University of Virginia Institutional Review Board - Health Sciences Research Guidelines for Researchers Using Gadolinium-Enhanced MRI in Research
|
|
|
- Ellen Dorsey
- 5 years ago
- Views:
Transcription
1 University of Virginia Institutional Review Board - Health Sciences Research Guidelines for Researchers Using Gadolinium-Enhanced MRI in Research Page 1 of 16
2 Table of Contents New Information:... 3 IRB-HSR Recommendations for past and future use of Gadolinium-enhanced MRI in Research:... 4 New exclusion criteria for protocol using gadolinium-enhanced MRI in research... 6 New risk language for the protocol using gadolinium-enhanced MRI in research... 7 Required Risk Language for the consent for New Studies or for studies that are Open to Enrollment but have not yet enrolled subjects:... 7 Addendum Template:... 9 Appendix A: FDA Public Health Advisory Appendix B: Recommendations from UVa Department of Nephrology for ESRD/dialysis patients requiring gadolinium as contrast Appendix C: Risk of Gadolinium Memo from the UVa Department of Radiology Dated: Thursday, April 19, Page 2 of 16
3 New Information: The FDA has recently released a warning about a new and rare disease called Nephrogenic Systemic Fibrosis (NSF) or Nephrogenic Fibrosing Dermopathy (NFD) that is linked with the use of Gadolinium in people with: 1. moderate chronic kidney disease (glomerular filtration rate (GFR) less than 60 ml/min) 2. end-stage kidney disease (GFR less than 15 ml/min). 3. receiving dialysis for acute or chronic kidney disease NSF/NFD causes fibrosis of the skin, connective tissues like muscles, tendons, ligaments, and blood vessels throughout the body. In addition, those who develop NSF/NFD may have scarring of their vital organs. The signs of NSF/NFD include: burning, itching, swelling, hardening and tightening of skin; red or dark patches on the skin; yellow spots on the whites of the eyes; stiffness in joints with trouble moving or straightening the arms, hands, legs or feet; pain deep in the hip bones or ribs; muscle weakness. In most of the cases reported to the FDA, symptoms of NSF/NFD started between 2 days to 18 months after exposure to the gadolinium-based contrast agent. NSF/NFD may be progressive (may worsen) and may be fatal. There is no known treatment for NSF/NFD. Skin biopsy is the only true means of diagnosis. A copy of the Public Health Advisory from the FDA is available in Appendix A of this document. On January 31, 2007, the UVa Department of Nephrology drafted recommendations related to the use of gadolinium in the presence of renal failure. These guidelines are available in Appendix B of this document and should be referenced for treating patients clinically. These recommendations were given to the UVa Department of Radiology. The Department of Radiology has established guidance for the use of gadolinium in clinical practice as well. The memo that was sent to clinicians earlier this year is provided for your reference in Appendix C of this document. All of these expert recommendations address the use of gadolinium in clinical practice; however these guidelines do not address the use of gadolinium in research. The purpose of this document to relate the IRB-HSR recommendation for the use of gadolinium in research. Page 3 of 16
4 IRB-HSR Recommendations for past and future use of Gadolinium-enhanced MRI in Research: 1. In general, the use of gadolinium-enhanced MRI for research purposes will not be approved in subjects with an estimated GFR of less than 60 ml/min. However, if the principal investigator wants to use gadolinium-enhanced MRI in research subjects with impaired renal function, the principal investigator must provide the IRB with a procedure to screen subjects, a risk/benefit analysis, a discussion of the potential for benefit of the MRI and a plan to minimize the risks. This information must be incorporated into the protocol. Based on review of this information, the IRB will then decide on a protocol by protocol basis whether there is an acceptable risk/benefit ratio. 2. For studies not yet approved by the IRB which utilize gadolinium-enhanced MRI the protocol and consent must list the risk of NSF/NFD and the protocol should list the new exclusion criteria unless otherwise approved by the IRB. Risk language has been approved by the Office of General Counsel and Health System Risk Management and is provided for you later in this document. 3. For studies previously approved by the IRB that utilized gadolinium-enhanced MRI for research the following steps must be taken:. If the study is Active ( e.g. not closed with the IRB) the following steps must be taken: o Add the new exclusion criteria and the recommended risk language and to the protocol (exact wording is given later in this document). o If the protocol is monitored by the GCRC Advisory Committee please contact them for review and approval. o If the protocol is investigator-initiated and overseen by the Cancer Center PRC please contact them to determine if PRC review is required. o Submit modified protocol to IRB for approval Page 4 of 16 o If the study is still open to enrollment: 1. Add the new risk section to the consent form (exact wording is given later in this document) 2. If the protocol is monitored by the GCRC Advisory Committee please contact them for review and approval. 3. If the protocol is investigator-initiated and overseen by the Cancer Center PRC please contact them to determine if PRC review is required. 4. Submit modified consent to IRB for approval 5. If subjects have already been enrolled take the following additional steps: 6. Create Consent Addendum (template attached to this document) 7. Submit Consent Addendum to IRB for approval 8. After IRB has approved consent addendum, send it to all subjects who have had a gadolinium-enhanced MRI within the last two years
5 9. The consent addendum should be mailed with a self-addressed envelope requesting that they sign the new addendum and return the form to the study team. The form should be signed by someone from the study team and a copy of the fully signed form should then be mailed to the participant. This should be done within 30 days following the modification approval. If the study is closed to enrollment and subjects were enrolled: 1. Create Consent Addendum (template attached to this document) 2. If the protocol is monitored by the GCRC Advisory Committee please contact them for review and approval. 3. If the protocol is investigator-initiated and overseen by the Cancer Center PRC please contact them to determine if PRC review is required. 4. Submit Consent Addendum to IRB for approval 5. After IRB has approved consent addendum, send it to all subjects who have had a gadolinium-enhanced MRI within the last two years 6. The consent addendum should be mailed with a self-addressed envelope requesting that they sign the new addendum and return the form to the study team. The form should be signed by someone from the study team and a copy of the fully signed form should then be mailed to the participant. This should be done within 30 days following the modification approval. If the study is Closed AND utilized gadolinium-enhanced MRI for research take the following steps: 1. Create Consent Addendum (template attached to this document) 2. If the protocol is monitored by the GCRC Advisory Committee please contact them for review and approval. 3. If the protocol is investigator-initiated and overseen by the Cancer Center PRC please contact them to determine if PRC review is required. 4. Submit Consent Addendum to IRB for approval 5. After IRB has approved consent addendum, mail it to all subjects who have had a gadolinium-enhanced MRI within the last two years 6. The consent addendum should be mailed with a self-addressed envelope requesting that they sign the new addendum and return the form to the study team. The form should be signed by someone from the study team and a copy of the fully signed form should then be mailed to the participant. This should be done within 30 days following the modification approval. The IRB administrative staff is available to help expedite the approval of the consent addendum. In addition the School of Medicine Clinical Trials Office is available to help with this process. Please contact Lori Elder at if assistance is needed. Page 5 of 16
6 New exclusion criteria for protocol using gadolinium-enhanced MRI in research Add the following recommended exclusion criteria to the protocol under the inclusion/exclusion criteria: Estimated GFR less than 60 ml/min based on a serum creatinine drawn within 90 days of the MRI if any of the following conditions exist: o Greater than 70 years of age o A history or indication of renal insufficiency o History of diabetes o History of paraproteinemia syndromes such as multiple myeloma o History of vascular disease (CAD, MI, carotid disease, PVD, or known visceral artery disease) Page 6 of 16
7 New risk language for the protocol using gadolinium-enhanced MRI in research 1. Required risk language for the protocol using gadolinium-enhanced MRI for research. Add the following recommended risk language to the protocol under the DSMP section 1.1: An extremely rare disease called Nephrogenic Systemic Fibrosis (NSF) or Nephrogenic Fibrosing Dermopathy (NFD) is linked with the use of gadolinium in people with: moderate chronic kidney disease (glomerular filtration rate (GFR) less than 60 ml/min) end-stage kidney disease (GFR less than 15 ml/min). receiving dialysis for acute or chronic kidney disease NSF/NFD causes hardening and thickening (fibrosis) of the skin, connective tissues like muscles, tendons, ligaments, and blood vessels throughout the body. In addition, those who develop NSF/NFD may have scaring of their body organs. NSF/NFD may be progressive (may worsen) and may be fatal. There is no known treatment for NSF/NFD. Skin biopsy is the only true means of diagnosis. Add the following recommended risk language to the protocol under the DSMP section 1.2: To minimize the risk of receiving gadolinium: In addition to the exclusion criteria in this protocol, all subjects will be screened by UVa Department of Radiology staff prior to receiving gadolinium. If radiology screening indicates that it might be unsafe for a subject to receive this contrast, then the subject should not be given the contrast for research purposes. If screening indicates that the subject may receive gadolinium, the subject will receive a brand of contrast that has thus far not been linked to reported cases of NSF per the FDA. Required Risk Language for the consent for New Studies or for studies that are Open to Enrollment but have not yet enrolled subjects: Place this information in the body of the consent form where risks of the study are discussed: Risk of using gadolinium: As part of the MRI/MRA you will receive a contrast called Gadolinium. This substance will help the tissues show up better. The FDA has received information about a new and extremely rare disease called Nephrogenic Systemic Fibrosis (NSF) or Nephrogenic Fibrosing Dermopathy (NFD) that is linked with the use of Gadolinium in people with moderate and severe kidney disease. Page 7 of 16
