Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells
|
|
|
- Barbra Montgomery
- 5 years ago
- Views:
Transcription
1 Biophysical Journal Volume 84 February Limiting Frequency of the Cochlear Amplifier Based on Electromotility of Outer Hair Cells Mark Ospeck, Xiao-xia Dong, and Kuni H. Iwasa Biophysics Section, National Institute on Deafness and Other Communication Disorders, National Institutes of Health, Bethesda, Maryland ABSTRACT Outer hair cells are the critical element for the sensitivity and sharpness of frequency selectivity of the ear. It is believed that fast motility (electromotility) of these cells is essential for this function. Indeed, force produced by outer hair cells follows their membrane potential very closely at least up to 60 khz. However, it has been pointed out that the cell s receptor potential is attenuated by a low-pass RC circuit inherent to these cells, with the RC roll-off frequencies significantly lower than their operating frequencies. This would render electromotility ineffective in producing force. To address this issue, we assume that multiple degrees of freedom and vibrational modes due to the complex structure of the organ of Corti provide optimal phases for outer hair cells force to cancel viscous drag. Our derived frequency limit depends on the drag-capacitance product, not directly on the RC time constant. With a reasonable assumption for the viscous drag, the estimated limit is khz, exceeding the RC corner frequency. Our analysis shows that a fast-activating potassium current can substantially extend the frequency limit by counteracting the capacitive current. INTRODUCTION The outer hair cell (OHC) is the key element for the sensitivity, dynamic range, and frequency selectivity of the mammalian ear (Liberman and Dodds, 1984; de Boer, 1991; Hubbard and Mountain, 1995). These characteristics of the ear can be realized if the hair cell functions as a feedback amplifier and produces force in such a way that amplifies weak signals. It was Gold (1948) who first pointed out the need for such an amplifier. He considered that the viscous damping of basilar membrane vibration was quite significant whereas the ear s performance appeared as if it was unaffected by such damping. Based on this observation, he predicted that the ear has a feedback amplifier canceling the effect of viscous drag. Although it is now thought that the motion of the basilar membrane is not as heavily damped as Gold expected (Rhode, 1971; Patuzzi, 1996), the presence of a feedback amplifier in the cochlea has been widely accepted (de Boer, 1991; Zweig, 1991; Patuzzi, 1996). In recent years it has been hypothesized that the voltage-dependent motility of the outer hair cell s cylindrical cell body, which is referred to as electromotility, make this mechanosensory cell function as the feedback amplifier (Brownell et al., 1985). The amplitude of this motility is ;5% of the cell length (Ashmore, 1987) and force production by the cell is ;0.1 nn/mv (Hallworth, 1995; Iwasa and Adachi, 1997). The motile mechanism of the outer hair cell is analogous to piezoelectricity in that it is based on direct electromechanical coupling (Iwasa, 1993; Submitted January 21, 2002, and accepted for publication August 12, Address reprint requests to Kuni H. Iwasa, National Institutes of Health, Building 50, Room 4152, 50 South Drive, MSC-8027, Bethesda, MD Tel.: ; Fax: ; iwasa@ nih.gov. Ó 2003 by the Biophysical Society /03/02/739/11 $2.00 Dallos et al., 1993; Iwasa, 1994; Gale and Ashmore, 1994; Kakehata and Santos-Sacchi, 1995; Iwasa, 2001), in which charge is transferred across the membrane (Ashmore, 1990; Santos-Sacchi, 1991; Iwasa, 1993). Such a motile mechanism can be fast because its speed is not limited by diffusion. Indeed, electromotility produces a phase-locked mechanical force without attenuation at least up to 60 khz (Frank et al., 1999). Moreover, prestin, a protein that reproduces most of the characteristics of the motor, has been identified (Zheng et al., 2000). These observations still do not definitively answer the question as to whether voltage-dependent motility is indeed responsible for the cell s functioning as a feedback amplifier. The main reason for this reservation is the so-called RC time constant problem. Because the mammalian hearing frequency range extends quite high (to 20 khz for humans and 40 khz for guinea pigs), at these higher frequencies the largest part of the mechanotransducer current must be used for charging and discharging the cell s membrane capacitance. It has been argued that the rate of charging and discharging is limited by the resistance of the cell, producing the RC time constant which determines the roll-off of the receptor potential. These roll-off frequencies are significantly lower than the cells operating frequencies (Housley and Ashmore, 1992). Inasmuch as the cell s force production is voltagedependent, the attenuation of its receptor potential due to the RC time constant problem will limit its force output. An additional reservation on the role of electromotility of the OHC is that it may not be the only motile mechanism which can counteract viscous drag. Nonmammalian hair cells, which do not possess somatic motility, produce active force in their hair bundles (Martin and Hudspeth, 1999; Ricci et al., 2000). Because this motile mechanism is directly associated with the hair bundle s mechanotransducer machinery, it does not depend on the cell s receptor potential, and thus experiences no RC time constant problem.
2 740 Ospeck et al. Although such force generation has not yet been observed in mammalian hair bundles, these observations provide reason to question the role of electromotility in outer hair cells. To address the RC time constant problem, here we describe only a local model, a segment of the cochlea that is resonating. Cochlear traveling waves are generated when lateral coupling through fluid is introduced between local segments. The significance of local resonance in the cochlea has been well recognized (Huxley, 1969; Robles et al., 1976), and local resonance can be considered as a limit where the traveling wave s group velocity substantially decreases while within a region of very light damping (Rhode, 1971; Zweig, 1976; Lighthill, 1981). We will show that OHC electromotility can indeed provide the amplifier function which accounts for the local resonance or light damping region by addressing the following questions: Is the outer hair cell s RC time constant really the limiting factor for the effectiveness of its voltagedriven motility? What is the frequency limit at which voltage-driven motility of the hair cell can function as an amplifier? Can ion channels improve the high-frequency performance of OHC motility? If they do, what would be the characteristics of such channels? AN OPTIMIZED PHASE APPROXIMATION We start by assuming that voltage-driven motility allows the OHC to function as a feedback amplifier. The effectiveness of outer hair cells in exerting force to modulate the vibration of the basilar membrane is determined by their receptor potential. The receptor potential is in turn determined by the bending of their hair bundles, which depends on the vibration of the cochlear partition. The effectiveness of the force generated will depend on the mode of vibration. To treat this rather complex system theoretically, various simplifying assumptions have been introduced. Most of those theories simplified the structure of the cochlear partition and limited the degrees of freedom of its motion to capture the dominant modes of vibration (see Patuzzi, 1996, for review). Instead of simplifying the structure, we simplify our treatment by assuming that this structure is optimally designed to counteract viscous drag. Specifically, we assume that the vibrational modes provide a feedback pathway with the correct phase to optimally exploit the electromotility of outer hair cells. With this approach, we can set an upper limit for the effectiveness of an amplifier that is based on the electromotility of outer hair cells. We assume that viscous drag due to shear between the tectorial membrane and the reticular lamina constitutes the major part of the drag (Allen, 1980) and that it is counteracted by the electromotility of outer hair cells (see discussion). Viscous drag that acts on the basilar membrane is thought to be less important because the boundary layer is thin at higher auditory frequencies (Freeman and Weiss, 1988; Keller and Neu, 1985). Assumptions The major specific assumptions of our approximation are as follows: 1. Bending amplitude of the hair bundles of outer hair cells is proportional to the displacement of the basilar membrane, and is of a similar magnitude. 2. Outer hair cells behave as piezoelectric elements. Their force production is controlled by their membrane potential. Currents elicited by mechanical force applied on these cells are proportional in magnitude with basilar membrane displacements but the phase may differ from that of the basilar membrane. 3. The multiple degrees of freedom in the vibration of the cochlear partition optimizes the phase of OHC feedback force so as to counter viscous drag. 4. The dominant viscous drag in the OHC feedback loop occurs between the tectorial membrane and the reticular lamina (gap drag). The justifications for and implications of these assumptions are discussed later in a section on cochlear mechanics. The model The assumptions that we made are now used to construct a minimal set of equations that describes an outer hair cell feedback loop in vivo. Let us start with membrane currents. The outer hair cell membrane is exposed to two media, endolymph and perilymph, which differ in their electrolyte compositions as well as in their electric potential (Fig. 1 A). The total membrane current consists of a mechanotransducer current at the apical membrane, which is exposed to potassium-rich endolymph at the endocochlear potential e ec, potassium currents at the basolateral membrane with the reversal potential e K, and current due to transferring motor charge (Fig. 1 B). The membrane potential V m satisfies C m dv m dt ¼ðP hb g hb þ a 1 P hb 9 g hb XÞðe ec ÿ V m Þ þðe K ÿ V m ÞPg K ÿ a 2 f dx dt : (1) Here, the hair bundle conductance is represented by the product of the maximum hair bundle conductance g hb and the (resting) open probability P hb of the transducer channels in the hair bundle. P 9 hb is the derivative of this open probability with respect to X, the displacement of the basilar
