Renal function measurements from MR renography and a simplified multicompartmental model
|
|
|
- Clarence Foster
- 6 years ago
- Views:
Transcription
1 Am J Physiol Renal Physiol 292: F1548 F1559, First published January 9, 2007; doi: /ajprenal Renal function measurements from MR renography and a simplified multicompartmental model Vivian S. Lee, 1 Henry Rusinek, 1 Louisa Bokacheva, 1 Ambrose J. Huang, 1 Niels Oesingmann, 2 Qun Chen, 1 Manmeen Kaur, 1 Keyma Prince, 1 Ting Song, 1,3 Elissa L. Kramer, 1 and Edward F. Leonard 4 1 Department of Radiology, New York University School of Medicine, Departments of 3 Biomedical Engineering and 4 Chemical Engineering, Columbia University, New York, New York; and 2 Siemens Medical Solutions, Malvern, Pennsylvania Submitted 31 August 2006; accepted in final form 1 January 2007 Lee VS, Rusinek H, Bokacheva L, Huang AJ, Oesingmann N, Chen Q, Kaur M, Prince K, Song T, Kramer EL, Leonard EF. Renal function measurements from MR renography and a simplified multicompartmental model. Am J Physiol Renal Physiol 292: F1548 F1559, First published January 9, 2007; doi: /ajprenal The purpose of this study was to determine the accuracy and sources of error in estimating singlekidney glomerular filtration rate (GFR) derived from low-dose gadolinium-enhanced T1-weighted MR renography. To analyze imaging data, MR signal intensity curves were converted to concentration vs. time curves, and a three-compartment, six-parameter model of the vascular-nephron system was used to analyze measured aortic, cortical, and medullary enhancement curves. Reliability of the parameter estimates was evaluated by sensitivity analysis and by Monte Carlo analyses of model solutions to which random noise had been added. The dominant sensitivity of the medullary enhancement curve to GFR 1 4 min after tracer injection was supported by a low coefficient of variation in model-fit GFR values (4%) when measured data were subjected to 5% noise. These analyses also showed the minimal effects of bolus dispersion in the aorta on parameter reliability. Single-kidney GFR from MR renography analyzed by the threecompartment model ( ml/min) agreed well with reference measurements from 99m Tc-DTPA clearance and scintigraphy (r 0.84, P 0.001). Bland-Altman analysis showed an average difference of 11.9 ml/min (95% confidence interval ml/min) between model and reference values. We conclude that a nephronbased multicompartmental model can be used to derive clinically useful estimates of single-kidney GFR from low-dose MR renography. multicompartmental modeling; magnetic resonance imaging; tracer kinetics; glomerular filtration rate; radionuclide scintigraphy; gadolinium chelates SERUM CREATININE LEVELS and creatinine clearance are commonly used to monitor renal function, since measurement of these parameters is relatively easy and safe. Estimates based on serum creatinine levels are derived from equations established from populations with known chronic kidney disease; these estimates may lead to a false-positive diagnosis of chronic kidney disease (35). Plasma clearance of substances such as inulin, which is freely filtered and neither resorbed nor secreted, is a better marker of renal function and glomerular filtration rate (GFR), but these measurements require multiple blood and urine collections. Moreover, clearance measurements of these tracers do not provide functional information for each individual kidney. Recently developed MR imaging (MRI) techniques monitor the transit of gadolinium contrast agents through the kidney. The behavior of gadolinium chelates, such as gadopentetate dimeglumine (Gd-DTPA), is similar to that of inulin; gadolinium chelates are freely filtered at the glomerulus without tubular secretion or resorption. Thus their kinetics can be used to determine physiological parameters such as GFR (10). Some laboratories have used MR to calculate the renal extraction fraction of gadolinium chelates on the basis of flow and concentration measurements in the renal artery and vein or inferior vena cava (9, 11, 13, 28). In a more direct approach, high-temporal-resolution T1-weighted three-dimensional imaging can be used to visualize the passage of gadolinium chelates through intrarenal regions in near real time. The physiological parameters are then determined from quantitative analysis of MR renography signal intensity data. Low doses of contrast material ensure that the signal loss that can be associated with concentrated gadolinium chelates (T2 effects) is avoided and make MR renography compatible with routine clinical contrast-enhanced imaging of the kidneys and renal arteries (24). MR renography analysis can be performed using methods of varying complexity, such as an analog of Gates method adapted from nuclear medicine (22, 23), approaches derived from two-compartment models (1, 5, 18), including the Patlak- Rutland method (17), and multicompartmental modeling methods (8, 20). Multicompartmental kinetic modeling is one of the most sophisticated and versatile tools for the analysis of biomedical systems (4, 37, 38). However, application of kinetic modeling to analysis of in vivo imaging using exogenous contrast agents is challenging. It requires that the model represent the biological system in terms of compartments that are functionally homogeneous, that specific compartments should correspond to regions of interest defined on images, and that the system of compartments be suitably parsimonious to allow for analytic solutions that are physiologically meaningful, numerically stable, and fit experimental data (21). Once these conditions are met, multicompartmental models have potential beyond alternative methods to elucidate broader physiological meaning from imaging data. We propose a technique for measuring renal function based on a multicompartmental model that recapitulates nephron physiology. We analyze the reliability of parameter determinations from the model and test the results of this approach Address for reprint requests and other correspondence: V. S. Lee, Dept. of Radiology, NYU School of Medicine, 560 First Ave., Rusk 225, New York, NY ( vivian.lee@med.nyu.edu). The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. F /07 $8.00 Copyright 2007 the American Physiological Society
2 against renal function measurements derived from 99m Tc- DTPA clearance and scintigraphy. RENAL MODEL MR renography produces information about tracer concentration vs. time for specific anatomic regions: cortex, medulla, and pelvis. The three-dimensional imaging volume includes both entire kidneys and the abdominal aorta and can be updated as frequently as every 3 s. A measured aortic enhancement vs. time curve serves as the input function for the model. The full multicompartmental model of the vascular-nephron system is briefly introduced, and a simplified and practical model is proposed for measurement of six functional parameters that include renal plasma flow (RPF) and GFR. Complete Model: Seven Compartments The full multicompartmental model (Fig. 1) divides the vascular-nephron unit into seven compartments: the intrarenal arteries and glomerular vessels (A), vasa recta and veins (V), proximal convoluted tubule (P), loop of Henle (L), distal convoluted tubule (D), collecting ducts (CD), and calyces and renal pelvis (U). Measurements of signal from the abdominal aorta serve as the aortic input function [Ao(t)]. Concentrations of gadolinium contrast material in each compartment X (mm) are a function of time [X(t)]. Concentrations are related by a system of linear differential equations that express the conservation of mass of the contrast material. The model assumes compartments have fixed volume and instantaneous mixing. For two compartments, concentrations can be directly estimated from imaging: U(t) by concentration measurements from the renal pelvis and V(t) from the renal vein. With extravascular gadolinium chelates used as tracers, the interstitium is assumed to be contained within the vascular (V and A) compartments. The regional concentration vs. time curves for the cortex and medulla are expressed as linear combinations of compartmental concentrations. The cortical region contains contributions from the A, V, P, D, and CD compartments; the medullary region consists of contributions from the A, V, L, and CD compartments. Each contribution is weighted by the relative volumes of each compartment in the two regions. The full 7-compartment model contains 17 parameters. Six parameters describe intercompartmental flows: RPF (ml/min), MULTICOMPARTMENTAL MODEL FOR MR RENOGRAPHY GFR (ml/min), and the flow rates at which the tracer-free filtrate in the various compartments is resorbed into the vasa recta (f P,f L,f D, and f CD ). All four tracer-free fluid resorption rates are expressed as fractions of GFR; for example, if 67% of the flow entering the proximal tubule is resorbed within the proximal tubule, 5% within the loop of Henle, 15% within the distal tubule, and 10% within the collecting ducts, then f P 0.67, f L 0.05, f D 0.15, and f CD Nine volume parameters describing six compartments (A, V, P, L, D, and CD) are required to fit measured renal cortical and medullary enhancement data. Three of the compartments are assumed to be fully contained in the renal cortex [P, with volume V P (ml), and D, with volume V D (ml)] or the medulla [L, with volume V L (ml)]. The A, V, and CD compartments are present in the cortex (Cx) and medulla (Med): V A V A,Cx V A,Med,V V V V,Cx V V,Med, and V CD V CD,Cx V CD,Med. Two additional parameters necessary for model fitting, the total volume of the cortex (V Cx, ml) and the volume of the medulla (V Med, ml), can be measured from the three-dimensional MR images. Simplified Model for Renal Function Measurements When the primary goal of the MR renography data analysis is to calculate single-kidney GFR (SKGFR), a simplified version of the model, in which only the A, P, and L compartments are included (Fig. 2), can be used. We describe this abbreviated model and explain the assumptions made to simplify it. All concentrations are expressed per volume of plasma or tubular fluid. Hence, to convert gadolinium concentrations measured in whole blood in the aorta to plasma concentration, we divide by the factor (1 Hct), where Hct is hematocrit. On the basis of conservation of mass for the indicator and with the assumption that the fluid resorbed from each tubular compartment is free of tracer, the concentrations of gadolinium contrast material in the A, P, and L compartments are expressed as follows da dt RPF Ao V A,Cx V A,Med 1 Hct A (1) dl dt dp dt F1549 GFR V P A 1 f P P (2) GFR V L 1 f P P 1 f P f L L (3) Fig. 1. Complete multicompartmental model of the kidney. With a gadolinium chelate used as tracer, plasma that enters the kidney [renal plasma flow (RPF)] is filtered at the level of the glomerulus [intrarenal arteries and glomerular vessels (A)], whereby it passes into the tubules [proximal convoluted tubule (P), loop of Henle (L), distal convoluted tubule (D), and collecting duct (CD)] or through the vasa recta and exits the kidneys via the veins [vasa recta and veins (V)] or ureter (U). Dashed arrows indicate tracer-free resorption of fluid from tubular compartments. Tubular resorption flows are expressed as a fraction of glomerular filtration rate (GFR); e.g., fluid resorbed at proximal tubules flows at a rate f PGFR. Model assumes that tracer is neither secreted nor resorbed by the tubules. Ao, aorta.