8 NSF/NFD causes hardening and thickening (fibrosis) of the skin, connective tissues like muscles, tendons, ligaments, and blood vessels throughout the body. In addition, those who develop NSF/NFD may have scaring of their body organs. The signs of NSF/NFD also include: burning, itching, swelling, hardening and tightening of skin; red or dark patches on the skin; yellow spots on the whites of the eyes; stiffness in joints with trouble moving or straightening the arms, hands, legs or feet; pain deep in the hip bones or ribs; Muscle weakness. In most of the cases reported to the FDA, symptoms of NSF/NFD started between 2 days to 18 months after a person received the Gadolinium-based contrast agent. NSF/NFD may get worse and may lead to death. There is no known treatment for NSF/NFD. IF you have any of the symptoms listed above after receiving Gadolinium-based contrast for a study MRI/MRA, please contact the study team immediately. The study team will review your symptoms and perhaps recommend a skin biopsy, which is the only way to determine if you actually have NSF/NDF. (Insert if appropriate) Because of this newly identified risk, study participants with moderate to severe renal failure will not receive Gadolinium based contrast agents before study MRI/MRA tests. (Insert if appropriate) Before you receive gadolinium/additional gadolinium for research: You will be screened by UVa Department of Radiology staff prior to getting gadolinium. If radiology screening shows that it might be unsafe for you to receive this contrast, then you will not be able to receive the contrast. (Add if necessary) You will not be allowed to enroll in the study. If your screening indicates that you may receive gadolinium, you will receive a brand(s) of contrast that has thus far not been linked to reported cases of NSF per the FDA. Page 8 of 16
9 Addendum Template: Use this template to communicate the risk for using gadolinium in research for studies that have enrolled subjects IRB-HSR #: Protocol Title Participant s Name Medical Record # Page 9 of 16 ADDENDUM TO CONSENT- Gadolinium This addendum to the consent form is to tell you about new information regarding the study in which you are enrolled. You received or are scheduled to receive a contrast agent called gadolinium before your study MRI/MRA exams. This contrast agent is given by IV and is useful because it helps the MRI/MRA pictures of the internal organs or blood vessels show up better. The FDA has received information about a new and extremely rare disease called Nephrogenic Systemic Fibrosis (NSF) or Nephrogenic Fibrosing Dermopathy (NFD) that is linked with the use of gadolinium in people with: moderate kidney failure (glomerular filtration rate (GFR) less than 60 ml/min) end-stage kidney disease (GFR less than 15 ml/min). NSF/NFD causes hardening and thickening (fibrosis) of the skin, connective tissues like muscles, tendons, ligaments, and blood vessels throughout the body. In addition, those who develop NSF/NFD may have scaring of their body organs. The signs of NSF/NFD also include: burning, itching, swelling, hardening and tightening of skin; red or dark patches on the skin; yellow spots on the whites of the eyes; stiffness in joints with trouble moving or straightening the arms, hands, legs or feet; pain deep in the hip bones or ribs; muscle weakness. In most of the cases reported to the FDA, symptoms of NSF/NFD started between 2 days to 18 months after exposure to the Gadolinium-based contrast agent. NSF/NFD may get worse and may lead to death. There is no known treatment for NSF/NFD. IF you had or have any of the symptoms listed above after receiving Gadolinium-based contrast for a study MRI/MRA, please contact the study team immediately. The study team will review your symptoms and perhaps recommend a skin biopsy, which is the only way to find out if you actually have NSF/NDF. The study team contact information is on page 2 of this document.
10 (Insert if appropriate) Because of this newly identified risk, study participants with moderate to severe renal failure will no longer receive Gadolinium based contrast agents before study MRI/MRA tests. (Insert if appropriate) Because of this newly identified risk, this study is being stopped, changed etc. Add details if study is being changed. Add information about final, or follow up visits. (Insert if appropriate) If you have not already received your MRI: You will be screened by UVa Department of Radiology staff prior to getting gadolinium. If radiology screening shows that it might be unsafe for you to receive this contrast, then you will not be able to receive the contrast. (Add if necessary) You will not be allowed to continue to be in the study. If your screening indicates that you may receive gadolinium, you will receive a brand(s) of contrast that has thus far not been linked to reported cases of NSF per the FDA. All other sections of the original consent form still apply. Please refer to it for any questions you might have. Contact Names and Numbers If you have any questions about this study, you should talk to (insert name and title) at (insert phone number) If you have any questions regarding research participants' rights, please contact the Chair of the Institutional Review Board for Health Sciences Research of the University of Virginia at (434) or the IRB-HSR staff at (434) Conclusion You will receive a signed copy of this form to keep. I HAVE READ, OR HAD READ TO ME, THE ABOVE INFORMATION BEFORE SIGNING THIS ADDENDUM. I HAVE BEEN OFFERED AN OPPORTUNITY TO ASK QUESTIONS AND HAVE RECEIVED ANSWERS THAT FULLY SATISFY THOSE QUESTIONS PARTICIPANT PARTICIPANT DATE (SIGNATURE) (PRINT) PERSON OBTAINING PERSON OBTAINING CONSENT DATE CONSENT (SIGNATURE) (PRINT) Page 10 of 16
11 Appendix A: FDA Public Health Advisory Update on Magnetic Resonance Imaging (MRI) Contrast Agents Containing Gadolinium and Nephrogenic Fibrosing Dermopathy The FDA has received additional information about a new disease, known as Nephrogenic Systemic Fibrosis or Nephrogenic Fibrosing Dermopathy (NSF/NFD), which may occur in patients with moderate to end-stage kidney disease after they have had a Magnetic Resonance Imaging (MRI) or Magnetic Resonance Angiography (MRA) scan with a gadolinium-based contrast agent. An MRI scan provides clear and detailed pictures of internal organs. An MRA test uses a gadolinium-based contrast agent to take detailed pictures of blood vessels. During some MRI scans and all MRA scans, a gadolinium-based contrast agent is injected into a patient s vein so blood vessels can be distinguished from other nearby tissues. As of December 21, 2006, FDA has received reports of 90 patients with moderate to end-stage kidney disease who developed NSF/NFD after they had an MRI or MRA with a gadolinium-based contrast agent. Their NSF/NFD began from 2 days to 18 months after exposure to the contrast agent. Many, but not all of these patients, received a high dose of the contrast agent; some received only one dose. In light of these reports, FDA is notifying health care providers and patients of the following: Patients with moderate to end-stage kidney disease who receive an MRI or MRA with a gadolinium-based contrast agent may get NSF/NFD which is debilitating and may cause death. Patients who believe they may have NSF/NFD should contact their doctor. Patients who develop NSF/NFD have areas of tight, rigid skin and may have scarring of their body organs. The signs of NSF/NFD also include: burning, itching, swelling, hardening and tightening of the skin; red or dark patches on the skin; yellow spots on the whites of the eyes; stiffness in joints with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness. When a patient with moderate to end-stage kidney disease needs an imaging study, select imaging methods other than MRI or MRA with a gadolinium-based contrast agent for the study whenever possible. If these patients must receive a gadolinium-based contrast agent, prompt dialysis following the MRI or MRA should be considered. The FDA asks health care professionals and patients to report possible cases of NSF/NFD to the FDA through the MedWatch program by phone (1-800-FDA-1088) or by the Internet at Worldwide, about 215 patients with NSF/NFD have been reported. Of these reports, the medical histories of 75 of these patients were reviewed in detail, and all of the patients had received a gadolinium-based contrast agent for an MRI or MRA. Researchers have identified gadolinium in skin biopsies of patients with NSF/NFD. Page 11 of 16
12 Why NSF/NFD occurs in patients with moderate to end-stage kidney disease who receive gadoliniumbased contrast agent is not yet known. The FDA is working with expert scientists to gather additional information about NSF/NFD. Currently there are five FDA approved gadolinium-based contrast agents, Magnevist, MultiHance, Omniscan, OptiMARK, and ProHance. These contrast agents are FDA approved for use during an MRI scan, but not for use during an MRA scan. Although NSF/NFD has been reported for only 3 of the 5 gadolinium-based contrast agents, FDA believes that there is a potential for NSF/NFD to occur with the use of any of the approved gadolinium-based contrast agents. You can find more details about NSF/NFD and gadolinium-based contrast agents in FDA s Information for Healthcare Professionals. Page 12 of 16
