. Therefore the potential at the anodal contacts is a little above zero. This explains the 350 mv isopotential line at the extreme left. The potential difference between the two anodes was 6.4 mv. C. Nerve Fiber Model The nerve fiber model was adopted from McNeal [20]. He used the FrankenhaeuserHuxley equations, derived from myelinated frog nerve, to describe the dynamic electric behavior of the membrane at the nodes of Ranvier. The properties of mammalian nerve fibers however, are different. Most notable is that voltage dependent potassium channels, shown in amphibians. are absent at the nodes of Ranvier in the mammal, and
4 416 EEE TRANSACTONS ON BOMEDCAL ENGNEERNG, VOL. 41, NO. 5, MAY 1994 TABLE 1 PARAMETERS FOR MYELNATED NERVE MODE PCC 0.7 Otn intra axonal restivity ( F~~~ 2 nodal membrane capacitance 1.5 pi17 nodal length LP 100 ratio of internodal distance to outer fiber diameter dp 0.7 ratio of axon to outer fiber diameter llllllllrll ll.,,,. 180 ~ ps that the membrane leak conductance is larger [9], [lo]. Therefore, we adapted the equations of membrane kinetics according to Chiu et al. [ 101. All temperaturedependent parameters were scaled to 37 C (for equations and parameters, see [31]). The ratio of the internodal length and the fiber diameter (LD) was set at 100, according to Schalow [27]. Other fiber parameters are summarized in Table 11. The responses of two fibers have been calculated with this model: one with a diameter of 4 pm, representing the parasympathetic fibers, and a 12 pm fiber representing the somatic fibers. The small axon was modeled with 51 excitable nodes and the large axon had 17 excitable nodes. n practice both the detrusor and the urethral sphincter are innervated by a small range of fiber diameters. Schalow [27], [28] showed a clear distinction between the two diameter ranges (detrusor: 25 pm, sphincter: 913 pm). Because small and large fibers are distributed throughout the whole nerve root, thresholds will vary as a function of the position inside the bundle. Therefore, threshold values of each fiber type were calculated at two extreme fiber positions: the axis and the edge of the nerve bundle. Rectangular monophasic current stimuli have been used for the excitation and blocking of the nerve fiber models, although it has been shown that blocking by this pulse shape may be ineffective, due to the phenomenon of anodal break excitation (see Section V). n order to determine how the electrode dimensions and stimulus current can be minimized, the effect of contact separation and cuff inner diameter on the thresholds for blocking and excitation have been simulated using a symmetrical cuff electrode. Using electrodes with the optimal dimensions, we simulated the influence of pulse duration and asymmetrical configurations to achieve maximum selectivity RESULTS Fig. 3 shows the response of a 4 pm fiber at the axis of the nerve bundle to the field shown in Fig. 2. Depolarization is shown with a bar above the line while hyperpolarization is indicated below the line. With increasing time there is successive depolarization of nodes at the distal, but not the proximal side, indicating a unidirectional propagating action potential. The hyperpolarization at the proximal anode is strong enough to arrest propagation (anodal block). A. Relation Between Contact Spacing and E,ui.itutionlBlockinR Threshold The reaction of a myelinated nerve fiber to extracellular stimulation is related to the second order difference quotient of the extracellular potential d, outside each node. This quotient llllllllrl.,,,~ 300 ps 360 ps lllllll n.... distal PM Fig. 3. Response of a 4 pm fiber to the field shown in Fig 3. t shows the deviation of the membrane potential from the resting potential at each node of Ranvier after initiation of a rectangular pulse of 320 ps. The nodal potentials are shown at 6 intervals of 60 /is after initiation of the pulse. At the top the cuff and the position of the contacts relative to the nodes of Ranvier are shown. A24/Az2 is called activating function by Rattay [24], [25]. Although fiber parameters also influence the amount of current needed for excitation, the activating function can be used to qualitatively study the influence of the geometry of multipolar electrodes. n a symmetrical tripolar configuration with identical anodal currents, the following qualitative relation between contact spacing and excitation threshold should theoretically be obtained. At large contact separations both the cathode and the two anodes can be considered as monopoles and the excitation threshold is fully determined by the cathodal field (Fig. 4(a)). When contact separation is decreased, the positive sidelobes of the anodes overlap with the positive central peak of the activating function of the cathode (Fig. 4(b)), which increases this peak value and decreases the excitation threshold. When contact separation is decreased further, the negative central peaks of the anodal activating functions overlap with the positive central peak of the cathodal activating function, which increases the excitation threshold (Fig. 4(c)). The qualitative relation between contact spacing and blocking threshold is identical to the relation between contact spacing and excitation threshold and can also be derived by the overlap of anodal and cathodal activating functions. n a symmetrical cuff configuration we calculated the influence of contact separation on the excitation and blocking thresholds of 4 pm and 12 pm fibers positioned at two extreme positions: at the axis of the nerve bundle and at its boundary. The cuff inner diameter was 2.0 mm. The circular contacts had zero width. When the excitation threshold was calculated, the fibers always had a node of Ranvier in the plane of the cathode. A pulse width of 200 ps was used. When the blocking threshold was calculated, a node of Ranvier was in the plane of the blocking anode. Because a symmetrical configuration was used, the behavior at the two anodes will be the same. The pulse width for blocking was 600 ps, which was long enough to achieve a minimum blocking threshold. The results in Fig. 5(a) show that the fibers at the axis of the bundle (curves b and d) exhibit the expected relationship. The fibers at the border of the bundle (a, c) are close to the contacts and the overlap of activating functions will therefore
5 RJKHOFF et a/ : SELECTVE STMULATON OF SACRAL NERVE ROOTS FOR BLADDER CONTROL 417 t res Fig. 4. Activating functions (AF s) of two anodes (A and A2) and a cathode (C) and the resulting AF (res) at three different contact separations. (a) Contact separation is relatively large and individual AF s can be considered an monopoles. The individual positive and negative peaks are as high as the resulting AF. (b) Contact separation decreased. Peaks of the resulting AF are increaned by the sidelobes of individual anodal and cathodal AF s. (c) Contact separation further decreased. Anodal and cathodal peaks overlap and decrease the peaks in the resulting AF. appear at smaller contact separations than shown in Fig. S(a). t is shown that the threshold of a fiber of a given diameter at the axis of the bundle is higher than at the border. When the contact separation is increased from 1.8 mm to 6.0 mm the ratio between threshold at axis and threshold at border for a 12 rm fiber decreases from 2.7 to 1.6 and for a 4 pm fiber from 4.3 to 2.9. Fig. 5(a) also shows that when a contact separation larger than 2.1 mm is used, activation of all 2 pm fibers without activation of any 4 pm fiber is possible. Fig. 5(b) shows the relation between contact spacing and blocking threshold. The threshold of a fiber with a given diameter is higher at the axis of the bundle than at its border. For a 12 pm fiber the ratio of threshold at axis to threshold at border decreases from 3.8 to 1.8 and for a 4 pm fiber from 5.9 to 3.3 when the electrode separation increases from 1.8 to 6.0 mm. Fig. S(b) also indicates the possibility of blocking all 12 pm fibers without blocking any 4 pm fiber when the contact separation is 2.1 mm or greater. To get an impression of the range of contact separations and stimulus currents which form an operating window for selective activation of small fibers with a tripolar cuff electrode, excitation and blocking threshold were plotted versus contact separation in Fig. S(c). The electrode had an asymmetry ratio of 2. All thresholds are shown as a function of cathodal current. f the asymmetry ratio equals 2, the cathodal current is three times the current at the distal anode. Thus, to present the blocking thresholds as a function of the cathodal current in Fig. S(c), all blocking thresholds of Fig. S(b) have been multiplied by 3. The operating window of this configuration is bordered by three lines. On the left, the window is bordered by solid line d (excitation threshold of 4 pm fiber at axis of nerve) and broken line b (block threshold of 12 pm fiber at axis), and on the right by broken line c (block threshold of 4 pm fibers at border of nerve bundle). The width of the window decreases from 0.82 ma (from 0.88 to 1.7 ma) at 6 mm separation to 0 ma at 2.2 mm contact separation. Decreasing the asymmetry ratio will decrease the window because the broken lines (threshold block) will move to the left, but the solid lines (threshold excitation) will not. With ratio = 1 (symmetrical) the width of the window is decreased to 0.26 ma (from 0.88 to 1.14 ma) at 6 mm contact separation. ncreasing the ratio above 2 will widen the window between broken lines b and c. However, the whole window moves to the right, so the electrode operates at a higher cathodal current. As shown in Fig. 5(c), the minimum cathodal current is determined by broken line b and solid line d. The largest window under the condition of minimum cathodal current is obtained if the ratio equals 2. One of the aims of the cuff electrode is to block all action potentials initiated at the cathode at the anode proximal to the cathode. n order to determine the minimum contact separation resulting in a complete block, it is sufficient to consider the fiber with the highest blocking threshold. Under the conditions of this study, this is a 4 rm fiber at the axis of the nerve bundle. Fig. S(d) shows the relation between contact separation and blocking threshold for a 4 pm fiber for three cuff diameters. When cuff diameter is decreased from 2.8 mm to 2.0 mm, the contact separation at which the minimum blocking threshold occurs decreases from 5.5 mm to 4 mm; however the minimum is not very pronounced. Threshold increases steeply below 3 mm contact separation. From these results we may conclude that the minimum contact separation for blocking all fibers at a low stimulus current is approximately 3 4 mm. We chose a separation of 3 mm because this makes the electrode smaller while the currents only increase 4.7(%, 5.1%; and 6.3% for 2.0 mm, 2.4 mm and 2.8 mm cuff inner diameter respectively. B. Relution Between Cuff nner Diameterand Esi,itutinnlBloc.liing Tlltcshold The influence of the cuff inner diameter on excitation and blocking threshold was calculated for a 6 mm contact separation and contact width of zero. The results in Fig. 6(a) show that excitation thresholds increase when the cuff inner diameter increases. The relative difference in threshold for a fiber at the axis and a fiber at the border of the bundle decreases with increasing cuff inner diameter. f the diameter increases from 2.0 mm to 3.6 mm, this relative difference decreases from 1.6 to 1.4 for a 12 pm fiber and from 2.9 to 1.8 for a 4 tm fiber. Moreover, the selectivity (stimulation of all 12 tm fibers and no 4 pm fibers) increases with increasing cuff diameter. f the cuff diameter increases from 2.0 mm to 3.6