3 F1550 MULTICOMPARTMENTAL MODEL FOR MR RENOGRAPHY Fig. 2. Simplified version of the model for determination of single-kidney GFR (SKGFR). Proximal convoluted tubules (P) are assumed to be confined to the renal cortex and the loops of Henle (L) to the medulla. The A compartment includes the entire vascular system, such as the arterioles, glomeruli, and vasa recta. Dashed lines represent tracer-free fluid resorption, again represented in terms of fractions of GFR. Measured from the MR images, the regional concentrations in the cortex [Cx(t)] and medulla [Med(t)] are expressed as linear combinations of the compartmental concentrations weighted by the contribution of each compartment to MR signal within that region Cx t V A,Cx V Cx A t V P V Cx P t (4) Med t V A,Med V Med A t V L V Med L t (5) where V A,Cx /V Cx reflects the volume fraction of arteries and glomeruli and V P /V Cx the volume fraction of proximal tubules in the cortex, V A,Med /V Med is the volume fraction of the arteries, and V L /V Med is the volume fraction for the loops of Henle in the medulla. As with the full version of the model, this abbreviated version assumes that all proximal tubules reside in the cortex and all loops of Henle are contained in the medulla. The A compartment spans the cortex and medulla, and the two volume parameters associated with the A compartment are V A,Cx and V A,Med. V Cx and V Med can be measured from three-dimensional MR image sets. Ao(t) is measured from MR renography. Equations 1 5 involve eight parameters: RPF, GFR, f P,f L, V P,V L,V A,Cx, and V A,Med. Since our preliminary work (see APPENDIX) revealed a high correlation between V P and f P and between V L and f L in this model, we chose to constrain two of the parameters: V P /V Cx is approximated to be 0.3, and V L / V Med is 0.5 (14, 30). We determined empirically that these ratios resulted in the best fits across all study subjects. Consequently, for this simplified version of the model, cortical and medullary concentration vs. time curves are fitted to derive six values: GFR, RPF, f P,f L,V A,Cx, and V A,Med. The rationale underlying the simplification of our full model is summarized as follows. First, the A and V compartments are combined into a single A compartment. Analysis of concentration vs. time curves for the aorta and renal vein demonstrated a modest difference between them at our imaging temporal resolution of 3 s that reflects extraction fractions that are typically 0.2 (Fig. 3). Second, the P and D compartments are combined into a single P compartment. For determination of flow parameters such as renal perfusion and glomerular filtration, which play a more important role early in the vascular-nephron system, this assumption is acceptable, provided fitting focuses on the earlier stages following filtration. Moreover, the smaller proportion of distal than proximal tubules [ratio 1:4 (14)] means that the effect of the distal tubules on GFR is small compared with the effect of the proximal tubules, which justifies omission of the D compartment from the simplified model. MATERIALS AND METHODS Human Subjects We examined 20 kidneys in 10 subjects (5 women and 5 men), [69.8 (SD 16.9)] yr of age, who were referred for suspected renovascular disease. Subjects had underlying diagnoses of chronic renal failure and hypertension (n 1) and hypertension alone (n 9). Serum creatinine levels were mg/dl. Written informed consent was obtained from all subjects, and the protocol was approved by our institutional review board. All subjects underwent 99m Tc-DTPA renography, the reference technique for SKGFR, and an abdominal MR examination in the same morning. The MR examination was conducted between the 1- and 3-h blood draws for the 99m Tc-DTPA renography. Immediately before each imaging study, subjects were asked to drink two small cups of water ( 300 ml) and to void. 99m Tc-DTPA Renography SKGFR and split renal function were measured using established 99m Tc-DTPA renography and plasma clearance methods in all subjects (31, 33). After subjects were given an intravenous bolus of 10 mci (370 MBq) of 99m Tc-DTPA, dynamic planar images were acquired with a large-field-of-view (FOV) gamma camera and lowenergy collimator with 20% energy window centered at 140 kev, a planar matrix of pixels, and pixel size of mm. Images were collected at 3-s intervals for 1.5 min and then at 60-s intervals for 60 min. For measurement of total (left right) GFR (TGFR) from plasma clearance of 99m Tc-DTPA, venous blood samples were collected from all subjects at 1 and 3 h after injection. On the basis of background- and attenuation-corrected renal uptake of 99m Tc-DTPA in the left and right kidneys (U left and U right) at 2 3 min, SKGFR (SKGFR left and SKGFR right) values were determined as follows: SKGFR left TGFR U left/(u left U right), and SKGFR right TGFR U right/(u left U right). MR Renography MR renography was performed for all subjects as part of a clinically indicated renal MRI study. Imaging was performed using a torso Fig. 3. Representative concentration vs. time curves for the main renal artery and vein demonstrate that the temporal pattern of enhancement of the renal vein coincides with that of the artery. Simplified 3-compartment model combines vascular (A and V) compartments into the A compartment. [Gd], gadolinium concentration. (Steady-state renal vein and arterial concentrations suggest an extraction fraction of 0.15.)
4 MULTICOMPARTMENTAL MODEL FOR MR RENOGRAPHY F1551 phased-array coil on a 1.5-T system with a maximum gradient strength of 45 mt/m (Avanto, Siemens Medical Solutions, Erlangen, Germany). The routine MRI protocol included breath-hold T1- and T2-weighted imaging and contrast-enhanced MR angiography. Before MR angiography, T1 measurements and low-dose gadolinium-enhanced MR renography were performed in all subjects. MR renography technique. MR renography was performed using a coronal interpolated three-dimensional spoiled GRE sequence with the following parameters: TR/TE/flip angle 2.84 ms/1.05 ms/12, partition matrix of interpolated to with a 425-mm FOV, 20 partitions interpolated to 40, resulting in an interpolated voxel size of mm, with parallel factor of 3, bandwidth of 650 Hz/voxel, and 3-s acquisition time. With parallel imaging, 24 reference lines were collected, and images were reconstructed using the generalized autocalibrating partially parallel acquisitions technique (15). To obtain a reliable baseline signal needed for an accurate estimate of tissue concentration, we acquired five threedimensional images during one 15-s breath hold before contrast administration. Then, at 8 s after the start of a bolus injection of 4 ml of Gd-DTPA (Magnevist, Berlex Laboratories, Wayne, NJ) at 2 ml/s (followed by a 20-ml saline flush), the three-dimensional acquisitions were repeated continuously for 30 s, during which the subject was asked to suspend respiration for as long as possible. Acquisitions were repeated during separate 3-s breath holds at multiple time points for 10 min thereafter. MR T1 measurements. For T1 measurements, a 6-mm-thick twodimensional axial slice through both kidneys was imaged in a single breath hold by a segmented, inversion recovery prepared-truefisp sequence (6) modified from a commercially available sequence that was used for myocardial infarct imaging (16) and had the following parameters: TR/TE/flip angle 4,000 ms/1.3 ms/10, 2.9 ms between excitation pulses, i ms (where i 1, 2, 3,...,40) inversion times, 975 Hz/voxel bandwidth, mm FOV, and imaging matrix. All the images were acquired within a single 20-s breath hold. The images were acquired before intravenous contrast material was injected. T1 values for the cortex and medulla were calculated by placing regions of interest over the cortex and medulla and fitting the resulting data to relaxation equations (6). MR renography image processing and analysis. Semiautomated image registration and segmentation of the three-dimensional renography data sets were performed using software developed in conjunction with Siemens Corporate Research (Princeton, NJ) to produce aortic, renal cortical, and renal medullary signal intensity vs. time curves (32). Registration and segmentation of each kidney required 20 min. Signal intensity vs. time curves were converted to gadolinium concentration vs. time curves on the basis of a technique previously reported (7). Briefly, the technique relies on careful prior calibration of the relation between T1 and signal intensity (SI) for the threedimensional gradient echo MR renography sequence SI kf T1 (6) where k is a scaling term that depends on a variety of factors, such as a subject habitus, system gain, and coil sensitivity, and f(t1) is a function that depends