13 Appendix B: Recommendations from UVa Department of Nephrology for ESRD/dialysis patients requiring gadolinium as contrast Introduction On 12/22/06 the FDA issued an alert stating that 90 cases of nephrogenic sclerosing fibrosis (NSFpreviously referred to as nephrogenic sclerosing dermopathy or nephrogenic fibrosing dermopathy) have been reported in patients with moderate chronic kidney disease or end stage kidney disease. All 90 patients had received gadolinium for MRI scans within 2 days to 18 months of developing the debilitating and potentially fatal condition. It is also possible that the most commonly used gadolinium preparation (gadodiamide-omniscan) is most likely to cause NSF Scattered cases of NSF have been previously reported, including 57 cases in Europe. All of these cases involved MRI. MRI, requires a high dose of gadolinium and it is thought that this combined with ESRD patients inability to rapidly excrete the agent likely contribute to the development of NSF. Gadolinium has been found in skin biopsies of patients with NSF. Investigation is ongoing. Gadolinium appears to be excreted totally by the kidney; hemodialysis is effective in removing gadolinium. The first hemodialysis treatment after gadolinium exposure removes approximately 70% of the gadolinium. The second and third post-exposure hemodialysis treatments remove 92% and 96% respectively (4). Recommendations--reducing the risk of NSF associated with MRI with gadolinium: 1. consider using another imaging modality or unenhanced MR in patients at risk of NSF ( ESRD patients and potentially those with GFR < 30) 2. Use gadolinium enhanced MRI only if clinically necessary 3. If using gadolinium in this population dialyze within 3 hours of the imaging procedure, and again at 24 and 48 hours. 4. Use the lowest possible dose of the agent that will still yield reliable diagnostic information 5. Inform patients in the target population of the risks and benefits and alternatives of the procedure 6. Report occurrences of NSF to nsf@esur.org and to the FDA Med Watch program. There is also an international NSF registry overseen by Shawn Cowper. (NFD/NSF website see REFERENCES 1.FDA (2006) Public Health Advisory: Gadolinium-containing Contrast Agents for Magnetic Resonance Imaging (MRI): Omniscan, Optimark, ProHance, and MultiHance. Available at 2 Nephrogenic Systemic Fibrosis (NSF): A disease recently associated with some of the Gadolinium contrast agents: a document prepared by the Contrast Medium Safety Committee of the European Society of Urogenital Radiology. Nov Cassels, C. Dialysis recommended to prevent NSF after Gadolinium based imaging in severe renal failure. Medscape medical news Information obtained from January 10, 2007 issue of the journal Radiology Page 13 of 16
14 4. Thomsen, H. S.et al Is there a causal relation between the administration of gadolinium based contrast media and the development of nephrogenic systemic fibrosis (NSF)? Clinical Radiology 2006; 61: Thomsen HS. Nephrogenic systemic fibrosis: a serious late adverse reaction to gadodiamide. Eur Radiol 2006; 16: Epub. 6. Maarckman P et al Nephrogenic systemic fibrosis: Suspected etiological role of gadodiamide used for contrast enhanced magnetic resonance imaging. J Am Soc Nephrol 2006; 17: University of Virginia Division of Nephrology January 31, 2007 JH/LN Page 14 of 16
15 Appendix C: Risk of Gadolinium Memo from the UVa Department of Radiology Dated: Thursday, April 19, 2007 Risks of gadolinium contrast Overall, MRI contrast agents have an excellent safety profile over 18 years and more than 200 million doses worldwide. However, recent reports have described an association between certain gadoliniumbased MR contrast agents and a rare but serious medial condition called Nephrogenic Systemic Fibrosis (NSF) or Nephrogenic Fibrosing Dermopathy (NFD). NSF/NFD was first described in 1997 and has only been observed as a side-effect in patients with chronic kidney disease. NSF/NFD causes thickening and hardening of the skin and may also affect the internal organs. The disease is progressive and may be fatal. There is currently no effective therapy for NSF/NFD. The cause and relationship to gadolinium based contrast agents are currently unknown. The following link provides some background on this topic: From the available and limited literature, with GFR or moderate kidney disease, it appears that the risk of developing NSF is greater than the general public, although this risk remains extremely low (<1% or less than 1 out of 100 patients). With GFR<30 or severe to end stage kidney disease, it again seems that the risk of developing NSF is very low, but more likely than either patients with no kidney disease, or moderate kidney disease, and perhaps as great as 1%. For patients on renal dialysis with renal failure, the risk of developing NSF seems much more significant, but is still likely to be low (less than 5%). For these patients, prompt dialysis is recommended following imaging studies using gadolinium-based contrast agents to reduce the contrast burden as a preventative measure. Implications In order to minimize the risk and ensure patients are well informed, it is recommended for all imaging studies that non-contrast protocols are optimized and contrast amount is reduced for protocols when this is feasible, while ensuring diagnostic integrity is maintained. For patients with existing renal disease, the decision and amount of contrast is a risk-benefit decision. In order to determine a patient's risk level, a screening form much like those currently used for CT contrast will be administered, and will identify those patients requiring labs. Additionally, this form will be used to document the radiologist's order for the administration of the contrast. For those patients requiring contrast, their risk will be stated for 3 distinct tiers: GFR 30-59, GFR <30 and Dialysis patients. For all patients in these 3 groups it is recommended that gadodiamide (Omniscan ) or gadoversetamide (OptiMARK ) not be used. Instead either gadopentetate dimeglumine (Magnevist ), gadoteridol (ProHance ), or gadobenate dimeglumine (MultiHance ) is recommended. When gadodiamide (Omniscan ) is used for patients without documented renal disease, it will be limited to weight based dosing (0.1mmol/kg) For Dialysis patients, the use of contrast is discouraged, but the choice to do so becomes a physician's risk-benefit assessment. Page 15 of 16
16 We will be implementing a consent form that will have a check box and description of risk for the 3 groups of relative increased risk. These consent forms will be "self administered" and the technologist will follow up with the patient to identify additional questions. Dialysis patients (for both CT and MRI) will be identified at time of scheduling along with when and where they are scheduled for dialysis and the scheduling of dialysis concurrent with the contrast administration will be facilitated by the radiology staff. Until we have a completed screening form and consent, it is recommended that patients identified as having potential or likely renal disease (based on technologist questioning) not be administered gadodiamide (Omniscan ). All patients on dialysis must be identified and cleared with the attending radiologist prior to contrast administration. For additional information please reference the UVa Department of Radiology Policy on Gadolinium Based Contrast ( Policy # PH006) Page 16 of 16
Gadolinium-Based Contrast Agents for Magnetic Resonance Imaging (marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance)
 Information for Healthcare Professionals Page 1 of 5 FDA ALERT [6/2006, updated 12/2006 and 5/23/2007: This updated Alert highlights FDA s request for addition of a boxed warning and new warnings about
Information for Healthcare Professionals Page 1 of 5 FDA ALERT [6/2006, updated 12/2006 and 5/23/2007: This updated Alert highlights FDA s request for addition of a boxed warning and new warnings about
SUBJECT: ASSESSMENT OF LAB RESULTS PRIOR TO INJECTING GADOLINIUM-BASED CONTRAST MEDIA.
 ASSESSMENT OF PATIENTS: IMAGING SERVICES Policy #: 300.02 Effective Date: 6/29/09 Last Revision Date: 10/07 Page 1 of 5 SUBJECT: ASSESSMENT OF LAB RESULTS PRIOR TO INJECTING GADOLINIUM-BASED CONTRAST MEDIA
ASSESSMENT OF PATIENTS: IMAGING SERVICES Policy #: 300.02 Effective Date: 6/29/09 Last Revision Date: 10/07 Page 1 of 5 SUBJECT: ASSESSMENT OF LAB RESULTS PRIOR TO INJECTING GADOLINIUM-BASED CONTRAST MEDIA
Gadolinium Based Contrast Agent Recommendations (Revised 06/07/2018)
 Gadolinium Based Contrast Agent Recommendations (Revised 06/07/2018) Introduction: Nephrogenic systemic fibrosis is the only known adverse health effect related to gadoliniumbased contrast agents (GBCAs).
Gadolinium Based Contrast Agent Recommendations (Revised 06/07/2018) Introduction: Nephrogenic systemic fibrosis is the only known adverse health effect related to gadoliniumbased contrast agents (GBCAs).
Global reports and findings of NSF and its effects
 Safety Forum: MR Safety Update - SMRT 2008 Sunday May 8, 2:25 p.m. Global reports and findings of NSF and its effects Tim Leiner, MD PhD Assistant Professor of Radiology, Department of Radiology, Maastricht
Safety Forum: MR Safety Update - SMRT 2008 Sunday May 8, 2:25 p.m. Global reports and findings of NSF and its effects Tim Leiner, MD PhD Assistant Professor of Radiology, Department of Radiology, Maastricht
Administration of Gadolinium Contrast in Adults Procedural Guideline
 Administration of Gadolinium Contrast in Adults Procedural Guideline This procedural guideline is designed to assist Radiologists by providing an analytical framework for the evaluation of gadolinium contrast
Administration of Gadolinium Contrast in Adults Procedural Guideline This procedural guideline is designed to assist Radiologists by providing an analytical framework for the evaluation of gadolinium contrast
Protocol for iv. iodine and gadolinium contrast studies
 Protocol for iv. iodine and gadolinium contrast studies Royal College of Radiologists Standard The individual administering the contrast agent must ensure that the patient understands that it is to be
Protocol for iv. iodine and gadolinium contrast studies Royal College of Radiologists Standard The individual administering the contrast agent must ensure that the patient understands that it is to be
DIVISION OF MEDICAL IMAGING AND HEMATOLOGY PRODUCTS
 DIVISION OF MEDICAL IMAGING AND HEMATOLOGY PRODUCTS MEMORANDUM TO THE FILE i. NDA: 20-123, 22-066 Product: Omniscan ii. NDA: 19-596, 21-037 Product: Magnevist iii. NDA: 20-976, 20-937,20-975 Product: OptiMARK
DIVISION OF MEDICAL IMAGING AND HEMATOLOGY PRODUCTS MEMORANDUM TO THE FILE i. NDA: 20-123, 22-066 Product: Omniscan ii. NDA: 19-596, 21-037 Product: Magnevist iii. NDA: 20-976, 20-937,20-975 Product: OptiMARK
Seeing is Believing... Look Beneath the Surface
 References 1. Idee JM, Port C, Raynal I, et al. Clinical and geological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundamental and Clinical Pharmacology.