6 ~ b ~ 418 EEE TRANSACTONS ON BOMEDCAL ENGNEERNG, VOL. 41, NO. 5, MAY k c Ei 1.5 m E s 1.0 jb \ h Contact separation [mm] (a) Contact separation [mm] (b) 7 Th. excit. (solid lines) Operating window selective activation small fibers b d, z E 4 Y 0 n i 3 \ 2.4 mm. \ i :, c ~ Th block (broken lines) Cathodal current [ma] (c 1 Contact separation [mm] Fig. 5. (a) Excitation threshold (cathodal current) as a function of contact separation. Zero contact width, cuff inner diameter: 2 mm, sacral root diameter: 1.4 mm. a: 12 pm fiber at border of nerve bundle, b: 12 pm fiber at axis of nerve bundle, c: 4 pm fiber at border of nerve bundle, d: 4 pm fiber at axis of nerve bundle. (b) Blocking threshold (anodal current) as a function of contact separation. Cuff inner diameter: 2 mm, zero contact width. ad: Fiber positions, see (a). (c) Excitation and blocking thresholds of small and large fibers at several contact separations as function of cathodal current for an asymmetrical tripolar cuff electrode. Hatched area is operating window for selective activation of small fibers. Asymmetry ratio: 2, cuff inner diameter: 2 mm, zero contact width. ad: Fiber positions, see (a). (d) Blocking threshold (anodal current) as a function of contact separation for a 4 jrm fiber positioned at the axis. Cuff inner diameters: 2.0, 2.4 and 2.8 mm. (d) mm, the ratio (threshold 4 pm fiber at nerve bordedthreshold 12 pm fiber at nerve axis) increases from 1.9 to 3.1. Fig. 6(b) shows that blocking thresholds also increase with increasing cuff inner diameter. Over the whole range of diameters it is possible to block all 12 pm fibers without blocking any 4 pm fiber. The selectivity ratio (threshold 4 pm fiber at nerve border/threshold 12 pm fiber at nerve axis) increases from 2.0 to 3.7 if the inner diameter increases from 2.0 mm to 3.6 mm. n Fig. 6(c) the data of Fig. 6(a) and (b) is combined in the same way as in Fig. 5(c) for a configuration with an asymmetry ratio of 2. t shows an operating window for selective activation of small fibers between solid line d (excitation threshold of 4 p,m fiber at axis of nerve) and broken line c (block threshold of 12 pm fiber at border). The width of the window increases from 0.82 ma ( ) to 9.9 ma ( ) if the cuff inner diameter is increased from 2.0 mm to 3.6 mm, at the cost of an increasing cathodal current. An asymmetry ratio above 2 will increase the width of the window but will also increase the cathodal current. C. Effect of Pulse Duration on Blocking Threshold The ratio of conduction velocities of a 4 pm fiber and a 12 pm fiber is about the same as their diameter ratio [4]. Therefore, it takes about twice as long for an action potential of a 4 pm fiber to propagate from cathode to anode as it takes for an action potential of a 12 pm fiber. Thus, it may be possible to block a 12 pm fiber and not a 4 pm fiber, solely by selecting the right pulse duration. To assess this duration we modeled two asymmetrical cuff configurations (cuff inner diameter: 2.4 mm), one having a ratio of 2 between contact separations (3 mm and 6 mm) and one with a ratio of 3 (3 mm
7 7 RJKHOFF e/ ul.: SELECTVE STMULATON OF SACRAL NERVE ROOTS FOR BLADDER CONTROL F 6 Y r Cuff inner diam. [mm] Cuff inner diam. [mm] (a) lab Th. excit. (solid lines) (b) 3.5 E E ; 3.0 c El. U L C Ab 'c d Th. block (broken lines) Cathodal current [ma] (C) Fig. 6. (a) Excitation threshold (cathodal current) as a function of cuff inner diameter. Contact separation: 6 mm. zero contact width. ad: Fiber positions, see Fig. 5(a). (b) Blocking threshold (anodal current) as a function of cuff inner diameter. Contact separation: 6 mm. a 4 Fiber positions, see Fig. 5(a). (c) Excitation and blocking thresholds of small and large fibers at several cuff inner diameters as a function of cathodal current for an asymmetrical tripolar cuff electrode. Hatched area is operating window for selective activation of small fibers. Asymmetry ratio: 2, cuff inner diameter: 2 mm, zero contact width. ad: Fiber positions, see Fig. 5(a). and 9 mmj. n both cases the proximal anode, used to produce a complete block, has been spaced 3 mm from the cathode, being the minimum separation for blocking all fibers with low stimulus current (see above). Fig. 7 shows the blocking thresholds at the distal anode for a 12 pm fiber (curve a1 and a2j at the axis of the nerve bundle and a 4 pm fiber (curve c 1 and c2j at its border. Lowest thresholds were calculated for pulse durations just long enough to block the action potential at the node in plane with the anode. For shorter pulses the action potential has to be blocked at a node closer to the cathode. Due to a lower value of the activating function at this node, a higher current is necessary to block the action potential. Therefore, the curves of threshold versus pulse duration show a stepwise increase of blocking threshold with decreasing pulse duration. f the distal anode is separated from the cathode by 6 mm, a pulse width of at least 220 ps is required to block the 12 bsm fiber (curve al) at minimum current while a pulse width of 390 ps is needed to block the 4 pm fiber (cl) as well. When taking into account the possibility of blocking at nodes closer to the cathode, there is a window of 190 ps (from 160 to 350 psj in which all 12 pm fibers can be blocked without blocking any 4 pm fiber. The time window can be increased if the separation of cathode and distal anode is increased. At a contact separation of 9 mm, 270 ps is needed to block the 12 pm fiber (a2j at minimum current, while a 530 ps pulse is required to block the 4 pm fiber (c2). When taking into account blocking at other nodes, the window will be 270 ps (from 230 to 500 ps). Apart from discriminating between large and small diameter fibers at the distal anode, the pulse duration should be adequate to block all fibers at the proximal anode at 3 mm from the cathode. This distance is the same in both configurations. The calculated values for a complete block at the proximal anode (4 pm fiber at the axis of the nerve bundle) are given by curve b in Fig. 7. t shows that a complete block requires a
8 420 EEE TRANSACTONS ON BOMEDCAL ENGNEERNG, VOL. 41, NO. 5. MAY a E 3 Y U n i * + 1 c2! i. 1.4 a E Y : 1.2 a.c + 1.o / Pulse duration [ps] Fig. 7. Blocking thresholds (anodal current) as a function of pulse duration for two asymmetrical cuff electrodes. Contact separations: 3 mm (curve b) and 6 mm (curve al and cl) and 9 mm (curve a2 and c2). Cuff inner diameter: 2.4 mm. al, a2 12 pm fiber at axis of nerve bundle, b: 4 pm at axis of nerve bundle, cl, c2: 4 pm at border of nerve bundle. pulse duration of at least 195 ps, reducing the window for a selective block from 190 ps to 155 ps in the configuration with an asymmetry ratio of 2. The window is not affected in case of an asymmetry ratio of 3, because a pulse duration of 230 ps required to block the 12 pm fibers exceeds the pulse duration required for a complete block. Therefore, a cuff electrode having a spacing ratio of 3 should be preferred. D. Effect of Contact Width and Nodal Position on Blocking Threshold The effects of contact width and nodal position on the blocking threshold will be most pronounced for a fiber close to the contacts. Therefore, a 4 pm fiber at the boundary of the bundle was considered. We observed that the main part of the current was injected into the medium at the edge of the anodal contact closest to the cathode. Thresholds are presented for two fiber positions exhibiting the largest difference in threshold: one having a node right under the edge of the anodal contact and another fiber shifted over half the intemodal distance, which is 0.2 mm for a 4 pm fiber. Fig. 8 shows the influence of contact width for the two positions. The fiber having a node in the plane of the contact edge has the lowest threshold (a). With increasing contact width both thresholds increase slightly. When the width is increased from 0.6 mm to 1.2 mm the thresholds only increased by 6%. The contact width had no influence on the absolute difference in blocking threshold between the two fiber positions. V. DSCUSSON The aim of this study was to determine the optimal geometric parameters for an asymmetric tripolar cuff electrode for the stimulation of sacral roots. Therefore, threshold currents for excitation and blocking of two nerve fibers with different diameters in the sacral root were calculated. We chose a 4 pm fiber and a 12 pm fiber diameter, representing fibers innervating the detrusor muscle and fibers innervating the external sphincter, respectively [27]. The proximal anode must Contact width [mm] Fig. 8. nfluence of contact width on the block thresholds (anodal current) of a 4 pm fiber at the border of the bundle. a: fiber