3 Limiting Frequency of Electromotility 741 FIGURE 1 The outer hair cell modeled as a capacitor containing a piezoelectric material which responds with mechanical force to voltage across its cell membrane. (A) Electromechanical feedback loop incorporating the outer hair cell (OHC). The OHC apical surface is a part of the reticular lamina (RL) and has three rows of stereocilia. The tips of the tallest stereocilia maintain firm contact with the tectorial membrane (TM). Its apical surface is bathed in endolymph, high in K þ and maintained at the endocochlear potential of ;þ80 mv. The OHC itself is also high in K þ with a resting potential of approximately ÿ70 mv. Its basolateral membrane is bathed in perilymph, low in K þ and at 0 mv potential. Acoustic stimulation displaces the basilar membrane (BM), which is described by the variable X. Displacement of the BM brings about shear motion between the RL and TM of the same order of magnitude. This shearing stimulates mechanotransduction channels in the stereocilia, producing the receptor potential V m. OHC force (V m ÿv 0 )f counteracts viscous drag ÿgdx/dt. We ignore direct drag on the basilar membrane itself inasmuch as the major drag originates in the gap between the RL and TM which is mechanically coupled to BM oscillation. This feedback loop consists of an electric part, which is described in more detail in (B), and a mechanical part, which is described in (C). (B) The electrical part of our model based on Eq. 1. Four currents charge and discharge outer hair cell capacitance C m. Standing current comes in through the transduction channels P hb g hb and goes out through the cell s K þ conductance P gk, establishing a voltage operating point near ÿ70 mv. A small voltage oscillation about that point is affected by mechanically oscillating the hair bundle conductance P hb 9 g hb x. The quantity f(dx/dt) is the small current necessary to operate the motors and a 1 and a 2 represent phases. (C) The mechanical part of our model based on Eqs. 2 and 3 in which gap drag force ÿa 1 gvx is coupled through an interaction force F int to the basilar membrane which is modeled as a simple harmonic oscillator. The oscillator consists of local BM mass m coupled to a local spring k. We ignore the smaller direct BM drag. This oscillator is driven by a voltagedependent motor force (V m ÿv 0 )f. Our optimized phase approximation states that the phases a 1 and a 3 provided by cochlear mechanics optimize OHC feedback force so as to counteract viscous resistance dominated by gap drag. membrane. g K is the maximum basolateral potassium conductance and P is the open probability of the potassium channel. f is a piezoelectric coefficient for the outer hair cell motor. Piezoelectric current at the lateral wall is proportional to the rate of length changes of the cell (Dong et al., 2002) and thus is proportional to the velocity of basilar membrane motion. To optimize phase relationships later on, we introduce adjustable phase factors a n with n ¼ 1, 2,..., which are complex numbers with an absolute value of unity, i.e., ja n j¼1. The displacement of the basilar membrane follows the equation, m d2 X dt 2 ¼ÿmv2XþFþF 0 int (2) because drag on the basilar membrane is not important (see section on cochlear mechanics). Here m is the mass that moves together with a local section of the basilar membrane, v 0 the natural resonance frequency of the basilar membrane, and F the force applied to the basilar membrane by fluid pressure due to acoustic stimulation (Fig. 1 C). F int represents interaction terms with other degrees of freedom or other modes of motion. In a steady state, energy loss due to gap drag is balanced by energy supplied by outer hair cells. Since we assume that the amplitude of basilar membrane motion is the same as the amplitude of gap shear, this energy balance can be translated into force balance if force that is produced by outer hair cells is utilized optimally. This balance leads to the equation, F int ¼ a 3 ðv m ÿ V 0 Þf ÿ a 1 g dx dt : (3) Here (V m V 0 )f is the force applied by the outer hair cell due to electromotility. The coefficient f in the equation is the same as in Eq. 1 because of the piezoelectric reciprocal relationship (Assumption 2) (Dong et al., 2002). The second term is gap drag. As we will discuss later, the boundary-layer thickness is always larger than the gap. Thus, gap drag is proportional to the drag coefficient g and to velocity, which is in turn proportional to frequency (Batchelor, 1967; Allen, 1980; Nobili and Mammano, 1993). What results is a one-degree-of-freedom description of basilar membrane vibration in which gap drag directly damps its oscillation (Fig. 1 C), analogous to a one-dimensional rendering (Kanis and de Boer, 1993) of a two-mass system (Neely and Kim, 1986). For example, it can be shown in a two-mass system that condition (Assumption 3) is realized when the ratio of drag force to coupling spring force is small. The argument of a 1 then becomes constrained to near zero by the requirement that gap drag in Eq. 3 functions as a damping factor on basilar membrane vibration. Gap drag ÿgdx/dt can be nullified by the effect of hair cell feedback force if their phases oppose whereas the magnitudes of the two terms agree. That is, a 1 g dx dt ¼ a 3 ðv m ÿ V 0 Þf: (4) For convenience, we call this Gold s condition, which he proposed in his pioneering work (Gold, 1948). Inasmuch as we are interested in finding the frequency limit of the system, we assume that it is driven by a small
4 742 Ospeck et al. acoustic stimulation at frequency v, and then solve for the steady-state solution. Thus we put, F ¼ a 5 f exp½ivtš; (5) V m ¼ V 0 þ y exp½ivtš; (6) X ¼ a 4 x exp½ivtš; (7) where the quantities expressed by lower case symbols are small. To proceed we need to know values for the parameters. We show the best estimates for these parameters in Tables 1 and 2. In the following, we examine the first-order terms with respect to these small quantities. Eq. 1 provides a relationship between y and x, y ¼ a 4 x a 1P9 hb g hb ðe ec ÿ V 0 Þÿia 2 fv ; (8) YðvÞ with the membrane admittance YðvÞ ¼ivC m þ P hb g hb þ Pg K : (9) Numerical examination shows that the second term ia 2 fv in the numerator of Eq. 8 can be ignored because it is much smaller than the first term (see Piezoelectric Resonance discussion). Next, we substitute for y from Eq. 8 into Gold s condition (Eq. 4) so that it reads fp9 hb g hb ðe ec ÿ V 0 Þ ¼ gv; (10) jyðvþj where the phase parameters have been adjusted so that OHC force opposes the drag. CAPACITANCE AND DRAG SET THE LIMITING FREQUENCY Eq. 10 shows that we need to know the admittance Y(v) of the cell membrane to determine the frequency that satisfies Gold s condition. The admittance Y(v) consists of a capacitive part, which is proportional to the frequency, and a conductive part. TABLE 1 Location-insensitive parameters Parameter Value e ec 80 mv* e K ÿ100 mv y V 0 ÿ70 mv z f 0.1 nn/mv *Békésy (1952); Basal turn more positive than apical turn by ;20 mv (Salt et al., 1989). y The Nernst potential with 140 mm K þ inside and 2.7 mm K þ outside the cell. z Cody and Russell (1987). Iwasa and Adachi (1997). TABLE 2 Location-sensitive parameters Apical Basal C m 45 pf* 15 pf* g K 2nS y 50 ns* 100 ns g hb 0.8 ns z 28 ns { P hb 9 g hb 0.03 ns/nm 1 ns/nm k g ÿ7 Ns/m ÿ7 Ns/m** *Housley and Ashmore (1992) y Mammano and Ashmore (1996) z Assumes that the equilibrium current through g K driven by V 0 ÿ e K 30 mv equals the current through half open g hb, driven by e ec ÿ V mv. Russell et al. (1986a) { The largest observed 9.2 ns hair bundle conductance in perilymph (Kros et al., 1992) multiplied by 3 to account for increased conductance in low Ca þ2 endolymph (Kros, 1996). k Obtained by multiplying the steepest slope for hair bundle open probability (25 nm) ÿ1 (Russell et al., 1986b) by the highest estimate for hair bundle conductance g hb 28 ns (Kros, 1996; Kros et al., 1992). We have assumed a 1:1 ratio between hair bundle displacement and basilar membrane displacement (see cochlear mechanics discussion). **Per outer hair cell values for the viscous drag of the gap between the tectorial membrane and reticular lamina (see text after Eq. 11). Let us examine the relative importance of the capacitive current in apical and basal hair cells. If the apical hair cell characterized in Tables 1 and 2 would operate at 100 Hz then its capacitive admittance is 28 ns, about an order-of-magnitude larger than its ionic conductance. A basal cell that operates at 10 khz has a capacitive admittance of ;1 ms, also about an order-of-magnitude larger than the cell s ionic conductance, which is much larger than that of apical cells. Thus, both apical and basal cells are dominated by capacitive admittance (jy(v)j vc m ). Gold s condition Eq. 10 becomes v 2 fp 9hbg hb ðe ec ÿ V 0 Þ ; (11) gc m which gives the frequency limit, above which the efficiency of the outer hair cell as a cochlear amplifier sharply declines. This limiting frequency does depend on the capacitance as expected but not directly on the RC. To obtain the frequency limit, viscous drag needs to be evaluated. Periodic motion in a fluid is characterized by a viscous boundary layer, which is defined by (Batchelor, 1967) rffiffiffiffiffi 2n d ¼ : (12) v Here n is the kinematic viscosity of the fluid, which is the viscosity h divided by the density r. For length scales smaller than d, corresponding to a Reynolds number less than one, drag force predominates over inertial force (Freeman and Weiss, 1988). The thickness of the boundary layer d is ;48 mm at 100 Hz and 5 mm at 10 khz with the value ÿ3 Pa Æ s for
5 Limiting Frequency of Electromotility 743 the viscosity of perilymphatic fluid (Dahl and Kleinfeldt, 1976). The gap between the tectorial membrane and the reticular lamina should be about the same as the length of the stereocilia, which is ;5 mm at the cochlear apex (100 Hz) and 1 mm near the base (10 khz) (Lim, 1980). The gap appears to be always shorter than the boundary layer (Freeman and Weiss, 1988). Thus we can evaluate its drag by the formula hsu/d, where S is the area of one of the confining plates, d is the gap, and U is the relative velocity of the two plates (Batchelor, 1967). For apical cells, the the gap is ;5 mm. One of the two confining plates is 60 mm long by 10 mm wide for a single row of three outer hair cells and one inner hair cell. Shear drag within this gap is shared by three outer hair cells so that the drag per OHC is equivalent to the Stokes drag on a sphere 4 mm in diameter. The resulting frequency limit is ;3 khz, much higher than cell s operating frequency of 100 Hz, which is yet higher than its RC corner frequency of ;10 Hz. This means that apical hair cells are capable of generating more active force than Gold s condition requires. In addition, the RC corner frequency does not directly limit their performance. For basal hair cells, however, capacitive current and gap drag do impose an upper limit. Although the area per outer hair cell is about the same as apical turns, the gap d is ;1 mm for the basal turn. Thus viscous drag is equivalent to a Stokes sphere 21 mm in diameter. Then our set of parameter values for basal hair cells, which could be regarded as somewhat favorable, leads to a limiting frequency of 13 khz. Although this frequency is much higher than the RC corner frequency of 1 khz, it is also much lower than 40 khz, the upper limit of the auditory range for guinea pigs. The so-called RC time constant problem is not relevant when outer hair cells are integrated into the cochlea where slope hair bundle conductance dominates the charging of the cell. Whereas the cell s capacitance C m is an important factor, the membrane resistance (or conductance) enters only indirectly. Instead, the factors important for the characteristic frequency are the sensitivity of hair bundle current to basilar membrane displacement, a piezoelectric parameter of the motor, and the viscous drag. Our analysis thus predicts that electromotility is sufficiently effective except for the basal turn, where the characteristic frequency is between 7 and 40 khz. To operate at the high frequency end of the basal turn, outer hair cells appear to require a mechanism in addition to electromotility. EFFECT OF LARGE NONLINEAR CURRENTS Are there any ways of reducing the capacitive current, which increases with frequency, to control the magnitude of the admittance Y(v)? If ion currents are linear (Ohmic), the ionic conductance increases the absolute value jy(v)j of the admittance function because the phases of the capacitive and conductive