References 1. Idee JM, Port C, Raynal I, et al. Clinical and geological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundamental and Clinical Pharmacology.
Contrast media Purpose of using contrast Contrast reaction Nephrotoxicity from contrast Nephrogenic systemic fibrosis When should contrast be used
 Contrast vs Non-Contrast When to Order Stephen McManus, M.D. Contrast media Purpose of using contrast Contrast reaction Nephrotoxicity from contrast Nephrogenic systemic fibrosis When should contrast be
Contrast vs Non-Contrast When to Order Stephen McManus, M.D. Contrast media Purpose of using contrast Contrast reaction Nephrotoxicity from contrast Nephrogenic systemic fibrosis When should contrast be
Policy #: 291 Latest Review Date: February 2013
 Effective for dates of service on or after April 1, 2013, refer to: https://www.bcbsal.org/providers/policies/carecore.cfm Name of Policy: Magnetic Resonance Angiography (MRA) of the Chest (excluding the
Effective for dates of service on or after April 1, 2013, refer to: https://www.bcbsal.org/providers/policies/carecore.cfm Name of Policy: Magnetic Resonance Angiography (MRA) of the Chest (excluding the
NSF Coming and Going
 NSF Coming and Going Martin R. Prince, MD, PhD Cornell and Columbia Universities Patent agreements: GE, Philips, Siemens, Hitachi, Medrad, Epix, Lantheus, Bayer, Bracco, Nemoto, Mallinckrodt and Topspins
NSF Coming and Going Martin R. Prince, MD, PhD Cornell and Columbia Universities Patent agreements: GE, Philips, Siemens, Hitachi, Medrad, Epix, Lantheus, Bayer, Bracco, Nemoto, Mallinckrodt and Topspins
A Safety Update on the Gadolinium Chelates
 Control # 1029 A Safety Update on the Gadolinium Chelates Val M. Runge, MD Universitätsinstitut für Diagnostische, Interventionelle und Pädiatrische Radiologie University Hospital Bern Allergic Reactions
Control # 1029 A Safety Update on the Gadolinium Chelates Val M. Runge, MD Universitätsinstitut für Diagnostische, Interventionelle und Pädiatrische Radiologie University Hospital Bern Allergic Reactions
Annex III. Amendments to relevant sections of the product information
 Annex III Amendments to relevant sections of the product information Note: These amendments to the relevant sections of the product information are the outcome of the referral procedure. The product information
Annex III Amendments to relevant sections of the product information Note: These amendments to the relevant sections of the product information are the outcome of the referral procedure. The product information
Information for Healthcare Professionals and other Stakeholders
 Information for Healthcare Professionals and other Stakeholders NEPHROGENIC SYSTEMIC FIBROSIS: AN UNCOMMON AND DEBILITATING DISEASE POSSIBLY ASSOCIATED WITH GADOLINIUM CHELATES Villepinte, February 2014
Information for Healthcare Professionals and other Stakeholders NEPHROGENIC SYSTEMIC FIBROSIS: AN UNCOMMON AND DEBILITATING DISEASE POSSIBLY ASSOCIATED WITH GADOLINIUM CHELATES Villepinte, February 2014
Please see Important Safety Information, including Boxed Warnings for Gadavist and Magnevist below.
 August 15 th, 2018 Dear Valued Customer: Thank you for your continued patience over the last several months as we have worked to resolve our contrast supply interruption. The purpose of this letter is
August 15 th, 2018 Dear Valued Customer: Thank you for your continued patience over the last several months as we have worked to resolve our contrast supply interruption. The purpose of this letter is
Important Update - Availability of Bayer s MRI (Magnetic Resonance Imaging) Contrast agents
 Important Update - Availability of Bayer s MRI (Magnetic Resonance Imaging) Contrast agents Certain batches of pharmaceutical products from our company s Supply Center in Berlin where final formulation
Important Update - Availability of Bayer s MRI (Magnetic Resonance Imaging) Contrast agents Certain batches of pharmaceutical products from our company s Supply Center in Berlin where final formulation
Beyond NSF: Acute GBCA adverse reactions
 Source images Beyond NSF: Acute GBCA adverse reactions Martin R. Prince, MD, PhD, FACR Disclosures Patent Agreements: GE, Siemens, Philips, Hitachi, Toshiba, Bayer, Bracco, Mallinckrodt, Medrad, Nemoto,
Source images Beyond NSF: Acute GBCA adverse reactions Martin R. Prince, MD, PhD, FACR Disclosures Patent Agreements: GE, Siemens, Philips, Hitachi, Toshiba, Bayer, Bracco, Mallinckrodt, Medrad, Nemoto,
William G. Bradley, Jr, MD, PhD, FACR
 William G. Bradley, Jr, MD, PhD, FACR Professor of Radiology Chair, Dept of Radiology University of California, San Diego Medical Center San Diego, California Learning Objectives Identify emerging safety
William G. Bradley, Jr, MD, PhD, FACR Professor of Radiology Chair, Dept of Radiology University of California, San Diego Medical Center San Diego, California Learning Objectives Identify emerging safety
Gadolinium-Based Contrast Media
 Gadolinium-Based Contrast Media Wm. Faulkner, B.S.,R.T.(R)(MR)CT, FSMRT, MRSO (MRSC ) Kristan Harrington, MBA, R.T.(R)(MR), MRSO (MRSC TM ) Gadolinium ( 64 Gd) 7 un-paired electrons chelate, any of a class
Gadolinium-Based Contrast Media Wm. Faulkner, B.S.,R.T.(R)(MR)CT, FSMRT, MRSO (MRSC ) Kristan Harrington, MBA, R.T.(R)(MR), MRSO (MRSC TM ) Gadolinium ( 64 Gd) 7 un-paired electrons chelate, any of a class
Please see Important Safety Information, including Boxed Warnings for Gadavist, Eovist and Magnevist on the following pages.
 Dear Valued Customer: As a valued partner we want to inform you that a technical issue occurred at our Berlin manufacturing center where final formulation and packaging of Bayer MRI (Magnetic Resonance
Dear Valued Customer: As a valued partner we want to inform you that a technical issue occurred at our Berlin manufacturing center where final formulation and packaging of Bayer MRI (Magnetic Resonance
Contrast Materials Patient Safety: What are contrast materials and how do they work?
 Contrast Materials Patient Safety: What are contrast materials and how do they work? Which imaging exams use contrast materials? How safe are contrast materials? How should I prepare for my imaging procedure
Contrast Materials Patient Safety: What are contrast materials and how do they work? Which imaging exams use contrast materials? How safe are contrast materials? How should I prepare for my imaging procedure
Instructions for the safe administration of gadolinium based contrast agents (GBCA). 1. PREADMINISTRATION PRECAUTIONS:
 POLICY NUMBER: RM 6-18 CATEGORY: Patient Care DATE: June 2016 NEXT REVIEW DATE: April 2017 SUBJECT: PURPOSE: POLICY: Intravenous Gadolinium Based Contrast Administration To prevent complications associated
POLICY NUMBER: RM 6-18 CATEGORY: Patient Care DATE: June 2016 NEXT REVIEW DATE: April 2017 SUBJECT: PURPOSE: POLICY: Intravenous Gadolinium Based Contrast Administration To prevent complications associated
Magnetic Resonance Imaging (MRI)
 (MRI) Disclaimer This film is an educational resource only and should not be used to make a decision on MRI. All such decisions must be made in consultation with a physician or licensed healthcare provider.
(MRI) Disclaimer This film is an educational resource only and should not be used to make a decision on MRI. All such decisions must be made in consultation with a physician or licensed healthcare provider.
MR Contrast Agents. Why Use Contrast Agents in MRI? Why Use Contrast Agents in MRI? US Agents. Understanding and Embracing Change
 Why Use Contrast Agents in MRI? Improve disease detection and characterization Increase sensitivity to extent of disease MR Contrast Agents Understanding and Embracing Change Kristan Harrington, MBA, RT
Why Use Contrast Agents in MRI? Improve disease detection and characterization Increase sensitivity to extent of disease MR Contrast Agents Understanding and Embracing Change Kristan Harrington, MBA, RT
Information for Healthcare Professionals and other Stakeholders
 Information for Healthcare Professionals and other Stakeholders NEPHROGENIC SYSTEMIC FIBROSIS: AN UNCOMMON AND DEBILITATING DISEASE POSSIBLY ASSOCIATED WITH GADOLINIUM CHELATES Villepinte, April 2013 Any
Information for Healthcare Professionals and other Stakeholders NEPHROGENIC SYSTEMIC FIBROSIS: AN UNCOMMON AND DEBILITATING DISEASE POSSIBLY ASSOCIATED WITH GADOLINIUM CHELATES Villepinte, April 2013 Any
Contrast-enhanced MRI: how do changing EU regulations impact daily practice?
