Scratching the Surface: A Review of SJS/TEN
|
|
|
- Jeremy Greene
- 5 years ago
- Views:
Transcription
1 Scratching the Surface: A Review of SJS/TEN Sarah Smith, PharmD Pharmacy Grand Rounds 2018 November 27, MFMER slide 1
2 Toxic Epidermal Necrolysis Cutaneous Anthrax Stevens Johnson Syndrome Necrotizing Fasciitis Toxic Shock Syndrome Rocky Mountain Spotted Fever Meningococcemia Did you know there are 7 types of dermatological emergencies? 2018 MFMER slide 2
3 Objectives 1. Review the pathophysiology of Stevens Johnson syndrome (SJS) and Toxic epidermal necrolysis (TEN) 2. Identify medications that may induce SJS/TEN 3. Discuss pharmacological interventions used to treat patients who present with SJS/TEN 2018 MFMER slide 3
4 Abbreviations SJS: Stevens Johnson syndrome TEN: Toxic epidermal necrolysis CBZ: carbamazepine APC: antigen presenting cell IVIG: intravenous immune globulin FasL: Fas ligand sfasl: soluble Fas ligand TNF α: Tumor necrosis factor alpha RCT: randomized control trial CTL: cytotoxic T lymphocytes SCARs: severe cutaneous adverse reactions 2018 MFMER slide 4
5 Patient Case 38 year old male PMH: epileptic seizures Developed high fever, sloughing of the epidermis, and clinical appearance of severe burn patient 2 weeks after starting a new antiepileptic medication 2018 MFMER slide 5
6 SJS/TEN Acute, life threatening hypersensitivity cutaneous reactions Characterized by full thickness epidermal necrosis Varying involvement of cutaneous, extracutaneous, and mucous membrane involvement Usatine RP, Sandy N. Dermatologic Emergencies, Jellinek. Am Fam Physician. 2010;82(7): Schneider JA, Cohen PR. Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing Supportive Measures. Adv Ther. 2017;34(6): MFMER slide 6
7 Distinguishing SJS vs TEN >30% BSA 10% BSA 10 30% BSA SJS SJS/TEN overlap TEN Percentage of BSA affected Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): Schneider JA, Cohen PR. Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing Supportive Measures. Adv Ther. 2017;34(6): MFMER slide 7
8 Clinical Course of SJS/TEN Prodrome: malaise, rash, fever, cough, myalgia 1 4 weeks after drug exposure Epidermal detachment progresses, large denuded areas Extreme pain, massive fluid & protein loss, hypothermia days Signs begin in mucous membranes Skin lesions start to manifest, progress to large blisters Re epithelialization of the epidermis begins May take up to 3 weeks Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): MFMER slide 8
9 Clinical Presentation Extracutaneous Nonspecific: Malaise, rash, fever, pain, cough, headache, N/V Respiratory Large ulcerations and epithelial necrosis of bronchial epithelium, respiratory distress, pulmonary edema, progressive respiratory failure Gastrointestinal Diarrhea, nausea, malabsorption, colonic perforation, melena Renal Proteinuria, hematuria, microalbuminuria Valeyrie Allanore L, Roujeau J. Chapter 40. Epidermal necrolysis (Stevens Johnson syndrome and toxic epidermal necrolysis). In: Goldsmith LA, Katz SI, Gilchrest BA et al, eds. Fitzpatrick s Dermatology in General Medicine. 8 th ed. New York, NY: McGraw Hill; 2012 Harr T, French LE. Toxic epidermal necrolysis and Stevens Johnson syndrome. Orphanet J Rare Dis. 2010;5(1):39. Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): Mockenhaupt M. The current understanding of Stevens Johnson syndrome and toxic epidermal necrolysis. Expert Rev Clin Immunol. 2011;7(6): MFMER slide 9
10 Clinical Presentation Cutaneous Initial Phase: Erythematous, dusky red, flat atypical target lesions with necrotic centers Lesions evenly distributed on face/trunk/proximal part of limbs Later phase: Lesions coalesce and evolve into flaccid blisters Epidermal detachment + Nikolsky sign Gentle lateral pressure causes lesional, detachable epidermis Early Phase Blistering, peeling skin with epidermal detachment Flat, dusky red atypical target lesions Later Phase Adapted from JAMA 2017; 153 (12): 1344 Valeyrie Allanore L, Roujeau J. Chapter 40. Epidermal necrolysis (Stevens Johnson syndrome and toxic epidermal necrolysis). In: Goldsmith LA, Katz SI, Gilchrest BA et al, eds. Fitzpatrick s Dermatology in General Medicine. 8 th ed. New York, NY: McGraw Hill; 2012 Harr T, French LE. Toxic epidermal necrolysis and Stevens Johnson syndrome. Orphanet J Rare Dis. 2010;5(1):39. Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): Mockenhaupt M. The current understanding of Stevens Johnson syndrome and toxic epidermal necrolysis. Expert Rev Clin Immunol. 2011;7(6): MFMER slide 10
11 Clinical Presentation Mucous membrane Ocular: Eyelid edema, redness, photophobia, discharge, lacrimation Buccal/Oral: Erosive, hemorrhagic lesions, grayish white pseudomembranes, crust on lips, nose involvement Genital Erosive hemorrhagic lesions, painful urination Ocular Redness, blisters, pain, and erosions of the lips and inside the mouth Redness, irritation, pain, and erosions of the eyelids Buccal/Oral Adapted from JAMA 2017; 153 (12): 1344 Valeyrie Allanore L, Roujeau J. Chapter 40. Epidermal necrolysis (Stevens Johnson syndrome and toxic epidermal necrolysis). In: Goldsmith LA, Katz SI, Gilchrest BA et al, eds. Fitzpatrick s Dermatology in General Medicine. 8 th ed. New York, NY: McGraw Hill; 2012 Harr T, French LE. Toxic epidermal necrolysis and Stevens Johnson syndrome. Orphanet J Rare Dis. 2010;5(1):39. Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): Mockenhaupt M. The current understanding of Stevens Johnson syndrome and toxic epidermal necrolysis. Expert Rev Clin Immunol. 2011;7(6): MFMER slide 11
12 Epidemiology US incidence: cases per 1 million people per year 1,178 cases reported to the FDA in 2017 More common in women Female to male ratio of 1.5:1 Ward KE, Archambault R, Mersfelder TL. Severe adverse skin reactions to nonsteroidal anti inflammatory drugs: a review of the literature. Am J Health Syst Pharm. 2010; 67: Four severe adverse events and the leading suspect drugs. ISMP Medication Safety Alert! Acute Care. 2018: 23 (18) MFMER slide 12
13 Mortality Mortality estimates: SJS: % SJS/TEN overlap: 19.4% TEN: 15 50% SCORTEN criteria Severity of illness score to predict mortality for SJS/TEN Measure on days 1 & 3 of hospitalization Wang C W, Yang L Y, Chen C B, et al. Randomized, controlled trial of TNF α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128(3): Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity and Mortality of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis in United States Adults. J Invest Dermatol. 2016;136(7): Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): Guegan S, Bastuji Garin S, Poszepczynska Guigne E, et al. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006; 126 (2): MFMER slide 13
14 SCORTEN criteria Age >40 years Malignancy BSA >10% Heart rate >120 bpm BUN >28 mg/dl Serum glucose >250 mg/dl Serum bicarbonate <20 mmol/l 1 point for each risk factor present # Risk Factors Predicted Mortality 0 1% 1 4% 2 12% 3 32% 4 62% 5 85% 6 95% 7 99% Guegan S, Bastuji Garin S, Poszepczynska Guigne E, et al. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006; 126 (2): Schneider JA, Cohen PR. Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing Supportive Measures. Adv Ther. 2017;34(6): Creamer D, Walsh SA, Dziewulski P, et al. UK guidelines for the management of Stevens Johnson syndrome/toxic epidermal necrolysis in adults J Plast Reconstr Aesthetic Surg. 2016;69(6):e119 e MFMER slide 14
15 Patient Case 38 year old male PMH: epileptic seizures Developed high fever, sloughing of the epidermis, and clinical appearance of severe burn patient 2 weeks after starting a new antiepileptic medication 2018 MFMER slide 15
16 Patient Case 80% BSA involvement Lab values HR: 132 bpm BUN: 36 mg/dl Serum creatinine: 1.2 mg/dl Glucose: 186 mg/dl Bicarbonate: 28 mmol/l 2018 MFMER slide 16
17 How would you classify this patient? a) SJS b) SJS/TEN overlap c) TEN 2018 MFMER slide 17