components are perpendicular in the complex plane and no destructive interference is possible. However, if ion currents have voltage-dependent kinetics, leading to delayed openings, then the phases of the ionic and capacitive currents can be opposed and, consequently, destructive interference can take place. Such destructive interference can reduce the admittance, raising the frequency limit. In the following, a couple of simple limiting examples are examined. Two-state channel Let us consider that a short OHC contains a voltagedependent potassium channel that has one open state and one closed state. Gating of this channel is represented schematically, 1 ÿ P ðclosedþ ƒƒ! kþ k ÿ P ðopenþ where k þ and k ÿ are transition rates. The probability that this channel is open at time t after the membrane potential is clamped to V m follows a Hodgkin-Huxley type differential equation, dpðv m ; tþ ¼ðP ðv m ÞÿPðV m ; tþþ=t; (13) dt where the relaxation time of the channel is given by t ¼ 1/(k þ þ k ÿ ). P (V m ) is the equilibrium open probability at membrane potential V m. Corresponding to an oscillating membrane potential V m (¼V 0 þ y exp[ivt]), the open probability P of the channel is divided into a steady-state component and an oscillating component, PðV m ; tþ ¼P ðv 0 Þþp exp½ivtš: (14) By substituting Eqs. 14 and 6 into Eq. 13, we obtain p ¼ P 9 y ivt þ 1 : (15) This term contains both a real part, which is in phase with the voltage, and an imaginary part, which lags behind the voltage. P 9 is the voltage derivative of P (V 0 ). By substituting Eq. 15 into Eq. 9, we find that the square of the absolute value of the admittance is 2 jyðvþj 2 tg ¼ v 2 g C m ÿ 1 þðvtþ 2 g 2 g þ P hb g hb þ P ðv 0 Þg K þ 1 þðvtþ ; (16) 2 where g g is the slope conductance of the potassium channel due only to its gating, ; g g ¼ g K P 9 ðv 0 ÿ e K Þ: (17)
6 744 Ospeck et al. Eq. 16 shows that the nonlinear current reduces the effective capacitive current, but also increases conductive current. Because the channel time constant can be chosen so that its effect on capacitive current is greater (vt. 1), the net result is a decrease of the magnitude of the admittance. With this mechanism, the magnitude of the admittance can be reduced by a factor of two and extend the frequency limit up to twofold (Fig. 2 C). From Eq. 16, we can obtain a two-state channel s equivalent inductance under a special condition. If vt is large, the imaginary part of a two-state channel s admittance is g g /(vt). Comparison with the 1/(vL) of an inductor provides that L ¼ t/g g. The corresponding resonance frequency is ðlc m Þ ÿ1=2 ; implying that L must be small to operate at high frequencies. This means that g g must be large and t must be small. How does this inductance compare with known values of other cells? The squid giant axon, with slow potassium channels at a relatively low density, has a specific inductance of 0.2 H cm 2 (Cole, 1968). Applying the specific inductance of the squid to a short OHC with a surface area of ;800 mm 2, we obtain ; H. Given the cell s capacitance this is several-orders-of-magnitude too large to support resonance above 10 khz. To estimate the inductance of OHC, substitute into L ¼ t/g g channel parameters which are appropriate to a short base coil OHC: (V 0 ÿ e K ) ¼ 30 mv, g K ¼ 100 ns, P 9 ¼ 0:1=mV for a voltage sensitivity s ¼ 3 mv, and t ¼ 50 ms. This leads to an inductance of 130 H. Although a two-state channel is not very effective in elevating the operating frequency, the channel does not have to be particularly fast compared to this operating frequency and its time constant does not have to be narrowly tuned. Channels with more complex gating In the following, we examine gating kinetics that are more favorable for sustaining a very high frequency electrical oscillation. One prominent difference between inductive current and current due to a two-state channel is in their instantaneous response to a voltage pulse. Whereas two-state channel current has a component that instantaneously increases on a voltage jump, inductive current does not FIGURE 2 Comparison of two-state and multi-state voltage-gated K þ channels in their ability to reduce OHC admittance and raise operating frequency. (A) Free energy profile of a two-state channel (2SC) and that of a hypothetical four-state channel (4SC). A positive jump in the membrane potential elicits an increase in the open probability of the 2SC with an exponential time course. For the illustrated 4SC, the immediate response is a transition from O3 toc2 on depolarization. This is followed by an increase of the O4 population at the expense of the C1 population. Thus, an initial closing response is followed by an opening upon depolarization, creating a delayed outward current. (B) Four currents elicited by a square voltage pulse: RES, resistive current; 2SC, a simple two-state, voltage-gated K þ channel; 4SC, a four-state, voltage-gated K þ channel; and IND, a perfect inductor in series with a small resistor. The 2SC has a resistive current component due to the channel being partly open at rest. The 4SC first passes current inwardly, effectively reducing the cell s real conductance, before its current turns outward. Thus it mimics an inductive current much better than a 2SC. (C) Performance of a 2SC current in reducing OHC admittance. Contour plot of the admittance Y against conductance g g and the channel s gating rate constant 1/t Æ g g is the slope conductance of the K þ channel due only to its gating. The Ohmic conductance of the cell at its operating point includes the dominant basolateral K þ conductance along with hair bundle conductance. v is the angular frequency. The frequency limit at which the OHC is able to counteract drag is inversely proportional to its admittance. (D) example showing the performance of a 4SC current in reducing OHC admittance. Along the 0.1 contour, virtually all capacitive admittance is eliminated. The 4SC contains an additional fast off current (negative conductance) which eliminates the conductance terms while leaving the slower inductance term in Eq. 16 virtually undisturbed.
7 Limiting Frequency of Electromotility 745 (Fig. 2 B). This feature can be mimicked by ion channels with multiple states. Let us consider a four-state potassium channel with two closed states, c1 and c2, and two open states, o3 and o4 (Fig. 2 A). Suppose the energy levels c1 and o3 go up and c2 and o4 go down on depolarization. Then, on depolarization, the open probability of the channel can quickly decrease before its increasing with a delay (Fig. 2 B). A current through this four-state channel considerably improves the high-frequency performance of short outer hair cells (Fig. 2 D). The more effective reduction of admittance by a four-state channel can be understood as reduction of the conductance term P (V 0 )g k by initial early negative conductance due to a fast gating process. The minimum of the admittance ratio is very low. In an example shown in Fig. 2 D, it is lower than Because the limiting frequency v and the admittance are related by Eq. 10, this means that the frequency limit can exceed 100 khz, high enough to make outer hair cells effective as an amplifier in the auditory range of all mammals even including bats provided that the motor is fast enough to be able to phase-lock to the membrane potential. A mechanism similar to this multiple-state channel is known in hair cells of the turtle and the frog (Crawford and Fettiplace, 1981; Hudspeth and Lewis, 1988). In these cells, a combination of two currents produce a phase delay. Voltage-gated calcium channels are colocalized with calcium-gated, large conductance potassium channels (Roberts et al., 1990). Upon depolarization, an inward current due to Ca 2þ is activated at first, but as Ca 2þ accumulates inside the cell the Ca 2þ -gated potassium channel is activated, eliciting an outward current. Eventually potassium current dominates, reversing the direction of the total current. This mechanism would be advantageous for hair cells of the frog and the turtle because these cells operate at relatively low frequencies (100 Hz). We speculate that this mechanism may not be suitable above 10 khz because it is dependent on Ca 2þ binding/unbinding kinetics, diffusion, and buffering. show that our system is poised on a Hopf bifurcation when Gold s condition is met. When Gold s condition is satisfied, i.e., the drag is exactly canceled by the membrane motor force, the basilar membrane s force-to-displacement transfer function take the shape of a cube-root (Fig. 3, A and B). As we show below, such behavior is a generic property of a Hopf bifurcation, a dynamical instability which occurs when a feedback oscillator has sufficient positive feedback so that it begins to ring spontaneously at small amplitude (Eguíluz et al., 2000). In the following we show the cube-root dependence of the basilar membrane s transfer function. First consider a simple harmonic oscillator, which is described by ðmv 2 0 ÿ mv2 þ igvþx ¼ F: (18) Here the oscillator with mass m is subjected to drag, which is characterized by a drag coefficient g, and driven by periodic external force F having frequency v. If the frequency of driving force is the same as the resonance frequency v 0 of the oscillator, F ¼ igv 0 x. Thus, the phase of the external force F must match that of the velocity iv 0 x. We apply a similar argument to match phases in our model and obtain, i gv ÿ fp9 g hb hb ðe ec ÿ V 0 Þ a 4 x ¼ a 5 f ; (19) vc m TRANSFER FUNCTION NEAR RESONATING POINT To determine the frequency limit, we have analyzed our basic Eqs. 1 and 2 by linear expansion. Then we imposed Gold s condition (Eq. 4) that viscous drag is counteracted by force produced by hair cells. For this purpose, we used the maximum value for displacement-to-conductance sensitivity of the hair bundle. The dominant nonlinearity of the system is the displacement-to-conductance transfer function of the hair bundle. In our linearized treatment, it was expanded to the first-order term (Eq. 11), giving rise to P hb 9 g hb ; where P hb 9 was considered constant. We now assume that the transfer function P hb is not a constant but a Boltzmann function of basilar membrane displacement X and return to Eqs. 1 and 2. We first solve these equations numerically. Then we analytically FIGURE 3 Simulation of our model s performance at high frequency (9.2 khz) when it includes a two-state, large, fast, voltage-dependent K þ conductance, placing the system very near to a Hopf bifurcation. By reducing the voltage-dependence of this conductance, the same system shows low-gain, linear performance. (A) BM amplitude plotted against external forcing. The system shows a compressive nonlinearity in its transfer function when it is poised on a Hopf bifurcation, and linear behavior when poised well below the bifurcation by turning off the voltage sensitivity of the K þ current. (B) Log log re-plot of (A). The slope near the zero-crossing is 1= 3, the dependence expected for a system poised on a Hopf bifurcation. Because the simulation cannot be poised exactly at the bifurcation, there is a small-amplitude forcing region with a linear transfer function. When the voltage-dependence of the fast K þ conductance is absent, the model meets Gold s condition, or poises on a Hopf bifurcation, at 6.2 khz. Increasing the voltage-sensitivity of the fast K þ conductance raised the frequency limit to 9.2 khz (The channel s gating time constant is 30 ms. Its voltage sensitivity s of e-fold current increase was changed from 100 to 2 mv).