 Safety considerations in contrast enhanced procedures Carlo Catalano Sapienza University of Rome Contrast-enhanced MRI: how do changing EU regulations impact daily practice? CE MRI: EU regulations - Initial
Safety considerations in contrast enhanced procedures Carlo Catalano Sapienza University of Rome Contrast-enhanced MRI: how do changing EU regulations impact daily practice? CE MRI: EU regulations - Initial
PATIENT CARE AND MRI SAFETY Module 7
 PATIENT CARE AND MRI SAFETY Module 7 1 Biological Considerations There are no reported adverse biological effects of extended exposure to MRI. However, several inconsequential and reversible effects of
PATIENT CARE AND MRI SAFETY Module 7 1 Biological Considerations There are no reported adverse biological effects of extended exposure to MRI. However, several inconsequential and reversible effects of
Lab Values Explained. working at full strength. Other possible causes of an elevated BUN include dehydration and heart failure.
 Patient Education Lab Values Explained Common Tests to Help Diagnose Kidney Disease Lab work, urine samples and other tests may be given as you undergo diagnosis and treatment for renal failure. The test
Patient Education Lab Values Explained Common Tests to Help Diagnose Kidney Disease Lab work, urine samples and other tests may be given as you undergo diagnosis and treatment for renal failure. The test
Update in Nephrology. Case: Question 1. Case presentation. Acute Kidney Injury. For her hypertension management, you decide to:
 Update in Nephrology Chronic Kidney Disease Renoprotection and Proteinuria, ACE and/or ARB Anemia management Update in Nephrology Renal artery stenosis Nephrogenic systemic fibrosis Division of Nephrology
Update in Nephrology Chronic Kidney Disease Renoprotection and Proteinuria, ACE and/or ARB Anemia management Update in Nephrology Renal artery stenosis Nephrogenic systemic fibrosis Division of Nephrology
What Your Kidneys Do
 UW MEDICINE PATIENT EDUCATION What Your Kidneys Do And what happens with kidney disease Class Goals 1. Understand what kidneys do. 2. Understand symptoms of uremia. 3. Know the common causes of kidney
UW MEDICINE PATIENT EDUCATION What Your Kidneys Do And what happens with kidney disease Class Goals 1. Understand what kidneys do. 2. Understand symptoms of uremia. 3. Know the common causes of kidney
Gadolinium-Based MR Contrast Agents
 Gadolinium-Based MR Contrast Agents Wm. Faulkner, BS,RT(R)(MR)(CT), FSMRT, MRSO (MRSC ) Kristan Harrington, MBA, RT(R)(MR) ARRT, MRSO (MRSC ) Magnetic Resonance Imaging, Vol 3, pp27-35, 1985 0.35 T 35/1600
Gadolinium-Based MR Contrast Agents Wm. Faulkner, BS,RT(R)(MR)(CT), FSMRT, MRSO (MRSC ) Kristan Harrington, MBA, RT(R)(MR) ARRT, MRSO (MRSC ) Magnetic Resonance Imaging, Vol 3, pp27-35, 1985 0.35 T 35/1600
Gadolinium and nephrogenic systemic fibrosis
 mini review http://www.kidney-international.org & 2007 International Society of Nephrology Gadolinium and nephrogenic systemic fibrosis T Grobner 1 and FC Prischl 2 1 2nd Department of Medicine/Nephrology,
mini review http://www.kidney-international.org & 2007 International Society of Nephrology Gadolinium and nephrogenic systemic fibrosis T Grobner 1 and FC Prischl 2 1 2nd Department of Medicine/Nephrology,
1 INDICATIONS AND USAGE 2 DOSAGE AND ADMINISTRATION. 1.1 Central Nervous System. 1.2 Extracranial/Extraspinal Tissues. 1.3 Body
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use MAGNEVIST safely and effectively. See full prescribing information for MAGNEVIST. MAGNEVIST (gadopentetate
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use MAGNEVIST safely and effectively. See full prescribing information for MAGNEVIST. MAGNEVIST (gadopentetate
PLEASE SCROLL DOWN FOR ARTICLE
 This article was downloaded by:[danish Veterinary and Agricultural Library] [Danish Veterinary and Agricultural Library] On: 24 June 2007 Access Details: [subscription number 773444395] Publisher: Informa
This article was downloaded by:[danish Veterinary and Agricultural Library] [Danish Veterinary and Agricultural Library] On: 24 June 2007 Access Details: [subscription number 773444395] Publisher: Informa
INTRODUCTION CLINICAL EXPERIENCE CLINICAL EFFICACY PEDIATRIC EXPERIENCE DOSING SUMMARY
 1 2 DOTAREM Administered Doses 3 Molecular Structure of DOTAREM DOTAREM offers high thermodynamic and kinetic stability provided by its macrocyclic and ionic structure* 3 *The clinical significance of
1 2 DOTAREM Administered Doses 3 Molecular Structure of DOTAREM DOTAREM offers high thermodynamic and kinetic stability provided by its macrocyclic and ionic structure* 3 *The clinical significance of
PRAC recommendations on signals for update of the product information
 Thursday 22 January 2015 EMA/PRAC/62352/2015 Pharmacovigilance Risk Assessment Committee PRAC recommendations on signals for update of the product information Adopted at the 6-9 January 2015 PRAC 1. Atorvastatin,
Thursday 22 January 2015 EMA/PRAC/62352/2015 Pharmacovigilance Risk Assessment Committee PRAC recommendations on signals for update of the product information Adopted at the 6-9 January 2015 PRAC 1. Atorvastatin,
Gadolinium-Based MR Contrast Agents
 Gadolinium-Based MR Contrast Agents Magnetic Resonance Imaging, Vol 3, pp27-35, 1985 Wm. Faulkner, BS,RT(R)(MR)(CT), FSMRT, MRSO (MRSC ) Kristan Harrington, MBA, RT(R)(MR) ARRT, MRSO (MRSC ) 0.35 T 0.35
Gadolinium-Based MR Contrast Agents Magnetic Resonance Imaging, Vol 3, pp27-35, 1985 Wm. Faulkner, BS,RT(R)(MR)(CT), FSMRT, MRSO (MRSC ) Kristan Harrington, MBA, RT(R)(MR) ARRT, MRSO (MRSC ) 0.35 T 0.35
Clinical Safety & Effectiveness Cohort 4-UTHSCSA. MRI Contrast Mis-administrations. May 21, 2010
 Clinical Safety & Effectiveness Cohort 4-UTHSCSA MRI Contrast Mis-administrations May 21, 2010 1 2 UTHSCSA/UHS The Team Ken Kist, MD (cohort member) Gilbert Cortez (cohort member) Kristi Hill-Herrera (cohort
Clinical Safety & Effectiveness Cohort 4-UTHSCSA MRI Contrast Mis-administrations May 21, 2010 1 2 UTHSCSA/UHS The Team Ken Kist, MD (cohort member) Gilbert Cortez (cohort member) Kristi Hill-Herrera (cohort
Chronic Kidney Disease. Dr Mohan B. Biyani A. Professor of Medicine University of Ottawa/Ottawa Hospital
 Chronic Kidney Disease Dr Mohan B. Biyani A. Professor of Medicine University of Ottawa/Ottawa Hospital Health Seminar Series Date 12 May 2013 Objectives Normal functioning of Kidneys. Risk factors to
Chronic Kidney Disease Dr Mohan B. Biyani A. Professor of Medicine University of Ottawa/Ottawa Hospital Health Seminar Series Date 12 May 2013 Objectives Normal functioning of Kidneys. Risk factors to
MRI Research Safety Form for IRB Approval
 MRI Research Safety Form for IRB Approval Please complete and e-mail to Julie Hanlon and Vera Kimbrell jahanlon@partners.org ;vkimbrell@partners.org Study Name: Contact: Anticipated Start Date: The MRI
MRI Research Safety Form for IRB Approval Please complete and e-mail to Julie Hanlon and Vera Kimbrell jahanlon@partners.org ;vkimbrell@partners.org Study Name: Contact: Anticipated Start Date: The MRI
PATIENT INFORMATION LEAFLET. DOTAREM mg/ml, solution for injection (Gadoteric acid)
 PATIENT INFORMATION LEAFLET DOTAREM 279.32 mg/ml, solution for injection (Gadoteric acid) Read all this leaflet carefully before you start using this medicine because it contains important information
PATIENT INFORMATION LEAFLET DOTAREM 279.32 mg/ml, solution for injection (Gadoteric acid) Read all this leaflet carefully before you start using this medicine because it contains important information
ATTORNEYS AND COUNSELLORS AT LAW
 ATTORNEYS AND COUNSELLORS AT LAW I. HEALTH CARE Medicare Our attorneys and staff are often asked questions about the qualifications and benefits under Medicare. According to a recent report by the Journal
ATTORNEYS AND COUNSELLORS AT LAW I. HEALTH CARE Medicare Our attorneys and staff are often asked questions about the qualifications and benefits under Medicare. According to a recent report by the Journal
ANGIOPLASTY AND STENTING
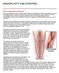 ANGIOPLASTY AND STENTING What is angioplasty and stenting? During an angioplasty, your vascular surgeon inflates a small balloon inside a narrowed blood vessel. This balloon helps to widen your blood vessel
ANGIOPLASTY AND STENTING What is angioplasty and stenting? During an angioplasty, your vascular surgeon inflates a small balloon inside a narrowed blood vessel. This balloon helps to widen your blood vessel
PRODUCT MONOGRAPH DOTAREM. (gadoterate meglumine) Injection. For Intravenous Use
 PRODUCT MONOGRAPH DOTAREM (gadoterate meglumine) Injection For Intravenous Use Gadolinium-Based Contrast Agent For Use with Magnetic Resonance Imaging (MRI) Guerbet LLC Date: August 26, 2013 120 W. 7 th
PRODUCT MONOGRAPH DOTAREM (gadoterate meglumine) Injection For Intravenous Use Gadolinium-Based Contrast Agent For Use with Magnetic Resonance Imaging (MRI) Guerbet LLC Date: August 26, 2013 120 W. 7 th
Gadolinium Use in Patients with Kidney Disease: A Cause for Concern
 Editorial Gadolinium Use in Patients with Kidney Disease: A Cause for Concern Mark A. Perazella* and Roger A. Rodby *Section of Nephrology, Department of Medicine, Yale University School of Medicine, New