only on sequence parameters. With our approach, k is determined separately for each region using the signal intensity from the unenhanced MR renography images and measured unenhanced T1 values. Aortic, cortical, and medullary signal intensities derived from the contrast-enhanced MR renography are converted to T1 values, and Eq. 7 is then used to solve for the concentration of gadolinium contrast [X (mm)] in each region 1 T1 region Gd 1 T1 region 1 T1 Gd 1 T1 region R 1 X (7) where T1 region Gd represents the T1 value of the contrast-enhanced renal region, T1 region is the T1 value of the unenhanced renal region, and R 1 is the relaxivity of gadolinium contrast (4.5 mm 1 s 1 ). Equation 7 applies to the cortex and medulla, each with its own measured T1 values. For the aorta, a T1 of 1,200 ms for unenhanced arterial blood is assumed (29). Analysis of MR Renography Data Using the Three-Compartment Renal Model To determine GFR, RPF, f P,f L,V A,Cx, and V A,Med for each kidney, we measured aortic, renal cortical, and renal medullary gadolinium concentration vs. time curves and locally developed software written in C to perform a fitting procedure. MR renography data up to 10 min after injection were analyzed. Our program combines the conventional simplex method (27) with a six-dimensional grid of starting parameter values to determine the best least-squares fit of measured data to the model, where, for example, grid steps were 5 ml/min per GFR step and 50 ml/min per RPF step. This approach avoids the limitation of many least-squares solvers, which, given noisy data and incorrect starting approximations, may provide an invalid local minimum solution. The residual [root mean square (RMS) error, with equal weights for cortical and medullary data points] was computed for all fits. Data Analysis for GFR Measurements Regression analysis and Bland-Altman plots were used to compare measurements of SKGFR by MR renography with reference values. Parameter Sensitivity and Monte Carlo Analyses of the Model We performed two analyses to assess the practical use of our model for measurement of renal functional parameters, given the presence of measurement errors in MR renography: 1) parameter sensitivity plots, to ensure that the clinically relevant changes in parameter values are reflected in a measurable change in observed data, and 2) Monte Carlo noise simulation analysis, to show that, despite data noise, all parameters can be recovered with clinically useful precision. Sensitivity analyses. We have plotted the relative (percent) change in Cx(t) and Med(t) due to a unit (1%) change in value for each parameter. The sensitivity function with respect to a parameter reflects the relative influence of that parameter on the model fit at each time point across the acquisition period. High sensitivity values are necessary for parameter optimization. The parameters used to generate model curves for the sensitivity analysis were derived from healthy subject 4, with left kidney SKGFR 68.3 ml/min, and subject 6, with left kidney SKGFR 17.7 ml/min (as determined by the 3-compartment model). Because differences in cardiac function and hemodynamics can dramatically affect the Ao(t) function, which in turn may also affect model fits, we also determined sensitivity functions for three representative cases of different Ao(t) functions reflecting different degrees of dispersion of the injection bolus (taken from experimental datasets from subjects 1, 3, and 6). The sensitivity functions were calculated using each of the input functions and a fixed set of model parameters selected to reflect a kidney with mild dysfunction: GFR 40.5 ml/min, corresponding to the right kidney of subject 6. Monte Carlo noise simulation analyses. To evaluate the precision, bias, and pairwise correlation of parameter estimates, the model was used to generate solutions in which we simulated random data noise, varied from 5% to 20% of the mean aortic, cortical, and medullary gadolinium concentrations. The model was then used to fit these pseudodata for which true values of the parameters were known. The variability or standard deviation of each of the fitted parameters in the presence of simulated noise was computed and expressed in terms of coefficient of variation (CV, %). This simulation was performed for the same sets of data used for sensitivity analysis (GFR 68.3 and 17.7 ml/min). Monte Carlo noise simulation analyses were repeated for the three Ao(t) functions used in the sensitivity analysis described above.
5 F1552 MULTICOMPARTMENTAL MODEL FOR MR RENOGRAPHY RESULTS Three-Compartment Model Fitting of MR Renography Data Representative images during transit of gadolinium contrast material (which causes signal to increase on the MR images) through the kidney as a result of filtration and excretion are shown in Fig. 4 for healthy subject 4 with normal renal function (serum creatinine 0.6 mg/dl) and for subject 8 with a history of hypertension and elevated serum creatinine (1.7 mg/dl). Each image is 1 slice from a set of 40 slices, which depicts the entire abdomen, including the abdominal aorta. Corresponding renal concentration vs. time curves for one kidney of each subject in Fig. 4, together with three-compartment model fits, are shown in Fig. 5. In all cases, cortical and medullary enhancement curves demonstrate an early vascular peak, higher in the cortex than medulla, reflecting the greater arterial perfusion of the cortex. In subject 4 (right kidney GFR 80.1 ml/min), a second peak in the cortex at 1 2 min reflects accumulation of contrast material in the glomeruli and proximal tubules. This peak is diminished in the case of renal insufficiency in subject 8 (left kidney GFR 11.3 ml/min). At 2 3 min after injection, medullary enhancement in the healthy kidney is also characterized by a prominent second, broader peak, which reflects the transit of filtered gadolinium contrast agent through the loops of Henle in the medulla. In renal insufficiency, this period of enhancement is delayed, and its amplitude is markedly diminished by comparison. The three-compartment model fits in Fig. 5 represent the sum of contributions from separate compartments in the model, namely, A and P compartments for the cortex and A and L compartments for the medulla. Each of those components is also plotted for the corresponding data in Fig. 5. The diminished second cortical and medullary peaks are reflected in decreased and delayed enhancement in the P and L compartments, as would be expected in the setting of decreased glomerular filtration. Fitted values for the six parameters in the model and RMS errors for model fits for the 20 kidneys are given in Table 1. Overall, the three-compartment model resulted in satisfactory fits of measured data. The overall RMS error for fits was mm, where concentrations in the cortex and medulla typically peaked at mm. Sensitivity Analysis of the Model Sensitivity functions of the model fits to changes in the fitting parameters are plotted for subject 4 with normal renal function (left kidney GFR 68.3 ml/min; Fig. 6, left) and subject 6 with diminished kidney function (left kidney GFR 17.7 ml/min; Fig. 6, right). These plots show the relative change in the model solutions for the cortex and medulla in response to a 1% change in the fitting parameters, except for parameters with values 0.1, where the increment was fixed at an absolute value of 0.01, and demonstrate the relative influence of each parameter at all time points in the acquisition. The sensitivity to RPF rises sharply to a peak of 0.9% in the cortex and medulla of the healthy kidney at 10 s (Fig. 6, left), which corresponds to the earliest arrival of the contrast material in the tissues (Fig. 5). The sensitivity to RPF then falls rapidly and remains low ( 0.1%) from 45 s until the end of the acquisition. The sensitivity functions for the two parameters that estimate renal blood volumes, V A,Cx and V A,Med, show maxima of opposite signs early in the acquisition, between 10 and 35 s. At later times (from 90 s), the sensitivity functions to V A,Cx and V A,Med remain nearly constant but have different values. The sensitivity of the cortex to V A,Cx at 90 s remains relatively high (0.3%), while sensitivity of the cortex to V A,Med is close to zero. In contrast, in the same time interval, the sensitivity of Fig. 4. A D: MR renography in healthy subject 4 (right kidney GFR 80.1 ml/min, left kidney GFR 75.0 ml/min). E H: MR renography in subject 8 (right kidney GFR 17.2 ml/min, left kidney GFR 11.3 ml/min). Images depict 1 of 40 slices through both kidneys without enhancement (A and E), at peak cortical enhancement (B and F), at peak medullary enhancement (C and G), and at final excretory phase (D and H).