8 746 Ospeck et al. from Eqs. 1, 2, 5, and 7. Now with Gold s condition, the left side vanishes if P hb 9 is treated as a constant. However, whereas P hb is in fact a Boltzmann function (Russell et al., 1986b), its higher order terms will become important, P9 hb ðxþ ¼ aeÿax ; (20) ð1 þ e ÿax Þ 2 where a is a constant. If this is expanded near its peak (x ¼ 0), we obtain P9 hb ðxþ ¼ a 4 ÿ a3 x 2 þ... (21) 8 2 Because Gold s condition drops the first (constant) term together with the drag, the second term in Eq. 21 dominates the left side of Eq. 19. Thus x 3 is proportional to external forcing f and we obtain the cube-root dependence of the transfer function expected for a system poised on a Hopf bifurcation. Our analysis demonstrates that on resonance our model, with or without fast potassium conductance, has a cube-root shaped force-to-basilar membrane displacement transfer function (Ruggero, 1992). This result is due to the saturating nonlinearity in the feedback loop, which is described by a Boltzmann function. Without fast potassium conductance the main source of nonlinearity is in the hair bundle s mechanical sensitivity. DISCUSSION Fast-activating currents Our theory predicts that voltage-dependent, large, fast currents should be present in outer hair cells that operate at frequencies above ;13 khz. These currents are unnecessary in hair cells that operate at lower frequencies. According to the frequency-location map (Greenwood, 1990), above 7 khz corresponds to the base turn of the guinea pig cochlea. Further we predict that the effectiveness of these currents will critically depend on their detailed time courses of activation. In the literature, there are two kinds of reports on ionic currents in basal outer hair cells. One of them uses whole-cell voltage clamp on isolated outer hair cells. Current records were obtained from an outer hair cell, isolated from the basal turn with enzyme digestion (Housley and Ashmore, 1992). The length of the cell is 22 mm. Its conductance is ;50 ns and weakly nonlinear. The other is an in situ current clamp experiment (Russell et al., 1986a). The resting conductance is ;100 ns and has a very strong outward rectification (e-fold conductance increase for s ¼ 3 mv), which would be consistent with our prediction. These two kinds of reports have been recognized as inconsistent (Kros, 1996). More recently, fast potassium currents have been found in isolated inner hair cells (Kros et al., 1998) making the absence of fast channels in isolated outer hair cells all the more counterintuitive and puzzling. As Kros has emphasized in his review, cell properties could be significantly altered by the isolation process, so that it is likely that the in situ data (Russell et al., 1986a) better reflects the in vivo condition. To resolve this problem, we are currently examining outer hair cells for fast activating currents. Our preliminary results indicate that short outer hair cells from the basal turn do in fact have fast-activating, outwardly-rectifying currents. The conductance we have observed is large (.100 ns) and it activates in much less than 1 ms at 228C (Ospeck et al., 2002). These fast-activating currents are absent in outer hair cells from more apical turns. Although those apical outer hair cells do have outwardly-rectifying currents, their activation times are greater than 10 ms (Mammano and Ashmore, 1996), much too slow to counteract capacitive current. Cochlear mechanics The local resonance model that we propose is based on four assumptions. Here, we briefly discuss issues in cochlear mechanics related to these assumptions. The significance of local resonance It has been shown that local resonance is an essential element of the mechanics of the cochlea (Huxley, 1969; Patuzzi, 1996). However, unlike a harmonic oscillator s classical resonance peak, the ear s resonant spectral shape is not symmetric with respect to a specific location s characteristic frequency but shows a steep high-frequency cutoff instead. The reason for the asymmetry is due to wave propagation which starts at the cochlea s high-frequency end. Cochlear traveling waves slow down considerably although growing in amplitude as they approach their respective resonance points and then diminish rapidly as they pass the resonance point (Zweig, 1976; Lighthill, 1981). Wave energy is absorbed at the resonance so that the cochlea behaves like a wave-guide with a high frequency cutoff. Shear flow in the gap and basilar membrane displacement The shearing motion of the fluid between the tectorial membrane and the reticular lamina is indispensable to the ear because the stereocilia of the inner hair cell is driven by this shear flow (Freeman and Weiss, 1988). We have already discussed how to evaluate the drag due to shear motion within the gap, assuming a 1:1 ratio of basilar membrane displacement to the shear (Table 2; Assumption 1). In the following we discuss the basis for this ratio. In a system with multiple degrees of freedom, the amplitude of one mode of motion does not necessarily determine the amplitude of another mode. However, the amplitude of basilar membrane displacement and neural output have a tight correlation (Ruggero, 1992). Also the relative movement of the plates that form the gap is the same
9 Limiting Frequency of Electromotility 747 as the deflection of the OHC hair bundle. We estimate a 1:1 ratio of hair bundle displacement to displacement of the basilar membrane based on the following comparison: The bundle is gated from full closed to full open by a deflection of only 80 nm (Russell et al., 1986a). The transfer function for the basilar membrane undergoes a transition from a compressively nonlinear regime to a linear one for ;100 nm peak-to-peak oscillations (Johnstone et al., 1986; Ruggero, 1992). Loss of compressive nonlinearity indicates that the amplifier is being turned off, which should approximately coincide with the hair bundle oscillating between its full closed and full open states hence the ratio of hair bundle deflection to basilar membrane displacement should be ;1:1. Significance of gap drag We assumed that gap drag is the dominant component of the drag in the cochlea and has significant effects on its vibration (Assumption 4). We will briefly discuss the validity of this assumption. It has been experimentally observed that the Q factor in the base coil is sensitive to the ear s condition Q is greatly diminished when the ear dies (see Patuzzi and Robertson, 1988, for review). Sensitivity also decreases reversibly near the characteristic frequency when the endocochlear potential is eliminated by furosemide (Ruggero and Rich, 1991). This reduction in the sensitivity is accompanied by phase lag of ;778. These experimental observations can be understood that drag is indeed important in the basal turn of the cochlea. To reduce gap drag by an active mechanism, more force is required in the basal turn than in the more apical turns because Eq. 2 indicates increased drag force at higher frequencies. In addition to the gap itself, the stereocilia of inner and outer hair cells can contribute to drag. Of the two, we can ignore drag on the stereocilia of outer hair cells. Because the tips of stereocilia of outer hair cells are embedded in the tectorial membrane, these stereocilia are bent at their roots by shear motion of the gap d. The velocity field of the fluid is linear because the gap is smaller than the thickness of the boundary layer d. Thus, the relative motion between the fluid and the stereocilia of outer hair cells is too small to result in significant drag. The hair bundles of inner hair cells may be a more important factor. Because they are freestanding, they bend depending on the balance between drag and bending stiffness. The upper bound of hair bundle drag can be evaluated by using an equivalent cylinder of 10 mm diameter in a shear field (Freeman and Weiss, 1988). Our estimates show that hair bundle drag of an inner hair cell is ;10% of gap drag for apical cells and only ;0.5% for basal cells. Drag that works on the basilar membrane has been modeled. The model indicates that basilar membrane drag is too small to affect cochlear mechanics significantly (Keller and Neu, 1985). Another important source of drag is the interior of the organ of Corti. This drag is very difficult to evaluate because detailed information on the mode of deformation of the organ is required. We can provide only a crude estimate. Assume that a quarter wavelength near the resonance point is of the cellular dimension of ;10 mm (Zweig, 1976) (according to a recent experimental report (Ren, 2002), the wavelength is ;150mm) and that basilar membrane vibration accompanies changes in thickness of the organ of Corti. Because organ of Corti is filled with fluid, changes in its thickness elicit fluid flow, resulting in drag due to exposed cells. For the sake of order estimation, we further assume that the amplitude of thickness change is the same as the amplitude of basilar membrane vibration and that the depth of the fluid space in the organ is the same as the length of the outer hair cell. The resulting drag per outer hair cell is dependent on the wavelength, the fluid viscosity, and the velocity of basilar membrane. The ratio of internal drag evaluated in this way to gap drag per outer hair cell is 3d/d, which is ;0.6 for the basal turn. Thus, this term can reduce the limiting frequency from 13 khz to 10 khz. In more apical turns, this correction is less significant. Our crude estimate appears reasonable from an evolutionary view point. The highly organized organ of Corti is a mammalian innovation that is associated with extending the hearing range into higher frequencies. Thus the structure of the organ of Corti likely minimizes its internal drag so as not to overwhelm gap drag, which is indispensable for the ear to function. Piezoelectric resonance The motile mechanism of outer hair cells is based on highly effective and nonlinear piezoelectricity. In this sense, the mechanism that we propose can be called piezoelectric resonance (Mountain and Hubbard, 1994). However, it is not pure piezoelectric resonance that is based upon an inductance in the equivalent circuits of piezoelectric elements (Ikeda, 1990). A combination of capacitance and inductance forms a resonating circuit, which can overcome the low pass RC filter in sustaining electric oscillation at frequencies higher than the RC roll-off frequency. However, our highfrequency analysis requires that hair cells function simply as an efficient motor and ionic currents function as the inductive element. Why doesn t the system behave as a much simpler piezoelectric resonator? In our treatment presented above, we ignored the piezoelectric reciprocal effect term in Eq. 8 because for basal OHC parameter values at 10 khz it is more than an order-of-magnitude smaller than the hair bundle conductance term. If instead we ignore the hair bundle term in favor of this reciprocal effect fv due to the motor, we obtain v f2 gc m ; (22)
10 748 Ospeck et al. instead of Eq. 11. Eq. 22 obviously represents the piezoelectric resonance frequency, which is only ;750 Hz for our basal OHC parameter values. Should the piezoelectric coefficient f be made larger by a factor of four without significantly increasing cell capacitance, then the reciprocal effect of the motor would be important at 10 khz. However, in order for piezoelectric resonance to prove useful for the ear of bats, which typically have thresholds below 0 db SPL at 80 khz (Kössl and Vater, 1995), a prohibitively large piezoelectric coefficient f would be required. CONCLUSIONS We propose an optimized phase approximation to characterize the outer hair cell feedback loops near to a local resonance inside the mammalian cochlea. This approximation provides the optimal condition for those hair cell s somatic electromotility to counteract viscous drag so as to establish a low loss, or high Q resonance. When we include the transduction channel s saturating nonlinearity in our model we can reproduce the compressive nonlinearity of the mammalian ear s transfer function. Such a nonlinearity is due to the outer hair cell feedback loop being poised on a Hopf bifurcation. Our estimate of the upper frequency limit at which electromotility is effective for the mammalian ear is between 10 and 13 khz. Outer hair cells, except those in the basal turn, should not need any additional mechanism that enhances the effect of electromotility. Those in the basal turn can have their frequency limit raised severalfold if electromotility is aided by fast, voltage-gated ion channels, which mimic inductance. We predict that such inductive channels are present in outer hair cells of the basal turn of the cochlea. REFERENCES Allen, J Cochlear micromechanics a physical model of transduction. J. Acoust. Soc. Am. 68: Ashmore, J. F A fast motile response in guinea-pig outer hair cells: the molecular basis of the cochlear amplifier. J. Physiol. (Lond.). 388: Ashmore, J. F Forward and reverse transduction in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. Neurosci. Res. Suppl. 12:S39 S50. Batchelor, G. K An Introduction to Fluid Dynamics. Cambridge University Press, Cambridge, UK. Békésy, G DC resting potentials inside the cochlear partition. J. Acoust. Soc. Am. 24: Brownell, W., C. Bader, D. Bertrand, and Y. Ribaupierre Evoked mechanical responses of isolated outer hair cells. Science. 227: Cody, A. R., and I. J. Russell The response of hair cells in the basal turn of the guinea-pig cochlea to tones. J. Physiol. (Lond.). 383: Cole, K. S Membranes, Ions, and Impulses. University of California Press, Berkeley, California. Crawford, A. C., and R. Fettiplace An electric tuning mechanism in turtle cochlear hair cells. J. Physiol. (Lond.). 312: Dahl, D., and D. Kleinfeldt Perilymph viscosity as a function of temperature and noise stress. Ann. Acad. Med. Stetin. 14: Dallos, P., R. Hallworth, and B. N. Evans Theory of electrically driven shape changes of cochlear outer hair cells. J. Neurophysiol. 70: de Boer, E Auditory physics. Physical principles in hearing theory. III. Phys. Rep. 203: Dong, X. X., M. Ospeck, and K. H. Iwasa Piezoelectric reciprocal relationship of the membrane motor in the cochlear outer hair cell. Biophys. J. 82: Eguíluz, V. M., M. Ospeck, Y. Choe, A. J. Hudspeth, and M. O. Magnasco Essential nonlinearities in hearing. Phys. Rev. Lett. 84: Frank, G., W. Hemmert, and A. W. Gummer Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proc. Natl. Acad. Sci. USA. 96: Freeman, D. M., and T. F. Weiss The role of fluid inertia in mechanical stimulation of hair cells. Hear. Res. 35: Gale, J. E., and J. F. Ashmore Charge displacement induced by rapid stretch in the basolateral membrane of the guinea-pig outer hair cell. Proc. Roy. Soc. (Lond.) B. Biol. Sci. 255: Gold, T Hearing. II. The physical basis of the action of the cochlea. Proc. Roy. Soc. (Lond.) B. Biol. Sci. 135: Greenwood, D. D A cochlear frequency-position function for several species 29 years later. J. Acoust. Soc. Am. 87: Hallworth, R Passive compliance and active force generation in the guinea pig outer hair cell. J. Neurophysiol. 74: Housley, G. D., and J. F. Ashmore Ionic currents of outer hair cells isolated from the guinea-pig cochlea. J. Physiol. (Lond.). 448: Hubbard, A. E., and D. C. Mountain Analysis and syhthesis of cochlear mechanical function using models. In Auditory Computation. H. L. Hawkins, T. A. McMullen, A. N. Popper, and R. R. Fay, editors. Springer, New York Hudspeth, A. J., and R. S. Lewis A model for electrical resonance and frequency tuning in saccular hair cells of the bull-frog, Rana Catesbeiana. J. Physiol. (Lond.). 400: Huxley, A. F Is resonance possible in the cochlea after all? Nature. 221: Ikeda, T Fundamentals of Piezoelectricity. Oxford University Press, Oxford, UK. Iwasa, K. H Effect of stress on the membrane capacitance of the auditory outer hair cell. Biophys. J. 65: Iwasa, K. H A membrane model for the fast motility of the outer hair cell. J. Acoust. Soc. Am. 96: Iwasa, K. H A two-state piezoelectric model for outer hair cell motility. Biophys. J. 81: Iwasa, K. H., and M. Adachi Force generation in the outer hair cell of the cochlea. Biophys. J. 73: Johnstone, B. M., R. Patuzzi, and G. K. Yates Basilar membrane measurements and the travelling wave. Hear. Res. 22: Kakehata, S., and J. Santos-Sacchi Membrane tension directly shifts voltage dependence of outer hair cell motility and associated gating charge. Biophys. J. 68: Kanis, L. J., and E. de Boer Self-suppression in a locally active nonlinear model of the cochlea. J. Acoust. Soc. Am. 64: Keller, J. B., and J. C. Neu Asymptotic analysis of a viscous cochlear model. J. Acoust. Soc. Am. 77: Kössl, M., and M. Vater Cochlear structure and function of bats. In Hearing by Bats. A. N. Popper, and R. R. Faye, editors. Springer, New York
THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA
 451 THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA DÁIBHID Ó MAOILÉIDIGH, FRANK JÜLICHER Max Planck Institute für Physik komplexer Systeme, Nöthnitzerstr. 38, 01187
451 THE INTERPLAY BETWEEN ACTIVE HAIR BUNDLE MECHANICS AND ELECTROMOTILITY IN THE COCHLEA DÁIBHID Ó MAOILÉIDIGH, FRANK JÜLICHER Max Planck Institute für Physik komplexer Systeme, Nöthnitzerstr. 38, 01187
HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier
 HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier 1 Cochlear Mechanics: Measures of Basilar Membrane Motion 2 Cochlear Mechanics: Measures of Basilar Membrane Motion Bekesy s experiments
HST 721 Lecture 4: Mechanics, electromotility and the cochlear amplifier 1 Cochlear Mechanics: Measures of Basilar Membrane Motion 2 Cochlear Mechanics: Measures of Basilar Membrane Motion Bekesy s experiments
Mechanical Properties of the Cochlea. Reading: Yost Ch. 7
 Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
Mechanical Properties of the Cochlea CF Reading: Yost Ch. 7 The Cochlea Inner ear contains auditory and vestibular sensory organs. Cochlea is a coiled tri-partite tube about 35 mm long. Basilar membrane,
Chapter 3: Anatomy and physiology of the sensory auditory mechanism
 Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Chapter 3: Anatomy and physiology of the sensory auditory mechanism Objectives (1) Anatomy of the inner ear Functions of the cochlear and vestibular systems Three compartments within the cochlea and membranes
Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics
 Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3676 3681, March 1999 Biophysics Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics PAUL J.
