RIG-I Detects Viral Genomic RNA during Negative-Strand RNA Virus Infection
|
|
|
- Damon Golden
- 5 years ago
- Views:
Transcription
1 RIG-I Detects Viral Genomic RNA during Negative-Strand RNA Virus Infection Jan Rehwinkel,,5 Choon Ping Tan,,2,5 Delphine Goubau, Oliver Schulz, Andreas Pichlmair,,4 Katja Bier, 2 Nicole Robb, 2 Frank Vreede, 2 Wendy Barclay, 3 Ervin Fodor, 2 and Caetano Reis e Sousa, * Immunobiology Laboratory, Cancer Research UK London Research Institute, 44 Lincoln s Inn Fields, London WC2A 3PX, UK 2 Sir William Dunn School of Pathology, University of Oxford, South Parks Road, Oxford OX 3RE, UK 3 Department of Virology, Imperial College, St. Mary s Campus, Norfolk Place, London W2 PG, UK 4 Present address: Research Center for Molecular Medicine of the Austrian Academy of Sciences (CeMM), Lazarettgasse 9/3, A-9 Vienna, Austria 5 These authors contributed equally to this work *Correspondence: caetano@cancer.org.uk DOI.6/j.cell.2..2 SUMMARY RIG-I is a key mediator of antiviral immunity, able to couple detection of infection by RNA viruses to the induction of interferons. Natural RIG-I stimulatory RNAs have variously been proposed to correspond to virus genomes, virus replication intermediates, viral transcripts, or self-rna cleaved by RNase L. However, the relative contribution of each of these RNA species to RIG-I activation and interferon induction in virus-infected cells is not known. Here, we use three approaches to identify physiological RIG-I agonists in cells infected with influenza A virus or Sendai virus. We show that RIG-I agonists are exclusively generated by the process of virus replication and correspond to full-length virus genomes. Therefore, nongenomic viral transcripts, short replication intermediates, and cleaved self-rna do not contribute substantially to interferon induction in cells infected with these negative strand RNA viruses. Rather, single-stranded RNA viral genomes bearing 5 -triphosphates constitute the natural RIG-I agonists that trigger cell-intrinsic innate immune responses during infection. INTRODUCTION Vertebrates possess a variety of defense mechanisms to detect, contain, and clear viral infections. Chief among these is the interferon (IFN) system, which plays a key role in inducing an antiviral state and contributes to the subsequent antigen specific adaptive immune response (Samuel, 2). Type I IFNs (IFN-a and -b, hereafter simply referred to as IFN) and type III IFNs are induced very rapidly in all cell types by receptors that monitor the cytosol for the presence of nucleic acids indicative of virus presence. Such receptors include RIG-I-like receptors (RLRs) that recognize RNA and are themselves IFN inducible (Yoneyama and Fujita, 29). The three members of this family RIG-I (retinoic acid-inducible gene I), MDA5 (melanoma differentiation factor-5) and LGP-2 (laboratory of genetics and physiology-2) all contain a DExD/H-box RNA helicase domain. RIG-I and MDA5 additionally posses two N-terminal caspase activation and recruitment domains that allow for interaction with the mitochondrial adaptor protein MAVS (Yoneyama and Fujita, 29). MAVS triggers the activation of NF-kB, IRF-3, and IRF-7, which in turn induce transcription of IFNs and other innate response genes. Notably, mice deficient in RIG-I, MDA5, or MAVS readily succumb to infection with RNA viruses, highlighting the importance of RLRs in antiviral defense (Gitlin et al., 26; Kato et al., 26; Kumar et al., 26). Total RNA extracted from virally infected cells can stimulate specific RLRs (Hornung et al., 26; Kato et al., 28; Pichlmair et al., 29). For example, RNA from cells infected with influenza A virus (flu) potently induces IFN-b when transfected into wild-type or MDA5-deficient, but not RIG-I-deficient mouse embryonic fibroblasts (Kato et al., 28). However, the actual stimulatory RNA molecules within these pools remain largely unidentified. Instead, RLR agonists have been defined with chemically or enzymatically synthesized nucleic acids (reviewed in Schlee et al., 29a). We and others identified RNAs transcribed in vitro by phage polymerases as potent RIG-I agonists (Hornung et al., 26; Pichlmair et al., 26). These RNAs carry a 5 -triphosphate (5 -PPP) moiety that is absolutely required for their activity (Hornung et al., 26; Pichlmair et al., 26). Other synthetic RIG-I agonists lack 5 -PPPs. These include poly I:C, which is prepared by annealing inosine and cytosine polymers that have monophosphate or diphosphate 5 ends (Grunberg-Manago et al., 955). Although long poly I:C activates MDA5 (Gitlin et al., 26; Kato et al., 26), short poly I:C (2 nt) is reported to trigger RIG-I (Kato et al., 28). Chemically synthesized RNA oligonucleotides 7 or 25 nt long and lacking 5 -PPPs also trigger RIG-I when annealed to a complementary strand (Kato et al., 28; Takahasi et al., 28). Thus, data obtained with synthetic RNAs suggest the possibility that there may be distinct types of RIG-I triggers in virally infected cells, including RNAs bearing 5 -PPPs or not and composed of either a single strand or two short complementary RNA strands. Cell 4, , February 5, 2 ª2 Elsevier Inc. 397
2 In addition to synthetic RNAs, some natural RNAs also serve as RIG-I agonists. For example, RIG-I-dependent IFN production can be observed in response to transfection with genomic RNA from viruses such as flu or rabies virus that bear 5 -PPPs but not genomes of viruses that have no 5 -phosphates, such as encephalomyocarditis virus, or that have a 5 -monophosphate, such as Hantaan virus, Crimean-Congo hemorrhagic fever virus, and Borna disease virus (Habjan et al., 28; Hornung et al., 26; Pichlmair et al., 26). This has led to the hypothesis that, in infected cells, RIG-I may be activated by viral genomes bearing 5 -PPPs. However, transfection of naked viral RNA does not mimic infection, and viral genomes in infected cells are in the form of viral ribonucleoprotein particles (vrnps) in which viral proteins may prevent access of RIG-I to the RNA. For example, the flu polymerase binds to the 5 end of the viral genome and is predicted to obscure the 5 -PPP necessary for RIG-I activation (Fodor et al., 994; Tiley et al., 994). Further doubt on the notion that RIG-I is primarily activated by viral genomes has come from reports that measles virus and Epstein-Barr virus transcripts (Plumet et al., 27; Samanta et al., 26), as well as products of host RNA cleavage by RNase L bearing 3 -monophosphates (Malathi et al., 27), serve as the triggers for RIG-I in virally infected cells. Thus, the identification of natural RNA molecules with the potential to activate RIG-I has not clarified the identity of the actual RIG-I stimulus responsible for initiating antiviral immunity. As such, there is a pressing need to study relevant RIG-I agonists isolated from virally infected cells as opposed to characterizing the types of synthetic or natural RNA that can activate RIG-I in experimental models. RIG-I is indispensable for IFN responses to negative-strand RNA viruses, including Sendai virus (SeV) and flu (Kato et al., 26). SeV has a nonsegmented genome consisting of a single RNA molecule and belongs to the paramyxoviridae family. This virus family includes important human pathogens such as measles, mumps, and respiratory syncytial virus. Flu is a segmented RNA virus, and annual flu epidemics result in an estimated 25, 5, deaths worldwide (Kilbourne, 26). In addition, the ability of flu to infect different mammalian and avian species and generate reassortants constantly poses the threat that a new highly pathogenic virus will emerge, leading to a pandemic outbreak. Notably, the virulence of some flu strains is due, at least in part, to a deregulation of the innate immune response (Maines et al., 28). Therefore, understanding how RIG-I becomes activated during infection with flu and other RNA viruses not only is of basic research interest but may also allow the development of new ways of containing viral spread and preventing disease. Here, we characterize the RNA species responsible for activating RIG-I in cells infected with flu or SeV. Reconstitution of flu vrnps in cell culture showed that only 5 -PPP-bearing viral genomic RNA triggered RIG-I. Furthermore, isolation of RIG-I complexes from infected cells revealed the presence of fulllength viral genomes that accounted for stimulatory activity. Taken together, our data show that 5 -PPP-bearing viral genomes rather than short double-stranded RNAs, viral transcripts, or cleaved self-rna constitute the physiological source of RIG-I stimulation and IFN induction during infection with negativestrand RNA viruses. RESULTS Reconstitution of Influenza A Virus vrnps Induces IFN-a/b To simplify the search for RIG-I agonists in flu-infected cells, we started with a mock infection system involving reconstitution of vrnps (Fodor et al., 22; Pleschka et al., 996) (Figure A). We confirmed expression of viral RNA (vrna), complementary RNA (crna), and messenger RNA (mrna) in vrnp reconstitution experiments with each of the eight PR8 genome segments (Figure SA available online). We then tested the IFN-inducing activity of total RNA from vrnp reconstitution experiments in an IFN-b promoter luciferase reporter assay (Figure B). RNA isolated from nontransfected cells (no TF) or from cells expressing the viral polymerase but no genome segment (no template) did not induce reporter activity (Figure B). However, RNA extracted from cells expressing the wild-type viral polymerase and any of the eight genome segments was stimulatory (Figure B), as reported for RNA isolated from flu-infected cells (Kato et al., 28). Cells expressing a polymerase mutated in its active site (PBa) did not accumulate stimulatory RNA (Figure C). In addition, the stimulatory activity of total cellular RNA from vrnp reconstitutions, like that of in vitro-transcribed (IVT) RNA, was RIG-I dependent (Figure D). The accumulation of stimulatory RNA in transfected cells was accompanied by RIG-I-dependent secretion of IFN-a/b into the culture medium, although this was not always detectable unless the cells were pretreated with IFN to upregulate RIG-I and downstream mediators prior to transfection (Figures E and F; see below for NS segment). In sum, like live infection, flu vrnp reconstitution induces RIG-I-dependent production of IFN and promotes the accumulation of RIG-I-stimulatory RNA in cells. Transcription of vrnps Is Dispensable for IFN Induction Stimulatory RNA accumulated in reconstitutions with a modified template (Pleschka et al., 996) in which the bacterial chloramphenicol acetyltransferase gene was flanked by a viral polymerase promoter composed of the 5 and 3 noncoding regions of the NS segment (vcat, Figure C). Therefore, apart from this short promoter, specific viral sequence elements are not required for the generation of stimulatory RNA. We next asked whether transcription and/or replication are necessary. We used point mutations in the PA subunit of the viral polymerase that selectively impair one or the other process (Hara et al., 26). As shown in Figure 2A, the PA-E4A polymerase (replication mutant) generated normal amounts of viral mrna from an NA template but vrna and crna levels were reduced about 3-fold. This was accompanied by a 3-fold reduction in IFN secretion by the transfected cells and by accumulation of lower amounts of stimulatory RNA (Figures 2B and 2C). In contrast, when transcription was selectively abrogated with the PA- D8A transcription mutant, mrna production was blocked, yet we did not see a loss but rather observed an increase in stimulatory RNA accumulation, as well as elevated IFN secretion (Figure 2). Similar results were obtained when the PB2 genome segment was used as the template (Figure S2). Therefore, in vrnp reconstitutions, transcription is dispensable for IFN induction and for the accumulation of stimulatory RNA, which 398 Cell 4, , February 5, 2 ª2 Elsevier Inc.
