Original Article. Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H 1, Tripathi C
|
|
|
- Gillian Owens
- 6 years ago
- Views:
Transcription
1
2 Original Article Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H 1, Tripathi C Department of Pharmacology, Government Medical College, Bhavnagar, 1 Department of Dermatology, Venerology and Leprosy, Sir Takhtsinhji General Hospital and Government Medical College, Bhavnagar, Gujarat, India Address for correspondence: Dr. C.B. Tripathi, cbrtripathi@yahoo. co.in Received : Review completed : Accepted : ABSTRACT Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare immunemediated severe cutaneous adverse reactions with incidence rate of 0.05 to 2 persons per million populations per year. Drugs are the most commonly implicated in 95% of cases. Aims: To audit the causative drugs, clinical outcome, and cost of management in SJS, TEN, and SJS-TEN overlap. Setting and Design: Tertiary care hospitals-based multicentric retrospective study (case series). Materials and Methods: Indoor case papers of SJS, TEN, and SJS-TEN overlap admitted between January 2006 and December 2009 in four tertiary care hospitals of Gujarat were scrutinized. Data were collected for demographic information, causative drugs, investigations, treatment given, duration of hospital stay, time interval between onset of symptoms and drug intake, clinical outcome, and complications. Data were analyzed to find out proportion of individual drugs responsible, major complications, and clinical outcome in SJS, TEN, and SJS-TEN overlap. Total cost of management was calculated by using cost of drugs, investigations, and consumables used during entire hospital stay. Statistical Analysis: One-way Analysis of Variance followed by Tukey-Kramer multiple comparison test was used for comparison of incubation period, duration of hospital stay, and cost of management. Results: Antimicrobials (50%), nonsteroidal anti-inflammatory drugs (22.41%), and antiseizure drugs (18.96%) were the most commonly associated groups. Nevirapine (28.12%) was the most common drug. Antiseizure drugs were more often associated with serious form of adverse reaction (TEN: 81.8%) than other drugs. Duration of hospital stay (20.6 vs 9.7 days) and cost of management (` 7 910/- vs ` 2 460/-) were significantly higher in TEN than SJS (P=0.020 and P<0.001, respectively). Time duration between drug intake and onset of symptoms (17.7 vs 27.5 days) was nonsignificantly lower in TEN as compared with SJS. Secondary infection (28.12%) was the most common complication noted. Mortality rate was 15.6% among all cases; 9% in SJS and 26.7% in TEN. Conclusion: Antimicrobial drugs are the most commonly implicated drugs and cost of managing these adverse drug reactions is higher than other serious ADRs. KEY WORDS: Adverse drug reaction, Stevens-Johnson syndrome, toxic epidermal necrolysis A dverse Quick Response Code: Introduction drug reactions (ADRs) hold special importance in healthcare as they account for 6% of total hospital Access this article online Website: DOI: / PubMed ID: Journal of Postgraduate Medicine April 2011 Vol 57 Issue 2 *** admissions, increase in economic burden on healthcare system, withdrawal of drugs from market, and death. [1-3] Among the various ADRs, cutaneous adverse reactions like skin rashes, urticaria, itching, fixed drug eruption, angioedema, erythema multiforme, Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN) are the common ones. [4,5] In this wide spectrum of cutaneous reactions, SJS and TEN are rare but serious form of ADRs affecting patient s life. [4] SJS and TEN are immune-mediated reactions, due to various etiological factors like drugs, infections, malignancy, and radiation therapy. [5] Drugs are the most commonly implicated in 95% of cases. [5] Incidence of SJS and TEN is 0.05 to 2 persons per million populations per year. [6,7] Incidence is higher in HIV patients than general 115
3 population. [8] Clinically, SJS and TEN are characterized by polymorphic lesions like erythematous macules, papules, plaque, vesicles, and bullae predilected to distal extremities with Nikolsky s sign positive. Target lesion with bull s eye appearance is characteristic of SJS and TEN. Oral, genital, and conjunctival mucosa is often involved in the form of erosion or ulceration. [9] The basic difference between SJS and TEN is the percentage of body surface area (BSA) involved: <10% in SJS; >30% in TEN; 10 to 30% in SJS-TEN overlap. [5,9] No confirmatory in vivo or in vitro tests are available for identifying offending agents in SJS and TEN. [9] Moreover, a rechallenge in a case is not ethically justified as these are the life-threatening reactions. So, to identify the drugs associated with risk of causing severe skin reactions, retrospective epidemiological data are required. This study was aimed to audit the causative drugs, clinical outcome, and economic burden due to these serious ADRs. This study also helped us to know the changing pattern of most common causative agents for SJS and TEN. Materials and Methods This multicentric study was started after approval from Institutional Review Board, Government Medical College, Bhavnagar, Gujarat. Permission for data collection at other centers was taken from the head of the respective institutions. Indoor case papers of SJS, TEN, and SJS-TEN overlap admitted between January 2006 and December 2009 in Sir Takhtsinhji General Hospital, Bhavnagar; Pandit Deendayal Upadhyay Civil Hospital, Rajkot; New Civil Hospital, Surat; and Sir Sayaji General Hospital, Vadodara were scrutinized. Data were collected for demographic information, causative drugs, time duration between drug intake and onset of symptoms, BSA involvement, duration of hospital stay, treatment given, complications, and clinical outcome. Cases with doubtful diagnosis and insufficient data were excluded from the study. Data were analyzed to find out the proportion of individual drugs involved, major complications, and cost of management for SJS, TEN, and SJS-TEN overlap. Total cost of management was calculated by using cost of drugs (average cost of drugs from IDR-triple i Drug Compendium), investigations, and consumables used during entire hospital stay. [10] Ward and nursing charges were not included in the management cost. SCORTEN score was calculated for each case at the time of admission and its relation with mortality was compared in the study. [11] Results are expressed as percentages and mean (95% confidence interval [CI]). Comparisons between the three groups were performed by using One-way Analysis of Variance (ANOVA) followed by Tukey-Kramer multiple comparison test. A P value of less than 0.05 was considered significant. All statistical analyses were performed with GraphPad InStat 3 (demo version). Results Of the 46 cases scrutinized, 32 cases were included in the study, whereas 14 cases were excluded from the study. Among the 32 cases, we found 11 cases of SJS, 15 cases of TEN, and 6 cases of SJS-TEN otverlap. Of the 32, 17 patients were male and 15 were female between age group of 7 to 60 years. Total 58 drugs were found as suspected causative agent. In 20 cases, multiple drugs were suspected, whereas in 12 cases, single drug was implicated. In all the 32 cases, drugs were the probable cause of eruptions, as determined by Naranjo s algorithm. [12] Antimicrobials (50%) were the most commonly associated group followed by nonsteroidal anti-inflammatory drugs (NSAIDs) (22.41%) and antiseizure drugs (18.96%) [Table 1]. Nevirapine (9 of 32 cases), paracetamol (8 of 32 cases), cotrimoxazole (7 of 32 cases), and phenytoin (7 of 32 cases) were found to be the most commonly associated drugs. Phenytoin (4 of 12 cases) was the most common drug among the single drug culprit cases. Associated diseases were present in 13 cases (HIV infection - 12 cases [37.5%] and malignancy - 1 case [3.1%]). In 12 HIV patients, nevirapine (9 of 12 cases), cotrimoxazole (6 of 12 cases), zidovudine (1 of 12 cases), and stavudine (1 of 12 cases) were found responsible for causing this reaction. Antiseizure drugs were more often associated with serious form of adverse reaction (TEN: 81.8%) than other drugs. Time duration between drug intake and onset of symptoms was 27.5 days (95% CI: ) in SJS, 17.7 days (95% CI: ) in TEN, and 15.3 days (95% CI: ) in SJS-TEN overlap. Table 1: Drug groups and individual drugs responsible for SJS, TEN, and SJS-TEN overlap Drugs SJS TEN SJS-TEN overlap Total Antimicrobials 12 (20.7) 10 (17.2) 07 (12) 29 (50) Cotrimoxazole Amoxicillin Erythromycin Quinine Ampicillin Cefixime Amikacin Gentamicin Levofloxacin Chloroquine Nevirapine Stavudine Zidovudine NSAIDs 03 (5.1) 07 (12) 03 (5.1) 13 (22.4) Paracetamol Nimesulide Diclofenac Aspirin Antiseizure 01 (1.7) 09 (15.5) 01 (1.7) 11 (19.0) Phenytoin Carbamazepine Phenobarbitone Others (8.6) (8.6) Ondansetron Domperidone Famotidine Pantoprazole Data are expressed in absolute numbers of cases and value expressed in () is in percentage; SJS: Stevens-Johnson syndrome; TEN: Toxic epidermal necrolysis; NSAIDs: Nonsteroidal anti-inflammatory drugs 116 Journal of Postgraduate Medicine April 2011 Vol 57 Issue 2