6 MULTICOMPARTMENTAL MODEL FOR MR RENOGRAPHY F1553 Fig. 5. Cortical (Cx) and medullary (Med) gadolinium concentration vs. time curves and associated model fit curves for 1 kidney from each subject in Fig. 4: healthy subject 4 (reference SKGFR 80.1 ml/min, model-derived SKGFR 71 ml/min) and subject 8 with diseased left kidney (reference SKGFR 11.3 ml/min, model-derived SKGFR 6.1 ml/min). Root mean square error for each fit was mm for healthy kidney and mm for diseased kidney. Early vascular peak in cortex and medulla of all cases reflects renal perfusion, as illustrated by corresponding model-derived enhancement curve for the A compartment. Reduced glomerular filtration in dysfunctional kidney is reflected by the diminished second peak of the cortical curve (decreased enhancement of the proximal tubules) and, more dramatically, the diminished and delayed second peak of the medullary curve (decreased enhancement of loops of Henle). the medulla to V A,Cx is nearly zero and the sensitivity to V A,Med remains nearly stable and does not exceed 0.17%. This difference helps ensure identifiability of both vascular volume parameters in fitting. Sensitivity functions confirm that the model is quite sensitive to changes in GFR. The wide peak in sensitivity to GFR functions extends between 8 and 150 s in the cortex and reaches the maximum of 0.4% at 35 s. Even greater is the sensitivity to GFR in the medulla, with a maximum spanning s and peaking at 1.1% at 50 s, where the medullary concentration reaches its maximum upslope. This time interval is consistent with the period in which the gadolinium chelate bolus travels from the renal cortex to the medulla through the loops of Henle. In the medulla, the sensitivity to GFR exceeds the sensitivity to all other parameters between 40 and 160 s. Tubular fluid resorption rates (f P and f L ) are associated with the delayed sensitivity maxima at 300 s, with a greater impact of changes in f L than f P on medullary curves and a negligible effect of f L on cortical curves. In comparison, for the kidney with lower GFR (Fig. 6, right), the peak in the sensitivity to GFR is broadened ( s) and the maximum sensitivity of the medulla to GFR at 80 s is delayed relative to that of a normal kidney. The amplitude of this peak is also decreased (0.9%). Nevertheless, the sensitivity of the medulla to GFR dominates the sensitivity to other parameters within the important time window of the medullary concentration rise at 1 4 min that enables identification of the GFR value using our model. Monte Carlo Simulations for Model Fits To further evaluate parameter identifiability, we performed a Monte Carlo simulation for parameter fitting for the two kidneys described above (Fig. 6): one with normal SKGFR and the other with diminished SKGFR. Table 2 shows the standard deviations of fitted parameter values when Ao(t), Cx(t), and Med(t) were subjected to 5% random noise. The 5% level was selected on the basis of typical signal-to-noise ratios of 20
7 F1554 MULTICOMPARTMENTAL MODEL FOR MR RENOGRAPHY Table 1. Fitted values for the six parameters in the model, including residual RMS fit and reference GFR values GFR, ml/min Subj No. RMS Error, mm RPF, ml/min VA,Cx, ml VA,Med, ml fp fl Model Nucl Right kidney Left kidney RMS, root mean square fit (cortex medulla); RPF, renal plasma flow; V A,Cx and V A,Med, Volumes of compartment A in cortex (Cx) and medulla (Med); f P and f L, fraction of tracer-free fluid resorbed at proximal tubule (P) and in the loop of Henle (L); GFR, glomerular filtration rate predicted by the model (Model) and measured by radionuclide renography (Nucl). with our MRI technique. At this noise level, estimates of GFR were stable, with a CV of 5% in the normal and dysfunctional cases. V A,Cx,V A,Med, and RPF demonstrated reasonable robustness, with CV of 10% or less. The tracer-free resorption fractions for the proximal tubules and loops of Henle, f P and f L, which are expressed as fractions of GFR, showed higher CV values, given their low unperturbed values. However, the absolute values of the standard deviations of each ( 0.06) are low. When the two parameters are combined into a single parameter, 1 (f P f L ), reflecting the filtered volume remaining after tubular resorption at the loop of Henle, the combined parameter was remarkably more robust and insensitive to noise, with CV 2.5% (Table 2). Effect of increased dispersion of aortic input. The model fits were also tested for sensitivity to different degrees of dispersion of the contrast bolus in the aorta. As shown in Fig. 7, separate sensitivity analyses were performed for a fixed set of kidney parameters and three Ao(t) functions (from subjects 1, 3, and 6), which reflect differences in cardiac output that may be independent of renal function (2, 3). The results demonstrate that, with a more dispersed Ao(t) function, the peak medullary sensitivity for GFR is slightly broader and diminished, but the peak remains 0.8%. Monte Carlo simulation was also performed to assess the effects of different Ao(t) functions on parameter identifiability. We found no difference in the behavior of the fitted parameters for the three Ao(t) functions. SKGFR Measurements Among the 10 subjects, SKGFR values derived from scintigraphic studies were ml/min. Three-compartment model-derived values were ml/min. The correlation between model-based SKGFR and reference values was high (r 0.84, P 0.001; Fig. 8). Images of one outlier kidney (left kidney of subject 5) revealed several large renal cysts, which may have interfered with the accurate determination of SKGFR by scintigraphy. Without that outlier kidney, the correlation was r Linear regression analysis gave y 0.76x 1.14 (y 0.93x 6.24 without the 1 outlier case). The Bland-Altman plot revealed that the modelbased SKGFR underestimated reference values by an average of 11.9 ml/min, with 95% confidence interval between 5.8 and 17.9 ml/min (Fig. 8). Without the outlier, the model values underestimated reference values by an average of 9.4 ml/min, with 95% confidence intervals between 5.5 and 13.3 ml/min. DISCUSSION Our three-compartment, six-parameter model of the nephron was able to fit a range of patterns of cortical and medullary enhancement in subjects whose SKGFR spanned the wide range typically encountered in clinical practice. RMS errors in fitted curves were typically mm. On the basis of Monte Carlo simulations, image noise contributes modestly to errors in determination of GFR. Other sources of errors include misregistration and blurring artifacts arising from respiratory motion and the simplifications inherent in the model, particularly the combination of arterial and venous (A and V) compartments into the A compartment and the combination of the proximal and distal convoluted tubule (P D) compartments into the P compartment. As illustrated in Fig. 5 (left), many of the fits underestimated measured data at later time points. We suspect that this occurs because the P compartment is unable to represent accurately the flow of contrast material in the distal tubule and collecting ducts.
8 MULTICOMPARTMENTAL MODEL FOR MR RENOGRAPHY F1555 Fig. 6. Sensitivity functions of cortical (top) and medullary (bottom) gadolinium concentration vs. time curves for the 6 model-fit parameters. Sensitivity functions depict percent changes in model solutions for each region in response to 1% change in a parameter. Sensitivity functions were determined using parameter values derived from fitted values for the left kidney from subject 4 [healthy kidney: model-derived SKGFR 68.3 ml/min, RPF ml/min, f P 0.19, f L 0.21, volume of A compartment in cortex (V A,Cx) 20.8 ml, volume of A compartment in medulla (V A,Med) 6.5 ml] and the dysfunctional left kidney from subject 6 (diseased kidney: model-derived SKGFR 17.7 ml/min, RPF 59.8 ml/min, f P 0.01, f L 0.15, V A,Cx 10.5 ml, V A,Med 4.6 ml). We found high correlation between the model-derived MR renographic measurements of SKGFR and reference radionuclide measurement (r 0.84). The model-derived SKGFR values tended to underestimate reference values by an average of 11.9 ml/min. The degree of underestimation did not correlate with renal function (Fig. 8) or with the goodness of fit (Table 1) and may reflect a limitation of the simplifying assumptions made to generate the six-parameter model. Other possible sources of discrepancies between the two measurements of SKGFR include physiological differences at the times of measurement. We attempted to minimize these by performing both studies on the same morning and by asking the subjects to drink the same amount of water before each study. For two kidneys (left kidneys of subjects 9 and 10), the MR estimate of SKGFR was zero, while the reference radionuclide measurement was finite (8.7 and 3.5 ml/min). Review of images confirmed visually detectable technetium tracer uptake on radionuclide images and the absence of delayed medullary enhancement after gadolinium administration. Additionally, segmentation of three-dimensional MR images is also confounded by low concentration of the contrast material. Improved accuracy in the estimate of low GFR values may require higher image contrast, for example, by imaging at 3 T or with higher injected doses of gadolinium chelate. The reference method for SKGFR, radionuclide renography, is also imperfect. With the two-sample approach for global GFR that we used, Rowell and colleagues (31) reported an error of 4 ml/min for global GFR measurements; reliance on scintigraphy to derive split renal function contributes an additional 2 4% CV (26). For our study, more precise determinations of SKGFR using inulin clearance were not considered feasible, given our subject population. Critical to the application of our multicompartmental model is the conversion of measured MR signal intensity to gadolinium concentration. Our technique is described by Bokacheva et al. (7). It presumes that the concentration of the contrast material does not exceed the level at which signal intensity declines with greater concentration (due to T2 effects), which is expected to hold true given the relatively low doses (4 ml or 0.02 mmol/kg body wt) of gadolinium injected intravenously and short echo times used in the renography sequence. It also assumes fixed relaxivity of the gadolinium and fast exchange of water molecules around the gadolinium. Other approaches have assumed that relative signal intensity changes vary lin-
9 F1556 MULTICOMPARTMENTAL MODEL FOR MR RENOGRAPHY Table 2. Monte Carlo noise sensitivity analysis Parameter Baseline Value SD CV, % Functional kidney (GFR 68.3 ml/min, subj 4 left kidney) RPF, ml/min GFR, ml/min V A,Cx, ml V A,Med, ml f P f L (f P f L) Dysfunctional kidney (GFR 17.7 ml/min, subj 6 left kidney) RPF, ml/min GFR, ml/min V A,Cx, ml V A,Med, ml f P f L (f P f L) Effect of 5% random noise added to Ao(t), Cx(t), and Med(t) curves on fitted parameter values for functional and dysfunctional kidney scenarios. CV, coefficient of variation. early with gadolinium concentration. In regions where gadolinium is concentrated, this assumption may fail. The simplified three-compartment, six-parameter model that we use is derived from a more complete 17-parameter model that recapitulates the functional units of the vascular-nephron system into seven separate compartments. For derivation of SKGFR, simplification allows for fitting of the cortical and medullary concentration vs. time curves with reasonable accuracy. Sensitivity and Monte Carlo analyses support the ability of the model to identify GFR and RPF with this method. Identifiability of the tubular fluid resorption parameters f P and f L is less robust; however, when combined into a single parameter, 1 (f P f L ), they are much less sensitive to noise. Other models have also been proposed for the analysis of MR renography and have generally focused on estimating GFR. For example, using a Patlak-Rutland approach, Hackstein et al. (17) simulated renal dynamics with two compartments [aorta to kidneys (renal tubules)] to model whole kidney enhancement. Annet et al. (1) used a cortical-compartment model with two compartments (glomeruli to renal tubules) and used only cortical enhancement to estimate GFR. Recently, using these two methods in human subjects, Buckley et al. (8) compared these GFR determinations and found that both highly correlated with reference values; however, both also overestimated GFR considerably. With a model that used separate determinations of cortical and medullary enhancement, Baumann and Rudin (5) modeled the cortex and medulla as distinct compartments, with the rate constant of flow between the two reflecting renal clearance. Other models (18, 36) have also been described. Our model resembles that of Baumann and Rudin, in that the transit of gadolinium from the renal cortex to the medulla is assumed to reflect GFR. However, our model expresses the functional compartments of the nephron explicitly, accounts for compartmental outflow, and has the potential to provide other useful parameters, such as renal perfusion and tubular function. Several assumptions underlie the proposed six-parameter model. First, the model assumes that the compartments, such as the glomeruli (A), proximal tubules (P), and loops of Henle (L), are well mixed. It ignores, for example, the heterogeneity of the fluid composition along the length of the tubules. The arterioles and glomerular capillaries are combined into one compartment, A, and the heterogeneity of these fluid-filled structures is ignored. Gadolinium contrast rapidly distributes into the interstitial compartment, and, hence, the interstitial compartment is effectively included in the vascular compartment. We observed that the fitted V A (arterial and venous volumes) exceeds what would be expected on the basis of histological data, most likely reflecting the interstitial contribution to the volume of distribution. Additionally, as discussed above, the vasa recta are combined into the A compartment in the simplified version (Fig. 3). The volumes of the P and L compartments are approximated using limited histological studies in Fig. 7. Effect of differences in dispersion of aortic input function (top) on GFR estimates based on sensitivity functions of cortical (middle) and medullary (bottom) gadolinium concentration vs. time curves for the 6 model-fit parameters. Parameters were unchanged for the 3 cases: model-derived SKGFR 40.5 ml/min, RPF 205 ml/min, f P 0.01, f L 0.34, V A,Cx 22.8 ml, and V A,Med 7.1 ml.
