1 Introduction. Kenneth Irungu 1 David Nyamu
|
|
|
- Loren Harvey
- 6 years ago
- Views:
Transcription
1 Drugs - Real World Outcomes (2017) 4:79 85 DOI /s x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to Kenyatta National Hospital: A Retrospective Cross-Sectional Study Kenneth Irungu 1 David Nyamu 2 Sylvia Opanga 2 Published online: 11 April 2017 Ó The Author(s) This article is an open access publication Abstract Background Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe cutaneous reactions. There is scant literature on the characteristics and causes of these conditions in Kenyatta National Hospital. Objective The aim of this study was to determine the prevalence, risk factors, and etiologies of SJS/TEN among patients admitted to Kenyatta National Hospital. Methods A retrospective cross-sectional study was done to find the characteristics and causes of severe cutaneous reactions among patients admitted to Kenyatta National Hospital. Universal sampling was employed, whereby all 115 patients with severe cutaneous reactions between June 2006 and June 2016 were studied. Information collected included participants sociodemographic variables, clinical characteristics of the disease, and the possible triggers. Data were analyzed using STATA version 13 at p B Results The mean age of patients was 31 years (±20). Low case numbers precluded statistically significant results; however, females represented 59.1% of patients, and 46.1% of patients were diagnosed between the ages of 21 and 40 years. SJS occurred in 47% of patients followed by TEN in 33.9% and SJS/TEN overlap in 19.1%. Drugs were determined to be the causative agent in 94.8% of the severe cutaneous reactions followed by infectious agents at 5.2%, principally HIV, herpes simplex virus 1, and mycoplasma. The most common drugs implicated were sulfonamides (26.1%) and nevirapine (15.7%). Conclusion Numerically, SJS was the most common subtype of SJS/TEN in Kenyatta National Hospital and was usually attributed to use of drugs, especially sulfonamides. Severe cutaneous reactions were observed more frequently in females and in patients aged between 21 and 40 years, indicating that emphasizing surveillance and medication counselling in these patient populations could be beneficial. Key Points Most of the drugs implicated in causing Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) were low-cost antibiotics commonly prescribed in our setting and drugs for treating HIV, malaria, and TB (infections which are common in our population), thus putting many people at risk of SJS/TEN. All patients of all ages should be informed of the symptoms of SJS/TEN and closely monitored for these when starting new medications, especially sulfonamide drugs. & Kenneth Irungu kariuki.kenneth@gmail.com 1 2 Ministry of Health, Karatina Sub-County Referral Hospital, P.O. Box , Karatina, Kenya Department of Pharmaceutics and Pharmacy Practice, School of Pharmacy, University of Nairobi, P.O. Box , Nairobi, Kenya 1 Introduction Stevens Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe bullous skin reactions considered medical emergencies due to their high mortality rate [1]. They are characterized by mucocutaneous tenderness, erythema, hemorrhagic erosions, and necrotic
2 80 K. Irungu et al. epidermal detachment [1]. They are both rare with SJS and TEN having a global incidence of 1 6 and cases per million person years, respectively [2]. Cutaneous drug reactions have a prevalence of 8.259% and these reactions range from urticaria and erythema to SJS and TEN [3]. The average age groups for TEN and SJS in West Germany have been documented as 63 and 25 years, respectively. Women in this region are at a higher risk for TEN than men at a ratio of 2:1, while the converse is true for SJS [4]. In Japan, the average ages for TEN and SJS were observed to be 56.6 and 55.1 years, respectively, with women being more predisposed to SJS than men at a ratio of 1:0.7. There was an equal sex ratio for TEN [5]. In a West African study, the average age group for both SJS and TEN was found to be 32.3 years with a female:male ratio of 1:0.6 [6]. Genetic factors also play a role, with human leukocyte antigens being implicated in SJS and TEN in patients of Asian ancestry. In addition, a slow N-acetylation phenotype predisposes to sulfonamide reactions [7]. Studies have revealed that 80% of the SJS/TEN cases are caused by drugs, mostly antibacterial sulfonamides, b- lactams, and also nevirapine [8]. The risk increases when there is co-infection with either mycoplasma pneumonia, herpes simplex virus 1 (HSV-1) or HIV virus, which are also independent etiologies for SJS/TEN [8]. There is scant published local data on the possible causes for SJS and TEN. The main objective of this study was to characterize SJS/TEN and identify their etiologies in Kenyatta National Hospital (KNH). 2 Methodology The study design was a retrospective cross-sectional study in the KNH medical records department. Patients who met the inclusion criteria (children and adults who were diagnosed and treated for SJS/TEN at KNH from June 2006 to June 2016) were included in the study. One hundred and fifteen patients met the criteria. In order to identify these patients, files of SJS/TEN patients were retrieved using the International Classification of Diseases Adapted Coding Modification 10 (ICDA-10) for L51.1 (SJS) and L51.2 (TEN). Only 89 patient files were obtained using this method. A further search for ICDA-10 skin diagnoses with bullous dermatologic erythematous conditions not otherwise specified (L51.9) yielded 26 more patients. Further review of the excluded files yielded no more SJS/TEN patient files. For a patient to be diagnosed as having SJS they must have had symptoms of acute erythematous mucosal and skin erosions or bullae which are target-like in appearance extending to \10% of the body surface area. For SJS/TEN overlap the patient should have had similar lesions covering 10 30% of the body surface area and for TEN they should have had extensive epidermal detachment of [30% body surface area in addition to the above symptoms. Patient medication histories and a short time lapse from intake of the drug to development of symptoms (2 14 days) confirmed the drugs as causative agents while the history of other illness confirmed, without intake of anti-retrovirals, HIV as the etiology. Furthermore, a Naranjo algorithm was used to determine causality of adverse drug reaction as had been indicated in the file [9]. Mycoplasma pneumonia was determined through laboratory tests and HSV-1 was determined through physical examination by a dermatologist. Pre-designed data collection forms were used to abstract participants socio-demographics, disease characteristics, subtype of the disease, and causative drugs. Twenty of the SJS/TEN patients medical files were randomly picked and data abstracted by an independent reviewer on separate data collection forms for comparison with our investigators forms. Discrepancies were sorted out by consensus. A database was created using MS Excel version 2007 software. This was exported to STATA software version 13 for analysis. Prevalence of the subtypes SJS, TEN, and SJS/TEN overlap was determined. The mean and median age of the disease at diagnosis were computed. Percentages were calculated for all of the patient characteristics and the causative agents. Inferential data analysis was done using the Chi-square test to compare for differences across causative agents and patient characteristics using STATA software version 13 (Statacorp, 4905 Lakeway Drive College Station, Texas USA: licensed to Major Nyamai University of Nairobi). The level of significance was set at 0.05 and p values B0.05 were considered statistically significant. 3 Results Of the 115 patients, the majority (68) was female. The mean and median ages were 31 (±20) and 32 (12 42) years, respectively. More patients were aged years (53) than younger or older (33 and 29, respectively) (Table 1). 3.1 Relative Frequency of Various Disease Subtypes Across the Study Population While insufficient cases were recorded to reach statistical significance, SJS was observed most frequently, occurring in 54 patients followed by TEN (n = 39) and SJS/TEN (n = 22). Among the total number of patients with severe cutaneous reactions, only 24 had a severity-of-illness score (SCORTEN) calculated, as shown in Table 2.