Editorial Gadolinium Use in Patients with Kidney Disease: A Cause for Concern Mark A. Perazella* and Roger A. Rodby *Section of Nephrology, Department of Medicine, Yale University School of Medicine, New
Imaging Patient Education. Magnetic Resonance Imaging (MRI)
 Magnetic Resonance Imaging (MRI) What you should know about your Body MRI exam: Purpose: Magnetic Resonance Imaging (MRI) uses radio waves and a strong magnetic field to provide clear and detailed images
Magnetic Resonance Imaging (MRI) What you should know about your Body MRI exam: Purpose: Magnetic Resonance Imaging (MRI) uses radio waves and a strong magnetic field to provide clear and detailed images
QUALITY OF LIFE WITH DIABETES AND CHRONIC KIDNEY DISEASE
 QUALITY OF LIFE WITH DIABETES AND CHRONIC KIDNEY DISEASE www.kidney.org National Kidney Foundation's Kidney Disease Outcomes Quality Initiative Did you know that the National Kidney Foundation's Kidney
QUALITY OF LIFE WITH DIABETES AND CHRONIC KIDNEY DISEASE www.kidney.org National Kidney Foundation's Kidney Disease Outcomes Quality Initiative Did you know that the National Kidney Foundation's Kidney
Public Assessment Report
 Public Assessment Report Increased risk of nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis and gadolinium-containing MRI contrast agents Executive summary 2 Introduction 3 Data assessed
Public Assessment Report Increased risk of nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis and gadolinium-containing MRI contrast agents Executive summary 2 Introduction 3 Data assessed
FDA Advisory Committee Briefing Document. Medical Imaging Drugs Advisory Committee. Meeting to be held on February 14, 2013
 FDA Advisory Committee Briefing Document Medical Imaging Drugs Advisory Committee Meeting to be held on February 14, 2013 New Drug Application 204-781 Gadoterate meglumine Injection (Dotarem ), sponsored
FDA Advisory Committee Briefing Document Medical Imaging Drugs Advisory Committee Meeting to be held on February 14, 2013 New Drug Application 204-781 Gadoterate meglumine Injection (Dotarem ), sponsored
Having a diagnostic catheter angiogram
 Having a diagnostic catheter angiogram This information leaflet will explain what an angiogram is and why you have been sent for one. Please read this leaflet carefully. If you have any questions or concerns
Having a diagnostic catheter angiogram This information leaflet will explain what an angiogram is and why you have been sent for one. Please read this leaflet carefully. If you have any questions or concerns
DIABETES AND CHRONIC KIDNEY DISEASE
 DIABETES AND CHRONIC KIDNEY DISEASE Stages 1 4 www.kidney.org National Kidney Foundation's Kidney Disease Outcomes Quality Initiative Did you know that the National Kidney Foundation's Kidney Disease Outcomes
DIABETES AND CHRONIC KIDNEY DISEASE Stages 1 4 www.kidney.org National Kidney Foundation's Kidney Disease Outcomes Quality Initiative Did you know that the National Kidney Foundation's Kidney Disease Outcomes
PRODUCT MONOGRAPH GADOVIST 1.0. gadobutrol injection 604 mg/ml (1.0 mmol/ml) For Intravenous Use
 PRODUCT MONOGRAPH GADOVIST 1.0 gadobutrol injection 604 mg/ml (1.0 mmol/ml) For Intravenous Use Contrast Enhancement Agent for Magnetic Resonance Imaging (MRI) Bayer Inc. 77 Belfield Road Toronto, ON M9W
PRODUCT MONOGRAPH GADOVIST 1.0 gadobutrol injection 604 mg/ml (1.0 mmol/ml) For Intravenous Use Contrast Enhancement Agent for Magnetic Resonance Imaging (MRI) Bayer Inc. 77 Belfield Road Toronto, ON M9W
Magnetic resonance imaging (MRI) scans are significantly
 Mini-Review Current Status of Gadolinium Toxicity in Patients with Kidney Disease Mark A. Perazella Department of Medicine, Section of Nephrology, Yale University School of Medicine, New Haven, Connecticut
Mini-Review Current Status of Gadolinium Toxicity in Patients with Kidney Disease Mark A. Perazella Department of Medicine, Section of Nephrology, Yale University School of Medicine, New Haven, Connecticut
Caring for your heart during and after Chronic Myeloid Leukemia (CML) treatment
 Caring for your heart during and after Chronic Myeloid Leukemia (CML) treatment For patients and families Reading this booklet can help you learn Why you were referred to the Ted Rogers Program in Cardiotoxicity
Caring for your heart during and after Chronic Myeloid Leukemia (CML) treatment For patients and families Reading this booklet can help you learn Why you were referred to the Ted Rogers Program in Cardiotoxicity
PRODUCT MONOGRAPH GADOVIST 1.0. gadobutrol injection 604 mg/ml (1.0 mmol/ml) For Intravenous Use
 PRODUCT MONOGRAPH GADOVIST 1.0 gadobutrol injection 604 mg/ml (1.0 mmol/ml) For Intravenous Use Contrast Enhancement Agent for Magnetic Resonance Imaging (MRI) For Professional Use Only Bayer Inc. Date
PRODUCT MONOGRAPH GADOVIST 1.0 gadobutrol injection 604 mg/ml (1.0 mmol/ml) For Intravenous Use Contrast Enhancement Agent for Magnetic Resonance Imaging (MRI) For Professional Use Only Bayer Inc. Date
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide. flow rate of approximately 2 ml/second for adults and 1-2 ml/second for pediatric
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DOTAREM safely and effectively. See full prescribing information for DOTAREM. DOTAREM (gadoterate
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DOTAREM safely and effectively. See full prescribing information for DOTAREM. DOTAREM (gadoterate
DOTAREM (gadoterate meglumine) Injection for intravenous use Initial U.S. Approval: 2013
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DOTAREM safely and effectively. See full prescribing information for DOTAREM. DOTAREM (gadoterate
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DOTAREM safely and effectively. See full prescribing information for DOTAREM. DOTAREM (gadoterate
Complications of nephrogenic systemic fibrosis following repeated exposure to gadolinium in a man with hypothyroidism: a case report
 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Complications of nephrogenic systemic fibrosis following repeated exposure to gadolinium in a man with hypothyroidism: a case report Arpita Aggarwal
JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access Complications of nephrogenic systemic fibrosis following repeated exposure to gadolinium in a man with hypothyroidism: a case report Arpita Aggarwal
Special Articles Original Research
 Special Articles Original Research Soulez et al. NSF in Patients With Stage 3 5 CKD Undergoing MRI With GBCA Special Articles Original Research Gilles Soulez 1 Daniel C. Bloomgarden 2,3 Neil M. Rofsky
Special Articles Original Research Soulez et al. NSF in Patients With Stage 3 5 CKD Undergoing MRI With GBCA Special Articles Original Research Gilles Soulez 1 Daniel C. Bloomgarden 2,3 Neil M. Rofsky
This copy is for personal use only. To order printed copies, contact Purpose: Materials and Methods: Results: Conclusion:
 This copy is for personal use only. To order printed copies, contact reprints@rsna.org No Incidence of Nephrogenic Systemic Fibrosis after Gadobenate Dimeglumine Administration in Patients Undergoing Dialysis
This copy is for personal use only. To order printed copies, contact reprints@rsna.org No Incidence of Nephrogenic Systemic Fibrosis after Gadobenate Dimeglumine Administration in Patients Undergoing Dialysis
Cholangiocarcinoma (Bile Duct Cancer)
 Cholangiocarcinoma (Bile Duct Cancer) The Bile Duct System (Biliary Tract) A network of bile ducts (tubes) connects the liver and the gallbladder to the small intestine. This network begins in the liver
Cholangiocarcinoma (Bile Duct Cancer) The Bile Duct System (Biliary Tract) A network of bile ducts (tubes) connects the liver and the gallbladder to the small intestine. This network begins in the liver
JYNARQUE REMS (RISK EVALUATION AND MITIGATION STRATEGY) PATIENT GUIDE
 JYNARQUE REMS (RISK EVALUATION AND MITIGATION STRATEGY) PATIENT GUIDE Patients: Your healthcare provider will go over this patient guide with you. It is important to ask any questions you may have. Keep
JYNARQUE REMS (RISK EVALUATION AND MITIGATION STRATEGY) PATIENT GUIDE Patients: Your healthcare provider will go over this patient guide with you. It is important to ask any questions you may have. Keep
Incidence of Immediate Gadolinium Contrast Media Reactions
 Health Care Policy and Quality Original Research Prince et al. Immediate Gadolinium Contrast Media Reactions Health Care Policy and Quality Original Research Martin R. Prince 1 Honglei Zhang Zhitong Zou
Health Care Policy and Quality Original Research Prince et al. Immediate Gadolinium Contrast Media Reactions Health Care Policy and Quality Original Research Martin R. Prince 1 Honglei Zhang Zhitong Zou
Patient Education Kidney Early Education Program (KEEP) Chapter 2 bjectives: Overview 1. Understand what kidneys do. 2. Understand symptoms
 Patient Education (KEEP) Chapter 2 What Your Kidneys Do And what happens when they fail Objectives: 1. Understand what kidneys do. 2. Understand symptoms of uremia and some ways to treat it. 3. Know the
Patient Education (KEEP) Chapter 2 What Your Kidneys Do And what happens when they fail Objectives: 1. Understand what kidneys do. 2. Understand symptoms of uremia and some ways to treat it. 3. Know the
Reference ID: MultiHance Injection, NDA , SAFETY LABELING CHANGES UNDER 505(o)(4) MultiHance PI to FDA_Revised Nov _CLEAN.