4 In treatment, systemic steroids were given in 8 of 11 cases of SJS, 12 of 15 cases of TEN, and 5 of 6 cases of SJS-TEN overlap, whereas intravenous immunoglobulin (IVIG) was given in 1 of 6 cases of SJS-TEN overlap. Duration of hospital stay was 9.7 days (95% CI: ) in SJS, 20.6 days (95% CI: ) in TEN, and 16.1 days (95% CI: ) in SJS-TEN overlap, while cost of management was ` 2 460/- (95% CI: ) in SJS, ` 7 910/-(95% CI: ) in TEN, and ` 4 857/- (95% CI: ) in SJS-TEN overlap. Duration of hospital stay and cost of management were significantly higher in TEN than in SJS (P=0.020 and P<0.001, respectively) [Table 2]. Various complications noted in cases of SJS, TEN, and SJS- TEN overlap were secondary infection, septicemia, altered liver function test, electrolytes imbalance, leucocytosis, leucopoenia, thrombocytopenia, hyperglycemia, respiratory distress, and acute renal failure. Secondary infection was the most common complication (9 of 32 cases) noted [Figure 1]. Mortality rate was 15.6% among all cases; 9% in SJS and 26.7% in TEN. Higher mortality rate was noticed in patients with higher SCORTEN score at the time of admission [Table 3]. Table 2: Comparison of incubation period, duration of hospital stay, and cost of management between SJS, TEN, and SJS-TEN overlap Groups Incubation period (days) Duration of hospital stay (days) Cost of management (`) SJS (n = 11) 27.5 ( ) 9.7 ( ) ( ) TEN (n = 15) 17.7 ( ) SJS-TEN overlap (n = 06) P value F (df) (ANOVA) 20.6 ( )* ( )** 15.3 ( ) 16.1 ( ) ( ) (2,29) (2,29) (2,29) Data expressed as mean (95% CI); *P=0.020 and **P<0.001 as compared with SJS by Tukey-Kramer multiple comparisons test; SJS: Stevens-Johnson syndrome; TEN: Toxic epidermal necrolysis Table 3: Comparison between SCORTEN score, mortality rate in present study, and expected mortality rate SCORTEN score at admission No. of cases Discussion We had male preponderance (53.1% male and 46.9% female), unlike female preponderance in earlier studies. [13-15] All the cases had same frequency of occurrence throughout the year, no seasonal variation was noted. [16] Three most common groups of drugs causing eruptions were antimicrobials, NSAIDs, and antiseizure drugs. This is in agreement with the previous reports. [13,14,17] Nevirapine (28.1%) was the most commonly associated drug in our study, as against phenytoin and carbamazepine which have been mentioned in previous study. [14,15] Antiseizure drugs were the most common drugs causing TEN in our study. They had the higher chance (81.8%) of causing severe eruption, that is, TEN than NSAIDs (53.84%) and antimicrobials (34.48%). This is higher as compared with the previous report (70%). [14] Average number of drugs to be found as causative for SJS, TEN, and SJS-TEN overlap was Though antimicrobials have been the most common group of drugs involved, there has been change in the individual drugs involved. Nevirapine and cotrimoxazole are the most common antimicrobial associated as compared with cephalosporins in an earlier study. [14] In all, 37.5% patients were HIV positive, which indicates increase in incidence of SJS and TEN in HIV patients, which was 7.3% in European study. [18] Nevirapine was the most common causative drug among the HIV patients as Mortality rate in study (%) Expected mortality rate [11] (%) or more >90 Figure 1: Complications noted in SJS, TEN, and SJS-TEN overlap (SJS: Stevens-Johnson syndrome; TEN: Toxic epidermal necrolysis; LFT: Liver function test) Journal of Postgraduate Medicine April 2011 Vol 57 Issue 2 117
5 compared with the cotrimoxazole. [8] However, of 7 culprit cases with cotrimoxazole, 6 patients were HIV positive. This shows higher incidence of SJS and TEN with cotrimoxazole in HIVpositive patients than in general populations. [8] In last few years, HIV infection might have changed the trend of antimicrobials causing SJS, TEN, and SJS-TEN overlap. In 4 HIV cases, reaction appeared after change in regimen of antiretroviral therapy from ZLE (Zidovudine, Lamivudine, Efavirenz) to ZLN (Zidovudine, Lamivudine, Nevirapine). Arrival of new drugs like nevirapine and lamotrigine have changed current scenario of causative drugs for SJS, TEN, and SJS-TEN overlap and risk of severe skin reaction increases with use of these drugs. [19] However, we did not have lamotrigine as culprit in our study. This may be due to its limited utilization or different environmental or genomic factors in our population. However, highest occurrence with nevirapine suggests the requirement of monitoring the newer drugs for such type of severe reactions. [19] Among NSAIDs, paracetamol was the most common to cause the reaction in our study and as described in other Indian study. [14] We did not find any oxicam derivative as culprit which is mentioned as the most commonly responsible among NSAIDs in American study. [20] This may be due to different genomic factors or drug utility pattern influencing both the populations. Duration of hospital stay in TEN is higher than in SJS, which is same as in previous study. [21] The risk of SJS, TEN, and SJS-TEN overlap appears to be greater in the first month of initiation of treatment as revealed by incubation period. Incubation period is nonsignificantly lower in the TEN as compared with SJS and SJS-TEN overlap. In our study, majority of cases were treated with systemic steroids, and mortality rate was found similar to previous study in which patients were treated with steroids. [14] Though the role of steroids is controversial in treatment of SJS and TEN, beneficial effect may be there if steroids are started early in treatment with proper dose. [22] IVIG was given in one patient with SCORTEN score 2 and had recovered within 8 days. We could not make any conclusion about the use of IVIG in treatment of SJS and TEN as it was used only in one patient in our study. Complications were seen more frequently in TEN group (63.4%), the most common being secondary infection (28.1%). One patient died in SJS group, whereas no mortality was noted in SJS-TEN overlap. Mortality rate in TEN group was 26.66%, which is equal to earlier study (26%). [14] Our study showed that mortality rate was higher in patients with higher SCORTEN score, which shows importance of SCORTEN score as an important tool for predicting the clinical outcome in patients of SJS, TEN, and SJS-TEN overlap. [11] Average cost of management of SJS (` 2 460/-) is nearly equal to average cost of management of other severe ADRs in India (` 2 820/-), but the cost of management of SJS-TEN overlap (` 4 857/-) and TEN (` 7 910/-) is much higher. [10] Being retrospective in nature, we were not able to calculate direct nonmedical and indirect costs. Thirty percent cases have been excluded from the study due to insufficient data or improper records. Proper record keeping is required for such type of study involving disease of low incidence rate. In conclusion, SJS, TEN, and SJS-TEN overlap are serious cutaneous adverse reactions most commonly caused by antimicrobials, NSAIDs, and antiseizure drugs. Their cost of management is higher than other serious ADRs. Acknowledgement Our sincere thanks to Dr. Neela Bhuptani, Professor and Head, Department of Dermatology, Venerology and Leprosy and Dr. R.B.Deshmukh, Medical Superintendent (P.D.U. Civil Hospital and Medical College, Rajkot); Dr. N.D.Kanthariya, Professor and Head, Department of Pharmacology and Dr. M.K.Vadel, Medical Superintendent (Government Medical College and New Civil Hospital, Surat) and Dr. Yogesh Marfatiya, Professor and Head, Department of Dermatology, Venerology and Leprosy and Dr. R.N. Deveshvar, Medical Superintendent (Medical College and S.S.G. Civil Hospital, Vadodara) for giving permission for the data collection at their respective centers. References 1. Patel KJ, Kedia MS, Bajpai D, Mehta SS, Kshirsagar NA, Gogtay NJ. Evaluation of the prevalence and economic burden of adverse drug reactions presenting to the medical emergency department of a tertiary referral centre: A prospective study. BMC Clin Pharmacol 2007;7:8. 