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 3676 3681, March 1999 Biophysics Comparing in vitro, in situ, and in vivo experimental data in a three-dimensional model of mammalian cochlear mechanics PAUL J.
What Drives Mechanical Amplification in the Mammalian Cochlea?
 What Drives Mechanical Amplification in the Mammalian Cochlea? Robert H. Withnell, Lauren A. Shaffer, and David J. Lilly The recent report by Peter Dallos and colleagues of the gene and protein responsible
What Drives Mechanical Amplification in the Mammalian Cochlea? Robert H. Withnell, Lauren A. Shaffer, and David J. Lilly The recent report by Peter Dallos and colleagues of the gene and protein responsible
The magnificent outer hair cell of Corti's organ
 The magnificent outer hair cell of Corti's organ Yale University J. Santos-Sacchi School of Medicine, New Haver, CT, USA Abstract The outer hair cell is one of two receptor cell types in the organ of Corti.
The magnificent outer hair cell of Corti's organ Yale University J. Santos-Sacchi School of Medicine, New Haver, CT, USA Abstract The outer hair cell is one of two receptor cell types in the organ of Corti.
Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression
 1398 Biophysical Journal Volume 106 March 2014 1398 1405 Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression Julien Meaud * and Karl Grosh Department
1398 Biophysical Journal Volume 106 March 2014 1398 1405 Effect of the Attachment of the Tectorial Membrane on Cochlear Micromechanics and Two-Tone Suppression Julien Meaud * and Karl Grosh Department
FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA. Invited Paper LINEAR RESPONSE OF THE COCHLEA
 FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA Invited Paper LINEAR RESPONSE OF THE COCHLEA David Alan Bies Visiting Research Fellow Department of Mechanical
FIFTH INTERNATIONAL CONGRESS ON SOUND AND VIBRATION DECEMBER 15-18, 1997 ADELAIDE, SOUTH AUSTRALIA Invited Paper LINEAR RESPONSE OF THE COCHLEA David Alan Bies Visiting Research Fellow Department of Mechanical
Auditory System Feedback
 Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
Feedback Auditory System Feedback Using all or a portion of the information from the output of a system to regulate or control the processes or inputs in order to modify the output. Central control of
Processing of sounds in the inner ear
 Processing of sounds in the inner ear Sripriya Ramamoorthy Associate Professor, IIT Bombay WiSSAP 2018 Cochlea converts sound into electrical signals [Picture courtesy of Northwestern University] von Bekesy
Processing of sounds in the inner ear Sripriya Ramamoorthy Associate Professor, IIT Bombay WiSSAP 2018 Cochlea converts sound into electrical signals [Picture courtesy of Northwestern University] von Bekesy
Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier
 Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier Sripriya Ramamoorthy 1 *, Alfred L. Nuttall 1,2 1 Oregon Hearing Research Center, Department of Otolaryngology, Oregon
Half-Octave Shift in Mammalian Hearing Is an Epiphenomenon of the Cochlear Amplifier Sripriya Ramamoorthy 1 *, Alfred L. Nuttall 1,2 1 Oregon Hearing Research Center, Department of Otolaryngology, Oregon
A truly remarkable aspect of human hearing is the vast
 AUDITORY COMPRESSION AND HEARING LOSS Sid P. Bacon Psychoacoustics Laboratory, Department of Speech and Hearing Science, Arizona State University Tempe, Arizona 85287 A truly remarkable aspect of human
AUDITORY COMPRESSION AND HEARING LOSS Sid P. Bacon Psychoacoustics Laboratory, Department of Speech and Hearing Science, Arizona State University Tempe, Arizona 85287 A truly remarkable aspect of human
An active cochlear model showing sharp tuning and high sensitivity
 Hearing Research, 9 (1983) 123-13 Elsevier Biomedical Press 123 An active cochlear model showing sharp tuning and high sensitivity Stephen T. Neely * and D.O. Kim Box 811, Washington Unitlersit}', St.
Hearing Research, 9 (1983) 123-13 Elsevier Biomedical Press 123 An active cochlear model showing sharp tuning and high sensitivity Stephen T. Neely * and D.O. Kim Box 811, Washington Unitlersit}', St.
HST 721 Efferent Control Lecture October 2004
 HST 721 Efferent Control Lecture October 2004 1 Stapedius Muscle Central Circuitry 2 Hypotheses for MEM Function A. Stapedius 1. Extend Dynamic Range - a gain control system 2. Protect the Inner Ear from
HST 721 Efferent Control Lecture October 2004 1 Stapedius Muscle Central Circuitry 2 Hypotheses for MEM Function A. Stapedius 1. Extend Dynamic Range - a gain control system 2. Protect the Inner Ear from
Cochlear anatomy, function and pathology II. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
Cochlear anatomy, function and pathology II Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (2) on the biophysics of the cochlea, the dual roles
Structure, Energy Transmission and Function. Gross Anatomy. Structure, Function & Process. External Auditory Meatus or Canal (EAM, EAC) Outer Ear
 Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
Gross Anatomy Structure, Energy Transmission and Function IE N O ME 1 Structure, Function & Process 4 External Auditory Meatus or Canal (EAM, EAC) Outer third is cartilaginous Inner 2/3 is osseous Junction
Reticular lamina and basilar membrane vibrations in living mouse cochleae
 Reticular lamina and basilar membrane vibrations in living mouse cochleae Tianying Ren a,1, Wenxuan He a, and David Kemp b a Oregon Hearing Research Center, Department of Otolaryngology, Oregon Health
Reticular lamina and basilar membrane vibrations in living mouse cochleae Tianying Ren a,1, Wenxuan He a, and David Kemp b a Oregon Hearing Research Center, Department of Otolaryngology, Oregon Health
Lecture 6 Hearing 1. Raghav Rajan Bio 354 Neurobiology 2 January 28th All lecture material from the following links unless otherwise mentioned:
 Lecture 6 Hearing 1 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
Lecture 6 Hearing 1 All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/purves_ch12_ch13_hearing
Optimal Electrical Properties of Outer Hair Cells Ensure Cochlear Amplification
 Optimal Electrical Properties of Outer Hair Cells Ensure Cochlear Amplification Jong-Hoon Nam 1,2 *, Robert Fettiplace 3 1 Department of Mechanical Engineering, Department of Biomedical Engineering, University
Optimal Electrical Properties of Outer Hair Cells Ensure Cochlear Amplification Jong-Hoon Nam 1,2 *, Robert Fettiplace 3 1 Department of Mechanical Engineering, Department of Biomedical Engineering, University
Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea
 Article Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea Wei Dong and Elizabeth S. Olson 2, * VA Loma Linda Health Care System and Otolaryngology/Head & Neck Surgery,
Article Two-Tone Suppression of Simultaneous Electrical and Mechanical Responses in the Cochlea Wei Dong and Elizabeth S. Olson 2, * VA Loma Linda Health Care System and Otolaryngology/Head & Neck Surgery,
P T P V U V I. INTRODUCTION. II. MODEL FORMULATION A. Cochlear fluid dynamics.
 Integration of outer hair cell activity in a one-dimensional cochlear model Azaria Cohen and Miriam Furst a) School of Electrical Engineering, Faculty of Engineering, Tel-Aviv University, Tel-Aviv 69978,
Integration of outer hair cell activity in a one-dimensional cochlear model Azaria Cohen and Miriam Furst a) School of Electrical Engineering, Faculty of Engineering, Tel-Aviv University, Tel-Aviv 69978,
The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992)
 The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992) by Pedro da Fonseca (pedrofon@mail.telepac.pt) Neuroscience course Presented in 17.12.99 to professor STEPHEN
The frequency analysis of the cochlea a review of Nobili et al (1998) and Ruggero et al (1992) by Pedro da Fonseca (pedrofon@mail.telepac.pt) Neuroscience course Presented in 17.12.99 to professor STEPHEN
What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components
 1 2 3 4 What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components as a function of time here during the production
1 2 3 4 What does it mean to analyze the frequency components of a sound? A spectrogram such as that shown here is the usual display of frequency components as a function of time here during the production
Systems Neuroscience Oct. 16, Auditory system. http:
 Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
Systems Neuroscience Oct. 16, 2018 Auditory system http: www.ini.unizh.ch/~kiper/system_neurosci.html The physics of sound Measuring sound intensity We are sensitive to an enormous range of intensities,
Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs
 Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs The Auditory Laboratory, Physiology University of Western Australia Greg O Beirne Dept. of Communication
Application of force to the cochlear wall: effect on auditory thresholds, OHC transduction, and DPOAEs The Auditory Laboratory, Physiology University of Western Australia Greg O Beirne Dept. of Communication
ENT 318 Artificial Organs Physiology of Ear
 ENT 318 Artificial Organs Physiology of Ear Lecturer: Ahmad Nasrul Norali The Ear The Ear Components of hearing mechanism - Outer Ear - Middle Ear - Inner Ear - Central Auditory Nervous System Major Divisions
ENT 318 Artificial Organs Physiology of Ear Lecturer: Ahmad Nasrul Norali The Ear The Ear Components of hearing mechanism - Outer Ear - Middle Ear - Inner Ear - Central Auditory Nervous System Major Divisions
THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS?