with node under inner edge of electrode, b: fiber shifted over half the intemodal distance. block the action potentials of all fibers initiated at the cathode, while the distal anode should only block the induced activity in the 12 pm fibers, without blocking the 4 pm fibers. At the distal side the difference (selectivity) between the blocking thresholds of 4 pm and 12 pm fibers should be as large as possible. Apart from these functional goals, the dimensions of the cuff and the currents should be minimized. To represent the biological volume conductor in a model with acceptable computational load, simplifying assumptions have been made with respect to the complexity and variability of the anatomy of the nerve roots. The nerve roots are considered to be cylindrical, while the nerve tissue is considered to be a homogeneous, anisotropic and frequencyindependent volume conductor. Structures influencing the electrical conductivity of the nerve (such as blood vessels and connective tissue) were not considered. The model used to simulate the electrical behavior of nerve fibers in a cuff electrode consists of two parts which are not coupled. As a consequence, any influence of currents generated by active fibers in the bundle on the electric field imposed on the fiber bundle by the applied currents has been neglected. These fiber currents will not influence excitation thresholds because most fibers will be inactive before excitation occurs. Therefore, only blocking thresholds will be affected. The extracellular potential gradients generated by active fibers will be larger when the nerve bundle is insulated than in a noninsulated situation [21]. n our configuration the bundle is only partially insulated by a cuff. The cuff ends are shortcircuited by the CSF surrounding the cuff. The current generated by active fibers runs partly through the CSF and decreases the potential field. The blocking occurs at the anode5 which are located at both cuff ends. At these ends we expect the extracellular potentials to be rather low because the anodes are very close to the shortcircuiting bulk. Therefore the effect of generated active currents is expected to be very small and can thus be neglected. n experiments on anodal blocking of peripheral nerves [12]. [17], excitation of large fibers occurs near the anode
9 RJKHOFF et ul : SELECTVE STMULATON OF SACRAL NERVE ROOTS FOR BLADDER CONTROL 42 1 at the end of a long rectangular blocking pulse. This socalled anodal break excitation does not occur in the nerve fiber model. A gradual decrease of the current, as in quasitrapezoidal pulses, to eliminate the anodal break excitation in experiments, was therefore not necessary in our simulations. We compared blocking thresholds using a rectangular pulse and a quasitrapezoidal one in a few cuff configurations, but the differences appeared to be negligible. n order to validate the model, the rectangular pulses can be extended by a slow decay if necessary, to avoid anodal break excitation [ 171. The propagation velocity in the model is 27 m/s and 67 m/s for the 4 pm and 12 pm fiber, respectively, if the nerve fibers are not affected by a depolarizing or hyperpolarizing electric field. These velocities were higher if the fiber was depolarized and lower if hyperpolarized. Moreover, the 4 pm fiber will have a larger delay before its excitation near the cathode than the 12 pm fiber. t is therefore difficult to relate the calculated pulse widths to the propagation velocities. Based on the velocities of the 12 pm and 4 pm fiber, it would take 45 and 11 1,us, respectively, to proceed 3 mm (between 6 mm and 9 mm contact separation). This is roughly in agreement with the SO and 140 ps delays from the simulations. Only a few papers on sacral root stimulation give experimental data useful for a comparison with our modeling results. These studies deal with excitation of fibers by monopolar or bipolar stimulation and not with tripolar cuff electrodes. All papers report the possibility of activating the urethral sphincter without activating the detrusor, which is in agreement with our modeling results. However, most data on thresholds for sphincter and detrusor contraction are given in voltages rather than currents. The stimulation voltages are largely dependent on electrode impedances and will, therefore, vary among experiments. Tanagho et al. [36] reported stimulation currents between 3 ma and 8 ma, which would be just above the threshold at which detrusor responds. They used helical electrodes placed extradurally, where the roots are surrounded by an epineurium. A helix has a larger leakage current than a cuff. This may explain the high threshold currents in comparison to our theoretical data (less than 2 ma). Using book electrodes (contacts mounted in grooves cut into a block of insulating material [S), Brindley et al. [7] measured 0.3 ma to 3.0 ma for the somatic threshold and S ma to 20 ma to achieve maximum detrusor response. No data is given on the electrode dimensions, although these are usually rather large. Their excitation threshold, however, fits a cuff diameter of 4.0 mm in Fig. 6(a) (after extrapolation of the curves). Using standard Brindley electrode books (rectangular cross section, 2 x 3 mm) we recently measured an initial bladder response in three patients at about 0.7 ma and a maximum bladder pressure at about 2 ma. f we assume that initial detrusor response occurs when only small fibers at the boundary of the bundle are activated and maximum bladder pressure occurs when all small fibers in the root are activated, then these data fit our theoretical values for a cuff diameter of 2.6 mm and 2.8 mm respectively (Fig. 6(a)). For a comparison of the modeling results on blocking with experimental results from literature, only a single paper could be found on blocking $acral roots [6]. n this paper results of blocking with electrode books in baboons were presented. Except for the contact separations, dimensions of the books are not given. For an electrode separation of 4.5 mm, blocking started at a total current of 4.6 ma. Since this current was applied to two anodes in parallel, the blocking threshold will have been 2.3 ma, being approximately 350% more than predicted by our model for a 12 pm fiber. This discrepancy may be due to the larger cross section of the book. They also found a minimum pulse duration for blocking over 200 ps. When contact separation was 6.0 mm we calculated a minimum pulse duration of 160 ps. This means that with 4.5 mm separation the minimum duration would even be shorter. Van den Honert et al. [ 171 measured the effect of electrode spacing on blocking in peripheral nerve. They found an increase in threshold if the spacing decreased from 9 mm to 3 mm. Our model predicts almost no threshold variation when spacing exceeds 4.0 mm (Fig. 5(b) and (d)). This difference may be due to the presence of a perineurium around peripheral nerves. This small layer with low conductivity increases the electrical distance between contacts and nerve fibers. t can therefore be interpreted in this case as an increased inner diameter of the cuff electrode (Fig. 5(d)). The model predicts that the smallest electrode separation to achieve a complete block at low stimulus current is 3 mm. To obtain a good selectivity between small and large fibers, the model predicts a large influence of pulse duration. The best selectivity occurs at a large electrode separation because in that case the time difference between the arrival at the anode of action potentials from large and small fibers will be large. According to the model a separation of 69 mm will be enough. This results in a maximum overall cuff length of 13 mm (contact width: 1 mm). n that case a pulse duration of 300 ps is sufficient. The current will depend on the ratio of the nerve root diameter and the cuff inner diameter. The contact width should be kept small (< 1 mm) because a large width increases thresholds. Because the model predicts a relatively large amplitude range and a large pulse duration window for the selective blocking of large fibers without blocking the small ones, we expect that selective activation of the detrusor muscle is feasible. Based on this modeling study, cuff electrodes have been developed and are currently tested in acute animal experiments. When applying this technique in patients, selective detrusor activation would result in more physiological bladder emptying at lower intravesical pressures as compared to other techniques. However, to avoid reflex incontinence, cutting the dorsal sacral roots to deafferent the bladder will still be necessary to abolish autonomic reflex contractions of the bladder. APPENDX The electric potential field generated by the cuff electrode in a volume conductor can be described by Poisson s equation where