10 MULTICOMPARTMENTAL MODEL FOR MR RENOGRAPHY F1557 Fig. 8. Relation between 3-compartment model-derived SKGFR measurements and nuclear medicine reference values. A: scatterplot with regression line depicting best linear fit for all data points. B: Bland-Altman plot showing difference between reference values and MR estimates of SKGFR. Difference between reference values and model-based SKGFR averaged 11.9 ml/min with 95% confidence interval between 5.8 and 17.9 ml/min. humans and animals. We recognize that these are likely to vary across individuals depending on age and disease state (14). The model assumes that fluids contain tracer or are tracer free. The effects of solutes that pass into and out of the nephron are ignored. The model assumes instantaneous mixing and ignores the tubular length and intratubular concentration variability. Also, the model assumes constant flow between the compartments. This may be reasonable when the modeling is performed over 5 10 min. However, we have observed some notable exceptions. For example, the flow of urine out of the collecting system has a temporal variation over 1 2 min that likely reflects ureteral peristalsis. For the purposes of determining GFR, these variations have a negligible effect on cortical and medullary curves. With respect to fitting our model to sampled data from MR images, we make some additional important assumptions. From the series of three-dimensional images, the detail afforded by MR renography enables segmentation of the kidney only into renal cortex, medulla, and collecting system (Fig. 4). We are unable to differentiate, for example, the proximal tubule from the distal tubule or the vasa recta from the loops of Henle. The task addressed here can be seen in general terms: to resolve information about the transient movement of a tracer through regions of interest to quantify an organ model consisting of functional compartments. This task is not straightforward when anatomic regions do not map uniquely onto the functional compartments. The essence of the solution is to apportion the transient changes measured experimentally in the region to the functional compartments, which overlap in space. Detail and completeness in the functional model, although desirable, must be balanced against limits on the spatial resolution of the region and variability of the relation between spatial and functional compartments among subjects. Consequently, for fitting our data, we express the regional concentrations as a composite of compartments. To do so, we make some simplifications. We assume that all glomeruli and proximal tubules are located within the cortex and that all loops of Henle are within the medulla. Image segmentation uses peak cortical enhancement to differentiate the cortex from the medulla; therefore, all regions of the kidney that demonstrate a similar perfusion pattern will be considered cortex. We assume that juxtamedullary glomeruli are included in the cortex. However, we recognize that volume averaging of the voxels that include the cortex and the medulla adds imprecision to our results. Preliminary analysis suggests that these errors result in 10% error in GFR measurements. Further work is needed. Despite these assumptions, the multicompartmental modeling approach has some distinct advantages. First, the model uses the measured aortic concentration vs. time curve as the input function for the multiple compartments, rather than a gamma variate fit (25). Recirculation of the tracer is inherently included in the fitting. Therefore, the tracer kinetic model can be used to study kidney function independent of cardiac output and vascular dynamics and allows the study to be performed using a minimally invasive intravenous injection of gadolinium contrast. Our analysis shows that measurements of GFR are not sensitive to dispersion of the bolus in the aorta. To derive an accurate aortic curve, our approach assumes the absence of flowrelated effects on the MR signal. This is achieved by positioning of our oblique coronal imaging slab in the plane of aortic flow. Second, because we use a large number of data points from a 10-min sampling interval, fits with the model are robust and less susceptible to the effects of noise or motion artifacts in the images. The technique, however, does rely on accurate registration and segmentation algorithms, which can be time consuming if not automated. Several laboratories continue to investigate the development of improved automated imageprocessing algorithms (12, 32, 34). Finally, and most importantly, the multicompartmental model has the advantage of broad applicability. Our approach has the potential to provide additional physiological information beyond GFR. Hsu (19) and others have argued that investigators studying renal function should think beyond GFR and explore other parameters that may better elucidate renal pathophysiology. Our approach has the potential for use in the study of RPF and renal blood volume, as well as tubular concentrating ability, expressed in terms of f P and f L, for example. For some parameters in our model, dedicated valida-
Assessment of Renal Function with Dynamic Contrast-Enhanced MR Imaging
 Assessment of Renal Function with Dynamic Contrast-Enhanced MR Imaging Louisa Bokacheva, PhD a, *,HenryRusinek,PhD a, Jeff L. Zhang, PhD a,vivian S. Lee, MD,PhD,MBA b KEYWORDS Dynamic contrast-enhanced
Assessment of Renal Function with Dynamic Contrast-Enhanced MR Imaging Louisa Bokacheva, PhD a, *,HenryRusinek,PhD a, Jeff L. Zhang, PhD a,vivian S. Lee, MD,PhD,MBA b KEYWORDS Dynamic contrast-enhanced
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Estimates of Glomerular Filtration Rate From MR Renography and Tracer Kinetic Models
 JOURNAL OF MAGNETIC RESONANCE IMAGING 29:371 382 (29) Original Research Estimates of Glomerular Filtration Rate From MR Renography and Tracer Kinetic Models Louisa Bokacheva, PhD,* Henry Rusinek, PhD,
JOURNAL OF MAGNETIC RESONANCE IMAGING 29:371 382 (29) Original Research Estimates of Glomerular Filtration Rate From MR Renography and Tracer Kinetic Models Louisa Bokacheva, PhD,* Henry Rusinek, PhD,
Chapter 23. The Nephron. (functional unit of the kidney
 Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Chapter 23 The Nephron (functional unit of the kidney Renal capsule The Nephron Renal cortex Nephron Collecting duct Efferent arteriole Afferent arteriole (a) Renal corpuscle: Glomerular capsule Glomerulus
Functional Assessment of the Kidney From Magnetic Resonance and Computed Tomography Renography: Impulse Retention Approach to a Multicompartment Model
 Magnetic Resonance in Medicine 59:278 288 (2008) Functional Assessment of the Kidney From Magnetic Resonance and Computed Tomography Renography: Impulse Retention Approach to a Multicompartment Model Jeff
Magnetic Resonance in Medicine 59:278 288 (2008) Functional Assessment of the Kidney From Magnetic Resonance and Computed Tomography Renography: Impulse Retention Approach to a Multicompartment Model Jeff
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
Bio 322 Human Anatomy Objectives for the laboratory exercise Urinary System Filtration Reabsorption Secretion Concentration
 Bio 322 Human Anatomy Objectives for the laboratory exercise Urinary System Required reading before beginning this lab: Saladin, KS: Human Anatomy 5 th ed (2017) Chapter 25 For this lab you will use parts
Bio 322 Human Anatomy Objectives for the laboratory exercise Urinary System Required reading before beginning this lab: Saladin, KS: Human Anatomy 5 th ed (2017) Chapter 25 For this lab you will use parts
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
Perfusion Physics. ICMRI2018 March 29-31, 2018 Grand Hilton Hotel, Seoul, Korea. Asian Forum Ⅱ: Perfusion MRI SY24-1.