3 A vrnp reconstitution ppoli-vrna (flu genome segment) pcdna-pb2, -PB, -PA (flu polymerase) pcdna-np (NP protein) transfect 293T 5 PPP 5 PPP vrna(-) (i) crna(+). mrna(+) C vcat vcat / PBa 8 ng 2 ng 5 ng 2.5 ng E pisre-luc. bioassay for type I IFN type I IFN (U/ml) (ii) viral protein without pre-treatment with pre-treatment PB2 PB PA HA NP NA M NS B * = not detectable * * * * * ** ** ** no templ no TF. D LL7 bioassay for type I IFN type I IFN (U/ml) poly I:C PB2PB PA HA NP NA M NS * * * * ** ** PB2 NA NS vrnp reconstitution no template no TF no template no TF 8 ng 2 ng 5 ng 2.5 ng RIG-I +/- RIG-I -/- * = not detectable F pisre-luc. bioassay for type I IFN without pre-treatment with pre-treatment * = not detectable type I IFN (U/ml) sirna: * ** ** ** ** cont. RIG-I cont. RIG-I cont. RIG-I PB2 PA no templ Figure. vrnp Reconstitution Induces IFN (A) Scheme of the vrnp reconstitution system. Individual segments of the flu genome (vrnas) are expressed off a promoter for RNA polymerase I, which generates uncapped RNA transcripts. These act as templates for the viral polymerase, which is expressed, together with the NP protein, from a different set of plasmids. The viral polymerase (i) replicates the negative sense genome segment via a positive sense crna intermediate (antigenome) and (ii) snatches short stretches of capped RNA (depicted in gray) from cellular mrnas to serve as primers for transcription of viral mrna, which is then translated into viral protein. (B) Each genome segment (PB2, PB, PA, HA, NP, NA, M, or NS) was used for the vrnp reconstitution or the genome segment was omitted (no template). Two days after transfection, total RNA was extracted and tested in an IFN-b promoter luciferase reporter assay. Results were normalized to a Renilla luciferase control and are shown as fold increase relative to cells treated with transfection reagent only. RNA extracted from non-transfected cells (no TF) and (Neo 99 ) were included as controls. (C) The bacterial CAT gene was flanked by viral 5 and 3 noncoding sequences (vcat) and expressed instead of an authentic viral genome segment. In one group, pcdna-pb was replaced by pcdna-pba that encodes a mutant abrogating polymerase activity (vcat/pba). Extracted RNA was tested as in (B). (D) Mouse embryonic fibroblasts of the indicated genotype were transfected with ng RNA extracted from vrnp reconstitutions with the PB2, NA, or NS genome segment. RNA from reconstitutions without a genome segment (no template), RNA from nontransfected cells (no TF), (Neo 99 ) and poly I:C were included as controls. After overnight culture, cell culture supernatants were tested for mouse IFN content. (E) Supernatants from vrnp reconstitutions were harvested 2 days after transfection. A bioassay was used to test for the presence of human IFN. Cells were pretreated or not with IFN-A/D ( units/ml overnight) prior to transfection. (F) sirna targeting RIG-I or a control sirna (cont.) were cotransfected with the plasmids for vrnp reconstitution. Supernatants were tested as in (E). Representative examples of three (A C and E) or two (D and F) independent experiments are shown. (D) (F) show average values and standard deviation of triplicate measurements. See also Figures S and S7. correlate instead with the amount of vrna and crna produced by viral replication. Full-Length Viral Genomes Bearing 5 -PPPs Trigger IFN Induction in vrnp Reconstitutions Resistance to DNase and susceptibility to RNase V+A treatments confirmed that extracted RNA accounted for stimulatory activity in vrnp reconstitutions (Figure 3A and Figure S3A). Digestion with Terminator (TER), a 5 -to-3 exonuclease that degrades RNA bearing 5 -monophosphate, led to disappearance of ribosomal RNAs but did not alter the potency of the preparations (Figure 3A and Figure S3A). In contrast, treatment with calf intestinal phosphatase (CIP), which removes 5 -phosphates, completely abolished the stimulatory activity (Figure 3A and Figure S3A). We then determined the size of the stimulatory RNA. RNA from vrnp reconstitutions using the PB2, NA, or NS segments was separated into eight fractions of decreasing size by agarose gel electrophoresis. Nucleic acid was extracted from each fraction and tested in the IFN-b reporter assay. As a control, we fractionated a 99 nt long and showed that its stimulatory activity was recovered exclusively in fraction 7, as expected (Figure 3B and Figure S3B). Notably, stimulatory RNA from vrnp reconstitutions with PB2, NA, and NS segments was recovered in fractions 2, 3, and 5/6, respectively, correlating with the respective size of these segments (234, 43, and 89 nt). This observation excludes a dominant role for short replication intermediates (or small stimulatory RNAs of self origin), which would elute in fractions 7 or 8 (Figure 3B and Figure S3B). In sum, RNAs corresponding in size to the viral genome or antigenome and bearing more than one 5 -phosphate account for the majority of the stimulatory RNA generated during vrnp reconstitution. Cell 4, , February 5, 2 ª2 Elsevier Inc. 399
4 A NA-mRNA (+) NA-cRNA (+) NA-vRNA (-) * B 5S rrna type I IFN (U/ml) primer extension PA-WT PA-D8A PA-E4A PA-WT PA-D8A PA-E4A pisre-luc. bioassay for type I IFN IFN-A/D pre-treatment without with WT-PA without pre-treatment with pre-treatment * ** * PA-D8A % of WT * = not detectable 33 PA-E4A 3 2 C IFN-A/D pre-treatment without with ** WT-PA * PA-D8A ** * WT-PA PA-D8A PA-E4A WT-PA PA-D8A PA-E4A PA-E4A NA-vRNA NA-cRNA NA-mRNA * = not detectable IFN-A/D pre-treatment without with WT-PA PA-D8A PA-E4A 8 ng 2 ng 5 ng 2.5 ng Figure 2. vrnp Replication but Not Transcription Induces IFN (A) Cells were pretreated or not with IFN-A/D as in Figure E and used for vrnp reconstitution with the NA genome segment in conjunction with an intact viral polymerase (WT), a polymerase lacking the PA subunit (), or with two point mutants, PA-D8A and PA-E4A. Extracted RNA was tested by primer extension for NA-cRNA, -mrna, and -vrna. A primer specific for 5S rrna was used as a control. Signals were quantified by phosphoimager, normalized to the 5S rrna control, and are expressed relative to WT polymerase. A nonspecific band is marked with an asterisk. (B) Supernatants from (A) were tested in the human IFN bioassay. (C) Total RNA extracted from (A) was tested in the IFN-b reporter assay. (A) is representative of two independent experiments, and (B) and (C) of four experiments. (B) shows average values and standard deviation of triplicate measurements. See Figure S2 for equivalent experiments using the PB2 genome segment. NS Inhibits IFN Induction in vrnp Reconstitutions and Associates with RIG-I and Stimulatory RNA The NS segment encodes the viral NS and NS2 proteins, the former of which binds RNA and, in the PR8 strain, acts as an inhibitor of IFN induction (Gack et al., 29; Guo et al., 27; Mibayashi et al., 27; Opitz et al., 27; Pichlmair et al., 26). Accordingly, we did not detect secretion of IFN in vrnp reconstitutions using the NS genome segment (Figure E) even though stimulatory RNA accumulated in these cells (Figure B and Figure S3A). However, when we modified the PR8 NS genome segment by introducing two point mutations (R38A and K4A) in the sequence encoding the RNA binding domain (Donelan et al., 23), vrnp reconstitution resulted in secretion of significant amounts of IFN (Figures SB SE). These results show that, as expected, NS inhibits IFN induction during vrnp reconstitution. To explore the possibility that IFN inhibition involves simultaneous binding of NS to stimulatory RNAs and to RIG-I, we used a two-step immunoprecipitation approach (Figure S4A). Lysates from cells expressing NS and -tagged RIG-I were combined with, and RIG-I complexes were precipitated with a- antibodies. As described (Pichlmair et al., 26), wild-type NS but not the NS R38A/K4A mutant associated with RIG-I (Figure S4B). Next, native complexes were eluted using peptide and were reprecipitated with a-ns antibody (Figure S4C). The majority of the stimulatory RNA was A phosphatase and nuclease treatments; PB2 template. untreated EtBr: nt: untreated CIP TER DNase RNase V+A CIP TER DNase RNase V+A 8 ng 2 ng 5 ng 2.5 ng 28S rrna 8S rrna B size-fractionation;. (99 nt) top PB2 (234 nt) NA (43 nt) NS (89 nt) fractions bottom Figure 3. Full-Length Viral Genomes Trigger IFN Induction in vrnp Reconstitutions (A) RNA extracted from reconstitutions using the PB2 genome segment was subjected to CIP, TER, DNase, or RNase A+V digestion. Parallel reactions with (+) and without ( ) enzyme were performed and RNAs were analyzed by gel electrophoresis and ethidium bromide staining (bottom) and in the IFN-b reporter assay (top). Extracted RNA without any further treatment was also included (untreated). (B) RNA extracted from reconstitutions using the PB2, NA, or NS genome segments was size fractionated on agarose gels (fractions to 8 from the pockets to the bottom). RNA was reisolated and tested as in (A). (Neo 99 ) was included in the fractionation. The length of this RNA and that of the viral genome segments is given in brackets. Data are representative of three independent experiments. See also Figure S3. 4 Cell 4, , February 5, 2 ª2 Elsevier Inc.