2. Brvar M, Fokter N, Bunc M, Mozina M. The frequency of adverse drug reaction related admissions according to method of detection, admission urgency and medical department specialty. BMC Clin Pharmacol 2009;9:8. 3. Suh DC, Woodall BS, Shin SK, Hermes-De Santis ER. Clinical and economic impact of adverse drug reactions in hospitalized patients. Ann Pharmacother 2000;34: Sharma VK, Sethuraman G, Kumar B. Cutaneous adverse drug reactions: Clinical pattern and causative agents - a 6 year series from Chandigarh, India. J Postgrad Med 2001;47: Sharma VK, Sethuraman G. Adverse cutaneous reactions to drugs: An overview. J Postgrad Med 1996;42: Borchers AT, Lee JL, Naguwa SM, Cheema GS, Gershwin ME. Stevens-Johnson syndrome and toxic epidermal necrolysis. Autoimmun Rev 2008;7: Li LF, MaC. Epidemiological study of severe cutaneous adverse drug reactions in a city district in China. Clin Exp Dermatol 2006;31: Khambaty MM, Hsu SS. Dermatology of the patient with HIV. Emerg Med Clin North Am 2010;28: Mockenhaupt M. Severe drug-induced skin reactions: Clinical pattern, diagnostics and therapy. J Dtsch Dermatol Ges 2009;7: Ramesh M, Pandit J, Parthasarathi G. Adverse drug reactions in a South Indian hospital- their severity and cost involved. Pharmacoepidemiol drug saf 2003;12: Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: A severity-of-illness score of Toxic Epidermal Necrolysis. J Investi Dermatol 2000;112: Naranjo CA, Busto U, Seller EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reaction. Clin. Pharmacol Ther 1981;30: Schopf E, Stuhmer A, Rzany B, Victor N, Zentgraf R, Kapp JF. Toxic epidermal necrolysis and Stevens-Johnson syndrome: An epidemiologic study from West Germany. Arch Dermatol 1991;127: Sharma VK, Sethuraman G, Minz A. Stevens Johnson syndrome, toxic epidermal necrolysis and SJS-TEN overlap: A retrospective study of causative drugs and clinical outcome. Indian J Dermatol Venereol Leprol 2008;74: Devi K, George S, Criton S, Suja V, Sridevi PK. Carbamazepine--the commonest cause of toxic epidermal necrolysis and Stevens- Johnson syndrome: A study of 7 years. Indian J Dermatol Venereol Leprol 2005;71: Wanat KA, Anadkat MJ, Klekotka PA. Seasonal variation of Stevens- Johnson syndrome and toxic epidermal necrolysis associated with trimethoprim-sulfamethoxazole. J Am Acad Dermatol 2009;60: Journal of Postgraduate Medicine April 2011 Vol 57 Issue 2
6 17. Leenutaphong V, Sivayathorn A, Suthipinittharm P, Sunthonpalin P. Stevens-Johnson syndrome and toxic epidermal necrolysis in Thailand. Int J Dermatol 1993;32: Fagot JP, Mockenhaupt M, Bouwes-Bavinck JN, Naldi L, Viboud C, Roujeau JC. Nevirapine and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. AIDS 2001;15: Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 2008;128: Ward KE, Archambault R, Mersfelder TL. Severe adverse skin reactions to nonsteroidal antiinflammatory drugs: A review of the literature. Am J Health Syst Pharm 2010;67: Yap FBB, Wahiduzzaman M, Pubalan M. Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review. Egyptian Dermatol Online J 2008; Kardaun SH, Jonkman MF. Dexamethasone pulse therapy for Stevens- Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol 2007;87: How to cite this article: Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H, Tripathi C. Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: A multicentric retrospective study. J Postgrad Med 2011;57: Source of Support: Nil, Conflict of Interest: None declared. Author Help: Online submission of the manuscripts Articles can be submitted online from For online submission, the articles should be prepared in two files (first page file and article file). Images should be submitted separately. 1) First Page File: Prepare the title page, covering letter, acknowledgement etc. using a word processor program. All information related to your identity should be included here. Use text/rtf/doc/pdf files. Do not zip the files. 2) Article File: The main text of the article, beginning with the Abstract to References (including tables) should be in this file. Do not include any information (such as acknowledgement, your names in page headers etc.) in this file. Use text/rtf/doc/pdf files. Do not zip the files. Limit the file size to 1 MB. Do not incorporate images in the file. If file size is large, graphs can be submitted separately as images, without their being incorporated in the article file. This will reduce the size of the file. 3) Images: Submit good quality color images. Each image should be less than 4096 kb (4 MB) in size. The size of the image can be reduced by decreasing the actual height and width of the images (keep up to about 6 inches and up to about 1800 x 1200 pixels). JPEG is the most suitable file format. The image quality should be good enough to judge the scientific value of the image. For the purpose of printing, always retain a good quality, high resolution image. This high resolution image should be sent to the editorial office at the time of sending a revised article. 4) Legends: Legends for the figures/images should be included at the end of the article file. Journal of Postgraduate Medicine April 2011 Vol 57 Issue 2 119
Prevalence and pattern of adverse cutaneous drug reactions presenting to a tertiary care hospital
 International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
International Journal of Research in Dermatology Janardhan B et al. Int J Res Dermatol. 2017 Mar;3(1):74-78 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20164248
CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES Ragesh SJIF Impact Factor 2.786 Volume 3, Issue 6, 1599-1604. Case Study ISSN 2278 4357 CARBAMAZEPINE INDUCED STEVENS JOHNSON SYNDROME- A CASE STUDY
Correspondence should be addressed to Wanjarus Roongpisuthipong; rr
 Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Dermatology Research and Practice, Article ID 237821, 5 pages http://dx.doi.org/10.1155/2014/237821 Research Article Retrospective Analysis of Corticosteroid Treatment in Stevens-Johnson Syndrome and/or
Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course
 Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
Southern Adventist Univeristy KnowledgeExchange@Southern Graduate Research Projects Nursing 12-4-2015 Stevens Johnson Syndrome: How Diagnosis Impacts Disease Course Sharon K. Hart Southern Adventist University,
1 Introduction. Kenneth Irungu 1 David Nyamu
 Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Drugs - Real World Outcomes (2017) 4:79 85 DOI 10.1007/s40801-017-0105-x ORIGINAL RESEARCH ARTICLE Characterization of Stevens Johnson Syndrome and Toxic Epidermal Necrolysis Among Patients Admitted to
Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review
 Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review Review question(s) To carry out a systematic review of the published evidence of the cutaneous adverse drug reactions in
Title: Cutaneous Adverse Drug Reactions in Indian population: A systematic review Review question(s) To carry out a systematic review of the published evidence of the cutaneous adverse drug reactions in
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review
 Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
Stevens - Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) In Sarawak: A Four Years Review Yap FBB, MRCP; Wahiduzzaman M, MBBS; Pubalan M, MRCP. Egyptian Dermatology Online Journal 4 (1): 1,
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME
 EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
EFAVIRENZ ASSOCIATED STEVENS-JOHNSON SYNDROME To the Editor: Persons with human immunodeficiency virus (HIV) infection are highly susceptible to adverse dermatological reactions to specific medications
Address for correspondence: Dr. Patel Raksha M. R-3, Doctor s Quarters, Jail Road, Vadodara- 01, Gujarat, India.
 Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
Clinical study of cutaneous drug eruptions in 200 patients Raksha M. Patel, Y. S. Marfatia Department of Skin and V.D, Medical College, Vadodara, Gujarat, India Address for correspondence: Dr. Patel Raksha
Analysis of Cutaneous Adverse Drug Reactions at a Tertiary Care Hospital a Prospective Study
 Tropical Journal of Pharmaceutical Research August 2011; 10 (4): 517-522 Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online
Tropical Journal of Pharmaceutical Research August 2011; 10 (4): 517-522 Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online
Department of Pharmacology & NIPER-Guwahati, Gauhati Medical College & Hospital, Guwahati , Assam, INDIA. 3
 J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
J Young Pharm, 2016; 8(2): 149-153 A multifaceted peer reviewed journal in the field of Pharmacy www.jyoungpharm.org www.phcog.net Short Communication A study on drug induced Stevens-Johnson Syndrome (SJS),
A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive Patients
 Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
Journal of US-China Medical Science 12 (2015) 85-89 doi: 10.17265/1548-6648/2015.02.008 D DAVID PUBLISHING A Retrospective Study of Spectrum of Nevirapine Induced Cutaneous Drug Reactions in HIV Positive
PHARMACOVIGILANCE CELL Government Medical College, Bhavnagar, Gujarat.
 PHARMACOVIGILANCE CELL Introduction Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drugrelated problems.
PHARMACOVIGILANCE CELL Introduction Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drugrelated problems.
Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients
 original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
original article korean j intern med 2012;27:203-210 pissn 1226-3303 eissn 2005-6648 Causes and Treatment Outcomes of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in 82 Adult Patients Hye-In
Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care center in South India
 Study Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care center in South India David Pudukadan, Devinder Mohan Thappa Department of Dermatology and STD, Jawaharlal
Study Adverse cutaneous drug reactions: Clinical pattern and causative agents in a tertiary care center in South India David Pudukadan, Devinder Mohan Thappa Department of Dermatology and STD, Jawaharlal
IJBCP International Journal of Basic & Clinical Pharmacology
 Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20171661 Original Research Article A study of cutaneous
Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20171661 Original Research Article A study of cutaneous
allergy Asia Pacific Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from
 pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
pissn -876 eissn -868 Original Article Asia Pac Allergy 6;6:-7 Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 9 Oki Suwarsa *, Wulan
A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome
 Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Open Journal of Clinical & Medical Case Reports Volume 2 (2016) Issue 11 A Case Report on Amoxicillin Induced Stevens- Johnson Syndrome Rajendra Singh Airee*; Aastha Rawal; Binu Mathew; H. Doddayya Abstract
Toxic Epidermal Necrolysis - A Case Report
 The Journal of Critical Care Medicine 2017;3(1):29-33 CASE REPORT Toxic Epidermal Necrolysis - A Case Report Laura Stătescu¹, Magda Constantin², Horia Silviu Morariu³ *, Laura Gheucă Solovăstru¹ ¹ Dermatology
The Journal of Critical Care Medicine 2017;3(1):29-33 CASE REPORT Toxic Epidermal Necrolysis - A Case Report Laura Stătescu¹, Magda Constantin², Horia Silviu Morariu³ *, Laura Gheucă Solovăstru¹ ¹ Dermatology
Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital
 Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
Original article: Drug-induced stevens-johnson syndrome : Experience from a tertiary care hospital *Arjun R 1,Mahalingeshwara Bhat K P 2, Sara C 1 1 PG student in General Medicine, KS Hegde Medical Acadamy,
Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
 Case Reports in Immunology Volume 2012, Article ID 931528, 4 pages doi:10.1155/2012/931528 Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
Case Reports in Immunology Volume 2012, Article ID 931528, 4 pages doi:10.1155/2012/931528 Case Report Cephazolin-Induced Toxic Epidermal Necrolysis Treated with Intravenous Immunoglobulin and N-Acetylcysteine
cipro loxacin; stevens-johnson syndrome; urinary tract infection; adverse drug reaction
 Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
Open Journal of Clinical & Medical Case Reports Volume 3 (2017) Issue 7 ISSN 2379-1039 Cipro loxacin induced Stevens Johnson syndrome: A case report on Dermatology department P Mohammed Sha i*; K Narotham
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC
 SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
SEVERE CUTANEOUS ADVERSE DRUG REACTIONS: STEVENS-JOHNSON SYNDROME AND TOXIC EPIDERMAL NECROLYSISA, A REPORT OF 4 CASES SEEN AT UMMC Shasha Khairullah, Rokiah Che Ismail Department of Medicine, Faculty
Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes
 Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Acta Derm Venereol 2012; 92: 62 66 CLINICAL REPORT Profile and Pattern of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in a General Hospital in Singapore: Treatment Outcomes Siew-Kiang Tan and
Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis: a case report
 Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Kasahara et al. BMC Nephrology (2017) 18:237 DOI 10.1186/s12882-017-0648-9 CASE REPORT Open Access Warfarin-induced toxic epidermal necrolysis in combination therapy of Henoch- Schönlein purpura nephritis:
Pediatric Dermatology
 Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
Pediatric Dermatology --------- Emergencies & Urgencies Nicholas V. Nguyen, M.D. Director, Pediatric Dermatology Disclosures In the past 12 months, I have had the following financial relationships with
The prevalence of Stevens Johnson Syndrome caused by antiretroviral in hospitalized patients at Dr. Hasan Sadikin General Hospital Bandung
 The prevalence of Stevens Johnson Syndrome caused by antiretroviral in hospitalized patients at Dr. Hasan Sadikin General Hospital Bandung Nurmilah Maelani*, Irna Sufiawati*, Hartati Purbo Darmadji** *Department
The prevalence of Stevens Johnson Syndrome caused by antiretroviral in hospitalized patients at Dr. Hasan Sadikin General Hospital Bandung Nurmilah Maelani*, Irna Sufiawati*, Hartati Purbo Darmadji** *Department
Dilantin (phenytoin) ROBERT A. SCHWARTZ
 Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Dilantin (phenytoin) ROBERT A. SCHWARTZ Bailey & Galyen Attorney in Charge, Mass Tort Litigation Managing Attorney, Houston 18333 Egret Bay Blvd., Suite 120 Houston, Texas 77058 Toll Free: (866) 715-1529
Stevens-Johnson syndrome (SJS) and toxic epidermal. necrolysis in Asian children
 Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children Mark Jean-Aan Koh, MD, and Yong-Kwang Tay, MD Singapore Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children Mark Jean-Aan Koh, MD, and Yong-Kwang Tay, MD Singapore Background: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis
International Journal of Pharma and Bio Sciences
 Original Research Article Pharmacology International Journal of Pharma and Bio Sciences ISSN 0975-6299 ASSESSMENT OF ECONOMIC IMPACT AND DEVELOPMENT OF A PREDICTOR MODEL FOR TOTAL COST DUE TO ADVERSE DRUG
Original Research Article Pharmacology International Journal of Pharma and Bio Sciences ISSN 0975-6299 ASSESSMENT OF ECONOMIC IMPACT AND DEVELOPMENT OF A PREDICTOR MODEL FOR TOTAL COST DUE TO ADVERSE DRUG
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS
 PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
PHENYTION, CARBAMAZEPINE, SODIUM VALPROATE AND LAMOTRIGINE INDUCED CUTANEOUS REACTIONS M. Ghaffarpour *, S. S. Hejazie, M. H. Harirchian and H. Pourmahmoodian Department of Neurology, Imam Khomeini Hospital,
Drug Allergy A Guide to Diagnosis and Management
 Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Drug Allergy A Guide to Diagnosis and Management (Version 1 April 2015 updated April 2018) Author: Jed Hewitt Chief Pharmacist, Governance & Professional Practice Date of Preparation: April 2015 Updated:
Emergency Dermatology Dr Melissa Barkham
 Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Emergency Dermatology Dr Melissa Barkham Spotlight Seminar 30 th September 2010 Why is this important? Urgent recognition and treatment of dermatologic emergencies can be life saving and prevent long term
Adverse Drug Reactions (ADRs) Outline
 Adverse Drug Reactions (ADRs) Outline 1. What are Adverse Drug Reactions (ADRs)? WHAT WHY HOW 2. How important are ADRs and are they preventable? 3. What are the classifications and mechanisms of ADRs?