 JOURNAL OF OTOLOGY THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS? LI Yi 1, He David Z 2 Abstract Cochlear outer hair cells (OHCs) are involved in a mechanical feedback loop in which
JOURNAL OF OTOLOGY THE COCHLEAR AMPLIFIER: IS IT HAIR BUNDLE MOTION OF OUTER HAIR CELLS? LI Yi 1, He David Z 2 Abstract Cochlear outer hair cells (OHCs) are involved in a mechanical feedback loop in which
What is the effect on the hair cell if the stereocilia are bent away from the kinocilium?
 CASE 44 A 53-year-old man presents to his primary care physician with complaints of feeling like the room is spinning, dizziness, decreased hearing, ringing in the ears, and fullness in both ears. He states
CASE 44 A 53-year-old man presents to his primary care physician with complaints of feeling like the room is spinning, dizziness, decreased hearing, ringing in the ears, and fullness in both ears. He states
SPHSC 462 HEARING DEVELOPMENT. Overview Review of Hearing Science Introduction
 SPHSC 462 HEARING DEVELOPMENT Overview Review of Hearing Science Introduction 1 Overview of course and requirements Lecture/discussion; lecture notes on website http://faculty.washington.edu/lawerner/sphsc462/
SPHSC 462 HEARING DEVELOPMENT Overview Review of Hearing Science Introduction 1 Overview of course and requirements Lecture/discussion; lecture notes on website http://faculty.washington.edu/lawerner/sphsc462/
SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS
 SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS Katherine Knisely, D. Bozovic University of California at Los Angeles It has long been observed that there is some form of amplification
SPONTANEOUS OSCILLATIONS IN HAIR CELLS OF THE BULLFROG SACCULUS Katherine Knisely, D. Bozovic University of California at Los Angeles It has long been observed that there is some form of amplification
Auditory Periphery! external middle inner. stapes movement initiates a pressure wave in cochlear fluid
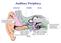 Auditory Periphery! external middle inner sound causes air pressure to increase at eardrum stapes movement initiates a pressure wave in cochlear fluid VIIIth nerve conveys neural signal to cochlear nucleus
Auditory Periphery! external middle inner sound causes air pressure to increase at eardrum stapes movement initiates a pressure wave in cochlear fluid VIIIth nerve conveys neural signal to cochlear nucleus
Can You Hear Me Now?
 An Introduction to the Mathematics of Hearing Department of Applied Mathematics University of Washington April 26, 2007 Some Questions How does hearing work? What are the important structures and mechanisms
An Introduction to the Mathematics of Hearing Department of Applied Mathematics University of Washington April 26, 2007 Some Questions How does hearing work? What are the important structures and mechanisms
Microstructures in the Organ of Corti Help Outer Hair Cells Form Traveling Waves along the Cochlear Coil
 2426 Biophysical Journal Volume 106 June 2014 2426 2433 Microstructures in the Organ of Corti Help Outer Hair Cells Form Traveling Waves along the Cochlear Coil Jong-Hoon Nam* Department of Mechanical
2426 Biophysical Journal Volume 106 June 2014 2426 2433 Microstructures in the Organ of Corti Help Outer Hair Cells Form Traveling Waves along the Cochlear Coil Jong-Hoon Nam* Department of Mechanical
Improving the diagnostic power of otoacoustic emissions. Arturo Moleti Physics Department University of Roma Tor Vergata
 Improving the diagnostic power of otoacoustic emissions Arturo Moleti Physics Department University of Roma Tor Vergata The human ear Ear canal: resonant cavity Middle ear: impedance adapter and pressure
Improving the diagnostic power of otoacoustic emissions Arturo Moleti Physics Department University of Roma Tor Vergata The human ear Ear canal: resonant cavity Middle ear: impedance adapter and pressure
Acoustics Research Institute
 Austrian Academy of Sciences Acoustics Research Institute Modeling Modelingof ofauditory AuditoryPerception Perception Bernhard BernhardLaback Labackand andpiotr PiotrMajdak Majdak http://www.kfs.oeaw.ac.at
Austrian Academy of Sciences Acoustics Research Institute Modeling Modelingof ofauditory AuditoryPerception Perception Bernhard BernhardLaback Labackand andpiotr PiotrMajdak Majdak http://www.kfs.oeaw.ac.at
The transformation of sound stimuli into electrical signals
 The transformation of sound stimuli into electrical signals Robert Fettiplace 2 1 Introduction Our sense of hearing depends on the correct performance of about 15 000 hair cells in each cochlea that serve
The transformation of sound stimuli into electrical signals Robert Fettiplace 2 1 Introduction Our sense of hearing depends on the correct performance of about 15 000 hair cells in each cochlea that serve
Cochlear anatomy, function and pathology I. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology I Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of these lectures Introduction to gross anatomy of the cochlea Focus (1) on
Cochlear anatomy, function and pathology I Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of these lectures Introduction to gross anatomy of the cochlea Focus (1) on
SOLUTIONS Homework #3. Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03
 SOLUTIONS Homework #3 Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03 Problem 1: a) Where in the cochlea would you say the process of "fourier decomposition" of the incoming
SOLUTIONS Homework #3 Introduction to Engineering in Medicine and Biology ECEN 1001 Due Tues. 9/30/03 Problem 1: a) Where in the cochlea would you say the process of "fourier decomposition" of the incoming
Modelling the micromechanics of the cochlea in Femlab
 Modelling the micromechanics of the cochlea in Femlab R.R.J.J. van Doorn DCT 27.7 Traineeship report Coach(es): Supervisor: Prof. S.J. Elliott Prof. P. Gardonio Prof. H. Nijmeijer Technische Universiteit
Modelling the micromechanics of the cochlea in Femlab R.R.J.J. van Doorn DCT 27.7 Traineeship report Coach(es): Supervisor: Prof. S.J. Elliott Prof. P. Gardonio Prof. H. Nijmeijer Technische Universiteit
Auditory System. Barb Rohrer (SEI )
 Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
Auditory System Barb Rohrer (SEI614 2-5086) Sounds arise from mechanical vibration (creating zones of compression and rarefaction; which ripple outwards) Transmitted through gaseous, aqueous or solid medium
BIONB/BME/ECE 4910 Neuronal Simulation Assignments 1, Spring 2013
 BIONB/BME/ECE 4910 Neuronal Simulation Assignments 1, Spring 2013 Tutorial Assignment Page Due Date Week 1/Assignment 1: Introduction to NIA 1 January 28 The Membrane Tutorial 9 Week 2/Assignment 2: Passive
BIONB/BME/ECE 4910 Neuronal Simulation Assignments 1, Spring 2013 Tutorial Assignment Page Due Date Week 1/Assignment 1: Introduction to NIA 1 January 28 The Membrane Tutorial 9 Week 2/Assignment 2: Passive
Comment by Delgutte and Anna. A. Dreyer (Eaton-Peabody Laboratory, Massachusetts Eye and Ear Infirmary, Boston, MA)
 Comments Comment by Delgutte and Anna. A. Dreyer (Eaton-Peabody Laboratory, Massachusetts Eye and Ear Infirmary, Boston, MA) Is phase locking to transposed stimuli as good as phase locking to low-frequency
Comments Comment by Delgutte and Anna. A. Dreyer (Eaton-Peabody Laboratory, Massachusetts Eye and Ear Infirmary, Boston, MA) Is phase locking to transposed stimuli as good as phase locking to low-frequency
The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons.
 1 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
1 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
AUDL GS08/GAV1 Signals, systems, acoustics and the ear. Pitch & Binaural listening
 AUDL GS08/GAV1 Signals, systems, acoustics and the ear Pitch & Binaural listening Review 25 20 15 10 5 0-5 100 1000 10000 25 20 15 10 5 0-5 100 1000 10000 Part I: Auditory frequency selectivity Tuning
AUDL GS08/GAV1 Signals, systems, acoustics and the ear Pitch & Binaural listening Review 25 20 15 10 5 0-5 100 1000 10000 25 20 15 10 5 0-5 100 1000 10000 Part I: Auditory frequency selectivity Tuning
Effects of Remaining Hair Cells on Cochlear Implant Function
 Effects of Remaining Hair Cells on Cochlear Implant Function N1-DC-2-15QPR1 Neural Prosthesis Program N. Hu, P.J. Abbas, C.A. Miller, B.K. Robinson, K.V. Nourski, F. Jeng, B.A. Abkes, J.M. Nichols Department
Effects of Remaining Hair Cells on Cochlear Implant Function N1-DC-2-15QPR1 Neural Prosthesis Program N. Hu, P.J. Abbas, C.A. Miller, B.K. Robinson, K.V. Nourski, F. Jeng, B.A. Abkes, J.M. Nichols Department
The Structure and Function of the Auditory Nerve
 The Structure and Function of the Auditory Nerve Brad May Structure and Function of the Auditory and Vestibular Systems (BME 580.626) September 21, 2010 1 Objectives Anatomy Basic response patterns Frequency
The Structure and Function of the Auditory Nerve Brad May Structure and Function of the Auditory and Vestibular Systems (BME 580.626) September 21, 2010 1 Objectives Anatomy Basic response patterns Frequency
Measurement of cochlear power gain in the sensitive gerbil ear
 Received 4 Nov 2 Accepted 3 Feb 2 Published Mar 2 DOI:.38/ncomms226 Measurement of cochlear power gain in the sensitive gerbil ear Tianying Ren,2, Wenxuan He & Peter G. Gillespie,3 The extraordinary sensitivity
Received 4 Nov 2 Accepted 3 Feb 2 Published Mar 2 DOI:.38/ncomms226 Measurement of cochlear power gain in the sensitive gerbil ear Tianying Ren,2, Wenxuan He & Peter G. Gillespie,3 The extraordinary sensitivity
OtoAcoustic Emissions (OAE s)
 OtoAcoustic Emissions (OAE s) Phenomenon and applications in audiological diagnostics Measurement procedures TEOAE and DPOAE Physiological backgound, functional models Acknowledgment: several illustrations
OtoAcoustic Emissions (OAE s) Phenomenon and applications in audiological diagnostics Measurement procedures TEOAE and DPOAE Physiological backgound, functional models Acknowledgment: several illustrations
to vibrate the fluid. The ossicles amplify the pressure. The surface area of the oval window is
 Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
Page 1 of 6 Question 1: How is the conduction of sound to the cochlea facilitated by the ossicles of the middle ear? Answer: Sound waves traveling through air move the tympanic membrane, which, in turn,
Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea
 Keywords: Cochlea, Basilar membrane, Hair cell 7006 Journal of Physiology (1998), 509.1, pp. 277 288 277 Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea Nigel P.
Keywords: Cochlea, Basilar membrane, Hair cell 7006 Journal of Physiology (1998), 509.1, pp. 277 288 277 Harmonic distortion on the basilar membrane in the basal turn of the guinea-pig cochlea Nigel P.