5 retained in the second immunoprecipitation with wild-type NS (Figure S4D), indicating that the viral protein traps stimulatory RNA and RIG-I in a trimolecular complex. However, RIG-I was not necessary for NS-dependent sequestration of stimulatory RNA as the latter still occurred in RIG-I-deficient cells (Figure S4E). A NS IP from infected cells; Western blot IP NS WB: a-ns ng 3 ng Association with NS Marks the Natural RIG-I Agonist during Flu Infection The above experiments suggested that NS immunoprecipitation might allow the isolation of RIG-I agonists from flu-infected cells. Indeed, nucleic acids extracted from NS pulldowns from flu-infected cells potently induced the IFN-b reporter (Figure 4A). This approach did not require artificial overexpression of any protein and allowed quantitative recovery of all relevant RIG-I agonists as evidenced by the fact that it depleted cell lysates from stimulatory activity (Figure 4B). The stimulatory activity of NS-associated RNAs was sensitive to RNase A treatment but not DNase digestion and was RIG-I dependent as it was greatly diminished by sirna depletion of mouse but not human RIG-I in NIH 3T3 cells and vice versa in HEK293T cells (Figure S4F and Figure 4C). We conclude that RIG-I agonistic RNAs generated during flu infection are associated with NS and can be purified from infected cells by NS immunoprecipitation. Flu Genomes Constitute the Physiological RIG-I Agonist NS-associated stimulatory activity was sensitive to CIP but not to TER treatment and encompassed RNA species ranging from.5 to 6 kb (Figures 5A and 5B). The phosphatase sensitivity and size characteristics suggested that stimulatory activity might be attributable to 5 -PPP-containing genomic or antigenomic RNA segments. Indeed, primer extension analysis revealed that all eight negative sense genome segments were highly enriched in the NS but not a control immunoprecipitate (Figures 5C and 5D). For example, PB2 vrna was 2-fold enriched among NS associated RNAs compared to RNA extracted from the lysate (Figures 5C and 5D). We also detected some viral crna and mrna in the NS precipitate, albeit not for all segments (Figures 5C and 5D). In northern blots with a full-length probe for M segment vrna, the NS-associated RNA migrated at around nt, corresponding to the size of the genome segment (27 nt, Figure 5E). Thus, full-length flu genomes are highly enriched in the NS-associated RIG-I stimulatory fraction. To validate these findings by an independent approach, we generated a cell line expressing -tagged RIG-I and infected these cells with PR8 flu or a mutant that does not express the NS protein (DNS). RIG-I was precipitated with a- antibody, and associated nucleic acids were tested in the IFN-b promoter reporter assay (Figure 6A). We recovered stimulatory RNA from the immunoprecipitation but not from a control reaction (Figure 6A and Figure S5A). Consistent with the observations from vrnp reconstitution and NS precipitation experiments, RIG-I-associated RNA lost its stimulatory activity after CIP but not TER treatment (Figure 6B). We characterized RNA from RIG-I precipitates by three approaches. First, we used oligonucleotides complementary to the PB2 and NA segments in primer extension experiments. We found that both vrnas and crnas were retained specifically in the RIG-I purification, B serial depletion; ; Western blot C RIG-I sirna; input Hu sirna NS NIH3T3 NS-IP NS-IP Mu sirna Hu sirna 5 ng 25 ng ng NS-IP NS-IP Mu sirna 5 ng ng 5 ng while contaminating 5S rrna was detectable in both RIG-I and control precipitates (Figure 6C). Second, in northern blots for vrna of the M segment, the RIG-I-associated RNA migrated at the size expected for the full-length genome segment (27 nt), and we did not detect faster-migrating RNA species (Figure 6D). Third, the size profile of RIG-I-associated stimulatory NS NS X 2X 3X Depletion NS WB: a-ns HEK293T Figure 4. NS Associates with the Actual RIG-I Agonist during Flu Infection (A) NS was immunoprecipitated from total lysate of MDCK cells infected with PR8 WT flu for 48 hr at a multiplicity of infection (MOI) of.. Nucleic acids associated with control or NS immunoprecipitates (IP) were extracted and tested in the IFN-b reporter assay. Western blot (WB) shows the presence of NS. (B) Total lysate of MDCK cells infected with flu was subjected to three rounds of mock or NS immunoprecipitation. Nucleic acids were extracted from the lysate and the depleted fractions and tested as in (A). The depletion of NS was monitored by WB. (C) Mouse NIH 3T3 or human HEK293T cells were cotransfected with the IFN-b promoter reporter plasmid and sirnas specific for human or mouse RIG-I. After 48 hr, cells were transfected with the indicated amount of or NS- associated RNA (NS-IP), and luciferase activity was measured 2 hr later. (A), (B), and (C) are representative of five, three, and two independent experiments, respectively. See also Figure S4. Cell 4, , February 5, 2 ª2 Elsevier Inc. 4
6 A nuclease and phosphatase treatments; B size fractionation; 75 ng 25 ng CIP TER (>) 2 (-6) 3 (2-6) 4 (-2) 5 (.5-) Fraction 6 (<.5) 7 (<<.5) C primer extension PB2 mrna (+) PB2 crna (+) PB2 vrna input NS IP D primer extension: phosphoimager quantification Segment vrna crna mrna PB PB PA HA NP NA M NS E Northern blot for M-vRNA IP: nt: 5 NS 5S rrna Figure 5. NS Binds 5 -PPP Influenza A Virus Genomes and Antigenomes (A) NS associated stimulatory nucleic acid (see Figure 4A) was subjected to CIP or TER treatment and tested in the IFN-b reporter assay. (B) Size profile of the NS-associated stimulatory RNA. NS-associated nucleic acid (see Figure 4A) was resolved by agarose gel electrophoresis. The gel was cut into seven fractions, and RNA was re-extracted and analyzed as in (A). The relative molecular weight of each fraction in nucleotides (3) is based on comparison with an RNA marker. (C) NS-associated RNA was immunoprecipitated from infected cells and PB2 mrna, crna, and vrna as well as 5S rrna were detected by primer extension. For all fractions, the same amount of RNA (4 ng) was used. (D) The primer extension shown in (C) was repeated with oligonucleotides complementary to all other segments and quantified by phosphoimager analysis. Results are expressed as fold enrichment in the NS precipitate compared to input RNA. HA and NS vrnas and some mrnas and crnas were present in the NS precipitate (denoted by the plus sign), but not detectable in the input material, hence fold enrichments could not be calculated. (E) Northern blot analysis of RNA associated with a-ns or control antibody using a full-length, internally labeled probe specific to M-vRNA. (A) (E) show representative examples of two independent experiments. RNA matched the size range of flu genome segments ( nt) and was not found in smaller fractions (Figure 6E). Thus, direct RIG-I precipitation reveals only the presence of flu viral genomes and not other stimulatory nucleic acids in flu-infected cells. RIG-I Is Triggered by Viral Genomic RNA during Sendai Virus Infection To extend our findings to other viruses sensed by RIG-I, we chose SeV. The SeV genome consists of a single negative sense 5 -PPP-bearing RNA molecule 5,384 nt long that serves as a template for the synthesis of capped mrnas and 5 -PPP antigenomes. Unlike flu, SeV makes short (5 nt long) leader and trailer 5 -PPP RNAs during infection. Thus, in SeV-infected cells, these RNAs could serve as RIG-I agonists, as proposed for the related measles virus (Plumet et al., 27). Total RNA from SeV-infected cells but not from uninfected cells potently induced the IFN-b promoter upon transfection into reporter cells (Figure 7A). As for flu, stimulatory activity was RIG-I dependent and sensitive to RNase, but not DNase treatment (Figures S6A and S6B) and was additionally sensitive 42 Cell 4, , February 5, 2 ª2 Elsevier Inc.
7 A B CIP and terminator nuclease; PR8 DNS PR8 WT ng 2.5 ng.6 ng.6 ng 2 ng 5 ng.3 ng.3 ng 2 ng 5 ng.3 ng.3 ng... - CIP + CIP + TER kda: WB: a- kda: WB: a- IP: IP: IP: C primer extension D Northern blot for M-vRNA IP: PB2-mRNA (+) PB2-cRNA (+) PR8 DNS PR8 WT IP: NA-mRNA (+) NA-cRNA (+) PR8 DNS PR8 WT IP: nt: PR8 DNS PR8 WT PB2-vRNA (-) NA-vRNA (-) 5 5S rrna E size fractionation 4% % 2.5%.6%. (>2) 2 (2-8) 3 (8-3.5) 4 (3.5-.5) 5 (.5-.3) 6 (.3-.5) 7 (<.5) Fraction Figure 6. Influenza A Virus Genomes and Antigenomes Trigger IFN Induction during Infection (A) HEK293 cells expressing -RIG-I were infected with flu PR8 WT or PR8 DNS at an MOI of. After 6 hr, RIG-I was precipitated with a- antibody. An isotope matched antibody () was used as a control. RNA extracted from the precipitates was tested in the IFN-b reporter assay (top). The precipitates were also tested by WB using a- antibodies (bottom). (B) RNA associated with -RIG-I (from PR8 DNS infection) was analyzed by CIP and TER treatment and tested as in (A). (C) Stimulatory RNA in the control and -RIG-I precipitates was analyzed by primer extension for the presence of PB2 and NA mrna, crna, and vrna, as well as 5S rrna. (D) Northern blot analysis of RNA bound to -RIG-I using a full-length probe specific for M-vRNA. (E) Size profile of the -RIG-I associated stimulatory RNA (from PR8 DNS infection) determined as in Figure 5B. (A) and (B) (E) are representative examples of four and two independent experiments, respectively. See also Figures S5 and S7. Cell 4, , February 5, 2 ª2 Elsevier Inc. 43
8 A total RNA from SeV infected cells; B CIP and terminator nuclease; C size fractionation; 2 ng 5 ng 2.5 ng 3. ng 2 ng 5 ng 2.5 ng 3. ng 4% % 2.5%.6%. no infection SeV infection. EtBr: nt: CIP TER 28S rrna 8S rrna. nt: > 3 IVT RNA < 3 > 3 < 3 SeV infection + CIP TER D NS IP; 6 ng 3 ng 5 ng E NS IP; primer extension anti-genomic RNA (+) mrna (+) genomic RNA (-) input IP NS IP F RIG-I and MDA5 IP; 8 ng 4 ng 2 ng G RIG-I and MDA5 IP; primer extension genomic RNA (-) 5S rrna RIG-I input IP MDA5 input IP. WT mut NS IP WB: a-ns 5S rrna. RIG-I MDA5 IP WT mut NS IP WB: a- RIG-I MDA5 IP Figure 7. Viral Genomes Constitute the Primary RIG-I Agonist during Sendai Virus Infection (A) HEK293 cells were infected or not with SeV at an MOI of 5. After 6 hr, RNA was extracted with TRIZOL and tested in the IFN-b reporter assay as in Figure B. (B) Stimulatory RNA extracted from SeV-infected cells was treated with CIP or TER. The minus sign denotes a control reaction without enzyme. Aliquots were tested as in (A) (top) and by agarose gel electrophoresis and ethidium bromide staining (bottom). (C) Size profile of stimulatory RNA extracted from SeV-infected cells. RNA from (A) was resolved by agarose gel electrophoresis. The gel was cut into two fractions, corresponding to RNAs migrating above or below 3 nt by comparison with an RNA marker. RNA was re-extracted and analyzed as in (A). IVT- RNA (Neo 99, 99 nt) was included as a control. (D) HEK293T cells were transfected with plasmids expressing NS or NS-R38A/K4A. After 24 hr, cells were infected with SeV at an MOI of 5. Cell lysates were prepared 2 hr later, and NS was precipitated. The precipitates were analyzed by WB (bottom) and nucleic acids were extracted and tested as in (A) (top). (E) Transiently transfected HEK293T expressing NS were infected with SeV and NS was precipitated as in (D). Nucleic acid extracted from the cell lysate (input) or associated with a-ns or control antibodies (IP) was analyzed for the presence of SeV genomic, antigenomic, and messenger RNA and 5S rrna by primer extension. (F) -RIG-I and -MDA5 were expressed in HEK293T cells by transient transfection, followed by infection with SeV after 24 hr. Cell lysates were prepared after 2 hr and RIG-I and MDA5 were precipitated using a- antibodies. Nucleic acids were extracted from precipitates (IP) and analyzed as in (A) (top panel). The bottom panel shows a WB using an aliquot of the IP. (G) Nucleic acids extracted from cell lysates (input) and precipitates (IP) as in Figure 7F were tested by primer extension for SeV genomic RNA and 5S rrna. Panels (A), (C), (F), and (G) and panels (B), (D), and (E) are a representative examples of three and two independent experiments, respectively. See also Figures S6 and S7. to CIP but not TER (Figure 7B). Size fractionation into large (>3 nt) and small (<3 nt) RNAs showed that stimulatory RNA from SeV infected cells was large, whereas that of a control 99 nt IVT RNA was small (Figure 7C). This excludes a major role for leader and trailer RNAs in triggering RIG-I and together with the CIP sensitivity suggests that genomic and/or antigenomic RNA is the primary RIG-I agonist during SeV infection. 44 Cell 4, , February 5, 2 ª2 Elsevier Inc.