 SY24-1 Perfusion Physics Hiroyuki Kabasawa MR Collaborations and Development, GE Healthcare, Tokyo, Japan Perfusion is referred as the blood supply to micro capillary in tissue. Perfusion parameter such
SY24-1 Perfusion Physics Hiroyuki Kabasawa MR Collaborations and Development, GE Healthcare, Tokyo, Japan Perfusion is referred as the blood supply to micro capillary in tissue. Perfusion parameter such
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
Angiotensin-Converting Enzyme Inhibitor-Enhanced MR Renography: Repeated Measures of GFR and RPF in Hypertensive Patients
 Articles in PresS. Am J Physiol Renal Physiol (January 21, 2009). doi:10.1152/ajprenal.90648.2008 1 Angiotensin-Converting Enzyme Inhibitor-Enhanced MR Renography: Repeated Measures of GFR and RPF in Hypertensive
Articles in PresS. Am J Physiol Renal Physiol (January 21, 2009). doi:10.1152/ajprenal.90648.2008 1 Angiotensin-Converting Enzyme Inhibitor-Enhanced MR Renography: Repeated Measures of GFR and RPF in Hypertensive
Magnetic Resonance Angiography
 Magnetic Resonance Angiography 1 Magnetic Resonance Angiography exploits flow enhancement of GR sequences saturation of venous flow allows arterial visualization saturation of arterial flow allows venous
Magnetic Resonance Angiography 1 Magnetic Resonance Angiography exploits flow enhancement of GR sequences saturation of venous flow allows arterial visualization saturation of arterial flow allows venous
Lecture-2 Review of the previous lecture:
 Lecture-2 Review of the previous lecture: -Kidney s function is to clean the blood by the removing of the waste plus adding some valuable substances -kidney failure will lead to death for many reasons,
Lecture-2 Review of the previous lecture: -Kidney s function is to clean the blood by the removing of the waste plus adding some valuable substances -kidney failure will lead to death for many reasons,
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Essentials of Clinical MR, 2 nd edition. 99. MRA Principles and Carotid MRA
 99. MRA Principles and Carotid MRA As described in Chapter 12, time of flight (TOF) magnetic resonance angiography (MRA) is commonly utilized in the evaluation of the circle of Willis. TOF MRA allows depiction
99. MRA Principles and Carotid MRA As described in Chapter 12, time of flight (TOF) magnetic resonance angiography (MRA) is commonly utilized in the evaluation of the circle of Willis. TOF MRA allows depiction
19. RENAL PHYSIOLOGY ROLE OF THE URINARY SYSTEM THE URINARY SYSTEM. Components and function. V BS 122 Physiology II 151 Class of 2011
 19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
Anatomical and Functional MRI of the Pancreas
 Anatomical and Functional MRI of the Pancreas MA Bali, MD, T Metens, PhD Erasme Hospital Free University of Brussels Belgium mbali@ulb.ac.be Introduction The use of MRI to investigate the pancreas has
Anatomical and Functional MRI of the Pancreas MA Bali, MD, T Metens, PhD Erasme Hospital Free University of Brussels Belgium mbali@ulb.ac.be Introduction The use of MRI to investigate the pancreas has
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
Urinary System. consists of the kidneys, ureters, urinary bladder and urethra
 Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
Urinary System 1 Urinary System consists of the kidneys, ureters, urinary bladder and urethra 2 Location of Kidneys The kidneys which are positioned retroperitoneally lie on either side of the vertebral
PERFUSION MRI CONTRAST BASED TECHNIQUES
 PERFUSION MRI CONTRAST BASED TECHNIQUES by Kenny K Israni Mar 28, 2006 PERFUSION - MRI Dynamic Susceptibility contrast Dynamic Relaxivity contrast STEADY-STATE STATE TECHNIQUES Steady-state Susceptibility
PERFUSION MRI CONTRAST BASED TECHNIQUES by Kenny K Israni Mar 28, 2006 PERFUSION - MRI Dynamic Susceptibility contrast Dynamic Relaxivity contrast STEADY-STATE STATE TECHNIQUES Steady-state Susceptibility
Angiotensin-converting enzyme inhibitor-enhanced MR renography: repeated measures of GFR and RPF in hypertensive patients
 Am J Physiol Renal Physiol 296: F884 F891, 2009. First published January 21, 2009; doi:10.1152/ajprenal.90648.2008. Angiotensin-converting enzyme inhibitor-enhanced MR renography: repeated measures of
Am J Physiol Renal Physiol 296: F884 F891, 2009. First published January 21, 2009; doi:10.1152/ajprenal.90648.2008. Angiotensin-converting enzyme inhibitor-enhanced MR renography: repeated measures of
Excretion Chapter 29. The Mammalian Excretory System consists of. The Kidney. The Nephron: the basic unit of the kidney.
 Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
Excretion Chapter 29 The Mammalian Excretory System consists of The Kidney 1. Vertebrate kidneys perform A. Ion balance B. Osmotic balance C. Blood pressure D. ph balance E. Excretion F. Hormone production
MR Advance Techniques. Vascular Imaging. Class II
 MR Advance Techniques Vascular Imaging Class II 1 Vascular Imaging There are several methods that can be used to evaluate the cardiovascular systems with the use of MRI. MRI will aloud to evaluate morphology
MR Advance Techniques Vascular Imaging Class II 1 Vascular Imaging There are several methods that can be used to evaluate the cardiovascular systems with the use of MRI. MRI will aloud to evaluate morphology
B108 BC Urinary System *
 OpenStax-CNX module: m62388 1 B108 BC Urinary System * Melodye Gold Based on Human Biology Chapter 12.2: Urinary System Anatomy and Function by OpenStax Willy Cushwa This work is produced by OpenStax-CNX
OpenStax-CNX module: m62388 1 B108 BC Urinary System * Melodye Gold Based on Human Biology Chapter 12.2: Urinary System Anatomy and Function by OpenStax Willy Cushwa This work is produced by OpenStax-CNX
A&P 2 CANALE T H E U R I N A R Y S Y S T E M
 A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
A&P 2 CANALE T H E U R I N A R Y S Y S T E M URINARY SYSTEM CONTRIBUTION TO HOMEOSTASIS Regulates body water levels Excess water taken in is excreted Output varies from 2-1/2 liter/day to 1 liter/hour
Renal Quiz - June 22, 21001
 Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
Renal Quiz - June 22, 21001 1. The molecular weight of calcium is 40 and chloride is 36. How many milligrams of CaCl 2 is required to give 2 meq of calcium? a) 40 b) 72 c) 112 d) 224 2. The extracellular
You should know the T max for any substance that you use and for PAH ; T max = mg / min
 Tubular function - What is clearance? o clearance referred to the theoretical volume of plasma from which a substance is cleared ( cleaned ) over a period of time and so its unit would be ((ml/min)) -
Tubular function - What is clearance? o clearance referred to the theoretical volume of plasma from which a substance is cleared ( cleaned ) over a period of time and so its unit would be ((ml/min)) -
Excretory Lecture Test Questions Set 1
 Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Non Contrast MRA. Mayil Krishnam. Director, Cardiovascular and Thoracic Imaging University of California, Irvine
 Non Contrast MRA Mayil Krishnam Director, Cardiovascular and Thoracic Imaging University of California, Irvine No disclosures Non contrast MRA-Why? Limitations of CTA Radiation exposure Iodinated contrast
Non Contrast MRA Mayil Krishnam Director, Cardiovascular and Thoracic Imaging University of California, Irvine No disclosures Non contrast MRA-Why? Limitations of CTA Radiation exposure Iodinated contrast
KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin
![KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin KD02 [Mar96] [Feb12] Which has the greatest renal clearance? A. PAH B. Glucose C. Urea D. Water E. Inulin](/thumbs/87/96174332.jpg) Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Renal Physiology MCQ KD01 [Mar96] [Apr01] Renal blood flow is dependent on: A. Juxtaglomerular apparatus B. [Na+] at macula densa C. Afferent vasodilatation D. Arterial pressure (poorly worded/recalled
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
Nephron Function and Urine Formation. Ms. Kula December 1, 2014 Biology 30S
 Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Nephron Function and Urine Formation Ms. Kula December 1, 2014 Biology 30S The Role of the Nephron In order for the body to properly function and maintain homeostasis, the amount of dissolved substances
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
Pediatric* MR Urography
 Abdominal Imaging Clinical Pediatric* MR Urography Richard A. Jones; Stephen Little; J. Damien Grattan-Smith Children s Healthcare of Atlanta, Department of Radiology, Atlanta, GA, USA MR urography represents
Abdominal Imaging Clinical Pediatric* MR Urography Richard A. Jones; Stephen Little; J. Damien Grattan-Smith Children s Healthcare of Atlanta, Department of Radiology, Atlanta, GA, USA MR urography represents
41B. Metabolism produces wastes that must be eliminated from the body. This. Renal System Physiology: Computer Simulation
 41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
41B E X E R C I S E Renal System Physiology: Computer Simulation O B J E C T I V E S 1. To define the following terms: glomerulus, glomerular capsule, renal corpuscle, renal tubule, nephron, proximal convoluted
PARTS OF THE URINARY SYSTEM
 EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
EXCRETORY SYSTEM Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid-base concentrations and metabolite concentrations 1 ORGANS OF EXCRETION Skin and
URINARY SYSTEM ANATOMY
 URINARY SYSTEM ANATOMY Adapted from Human Anatomy & Physiology Marieb and Hoehn (9 th ed.) OVERVIEW Metabolism of nutrients by the body produces wastes that must be removed from the body. Although excretory
URINARY SYSTEM ANATOMY Adapted from Human Anatomy & Physiology Marieb and Hoehn (9 th ed.) OVERVIEW Metabolism of nutrients by the body produces wastes that must be removed from the body. Although excretory
Functional morphology of kidneys Clearance
 Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Functional morphology of kidneys Clearance Assoc. Prof. MUDr. Markéta Bébarová, Ph.D. Department of Physiology Faculty of Medicine, Masaryk University This presentation includes only the most important
Functions of the kidney
 Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
Physiology of Urinary tract Kidney, Ureter, Urinary bladder Urethra Kidney function Excretion Physiology of volume regulation Functions of the kidney Excretion of dangerous substances endogenous (metabolites):
General Anatomy of Urinary System
 General Anatomy of Urinary System URINARY SYSTEM ORGANS Kidneys (2) Ureters (2) Urinary bladder Urethra KIDNEY FUNCTIONS Control blood volume and composition KIDNEY FUNCTIONS Filter blood plasma, eliminate
General Anatomy of Urinary System URINARY SYSTEM ORGANS Kidneys (2) Ureters (2) Urinary bladder Urethra KIDNEY FUNCTIONS Control blood volume and composition KIDNEY FUNCTIONS Filter blood plasma, eliminate
The kidneys are excretory and regulatory organs. By
 exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
exercise 9 Renal System Physiology Objectives 1. To define nephron, renal corpuscle, renal tubule, afferent arteriole, glomerular filtration, efferent arteriole, aldosterone, ADH, and reabsorption 2. To
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Chapter 44: Osmoregulation and Excretion
 Name Period The steady-state physiological condition that organisms must maintain is termed homeostasis. Osmoregulation and excretion are frequently cited examples of homeostasis and are the central ideas
Name Period The steady-state physiological condition that organisms must maintain is termed homeostasis. Osmoregulation and excretion are frequently cited examples of homeostasis and are the central ideas
Osmotic Regulation and the Urinary System. Chapter 50
 Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Osmotic Regulation and the Urinary System Chapter 50 Challenge Questions Indicate the areas of the nephron that the following hormones target, and describe when and how the hormones elicit their actions.
Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion.
 The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
The Kidney Vertebrates possess kidneys: internal organs which are vital to ion and water balance and excretion. The kidney has 6 roles in the maintenance of homeostasis. 6 Main Functions 1. Ion Balance
MAJOR FUNCTIONS OF THE KIDNEY
 MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
MAJOR FUNCTIONS OF THE KIDNEY REGULATION OF BODY FLUID VOLUME REGULATION OF OSMOTIC BALANCE REGULATION OF ELECTROLYTE COMPOSITION REGULATION OF ACID-BASE BALANCE REGULATION OF BLOOD PRESSURE ERYTHROPOIESIS
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1)
 RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
RENAL PHYSIOLOGY, HOMEOSTASIS OF FLUID COMPARTMENTS (1) Dr. Attila Nagy 2017 Functional role of the kidney 1.Homeostasis of fluid compartments (isosmia, isovolemia, isoionia, isohydria,) 2. Elimination
Urinary bladder provides a temporary storage reservoir for urine
 Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
Urinary System Organs Kidney Filters blood, allowing toxins, metabolic wastes, and excess ions to leave the body in urine Urinary bladder provides a temporary storage reservoir for urine Paired ureters
CHAPTER 25 URINARY. Urinary system. Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1. functions
 CHAPTER 25 URINARY Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1 fluid waste elimination secretion of wastes control blood volume and BP control blood ph electrolyte levels RBC levels hormone production
CHAPTER 25 URINARY Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1 fluid waste elimination secretion of wastes control blood volume and BP control blood ph electrolyte levels RBC levels hormone production
PHYSICS OF MRI ACQUISITION. Alternatives to BOLD for fmri
 PHYSICS OF MRI ACQUISITION Quick Review for fmri HST-583, Fall 2002 HST.583: Functional Magnetic Resonance Imaging: Data Acquisition and Analysis Harvard-MIT Division of Health Sciences and Technology
PHYSICS OF MRI ACQUISITION Quick Review for fmri HST-583, Fall 2002 HST.583: Functional Magnetic Resonance Imaging: Data Acquisition and Analysis Harvard-MIT Division of Health Sciences and Technology
April 08, biology 2201 ch 11.3 excretion.notebook. Biology The Excretory System. Apr 13 9:14 PM EXCRETORY SYSTEM.
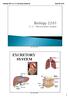 Biology 2201 11.3 The Excretory System EXCRETORY SYSTEM 1 Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid base concentrations and metabolite concentrations
Biology 2201 11.3 The Excretory System EXCRETORY SYSTEM 1 Excretory System How does the excretory system maintain homeostasis? It regulates heat, water, salt, acid base concentrations and metabolite concentrations
Kidney Structure. Renal Lobe = renal pyramid & overlying cortex. Renal Lobule = medullary ray & surrounding cortical labryinth.
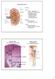 Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
A. Correct! Flushing acids from the system will assist in re-establishing the acid-base equilibrium in the blood.
 OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
12/7/10. Excretory System. The basic function of the excretory system is to regulate the volume and composition of body fluids by:
 Excretory System The basic function of the excretory system is to regulate the volume and composition of body fluids by: o o removing wastes returning needed substances to the body for reuse Body systems
Excretory System The basic function of the excretory system is to regulate the volume and composition of body fluids by: o o removing wastes returning needed substances to the body for reuse Body systems
URINARY SYSTEM. These organs lie posterior or inferior to the. (membrane).
 URINARY SYSTEM I. INTRODUCTION Each kidney is made up of about a million tiny tubules called nephrons. Each nephron individually filters the blood and makes urine and it does the job completely, from start
URINARY SYSTEM I. INTRODUCTION Each kidney is made up of about a million tiny tubules called nephrons. Each nephron individually filters the blood and makes urine and it does the job completely, from start
Outline Urinary System. Urinary System and Excretion. Urine. Urinary System. I. Function II. Organs of the urinary system
 Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
1. Urinary System, General
 S T U D Y G U I D E 16 1. Urinary System, General a. Label the figure by placing the numbers of the structures in the spaces by the correct labels. 7 Aorta 6 Kidney 8 Ureter 2 Inferior vena cava 4 Renal
S T U D Y G U I D E 16 1. Urinary System, General a. Label the figure by placing the numbers of the structures in the spaces by the correct labels. 7 Aorta 6 Kidney 8 Ureter 2 Inferior vena cava 4 Renal
Urinary System VASTACCESS, INC.
 Urinary System www.vastaccess.com 2 Urinary Tract Kidney Ureter Urinary Bladder Urethra Prostate (male) Membranous (male) Spongy (male) 3 Kidney Relations Suprarenal (Adrenal) Glands Liver Duodenum Transverse
Urinary System www.vastaccess.com 2 Urinary Tract Kidney Ureter Urinary Bladder Urethra Prostate (male) Membranous (male) Spongy (male) 3 Kidney Relations Suprarenal (Adrenal) Glands Liver Duodenum Transverse
Mr. Epithelium s Anatomy and Physiology Test SSSS
 Mr. Epithelium s Anatomy and Physiology Test SSSS You have 50 minutes to complete this test packet. One 8.5 x 11 cheat sheet is allowed, along with 1 non-programmable calculator dedicated to computation.
Mr. Epithelium s Anatomy and Physiology Test SSSS You have 50 minutes to complete this test packet. One 8.5 x 11 cheat sheet is allowed, along with 1 non-programmable calculator dedicated to computation.
Histology Urinary system
 Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
Histology Urinary system Urinary system Composed of two kidneys, two ureters, the urinary bladder, and the urethra, the urinary system plays a critical role in: 1- Blood filtration,(filtration of cellular
BIOH122 Human Biological Science 2
 BIOH122 Human Biological Science 2 Session 16 Urinary System 1 The Kidneys Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Functions of Urinary system o The Kidneys:
BIOH122 Human Biological Science 2 Session 16 Urinary System 1 The Kidneys Bioscience Department Endeavour College of Natural Health endeavour.edu.au Session Plan o Functions of Urinary system o The Kidneys:
Urinary System and Fluid Balance. Urine Production
 Urinary System and Fluid Balance Name Pd Date Urine Production The three processes critical to the formation of urine are filtration, reabsorption, and secretion. Match these terms with the correct statement
Urinary System and Fluid Balance Name Pd Date Urine Production The three processes critical to the formation of urine are filtration, reabsorption, and secretion. Match these terms with the correct statement
RADIO-NUCLIDE STUDIES FOR THE EVALUATION OF KIDNEY FUNCTIONS. DR RIANA NEL NUCLEAR MEDICINE DEPT 27 Sept 2010
 RADIO-NUCLIDE STUDIES FOR THE EVALUATION OF KIDNEY FUNCTIONS DR RIANA NEL NUCLEAR MEDICINE DEPT 27 Sept 2010 Alive patient RA + tracers Tc-99m(86%) + MAG 3 DMSA (DTPA) T½ = 6,5h Gamma camera ect. Radio-active
RADIO-NUCLIDE STUDIES FOR THE EVALUATION OF KIDNEY FUNCTIONS DR RIANA NEL NUCLEAR MEDICINE DEPT 27 Sept 2010 Alive patient RA + tracers Tc-99m(86%) + MAG 3 DMSA (DTPA) T½ = 6,5h Gamma camera ect. Radio-active
November 30, 2016 & URINE FORMATION
 & URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
& URINE FORMATION REVIEW! Urinary/Renal System 200 litres of blood are filtered daily by the kidneys Usable material: reabsorbed back into blood Waste: drained into the bladder away from the heart to the
Urinary System and Excretion. Bio105 Lecture 20 Chapter 16
 Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
Urinary System and Excretion Bio105 Lecture 20 Chapter 16 1 Outline Urinary System I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of the urinary system
Non-Invasive MR-based Evaluation of Kidney Function without Exogenous Contrast Agent. Xiang He, PhD Department of Radiology University of Pittsburgh
 Non-Invasive MR-based Evaluation of Kidney Function without Exogenous Contrast Agent Xiang He, PhD Department of Radiology University of Pittsburgh Contents MR-based non-invasive estimation of single kidney
Non-Invasive MR-based Evaluation of Kidney Function without Exogenous Contrast Agent Xiang He, PhD Department of Radiology University of Pittsburgh Contents MR-based non-invasive estimation of single kidney
The functions of the kidney:
 The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The functions of the kidney: After reading this lecture you should be able to.. 1. List the main functions of the kidney. 2. Know the basic physiological anatomy of the kidney and the nephron 3. Describe
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Urine Formation by the Kidneys: I. Glomerular Filtration, Renal Blood Flow and Their Control.