3 SJS/TEN in Kenyatta Hospital 81 Table 1 Socio-demographic characteristics of the study participants Characteristic Age category Mean age, years (SD) 31 (±20) Median age, years (range) 32 (12 42) years 53 (46.1) years 22 (19.1) [60 years 7 (6.1) Sex Male 47 (40.9) Female 68 (59.1) Occupation Salaried 24 (21.1) Self-employed 35 (30.7) Unemployed 55 (48.3) Education level Non-formal 25 (23.2) Primary 43 (39.8) Secondary 30 (27.8) College/university 10 (9.3) Year of diagnosis (14.9) (36.5) (48.7) Residence Nairobi 27 (23.5) Central 24 (20.9) Eastern 21 (18.3) Nyanza 14 (12.2) Rift Valley 13 (11.3) Western 12 (10.4) North Eastern 3 (2.6) Coast 1 (0.9) In keeping with total cases in 21- to 40-year-olds being more frequent than in other age groups, there were greater numbers of cases of each subtype within this age group than in other age brackets (see Table 4). Numerically, more cases of SJS and TEN occurred in females and more cases of SJS/TEN overlap occurred in males (Table 4). 3.2 Relative Frequency of Exposure to Agents Known to Cause SJS/TEN in the Study Population One hundred and nine (94.8%) of the SJS/TEN cases were thought to be caused by use of drugs. Twelve (10.4%) of the patients in the study had SJS/TEN attributable to intake of more than one drug. The remaining six cases of SJS/ Table 2 Disease characteristics of the study population Characteristic Subclass of the disease (n = 115) SJS 54 (47.0) SJS/TEN overlap 22 (19.1) TEN 39 (33.9) SCORTEN 1 5 (20.8) 2 11 (45.8) 3 5 (20.8) 4 2 (8.3) 6 1 (4.2) Method of diagnosis (n = 115) Histopathological 2 (1.7) Clinical 113 (98.3) Origin of the skin reaction (n = 115) Home 105 (91.3) Hospital 10 (8.7) Family history (n = 115) Positive 2 (1.7) Negative 113 (98.3) Median latency time from exposure to drug to 4 days (range 2 14) onset of symptoms TEN were thought to be caused by infectious agents, namely HIV (n = 4), HSV1 (n = 1), and mycoplasma pneumonia (n = 1). As seen in Table 3, use of sulfonamides accounted for the highest number of cases at 25 (22.9%), followed by nevirapine at 18 (16.5%), ciprofloxacin at 13 (11.9%), and penicillins at 8 (7.3%). Other drugs implicated included metronidazole (n = 3), lamotrigine (n = 1), phenobarbitone (n = 1), efavirenz (n = 2), meloxicam (n = 1), hydrochlorothiazide (n = 1), paracetamol (n = 2), allopurinol (n = 1), rifampicin/isoniazid/pyrazinamide/ethambutol (n = 3), quinine (n = 2), magnesium sulphate (n = 1), griseofulvin (n = 1), streptomycin (n = 1), herbal medication (n = 2),oral polio vaccine (n = 1), and unknown medication (n = 11). 3.3 Association Between Demographic Characteristics and Development of Various Disease Subtypes Table 4 compares the demographic characteristics and development of disease subtypes. There were no statistically significant associations between patient s age, gender, and education level on the development of various disease subclasses.
4 82 K. Irungu et al. Table 3 Probable causative agent of SJS/TEN in Kenyatta Hospital patients where a drug was suspected (N = 109) Drug Sulfamethoxazole/ 25 (22.9) trimethoprim Nevirapine 18 (16.5) Ciprofloxacin 13 (11.9) Penicillins 8 (7.3) Cephalosporins 6 (5.5) Artemether/lumefantrine 6 (5.5) Sulfadoxine/pyrimethamine 5 (4.6) Carbamazepine 5 (4.6) Others 23 (21.1) Total 109 (100) SJS/TEN cases attributable to drug, 3.4 Association Between the Prevalence of Various Drugs Implicated in Causation of Disorders and Development of Disease Subtypes Table 5 shows the association between the prevalence of various drugs implicated in causation of disorders and development of disease subtypes. Notably, most of the drugs implicated in causing SJS/TEN disease subtypes were antibiotics and drugs used in the treatment of HIV and malaria. 4 Discussion There were a total of 115 patients treated for severe cutaneous reactions over a period of 10 years, representing a prevalence of 11 cases per year. This result mirrors the scarcity of this serious toxidermia observed in other studies [1, 2, 4]. While cases were too infrequent for comparisons to reach statistical significance, 59.1% of cases were in women, while 40.9% were in men. This female predominance is similar to findings from other studies done by Saka et al. [6] and Chan [10]. Numerically, there were more females than males with SJS and TEN disease subtypes at 30.4 versus 16.5% and 20.0 versus 13.9%, respectively. This trend has been seen in another study, where more females had SJS and TEN disease subtypes at 28.2 versus 16.2% and 29.1 versus 26.5%, respectively [11]. There were, however, no statistically significant associations between patients sex on the development of various disease subclasses. The largest number of patients was those aged between 21 and 40 years (46.1%), compared with 0 20 years (28.7%), years (19.1%), or[60 years (6.1%). There were also more patients in this age category across the various disease subtypes, a finding that was replicated in the study by Saka et al. [6]. This does not match all-cause age distribution for patients at the hospital, so could possibly be attributed to frequent self-prescribing of medicine in this age group. Our results on the most prevalent age Table 4 Relationship between demographics and SJS/TEN disease subtype Demographic characteristic Disease subclass p value SJS SJS/TEN TEN Age category (years) (13) 6 (5.2) 12 (10.4) (25.2) 8 (7) 16 (13.9) (7) 5 (4.3) 9 (7.8) [60 2 (1.7) 3 (2.6) 2 (1.7) Sex Male 19 (16.5) 12 (10.4) 16 (13.9) Female 35 (30.4) 10 (8.7) 23 (20) Occupation Salaried 8 (7.0) 6 (5.2) 10 (8.7) Self-employed 18 (15.7) 6 (5.2) 11 (9.6) Unemployed 28 (24.3) 9 (7.8) 18 (15.7) Education level Non-formal 11 (9.6) 4 (3.5) 10 (8.7) Primary 23 (20) 6 (5.2) 14 (12.2) Secondary 11 (9.6) 8 (7) 11 (9.6) College/university 7 (6.1) 3 (2.6) 0 (0)
5 SJS/TEN in Kenyatta Hospital 83 Table 5 Relationship between frequency of drugs being implicated in SJS/TEN causation and disease subtypes Drug SJS SJS/TEN overlap TEN p value Sulfonamides 16 (13.9) 7 (6.1) 15 (13) Nevirapine 10 (8.7) 4 (3.5) 4 (3.5) Ciprofloxacin 4 (3.5) 2 (1.7) 7 (6.1) Unknown drug 6 (5.2) 1 (0.9) 4 (3.5) b-lactam antibiotics a 7 (6.1) 3 (2.6) 4 (3.5) Artemether/lumefantrine 1 (0.9) 5 (4.3) 0 (0) Carbamazepine 4 (3.5) 0 (0) 1 (0.9) a b-lactam antibiotics penicillins and cephalosporins group conflict with other studies that show most patients being elderly and attributing this to reduced drug clearance, or to most patients being children because of poor immune status. For instance, one study [10] found more SJS/TEN patients in the \5-years-old category, attributing it to viral causes that are more prevalent in this age group, while some findings in West Germany found more TEN in patients aged above 63 years [4]. Nearly half of the patients were seen in the last 2 years because of improved referral systems from different health facilities and reception to our study site. These were put in place by the public health administration that facilitated more patients reaching our study site. Kenyatta National Hospital is the largest referral center in East Africa. The site was appropriate because it is the largest public hospital that provides specialized medical management and treatment services for all conditions and for all ages, both children and adults. It is also the facility with the top medical experts in the country, including dermatologists who are involved in the management of SJS/TEN, hence justifying the referrals to the hospital. SJS/TEN patients from all over the country are also referred here because management of this condition can be appropriately carried out in either the critical care unit or the burns unit if necessary. Nairobi and Central provinces contributed the highest percentage of patients at 23.5 and 20.9%, respectively. This could be attributable to the proximity of the provinces to the study site and as such most patients were referred for further management owing to the life-threatening nature of the toxidermia. More