9 To capture the physiologically relevant RIG-I agonist during SeV infection, we again made use of flu NS, which is able to inhibit IFN responses to SeV (Wang et al., 2). As predicted, WT NS but not the NS mutant was able to precipitate stimulatory RNAs from extracts of transiently transfected cells infected with SeV (Figure 7D). We next tested by primer extension if NS-associated stimulatory RNA contains SeV genomic, antigenomic, and/or messenger RNA (Figure S6D). Compared to RNA extracted from the cell lysate, SeV genomic RNA was enriched between 6.6- and.5-fold in the NS precipitate, while the antigenome and N-mRNA were not detectable (Figure 7E and Figure S6C). We validated these results by precipitating - RIG-I (or -MDA5 as a control) from transiently transfected cells infected with SeV. Stimulatory RNA was recovered only in the -RIG-I immunoprecipitation and SeV genomic RNA was enriched between 7.4- and 7-fold in the RIG-I precipitate compared to cell lysate (Figures 7F and 7G). Thus, flu NS or RIG-I precipitation selectively enriches for SeV viral genomes. Taken together with the size characteristics of the stimulatory RNA and the CIP sensitivity, these observations show that 5 -PPP bearing genomic RNA is the main trigger for RIG-I during SeV infection. DISCUSSION Sensing of virus presence and cytokine induction via the RIG-I pathway are crucial for successful host defense against infections with RNA viruses (Pichlmair and Reis e Sousa, 27; Yoneyama and Fujita, 29). Although the signaling cascade from RIG-I to IFN induction is well defined, the identity and properties of RIG-I agonists and the mechanisms that allow the helicase to be activated specifically in infected cells are controversial. Viral genomes, shorter viral transcripts, double-stranded RNA, or cellular RNA cleaved by RNase L have all been suggested to trigger RIG-I (Habjan et al., 28; Hausmann et al., 28; Hornung et al., 26; Kato et al., 28; Malathi et al., 27; Pichlmair et al., 26; Plumet et al., 27; Ranjith-Kumar et al., 29; Samanta et al., 26; Takahasi et al., 28). Such RNAs have been variably defined as containing no phosphates, 5 -monophosphates, 5 -triphosphates, or 3 -monophosphates (Hornung et al., 26; Kato et al., 28; Malathi et al., 27; Pichlmair et al., 26; Takahasi et al., 28) and, in some cases, to require specific structural determinants or sequence motifs (Marques et al., 26; Saito et al., 28; Schlee et al., 29b; Schmidt et al., 29; Uzri and Gehrke, 29). Most of these studies, however, have been limited to the analysis of RIG-I activation by defined RNAs, including synthetic RNAs made by chemical or enzymatic synthesis or vrnas isolated from virus particles. Although such studies have been instrumental in defining the range of RNAs that can activate RIG-I, they have fallen short of identifying physiological RIG-I agonists that are actually responsible for activating RIG-I and triggering IFN production in virus-infected cells. Here, we analyze the properties of relevant RIG-I agonists in cells infected with flu or SeV. Using three complementary approaches, we find that genomic RNA generated by viral replication constitutes the major trigger for RIG-I and conclude that viral transcripts, RNase L cleavage products, and/or other RNA species make only a minor contribution to cell-intrinsic antiviral innate immunity. We started with a mock infection system that allows the reconstitution of flu vrnp complexes and leads to IFN induction and accumulation of stimulatory RNA (Figure ). Using this system, we found that an artificial genome segment that retains the viral promoter but otherwise lacks viral sequences behaved similarly to bona fide flu vrna segments (Figure C). Therefore, RIG-I activation in this setting is largely sequence independent. This is in contrast to recent reports suggesting that a polyuridine motif in the hepatitis C virus 3 untranslated region is required for triggering RIG-I (Saito et al., 28; Uzri and Gehrke, 29). Importantly, those conclusions were based on cellular responses to transfected s, whereas the vrnp reconstitution system used here allowed us to look at RNAs made endogenously by the mock-infected cell. Nevertheless, it remains possible that sequence motifs may facilitate RIG-I activation in some instances. Indeed, such motifs could contribute to the observed quantitative differences in accumulation of stimulatory RNA and IFN secretion depending on which of eight flu genome segments was used for reconstitution (Figures B and E). Alternatively, those differences may be due to the expression of viral proteins associated with the viral genome (such as the PB2, PB, PA, NP, M, NS, and NS2 proteins), which may inhibit or facilitate the access and/or function of RIG-I. In a particularly striking example of the latter point, the viral NS protein completely blocked IFN induction during vrnp reconstitution (Figures SB SE). NS can interact with RIG-I (Mibayashi et al., 27), especially in the presence of stimulatory RNA through formation of a trimeric complex (Figures S4A S4D) (Pichlmair et al., 26). This activity of NS is dependent on the integrity of the RNA binding domain, which is reported to bind double-stranded RNA (Hatada and Fukuda, 992). Interestingly, recent studies demonstrate that, in addition to the 5 -PPP, synthetic RNAs require base pairing at the 5 end in order to trigger RIG-I (Schlee et al., 29b; Schmidt et al., 29). Such 5 base-paired regions can be found within the genomes of flu and SeV (Knipe and Howley, 27). We therefore envisage that one mechanism of NS action may be to bind to the base-paired region at the 5 end of viral genomes. This does not prevent RIG-I binding to the 5 -PPP via its C-terminal domain (Cui et al., 28; Takahasi et al., 28) but may block translocation along the base paired stretch, which has been proposed to be necessary for signaling (Myong et al., 29). This model (Figure S4G) therefore suggests that the ability of NS to associate with stimulatory RNAs is due to its propensity to recognize RNA secondary structure determinants important for RIG-I activation (Schlee et al., 29b; Schmidt et al., 29) and is consistent with the finding that NS binds agonistic RNA in the absence of functional RIG-I (Figure S4E). This model does not exclude additional modes of NS action, such as inhibition of TRIM25-mediated RIG-I ubiquitination (Gack et al., 29). Given the finding that NS associates with stimulatory RNA, we used it as one of our strategies to purify RIG-I agonists from infected cells. As a complementary approach, we immunoprecipitated epitope-tagged RIG-I from infected cells. Both precipitations enriched for flu and SeV genomic RNAs. Furthermore, NS- or RIG-I-associated stimulatory RNA matched the Cell 4, , February 5, 2 ª2 Elsevier Inc. 45
10 size of vrna and required 5 -phosphates for stimulatory activity. Thus, viral genomic RNAs represent the major RIG-I agonist in flu- and SeV-infected cells. Antigenomes, which have an identical size to the genome and also bear 5 -PPP, may also contribute to IFN induction. Indeed, flu crnas were present in the NS and RIG-I immunoprecipitates (Figures 5C, 5D, and 6C). Their contribution, however, is likely to be minor, as crna accumulates to much lower levels compared to vrna (Robb et al., 29) (Figure SA and Figure 5C). In contrast to vrna and crna, flu or SeV transcripts do not appear to trigger RIG-I, based on the size distribution of the stimulatory RNA, the fact that the transcription-defective PA-D8A mutant flu polymerase was fully capable of inducing IFN in vrnp reconstitution experiments, and the fact that viral mrnas, like cellular mrnas, are capped. These findings do not exclude a role for viral transcripts in activating RIG-I in other virus infections such as measles virus and Epstein-Barr virus (Plumet et al., 27; Samanta et al., 26) as those viruses use mechanisms for transcription that can result in transcripts bearing 5 -triphosphates. However, it is worth noting that measlesrelated SeV also generates uncapped short 5 -triphosphatebearing leader and trailer RNA transcripts, yet our size fractionation experiments exclude a role for these short RNAs in RIG-I stimulation in SeV-infected cells (Figure 7C). We speculate that leader and trailer RNAs lack a sufficient degree of secondary structure to potently trigger RIG-I and/or are sequestered by association with cellular proteins. Similarly, during infection with another paramyxovirus, respiratory syncytial virus, leader RNAs do not play an important role in IFN induction (Bitko et al., 28). Thus, the ability of a virus to generate uncapped transcripts during its life cycle does not necessarily mean that these will act as RIG-I agonists. Our results also appear to exclude RNase L cleavage products as major RIG-I agonists during infection with negative-strand RNA viruses. Such cleavage products have 5 -hydroxyl and 3 -monophosphate ends and are expected to be shorter than 2 nt (Malathi et al., 27; Wreschner et al., 98). Yet we found that the stimulatory activity of RNA isolated from both vrnp reconstitutions and infected cells strictly required 5 -phosphates and was longer than 2 nt. It may therefore be the case that RNase L-cleaved self or viral RNAs are not obligate RIG-I agonists but primarily serve to amplify RIG-I activation driven by vrna. Consistent with such a model, RNase L-deficient mice show only a 6-fold reduction in serum IFN-b after infection with SeV (Malathi et al., 27). Virus entry into cells can induce innate immune responses in the absence of replication (Collins et al., 24) and, in fact, 56 C-inactivated influenza virus was originally used to discover IFNs (Isaacs and Lindenmann, 957). In retrospect, the latter observations may be explained by RIG-I-mediated recognition of incoming viral genomes delivered by high doses of fusogenic virus. However, the fact that NS, a nonstructural protein only produced after infection, can effectively prevent IFN induction by flu indicates that the incoming genomes of virus particles are not the major triggers of RIG-I activation during live infection. Consistent with that notion, infection in the presence of drugs that block translation (and, consequently, inhibit the virus life cycle) prevents accumulation of stimulatory RNA in the cytoplasm of flu-infected cells (Figure S7A). Therefore, we believe that progeny genomes are the likely source of RIG-I stimulatory activity. However, flu replication is confined to the nucleus (Jackson et al., 982; Krug et al., 987), raising the question of how progeny viral genomic RNA is sensed in the cytoplasmic compartment monitored by RIG-I. It is clear that progeny genomes traverse the cytoplasm for assembly of new virions, but these genomes are bound at the ends by the flu polymerase and along their length by the NP protein. For RIG-I to interact with the genome and the critical 5 -PPP moiety, these viral proteins need either to be displaced or to dissociate from the vrna. The former process could be facilitated by the ATP-driven helicase activity of RIG-I (Takahasi et al., 28), whereas the latter may occur naturally as part of an equilibrium reaction. Indeed, viral RNA within vrnps is accessible to nucleases (Duesberg, 969) and might therefore also permit RIG-I docking, at least for a fraction of the estimated, viral genome segments present within an infected cell. Here, we report that viral genomes are the major trigger for RIG-I in cells infected with negative-sense single-stranded RNA viruses. Our findings confirm earlier suggestions that single stranded RNAs bearing 5 -PPPs constitute effective agonists for RIG-I (Hornung et al., 26; Pichlmair et al., 26). It is worth noting that single-strandedness does not mean absence of base pairing. Flu genome segments and SeV genomic RNA adopt a panhandle conformation by pairing of complementary 5 and 3 ends (Knipe and Howley, 27). Interestingly, Myong et al. showed that RIG-I translocates on synthetic doublestranded RNA molecules and that this movement is enhanced the presence of 5 -PPP (Myong et al., 29). Notably, treatment of the stimulatory RNAs studied here with the double-stranded RNA specific nuclease RNase III abolishes RIG-I stimulatory activity (Figure S7B), which indicates that these RNAs contain base-paired regions. Therefore, we envisage that base-pairing within the panhandle structure of single-stranded flu and SeV genomic RNAs acts in cooperation with the presence of 5 -PPP to allow for potent RIG-I activation (Pichlmair et al., 26). This model is likely to apply to other viruses sensed by RIG-I as panhandle structures are found in many single stranded RNA virus genomes (Schlee et al., 29b). Thus, RIG-I integrates RNA secondary structure determinants and the presence of a 5 -PPP to effectively discriminate viral genomes from self-rna. EXPERIMENTAL PROCEDURES Reconstitution of Flu vrnps One million HEK293T cells were transiently transfected using lipofectamine 2 (Invitrogen) with mg each of pcdna-pb2, -PB, -PA, and -NP (all from the flu WSN strain) and a ppoli construct expressing a flu genome segment (derived from the flu A/PR/8/34 [PR8] strain). Two days after transfection, cell culture supernatants were collected and total RNA was extracted with TRIZOL (Invitrogen). RNA Analysis CIP (New England Biolabs), TER (Epicenter Biotechnologies), RQ DNase (Promega), and RNase V (Ambion) combined with RNase A (Sigma) or RNase III (Ambion) were used according to manufacturer recommendations. A control reaction omitting the enzyme was carried out in parallel. RNA was recovered by extraction with phenol:chloroform:isoamylalcohol (25:24:), followed by chloroform extraction and precipitation with ethanol and sodium acetate in 46 Cell 4, , February 5, 2 ª2 Elsevier Inc.