18 How would you classify this patient? a) SJS b) SJS/TEN overlap c) TEN 2018 MFMER slide 18
19 Etiology Known causes: Drugs Most common 50% of SJS cases; 80 90% of TEN cases Vaccinations MMR, varicella, tetanus, influenza, hantavirus (HFRS) Sunlight exposure Pregnancy Harr T, French LE. Toxic epidermal necrolysis and Stevens Johnson syndrome. Orphanet J Rare Dis. 2010;5(1):39. Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): Usatine RP, Sandy N. Dermatologic Emergencies, Jellinek. Am Fam Physician. 2010;82(7): MFMER slide 19
20 Etiology Known causes: Infectious agents Herpes virus, Mycoplasma pneumoniae, HIV, Hepatitis A Noninfectious conditions: Cancer, radiation, lupus erythematosus, collagen vascular disease Bone marrow/solid organ transplants Idiopathic Harr T, French LE. Toxic epidermal necrolysis and Stevens Johnson syndrome. Orphanet J Rare Dis. 2010;5(1):39. Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): Usatine RP, Sandy N. Dermatologic Emergencies, Jellinek. Am Fam Physician. 2010;82(7): MFMER slide 20
21 Genetic Factors HLA B*15:02 Carbamazepine, lamotrigine, oxcarbazepine, & phenytoin related SJS/TEN Asian ancestry HLA B*58:01 Allopurinolrelated SJS/TEN Asian & Non Asian populations HLA A*31:01 Carbamazepinerelated SJS/TEN Japanese, Indian, & European ancestry Other possible associations: HLA B*15:08, HLA B*15:11, HLA B*15:18 & HLA B*51:01 Schneider JA, Cohen PR. Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing Supportive Measures. Adv Ther. 2017;34(6): MFMER slide 21
22 SJS/TEN Inducing Medications Most Common Agents Allopurinol Carbamazepine Lamotrigine Nevirapine Oxicam NSAIDS Phenobarbital Phenytoin Sulfamethoxazole and other sulfur antibiotics Sulfasalazine Other Suspected Agents Antibiotics: Aminopencillins, Cephalosporins, Quinolones, Tetracyclines, Macrolides Valproicacid Oxcarbazepine Abacavir Diclofenac Zonisamide Lenalidomide Acetazolamide Ethambutol Mirtazapine Oseltamivir Creamer D, Walsh SA, Dziewulski P, et al. UK guidelines for the management of Stevens Johnson syndrome/toxic epidermal necrolysis in adults J Plast Reconstr Aesthetic Surg. 2016;69(6):e119 e153. Ruminski MA, Wisneski SS, Dugan SE. Stevens Johnson syndrome: what a pharmacist should know. US Pharm. 2013; 38(7): MFMER slide 22
23 Drug-Induced SJS/TEN Most often occurs within 4 28 days of 1 st exposure to suspect drug May also occur: Within hours upon rechallenge Up to 8 weeks post exposure ALDEN (ALgorithm of Drug causality for Epidermal Necrolysis) Assessment tool for drug causality in SJS/TEN Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): Creamer D, Walsh SA, Dziewulski P, et al. UK guidelines for the management of Stevens Johnson syndrome/toxic epidermal necrolysis in adults J Plast Reconstr Aesthetic Surg. 2016;69(6):e119 e MFMER slide 23
24 Pathophysiology SJS/TEN characterized by: Apoptotic keratinocyte cell death in the epidermis Epidermal detachment and necrosis Exact pathogenesis unknown Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): Ruminski MA, Wisneski SS, Dugan SE. Stevens Johnson syndrome: what a pharmacist should know. US Pharm. 2013; 38(7): MFMER slide 24
25 One Proposed Theory of Pathogenesis: CBZ + protein CBZ + protein APC APC Tcell release of cytokines CBZ + protein APC T cell Granulysin, sfasl, Perforin, Granzyme B CBZ = carbamazepine, APC = antigen presenting cell, sfasl = soluble Fas ligand Valeyrie Allanore L, Roujeau J. Chapter 40. Epidermal necrolysis (Stevens Johnson syndrome and toxic epidermal necrolysis). In: Goldsmith LA, Katz SI, Gilchrest BA et al, eds. Fitzpatrick s Dermatology in General Medicine. 8 th ed. New York, NY: McGraw Hill; 2012 Ruminski MA, Wisneski SS, Dugan SE. Stevens Johnson syndrome: what a pharmacist should know. US Pharm. 2013; 38(7): Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14(12): MFMER slide 25
26 Complications Secondary skin infections Bloodstream infections Eye problems Persistent respiratory sequelae Permanent skin damage Abnormal bumps, coloring, possible scarring Hair loss, nail deformities Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): MFMER slide 26
27 Patient Case 38 year old male PMH: epileptic seizures Developed high fever, sloughing of the epidermis, and clinical appearance of severe burn patient 2 weeks after starting a new antiepileptic medication 2018 MFMER slide 27
28 Patient Case 80% BSA involvement Lab values HR: 132 bpm BUN: 36 mg/dl Glucose: 186 mg/dl Bicarbonate: 28 mmol/l 2018 MFMER slide 28
29 What medication most likely caused this patient s reaction? a) Lamotrigine b) Valproic acid c) Oxcarbazepine 2018 MFMER slide 29
30 What medication most likely caused this patient s reaction? a) Lamotrigine b) Valproic acid c) Oxcarbazepine 2018 MFMER slide 30
31 Treatment Approach No formal US Guidelines available Early drug withdrawal Transfer to burn ICU Supportive care Room temp C Consult specialties Dermatology Ophthalmology Urology Calculate SCORTEN Days 1 & 3 Consider adjuvant therapies Within h Creamer D, Walsh SA, Dziewulski P, et al. UK guidelines for the management of Stevens Johnson syndrome/toxic epidermal necrolysis in adults J Plast Reconstr Aesthetic Surg. 2016;69(6):e119 e153. Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): Schneider JA, Cohen PR. Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing Supportive Measures. Adv Ther. 2017;34(6): MFMER slide 31
32 Supportive Care Pain Control Fluids Antibiotics No current guidelines PCAs may be difficult due to hand involvement Avoid morphine when able Aggressive fluid & electrolyte replacement Maintain urine output ml/kg/h Only if infection is present May lead to drug resistance Potential to worsen skin toxicity Creamer D, Walsh SA, Dziewulski P, et al. UK guidelines for the management of Stevens Johnson syndrome/toxic epidermal necrolysis in adults J Plast Reconstr Aesthetic Surg. 2016;69(6):e119 e153. Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): Schneider JA, Cohen PR. Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing Supportive Measures. Adv Ther. 2017;34(6): MFMER slide 32
33 Supportive Care Eye Care Eye emollients Antiseptic eye drops Topical antibiotics Topical steroids Severe cases: amniotic membrane transplantation Wound Care Disinfecting mouthwashes (chlorhexidine) Mild ointments (white petrolatum) Avoid topical antiinfectives with a sulfa moiety Creamer D, Walsh SA, Dziewulski P, et al. UK guidelines for the management of Stevens Johnson syndrome/toxic epidermal necrolysis in adults J Plast Reconstr Aesthetic Surg. 2016;69(6):e119 e153. Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): Schneider JA, Cohen PR. Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing Supportive Measures. Adv Ther. 2017;34(6): MFMER slide 33
34 Treatment Consider adjuvant therapies Systemic corticosteroids IVIG Cyclosporine Infliximab Etanercept Plasmapheresis 2018 MFMER slide 34
35 Corticosteroids Proposed mechanism: Ability to modify inflammatory and immune responses 2018 MFMER slide 35
36 Corticosteroids Author & year Study type Number patients Halebian et al. Retrospective comparative trial Treatment 30 Hydrocortisone 240 1,000 mg over max 7 days Mortality with/without steroids 66% / 33% Other Kelemen et al. Retrospective 51 NR 50% / 3% Infection, hospitalization, & mortality reduced if <48 h steroids Kakourou et al. Retrospective 16 Methylprednisolone mg/kg/day 0% / 0% Shorter period of fever with steroids Forman et al. Retrospective 39 NR 3.6% / 21% complications Kardaun and Jonkman Retrospective 12 Dexamethasone 100 mg or 1.5 mg/kg x 3 days Yamane et al. Retrospective 117 Prednisolone mg/day Schneck et al. Retrospective multicenter Yang et al. Retrospective TEN SJS % / 3.6% / 16.6% 281 NR 17.6% / 27.8% No significant benefit to any treatment Methylprednisolone mg/kg/day 27% / 16.7% / 16% more likely to die with steroids Adapted from: Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): MFMER slide 36