 MultiHance Injection, NDA 21-357, SAFETY LABELING CHANGES UNDER 505(o)(4) MultiHance PI to FDA_Revised Nov-18-2010_CLEAN.doc HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the
MultiHance Injection, NDA 21-357, SAFETY LABELING CHANGES UNDER 505(o)(4) MultiHance PI to FDA_Revised Nov-18-2010_CLEAN.doc HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the
Nuclear Medicine Bone Imaging
 Nuclear Medicine Bone Imaging An Introductory Guide For Patients And Their Families You may be wondering why your doctor ordered a nuclear medicine bone scan. You might have questions such as: How does
Nuclear Medicine Bone Imaging An Introductory Guide For Patients And Their Families You may be wondering why your doctor ordered a nuclear medicine bone scan. You might have questions such as: How does
Interventional Radiology (IR)
 Interventional Radiology (IR) Risk information for inpatients UHN Read this brochure to learn about: Interventional Radiology (IR) procedures The risks of IR procedures Problems to what to watch for Please
Interventional Radiology (IR) Risk information for inpatients UHN Read this brochure to learn about: Interventional Radiology (IR) procedures The risks of IR procedures Problems to what to watch for Please
Update. Drug Safety. Welcome to the first issue of the newly launched Drug Safety Update. This
 Latest advice for medicines users The monthly newsletter from the Medicines and Healthcare products Regulatory Agency and its independent advisor the Commission on Human Medicines Volume 1, Issue 1 August
Latest advice for medicines users The monthly newsletter from the Medicines and Healthcare products Regulatory Agency and its independent advisor the Commission on Human Medicines Volume 1, Issue 1 August
3 generations of ADPKD. 1 long-awaited treatment.
 3 generations of ADPKD. 1 long-awaited treatment. HOPE is finally here now that treatment is available for people with autosomal dominant polycystic kidney disease (ADPKD). JYNARQUE is proven to slow kidney
3 generations of ADPKD. 1 long-awaited treatment. HOPE is finally here now that treatment is available for people with autosomal dominant polycystic kidney disease (ADPKD). JYNARQUE is proven to slow kidney
Department of Vascular Surgery, Maastricht University, Maastricht - The Netherlands 2
 The Journal of Vascular Access 2007; 8: 281-286 ORIGINAL ARTICLE Accessory veins and radial-cephalic arteriovenous fistula non-maturation: a prospective analysis using contrast-enhanced magnetic resonance
The Journal of Vascular Access 2007; 8: 281-286 ORIGINAL ARTICLE Accessory veins and radial-cephalic arteriovenous fistula non-maturation: a prospective analysis using contrast-enhanced magnetic resonance
PRODUCT MONOGRAPH PRIMOVIST. gadoxetate disodium injection mg/ml (0.25 mmol/ml)
 PRODUCT MONOGRAPH PRIMOVIST gadoxetate disodium injection 181.43 mg/ml (0.25 mmol/ml) Intravenous contrast enhancement agent for magnetic resonance imaging (MRI) For Professional Use Only Distributed and
PRODUCT MONOGRAPH PRIMOVIST gadoxetate disodium injection 181.43 mg/ml (0.25 mmol/ml) Intravenous contrast enhancement agent for magnetic resonance imaging (MRI) For Professional Use Only Distributed and
GPA & MPA GRANULOMATOSIS WITH POLYANGIITIS &MICROSCOPIC POLYANGIITIS. What you need to know about GPA & MPA
 GRANULOMATOSIS WITH POLYANGIITIS &MICROSCOPIC POLYANGIITIS GPA & MPA This medical guide is designed for educational purposes to help patients understand GPA & MPA. Please consult your doctor on specific
GRANULOMATOSIS WITH POLYANGIITIS &MICROSCOPIC POLYANGIITIS GPA & MPA This medical guide is designed for educational purposes to help patients understand GPA & MPA. Please consult your doctor on specific
Duplex Ultrasound. A Detailed Look at Your Blood Vessels
 Duplex Ultrasound A Detailed Look at Your Blood Vessels What Is Duplex Ultrasound? Ultrasound is a test that uses sound waves to create detailed pictures of the inside of your body. Duplex ultrasound is
Duplex Ultrasound A Detailed Look at Your Blood Vessels What Is Duplex Ultrasound? Ultrasound is a test that uses sound waves to create detailed pictures of the inside of your body. Duplex ultrasound is
Information for patients undergoing Angiography (Angiogram) or Arteriography (Arteriogram) Patient Information
 Information for patients undergoing Angiography (Angiogram) or Arteriography (Arteriogram) Patient Information Author ID: SH Leaflet Number: X-ray 004 Version: 6 Name of Leaflet: Information for patients
Information for patients undergoing Angiography (Angiogram) or Arteriography (Arteriogram) Patient Information Author ID: SH Leaflet Number: X-ray 004 Version: 6 Name of Leaflet: Information for patients
ALL PRINCIPAL INVESTIGATORS/NURSES/DATA MANAGERS RE: PROTOCOL GOG-0233 ACRIN 6671, REVISION # 9 & #10
 TO: FROM: ALL PRINCIPAL INVESTIGATORS/NURSES/DATA MANAGERS LEAH MADDEN PROTOCOL SECTION DATE: JUNE 27, 2011 RE: PROTOCOL GOG-0233 ACRIN 6671, REVISION # 9 & #10 Protocol Title: Utility of Preoperative
TO: FROM: ALL PRINCIPAL INVESTIGATORS/NURSES/DATA MANAGERS LEAH MADDEN PROTOCOL SECTION DATE: JUNE 27, 2011 RE: PROTOCOL GOG-0233 ACRIN 6671, REVISION # 9 & #10 Protocol Title: Utility of Preoperative
Index. mri.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Angiogenesis, and cancer of prostate, 689 690 Angiography, MR. See MR angiography. Apoptosis, MR imaging of, 637 Apparent diffusion coefficient,
Index Note: Page numbers of article titles are in boldface type. A Angiogenesis, and cancer of prostate, 689 690 Angiography, MR. See MR angiography. Apoptosis, MR imaging of, 637 Apparent diffusion coefficient,
FULL PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use MAGNEVIST safely and effectively. See full prescribing information for MAGNEVIST. MAGNEVIST (gadopentetate
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use MAGNEVIST safely and effectively. See full prescribing information for MAGNEVIST. MAGNEVIST (gadopentetate
Annex II. Scientific conclusions
 Annex II Scientific conclusions 31 Scientific conclusions In accordance with Article 107k of Directive 2001/83/EC, the CHMP considered the PRAC recommendation adopted on 6 July 2017. Overall summary of
Annex II Scientific conclusions 31 Scientific conclusions In accordance with Article 107k of Directive 2001/83/EC, the CHMP considered the PRAC recommendation adopted on 6 July 2017. Overall summary of
Nephrogenic Systemic Fibrosis: Possible Association with a Predisposing Infection
 MR Imaging Original Research MR Imaging Original Research Lauren Parks Golding 1,2 James M. Provenzale 3 Golding LP, Provenzale JM Keywords: connective tissue disorders, contrast media, dialysis, infections,
MR Imaging Original Research MR Imaging Original Research Lauren Parks Golding 1,2 James M. Provenzale 3 Golding LP, Provenzale JM Keywords: connective tissue disorders, contrast media, dialysis, infections,
Clinical Study Nephrogenic Systemic Fibrosis Risk and Liver Disease
 International Nephrology, Article ID 679605, 6 pages http://dx.doi.org/10.1155/2014/679605 Clinical Study Nephrogenic Systemic Fibrosis Risk and Liver Disease Robert F. Hanna, 1 Lee A. Finkelstone, 1 Daniel
International Nephrology, Article ID 679605, 6 pages http://dx.doi.org/10.1155/2014/679605 Clinical Study Nephrogenic Systemic Fibrosis Risk and Liver Disease Robert F. Hanna, 1 Lee A. Finkelstone, 1 Daniel
Extracellular gadolinium-based contrast media: An overview
 European Journal of Radiology 66 (2008) 160 167 Extracellular gadolinium-based contrast media: An overview Marie-France Bellin, Aart J. Van Der Molen University Paris-Sud 11, Department of Radiology, University
European Journal of Radiology 66 (2008) 160 167 Extracellular gadolinium-based contrast media: An overview Marie-France Bellin, Aart J. Van Der Molen University Paris-Sud 11, Department of Radiology, University
NATIONAL KIDNEY MONTH
 NATIONAL KIDNEY MONTH According to the WebMD website, kidneys have several specific roles: Maintain your body s balance of water and concentration of minerals, such as sodium, potassium, magnesium and
NATIONAL KIDNEY MONTH According to the WebMD website, kidneys have several specific roles: Maintain your body s balance of water and concentration of minerals, such as sodium, potassium, magnesium and
Information for patients having an isotope kidney (renal) scan (also known as a DMSA scan)
 Information for patients having an isotope kidney (renal) scan (also known as a DMSA scan) The leaflet tells you about having an isotope kidney (renal) scan. It is also known as a DMSA scan. It explains
Information for patients having an isotope kidney (renal) scan (also known as a DMSA scan) The leaflet tells you about having an isotope kidney (renal) scan. It is also known as a DMSA scan. It explains
Intravenous Pyelogram (IVP)
 Scan for mobile link. Intravenous Pyelogram (IVP) Intravenous pyelogram (IVP) is an x-ray exam that uses an injection of contrast material to evaluate your kidneys, ureters and bladder and help diagnose
Scan for mobile link. Intravenous Pyelogram (IVP) Intravenous pyelogram (IVP) is an x-ray exam that uses an injection of contrast material to evaluate your kidneys, ureters and bladder and help diagnose
Elements for a public summary
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology HIV is a virus that attacks the immune system (the body s natural defences) and weakens it by destroying certain white blood cells
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology HIV is a virus that attacks the immune system (the body s natural defences) and weakens it by destroying certain white blood cells
Kidney Biopsy. Table of Contents. Test Overview. Why It Is Done. Test Overview. Why It Is Done. How to Prepare. How It Is Done.