11 the presence of glycogen. For size fractionation, RNAs were separated on.75% TBE-agarose gels at 7 V for 3 hr. Gels were cut into slices (including the well and bottom of the gel) and RNA was recovered from gel pieces with Quantum Prep Freeze N Squeeze Spin Columns (Bio-Rad) and precipitation (as above). Primer extension and northern blot assays are described in the Extended Experimental Procedures. Oligonucleotide sequences are given in Table S. Immunoprecipitation from Virally Infected Cells Lysates from cells infected with flu PR8, flu PR8 DNS, or SeV were used for immunoprecipitation as detailed in the Extended Experimental Procedures. Aliquots of the beads were boiled in SDS sample buffer for western blot analysis or RNA was recovered from the beads by extraction and precipitation as described above. SUPPLEMENTAL INFORMATION Supplemental Information includes Extended Experimental Procedures, seven figures, and one table and can be found with this article online at doi:.6/j. cell ACKNOWLEDGMENTS We thank Peter Palese and Shizuo Akira for reagents and George Brownlee and all members of the Immunobiology laboratory for advice and critical discussions. J.R. is a recipient of FEBS and Human Frontier Science Program long-term fellowships. D.G. is a recipient of a Baxter and Alma Ricard Foundation scholarship. K.B. is a recipient of a Biotechnology and Biological Sciences Research Council studentship. E.F. is supported by grants from the MRC, Wellcome Trust, and the European Union (FLUINNATE). Work in the C.R.S. laboratory is funded by Cancer Research UK and a prize from Fondation Bettencourt-Schueller. Received: June, 29 Revised: November, 29 Accepted: January 6, 2 Published: February 4, 2 REFERENCES Bitko, V., Musiyenko, A., Bayfield, M.A., Maraia, R.J., and Barik, S. (28). Cellular La protein shields nonsegmented negative-strand RNA viral leader RNA from RIG-I and enhances virus growth by diverse mechanisms. J. Virol. 82, Collins, S.E., Noyce, R.S., and Mossman, K.L. (24). Innate cellular response to virus particle entry requires IRF3 but not virus replication. J. Virol. 78, Cui, S., Eisenächer, K., Kirchhofer, A., Brzózka, K., Lammens, A., Lammens, K., Fujita, T., Conzelmann, K.K., Krug, A., and Hopfner, K.P. (28). The C-terminal regulatory domain is the RNA 5 -triphosphate sensor of RIG-I. Mol. Cell 29, Donelan, N.R., Basler, C.F., and García-Sastre, A. (23). A recombinant influenza A virus expressing an RNA-binding-defective NS protein induces high levels of beta interferon and is attenuated in mice. J. Virol. 77, Duesberg, P.H. (969). Distinct subunits of the ribonucleoprotein of influenza virus. J. Mol. Biol. 42, Fodor, E., Pritlove, D.C., and Brownlee, G.G. (994). The influenza virus panhandle is involved in the initiation of transcription. J. Virol. 68, Fodor, E., Crow, M., Mingay, L.J., Deng, T., Sharps, J., Fechter, P., and Brownlee, G.G. (22). A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase inhibits endonucleolytic cleavage of capped RNAs. J. Virol. 76, Gack, M.U., Albrecht, R.A., Urano, T., Inn, K.S., Huang, I.C., Carnero, E., Farzan, M., Inoue, S., Jung, J.U., and García-Sastre, A. (29). Influenza A virus NS targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5, Gitlin, L., Barchet, W., Gilfillan, S., Cella, M., Beutler, B., Flavell, R.A., Diamond, M.S., and Colonna, M. (26). Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 3, Grunberg-Manago, M., Oritz, P.J., and Ochoa, S. (955). Enzymatic synthesis of nucleic acidlike polynucleotides. Science 22, Guo, Z., Chen, L.M., Zeng, H., Gomez, J.A., Plowden, J., Fujita, T., Katz, J.M., Donis, R.O., and Sambhara, S. (27). NS protein of influenza A virus inhibits the function of intracytoplasmic pathogen sensor, RIG-I. Am. J. Respir. Cell Mol. Biol. 36, Habjan, M., Andersson, I., Klingström, J., Schümann, M., Martin, A., Zimmermann, P., Wagner, V., Pichlmair, A., Schneider, U., Mühlberger, E., et al. (28). Processing of genome 5 termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PLoS ONE 3, e232. Hara, K., Schmidt, F.I., Crow, M., and Brownlee, G.G. (26). Amino acid residues in the N-terminal region of the PA subunit of influenza A virus RNA polymerase play a critical role in protein stability, endonuclease activity, cap binding, and virion RNA promoter binding. J. Virol. 8, Hatada, E., and Fukuda, R. (992). Binding of influenza A virus NS protein to dsrna in vitro. J. Gen. Virol. 73, Hausmann, S., Marq, J.B., Tapparel, C., Kolakofsky, D., and Garcin, D. (28). RIG-I and dsrna-induced IFNbeta activation. PLoS ONE 3, e3965. Hornung, V., Ellegast, J., Kim, S., Brzózka, K., Jung, A., Kato, H., Poeck, H., Akira, S., Conzelmann, K.K., Schlee, M., et al. (26). 5 -Triphosphate RNA is the ligand for RIG-I. Science 34, Isaacs, A., and Lindenmann, J. (957). Virus interference. I. The interferon. Proc. R Soc. Lond. B Biol. Sci. 47, Jackson, D.A., Caton, A.J., McCready, S.J., and Cook, P.R. (982). Influenza virus RNA is synthesized at fixed sites in the nucleus. Nature 296, Kato, H., Takeuchi, O., Sato, S., Yoneyama, M., Yamamoto, M., Matsui, K., Uematsu, S., Jung, A., Kawai, T., Ishii, K.J., et al. (26). Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 44, 5. Kato, H., Takeuchi, O., Mikamo-Satoh, E., Hirai, R., Kawai, T., Matsushita, K., Hiiragi, A., Dermody, T.S., Fujita, T., and Akira, S. (28). Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-i and melanoma differentiation-associated gene 5. J. Exp. Med. 25, 6 6. Kilbourne, E.D. (26). Influenza pandemics of the 2th century. Emerg. Infect. Dis. 2, 9 4. Knipe, D.M., and Howley, P.M. (27). Fields Virology, Fifth Edition (Philadelphia: Lippincott Williams & Wilkins). Krug, R.M., St Angelo, C., Broni, B., and Shapiro, G. (987). Transcription and replication of influenza virion RNA in the nucleus of infected cells. Cold Spring Harb. Symp. Quant. Biol. 52, Kumar, H., Kawai, T., Kato, H., Sato, S., Takahashi, K., Coban, C., Yamamoto, M., Uematsu, S., Ishii, K.J., Takeuchi, O., and Akira, S. (26). Essential role of IPS- in innate immune responses against RNA viruses. J. Exp. Med. 23, Maines, T.R., Szretter, K.J., Perrone, L., Belser, J.A., Bright, R.A., Zeng, H., Tumpey, T.M., and Katz, J.M. (28). Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol. Rev. 225, Malathi, K., Dong, B., Gale, M., Jr., and Silverman, R.H. (27). Small self-rna generated by RNase L amplifies antiviral innate immunity. Nature 448, Marques, J.T., Devosse, T., Wang, D., Zamanian-Daryoush, M., Serbinowski, P., Hartmann, R., Fujita, T., Behlke, M.A., and Williams, B.R. (26). A structural basis for discriminating between self and nonself double-stranded RNAs in mammalian cells. Nat. Biotechnol. 24, Cell 4, , February 5, 2 ª2 Elsevier Inc. 47
IFN TLR TLR TLR. Toll-like receptor TLR. IFN Retinoic acid inducible gene-i RIG-I RIG-I RNA RIG-I RIG-I RIG-I
 58 2 pp.97-104 2008 1. RNA RIG-I 1 2 3 RIG-I RIG-I RNA RNA IFN RIG-I RNA RIG-I RNA RNA RIG-I RNA RIG-I 1 2 Toll-like receptor TLR I 606-8507 53 TEL : 075-751-4031 FAX : 075-751-4031 E-mail: sgo@virus.kyoto-u.ac.jp
58 2 pp.97-104 2008 1. RNA RIG-I 1 2 3 RIG-I RIG-I RNA RNA IFN RIG-I RNA RIG-I RNA RNA RIG-I RNA RIG-I 1 2 Toll-like receptor TLR I 606-8507 53 TEL : 075-751-4031 FAX : 075-751-4031 E-mail: sgo@virus.kyoto-u.ac.jp
The accumulation of influenza A virus segment 7 spliced mrnas is regulated by the NS1 protein
 Journal of General Virology (2012), 93, 113 118 DOI 10.1099/vir.0.035485-0 Short Communication Correspondence Ervin Fodor ervin.fodor@path.ox.ac.uk Received 24 June 2011 Accepted 12 September 2011 The
Journal of General Virology (2012), 93, 113 118 DOI 10.1099/vir.0.035485-0 Short Communication Correspondence Ervin Fodor ervin.fodor@path.ox.ac.uk Received 24 June 2011 Accepted 12 September 2011 The
Supplementary Fig. S1. Schematic diagram of minigenome segments.
 open reading frame 1565 (segment 5) 47 (-) 3 5 (+) 76 101 125 149 173 197 221 246 287 open reading frame 890 (segment 8) 60 (-) 3 5 (+) 172 Supplementary Fig. S1. Schematic diagram of minigenome segments.
open reading frame 1565 (segment 5) 47 (-) 3 5 (+) 76 101 125 149 173 197 221 246 287 open reading frame 890 (segment 8) 60 (-) 3 5 (+) 172 Supplementary Fig. S1. Schematic diagram of minigenome segments.
Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid.