patients were also less educated (63.0%), and a high percentage were unemployed (48.3%).These are factors that affect proper medication use and good access to safe efficacious medications [12]. The causative agents of SJS/TEN were determined by checking the original patient medical files since it was a retrospective study. These were considered accurate due to the following reasons: the drugs implicated in our study were consistent with those in other studies. For other drugs like anti-tuberculosis drugs and anti-malarials there is literature implicating them to cause SJS/TEN [6]. There was a reasonable time lapse between exposure to the drugs and development of symptoms. All our patients during their stay at the hospital had been reviewed by teams of dermatologists and medical experts whose acumen we thought was adequate to have determined the causative agents. Also, during data collection, the medical files had been reviewed by two independent reviewers to ascertain that information obtained from the files was accurate. Our study revealed the probable causes of SJS/TEN to be mostly drugs, accounting for over 90%. Twelve of our patients had SJS/TEN attributable to use of more than one drug. For each of these drugs, a probability of causality of 0.5 was assigned and the latency period for onset of symptoms was determined from intake of the first drug. The median latency for development of symptoms after exposure to the drugs was 4 days with a range of 2 14 days, matching other studies [7, 13]. This is consistent with the latency period for type IV hypersensitivity reaction mediated by T lymphocytes, the reaction responsible for causing SJS/TEN [2, 7]. The high prevalence of probable drug causes correlates with studies by Roujeau et al. [1], Haddad et al. [14], and Levi et al. [15]. HIV as a cause accounted for 3.5% of the cases and in a regional study by Saka et al., an association was found between HIV and SJS/TEN [6]. HSV-1 and mycoplasma pneumonia were also confirmed to be associated with SJS/TEN as in other studies [16, 17]. Of particular importance is the fact that the drugs mostly implicated to cause SJS/TEN in our study are antibiotics, anti-retrovirals, anti-tuberculosis drugs, and anti-malarials. All of these are commonly used locally, due to the high prevalence of relevant infectious diseases in this region, suggesting that many people are at risk of SJS/TEN because of using these drugs. While antiretrovirals and anti-tuberculosis drugs are used more frequently in patients aged years, antibiotics and antimalarials are more frequently used in patients aged 0 20 years [18, 19]. As such, the region s unique
6 84 K. Irungu et al. prescribing patterns likely do not account for the prevalence of SJS/TEN in patients aged years as opposed to different age groups, as observed in some other studies [4, 10]. Most of the severe cutaneous reactions were attributed to sulfonamides (27.5%) followed by ciprofloxacin (11.9%), penicillins (7.3%), and cephalosporins. The multicentric European study by Roujeau et al. found a significant association between SJS/TEN and antibacterial sulfonamides, aminopenicillins, and quinolones [1]. Nevirapine use was also implicated at 16.5%, as was found by Mockenhaupt et al. in the EuroScar study [20]. Roujeau et al. implicated anticonvulsant medications carbamazepine, phenobarbitone, and phenytoin to cause SJS/TEN, but this did not match our study [1].This could have been due to a lower prevalence of convulsive disorders in our population or differences in study methodologies. This could also be because of genetic differences between our population and the Western and Asian countries. Mockenhaupt et al. had also found significant association between SJS/TEN and lamotrigine, whereas we found only one case linked to its use in our study [20]. This could be attributed to the infrequent use of this drug among the local population since it is not in our treatment guidelines as first-line management of seizures [21]. Anti-tuberculosis medications and antimalarial drugs were notably missing as probable causes of SJS/TEN in the studies done in Europe and Asia, but have featured in our study and also in a multicentric study done by Saka et al. in West Africa [6]. This could be explained by the high prevalence of tuberculosis and malaria in Africa as compared with the other two continents. Herbal medications sought from traditional healers may have unknown ingredients owing to adulteration and undocumented phytochemical extracts. These herbal medications have been shown to cause SJS/TEN [6] and in our study they were implicated in two cases. This was minimal considering the rampant use of herbal medicines locally. We attributed this to under-reporting of their use by patients to clinicians. There is a paucity of published data on associations between metronidazole and SJS/TEN. However, in our study it accounted for two cases. This prevalence was low because local anecdotal information on the association between metronidazole and SJS/TEN is high and may have alerted clinicians on its use. Other drugs implicated in our study and not in previous research included magnesium sulfate and griseofulvin. However, it was observed that the patient who reacted to magnesium sulfate had been receiving penicillins earlier, which might have been a confounder. It was also observed that the patient who reacted to griseofulvin had been taking sulfonamides, a possible confounder. There was a high number of SJS/TEN cases (n = 11) caused by unidentified drugs. It was observed that these had been prescribed from far-flung hospitals and no records were available to identify them. 4.1 Study Limitations Information bias arose since the cases were already classified as SJS or TEN according to the medical records. We relied on reported data and the verification was difficult because some of these patients may have suffered from other skin illnesses. This bias was minimized by using a standardized data collection tool that indicated the symptoms required for diagnosis of SJS/TEN. Additionally, twenty random medical files were selected, reviewed, and data extracted to a separate data collection tool by an independent dermatology resident who was not affiliated to the study. Comparisons were made for the two data sets and differences sorted out by consensus. There was also a small sample size which we tried to increase by going back in time over 10 years. 5 Conclusion and Recommendation SJS/TEN are rare severe cutaneous reactions and the most frequently observed disease subtype in our study was SJS. Women and persons aged years were the most affected groups within our study population, and drugs, especially sulfur-based medications, were deemed to be the causative agent in over 90% of the cases. The findings suggest that clinicians should be aware of the side effects of sulfur-based drugs for effective pharmacovigilance among patients who have these medicines prescribed for them. Due to the severity of SJS/TEN, all patients of all age groups taking new medications should undergo close drug monitoring and, based on our study results, this is especially important for women aged years. Further studies are warranted in greater sample sizes across Kenya, comparing patient characteristics with the general population, in order to reach statistical significance and to determine any other predisposing factors to SJS/TEN. Acknowledgements We thank the following for their support: Dr Peter Karimi, Dr Eric Guantai, Samantha Irungu, and the Kenyatta National Hospital medical records staff. Author s Contributions All authors were involved in drafting the protocol and the interpretation of results. Data were extracted from patient files by KI. Data analysis was carried out by KI with the help of DG and a biostatistician. All authors were involved in the drafting and approval of the final review, with KI being the coordinating author. All authors read and approved the final manuscript.