37 IVIG Proposed mechanism: Ability to block Fas and subsequent FasL mediated apoptosis of keratinocytes Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): MFMER slide 37
38 IVIG Author & year Study type Number patients Bachot et al. Prospective open trial Treatment average total IVIG dose (g/kg) (3 patients) 2.0 (31 patients) Mortality with/without IVIG 32% / Other Metry et al. Retrospective % / Early treatment correlated with longer time to response Brown et al. Retrospective % / 28.6% Yeung et al. Prospective/ retrospective controls % / 10% Shorter time to cessation of progression and reepithelialization with IVIG Gravante et al. Retrospective % / 27% Stella et al. Retrospective % / 75% Yamane et al. Retrospective 117 Max 1.2 9% / 3% Schneck et al Retrospective % / 20.8% No significant benefit from any treatment Yang et al. Retrospective % / 22.8% Nonsignificant reductions in mortality, time of progression, and Adapted from: Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): MFMER slide 38
39 The role of intravenous immunoglobulin in toxic epidermal necrolysis: a retrospective analysis of 64 patients managed in a specialized centre Lee HY, Lim YL, Thirumoorthy T, and Pang SM. British Journal of Dermatology MFMER slide 39
40 Study Design Primary Endpoint Evaluate the risk of in hospital mortality Study Methods Single center, retrospective analysis 64 patients included from Lee HY, Lim YL, Thirumoorthy T, and Pang SM. The role of intravenous immunoglobulin in toxic epidermal necrolysis: a retrospective analysis of 64 patients managed in a specialized centre. British Journal of Dermatology ; 169: MFMER slide 40
41 Enrollment Inclusion Diagnosed with SJS/TEN overlap or TEN Treated with IVIG Exclusion Exclusion Criteria Diagnosed with SJS No progression of disease Primary treatment with corticosteroids Lee HY, Lim YL, Thirumoorthy T, and Pang SM. The role of intravenous immunoglobulin in toxic epidermal necrolysis: a retrospective analysis of 64 patients managed in a specialized centre. British Journal of Dermatology ; 169: MFMER slide 41
42 Clinical Characteristics Characteristic IVIG (n = 64) Sex male, n (%) 26 (41) Age (years) 57 ± 19 Ethnicity, n (%) Chinese 42 (66) Malay 18 (28) Indian 4 (6) SCORTEN overall 2.6 ± 1.2 Cumulative dose IVIG (g/kg) 2.4 ± 0.8 Daily dosage (g/kg/day) 0.6 ± 0.2 Duration of administration (days) 4.0 ± 1.3 Lee HY, Lim YL, Thirumoorthy T, and Pang SM. The role of intravenous immunoglobulin in toxic epidermal necrolysis: a retrospective analysis of 64 patients managed in a specialized centre. British Journal of Dermatology ; 169: MFMER slide 42
43 Results Primary Predicted mortality, n Observed mortality, n Standardized mortality (95% CI) n = ( ) Secondary Analyses Parameter Survivors (n = 44) Non survivors (n = 20) p value SCORTEN 2.2 ± ± 1.0 <0.001 Secondary Analyses Parameter Low dose IVIG <3 g/kg (n = 42) SCORTEN 2.6 ± ± 0.9 High dose IVIG 3 g/kg (n = 19) p value Observed mortality, n (%) 13 (31) 5 (26) 0.71 Lee HY, Lim YL, Thirumoorthy T, and Pang SM. The role of intravenous immunoglobulin in toxic epidermal necrolysis: a retrospective analysis of 64 patients managed in a specialized centre. British Journal of Dermatology ; 169: MFMER slide 43
44 Conclusions Limitations: retrospective design, varied dosing strategies Conclusions: IVIG does not confer a significant survival benefit Lee HY, Lim YL, Thirumoorthy T, and Pang SM. The role of intravenous immunoglobulin in toxic epidermal necrolysis: a retrospective analysis of 64 patients managed in a specialized centre. British Journal of Dermatology ; 169: MFMER slide 44
45 Cyclosporine Proposed mechanism: A calcineurin inhibitor with the ability to block the function of T cells Schneider JA, Cohen PR. Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing Supportive Measures. Adv Ther. 2017;34(6): Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens Johnson syndrome: a review. Crit Care Med. 2011;39(6): MFMER slide 45
46 Cyclosporine Author & year Study type Number patients Valeyrie Allanore et al Singh et al Kirchof et al Lee et al Open phase II trial Prospective open trial Cyclosporine treatment 29 3 mg/kg x 10 d, then 2 mg/kg x 10 d, then 1 mg/kg x 10 d 11 3 mg/kg x 7d, then 2 mg/kg x 7 d Mortality, n Other 0 Progression and death rate lower than expected 0 May have encouraging role Retrospective mg/kg x 7 d 1 May have mortality benefit over IVIG Retrospective 44 3 mg/kg x 10 d, then 2 mg/kg x 10 d, then 1 mg/kg x 10 d 3 Statistically insignificant survival benefit compared to supportive care Lee HY, Fook Chong S, Koh HY, Thirumoorthy T, Pang SM. Cyclosporine treatment for Stevens Johnson syndrome/toxic epidermal necrolysis: Retrospective analysis of a cohort treated in a specialized referral center. J Am Acad Dermatol. 2017;76(1): MFMER slide 46
47 TNF-α Inhibitors Proposed mechanism: Ability to inhibit granulysin and TNF α secretion from blister cells 2018 MFMER slide 47
48 TNF-α Inhibitors - Infliximab Author & year Study type Number patients Zarate Correa et al Wojtkiewicz et al Patmanidis et al Case series Case report Case report Treatment Mortality Other 4 Single dose 300 mg infliximab on Day 1 or 2 after admission 1 Single dose 5 mg/kg infliximab after IVIG failure mg methylprednisolone IV bolus followed by 5 mg/kg infliximab IVIG 2g/kg x5 days 0% Disease progression halted in all 4 patients 0% Within 24 hours of infliximab dose onset of new blisters stopped 0% Skin condition markedly stabilized by day 2 of admission Zárate Correa LC, Carrillo Gómez DC, Ramírez Escobar AF, Serrano Reyes C. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23(1): Wojtkiewicz A, Wysocki M, Fortuna J, Chrupek M, Matczuk M, Koltan A. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88(4): Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012: MFMER slide 48
49 Randomized, controlled trial of TNF-α antagonist in CTLmediated severe cutaneous adverse reactions Wang CW, Yang LY, Chen CB, et al.; and the Taiwan Severe Cutaneous Adverse Reaction (TSCAR) Consortium. J Clin Invest. 2018;128(3): MFMER slide 49
50 Objectives Primary endpoint: Time required to heal skin erosions and oral mucosa and to begin re epithelialization Secondary monitoring parameters: Adverse events Mortality rates Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128(3): MFMER slide 50
51 Study Methods Single center, prospective, open label, randomized controlled unblinded trial Randomized 1:1 25 mg (or 50 mg if >65 kg) etanercept SQ injection twice a week mg/kg/day prednisolone IV Treated until skin lesions healed 96 patients randomized, 71 completed to efficacy analysis Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128(3): MFMER slide 51
52 Enrollment Inclusion Older than 4 years Diagnosed with probably/definite SJS/TEN Exclusion Pregnant or breastfeeding Allergy to any TNF α inhibitor Active/latent tuberculosis Severe, active infection and septicemia Carriers of active hepatitis B or C Suspected carriers of HIV with CD4 Tcell count <200 Poor compliance or safety concerns Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128(3): MFMER slide 52
53 Baseline Characteristics Characteristic All n= 91 Etanercept n = 48 Corticosteroid n = 43 p value Age, years ± ± ± Sex, n (%) Male 40 (44) 20 (41.7) 20 (46.5) Female 51 (56) 28 (58.3) 23 (53.5) Skin detachment, n (%) BSA 10%, n (%) 35 (38.5) 18 (37.5) 17 (39.5) BSA <10%, n (%) 56 (61.5) 30 (62.5) 26 (60.5) SCORTEN mean 1.85 ± ± Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128(3): MFMER slide 53
54 Results Parameter Etanercept n = 38 Corticosteroid n = 33 p value Median time for skin healing, d Predicted mortality, %, 17.7 ± ± mean Observed mortality, n 4 (8.3) 7 (16.3) (%) GI hemorrhage, % Serious adverse events, n 5 9 Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128(3): MFMER slide 54