Adverse Drug Reactions (ADRs) Outline 1. What are Adverse Drug Reactions (ADRs)? WHAT WHY HOW 2. How important are ADRs and are they preventable? 3. What are the classifications and mechanisms of ADRs?
Influence of Diet and NSAIMs in Allergic Skin Reactions
 Influence of Diet and NSAIMs in Allergic Skin Reactions Dixon Thomas, Molly Mathew, C. Vijay Raghavan Vol. 3 No. 6 (June 2011) International Journal of Collaborative Research on Internal Medicine & Public
Influence of Diet and NSAIMs in Allergic Skin Reactions Dixon Thomas, Molly Mathew, C. Vijay Raghavan Vol. 3 No. 6 (June 2011) International Journal of Collaborative Research on Internal Medicine & Public
Adverse Drug Reactions pattern in a tertiary level teaching hospital: A Retrospective Study
 Original Article Adverse Drug Reactions pattern in a tertiary level teaching hospital: A Retrospective Study Anita Gupta, Anjleen Kaur, Prashant Shukla*, Hema Chhabra Department of Pharmacology, Government
Original Article Adverse Drug Reactions pattern in a tertiary level teaching hospital: A Retrospective Study Anita Gupta, Anjleen Kaur, Prashant Shukla*, Hema Chhabra Department of Pharmacology, Government
HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review
 HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
HIV Clinical Management HIV-Associated Toxic Epidermal Necrolysis at San Francisco General Hospital: A 13-Year Retrospective Review Journal of the International Association of Providers of AIDS Care 2017,
Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from 2000 to 2006 Yumiko Yamane 1, Michiko Aihara 1 and Zenro Ikezawa 1
 Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
Allergology International. 7;:9- DOI:. allergolint.o-7- ORIGINAL ARTICLE Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from to Yumiko Yamane, Michiko Aihara and Zenro Ikezawa
REVIEW ARTICLES ADVERSE DRUG REACTION: A COMMON DERMATOLOGICAL EMERGENCY
 REVIEW ARTICLES ADVERSE DRUG REACTION: A COMMON DERMATOLOGICAL EMERGENCY LUBNA KHONDKER 1, MD ABDUL WAHAB 2, MD SHIRAJUL ISLAM KHAN 3. Abstract In the evaluation of patients with a history of adverse cutaneous
REVIEW ARTICLES ADVERSE DRUG REACTION: A COMMON DERMATOLOGICAL EMERGENCY LUBNA KHONDKER 1, MD ABDUL WAHAB 2, MD SHIRAJUL ISLAM KHAN 3. Abstract In the evaluation of patients with a history of adverse cutaneous
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update
 Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
Stevens-Johnson s Syndrome / Toxic Epidermal Necrolysis: An update Robert G. Micheletti, MD Assistant Professor of Dermatology and Medicine Director, Cutaneous Vasculitis Clinic, Penn Vasculitis Center
GENERAL PROFILE AND SURVIVAL PROBABILITIES OF HIV PATIENTS REGISTERED AT ANTI RETROVIRAL THERAPY CENTRE, NEW CIVIL HOSPITAL, SURAT, GUJARAT
 Original article GENERAL PROFILE AND SURVIVAL PROBABILITIES OF HIV PATIENTS REGISTERED AT ANTI RETROVIRAL THERAPY CENTRE, NEW CIVIL HOSPITAL, SURAT, GUJARAT Sridhar P Ryavanki 1, J K Kosambiya 2, Alap
Original article GENERAL PROFILE AND SURVIVAL PROBABILITIES OF HIV PATIENTS REGISTERED AT ANTI RETROVIRAL THERAPY CENTRE, NEW CIVIL HOSPITAL, SURAT, GUJARAT Sridhar P Ryavanki 1, J K Kosambiya 2, Alap
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT
 OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
OXCARBAZEPINE-INDUCED STEVENS-JOHNSON SYNDROME: A CASE REPORT Lung-Chang Lin, 1,2 Ping-Chin Lai, 3 Sheau-Fang Yang, 4 and Rei-Cheng Yang 1,5 Departments of 1 Pediatrics and 4 Pathology, Kaohsiung Medical
The liver and skin are two of the commonest
 AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 3, 2016 LIVER INJURY/REGENERATION Drug-Induced Liver Injury Associated With Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis:
PEER REVIEW HISTORY ARTICLE DETAILS VERSION 1 - REVIEW
 PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
PEER REVIEW HISTORY BMJ Paediatrics Open publishes all reviews undertaken for accepted manuscripts. Reviewers are asked to complete a checklist review form and are provided with free text boxes to elaborate
Severe cutaneous adverse reactions due to isoniazid in a HIV Positive patient
 Indian J Lepr 202, 84 : 227-232 Hind Kusht Nivaran Sangh, New Delhi http://www.ijl.org.in Case Report Severe cutaneous adverse reactions due to isoniazid in a HIV Positive patient BK Viswanath, P Ranka,
Indian J Lepr 202, 84 : 227-232 Hind Kusht Nivaran Sangh, New Delhi http://www.ijl.org.in Case Report Severe cutaneous adverse reactions due to isoniazid in a HIV Positive patient BK Viswanath, P Ranka,
Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review
 140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
140 Taiwanese Journal of Psychiatry (Taipei) Vol. 25 No. 3 2011 Overview Risk Factors and Management of Mood Stabilizer-associated Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Mini-review Gen-Tang
A Case Report on Paracetamol Induced Generalized Fixed Drug Eruptions
 Human Journals Case report November 2016 Vol.:7, Issue:4 All rights are reserved by Dr. C Sugunakar Raju et al. A Case Report on Paracetamol Induced Generalized Fixed Drug Eruptions Keywords: Skin Eruptions,
Human Journals Case report November 2016 Vol.:7, Issue:4 All rights are reserved by Dr. C Sugunakar Raju et al. A Case Report on Paracetamol Induced Generalized Fixed Drug Eruptions Keywords: Skin Eruptions,
Severe cutaneous adverse reactions (SCAR) are life-threatening
 Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Stevens-Johnson syndrome and toxic epidermal necrolysis: clinical patterns, diagnostic considerations, etiology, and therapeutic management Maja Mockenhaupt, MD, PhD n Abstract Severe cutaneous adverse
Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate
 Original Research Article Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate Manab Nandy 1, Sangeeta De 2*, Mustafa Asad 2, Nirmal
Original Research Article Analysis of causation of Stevens Johnson Syndrome in a patient of rheumatoid arthritis with increased dose of methotrexate Manab Nandy 1, Sangeeta De 2*, Mustafa Asad 2, Nirmal
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES. R e g i S C A R PATIENT'S DATA. Age country of birth
 REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
REGISTRY OF SEVERE CUTANEOUS ADVERSE REACTIONS TO DRUGS AND COLLECTION OF BIOLOGICAL SAMPLES R e g i S C A R PATIENT'S DATA Initials of the patient date of birth Age country of birth Gender male female
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea
 Original Article Yonsei Med J 2019 Feb;60(2):208-215 pissn: 0513-5796 eissn: 1976-2437 Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea Min-Gyu Kang 1,2,3, Kyung-Hee
Original Article Yonsei Med J 2019 Feb;60(2):208-215 pissn: 0513-5796 eissn: 1976-2437 Analysis of Individual Case Safety Reports of Severe Cutaneous Adverse Reactions in Korea Min-Gyu Kang 1,2,3, Kyung-Hee
Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics
 MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
MAJOR ARTICLE Severe Cutaneous Adverse Reactions Related to Systemic Antibiotics Ying-Fang Lin, 1 Chih-Hsun Yang, 2,3 Hu Sindy, 1,3 Jing-Yi Lin, 2,3 Chung-Yee Rosaline Hui, 2,3 Yun-Chen Tsai, 2 Ting-Shu
Drug eruptions and hepatic involvement: a study
 International Journal of Research in Dermatology http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20183819 Drug eruptions and hepatic involvement:
International Journal of Research in Dermatology http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20183819 Drug eruptions and hepatic involvement:
A study of adverse reaction algorithms in a drug surveillance program
 A study of adverse reaction algorithms in a drug surveillance program To improve agreement among observers, several investigators have recently proposed methods (algorithms) to standardize assessments
A study of adverse reaction algorithms in a drug surveillance program To improve agreement among observers, several investigators have recently proposed methods (algorithms) to standardize assessments
Scratching the Surface: A Review of SJS/TEN
 Scratching the Surface: A Review of SJS/TEN Sarah Smith, PharmD Pharmacy Grand Rounds 2018 November 27, 2018 2018 MFMER slide 1 Toxic Epidermal Necrolysis Cutaneous Anthrax Stevens Johnson Syndrome Necrotizing
Scratching the Surface: A Review of SJS/TEN Sarah Smith, PharmD Pharmacy Grand Rounds 2018 November 27, 2018 2018 MFMER slide 1 Toxic Epidermal Necrolysis Cutaneous Anthrax Stevens Johnson Syndrome Necrotizing
Cutaneous adverse drug reactions in a tertiary care hospital
 Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 202, 4 (2):408-43 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-507 USA CODEN: DPLEB4 Cutaneous
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 202, 4 (2):408-43 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-507 USA CODEN: DPLEB4 Cutaneous
Stevens-Johnson Syndrome : A Case Report
 https://doi.org/10.5933/jkapd.2017.44.4.455 J Korean Acad Pediatr Dent 44(4) 2017 ISSN (print) 1226-8496 ISSN (online) 2288-3819 Stevens-Johnson Syndrome : A Case Report Yongho Song, Nanyoung Lee, Sangho
https://doi.org/10.5933/jkapd.2017.44.4.455 J Korean Acad Pediatr Dent 44(4) 2017 ISSN (print) 1226-8496 ISSN (online) 2288-3819 Stevens-Johnson Syndrome : A Case Report Yongho Song, Nanyoung Lee, Sangho
A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia
 ORIGINAL ARTICLE A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Chai Har Loo, MRCP, Wooi Chiang Tan, AdvMDerm, Yek Huan Khor, AdvMDerm,
ORIGINAL ARTICLE A 10-years retrospective study on Severe Cutaneous Adverse Reactions (SCARs) in a tertiary hospital in Penang, Malaysia Chai Har Loo, MRCP, Wooi Chiang Tan, AdvMDerm, Yek Huan Khor, AdvMDerm,
Research Pattern of adverse drug reactions reported to the regional Pharmacovigilance center at Nepal Medical College and Teaching Hospital, Kathmandu
 Research Pattern of adverse drug reactions reported to the regional Pharmacovigilance center at Nepal Medical College and Teaching Hospital, Kathmandu Durga Bista 1*, Bal Ram Shrestha 2, Prakash Rai 1,
Research Pattern of adverse drug reactions reported to the regional Pharmacovigilance center at Nepal Medical College and Teaching Hospital, Kathmandu Durga Bista 1*, Bal Ram Shrestha 2, Prakash Rai 1,
Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening
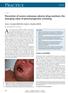 CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
CMAJ Cases Prevention of severe cutaneous adverse drug reactions: the emerging value of pharmacogenetic screening Suran L. Fernando MB BS PhD, Andrew J. Broadfoot MB BS Previously published at www.cmaj.ca
Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients
 Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Bhargavi
Spectrum of Cutaneous Adverse Reactions to Levetiracetam and Human Leukocyte Antigen Typing in North-Indian Patients Original Article Journal of Epilepsy Research pissn 2233-6249 / eissn 2233-6257 Bhargavi
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW
 STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
STEVEN JOHNSON S SYNDROME: A BRIEF REVIEW G. Ramya sree*, Sreeram Vandavasi Guru 1, E. Sam Jeeva Kumar 2 * Pharm D, Intern, P. Rami Reddy Memorial College of Pharmacy, Kadapa. 1 Pharm-D Intern, P. Rami
PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN
 PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN Summary of Risk Management Plan for REZOLSTA This is a summary of the risk management plan (RMP) for REZOLSTA. The RMP details important risks of REZOLSTA,
PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN Summary of Risk Management Plan for REZOLSTA This is a summary of the risk management plan (RMP) for REZOLSTA. The RMP details important risks of REZOLSTA,
A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit
 J Korean Surg Soc 0;:133-139 DOI:.414/jkss.0..3.133 원 저 A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit Departments of Surgery
J Korean Surg Soc 0;:133-139 DOI:.414/jkss.0..3.133 원 저 A Clinical Study of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: Efficacy of Treatment in Burn Intensive Care Unit Departments of Surgery
Cutaneous manifestations and systemic correlation in patients with lupus erythematosus and its subsets: a study of 40 cases
 International Journal of Research in Dermatology Mahajan R et al. Int J Res Dermatol. 2018 Nov;4(4):479-483 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20183407
International Journal of Research in Dermatology Mahajan R et al. Int J Res Dermatol. 2018 Nov;4(4):479-483 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20183407
Skin Manifestations of Drug Reactions
 Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Skin Manifestations of Drug Reactions Dr Carol Hlela, Division of Dermatology Department of Medicine, University of Cape Town and Red Cross Children s Hospital What are the Skin Manifestations of Drug
Cutaneous drug reactions
 Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Maintenance of Certification clinical management series Series editor: James T. Li, MD, PhD Cutaneous drug reactions David A. Khan, MD Dallas, Tex INSTRUCTIONS Credit can now be obtained, free for a limited
Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis
 Acta Derm Venereol 2007; 87: 144 148 CLINICAL REPORT Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis Sylvia H. Kardaun and Marcel F. Jonkman Center for Blistering Diseases,
Acta Derm Venereol 2007; 87: 144 148 CLINICAL REPORT Dexamethasone Pulse Therapy for Stevens-Johnson Syndrome/ Toxic Epidermal Necrolysis Sylvia H. Kardaun and Marcel F. Jonkman Center for Blistering Diseases,
Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions
 Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions Jeremy A. Schneider, MD Assistant Clinical Professor UC San Diego Department of Dermatology Objectives Brief overview of some of
Inpatient Consultative Dermatology and Severe Cutaneous Adverse Reactions Jeremy A. Schneider, MD Assistant Clinical Professor UC San Diego Department of Dermatology Objectives Brief overview of some of
A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash
 CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
CLINICAL CASE OF THE MONTH A Middle-Aged Man with Newly Diagnosed HIV Infection and Rash Patrick Njoku, MD; Temeka Tate, MD; Sousan Zadeh, MD; Erin Hauck, MD; Anila Chaudhry, MD; Betty Lo-Blais, MD; Lee
Toxic epidermal necrolysis and Stevens Johnson syndrome in South Africa: a 3-year prospective study
 Q J Med 2012; 105:839 846 doi:10.1093/qjmed/hcs078 Advance Access Publication 28 April 2012 Toxic epidermal necrolysis and Stevens Johnson syndrome in South Africa: a 3-year prospective study S.M.H. KANNENBERG
Q J Med 2012; 105:839 846 doi:10.1093/qjmed/hcs078 Advance Access Publication 28 April 2012 Toxic epidermal necrolysis and Stevens Johnson syndrome in South Africa: a 3-year prospective study S.M.H. KANNENBERG
Future of Pediatrics: Blisters, Hives and Other Tales from the Emergency Room June 14 th, 2016
 A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
A. Yasmine Kirkorian MD Assistant Professor of Dermatology & Pediatrics Children s National Health System George Washington University School of Medicine & Health Sciences Future of Pediatrics: Blisters,
Toxic epidermal necrolysis
 REVIEW Anaesthesiology Intensive Therapy 2015, vol. 47, no 3, 257 262 ISSN 0209 1712 10.5603/AIT.2015.0037 www.ait.viamedica.pl Toxic epidermal necrolysis Joanna Hinc-Kasprzyk, Agnieszka Polak-Krzemińska,
REVIEW Anaesthesiology Intensive Therapy 2015, vol. 47, no 3, 257 262 ISSN 0209 1712 10.5603/AIT.2015.0037 www.ait.viamedica.pl Toxic epidermal necrolysis Joanna Hinc-Kasprzyk, Agnieszka Polak-Krzemińska,
Oral Acetazolamide after Boston Keratoprosthesis in Stevens Johnson Syndrome
 Oral Acetazolamide after Boston Keratoprosthesis in Stevens Johnson Syndrome The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters
Oral Acetazolamide after Boston Keratoprosthesis in Stevens Johnson Syndrome The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters
Stevens-Johnson Syndrome in patients receiving cranial irradiation and concomitant diphenylhydantoin
 Turkish Journal of Cancer Vol. 32/ No. 4/2002 Stevens-Johnson Syndrome in patients receiving cranial irradiation and concomitant diphenylhydantoin ERKAN TOPKAN, FARUK ZORLU, MURAT GÜRKAYNAK Hacettepe University
Turkish Journal of Cancer Vol. 32/ No. 4/2002 Stevens-Johnson Syndrome in patients receiving cranial irradiation and concomitant diphenylhydantoin ERKAN TOPKAN, FARUK ZORLU, MURAT GÜRKAYNAK Hacettepe University
To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving
 Present : Dr Pham Thi Minh Rang Internal Department No2-Hospital for children No2 AIMS To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving To
Present : Dr Pham Thi Minh Rang Internal Department No2-Hospital for children No2 AIMS To update the use of IVIG and CORTICOIDS IN management of SJS/ TEN To remind Doctors being careful when giving To
Emergency Dermatology. Emergency Dermatology
 Emergency Dermatology These are rapidly progressive skin conditions and some are potentially lifethreatening. Early recognition is important to implement prompt supportive care and therapy. Some are drug
Emergency Dermatology These are rapidly progressive skin conditions and some are potentially lifethreatening. Early recognition is important to implement prompt supportive care and therapy. Some are drug
Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: Assessment of Medication Risks with Emphasis on Recently Marketed Drugs. The EuroSCAR-Study
 ORIGINAL ARTICLE Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: Assessment of Medication Risks with Emphasis on Recently Marketed Drugs. The EuroSCAR-Study Maja Mockenhaupt 1, Cecile Viboud 2,
ORIGINAL ARTICLE Stevens Johnson Syndrome and Toxic Epidermal Necrolysis: Assessment of Medication Risks with Emphasis on Recently Marketed Drugs. The EuroSCAR-Study Maja Mockenhaupt 1, Cecile Viboud 2,
Cutaneous Adverse Drug Reactions in Domestic Animals. Katherine Doerr, DVM, Dip. ACVD. Veterinary Dermatology Center
 Cutaneous Adverse Drug Reactions in Domestic Animals Katherine Doerr, DVM, Dip. ACVD Veterinary Dermatology Center Maitland, Rockledge, Waterford Lakes, FL Not highly studied in veterinary medicine Unknown
Cutaneous Adverse Drug Reactions in Domestic Animals Katherine Doerr, DVM, Dip. ACVD Veterinary Dermatology Center Maitland, Rockledge, Waterford Lakes, FL Not highly studied in veterinary medicine Unknown
Original Research Article. Ishan Pandya 1, Purna Pandya 2 *, Neha Pethani 3, Rima Bharatbhai Shah 2
 International Journal of Research in Dermatology Pandya I et al. Int J Res Dermatol. 2017 Jun;3(2):228-233 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20172202
International Journal of Research in Dermatology Pandya I et al. Int J Res Dermatol. 2017 Jun;3(2):228-233 http://www.ijord.com Original Research Article DOI: http://dx.doi.org/10.18203/issn.2455-4529.intjresdermatol20172202
DRUG UTILIZATION STUDIES TO GUIDE BETTER HOSPITAL PHARMACEUTICAL POLICY
 DRUG UTILIZATION STUDIES TO GUIDE BETTER HOSPITAL PHARMACEUTICAL POLICY Dr. G. Parthasarathi Professor, Pharmacy Practice JSS University, Mysore 30 October 2011 ACPE6 Beijing, 2011 1 Presentation Outline
DRUG UTILIZATION STUDIES TO GUIDE BETTER HOSPITAL PHARMACEUTICAL POLICY Dr. G. Parthasarathi Professor, Pharmacy Practice JSS University, Mysore 30 October 2011 ACPE6 Beijing, 2011 1 Presentation Outline
A retrospective study of the pattern of sexually transmitted diseases during a ten-year period METHODS
 Study A retrospective study of the pattern of sexually transmitted diseases during a ten-year period Beena Narayanan Department of Dermatology and Venereology, Medical College Hospital, Kottayam, Kerala,
Study A retrospective study of the pattern of sexually transmitted diseases during a ten-year period Beena Narayanan Department of Dermatology and Venereology, Medical College Hospital, Kottayam, Kerala,
Prescribing for substance misuse: alcohol detoxification. Clinical background
 Prescribing for substance misuse: alcohol detoxification POMH-UK Quality Improvement Programme. Topic 14a: baseline Clinical background 1 2014 The Royal College of Psychiatrists. For further information
Prescribing for substance misuse: alcohol detoxification POMH-UK Quality Improvement Programme. Topic 14a: baseline Clinical background 1 2014 The Royal College of Psychiatrists. For further information
Your letter Your reference The Hague, Subject Request for change in the product information following the PhVWP/CMDh decision