10 422 EEE TRANSACTONS ON BOMEDCAL ENGNEERNG, VOL. 41, NO. 5, MAY 1994 o = conductivity tensor 4 = electric potential + j, = source current density. Considering the current source as point sources, each point source i can be described with an amplitude i multiplied with a delta function. Writing out the lefthand side of (1) in full and expressing the righthand side as a summation of point sources gives r, 1 J. a The volume conductor is rotation symmetric with the axis of the cuff electrode and nerve being equal to the symmetry axis of the model. Therefore the lefthand side of (2) can be expressed in cylindrical coordinates using the coordinate transformations of (3). When assuming (4), the result can be written as the lefthand side of (5). The righthand side of (2) resembles N infinitely thin current rings with current, and radius R, which can also be expressed in cylindrical coordinates (5). r = q m B = arctan( 5) z=z (3) ox = oy or. (4) The third term of the lefthand side of (5) equals zero because of rotational symmetry and vanishes. This gives the analytical expression (6) for the electrical potential 4. &(or$) + 1 a4 + 7% l a (or$) + & ( g z g ) Fig 9 < > h, 2, 1 z, Definitions of spacing and conductivities in grid in rdirection: Cj = rj rj1 (j = 1..& 1) Q is number of grid points in rdirection. Equation (7) is the result of the first order discretization of (6). where (7) where N &6(r RrL? z z,) (5) n=l 4 : electric potential r : radius (T, = O,.(T, z) crz = a,(r. z) 11, RlL : radius of ring 71. h(r R,,, z z,) : delta function. : conductivity in rdirection : conductivity in zdirection : current injected by ring 71, Because of inhomogeneities of the volume, no analytical solution of (6) can be found. To solve (6) numerically we used a finite difference technique in a grid with variable spacing. This involves discretization of (6) with spacing 11 and k defined as in zdirection: h, = z, z,1 (i = l..p 1) P is number of grid points in zdirection The discrete conductivities a:,, and of,j are defined as shown in Fig. 9. From (7) the potential qhz,j can be isolated and expressed in the potentials of the neighboring grid points plus the current injected in point (z,j). The result is shown in (9) and (10). As for boundary conditions, a Neumann condition (a4/8~ = 0) was used at the symmetry axis (r = 0) while a Dirichlet condition (4 = 0) was applied at the other three boundaries. The set of linear equations (9) has been solved with the (iterative) successive relaxation method using a relaxation factor of 1.8. The used dimensions were: 60 x 80 points, 40 x 18 mm, i.e., a cylinder with 40 mm length and 18 mm radius. The smallest spacing (0.3 mm in zdirection, 0.1 mm in rdirection) was used in the volume confined by the cuff. A solution which meets our accuracy criterion (1 1) requires 5000 iterations (M: total grid points, C: iterations done). dlj = '?4?1,J + /j$l+1,j + (7 t)4'b,j1 f (6 f )4L,,fl + d n + [ + y + 6 (9)
11 RUKHOFF er al.: SELECTVE STMULATON OF SACRAL XERVE ROOTS FOR BLADDER CONTROL 423 REFERENCES N. Accornero, G. Bini, G. L. Lenzi and M. Manfredi, Selective activation of peripheral nerve fibre groups of different diameter by triangular shaped stimulus pulses, J. Physiol., vol. 273, pp, , W. K. Altman and R. Plonsey, A twopart model for determining the electromagnetic and physiologic behavior of cuff electrode nerve stimulators, ZEEE Trans. Biomed. Eng., vol. BME33, pp , W. H. Boyce, J. E. Lathem and L. D. Hundt, Research related to the development of an artificial electrical stimulator for the paralyzed human bladder: A review, J. Urol., vol. 91, pp. 4151, A. Boyd and M. R. Davey, Composition of peripheral nerves. Edinburgh: Livingstone, G. S. Brindley, An implant to empty the bladder or close the urethra, J. Neurol. Neurosurg. Psych., vol. 40, pp , G. S. Brindley and M. D. Craggs, A technique for anodally blocking large nerve fibres through chronically implanted electrodes, J. Neurol. Neurosurg. Psych., vol. 43, pp , G. S. Brindley, C. E. Polkey and D. N. Rushton, Sacral anterior root stimulators for bladder control in paraplegia, Paraplegia, vol. 20, pp , G. S. Brindley, C. E. Polkey, D. N. Rushton and L. Cardozo, Sacral anterior root stimulators for bladder control in paraplegia: The first 50 cases, J. Neurol. Neurosurg. Psych., vol. 49, pp. 110&1114, T. Brismar, Potential clamp analysis of membrane currents in rat myelinated nerve fibres, J. Physiol.. vol. 298, pp , S. Y. Chiu, J. M. Ritchie, R. B. Rogart and D. Stagg, A quantitative description of membrane currents in rabbit myelinated nerve, J. Physiol., vol. 292, pp , R. E. Coggeshall, Law of separation of function of spinal roots, Physiol. Reviews, vol. 60, pp , Z.P. Fang and J. T. Mortimer, Selective activation of small motor axons by quasitrapezoidal current pulses, ZEEE Trans. Biomed. Eng., vol. 38, pp , A. S. Ferguson, J. D. Sweeney, D. Durand and J. T. Mortimer, Finite difference modelling of nerve cuff electric fields, Proc. 9th Annu. nt. Conf. EEEEMBS, pp , L. A. Geddes and L. E. Baker, The specific resistance of biological materiala compendium of data for the biomedical engineer and physiologist, Med. 5: Bid. Eng., vol. 5, pp , D. B. Halverstadt and W. L. Parry, Electronic stimulation of the human bladder: 9 years later, J. Urol., vol. 113, pp , B. Holmquist and 0. Tord, Electromicturition in male dogs at pelvic nerve stimulation: an urethrocystographic study, Scand. J. Uml. Nephol., vol. 2 (suppl. 2). pp. 115, C. van den Honert and J. T. Mortimer, A technique for collision block of peripheral nerve: Single stimulus analysis, EEE Trans. Biomed. Eng., vol. BME28, pp , U. Jonas and E. A. Tanagho, Studies on the feasibility of urinary bladder evacuation by direct spinal cord stimulation. 11. Post stimulus voiding: a way to overcome outflow resistance. nvesr. Urol.. vol. 13, pp. 151, P. E. V. van Kerrebroeck, E. Koldewijn, H. Wijkstra and F. M. J. Debruyne, ntradural sacral rhizotomies and implantation of an anterior sacral root stimulator in the treatment of neurogenic bladder dysfunction after spinal cord injury; Surgical technique and complications, World J. Urol., vol. 9, pp , [20] D. R. McNeal, Analysis of a model for excitation of myelinated nerve, EEE Trans. Biomed. Eng., vol. BME23, pp , [21] J. H. Meier, Selectivity and design of neuroelectronic interfaces, Ph.D. Thesis, University of Twente, C. W. Morgan, W. C. de Groat, L. A. Felkins and S. J. Zhang, Axon collaterals indicate broad intraspinal role for preganglionic neurons, Proc. Natl. Acad. Sci. USA, vol. 88, pp , [23] T. Morita, 0. Nishizawa, H. Noto and S. Tsuchida, Pelvic nerve innervation of the external sphincter of the urethra as suggested by urodynamic and horseradish peroxidase studies, J. Urol., vol. 131, pp , F. Rattay, Ways to approximate currentdistance relations for electrically stimulated fibers, J. Theor. Biol., vol. 125, pp , [25] F. Rattay, Analysis of models for extracellular fiber stimulation, EEE Trans. Biomed. Ens., vol. 36, pp , July [26] G. Schalow, The problem of cauda equina nerve root identification, Zbl. Neurochirurgie, vol. 46, pp , [27] G. Schalow, Efferent and afferent fibres in human sacral ventral nerve roots: basic research and clinical implications, Electiomyogr. Clin. Neurophysiol., vol. 29, pp. 3353, [28] G. Schalow. Oscillatory firing of single human sphincteric a2 en a3 motoneurons reflexly activated for the continence of urinary bladder and rectum. Restoration of bladder function in paraplegia, Electromyogr. Clin. Neurophysiol.. vol. 31. pp , [29] G. Schalow, Number of fibres and fibre diameter distributions of nerves innervating the urinary bladder in humans. Acceptor nerve analysis. 1 (V), Electromyogr. Clin. Neurophysiol., vol. 32, pp [30] R. A. Schmidt, H. Bruschini and E. A. Tanagho, Sacral root stimulation in controlled micturition. (Peripheral somatic neurotomy and stimulated voiding), nvest. Urol., vol. 17, pp , [31] J. J. Struijk, J. Holsheimer, G. G. van der Heide and H. B. K. Boom, Recruitment of dorsal column fibers in spinal cord stimulation: nfluence of collateral branching. EEE Trans. Biomed. Eng., vol. 39, pp , [32] J. D. Sweeney and J. T. Mortimer, An asymmetric two electrode cuff for generation of unidirectionally propagated action potentials, EEE Trans. Biomed. Eng., vol. BME33, pp , [33] J. D. Sweeney, J. T. Mortimer and D. R. Bodner, Acute animal studies on electrically induced collision block for motor activity, Neurourol. Urodyn., vol. 8, pp , [34] J. D. Sweeney, J. T. Mortimer and D. R. Bodner, Animal study of pudendal nerve cuff implants for collision block of motor activity, Neurourol. Urodyn., vol. 9, pp. 8393, [35] E. A. Tanagho and R. A. Schmidt, Electrical stimulation in the clinical management of the neurogenic bladder, J. Urd., vol pu , r ~ E. A. Tanagho. R. A. Schmidt and B. R. Orvis. Neural stimulation for the control of voiding dysfunction; A preliminary report in 22 patients with serious neuropathic voiding disorders, J. Urol., vol. 142, pp , [37] J. W. Thuroff, M. A. Bazeed, R. A. Schmidt, D. M. Wiggin and E. A. Tanagho, Functional pattern of sacral root stimulation in dogs:. Micturition, J. Urd., vol. 127, pp , [38] J. S. Walter, J. S. Wheeler, C. J. Robinson and R. D. Wurster, Surface stimulation techniques for the bladder management in the spinal dog, J. Urd., vol. 141, pp , [39] H. Wijkstra, N. J. M. Rijkhoff, J. Holsheimer, P. E. V. A. van Kerrebroeck, E. L. Koldewijn. H. B. K. Boom and F. M. J. Debruyne, Selective stimulation and blocking of sacral nerves: research setup and preliminary results, Pioc. 13th. Annu. nt. Con5 ZEEEEMBS, pp , Nico J. M. Rijkhoff was born in Opneer, The Netherlands, in He received the M.Sc. degree in electrical and biomedical engineering in 1990 from the University of Twente, Enschede, The Netherlands. Since 1991, he has been working towards the Ph.D. degree at the Biomedical Engineering Group of the Department of Urology at the University Hospital Nijmegen, The Netherlands. His research interests are related to electrical stimulation of the sacral nerve roots for restoration of urinary bladder control in spinal cord injury.
Technologies and architectures" Stimulator, electrodes, system flexibility, reliability, security, etc."
 March 2011 Introduction" Basic principle (Depolarization, hyper polarization, etc.." Stimulation types (Magnetic and electrical)" Main stimulation parameters (Current, voltage, etc )" Characteristics (Muscular
March 2011 Introduction" Basic principle (Depolarization, hyper polarization, etc.." Stimulation types (Magnetic and electrical)" Main stimulation parameters (Current, voltage, etc )" Characteristics (Muscular
Electrostimulation Part 3: Bladder dysfunctions
 GBM8320 Dispositifs Médicaux Intelligents Electrostimulation Part 3: Bladder dysfunctions Mohamad Sawan et al Laboratoire de neurotechnologies Polystim!!! http://www.cours.polymtl.ca/gbm8320/! mohamad.sawan@polymtl.ca!
GBM8320 Dispositifs Médicaux Intelligents Electrostimulation Part 3: Bladder dysfunctions Mohamad Sawan et al Laboratoire de neurotechnologies Polystim!!! http://www.cours.polymtl.ca/gbm8320/! mohamad.sawan@polymtl.ca!
GBM8320 Dispositifs Médicaux Intelligents. Electrostimulation. Part 3: Bladder dysfunctions
 GBM8320 Dispositifs Médicaux Intelligents Electrostimulation Part 3: Bladder dysfunctions Mohamad Sawan et al Laboratoire de neurotechnologies Polystim!!! http://www.cours.polymtl.ca/gbm8320/! mohamad.sawan@polymtl.ca!
GBM8320 Dispositifs Médicaux Intelligents Electrostimulation Part 3: Bladder dysfunctions Mohamad Sawan et al Laboratoire de neurotechnologies Polystim!!! http://www.cours.polymtl.ca/gbm8320/! mohamad.sawan@polymtl.ca!