55 Conclusion Strengths: randomized controlled trial Limitations: unblinded Conclusion: Etanercept reduced predicted mortality, rates of GI hemorrhage, and time to skin healing in moderate to severe SJS/TEN compared to corticosteroids Further studies in combination with other treatment strategies needed Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128(3): MFMER slide 55
56 Summary of Adjuvant Therapies Steroids Have been associated with increased infection rates, duration of stay, and mortality Possible beneficial role with short term therapy or as addition to other therapies IVIG Conflicting evidence Most recent studies show no survival benefit over supportive care Cyclosporine Most recent study shows no survival benefit over supportive care More research required TNF α inhibitors Best evidence for mortality benefit Further research required to determine ideal treatment regimen 2018 MFMER slide 56
57 Plasmapheresis Proposed mechanism: Can enhance the removal of medications and activated immune cells from plasma Studies have not consistently shown benefit May be considered if other treatments are failing Ruminski MA, Wisneski SS, Dugan SE. Stevens Johnson syndrome: what a pharmacist should know. US Pharm. 2013; 38(7): Narita YM, Hirahara K, Mizukawa Y, et al. Efficacy of plasmapheresis for the treatment of severe toxic epidermal necrolysis: is cytokine expression analysis useful in predicting its therapeutic efficacy? J Dermatol. 2011; 38: Creamer D, Walsh SA, Dziewulski P, et al. UK guidelines for the management of Stevens Johnson syndrome/toxic epidermal necrolysis in adults J Plast Reconstr Aesthetic Surg. 2016;69(6):e119 e MFMER slide 57
58 Thalidomide Use is contraindicated in SJS/TEN Double blind, randomized placebo controlled trial (n =12) Mortality increased in thalidomide group vs placebo (83% vs 30%) Trial was discontinued early Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomized comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet. 1998; 352: MFMER slide 58
59 Optimal treatment of SJS/TEN includes supportive care and extended duration corticosteroids. a) True b) False 2018 MFMER slide 59
60 Optimal treatment of SJS/TEN includes supportive care and extended duration corticosteroids. a) True b) False 2018 MFMER slide 60
61 Summary SJS/TEN characterized by full thickness epidermal necrosis and keratinocyte apoptosis Medications are the most common cause of SJS/TEN There is no gold standard of treatment Stop offending agent Supportive care 2018 MFMER slide 61
62 Discussion & Questions 2018 MFMER slide 62
63 Study References 1. Halebian PH, Corder VJ, Madden MR, Finklestein JL, Shires GT. Improved burn center survival of patients with toxic epidermal necrolysis managed without corticosteroids. Ann Surg. 1986;204(5): Kelemen JJ III, Cioffi WG, McManus WF, et al: Burn center care for patients with toxic epidermal necrolysis. J Am Coll Surg 1995; 180: Kakourou T, Klontza D, Soteropoulou F, et al: Corticosteroid treatment of erythema multiforme major (Stevens Johnson syndrome) in children. Eur J Pediatr 1997;156: Forman R, Koren G, Shear NH: Erythema multiforme, Stevens Johnson syndrome and toxic epidermal necrolysis in children: A review of 10 years experience. Drug Saf 2002; 25: Kardaun SH, Jonkman MF: Dexamethasone pulse therapy for Stevens Johnson syndrome toxic epidermal necrolysis. Acta Derm Venereol 2007; 87: Yamane Y, Aihara M, Ikezawa Z: Analysis of Stevens Johnson syndrome and toxic epidermal necrolysis in Japan from 2000 to Allergol Int 2007; 56: Schneck J, Fagot J P, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens Johnson syndrome and toxic epidermal necrolysis: A retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008;58(1): Yang Y, Xu J, Li F, et al: Combination therapy of intravenous immunoglobulin and corticosteroid in the treatment of toxic epidermal necrolysis and Stevens Johnson syndrome: A retrospective comparative study in China. Int J Dermatol 2009; 48: Bachot N, Revuz J, Roujeau JC: Intravenous immunoglobulin treatment for Stevens Johnson syndrome and toxic epidermal necrolysis: A prospective noncomparative study showing no benefit on mortality or progression. Arch Dermatol 2003; 139: Metry DW, Jung P, Levy ML: Use of intravenous immunoglobulin in children with stevens johnson syndrome and toxic epidermal necrolysis: Seven cases and review of the literature. Pediatrics 2003; 112: Brown KM, Silver GM, Halerz M, et al: Toxic epidermal necrolysis: Does immunoglobulin make a difference? J Burn Care Rehabil 2004; 25: Yeung CK, Lam LK, Chan HH: The timing of intravenous immunoglobulin therapy in Stevens Johnson syndrome and toxic epidermal necrolysis. Clin Exp Dermatol 2005; 30: Gravante G, Delogu D, Marianetti M, et al: Toxic epidermal necrolysis and Steven Johnson syndrome: 11 years experience and outcome. Eur Rev Med Pharmacol Sci 2007; 11: Stella M, Clemente A, Bollero D, et al: Toxic epidermal necrolysis (TEN) and Stevens Johnson syndrome (SJS): Experience with highdose intravenous immunoglobulins and topical conservative approach. A retrospective analysis. Burns 2007; 33: MFMER slide 63
allergy Asia Pacific Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from
 pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course
 Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update
 Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
Correspondence should be addressed to Wanjarus Roongpisuthipong; rr
 Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Dilantin (phenytoin) ROBERT A. SCHWARTZ
 Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis: a case report
 Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving
 Present : Dr Pham Thi Minh Rang Internal Department No2-Hospital for children No2 AIMS To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving To
Present : Dr Pham Thi Minh Rang Internal Department No2-Hospital for children No2 AIMS To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving To
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW
 SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT
 OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital
 Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review
 140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
 Case Reports in Immunology Volume 2012, Article ID 931528, 4 pages doi:10.1155/2012/931528 Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
Case Reports in Immunology Volume 2012, Article ID 931528, 4 pages doi:10.1155/2012/931528 Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
Pediatric Dermatology
 Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
Cutaneous Conditions Associated with Systemic Disease
 Cutaneous Conditions Associated with Systemic Disease Johnnie M Woodson, M.D., F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates,
Cutaneous Conditions Associated with Systemic Disease Johnnie M Woodson, M.D., F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates,
A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome
 Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
Toxic Epidermal Necrolysis - A Case Report
 The Journal of Critical Care Medicine 2017;3(1):29-33 CASE REPORT Toxic Epidermal Necrolysis - A Case Report Laura Stătescu¹, Magda Constantin², Horia Silviu Morariu³ *, Laura Gheucă Solovăstru¹ ¹ Dermatology
The Journal of Critical Care Medicine 2017;3(1):29-33 CASE REPORT Toxic Epidermal Necrolysis - A Case Report Laura Stătescu¹, Magda Constantin², Horia Silviu Morariu³ *, Laura Gheucă Solovăstru¹ ¹ Dermatology
Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions
 Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions Jeremy A. Schneider, MD Assistant Clinical Professor UC San Diego Department of Dermatology Objectives Brief overview of some of
Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions Jeremy A. Schneider, MD Assistant Clinical Professor UC San Diego Department of Dermatology Objectives Brief overview of some of
Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes
 Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Stevens-Johnson syndrome (SJS) and toxic epidermal. necrolysis in Asian children
 Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children Mark Jean-Aan Koh, MD, and Yong-Kwang Tay, MD Singapore Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children Mark Jean-Aan Koh, MD, and Yong-Kwang Tay, MD Singapore Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS IN CHILDREN: A LITERATURE REVIEW OF CURRENT TREATMENTS
 STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS IN CHILDREN: A LITERATURE REVIEW OF CURRENT TREATMENTS *Blanca R. Del Pozzo-Magaña, 1 Alejandro Lazo-Langner 2,3 1. Department of Pediatrics, University
STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSIS IN CHILDREN: A LITERATURE REVIEW OF CURRENT TREATMENTS *Blanca R. Del Pozzo-Magaña, 1 Alejandro Lazo-Langner 2,3 1. Department of Pediatrics, University
Skin Manifestations of Drug Reactions
 Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening
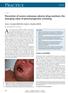 CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES. R e g i S C A R PATIENT'S DATA. Age country of birth
 REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
Tumor Necrosis Factor α Inhibitors in the Treatment of Toxic Epidermal Necrolysis
 ORIGINAL RESEARCH Tumor Necrosis Factor α Inhibitors in the Treatment of Toxic Epidermal Necrolysis Katelyn F. Woolridge, MD; Patrick L. Boler, MD, PharmD; Brian D. Lee, MD PRACTICE POINTS Controversy
ORIGINAL RESEARCH Tumor Necrosis Factor α Inhibitors in the Treatment of Toxic Epidermal Necrolysis Katelyn F. Woolridge, MD; Patrick L. Boler, MD, PharmD; Brian D. Lee, MD PRACTICE POINTS Controversy
Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection
 Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
Bugs and Drugs: What s New in Hypersensitivity Reactions?
 Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash
 CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME
 EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis
 Acta Derm Venereol 2007; 87: 144 148 CLINICAL REPORT Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis Sylvia H. Kardaun and Marcel F. Jonkman Center for Blistering Diseases,
Acta Derm Venereol 2007; 87: 144 148 CLINICAL REPORT Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis Sylvia H. Kardaun and Marcel F. Jonkman Center for Blistering Diseases,
Future of Pediatrics: Blisters, Hives and Other Tales from the Emergency Room June 14 th, 2016
 A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients
 original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit
 J Korean Surg Soc 0;:133-139 DOI:.414/jkss.0..3.133 원 저 A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit Departments of Surgery
J Korean Surg Soc 0;:133-139 DOI:.414/jkss.0..3.133 원 저 A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit Departments of Surgery
A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive Patients
 Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
Five things not to miss in Dermatology. Dr Judy Wismer Associate Clinical Professor Michael G DeGroote School of Medicine
 Five things not to miss in Dermatology Dr Judy Wismer Associate Clinical Professor Michael G DeGroote School of Medicine Key Descriptives Fever, skin pain Purpura, necrosis Bullae, Mucosal, Skin sloughing
Five things not to miss in Dermatology Dr Judy Wismer Associate Clinical Professor Michael G DeGroote School of Medicine Key Descriptives Fever, skin pain Purpura, necrosis Bullae, Mucosal, Skin sloughing
1 Introduction. Kenneth Irungu 1 David Nyamu
 Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review
 Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review
 HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
Emergency Dermatology Dr Melissa Barkham
 Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
ABSTRACT. Keywords: Corticosteroids; Cyclosporine; Dermatology; Etanercept; Immunoglobulin; Necrolysis; Johnson; Stevens; Syndrome REVIEW
 Adv Ther (2017) 34:1235 1244 DOI 10.1007/s12325-017-0530-y REVIEW Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing
Adv Ther (2017) 34:1235 1244 DOI 10.1007/s12325-017-0530-y REVIEW Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing
Review Article Treatments for Severe Cutaneous Adverse Reactions
 Hindawi Journal of Immunology Research Volume 2017, Article ID 1503709, 9 pages https://doi.org/10.1155/2017/1503709 Review Article Treatments for Severe Cutaneous Adverse Reactions Yung-Tsu Cho and Chia-Yu
Hindawi Journal of Immunology Research Volume 2017, Article ID 1503709, 9 pages https://doi.org/10.1155/2017/1503709 Review Article Treatments for Severe Cutaneous Adverse Reactions Yung-Tsu Cho and Chia-Yu
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW
 STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
Toxic epidermal necrolysis
 REVIEW Anaesthesiology Intensive Therapy 2015, vol. 47, no 3, 257 262 ISSN 0209 1712 10.5603/AIT.2015.0037 www.ait.viamedica.pl Toxic epidermal necrolysis Joanna Hinc-Kasprzyk, Agnieszka Polak-Krzemińska,
REVIEW Anaesthesiology Intensive Therapy 2015, vol. 47, no 3, 257 262 ISSN 0209 1712 10.5603/AIT.2015.0037 www.ait.viamedica.pl Toxic epidermal necrolysis Joanna Hinc-Kasprzyk, Agnieszka Polak-Krzemińska,
Sidney Miller, MD, FACS Professor of Surgery Director of Research and Development Ohio State University Burn Center
 Management of the Burn Patient Sidney Miller, MD, FACS Professor of Surgery Director of Research and Development Ohio State University Burn Center American Burn Association Transfer Criteria Burn > 10%
Management of the Burn Patient Sidney Miller, MD, FACS Professor of Surgery Director of Research and Development Ohio State University Burn Center American Burn Association Transfer Criteria Burn > 10%
Mark A. Bechtel, MD Clinical Associate Professor Division Director, Dermatology Ohio State University Medical Center
 Dermatologic Emergencies Mark A. Bechtel, MD Clinical Associate Professor Division Director, Dermatology Ohio State University Medical Center Clinical Features of SJS/TEN Initial symptoms Fever, stinging
Dermatologic Emergencies Mark A. Bechtel, MD Clinical Associate Professor Division Director, Dermatology Ohio State University Medical Center Clinical Features of SJS/TEN Initial symptoms Fever, stinging
Emergency Dermatology. Emergency Dermatology
 Emergency Dermatology These are rapidly progressive skin conditions and some are potentially lifethreatening. Early recognition is important to implement prompt supportive care and therapy. Some are drug
Emergency Dermatology These are rapidly progressive skin conditions and some are potentially lifethreatening. Early recognition is important to implement prompt supportive care and therapy. Some are drug
Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea 2
 pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2013;27(5):331-340 http://dx.doi.org/10.3341/kjo.2013.27.5.331 Original Article Effect of Age and Early Intervention with a Systemic Steroid, Intravenous
pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2013;27(5):331-340 http://dx.doi.org/10.3341/kjo.2013.27.5.331 Original Article Effect of Age and Early Intervention with a Systemic Steroid, Intravenous
Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from 2000 to 2006 Yumiko Yamane 1, Michiko Aihara 1 and Zenro Ikezawa 1
 Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
DERMATOLOGIC EMERGENCIES. Mary Evers D.O., F.A.O.C.D. Georgetown, Texas
 DERMATOLOGIC EMERGENCIES Mary Evers D.O., F.A.O.C.D. Georgetown, Texas SKIN EMERGENCIES??? Subclassifications: Autoimmune (Anaphylaxis, Vasculitis, Pemphigus) Erythroderma (AGEP, DRESS, SJS, TEN) Infectious
DERMATOLOGIC EMERGENCIES Mary Evers D.O., F.A.O.C.D. Georgetown, Texas SKIN EMERGENCIES??? Subclassifications: Autoimmune (Anaphylaxis, Vasculitis, Pemphigus) Erythroderma (AGEP, DRESS, SJS, TEN) Infectious
Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis.
 Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Supplementary online-material Supplementary Table S1: Detailed descriptive analysis
Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Supplementary online-material Supplementary Table S1: Detailed descriptive analysis
Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review
 Case Report Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review Deepali P. Mohite*, Satyajitraje Tekade*, Amol Gadbail*, M. S. Chaudhary** Abstract : Toxic epidermal necrolysis (TEN) and Stevens
Case Report Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review Deepali P. Mohite*, Satyajitraje Tekade*, Amol Gadbail*, M. S. Chaudhary** Abstract : Toxic epidermal necrolysis (TEN) and Stevens
Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate
 Original Research Article Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate Manab Nandy 1, Sangeeta De 2*, Mustafa Asad 2, Nirmal
Original Research Article Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate Manab Nandy 1, Sangeeta De 2*, Mustafa Asad 2, Nirmal
Severe cutaneous adverse reactions (SCAR) are life-threatening
 Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Big rashes in little patients:
 ! Big rashes in little patients: Severe drug eruptions and cutaneous infections!! Marcia Hogeling, MD, FAAD Assistant Clinical Professor Director, Pediatric Dermatology Division of Dermatology David Geffen
! Big rashes in little patients: Severe drug eruptions and cutaneous infections!! Marcia Hogeling, MD, FAAD Assistant Clinical Professor Director, Pediatric Dermatology Division of Dermatology David Geffen
Diagnosis and Management of Drug-induced Stevens-Johnson Syndrome: Report of Two Cases
 10.5005/jp-journals-10011-1189 CASE REPORT JIAOMR Diagnosis and Management of Drug-induced Stevens-Johnson Syndrome: Report of Two Cases 1 M Venkateshwarlu, 2 B Radhika 1 Professor and Head, Department
10.5005/jp-journals-10011-1189 CASE REPORT JIAOMR Diagnosis and Management of Drug-induced Stevens-Johnson Syndrome: Report of Two Cases 1 M Venkateshwarlu, 2 B Radhika 1 Professor and Head, Department
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC
 SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
PedsCases Podcast Scripts
 PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
Accepted Manuscript. Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need
 Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
DOSING AND INFORMATION GUIDE LEAPS AHEAD
 DOSING AND INFORMATION GUIDE In patients with WT RAS* mcrc 1 VECTIBIX (panitumumab) LEAPS AHEAD 5.6-month increase in median OS with FOLFOX vs FOLFOX alone 1 Spot the difference. CHOOSE VECTIBIX PRIME
DOSING AND INFORMATION GUIDE In patients with WT RAS* mcrc 1 VECTIBIX (panitumumab) LEAPS AHEAD 5.6-month increase in median OS with FOLFOX vs FOLFOX alone 1 Spot the difference. CHOOSE VECTIBIX PRIME
Original Article. Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H 1, Tripathi C
 Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
NEOFEN 60 mg suppository
 PACKAGE LEAFLET: INFORMATION FOR THE USER NEOFEN 60 mg suppository IBUPROFEN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER NEOFEN 60 mg suppository IBUPROFEN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
Severe cutaneous reactions caused by barbiturates in seven Iranian children
 Pharmacology and therapeutics Severe cutaneous reactions caused by barbiturates in seven Iranian children Setareh Mamishi, MD, Fatemeh Fattahi, MD, Zahra Pourpak, MD, PhD, Farzaneh Mirza Aghaee, MD, Zeinab
Pharmacology and therapeutics Severe cutaneous reactions caused by barbiturates in seven Iranian children Setareh Mamishi, MD, Fatemeh Fattahi, MD, Zahra Pourpak, MD, PhD, Farzaneh Mirza Aghaee, MD, Zeinab
DERMATOLOGICAL EMERGENCIES. DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE
 DERMATOLOGICAL EMERGENCIES DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE Dermatological Emergencies INFECTIONS ERYTHRODERMA DRUG ERUPTIONS STEVENS-JOHNSON
DERMATOLOGICAL EMERGENCIES DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE Dermatological Emergencies INFECTIONS ERYTHRODERMA DRUG ERUPTIONS STEVENS-JOHNSON
STEVENS-JOHNSON SYNDROME: A CASE REPORT
 STEVENS-JOHNSON SYNDROME: A CASE REPORT Castana O., Rempelos G., Anagiotos G., Apostolopoulou C., Dimitrouli A., Alexakis D. Department of Plastic and Reconstructive Surgery, Evangelismos General Hospital,
STEVENS-JOHNSON SYNDROME: A CASE REPORT Castana O., Rempelos G., Anagiotos G., Apostolopoulou C., Dimitrouli A., Alexakis D. Department of Plastic and Reconstructive Surgery, Evangelismos General Hospital,
Impavido. (miltefosine) New Product Slideshow
 Impavido (miltefosine) New Product Slideshow Introduction Brand name: Impavido Generic name: Miltefosine Pharmacological class: Antileishmanial agent Strength and Formulation: 50mg; hard gel capsules Manufacturer:
Impavido (miltefosine) New Product Slideshow Introduction Brand name: Impavido Generic name: Miltefosine Pharmacological class: Antileishmanial agent Strength and Formulation: 50mg; hard gel capsules Manufacturer:
SEVERE CUTANEUS ADVERSE DRUGS EFFECTS MANIFESTED AS TOXIC EPIDERMAL NECROLYSIS (TEN) DUE TO ORAL ANTITUBERCULOSIS DRUGS AND REVIEW OF THE LITERATURE
 SEVERE CUTANEUS ADVERSE DRUGS EFFECTS MANIFESTED AS TOXIC EPIDERMAL NECROLYSIS (TEN) DUE TO ORAL ANTITUBERCULOSIS DRUGS AND REVIEW OF THE LITERATURE * HM Nataprawira, ** M Soepriadi * Respirology Division,
SEVERE CUTANEUS ADVERSE DRUGS EFFECTS MANIFESTED AS TOXIC EPIDERMAL NECROLYSIS (TEN) DUE TO ORAL ANTITUBERCULOSIS DRUGS AND REVIEW OF THE LITERATURE * HM Nataprawira, ** M Soepriadi * Respirology Division,
Stressed Out: Evaluating the Need for Stress Ulcer Prophylaxis in the ICU
 Stressed Out: Evaluating the Need for Stress Ulcer Prophylaxis in the ICU Josh Arnold, PharmD PGY1 Pharmacy Resident Pharmacy Grand Rounds November 8, 2016 2016 MFMER slide-1 Objectives Identify the significance
Stressed Out: Evaluating the Need for Stress Ulcer Prophylaxis in the ICU Josh Arnold, PharmD PGY1 Pharmacy Resident Pharmacy Grand Rounds November 8, 2016 2016 MFMER slide-1 Objectives Identify the significance
Stevens-Johnson Syndrome : A Case Report
 https://doi.org/10.5933/jkapd.2017.44.4.455 J Korean Acad Pediatr Dent 44(4) 2017 ISSN (print) 1226-8496 ISSN (online) 2288-3819 Stevens-Johnson Syndrome : A Case Report Yongho Song, Nanyoung Lee, Sangho
https://doi.org/10.5933/jkapd.2017.44.4.455 J Korean Acad Pediatr Dent 44(4) 2017 ISSN (print) 1226-8496 ISSN (online) 2288-3819 Stevens-Johnson Syndrome : A Case Report Yongho Song, Nanyoung Lee, Sangho
Severe Desquamating Disorder After Liver Transplant: Toxic Epidermal Necrolyis or Graft Versus Host Disease?