7 SJS/TEN in Kenyatta Hospital 85 Compliance with Ethical Standards Funding for the study This study was funded by the authors. Conflict of interest Kenneth Irungu declares no conflict of interest, David Nyamu declares no conflict of interest, Sylvia Opanga declares no conflict of interest. Ethical clearance Ethical approval was sought from the Kenyatta National Hospital/University of Nairobi Ethics and Research committee (KNH/UON-ERC) and approval (Ref: KNH-ERC/A/119) was granted on 4th April Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License ( which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. References 1. Roujeau J, Kelly J, Naldi L, Rzany B, Stern R, Anderson T, et al. Medication use and the risk of Stevens Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333: Harr T, French L. Toxic epidermal necrolysis and Stevens Johnson syndrome. Orphanet J Rare Dis. 2010;5: Patel T, Thakkah S, Sharmar D. Cutaneous adverse drug reactions in Indian population: a systematic review. Indian Dermatol Online J. 2014;5: Schopf E, Stuhmer A, Rzany B, Victor N, Zentgraf R, Kapp J. Toxic epidermal necrolysis and Stevens Johnson syndrome: an epidemiologic study from West Germany. Arch Dermatol. 1991;127: Yamane Y, Matsukura S, Watanabe Y, Yamaguchi Y. Retrospective analysis of Stevens Johnson syndrome and toxic epidermal necrolysis in 87 Japanese patients: treatment and outcome. Allergol Int. 2016;65: Saka B, Barro-traore F, Atadokpe FA, Niamba PA, Ade H, Pitche VP, et al. Tropical medicine rounds Stevens Johnson syndrome and toxic epidermal necrolysis in sub-saharan Africa: a multicentric study in four countries. Int J Dermatol. 2013;52: Clinard V. Drug-induced skin disorders. In: US Pharmacist Accessed 10 Dec Roujeau J, Stern R. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994;331: Naranjo C, Busto U, Sellers E, Sandor P, Ruiz I, Roberts E, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30: Chan H-L. The incidence of erythema multiforme, Stevens Johnson syndrome, and toxic epidermal necrolysis. Arch Dermatol Am Med Assoc. 1990;126: Yamane Y, Aihara M, Ikezawa Z. Analysis of Stevens Johnson syndrome and toxic epidermal necrolysis in Japan from 2000 to Allergol Int. 2007;56: Kizito M. Assessment of the patient factors impacting on oral anticoagulation therapy among adult outpatients at Kenyatta national hospital. [MPharm Thesis]. University of Nairobi, Bastuji-garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau J. Clinical classification of cases of toxic epidermal necrolysis, Stevens Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129: Haddad C, Sidoroff A, Kardaun SH, Mockenhaupt M, Creamer D, Dunant A, et al. Stevens Johnson syndrome/toxic epidermal necrolysis: are drug dictionaries correctly informing physicians regarding the risk? Drug Saf. 2013;36: Levi N, Bastuji-Garin S, Mockenhaupt M, Roujeau J-C, Flahault A, Kelly JP, et al. Medications as risk factors of Stevens Johnson syndrome and toxic epidermal necrolysis in children: a pooled analysis. Pediatrics. 2009;123: Sontheimer R, Garibaldi R, Gerald G, Krueger M. Stevens Johnson syndrome associated with Mycoplasma pneumoniae infections. Arch Dermatol. 1978;114: Garcia-Doval I, LeCleach L, Bocquet H, Otero X, Roujeau J-C. Toxic epidermal necrolysis and Stevens Johnson syndrome. Arch Dermatol Am Med Assoc. 2000;136: Kilmarx PH. Global epidemiology of HIV. Curr Opin I HIV AIDS. 2009;4: Malaria Control Programme. Epidemiology of malaria in Kenya. Afr J Med Pract. 1994;1: Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Investig Dermatol. The Society for Investigative Dermatology, Inc. 2008;128: Wafula E, Abinya N, Karanja J, Kaseje D, Musoke R. Clinical guidelines for management and referral of common conditions at levels 4 6 hospitals, vol. III. Nairobi: Ministry of Medical Services and Ministry of Public Health and Sanitation; 2009.
Correspondence should be addressed to Wanjarus Roongpisuthipong; rr
 Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Prevalence and pattern of adverse cutaneous drug reactions presenting to a tertiary care hospital
 International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME
 EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course
 Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Original Article. Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H 1, Tripathi C
 Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah
CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
PEER REVIEW HISTORY ARTICLE DETAILS VERSION 1 - REVIEW
 PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive Patients
 Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome
 Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Department of Pharmacology & NIPER-Guwahati, Gauhati Medical College & Hospital, Guwahati , Assam, INDIA. 3
 J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review
 Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
ADVERSE DRUG REACTIONS AMONG HIV INFECTED AND UNINFECTED ADULTS RECEIVING ANTi-TUBERCULOUS THERAPY AT KENYATTA NATIONAL HOSPITAL
 October 2011 Ea s t Af r i c a n Me d i c a l Jo u r n a l 327 East African Medical Journal Vol. 88 No. 10 October 2011 ADVERSE DRUG REACTIONS AMONG HIV INFECTED AND UNINFECTED ADULTS RECEIVING ANTI- TUBERCULOUS
October 2011 Ea s t Af r i c a n Me d i c a l Jo u r n a l 327 East African Medical Journal Vol. 88 No. 10 October 2011 ADVERSE DRUG REACTIONS AMONG HIV INFECTED AND UNINFECTED ADULTS RECEIVING ANTI- TUBERCULOUS
cipro loxacin; stevens-johnson syndrome; urinary tract infection; adverse drug reaction
 Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
The liver and skin are two of the commonest
 AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
 Case Reports in Immunology Volume 2012, Article ID 931528, 4 pages doi:10.1155/2012/931528 Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
Case Reports in Immunology Volume 2012, Article ID 931528, 4 pages doi:10.1155/2012/931528 Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
allergy Asia Pacific Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from
 pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
Dilantin (phenytoin) ROBERT A. SCHWARTZ
 Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review
 HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis: a case report
 Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review
 Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review Review question(s) To carry out a systematic review of the published evidence of the cutaneous adverse drug reactions in
Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review Review question(s) To carry out a systematic review of the published evidence of the cutaneous adverse drug reactions in
Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes
 Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Pediatric Dermatology
 Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital
 Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review
 140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from 2000 to 2006 Yumiko Yamane 1, Michiko Aihara 1 and Zenro Ikezawa 1
 Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
PREVALENCE OF HIV INFECTION AND RISK FACTORS OF TUBERCULIN INFECTION AMONG HOUSEHOLD CONTACTS IN AN HIV EPIDEMIC AREA: CHIANG RAI PROVINCE, THAILAND
 JOURNAL OF SCIENCE, Hue University, N 0 61, 2010 PREVALENCE OF HIV INFECTION AND RISK FACTORS OF TUBERCULIN INFECTION AMONG HOUSEHOLD CONTACTS IN AN HIV EPIDEMIC AREA: CHIANG RAI PROVINCE, THAILAND Pornnapa
JOURNAL OF SCIENCE, Hue University, N 0 61, 2010 PREVALENCE OF HIV INFECTION AND RISK FACTORS OF TUBERCULIN INFECTION AMONG HOUSEHOLD CONTACTS IN AN HIV EPIDEMIC AREA: CHIANG RAI PROVINCE, THAILAND Pornnapa
Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients
 original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT
 OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
Stevens-Johnson Syndrome in patients receiving cranial irradiation and concomitant diphenylhydantoin
 Turkish Journal of Cancer Vol. 32/ No. 4/2002 Stevens-Johnson Syndrome in patients receiving cranial irradiation and concomitant diphenylhydantoin ERKAN TOPKAN, FARUK ZORLU, MURAT GÜRKAYNAK Hacettepe University
Turkish Journal of Cancer Vol. 32/ No. 4/2002 Stevens-Johnson Syndrome in patients receiving cranial irradiation and concomitant diphenylhydantoin ERKAN TOPKAN, FARUK ZORLU, MURAT GÜRKAYNAK Hacettepe University
The relationship between adherence to clinic appointments and year-one mortality for HIV infected patients at a Referral Hospital in Western Kenya
 The relationship between adherence to clinic appointments and year-one mortality for HIV infected patients at a Referral Hospital in Western Kenya Muthusi, K; Burmen, B 8th International Workshop on HIV
The relationship between adherence to clinic appointments and year-one mortality for HIV infected patients at a Referral Hospital in Western Kenya Muthusi, K; Burmen, B 8th International Workshop on HIV
Cutaneous drug reactions
 Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea
 Original Article Yonsei Med J 2019 Feb;60(2):208-215 pissn: 0513-5796 eissn: 1976-2437 Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea Min-Gyu Kang 1,2,3, Kyung-Hee
Original Article Yonsei Med J 2019 Feb;60(2):208-215 pissn: 0513-5796 eissn: 1976-2437 Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea Min-Gyu Kang 1,2,3, Kyung-Hee
Erythema Multiforme with Reference to Atypical Presentation in an HIV-Positive Patient Following Antiretroviral Therapy Discontinuation
 2009;17(1):9-15 CLINICAL ARTICLE Erythema Multiforme with Reference to Atypical Presentation in an HIV-Positive Patient Following Antiretroviral Therapy Discontinuation Liborija Lugović Mihić, Marija Buljan,
2009;17(1):9-15 CLINICAL ARTICLE Erythema Multiforme with Reference to Atypical Presentation in an HIV-Positive Patient Following Antiretroviral Therapy Discontinuation Liborija Lugović Mihić, Marija Buljan,
Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics
 MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
 BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
BRIEF COMMUNICATION http://dx.doi.org/10.4110/in.2016.16.4.256 pissn 1598-2629 eissn 2092-6685 Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Associated with Acetaminophen Use during Viral Infections
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC
 SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
Determinants of Discontinuation of Contraceptive Methods among Women at Kenyatta National Hospital, Kenya
 African Journal of Pharmacology and Therapeutics Vol. 5 No. 1 Pages 28-34, 2016 Open Access to full text available at http://journals.uonbi.ac.ke/ajpt Research Article Determinants of Discontinuation of
African Journal of Pharmacology and Therapeutics Vol. 5 No. 1 Pages 28-34, 2016 Open Access to full text available at http://journals.uonbi.ac.ke/ajpt Research Article Determinants of Discontinuation of
Emergency Dermatology Dr Melissa Barkham
 Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Stevens-Johnson syndrome (SJS) and toxic epidermal. necrolysis in Asian children
 Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children Mark Jean-Aan Koh, MD, and Yong-Kwang Tay, MD Singapore Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children Mark Jean-Aan Koh, MD, and Yong-Kwang Tay, MD Singapore Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update
 Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
Adverse Drug Reactions (ADRs) Outline
 Adverse Drug Reactions (ADRs) Outline 1. What are Adverse Drug Reactions (ADRs)? WHAT WHY HOW 2. How important are ADRs and are they preventable? 3. What are the classifications and mechanisms of ADRs?
Adverse Drug Reactions (ADRs) Outline 1. What are Adverse Drug Reactions (ADRs)? WHAT WHY HOW 2. How important are ADRs and are they preventable? 3. What are the classifications and mechanisms of ADRs?
Prevalence of tuberculosis (TB) infection and disease among adolescents western Kenya: preparation for future TB vaccine trials
 Prevalence of tuberculosis (TB) infection and disease among adolescents western Kenya: preparation for future TB vaccine trials Videlis Nduba 1,2, Peter Onyango 1, Anja Van t Hoog 1,3, Anthony Hawkridge
Prevalence of tuberculosis (TB) infection and disease among adolescents western Kenya: preparation for future TB vaccine trials Videlis Nduba 1,2, Peter Onyango 1, Anja Van t Hoog 1,3, Anthony Hawkridge
Dr. Mercy Maina, Bpharm Pharmacovigilance Pharmacist USAID- AMPATH 1/17/2014 1
 Dr. Mercy Maina, Bpharm Pharmacovigilance Pharmacist USAID- AMPATH 1 Provide a brief description of AMPATH Give a background and description of the project Review the preliminary results Discuss the challenges
Dr. Mercy Maina, Bpharm Pharmacovigilance Pharmacist USAID- AMPATH 1 Provide a brief description of AMPATH Give a background and description of the project Review the preliminary results Discuss the challenges
Future of Pediatrics: Blisters, Hives and Other Tales from the Emergency Room June 14 th, 2016
 A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW
 STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
Scaling up priority HIV/AIDS interventions in the health sector
 TOWARDS UNIVERSAL ACCESS? Scaling up priority HIV/AIDS interventions in the health sector Yves Souteyrand, WHO October 2011 Towards universal access targets UN General Assembly High level Meeting June
TOWARDS UNIVERSAL ACCESS? Scaling up priority HIV/AIDS interventions in the health sector Yves Souteyrand, WHO October 2011 Towards universal access targets UN General Assembly High level Meeting June
A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash
 CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS
 PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
Risk of Stevens Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics
 Risk of Stevens Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics Maja Mockenhaupt, MD; John Messenheimer, MD; Pat Tennis, PhD; and Juergen Schlingmann Abstract Background:
Risk of Stevens Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics Maja Mockenhaupt, MD; John Messenheimer, MD; Pat Tennis, PhD; and Juergen Schlingmann Abstract Background:
Bugs and Drugs: What s New in Hypersensitivity Reactions?
 Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Bugs and Drugs: What s New in Hypersensitivity Reactions? Erin Mathes, MD Associate Professor of Dermatology and Pediatrics University of California, San Francisco DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY
Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: Assessment of Medication Risks with Emphasis on Recently Marketed Drugs. The EuroSCAR-Study
 ORIGINAL ARTICLE Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: Assessment of Medication Risks with Emphasis on Recently Marketed Drugs. The EuroSCAR-Study Maja Mockenhaupt 1, Cecile Viboud 2,
ORIGINAL ARTICLE Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: Assessment of Medication Risks with Emphasis on Recently Marketed Drugs. The EuroSCAR-Study Maja Mockenhaupt 1, Cecile Viboud 2,
Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015)
 1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
1 of 5 Agenda* The 9th International Congress on Cutaneous Adverse Drug Reactions (iscar 2015) Co-chairs: Dr. Neil H. Shear at the 23rd World Congress of Dermatology in Vancouver, Canada. Division of Dermatology,
AOU Ospedali Riuniti - Ancona
 AOU Ospedali Riuniti - Ancona Ospedale Materno-Infantile di Alta Specializzazione G. Salesi UOC Pediatria Allergia a farmaci e infezioni: tra coesistenza e casualità fabrizio franceschini Drug Hypersensitivity
AOU Ospedali Riuniti - Ancona Ospedale Materno-Infantile di Alta Specializzazione G. Salesi UOC Pediatria Allergia a farmaci e infezioni: tra coesistenza e casualità fabrizio franceschini Drug Hypersensitivity
Research Article Challenges in Assessing Outcomes among Infants of Pregnant HIV-Positive Women Receiving ART in Uganda
 Hindawi AIDS Research and Treatment Volume 2017, Article ID 3202737, 4 pages https://doi.org/10.1155/2017/3202737 Research Article Challenges in Assessing Outcomes among Infants of Pregnant HIV-Positive
Hindawi AIDS Research and Treatment Volume 2017, Article ID 3202737, 4 pages https://doi.org/10.1155/2017/3202737 Research Article Challenges in Assessing Outcomes among Infants of Pregnant HIV-Positive
A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit
 J Korean Surg Soc 0;:133-139 DOI:.414/jkss.0..3.133 원 저 A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit Departments of Surgery
J Korean Surg Soc 0;:133-139 DOI:.414/jkss.0..3.133 원 저 A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit Departments of Surgery
Influenza Update N 159
 Influenza Update N 159 10 May 2012 Summary The seasonal peak for influenza has passed in most countries in the temperate regions of the northern hemisphere. Different viruses have predominated in different
Influenza Update N 159 10 May 2012 Summary The seasonal peak for influenza has passed in most countries in the temperate regions of the northern hemisphere. Different viruses have predominated in different
Skin Manifestations of Drug Reactions
 Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection
 Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
Case Report Carbamazepine-Induced Incomplete Stevens-Johnson Syndrome: Report of a Case in Children without Mycoplasma pneumoniae Infection J Med Assoc Thai 2015; 98 (Suppl. 7): S243-S247 Full text. e-journal:
A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia
 ORIGINAL ARTICLE A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Chai Har Loo, MRCP, Wooi Chiang Tan, AdvMDerm, Yek Huan Khor, AdvMDerm,
ORIGINAL ARTICLE A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Chai Har Loo, MRCP, Wooi Chiang Tan, AdvMDerm, Yek Huan Khor, AdvMDerm,
Spontaneous reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antiepileptic drugs
 European Review for Medical and Pharmacological Sciences Spontaneous reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antiepileptic drugs L. ORDOÑEZ, E. SALGUEIRO, F.J.
European Review for Medical and Pharmacological Sciences Spontaneous reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antiepileptic drugs L. ORDOÑEZ, E. SALGUEIRO, F.J.
Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate
 Original Research Article Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate Manab Nandy 1, Sangeeta De 2*, Mustafa Asad 2, Nirmal
Original Research Article Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate Manab Nandy 1, Sangeeta De 2*, Mustafa Asad 2, Nirmal
Chapter 6 PHARMACOVIGELANCE. M.G.Rajanandh, Department of Pharmacy Practice, SRM College of Pharmacy, SRM University.
 Chapter 6 PHARMACOVIGELANCE M.G.Rajanandh, Department of Pharmacy Practice, SRM College of Pharmacy, SRM University. DEFENITION: Pharmacovigilance (abbreviated PV or PhV) is the Pharmacological science
Chapter 6 PHARMACOVIGELANCE M.G.Rajanandh, Department of Pharmacy Practice, SRM College of Pharmacy, SRM University. DEFENITION: Pharmacovigilance (abbreviated PV or PhV) is the Pharmacological science
Drug Allergy A Guide to Diagnosis and Management
 Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
THE PATTERN OF HEARING DISORDERS IN HIV POSITIVE PATIENTS ON ANTI - RETROVIRALS AT KENYATTA NATIONAL HOSPITAL
 October 2010 Ea s t Af r i c a n Me d i c a l Jo u r n a l 425 East African Medical Journal Vol. 87 No. 10 October 2010 THE PATTERN OF HEARING DISORDERS IN HIV POSITIVE PATIENTS ON ANTI - RETROVIRALS AT
October 2010 Ea s t Af r i c a n Me d i c a l Jo u r n a l 425 East African Medical Journal Vol. 87 No. 10 October 2010 THE PATTERN OF HEARING DISORDERS IN HIV POSITIVE PATIENTS ON ANTI - RETROVIRALS AT
Unsupervised activity is a major risk factor for traumatic coma and its age-specific
 The assessment of patients in coma is a medical emergency. The cause should be identified and, where possible, corrected and the brain provided with appropriate protection to reduce further damage. It
The assessment of patients in coma is a medical emergency. The cause should be identified and, where possible, corrected and the brain provided with appropriate protection to reduce further damage. It
Stevens-Johnson Syndrome as an Unusual Adverse Effect of Azithromycin
 2006;14(1):40-45 CASE REPORT Stevens-Johnson Syndrome as an Unusual Adverse Effect of Azithromycin Neva Brkljačić, Sonja Gracin, Ingrid Prkačin, Mirjana Sabljar-Matovinović, Anna Mrzljak, Zlatka Nemet
2006;14(1):40-45 CASE REPORT Stevens-Johnson Syndrome as an Unusual Adverse Effect of Azithromycin Neva Brkljačić, Sonja Gracin, Ingrid Prkačin, Mirjana Sabljar-Matovinović, Anna Mrzljak, Zlatka Nemet
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW
 SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
SKIN REACTIONS WITH PSYCHOTROPICS: A SYSTEMATIC REVIEW *Anderson Isaac, PharmD Candidate, 2019 Pooja Patel, PharmD Candidate, 2019 Katelyn Thomasson, PharmD Candidate, 2019 Erika Tillery, PharmD, BCPP,
PedsCases Podcast Scripts
 PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on Drug Allergy. These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio
Cutaneous Conditions Associated with Systemic Disease
 Cutaneous Conditions Associated with Systemic Disease Johnnie M Woodson, M.D., F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates,
Cutaneous Conditions Associated with Systemic Disease Johnnie M Woodson, M.D., F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates,
Address for correspondence: Dr. Patel Raksha M. R-3, Doctor s Quarters, Jail Road, Vadodara- 01, Gujarat, India.
 Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
STEVENS-JOHNSON SYNDROME: A CASE REPORT
 STEVENS-JOHNSON SYNDROME: A CASE REPORT Castana O., Rempelos G., Anagiotos G., Apostolopoulou C., Dimitrouli A., Alexakis D. Department of Plastic and Reconstructive Surgery, Evangelismos General Hospital,
STEVENS-JOHNSON SYNDROME: A CASE REPORT Castana O., Rempelos G., Anagiotos G., Apostolopoulou C., Dimitrouli A., Alexakis D. Department of Plastic and Reconstructive Surgery, Evangelismos General Hospital,
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES. R e g i S C A R PATIENT'S DATA. Age country of birth
 REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
Global, National, Regional
 Epidemiology of TB: Global, National, Regional September 13, 211 Edward Zuroweste, MD Chief Medical Officer Migrant Clinicians Network Assistant Professor of Medicine Johns Hopkins School of Medicine Epidemiology
Epidemiology of TB: Global, National, Regional September 13, 211 Edward Zuroweste, MD Chief Medical Officer Migrant Clinicians Network Assistant Professor of Medicine Johns Hopkins School of Medicine Epidemiology
University of Groningen
 University of Groningen Toxic epidermal necrolysis, DRESS, AGEP Bouvresse, Sophie; Valeyrie-Allanore, Laurence; Ortonne, Nicolas; Konstantinou, Marie Pauline; Kardaun, Sylvia H.; Bagot, Martine; Wolkenstein,
University of Groningen Toxic epidermal necrolysis, DRESS, AGEP Bouvresse, Sophie; Valeyrie-Allanore, Laurence; Ortonne, Nicolas; Konstantinou, Marie Pauline; Kardaun, Sylvia H.; Bagot, Martine; Wolkenstein,
Date of study period: April 12 May 7, 2010 and August September, 2010
 Classification of antiretroviral therapy failure using immunologic and clinical versus virologic monitoring in HIV-infected children and adolescents in Cambodia Date of study period: April 12 May 7, 2010
Classification of antiretroviral therapy failure using immunologic and clinical versus virologic monitoring in HIV-infected children and adolescents in Cambodia Date of study period: April 12 May 7, 2010
PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN
 PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN Summary of Risk Management Plan for REZOLSTA This is a summary of the risk management plan (RMP) for REZOLSTA. The RMP details important risks of REZOLSTA,
PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN Summary of Risk Management Plan for REZOLSTA This is a summary of the risk management plan (RMP) for REZOLSTA. The RMP details important risks of REZOLSTA,
International Journal of Basic & Clinical Pharmacology. Profile of cutaneous adverse drug reactions of carbamazepine
 Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20175211 Original Research Article Profile of cutaneous
Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20175211 Original Research Article Profile of cutaneous
Severe cutaneous adverse reactions (SCAR) are life-threatening
 Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Conference Item (paper)
 Eliud Wekesa and Ernestina Coast "Just like a taste of water which is too little to quench the thirst": condom use among people living with HIV/AIDS in Nairobi urban slums Conference Item (paper) Original
Eliud Wekesa and Ernestina Coast "Just like a taste of water which is too little to quench the thirst": condom use among people living with HIV/AIDS in Nairobi urban slums Conference Item (paper) Original
Toxic epidermal necrolysis and Stevens Johnson syndrome in South Africa: a 3-year prospective study
 Q J Med 2012; 105:839 846 doi:10.1093/qjmed/hcs078 Advance Access Publication 28 April 2012 Toxic epidermal necrolysis and Stevens Johnson syndrome in South Africa: a 3-year prospective study S.M.H. KANNENBERG
Q J Med 2012; 105:839 846 doi:10.1093/qjmed/hcs078 Advance Access Publication 28 April 2012 Toxic epidermal necrolysis and Stevens Johnson syndrome in South Africa: a 3-year prospective study S.M.H. KANNENBERG
Repellent Soap. The Jojoo Mosquito. Africa s innovative solution to Malaria prevention. Sapphire Trading Company Ltd
 The Jojoo Mosquito Repellent Soap Africa s innovative solution to Malaria prevention Sapphire Trading Company Ltd P.O.Box: 45938-00100 Nairobi, Kenya. Tel: +254 735 397 267 +254 733 540 868 +254 700 550
The Jojoo Mosquito Repellent Soap Africa s innovative solution to Malaria prevention Sapphire Trading Company Ltd P.O.Box: 45938-00100 Nairobi, Kenya. Tel: +254 735 397 267 +254 733 540 868 +254 700 550
Cutaneous Drug Reactions
 Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
Long-term Sequelae of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis
 Acta Derm Venereol 2016; 96: 525 529 CLINICAL REPORT Long-term Sequelae of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis Che-Wen YANG 1, Yung-Tsu CHO 1, Kai-Lung CHEN 1, Yi-Chun CHEN 1,2, Hsiang-Lin
Acta Derm Venereol 2016; 96: 525 529 CLINICAL REPORT Long-term Sequelae of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis Che-Wen YANG 1, Yung-Tsu CHO 1, Kai-Lung CHEN 1, Yi-Chun CHEN 1,2, Hsiang-Lin
Journal of Global Trends in Pharmaceutical Sciences
 An Elsevier Indexed Journal ISSN-2230-7346 Journal of Global Trends in Pharmaceutical Sciences A CASE STUDY ON HERBAL THERAPY INDUCED ERYTHEMA MULTIFORME S. Showkath Ali * 1, S. Priyanka 2, Bandla Aswani
An Elsevier Indexed Journal ISSN-2230-7346 Journal of Global Trends in Pharmaceutical Sciences A CASE STUDY ON HERBAL THERAPY INDUCED ERYTHEMA MULTIFORME S. Showkath Ali * 1, S. Priyanka 2, Bandla Aswani
TITLE: SAFE USE OF MEDICINES IN ZANZIBAR A
 Table of content Acknowledgements Introduction TITLE: SAFE USE OF MEDICINES IN ZANZIBAR A guide for health professionals in detecting and reporting adverse drug reaction. (Beautiful photograph tablets
Table of content Acknowledgements Introduction TITLE: SAFE USE OF MEDICINES IN ZANZIBAR A guide for health professionals in detecting and reporting adverse drug reaction. (Beautiful photograph tablets
Clinical profile of skin diseases in accident and emergency department attenders
 Hong Kong J. Dermatol. Venereol. (2007) 15, 4-9 Original Article Clinical profile of skin diseases in accident and emergency department attenders CY Chan, KL Kam, CA Graham, TH Rainer, NM Luk Skin problems