Quantitative Electrophysiology
 ECE 795: Quantitative Electrophysiology Notes for Lecture #10 Wednesday, November 22, 2006 14. FUNDAMENTALS OF FUNCTIONAL ELECTRICAL STIMULATION (FES) We will look at: Design issues for FES Subthreshold
ECE 795: Quantitative Electrophysiology Notes for Lecture #10 Wednesday, November 22, 2006 14. FUNDAMENTALS OF FUNCTIONAL ELECTRICAL STIMULATION (FES) We will look at: Design issues for FES Subthreshold
SELECTIVE BLOCK TECHNIQUES OF URETHRAL SPHINCTER CONTRACTIONS IN SACRAL ANTERIOR ROOT STIMULATION
 SELECTIVE BLOCK TECHNIQUES OF URETHRAL SPHINCTER CONTRACTIONS IN SACRAL ANTERIOR ROOT STIMULATION S. Schumacher, S. Bross*, J.R. Scheepe*, G. Böhler*, K.P. Jünemann*, S.C. Müller and P. Alken* Department
SELECTIVE BLOCK TECHNIQUES OF URETHRAL SPHINCTER CONTRACTIONS IN SACRAL ANTERIOR ROOT STIMULATION S. Schumacher, S. Bross*, J.R. Scheepe*, G. Böhler*, K.P. Jünemann*, S.C. Müller and P. Alken* Department
Nerve. (2) Duration of the stimulus A certain period can give response. The Strength - Duration Curve
 Nerve Neuron (nerve cell) is the structural unit of nervous system. Nerve is formed of large numbers of nerve fibers. Types of nerve fibers Myelinated nerve fibers Covered by myelin sheath interrupted
Nerve Neuron (nerve cell) is the structural unit of nervous system. Nerve is formed of large numbers of nerve fibers. Types of nerve fibers Myelinated nerve fibers Covered by myelin sheath interrupted
EE 791 Lecture 2 Jan 19, 2015
 EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
SPINAL cord stimulation (SCS) is the most common
 Computational Analysis of Kilohertz Frequency Spinal Cord Stimulation for Chronic Pain Management Scott F. Lempka, Ph.D., Cameron C. McIntyre, Ph.D., Kevin L. Kilgore, Ph.D., Andre G. Machado, M.D., Ph.D.
Computational Analysis of Kilohertz Frequency Spinal Cord Stimulation for Chronic Pain Management Scott F. Lempka, Ph.D., Cameron C. McIntyre, Ph.D., Kevin L. Kilgore, Ph.D., Andre G. Machado, M.D., Ph.D.
Physiologic Anatomy and Nervous Connections of the Bladder
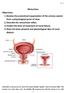 Micturition Objectives: 1. Review the anatomical organization of the urinary system from a physiological point of view. 2. Describe the micturition reflex. 3. Predict the lines of treatment of renal failure.
Micturition Objectives: 1. Review the anatomical organization of the urinary system from a physiological point of view. 2. Describe the micturition reflex. 3. Predict the lines of treatment of renal failure.
Cellular Bioelectricity
 ELEC ENG 3BB3: Cellular Bioelectricity Notes for Lecture #30 Thursday, March 30, 2006 Nerve excitation: To evaluate the pattern of nerve activation that is produced by a particular electrode configuration,
ELEC ENG 3BB3: Cellular Bioelectricity Notes for Lecture #30 Thursday, March 30, 2006 Nerve excitation: To evaluate the pattern of nerve activation that is produced by a particular electrode configuration,
Analysis of nerve conduction block induced by direct current
 J Comput Neurosci (29) 27:21 21 DOI 1.17/s1827-9-137-7 Analysis of nerve conduction block induced by direct current Changfeng Tai & James R. Roppolo & William C. de Groat Received: 24 November 28 /Revised:
J Comput Neurosci (29) 27:21 21 DOI 1.17/s1827-9-137-7 Analysis of nerve conduction block induced by direct current Changfeng Tai & James R. Roppolo & William C. de Groat Received: 24 November 28 /Revised:
Stimulation of the Sacral Anterior Root Combined with Posterior Sacral Rhizotomy in Patients with Spinal Cord Injury. Original Policy Date
 MP 7.01.58 Stimulation of the Sacral Anterior Root Combined with Posterior Sacral Rhizotomy in Patients with Spinal Cord Injury Medical Policy Section Issue 12:2013 Original Policy Date 12:2013 Last Review
MP 7.01.58 Stimulation of the Sacral Anterior Root Combined with Posterior Sacral Rhizotomy in Patients with Spinal Cord Injury Medical Policy Section Issue 12:2013 Original Policy Date 12:2013 Last Review
Mathematical Modelling of Intra- and Extracellular Potentials Generated by Active Structures with Short Regions of Increased Diameter
 Gen. Physiol. Biophys. (1988). 7, 401 412 401 Mathematical Modelling of Intra- and Extracellular Potentials Generated by Active Structures with Short Regions of Increased Diameter N. DlMITROVA CLBA, Centre
Gen. Physiol. Biophys. (1988). 7, 401 412 401 Mathematical Modelling of Intra- and Extracellular Potentials Generated by Active Structures with Short Regions of Increased Diameter N. DlMITROVA CLBA, Centre
BI 232: Human Anatomy & Physiology
 BI 232: Human Anatomy & Physiology Roster Business Course Introduction and Syllabus Notecard Name E-mail Why you are taking the course Something interesting you did over break Lecture Tips Use the Study
BI 232: Human Anatomy & Physiology Roster Business Course Introduction and Syllabus Notecard Name E-mail Why you are taking the course Something interesting you did over break Lecture Tips Use the Study
Chapter 8 Nervous System
 Chapter 8 Nervous System Two message centers: Functions of these systems: 1. * 2. * Overview of the Nervous System Parts: General Functions: Functions Sensory input: Sensation via nerves Integration: interpretation
Chapter 8 Nervous System Two message centers: Functions of these systems: 1. * 2. * Overview of the Nervous System Parts: General Functions: Functions Sensory input: Sensation via nerves Integration: interpretation
Renal Physiology: Filling of the Urinary Bladder, Micturition, Physiologic Basis of some Renal Function Tests. Amelyn R.
 Renal Physiology: Filling of the Urinary Bladder, Micturition, Physiologic Basis of some Renal Function Tests Amelyn R. Rafael, MD 1 Functions of the Urinary Bladder 1. storage of urine 150 cc 1 st urge
Renal Physiology: Filling of the Urinary Bladder, Micturition, Physiologic Basis of some Renal Function Tests Amelyn R. Rafael, MD 1 Functions of the Urinary Bladder 1. storage of urine 150 cc 1 st urge
NEURONS Chapter Neurons: specialized cells of the nervous system 2. Nerves: bundles of neuron axons 3. Nervous systems
 NEURONS Chapter 12 Figure 12.1 Neuronal and hormonal signaling both convey information over long distances 1. Nervous system A. nervous tissue B. conducts electrical impulses C. rapid communication 2.
NEURONS Chapter 12 Figure 12.1 Neuronal and hormonal signaling both convey information over long distances 1. Nervous system A. nervous tissue B. conducts electrical impulses C. rapid communication 2.
New Neurostimulation and Blockade Strategy to. Enhance Bladder Voiding in Paraplegics
 Contemporary Engineering Sciences, Vol. 3, 2010, no. 7, 321-337 New Neurostimulation and Blockade Strategy to Enhance Bladder Voiding in Paraplegics Fayçal Mounaïm 1, Ehab Elzayat 2, Mohamad Sawan 1, Jacques
Contemporary Engineering Sciences, Vol. 3, 2010, no. 7, 321-337 New Neurostimulation and Blockade Strategy to Enhance Bladder Voiding in Paraplegics Fayçal Mounaïm 1, Ehab Elzayat 2, Mohamad Sawan 1, Jacques
Neurophysiology scripts. Slide 2
 Neurophysiology scripts Slide 2 Nervous system and Endocrine system both maintain homeostasis in the body. Nervous system by nerve impulse and Endocrine system by hormones. Since the nerve impulse is an
Neurophysiology scripts Slide 2 Nervous system and Endocrine system both maintain homeostasis in the body. Nervous system by nerve impulse and Endocrine system by hormones. Since the nerve impulse is an
10.1: Introduction. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial cells) Dendrites.