 Severe Desquamating Disorder After Liver Transplant: Toxic Epidermal Necrolyis or Graft Versus Host Disease? John T. Schulz III, MD, PhD, FACS, and Robert L. Sheridan, MD, FACS Bridgeport Hospital, Bridgeport,
Severe Desquamating Disorder After Liver Transplant: Toxic Epidermal Necrolyis or Graft Versus Host Disease? John T. Schulz III, MD, PhD, FACS, and Robert L. Sheridan, MD, FACS Bridgeport Hospital, Bridgeport,
November/December Issue
 In This Issue In luenza Vaccine Update 2016-2017 Treatment Update for Stevens-Johnson and TEN Syndromes Formulary Update Marcia J. Wyman, Pharm.D., BCPS Drug Information Pharmacist Editor Mandy C. Leonard,
In This Issue In luenza Vaccine Update 2016-2017 Treatment Update for Stevens-Johnson and TEN Syndromes Formulary Update Marcia J. Wyman, Pharm.D., BCPS Drug Information Pharmacist Editor Mandy C. Leonard,
Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
 BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics
 MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Chronic lymphocytic leukaemia 1 Chronic lymphocytic leukemia (CLL) is a condition characterized by a progressive accumulation
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Chronic lymphocytic leukaemia 1 Chronic lymphocytic leukemia (CLL) is a condition characterized by a progressive accumulation
cipro loxacin; stevens-johnson syndrome; urinary tract infection; adverse drug reaction
 Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
SJS/TEN spectrum. Stevens-Johnson syndrome (SJS) /Toxic Epidermal Necrolysis (TEN) 10/7/2016
 Jesse Keller MD Assistant Professor Oregon Health & Science University Stevens-Johnson syndrome (SJS) /Toxic Epidermal Necrolysis (TEN) Drug induced dermemergencies that exist on a spectrum Delayed reaction:
Jesse Keller MD Assistant Professor Oregon Health & Science University Stevens-Johnson syndrome (SJS) /Toxic Epidermal Necrolysis (TEN) Drug induced dermemergencies that exist on a spectrum Delayed reaction:
건강한성인에서의오진하기쉬운포도구균성열상피부증후군의치험례. Staphylococcal Scalded Skin Syndrome in a Healthy Adult: Easy to Misdiagnose
 Archives of Hand and Microsurgery Arch Hand Microsurg 2018;23(4):271-276. https://doi.org/10.12790/ahm.2018.23.4.271 pissn 2586-3290 eissn 2586-3533 Case Report 건강한성인에서의오진하기쉬운포도구균성열상피부증후군의치험례 김홍일ㆍ곽찬이ㆍ박언주
Archives of Hand and Microsurgery Arch Hand Microsurg 2018;23(4):271-276. https://doi.org/10.12790/ahm.2018.23.4.271 pissn 2586-3290 eissn 2586-3533 Case Report 건강한성인에서의오진하기쉬운포도구균성열상피부증후군의치험례 김홍일ㆍ곽찬이ㆍ박언주
Dr. Venkateswari. R. Dr. Janani Sankar s unit Kanchi Kamakoti CHILDS Trust Hospital
 Dr. Venkateswari. R. Dr. Janani Sankar s unit Kanchi Kamakoti CHILDS Trust Hospital Acknowledgements: KKCTH Dr. Ramkumar Consultant Dermatologist Dr. Ramprakash Consultant Ophthalmologist Dr. Prasad Manne
Dr. Venkateswari. R. Dr. Janani Sankar s unit Kanchi Kamakoti CHILDS Trust Hospital Acknowledgements: KKCTH Dr. Ramkumar Consultant Dermatologist Dr. Ramprakash Consultant Ophthalmologist Dr. Prasad Manne
Effective pain management begins with OFIRMEV (acetaminophen) injection FIRST Proven efficacy with rapid reduction in pain 1
 Effective pain management begins with OFIRMEV (acetaminophen) injection FIRST Proven efficacy with rapid reduction in pain 1 Fast onset of pain relief with 7% reduction in visual analog scale (VAS) scores
Effective pain management begins with OFIRMEV (acetaminophen) injection FIRST Proven efficacy with rapid reduction in pain 1 Fast onset of pain relief with 7% reduction in visual analog scale (VAS) scores
Several different manifestations
 Toxic epidermal necrolysis and Stevens-Johnson syndrome: A review* Roland Gerull, MD; Mathias Nelle, MD; Thomas Schaible, MD Objectives: The aims of this review are to summarize the definitions, causes,
Toxic epidermal necrolysis and Stevens-Johnson syndrome: A review* Roland Gerull, MD; Mathias Nelle, MD; Thomas Schaible, MD Objectives: The aims of this review are to summarize the definitions, causes,
Your letter Your reference The Hague, Subject Request for change in the product information following the PhVWP/CMDh decision
 Registratiehouder ( t.a.v. registratieafdeling) Your letter Your reference The Hague, -- --.. Case number Our reference../../ Case manager Telephone number Subject Request for change in the product information
Registratiehouder ( t.a.v. registratieafdeling) Your letter Your reference The Hague, -- --.. Case number Our reference../../ Case manager Telephone number Subject Request for change in the product information
Cutaneous Drug Reactions
 Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
GOOD MORNING! AUGUST 5, 2014
 GOOD MORNING! AUGUST 5, 2014 PREP QUESTION During the health supervision visit of a term newborn boy, his mother relates that a cousins child died at age 4 months from sudden infant death syndrome. She
GOOD MORNING! AUGUST 5, 2014 PREP QUESTION During the health supervision visit of a term newborn boy, his mother relates that a cousins child died at age 4 months from sudden infant death syndrome. She
ABACAVIR HYPERSENSITIVITY REACTION
 ABACAVIR HYPERSENSITIVITY REACTION Key Risk Minimisation Points: Abacavir Hypersensitivity Reaction (HSR) Abacavir is associated with a risk for hypersensitivity reactions (HSR) characterised by fever
ABACAVIR HYPERSENSITIVITY REACTION Key Risk Minimisation Points: Abacavir Hypersensitivity Reaction (HSR) Abacavir is associated with a risk for hypersensitivity reactions (HSR) characterised by fever
Elements for a Public Summary
 VI.2 VI.2.1 Elements for a Public Summary Overview of Disease Epidemiology Non-Small Cell Lung Cancer (NSCLC): Non-small cell lung cancer accounts for 85 90% of lung cancers [Reck, 2014] and is the leading
VI.2 VI.2.1 Elements for a Public Summary Overview of Disease Epidemiology Non-Small Cell Lung Cancer (NSCLC): Non-small cell lung cancer accounts for 85 90% of lung cancers [Reck, 2014] and is the leading
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients
Rayos Prior Authorization Program Summary
 Rayos Prior Authorization Program Summary FDA APPROVED INDICATIONS AND DOSAGE FDA-Approved Indications: 1 Agent Indication Dosage Rayos (prednisone delayedrelease tablet) as an anti-inflammatory or immunosuppressive
Rayos Prior Authorization Program Summary FDA APPROVED INDICATIONS AND DOSAGE FDA-Approved Indications: 1 Agent Indication Dosage Rayos (prednisone delayedrelease tablet) as an anti-inflammatory or immunosuppressive
Ninlaro. (ixazomib) New Product Slideshow
 Ninlaro (ixazomib) New Product Slideshow Introduction Brand name: Ninlaro Generic name: Ixazomib Pharmacological class: Proteasome inhibitor Strength and Formulation: 2.3mg, 3mg, 4mg; gel caps Manufacturer:
Ninlaro (ixazomib) New Product Slideshow Introduction Brand name: Ninlaro Generic name: Ixazomib Pharmacological class: Proteasome inhibitor Strength and Formulation: 2.3mg, 3mg, 4mg; gel caps Manufacturer:
To provide guidance on prevention and control of illness caused by varicella-zoster virus (VZV).