 Urine Formation by the Kidneys: I. Glomerular Filtration, Renal Blood Flow and Their Control. Chapter 26 Yanal A Shafagoj. MD. PhD Lecture-1 Introduction 31/3/2015 1 University of Jordan Faculty of Medicine
Urine Formation by the Kidneys: I. Glomerular Filtration, Renal Blood Flow and Their Control. Chapter 26 Yanal A Shafagoj. MD. PhD Lecture-1 Introduction 31/3/2015 1 University of Jordan Faculty of Medicine
Renal Physiology. April, J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine.
 Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Renal Physiology April, 2011 J. Mohan, PhD. Lecturer, Physiology Unit, Faculty of Medical Sciences, U.W.I., St Augustine. Office : Room 105, Physiology Unit. References: Koeppen B.E. & Stanton B.A. (2010).
Clearance and Reabsorption. Use the following data to answer these questions. a. plasma flow rate in the afferent arterioles:
 Bio390 thanks to Dr. J.F. Anderson, Dept Zoology Univ. of Florida, Gainesville Clearance and Reabsorption Use the following data to answer these questions. renal inulin clearance: [inulin] arterial plasma
Bio390 thanks to Dr. J.F. Anderson, Dept Zoology Univ. of Florida, Gainesville Clearance and Reabsorption Use the following data to answer these questions. renal inulin clearance: [inulin] arterial plasma
The Urinary System 15PART A. PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Urinary System 15PART A Functions of the Urinary System Elimination of waste products Nitrogenous
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Urinary System 15PART A Functions of the Urinary System Elimination of waste products Nitrogenous
Renal Clearance. Dr. Eman El Eter
 Renal Clearance Dr. Eman El Eter Concept of clearance Clearance is the volume of plasma that is completely cleared of a substance each minute. Example: Renal clearance of Substance X is defined as the
Renal Clearance Dr. Eman El Eter Concept of clearance Clearance is the volume of plasma that is completely cleared of a substance each minute. Example: Renal clearance of Substance X is defined as the
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Chapter 44. Osmoregulation and Excretion
 Chapter 44 Osmoregulation and Excretion Overview: A Balancing Act Physiological systems of animals operate in a fluid environment Relative concentrations of water and solutes must be maintained within
Chapter 44 Osmoregulation and Excretion Overview: A Balancing Act Physiological systems of animals operate in a fluid environment Relative concentrations of water and solutes must be maintained within
1. a)label the parts indicated above and give one function for structures Y and Z
 Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Excretory System 1 1. Excretory System a)label the parts indicated above and give one function for structures Y and Z W- renal cortex - X- renal medulla Y- renal pelvis collecting center of urine and then
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
DIRECT RENIN INHIBITOR (DRI) EFFECT ON GFR AND USE IN RENAL ARTERY STENOSIS SCREENING
 DIRECT RENIN INHIBITOR (DRI) EFFECT ON GFR AND USE IN RENAL ARTERY STENOSIS SCREENING Harold Thomas Pretorius, MD, PhD, Nichole Richards, and Michael Harrell Abstract Objective: A new method using a nuclear
DIRECT RENIN INHIBITOR (DRI) EFFECT ON GFR AND USE IN RENAL ARTERY STENOSIS SCREENING Harold Thomas Pretorius, MD, PhD, Nichole Richards, and Michael Harrell Abstract Objective: A new method using a nuclear
Kidney Physiology. Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed
 Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
PHGY210 Renal Physiology
 PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
PHGY210 Renal Physiology Tomoko Takano, MD, PhD *Associate Professor of Medicine and Physiology McGill University *Nephrologist, McGill University Health Centre Lecture plan Lecture 1: Anatomy, basics
Basic Functions of the Kidneys
 Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Dr. Adelina Vlad Basic Functions of the Kidneys Eliminate plasma METABOLIC WASTE PRODUCTS and FOREIGN COMPOUNDS The kidney are the primary means for eliminating metabolic waste products (urea, creatinine,
Controversies around antenatally detected PUJ syndrom. Amy Piepsz, CHU St Pierre, Brussels, Belgium
 Controversies around antenatally detected PUJ syndrom Amy Piepsz, CHU St Pierre, Brussels, Belgium Editors : Anthony Caldamone, USA Pierre Mouriquand, France Newborn boy History of prenatally diagnosed
Controversies around antenatally detected PUJ syndrom Amy Piepsz, CHU St Pierre, Brussels, Belgium Editors : Anthony Caldamone, USA Pierre Mouriquand, France Newborn boy History of prenatally diagnosed
Physiology (6) 2/4/2018. Rahmeh Alsukkar
 Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
Physiology (6) 2/4/2018 Rahmeh Alsukkar **unfortunately the sheet does not involve the slides. ** the doctor repeat a lot of things from the previous lecture so this sheet will begin from slide 139 to
Urinary System. Chapter 17 7/19/11. Introduction
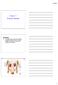 7/19/11 Chapter 17 Urinary System Introduction A. The urinary system consists of two kidneys that filter the blood, two ureters, a urinary bladder, and a urethra to convey waste substances to the outside.
7/19/11 Chapter 17 Urinary System Introduction A. The urinary system consists of two kidneys that filter the blood, two ureters, a urinary bladder, and a urethra to convey waste substances to the outside.
Lesson 14.1: Learning the Key Terms
 209 Lesson 14.1: Learning the Key Terms Directions: Place the letter of the best definition next to each key term. 1. collecting duct 2. distal convoluted tubule 3. glomerulus 4. nephron 5. nephron loop
209 Lesson 14.1: Learning the Key Terms Directions: Place the letter of the best definition next to each key term. 1. collecting duct 2. distal convoluted tubule 3. glomerulus 4. nephron 5. nephron loop
Unit #4 Waste and Excretion. The Kidneys
 Unit #4 Waste and Excretion The Kidneys Renal Hilus (Hilus) the doorway of the kidney Ureter leaves this region blood and lymphatic vessels enter and exit here Renal Capsule (Capsule) smooth fibrous tissue
Unit #4 Waste and Excretion The Kidneys Renal Hilus (Hilus) the doorway of the kidney Ureter leaves this region blood and lymphatic vessels enter and exit here Renal Capsule (Capsule) smooth fibrous tissue
Animals. Male C57Bl/6 mice (n=27) were obtained from Charles River (Sulzfeld, Germany) and
 Supplemental Methods Animals. Male C57Bl/6 mice (n=27) were obtained from Charles River (Sulzfeld, Germany) and housed in groups of 5-10 in individually ventilated BCU cages within a temperature controlled
Supplemental Methods Animals. Male C57Bl/6 mice (n=27) were obtained from Charles River (Sulzfeld, Germany) and housed in groups of 5-10 in individually ventilated BCU cages within a temperature controlled
Class XI - Biology Excretory Products and their Elimination
 Class XI - Biology Excretory Products and their Elimination MULTIPLE CHOICE QUESTIONS 1. The following substances are the excretory products in animals. Choose the least toxic form among them? a. Urea
Class XI - Biology Excretory Products and their Elimination MULTIPLE CHOICE QUESTIONS 1. The following substances are the excretory products in animals. Choose the least toxic form among them? a. Urea
1Pulse sequences for non CE MRA
 MRI: Principles and Applications, Friday, 8.30 9.20 am Pulse sequences for non CE MRA S. I. Gonçalves, PhD Radiology Department University Hospital Coimbra Autumn Semester, 2011 1 Magnetic resonance angiography
MRI: Principles and Applications, Friday, 8.30 9.20 am Pulse sequences for non CE MRA S. I. Gonçalves, PhD Radiology Department University Hospital Coimbra Autumn Semester, 2011 1 Magnetic resonance angiography
Figure 26.1 An Introduction to the Urinary System
 Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
Chapter 26 Figure 26.1 An Introduction to the Urinary System Components of the Urinary System Kidney Produces urine Ureter Transports urine toward the urinary bladder Urinary Bladder Temporarily stores
describe the location of the kidneys relative to the vertebral column:
 Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
The Excretory System. Biology 20
 The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
The Excretory System Biology 20 Introduction Follow along on page 376 What dangers exist if your body is unable to regulate the fluid balance of your tissues? What challenged would the body have to respond
Human Anatomy Unit 3 URINARY SYSTEM
 Human Anatomy Unit 3 URINARY SYSTEM In Anatomy Today Components Kidneys Ureters Urinary bladder Urethra Functions Storage of urine Bladder stores up to 1 L of urine Excretion of urine Transport of urine
Human Anatomy Unit 3 URINARY SYSTEM In Anatomy Today Components Kidneys Ureters Urinary bladder Urethra Functions Storage of urine Bladder stores up to 1 L of urine Excretion of urine Transport of urine
Lab 19 The Urinary System
 Lab 19 The Urinary System Laboratory Objectives Identify and describe the micro- and macroscopic anatomy of the kidney. Track the blood flow in and out of the kidney. Compare blood, glomerular filtrate,
Lab 19 The Urinary System Laboratory Objectives Identify and describe the micro- and macroscopic anatomy of the kidney. Track the blood flow in and out of the kidney. Compare blood, glomerular filtrate,
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM
 RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
RENAL PHYSIOLOGY WESTMEAD PRIMARY EXAM RENAL PHYSIOLOGY - ANATOMY Glomerulus + renal tubule Each kidney has 1.3 million nephrons Cortical nephrons (85%) have shorter Loop of Henle than Juxtamedullary nephrons