 10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
10.1: Introduction Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Cell types in neural tissue: Neurons Neuroglial cells (also known as neuroglia, glia, and glial
BIOLOGY 2050 LECTURE NOTES ANATOMY & PHYSIOLOGY I (A. IMHOLTZ) FUNDAMENTALS OF THE NERVOUS SYSTEM AND NERVOUS TISSUE P1 OF 5
 P1 OF 5 The nervous system controls/coordinates the activities of cells, tissues, & organs. The endocrine system also plays a role in control/coordination. The nervous system is more dominant. Its mechanisms
P1 OF 5 The nervous system controls/coordinates the activities of cells, tissues, & organs. The endocrine system also plays a role in control/coordination. The nervous system is more dominant. Its mechanisms
The Nervous System. Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output
 The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
Physiology of the nerve
 Physiology of the nerve Objectives Transmembrane potential Action potential Relative and absolute refractory period The all-or-none law Hoorweg Weiss curve Du Bois Reymond principle Types of nerve fibres
Physiology of the nerve Objectives Transmembrane potential Action potential Relative and absolute refractory period The all-or-none law Hoorweg Weiss curve Du Bois Reymond principle Types of nerve fibres
Major Structures of the Nervous System. Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors
 Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
Major Structures of the Nervous System Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Nervous System Divisions Central Nervous System (CNS) consists
Chapter 17 Nervous System
 Chapter 17 Nervous System 1 The Nervous System Two Anatomical Divisions Central Nervous System (CNS) Brain and Spinal Cord Peripheral Nervous System (PNS) Two Types of Cells Neurons Transmit nerve impulses
Chapter 17 Nervous System 1 The Nervous System Two Anatomical Divisions Central Nervous System (CNS) Brain and Spinal Cord Peripheral Nervous System (PNS) Two Types of Cells Neurons Transmit nerve impulses
Chapter 11: Nervous System and Nervous Tissue
 Chapter 11: Nervous System and Nervous Tissue I. Functions and divisions of the nervous system A. Sensory input: monitor changes in internal and external environment B. Integrations: make decisions about
Chapter 11: Nervous System and Nervous Tissue I. Functions and divisions of the nervous system A. Sensory input: monitor changes in internal and external environment B. Integrations: make decisions about
Chapter 7. The Nervous System: Structure and Control of Movement
 Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
Chapter 7. Objectives
 Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
Chapter 7 The Nervous System: Structure and Control of Movement Objectives Discuss the general organization of the nervous system Describe the structure & function of a nerve Draw and label the pathways
The Nervous System PART A
 7 The Nervous System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Structural Classification
7 The Nervous System PART A PowerPoint Lecture Slide Presentation by Jerry L. Cook, Sam Houston University ESSENTIALS OF HUMAN ANATOMY & PHYSIOLOGY EIGHTH EDITION ELAINE N. MARIEB Structural Classification
The Praxis FES System and Bladder/Bowel Management in Patients with Spinal Cord Injury
 The Praxis FES System and Bladder/Bowel Management in Patients with Spinal Cord Injury Brian J. Benda 1, Thierry Houdayer 2, Graham Creasey 3, Randal R. Betz 1, Brian T. Smith 1 *, Therese E. Johnston
The Praxis FES System and Bladder/Bowel Management in Patients with Spinal Cord Injury Brian J. Benda 1, Thierry Houdayer 2, Graham Creasey 3, Randal R. Betz 1, Brian T. Smith 1 *, Therese E. Johnston
Simulation of myelinated neuron with focus on conduction speed and changeable excitability
 Simulation of myelinated neuron with focus on conduction speed and changeable excitability Pengfei Chen Sung Min Kim Abstract In this paper we focus on the two particular properties of myelinated neuron,
Simulation of myelinated neuron with focus on conduction speed and changeable excitability Pengfei Chen Sung Min Kim Abstract In this paper we focus on the two particular properties of myelinated neuron,
Nervous System. Unit 6.6 (6 th Edition) Chapter 7.6 (7 th Edition)
 Nervous System Unit 6.6 (6 th Edition) Chapter 7.6 (7 th Edition) 1 Learning Objectives Identify the main parts (anatomy) of a neuron. Identify the 2 divisions of nervous system. Classify the major types
Nervous System Unit 6.6 (6 th Edition) Chapter 7.6 (7 th Edition) 1 Learning Objectives Identify the main parts (anatomy) of a neuron. Identify the 2 divisions of nervous system. Classify the major types
Fundamentals of the Nervous System and Nervous Tissue. Nervous System. Basic Divisions of the Nervous System C H A P T E R 12.
 C H A P T E R 12 Fundamentals of the Nervous System and Nervous Tissue Nervous System Sensory input Integration Motor output Figure 12.1 Basic Divisions of the Nervous System Brain CNS Spinal cord Nerves
C H A P T E R 12 Fundamentals of the Nervous System and Nervous Tissue Nervous System Sensory input Integration Motor output Figure 12.1 Basic Divisions of the Nervous System Brain CNS Spinal cord Nerves
Nervous Systems: Diversity & Functional Organization
 Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Chapter 7 Nerve Cells and Electrical Signaling
 Chapter 7 Nerve Cells and Electrical Signaling 7.1. Overview of the Nervous System (Figure 7.1) 7.2. Cells of the Nervous System o Neurons are excitable cells which can generate action potentials o 90%
Chapter 7 Nerve Cells and Electrical Signaling 7.1. Overview of the Nervous System (Figure 7.1) 7.2. Cells of the Nervous System o Neurons are excitable cells which can generate action potentials o 90%
Regulation of the Urinary Bladder Chapter 26
 Regulation of the Urinary Bladder Chapter 26 Anatomy 1. The urinary bladder is smooth muscle lined internally by transitional epithelium and externally by the parietal peritoneum. Contraction of the smooth
Regulation of the Urinary Bladder Chapter 26 Anatomy 1. The urinary bladder is smooth muscle lined internally by transitional epithelium and externally by the parietal peritoneum. Contraction of the smooth
35-2 The Nervous System Slide 1 of 38
 1 of 38 35-2 The Nervous System The nervous system controls and coordinates functions throughout the body and responds to internal and external stimuli. 2 of 38 Neurons Neurons The messages carried by
1 of 38 35-2 The Nervous System The nervous system controls and coordinates functions throughout the body and responds to internal and external stimuli. 2 of 38 Neurons Neurons The messages carried by
Ameen Alsaras. Ameen Alsaras. Mohd.Khatatbeh
 9 Ameen Alsaras Ameen Alsaras Mohd.Khatatbeh Nerve Cells (Neurons) *Remember: The neural cell consists of: 1-Cell body 2-Dendrites 3-Axon which ends as axon terminals. The conduction of impulse through
9 Ameen Alsaras Ameen Alsaras Mohd.Khatatbeh Nerve Cells (Neurons) *Remember: The neural cell consists of: 1-Cell body 2-Dendrites 3-Axon which ends as axon terminals. The conduction of impulse through
Signal transduction underlying the control of urinary bladder smooth muscle tone Puspitoayu, E.
 UvA-DARE (Digital Academic Repository) Signal transduction underlying the control of urinary bladder smooth muscle tone Puspitoayu, E. Link to publication Citation for published version (APA): Puspitoayu,
UvA-DARE (Digital Academic Repository) Signal transduction underlying the control of urinary bladder smooth muscle tone Puspitoayu, E. Link to publication Citation for published version (APA): Puspitoayu,
Nervous System. Master controlling and communicating system of the body. Secrete chemicals called neurotransmitters
 Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Nervous System Master controlling and communicating system of the body Interacts with the endocrine system to control and coordinate the body s responses to changes in its environment, as well as growth,
Neuromodulation and neurostimulation: overview and future potential
 Perspective Neuromodulation and neurostimulation: overview and future potential Emil A. Tanagho Department of Urology, University of California, San Francisco Medical Center, San Francisco, California,
Perspective Neuromodulation and neurostimulation: overview and future potential Emil A. Tanagho Department of Urology, University of California, San Francisco Medical Center, San Francisco, California,
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 TEXTBOOK AND REQUIRED/RECOMMENDED
Chapter Six Review Sections 1 and 2
 NAME PER DATE Chapter Six Review Sections 1 and 2 Matching: 1. afferent nerves 2. autonomic nervous system 3. cell body 4. central nervous system (CNS) 5. dendrites 6. efferent nerves 7. myelin sheath
NAME PER DATE Chapter Six Review Sections 1 and 2 Matching: 1. afferent nerves 2. autonomic nervous system 3. cell body 4. central nervous system (CNS) 5. dendrites 6. efferent nerves 7. myelin sheath
Neural Control of Lower Urinary Tract Function. William C. de Groat University of Pittsburgh Medical School
 Neural Control of Lower Urinary Tract Function William C. de Groat University of Pittsburgh Medical School Disclosures Current funding: NIH Grants, DK093424, DK-091253, DK-094905, DK-090006. Other financial
Neural Control of Lower Urinary Tract Function William C. de Groat University of Pittsburgh Medical School Disclosures Current funding: NIH Grants, DK093424, DK-091253, DK-094905, DK-090006. Other financial
Storage is accomplished through the following mechanisms:
 NROSCI/BIOSC 1070 and MSNBIO 2070 September 13, 2017 Examples of Coordinated Autonomic and Motor Responses and Return to the Cardiovascular System 1) Micturition Micturition, or the process of emptying
NROSCI/BIOSC 1070 and MSNBIO 2070 September 13, 2017 Examples of Coordinated Autonomic and Motor Responses and Return to the Cardiovascular System 1) Micturition Micturition, or the process of emptying
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Principles of Electrical Currents. HuP 272
 Principles of Electrical Currents HuP 272 Electricity is an element of PT modalities most frightening and least understood. Understanding the basis principles will later aid you in establishing treatment
Principles of Electrical Currents HuP 272 Electricity is an element of PT modalities most frightening and least understood. Understanding the basis principles will later aid you in establishing treatment
Introduction to Neurobiology
 Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
Biology 240 General Zoology Introduction to Neurobiology Nervous System functions: communication of information via nerve signals integration and processing of information control of physiological and
NERVOUS SYSTEM 1 CHAPTER 10 BIO 211: ANATOMY & PHYSIOLOGY I
 BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A This set Ch 10 B CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A This set Ch 10 B CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
Overview of the Nervous System A. Subdivisions of the Nervous System: 1. The two major subdivisions of the nervous system:
 BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A This set Ch 10 B CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A This set Ch 10 B CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
The Spinal Cord & Spinal Nerves
 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward and downward travel
The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward and downward travel
Functions of the Nervous System. Fundamentals of the Nervous System & Nervous Tissue
 Fundamentals of the Nervous System & Nervous Tissue Overview Structure cell types & structures Neurophysiology membrane potential Synapse, neurotransmitters & receptors Functions of the Nervous System
Fundamentals of the Nervous System & Nervous Tissue Overview Structure cell types & structures Neurophysiology membrane potential Synapse, neurotransmitters & receptors Functions of the Nervous System
Chapter 7 Nervous System
 Chapter 7 Nervous System Two message centers: Functions of these systems: 1. * 2. * Overview of the Nervous System Parts: General Functions: Functions Sensory input: Sensation via nerves Integration: interpretation
Chapter 7 Nervous System Two message centers: Functions of these systems: 1. * 2. * Overview of the Nervous System Parts: General Functions: Functions Sensory input: Sensation via nerves Integration: interpretation
Impact of Demyelination Disease on Neuronal Networks
 Impact of Demyelination Disease on Neuronal Networks Sandeep Adem Chiyuan Chang Mark Fleming sadem@eng.ucsd.edu chc418@eng.ucsd.edu m3flemin@eng.ucsd.edu 1. Abstract Demyelination has a detrimental impact
Impact of Demyelination Disease on Neuronal Networks Sandeep Adem Chiyuan Chang Mark Fleming sadem@eng.ucsd.edu chc418@eng.ucsd.edu m3flemin@eng.ucsd.edu 1. Abstract Demyelination has a detrimental impact
BIONB/BME/ECE 4910 Neuronal Simulation Assignments 1, Spring 2013
 BIONB/BME/ECE 4910 Neuronal Simulation Assignments 1, Spring 2013 Tutorial Assignment Page Due Date Week 1/Assignment 1: Introduction to NIA 1 January 28 The Membrane Tutorial 9 Week 2/Assignment 2: Passive
BIONB/BME/ECE 4910 Neuronal Simulation Assignments 1, Spring 2013 Tutorial Assignment Page Due Date Week 1/Assignment 1: Introduction to NIA 1 January 28 The Membrane Tutorial 9 Week 2/Assignment 2: Passive
APPLICATION OF HIGH FREQUENCY ELECTRICAL BLOCK ON THE EFFERENT NERVES TO THE LOWER URINARY TRACT FOR BLADDER VOIDING.