 Effective Date: 04/18 Replaces: 0 4 / 1 3 / 1 7 Page 1 of 4 POLICY: To provide guidance on prevention and control of illness caused by varicella-zoster virus (VZV). DEFINITIONS Two syndromes occur from
Effective Date: 04/18 Replaces: 0 4 / 1 3 / 1 7 Page 1 of 4 POLICY: To provide guidance on prevention and control of illness caused by varicella-zoster virus (VZV). DEFINITIONS Two syndromes occur from
In the past few years, beta-lactam-resistant gram-positive
 Early Diagnosis Is Key in Vancomycin-Induced Linear IgA Bullous Dermatosis and Stevens-Johnson Syndrome Douglas H. Jones, MS Michael Todd, MD Timothy J. Craig, DO Background: With the emergence of highly
Early Diagnosis Is Key in Vancomycin-Induced Linear IgA Bullous Dermatosis and Stevens-Johnson Syndrome Douglas H. Jones, MS Michael Todd, MD Timothy J. Craig, DO Background: With the emergence of highly
CUTANEOUS DRUG REACTIONS OR I WOULDN T HAVE SEEN IT, IF I HADN T BELIEVED IT Edmund J. Rosser Jr., DVM, DACVD
 CUTANEOUS DRUG REACTIONS OR I WOULDN T HAVE SEEN IT, IF I HADN T BELIEVED IT Edmund J. Rosser Jr., DVM, DACVD DERMATOLOGY Pathogenesis Immunologic: can involve Type I, II, III, IV hypersensitivity reactions.
CUTANEOUS DRUG REACTIONS OR I WOULDN T HAVE SEEN IT, IF I HADN T BELIEVED IT Edmund J. Rosser Jr., DVM, DACVD DERMATOLOGY Pathogenesis Immunologic: can involve Type I, II, III, IV hypersensitivity reactions.
CLINICAL GUIDELINES. Summary of Literature and Recommendations Concerning Immunization and Steroid Injections Thomas J. Gilbert M.D., M.P.P.
 CLINICAL GUIDELINES Summary of Literature and Recommendations Concerning Immunization and Steroid Injections Thomas J. Gilbert M.D., M.P.P. 11/2/15 Several practices routinely delay steroid injections
CLINICAL GUIDELINES Summary of Literature and Recommendations Concerning Immunization and Steroid Injections Thomas J. Gilbert M.D., M.P.P. 11/2/15 Several practices routinely delay steroid injections
Checkpoint inhibitors: Strategies to checkmate T-cell mediated toxicity. Disclosure Statement. Learning Objectives
 Checkpoint inhibitors: Strategies to checkmate T-cell mediated toxicity Adam J. DiPippo, PharmD Clinical Pharmacy Specialist Leukemia Texas Society of Health-System Pharmacists 2017 Annual Seminar April
Checkpoint inhibitors: Strategies to checkmate T-cell mediated toxicity Adam J. DiPippo, PharmD Clinical Pharmacy Specialist Leukemia Texas Society of Health-System Pharmacists 2017 Annual Seminar April
Case Report: SUCCESSFUL TREATMENT OF STEVENS JOHNSON SYNDROME -TOXIC EPIDERMAL NECROLYSIS WITH INTRAVENOUS IMMUNOGLOBULIN
 Folia Medica Indonesiana Vol. 43 No. 2 April-June 2007 : 105-112 Successful Treatment of Stevens Johnson Syndrome (Bony Pramono, Ariyanto Harsono) Case Report: SUCCESSFUL TREATMENT OF STEVENS JOHNSON SYNDROME
Folia Medica Indonesiana Vol. 43 No. 2 April-June 2007 : 105-112 Successful Treatment of Stevens Johnson Syndrome (Bony Pramono, Ariyanto Harsono) Case Report: SUCCESSFUL TREATMENT OF STEVENS JOHNSON SYNDROME
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 3 October 2012
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 October 2012 REMICADE 100 mg, powder for concentrate for solution for infusion B/1 vial (CIP code: 562 070-1) Applicant:
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 3 October 2012 REMICADE 100 mg, powder for concentrate for solution for infusion B/1 vial (CIP code: 562 070-1) Applicant:
Back to the Future: Updated Guidelines for Evaluation and Management of Adrenal Insufficiency in the Critically Ill
 Back to the Future: Updated Guidelines for Evaluation and Management of Adrenal Insufficiency in the Critically Ill Joe Palumbo PGY-2 Critical Care Pharmacy Resident Buffalo General Medical Center Disclosures
Back to the Future: Updated Guidelines for Evaluation and Management of Adrenal Insufficiency in the Critically Ill Joe Palumbo PGY-2 Critical Care Pharmacy Resident Buffalo General Medical Center Disclosures
Genetic susceptibility for Stevens-Johnson syndrome/toxic epidermal necrolysis with mucosal involvements
 249 Special Issue: Inflammation in Ophthalmology Review Article Genetic susceptibility for Stevens-Johnson syndrome/toxic epidermal necrolysis with mucosal involvements 1, 2, ) Mayumi Ueta 1) Department
249 Special Issue: Inflammation in Ophthalmology Review Article Genetic susceptibility for Stevens-Johnson syndrome/toxic epidermal necrolysis with mucosal involvements 1, 2, ) Mayumi Ueta 1) Department
Trust Guideline. for Ciclosporin Treatment & Monitoring for Adult* Patients with Acute, Severe Ulcerative Colitis. (*ie aged 16 years and over)
 Trust Guideline for Ciclosporin Treatment & Monitoring for Adult* Patients with Acute, Severe Ulcerative Colitis (*ie aged 16 years and over) abc A guideline recommended for use In: Gastroenterology/Medical
Trust Guideline for Ciclosporin Treatment & Monitoring for Adult* Patients with Acute, Severe Ulcerative Colitis (*ie aged 16 years and over) abc A guideline recommended for use In: Gastroenterology/Medical
GROUP A STREPTOCOCCUS (GAS) INVASIVE
 GROUP A STREPTOCOCCUS (GAS) INVASIVE Case definition CONFIRMED CASE Laboratory confirmation of infection with or without clinical evidence of invasive disease: isolation of group A streptococcus (Streptococcus
GROUP A STREPTOCOCCUS (GAS) INVASIVE Case definition CONFIRMED CASE Laboratory confirmation of infection with or without clinical evidence of invasive disease: isolation of group A streptococcus (Streptococcus
Evolving therapies for posterior uveitis. Infliximab (Remicade) Infliximab: pharmacology. FDA-approved monoclonal antibody therapy Target
 Evolving therapies for posterior uveitis Sam Dahr, M.D. September 17, 2005 Midwest Ophthalmology Conference Infliximab (Remicade) FDA approved for Crohn s disease, rheumatoid arthritis, and psoriatic arthritis
Evolving therapies for posterior uveitis Sam Dahr, M.D. September 17, 2005 Midwest Ophthalmology Conference Infliximab (Remicade) FDA approved for Crohn s disease, rheumatoid arthritis, and psoriatic arthritis
Dermergency! An Approach to Identification and Management of Life-Threatening Rashes
 Dermergency! An Approach to Identification and Management of Life-Threatening Rashes Gabby Anderson, PharmD PGY2 Emergency Medicine Pharmacy Resident anderson.gabrielle@mayo.edu Pharmacy Grand Rounds January
Dermergency! An Approach to Identification and Management of Life-Threatening Rashes Gabby Anderson, PharmD PGY2 Emergency Medicine Pharmacy Resident anderson.gabrielle@mayo.edu Pharmacy Grand Rounds January