 Kidney Biopsy Table of Contents Test Overview Why It Is Done How to Prepare How It Is Done Results What Affects the Test? Test Overview The two kidneys {IMAGE LINK 1} are found on either side of the spine,
Kidney Biopsy Table of Contents Test Overview Why It Is Done How to Prepare How It Is Done Results What Affects the Test? Test Overview The two kidneys {IMAGE LINK 1} are found on either side of the spine,
Hepatobiliary Contrast Agents for Liver MRI
 Hepatobiliary Contrast Agents for Liver MRI Scott B. Reeder, MD, PhD International Society for Magnetic Resonance in Medicine Sociedad Mexicana de Radiologia e Imagen (SMRI) Mexico City June 4, 2014 Department
Hepatobiliary Contrast Agents for Liver MRI Scott B. Reeder, MD, PhD International Society for Magnetic Resonance in Medicine Sociedad Mexicana de Radiologia e Imagen (SMRI) Mexico City June 4, 2014 Department
UW MEDICINE PATIENT EDUCATION. Hemodialysis. A treatment option for kidney disease. Treatment Options for Kidney Disease
 UW MEDICINE PATIENT EDUCATION Hemodialysis A treatment option for kidney disease Class Goals 1. Understand the purpose and care of blood access. 2. Understand the purpose and basic principles of hemodialysis.
UW MEDICINE PATIENT EDUCATION Hemodialysis A treatment option for kidney disease Class Goals 1. Understand the purpose and care of blood access. 2. Understand the purpose and basic principles of hemodialysis.
Side Effects and Complications of Magnetic Resonance Contrast Media
 HOSPITAL CHRONICLES 2012, 7(4): 208 214 Review Side Effects and Complications of Magnetic Resonance Contrast Media Ekaterini Tavernaraki, MD, PhD, Anna Skoula MD, Stylianos Benakis, MD, Dimitrios Exarhos
HOSPITAL CHRONICLES 2012, 7(4): 208 214 Review Side Effects and Complications of Magnetic Resonance Contrast Media Ekaterini Tavernaraki, MD, PhD, Anna Skoula MD, Stylianos Benakis, MD, Dimitrios Exarhos
MR Angiography (MRA)
 MR Angiography (MRA) What is MR Angiography? What are some common uses of the procedure? How should I prepare? What does the equipment look like? How does the procedure work? How is the procedure performed?
MR Angiography (MRA) What is MR Angiography? What are some common uses of the procedure? How should I prepare? What does the equipment look like? How does the procedure work? How is the procedure performed?
Radiology Coding. Copyright. Today s Goal 8/17/2010. Answer your questions! Melody W. Mulaik CODING
 Radiology Coding Tips & Traps Melody W. Mulaik 1 877 6 CODING melody.mulaik@codingstrategies.com The material contained in this presentation and handout are distributed under copyright by Coding Strategies,
Radiology Coding Tips & Traps Melody W. Mulaik 1 877 6 CODING melody.mulaik@codingstrategies.com The material contained in this presentation and handout are distributed under copyright by Coding Strategies,
Title of Research Study: Discovery and Validation of Biomarkers for Lichen Sclerosus
 Page 1 of 8 Informed Consent for Participation in a Research Study Title of Research Study: Discovery and Validation of Biomarkers for Lichen Sclerosus Investigator Contact Information: Principal Investigator:
Page 1 of 8 Informed Consent for Participation in a Research Study Title of Research Study: Discovery and Validation of Biomarkers for Lichen Sclerosus Investigator Contact Information: Principal Investigator:
REGISTRATION INFORMATION
 REGISTRATION INFORMATION PATIENT INFORMATION (PLEASE USE FULL LEGAL NAME) Last: First: MI: Sex: DOB: SSN# Marital Status: Home Phone: Address: Cell Phone: City: State: Zip: Email: Employer: Work Phone:
REGISTRATION INFORMATION PATIENT INFORMATION (PLEASE USE FULL LEGAL NAME) Last: First: MI: Sex: DOB: SSN# Marital Status: Home Phone: Address: Cell Phone: City: State: Zip: Email: Employer: Work Phone:
Overview
 Overview Tutorial Script 1 Script for the Virtual Stroke Lab Tutorial Overview http://www.ilstu.edu Background and Vocabulary This podcast provides an overview of The Virtual Stroke Lab, a free virtual
Overview Tutorial Script 1 Script for the Virtual Stroke Lab Tutorial Overview http://www.ilstu.edu Background and Vocabulary This podcast provides an overview of The Virtual Stroke Lab, a free virtual
Ureteral Stenting and Nephrostomy
 Scan for mobile link. Ureteral Stenting and Nephrostomy Ureteral stenting and nephrostomy help restore urine flow through blocked ureters and return the kidney to normal function. Ureters are long, narrow
Scan for mobile link. Ureteral Stenting and Nephrostomy Ureteral stenting and nephrostomy help restore urine flow through blocked ureters and return the kidney to normal function. Ureters are long, narrow
Intermittent Claudication
 Intermittent Claudication Exceptional healthcare, personally delivered Ask 3 Questions Preparation for your Appointments We want you to be active in your healthcare. By telling us what is important to
Intermittent Claudication Exceptional healthcare, personally delivered Ask 3 Questions Preparation for your Appointments We want you to be active in your healthcare. By telling us what is important to
PRESCRIBER SAFETY BROCHURE; IMPORTANT SAFETY INFORMATION FOR THE HEALTHCARE PROVIDER
 PRESCRIBER SAFETY BROCHURE; IMPORTANT SAFETY INFORMATION FOR THE HEALTHCARE PROVIDER BEFORE STARTING YOUR PATIENTS ON SOLIRIS Important safety information for the healthcare provider Prior to initiating
PRESCRIBER SAFETY BROCHURE; IMPORTANT SAFETY INFORMATION FOR THE HEALTHCARE PROVIDER BEFORE STARTING YOUR PATIENTS ON SOLIRIS Important safety information for the healthcare provider Prior to initiating
Having a Breast Biopsy. A Review of the Research for Women and Their Families
 Having a Breast Biopsy A Review of the Research for Women and Their Families e Is This Information Right For Me? This information is right for you if: You are a woman. The information in this summary is
Having a Breast Biopsy A Review of the Research for Women and Their Families e Is This Information Right For Me? This information is right for you if: You are a woman. The information in this summary is
Contrast between Scar and Recurrent Herniated Disk on Contrast-Enhanced MR Images
 AJNR Am J Neuroradiol 23:1652 1656, November/December 2002 Contrast between Scar and Recurrent Herniated Disk on Contrast-Enhanced MR Images Victor Haughton, Ken Schreibman, and Arthur De Smet BACKGROUND
AJNR Am J Neuroradiol 23:1652 1656, November/December 2002 Contrast between Scar and Recurrent Herniated Disk on Contrast-Enhanced MR Images Victor Haughton, Ken Schreibman, and Arthur De Smet BACKGROUND