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
5 -Triphosphate RNA Is the Ligand for RIG-I
 5 -Triphosphate RNA Is the Ligand for RIG-I Veit Hornung, 1 Jana Ellegast, 1 Sarah Kim, 1 Krzysztof Brzózka, 3 Andreas Jung, 2 Hiroki Kato, 2 Hendrik Poeck, 1 Shizuo Akira, 2 Karl-Klaus Conzelmann, 3 Martin
5 -Triphosphate RNA Is the Ligand for RIG-I Veit Hornung, 1 Jana Ellegast, 1 Sarah Kim, 1 Krzysztof Brzózka, 3 Andreas Jung, 2 Hiroki Kato, 2 Hendrik Poeck, 1 Shizuo Akira, 2 Karl-Klaus Conzelmann, 3 Martin
Intrinsic cellular defenses against virus infection
 Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
Innate Immunity & Inflammation
 Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Patricia Fitzgerald-Bocarsly
 FLU Patricia Fitzgerald-Bocarsly October 23, 2008 Orthomyxoviruses Orthomyxo virus (ortho = true or correct ) Negative-sense RNA virus (complementary to mrna) Five different genera Influenza A, B, C Thogotovirus
FLU Patricia Fitzgerald-Bocarsly October 23, 2008 Orthomyxoviruses Orthomyxo virus (ortho = true or correct ) Negative-sense RNA virus (complementary to mrna) Five different genera Influenza A, B, C Thogotovirus
Mutational Analysis of the Influenza Virus crna Promoter and Identification of Nucleotides Critical for Replication
 JOURNAL OF VIROLOGY, June 2004, p. 6263 6270 Vol. 78, No. 12 0022-538X/04/$08.00 0 DOI: 10.1128/JVI.78.12.6263 6270.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. Mutational
JOURNAL OF VIROLOGY, June 2004, p. 6263 6270 Vol. 78, No. 12 0022-538X/04/$08.00 0 DOI: 10.1128/JVI.78.12.6263 6270.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved. Mutational
Reoviruses. Virion. Genome. Genes and proteins. Viruses and hosts. Diseases. Distinctive characteristics
 Reoviruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Naked icosahedral capsid (T=13), diameter 60-85 nm Capsid consists of two or three concentric protein
Reoviruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Naked icosahedral capsid (T=13), diameter 60-85 nm Capsid consists of two or three concentric protein
Influenza viruses. Virion. Genome. Genes and proteins. Viruses and hosts. Diseases. Distinctive characteristics
 Influenza viruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Enveloped particles, quasi-spherical or filamentous Diameter 80-120 nm Envelope is derived
Influenza viruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Enveloped particles, quasi-spherical or filamentous Diameter 80-120 nm Envelope is derived
Viral vaccines. Lec. 3 أ.د.فائزة عبد هللا مخلص
 Lec. 3 أ.د.فائزة عبد هللا مخلص Viral vaccines 0bjectives 1-Define active immunity. 2-Describe the methods used for the preparation of attenuated live & killed virus vaccines. 3- Comparison of Characteristics
Lec. 3 أ.د.فائزة عبد هللا مخلص Viral vaccines 0bjectives 1-Define active immunity. 2-Describe the methods used for the preparation of attenuated live & killed virus vaccines. 3- Comparison of Characteristics
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer. Application Note
 Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer Application Note Odile Sismeiro, Jean-Yves Coppée, Christophe Antoniewski, and Hélène Thomassin
Analysis of small RNAs from Drosophila Schneider cells using the Small RNA assay on the Agilent 2100 bioanalyzer Application Note Odile Sismeiro, Jean-Yves Coppée, Christophe Antoniewski, and Hélène Thomassin
Regulation of cell signaling cascades by influenza A virus
 The Second International Symposium on Optimization and Systems Biology (OSB 08) Lijiang, China, October 31 November 3, 2008 Copyright 2008 ORSC & APORC, pp. 389 394 Regulation of cell signaling cascades
The Second International Symposium on Optimization and Systems Biology (OSB 08) Lijiang, China, October 31 November 3, 2008 Copyright 2008 ORSC & APORC, pp. 389 394 Regulation of cell signaling cascades
Supplementary Figure 1
 Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Species-Specific Inhibition of RIG-I Ubiquitination and IFN Induction by the Influenza A Virus NS1 Protein
 Species-Specific Inhibition of RIG-I Ubiquitination and IFN Induction by the Influenza A Virus NS1 Protein Ricardo Rajsbaum 1,2, Randy A. Albrecht 1,2, May K. Wang 3, Natalya P. Maharaj 3, Gijs A. Versteeg
Species-Specific Inhibition of RIG-I Ubiquitination and IFN Induction by the Influenza A Virus NS1 Protein Ricardo Rajsbaum 1,2, Randy A. Albrecht 1,2, May K. Wang 3, Natalya P. Maharaj 3, Gijs A. Versteeg
7.012 Quiz 3 Answers
 MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh-
 1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
Oncolytic virus strategy
 Oncolytic viruses Oncolytic virus strategy normal tumor NO replication replication survival lysis Oncolytic virus strategy Mechanisms of tumor selectivity of several, some of them naturally, oncolytic
Oncolytic viruses Oncolytic virus strategy normal tumor NO replication replication survival lysis Oncolytic virus strategy Mechanisms of tumor selectivity of several, some of them naturally, oncolytic
ISG15 sirna # Ctrl sirna+ifn+wt. Virus titer (Pfu/ml) hours post infection. d USP18 sirna #2 IFN
 a ISG15 sirna Ctrl #1 #2 ISG15 conjugates IB: ISG15 Virus titer (Pfu/ml) b ISG15 sirna #2 10 7 Ctrl sirna+ifn+wt Ctrl sirna+ifn+67 10 6 ISG15 sirna+ifn+wt ISG15 sirna+ifn+67 10 5 10 4 10 3 Free ISG15 10
a ISG15 sirna Ctrl #1 #2 ISG15 conjugates IB: ISG15 Virus titer (Pfu/ml) b ISG15 sirna #2 10 7 Ctrl sirna+ifn+wt Ctrl sirna+ifn+67 10 6 ISG15 sirna+ifn+wt ISG15 sirna+ifn+67 10 5 10 4 10 3 Free ISG15 10
number Done by Corrected by Doctor Ashraf
 number 4 Done by Nedaa Bani Ata Corrected by Rama Nada Doctor Ashraf Genome replication and gene expression Remember the steps of viral replication from the last lecture: Attachment, Adsorption, Penetration,
number 4 Done by Nedaa Bani Ata Corrected by Rama Nada Doctor Ashraf Genome replication and gene expression Remember the steps of viral replication from the last lecture: Attachment, Adsorption, Penetration,
Different De Novo Initiation Strategies Are Used by Influenza Virus RNA Polymerase on Its crna and Viral RNA Promoters during Viral RNA Replication
 JOURNAL OF VIROLOGY, Mar. 2006, p. 2337 2348 Vol. 80, No. 5 0022-538X/06/$08.00 0 doi:10.1128/jvi.80.5.2337 2348.2006 Copyright 2006, American Society for Microbiology. All Rights Reserved. Different De
JOURNAL OF VIROLOGY, Mar. 2006, p. 2337 2348 Vol. 80, No. 5 0022-538X/06/$08.00 0 doi:10.1128/jvi.80.5.2337 2348.2006 Copyright 2006, American Society for Microbiology. All Rights Reserved. Different De
316 Cell 141, , April 16, 2010 ª2010 Elsevier Inc.
 Figure 1. In Vitro Reconstitution of the RIG-I Pathway and Regulation of RIG-I by RNA and Ubiquitination (A) Purification of RIG-I protein from Sendai virus-infected (+SeV) or untreated ( SeV) HEK293T
Figure 1. In Vitro Reconstitution of the RIG-I Pathway and Regulation of RIG-I by RNA and Ubiquitination (A) Purification of RIG-I protein from Sendai virus-infected (+SeV) or untreated ( SeV) HEK293T
Unexpected complexity in the interference activity of a cloned influenza defective interfering RNA
 Meng et al. Virology Journal (2017) 14:138 DOI 10.1186/s12985-017-0805-6 RESEARCH Unexpected complexity in the interference activity of a cloned influenza defective interfering RNA Open Access Bo Meng
Meng et al. Virology Journal (2017) 14:138 DOI 10.1186/s12985-017-0805-6 RESEARCH Unexpected complexity in the interference activity of a cloned influenza defective interfering RNA Open Access Bo Meng
Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v)
 SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
SUPPLEMENTARY MATERIAL AND METHODS Soft Agar Assay. For each cell pool, 100,000 cells were resuspended in 0.35% (w/v) top agar (LONZA, SeaKem LE Agarose cat.5004) and plated onto 0.5% (w/v) basal agar.
RIG-I dependent sensing of poly(da-dt) via the induction of an RNA
 RI-I dependent sensing of via the induction of an RNA polymerase III transcribed RNA intermediate Andrea Ablasser 1*, Franz Bauernfeind 1*, unther Hartmann 1, Eicke Latz 2, Katherine A. Fitzgerald 2* and
RI-I dependent sensing of via the induction of an RNA polymerase III transcribed RNA intermediate Andrea Ablasser 1*, Franz Bauernfeind 1*, unther Hartmann 1, Eicke Latz 2, Katherine A. Fitzgerald 2* and
Unidirectional RNA polymerase I polymerase II transcription system for the generation of influenza A virus from eight plasmids
 Journal of General Virology (2000), 81, 2843 2847. Printed in Great Britain... SHORT COMMUNICATION Unidirectional RNA polymerase I polymerase II transcription system for the generation of influenza A virus
Journal of General Virology (2000), 81, 2843 2847. Printed in Great Britain... SHORT COMMUNICATION Unidirectional RNA polymerase I polymerase II transcription system for the generation of influenza A virus
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature14008 Supplementary Figure 1. Sequence alignment of A/little yellow-shouldered bat/guatemala/060/2010 (H17N10) polymerase with that of human strain A/Victoria/3/75(H3N2). The secondary
doi:10.1038/nature14008 Supplementary Figure 1. Sequence alignment of A/little yellow-shouldered bat/guatemala/060/2010 (H17N10) polymerase with that of human strain A/Victoria/3/75(H3N2). The secondary
1. TLR. TLR Toll-like receptors. Toll Toll-like receptor, TLR TLR TLR TLR. type I TLR TLR. Toll
 54pp.145 152 2004 1. TLR T B TLR Toll-like receptors TLR TLR I IFN TLR T B B T Toll NF- B 1996 565-0871 3-1 TEL 06-6879-8303 FAX 06-6879-8305 E-mail uemattsu@biken.osaka-u.ac.jp Toll Toll-like receptor,
54pp.145 152 2004 1. TLR T B TLR Toll-like receptors TLR TLR I IFN TLR T B B T Toll NF- B 1996 565-0871 3-1 TEL 06-6879-8303 FAX 06-6879-8305 E-mail uemattsu@biken.osaka-u.ac.jp Toll Toll-like receptor,
Materials and Methods , The two-hybrid principle.
 The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
Hepatitis B Antiviral Drug Development Multi-Marker Screening Assay
 Hepatitis B Antiviral Drug Development Multi-Marker Screening Assay Background ImQuest BioSciences has developed and qualified a single-plate method to expedite the screening of antiviral agents against
Hepatitis B Antiviral Drug Development Multi-Marker Screening Assay Background ImQuest BioSciences has developed and qualified a single-plate method to expedite the screening of antiviral agents against
Structural vs. nonstructural proteins
 Why would you want to study proteins associated with viruses or virus infection? Receptors Mechanism of uncoating How is gene expression carried out, exclusively by viral enzymes? Gene expression phases?
Why would you want to study proteins associated with viruses or virus infection? Receptors Mechanism of uncoating How is gene expression carried out, exclusively by viral enzymes? Gene expression phases?