Efferent-mediated control of basilar membrane motion
 J Physiol 576. (2006) pp 49 54 49 Topical Review Efferent-mediated control of basilar membrane motion N. P. Cooper and J. J. Guinan Jr 2 School of Life Sciences, Keele University, Keele, Staffordshire
J Physiol 576. (2006) pp 49 54 49 Topical Review Efferent-mediated control of basilar membrane motion N. P. Cooper and J. J. Guinan Jr 2 School of Life Sciences, Keele University, Keele, Staffordshire
Neuroscience 201A Problem Set #1, 27 September 2016
 Neuroscience 201A Problem Set #1, 27 September 2016 1. The figure above was obtained from a paper on calcium channels expressed by dentate granule cells. The whole-cell Ca 2+ currents in (A) were measured
Neuroscience 201A Problem Set #1, 27 September 2016 1. The figure above was obtained from a paper on calcium channels expressed by dentate granule cells. The whole-cell Ca 2+ currents in (A) were measured
In vivo outer hair cell length changes expose the active process in the cochlea
 In vivo outer hair cell length changes expose the active process in the cochlea Dingjun Zha, Fangyi Chen, Sripriya Ramamoorthy, Anders Fridberger, Niloy Choudhury, Steven L Jacques, Ruikang K Wang and
In vivo outer hair cell length changes expose the active process in the cochlea Dingjun Zha, Fangyi Chen, Sripriya Ramamoorthy, Anders Fridberger, Niloy Choudhury, Steven L Jacques, Ruikang K Wang and
Required Slide. Session Objectives
 Auditory Physiology Required Slide Session Objectives Auditory System: At the end of this session, students will be able to: 1. Characterize the range of normal human hearing. 2. Understand the components
Auditory Physiology Required Slide Session Objectives Auditory System: At the end of this session, students will be able to: 1. Characterize the range of normal human hearing. 2. Understand the components
Consequences of Location-Dependent Organ of Corti Micro-Mechanics
 RESEARCH ARTICLE Consequences of Location-Dependent Organ of Corti Micro-Mechanics Yanju Liu 1, Sheryl M. Gracewski 1,2, Jong-Hoon Nam 1,2 * 1 Department of Mechanical Engineering, University of Rochester,
RESEARCH ARTICLE Consequences of Location-Dependent Organ of Corti Micro-Mechanics Yanju Liu 1, Sheryl M. Gracewski 1,2, Jong-Hoon Nam 1,2 * 1 Department of Mechanical Engineering, University of Rochester,
sensitivity (db) frequency (khz) I: MONGOLIAN GERBIL FREQUENCY HEARING CHARACTERISTICS
 Supplemental materials to Frequency sensitivity in mammalian hearing from a fundamental nonlinear physics model of the inner ear, by Kanders, K., Lorimer, T., Gomez, F. & Stoop, R. -6-5 sensitivity (db)
Supplemental materials to Frequency sensitivity in mammalian hearing from a fundamental nonlinear physics model of the inner ear, by Kanders, K., Lorimer, T., Gomez, F. & Stoop, R. -6-5 sensitivity (db)
Prestin-Driven Cochlear Amplification Is Not Limited by the Outer Hair Cell Membrane Time Constant
 Article Prestin-Driven Cochlear Amplification Is Not Limited by the Outer Hair Cell Membrane Time Constant Stuart L. Johnson, 1 Maryline Beurg, 2 Walter Marcotti, 1, * and Robert Fettiplace 3, * 1 Department
Article Prestin-Driven Cochlear Amplification Is Not Limited by the Outer Hair Cell Membrane Time Constant Stuart L. Johnson, 1 Maryline Beurg, 2 Walter Marcotti, 1, * and Robert Fettiplace 3, * 1 Department
Hearing Research 273 (2011) 109e122. Contents lists available at ScienceDirect. Hearing Research. journal homepage:
 Hearing Research 273 (2011) 109e122 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares Somatic motility and hair bundle mechanics, are both necessary
Hearing Research 273 (2011) 109e122 Contents lists available at ScienceDirect Hearing Research journal homepage: www.elsevier.com/locate/heares Somatic motility and hair bundle mechanics, are both necessary
Acoustics, signals & systems for audiology. Psychoacoustics of hearing impairment
 Acoustics, signals & systems for audiology Psychoacoustics of hearing impairment Three main types of hearing impairment Conductive Sound is not properly transmitted from the outer to the inner ear Sensorineural
Acoustics, signals & systems for audiology Psychoacoustics of hearing impairment Three main types of hearing impairment Conductive Sound is not properly transmitted from the outer to the inner ear Sensorineural
Auditory Physiology PSY 310 Greg Francis. Lecture 30. Organ of Corti
 Auditory Physiology PSY 310 Greg Francis Lecture 30 Waves, waves, waves. Organ of Corti Tectorial membrane Sits on top Inner hair cells Outer hair cells The microphone for the brain 1 Hearing Perceptually,
Auditory Physiology PSY 310 Greg Francis Lecture 30 Waves, waves, waves. Organ of Corti Tectorial membrane Sits on top Inner hair cells Outer hair cells The microphone for the brain 1 Hearing Perceptually,
A Bidirectional Analog VLSI Cochlear Model
 A Bidirectional Analog VLSI Cochlear Model Lloyd Watts Richard F. Lyon 1 Carver Mead Department of Computer Science California Institute of Technology Pasadena, CA USA 91125 Abstract A novel circuit is
A Bidirectional Analog VLSI Cochlear Model Lloyd Watts Richard F. Lyon 1 Carver Mead Department of Computer Science California Institute of Technology Pasadena, CA USA 91125 Abstract A novel circuit is
Auditory Physiology Richard M. Costanzo, Ph.D.
 Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
Auditory Physiology Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture, the student should be able to: 1. Describe the morphology and function of the following structures:
NEURONS Chapter Neurons: specialized cells of the nervous system 2. Nerves: bundles of neuron axons 3. Nervous systems
 NEURONS Chapter 12 Figure 12.1 Neuronal and hormonal signaling both convey information over long distances 1. Nervous system A. nervous tissue B. conducts electrical impulses C. rapid communication 2.
NEURONS Chapter 12 Figure 12.1 Neuronal and hormonal signaling both convey information over long distances 1. Nervous system A. nervous tissue B. conducts electrical impulses C. rapid communication 2.
The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons.
 1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
1 2 The mammalian cochlea possesses two classes of afferent neurons and two classes of efferent neurons. Type I afferents contact single inner hair cells to provide acoustic analysis as we know it. Type
Deafness and hearing impairment
 Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
Auditory Physiology Deafness and hearing impairment About one in every 10 Americans has some degree of hearing loss. The great majority develop hearing loss as they age. Hearing impairment in very early
Advanced otoacoustic emission detection techniques and clinical diagnostics applications
 Advanced otoacoustic emission detection techniques and clinical diagnostics applications Arturo Moleti Physics Department, University of Roma Tor Vergata, Roma, ITALY Towards objective diagnostics of human
Advanced otoacoustic emission detection techniques and clinical diagnostics applications Arturo Moleti Physics Department, University of Roma Tor Vergata, Roma, ITALY Towards objective diagnostics of human
Voltage-clamp errors cause anomalous interaction between independent ion channels
 AUDITORYAND VESTIBULAR SYSTEMS Voltage-clamp errors cause anomalous interaction between independent ion channels Hamilton E. Farris CA and Anthony J. Ricci Neuroscience Center and Kresge Hearing Labs,
AUDITORYAND VESTIBULAR SYSTEMS Voltage-clamp errors cause anomalous interaction between independent ion channels Hamilton E. Farris CA and Anthony J. Ricci Neuroscience Center and Kresge Hearing Labs,
FOUR COUNTER-ARGUMENTS FOR SLOW-WAVE OAEs
 FOUR COUNTER-ARGUMENTS FOR SLOW-WAVE OAEs CHRISTOPHER A. SHERA Eaton-Peabody Laboratory, Boston, MA 02114, USA email: shera@epl.meei.harvard.edu ARNOLD TUBIS Institute for Nonlinear Science, La Jolla,
FOUR COUNTER-ARGUMENTS FOR SLOW-WAVE OAEs CHRISTOPHER A. SHERA Eaton-Peabody Laboratory, Boston, MA 02114, USA email: shera@epl.meei.harvard.edu ARNOLD TUBIS Institute for Nonlinear Science, La Jolla,
Chapter 11: Sound, The Auditory System, and Pitch Perception
 Chapter 11: Sound, The Auditory System, and Pitch Perception Overview of Questions What is it that makes sounds high pitched or low pitched? How do sound vibrations inside the ear lead to the perception
Chapter 11: Sound, The Auditory System, and Pitch Perception Overview of Questions What is it that makes sounds high pitched or low pitched? How do sound vibrations inside the ear lead to the perception
MECHANISM OF HEARING
 MECHANISM OF HEARING Sound: Sound is a vibration that propagates as an audible wave of pressure, through a transmission medium such as gas, liquid or solid. Sound is produced from alternate compression
MECHANISM OF HEARING Sound: Sound is a vibration that propagates as an audible wave of pressure, through a transmission medium such as gas, liquid or solid. Sound is produced from alternate compression
Feed-Forward and Feed-Backward Amplification Model from Cochlear Cytoarchitecture: An Interspecies Comparison
 Biophysical Journal Volume 100 January 2011 1 10 1 Feed-Forward and Feed-Backward Amplification Model from Cochlear Cytoarchitecture: An Interspecies Comparison Yong-Jin Yoon, Charles R. Steele, and Sunil
Biophysical Journal Volume 100 January 2011 1 10 1 Feed-Forward and Feed-Backward Amplification Model from Cochlear Cytoarchitecture: An Interspecies Comparison Yong-Jin Yoon, Charles R. Steele, and Sunil
COCHLEAR MODEL FOR NORMAL AND DAMAGED EARS
 TEL AVIV UNIVERSITY THE IBY AND ALADAR FLEISCHMAN FACULTY OF ENGINEERING The Zandman-Slaner Graduate School of Engineering COCHLEAR MODEL FOR NORMAL AND DAMAGED EARS By Azaria Cohen THESIS SUBMITTED FOR
TEL AVIV UNIVERSITY THE IBY AND ALADAR FLEISCHMAN FACULTY OF ENGINEERING The Zandman-Slaner Graduate School of Engineering COCHLEAR MODEL FOR NORMAL AND DAMAGED EARS By Azaria Cohen THESIS SUBMITTED FOR
Sound and its characteristics. The decibel scale. Structure and function of the ear. Békésy s theory. Molecular basis of hair cell function.
 Hearing Sound and its characteristics. The decibel scale. Structure and function of the ear. Békésy s theory. Molecular basis of hair cell function. 19/11/2014 Sound A type of longitudinal mass wave that
Hearing Sound and its characteristics. The decibel scale. Structure and function of the ear. Békésy s theory. Molecular basis of hair cell function. 19/11/2014 Sound A type of longitudinal mass wave that
Detection of Cochlear Amplification and Its Activation
 Biophysical Journal Volume 5 August 23 67 78 67 Detection of Cochlear Amplification and Its Activation Wei Dong and Elizabeth S. Olson * Otolaryngology, Head and Neck Surgery and Biomedical Engineering,
Biophysical Journal Volume 5 August 23 67 78 67 Detection of Cochlear Amplification and Its Activation Wei Dong and Elizabeth S. Olson * Otolaryngology, Head and Neck Surgery and Biomedical Engineering,
A Critique of the Critical Cochlea: Hopf a
 A Critique of the Critical Cochlea: Hopf a Bifurcation Is Better Than None A. J. Hudspeth, Frank Jülicher and Pascal Martin J Neurophysiol 104:1219-1229, 2010. First published 10 June 2010; doi:10.1152/jn.00437.2010
A Critique of the Critical Cochlea: Hopf a Bifurcation Is Better Than None A. J. Hudspeth, Frank Jülicher and Pascal Martin J Neurophysiol 104:1219-1229, 2010. First published 10 June 2010; doi:10.1152/jn.00437.2010
Unit VIII Problem 9 Physiology: Hearing
 Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
Unit VIII Problem 9 Physiology: Hearing - We can hear a limited range of frequency between 20 Hz 20,000 Hz (human hearing acuity is between 1000 Hz 4000 Hz). - The ear is divided into 3 parts. Those are:
Representation of sound in the auditory nerve
 Representation of sound in the auditory nerve Eric D. Young Department of Biomedical Engineering Johns Hopkins University Young, ED. Neural representation of spectral and temporal information in speech.