Hong Kong J. Dermatol. Venereol. (2007) 15, 4-9 Original Article Clinical profile of skin diseases in accident and emergency department attenders CY Chan, KL Kam, CA Graham, TH Rainer, NM Luk Skin problems
Accepted Manuscript. Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need
 Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
Antibiotic Consumption in the Community in Ireland REPORT FOR First Half of 2013
 Antibiotic Consumption in the Community in Ireland REPORT FOR First Half of 2013 1 Summary: Community Antibiotic Use The rate for Quarter 1 of 2013 (1 st January to 31 st March) was 27.4 Defined Daily
Antibiotic Consumption in the Community in Ireland REPORT FOR First Half of 2013 1 Summary: Community Antibiotic Use The rate for Quarter 1 of 2013 (1 st January to 31 st March) was 27.4 Defined Daily
DETERMINANTS OF CERVICAL CANCER SCREENING PRACTICE AMONG WOMEN OF REPRODUCTIVE AGE IN NYARIBARI CHACHE SUB-COUNTY
 DETERMINANTS OF CERVICAL CANCER SCREENING PRACTICE AMONG WOMEN OF REPRODUCTIVE AGE IN NYARIBARI CHACHE SUB-COUNTY 1 Godner Bwari Peter, 1 Rose Olayo (PhD), 1 George Ayodo (PhD) 1 School of Health Science,
DETERMINANTS OF CERVICAL CANCER SCREENING PRACTICE AMONG WOMEN OF REPRODUCTIVE AGE IN NYARIBARI CHACHE SUB-COUNTY 1 Godner Bwari Peter, 1 Rose Olayo (PhD), 1 George Ayodo (PhD) 1 School of Health Science,
Herpes Simplex Viruses: Disease Burden. Richard Whitley The University of Alabama at Birmingham Herpes Virus Infection and Immunity June 18-20, 2012
 Herpes Simplex Viruses: Disease Burden Richard Whitley The University of Alabama at Birmingham Herpes Virus Infection and Immunity June 18-20, 2012 Mucocutaneous HSV Infections Life-Threatening HSV Diseases
Herpes Simplex Viruses: Disease Burden Richard Whitley The University of Alabama at Birmingham Herpes Virus Infection and Immunity June 18-20, 2012 Mucocutaneous HSV Infections Life-Threatening HSV Diseases
DERMATOLOGIC EMERGENCIES. Mary Evers D.O., F.A.O.C.D. Georgetown, Texas
 DERMATOLOGIC EMERGENCIES Mary Evers D.O., F.A.O.C.D. Georgetown, Texas SKIN EMERGENCIES??? Subclassifications: Autoimmune (Anaphylaxis, Vasculitis, Pemphigus) Erythroderma (AGEP, DRESS, SJS, TEN) Infectious
DERMATOLOGIC EMERGENCIES Mary Evers D.O., F.A.O.C.D. Georgetown, Texas SKIN EMERGENCIES??? Subclassifications: Autoimmune (Anaphylaxis, Vasculitis, Pemphigus) Erythroderma (AGEP, DRESS, SJS, TEN) Infectious
The human immunodeficiency virus/acquired immune
 Vol. 7(4), pp. 44-48, May 2015 DOI: 10.5897/JAHR2014.0320 Article Number: 70F676253212 ISSN 2141-2359 Copyright 2015 Author(s) retain the copyright of this article http://www.academicjournals.org/jahr
Vol. 7(4), pp. 44-48, May 2015 DOI: 10.5897/JAHR2014.0320 Article Number: 70F676253212 ISSN 2141-2359 Copyright 2015 Author(s) retain the copyright of this article http://www.academicjournals.org/jahr
Alvarado vs Lintula Scoring Systems in Acute Appendicitis
 ORIGINAL ARTICLE Alvarado vs Lintula Scoring Systems in Acute Appendicitis Daniel Ojuka, Mike Sangoro School of Medicine, University of Nairobi Correspondence to: Dr. Daniel Ojuka, P.O. Box 19762 00202,
ORIGINAL ARTICLE Alvarado vs Lintula Scoring Systems in Acute Appendicitis Daniel Ojuka, Mike Sangoro School of Medicine, University of Nairobi Correspondence to: Dr. Daniel Ojuka, P.O. Box 19762 00202,
Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review
 Case Report Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review Deepali P. Mohite*, Satyajitraje Tekade*, Amol Gadbail*, M. S. Chaudhary** Abstract : Toxic epidermal necrolysis (TEN) and Stevens
Case Report Toxic Epidermal Necrolysis and SJS : Case Reports & Brief Review Deepali P. Mohite*, Satyajitraje Tekade*, Amol Gadbail*, M. S. Chaudhary** Abstract : Toxic epidermal necrolysis (TEN) and Stevens
Impact of Counseling on Patient Caretaker s Knowledge and Medication Adherence to Paediatric Antiepileptic Drug Therapy
 International Journal of Science and Healthcare Research Vol.3; Issue: 4; Oct.-Dec. 2018 Website: www.ijshr.com Original Research Article ISSN: 2455-7587 Impact of Counseling on Patient Caretaker s Knowledge
International Journal of Science and Healthcare Research Vol.3; Issue: 4; Oct.-Dec. 2018 Website: www.ijshr.com Original Research Article ISSN: 2455-7587 Impact of Counseling on Patient Caretaker s Knowledge
Guidelines for management of suspected sepsis in young infants where referral is not possible
 Guidelines for management of suspected sepsis in young infants where referral is not possible Kenya Paediatrics Association Conference, 26-29 April 2016, Eldoret Kenya 1 Kenya Paediatrics Association Conference,
Guidelines for management of suspected sepsis in young infants where referral is not possible Kenya Paediatrics Association Conference, 26-29 April 2016, Eldoret Kenya 1 Kenya Paediatrics Association Conference,
Global, National, Regional
 Epidemiology of TB: Global, National, Regional September 13, 211 Edward Zuroweste, MD Chief Medical Officer Migrant Clinicians Network Assistant Professor of Medicine Johns Hopkins School of Medicine Epidemiology
Epidemiology of TB: Global, National, Regional September 13, 211 Edward Zuroweste, MD Chief Medical Officer Migrant Clinicians Network Assistant Professor of Medicine Johns Hopkins School of Medicine Epidemiology
Toxic epidermal necrolysis
 REVIEW Anaesthesiology Intensive Therapy 2015, vol. 47, no 3, 257 262 ISSN 0209 1712 10.5603/AIT.2015.0037 www.ait.viamedica.pl Toxic epidermal necrolysis Joanna Hinc-Kasprzyk, Agnieszka Polak-Krzemińska,
REVIEW Anaesthesiology Intensive Therapy 2015, vol. 47, no 3, 257 262 ISSN 0209 1712 10.5603/AIT.2015.0037 www.ait.viamedica.pl Toxic epidermal necrolysis Joanna Hinc-Kasprzyk, Agnieszka Polak-Krzemińska,
Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening
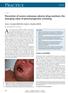 CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
Running head: MEDIA AND HIV/AIDS STIGMA IN KENYA. Mass media and HIV/AIDS stigma among women in urban and rural areas: Findings from
 Running head: MEDIA AND HIV/AIDS STIGMA IN KENYA Mass media and HIV/AIDS stigma among women in urban and rural areas: Findings from the 2008/09 Kenya Demographic and Health Survey Elijah O. Onsomu 1 *,
Running head: MEDIA AND HIV/AIDS STIGMA IN KENYA Mass media and HIV/AIDS stigma among women in urban and rural areas: Findings from the 2008/09 Kenya Demographic and Health Survey Elijah O. Onsomu 1 *,