LETTERS. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses
 doi:10.1038/nature04734 Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses Hiroki Kato 1,3 *, Osamu Takeuchi 1,3 *, Shintaro Sato 3, Mitsutoshi Yoneyama 4, Masahiro Yamamoto
doi:10.1038/nature04734 Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses Hiroki Kato 1,3 *, Osamu Takeuchi 1,3 *, Shintaro Sato 3, Mitsutoshi Yoneyama 4, Masahiro Yamamoto
The RNA Polymerase of Influenza Virus, Bound to the 5 End of Virion RNA, Acts in cis To Polyadenylate mrna
 JOURNAL OF VIROLOGY, Oct. 1998, p. 8214 8219 Vol. 72, No. 10 0022-538X/98/$04.00 0 Copyright 1998, American Society for Microbiology. All Rights Reserved. The RNA Polymerase of Influenza Virus, Bound to
JOURNAL OF VIROLOGY, Oct. 1998, p. 8214 8219 Vol. 72, No. 10 0022-538X/98/$04.00 0 Copyright 1998, American Society for Microbiology. All Rights Reserved. The RNA Polymerase of Influenza Virus, Bound to
Islamic University Faculty of Medicine
 Islamic University Faculty of Medicine 2012 2013 2 RNA is a modular structure built from a combination of secondary and tertiary structural motifs. RNA chains fold into unique 3 D structures, which act
Islamic University Faculty of Medicine 2012 2013 2 RNA is a modular structure built from a combination of secondary and tertiary structural motifs. RNA chains fold into unique 3 D structures, which act
Role for the Paramyxovirus Genomic Promoter in Limiting Host Cell Antiviral Responses and Cell Killing
 JOURNAL OF VIROLOGY, Sept. 2009, p. 9057 9067 Vol. 83, No. 18 0022-538X/09/$08.00 0 doi:10.1128/jvi.01055-09 Copyright 2009, American Society for Microbiology. All Rights Reserved. Role for the Paramyxovirus
JOURNAL OF VIROLOGY, Sept. 2009, p. 9057 9067 Vol. 83, No. 18 0022-538X/09/$08.00 0 doi:10.1128/jvi.01055-09 Copyright 2009, American Society for Microbiology. All Rights Reserved. Role for the Paramyxovirus
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Coronaviruses. Virion. Genome. Genes and proteins. Viruses and hosts. Diseases. Distinctive characteristics
 Coronaviruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Spherical enveloped particles studded with clubbed spikes Diameter 120-160 nm Coiled helical
Coronaviruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Spherical enveloped particles studded with clubbed spikes Diameter 120-160 nm Coiled helical
Supplementary information
 Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
October 26, Lecture Readings. Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell
 October 26, 2006 Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell 1. Secretory pathway a. Formation of coated vesicles b. SNAREs and vesicle targeting 2. Membrane fusion a. SNAREs
October 26, 2006 Vesicular Trafficking, Secretory Pathway, HIV Assembly and Exit from Cell 1. Secretory pathway a. Formation of coated vesicles b. SNAREs and vesicle targeting 2. Membrane fusion a. SNAREs
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Lecture 2: Virology. I. Background
 Lecture 2: Virology I. Background A. Properties 1. Simple biological systems a. Aggregates of nucleic acids and protein 2. Non-living a. Cannot reproduce or carry out metabolic activities outside of a
Lecture 2: Virology I. Background A. Properties 1. Simple biological systems a. Aggregates of nucleic acids and protein 2. Non-living a. Cannot reproduce or carry out metabolic activities outside of a
Mismatches in Influenza A virus vrna panhandle prevent. RIG-I sensing by impairing RNA/RIG-I complex formation
 JVI Accepted Manuscript Posted Online 7 October 215 J. Virol. doi:1.1128/jvi.1671-15 Copyright 215, American Society for Microbiology. All Rights Reserved. 1 2 Mismatches in Influenza A virus vrna panhandle
JVI Accepted Manuscript Posted Online 7 October 215 J. Virol. doi:1.1128/jvi.1671-15 Copyright 215, American Society for Microbiology. All Rights Reserved. 1 2 Mismatches in Influenza A virus vrna panhandle
Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS
 Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS nucleotide sequences (a, b) or amino acid sequences (c) from
Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS nucleotide sequences (a, b) or amino acid sequences (c) from
Short Communication. Southwestern Medical Center, Dallas, TX, USA
 Journal of General Virology (2009), 90, 74 78 DOI 10.1099/vir.0.005389-0 Short Communication Correspondence Søren R. Paludan srp@microbiology.au.dk Herpes simplex virus infection is sensed by both Toll-like
Journal of General Virology (2009), 90, 74 78 DOI 10.1099/vir.0.005389-0 Short Communication Correspondence Søren R. Paludan srp@microbiology.au.dk Herpes simplex virus infection is sensed by both Toll-like
Supplementary Figure 1
 Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
Translation. Host Cell Shutoff 1) Initiation of eukaryotic translation involves many initiation factors
 Translation Questions? 1) How does poliovirus shutoff eukaryotic translation? 2) If eukaryotic messages are not translated how can poliovirus get its message translated? Host Cell Shutoff 1) Initiation
Translation Questions? 1) How does poliovirus shutoff eukaryotic translation? 2) If eukaryotic messages are not translated how can poliovirus get its message translated? Host Cell Shutoff 1) Initiation
Life Sciences 1A Midterm Exam 2. November 13, 2006
 Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
hemagglutinin and the neuraminidase genes (RNA/recombinant viruses/polyacrylamide gel electrophoresis/genetics)
 Proc. Natl. Acad. Sci. USA Vol. 73, No. 6, pp. 242-246, June 976 Microbiology Mapping of the influenza virus genome: Identification of the hemagglutinin and the neuraminidase genes (RNA/recombinant viruses/polyacrylamide
Proc. Natl. Acad. Sci. USA Vol. 73, No. 6, pp. 242-246, June 976 Microbiology Mapping of the influenza virus genome: Identification of the hemagglutinin and the neuraminidase genes (RNA/recombinant viruses/polyacrylamide
Plasmid-Driven Formation of Influenza Virus-Like Particles
 JOURNAL OF VIROLOGY, Jan. 2000, p. 547 551 Vol. 74, No. 1 0022-538X/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Plasmid-Driven Formation of Influenza Virus-Like
JOURNAL OF VIROLOGY, Jan. 2000, p. 547 551 Vol. 74, No. 1 0022-538X/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Plasmid-Driven Formation of Influenza Virus-Like
LESSON 4.4 WORKBOOK. How viruses make us sick: Viral Replication
 DEFINITIONS OF TERMS Eukaryotic: Non-bacterial cell type (bacteria are prokaryotes).. LESSON 4.4 WORKBOOK How viruses make us sick: Viral Replication This lesson extends the principles we learned in Unit
DEFINITIONS OF TERMS Eukaryotic: Non-bacterial cell type (bacteria are prokaryotes).. LESSON 4.4 WORKBOOK How viruses make us sick: Viral Replication This lesson extends the principles we learned in Unit
 http://nmhm.washingtondc.museum/collections/archives/agalleries/1918flu/ncp1603.jpg 1 https://assets-production-webvanta-com.s3-us-west- 2 2.amazonaws.com/000000/47/62/original/images/img_109_influenza/Spanish_flu_death_chart.jpg
http://nmhm.washingtondc.museum/collections/archives/agalleries/1918flu/ncp1603.jpg 1 https://assets-production-webvanta-com.s3-us-west- 2 2.amazonaws.com/000000/47/62/original/images/img_109_influenza/Spanish_flu_death_chart.jpg
Cellular cap-binding proteins associate with influenza virus mrnas
 Journal of General Virology (2011), 92, 1627 1634 DOI 10.1099/vir.0.029231-0 Cellular cap-binding proteins associate with influenza virus mrnas Katja Bier, Ashley York and Ervin Fodor Correspondence Ervin
Journal of General Virology (2011), 92, 1627 1634 DOI 10.1099/vir.0.029231-0 Cellular cap-binding proteins associate with influenza virus mrnas Katja Bier, Ashley York and Ervin Fodor Correspondence Ervin
Overview: Chapter 19 Viruses: A Borrowed Life
 Overview: Chapter 19 Viruses: A Borrowed Life Viruses called bacteriophages can infect and set in motion a genetic takeover of bacteria, such as Escherichia coli Viruses lead a kind of borrowed life between
Overview: Chapter 19 Viruses: A Borrowed Life Viruses called bacteriophages can infect and set in motion a genetic takeover of bacteria, such as Escherichia coli Viruses lead a kind of borrowed life between
Basis and Clinical Applications of Interferon
 Interferon Therapy Basis and Clinical Applications of Interferon JMAJ 47(1): 7 12, 2004 Jiro IMANISHI Professor, Kyoto Prefectural University of Medicine Abstract: Interferon (IFN) is an antiviral substance
Interferon Therapy Basis and Clinical Applications of Interferon JMAJ 47(1): 7 12, 2004 Jiro IMANISHI Professor, Kyoto Prefectural University of Medicine Abstract: Interferon (IFN) is an antiviral substance
Technology Overview. Summary
 Live Attenuated Influenza Vaccines with Altered NS1 Technology Overview Summary Transformative Technology: Live attenuated influenza vaccines (LAIVs) with precise, genetically stable truncations of the
Live Attenuated Influenza Vaccines with Altered NS1 Technology Overview Summary Transformative Technology: Live attenuated influenza vaccines (LAIVs) with precise, genetically stable truncations of the
Activation of MDA5 Requires Higher-Order RNA Structures Generated during Virus Infection
 JOURNAL OF VIROLOGY, Oct. 2009, p. 10761 10769 Vol. 83, No. 20 0022-538X/09/$08.00 0 doi:10.1128/jvi.00770-09 Copyright 2009, American Society for Microbiology. All Rights Reserved. Activation of MDA5
JOURNAL OF VIROLOGY, Oct. 2009, p. 10761 10769 Vol. 83, No. 20 0022-538X/09/$08.00 0 doi:10.1128/jvi.00770-09 Copyright 2009, American Society for Microbiology. All Rights Reserved. Activation of MDA5
10th International Rotavirus Symposium Bangkok, Thailand
 Rotavirus Host Range Restriction and Innate Immunity: Mechanisms of Vaccine Attenuation Harry Greenberg MD Stanford University 10th International Rotavirus Symposium Bangkok, Thailand 09/19/12 B dsrna
Rotavirus Host Range Restriction and Innate Immunity: Mechanisms of Vaccine Attenuation Harry Greenberg MD Stanford University 10th International Rotavirus Symposium Bangkok, Thailand 09/19/12 B dsrna
Reverse Genetics of RNA Viruses
 Reverse Genetics of RNA Viruses Reverse Genetics (RG) he creation of a virus with a fulllength copy of the viral genome he most powerful tool in modern virology RG of RNA viruses Generation or recovery
Reverse Genetics of RNA Viruses Reverse Genetics (RG) he creation of a virus with a fulllength copy of the viral genome he most powerful tool in modern virology RG of RNA viruses Generation or recovery
As the first line of host defense, the innate
 HEPATOLOGY, VOL. 65, NO. 5, 2017 AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES Laboratory of Genetics and Physiology 2 (LGP2) Plays an Essential Role in Hepatitis C Virus Infection-Induced Interferon
HEPATOLOGY, VOL. 65, NO. 5, 2017 AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES Laboratory of Genetics and Physiology 2 (LGP2) Plays an Essential Role in Hepatitis C Virus Infection-Induced Interferon
Supplementary information. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins
 Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells
 MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells Margaret S Ebert, Joel R Neilson & Phillip A Sharp Supplementary figures and text: Supplementary Figure 1. Effect of sponges on
Human Genome: Mapping, Sequencing Techniques, Diseases
 Human Genome: Mapping, Sequencing Techniques, Diseases Lecture 4 BINF 7580 Fall 2005 1 Let us review what we talked about at the previous lecture. Please,... 2 The central dogma states that the transfer
Human Genome: Mapping, Sequencing Techniques, Diseases Lecture 4 BINF 7580 Fall 2005 1 Let us review what we talked about at the previous lecture. Please,... 2 The central dogma states that the transfer
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
The Early Interferon Response to Rotavirus Is Regulated by PKR and Depends on MAVS/IPS-1, RIG-I, MDA-5, and IRF3
 JOURNAL OF VIROLOGY, Apr. 2011, p. 3717 3732 Vol. 85, No. 8 0022-538X/11/$12.00 doi:10.1128/jvi.02634-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. The Early Interferon Response
JOURNAL OF VIROLOGY, Apr. 2011, p. 3717 3732 Vol. 85, No. 8 0022-538X/11/$12.00 doi:10.1128/jvi.02634-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. The Early Interferon Response
A Yersinia-Secreted Effector Protein Promotes Virulence by Preventing Inflammasome Recognition of the Type III Secretion System
 Cell Host & Microbe, Volume 7 Supplemental Information A Yersinia-Secreted Effector Protein Promotes Virulence by Preventing Inflammasome Recognition of the Type III Secretion System Igor E. Brodsky, Noah
Cell Host & Microbe, Volume 7 Supplemental Information A Yersinia-Secreted Effector Protein Promotes Virulence by Preventing Inflammasome Recognition of the Type III Secretion System Igor E. Brodsky, Noah
Viral Genetics. BIT 220 Chapter 16
 Viral Genetics BIT 220 Chapter 16 Details of the Virus Classified According to a. DNA or RNA b. Enveloped or Non-Enveloped c. Single-stranded or double-stranded Viruses contain only a few genes Reverse
Viral Genetics BIT 220 Chapter 16 Details of the Virus Classified According to a. DNA or RNA b. Enveloped or Non-Enveloped c. Single-stranded or double-stranded Viruses contain only a few genes Reverse
AP Biology. Viral diseases Polio. Chapter 18. Smallpox. Influenza: 1918 epidemic. Emerging viruses. A sense of size
 Hepatitis Viral diseases Polio Chapter 18. Measles Viral Genetics Influenza: 1918 epidemic 30-40 million deaths world-wide Chicken pox Smallpox Eradicated in 1976 vaccinations ceased in 1980 at risk population?
Hepatitis Viral diseases Polio Chapter 18. Measles Viral Genetics Influenza: 1918 epidemic 30-40 million deaths world-wide Chicken pox Smallpox Eradicated in 1976 vaccinations ceased in 1980 at risk population?