Representation of sound in the auditory nerve Eric D. Young Department of Biomedical Engineering Johns Hopkins University Young, ED. Neural representation of spectral and temporal information in speech.
FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS. Hans P. Zenner, Ulrike Zimmermann and Alfred H. Gitter
 Vol. 149,No. 1, 1987 November 30, 1987 BlOCHEMlCALANDBlOPHYSlCALRESEARCHCOMMUNlCATlONS Pages 304-308 FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS Hans P. Zenner, Ulrike Zimmermann and Alfred
Vol. 149,No. 1, 1987 November 30, 1987 BlOCHEMlCALANDBlOPHYSlCALRESEARCHCOMMUNlCATlONS Pages 304-308 FAST MOTILITY OF ISOLATED MAMMALIAN AUDITORY SENSORY CELLS Hans P. Zenner, Ulrike Zimmermann and Alfred
Force Transmission in the Organ of Corti Micromachine
 Biophysical Journal Volume 98 June 2010 2813 2821 2813 Force Transmission in the Organ of Corti Micromachine Jong-Hoon Nam and Robert Fettiplace* Department of Physiology, University of Wisconsin Medical
Biophysical Journal Volume 98 June 2010 2813 2821 2813 Force Transmission in the Organ of Corti Micromachine Jong-Hoon Nam and Robert Fettiplace* Department of Physiology, University of Wisconsin Medical
Biological Basis of Hearing-Aid Design
 Annals of Biomedical Engineering, Vol. 30, pp. 157 168, 2002 Printed in the USA. All rights reserved. 0090-6964/2002/30 2 /157/12/$15.00 Copyright 2002 Biomedical Engineering Society Biological Basis of
Annals of Biomedical Engineering, Vol. 30, pp. 157 168, 2002 Printed in the USA. All rights reserved. 0090-6964/2002/30 2 /157/12/$15.00 Copyright 2002 Biomedical Engineering Society Biological Basis of
Mechanoelectric Transduction of Adult Inner Hair Cells
 1006 The Journal of Neuroscience, January 31, 2007 27(5):1006 1014 Cellular/Molecular Mechanoelectric Transduction of Adult Inner Hair Cells Shuping Jia, 1 Peter Dallos, 2 and David Z. Z. He 1 1 Hair Cell
1006 The Journal of Neuroscience, January 31, 2007 27(5):1006 1014 Cellular/Molecular Mechanoelectric Transduction of Adult Inner Hair Cells Shuping Jia, 1 Peter Dallos, 2 and David Z. Z. He 1 1 Hair Cell
Auditory nerve model for predicting performance limits of normal and impaired listeners
 Heinz et al.: Acoustics Research Letters Online [DOI 1.1121/1.1387155] Published Online 12 June 21 Auditory nerve model for predicting performance limits of normal and impaired listeners Michael G. Heinz
Heinz et al.: Acoustics Research Letters Online [DOI 1.1121/1.1387155] Published Online 12 June 21 Auditory nerve model for predicting performance limits of normal and impaired listeners Michael G. Heinz
Evidence and synthesis
 CHAPTER D 9 (Discussion continued) Evidence and synthesis 9.1 Synthesis 9.1/a Outer hair cells as dual detectors Pressure detection and transduction currents The silent current 9.1/b Cancellation effects
CHAPTER D 9 (Discussion continued) Evidence and synthesis 9.1 Synthesis 9.1/a Outer hair cells as dual detectors Pressure detection and transduction currents The silent current 9.1/b Cancellation effects
Before we talk about the auditory system we will talk about the sound and waves
 The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
The Auditory System PHYSIO: #3 DR.LOAI ZAGOUL 24/3/2014 Refer to the slides for some photos. Before we talk about the auditory system we will talk about the sound and waves All waves have basic characteristics:
Intro to Audition & Hearing
 Intro to Audition & Hearing Lecture 16 Chapter 9, part II Jonathan Pillow Sensation & Perception (PSY 345 / NEU 325) Fall 2017 1 Sine wave: one of the simplest kinds of sounds: sound for which pressure
Intro to Audition & Hearing Lecture 16 Chapter 9, part II Jonathan Pillow Sensation & Perception (PSY 345 / NEU 325) Fall 2017 1 Sine wave: one of the simplest kinds of sounds: sound for which pressure
Sound and Hearing. Decibels. Frequency Coding & Localization 1. Everything is vibration. The universe is made of waves.
 Frequency Coding & Localization 1 Sound and Hearing Everything is vibration The universe is made of waves db = 2log(P1/Po) P1 = amplitude of the sound wave Po = reference pressure =.2 dynes/cm 2 Decibels
Frequency Coding & Localization 1 Sound and Hearing Everything is vibration The universe is made of waves db = 2log(P1/Po) P1 = amplitude of the sound wave Po = reference pressure =.2 dynes/cm 2 Decibels
Medical Electronics Dr. Neil Townsend Michaelmas Term 2001 (www.robots.ox.ac.uk/~neil/teaching/lectures/med_elec) The story so far
 Medical Electronics Dr. Neil Townsend Michaelmas Term 2001 (www.robots.ox.ac.uk/~neil/teaching/lectures/med_elec) The story so far The heart pumps blood around the body. It has four chambers which contact
Medical Electronics Dr. Neil Townsend Michaelmas Term 2001 (www.robots.ox.ac.uk/~neil/teaching/lectures/med_elec) The story so far The heart pumps blood around the body. It has four chambers which contact
Distortion product emissions from a cochlear model with nonlinear mechanoelectrical transduction in outer hair cells
 Distortion product emissions from a cochlear model with nonlinear mechanoelectrical transduction in outer hair cells Yi-Wen Liu and Stephen T. Neely Boys Town National Research Hospital, 555 North 3th
Distortion product emissions from a cochlear model with nonlinear mechanoelectrical transduction in outer hair cells Yi-Wen Liu and Stephen T. Neely Boys Town National Research Hospital, 555 North 3th
Nonlinear Cochlear Signal Processing
 Nonlinear Cochlear Signal Processing Jont B. Allen Florham Park, NJ March 13, 2001 Contents 1 Macromechanics 5 1.1 The early history of cochlear modeling...................... 6 1.2 The 1 model of the
Nonlinear Cochlear Signal Processing Jont B. Allen Florham Park, NJ March 13, 2001 Contents 1 Macromechanics 5 1.1 The early history of cochlear modeling...................... 6 1.2 The 1 model of the
Signals, systems, acoustics and the ear. Week 5. The peripheral auditory system: The ear as a signal processor
 Signals, systems, acoustics and the ear Week 5 The peripheral auditory system: The ear as a signal processor Think of this set of organs 2 as a collection of systems, transforming sounds to be sent to
Signals, systems, acoustics and the ear Week 5 The peripheral auditory system: The ear as a signal processor Think of this set of organs 2 as a collection of systems, transforming sounds to be sent to
Electrophysiology. General Neurophysiology. Action Potentials
 5 Electrophysiology Cochlear implants should aim to reproduce the coding of sound in the auditory system as closely as possible, for best sound perception. The cochlear implant is in part the result of
5 Electrophysiology Cochlear implants should aim to reproduce the coding of sound in the auditory system as closely as possible, for best sound perception. The cochlear implant is in part the result of
Cochlear anatomy, function and pathology III. Professor Dave Furness Keele University
 Cochlear anatomy, function and pathology III Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (3) on the cochlear lateral wall and Reissner s membrane:
Cochlear anatomy, function and pathology III Professor Dave Furness Keele University d.n.furness@keele.ac.uk Aims and objectives of this lecture Focus (3) on the cochlear lateral wall and Reissner s membrane:
Possible mechanisms of cochlear two-tone suppression represented by vector subtraction within a model
 Acoust. Sci. & Tech. 39, 1 (018) PAPER #018 The Acoustical Society of Japan Possible mechanisms of cochlear two-tone suppression represented by vector subtraction within a model Yasuki Murakami 1; and
Acoust. Sci. & Tech. 39, 1 (018) PAPER #018 The Acoustical Society of Japan Possible mechanisms of cochlear two-tone suppression represented by vector subtraction within a model Yasuki Murakami 1; and
A cochlear AGC model, proposing a new type of cochlear non-linearity
 TEL-AVIV UNIVERSITY The Iby and Aladar Fleischman faculty of engineering The Zandman-Slaner school of Graduate studies A cochlear AGC model, proposing a new type of cochlear non-linearity A thesis submitted
TEL-AVIV UNIVERSITY The Iby and Aladar Fleischman faculty of engineering The Zandman-Slaner school of Graduate studies A cochlear AGC model, proposing a new type of cochlear non-linearity A thesis submitted
So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to
 So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to emphasize the intricate and well-protected structure of the
So now to The Ear. Drawings from Max Brodel, an Austrian artist who came to Johns Hopkins in the 1920s. My point in showing this figure is to emphasize the intricate and well-protected structure of the
Overview of ISVR CAV Workshop 2011
 Overview of ISVR CAV Workshop 2011 New Faculty Structure FACULTY ST UDENT S (FTE) ACADEMIC STAFF (FT E) NON- ACADEMIC ST A FF (FT E) TURNOVER ( M) Hum anities 2721 199 72 18 Business & Law 1726 96 41 11
Overview of ISVR CAV Workshop 2011 New Faculty Structure FACULTY ST UDENT S (FTE) ACADEMIC STAFF (FT E) NON- ACADEMIC ST A FF (FT E) TURNOVER ( M) Hum anities 2721 199 72 18 Business & Law 1726 96 41 11
A computer model of medial efferent suppression in the mammalian auditory system
 A computer model of medial efferent suppression in the mammalian auditory system Robert T. Ferry a and Ray Meddis Department of Psychology, University of Essex, Colchester, CO4 3SQ, United Kingdom Received
A computer model of medial efferent suppression in the mammalian auditory system Robert T. Ferry a and Ray Meddis Department of Psychology, University of Essex, Colchester, CO4 3SQ, United Kingdom Received
Modeling auditory-nerve responses for high sound pressure levels in the normal and impaired auditory periphery
 PREPRINT Accepted for publication in J. Acoust. Soc. Am., June 19, 26 Modeling auditory-nerve responses for high sound pressure levels in the normal and impaired auditory periphery Zilany, Muhammad S.
PREPRINT Accepted for publication in J. Acoust. Soc. Am., June 19, 26 Modeling auditory-nerve responses for high sound pressure levels in the normal and impaired auditory periphery Zilany, Muhammad S.
Music and Hearing in the Older Population: an Audiologist's Perspective
 Music and Hearing in the Older Population: an Audiologist's Perspective Dwight Ough, M.A., CCC-A Audiologist Charlotte County Hearing Health Care Centre Inc. St. Stephen, New Brunswick Anatomy and Physiology
Music and Hearing in the Older Population: an Audiologist's Perspective Dwight Ough, M.A., CCC-A Audiologist Charlotte County Hearing Health Care Centre Inc. St. Stephen, New Brunswick Anatomy and Physiology