 i APPLICATION OF HIGH FREQUENCY ELECTRICAL BLOCK ON THE EFFERENT NERVES TO THE LOWER URINARY TRACT FOR BLADDER VOIDING By Adam Sprott Boger Submitted in partial fulfillment of the requirements for the
i APPLICATION OF HIGH FREQUENCY ELECTRICAL BLOCK ON THE EFFERENT NERVES TO THE LOWER URINARY TRACT FOR BLADDER VOIDING By Adam Sprott Boger Submitted in partial fulfillment of the requirements for the
2/27/2019. Functions of the Nervous System. Nervous Tissue and Neuron Function. Fundamentals Of The Nervous System And Nervous Tissue
 Nervous Tissue and Neuron Function Fundamentals Of The Nervous System And Nervous Tissue Learn and Understand 1. Like muscle cells, neurons use membrane polarity upset (AP) as a signal therefore keeping
Nervous Tissue and Neuron Function Fundamentals Of The Nervous System And Nervous Tissue Learn and Understand 1. Like muscle cells, neurons use membrane polarity upset (AP) as a signal therefore keeping
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM
 STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
STRUCTURAL ELEMENTS OF THE NERVOUS SYSTEM STRUCTURE AND MAINTENANCE OF NEURONS (a) (b) Dendrites Cell body Initial segment collateral terminals (a) Diagrammatic representation of a neuron. The break in
The Nervous System 12/11/2015
 The Nervous System Biology 12 Unit 3: Homeostasis December 11, 2015 The nervous system is an elaborate communication system that contains more than 100 billion nerve cells in the brain alone There are
The Nervous System Biology 12 Unit 3: Homeostasis December 11, 2015 The nervous system is an elaborate communication system that contains more than 100 billion nerve cells in the brain alone There are
Shock-induced termination of cardiac arrhythmias
 Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Cardiac arrhythmias, also known as irregular heartbeat, occur
Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Cardiac arrhythmias, also known as irregular heartbeat, occur
Endocrine System Nervous System
 Cells Endocrine System Nervous System Tissues Controls Organs Nervous System vs Endocrine System Electrical signals (graded potentials and action potentials) and chemical signals (neurotransmitters) Fast
Cells Endocrine System Nervous System Tissues Controls Organs Nervous System vs Endocrine System Electrical signals (graded potentials and action potentials) and chemical signals (neurotransmitters) Fast
Spinal Cord Protection. Chapter 13 The Spinal Cord & Spinal Nerves. External Anatomy of Spinal Cord. Structures Covering the Spinal Cord
 Spinal Cord Protection Chapter 13 The Spinal Cord & Spinal Nerves We are only going to cover Pages 420-434 and 447 Together with brain forms the CNS Functions spinal cord reflexes integration (summation
Spinal Cord Protection Chapter 13 The Spinal Cord & Spinal Nerves We are only going to cover Pages 420-434 and 447 Together with brain forms the CNS Functions spinal cord reflexes integration (summation
TREATMENT METHODS FOR DISORDERS OF SMALL ANIMAL BLADDER FUNCTION
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk TREATMENT METHODS FOR DISORDERS OF SMALL ANIMAL BLADDER FUNCTION Author : SIMONA T RADAELLI Categories : Vets Date : July
Vet Times The website for the veterinary profession https://www.vettimes.co.uk TREATMENT METHODS FOR DISORDERS OF SMALL ANIMAL BLADDER FUNCTION Author : SIMONA T RADAELLI Categories : Vets Date : July
Neurophysiology of Nerve Impulses
 M52_MARI0000_00_SE_EX03.qxd 8/22/11 2:47 PM Page 358 3 E X E R C I S E Neurophysiology of Nerve Impulses Advance Preparation/Comments Consider doing a short introductory presentation with the following
M52_MARI0000_00_SE_EX03.qxd 8/22/11 2:47 PM Page 358 3 E X E R C I S E Neurophysiology of Nerve Impulses Advance Preparation/Comments Consider doing a short introductory presentation with the following
Nervous System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University
 Nervous System Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly adopted from the
Nervous System Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly adopted from the
A. Subdivisions of the Nervous System: 1. The two major subdivisions of the nervous system:
 BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A Ch 10 B CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
BIO 211: ANATOMY & PHYSIOLOGY I 1 Ch 10 A Ch 10 B CHAPTER 10 NERVOUS SYSTEM 1 BASIC STRUCTURE and FUNCTION Dr. Lawrence G. Altman www.lawrencegaltman.com Some illustrations are courtesy of McGraw-Hill.
Lecture 14: The Spinal Cord
 Lecture 14: The Spinal Cord M/O Chapters 16 69. Describe the relationship(s) between the following structures: root, nerve, ramus, plexus, tract, nucleus, and ganglion. 70. Trace the path of information
Lecture 14: The Spinal Cord M/O Chapters 16 69. Describe the relationship(s) between the following structures: root, nerve, ramus, plexus, tract, nucleus, and ganglion. 70. Trace the path of information
Outline. Neuron Structure. Week 4 - Nervous System. The Nervous System: Neurons and Synapses
 Outline Week 4 - The Nervous System: Neurons and Synapses Neurons Neuron structures Types of neurons Electrical activity of neurons Depolarization, repolarization, hyperpolarization Synapses Release of
Outline Week 4 - The Nervous System: Neurons and Synapses Neurons Neuron structures Types of neurons Electrical activity of neurons Depolarization, repolarization, hyperpolarization Synapses Release of
Chapter 11: Functional Organization of Nervous Tissue
 Chapter 11: Functional Organization of Nervous Tissue I. Functions of the Nervous System A. List and describe the five major nervous system functions: 1. 2. 3. 4. 5. II. Divisions of the Nervous System
Chapter 11: Functional Organization of Nervous Tissue I. Functions of the Nervous System A. List and describe the five major nervous system functions: 1. 2. 3. 4. 5. II. Divisions of the Nervous System
Nervous System. Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI
 Nervous System Overview.I Histology.II Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI Repairs.VII Pathology.VIII.IV 1 Controls and integrates all body activities
Nervous System Overview.I Histology.II Electrical Signals.III Signal Transmission at Synapses Neurotransmitters.V Neural Circuits.VI Repairs.VII Pathology.VIII.IV 1 Controls and integrates all body activities
Compound Action Potential, CAP
 Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
Stimulus Strength UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY INTRODUCTION TO NEUROPHYSIOLOGY Spring, 2013 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition
Functions of Nervous System Neuron Structure
 Chapter 10 Nervous System I Divisions of the Nervous System Cell Types of Neural Tissue neurons neuroglial cells Central Nervous System brain spinal cord Peripheral Nervous System nerves cranial nerves
Chapter 10 Nervous System I Divisions of the Nervous System Cell Types of Neural Tissue neurons neuroglial cells Central Nervous System brain spinal cord Peripheral Nervous System nerves cranial nerves
Objectives. Key Outlines:
 Objectives! Iden8fy and describe the Func8onal Anatomy of Urinary Bladder! Describe the mechanism of filling and emptying of the urinary bladder! Cystometrogram! Appreciate neurogenic control of the mechanism
Objectives! Iden8fy and describe the Func8onal Anatomy of Urinary Bladder! Describe the mechanism of filling and emptying of the urinary bladder! Cystometrogram! Appreciate neurogenic control of the mechanism
Chapter 23. Micturition and Renal Insufficiency
 Chapter 23 Micturition and Renal Insufficiency Voiding Urine Between acts of urination, the bladder is filling. detrusor muscle relaxes urethral sphincters are tightly closed accomplished by sympathetic
Chapter 23 Micturition and Renal Insufficiency Voiding Urine Between acts of urination, the bladder is filling. detrusor muscle relaxes urethral sphincters are tightly closed accomplished by sympathetic
D) around, bypassing B) toward
 Nervous System Practice Questions 1. Which of the following are the parts of neurons? A) brain, spinal cord, and vertebral column B) dendrite, axon, and cell body C) sensory and motor D) cortex, medulla
Nervous System Practice Questions 1. Which of the following are the parts of neurons? A) brain, spinal cord, and vertebral column B) dendrite, axon, and cell body C) sensory and motor D) cortex, medulla
Development of mathematical model for lower urinary tract dysfunctions
 Development of mathematical model for lower urinary tract dysfunctions Mohanad A.Deaf 1, Mohamed A.A.Eldosoky 1, Ahmed M. El-Garhy 2, Hesham W.Gomma 2, Ahmed S.El-Azab 3 Dept. of Biomedical Engineering,
Development of mathematical model for lower urinary tract dysfunctions Mohanad A.Deaf 1, Mohamed A.A.Eldosoky 1, Ahmed M. El-Garhy 2, Hesham W.Gomma 2, Ahmed S.El-Azab 3 Dept. of Biomedical Engineering,
Systems Neuroscience November 21, 2017 The autonomic nervous system
 Systems Neuroscience November 21, 2017 The autonomic nervous system Daniel C. Kiper kiper@ini.phys.ethz.ch http: www.ini.unizh.ch/~kiper/system_neurosci.html How is the organization of the autonomic nervous
Systems Neuroscience November 21, 2017 The autonomic nervous system Daniel C. Kiper kiper@ini.phys.ethz.ch http: www.ini.unizh.ch/~kiper/system_neurosci.html How is the organization of the autonomic nervous
Chapter 12 Nervous Tissue. Copyright 2009 John Wiley & Sons, Inc. 1
 Chapter 12 Nervous Tissue Copyright 2009 John Wiley & Sons, Inc. 1 Terms to Know CNS PNS Afferent division Efferent division Somatic nervous system Autonomic nervous system Sympathetic nervous system Parasympathetic
Chapter 12 Nervous Tissue Copyright 2009 John Wiley & Sons, Inc. 1 Terms to Know CNS PNS Afferent division Efferent division Somatic nervous system Autonomic nervous system Sympathetic nervous system Parasympathetic
Information Processing During Transient Responses in the Crayfish Visual System
 Information Processing During Transient Responses in the Crayfish Visual System Christopher J. Rozell, Don. H. Johnson and Raymon M. Glantz Department of Electrical & Computer Engineering Department of
Information Processing During Transient Responses in the Crayfish Visual System Christopher J. Rozell, Don. H. Johnson and Raymon M. Glantz Department of Electrical & Computer Engineering Department of
Questions. Question 1!