Influence of Host Cell Defence during Influenza Vaccine Production in MDCK Cells
 Vaccine Technology III, 07 June 2010 Puerto Vallarta/Mexico Influence of Host Cell Defence during Influenza Vaccine Production in MDCK Cells T. Frensing 1, C. Seitz 1, B. Heynisch 2 and U. Reichl 1/2 1
Vaccine Technology III, 07 June 2010 Puerto Vallarta/Mexico Influence of Host Cell Defence during Influenza Vaccine Production in MDCK Cells T. Frensing 1, C. Seitz 1, B. Heynisch 2 and U. Reichl 1/2 1
Size nm m m
 1 Viral size and organization Size 20-250nm 0.000000002m-0.000000025m Virion structure Capsid Core Acellular obligate intracellular parasites Lack organelles, metabolic activities, and reproduction Replicated
1 Viral size and organization Size 20-250nm 0.000000002m-0.000000025m Virion structure Capsid Core Acellular obligate intracellular parasites Lack organelles, metabolic activities, and reproduction Replicated
SUPPLEMENT ARTICLE. Valeria Cannas, Gian Luca Daino, Angela Corona, Francesca Esposito, and Enzo Tramontano
 SUPPLEMENT ARTICLE A Luciferase Reporter Gene Assay to Measure Ebola Virus Viral Protein 35 Associated Inhibition of Double-Stranded RNA Stimulated, Retinoic Acid Inducible Gene 1 Mediated Induction of
SUPPLEMENT ARTICLE A Luciferase Reporter Gene Assay to Measure Ebola Virus Viral Protein 35 Associated Inhibition of Double-Stranded RNA Stimulated, Retinoic Acid Inducible Gene 1 Mediated Induction of
Cell Quality Control. Peter Takizawa Department of Cell Biology
 Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
The reovirus genome comprises 10 segments of doublestranded
 Reovirus reverse genetics: Incorporation of the CAT gene into the reovirus genome Michael R. Roner* and Wolfgang K. Joklik *Department of Biological Sciences, Center for Molecular Biology and Biotechnology,
Reovirus reverse genetics: Incorporation of the CAT gene into the reovirus genome Michael R. Roner* and Wolfgang K. Joklik *Department of Biological Sciences, Center for Molecular Biology and Biotechnology,
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
MDA5 Detects the Double-Stranded RNA Replicative Form in Picornavirus-Infected Cells
 Cell Reports Report MDA5 Detects the Double-Stranded RNA Replicative Form in Picornavirus-Infected Cells Qian Feng, 1,4,5 Stanleyson V. Hato, 1,4,6 Martijn A. Langereis, 1,5 Jan Zoll, 1 Richard Virgen-Slane,
Cell Reports Report MDA5 Detects the Double-Stranded RNA Replicative Form in Picornavirus-Infected Cells Qian Feng, 1,4,5 Stanleyson V. Hato, 1,4,6 Martijn A. Langereis, 1,5 Jan Zoll, 1 Richard Virgen-Slane,
7.012 Problem Set 6 Solutions
 Name Section 7.012 Problem Set 6 Solutions Question 1 The viral family Orthomyxoviridae contains the influenza A, B and C viruses. These viruses have a (-)ss RNA genome surrounded by a capsid composed
Name Section 7.012 Problem Set 6 Solutions Question 1 The viral family Orthomyxoviridae contains the influenza A, B and C viruses. These viruses have a (-)ss RNA genome surrounded by a capsid composed
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/406/ra126/dc1 Supplementary Materials for The microrna mir-485 targets host and influenza virus transcripts to regulate antiviral immunity and restrict viral
www.sciencesignaling.org/cgi/content/full/8/406/ra126/dc1 Supplementary Materials for The microrna mir-485 targets host and influenza virus transcripts to regulate antiviral immunity and restrict viral
DNA codes for RNA, which guides protein synthesis.
 Section 3: DNA codes for RNA, which guides protein synthesis. K What I Know W What I Want to Find Out L What I Learned Vocabulary Review synthesis New RNA messenger RNA ribosomal RNA transfer RNA transcription
Section 3: DNA codes for RNA, which guides protein synthesis. K What I Know W What I Want to Find Out L What I Learned Vocabulary Review synthesis New RNA messenger RNA ribosomal RNA transfer RNA transcription
Relative activity (%) SC35M
 a 125 Bat (H17N) b 125 A/WSN (H1N1) Relative activity (%) 0 75 50 25 Relative activity (%) 0 75 50 25 0 Pos. Neg. PA PB1 Pos. Neg. NP PA PB1 PB2 0 Pos. Neg. NP PA PB1 PB2 SC35M Bat Supplementary Figure
a 125 Bat (H17N) b 125 A/WSN (H1N1) Relative activity (%) 0 75 50 25 Relative activity (%) 0 75 50 25 0 Pos. Neg. PA PB1 Pos. Neg. NP PA PB1 PB2 0 Pos. Neg. NP PA PB1 PB2 SC35M Bat Supplementary Figure
Avian Influenza Virus H7N9. Dr. Di Liu Network Information Center Institute of Microbiology Chinese Academy of Sciences
 Avian Influenza Virus H7N9 Dr. Di Liu Network Information Center Institute of Microbiology Chinese Academy of Sciences Avian Influenza Virus RNA virus, Orthomyxoviruses Influenza A virus Eight Gene segments
Avian Influenza Virus H7N9 Dr. Di Liu Network Information Center Institute of Microbiology Chinese Academy of Sciences Avian Influenza Virus RNA virus, Orthomyxoviruses Influenza A virus Eight Gene segments
MedChem 401~ Retroviridae. Retroviridae
 MedChem 401~ Retroviridae Retroviruses plus-sense RNA genome (!8-10 kb) protein capsid lipid envelop envelope glycoproteins reverse transcriptase enzyme integrase enzyme protease enzyme Retroviridae The
MedChem 401~ Retroviridae Retroviruses plus-sense RNA genome (!8-10 kb) protein capsid lipid envelop envelope glycoproteins reverse transcriptase enzyme integrase enzyme protease enzyme Retroviridae The
Enzyme-coupled Receptors. Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors
 Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Enzyme-coupled Receptors Cell-surface receptors 1. Ion-channel-coupled receptors 2. G-protein-coupled receptors 3. Enzyme-coupled receptors Cell-surface receptors allow a flow of ions across the plasma
Tel: ; Fax: ;
 Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
Multifunctional Adaptive NS1 Mutations Are Selected upon Human Influenza Virus Evolution in the Mouse
 Multifunctional Adaptive NS1 Mutations Are Selected upon Human Influenza Virus Evolution in the Mouse Nicole E. Forbes 1,2, Jihui Ping 1,2, Samar K. Dankar 1,2, Jian-Jun Jia 1,2, Mohammed Selman 1,2, Liya
Multifunctional Adaptive NS1 Mutations Are Selected upon Human Influenza Virus Evolution in the Mouse Nicole E. Forbes 1,2, Jihui Ping 1,2, Samar K. Dankar 1,2, Jian-Jun Jia 1,2, Mohammed Selman 1,2, Liya
Problem Set 8 Key 1 of 8
 7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
7.06 2003 Problem Set 8 Key 1 of 8 7.06 2003 Problem Set 8 Key 1. As a bright MD/PhD, you are interested in questions about the control of cell number in the body. Recently, you've seen three patients
Page 32 AP Biology: 2013 Exam Review CONCEPT 6 REGULATION
 Page 32 AP Biology: 2013 Exam Review CONCEPT 6 REGULATION 1. Feedback a. Negative feedback mechanisms maintain dynamic homeostasis for a particular condition (variable) by regulating physiological processes,
Page 32 AP Biology: 2013 Exam Review CONCEPT 6 REGULATION 1. Feedback a. Negative feedback mechanisms maintain dynamic homeostasis for a particular condition (variable) by regulating physiological processes,
Virus and Prokaryotic Gene Regulation - 1
 Virus and Prokaryotic Gene Regulation - 1 We have discussed the molecular structure of DNA and its function in DNA duplication and in transcription and protein synthesis. We now turn to how cells regulate
Virus and Prokaryotic Gene Regulation - 1 We have discussed the molecular structure of DNA and its function in DNA duplication and in transcription and protein synthesis. We now turn to how cells regulate
The Coxsackievirus B 3C pro Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling
 The Coxsackievirus B 3C pro Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling Amitava Mukherjee 1, Stefanie A. Morosky 2, Elizabeth Delorme-Axford 1, Naomi Dybdahl-Sissoko
The Coxsackievirus B 3C pro Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling Amitava Mukherjee 1, Stefanie A. Morosky 2, Elizabeth Delorme-Axford 1, Naomi Dybdahl-Sissoko
S1a S1b S1c. S1d. S1f S1g S1h SUPPLEMENTARY FIGURE 1. - si sc Il17rd Il17ra bp. rig/s IL-17RD (ng) -100 IL-17RD
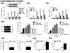 SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
Follow this and additional works at: Part of the Medicine and Health Sciences Commons
 Washington University School of Medicine Digital Commons@Becker Open Access Publications 2009 Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2009 Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature05732 SUPPLEMENTARY INFORMATION Supplemental Data Supplement Figure Legends Figure S1. RIG-I 2CARD undergo robust ubiquitination a, (top) At 48 h posttransfection with a GST, GST-RIG-I-2CARD
doi: 10.1038/nature05732 SUPPLEMENTARY INFORMATION Supplemental Data Supplement Figure Legends Figure S1. RIG-I 2CARD undergo robust ubiquitination a, (top) At 48 h posttransfection with a GST, GST-RIG-I-2CARD
The early interferon response to rotavirus is regulated by PKR and depends on
 JVI Accepts, published online ahead of print on February 0 J. Virol. doi:./jvi.0- Copyright 0, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved. 1 The early
JVI Accepts, published online ahead of print on February 0 J. Virol. doi:./jvi.0- Copyright 0, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved. 1 The early
Chapter 3 The Induced Responses of Innate Immunity
 Chapter 3 The Induced Responses of Innate Immunity Pattern recognition by cells of the innate immune system Pattern recognition by cells of the innate immune system 4 main pattern recognition receptors
Chapter 3 The Induced Responses of Innate Immunity Pattern recognition by cells of the innate immune system Pattern recognition by cells of the innate immune system 4 main pattern recognition receptors
Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, 7 Center Drive MSC 0720, Bethesda, MD
 Proc. Natl. Acad. Sci. USA Vol. 96, pp. 11259 11264, September 1999 Biochemistry The M2 2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 11259 11264, September 1999 Biochemistry The M2 2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication
The X protein of Borna disease virus serves essential functions in ACCEPTED. Department of Virology, University of Freiburg, Freiburg, Germany
 JVI Accepts, published online ahead of print on 11 April 07 J. Virol. doi:.1128/jvi.02468-06 Copyright 07, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved.
JVI Accepts, published online ahead of print on 11 April 07 J. Virol. doi:.1128/jvi.02468-06 Copyright 07, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved.
CDC website:
 Hepatitis B virus CDC website: http://www.cdc.gov/ncidod/diseases/hepatitis/slideset/hep_b/slide_1.htm Relevance Key Features es of Hepatitis t B Virus 250 million people infected worldwide. In areas of
Hepatitis B virus CDC website: http://www.cdc.gov/ncidod/diseases/hepatitis/slideset/hep_b/slide_1.htm Relevance Key Features es of Hepatitis t B Virus 250 million people infected worldwide. In areas of
Gene Vaccine Dr. Sina Soleimani
 Gene Vaccine Dr. Sina Soleimani Human Viral Vaccines Quality Control Laboratory (HVVQC) Titles 1. A short Introduction of Vaccine History 2. First Lineage of Vaccines 3. Second Lineage of Vaccines 3. New
Gene Vaccine Dr. Sina Soleimani Human Viral Vaccines Quality Control Laboratory (HVVQC) Titles 1. A short Introduction of Vaccine History 2. First Lineage of Vaccines 3. Second Lineage of Vaccines 3. New
numbe r Done by Corrected by Doctor
 numbe r 5 Done by Mustafa Khader Corrected by Mahdi Sharawi Doctor Ashraf Khasawneh Viral Replication Mechanisms: (Protein Synthesis) 1. Monocistronic Method: All human cells practice the monocistronic
numbe r 5 Done by Mustafa Khader Corrected by Mahdi Sharawi Doctor Ashraf Khasawneh Viral Replication Mechanisms: (Protein Synthesis) 1. Monocistronic Method: All human cells practice the monocistronic