 Registratiehouder ( t.a.v. registratieafdeling) Your letter Your reference The Hague, -- --.. Case number Our reference../../ Case manager Telephone number Subject Request for change in the product information
Registratiehouder ( t.a.v. registratieafdeling) Your letter Your reference The Hague, -- --.. Case number Our reference../../ Case manager Telephone number Subject Request for change in the product information
Changing Trends of Leprosy in Post Elimination Era - A Study from an Endemic Area
 Indian J Lepr 2017, 89 : 23-27 Hind Kusht Nivaran Sangh, New Delhi http://www.ijl.org.in Original Article Changing Trends of Leprosy in Post Elimination Era - A Study from an Endemic Area 1 2 3 R Murugaiyan,
Indian J Lepr 2017, 89 : 23-27 Hind Kusht Nivaran Sangh, New Delhi http://www.ijl.org.in Original Article Changing Trends of Leprosy in Post Elimination Era - A Study from an Endemic Area 1 2 3 R Murugaiyan,
nevirapine, stavudine and lamivudine
 nevirapine, stavudine and lamivudine Information on starting treatment with: nevirapine (NVP) + stavudine (d4t) + lamivudine (3TC) Nevirapine, stavudine and lamivudine (non-fixed dose) 2 Lead in dosing
nevirapine, stavudine and lamivudine Information on starting treatment with: nevirapine (NVP) + stavudine (d4t) + lamivudine (3TC) Nevirapine, stavudine and lamivudine (non-fixed dose) 2 Lead in dosing
Cutaneous Conditions Associated with Systemic Disease
 Cutaneous Conditions Associated with Systemic Disease Johnnie M Woodson, M.D., F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates,
Cutaneous Conditions Associated with Systemic Disease Johnnie M Woodson, M.D., F.A.A.D. Assistant Professor of Dermatology University of Nevada School of Medicine Director of J. Woodson Dermatology & Associates,
Severe cutaneous adverse reactions: an evidence based approach
 Original Article 21 Severe cutaneous adverse reactions: an evidence based approach D.P. Shrestha, D. Gurung, A. Kumar Department of Dermatology and Venereology, Maharagunj Campus, Institute of Medicine,
Original Article 21 Severe cutaneous adverse reactions: an evidence based approach D.P. Shrestha, D. Gurung, A. Kumar Department of Dermatology and Venereology, Maharagunj Campus, Institute of Medicine,
Accepted Manuscript. Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need
 Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
Accepted Manuscript Wound Management Strategies in Stevens-Johnson syndrome/toxic Epidermal Necrolysis: An unmet need Lee Haur Yueh, MBBS, MRCP, MMed, FAMS PII: S0190-9622(18)32138-8 DOI: 10.1016/j.jaad.2018.05.1258
IJBCP International Journal of Basic & Clinical Pharmacology
 Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20162852 Research Article Prescription audit and
Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20162852 Research Article Prescription audit and
Clinico-etiological study of pyodermas in a tertiary care hospital
 Original Research Article Purnachandra Badabagni 1, Shashikant Malkud 2,* 1 Associate Professor, 2 Assistant Professor, Dept. of Dermatology, Venereology and Leprosy, MNR Medical College & Hospital, Sangareddy,
Original Research Article Purnachandra Badabagni 1, Shashikant Malkud 2,* 1 Associate Professor, 2 Assistant Professor, Dept. of Dermatology, Venereology and Leprosy, MNR Medical College & Hospital, Sangareddy,
JMSCR Vol 05 Issue 10 Page October 2017
 www.jmscr.igmpublication.org Impact Factor 5.84 Index Copernicus Value: 71.58 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v5i10.125 Histomorphological Study of Lichen Planus
www.jmscr.igmpublication.org Impact Factor 5.84 Index Copernicus Value: 71.58 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v5i10.125 Histomorphological Study of Lichen Planus
Clinical profile of skin diseases in accident and emergency department attenders
 Hong Kong J. Dermatol. Venereol. (2007) 15, 4-9 Original Article Clinical profile of skin diseases in accident and emergency department attenders CY Chan, KL Kam, CA Graham, TH Rainer, NM Luk Skin problems
Hong Kong J. Dermatol. Venereol. (2007) 15, 4-9 Original Article Clinical profile of skin diseases in accident and emergency department attenders CY Chan, KL Kam, CA Graham, TH Rainer, NM Luk Skin problems
Department of Dermatology, Christian Medical College and Hospital, Ludhiana, Punjab, India.
 Bullous pemphigoid mimicking granulomatous inflammation Abhilasha Williams, Emy Abi Thomas. Department of Dermatology, Christian Medical College and Hospital, Ludhiana, Punjab, India. Egyptian Dermatology
Bullous pemphigoid mimicking granulomatous inflammation Abhilasha Williams, Emy Abi Thomas. Department of Dermatology, Christian Medical College and Hospital, Ludhiana, Punjab, India. Egyptian Dermatology
Cutaneous Drug Reactions
 Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
Cutaneous Drug Reactions Andrei Metelitsa, MD, FRCPC, FAAD Co-Director, Institute for Skin Advancement Clinical Associate Professor, Dermatology University of Calgary, Canada Copyright 2017 by Sea Courses
DERMATOLOGICAL EMERGENCIES. DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE
 DERMATOLOGICAL EMERGENCIES DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE Dermatological Emergencies INFECTIONS ERYTHRODERMA DRUG ERUPTIONS STEVENS-JOHNSON
DERMATOLOGICAL EMERGENCIES DR. Ian Hoyle MBBS DIP IMC RCS (Ed), DA (UK),FRACGP,FACRRM,DIP DERM(Wales) TASMANIAN SKIN AND BODY CENTRE Dermatological Emergencies INFECTIONS ERYTHRODERMA DRUG ERUPTIONS STEVENS-JOHNSON
A Drug Utilization Study in the Indoor Ward of the Surgery Department of a Tertiary Care Hospital of Eastern India.
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 14, Issue 10 Ver. VIII (Oct. 2015), PP 42-47 www.iosrjournals.org A Drug Utilization Study in the Indoor
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 14, Issue 10 Ver. VIII (Oct. 2015), PP 42-47 www.iosrjournals.org A Drug Utilization Study in the Indoor
Determination of Incidence and Characteristics of Preventable Adverse Drug Reactions : A Study in Phrae Hospital
 55 Determination of Incidence and Characteristics of Preventable Adverse Drug Reactions : A Study in Phrae Hospital Kannika Thiankhanithikun* and Sayam Kaewvichit Faculty of Pharmacy, Chiang Mai University,
55 Determination of Incidence and Characteristics of Preventable Adverse Drug Reactions : A Study in Phrae Hospital Kannika Thiankhanithikun* and Sayam Kaewvichit Faculty of Pharmacy, Chiang Mai University,
International Journal of Basic & Clinical Pharmacology. Profile of cutaneous adverse drug reactions of carbamazepine
 Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20175211 Original Research Article Profile of cutaneous
Print ISSN: 2319-2003 Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20175211 Original Research Article Profile of cutaneous