 Questions Question 1 In a laboratory, scientists often study neurons in isolation, outside of a living creature, in a dish. In this setting, one can have a good deal of control over the local ionic environment
Questions Question 1 In a laboratory, scientists often study neurons in isolation, outside of a living creature, in a dish. In this setting, one can have a good deal of control over the local ionic environment
You can follow the path of the neural signal. The sensory neurons detect a stimulus in your finger and send that information to the CNS.
 1 Nervous system maintains coordination through the use of electrical and chemical processes. There are three aspects: sensory, motor, and integrative, which we will discuss throughout the system. The
1 Nervous system maintains coordination through the use of electrical and chemical processes. There are three aspects: sensory, motor, and integrative, which we will discuss throughout the system. The
Omar Sami. Muhammad Abid. Muhammad khatatbeh
 10 Omar Sami Muhammad Abid Muhammad khatatbeh Let s shock the world In this lecture we are going to cover topics said in previous lectures and then start with the nerve cells (neurons) and the synapses
10 Omar Sami Muhammad Abid Muhammad khatatbeh Let s shock the world In this lecture we are going to cover topics said in previous lectures and then start with the nerve cells (neurons) and the synapses
Introduction to Physiological Psychology
 Introduction to Physiological Psychology Review Kim Sweeney ksweeney@cogsci.ucsd.edu www.cogsci.ucsd.edu/~ksweeney/psy260.html Today n Discuss Final Paper Proposal (due 3/10) n General Review 1 The article
Introduction to Physiological Psychology Review Kim Sweeney ksweeney@cogsci.ucsd.edu www.cogsci.ucsd.edu/~ksweeney/psy260.html Today n Discuss Final Paper Proposal (due 3/10) n General Review 1 The article
EE 791 Lecture 10. FES April 1, EE 791 Lecture 10 1
 EE 791 Lecture 10 FES April 1, 2013 EE 791 Lecture 10 1 Normal Functional Control EE 791 Lecture 10 2 Current uses of FES Cardiovascular Exercise Breathing assist Grasping and Reaching Transfer and Standing
EE 791 Lecture 10 FES April 1, 2013 EE 791 Lecture 10 1 Normal Functional Control EE 791 Lecture 10 2 Current uses of FES Cardiovascular Exercise Breathing assist Grasping and Reaching Transfer and Standing
HUMAN MOTOR CONTROL. Emmanuel Guigon
 HUMAN MOTOR CONTROL Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris, France emmanuel.guigon@upmc.fr e.guigon.free.fr/teaching.html
HUMAN MOTOR CONTROL Emmanuel Guigon Institut des Systèmes Intelligents et de Robotique Université Pierre et Marie Curie CNRS / UMR 7222 Paris, France emmanuel.guigon@upmc.fr e.guigon.free.fr/teaching.html
Development of Ultrasound Based Techniques for Measuring Skeletal Muscle Motion
 Development of Ultrasound Based Techniques for Measuring Skeletal Muscle Motion Jason Silver August 26, 2009 Presentation Outline Introduction Thesis Objectives Mathematical Model and Principles Methods
Development of Ultrasound Based Techniques for Measuring Skeletal Muscle Motion Jason Silver August 26, 2009 Presentation Outline Introduction Thesis Objectives Mathematical Model and Principles Methods
(10) Patent No.: US 6,836,684 B1
 (12) United States Patent Rijkhoff et al. US006836684B1 (10) Patent No.: US 6,836,684 B1 (45) Date of Patent: Dec. 28, 2004 (54) (75) (73) (*) (21) (22) (86) (87) (51) (52) (58) (56) METHOD TO CONTROLAN
(12) United States Patent Rijkhoff et al. US006836684B1 (10) Patent No.: US 6,836,684 B1 (45) Date of Patent: Dec. 28, 2004 (54) (75) (73) (*) (21) (22) (86) (87) (51) (52) (58) (56) METHOD TO CONTROLAN
sensory input receptors integration Human Anatomy motor output Ch. 7 effectors Structural classification
 Human Anatomy Ch. 7 I. The Nervous System A. General characteristics 1. body s control & communication center a. 3 overlapping functions 1) sensory input: receptors monitor stimuli 2) integration: processes,
Human Anatomy Ch. 7 I. The Nervous System A. General characteristics 1. body s control & communication center a. 3 overlapping functions 1) sensory input: receptors monitor stimuli 2) integration: processes,
NERVOUS SYSTEM CELLS. a. afferent division CHAPTER 12 ORGANIZATION OF THE NERVOUS SYSTEM. Student Name
 Student Name CHAPTER 12 NERVOUS SYSTEM CELLS T he nervous system organizes and coordinates the millions of impulses received each day to make communication with and enjoyment of our environment possible.
Student Name CHAPTER 12 NERVOUS SYSTEM CELLS T he nervous system organizes and coordinates the millions of impulses received each day to make communication with and enjoyment of our environment possible.
(Received 10 April 1956)
 446 J. Physiol. (I956) I33, 446-455 A COMPARISON OF FLEXOR AND EXTENSOR REFLEXES OF MUSCULAR ORIGIN BY M. G. F. FUORTES AND D. H. HUBEL From the Department ofneurophysiology, Walter Reed Army Institute
446 J. Physiol. (I956) I33, 446-455 A COMPARISON OF FLEXOR AND EXTENSOR REFLEXES OF MUSCULAR ORIGIN BY M. G. F. FUORTES AND D. H. HUBEL From the Department ofneurophysiology, Walter Reed Army Institute
PNS and ANS Flashcards
 1. Name several SOMATIC SENSES Light touch (being touched by a feather), heat, cold, vibration, pressure, pain are SOMATIC SENSES. 2. What are proprioceptors; and how is proprioception tested? PROPRIOCEPTORS
1. Name several SOMATIC SENSES Light touch (being touched by a feather), heat, cold, vibration, pressure, pain are SOMATIC SENSES. 2. What are proprioceptors; and how is proprioception tested? PROPRIOCEPTORS
BIOL241 - Lecture 12a
 Cranial Nerves, source: training.seer.cancer.gov Nervous System Overview BIOL241 - Lecture 12a 1 Topics Divisions of the NS: CNS and PNS Structure and types of neurons Synapses Structure and function of
Cranial Nerves, source: training.seer.cancer.gov Nervous System Overview BIOL241 - Lecture 12a 1 Topics Divisions of the NS: CNS and PNS Structure and types of neurons Synapses Structure and function of
211MDS Pain theories
 211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
Warm-Up. Label the parts of the neuron below.
 Warm-Up Label the parts of the neuron below. A B C D E F G Warm-Up 1. One neuron transmits a nerve impulse at 40 m/s. Another conducts at the rate of 1 m/s. Which neuron has a myelinated axon? 2. List
Warm-Up Label the parts of the neuron below. A B C D E F G Warm-Up 1. One neuron transmits a nerve impulse at 40 m/s. Another conducts at the rate of 1 m/s. Which neuron has a myelinated axon? 2. List
Chapter 13. The Spinal Cord & Spinal Nerves. Spinal Cord. Spinal Cord Protection. Meninges. Together with brain forms the CNS Functions
 Spinal Cord Chapter 13 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward
Spinal Cord Chapter 13 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward
Minimal invasive electrode implantation for conditional stimulation of the dorsal genital nerve in neurogenic detrusor overactivity
 (2011) 49, 566 572 & 2011 International Society All rights reserved 1362-4393/11 $32.00 www.nature.com/sc ORIGINAL ARTICLE Minimal invasive electrode implantation for conditional stimulation of the dorsal
(2011) 49, 566 572 & 2011 International Society All rights reserved 1362-4393/11 $32.00 www.nature.com/sc ORIGINAL ARTICLE Minimal invasive electrode implantation for conditional stimulation of the dorsal
Functions of the Nervous System
 The Nervous System Functions of the Nervous System 1. Control center for all body activities 2. Responds and adapts to changes that occur both inside and outside the body (Ex: pain, temperature, pregnancy)
The Nervous System Functions of the Nervous System 1. Control center for all body activities 2. Responds and adapts to changes that occur both inside and outside the body (Ex: pain, temperature, pregnancy)
